Abstract
Individual variation in social behavior offers an opportunity to explore gene-by-environment interactions that could contribute to adaptative or atypical behavioral profiles (e.g., autism spectrum disorders). Outbred, socially monogamous prairie voles provide an excellent model to experimentally explore how natural variations in rearing and genetic diversity interact to shape reproductive and nonreproductive social behavior. In this study, we manipulated rearing (biparental versus dam-only), genotyped the intronic NT213739 single nucleotide polymorphism (SNP) of the oxytocin receptor gene (Oxtr), and then assessed how each factor and their interaction related to reciprocal interactions and partner preference in male and female adult prairie voles. We found that subjects reared biparentally and carrying the C/T version of NT213739 formed more robust partner preferences than T/T subjects. In general, dam-only reared animals huddled less with a conspecific in reproductive and nonreproductive contexts, but the effect of rearing was more pronounced in T/T carrying animals. In line with previous literature, C/T animals exhibited higher densities of oxytocin receptor (OXTR) in the striatum (caudoputamen, nucleus accumbens) compared to T/T subjects. There was also a gene-by-rearing interaction in the striatum and insula of females: In the insula, T/T females expressed varying OXTR densities depending on rearing. Overall, this study demonstrates that significant differences in adult reproductive and nonreproductive social behavior and OXTR density can arise due to natural differences in Oxtr, experimental manipulations of rearing, and their interaction.
Keywords: Social behavior, prairie vole, oxytocin receptor, rearing, gene-by-environment interaction, partner preference, reciprocal interaction, Oxtr single-nucleotide polymorphism
1. Introduction
Individual differences in social behavior appear across species (Stevenson et al., 2018). Identifying the mechanisms that produce this variation is the subject of intense study in evolutionary ecology (Bell, 2007; Sih et al., 2004), behavioral genetics (Tikhodeyev and Shcherbakova, 2019), and clinical neuroscience (Kendrick et al., 2018; Loth et al., 2014; Notzon et al., 2016).
There is strong evidence that genetic variation in the oxytocin (OXT) system gives rise to variation in social behavior, from facial recognition to affiliation, pair bonding, and parenting (Rilling and Young, 2014; Skuse et al., 2014; Walum et al., 2012). In some cases, genetic variability has been linked to more extreme forms of behavioral variation, such as those associated with autism spectrum disorders (ASD), schizophrenia, and other disorders (Kendrick et al., 2018). Early rearing environment also appears to shape individual differences in social behavior (Barrett et al., 2015; Meaney, 2001; Palumbo et al., 2018), with the OXT system being sensitive to experience (Ahern and Young, 2009; Francis et al., 2000; Johnson and Buisman-Pijlman, 2016). Indeed, there is growing evidence that polymorphisms in the oxytocin receptor gene (OXTR in humans, Oxtr in rodents) may interact with adverse experiences to shape social behavior and emotional regulation in humans (Hostinar et al., 2014; Loth et al., 2014; Smearman et al., 2015; Thompson et al., 2011).
Prairie voles (Microtus ochrogaster) provide an excellent animal model to experimentally examine how genetics, early rearing, and gene-by-environment interactions shape individual differences in social behavior (Barrett et al., 2015; McGraw and Young, 2009). Prairie voles are small, monogamous rodents that typically form long-term bonds between mates and rear offspring biparentally (Ahern et al., 2011; Ahern and Young, 2009; Carter et al., 1995; Getz et al., 1981). Further, like humans, prairie voles also exhibit marked individual variation in behavior, genetics, and neurobiology, even in the laboratory (Barrett et al., 2015; Hammock and Young, 2005; King et al., 2016; Okhovat et al., 2015; Young and Wang, 2004). These individual differences can be leveraged to tease apart the contribution of Oxtr variants, the environment, and their interaction.
Recently, the Oxtr intronic single nucleotide polymorphism (SNP) NT213739 was strongly associated in prairie voles with striatal OXT receptor (OXTR) density and adult partner preference formation (King et al., 2016). C/C, C/T, and T/T variants predicted high, mid-range, and low OXTR densities in the caudoputamen (CP) and nucleus accumbens (NAcc). Importantly, the C/C variant and high NAcc OXTR were associated with more rapid partner preference formation (King et al., 2016). Whether Oxtr variants influenced the standard onset (post 24 hrs cohabitation) of partner preference formation was not reported.
In parallel, other studies have shown that adult prairie vole partner preference formation is sensitive to differences in rearing (Ahern and Young, 2009; Arias Del Razo and Bales, 2016; Bales et al., 2007; Barrett et al., 2015; Getz and McGuire, 1997). For example, we previously showed that biparental vs dam-only rearing, which both occur in nature, affected the quantity of early social interactions and, in turn, adult bonding and parenting behaviors of offspring in adulthood (Ahern and Young, 2009). Likewise, neglect-like rearing inhibits female partner preference formation (Barrett et al., 2015).
In the current study, we investigated whether the cis intronic Oxtr SNP NT213739 identified by King et al. (2016) might interact with naturalistic rearing differences to influence adult social behavior and possibly NAcc OXTR expression. We explored these relationships in males and females, in three different forebrain regions, and in both reproductive and nonreproductive social contexts. Inclusion of nonreproductive contexts is critical, because much of the human literature focuses on social behaviors in nonreproductive contexts, while most of the prairie vole literature has focused on reproductive-related social behaviors.
We hypothesized that biparentally reared C-carrying animals would exhibit the most robust partner preferences and the highest OXTR densities in the NAcc. Prairie voles reared by the dam only or that carried T/T would show non-significant partner preferences and lower OXTR densities, and dam-only reared T/T animals would exhibit no partner preference, the least huddling, and the lowest OXTR densities. Further, we hypothesized that similar differences would occur in nonreproductive social contexts, with BP-reared C-carrying animals exhibiting more time being prosocial than T/T and singledam reared animals in the nonreproductive social context.
2. Methods and Materials
2.1. Animals
Prairie voles (Microtus ochrogaster) originally derived from Illinois were bred and reared until 21 d in standard rat cages (43 x 20 x 20 cm) containing corn cob bedding (1/4″, Teklad, 7097), ad libitum access to water and food (LabDiet, 5263-4), and a cotton nestlet (Ancare, NES3600QTY 59CS). Weanlings were then housed in mouse cages (25 x 15 x 13 cm), also with corn cob bedding, a nestlet, and ad libitum access to food and water. Ambient temperature and humidity were maintained at ~20-25C and 35-60%, respectively, with a 14:10 light:dark cycle. This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, NIH. The protocol was approved by the Institutional Animal Care and Use Committee of Quinnipiac University.
Breeders were used to produce 12 male and 12 female offspring in three cohorts, for a total of 36 males and 36 females. Following Ahern et al. (2009), pups were reared either biparentally (BP) or dam-only (DO). These differences in rearing occur naturally in the wild and we have shown they lead to significant differences in the amount of care pups receive (e.g., licking and grooming, time exposed, etc.) over the first 10 postnatal days (Ahern et al., 2011; Ahern and Young, 2009; Getz et al., 1981); parenting behavior was not scored in this study. At 21 d of age, pups were weaned into same-sex pairs, with males and females housed in separate cages and rooms. When necessary, weanlings were ear punched for identification.
In adulthood (70-105 d of age), social behavior of 72 animals was examined using repeated reciprocal interaction tests and the partner preference test. Tissue was then collected for brain and genetic analysis.
2.2. Social Behavior Testing Overview
The partner preference test (PPT) is the gold standard for assessing social affiliation and bonding behavior in prairie voles (Ahern et al., 2009; Beery et al., 2018; Williams et al., 1992). Importantly, this test is often used as a proxy to quantify male-female bonding, so it represents social behavior in a reproductive, choice-based context.
To complement this measure of sociality, we also tested prairie voles in long-duration, same-sex reciprocal interaction tests. These tests parallel the length of the PPT (3-hours) and the ability of subjects to fully interact (Ahern et al., 2019), neither of which is possible in many other standard rodent tests of sociality (Beery et al., 2018). The reciprocal interaction tests also allow repetitive testing in a way that is not typically possible with the PPT.
