Abstract
OBJECTIVE
Prospective associations between n-3 fatty acid biomarkers and type 2 diabetes (T2D) risk are not consistent in individual studies. We aimed to summarize the prospective associations of biomarkers of α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) with T2D risk through an individual participant-level pooled analysis.
RESEARCH DESIGN AND METHODS
For our analysis we incorporated data from a global consortium of 20 prospective studies from 14 countries. We included 65,147 participants who had blood measurements of ALA, EPA, DPA, or DHA and were free of diabetes at baseline. De novo harmonized analyses were performed in each cohort following a prespecified protocol, and cohort-specific associations were pooled using inverse variance–weighted meta-analysis.
RESULTS
A total of 16,693 incident T2D cases were identified during follow-up (median follow-up ranging from 2.5 to 21.2 years). In pooled multivariable analysis, per interquintile range (difference between the 90th and 10th percentiles for each fatty acid), EPA, DPA, DHA, and their sum were associated with lower T2D incidence, with hazard ratios (HRs) and 95% CIs of 0.92 (0.87, 0.96), 0.79 (0.73, 0.85), 0.82 (0.76, 0.89), and 0.81 (0.75, 0.88), respectively (all P < 0.001). ALA was not associated with T2D (HR 0.97 [95% CI 0.92, 1.02]) per interquintile range. Associations were robust across prespecified subgroups as well as in sensitivity analyses.
CONCLUSIONS
Higher circulating biomarkers of seafood-derived n-3 fatty acids, including EPA, DPA, DHA, and their sum, were associated with lower risk of T2D in a global consortium of prospective studies. The biomarker of plant-derived ALA was not significantly associated with T2D risk.
Introduction
n-3 polyunsaturated fatty acids (n-3 PUFAs), especially those from marine sources, can improve cardiometabolic risk factors and may have a role in the prevention of type 2 diabetes (T2D) (1,2). Meta-analyses of short-term randomized controlled trials (RCTs) have indicated that fish oil supplementation may reduce adiposity, increase adiponectin, lower circulating triglycerides and inflammatory markers (3,4), and modestly improve glycemic control (4,5). However, observational studies on fish/seafood intake and T2D risk have been conflicting (6). In particular, fish/seafood intake was positively associated with T2D risk in North America, whereas an inverse association was observed in Asia (6).
Compared with self-reported dietary consumption, circulating n-3 PUFA biomarkers are not subject to recall bias and allow for objective assessment of individual n-3 PUFAs (1). In addition, biomarkers represent the combined influence of diet and metabolism and thus may better reflect bioavailable n-3 PUFA intake. However, in contrast with studies of self-reported dietary habits, fewer studies have examined objective n-3 PUFA biomarkers with incident T2D (7,8), and existing evidence is inconclusive. In addition to sample size limitations in certain biomarker studies, publication bias and inability to assess heterogeneity by participant characteristics also hinder the further understanding of potentially important associations between n-3 PUFAs and T2D. Clearly, additional research that addresses these limitations is warranted. The importance of fish/seafood consumption in many populations (9), the increased availability of n-3–fortified foods such as dairy products and eggs (10), and increasing use of fish oil supplements (11) all render the relationship of n-3 PUFAs with T2D an important scientific, clinical, and public health question (12,13).
To fill this knowledge gap, we compiled data from 20 prospective studies participating in the Fatty Acids and Outcomes Research Consortium (FORCE) to evaluate seafood- or plant-derived biomarkers of n-3 PUFAs in relation to incident T2D. We hypothesized that higher seafood- or plant-derived n-3 PUFA biomarker levels are associated with lower T2D risk.
Research Design and Methods
FORCE
FORCE (https://force.nutrition.tufts.edu/) originated from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. Detailed information has previously been published (14,15). Twenty cohorts agreed to be included in the present analysis and were selected based on prospective study design (cohort or case-cohort), availability of fatty acid biomarkers of interest, and ascertainment of T2D.
We included adults (age ≥18 years) with measurements for one or more of α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), or docosahexaenoic acid (DHA) biomarkers and who were free of diabetes at baseline. Each participating study received corresponding approval from their institutional review boards, and all participants provided informed consent.
Fatty Acid Assessments
Fatty acid measurements in each cohort were performed with use of gas chromatography in varying lipid compartments including adipose tissue, erythrocyte/plasma phospholipids, total plasma/serum, cholesterol ester, and plasma triglycerides. Cohort-specific protocols for the measurement and quantification of fatty acids can be found in Supplementary Approaches. Concentrations of each fatty acid were expressed as a percentage of total fatty acids in their respective lipid compartments. Prior analyses have demonstrated reasonable long-term reproducibility of n-3 fatty acid measurements over the span of 6–13 years, with Spearman coefficients of 0.40–0.65 for ALA, 0.59–0.76 for EPA, 0.63–0.78 for DPA, and 0.71–0.80 for DHA (16).
Assessment of Incident T2D
The outcome of T2D was ascertained with one or more of the following definitions: 1) fasting glucose ≥126 mg/dL (7.0 mmol/L), 2) HbA1c ≥6.5%, 3) 2-h oral glucose tolerance test ≥200 mg/dL (11.1 mmol/L), 4) self-reported use of oral hypoglycemic medications or insulin, and 5) self-reported physician diagnosis or linkage to disease registries. Cohort-specific details regarding diabetes ascertainment are presented in Supplementary Approaches.
Statistical Analyses
Pearson correlation coefficients were calculated between each individual n-3 fatty acid as well as with the sum of EPA, DPA, and DHA for assessment of the degree of correlation between each biomarker. For the two studies that used a case-cohort design (European Prospective Investigation into Cancer and Nutrition InterAct Consortium [EPIC-InterAct] and Melbourne Collaborative Cohort Study [MCCS]), correlation coefficients were obtained among participants in the subcohort. Individual participant–level de novo analysis was performed in each cohort with use of a prespecified harmonized protocol. For prospective cohort studies, Cox proportional hazards models were used to calculate hazard ratio (HR) and corresponding 95% CI. Follow-up duration was calculated from the time of fatty acid measurement to the time of incident T2D diagnosis, death, loss to follow-up, or the end of follow-up—whichever came first. For prospective case-cohort studies, HRs and 95% CIs were obtained after application of the appropriate sampling weights. In the MCCS, time-to-event information was not available, and therefore logistic regression was used to calculate the odds ratios as an estimate of the HR.
For facilitation of comparability across different lipid compartments, the concentration of each fatty acid biomarker was standardized to the interquintile range (defined as the difference between the 10th and 90th percentile of fatty acid concentrations). Potential nonlinearity was examined for each biomarker with use of cohort-specific quintiles.
