Summary
The LRK10‐like receptor kinases (LRK10L‐RLKs) are ubiquitously present in higher plants, but knowledge of their expression and function is still limited. Here, we report expression and functional analysis of TtdLRK10L‐1, a typical LRK10L‐RLK in durum wheat (Triticum turgidum L. ssp. durum). The introns of TtdLRK10L‐1 contained multiple kinds of predicted cis‐elements. To investigate the potential effect of these cis‐elements on TtdLRK10L‐1 expression and function, two types of transgenic wheat lines were prepared, which expressed a GFP‐tagged TtdLRK10L‐1 protein (TtdLRK10L‐1:GFP) from the cDNA or genomic DNA (gDNA) sequence of TtdLRK10L‐1 under the native promoter. TtdLRK10L‐1:GFP expression was up‐regulated by the powdery mildew pathogen Blumeria graminis f. sp. tritici (Bgt) in both types of transgenic plants, with the scale of the elevation being much stronger in the gDNA lines. Both types of transgenic plants exhibited enhanced resistance to Bgt infection relative to wild type control. Notably, the Bgt defence activated in the gDNA lines was significantly stronger than that in the cDNA lines. Further analysis revealed that a putative MYB transcription factor binding site (MYB‐BS, CAGTTA) located in TtdLRK10L‐1 intron I was critical for the efficient expression and function of TtdLRK10L‐1 in Bgt defence. This MYB‐BS could also increase the activity of a superpromoter widely used in ectopic gene expression studies in plants. Together, our results deepen the understanding of the expression and functional characteristics of LRK10L‐RLKs. TtdLRK10L‐1 is likely useful for further dissecting the molecular processes underlying wheat defence against Bgt and for developing Bgt resistant wheat crops.
Keywords: intron, MYB binding site, powdery mildew, receptor‐like kinase, TtdLRK10L‐1, wheat
Introduction
Plants have evolved a sophisticated innate immune system to defend against pathogen attack, which functions in two forms, pathogen‐associated molecular pattern (PAMP)‐triggered immunity (PTI) and effector‐triggered immunity (ETI) (Jones and Dangl, 2006; Zhou and Zhang, 2020). Many plant receptor‐like kinases (RLKs) have been shown to perceive and process the signals from invading pathogens in diverse pathosystems (Tang et al., 2017). They often serve as pattern‐recognizing receptors (PRRs) in PTI and are rapidly activated by specific pathogen effectors (Dardick et al., 2012; Tang et al., 2017; Zhou and Zhang, 2020; Zhou et al., 2019).
In general, a typical RLK protein contains a variable ectodomain responsible for ligand binding, a single transmembrane domain, an intracellular juxtamembrane domain, and a cytoplasmic kinase domain (Shiu and Bleecker, 2003). Based on the structural features of extracellular regions, the plant RLK family has been divided into 46 different subfamilies (Shiu and Bleecker, 2003; Shiu et al., 2004). According to the presence or absence of the RD motif, which consists of a highly conserved positively charged arginine (R) located adjacent to the key negatively charged catalytic aspartate (D) in the kinase domain, plant and animal RLKs can be classified as RD and non‐RD kinases (Dardick and Ronald, 2006; Dardick et al., 2012). The function of RLKs in plant immune response involves specific phosphorylation events within and outside of the kinase domain, which leads to altered kinase activity and thus immune signal transduction (Dardick et al., 2012; Tang et al., 2017; Zhou and Zhang, 2020). To date, the function of several RLKs in pathogen resistance has been extensively studied in the model plants with simpler genomes, for example, rice and Arabidopsis (Tang et al., 2017; Zhou et al., 2019). The examples include XA21 (LRR XII subfamily) and XA26 (LRR XII subfamily) from rice (Chen et al., 2014; Song et al., 1995; Sun et al., 2004; Tang et al., 2019) and PR5K (LRK10L‐2 subfamily) and FLS2 (LRR XII subfamily) from Arabidopsis (Dunning et al., 2007; Lu et al., 2011; Wang et al., 1996). In contrast, much less progress is made in understanding the RLKs functioning in the disease resistance of the crop plants with complex genomes.
The powdery mildew disease caused by Blumeria graminis f. sp. tritici (Bgt) severely reduces the grain yield and quality of worldwide wheat crops including hexaploid common wheat (Triticum aestivum, AABBDD) and tetraploid durum wheat (Triticum turgidum L. ssp. durum, AABB) (Figueroa et al., 2018; Singh et al., 2016). Breeding and utilizing Bgt resistant wheat cultivars remain the most effective, economic and environmentally friendly approach to combat this disease (Singh et al., 2016; Zou et al., 2018). At present, two major types of powdery mildew resistance have been characterized, which are controlled by R genes and negative regulators of cellular defence, respectively (Zou et al., 2018). Some Pm resistance genes have been isolated by map‐based cloning that encode classic R proteins, but the resistance mediated by them tends to be Bgt race‐specific (Bourras et al., 2019; He et al., 2018; Hurni et al., 2013; Krattinger et al., 2009; Lu et al., 2020; Moore et al., 2015; Singh et al., 2018; Xie et al., 2020; Xing et al., 2018; Yahiaoui et al., 2004; Zou et al., 2018). On the other hand, the powdery mildew resistance conferred by mutations in the negative regulators, for example, mildew resistance locus (MLO) and enhanced disease resistance 1 (EDR1), appears to be broad‐spectrum (Acevedo‐Garcia et al., 2017; Wang et al., 2014; Zhang et al., 2017). In addition, several RLKs, namely, TaRLK1 and TaRLK2 from wheat (Chen et al., 2016) and RLK‐V and LecRK‐V from Haynaldia villosa (Hu et al., 2018; Wang et al., 2018), have been shown to regulate wheat defence to Bgt. TaRLK1 and TaRLK2 are leucine‐rich‐repeat (LRR) RLKs; their overexpression in transgenic wheat leads to enhanced Bgt resistance via increased production of reactive oxygen species (ROS) in fungal penetration sites (Chen et al., 2016). RLK‐V is a malectin‐like/LRR‐RLK that regulates both basal defence and Pm21‐mediated resistance to Bgt with the involvement of elevated ROS accumulation (Hu et al., 2018). LecRK‐V is a lectin type receptor‐like kinase, whose overexpression confers resistance to Bgt through enhancing ROS and cell death upon Bgt infection (Wang et al., 2018). TaRLK1, TaRLK2, RLK‐V and LecRK‐V are all RD kinases (Chen et al., 2016; Hu et al., 2018; Wang et al., 2018).
LRK10‐like RLKs (LRK10L‐RLKs) were firstly reported in wheat and relatives, but later studies indicate that these proteins are widespread in higher plants and constitute a distinct subfamily (LRK10L‐2) of plant RLKs (Cheng et al., 2003; Dezhsetan, 2017; Feuillet et al., 1997, 1998; Sakamoto et al., 2012; Shiu and Bleecker, 2003; Shiu et al., 2004). The primary structure of LRK10 proteins includes a signal peptide, a cysteine‐rich extracellular domain, a transmembrane domain and a predicted intracellular serine/threonine kinase domain. They are targeted to the plasma membrane and belong to the non‐RD kinases (Dardick et al., 2012; Feuillet et al., 1997, 1998; Zhou et al., 2007). Furthermore, one LRK10 member, TaRLK‐R3, has been shown to possess auto‐phosphorylation activity in vitro (Zhou et al., 2007). The expression of LRK10 genes is regulated both developmentally and environmentally. In wheat, they are inducible by light, more highly expressed in mature leaves, and up‐regulated by diverse abiotic and biotic stresses (Feuillet et al., 1997, 1998; Zhou et al., 2007). The complex regulation of LRK10 gene expression correlates with the presence of numerous putative cis‐elements in the promoter and intronic regions (Zhou et al., 2007), although no experimental evidence has yet been obtained to validate the function of these cis‐elements. Importantly, three LRK10L‐RLKs (TaRLK‐R1, ‐R2 and ‐R3) have been demonstrated to contribute positively to wheat hypersensitive resistance to the stripe rust fungus (Puccinia striiformis f. sp. tritici) (Zhou et al., 2007). However, it is still unknown if LRK10L‐RLKs may participate in wheat defence response to Bgt infection.
From the information presented above, the main objectives of this work were to investigate if LRK10L‐RLKs may function in wheat defence against Bgt and whether the intronic cis‐elements may affect the expression and function of LRK10L‐RLKs. Towards this end, we studied the expression and function of TtdLRK10L‐1 in wheat defence against Bgt infection. TtdLRK10L‐1 was identified in the durum wheat cultivar Stewart and resembled highly LRK10 in both amino acid sequence and primary structure. Like the genes encoding TaRLK‐R1, ‐R2 and ‐R3, TtdLRK10L‐1 carried abundantly predicted cis‐elements in its introns. Central to this study, we prepared two types of transgenic wheat lines that ectopically expressed GFP‐tagged TtdLRK10L‐1 protein (i.e. TtdLRK10L‐1:GFP) from the cDNA coding sequence of TtdLRK10L‐1 or its genomic open reading frame (ORF) under the native promoter. The results from analysing these transgenic plants, together with the data obtained from single‐cell Bgt defence tests and transient luciferase (LUC) reporter assays, allowed us to reveal the contribution of TtdLRK10L‐1 to durum wheat resistance against Bgt infection as well as the positive role of an intronic putative MYB binding site (MYB‐BS) in the expression and function of TtdLRK10L‐1. Remarkably, the presence of this MYB‐BS could also enhance the activity of a superpromoter, which was developed by combining a trimer of the octopine synthase transcriptional activating element and the activator‐promoter region of mannopine synthase2′ and has been highly useful for ectopic gene expression studies in both monocot and dicot plants (Lee et al., 2007). Thus, our work provides new insight into the function of TtdLRK10L‐1 and enriches the knowledge of gene expression regulation by intron‐located cis‐elements.
Results
Structural and expression features of TtdLRK10L‐1
Previously, we conducted a preliminary functional analysis of three LRK10L‐RLKs (TaLRK‐R1, ‐R2 and ‐R3) in hexaploid common wheat (Zhou et al., 2007). But further molecular and mechanistic studies of these RLKs are hampered by the difficulty in genetically transforming the common wheat varieties (Suwon 11, Chinese Spring, and Xiaoyan 54) used in the original work. To deepen our research, we decided to analyse LRK10L‐RLKs in the spring durum wheat cultivar Stewart that is amenable to Agrobacterium‐mediated genetic transformation (He et al., 2010).