2.3. Reciprocal Interaction Testing
The long-duration reciprocal interaction tests used in this study have been described fully in (Ahern et al., 2019). In brief, approximately 2 days prior to the first test session, one animal from each weanling pair was marked by shaving the rump to reveal the lighter skin underneath (~2 inches anterior-to-posterior by ~1-2 inches right-to-left) and loosely collared with a bright yellow zip-tie (Commercial Electric, #826 843). Males and females within each cohort were tested separately. Therefore, each cohort had a male group consisting of 6 marked animals and 6 unmarked animals and a female group consisting of 6 marked animals and 6 unmarked animals.
All reciprocal interaction tests occurred in the same 3-chamber testing boxes that are normally used for the PPT (Ahern et al., 2009). Each test consisted of one marked animal and one unmarked animal. Unlike the PPT, both subjects are free roaming. At the beginning of each test, the two animals were introduced into separate chambers within the 3-chambered box. The entire 3-hour session was digitally recorded.
Each cohort of same-sex animals was then tested using a partial round-robin design, with each marked male tested with its unmarked cagemate twice (SS-CM tests 1-2) and with four different strangers once each (SS-S tests 1-4; Figure 1). Because each animal served as both test animal and stimulus animal, testing the animals in this fashion ensured animals served as a stranger multiple times and as a cagemate twice. The design was only a partial round-robin, however, because marked animals were never tested with other marked animals and unmarked animals were never tested with other unmarked animals. SS-CM and SS-S test sessions were pseudo-counter-balanced to avoid temporal and spatial positioning effects (see Figure 1). For this study, we averaged the behavior from the two cagemate tests (SS-CM 1-2) and first two stranger tests (SS-S 1-2) to compare social behavior in response to familiar vs unfamiliar same-sex conspecifics.
Figure 1.
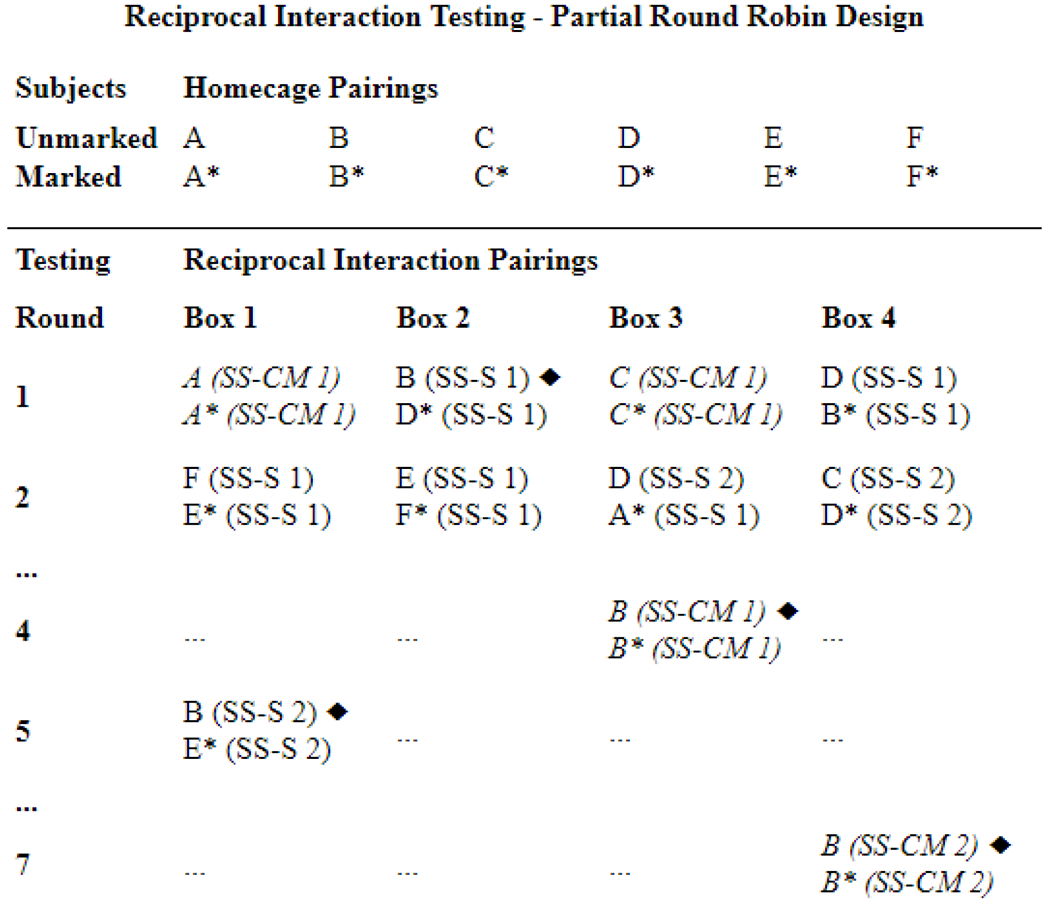
Example reciprocal interaction partial round robin testing design. Animals were weaned into homecage pairs; one was “marked” prior to testing. Reciprocal interaction tests involved multiple rounds of assessing Same-Sex Stranger (SS-S) and Same-Sex Cagemate (SS-CM) pairings. Pairs were recorded for 3 hours. Each subject interacted with multiple strangers (e.g., pairing SS-S 1, SS-S 2) and their cagemate twice (pairing SS-CM 1, SS-CM 2). Counterbalancing avoided temporal and spatial positioning effects. To illustrate testing progression, subject “B” pairings are highlighted (♦). After round 2, non-”B” rounds and pairings are represented by a “…” to allow easier visualization.
After all same-sex reciprocal interaction tests had been completed, male and female pairs were created by pairing a marked animal with an unmarked animal. Sibling pairs were avoided. The first 3 hours of male-female cohabitation served as an opposite-sex stranger (OS-S) reciprocal interaction test. After the OS-S session, pairs were moved to a homecage to cohabitate for ~21 more hours (for a total of ~24 hours).
2.4. Partner Preference Testing
PPTs have been described extensively elsewhere (Williams et al., 1992) and we followed the same procedure as Ahern et al. (2009) to allow automated scoring of affiliative huddling. In brief, after 24 hours of cohabitation (3 hours of OS-S testing plus ~21 more hours in a homecage), females were tethered at each end of the PPT testing boxes and allowed to acclimate for >5 min. The female that had cohabitated with the test male was the partner; a female of similar sociosexual experience served as the stranger. The locations of partners and strangers were counterbalanced. Males were then placed in the central chamber and allowed to roam freely for 3 hours.
After testing, males and females were returned to their homecages, and pairs were allowed to cohabitate again overnight for a total of ~48 hours since initial pairing. Males were then tethered as stimulus animals and female subjects were PPT tested for 3 hours.
Thus, male PPT behavior represents 24 hours of cohabitation; female PPT behavior represents 48 hours of cohabitation.
2.5. Behavior Quantification
All behavior tests were recorded by a digital surveillance system (QSee). Video files were processed and behavior scored automatically by TopScan’s SocialScan 2.0 and AgressionScan modules (Clever Sys Inc., Reston, VA), using the settings described in (Ahern et al., 2019). Animals were marked or unmarked to allow the program to track individuals. For the reciprocal interaction tests, time spent huddling and distance moved were the outcome measures. For the PPT, time spent being social, time being nonsocial, time huddling with the partner and stranger, and distance moved were the dependent measures. Huddling was defined programmatically as the amount of time in immobile social contact with a conspecific, given an immobility threshold of 0.05. Being social was defined as being able to socially interact; while being nonsocial was defined as the time spent in the neutral chamber outside the reach of the tethered stimulus animals. Inter-rater reliability with a trained human scorer was high (Ahern et al., 2019). The total distance moved represented the distance traveled (in mm) of the animal’s center point after calibrating the program to the size of the test boxes. In the reciprocal interaction tests, all distance data was analyzed. In the PPT, distance was only quantified for the test animal in the nonsocial areas of the testing box (i.e., the areas beyond the reach of the tethered stimulus animals) to avoid applying distances to the wrong animal.
2.6. Tissue Collection
After the female PPT sessions, males and females were returned to their homecages and then sacrificed to collect brain and liver tissue. Subjects were euthanized using an overdose of CO2 and then rapidly decapitated. Brains were quickly removed, split into right and left hemispheres, and snap frozen by burying them in crushed dry-ice. Portions of the liver were also removed and placed into 1.5 mL tubes. Brains and livers were stored at −80C until processed.
