This cross-sectional study describes the development of dementia and the level of cognitive decline among individuals with varying socioeconomic backgrounds who underwent a diagnostic evaluation for dementia.
Key Points
Question
Is socioeconomic status, as defined by household income, associated with dementia diagnosis and cognitive severity at diagnosis?
Findings
In this cross-sectional study of 10 191 individuals in Denmark with a first-time referral for diagnostic evaluation for dementia, those with a household income in the upper tertile were less likely to receive a dementia diagnosis after referral and had a less severe cognitive stage at diagnosis compared with individuals with a household income in the middle and lower tertiles.
Meaning
Findings from this study suggest that, in Denmark, affluent individuals may have the advantage of receiving earlier dementia diagnosis.
Abstract
Importance
Low socioeconomic status (SES) has been identified as a risk factor for the development of dementia. However, few studies have focused on the association between SES and dementia diagnostic evaluation on a population level.
Objective
To investigate whether household income (HHI) is associated with dementia diagnosis and cognitive severity at the time of diagnosis.
Design, Setting, and Participants
This population- and register-based cross-sectional study analyzed health, social, and economic data obtained from various Danish national registers. The study population comprised individuals who received a first-time referral for a diagnostic evaluation for dementia to the secondary health care sector of Denmark between January 1, 2017, and December 17, 2018. Dementia-related health data were retrieved from the Danish Quality Database for Dementia. Data analysis was conducted from October 2019 to December 2020.
Exposures
Annual HHI (used as a proxy for SES) for 2015 and 2016 was obtained from Statistics Denmark and categorized into upper, middle, and lower tertiles within 5-year interval age groups.
Main Outcomes and Measures
Dementia diagnoses (Alzheimer disease, vascular dementia, mixed dementia, dementia with Lewy bodies, Parkinson disease dementia, or other) and cognitive stages at diagnosis (cognitively intact; mild cognitive impairment but not dementia; or mild, moderate, or severe dementia) were retrieved from the database. Univariable and multivariable logistic and linear regressions adjusted for age group, sex, region of residence, household type, period (2017 and 2018), medication type, and medical conditions were analyzed for a possible association between HHI and receipt of dementia diagnosis.
Results
Among the 10 191 individuals (mean [SD] age, 75 [10] years; 5476 women [53.7%]) included in the study, 8844 (86.8%) were diagnosed with dementia. Individuals with HHI in the upper tertile compared with those with lower-tertile HHI were less likely to receive a dementia diagnosis after referral (odds ratio, 0.65; 95% CI, 0.55-0.78) and, if diagnosed with dementia, had less severe cognitive stage (β, −0.16; 95% CI, −0.21 to −0.10). Individuals with middle-tertile HHI did not significantly differ from those with lower-tertile HHI in terms of dementia diagnosis (odds ratio, 0.92; 95% CI, 0.77-1.09) and cognitive stage at diagnosis (β, 0.01; 95% CI, −0.04 to 0.06).
Conclusions and Relevance
The results of this study revealed a social inequality in dementia diagnostic evaluation: in Denmark, people with higher income seem to receive an earlier diagnosis. Public health strategies should target people with lower SES for earlier dementia detection and intervention.
Introduction
Dementia is a medical condition characterized by cognitive functional decline that affects daily life and social activities.1 Approximately 50 million people worldwide live with dementia, and this number is expected to triple by 2050 with the aging of the population presenting a substantial challenge to patients, families, and society.2
Several risk factors for the development of dementia have been identified, including biological, lifestyle, environmental, and pathological factors associated with certain medical conditions and diseases.3,4,5,6,7 Socioeconomic status (SES), commonly measured by educational level, income level, and occupation, has been recognized as a risk factor for dementia and dementia-related death given that low SES was found to be associated with an increased risk for both.8,9,10,11,12,13
Aside from studies on the risk of developing dementia, few studies have focused on SES and its association with dementia diagnosis and cognitive severity at diagnosis. Findings of such studies generally showed that lower SES was associated with a higher risk of receiving a dementia diagnosis and lower cognitive function at the time of diagnosis.14,15,16 Although SES measures in these previous studies varied, the limitations in data collection and sample size in these studies as well as the discrepancies in health care access (eg, using free and universal or fee-based insurance) in Denmark compared to these countries may result in differences in association estimation. Moreover, most studies on SES have focused primarily on education and occupation as measures; however, household income (HHI) and wealth may be better indicators of SES for examining the health outcomes of older people who are retired or close to retirement, especially because education is typically pursued early in life.12,17,18,19 However, to our knowledge, no register-based nationwide study has been conducted on HHI and its association with the diagnostic evaluation for dementia.
Denmark offers universal, free health care services to all citizens regardless of their social or economic position.20 Danish national registries record health, social, and economic data and can be linked at the individual level.21 Herein, we conducted a nationwide study of individuals in Denmark who had a referral for a first-time diagnostic evaluation for dementia in 2017 to 2018. Our objective was to investigate whether HHI is associated with dementia diagnosis and cognitive severity at time of diagnosis.