Prespecified covariates included sex (male, female), age (years), field site, race/ethnicity (with White as the reference group), education (less than high school, high school graduate, college or higher, or cohort-specific categories), smoking status (never, former, current), physical activity (in kcal/week, METs/week, or h/day), alcohol consumption (drinks or servings/day, g/day, or mL/day), treatment for or presence of hypertension (yes, no), treatment for or presence of hypercholesterolemia (yes, no), prevalent coronary heart disease (yes, no), BMI (kg/m2), waist circumference (cm), and circulating linoleic acid (LA) (18:2n-6) and trans fatty acids (total t-18:1 and t-18:2) (as % of total fatty acids). Individuals with missing categorical covariates were included with use of a missing indicator. In secondary analyses, additional adjustments were made for circulating triglycerides (mg/dL or mmol/L) as well as fish/seafood intake (servings/week or otherwise defined in each cohort, as measured by dietary questionnaires). To examine the robustness of associations, we conducted sensitivity analyses by excluding T2D diagnosed within the first 2 years of follow-up to minimize reverse causation biases (17) and restricting to the first 6 years of follow-up to reduce misclassification due to within-person changes in fatty acid concentrations over time.
Estimates of relative risks (RRs) (including HRs or odds ratios) and corresponding SEs from individual studies were pooled with use of inverse variance–weighted meta-analysis. To assess the robustness of our findings, we also used a random-effects model. Since several studies had measured fatty acids in multiple lipid compartments, the risk estimate from a single compartment was selected for the pooled analysis. We chose the lipid compartment that can best reflect long-term dietary intake in the following sequence: adipose tissue > erythrocyte phospholipids > plasma phospholipids > total plasma/serum > cholesterol esters > plasma triglycerides (18,19). The consistency of associations across different lipid compartments was also assessed. Heterogeneity in the overall and compartment-specific analyses was evaluated with use of the I2 statistic.
Several prespecified subgroup analyses were conducted by global region, age, sex, race/ethnicity, BMI, LA biomarkers, and triglycerides. Heterogeneity between subgroups was assessed with inverse variance–weighted meta-regression. For these exploratory analyses, a more stringent Bonferroni-adjusted P value of <0.0014 (5 fatty acids × 7 subgroups) was used to denote statistical significance. In six cohorts (Framingham Heart Study [FHS], Atherosclerosis Risk in Communities study [ARIC], Hisayama Study, Three City Study [3C], Finnish Diabetes Prevention Study [FDPS], and Women’s Health Initiative Memory Study [WHIMS]), we calculated a weighted T2D genetic risk score (GRS) using 35 single nucleotide polymorphisms found to be significantly associated with T2D risk in prior genome-wide association studies (20) (Supplementary Approaches). Interactions between n-3 biomarkers and the GRS were examined.
In sensitivity analyses, we performed a dose-response meta-analysis within individual lipid compartments to assess for potential nonlinearity between each biomarker and risk of T2D. Restricted cubic splines that used three knots (at the 10th, 50th, and 90th percentiles of fatty acids in each compartment) were used to model the association.
Statistical analyses were performed with Stata 15.1 (StataCorp, College Station, TX). P values <0.05 were deemed to be statistically significant unless otherwise specified.
Results
Select baseline characteristics for the studies and participants are presented in Table 1. We included 18 prospective cohorts and 2 prospective case-cohorts from 14 countries in North America, Europe/Australia, and Asia. The majority of participants were of European ancestry. The cohort-specific mean ages ranged from 49.7 to 75.5 years and mean BMI ranged from 23.1 to 31.1 kg/m2. Additional baseline characteristics can be found in Supplementary Tables 1 and 2.
Table 1.
Baseline characteristics of studies in the pooled analysis of n-3 fatty acid biomarkers with 65,147 participants and 16,693 incident cases of T2D
| Study | Country | Study design | Baseline year | Total N/n diabetes cases | Maximum follow-up, years | Age, years | Women, % | BMI, kg/m2 | Biomarker compartment | Fatty acids assessed, n |
|---|---|---|---|---|---|---|---|---|---|---|
| AGES-R | Iceland | Prospective cohort | 2002–2006 | 753/28 | 7.8 | 75.5 (5.2) | 59.5 | 27.0 (4.0) | Plasma phospholipids | 41 |
| AOC | The Netherlands | Prospective cohort | 2002–2006 | 779/38 | 4.8 | 68.9 (5.6) | 20.8 | 27.3 (3.6) | Plasma phospholipids | 38 |
| ARIC | U.S. | Prospective cohort | 1987–1989 | 3,273/512 | 9.0 | 54.4 (5.9) | 45.5 | 26.0 (4.6) | Plasma phospholipids | 29 |
| CCCC | Taiwan | Prospective cohort | 1992–1995 | 1,443/651 | 10.4 | 60.1 (10.1) | 43.2 | 23.1 (3.3) | Total plasma | 29 |