In the genomic sequence of the hexaploid wheat variety Chinese Spring (CS), seven LRK10 genes were annotated, which were located on 1A (TraesCS1A02G018000 and TraesCS1A02G018600), 1B (TraesCS1B02G020700 and TraesCS1B02G022400) and 1D (TraesCS1D02G015300, TraesCS1D02G015900 and TraesCS1D02G017800) chromosomes, respectively (Table 1). Nucleic acid and amino acid sequence comparisons indicated that TraesCS1A02G018600 corresponded to LRK10, whereas TraesCS1B02G022400, TraesCS1D02G017800 and TraesCS1B02G020700 were TaLRK‐R1, ‐R2 and ‐R3, respectively (Table 1). In the genome sequence of the durum wheat cultivar Svevo, four LRK10 genes have been annotated, which were located on 1A (TRITD1Av1G004220) and 1B (TRITD1Bv1G004010 and TRITD1Bv1G004020) chromosomes or remained to be assigned to a specific chromosome (TRITD0Uv1G102600) (Table 1). Three to four LRK10 genes were also present in the annotated genome sequence of T. urartu, Ae. tauschii or wild emmer wheat (Table 1). The deduced protein of TRITD1Av1G004220 was highly similar to LRK10 and TaRLK‐R1, ‐R2 and ‐R3 in both amino acid sequence and primary structure (Figure S1). In phylogenetic analysis, TaRLK‐R1, TaRLK‐R2, TaRLK‐R3 and TRITD1Av1G004220 clustered in different sub‐branches (Figure S2), indicating that the four genes may have similar but not identical functions. Thus, we focused on TRITD1Av1G004220, which encodes TtdLRK10L‐1, to facilitate subsequent experimentation.
Table 1.
TtdLRK10L‐1 and its homologs annotated in the genomic sequence of common wheat and closely related Triticeae species
|
Species (Genotype) |
Chromosome | Locus | Correspondence to the genes investigated | Size of genomic ORF (bp) | Size of intron I (bp) | Size of intron II (bp) |
|---|---|---|---|---|---|---|
| Common wheat (Chinese Spring) | 1A | TraesCS1A02G018000 | ‐ | 4491 | 2430 | 156 |
| 1A | TraesCS1A02G018600 | LRK10 | 2950 | 831 | 181 | |
| 1B | TraesCS1B02G020700 | TaRLK‐R3 | 3177 | 1096 | 185 | |
| 1B | TraesCS1B02G022400 | TaRLK‐R1 | 2925 | 824 | 178 | |
| 1D | TraesCS1D02G015300 | ‐ | 3081 | 1007 | 169 | |
| 1D | TraesCS1D02G015900 | ‐ | 3381 | 1315 | 182 | |
| 1D | TraesCS1D02G017800 | TaRLK‐R2 | 2912 | 820 | 178 | |
| Durum wheat (Svevo) | 1A | TRITD1Av1G004220 | TtdLRK10L‐1 | 3108 | 1015 | 191 |
| 1B | TRITD1Bv1G004010 | ‐ | 3489 | 1141 | 178 | |
| 1B | TRITD1Bv1G004020 | ‐ | 2725 | 598 | 186 | |
| Un | TRITD0Uv1G102600 | ‐ | 2724 | 593 | 181 | |
| Wild emmer wheat (Zavitan) | 1A | TRIDC1AG001890 | ‐ | 3431 | 1339 | 178 |
| 1A | TRIDC1AG001960 | ‐ | 2761 | 616 | 186 | |
| Un | TRIDC0UG001810 | ‐ | 2740 | 598 | 186 | |
| Un | TRIDC0UG021350 | ‐ | 3269 | 1111 | 184 | |
|
T. urartu (G1812) |
1A | TuG1812G0100000266 | ‐ | 3183 | 594 | 350 |
| Un | TuG1812S0002479500 | ‐ | 2878 | 592 | 348 | |
| Un | TuG1812S0003465600 | ‐ | 2718 | 599 | 181 | |
| Ae. tauschii (AL8/78) | 1D | AET1Gv20037000 | ‐ | 3437 | 1361 | 168 |
| 1D | AET1Gv20040600 | ‐ | 3018 | 847 | 178 | |
| 1D | AET1Gv20040700 | ‐ | 2741 | 588 | 185 |
ORF, open reading frame. Un, unassigned to specific chromosomes.
The TtdLRK10L‐1 cDNA sequence isolated from Stewart was 100% identical to that of TRITD1Av1G004220. The deduced protein of TtdLRK10L‐1 contained a signal peptide, a cysteine‐rich extracellular domain, a transmembrane domain and an intracellular kinase domain containing the 12 subdomains shared by plant serine/threonine protein kinases (Figure S1). The genomic sequence of TtdLRK10L‐1 had two introns and three exons, with the size of the first intron (intron I, 1015 bp) being substantially larger than that of the second intron (intron II, 191 bp) (Figure 1a). When analysed in the NEW PLACE database (Higo et al., 1999), multiple putative cis‐elements were found in the two introns of TtdLRK10L‐1 (Table S1). These cis‐elements were likely involved in transcription factor binding, abiotic stress response, tissue‐specific expression and phytohormone induction. Notably, an MYB‐BS containing the consensus MYB transcription factor recognition sequence (TAACTG) and two W‐box transcription factor binding elements were present in only intron I (Table S1). Like what was recorded for LRK10L‐RLKs by previous studies (Feuillet et al., 1997, 1998; Zhou et al., 2007), TtdLRK10L‐1 expression was induced by light and highly abundant in wheat leaf tissues (Figure S3).
Figure 1.

Characterization of TtdLRK10L‐1 genomic sequence and two types of transgenic plants. (a) Exon and intron structure of TtdLRK10L‐1 as compared to that of LRK10, TaRLK‐R1, ‐R2 and –R3. Exons and introns were represented by black rectangles and lines, respectively. The values depict the lengths of exons and introns (bp). ATG, start codon; TAA, stop codon. (b) Schematic representation of the T‐DNA region in the transformation constructs pNP:TtdLRK10L‐1cDNA‐GFP (top panel) or pNP:TtdLRK10L‐1gDNA‐GFP (lower panel). (c) Molecular identification of six transgenic lines developed using pNP:TtdLRK10L‐1cDNA‐GFP (CL‐1, −2 and −3) or pNP:TtdLRK10L‐1gDNA‐GFP (GL‐1, −2 and −3), with wild type (WT) Stewart and a transgenic null segregant (TNS) as negative controls. Transgene presence and transcription were specifically detected in the six transgenic lines by genomic and RT‐PCR assays, respectively. The GFP‐tagged TtdLRK10L‐1, with an expected molecular mass of 97 kD (70 kD TtdLRK10L‐1 + 27 kD GFP) was specifically detected by immunoblotting with a GFP antibody. The relative intensities of TtdLRK10L‐1:GFP bands, reflecting TtdLRK10L‐1:GFP accumulation levels, are quantified using ImageJ (https://imagej.nih.gov/ij/) and shown above the graph. Immunodetection of plant actin protein (~ 43 kD) served as a control for equal loading. Ponceau S staining of Rubisco large subunit (~ 55 kD) also demonstrated equal loading across the compared samples. This set of assays was each repeated three times with nearly identical results obtained. (d) Targeting of TtdLRK10L‐1:GFP fusion protein to the plasma membrane in the leaf cells of the cDNA and gDNA transgenic lines but not the TNS control. Scale bars, 50 μm. The images shown were representative of three separate confocal microscopic examinations, with at least four different plants examined per line per assay.
Development and characterization of two types of transgenic lines
To facilitate further expression and functional investigations, we prepared two types of transgenic Stewart wheat lines using TtdLRK10L‐1 native promoter. Three homozygous and independent cDNA lines (CL‐1, CL‐2 and CL‐3) expressed a TtdLRK10L‐1‐GFP transgene from the cDNA coding sequence devoid of introns I and II; another three homozygous and independent gDNA lines (GL‐1, GL‐2 and GL‐3) expressed the same transgene but from the genomic sequence containing both introns (Figure 1b). Real‐time TaqMan PCR assays showed that CL‐1, CL‐2, CL‐3, GL‐1, GL‐2 and GL‐3 each harboured a single copy of TtdLRK10L‐1‐GFP transgene (Figure S4). Consistent with these results, genomic PCR, RT‐PCR and protein blot assays revealed the presence and active transcription and translation of TtdLRK10L‐1‐GFP in the cDNA and gDNA transgenic lines (Figure 1c). Notably, the protein level of TtdLRK10L‐1:GFP was higher in GL‐1, GL‐2 and GL‐3 than in CL‐1, CL‐2 and CL‐3 (Figure 1c, lower panel). Confocal microscopy indicated that TtdLRK10L‐1:GFP was targeted to the plasma membrane in the leaf epidermal cells of both cDNA and gDNA transgenic lines (Figure 1d). Consistent with this finding, the TtdLRK10L‐1:GFP fusion protein expressed in wheat mesophyll protoplasts was located in the plasma membrane (Figure S5).
Higher transgene expression and stronger Bgt defence in gDNA transgenic lines
The cDNA and gDNA transgenic lines were inoculated with Bgt at the seedling stage. Quantitative RT‐PCR assay showed that the transcript level of endogenous TtdLRK10L‐1 was gradually increased by Bgt infection (Figure S6). Likewise, the TtdLRK10L‐1‐GFP transgene was significantly up‐regulated by Bgt infection at 24 and 48 hours post‐inoculation (hpi), with the magnitude of the elevation being much stronger in the three gDNA lines (GL‐1, GL‐2 and GL‐3) (Figure 2a). The protein level of TtdLRK10L‐1:GFP was also enhanced by Bgt infection, with the scale of the increase being larger for the gDNA lines GL‐1, GL‐2 and GL‐3 (Figure 2b). At 72 hpi, both the cDNA and gDNA transgenic lines developed fewer Bgt microcolonies than the controls, which included wild type (WT) Stewart and a transgene null segregant (TNS) (Figure 2c). The six transgenic lines also had decreased Bgt colonies on their leaves than WT Stewart and TNS at seven days post‐inoculation (dpi) (Figure 2d and e). Importantly, GL‐1, GL‐2 and GL‐3 consistently exhibited significantly lower Bgt growth than CL‐1, CL‐2 and CL‐3 (Figure 2c, d and e), indicating the occurrence of more potent Bgt defence in the three gDNA transgenic lines compared to the three cDNA lines.
Figure 2.
