Abstract
The synthetic progestin 17-α-hydroxyprogesterone caproate (17-OHPC) is commonly prescribed to pregnant women with a history of preterm delivery, despite little evidence of efficacy. The timing of 17-OHPC administration coincides with fetal mesocortical dopamine pathway development, yet the potential effects on cortical development and cognition are almost unknown. In rodent models, exposure to 17-OHPC significantly increased dopaminergic innervation of the medial prefrontal cortex (mPFC), an aberrant pattern of connectivity that may underlie deficits in cognitive flexibility observed in adulthood. In the present study, tyrosine hydroxylase (TH) immunoreactivity was used to determine whether 17-OHPC altered dopaminergic innervation of the mPFC during a neonatal period of synaptogenesis in males and females. Although there were no differences in the amount of TH-immunoreactive (−IR) fibres, there was a sex difference in TH-IR fibre distribution in deep layers of the prelimbic area (PL) mPFC; males had a narrower pattern of dopaminergic innervation than females. 17-OHPC exposure abolished these sex-specific patterns, such that 17-OHPC females had a narrower pattern in the PL than control females. In the infralimbic mPFC (IL), 17-OHPC males had a broader pattern of distribution of TH-immunoreactivity than control males with no differences in the amount of TH-IR fibres. 17-OHPC also created a sex difference in which males had a lower TH-IR fibre density than females. We also examined microglia, brain macrophages that play a key role in sculpting dopaminergic axon outgrowth in development, using phenotype as an indirect measure of microglial activity. Females had a greater number of reactive stout microglia compared to males in the PL, and males had more active round microglia than females in the IL. 17-OHPC treatment abolished the sex differences in both regions. These findings demonstrate that developmental exposure to 17-OHPC can exert differential effects in males and females and may diminish sex differences in cortical maturation.
Keywords: 17α-hydroxyprogesterone caproate, infralimbic area, mesocortical dopamine pathway, prelimbic area, tyrosine hydroxylase
1 |. INTRODUCTION
Preterm birth, defined as parturition prior to 37 weeks of gestation, occurs in approximately one in 10 pregnancies in the USA.1 Although the exact cause is unknown, preterm birth may result from a confluence of genetic and environmental factors.2 In an attempt to reduce the potential risk for another preterm delivery, the synthetic progestin 17-alpha-hydroxyprogesterone caproate (17-OHPC) has been recommended for all women with a history of preterm delivery that did not result from known obstetrical, medical or fetal causes.3 Treatment with 17-OHPC begins between gestational weeks 16 and 20, and continues to week 37.4,5 17-OHPC can cross from the maternal unit to the fetal unit of the placenta in situ6,7 and has been detected in neonatal plasma 44 days after the last maternal injection,7 indicating that the fetus may be exposed to the synthetic progestin.
The timing of 17-OHPC administration coincides with critical periods of mesocortical dopamine pathway development in the fetus. Comprised of dopaminergic neurones of the ventral tegmental area (VTA) that innervate the medial prefrontal cortex (mPFC), the mesocortical dopamine pathway mediates complex cognitive behaviours. Treatment with 17-OHPC begins in the second trimester, when early dopaminergic afferents are arriving to the prefrontal cortex.8 In rodent models, the developing mesocortical dopamine pathway is sensitive to progestins because progesterone receptor (PR) is transiently expressed in both the VTA and mPFC.9,10 Indeed, approximately 90% of the PR-expressing cells in the VTA are dopaminergic and project directly to the mPFC in rats.9 Nuclear steroid hormone receptors are powerful transcription factors, and 17-OHPC has a strong binding affinity for PR,11 suggesting that 17-OHPC exposure may fundamentally alter the development of the mesocortical dopamine pathway. Indeed, in rodent models, 17-OHPC exposure during development significantly increased the density of dopaminergic innervation of layers I/II of the prelimbic area (PL) of the juvenile mPFC.12 This altered dopaminergic innervation is hypothesised to underlie the functional deficits in cognitive flexibility and increased perseveration in adulthood following developmental exposure to 17-OHPC,12 both dopamine-mediated behaviours.13–16 Specifically, 17-OHPC-treated rats were slower to shift to a change in reward contingency compared to controls in the set shift assay, which was the result of an increase in perseverative errors, in which 17-OHPC treated rats continued to rely on the previous rule significantly longer than controls.12 Thus, there is evidence to support that 17-OHPC exposure during critical periods of mPFC development can alter dopaminergic neural circuitry and have long-term impacts on subsequent behaviour.
The present study investigated whether increases in dopaminergic innervation in the mPFC previously observed at periadolescence following postnatal exposure to 17-OHPC emerge during the first postnatal week. Not only is the perinatal period characterised by exuberant synapse formation,17 but also there is peak PR expression in the VTA and detectable PR in the mPFC. Additionally, within the first postnatal week, subregions of the mPFC, such as the PL and infralimbic area (IL), are discernable.18,19 Although the PL and IL play a role in emotional and cognitive processes, and both receive dopaminergic input from the VTA, the PL is more directly involved in mediating cognitive behaviours, whereas the IL is more involved in mediating visceromotor activity.20 Dopaminergic innervation in both PL and IL was examined in the present study. Additionally, microglia, the resident macrophages of the brain, were also examined because they play a critical role in establishing dopaminergic circuitry of the forebrain.21
2 |. MATERIALS AND METHODS
2.1 |. Animals and 17-OHPC treatment
Although the mesocortical dopamine pathway develops prenatally in humans, this pathway develops postnatally in rats,22,23 which were used here as a model of cortical development. Peak PR expression in the rodent mPFC also occurs within the first postnatal week.9 Thus, rats were administered 17-OHPC postnatally during this critical window of mesocortical dopamine pathway maturation. Tyrosine hydroxylase (TH)-immunoreactivity was used as a marker to discern developmental changes in dopaminergic innervation of the mPFC. The commonly used marker for microglia, ionised calcium binding adaptor molecule 1 (Iba-1), was used here to determine whether morphological changes in microglia, an indirect measure of activation, coincide with changes in TH innervation patterns, as a possible mechanism by which 17-OHPC may exert its effects on mesocortical dopamine pathway development.
Timed-pregnant female Sprague-Dawley rats were purchased from Taconic Laboratories (Germantown, NY, USA) and were allowed to deliver pups normally. The day of birth was designated as postnatal day 1 (P1). Male and female pups received injections of 17-OHPC (0.5mg/kg, s.c.; MP Biomedicals, Santa Ana, CA, USA) in sesame oil (17-OHPC) or an equal volume of the oil vehicle alone (controls) from P1 to P7. This dose of 17-OHPC is the per kg equivalent of the dose commonly administered to pregnant women. For the TH immunohistochemistry study, five litters were represented for the control condition (n = 9 females, n = 8 males) and four litters were represented for the 17-OHPC treatment group (n = 8 females, n = 9 males). In the Iba-1 study, there were four litters each for the control condition (n = 8 females, 10 males) and the 17-OHPC treatment group (n = 11 females, 12 males). All animals were housed under a reverse 12:12 hour light/dark photocycle at a constant temperature of 25 ± 2°C, with food and water available ad lib. All procedures were approved by the Institutional Animal Care and Use Committee at University at Albany.
2.2 |. Tissue collection
For TH immunohistochemistry, animals were anaesthetised by hypothermia and killed by decapitation on P7. Brains were removed and immersion fixed in 4% acrolein in 0.87% NaCl in 0.1 mol L−1 phosphate buffered saline (pH 7.4) for 6 hours and cryoprotected in 30% sucrose in 0.1 mol L−1 phosphate buffer for 72 hours.
For Iba-1 immunohistochemistry, animals were anaesthetised by hypothermia, followed by a transcardial perfusion first with 0.9% saline and then by 4% paraformaldehyde on P7. Brains were removed and immersed in 4% paraformaldehyde for 24 hours and then cryoprotected in 30% sucrose in 0.1 mol L−1 phosphate buffer for 72 hours.