Methods
Design, Data Sources, and Population
This population- and register-based cross-sectional study used data from Danish national registers. In compliance with European data protection rules, the University of Southern Denmark registered this project. According to Danish law, review by an ethics board and patient informed consent are not required for purely register-based studies. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.22
Dementia-related health data, including diagnosis and cognitive stage at diagnosis, were retrieved from the Danish Quality Database for Dementia (DANDEM).23 The purpose of DANDEM is to improve and monitor the quality of diagnosis for all persons with a referral for elective dementia assessments to the Danish secondary health care sector, including memory clinics and dementia assessment units. DANDEM was established in January 2016, and the first year of data collection was considered as a trial period. Using the unique civil registration number assigned to Danish citizens at birth and to persons with a Danish residence permit on immigration, we linked individuals’ health data in DANDEM with HHI and household type from Statistics Denmark,24 demographic characteristics (age, sex, region of residency, and vital status, such as date of death) from the Danish Civil Registration System,25 educational level from the Population Education Register,26 history of medical conditions from the Danish National Patient Registry,27 and history of medications from the Danish National Prescription Registry.28
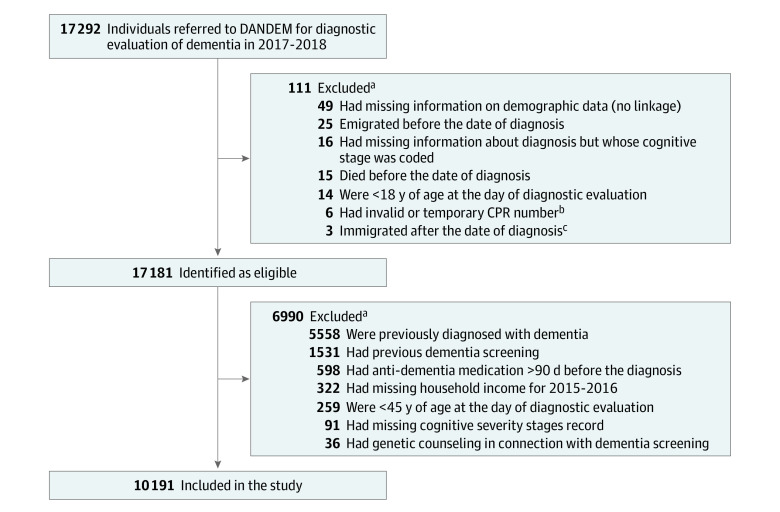
The study population was selected from 17 292 individuals with a referral to memory clinics or dementia assessment units across all regions of Denmark for a dementia diagnostic evaluation between January 1, 2017, and December 17, 2018. For this analysis, we included only individuals aged 45 years or older with a first-time referral for dementia evaluation and for whom complete data were registered. Complete data included dementia diagnosis, cognitive severity stage, and HHI during the study period (Figure). Data analysis was conducted from October 2019 to December 2020.
Figure. Flowchart of Population Selection .
aSome individuals satisfied more than 1 exclusion criteria.
bTemporary civil registration number (CPR) refers to the identification number recorded in the Danish Quality Database for Dementia (DANDEM) that was a mix of letters and numbers.
cData were missing before the date of diagnosis.
Assessment of Household Income, Dementia Diagnosis, and Cognitive Stage at Diagnosis
Annual HHI registered by Statistics Denmark is based on equivalized disposable income for the household.29 We used HHI as a proxy for SES. To avoid an illness factor in income, we retrieved HHI at 2 years before the dementia evaluation: 2015 HHI data were used for people with a referral for dementia evaluation in 2017, and 2016 HHI data were used for those with a referral in 2018. We categorized individuals according to income tertiles (lower, medium, or upper) within age groups. We used a different method to define personal income to investigate the referral rate (eMethods in the Supplement).
The date of dementia diagnosis, whether a dementia diagnosis was received (yes or no), type of dementia, and cognitive stage at diagnosis were retrieved from DANDEM. The latter 3 data points followed the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision dementia diagnostic criteria.30 The types of dementia diagnosis in DANDEM are recorded as follows: Alzheimer disease, vascular dementia, mixed dementia (Alzheimer disease and vascular dementia), dementia with Lewy bodies, Parkinson disease dementia, or other types. Stages of cognitive severity in DANDEM are graded as follows: cognitively intact, mild cognitive impairment but not dementia, mild dementia, moderate dementia, or severe dementia. For the purposes of analysis, we extracted these stages and assigned a level to each stage as follows: level 1 was cognitively intact, level 2 was mild cognitive impairment but not dementia, level 3 was mild dementia, level 4 was moderate dementia, and level 5 was severe dementia.
Assessment of Other Covariates
Based on previous findings,31,32,33,34,35 we included the baseline covariates of age, sex, household type, length of education (in years), region of residence, medication type, and medical conditions. We divided individuals into 6 age groups within 5-year intervals (<65, 65-69, 70-74, 75-79, 80-84, or ≥85 years). Household type was either living alone or living with someone. In accordance with the highest educational level recorded in the Population Education Register,26 we used the following categories for length of education: short term (≤10 years), medium term (11-15 years), or long term (>15 years).
Using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes, we extracted from the Danish National Patient Registry each individual’s medical conditions for 10 years before the index date.27 Fourteen medical conditions were identified: type 2 diabetes, chronic obstructive pulmonary disease, ischemic heart disease, depression, hypertension, stroke, atrial fibrillation, cancer, fractures, peripheral vascular disease, hemorrhage, cerebrovascular disease, kidney disease, and rheumatic disease. These medical conditions have been found to be associated with dementia and household economics.36,37,38,39,40,41 Based on the presence of these medical conditions during the 10-year period, we grouped individuals according to the number of medical conditions they had (0, 1, 2, 3, or ≥4) in any combination.
We assessed 5 types of prescription medications registered in the Danish National Prescription Registry at 1 year before the index date28: antipsychotics, antianxiety, hypnotics and sedatives, antidepressants, and opioids. Because of the behavioral and psychological symptoms of dementia, these medicines are frequently prescribed to individuals with dementia.42,43,44
Statistical Analysis
The frequency of the sample’s baseline characteristics as well as the dementia diagnosis and cognitive stage at diagnosis were described by HHI levels. We applied 4 logistic regression models to estimate the association between HHI (with the lower-tertile HHI being the reference group) and receipt of dementia diagnosis (yes or no). Model 1 was the crude analysis of HHI for dementia diagnosis. Model 2 started with model 1 and was adjusted for confounders (age group, sex, region of residence, household type, and year 2017 and 2018). Model 3 used model 2 as the basis and was adjusted for the 5 selected medication types. Model 4 was model 3 but adjusted for the 14 medical conditions and the number of medical conditions. Odds ratios (ORs) with 95% CIs were reported. We conducted supplementary analyses of the equality of estimation coefficients for HHI groups between model 1 and model 4, and we looked into the misclassification errors using model 4 as the sample data with and without HHI.
