Abstract
Background
Nausea and vomiting are distressing symptoms which are experienced commonly during caesarean section under regional anaesthesia and in the postoperative period.
Objectives
To assess the efficacy of pharmacological and non‐pharmacological interventions versus placebo or no intervention given prophylactically to prevent nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (16 April 2020), and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials (RCTs) of studies and conference abstracts, and excluded quasi‐RCTs and cross‐over studies.
Data collection and analysis
Review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. Our primary outcomes are intraoperative and postoperative nausea and vomiting. Data entry was checked. Two review authors independently assessed the certainty of the evidence using the GRADE approach.
Main results
Eighty‐four studies (involving 10,990 women) met our inclusion criteria. Sixty‐nine studies, involving 8928 women, contributed data. Most studies involved women undergoing elective caesarean section. Many studies were small with unclear risk of bias and sometimes few events. The overall certainty of the evidence assessed using GRADE was moderate to very low.
5‐HT3 antagonists: We found intraoperative nausea may be reduced by 5‐HT3 antagonists (average risk ratio (aRR) 0.55, 95% confidence interval (CI) 0.42 to 0.71, 12 studies, 1419 women, low‐certainty evidence). There may be a reduction in intraoperative vomiting but the evidence is very uncertain (aRR 0.46, 95% CI 0.29 to 0.73, 11 studies, 1414 women, very low‐certainty evidence). There is probably a reduction in postoperative nausea (aRR 0.40, 95% CI 0.30 to 0.54, 10 studies, 1340 women, moderate‐certainty evidence), and these drugs may show a reduction in postoperative vomiting (aRR 0.47, 95% CI 0.31 to 0.69, 10 studies, 1450 women, low‐certainty evidence).
Dopamine antagonists: We found dopamine antagonists may reduce intraoperative nausea but the evidence is very uncertain (aRR 0.38, 95% CI 0.27 to 0.52, 15 studies, 1180 women, very low‐certainty evidence). Dopamine antagonists may reduce intraoperative vomiting (aRR 0.41, 95% CI 0.28 to 0.60, 12 studies, 942 women, low‐certainty evidence) and postoperative nausea (aRR 0.61, 95% CI 0.48 to 0.79, 7 studies, 601 women, low‐certainty evidence). We are uncertain if dopamine antagonists reduce postoperative vomiting (aRR 0.63, 95% CI 0.44 to 0.92, 9 studies, 860 women, very low‐certainty evidence).
Corticosteroids (steroids): We are uncertain if intraoperative nausea is reduced by corticosteroids (aRR 0.56, 95% CI 0.37 to 0.83, 6 studies, 609 women, very low‐certainty evidence) similarly for intraoperative vomiting (aRR 0.52, 95% CI 0.31 to 0.87, 6 studies, 609 women, very low‐certainty evidence). Corticosteroids probably reduce postoperative nausea (aRR 0.59, 95% CI 0.49 to 0.73, 6 studies, 733 women, moderate‐certainty evidence), and may reduce postoperative vomiting (aRR 0.68, 95% CI 0.49 to 0.95, 7 studies, 793 women, low‐certainty evidence).
Antihistamines: Antihistamines may have little to no effect on intraoperative nausea (RR 0.99, 95% CI 0.47 to 2.11, 1 study, 149 women, very low‐certainty evidence) or intraoperative vomiting (no events in the one study of 149 women). Antihistamines may reduce postoperative nausea (aRR 0.44, 95% CI 0.30 to 0.64, 4 studies, 514 women, low‐certainty evidence), however, we are uncertain whether antihistamines reduce postoperative vomiting (average RR 0.48, 95% CI 0.29 to 0.81, 3 studies, 333 women, very low‐certainty evidence).
Anticholinergics: Anticholinergics may reduce intraoperative nausea (aRR 0.67, 95% CI 0.51 to 0.87, 4 studies, 453 women, low‐certainty evidence) but may have little to no effect on intraoperative vomiting (aRR 0.79, 95% CI 0.40 to 1.54, 4 studies; 453 women, very low‐certainty evidence). No studies looked at anticholinergics in postoperative nausea, but they may reduce postoperative vomiting (aRR 0.55, 95% CI 0.41 to 0.74, 1 study, 161 women, low‐certainty evidence).
Sedatives: We found that sedatives probably reduce intraoperative nausea (aRR 0.65, 95% CI 0.51 to 0.82, 8 studies, 593 women, moderate‐certainty evidence) and intraoperative vomiting (aRR 0.35, 95% CI 0.24 to 0.52, 8 studies, 593 women, moderate‐certainty evidence). However, we are uncertain whether sedatives reduce postoperative nausea (aRR 0.25, 95% CI 0.09 to 0.71, 2 studies, 145 women, very low‐certainty evidence) and they may reduce postoperative vomiting (aRR 0.09, 95% CI 0.03 to 0.28, 2 studies, 145 women, low‐certainty evidence).
Opioid antagonists: There were no studies assessing intraoperative nausea or vomiting. Opioid antagonists may result in little or no difference to the number of women having postoperative nausea (aRR 0.75, 95% CI 0.39 to 1.45, 1 study, 120 women, low‐certainty evidence) or postoperative vomiting (aRR 1.25, 95% CI 0.35 to 4.43, 1 study, 120 women, low‐certainty evidence).
Acupressure: It is uncertain whether acupressure/acupuncture reduces intraoperative nausea (aRR 0.55, 95% CI 0.41 to 0.74, 9 studies, 1221 women, very low‐certainty evidence). Acupressure may reduce intraoperative vomiting (aRR 0.52, 95% CI 0.33 to 0.80, 9 studies, 1221 women, low‐certainty evidence) but it is uncertain whether it reduces postoperative nausea (aRR 0.46, 95% CI 0.27 to 0.75, 7 studies, 1069 women, very low‐certainty evidence) or postoperative vomiting (aRR 0.52, 95% CI 0.34 to 0.79, 7 studies, 1069 women, very low‐certainty evidence).
Ginger: It is uncertain whether ginger makes any difference to the number of women having intraoperative nausea (aRR 0.66, 95% CI 0.36 to 1.21, 2 studies, 331 women, very low‐certainty evidence), intraoperative vomiting (aRR 0.62, 95% CI 0.38 to 1.00, 2 studies, 331 women, very low‐certainty evidence), postoperative nausea (aRR 0.63, 95% CI 0.22 to 1.77, 1 study, 92 women, very low‐certainty evidence) and postoperative vomiting (aRR 0.20, 95% CI 0.02 to 1.65, 1 study, 92 women, very low‐certainty evidence).
Few studies assessed our secondary outcomes including adverse effects or women's views.
Authors' conclusions
This review indicates that 5‐HT3 antagonists, dopamine antagonists, corticosteroids, sedatives and acupressure probably or possibly have efficacy in reducing nausea and vomiting in women undergoing regional anaesthesia for caesarean section. However the certainty of evidence varied widely and was generally low. Future research is needed to assess side effects of treatment, women's views and to compare the efficacy of combinations of different medications.
Plain language summary
Reducing nausea and vomiting in women having a caesarean birth with regional anaesthesia
What is the issue?
The aim of this Cochrane Review was to find out from randomised controlled trials how effective drugs and other treatments are for reducing nausea and vomiting during and after caesarean section with epidural or spinal anaesthesia, when compared with an inactive control. We searched for all relevant studies to answer our review question (April 2020).
Why is this important?
Women often prefer to be awake for the birth of their child, so when possible, a caesarean is performed under regional anaesthesia (spinal or epidural). Nausea and vomiting are commonly experienced during and immediately after caesarean section with regional anaesthesia. This is distressing for women. Vomiting during surgery can also challenge the operating surgeon and put the mother at risk of fluids from the stomach going into her windpipe.
Several drugs are commonly used to reduce nausea and vomiting. There are also some non‐drug approaches such as acupressure/acupuncture and ginger. Possible side effects include headaches, dizziness, low blood pressure and itching.
What evidence did we find?
We identified 69 randomised controlled studies (involving 8928 women) that provided data. Data were mostly on non‐emergency caesareans and most findings were supported only by low or very low‐certainty evidence. This was due to many of the studies being old, with small numbers of participants or unclear methodology. A few outcomes had moderate‐certainty evidence.
5‐HT3 antagonists (like ondansetron, granisetron): these probably reduce nausea after surgery, and they may also reduce nausea during surgery (low‐certainty evidence) and vomiting after surgery, but any effect on vomiting during surgery is unclear.
Dopamine antagonists (like metoclopramide, droperidol): these may reduce vomiting during surgery and nausea after surgery, but it is unclear whether they reduce nausea during surgery and vomiting after surgery.
Steroids (like dexamethasone): these probably reduce nausea after surgery and may reduce vomiting after surgery, but it is unclear whether steroids reduce nausea and vomiting during surgery.
Antihistamines (like dimenhydrinate, cyclizine): these may reduce nausea after surgery, but they make little or no difference to nausea and vomiting during surgery and vomiting after surgery.
Anticholinergics (like glycopyrrolate, scopolamine): these may reduce nausea during surgery and vomiting after surgery, but they may make little to no difference to vomiting during surgery. There were no studies on nausea after surgery,
Sedatives (like propofol, midazolam, ketamine): these probably reduce nausea and vomiting during surgery and may reduce vomiting after surgery, but it is uncertain whether they reduce nausea after surgery.
Opioid antagonists (like nalbuphine): only one small study provided data on nausea and vomiting after surgery, and found they may make little or no difference.
Acupressure/acupuncture: this may reduce vomiting during surgery but it is uncertain if it reduces nausea during surgery or nausea and vomiting after surgery.
Ginger: it is unclear if ginger reduces nausea and vomiting during surgery or nausea and vomiting after surgery.
Few studies assessed women's views. What limited data there were on side effects did not find any differences.
What does this mean?
Several classes of drugs may help to reduce the number of women who experience nausea and vomiting during and after regional anaesthesia for caesarean births, although more data are needed. Acupressure may also help but we did not find enough data on ginger. Very few studies looked at women’s views and overall, there were not enough data on possible side effects.
Summary of findings
Summary of findings 1. 5‐HT3 antagonists compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
| 5‐HT3 antagonists compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section | ||||||
| Patient or population: women undergoing regional anaesthesia for caesarean section Setting: hospitals across low‐, middle‐ and high‐income countries Intervention: 5‐HT3 antagonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with 5‐HT3 antagonists | |||||
| Nausea ‐ intraoperative | Study population | RR 0.55 (0.42 to 0.71) | 1419 (12 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 479 per 1000 | 263 per 1000 (201 to 340) | |||||
| Vomiting ‐ intraoperative | Study population | RR 0.46 (0.29 to 0.73) | 1414 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 5 | ||
| 241 per 1000 | 111 per 1000 (70 to 176) | |||||
| Nausea ‐ postoperative | Study population | RR 0.40 (0.30 to 0.54) | 1340 (10 RCTs) | ⊕⊕⊕⊝ MODERATE 6 | ||
| 338 per 1000 | 135 per 1000 (101 to 183) | |||||
| Vomiting ‐ postoperative | Study population | RR 0.47 (0.31 to 0.69) | 1450 (10 RCTs) | ⊕⊕⊝⊝ LOW 5 7 | ||
| 228 per 1000 | 107 per 1000 (71 to 157) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 1 for risk of bias: > 90% of data comes from studies with unclear selection bias
2 Downgrade 1 for inconsistency: there may be substantial heterogeneity I2 = 65%, Chi2 P = 0.0009.
3 Downgrade 1 for risk of bias: > 80% of data comes from studies with unclear selection bias
4 Downgrade 1 for inconsistency: there may be substantial heterogeneity I2 = 58%, Chi2 P = 0.008.
5 Downgrade 1 for publication bias: there is some evidence of possible publication bias in the funnel plot.
6 Downgrade 1 for risk of bias: > 65% of data comes from studies with unclear selection bias
7 Downgrade 1 for risk of bias: > 70% of data comes from studies with unclear selection bias
Summary of findings 2. Dopamine antagonists compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
| Dopamine antagonists compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section | ||||||
| Patient or population: women undergoing regional anaesthesia for caesarean section Setting: hospitals across low‐, middle‐ and high‐income countries Intervention: dopamine antagonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with dopamine antagonists (B) | |||||
| Nausea ‐ intraoperative | Study population | RR 0.38 (0.27 to 0.52) | 1180 (15 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 444 per 1000 | 169 per 1000 (120 to 231) | |||||
| Vomiting ‐ intraoperative | Study population | RR 0.41 (0.28 to 0.60) | 942 (12 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 211 per 1000 | 87 per 1000 (59 to 127) | |||||
| Nausea ‐ postoperative | Study population | RR 0.61 (0.48 to 0.79) | 601 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 393 per 1000 | 240 per 1000 (189 to 311) | |||||
| Vomiting ‐ postoperative | Study population | RR 0.63 (0.44 to 0.92) | 860 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| 264 per 1000 | 167 per 1000 (116 to 243) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 2 for risk of bias: all the data come from studies with unclear risk of selection bias.
2 Downgrade 1 for inconsistency. Moderate to substantial heterogeneity, I2 = 54%, Chi2 P = 0.005
3 Downgrade 1 for publication bias: evidence of some publication bias in the funnel plot
Summary of findings 3. Corticosteroids compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
| Corticosteroids compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section | ||||||
| Patient or population: women undergoing regional anaesthesia for caesarean section Setting: hospitals across low‐, middle‐ and high‐income countries Intervention: corticosteroids Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with corticosteroids (C) | |||||
| Nausea ‐ intraoperative | Study population | RR 0.56 (0.37 to 0.83) | 609 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 403 per 1000 | 226 per 1000 (149 to 334) | |||||
| Vomiting ‐ intraoperative | Study population | RR 0.52 (0.31 to 0.87) | 609 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| 141 per 1000 | 73 per 1000 (44 to 123) | |||||
| Nausea ‐ postoperative | Study population | RR 0.59 (0.49 to 0.73) | 733 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | ||
| 491 per 1000 | 290 per 1000 (240 to 358) | |||||
| Vomiting ‐ postoperative | Study population | RR 0.68 (0.49 to 0.95) | 793 (7 RCTs) | ⊕⊕⊝⊝ LOW 5 6 | ||
| 355 per 1000 | 241 per 1000 (174 to 337) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 2 for risk of bias: all the data comes from studies with unclear risk of selection bias
2 Downgrade 1 for inconsistency: there is moderate heterogeneity I2 = 50% and Chi2 P = 0.06.
3 Downgrade 1 for imprecision: Wide CI close to line of no difference. Only 61 events out of 609 women.
4 Downgrade 1 for risk of bias: 69% of data comes from studies with unclear risk of selection bias.
5 Downgrade 1 for risk of bias: 83%% of data comes from studies with unclear risk of selection bias.
6 Downgrade 1 for inconsistency: may show moderate heterogeneity. I2 = 52%. Chi2 P = 0.03.
Summary of findings 4. Antihistamines compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
| Antihistamines compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section | ||||||
| Patient or population: preventing nausea and vomiting Setting: in women undergoing regional anaesthesia for caesarean section Intervention: antihistamines Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with antihistamines | |||||
| Nausea ‐ intraoperative | Study population | RR 0.99 (0.47 to 2.11) | 149 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 155 per 1000 | 153 per 1000 (73 to 327) | |||||
| Vomiting ‐ intraoperative | Study population | not estimable | 149 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 |
Only one RCT with no intraoperative vomiting events | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Nausea ‐ postoperative | Study population | RR 0.44 (0.30 to 0.64) | 514 (4 RCTs) | ⊕⊕⊝⊝ LOW 4 | ||
| 309 per 1000 | 136 per 1000 (93 to 198) | |||||
| Vomiting ‐ postoperative | Study population | RR 0.48 (0.29 to 0.81) | 333 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 5 | ||
| 189 per 1000 | 91 per 1000 (55 to 153) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 2 for risk of bias; only one study with unclear risk of bias across 6 domains and high risk for one domain
2 Downgrade 2 for imprecision: wide CI, only 23 events out of 149 women in a single study.
3 Downgrade 2 for imprecision: there are no events.
4 Downgrade 2 for risk of bias: all data from studies with unclear risk of selection bias
5 Downgrade 1 for imprecision: only 45 events out of 333 women.
Summary of findings 5. Anticholinergics compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
| Anticholinergics compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section | ||||||
| Patient or population: women undergoing regional anaesthesia for caesarean section Setting: hospitals across low‐, middle‐ and high‐income countries Intervention: anticholinergics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with anticholinergics | |||||
| Nausea ‐ intraoperative | Study population | RR 0.67 (0.51 to 0.87) | 453 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 665 per 1000 | 446 per 1000 (339 to 579) | |||||
| Vomiting ‐ intraoperative | Study population | RR 0.79 (0.40 to 1.54) | 453 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 304 per 1000 | 240 per 1000 (122 to 468) | |||||
| Nausea ‐ postoperative | Study population | ‐ | (0 RCTs) | ‐ | ||
| see comment | see comment | |||||
| Vomiting ‐ postoperative | Study population | RR 0.55 (0.41 to 0.74) | 161 (1 RCT) | ⊕⊕⊝⊝ LOW 4 5 | ||
| 728 per 1000 | 401 per 1000 (299 to 539) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 2 for risk of bias: all the data from studies with unclear selection bias.
2 Downgrade 1 for inconsistency: there may be moderate heterogeneity I2 = 52% Chi2 P = 0.10.
3 Downgrade 1 for imprecision: wide CI, crossing the line of no difference. 120 events out of 453 women participants.
4 Downgrade 1 for risk of bias: only one study with unclear allocation concealment but adequate sequence generation
5 Downgrade 1 for imprecision: a single study shows a wide confidence interval away from the line of no difference but with 91 events out of 161 women participants.
Summary of findings 6. Sedatives compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
| Sedatives compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section | ||||||
| Patient or population: women undergoing regional anaesthesia for caesarean section Setting: hospitals across low‐, middle‐ and high‐income countries Intervention: sedatives Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with sedatives (F) | |||||
| Nausea ‐ intraoperative | Study population | RR 0.65 (0.51 to 0.82) | 593 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 375 per 1000 | 244 per 1000 (191 to 308) | |||||
| Vomiting ‐ intraoperative | Study population | RR 0.35 (0.24 to 0.52) | 593 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | ||
| 294 per 1000 | 103 per 1000 (71 to 153) | |||||
| Nausea ‐ postoperative | Study population | RR 0.25 (0.09 to 0.71) | 145 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | ||
| 441 per 1000 | 110 per 1000 (40 to 313) | |||||
| Vomiting ‐ postoperative | Study population | RR 0.09 (0.03 to 0.28) | 145 (2 RCTs) | ⊕⊕⊝⊝ LOW 5 | ||
| 356 per 1000 | 32 per 1000 (11 to 100) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 1 for risk of bias: 56% of data from studies with low risk of selection bias.
2 Downgrade 1 for risk of bias: 75% of data were from studies with unclear selection bias.
3 Downgrade 1 for inconsistency: moderate heterogeneity. I2 = 58%. Chi2 P = 0.09.
4 Downgrade 2 for imprecision: low number of events ‐ 37 and low number of participants 145. Wide CI though a reasonable distance from line of no difference.
5 Downgrade 2 for imprecision: low number of events ‐ 23 and low number of participants 145. Wide CI but a good distance from the line of no difference although the data of high effectiveness comes from just one study of 44 women.
Summary of findings 7. Opioid antagonists compared to placebo for preventing nausea and vomiting.
| Opioid antagonists compared to placebo for preventing nausea and vomiting | ||||||
| Patient or population: preventing nausea and vomiting Setting: in women undergoing regional anaesthesia for caesarean section Intervention: opioid antagonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with opioid antagonists | |||||
| Nausea ‐ intraoperative | Study population | ‐ | (0 studies) | ‐ | ||
| see comment | see comment | |||||
| Vomiting ‐ intraoperative | Study population | ‐ | (0 study) | ‐ | ||
| see comment | see comment | |||||
| Nausea ‐ postoperative | Study population | RR 0.75 (0.39 to 1.45) | 120 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 267 per 1000 | 200 per 1000 (104 to 387) | |||||
| Vomiting ‐ postoperative | Study population | RR 1.25 (0.35 to 4.43) | 120 (1 RCT) | ⊕⊕⊝⊝ LOW 2 | ||
| 67 per 1000 | 83 per 1000 (23 to 295) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 2 for imprecision. Only 28 events out of 120 women in one study. Wide CI crossing line of no difference.
2 Downgrade 2 for imprecision. Only 9 events out of 120 women in one study. Wide CI crossing line of no difference.
Summary of findings 8. Acupressure/acupuncture compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
| Acupressure/acupuncture compared to placebo for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section | ||||||
| Patient or population: women undergoing regional anaesthesia for caesarean section Setting: hospitals across low‐, middle‐ and high‐income countries Intervention: acupressure/acupuncture Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with acupressure/acupuncture (K) | |||||
| Nausea ‐ intraoperative | Study population | RR 0.55 (0.41 to 0.74) | 1221 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 466 per 1000 | 256 per 1000 (191 to 345) | |||||
| Vomiting ‐ intraoperative | Study population | RR 0.52 (0.33 to 0.80) | 1221 (9 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 236 per 1000 | 123 per 1000 (78 to 189) | |||||
| Nausea ‐ postoperative | Study population | RR 0.46 (0.27 to 0.75) | 1069 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| 411 per 1000 | 189 per 1000 (111 to 308) | |||||
| Vomiting ‐ postoperative | Study population | RR 0.52 (0.34 to 0.79) | 1069 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 | ||
| 302 per 1000 | 157 per 1000 (103 to 239) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 2 for risk of bias: all the data comes from studies which are unclear risk of selection bias.
2 Downgrade 1 for inconsistency: substantial heterogeneity I2 = 69% Chi2 P = 0.0010.
3 Downgrade 1 for inconsistency: substantial heterogeneity. I2 = 81% and Chi2 P = < 0.0001. Could be downgrade by 2, borderline decision
4 Downgrade 1 for inconsistency: moderate heterogeneity. I2 = 62% and Chi2 P = 0.01.
Summary of findings 9. Ginger compared to placebo for preventing nausea and vomiting.
| Ginger compared to placebo for preventing nausea and vomiting | ||||||
| Patient or population: preventing nausea and vomiting Setting: in women undergoing regional anaesthesia for caesarean section? Intervention: ginger Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with ginger | |||||
| Nausea ‐ intraoperative | Study population | RR 0.66 (0.36 to 1.21) | 331 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 586 per 1000 | 387 per 1000 (211 to 709) | |||||
| Vomiting ‐ intraoperative | Study population | RR 0.62 (0.38 to 1.00) | 331 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 | ||
| 408 per 1000 | 253 per 1000 (155 to 408) | |||||
| Nausea ‐ postoperative | Study population | RR 0.63 (0.22 to 1.77) | 92 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5 6 | ||
| 174 per 1000 | 110 per 1000 (38 to 308) | |||||
| Vomiting ‐ postoperative | Study population | RR 0.20 (0.02 to 1.65) | 92 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5 7 | ||
| 109 per 1000 | 22 per 1000 (2 to 179) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade 2 for risk of bias: Only 2 studies both with unclear risk of selection bias
2 Downgrade 1 for inconsistency. Substantial heterogeneity. I2 = 74%, Chi2 P = 0.05
3 Downgrade 1 for imprecision. Very wide CI crossing the line of no difference. 170 events and 331 women participants
4 Downgrade 1 for imprecision: Wide CI. meeting the line of no difference. 112 events and 331 women participating
5 Downgrade 2 for risk of bias: Only 1 study with unclear risk of selection bias
6 Downgrade 2 for imprecision: Wide CI. Only 6 events out of 92 women
7 Downgrade 2 for imprecision: Wide CI crosses line of no difference. 5 events only and just 92 women included
Background
Nausea and vomiting are unpleasant symptoms commonly experienced by pregnant women during caesarean section under regional anaesthesia, and may also occur in the postpartum period following a caesarean under either regional or general anaesthesia. Nausea and vomiting around the time of the birth of a baby can be uncomfortable and distressing for the woman. If vomiting occurs intraoperatively during the caesarean under regional anaesthesia, it offers significant challenges to the operating surgeon, may increase the duration of surgery, the risk of bleeding, the risk of inadvertent surgical trauma and the risk of aspiration of gastric contents (Paranjothy 2014).
Caesarean section is one of the most commonly performed surgical procedures. World Health Organization data indicate that worldwide around 140 million babies are born each year. Globally caesarean rates vary widely; from less than 5% of births in low‐income countries (e.g. Zimbabwe) to above 30% in high‐income countries (e.g. Germany) (Boerma 2018) and in one study of NHS trusts the caesarean section rate ranged from 14.9% to 32.1% (Bragg 2010). These figures suggest the number of caesareans worldwide is at least 10 to 20 million per year. Caesarean section rates have also risen considerably in many countries in recent years and this trend is continuing (Chen 2018). There are several reasons why general anaesthesia should be avoided if possible in the later stages of pregnancy, and most women want to be awake for the birth of their child, so except where there is a contraindication or in some emergency situations, most caesareans are carried out under regional anaesthesia using spinal or epidural techniques.
Many factors can contribute to the development of nausea and vomiting at caesarean section. While some causes of nausea and vomiting are common to other non‐obstetric surgical procedures, many are unique to caesarean sections. There is a body of published literature, including consensus guidelines (Gan 2019), to help anaesthetists reduce the risk of postoperative nausea and vomiting. However, because some of the underlying causes of nausea and vomiting during caesarean section may be specific to the procedure, it is reasonable to assume that the choice of effective treatments may also differ from other types of surgery. Anaesthetists need to consider specific evidence in this setting. Since all interventions are associated with increased healthcare costs and potential risks to the woman (and potentially to the neonate, via either placental transfer or breastfeeding) it is clear that antiemetic use should be evidence‐based.
In some countries, for example in the United Kingdom, there is a recommendation that to reduce nausea and vomiting at caesarean delivery the routine administration of drugs (antiemetics ‐ drugs to reduce nausea and vomiting) or acupressure should be considered (NICE 2011). However, many anaesthetists may choose to give antiemetic medication only when nausea and vomiting occur (treatment) rather than as prophylaxis (prevention). It is not known to what extent medications which have been shown to be efficacious as treatment are also efficacious as prophylaxis (and vice versa).
The aim of this review is to assess the effectiveness of interventions to prevent nausea and vomiting given as prophylaxis during caesarean section under regional anaesthesia. Future reviews will be required to assess studies on interventions for treatment (rather than prevention) of nausea and vomiting, procedures performed as emergencies and caesarean deliveries performed under general anaesthesia.
Description of the condition
Nausea is the unpleasant subjective urge to vomit, while vomiting is the physiological process associated with propulsive abdominal muscular spasms leading to the expulsion of gastric contents. Retching involves the same propulsive muscular spasms as vomiting but without the expulsion of any gastric contents.
There are several aetiological factors (factors causing or contributing to the development of a condition or disease) which may contribute to the development of nausea and vomiting during caesarean section. These may include the following.
Haemodynamic changes (i.e. changes in blood flow) such as hypotension (low blood pressure ‐ a frequent side effect of regional anaesthesia, Chooi 2017) and reduced cardiac output from aorto‐caval compression resulting from placing the woman on her back (supine position) (Cooke 1979).
Surgical stimulation from visceral traction such as manual delivery of the baby and in particular, exteriorisation of the uterus(temporary removal of the uterus from the abdominal cavity to facilitate repairing the incision), Wahab 1999).
Intraoperative medications may contribute to nausea and include opiates, antibiotics and administered uterotonics such as oxytocin and particularly ergometrine (De Groot 1998).
Psychological factors such as stress, anxiety, fatigue and prolonged starvation should not be underestimated as contributors to nausea and vomiting. This may particularly be the case with emergency caesarean delivery.
Medications given prior to the caesarean, such as medications to reduce the risk of aspiration (Paranjothy 2014). If the woman has been in labour prior to surgery then pain relief already provided such as opioids and nitrous oxide may also have residual emetogenic effects.
Few prospective observational studies or audit data have been published and so the underlying incidence of nausea and vomiting during caesarean section is uncertain. It is also likely that the baseline rate will vary considerably depending on the anaesthetic, analgesic and vasopressor regimen that is being used. However, it would seem reasonable to use the rates in the placebo arms of well‐designed randomised trials as an indication of the baseline rate of nausea and vomiting. Published placebo data show rates of intraoperative nausea in the order of 48% (Habib 2013) to 79% (Abouleish 1999). Vomiting rates are typically lower than the rates of nausea, in the order of 15% (Voigt 2013) to 38% (El‐Deeb 2011a). Most studies recruit women in the setting of elective caesarean section, and it is likely that rates are higher in the setting of emergency caesarean section.
Nausea and vomiting in the postpartum period are also common, and can affect women who received either regional or general anaesthesia. In most types of surgery, the use of regional anaesthesia is thought to be associated with lower rates of postoperative nausea and vomiting than general anaesthesia (Gan 2019); however, this difference may not be apparent following caesarean delivery. Almost all postoperative analgesia regimens involve the use of opioid type medications, either by oral, intravenous or neuraxial (spinal or epidural) routes, all of which can contribute to nausea and vomiting.
Description of the intervention
In this review, we have included pharmacological and non‐pharmacological interventions given specifically for the purpose of preventing nausea and vomiting in women undergoing caesarean under regional anaesthesia. Whilst hypotension is an important cause of these symptoms during a caesarean, treatment for hypotension during regional anaesthesia has already been specifically addressed in another Cochrane Review (Chooi 2017). Similarly, interventions to reduce the risk of acid aspiration may well affect nausea and vomiting and these interventions have also been addressed in another Cochrane Review (Paranjothy 2014).
The pharmacological interventions available include medications from a wide range of drug classes including serotonin and dopamine receptor antagonists, corticosteroids, antihistamines, sedatives and anticholinergics (Flake 2004). A number of non‐pharmacological approaches have also been used traditionally to treat nausea in pregnancy, and some of these have been studied in this setting. These include acupuncture or acupressure (Ho 2006) and oral ginger (Kalava 2013).
How the intervention might work
Pharmacological interventions
For many of the recognised interventions used for the prevention of nausea and vomiting, the mechanism of action is not well understood. However, most treatments can be classed pharmacologically based on their biochemical receptor target. Nausea and vomiting caused by visceral stimulation is thought to be mediated predominantly via serotonin (5‐HT) and dopamine receptors. The chemoreceptor trigger zone (CTZ) is a small region within the brainstem responsible for the symptoms of medication and toxin related emesis, including post anaesthetic nausea and vomiting, and is also mediated by serotonin and dopamine. In contrast, nausea and vomiting caused by central nervous system and vestibular mechanisms, such as motion sickness, are thought to be mediated mainly via histamine and acetylcholine.
The main classes of medications in use include the following (Flake 2004; Gan 2003).
Serotonin (5‐HT3) receptor subtype‐3 antagonists (e.g. ondansetron, granisetron) antagonise the emetic effects of serotonin in the small bowel, vagus nerve and CTZ (Peixoto 2006). They are effective (George 2009) and have few side effects.
Dopamine receptor antagonists (e.g. metoclopramide, prochlorperazine, droperidol, domperidone) antagonise the effects of dopamine at the D2 receptors in the CTZ. They have a wide variety of associated side effects including sedation, agitation, and extra‐pyramidal effects (Chestnut 1987). Droperidol has been associated with very rare, but potentially life‐threatening, cardiac arrhythmias.
Corticosteroids also known as steroids (most commonly dexamethasone) are regarded as being highly effective, but their mechanism of action is unclear (Tzeng 2000). Whilst long‐term steroid use can lead to a wide variety of side effects such as fluid and electrolyte changes, obesity, and diabetes, single antiemetic doses are well tolerated, even in diabetics.
Antihistamines (e.g. promethazine and cyclizine (Nortcliffe 2003) can cause a variety of adverse effects including sedation and dry mouth.
Anticholinergic agents (e.g. glycopyrrolate (Ure 1999) and scopolamine (Kotelko 1989) are mainly useful for nausea and vomiting caused via the vestibular system, i.e. motion sickness. They can also cause a dry mouth and potentially urinary retention.
Sedatives. Very low doses of sedatives such as midazolam or propofol (Mukherjee 2006; Tarhan 2007) seem to have antiemetic efficacy. The mechanism of action is unclear, but may relate to the contribution of psychological factors such as stress and anxiety to the incidence of emetic symptoms.
Opioids antagonists or partial agonists. A number of studies have attempted to demonstrate the beneficial effects of opioids. Whilst opioids would generally be considered a cause, rather than a treatment, of nausea and vomiting, it is possible that when two opioids are administered together, one of them may reduce the opioid‐induced emetic symptoms caused by the other. If one drug is an opioid antagonist or partial agonist (such as naloxone or nalbuphine) (Charuluxananan 2003), then it may reduce the opioid‐related side effects (such as nausea, itch and constipation) without unduly reducing the analgesic benefits.
Non‐pharmacological interventions
Acupuncture or acupressure: acupressure or acupuncture at the P6 point at the wrist has long been a traditional treatment for nausea, particularly sea sickness. The mechanism of action of acupuncture and acupressure is not well understood (Duggal 1998; Harmon 2000). Potential adverse effects of acupuncture include infection or trauma from acupuncture needles.
Alternative natural therapies such as ginger (Kalava 2013; Zeraati 2016) and peppermint (Lane 2012; Niaki 2016) also have long histories of use as traditional treatments for reducing nausea in pregnancy. Although associated with minimal side effects, their efficacy is uncertain (Matthews 2015).
Why it is important to do this review
Nausea and vomiting are very common symptoms experienced both during and following caesarean section, may increase morbidity, and can be very distressing for women and their families. Many interventions are available and routine prophylactic treatment has been proposed (NICE 2011). The available interventions have widely varying cost and significant side‐effect profiles. Whilst guidelines exist for the prevention of nausea and vomiting after general anaesthesia in non‐pregnant patients (Gan 2019), the aetiology of emetic symptoms at caesarean section are clearly multifactorial and the current literature may not be directly applicable. This review is important to ensure that women undergoing caesarean section are offered interventions to prevent nausea and vomiting which are safe, efficacious and cost‐effective.
Objectives
To assess the efficacy of pharmacological and non‐pharmacological interventions versus placebo or no intervention given prophylactically to prevent nausea and vomiting in women undergoing regional anaesthesia for caesarean section.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs), including conference abstracts. We planned to include cluster‐randomised trials, but none were identified. Quasi‐RCTs and cross‐over studies were excluded.
Types of participants
Pregnant women undergoing elective or emergency caesarean section under regional anaesthesia.
Types of interventions
In this updated review, we have included studies where the participants were women undergoing caesarean section under regional anaesthesia (either spinal, epidural or both) comparing interventions for nausea and vomiting against placebo or no intervention. Intervention versus intervention comparisons were excluded. We included studies where the intervention was given with the express purpose of preventing nausea and vomiting, either intraoperative, postoperative, or both.
Interventions included the following categories.
Serotonin (5‐HT3) receptor antagonists (e.g. ondansetron, granisetron).
Dopamine receptor antagonists (e.g. metoclopramide, prochlorperazine, droperidol, domperidone).
Corticosteroids (e.g. dexamethasone).
Antihistamines (e.g. promethazine, cyclizine).
Anticholinergic agents (e.g. glycopyrrolate, scopolamine).
Sedatives (e.g. midazolam, propofol).
Opioids antagonists or partial agonists (e.g. nalbuphine).
Acupressure/acupuncture.
Alternative therapies such as ginger or peppermint.
We compared the different drug classes against placebo, setting out individual drugs and doses as subgroups.
We excluded:
studies where the authors were comparing two different treatments (unless there was also a control/placebo arm) and studies investigating combinations of treatments;
studies where the intervention was for reducing aspiration pneumonitis, as this is the subject of another review (Paranjothy 2014);
studies where the express purpose was to treat another problem which may impact upon the development of nausea or vomiting, such as studies assessing agents for treating hypotension. This has also been studied in another review (Chooi 2017);
studies where a recognised antiemetic was given, but the focus of the study was on another effect of that medication (for example, studies on the haemodynamic effects of ondansetron);
studies which assessed the efficacy of interventions for treatment, rather than prevention, of nausea and vomiting. This may be the subject of a separate future review;
studies where the intervention was not recognised as an antiemetic and did not have a reasonable theoretical justification for affecting nausea and vomiting, e.g. supplemental oxygen; intravenous fluids; anticonvulsants; antidepressants, opioid agonists.
Types of outcome measures
Primary outcomes
Nausea intraoperatively.
Vomiting (and/or retching) intraoperatively.
Nausea postoperatively.
Vomiting (and/or retching) postoperatively.
Secondary outcomes
Nausea plus vomiting/retching.
Maternal adverse effects: e.g. sedation, restlessness, extra‐pyramidal effects, surgical bleeding, hypotension, atonic uterus.
Neonatal morbidity: e.g. Apgar scores less than seven at five minutes.
Initiation of breastfeeding.
Duration of exclusive breastfeeding.
Maternal satisfaction (using a validated questionnaire).
In this review, when authors reported retching and vomiting separately, we combined these data, as we believe retching is more pathophysiologically analogous to vomiting than nausea. In this update, we clarified our approach to the postoperative data. Where a paper reports a number of time epochs (for example, zero to four hours, four to eight hours, etc), we have included data from the earliest reported time period because we believe these data were most likely to reflect the efficacy of the intervention.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (16 April 2020).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies, Excluded studies, Studies awaiting classification or Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (1 April 2020) using the search methods described in Appendix 1.
Searching other resources
We searched for further studies in the reference list of the studies identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeGriffiths 2012.
For this update, the following methods were used for assessing the 174 new studies that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2020) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2019). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals. Where a random‐effects model has been used, we report this as an average risk ratio (aRR).
Continuous data
We planned to use mean difference if outcomes were measured in the same way between trials and standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials, but did not identify any. Had we identified any, we would have adjusted their standard error using the methods described in the Handbook[Section16.3.4 and 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we would have reported this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identify both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would have considered it reasonable to combine the results from both if there is little heterogeneity between the study designs. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We excluded cross‐over trials.
Other unit of analysis issues
Where we found multi‐arm studies, we assessed which arms were relevant to our question and included data taking care not to double count the data in the placebo group by dividing the placebo data equally amongst the relevant comparisons such that when the data were pooled, the correct number of events and participants were included.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as reported in the Cochrane Handbook (Higgins 2019):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity*;
50% to 90%: may represent substantial heterogeneity*;
75% to 100%: considerable heterogeneity*.
and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2020).
We used random‐effects meta‐analyses for combining data because we considered that there would be heterogeneity sufficient to expect that the underlying treatment effects would differ between trials because our question is around groups of drugs and so we are combining data from different drugs and different doses within the meta‐analyses.
The random‐effects summary was treated as the average range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. The results are presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Different drugs with the same group of drugs
Difference doses of the drugs within the group of drugs
The following four primary outcomes were used in subgroup analyses.
intraoperative nausea
intraoperative vomiting
postoperative nausea
postoperative vomiting
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2020). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality assessed by selection bias (sequence generation and allocation concealment) and attrition bias (incomplete outcome data), with poor‐quality studies (either high risk or unclear risk) being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Summary of findings and assessment of the certainty of the evidence
For this update, the certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons. All nine comparisons were chosen as a specific focus as they represent the most clinically‐relevant comparisons in this updated review.
Comparisons for GRADE and Summary of findings
5‐HT3 antagonists versus placebo
Dopamine antagonists versus placebo
Corticosteroids versus placebo
Antihistamines versus placebo
Anticholinergics versus placebo
Sedatives versus placebo
Opioid antagonists/partial agonists versus placebo
Acupressure/acupuncture versus placebo
Ginger versus placebo
Outcomes for GRADE and Summary of findings
Incidence of intraoperative nausea
Incidence of intraoperative vomiting/retching
Incidence of postoperative nausea
Incidence of postoperative vomiting/retching
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2020) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of certainty each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, indirectness, imprecision and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, serious inconsistency, indirectness of evidence, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
We assessed 218 new trial reports, plus the change in scope meant we also reassessed the 204 trial reports referenced in the previous version of the review.
All in all in this 2021 update, there are 84 included studies (112 reports) (Characteristics of included studies) and 236 excluded studies (269 reports) (Characteristics of excluded studies). Ten studies are awaiting classification (Characteristics of studies awaiting classification). These are predominantly conference abstracts where we have been unable to contact the authors or studies in a non‐English language where we have been unable to obtain a translation as yet. There are 27 studies identified as ongoing (31 reports) (Characteristics of ongoing studies).
The change in scope meant we excluded nine studies from the 2012 publication, six of these studies had provided data (Chestnut 1989; Gaiser 2002; Owczarzak 1997; Pecora 2009; Phillips 2007; Shahriari 2009), and three had provided no data (Biwas 2002; Chaudhuri 2004; Manullang 2000).
In addition, there were eight comparisons in multi‐arm studies where, due to our change in scope, some arms were now excluded and the data from these women were not included in our review (Abdollahpour 2015; Habib 2013; Khalayleh 2005; Levin 2019; Mokini 2014; Shen 2012; Voigt 2013; Wu 2007).
(See: Figure 1)
1.
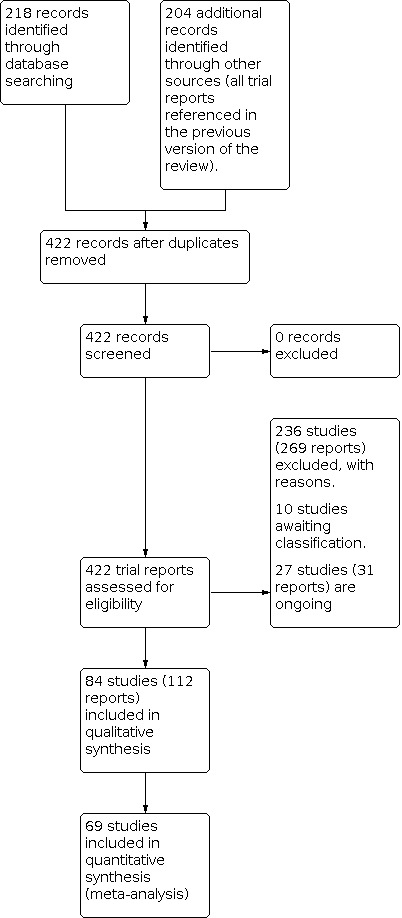
Study flow diagram.
Included studies
Of the 84 included studies (involving 10,990 women), 69 studies involving 8928 women provided usable data for this review, taking into account the arms of the multi‐arm studies which are not included in our inclusion criteria (Abdel‐Aleem 2012; Abdollahpour 2015; Abouleish 1999; Ahn 2002; Apiliogullari 2007; Baciarello 2011; Biswas 2003; Caba 1997; Cardoso 2013; Carvalho 2010; Charuluxananan 2003; Cherian 2001; Chestnut 1987; Choi 1999; Dasgupta 2012; Direkvand‐Moghadam 2013; Duggal 1998; Duman 2010; El‐Deeb 2011a; Garcia‐Miguel 2000; Habib 2006; Habib 2013; Harmon 2000; Harnett 2007; Hassanein 2015; Ho 1996; Ho 2006; Huang 1992; Ibrahim 2019; Jaafarpour 2008; Kalava 2013; Kampo 2019; Kasodekar 2006; Khalayleh 2005; Koju 2015; Kotelko 1989; Levin 2019; Li 2012; Lussos 1992; Mandell 1992; Maranhao 1988; Mohammadi 2015; Mokini 2014; Mukherjee 2006; Munnur 2008; Niu 2018; Noroozinia 2013; Nortcliffe 2003; Pan 1996; Pan 2001; Pan 2003; Parra‐Guiza 2018; Peixoto 2006; Rasooli 2014; Rudra 2004a; Sahoo 2012; Selzer 2020; Shabana 2012; Shen 2012; Stein 1997; Tarhan 2007; Tkachenko 2019; Tzeng 2000; Uerpairojkit 2017; Ure 1999; Voigt 2013; Wang 2001; Wu 2007; Zeraati 2016).
Fifteen studies are included but do not contribute data to the meta‐analysis because the data were either presented in a graphical format only, or there was no information on the number of women in each outcome group (Birnbach 1993; Boone 2002; ; Imbeloni 1986; Jang 1997; Kim 1999; Lee 2002; Lim 2001a; Lim 2001b; Liu 2015a; Modir 2019; Pazoki 2018; Quiney 1995; Sanansilp 1998; Weiss 1995; Yazigi 2002). We have written to these authors requesting further information.
Multi‐arm studies
There are 41 multi‐arm studies, 31 are three‐arm studies (Abdollahpour 2015; Apiliogullari 2007; Baciarello 2011; Birnbach 1993; Choi 1999; Direkvand‐Moghadam 2013; Duman 2010; El‐Deeb 2011a; Garcia‐Miguel 2000; Habib 2013; Harnett 2007; Hassanein 2015; Kampo 2019; Khalayleh 2005; Levin 2019; Li 2012; Maranhao 1988; Modir 2019; Munnur 2008; Nortcliffe 2003; Pan 1996; Pan 2001; Parra‐Guiza 2018; Pazoki 2018; Peixoto 2006; Rasooli 2014; Sanansilp 1998; Stein 1997; Tarhan 2007; Tkachenko 2019; Tzeng 2000; Voigt 2013) and 10 studies are four‐arm studies (Ahn 2002; Biswas 2003; Charuluxananan 2003; Lee 2002; Mokini 2014; Mukherjee 2006; Shen 2012; Voigt 2013; Wang 2001; Wu 2007). Where two or more arms of a study fell within the same comparison, we treated the data as described in the Unit of analysis issues.
Of the multi‐arm studies which provided data, 18 compared more than one drug against placebo but the drugs were in different categories and so in different comparisons (Biswas 2003; Choi 1999; Direkvand‐Moghadam 2013; Duman 2010; El‐Deeb 2011a; Garcia‐Miguel 2000; Harnett 2007; Hassanein 2015; Kampo 2019; Nortcliffe 2003; Pan 1996; Pan 2001; Parra‐Guiza 2018; Peixoto 2006; Shen 2012; Stein 1997; Tzeng 2000; Wu 2007). Six multi‐arm studies providing data included arms with one of our excluded drugs or a combination of drugs, so data from these arms were excluded (Abdollahpour 2015; Habib 2013; Khalayleh 2005; Levin 2019; Li 2012; Voigt 2013). Seven multi‐arm studies providing data looked at different concentrations of the same drug or different routes of administration and we adjusted the placebo data accordingly (Ahn 2002; Apiliogullari 2007; Baciarello 2011; Lee 2002; Mukherjee 2006; Tkachenko 2019; Wang 2001). Four multi‐arm studies looked at different drugs from the same category and so were in the same comparison and here we adjusted the placebo data accordingly (Maranhao 1988; Munnur 2008; Rasooli 2014; Tarhan 2007). One four‐arm study looked at two drugs from different categories and for one of these drugs looked at two doses, the placebo data was dealt with accordingly (Charuluxananan 2003) and another four‐arm study one arm was excluded as it was a combination of drugs and the other two arms were drugs in different categories (Mokini 2014). Four of the multi‐arm studies provided no data that we could use in this review (Birnbach 1993; Pazoki 2018; Sanansilp 1998; Modir 2019).
Populations
The included studies covered women undergoing elective and emergency caesarean sections under regional anaesthesia, with either spinal or epidural anaesthesia. Most studies reported women in American Society of Anesthesiologists physical status classification (ASA) Grade 1 to 2, and so generally with no medical problems (Characteristics of included studies)
Interventions
The studies covered drugs in seven different classes of drugs. For 5‐HT3 antagonists (e.g. ondansetron, granisetron) there were 21 studies involving providing data on 2686 women; for dopamine antagonists (e.g. metoclopramide, droperidol) there were 20 studies providing data on 1880 women; for corticosteroids (e.g. dexamethasone) there were 12 studies providing data on 1182 women; for antihistamines (e.g. dimenhydrinate, cyclizine) there were four studies providing data on 514 women; for anticholinergics (e.g. glycopyrrolate, scopolamine) there were six studies providing data on 787 women; for sedatives (e.g. propofol, midazolam) there were 13 studies providing data on 1265 women; and for opioid antagonists/partial agonists (nalbuphine) there were two studies providing data on 197 women. Ten studies on acupressure/acupuncture provided data on 1401 women and two studies on ginger which provided data on 365 women (Characteristics of included studies).
Outcomes
Most studies reported intraoperative nausea, intraoperative vomiting, postoperative nausea and postoperative vomiting separately, but a few reported combines nausea and vomiting both intraoperative and postoperative. Some studies reported looking for side effects/adverse effects such as hypotension, itching, dizziness. Few studies looked at women's satisfaction (Characteristics of included studies).
Settings
The 84 studies were undertaken in a wide range of countries across the world (see Characteristics of included studies):
Americas (24 studies) ‐ USA 18 studies, South America four studies (including one from Columbia and two from Brazil), Canada two studies;
Asia (24 studies) ‐ India five studies, China five studies, Nepal one study, Thailand three studies, Taiwan three studies; South Korea five studies, Singapore two studies;
Middle East (14 studies) ‐ Iran 10 studies, Lebanon one study, Turkey three studies;
UK/Europe (10 studies) ‐ UK four studies, Germany one study, Ireland one study, Italy one study, Spain two studies; Ukraine one study;
Africa (seven studies) ‐ Egypt six studies, Ghana one study.
For two studies, there was no information provided on the setting, and three studies were conducted across multiple countries (e.g. USA and UK).
Dates of included studies
Fifty‐nine studies did not report the dates over which their studies were undertaken. The studies which reported dates covered 2001 to 2017 and publication dates range from 1987 to 2020 (Characteristics of included studies).
Funding sources of included studies
Seventy‐one studies did not report funding sources. Of the studies reporting this information, two studies reported commercial company funding (Abouleish 1999; Duggal 1998), one study specifically reported no commercial funding (Cherian 2001), nine studies reported finding from universities, hospitals and public funding bodies (Abdollahpour 2015; Cardoso 2013; Direkvand‐Moghadam 2013;Duggal 1998; Modir 2019; Parra‐Guiza 2018; Pazoki 2018; Selzer 2020; Zeraati 2016), and two studies reported specifically that they had no funding (Kampo 2019; Levin 2019).
Declarations of interest of authors of included studies
Seventy‐three studies did not report on declarations of interest of the authors. Eleven studies reported no conflict of interest for their authors (Abdel‐Aleem 2012; Abouleish 1999; Cardoso 2013;Kampo 2019; Koju 2015; Levin 2019; Niu 2018; Parra‐Guiza 2018; Selzer 2020; Uerpairojkit 2017; Voigt 2013).
Elective versus emergency caesarean sections
Of all our included studies, the vast majority were specifically restricted to elective caesarean sections. Only one study mentioned including both elective and emergency caesareans but they did not present the data separately (Caba 1997). One other study specifically included only women undergoing emergency caesarean section (Huang 1992). Most of the remaining studies did not mention whether they included elective or emergency caesareans. We have, therefore, not been able to consider the subgroup comparison of elective versus emergency caesarean section.
Excluded studies
Excluded studies
We have excluded a total of 236 studies (269 reports). The excluded studies are listed in the reference section under excluded studies and the table Characteristics of excluded studies states the reasons for exclusion from this review. Studies were excluded for a wide variety of reasons. Some studies were excluded for multiple reasons. Many studies that were excluded, assessed interventions for reducing the risk of aspiration pneumonitis at caesarean section rather than reducing the risk of nausea and vomiting, as the search strategy included both these circumstances in the original protocol, which remains part of the aspiration pneumonitis review (Paranjothy 2004). Fifty‐four studies looking at aspiration prophylaxis and are included in the review of interventions for reducing aspiration prophylaxis at caesarean section (Paranjothy 2014). Seven studies (Fujii 1998a; Fujii 1998b; Fujii 1999; Fujii 2002; Fujii 2004; Numazaki 2000; Numazaki 2003) were excluded following investigation into research authenticity (Carlisle 2012).
Although our review assesses interventions for prevention (rather than treatment) of nausea and vomiting, our current search would identify treatment studies too. There were only three randomised controlled trials (RCTs) identified which specifically assessed interventions for treatment (rather than prevention) of nausea and vomiting (Fazel 2017; Kimura 2011; Lane 2012). Four studies were excluded because the women had their caesarean sections with general anaesthesia (Abadi 2018; Huseyinogclu 2016; Hussain 2014; Kocamanoglu 2005).
One hundred and seven studies were excluded because they studied aspects of anaesthesia other than interventions given for the prevention of nausea and vomiting. Many studies assessed antiemetic medication but were focused on the haemodynamic effects of the medication, or the quality and duration of anaesthesia (rather than the antiemetic effect). Some studies examined other medication such as analgesics, antidepressants or anticonvulsants, again not focused on their antiemetic effects. Some other studies compared different surgical techniques, or other interventions such as supplemental oxygen, intravenous fluids or prolonged fasting. These were also outside the inclusion criteria for our review (Characteristics of excluded studies).
Fifty studies were excluded as they compared different treatments, or combinations of treatments, without a placebo or control group.
Thirteen studies were excluded as they were deemed not to be an RCT (Atkinson 1980; Boschi 1984; Brock‐Utne 1989; Chen 2005; Colman 1988; Datta 1982; Dewan 1982; Dundee 1979; Fazel 2017; Qvist 1983; Santos 1984; Sultan 2014; Tanaka 2007).
Seven studies were excluded as the publications had been retracted since our previous review was published.
Risk of bias in included studies
Overall risk of bias is reported in Figure 2 and Figure 3.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Of the 84 studies included in the review, random sequence generation was judged to be of low risk of bias in 38 studies, with 46 studies being judged of unclear risk. Many studies simply stated that "patients were randomised" without providing any further details.
Allocation concealment was generally poorly described. It was judged to be of low risk of bias in five studies and of unclear risk in 79 studies. There were only five studies where both sequence generation and allocation concealment were judged to be of low risk (Abdel‐Aleem 2012; Charuluxananan 2003; Cherian 2001; Tarhan 2007; Uerpairojkit 2017).
Blinding
Blinding was assessed in more detail in this updated review. Blinding was sometimes described poorly, with many studies simply describing a "double blind" design.
We judged blinding of participants and clinicians as of low risk of bias in 30 studies,and of unclear bias in 50studies. Blinding was considered at high risk of bias in four studies because the treating anaesthetist was likely not blinded to the study drug (Direkvand‐Moghadam 2013; Mokini 2014; Rasooli 2014; Rudra 2004a). In all these studies, it seemed some effort at blinding the treating clinician could have been made.
Blinding of outcome assessors was variably described, with 36 studies judged to be of low risk and 46 studies judged to be of unclear risk. Two studies were considered to be of high risk of bias in this regard (Ahn 2002; Mokini 2014).
Incomplete outcome data
Incomplete outcome data were addressed adequately and so at low risk of bias in 47 studies. In 34 studies, it was judged to be of unclear bias, and at high risk of bias in three studies (Baciarello 2011; Cardoso 2013; Duman 2010). In these three studies, data on a significant number of participants were excluded and we were unable to be re‐include on an intention‐to‐treat basis.
Selective reporting
As we were generally not able to assess study protocols, 76 studies were judged to be unclear about selective reporting bias with just one study assessed as low risk (Kalava 2013).. However, seven studies were judged to show a high risk of bias (Ahn 2002; Carvalho 2010; Ibrahim 2019; Jang 1997; Selzer 2020; Tkachenko 2019; Voigt 2013), generally because they did not report outcomes which were pre‐specified in the study methods.
Other potential sources of bias
Thirty‐six studies were judged to be free of other potential sources of bias, with 46 being unclear. Two studies were judged to be at high risk of bias (Habib 2013; Hassanein 2015). One study was conducted at two different centres. There seemed to be many differences in practice between the two centres and the study seemed poorly controlled (Habib 2013). Another study included an unspecified number of women undergoing additional surgical procedures (such as tubal ligation (Hassanein 2015).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
1) 5‐HT3 receptor antagonists versus placebo (25 studies, 3942 women, Comparison 1)
Whilst 25 studies assessed this comparison, only 21 provided usable data on outcomes involving 2686 women (Abouleish 1999; Charuluxananan 2003; Cherian 2001; Dasgupta 2012; El‐Deeb 2011a; Garcia‐Miguel 2000; Harnett 2007; Kasodekar 2006; Koju 2015; Mohammadi 2015; Munnur 2008; Pan 1996; Pan 2001; Pan 2003; Parra‐Guiza 2018; Peixoto 2006, Sahoo 2012; Shen 2012; Uerpairojkit 2017; Voigt 2013; Yazigi 2002). Four studies provided no data which could be included in our analyses (Boone 2002; Lee 2002; Pazoki 2018; Yazigi 2002). Of the studies that provided data, 17 studied ondansetron (Abouleish 1999; Charuluxananan 2003; Cherian 2001; El‐Deeb 2011a; Garcia‐Miguel 2000; Harnett 2007; Koju 2015; Munnur 2008; Pan 1996; Pan 2001; Pan 2003; Parra‐Guiza 2018; Peixoto 2006, Sahoo 2012; Shen 2012; Uerpairojkit 2017; Yazigi 2002), five examined granisetron (Dasgupta 2012; Kasodekar 2006; Lee 2002; Mohammadi 2015; Munnur 2008) and one studied tropisotron (Voigt 2013).
The studies which provided data were undertaken in: USA (six studies); Egypt (two studies); India (two studies); Iran (one study); Iran (one study); Spain (one study); Thailand (one study); and UK (one study).
Of the 21 studies providing data, only three were judged to have had both adequate sequence generation and allocation concealment (Abouleish 1999; Charuluxananan 2003; Cherian 2001). The remaining are unclear. Five studies were considered to have adequate and well‐described blinding (Dasgupta 2012; Mohammadi 2015; Pan 1996; Pan 2001; Peixoto 2006). The remainder are unclear in at least one element (seeFigure 2 and Figure 3).
Primary outcomes
Intraoperative nausea
5‐HT3 antagonists may reduce the number of women having intraoperative nausea (average risk ratio (RR) 0.55, 95% confidence interval (CI) 0.42 to 0.71), 12 studies, 1419 women, random‐effects (T2 = 0.11; Chi2 P = 0.0009; I2 = 65%), Analysis 1.1). The certainty of the evidence was low, downgraded for serious risk of bias and serious inconsistency (Table 1).
1.1. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 1: Nausea ‐ intraoperative
In a subgroup analysis by drug and dose, there was significant difference in treatment effect between the subgroups (Chi² = 7.72, P = 0.05, I² = 61.1%).
The sensitivity analysis left only one study (with 81 women) at low risk of bias across selection and attrition bias and this showed no reduction and a wide CI crossing the line of no difference (average RR 1.11, 95% CI 0.45 to 2.79).
Intraoperative vomiting
5‐HT3 antagonists may lead to a reduction in the number of women having intraoperative vomiting, but the results are very uncertain (average RR 0.46, 95% CI 0.29 to 0.73, 11 studies, 1414 women, random‐effects (T² = 0.27, Chi² P = 0.008, I² = 58%), Analysis 1.2).The certainty of the evidence is very low, downgraded for serious risk of bias, serious inconsistency and some evidence of publication bias (Table 1).
1.2. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 2: Vomiting ‐ intraoperative
In the subgroup analysis by dose of drug, there was no evidence of differences in treatment effect between the subgroups (Chi² = 4.33, P = 0.22, I² = 32.2%).
The sensitivity analysis left only one study (with 81 women) at low risk of bias across selection and attrition bias, it showed a similar result to the main analysis but a wider CI (RR 0.38, 95% CI 0.18 to 0.81).
Postoperative nausea
5‐HT3 antagonists probably reduce the number of women having postoperative nausea (average RR 0.40, 95% CI 0.30 to 0.54, 10 studies, 1340 women, random‐effects (T² = 0.09, Chi² P = 0.10, I² = 37%) (Analysis 1.3). The certainty of the evidence was moderate, downgraded for serious risk of bias (Table 1).
1.3. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 3: Nausea ‐ postoperative
The subgroup analysis by drug and dose did not identify any heterogeneity (Chi² = 0.15, df = 3 (P = 0.99), I² = 0%).
The sensitivity analysis left only two studies (with 338 women) at low risk of bias across selection and attrition bias and this showed similar finding, with a wider CI (average RR 0.56, 95% CI 0.38 to 0.83).
Postoperative vomiting
5‐HT3 antagonists may reduce the number of women having postoperative vomiting (average RR 0.47, 95% CI 0.31 to 0.69, 10 studies, 1450 women, random‐effects (T² = 0.13, Chi² P = 0.10, I² = 37%), Analysis 1.4). The certainty of the evidence was low, downgraded for serious risk of bias and some evidence of publication bias (Table 1).
1.4. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 4: Vomiting ‐ postoperative
The subgroup analysis by drug and dose did not identify any differences (Chi² = 1.15, df = 3 (P = 0.76), I² = 0%).
The sensitivity analysis left only two studies (with 338 women) at low risk of bias across selection and attrition bias and this showed a wider CI crossing the line of no difference (average RR 0.94, 95% CI 0.53 to 1.67).
Secondary outcomes
Intraoperative nausea + vomiting
Only one small study (Voigt 2013) looked at this outcome and so there are insufficient data to make any judgement (Analysis 1.5).
1.5. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 5: 'Nausea + Vomiting' ‐ intraoperative (not pre‐specified)
Postoperative nausea + vomiting
5HT3 antagonists may reduce the number of women having postoperative nausea plus vomiting (RR 0.57, 95% CI 0.41 to 0.80, five studies, 576 women), however, the certainty of the evidence is low due to unclear risk of bias on most aspects including selection and attrition bias. (Analysis 1.6).
1.6. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 6: 'Nausea + Vomiting' ‐ postoperative ‐ (not pre‐specified)
Maternal satisfaction
We identified two differing results in women's satisfaction between the 5‐HT3 receptor antagonist ondansetron and placebo. One study showed a benefit from the ondansetron (RR 1.99, 95%CI 1.35 to 2.94, 1 study, 105 women) (Pan 2001), and the other showed no difference (RR 0.98, 95% CI 0.82 to 1.16, 1 study 81 women) (Cherian 2001) (Analysis 1.7).
1.7. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 7: Maternal satisfaction
Adverse effects and side effects
There were no events in the one study involving 100 women that assessed a composite outcome of adverse effects. There was no indication of adverse effects for a number of outcome measures: headaches/dizziness (average RR 1.04, 95% CI 0.60 to 1.79, 4 studies, 433 women, Analysis 1.9); hypotension (average RR 1.22, 95% CI 0.72 to 2.08, 3 studies 290 women, Analysis 1.10); and pruritis/itching (RR 0.85, 95% CI 0.69 to 1.05, 4 studies 488 women, ,Analysis 1.11); dry mouth (RR 0.75, 95% CI 0.17 to 3.22, 1 study, 130 women, Analysis 1.12); drowsiness/sedation (RR 3.94, 95% CI 0.45 to 34.63, 2 studies, 170 women, Analysis 1.13). .
1.9. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 9: Headache/dizziness/vertigo
1.10. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 10: Hypotension
1.11. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 11: Pruritus/itching
1.12. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 12: Dry mouth
1.13. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 13: Drowsiness/sedation
Rescue antiemetics used: 5HT3 antagonist ondansetron may reduce the use of rescue antiemetics (RR 0.32, 95% CI 0.11 to 0.93, 1 study, 158 women, Analysis 1.14) but more data are needed.
1.14. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 14: Rescue antiemetic (not pre‐specified)
2) Dopamine antagonists versus placebo (24 studies, 2965 women, Comparison 2)
Twenty‐four studies compared dopamine antagonists with placebo, of which 20 studies provided data for analysis involving 1880 women (Biswas 2003; Chestnut 1987; Choi 1999; Direkvand‐Moghadam 2013; Duman 2010; Garcia‐Miguel 2000; Habib 2013; Huang 1992; Kampo 2019; Khalayleh 2005; Lussos 1992; Mandell 1992; Maranhao 1988; Mokini 2014; Pan 1996; Pan 2001; Peixoto 2006; Stein 1997; Tzeng 2000; Wu 2007). Four studies provided no data which could be included in our analyses (Birnbach 1993; Imbeloni 1986; Kim 1999; Sanansilp 1998). Of the studies providing data, 15 studied metoclopramide (Biswas 2003; Chestnut 1987; Choi 1999; Direkvand‐Moghadam 2013; Duman 2010; Garcia‐Miguel 2000; Habib 2013; Huang 1992; Kampo 2019; Khalayleh 2005; Lussos 1992; Maranhao 1988; Mokini 2014; Pan 2001; Stein 1997). Five examined droperidol (Mandell 1992; Pan 1996; Peixoto 2006; Tzeng 2000; Wu 2007).
The 20 studies which provided data were undertaken in: USA (seven studies); one in the USA and Canada, Taiwan (two studies); India (one study); Iran (two studies); Spain (one study); Turkey (one study); Africa (one study) South America (two studies) and two studies where the setting was not described.
Overall, the studies were of uncertain or variable quality. Of the 20 studies which provided data, only three were judged to have had both adequate random sequence generation and allocation concealment (Chestnut 1987; Habib 2013; ; Stein 1997). Nine studies were judged to have had adequately described blinding (Chestnut 1987; Duman 2010; Habib 2013; ; Pan 1996; Pan 2001; Peixoto 2006; Stein 1997; Tzeng 2000; Wu 2007). All the remaining being unclear (Figure 2) except for one study where it seemed likely that the patient and clinicians would both have been aware of the group allocation (Direkvand‐Moghadam 2013). study appeared at high risk of bias due to missing data, where substantial numbers of patients were excluded after randomisation and weren't able to be re‐included (Duman 2010).
Primary outcomes
Intraoperative nausea
Dopamine antagonists may reduce the number of women having intraoperative nausea but the results are very uncertain (average RR 0.38, 95% CI 0.27 to 0.52, 15 studies, 1180 women, random‐effects (T² = 0.19, Chi² P = 0.005, I² = 54%), Analysis 2.1). The certainty of the evidence was very low, downgraded for very serious risk of bias and serious inconsistency (Table 2).
2.1. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 1: Nausea ‐ intraoperative
In the subgroup analysis by dose and drug, there was no evidence of differences in treatment effects between the subgroups (Chi² = 0.79, df = 6 (P = 0.99), I² = 0%)..
We could not undertake a sensitivity analysis because none of the studies providing data were at low risk of bias across selection and attrition bias. .
Intraoperative vomiting
Dopamine antagonists may reduce the number of women with intraoperative vomiting (average RR 0.41, 95% CI 0.28 to 0.60, 12 studies, 942 women, random‐effects, T² = 00.02, Chi² P = 0.40, I² = 5%, Analysis 2.2). The certainty of the evidence was low, downgraded for very serious risk of bias.
2.2. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 2: Vomiting ‐ intraoperative
In the subgroup analysis by drug and dose, there was no evidence of differences in treatment effects between the subgroups Chi² = 2.70, df = 5 (P = 0.75), I² = 0%.
We could not undertake a sensitivity analysis because none of the studies providing data were at low risk of bias across selection and reporting bias, and this also showed a reduced relative risk but a CI that crossed the line of no difference (average RR 0.34, 95% CI 0.10 to 1.23).
Postoperative nausea
Dopamine antagonists may reduce the number of women with postoperative nausea (average RR 0.61, 95% CI 0.48 to 0.79, 7 studies, 601 women, random‐effects, T² = 0.01, Chi² P = 0.35, I² = 10%, Analysis 2.3). The certainty of the evidence is low, downgraded for very serious risk of bias (Table 2).
2.3. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 3: Nausea ‐ postoperative
In the subgroup analysis by drug and dose, there was no evidence of differences in treatment effects between subgroups (Chi² = 2.84, df = 2 (P = 0.24), I² = 29.5%)
We could not undertake a sensitivity analysis because none of the studies providing data were at low risk of bias across selective and reporting bias,
Postoperative vomiting
Dopamine antagonists may lead to a reduction in the number of women having postoperative vomiting but the results are very uncertain (average RR 0.63, 95% CI 0.44 to 0.92, 9 studies, 860 women, T² = 0.13, Chi² P = 0.08, I² = 43%, Analysis 2.4). The certainty of the evidence is very low due to very serous risk of bias and some evidence of publication bias. (Table 2), . These findings were broadly consistent when the individual interventions of metoclopramide and droperidol were assessed separately.
2.4. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 4: Vomiting ‐ postoperative
In the subgroup analysis by type and dose of drug, there was no evidence of differences in treatment effects between subgroups (Chi² = 2.03, df = 2 (P = 0.36), I² = 1.3%).
We could not undertake a sensitivity analysis because none of the studies providing data were at low risk of bias across selective and reporting bias.
Secondary outcomes
Intraoperative nausea + vomiting
There is only one small study with 98 women so the findings are very uncertain (average RR 0.12, 95% CI 0.02 to 0.88, Analysis 2.5).
2.5. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 5: 'Nausea + vomiting' ‐ intraoperative (not pre‐specfied)
Postoperative nausea + vomiting
There are four studies involving 450 women, so the findings are uncertain (average RR 0.23, 95% CI 0.05 to 1.02, Analysis 2.6). However, one study has a very extreme result (Kampo 2019). We have checked the paper and can find nothing to explain this result, so we also report the findings as well excluding these data (average (RR 0.49, 95% CI 0.32 to 0.75, 3 studies, 220 women).
2.6. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 6: 'Nausea + vomiting' ‐ postoperative (not pre‐specified)
Maternal satisfaction
We identified no overall difference in women's satisfaction between dopamine antagonists and placebo (RR 1.42, 95% CI 0.91 to 2.21, 1 study, 102 women, Analysis 2.7).
2.7. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 7: Maternal satisfaction
Adverse effects and side effects
Although there were no estimates of a composite outcome of adverse effects, a few studies did measure anxiety, headaches/dizziness, hypotension and pruritus. There were no differences identified (Analysis 2.8; Analysis 2.9; Analysis 2.10; Analysis 2.13).
2.8. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 8: Anxiety
2.9. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 9: Headache/dizziness
2.10. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 10: Hypotension
2.13. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 13: Pruritus/itching
Subgroup analyses
For possible variations between individual drugs, seeAnalysis 2.1 to Analysis 2.7.
3) Corticosteroids versus placebo (15 studies, 1830 women, Comparison 3)
Fifteen studies looked at corticosteroids versus placebo, of which 12 studies involving 1182 women provided data for the review. Studies compared corticosteroids against placebo, all studied dexamethasone but in various doses from 2.5 mg to 10 mg (Abdel‐Aleem 2012; Biswas 2003; Cardoso 2013; Hassanein 2015; Jaafarpour 2008; Nortcliffe 2003; Parra‐Guiza 2018; Selzer 2020; Tkachenko 2019; Tzeng 2000; Wang 2001; Wu 2007). Two included studies provided no data for the review (Lim 2001b; Modir 2019).
The studies were undertaken in: Taiwan (three studies); Egypt (two studies); Brazil (one study); India (one study); Iran (one study), Colombia (one study), Ukraine (one study), USA (one study)and UK (one study).
The studies were of questionable quality with only one being judged as having adequate sequence generation and allocation concealment (Abdel‐Aleem 2012; ). Six had adequate blinding (Abdel‐Aleem 2012; Hassanein 2015; Selzer 2020; Tzeng 2000; Wang 2001; Wu 2007). One study excluded many women after randomisation if they suffered intraoperative nausea or vomiting and this amounted to 31% of the enrolled subjects (Abdel‐Aleem 2012). Another study excluded 46% of women after randomisation if they were not the first patient of the day (Cardoso 2013).
Primary outcomes
Intraoperative nausea
Dexamethasone may reduce the number of women having intraoperative nausea but the results are very uncertain (average RR 0.56, 95% CI 0.37 to 0.83, 6 studies, 609 women, random‐effects (T² = 0.12, Chi² P = 0.06, I² = 50%), Analysis 3.1), The certainty of the evidence was very low, downgraded for very serious risk of bias and serious inconsistency (Table 3).
3.1. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 1: Nausea ‐ intraoperative
In the subgroup analysis by dose of drug and route of administration (intravenous and intrathecal) , there was evidence of differences between the various doses and routes of administration (Chi² = 7.54, df = 2 (P = 0.02), I² = 73.5%).
The sensitivity analysis could not be undertaken as none of the included studies were low risk for selection.and attrition bias.
Intraoperative vomiting
Dexamethasone may reduce the number of women having intraoperative vomiting but the results are very uncertain (average RR 0.52, 95% CI 0.31 to 0.87, 6 studies, 609 women, random‐effects (T² = 0.00, Chi² P = 0.69, I² = 0%, Analysis 3.2). The certainty of the evidence being very low, downgraded for very serious risk of bias, and serious imprecision (Table 3).
3.2. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 2: Vomiting ‐ intraoperative
The subgroup analysis by dose of drug and route of administration (intravenous and intrathecal showed no difference between the subgroups (Chi² = 1.80, df = 2 (P = 0.41), I² = 0%).
The sensitivity analysis could not be undertaken as none of the included studies were low risk for selection and attrition bias.
Postoperative nausea
Dexamethasone probably reduces the number of women having postoperative vomiting (average RR 0.59, 95% CI 0.49 to 0.73, 6 studies, 733 women, random‐effects (T² = 0.01, Chi² P = 0.36, I² = 9%), Analysis 3.3). The certainty of the evidence was moderate, downgraded for serious risk of bias.
3.3. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 3: Nausea ‐ postoperative
The subgroup analyses by dose of drug and route of administration (intravenous and intrathecal) showed no difference between the subgroups (Chi² = 6.87, df = 5 (P = 0.23), I² = 27.2%).
The sensitivity analysis could not be undertaken as none of the included studies were low risk for selection.and attrition bias. .
Postoperative vomiting
Dexamethasone may reduce the number of women having postoperative vomiting (average RR 0.68, 95% CI 0.49 to 0.95, 7 studies, 793 women, random‐effects (T² = 0.11, Chi² P = 0.03, I² = 52%) Analysis 3.4). The certainty of the evidence was low, downgraded for serious risk of bias and serious inconsistency (Table 3).
3.4. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 4: Vomiting ‐ postoperative
The subgroup analysis by dose of drug and route of administration (intravenous and intrathecal) showed some variation (Chi² = 14.65, df = 5 (P = 0.01), I² = 65.9%).
The sensitivity analysis could not be undertaken as none of the included studies were low risk for selection.and attrition bias..
Secondary outcomes
Intraoperative nausea + vomiting
We identified only one study of 108 women (Selzer 2020) so the findings are very uncertain (average RR 1.65, 95% CI 0.96 to 2.84, Analysis 3.5).
3.5. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 5: 'Nausea + Vomiting' ‐ intraoperative (not pre‐specified)
Postoperative nausea + vomiting
We identified only one study of 108 women (Selzer 2020) so the findings are very uncertain (RR 0.94, 95% CI 0.79 to 1.12, Analysis 3.6).
3.6. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 6: 'Nausea + Vomiting' ‐ postoperative ‐ (not pre‐specified)
Adverse effects and side effects
Although there were no estimates of a composite outcome of adverse effects, a few studies did measure hypotension and pruritis but there were insufficient data to make any firm statement about adverse effects (Analysis 3.7; to Analysis 3.10).
3.7. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 7: Hypotension
3.10. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 10: Rescue antiemetics (not pre‐specified)
4) Antihistamines versus placebo (4 studies, 654 women, Comparison 4)
Four studies compared antihistamines with placebo, all studies providing data on 514 women (Apiliogullari 2007; Carvalho 2010; Duman 2010; Nortcliffe 2003).
The studies were undertaken in: Iran (one study); Turkey (one study) Canada (one study) and UK (one study).
Only one study had adequate sequence generation (Duman 2010). All four studies were unclear with regard to allocation concealment. Only one study had adequate blinding (Duman 2010). Two studies were assessed as high risk of bias ‐ one due to missing data that could not be re‐included (Duman 2010) and one due to pre‐specified outcomes not reported (Carvalho 2010).
Primary outcomes
Intraoperative nausea
Antihistamines (e.g. dimenhydrinate ) may make little of no difference to intraoperative nausea (RR 0.99, 95% CI 0.47 to 2.11, 1 study, 149 women, Analysis 4.1). The certainty of the evidence was very low, downgraded for very serious risk of bias, and very serious imprecision Table 4).
4.1. Analysis.