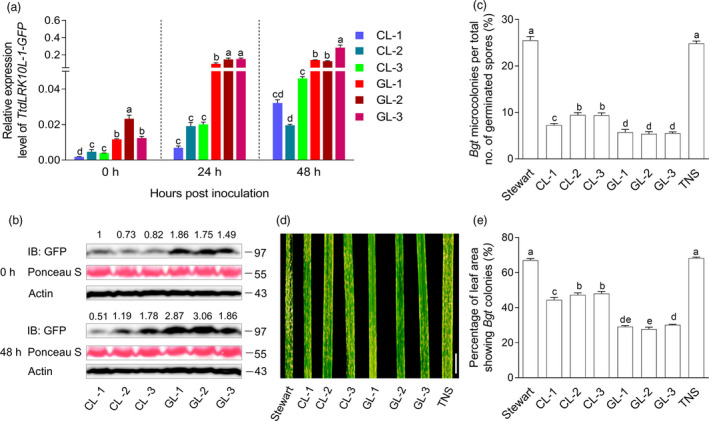
Comparative analysis of TtdLRK10L‐1 cDNA (CL‐1, −2 and −3) and gDNA (GL‐1, −2 and −3) transgenic lines in their response to powdery mildew (Bgt) infection. (a) Expression levels of TtdLRK10L‐1‐GFP transgene in the cDNA and gDNA transgenic lines before Bgt inoculation (0 h) and at two time points (24 h and 48 h) post‐Bgt inoculation as assessed by qRT‐PCR. Each data point was the mean (± SD) of the expression values obtained from four separate plants. The assay was repeated three times with similar results generated. (b) Detection of TtdLRK10L‐1:GFP fusion protein (~ 97 kD) in the six transgenic lines at 0 and 48 h post‐Bgt inoculation by immunoblotting with a GFP specific antibody. The relative intensities of TtdLRK10L‐1:GFP bands, reflecting TtdLRK10L‐1:GFP accumulation levels, are quantified using ImageJ (https://imagej.nih.gov/ij/) and shown above the graph. Equal loading of protein samples was demonstrated by Ponceau S staining of Rubisco large subunit (~ 55 kD) or by immunodetection of plant actin protein (~ 43 kD). The data shown were representative of three independent experiments. (c) Percentage of microcolonies formed from the total number of germinated spores of Bgt determined for the six transgenic lines and the two control materials, WT Stewart and transgene null segregant (TNS), at 72 h post‐Bgt inoculation. Each data point was the mean (± SD) of three separate Bgt inoculation assays, with at least 600 germinated spores examined per genotype per assay. (d) Bgt colonies developed on the leaf surface of the six transgenic lines and the two controls (WT Stewart and TNS) at seven days post‐inoculation. The images displayed were representative of three independent inoculation experiments. Scale bars, 1 cm. (e) Quantification of the percentages of leaf areas with Bgt colonies using ImageJ in the different leaf samples in (d). In (a), (c) and (e), each data point was the mean ( SD) of three separate measurements. The means were analysed using Duncan’s multiple comparison test, with those marked by different letters being statistically significant (P < 0.05).
Oxidative bursts at Bgt penetration site (type a) or throughout a Bgt‐infected cell (type b) constitute an important early event of strong host cell defence to powdery mildew infection (Chen et al., 2016; Hu et al., 2018; Wang et al., 2018). The two types of oxidative bursts can be reliably detected by histological staining of H2O2 accumulation with 3,3’‐diaminobenzidine (DAB) (Figure 3a). At 24 hpi, the percentages of cells showing the two types of oxidative bursts were lowest in WT Stewart (4.17% and 0.66%) and TNS control (4.34% and 0.83%) plants, intermediate in the three cDNA transgenic lines (CL‐1: 12.17% and 2.16% ; CL‐2: 11.17% and 3.5%; CL‐3: 11.67% and 3.66%), and highest in the three gDNA lines (GL‐1: 16.50% and 7.67%; GL‐2: 18.50% and 6.50% ; GL‐3: 16.50% and 7.00%) (Figure 3b). On the other hand, the percentage of cells with no oxidative burst (type c), which indicates a susceptible response, was highest in WT Stewart and TNS control plants, intermediate in the three cDNA lines, and lowest in the three gDNA lines (Figure 3b).
Figure 3.

Analysis of Bgt‐induced oxidative bursts in the cDNA (CL‐1, −2 and −3) and gDNA (GL‐1, −2 and −3) transgenic lines and the two control materials, WT Stewart and transgene null segregant (TNS), at 48 h post‐pathogen inoculation. (a) Three types of cellular oxidative bursts as revealed by staining with 3,3’‐diaminobenzidine. Type a, oxidative burst at Bgt penetration site. Type b, oxidative burst distributed throughout the cell. Type c, no detectable oxidative burst. Scale bars, 50 μm. (b) Quantitative comparison of the percentages of cells showing types a, b or c oxidative bursts in the transgenic and control lines. Each mean (± SD) was calculated with the data obtained from four separate plants. The means were statistically compared using Duncan’s multiple comparison test, with those marked by different letters being significantly different (P < 0.05). The staining assay was performed twice with similar results obtained.
Effect of intron I on TtdLRK10L‐1 mediated defence against Bgt
The findings that the three gDNA lines displayed higher expression of TtdLRK10L‐1‐GFP transgene and stronger Bgt defence than the three cDNA lines indicated the potential involvement of introns in regulating TtdLRK10L‐1 expression and function. To test this possibility, the cDNA coding sequence and the genomic ORF of TtdLRK10L‐1, as well as the four mutants with only intron I, intron II, intron I with mutant MYB binding site (intron I/mMYB‐BS) or intron I with the two W‐box elements mutated (intron I/mW‐box) (Figure 4a), were used to perform single‐cell Bgt defence assays in one‐week‐old leaves of Stewart seedlings. Bgt haustorium index (HI) in the transiently transformed cells, which is an effective indicator of the defence to Bgt infection (Shen et al., 2003, 2007; Zhang et al., 2016), was scored, with the HI values of the examined constructs statistically compared. As shown in Figure 4b, the highest HI values were obtained for pHZ206(VC) and pUbi:TtdLRK10L‐1intron II, whereas the lowest HI values were recorded for pUbi:TtdLRK10L‐1gDNA, pUbi:TtdLRK10L‐1intron I and pUbi:TtdLRK10L‐1intron I/mW‐box, with the HI values determined for pUbi:TtdLRK10L‐1cDNA and pUbi:TtdLRK10L‐1intron I/mMYB‐BS being intermediate. Together, these data indicated that lacking intron I (as in pUbi:TtdLRK10L‐1intron II) substantially lowered TtdLRK10L‐1 mediated Bgt defence, removing intron II (pUbi:TtdLRK10L‐1intron I) or mutating the W‐boxes in intron I (pUbi:TtdLRK10L‐1intron I/mW‐box) had no significant effect on TtdLRK10L‐1 function in Bgt defence, and mutating the MYB‐BS in intron I (pUbi:TtdLRK10L‐1intron I/mMYB‐BS) further debilitated the promotion of Bgt defence by TtdLRK10L‐1.
Figure 4.

Analysis of intron involvement in the expression and function of TtdLRK10L‐1 using single‐cell Bgt defence tests. (a) A diagram showing WT and mutant ORFs of TtdLRK10L‐1 used in the assays. The putative wild type MYB‐BS and W‐box elements, unique to intron I, were represented by filled red and green diamonds, respectively. Dashed lines indicate specific sequences removed in different mutants. The mutant MYB‐BS, created by mutating CAGTTA to AAAAAA, is marked by an empty red diamond. The two empty green diamonds indicate mutant W‐box elements developed by changing GGTCA to AAAAA. (b) Haustorium index values conferred by expressing WT and mutant ORFs of TtdLRK10L‐1 in single‐cell Bgt defence assays. The six ORFs were all expressed under the ubiquitin gene promoter (pUbi). The pHZ206 plasmid, carrying an empty pUbi expression cassette, served as a vector control (VC) during the tests. Each haustorium index value was mean (± SD) of the data generated in three independent replicates, with ≥ 40 interactive cells examined in each replicate. The means were statistically analysed using Duncan’s multiple comparison test, with those marked by different letters being significantly different (P < 0.05). The data shown were representative of three separate tests.
Enhancement of superpromoter activity by the putative MYB‐BS in TtdLRK10L‐1 intron I
Further to the above experiment, we examined if the MYB‐BS (CAGTTA) in TtdLRK10L‐1 intron I might increase the activity of the superpromoter (SP), which is less susceptible to silencing and has been extensively used in the ectopic gene expression studies of plants (Lee et al., 2007). For this investigation, the T‐DNA vector SP1300:LUC, which carries a superpromoter directed LUC expression cassette, was used to create the desired reporter constructs. These constructs harboured TtdLRK10L‐1gDNA, TtdLRK10L‐1cDNA, TtdLRK10L‐1intron I, TtdLRK10L‐1intron II, TtdLRK10L‐1intron I/mMYB‐BS or TtdLRK10L‐1intron I/mW‐box upstream of the coding sequence of LUC (Figure S7). After confirming in‐frame fusion with the LUC coding sequence in each construct, three sets of agroinfiltration assays were executed in tobacco (Nicotiana benthamiana) leaves. In the first set, the LUC signals elicited by SP:TtdLRK10L‐1cDNA‐LUC, SP:TtdLRK10L‐1gDNA‐LUC, SP:TtdLRK10L‐1intron I‐LUC or SP:TtdLRK10L‐1intron II‐LUC were compared (Figure 5a, d), which showed that the presence of intron I (as in SP:TtdLRK10L‐1gDNA‐LUC and SP:TtdLRK10L‐1intron I‐LUC) was necessary and sufficient for achieving a higher level of LUC signals. In the second set, the LUC signals produced by SP:TtdLRK10L‐1cDNA‐LUC, SP:TtdLRK10L‐1gDNA‐LUC, SP:TtdLRK10L‐1intron I/mMYB‐BS‐LUC or SP:TtdLRK10L‐1intron I/mW‐box‐LUC were compared (Figure 5b, e), which indicated that the existence of MYB‐BS, but not the W‐box elements, was required for the higher LUC signals elicited by SP:TtdLRK10L‐1gDNA‐LUC. In the third set, the LUC signals derived from SP:TtdLRK10L‐1intron I‐LUC, SP:TtdLRK10L‐1intron I/mMYB‐BS‐LUC or SP:TtdLRK10L‐1intron I/mW‐box‐LUC were evaluated (Figure 5c, f). The result of this set, together with that obtained in the second set (Figure 5b, d and e), confirmed the importance of the MYB‐BS, but not the W‐box elements, in yielding the higher LUC signals by SP:TtdLRK10L‐1gDNA‐LUC or SP:TtdLRK10L‐1intron I‐LUC. Considering that SP was used to create all six examined cassettes, it was logic to suggest that the putative MYB‐BS in TtdLRK10L‐1 intron I enhanced the activity of SP in SP:TtdLRK10L‐1gDNA‐LUC, SP:TtdLRK10L‐1intron I‐LUC and SP:TtdLRK10L‐1intron I/mW‐box‐LUC, thus enabling them to produce higher levels of LUC signals than SP:TtdLRK10L‐1cDNA‐LUC, SP:TtdLRK10L‐1intron II‐LUC and SP:TtdLRK10L‐1intron I/mMYB‐BS‐LUC, in which the normal MYB‐BS (CAGTTA) was either absent or mutated to AAAAAA.
