Abstract
Chromium (Cr) causes toxic effects in plants by generating reactive oxygen species (ROS) which create oxidative environment. Azotobacter vinelandii helps in growth and development of many crops; however, its role in Cr stress tolerance in rice has not been explored. Here, we report the new function of Azotobacter vinelandii strain SRI Az3 (Accession number JQ796077) in providing Cr stress tolerance in Oryza sativa (var. IR64). The efficiency of the strain was checked under different concentrations (50, 100, 150, 200 and 250 µM) of Cr stress and it was observed that it provides stress tolerance to rice plant up to 200 µM concentration. Different agronomic growth parameters were found to be better in this strain of Azotobacter vinelandii-inoculated rice plants as compared to un-inoculated one. The agronomic growth and photosynthetic characteristics such as net photosynthetic rate (PN), stomatal conductance (gs), intercellular CO2 (Ci) were also found to be significantly increased with increasing concentration of Azotobacter vinelandii inoculation. The activities of antioxidant enzymes were significantly higher (35%) in rice plants inoculated with Azotobacter vinelandii as compared with un-inoculated rice plant. All these positive effects of Azotobacter vinelandii help rice to survive from the toxic effect of Cr.
Keywords: Antioxidant enzymes, Azotobacter vinelandii, Chromium stress tolerance, Rice, Heavy metal stress
Introduction
Crop plants are constantly exposed to various environmental stresses including toxic heavy metals which negatively affect the growth and development resulting in significant reduction of their productivity (Sahoo et al. 2014; Foucault et al. 2013; Saifullah et al. 2015; Sabir et al. 2015; Rai et al. 2019). Among heavy metals, Cr is one of the most toxic heavy metal affecting soil, water and plants, it has not any essential metabolic function in plants (Jun et al. 2009; Shahid et al. 2017; Zaheer et al. 2020). Toxic effects of Cr on crops are due to its induction of ROS which caused oxidative stress (Sharma et al. 2020). Recently, it has been reported that Cr-induced ROS are responsible for causing cytotoxic, genotoxic and photosynthetic changes in plants (Wakeel et al. 2020). The negative impact of Cr is mainly dependent on its valence state. Hexavalent chromium [Cr(VI)] is conceived as the most toxic form, highly soluble in water, which usually occurs amalgamated with oxygen as chromate (CrO42−) or dichromate (Cr2O72−) oxyanion. Cr(III) is less toxic, less mobile, and is mainly found coalesced to organic matter in soil and aquatic environment (Becquer et al. 2003; Bakshi and Panigrahi 2018). Some microorganisms show resistance to this heavy metal, despite the toxicity of Cr(VI), showing the ability to reduce Cr(VI) to Cr(III), as was first documented for Pseudomonas spp., and a characterization of bacteria capable of reducing Cr(VI) was successively reported in 1979 (Romanenko and Korenkov 1977). Numerous bacteria have then been reported to reduce Cr(VI) to Cr(III) as a mechanism of resistance to Cr(VI) (Camargo et al. 2003; He et al. 2011). Since Cr(III) and Cr(VI) may interconvert in the soil, therefore, it is difficult to valuate separately the consequence of the two types of Cr on plants. Consequently, it might be appropriate to use the term Cr toxicity in plants rather than toxicity of Cr(III) or Cr(VI) (Arun et al. 2005; Chowdhury et al. 2018). Since plants lack a specific transport system for Cr, it is transported by carriers of essential ions such as sulfate or iron. Toxic effects of Cr on plant growth and development include alterations in the germination process as well as in the growth of roots, stems and leaves, which may affect total dry matter production and yield. Chromium also causes deleterious effects on physiological processes of plants such as photosynthesis, water relations and mineral nutrition (Shanker et al. 2005; Sharma et al. 2020). Dixit et al. (2002) studied the effect of Cr(VI) on the electron transport system of pea (Pisum sativum L.cv. Azad) and found that at 200 µM concentration, Cr ion inactivates electron transport and enhances superoxide generation. The effect of different concentration (50, 100, 200, 300 and 400 µM) of Cr(VI) on citrullus plants was tested and it has been reported that the Cr(VI) levels greater than 200 µM concentration reduces growth with chlorosis and loss of turgor of middle leaves (Dube et al. 2003). The effects of Cr(VI) on the growth and development of Arabidopsis thaliana, were studied, and concentrations of Cr(VI) greater than 200 µM were reported to be toxic to plants which inhibit the growth of roots and shoots and the development of chlorosis in leaves (Castro et al. 2007). In this concentration, the growth of primary root was totally inhibited but the plants continued their growth by alliterating the development of root (Castro et al. 2007). Cr(VI) at concentration 250 and 500 μM caused interveinal chlorosis in both young and middle leaves of Lolium perenne after 30 and 15 days of exposure, respectively (Vernay et al. 2007). The fall of older leaves and wilting of younger leaves of Datura innoxia was observed after exposure of plants to 200 and 500 μM Cr(VI) (Vernay et al. 2008). The Cr stress severely affects the growth of rice plant (Ahmad et al. 2011). The growth of total leaf area, shoot weight, root weight, dry weight and the yield of the paddy gradually decreased with increasing Cr concentration (Sundaramoorthy et al. 2010; Zhang et al. 2010; Nagarajan and Ganesh 2015).
Phytoremediation is now accepted as an effective technique for plants to clean up hazardous contaminants from contaminated areas (Yu and Gu 2007). The use of hyperaccumulator plants has also been regarded as environmentally friendly to extract Cr(VI) from polluted spheres (Salt et al. 1998). By adding chelate compounds in a process called induced phytoextraction, the phytoextraction capability of a plant other than hyperaccumulator species can be enhanced (Salt et al. 1998). Chelating agents, such as EDTA, DTPA, and CA, are often used to increase the bioavailability and absorption of heavy metals by plants.
Present upsurge in Cr(VI) reduction-potential recovery of novel PGPRs has led to the reduction of Cr toxicity and increase of plant biomass in Cr-stressed soils (Maqbool et al 2015; Soni et al. 2014). Several PGPB-reducing Cr(VI) bacterial genera have been isolated from soils, such as Ochrobactrum (Faisal and Hasnain 2005), Delftia (Morel et al. 2011), Pseudomonas (Rajkumar et al. 2005), Bacillus (Karupiaah and Rajaram 2011), Cellulosimicrobium (Chatterjee et al. 2009), Mesorhizobium (Wani et al. 2008), and Rhodococcus (Trivedi et al. 2007). As toxic Cr derivatives are converted into environmentally less harmful products by processes of Cr(VI) reduction, the bacterial feature of reductive immobilization of Cr has a special significance. As these beneficial bacteria induce changes in plant metabolism (e.g., extensive proliferation in roots for better nutrient absorption, increased bacterial siderophore-mediated iron uptake, and upregulation of genes involved in stress mitigation, etc.), plants inoculated with PGPB exhibiting Cr(VI) reducing property have shown better adaptation while growing in Cr-stressed soils.