Comparison 4: Antihistamines vs placebo, Outcome 1: Nausea ‐ intraoperative
It was not possible to undertake subgroup analysis as only one study assessed this outcome (Carvalho 2010).
It was not possible to undertake sensitivity analysis as only one study (with unclear risk of selection and reporting bias) assessed this outcome (Carvalho 2010).
Intraoperative vomiting
Antihistamines (e.g. dimenhydrinate ) may make little of no difference to intraoperative nausea as there were no events in the one study of 149 women looking at this outcome and the GRADE assessment of this study was very low due to very serious risk of bias and very serious imprecision (Analysis 4.2, Table 4).
4.2. Analysis.

Comparison 4: Antihistamines vs placebo, Outcome 2: Vomiting ‐ intraoperative
It was not possible to undertake subgroup analysis as only one study assessed this outcome (Carvalho 2010).
It was not possible to undertake sensitivity analysis as only one study (with unclear risk of selection and reporting bias) assessed this outcome (Carvalho 2010).
Postoperative nausea
Antihistamines may lead to a reduction in postoperative nausea (average RR 0.44, 95% CI 0.30 to 0.64, 4 studies, 514 women, random‐effects (T² = 0.02, Chi² P = 0.34, I² = 11%), Analysis 4.3) as the certainty of the evidence is low, downgraded for very serious risk of bias. (Table 4).
4.3. Analysis.

Comparison 4: Antihistamines vs placebo, Outcome 3: Nausea ‐ postoperative
In the subgroup analysis by type and dose of drug, there was no evidence of differences in treatment effects between subgroups (Chi² = 3.98, df = 3 (P = 0.26), I² = 24.6%).
In the sensitivity analysis there were no studies with low risk of selection and attrition bias.
Postoperative vomiting
Antihistamines may lead to a reduction in postoperative vomiting but the results are very uncertain (average RR 0.48, 95% CI 0.29 to 0.81, 3 studies, 333 women, random‐effects (T² = 0.00, Chi² P = 0.66, I² = 0%), Analysis 4.4), The certainty of the evidence is very low, downgraded for very serious risk of bias and serious imprecision (Table 4).
4.4. Analysis.

Comparison 4: Antihistamines vs placebo, Outcome 4: Vomiting ‐ postoperative
The subgroup analysis by type of drug or dose showed no difference between the subgroups (Chi² = 0.79, df = 2 (P = 0.67), I² = 0%).
In the sensitivity analysis there were no studies with low risk of selection and attrition bias.
Secondary outcomes
None of the studies looked at intraoperative 'nausea + vomiting nor postoperative 'nausea + vomiting'.
Adverse effects and side effects
Although there were no estimates of a composite outcome of adverse effects, one study involving 149 women looked at hypotension (Carvalho 2010, Analysis 4.5), but there were insufficient data to make any firm statement about hypotension.
4.5. Analysis.

Comparison 4: Antihistamines vs placebo, Outcome 5: Hypotension
5) Anticholenergics versus placebo (7 studies, 1088 women, Comparison 5)
Seven studies compared anticholinergics with placebo, with six of these studies (involving 787 women) reporting data that we could use in the review (Baciarello 2011; Biswas 2003; Harnett 2007; Kotelko 1989; Shen 2012; Ure 1999). One study provides no data for the review as they present data across multiple time periods and it is unclear if women have been counted multiple times (Quiney 1995).
The studies which provided data were undertaken in: USA (two studies); India (one study), Italy (one study), China (one study) and UK (one study).
Two studies had adequate sequence generation (Baciarello 2011; Harnett 2007). The other four were unclear. One study had adequate allocation concealment (Baciarello 2011) the others studies were unclear. Only one study described adequate blinding of participants and outcome assessment (Baciarello 2011). One study was judged to be at high risk of income data as 12 participants were excluded after randomisation (Baciarello 2011).
Primary outcomes
Intraoperative nausea
Anticholinergics may reduce intraoperative nausea (average RR 0.67, 95% CI 0.51 to 0.87, 4 studies, 453 women, random‐effects (T² = 0.03, Chi² P = 0.13, I² = 47%), Analysis 5.1). The certainty of the evidence was low, downgraded for very serious risk of bias (Analysis 5.1).
5.1. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 1: Nausea ‐ intraoperative
The subgroup analyses by type of drug or dose showed no difference between the subgroups (Chi² = 0.70, df = 1 (P = 0.40), I² = 0%).
In the sensitivity analysis there were no studies with low risk of selection and attrition bias.
Intraoperative vomiting
Anticholenergics may make little or no difference to intraoperative vomiting (average RR 0.79, 95% CI 0.40 to 1.54, 4 studies, 453 women, random‐effects (T² = 0.22, Chi² P = 0.10, I² = 52%), Analysis 5.2). The certainty of the evidence is very low, downgraded for very serious risk of bias, serious inconsistency and serious imprecision (Table 5).
5.2. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 2: Vomiting ‐ intraoperative
These findings were persistent in the scopolamine subgroup, but not the glycopyrrolate subgroup, however, there were much smaller numbers in the glycopyrrolate subgroup.
In a subgroup analysis by type of drug and dose, showed no difference between the subgroups (Chi² = 0.86, df = 1 (P = 0.35), I² = 0%)
In the sensitivity analysis there were no studies with low risk of selection and attrition bias.
Postoperative nausea
None of the studies assessed postoperative nausea.
Postoperative vomiting
Only one study of 161 women looked at this outcome (Harnett 2007). So we are very uncertain whether anticholinergics reduce postoperative vomiting (RR 0.55, 95% CI 0.41 to 0.74, 1 study, 161 women, (Analysis 5.4). The certainty of the evidence was low, downgraded for serious risk of bias and serious imprecision (Table 5).
5.4. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 4: Vomiting ‐ postoperative
There were no assessments on subgroups nor sensitivity because there was only one study..
Secondary outcomes
Intraoperative 'nausea + vomiting'
None of the studies assessed this outcome.
Postoperative 'nausea + vomiting'
Anticholinergics may reduce the number of women with postoperative nausea and vomiting (average RR 0.46, 95% CI 0.25 to 0.85, 2 studies, 334 women, Analysis 5.6) but the certainty of the evidence is very low coming from just two small studies, so overall anticholinergics may make little or no difference.
5.6. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 6: 'Nausea + vomiting' ‐ postoperative (not pre‐specified)
Adverse effects and side effects
Although there were no estimates of a composite outcome of adverse effects, a few studies did measure a number of adverse and side effects, including blurred vision, anxiety/disorientation and dizziness, (Analysis 5.7 to Analysis 5.13) but we feel there are insufficient data to make any firm statement about adverse or side effects.
5.7. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 7: Blurred vision
5.13. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 13: Drowsiness
6) Sedatives versus placebo (17 studies, 1730 women, Comparison 6)
Seventeen studies assessed sedatives versus placebo, with 13 providing analysable data on 1265 women. Most studies assessed propofol but at differing doses (Ahn 2002; Caba 1997; Kampo 2019; Mokini 2014; Mukherjee 2006; Niu 2018; Rasooli 2014; Rudra 2004a; Tarhan 2007). Two studies assessed midazolam, two intravenous (Rasooli 2014; Tarhan 2007) and one intrathecal (Abdollahpour 2015). Two studies assessed ketamine (Hassanein 2015; Shabana 2012). One study provided data in graphical form only (Weiss 1995) and we have written to the authors to obtain the numerical data. One study provided outcome data as percentages and it was unclear how many women were in each group (Modir 2019).
The studies which provided data were undertaken in: India (two studies); Iran (two studies); Korea (one study) Egypt (two studies); Spain (one study) Ghana (one study), China (one study) and Turkey (one study). One study did not specify where it was conducted.
The 12 studies providing data appeared to be of reasonable quality with eight studies having adequate random sequence generation but only three having adequate allocation concealment. Only four of the 12 studies had adequate blinding (Hassanein 2015; Niu 2018; Mukherjee 2006; Tarhan 2007). Four studies were rated as inadequate blinding, where it was highly likely that the participants and/or clinicians would have been aware of the group allocation (Ahn 2002; Mokini 2014; Rasooli 2014; Rudra 2004a).
Primary outcomes
Intraoperative nausea
Sedatives probably reduce the number of women having intraoperative nausea (average RR 0.65, 95% CI 0.51 to 0.82, 8 studies, 593 women, random‐effects (T² = 0.00, Chi² P = 0.64, I² = 0%), Analysis 6.1). The certainty of the evidence was moderate, downgraded for serious risk of bias (Table 6).
6.1. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 1: Nausea ‐ intraoperative
Subgroup analysis by type of drug and dose showed no difference between the subgroups (Chi² = 5.78, df = 7 (P = 0.57), I² = 0%).
The sensitivity analysis included one study (with 88 women) at low risk of bias of selection and attrition bias, and this showed similar findings to the main analysis although the lower CI now crosses the line of no difference (RR 0.76, 95% CI 0.53 to 1.08).
Intraoperative vomiting
Sedatives probably reduce the number of women with intraoperative vomiting (average RR 0.35, 95% CI 0.24 to 0.52, 8 studies, 593 women, random‐effects (T² = 0.00, Chi² P = 0.55, I² = 0%), Analysis 6.2). The certainty of the evidence was moderate, downgraded for serious risk of bias (Table 6).
6.2. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 2: Vomiting ‐ intraoperative
Subgroup analysis by type of drug and dose showed no difference between the subgroups (Chi² = 3.97, df = 7 (P = 0.78), I² = 0%).
The sensitivity analysis included one (with 88 women) at low risk of bias of selection and attrition bias, and this showed similar findings to the main analysis (RR 0.43, 95% CI 0.20 to 0.95).
Postoperative nausea
Sedatives may reduce the number of women with postoperative nausea but the results are very uncertain (average RR 0.25, 95% CI 0.09 to 0.71, 2 studies, 145 women, (T² = 0.47, Chi² P = 0.09, I² = 58%), Analysis 6.3) The certainty of the evidence was very low, downgraded for serious inconsistency and very serious imprecision (Table 6).
6.3. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 3: Nausea ‐ postoperative
Subgroup analysis by type of drug and dose showed some difference between the groups (Chi² = 4.64, df = 2 (P = 0.10), I² = 56.9%).
The sensitivity analysis included one study (with 88 women) at low risk of bias of selection and attrition bias, and this showed similar findings to the main analysis although the upper CI is further away from the line of no difference ( RR 0.17, 95% CI 0.09 to 0.31).
Postoperative vomiting
Sedatives may reduce the number of women with postoperative vomiting (average RR 0.09, 95% CI 0.03 to 0.28, 2 studies, 145 women, (T² = 0.00, Chi² P = 0.39, I² = 0%), Analysis 6.4). The certainty of the evidence was low, downgraded for very serious imprecision (Table 6).
6.4. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 4: Vomiting ‐ postoperative
Subgroup analysis by type of drug and dose showed no difference between the groups (Chi² = 1.77, df = 2 (P = 0.41), I² = 0%).
The sensitivity analysis included one study (with 88 women) at low risk of bias of selection and attrition bias, and this showed similar findings to the main analysis although the upper CI is further away from the line of no difference ( (RR 0.07, 95% CI 0.02 to 0.24).
Secondary outcomes
Intraoperative 'nausea & vomiting'
None of the studies assessed this outcome.
Postoperative 'nausea & vomiting'
Sedatives may reduce the incidence of postoperative nausea and vomiting (average RR 0.06, 95% CI 0.02 to 0.22, 2 studies, 348 women, Analysis 6.6) . However, one study has a very extreme result (Kampo 2019). We have checked the paper and can find nothing to explain this result, so we also report the findings, as well excluding these data (average (RR 0.12, 95% CI 0.04 to 0.36, 1 study, 118 women).
6.6. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 6: 'Nausea + vomiting' ‐ postoperative (not pre‐specified)
Adverse and side effects:
Although there were no estimates of a composite outcome of adverse effects, a few studies did measure a number of maternal adverse and side effects (Analysis 6.7 to Analysis 6.9).
6.7. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 7: Pruritis/itching
6.9. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 9: Shivering
One study of 80 women (Niu 2018) reported no babies had Apgar scores less than seven at five minutes in either group. Also that all women in both groups initiated breastfeeding.
7) Opioids antagonists versus placebo (4 studies, 380 women, Comparison 7)
Four studies were eligible for inclusion in this comparison (Abdollahpour 2015; Charuluxananan 2003; Ibrahim 2019; Jang 1997), but only two provided data for analysis involving 197 women (Charuluxananan 2003; Ibrahim 2019). Three studies compared opioid antagonists with placebo (Abdollahpour 2015; Charuluxananan 2003; Ibrahim 2019): two studies assessed nalbuphine, one intravenously (Charuluxananan 2003) and the other intrathecally (Ibrahim 2019); and one study assessed intrathecal sufentanil (Abdollahpour 2015). The fourth study assessed butorphanol, and although the abstract was in English, we have been unable to get the full paper translated from Korean to analyse any of the data (Jang 1997).
The studies were undertaken in Iran, Thailand, Egypt and South Korea.
Both studies providing data were judged to have adequate random sequence generation, although blinding was not well described and judged to be unclear in both studies. The studies were also judged to be of unclear risk for other biases.
Primary outcomes
Intraoperative nausea
None of the studies assessed this outcome.
Intraoperative vomiting
None of the studies assessed this outcome.
Postoperative nausea
It is uncertain whether opioid antagonists may reduce, increase or may make little no difference to the number of women having postoperative nausea (RR 0.75, 95% CI 0.39 to 1.45, 1 study, 120 women, Analysis 7.3). The certainty of the evidence was low, downgraded due to very serious imprecision (Table 7).
7.3. Analysis.

Comparison 7: Opioid antagonist/partial agonist vs placebo, Outcome 3: Nausea ‐ postoperative
It was not possible to undertake a subgroup analysis by drug and dose because there was only one study reporting this outcome.,
It was not possible to undertake a sensitivity analysis as there was only one study assessing postoperative nausea and vomiting and this study was low risk for selection and attrition bias.
Postoperative vomiting
It is uncertain whether opioid antagonists may reduce the number of women having intraoperative vomiting (RR 1.25, 95% CI 0.35 to 4.43, 1 study, 120 women, Analysis 7.4). The certainty of the evidence was low, downgraded for very serious imprecision (Table 7).
7.4. Analysis.

Comparison 7: Opioid antagonist/partial agonist vs placebo, Outcome 4: Vomiting ‐ postoperative
It was not possible to undertake a subgroup analysis by drug and dose because there was only one study reporting this outcome.,
It was not possible to undertake a sensitivity analysis as there was only one study assessing postoperative nausea and vomiting and this study was low risk for selection and attrition bias.
Secondary outcomes
Intraoperative 'nausea & vomiting'
No studies assessed this outcome.
Postoperative 'nausea & vomiting'
Nalbuphine (an opioid antagonist) may reduce postoperative nausea & vomiting (RR 0.09, 95% CI 0.02 to 0.37, 1 study, 77 women, nAnalysis 7.6), but the certainty of the evidence is very low and further data are needed.
7.6. Analysis.

Comparison 7: Opioid antagonist/partial agonist vs placebo, Outcome 6: 'Nausea + vomiting' ‐ postoperative (not pre‐specified)
Adverse effects and side effects
Only two studies assessed pruritis as a side effect (Analysis 7.7) with markedly different results, so we do not have enough data on which to make a meaningful assessment.
7.7. Analysis.

Comparison 7: Opioid antagonist/partial agonist vs placebo, Outcome 7: Pruritus/itching
8) Acupressure/acupuncture versus placebo (14 studies, 1818 women, Comparison 8)
Fourteen studies compared acupressure/acupuncture with placebo, with 11 studies providing data on 1401 women (Direkvand‐Moghadam 2013; Duggal 1998; El‐Deeb 2011a; Habib 2006; Harmon 2000; Ho 1996; Ho 2006; Levin 2019; Li 2012; Noroozinia 2013; Stein 1997). One study addressed this question but provided graphical data only (Birnbach 1993). Data from two studies were not included as it was unclear how many women were allocated to each group (Lim 2001a; Lim 2001b). All eleven studies looked at acupressure, and none studies acupuncture.
The studies which provided data were undertaken in: USA (three studies); Iran (two studies); Canada (one study); China (two studies); Egypt (one study), Ireland (one study) and one study in the USA and Canada.
The studies providing data were of borderline quality with only four out of 11 describing adequate blinding of all relevant parties, and a further two studies providing an incomplete description of blinding. One study was judged at high risk of bias as it seemed likely the participants and treating clinicians were not blinded to group allocation (Direkvand‐Moghadam 2013). However, only four of the nine studies described adequate random sequence generation and only four adequate allocation concealment. We assessed the studies as low quality using GRADE criteria on the basis of inconsistency and imprecision.
Primary outcomes
Intraoperative nausea
Acupressure/acupuncture may reduce the number of women having intraoperative nausea but the results are very uncertain (average RR 0.55, 95% CI 0.41 to 0.74), 9 studies, 1221 women, random‐effects (T² = 0.12, Chi² P = 0.0001, I² = 69%), Analysis 8.1). The certainty of the evidence is very low, downgraded for very serious risk of bias and serious inconsistency (Table 8).
8.1. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 1: Nausea ‐ intraoperative
It was not possible to undertake a subgroup analysis as all the studies used acupressure and we did not differentiate between the different types of acupressure.
It was not possible to undertake a sensitivity analysis as none of the studies providing data were at low risk of selection and reporting bias.
Intraoperative vomiting
Acupressure/acupuncture may reduce the number of women having intraoperative vomiting (average RR 0.52, 95% CI 0.33 to 0.80, 9 studies, 1221 women) (random‐effects (T² = 0.18, Chi² P = 0.05, I² = 47%), Analysis 8.2) . The certainty of the evidence is low, downgraded for very serious risk of bias (Table 8).
8.2. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 2: Vomiting ‐ intraoperative
It was not possible to undertake a subgroup analysis as all the studies used acupressure and we did not differentiate between the different types of acupressure.
It was not possible to undertake a sensitivity analysis as none of the studies providing data were at low risk of selection and reporting bias.
Postoperative nausea
Acupressure/acupuncture may reduce the number of women having postoperative nausea but the results are very uncertain (average RR 0.46, 95% CI 0.27 to 0.75, 7 studies, 1069 women, random‐effects (T² = 0.32, Chi² P = 0.0001, I² = 81%), Analysis 8.3). The certainty of the evidence is very low downgraded for very serious risk of bias and serious inconsistency (Table 8).
8.3. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 3: Nausea ‐ postoperative
It was not possible to undertake a subgroup analysis as all the studies used acupressure and we did not differentiate between the different types of acupressure.
It was not possible to undertake a sensitivity analysis as none of the studies were low risk for selection and attrition bias.
Postoperative vomiting
Acupressure/acupuncture may reduce the number of women having postoperative vomiting but the results are very uncertain (average RR 0.52, 95% CI 0.34 to 0.79, 7 studies, 1069 women, random‐effects (T² = 0.17, Chi² P = 0.01, I² = 62%), Analysis 8.4). The certainty of the evidence is very low downgraded for very serous risk of bias and serious inconsistency (Table 8).
8.4. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 4: Vomiting ‐ postoperative
It was not possible to undertake a subgroup analysis as all the studies used acupressure and we did not differentiate between the different types of acupressure.
It was not possible to undertake a sensitivity analysis as none of the studies were low risk for selection and attrition bias.
Secondary outcomes
None of the studies reported intraoperative 'nausea + vomiting' nor postoperative 'nausea + vomiting'.
Adverse effects and side effects
It is uncertain whether acupressure/acupuncture increases side effects of anxiety, dizziness, hypotension or itching because the certainty of the evidence is very low (Analysis 8.5 to Analysis 8.8). Acupressure/acupuncture may reduce the use of rescue antiemetics (average RR 0.50, 95% CI 0.36 to 0.71, 2 studies, 240 women, Analysis 8.9) but the certainty of the evidence is very low downgraded for very severs risk of bias and very severe imprecision (Table 8).
8.5. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 5: Anxiety
8.8. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 8: Pruritus/itching
8.9. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 9: Rescue antiemetic (not pre‐specified)
9) Ginger versus placebo (2 studies, 365 women, Comparison 9)
Two studies compared oral ginger with placebo included 365 women both provided data for the review (Kalava 2013; Zeraati 2016). One study described adequate random sequence generation, but both were unclear regarding allocation concealment and blinding. They were judged to be of low or unclear risk for other biases.
One study was undertaken in USA and one in Iran.
Primary outcomes
Intraoperative nausea
It is uncertain whether ginger reduces, increases or makes little to no difference in the number of women having intraoperative nausea (average RR 0.66, 95% CI 0.36 to 1.21, 2 studies, 331 women, random‐effects (T² = 0.15, Chi² P = 0.05, I² = 74%), Analysis 9.1. The certainty of the evidence is very low, downgraded for very serious risk of bias, serious inconsistency and serious imprecision (Table 9).
9.1. Analysis.

