SUMMARY
Background:
We compared the safety and efficacy of three antiretroviral regimens started in pregnancy: dolutegravir (DTG)+emtricitabine (FTC)/tenofovir alafenamide fumarate (TAF); DTG+FTC/tenofovir disoproxil fumarate (TDF); and efavirenz (EFV)/FTC/TDF.
Methods:
Women with HIV-1 infection at 22 sites in 9 countries were randomized 1:1:1 at 14–28 weeks’ gestation to start each regimen (open-label). The primary efficacy outcome was virologic non-inferiority at delivery (−10% margin) of the combined DTG groups versus the EFV group, with superiority tested in a pre-planned secondary analysis. Primary safety outcomes, compared pairwise among treatment groups, were occurrence of any adverse pregnancy outcome (preterm delivery, small for gestational age, stillbirth, or spontaneous abortion) and occurrence of maternal and infant adverse events, grade ≥3. Primary analyses were intention-to-treat (ITT).
Findings:
We enrolled 643 women: 217 DTG+FTC/TAF, 215 DTG+FTC/TDF, 211 EFV/FTC/TDF. Enrollment medians were 22 weeks’ gestation, HIV-1 RNA 903 copies/mL (28% <200 copies/mL), and CD4 count 466 cells/uL. Delivery HIV-1 RNA was available for 605 (94%) women: 395 (98%) in the combined DTG groups had delivery HIV-1 RNA <200 copies/mL vs. 182 (91%) in the EFV group (difference 7% [95%CI 2%, 11%]; p=0·005). Fewer women assigned to DTG+FTC/TAF (24%) experienced the composite adverse pregnancy outcome than women assigned to either DTG+FTC/TDF (33%, difference −9% [95% CI −17%, −0·3%]; p=0·043) or EFV/FTC/TDF (33%, difference −9% [95%CI −17%, −0·1%]; p=0·047). Grade ≥3 maternal and infant adverse events did not differ by group. Preterm delivery was significantly lower with DTG+FTC/TAF (6%) than EFV/FTC/TDF (12%, difference −6% [95%CI −12%, −0·9%]; p-value = 0·023). Stillbirth was more frequent with DTG (non-significant, p=0.064). Neonatal mortality was more frequent with EFV/FTC/TDF (5%) than DTG+FTC/TAF (1·0%, p=0·019) or DTG+FTC/TDF (1·5%, p=0·050).
Interpretation:
When started in pregnancy, DTG-containing regimens had superior virologic efficacy at delivery compared with EFV/FTC/TDF. DTG+FTC/TAF had the lowest frequency of the composite adverse pregnancy outcome and of neonatal death.
BACKGROUND
Over 1·3 million women living with HIV-1 infection become pregnant each year.1 Antiretroviral treatment (ART) during pregnancy is critical for both maternal health and prevention of perinatal HIV-1 transmission. Unfortunately, high-quality safety and efficacy data in pregnancy are scarce for most antiretrovirals.
In 2018, WHO guidelines for first-line treatment of adults (including in pregnancy) replaced efavirenz (EFV) with dolutegravir (DTG), a potent and well-tolerated integrase inhibitor with a high barrier to development of drug resistance.2 Along with lamivudine (3TC) or emtricitabine (FTC), tenofovir disoproxil fumarate (TDF) remains a component of WHO-recommended first-line ART, but tenofovir alafenamide fumarate (TAF) is now recommended in first-line HIV treatment regimens in several countries. TAF is as effective as TDF at treating HIV, may have less renal and bone toxicity, and has lower manufacturing costs.3-5 DTG is already used by large numbers of women with HIV who are pregnant or of reproductive potential, and TAF is being considered for use in first-line treatment globally. We therefore conducted a clinical trial to compare the safety and virologic efficacy of DTG- and TAF-containing ART started in pregnancy.
METHODS
Trial overview
IMPAACT 2010 (“VESTED: Virologic Efficacy and Safety of ART Combinations with TAF/TDF, EFV, and DTG”) is an open-label, Phase III randomized trial comparing the virologic efficacy and safety of three ART regimens (DTG+FTC/TAF, DTG+FTC/TDF, or EFV/FTC/TDF) for pregnant women with HIV and their infants (NCT03048422). Participants enrolled from January 2018 to February 2019 at 22 sites in 9 countries (Table 1). Enrollment was paused May–October 2018 following report that DTG at conception might increase risk of neural tube defects.6 At the time of analysis, all mothers had a pregnancy outcome but maternal and infant postpartum follow-up were still underway. Here we summarize maternal events through 14 days postpartum and infant events through 28 days of life rather than through 50 weeks postpartum. Data on longer-term follow-up will be reported when available.
Table 1.
Maternal baseline characteristics by randomized group
| DTG+FTC/TAF | DTG+FTC/TDF | EFV/FTC/TDF | Total | |
|---|---|---|---|---|
| (N = 217) | (N = 215) | (N = 211) | (N = 643) | |
| Age (median years, range) | 26·8 (18·1–44·5) | 26·0 (18·1–44·0) | 26·6 (18·3–42·7) | 26·6 (18·1–44·5) |
| Country | ||||
| Zimbabwe | 82 (37·8%) | 84 (39·1%) | 83 (39·3%) | 249 (38·7%) |
| South Africa | 37 (17·1%) | 37 (17·2%) | 37 (17·5%) | 111 (17·3%) |
| Uganda | 37 (17·1%) | 37 (17·2%) | 36 (17·1%) | 110 (17·1%) |
| Brazil | 21 (9·7%) | 19 (8·8%) | 17 (8·1%) | 57 (8·9%) |
| Botswana | 16 (7·4%) | 18 (8·4%) | 17 (8·1%) | 51 (7·9%) |
| Tanzania | 15 (6·9%) | 13 (6·0%) | 15 (7·1%) | 43 (6·7%) |
| Thailand | 5 (2·3%) | 4 (1·9%) | 6 (2·8%) | 15 (2·3%) |
| United States | 2 (0·9%) | 2 (0·9%) | 0 (0·0%) | 4 (0·6%) |
| India | 2 (0·9%) | 1 (0·5%) | 0 (0·0%) | 3 (0·5%) |
| Race | ||||
| Black | 195 (89·9%) | 196 (91·2%) | 194 (91·9%) | 585 (91·0%) |
| Asian | 7 (3·2%) | 5 (2·3%) | 6 (2·8%) | 18 (2·8%) |
| White | 5 (2·3%) | 7 (3·3%) | 7 (3·3%) | 19 (3·0%) |
| Other | 10 (4·6%) | 6 (2·8%) | 4 (1·9%) | 20 (3·1%) |
| Unknown | 0 (0·0%) | 1 (0·5%) | 0 (0·0%) | 1 (0·2%) |
| Gestational age at study entry (median weeks, Q1, Q3) | 22·1 (18·4, 25·0) | 21·3 (18·1, 25·1) | 22·1 (18·3, 25·6) | 21·9 (18·3, 25·3) |
| Gestational age at study entry (categorized) | ||||
| 14–18 weeks | 58 (26·7%) | 64 (29·8%) | 59 (28·0%) | 181 (28·1%) |
| 19–23 weeks | 93 (42·9%) | 83 (38·6%) | 77 (36·5%) | 253 (39·3%) |
| 24–28 weeks | 66 (30·4%) | 68 (31·6%) | 75 (35·5%) | 209 (32·5%) |
| Hepatitis B surface antigen positive | 3 (1·4%) | 6 (2·8%) | 4 (1·9%) | 13 (2·0%) |
| Log10 HIV-1 RNA (median copies/mL, Q1, Q3) | 2·9 (2·2, 3·8) | 2·9 (2·1, 3·6) | 3·1 (2·3, 3·7) | 3·0 (2·2, 3·7) |
| HIV-1 RNA (median copies/mL, Q1, Q3) | 781·0 (147·0, 5,733·0) | 715·0 (128·0, 4,304·0) | 1,357·0 (198·0, 5,125·0) | 902·5 (152·0, 5,182·5) |
| HIV-1 RNA (copies/mL, categorized) | ||||
| <50 | 36 (16·7%) | 37 (17·3%) | 27 (13·0%) | 100 (15·7%) |
| <200 | 62 (28·7%) | 66 (30·7%) | 53 (25·4%) | 181 (28·3%) |
| CD4 (median cells/uL, Q1, Q3) | 467 (324, 624) | 481 (332, 642) | 439 (300, 616) | 466 (308, 624) |
| CD4 (cells/uL, categorized) | ||||
| <50 | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) |
| 50-349 | 64 (29·8%) | 60 (27·9%) | 73 (35·1%) | 197 (30·9%) |
| 350-499 | 56 (26·0%) | 53 (24·7%) | 50 (24·0%) | 159 (24·9%) |
| 500-750 | 68 (31·6%) | 67 (31·2%) | 59 (28·4%) | 194 (30·4%) |
| > 750 | 27 (12·6%) | 34 (15·8%) | 26 (12·5%) | 87 (13·6%) |
| Weight (median kg, Q1, Q3) | 65·0 (56·7, 77·1) | 63·0 (56·3, 72·0) | 61·4 (55·4, 71·2) | 63·0 (56·2, 73·0) |
| BMI (median kg/cm2, Q1, Q3) | 25·1 (22·5, 29·4) | 24·5 (22·0, 28·1) | 24·3 (21·5, 28·3) | 24·7 (22·0, 28·4) |
| Creatinine Clearance (mean mL/min, SD) | 192·1 (59·6) | 186·6 (65·0) | 182·6 (56·2) | 187·2 (60·4) |
| Creatinine (mean mg/dL, SD) | 0·49 (0·09) | 0·49 (0·09) | 0·49 (0·10) | 0·49 (0·10) |
| Took prior TDF or (FTC/TDF) pre-exposure prophylaxis* | 1 (0·5%) | 2 (1·0%) | 0 (0·0%) | 3 (0·5%) |
| Received ART during a previous pregnancy / breastfeeding* | 1 (0·5%) | 0 (0·0%) | 1 (0·5%) | 2 (0·3%) |
| Received ART during current pregnancy prior to enrollment | 176 (81·1%) | 180 (83·7%) | 176 (83·4%) | 532 (82·7%) |
| Median # days of ART (range) | 6 (1–15) | 6 (1-14) | 6 (1-14) | 6 (1-15) |
| EFV/XTC/TDF** | 166 (76·5%) | 165 (76·7%) | 165 (78·2%) | 496 (77·1%) |
| DTG/XTC/TDF or TAF** | 7 (3·2%) | 8 (3·7%) | 6 (2·8%) | 21 (3·3%) |
| Other regimen | 3 (1·4%) | 7 (3·3%) | 5 (1·4%) | 14 (2·2%) |
All 3 women took less than 1 week of pre-exposure prophylaxis.
