Abstract
Many plant disease resistance (R) genes function specifically in reaction to the presence of cognate effectors from a pathogen. Xanthomonas oryzae pathovar oryzae (Xoo) uses transcription activator-like effectors (TALes) to target specific rice genes for expression, thereby promoting host susceptibility to bacterial blight. Here, we report the molecular characterization of Xa7, the cognate R gene to the TALes AvrXa7 and PthXo3, which target the rice major susceptibility gene SWEET14. Xa7 was mapped to a unique 74-kb region. Gene expression analysis of the region revealed a candidate gene that contained a putative AvrXa7 effector binding element (EBE) in its promoter and encoded a 113-amino-acid peptide of unknown function. Genome editing at the Xa7 locus rendered the plants susceptible to avrXa7-carrying Xoo strains. Both AvrXa7 and PthXo3 activated a GUS reporter gene fused with the EBE-containing Xa7 promoter in Nicotiana benthamiana. The EBE of Xa7 is a close mimic of the EBE of SWEET14 for TALe-induced disease susceptibility. Ectopic expression of Xa7 triggers cell death in N. benthamiana. Xa7 is prevalent in indica rice accessions from 3000 rice genomes. Xa7 appears to be an adaptation that protects against pathogen exploitation of SWEET14 and disease susceptibility.
Keywords: Xa7, TAL effector, Xanthomonas, bacterial blight, disease resistance, SWEET14
Bacterial TAL effectors hijack host gene expression to mediate disease susceptibility, and host plants counteract TAL effectors with resistance genes to thwart pathogen infection. This study reports the cloning of Xa7, demonstrates its induction by the essential virulence TAL effectors AvrXa7 and PthXo3, and documents its prevalence in indica rice cultivars.
Introduction
Crop plants suffer detrimental effects from plant diseases and pests, which cause global yield losses of about 20% each year (Oerke 2006; Savary et al., 2019). To counteract disease, host plants have evolved innate immunity mechanisms that work against pathogens mainly through a diverse array of plant genes and gene products that recognize molecular signals from the pathogens (Spoel and Dong 2012). Conceptually, resistance is mediated by two general pathways. In the first pathway, membrane-bound receptors recognize conserved small molecules, often of pathogen origin, the so-called pathogen-associated molecular patterns, and trigger basal and broad immunity against the invading pathogens (Jones and Dangl 2006). Many host- and cultivar-specific pathogenic fungi and proteobacteria have evolved processes that suppress basal immunity (Cook et al., 2015; Monteiro and Nishimura 2018). Plants, in turn, have evolved a second layer of defense, the so-called effector triggered immunity (ETI), which involves the specific recognition of immunity-suppressive effectors (Jones and Dangl 2006). Major resistance (R) genes are adaptive components of the plant defense system that arise from selective pressure exerted by virulent pathogen populations. In some cases, pathogens can overcome ETI through mutation or loss of R gene-specific effector genes or the acquisition of new effectors that, in turn, suppress ETI (Jackson et al., 1999; Feng and Zhou 2012).
Bacterial blight (BB) of rice, caused by the γ-proteobacterium Xanthomonas oryzae pathovar oryzae (Xoo), is among the most damaging diseases in a wide range of South Asian rice-producing areas and also poses a threat in some African countries (Niño-Liu et al., 2006). R gene deployment is the most economically sound and environmentally friendly means to control BB, and many BB-specific R genes have been identified and characterized at the molecular level (Song et al., 1995; Yoshimura et al., 1998; Yang et al., 2000; Iyer and McCouch 2004; Sun et al., 2004; Gu et al., 2005; Chu et al., 2006; Xiang et al., 2006; Liu et al., 2011; Tian et al., 2014; Wang et al., 2015; Hu et al., 2017; Ji et al., 2020; Zhang et al., 2020). The cognate effectors that recognize R genes, as in other proteobacterial disease complexes, are commonly effectors of the type III secretion pathway. Historically, type III effectors with a cognate R gene are named Avr effectors (Leach and White 1996). All the known cognate type III Avr effector/R gene pairs in BB involve a subset of type III effectors known as transcription activator-like effectors (TALes).
TALes of Xanthomonas represent the largest subgroup of type III effector proteins in plant pathogenic bacteria. For the most part, they function as transcription factors that promote the expression of host genes by binding to sequence-specific promoter segments, referred to here as effector binding elements (EBEs). Consequentially, expression of the host susceptibility (S) gene enhances the disease process. TALes of Xoo that have a dramatic effect on virulence and host susceptibility are referred to as major TALes and are known to target three members of the sucrose transporter, or SWEET, gene family. In the absence of SWEET gene expression, Xoo strains are virtually nonpathogenic, and every Xoo strain examined to date has at least one gene for a major TALe (Oliva et al., 2019). Rice cultivars have adapted to TALe-mediated virulence by the acquisition of a genetically dominant R gene class defined by TALe-specific expression that triggers a state of resistance. Rice R genes with TALe-specific expression include Xa27, Xa10, and Xa23 (Gu et al., 2005; Tian et al., 2014; Wang et al., 2015). TALe-mediated R gene expression has also been demonstrated for Bs3 and Bs4C-R in pepper (Capsicum sp.) (Römer et al., 2009; Strauss et al., 2012).
Xa7 is a dominant R gene of rice that confers resistance to Xoo strains that harbor the cognate major TALe AvrXa7 (Hopkins et al., 1992). The AvrXa7 effector has a dual function: as a virulence factor, it induces the rice S gene SWEET14, which encodes a sucrose efflux transporter, and as an avirulence factor, it also triggers Xa7-mediated resistance. AvrXa7 targets an overlapping EBE of the S gene SWEET14 with a second major TALe, PthXo3. Although the identity and mechanism of Xa7 are unknown, it has been shown to confer resistance to all six Japanese Xoo races or sub-races and 4 of 10 Philippine Xoo races (Ogawa et al., 1991). Xoo races are defined by the set of R genes in a given group of rice cultivars with which the strains are incompatible. The broad spectrum of Xa7 makes it an important R gene in rice breeding programs (Ogawa et al., 1991; Hsu et al., 2020). The pathogen gene avrXa7 was found in 11 of 33 fully sequenced Asian Xoo strains, whereas pthXo3 was found in 12 of the 33 strains. No strain contained both avrXa7 and pthXo3 (Oliva et al., 2019). Xa7 has been found to retain effectiveness under field conditions (Bai et al., 2000) and to perform better at high temperatures (Webb et al., 2010; Dossa et al., 2020), which are reported to compromise the function of some R genes. Efforts to map Xa7 have placed the gene on chromosome 6 (Kaji and Ogawa 1995; Porter et al., 2003; Chen et al., 2008; Zhang et al., 2009). In this study, we present evidence for the identity of Xa7 based on fine mapping, gene expression assays, and CRISPR-mediated gene editing.
