Abstract
PROBLEM
The Reduced Uterine Perfusion Pressure (RUPP) rat model of placental ischemia recapitulates many characteristics of preeclampsia including maternal hypertension, intrauterine growth restriction (IUGR), and increased cytolytic natural killer cells (cNKs). While we have previously shown a 5-fold higher cytotoxicity of RUPP NKs vs normal pregnant NKs, their role in RUPP pathophysiology remains unclear. In this study, we tested the hypotheses that (1) adoptive transfer of RUPP-stimulated NKs will induce maternal hypertension and IUGR in normal pregnant control (Sham) rats and (2) adoptive transfer of Sham NKs will attenuate maternal hypertension and IUGR in RUPP rats.
METHOD OF STUDY
On gestation day (GD)14, vehicle or 5×106 RUPP NKs were infused i.v. into a subset of Sham rats (Sham+RUPP NK), and vehicle or 5×106 Sham NKs were infused i.v. into a subset of RUPP rats (RUPP+Sham NK; n=12/group). On GD18, Uterine Artery Resistance Index (UARI) was measured. On GD19 mean arterial pressure (MAP) was measured, animals were sacrificed, and blood and tissues were collected for analysis.
RESULTS
Adoptive transfer of RUPP NK cells into Sham rats resulted in elevated NK activation, UARI, placental oxidative stress, and preproendothelin expression as well as reduced circulating nitrate/nitrite. This led to maternal hypertension and IUGR. RUPP recipients of Sham NK cells demonstrated normalized NK activation, sFlt-1, circulating and placental VEGF, and UARI, which led to improved maternal blood pressure and normal fetal growth.
CONCLUSIONS
These data suggest a direct role for cNKs in causing preeclampsia pathophysiology and a role for normal NKs to improve maternal outcomes and IUGR during late gestation.
Keywords: Preeclampsia, Natural Killer Cells, Hypertension, Pregnancy
INTRODUCTION
Preeclampsia (PE) is a multisystem obstetric disorder that affects around 2–8% of pregnancies and remains one of the leading causes of maternal and fetal morbidity worldwide.[1] Preeclampsia is characterized by maternal hypertension, intrauterine growth restriction (IUGR) and end organ damage that can manifest as proteinuria, liver dysfunction, or cerebral disturbances.[2] The disorder can also progress to eclampsia, a life-threatening condition characterized by unexplained seizures and multi-organ failure.[3, 4] Together, PE and eclampsia contribute to 10%−15% of maternal deaths.[5] Despite decades of research, the only cure for PE is the delivery, often times early, of the fetus and placenta, which contributes significantly to negative fetal outcomes. [2, 3]
One reason for the lack of treatments is that the pathogenesis of PE is still not completely understood.[6] It is widely believed that PE occurs because of insufficient remodeling of the utero-placental vasculature by cytotrophoblasts, leading to reduced blood flow to the fetus and placenta.[3, 4, 7] The resultant ischemia promotes placental release of pro-inflammatory cytokines such as Tumor Necrosis Factor (TNF)-α and Interleukin (IL)-17 as well as antiangiogenic factors such as soluble fms-like tyrosine kinase-1 (sFlt-1) into the maternal circulation.[2–4] This is thought to lead to the maternal endothelial dysfunction, oxidative stress, and increased immune activation that characterize PE.[3, 4]
Natural Killer (NK) cells play an important role in pregnancy, and have been implicated in several pregnancy disorders including PE.[8, 9] In the circulation, these innate lymphocytes primarily function to destroy virally infected or cancerous cells through the release of cytolytic enzymes.[10] However, during normal human pregnancy, a subset of NK cells known as decidual NK cells (dNK) display little cytotoxicity and instead play a supportive role. dNKs make up 70% of decidual cells and function as important immune regulators at the maternal-fetal interface in human pregnancies.[11–13] dNK cells also play important roles in placentation by regulating trophoblast invasion and promoting spiral artery remodeling.[9, 14, 15] Importantly, several clinical studies have documented a Type 1 shift in NK cells towards an activated phenotype in women with pregnancy disorders including recurrent spontaneous abortion (RSA) (early gestation) and PE (late gestation).[7, 8, 16] These cytolytic NK cells (cNKs) display increased secretion of TNF-α and Interferon (IFN)-y, decreased secretion of vascular endothelial growth factor (VEGF), and increased cytotoxicity.[7, 14]
The Reduced Uterine Perfusion Pressure (RUPP) model of placental ischemia has been extensively used to investigate the role of various immune cell types in the development and pathophysiology of PE. [17–19] This well-established animal model recapitulates many of the aforementioned characteristics observed in preeclamptic women including hypertension, IUGR, increased sFlt-1, and chronic immune cell activation.[20] We have previously published that cNKs are significantly increased in RUPP rats compared to their normal pregnant counterparts.[21, 22] We also showed a 5-fold increase in cytotoxicity of placental NK cells from RUPP compared to those of their normal pregnant counterparts.[22] Additionally, we previously depleted NK cells in RUPP and saw a significant reduction in maternal blood pressure and improved fetal weight.[21] However, because this study depleted all subsets of NK cells, it did not reveal the precise role that cNK cells play in the pathophysiology observed in RUPP animals. Therefore, in this study, we aimed to test the following hypotheses: 1.) RUPP-stimulated NKs play a direct role in causing maternal HTN and IUGR in pregnant rats by inducing exaggerated inflammation, oxidative stress, and endothelin-1 (ET-1), while reducing nitric oxide (NO) to cause endothelial dysfunction and increased vascular resistance; 2.) Normal pregnant control (Sham) NKs attenuate maternal HTN and IUGR in RUPP rats by suppressing inflammation, oxidative stress, and ET-1, while stimulating NO to improve endothelial function and decrease vascular resistance. To test this, we adoptively transferred NK cells from RUPP rats into Sham rats and evaluated changes in NK cell activation, UARI, sFlt-1, eNOS expression and phosphorylation, preproendothelin (PPET) transcription, and oxidative stress as mechanisms to increase MAP and IUGR. Additionally, we transferred placental NK cells from Sham rats into RUPP rats to determine if the normally supportive nature of these cells can improve the pathophysiology observed in RUPP rats.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files.
Animals
12–13 week old, timed pregnant Sprague-Dawley rats were purchased from Envigo (Indianapolis, IN). The animals were delivered to the Center for Comparative Research at the University of Mississippi Medical Center on day 10 or 11 of their gestation and weighed approximately 250–260g upon delivery. The animals were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle and maintained on a normal diet. Rats were randomly assigned to experimental groups.The rats were group housed until after surgery.All experimental procedures were carried out in accordance with the National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Reduction in uterine perfusion pressure.
On Day 14 of gestation (GD14), animals underwent either RUPP or Sham surgeries under 2% isoflurane anesthesia delivered by a vaporizer (Ohio Medical Products, Madison, WI). The RUPP surgery was performed to induce placental ischemia as previously described.[23, 24] The animals received carprofen immediately following surgery and 24 hours post-surgery (5 mg/kg) to control for post-operative pain. Sham animals were subjected to the surgery without application of clips. Rats were excluded when the procedure resulted in total reabsorption of all fetuses.
NK cell isolation
On the day of harvest, placentas from Sham and RUPP rats were collected and homogenized in RPMI (10% FBS). The homogenate was passed through a 70 μm filter and lymphocytes were isolated by centrifugation on a cushion of Ficoll-Hypaque according to the instructions of the manufacturer. The isolated lymphocytes were incubated with the biotin labeled anti-CD3 and anti-biotin microbeads. The CD3+ population was depleted using (LD) columns (Miltenyi Biotec) according to the manufacturer’s instructions. The flow-through containing the CD3− population of lymphocytes was then incubated with PE-labeled anti-CD161 antibody and subsequently incubated with anti-PE microbeads (Miltenyi Biotec). CD3− CD161+ NK cells were isolated using a MS column (Miltenyi Biotec) according to the manufacturer’s protocol prior to culture and expansion. The collected cells were resuspended in NK cell activation media (RPMI, 10% FBS, 1% Pen/Strep, 2 ng/mL IL-2) [25–27], and allowed to expand in culture for 48 hrs.