XTC indicates either FTC or lamivudine (3TC).
Study outcomes
The primary efficacy outcome was maternal HIV-1 RNA <200 copies/mL at the delivery visit. This HIV-1 RNA threshold was chosen as it was the lowest threshold for the validated HIV-1 RNA assay (Abbott RealTime viral load assay) used at all participating sites.
We present three safety outcome measures. The first safety outcome was a composite adverse pregnancy outcome, defined as occurrence of any of the following: spontaneous abortion (<20 weeks of gestation), stillbirth (≥20 weeks of gestation), preterm delivery (<37 weeks of gestation in live-born babies), or small for gestational age (SGA; weight <10th percentile for GA, adjusted for sex).7,8 The composite adverse pregnancy outcome was a primary study outcome. The second safety outcome was occurrence of maternal grade 3 or higher adverse events through 14 days postpartum. The third safety outcome was occurrence of infant grade 3 or higher adverse events 28 days after birth. Maternal and infant adverse events through 50 weeks postpartum/after birth are also primary safety outcomes.
Study population
Pregnant women ≥18 years with confirmed HIV-1 enrolled from 14 to 28 weeks of gestation. Participants were ART-naïve with these exceptions: up to 14 days of ART during current pregnancy; prior TDF or TDF/FTC pre-exposure prophylaxis; or ART during prior pregnancies (last dose ≥6 months previously). Women were excluded if pregnant with a fetus with known anomaly or multiple fetuses; receiving medication for psychiatric illness (or history of suicidal ideation/attempt); hospitalization/acute significant illness in preceding 14 days; active tuberculosis; ALT/AST ≥2·5x upper limit of normal; or estimated creatinine clearance <60 mL/minute (full inclusion/exclusion criteria in Supplementary Appendix).
Randomization and masking
Eligible women were randomly assigned in a 1:1:1 ratio to receive DTG+FTC/TAF, DTG+FTC/TDF, or EFV/FTC/TDF. Study drugs were open-label. Randomization was stratified by gestational age (14-18 weeks, 19-23 weeks, 24-28 weeks) and country. Permuted blocks of size 6 were generated by a central computerized randomization system that was maintained by a data management group independent of the study team. Each permuted block contained 6 treatment allocations: 2 for each treatment group. A file of permuted blocks was generated separately for each combination of stratification levels.9 Site pharmacists received treatment information from the randomization system and dispensed study drug. Local study staff and participants were unmasked to study treatment assignment. The statisticians had access to unmasked data.
Study procedures
Women underwent fetal ultrasound before or within 14 days of enrollment to estimate gestational age. Gestational age was estimated using the American College of Obstetricians and Gynecologists’ recommendation of redating the estimated delivery date from the last menstrual period with the estimated delivery date based on the earliest ultrasound exam.10 For ultrasounds we collected fetal biometry measures and centrally re-estimated the delivery date based on biometry.11,12 Following randomization, antepartum study visits occurred every 4 weeks and at delivery. Maternal HIV-1 RNA (Abbott RealTime), ALT/AST, and creatinine were measured before randomization and regularly throughout follow-up. In infants, at the birth visit HIV nucleic acid test (NAT, RNA or DNA), ALT, creatinine, and complete blood count were performed.
Study drugs were administered orally as followed: DTG as one 50mg tablet daily; FTC 200mg/TAF 25mg and FTC 200mg/TDF 300mg each as one fixed-dose combination tablet daily; and EFV 600mg/FTC 200mg/TDF 300mg as one fixed-dose combination tablet daily.
In cases of virologic failure (two consecutive HIV-1 RNA ≥200 copies/mL, obtained ≥24 weeks on study) or drug toxicity, site investigators consulted with the trial’s Clinical Management Committee and could prescribe alternative antiretroviral regimens (with real-time HIV-1 drug resistance genotyping).
Major congenital anomaly was defined as structural abnormality with surgical, medical, or cosmetic importance. The following were not to be considered major congenital anomalies: genetic disorders, chromosome abnormalities, minor anomalies, birth marks, positional deformities, prematurity-related findings, prenatal ultrasound-only findings (i.e., findings only identified by ultrasound and not by the examining pediatrician), and polydactyly (postaxial, type B).
Infant HIV infection was defined as two positive NATs from different dates (first sample drawn within 14 days), or single positive NAT in an infant who died before re-testing.
Statistical analysis
A sample size of 639 pairs was selected to provide 80% power for a −10% non-inferiority margin for virologic efficacy at delivery of the combined DTG vs. EFV/FTC/TDF groups, assuming that 10% of data for the primary outcomes would be missing.
Using the sample size determined from the primary efficacy outcome, it was assumed if 27% of the EFV-containing group experienced the composite adverse pregnancy outcome, a difference in 14% (to 41%) or −12% (to 15%) in either of the DTG-containing groups would result in 80% power.13
Binary outcomes were analyzed with two-sample tests of proportions with normal approximation, survival outcomes with log-rank test, and continuous outcomes with a two-sample t-test. Longitudinal analyses of rate of change over time used generalized estimating equations with an identity link, an exchangeable working correlation matrix, and main effects for study-time and arm plus a study-time by arm interaction term. No adjustments were made for multiple comparisons of different outcome measures. P-values were 2-sided; p-values <0·05 were considered statistically significant.
All comparisons were made using principle of ITT. The ITT population included all randomized participants. Per-protocol analyses were also performed for selected outcomes. Per-protocol analyses excluded all data on participants who did not remain on the randomized regimen up to the time of the measured outcome, with the exception of switches made due to a requirement for other concomitant medications.
Analyses were performed using SAS version 9.4.
Virologic efficacy through delivery
The primary efficacy analyses compared the EFV group to the combined DTG groups as a binary outcome, with success (viral suppression) defined as HIV-1 RNA <200 copies/mL at delivery (or within 14 days after). Estimates, confidence intervals (CIs), and p-values for the primary efficacy analyses were adjusted for two interim efficacy analyses using the O’Brien-Fleming like error spending function and the time-ordered definition of extremity. We first evaluated whether treatment initiated during pregnancy with a DTG-containing regimen was non-inferior to EFV/FTC/TDF in achieving HIV-1 RNA <200 copies/mL at delivery. After establishment of non-inferiority, superiority of the DTG groups over the EFV-containing group was tested, per pre-planned analysis. Pre-specified sensitivity analyses were conducted using multiple imputation to handle missing viral load data (see Appendix), and the primary outcome was stratified by baseline HIV-1 RNA (<200 copies/mL vs. ≥200 copies/mL) to understand the effect of baseline virologic suppression on the study conclusions. ITT and per-protocol analyses were performed.
Pre-specified secondary analyses related to virologic efficacy included time-to-viral suppression, viral suppression at delivery using the FDA Snapshot algorithm14 (but using HIV-1 RNA <200 copies/mL).
An additional pre-specified analysis evaluated delivery viral suppression of HIV-1 RNA <50 copies/mL. Per protocol, this secondary efficacy outcome was to be measured using a centralized lab, in contrast to the primary analysis of <200 copies/mL using clinic real-time results. At the time of manuscript submission, approximately 10% of the expected results were missing from the centralized lab. Given the interest of <50 copies/mL results, real-time viral loads were used in place for those who did not yet have a centralized viral load. This analysis is not the final analysis for this secondary analysis.
Secondary efficacy and sensitivity analyses were not adjusted for the two interim analyses.
Safety outcomes
We determined whether the composite adverse pregnancy outcome (a primary safety outcome), maternal grade 3 or higher adverse events through 14 days postpartum, and infant grade 3 or higher adverse events through 28 days after birth,15 differed for any pairwise regimen comparison in pre-specified analyses. While follow-up is ongoing, maternal and infant (live-born) grade 3 or higher adverse events15 were analyzed as a survival outcome as a pre-specified analysis; creatinine clearance as continuous outcome; and average weekly weight change analyzed longitudinally. In secondary analysis, major congenital anomalies16 were added to the composite outcome. Neonatal death (overall and by arm) was analyzed in post-hoc analyses. ITT analyses were performed for all safety outcomes and per-protocol analyses were performed for analyses of weight gain. Safety analyses were not adjusted for the two interim analyses.
Trial ethics and oversight
The study was approved by Institutional Review Boards at each site. All maternal participants provided written informed consent. The study was monitored by an independent Data and Safety Monitoring Board.
Role of the funding source
Representatives of the funding source (U.S. National Institutes of Health) participated in study design and writing of the report, but the corresponding author made the final independent decision to submit the manuscript for publication in its current form. All authors had full access to all the data in the study.