Figure 5.

Effect of the putative MYB binding site (MYB‐BS) in TtdLRK10L‐1 intron I on superpromoter activity analysed using LUC reporter assays conducted with the leaves of Nicotiana benthamiana. (a‐c) LUC signals generated in three sets of reporter assays that were initiated by different combinations of the constructs expressing WT or mutant TtdLRK10L‐1 ORFs (as explained in Figure 4a). The ORFs were all expressed under the direction of the superpromoter. (d‐f) Quantitative analysis of LUC signals generated in the assays depicted in (a), (b) and (c), respectively. Each data point was mean (± SD) of the LUC signals generated from four different plants. The means were statistically compared using Duncan’s multiple comparison test, with those labelled by different letters being significantly different (P < 0.05). The whole dataset shown was typical of three independent experiments.
Discussion
Owing to their wide presence in higher plants, LRK‐RLKs may well play important roles in the growth and development of plants and their responses to biotic and abiotic responses, although there is still little functional information on these RLKs. Further to the original observations made on LRK10L‐RLKs in wheat and related Triticeae species (Cheng et al., 2003; Feuillet et al., 1997, 1998; Zhou et al., 2007), we here studied in more detail the expression and function of TtdLRK10L‐1, a typical LRK10L‐RLK from durum wheat.
TtdLRK10L‐1 functions in wheat defence against Bgt via positively regulating oxidative burst
As revealed by functional genomics studies (Fu et al., 2016; Li et al., 2016; Xin et al., 2012; Xing et al., 2017; Zhang et al., 2019; Zhang et al., 2016), numerous genes and multiple defence processes are activated in wheat responses to Bgt infection in both compatible and incompatible interactions, whose dissection is essential for understanding and improving wheat resistance to powdery mildew disease. Based on the evidence gathered here, we suggest that TtdLRK10L‐1 functions in wheat defence against Bgt infection by promoting oxidative burst. As shown by analysing the expression of TtdLRK10L‐1‐GFP transgene directed by the native promoter (Figures 1 and 2), Bgt infection can trigger the active transcription and translation of TtdLRK10L‐1, with the resultant proteins targeted to the plasma membrane, a cellular location likely conducive for the proper action of TtdLRK10L‐1. Compelling evidence for the function of TtdLRK10L‐1 in Bgt defence comes from the finding that the expression of TtdLRK10L‐1‐GFP transgene, from either the cDNA coding sequence or the genomic ORF, strongly decreased Bgt growth and development relative to that observed for WT Stewart and TNS controls (Figure 2). Because transgene expression was directed under the native promoter, it is highly likely that the functional information obtained from analysing TtdLRK10L‐1‐GFP reflects the authentic action of endogenous TtdLRK10L‐1. Thus, TtdLRK10L‐1, a canonic LRK10L‐RLK by its primary structure, joins the previously described LRR‐RLKs (TaRLK1 and TaRLK2), malectin‐like/LRR‐RLK (RLK‐V) and lectin‐RLK (LecRK‐V) in being able to positively contribute to wheat defence to Bgt. Since TtdLRK10L‐1 is a putative non‐RD kinase, and TaRLK1, TaRLK2, RLK‐V and LecRK‐V are all predicted to be RD kinases (Chen et al., 2016; Hu et al., 2018; Wang et al., 2018), we deduce that a wide spectrum of RLKs may participate in wheat defence processes to Bgt infection.
Oxidative burst, which is important in the process of plant defence, was observed in TaRLK1, TaRLK2, RLK‐V and LecRK‐V mediated wheat defence to Bgt infection (Chen et al., 2016; Hu et al., 2018; Wang et al., 2018). Consistently, we found that the enhanced Bgt defence response seen in the two types of transgenic lines was associated with increased oxidative bursts at both the Bgt penetration sites and the entire Bgt‐infected cells, with the degree of the increase positively correlating with the amount of TtdLRK10L‐1:GFP protein and the level of Bgt defence (Figures 2 and 3). Thus the regulation of oxidative burst is an integral component of the Bgt defence processes regulated by TtdLRK10L‐1. A plant NADPH oxidase, known as respiratory burst oxidase homolog D (RBOHD), has been shown to play a crucial role in pathogen infection‐induced oxidative burst (Couto and Zipfel, 2016; Kimura et al., 2017, 2020). Some plant kinases, including RLKs, have been shown to directly phosphorylate RBOHD, thereby regulating the production of ROS in response to pathogen attack or PAMP treatment (Kadota et al., 2014; Kimura et al., 2017, 2020; Lee et al., 2020; Li et al., 2014; Zhang et al., 2018). For example, Arabidopsis CRK2, an RLK with a cysteine‐rich ectodomain, directly phosphorylated a serine residue (S703) at the C‐terminal region of RBOHD, thus stimulating ROS burst in the cells infected by the bacterial pathogen Pseudomonas syringae pv tomato DC3000 (Pst DC3000); functional deficiency of CRK2 impaired ROS production and Arabidopsis defence to Pst DC3000 (Kimura et al., 2020). As TtdLRK10L‐1 extracellular domain is also rich in cysteine, and ROS burst and Bgt defence were both enhanced in the TtdLRK10L‐1:GFP expressing transgenic wheat plants, it will be interesting to examine if TtdLRK10L‐1 may modulate the phosphorylation status, and thus the function of RBOHD, in further research.
An intronic MYB‐BS is required for efficient expression and function of TtdLRK10L‐1 in wheat defence against Bgt
Intron‐mediated regulation (IMR) is an important mechanism for controlling gene expression levels in eukaryotic organisms (Gallegos and Rose, 2015, 2019; Laxa, 2017; Shaul, 2017). In higher plants, recent studies suggest that IMR has two forms, intron‐mediated enhancement (IME) and intron‐mediated suppression (IMS). A well‐analysed example of IME concerns the binding of two MYB transcription factors (PHR1 and PHL1) to the PHR1‐binding site located in the first intron of AtP5CS1 (Aleksza et al., 2017), which encodes Δ1‐pyrroline‐carboxylate synthetase crucial for proline biosynthesis from glutamic acid (Fichman et al., 2015). This IME regulates the expression and function of AtP5CS1 in phosphate depletion‐induced proline accumulation in Arabidopsis (Aleksza et al., 2017). A thoroughly studied example of IMS relates to the intronic cis‐element SE1, which recruits trans‐acting repressors to suppress the expression of rice Eui1 gene that encodes a gibberellic acid (GA) deactivating enzyme; loss of SE1 promotes Eui1 expression, leading to a decrease of bioactive GA and a dwarfing plant phenotype (Xie et al., 2018). To our knowledge, neither IME nor IMS has previously been analysed in detail for LRK10L members or other plant RLK genes. Here, by comparatively analysing two types of transgenic lines developed with the absence or presence of TtdLRKL10‐1 introns in the native promoter directed transgene (Figures 1, 2, 3), coupled with additional single single‐cell Bgt defence tests and LUC reporter assays (Figures 4 and 5), we stepwisely delineated a putative MYB‐BS (CAGTTA) in the first intron of TtdLRKL10‐1 that is required for efficient expression and function of TtdLRKL10‐1 in wheat defence to Bgt infection. However, further work is needed to identify the trans‐acting factor(s) that can bind to the MYB‐BS. As demonstrated by previous studies (e.g. Aleksza et al., 2017; Xie et al., 2018), this future goal is achievable using a combination of molecular genetic and biochemical approaches.
Judging from the LUC reporter assays, the intron I located MYB‐BS of TtdLRKL10‐1 can significantly enhance the activity of the superpromoter (Lee et al., 2007). This is remarkable because Lee et al. (2007) showed that it was not possible to increase the activity of this superpromoter using the leader intron of maize ubiquitin gene (UbiI) in either transiently transfected protoplasts or stably transformed plants, although UbiI could effectively elevate the activity of pUbi. The same study also found that superpromoter activity could not be increased by an intron from the ST‐LS1 gene of potato either. Given these results, we speculate that the putative MYB‐BS and TtdLRKL10‐1 intron I may be employed to further increase the efficacy and versatility of the widely used superpromoter.
Apart from intron‐located cis‐elements, other factors have also been reported to affect the expression and function of plant genes including those encoding RLKs. For example, the malectin‐like/leucine‐rich repeat RLK, RLK‐V, has two alternatively spliced transcripts, with only one of them being functional in regulating wheat defence to Bgt (Hu et al., 2018). The 3′‐untranslated region (3′‐UTR) may also contain different types of cis‐elements (including miniature inverted‐repeat transposable element) capable of regulating plant gene expression at post‐transcriptional and/or translational levels (Bernardes and Menossi, 2020; Shen et al., 2017). Because of these facts, it will be interesting to investigate whether alternative mRNA splicing and 3′‐UTR may affect the expression and function of TtdLRK10L‐1 in further research. Finally, it is worth noting that we used TtdLRK10L‐1:GFP fusion protein to investigate the function of TtdLRK10L‐1 in this work. Although many studies in the past have used GFP fusions to examine the function of plant RLKs (e.g. Campos et al., 2020; Fàbregas et al., 2013; Park et al., 2013), there is a possibility that GFP fusion proteins of RLKs may differ from the native proteins to some extent. Consequently, further work is necessary to validate the function of TtdLRK10L‐1 in wheat defence to Bgt using the wheat lines expressing native TtdLRK10L‐1 protein.