Adoptive Transfer of Natural Killer Cells
On GD14, vehicle or 5×106 expanded RUPP NK cells were infused i.v. via the femoral vein into a subset of Sham rats (Sham n=12, Sham+RUPP NK, n=12), and vehicle or 5×106 expanded Sham NK cells were infused i.v. via the femoral vein into a subset of RUPP rats (RUPP n=12, RUPP+Sham NK, n=12).
Determination of Uterine Artery Resistance Index
On GD 18, rats from all 4 groups were shaved and all abdominal hair was removed with a depilation cream. Power Doppler velocimetry measurements were performed on anesthetized pregnant dams at an imaging station with a Vevo 770 unit (Visual Sonics, Toronto, Canada) using a 30 Hz transducer and an insonating angle <30° as previously described.[28] The peak systolic flow velocity (PSV) and end diastolic flow velocity (EDV) were measured bilaterally. The uterine artery resistance index was calculated using the following formula: UARI= (PSV-EDV)/PSV. Uterine artery resistance index was determined using the mean measurements of three waveforms per side.
Mean Arterial Pressure measurement and sample collection
Under isoflurane anesthesia, 0.58 mm I.D. x 0.99 mm O.D vinyl catheter tubing (Scientific Commodities Inc., Lake Havasu City, AZ) was implanted into the carotid arteries and tunneled to the back of the neck on GD18 for the measurement of mean arterial pressure. On GD19, rats were placed in individual restrainers and conscious mean arterial pressure (MAP) was monitored with a pressure transducer (Powerlab, AD Instruments, Colorado Springs, CO). The MAP was recorded for 30 minutes following a 30-minute stabilization period. After MAP measurement, the animals were anesthetized for blood and tissue collection. Placental and fetal weights were recorded for each dam and averaged. Randomly selected placentas were processed immediately or snap frozen in liquid nitrogen and stored at −80°C until analysis.
Determination of circulating and placental NK cell populations by flow cytometry
Single-cell suspensions of placental leukocytes were prepared as previously described[21, 22, 29]. Single-cell suspensions (1 × 106 cells) were stained for flow cytometry after being blocked with 10% goat and mouse serum. Antibodies used for flow cytometry were as follows: VioGreen anti-CD3, anti-ANK61 antibody, anti-mouse FITC, anti-ANK44, and anti-mouse Alexa Fluor 405. Flow cytometry was performed on the MACSQuant Analyzer 10 (Miltenyi Biotec) and analyzed using FlowLogic software (Innovai, Sydney, Australia). Lymphocytes were gated in the forward- and side-scatter plot. CD3- ANK61+ cells identified the total NK cell population and CD3- ANK61+ ANK44+ cells identified cNK cells. After doublet exclusion, additional gates were set using fluorescence-minus-one controls. Results are expressed as percentage of cells in the gated lymphocyte population.
Determination of systemic and placental oxidative stress
Plasma 8-isoprostane (8-isoPGF2α) concentrations were measured in duplicate with commercially available enzyme linked immunosorbent (ELISA) assays kits (#516351, Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. Superoxide production in the placenta was measured using the lucigenin technique as previously described.[22–24, 30] Each sample was run in triplicate and the average was used for data transformation. The protein concentration was measured using a protein assay with BSA standards (Pierce, Rockford, IL), and the data are expressed as RLUs/min/mg.
Determination of total Nitrate/Nitrite and cGMP levels
Blood was collected in EDTA tubes and spun at 3000 x g for 10 minutes at 4o C. The collected plasma was assessed in duplicate using the Nitrate/Nitrite Colorimetric Assay Kit (Catalog# 78001, Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions. Levels of cGMP were measured in plasma and placental homogenates in duplicate using the Cyclic GMP ELISA Kit (# 581021, Cayman Chemical) according to the manufacturer’s instructions. The protein concentration of the placental homogenates was measured using a protein assay with BSA standards. All placental data were normalized to protein and are expressed as pmol/mg.
Determination of Placental PreProEndothelin (PPET) mRNA levels.
RNA extraction from the placenta was done via the RNeasy Mini Kit (Qiagen, Valencia CA) according to the manufacturer’s protocol. 1ug of the extracted RNA was then used to synthesize cDNA, then transformed to cDNA via the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative Real-time PCR (qRT-PCR) was used to express preproendothelin-1 (PPET-1) via the Bio-Rad iQ SYBR Green Supermix and the CFX96 Touch Real-Time PCR Detection System was used for analysis. The following primers were used: Forward primer: CTAGGTCTAAGCGATCCTTG and reverse primer: TCTTTGTCTGCTTGGC[31]. PPET mRNA levels were calculated according to the following formula 2−ΔΔCt (2avg.Ct gene of interest−avg Ct beta actin) as recommended by Applied Biosystems (Foster City, CA).
Determination of placental and circulating cytokines and angiogenic factors
Circulating and placental IL-17, TNF-α, IFN-γ, VEGF, and circulating IL-6 were measured using the Bio-Plex Pro Rat Cytokine Immunoassay Kit (Bio-Rad) according to the manufacturer’s instructions. Circulating sFlt-1 levels were measured in collected plasma via ELISA (Catalog# MVR100 R&D systems, Minneapolis, MN) according to the manufacturer’s instructions. All sample analyses were performed in duplicate. Protein concentration of the placental homogenates was measured using a protein assay with BSA standards. All placental data were normalized to protein and are expressed as pg/mg.
Western blot analysis of endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS (peNOS).
Placental eNOS and peNOS expression was assessed in placental tissue via Western blot as previously described. [28] Briefly, placentas were homogenized in cold RIPA-buffer and protein extracts, separated by SDS-PAGE using a polyacrylamide gel (4–20%). Protein was transferred onto nitrocellulose membranes (Bio-Rad) and blocked with 5% Blotting-Grade Nonfat Dry milk (Bio-Rad) for 1 h at room temperature. Membranes were incubated overnight at 4ºC with primary antibody directed against eNOS or eNOS (pS1177) and β-actin, followed by anti-mouse IgG IRDye® 800Dx conjugated secondary antibody for 1 hr at room temperature and scanned using the Odyssey CLx Imager (LI-COR Biosciences, Lincoln, NE). The intensity of specific bands was quantified by densitometry using Image J (National Institutes of Health, USA) and placental eNOS or peNOS expression was normalized to β-actin.
Antibodies
Specific information for antibodies used in Flow Cytometry, NK isolations, and Western Blot is located in the Major Resources Table.
Major Resources Tables.
| Animals (in vivo studies) | ||||
| Species | Vendor or Source | Background Strain | Sex | |
| Rat | Envigo (Indianapolis, IN) | Sprague Dawley | Female | |
| Antibodies | ||||
| Target antigen | Vendor or Source | Catalog # | Working concentration | Lot # (preferred but not required) |
| Rat CD3 - Biotin | Miltenyi Biotec, (Auburn, CA) | 130101973 | 1:10 | |
| Rat CD3-APCVio770 | Miltenyi Biotec | 130102675 | 1:10 | |
| Rat CD161-PE | Miltenyi Biotec | 130102712 | 1:10 | |
| Rat ANK 61 | Abcam (Cambridge, MA) | ab36392 | 1:50 | |
| Mouse IgG-Alexa Flour 405 | Abcam | Ab175663 | 1:100 | |
| Rat ANK 44 | Abcam | Ab36388 | 1:100 | |
| Mouse IgG-FITC | Abcam | Ab97230 | 1:50 | |
| Rat Beta-actin | MilliporeSigma (Darmstadt, Germany) | A1978 | 1:2,000 | |
| Human eNOS | BD Transduction Laboratories (San Jose, CA) | 610296 | 1:250 | |
| Mouse IgG IRDye® 800CW | Rockland (Gilbertsville, PA) | 610731002 | 1:10,000 | |
Statistical analysis
All of the data are expressed as mean ± SEM. Statistical analyses were performed with one-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test using GraphPad Prism (Version 8.1.2). A value of p < 0.05 was considered statistically significant.