We applied linear regression models for estimating the association between HHI and cognitive severity stage at diagnosis in the crude (univariable) and multivariable models, adjusting for covariates in the 4 models. Coefficient (β) estimates with 95% CI were reported. All analyses were performed using Stata, version 16 (StataCorp LLC). A 2-tailed P < .05 was considered to be statistically significant.
Results
Of 17 292 individuals with a first-time referral for diagnostic evaluation for dementia in 2017 to 2018, a total of 10 191 individuals (mean [SD] age, 75 [10] years; 5476 women [53.7%] and 4715 men [46.3%]) were eligible for analysis (Figure and Table 1). The number of individuals with HHI in the lower, middle, and upper tertiles was similar across age groups. However, families with higher SES had the lowest proportion of referrals for diagnostic evaluation for dementia (eTable 1 in the Supplement). Overall, individuals with HHI in the lower tertile vs the upper tertile were more likely to be women (2048 [60.3%] vs 1572 [46.3%]), to have a lower educational level (short term: 1716 [50.5%] vs 511 [15.1%]), to live alone (2093 [61.6%] vs 1121 [33.0%]), and to have multiple medical conditions (≥4: 717 [21.1%] vs 606 [17.9%]) (Table 1).
Table 1. Characteristics of Individuals With a First-Time Referral for Dementia Diagnostic Evaluationa.
| Variable | No. (%) | |||
|---|---|---|---|---|
| All (N = 10 191) | Household incomeb | |||
| Lower tertile (n = 3399) | Middle tertile (n = 3398) | Upper tertile (n = 3394) | ||
| Age, mean (SD), y | 75 (10) | 75 (10) | 75 (10) | 75 (10) |
| Age group, y | ||||
| <65 | 1523 (14.9) | 508 (14.9) | 508 (14.9) | 507 (14.9) |
| 65-69 | 987 (9.7) | 329 (9.7) | 329 (9.7) | 329 (9.7) |
| 70-74 | 1804 (17.7) | 602 (17.7) | 601 (17.7) | 601 (17.7) |
| 75-79 | 2075 (20.4) | 692 (20.4) | 692 (20.4) | 691 (20.4) |
| 80-84 | 2057 (20.2) | 686 (20.2) | 686 (20.2) | 685 (20.2) |
| ≥85 | 1745 (17.1) | 582 (17.1) | 582 (17.1) | 581 (17.1) |
| Sex | ||||
| Male | 4715 (46.3) | 1351 (39.7) | 1542 (45.4) | 1822 (53.7) |
| Female | 5476 (53.7) | 2048 (60.3) | 1856 (54.6) | 1572 (46.3) |
| Period | ||||
| 2017 | 4302 (42.2) | 1534 (45.1) | 1373 (40.4) | 1395 (41.1) |
| 2018 | 5889 (57.8) | 1865 (54.9) | 2025 (59.6) | 1999 (58.9) |
| Length of education | ||||
| Short term (≤10 y) | 3555 (34.9) | 1716 (50.5) | 1328 (39.1) | 511 (15.1) |
| Medium term (11-15 y) | 4685 (46.0) | 1411 (41.5) | 1641 (48.3) | 1633 (48.1) |
| Long term (>15 y) | 1718 (16.9) | 166 (4.9) | 361 (10.6) | 1191 (35.1) |
| Unknown or missing data | 233 (2.3) | 106 (3.1) | 68 (2.0) | 59 (1.7) |
| Household type | ||||
| Living alone | 4914 (47.2) | 2093 (61.6) | 1600 (47.1) | 1121 (33.0) |
| Living with someone | 5377 (52.8) | 1306 (38.4) | 1798 (52.9) | 2273 (67.0) |
| Region of residence | ||||
| Region of Northern Denmark | 648 (6.4) | 268 (7.9) | 203 (6.0) | 177 (5.2) |
| Central Denmark Region | 2025 (19.9) | 647 (19.0) | 716 (21.1) | 662 (19.5) |
| Region of Southern Denmark | 2990 (29.3) | 1204 (35.4) | 1042 (30.7) | 744 (21.9) |
| Capital Region of Denmark | 3328 (32.7) | 904 (26.6) | 1000 (29.4) | 1424 (42.0) |
| Region Zealand | 1200 (11.8) | 376 (11.1) | 437 (12.9) | 387 (11.4) |
| Medical conditions | ||||
| COPD | 1106 (10.9) | 433 (12.7) | 401 (11.8) | 272 (8.0) |
| Type 2 diabetes | 1300 (12.8) | 513 (15.1) | 439 (12.9) | 348 (10.3) |
| Cancer | 1168 (11.5) | 352 (10.4) | 400 (11.8) | 416 (12.3) |
| Hypertension | 3650 (35.8) | 1328 (39.1) | 1232 (36.3) | 1090 (32.1) |
| Depression | 1172 (11.5) | 438 (12.9) | 408 (12.0) | 326 (9.6) |
| Fractures | 5215 (51.2) | 1699 (50.0) | 1752 (51.6) | 1764 (52.0) |
| Stroke | 961 (9.4) | 324 (9.5) | 325 (9.6) | 312 (9.2) |
| Ischemic heart condition | 1511 (14.8) | 531 (15.6) | 524 (15.4) | 456 (13.4) |
| Atrial fibrillation | 1447 (14.2) | 493 (14.5) | 483 (14.2) | 471 (13.9) |
| Peripheral vascular disease | 479 (4.7) | 180 (5.3) | 156 (4.6) | 143 (4.2) |
| Hemorrhage | 1102 (10.8) | 379 (11.2) | 358 (10.5) | 365 (10.8) |
| Cerebrovascular disease | 1748 (17.2) | 590 (17.4) | 594 (17.5) | 564 (16.6) |
| Kidney disease | 342 (3.4) | 137 (4.0) | 109 (3.2) | 96 (2.8) |
| Rheumatic disease | 369 (3.6) | 121 (3.6) | 127 (3.7) | 121 (3.6) |
| No. of medical conditions | ||||
| 0 | 1727 (16.9) | 545 (16.0) | 558 (16.4) | 624 (18.4) |
| 1 | 2722 (26.7) | 841 (24.7) | 891 (26.2) | 990 (29.2) |
| 2 | 2116 (20.8) | 745 (21.9) | 694 (20.4) | 677 (19.9) |
| 3 | 1528 (15.0) | 533 (15.7) | 517 (15.2) | 478 (14.1) |
| ≥4 | 2036 (20.0) | 717 (21.1) | 713 (21.0) | 606 (17.9) |
| Unknown or missing data | 62 (0.6) | 18 (0.5) | 25 (0.7) | 19 (0.6) |
| Medication type | ||||
| Antipsychotics | 602 (5.9) | 244 (7.2) | 203 (6.0) | 155 (4.6) |
| Antianxiety | 502 (4.9) | 200 (5.9) | 177 (5.2) | 125 (3.7) |
| Hypnotics and sedatives | 972 (9.5) | 315 (9.3) | 331 (9.7) | 326 (9.6) |
| Antidepressants | 2489 (24.4) | 895 (26.3) | 878 (25.8) | 716 (21.1) |
| Opioids | 1439 (14.1) | 536 (15.8) | 513 (15.1) | 390 (11.5) |
Abbreviation: COPD, chronic obstructive pulmonary disease.