| CHS | U.S. | Prospective cohort | 1992–1993 | 3,007/291 | 18.0 | 75.1 (5.3) | 59.8 | 26.4 (4.6) | Plasma phospholipids | 42 |
| EPIC-InterAct | 8 European countries | Prospective case-cohort | 1993–1997 | 27,296/12,132 | 17.5 | 52.3 (9.2) | 62.3 | 26.0 (4.2) | Plasma phospholipids | 37 |
| FDPS | Finland | Prospective cohort | 1993–1998 | 396/161 | 16.0 | 55.5 (7.2) | 67.9 | 31.1 (4.7) | Total serum | 30 |
| FHS | U.S. | Prospective cohort | 2005–2008 | 1,872/95 | 9.0 | 64.5 (8.3) | 57.5 | 27.7 (5.0) | Erythrocyte phospholipids | 33 |
| Hisayama | Japan | Prospective cohort | 2002–2003 | 2,172/222 | 7.0 | 58.5 (10.5) | 60.0 | 23.1 (3.2) | Total serum | 24 |
| HPFS | U.S. | Prospective cohort | 1994 | 1,491/108 | 20.2 | 64.5 (8.6) | 0.0 | 25.8 (3.2) | Total plasma, erythrocyte phospholipids | 37 |
| KIHD | Finland | Prospective cohort | 1984–1989 for men, 1998–2001 for women | 3,389/595 | 26.8 | 55.5 (7.1) | 29.5 | 27.1 (3.9) | Total serum | 14 |
| MCCS | Australia | Prospective case-cohort | 1990–1994 | 4,034/335 | 9.9 | 55.0 (8.6) | 55.5 | 26.7 (4.3) | Plasma phospholipids | 53 |
| MESA | U.S. | Prospective cohort | 2000–2002 | 2,099/285 | 11.2 | 61.0 (12.0) | 54.7 | 27.6 (5.4) | Plasma phospholipids | 27 |
| METSIM | Finland | Prospective cohort | 2006–2010 | 1,302/101 | 7.9 | 55.0 (5.6) | 0.0 | 26.4 (3.5) | Plasma phospholipids, erythrocyte phospholipids, cholesterol esters, triglycerides | 22 |
| NHS | U.S. | Prospective cohort | 1990 | 1,446/149 | 24.8 | 60.4 (6.4) | 100.0 | 25.3 (4.4) | Total plasma, erythrocyte phospholipids | 37 |
| PIVUS | Sweden | Prospective cohort | 2001–2004 | 872/69 | 10.9 | 70.2 (0.2) | 51.1 | 26.8 (4.1) | Plasma phospholipids, cholesterol ester | 16 |
| 3C | France | Prospective cohort | 1999–2000 | 1,218/83 | 13.0 | 74.4 (4.8) | 61.8 | 26.0 (4.0) | Total plasma | 35 |
| ULSAM-50 | Sweden | Prospective cohort | 1970–1973 | 1,899/249 | 42.3 | 49.7 (0.6) | 0.0 | 25.0 (3.2) | Cholesterol ester | 17 |
| ULSAM-70 | Sweden | Prospective cohort | 1991–1995 | 738/99 | 21.5 | 71.0 (0.6) | 0.0 | 26.2 (3.2) | Adipose tissue | 17 |
| WHIMS | U.S. | Prospective cohort | 1995 | 5,668/490 | 14.1 | 70.1 (3.8) | 100.0 | 28.1 (5.5) | Erythrocyte phospholipids | 22 |
Data are means ± SD unless otherwise indicated. Studies with measurements in multiple lipid compartments included the Health Professionals Follow-up Study (HPFS), Metabolic Syndrome in Men (METSIM) study, Nurses’ Health Study (NHS), and Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). AOC, Alpha Omega Cohort; CCCC, Chin-Shan Community Cardiovascular Cohort study; CHS, Cardiovascular Health Study; Hisayama, Hisayama Study; HPFS, Health Professionals Follow-up Study; KIHD, Kuopio Ischemic Heart Disease Risk Factor Study; MESA, Multi-Ethnic Study of Atherosclerosis; METSIM, Metabolic Syndrome in Men Study; NHS, Nurses’ Health Study; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors.
Lipid compartments measured across the studies included phospholipids (13 studies), total plasma/serum (6 studies), cholesterol esters (3 studies), triglycerides (1 study), and adipose tissue (1 study). Four studies measured fatty acids in multiple lipid compartments (Table 1 footnote and Supplementary Fig. 1). Pearson correlations between ALA and the other n-3 PUFAs were generally weak to modest (|r| < 0.3) (Supplementary Table 3). The correlations of EPA, DPA, and DHA were stronger, ranging from 0.4 to 0.8. Fish oil supplement usage was rare (<5%), except in the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-R) with 69.4% ever use (Supplementary Table 1). Habitual fish/seafood intake was quantified differently across cohorts (e.g., servings per day or grams per day) but tended to be higher in East Asian and Nordic countries (Supplementary Table 2).
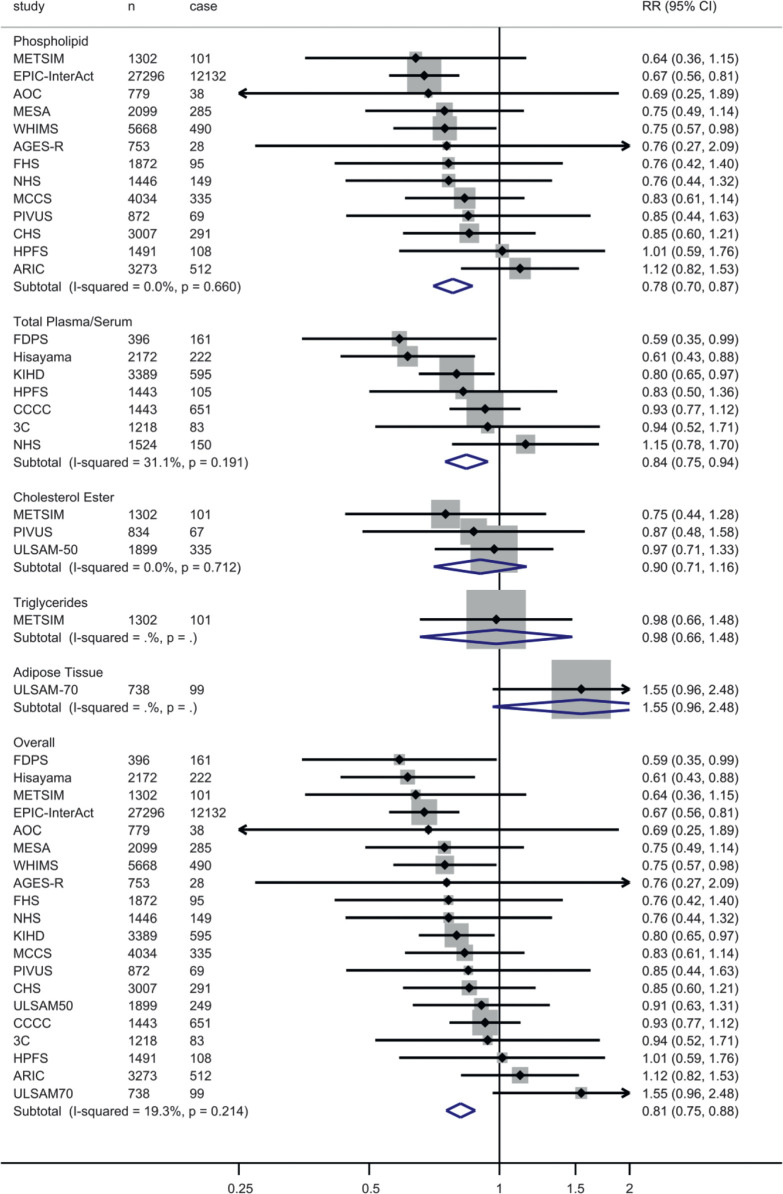
After 586,497 person-years of follow-up among 65,147 participants (median follow-up ranged from 2.5 to 21.2 years), a total of 16,693 incident cases of T2D were ascertained. In the primary pooled analysis, ALA was not significantly associated with T2D (RR per interquintile range 0.97 [95% CI 0.92, 1.02; I2 = 48.6%]) (Supplementary Fig. 2A and Table 2). In contrast, higher EPA (RR 0.92 [95% CI 0.87, 0.96]), DPA (0.79 [0.73, 0.85]), DHA (0.82 [0.76, 0.89]), and EPA + DPA + DHA (0.81 [0.75, 0.88]) were associated with lower diabetes incidence (Supplementary Fig. 2B–D, Fig. 1, and Table 2). There was moderate heterogeneity for the associations of EPA (I2 = 39.0%) and DPA (I2 = 44.5%) and low heterogeneity for DHA (I2 = 27.8%) and EPA + DPA + DHA (I2 = 19.3%). Heterogeneity across different lipid compartments was not appreciable (Table 2). A positive association was seen between adipose tissue DPA and T2D (RR 1.69 [95% CI 1.03, 2.77]) in the Uppsala Longitudinal Study of Adult Men (ULSAM)-70. In post hoc analysis, exclusion of the cohort contributing the largest weight (EPIC-InterAct) did not materially alter our findings (Supplementary Table 4). A similar pattern of associations was observed in comparison of extreme quintiles of fatty acids or when a random-effects model was used (Table 2).