RESULTS
Effects of NK Adoptive Transfer on Circulating and Placental NK populations
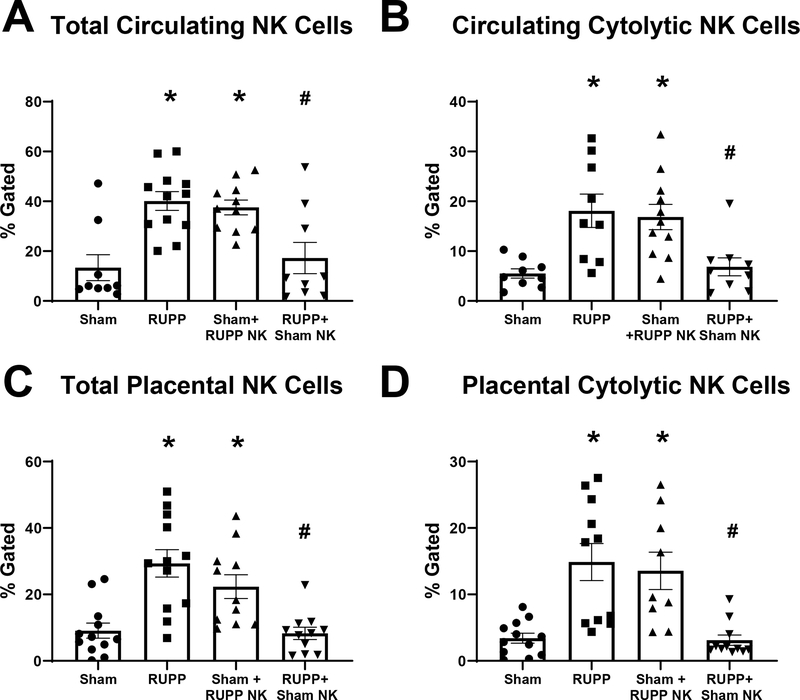
Similar to what we have previously shown[21, 22], total circulating NK cells were elevated in RUPP with a value of 40±4% compared to 13±5% in Sham (p=0.0008 vs Sham). Total circulating NK cells were elevated in Sham rats that received RUPP NKs at 38±3% (p=0.0032 vs Sham) and were reduced in RUPP rats that received Sham NKs at 17±6% (p=0.005 vs RUPP; Figure 1A). Similarly, cNKs in the circulation were elevated in RUPP with 18±3% gated compared to 6±1% in Sham (p=0.005 vs Sham). Circulating cNKs were also elevated in Sham+RUPP NK with 17±3% of gated lymphocytes (p=0.008 vs Sham). Additionally, circulating cNK were reduced in RUPP+Sham NK compared to RUPP at 7±2%. (p=0.01 vs RUPP; Figure 1B).
Figure 1. Effects of Natural Killer (NK) Cell Adoptive Transfer on Circulating and Placental NK Populations in Pregnant Rats.
On Gestation Day (GD) 14, vehicle or 5×106 Reduced Uterine Perfusion Pressure (RUPP) NKs were infused i.v. into a subset of Sham rats and vehicle or 5×106 Sham NKs were infused i.v. into a subset of RUPP rats. On GD19, blood and placentas were collected, processed, and analyzed via flow cytometry to obtain levels of (A) circulating total NK cells and (B) circulating cytolytic NK cells along with (C) placental total NK cells, and (D) placental cytolytic NK cells. Sham n=9–12; RUPP n=9–12; Sham+RUPP NK n=9–11; RUPP+Sham NK n=9–11. All data are expressed as mean ± SEM. Statistical analyses were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Within the placenta, there was a similar trend. Total placental NK cells were elevated in RUPP at 29±4% compared to 9±2% in Sham (p=0.0002 vs Sham). Total NKs were elevated in Sham recipients of RUPP NKs at 22±4% (p=0.02 vs Sham) and were reduced in RUPP recipients of Sham NKs at 8±2% in (p=0.0001 vs RUPP; Figure 1C). Placental cNKs cells were elevated in RUPP at 15±3% compared to 3±1% in Sham (p=0.0006 vs Sham) and were also elevated in Sham+RUPP NK at 14±3% (p=0.004 vs Sham). These cells were reduced in RUPP+Sham NK at 3±1% (p=0.0005 vs RUPP; Figure 1D). The gating strategy for NK cell analysis is shown in Figure S1.
Effects of NK Adoptive Transfer on UARI and placental PPET
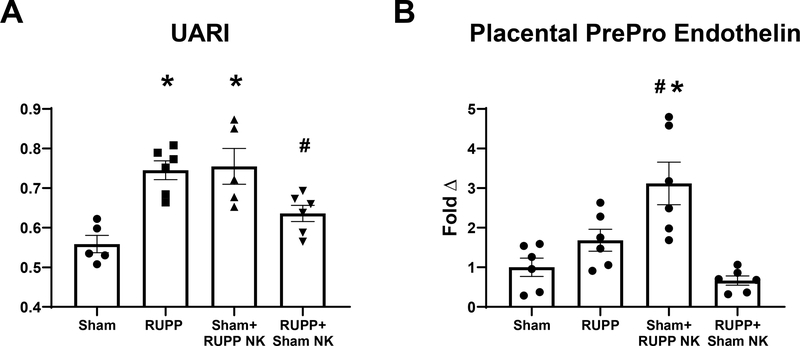
Similar to what has been previously published[17, 28] UARI was elevated in RUPP at 0.75±0.02 compared to 0.56±0.02 in Sham (p=0.001 vs Sham). UARI was elevated in Sham+ RUPP NK at 0.76±0.05 (p=0.001 vs Sham) and was reduced in RUPP recipients of Sham NKs at 0.63±0.02 (p=0.049 vs RUPP; Figure 2A). Placental PPET expression was 3 fold higher in Sham+RUPP NK compred to Sham (p= 0.0010 vs Sham). Expression was 1.7±0.3 in RUPP (p=0.48 vs Sham) and and 0.67±0.11 in RUPP+Sham NK. (p=0.16 vs RUPP; Figure 2B).
Figure 2. Effects of Natural Killer (NK) Cell Adoptive Transfer on Maternal Uterine Artery Resistance Index (UARI) and placental PrePro Endothelin (PPET).
On Gestation Day (GD) 14, vehicle or 5×106 Reduced Uterine Perfusion Pressure (RUPP) NKs were infused i.v. into a subset of Sham rats and vehicle or 5×106 Sham NKs were infused i.v. into a subset of RUPP rats. On GD18, (A) UARI was measured using Doppler ultrasound.. On GD19 animals were sacrificed and placentas were collected and frozen for analysis of (B) PPET mRNA levels. Sham: n=5–6, RUPP: n=6, Sham+RUPP NK: n=6, RUPP+Sham NK: n=5–6. All data are expressed as mean± SEM. Statistical analyses were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Effects of NK Adoptive Transfer on MAP and IUGR
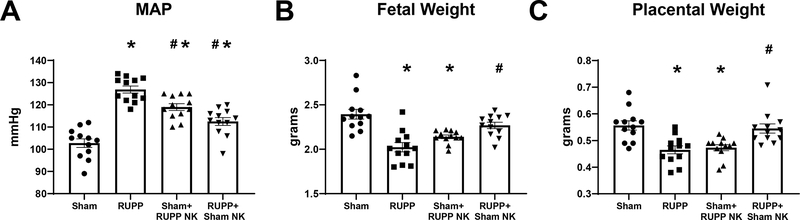
MAP was also elevated in RUPP at 127±2mmHg in RUPP compared to 103±2 mmHg in Sham (p<0.0001 vs Sham) and was elevated in Sham recipients of RUPP NKs at 119±2 mmHg (p<0.0001 vs Sham). MAP was reduced in RUPP recipients of Sham NKs at 113±2 mmHg (p<0.0001 vs RUPP), although it remained elevated compared to Sham (p=0.001 vs Sham; Figure 3A). Mean pup weight was reduced in RUPP at 2.0±0.05 g compared to 2.4±0.06 g in Sham (p<0.0001 vs Sham). Similarly, mean pup weight of Sham recipients of RUPP NKs was reduced at 2.1±0.07 g (p=0.0008 vs Sham) and was normalized to 2.3±0.03g in RUPP recipients of Sham NKs. (p=0.001 vs RUPP; Figure 3B). Mean placental weight was also reduced in RUPP at 0.47±0.01 g compared to 0.56±0.02 g in Sham (p=0.0007 vs Sham). Sham+RUPP NK placental weight was reduced at 0.47±0.01 g (p=0.002 vs Sham) and RUPP+Sham NK placental weight was elevated at 0.55±0.02 g (p=0.004 vs RUPP; Figure 3C)
Figure 3. Effects of Natural Killer (NK) Cell Adoptive Transfer on, Maternal Mean Arterial Pressure (MAP), and Fetal and Placental Weight.