All brains were sectioned through the extent of the mPFC at 40 μm in the coronal plane on a freezing microtome. Sections were stored at −20°C in cryoprotectant (30% sucrose, 0.1% polyvinylpyrrolidone-40 in ethylene glycol in 0.1 mol L−1 phosphate buffer until further processing.
2.3 |. TH immunohistochemistry
Immunohistochemical procedures were identical to those described previously.9,12 Free-floating sections of the mPFC were rinsed with Tris-buffered saline (TBS pH 7.6) three times (5 minutes each) to remove cryoprotectant. Sections were incubated in 20% normal goat serum (NGS), 1% bovine serum albumin and 1% hydrogen peroxide in TBS for 30 minutes. Sections were incubated in primary antisera against TH (dilution 1:1000; Millipore, Burlington, MA, USA; catalogue no. AB152; RRID:AB_390204) in TBS containing 0.3% Triton-X-100 and 2% NGS (TTG) for 48 hours at 4°C. According to the manufacturer, the immunogen for this primary antibody is denatured TH from rat pheochromocytoma and has been shown by western blot analysis not to recognise other monoaminergic synthetic enzymes.
Sections were rinsed out of primary antisera with TTG three times (5 minutes each), then incubated in 5 μg mL−1 goat anti-rabbit biotinylated secondary antibody (Vector Laboratories Inc., Burlingame, CA, USA) in TTG for 90 minutes. The sections were rinsed out of the secondary antibody with two rinses of TTG (5 minutes each) followed by 2 rinses of TBS (5 minutes each). Sections were then incubated with Avidin Biotin Complex in TBS (Vectastain Elite Kit; Vector Laboratories Inc., Burlingame, CA, USA) for 60 minutes. After sections were rinsed three times (5 minutes each) with TBS, immunoreactivity was visualised with 0.05% 3,3′-diaminobenzidine (Sigma-Aldrich, St Louis, MO, USA) and 0.02% hydrogen peroxide in TBS. Sections were mounted on gelatin-coated slides and covers-lipped with Permount (Fisher Scientific, Waltham, MA, USA).
2.4 |. Iba-1 immunohistochemistry
The immunohistochemical procedures were the same as described above, except that sections were incubated in primary antisera against ionised calcium binding adaptor molecule 1 (Iba-1) (dilution 1:5000; Wako Pure Chemical Industries, Tokyo, Japan; catalogue no. 019–19741; RRID:AB_839504), a microglia-specific marker for 48 hours at 4°C. According to the manufacturer, rabbit polyclonal anti-Iba-1 was raised against a synthetic peptide corresponding to the C-terminal fragment of rat Iba-1, purified by using antigen-affinity chromatography from rabbit antisera, and recognises a single 17-kDa band that corresponds to Iba protein on western blot analysis of rat brain.
2.5 |. Image analysis
Anatomically matched sections of the mPFC were selected from each animal for image analysis. The mPFC was identified using the presence of the anterior forceps of the corpus callosum and the anterior commissure as structural landmarks.19,24 Images for TH-immunoreactive (−IR) analysis were captured at 4× and 10× magnification in PL and IL mPFC on a Eclipse E600 microscope (Nikon, Tokyo, Japan) with a darkfield condenser, fitted with a SPOT Insight camera (Diagnostic Instruments Inc, Sterling Heights, MI, USA) connected to a computer (Dell Inc., Round Rock, TX, USA). Similarly, Iba-1 images were captured at 10× and 20× in PL and IL mPFC with a brightfield condenser. ImageJ software (https://imagej.nih.gov/ij) was used to process TH-IR and Iba-1 photomicrographs for analysis.
2.6 |. TH immunoreactivity analysis
Darkfield images of the PL and IL mPFC (Figure 1A) were skeletonised, such that all were made black with a white background, and fibres were standardised to 1 pixel in width to control for variability in fibre thickness. The mediolateral distribution of TH-IR fibres was measured using the width of a rectangle of a constant height placed over the densest part of the TH-IR fibres within the deep layers in the dorsoventral extent of either the PL or the IL mPFC. It was observed by an experimenter blind to treatment group that some animals differed in the width of the distribution of the densest band of TH-IR fibres. To objectively and quantitatively determine whether these differences could be attributed to treatment group or sex, the width of the rectangle was set for each animal by an experimenter blind to experimental group based on specific criteria. The most superficial layer border of the rectangle was placed at the point at which the TH-IR fibres distinctly changes orientation, with TH-IR fibres on the outside of the rectangle running in a medial-lateral direction. The deeper layer border of the rectangle was placed at the point at which the density of the TH-IR fibres markedly decreases. Objective criterion for the deep layer border of the rectangle was set at the point at where fibre density dropped by at least 70% (Figure 1B), thus providing a quantitative measure of the distribution of the denest band of TH-IR fibres. The mediolateral distribution of TH-IR fibres was quantified as the width (μm) of the rectangle (TH-IR fibre distribution). The relative total amount of TH-IR fibres was quantified as the area (μm2) covered by black pixels in the skeletonised images within the rectangle. The relative density of TH-IR fibres was calculated as the total amount of TH-IR fibres divided by the area (μm2) of the rectangle. The relative total amount of TH-IR fibres was also measured in the superficial layers PL and IL using a rectangle of constant area. To rule out the possibility that group differences in cortical thickness was contributing to group differences in the medio-lateral distribution of TH-IR fibres, a polygon was placed within the boundaries of the PL and the IL based on cytoarchitecture17 at 4× magnification. The length (μm) of a line from the white matter to the cortical surface, representing the maximum width of the polygon, was measured to determine cortical thickness of all layers. The Shapiro-Wilk test was used to evaluate normality. Statistical analyses were conducted for each measure using a two-way ANOVA (sex × treatment). Preplanned, post-hoc comparisons were made for both the PL and IL using the Student-Newman-Keuls test (P < 0.05) for both the PL and IL.
FIGURE 1.