Table 2.
RRs of n-3 fatty acid biomarkers and incident T2D
| Exposure | Studies, n† | Cases, n† | Analysis per interquintile range | Analysis comparing Q5 vs. Q1 | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI), fixed effect‡ | RR (95% CI), random effect‡ | I2 (%) | RR (95% CI), fixed effect‡ | RR (95% CI), random effect‡ | I2 (%) | |||
| ALA | ||||||||
| Phospholipids | 13 | 14,633 | 0.96 (0.91, 1.02) | 0.99 (0.88, 1.11) | 55.3 | 0.90 (0.80, 1.02) | 0.94 (0.80, 1.12) | 31.2 |
| Total plasma/serum | 7 | 1,967 | 1.03 (0.93, 1.14) | 1.06 (0.90, 1.25) | 52.8 | 1.07 (0.92, 1.24) | 1.07 (0.92, 1.24) | 0.0 |
| Cholesterol esters | 3 | 503 | 0.91 (0.72, 1.16) | 0.91 (0.72, 1.16) | 0.0 | 0.81 (0.60, 1.10) | 0.81 (0.60, 1.10) | 0.0 |
| Triglycerides | 1 | 101 | 0.92 (0.46, 1.83) | 0.92 (0.46, 1.83) | — | 0.80 (0.36, 1.75) | 0.80 (0.36, 1.75) | — |
| Adipose tissue | 1 | 99 | 0.65 (0.36, 1.16) | 0.65 (0.36, 1.16) | — | 0.52 (0.24, 1.10) | 0.52 (0.24, 1.10) | — |
| Overall | 20 | 16,693 | 0.97 (0.92, 1.02) | 0.98 (0.90, 1.08) | 48.6 | 0.94 (0.85, 1.03) | 0.96 (0.87, 1.07) | 16.7 |
| EPA | ||||||||
| Phospholipids | 13 | 14,633 | 0.92 (0.86, 0.97) | 0.94 (0.83, 1.07) | 50.2 | 0.82 (0.73, 0.92) | 0.81 (0.72, 0.92) | 0.0 |
| Total plasma/serum | 7 | 1,967 | 0.93 (0.86, 1.00) | 0.90 (0.79, 1.02) | 42.8 | 0.81 (0.70, 0.94) | 0.81 (0.64, 1.03) | 54.4 |
| Cholesterol esters | 3 | 503 | 0.91 (0.72, 1.17) | 0.92 (0.72, 1.17) | 0.0 | 0.90 (0.66, 1.25) | 0.90 (0.66, 1.25) | 0.0 |
| Triglycerides | 1 | 101 | 1.05 (0.79, 1.39) | 1.05 (0.79, 1.39) | — | 0.92 (0.49, 1.72) | 0.92 (0.49, 1.72) | — |
| Adipose tissue | 1 | 99 | 1.12 (0.59, 2.13) | 1.12 (0.59, 2.13) | — | 0.91 (0.44, 1.89) | 0.91 (0.44, 1.89) | — |
| Overall | 20 | 16,693 | 0.92 (0.87, 0.96) | 0.92 (0.85, 0.996) | 39.0 | 0.82 (0.74, 0.89) | 0.81 (0.74, 0.89) | 0.0 |
| DPA | ||||||||
| Phospholipids | 13 | 14,633 | 0.78 (0.71, 0.85) | 0.83 (0.72, 0.94) | 32.1 | 0.78 (0.70, 0.87) | 0.78 (0.70, 0.87) | 0.0 |
| Total plasma/serum | 6 | 1,316 | 0.93 (0.84, 1.04) | 0.80 (0.62, 1.03) | 75.9 | 0.73 (0.61, 0.88) | 0.73 (0.60, 0.89) | 9.1 |
| Triglycerides | 1 | 101 | 0.62 (0.31, 1.24) | 0.62 (0.31, 1.24) | — | 0.54 (0.27, 1.05) | 0.54 (0.27, 1.05) | — |
| Adipose tissue | 1 | 99 | 1.69 (1.03, 2.77) | 1.69 (1.03, 2.77) | — | 1.87 (0.92, 3.79) | 1.87 (0.92, 3.79) | — |
| Overall | 18 | 15,793 | 0.79 (0.73, 0.85) | 0.83 (0.74, 0.94) | 44.5 | 0.77 (0.70, 0.85) | 0.77 (0.70, 0.85) | 0.0 |
| DHA | ||||||||
| Phospholipids | 13 | 14,633 | 0.80 (0.72, 0.89) | 0.80 (0.72, 0.89) | 0.0 | 0.76 (0.66, 0.87) | 0.76 (0.66, 0.88) | 3.6 |
| Total plasma/serum | 7 | 1,967 | 0.81 (0.72, 0.91) | 0.81 (0.70, 0.93) | 17.3 | 0.76 (0.65, 0.88) | 0.76 (0.65, 0.88) | 0.0 |
| Cholesterol esters | 3 | 503 | 0.93 (0.73, 1.17) | 0.93 (0.73, 1.17) | 0.0 | 0.85 (0.64, 1.15) | 0.85 (0.64, 1.15) | 0.0 |
| Triglycerides | 1 | 101 | 1.00 (0.69, 1.46) | 1.00 (0.69, 1.46) | — | 0.67 (0.37, 1.19) | 0.67 (0.37, 1.19) | — |
| Adipose tissue | 1 | 99 | 1.53 (0.99, 2.37) | 1.53 (0.99, 2.37) | — | 1.81 (0.89, 3.67) | 1.81 (0.89, 3.67) | — |
| Overall | 20 | 16,693 | 0.82 (0.76, 0.89) | 0.83 (0.75, 0.92) | 27.8 | 0.77 (0.70, 0.85) | 0.78 (0.70, 0.88) | 14.7 |
| EPA + DPA + DHA§ | ||||||||
| Phospholipids | 13 | 14,633 | 0.78 (0.70, 0.87) | 0.78 (0.70, 0.87) | 0.0 | 0.71 (0.62, 0.80) | 0.71 (0.62, 0.81) | 0.0 |
| Total plasma/serum | 7 | 1,967 | 0.84 (0.75, 0.94) | 0.83 (0.71, 0.97) | 31.1 | 0.77 (0.66, 0.89) | 0.77 (0.66, 0.91) | 7.4 |
| Cholesterol esters | 3 | 503 | 0.90 (0.71, 1.16) | 0.90 (0.71, 1.16) | 0.0 | 0.87 (0.64, 1.20) | 0.87 (0.64, 1.20) | 0.0 |
| Triglycerides | 1 | 101 | 0.98 (0.66, 1.48) | 0.98 (0.66, 1.48) | — | 0.68 (0.36, 1.26) | 0.68 (0.36, 1.26) | — |
| Adipose tissue | 1 | 99 | 1.55 (0.96, 2.48) | 1.55 (0.96, 2.48) | — | 2.11 (1.01, 4.41) | 2.11 (1.01, 4.41) | — |
| Overall | 20 | 16,693 | 0.81 (0.75, 0.88) | 0.82 (0.75, 0.90) | 19.3 | 0.74 (0.67, 0.82) | 0.77 (0.68, 0.86) | 20.0 |
Q, quintile. Multiple lipid fractions were available for some studies, but only one lipid fraction was used for the overall analysis.