On Gestation Day (GD) 14, vehicle or 5×106 Reduced Uterine Perfusion Pressure (RUPP) NKs were infused i.v. into a subset of Sham rats and vehicle or 5×106 Sham NKs were infused i.v. into a subset of RUPP rats. On GD19 (A) conscious mean arterial pressure was measured, and (B) fetal and (C) placental weights were recorded under isoflurane anesthesia. Sham: n=12, RUPP: n=12, Sham+RUPP NK: n=12, RUPP+Sham NK: n=12. All data are expressed as mean± SEM. Statistical analyses were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Effects of NK Adoptive Transfer on Circulating and Placental VEGF and sFlt-1
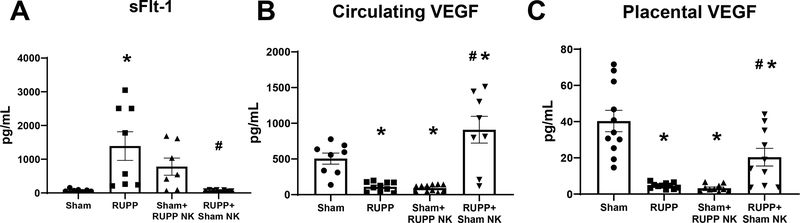
sFlt-1 was elevated in RUPP at 1391±424 pg/mL compared to 76±15 pg/mL in Sham (p=0.007 vs Sham) and was 780±256 pg/mL in Sham recipients of RUPP NKs (p=0.26 vs Sham). Infusion of Sham NKs into RUPP rats normalized sFlt-1 to 67±8 pg/mL (p=0.005 vs RUPP; Figure 4A). Circulating VEGF was reduced in RUPP at 112±21 pg/mL compared to 506±78 pg/mL in Sham (p=0.02 vs Sham). Circulating VEGF was reduced in Sham+RUPP NK at 92±15 pg/mL (p= 0.01 vs Sham) and was elevated in RUPP+Sham NK at 910±187 pg/mL (p<0.0001 vs RUPP; Figure 4B). Similarly, placental VEGF was reduced in RUPP at 4.6±0.5 pg/mg compared to 40.3±5.9 pg/mg in Sham (p<0.0001 vs Sham). Placental VEGF was also reduced in Sham+RUPP NK at 3.2±0.6 pg/mg (p<0.0001 vs Sham) and was elevated in RUPP+Sham NK at 20.4±4.9 pg/mg (p=0.03 vs RUPP) although the levels were still reduced compared to Sham (p=0.0045 vs Sham; Figure 4C).
Figure 4. Effects of Natural Killer (NK) Cell Adoptive Transfer on Circulating and Placental Angiogenic Factors.
Gestation Day (GD) 14, vehicle or 5×106 Reduced Uterine Perfusion Pressure (RUPP) NKs were infused i.v. into a subset of Sham rats and vehicle or 5×106 Sham NKs were infused i.v. into a subset of RUPP rats. On GD19, blood and placentas were collected and processed for further analysis. (A) Plasma levels of sFlt-1 was assessed using a commercial ELISA. Circulating (B) and Placental (C) levels of VEGF were measured using the Bio-Plex Pro Rat Cytokine Immunoassay Kit. All data are expressed as mean ± SEM. Sham n=7–11, RUPP n=8–11, Sham+RUPP NK n=7–11, RUPP+Sham NK n=8–10. Statistical analyses were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
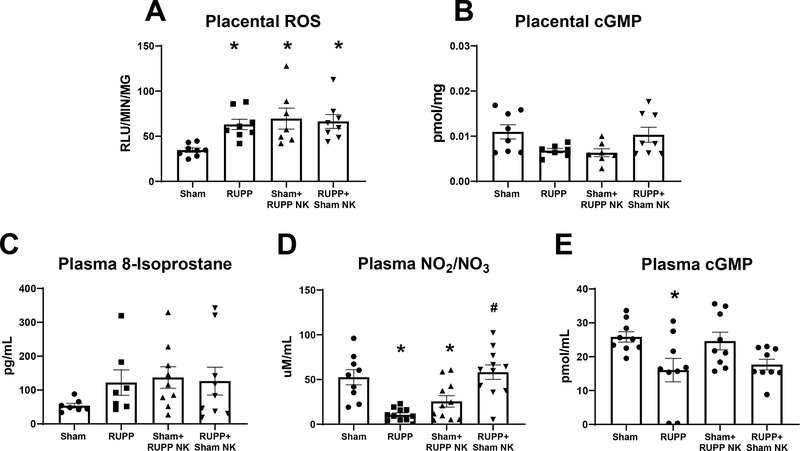
Effects of NK Adoptive Transfer on Placental and Circulating Oxidative Stress, cGMP, and Circulating Nitrate/Nitrite.
Placental ROS was elevated in RUPP at 63±6 RLU/min/mg compared to 35±2 RLU/min/mg in Sham (p=0.02 vs Sham). It was also elevated in Sham+RUPP NKs at 60±5 RLU/min/mg (p=0.005 vs Sham) and remained elevated at 70±12 RLU/min/mg in RUPP+Sham NK (p=0.98 vs RUPP; Figure 5A). Placental levels of cGMP were 0.01±0.002 pmol/mg in Sham, 0.006±0.0005 pmol/mg in RUPP, 0.006±0.0009 pg/mol in Sham+RUPP NK and 0.01± 002 pmol/mg in RUPP+Sham NK (p>0.05 for all comparisons; Figure 5B).
Figure 5. Effects of Natural Killer (NK) Cell Adoptive Transfer on Placental and Circulating Oxidative Stress, Cyclic GMP, and Circulating Nitrate/Nitrite levels in Pregnant Rats.
On Gestation Day (GD) 14, vehicle or 5×106 Reduced Uterine Perfusion Pressure (RUPP) NKs were infused i.v. into a subset of Sham rats and vehicle or 5×106 Sham NKs were infused i.v. into a subset of RUPP rats. On GD19, blood and placentas were collected following sacrifice and processed for further analysis. (A) Reactive oxygen species in placental homogenates were assessed using lucigenin and (B) placental cyclic GMP was measured via ELISA. (C) Circulating levels of 8-isoprostane were measured via ELISA. (D) Plasma total nitrate/nitrite was assessed using a colorimetric assay and (E) plasma cyclic GMP was measured via ELISA. All data are expressed as mean ± SEM. Sham n=7–9, RUPP n=7–11, Sham+RUPP NK n=7–10, RUPP+Sham NK n=7–10. Statistical analyses were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Circulating levels of Isoprostane-8 were 54±7 pg/mL in Sham, 122±37 pg/mL in RUPP, 137±32 pg/mL in Sham+ RUPP NK, and 126±41 pg/mL in RUPP+Sham NK (p>0.05 for all comparisons; Figure 5C). Total levels of plasma nitrate/nitrite were reduced in RUPP at 10.6±2.1 μM/mL compared to 47.5±8.9 μM/mL in Sham (p= 0.0008 vs Sham). The levels were also reduced in Sham+RUPP NK at 22.1±5.9 μM/mL (p=0.03 vs Sham) but were elevated in RUPP+Sham NK at 63.5±6.8 μM/mL (p<0.0001 vs RUPP; Figure 5D). Plasma levels of cGMP decreased from 26±2 pmol/mL in Sham to 16±3 pmol/mL in RUPP (p=0.04 vs Sham). cGMP was 25±3 pmol/mL in Sham+RUPP NK (p=0.98 vs Sham) and 18±2 pmol/mL in RUPP+Sham NK (p=0.97 vs RUPP; Figure 5E).