RESULTS
Enrollment, study withdrawals and treatment changes
We screened 810 and enrolled 643 pregnant women (Figure 1), with similar baseline characteristics across groups (Table 1). Most women (83%) took antiretrovirals during the current pregnancy before enrollment, primarily EFV-based regimens (median 6 days, range 1–15). Enrollment HIV-1 RNA, available for 640/643 (99·5%) women, was low (median 903 copies/mL; 181 [28%] had HIV-1 RNA <200 copies/mL at entry). Seven sites were able to collect viral load information for 43 women at both screening and the day of randomization. Forty of the 43 women (93%) had a viral load ≥200 copies/mL at screening; 32 (74%) had viral load ≥1,000 copies/mL at screening.
Figure 1:
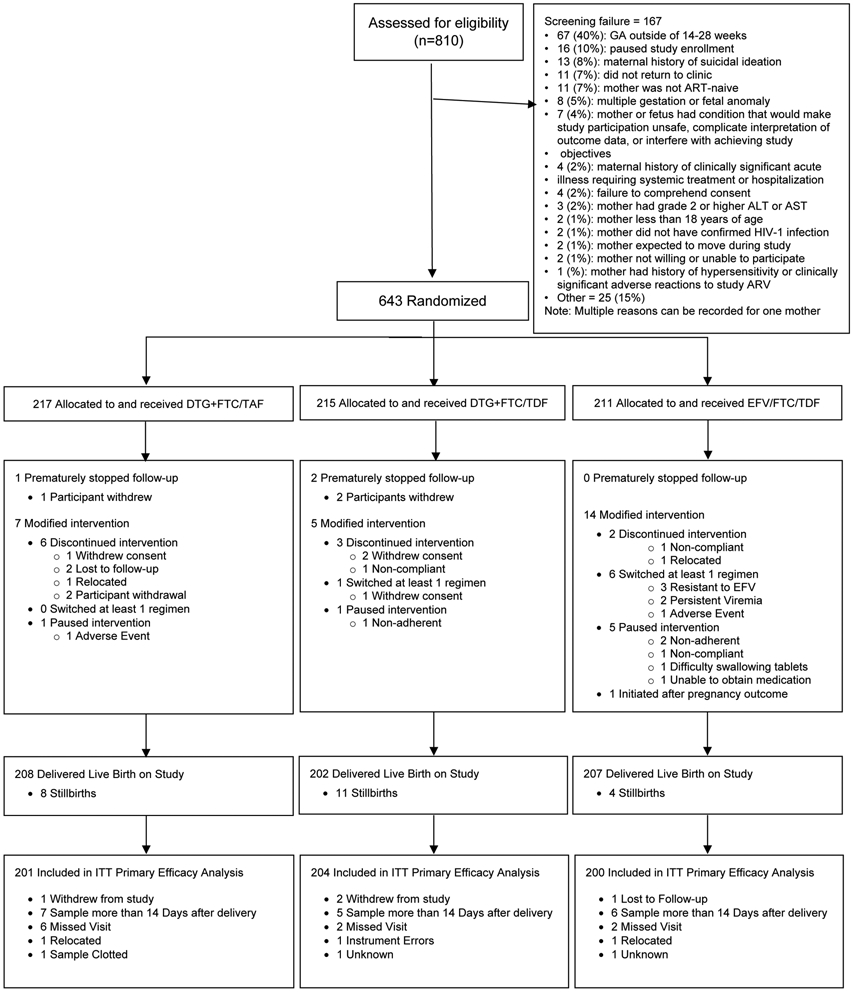
CONSORT IMPAACT 2010 Diagram
Over the median 17·4 weeks between randomization and pregnancy outcome, 3 women withdrew from the study and 623 (97%) did not miss any study visits. Twenty-six (4%) women had their randomized treatment modified before delivery: 7 (3%) in DTG+FTC/TAF, 5 (2%) in DTG+FTC/TDF, and 14 (7%) in EFV/FTC/TDF groups (Figure 1, Appendix Tables S1 and S2).
Virologic efficacy
Primary efficacy analyses
Six hundred and five (94%) of 643 enrolled women had delivery HIV-1 RNA result available, 577 (95%) of whom had viral suppression: 395/405 (98%) in the combined DTG groups versus 182/200 (91%) in the EFV/FTC/TDF group (estimated difference [95%CI] 7% [2%, 11%], excluding the non-inferiority margin of −10%) (Figure 2a, Table 2). The combined DTG regimens met pre-specified criteria for virologic superiority compared with EFV/FTC/TDF (p=0·005). Per-protocol analysis showed similar results.
Figure 2.
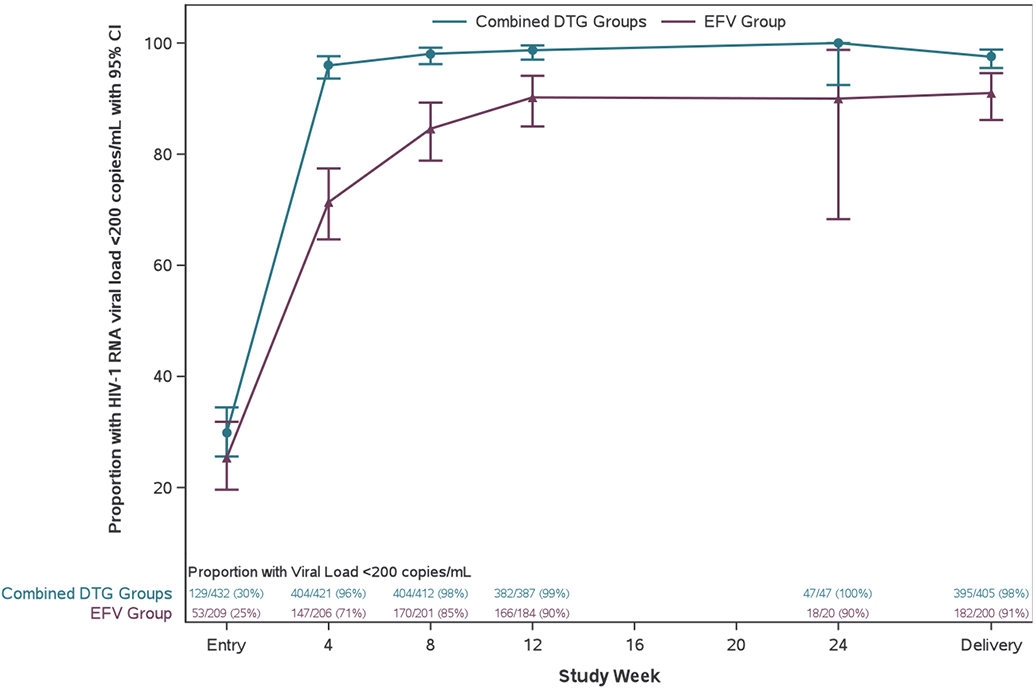
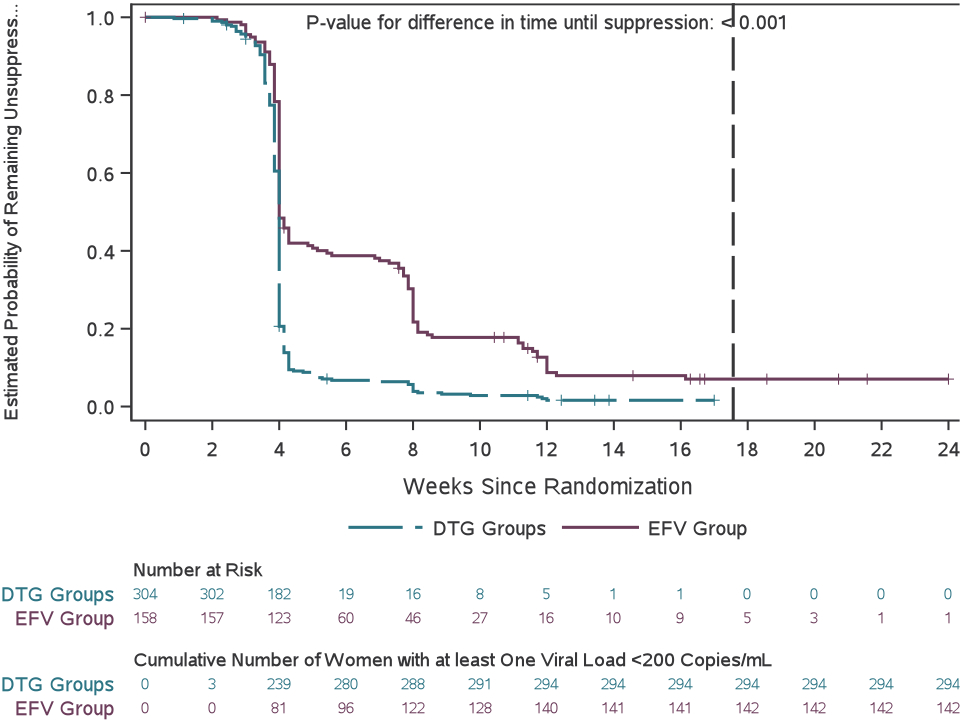
Viral suppression across study weeks before delivery
(A) Proportion of participants with viral suppression (defined as a HIV-1 concentration of <200 copies per mL) from study entry to delivery. Error bars show 95% CIs. (B) Estimated probability of not achieving viral suppression between randomisation and delivery. The grey dashed line indicates overall mean duration of follow-up from randomisation to delivery.
Table 2.