Rice is a staple food on which half of the world population is dependent. Due to the toxic effects of Cr in soil, the growth and development of rice plants are affected (Solanki and Dhankhar 2011). Therefore, improvement of rice tolerance against Cr toxicity is essential. Many soil microbes (plant growth-promoting rhizobacteria, PGPRs) play important role in promoting plant growth and development under normal and adverse conditions, and therefore, help in sustaining agricultural productivity (Das et al. 2013). Azotobacter vinelandii participates in diverse metabolic functions owing to its capacity to produce vitamins and hormones and promotes plant growth and development in adverse conditions (Wani et al. 2013). We previously reported that Azotobacter vinelandii, a Gram-negative diazotroph, is a free-living N2 fixer found in soil, and it plays an important role during salinity stress tolerance and improves the productivity of rice crop under salinity stress (Sahoo et al. 2014).
The role of Azotobacter vinelandii on the Cr(VI) stress tolerance in rice has not been explored yet. Most of the earlier reports on toxicity of Cr(VI) mentioned above, state that the concentration of Cr(VI) greater than 200 µM is toxic which inhibits agronomic growth of the plant. Therefore, we decided to evaluate the role of Azotobacter vinelandii in providing tolerance to rice plants against the Cr(VI) stress (200 µM) in pot culture experiments.
Materials and methods
Preparation of seedlings, pots and treatments
The seeds of rice genotype IR64 were obtained from International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India. The rice seeds (Oryza sativa L. var IR64) were placed in hydroponics system for 21 days in the green house of ICGEB, New Delhi, India. The temperature inside green house was 28±2 °C and 16 h light and 8 h dark was maintained for growth of seedlings. Azotobacter vinelandii strain SRIAz3 were used in this study. The Azotobacter vinelandii strain SRI Az3 was isolated by us from System of Rice Intensification (SRI) field of Odisha University of Agriculture and Technology, Bhubaneswar, Odisha, India. The accession number of the strain is cataloged as JQ796077. Different concentrations (10%, 15% and 20%) of LB (Luria–Bertani) culture (109 cfu/mL) were used. The bacterial strain (Azotobacter vinelandii SRIAz3) was allowed to grow in LB broth medium for 48 h at 30 °C. Then, the optical density at 600 nm of bacterial culture was measured. When the O.D reached in between 0.8 and 1.0, then the bacterial culture was used for further study. The 21-day-old healthy rice (Oryza sativa L. var IR64) seedling were dipped separately in bacterial suspensions (10%, 15% and 20% v/v, i.e., 2 × 109 cfu/mL) for 2 h as recommended for commercial formulations by Bureau of Indian Standards (BIS) and transplanted in different pots with three replications each under defined treatments (T) viz., T1, inoculation with 10% concentration of Azotobacter vinelandii; T2, with 15% inoculation; T3, with 20% inoculation and plants without any inoculation taken as control (C).
Relative expression of antioxidant genes in different concentration of chromium
Chromium stress-tolerance level for three treatments along with control were checked by measuring the fold change for antioxidant genes, i.e., ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR). The relative expression of these genes were estimated under different concentrations (50, 100, 150, 200 and 250 μm) of Cr (VI) (K2Cr2O7) stress condition, with respect to OsActin1 gene as internal control. Leaf samples of all the 3 treatments and control (T1, T2, T3 and C) were analyzed to study the expression of antioxidant genes, the data were collected from three independent technical repeats. The following gene-specific primers were used in this experiment. For CAT gene, Forward 5´-GAAGCCAAGCATGTGAAGAAAC-3´; Reverse 5´-GCCCAACGACAACAGAAGA-3´ primers were used. For APX gene, primers were Forward 5´- GCCCGTGGTACTCTTGTTT-3´; Reverse 5´-CAACGTACTGAGGATGCCATAG-3´ and for GR gene, Forward 5´-CTATCAGTAGTGGGCTTGAGTG-3´; and Reverse 5´-TCTCCTGCCGTTTGGATATG-3´ primers were used in this study.
Chromium stress-tolerance assays
Rice plants after 6 weeks DAS were subjected to Cr(VI) stress. Cr (VI) stress was induced by incubating plants in ½ strength Hoagland’s nutrient solution containing Cr (VI) at concentration of 200 µM. All the pots (T1, T2, T3 and C) were kept in one big tank filled with Hoagland’s nutrient solution containing Cr (VI) at concentration of 200 µM. The plants were grown in the green house and the white light was provided (16 h photo period) by white fluorescent tubes (36 W Philips TLD) with a photon flux density of 52 μ/m2 s (PAR). Harvesting was done after maturity (90 DAS)
Observations of agronomic growth parameters
Growth parameters such as plant height (cm), root length (cm), root dry weight (g), and leaf area (cm2) were studied and recorded according to the method described earlier (Sahoo et al. 2014).
Extraction and estimation of total protein
To extract total proteins, rice plants were crushed in liquid nitrogen with the help of mortar and pestle. 1 g of the powdered tissues was taken and 1 ml of extraction buffer containing 0.1% sodium lauryl sarcosine, 0.1% Triton X-100, 0.01 M ethylenediaminetetraacetic (EDTA), 0.05 M Na2HPO4 and 0.01 M β-mercaptoethanol was added to it. The homogenate was transferred to microcentrifuge tube and centrifuged at 13,000 rpm for 10 min. Supernatant were stored at − 80 °C for further experiment. The concentrations of protein in the supernatants were measured by Bradford method (Bradford 1976). Bradford’s reagent containing 50 mL of 95% ethanol with Coomassie brilliant blue G-250, 100 mL 85% phosphoric acid. The solution was prepared with constant stirring. Distilled water was used to adjust the final volume. Protein concentration was measured at 595 nm, using Shimadzu UV-160A spectrophotometer. BSA was used as standard for plotting a standard curve.
Estimation of total chlorophyll
Leaf samples from each treatment were cut into pieces. 100 mg leaf of each treatment was taken. The samples were immersed in 15 mL of 80% acetone in a 50 mL conical flask and kept in darkness for extraction of chlorophyll. Thereafter, the chlorophyll extracts were decanted off and optical density (O.D) of the chlorophyll extract was measured at 645 nm and 663 nm under a colorimeter. The amount of chlorophyll a, chlorophyll b and total chlorophyll were calculated in mg/g (Arnon 1949).
Measurement of photosynthetic characteristics
An infra-red gas analyzer (IRGA, LiCor, Lincoln, NE, USA) was used on a sunny day between 10:00 and 12:00 h to estimate net photosynthetic rate (Pn), stomatal conductance (gs) and intercellular CO2 concentration (Ci) on the fourth and fifth fully expanded leaves of treated and the control plants. The atmospheric conditions during the measurement were photosynthetically active radiation (PAR), 1050 ± 7l mol/m2/s, relative humidity 66 ± 4%, atmospheric temperature 24 ± 2 °C and atmospheric CO2, 350 µM mol-1.