Results
Fine mapping of Xa7 from IRBB7
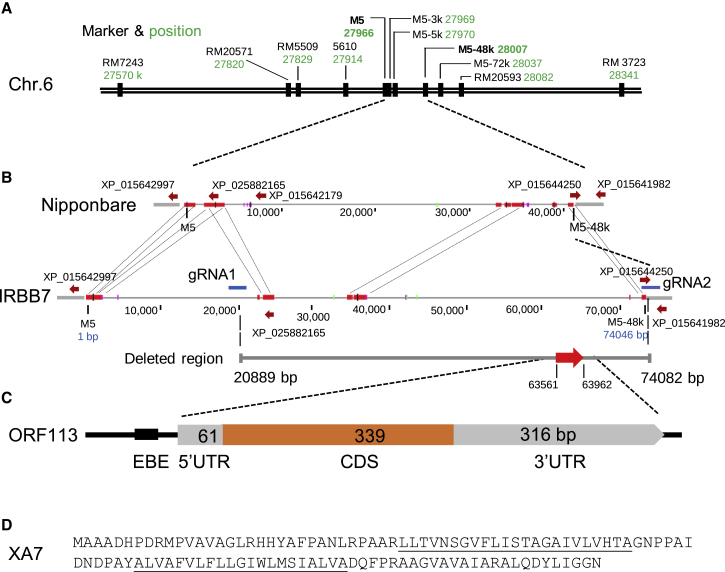
To fine map Xa7 in IRBB7 (an indica rice variety carrying Xa7), the first mapping population was created by crossing the near-isogenic line IRBB7 with the recurrent parental cultivar IR24 (Ogawa et al., 1991). For mapping, 220 F2 plants were phenotyped by inoculation with the avrXa7-carrying Xoo strain PXO86,and genotyped using the previously reported Xa7-linked marker M5 (Porter et al., 2003). A set of 10 newly developed molecular markers linked to M5 were also identified and used to further genotype the plants (see Supplemental Table 1 for markers and Supplemental Table 2 for oligonucleotides). Xa7 was mapped to an interval defined by the markers RM7243 (three recombinants) and RM20593 (two recombinants) and was shown to co-segregate with the markers M5, M5-5k, and M5-48k (Figure 1A). These results indicate that Xa7 is located within a region of 512 kilobases (kb) between RM7243 and RM20593 relative to the reference genome of the cultivar Nipponbare. An additional 17 000 members from an F2 population of IRBB7 and Nipponbare were screened for recombinants between RM7243 and RM20593, and the recombinants were phenotyped for genetic association analysis. Based on a number of polymorphic markers (Supplemental Table 1), Xa7 was shown to reside within a region corresponding to the 41.3-kb region between M5 and M5-48k on the Nipponbare reference genome (Figure 1B).
Figure 1.
Fine mapping of Xa7.
(A) Physical map of Xa7 locus and associated markers on chromosome 6. The individual molecular markers are indicated as the genomic coordinates (in kilobases and in green) on chromosome 6 of the Nipponbare reference genome.
(B) Genomic regions delimited by two markers (M5 and M5-48k) in Nipponbare and IRBB7. Annotated genes in Nipponbare are shown with red arrows, and those in IRBB7 are shown in blue. Red bars linking Nipponbare and IRBB7 indicate syntenic regions. Two Cas9 guide RNAs for the deletion of a 53-kb region within the Xa7 candidate gene in IRBB7 are denoted by red arrows.
(C) Schematic graph of Xa7. The gene structure is depicted as EBE for AvrXa7, 5′ UTR for the 5′ untranslated region, and 3′ UTR for the 3′ untranslated region. CDS, coding sequence. (D) XA7 amino acids. Single letters of amino acids are used. The transmembrane sequences are underlined.
Short and long sequencing reads obtained from IRBB7 DNA by Illumina and Nanopore sequencing were used for de novo assembly of the Xa7 region across markers M5 and M5-48k, resulting in a genomic sequence of 74 kb. PCR amplification and sequencing of the amplicons were also performed to validate the accuracy of the sequencing data, and the sequence was aligned with the related region from Nipponbare (Figure 1B). The regions are syntenic and include homologous genes that encode the IRGSP GenBank protein accessions XP_025882165.1 (only the 402 amino acid [aa] C-terminal exon is conserved in IRBB7), XP_015642179.1 (not present in IRBB7), XP_015644250.1 (perfectly conserved), and XP_015641982.1 (perfectly conserved). All proteins are annotated as “uncharacterized.” XP_015641982.1 contains a common protein–protein interaction motif of about 100 aa, known as the BTB/POZ domain. The IRBB7 contig has a GC content of approximately 45%, and about 62% of the sequence matches transposable elements as determined by RepeatMasker (version open-4.0, http://www.repeatmasker.org) using the RITE database (Copetti et al., 2015).
Deletion of the Xa7 region
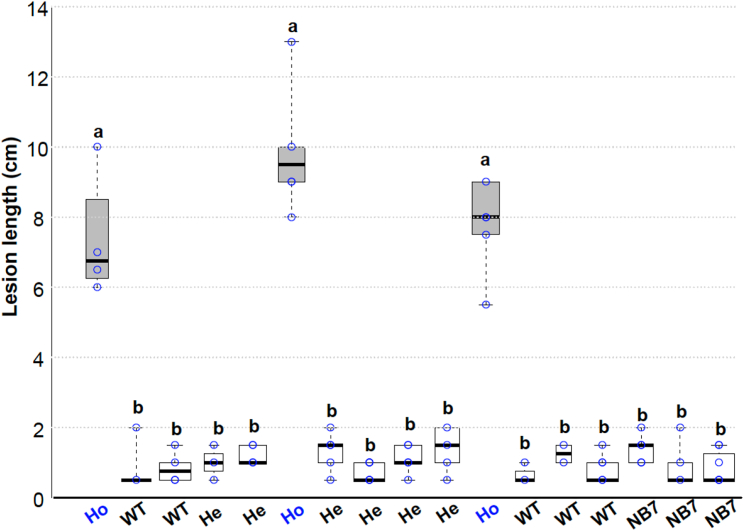
To further confirm the location of Xa7, a line named NB7, which was derived from an IRBB7 and Nipponbare cross and is resistant to PXO86, was used to delete 53 kb of the IRBB7 Xa7 region using CRISPR-Cas9 with two guide RNAs (Figure 1B, gRNA1 and gRNA2). NB7 is transformable due to its Nipponbare genetic background. PCR with deletion-specific and internal primers showed that one transgenic line, nb7-1, contained a large 53-kb deletion delimited by the two guide RNAs in one of its chromosomal copies (Supplemental Figure 1; see Supplemental Table 2 for oligonucleotide information). The nb7-1 line was resistant to disease after inoculation with Xoo strain PXO86, indicating that it retained a copy of Xa7 and was heterozygous for the deletion. Susceptibility was shown to co-segregate with the homozygous deletion in the T1 population (n = 5/24) using primers that could detect the wild-type and deleted regions (Figure 2).
Figure 2.
Disease phenotypes of progeny from the NB7 CRISPR T0 line.
Individual plants with genotypes homozygous (Ho) and heterozygous (He) for the 53-kb deletion and wild-type (WT) plants were inoculated with PXO86. Lesion lengths were measured 12 days after inoculation on three to five leaves of individual plants. Individual segregants were genotyped for the presence or absence of the 53-kb chromosomal fragment deletion by PCR. Ho, segregants homozygous for the 53-kb deletion; He, segregants heterozygous for the 53-kb deletion; WT, progeny homozygous for the WT genotype; NB7, the parent Xa7 isogenic line. Center lines show the medians; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend to the minimum and maximum values; box width is proportional to the square root of the sample size; and data points are plotted as open circles. n = 3 to 8 sample points. Treatments with the same lowercase letter are not significantly different at p < 0.05.
Gene expression from the Xa7 region
We hypothesized that Xa7 could be distinguished from the other annotated genes in the 53-kb region based on its TALe-mediated expression. RNA samples from IRBB7 infected with PXO86 and the avrXa7 mutant MX53 were subjected to RAMPAGE analysis, which combines RNA annotation and mapping of the respective promoters (Batut and Gingeras 2013; Raborn and Brendel 2019). The transcription start site (TSS)-adjacent sequences of transcribed genes in the unique 53-kb region were captured, and the RAMPAGE reads were used to project the transcript abundance of individual genes in the two treatments (with and without avrXa7). We used the Bioconductor TSRchitect package (Raborn et al., 2017) to identify transcription start regions (TSRs) in the contig, and the edgeR package (Robinson et al., 2010; McCarthy et al., 2012) to assess differential expression. Only one strongly induced TSR was found in the 53-kb region, in positions 63 494–63 513 (predominant TSS at 63 503; Figure 1), and it showed a 147-fold increase in expression after treatment with PXO86 relative to MX53 (Supplemental Figure 2). We refer to this transcript as R-Xa7 (Figure 1B, red arrow).