Effects of NK Adoptive Transfer on Circulating and Placental Cytokines
All circulating and placental cytokine data are summarized in Table 1. Circulating IL-6 was elevated in RUPP at 426±56 pg/mL compared to 138±21 pg/mL in Sham (p=0.0004 vs Sham) and was also elevated in Sham+RUPP NK at 338±40 pg/mK (p=0.02 vs Sham). Compared to RUPP, circulating IL-6 was reduced in RUPP+Sham NK at 245±56 pg/mL (p=0.042 vs RUPP). Circulating IL-17 was elevated in RUPP at 30.5±4.9 pg/mL compared to 6.8±1.6 pg/mL in Sham (p=0.008 vs Sham) and remained elevated in RUPP+Sham NKs at 45.5±8.9 pg/mL (p=0.19 vs RUPP). There was a trend toward elevated circulating IL-17 in Sham+RUPP NK at 23.8±3.7 pg/mL (p=0.08 vs Sham). Circulating TNF-α was elevated in RUPP at 152±17 pg/mL compared to 30.3±5.0 pg/mL in Sham (p<0.0001 vs Sham), was elevated in Sham+RUPP NK at 109±14 pg/mL (p=0.002), and remained elevated in RUPP+Sham NK at 115.4±17 pg/mL (p=0.27 vs RUPP). Levels of circulating IFN-γ were elevated in RUPP at 95±15 pg/mL compared to 19±3 pg/mL in Sham (p<0.0001 vs Sham) and remained elevated at 75±13 pg/mL in RUPP+Sham NK (p=0.53 vs RUPP). The levels remained normal in Sham+RUPP NK at 45±7 pg/mL (p=0.36 vs Sham).
Table 1.
Effects of Natural Killer (NK) Cell Adoptive Transfer on Circulating and Placental Cytokines in Pregnant Rats.
| Circulating Cytokines | |||||
| Cytokine (pg/mL) | Sham | RUPP | Sham+RUPP NK | RUPP+Sham NK | n/group |
| IL-6 | 138±21 | 426±56* | 338±40* | 245±56# | 8–9 |
| IL-17 | 6.8±1.6 | 30.5±4.9* | 23.8±3.7 | 45.5±8.9* | 8–10 |
| TNF-α | 30.3±5.0 | 152±16.6* | 108.7±13.6* | 115.4±17* | 10–11 |
| IFN- γ | 19.3±3.1 | 95.2±14.8* | 44.6±6.5# | 74.7±12.8* | 10–11 |
| Placental Cytokines | |||||
| Cytokine (pg/mg) | Sham | RUPP | Sham+RUPP NK | RUPP+Sham NK | n/ group |
| IL-17 | 0.63±0.24 | 4.1±0.72* | 0.57±0.22# | 5.1±0.73* | 10–12 |
| TNF-α | 36.3±4.0 | 64.3±7.2* | 46.3±4.5 | 71.7±5.4* | 9–11 |
| IFN-γ | 5.1±1.4 | 15.5±3.0* | 14.6±2.0* | 5.9±1.8# | 11–12 |
On GD14, vehicle or 5x106 RUPP NKs were infused i.v. into a subset of Sham rats and vehicle or 5x106 Sham NKs were infused i.v. into a subset of RUPP rats. On GD19, blood and placentas were collected following sacrifice and processed for further analysis. Levels of cytokines were measured in blood and placental homogenates of each group using the Bio-Plex Pro Rat Cytokine Immunoassay Kit. Placental data are normalized to total protein. All data are expressed as mean±SEM. Statistical analyses were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post-hoc correction.
p<0.05 vs. Sham
p<0.05 vs RUPP.
In the placenta, levels of IL-17 were elevated in RUPP at 4±0.7 pg/mg compared to 0.6±0.2 in Sham (p=0.0001 vs Sham) and remained elevated at 5±0.7 pg/mg in RUPP+Sham NK (p=0.53 vs RUPP). Placental IL-17 remained normal in Sham+RUPP NK at 0.6±0.2 pg/mg (p=0.9997 vs Sham). Similarly, placental TNF-α was elevated in RUPP at 64±7 pg/mg compared to 36±4 pg/mg in Sham (p=0.007 vs Sham) and remained elevated at 72±5 pg/mg in RUPP+Sham NK (p=0.77 vs RUPP). Placental TNF- α remained normal in Sham+RUPP NK at 46±5 pg/mg (p=0.63 vs Sham). Placental IFN-γ was elevated in RUPP at 16±3 pg/mg compared to 5±1 pg/mg in Sham (p=0.007 vs Sham) and was also elevated in Sham+RUPP NK at 15±2 pg/mg (p=0.02 vs Sham). In RUPP+Sham NK, levels of placental IFN-γ were normalized at 6±2 pg/mg (p=0.01 vs RUPP).
Effects of NK Adoptive Transfer on Placental eNOS expression and phosphorylation
Placental eNOS expression was reduced by 30% in RUPP (0.71±0.09) compared to Sham, although this was not significantly different (p=0.08 vs Sham). Placental eNOS expression was 0.86±0.09 in Sham+RUPP NK (p= 0.58 vs Sham) and 0.83±0.10 in RUPP+Sham NK. (p= 0.77 vs RUPP; Figure S2). Placental phosphorylated eNOS (pS1177) was not different amoung the groups (Sham =1.0±0.04, Rupp = 0.97±0.08, Sham+RUPP NK = 0.87±0.10, RUPP+Sham NK = 0.99±0.05; p>0.05 for all comparisons; Figure S3).
DISCUSSION
Several clinical studies have reported a shift toward activated NK cells in PE. [32–35] However, the precise role of different NK cell phenotypes in PE pathophysiology has not been studied in vivo. Therefore, in this study we tested the hypotheses that (1. RUPP stimulated NKs play a direct role in causing maternal HTN and IUGR in pregnant rats and (2. Sham NKs attenuate maternal HTN and IUGR in RUPP rats. As we have previously published, there was a significant increase in both total NK cells and cNKs in the circulation and placenta of RUPP rats compared to Sham rats. We also found that RUPP-stimulated NK cells caused increased NK cell activation in the circulation and placentas of Sham recipient rats, and increased MAP, IUGR, UARI, and placental ROS. Furthermore, we observed that adoptive transfer of Sham NK cells into RUPP rats normalized NK cell activation, attenuated the increased MAP, improved IUGR, and reduced UARI. Thus, we have demonstrated that cNK cells play a direct role in PE pathophysiology, and that the polarization of NK cell phenotypes can influence pregnancy outcomes.
The results of this study not only suggest the direct contribution of placental ischemia-stimulated cNK cells in the pathophysiology of the RUPP model, but also submit for the first time, that normal placental NK cells can ameliorate RUPP induced pathophysiology. While the role of placental NK cells in pregnancy has not been thourougly researched, the beneficial activities of dNK cells during pregnancy have been widely established.[11–13] There have been several in vitro studies showing that human dNK assist in trophoblast migration, spiral artery remodeling, and fetal growth via secretion of growth factors.[11, 12, 36, 37] Likewise, experiments in animal models have also shown the importance of NK cells in normal pregnancy with NK cell deficiency or depletion resulting in altered placental structure, incomplete vascular remodeling, and fetal growth restriction.[37, 38] Importantly, there is increasing evidence that pregnancy outcomes can depend greatly on the phenotype of the NK cells present.[36, 39, 40] This current study suggests that this may apply to placental NK cells as well as dNKs.
Activated dNK cells have been implicated in several pregnancy disorders including RSA, fetal/neonatal alloimmune thrombocytopenia (FNAIT), and placental insufficiency.[9, 16, 41] While dNKs normally have little cytotoxic activity, their granules contain large amounts of cytolytic enzymes, and they have the potential to become activated, with enhanced cytotoxic activity. [16, 42, 43] Similarly, in our previous in vitro study, we found a 5-fold increase in cytotoxicity of placental NK cells from RUPP compared to those isolated from Sham rats.[22] We hypothesized that the observed shift in NK cell phenotype contributes to the pathophysiology observed in RUPP rats.
While we have previously shown that total NK cell depletion from GD14-GD19 significantly improved the phenotype observed in RUPP rats, that study did not specifically address the role of different NK cell phenotypes. By using adoptive transfer techniques, we are able to distinguish the direct roles of both RUPP and Sham placental NK cells in vivo. Additionally, we assessed the resulting NK populations in the circulation and placenta using flow cytometry. With the PE-like phenotype observed in the Sham+RUPP NK rats, the increased NK activation reinforces the possible role of cNKs in PE pathophysiology. Moreover, we observed significant attenuation of NK activation in RUPP recipients of Sham NKs along with marked improvement in their pathophysiology. This suggests that shifting the NK phenotype may be a potential therapeutic strategy for PE treatment.