From January 1, 2017, to December 17, 2018.
Household income in tertiles within age groups.
Table 2 shows that, among the 8844 of 10 191 individuals (86.8%) who received a dementia diagnosis, fewer had an HHI in the upper tertile (2839 [83.6%]) than in the middle (2989 [88.0%]) and lower (3016 [88.7%]) tertiles. Also, fewer individuals with upper-tertile HHI presented with moderate (748 [22.0%]) or severe (147 [4.3%]) dementia at the time of diagnosis compared with individuals with middle-tertile (moderate dementia: 882 [26.0%]; severe dementia: 185 [5.4%]) and lower-tertile (moderate dementia: 902 [26.5%]; severe dementia: 193 [5.7%]) HHI.
Table 2. Distribution of Individuals by Dementia Diagnosis, Dementia Type, and Cognitive Severity Stage at Diagnosisa.
| Variable | No. (%) | |||
|---|---|---|---|---|
| All (N = 10 191) | Household incomeb | |||
| Lower tertile (n = 3399) | Middle tertile (n = 3398) | Upper tertile (n = 3394) | ||
| Received a dementia diagnosis? | ||||
| No | 1347 (13.2) | 383 (11.3) | 409 (12.0) | 555 (16.4) |
| Yes | 8844 (86.8) | 3016 (88.7) | 2989 (88.0) | 2839 (83.6) |
| Dementia type | ||||
| Alzheimer disease | 3204 (31.4) | 1042 (30.7) | 1120 (33.0) | 1042 (30.7) |
| Vascular dementia | 908 (10.3) | 300 (9.9) | 332 (11.1) | 276 (9.7) |
| Mixed dementiac | 765 (7.5) | 275 (8.1) | 267 (7.9) | 223 (6.6) |
| Dementia with Lewy bodies | 280 (2.7) | 82 (2.4) | 82 (2.4) | 116 (3.4) |
| PD dementia | 127 (1.2) | 34 (1.0) | 52 (1.5) | 41 (1.2) |
| Frontotemporal dementia | 179 (1.8) | 49 (1.4) | 52 (1.5) | 78 (2.3) |
| Normal pressure hydrocephalus | 175 (1.7) | 50 (1.5) | 66 (1.9) | 59 (1.7) |
| Huntington disease | 24 (0.2) | 7 (0.2) | 6 (0.2) | 11 (0.3) |
| Other neurodegenerative disease | 154 (1.5) | 43 (1.3) | 41 (1.2) | 70 (2.1) |
| Unresolved cause | 1555 (15.3) | 520 (15.3) | 514 (15.1) | 521 (15.4 |
| Alcohol-related dementia | 258 (2.5) | 134 (3.9) | 72 (2.1) | 52 (1.5) |
| Other not neurodegenerative disease | 712 (7.0) | 273 (8.0) | 219 (6.4) | 220 (6.5) |
| Affective disorder | 503 (4.9) | 207 (6.1) | 166 (4.9) | 130 (3.8) |
| Cognitive severity stage at diagnosis | ||||
| Cognitively intact | 1430 (14.0) | 407 (12.0) | 423 (12.4) | 600 (17.7) |
| MCI but not dementia | 2943 (28.9) | 1028 (30.2) | 937 (27.6) | 978 (28.8) |
| Mild dementia | 2761 (27.1) | 869 (25.6) | 971 (28.6) | 921 (27.1) |
| Moderate dementia | 2532 (24.8) | 902 (26.5) | 882 (26.0) | 748 (22.0) |
| Severe dementia | 525 (5.2) | 193 (5.7) | 185 (5.4) | 147 (4.3) |
Abbreviations: MCI, mild cognitive impairment; PD, Parkinson disease.
From January 1, 2017, to December 17, 2018.
Household income in tertiles within age groups.
Alzheimer disease and vascular dementia.
Logistic regression analyses showed that, compared with individuals with lower-tertile HHI, the odds ratio (OR) for receiving a dementia diagnosis was 0.90 (95% CI, 0.77-1.05) for individuals with HHI in the middle tertile and was 0.66 (95% CI, 0.57-0.77) for those in the upper tertile (Table 3). After successive adjustment for the covariates (model 4), the ORs were attenuated but remained similar, indicating a significantly lower risk for those with upper-tertile HHI (OR, 0.65; 95% CI, 0.55-0.78) and not significantly higher risk for individuals with middle-tertile HHI (OR, 0.92; 95% CI, 0.77-1.09). Furthermore, supplementary analyses showed no statistical difference between the estimated coefficients for HHI groups between model 1 and model 4. Misclassification error analysis using model 4 as the sample data indicated that HHI was a relevant factor for dementia diagnosis in this study population but was not a main factor (eTable 2 and eFigure in the Supplement).