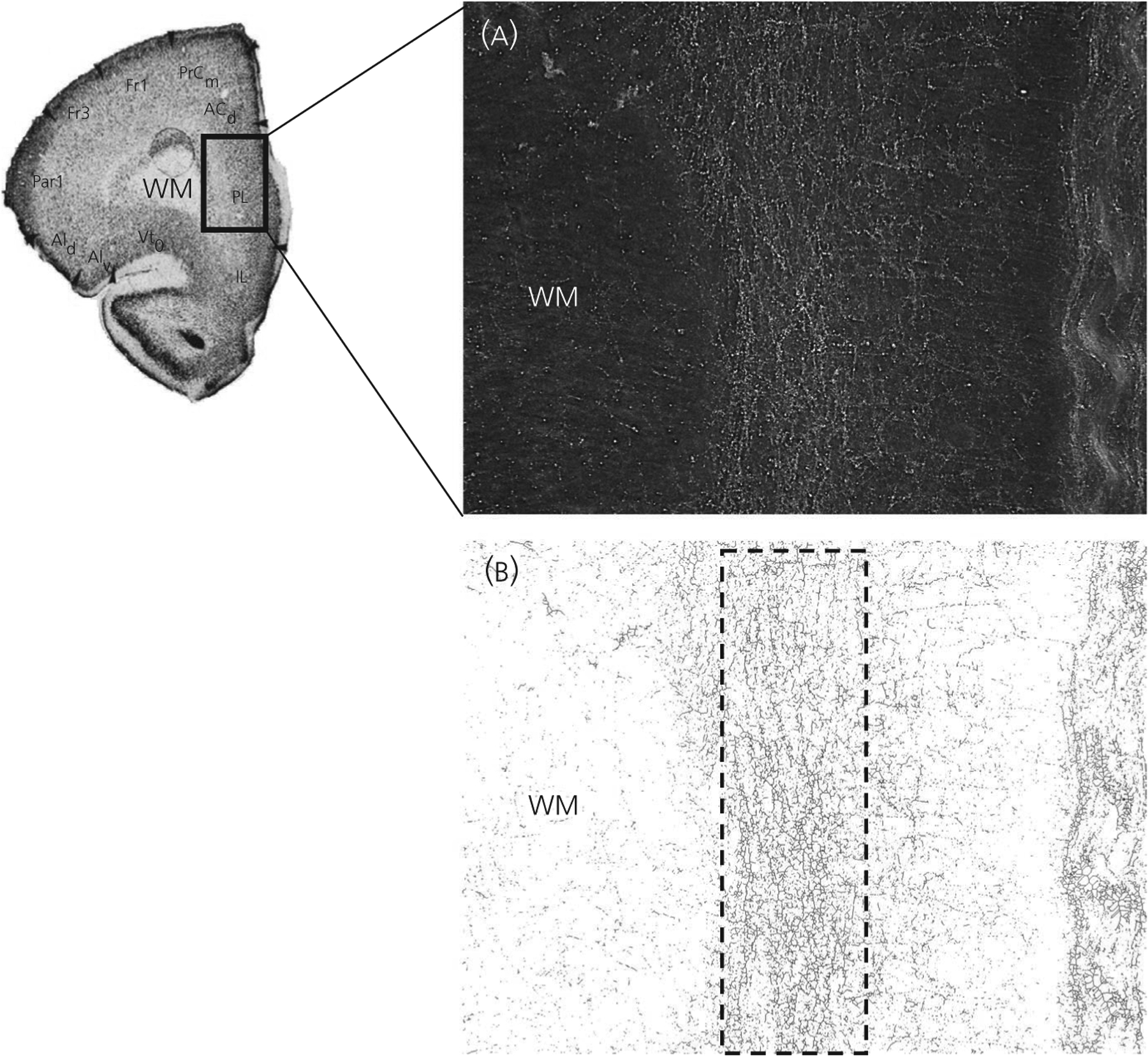
Tyrosine hydroxylase-immunoreactive (TH-IR) fibre analysis in the prelimbic (PL) medial prefrontal cortex (mPFC). Original photomicrographs were obtained under darkfield illumination (A) and images were then skeletonised for analysis (B). A rectangle with a standardised height was placed over the densest part of the TH-IR fibres in the dorsoventral extent of either the PL or the IL mPFC (deep layers pictured). The width of the rectangle was used to measure the mediolateral distribution of TH-IR fibres. The superficial boundary of the rectangle was placed where the TH-IR fibres distinctly changed orientation, and the deep layer border was determined based on at least a 70% decrease in TH-IR fibre density from the inside to the outside of the rectangle. The image of the mPFC section was taken from Van Eden and Uylings.19 WM, white matter
2.7 |. Iba-1 analysis
Iba-1-IR microglia from PL and IL mPFC were sorted into four distinct morphological categories as reported previously, and are considered to be indicators of activational state.25–28 Microglia were classified into the categories: (i) round/amoeboid (round); (ii) microglia with stout processes (stout); (iii) microglia with thick long processes (thick); and (iv) microglia with thin ramified processes (thin). The number of microglia in each category was quantified each for the PL and IL mPFC. The Shapiro-Wilk test was used to evaluate normality. Statistical analyses of microglia were performed using a two-way ANOVA (sex × treatment) for each morphological category, followed by a preplanned post-hoc, stepwise test that allows for multiple pairwise comparisons.
3 |. RESULTS
3.1 |. TH-IR in prelimbic mPFC
For TH-IR fibre distribution in PL mPFC (Figure 2), there was a significant interaction (F1,30 = 5.76, P < 0.05) between sex and treatment (Figure 3A). Control males had a narrower distribution pattern in the deep layers compared to control females (P < 0.01). 17-OHPC exposure abolished this sex difference by significantly narrowing the distribution pattern in treated females compared to control females (P < 0.05) to a level similar to that observed in males, although it had no significant effects in males.
FIGURE 2.
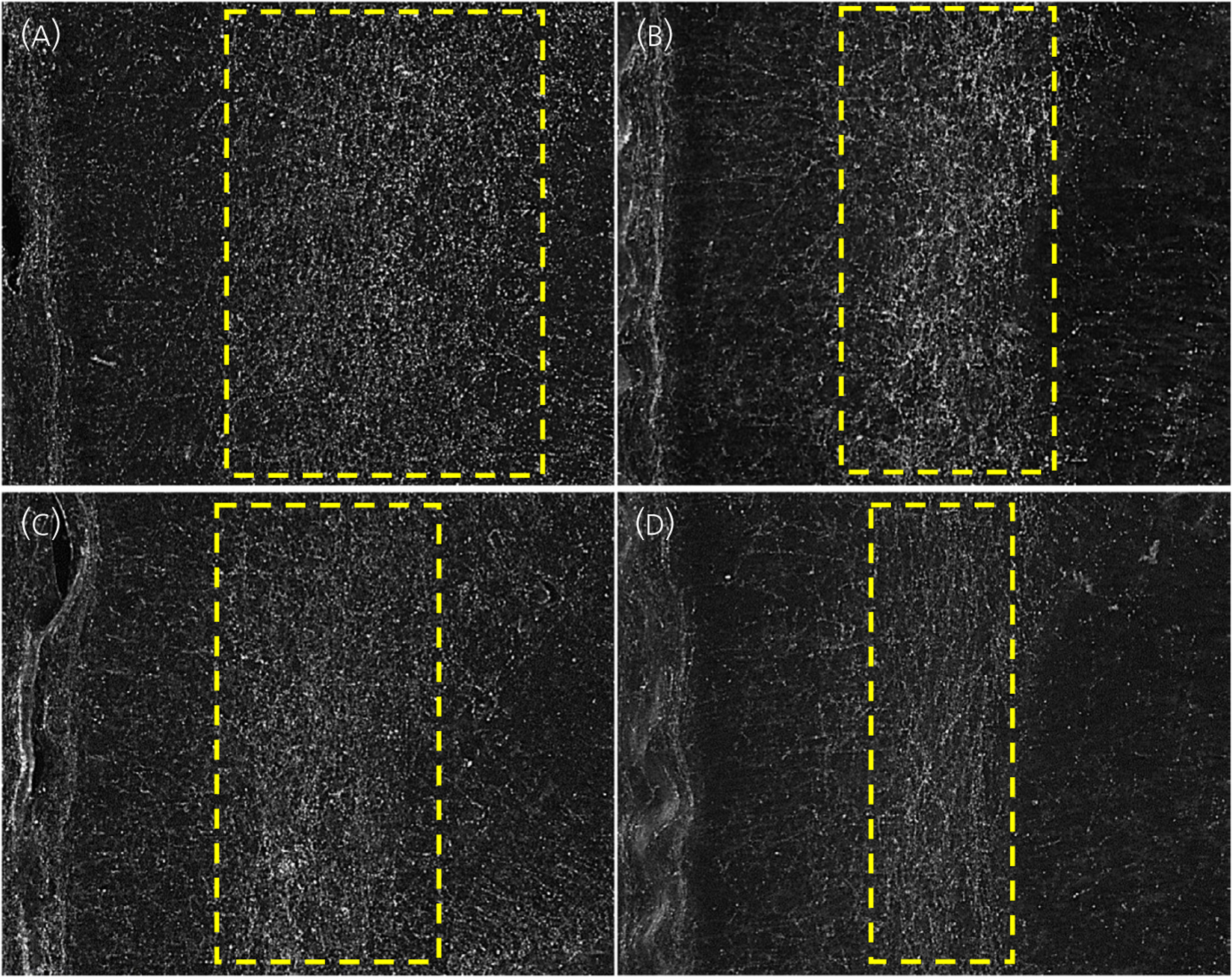
Two distinct patterns in the distribution of tyrosine hydroxylase-immunoreactive (TH-IR) fibres in the prelimbic medial prefrontal cortex; a broad pattern of TH-IR fibres in (A) control females, or a narrower pattern in (B) control males, (C) 17-α-hydroxyprogesterone caproate (17-OHPC)-treated females and (D) 17-OHPC-treated males
FIGURE 3.