2.7. Genotyping
DNA for NT213739 genotyping was isolated from frozen livers using the Qiagen DNeasy Kit (Qiagen, #69506). In King et al. (2016), they used forward (5’- GGGACGTTCACGTTACATGG -3’) and reverse (5’- AGACGGGACAGAGTCTCCAG -3’) primers to amplify a 117 bp amplicon around the intronic SNP. The amplicon was then restriction enzyme digested using BsiHKAI (NEB, #RO570S), which cuts the C-allele but not the T-allele. BsiHKAI splits the amplicon approximately in half.
We had difficulty clearly distinguishing cut vs uncut amplicons, so we designed our own forward (5’ - CTCCTATTCAGCCCTCAGAAAC - 3’) and reverse (5’ - TGAACCCTTGGTGAGGAAAC - 3’) primers. These new primers produce a 644 bp amplicon and BsiHKAI cuts the C-allele to produce bands of 492 bp and 152 bp.
With our new primers, we used Ilustra PuRe Taq Ready-to-Go PCR Beads (GE, #27-9557-01), a PCR cycler (BioRad) set to 35 cycles (94C denature, 55C annealing, 72C elongation), and then a BsiHKAI restriction digest for 1.5 hours before visualization using a 3% agarose gel (Hoefer, #GR140-500) infused with SYBR green, run for ~1 hr at ~100V. See Supplemental Materials for additional details.
Of the 72 animals that underwent behavioral testing, only 69 were successfully genotyped (Supplementary Figure S1). Of those 69, unfortunately only 3 were C/C, so C/C animals were excluded, leaving 66 C/T and T/T subjects (Male BP-reared C/T = 7; Male BP-reared T/T = 11; Male DO-reared C/T = 7; Male DO-Reared T/T = 8; Female BP-reared C/T = 8; Female BP-reared T/T = 7; Female DO-reared C/T = 11; Female DO-Reared T/T = 7). All genotype comparisons were between C/T and T/T. Importantly, while King et al. (2016) focused on C/C vs T/T, they do report distinct OXTR binding densities between C/T and T/T as well.
2.8. OXTR Autoradiography
The left hemisphere from each test animal was cryostat sectioned into 6 coronal series at 20 μm. Sections were thaw-mounted onto Superfront Plus slides (Denville) and stored at −80C. One series was used for OXTR autoradiography using a previously published procedure (Beery and Zucker, 2010) using the 125I-omithine vasotocin analog vasotocin, d(CH2)5[Tyr(Me)2,Thr4,Orn8,(125I)Tyr9-NH2] (1251-OVTA, PerkinElmer, #NEX254010UC). Non-specific binding was assessed in a small sampling of parallel slides using (Thr4,Gly7)-oxytocin (Bachem, El-7710), a selective OXTR agonist with > 16,000-fold higher selectivity for the oxytocin receptor over either the V1a or V1b receptor (Manning et al., 2012). After completion of the receptor autoradiographic assay, slides were apposed to Kodak BioMax MR film (Carestream Health, #870-1302) for 72 hours. All brains from the 3 cohorts were assayed simultaneously, allowing for within assay group comparisons. The film was subsequently developed and scanned at 1200dpi (Mustek ScanExpress A3 USB 2400 Pro) at uniform settings.
2.9. OXTR Density Quantification Autoradiography
ImageJ (https://imagej.nih.gov/ij/) was used to quantify the digital scans OXTR in 3 regions of interest (ROIs): the NAcc (shell and core combined, as in King et al., 2016), the adjacent caudoputamen (CP), and the Insula (Ins). The Allen Mouse Brain reference atlas was used as a reference for delimiting structures (Lein et al., 2007). Quantification assessed relative optical desnity of OXTR, as in other studies (Ondrasek et al., 2018; Parker et al., 2001; Zoicas et al., 2014). Scorers were blind to rearing, sex, and genotype, and they showed high inter-rater reliability (e.g., NAcc: r = 0.98, p < 0.05, N = 46 subjects). For the NAcc, every section with a clearly delimitable structure was traced and quantified. In most cases 8-10 tracings were averaged to produce a mean relative optical density for each subject. The CP and Ins were traced on the same sections as the NAcc and all tracings within each ROI were averaged. The average non-specific binding optical density was then subtracted from these averages to produce the scores that were used in the analysis.
2.10. Data Analysis
Animal identities were decoded and outcome measures calculated in a spreadsheet and then imported into SPSS (v. 23, IBM) to calculate descriptive and inferential statistics. SPSS’s general linear modeling (GLM) was used to analyze the effect of the manipulated (e.g., rearing, type of stimulus, test type) and measured factors (e.g., sex, genotype) on prairie vole behavior and relative optical density of OXTR. We also calculated Pearson’s correlation coefficients to analyze the relationship between behaviors across tests.
Statistical significance was based on an alpha level of 0.05, and all tests were two-tailed. Post hoc tests were conducted after significant main effects or interactions.
3. Results
3.1. Genotype and Rearing Influenced Partner Preference Test Behavior
First, we tested the hypothesis that genotype, rearing, and genotype-by-rearing interactions influence adult partner preference behavior. We analyzed PPT social and nonsocial behavior using a mixed general linear model (GLM), which accounted for sex (male, female), rearing (BP-reared, DO-reared), genotype (C/T, T/T), and type of social behavior (repeated-measure: being social with the partner, being social with the stranger, and being nonsocial in the neutral chamber) on duration of behavior. The multivariate test revealed a significant within-subjects effect of behavior-type (F1,58 = 79.43, p = 1.85E-12) and multiple interactions, including behavior-type-by-rearing (F1,58 = 5.05, p = 0.029), behavior-type-by-sex (F1,58 = 11.44, p = 0.001), behavior-type-by-genotype-by-sex (F1,58 = 4.66, p = 0.035), and a behavior-type-by-rearing-by-genotype interaction (F1,58 = 3.82, p = 0.049), We also found significant between-subjects effects of genotype (F1,58 = 4.14, p = 0.046), sex (F1,58 = 4.13, p = 0.047), and a genotype-by-sex interaction (F1,58 = 5.20, p = 0.026).
We then used a second mixed GLM to analyze huddling specifically, with sex (male, female), rearing (BP-reared, DO-reared), genotype (C/T, T/T), and stimulus (repeated-measure: partner, stranger) on duration of huddling as the outcome measure. We found a significant between-subjects effect of rearing (F1,58 = 8.00, p = 0.006; Figure 2), a within-subjects effect of stimulus (F1,58 = 58.61, p = 2.316E-10), and significant stimulus-by-rearing (F1,58 = 4.84, p = 0.032) and stimulus-by-sex (F1,58 = 5.15, p = 0.027) interactions.
Figure 2.
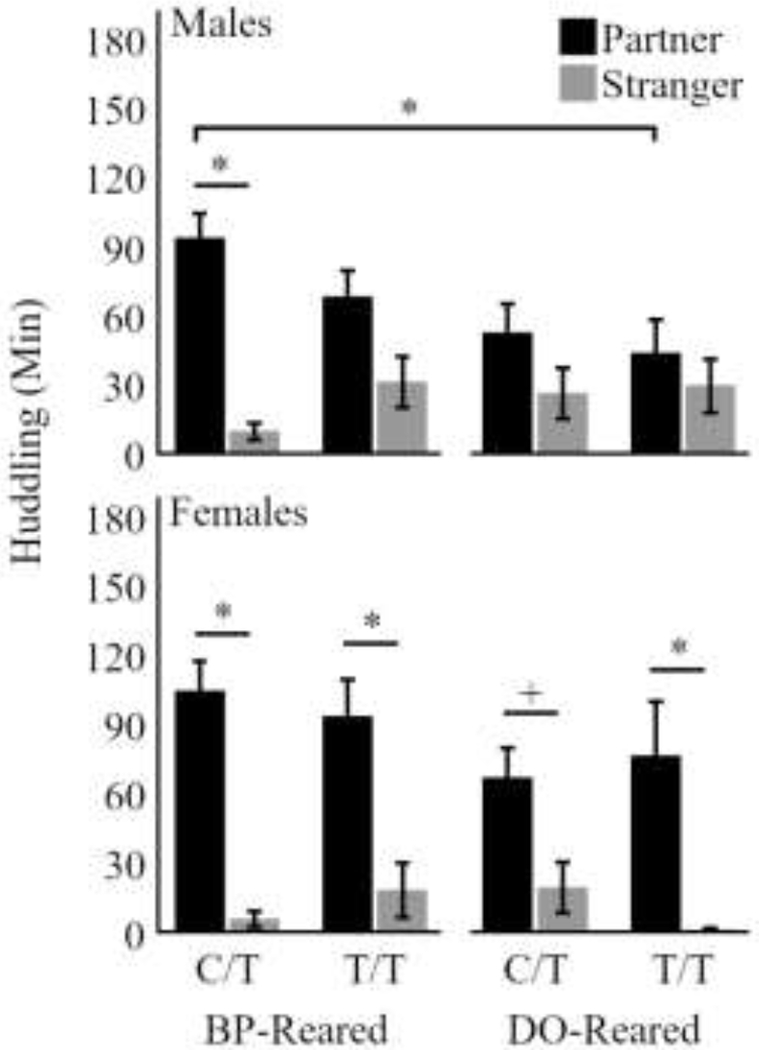
Duration of huddling with the partner (black) and stranger (gray) for eight subgroups. Males are represented in the top row and show that BP-reared C/T males exhibit a robust partner preference (p < 0.05), whereas BP-reared T/T males and DO-reared males of both genotypes do not. Further, DO-Reared T/T males huddled significantly less with the partner than BP-Reared C/T males (p < 0.05). Females are represented in the bottom row and they exhibited a significant (or trending) partner preference in all subgroups. Bars represent means ± SEM. * p < 0.05, + p < 0.1.