HIV-1 RNA suppression to <200 copies/mL at delivery visit in women in the DTG groups vs. the EFV group, among all women and by entry HIV-1 RNA result
| DTG groups n/N (%) |
EFV group n/N (%) |
Difference in proportions |
P-value | |
|---|---|---|---|---|
| All women | ||||
| Intention-to treat, <200 cp/mL | 395/405 (97·5%) | 182/200 (91·0%) | 6·5% (2·0%, 10·7%) | 0·005* |
| Per-protocol, <200 cp/mL | 389/399 (97·5%) | 171/187 (91·4%) | 6·0% (1·6%, 10·3%) | 0·008* |
| FDA snapshot, <200 cp/mL | 389/432 (90·0%) | 171/211 (81·0%) | 9·0% (3·0%, 15·0%) | 0·003 |
| Intention-to-treat, <50 cp/mL | 387/407 (95·1%) | 160/201 (79·6%) | 15·5% (9·5%, 21·4%) | <0·001 |
| Per-protocol, <50 cp/mL | 381/401 (95·0%) | 151/188 (80·3%) | 14·7% (8·6%, 20·8%) | <0·001 |
| Stratified by entry HIV-1 RNA (to <200 cp/mL, intention-to-treat) | ||||
| Women with entry HIV-1 RNA ≥200 copies/mL | 275/285 (96·5%) | 130/148 (87·8%) | 8·7% (3·0%, 14·3%) | 0·003 |
| Women with entry HIV-1 RNA <200 copies/mL | 119/119 (100·0%) | 50/50 (100·0%) |
P-value has been corrected for interim analyses.
Intention-to-treat (ITT) comparisons were based on the randomized group. Per-protocol analyses excluded viral loads from participants who switched, added, stopped, or did not start any of the ARVs in the randomized regimen before the delivery viral load was sampled.
Secondary efficacy analyses
Women randomized to a DTG-containing regimen had significantly shorter time to viral suppression <200 copies/mL than the EFV group (p<0·001, Figure 2b). Women in the combined DTG groups also shorter time to viral suppression <400 copies/mL (p<0·001) and <1,000 copies/mL (p=0·018) than women in the EFV group (Table S3 and Figures S1 and S2). The proportions of women with HIV-1 RNA <50 copies/mL at delivery were 387/407 (95%) in the combined DTG groups vs. 160/201 (80%) in the EFV group (estimated difference [95%CI] 16% [10%, 21%]) (Table 2).
Sensitivity analyses of virologic efficacy at delivery
The two DTG groups showed similar efficacy to one another, including when analyzed by the modified FDA Snapshot approach (Table S4). Virologic efficacy results using multiple imputation to account for missing HIV-1 RNA were similar to primary results (Table S5).
Adverse pregnancy outcomes
Safety
Primary composite adverse pregnancy outcome
Six hundred forty out of 643 [99·5%]) women had pregnancy outcome recorded (Figure 1): 617/640 (96%) were live births and 23/640 (4%) stillbirths (no spontaneous abortions). Among live births, 56/617 (9%) were preterm and 119/602 (20%) SGA; 7/602 (1%) were both preterm and SGA.
The composite adverse pregnancy outcome was experienced by 191/640 (30%) mother-infant pairs (Figure 3a). Significantly fewer women in the DTG+FTC/TAF group (52/216, 24%) had the composite adverse pregnancy outcome compared with the DTG+FTC/TDF group (70/213, 33%, difference −9% [95% CI −17%, −0·3%]; p=0·043) or the EFV/FTC/TDF group (69/211, 33%, difference −9% [95%CI −17%, −0·1%]; p=0·047). There was no apparent difference in the frequency of the composite adverse pregnancy outcome between the DTG+FTC/TDF and EFV/FTC/TDF groups. Analyses counting early study discontinuations as failures and adding congenital anomalies yielded similar findings (Figure S3).
Figure 3.
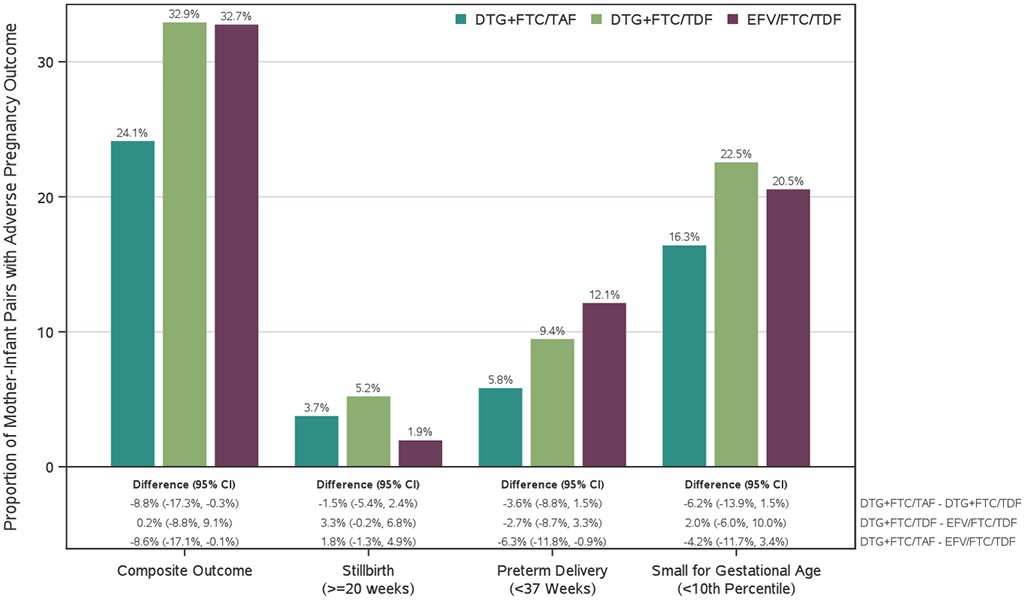
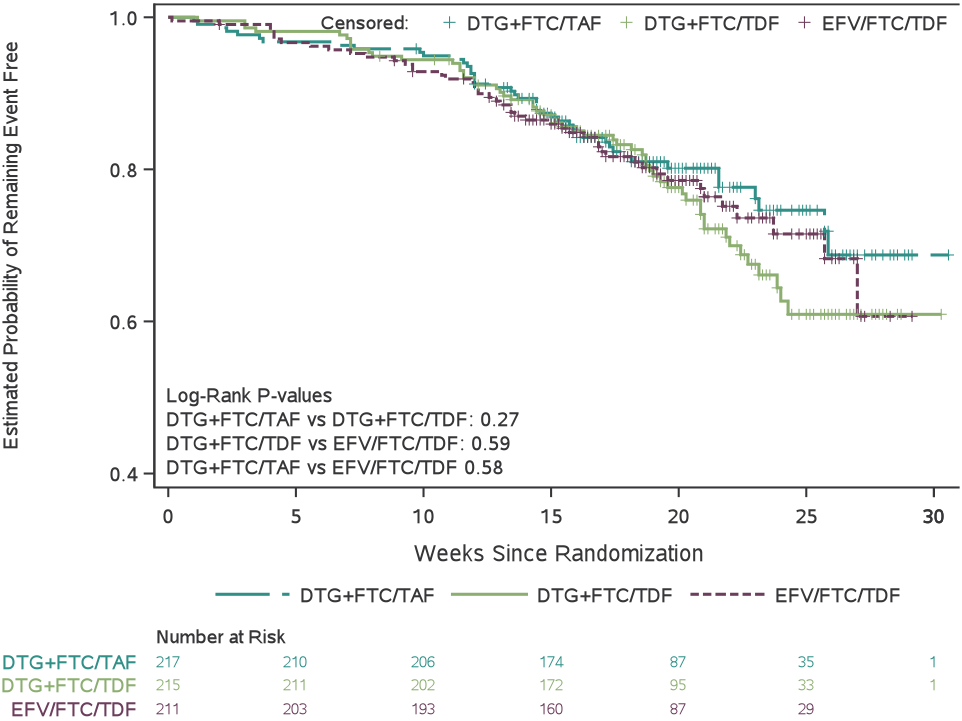
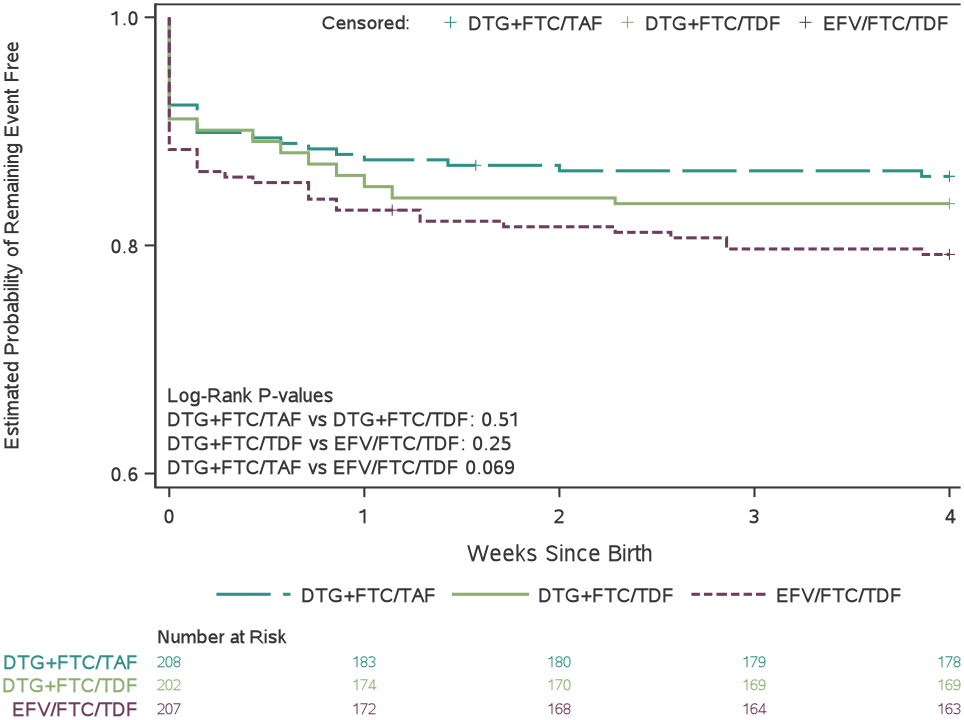
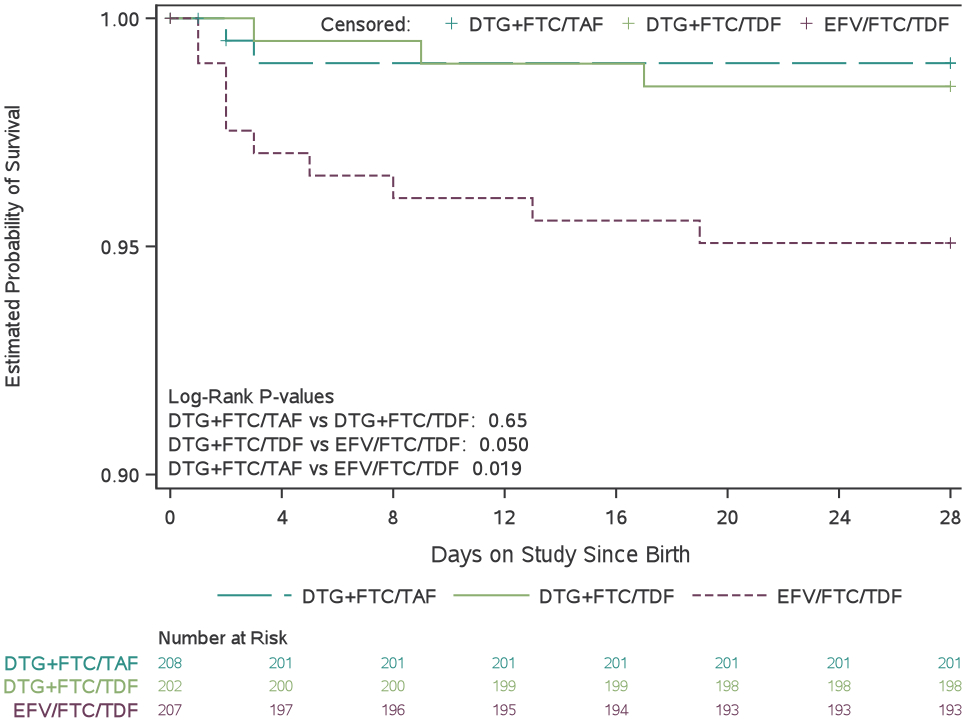
Adverse pregnancy outcomes and grade 3 or higher adverse events by treatment group
(A) Proportion of mother–infant pairs who had a composite adverse pregnancy outcome, defined as the occurrence of any of the following: spontaneous abortion (at <20 weeks’ gestation), stillbirth (at ≥20 weeks’ gestation), preterm delivery (at <37 weeks’ gestation), or small for gestational age (birthweight <10th percentile for gestational age, adjusted for sex). (B) Estimated probability of mothers remaining free of a grade 3 or higher adverse event between randomisation and 14 days postpartum. (C) Estimated probability of infants remaining free of a grade 3 or higher adverse event between birth and age 4 weeks. (D) Estimated probability of infant survival between birth and age 28 days. DTG=dolutegravir. FTC=emtricitabine. TAF=tenofovir alafenamide fumarate. TDF=tenofovir disoproxil fumarate. EFV=efavirenz.
In a secondary analysis that combined the DTG groups, 122/429 (28%) of mother-infant pairs in the DTG and 69/211 (33%) in the EFV/FTC/TDF groups experienced the composite adverse pregnancy outcome (p=0·27).
Preterm delivery among live-born babies was significantly less frequent in the DTG+FTC/TAF (12/208, 6%) than the EFV/FTC/TDF group (25/207, 12%, difference −6% [95%CI −12%, −0·9%]; p=0·023) (Figure 3a). While there was no significant difference between the two DTG groups, higher numbers of preterm births were observed with DTG+FTC/TDF (19/202, 9%) than DTG+FTC/TAF (12/208, 6%, p=0·16) (see Table S6 for preterm delivery including live-born and stillborn babies). One (0·5%) infant in DTG+FTC/TAF, 2 (1%) in DTG+FTC/TDF, and 6 (3%) in EFV/FTC/TDF groups were very preterm (<32 weeks).