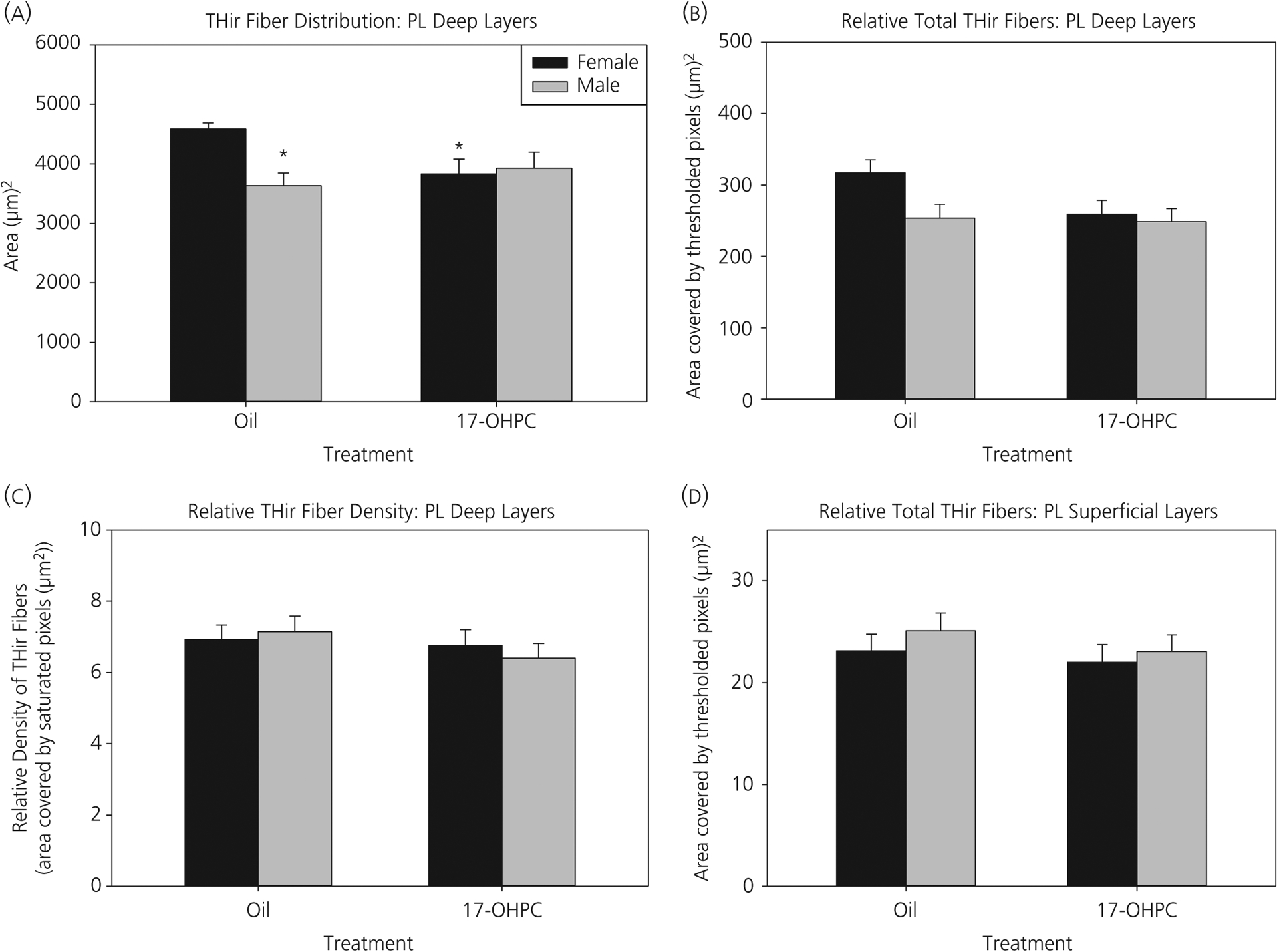
Prelimbic (PL) medial prefrontal cortex (mPFC): 17-α-hydroxyprogesterone caproate (17-OHPC) reduced the tyrosine hydroxylase-immunoreactive (TH-IR) fibre distribution in females, abolishing a novel sex difference. (A) TH-IR fibre distribution, (B) relative total amount of TH-IR fibres and (C) relative density of TH-IR fibres in the deep layers or (D) relative total TH-IR fibres in the superficial layers of the prelimbic mPFC at postnatal day 7 in control or 17-OHPC males and females. *Significantly different from control females (P < 0.05)
The relative total amount of TH-IR fibres in the deep layers of the PL, although not statistically different among the groups, had a strikingly similar pattern to that observed in the TH-IR fibre distribution measure (Figure 3B). There were no significant differences in the relative TH-IR fibre density (Figure 3C). In the superficial PL layers, the relative total amount of TH-IR fibres was not statistically different between the groups (Figure 3D).
3.2 |. TH-IR in infralimbic mPFC
For TH-IR fibre distribution in the deep layers of the IL mPFC (Figure 4A), there was a main effect of treatment (F1,30 = 4.95, P < 0.05). Post-hoc comparisons demonstrated that 17-OHPC males had a significantly broader distribution pattern than control males (P < 0.01). There was no significant effect of 17-OHPC in females. There were no significant differences in the relative total amount of TH-IR fibres in the deep IL layers (Figure 4B). For relative density of TH-IR fibres within the deep IL layers, there was a significant interaction (F1,30 = 5.96, P < 0.05) (Figure 4C). Post-hoc comparisons revealed a sex difference only in 17-OHPC treated animals (P < 0.05). 17-OHPC males had a significantly lower relative density of TH-IR fibres compared to 17-OHPC females (P < 0.05). There were no significant sex differences in controls.
FIGURE 4.