Sixty-eight base pairs downstream of the predominant R-Xa7 TSS site is an open reading frame (ORF) of 342 bp (including the stop codon) designated ORF113. A 726-bp cDNA, encompassing ORF113, a 68-bp 5′ UTR, and a 316-bp 3′ UTR, was isolated by screening a cDNA library derived from IRBB7 infected with PXO86 (Figure 1C, Supplemental Information 1). ORF113 was predicted to encode a small protein of 113 aa that showed no significant similarity to known R proteins in the database (Figure 1D). The gene from IRBB7 is hereafter tentatively referred to as Xa7.
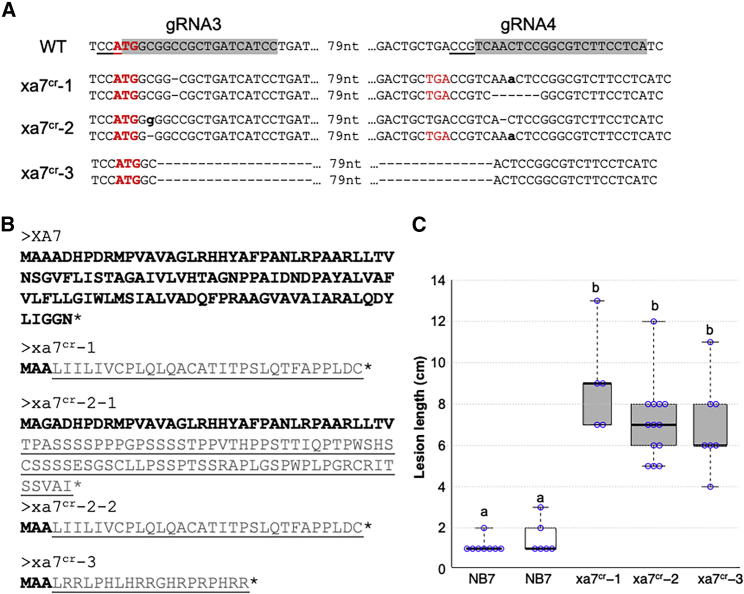
To corroborate its involvement in Xa7-mediated resistance, two sites in Xa7 were targeted by CRISPR in NB7. Two CRISPR target sites in Xa7 were chosen to construct two guide RNAs for the transformation of NB7 (Figure 3A, gRNA3 and gRNA4). Sequence analysis of 15 T0 transgenic plants revealed three independent T0 plants in which both alleles were knocked out: xa7cr-1, xa7cr-2, and xa7cr-3. The mutations (1-bp deletion) at the first guide RNA target in xa7cr-1 led to a premature stop codon; the mutations (79-bp deletion) in xa7cr-2 also led to a premature stop codon in Xa7; and the two alleles in xa7cr-3 also led to two null mutations in Xa7 (Figure 3A and 3B). All three altered lines were susceptible to PXO86, indicating that Xa7-mediated resistance to PXO86 requires functional Xa7 (Figure 3C).
Figure 3.
Disease phenotypes of Xa7 mutant lines.
(A) Genotypes of three CRISPR lines with deletions and insertions at two guide RNA target sites. Guide RNA target sequences are shaded, and the adjacent Cas9 PAM sequences are underlined. Start codons are in red and bold, and stop codons are in red. Inserted nucleotides are in bold lowercase letters, and dashed lines indicate deleted nucleotides.
(B) Genotypes of three CRISPR lines with Xa7 knocked out. Predicted amino acids encoded by different alleles are shown, with WT sequences in bold and new amino acids due to frameshift mutations underlined.
(C) The isogenic line NB7 and three CRISPR lines containing Xa7 knockout mutations were inoculated with PXO86. Lesion lengths were measured as for Figure 2. Treatments with the same lowercase letter are not significantly different at p < 0.05.
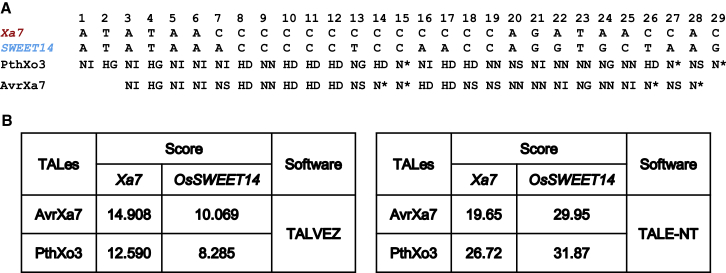
To identify the putative EBE for AvrXa7 in Xa7 (designated EBEAvrXA7), the sequence upstream of the Xa7 cDNA was analyzed by the EBE prediction programs TALVEZ and TALE-NT (Doyle et al., 2012; Booher and Bogdanove 2014). Both programs predicted consensus sequences of 26 nucleotides for AvrXa7 and 29 nucleotides for PthXo3 located 134–109 and 136–107 bp upstream of the Xa7 ATG that exhibited the DNA binding specificity predicted for the repeat regions of AvrXa7 and PthXo3, respectively (Figure 4A). The EBEs of Xa7 for AvrXa7 and PthXo3 are similar to the corresponding EBEs of SWEET14 (Figure 4A), and their predicted binding scores are comparable to those of the previously characterized overlapping SWEET14 EBEs for AvrXa7 and PthXo3 (Figure 4B) (Antony et al., 2010).
Figure 4.
AvrXa7 and PthXo3 are predicted to target two overlapping EBEs in Xa7.
(A) Individual RVDs of the AvrXa7 and PthXo3 central repeats match single nucleotides of the predicted EBE regions in the Xa7 promoter. Single letters are used for amino acids at the 12th and 13th positions of individual repeats. ∗ The missing amino acid at the 13th position of a particular repeat. The EBE regions for AvrXa7 and PthXo3 in the S gene SWEET14 are shown for comparison.
(B) The scores of matches between the DNA sequences of putative Xa7 and SWEET14 (as controls) and the repeats of AvrXa7 and PthXo3 as predicted by two programs (TALE-NT and TALVEZ). A lower score indicates a higher binding affinity between the RVDs and the target sequence.
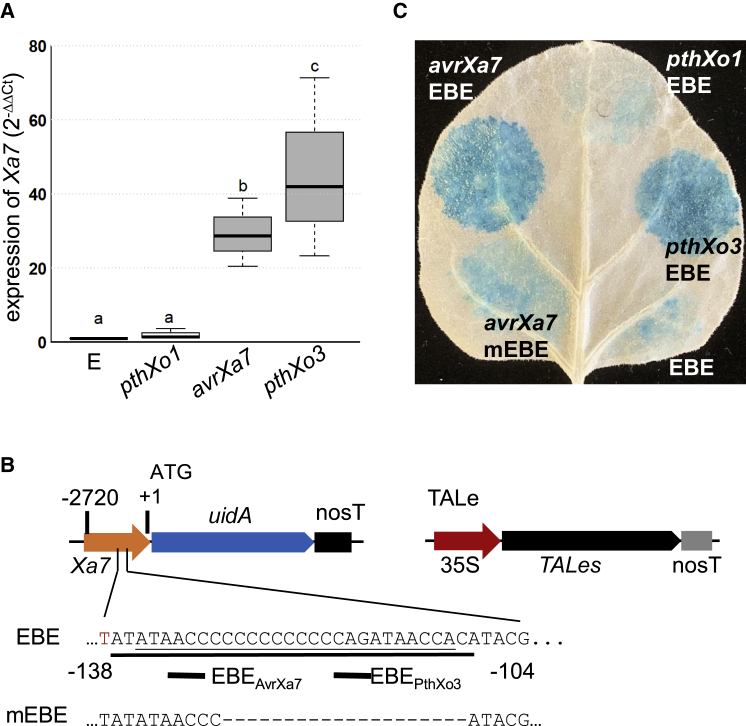
AvrXa7 and PthXo3 induce Xa7 expression in an EBEAvrXa7-dependent manner
To examine the expression of Xa7 in response to Xoo inoculation, qRT-PCR was performed using RNA extracted from inoculated rice leaf tissue and specific primers in Xa7 after inoculation with Xoo strains that varied in major TALe gene content. The strain ME2 is a mutant of PXO99A that has no major TALe gene (Yang and White 2004). Individual major TALe genes were introduced into ME2, and these strains were inoculated on IRBB7. Xa7 was induced after inoculation with ME2(avrXa7) and ME2(pthXo3) (Figure 5A). Expression was not observed after inoculation with ME2(pthXo1) or ME2, indicating that Xa7 induction is specific to AvrXa7 and PthXo3 (Figure 5A). TALe-dependent Xa7 promoter activity was assayed by transient expression using a β-glucuronidase (GUS) reporter in Nicotiana benthamiana. A 2.7-kb fragment upstream of the ATG of Xa7 was ligated with the GUS reporter gene (Figure 5B), and the construct (labeled EBE) was co-transferred with CaMV 35S-driven avrXa7 into N. benthamiana leaf cells by agroinfiltration (Figure 5C). Similarly, the reporter construct was co-delivered with pthXo1 or pthXo3. Sites inoculated with avrXa7 and pthXo3 displayed high GUS activity (Figure 5C), whereas sites inoculated with the GUS reporter construct (EBE), an empty construct, or 35S-driven pthXo1 displayed very low GUS activity. When a promoter containing a mutated EBE (labeled mEBE with a 20-bp deletion) was used, GUS activity with avrXa7 was reduced (Figure 5C).