Comparison 9: Ginger vs placebo, Outcome 1: Nausea ‐ intraoperative
In a subgroup analysis by dose, there was evidence of subgroup difference ( Chi² = 3.70, df = 1 (P = 0.05), I² = 73.0%).
The sensitivity analysis was not undertaken as neither of the studies were at low risk of bias of selection and attrition bias,
Intraoperative vomiting
Ginger may reduce the number of women having intraoperative vomiting or may make little or no difference (average RR 0.62, 95% CI 0.38 to 1.00, 2 studies, 331 women, random‐effects (T² = 0.06, Chi² P = 0.16, I² = 49%), Analysis 9.2). The certainty of the evidence was very low, downgraded for very serious risk of bias, and serious imprecision (Table 9).
9.2. Analysis.

Comparison 9: Ginger vs placebo, Outcome 2: Vomiting ‐ intraoperative
In a subgroup analysis by dose there was no evidence of a difference but more data are needed to be sure (Chi² = 1.97, df = 1 (P = 0.16), I² = 49.2%).
The sensitivity analysis was not undertaken as neither of the studies were at low risk of bias of selection and attrition bias,
Postoperative nausea
It is uncertain whether ginger reduces, increases or makes little to no difference to the number of women having postoperative nausea (average RR 0.63, 95% CI 0.22 to 1.77, 1 study, 92 women, Analysis 9.3). The certainty of the evidence was very low, downgraded for very serious risk of bias, and very serious imprecision (Table 9).
9.3. Analysis.

Comparison 9: Ginger vs placebo, Outcome 3: Nausea ‐ postoperative
There is no subgroup analysis nor sensitivity analysis as there was only one study.
Postoperative vomiting
It is uncertain whether ginger reduces increases, decreases or makes little to no difference to the number of women having postoperative vomiting (average RR 0.20, 95% CI 0.02 to 1.65, 1 study, 92 women, Analysis 9.4). The certainty of the evidence was very low, downgraded for very serious risk of bias and very serious imprecision (Table 9).
9.4. Analysis.

Comparison 9: Ginger vs placebo, Outcome 4: Vomiting ‐ postoperative
There is no subgroup analysis nor sensitivity analysis as there was only one study.
Secondary outcomes
The studies did not report any of our secondary outcomes.
Discussion
Summary of main results
We found 84 included studies involving 10,990 women with 69 studies providing useable data on 8928 women, and this covered nine comparisons. The certainty of the data was generally low and very low, mainly due to many of the studies being quite old and undertaken in times when methodological information was not required in publications, hence risk of bias is generally unclear and also many studies are small.
Placebo‐controlled studies
1. 5‐HT3 antagonists. In the 21 studies (involving 2686 women) that provided data, overall, we found that 5‐HT3 antagonists (mainly ondansetron and granisetron) probably reduces postoperative nausea (moderate‐certainty evidence), may be effective in reducing intraoperative nausea and postoperative vomiting (low‐certainty evidence), but the effect on intraoperative vomiting is uncertain (very low‐certainty evidence) . There were no indications of adverse effects such as headaches, dizziness, hypotension and itchiness, although more data are needed.
2. Dopamine antagonists. In 20 studies (involving 1880 women) that provided data, we found that dopamine antagonists (both metoclopramide and droperidol) may be effective in reducing intraoperative vomiting and postoperative nausea (low‐certainty evidence), but it is uncertain whether they reduce intraoperative nausea and postoperative vomiting (very low‐certainty evidence). These results were broadly consistent with both metoclopramide and droperidol. However, there were insufficient data to determine if there were significant adverse effects like headaches, dizziness, hypotension and pruritus.
3. Corticosteroids. In 12 studies (involving 1182 women) that provided data, corticosteroids probably reduce postoperative nausea (moderate‐certainty evidence) and may reduce postoperative vomiting (low‐certainty evidence). We are uncertain whether corticosteroids reduce intraoperative nausea and vomiting (very low‐certainty evidence). There were limited data on adverse effects.
4. Antihistamines. In four studies (involving 514 women) that provided data, antihistamines (mainly dimenhydrinate and cyclizine) may reduce postoperative nausea (low‐certainty evidence) but may make little to no difference to intraoperative nausea, intraoperative vomiting (no events in the 149 women where this outcome was assessed) and postoperative vomiting (very low‐certainty evidence). Only one small study looked at the adverse effect of hypotension.
5. Anticholenergic drugs. In the six studies (involving 787 women) that provided data, we found that anticholinergic drugs (mainly glycopyrrolate and scopolamine) may be effective at reducing intraoperative nausea and postoperative vomiting (low‐certainty evidence) but may have little or no effect on intraoperative vomiting (very low‐certainty evidence). No study assessed postoperative nausea. There were few data on adverse effects.
6. Sedatives. In 13 studies (involving 1265 women) provided data and addressed sedatives as an intervention. Most studies included propofol, but some included midazolam and one study used ketamine. Overall, the use of sedatives probably reduces intraoperative nausea and intraoperative vomiting (moderate‐certainty evidence) and may reduce postoperative vomiting (low‐certainty evidence). It is uncertain if sedatives reduce postoperative nausea (very low‐certainty evidence). Reports generally provided insufficient data on potential adverse effects,in particular sedation.
7. Opioid antagonists/partial agonists. There were two studies that provided data involving 197 women assessing opioid antagonists used specifically to reduce nausea and vomiting. We found little to no difference in postoperative nausea or vomiting with these interventions (low‐certainty evidence) and there were no studies assessing intraoperative nausea and vomiting. Studies only looked at the side effect of itching and found no difference on limited data.
8. Acupressure/acupuncture. In the 10 studies (involving 1401 women) that provided data, we found acupressure/acupuncture may reduce the number of women having intraoperative vomiting (low‐certainty evidence) but it uncertain whether there is a reduction in intraoperative nausea, postoperative nausea and postoperative vomiting (very low‐certainty evidence). There were insufficient data on potential adverse effects. .
9. Ginger. In the two studies (involving 365 women) that provided data and compared ginger with placebo, it is uncertain whether ginger reduces, increases, or has no effect on intraoperative nausea and vomiting and postoperative nausea and vomiting (all very low‐certainty evidence). No side effects were assessed in either study.
Overall completeness and applicability of evidence
There are good data assessing the efficacy of most standard classes of antiemetic compared with placebo in preventing nausea and vomiting during and following caesarean section under regional anaesthesia. However, most of the trials are small and the certainty of evidence is generally low.
In this updated review we did not attempt to compare different classes of antiemetics or different combinations of therapy.
We excluded several studies that assessed the efficacy of antiemetics given for treatment (rather than prevention) of established nausea and vomiting and these would need to be dealt with in a separate review.
Quality of the evidence
The quality of the evidence in this review varied widely. Considering just the 69 studies providing data for the review, on standard 'Risk of bias' assessment, only five studies were rated as low risk on all criteria (apart from Selective Bias). In comparison, 14 studies were rated as 'unclear' or 'high risk' on some or all criteria. Seventeen studies were rated as high risk on at least one criteria ‐ including for management of missing data (Abdel‐Aleem 2012, Baciarello 2011; Cardoso 2013; Duman 2010) inadequate blinding (Ahn 2002; Direkvand‐Moghadam 2013; Levin 2019; Mokini 2014; Rasooli 2014; Rudra 2004a) or selective reporting (Ahn 2002; Carvalho 2010; Ibrahim 2019; Jang 1997; Selzer 2020; Tkachenko 2019; Voigt 2013). One study was rated as high risk for other bias due to a poorly controlled study protocol (Habib 2013).
Of the 84 studies providing data, 37 described adequate random sequence generation, however only 18 described adequate allocation concealment.
Using GRADE criteria for assessing the certainty of the evidence, we found the following.
Comparison 1: 5HT3 antagonists: moderate‐certainty evidence for postoperative nausea (downgraded for serious risk of bias); low‐certainty evidence for intraoperative nausea (downgraded for serious risk of bias and serious inconsistency) and postoperative vomiting (downgraded for serious risk of bias and possible publication bias); very low‐certainty evidence for intraoperative vomiting (downgraded for serious risk of bias, serious inconsistency and possible publication bias). (Table 1).
Comparison 2: dopamine antagonists: low‐certainty evidence for intraoperative vomiting and postoperative nausea and very low‐certainty evidence for intraoperative nausea and postoperative vomiting. Intraoperative nausea (downgraded for serious risk of bias and serious inconsistency; intraoperative vomiting (downgraded for serious risk of bias); postoperative nausea (downgraded for serious risk of bias) and postoperative vomiting (downgraded for serious risk of bias and possible publication bias) (Table 2).
Comparison 3: corticosteroids: moderate‐certainty evidence for postoperative nausea (downgraded for serious risk of bias); low‐certainty evidence for postoperative vomiting (downgraded for serious risk of bias and serious inconsistency); very low‐certainty for intraoperative nausea (downgraded for very serious risk of bias and serious inconsistency) and intraoperative vomiting (downgraded for very serious risk of bias and serious imprecision) (Table 3).
Comparison 4: antihistamines: very low‐certainty evidence for intraoperative nausea, intraoperative vomiting and postoperative vomiting (all downgraded for very serious risk of bias and very serious imprecision); and low‐certainty evidence for postoperative nausea (downgraded for very serious risk of bias) (Table 4).
Comparison 5: anticholinergics: low‐certainty evidence for intraoperative nausea (downgraded for very serious risk of bias) and postoperative vomiting (downgraded for serious risk of bias and serious imprecision); very low‐certainty evidence for intraoperative vomiting (downgraded for very serious risk of bias, serious inconsistency and serious imprecision) (Table 5).
Comparison 6: sedatives: moderate‐certainty evidence for intraoperative nausea (downgraded for serious risk of bias) and intraoperative vomiting (downgraded for serious risk of bias); low‐certainty evidence for postoperative vomiting (downgraded for very serious imprecision); very low‐certainty evidence for postoperative nausea (downgraded for serious inconsistence and very serious imprecision) (Table 6).
Comparson 7: opioid antagonists: low‐certainty evidence for postoperative nausea (downgraded for very serious imprecision) and postoperative vomiting (downgraded for very serious imprecision) (Table 7).
Comaprison 8: acupressure/acupuncture: low‐certainty evidence for intraoperative vomiting (downgraded for very serious risk of bias); very low‐certainty evidence for intraoperative nausea (downgraded for very serious risk of bias and serious inconsistency), postoperative nausea (downgraded for very serious risk of bias and serious inconsistency and postoperative vomiting (downgraded for very serious risk of bias and serious inconsistency) (Table 8).
Comparison 9: ginger: very low‐certainty evidence for: intraoperative nausea (downgraded for risk of bias, serious inconsistency and serious imprecision), intraoperative vomiting (downgraded for risk of bias and serious imprecision); postoperative nausea (downgraded for serious risk of bias and very serious imprecision and postoperative vomiting (downgraded for serious risk of bias and very serious imprecision) (Table 9).
Potential biases in the review process
The possibility of introducing bias was present at every stage of the review process. We attempted to minimise bias in a number of ways; two review authors assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements.
Agreements and disagreements with other studies or reviews
The findings of this study are broadly consistent with previously published reviews of nausea and vomiting at caesarean section (Balki 2005). Systematic reviews assessing specific medications such as ondansetron (George 2009; Zhou 2018) and metoclopramide (Mishriky 2012) have demonstrated efficacy, however a meta‐analysis of acupressure did not show a positive effect (Allen 2008).
Authors' conclusions
Implications for practice.
This study indicates that many agents, from a diverse range of pharmacological classes, may have efficacy in preventing intraoperative and postoperative emetic symptoms at caesarean section. This is perhaps consistent with the multi‐factorial pathogenesis of the condition. Of the included interventions, 5HT3 antagonists, dopamine antagonists, corticosteroids, sedatives and acupressure all showed a reduction in all of our primary outcomes. However, the certainty of evidence was generally low/very low.
Several other classes of drugs and interventions show effects on some of these outcomes only, for example, antihistamines and anticholinergics. This may reflect the amount of data available.
The studies suggest that emetic symptoms are very common both during and following caesarean section. Placebo arms of trials included in this review suggest an intraoperative incidence of nausea in the order of 20% to 60%. This gives some weight to published guidelines recommending prophylaxis rather than treatment of emesis at caesarean section (NICE 2011).
Implications for research.
Whilst this review provides evidence that many single agents are efficacious in preventing nausea and vomiting much of it is low/very low in certainty. A network meta‐analysis might be undertaken to compare different drugs and drug groups. There are no data comparing the efficacy of agents for treatment of established nausea and vomiting although these would be dealt with in a separate review. Future studies should assess potential adverse effects and women's views.
What's new
| Date | Event | Description |
|---|---|---|
| 16 April 2020 | New search has been performed | Due to the increasing complexity of the review, we are now restricting the scope of the review to interventions compared with placebo or no treatment only. We have removed intervention versus intervention comparisons and studies investigating combinations of treatments. We have separated the blinding assessment for performance and detection bias to separate assessments of performance bias and detection bias as per Cochrane updated methodology. We have assessed the certainty of evidence using GRADE criteria and now have nine 'Summary of findings' tables in this update. Clarified definition of a primary outcome: When a study reported postoperative data into multiple time epochs, we have extracted the earliest postoperative data for inclusion in our review. When overall data were provided, we have used this to reflect intraoperative data. We removed comparisons where no identified study published usable data on that comparison |
| 16 April 2020 | New citation required and conclusions have changed | Search updated and 218 new trial reports assessed, plus 204 trial reports from the previous version of this review have been reassessed due to the change in scope. This update includes a total of 84 studies, with 69 providing data. In addition to the effective interventions identified in the previous version, in this update, corticosteroids were found to be effective in all our primary outcomes. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 9, 2012
Acknowledgements
Dr Eugene HC Liu Department of Anaesthesia, National University Hospital, Singapore.
Eugene Liu (EL) and Shantini Paranjothy (SP) wrote the first drafts of the combined protocol (Drugs at caesarean section for preventing nausea, vomiting and aspiration pneumonitis), which was subsequently split into this review and Paranjothy 2014.
Several papers considered for inclusion in the review were not reported in English. Thanks to Edgardo Abalos for translating Caba 1997; Liza Abraham for translating Maranhao 1988; Julie Arbon for translating Jasson 1987; Alex Balistreri for translating Karamanlioglu 1995, von Braun 1994 and Zoroglu 1999a; Elizabeth Dumford Chavez for translating Imbeloni 1986; Samiye Godel for translating Ozkan 2000; Gillian Kenyon for translating Tryba 1983; Alison Ledward for translating Jasson 1989b and Zue 1999; Almira Opardija for translating Avramovic 1979; Sara Roden‐Scott for translating Bifarini 1990 and Bifarini 1992 and Aidan Tan for translating.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
As part of the prepublication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Professor Anne Matthews, Dublin City University, Ireland; and Polly Griffith, Cochrane Consumer Network, UK.
Appendices
Appendix 1. ICTRP and ClinicalTrials.gov ‐ search methods
ICTRP
Each line was run separately.
nausea AND cesarean
nausea AND caesarean
vomiting AND cesarean
vomiting AND caesarean
antiemetics AND cesarean
antiemetics AND caesarean
ClinicalTrials.gov
Advanced search
cesarean section | Interventional studies | vomiting
cesarean section | Interventional studies | nausea
cesarean section | Interventional studies | antiemetics
Data and analyses
Comparison 1. 5‐HT3 antagonists vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Nausea ‐ intraoperative | 12 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.71] |
| 1.1.1 Ondansetron ‐ 4 mg | 9 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 1.1.2 Ondansetron ‐ 8 mg | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.20, 1.01] |
| 1.1.3 Granisetron ‐ 1 mg | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.59, 2.20] |
| 1.1.4 Granisetron 3 mg | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.61] |
| 1.2 Vomiting ‐ intraoperative | 11 | 1414 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.29, 0.73] |
| 1.2.1 Ondansetron ‐ 4 mg | 8 | 1059 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.31, 0.84] |
| 1.2.2 Ondansetron ‐ 8 mg | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.03] |
| 1.2.3 Granisetron ‐ 1 mg | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.28] |
| 1.2.4 Tropisotron 2 mg | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.67] |
| 1.3 Nausea ‐ postoperative | 10 | 1340 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.54] |
| 1.3.1 Ondansetron ‐ 4 mg | 8 | 1023 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.58] |
| 1.3.2 Ondansetron ‐ 8 mg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.18, 1.09] |
| 1.3.3 Granisetron ‐ 40 mcg/kg | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.90] |
| 1.3.4 Tropisotron ‐ 2 mg | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.71] |
| 1.4 Vomiting ‐ postoperative | 10 | 1450 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.69] |
| 1.4.1 Ondansetron ‐ 4 mg | 8 | 1133 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.71] |
| 1.4.2 Ondansetron ‐ 8 mg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.19, 5.15] |
| 1.4.3 Granisetron ‐ 40 mcg/kg | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.12, 1.54] |
| 1.4.4 Tropisotron ‐ 2 mg | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.38] |
| 1.5 'Nausea + Vomiting' ‐ intraoperative (not pre‐specified) | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.97] |
| 1.5.1 Tropisotron ‐ 2 mg | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.97] |
| 1.6 'Nausea + Vomiting' ‐ postoperative ‐ (not pre‐specified) | 5 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.80] |
| 1.6.1 Ondansetron ‐ 4 mg | 3 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.40, 1.13] |
| 1.6.2 Ondansetron ‐ 8 mg | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.72] |
| 1.6.3 Tropisotron ‐ 2 mg | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.71] |
| 1.6.4 Granisetron ‐ 0.1 mg | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.22, 2.31] |
| 1.7 Maternal satisfaction | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 Ondansetron ‐ 4 mg | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 Ondansetron ‐ 8 mg | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.3 Granisetron ‐ 1 mg | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.4 Granisetron ‐ 3 mg | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8 Maternal adverse outcomes | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.8.1 Ondansetron ‐ 8 mg | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Headache/dizziness/vertigo | 4 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.79] |
| 1.9.1 Ondansetron ‐ 4 mg | 4 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.79] |
| 1.10 Hypotension | 3 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.72, 2.08] |
| 1.10.1 Ondansetron ‐ 4 mg | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.48, 4.34] |
| 1.10.2 Granisetron ‐ 1 mg | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 72.65] |
| 1.11 Pruritus/itching | 4 | 488 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 1.11.1 Ondansetron ‐ 4 mg | 3 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.14] |
| 1.11.2 Ondansetron ‐ 8 mg | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 1.12 Dry mouth | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.17, 3.22] |
| 1.12.1 Ondansetron ‐ 4 mg | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.17, 3.22] |
| 1.13 Drowsiness/sedation | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.45, 34.63] |
| 1.13.1 Ondansetron ‐ 4 mg | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.45, 34.63] |
| 1.14 Rescue antiemetic (not pre‐specified) | 1 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.93] |
1.8. Analysis.

Comparison 1: 5‐HT3 antagonists vs placebo, Outcome 8: Maternal adverse outcomes
Comparison 2. Dopamine antagonists vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Nausea ‐ intraoperative | 15 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.27, 0.52] |
| 2.1.1 Metoclopramide ‐ 10 mg | 10 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.62] |
| 2.1.2 Metoclopramide ‐ 20 mg | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.10, 0.75] |
| 2.1.3 Metoclopramide ‐ 0.15 mg/kg | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.90] |
| 2.1.4 Droperidol ‐ 0.5 mg | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.17, 0.65] |
| 2.1.5 Droperidol ‐ 0.625 mg | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.15, 0.90] |
| 2.1.6 Droperidol ‐ 1.25 mg | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.17, 1.15] |
| 2.1.7 Droperidol ‐ 5 mg | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.01] |
| 2.2 Vomiting ‐ intraoperative | 12 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.60] |
| 2.2.1 Metoclopramide ‐ 10 mg | 8 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.27, 0.76] |
| 2.2.2 Metoclopramide ‐ 0.15 mg/kg | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.54] |
| 2.2.3 Droperidol ‐ 0.5 mg | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.23] |
| 2.2.4 Droperidol ‐ 0.625 mg | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.07, 1.17] |
| 2.2.5 Droperidol ‐ 1.25 mg | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.27] |
| 2.2.6 Droperidol ‐ 5 mg | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.98] |
| 2.3 Nausea ‐ postoperative | 7 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.48, 0.79] |
| 2.3.1 Metoclopramide ‐ 10 mg | 5 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.80] |
| 2.3.2 Metoclopramide ‐ 0.15 mg/kg | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.16, 1.02] |
| 2.3.3 Droperidol ‐ 1.25 mg | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.43, 6.51] |
| 2.4 Vomiting ‐ postoperative | 9 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.92] |
| 2.4.1 Metoclopramide ‐ 10 mg | 6 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.20] |
| 2.4.2 Metoclopramide ‐ 0.15 mg/kg | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.90] |
| 2.4.3 Droperidol ‐ 1.25 mg | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.32, 0.94] |
| 2.5 'Nausea + vomiting' ‐ intraoperative (not pre‐specfied) | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.88] |
| 2.5.1 Metoclopramide ‐ 10 mg | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.88] |
| 2.6 'Nausea + vomiting' ‐ postoperative (not pre‐specified) | 3 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.05, 1.02] |
| 2.6.1 Metoclopramide ‐ 10 mg | 3 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.71] |
| 2.6.2 Droperidol ‐ 0.625 mg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.75] |
| 2.7 Maternal satisfaction | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.91, 2.21] |
| 2.7.1 Metoclopramide ‐ 10 mg | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.91, 2.21] |
| 2.8 Anxiety | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 4.00 [0.48, 33.33] |
| 2.8.1 Metoclopramide ‐ 10 mg | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 4.00 [0.48, 33.33] |
| 2.9 Headache/dizziness | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.00] |
| 2.9.1 Metoclopramide ‐ 10 mg | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.00] |
| 2.10 Hypotension | 6 | 563 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.90, 1.30] |
| 2.10.1 Metoclopramide ‐ 10 mg | 4 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.77, 1.28] |
| 2.10.2 Metoclopramide ‐ 0.15 mg/kg | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.66, 3.95] |
| 2.10.3 Droperidol ‐ 0.5 mg | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.60] |
| 2.10.4 Droperidol ‐ 0.625 mg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.69, 2.25] |
| 2.11 Rescue antiemetics (not pre‐specified) | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.25] |
| 2.12 Sedation | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 5.54 [2.78, 11.06] |
| 2.12.1 Metoclopramide ‐ 10 mg | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 4.24 [1.73, 10.41] |
| 2.12.2 Droperidol ‐ 0.625 mg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 8.17 [2.77, 24.05] |
| 2.13 Pruritus/itching | 3 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.03] |
| 2.13.1 Metoclopramide ‐ 10 mg | 2 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.03] |
| 2.13.2 Droperidol ‐ 1.25 mg | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.55, 1.53] |
2.11. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 11: Rescue antiemetics (not pre‐specified)
2.12. Analysis.

Comparison 2: Dopamine antagonists vs placebo, Outcome 12: Sedation
Comparison 3. Corticosteroids vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Nausea ‐ intraoperative | 6 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.83] |
| 3.1.1 Dexamethasone ‐ 4 mg IV | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.17] |
| 3.1.2 Dexamethasone ‐ 4 mg IT | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.15, 0.75] |
| 3.1.3 Dexamethasone ‐ 8 mg IV | 5 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.82] |
| 3.2 Vomiting ‐ intraoperative | 6 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 3.2.1 Dexamethasone ‐ 4 mg IV | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.33, 2.32] |
| 3.2.2 Dexamethasone ‐ 4 mg IT | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.02, 2.47] |
| 3.2.3 Dexamethasone ‐ 8 mg IV | 5 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.23, 0.83] |
| 3.3 Nausea ‐ postoperative | 6 | 733 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.49, 0.73] |
| 3.3.1 Dexamethasone ‐ 2.5 mg IV | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.63] |
| 3.3.2 Dexamethasone ‐ 4 mg IV | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.88] |
| 3.3.3 Dexamethasone ‐ 5 mg IV | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.38] |
| 3.3.4 Dexamethasone ‐ 8 mg IV | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.60, 1.13] |
| 3.3.5 Dexamethasone ‐ 8 mg IT | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.74] |
| 3.3.6 Dexamethasone ‐ 10 mg IV | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.69] |
| 3.4 Vomiting ‐ postoperative | 7 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.49, 0.95] |
| 3.4.1 Dexamethasone ‐ 2.5 mg IV | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.22, 2.49] |
| 3.4.2 Dexamethasone ‐ 4 mg IV | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.73] |
| 3.4.3 Dexamethasone ‐ 5 mg IV | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.08, 1.51] |
| 3.4.4 Dexamethasone ‐ 8 mg IV | 3 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.79, 1.31] |
| 3.4.5 Dexamethasone ‐ 8 mg IT | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.31, 0.81] |
| 3.4.6 Dexamethasone ‐ 10 mg IV | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.73] |
| 3.5 'Nausea + Vomiting' ‐ intraoperative (not pre‐specified) | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.96, 2.84] |
| 3.5.1 Dexamethasone ‐ 8 mg IV | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.96, 2.84] |
| 3.6 'Nausea + Vomiting' ‐ postoperative ‐ (not pre‐specified) | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.12] |
| 3.6.1 Dexamethasone ‐ 8 mg IV | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.12] |
| 3.7 Hypotension | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.34, 1.12] |
| 3.7.1 Dexamethasone ‐ 4 mg IT | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.25, 0.78] |
| 3.7.2 Dexamethasone ‐ 8 mg IV | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.53, 1.22] |
| 3.8 Bradycardia | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.30, 1.16] |
| 3.8.1 Dexamethasone ‐ 4 mg IT | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.14, 1.15] |
| 3.8.2 Dexamethasone ‐ 8 mg IV | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.32, 1.87] |
| 3.9 Shivering | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.41, 1.05] |
| 3.9.1 Dexamethasone ‐ 4 mg IT | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.26, 1.09] |
| 3.9.2 Dexamethasone ‐ 8 mg IV | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.42, 1.40] |
| 3.10 Rescue antiemetics (not pre‐specified) | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.57] |
| 3.10.1 Dexamethasone 8 mg IV | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.57] |
3.8. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 8: Bradycardia
3.9. Analysis.

Comparison 3: Corticosteroids vs placebo, Outcome 9: Shivering
Comparison 4. Antihistamines vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Nausea ‐ intraoperative | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.47, 2.11] |
| 4.1.1 Dimenhydrinate ‐ 25 mg | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.47, 2.11] |
| 4.2 Vomiting ‐ intraoperative | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.2.1 Dimenhydrinate ‐ 25 mg | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.3 Nausea ‐ postoperative | 4 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.30, 0.64] |
| 4.3.1 Dimenhydrinate ‐ 25 mg | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.52] |
| 4.3.2 Dimenhydrate ‐ 50 mg | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.69] |
| 4.3.3 Dimenhydrate ‐ 100 mg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.57] |
| 4.3.4 Cyclizine ‐ 50 mg | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.88] |
| 4.4 Vomiting ‐ postoperative | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.81] |
| 4.4.1 Dimenhydrinate ‐ 25 mg | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.48] |
| 4.4.2 Dimenhydrate ‐ 50 mg | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.19, 1.42] |
| 4.4.3 Cyclizine ‐ 50 mg | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.93] |
| 4.5 Hypotension | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.90, 2.40] |
| 4.5.1 Dimenhydrinate ‐ 25 mg | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.90, 2.40] |
Comparison 5. Anticholinergics vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Nausea ‐ intraoperative | 4 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.51, 0.87] |
| 5.1.1 Glycopyrrolate ‐ 0.2 mg | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.09] |
| 5.1.2 Scopolamine patch | 2 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 5.2 Vomiting ‐ intraoperative | 4 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.54] |
| 5.2.1 Glycopyrrolate ‐ 0.2 mg | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.12, 1.62] |
| 5.2.2 Scopolamine patch | 2 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.36, 2.59] |
| 5.3 Nausea ‐ postoperative | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.4 Vomiting ‐ postoperative | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 5.4.1 Scopolamine patch | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 5.5 'Nausea + Vomiting' ‐ intraoperative (not pre‐specified) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.6 'Nausea + vomiting' ‐ postoperative (not pre‐specified) | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.25, 0.85] |
| 5.6.1 Atropine ‐ 100 mcg IT | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.14, 0.52] |
| 5.6.2 Atropine ‐ 100 mcg IV | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.45, 1.15] |
| 5.6.3 Scopolamine 0.3 mg | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 1.01] |
| 5.7 Blurred vision | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.21, 3.40] |
| 5.7.1 Scopolamine patch | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.37, 10.57] |
| 5.7.2 Atropine ‐ 100 mcg intrathecal | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.18, 4.76] |
| 5.7.3 Atropine ‐ 100 mcg intravenous | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.00, 1.96] |
| 5.8 Anxiety/Disorientation | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.35, 2.58] |
| 5.8.1 Scopolamine patch | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.12, 72.08] |
| 5.8.2 Atropine ‐ 100 mcg intrathecal | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.19, 3.01] |
| 5.8.3 Atropine ‐ 100 mcg intravenous | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.18, 4.95] |
| 5.9 Dizziness | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.37, 2.24] |
| 5.9.1 Scopolamine patch | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.39, 2.54] |
| 5.9.2 Scopolamine 0.3 mg/5 mL IV | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.03] |
| 5.10 Hypotension | 3 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.13] |
| 5.10.1 Glycopyrrolate ‐ 0.2 mg | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.50, 1.26] |
| 5.10.2 Atropine ‐ 100 mcg intrathecal | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.07, 3.11] |
| 5.10.3 Atropine ‐ 100 mcg intravenous | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.09, 10.15] |
| 5.11 Pruritus/itching | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.18] |
| 5.11.1 Scopolamine patch | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.28] |
| 5.11.2 Atropine ‐ 100 mcg intrathecal | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.19, 1.47] |
| 5.11.3 Atropine ‐ 100 mcg intravenous | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.36, 2.16] |
| 5.12 Xerostomia/dry mouth | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.53, 1.27] |
| 5.12.1 Atropine ‐ 100 mcg intrathecal | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.43, 1.59] |
| 5.12.2 Atropine ‐ 100 mcg intravenous | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.41] |
| 5.12.3 Scopolamine 0.3 mg/5 mL IV | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.35, 4.45] |
| 5.13 Drowsiness | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 72.31] |
| 5.13.1 Scopolamine 0.3 mg/5 mL IV | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 72.31] |
5.3. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 3: Nausea ‐ postoperative
5.5. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 5: 'Nausea + Vomiting' ‐ intraoperative (not pre‐specified)
5.8. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 8: Anxiety/Disorientation
5.9. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 9: Dizziness
5.10. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 10: Hypotension
5.11. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 11: Pruritus/itching
5.12. Analysis.

Comparison 5: Anticholinergics vs placebo, Outcome 12: Xerostomia/dry mouth
Comparison 6. Sedatives vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Nausea ‐ intraoperative | 8 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.51, 0.82] |
| 6.1.1 Propofol ‐ 0.5 mg/kg/hr | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.19, 2.93] |
| 6.1.2 Propofol ‐ 1.0 mg/kg/hr | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.26, 1.20] |
| 6.1.3 Propofol ‐ 1.5 mg/kg/hr | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.09, 1.35] |
| 6.1.4 Propofol ‐ 10 mg IV ‐ single dose | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.39, 3.67] |
| 6.1.5 Propofol ‐ 20 mg + 1.0 mg/kg/hr | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.32] |
| 6.1.6 Propofol TCI target 1 ug/ml | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.23, 0.75] |
| 6.1.7 Midazolam ‐ 1.0 mg + 1.0 mg/hr | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.48, 1.20] |
| 6.1.8 Ketamine 0.4.mg/kg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.11] |
| 6.1.9 Intrathecal midazolam 2 mg vs placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.2 Vomiting ‐ intraoperative | 8 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.24, 0.52] |
| 6.2.1 Propofol ‐ 0.5 mg/kg/hr | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.24, 3.35] |
| 6.2.2 Propofol ‐ 1.0 mg/kg/hr | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.15, 0.65] |
| 6.2.3 Propofol ‐ 1.5 mg/kg/hr | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.05, 1.12] |
| 6.2.4 Propofol ‐ 10 mg IV ‐ single dose | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.06, 6.21] |
| 6.2.5 Propofol ‐ 20 mg + 1.0 mg/kg/hr | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.65] |
| 6.2.6 Propofol TCI target 1 ug/ml | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.10, 1.14] |
| 6.2.7 Midazolam ‐ 1.0 mg + 1.0 mg/hr | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.86] |
| 6.2.8 Ketamine 0.4 mg/kg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.15, 1.34] |
| 6.2.9 Intrathecal midazolam 2 mg vs placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.3 Nausea ‐ postoperative | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.71] |
| 6.3.1 Propofol ‐ 10 mg IV ‐ single dose | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.23, 24.83] |
| 6.3.2 Propofol ‐ 20 mg + 1.0 mg/kg/hr | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.06, 0.40] |
| 6.3.3 Midazolam ‐ 1.0 mg + 1.0 mg/hr | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.08, 0.40] |
| 6.4 Vomiting ‐ postoperative | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.03, 0.28] |
| 6.4.1 Propofol ‐ 10 mg IV ‐ single dose | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.02, 9.31] |
| 6.4.2 Propofol ‐ 20 mg + 1.0 mg/kg/hr | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.37] |
| 6.4.3 Midazolam ‐ 1.0 mg + 1.0 mg/hr | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.37] |
| 6.5 'Nausea + vomiting' ‐ intraoperative (not pre‐specified) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.6 'Nausea + vomiting' ‐ postoperative (not pre‐specified) | 2 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.02, 0.22] |
| 6.6.1 Propofol ‐ 0.5 mg/kg | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.01, 0.08] |
| 6.6.2 Propofol TCI target 1 ug/ml | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.93] |
| 6.6.3 Propofol TCI target 1.5 ug/ml | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.80] |
| 6.6.4 Propofol TCI target 2 ug/ml | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.01, 0.66] |
| 6.7 Pruritis/itching | 2 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.02, 0.12] |
| 6.7.1 Propofol ‐ 0.5 mg/kg | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.01, 0.09] |
| 6.7.2 Propofol TCI target 1 ug/ml | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.78] |
| 6.7.3 Propofol TCI target 1.5 ug/ml | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.78] |
| 6.7.4 Propofol TCI target 2 ug/ml | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.69] |
| 6.8 Hypotension | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.86, 1.93] |
| 6.8.1 Propofol TCI target 1 ug/ml | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.83, 2.03] |
| 6.8.2 Propofol TCI target 1.5 ug/ml | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.20, 3.76] |
| 6.8.3 Propofol TCI target 2 ug/ml | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.44, 6.36] |
| 6.9 Shivering | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.08, 0.72] |
| 6.9.1 Propofol TCI target 1 ug/ml | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.06, 2.14] |
| 6.9.2 Propofol TCI target 1.5 ug/ml | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.70] |
| 6.9.3 Propofol TCI target 2 ug/ml | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.04, 1.15] |
| 6.10 Apgar score < 7 at 5 mins | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.10.1 Propofol TCI target 1 ug/ml | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.11 Initiation of breastfeeding | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 6.11.1 Propofol TCI target 1 ug/ml | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
6.5. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 5: 'Nausea + vomiting' ‐ intraoperative (not pre‐specified)
6.8. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 8: Hypotension
6.10. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 10: Apgar score < 7 at 5 mins
6.11. Analysis.

Comparison 6: Sedatives vs placebo, Outcome 11: Initiation of breastfeeding
Comparison 7. Opioid antagonist/partial agonist vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Nausea ‐ intraoperative | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 7.2 Vomiting ‐ intraoperative | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 7.3 Nausea ‐ postoperative | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 7.3.1 Nalbuphine ‐ 4 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 7.4 Vomiting ‐ postoperative | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.35, 4.43] |
| 7.4.1 Nalbuphine ‐ 4 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.35, 4.43] |
| 7.5 'Nausea + vomiting' ‐ intraoperative (not pre‐specified) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 7.6 'Nausea + vomiting' ‐ postoperative (not pre‐specified) | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.37] |
| 7.6.1 Nalbuphine ‐ 0.5 mg | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.37] |
| 7.7 Pruritus/itching | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.02, 5.27] |
| 7.7.1 Nalbuphine ‐ 0.5 mg | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.04, 0.39] |
| 7.7.2 Nalbuphine ‐ 4 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.74, 0.99] |
7.1. Analysis.

Comparison 7: Opioid antagonist/partial agonist vs placebo, Outcome 1: Nausea ‐ intraoperative
7.2. Analysis.

Comparison 7: Opioid antagonist/partial agonist vs placebo, Outcome 2: Vomiting ‐ intraoperative
7.5. Analysis.

Comparison 7: Opioid antagonist/partial agonist vs placebo, Outcome 5: 'Nausea + vomiting' ‐ intraoperative (not pre‐specified)
Comparison 8. Acupressure/acupuncture vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Nausea ‐ intraoperative | 9 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 8.1.1 Acupressure | 9 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 8.2 Vomiting ‐ intraoperative | 9 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.33, 0.80] |
| 8.2.1 Acupressure | 9 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.33, 0.80] |
| 8.3 Nausea ‐ postoperative | 7 | 1069 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.75] |
| 8.3.1 Acupressure | 7 | 1069 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.75] |
| 8.4 Vomiting ‐ postoperative | 7 | 1069 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.79] |
| 8.4.1 Acupressure | 7 | 1069 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.79] |
| 8.5 Anxiety | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.07, 15.12] |
| 8.5.1 Acupressure | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.07, 15.12] |
| 8.6 Dizziness | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.07, 15.26] |
| 8.6.1 Acupressure | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.07, 15.26] |
| 8.7 Hypotension | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.54, 1.16] |
| 8.7.1 Acupressure | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.54, 1.16] |
| 8.8 Pruritus/itching | 3 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.85, 1.55] |
| 8.8.1 Acupressure | 3 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.85, 1.55] |
| 8.9 Rescue antiemetic (not pre‐specified) | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.36, 0.71] |
8.6. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 6: Dizziness
8.7. Analysis.