No significant between-group differences were observed for SGA or very SGA. SGA occurred in 33/202 (16%) of the DTG+FTC/TAF group, 45/200 (23%) of the DTG+FTC/TDF group, and 41/200 (21%) of the EFV/FTC/TDF group (p≥0·05 for all comparisons). Twelve (6%) infants in DTG+FTC/TAF, 24 (12%) in DTG+FTC/TDF, and 22 (11%) in EFV/FTC/TDF groups were very SGA (<5th percentile in weight for GA). Of infants born at term, 32/190 (17%) in DTG+FTC/TAF, 40/181 (22%) in DTG+FTC/TDF, and 40/176 (23%) in the EFV/FTC/TDF groups were SGA.
Although differences did not reach statistical significance, stillbirth occurred in more women in each of the DTG groups (8/216 [4%] in DTG+FTC/TAF and 11/213 [5%] in DTG+FTC/TDF groups) than in the EFV group (4/211 [2%], p=0.064), with 74% of all stillbirths occurring at <37 weeks’ gestation. The circumstances surrounding stillbirths varied from obstetric complications to premature delivery of a macerated fetus (stillbirth details, Table S7).
Maternal grade 3 or higher adverse events through 14 days after delivery
We did not observe any significant between-group differences in time to grade ≥3 adverse events in the 643 women randomized in the trial (Figure 3b). One hundred forty-eight (23%) of 643 women experienced at least one grade ≥3 adverse event through 14 days postpartum (Table 3): 45/217 (21%) of DTG+FTC/TAF, 56/215 (26%) of DTG+FTC/TDF, and 47/211 (22%) of EFV/FTC/TDF groups. One woman in the DTG+FTC/TAF group died of sepsis 2 weeks after cesarean delivery.
Table 3.
Maternal grade 3 or higher adverse events, and infant baseline characteristics and infant grade 3 or higher adverse events*
| DTG+FTC/TAF | DTG+FTC/TDF | EFV/FTC/TDF | |
|---|---|---|---|
| Maternal outcome through 14 days after delivery | N = 217 | N = 215 | N = 211 |
| Women with any grade 3 or higher clinical or laboratory adverse event | 45 (20·7%) | 56 (26·0%) | 47 (22·3%) |
| Death** | 1 (0·5%) | 0 (0%) | 0 (0%) |
| Any grade 3 or higher clinical adverse event | 40 (18·4%) | 40 (18·6%) | 38 (18·0%) |
| Infection | 5 (2·3%) | 5 (2·3%) | 8 (3·8)% |
| Pregnancy/perinatal complication (excluding SB and PTD) | 25 (11·5%) | 28 (13·0%) | 27 (12·8%) |
| Gestational hypertension | 5 (2·3%) | 5 (2·3%) | 7 (3·3%) |
| Pre-eclampsia or eclampsia | 5 (2·3%) | 3 (1·4%) | 1 (0·5%) |
| Gestational diabetes | 0 (0%) | 1 (0·5%) | 0 (0%) |
| Premature rupture of membranes (term and preterm) | 5 (2·3%) | 5 (2·3%) | 5 (2·4%) |
| Hemorrhage (antepartum to 14 days postpartum) | 4 (1·8%) | 2 (0·9%) | 4 (1·9%) |
| Other pregnancy complication | 8 (3·7%) | 13 (6·0%) | 11 (5·2%) |
| Any grade 3 or higher laboratory-based adverse event | 9 (4·1%) | 20 (9·3%) | 15 (7·1%) |
| Low hemoglobin or reported anemia | 8 (3·7%) | 17 (7·9%) | 11 (5·2%) |
| Low creatinine clearance† | 1 (0·5%) | 1 (0·5%) | 2 (0·9%) |
| AST | 0 (0%) | 1 (0·5%) | 1 (0·5%) |
| Other maternal outcomes | |||
| Estimated creatinine clearance at delivery (mean mL/min)¶ | 148·5 | 134·9 | 155·3 |
| Creatinine at delivery (mean mg/dL) | 0·64 | 0·68 | 0·57 |
| Weekly weight gain (mean kg) | 0·378 | 0·319 | 0·291 |
| Weekly weight gain standardized to GA (mean kg) | 0·371 | 0·332 | 0·289 |
| Infant outcome, through 28 days of life | (N = 208) | (N = 202) | (N = 207) |
| Infants with any grade 3 or higher adverse event | 29 (13·9%) | 33 (16·3%) | 43 (20·8%) |
| Infection | 3 (1·4%) | 10 (5·0%) | 9 (4·3%) |
| Nervous system disorder# | 3 (1·4%) | 0 (0%) | 7 (3·4%) |
| Respiratory tract disorder | 11 (5·3%) | 6 (3·0%) | 10 (4·8%) |
| Hypoglycemia | 4 (1·9%) | 4 (2·0%) | 4 (1·9%) |
| Elevated creatinine | 2 (1·0%) | 5 (2·5%) | 4 (1·9%) |
| Elevated bilirubin | 1 (0·5%) | 1 (0·5%) | 0 (0%) |
| Other infant outcomes | |||
| Gestational age at birth (median weeks, range) | 39·7 (31·1, 43·8) | 39·9 (28·1, 43·9) | 39·6 (25·1, 44·4) |
| Birth weight (median grams) | 3,160 | 3,065 | 3,000 |
| Low birth weight (<2500 grams) | 13 (6.4%) | 19 (9.5%) | 24 (12%) |
| Very low birth weight (<1500 grams) | 0 | 1 (0.5%) | 2 (1.0%) |
| Birth weight >4kg | 8 (4·0%) | 3 (1·5%) | 4 (2·0%) |
| Died by 28 days after birth& | 2 (1·0%) | 3 (1·5%) | 10 (4·8%) |
| Born <37 weeks, of infants who died by 28 days | 1 (0·5%) | 0 (0%) | 3 (1·4%) |
| SGA, of infants who died by 28 days | 2 (1·0%) | 2 (1·0%) | 3 (1·5%) |
| Creatinine clearance at birth (mean mL/min)‡ | 52·5 | 53·3 | 49·6 |
| Creatinine at birth (mean mg/dL) | 0·62 | 0·56 | 0·50 |
Table 3 presents the numbers of women (and infants) with grade 3 or higher events; some women and infants may have each had more than 1 event, hence not all columns will total. Participants who experienced multiple grade 3 or higher events were reported at the highest-grade event in each row. Only the most frequent or relevant specific clinical events are listed; please see Tables S8 and S15 for detailed listings of reported events.