In summary, this work has improved the understanding of LRK10L‐RLKs through discovering the function of TtdLRK10L‐1 in wheat defence against powdery mildew disease and by revealing the importance of an intron‐located MYB‐BS in enhancing the expression of TtdLRK10L‐1. TtdLRK10L‐1 may provide a new avenue for more systematically dissecting the molecular and physiological processes functioning in wheat defence against Bgt. This gene might also be useful for developing Bgt resistant wheat crops using molecular breeding methods including transgenic breeding and marker assisted selection of elite allele. In line with this view, we found TtdLRK10L‐1 orthologs in both wild emmer wheat and 10 diverse common wheat cultivars sequenced by the 10 + wheat genomes project (http://www.10wheatgenomes.com/) (Figure S8). Interestingly, there are molecular variations in exons and introns, as well as the putative MYB‐BS, among TaLRK10L‐1 alleles from the 10 common wheat cultivars (Figure S8), indicating the possibility of selecting superior alleles for breeding applications. Lastly, the putative MYB‐BS and its hosting intron in TtdLRK10L‐1, which showed a strong IME effect, may be harnessed to boost ectopic gene expression in future plant biotechnology research.
Experimental procedures
Plant materials
The spring type durum wheat cultivar Stewart and a laboratory strain of N. benthamiana were used in this work. Wheat plants were raised in a greenhouse under a 16‐h light/8‐h dark photoperiod, a day/night temperature cycle of 24/18 °C, and 60‐70% relative humidity. N. benthamiana plants were cultured in a growth room with the following environmental settings, a 16‐h light/8‐h dark photoperiod, a day/night temperature cycle of 23/20 °C, 60‐70% relative humidity, and a light intensity of approximately 130 μmol m‐2 s‐1.
DNA and RNA extractions and PCR assays
Genomic DNA was prepared using a CTAB‐based protocol as described previously (Saghai‐Maroof et al., 1984). Total RNA was isolated from the desired samples using TriPure Isolation Reagent (Roche, Indianapolis, IN, USA) and treated with RNase‐free DNase (Qiagen, Hilden, Germany) to eliminate contaminating genomic DNA according to the manufacturer’s instructions. Genomic PCR was conducted using the high‐fidelity Transstart FastPfu Fly DNA Polymerase (Transgen, Beijing, China). For RT‐PCR, first‐strand cDNA was synthesized using GoScriptTM Reverse Transcription System (Promega, Madison, WI, USA). The resulting cDNAs were subjected to RT‐PCR or qRT‐PCR assays as described previously (Zhou et al., 2007). Transstart FastPfu Fly DNA Polymerase was used in all RT‐PCR assays. The qRT‐PCR was performed using three independent RNA preparations as biological replicates with a LightCycler 480 Real‐time PCR system (Roche). The relative transcript levels of TtdLRK10L‐1 or TtdLRK10L‐1‐GFP were normalized to that of the reference gene Actin (GenBank accession AB181991.1) and calculated using the 2‐∆∆CT method (Livak and Schmittgen, 2001). Details of the oligonucleotide primers used in PCR assays are listed in Table S2.
Bioinformatic search of LRK10 genes, isolation of TtdLRK10L‐1 and cis‐element prediction
It is well known that LRK10 genes are located on group 1 chromosomes and exist as a multigene family in wheat and relatives (Feuillet et al., 1997, 1998; Zhou et al., 2007). To efficiently identify TtdLRK10L‐1 and its homologs in this work, we used the genomic sequence information of common wheat (IWGSC et al., 2018), durum wheat (Maccaferri et al., 2019), wild emmer wheat (Avni et al., 2017), T. urartu (Ling et al., 2018) and Aegilops tauschii (Luo et al., 2017). The last two species are progenitors of the A or D subgenomes of hexaploid and tetraploid wheat (Marcussen et al., 2014). Hence, the deduced amino acid sequence of LRK10 (GenBank accession U51330.1) was used to search the genomic databases of Chinese Spring (common wheat), Svevo (durum wheat), Zavitan (wild emmer wheat), G1812 (T. urartu) and AL8/78 (Ae. tauschii) at the Triticeae Multi‐omics centre (http://202.194.139.32), with the homologous genes identified using stringent identity (≥ 80%) and coverage (≥ 95%) cut‐offs. The deduced amino acid sequences of LRK10 homologs and Arabidopsis PR5K were aligned and used for constructing a phylogenetic tree using the neighbour‐joining method installed in the MEGA 5 package (Tamura et al., 2011). Subsequently, the cDNA coding sequence and genomic ORF of TtdLRK10L‐1 were isolated from Stewart by RT‐PCR and genomic PCR assays, respectively (see above). The New PLACE database (https://www.dna.affrc.go.jp/PLACE/?action=newplace; Higo et al., 1999) was used to predict putative cis‐elements in the two introns of TtdLRK10L‐1 (Table S1).
Development of transgenic lines and characterization of transgene copy number
Two constructs, pNP:TtdLRK10L‐1cDNA‐GFP and pNP:TtdLRK10L‐1gDNA‐GFP, were developed for transforming Stewart using the dual binary pGreenII‐UB/pSoup vector system as reported in previous studies (He et al., 2010; Hellens et al., 2000; Wang et al., 2016). For developing pNP:TtdLRK10L‐1cDNA‐GFP, the native promoter (NP, 1082 bp) and cDNA coding sequence (1899 bp, without stop codon) were amplified by PCR and cloned in two steps into an in‐house prepared plasmid vector pAct1:GFP that carried a GFP expression cassette under the direction of rice Act1 gene promoter. The first step replaced the Act1 promoter by NP, and the second step fused TtdLRK10L‐1 cDNA sequence with that of GFP. Subsequently, the NP:TtdLRK10L‐1cDNA‐GFP fragment was amplified using high‐fidelity PCR with attB containing primers, followed by cloning into the Gateway compatible vector pGreenII‐UB through BP and LR clonase reactions (Hartley et al., 2000), thus yielding pNP:TtdLRK10L‐1cDNA‐GFP. The construct pNP:TtdLRK10L‐1gDNA‐GFP was prepared similarly except that TtdLRK10L‐1 gDNA sequence (3105 bp, without stop codon) was used to fuse with GFP. After confirming in‐frame fusion between TtdLRK10L‐1 cDNA (gDNA) coding sequence with that of GFP by DNA sequencing, pNP:TtdLRK10L‐1cDNA‐GFP (pNP:TtdLRK10L‐1gDNA‐GFP) and the pSoup vector (pAL154) were co‐transformed into the Agrobacterium strain AGL1 for wheat transformation. The T0 positive plants were identified and propagated through T1 to T4. Eventually, three independent T4 homozygous cDNA lines (CL‐1, −2 and −3) and another three independent T4 homozygous gDNA lines (GL‐1, −2 and −3) were obtained with sufficient seeds for the different analyses performed in this work. A TNS plant identified in the T2 population was kept as a negative control in the study. The primers used in the cloning experiment are listed in Table S2.
Real‐time TaqMan assays were conducted to analyse transgene copy number in the six transgenic lines, with WT Stewart and the TNS line employed as negative controls. The assays were executed following the method detailed by Gadaleta et al. (2011) with some modifications. Briefly, two primers and one TaqMan probe, specific for TtdLRK10L‐1cDNA‐GFP and TtdLRK10L‐1gDNA‐GFP fusion cistrons (Figure S4), were designed and synthesized. The amplicon generated by the two primers was 86 bp; the TaqMan probe was 15 bp and labelled by FAM and BHQ1 moieties at its 5′‐ and 3′‐ends, respectively (Table S2). Two standard calibration curves (Figure S4), suitable for determining transgene copy number via real‐time absolute quantification of DNA, were developed using serial dilutions of the purified plasmids of pNP:TtdLRK10L‐1cDNA‐GFP or pNP:TtdLRK10L‐1gDNA‐GFP. TaqMan reactions were carried out in the StepOne Plus Real‐time PCR System (Thermo Fisher, Rockford, IL, USA) using 96 well reaction plates. Triplicate samples were analysed for each transgenic line, and the mean Ct value obtained was compared to that produced by a known starting amount of DNA in the calibration curve to infer transgene copy number in the analysed transgenic material (Figure S4). Two independent sets of TaqMan assays were performed with highly similar results obtained.
Detection of TtdLRK10L‐1:GFP fusion protein in transgenic wheat plants
Two sets of immunoblotting assays were performed to detect the accumulation of TtdLRK10L‐1:GFP in the two types of transgenic wheat with WT Stewart and the TNS line as negative controls. The first set used leaf samples from the plants grown under normal conditions, whereas the second set used those collected at different time points post‐Bgt inoculation. Total membrane proteins were isolated from each leaf sample (~ 0.7 g) using the Minute Plasma Membrane Protein Isolation Kit for Plants (Invent, Plymouth, MN, USA) following the supplier’s instruction. The resultant membrane proteins were separated on 12% SDS‐PAGE gel, and subsequently electroblotted onto Amersham Protran 0.45 μm NC membrane (GE Healthcare, Chicago, IL, USA). TtdLRK10L‐1:GFP was detected using a GFP specific antibody (Roche Diagnostics, catalog number: 11814460001) at 1:5000 dilution. The blots were then developed with HRP‐conjugated secondary antibody at 1:5000 dilution and the SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA). Detection of actin proteins, which used an Anti‐Plant‐actin Mouse Monoclonal Antibody (Easybio, Beijing, China), served as a control for equal protein loading. Plasma membrane targeting of TtdLRK10L‐1:GFP in the leaf cells of the two types of transgenic wheat grown under normal conditions was detected under a Zeiss LSM 710 confocal microscope.
Subcellular localization of TtdLRK10L‐1
The full‐length TtdLRK10L‐1 cDNA (TtdLRK10L‐1cDNA) and genomic ORF (TtdLRK10L‐1gDNA) without stop codon were each fused with GFP coding sequence to express TtdLRK10L‐1:GFP fusion protein in wheat protoplasts under the direction of maize ubiquitin gene promoter pUbi in the plasmid vector pHZ202. The cloning step used the restriction enzymes BamHI and KpnI and employed an In‐Fusion HD Cloning kit (Clontech, Mountain View, CA, USA). The resulting constructs (pUbi:TtdLRK10L‐1cDNA‐GFP, pUbi:TtdLRK10L‐1gDNA‐GFP) and pHZ202 (pUbi:GFP) were each transformed into the mesophyll protoplasts prepared from the wheat cultivar Kenong199. The plasma membrane was stained with 50 μΜ FM 4‐64 (Invitrogen, CA, USA) according to the method detailed by Ueda et al. (2001) with some modifications. Briefly, after 24 h incubation in W5 solution, transformed wheat protoplasts were collected by centrifugation, gently resuspended in W5 solution containing 50 μΜ FM 4‐64, and placed on ice for 10 min. The stained protoplasts were washed and resuspended in the W5 solution without FM 4‐64. After incubation for 30 min at room temperature, the protoplasts were visualized under a Zeiss LSM 710 confocal microscope.