Table 3. Association Between Household Income and Dementia Diagnosis and Cognitive Severity Stage at Diagnosisa.
| Household income | Model 1b | Model 2c | Model 3d | Model 4e |
|---|---|---|---|---|
| Logistic regressions for dementia diagnosis, OR (95% CI) | ||||
| Lower tertile | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Middle tertile | 0.90 (0.77 to 1.05) | 0.92 (0.78 to 1.09) | 0.92 (0.78 to 1.09) | 0.92 (0.77 to 1.09) |
| Upper tertile | 0.66 (0.57 to 0.77) | 0.67 (0.56 to 0.79) | 0.67 (0.57 to 0.80) | 0.65 (0.55 to 0.78) |
| Linear regressions for cognitive severity stage at diagnosis, β (95% CI) | ||||
| Lower tertile | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Middle tertile | −0.00 (−0.06 to 0.05) | 0.00 (−0.05 to 0.05) | 0.01 (−0.04 to 0.06) | 0.01 (−0.04 to 0.06) |
| Upper tertile | −0.16 (−0.21 to −0.10) | −0.15 (−0.20 to −0.10) | −0.15 (−0.20 to −0.10) | −0.16 (−0.21 to −0.10) |
| Constant | 1.85 (1.81 to 1.89) | −1.40 (−1.88 to −0.92) | −1.42 (−1.90 to −0.94) | −1.29 (−1.77 to 0.81) |
Abbreviation: OR, odds ratio.
Among 9203 individuals; see model 4 description for the complete information on any covariates.
Model 1 was the crude model of household income for dementia diagnosis.
Model 2 was model 1 adjusted for age group, sex, region of residence, household type, and period (2017 and 2018).
Model 3 was model 2 adjusted for 5 types of medications (antipsychotics, antianxiety, hypnotics and sedatives, antidepressants, and opioids).
Model 4 was model 3 adjusted for 14 medical conditions (type 2 diabetes, chronic obstructive pulmonary disease, ischemic heart disease, depression, hypertension, stroke, atrial fibrillation, cancer, fractures, peripheral vascular disease, hemorrhage, cerebrovascular disease, kidney disease, and rheumatic disease) and number of medical conditions (0, 1, 2, 3, or ≥4) in any combination.
When compared with lower-tertile HHI, middle-tertile HHI was not associated with cognitive severity at diagnosis (β, 0.01; 95% CI, −0.04 to 0.06); however, upper-tertile HHI was significantly inversely associated with cognitive severity stage at diagnosis (β, −0.16; 95% CI, −0.21 to −0.10) (model 4 in Table 3). These associations remained similar when counting only the individuals who were diagnosed with mild, moderate, or severe dementia and excluding individuals with other cognitive stages that were present at time of diagnosis (eTable 3 in the Supplement).
Discussion
Using HHI as a proxy for SES, we found that individuals with higher SES were less likely to be diagnosed with dementia and, if diagnosed, had less severe dementia stage. Individuals with mid-level SES did not differ from individuals with lower SES in terms of dementia diagnosis and cognitive severity stage at diagnosis.
Previous studies frequently reported that lower SES was associated with a higher risk of dementia diagnosis and a later stage of dementia at diagnosis and that individuals with higher SES were less likely to be diagnosed with dementia but, if diagnosed, had less severe dementia.14,15,16,45,46 A small Canadian study (262 patients from a memory clinic) reported that individuals with lower SES presented at the clinic later and with more advanced dementia compared with those with higher SES.14 In a larger study from the US (1658 individuals with Alzheimer disease), having fewer years of education was associated with later detection of the disease and a more severe stage at diagnosis.15 A study from England (1420 patients with new referrals) showed that socioeconomic deprivation (based on residential postal codes) was associated with lower cognitive function compared with living in more affluent areas.16
Discrepancies in study findings may be a natural consequence of the different measurement protocols for SES. The aforementioned studies often used educational level as a measure for SES. In a Swedish study, Darin-Mattsson and colleagues19 reported that income was associated with late-life health that was independent of all other SES indicators, including educational level, occupational complexity, social class, and SES index (the summary of all 5 indicators). In the present study, most of the individuals were pensioners, and the tax-registered pension was the primary income. We used HHI as a measurement of SES and found that, although lower HHI was a risk factor for dementia diagnosis and severity stage at diagnosis, people with higher HHI did benefit from health inequality; they received earlier referrals and earlier diagnosis, which again benefitted them as they were able to get earlier treatment and potentially slow the disease progression compared with individuals with lower SES. Adding education into this analysis did not change these findings (eTable 4 in the Supplement). Although education has been associated with cognitive function and has been suggested to supply cognitive reserve,47 judging from current empirical evidence, its contribution to cognitive reserve or decline is limited.48,49
People with higher SES often have higher educational attainment and higher paying jobs that require certain levels of intellectual function; perhaps individuals with more cognitively demanding jobs can more easily perceive their own cognitive changes, thus leading them to consult with a physician earlier. In addition, higher educational level not only has been associated with a reduced risk of dementia but is believed to lead to better understanding of dementia and better awareness of the symptoms for early detection and diagnosis.14,50 Systematic reviews have found that educational deficits were a major factor in dementia diagnosis delay51 and that other factors, such as fear of treatment result, denial, dementia stigma, and living alone, can also delay individuals from seeking a diagnostic evaluation for dementia.52,53,54 Moreover, lack of awareness and/or lack of knowledge of dementia may also be factors in greater severity at the time of diagnosis for people with lower SES.55,56
In addition, we conducted another analysis that showed that families with higher SES had the lowest proportion of referrals for diagnostic evaluation for dementia (eTable 1 in the Supplement). Although referrals can be changed by health care systems,57 referrals are commonly the product of the assessment by a general practitioner combined with disease symptoms, patient preference, and patient ability to share information and derive maximum value from communication, examination, and experiences.58 It has been argued that studies based on patient referrals to secondary or tertiary care centers may have severe selection bias.59
Although we conducted a population-based study using dementia diagnoses from all memory clinics and dementia assessment units located in all regions of Denmark, it is unknown how many individuals were diagnosed with dementia by their general practitioner without need for a further referral. Many health care professionals hypothesized that dementia would be undetectable and underdiagnosed in many countries, including Denmark,60,61 and that a higher proportion of such undetected cases would likely consist of individuals with lower SES.62 However, this hypothesis needs to be investigated further.