Maternal cause of death: one mother died of sepsis approximately 2 weeks following Cesarean section.
Defined as creatinine >1·8 x upper limit of normal or estimated creatinine clearance <60 mL/min by Cockgroft-Gault.
By Cockgroft-Gault.
Nervous system disorders: in DTG+FTC/TAF group: 2 infants with hypoxic-ischemic encephalopathy and 1 with seizure. In EFV/FTC/TDF group: 1 infant with bulging fontanelle, 1 with hydrocephalus and intraventricular hemorrhage, and 5 with hypoxic-ischemic encephalopathy.
Infant cause of death: DTG+FTC/TAF group: 1 Hypoxic ischemic encephalopathy. 1 Birth asphyxia. DTG+FTC/TDF group: 1 Birth asphyxia. 1 Probable pneumonia. 1 Unknown. EFV/FTC/TDF group: 3 Hypoxic ischemic encephalopathy. 1 Severe prematurity. 3 Neonatal sepsis. 1 Neonatal respiratory distress syndrome. 1 Fetal distress due to prolonged labor. 1 Unknown.
By Schwartz formula.
The most common type of grade ≥3 adverse events was pregnancy complication (80 women, Table 3), including gestational hypertension (17/643 [3%] women, similar numbers in the 3 groups) and grade ≥3 pre-eclampsia or eclampsia (9/643 [1%] women, 8 in the combined DTG groups and 1 in the EFV group). Only 1 woman (DTG+FTC/TDF group) was diagnosed with gestational diabetes. No women experienced grade ≥3 headache, insomnia, or depression.
Renal safety events were rare (4 women had grade 3 creatinine clearance, 1 in each DTG group, 2 in the EFV group).
Neonatal adverse events grade 3 or higher through 28 days after birth
All 617 live-born babies contributed follow-up data for this analysis; 15 (2%) died (Table 3) and 2 moved out of area. Infant characteristics at birth were similar by group with the exception of birthweight (lowest in the EFV group, Table 2, consistent with pattern of preterm delivery).
Overall, 105 (17%) live-born infants experienced at least one grade ≥3 event (including death) through 28 days (Table 3). We did not observe any significant between-group differences in time to grade ≥3 adverse events in neonates (Figure 3c). A sensitivity analysis that counted early study discontinuations as failures yielded similar findings (Table S15).
The proportions of mother-infant pairs who did not experience a composite adverse pregnancy outcome or a grade ≥3 adverse event were: DTG+FTC/TAF 59%, DTG+FTC/TDF 46%, and EFV/FTC/TDF 47%.
Other maternal outcomes
Women in the DTG+FTC/TAF group had significantly greater average weekly weight gain (0·378 kg/week) compared to women in the DTG+FTC/TDF group (0·319 kg/week, difference +0·058 kg/week, 95%CI: 0·013, 0·103, p=0·011) and EFV/FTC/TDF group (0·291 kg/week, difference +0·086 kg/week, 95%CI: 0·040, 0·133, p<0·001). There was no significant difference in weekly weight gain between women in the DTG+FTC/TDF and EFV/FTC/TDF groups (details in Table S11). Mean weight gain in all three groups was lower than the 0·42 kg/week recommended by the Institute of Medicine during the 2nd and 3rd trimesters.17
Estimated maternal creatinine clearance at delivery was significantly lower in the DTG+FTC/TDF group (135 mL/min) than in the DTG+FTC/TAF group (149 mL/min, p=0·005) or the EFV/FTC/TDF group (155 mL/min, p<0·001) (Table 3 and Table S10). Similarly, absolute maternal creatinine at delivery was significantly higher in the DTG+FTC/TDF group (0·68 mg/dL) than in the DTG+FTC/TAF group (0·64 mg/dL, p=0·018) or the EFV/FTC/TDF group (0·57 mg/dL, p< 0·001); and absolute creatinine was also higher in the DTG+FTC/TAF group than the EFV/FTC/TDF group (p<0.001) (Table 3 and Table S10).
Other neonatal outcomes
Neonatal mortality
In post-hoc analysis, neonatal mortality was higher in the EFV/FTC/TDF (10/207 [5%]) than in DTG+FTC/TAF (2/208 [1·0%], p=0·019) or DTG+FTC/TDF (3/202 [1·5%], p=0·050) groups, with no significant difference between the DTG+FTC/TAF and DTG+FTC/TDF groups (p=0·65) (Figure 3d, Tables S17-S18). Most infants who died were born at term (11/15 [73%]); median time to death was 2 days, and 11 (73%) died in the first week of life. In post-hoc analysis, either stillbirth or neonatal death (combined) occurred in 5% in the DTG+FTC/TAF group and 7% in each of the other groups (no significant differences, Table S19).
Congenital anomalies
Three major congenital anomalies were reported: talipes equinovarus of one foot (DTG+FTC/TAF group), duodenal atresia/ileal stenosis (EFV/FTC/TDF group), and subgaleal cyst (EFV/FTC/TDF group). Twenty additional infants were reported to have a potential anomaly or genetic abnormality, when including events that did not meet criteria for major anomalies:16 8 (4%) children in each of the DTG groups and 4 (2%) in the EFV group (Tables S20-S21). No patterns of abnormalities were observed.
HIV infection
Five hundred and sixty-one (91%) of 617 live-born infants had at least one HIV NAT result available. Two (0·4%) of these 561 infants had at least one positive HIV-1 NAT (Table S22). One was in the DTG+FTC/TAF group; the mother had detectable HIV-1 RNA at enrollment (26 weeks’ gestation), delivery (39 weeks’ gestation), and each antepartum visit. The second infant was in the DTG+FTC/TDF group; the mother had HIV-1 RNA <40 copies/mL at enrollment (25 weeks’ gestation) and at delivery (41 weeks’ gestation), with highest measured HIV-1 RNA (42 copies/mL) 4 weeks after enrollment.
DISCUSSION
When started between 14 and 28 weeks of pregnancy, all three ART regimens studied in this trial led to high rates of virologic suppression. DTG-based regimens had a higher rate of viral suppression at delivery and shorter time to viral suppression than EFV-based regimens. DTG+FTC/TAF had the most favorable safety profile with significantly fewer composite adverse pregnancy outcomes (stillbirth, preterm delivery, or SGA) compared with either DTG+FTC/TDF or EFV/FTC/TDF. No significant differences were observed in occurrence of grade ≥3 maternal or infant events. We also observed significantly fewer neonatal deaths with DTG-based than with EFV-based ART. Notably, this study represents the largest prospective evaluation of FTC/TAF in pregnancy.
Trials in non-pregnant adults from different continents have shown equal or greater efficacy and shorter time to viral suppression with DTG-containing ART compared with EFV-containing ART.18-20 Our virologic efficacy findings are consistent with these results. In prior studies, EFV-containing regimens were associated with more adverse events than DTG regimens, explaining at least some of their reported differences in efficacy (particularly in studies using the FDA Snapshot efficacy endpoint). In our trial, more women in the EFV than the DTG groups modified treatment prior to delivery (with low rates of modification in all groups); however, rates of maternal adverse events did not differ by group, missed visits were infrequent and similar between groups, and results of our ITT and per-protocol analyses were very similar. In addition, time to viral suppression was significantly shorter with DTG- vs. EFV-based ART in prior trials,18-20 in our trial, and in the DolPHIN-2 trial, which compared the virologic efficacy of DTG- vs. EFV-containing ART started late in pregnancy in total of 268 women.21 Finally, some women participating in IMPAACT 2010 might have had pre-existing EFV-resistant HIV-1: nearly 90% of our participants enrolled in sub-Saharan Africa, and drug resistance surveys from the region show that approximately 9-20% of women from countries in this region have pre-ART non-nucleoside reverse transcriptase resistance (i.e. to EFV).22,23 Taken together, these results collectively suggest that the greater virologic efficacy seen with DTG-ART vs. EFV-ART (in prior studies as well as our trial) is due to a combination of better tolerability of DTG compared with EFV; higher potency of DTG (with more rapid HIV-1 decline after starting DTG); and possible pre-treatment EFV resistance. Our participants had low HIV-1 RNA level at entry, likely in part related to the median 6 days of ART taken prior to enrollment; roughly one quarter of women had HIV-1 RNA<200 copies/mL, all of whom maintained viral suppression until delivery.