Bgt inoculation and histochemical detection of H2O2
The Bgt race E09, maintained on the susceptible wheat cultivar Kenong 199, was used to inoculate the seedlings of the six transgenic lines and the two negative controls (Stewart and TNS) at the four‐leaf stage as reported previously (Wang et al., 2014; Zou et al., 2018). At 72 hpi, 10 leaves were harvested from each inoculated line and subjected to microcolony staining using Coomassie Brilliant Blue R 250 (Wang et al., 2014). For each line, at least 2000 germinated Bgt spores were examined with three independent replicates, which were used to calculate the percentage of microcolonies formed from the total number of germinated Bgt spores. Bgt colonies developed in the infected leaves of the compared lines were photographed at 7 dpi. The Bgt inoculation experiment was repeated three times with highly similar results obtained.
Histochemical detection of H2O2 was carried out at 24 hpi of Bgt. For each of the 8 examined lines, 8 to 10 leaves detached from 4 different plants were stained with 3,3’‐diaminobenzidine (Bio Basic Inc., Shanghai, China) following the procedure described by Chen et al. (2016). The division of oxidative burst into three types was performed according to Wang et al. (2018). Two independent H2O2 detection experiments were accomplished with highly similar findings made.
Single‐cell Bgt defence tests
Four mutant TtdLRK10L‐1 sequences, that is, TtdLRK10L‐1intron I, TtdLRK10L‐1intron II, TtdLRK10L‐1intron I/mMYB‐BS and TtdLRK10L‐1intron I/mW‐box, were synthesized commercially (Genewiz Ltd., Suzhou, China). In intron I/mMYB‐BS, the putative MYB‐BS (CAGTTA) unique to intron I was mutated to AAAAAA, while in intron I/mW‐box, the two predicted W‐box (GGTCA) elements specifically present in intron I were both changed to AAAAA. The four mutants, as well as the cDNA coding sequence (TtdLRK10L‐1cDNA) and genomic ORF (TtdLRK10L‐1gDNA) of TtdLRK10L‐1, were each transferred into the plasmid vector pHZ206 that contained an empty pUbi cassette. This step used the restriction enzymes BamHI and KpnI and employed an In‐Fusion HD Cloning kit (Clontech, Mountain View, CA, USA). The resultant constructs, pUbi:TtdLRK10L‐1gDNA, pUbi:TtdLRK10L‐1cDNA, pUbi:TtdLRK10L‐1intron I, pUbi:TtdLRK10L‐1intron II, pUbi:TtdLRK10L‐1intron I/mMYB‐BS , pUbi:TtdLRK10L‐1intron I/mW‐box, as well as an empty vector control (VC) pHZ206 and a reporter construct expressing the marker gene β‐glucuronidase (Zheng et al., 2020), were used to conduct single‐cell Bgt defence tests by biolistic delivery of plasmid DNA into the epidermal cells of one‐week‐old Stewart leaves as detailed previously (Shen et al., 2003, 2007; Wang et al., 2014). Following Bgt inoculation, the interactive epidermal cells, which not only were successfully transfected by plasmid constructs and but also had germinating Bgt spores, were microscopically examined for haustorium growth. Three independent replicates were checked for each construct, with at least 40 interactive cells observed in each replicate. The cells with haustorium growth were recorded and used to calculate a haustorium index against the total number of interactive cells examined. Three separate single‐cell Bgt defence tests were executed with similar sets of HI data generated.
LUC reporter assays
The four mutants of TtdLRK10L‐1 as well as its cDNA and gDNA coding regions were amplified by PCR, digested with XbaI and KpnI, and cloned into the binary vector SP1300:LUC, which carried a superpromoter directed LUC expression cassette in pCAMBIA1300 plasmid. This superpromoter, developed using octopine synthase transcriptional activating element and the activator‐promoter region of mannopine synthase2′, has a stronger activity than the enhanced double CaMV 35S (Lee et al., 2007). The cloning step, executed using the In‐Fusion HD Cloning kit (Clontech, Mountain View, CA, USA), produced pSP:TtdLRK10L‐1gDNA, pSP:TtdLRK10L‐1cDNA, pSP:TtdLRK10L‐1intron I, pSP:TtdLRK10L‐1intron II, pSP:TtdLRK10L‐1intron I/mMYB‐BS and pSP:TtdLRK10L‐1intron I/mW‐box (Figure S7). The six constructs were separately introduced into the cells of Agrobacterium tumefaciens (strain GV3101), and then used to perform three sets of reporter assays (Figure 5) as outlined by Yang et al. (2017). Briefly, GV3101 cells carrying the above constructs were grown in LB medium, harvested and resuspended, followed by infiltration into the leaves of 5‐week‐old N. benthamiana plants. The infiltrated leaves were sprayed with 100 μM luciferin (Promega) at 48 h post‐infiltration and kept in dark for 5 min before recording luminescence using the Nightshade LB 985 In vivo Plant Imaging System (Berthold Technologies, Bad Wildbad, Germany). Three independent biological replicates were examined for each set of the assay, with each replicate involving the examination of four leaves from four separate plants. The LUC reporter assays were repeated three times with highly similar data obtained.
Statistical analysis
Numerical values were presented as means ± SD. Statistical comparison of the means was performed using Duncan’s multiple comparison tests in the SAS software package (version 9.2, SAS Institute, Cary, NC, USA).
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
K.Z., D.W. and G.L. perceived and designed the project. T.X. and Y.Y. conducted the main experiments. H.Z., X.H., H. J. and Z.X. participated in data analysis, Bgt infection phenotype scoring, and Bgt culture maintenance. W.Q. developed the vector SP1300:LUC and assisted the preparation of derivative constructs. L.X. took part in the development of transgenic lines. T.X. and X.J. conducted subcellular localization of TtdLRK10L‐1. T.X., D.W. and K.Z. wrote the manuscript.
Accession number
The promoter, cDNA and genomic DNA sequences of TtdLRK10L‐1 from the durum wheat cultivar Stewart have been deposited in GenBank under the accession number MN597454.
Supporting information
Figure S1 Comparison of the deduced amino acid sequences of TtdLRK10L‐1 and representative homologs from common wheat.
Figure S2 Phylogenetic tree of TtdLRK10L‐1 and its homologs annotated in the genomes of common wheat and closely related Triticeae species.
Figure S3 Investigation of TtdRLK10L‐1 expression profiles in Stewart by qRT‐PCR assays.
Figure S4 Analysis of transgene copy number using real‐time TaqMan PCR assays.
Figure S5 Subcellular localization of TtdLRK10L‐1 in wheat protoplasts.
Figure S6 Up‐regulation of TtdLRK10L‐1 expression in response to Bgt infection.
Figure S7 Schematic representation of the T‐DNA region in the transformation constructs SP:TtdLRK10L‐1gDNA‐LUC, SP:TtdLRK10L‐1cDNA‐LUC, SP:TtdLRK10L‐1intron I‐LUC, SP:TtdLRK10L‐1intron II‐LUC, SP:TtdLRK10L‐1intron I/mMYB‐BS‐LUC and SP:TtdLRK10L‐1intron I/mW‐box‐LUC.
Figure S8 Presence of TtdLRK10L‐1 orthologs in wild emmer wheat and common wheat.
Table S1 Cis‐elements predicted in introns I and II of TtdLRK10L‐1
Table S2 A list of PCR primers and the TaqMan probe in this study
Acknowledgements
We thank Dr. Huanbin Zhou (Chinese Academy of Agricultural Sciences, Beijing, China) for providing the plasmids pHZ206 and pHZ202 and Professor Yun Zhou (Henan University, Kaifeng, China) for help on confocal microscopy. This work was supported by the key research and development programme of the Ministry of Science and Technology of China (2016YFD0101004, 2017YFD0100600).
Xia, T. , Yang, Y. , Zheng, H. , Han, X. , Jin, H. , Xiong, Z. , Qian, W. , Xia, L. , Ji, X. , Li, G. , Wang, D. and Zhang, K. (2021) Efficient expression and function of a receptor‐like kinase in wheat powdery mildew defence require an intron‐located MYB binding site. Plant Biotechnol J, 10.1111/pbi.13512
Contributor Information
Guangwei Li, Email: wheatwill@163.com.
Daowen Wang, Email: dwwang@henau.edu.cn.
Kunpu Zhang, Email: zkp66@126.com.