Comparison 8: Acupressure/acupuncture vs placebo, Outcome 7: Hypotension
Comparison 9. Ginger vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Nausea ‐ intraoperative | 2 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.36, 1.21] |
| 9.1.1 Ginger ‐ 1 g oral | 1 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.06] |
| 9.1.2 Ginger ‐ 25 drops oral | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.82] |
| 9.2 Vomiting ‐ intraoperative | 2 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.38, 1.00] |
| 9.2.1 Ginger 1 g oral | 1 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.52, 1.10] |
| 9.2.2 Ginger ‐ 25 drops oral | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.82] |
| 9.3 Nausea ‐ postoperative | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.77] |
| 9.3.1 Ginger ‐ 25 drops oral | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.77] |
| 9.4 Vomiting ‐ postoperative | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.65] |
| 9.4.1 Ginger ‐ 25 drops oral | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Aleem 2012.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: corticosteroid (Comparison 3)
Comparator: placebo
|
|
| Outcomes | Nausea, vomiting, number of vomiting attacks, need for anti‐emetics, sedation, itch, respiratory depression, pain, satisfaction (retching classified as vomiting). | |
| Notes | Setting: Assiut University Hospital, Egypt. Dates: February 2008 to December 2009. Funding source: not reported Declaration of interest: none declared. We wrote to authors for clarification on whether the 53 women were excluded pre or post randomisation as it is unclear from the text of the publication. Spinal with bupivacaine and 200 mcg IT morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer based random allocation table" |
| Allocation concealment (selection bias) | Low risk | Quote:“sealed opaque envelopes consecutively numbered and coded” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | the patient ... was unaware of which intervention had been received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those assessing the outcomes were also unaware of which intervention had been received. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 53 patients were excluded enrolment although it is unclear if this was before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to assess the trial protocol. |
| Other bias | Low risk | Similar baseline characteristics |
Abdollahpour 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: sedative (Comparison 6)
Intervention: opioid (excluded from our synthesis)
Comparator: placebo
|
|
| Outcomes |
Pre‐specified outcomes: Analgesia quality: ‐ Onset (sensory, motor) ‐ Recovery (sensory, motor) ‐ Time to request additional analgesia Complications: ‐ Nausea ‐ Vomiting ‐ Shivering ‐ Hypotension |
|
| Notes | Setting: Semnan University of Medical Sciences, Semnan, Iran. Dates: 2012 to 2013, Funding source: the study supported by Semnan University of Medical Sciences, Semnan, Iran. Declaration of interest: not reported. Spinal anaesthesia with bupivacaine We include only the data comparing midazolam vs placebo as sufentanil is an opioid |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"random block method" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described |
| Selective reporting (reporting bias) | Unclear risk | We were unable to assess the trial protocol |
| Other bias | Unclear risk | Baseline characteristics (except age) not described |
Abouleish 1999.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 74 women 18‐40 years undergoing elective CS at term under spinal anaesthesia, ASA 1‐2, no significant maternal medical conditions. | |
| Interventions | Intervention: 5‐HT3antagonist (Comparison 1)
Comparator: placebo
|
|
| Outcomes | Nausea ‐ presence/absence and severity. Vomiting ‐ severity, frequency. |
|
| Notes | Setting: Texas, USA. The Middlesex Hospital, London, UK. Dates: not reported Funding source: funding was received from Glaxo‐Wellcome and NEI Vision Core Declaration of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Syringes produced by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "Double blind" but no other details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "Double blind" but no other details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants withdrawn, 5 withdrew consent, 2 had an exclusion criteria (oesophageal reflux), 1 failed spinal converted to GA. Data not re‐included. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Mild increase in BP in the control group, not significant. |
Ahn 2002.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | Women undergoing elective CS under CSE anaesthesia. N = 120 |
|
| Interventions | Intervention 1: sedative (Comparison 6)
Intervention 2: sedative (Comparison 6)
Intervention 3: sedative (Comparison 6)
Comparator: placebo
|
|
| Outcomes | Nausea, vomiting, sedation, satisfaction, pruritis, abdominal discomfort. | |
| Notes | Setting: Korea Dates: not reported Funding source: not reported Declaration of interest: not reported English abstract with main paper in Korean. We will attempt to get a translation. Drugs are in separate subgroups but then pooled in our analysis, so the placebo data are dealt with according to our methods (Unit of analysis issues). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomly allocated" but no information on how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Authors make no mention of blinding, intraoperative data collectors (at least) likely to be aware of intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2% data loss, 118/120 women provide data |
| Selective reporting (reporting bias) | High risk | Trial not pre‐registered. A number of pre‐specified outcomes not reported (shivering, amnesia and hypotension) |
| Other bias | Unclear risk | Abstract only (in English), insufficient information to assess |
Apiliogullari 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 181 women. Spinal anaesthesia for CS. | |
| Interventions | Intervention 1: antihistamine (Comparison 4)
Intervention 2: antihistamine (Comparison 4)
Comparator: placebo
|
|
| Outcomes | PONV, sedation, side effects. | |
| Notes | Setting: Dr. Faruk Sukan Hospital and Selcuk University, Medical Faculty, Konya, Turkey. Dates: not described Funding source: not reported. Declaration of interest: not reported Abstracts only. Unpublished data provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as double‐blind, no other details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as double‐blind, no other details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to assess the trial protocol. |
| Other bias | Unclear risk | No information. |
Baciarello 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 204 ASA 1‐2 women undergoing CS under spinal anaesthesia. N = 216 women randomised, 204 analysed |
|
| Interventions | Intervention 1: anticholinergic (Comparison 5)
Intervention 2: anticholinergic(Comparison 5)
Comparator: placebo
|
|
| Outcomes | PONV, pain. | |
| Notes | Setting: University Hospital of Parma, Parma, Italy and University Hospital of Messina, Messina, Italy. Dates: April 2007 to October 2008 Funding source: not reported Declaration of interest: not reported We will write to authors for further information. Drugs are in separate subgroups but then pooled in our analysis, so the placebo data are dealt with according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'random sequence from random.org' |
| Allocation concealment (selection bias) | Unclear risk | 'sealed envelope' but authors do not mention opaque nor consecutively numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:“Anaesthesiologist in charge of patient read her group assignment from sealed envelope immediately before performing anaesthesia. All other involved personal were kept blind … except the nurse assisting the physician" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:“assessor was not involved in intraoperative care” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 patients excluded and data not able to be re‐included. |
| Selective reporting (reporting bias) | Unclear risk | We were not able to assess the study protocol. |
| Other bias | Unclear risk | No information provided |
Birnbach 1993.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 60 women undergoing elective CS with spinal anaesthesia, no history of nausea or vomiting within 24 hours or after previous anaesthetics. N = 60 |
|
| Interventions | Intervention 1: acupuncture/acupressure (Comparison 8)
Intervention 2: dopamine antagonist (Comparison 2)
Comparator: placebo
|
|
| Outcomes | VAS nausea and vomiting. | |
| Notes | Setting: St Lukes/Roosevelt’s Hospital, New York, USA. Dates: not reported Funding source: not reported Declaration of interest: not reported This conference abstract currently provides no data for the review because we were unable to assess results from graphical data. We wrote to the authors in 2010 but did not receive a reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Methods not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant was blinded, but unclear whether the assessor was blinded or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participant was blinded, but unclear whether the assessor was blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No apparent problems. |
Biswas 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 80 women undergoing spinal anaesthesia for CS, ASA 1, 20‐35 years, without a history of nausea/vomiting, motion sickness, GI or liver disease, current antiemetic use. | |
| Interventions | Intervention 1: anticholinergic (Comparison 5)
Intervention 2: corticosteroid (Comparison 3)
Intervention 3: dopamine antagonist (Comparison 2)
Comparator: placebo
|
|
| Outcomes | Nausea, vomiting, cardiovascular instability, Apgar scores. | |
| Notes | Setting: Calcutta National Medical College and Hospitals, Calcutta, India. Dates: not reported Funding source: not reported Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Outcome assessor blinded, others unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded, others unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not assessed. |
| Other bias | Unclear risk | Nil apparent. |
Boone 2002.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
|
|
| Interventions | Intervention: 5‐HT3antagonist (Comparison 1)
Comparator: no treatment
|
|
| Outcomes | Nausea/retching/vomiting ‐ presented as a combined score. | |
| Notes | Setting: Texas Tech University Health Sciences Center, El Paso, Texas, USA. Dates: not reported Funding source: not reported Declaration of interest: not reported Conference abstract only. This study currently provides no data for the review. We wrote to the authors in 2010 requesting separated outcome data but did not receive a reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomised." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | None apparent. |
Caba 1997.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusions: quote:"any contraindication to spinal anaesthesia, pre‐eclampsia, clear background of hypertension and NVPO (post operative nausea and vomiting), body mass index greater than 45, and less than 6 hour fasting…” |
|
| Interventions | Intervention: sedative (Comparison 6)
Comparator: placebo
|
|
| Outcomes | Nausea and vomiting before and after intervention, and postoperative; change in haemodynamics; sedation. | |
| Notes | Setting: University Hospital Seville, Spain. Dates: not reported Funding source: not reported Declaration of interest: not reported In Spanish. Translated by Edgardo Abalos |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘…through a table of randomisation …’ |
| Allocation concealment (selection bias) | Unclear risk | Prepared by hospital pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specifically described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specifically described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 women excluded from the Protofol group, 2 had GA due to inadequate block, 1 had anaphylaxis. There appeared to be no other loss of data, but overall we felt uncertain about this. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Study was not stopped early. Baseline data were similar on age, height, weight, BMI, gestational age, birthweight, previous medication for dilatation, surgical time (minutes), other associated interventions, surgical incision: vertical intraumbilical, Pfannenstiel. |
Cardoso 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: corticosteroid (Comparison 3)
Comparator: placebo
Administered prior to start of surgery |
|
| Outcomes | Incidence nausea and vomiting in first 24 hours postop, at 1, 2, 3, 6, 12, 24 hours | |
| Notes | Setting: Santa Case de Misericordia, Sao Paulo, Brazil. Dates: 1 January to 30 June 2008 Funding source: Department of Anaesthesiology of same hospital. Declaration of interest: authors declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“used a computer generated table” |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating anaesthesiologist probably knew allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Different anaesthesiologist |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 61/131 (46%) women were excluded after randomisation as they were not the first women of the day |
| Selective reporting (reporting bias) | Unclear risk | Not assessed |
| Other bias | Unclear risk | Demographic data similar but there is insufficient methodology to assess other biases. |
Carvalho 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: antihistamine (Comparison 4)
Comparator: placebo
|
|
| Outcomes | Incidence of pre or post‐delivery nausea as reported by the women; etc. | |
| Notes | Setting: Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, M5G 1X5 Dates: not reported Funding source: not reported Declaration of interest: not reported Trial registration: NCT00791960 Conference abstract only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many women were actually randomised |
| Selective reporting (reporting bias) | High risk | Apgar scores and other neonatal data not reported |
| Other bias | Unclear risk | Insufficient methodology in the conference abstract to be able to assess other possible biases |
Charuluxananan 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 240 ASA 1‐2 women undergoing CS under spinal anaesthesia without allergy to study drugs, pruritis, skin disease. | |
| Interventions | Intervention 1: opioid antagonist (Comparison 7)
Intervention 2: 5‐HT3antagonist (Comparson 1)
Intervention 3: 5‐HT3antagonist (Comparson 1)
Comparator: placebo.
|
|
| Outcomes | Pruritis, post‐op nausea and vomiting, adverse effects. | |
| Notes | Setting: King Chulalongkorn Memorial Hospital, Bangkok, Thailand. Dates: not reported Funding source: not reported Declaration of interest: not reported In Comparison 1, data in Groups 2 and 3 were entered as subgroups and combined with overall data for treatment effect with the required adjustment of the placebo data for the 2 subgroups according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes and randomly allocated coded syringes were prepared by a nurse anaesthetist not involved with the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote:"double blind" but not specifically described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote:"double blind" but not specifically described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No baseline differences. |
Cherian 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 81 women undergoing elective CS at term under spinal anaesthesia. | |
| Interventions | Intervention: 5‐HT3 antagonist (Comparison 1)
Comparator: placebo
|
|
| Outcomes | Nausea ‐ mild/severe, vomiting, pain, sedation, women's satisfaction. | |
| Notes | Setting: North Staffordshire Hospital, Stoke‐on‐Trent, Staffordshire, England, UK. Dates: not reported. Funding source: no commercial funding reported. Declaration of interest: not reported. Combined "mild" and "severe" to form overall incidence of nausea. Combine "moderate" and "poor" satisfaction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐allocated numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unclear what placebo was given, although states participants were "unaware" of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear what placebo was given, although states participants were "unaware" of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of data described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance. |
Chestnut 1987.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 69 ASA 1‐2 women undergoing lumbar epidural for elective CS, with no history of nausea or vomiting in the 24 hours before surgery (or any adjunctive surgery apart from tubal ligation). | |
| Interventions | Intervention 1: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Intraoperative and PONV, anxiety, sedation. | |
| Notes | Setting: University of Iowa College of Medicine, Iowa, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Pharmacy undertook preparation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"The patient, anaesthesiologist, obstetrician and nursing staff were unaware of the identity of the study solution" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"The patient, anaesthesiologist, obstetrician and nursing staff were unaware of the identity of the study solution" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Similar baseline variables. |
Choi 1999.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women undergoing CS under regional anaesthesia N = 180 |
|
| Interventions | Intervention 1: dopamine antagonist (Comparison 2)
Intervention 2: dopamine antagonist (Comparison 2)
Comparator: placebo
Women were initially randomised to epidural (N = 90) or spinal (N = 90) anaesthesia. We will pool the data for these 2 types of anaesthesia |
|
| Outcomes | Nausea and vomiting, sedation, adverse effects. | |
| Notes | Setting: Samsung Medical Centre, Sungkyunkwan University Hospital, Seoul, South Korea Dates: not reported in English abstract Funding source: none reported in English abstract Declaration of interest: none reported in English abstract Abstract in English, rest of paper in Korean. Drugs are in separate subgroups but then pooled in our analysis, so the placebo data are dealt with according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail, just reported as "... randomly assigned ..." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be no loss of data |
| Selective reporting (reporting bias) | Unclear risk | Although outcomes listed in the methods were reported, but we did not assess the trial protocol |
| Other bias | Unclear risk | There was insufficient methodology in the abstract to judge on other potential biasses. We would not expect exactly 30 women to be randomised to each of 6 groups |
Dasgupta 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 80 women undergoing elective CS, Calcutta medical college India, spinal anaesthesia, ASA 1‐2 | |
| Interventions | Intervention: 5‐HT3 antagonist (Comparison 1)
Comparator: placebo
|
|
| Outcomes | Postoperative nausea, retching and vomiting (retching = vomiting), nausea VAS 0‐10. Adverse effects | |
| Notes | Setting: Calcutta Medical College, Kolkata, India. Dates: January 2007 to January 2008 Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not described. Patients were quote:"matched for age and BMI" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:“study drugs were prepared by personnel not involved in the study … anaesthetists, patients and investigators who collected post‐delivery data were blinded to the study drug administered” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:“study drugs were prepared by personnel not involved in the study … anaesthetists, patients and investigators who collected post‐delivery data were blinded to the study drug administered” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nil apparent |
| Selective reporting (reporting bias) | Unclear risk | We were not able to assess the study protocol |
| Other bias | Unclear risk | It is not clear what was meant by "patients were matched by age and BMI", potential risk for allocation/selection bias? |
Direkvand‐Moghadam 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
|
|
| Interventions | Intervention 1: dopamine antagonist (Comparison 2)
Intervention 2: acupuncture/acupressure (Comparison 8)
Comparison: placebo (no intervention)
|
|
| Outcomes | Incidence of nausea and vomiting intra‐op, then 30, 60, 90, 120, 240 and 360 min after surgery | |
| Notes | Setting: Mustafa University Hospital of Ilam, West Iran. Dates: Septemebr 2011 to October 2012. Funding source: Ilam University of Medical Sciences. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"random number table" |
| Allocation concealment (selection bias) | Unclear risk | Allocation undertaken by midwife prior to anaesthesia |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patient aware of which allocation group patient was in |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"researcher not aware of grouping of patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data missing |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Low risk | Nil apparent |
Duggal 1998.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 244 women ASA 1‐2 undergoing elective CS under spinal anaesthesia with no hyperemesis or antiemetic use in the previous 48 hours. | |
| Interventions | Intervention: acupuncture/acupressure (Comparison 8)
Comparator: placebo
|
|
| Outcomes | Nausea or vomiting intraoperative and up to 10 hours postoperatively. | |
| Notes | Setting: BC Women’s Hospital and Health Centre Society, Vancouver, British Columbia, Canada. Dates: not reported. Funding source: Grant from BC Medcal Services Foundation and wristbands donated from Sea Band UK Limited. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"group P a pair of similar‐looking placebo wristbands from which the plastic studs were missing" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nature of the bands was therefore unknown to the patient, anaesthetist and investigators for the duration of the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Many exclusions ‐ not ITT. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance. |
Duman 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention 1: antihistamine (Comparison 4)
Intervention 2: dopamine antagonist (Comparson 2)
Comparison: placebo
|
|
| Outcomes | PONV in 1st 24 hours after surgery; nausea; vomiting; severe nausea; rescue antiemetic; pruritus; sedation. | |
| Notes | Setting: Konya, Turkey. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. This study initially provided very limited information in abstract form. We wrote to the authors and obtained further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:“The patients were unaware of to which group they were randomised. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"Nursing staff were blinded to randomisation process and selection of the drugs used in this study.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Women were excluded if they received intraoperative propofol quote:“due to propofol’s known antiemetic properties". The number excluded were: Dimenhydrinate (Gp D) excluded 9/70 = 13%, Metoclopramide (Gp M) excluded 12/70 = 17%, placebo (Gp P) excluded 7/70 = 10%. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No problems apparent. |
El‐Deeb 2011a.
| Study characteristics | ||
| Methods | Individual RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: 5‐HT3 antagonist (Comparison 1)
Intervention 2: acupressure (Comparison 8)
Placebo
|
|
| Outcomes | Nausea and vomiting per 10 minutes intraoperative. Nausea and vomiting at 2, 4, 6, 12, 24 hours postoperative 4 mg ondansetron as rescue Adverse effects Women's satisfaction |
|
| Notes | Setting: Mansoura, Egypt. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unspecified. Sham needling was performed but no details on blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluation postoperatively by an independent anaesthetist who was blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unspecified |
| Selective reporting (reporting bias) | Unclear risk | Unspecified |
| Other bias | Low risk | Nil apparent |
Garcia‐Miguel 2000.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 150 ASA 1‐2 women undergoing CS under spinal anaesthesia, although three women required general anaesthesia and were excluded. | |
| Interventions | Intervention 1: 5HT3 antagonist (Comparison 1)
Intervention 2: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Intraoperative nausea and vomiting | |
| Notes | Setting: Hospital General de Segovia, Spain. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote:"double blind" otherwise unspecified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unspecified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 150 recruited, only 147 results, 3 required GA due to inadequate spinal |
| Selective reporting (reporting bias) | Unclear risk | We were not able to review the study protocol |
| Other bias | Low risk | Similar baseline characteristics |
Habib 2006.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | 91 women scheduled for elective CS under spinal anaesthesia without previous experience of acupuncture or acu‐stimulation or had experienced nausea or vomiting or taken antiemetics or glucocorticoids within 24 hours before surgery or who had an implanted pacemaker or defibrillator device. | |
| Interventions | Intervention: acupuncture/acupressure (K)
Comparison: placebo
|
|
| Outcomes | Nausea and vomiting (intra‐ and postoperative), intraoperative and postoperative antiemetic use and pruritis. | |
| Notes | Setting: Duke University Medical Center, Durham, North Carolina, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A separate researcher who was unaware of the patient’s randomisation collected the data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 women excluded for protocol violations post randomisation. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline differences. |
Habib 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: 5HT3 antagonist + dopamine antagonist ‐ not included as combination of drugs
Intervention 2: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Nausea intra‐op (using nausea score) at 5, 10 minutes and then 10 minutely intra‐op; vomiting episodes; rescue ant‐emetics; pruritus and opioid consumption post‐op | |
| Notes | Setting: Duke University Medical Center, Durham, North Carolina; IWK Health Centre, Halifax, Canada; and Carver College of Medicine, Iowa, USA. Dates: Decemebr 2008 to January 2011. Funding source: not reported. Declaration of interest: not reported. Complex methodology; anti‐emetic use varied between the 2 centres where study conducted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes, but there is no mention of the envelopes being sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants unaware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collector unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nil |
| Selective reporting (reporting bias) | Unclear risk | We were unable to assess the study protocol |
| Other bias | High risk | 2 centres with different practices, protocol poorly controlled |
Harmon 2000.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 94 ASA 1 women 18‐40 years scheduled for elective CS under spinal anaesthesia, with no previous history of nausea and vomiting postoperatively or in the previous 24 hours, obesity, diabetes, previous experience of acupuncture or acupressure. | |
| Interventions | Intervention: acupuncture/acupressure (K)
Comparison: placebo
|
|
| Outcomes | Nausea and vomiting during and up to 24 hours postoperative. | |
| Notes | Setting: University College Hospital, Galway, Ireland. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomly allocated.' |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if/how the participant was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Post‐op assessor blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants excluded, but unclear pre or post randomisation. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance described. |
Harnett 2007.
| Study characteristics | ||
| Methods | Individual RCT. | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Group 1: anticholinergic (Comparison 5)
Group 2: 5‐HT3 antagonist(Comparison 1)
Group 3: placebo
|
|
| Outcomes | Intraoperative nausea, vomiting, post‐op N or V at 0‐2, 2‐6, 6‐24 hours, rescue antiemetics. | |
| Notes | Setting: Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Postoperative nausea reported as a VAS score, and therefore were not usable data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated scheme." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The treating anaesthesiologist remained blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent exclusions or dropouts, ITT. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance. |
Hassanein 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Excluson criteria: |
|
| Interventions | Group 1: sedative (Comparison 6)
Group 2: steroids (Comparison 3)
Group 3: placebo
|
|
| Outcomes | Intra‐operative nausea and vomiting, sedation scores | |
| Notes | Setting: Al‐Minia University, Egypt. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. In some cases, but not all, tubal ligation was performed. These were not analysed separately. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Syringes were given by the second anaesthetist to the anaesthetist who was unaware of the content of the syringe |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nausea, retching and vomiting episodes were recorded by an anaesthetist who was blinded to the drug administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients excluded due to inadequate spinal anaesthesia |
| Selective reporting (reporting bias) | Unclear risk | We were unable to assess the study protocol |
| Other bias | High risk | Some patients had tubal ligation as well, number unspecified |
Ho 1996.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | 60 women ASA 1, aged 21‐35, undergoing elective CS, under spinal anaesthesia with epidural morphine, no history of carpal tunnel syndrome or nausea or vomiting within 24 hours of CS. | |
| Interventions | Intervention: acupuncture/acupressure (K)
Comparison: placebo
|
|
| Outcomes | Nausea, vomiting, pruritis, dizziness. | |
| Notes | Setting: Veteran's General Hospital, Taipei, and National Yang‐Ming University, Taiwan, China. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but it unclear if they were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and treatment wristbands identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed by anaesthetists not involved in intraoperative care, blinded to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | None apparent. |
Ho 2006.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | 110 women scheduled for elective CS under spinal anaesthesia, ASA 1‐2, between 23 and 40 years, without carpal tunnel syndrome or nausea and vomiting in the previous 24 hours. | |
| Interventions | Intervention: acupuncture/acupressure (K)
Comparison: placebo
|
|
| Outcomes | Nausea and vomiting ‐ 1. spinal ‐ skin incision, 2. incision to delivery, 3. delivery to skin closure, 4 skin closure to PACU. | |
| Notes | Setting: Taipei Veterans General Hospital and Mackay Memorial Hospital, Taipei, Taiwan. Dates: not reported. Funding source: not described. Declaration of interest: not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using quote: "envelope system". |
| Allocation concealment (selection bias) | Unclear risk | Type of envelope unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, anesthesiologists, obstetricians, and nurses were all blinded to treatment group". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, anesthesiologists, obstetricians, and nurses were all blinded to treatment group". No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance. |
Huang 1992.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention: dopamine antagonist (Comparson 2)
Comparison: no treatment
|
|
| Outcomes | Nausea, hypotension, bradycardia. | |
| Notes | Setting: study location (hospital, city, country) not described. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessor described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | None described. |
Ibrahim 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: opioid antagonist (Comparison 7)
Comparator: placebo
Both groups received bupivacaine and morphine as part of the spinal anaesthesia |
|
| Outcomes | Nausea and vomiting | |
| Notes | Setting: Womens Health Hospital, Assiut University, Faculty of Medicine, Egypt Dates: July 2016 ‐ August 2017 Funding source: not reported in English translation of abstract Declaration of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer generated..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "... placed in a sealed envelope prior to study initiation...." but no mention of envelopes being opaque or serially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/80 (4%) of women were excluded after randomisation |
| Selective reporting (reporting bias) | High risk | Methods say they will report itching, hypotension, bradycardia but these outcomes not reported. Paracetamol use reported but not listed in outcomes |
| Other bias | Unclear risk | Baseline data similar but unclear of there may be other biases |
Imbeloni 1986.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
|
|
| Interventions | Group 1: dopamine antagonist (Comparison 2)
Group 2: placebo
|
|
| Outcomes | Nausea and vomiting. | |
| Notes | Setting: Rio de Janeiro, Brazil. Dates: not reported in English abstract Funding source: not reported in English abstract. Declaration of interest: not reported in English abstract. This study currently provides no data for the review because for 'nausea + vomiting' data are unclear if the data are for intraoperative or postoperative. We wrote to the authors in 2009 to request further information but had no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Women were divided into groups randomly." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No apparent baseline differences. |
Jaafarpour 2008.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 80 women undergoing elective CS under spinal anaesthesia in a university hospital in Iran. Contraindications: use of antiemetics within 24 hours, contraindication to regional anaesthesia, allergy to dexamethasone, GI disease, HT or glucose intolerance, PONV or motion sickness. | |
| Interventions | Group 1: steroid (Comparison 3)
Group 2: placebo
|
|
| Outcomes | Intraoperative nausea, vomiting, retching and pain. | |
| Notes | Setting: Ilam Shahid Mostafa Khomeini Hospital, Iran. Dates: 2008 Funding source: not reported. Declaration of interest: not reported. Retching data were combined with vomiting. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned" ‐ no other details. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sealed envelopes" ‐ no other details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind" ‐ no other details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unspecified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss to follow‐up or exclusions, appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | We have not assessed the trial protocol. |
| Other bias | Low risk | No apparent baseline imbalance. |
Jang 1997.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention: opioid antagonist (Comparson 7)
Comparator: placebo
Followed by infusion. |
|
| Outcomes | Nausea, vomiting, analgesia, pruritis and other side effects. | |
| Notes | Setting: not reported, but authors from Seoul, South Korea Dates: not reported Funding source: not reported Declaration of interest: not reported Abstract in English and tables, rest of the paper in Korean. No data available for the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information except to say quote: "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Methods say they will assess analgesic effects and side effects. They report satisfaction but this is not in the methods. We did not assess the trial protocol |
| Other bias | Unclear risk | We only have an English translation of the abstract so cannot assess this |
Kalava 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention: ginger (Comparison 9)
Comparator: placebo:
|
|
| Outcomes | Post op – 2, 2.5, 24 hours – nausea, pain, itch 72 hours – side effects |
|
| Notes | Setting: New York Methodist Hospital, Brooklyn, New York, USA. Dates: June 2010 to April 2011. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated table" |
| Allocation concealment (selection bias) | Unclear risk | Unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34 women (16%) were excluded post randomisation (13 placebo, 21 ginger) for a variety of reasons unlikely to be related to outcome. |
| Selective reporting (reporting bias) | Low risk | We have not assessed the trial protocol. |
| Other bias | Unclear risk | Baseline demographic differences (non stat sig) |
Kampo 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: sedative (Comparison 6)
Intervention 2: dopamine antagonist (Comparison 2)
Comparator: placebo
|
|
| Outcomes | PONV (early and late), rescue antiemetic, pain, pruritis, satisfaction | |
| Notes | Setting: Tamale Teaching Hospital, Tamale, Ghana Dates: April 2016 to May 2017 Funding source: authors report no funding Declaration of interest: authors declare no competing interests. Data on 'nausea + vomiting' in Comparison 2 (2.6) and Comparison 6 (6.6) seem very extreme but we cannot find a possible explanation in the publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...a computer generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed opaque envelope" but no mention of sequential numbering |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although reported as a double‐blind RCT, one drug would have a milky appearance and the other drug and control would be clear, and it is not described how the participants and personnel were blinded. Also the dose of propofol is sufficient to cause noticeable sedation in most women, so it would be obvious to the clinician if the women was given propofol. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 out of 360 (4%) were excluded and therefore lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The outcomes listed in the methods were reported in the results, but we did not assess the trial protocol |
| Other bias | Unclear risk | It is unclear how many women were randomised as the envelope was opened 15 minutes before end of surgery and women were excluded due to PPH |
Kasodekar 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 176 women undergoing elective CS under spinal anaesthesia. | |
| Interventions | Intervention: 5‐HT3antagonist (A)
Comparison: placebo
|
|
| Outcomes | Intraoperative nausea, vomiting, retching, rescue antiemetic, hypotension, pain. | |
| Notes | Setting: Mansoura University, Egypt. Dates: not reported Funding source: not reported. Declaration of interest: not reported. Conference abstract only. Retching data combined with vomiting. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unspecified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance. |
Khalayleh 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Incluson criteria:
Excluson criteria: |
|
| Interventions | Intervention 1: opioid ‐ excluded from the review
Intervention 2: dopamine antagonist (Comparison 2)
Comparator: placebo
|
|
| Outcomes | intraoperative and PONV, rescue droperidol | |
| Notes | Setting: Iran Dates: Jan 2002‐Dec 2003 Funding source: not reported Declaration of Interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "intraoperative and post operative emetic episodes were recorded by doctor who had no knowledge of which study drug the patient had received" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients lost out of 150 |
| Selective reporting (reporting bias) | Unclear risk | They report no side effects in results (but do not specify which side effects), also do not mention side effects in the methods |
| Other bias | Unclear risk | The fact the timing of the interventions are not clear in the Methods raises the risk of other biases |
Kim 1999.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: dopamine antagonist (Comparison 2)
Intervention 2: 5HT3 antagonist (Comparison 1)
Comparator: placebo
|
|
| Outcomes | Nausea, vomiting, satisfaction, side effects. | |
| Notes | Setting: Eulgi General Hospital, Seoul, South Korea Dates: not reported Funding source: not reported in abstract Declaration of interest: not reported in abstract English abstract only, rest of paper in Korean. We will attempt to get translation No data for the review in the abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just says "randomly" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided in abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 women recruited but nothing to say how many analysed |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported in results, but not mentioned in Methods. Need a translation of the full paper |
| Other bias | Unclear risk | Very little methodology in the Abstract to assess for other biases |
Koju 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 50 women for elective CD under spinal | |
| Interventions | Intervention: 5‐HT3antagonist (A)
Comparison: placebo
|
|
| Outcomes | PONV on 4 point scale every 15 in for 4 hours, then at 4, 8 and 24 hours | |
| Notes | Setting: Patan Hospital, Patan, Lalitpur, Nepal. Dates: August 2008 to January 2009 Funding source: not reported. Declaration of interest: state no conflict of interest both financial and non‐financial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unspecified:quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Only reports that women and clinicians unaware of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Nurse drew up drugs, Dr administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Resident (junior) doctors performed data collection |
| Other bias | Unclear risk | No baseline imbalance |
Kotelko 1989.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 203 ASA 1‐2 women undergoing elective CS, 18‐38 years with epidural anaesthesia and epidural morphine. | |
| Interventions | Intervention: anticholinergic (Comparison 5)
Comparison: placebo
|
|
| Outcomes | Nausea, vomiting, retching, rescue antiemetics required, itch, pain, adverse effects. | |
| Notes | Setting: Cedars‐Sinai Medical Center, Los Angeles, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Various adverse effects listed, pruritis used as most common side effect. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None identified. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Quote: "No significant differences between groups." |
Lee 2002.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: 5HT3 antagonist (Comparison 1)
Intervention 2: 5HT3 antagonist (Comparison 1)
Intervention 3: 5HT3 antagonist (Comparison 1)
Comparator: placebo
|
|
| Outcomes | VAS pain scores, emetic episodes, emesis scores, side effects | |
| Notes | Setting: Samsung Cheil Hospital, Seoul, South Korea. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Full paper in English. There were no data for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised according to computerised list" |
| Allocation concealment (selection bias) | Unclear risk | Unrelated anaesthetist prepared syringes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "post operative emetic scores were recorded by anesthesiologist blinded to which antiemetic each patient had received" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear if any data incomplete/missing |
| Selective reporting (reporting bias) | Unclear risk | outcomes appear to be consistent with methods, but we did not assess the trial protocol |
| Other bias | Unclear risk | Nil apparent |
Levin 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: acupressure P6
Intervention 2: drug combination ‐ excluded from the review
Comparator: no treatment
|
|
| Outcomes | Primary outcome: nausea and vomiting | |
| Notes | Setting: Rutgers‐Robert Wood Johnson Medical School, New Jersey, USA Dates: July 2015 to March 2016. Funding source: authors state no funding. Declaration of interest: authors state no conflict. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No described blinding at all |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No described blinding at all |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Authors appear to report the outcomes intended, but we did not assess the trial protocol |
| Other bias | Unclear risk | Very limited detail in abstract/e‐poster, more detail in full paper |
Li 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women undergoing elective CS with epidural anaesthesia N = 180 |
|
| Interventions | Intervention 1: acupressure/acupuncture (Comparison 8)
Intervention 2: acupressure/acupuncture ‐ excluded at present
Comparator: no stimulation
|
|
| Outcomes | PONV at 48 hours | |
| Notes | Setting: 2 separate regions in TsingDao, Qingdao municipal hospital and Qingdao Hiser Medical Center Dates: Nov 2011 to March 2012. Funding source: not reported. Declaration of interest: not reported. Abstract is in English, the paper in Chinese and we have a translation form. Intervention 2 is excluded at present because we are unsure whether this is an additional intervention or a sham control. We will write to the authors for clarification. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random numbers" but no detail on how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants likely aware if they were receiving the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on whether outcome assessor was unaware |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 180 participants provided data |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes appear to have been reported, but we did not assess the trial protocol |
| Other bias | Unclear risk | Difficult to assess as we were assessing a translation not the original paper |
Lim 2001a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: acupuncture/acupressure (Comparison 8)
Comparator: placebo
|
|
| Outcomes | Pre‐specified:
Reported:
|
|
| Notes | Setting: not described but authors from KK Women’s and Children’s Hospital, Singapore Dates: not reported Funding source: not reported Declaration of interest: not reported Conference abstract only. Wrote to authors in July 2009 for further details. No response received. No data for this review as no information on the number of women in each group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information just “…were randomised…” |
| Allocation concealment (selection bias) | Unclear risk | No information just “…were randomised…” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although there was a placebo, the women would, we think, have felt if there was electrical stimulation or not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although it is reported that the investigator was blinded, the women reporting on nausea and pain would probably have known – the assessor observed the vomiting. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information is reported |
| Selective reporting (reporting bias) | Unclear risk | The authors report on the 3 outcomes listed in Methods but also satisfaction, however this is only a conference abstract. We did not assess the trial protocol. |
| Other bias | Unclear risk | State similar demographics but no detail. No methodological information provided so not possible to assess this. |
Lim 2001b.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: corticosteroid (Comparison 3)
Comparator: placebo
|
|
| Outcomes | Pre‐specified:
Reported:
|
|
| Notes | Setting: not reported but authors from KK Women’s and Children’s Hospital, Singapore Dates: not reported Funding source: not reported Declaration of interest: not reported No data for this reviewas no information on the number of women in each group Conference abstract only. Wrote to authors in July 2009 but received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says investigator was blinded – women were probably also but there is no information on how blinding was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Says investigator was blinded but it is not clear if women were blinded and they were being asked about nausea. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | Only reported nausea and vomiting but likely they collected other data. We did not assess the trial protocol |
| Other bias | Unclear risk | States demographically similar but no detail. No methodology reported so not possible to assess |
Liu 2015a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women elective CS under regional anaesthesia N = 90 |
|
| Interventions | Intervention: acupoint electrostimulation
Comparator 1: sham stimulation
Comparator 2: placebo
|
|
| Outcomes | Haemodynamics, VAS nausea score, plasma 5‐HT concentrations. Bleeding, administration of oxytocin, ephedrine and atropine |
|
| Notes | Setting: not stated but authors from Qingdao Regional Hospital, Shandong Province, China Dates: not reported Funding source: not reported Declaration of interest: not reported. Chinese paper with English abstract. No data for this review in the abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Sites covered with sticking plaster, but unclear if participants aware |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear from abstract |
| Selective reporting (reporting bias) | Unclear risk | Unclear from abstract |
| Other bias | Unclear risk | Unclear from abstract |
Lussos 1992.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 42 women undergoing elective CS under spinal anaesthesia, without treatment of nausea and vomiting in the week before surgery, diabetes or uteroplacental insufficiency. | |
| Interventions | Intervention: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Nausea and vomiting pre and postdelivery. | |
| Notes | Setting: Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA. Dates: not reported Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Similar baseline characteristics. |
Mandell 1992.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 128 healthy women at term, singleton pregnancies, elective (or non‐emergent) CS, with regional anaesthesia, with no other anaesthetic adjuvants or the use of adjuvants. | |
| Interventions | Intervention: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Incidence of nausea and vomiting, narcotic administration, hypotension. | |
| Notes | Setting: Winston‐Salem, North Carolina, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Results were provided as a percentage of the overall number of participants in each group. Actual participant numbers with each outcome calculated by multiplication of this percentage by the number of participants in each group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind fashion". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unspecified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, 7 participants lost before intervention. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Only reports on those women who completed the treatment. |
Maranhao 1988.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women undergoing elective CS, under subarachnoid block. | |
| Interventions | Intervention 1: dopamine antagonist (Comparison 2)
Intervention 2: dopamine antagonist (Comparison 2)
Comparator: placebo
|
|
| Outcomes | Nausea and vomiting | |
| Notes | Setting: not clear on the photocopy, but likely South America Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Abstract in English, main paper probably Portuguese. We now have translation forms. We wrote to the authors in 2009 but have received no reply. Data in Interventions 1 and 2 were entered as subgroups and combined with overall data for treatment effect with the required adjustment of the placebo data for the 2 subgroups according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated by chance" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding at all |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding at all |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appear to have provided data |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes appear to have been reported but we did not assess the trial protocol |
| Other bias | Unclear risk | Unable to assess from our translation |
Modir 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women having elective CS under spinal anaesthesia N = 140 |
|
| Interventions | Intervention 1: corticosteroid (Comparison 3)
Intervention 2: sedative (Comparison 6)
Intervention 3: (Comparison 6)
Comparator: placebo
|
|
| Outcomes | Nausea and vomiting | |
| Notes | Setting: Valiasr Hospital, Arak, Iran, presumably, but not stated specifically Dates: not reported Funding source: Arak University of Medical Sciences Declaration of interest: not reported. No data for the review as it is unclear how many women in each group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block random allocation method used but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although described as double blind ‐ no discussion of blinding participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “… second project executive, who was unaware of the grouping assignment, recorded the data.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many women provided data in each group |
| Selective reporting (reporting bias) | Unclear risk | The same outcomes in the methods are reported in the results but we did not assess the trial protocol |
| Other bias | Unclear risk | Women were excluded (presumably after randomisation) if they were vomiting intra‐op or if dissatisfied – unclear why these women were to be excluded. |
Mohammadi 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention: 5HT3 antagonist (Comparison 1)
Comparator: placebo
|
|
| Outcomes | Shivering, nausea and vomiting | |
| Notes | Setting: Dr. Shariati Hospital, Tehran, Iran. Dates: March to September 2013. Funding source: not reported. Declaration of interest: not reported. Recorded presence of nausea and vomiting, but results only shown for intra‐op |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes, hidden |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Separate researcher and clinician. Patients blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Separate researcher and clinician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the study protocol |