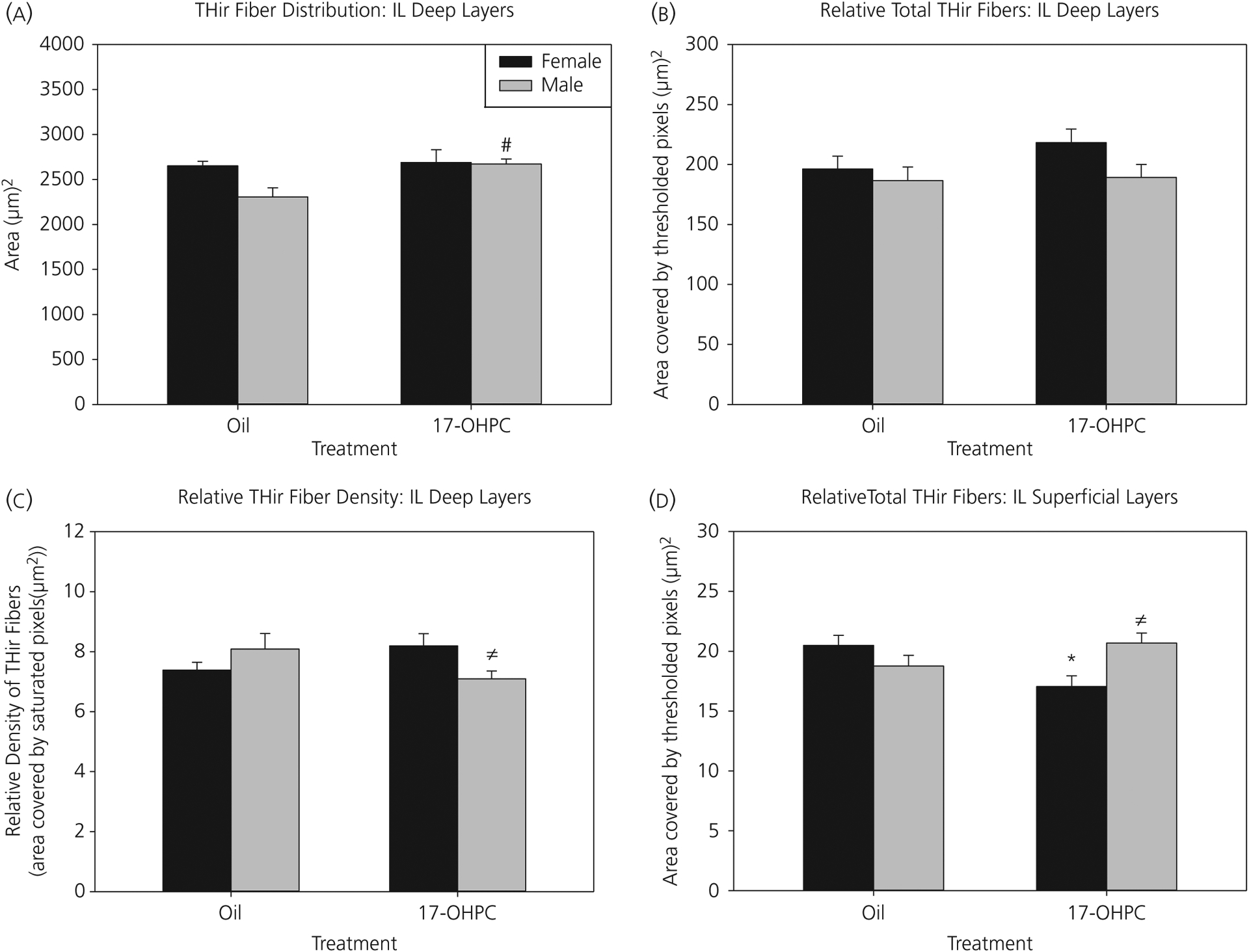
Infralimbic (IL) medial prefrontal cortex (mPFC): 17-α-hydroxyprogesterone caproate (17-OHPC) increased the tyrosine hydroxylase-immunoreactive (TH-IR) fibre distribution in males, but had no effect in females. (A) TH-IR fibre distribution, (B) relative total amount of TH-IR fibres and (C) relative density of TH-IR fibres in deep layers or (D) relative total TH-IR fibres in superficial layers of the infralimbic mPFC at postnatal day 7 in control or 17-OHPC males and females. #Significantly different from control males (P < 0.01). ≠Significantly different from 17-OHPC females (P < 0.01). *Significantly different from control females (P < 0.05)
For the relative total amount of TH-IR fibres in the superficial layers of IL, there was a significant interaction (F1,30 = 9.60, P < 0.01) (Figure 4D). Post-hoc analysis demonstrated that 17-OHPC females had a significant reduction in relative total TH-IR fibres compared to control females (P < 0.01). Additionally, there was a sex difference in 17-OHPC exposed animals, in which males had significantly greater relative total TH-IR fibres compared to females (P < 0.01). There was no significant sex difference in controls.
3.3 |. Cortical thickness
The significant differences in the pattern of distribution of TH-immunoreactivity were not influenced by differences in cortical thickness. In both the PL and IL mPFC, there were no main effects of sex or treatment and no significant interactions on cortical thickness (Figure 5).
FIGURE 5.
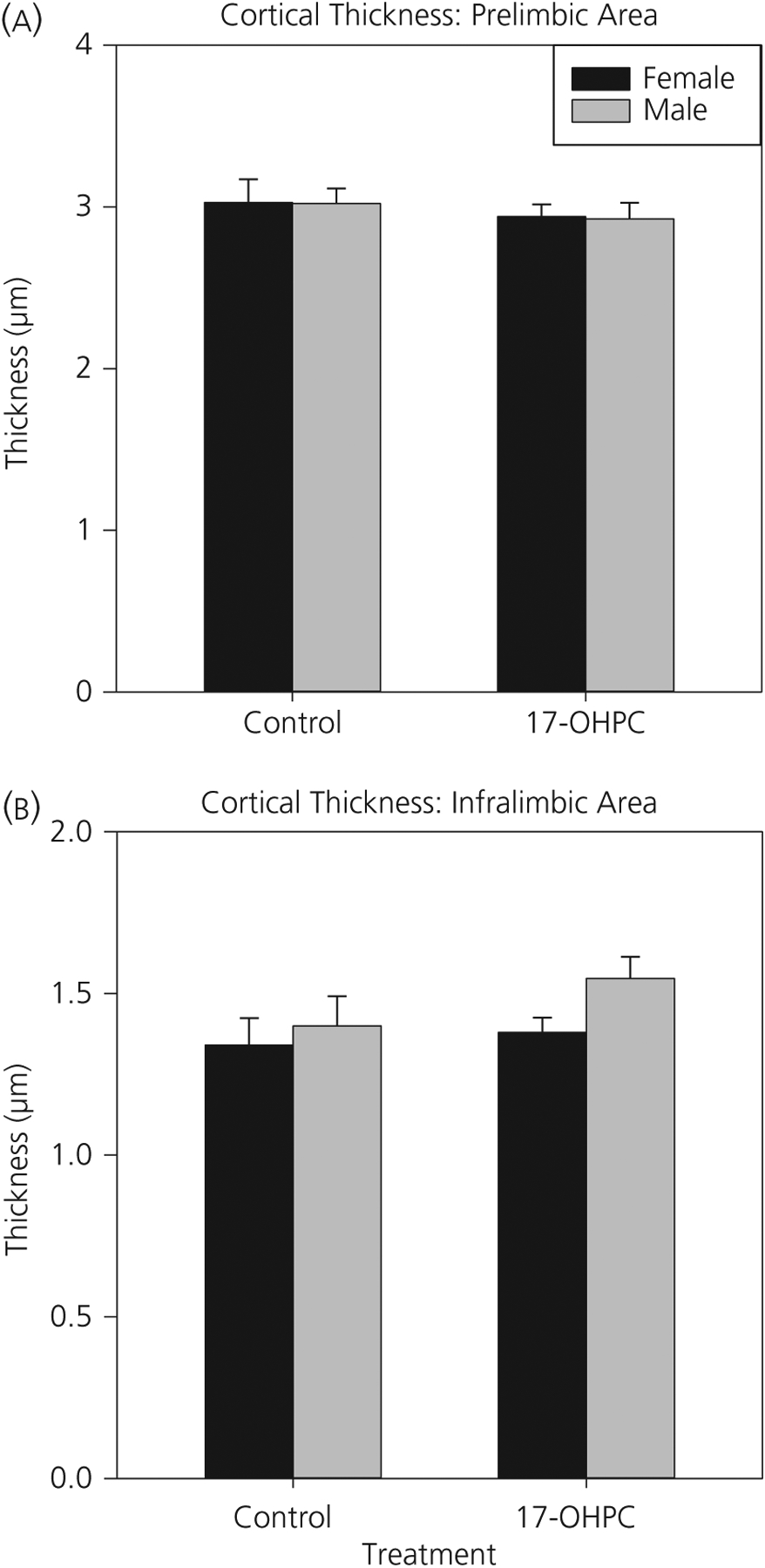
Cortical thickness is not altered by sex or 17-α-hydroxyprogesterone caproate (17-OHPC). Cortical thickness (micrometers) of the (A) prelimbic and (B) infralimbic medial prefrontal cortex in postnatal day 7 control or 17-OHPC males and females is shown. There were no significant sex or treatment differences
3.4 |. Microglia in prelimbic mPFC
There was a significant interaction (F1,37 = 6.87, P < 0.05) between sex and treatment on the number of stout microglia in PL mPFC (Figure 6). Post-hoc tests revealed a sex difference in which control males had significantly fewer stout microglia than control females (P < 0.01). Additionally, 17-OHPC treatment reduced the number of stout microglia in females (P < 0.05) but had no significant effect in males. There were no significant effects of sex or treatment and no interactions for round, thick or thin microglia.