Strengths and Limitations
This study has some strengths. To our knowledge, this analysis is the first population- and register-based study of HHI as a proxy for SES and its association with diagnostic evaluation for dementia. By using different degrees of analysis models, we found an association between HHI and dementia diagnosis and cognitive severity stage at diagnosis in the context of a universal health care system.
This study also has some limitations. The health data related to referrals, dementia diagnosis, and cognitive severity stage were obtained from DANDEM, a high-quality national database for dementia established in 2016 to improve and monitor the quality of diagnosis at memory clinics or dementia assessment units throughout Denmark. A 2015 study of the memory clinics in the Capital Region of Denmark reported that limited resources in memory clinics may have been a factor in the impaired precision of dementia diagnosis.63 To our knowledge, no study has yet performed a comprehensive evaluation or validation of DANDEM for the diagnoses recorded by these memory clinics and assessment units; further research is, therefore, warranted.
We did not adjust for lifestyle factors, such as smoking, diet, alcohol consumption, and physical activity, because Denmark generally does not register such information in the national registers; however, these factors are associated with SES and are often considered to be modifiable factors in the risk of dementia.64,65 Adding these factors into analysis when they become available may strengthen the accuracy of association estimation.
The data set we used included only cross-sectional data on individuals who received a first-time referral for a diagnostic evaluation for dementia in the secondary health care sector in 2017 to 2018 and registered in DANDEM. Furthermore, we had no information on onset of symptoms, date of physician visit, or date of referral. Therefore, the data did not allow longitudinal follow-up, including the 4-stage timeline of disease progression from neuropathology with no clinical signs to late-stage disease.66 A 2014 survey-based report of participant responses from 24 European countries revealed that 81% of the respondents spent an average of at least 8 weeks from time of referral to specialist visit for assessment; the other respondents reported 4 weeks or less.52 However, it may take up to 2 years for family members to notice the first symptoms of dementia before the consultation and about 3 years before diagnosis.67 We were unclear about the implication of SES for the 4 stages of dementia progression in the Danish context. Further studies should take this impact into consideration.
Conclusions
In this population- and register-based cross-sectional study of individuals with referrals for a dementia diagnostic evaluation in Denmark, SES was associated with dementia diagnosis and cognitive severity at diagnosis, especially for those in higher income households. Individuals with higher SES were less likely to receive a dementia diagnosis and, among those diagnosed with dementia, had less severe cognitive stage at diagnosis compared with individuals with lower SES. These results reveal a social inequity in diagnostic evaluation for dementia in Denmark: affluent individuals seem to have the advantage of receiving earlier diagnosis. Public health strategies should target people with lower SES for earlier detection and intervention for dementia.
eMethods. Statistical Methods for Defining Individual Personal Income Using Household Income
eTable 1. Referral Rates by Adjustable Household Income Group
eTable 2. Estimated Mean Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, and Misclassification Error Based on 10-Fold Cross-Validation for Logistic Model 4 With and Without Household Income (HHI)
eTable 3. Linear Regression of the Association Between Household Income and Cognitive Severity Stage at Diagnosis
eTable 4. Association Between Household Income and Dementia Diagnosis and Severity at Diagnosis in the Full Adjusted Model
eFigure. ROC Curve for Misclassification Error Using Model 4 Dataset With and Without Household Income
References
- 1.Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88(10):1452-1456. doi: 10.2105/AJPH.88.10.1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Dementia: key facts. Published 2019. Accessed April 4, 2020. https://www.who.int/news-room/fact-sheets/detail/dementia
- 3.Loy CT, Schofield PR, Turner AM, Kwok JB. Genetics of dementia. Lancet. 2014;383(9919):828-840. doi: 10.1016/S0140-6736(13)60630-3 [DOI] [PubMed] [Google Scholar]
- 4.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165(3):321-326. doi: 10.1001/archinte.165.3.321 [DOI] [PubMed] [Google Scholar]
- 5.Killin LO, Starr JM, Shiue IJ, Russ TC. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016;16(1):175. doi: 10.1186/s12877-016-0342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589-1599. doi: 10.1001/jama.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaffe K. Modifiable risk factors and prevention of dementia: what is the latest evidence? JAMA Intern Med. 2018;178(2):281-282. doi: 10.1001/jamainternmed.2017.7299 [DOI] [PubMed] [Google Scholar]
- 8.Kivimäki M, Batty GD, Pentti J, et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5(3):e140-e149. doi: 10.1016/S2468-2667(19)30248-8 [DOI] [PubMed] [Google Scholar]
- 9.Murayama H, Sugiyama M, Inagaki H, et al. The differential effects of age on the association between childhood socioeconomic disadvantage and subjective symptoms of dementia among older Japanese people. J Epidemiol. 2019;29(7):241-246. doi: 10.2188/jea.JE20180002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol. 2017;186(7):805-814. doi: 10.1093/aje/kwx155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korhonen K, Einiö E, Leinonen T, Tarkiainen L, Martikainen P. Midlife socioeconomic position and old-age dementia mortality: a large prospective register-based study from Finland. BMJ Open. 2020;10(1):e033234. doi: 10.1136/bmjopen-2019-033234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadar D, Lassale C, Davies H, Llewellyn DJ, Batty GD, Steptoe A. Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-year follow-up in the English Longitudinal Study of Ageing. JAMA Psychiatry. 2018;75(7):723-732. doi: 10.1001/jamapsychiatry.2018.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25(4):289-304. doi: 10.1097/WAD.0b013e318211c83c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian W, Schweizer TA, Fischer CE. Impact of socioeconomic status on initial clinical presentation to a memory disorders clinic. Int Psychogeriatr. 2014;26(4):597-603. doi: 10.1017/S1041610213002299 [DOI] [PubMed] [Google Scholar]