Measurement of soluble sugar
Glucose and fructose content in leaves of three Azotobacter treated plants as well as WT were measured after Cr(VI) stress for 24 h using the method described by Karkacier et al. (2003).
Estimation of endogenous ion content
Endogenous ion such as nitrogen, phosphorus, and potassium was estimated from each plant tissue. The samples were kept at 80 ± 5 °C for 48 h and the dry weight of each sample was recorded. Total nitrogen content in plant material was determined according to Micro Kjeldahl method (Jackson 1973). The phosphorus content of plant samples was calculated in percentage using spectrophotometer described earlier (Gupta 2004). Potassium was estimated through the flame photometer (Champman and Pratt 1982) following standard protocol.
Estimation of IAA, GA3 and zeatin from plant tissues
The extraction of endogenous plant hormones was carried out according to Chen et al. (1996). About 0.5–1.0 g of fresh plant samples was weighed and ground to powder and 5 mL of 80% methyl alcohol solution was added to a ratio of 1:10–20 (w:v). The extract was kept at 4 °C for 12 h, then centrifuged for 30 min at 2000 rpm. The leached solution was removed, and 3 mL (80%) cold methyl alcohol solution was added and shaken for several hours, then centrifuged for 20 min. The supernatant solution was dried with Nitrogen in a water bath until half solution evaporated. Petroleum ether and distill liquid (supernatant solution) at ratio of 1:1 were shaken until the distinct differences were observed. The solution was left to settle and the petrol ether was removed and the methyl alcohol solution was kept. The methyl alcohol extract was dried with nitrogen on the water bath at pH 2.0 and extracted three times with equal volume of glacial acetic acid and shaken on a mechanical shaker. All the methanol organic phase was combined and adjusted the water phase to pH 2.8. Two milliliters of glacial acetic acid and ethyl acetate were added to it and shaken. Extraction was carried out three times with 2 mL of ethyl acetate. The entire ethyl acetate phase combined and dried with nitrogen on water bath at 40 °C and extracted three times with 2 mL butanol, and dried with nitrogen on water bath until it reduced to 1 mL. The filtrate passed through 0.45 µm membrane and 0.1 µL samples were analyzed by HPLC to separate and determine the concentration of indole-3-acetic acid, gibberellic acid and zeatin endogenous hormones concentration in samples with mobile phase mixture of acetonitrile and water (volume ratio 4:6) at flow rate of 1 mL per min with an injection volume of 0.1 µL detector wavelength set at 254 nm.
Assay of antioxidant enzymes of rice plants with different treatments
Activities of different antioxidant enzymes including ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), guaiacol peroxidise (GPX) and proline content were estimated using standard methods described earlier (Garg et al. 2012). Estimation of ion leakage, relative water content (RWC) was measured by the method described earlier (Tuteja et al. 2013).
Ascorbate peroxidase
For ascorbate peroxidase (APX) activity, the homogenized plant tissues were mixed with buffer solution containing 100 mM phosphate buffer (pH 7.0), 0.1 mM EDTA, 1.0 mM ascorbate and 1 mM DTT. APX activity was determined by calculating the rate of hydrogen peroxide dependent oxidation of ascorbic acid in buffer containing 50 mM phosphate buffer (pH 7.0), 0.5 mM ascorbate and enzyme extract, in a total volume of 1 mL (Chen and Asada 1999). The rate of ascorbic acid oxidation was initiated by adding 10 μL of 10 % (v/v) H2O2 and the decrease in absorbance was monitored at 290 nm (ε0 2.8/mM/cm) for 2 min. One unit of enzyme activity was defined as amount of enzyme required to oxidize 1 μM of ascorbate per min.
Catalase
For catalase activity, plant samples were homogenized in 50 mM phosphate buffer (pH 7.0) and 1 mM DTT (dithiothreitol). CAT activity was measured using assay solution containing 50 mM phosphate buffer (pH 7.0), 33.5 mM H2O2 and 0.1 mL enzyme extract. Decrease in absorbance of H2O2 (ε039.4/mM/cm) was recorded within 2 min at 240 nm (Aebi 1984). One unit of CAT activity was defined as the amount of enzyme required to oxidize 1 μmol of H2O2 per minute.
Glutathione reductase
The homogenized tissues were mixed in extraction buffer containing 100 mM phosphate buffer (pH 7.5) and 0.5 mM EDTA, 0.75 mM DTNB, 0.1 mM NADPH. The reaction was initiated by adding 1.0 mM oxidized glutathione (GSSG) when 5,5-dithiobis (2 nitrobenzoic acid) (DTNB) was reduced by glutathione (GSH) to form TNB (Smith et al. 1988). Glutathione reductase was assayed by monitoring the increase in absorbance at 412 nm (ε06.22/mM/cm). One unit of enzyme was defined by amount of enzyme required to form 1 μ mol of GS-TNB min−1 by the reduction of DTNB.
Guaiacol peroxidase
The leaf tissues were homogenized thoroughly (1.0 g) in liquid nitrogen with 0.1 M potassium phosphate buffer (pH 7.0) under cold condition. The homogenate was centrifuged at 15,000×g, at 4 °C for 15 min. The supernatant was concentrated using 80 % ammonium sulfate (NH4)2SO4 precipitation followed by dialysis and lyophilization (Sambrook and Russell 2001). The concentrated protein samples were incubated in a mixture of 0.1 M phosphate buffer, pH 6.5; 1.5 mM O-dianisidine; 0.2 M H2O2; 50 μg of protein at 37 °C. The absorbance was recorded at 430 nm. The enzyme activity was determined as amount of enzyme required to change the absorbance by 0.1 per unit time (Heu et al. 2009).
Proline estimation
Proline content in plant tissue was determined as described by Bates et al. (1973). 500 mg of homogenized plant samples was mixed in 10 ml of 3% sulfosalicyclic acid (w/v) with pestle and mortar in ice cold bath. Then, it was centrifuged at 10,000g for 15 min followed by filtration. 2 mL of filtrate was taken and then mixed with 2 mL of acid ninhydrin and glacial acetic acid. The mixture was kept at 100 °C for 1 h until the development of colored complex. Then, the mixture was kept in ice for cooling. Twice the amount of toluene was added to it and vortexed for 15–20 s. Optical density at 520 nm was documented. The proline content was determined using standard curve of l-Proline.