FIGURE 6.
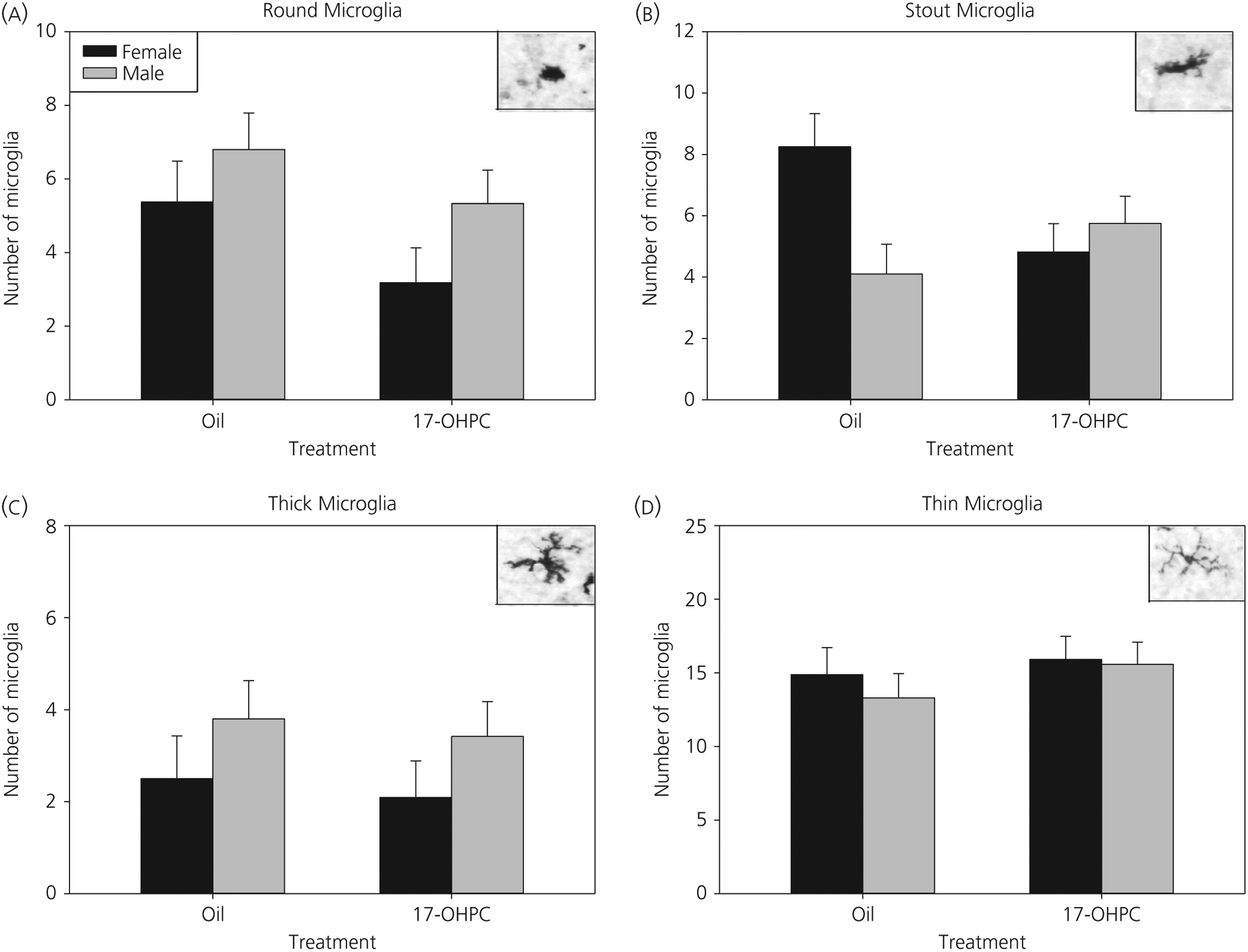
Prelimbic medial prefrontal cortex (mPFC): 17-α-hydroxyprogesterone caproate (17-OHPC) decreases stout microglia in females, abolishing a sex difference. Microglia phenotype analysis in the prelimbic medial mPFC at postnatal day 7 (P7). The number of microglia with round (A), stout (B), thick (C) or thin (D) process phenotypes in P7 males and females treated with oil or 17-OHPC is shown. *Significantly different from control females (P < 0.05)
3.5 |. Microglia in infralimbic mPFC
In the IL mPFC, there was a significant interaction between sex and treatment for the total number of microglia (all phenotypes) (F1,37 = 5.27, P < 0.05). Post-hoc tests demonstrated a significant sex difference in control animals (P < 0.05), where males had significantly fewer microglia than females (males: 27.4 ± 1.53; females: 37.13 ± 2.74). Therefore, to determine the distribution of microglia within each phenotype, the percentage of round, stout, thick and thin microglia of the total number of microglia was analysed (analyses for the raw cell numbers are provided below).
There was a significant interaction in the percentage of round microglia (F1,37 = 4.55, P < 0.05) (Figure 7A). Post-hoc analysis demonstrated that control males had a greater percentage of round microglia compared to females (P < 0.05), although there was no significant effect of sex in 17-OHPC treated animals. For percentage stout microglia in the IL, there was a significant effect of treatment (F1,37 = 5.33, P < 0.05). 17-OHPC exposure significantly decreased the percentage of stout microglia compared to control animals (Figure 7B). There were no significant effects in the percentage of thick (Figure 7C) or thin (Figure 7D) microglia.
FIGURE 7.