- 15.Moritz DJ, Petitti DB. Association of education with reported age of onset and severity of Alzheimer’s disease at presentation: implications for the use of clinical samples. Am J Epidemiol. 1993;137(4):456-462. doi: 10.1093/oxfordjournals.aje.a116694 [DOI] [PubMed] [Google Scholar]
- 16.Park MH, Smith SC, Neuburger J, Chrysanthaki T, Hendriks AAJ, Black N. Sociodemographic characteristics, cognitive function, and health-related quality of life of patients referred to memory assessment services in England. Alzheimer Dis Assoc Disord. 2017;31(2):159-167. doi: 10.1097/WAD.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 17.Daly MC, Duncan GJ, McDonough P, Williams DR. Optimal indicators of socioeconomic status for health research. Am J Public Health. 2002;92(7):1151-1157. doi: 10.2105/AJPH.92.7.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. 2006;60(1):7-12. doi: 10.1136/jech.2004.023531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darin-Mattsson A, Fors S, Kåreholt I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int J Equity Health. 2017;16(1):173. doi: 10.1186/s12939-017-0670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health . Healthcare in Denmark: An Overview. Ministry of Health; 2016. [Google Scholar]
- 21.Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 suppl):12-16. doi: 10.1177/1403494811399956 [DOI] [PubMed] [Google Scholar]
- 22.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 23.RKKP . The Danish Clinical Quality Program-National Clinical Registries (RKKP). Accessed April 23, 2020. https://www.rkkp.dk/in-english/
- 24.Statistics Denmark . Subjects. Accessed July 17, 2020. https://www.dst.dk/en
- 25.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22-25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 26.Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7 suppl):91-94. doi: 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7)(suppl):38-41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 29.Eurostat. Glossary: equivalised disposable income. Accessed December 3, 2020. https://ec.europa.eu/eurostat/statistics-explained/index.php/Glossary:Equivalised_disposable_income
- 30.ICD-10 Version:2016. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Accessed February 15, 2021. https://icd.who.int/browse10/2016/en
- 31.Launer LJ, Andersen K, Dewey ME, et al. ; EURODEM Incidence Research Group and Work Groups . Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology. 1999;52(1):78-84. doi: 10.1212/WNL.52.1.78 [DOI] [PubMed] [Google Scholar]
- 32.Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13(4):406-418. doi: 10.1016/j.jalz.2016.07.152 [DOI] [PubMed] [Google Scholar]
- 33.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315-1319. doi: 10.1016/S0140-6736(00)02113-9 [DOI] [PubMed] [Google Scholar]
- 34.Russ TC, Batty GD, Hearnshaw GF, Fenton C, Starr JM. Geographical variation in dementia: systematic review with meta-analysis. Int J Epidemiol. 2012;41(4):1012-1032. doi: 10.1093/ije/dys103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jutkowitz E, MacLehose RF, Gaugler JE, Dowd B, Kuntz KM, Kane RL. Risk factors associated with cognitive, functional, and behavioral trajectories of newly diagnosed dementia patients. J Gerontol A Biol Sci Med Sci. 2017;72(2):251-258. doi: 10.1093/gerona/glw079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718-726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Cai X, Shi X, et al. Chronic obstructive pulmonary disease as a risk factor for cognitive dysfunction: a meta-analysis of current studies. J Alzheimers Dis. 2016;52(1):101-111. doi: 10.3233/JAD-150735 [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Jung SH, Lee SU, Lim JY, Yoon KS, Lee SY. Postoperative delirium after hip surgery is a potential risk factor for incident dementia: a systematic review and meta-analysis of prospective studies. Arch Gerontol Geriatr. 2020;87:103977. doi: 10.1016/j.archger.2019.103977 [DOI] [PubMed] [Google Scholar]
- 39.Deckers K, Camerino I, van Boxtel MP, et al. Dementia risk in renal dysfunction: a systematic review and meta-analysis of prospective studies. Neurology. 2017;88(2):198-208. doi: 10.1212/WNL.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sood A, Raji MA. Cognitive impairment in elderly patients with rheumatic disease and the effect of disease-modifying anti-rheumatic drugs. Clin Rheumatol. 2021;40(4):1221-1231. doi: 10.1007/s10067-020-05372-1 [DOI] [PubMed] [Google Scholar]
- 41.Busse R, Blümel M, Scheller-Kreinsen D, Zentner A; European Observatory on Health Systems and Policies. Tackling Chronic Disease in Europe: Strategies, Interventions and Challenges. World Health Organization; 2010. [Google Scholar]
- 42.Jensen-Dahm C, Gasse C, Astrup A, Mortensen PB, Waldemar G. Frequent use of opioids in patients with dementia and nursing home residents: a study of the entire elderly population of Denmark. Alzheimers Dement. 2015;11(6):691-699. doi: 10.1016/j.jalz.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 43.Nørgaard A, Jensen-Dahm C, Gasse C, Hansen ES, Waldemar G. Psychotropic polypharmacy in patients with dementia: prevalence and predictors. J Alzheimers Dis. 2017;56(2):707-716. doi: 10.3233/JAD-160828 [DOI] [PubMed] [Google Scholar]
- 44.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer C, Yeung E, Hansen T, et al. Impact of socioeconomic status on the prevalence of dementia in an inner city memory disorders clinic. Int Psychogeriatr. 2009;21(6):1096-1104. doi: 10.1017/S1041610209990846 [DOI] [PubMed] [Google Scholar]
- 46.Barrett S, Savage G, Coen R, et al. A comparison of people presenting with symptoms of dementia in Northern Ireland and the Republic of Ireland. Methodology. 2012;4(4):4.5.