Statistical analysis
The means of three separate experiments under the same environmental conditions are all the experimental data collected, and the results are expressed as mean with standard deviation (mean ± SD). To test significance between mean values of control and stressed plants, one-way variance analysis (ANOVA) was used and comparison between means was performed using Tukey–Kramer multiple comparison tests with the aid of Graph Pad InStat software (version 3.0). Cultivars were found to be statistically relevant at P < 0.05, P < 0.01 and P < 0.001.
Results
Establishment of chromium stress-tolerance level for plants
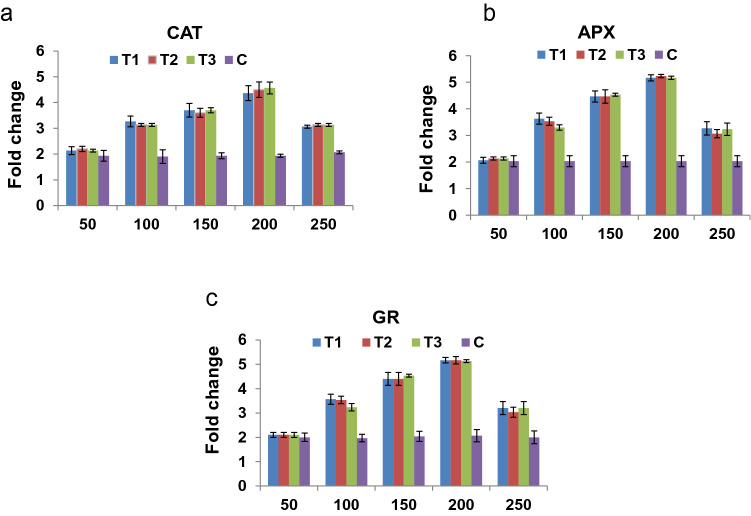
The relative expression of antioxidant genes such as CAT, APX and GR in the presence of different concentrations of Cr showed the up regulation at 200 µM. But the down regulation of these genes was observed at 250 µM Cr(VI) stress. It was an indication of the Cr(VI) stress-tolerance level for different treatments (Fig. 1a–c)
Fig. 1.
Relative gene expression of antioxidant genes under different concentration of chromium stress. a Relative gene expression of catalase (CAT), b ascorbate peroxidise (APX), and c glutathione reductase (GR) genes in T1, T2, T3 and C under 50, 100, 150, 200 and 250 µM chromium stress for 24 h. The catalase expression was increasing from 2-fold (50 μM) to 4.5-fold (200 μM) in all treatments except control. Then, at 250 μM, the fold change was dropped down to 3.5-fold. Similarly, in case of APX and GR, the same trend was observed
Agronomic performance of rice plants under chromium stress conditions
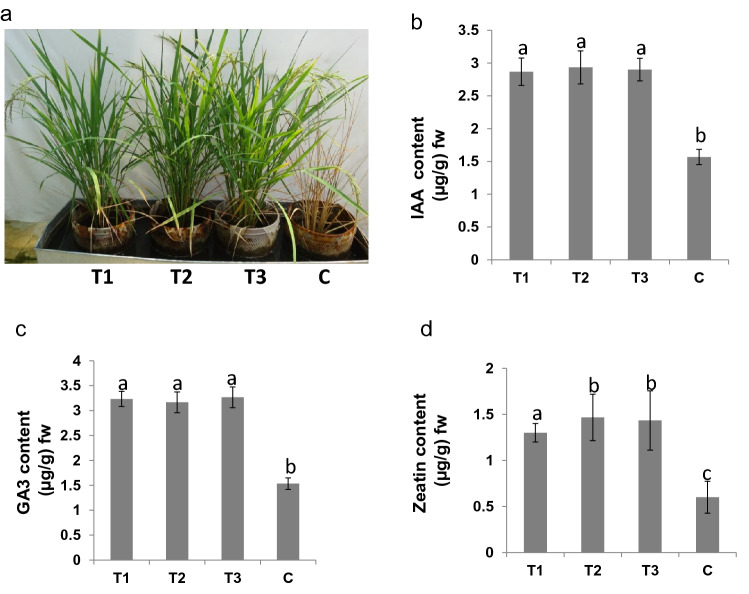
The agronomic characteristics of rice plants in all the 3 treatments and control (T1, T2, T3 and C) were recorded (Fig. 2a). There was a significant difference in agronomic parameters of rice plants after 3 different treatments (T1, T2 and T3) when compared with the control plants. Better agronomic characteristics were observed in all the treatments under 200 µm Cr(VI) stress except control (Table 1). The control rice plants of C pot died due to toxic stress of Cr(VI). But the treated plants (T1, T2 and T3) survived up to maturity.
Fig. 2.
Chromium stress-tolerance assay. a Azotobacter vinelandii treated (T1, T2 and T3) and non-treated (C) rice plants under 200 µM chromium stress for 15 days. T1, inoculation with 10% concentration of A. vinelandii; T2, with 15% inoculation; T3, with 20% (v/v, i.e., 2 × 109 cfu/ml) inoculation and plants without any inoculation taken as control (C). b Endogenous IAA content in all plants. c Endogenous content of GA3. d Endogenous content of IAA in all plants. Higher endogenous hormone content was found in all treatments when compared with control
Table 1.
Growth (plant height, root length, root dry weight, and leaf area), photosynthesis (total chlorophyll content; net photosynthetic rate, stomatal conductance, and internal CO2 concentration, and total protein); nutrients (nitrogen, phosphorus, potassium, and sodium) of rice plants at different treatments (T1, T2, T3) and control (C) after 15-day chromium stress
| Attributes | T1 (10% A. vinelandii) | T2 (15% A. vinelandii) | T3 (20% A. vinelandii) | C (Control, 0% A. vinelandii) |
|---|---|---|---|---|
| Plant height (cm) | 75±3.2a | 79±3.1a | 81±3.1b | 63±3.0c |
| Root length (cm) | 31±0.8a | 32±1.2a | 32±1.1a | 22±1.1b |
| Root dry weight (g) | 2.5±0.12a | 2.7±0.1a | 2.8±0.1a | 2.1±0.12b |
| Leaf area (cm2/plant) | 92±2.4a | 92±1.6a | 98±1.5b | 49±1.0c |
| Total chlorophyll (mg/g f wt) | 9.05±0.22a | 9.15±0.3a | 9.15±0.4a | 4.65±0.5b |
| Total protein (mg/g f wt) | 1.75±0.53a | 1.74±0.82a | 1.78±0.55a | 1.63±0.91b |
| Net photosynthetic rate (PN, µ mol CO2 m-2s-1) | 9.25±0.5a | 9.11±0.2a | 9.05±0.3a | 8.01±0.4b |
| Stomatal conductance (gs, m mol m−2 s−1) | 246±11.4a | 248±10.9a | 255±10.2b | 213±11.5c |
| Intracellular CO2 (Ci, µ mol mol−1) | 222±11.2a | 224±11.4a | 225±10.4a | 214±10.5b |
| Nitrogen (%) | 0.285±0.011a | 0.286±0.012a | 0.312±0.011b | 0.275±0.011c |
| Phosphorus (%) | 0.243±0.011a | 0.242±0.011a | 0.247±0.011a | 0.222±0.011b |
| Potassium (%) | 0.165±0.003a | 0.168±0.002a | 0.163±0.001a | 0.128±0.001b |
| Sodium (%) | 0.042±0.001a | 0.046±0.001a | 0.045±0.001a | 0.047±0.001a |
The superscript letters a, b and c indicate significant differences at P > 0.05 level as determined by Duncan’s multiple range test (DMRT)
Higher photosynthetic characteristics and endogenous ion contents in Azotobacter vinelandii-inoculated rice plants
The photosynthetic characteristics of rice plants were recorded after 15 days of Cr(VI) stress. The photosynthetic rate declined by 37% in control plants as compared to T1, T2 and T3 rice plants. There are no significant differences among T1, T2 and T3 plants during Cr(VI) stress. The net photosynthetic rate, stomatal conductance, and intracellular CO2 were also higher in plants of T1, T2 and T3 pots as compared to the control plants (Table 1). The photosynthetic characteristics of T3 plants were found to be higher among inoculated treatments, i.e., T1 and T2. Rice plants of T1, T2 and T3 pots possess higher endogenous hormone content when compared with control plants (Fig. 2b–d). IAA, zeatin and GA3 contents in T2 plants were higher among all the plants.