| Other bias | Unclear risk | Similar baseline characteristics |
Mokini 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: sedative (Comparison 6)
Intervention 2: dopamine antagonist (Comparison 2)
Intervention 3: sedative + dopamine antagonist ‐ excluded from this review
Comparator: placebo
|
|
| Outcomes | Nausea and vomiting | |
| Notes | Setting: authors are Italian but no details Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Conference abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding (and one intervention is white in colour) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding (and one intervention is white in colour) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many women were randomised and provided data |
| Selective reporting (reporting bias) | Unclear risk | Inadequate info in abstract and we did not assess the trial protocol |
| Other bias | Unclear risk | Inadequate info in abstract |
Mukherjee 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 80 ASA 1‐2, age 20‐34, undergoing CS under spinal anaesthesia, with no antiemetic drugs within 24 hours. | |
| Interventions | Intervention 1: sedative (Comparison 6)
Intervention 2: sedative (Comparison 6)
Intervention 3: sedative (Comparison 6)
Comparison: placebo
|
|
| Outcomes | Intraoperative nausea, vomiting, retching, adverse events. | |
| Notes | Setting: Calcutta National Medical College and Hospital, Kolkata, India. Dates: not reported Funding source: not reported. Declaration of interest: not reported. Retching combined with vomiting, dosage groups combined to yield overall treatment effect. Drugs are in separate subgroups but then pooled in our analysis, so the placebo data are dealt with according to our methods (Unit of analysis issues). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation chart. |
| Allocation concealment (selection bias) | Unclear risk | Identical syringes prepared by uninvolved anaesthetist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blinded manner". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by anaesthetist unaware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No differences in baseline groups. |
Munnur 2008.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: 5‐HT3antagonist (Comparison 1)
Intervention 2: 5‐HT3antagonist (Comparison 1)
Comparison: placebo
|
|
| Outcomes | Composite PONV. | |
| Notes | Setting: Baylor College of Medicine, Houston, Texas, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Conference abstract. Drugs are in separate subgroups but then pooled in our analysis, so the placebo data are dealt with according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..randomised..." ‐ no further information. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear who administered the study drug and if they (or the patients) were blinded. Study is described as “double blind” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Post‐op nurses were unaware, but it does not actually say that they did the data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or lost data reported. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No information provided on baseline data. Only a conference abstract so not able to assess if there were other biases. |
Niu 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
|
|
| Interventions | Intervention: sedative ( Comparison 6)
Comparator: placebo
|
|
| Outcomes | Nausea and vomiting | |
| Notes | Setting: Aviation General Hospital, Beijing, China Dates: October 2016 to February 2017 Funding source: not reported Declaration of interest: stated that authors have no conflicts of interest Authors also reported on maternal pain and neonatal behavioral neurological assessment (NBNA), reporting no difference between the groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Allocated using random number by research fellow A 1:1 ratio – who was not involved with assessment or pt instructions (that was RF B). After obtaining consent, patients were allocated to either the propofol or the placebo group by opening a sealed opaque envelope.” However, it is unclear if the envelopes were sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The parturient head site was covered by surgical drapes during the operation so they could not see the infusion line.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed by independent Research Fellow that was not involved with randomisation – different research fellows assessed different outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included in the analysis. 92 women were scheduled for CS – 12 were excluded, the remaining 80 women completed the trial |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from the methods section were reported on but we did not assess the trial protocol.. |
| Other bias | Unclear risk | It is not clear if there may have been other biases |
Noroozinia 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 152 women ASA 1 or 2 undergoing elective CD under spinal anaesthesia | |
| Interventions | Intervention: acupuncture/acupressure (Comparison 8)
Comparison: placebo
|
|
| Outcomes | Intra‐op and post‐op (in first, second and third 2‐hour periods) nausea and vomiting | |
| Notes | Setting: Imam Khomeini Training Hospital, Urmia, Iran. Dates: 2010 Funding source: not reported. Declaration of interest: not reported. VAS scoring of nausea performed at unspecified times (esp intra‐op, no info). Post‐op incidence of vomiting performed in PACU,quote: "0‐2 hrs", "2‐4 hrs" and "4‐6 hrs". For comparisons in this review the post‐op N& V incidence was averaged over the 4 time epochs. Revman does not permit decimal entries (i.e. 1.25, 1.5), so 1.25 rounded down to 1; 1.5 rounded up to 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unspecifed:quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Elastic band on wrist used in all women but those with acupressure point may have known they had it |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding difficult for pressure sensitive area |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Chart assessment", otherwise unspecified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts? |
| Selective reporting (reporting bias) | Unclear risk | Unspecified |
| Other bias | Unclear risk | No sample size calculation, no VAS results presented |
Nortcliffe 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: antihistamine (Comparison 4)
Intervention 2: steroid (Comparison 3)
Comparison: placebo
|
|
| Outcomes | Incidence of nausea and vomiting in the 24 hours postsurgery, requirement for rescue antiemetic medication, women's satisfaction. | |
| Notes | Setting: University Hospitals of Leicester, UK. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomisation is groups of 9, unclear how produced. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but no mention of their being sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication prepared by the anaesthetist who did not collect data. Patients unaware of medication administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medication prepared by the anaesthetist who did not collect data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All missing data accounted for. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline differences in groups. |
Pan 1996.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 48 women undergoing elective CS under epidural anaesthesia, ASA 1‐2, not planning to breast feed, no psychiatric disease or motion sickness. | |
| Interventions | Intervention 1: 5‐HT3antagonist (Comparison 1)
Intervention 2: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Intraoperative nausea, vomiting (combined to make a cumulative score). | |
| Notes | Setting: University Medical Centre, Virginia, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Who made up the syringes was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Assessor unaware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor unaware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the protocol. |
| Other bias | Low risk | No baseline differences. |
Pan 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 156 ASA 1‐2 undergoing elective CS under epidural anaesthesia with no history of psychiatric disease or breastfeeding. | |
| Interventions | Intervention 1: 5‐HT3antagonist (Comparison 1)
Intervention 2: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Nausea or vomiting intraoperative or up to 24 hours postoperative, sedation score. | |
| Notes | Setting: University Medical Centre, Virginia, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. All interventions given in 10 mL after clamping cord. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Explicitly stated that all were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Explicitly stated that all were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants were excluded after randomisation and could not be re‐included. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline differences. |
Pan 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 40 women undergoing elective CS under epidural anaesthesia. Women were excluded if the had a recent history of GI disease, nausea and vomiting or a quote: "maternal history of chronic utero‐placental insufficiency". |
|
| Interventions | Intervention: 5‐HT3antagonist (Comparison 1)
Comparison: placebo
|
|
| Outcomes | Nausea ‐ before/after birth, vomiting/retching before/after birth. | |
| Notes | Setting: University Medical Center, Virginia, Richmond, Virginia, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Retching data included in vomiting. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of data described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No other bias apparent. |
Parra‐Guiza 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclussion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: 5HT3 antagonist (Comparison 1)
Intervention 2: corticosteroid (Comparison 3)
Comparator: placebo
|
|
| Outcomes | Nausea and vomiting, etc. | |
| Notes | Setting: Columbia, South America Dates: February 2014 to September 2016 Funding source: Industrial University of Santander (Universidad Industrial de Santander) (from trial reg) Declaration of interest: authors declared no conflicts of interest (reported in Spanish at end or publication) No data available in the Abstract. Publication in Portuguese/Spanish (?) with abstract in English. No translation as yet Trial registration form reported under Guiza 2016, but publication indicated the first author should be Parra‐Guiza We will try to get a translation or contact the authors for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in the abstract translation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in the abstract translation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported in the translated abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in the translated abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No apparent loss of data after randomisation |
| Selective reporting (reporting bias) | Unclear risk | They do not report on pruritus in the abstract, yet this is the secondary outcome in the trial registration form. However, we do not have a translation of the paper yet, nor have we seen the trial protocol. |
| Other bias | Unclear risk | We were only able to assess the English abstract and so were unable to assess the whole paper. |
Pazoki 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women having CS under spinal anaesthesia N = 195 women randomised, 191 women had their data analysed |
|
| Interventions | Intervention 1: 5 HT3 antagonist (Comparison 1)
Intervention 2: 5 HT3 antagonist (Comparison 1)
Comparator; placebo
|
|
| Outcomes | Headache and nausea and vomiting | |
| Notes | Setting: Taleghani Hospital, Arak, Iran Dates: not reported Funding source: the work was supported by a grant from Arak University of Medical Sciences Declaration of interest: not reported No data included in the reviewuntil we contact authors to check the apparently conflicting information on the denominators |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications were coded and administered to three groups A, B and C by an anesthetist who was not involved in the data collection, whilst the patients and the resident collecting information were unaware of patient grouping |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medications were coded and administered to three groups A, B and C by an anesthetist who was not involved in the data collection, whilst the patients and the resident collecting information were unaware of patient grouping |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/195 (2%) women were excluded |
| Selective reporting (reporting bias) | Unclear risk | Study just aiming at assessing headache and nausea and vomiting but we did not assess hr trial protocol and there may have been other outcomes listed. |
| Other bias | Unclear risk | Baselne characteristics were similar between the groups but there is very little methodology reported in the paper so assessment was unclear |
Peixoto 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 120 women undergoing elective CS under spinal anaesthesia, ASA 1‐2, no pre‐op emesis or antiemetic medications within 24 hours. | |
| Interventions | Intervention 1: 5‐HT3antagonist (Comparison 1)
Intervention 2: dopamine antagonist (Comparson 2)
Comparison: placebo
|
|
| Outcomes | Nausea and vomiting up to 24 hours postoperative, adverse events. | |
| Notes | Setting: Erechim, Brazil and Yale, Conneticut, USA. Dates: April 2001 to August 2003 Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation code. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, syringes prepared by member of research team not involved in care. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All personnel unaware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All personnel unaware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nil. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No significant differences reported on. |
Quiney 1995.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Pregnant women undergoing elective CS under spinal anaesthesia. N = 40 |
|
| Interventions | Intervention: anticholinergic (E)
Comparison: placebo
|
|
| Outcomes | Hypotension, emetic symptoms, pain. | |
| Notes | Setting: Southmead Hospital, Bristol, UK. Dates: not reported Funding source: not reported. Declaration of interest: not reported. Conference abstract. This study currently provides no data for the review because we cannot be sure women are not counted more than once because of the 3 time periods of assessment. We wrote to authors in 2009 for clarification but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" ‐ but no further detail provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Not described. |
Rasooli 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusiom criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: sedative (Comparison 6)
Intervention 2: sedative (Comparison 6)
Comparison: placebo
|
|
| Outcomes | nausea and vomiting and retching on 4‐point score | |
| Notes | Setting: Al‐Zahra Obstetrics and Gynecology Educational Hospital, Tabriz, Iran. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Looks like N&V only examined intra‐op. Authors do not state if post‐op N&V specifically looked at. Drugs are in separate subgroups but then pooled in our analysis, so the placebo data are dealt with according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unspecified: quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Solutions were prepared by an assistant not involved in care |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Double blind". However patients could see what infusion they were receiving (propofol) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments by blinded third party |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Unclear risk | Specified endpoints; possibly low risk? |
| Other bias | Unclear risk | VAS scores apparently taken but not reported |
Rudra 2004a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 60 ASA 1‐2 women scheduled for CS with spinal anaesthesia, no GI/liver/ear disease, hyperemesis, hyperlipidaemia, antiemetics within 24 hours. | |
| Interventions | Intervention: sedative (Comparison 6)
Comparison: placebo
|
|
| Outcomes | Nausea, retching, vomiting, rescue antiemetics. | |
| Notes | Setting: Calcutta National Medical College, Kolkata, India. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Retching data combined with vomiting. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treating anaesthetist not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No major differences in baseline characteristics. |
Sahoo 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 52 women ASA 1‐2 undergoing elective CS with spinal anaesthesia, with no contraindications to spinal anaesthesia. | |
| Interventions | Intervention: 5HT3 antagonist (Comparison 1)
Compaison: placebo
|
|
| Outcomes | Heart rate, BP, oxygen saturations, nausea, vomiting, pain. | |
| Notes | Setting: Kolkata, India. Dates: September to December 2008 Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No additional information about participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observations were made by an anaesthetist blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None apparent. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the study protocol. |
| Other bias | Low risk | Nil apparent. |
Sanansilp 1998.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 97 women undergoing CS under epidural, ASA 1‐2, without history of convulsions, Parkinsonism, drug abuse and psychiatric problems. | |
| Interventions | Group 1: dopamine antagonist (Comparison 2)
Group 2: dopamine antagonist (Comparison 2)
Group 3. placebo
|
|
| Outcomes | Pruritis, nausea, vomiting, sedation, pain. | |
| Notes | Setting: Siriraj Hospital, Bangkok, Thailand. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. This study currently provides no data for the review because the data are presented graphically. We have wrote to the authors in 2009 requesting the specific data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote; "double blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specifically described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no exclusions or loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No evidence of other biases. |
Selzer 2020.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women undergoing elective CS under spinal anaesthesia (IT morphine) N = 122 women randomised |
|
| Interventions | Intervention: corticosteroid (Comparison 3)
Group 2: placebo.
|
|
| Outcomes | Nausea and vomiting (intra‐ and post‐op), pain, satisfaction. | |
| Notes | Setting: New York‐Presbyterian Hospital/Weill Cornell Medicine in New York, NY, USA Dates: November 2012 to September 2014 Funding source: this study was funded by departmental support from the Department of Anesthesiology of Weill Cornell Medicine. There are no additional commercial or non‐commercial affiliations, associations, or sources of funding to disclose. Declaration of interest: authors declared none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…a computer‐generated simple (non‐blocked) random number sequence…” |
| Allocation concealment (selection bias) | Unclear risk | No information on whether allocation was concealed it just reports: quote: “After randomization, the study drug…or placebo … was prepared by an unblinded investigator who had no further involvement in the study.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “All subjects, care providers, and data collectors were blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All subjects, care providers, and data collectors were blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 122 women were randomised and data from 108 analysed (11% loss of data) |
| Selective reporting (reporting bias) | High risk | The method section said data would be collected at 0, 1, 3, 6, 24 and 48 hours, but the results section only reports one overall incidence throughout the 48 hours. It is unclear how this overall data were calculated. |
| Other bias | Unclear risk | They excluded women after randomisation if they did not receive the intervention of had a PPH, these women should have still been included, but only amount to 11% loss. It is unclear if there might be other biases. |
Shabana 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 220 women undergoing caesarean delivery under spinal anaesthesia | |
| Interventions | Intervention: sedative (F)
Comparison: placebo
|
|
| Outcomes | Nausea, vomiting, haemodynamics, adverse effects (hallucinations, sedation) | |
| Notes | Setting: Mansoura University Hospitals, Egypt. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Authors reported the number of nausea and vomiting episodes, rather than the number of patients who had nausea and vomiting. We will attempt to contact the authors to clarify. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specifically described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The treating anaesthetist was unaware of the group allocation and recorded intraoperative data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 patients were excluded from the study after enrolment, it is unclear if this was before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | We were not able to examine the study protocol |
| Other bias | Low risk | Nil apparent |
Shen 2012.
| Study characteristics | ||
| Methods | RCT. | |
| Participants | Incluson criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: anticholinergic (Comparison 5)
Intervention 2: 5HT3 antagonist (Comparison 1)
Intervention 3: anticholinergic + 5HT3 antagonist ‐ exclude as a combination of drugs
Comparator: placebo
|
|
| Outcomes | Nausea and vomiting | |
| Notes | Setting: not reported but authors from China‐Japan Frienship Hospital, Beijing, China Dates: not reported Funding source: not reported. Declaration of interest: not reported. English abstract only assessed, we will attempt to locate the full paper in English. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported as “…randomly divided…” |
| Allocation concealment (selection bias) | Unclear risk | Not reported in abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported in abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in abstract |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seems clear that all participants provided data (e.g.. in table 3 the percentages are in brackets) |
| Selective reporting (reporting bias) | Unclear risk | Not possible to assess as we only have the English abstract at this point |
| Other bias | Unclear risk | Not possible to assess as we only have the English abstract at this point |
Stein 1997.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 75 healthy pregnant women undergoing elective CS under spinal anaesthesia, without history of diabetes, morbid obesity previous postoperative nausea or vomiting, nausea or vomiting in the previous 24 hours. | |
| Interventions | Intervention 1: acupuncture/acupressure (Comparison 8)
Intervention 2: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Hypotension, sedation, nausea and vomiting, Apgar score. | |
| Notes | Setting: St. Luke's‐Roosevelt Hospital Center, New York, New York, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospectively randomised using envelope system." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "prospectively randomised using envelope system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, anaesthesiologists, nurses and obstetricians were all blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, anaesthesiologists, nurses and obstetricians were all blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | None apparent. |
Tarhan 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 88 ASA 1‐2, 20‐38 year old women undergoing elective CS under spinal anaesthesia with no history of GI disease, recent antiemetic use or contraindication for regional anaesthesia. | |
| Interventions | Intervention 1: sedative (Comparison 6)
Intervention 2: sedative (Comparison 6)
Comparison: placebo
|
|
| Outcomes | Nausea, vomiting, retching ‐ intraoperative and postoperative. | |
| Notes | Setting: Ankara, Turkey. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Retching combined with vomiting. In Table 2, Column 4, reports that the control group consisted of n = 28, but then in the body of the table, it states n = 30. Email correspondence with author confirms that 28 participants were actually enrolled in the control group. Drugs are in separate subgroups but then pooled in our analysis so the placebo data are dealt with according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Drugs prepared and covered according to a random number list by a personnel member who was not aware of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". No additional information about participant blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative outcomes assessed by blinded clinician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline differences. |
Tkachenko 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Incclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention 1: corticosteroid (Comparison 3)
Intervention 2: corticosteroid (Comparison 3)
Comparator: placebo
We pooled the data from the 2 routes of administration of dexamethasone |
|
| Outcomes | Nausea & vomiting | |
| Notes | Setting: Kyiv City Centre of Reproductive and Perinatal Medicine, Kyiv Ukraine Dates: not reported. Funding source: not reported Declaration of interest: not reported. Conference abstract. Drugs are in separate subgroups but then pooled in our analysis so the placebo data are dealt with according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote:: “double blind” – but treating doctors unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote:“double blind” – but treating doctors unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 124 women provided data, unclear how many recruited |
| Selective reporting (reporting bias) | High risk | Authors say they will report “The patients were evaluated for blood pressure, heart rate, nausea, vomiting, shivering or other complications during intra‐ or postoperative period (24h).” but they only report 1 set of data and do not say if this is intra‐ or post‐operative |
| Other bias | Unclear risk | Inadequate info to assess |
Tzeng 2000.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 113 women undergoing elective CS under epidural anaesthesia, ASA 1‐2, age 20‐35 years. | |
| Interventions | Intervention 1: steroid (Comparison 3)
Intervention 2: dopamine antagonist (Comparison 2)
Comparison: placebo
|
|
| Outcomes | Nausea, vomiting, need for antiemetic rescue medication. | |
| Notes | Setting: Taipei and Tainan, Taiwan. Dates: not reported Funding source: not reported. Declaration of interest: not reported. Pruritis (local/generalised) was the only adverse event listed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation table." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The randomisation process and the identity of the study drugs were blinded from the parturients, the anesthesiologists during surgery, and the investigators who collected the postoperative data". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The randomizations process and the identity of the study drugs were blinded from the parturients, the anesthesiologists during surgery, and the investigators who collected the postoperative data". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 women were withdrawn due to failed regional anaesthesia. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the study protocol. |
| Other bias | Low risk | No baseline difference in groups. |
Uerpairojkit 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention: 5HT3 antagonist (Comparison 1)
Comparator: placebo
|
|
| Outcomes | Nausea presence and severity, during first breast feed and first 24 hours, vomiting, vertigo and itching, rescue antiemetics | |
| Notes | Setting: King Chulalongkorn Memorial Hospital and Yala Regional Hospital, Thailand Dates: 2012 to 2015 Funding source: the authors thank the Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University and Yala Regional Hospital, Yala, Thailand for their assistance and cooperation but do not mention funding as such Declaration of interest: authors reported no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…computer random number generator…” |
| Allocation concealment (selection bias) | Low risk | Quote: "Corresponding code according to random number table" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The patients were also blinded to the study medication.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “The study drug was given blindly according to the corresponding codes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/160 women were excluded after randomisation |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods were reported on but we did not assess the trial protocol |
| Other bias | Unclear risk | Baseline data were similar, but there is very little methodology reported so it is unclear if there might have been other bases. |
Ure 1999.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 49 ASA 1‐2, singleton pregnancies, elective CS at term under spinal anaesthesia, without pregnancy‐induced hypertension, placenta praevia, diabetes, coagulopathy, neurological or cardiac disease. | |
| Interventions | Intervention: anticholinergic (Comparison 5)
Comparison: placebo
|
|
| Outcomes | Nausea and vomiting, hypotension, Apgar scores. | |
| Notes | Setting: Glasgow Royal Infirmary, Glasgow, UK. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Apgar scores not provided as median and range. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawn prior to intervention. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | None described. |
Voigt 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | 4 groups: Intervention 1 (group 2): 5HT3 antagonist + dopamine antagonist ‐ not used in this review
Intervention 2 (group 3): antihistamine+corticosteroid ‐ not used in this review
Intervention 3 (group 4): 5HT3 antagonist (Comparison 1)
Comparator (group 1): placebo
We include only group 1 (control) and group 4 Tropisetron alone |
|
| Outcomes | Nausea and vomiting: intra‐operative; early post‐operative (0–2 hours); late post‐operative (2–24 hours). We include intra‐operative and early post‐operative as per our Methods (data from the earliest time point). | |
| Notes | Setting: Evangelian Deaconry Hospital, Freiburg, Germany. Dates: 2010 to 2012 Funding source: not reported Declaration of interest: authors stated “None of the authors have any financial relationships with commercial companies involved with a product in this study”. We will write to the authors and request data separated into nausea and vomiting and for information on the risk of bias assessments. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method poorly described ("sealed envelopes") |
| Allocation concealment (selection bias) | Unclear risk | Although authors report quote: "Sealed opaque envelopes opened by anaesthetist immediately prior to surgery" it is not reported if they were serially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collected by quote: "trained investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All women appeared to be included in outcome data |
| Selective reporting (reporting bias) | High risk | Satisfaction and complications do not appear to be reported as per the Methods. We did not assess the trial protocol. |
| Other bias | Unclear risk | Limited methodological information reported. |
Wang 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 175 ASA 1‐2 women (body wt 50‐90 kg) undergoing elective CS under epidural anaesthesia without a history of PONV, GI disorder. | |
| Interventions | Intervention 1: steroid (Comparison 3)
Intervention 2: steroid (Comparison 3)
Intervention 3: steroid (Comparison 3)
Comparison: placebo
|
|
| Outcomes | Incidence of nausea, vomiting, severe vomiting (> 4 episodes), rescue antiemetics, total proportion with no nausea or vomiting. | |
| Notes | Setting: Taipei, Taiwan. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Dose groups combined to yield overall treatment effect for dexamethasone (steroid). Drugs are in separate subgroups but then pooled in our analysis, so the placebo data are dealt with according to our methods (Unit of analysis issues) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The randomisation process and the identity of the study drugs were blinded from the parturients, the anesthesiologists during surgery, and the investigators who collected the postoperative data". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The randomisation process and the identity of the study drugs were blinded from the parturients, the anesthesiologists during surgery, and the investigators who collected the postoperative data". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 excluded for missing data. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Baseline data similar. |
Weiss 1995.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 74 women undergoing spinal anaesthesia for CS. | |
| Interventions | Intervention: sedative (Comparison 6)
Comparison: placebo
|
|
| Outcomes | Nausea/pruritis scales at various postoperative times. | |
| Notes | Setting: Long Island Jewish Medical Centre, New York, USA. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. This study currently provides no data for the review because the data are presented only in graphical form. We wrote to the authors in 2009 to request the specific data. Conference abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators blinded. Treatment identical in appearance to placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Nil apparent |
Wu 2007.
| Study characteristics | ||
| Methods | Individual RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Group 1: steroid (Comparison 3)
Group 2: dopamine antagonist (Comparison 2)
Group 3: steroid + Dopamine antagonist ‐ data nor used in this review
Group 4: Comparison: placebo
|
|
| Outcomes | Composite outcome of nausea and vomiting | |
| Notes | Setting: Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. Only useable data are postoperative vomiting. Other outcomes are amalgamated in composite score. We have written to the authors and attempt to get separated data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications made up by uninvolved anaesthetist blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers blinded to allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up, ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance. |
Yazigi 2002.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 100 ASA 1‐2 women undergoing elective CS. | |
| Interventions | Intervention: 5‐HT3antagonist (Compaeison 1)
Comparison: placebo
|
|
| Outcomes | Pruritis, nausea, vomiting, pain, side effects. | |
| Notes | Setting: Hotel‐Dieu de France University Hospital, Beirut, Lebanon. Dates: not reported. Funding source: not reported. Declaration of interest: not reported. This study currently provides no data for the review because the outcomes are reported as combined "Nausea and Vomiting". We have written to the authors requesting separated data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Drug solutions were given according to a double blinded protocol" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessment was made by an anesthesia resident who was blinded to the treatment groups”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes in methods reported in the results but we did not assess the trial protocol. |
| Other bias | Unclear risk | Similar baseline characteristics but insufficient methodology reported to be sure of no further bias. |
Zeraati 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria:
Exclusion criteria: |
|
| Interventions | Intervention: ginger (Comparison 9)
Comparator: no ginger
|
|
| Outcomes | Nausea and vomiting | |
| Notes | Setting: Bojnoord Bentolhoda Hospital, Iran Dates: 2014 Funding source: North Khorasan University of Medical Sciences Declaration of interest: not reported. We pooled the data for mild, moderate and severe nausea and the same for vomiting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no detail |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double blind but ginger has a taste so women likely to be aware but not clear as there is a possibility of tasteless ginger |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double blind but ginger has a taste women likely to be aware. Outcomes "self‐assessed" by women, who may or may not have known. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients appeared to provide data |
| Selective reporting (reporting bias) | Unclear risk | Outcomes described in methods appeared to be reported but we did not assess the study protocol |
| Other bias | Unclear risk | No other apparent bias |
ASA: American Society of Anaesthesiologists; BMI: body mass index;BP: blood pressure; CS: caesarean section; CSE: combined spinal epidural;GI: gastrointestinal; GA: general anaesthesia; IT: intrathecal;GIT: gastrointestinal disease;ITT: intention‐to‐treat; IV: intravenous; LUSCS: Lower Uterine Segment Caesarean Section; NSAID: non‐steroidal anti‐inflammatory; PACU: post anaesthesia care unit; PCA: patient controlled analgesia; PONV: postoperative nausea and vomiting; PPH: postpartum hemorrhage; RCT: randomised controlled trial; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abadi 2018 | Study of acupressure vs placebo for women having CS, but under general anaesthesia. |
| Abboud 1984 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Abdalla 2019 | No placebo group. Study on dexamethasone vs atropine vs dexamethasone + atropine for nausea and vomiting at CS |
| Abdellah 2018 | Study comparing 2 surgical techniques (exterioration vs intraperitoneal) for CS under spinal anaesthesia. |
| Ackerman 1987 | Study assessed lipophilic opioids drugs given predominantly for analgesia. |
| Ackerman 1988 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Ackerman 1989 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Afsargharehbagh 2018 | No placebo group. Study looked at women having CS and compared ondansetron vs metoclopramide. Includes trial registration |
| Alghanem 2019 | Focus of study is effects of ondansetron vs placebo on haemodynamics and not nausea and vomiting. Previous study name: NCT04140058 2019 |
| Alipour 2017 | Study looked at women having CS and compared midazolam vs ondansetron ‐ no placebo group |
| Allen 2013 | Dexamethazone for analgesia for post CS pain not for nausea and vomiting. |
| Ananthakrishnan 2004 | Study compared fasting overnight with a light breakfast ‐ not considered within our review question. |
| Anonymous 2010 | Focus of study was phenylephrine vs placebo in women having a CS under spinal anaesthesia assessing the incidence of nausea. However, there are no names associated with this trial registration which is now 10 years old. |
| Askar 2017 | Study assessed combination of azithromycin + dexamethasone vs dexamethasone at CS under spinal anaesthesia, no placebo group. |
| Atalay 2010 | The study investigated different anaesthetic techniques and their side effects, not interventions for PONV as such. |
| Atkinson 1980 | Not an RCT. |
| Avramovic 1979 | Study assesses an intervention given to reduce abdominal discomfort, not to reduce nausea and vomiting. |
| Ayorinde 2001 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Banihashem 2011 | No placebo: study looking at dexamethasone vs ondansetron in women having CS under spinal anaesthesia. |
| Belzarena 1993 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Bifarini 1990 | Following translation, study assessed drugs given for aspiration prophylaxis, not for prevention of nausea and vomiting. |
| Bifarini 1992 | Following translation, study assessed drugs given for aspiration prophylaxis, not for prevention of nausea and vomiting. |
| Biwas 2002 | No placebo group. Study looked at metoclopramide vs glycopyrrolate vs 'metoclopramide + glycopyrrolate' |
| Bonhomme 2002 | Study on droperidol vs placebo, but interventions not given at CS but given postnatally |
| Boschi 1984 | Not an RCT; women were divided into groups. |
| Briao 2015 | This study examines pruritis ‐ not nausea and vomiting ‐ as the main outcome. |
| Brock‐Utne 1989 | Not an RCT; women were allocated to groups. |
| Brody 2008 | Study relates to the use of IM ephedrine (predominantly for preventing hypotension rather than PONV). |
| Bylsma‐Howell 1983 | Study was assessing the effect of metoclopramide on gastric emptying and aspiration prophylaxis not for reducing nausea and vomiting. |
| Chan 1992 | Study was a quasi‐RCT. |
| Chan 1997 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Chang 2011 | Study on mitazapine, an antidepressant, so not included. |
| Chattopadhyay 2015 | No placebo: study on palonosetron vs ramosetron in women undergoing CS under spinal anaesthesia. |
| Chaudhuri 2004 | No placebo. Study looked at 'dolasetron + metoclopramide' vs metoclopramide at CS under regional anaesthesia |
| Chauhan 2014 | No placebo group. Looked at nausea and vomiting with ramosetron vs ondansetron in women having CS under regional anaesthesia |
| Chen 2005 | Not an RCT; women were assigned to groups. |
| Chestnut 1989 | No placebo group. Study looked at nausea and vomiting with metoclopramide vs droperidol in women having CS under epidural anaesthesia |
| Chung 1998 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Cohen 1984 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. No information on how women were allocated to groups. |
| Cohen 2016a | Comparing different interventions and no placebo group. Scopolamine patch vs acupressure point P6 vs scopolamine + acupressure point P6. |
| Colman 1988 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. No information on how women were allocated to groups. |
| Connelly 1997 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Cooper 2002 | Study assessed drugs given for manipulation of blood pressure, not for prevention of nausea and vomiting. |
| Cowan 2002 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Dahlgren 1997 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Dailey 1988 | Studied effect of cimetidine and ranitidine on lignocaine concentrations in the blood. |
| Datta 1982 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. No information on how women were allocated to groups. |
| Demirhan 2013 | No placebo group. Study looked at nausea and vomiting with ondansetron vs dexamethasone vs 'ondansetron + dexamethasone' in women having CS under spinal anaesthesia |
| Dereu 2019 | No placebo group. Study compared IT morphine with TAP block with ropivacaine + clonidine |
| Dewan 1982 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not described as an RCT; women were assigned to groups. |
| Dewan 1984 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Dewan 1985 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Dundee 1979 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. No information on how women were allocated to groups. |
| El Khouly 2016 | Interventon (ondansetron) for reducing hypotension not nausea and vomiting. Trial registration reference: PACTR201601001397193 2015 |
| El Saied Hafez 2017 | Study is looking at the effect of gabapentin for pain relief at CS under spinal anaesthesia. Gabapentin is an anticonvulsant with side effect of nausea and vomiting and we are excluding anticonvulsant drugs. |
| El‐Deeb 2011 | Combination drugs. Study on ‘granisetron (5‐HT3 antagonist) + dexamethasone (corticosteroid)’ vs ‘midazolam (sedative) + dexamethasone (corticosteroid)’ vs placebo in women having CS under spinal anaesthesia. |
| Elhakim 2005 | Study assess medication given for the purpose of pain relief not reduction of nausea and vomiting. |
| Elmetwally 2019 | Study assessing effects of atropine vs metoclopramide on nausea and vomiting at CS, but no placebo group. Previous study name: NCT03932578 2019 |
| Ewart 1990 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Fan 1994 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Farzi 2017 | Primary focus was analgesia and block duration, not specifically nausea and vomiting. Drugs included: fentanyl vs sufentanil vs placebo. |
| Fattahi 2015 | Study assesses incidence of headache not nausea and vomiting. |
| Fazel 2017 | The study is described as a 'double‐blind clinical trial' with no mention of randomisation nor of type of anaesthesia used. Also, the oil is given to women with nausea ‐ so it appears to be a treatment study not a prevention study. The publication is in Iranian and we only have the English translation of the abstract to consider. |
| Flynn 1989a | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Study of effect of 400 mg cimetidine or 150 mg ranitidine or placebo on plasma levels of bupivacaine at CS. |
| Flynn 1989b | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Study of effect of 200 mg cimetidine plasma levels of lignocaine at CS. |
| Flynn 1989c | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Study of effect of 400 mg cimetidine or 150 mg ranitidine or placebo on plasma levels of lidocaine at CS. |
| Fogarty 1992 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Frank 1984 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Freeman 1999 | Study assessed the use of sodium citrate (normally used for reducing aspiration pneumonitis during general anaesthesia) for spinal anaesthesia at CS. Although the study assessed nausea and vomiting, this was not the primary purpose. |
| Fujii 1998a | This study was retracted by the publishing journal after uncertainty was raised as to the validity of the data (Carlisle 2012). |
| Fujii 1998b | This study was retracted by the publishing journal after uncertainty was raised as to the validity of the data (Carlisle 2012). |
| Fujii 1999 | This study was retracted by the publishing journal after uncertainty was raised as to the validity of the data (Carlisle 2012). |
| Fujii 2002 | This study was retracted by the publishing journal after uncertainty was raised as to the validity of the data (Carlisle 2012). |
| Fujii 2004 | This study was retracted by the publishing journal after uncertainty was raised as to the validity of the data (Carlisle 2012). |
| Gaiser 2002 | IV fluids is not an intervention we are covering in this review. Study was on IV fluids vs placebo in women having CS under regional anaesthesia. |
| Galehdar 2011 | No placebo: study looking at high vs low oxygen in women having CS under spinal anaesthesia and oxygen is not generally given during spinal anaesthesia. |
| Galehdar 2016 | No placebo group, studying ondansetron vs acupressure for nausea and vomiting in women having elective CS. |
| Gangadhara Gowda 2014 | No placebo. Study compares IT fentanyl versus IV ondansetron for nausea and vomiting at CS under spinal anaesthesia. |
| George 2018 | No placebo and comparing two methods of administration of phenylephrine (infusion vs bolus). |
| Ghods 2005a | Intervention was given in the postoperative period for prevention of nausea and vomiting. Our protocol includes studies only where interventions were given during the CS. |
| Gunka 2013 | Study assessed effect of ondansetron on cardiac output, not for prevention of nausea and vomiting. Previous study name: NCT01841606 2013 |
| Gutsche 1976 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Habib 2019 | Study looking at cyclizine versus dexamethasone for nausea and vomiting but no placebo group. Previous study name: NCT03931135 2019 |
| Hackworth 2010 | Focus is hypotension and bradycardia, not nausea and vomiting: study of women having CS under spinal anaesthesia with nausea and vomiting only secondary outcomes. |
| Hafez 2017 | The focus of this study was the effect of gabapentin (an anticonvulsant) on analgesia with a passing mention of nausea and vomiting. |
| Hajian 2016 | Study focused on ondansetron's effect on haemodynamics, assessing blood pressure and heart rate primarily and only nausea and vomiting as additional information. |
| Hamzei 2015 | The intervention in this study is not given primarily to prevent nausea and vomiting. |
| Han 2007 | Study is assessing different routes of administration, not the efficacy of the medication itself. |
| Hildyard 2000 | Data presented graphically, but without numerical results. We wrote to the authors in June 2008 but received no response. |
| Hodgkinson 1983 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not ITT analysis for primary outcome and high exclusion rates post randomisation. |
| Holdsworth 1974 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not an RCT. |
| Holdsworth 1978 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Quasi‐RCT. |
| Holdsworth 1980 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not an RCT. Assessing women's positions for GA. |
| Hong 2004 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Hunt 1989 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Husemeyer 1980 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Huseyinogclu 2016 | Women all had general anaesthetic. |
| Hussain 2011 | Study assessed interventions for improving gastric emptying not nausea and vomiting. |
| Hussain 2014 | Study was with women having general anaesthetic for CS and not spinal anaesthesia, also the focus was on reducing acidity, and not nausea and vomiting. |
| Imeh 2014 | No placebo group. Study looked at nausea and vomiting with dexamethasone vs ondansetron in women having CS under spinal anaesthesia. |
| Iqbal 2000 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Ishiyama 2001 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Jabalameli 2011 | No placebo group. Women having CS under general anaesthesia: study looking at remifentanil vs fentanyl vs fentanyl + morphine for nausea and vomiting. |
| Jabalameli 2012 | No placebo group: study compared midazoalm vs ondansetron vs midazoalm + ondansetron for nausea and vomiting at CS under spinal anaesthesia. |
| Jacquemyn 2013 | Drugs are not antiemetics. Main focus of study was carbitocin and oxytocin for postpartum haemorrhage and their influence on nausea and vomiting. Prevous study name: ISRCTN95504420 2013 |
| Jain 2015 | No placebo group. Study on nausea and vomiting with ondansetron vs glycopyrrolate in women having CS under spinal anaesthesia |
| Jain 2017 | Study drugs not antiemetics. Study on women at < 36 weeks' gestation having elective CS, looking at use of rabeprazole and ranitidine for reducing stomach volume. Previous name: CTRI/2017/11/010517 2017 |
| Jasson 1989a | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Jeon 2000 | Study randomised women having CS under regional anaesthesia to spinal vs epidural, then investigated effects of propofol on sedation with nausea and vomiting as side effects. |
| Kang 1982 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Kangas‐Saarela 1990 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Karamanlioglu 1995 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Karaoren 2019 | Study on effects of sitting vs supine positions during CS on hypotension Previous study name: NCT03834259 2019 |
| Karimi 2020 | Study compared dexamethasone vs ondansetron with no placebo |
| Kiasari 2017 | Study comparing two routes of administration of the same drug, dexamethazone, intravenous vs intrathecal. No placebo group. Previous study name: IRCT2016112631095N1 2017 |
| Kimura 2011 | This was a study of treatment not prevention. Intervention was only administered if the woman developed nausea. |
| King 1998 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Kita 2018 | Study appears to allocate to different types of regional anaesthesia and then assess nausea and vomiting but difficult to assess. |
| Kjaer 2006 | In this review, we are assessing the efficacy of medication given for the specific purpose of reducing nausea and vomiting at CS. In this study, the medication is given for the purpose of aspiration prophylaxis, and the incidence of emetic side effects is being studied. |
| Kocamanoglu 2005 | Study looked at prevention of postoperative nausea and vomiting after general anaesthesia for cesarean section not regional anaesthesia. |
| Kumar 2017 | The main focus of this study is on the effect of ondansetron on blood pressure and heart rate. |
| Lal 2018 | Looking at medication for blood pressure not nausea and vomiting. Drugs included propofol + ranitidine + metoclopramide vs ranitidine + metoclopramide. Previous study name: CTRI/2018/03/012692 2018 |
| Landa 2016 | Study looked at ketorolac (a nonsteroidal anti‐inflammatory drug (NSAID) vs placebo. This group of drugs is not included in this review. |
| Lane 2012 | This study investigates treatment (rather than prophylaxis). |
| Lee 1992 | Looking at opioid (nalbuphine) as analgesic in women having CS under epidural anaesthesia, opioids are excluded from this review. |
| Lee 2001 | No placebo and focus is on analgesia. Study looked at the effect morphine vs nalbuphine on analgesia for women having a CS under regional anaesthesia. |
| Levin 2018 | No placebo group. Study looked at women having CS under spinal anaesthesia and compared scopolamine versus acupuncture P6 versus ‘scopolamine + acupuncture P6’ for nausea and vomiting. |
| Lim 1991 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not randomised. |
| Lin 1996 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Liu 2015 | This study did not appear to report any of our primary outcomes. |
| Loughrey 2002 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Madhumala 2019 | No placebo group, Comparing chewing gum vs ondansetron for nausea and vomiting. Prevous study name: CTRI/2019/06/019923 2019 |
| Malekianzadeh 2012 | Focus is on reducing hypotension with spinal anaesthesia for CS, not on nausea and vomiting. Previous study name: IRCT201111138090N1 2012 |
| Manullang 2000 | No placebo group. Study on nausea and vomiting with fentanyl vs ondansetron in women having CS under spinal anaesthesia. |
| McCaughey 1981 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not a randomised study. |
| Mebazaa 2003 | Study assessing the anaesthetic drug, not intervention for prevention of nausea and vomiting. |
| Memari 2015 | We are not including anticonvulsant drugs in this review. The study was on nausea and vomiting with gabapentin versus placebo in women having CS under spinal anaesthesia |
| Mofrad 2019 | The main focus of the study is blood pressure. Previous study name: IRCT20191015045121N1 2019 |
| Mokhtar 2016 | Study on ondansetron vs placebo but focusing on shivering with nausea and vomiting only secondary outcomes Previous study name: PACTR201612001896411 2016 |