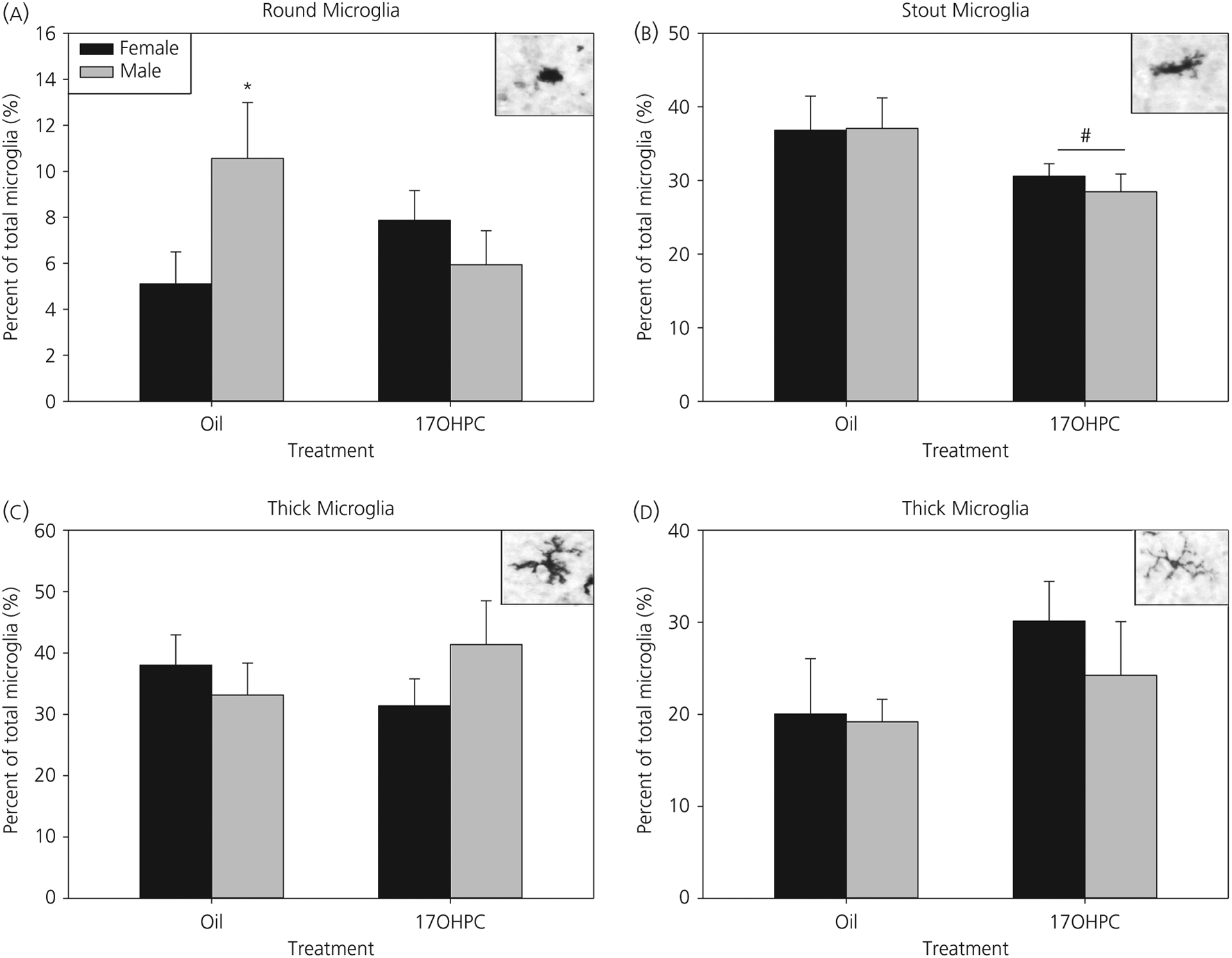
Infralimbic medial prefrontal cortex (mPFC): 17-α-hydroxyprogesterone caproate (17-OHPC) abolishes a sex difference in round microglia. Microglia phenotype analysis in the infralimbic mPFC at postnatal day 7 (P7). The percentage of microglia with round (A), stout (B), thick (C) or thin (D) process phenotypes in P7 males and females treated with oil or 17-OHPC is shown. *Significantly different from control females (P < 0.05). #Significantly different from control group (P < 0.05)
In the analysis of raw microglia cell numbers, there was a main effect of treatment (F1,37 = 4.21, P = 0.047) in the number of stout microglia in the IL mPFC (data not shown). 17-OHPC treatment reduced the number of stout microglia. There were no significant effects for round, thick or thin microglia.
4 |. DISCUSSION
The results of the present study reveal several novel sex differences in mPFC development, which were eliminated following exposure to the clinically used synthetic progestin, 17-OHPC. In both the PL and the IL mPFC, there were significant sex differences in dopaminergic innervation patterns, in which males had a markedly narrower distribution of TH-IR fibres in deep layers compared to females in the absence of sex differences in overall cortical thickness. The effects of 17-OHPC were sex-specific based on mPFC sub-region. In the PL, 17-OHPC reduced the TH-IR fibre distribution in females, but not males, whereas, in the IL, 17-OHPC increased TH-IR fibre distribution in males, but not females, thereby eliminating the sex difference in both regions. A potential mechanism by which 17-OHPC exerts its effects may be through indirect activation of microglia, which are known to play a critical role in synaptic pruning during development. There were sex differences in the number of microglia with reactive phenotypes (females > males in PL; males > females in IL). 17-OHPC exposure decreased the number of reactive microglia in females compared to controls in the PL, whereas this treatment decreased the percentage of reactive microglia in males in the IL. However, 17-OHPC abolished the sex differences in reactive microglia in both regions. Taken together, the present findings suggest that 17-OHPC, a progestin commonly administered during pregnancy to women with a history of preterm delivery, may alter fundamental processes of development within the prefrontal cortex in a sex-specific manner.
Sex differences in developing mPFC have not been widely reported to date, making the current findings among the first in this respect. The mechanism by which these sex differences in TH-IR fibre distribution in postnatal PL and IL mPFC arise during development is unknown. Many sex differences in the brain are the result of differential exposure of males and females to testosterone during critical windows of development,29,30 making this a possibility in the mPFC. Androgen receptors are indeed expressed in the mesocortical dopamine pathway31–34 and gonadectomy on the day of birth reduced the density of TH-IR fibres in the cingulate, motor and somatosensory cortices in adult males, an effect prevented by testosterone supplementation.35 However, the nature of 17-OHPC treatment effects on these sex differences may argue against a hormonal basis, and rather suggest other interesting possibilities.
Exposure to 17-OHPC during the first postnatal week abolished sex differences in dopaminergic innervation patterns in the mPFC but did so in a sex- and region-dependent manner, making simple differences in circulating gonadal hormones a less parsimonious explanation for the observed sex and treatment effects. Rather, sex- and regional differences in the rate of mPFC maturation may be a more likely hypothesis. For example, females had higher levels of endogenous dopamine at P10 in anterior cortex compared to males,36 suggesting that dopaminergic innervation of the female mPFC may precede that of males during early life. Furthermore, the early postnatal period is characterised by an initial exuberance of cortical projections17 leading to the hypothesis that the diffuse TH-IR fibre distribution pattern observed in P7 females represents a more advanced state of maturation compared to males. Additionally, dopaminergic innervation of the IL matures prior to that of the PL; IL exhibited an adult pattern of dopaminergic innervation by P6, whereas, in PL, dopaminergic innervation was modest in layer V and almost absent in layer I, representing a more immature state at the same chronological age.18 Therefore, the sex difference in TH-IR fibre distribution observed in controls at P7 in both PL and IL may represent ‘snapshots’ in the time of mPFC development, in which females were captured in a more mature state compared to males. The fact that 17-OHPC decreased TH-IR fibre distribution in deep layers of the female PL but increased the distribution of these same layers in male IL is consistent with the idea that synthetic progestin exposure may abolish normal sex differences in the pace of mPFC maturation.
The attenuation of endogenous sex differences in dopaminergic innervation patterns by 17-OHPC has potential clinical implications. These sex differences appear to be developmentally transient because TH-IR fibre density and distribution patterns are similar in males and females by peri-adolesence and adulthood.12,37,38 However, De Vries39 has postulated that some sex differences in the brain may arise to compensate for sex differences in the underlying physiology that are capable of producing suboptimal sex differences later in life. In humans, longitudinal MRI studies demonstrated that girls achieved peak levels in cerebral volume at age 11 years, whereas boys did not reach peak volume until age 15 years, suggesting that males trail females in cortical maturation.40 In this context, the finding that 17-OHPC exposure abolishes developmental sex differences in mPFC innervation has implications for the potential effects of 17-OHPC on human male and female brain development and subsequent behavioural outcomes in boys and girls.
The number of microglia with reactive phenotypes is both sex-dependent and region-specific in the mPFC of controls at P7. The number of stout microglia was higher in females compared to males in the PL, although the percentage of round microglia was higher in males in the IL. Microglia in the active state play a critical role in synaptogenesis,41,42 synaptic refinement43 and synaptic pruning,44 but, more specifically, they have been shown to play a role in sculpting dopaminergic innervation patterns of the forebrain.21 Similar to its effects on TH-IR fibres, 17-OHPC abolished sex differences in the number of reactive microglia by exerting differential effects in males and females depending on region. 17-OHPC reduced stout microglia in females in PL but reduced the percentage of round microglia in males in IL. These findings suggest that exposure to this synthetic progestin during development may disrupt normal microglia-mediated developmental processes and could potentially explain the aberrant dopaminergic innervation patterns that we observed in the present study. However, microglia do not generally express PR45 and, specifically, microglia in the postnatal PL and IL mPFC do not contain PRir (J. Willing & C. K. Wagner). Therefore, 17-OHPC may exert its effects on microglia through disrupted communication between PR-expressing pyramidal neurones of the developing mPFC and local microglia; for example, via complement or fractalkine signalling pathways.46–48 These pathway disruptions during critical periods in development could alter synaptic formation and elimination through reduced microglial phagocytosis to alter functional connectivity in a sex-dependent manner. Indeed, in males and females treated with 17-OHPC during development, there were no sex-differences in TH-IR fibre density at P25, yet there were sex-specific deficits in cognitive flexibility in adulthood,12 suggesting that altering microglia-neurone cross-talk during development may have significant implications later in life with differential outcomes for males and females.