We followed these significant main effects and interactions with post hoc t-tests of partner-vs-stranger huddling within each subgroup. BP-reared C/T males exhibited a significant partner preference (p = 0.02; Figure 2 Males), whereas DO-reared C/T, BP-reared T/T, and DO-reared T/T males did not (p = 0.11, p = 0.31, and p = 0.58, respectively). Female BP-reared C/T, T/T, and DO-reared T/T animals demonstrated significant partner preferences (p = 0.01, p = 0.04, and p = 0.03; Figure 2 Females), whereas DO-reared C/T females only demonstrated a trend (p = 0.08).
Lastly, based on our expectations from Ahern et al. (2009) and King et al. (2016), we assessed the combined contribution of rearing and genotype to test the hypothesis that animals would huddle the least. Post hoc tests revealed a significant difference in the duration of partner huddling (p = 0.02), but not a significant difference in stranger huddling (p = 0.14), when comparing BP-reared C/T and DO-reared T/T males. The same comparison did not reveal significant differences in females (p > 0.24 for both).
3.2. Genotype and a Genotype-by-Rearing Interaction Affected Striatal OXTR Density
We next tested the hypothesis that genotype and a genotype-by-rearing interaction would influence relative optical density of OXTR in the striatum (NAcc and CP), but not the insula (Ins). For each ROI we conducted a separate univariate GLM, with rearing (BP-reared, DO-reared), genotype (C/T, T/T), and sex (male, female) as factors.
For the NAcc, the GLM revealed a significant effect of genotype (F1,56 = 90.14, p = 2.90E-13), but no effect of rearing (F1,56 = 0.25, p = 0.62) nor sex (F1,56 = 0.10, p = 0.76; Figure 3). The genotype-by-rearing interaction was not significant (F1,56 = 2.02, p = 0.16), nor were any other interactions (p > 0.275 for all). In the CP, we found a significant association with genotype (F1,57 = 102.63, p = 2.33E-14) and a genotype-by-rearing interaction (F1,57 = 4.84, p = 0.032; Figure 3). The same analysis for the Ins found a significant gene-by-rearing interaction (F1,57 = 6.33, p = 0.02; Figure 3), but no effects of sex, rearing, nor genotype (p > 0.13 for all). Figure 4 illustrates the differences in OXTR relative optical density observed between C/T and T/T animals in the forebrain.
Figure 3.
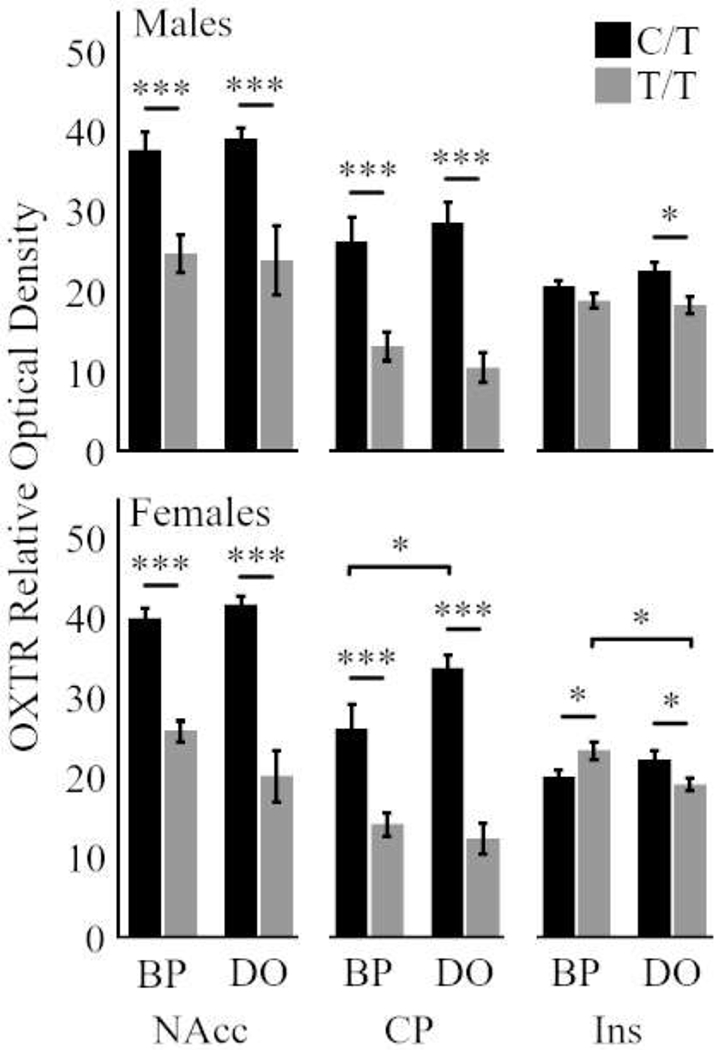
Relative optical density of oxytocin receptor (OXTR) in three forebrain regions of interest: nucleus accumbens (NAcc), caudoputamen (CP), and insula (Ins). Males are represented in the top row, Females in the bottom row. Striatal NAcc and CP OXTR density is significantly higher in C/T animals than in T/T, regardless of rearing (Males and Females). In Females, the CP and Ins exhibited a genotype-by-rearing interaction; NAcc exhibited a trend (p = 0.056). Bars represent means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 4.
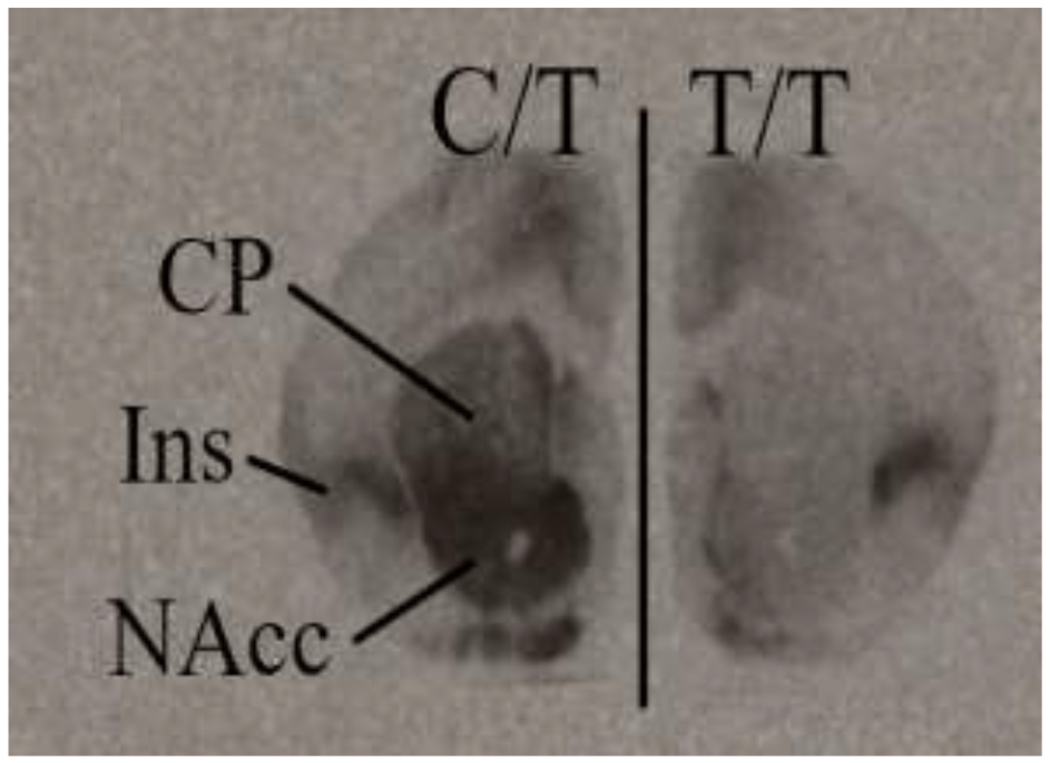
Oxytocin receptor (OXTR) autoradiographic images of prairie vole forebrains carrying the C/T (left) vs T/T (right) versions of the NT213739 single nucleotide polymorphism (SNP). As shown previously in King et al. (2016) and quantified in Figure 3, C/T carrying prairie voles have significantly more OXTR in the striatum (CP and NAcc) than T/T carrying prairie voles. Variation in other regions (e.g., Ins) is less pronounced.