References
- Acevedo‐Garcia, J. , Spencer, D. , Thieron, H. , Reinstädler, A. , Hammond‐Kosack, K. , Phillips, A.L. and Panstruga, R. (2017) mlo‐based powdery mildew resistance in hexaploid bread wheat generated by a non‐transgenic TILLING approach. Plant Biotechnol. J. 15, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksza, D. , Horváth, G.V. , Sándor, G. and Szabados, L. (2017) Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol. 175, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Wheat Genome Sequencing Consortium , Appels, Rudi , Eversole, Kellye , Stein, Nils , Feuillet, C. , Keller, B. , Rogers, J. , Pozniak, C. J. et al. (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science, 361, eaar7191. [DOI] [PubMed] [Google Scholar]
- Avni, R. , Nave, M. , Barad, O. , Baruch, K. , Twardziok, S.O. , Gundlach, H. , Hale, I. et al. (2017) Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 357, 93–97. [DOI] [PubMed] [Google Scholar]
- Bernardes, W.S. and Menossi, M. (2020) Plant 3' regulatory regions from mRNA‐encoding genes and their uses to modulate expression. Front Plant Sci. 11, 1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourras, S. , Kunz, L. , Xue, M.F. , Praz, C.R. , Müller, M.C. , Kälin, C. , Schläfli, M. et al. (2019) The AvrPm3‐Pm3 effector‐NLR interactions control both race‐specific resistance and host‐specificity of cereal mildews on wheat. Nat. Commun. 10, 2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, R. , Goff, J. , Rodriguez‐Furlan, C. and Van Norman, J.M. (2020) The Arabidopsis receptor kinase IRK is polarized and represses specific cell divisions in roots. Dev. Cell 52, 183–195. [DOI] [PubMed] [Google Scholar]
- Chen, T.T. , Xiao, J. , Xu, J. , Wan, W.T. , Qin, B. , Cao, A.Z. , Chen, W. et al. (2016) Two members of TaRLK family confer powdery mildew resistance in common wheat. BMC Plant Biol. 16, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X.W. , Zuo, S.M. , Schwessinger, B. , Chern, M. , Canlas, P.E. , Ruan, D.L. , Zhou, X.G. et al. (2014) An XA21‐associated kinase (OsSERK2) regulates immunity mediated by the XA21 and XA3 immune receptors. Mol. Plant 7, 874–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, D.W. , Armstrong, K.C. , Drouin, G. , McElroy, A. , Fedak, G. and Molnar, S.D. (2003) Isolation and identification of Triticeae chromosome 1 receptor‐like kinase genes (Lrk10) from diploid, tetraploid, and hexaploid species of the genus Avena . Genome 46, 119–127. [DOI] [PubMed] [Google Scholar]
- Couto, D. and Zipfel, C. (2016) Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. [DOI] [PubMed] [Google Scholar]
- Dardick, C. and Ronald, P. (2006) Plant and animal pathogen recognition receptors signal through non‐RD kinases. PLoS Pathog. 2, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardick, C. , Schwessinger, B. and Ronald, P. (2012) Non‐arginine‐aspartate (non‐RD) kinases are associated with innate immune receptors that recognize conserved microbial signatures. Curr. Opin. Plant Biol. 15, 358–366. [DOI] [PubMed] [Google Scholar]
- Dezhsetan, S. (2017) Genome scanning for identification and mapping of receptor‐like kinase (RLK) gene superfamily in Solanum tuberosum . Physiol. Mol. Biol. Pla. 23, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning, F.M. , Sun, W.X. , Jansen, K.L. , Helft, L. and Bent, A.F. (2007) Identification and mutational analysis of Arabidopsis FLS2 leucine‐rich repeat domain residues that contribute to flagellin perception. Plant Cell 19, 3297–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fàbregas, N. , Li, N. , Boeren, S. , Nash, T.E. , Goshe, M.B. , Clouse, S.D. , de Vries, S. et al. (2013) The brassinosteroid insensitive1‐like3 signalosome complex regulates Arabidopsis root development. Plant Cell 25, 3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet, C. , Reuzeau, C. , Kjellbom, P. and Keller, B. (1998) Molecular characterization of a new type of receptor‐like kinase (wlrk) gene family in wheat. Plant Mol. Biol. 37, 943–953. [DOI] [PubMed] [Google Scholar]
- Feuillet, C. , Schachermayr, G. and Keller, B. (1997) Molecular cloning of a new receptor‐like kinase gene encode at the Lr10 disease resistance locus of wheat. Plant J. 11, 45–52. [DOI] [PubMed] [Google Scholar]
- Fichman, Y. , Gerdes, S.Y. , Kovács, H. , Szabados, L. , Zilberstein, A. and Csonka, L.N. (2015) Evolution of proline biosynthesis: enzymology, bioinformatics, genetics, and transcriptional regulation. Biol. Rev. Camb. Philos. Soc. 90, 1065–1099. [DOI] [PubMed] [Google Scholar]
- Figueroa, M. , Hammond‐Kosack, K.E. and Solomon, P.S. (2018) A review of wheat diseases‐a field perspective. Mol. Plant Pathol. 19, 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y. , Zhang, H. , Mandal, S.N. , Wang, C.Y. , Chen, C.H. and Ji, W.Q. (2016) Quantitative proteomics reveals the central changes of wheat in response to powdery mildew. J. Proteomics 130, 108–119. [DOI] [PubMed] [Google Scholar]
- Gadaleta, A. , Giancaspro, A. , Cardone, F.M. and Blanco, A. (2011) Real‐time PCR for the detection of precise transgene copy number in durum wheat. Cell. Mol. Biol. Lett. 16, 652–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos, J.E. and Rose, A.B. (2015) The enduring mystery of intron‐mediated enhancement. Plant Sci. 237, 8–15. [DOI] [PubMed] [Google Scholar]
- Gallegos, J.E. and Rose, A.B. (2019) An intron‐derived motif strongly increases gene expression from transcribed sequences through a splicing independent mechanism in Arabidopsis thaliana . Sci. Rep. 9, 13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, J.L. , Temple, G.F. and Brasch, M.A. (2000) DNA cloning using in vitro site‐specific recombination. Genome Res. 10, 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Jones, H.D. , Chen, S. , Chen, X.M. , Wang, D.W. , Li, K.X. , Wang, D.S. et al. (2010) Agrobacterium‐mediated transformation of durum wheat (Triticum turgidum L. var. durum cv Stewart) with improved efficiency. J. Exp. Bot. 61, 1567–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, H.G. , Zhu, S.Y. , Zhao, R.H. , Jiang, Z.N. , Ji, Y.Y. , Ji, J. , Qiu, D. et al. (2018) Pm21, encoding a typical CC‐NBS‐LRR protein, confers broad‐spectrum resistance to wheat powdery mildew disease. Mol. Plant 11, 879–882. [DOI] [PubMed] [Google Scholar]
- Hellens, R.P. , Edwards, E.A. , Leyland, N.R. , Bean, S. and Mullineaux, P.M. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium‐mediated plant transformation. Plant Mol. Biol. 42, 819–832. [DOI] [PubMed] [Google Scholar]
- Higo, K. , Ugawa, Y. , Iwamota, M. and Korenaga, T. (1999) Plant cis‐acting regulatory DNA elements (PLACE) database. Nucleic Acids Res. 27, 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, P. , Liu, J.Q. , Xu, J.F. , Zhou, C.Y. , Cao, S. , Zhou, W.H. , Huang, Z.P. et al. (2018) A malectin‐like/leucine‐rich repeat receptor protein kinase gene, RLK‐V, regulates powdery mildew resistance in wheat. Mol. Plant Pathol. 19, 2561–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurni, S. , Brunner, S. , Buchmann, G. , Herren, G. , Jordan, T. , Krukowski, P. , Wicker, T. et al. (2013) Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 76, 957–969. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kadota, Y. , Sklenar, J. , Derbyshire, P. , Stransfeld, L. , Asai, S. , Ntoukakis, V. , Jones, J.D. et al. (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR‐associated kinase BIK1 during plant immunity. Mol. Cell 54, 43–55. [DOI] [PubMed] [Google Scholar]
- Kimura, S. , Hunter, K. , Vaahtera, L. , Tran, H.C. , Citterico, M. , Vaattovaara, A. , Rokka, A. et al. (2020) CRK2 and C‐terminal phosphorylation of NADPH oxidase RBOHD regulate reactive oxygen species production in Arabidopsis . Plant Cell 32, 1063–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, S. , Waszczak, C. , Hunter, K. and Wrzaczek, M. (2017) Bound by fate: the role of reactive oxygen species in receptor‐like kinase signaling. Plant Cell 29, 638–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger, S.G. , Lagudah, E.S. , Spielmeyer, W. , Singh, R.P. , Huerta‐Espino, J. , McFadden, H. , Bossolini, E. et al. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323, 1360–1363. [DOI] [PubMed] [Google Scholar]
- Laxa, M. (2017) Intron‐mediated enhancement: a tool for heterologous gene expression in plants? Front. Plant Sci. 7, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L.Y. , Kononov, M.E. , Bassuner, B. , Frame, B.R. , Wang, K. and Gelvin, S.B. (2007) Novel plant transformation vectors containing the superpromoter. Plant Physiol. 145, 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. , Lal, N.K. , Lin, Z.D. , Ma, S.S. , Liu, J. , Castro, B. , Toruño, T. et al. (2020) Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 11, 1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Li, M. , Yu, L.P. , Zhou, Z.Y. , Liang, X.X. , Liu, Z.X. , Cai, G.H. et al. (2014) The FLS2‐associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15, 329–338. [DOI] [PubMed] [Google Scholar]
- Li, Q.Q. , Niu, Z.B. , Bao, Y.G. , Tian, Q.J. , Wang, H.G. , Kong, L.R. and Feng, D.S. (2016) Transcriptome analysis of genes related to resistance against powdery mildew in wheat‐thinopyrum alien addition disomic line germplasm SN6306. Gene 590, 5–17. [DOI] [PubMed] [Google Scholar]
- Ling, H.Q. , Ma, B. , Shi, X.L. , Liu, H. , Dong, L.L. , Sun, H. , Cao, Y.H. et al. (2018) Genome sequence of the progenitor of wheat A subgenome Triticum urartu . Nature 557, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu, P. , Guo, L. , Wang, Z.Z. , Li, B.B. , Li, J. , Li, Y.H. , Qiu, D. et al. (2020) A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 11, 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D.P. , Lin, W.W. , Gao, X.Q. , Wu, S.J. , Cheng, C. , Avila, J. , Heese, A.A. et al. (2011) Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332, 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M.C. , Gu, Y.Q. , Puiu, D. , Wang, H. , Twardziok, S.O. , Deal, K.R. , Huo, N.X. et al. (2017) Genome sequence of the progenitor of the wheat D genome Aegilops tauschii . Nature 55, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri, M. , Harris, N.S. , Twardziok, S.O. , Pasam, R.K. , Gundlach, H. , Spannagl, M. , Ormanbekova, D. et al. (2019) Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 51, 885–895. [DOI] [PubMed] [Google Scholar]