| Murphy 1984 | Study assessed effect of metoclopramide on gastric emptying for reducing nausea and vomiting. |
| Nado 2017 | Study looked at a combination of drugs, propofol (sedative) + dexamethasone (steroid), vs placebo. Combinations of drugs are excluded from this review |
| Naja 2016 | Study assessed effect of ondansetron vs ondansetron + dexamethasone on relieving side effects of morphine, primary outcome is pruritus, and not for prevention of nausea and vomiting. Previous study name: NCT02793843 2016 |
| Nallam 2017 | Main focus is on ondansetron vs placebo for shivering. |
| Nantasupha 2016 | This study does not assess an intervention given at CS to reduce nausea and vomiting. |
| Ngan 2000 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Ngan 2001 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Ngan 2004a | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Ngan 2004b | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Niaki 2016 | No placebo group. Compared cumin vs peppermint vs milk of magnesia for gastrointestinal complications at CS. |
| Nivatpumin 2009 | Studies metoclopramide vs ondansetron vs dexamethasone + metoclopramide vs dexamethasone + ondansetron for nausea and vomiting at CS, but no control group |
| Nivatpumin 2016 | Interventions in this study are not given for the purpose of preventing nausea and vomiting. |
| Numazaki 2000 | This study was retracted by the publishing journal after uncertainty was raised as to the validity of the data (Carlisle 2012). |
| Numazaki 2003 | This study was retracted by the publishing journal after uncertainty was raised as to the validity of the data (Carlisle 2012). |
| O'Sullivan 1985 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| O'Sullivan 1988 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Olsen 1994 | Study assessing interventions for blood pressure control, not prevention of nausea and vomiting. |
| Ormezzano 1990 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Orr 1993 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Osman 1995 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. No information on the number of women in each group. |
| Ostheimer 1982 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Ouyang 2002 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Owczarzak 1997 | No placebo group. Study on nausea and vomiting with ondansetron vs metoclopramide in women having CS under epidural anaesthesia |
| Ozkan 2000 | Study investigates medication given for aspiration prophylaxis not nausea and vomiting prevention. |
| Pakniat 2017 | Study on ginger vs metoclopramide for nausea and vomiting at CS but no placebo group. |
| Palmer 1991 | Study investigates medication given for aspiration prophylaxis not nausea and vomiting prevention. |
| Palmer 1995 | Study assessing fentanyl for pain relief. |
| Pecora 2009 | Study on supplemental oxygen vs placebo ‐ not an intervention in this review. |
| Peivandi 2019 | Study looked at women having an elective CS under spinal anaesthesia and compared naloxone with placebo focusing on pain management. Nausea was only an additional outcome. |
| Pellegrini 2001 | Study assessed drugs given with regard to analgesia, not for prevention of nausea and vomiting. Opioid antagonists were not included in the protocol as possible interventions for reducing nausea and vomiting. |
| Phillips 2007 | Study on supplemental oxygen vs placebo ‐ not an intervention in this review |
| Pickering 1980 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Pogodin 2012 | Study looking at oxygen for nausea and vomiting at CS under spinal anaesthesia, but there is no good theoretical basis for this intervention. |
| Popivanov 2019 | Study assessing effects of chewing gum vs ondansetron for nausea and vomiting at CS but no placebo group. Previous study name: NCT04191694 2019 |
| Prakash 2006 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Prakash 2019 | Primary focus is reducing high blood pressure with ondansetron not on nausea and vomiting Previous study name: CTRI/2019/02/017489 2019 |
| Qvist 1983 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not randomised. |
| Qvist 1985 | Studied effect of placental transfer of cimetidine given prior to induction of anaesthesia. |
| Rahman 2018 | Study on peppermint + granisetron + dexamethasone vs granisetron + dexamethasone, so no placebo group. Additional previous study name: NCT03434340 2018 |
| Ramanathan 1983 | Study assessing interventions given for blood pressure control, not prevention of nausea and vomiting. |
| Ramin 1994 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Rasooli 2019 | No placebo. Study looked at women having CS under spinal anaesthesia and compared 'phenylephrine/metoclopramide + ondansetron' vs 'phenylephrine/metoclopramide' with primary outcome of nausea and vomiting. |
| Ravindranathan 2018 | No comparison vs placebo, propofol + ranitidine + metoclopramide vs ranitidine + metoclopramide with women having CS under spinal anaesthesia Previous study name: CTRI/2018/05/013610 2018 |
| Razanejadi 2018 | No placebo group, only dexamethasone vs ondansetron. Previous study name: IRCT20180210038681N1 2018 |
| Rocke 1994 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Rout 1992 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Rout 1993 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Rudra 2004b | Assesses medication given predominantly for the purpose of achieving surgical analgesia, not for the purpose of reducing nausea and vomiting. |
| Sadeh 2019 | No full placebo group. 4 mg ondansetron + 4 mg dexamethasone vs 4 mg ondansetron + 8 mg dexamethasone vs 4 mg ondansetron + placebo. Previous study name: IRCT20190409043219N2 2019 |
| Saem 2017 | No placebo and focus is hypotension not nausea and vomiting. Study looking at women having CS under spinal anaesthesia and comparing lidocaine + pethidine vs lidocaine vs bupivacaine. Previous correct study name: IRCT20170417033491N2 2017 |
| Sahare 2013 | No placebo. Study looking at women having CS under spinal anaesthesia comparing ondansetron vs granisetron vs ramosetron. |
| Sane 2015 | No placebo group. Study on nausea and vomiting with ondansetron vs dexamethasone vs 'ondansetron + dexamethasone' in women having CS under spinal anaesthesia. |
| Sane 2016 | Study looked at midazolam vs ondansetron vs midazolam + ondansetron for nausea and vomiting at CS under spinal anaesthesia ‐ no placebo group. |
| Sane 2017a | Study looking at ondansetron vs propofol in women having CS under spinal anaesthesia, no placebo group and focus is pruritus not nausea and vomiting. Previous study name: IRCT2017041527677N7 2017. Different studies from Sane 2017 and 2016 |
| Santos 1984 | Study did not appear to randomise women to each intervention. |
| Seidy 2010 | No placebo. Study looked at women having CS (though unclear the type of anaesthesia) comparing supplemental oxygen 80% versus 30%. |
| Sen 2001 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Seyedhejazi 2007 | Assess medication given for the purpose of achieving surgical anaesthesia, not for the purpose of reducing nausea and vomiting. |
| Shafaeiyan 2018 | Study assessing effect of warming fluids at CS under spinal anaesthesia on pain. |
| Shafeinia 2020 | The main focus of the study is effect of phenylephrine on haemodynamic changes and blood pressure. Now has full publication under Shafeinia 2020. Previous study name: IRCT20191007045023N1 2019 |
| Shahriari 2009 | No placebo group. Study on N&V with midazolam vs metoclopramide in women having CS under spinal anaesthesia. |
| Shaikh 2015 | No placebo group. Study om nausea and vomiting with midazolam vs fentanyl in women having CS under spinal anaesthesia. |
| Shende 1998 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Shifman 2010 | The study compared 2 different doses of the same drug (4 mg vs 12 mg dexamethasone). |
| Shin 2019 | Study of women at CS under regional anaesthesia comparing midazolam + fentanyl vs midazolam where nausea and vomiting is a side effect of midazolam ‐ no placebo group. |
| Siddik‐Sayyid 2002 | Study assessed drugs given for analgesia, not for prevention of nausea and vomiting. |
| Singh 2018 | Focus is shivering not nausea and vomiting which are only secondary outcomes. Study looking at women having CS under spinal anaesthesia, comparing granisetron vs placebo. |
| Stuart 1996 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not ITT. |
| Sultan 2014 | Not an RCT but a pharmacokinetic study. |
| Sutanto 2013 | Looking at affect of oral supplements on metabolic stress. Study of women having CS (type of anaesthesia not reported) comparing: oral nutritional supplement (carbohydrate, protein and fat) 200 mL at 1 kcal/mL + vitamin & minerals – no fibre vs tea with sugar 40 k cals carbohydrate. |
| Swaro 2018 | Study assessed effect of palonosetron vs dexamethasone vs palonosetron + dexamethasone on nausea and vomiting in women having CS under spinal anaesthesia ‐ no placebo group |
| Tanaka 2007 | Not an RCT but a dose‐response study. |
| Taylor 1966 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Not an RCT. |
| Tekyeh 2013 | Study examines different doses of anaesthetic rather than an intervention for preventing nausea and vomiting. |
| Terui 2014 | Study on acetaminophen (paracetamol) vs placebo, a drug not included in this review. |
| Tettambel 1983 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Quasi‐RCT. |
| Tianthong 2018 | Study focuses on ginger for abdominal extension in women having CS. |
| Trabelsi 2015 | The focus of this study is on hypotension not nausea and vomiting |
| Tripathi 1995 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Tryba 1983 | Assesses medication given for the purpose of aspiration prophylaxis not nausea and vomiting. |
| Tshibangu 2010 | The study assessed the impact of IT morphine given primarily for postoperative analgesia. |
| Varshney 2019 | Main focus is shivering not nausea and vomiting. Study is on ramosetron vs placebo in women having CS under spinal anaesthesia. |
| Vercauteren 2000 | Study assessing drugs given for blood pressure control, not prevention of nausea and vomiting. |
| Viney 2012 | Study of surgical techniques. Study looked at women having CS and compared intra‐abdominal irrigation with no irrigation assessing nausea as the main outcome. |
| von Braun 1994 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. Number of women in each group not reported. |
| Wang 2014 | No placebo. Study on women at high risk of PPH needing hemabate during CS looking at droperidol vs dexamethasone. |
| Wani 2015 | Study looked at women having CS under subarachnoid block comparing ondansetron vs P6 acupressure for carboprost induced nausea and vomiting ‐ no placebo |
| Wig 1987 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Xu 2018 | Study focuses on effects of phenylephrine (a decongestant) vs bupivacaine vs placebo on maternal haemodynamics and cord blood gasses. Previous study name: NCT03507387 2018 |
| Yang 2018 | Study looking at women having CS (but no information on type of anaesthesia) comparing 50 mL of water with no water ‐ intervention to within our remit. |
| Yau 1992 | Study assessed interventions for reducing aspiration pneumonitis at CS not for reducing nausea and vomiting. |
| Yazdi 2015 | Study is assessing different levels of oxygen with no placebo group and also the main focus is arterial oxygen saturation and not nausea and vomiting. |
| Zabetian 2014 | Study looked at midazolam vs propofol in women having CS under spinal anaesthesia but there was no placebo group. |
| Zarief 2018 | No placebo. Study looking at effect of IT atropine vs dexamethasone on nausea and vomiting. Previous study name: NCT03387956 2018 |
| Zoroglu 1999 | Study assessed interventions for reducing aspiration pneumonitis at caesarean section not for reducing nausea and vomiting. |
| Zue 1999 | Study assessed interventions for reducing aspiration pneumonitis at caesarean section not for reducing nausea and vomiting. |
| Ünlügenç 2016 | Study assessed ondansetron on cardiac effects, not for prevention of nausea and vomiting. Previous study name: NCT02928601 2016 |
CS: caesarean section;GA: gestational age;IT: intrathecal;ITT: intention to treat;IV: intravenous; RCT: randomised controlled trial; PONV: postoperative nausea and vomiting; PPH: postpartum hemorrhage; vs: versus
Characteristics of studies awaiting classification [ordered by study ID]
Ahn 2017.
| Methods | Unclear. The authors report: "This study used a non‐equivalent control pre‐post quasi‐experimental design.” which suggests they did not randomise yet they say they "...randomly assigned...' . |
| Participants |
|
| Interventions | Intervention: acupressure
Comparator: no intervention
|
| Outcomes | Nausea, vomiting and pain |
| Notes | Setting: Korea Dates: not reported Funding source: no specific funding Declaration of interest: reported as none New 2020. We will write to the authors for clarification. |
Biswas 2002.
| Methods | |
| Participants | Comparison between IV metoclopramide and IT fentanyl to prevent intraoperative and early PONV in patients undergoing caesarean delivery under spinal anaesthesia. |
| Interventions | IV metoclopramide and IT fentanyl, unclear if there is a placebo or not |
| Outcomes | Nausea and vomiting |
| Notes | We are trying to locate this paper. |
Gamermann 2015.
| Methods | RCT. |
| Participants | Women undergoing CS under spinal anaesthesia. N = 56 |
| Interventions | Intervention: acupuncture
Comparator: placebo
|
| Outcomes | Nausea, vomiting, pain scores 24 and 48 hours post‐op. |
| Notes | Setting: Dates: Funding source: Declaration of interest: Abstract only. We will write to the authors seeking the full paper. We are trying to locate the publication in Acupuncture & Related Therapies 2015 (Feb) 3(1) 11‐14. |
Litchfield 2007.
| Methods | RCT. |
| Participants | Women undergoing elective CS under spinal anaesthesia. N = |
| Interventions | Intervention: antihistamine
Comparator: placebo
|
| Outcomes | Nausea and pruritus |
| Notes | Conference abstract only. It is unclear if this is a randomised trial or not. No data available for our review as authors are reporting scores. We will attempt to contact the author to obtain the full paper or further information and data. Setting: Naval Medical Center, Portsmouth, Virginia, USA |
Malhotra 2019.
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia N = |
| Interventions | Intervention 1: anticholinergic
Intervention 2: corticosteroid
Intervention 3: dopamine antagonist
Comparator: placebo
|
| Outcomes | Nausea and vomiting |
| Notes | Setting: Dates: Funding source: Declaration of interest: Conference abstract with no information on the institution nor numbers of women allocate to the groups. We will try and contact the authors. |
Salman 2016.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | We are trying to locate this paper |
Sane 2017.
| Methods | Unclear |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Intervention: granisetron Comparator: placebo |
| Outcomes | Pruritis |
| Notes | It is unclear this is an RCT and abstract doesn't mention nausea and vomiting, only pruritis (though nausea and vomiting in title). Only have Engllsh abstract and the rest of paper in Iranian. We will try to get a translation and/or write to authors. |
Sarat 2007.
| Methods | RCT ‐ but not clear |
| Participants | Women undergoing elective caesarean delivery. |
| Interventions | Glycopyrrolate, metoclopramide, ondansetron. |
| Outcomes | |
| Notes | Abstract only, awaiting full paper. |
Shah 2016.
| Methods | Unclear |
| Participants | Women having a CS under combined spinal epidural anaesthesia |
| Interventions | Bilateral acupoint stimulation at P6 vs unilateral acupoint stimulation at P6 ‐ unclear if there is a no stimulation group |
| Outcomes | Nausea and vomiting |
| Notes | We only have the conference abstract title: 'Is bilateral nei‐guan point (P6) stimulation more effective than unilateral P6 stimulation in reducing nausea and vomiting (N/V) during and after caesarean section (C/S) with combined spinal epidural (CSE) anaesthesia?'. We will write to authors for further information. |
Zakeri 2014.
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Pethidine vs midazolam vs placebo |
| Outcomes | Analgesia, haemodynamics with nausea and vomiting 5th primary outcome |
| Notes | A clinical trial 0f analgesic effects, hemodynamic changes and PONV after IT injection of bupivacaine plus midazolam, bupivacaine plus pethidine or bupivacaine alone in elective cesarean Ssction Scientific enquiries: Habib Zakeri ‐ email: zakerihabib@gmail.com We are unsure of the primary focus as nausea and vomiting is the 5th primary outcome. We will asses for classification when the full paper is published and then decide what the primary focus is. https://en.irct.ir/trial/17203 IRCT2014102719145N2 2014 |
CS: caesarean section;IM: intramuscular; IT: intrathecal;IV: intravenous;PONV: postoperative nausea and vomiting; RCT: randomised controlled trial; vs: versus.
Characteristics of ongoing studies [ordered by study ID]
Abramovitz 2007.
| Study name | A double‐blind, placebo‐controlled study to evaluate the effect of intramuscular ephedrine on the incidence of perioperative nausea and vomiting during elective caesarean section |
| Methods | RCT |
| Participants | Women having CS with spinal anaesthesia |
| Interventions | Ephedrine vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | February 2007 |
| Contact information | Sharon Abramovitz, email: sea2003@med.cornell.edu and Vanessa J Pressimone, email: vjp2001@med.cornell.edu |
| Notes |
https://clinicaltrials.gov/ct2/show/NCT00432991 New York Presbyterian Hospital, USA |
Amini 2019.
| Study name | Comparison of the effect of acupressure and ondansetron on prevention of nausea and vomiting among patients undergoing cesarean section under spinal anaesthesia |
| Methods | RCT |
| Participants | Women having elective CS under spinal anaesthesia |
| Interventions | Acupressure vs ondansetron vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 13 March 2019 (expected start date) |
| Contact information | Amir Amini, email: aminiamir150@yahoo.com |
| Notes | Setting: Khorramdarh Booali Hospital, Emam Hossain Square, Khorramdarh, Zanjan, Iran Previous study name: IRCT20190127042519N1 2019 |
An 2016.
| Study name | The antiemetic efficacy and safety of subhypnotic dose of propofol for decreasing the incidence of intraoperative nausea and vomiting during caesarean section |
| Methods | RCT |
| Participants | Inclusion criteria: 1. ASA I‐II; 2. elective caesarean section; 3. gestation period ≥ 37 weeks; 4. breastfeeding. Exclusion criteria: 1. who had gastrointestinal diseases; 2. who had history of motion sickness and/or previous emesis 24 hours before surgery; 3. who are allergic to propofol; 4. whose body weight 2 times than normal; 5. who had a history of emesis in an intraoperative, post‐delivery period; 6. who had received any antiemetic medication within 24 hours before surgery. |
| Interventions | Propofol vs placebo |
| Outcomes | Nausea, retching, vomiting |
| Starting date | 20 Oct 2016 to 28 Feb 2017 |
| Contact information | Jianxiong An: email ‐ anjianxiong@yeah.net |
| Notes | http://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR-INR-16009539 |
Barzanji 2019.
| Study name | Assessment of pyridoxine (vitamin B6) effects on the nausea and vomiting rates in patients candidate for elective cesarean section |
| Methods | RCT |
| Participants | Women having elective CS |
| Interventions | Vitamin B6 (25 mg) vs vitamin B6 (50 mg) vs vitamin B6 (100 mg) vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | Expected 5 May 2019 |
| Contact information | Seyed Arvin Barzanji email: besathospital@muk.ac.ir |
| Notes | IRCT20171216037910N1 Previous study name: IRCT20171216037910N1 2019 |
Bi 2017.
| Study name | Effect of propofol for prevention of post‐delivery nausea and vomiting during elective cesarean delivery under spinal anaesthesia |
| Methods | RCT |
| Participants |
Inclusion: patients aged 20‐40 years, with physical statues I and II with full term pregnancy undergoing elective CS under spinal anaesthesia. N = Excusion: patients with allergy or hypersensitivity to granisetron, propofol or fat emulsion; history of nausea and vomiting within 24 hours before CD; history of gastrointestinal or psychiatric disease, motion sickness, smoking,PONV; morbid obesity(weight > 85 kg); consumption of drugs such as opioids, antiemetics, H2‐antagonist, phenothiazines and/or corticosteroids within 24 hours before the study period; any chronic medical or surgical disorders complicating the pregnancy; and conditions contraindicating regional anaesthesia |
| Interventions | Intervention: propofol
Comparator 1: placebo ‐ lipid emulsion
Comparator 2: placebo
Both groups given hemabate as prophylactic for PPH |
| Outcomes | Primary outcome is the presence of post‐delivery intra‐operative nausea and vomiting. Secondary outcome include the need for rescue antiemetic, the presence of hypertension, chest pain, headache, and other adverse outcome caused by hemabate. |
| Starting date | 1 March 2017 |
| Contact information | Yanmei Bi, Email: scutanling@163.com. West China Second Hospital, Sichuan University, West China Women's and Children's Hospital. |
| Notes | Dates: 01/03/2017 to 30/09/2017 Trial registration: NCT03185156 and ChiCTR‐IOR‐17010491. JG+GG: include as on‐going study ‐ propofol vs placebo. |
Cohen 2016b.
| Study name | Is Intra‐operative acupuncture point P6 stimulation as effective as traditional pharmacotherapy in reducing nausea and vomiting during cesarean section with regional anaesthesia? |
| Methods | RCT. Ongoing study for acupuncture vs placebo data |
| Participants | Inclusion criteria:
Excluson criteria:
|
| Interventions | Intervention 1:
Interventon 2:
Comparator:
|
| Outcomes | Nausea and vomiting, etc. |
| Starting date | Juky 2015 |
| Contact information | Shaul Cohen ‐ email: cohensh@rwjms.rutgers.edu |
| Notes | Setting: Robert Wood Johnson University Hospital, New Brunswick, New Jersey, USA, 08901 Dates; Funding: Declaration of interests: Study results in Trial reg website ‐ so look for publication. |
Fanelli 2009.
| Study name | IntrathecalaAtropine to prevent nausea and vomiting after spinal anaesthesia with morphine for elective caesarean section: a randomised controlled trial |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Atropne IV vs atropine intrathecal vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | May 2007‐May 2008 |
| Contact information | Contact: Guido Fanelli: University and Hospital of Parma, Parma, PR, Italy 43126 |
| Notes | Over 10 years old. |
Farokhi 2016.
| Study name | Comparison of dexamethasone‐ketamine and dexmedetomidine for prevention of postoperative nausea and vomiting during and after cesarean section under spinal anaesthesia |
| Methods | RCT |
| Participants | Women having an elective CS under spinal anaesthesia |
| Interventions | Dexamethasone vs ketamine vs dexmedetomidine (dexamethazone + ketamine) vs placebo |
| Outcomes | Nausea and vomiting, etc |
| Starting date | Expected start date 22 June 2017 to expected end date 22 June 2019 |
| Contact information | Fariba Farokhi email: f.farokhi@arakmu.ac.ir and Nilufar Shams email: alikamaliir@yahoo.com |
| Notes | Setting: Taleghani Hospital, Emam Khomeini Street, Arak, Iran |
Gazi 2018.
| Study name | A comparative study on effect of low dose ketamine VS dexamethasone on intraoperative nausea and vomiting during cesarean section under spinal anaesthesia |
| Methods | RCT. |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Ketamine IV vs dexamethasone vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | April 2018 |
| Contact information | Ahmad Gazi, email: dr.ghaziahmad@gmail.com and Sona Emami, email: sona_emami@yahoo.com |
| Notes | Setting: Alavi Hospital, Moadi Street, Ardabil, 5615783134, Ardabil, Iran GG: managed to locate at https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20150808023559N18 Previous study name: IRCT20150808023559N18 2018 |
Hao 2019.
| Study name | A randomised controlled study for acupoint stimulation using subacupuncture to relieve postoperative nausea and vomiting in patients undergoing gynecological and obstetrical surgery |
| Methods | RCT. GG: on‐going study but will need data on women having CS only, also need to know type of anaesthesia at CS |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Interventon:
Comparator:
|
| Outcomes | Nausea, vomiting etc. |
| Starting date | Not reported |
| Contact information | Study leader: Wei Hao ‐ email: zhang6620072@163.com. Applicant Chunlei Zhang ‐ email: zhang6620072@163.com |
| Notes | Setting: Hebei Hospital of Traditional Chinese Medicine, Chang'an District, Shijiazhuang, Hebei, China Trial registration: ChiCTR1900026709 Web link: http://www.chictr.org.cn/showproj.aspx?proj=43770 (last accessed 8 July 2020) |
Hess 2017.
| Study name | Dexmedetomidine after cesarean for the treatment of nausea and shivering |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Dexmedetomidine (a sedative) vs placebo |
| Outcomes | Nausea and vomiting, shivering |
| Starting date | 18 February 2020 |
| Contact information | Philip E Hess, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States, 02215. email: phess@bidmc.harvard.edu |
| Notes | Setting: Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA Previous study name: NCT03370562 2017 |
Hosseini 2020.
| Study name | The effect of chamomile aromatherapy with and without oxygen on severity of pain, bloating and nausea in women after cesarean section with spinal anaesthesia |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Chamomile + oxygen vs oxygen vs chamomile vs control |
| Outcomes | Nausea and vomiting |
| Starting date | 12 October 2019 |
| Contact information | Dr. Nazafarin Hosseini, email: hosseinichenar@yahoo.com |
| Notes | Trial registration: IRCT20141222020401N7 Web link: https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20141222020401N7 Previous study name: IRCT20141222020401N7 2020 |
Jamilian 2014.
| Study name | Comparison the effect of oral gabapentin and oral ondansetron and oral ginger to prevention nausea and vomiting after cesarean section by spinal anesthesia |
| Methods | RCT |
| Participants | Women undergoing CS under spinal anaesthesia |
| Interventions | Gabapentin vs ondansetron vs ginger vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 24 December 2013 |
| Contact information | Dr.Mehri Jamilian, email: mjamilian@arakmu.ac.ir |
| Notes | Setting: Arak University of Medical Sciences, Taleghani Hospital, Arak, Iran |
Khatiban 2017.
| Study name | Effects of cardamoms inhalation aromatherapy on the mothers' nausea and vomiting in peri‐ and post‐operation of the elective cesarean section |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Cardamoms inhalation aromatherapy vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 22 May 2017 |
| Contact information | Mahnaz Khatiban, email: m‐khatiban@umsha.ac.ir; mahnaz.khatiban@gmail.com |
| Notes | Setting: Hamadan University of Medical Sciences, Hamadan, Iran Also: IRCT2017050333794N1 |
Khojasteh 2016.
| Study name | The effect of metoclopramide, propofol and dexamethasone in controlling nausea and vomiting during spinal anaesthesia for cesarean section |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Propofol vs metoclopramide vs dexamethasone vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 22 November 2015 |
| Contact information | Lila Khojasteh, email: drlilakhojaste@yahoo.com |
| Notes | Setting: Azad University Hospitals of Tehran, Iran |
Kotfis 2019.
| Study name | An analysis of risk factors and implementation of strategies to prevent nausea and vomiting in patients undergoing regional anaesthesia for CS |
| Methods | RCT |
| Participants | Women undergoing CS under regional anaesthesia |
| Interventions | Carbohydrate supplement v placebo |
| Outcomes | Nausea and vomiting. |
| Starting date | 3 September 2019 |
| Contact information | Katarzyna L Kotfis, email: katarzyna.kotfis@pum.edu.pl |
| Notes | Setting: Pomeranian Medical University, Szczecin, Poland, 70‐111 Previous study name: NCT04069806 2019 |
Mendonca 2015.
| Study name | Prophylactic antiemetic efficacy of palonosetron vs ondansetron for cesarean sections under regional anaesthesia (PONV) |
| Methods | RCT |
| Participants | Women having caesarean under spinal anaesthesia |
| Interventions | Ondansetron vs palonosetron vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | October 2015 |
| Contact information | Fabricio T Mendonca, Hospital de Base do Distrito Federal, Brasilia, DF, Brazil, 70680250 |
| Notes | Setting: Maternal and Child Hospital of Brasilia, Federal District, Brazil |
Mousavi 2019.
| Study name | Study of the effect of auriculotherapy on nausea and vomiting in women following elective cesarean section |
| Methods | RCT |
| Participants | Women with singleton pregnancy, > 37 weeks' gestation having elective CS under spinal anaesthesia |
| Interventions | Auriculotherapy (ear acupuncture) vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 9 May 2016 |
| Contact information | Fatemeh Sadat Mousavi: email: fmousavi@muq.ac.ir and Nahid Golmakani: email golmakanin@mums.ac.ir |
| Notes | Setting: School of Nursing and Midwifery, Ibn Sina street,Danesgah Street, Mashhad, Iran Previous study name: IRCT20180526039845N1 2019 |
Norouzi 2013.
| Study name | Comparison of oral and intravenous ondansetron in the prevention of postoperative nausea and vomiting in cesarean section with spinal anaesthesia |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Interevntion 1: ondansetron (8 mg in 2 tablets) + 2 mL distilled water IV Intervention 2: 2 placebo tablets + ondansetron IV Comparator: 2 placebo tablets + 2ml distilled water |
| Outcomes | Primary outcome nausea and vomiting |
| Starting date | 21 December 2012 |
| Contact information | Dr Afsaneh Norouzi, email: norouzi.a@arakmu.ac.ir; norouzi43@yahoo.com |
| Notes | Setting: Talghani Hospital, Arak, Iran |
Norouzi 2014.
| Study name | Gabapentin effect on preventing of nausea and vomiting in caesarean |
| Methods | RCT |
| Participants | Women having elective CS ‐ but will need to check type of anaesthesia before including |
| Interventions | Gabapentin vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 4 December 2012 |
| Contact information | Dr.Afsaneh Norouzi, email: norouzi43@yahoo.com |
| Notes | Setting: Amirkabir Hospital, Arak, Iran |
Okutani 2012.
| Study name | Effect of oral fluid infusion before cesarean section on intraoperative metabolism, haemodynamic changes and postoperative nausea and vomiting |
| Methods | RCT |
| Participants | Women undergoing elective CS under spinal anaesthesia |
| Interventions | Oral fluid three hours prior to CS vs no intervention |
| Outcomes | Nausea and vomiting |
| Starting date | 20 July 2012 |
| Contact information | Ryu Okutani, email: ryuokutani0909@ybb.ne.jp |
| Notes | Setting: Osaka City General Hospital and Children's Hospital, Osaka, Japan |
Rassoli 2013.
| Study name | The effect of subhypnotic doses of propofol and midazolam to prevent nausea and vomiting during spinal anaesthesia for elective CS |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthetise |
| Interventions | Propofol vs midazolam vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 20 March 2011 |
| Contact information | Dr Sousan Rasooli, email: rasoolis@tbzmed.ac.ir and rasooli_s@yahoo.com |
| Notes | Setting: Alzahra Hospital, Tabriz University of Medical Sciences, Tabriz, Iran. |
Shahinfar 2016.
| Study name | The effect of ginger extract on the incidence and severity of nausea and vomiting after cesarean section under spinal anaesthesia |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Ginger vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 20 March 2016 |
| Contact information | Javad Shahinfar, email: dr.jshahinfar@gmail.com |
| Notes | Setting: Bentolhoda Hospital, ZAyeshgah Ave, Bojnurd, Iran |
Soltani 2014.
| Study name | Effect of capsaicin ointment in K‐K9 point, capsaicin ointment in K‐D2 point and placebo ointment (Vaseline) in K‐K9 point on nausea and vomiting during and postoperative cesarean section with spinal anaesthesia |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Capsaicin at acupressure points vs placebo at acupressure points |
| Outcomes | Nausea and vomiting |
| Starting date | 6 August 2014 |
| Contact information | Narges Soltani. email: soltani.n@bums.ac.ir |
| Notes | Setting: Omolbanin Gynecological Hospital, Khorasan Razavi, Mashhad, Iran |
Thenuwara 2017.
| Study name | Randomised double control study to assess the efficacy of administering 1 mL of Glycopyrrolate with the spinal dose in minimizing nausea and vomiting in patients undergoing cesarean section under spinal anaesthesia |
| Methods | RCT |
| Participants | Women having CS under spinal anaesthesia |
| Interventions | Glycopyrrolatevs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | 15 May 2015 |
| Contact information | Kokila N Thenuwara MD, University of Iowa, Iowa City, Iowa, United States, 52242 |
| Notes | Setting: Iowa, USA |
Tindimwebwa 2009.
| Study name | Antiemetic efficacy and safety of dexamethasone in patients undergoing CSs at Mulago Hospital |
| Methods | RCT |
| Participants | Women having CS under either spinal or general anaesthesia |
| Interventions | Dexamethasone vs placebo |
| Outcomes | Nausea and vomiting |
| Starting date | March 2010 |
| Contact information | Dr JVB Tindimwebwa Mulago National Refferal Hospital, Kampala, Uganda, 00256 and Dr Arthur Kwizera, Department of Anaesthesia, Makerere University, College of Health Sciences, Kampala, Uganda, |
| Notes | Setting: Mulago National Refferal Hospital, Kampala, Uganda, 00256 We want only spinal anaesthesia data |
Yulin 2019.
| Study name | Efficacy and safety of electroacupuncture combined with tropisetron in treating carboprost tromethamine–induced nausea and vomiting during cesarean section:a prospective, randomised, controlled clinical trial (ChiCTR1900021396) |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Interventions 1:
Intervention 2;
Intervention 3
Comparator:
|
| Outcomes | Nausea, vomiting etc. |
| Starting date | 1 October 2015 |
| Contact information | Study leader: Chang Yulin email: 17631695925@163.com. Also Yu Lili ‐ email: 18713057030@163.com |
| Notes | Setting: Cangzhou Central Hospital, Cangzhou, Hebei, China Tropisetron is a serotonin 5‐HT3 receptor antagonist Trial registration: ChiCTR1900021396 Previous study name: ChiCTR1900021396 2019 Web link: Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900021396 Gill's webline: http://www.chictr.org.cn/com/25/hvshowproject.aspx?id=17613 (last accessed 8 July 2020) |
ASA: American Society of Anaesthesiologists physical status classification; CS: caesarean section;IV: intravenous;RCT: randomised controlled trial; PONV: postoperative nausea and vomiting;PPH: postpartum haemorrhage;vs: versus
Differences between protocol and review
We have changed the outcomes of 'Maternal adverse effects' and 'Neonatal morbidity' from being primary outcomes to secondary outcomes.
We have modified the wording in the methods sections for Assessment of heterogeneity, Assessment of reporting biases and Data synthesis to update them with the new methods being used by the group, developed in conjunction with the group's statistician, Simon Gates, and Richard Riley. We have used these new methods in the review.
We have added alternative therapies such as ginger or peppermint to the list of interventions.
We have clarified that this review is specifically assessing the efficacy of interventions for the prevention (rather than treatment) of nausea and vomiting. Treatment interventions will be assessed in a subsequent review.
We have changed the title of the review from that of the protocol which was 'Interventions for reducing nausea and vomiting at caesarean section'. We have done this to clarify for readers that we are looking at prevention and not treatment interventions. Also we have determined that this review should only assess studies where the caesarean section was performed under regional anaesthesia. Studies where general anaesthesia was performed will be assessed in a separate subsequent review.
We are now including subgroup analyses bases on type and doses of drugs used.
We have clarified the time‐frame over which postoperative symptoms will be assessed.
For the 2020 update, we added in a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
For the 2020 update, due to the increasing complexity of the review, we have revised the scope to include studies comparing interventions with placebo or no treatment, and have removed intervention versus intervention comparisons which will be better assessed in a network meta‐analysis. We also excluded reviews of combination interventions.
For the 2020 update we have added data on the outcomes 'Nausea plus vomiting' and 'Rescue antiemetic'.
Contributions of authors
Shantini Paranjothy (SP) wrote the first drafts of the combined protocol (Drugs at caesarean section for preventing nausea, vomiting and aspiration pneumonitis) with input from Eugene Liu (EL), Heather Brown (HB) and Jane Thomas (JT). SP combined the drafts for editorial consideration with input from EL, HB and JT. The revisions in response to the editorial feedback were made by SP, EL, HB, and JT commented on the revised version. In 2007, James Griffiths (JG), Gill Gyte (GG) and Hannah Broughton (HKB) joined the review team. After the initial searches and papers were assessed for eligibility, it was agreed to split the primary review into two separate reviews. JG was appointed as contact person on the nausea and vomiting review. SP remained contact person for the aspiration pneumonitis review (Paranjothy 2014).
For this review, JG drafted the Background section with comments and input from other authors. In 2016, Phil Popham (PP) and Kacey Williams (KW) joined the review team. In this 2020 update, JG and PP revised and updated the background section; JG, GG, SP, HKB, KW, PP and JT contributed to data extraction, both extracting data and checking. JG, PP and GG entered the data and JG and GG analysed the data and prepared the first draft of this review. GG and JG checked data entry. All authors contributed to the interpretation of the data and the final draft of the review.
Sources of support
Internal sources
The University of Liverpool, UK
External sources
-
National Institute for Health Research, UK
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS‐prioritised centrally‐managed, pregnancy and childbirth systematic reviews: CPGS02
Declarations of interest
James D Griffiths: none known.
Gillian ML Gyte: GG received royalties from John Wiley & Sons in respect of ‘A Cochrane Pocketbook – Pregnancy and Childbirth’ Hofmeyr GJ et al. 2008.
Phil A Popham: none known.
Kacey Williams: none known.
Shantini Paranjothy: none known.
Hannah K Broughton: none known.
Jane Thomas: none known.
Heather C Brown: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdel‐Aleem 2012 {published data only}
- Abdel-Aleem M, Osman A, Morsy K. Effect of coadministration of dexamethasone with intrathecal morphine on postoperative outcomes after cesarean delivery. International Journal of Gynecology and Obstetrics 2012;116(2):158-61. [DOI] [PubMed] [Google Scholar]
Abdollahpour 2015 {published data only}
- Abdollahpour A, Azadi R, Bandari R, Mirmohammadkhani M. Effects of adding midazolam and sufentanil to intrathecal bupivacaine on analgesia quality and postoperative complications in elective cesarean section. Anesthesiology and Pain Medicine 2015;5(4):e23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abouleish 1999 {published data only}
- Abouleish EI, Rashid S, Haque S, Giezentanner A, Joynton P, Chuang AZ. Ondansetron versus placebo for the control of nausea and vomiting during caesarean section under spinal anaesthesia. Anaesthesia 1999;54:479-82. [DOI] [PubMed] [Google Scholar]
Ahn 2002 {published data only}
- Ahn HJ, Kim YA, Choi DH. The effects of sedation using propofol TCI on the satisfaction level and side effects of parturients during regional anesthesia for a cesarean section. Korean Journal of Anesthesiology 2002;43(1):66-72. [Google Scholar]
Apiliogullari 2007 {published and unpublished data}
- Apiliogullari S, Duman A, Gok F. Prophylactic dimenhydrinate (dramamine) is effective in the treatment of nausea-vomiting with intrathecal morphine [abstract]. Regional Anesthesia and Pain Medicine 2007;32(5 Suppl):87. [Google Scholar]
Baciarello 2011 {published data only}
- Baciarello M, Cornini A, Zasa M, Pedrona P, Scrofani G, Venuti FS, et al. Intrathecal atropine to prevent postoperative nausea and vomiting after cesarean section: a randomized, controlled trial. Minerva Anestesiologica 2011;77(8):781-8. [PubMed] [Google Scholar]
Birnbach 1993 {published data only}
- Birnbach DJ, Stein DJ, Saunders TA, Grunebaum A, Kitain EM. Prophylactic use of acupressure for the prevention of nausea and vomiting during spinal anesthesia for cesarean section. Anesthesiology 1993;79:A1020. [Google Scholar]
Biswas 2003 {published data only}
- Biswas BN, Rudra A, Das SK, Nath S, Biswas SC. A comparative study of glycopyrrolate, dexamethasone and metoclopramide in control of post-operative nausea and vomiting after spinal anaesthesia for caesarean delivery. Indian Journal of Anaesthesia 2003;47(3):198-200. [Google Scholar]
Boone 2002 {published data only}
- Boone JB, Gao GG, Clark CP, Chaudhuri S, Chaudhuri K. Dolasetron for prevention of perioperative nausea and vomiting during cesarean section under regional anesthesia [abstract]. Anesthesiology 2002;96 Suppl:A1069. [Google Scholar]
Caba 1997 {published data only}
- Caba F, Bernal L, Pallares JA, Echevarria M, Rodriguez R. Prophylaxis for intraoperative nausea and vomiting with subhypnotic doses of propofol during intradural anaesthesia for caesarean section [Profilaxis de nauseas y vomitos intraoperatorios con dosis subhipnoticas de propofol durante anestesia intradural en cesareas]. Revista Espanola de Anestesiologia y Reanimacion 1997;44(Suppl 1):122. [CENTRAL: CN-00400431] [PubMed] [Google Scholar]
- Caba F, Echevarria M, Bernal-Davalos L, Pallares-Gonzalez JA, Rodriguez-Rodriguez R. Prophylaxis for intraoperative nausea and vomiting with nonhypnotic doses of propofol during intradural anesthesia for cesarean delivery [Profilaxis de las nauseas y los vomitos intraoperatorios con dosis subhipnoticas de propofol durante la anestesia intradural en cesareas]. Revista Espanola De Anestesiologia y Reanimacion 1997;44:262-6. [PubMed] [Google Scholar]
Cardoso 2013 {published data only}
- Cardoso MM, Leite AO, Santos EA, Gozzani JL, Mathias LA. Effect of dexamethasone on prevention of postoperative nausea, vomiting and pain after caesarean section: a randomised, placebo-controlled, double-blind trial. European Journal of Anaesthesiology 2013;30:102-5. [DOI] [PubMed] [Google Scholar]
Carvalho 2010 {published data only}
- Carvalho JC, Nair GS, Downey K, Balki M. Prophylactic dimenhydrinate does not reduce nausea and vomiting during cesarean section delivery under spinal anesthesia: a randomized, double-blind, placebo-controlled trial. In: Society for Obstetric Anesthesia and Perinatology (SOAP) 42nd Annual Meeting; 2010 May 12-16, San Antonio, USA. 2010.
- NCT00791960. Prophylactic dimenhydrinate for intraoperative nausea and vomiting. Https://clinicaltrials.gov/show/NCT00791960 (first received 2008 Nov 13). [CENTRAL: CN-01485523]
Charuluxananan 2003 {published data only}
- Charuluxananan S, Kyokong O, Somboonviboon W, Narasethakamol A, Promlok P. Nalbuphine versus ondansetron for prevention of intrathecal morphine-induced pruritus after cesarean delivery. Anesthesia and Analgesia 2003;96(6):1789-93. [DOI] [PubMed] [Google Scholar]
Cherian 2001 {published data only}
- Cherian VT, Smith I. Prophylactic ondansetron does not improve patient satisfaction in women using PCA after caesarean section. British Journal of Anaesthesia 2001;87(3):502-4. [DOI] [PubMed] [Google Scholar]
Chestnut 1987 {published data only}
- Chestnut DH, Bates JN, Choi WW, Vandewalker GE. Administration of metoclopramide for prevention of nausea and vomiting during regional anesthesia for cesarean section: a randomized, double blind, placebo controlled study. Anesthesiology 1986;65:390. [DOI] [PubMed] [Google Scholar]
- Chestnut DH, Vandewalker GE, Owen CL, Bates JN, Choi WW. Administration of metoclopramide for prevention of nausea and vomiting during epidural anesthesia for elective cesarean section. Anesthesiology 1987;66:563-6. [DOI] [PubMed] [Google Scholar]
Choi 1999 {published data only}
- Choi DH, Kim SC, Sim WS. Prevention of nausea and vomiting during spinal or epidural anesthesia for cesarean section - the efficacy of metoclopramide and droperidol. Korean Journal of Anesthesiology 1999;37(6):1054-9. [Google Scholar]
Dasgupta 2012 {published data only}
- Dasgupta M, Biswas BN, Chatterjee S, Mazumder P, Bhanja M. Randomized, placebo-controlled trial of granisetron for control of nausea and vomiting during cesarean delivery under spinal anesthesia. Journal of Obstetrics and Gynecology of India 2012;62(4):419-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Direkvand‐Moghadam 2013 {published data only}
- Direkvand-Moghadam A, Khosravi A. Effect of acupressure on post-operative nausea and vomiting in cesarean section: a randomised controlled trial. Journal of Clinical and Diagnostic Research 2013;7(10):2247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT201111146575N4. Comparing the effect of acupressure and metoclopramide on nausea and vomiting in women with spinal anesthesia for cesarean. http://en.irct.ir/trial/7020 (21 December 2011). [CENTRAL: CN-01849415]
Duggal 1998 {published data only}
- Duggal KN, Douglas MJ, Peter EA, Merrick PM. Acupressure for intrathecal narcotic-induced nausea and vomiting after caesarean section. International Journal of Obstetric Anesthesia 1998;7(4):231-6. [DOI] [PubMed] [Google Scholar]
Duman 2010 {published data only}
- Apiliogullari S, Gok F, Canpolat A, Soysal S, Toy H, Sukan F. Comparison of prophylactic dimenhydrinate and metoclopramide for prevention of nausea-vomiting caused by intrathecal opioids in parturients undergoing caesarean delivery. Regional Anesthesia and Pain Medicine 2008;33(5 Suppl 1):131. [Google Scholar]
- Duman A, Apiliogullari S, Gok F, Sutcu E, Soysal S, Toy H. A randomized comparison of dimenhydrinate, metoclopramide and placebo for the prevention of nausea and vomiting following intrathecal fentanyl and morphine in cesarean delivery. Nobel Medicus 2010;6(3):13-9. [Google Scholar]
- Duman A, Apiliogullari S, Gook F, Sutcu E, Soysal S, Toy H. A randomized comparison of dimenhydrinate, metoclopramide and placebo for the prevention of nausea and vomiting following intrathecal fentanyl and morphine for cesarean delivery. Data on file.
El‐Deeb 2011a {published data only}
- El-Deeb AM, Ahmady MS. Effect of acupuncture on nausea and/or vomiting during and after cesarean section in comparison with ondansetron. Journal of Anesthesia 2011;25(5):698-703. [DOI] [PubMed] [Google Scholar]
Garcia‐Miguel 2000 {published data only}
- Garcia-Miguel FJ, Montano E, Mart-Vicente V, Fuentes AL, Alsina FJ, San Jose JA. Prophylaxis against intraoperative nausea and vomiting during spinal anesthesia for cesarean section: a comparative study of ondansetron versus metoclopramide. Internet Journal of Anesthesiology 2000;4(2).
Habib 2006 {published data only}
- Habib AS, Itchon-Ramos N, Phillips-Bute BG, Gan TJ, the Duke Women's Anesthesia (DWA) Research Group. Transcutaneous acupoint electrical stimulation with the ReliefBand for the prevention of nausea and vomiting during and after cesarean delivery under spinal anesthesia. Anesthesia and Analgesia 2006;102(2):581-4. [DOI] [PubMed] [Google Scholar]
Habib 2013 {published data only}
- Habib AS, George RB, McKeen DM, White WD, Ituk US, Megalla SA, et al. Antiemetics added to phenylephrine infusion during cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2013;121(3):615-23. [DOI] [PubMed] [Google Scholar]
- NCT01216410. Prevention of intraoperative nausea and vomiting during cesarean section. Https://clinicaltrials.gov/show/NCT01216410 (first received 2010 Oct 6). [CENTRAL: CN-02025773]
Harmon 2000 {published data only}
- Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and prevention of nausea and vomiting during and after spinal anaesthesia for caesarean section. British Journal of Anaesthesia 2000;84(4):463-7. [DOI] [PubMed] [Google Scholar]
- Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and the prevention of nausea and vomiting during and after spinal anaesthesia for caesarean section. British Journal of Anaesthesia 1999;82 Suppl:161. [DOI] [PubMed] [Google Scholar]
Harnett 2007 {published data only}
- Harnett MJ, O'Rourke N, Walsh M, Carabuena JM, Segal S. Transdermal scopolamine for prevention of intrathecal morphine-induced nausea and vomiting after cesarean delivery. Anesthesia and Analgesia 2007;105(3):764-9. [DOI] [PubMed] [Google Scholar]
Hassanein 2015 {published data only}
- Hassanein A, Mahmoud E. Effect of low dose ketamine versus dexamethasone on intraoperative nausea and vomiting during cesarean section under spinal anesthesia. Egyptian Journal of Anaesthesia 2015;31(1):59-63. [Google Scholar]
Ho 1996 {published data only}
- Ho CM, Hseu SS, Tsai SK, Lee TY. Effect of P-6 acupressure on prevention of nausea and vomiting after epidural morphine for post-Cesarean section pain relief. Acta Anaesthesiologica Scandinavica 1996;40:372-5. [DOI] [PubMed] [Google Scholar]
Ho 2006 {published data only}
- Ho CM, Tsai HJ, Chan KH, Tsai SK. P6 acupressure does not prevent emesis during spinal anesthesia for cesarean delivery. Anesthesia and Analgesia 2006;102(3):900-3. [DOI] [PubMed] [Google Scholar]
- Ho CM, Tsai SK. P-6 acupressure do not prevent emesis during cesarean section [abstract]. Canadian Journal of Anesthesia 2002;49(6 Suppl):A51. [Google Scholar]
Huang 1992 {published data only}
- Huang WP. Clinical observation of using Metoclopramide to relieve dragging reaction during baby deliver in caesarean operation. Chinese Journal of Anaesthesiology 1992;12(1):33. [Google Scholar]
Ibrahim 2019 {published data only}
- Ibrahim AS, Aly MG, Thabet ME, Abdelaziz MR. Effect of adding nalbuphine to intrathecal bupivacaine with morphine on postoperative nausea and vomiting and pruritus after elective cesarean delivery: a randomized double blinded study. Minerva Anestesiologica 2019;85(3):255-62. [CENTRAL: CN-01939130] [EMBASE: 2001726956] [PMID: ] [DOI] [PubMed] [Google Scholar]
Imbeloni 1986 {published data only}
- Imbeloni LE, Santos JM, Santos MM. Prophylactic use of metoclopramide for control of nausea and vomiting during epidural anesthesia for cesarean section [Uso profilatico da metoclopramida no controle de nauseas e vomitos durante anestesia peridural para cesariana]. Revista Brasileira de Anestesiologia 1986;36(4):295-8. [Google Scholar]
Jaafarpour 2008 {published data only}
- Jaafarpour M, Khani A, Dyrekvandmoghadam A, Khajavikhan J, Saadipour KH. The effect of dexamethasone on nausea, vomiting and pain in parturients undergoing caesarean delivery. Journal of Clinical and Diagnostic Research 2008;2(3):854-8. [Google Scholar]
Jang 1997 {published data only}
- Jang DG, Chang WY, Yoon SY, Kim KB. Epidural butorphanol reduces the side effects from epidural morphine after cesarean section. Korean Journal of Anesthesiology 1997;33(2):297-303. [Google Scholar]
Kalava 2013 {published data only}
- Kalava A, Darji SJ, Kalstein A, Yarmush JM, SchianodiCola J, Weinberg J. Efficacy of ginger on intraoperative and postoperative nausea and vomiting in elective cesarean section patients. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;169(2):184-8. [DOI] [PubMed] [Google Scholar]
- Kalava A, Weinberg J, Jayakumar M, Yarmush JM, SchianodiCola JJ. Efficacy of ginger on intraoperative and postoperative nausea and vomiting in elective cesarean section patients. In: http://www.asaabstracts.com/strands/asaabstracts/abstract.htm;jsessionid=0507CB47FEB444D841EEF290BF35A8BF?year=2011&index=13&absnum=4726 (accessed 1 Febraury 2012). 2011. [DOI] [PubMed]
- NCT01733212. Efficacy of ginger on intraoperative and postoperative nausea and vomiting in elective cesarean section patients. Https://clinicaltrials.gov/show/NCT01733212 2012 Nov 26. [CENTRAL: CN-02041676] [DOI] [PubMed]
Kampo 2019 {published data only}
- ISRCTN15475205. Does propofol prevent morphine-induced nausea, vomiting and itching in women who have had a Caesarean section? Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN15475205 (first received 2019). [CENTRAL: CN-01974804]
- Kampo S, Afful AP, Mohammed S, Ntim M, Buunaaim AD, Anabah TW. Sub-hypnotic dose of propofol as antiemetic prophylaxis attenuates intrathecal morphine-induced postoperative nausea and vomiting, and pruritus in parturient undergoing cesarean section - a randomized control trial. BMC Anesthesiology 2019;19(1):177. [CENTRAL: CN-01987099] [EMBASE: 629299629] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasodekar 2006 {published data only}
- Kasodekar S, Dhumne S, Balki M, Carvalho J. Prophylactic granisetron does not prevent nausea and vomiting during elective cesarean section under spinal anesthesia [abstract]. Anesthesiology 2006;104(Suppl 1):9. [DOI] [PubMed] [Google Scholar]
Khalayleh 2005 {published data only}
- Khalayleh K, Omar AA. Comparitive antiemetic efficacy of intrathecal fentanyl and intravenous metoclopromide, during Caesarean Section under spinal anaesthesia. Journal of the Bahrain Medical Society 2005;17(4):243-7. [Google Scholar]
Kim 1999 {published data only}
- Kim MO, Suh KS. A comparison of the antiemetic effect of ondansetron and metoclopramide on nausea and vomiting associated with epidural buprenorphine. Korean Journal of Anesthesiology 1999;37(4):656-61. [Google Scholar]
Koju 2015 {published data only}
- Koju RB, CTRI/2015/01/005362. Prophylactic administration of ondansetron in prevention of intrathecal morphine induced pruritus and post-operative nausea and vomiting in patients undergoing caesarean section. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10813 (07 January 2015). [DOI] [PMC free article] [PubMed]
- Koju RB, Gurung BS, Dongol Y. Prophylactic administration of ondansetron in prevention of intrathecal morphine-induced pruritus and post-operative nausea and vomiting in patients undergoing caesarean section. BMC Anesthesiology 2015;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotelko 1989 {published data only}
- Kotelko DM, Rottman RL, Wright WC, Stone JJ, Yamashiro AY, Rosenblatt RM. Transderm SCOP decreases post-cesarean nausea and vomiting in patients receiving epidural morphine. Anesthesiology 1988;69:A666. [DOI] [PubMed] [Google Scholar]