Figure 5.
Transient assay of interactions between TALes and a reporter gene fused with the promoter elements of Xa7.
(A) Induction of the Xa7 candidate gene in IRBB7 by ME2 carrying different TALe genes as revealed by qRT-PCR.
(B) Constructs of the β-glucuronidase (GUS) gene fused with a promoter fragment (EBE) or a mutated element (mEBE) and of TALe genes under the 35S promoter.
(C) GUS staining of N. benthamiana leaf infiltrated with Agrobacterium strains containing different constructs from (A). The leaf was stained with X-Gluc for 1 h and destained with ethanol for 3 days.
Ectopic expression of Xa7 induces cell death in N. benthamiana
Although all the TALe-dependent executor R proteins of rice share some amino acid identities, it is unclear whether the proteins are phylogenetically related, with the exception of XA10 and XA23 (Figure 6A). Ectopic expression of Xa10 and Xa23 triggers cell death in N. benthamiana (Tian et al., 2014; Wang et al., 2015), but the effect of Xa27 and Xa7 is unknown. To investigate whether Xa7 can function in N. benthamiana, the 35S promoter (35S) was placed immediately upstream of the translation start codon of the Xa7, Xa10, Xa23, and Xa27 ORFs, and each construct was delivered into N. benthamiana leaves by agroinfection. A weak HR (hypersensitive response) for Xa7, Xa10, and Xa23 was visible at 16 h after infiltration, and HR was pronounced at 48 h after infiltration, although the degree of cell death induced by Xa7 appeared lower than that induced by Xa10 and Xa23 (Figure 6B). No visible HR occurred after the transient expression of Xa27 (Figure 6B).
Figure 6.
Transient expression of TALe-induced R genes in N. benthamiana.
(A) Alignment of predicted amino acids encoded by four executor R genes (Xa7, Xa27, Xa23, and Xa10). Conserved amino acids are shaded in blue. Transmembrane regions are underlined as predicted by TMpred (https://embnet.vital-it.ch/software/TMPRED_form.html). The NCBI COBALT multiple alignment tool was used. Sequences of XA10, XA23, and XA27 are from NCBI accessions AGE45112.1, AIX09985.1, and AYY54165.1, respectively.
(B) Cell death (rapid localized cell collapse within 48 h) in N. benthamiana leaves. The four genes were placed under the control of the 35S promoter and transiently expressed through agroinfiltration. Different time points are indicated above each panel.
Spectrum of Xa7 resistance against various Xoo strains
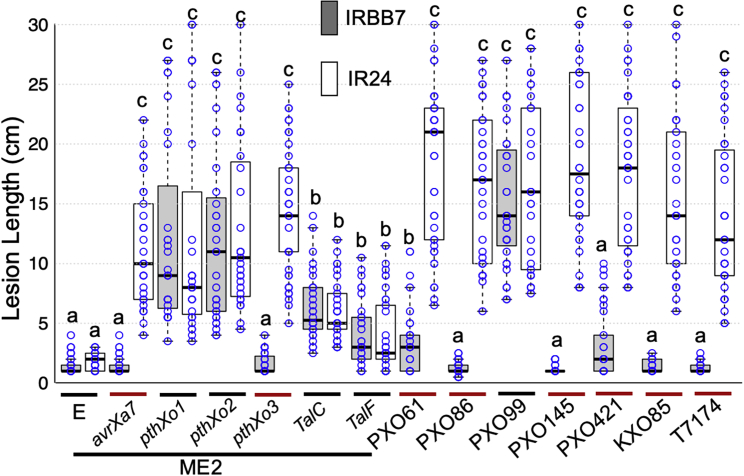
We next examined the spectrum of Xa7 resistance against all known major TALe genes and representative Xoo strains. Six major TALe genes (avrXa7, pthXo1, pthXo2, pthXo3, TalC, and TalF) that target three cognate SWEET-related S genes in rice were transferred into ME2. ME2 is not pathogenic in either IR24 or IRBB7 due to loss of the endogenous copy of pthXo1 and the resulting inability to induce any SWEET gene. Individual ME2 complementation strains carrying avrXa7 and pthXo3 caused susceptibility in IR24 and resistance in IRBB7, whereas the other four TALe genes in ME2 caused susceptibility in both IR24 and IRBB7, indicating that Xa7 is specific to avrXa7 and pthXo3 (Figure 7). Seven representative field isolates of Xoo are known to carry avrXa7 or pthXo3 or lack either gene (Oliva et al., 2019). Only isolates carrying avrXa7 or pthXo3 triggered resistance (Figure 7). ME2(pthXo3) resulted in an incompatible interaction, and PXO61, which contains pthXo3, was scored as moderately susceptible in comparison to the PXO99 and PXO86 reactions. IRBB7 has been scored as moderately susceptible to resistant to PXO61 in previous tests (Institute 2006).
Figure 7.
Xa7 confers resistance to Xoo strains containing avrXa7 or pthXo3.
Rice plants of IR24 (clear boxes) and IRBB7 (filled boxes) were inoculated with strain ME2 carrying different major TALe genes. Individual strains are identified on the basis of the major TALe gene. Field isolates are specified below individual graphs to the left of the ME2-derived strains. Center lines indicate the median lesion length; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend to the minimum and maximum values; box width is proportional to the square root of the sample size; and data points (n = 26–42) are plotted as open circles. Treatments with the same lowercase letter are not significantly different at p < 0.05.
Prevalence of Xa7 locus in other species and rice cultivars
Xa7 homologs have been identified in other species. Homologs exist in wild rice species (O. punctata and O. longistaminata), sorghum, Setaria, and panicgrasses (P. hallii and D. oligosanthes). The C termini of XA7 homologs are more conserved than the N termini (Supplemental Figure 3A and 3B). To determine whether representative genes could cause cell death in N. benthamiana, the coding sequences of six homologous genes were each constructed under the control of the 35S promoter and expressed ectopically by agroinfiltration of N. benthamiana (Supplemental Information 1). Among these constructs, only Ol_Xa7 resulted in an HR at 48 h. Op_Xa7 appeared to cause a weak cell death response compared with that of Xa7 (Supplemental Figure 3C). No obvious cell death phenotype was observed with the remaining homologs (Supplemental Figure 3C).