- Marcussen, T. , Sandve, S.R. , Heier, L. , Spannagl, M. , Pfeifer, M. , Jakobsen, K.s , Wulff, B.B.H. , et al. (2014) Ancient hybridizations among the ancestral genomes of bread wheat. Science, 345, 1250092. [DOI] [PubMed] [Google Scholar]
- Moore, J.W. , Herrera‐Foessel, S. , Lan, C.X. , Schnippenkoetter, W. , Ayliffe, M. , Huerta‐Espino, J. , Lillemo, M. et al. (2015) A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 47, 1494–1498. [DOI] [PubMed] [Google Scholar]
- Park, C.J. , Sharma, R. , Lefebvre, B. , Canlas, P.E. and Ronald, P.C. (2013) The endoplasmic reticulum‐quality control component SDF2 is essential for XA21‐mediated immunity in rice. Plant Sci. 210, 53–60. [DOI] [PubMed] [Google Scholar]
- Saghai‐Maroof, M.A. , Soliman, K.M. , Jorgensen, R.A. and Allard, R.W. (1984) Ribosomal DNA spacer‐length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81, 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto, T. , Deguchi, M. , Brustolini, O.J.B. , Santos, A.A. , Silva, F.F. and Fontes, E.P.B. (2012) The tomato RLK superfamily: phylogeny and functional predictions about the role of the LRRII‐RLK subfamily in antiviral defense. BMC Plant Biol. 12, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul, O. (2017) How introns enhance gene expression. Int. J. Biochem. Cell Biol. 91, 145–155. [DOI] [PubMed] [Google Scholar]
- Shen, J.Q. , Liu, J.H. , Xie, K.B. , Xing, F. , Xiong, F. , Xiao, J.H. , Li, X.H. et al. (2017) Translational repression by a miniature inverted‐repeat transposable element in the 3' untranslated region. Nat. Commun. 8, 14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Q.H. , Saijo, Y. , Mauch, S. , Biskup, C. , Bieri, S. , Keller, B. , Seki, H. et al. (2007) Nuclear activity of MLA immune receptors links isolate‐specific and basal disease‐resistance responses. Science 315, 1098–1103. [DOI] [PubMed] [Google Scholar]
- Shen, Q.H. , Zhou, F. , Bieri, S. , Haizel, T. , Shirasu, K. and Schulze‐Lefert, P. (2003) Recognition specificity and RAR1/SGT1 dependence in barley Mla disease resistance genes to the powdery mildew fungus. Plant Cell 15, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu, S.H. and Bleecker, A.B. (2003) Expansion of the receptor‐like kinase/pelle gene family and receptor‐like proteins in Arabidopsis . Plant Physiol. 132, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu, S.H. , Karlowski, W.M. , Pan, R.S. , Tzeng, Y.H. , Mayer, K.F.X. and Li, W.H. (2004) Comparative analysis of the receptor‐like kinase family in Arabidopsis and Rice. Plant Cell 16, 1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S.P. , Hurni, S. , Ruinelli, M. , Brunner, S. , Sanchez‐Martin, J. , Krukowski, P. , Peditto, D. et al. (2018) Evolutionary divergence of the rye Pm17 and Pm8 resistance genes reveals ancient diversity. Plant Mol. Biol. 98, 249–260. [DOI] [PubMed] [Google Scholar]
- Singh, R.P. , Singh, P.K. , Rutkoski, J. , Hodson, D.P. , He, X.Y. , Jørgensen, L.N. , Hovmøller, M.S. et al. (2016) Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 54, 303–322. [DOI] [PubMed] [Google Scholar]
- Song, W.Y. , Wang, G.L. , Chen, L.L. , Kim, H.S. , Pi, L.Y. , Holsten, T. , Gardner, J. et al. (1995) A receptor kinase‐like protein encoded by the rice disease resistance gene, Xa21 . Science 270, 1804–1806. [DOI] [PubMed] [Google Scholar]
- Sun, X.L. , Cao, Y.L. , Yang, Z.F. , Xu, C.G. , Li, X.H. , Wang, S.P. and Zhang, Q.F. (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase‐like protein. Plant J. 37, 517–527. [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. , Nei, M. and Kumar, S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J.Y. , Wang, Y.Q. , Yin, W.C. , Dong, G.J. , Sun, K. , Teng, Z.F. , Wu, X.J. et al. (2019) Mutation of a nucleotide‐binding leucine‐rich repeat immune receptor‐type protein disrupts immunity to bacterial blight. Plant Physiol. 181, 1295–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D.Z. , Wang, G.X. and Zhou, J.M. (2017) Receptor kinases in plant‐pathogen interactions: more than pattern recognition. Plant Cell 29, 618–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, T. , Yamaguchi, M. , Uchimiya, H. and Nakano, A. (2001) Ara6, a plant‐unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana . EMBO J. 20, 4730–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.K. , Cheng, J.Y. , Fan, A. , Zhao, J. , Yu, Z.Y. , Li, Y.B. , Zhang, H. et al. (2018) LecRK‐V, an L‐type lectin receptor kinase in Haynaldia villosa, plays positive role in resistance to wheat powdery mildew. Plant Biotechnol. J. 16, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.P. , Cheng, X. , Shan, Q.W. , Zhang, Y. , Liu, J.X. , Gao, C.X. and Qiu, J.L. (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32, 947–951. [DOI] [PubMed] [Google Scholar]
- Wang, G.P. , Yu, X.D. , Sun, Y.W. , Jones, H.D. and Xia, L.Q. (2016) Generation of marker‐ and/or backbone‐free transgenic wheat plants via Agrobacterium‐mediated transformation. Front. Plant Sci. 7, 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.Q. , Zafian, P. , Choudhary, M. and Lawton, M. (1996) The PR5K receptor protein kinase from Arabidopsis thaliana is structurally related to a family of plant defense proteins. Proc. Natl. Acad. Sci. USA 93, 2598–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J.Z. , Guo, G.H. , Wang, Y. , Hu, T.Z. , Wang, L.L. , Li, J.T. , Qiu, D. et al. (2020) A rare single nucleotide variant in Pm5e confers powdery mildew resistance in common wheat. New Phytol. 228, 1011–1026. [DOI] [PubMed] [Google Scholar]
- Xie, Y.Y. , Zhang, Y.L. , Han, J.L. , Luo, J.K. , Li, G.S. , Huang, J.L. , Wu, H.B. et al. (2018) The intronic cis element SE1 recruits trans‐acting repressor complexes to repress the expression of ELONGATED UPPERMOST INTERNODE1 in rice. Mol. Plant 11, 720–735. [DOI] [PubMed] [Google Scholar]
- Xin, M.M. , Wang, X.F. , Peng, H.R. , Yao, Y.Y. , Xie, C.J. , Han, Y. , Ni, Z.F. et al. (2012) Transcriptome comparison of susceptible and resistant wheat in response to powdery mildew infection. Genomics Proteomics Bioinformatics 10, 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, L.P. , Hu, P. , Liu, J.Q. , Witek, K. , Zhou, S. , Xu, J.F. , Zhou, W.H. et al. (2018) Pm21 from Haynaldia villosa encodes a CC‐NBS‐LRR protein conferring powdery mildew resistance in wheat. Mol. Plant 11, 874–878. [DOI] [PubMed] [Google Scholar]
- Xing, P. , Zhang, X. , Bao, Y. , Wang, Y. , Wang, H. and Li, X. (2017) Comparative transcriptome analyses of resistant and susceptible near‐isogenic wheat lines following inoculation with Blumeria graminis f. sp. tritici. Int. J. Genomics, 2017, 7305684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiaoui, N. , Srichumpa, P. , Dudler, R. and Keller, B. (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 37, 528–538. [DOI] [PubMed] [Google Scholar]
- Yang, Q.H. , Li, X.Y. , Tu, H.T. and Pan, S.Q. (2017) Agrobacterium‐delivered virulence protein VirE2 is trafficked inside host cells via a myosin XI‐K‐powered ER/actin network. Proc. Natl. Acad. Sci. USA 114, 2982–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.W. , Bai, Y. , Wu, G.H. , Zou, S.H. , Chen, Y.F. , Gao, C.X. and Tang, D.Z. (2017) Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 91, 717–724. [DOI] [PubMed] [Google Scholar]
- Zhang, M.X. , Chiang, Y.H. , Toruño, T.Y. , Lee, D. , Ma, M.M. , Liang, X.X. , Lal, N.K. et al. (2018) The MAP4 kinase SIK1 ensures robust extracellular ROS burst and antibacterial immunity in plants. Cell Host Microbe 24, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Mao, R. , Wang, Y.Z. , Zhang, L. , Wang, C.Y. , Lv, S.K. , Liu, X.L. et al. (2019) Transcriptome‐wide alternative splicing modulation during plant‐pathogen interactions in wheat. Plant Sci. 288, 110160. [DOI] [PubMed] [Google Scholar]
- Zhang, J.C. , Zheng, H.Y. , Li, Y.W. , Li, H.J. , Liu, X. , Qin, H.J. , Dong, L.L. et al. (2016) Coexpression network analysis of the genes regulated by two types of resistance responses to powdery mildew in wheat. Sci. Rep. 6, 23805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H.Y. , Dong, L.L. , Han, X.Y. , Jin, H.B. , Yin, C.C. , Han, Y.L. , Li, B. et al. (2020) The TuMYB46L‐TuACO3 module regulates ethylene biosynthesis in einkorn wheat defense to powdery mildew. New Phytol. 225, 2526–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H.B. , Li, S.F. , Deng, Z.Y. , Wang, X.P. , Chen, T. , Zhang, J.S. , Chen, S.Y. et al. (2007) Molecular analysis of three new receptor‐like kinase genes from hexaploid wheat and evidence for their participation in the wheat hypersensitive response to stripe rust fungus infection. Plant J. 52, 420–434. [DOI] [PubMed] [Google Scholar]
- Zhou, J.M. and Zhang, Y.L. (2020) Plant immunity: danger perception and signaling. Cell 181, 978–989. [DOI] [PubMed] [Google Scholar]
- Zhou, Z.Y. , Zhao, Y. , Bi, G.Z. , Liang, X.X. and Zhou, J.M. (2019) Early signaling mechanisms underlying receptor kinase‐mediated immunity in plants. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 374, 20180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, S.H. , Wang, H. , Li, Y.W. , Kong, Z.S. and Tang, D.Z. (2018) The NB‐LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol. 218, 298–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of the deduced amino acid sequences of TtdLRK10L‐1 and representative homologs from common wheat.
Figure S2 Phylogenetic tree of TtdLRK10L‐1 and its homologs annotated in the genomes of common wheat and closely related Triticeae species.
Figure S3 Investigation of TtdRLK10L‐1 expression profiles in Stewart by qRT‐PCR assays.
Figure S4 Analysis of transgene copy number using real‐time TaqMan PCR assays.
Figure S5 Subcellular localization of TtdLRK10L‐1 in wheat protoplasts.
Figure S6 Up‐regulation of TtdLRK10L‐1 expression in response to Bgt infection.
Figure S7 Schematic representation of the T‐DNA region in the transformation constructs SP:TtdLRK10L‐1gDNA‐LUC, SP:TtdLRK10L‐1cDNA‐LUC, SP:TtdLRK10L‐1intron I‐LUC, SP:TtdLRK10L‐1intron II‐LUC, SP:TtdLRK10L‐1intron I/mMYB‐BS‐LUC and SP:TtdLRK10L‐1intron I/mW‐box‐LUC.
Figure S8 Presence of TtdLRK10L‐1 orthologs in wild emmer wheat and common wheat.
Table S1 Cis‐elements predicted in introns I and II of TtdLRK10L‐1
Table S2 A list of PCR primers and the TaqMan probe in this study