- Kotelko DM, Rottman RL, Wright WC, Stone JJ, Yamashiro AY, Rosenblatt RM. Transdermal scopolamine decreases nausea and vomiting following cesarean section in patients receiving epidural morphine. Anesthesiology 1989;71:675-8. [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee YW, Hong JY, Yoon HJ, Kim SM. Could emesis from epidural anesthesia for a cesarean section be controlled by prophylactic low-dose granisetron? Korean Journal of Anesthesiology 2002;43(6):s13-9. [Google Scholar]
Levin 2019 {published data only}
- Levin D, Cohen S, Mellender S, Mohiuddin A, Zhao R, Kiss G, et al. Transcutaneous P6 acupoint electrical stimulation prevents both nausea and vomiting during elective cesarean section under combined spinal-epidural: a randomized control clinical trial. Anesthesia and Analgesia 2018;126(4):421-2. [CENTRAL: CN-01789975] [EMBASE: 626090330] [Google Scholar]
- Levin D, Cohen S, Mellender S, Shah U, Kang P, Mohiuddin A, et al. Effectiveness of p6 stimulation for reduction of nausea and vomiting during caesarean section under combined spinal-epidural anaesthesia: a randomised controlled trial. Turkish Journal of Anaesthesiology and Reanimation 2019;47(2):120-7. [CENTRAL: CN-02089475] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D, Cohen S, Mohiuddin A, Naftalovich R, Kang P, Mellender S, et al. How does acupressure point P6 stimulation compare to pharmacological therapy in reduction of nausea and vomiting during cesarean section with neuraxial anesthesia? Randomized control trial. Regional Anesthesia and Pain Medicine 2017;42(6):no pagination. [CENTRAL: CN-01441790] [EMBASE: 619777950] [Google Scholar]
Li 2012 {published data only}
- Li JZ, Li XZ, Wang MS, Li JP, Shi F, Yu HF. [Effects of transcutaneous electrical stimulation of auricular Shenmen point on postoperative nausea and vomiting and patient-controlled epidural analgesia in cesarean section]. [Chinese]. Chung-Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2012;92(27):1892-5. [PubMed] [Google Scholar]
Lim 2001a {published data only}
- Lim TJ, Chiu JW, Tan HM. Transcutaneous acupoint electrical stimulation for prevention of perioperative nausea and vomiting during cesarean section with spinal anesthesia [abstract]. Anesthesiology 2001;95 Suppl:A1078. [Google Scholar]
Lim 2001b {published data only}
- Lim TJ, Lim E, Chiu JW, Tan HM. Perioperative antiemetic efficacy of dexamethasone 4mg in cesarean section under spinal anaesthesia [abstract]. Anesthesiology 2001;95 Suppl:A466. [Google Scholar]
Liu 2015a {published data only}
- Liu Y, Wang M, Li Q, Wang L, Li J. Impacts of transcutaneous acupoint electric stimulation on the postoperative nausea and vomiting and plasma 5-ht concentration after cesarean section. Chinese Acupuncture & Moxibustion 2015;35(10):1039-43. [PubMed] [Google Scholar]
Lussos 1992 {published data only}
- Lussos S, Bader A, Thornhill M, Datta S. The antiemetic efficacy of prophylactic metoclopramide for cesarean delivery. Anesthesiology 1991;75:A1081. [PubMed] [Google Scholar]
- Lussos SA, Bader AM, Thornhill ML, Datta S. The antiemetic efficacy and safety of prophylactic metoclopramide for elective cesarean delivery during spinal anesthesia. Regional Anesthesia 1992;17:126-30. [PubMed] [Google Scholar]
Mandell 1992 {published data only}
- Mandell G, Dewan DM, Howard G, Floyd HM. The effectiveness of low dose droperidol in controlling nausea and vomiting during epidural anesthesia for cesarean section. Anesthesiology 1986;65:391. [DOI] [PubMed] [Google Scholar]
- Mandell GL, Dewan DM, Howard G, Floyd HM. The effectiveness of low dose droperidol in controlling nausea and vomiting during epidural anesthesia for cesarean section. International Journal of Obstetric Anesthesia 1992;1(2):65-8. [DOI] [PubMed] [Google Scholar]
Maranhao 1988 {published data only}
- Maranhao MV, Coelho VV, Ivo CM, Maranhao MH, Amaral EB. A comparative study between metoclopramide and droperidol in the control of nausea and vomit in patients subjected to spinal blockade during cesarean section [Estudo comparativo entre a metoclopramida e o droperidol no controle de nauseas e vomitos na operacao cesariana em gestantes submetidas a bloqueio subaracnoideo]. Revista Brasileira de Anestesiologia 1988;38(4):245-9. [Google Scholar]
Modir 2019 {published data only}
- Modir H, Moshiri E, Kamali A, Shokrpour M, Shams N. Prophylatic efficacy of dexamethasone, ketamine and dexmedetomidine against intra- and postoperative nausea and vomiting under spinal anesthesia. Formosan Journal of Surgery 2019;52(1):17-23. [CENTRAL: CN-01790839] [EMBASE: 626475964] [Google Scholar]
Mohammadi 2015 {published data only}
- Mohammadi SS, Jabbarzadeh S, Movafegh A. Efficacy of granisetron on prevention of shivering, nausea and vomiting during cesarean delivery under spinal anesthesia: a randomized double-blinded clinical trial. Journal of Obstetric Anaesthesia and Critical Care 2015;5(1):22-6. [Google Scholar]
Mokini 2014 {published data only}
- Mokini Z, Fabbri E, Dell'Atti I, Di Martino R, D'Ettorre M, Petrini F. A randomized, controlled, double blind trial on the incidence of nausea and vomiting with prophylactic subhypnotic propofol vs metoclopramide and in combination therapy in cesarean section with subarachnoid anesthesia. European Journal of Anaesthesiology 2014;31:192. [Google Scholar]
- NCT01781377. Prophylactic subhypnotic propofol for nausea and vomiting during for cesarean section under subarachnoid anesthesia. Https://clinicaltrials.gov/show/NCT01781377 (first received 2013 Jan 25). [CENTRAL: CN-01540221]
Mukherjee 2006 {published data only}
- Mukherjee S, Rudra A, Mandel M, Kumar P. Optimum dose of propofol for prevention of emetic episodes during caesarean delivery: an evaluation. Journal of Anaesthesia and Clinical Pharmacology 2006;22(2):139-43. [Google Scholar]
Munnur 2008 {published data only}
- Munnur U, Tran C, Zambare SN, Lozano-Gorena M, Suresh MS. Post operative nausea and vomiting following cesarean section with intrathecal morphine. Anesthesiology 2008;109:A1113. [Google Scholar]
Niu 2018 {published data only}
- Niu K, Liu H, Chen R-W, Fang Q-W, Wen H, Guo S-M, et al. Use of propofol for prevention of post-delivery nausea during cesarean section: a double-blind, randomized, placebo-controlled trial. Journal of Anesthesia 2018;32(5):748-55. [CENTRAL: CN-02000278] [EMBASE: 623932583] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noroozinia 2013 {published data only}
- Noroozinia H, Mahoori A, Hasani E, Gerami-Fahim M, Sepehrvand N. The effect of acupressure on nausea and vomiting after cesarean section under spinal anesthesia. Acta Medica Iranica 2013;51(3):163-7. [PubMed] [Google Scholar]
Nortcliffe 2003 {published data only}
- Nortcliffe SA, Buggy DJ. Prophylaxis of nausea and vomiting after spinal morphine analgesia for caesarean section: comparison between cyclizine, dexamethasone, and placebo [abstract]. British Journal of Anaesthesia 2001;87(4):657P. [DOI] [PubMed] [Google Scholar]
- Nortcliffe SA, Shah J, Buggy DJ. Prevention of postoperative nausea and vomiting after spinal morphine for caesarean section: comparison of cyclizine, dexamethasone and placebo. British Journal of Anaesthesia 2003;90(5):665-70. [DOI] [PubMed] [Google Scholar]
Pan 1996 {published data only}
- Pan PH, Moore CH. Intraoperative antiemetic efficacy of prophylactic ondansetron versus droperidol for cesarean section patients under epidural anesthesia. Anesthesia and Analgesia 1996;83(5):982-6. [DOI] [PubMed] [Google Scholar]
Pan 2001 {published data only}
- Pan P, Moore C, Fragneto R, Ross V, Justis G. Is antiemetic prophylaxis cost-effective for cesarean section patients [abstract]. Anesthesiology 2000;93(3A):A1092. [Google Scholar]
- Pan PH, Moore C, Fragneto R, Ross V, Justis G. Efficacy and cost-effectiveness of prophylactic ondansetron versus metoclopramide for cesarean section patients under epidural anesthesia [abstract]. Anesthesiology 2000;92 Suppl:A40. [Google Scholar]
- Pan PH, Moore CH. Comparing the efficacy of prophylactic metoclopramide, ondansetron, and placebo in cesarean section patients given epidural anesthesia. Journal of Clinical Anesthesia 2001;13(6):430-5. [DOI] [PubMed] [Google Scholar]
- Pan PH, Moore CM. Efficacy and cost-effectiveness of prophylactic ondansetron versus metoclopramide for cesarean section patients under epidural anesthesia [abstract]. Anesthesia and Analgesia 2000;90 Suppl:S295. [DOI] [PubMed] [Google Scholar]
Pan 2003 {published data only}
- Pan AK, Rudra A. Prophylactic single dose intravenous administration of ondansetron in the prevention of postoperative emetic symptoms during spinal anaesthesia for caesarean delivery. Indian Journal of Anaesthesia 2003;47(3):178-80. [Google Scholar]
Parra‐Guiza 2018 {published data only}
- ISRCTN57227250. Prevention of nausea and vomiting comparing ondansetron and dexamethasone versus placebo in cesarean section under regional anesthesia. http://www.isrctn.com/ISRCTN57227250 (7 July 2016). [CENTRAL: CN-01812416]
- Parra-Guiza R, Melendez HJ, Ochoa ME. Prophylactic efficacy of ondansetron and dexamethasone in nausea and vomit in patients undergoing cesarean section with neuraxial opioids. Controlled clinical trial. Medicas UIS 2018;31(1):31-8. [DOI: 10.18273/revmed.v31n1-2018004] [DOI] [Google Scholar]
Pazoki 2018 {published data only}
- Pazoki S, Modir H, Kamali A, Zamani A, Shahidani M. Ondansetron 8 mg and 4 mg with normal saline against post-operative headache and nausea/vomiting after spinal anesthesia: a randomized double-blind trial. Medical Gas Research 2018;8(2):48-53. [CENTRAL: CN-01617025] [EMBASE: 622985422] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peixoto 2006 {published data only}
- Peixoto AJ, Celich MF, Zardo L, Peixoto Filho AJ. Ondansetron or droperidol for prophylaxis of nausea and vomiting after intrathecal morphine. European Journal of Anaesthesiology 2006;23(8):670-5. [DOI] [PubMed] [Google Scholar]
Quiney 1995 {published data only}
- Quiney NF, Murphy PG. The effect of pretreatment with glycopyrrolate on emetic and hypotensive problems during caesarean section conducted under spinal anaesthesia [abstract]. International Journal of Obstetric Anesthesia 1995;4:66-7. [Google Scholar]
Rasooli 2014 {published data only}
- Rasooli S, Moslemi F, Khaki A. Effect of sub hypnotic doses of propofol and midazolam for nausea and vomiting during spinal anesthesia for cesarean section. Anesthesiology and Pain Medicine 2014;4(4):e19384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudra 2004a {published data only}
- Rudra A, Halder R, Sen A, Kundu S. Efficacy of low dose propofol for control of emetic episodes during caesarean delivery with spinal anaesthesia. Indian Journal of Anaesthesia 2004;48(1):31-4. [Google Scholar]
Sahoo 2012 {published data only}
- Sahoo T, SenDasgupta C, Goswami A, Hazra A. Reduction in spinal-induced hypotension with ondansetron in parturients undergoing caesarean section: A double-blind randomised, placebo-controlled study. International Journal of Obstetric Anesthesia 2012;21(1):24-8. [DOI] [PubMed] [Google Scholar]
Sanansilp 1998 {published data only}
- Sanansilp V, Areewatana S, Tonsukchai N. Droperidol and the side effects of epidural morphine after cesarean section. Anesthesia & Analgesia 1998;86(3):532-7. [DOI] [PubMed] [Google Scholar]
Selzer 2020 {published data only}
- Kjaer K. Dexamethasone for the prevention of post-operative nausea and vomiting in patients undergoing cesarean sections. https://clinicaltrials.gov/ct2/show/results/NCT01734161 (27 November 2012). [CENTRAL: CN-01538899]
- Selzer A, Pryor KO, Tangel V, O'Connell K, Kjaer K. The effect of intravenous dexamethasone on postoperative nausea and vomiting after Cesarean delivery with intrathecal morphine: a randomized-controlled trial [Effet de la dexamethasone intraveineuse sur les nausees et vomissements postoperatoires apres un accouchement par cesarienne avec morphine intrathecale: un essai randomise controle]. Canadian Journal of Anesthesia 2020;67:817-26. [CENTRAL: CN-02072818] [EMBASE: 2004140352] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Selzer AR, Pick J, Abramovitz S, Gadalla F, Darwich A, Kjaer K. The effect of a single dose of intravenous dexamethasone on nausea and vomiting when administered prior to intrathecal morphine for cesarean section: a randomized, placebo-controlled, double-blinded trial. In: Society for Obstetric Anesthesia and Perinatology (SOAP) 47th Annual Meeting; 2015 May 13-17; Colorado, USA. 2015:T-03.
Shabana 2012 {published data only}
- Shabana AM, Nasr ES, Moawad HE. Effect of ketamine on intraoperative nausea and vomiting during elective caesarean section under spinal anaesthesia: a placebo-controlled prospective randomized double blinded study. Egyptian Journal of Anaesthesia 2012;28(2):169-74. [Google Scholar]
Shen 2012 {published data only}
- Shen YJ, Yin YQ, Zhang YJ, Zhu Q, Zhang JH, Zhao W, et al. Efficacy of intravenous scopolamine for preventing postoperative nausea and vomiting after cesarean section. Acta Academiae Medicinae Sinicae 2012;34(1):32-7. [PubMed] [Google Scholar]
Stein 1997 {published data only}
- Stein DJ, Birnbach DJ, Danzer BI, Kuroda MM, Grunebaum A, Thys DM. Acupressure versus intravenous metoclopramide to prevent nausea and vomiting during spinal anesthesia for cesarean section. Anesthesia and Analgesia 1997;84(2):342-5. [DOI] [PubMed] [Google Scholar]
Tarhan 2007 {published data only}
- Tarhan O, Canbay O, Celebi N, Uzun S, Sahin A, Coskun F, et al. Subhypnotic doses of midazolam prevent nausea and vomiting during spinal anesthesia for cesarean section. Minerva Anestesiologica 2007;73(12):629-33. [PubMed] [Google Scholar]
Tkachenko 2019 {published data only}
- Tkachenko R, Pyasetska N. The efficiency of intrathecal dexamethasone for spinal anaesthesia in elective caesarean section. Regional Anesthesia and Pain Medicine 2019;44(10):A192. [CENTRAL: CN-01999210] [EMBASE: 629475510] [Google Scholar]
Tzeng 2000 {published data only}
- Tzeng JI, Wang JJ, Ho ST, Tang CS, Liu YC, Lee SC. Dexamethasone for prophylaxis of nausea and vomiting after epidural morphine for post-Caesarean section analgesia: comparison of droperidol and saline. British Journal of Anaesthesia 2000;85(6):865-8. [DOI] [PubMed] [Google Scholar]
Uerpairojkit 2017 {published data only}
- TCTR20160216003. Ondansetron for prophylaxis of spinal morphine induced nausea during early rooming in breastfeeding: a randomized placebo controlled trial. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1734 (16 February 2016). [CENTRAL: CN-01882139]
- Uerpairojkit K, Chesoh A, Budcharoentong D. Ondansetron for prophylaxis of spinal morphine induced nausea during early rooming in breastfeeding: a randomized placebo controlled trial. Anesthesia and Analgesia 2016;123(3 Suppl):286-7. [CENTRAL: CN-01783979] [EMBASE: 612648638] [Google Scholar]
- Uerpairojkit K, Chesoh A, Budcharoentong D. Ondansetron for prophylaxis of spinal morphine induced nausea during early rooming in breastfeeding: a randomized placebo controlled trial. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2017;100(12):1283-9. [CENTRAL: CN-01451211] [EMBASE: 620181091] [Google Scholar]
Ure 1999 {published data only}
- Ure D, James KS, McNeill M, Booth JV. Glycopyrrolate reduces nausea during spinal anaesthesia for caesarean section without affecting neonatal outcome. British Journal of Anaesthesia 1999;82(2):277-9. [DOI] [PubMed] [Google Scholar]
Voigt 2013 {published data only}
- Voigt M, Frohlich CW, Huttel C, Kranke P, Mennen J, Boessneck O, et al. Prophylaxis of intra- and postoperative nausea and vomiting in patients during cesarean section in spinal anesthesia. Medical Science Monitor 2013;19:993-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2001 {published data only}
- Wang JJ, Ho ST, Wong CS, Tzeng JI, Liu HS, Ger LP. Dexamethasone prophylaxis of nausea and vomiting after epidural morphine for post-cesarean analgesia. Canadian Journal of Anaesthesia 2001;48(2):185-90. [DOI] [PubMed] [Google Scholar]
Weiss 1995 {published data only}
- Weiss MD, Diaz F, Zuberi F, Milman B, Pasricha S. Subhypnotic doses of propofol for prevention of postoperative pruritus and nausea in cesarean section patients given spinal opioids. Anesthesiology 1995;83(3A):A960. [Google Scholar]
Wu 2007 {published data only}
- Wu JI, Lo Y, Chia YY, Liu K, Fong WP, Yang LC, et al. Prevention of postoperative nausea and vomiting after intrathecal morphine for cesarean section: a randomized comparison of dexamethasone, droperidol, and a combination. International Journal of Obstetric Anesthesia 2007;16(2):122-7. [DOI] [PubMed] [Google Scholar]
Yazigi 2002 {published data only}
- Yazigi A, Chalhoub V, Madi-Jebara S, Haddad F, Hayek G. Prophylactic ondansetron is effective in the treatment of nausea and vomiting but not on pruritis after cesarean delivery with intrathecal sufentanil-morphine. Journal of Clinical Anesthesia 2002;14:183-6. [DOI] [PubMed] [Google Scholar]
Zeraati 2016 {published data only}
- Zeraati H, Shahinfar J, Hesari SI, Masrorniya M, Nasimi F. The effect of ginger extract on the incidence and severity of nausea and vomiting after cesarean section under spinal anesthesia. Anesthesiology and Pain Medicine 2016;6(5):e38943. [CENTRAL: CN-01760273] [EMBASE: 612722027] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abadi 2018 {published data only}
- Abadi F, IRCT20171015036784N3. The effect of acupressure on anxiety before cesarean section and nausea, vomiting and pain after cesarean section in woman admitted to hospital. http://en.irct.ir/trial/27409 (29 January 2018).
Abboud 1984 {published data only}
- Abboud TK, Curtis J, Earl S, Henriksen EH, Hughes SC, Levinson G, et al. Efficacy of clear antacid prophylaxis in obstetrics. Acta Anaesthesiologica Scandinavica 1984;28:301-4. [DOI] [PubMed] [Google Scholar]
Abdalla 2019 {published data only}
- Abdalla E, Kamel EZ, Farrag WS. Intravenous dexamethasone combined with intrathecal atropine to prevent morphine-related nausea and vomiting after cesarean delivery: a randomized double-blinded study. Egyptian Journal of Anaesthesia 2019;35(1):65-70. [CENTRAL: CN-02049281] [EMBASE: 2003858082] [Google Scholar]
Abdellah 2018 {published data only}
- Abdellah MS, Abbas AM, Ali MK, Mahmoud A, Abdullah SA. Uterine exteriorization versus intraperitoneal repair: effect on intraoperative nausea and vomiting during repeat cesarean delivery - a randomized clinical trial. Facts, Views & Vision in ObGynObstetrics and Gynaecology 2018;10(3):131-7. [CENTRAL: CN-02089474] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Ackerman 1987 {published data only}
- Ackerman WE, Colclough GW, Guiler JM, Guilder DS, Akin JM. Epidural lipophilic opioids administered prophylactically for control of nausea, vomiting and pain during cesarean section. In: Proceedings of 19th Annual Meeting of Society for Obstetric Anesthesia and Perinatology; 1987 May 20-23; Halifax, Nova Scotia, Canada. 1987:143.
Ackerman 1988 {published data only}
- Ackerman WE, Juneja MM, Colclough GW, Kaczorowski DM. Epidural fentanyl significantly decreases nausea and vomiting during uterine manipulation in awake patients undergoing cesarean section. Anesthesiology 1988;69:A679. [Google Scholar]
Ackerman 1989 {published data only}
- Ackerman WE, Juneja MM, Colclough GW, Kaczorowski DM. Epidural lipophilic opioids significantly decrease nausea and emesis during uterine manipulation in awake patients undergoing cesarean section. Regional Anesthesia 1989;14(2):23. [Google Scholar]
Afsargharehbagh 2018 {published data only}
- Afsargharehbagh R, Mosaed S, Nasiri A, Afshari M, Moosazadeh M. Comparison of the effects of intravenous metoclopramide and ondansetron on prevention of nausea and vomiting after cesarean section. Biomedical Research (India) 2018;29(15):3043-6. [CENTRAL: CN-01645172] [EMBASE: 623939714] [Google Scholar]
- Afsargharehbagh R. Comparison of the effects of intravenous metoclopramide and ondansetron on prevention of nausea and vomiting after cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20170502033762N2 2018 2018:[CENTRAL: CN-01895567; CRS: 10795276; CRSREP: 10795276]. [CENTRAL: CN-01895567]
Alghanem 2019 {published data only}
- NCT04140058. Hemodynamic protection of preoperative ondansetron 15 minutes before spinal anaesthesia in caesarean. Https://clinicaltrials.gov/show/NCT04140058 2019 Oct 25. [CENTRAL: CN-01994383]
Alipour 2017 {published data only}
- IRCT2017021112251N4. effects of midazolam and ondansetron on nausea and vomiting after spinal anesthesia [Comparison effects of intravenous midazolam and ondansetron on nausea and vomiting after spinal anesthesia for cesarean section]. http://en.irct.ir/trial/12347 (27 May 2017). [CENTRAL: CN-01888925]
Allen 2013 {published data only}
- Allen T, NCT01812057. Dexamethasone as an analgesic adjunct for post-cesarean delivery pain relief. https://clinicaltrials.gov/ct2/show/results/NCT01812057 (15 March 2013).
Ananthakrishnan 2004 {published data only}ISRCTN66467815
- Ananthakrishnan A, ISRCTN66467815. Preoperative fasting and postoperative nausea and vomiting after elective caesarean section under spinal anaesthesia: a randomised controlled study. http://www.isrctn.com/ISRCTN66467815 (30 September 2004).
Anonymous 2010 {published data only}
- EUCTR2009-013543-11-GB. A comparison of nausea with two routine ways of managing low blood pressure after delivery of the baby during spinal anaesthesia for Caesarean section. - nausea during Caesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009-013543-11-GB 2010. [CENTRAL: CN-01883407]
Askar 2017 {published data only}
- NCT03165123. Effect of using erythromycin a versus placebo with dexamethasone in prevention of post-spinal nausea and vomiting. Https://clinicaltrials.gov/show/nct03165123 2017 May 15. [CENTRAL: CN-01575273]
Atalay 2010 {published data only}
- Atalay C, Aksoy M, Aksoy AN, Dogan N, Kursad H. Combining intrathecal bupivacaine and meperidine during caesarean section to prevent spinal anaesthesia-induced hypotension and other side-effects. Journal of International Medical Research 2010;38(5):1626-36. [DOI] [PubMed] [Google Scholar]
Atkinson 1980 {published data only}
- Atkinson RE, Thomas T, Nicholas AD, Anderson I. An antacid comparison trial. Anaesthesia 1980;35:1126-30. [Google Scholar]
Avramovic 1979 {published data only}
- Avramovic D, Sulovic V, Lazarevic B, Cvetkovic M, Milacic D. Prevention of postoperative abdominal discomfort and gastrointestinal distension after cesarean section by use of simeticon. Jugoslavenska Ginekologija i Opstetricija 1979;19(5-6):307-11. [PubMed] [Google Scholar]
Ayorinde 2001 {published data only}
- Ayorinde B, Brown J, Buczkowski P, Shah J, Buggy DJ. Prevention of spinal anaesthesia-induced hypotension during caesarean section: comparative study of pre-emptive vasopressors. British Journal of Anaesthesia 2000;84(2):277P-288P. [DOI] [PubMed] [Google Scholar]
- Ayorinde BT, Buczkowski P, Brown J, Shah J, Buggy DJ. Evaluation of pre-emptive intramuscular phenylephrine and ephedrine for reduction of spinal anaesthesia-induced hypotension during caesarean section. British Journal of Anaesthesia 2001;86(3):372-6. [DOI] [PubMed] [Google Scholar]
Banihashem 2011 {published data only}
- Banihashem N, Hassannasab B, Naziri F, Rahimifar AR, Hosseini V, Shirkhani Z. Comparison of the prophylactic effect of ondansetron and dexamethasone on postoperative nausea and vomiting after intrathecal meperidine in women scheduled for elective cesarean section. Journal of Babol University of Medical Sciences 2011;13(3):30-3. [Google Scholar]
Belzarena 1993 {published data only}
- Belzarena SD. Addition of epinephrine to a combination of bupivacaine and fentanyl in spinal anesthesia for cesarean section. Regional Anesthesia 1993;18S1:15. [Google Scholar]
Bifarini 1990 {published data only}
- Bifarini G, Trabalza N, Falconi S, Mala L, Favetta P. Effects on newborn of pharmacological prophylaxis for Mendelson's syndrome. Minerva Anestesiologica 1990;56:1447-50. [PubMed] [Google Scholar]
Bifarini 1992 {published data only}
- Bifarini G, Favetta P, Ciammiti B, Cirulli P, Del Sindaco F, Pasqualucci A. Pharmacological prevention of Mendelson's syndrome: controlled clinical trial. Minerva Anestesiologica 1992;58(3):95-9. [PubMed] [Google Scholar]
Biwas 2002 {published data only}
- Biwas SC, Biswas BN, Rudra A, Pan A. A comparative study of metoclopramide, glycopyrrolate and combination of metoclopramide and glycopyrrolate in control of intraoperative and postoperative nausea and vomiting after spinal anaesthesia for caesarean section. Journal of Obstetrics and Gynecology of India 2002;52(3):73-5. [Google Scholar]
Bonhomme 2002 {published data only}
- Bonhomme V, Brichant JF, Wuilmart M, Dewandre PY, Hans P. Droperidol reduces nausea after caesarean section but alters the neurological status of the breastfed infants [abstract]. Anesthesiology 2002;96:A1044. [Google Scholar]
Boschi 1984 {published data only}
- Boschi S, Di MMG, Pigna A, Rossi R. The effect of ranitidine on gastric ph and volume in patients undergoing cesarean section: Possible relationship to Mendelson's syndrome. Current Therapeutic Research, Clinical and Experimental 1984;35(4):654-62. [Google Scholar]
Briao 2015 {published data only}
- Briao FF, Horta ML, Horta BL, Barros GA, Behrensdorf AP, Severo I, et al. Comparison of droperidol and ondansetron prophylactic effect on subarachnoid morphine-induced pruritus [Comparacao dos efeitos profilaticos do droperidol e do ondansetron sobre o prurido provocado pela morfina subaracnoidea]. Revista Brasileira De Anestesiologia 2015;65(4):244-8. [DOI] [PubMed] [Google Scholar]
Brock‐Utne 1989 {published data only}
- Brock-Utne JG, Rout C, Moodley J, Mayat N. Influence of preoperative gastric aspiration on the volume and pH of gastric contents in obstetric patients undergoing caesarean section. British Journal of Anaesthesia 1989;62:397-401. [DOI] [PubMed] [Google Scholar]
Brody 2008 {published data only}
- Brody R. Study of the effect of intramuscular ephedrine on the incidence of nausea and vomiting during elective cesarean section. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Bylsma‐Howell 1983 {published data only}
- Bylsma-Howell M, McMorland GH, Rurak DW, McErlane B, Axelson JE. Placental transport of metoclopramide: assessment of maternal and neonatal effects. Canadian Anaesthetists Society Journal 1983;30:487-92. [DOI] [PubMed] [Google Scholar]
Chan 1992 {published data only}
- Chan YK. A comparison of sodium citrate and sodium citrate/ranitidine combination for acid aspiration prophylaxis. Medical Journal of Malaysia 1992;47(1):27-30. [PubMed] [Google Scholar]
Chan 1997 {published data only}
- Chan WS, Irwin MG, Tong WN, Lam YH. Prevention of hypotension during spinal anaesthesia for caesarean section: ephedrine infusion versus fluid preload. Anaesthesia 1997;52(9):908-13. [DOI] [PubMed] [Google Scholar]
Chang 2011 {published data only}
- Chang FL, Sheen MJ. Efficacy of mirtazapine in preventing intrathecal morphine-induced nausea and vomiting after cesarean section. Regional Anesthesia and Pain Medicine 2011;36(5):511. [DOI] [PubMed] [Google Scholar]
Chattopadhyay 2015 {published data only}
- Chattopadhyay S, Goswami S. Palonosetron versus ramosetron prophylaxis for control of postoperative nausea and vomiting after cesarean delivery under spinal anesthesia. Journal of Obstetrics and Gynecology of India 2015;65(1):28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhuri 2004 {published data only}
- Chaudhuri K, Chaudhuri S, Lampe C, Rivera J, Solis-Estrada J. Does addition of dolasetron to metoclopramide alter nausea and vomiting in cesarean section patients under regional anesthesia [abstract]. Anesthesiology 2004;101 Suppl:A1234. [Google Scholar]
Chauhan 2014 {published data only}
- Chauhan D, Bhavsar M. Comparison of ramosetron and ondansetron for prevention of nausea and vomiting after carboprost in LSCS patients under spinal anesthesia. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2014;3(3):581-4. [Google Scholar]
Chen 2005 {published data only}
- Chen HM, Chang FY, Hsu CT. Effect of acupressure on nausea, vomiting, anxiety and pain among post-cesarean section women in Taiwan. Kaohsiung Journal of Medical Sciences 2005;21(8):341-50. [DOI] [PubMed] [Google Scholar]
Chestnut 1989 {published data only}
- Chestnut DH, Owen CL, Geiger M, Bates JN, Choi WW, Ostman PL. Metoclopramide versus droperidol for prevention of nausea and vomiting during epidural anesthesia for cesarean section. Southern Medical Journal 1989;82(10):1224-7. [DOI] [PubMed] [Google Scholar]
- Chestnut DH, Owen CL, Ostman LG, Bates JN, Choi WW. A comparison of metoclopramide and droperidol for prevention of nausea and vomiting during and after elective cesarean section with epidural anesthesia. In: Proceedings of 19th Annual Meeting of Society for Obstetric Anesthesia and Perinatology; 1987 May 20-23; Halifax, Nova Scotia, Canada. 1987:118.
Chung 1998 {published data only}
- Chung CJ, Kim JS, Park HS, Chin YJ. The efficacy of intrathecal neostigmine, intrathecal morphine, and their combination for post-cesarean section analgesia. Anesthesia and Analgesia 1998;87(2):341-6. [DOI] [PubMed] [Google Scholar]
Cohen 1984 {published data only}
- Cohen SE, Barrier G. Does metoclopramide decrease gastric volume in cesarean section patients? Anesthesiology 1983;59:A403. [DOI] [PubMed] [Google Scholar]
- Cohen SE, Jasson J, Talafre ML, Chauvelot-Moachon L, Barrier G. Does metoclopramide decrease the volume of gastric contents in patients undergoing cesarean section? Anesthesiology 1984;61:604-7. [DOI] [PubMed] [Google Scholar]
Cohen 2016a {published data only}
- NCT02960113. Scopolamine patch and acupressure point P6 stimulation for reduction of nausea and vomiting during cesarean section. Https://clinicaltrials.gov/show/NCT02960113 (first received 2016 Nov 7). [CENTRAL: CN-01559905]
Colman 1988 {published data only}
- Colman RD, Frank M, Loughnan BA, Cohen DG, Cattermole R. Use of i.m. ranitidine for the prophylaxis of aspiration pneumonitis in obstetrics. British Journal of Anaesthesia 1988;61:720-9. [DOI] [PubMed] [Google Scholar]
Connelly 1997 {published data only}
- Connelly NR, Rahimi A, Parker RK. Nalmefene or naloxone for preventing intrathecal opioid mediated side effects in cesarean delivery patients. International Journal of Obstetric Anesthesia 1997;6:231-4. [DOI] [PubMed] [Google Scholar]
Cooper 2002 {published data only}
- Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean section. Anesthesiology 2002;97:1582-90. [DOI] [PubMed] [Google Scholar]
Cowan 2002 {published data only}
- Cowan CM, Kendall JB, Barclay PM, Wilkes RG. Comparison of intrathecal fentanyl and diamorphine in addition to bupivacaine for caesarean section under spinal anaesthesia. British Journal of Anaesthesia 2002;89(3):452-8. [PubMed] [Google Scholar]
Dahlgren 1997 {published data only}
- Dahlgren G, Hultstrand C, Jakobsson J, Norman M, Eriksson EW, Martin H. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesthesia and Analgesia 1997;85(6):1288-93. [DOI] [PubMed] [Google Scholar]
Dailey 1988 {published data only}
- Dailey PA, Hughes SC, Rosen MA, Healy K, Cheek DB, Pytka S, et al. Lidocaine levels during cesarean section after pretreatment with ranitidine or cimetidine. Anesthesiology 1985;63:444. [Google Scholar]
- Dailey PA, Hughes SC, Rosen MA, Healy K, Cheek DB, Shnider SM. Effect of cimetidine and ranitidine on lidocaine concentrations during epidural anesthesia for cesarean section. Anesthesiology 1988;69:1013-7. [DOI] [PubMed] [Google Scholar]
Datta 1982 {published data only}
- Datta S, Alper MH, Ostheimer GW, Weiss JB. Method of ephedrine administration and nausea and hypotension during spinal anesthesia for cesarean section. Anesthesiology 1982;56:68-70. [DOI] [PubMed] [Google Scholar]
Demirhan 2013 {published data only}
- Demirhan A, Tekelioglu YU, Akkaya A, Ozlu T, Yildiz I, Bayir H, et al. Antiemetic effects of dexamethasone and ondansetron combination during cesarean sections under spinal anaesthesia. African Health Sciences 2013;13(2):475-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dereu 2019 {published data only}
- Dereu D, Savoldelli G L, Mercier Y, Combescure C, Mathivon S, Rehberg B. The impact of a transversus abdominis plane block including clonidine vs. intrathecal morphine on nausea and vomiting after caesarean section: a randomised controlled trial. European Journal of Anaesthesiology 2019;36(8):575-82. [CENTRAL: CN-01978373] [EMBASE: 628756444] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dewan 1982 {published data only}
- Dewan DM, Wheeler AS, James FM III, Floyd HM, Rhyne L. Antacid anticholinergic regimens in patients undergoing elective caesarean section. Canadian Anaesthetists Society Journal 1982;29:27-30. [DOI] [PubMed] [Google Scholar]
Dewan 1984 {published data only}
- Dewan DM, Floyd HM, Thistlewood JM, Bogard TD. Sodium citrate premedication for elective cesarean section. Anesthesia and Analgesia 1984;63:205. [PubMed] [Google Scholar]
Dewan 1985 {published data only}
- Dewan DM, Floyd HM, Thistlewood JM, Bogard TD, Spielman FJ. Sodium citrate pretreatment in elective cesarean section patients. Anesthesia and Analgesia 1985;64:34-7. [PubMed] [Google Scholar]
Dundee 1979 {published data only}
- Dundee JW, Howe JP, Moore J, McCaughey W. Effect of cimetidine on gastric pH in women undergoing elective caesarean section [proceedings]. British Journal of Clinical Pharmacology 1979;8:391P-2P. [DOI] [PubMed] [Google Scholar]
El‐Deeb 2011 {published data only}
- El-Deeb A, Abd el Motlb E. Prophylactic multimodal antiemetic in women undergoing cesarean section under spinal anesthesia. Egyptian Journal of Anaesthesia 2011;27(2):107-11. [Google Scholar]
Elhakim 2005 {published data only}
- Elhakim M, El-Megid WA, Metry A, El-hennawy A, El-Queseny K. Analgesic and antacid properties of i.m. tramadol given before caesarean section under general anaesthesia. British Journal of Anaesthesia 2005;95(6):811-5. [DOI] [PubMed] [Google Scholar]
El Khouly 2016 {published data only}
- El Khouly NI, Meligy AM. Randomized controlled trial comparing ondansetron and placebo for the reduction of spinal anesthesia-induced hypotension during elective cesarean delivery in Egypt. International Journal of Gynaecology and Obstetrics 2016;135(2):205-9. [CENTRAL: CN-01368664] [DOI: 10.1016/j.ijgo.2016.06.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
- PACTR201601001397193. Ondasterone during spinal anaesthesia for elective caeserian section [tTe effect of ondasterone versus placebo in reduction of hypotension during spinal anesthesia for elective caesarean section:a prospective randomises trial]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201601001397193 2015. [CENTRAL: CN-01824697]
Elmetwally 2019 {published data only}
- NCT03932578. Intrathecal atropine vs IV metoclopramide for nausea & vomiting during CS. Https://clinicaltrials.gov/show/NCT03932578 (first received 2019 April 30). [CENTRAL: CN-01931707]
El Saied Hafez 2017 {published data only}
- El Saied Hafez MH, El Din Abdelhamid MH, Youssef MM, Abdelrahim IK. Randomized controlled trial of two oral regimens of gabapentin versus placebo in patients for cesarean section under spinal anesthesia regarding postoperative pain, sedation, nausea and vomiting. Egyptian Journal of Anaesthesia 2017;33(1):59-65. [CENTRAL: CN-01332566] [EMBASE: 613962944] [Google Scholar]
Ewart 1990 {published data only}
- Ewart MC, Yau G, Gin T, Kotur CF, Oh TE. A comparison of oral omeprazole and ranitidine as premedication for elective caesarean section. In: Proceedings of Spring Meeting of Obstetric Anaesthetists Associationl; 1990; London, UK. 1990:33.
- Ewart MC, Yau G, Gin T, Kotur CF, Oh TE. A comparison of the effects of omeprazole and ranitidine on gastric secretion in women undergoing elective caesarean section. Anaesthesia 1990;45:527-30. [DOI] [PubMed] [Google Scholar]
Fan 1994 {published data only}
- Fan SZ, Susetio L, Wang YP, Cheng YJ, Liu CC. Low dose of intrathecal hyperbaric bupivacaine combined with epidural lidocaine for cesarean section - a balance block technique. Anesthesia and Analgesia 1994;78:474-7. [DOI] [PubMed] [Google Scholar]
Farzi 2017 {published data only}
- Farzi F, Ghazanfar Tehran S, Mirmansouri A, Naderi Nabi B, Atrkar Roushan Z, Nematollahi Sani M, et al. Comparing the effect of adding fentanyl, sufentanil, and placebo with intrathecal bupivacaine on duration of analgesia and complications of spinal anesthesia in patients undergoing cesarean section. Anesthesiology and Pain Medicine 2017;7(5):e12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fattahi 2015 {published data only}
- Fattahi Z, Hadavi SM, Sahmeddini MA. Effect of ondansetron on post-dural puncture headache (PDPH) in parturients undergoing cesarean section: a double-blind randomized placebo-controlled study. Journal of Anesthesia 2015;29(5):702-7. [CENTRAL: CN-01256596] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fazel 2017 {published data only}
- Fazel N, Pejhan A, Taghizadeh M, Tabarayi Y, Rivadeh F, Farkhondeh L. The effect of anethum graveolens L. oil consumption on the intensity of nausea after cesarean section. Iranian Journal of Obstetrics, Gynecology and Infertility 2017;20(7):18-24. [CENTRAL: CN-01440150] [EMBASE: 619421387] [Google Scholar]
Flynn 1989a {published data only}
- Flynn RJ, Moore J, Collier PS, McClean E. Does pretreatment with cimetidine and ranitidine affect the disposition of bupivacaine? British Journal of Anaesthesia 1989;62:87-91. [DOI] [PubMed] [Google Scholar]
Flynn 1989b {published data only}
- Flynn RJ, Moore J, Collier PS, Howard PJ. Effect of intravenous cimetidine on lignocaine disposition during extradural caesarean section. Anaesthesia 1989;44:739-41. [DOI] [PubMed] [Google Scholar]
- Flynn RJ, Moore J. Does cimetidine effect plasma lidocaine levels in the parturient? Anesthesia and Analgesia 1989;68:S87. [DOI] [PubMed] [Google Scholar]
Flynn 1989c {published data only}
- Flynn RJ, Moore J, Collier PS, Howard PJ. Single dose oral H2-antagonists do not affect plasma lidocaine levels in the parturient. Acta Anaesthesiologica Scandinavica 1989;33:593-6. [DOI] [PubMed] [Google Scholar]
- Flynn RJ, Moore J. Effect of H2-receptor antagonists on plasma lignocaine concentrations in the obstetric patient. British Journal of Anaesthesia 1988;61:512P. [Google Scholar]
- Flynn RJ, Moore J. Lack of effect of cimetidine and ranitidine on lidocaine disposition in the parturient. Anesthesiology 1988;69:A656. [Google Scholar]
Fogarty 1992 {published data only}
- Fogarty DJ, Moore J. Further investigations of omeprazole in obstetric patients. International Journal of Obstetric Anesthesia 1992;1:180. [Google Scholar]
Frank 1984 {published data only}
- Frank M, Evans M, Flynn P, Aun C. Comparison of the prophylactic use of magnesium trisilicate mixture BPC, sodium citrate mixture or cimetidine in obstetrics. British Journal of Anaesthesia 1984;56:355-61. [DOI] [PubMed] [Google Scholar]
Freeman 1999 {published data only}
- Freeman J, Lafreniere L, Watkins E, Dresner M. Does sodium citrate increase sickness associated with spinal anaesthesia for caesarean section? British Journal of Anaesthesia 1999;82 Suppl:160. [Google Scholar]
Fujii 1998a {published data only}
- Editors. Retraction. Granisetron prevents nausea and vomiting during spinal anaesthesia for caesarean section. Acta Anaesthesiologica Scandinavica 2013;57(4):535. [PubMed] [Google Scholar]
- Fujii Y, Tanaka H, Toyooka H. Granisetron prevents nausea and vomiting during spinal anaesthesia for caesarean section. Acta Anaesthesiologica Scandinavica 1998;42(3):312-5. [DOI] [PubMed] [Google Scholar]
Fujii 1998b {published data only}
- Editors. Retraction. Prevention of nausea and vomiting with granisetron, droperidol and metoclopramide during and after spinal anaesthesia for caesarean section: a randomized, doubleblind, placebo-controlled trial. Acta Anaesthesiologica Scandinavica 2013;57(4):537. [PubMed] [Google Scholar]
- Fujii Y, Tanaka H, Toyooka H. Prevention of nausea and vomiting with granisetron, droperidol and metoclopramide during and after spinal anaesthesia for caesarean section: a randomized, double-blind, placebo-controlled trial. Acta Anaesthesiologica Scandinavica 1998;42(8):921-5. [DOI] [PubMed] [Google Scholar]
Fujii 1999 {published data only}
- Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Granisetron/dexamethasone combination for reducing nausea and vomiting during and after spinal anesthesia for cesarean section. Anesthesia and Analgesia 1999;88(6):1346-50. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Granisetron/dexamethasone combination for reducing nausea and vomiting during and after spinal anesthesia for cesarean section. Anesthesia and Analgesia 1999;88:1346-50. Retraction. Anesthesia and Analgesia 2013;116(3):744. [DOI] [PubMed] [Google Scholar]
Fujii 2002 {published data only}
- Editors. Notice of retraction: "dexamethasone for the prevention of nausea and vomiting after dilatation and curettage: a randomized controlled trial" (fujii and uemura) and "dose-range effects of propofol for reducing emetic symptoms during cesarean delivery" (fujii and numazaki). Obstetrics and Gynecology 2013;121(6):1362. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Numazaki M. Dose-range effects of propofol for reducing emetic symptoms during cesarean delivery. Obstetrics & Gynecology 2002;99:75-9. [DOI] [PubMed] [Google Scholar]
Fujii 2004 {published data only}
- Fujii Y, Numazaki M. RETRACTED: Randomized, double-blind comparison of subhypnotic-dose propofol alone and combined with dexamethasone for emesis in parturients undergoing cesarean delivery. Clinical Therapeutics 2004;26(8):1286-91. [DOI] [PubMed] [Google Scholar]
Gaiser 2002 {published data only}
- Gaiser RR, Dong Y, Cheek TG, Gutsche BB. Does increased intravenous hydration decrease the incidence of nausea/vomiting following cesarean section? [abstract]. Anesthesiology 2002;96(Suppl 1):P83. [Google Scholar]
Galehdar 2011 {published data only}
- IRCT201101125596N1. Effect of oxygen therapy on nausea and vomiting [Comparison of administration of supplemental oxygen on nausea and vomiting in patients undergoing spinal anesthesia for elective cesarean section]. http://en.irct.ir/trial/6012 (12 July 2011). [CENTRAL: CN-01852117]
Galehdar 2016 {published data only}
- IRCT201603155596N3. Comparative effect of Ondansetron and acupressure on nausea and vomiting. http://en.irct.ir/trial/6013 (12 April 2016). [CENTRAL: CN-01816375]
Gangadhara Gowda 2014 {published data only}
- Gangadhara Gowda KG, Dhorigol MG. Comparision of intrathecal fentanyl and intravenous ondansetron for prevention of nausea -vomiting during cesarean delivery with spinal anaesthesia. Indian Journal of Public Health Research and Development 2014;5(4):147-50. [CENTRAL: CN-01084135] [EMBASE: 604818070] [Google Scholar]
George 2018 {published data only}
- George RB, McKeen DM, Dominguez JE, Allen TK, Doyle PA, Habib AS. A randomized trial of phenylephrine infusion versus bolus dosing for nausea and vomiting during cesarean delivery in obese women [Une etude randomisee comparant une perfusion versus un bolus de phenylephrine chez des femmes obeses pour traiter les nausees et vomissements pendant un accouchement par cesarienne]. Canadian Journal of Anesthesia 2018;65(3):254-62. [CENTRAL: CN-01442476] [EMBASE: 619600558] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01481740. A double blind randomized controlled trial of phenylephrine for the prevention of spinal induced hypotension in obese parturients. https://clinicaltrials.gov/ct2/show/NCT01481740.
Ghods 2005a {published data only}
- Ghods AA, Soleimani M, Narimani M. Effect of postoperative supplemental oxygen on nausea and vomiting after cesarean birth. Journal of Perianesthesia Nursing 2005;20(3):200-5. [DOI] [PubMed] [Google Scholar]
Gunka 2013 {published data only}
- NCT01841606. The effect of ondansetron on cardiac output in elective cesarean deliveries. Https://clinicaltrials.gov/show/NCT01841606 (first received 2013 Mar 11). [CENTRAL: CN-01541847]
Gutsche 1976 {published data only}
- Gutsche BB. Prophylactic ephedrine preceding spinal analgesia for cesarean section. Anesthesiology 1976;45:462-5. [DOI] [PubMed] [Google Scholar]
Habib 2019 {published data only}
- NCT03931135. Cyclizine Vs. dexamethasone for nausea and vomiting following intrathecal morphine in cases of cesarean section. Https://clinicaltrials.gov/show/nct03931135 2019 Apr 30. [CENTRAL: CN-01931679]
Hackworth 2010 {published data only}
- NCT01414777. Intravenous ondansetron to attenuate the hypotensive, bradycardic response to spinal anesthesia in healthy parturients. Https://clinicaltrials.gov/show/NCT01414777 2010 Sep 16. [CENTRAL: CN-02018275]
Hafez 2017 {published data only}
- Hafez MH, Abdelhamid MH, Youssef MM, Abdelrahim IK. Randomized controlled trial of two oral regimens of gabapentin versus placebo in patients for Cesarean section under spinal anesthesia regarding postoperative pain, sedation, nausea and vomiting. Egyptian Journal of Anaesthesia 2017;33(1):59-65. [Google Scholar]
Hajian 2016 {published data only}
- Hajian P, Malekianzadeh B, Davoudi M. Efficacy of intravenous ondansetron on hemodynamic complications in women undergoing spinal anesthesia for cesarean section: a randomized placebo controlled clinical trial. Galen Medical Journal 2016;5(1):13-8. [CENTRAL: CN-01329765] [EMBASE: 614203795] [Google Scholar]
Hamzei 2015 {published data only}
- Hamzei A, Nazemi SH, Alami A, Gochan AD, Kazemi A. Comparing different epinephrine concentrations for spinal anesthesia in cesarean section: A double-blind randomized clinical trial. Iranian Journal of Medical Sciences 2015;40(4):302-8. [PMC free article] [PubMed] [Google Scholar]
Han 2007 {published data only}
- Han DW, Hong SW, Kwon JY, Lee JW, Kim KJ. Epidural ondansetron is more effective to prevent postoperative pruritus and nausea than intravenous ondansetron in elective cesarean delivery. Acta Obstetricia et Gynecologica Scandinavica 2007;86:683-7. [DOI] [PubMed] [Google Scholar]
Hildyard 2000 {published data only}
- Hildyard C, Freeman J, Dresner M. Reducing the incidence of postoperative nausea and vomiting following elective caesarean section under spinal anaesthesia [abstract]. International Journal of Obstetric Anesthesia 2000;9:199. [Google Scholar]
- Hildyard CL, Quinn A, Dresner M, Freeman J. Cyclizine reduces the incidence of nausea and vomiting after elective caesarean section with intrathecal diamorphine [abstract]. British Journal of Anaesthesia 2001;87(4):672P. [Google Scholar]
Hodgkinson 1983 {published data only}
- Hodgkinson R, Glassenberg R, Joyce TH, Coombs DW, Ostheimer GW, Gibbs CP. Comparison of cimetidine (Tagamet) with antacid for safety and effectiveness in reducing gastric acidity before elective cesarean section. Anesthesiology 1983;59:86-90. [DOI] [PubMed] [Google Scholar]
Holdsworth 1974 {published data only}
- Holdsworth JD, Furness RM, Roulston RG. A comparison of apomorphine and stomach tubes for emptying the stomach before general anaesthesia in obstetrics. British Journal of Anaesthesia 1974;46:526-9. [DOI] [PubMed] [Google Scholar]
Holdsworth 1978 {published data only}
- Holdsworth JD. The place of apomorphine prior to obstetric analgesia. Journal of International Medical Research 1978;6:26-32. [PubMed] [Google Scholar]
Holdsworth 1980 {published data only}
- Holdsworth JD, Johnson K, Mascall G, Gwynne Roulston R, Tomlinson PA. Mixing of antacids with stomach contents. Anaesthesia 1980;35:641-50. [DOI] [PubMed] [Google Scholar]
Hong 2004 {published data only}
- Hong JY. Effect of dextrose on preoperative gastric content and serum gastrin [abstract]. Canadian Journal of Anesthesia 2004;51(Suppl 1):A57. [Google Scholar]
Hunt 1989 {published data only}
- Hunt CO, Naulty JS, Bader AM, Hauch MA, Vartikar JV, Datta S, et al. Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology 1989;71:535-40. [DOI] [PubMed] [Google Scholar]
Husemeyer 1980 {published data only}
- Husemeyer RP, Davenport HT. Prophylaxis for Mendelson's syndrome before elective caesarean section. A comparison of cimetidine and magnesium trisilicate mixture regimens. British Journal of Obstetrics and Gynaecology 1980;87:565-70. [DOI] [PubMed] [Google Scholar]
Huseyinogclu 2016 {published data only}
- Huseyinogclu U, Ulker K. Preoperative use of 10-mg metoclopramide and 50-mg dimenhydrinate in the prophylaxis of postoperative nausea and vomiting in elective caesarean births: a prospective randomized clinical study. Journal of Obstetrics and Gynecology of India 2016;66(4):252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussain 2011 {published data only}
- Hussain S, Khan RA, Iqbal M, Shafiq M, Khan FA. A comparison of the effects of erythromycin and metoclopramide on gastric fluid volume and pH in patients undergoing elective caesarean section. Anaesthesia, Pain and Intensive Care 2011;15(3):148-52. [Google Scholar]
Hussain 2014 {published data only}
- Hussain M, Ishaq N, Yusaf A. Comparison of acid reducing properties of tramadol and ranitidine given before caesarean section under general anaesthesia. Pakistan Journal of Medical and Health Sciences 2014;8(1):129-32. [Google Scholar]
Imeh 2014 {published data only}
- Imeh A, Olaniyi O, Simeon O, Omotola O. Dexamethasone versus a combination of dexamethasone and ondansetron as prophylactic antiemetic in patients receiving intrathecal morphine for caesarean section. African Health Sciences 2014;14(2):453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal 2000 {published data only}
- Iqbal MS, Ashfaque M, Akram M. Gastric fluid volume and pH: a comparison of effects of ranitidine alone with combination of ranitidine and metoclopramide in patients undergoing elective caesarean section. Annals of King Edward Medical College 2000;6(2):189-91. [Google Scholar]
Ishiyama 2001 {published data only}
- Ishiyama T, Yamaguchi T, Kashimoto S, Kumazawa T. Effects of epidural fentanyl and intravenous flurbiprofen for visceral pain during cesarean section under spinal anesthesia. Journal of Anesthesia 2001;15:69-73. [DOI] [PubMed] [Google Scholar]
Jabalameli 2011 {published data only}
- IRCT201010232405N5. The effect of remifentanil or fentanyl on postoperative nausea and vomiting after cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201010232405N5 2010. [CENTRAL: CN-01847612]
- Jabalameli M, Rouholamin S, Gourtanian F. A comparison of the effects of fentanyl and remifentanil on nausea, vomiting, and pain after cesarean section. IRCS Journal of Medical Science 2011;30(3):183-7. [PMC free article] [PubMed] [Google Scholar]
Jabalameli 2012 {published data only}
- IRCT201201302405N10. Comparison of midazoalm, ondansetron, and a combination for treatment of postoperative nausea and vomiting after spinal anesthesia for cesarean delivery. http://en.irct.ir/trial/2107 (16 February 2012). [CENTRAL: CN-01869823] [DOI] [PMC free article] [PubMed]
- IRCT201201308877N1. Treatment of postoperative nausea and vomiting after spinal anesthesia for cesarean delivery: a randomized comparison of midazoalm, ondansetron, and a combination. http://en.irct.ir/trial/9360 (5 February 2012). [CENTRAL: CN-01873670] [DOI] [PMC free article] [PubMed]
Jacquemyn 2013 {published data only}
- ISRCTN95504420. A study on side effects such as nausea and vomitus after medication to prevent excessive bleeding after a caesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN95504420 2013. [CENTRAL: CN-01851736]
Jain 2015 {published data only}
- Jain R, Sharma R. A comparative study of effects of glycopyrrolate and ondansetron on nausea and vomiting in cesarean section under spinal anesthesia. Anesthesia, Essays and Researches 2015;9(3):348-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2017 {published data only}
- CTRI/2017/11/010517. Effect of rabeprazole and ranitidine in reducing stomach volume in women undergoing cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/11/010517 2017. [CENTRAL: CN-01887815]
Jasson 1989a {published data only}
- Jasson J, Lefevre G, Talaffre ML, Legagneux F, Guerin JP, Vassilieff N, et al. Immediate pre-anesthetic administration of sodium citrate in cesarean interventions [abstract] [Administration immediate pre-anesthesique de citrate de sodium pour intervention cesarienne programmee]. Annales Francaises d'Anesthesie et de Reanimation 1987;6:R155. [Google Scholar]
- Jasson J, Lefevre G, Tallet F, Talafre ML, Legagneux F, Conseiller C. Oral sodium citrate before general anaesthesia for elective caesarean section Effects of pH and volume of gastric content [Ingestion de citrate de sodium avant l'anesthesie generale pour cesarienne programmee. Effet sur le pH et le volume du contenu gastrique.]. Annales Francaises d Anesthesie et de Reanimation 1989;8:12-8. [DOI] [PubMed] [Google Scholar]
Jeon 2000 {published data only}
- Jeon WJ, Kim DH, Choi DH. Patient controlled sedation using propofol during regional anesthesia for cesarean section. Korean Journal of Anesthesiology 2000;39(4):534-41. [Google Scholar]
Kang 1982 {published data only}
- Kang YG, Abouleish E, Caritis S. Prophylactic intravenous ephedrine infusion during spinal anesthesia for cesarean section. Anesthesia and Analgesia 1982;61:839-42. [PubMed] [Google Scholar]
Kangas‐Saarela 1990 {published data only}
- Kangas-Saarela T, Hollmen AI, Tolonen U, Eskelinen P, Alahuhta S, Jouppila R, et al. Does ephedrine influence newborn neurobehavioural responses and spectral EEG when used to prevent maternal hypotension during caesarean section? Acta Anaesthesiologica Scandinavica 1990;34:8-16. [DOI] [PubMed] [Google Scholar]
Karamanlioglu 1995 {published data only}
- Karamanlioglu B, Canogullari M, Arslan G, Alagol A, Sengonul O. The value of omeprazole and h2 receptor blockers in the prophylaxis of aspiration in cesarean operation [Sezaryen ameliyatlarinda omeprazol ve H2 reseptor blokerlerinin aspirasyon pnomonisi profilaksisindeki degeri]. Turk Anesteziyoloji Ve Reanimasyon 1995;23(3):338-42. [Google Scholar]
Karaoren 2019 {published data only}
- NCT03834259. The effects of keeping the patient in a sitting position for one minute after spinal anesthesia. Https://clinicaltrials.gov/show/nct03834259 2019 Feb 7. [CENTRAL: CN-01702416]
Karimi 2020 {published data only}
- Karimi A, Nejadi J, Shamseh M, Ronasi N, Birjandi M. Comparison of the effects of dexamethasone and ondansetron on the reduction of postoperative nausea and vomiting following cesarean section under spinal anesthesia. Current Clinical Pharmacology 2020;2 March:Epub ahead of print. [DOI: 10.2174/1574884715666200302122800] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kiasari 2017 {published data only}
- IRCT2016112631095N1. Clinical trial of comparison of intrathecal with intravenous Dexamethasone to reduce post operation complications after cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016112631095N1 2017. [CENTRAL: CN-01882270]
Kimura 2011 {published data only}
- Kimura M, Okamoto T, Sukagoshi H, Sato J, Saito S. Effect of flurbiprofen, metoclopramide and droperidol for nausea and emesis during cesarean section under spinal anesthesia. Journal of Anesthesia 2011;25(5):692-7. [DOI] [PubMed] [Google Scholar]
King 1998 {published data only}
- King SW, Rosen MA. Prophylactic ephedrine and hypotension associated with spinal anesthesia for cesarean delivery. International Journal of Obstetric Anesthesia 1998;7:18-22. [DOI] [PubMed] [Google Scholar]