Scavenging capacity of ROS in rice plants
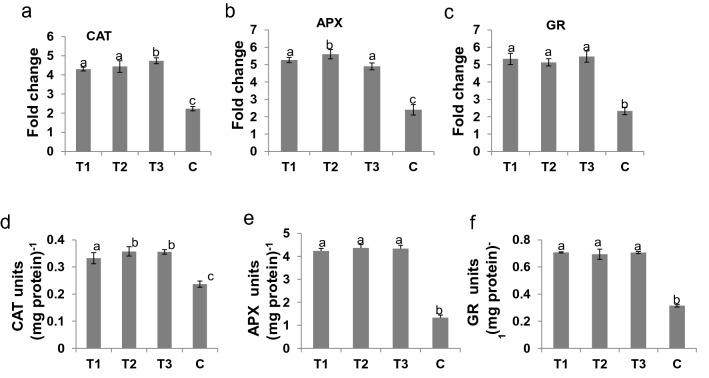
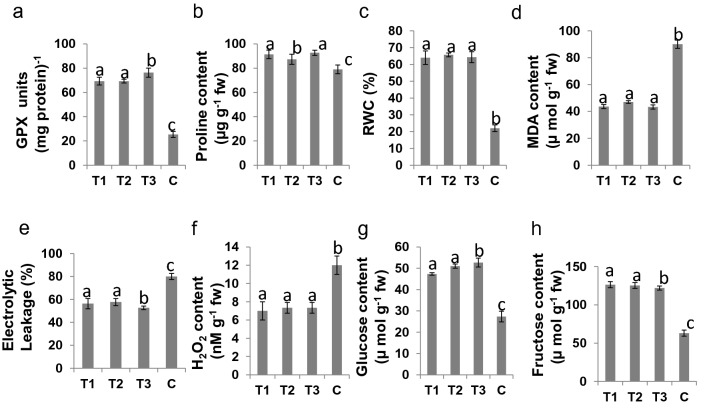
We determined the relative expression of some of the antioxidant marker genes such as catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) in plants of all the 4 treatments under Cr(VI) stress (200 μM) conditions (Fig. 3a–c). The catalase expression increased from 2-fold (50 μM) to 4.5-fold (200 μM) in all treatments except control. Then, at 250 μM, the fold change dropped down to 3.5-fold. Similarly, in case of APX and GR, the same trend was observed. The reduced expression of antioxidant marker genes were found in control plants, whereas higher expression was found in other treatments (T1, T2 and T3). In addition, enzymatic activities of the CAT, APX, and GR were significantly higher in all the 3 treatments (T1, T2 and T3) when compared to the control plants under stress condition (Fig. 3d–f). Similarly, the guaiacol peroxidase (GPX), proline and relative water content (RWC) were significantly higher in plants of Azotobacter vinelandii-inoculated pots (T1, T2 and T3) when compared to the plants of un-inoculated control pot C under Cr(VI) stress (Fig. 4a–c). In addition, reduction in MDA content, H2O2 production, and ion leakage were observed in the plants of 3 treatments (T1, T2 and T3) when compared with control plants of pot C (Fig. 4d–f).
Fig. 3.
Relative gene expression and antioxidant enzyme content of rice plants after 200 µM chromium stress. a Relative gene expression of catalase (CAT), b ascorbate peroxidise (APX), and c glutathione reductase (GR) genes in T1, T2, T3 and C rice plants after 15 days chromium stress. Similarly, activity of d catalase (CAT), (e) ascorbate peroxidase (APX) and (f) glutathione reductase (GR) enzymes in rice lines. The experiments were independently repeated three times with minimum three technical replicates. Graphs show mean values ± standard error. Values with different letters are significantly different at P < 0.05 (estimated using one-way ANOVA)
Fig. 4.
Biochemical analysis of rice plants of all treatments T1, T2, T3 and C after 200 µM chromium stress. a Guaiacol peroxidase (GPX) activity. b Level of proline accumulation. c Percent relative water content (RWC). d Lipid peroxidation expressed in terms of MDA content. e Electrolytic leakage. f Hydrogen peroxide (H2O2) content. g Glucose content in plants. h Fructose content in plants
The higher soluble sugar content in Azotobacter-inoculated plants
The rice plants of 3 treatments (T1, T2 and T3) possessed higher soluble sugar content, i.e., glucose and fructose than control plants under Cr(VI) stress conditions (Fig. 4g, h).
Population of Azotobacter vinelandii in different pots
The population dynamics of Azotobacter vinelandii in all the pots (T1, T2 and T3) were found to be varying and there was no significant reduction in their population even after 15 days of Cr(VI) stress. The population of Azotobacter vinelandii was 0.70× 106 cfu/g, 0.68× 106 cfu/g and 0.92× 106 cfu/g in T1, T2 and T3 pots, respectively.