The role of the OXT system in prairie vole social behavior is usually more emphasized in females (for review, see Young and Wang, 2004; but also see, Johnson et al., 2017). We therefore split by sex and ran three follow-up GLMs, with an a priori focus on females. Again, genotype exerted a significant effect in the NAcc (F1,28 = 94.193, p = 1.86E-10) and CP (F1,8 = 58.33, p = 2.55E-8), but not the Ins (F1,28 = 0.006, p = 0.94). All three ROIs – including the Ins – revealed a significant or trending genotype-by-rearing interaction in each ROI (NAcc: F1,28 = 3.972, p = 0.056 ; CP: F1,28 = 4.609, p = 0.041; Ins: F1,28 = 8.774, p = 0.006; Figure 3 Females).
King et al. (2016), however, focused on males and, in this study (see above), males appeared sensitive to genotype and rearing factors in the PPT, so we examined males as well. Males also demonstrated the genotype effects in the NAcc (F1,28 = 25.18, p = 0.00003), CP ( F1,28 = 58.33, p = 2.56E-8), and in the Ins (F1,29 = 5.09, p = 0.03), but there were no genotype-by-rearing interactions (p > 0.329 for all; Figure 3 Males).
For both sexes and rearing conditions, post hoc tests revealed a significant influence of genotype in all C/T vs T/T comparisons in the NAcc and CP (p < 0.05 for all). In the Ins, there was not an overall significant difference in OXTR between the two genotypes. In females, post hoc t-tests revealed significant differences in OXTR between genotypes in both rearing conditions, but in the opposite directions (BP: C/T < T/T, p = 0.03; DO: C/T > T/T, p = 0.04; Figure 3 Females). This last finding corresponds to a gene-by-rearing interaction.
3.3. Genotype and Rearing Affect Nonreproductive Social Behavior
Third, we tested the hypothesis that nonreproductive social behavior would also be influenced by rearing and genotype. One advantage of the same-sex reciprocal interaction tests is that they can be repeated, so a behavioral average can be calculated for each animal for each test type. We employed the SS-CM 1-2 and SS-S 1-2 averages as the outcome measure in this mixed-model GLM, again looking at the between-subjects effects of rearing (BP-reared, DO-reared), genotype (C/T, T/T), and sex (male, female), as well as the within-subjects factor of test-type (SS-CM, SS-S) on the duration of huddling behavior. This analysis revealed a significant effect of test-type (F1,56 = 19.30, p = 0.0005; Figures 5) and a test-type-by-genotype interaction (F1,56 = 4.18, p = 0.046), but no test-type-by-rearing interaction (F1,56 = 0.2, p = 0.66). Between-subjects, there was a significant effect of rearing (F1,56 = 5.80, p = 0.02) and a rearing-by-genotype interaction (F1,56 = 5.71, p = 0.02). Sex did not exert a significant effect (p = 0.76 for sex alone and p > 0.32 for all interactions), so males and females are combined in Figure 5.
Figure 5.
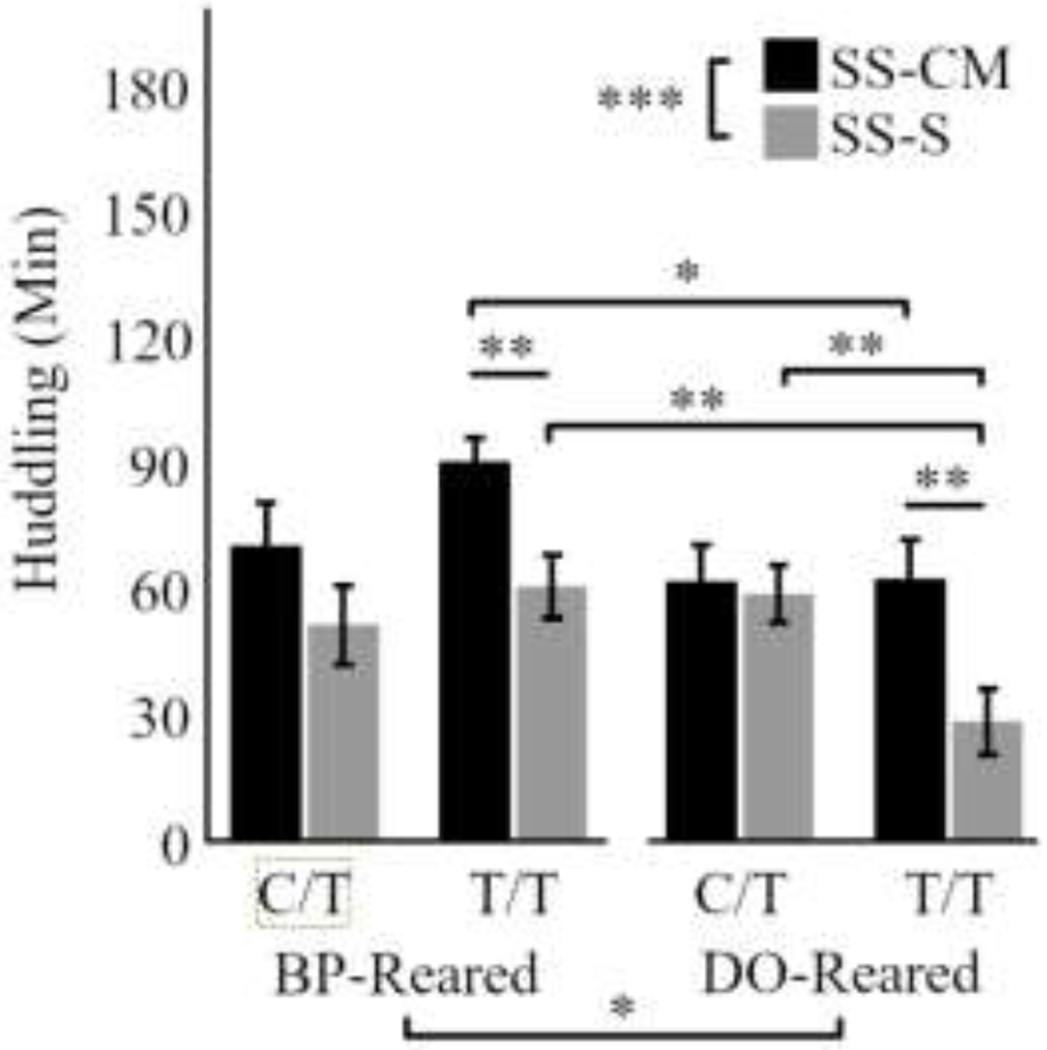
For both sexes (combined above), more huddling occurred between cagemates (SS-CM) than strangers (SS-S), and BP-reared animals huddled more than DO-reared animals. There was also a significant genotype-by-rearing interaction, such that BP-reared T/T animals huddled more with cagemates (SS-CM) and DO-reared T/T animals huddled significantly less with strangers (SS-S) than in other subgroups. Bars represent means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001.