Effect estimates were pooled with use of inverse variance–weighted fixed-effects or random-effects meta-analysis.
For Uppsala Longitudinal Study of Adult Men-50 (ULSAM-50) and Chin-Shan Community Cardiovascular Cohort study (CCCC), DPA measurements were not available. The risk estimates for EPA + DHA were included in this pooled result.
Figure 1.
Pooled RRs of T2D according to interquintile range (difference between 90th and 10th percentiles) of the sum of EPA, DPA, and DHA biomarkers. The association between the sum of EPA, DPA, and DHA and T2D was assessed in multivariable models for each cohort, and the results were pooled with use of inverse variance–weighted fixed-effects meta-analysis. In each cohort, multivariate RR was assessed with adjustment for sex, age, field site (if appropriate), race, socioeconomic status (education, occupation), smoking status, physical activity, alcohol consumption, treatment for hypertension, treatment for hypercholesterolemia, prevalent coronary heart disease, BMI, waist circumference, and biomarkers of LA (18:2n-6) and trans fatty acids (total t-18:1 and t-18:2). If multiple biomarkers were available for a study, only one was used for the overall analysis based on the best ability to reflect long-term dietary intake (in the following order of preference): adipose tissue, erythrocyte phospholipids, plasma phospholipids, total plasma/serum, cholesterol esters, and triglycerides. For studies not mentioned elsewhere in the text, the expansions for study acronyms can be found in the legend to Table 1. n, total number of participants in a particular study; case, number of T2D cases.
Sensitivity analyses using restricted cubic splines demonstrated similar patterns of association with little evidence for nonlinearity (Supplementary Fig. 3A–S). Among different lipid compartments, a significant inverse association was observed for plasma phospholipid ALA and T2D risk (nine cohorts). Findings for EPA, DPA, DHA, and EPA + DPA + DHA were similar to the main findings for phospholipids and total plasma/serum compartments, with no significant findings in cholesterol esters (three cohorts).
In the prespecified subgroup analyses, there was no significant effect modification by baseline participant characteristics (Table 3), although we observed nominally significant heterogeneity by global region for EPA (stronger inverse association in Europe and Australia) and by baseline BMI for DHA (stronger inverse association for BMI >30 kg/m2). There was also a trend toward a stronger inverse association among individuals with BMI >30 kg/m2 for EPA + DPA + DHA. No significant differences in associations were observed for studies that used a case-cohort versus a prospective cohort design (data not shown). There was no significant interaction with the T2D GRS (Supplementary Table 5).
Table 3.
Stratified analysis of n-3 fatty acid biomarkers by prespecified sources of heterogeneity
| ALA | EPA | DPA | DHA | EPA + DPA + DHA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P het | RR (95% CI) | P het | RR (95% CI) | P het | RR (95% CI) | P het | RR (95% CI) | P het | |
| Overall estimate | 0.97 (0.92, 1.02) | 0.92 (0.87, 0.96) | 0.79 (0.73, 0.85) | 0.82 (0.76, 0.89) | 0.81 (0.75, 0.88) | |||||
| Global region | 0.35 | 0.009 | 0.05 | 0.68 | 0.69 | |||||
| North America | 1.04 (0.94, 1.16) | 1.07 (0.96, 1.21) | 0.86 (0.75, 0.99) | 0.87 (0.75, 1.00) | 0.86 (0.74, 0.99) | |||||
| Europe | 0.93 (0.88, 1.00) | 0.88 (0.82, 0.93) | 0.76 (0.69, 0.84) | 0.80 (0.71, 0.89) | 0.77 (0.69, 0.87) | |||||
| Asia | 0.98 (0.86, 1.13) | 0.94 (0.85, 1.03) | 0.65 (0.44, 0.96) | 0.83 (0.69, 1.00) | 0.85 (0.72, 1.00) | |||||
| Australia | 1.03 (0.80, 1.33) | 0.73 (0.53, 0.998) | 1.16 (0.80, 1.67) | 0.90 (0.66, 1.24) | 0.83 (0.61, 1.14) | |||||
| Age, years | 0.27 | 0.82 | 0.86 | 0.57 | 0.81 | |||||
| <60 | 0.94 (0.88, 1.02) | 0.92 (0.84, 0.997) | 0.78 (0.69, 0.88) | 0.78 (0.69, 0.88) | 0.79 (0.70, 0.89) | |||||
| ≥60 | 1.00 (0.93, 1.09) | 0.93 (0.87, 0.99) | 0.79 (0.72, 0.88) | 0.81 (0.74, 0.90) | 0.80 (0.73, 0.88) | |||||
| Sex | 0.96 | 0.55 | 0.78 | 0.11 | 0.11 | |||||
| Male | 0.98 (0.92, 1.05) | 0.90 (0.84, 0.96) | 0.77 (0.69, 0.87) | 0.86 (0.77, 0.95) | 0.85 (0.77, 0.93) | |||||
| Female | 0.98 (0.88, 1.09) | 0.93 (0.86, 0.99) | 0.79 (0.71, 0.88) | 0.75 (0.67, 0.85) | 0.75 (0.67, 0.84) | |||||
| Race/ethnicity | 0.62 | 0.04 | 0.29 | 0.60 | 0.32 | |||||
| White | 0.98 (0.92, 1.03) | 0.88 (0.83, 0.93) | 0.77 (0.71, 0.84) | 0.83 (0.76, 0.91) | 0.82 (0.75, 0.89) | |||||
| Black | 0.97 (0.65, 1.44) | 1.12 (0.91, 1.38) | 1.06 (0.77, 1.47) | 0.97 (0.65, 1.44) | 0.98 (0.68, 1.42) | |||||
| East Asian | 0.97 (0.85, 1.10) | 0.95 (0.87, 1.04) | 0.73 (0.52, 1.02) | 0.82 (0.68, 0.99) | 0.85 (0.72, 0.996) | |||||
| Hispanic | 0.68 (0.40, 1.15) | 0.53 (0.26, 1.07) | 0.78 (0.43, 1.44) | 0.44 (0.13, 1.44) | 0.39 (0.12, 1.23) | |||||
| BMI, kg/m2 | 0.17 | 0.25 | 0.49 | 0.03 | 0.07 | |||||
| <30 | 0.95 (0.88, 1.01) | 0.91 (0.87, 0.96) | 0.79 (0.72, 0.87) | 0.86 (0.78, 0.94) | 0.84 (0.77, 0.91) | |||||