We observed a significantly elevated UARI in Sham+RUPP NK rats compared to Sham, as well as attenuated UARI in RUPP+Sham NK rats compared to RUPP. Measurement of uterine artery resistance via Doppler ultrasound is a useful screening tool to predict the development of some pregnancy complications including PE and IUGR.[44, 45] Uterine artery resistance typically decreases as pregnancy progresses and this is largely attributed to spiral artery remodeling by trophoblasts.[46–48] Thus, increased UARI observed in preeclamptic women is thought to be due to poorly remodeled vessels, and this has been reflected in rodent models, including the RUPP rat.[37, 45, 49, 50] While the placenta is largely established by GD12 in rats, there is an additional wave of trophoblast invasion that occurs around GD13.5 [51, 52] and introduction of RUPP NK cells into Sham rats on GD14 could inhibit this invasion. This may impair further spiral artery remodeling and be the cause of the increased UARI we observed in this group. Alternatively, the addition of normal NK cells into the RUPP animals may promote the additional wave of trophoblast invasion, therefore causing the decreased UARI observed in RUPP+Sham NK. Another factor that has been shown to influence UARI is nitric oxide (NO) induced vasodilation.[53, 54] Therefore, we evaluated changes in placental eNOS expression as well as total Nitrate/Nitrite as an indication of NO bioavailability. Although we saw no changes in eNOS expression between groups, plasma nitrate/nitrite was reduced in Sham+RUPP NK rats compared to Sham and was elevated in RUPP+Sham NK rats compared to RUPP. These data may explain the respective alterations in UARI observed in each group.
Because increased vascular resistance impedes blood flow to the placenta and placental under-perfusion deprives the fetus of oxygen and nutrients,[55] increased UARI is also associated with IUGR.[55, 56] We observed that changes in fetal and placental weight corresponded with changes in UARI with decreased fetal and placental weight in Sham+RUPP NK which provides additional evidence that cNKs may contribute to IUGR in PE women. Similarly, we observed increased fetal and placental weight in RUPP+Sham NK which suggests that NK mediated reductions in UARI may improve fetal outcomes in response to placental ischemia.
NK cell adoptive transfer altered inflammatory cytokines in the recipient groups. Activated NK cells secrete several cytokines with the main ones being TNF-α and IFN-γ [57–59], and these two cytokines have also been observed to be elevated in PE women and RUPP rats.[21, 60–62] In this study, adoptive transfer of RUPP NKs into Sham rats resulted in higher circulating TNF-α and placental IFN-γ. There are several mechanisms by which these cytokines could contribute to the elevated MAP and IUGR observed in this group. Both TNF-α and IFN-γ have been shown to decrease NO production in cultured endothelial cells,[63, 64] and TNF- α infusion has been shown to reduce NO bioavailability in humans.[65] Elevated TNF-α has also been shown to cause human trophoblast apoptosis and endothelial dysfunction in vitro [66] and a PE-like phenotype in mice, rats, and baboons.[67–69] TNF-α infusion into pregnant rats has also been shown to increase PPET transcription[31] which was also observed in the Sham+RUPP NK group. Endothelin, a potent vasoconstrictor, is increased in both PE women and animal models, and has been identified as an important pathway in PE pathophysiology.[70, 71] Regarding IFN-γ, studies in mice have shown that it is indispensable for spiral artery formation and its absence causes increased rates of fetal loss.[38] However, exposure to excessive IFN-γ is associated with increased fetal resorption in mice[72] and increased placental apoptosis in rabbits[73]. Furthermore, in vitro administration of IFN- γ increased apoptosis of human cytotrophoblasts[74] and decreased VEGF secretion from human endothelial stromal cells.[75] In this study, placental IFN-γ was normalized in RUPP+Sham NK, which may be another mechanism by which normal NKs cause restored normal placental and pup weights in the RUPP+Sham NK group. This also points to activated NK cells as a significant source of IFN-γ in the placenta during placental ischemia. These data highlight a need for further examination of the role of IFN-γ in RUPP pathophysiology and PE.
Circulating IL-6 is another cytokine that is elevated in PE women, and has been shown to contribute to several aspects of PE pathophysiology including endothelial dysfunction and oxidative stress.[76, 77] In the current study we found IL-6 elevated in the Sham recipients of RUPP NK, which may contribute to the increased MAP, placental oxidative stress, and UARI observed in the group. Additionally, IL-6 was reduced in RUPP recipients of Sham NKs, and may contribute to their decreased MAP and improved UARI. IL-17, a significant pro-inflammatory cytokine in PE women[78], contributes to the HTN and IUGR observed in RUPP rats.[79, 80] We have previously shown that IL-17 infusion into pregnant rats causes increased NK cell activation.[22, 29] In this study, circulating and placental levels of IL-17 were unchanged in Sham recipients of RUPP NKs compared to Sham and remained elevated in the RUPP+Shan NK group. Interestingly, NK activation was reduced in RUPP+Sham NK rats despite elevated circulating and placental IL-17 levels. This suggests that the introduction of Sham NK cells at the time of the RUPP procedure was sufficient to prevent IL-17 induced NK activation, and indicates that further investigation regarding this mechanism is needed.
The observed changes in circulating levels of the antiangiogenic factor sFlt-1 following adoptive transfer of Sham NK cells into RUPP rats also gives insight into the mechanism by which Sham NK cells blunted MAP in RUPP recipients. sFlt-1 is a splice variant of the VEGF receptor and acts as an antagonist to VEGF and placental growth factor (PLGF).[81, 82] Several studies have shown increased circulating sFlt-1 levels in preeclamptic women when compared to their normal pregnant counterparts.[82–84] sFlt-1 is also significantly increased in the RUPP rat model of PE[85, 86] and several investigators have found that infusion of sFlt-1 into pregnant rats causes a PE-like phenotype.[85–89] While we still do not fully understand mechanisms by which Sham NK cells prevented an increase sFlt-1 in RUPP recipients, we suspect it may be due to increased VEGF production. dNK cells are known to secrete this angiogenic factor [11, 90, 91], and dNK cells from women with RSA produce less.[92] In the current study, we saw that circulating and placental VEGF levels were reduced in Sham recipients of RUPP NKs and elevated in RUPP recipients of Sham NKs. This suggests that RUPP stimulated NKs secrete fewer pro-angiogenic factors than their normal counterparts. Several investigators have administered recombinant VEGF and PLGF to PE animal models and saw decreased circulating sFlt-1 levels, decreased maternal MAP, and increased fetal weight. [93, 94] Thus, the reduction in circulating sFlt-1 is a possible mechanism by which IUGR was attenuated and MAP was reduced in RUPP+Sham NKs. Furthermore, the reduction in VEGF is possible mechanism by which IUGR was induced and MAP elevated in the Sham+RUPP NK group.
While these results have provided more information concerning NK polarization in PE, there are some limitations to the current study. Although we observe significantly higher placental ROS in Sham+RUPP NK animals, there was no change in placental ROS in RUPP+Sham NK suggesting that the mechanisms responsible are independent of cNKs and warrant further study. Additionally, RUPP recipients of Sham NKs still displayed elevated levels of circulating and placental TNF-α and circulating IFN-γ. Since both total and activated NK cells in the circulation and placenta were normalized in RUPP+Sham NK, this points to an additional cellular source of these cytokines in RUPPs. Inflammatory CD4+ T cells are known to also produce these cytokines[95, 96] and these cells are also elevated in RUPPs [97], making them them a possible source. Finally, continued examination into the precise mechanisms by which RUPP NK cells induced a PE like phenotype in Sham rats is needed, particularly the role of cNK associated cytokines TNF- α and IFN-γ. While previous studies in the RUPP have shown that TNF-α inhibition decreases MAP, additional investigation into the mechanism of this improvement is warranted. Furthermore, the role of IFN-γ in RUPP pathophysiology has not been explored and further study could reveal the pathways involved. Despite these limitations, the results of this study provide significant insight on the role of NK cell polarization in PE pathophysiology.
In this study, adoptive transfer of RUPP NK cells into Sham pregnant rats increased placental ROS, circulating TNF-α, placental IFN-γ, and decreased circulating and placental VEGF. This resulted in a PE like phenotype with significantly elevated NK cell activation, MAP, UARI, and IUGR. Alternatively, transfer of Sham NK cells into RUPP rats normalized circulating and placental NK cell populations, sFlt-1, circulating and placental VEGF, and placental IFN-γ. This led to significantly lower MAP, improved IUGR, and decreased UARI compared to RUPPs. Research focused on identifying factors involved in NK cell activation as well as those that can normalize the placental NK populations could provide therapeutic targets to prevent or treat PE in women.