Discussion
Chromium (Cr) is a toxic element for plants, which causes oxidative damages to DNA, RNA, proteins, and pigments (Yadav et al. 2010; Sharma et al. 2011; Dhali et al. 2020). Plants contain unique setup of antioxidant enzymes against such oxidative stress (Choudhary et al. 2012). Azotobacter vinelandii has very important role during environmental stresses. It helps plants to survive during stress conditions (Sahoo et al. 2014). The stress-tolerance level of different treatments (T1, T2, and T3) at different Cr(VI) concentrations (50, 100, 150, 200 and 250 µM) were checked and the higher antioxidant gene expression levels were found at 200 µm Cr(VI) stress conditions for all the treatments. The relative gene expression level was found lower at 250 µm Cr(VI) stress conditions. On the basis of the expression level of antioxidant genes, we performed all our studies at 200 µM Cr(VI) stress. Here, rice plants inoculated with different concentrations (10%, 15% and 20%) of Azotobacter vinelandii revealed better growth under 200 µM Cr(VI) stresses for 15 days. In contrast, the rice plants without Azotobacter vinelandii inoculation could not survive under Cr(VI) stress. The higher expression of antioxidant enzymes such as CAT, APX and GR and the relative expression of these antioxidant genes (CAT, APX and GR) were found to be higher in Azotobacter vinelandii-inoculated rice plants (T1, T2 and T3) whereas less expression was observed in un-inoculated control plants (C). This observation provides strong evidence that Azotobacter vinelandii helps plants to survive and withstand in continuous Cr(VI) stress. Proline has been identified as a molecule which performs a variety of functions, accumulating in elevated level in response to diverse stresses (Liang et al. 2013). Proline homeostasis is essential for meristematic cells owing to its function to retain sustainability of plant growth under prolong stress and proline could have a protective function (Kavi-Kishor and Sreenivasulu 2014). The parallel evidence involving increased proline content in Azotobacter vinelandii-inoculated rice plants suggests that Azotobacter vinelandii also has role in stimulation of proline during Cr(VI) stress. Lipid peroxidation has been reported to be increased after prolonged exposure to stress (Soliman et al. 2011). Here, we observed higher MDA content and H2O2 production in control rice plants as compared to treated plants (T1, T2 and T3) under Cr(VI) stress. This result provides further evidence that Azotobacter vinelandii contributes a strong support for tolerance against prolonged Cr(VI) stress. Plant hormones such as IAA, GA3 and zeatin play an important role in plant growth and development and also in adaptation to different stresses (Peleg et al. 2011). In the present study, we found higher IAA, GA3 and zeatin content in T1, T2 and T3 rice plants as compared to un-inoculated control rice plants. Therefore, these data support the role of Azotobacter vinelandii in growth and development of rice plant under Cr(VI) stress. According to previous report, the biomass of plant was increased in the presence of growth-promoting microorganisms under Cr(VI) stress (Fan et al. 2011). In our study, the same trend was observed. Here, better biomass of plants in T1, T2 and T3 as compared to control is evidence that Azotobacter vinelandii promotes better growth during 200 µm Cr stress condition. Significant improvement of rice plants after Cr stress was reported by increasing macronutrients indicating change in nutrient status in plants that is correlated with improved tolerance to Cr(VI) stress (Panda and Choudhury 2005). Here, we observed an improved macronutrients profile in T1, T2 and T3 rice plants under Cr(VI) stress with respect to that of un-inoculated control plants.
Sugars may play key roles in stress defense mechanisms, including membrane stability, via interaction with phospholipid head groups and ROS detoxification (Bohnert and Jensen 1996; Bentsink et al. 2000; Roy et al. 2005; Tuteja et al. 2014). In this study, we found that glucose and fructose content were higher in T1, T2 and T3 rice plants than control plants. The findings of present investigation suggest that A. vinelandii potentially contributes to rice plants to maintain higher level of compatible solute, plant hormones and macronutrients, leading to better growth of root and shoots and thereby improved tolerance to Cr(VI) stress. We observed that the 20% Azotobacter vinelandii-inoculated rice plants (T3) have more potency to tolerate the toxicity of prolonged 200 µM Cr(VI) stress. Our findings suggest that the increased population of Azotobacter vinelandii synthesizes more growth-promoting hormones, stimulates more detoxification of ROS and more stabilization of antioxidant machinery during stress. These findings are in agreement with earlier reports (Bhardwaj et al. 2014).
It can be concluded from the current study that Azotobacter vinelandii due to their enhanced activity of several plant growth-promoting mechanisms has the potential to boost phytoextraction of heavy metals from contaminated soil. Moreover, combining stress alleviator alleviates the Cr(VI) inducing oxidative stress by activating antioxidant defense system, as evidenced by the decreased accumulation of MDA, reduced H2O2 and less electrolytic leakage. In addition, Azotobacter vinelandii inoculation maintains the cellular redox homeostasis, thus enabling the growing rice plants to cope with better Cr(VI) stress. Furthermore, these studies indicate that effectiveness of metal tolerant for metal detoxification from soil and improved plant growth in metal stress condition could further be enhanced by combining these bacteria with suitable stress alleviator. Overall this study suggests the novel role of Azotobacter vinelandii in Cr(VI) stress combating that also increases its importance in improving other crops of interest.
Acknowledgements
We are thankful to Dr. Madhusmita Pradhan for help in preparation of the manuscript.
Author contributions
RKS performed all the experiments and wrote the first draft of the manuscript. VR helped RKS during performing experiments. NT supervised all the experiments and wrote the final manuscript. All the authors have read and approved the manuscript.