| ≥30 | 1.03 (0.93, 1.14) | 0.85 (0.77, 0.94) | 0.75 (0.64, 0.87) | 0.70 (0.59, 0.82) | 0.71 (0.60, 0.83) | |||||
| LA (% of fatty acids) | 0.34 | 0.05 | 0.56 | 0.42 | 0.32 | |||||
| <Median | 0.95 (0.89, 1.02) | 0.89 (0.85, 0.94) | 0.79 (0.72, 0.87) | 0.83 (0.75, 0.91) | 0.83 (0.75, 0.90) | |||||
| ≥Median | 1.01 (0.92, 1.10) | 1.00 (0.91, 1.10) | 0.75 (0.65, 0.87) | 0.77 (0.68, 0.88) | 0.76 (0.66, 0.87) | |||||
| Triglycerides, mg/dL | 0.55 | 0.66 | 0.98 | 0.77 | 0.33 | |||||
| <150 | 0.99 (0.92, 1.07) | 0.94 (0.89, 0.999) | 0.75 (0.67, 0.84) | 0.83 (0.75, 0.92) | 0.84 (0.77, 0.93) | |||||
| ≥150 | 0.95 (0.87, 1.05) | 0.92 (0.85, 0.997) | 0.75 (0.66, 0.86) | 0.81 (0.71, 0.93) | 0.78 (0.68, 0.89) | |||||
Multiple lipid fractions were available for some studies, but only one lipid fraction was used for the overall analysis. Effect estimates were pooled with use of inverse variance–weighted fixed-effects meta-analysis. Phet, Pheterogeneity.
In models with additional adjustment for circulating triglycerides and fish intake, the risk estimates for each biomarker remained essentially unchanged (Supplementary Fig. 4). Moreover, similar associations were found in sensitivity analyses for which cases of T2D ascertained in the first 2 years of follow-up were excluded or follow-up was restricted to the first 6 years (Supplementary Table 4).
Conclusions
In this pooled analysis of 65,147 adults from 20 prospective studies, seafood-derived n-3 fatty acid biomarkers including EPA, DPA, DHA, and their sum were associated with lower risk of T2D. Plant-derived ALA was not significantly associated with T2D. Our findings were robust across subgroups and sensitivity analyses and upon extensive adjustment for sociodemographic factors, lifestyle habits, medical diagnoses, and adiposity. To our knowledge, the current study represents the most comprehensive assessment of n-3 fatty acid biomarkers and risk of T2D.
Several plausible physiologic mechanisms support our observations. In long-term prospective observational studies and meta-analyses of RCTs, higher fish and n-3 fatty acid intake was associated with less long-term weight gain and lower waist circumference, BMI, and body fat percentage, all of which have been identified as risk factors for T2D (3,21). Moreover, n-3 fatty acid supplementation increases adiponectin levels, a marker of improved insulin sensitivity, lower inflammation, and reduced diabetes risk (22,23). n-3 supplementation may improve insulin sensitivity in certain populations including women or individuals with metabolic disorders and has been shown to marginally improve glycemic control in patients with T2D, though findings were not entirely consistent (4,24). Furthermore, n-3 fatty acids downregulate triglyceride synthesis and hepatic de novo lipogenesis and increase fat oxidation, and all of these effects may lead to reduced metabolic risk (8,25). n-3 fatty acids have also been shown to exert insulin-sensitizing and anti-inflammatory effects through the GPR120 signaling pathway as well as through their metabolism into resolvins and protectins (26,27). Lastly, n-3 fatty acid biomarkers may also represent a marker for other beneficial bioactive compounds in fish/seafood, specifically taurine, which may improve glucose metabolism (28,29).
A recent meta-analysis of RCTs with ALA or marine n-3 fatty acid supplementation did not find an overall effect on T2D incidence (5), although there were several notable differences between this study and our pooled analysis. First, the vast majority of the RCTs tested fish oil supplements, in contrast to our study, which assessed biomarkers that are more reflective of habitual dietary intakes (given the low usage of fish oil supplements). Moreover, the control arms in the RCTs often included other bioactive compounds, such as olive oil or n-6-rich oils, which may have metabolic benefits in their own respect (30), whereas in our primary model, with the adjustment for circulating LA and trans fats, we assessed the impact of replacing saturated, monounsaturated, and non-LA PUFAs with n-3 PUFAs. Similarly, the Prevención con Dieta Mediterránea (PREDIMED) trial, where the Mediterranean diet arms significantly increased dietary ALA and marine n-3 fatty acid intake, demonstrated significantly lower T2D incidence in comparison with the low-fat arm (31). However, since PREDIMED led to multiple dietary changes, we cannot readily attribute these effects to n-3 PUFAs per se. Finally, most existing supplement trials have focused on patients with existing cardiovascular disease or those at high cardiovascular risk, with relatively short follow-up duration, whereas our studies examined generally healthy populations with substantially longer follow-up time (32). Therefore, future intervention studies should explore whether higher intakes of n-3 PUFA-rich foods, particularly when used to replace other dietary components, may be beneficial for the prevention of T2D.