Supplementary Material
ACKNOWLEDGMENTS
OKT, CHB, GAT, LMA, CJ, MG, CAG, TI, OTH, JMW, and DCC gave final approval of the version to be published and agreed to be accountable for all alspects of the work in ensuring the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
OKT, CHB, GAT, LMA, CJ, MG, CAG, TI, OTH JMW, and DCC, made substantial contributions to the conception and design of acquisition of data or analysis and intetpretation of data. O
OKT and DCC drafted the manuscript and revised it critically for important intellectural content.
SOURCES OF FUNDING
This work was funded by NIH Grants: T32HL105324 and F31HL149257 awarded to OKT, R01DK109133 awarded to JMW, and R00HL130456, P20GM104357, and R01HL151407 awarded to DCC..
Footnotes
References
- 1.Jeyabalan A, Epidemiology of preeclampsia: impact of obesity. Nutr Rev, 2013. 71 Suppl 1: p. S18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uzan J, et al. , Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag, 2011. 7: p. 467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hladunewich M, Karumanchi SA, and Lafayette R, Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol, 2007. 2(3): p. 543–9. [DOI] [PubMed] [Google Scholar]
- 4.Young BC, Levine RJ, and Karumanchi SA, Pathogenesis of preeclampsia. Annu Rev Pathol, 2010. 5: p. 173–92. [DOI] [PubMed] [Google Scholar]
- 5.Ezeigwe CO, et al. , Placental Peripartum Pathologies in Women with Preeclampsia and Eclampsia. Obstet Gynecol Int, 2018. 2018: p. 9462938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayrink J, Costa ML, and Cecatti JG, Preeclampsia in 2018: Revisiting Concepts, Physiopathology, and Prediction. ScientificWorldJournal, 2018. 2018: p. 6268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukui A, et al. , Changes of NK cells in preeclampsia. Am J Reprod Immunol, 2012. 67(4): p. 278–86. [DOI] [PubMed] [Google Scholar]
- 8.Fukui A, et al. , Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol, 2011. 90(1): p. 105–10. [DOI] [PubMed] [Google Scholar]
- 9.Wallace AE, et al. , Decidual natural killer cell interactions with trophoblasts are impaired in pregnancies at increased risk of preeclampsia. Am J Pathol, 2013. 183(6): p. 1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristiani CM, et al. , Human NK Cell Subsets in Pregnancy and Disease: Toward a New Biological Complexity. Front Immunol, 2016. 7: p. 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna J, et al. , Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med, 2006. 12(9): p. 1065–74. [DOI] [PubMed] [Google Scholar]
- 12.Jabrane-Ferrat N and Siewiera J, The up side of decidual natural killer cells: new developments in immunology of pregnancy. Immunology, 2014. 141(4): p. 490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croy BA, et al. , Decidual natural killer cells: key regulators of placental development (a review). J Reprod Immunol, 2002. 57(1–2): p. 151–68. [DOI] [PubMed] [Google Scholar]
- 14.Saito S, et al. , The role of the immune system in preeclampsia. Mol Aspects Med, 2007. 28(2): p. 192–209. [DOI] [PubMed] [Google Scholar]
- 15.Bulmer JN, Williams PJ, and Lash GE, Immune cells in the placental bed. Int J Dev Biol, 2010. 54(2–3): p. 281–94. [DOI] [PubMed] [Google Scholar]
- 16.Yougbare I, et al. , Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat Commun, 2017. 8(1): p. 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amaral LM, et al. , Continued Investigation Into 17-OHPC: Results From the Preclinical RUPP Rat Model of Preeclampsia. Hypertension, 2017. 70(6): p. 1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Bai C, and Liu Y, Interleukin-6 contributes to myocardial damage in pregnant rats with reduced uterine perfusion pressure. Braz J Med Biol Res, 2018. 51(8): p. e6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh SK, et al. , Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization. Hypertension, 2009. 54(2): p. 345–51. [DOI] [PubMed] [Google Scholar]
- 20.Li J, LaMarca B, and Reckelhoff JF, A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. AJP: Heart and Circulatory Physiology, 2012. 303(1): p. H1–H8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elfarra J, et al. , Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure. Clin Sci (Lond), 2017. 131(23): p. 2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travis OK, et al. , Interleukin-17 Signaling Mediates Cytolytic Natural Killer Cell Activation in Response to Placental Ischemia. Am J Physiol Regul Integr Comp Physiol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields CA, et al. , Placental ischemia-stimulated T-helper 17 cells induce preeclampsia-associated cytolytic natural killer cells during pregnancy. Am J Physiol Regul Integr Comp Physiol, 2018. 315(2): p. R336–R343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornelius DC, et al. , Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Physiol Regul Integr Comp Physiol, 2016. 311(6): p. R1192–R1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng BG, et al. , Expansion and activation of natural killer cells from PBMC for immunotherapy of hepatocellular carcinoma. World J Gastroenterol, 2004. 10(14): p. 2119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv LH, et al. , Functional distinction of rat liver natural killer cells from spleen natural killer cells under normal and acidic conditions in vitro. Hepatobiliary Pancreat Dis Int, 2012. 11(3): p. 285–93. [DOI] [PubMed] [Google Scholar]
- 27.Travis OK, et al. , Interleukin-17 signaling mediates cytolytic natural killer cell activation in response to placental ischemia. Am J Physiol Regul Integr Comp Physiol, 2020. 318(6): p. R1036–R1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaral LM, et al. , 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension, 2015. 65(1): p. 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travis OK, et al. , Chronic infusion of interleukin-17 promotes hypertension, activation of cytolytic natural killer cells, and vascular dysfunction in pregnant rats. Physiol Rep, 2019. 7(7): p. e14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace K, et al. , CD4(+) T Cells Are Important Mediators of Oxidative Stress That Cause Hypertension in Response to Placental Ischemia. Hypertension, 2014. 64(5): p. 1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaMarca BB, et al. , Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension, 2005. 46(4): p. 1022–5. [DOI] [PubMed] [Google Scholar]
- 32.Borzychowski AM, et al. , Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. European Journal of Immunology, 2005. 35(10): p. 3054–3063. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, et al. , Studies on activity of NK cells in preeclampsia patients. J Huazhong Univ Sci Technolog Med Sci, 2004. 24(5): p. 473–5. [DOI] [PubMed] [Google Scholar]
- 34.Bachmayer N, et al. , Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am J Reprod Immunol, 2006. 56(5–6): p. 292–301. [DOI] [PubMed] [Google Scholar]
- 35.Wilczynski JR, et al. , Lymphocyte subset distribution and cytokine secretion in third trimester decidua in normal pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol, 2003. 109(1): p. 8–15. [DOI] [PubMed] [Google Scholar]
- 36.Moffett A and Colucci F, Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest, 2014. 124(5): p. 1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer N, Schuler T, and Zenclussen AC, Simultaneous Ablation of Uterine Natural Killer Cells and Uterine Mast Cells in Mice Leads to Poor Vascularization and Abnormal Doppler Measurements That Compromise Fetal Well-being. Front Immunol, 2017. 8: p. 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashkar AA, et al. , Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol, 2003. 171(6): p. 2937–44. [DOI] [PubMed] [Google Scholar]
- 39.Mosimann B, et al. , Natural Killer Cells and Their Activation Status in Normal Pregnancy. International Journal of Reproductive Medicine, 2013. 2013: p. 906813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dosiou C and Giudice LC, Natural Killer Cells in Pregnancy and Recurrent Pregnancy Loss: Endocrine and Immunologic Perspectives. Endocrine Reviews, 2005. 26(1): p. 44–62. [DOI] [PubMed] [Google Scholar]
- 41.Nakashima A, et al. , Granulysin produced by uterine natural killer cells induces apoptosis of extravillous trophoblasts in spontaneous abortion. Am J Pathol, 2008. 173(3): p. 653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma S, Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol, 2014. 58(2–4): p. 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fauriat C, et al. , Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood, 2010. 115(11): p. 2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridder A, et al. , Preeclampsia: The Relationship between Uterine Artery Blood Flow and Trophoblast Function. Int J Mol Sci, 2019. 20(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cnossen JS, et al. , Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ, 2008. 178(6): p. 701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giordano R, et al. , Uterine artery Doppler flow studies in obstetric practice. J Prenat Med, 2010. 4(4): p. 59–62. [PMC free article] [PubMed] [Google Scholar]