For C/T carrying animals, post hoc t-tests revealed no difference between the BP-reared and DO-reared condition in huddling test types (t < 1.9, p > 0.05 for both), whereas for T/T carrying animals, there was significantly more huddling within the cagemate (SS-CM) versus the stranger (SS-S) tests for both rearing conditions (p < 0.006 for both; Figure 5). This difference, however, manifested differently in each rearing condition: e.g., DO-reared T/T animals huddled significantly less in the SS-S test (p = 0.006); whereas the BP-reared T/T animals huddled more in the SS-CM test (p = 0.02).
Lastly, we examined whether huddling behavior exhibited in nonreproductive contexts (SS-CM and SS-S) predict huddling in reproductive contexts (OS-S and PPT). Neither SS-CM nor SS-S huddling correlated significantly with OS-S nor PPT huddling behavior (see Supplemental Materials, Figure S2).
4. Discussion
We show that natural variation in Oxtr and naturalistic manipulation of early rearing interact, and work independently, to influence adult prairie vole social behavior in multiple social contexts in both sexes. Corroborating previous work, offspring reared only by the dam engaged in less PPT huddling and exhibited less robust partner preferences after ≥24 hrs cohabitation compared to BP-reared peers (Figure 2) (Ahern and Young, 2009; Wang and Novak, 1992). We also showed that the C/T genotype of the NT213739 SNP is associated with significantly more OXTR expression in the striatum (CP and NAcc) than the T/T genotype, replicating work by King et al. (2016). Most importantly, however, is our demonstration that the naturalistic manipulation of rearing interacted with Oxtr SNP variants to shape adult social behavior in both reproductive (partner preference; Figure 2) and nonreproductive contexts (same-sex reciprocal interaction tests; Figure 5), as well as alter OXTR density in the forebrain (CP, Ins, and possibly the NAcc; Figure 3).
These findings align with other prairie vole studies showing how natural variation in early parental care, such as amount of parental contact, can influence alloparenting and adult partner preference formation (Arias Del Razo and Bales, 2016). Further, certain types of early experience, such as handling, is known to alter OXTR binding in the NAcc via methylation of Oxtr (Perkeybile et al., 2019), although other manipulations of early rearing have not noted differences in NAcc OXTR binding (Ahern et al., 2009; Barrett et al., 2015). In this study, the interaction between rearing and Oxtr did not significantly alter OXTR density in the NAcc overall, but in females, there was a gene-by-rearing interaction in both the CP and Ins and a trend in the NAcc (Figure 3). For the striatum, DO-rearing of females seemed to enhance the difference in OXTR binding between genotypes, driving binding up in C/T and down in T/T females. This was not seen in males.
Another potentially interesting sex difference is that Oxtr SNP variants and rearing might exert a greater influence on social behavior in males than in females (Figure 2). This, however, should be interpreted cautiously. Males had cohabited for 24 hrs prior to the PPT, while females had cohabitated for 48 hrs. In Ahern et al. (2009), PPT behavior and the effect of rearing appeared similar after 24 and 48 hrs cohabitation in both sexes, but it is unclear how robust that consistency is. In this study, the difference in cohabitation between sexes in this study may explain why males and females showed different patterns of partner preference formation across subgroups. We also did not see a significant sex difference in the nonreproductive social tests.
Overall, however, our findings have important implications for understanding how genetic and social-environmental variation can contribute to individual differences in both sexes, and they provide a model for experimentally examining how rearing shapes behavior and OXTR receptor binding in an allele-specific and, in some cases, a sex-specific way.
As an animal model of social behavior, prairie voles exhibit robust individual differences that are observed in other outbred species, including humans (Barrett et al., 2015; Bosch and Young, 2018). The data presented here provide support for two complementary theories of individual variation. First, individual differences are not necessarily “noise” surrounding an optimal behavioral profile (Daly and Wilson, 1999; Sloan Wilson et al., 1994). Instead, individual differences arise from distinct gene-by-environment interactions that promote behavioral diversity in the population. This behavioral diversity then in turn can promote future genetic diversity (Okhovat et al., 2015; Young and Wang, 2004).
For example, variation in the arginine vasopressin (AVP) receptor gene, avprla, and the resulting expression of AVP receptor la in the brain are promoted through trade-offs between mate fidelity and infidelity in prairie vole populations (Okhovat et al., 2015). Given the deep biological similarities between the AVP and OXT systems, it seems likely that Oxtr is under similar selection pressure (Barrett et al., 2015; King et al., 2016; Ophir et al., 2012) and that variation in rearing moderates this process by enhancing or inhibiting genetically driven differences in behavior. Related to the current study, BP- and DO-rearing each occur about one-third of the time in the wild (Getz and Carter, 1996). Conditions that promote the DO-rearing, such as increased predation, might push the behavior of adult offspring toward slower bond formation (Ahern and Young, 2009) and lower engagement with same-sex conspecifics, particularly in T/T males. Increasing the number of individuals that exhibit this behavioral profile may enhance reproductive success of the population in that local environment. Indeed, Ophir and colleagues have shown that females can distinguish males that exhibit distinct patterns of initial aggression, alloparenting, and commitment, which leads to mate preferences (Ophir et al., 2008).
More broadly, understanding how genetic predisposition is shaped by environmental input is paramount to understanding how individual differences in human personality and psychopathology arise (Cataldo et al., 2018; Meaney, 2001; Sherman et al., 2013; Williams and Thompson, 1993). Studying the OXT system in a highly social model species that allows experimental manipulation of the environment fits this focus.
Many studies demonstrate that the OXT system is important for regulating social behavior, and associations have been shown between human OXTR SNPs and social recognition (Kalyoncu et al., 2017), empathy (Gong et al., 2017), bonding and attachment (Notzon et al., 2016), and parental sensitivity (Bakermans-Kranenburg and van Ijzendoorn, 2008; Mehta et al., 2016). There is also a growing literature demonstrating the ways OXTR SNPs and childhood experiences interact in depression, anxiety, post-traumatic stress disorder, and attachment disorders (Hostinar et al., 2014; Loth et al., 2014; Schneider-Hassloff et al., 2016; Thompson et al., 2011; Verona et al., 2018). But these studies are necessarily correlational. Prairie voles and the tests presented here provide an experimental approach for studying how natural variations in genes and rearing interact to produce variation. They also may provide a model to test pharmacotherapies.
Indeed, one of the key findings of the present study is that Oxtr and rearing interact to alter social behavior not only within choice-based reproductive contexts, but also in non-choice, nonreproductive contexts. Previous prairie vole studies have shown selective social preferences for familiar same-sex peers (Beery et al., 2018; DeVries et al., 1997; Lee et al., 2019), but it was not clear if familiar and unfamiliar same-sex pairs would behave in quantifiably different ways in non-choice tests. Figure 5 demonstrates how familiar cagemates generally huddle more than strangers and how rearing, genotype, and test type can interact to produce different behavioral profiles. In the future, we could use these differences to test if a pharmacotherapy that is known increase OXT signaling, e.g., a central melanocortin receptor 4 agonist, will increase stranger-directed huddling behavior when administered prior to testing or administered in early life, as have been done for tests of reproductively focused partner preference formation (Barrett et al., 2015; Modi et al., 2015). Further, accounting for the genetic and rearing elements simultaneously will provide an opportunity to test interventions that are subgroup dependent (i.e., more individualized). For example, DO-reared T/T animals show markedly lower same-sex stranger huddling (Figure 5) and thus may be sensitive to a treatment that does not affect BP-reared C/T animals. Further, the finding here that there was no correlation between reproductive (PPT and OS-S) vs nonreproductive (SS-CM and SS-S) social behavior (see Supplemental Materials, Figure S2) suggests partner preference behavior may not predict non-reproductive social behavior, which potentially aligns better with models of psychopathologies. Thus, testing in the nonreproductive contexts described here may provide an additional pre-clinical screening of pharmacotherapies that target sociality.
Another result to investigate further is the unexpected finding that OXTR in the Ins was sensitive to a genotype-by-rearing interaction. In King et al. (2016), the Ins was found to express OXTR at approximately equal densities across NT213739 genotypes. We originally included the Ins as a type of negative control. Instead, we found significant differences driven by genotype and rearing: in males, the Ins OXTR density in C/T subjects was higher than in T/T subjects; in females, there was a genotype-by-rearing interaction, such that C/T animals remained stable across rearing conditions, but T/T animals were sensitive to rearing (Figure 4). Differences in Ins activity have been identified in a few human OXTR studies of brain activity (Wang et al., 2017; Zimmermann et al., 2018) and it might relate to sociality through its role in interoceptive, emotional, and cognitive reward processing (Craig, 2002; Ophir et al., 2012; Poeppl et al., 2019). Future research will need to explore this Ins gene-by-environment interaction and its relevance to reproductive (Ophir et al., 2012) and nonreproductive social behavior in prairie voles. Based on work by Ophir et al. (2012), we would predict that the rearing-by-genotype interaction for the Ins OXTR observed here might shift mating decisions and mating success under naturalistic conditions.