A total of 294 accessions from a scan of 3000 rice genome sequences contained Xa7 coding sequences (Supplemental Data 1). A 3171-bp region of the IRBB7 Xa7 locus, including 842 bp upstream and 2014 bp downstream of the Xa7 coding sequence, was used as the reference to assemble reads from these 294 accessions, generating 294 contigs that contained Xa7 and its flanking sequences. Due to the lower sequencing coverage of some accessions and the limitations of next generation sequencing technology to reveal 13 consecutive Cs, only nine contigs contained the Xa7 EBEs for AvrXa7 and PthXo3. The EBEs could not be unambiguously identified in the majority of contigs (Supplemental Data 1).
Among the 294 accessions, the majority (n = 185, 63%) are indica, 72 are japonica (24%), 22 are Aus/boro, 14 are Basmati/sadri, and seven are intermediate types (Supplemental Data 1). Geographically, India has the most accessions (n = 58), followed by China (n = 41), Bangladesh (n = 34), Indonesia (n = 26), the Philippines (n = 22), and Cambodia (n = 19) (Supplemental Data 1).
Discussion
Xa7 has mysteriously evaded cloning endeavors for the past 20 years. Here, multiple approaches provide evidence that our postulated candidate gene is indeed the cognate R gene for the TALe AvrXa7. Three critical observations are that (1) the gene lies within the region of markers previously associated with the Xa7 locus; (2) the deletion of the region and specific mutations in ORF113 eliminate Xa7-mediated resistance; and (3) the gene is expressed in an AvrXa7-dependent manner upon infection. That the AvrXa7-mediated expression of Xa7 is directed by a sequence-specific element in its promoter was corroborated by transient expression assays in N. benthamiana leaves. The GUS fusion was not expressed in the absence of AvrXa7 or another TALe (PthXo1) that has an alternate binding specificity. Disruption of the EBE by a 20-bp deletion also interfered with AvrXa7-dependent transient expression. The experiments also revealed that the TALe PthXo3 functions as an allele of AvrXa7 and directs Xa7-mediated resistance. The PthXo3-dependent activity of Xa7 explains its relatively broad activity against extant Asian strains of Xoo. The gene for PthXo3 was originally cloned from PXO61, a strain isolated in the Philippines. IRBB7 has been scored variously as moderately susceptible (or resistant) to resistant to PXO61 (Ogawa et al., 1991; Lee et al., 2003; Zhang et al., 2009; Xu et al., 2012). Here, we demonstrate that a variety of strains carrying the endogenous and transferred pthXo3 are associated with Xa7-mediated resistance. Genes for AvrXa7 and PthXo3 are found in many extant strains of Xoo (Oliva et al., 2019). Identification of Xa7 not only advances our understanding of molecular and genetic mechanisms of disease resistance but also facilitates the marker-assisted breeding of Xa7 into elite rice cultivars for broad resistance.
Cloning of Xa7 increases the number of executor R genes to four (Xa10, Xa23, Xa27, and Xa7). These genes form a group of unique R genes in rice and can be further divided into three subgroups with Xa10 and Xa23 in one group and Xa27 and Xa7 as single members of two additional groups. Xa27 has four Xa27-like genes (Os06g39810, Os06g07150, Os06g39800, and Os06g39860) in Nipponbare alone (Li et al., 2013). Xa10/Xa23 has two homologous genes (Os11g37620 and Os11g37570) in the Nipponbare genome (Wang et al., 2017). There is only one Xa7 homolog (Os11g26900) in the Nipponbare genome, and the two share 57% identity at the amino acid level. No evidence has been found to indicate that the TALe-induced R genes serve any purpose other than disease resistance. Conversely, the cognate TALe genes avrXa10 (for Xa10), avrXa23 (for Xa23), and avrXa27 (Xa27) lack a detectable virulence contribution to their respective Xoo isolates. AvrXa7 and PthXo3, on the other hand, are major virulence determinants for their respective Xoo isolates (Yang et al., 2000; Yang and White 2004). Inactivation of the corresponding genes, avrXa7 or pthXo3, renders these isolates almost nonpathogenic (Hopkins et al., 1992; Yang and White 2004). The alteration of such virulence TALes to evade recognition by cognate R genes imposes a physiological and ecological fitness penalty on Xoo strains due, in this case, to the loss of elevated SWEET14 expression. Xa7 is thus far unique in that its EBE mimics that of the S gene, guarding against pathogen exploitation of SWEET14 in healthy cells by triggering the death of infected cells that are injected with either of the two major TALes.
Several examples show that the transient overexpression of executor R genes in N. benthamiana can induce cell death, which may mimic the HR phenotype in the host plant/microbe interaction (Romer et al., 2009; Strauss et al., 2012; Tian et al., 2014; Wang et al., 2015; Wang et al., 2017; Wang et al., 2018). In this study, we compared the ability of four executor genes cloned from rice to induce cell death in N. benthamiana. The appearance of the HR showed that Xa7 has a moderate ability to induce cell death relative to Xa10 and Xa23, both of which trigger cell death much more rapidly and strongly. No visible cell death was observed in N. benthamiana when Xa27 was overexpressed (Figure 6B) (Tian et al., 2014). Xa27 was reported as an R gene that triggered a strong HR in response to AvrXa27 in rice (Gu et al., 2005), and Xa27-like genes activated by designer TALes that targeted the promoters of Xa27-like genes also mediated strong HR in rice (Li et al., 2013). Together with the observation of the diverse abilities of six Xa7 homologs to induce cell death in N. benthamiana, our results therefore show that the mechanism of cell death induction in N. benthamiana may differ from the HR triggered in rice by the corresponding avirulence TALe gene.
All four executor R genes in rice encode small proteins: XA27, XA23, and XA7 consist of 113 aa, and XA10 consists of 126 aa (Figure 6A). XA27, the first executor R gene identified, is predicted to contain two transmembrane domains (Gu et al., 2005). The N terminus of XA27 is also predicted to contain a signal anchor-like sequence, leading to the cellular localization of XA27-green fluorescent protein to the apoplast and wall of xylem cells. Alterations to the hydrophobic nature of the signal anchor-like sequence change the location and resistance activity of XA27 to an avirulent strain of Xoo (Wu et al., 2008). On the other hand, XA10 is predicted to contain four transmembrane domains and localizes in the endoplasmic reticulum (ER) (Tian et al., 2014). XA10 was found to be associated with ER Ca2+ depletion in plant and HeLa cells. Mutations that render the protein unable to deplete ER Ca2+ and to cause cell death in N. benthamiana concomitantly abolish Xa10-mediated resistance in rice (Tian et al., 2014). Similarly, Bs4C-R, induced by AvrBs4 for the resistance of pepper to Xanthomonas campestris pathovar vesicatoria, encodes a 164-aa protein of unknown function (Strauss et al., 2012). Bs4C-R is predicted to contain four transmembrane motifs, and a fluorescent fusion protein of BS4C-R was shown to localize in the ER membrane in N. benthamiana. BS4C-R causes cell death in N. benthamiana when ectopically expressed (Wang et al., 2018). Significantly, Xa10 promoter-Bs4C genes confer rice resistance to Xoo strains carrying avrXa10 (Wang et al., 2018). One exception to the small TALe-induced R proteins is Bs3 of pepper, which encodes 342 amino acids. Its gene product is homologous to the flavin-dependent monooxygenases (Römer et al., 2007), a group of enzymes that catalyze a wide range of chemo-, regio-, and enantio-selective oxygenation reactions (Huijbers et al., 2014). In this study, XA7 is predicted to possess two transmembrane domains (Figure 6A), but its cellular localization remains to be characterized. Genome editing, especially base editing that induces DNA substitutions (Anzalone et al., 2020), will provide a robust tool with which to dissect the structure-function requirements of XA7 in an endogenous context.