Declarations
Conflict of interest
The authors have nothing to disclose.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Wahid A, Ahmad SS, Butt ZA, Tariq M. Ecophysiological responses of rice (Oryza sativa l.) To hexavalent chromium. Pak J Bot. 2011;43:2853–2859. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts in polyphenoloxidase in beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun KS, Cervantes C, Herminia L-T, Avudainayagamd S. Chromium toxicity in plants. Environ Int. 2005;31:739–753. doi: 10.1016/j.envint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Bakshi A, Panigrahi AK. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol Rep. 2018;5:440–447. doi: 10.1016/j.toxrep.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of proline in water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Becquer T, Quantin C, Sicot M, Boudot JP. Chromium availability in ultramafic soils from New Caledonia. Sci Total Environ. 2003;301:251–261. doi: 10.1016/s0048-9697(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Bentsink L, Alonso-Blanco C, Vreugdenhil D, Tesnier K, Groot SPC, Koornneef M. Genetic analysis of seed soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol. 2000;124:1595–1604. doi: 10.1104/pp.124.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact. 2014;13:66. doi: 10.1186/1475-2859-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bohnert HJ, Jensen RG. Strategies for engineering water stress tolerance in plants. Trends Plant Sci. 1996;14:89–97. [Google Scholar]
- Camargo FA, Okeke BC, Bento FM, Frankenberger WT. In vitro reduction of hexavalent chromium by a cell-free extract of Bacillus sp. ES 29 stimulated by Cu2+ Appl Microbiol Biot. 2003;62:569–573. doi: 10.1007/s00253-003-1291-x. [DOI] [PubMed] [Google Scholar]
- Castro RO, Trujillo MM, Bucio JL, Cervantes C, Dubrovsky J. Effects of dichromate ongrowth and root system architecture of Arabidopsis thaliana seedlings. Plant Sci. 2007;172:684–691. [Google Scholar]
- Chapman HD, Pratt PF. Method and of analysis of soil, plant and water. 2. California University Agricultural Division; 1982. p. 170. [Google Scholar]
- Chatterjee S, Sau GB, Mukherjee SK. Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J Microbiol Biotechnol. 2009;25:1829–1836. doi: 10.1007/s11274-009-0084-5. [DOI] [Google Scholar]
- Chen GX, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1999;30:987–998. [Google Scholar]
- Chen Y, Li F, Ma Y, Chong K, Xu Y. Overexpression of OrbHLH001, a putative helix-loop-helix transcription factor, causes increased expression of AKT1 and maintains ionic balance under salt stress in rice. J Plant Physiol. 2013;170:93–100. doi: 10.1016/j.jplph.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Choudhary SP, Kanwar M, Bhardwaj R, Yu JQ, Tran LSP. Chromium stress mitigation by polyaminebrassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE. 2012;7:e33210. doi: 10.1371/journal.pone.0033210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury AR, Datta R, Sarkar D. Heavy metal pollution and remediation. Green Chem. 2018;3:359–373. [Google Scholar]
- Das AJ, Kumar M, Kumar R. Plant growth promoting rhizobacteria (pgpr): an alternative of chemical fertilizer for sustainable, environment friendly agriculture. Res J Agric Fores Sci. 2013;1:21–23. [Google Scholar]
- Dhali S, Pradhan M, Sahoo RK, Pradhan C, Mohanty S. Growth and biochemical variations in Macrotyloma uniflorum var. madhu under chromium stress. Indian J Agric Sci. 2020;54:95–100. [Google Scholar]
- Dixit V, Pandey V, Shyam R. Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv. Azad) root mitochondria. Plant Cell Environ. 2002;25:687–693. [Google Scholar]
- Dube BK, Tewari K, Chatterjee J, Chatterjee C. Excess chromium alters uptake and translocation of certain nutrients in Citrullus. Chemosphere. 2003;53:1147–1153. doi: 10.1016/S0045-6535(03)00570-8. [DOI] [PubMed] [Google Scholar]
- Faisal M, Hasnain S. Bacterial Cr (VI) reduction concurrently improves sunflower (Helianthus Annuus L.) growth. Biotechnol Lett. 2005;27:943–947. doi: 10.1007/s10529-005-7188-2. [DOI] [PubMed] [Google Scholar]
- Fan L, Ma Z, Liang J, Li H, Wang E, Wei G. Characterization of a copper-resistant symbiotic bacterium isolated from Medicago lupulina growing in mine tailings. Bioresour Technol. 2011;102:703–709. doi: 10.1016/j.biortech.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Foucault Y, Leveque T, Xiong T, Schreck E, Austruy A, Shahid M, Dumat C. Green manure plants for remediation of soils polluted by metals and metalloids: ecotoxicity and human bioavailability assessment. Chemosphere. 2013;93:1430–1435. doi: 10.1016/j.chemosphere.2013.07.040. [DOI] [PubMed] [Google Scholar]
- Garg B, Jaiswal JP, Misra S, Tripathi BN, Prasad MA. A comprehensive study on dehydration-induced antioxidative responses during germination of Indian bread wheat (Triticum aestivum L. em Thell) cultivars collected from different agroclimatic zones. Physiol Mol Biol Plants. 2012;18:217–228. doi: 10.1007/s12298-012-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK. Methods in environmental analysis water, soil and air. India: Agrobios; 2004. pp. 242–245. [Google Scholar]
- He MY, Li XY, Liu HL, Miller SJ, Wang GJ, Rensing C. Characterization and genomic analysis of a chromate resistant and reducing bacterial strain Lysinibacillus fusiformis ZC1. J Hazard Mater. 2011;185:682–688. doi: 10.1016/j.jhazmat.2010.09.072. [DOI] [PubMed] [Google Scholar]
- Heu Y, Ge Y, Zang C, Zu T, Cheng W. Cd toxicity and translocation in rice seedlings are reduced by hydrogen peroxide treatments. Plant Growth Regul. 2009;5(1):51–61. doi: 10.1007/s10725-009-9387-7. [DOI] [Google Scholar]
- Jackson TL. Soil chemical analysis. New Delhi: Prentice-Hall of India Private Limited; 1973. p. 498. [Google Scholar]
- Jun R, Ling T, Guanghua Z. Effects of chromium on seed germination, root elongation and coleoptiles growth in six pulses. Inter J Environ Sci Technol. 2009;6:571–578. [Google Scholar]
- Karkacier M, Erbas M, Uslu MK, Aksu M. Comparison of different extraction and detection methods for sugars using amino-bonded phase HPLC. J Chromatogr Sci. 2003;41:331–333. doi: 10.1093/chromsci/41.6.331. [DOI] [PubMed] [Google Scholar]
- Karuppiah PS, Rajaram J. Exploring the potential of chromium reducing Bacillus sp. and there plant growth promoting activities. Microbiol Res. 2011;1:17–23. [Google Scholar]
- Kavi-Kishor PB, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37:300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- Khatun MR, Mukta RH, Islam MA, Huda AKMN. Insight into citric acid-induced chromium detoxification in rice (Oryza sativa. L) Int J Phytoremediation. 2019;31:1–7. doi: 10.1080/15226514.2019.1619162. [DOI] [PubMed] [Google Scholar]
- Liang X, Zhang L, Natarajan SK, Becker DF. Proline mechanisms of stress survival. Antioxid Redox Sig. 2013;19:998–1011. doi: 10.1089/ars.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool Z, Asghar HN, Shahzad T, Hussain S, Riaz M, Ali S, Arif MS, Maqsood M. Isolating, screening and applying chromium reducing bacteria to promote growth and yield of okra (Hibiscus esculentus L) in chromium contaminated soils. Ecotoxicol Environ Saf. 2015;114:343–349. doi: 10.1016/j.ecoenv.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Medda S, Mondal NK. Chromium toxicity and ultrastructural deformation of Cicer arietinum with special reference of root elongation and coleoptile growth. Ann Agrar Sci. 2017;15:396–401. [Google Scholar]