Contrary to prior studies (7,8), our analysis did not show an overall beneficial association between ALA biomarkers and T2D risk. ALA is rapidly oxidized following ingestion, which may explain the generally low correlations between ALA intake and circulating levels (33). Hence, while foods rich in ALA such as walnuts or flaxseeds may be linked to lower risk of diabetes (34), ALA biomarkers may not adequately capture intakes of these foods (35). On the other hand, other dietary constituents present in ALA-rich foods, including fiber, magnesium, and phenolics, may be mediating the beneficial associations seen in observational studies (34). Our models also adjusted for levels of LA, an n-6 fatty acid that is often present in foods high in ALA and previously found to be associated with lower T2D incidence (15). Lack of adjustment for circulating LA biomarkers may have led to the observed inverse associations for ALA with incident T2D (7). It is worth noting that although, when data across all compartments were pooled, ALA levels were not associated with T2D in the current analysis, we observed a significant inverse association between plasma phospholipid ALA levels and T2D, based on nine cohorts in our consortium. Hence, we cannot preclude the possibility that ALA may mitigate T2D risk through certain metabolic pathways, though this requires further confirmation.
Our findings are consistent with studies of self-reported fish and long-chain n-3 PUFA intake in Asia but not for those in North America or Europe (6). There are several potential explanations. First, circulating n-3 PUFA biomarkers represent an objective measurement free of recall/memory biases compared with self-reported intakes. Our observations are robust in sensitivity analyses, including the exclusion of the largest contributing study, EPIC-InterAct, which independently reported borderline inverse associations for DPA and DHA (7). The reasons for discrepancies in the association observed for ALA and EPA in our study compared with EPIC-InterAct are unknown, although differences in the adjusted covariates, particularly other fatty acids, may play a role. Adjustments for circulating LA and trans fatty acids could further reduce dietary confounding, particularly since deep-frying and broiling (with oils high in n-6 or trans fats) are common preparation methods for fish/seafood in some Western populations (14). Furthermore, biomarkers represent the summed influence of both diet and metabolism, which may be more relevant to biologic effects than diet alone. This is particularly true for DPA, which is mostly derived from endogenous elongation of EPA and may be influenced by multiple factors including EPA intake (36), FADS1/2 and ELOVL2 variants (37), and hormonal regulation (38). Our results support the need for future studies examining the precise metabolic pathways through which EPA, DPA, and DHA may act to modify T2D risk.
Previous findings from FORCE demonstrated stronger inverse associations of n-3 fatty acid biomarkers in the phospholipid and total plasma compartments with incident coronary heart disease, as well as borderline or lack of association for these biomarkers in the cholesterol ester or adipose tissue compartments (14). We observed a similar pattern of associations in our analysis on incident T2D, providing further support that the concentration of n-3 fatty acids in these compartments may be most relevant for cardiometabolic risk. Prior experimental studies have demonstrated that n-3 fatty acids, particularly EPA and DHA, tend to be most concentrated in phospholipids, and the n-3 fatty acid concentrations in this compartment are most responsive to changes in dietary intakes or supplementation (14). This may be due to the fact that phospholipid (for both plasma and erythrocytes) n-3 fatty acids can most readily exert membrane-stabilizing effects and interact with cell membrane proteins, including enzymes responsible for eicosanoid production, as well as influence gene transcription by binding to nuclear receptors such as the peroxisome proliferator–activated receptors (39). Additionally, we observed an inverse trend for triglyceride DPA and incident T2D in a single cohort but no association for the other n-3 fatty acid biomarkers. Additional studies of n-3 fatty acids in triglycerides are warranted to confirm this finding.
Our novel findings of no significant interaction between n-3 PUFA biomarkers and a T2D GRS build on and expand a recent pooled analysis conducted among adults of European ancestry that did not show a significant interaction between self-reported estimates of dietary n-3 PUFAs and a T2D GRS (40). However, a limitation of this study and our present genetic interaction analysis is that the GRS used was derived from populations of European ancestry, and, hence, our findings do not preclude potential gene–fatty acid interactions among individuals of other ancestries. Additional studies are warranted to confirm whether specific individuals may derive greater long-term cardiometabolic benefits from n-3 PUFAs.
Our study has several strengths. In comparison with prior studies (7), we included nearly all known studies with measurements of n-3 biomarkers and incident T2D, thus reducing the likelihood of publication bias and increasing statistical power for identifying associations and assessing sources of heterogeneity. Exposures, outcomes, covariates, and analytical methods were harmonized in de novo participant-level analyses, reducing heterogeneity. In prior studies investigators were often only able to assess fatty acids in a single lipid compartment, while we examined multiple compartments, with largely consistent associations. Including participants from multiple countries with varying dietary cultures helps to enhance generalizability.
Potential limitations warrant consideration. There were relatively few cohorts with measurements in adipose tissue, plasma triglycerides, or cholesterol esters, which reduced the statistical power to detect associations for these compartments. The majority of participants were of European or East Asian ancestry; thus, the generalizability of our findings to other racial/ethnic groups could be limited. Associations were based on a single measurement of n-3 biomarkers, and changes over time may tend to attenuate associations toward the null. Prior studies have shown high reproducibility of n-3 fatty acids over time; thus, a single measurement may be adequate for estimating their long-term concentrations (16). Due to the observational nature of this study, residual or unmeasured confounding cannot be ruled out. However, the relative consistency of our findings across diverse populations, robustness in sensitivity analyses, and supporting biologic plausibility from effects on intermediary risk factors collectively suggest that the associations were unlikely to be solely due to confounding. The additional adjustments for circulating LA and trans fatty acid biomarkers may make it harder to infer health benefits of specific n-3-rich foods, particularly those high in ALA, due to the frequent presence of both ALA and LA in the same food. We did not adjust for dietary factors besides fish/seafood intake, though the consistent findings of n-3 biomarkers across populations with varying dietary patterns suggest that residual confounding from diet was likely small. Nevertheless, the VITamin D and OmegA-3 TriaL (VITAL), which seeks to examine the effects of EPA + DHA supplementation on chronic diseases, may be able to shed light on the role for increasing specific n-3 PUFAs in the primary prevention of T2D.
In conclusion, our study suggests that higher circulating levels of seafood-derived n-3 fatty acids, namely, EPA, DPA, and DHA and their sum, are related to lower T2D risk, whereas plant-derived ALA was not associated with risk.