Methods
Plant materials, bacterial strains, medium, and growth conditions
The indica rice variety IR24, the recurrent near-isogenic line IRBB7 with R gene Xa7, and the japonica variety Nipponbare were kindly provided by the International Rice Research Institute and the U.S. National Small Grains Collection. The rice line NB7 was a segregant from a cross of IRBB7 and Nipponbare that exhibited Xa7-mediated resistance activity and the tissue culture trait of Nipponbare. N. benthamiana seeds were kindly provided by Dr. Gregory Martin. Xoo strains PXO86, PXO99A, and PXO99A mutant ME2 and transformants ME2(avrXa7), ME2(pthXo3), and ME2(pthXo1) were from the collection of the Yang laboratory.
All rice plants were grown in the greenhouse and growth chambers with a 12-h 30°C light period and a 12-h 28°C dark period at 60%–75% relative humidity. Escherichia coli strains were grown in Luria-Bertani medium supplemented with appropriate antibiotics at 37°C. Agrobacterium tumefaciens strains were grown at 30°C. All Xoo strains were grown at 28°C on TSA (10 g/l tryptone, 10 g/l sucrose, 1 g/l glutamic acid, 1.5% Difco agar). Antibiotics were used at the following concentrations if required: 100 μg/ml ampicillin, 10 μg/ml cephalexin, 25 μg/ml rifampin, 25 μg/ml kanamycin, and 100 μg/ml spectinomycin.
Disease assays
The leaf tip-clipping method was used to measure the lesion lengths of blight disease as described previously (Yang and Bogdanove 2013). In brief, an aliquot of the appropriate Xoo glycerol stock, stored at −80°C, was streaked onto TSA containing appropriate antibiotics and grown at 28°C for about 3 days. The bacterial cells were harvested from plates, suspended in sterile water, washed twice with water, and resuspended in water; the solution was adjusted to an optical density of 0.5 at 600 nm. Scissor blades were immersed in the Xoo suspension and used to clip the tip of a fully expanded leaf. The lesion lengths were measured at 14 days or at the specified days after inoculation. Three replicates with multiple leaves per replicate were examined for each Xoo strain. Data were plotted using BoxPlotR (http://shiny.chemgrid.org/boxplotr/). One-way analysis of variance was performed on all measurements. The Tukey honestly significant difference test was used for post-ANOVA pair-wise tests for significance (p<0.05).
CRISPR-Cas9-based gene editing in rice
The CRISPR-Cas9 system used to generate a large chromosomal deletion in the Xa7 locus and mutations within the Xa7 coding region was described previously (Zhou et al., 2014). Two guide RNA genes (gRNA1 and gRNA2) targeting two sites spanning the 53-kb Xa7 locus were constructed in the intermediate guide RNA vector pgRNA-1. The guide RNA gene cassette was mobilized into the Cas9 destination vector pBY02-Cas9-GW through the Gateway reaction using LR clonase (Thermo Fisher Scientific), resulting in pCas9-gRNA1+2. Similarly, two guide RNA genes targeting two sites (gRNA-3 and gRNA-4) in the Xa7 coding region were combined into pBY02-Cas9-GW, resulting in pCas9-gRNA3+4. Both constructs were transferred into the Xa7 isogenic line NB7 through the biolistic particle bombardment DNA delivery method. Rice tissue culture and regeneration were performed using methods described previously (Hiei et al., 1994). Genotyping the CRISPR plants from the T0 and T1 generations was performed by PCR amplification of relevant regions and Sanger sequencing of the amplicons.
Gene expression assays
For RAMPAGE experiments, young leaves of IRBB7 were inoculated with PXO86 and the avrXa7 knockout mutant MX53 (Hopkins et al., 1992). Total RNA was extracted using the TRIzol reagent (Thermo Fisher Scientific) 24 h after inoculation. Three replicates for each Xoo strain were used to construct RAMPAGE libraries for paired-end sequencing as described previously (Batut and Gingeras 2013; Raborn and Brendel 2019). In brief, total RNA was subjected to DNase I treatment, ribosomal RNA depletion, reverse complementary DNA synthesis with custom RAMPAGE-specific oligos, cap-trapping of the 5′-complete cDNA and RNA double-stranded DNA/RNA, streptavidin-based pull-down of the biotinylated DNA/RNA, PCR amplification and size selection of double-stranded DNA, and Illumina-based paired-end sequencing. Library quality was assessed using the Agilent 2200 TapeStation instrument (Agilent Technologies, Santa Clara, CA, USA) at the Indiana University Center for Genomics and Bioinformatics. All computational analyses are documented for reproducibility at https://github.com/BrendelGroup/AllRice following the guidelines proposed in Brendel (2018).
Transient TALe-specific Xa7 promoter activity in N. benthamiana
Xa7 promoter fusions to GUS reporter constructs were made using the 2.7-kb promoter region upstream of the Xa7 ATG after amplification with the oligos Pro2.7kHind-F and Xa7ATG-R from IRBB7 genomic DNA. The amplicon was cloned into pCAMBIA1305 at HindIII and NcoI through Gibson cloning (Gibson et al., 2009). To construct the Xa7 promoter-GUS reporter with a mutant AvrXa7 binding element, two fragments of the promoter were amplified with Pro2.7kHind-F and DelEBE-R3 and DelEBE-F3 and Xa7ATG-R from IRBB7 genomic DNA and inserted into pCAMBIA1305 at HindIII and NcoI. The constructs were transferred into the Agrobacterium strain EHA105. The TALe expression constructs were made by cloning the coding regions of avrXa7, pthXo3, and pthXo1 under the 35S promoter in pCAMBIA1300 at BamHI and SpeI sites. N. benthamiana plants were grown under 12 h of light and 12 h of darkness at 25°C and approximately 40%–60% relative humidity. Leaves of 4-week-old N. benthamiana plants were used for infiltration with a 1-ml needleless syringe. Agrobacterium strain EHA105 that harbored the construct of interest was cultured in Luria-Bertani medium containing 25 mg/l rifampin, 25 mg/l kanamycin, and 100 μM acetosyringone. The bacterial cells were collected through centrifugation and resuspended in Murashige and Skoog medium containing 100 μM acetosyringone, pH 5.8. The cell suspension was adjusted to an OD600 of 0.2 for infiltration. For co-inoculation, cells of two Agrobacterium strains were mixed in equal volume before infiltration into N. benthamiana leaves.
Sequencing and annotation of the Xa7 region
Genomic DNA of IRBB7 was extracted using the CTAB method (Porebski et al., 1997). Sequencing was conducted using long-read Oxford Nanopore and Illumina technologies. The assembler Flye (Kolmogorov et al., 2019) was used to create a de novo assembly of the IRBB7 genome. The resulting contigs were first corrected by re-mapping Nanopore reads and correcting with the medaka tool. A second correction was performed by mapping highly accurate Illumina reads using Bowtie 2 (Langmead and Salzberg 2012) and the Pilon correction tool (Walker et al., 2014). Gene structure annotation was based on spliced alignment of homologous proteins and transcripts using GenomeThreader (Gremme et al., 2005).
Survey of the Xa7 locus from 3000 rice genomes
The complete 3000 rice genome project (3K RGP) database was downloaded from http://gigadb.org/dataset/200001. The database contains ∼11 Tb of raw paired-end Illumina reads from 3010 diverse cultivated rice (Oryza sativa L.) accessions. Reads were mapped against the 4-kb region spanning the Xa7 locus using Bowtie 2 (Langmead and Salzberg 2012). The mapped reads were retrieved from 294 rice lines (including IRBB7), compressed as bam files, and sorted. A consensus fasta file of the Xa7 region from each rice line was created using SAMtools and BCFtools (Li et al., 2009; Li 2011). The process was performed using a customized Unix pipeline (Supplemental Information 2).