The safety and toxicity of widely used ART regimens during pregnancy are of global interest. Especially in low and middle-income regions, preterm delivery and SGA are associated with substantial childhood morbidity and mortality.24-26 We observed significantly fewer neonatal deaths (but a non-significant trend toward more stillbirths) in the DTG groups compared with the EFV group. The majority of neonatal deaths occurred in full-term babies within 1 week of birth, often due to pregnancy/delivery complications. The combined frequency of either stillbirth or neonatal death was reassuringly similar in all three groups. A large observational study in Botswana found similar rates of stillbirth and of neonatal death in women initiating DTG-based and EFV-based ART in pregnancy.27
The reasons for the lower rate of the composite adverse pregnancy outcome with DTG+FTC/TAF (and of preterm delivery with DTG+FTC/TAF compared with EFV/FTC/TDF) are not known. Women in the DTG+FTC/TAF group had the highest weekly 2nd- and 3rd-trimester weight gain (DTG+FTC/TAF was also found to be associated with the greatest weight gain of the three same regimens that were studied in the ADVANCE trial).20 This weight gain was closer to (and still less than) the recommended weight gain in pregnancy (0·42 kg/week).17 A prior observational study in Botswana found that inadequate weekly weight gain in pregnancy was significantly more frequent in women starting EFV/FTC/TDF than DTG+FTC/TDF, and both groups had lower weight gain than women without HIV.28 Inadequate weight gain in pregnancy is associated with preterm birth and SGA.29 Tenofovir and/or EFV may also lead to adverse pregnancy outcomes through mechanisms other than inadequate weight gain. TDF yields free plasma levels of tenofovir that are approximately 10-fold higher than levels with TAF (but lower intracellular tenofovir levels).30 The PROMISE trial showed significantly higher rates of both severe adverse pregnancy outcome and early infant death in mothers starting TDF/FTC-containing vs. zidovudine-containing ART (each with lopinavir-ritonavir [LPV/r]);31 it is unclear whether these adverse outcomes were related to maternal LPV/r, TDF, the combination thereof, or unrelated to maternal regimen. In the Botswana Tsepamo birth outcomes surveillance study (which included data from 5,780 women taking ART from conception), rates of severe adverse birth outcome were lowest in women taking EFV/FTC/TDF (36%) and highest in women taking LPV/r/FTC/TDF (49%).32 The Tsepamo study also detected an association between DTG use from conception and neural tube defects (although the association has become less strong over time).6,33,34
Rates of grade 3 or higher toxicities did not differ between regimen groups in either mothers (through delivery) or neonates (through day 28 of life). Maternal estimated creatinine clearance was lower with DTG than EFV and was significantly lower in the DTG+FTC/TDF group (135 mL/min) than in the other two treatment groups. DTG is known to cause a reversible non-pathological increase (of 10-15%) in creatinine levels mediated by inhibition of OCT-2; TDF is associated with slightly lower blood creatinine values compared with TAF due to higher plasma tenofovir levels. However, the differences in estimated creatinine clearance that we observed were small and not of clinical importance in the study participants. Clinically relevant creatinine elevation was infrequent in mothers and infants and did not appear to differ by arm (although the study was not powered to compare this outcome between arms).
Our trial has several strengths. VESTED is one of very few randomized ART trials in pregnancy.21,35,36 Retention and data completeness were very high. Our study also had a number of limitations. We enrolled women starting at 14 weeks gestation and could not evaluate effects of drug exposure at conception/early in pregnancy on adverse pregnancy outcomes (including neural tube defects33 or spontaneous abortion)37 in women conceiving on ART, who now represent the majority of pregnant women with HIV-1. The study was open-label, used EFV at 600mg (rather than 400mg) which may be associated with more side effects, and did not screen for pre-existing drug resistance. Four fifths of women took a median of 6 days of ART prior to enrollment (permitted per protocol for ethical reasons), and 28% of women had HIV1 RNA <200 copies/mL at study entry; this could have led to higher rates of viral suppression at delivery. However, results of analyses restricted to women with HIV-1 RNA ≥200 copies/mL at entry were very similar to primary results, showing high rates of virologic suppression and a slightly larger difference in proportions of women with viral suppression at delivery (favoring DTG-ART). We excluded women with multiple gestation or known fetal anomaly or other medical conditions, which may have led to lower overall rates of adverse pregnancy outcome. Gestational age was estimated using 2nd trimester fetal ultrasound which is not as accurate for dating as 1st trimester ultrasound (and could have led to misclassification in small for gestational age and pre term outcomes); however, this was one of few trials conducted in primarily resource-constrained settings in which all women underwent fetal ultrasound. This paper only reports maternal and child outcomes through 14 days postpartum / 28 days of life, respectively. Finally, the study was not powered to detect differences in rare adverse outcomes nor in perinatal HIV-1 transmission.
The VESTED trial demonstrated that the efficacy and safety observed with DTG-based ART in non-pregnant adults are also evident when started in pregnancy and can lead to improved HIV-1 suppression at delivery. We also observed an unexpected decrease in the composite adverse pregnancy outcome and neonatal death with DTG+FTC/TAF. These findings affirm the WHO recommendation to use DTG in all populations,38 including after the first trimester of pregnancy, and suggest that TAF may be preferred over TDF when started during pregnancy (when taken with DTG and FTC) due to lower risk of adverse pregnancy outcomes.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study4
More than one million women with HIV become pregnant each year. Prompt initiation of antiretroviral treatment (ART) is recommended for pregnant women to optimize their health outcomes and minimize perinatal HIV-1 transmission. Unfortunately, high-quality pregnancy safety and efficacy data are lacking for most antiretrovirals, including those recommended by the World Health Organization (WHO) for first-line treatment of adults with HIV: dolutegravir (DTG) plus [emtricitabine (FTC) or lamivudine (3TC)] plus tenofovir disoproxil fumarate (TDF) (or tenofovir alafenamide fumarate [TAF] rather than TDF, per International AIDS Society [IAS-USA] guidelines). Prior trials of modest size have demonstrated high virologic efficacy of DTG-containing ART in pregnancy. However, these trials were not powered to evaluate adverse pregnancy outcomes with DTG. A small number of observational studies have described pregnancy outcomes with DTG, but these lack detailed data and are subject to biases. Furthermore, almost no pregnancy data exist regarding the safety and efficacy of TAF, a drug that may replace TDF in first-line treatment due to lower bone and renal toxicity and lower manufacturing cost. Prior evidence was sought in PubMed and abstracts from major HIV-related conferences from 2000 to 2020 using the search terms HIV, pregnancy, dolutegravir, efavirenz, tenofovir alafenamide fumarate and TAF, tenofovir disoproxil fumarate and TDF, safety, efficacy, viral suppression, pregnancy outcome, adverse events, randomized, trial.
Added value of this study
This is one of very few randomized trials to compare the safety and efficacy of HIV treatment regimens started in pregnancy. Pregnant women with HIV from 9 countries were randomized 1:1:1 at 14–28 weeks’ gestation to start one of three regimens: 217 women to DTG plus FTC/TAF (DTG+FTC/TAF); 215 to DTG plus FTC/TDF (DTG+FTC/TDF); and 211 to efavirenz/FTC/TDF (EFV/FTC/TDF). Rates of HIV-1 RNA suppression (to <200 copies/mL) at delivery were high in all study groups (98% in the combined DTG groups and 91% in the EFV/FTC/TDF group), with the combined DTG regimens meeting criteria for virologic superiority at delivery compared with EFV/FTC/TDF (difference 7% [95%CI 2%, 11%]; p=0·005). The composite adverse pregnancy outcome (occurrence of preterm delivery, small for gestational age, stillbirth, or spontaneous abortion) was observed in significantly fewer women assigned to DTG+FTC/TAF (24%) compared with women assigned to either DTG+FTC/TDF (33%, p=0·043) or EFV/FTC/TDF (33%, p=0·047). Women in the EFV/FTC/TDF group experienced significantly higher rates of preterm delivery than women in the DTG+FTC/TAF group. Neonatal mortality (but not stillbirth) was also more frequent with EFV/FTC/TDF (5%) than with DTG+FTC/TAF (1·0%, p=0·019) or DTG+FTC/TDF (1·5%, p=0·050). Grade 3 or higher maternal and infant adverse events did not differ by group.
Implications of all the available evidence
We evaluated the safety and virologic efficacy in pregnancy of antiretroviral regimens that are likely to be used by large numbers of women initiating treatment for HIV during pregnancy, but for which minimal rigorous pregnancy safety and efficacy data exist. We demonstrated that DTG-based ART started in pregnancy has superior virologic efficacy at delivery compared with EFV/FTC/TDF. We also observed with DTG+FTC/TAF, compared with DTG+FTC/TDF or with EFV/FTC/TDF, an unexpected decrease in the cumulative rate of the composite adverse pregnancy outcome and a lower rate of neonatal death. Findings from this trial affirm the WHO recommendation to use DTG in all populations, including women starting antiretroviral treatment during pregnancy, and suggest that it may be preferable to initiate TAF rather than TDF during pregnancy (in combination with DTG and FTC), due to lower risk of adverse pregnancy outcomes.
Acknowledgements
We thank and acknowledge the trial participants; the site investigators, site staff and collaborating institutions, and the local/IMPAACT Community Advisory Board members who supported this trial; IMPAACT network leadership; the National Institute of Allergy and Infectious Diseases African Data and Safety Monitoring Board members; the National Institute of Allergy and Infectious Diseases Therapeutics and Prevention Data and Safety Monitoring Board members; and the Safety Review Group members.