- Morel MA, Ubalde MC, Braña V, et al. Delftia sp. JD2: a potential Cr (VI)-reducing agent with plant growth-promoting activity. Arch Microbiol. 2011;193:63–68. doi: 10.1007/s00203-010-0632-2. [DOI] [PubMed] [Google Scholar]
- Nagarajan M, Ganesh SK. Toxic effects of chromium on growth of some paddy varieties. Intl Lett Nat Sci. 2015;35:36–44. [Google Scholar]
- Panda SK, Choudhury S. Chromium stress in plants. Braz J Plant Physiol. 2005;17:95–102. [Google Scholar]
- Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J. 2011;9:747–758. doi: 10.1111/j.1467-7652.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int. 2019;125:365–385. doi: 10.1016/j.envint.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Rajkumar M, Nagendran R, Lee KJ, Lee WH. Characterization of a novel Cr 6+ reducing Pseudomonas sp. with plant growth-promoting potential. Curr Microbiol. 2005;50:266–271. doi: 10.1007/s00284-005-4470-4. [DOI] [PubMed] [Google Scholar]
- Romanenko VI, Koren’kov VN. A pure culture of bacteria utilizing chromates and bichromates as hydrogen acceptors in growth under anaerobic conditions. Mikrobiologiya. 1977;46:414–417. [PubMed] [Google Scholar]
- Roy P, Niyogi K, SenGupta DN, Ghosh B. Spermidine treatment to rice seedlings recovers salinity stressinduced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and saltsensitive rice cultivars. Plant Sci. 2005;168:583–591. [Google Scholar]
- Sabir M, Waraich EA, Hakeem KR, Ozturk M, Ahmad HR, Shahid M. Phytoremediation: mechanisms and adaptations. Soil Remediat Plants. 2015;4:85–105. [Google Scholar]
- Sahoo RK, Ansari MW, Dangar TK, Mohanty S, Tuteja N. Phenotypic and molecular characterization of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma. 2014;251:511–523. doi: 10.1007/s00709-013-0547-2. [DOI] [PubMed] [Google Scholar]
- Saifullah SM, Zia-Ur-Rehman M, Sabir M, Ahmad HR. Phytoremediation of Pb contaminated soils using synthetic chelates. In: Hakeem K, Sabir M, Ozturk M, Murmet A, editors. Soil remediation and plants. San Diego: Elsevier; 2015. pp. 397–414. [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harb Lab Press. 2001;3:114–115. [Google Scholar]
- Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere. 2017;178:513–533. doi: 10.1016/j.chemosphere.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S. Chromium toxicity in plants. Environ Int. 2005;31:739–753. doi: 10.1016/j.envint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Sharma I, Pati PK, Bhardwaj R. Effect of 28- homobrassinolide on antioxidant defence system in Raphanus sativus L. under chromium toxicity. Ecotoxicology. 2011;20:862–874. doi: 10.1007/s10646-011-0650-0. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D. Chromium bioaccumulation and its impacts on plants: an overview. Plants. 2020;9:100. doi: 10.3390/plants9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5, 5 -dithiobis (2- nitrobenzoic acid) Ann Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Solanki R, Dhankhar R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia. 2011;66:195–204. [Google Scholar]
- Soliman W, Fujimori M, Tase K, Sugiyama S. Oxidative stress and physiological damage under prolonged heat stress in C3 grass Lolium perenne. Grassland Sci. 2011;57:101–106. [Google Scholar]
- Soni SK, Singh R, Singh M, Awasthi A, Wasnik K, Kalra A. Pretreatment of Cr (VI)-amended soil with chromate-reducing rhizobacteria decreases plant toxicity and increases the yield of Pisum sativum. Arch Environ Contam Toxicol. 2014;66:616–627. doi: 10.1007/s00244-014-0003-0. [DOI] [PubMed] [Google Scholar]
- Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L. Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. C R Biol. 2010;333:597–607. doi: 10.1016/j.crvi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Pandey A, Sa T. Chromate reducing and plant growth promoting activities of psychrotrophic Rhodococcus erythropolis MtCC 7,905. J Basic Microbiol. 2007;47(6):513–517. doi: 10.1002/jobm.200700224. [DOI] [PubMed] [Google Scholar]
- Tuteja N, Sahoo RK, Garg B, Tuteja R. OsSUV3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR64) Plant J. 2013;76:115–127. doi: 10.1111/tpj.12277. [DOI] [PubMed] [Google Scholar]
- Tuteja N, Banu MSA, Huda KMK, Gill SS, Jain P, Pham XH, Tuteja R. Pea p68, a DEAD-box helicase, provides salinity stress tolerance in transgenic tobacco by reducing oxidative stress and improving photosynthesis machinery. PLoS ONE. 2014;9(5):e98287. doi: 10.1371/journal.pone.0098287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernay P, Gauthier-Moussard C, Hitmi A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere. 2007;68:1563–1575. doi: 10.1016/j.chemosphere.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Vernay P, Gauthier-Moussard C, Jean L, Bordas F, Faure O, Ledoigt G, Hitmi A. Effect of chromium species on phytochemical and physiological parameters in Datura innoxia. Chemosphere. 2008;72:763–771. doi: 10.1016/j.chemosphere.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Wakeel A, Xu M, Gan Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int J Mol Sci. 2020;21:728. doi: 10.3390/ijms21030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani PA, Khan MS, Zaidi A. Chromium-reducing and plant growth-promoting Mesorhizobium improves chickpea growth in chromium-amended soil. Biotechnol Lett. 2008;30:159–163. doi: 10.1007/s10529-007-9515-2. [DOI] [PubMed] [Google Scholar]
- Wani SA, Chand S, Ali T. Potential use of Azotobacter chroococcum in crop production: an overview. Curr Agric Res J. 2013;1:35–38. [Google Scholar]
- Yadav SK, Dhote M, Kumar P, Sharma J, Chakrabarti T, Juwarkar AA. Differential antioxidative enzyme responses of Jatropha curcas L. to chromium stress. J Hazard Mater. 2010;180:609–615. doi: 10.1016/j.jhazmat.2010.04.077. [DOI] [PubMed] [Google Scholar]
- Yu XZ, Gu JD. Accumulation and distribution of trivalent chromium and effects on hybrid willow (Salix matsudana Koidz x alba L.) metabolism. Arch Environ Contam Toxicol. 2007;52(4):503–511. doi: 10.1007/s00244-006-0155-7. [DOI] [PubMed] [Google Scholar]
- Yu XZ, Lin YJ, Zhang Q. Metallothioneins enhance chromium detoxification through scavenging ROS and stimulating metal chelation in Oryza sativa. Chemosphere. 2019;220:300–313. doi: 10.1016/j.chemosphere.2018.12.119. [DOI] [PubMed] [Google Scholar]
- Zaheer IE, Ali S, Saleem MH, Arslan Ashraf M, Ali Q, Abbas Z, Rizwan M, El-Sheikh MA, Alyemeni MN, Wijaya L. Zinc-lysine supplementation mitigates oxidative stress in rapeseed (Brassica napus l.) by preventing phytotoxicity of chromium, when irrigated with tannery wastewater. Plants. 2020;9(9):E1145. doi: 10.3390/plants9091145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lin L, Zhu X, Liu Q, Yang Y, Yuan W, Shao J (2010) Eco-physiological responses of rice (Oryza sativa L.) roots to zinc, chromium stress. In: 4th International Conference on bioinformatics and biomedical engineering, Chengdu, China.