Article Information
Funding. Funding for this work was supported by National Heart, Lung, and Blood Institute, National Institutes of Health, research grants R01HL034594 and R01HL088521 and by research grants U01CA167552 and R01HL35464 from the National Institutes of Health. Funding for individual cohorts is listed in the Supplementary Appendix. D.M. reports (all outside the submitted work) research funding from the National Institutes of Health and the Bill & Melinda Gates Foundation and personal fees from the Cleveland Clinic Foundation.
Duality of Interest. W.S.H. holds stock in OmegaQuant Analytics, LLC, and is a member of the RB Schiff Science and Innovation Advisory Board. D.M. reports (all outside the submitted work) personal fees from GOED, Nutrition Impact, Bunge, Indigo Agriculture, Motif FoodWorks, Amarin, Acasti Pharma, America’s Test Kitchen, and Danone; serving on the scientific advisory board for Brightseed, DayTwo, Elysium Health, Filtricine, HumanCo, and Tiny Organics; and chapter royalties from UpToDate. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors contributed to the study conception and design. F.Q., A.V.A.K., F.I., M.M., N.T., X.Z., J.K.B., H.L., Y.H., K.-L.C., A.C.W., M.L., R.A.M., C.S., J.M.G., V.D.d.M., and W.G. conducted the data analysis. All authors interpreted the data. F.Q., A.V.A.K., F.I., N.G.F., J.H.Y.W., R.N.L., R.M., D.M., and Q.S. wrote the first draft of the article. All authors reviewed and edited the manuscript and approved the final version for submission. F.Q. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Heart Association EPI|Lifestyle 2019 Scientific Sessions, Houston, TX, 5–8 March 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13724110.
D.M. and Q.S. contributed equally as senior authors.
References
- 1. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011;58:2047–2067 [DOI] [PubMed] [Google Scholar]
- 2. Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem 2013;69:633–651 [DOI] [PubMed] [Google Scholar]
- 3. Bender N, Portmann M, Heg Z, Hofmann K, Zwahlen M, Egger M. Fish or n3-PUFA intake and body composition: a systematic review and meta-analysis. Obes Rev 2014;15:657–665 [DOI] [PubMed] [Google Scholar]
- 4. O’Mahoney LL, Matu J, Price OJ, et al. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovasc Diabetol 2018;17:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A; PUFAH Group . Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ 2019;366:l4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallin A, Di Giuseppe D, Orsini N, Patel PS, Forouhi NG, Wolk A. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes: systematic review and meta-analysis of prospective studies. Diabetes Care 2012;35:918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forouhi NG, Imamura F, Sharp SJ, et al. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med 2016;13:e1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107(Suppl. 2):S214–S227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. GBD 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;393:1958–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganesan B, Brothersen C, McMahon DJ. Fortification of foods with omega-3 polyunsaturated fatty acids. Crit Rev Food Sci Nutr 2014;54:98–114 [DOI] [PubMed] [Google Scholar]
- 11. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012. JAMA 2016;316:1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol 2011;8:228–236 [DOI] [PubMed] [Google Scholar]
- 13. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Gobbo LC, Imamura F, Aslibekyan S, et al.; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) . ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies [published correction appears in JAMA Intern Med 2016;176:1727–1728; JAMA Intern Med 2016;176:1728; JAMA Intern Med 2019;179:457]. JAMA Intern Med 2016;176:1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu JHY, Marklund M, Imamura F, et al.; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol 2017;5:965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai HT, de Oliveira Otto MC, Lemaitre RN, et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ 2018;363:k4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. I S Sobczak A, A Blindauer C, J Stewart A. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 2019;11:2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 1997;38:2012–2022 [PubMed] [Google Scholar]
- 19. Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74–81 [DOI] [PubMed] [Google Scholar]
- 20. Kwak SH, Park KS. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp Mol Med 2016;48:e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu X, Li Y, Tobias DK, et al. Changes in types of dietary fats influence long-term weight change in US women and men. J Nutr 2018;148:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr 2012;142:592S–599S [DOI] [PubMed] [Google Scholar]
- 23. Wu JH, Cahill LE, Mozaffarian D. Effect of fish oil on circulating adiponectin: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2013;98:2451–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao H, Geng T, Huang T, Zhao Q. Fish oil supplementation and insulin sensitivity: a systematic review and meta-analysis. Lipids Health Dis 2017;16:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014;510:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oh DY, Walenta E, Akiyama TE, et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 2014;20:942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 2014;19:21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dale HF, Madsen L, Lied GA. Fish-derived proteins and their potential to improve human health. Nutr Rev 2019;77:572–583 [DOI] [PubMed] [Google Scholar]
- 29. Zheng Y, Ceglarek U, Huang T, et al. Plasma taurine, diabetes genetic predisposition, and changes of insulin sensitivity in response to weight-loss diets. J Clin Endocrinol Metab 2016;101:3820–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imamura F, Micha R, Wu JH, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med 2016;13:e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014;160:1–10 [DOI] [PubMed] [Google Scholar]
- 32. Satija A, Stampfer MJ, Rimm EB, Willett W, Hu FB. Perspective: are large, simple trials the solution for nutrition research? Adv Nutr 2018;9:378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pertiwi K, Kok DE, Wanders AJ, de Goede J, Zock PL, Geleijnse JM. Circulating n-3 fatty acids and linoleic acid as indicators of dietary fatty acid intake in post-myocardial infarction patients. Nutr Metab Cardiovasc Dis 2019;29:343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pan A, Sun Q, Manson JE, Willett WC, Hu FB. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr 2013;143:512–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E, Davy BM. Dietary biomarkers: advances, limitations and future directions. Nutr J 2012;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Superko HR, Superko SM, Nasir K, Agatston A, Garrett BC. Omega-3 fatty acid blood levels: clinical significance and controversy. Circulation 2013;128:2154–2161 [DOI] [PubMed] [Google Scholar]
- 37. Smith CE, Follis JL, Nettleton JA, et al. Dietary fatty acids modulate associations between genetic variants and circulating fatty acids in plasma and erythrocyte membranes: meta-analysis of nine studies in the CHARGE consortium. Mol Nutr Food Res 2015;59:1373–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr 2004;79:765–773 [DOI] [PubMed] [Google Scholar]
- 39. Sheikh O, Vande Hei AG, Battisha A, Hammad T, Pham S, Chilton R. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: as it pertains to the recently published REDUCE-IT trial. Cardiovasc Diabetol 2019;18:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Merino J, Guasch-Ferré M, Ellervik C, et al. Quality of dietary fat and genetic risk of type 2 diabetes: individual participant data meta-analysis. BMJ 2019;366:l4292. [DOI] [PMC free article] [PubMed] [Google Scholar]