Sources of support/funding
This study was funded and sponsored by the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network. Overall support for the IMPAACT Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. SH was supported by the Developing Research Innovation, Localisation and Leadership (DRILL) program, funded under Award Number D43TW010131 by the National Institutes of Health (NIH) through the Fogarty International Center (FIC); the NIH Common Fund and the Office of AIDS Research, Office of the Director; and NIMH. Study drugs were provided by ViiV Healthcare, Gilead Sciences, and Mylan Pharmaceuticals.
Appendix
IMPAACT Study 2010 Team members, in addition to co-authors: Brookie M. Best, PharmD, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego; Cheryl Blanchette, MS, FHI 360; Renee Browning, MSN, Henry M. Jackson Foundation, National Institute for Allergy and Infectious Diseases; US National Institutes of Health; Yao Cheng, PhD, Medicines Patent Pool; Andee Fox, MPH, Frontier Science & Technology Research Foundation; Nagawa Jaliaah, Mulago Hospital; Kevin Knowles, PhD, Frontier Science & Technology Research Foundation; Mark Mirochnick, MD, Boston University School of Medicine/Boston Medical Center; William A. Murtaugh, MPH, UCLA Health; Emmanuel Patras, Mylan Laboratories Limited; Mauricio Pinilla, MS, Statistical and Data Analysis Center, Harvard T.H. Chan School of Public Health; Jean van Wyk, MBChB, ViiV Healthcare; and Frances Whalen, MPH, Pediatric Infectious Diseases Division, University of California, Los Angeles.
Footnotes
Declaration of interests
JDM has received research support from Gilead, paid to his institution. NKT is an employee of ViiV Healthcare. JFR is an employee and stockholder of Gilead Sciences. KRA has received fees from Gilead Sciences for expert consultation. JC has received fees from Merck & Co. for service on a Scientific Advisory Board. PES has received research support, and fees for service on Scientific Advisory Boards, from Gilead Sciences and ViiV Healthcare. The other authors declare no competing interests.
Data sharing statement
This study’s data cannot be made publicly available due to ethical restrictions in the study’s informed consent documents and in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data, including participant data with partially identifying information, are available to interested researchers upon request to the IMPAACT Statistical and Data Management Center’s Data Access Committee (sdac.data@fstrf.org) with the agreement of the IMPAACT Network.
Contributor Information
Shahin Lockman, Division of Infectious Disease, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA; Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Sean S. Brummel, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA
Lauren Ziemba, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Lynda Stranix-Chibanda, College of Health Sciences, University of Zimbabwe, Harare, Zimbabwe.
Katie McCarthy, FHI 360, Durham, North Carolina, USA.
Anne Coletti, FHI 360, Durham, North Carolina, USA.
Patrick Jean-Philippe, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Ben Johnston, Frontier Science Foundation, Amherst, New York, USA.
Chelsea Krotje, Frontier Science Foundation, Amherst, New York, USA.
Lee Fairlie, Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Risa M. Hoffman, David Geffen School of Medicine, Division of Infectious Diseases, University of California, Los Angeles, Los Angeles, California, USA
Paul E. Sax, Division of Infectious Disease, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Sikhulile Moyo, Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA; Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Nahida Chakhtoura, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA.
Jeffrey S. A. Stringer, Division of Global Women’s Health, Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Gaerolwe Masheto, Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA; Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Violet Korutaro, Baylor College of Medicine Children’s Foundation, Kampala, Uganda.
Haseena Cassim, Perinatal HIV Research Unit, University of the Witwatersrand, Johannesburg, South Africa.
Blandina T. Mmbaga, Kilimanjaro Clinical Research Institute, Kilimanjaro Christian Medical Centre and Kilimanjaro Christian Medical University College, Moshi, Tanzania
Esau João, Hospital Federal dos Servidores do Estado, Rio de Janeiro, Brazil.
Sherika Hanley, Centre for the AIDS Programme of Research in South Africa, University of KwaZulu-Natal, Umlazi, South Africa.
Lynette Purdue, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Lewis B. Holmes, MassGeneral Hospital for Children, Boston, Massachusetts, USA.
Jeremiah D. Momper, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, La Jolla, California, USA
Roger L. Shapiro, Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA; Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana
Navdeep K. Thoofer, ViiV Healthcare, Brentford, Middlesex, UK
James F. Rooney, Gilead Sciences, Foster City, California, USA
Lisa M. Frenkel, Departments of Pediatrics, Laboratory Medicine, Global Health, and Medicine, University of Washington, and Seattle Children’s Research Institute, Seattle, Washington, USA.
K. Rivet Amico, Department of Health Behavior and Health Education, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Lameck Chinula, Division of Global Women’s Health, Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill, Chapel Hill, Lilongwe, Malawi.
Judith Currier, David Geffen School of Medicine, Division of Infectious Diseases, University of California, Los Angeles, Los Angeles, California, USA.
References
- 1.World Health Organization (WHO). Prevention of mother-to-child transmission: estimates by WHO region. 2019. http://apps.who.int/gho/data/view.main.23500REG?lang=en (accessed 22 June 2020).
- 2.World Health Organization (WHO). Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization, 2018. https://www.who.int/hiv/pub/guidelines/ARV2018update/en/. [Google Scholar]
- 3.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385(9987): 2606–15. [DOI] [PubMed] [Google Scholar]
- 4.Ripin D, Prabhu VR. A cost-savings analysis of a candidate universal antiretroviral regimen. Curr Opin HIV AIDS 2017; 12(4): 403–7. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS 2019; 33(9): 1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 2018; 379(10): 979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384(9946): 857–68. [DOI] [PubMed] [Google Scholar]
- 8.Villar J, Giuliani F, Fenton TR, et al. INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016; 387(10021): 844–5. [DOI] [PubMed] [Google Scholar]
- 9.Zelen M The randomization and stratification of patients to clinical trials. J Chronic Dis 1974; 27(7-8): 365–75. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Obstetric Practice, American Institute of Ultrasound in Medicine, Society for Maternal-Fetal Medicine. Committee opinion no 700: methods for estimating the due date. Obstet Gynecol 2017; 129(5): e150–e4. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano R, Dhami J, Ohuma EO, et al. Pregnancy dating by fetal crown-rump length: a systematic review of charts. BJOG 2014; 121(5): 556–65. [DOI] [PubMed] [Google Scholar]
- 12.Papageorghiou AT, Kemp B, Stones W, et al. Ultrasound-based gestational-age estimation in late pregnancy. Ultrasound Obstet Gynecol 2016; 48(6): 719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zash R, Souda S, Chen JY, et al. Reassuring birth outcomes with tenofovir/emtricitabine/efavirenz used for prevention of mother-to-child transmission of HIV in Botswana. J Acquir Immune Defic Syndr 2016; 71(4): 428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Center for Drug Evaluation and Research (CDER). Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment: guidance for industry. Clinical/antimicrobial revision 1. Silver Spring, MD: Food and Drug Administration (FDA), US Department of Health and Human Services, November 2015. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-immunodeficiency-virus-1-infection-developing-antiretroviral-drugs-treatment. [Google Scholar]
- 15.Division of AIDS. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1. Bethesda, MD: National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services, July 2017. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. [Google Scholar]
- 16.Holmes LB, Westgate MN. Inclusion and exclusion criteria for malformations in newborn infants exposed to potential teratogens. Birth Defects Res A Clin Mol Teratol 2011; 91(9): 807–12. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria Siega-Riz A. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol 2010; 116(5): 1191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 2012; 12(2): 111–8. [DOI] [PubMed] [Google Scholar]
- 19.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369(19): 1807–18. [DOI] [PubMed] [Google Scholar]
- 20.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381(9): 803–15. [DOI] [PubMed] [Google Scholar]
- 21.Kintu K, Malaba TR, Nakibuka J, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV 2020; 7(5): e332–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chimukangara B, Lessells RJ, Rhee SY, et al. Trends in pretreatment HIV-1 drug resistance in antiretroviral therapy-naive adults in South Africa, 2000-2016: a pooled sequence analysis. EClinicalMedicine 2019; 9: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO). HIV drug resistance report 2019. Geneva: World Health Organization, 2019. https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/. [Google Scholar]
- 24.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388(10063): 3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med 2016; 21(2): 74–9. [DOI] [PubMed] [Google Scholar]
- 26.Lee AC, Kozuki N, Cousens S, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ 2017; 358: j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zash R, Jacobson DL, Diseko M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health 2018; 6(7): e804–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caniglia E, Shapiro R, Diseko M, et al. Weight gain during pregnancy among women initiating dolutegravir in Botswana. IAS 2019: 10th IAS Conference on HIV Science; 21-24 July 2019; Mexico City. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group, Voerman E, Santos S, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019; 321(17): 1702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr 2013; 63(4): 449–55. [DOI] [PubMed] [Google Scholar]
- 31.Fowler MG, Qin M, Fiscus SA, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375(18): 1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zash R, Jacobson DL, Diseko M, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017; 171(10): e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019; 381(9): 827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zash R, Holmes L, Diseko M, et al. Update on neural tube defects with antiretroviral exposure in the Tsepamo study, Botswana. AIDS 2020: 23rd International AIDS Conference; 6-10 July 2020; Virtual. Abstract no. OAXLB0102. [Google Scholar]
- 35.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010; 362(24): 2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joao EC, Morrison RL, Shapiro DE, et al. Raltegravir versus efavirenz in antiretroviral-naive pregnant women living with HIV (NICHD P1081): an open-label, randomised, controlled, phase 4 trial. Lancet HIV 2020; 7(5): e322–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theron G, Brummel S, Fairlie L, et al. Pregnancy outcomes of women conceiving on antiretroviral therapy (ART) compared to those commenced on ART during pregnancy. Clin Infect Dis 2020. June 20: ciaa805. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO). Update of recommendations on first- and second-line antiretroviral regimens. Geneva: World Health Organization, July 2019. https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