Lastly, this gene-by-environment model may help identify how Oxtr is regulated. King et al. (2016) focused on NT213739 because it was the only one of 15 SNPs in perfect linkage disequilibrium to be associated with OXTR density in the striatum and also show consistent transcriptional activity. To our knowledge, it is not yet known which transcription factor (possibly CTCF) is responsible for the striking differences in OXTR expression between C/T and T/T carrying prairie voles. Here our experimental results suggest that the regulatory mechanism can be influenced by rearing, at least in females, but the mechanisms driving the large difference in OXTR binding between genotypes are not highly sensitive to early rearing.
Social behavior is profoundly important for the survival and health of individuals and whole species. But it has become increasingly clear that there is not just one sociobehavioral profile that represents optimality. This study provides a novel approach to studying gene-by-environment interactions in a rodent model that mimics the wide variation observed in other outbred species including human populations. It also showed that natural variations in Oxtr gene variants can interact with differences in rearing to shift OXTR expression in the forebrain and shape sociobehavioral profiles across reproductive and nonreproductive contexts in both sexes.
Supplementary Material
Highlights.
Naturalistic differences in rearing interacted with oxytocin receptor gene variants to alter social behavior
Social behavior was altered in reproductive (male-female) and nonreproductive (same-sex) contexts in both sexes.
Oxytocin receptor density in the striatum was strongly associated with Oxtr SNP variant
Oxytocin receptor densities in the striatum (caudoputamen, nucleus accumbens) and insula were sensitive to this rearing-by-gene interaction in females.
Contribution to the Field Statement.
Individual variation in social behavior is relevant to evolutionary biology and translational neuroscience. Identifying the factors that contribute to behavioral diversity will help predict adaptation to changing environments and offer insight into atypical behavior (e.g., disorders). Oxytocin receptor signaling and differences in early social environment appear to be important factors in shaping stable individual differences in social behavior. In this study, we used outbred prairie voles to experimentally examine how natural variants of the oxytocin receptor gene interact with naturalistic manipulations of rearing to influence adult social behavior. We replicated the findings of two previous studies, one demonstrating that differences in early rearing structure can alter adult partner preference formation, and the second showing the contribution of genetic differences to oxytocin receptor expression, particularly in the nucleus accumbens. We then demonstrated for the first time that naturalistic differences in rearing interact with oxytocin receptor gene variants to alter social behavior in reproductive (male-female) and nonreproductive (same-sex) contexts in both sexes. Oxytocin receptor densities in the insula were also sensitive to this rearing-by-gene interaction. These data provide experimental evidence that variation in early rearing can interact with known genetic variants to produce significant differences in adult social behavior.
Acknowledgements
We thank Holli Zampano, Lena Grahm, and Emily Lucibella for their excellent animal care, and Melissa Boucher for her work in helping explore different ways to conduct and quantify reciprocal interaction tests. I also thank the referees.
Funding
This work was supported by Quinnipiac University’s College of Arts & Sciences (TA) and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (TA). AB was supported by a grant from the National Institute of Mental Health (Award R15MH113085)
Abbreviations
- AVP
arginine vasopressin
- avpr1a
arginine vasopressin 1a receptor gene
- CP
caudoputamen
- GLM
general linear model
- Ins
Insula
- NAcc
nucleus accumbens
- OS-S
same-sex stranger
- OXT
oxytocin
- OXTR
oxytocin receptor
- OXTR
human oxytocin receptor gene
- Oxtr
non-human oxytocin receptor gene
- PPT
partner preference test
- ROI
region of interest
- SNP
single nucleotide polymorphism
- SS-CM
same-sex cagemate
- SS-S
same-sex stranger.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: None
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahern TH, Hammock EAD, Young LJ, 2011. Parental Division of Labor, Coordination, and the Effects of Family Structure on Parenting in Monogamous Prairie Voles (Microtus ochrogaster). Dev Psychobiol 53, 118–131. 10.1002/dev.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Modi ME, Burkett JP, Young LJ, 2009. Evaluation of two automated metrics for analyzing partner preference tests. J. Neurosci. Methods. 10.1016/j.jneumeth.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Ophir A, Bum D, 2019. Evaluating the stability of individual variation in social and nonsocial behavioural types using prairie voles (Microtus ochrogaster). Behav. Processes 169, 103961. 10.1016/j.beproc.2019.103961 [DOI] [PubMed] [Google Scholar]
- Ahern TH, Young LJ, 2009. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster). Front Behav Neurosci 3, 17. 10.3389/neuro.08.017.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias Del Razo R, Bales KL, 2016. Exploration in a dispersal task: Effects of early experience and correlation with other behaviors in prairie voles (Microtus ochrogaster). Behav Processes 132, 66–75. 10.1016/j.beproc.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoom MH, 2008. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci 3, 128–134. 10.1093/scan/nsn004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS, 2007. Early experience affects the traits of monogamy in a sexually dimorphic manner. Developmental Psychobiology 49, 335–42. 10.1002/dev.20216 [DOI] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ, 2015. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry 5, e606. 10.1038/tp.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, Blandino KL, 2018. Specificity in Sociality: Mice and Prairie Voles Exhibit Different Patterns of Peer Affiliation. Front Behav Neurosci 12. 10.3389/fnbeh.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker L, 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169, 665–673. 10.1016/j.neuroscience.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Bell AM, 2007. Future directions in behavioural syndromes research. Proc Biol Sci 274, 755–761. 10.1098/rspb.2006.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Young LJ, 2018. Oxytocin and Social Relationships: From Attachment to Bond Disruption. Curr Top Behav Neurosci 35, 97–117. 10.1007/7854_2017_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL, 1995. Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience and Biobehavioral Reviews 19, 303–14. https://doi.org/7630584 [DOI] [PubMed] [Google Scholar]
- Cataldo L, Azhari A, Lepri B, Esposito G, 2018. Oxytocin receptors (OXTR) and early parental care: An interaction that modulates psychiatric disorders. Res Dev Disabil 82, 27–38. 10.1016/j.ridd.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Craig AD, 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3, 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Daly M, Wilson MI, 1999. Human evolutionary psychology and animal behaviour. Anim Behav 57, 509–519. 10.1006/anbe.1998.1027 [DOI] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS, 1997. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can. J. Zool 75, 295–301. 10.1139/z97-037 [DOI] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ, 2000. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol 12, 1145–8. [DOI] [PubMed] [Google Scholar]
- Getz L, McGuire B, 1997. Communal nesting in prairie voles (Microtus ochrogaster): Formation, composition, and persistence of communal groups. Canadian Journal of Zoology-Revue Canadienne de Zoologie 75, 525–534. [Google Scholar]
- Getz LL, Carter CS, 1996. Prairie-vole partnerships. American Scientist 84, 56–62. [Google Scholar]
- Getz LL, Carter CS, Gavish L, 1981. The Mating System of the Prairie Vole, Microtus ochrogaster: Field and Laboratory Evidence for Pair-Bonding. Behavioral Ecology and Sociobiology 8, 189–194. [Google Scholar]