In summary, the findings obtained in the present study demonstrate that 17-OHPC, a drug in clinical use in pregnant women, may influence the development of the mesocortical dopamine pathway in a sex- and region-specific manner. A sex difference in TH-IR fibre distribution was abolished with 17-OHPC exposure, perhaps by altering the maturational rate of dopaminergic innervation and altering microglial activity. The present rodent model of development can inform the risks and benefits of the use of 17-OHPC in human pregnancy. To date, only a single, limited study exists examining medical records of children whose mothers received 17-OHPC during pregnancy. Although there were no differences in the incidence of behavioural disorder outcomes, gender-specific changes were not factored into the analysis of the small sample.49 We offer the suggestion that 17-OHPC exposure may differentially affect males and females, perhaps increasing the vulnerability of males and females to typically male-biased behavioural disorders, some of which may be associated with disruption of prefrontal cortex maturation. Further investigation of the potential developmental and long-term impacts of the maternal administration of 17-OHPC on boys and girls is needed.
ACKNOWLEDGEMENTS
This study was supported by NIH HD076430 and NIH HD093907 to CKW.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: HD076430 and HD093907
Footnotes
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Martin JA, Osterman MJK. Describing the increase in preterm births in the United States, 2014–2016 key findings data from the national vital statistics system. NCHS Data Brief. 2014;312:1–29. [PubMed] [Google Scholar]
- 2.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science (80-). 2014;345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iams JD, Berghella V. Care for women with prior preterm birth. Am J Obstet Gynecol. 2010;203:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berghella V Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–386. [DOI] [PubMed] [Google Scholar]
- 5.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. [DOI] [PubMed] [Google Scholar]
- 6.Hemauer SJ, Yan R, Patrikeeva SL, et al. Transplacental transfer and metabolism of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2008;199:169.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caritis SN, Sharma S, Venkataramanan R, et al. Pharmacology and placental transport of 17-hydroxyprogesterone caproate in singleton gestation. Am J Obstet Gynecol. 2012;207:398.e1–398.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verney C, Milosevic A, Alvarez C, Berger B. Immunocytochemical evidence of well-developed dopaminergic and noradrenergic innervations in the frontal cerebral cortex of human fetuses at midgestation. J Comp Neurol. 1993;336:331–344. [DOI] [PubMed] [Google Scholar]
- 9.Willing J, Wagner CK. Progesterone receptor expression in the developing mesocortical dopamine pathway: importance for complex cognitive behavior in adulthood. Neuroendocrinology. 2016;103:207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López V, Wagner CK. Progestin receptor is transiently expressed perinatally in neurons of the rat isocortex. J Comp Neurol. 2009;512:124–139. [DOI] [PubMed] [Google Scholar]
- 11.Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN. Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins. Am J Obstet Gynecol. 2007;197:599.e1–599.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willing J, Wagner CK. Exposure to the synthetic progestin, 17α-hydroxyprogesterone caproate during development impairs cognitive flexibility in adulthood. Endocrinology. 2016;157:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pezze M, McGarrity S, Mason R, Fone KC, Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J Neurosci. 2014;34:7931–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: The critical role of early developmental events on prefrontal function. Behav Brain Res. 2003;146:43–55. [DOI] [PubMed] [Google Scholar]
- 16.Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. [DOI] [PubMed] [Google Scholar]
- 18.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. [DOI] [PubMed] [Google Scholar]
- 19.Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:253–267. [DOI] [PubMed] [Google Scholar]
- 20.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. [DOI] [PubMed] [Google Scholar]
- 21.Squarzoni P, Oller G, Hoeffel G, et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. [DOI] [PubMed] [Google Scholar]
- 22.Adlard BP, Dobbing J, Smart JL. An alternative animal model for the full-term small-for-dates human baby. Biol Neonate. 1973;23:95–108. [DOI] [PubMed] [Google Scholar]
- 23.Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26:61–67. [DOI] [PubMed] [Google Scholar]
- 24.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Pouchoulen M, VanRyzin JW, McCarthy MM. Morphological and phagocytic profile of microglia in the developing rat cerebellum. eNeuro. 2015;2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;45:948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bollinger JL, Bergeon Burns CM, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun. 2016;52:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkins AE, Piazza MK, Deak T. Stereological analysis of microglia in aged male and female Fischer 344 rats in socially relevant brain regions. Neuroscience. 2018;377:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenz KM, McCarthy MM. Organized for sex - steroid hormones and the developing hypothalamus. Eur J Neurosci. 2010;32:2096–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy MM, Herold K, Stockman SL. Fast, furious and enduring: sensitive versus critical periods in sexual differentiation of the brain. Physiol Behav. 2017;2018:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kritzer MF, Adler A, Marotta J, Smirlis T. Regionally selective effects of gonadectomy on cortical catecholamine innervation in adult male rats are most disruptive to afferents in prefrontal cortex. Cereb Cortex. 1999;9:507–518. [DOI] [PubMed] [Google Scholar]
- 32.Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: Localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated. Cereb Cortex. 2012;22:1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low KL, Ma C, Soma KK. Tyramide signal amplification permits immunohistochemical analyses of androgen receptors in the rat prefrontal cortex. J Histochem Cytochem. 2017;65:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kritzer MF. Perinatal gonadectomy exerts regionally selective, lateralized effects on the density of axons immunoreactive for tyrosine hydroxylase in the cerebral cortex of adult male rats. J Neurosci. 1998;18:10735–10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart J, Kühnemann S, Rajabi H. Neonatal exposure to gonadal hormones affects the development of monoamine systems in rat cortex. J Neuroendocrinol. 1991;3:85–93. [DOI] [PubMed] [Google Scholar]
- 37.Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willing J, Cortes LR, Brodsky JM, Kim T, Juraska JM. Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Dev Psychobiol. 2017;59:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Vries GJ. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. [DOI] [PubMed] [Google Scholar]
- 40.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through BDNF. Cell. 2014;155:1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto A, Wake H, Ishikawa AW, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sominsky L, De Luca S, Spencer SJ. Microglia: Key players in neurodevelopment and neuronal plasticity. Int J Biochem Cell Biol. 2017;2018:56–60. [DOI] [PubMed] [Google Scholar]
- 44.Zhan Y, Paolicelli RC, Sforazzini F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. [DOI] [PubMed] [Google Scholar]
- 45.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. [DOI] [PubMed] [Google Scholar]
- 46.Pósfai B, Cserép C, Orsolits B, Dénes Á. New insights into microglia-neuron interactions: a neuron’s perspective. Neuroscience. 2019;405:103–117. [DOI] [PubMed] [Google Scholar]
- 47.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokolowski JD, Chabanon-Hicks CN, Han CZ, Heffron DS, Mandell JW. Fractalkine is a “find-me” signal released by neurons undergoing ethanol-induced apoptosis. Front Cell Neurosci. 2014;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Northen AT, Norman GS, Anderson K, et al. Follow-up of children exposed in utero to 17 alpha-hydroxyprogesterone caproate compared with placebo. Obstet Gynecol. 2007;110:865–872. [DOI] [PubMed] [Google Scholar]