Funding
This work was partially supported by the United States Department of Agriculture National Institute of Agriculture and Food (2017-67013-26521 to B.Y.), the National Science Foundation (1238189 to F.F.W., V.P.B., and B.Y.; 1741090 to F.F.W.), and subawards to University of Missouri and University of Florida from the Heinrich Heine University Düsseldorf funded by the Bill & Melinda Gates Foundation [OPP1155704] (B.Y. and F.F.W.).
Author contributions
B.Y. and D.L. conceived the experiments. D.L. performed the experiments. J.C.H.-T. and F.F.W. conducted Nanopore sequencing of IRBB7, performed genome assembly, and analyzed the 3000 rice genomes for Xa7 prevalence. R.T.R. constructed and sequenced the RAMPAGE and RNA sequencing libraries. V.P.B. analyzed the RAMPAGE and RNA sequencing data. D.L. and B.Y. wrote the manuscript, and F.F.W. edited the manuscript with input from all other co-authors.
Acknowledgments
We are grateful to the US National Small Grains Collection (NSGC) and the International Rice Research Institute for providing rice accessions, and to Drs. Xingu Mao and Lifeng Zhao for help with Xa7 mapping experiments. No conflict of interest declared.
Published: January 19, 2021
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Accession numbers
The NCBI GenBank accession numbers are MW467883 for the Xa7 cDNA, MW561276 for the Xa7 contig, and GSE165583 for the RAMPAGE data. All computational analyses are documented for reproducibility at https://github.com/BrendelGroup/AllRice.
Supplemental information
References
- Antony G., Zhou J., Huang S., Li T., Liu B., White F., Yang B. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell. 2010;22:3864–3876. doi: 10.1105/tpc.110.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A.V., Koblan L.W., Liu D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020;38:824–844. doi: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- Bai J., Choi S.H., Ponciano G., Leung H., Leach J.E. Xanthomonas oryzae pv. oryzae avirulence genes contribute differently and specifically to pathogen aggressiveness. Mol. Plant Microbe Interact. 2000;13:1322–1329. doi: 10.1094/MPMI.2000.13.12.1322. [DOI] [PubMed] [Google Scholar]
- Batut P., Gingeras T.R. RAMPAGE: promoter activity profiling by paired-end sequencing of 5'-complete cDNAs. Curr. Protoc. Mol. Biol. 2013;104 doi: 10.1002/0471142727.mb25b11s104. Unit 25B.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher N.J., Bogdanove A.J. Tools for TAL effector design and target prediction. Methods. 2014;69:121–127. doi: 10.1016/j.ymeth.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V.P. From small RNA discoveries to a new paradigm in computational genomics? New Phytol. 2018;220:659–660. doi: 10.1111/nph.15478. [DOI] [PubMed] [Google Scholar]
- Chen S., Huang Z.G., Zeng L.X., Yang J.Y., Liu Q.G., Zhu X.Y. High-resolution mapping and gene prediction of Xanthomonas oryzae pv. oryzae resistance gene Xa7. Mol. Breed. 2008;22:433–441. [Google Scholar]
- Chu Z., Yuan M., Yao J., Ge X., Yuan B., Xu C., Li X., Fu B., Li Z., Bennetzen J.L. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006;20:1250–1255. doi: 10.1101/gad.1416306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D.E., Mesarich C.H., Thomma B.P. Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 2015;53:541–563. doi: 10.1146/annurev-phyto-080614-120114. [DOI] [PubMed] [Google Scholar]
- Copetti D., Zhang J., El Baidouri M., Gao D., Wang J., Barghini E., Cossu R.M., Angelova A., Maldonado L C.E., Roffler S. RiTE database: a resource database for genus-wide rice genomics and evolutionary biology. BMC Genomics. 2015;16:538. doi: 10.1186/s12864-015-1762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossa G.S., Quibod I., Atienza-Grande G., Oliva R., Maiss E., Vera Cruz C., Wydra K. Rice pyramided line IRBB67 (Xa4/Xa7) homeostasis under combined stress of high temperature and bacterial blight. Sci. Rep. 2020;10:683. doi: 10.1038/s41598-020-57499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle E.L., Booher N.J., Standage D.S., Voytas D.F., Brendel V.P., Vandyk J.K., Bogdanove A.J. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40:W117–W122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F., Zhou J.M. Plant-bacterial pathogen interactions mediated by type III effectors. Curr. Opin. Plant Biol. 2012;15:469–476. doi: 10.1016/j.pbi.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A., Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Gremme G., Brendel V., Sparks M.E., Kurtz S. Engineering a software tool for gene structure prediction in higher organisms. Inf. Softw. Technol. 2005;47:965–978. [Google Scholar]
- Gu K., Yang B., Tian D., Wu L., Wang D., Sreekala C., Yang F., Chu Z., Wang G.L., White F.F. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature. 2005;435:1122–1125. doi: 10.1038/nature03630. [DOI] [PubMed] [Google Scholar]
- Hiei Y., Ohta S., Komari T., Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- Hopkins C.M., White F.F., Choi S.H., Guo A., Leach J.E. Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 1992;5:451–459. doi: 10.1094/mpmi-5-451. [DOI] [PubMed] [Google Scholar]
- Hsu Y.C., Chiu C.H., Yap R., Tseng Y.C., Wu Y.P. Pyramiding bacterial blight resistance genes in Tainung82 for broad-spectrum resistance using marker-assisted selection. Int. J. Mol. Sci. 2020;21:1281. doi: 10.3390/ijms21041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Cao J., Zhang J., Xia F., Ke Y., Zhang H., Xie W., Liu H., Cui Y., Cao Y. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants. 2017;3:17009. doi: 10.1038/nplants.2017.9. [DOI] [PubMed] [Google Scholar]
- Huijbers M.M., Montersino S., Westphal A.H., Tischler D., van Berkel W.J. Flavin dependent monooxygenases. Arch. Biochem. Biophys. 2014;544:2–17. doi: 10.1016/j.abb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Institute I.R.R. Breeding for disease resistance in rice: bacterial blight. 2006. http://www.knowledgebank.irri.org/ricebreedingcourse/Breeding_for_disease_resistance_Blight.htm
- Iyer A.S., McCouch S.R. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant Microbe Interact. 2004;17:1348–1354. doi: 10.1094/MPMI.2004.17.12.1348. [DOI] [PubMed] [Google Scholar]
- Jackson R.W., Athanassopoulos E., Tsiamis G., Mansfield J.W., Sesma A., Arnold D.L., Gibbon M.J., Murillo J., Taylor J.D., Vivian A. Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola. Proc. Natl. Acad. Sci. U S A. 1999;96:10875–10880. doi: 10.1073/pnas.96.19.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C., Ji Z., Liu B., Cheng H., Liu H., Liu S., Yang B., Chen G. Xa1 Allelic R genes activate rice blight resistance suppressed by interfering TAL effectors. Plant Commun. 2020;1:100087. doi: 10.1016/j.xplc.2020.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kaji K., Ogawa T. Identification of the located chromosome of the resistance gene. Xa-7 to bacterial leaf blight in rice. Breed. Sci. 1995;45:79. [Google Scholar]
- Kolmogorov M., Yuan J., Lin Y., Pevzner P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J.E., White F.F. Bacterial avirulence genes. Annu. Rev. Phytopathol. 1996;34:153–179. doi: 10.1146/annurev.phyto.34.1.153. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Rasabandith S., Angeles E.R., Khush G.S. Inheritance of resistance to bacterial blight in 21 cultivars of rice. Phytopathology. 2003;93:147–152. doi: 10.1094/PHYTO.2003.93.2.147. [DOI] [PubMed] [Google Scholar]
- Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., Subgroup G.P.D.P. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Huang S., Zhou J., Yang B. Designer TAL effectors induce disease susceptibility and resistance to Xanthomonas oryzae pv. oryzae in rice. Mol. Plant. 2013;6:781–789. doi: 10.1093/mp/sst034. [DOI] [PubMed] [Google Scholar]