- Gong P, Fan H, Liu J, Yang X, Zhang K, Zhou X, 2017. Revisiting the impact of OXTR rs53576 on empathy: A population-based study and a meta-analysis. Psychoneuroendocrinology 80, 131–136. 10.1016/j.psyneuen.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Hammock EAD, Young LJ, 2005. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 308, 1630–4. https://doi.org/308/5728/1630 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Cicchetti D, Rogosch FA, 2014. Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Dev. Psychopathol 26, 465–477. 10.1017/S0954579414000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Buisman-Pijlman FTA, 2016. Adversity impacting on oxytocin and behaviour: timing matters. Behav Pharmacol 27, 659–671. 10.1097/FBP.0000000000000269 [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Ricfkohl PC, Young LJ, 2017. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm Behav 87, 16–24. 10.1016/j.yhbeh.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyoncu T, Özbaran B, Köse S, Onay H, 2017. Variation in the Oxytocin Receptor Gene Is Associated With Social Cognition and ADHD. J Atten Disord 1087054717706757. 10.1177/1087054717706757 [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Guastella AJ, Becker B, 2018. Overview of Human Oxytocin Research. Curr Top Behav Neurosci 35, 321–348. 10.1007/7854_2017_19 [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ, 2016. Variation in the Oxytocin Receptor Gene Predicts Brain Region Specific Expression and Social Attachment. Biol Psychiatry 80, 160–169. 10.1016/j.biopsych.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, Beery AK, 2019. Affiliation, Aggression, and Selectivity of Peer Relationships in Meadow and Prairie Voles. Front Behav Neurosci 13. 10.3389/fnbeh.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen Lin, Chen Li, Chen T-M, Chi Chin M, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong H-W, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Samo NR, Schaffnit K, Shapovalova NV, Sivisay T, Slsaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf K-R, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Vamam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Feng Yuan X, Zhang B, Zwingman TA, Jones AR, 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Loth E, Poline J-B, Thyreau B, Jia T, Tao C, Lourdusamy A, Stacey D, Cattrell A, Desrivieres S, Ruggeri B, Fritsch V, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, Carvalho FM, Conrod PJ, Fauth-Buehler M, Flor EL, Gallinat J, Garavan EL, Heinz A, Bruehl R, Lawrence C, Mann K, Martinot J-L, Nees F, Paus T, Pausova Z, Poustka L, Rietschel M, Smolka M, Struve M, Feng J, Schumann G, IMAGEN Consortium, 2014. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol. Psychiatry 76, 367–376. 10.1016/j.biopsych.2013.07.043 [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G, 2012. Oxytocin and Vasopressin Agonists and Antagonists as Research Tools and Potential Therapeutics. Journal of Neuroendocrinology 24, 609–628. 10.1111/j.1365-2826.2012.02303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Young LJ, 2009. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 10.1016/j.tins.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci 24, 1161–92. https://doi.org/11520931 [DOI] [PubMed] [Google Scholar]
- Mehta D, Eapen V, Kohlhoff J, Mendoza Diaz A, Barnett B, Silove D, Dadds MR, 2016. Genetic Regulation of Maternal Oxytocin Response and Its Influences on Maternal Behavior. Neural Plast. 2016, 5740365. 10.1155/2016/5740365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, Young LJ, 2015. Melanocortin Receptor Agonists Facilitate Oxytocin-Dependent Partner Preference Formation in the Prairie Vole. Neuropsychopharmacology 40, 1856–1865. 10.1038/npp.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notzon S, Domschke K, Holitschke K, Ziegler C, Arolt V, Pauli P, Reif A, Deckert J, Zwanzger P, 2016. Attachment style and oxytocin receptor gene variation interact in influencing social anxiety. World J. Biol. Psychiatry 17, 76–83. 10.3109/15622975.2015.1091502 [DOI] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM, 2015. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science 350, 1371–1374. 10.1126/science.aac5791 [DOI] [PubMed] [Google Scholar]
- Ondrasek NR, Freeman SM, Bales KL, Calisi RM, 2018. Nonapeptide Receptor Distributions in Promising Avian Models for the Neuroecology of Flocking. Front Neurosci 12, 713. 10.3389/fnins.2018.00713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Crino OL, Wilkerson QC, Wolff JO, Phelps SM, 2008. Female-directed aggression predicts paternal behavior, but female prairie voles prefer affiliative males to paternal males. Brain Behav. Evol 71, 32–40. https://doi.org/000108609 [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng D-J, Phelps SM, 2012. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav 61, 445–453. 10.1016/j.yhbeh.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo S, Mariotti V, Iofrida C, Pellegrini S, 2018. Genes and Aggressive Behavior: Epigenetic Mechanisms Underlying Individual Susceptibility to Aversive Environments. Front Behav Neurosci 12, 117. 10.3389/fnbeh.2018.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Kinney LF, Lee TM, 2001. Day length and sociosexual cohabitation alter central oxytocin receptor binding in female meadow voles (Microtus pennsylvanicus). Behav Neurosci 115, 1349–1356. [PubMed] [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, Karaoli T, Gregory SG, Mohammadi N, Epstein L, Bales KL, Connelly JJ, 2019. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99, 128–136. 10.1016/j.psyneuen.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Donges MR, Mokros A, Rupprecht R, Fox PT, Laird AR, Bzdok D, Langguth B, Eickhoff SB, 2019. A view behind the mask of sanity: meta-analysis of aberrant brain activity in psychopaths. Mol. Psychiatry 24, 463–470. 10.1038/s41380-018-0122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ, 2014. The biology of mammalian parenting and its effect on offspring social development. Science 345, 771–776. 10.1126/science.1252723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Hassloff EL, Straube B, Jansen A, Nuscheler B, Wemken G, Witt SH, Rietschel M, Kircher T, 2016. Oxytocin receptor polymorphism and childhood social experiences shape adult personality, brain structure and neural correlates of mentalizing. Neuroimage 134, 671–684. 10.1016/j.neuroimage.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Sherman RA, Figueredo AJ, Funder DC, 2013. The behavioral correlates of overall and distinctive life history strategy. J Pers Soc Psychol 105, 873–888. 10.1037/a0033772 [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC, 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. (Amst.) 19, 372–378. 10.1016/j.tree.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimäki T, Binder EB, Young LJ, 2014. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci U S A 111, 1987–1992. 10.1073/pnas.1302985111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan Wilson D, Clark AB, Coleman K, Dearstyne T, 1994. Shyness and boldness in humans and other animals. Trends Ecol. Evol. (Amst.) 9, 442–446. 10.1016/0169-5347(94)90134-1 [DOI] [PubMed] [Google Scholar]
- Smearman EL, Winiarski DA, Brennan PA, Najman J, Johnson KC, 2015. Social stress and the oxytocin receptor gene interact to predict antisocial behavior in an at-risk cohort. Dev. Psychopathol 27, 309–318. 10.1017/S0954579414000649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Alward BA, Ebling FJP, Femald RD, Kelly A, Ophir AG, 2018. The Value of Comparative Animal Research: Krogh’s Principle Facilitates Scientific Discoveries. Policy Insights from the Behavioral and Brain Sciences 5, 118–125. 10.1177/2372732217745097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH, 2011. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology 36, 144–147. 10.1016/j.psyneuen.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhodeyev ON, Shcherbakova OV, 2019. The Problem ofNon-Shared Environment in Behavioral Genetics. Behav. Genet, 10.1007/sl0519-019-09950-l [DOI] [PubMed] [Google Scholar]
- Verona E, Murphy B, Bresin K, 2018. Oxytocin-related single-nucleotide polymorphisms, family environment, and psychopathic traits. Personal Disord 9, 584–589. 10.1037/per0000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Pedersen NL, Anckarsäter H, Larsson H, Westberg L, 2012. Variation in the oxytocin receptor gene (OXTR) is associated with pair-bonding and social behavior. Biol Psychiatry 71, 419–426. 10.1016/j.biopsych.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Braskie MN, Hafzalla GW, Faskowitz J, McMahon KL, de Zubicaray GI, Wright MJ, Yu C, Thompson PM, 2017. Relationship of a common OXTR gene variant to brain structure and default mode network function in healthy humans. Neuroimage 147, 500–506. 10.1016/j.neuroimage.2016.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Novak M, 1992. Influence of the social-environment on parental behavior and pup development of meadow voles (Microtus pennsylvanicus) and prairie voles (Microtus-ochrogaster). Journal of Comparative Psychology 106, 163–171. [Google Scholar]
- Williams DE, Thompson JK, 1993. Biology and behavior. A set-point hypothesis of psychological functioning. Behav Modif 17, 43–57. 10.1177/01454455930171004 [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T, 1992. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci 652, 487–9. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, 2004. The neurobiology of pair bonding. Nat. Neurosci 7, 1048–54. https://doi.org/15452576 [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Deris N, Montag C, Reuter M, Felten A, Becker B, Weber B, Markett S, 2018. A common polymorphism on the oxytocin receptor gene (rs2268498) and resting-state functional connectivity of amygdala subregions - A genetic imaging study. Neuroimage 179, 1–10. 10.1016/j.neuroimage.2018.06.014 [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID, 2014. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology 39, 3027–3035. 10.1038/npp.2014.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