- 47.Harrison SL, Sajjad A, Bramer WM, Ikram MA, Tiemeier H, Stephan BC. Exploring strategies to operationalize cognitive reserve: a systematic review of reviews. J Clin Exp Neuropsychol. 2015;37(3):253-264. doi: 10.1080/13803395.2014.1002759 [DOI] [PubMed] [Google Scholar]
- 48.Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett DA. Education and cognitive reserve in old age. Neurology. 2019;92(10):e1041-e1050. doi: 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seblova D, Berggren R, Lövdén M. Education and age-related decline in cognitive performance: systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2020;58:101005. doi: 10.1016/j.arr.2019.101005 [DOI] [PubMed] [Google Scholar]
- 50.Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS; CoSTREAM Consortium, on behalf of the International Genomics of Alzheimer’s Project . Modifiable pathways in Alzheimer’s disease: mendelian randomisation analysis. BMJ. 2017;359:j5375. doi: 10.1136/bmj.j5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306-314. doi: 10.1097/WAD.0b013e3181a6bebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brooker D, La Fontaine J, Evans S, Bray J, Saad K. Public health guidance to facilitate timely diagnosis of dementia: Alzheimer’s cooperative valuation in Europe recommendations. Int J Geriatr Psychiatry. 2014;29(7):682-693. doi: 10.1002/gps.4066 [DOI] [PubMed] [Google Scholar]
- 53.Lian Y, Xiao LD, Zeng F, Wu X, Wang Z, Ren H. The experiences of people with dementia and their caregivers in dementia diagnosis. J Alzheimers Dis. 2017;59(4):1203-1211. doi: 10.3233/JAD-170370 [DOI] [PubMed] [Google Scholar]
- 54.Chrisp TA, Tabberer S, Thomas BD, Goddard WA. Dementia early diagnosis: triggers, supports and constraints affecting the decision to engage with the health care system. Aging Ment Health. 2012;16(5):559-565. doi: 10.1080/13607863.2011.651794 [DOI] [PubMed] [Google Scholar]
- 55.Aalten P, van Valen E, de Vugt ME, Lousberg R, Jolles J, Verhey FR. Awareness and behavioral problems in dementia patients: a prospective study. Int Psychogeriatr. 2006;18(1):3-17. doi: 10.1017/S1041610205002772 [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Abdin E, Vaingankar JA, et al. The relationship among unawareness of memory impairment, depression, and dementia in older adults with memory impairment in Singapore. Psychogeriatrics. 2017;17(6):430-438. doi: 10.1111/psyg.12270 [DOI] [PubMed] [Google Scholar]
- 57.Winkelmayer WC, Glynn RJ, Levin R, Owen WF Jr, Avorn J. Determinants of delayed nephrologist referral in patients with chronic kidney disease. Am J Kidney Dis. 2001;38(6):1178-1184. doi: 10.1053/ajkd.2001.29207 [DOI] [PubMed] [Google Scholar]
- 58.Balogh EP, Miller BT, Ball JR, eds. Improving Diagnosis in Health Care. National Academies Press; 2015. [PubMed] [Google Scholar]
- 59.Kokmen E, Ozsarfati Y, Beard CM, O’Brien PC, Rocca WA. Impact of referral bias on clinical and epidemiological studies of Alzheimer’s disease. J Clin Epidemiol. 1996;49(1):79-83. doi: 10.1016/0895-4356(95)00031-3 [DOI] [PubMed] [Google Scholar]
- 60.Connolly A, Gaehl E, Martin H, Morris J, Purandare N. Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health. 2011;15(8):978-984. doi: 10.1080/13607863.2011.596805 [DOI] [PubMed] [Google Scholar]
- 61.Phung TK, Waltoft BL, Kessing LV, Mortensen PB, Waldemar G. Time trend in diagnosing dementia in secondary care. Dement Geriatr Cogn Disord. 2010;29(2):146-153. doi: 10.1159/000269933 [DOI] [PubMed] [Google Scholar]
- 62.Savva GM, Arthur A. Who has undiagnosed dementia? a cross-sectional analysis of participants of the Aging, Demographics and Memory Study. Age Ageing. 2015;44(4):642-647. doi: 10.1093/ageing/afv020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fereshtehnejad SM, Johannsen P, Waldemar G, Eriksdotter M. Dementia diagnosis, treatment, and care in specialist clinics in two Scandinavian countries: a data comparison between the Swedish Dementia Registry (SveDem) and the Danish Dementia Registry. J Alzheimers Dis. 2015;48(1):229-239. doi: 10.3233/JAD-150144 [DOI] [PubMed] [Google Scholar]
- 64.Di Marco LY, Marzo A, Muñoz-Ruiz M, et al. Modifiable lifestyle factors in dementia: a systematic review of longitudinal observational cohort studies. J Alzheimers Dis. 2014;42(1):119-135. doi: 10.3233/JAD-132225 [DOI] [PubMed] [Google Scholar]
- 65.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14(1):643. doi: 10.1186/1471-2458-14-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prince M, Bryce R, Ferri C. World Alzheimer Report 2011: The Benefits of Early Diagnosis and Intervention. Alzheimer’s Disease International; 2011. [Google Scholar]
- 67.Speechly CM, Bridges-Webb C, Passmore E. The pathway to dementia diagnosis. Med J Aust. 2008;189(9):487-489. doi: 10.5694/j.1326-5377.2008.tb02140.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Statistical Methods for Defining Individual Personal Income Using Household Income
eTable 1. Referral Rates by Adjustable Household Income Group
eTable 2. Estimated Mean Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, and Misclassification Error Based on 10-Fold Cross-Validation for Logistic Model 4 With and Without Household Income (HHI)
eTable 3. Linear Regression of the Association Between Household Income and Cognitive Severity Stage at Diagnosis
eTable 4. Association Between Household Income and Dementia Diagnosis and Severity at Diagnosis in the Full Adjusted Model
eFigure. ROC Curve for Misclassification Error Using Model 4 Dataset With and Without Household Income