- Liu Q., Yuan M., Zhou Y., Li X., Xiao J., Wang S. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ. 2011;34:1958–1969. doi: 10.1111/j.1365-3040.2011.02391.x. [DOI] [PubMed] [Google Scholar]
- McCarthy D.J., Chen Y., Smyth G.K. Differential expression analysis of multifactor RNA-seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro F., Nishimura M.T. Structural, functional, and genomic diversity of plant NLR proteins: an evolved resource for rational engineering of plant immunity. Annu. Rev. Phytopathol. 2018;56:243–267. doi: 10.1146/annurev-phyto-080417-045817. [DOI] [PubMed] [Google Scholar]
- Niño-Liu D.O., Ronald P.C., Bogdanove A.J. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 2006;7:303–324. doi: 10.1111/j.1364-3703.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Oerke E.C. Crop losses to pests. J. Agric. Sci. 2006;144:13. [Google Scholar]
- Ogawa T., Yamaoto T., Khush G.S., Mew T.-W. Breeding of near-isogenic lines of rice with single genes for resistance to bacterial blight pathogen (Xanthomonas campestris pv. oryzae) Japan. J. Breed. 1991;41:523–529. [Google Scholar]
- Oliva R., Ji C., Atienza-Grande G., Huguet-Tapia J.C., Perez-Quintero A., Li T., Eom J.S., Li C., Nguyen H., Liu B. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019;37:1344–1350. doi: 10.1038/s41587-019-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porebski S., Bailey L.G., Baum B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997;15:8. [Google Scholar]
- Porter B.W., Chittoor J.M., Yano M., Sasaki T., White F.F. Development and mapping of markers linked to the rice bacterial blight resistance gene Xa7. Crop Sci. 2003;43:1484–1492. [Google Scholar]
- Raborn R.T., Brendel V.P. Using RAMPAGE to identify and annotate promoters in insect genomes. Methods Mol. Biol. 2019;1858:99–116. doi: 10.1007/978-1-4939-8775-7_9. [DOI] [PubMed] [Google Scholar]
- Raborn RT, Sridharan K, Brendel VP (2017). TSRchitect: Promoter identification from large-scale TSS profiling data. 10.18129/B9.bioc.TSRchitect. [DOI]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer P., Recht S., Lahaye T. A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens. Proc. Natl. Acad. Sci. U S A. 2009;106:20526–20531. doi: 10.1073/pnas.0908812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer P., Hahn S., Jordan T., Strauss T., Bonas U., Lahaye T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318:645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- Römer P., Recht S., Lahaye T. A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens. Proc. Natl. Acad. Sci. U S A. 2009;106:20526–20531. doi: 10.1073/pnas.0908812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- Song W.Y., Wang G.L., Chen L.L., Kim H.S., Pi L.Y., Holsten T., Gardner J., Wang B., Zhai W.X., Zhu L.H. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Spoel S.H., Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012;12:89–100. doi: 10.1038/nri3141. [DOI] [PubMed] [Google Scholar]
- Strauss T., van Poecke R., Strauss A., Romer P., Minsavage G., Singh S., Wolf C., Kim S., Lee H., Yeom S. RNA-seq pinpoints a Xanthomonas TAL-effector activated resistance gene in a large-crop genome. Proc. Natl. Acad. Sci. U S A. 2012;109:19480–19485. doi: 10.1073/pnas.1212415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Cao Y., Yang Z., Xu C., Li X., Wang S., Zhang Q. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 2004;37:517–527. doi: 10.1046/j.1365-313x.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Tian D., Wang J., Zeng X., Gu K., Qiu C., Yang X., Zhou Z., Goh M., Luo Y., Murata-Hori M. The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell. 2014;26:497–515. doi: 10.1105/tpc.113.119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B.J., Abeel T., Shea T., Priest M., Abouelliel A., Sakthikumar S., Cuomo C.A., Zeng Q., Wortman J., Young S.K. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhang X., Fan Y., Gao Y., Zhu Q., Zheng C., Qin T., Li Y., Che J., Zhang M. XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Mol. Plant. 2015;8:290–302. doi: 10.1016/j.molp.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Wang J., Tian D., Gu K., Yang X., Wang L., Zeng X., Yin Z. Induction of Xa10-like genes in rice cultivar nipponbare confers disease resistance to rice bacterial blight. Mol. Plant Microbe Interact. 2017;30:466–477. doi: 10.1094/MPMI-11-16-0229-R. [DOI] [PubMed] [Google Scholar]
- Wang J., Zeng X., Tian D., Yang X., Wang L., Yin Z. The pepper Bs4C proteins are localized to the endoplasmic reticulum (ER) membrane and confer disease resistance to bacterial blight in transgenic rice. Mol. Plant Pathol. 2018;19:2025–2035. doi: 10.1111/mpp.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb K.M., Oña I., Bai J., Garrett K.A., Mew T., Vera Cruz C.M., Leach J.E. A benefit of high temperature: increased effectiveness of a rice bacterial blight disease resistance gene. New Phytol. 2010;185:568–576. doi: 10.1111/j.1469-8137.2009.03076.x. [DOI] [PubMed] [Google Scholar]
- Wu L., Goh M.L., Sreekala C., Yin Z. XA27 depends on an amino-terminal signal-anchor-like sequence to localize to the apoplast for resistance to Xanthomonas oryzae pv. oryzae. Plant Physiol. 2008;148:1497–1509. doi: 10.1104/pp.108.123356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Cao Y., Xu C., Li X., Wang S. Xa3, conferring resistance for rice bacterial blight and encoding a receptor kinase-like protein, is the same as Xa26. Theor. Appl. Genet. 2006;113:1347–1355. doi: 10.1007/s00122-006-0388-x. [DOI] [PubMed] [Google Scholar]
- Xu J., Jiang J., Dong X., Ali J., Mou T. Introgression of bacterial blight (BB) resistance genes Xa7 and Xa21 into popular restorer line and their hybrids by molecular marker-assisted backcross (MABC) selection scheme. Afr. J. Biotechnol. 2012;11:9. [Google Scholar]
- Yang B., Bogdanove A. Inoculation and virulence assay for bacterial blight and bacterial leaf streak of rice. Methods Mol. Biol. 2013;956:249–255. doi: 10.1007/978-1-62703-194-3_18. [DOI] [PubMed] [Google Scholar]
- Yang B., White F.F. Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol. Plant Microbe Interact. 2004;17:1192–1200. doi: 10.1094/MPMI.2004.17.11.1192. [DOI] [PubMed] [Google Scholar]
- Yang B., Zhu W., Johnson L., White F. The virulence factor AvrXa7 of Xanthomonas oryzae pv. oryzae is a type III secretion pathway-dependent nuclear-localized double-stranded DNA-binding protein. Proc. Natl. Acad. Sci. U S A. 2000;97:9807–9812. doi: 10.1073/pnas.170286897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Yamanouchi U., Katayose Y., Toki S., Wang Z.X., Kono I., Kurata N., Yano M., Iwata N., Sasaki T. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. U S A. 1998;95:1663–1668. doi: 10.1073/pnas.95.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhang H., Li F., Ouyang Y., Yuan M., Li X., Xiao J., Wang S. Multiple alleles encoding atypical NLRs with unique central tandem repeats in rice confer resistance to Xanthomonas oryzae pv. oryzae. Plant Commun. 2020;1:100088. doi: 10.1016/j.xplc.2020.100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.C., Wang J.F., Pan J.W., Gu Z.M., Chen X.F., Jin Y., Liu F., Zhang H.S., Ma B.J. Identification and molecular mapping of the rice bacterial blight resistance gene allelic to Xa7 from an elite restorer line Zhenhui 084. Eur. J. Plant Pathol. 2009;125:235–244. [Google Scholar]
- Zhou H., Liu B., Weeks D.P., Spalding M.H., Yang B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 2014;42:10903–10914. doi: 10.1093/nar/gku806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.