- 47.Prefumo F, Sebire NJ, and Thilaganathan B, Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum Reprod, 2004. 19(1): p. 206–9. [DOI] [PubMed] [Google Scholar]
- 48.Fraser R, et al. , Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol, 2012. 228(3): p. 322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillis EE, et al. , The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol, 2015. 309(1): p. R62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavalli RC, et al. , Induced Human Decidual NK-Like Cells Improve Utero-Placental Perfusion in Mice. PLoS One, 2016. 11(10): p. e0164353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva JF and Serakides R, Intrauterine trophoblast migration: A comparative view of humans and rodents. Cell Adh Migr, 2016. 10(1–2): p. 88–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soares MJ, et al. , Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta, 2012. 33(4): p. 233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdel-Razik M, El-Berry S, and Mostafa A, The Effects of Nitric Oxide Donors on Uterine Artery and Sub-endometrial Blood Flow in Patients with Unexplained Recurrent Abortion. J Reprod Infertil, 2014. 15(3): p. 142–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Kulandavelu S, et al. , Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension, 2012. 60(1): p. 231–8. [DOI] [PubMed] [Google Scholar]
- 55.Krishna U and Bhalerao S, Placental insufficiency and fetal growth restriction. J Obstet Gynaecol India, 2011. 61(5): p. 505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLeod L, How useful is uterine artery Doppler ultrasonography in predicting pre-eclampsia and intrauterine growth restriction? CMAJ, 2008. 178(6): p. 727–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woo CH, et al. , Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem, 2000. 275(41): p. 32357–62. [DOI] [PubMed] [Google Scholar]
- 58.Kim JJ, et al. , TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L). Cell Death Differ, 2010. 17(9): p. 1420–34. [DOI] [PubMed] [Google Scholar]
- 59.Mosovsky K, et al. , Interaction of Interferon gamma-induced reactive oxygen species with ceftazidime leads to synergistic killing of intracellular Burkholderia pseudomallei. Antimicrob Agents Chemother, 2014. 58(10): p. 5954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Udenze I, et al. , The role of cytokines as inflammatory mediators in preeclampsia. Pan Afr Med J, 2015. 20: p. 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raghupathy R, Cytokines as key players in the pathophysiology of preeclampsia. Med Princ Pract, 2013. 22 Suppl 1: p. 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaMarca B, et al. , Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension, 2008. 52(6): p. 1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodwin BL, et al. , Tumor necrosis factor-alpha reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. Am J Physiol Heart Circ Physiol, 2007. 293(2): p. H1115–21. [DOI] [PubMed] [Google Scholar]
- 64.Javanmard SH and Dana N, The effect of interferon gamma on endothelial cell nitric oxide production and apoptosis. Adv Biomed Res, 2012. 1: p. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura M, et al. , Effects of tumor necrosis factor-alpha on basal and stimulated endothelium-dependent vasomotion in human resistance vessel. J Cardiovasc Pharmacol, 2000. 36(4): p. 487–92. [DOI] [PubMed] [Google Scholar]
- 66.Azizieh FY and Raghupathy RG, Tumor necrosis factor-alpha and pregnancy complications: a prospective study. Med Princ Pract, 2015. 24(2): p. 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sunderland NS, et al. , Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine, 2011. 56(2): p. 192–9. [DOI] [PubMed] [Google Scholar]
- 68.Bobek G, et al. , Placental Regulation of Inflammation and Hypoxia after TNF-α Infusion in Mice. American Journal of Reproductive Immunology, 2015. 74(5): p. 407–418. [DOI] [PubMed] [Google Scholar]
- 69.Alexander BT, et al. , Tumor necrosis factor–α–induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression*. American Journal of Hypertension, 2002. 15(2): p. 170–175. [DOI] [PubMed] [Google Scholar]
- 70.Saleh L, et al. , The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther Adv Cardiovasc Dis, 2016. 10(5): p. 282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verdonk K, et al. , Association Studies Suggest a Key Role for Endothelin-1 in the Pathogenesis of Preeclampsia and the Accompanying Renin–Angiotensin–Aldosterone System Suppression. Hypertension, 2015. 65(6): p. 1316–1323. [DOI] [PubMed] [Google Scholar]
- 72.Liu HY, et al. , High-dose interferon-gamma promotes abortion in mice by suppressing Treg and Th17 polarization. J Interferon Cytokine Res, 2014. 34(5): p. 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z, et al. , The effect on MHC class II expression and apoptosis in placenta by IFNγ administration. Contraception, 2002. 65(2): p. 177–184. [DOI] [PubMed] [Google Scholar]
- 74.Sun QH, et al. , IFN-gamma promotes apoptosis of the uterus and placenta in pregnant rat and human cytotrophoblast cells. J Interferon Cytokine Res, 2007. 27(7): p. 567–78. [DOI] [PubMed] [Google Scholar]
- 75.Kawano Y, et al. , Effects of interferon-gamma on secretion of vascular endothelial growth factor by endometrial stromal cells. Am J Reprod Immunol, 2000. 43(1): p. 47–52. [DOI] [PubMed] [Google Scholar]
- 76.Lamarca B, Brewer J, and Wallace K, IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? International journal of interferon, cytokine and mediator research, 2011. 2011(3): p. 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lockwood CJ, et al. , Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. The American journal of pathology, 2008. 172(6): p. 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Shahaway AA, et al. , Role of maternal serum interleukin 17 in preeclampsia: diagnosis and prognosis. Journal of inflammation research, 2019. 12: p. 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhillion P, et al. , IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. American journal of physiology. Regulatory, integrative and comparative physiology, 2012. 303(4): p. R353–R358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cornelius DC, et al. , Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension (Dallas, Tex. : 1979), 2013. 62(6): p. 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roberts JM and Rajakumar A, Preeclampsia and soluble fms-like tyrosine kinase 1. J Clin Endocrinol Metab, 2009. 94(7): p. 2252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maynard SE, et al. , Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest, 2003. 111(5): p. 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koga K, et al. , Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab, 2003. 88(5): p. 2348–51. [DOI] [PubMed] [Google Scholar]
- 84.Tsatsaris V, et al. , Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab, 2003. 88(11): p. 5555–63. [DOI] [PubMed] [Google Scholar]
- 85.Gilbert JS, Babcock SA, and Granger JP, Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension, 2007. 50(6): p. 1142–7. [DOI] [PubMed] [Google Scholar]
- 86.Zhu M, et al. , Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am J Physiol Regul Integr Comp Physiol, 2016. 311(3): p. R505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bridges JP, et al. , Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens, 2009. 22(5): p. 564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li F, et al. , eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol, 2012. 23(4): p. 652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy SR, et al. , Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension, 2010. 55(2): p. 394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kopcow HD and Karumanchi SA, Angiogenic factors and natural killer (NK) cells in the pathogenesis of preeclampsia. J Reprod Immunol, 2007. 76(1–2): p. 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu B, et al. , Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity, 2017. 47(6): p. 1100–1113 e6. [DOI] [PubMed] [Google Scholar]
- 92.Guo W, et al. , Decreased Human Leukocyte Antigen-G Expression by miR-133a Contributes to Impairment of Proinvasion and Proangiogenesis Functions of Decidual NK Cells. Frontiers in Immunology, 2017. 8(741). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spradley FT, et al. , Placental Growth Factor Administration Abolishes Placental Ischemia-Induced Hypertension. Hypertension, 2016. 67(4): p. 740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilbert JS, et al. , Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension, 2010. 55(2): p. 380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biron CA, Cytokines in the generation of immune responses to, and resolution of, virus infection. Current Opinion in Immunology, 1994. 6(4): p. 530–538. [DOI] [PubMed] [Google Scholar]
- 96.Raphael I, et al. , T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine, 2015. 74(1): p. 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novotny S, et al. , CD4(+) T Cells Play a Critical Role in Mediating Hypertension in Response to Placental Ischemia. J Hypertens (Los Angel), 2013. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.