Kita 2018 {published data only}
- JMA-IIA00398. The efficacy of epidural anesthesia for the prevention of intraoperative nausea and vomiting during elective cesarean section [The efficacy of epidural anesthesia for the prevention of intraoperative nausea and vomiting during elective cesarean section under combined epidural and spinal anesthesia]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JMA-IIA00398 2018. [CENTRAL: CN-01950126]
Kjaer 2006 {published data only}
- Kjaer K, Comerford M, Kondilis L, DiMaria L, Abramovitz S, Kiselev M, et al. Oral sodium citrate increases nausea amongst elective cesarean delivery patients. Canadian Journal of Anaesthesia 2006;53(8):776-80. [DOI] [PubMed] [Google Scholar]
Kocamanoglu 2005 {published data only}
- Kocamanoglu IS, Baris S, Karakaya D, Sener B, Tur A, Cetinkaya M. Effects of granisetron with droperidol or dexamethasone on prevention of postoperative nausea and vomiting after general anesthesia for cesarean section. Methods and Findings in Experimental and Clinical Pharmacology 2005;27(7):489-93. [DOI] [PubMed] [Google Scholar]
Kumar 2017 {published data only}
- CTRI/2017/10/009964. Effect of Ondansetron - a preventer of nausea and vomiting on change in blood pressure and heart rate after spinal anesthesia in patients undergoing cesarean section. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19014 (first received 3 October 2017). [CENTRAL: CN-01894769]
Lal 2018 {published data only}
- CTRI/2018/03/012692. A study to evaluate the effect of ondansetron on blood pressure in pregnant females undergoing caesarean delivery under spinal anaesthesia. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/03/012692 2018. [CENTRAL: CN-01901385]
Landa 2016 {published data only}
- Landa SE, Costa D, Markley J, Woglom A, Jiang Y, Hormozi L. Ketorolac prevents nausea and vomiting related to uterine exteriorization during cesarean section: a randomized, controlled double-blinded study. Anesthesia and Analgesia 2016;123(3 Suppl):S-187. [Google Scholar]
- Landa SE, Costa D, Markley J, Woglom A, Jiang Y, Hormozi L. Ketorolac prevents nausea and vomiting related to uterine exteriorization during cesarean section: a randomized, controlled double-blinded study. Anesthesia and Analgesia 2016;123(3 Suppl):S-187. [Google Scholar]
Lane 2012 {published data only}
- Lane B, Cannella K, Bowen C, Copelan D, Nteff G, Barnes K, et al. Examination of the effectiveness of peppermint aromatherapy on nausea in women post C-section. Journal of Holistic Nursing 2012;30(2):90-104. [DOI] [PubMed] [Google Scholar]
Lee 1992 {published data only}
- Lee YW, Lee JW, Yoon DM, Oh HK. A comparison of the analgesic and side effects of epidural morphine and nalbuphine-morphine mixture in post-cesarean section patients. Journal of the Korean Pain Society 1992;5(2):221-8. [Google Scholar]
Lee 2001 {published data only}
- Lee SM, Lee JU, Shin HB, Lee WH. A comparison of the analgesic and side effects of continuous epidural morphine and nalbuphine after a cesarean section. Korean Journal of Anesthesiology 2001;41(1):59-65. [Google Scholar]
Levin 2018 {published data only}
- Levin D, Cohen S, Mellender S, Mohiuddin A, Shah U, Zhao R, et al. Does the application of scopolamine patch with intra-operative acupressure point P6 stimulation increase parturients' intra-cesarean satisfaction? a prospective randomized clinical trial. Anesthesia and Analgesia 2018;126(4):409-10. [CENTRAL: CN-01787567] [EMBASE: 626089892] [Google Scholar]
Lim 1991 {published data only}
- Lim SK, Elegbe EO. The use of single dose of sodium citrate as a prophylaxis against acid aspiration syndrome in obstetric patients undergoing caesarean section. Medical Journal of Malaysia 1991;46(4):349-55. [PubMed] [Google Scholar]
Lin 1996 {published data only}
- Lin CJ, Huang CL, Hsu HW, Chen TL. Prophylaxis against acid aspiration in regional anesthesia for elective cesarean section: a comparison between oral single-dose ranitidine, famotidine and omeprazole assessed with fiberoptic gastric aspiration. Acta Anaesthesiologica Sinica 1996;34(4):179-84. [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu Y, Chen HX, Kang DL, Kuang XH, Liu WX, Ni J. Influence of dexmedetomidine on incidence of adverse reactions introduced by hemabate in postpartum hemorrhage during cesarean section. International Journal of Clinical and Experimental Medicine 2015;8(8):13776-82. [PMC free article] [PubMed] [Google Scholar]
Loughrey 2002 {published data only}
- Loughrey JP, Walsh F, Gardiner J. Prophylactic intravenous bolus ephedrine for elective caesarean section under spinal anaesthesia. European Journal of Anaesthesiology 2002;19(1):63-8. [DOI] [PubMed] [Google Scholar]
Madhumala 2019 {published data only}
- CTRI/2019/06/019923. Ondansetron (Anti - emetic) vs chewing gum for prevention of nausea and vomiting after caesarean delivery. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/06/019923 2019. [CENTRAL: CN-02065830]
Malekianzadeh 2012 {published data only}
- IRCT201111138090N1. Ondansetron effect in cesarean [Effect of intravenous ondansetron in preventing hypotension in elective cesarean patients under spinal anesthesia]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201111138090N1 2012. [CENTRAL: CN-01815470]
Manullang 2000 {published data only}
- Manullang TR, Viscomi CM, Pace NL. Intrathecal fentanyl is superior to intravenous ondansetron for the prevention of perioperative nausea during cesarean delivery with spinal anesthesia. Anesthesia and Analgesia 2000;90:1162-6. [DOI] [PubMed] [Google Scholar]
McCaughey 1981 {published data only}
- McCaughey W, Howe JP, Moore J, Dundee JW. Cimetidine in elective caesarean section. Effect on gastric acidity. Anaesthesia 1981;36:167-72. [DOI] [PubMed] [Google Scholar]
Mebazaa 2003 {published data only}
- Mebazaa MS, Meftah RB, Abassi M, Miraoui W, Bessa Z, Ammar MS. Post-operative nausea and vomiting after spinal anesthesia for cesarean section [abstract]. Anesthesiology 2003;99:A1201. [Google Scholar]
Memari 2015 {published data only}
- Memari F, Jadidi R, Noroozi A, Mohammadbeigi A, Falahati J. Protecting effect of gabapentin for nausea and vomiting in the surgery of cesarean after spinal anesthesia. Anesthesia, Essays and Researches 2015;9(3):401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mofrad 2019 {published data only}
- IRCT20191015045121N1. Effect of ondansetron in prevention of hypotension following spinal anesthesia in cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20191015045121N1 2019. [CENTRAL: CN-02069874]
Mokhtar 2016 {published data only}
- Mokhtar A. Ondansetron for post-spinal shivering [The role of ondansetron in prevention of post-spinal shivering (PSS) in the obstetric patient: a double blind randomized controlled trial]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201612001896411 2016. [CENTRAL: CN-01829410]
Murphy 1984 {published data only}
- Murphy DF, Nally B, Gardiner J, Unwin A. Effect of metoclopramide on gastric emptying before elective and emergency caesarean section. British Journal of Anaesthesia 1984;56:1113-6. [DOI] [PubMed] [Google Scholar]
Nado 2017 {published data only}
- Nado H, Singh NR, Laithangbam PK, Shanti Devi RK, Alemwapang O, Gangmei FL. A comparative study between propofol and propofol plus dexamethasone as antiemetic during cesarean section under spinal anesthesia. JMS - Journal of Medical Society 2017;31(3):174-7. [CENTRAL: CN-01404509] [EMBASE: 617897246] [Google Scholar]
Naja 2016 {published data only}
- NCT02793843. Ondansetron vs ondansetron plus dexamethasone for relieving intrathecal morphine side effects after C-section. Https://clinicaltrials.gov/show/NCT02793843 (first received 2016 Jun 3). [CENTRAL: CN-01558842]
Nallam 2017 {published data only}
- Nallam SR, Cherukuru K, Sateesh G. Efficacy of intravenous ondansetron for prevention of postspinal shivering during lower segment cesarean section: a double-blinded randomized trial. Anesthesia, Essays and Researches 2017;11(2):508-13. [CENTRAL: CN-02089477] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nantasupha 2016 {published data only}
- Nantasupha C, Ruengkhachorn I, Ruangvutilert P. Effect of conventional diet schedule, early feeding and early feeding plus domperidone on postcesarean diet tolerance: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2016;42(5):519-25. [DOI] [PubMed] [Google Scholar]
Ngan 2000 {published data only}
- Ngan Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesthesia and Analgesia 2000;90:1390-5. [DOI] [PubMed] [Google Scholar]
Ngan 2001 {published data only}
- Ngan Kee WD, Lau TK, Khaw KS, Lee BB. Comparison of metaraminol and ephedrine infusions for maintaining arterial pressure during spinal anesthesia for elective cesarean section. Anesthesiology 2001;95:307-13. [DOI] [PubMed] [Google Scholar]
Ngan 2004a {published data only}
- Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for caesarean section. British Journal of Anaesthesia 2004;92(4):469-74. [DOI] [PubMed] [Google Scholar]
Ngan 2004b {published data only}
- Ngan Kee WD, Khaw KS, Ng FF, Lee BB. Prophylactic phenylephedrine infusion for preventing hypotension during spinal anesthesia for cesarean delivery. Anesthesia and Analgesia 2004;98:815-21. [DOI] [PubMed] [Google Scholar]
Niaki 2016 {published data only}
- Niaki MT, Atarod Z, Omidvar S, Zafari M, Aghamohammadi A, Asadi T, et al. Comparing the effects of cumin, peppermint, and milk of magnesia on gastrointestinal complications after caesarean section. Global Journal of Health Science 2016;8(12):78-86. [Google Scholar]
Nivatpumin 2009 {published data only}
- NCT00892996. Efficacy of prevention for postoperative nausea and vomiting after intrathecal morphine in cesarean section. Https://clinicaltrials.gov/show/NCT00892996 (first received 2009 May 3). [CENTRAL: CN-01500726]
Nivatpumin 2016 {published data only}
- Nivatpumin P, Thamvittayakul V. Ephedrine versus ondansetron in the prevention of hypotension during cesarean delivery: a randomized, double-blind, placebo-controlled trial. International Journal of Obstetric Anesthesia 2016;27:25-31. [DOI] [PubMed] [Google Scholar]
Numazaki 2000 {published data only}
- Gibbs N. Editor's note: notice of retraction. Anaesthesia and Intensive Care 2013;41(2):275. [PubMed] [Google Scholar]
- Numazaki M, Fujii Y. Subhypnotic dose of propofol for the prevention of nausea and vomiting during spinal anaesthesia for caesarean section. Anaesthesia and Intensive Care 2000;28:262-5. [PubMed] [Google Scholar]
Numazaki 2003 {published data only}
- Editors. Retraction notice to "reduction of emetic symptoms during cesarean delivery with antiemetics: propofol at subhypnotic dose versus traditional antiemetics" (j clin anesth 2003;15:423-27). Journal of Clinical Anesthesia 2013;25(4):353. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Fujii Y. Reduction of emetic symptoms during cesarean delivery with antiemetics: propofol at subhypnotic dose versus traditional antiemetics. Journal of Clinical Anesthesia 2003;15:423-7. [DOI] [PubMed] [Google Scholar]
O'Sullivan 1985 {published data only}
- O'Sullivan G, Sear JW, Bullingham RE, Carrie LE. The effect of magnesium trisilicate mixture, metoclopramide and ranitidine on gastric ph, volume and serum gastrin. Anaesthesia 1985;40(3):246-53. [DOI] [PubMed] [Google Scholar]
O'Sullivan 1988 {published data only}
- O'Sullivan GM, Smith M, Morgan B, Brighouse D, Reynolds F. H2 antagonists and bupivacaine clearance. Anaesthesia 1988;43:93-5. [DOI] [PubMed] [Google Scholar]
Olsen 1994 {published data only}
- Olsen KS, Feilberg VL, Hansen CL, Rudkjobing O, Pedersen T, Kyst A. Prevention of hypotension during spinal anaesthesia for caesarean section. International Journal of Obstetric Anesthesia 1994;3:20-4. [DOI] [PubMed] [Google Scholar]
Ormezzano 1990 {published data only}
- Ormezzano X, Francois TP, Viaud JY, Bukowski JG, Bourgeonneau MC, Cottron D, et al. Aspiration pneumonitis prophylaxis in obstetric anaesthesia: comparison of effervescent cimetidine-sodium citrate mixture and sodium citrate. British Journal of Anaesthesia 1990;64:503-6. [DOI] [PubMed] [Google Scholar]
Orr 1993 {published data only}
- Orr DA, Bill KM, Gillon KR, Wilson CM, Fogarty DJ, Moore J. Effects of omeprazole, with and without metoclopramide, in elective obstetric anaesthesia. Anaesthesia 1993;48:114-9. [DOI] [PubMed] [Google Scholar]
Osman 1995 {published data only}
- Osman H. Ranitidine versus cimetidine prior to emergency obstetric anesthesia. Middle East Journal of Anesthesiology 1995;13(2):205-11. [PubMed] [Google Scholar]
Ostheimer 1982 {published data only}
- Ostheimer GW, Morrison JA, Lavoie C, Sepkoski C, Hoffman J, Datta S. The effect of cimetidine on the mother, newborn and neonatal neurobehavior. Anesthesiology 1982;57:A405. [Google Scholar]
Ouyang 2002 {published data only}
- Ouyang MW, Gu MN, Lin CS. Inhibiting effect of intrathecal fentanyl on intraoperative vomiting during cesarean delivery under epidural anesthesia. Journal of the First Military Medical University (Di Yi Junyi Daxue Xuebao) 2002;22(11):1037-8. [PubMed] [Google Scholar]
Owczarzak 1997 {published data only}
- Owczarzak D, Ghellar MR, Oliveira GR, Semeghini MM. Ondansetron and epidural morphine-induced pruritus and emesis after caesarean section: comparison with metoclopramide [abstract]. British Journal of Anaesthesia 1997;78 Suppl 1:110. [Google Scholar]
Ozkan 2000 {published data only}
- Ozkan T, Senturk M, Yavru A, Celebi S, Pembeci K. Does preoperative fluid fasting have a benefit on aspiration prophylaxis in obstetric anaesthesia. Turk Anesteziyoloji Ve Reanimasyon 2000;28:425-9. [Google Scholar]
Pakniat 2017 {published data only}
- IRCT201611028611N3. The effect of ginger versus metoclopramide in prevention of nausea and vomiting in patients undergoing cesarean section with spinal anaesthesia. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201611028611N3 2017. [CENTRAL: CN-01878702]
- Pakniat H, IRCT201611028611N3. The effect of ginger versus metoclopramid in prevention of nausea and vomiting in patients undergoing cesarean section with spinal anaesthesia. http://en.irct.ir/trial/9112 (31 January 2017).
Palmer 1991 {published data only}
- Palmer AW, Waugaman WR, Conklin KA, Kotelko DM. Does the administration of oral Bicitra before elective cesarean section affect the incidence of nausea and vomiting in the parturient? Nurse Anesthesia 1991;2(3):126-33. [PubMed] [Google Scholar]
Palmer 1995 {published data only}
- Palmer CM, Voulgaropoulos D, Alves D. Subarachnoid fentanyl augments lidocaine spinal anesthesia for cesarean delivery. Regional Anesthesia 1995;20(5):389-94. [PubMed] [Google Scholar]
Pecora 2009 {published data only}
- Pecora FS, Malbouisson LM, Torres ML. Supplemental oxygen and the incidence of perioperative nausea and vomiting in cesarean sections under subarachnoid block [Oxigenio suplmentar e incidencia de nauseas e vomitos perioperatorios no parto cesariano sob anestesia subaracnoidnea]. Revista Brasileira de Anestesiologia 2009;59(5):558-69. [DOI] [PubMed] [Google Scholar]
Peivandi 2019 {published data only}
- Peivandi S, Habibi MR, Baradari AG, Gholinataj A, Foladi F, Habibi A, et al. The effect of adding low-dose naloxone to intrathecal morphine on postoperative pain and morphine related side effects after cesarean section: a double-blind, randomized, clinical trial. Open Access Macedonian Journal of Medical Sciences 2019;7(23):3979-83. [CENTRAL: CN-02089499] [EMBASE: 2003849562] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellegrini 2001 {published data only}
- Pellegrini JE, Bailey SL, Graves J, Paice JA, Shott S, Faut-Callahan M. The impact of nalmefene on side effects due to intrathecal morphine at cesarean section. AANA Journal 2001;69(3):199-205. [PubMed] [Google Scholar]
Phillips 2007 {published data only}
- Phillips TW Jr, Broussard DM, Sumrall WD 3rd, Hart SR. Intraoperative oxygen administration does not reduce the incidence or severity of nausea or vomiting associated with neuraxial anesthesia for cesarean delivery. Anesthesia and Analgesia 2007;105(4):1113-7. [DOI] [PubMed] [Google Scholar]
- Phillips TW, Hart SR, Broussard D, Sumrall WD. Effects of supplemental oxygen administration on the incidence of nausea/vomiting during regional anesthesia for cesarean section [abstract]. Anesthesiology 2005;102(Suppl 1):28. [Google Scholar]
Pickering 1980 {published data only}
- Pickering BG, Palahniuk RJ, Cumming M. Cimetidine premedication in elective caesarean section. Canadian Anaesthetists Society Journal 1980;27:33-5. [DOI] [PubMed] [Google Scholar]
Pogodin 2012 {published data only}
- Pogodin A, Shifman E. The influence of oxygen inhalation on frequency of intraoperative nausea and vomiting in parturients undergoing caesarean section under spinal anaesthesia. Regional Anesthesia and Pain Medicine 2012;37(5 Suppl 1):E209-10. [Google Scholar]
Popivanov 2019 {published data only}
- NCT04191694. Chewing gum to prevent nausea and vomiting after caesarean section Under spinal anaesthesia. Https://clinicaltrials.gov/show/NCT04191694 2019 Dec 10. [CENTRAL: CN-02053022]
Prakash 2006 {published data only}
- Prakash S, Joshi N, Gogia AR, Prakash S, Singh R. Analgesic efficacy of two doses of intrathecal midazolam with bupivacaine in patients undergoing cesarean delivery. Regional Anesthesia and Pain Medicine 2006;31(3):221-6. [DOI] [PubMed] [Google Scholar]
Prakash 2019 {published data only}
- CTRI/2019/02/017489. Effect of ondansetron in decreasing occurrence of low blood pressure during caesarean operation in pregnant women with high blood pressure. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/02/017489 2019. [CENTRAL: CN-01972137]
Qvist 1983 {published data only}
- Qvist N, Storm K. Cimethidine pre-anesthetic - a prophylactic method against Mendelson's syndrome in cesarean section. Acta Obstetricia et Gynecologica Scandinavica 1983;62:157-9. [DOI] [PubMed] [Google Scholar]
Qvist 1985 {published data only}
- Qvist N, Storm K, Holmskov A. Cimetidine as pre-anesthetic agent for cesarean section: perinatal effects on the infant, the placental transfer of cimetidine and its elimination in the infants. Journal of Perinatal Medicine 1985;13:179-83. [DOI] [PubMed] [Google Scholar]
Rahman 2018 {published data only}
- NCT03434340. Aromatherapy for prevention of intrathecal morphine induced nausea and vomiting. Https://clinicaltrials.gov/show/NCT03434340 (first received 2018 Jan 24). [CENTRAL: CN-01523006]
- Rahman RA, NCT03434340. Aromatherapy as prophylaxis for prevention of intrathecal morphine induced nausea and vomiting in lower segment cesarean section. https://clinicaltrials.gov/ct2/show/record/NCT03434340 (15 February 2018).
Ramanathan 1983 {published data only}
- Ramanathan S, Masih A, Rock I, Chalon J, Turndorf H. Maternal and fetal effects of prophylactic hydration with crystalloids or colloids before epidural anesthesia. Anesthesia and Analgesia 1983;62:673-8. [PubMed] [Google Scholar]
Ramin 1994 {published data only}
- Ramin SM, Ramin KD, Cox K, Magness RR, Shearer VE, Grant NF. Comparison of prophylactic angiotensin II versus ephedrine infusion for prevention of maternal hypotension during spinal anesthesia. American Journal of Obstetrics and Gynecology 1994;171:734-9. [DOI] [PubMed] [Google Scholar]
Rasooli 2019 {published data only}
- IRCT2013042210765N2. Comparison of metoclopramide and ondansetrone with prophylactic phenylephrine infusion effects on management of nausea and vomiting associated with spinal anesthesia for cesarean section. http://en.irct.ir/trial/11174 (14 January 2014). [CENTRAL: CN-01866833]
- Rasooli S, Moslemi F, Gogazadeh M. Preventing nausea and vomiting using ondansetron and metoclopramide-phenylephrine in cesarean section using spinal anesthesia. Crescent Journal of Medical and Biological Sciences 2019;6(1):61-5. [CENTRAL: CN-01791378] [EMBASE: 626206102] [Google Scholar]
Ravindranathan 2018 {published data only}
- CTRI/2018/05/013610. Combination of three commonly used anesthetic drugs -ranitidine, metoclopramide and propofol given in different combinations to prevent nausea and vomiting during caesarian section in pregnancy. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/05/013610 2018. [CENTRAL: CN-01895891]
Razanejadi 2018 {published data only}
- Razanejadi. The effect of dexamethasone and endonestrone premedication on the incidence of nausea and vomiting after cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180210038681N1 2018.
Rocke 1994 {published data only}
- Rocke DA, Rout CC, Gouws E. Intravenous administration of the proton pump inhibitor omeprazole reduces the risk of acid aspiration at emergency cesarean section. Anesthesia and Analgesia 1994;78:1093-8. [DOI] [PubMed] [Google Scholar]
Rout 1992 {published data only}
- Rout CC, Rocke DA, Brijball R, Koovarjee RV. Prophylactic intramuscular ephedrine prior to caesarean section. Anaesthesia and Intensive Care 1992;20:448-52. [DOI] [PubMed] [Google Scholar]
Rout 1993 {published data only}
- Rout CC, Rocke DA, Gouws E. Intravenous ranitidine reduces the risk of acid aspiration of gastric contents at emergency cesarean section. Anesthesia and Analgesia 1993;76:156-61. [DOI] [PubMed] [Google Scholar]
Rudra 2004b {published data only}
- Rudra P, Rudra A. Comparison of intrathecal fentanyl and midazolam for prevention of nausea-vomiting during caesarean delivery under spinal anaesthesia. Indian Journal of Anaesthesia 2004;48(6):461-4. [CENTRAL: CN-01094191] [Google Scholar]
Sadeh 2019 {published data only}
- IRCT20190409043219N2. Determination the effect of low dose of dexamethasone on prevention of post operative nausea and vomiting after elective cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190409043219N2 2019. [CENTRAL: CN-02069440]
Saem 2017 {published data only}
- IRCT20170417033491N2. Study the effect of Bupivacaine 0.5%, Lidocaine 5%, Pethidine, Lidocaine 5% & intratecal on the incidence of decrease blood pressure, nausea and vomiting for elective caesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20170417033491N2 2018. [CENTRAL: CN-01906003]
Sahare 2013 {published data only}
- Sahare KK, Naik R, Agrawal S. Intravenous ondansetron granisetron and ramosetron for prevention of postoperative nausea and vomiting in patients undergoing LSCS a comparative study. In: Anesthesia and Analgesia. Vol. 116. Lippincott Williams and Wilkins, 2013. [CENTRAL: CN-01062680] [EMBASE: 71325753]
Sane 2015 {published data only}
- Sane S, Hasanlui MV, Abbasivash R, Mahoori A, Hashemi ST, Rafiei F. Comparing the effect of intravenous dexamethasone, intravenous ondansetron, and their combination on nausea and vomiting in cesarean section with spinal anesthesia. Advanced Biomedical Research 2015;4:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sane 2016 {published data only}
- IRCT2016073027677N2. Clinical trial effect of midazolam and ondansetron on nausea and vomiting in parturient under cesarean with spinal anesthesia. http://en.irct.ir/trial/22619 (19 October 2016). [CENTRAL: CN-01845722]
Sane 2017a {published data only}
- IRCT2017041527677N7. Clinical trial the effect of intravenous ondansetron and sub-hypnotic propofol dose on pruritus after intrathecal opioid in patient with elective cesarean surgery. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017041527677N7; https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017041527677N7 2017. [CENTRAL: CN-01886941]
Santos 1984 {published data only}
- Santos A, Datta S. Prophylactic use of droperidol for control of nausea and vomiting during spinal anesthesia for cesarean section. Anesthesia and Analgesia 1984;63:85-7. [PubMed] [Google Scholar]
Seidy 2010 {published data only}
- Seidy J, Farhadifar F, Ghadami N, Zandvakili F, Roshani D, Taifoori L, et al. [Effect of supplemental oxygen on the incidence and severity of nausea and vomiting in the patients after cesarean surgery under spinal anesthesia]. Scientific Journal of Kurdistan University of Medical Sciences 2010;15(2):26-35. [Google Scholar]
Sen 2001 {published data only}
- Sen A, Rudra A, Sarkar SK, Biswas B. Intrathecal midazolam for postoperative pain relief in caesarean section delivery. Journal of the Indian Medical Association 2001;99(12):683-4, 686. [PubMed] [Google Scholar]
Seyedhejazi 2007 {published data only}
- Seyedhejazi M, Madarek E. The effect of small dose bupivacaine-fentanyl in spinal anesthesia on hemodynamic nausea and vomiting in cesarean section. Pakistan Journal of Medical Sciences 2007;23(5):747-50. [Google Scholar]
Shafaeiyan 2018 {published data only}
- Shafaeiyan M, Ghods F, Rahbar M, Daneshi Z, Sadati L, Mashak B, et al. The effect of warm intravenous fluid on postoperative pain: a double-blind clinical trial. Preventive Care in Nursing & Midwifery Journal 2018;8(4):61-22. [Google Scholar]
- Torkmandi H, IRCT2017020232361N1. Comparison effect of warm serum and hot air radiant on prevention of Headache - nausea and vomiting after cesarean surgery. http://en.irct.ir/trial/25240 (27 June 2017).
Shafeinia 2020 {published data only}
- IRCT20191007045023N1. The effect of phenylephrine on blood pressure and nausea and vomiting in pregnant women. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20191007045023N1 2019. [CENTRAL: CN-02069845]
- Shafeinia A, Ghaed MA, Nikoubakht N. The effect of phenylephrine infusion on maternal hemodynamic changes during spinal anesthesia for cesarean delivery. Anesthetic Pain Medicine 2020;10(1):e99094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahriari 2009 {published data only}
- Shahriari A, Khooshideh M, Heidari MH. Prevention of nausea and vomiting in caesarean section under spinal anaesthesia with midazolam or metoclopramide? JPMA - Journal of the Pakistan Medical Association 2009;59(11):756-9. [PubMed] [Google Scholar]
Shaikh 2015 {published data only}
- Shaikh SI, Govindaraju C, Hegade G. Comparison of intrathecal fentanyl and midazolam for prevention of nausea-vomiting during cesarean section under spinal anesthesia. Anaesthesia, Pain and Intensive Care 2015;19(2):124-9. [Google Scholar]
Shende 1998 {published data only}
- Shende D, Cooper GM, Bowden MI. The influence of intrathecal fentanyl on the characteristics of subarachnoid block for caesarean section. Anaesthesia 1998;53(7):706-10. [CENTRAL: CN-00155609] [EMBASE: 1998248556] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shifman 2010 {published data only}
- Shifman E, Pogodin A. A comparison of different doses of dexamethasone for prevention of intraoperative nausea and vomiting during spinal anaesthesia for caesarean section. Regional Anesthesia and Pain Medicine 2010;35(3):E175. [Google Scholar]
Shin 2019 {published data only}
- KCT0002424. Comparison of intravenous midazolam with fentanyl for sedative effect and nausea & vomiting for elective cesarean section under spinal anesthesia. http://cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=11034 (6 April 2016). [CENTRAL: CN-01894486]
- Shin DW, Kim Y, Hong B, Yoon S-H, Lim CS, Youn S. Effect of fentanyl on nausea and vomiting in cesarean section under spinal anesthesia: a randomized controlled study. Journal of International Medical Research 2019 Aug;47(10):4798-807. [CENTRAL: CN-01990639] [EMBASE: 629176824] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddik‐Sayyid 2002 {published data only}
- Siddik-Sayyid SM, Aouad MT, Jalbout MI, Zalaket MI, Berzina CE, Baraka AS. Intrathecal versus intravenous fentanyl for supplementation of subarachnoid block during cesarean section. Anesthesia and Analgesia 2002;95:209-13. [DOI] [PubMed] [Google Scholar]
Singh 2018 {published data only}
- CTRI/2018/12/016756. A clinical trial to study the effectiveness of a drug Granisetron to prevent shivering, nausea and vomiting, which occurs after giving spinal anaesthesia in pregnant females undergoing cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/12/016756 2018. [CENTRAL: CN-01948406]
Stuart 1996 {published data only}
- Gin T, Stuart JC, Kan AF, Rowbottom SJ, Yau G. IV ranitidine or omeprazole for emergency caesarean section. Anaesthesia and Intensive Care 1994;22:483. [Google Scholar]
- Stuart JC, Kan AF, Rowbottom SJ, Yau G, Gin T. Acid aspiration prophylaxis for emergency caesarean section. Anaesthesia 1996;51(5):415-21. [DOI] [PubMed] [Google Scholar]
Sultan 2014 {published data only}
- Sultan P, Elkomy M, Peltz G, Clavijo C, Wu M, Galinkin JL, et al. Pharmacokinetics of ondansetron in non-pregnant and pregnant women. International Journal of Obstetric Anesthesia 2014;23(Suppl 1):S9. [Google Scholar]
Sutanto 2013 {published data only}
- Sutanto LB, Bardosono S, Aziz MF, Madjid A, Purba JS. Oral nutritional supplements before surgery reduced the post-operative metabolic stress factors in cesarean section. Clinical Nutrition (Edinburgh, Scotland) 2013;32(Suppl 1):S124. [CENTRAL: CN-01073911] [EMBASE: 71797737] [Google Scholar]
Swaro 2018 {published data only}
- Swaro S, Karan D, Banerjee A. Comparison of palonosetron, dexamethasone, and palonosetron plus dexamethasone as prophylactic antiemetic and antipruritic drug in patients receiving intrathecal morphine for lower segment cesarean section. Anesthesia, Essays and Researches 2018;12(2):322-7. [CENTRAL: CN-02089476] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2007 {published data only}
- Tanaka M, Balki M, Parkes R, Carvalho J. ED95 of phenylephrine to prevent hypotension and nausea/vomiting after spinal anesthesia for cesarean section [abstract]. Anesthesiology 2007;106(Suppl 1):55. [Google Scholar]
Taylor 1966 {published data only}
- Taylor G, Pryse-Davies J. The prophylactic use of antacids in the prevention of the acid-pulmonary-aspiration syndrome (Mendelson's syndrome). Lancet 1966;1:288-91. [DOI] [PubMed] [Google Scholar]
Tekyeh 2013 {published data only}
- Tekyeh SM, Tabari M, Jahanian V. Investigation the effects and side effects of different dosage of bupivacaine in combination with sufentanil for spinal anesthesia in caesarean section. [Persian]. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;16(66):1-9. [Google Scholar]
Terui 2014 {published data only}
- UMIN000014020. Prevention study of the postoperative nausea and vomiting by intraoperative intravenous acetaminophen in patients during cesarean section in regional anesthesia. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000016324 (21 May 2014). [CENTRAL: CN-01826909]
Tettambel 1983 {published data only}
- Tettambel MA. Preoperative use of antacids to prevent Mendelson's syndrome in cesarean section: a pilot study. Journal of the American Osteopathic Association 1983;82:858-60. [PubMed] [Google Scholar]
Tianthong 2018 {published data only}
- Tianthong W, Phupong V. A randomized, double-blind, placebo-controlled trial on the efficacy of ginger in the prevention of abdominal distention in post cesarean section patients. Scientific Reports 2018;8(1):6835. [CENTRAL: CN-01987827] [EMBASE: 629340476] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trabelsi 2015 {published data only}
- ACTRN12613000036718. The effect of ondansetron on hypotension due to spinal anesthesia for caesarean section. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613000036718 2013. [CENTRAL: CN-01836126]
- Trabelsi W, Romdhani C, Elaskri H, Sammoud W, Bensalah M, Labbene I, et al. Effect of ondansetron on the occurrence of hypotension and on neonatal parameters during spinal anesthesia for elective caesarean section: a prospective, randomized, controlled, double-blind study. Anesthesiology Research and Practice 2015;2015:Article ID 158061. [DOI: 10.1155/2015/158061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripathi 1995 {published data only}
- Tripathi A, Somwanshi M, Singh B, Bajaj P. A comparison of intravenous ranitidine and omeprazole on gastric volume and ph in women undergoing emergency caesarean section [see comments]. Canadian Journal of Anaesthesia 1995;42(9):797-800. [DOI] [PubMed] [Google Scholar]
Tryba 1983 {published data only}
- Tryba M, Burkert W, Husch M, Zenz M. Effectiveness of cimetidine in the prevention of aspiration pneumonia in obstetrics [Wirksamkeit von Cimetidin zur Prophylaxe der Aspirationspneumonie in der Geburtshilfe]. Fortschritte der Medizin 1983;101:1757-61. [PubMed] [Google Scholar]
Tshibangu 2010 {published data only}
- Tshibangu NA, Motte Neuville F, Gepts E, Bailly A, Nguyen T, Hirsoux L. Impact of intrathecal morphine on the tolerance of early feeding after cesarean section. Annales Francaises d'Anesthesie et de Reanimation 2010;29(2):113-6. [DOI] [PubMed] [Google Scholar]
Ünlügenç 2016 {published data only}
- NCT02928601. Norepinephrine consumption after prophylactic ondansetron. Https://clinicaltrials.gov/show/NCT02928601 2016 Oct 10. [CENTRAL: CN-02037565]
Varshney 2019 {published data only}
- Varshney RK, Garg M, Kapoor K, Jheetay GS. The role of ramosetron in the prevention of post-spinal shivering in obstetric patients. A prospective randomized double blind study. Romanian Journal of Anaesthesia and Intensive Care 2019;26(1):37-43. [CENTRAL: CN-01959322] [EMBASE: 628323044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vercauteren 2000 {published data only}
- Vercauteren MP, Coppejans HC, Hoffmann VH, Mertens E, Adriaensen HA. Prevention of hypotension by a single 5-mg dose of ephedrine during small-dose spinal anesthesia in prehydrated cesarean delivery patients [see comments]. Anesthesia and Analgesia 2000;90:324-7. [DOI] [PubMed] [Google Scholar]
Viney 2012 {published data only}
- NCT01479712. Intra-abdominal at cesarean section: a randomized controlled trial. Https://clinicaltrials.gov/show/NCT01479712 2011 Nov 22. [CENTRAL: CN-02018754]
- Viney R, Isaacs C, Chelmow D. Intra-abdominal irrigation at cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2012;119(6):1106-11. [DOI] [PubMed] [Google Scholar]
von Braun 1994 {published data only}
- Braun GG, Koppert W, Martus P, Schywalsky M, Schmitt H, Mogendorf F, et al. Drug induced prevention of the acid aspiration syndrome in non-elective cesarean section. Anaesthesiologie und Reanimation 1994;19:37-42. [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang J, ChiCTR-IPR-14005749. A randomised double-blind trial combining low-dose droperidol and dexamethasone to prevent gastrointestinal adverse interaction induced by hemabate during cesarean delivery. http://www.chictr.org.cn/showproj.aspx?proj=10171 (27 December 2014).
Wani 2015 {published data only}
- Wani SA, Chadha M, Prakash A, Aggarwal S. A comparison of ondansetron and P6 point acupuncture stimulation in prevention of carboprost induced nausea and vomiting in patients undergoing cesarean section under subarachnoid block. Anaesthesia, Pain and Intensive Care 2015;19(1):24-7. [CENTRAL: CN-01083864] [EMBASE: 604798011] [Google Scholar]
Wig 1987 {published data only}
- Wig J, Biswas GC, Malhotra SK, Gupta AN. Comparison of sodium citrate with magnesium trisilicate as pre-anaesthetic antacid in emergency caesarean sections. Indian Journal of Medical Research 1987;85:306-10. [PubMed] [Google Scholar]
Xu 2018 {published data only}
- NCT03507387. Preventive intramuscular phenylephrine versus placebo in elective cesarean section under spinal anesthesia. Https://clinicaltrials.gov/show/NCT03507387 (first received 2018 Apr 7). [CENTRAL: CN-01568343]
Yang 2018 {published data only}
- ChiCTR1800016643. Effect of preoperative 50ml water on maternal satisfaction and postoperative nausea and vomiting. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800016643 2018. [CENTRAL: CN-01908982]
Yau 1992 {published data only}
- Yau G, Kan AF, Gin T, Oh TE. A comparison of omeprazole and ranitidine for prophylaxis against aspiration pneumonitis in emergency caesarean section. Anaesthesia 1992;47:101-4. [DOI] [PubMed] [Google Scholar]
Yazdi 2015 {published data only}
- Yazdi K, IRCT2014080218649N1. Comparison the effect of oxygen therapy with face mask and nasal cannula on blood oxygen saturation, nausea, vomiting and patient comfort in patients undergoing spinal anesthesia during caesarean section at Shohada hospital in Gonbad. http://en.irct.ir/trial/16846 (14 April 2015).
Zabetian 2014 {published data only}
- IRCT2014031717039N1. Comparison of Midazolam and Propofol on nausea and vomiting induced during cesarean section with spinal anesthesia. http://en.irct.ir/trial/15774 (30 July 2014). [CENTRAL: CN-01803823]
Zarief 2018 {published data only}
- NCT03387956d. Intravenous dexamethasone combined with intrathecal atropine to prevent morphine-related nausea and vomiting after cesarean delivery: a randomized double-blinded study. Https://clinicaltrials.gov/show/NCT03387956 (January 2, 2018). [CENTRAL: CN-01567125]
Zoroglu 1999 {published data only}
- Zoroglu F, Karamanlioglu B, Pamukcu Z. The value of nizatidine and famotidine in the prophylaxis of acid aspiration pneumonia in cesarean operation [Elektif sezaryen ameliyatlarinda nizatidin ve famotidinin asit aspirasyon pnomonisi profilaksisindeki degeri]. Turk Anesteziyoloji Ve Reanimasyon 2000;28(10):521-6. [Google Scholar]
- Zoroglu F, Karamanlioglu B, Unal C, Pamukcu Z. The value of nizatidine and famotidine in prophylaxis of aspiration pneumonia in caesarean section. British Journal of Anaesthesia 1999;82 Suppl:159-60. [Google Scholar]
Zue 1999 {published data only}
- Zue AS, Meye JF, Nguema PN, Nsafu DN, Milama EN. Effervescent ranitidine before urgent caesarean section: effect on gastric pH [Ranitidine effervescente avant cesarienne en urgence: effet sur le pH gastrique]. Cahiers d'Anesthesiologie 1999;47:1-3. [Google Scholar]
References to studies awaiting assessment
Ahn 2017 {published data only}
- Ahn NY, Park HJ. Effects of Korean hand acupressure on opioid-related nausea and vomiting, and pain after caesarean delivery using spinal anaesthesia. Complementary Therapies in Clinical Practice 2017;28:101-7. [CENTRAL: CN-01615610] [PMID: ] [DOI] [PubMed] [Google Scholar]
Biswas 2002 {published data only}
- Biswas BN, Rudra A, Nath S. Comparison between intravenous metoclopramide and intrathecal fentanyl to prevent intraoperative and early postoperative nausea and vomiting in patients undergoing caesarean delivery under spinal anaesthesia. Journal of Anaesthesiology, Clinical Pharmacology 2002;18(1):47-51. [CENTRAL: CN-01750529] [EMBASE: 40385205] [Google Scholar]
Gamermann 2015 {published data only}
- Gamermann PW, Martins AL, Rosa L, Ribeiro HD, Borba DL, Antoniazi V, et al. Acupuncture as a complement to the pharmacological management of pain, nausea and vomiting after cesarean section: a randomized clinical trial. Acupuncture and Related Therapies 2015;3:11-4. [Google Scholar]
Litchfield 2007 {published data only}
- Litchfield JS, Pronk CR, Nezat GG, Pellegrini J. Efficacy of prophylactic administration of intramuscular promethazine in a parturient population scheduled for cesarean section with intrathecal analgesia. AANA Journal 2007;75(5):369. [Google Scholar]
Malhotra 2019 {published data only}
- Malhotra G, Suresh YV, Anupama Suresh Y, Osmani SG, Prabhu K. Comparison prophylactic glycopyrrolate, dexamethasone, metoclopramide in control of nausea and vomiting after spinal anaesthesia for caesarean delivery. Indian Journal of Public Health Research and Development 2019;10(7):261-6. [CENTRAL: CN-01997800] [EMBASE: 2002831996] [Google Scholar]
Salman 2016 {published data only}
- Salman N, Sekerci S, Akarsu S, Olgun Keles B, Zagpusat E. Comparison of anti-emetic effects of ketamine and propofol sedation used for cesarean deliveries under spinal anesthesia [Spinal anestezi altinda yapilan sezaryenlerde ketamin ve propofol sedasyonunun bulanti ve kusmayi onleyici etkilerinin karsilastirilmasi]. Anestezi Dergisi 2016;24(4):257-62. [CENTRAL: CN-01297271] [EMBASE: 614030565] [Google Scholar]
Sane 2017 {published data only}
- Sane S, Mahoori A, Vash RA, Rezai H, Fazlifard S. Effects of granisetron on pruritus, nausea, and vomiting induced by intrathecal opioid in cesarean section under spinal anesthesia. Journal of Mazandaran University of Medical Sciences 2017;27(147):150-8. [CENTRAL: CN-01365134] [EMBASE: 615440912] [Google Scholar]
Sarat 2007 {published data only}
- Sarat Singh S, Upendra Singh K, Ratan Singh N, Rajkumar G, Maniram Singh Kh, Deban Singh L. Prevention of nausea and vomiting in caesarean delivery under spinal anaesthesia with glycopyrrolate, metoclopramide and ondansetron - a comparative study. JMS - Journal of Medical Society 2007;21(3):138-42. [Google Scholar]
Shah 2016 {published data only}
- Shah S, Zhao R, Cohen S, Patel P, Mellender S. Is bilateral nei-guan point (P6) stimulation more effective than unilateral P6 stimulation in reducing nausea and vomiting (N/V) during and after cesarean section (C/S) with combined spinal epidural (CSE) anesthesia? Regional Anesthesia and Pain Medicine 2016;41(5):no pagination. [CENTRAL: CN-01333663] [EMBASE: 614572270] [Google Scholar]
Zakeri 2014 {published data only}
- IRCT2014102719145N2. Effect of intrathecal midazolam, pethidine and bupivacaine on analgesia, hemodynamic status and nausea and vomiting after elective cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014102719145N2 2014. [CENTRAL: CN-01864627]
References to ongoing studies
Abramovitz 2007 {published data only}
- Abramovitz S, NCT00432991. A double-blind, placebo-controlled study to evaluate the effect of intramuscular ephedrine on the incidence of perioperative nausea and vomiting during elective cesarean section. https://clinicaltrials.gov/ct2/show/results/NCT00432991 (9 February 2007).
Amini 2019 {published data only}
- IRCT20190127042519N1. Effect of acupressure on prevention of nausea and vomiting [Comparison of the effect of acupressure and ondansetron on prevention of nausea and vomiting among patients undergoing cesarean section under spinal anesthesia]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190127042519N1 2019. [CENTRAL: CN-01975533]
An 2016 {published data only}
- ChiCTR-INR-16009539. The antiemetic efficacy and safety of subhypnotic dose of propofol for decreasing the incidence of intraoperative nausea and vomiting during cesarean section. http://www.chictr.org.cn/showproj.aspx?proj=16238 (21 October 2016). [CENTRAL: CN-01848685]
Barzanji 2019 {published data only}
- Barzanji. Vitamin B6 in elective cesarean section [Assessment of pyridoxine (vitamin B6) effects on the nausea and vomiting rates in patients candidate for elective cesarean section]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20171216037910N1 2019.
Bi 2017 {published data only}
- ChiCTR-IOR-17010491. Effect of propofol for prevention of post-delivery nausea and vomiting during elective cesarean delivery under spinal anesthesia. http://www.chictr.org.cn/showproj.aspx?proj=17452 (20 January 2017). [CENTRAL: CN-01817011]
- NCT03185156. The preventive effects of sub hypnotic dose of propofol for nausea and vomiting induced by hemabate. Https://clinicaltrials.gov/show/nct03185156 (first received 2017 Jun 10). [CENTRAL: CN-01494926]
Cohen 2016b {published data only}
- NCT02959840. Acupuncture point P6 stimulation for reduction of nausea and vomiting during cesarean. https://clinicaltrials.gov/ct2/show/results/NCT02959840 (first received 2016 Oct 10). [CENTRAL: CN-01559899]
Fanelli 2009 {published data only}
- NCT00921102. Atropine to prevent nausea and vomiting after spinal anesthesia for caesarean section. Https://clinicaltrials.gov/show/NCT00921102 (first received 2009 Jun 15). [CENTRAL: CN-01524382]
Farokhi 2016 {published data only}
- IRCT2016091320258N10. Comparison of dexamethasone- ketamine and dexmedetomidine for prevention of postoperative nausea and vomiting during and after cesarean section under spinal anesthesia. http://en.irct.ir/trial/17949 (8 November 2016). [CENTRAL: CN-01854890]
Gazi 2018 {published data only}
- IRCT20150808023559N18. Comparison of the effect of low-dose Ketamine and dexamethasone on the control of nausea and vomiting. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20150808023559N18 2018. [CENTRAL: CN-01904180]
Hao 2019 {published data only}
- ChiCTR1900026709. A randomized controlled study for acupoint stimulation using subacupuncture to relieve postoperative nausea and vomiting in patients undergoing gynecological and obstetrical surgery. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900026709 2019. [CENTRAL: CN-02065256]
Hess 2017 {published data only}
- Hess PE, NCT03370562. Dexmedetomidine after cesarean for the treatment of nausea and shivering. Https://clinicaltrials.gov/show/NCT03370562 (first received 2017 Nov 27). [CENTRAL: CN-01566616]
Hosseini 2020 {published data only}
- IRCT20141222020401N7. The effect of chamomile aromatherapy with and without oxygen on severity of pain, bloating and nausea in women after cesarean section with spinal anesthesia. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20141222020401N7 2020. [CENTRAL: CN-02069056]
Jamilian 2014 {published data only}
- IRCT201401014686N10. Comparison the effect of oral gabapentin and oral ondansetron and oral ginger to prevention nausea and vomiting after cesarean section by spinal anesthesia. http://en.irct.ir/trial/5023 (3 January 2014). [CENTRAL: CN-01858416]
Khatiban 2017 {published data only}
- IRCT2017050333794N1. Cardamom aromatherapy on the women's nausea and vomiting in cesarean section operation. http://en.irct.ir/trial/25980 (5 May 2017). [CENTRAL: CN-01893493]
Khojasteh 2016 {published data only}
- IRCT2016072729103N1. The effect of metoclopramide, propofol and dexamethasone in controlling nausea and vomiting for cesarean section. http://en.irct.ir/trial/23477 (18 September 2016). [CENTRAL: CN-01862986]
Kotfis 2019 {published data only}
- NCT04069806. Preoperative oral carbohydrate for nausea and vomiting prevention during caesarian section. Https://clinicaltrials.gov/show/nct04069806 2019 Aug 27. [CENTRAL: CN-01983790]
Mendonca 2015 {published data only}
- Mendonca FT, NCT02468323. Prophylactic antiemetic efficacy of palonosetron versus ondansetron for cesarean sections under regional anesthesia: study single center, prospective, double-blind, randomized, placebo controlled. https://clinicaltrials.gov/ct2/show/record/NCT02468323 (10 June 2015).
Mousavi 2019 {published data only}
- IRCT20180526039845N1. Effect of auriculotherapy on nausea and vomiting in women following elective cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180526039845N1 2019. [CENTRAL: CN-01971333]
Norouzi 2013 {published data only}
- IRCT201212162080N13. Comparison of oral and intravenous ondansetron in the prevention of postoperative nausea and vomiting in cesarean section with spinal anesthesia. http://en.irct.ir/trial/1687 (9 June 2013). [CENTRAL: CN-01816126]
Norouzi 2014 {published data only}
- IRCT2013031211581N3. The effect of Gabapentin on preventing the nausea and vomiting of patients after spinal anesthesia in the surgery of cesarean. http://en.irct.ir/trial/11792 (27 September 2014). [CENTRAL: CN-01834488]
- Norouzi A, IRCT2013031211581N3. Gabapentin effect on preventing of nausea and vomiting in cesarean. http://en.irct.ir/trial/11792 (27 September 2014).
Okutani 2012 {published data only}
- Okutani R, UMIN000008443. Effect of oral fluid infusion before cesarean section on intraoperative metabolism, hemodynamic changes and postoperative nausea and vomiting. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000009906 (20 July 2012).
- UMIN000008443. Effect of oral fluid infusion before cesarean section on intraoperative metabolism, hemodynamic changes and postoperative nausea and vomiting. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000009906 (20 July 2012). [CENTRAL: CN-01820120]
Rassoli 2013 {published data only}
- IRCT2012120810765N1. Effect of low dose propofol and midazolam to prevent nausea and vomiting during caesarean section. http://en.irct.ir/trial/11173 (6 August 2013). [CENTRAL: CN-01857701]
- Rassoli S, IRCT2012120810765N1. Effect of low dose propofol and midazolam to prevent nausea and vomiting during caesarean section. http://en.irct.ir/trial/11173 (6 August 2013).
Shahinfar 2016 {published data only}
- IRCT2016042719359N3. The effect of ginger extract on the incidence and severity of nausea and vomiting [The effect of ginger extract on the incidence and severity of nausea and vomiting after cesarean section under spinal anesthesia]. http://en.irct.ir/trial/17323 (13 May 2016). [CENTRAL: CN-01867863]
Soltani 2014 {published data only}
- IRCT2014062918269N1. Effect of capsaicin ointment on nausea and vomiting. http://en.irct.ir/trial/16625 (1 January 2014). [CENTRAL: CN-01839979]
Thenuwara 2017 {published data only}
- NCT02872935. Minimizing nausea and vomiting during spinals for CS. Https://clinicaltrials.gov/show/NCT02872935 (first received 2016 Aug 5). [CENTRAL: CN-01520310]
Tindimwebwa 2009 {published data only}
- NCT01028547. Antiemetic efficacy and safety of dexamethasone in obstetric surgical patients. Https://clinicaltrials.gov/show/NCT01028547 (first received 2009 Dec 7). [CENTRAL: CN-01527137]
Yulin 2019 {published data only}
- ChiCTR1900021396. A randomized controlled trial for western medicine combined with traditional Chinese medicine in the treatment of nausea and vomiting during and after cesarean section. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900021396 2019. [CENTRAL: CN-01972854]
Additional references
Allen 2008
- Allen TK, Habib AS. P6 stimulation for the prevention of nausea and vomiting associated with cesarean delivery under neuraxial anesthesia: a systematic review of randomized controlled trials. Anesthesia & Analgesia 2008;107(4):1308-12. [DOI] [PubMed] [Google Scholar]
Balki 2005
- Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. International Journal of Obstetric Anesthesia 2005;14:230-41. [DOI] [PubMed] [Google Scholar]
Boerma 2018
- Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet 2018;392:1341-8. [DOI] [PubMed] [Google Scholar]
Bragg 2010
- Bragg F, Cromwell DA, Edozien LC, Gurol-Urganci I, Mahmood TA, Templeton A, et al. Variation in rates of caesarean section among English NHS trusts after accounting for maternal and clinical risk: cross sectional study. BMJ 2010;341:5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlisle 2012
- Carlisle, JB. The analysis of 168 randomised controlled trials to test data integrity. Anaesthesia 2012;67:512-37. [DOI: 10.1111/j.1365-2044.2012.07128.x] [DOI] [PubMed] [Google Scholar]
Chen 2018
- Chen I, Opiyo N, Tavender E, Mortazhejri S, Rader T, Petkovic J, et al. Non-clinical interventions for reducing unnecessary caesarean section. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD005528. [DOI: 10.1002/14651858.CD005528.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chooi 2017
- Chooi C, Cox JJ, Lumb RS, Middleton P, Chemali M, Emmett RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD002251. [DOI: 10.1002/14651858.CD002251.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooke 1979
- Cooke RD, Comyn DJ, Ball RW. Prevention of postoperative nausea and vomiting by domperidone: a double-blind randomized study using domperidone, metoclopramide and a placebo. South African Medical Journal 1979;56(21):827-9. [PubMed] [Google Scholar]
De Groot 1998
- De Groot AN, Van Dongen PW, Vree TB, Hekster YA, Van Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998;54(4):523-35. [DOI] [PubMed] [Google Scholar]
Flake 2004
- Flake ZA, Scalley RD, Bailey AG. Practical selection of antiemetics. American Family Physician 2004;69(5):1169-74. [PubMed] [Google Scholar]
Gan 2003
- Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesthesia and Analgesia 2003;97(1):62-71. [DOI] [PubMed] [Google Scholar]
Gan 2019
- Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia & Analgesia 2019;131(2):411-48. [DOI] [PubMed] [Google Scholar]
George 2009
- Ronald GB, Allen TK, Habib AS. Serotonin receptor antagonists for the prevention and treatment of pruritus, nausea, and vomiting in women undergoing cesarean delivery with intrathecal morphine: a systematic review and meta-analysis. Anesthesia & Analgesia 2009;109(1):174-82. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Oxford: Wiley & Sons Ltd, 2019. [Google Scholar]
Jasson 1987
- Jasson J, Lefevre G, Talaffre ML, Legagneux F, Guerin JP, Vassilieff N, et al. Immediate pre-anesthetic administration of sodium citrate in cesarean interventions [abstract] [Administration immediate pre-anesthesique de citrate de sodium pour intervention cesarienne programmee]. Annales Francaises d'Anesthesie et de Reanimation 1987;6:R155. [Google Scholar]
Jasson 1989b
- Jasson J, Lefevre G, Tallet F, Talafre ML, Legagneux F, Conseiller C. Oral sodium citrate before general anaesthesia for elective caesarean section Effects of pH and volume of gastric content [Ingestion de citrate de sodium avant l'anesthesie generale pour cesarienne programmee. Effet sur le pH et le volume du contenu gastrique]. Annales Francaises d Anesthesie et de Reanimation 1989;8:12-8. [DOI] [PubMed] [Google Scholar]
Matthews 2015
- Matthews A, Haas DM, O'Mathúna DP, Dowswell T. Interventions for nausea and vomiting in early pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD007575. [DOI: 10.1002/14651858.CD007575.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishriky 2012
- Mishriky BM, Habib AS. Metoclopramide for nausea and vomiting prophylaxis during and after Caesarean delivery: a systematic review and meta-analysis. British Journal of Anaesthesia 2012;108(3):374-83. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Collaborating Centre for Women's and Children's Health. Caesarean section: NICE clinical guideline. 2nd edition. London: RCOG Press, 2011. [Google Scholar]
Paranjothy 2014
- Paranjothy S, Griffiths JD, Broughton HK, Gyte GM, Brown HC, Thomas J. Interventions at caesarean section for reducing the risk of aspiration pneumonitis. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD004943. [DOI: 10.1002/14651858.CD004943.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Wahab 1999
- Wahab MA, Karantzis P, Eccersley PS, Russell IF, Thompson JW, Lindow SW. A randomised, controlled study of uterine exteriorisation and repair at caesarean section. British Journal of Obstetrics and Gynaecology 1999;106(9):913-6. [DOI] [PubMed] [Google Scholar]
Zhou 2018
- Zhou C, Zhu Y, Bao Z, Wang X, Liu Q. Efficacy of ondansetron for spinal anesthesia during cesarean section: a meta-analysis of randomized trials. Journal of International Medical Research 2018;46(2):654-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Griffiths 2009
- Griffiths JD, Gyte GM, Paranjothy S, Brown HC, Broughton HK, Thomas J. Interventions for reducing nausea and vomiting at caesarean section. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007579. [DOI: 10.1002/14651858.CD007579] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2012
- Griffiths JD, Gyte GM, Paranjothy S, Brown HC, Broughton HK, Thomas J. Interventions for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD007579. [DOI: 10.1002/14651858.CD007579.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paranjothy 2004
- Paranjothy S, Liu E, Brown H, Thomas J. Drugs at caesarean section for preventing nausea, vomiting and aspiration pneumonitis. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD004943. [DOI: 10.1002/14651858.CD004943] [DOI] [Google Scholar]


