Abstract
Aim:
To investigate the degree to which psychological stress, self-reported pain scores, and pain sensitivity during an acute state of low back pain (LBP) predict the development of persistent LBP trajectories.
Background:
Identifying which factors influence LBP trajectories is critical to understand why some individuals experience persistent LBP and to illuminate areas for nursing intervention.
Methods:
A secondary data analysis of a prospective study examining trajectories of LBP was conducted. The sample was comprised of 217 adults with acute-onset LBP recruited from the community and followed over 24 weeks. Variables of interest included demographic data, perceived stress scores, self-reported pain scores, and somatosensory characteristics collected within the first 4 weeks of LBP onset. The data were analyzed using non-parametric bivariate comparisons and a semi-parametric Cox proportional hazards model with interval-censoring.
Results:
Individuals with higher psychological stress scores were less likely to experience pain resolution (Hazard ratio [HR] = 0.555, 95% confidence interval [CI] = 0.36–0.85, p = 0.02). After adjustment for covariates in the final model, analysis revealed household income (HR = 2.79, 95% CI [1.63–4.67], p < 0.001) to be the dominant predictor of LBP persistence in this sample.
Conclusion:
Heightened psychological stress and pain severity as well as decreased pressure pain thresholds were indicated as influential factors of LBP trajectories, although household income was identified as the dominant predictor. Strategies which integrate assessment of stress, self-reported pain scores, pain sensitivity, and social determinants for patients experiencing pain are needed to advance nursing care.
Keywords: Low back pain, chronic pain, psychological stress, social determinants of health
Background
Non-specific persistent low back pain (pLBP) has garnered frequent attention in the last few decades as a top cause of mobility limitation in adults worldwide (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). In the United States (US), pLBP is estimated to effect over 13% of the population at any given time, with an estimated annual financial burden of $30.3 billion as of 2010 (Gaskin & Richard, 2012; Shmagel, Foley, & Ibrahim, 2016). pLBP, defined as idiopathic low back pain lasting more than three months, is included in the recently designated “primary pain syndrome” category of the International Classification of Diseases Version 11 (Treede et al., 2019). Primary pain syndromes are conditions in which pain is the disease rather than a symptom of another ongoing disease process.
Various biopsychosocial processes contribute to underlying mechanisms which induce pain persistence (Gatchel, Peng, Peters, Fuchs, & Turk, 2007). Previous research has pointed to stress, depression, and maladaptive coping behaviors as contributing factors to the persistence of chronic pain (Allegri et al., 2016; Lunde & Seiberg, 2020; Mun et al., 2019; Meints & Edwards, 2018). These cognitive and emotional processes may help to drive and maintain ongoing pain states by mediating pain responses and outcomes, including pain severity and sensitivity (Edwards, Dworkin, Sullivan, Turk, & Wasan, 2016; Meints & Edwards, 2018).
Deconstructing the relationship between psychological stress and the development of pain persistence may shed light on the mechanisms that are engaged in difficult to treat primary pain syndromes. It has been speculated that chronic stress is a mediating factor in persistent pain states which contributes to pathophysiological modifications in nociceptive pathways leading to a heightened pain sensitivity and response to noxious stimuli (i.e. hyperalgesia) (Jennings, Okine, Roche, & Finn, 2014). Moreover, the size and direction of stress effects on chronic pain has been proposed to be heavily influenced by individual differences in the physiological stress response (Vachon-Presseau, 2018). Changes in emotional regulation can promote increases in sensitivity of the central and peripheral neurons, resulting in localized pain at the site of the noxious stimulus as well as at distal sites (Egloff, Hirschi, & von Kanel, 2013; Imbe, Iwai-Liao, & Senba, 2006; Lunde & Seiberg, 2020; Jennings et al., 2014). Widespread changes in peripheral and central pain pathways have been documented through the use of quantitative sensory testing (QST) and functional magnetic resonance imaging, showing that individuals with pLBP experience increased pain sensitivity and hyperactivity of neuronal signaling (Starkweather et al., 2016b; Starkweather et al., 2016c; Marcuzzi, Wrigley, Dean, Graham, & Hush, 2018). However, the direct influence of stress, pain severity, and pain sensitivity as mechanistic factors in developing pain trajectories prior to a chronic pain diagnosis is not well established (Lunde & Seiberg, 2020). A recent review suggested that stressful events in childhood may contribute to the development of pLBP in adulthood, although limitations related to the rigor of the reviewed primary studies contributed to challenges in identifying definitive associations (Buscemi, Chang, Liston, McAuley, & Schabrun, 2019). Further investigations outlining the relationship between psychological stress and LBP trajectories are warranted.
Stress is particularly relevant to nursing as it represents a potentially modifiable factor that can be assessed throughout all levels of clinical care and for which interventions could happen in clinical settings or the community. Understanding how stress influences an individual’s pain trajectory will assist nurses to identify key risk factors at pain onset and develop appropriately targeted interventions. Thus, the objectives of this study were twofold: (1) to explore the degree to which increased psychological stress, self-reported pain scores, and pain sensitivity (primary risk factors) during a phase of acute LBP are independently predictive of pain trajectories over time; and (2) to identify if these primary risk factors, taken together at baseline, present a model predicting the trajectory of low back pain. Our hypothesis was that individuals with heightened psychological stress scores, self-reported pain scores, and pain sensitivity at baseline would be less likely to experience pain resolution.
Methods
Design and Sample
This study presents a secondary analysis of an existing data set which measured prospective changes in pain characteristics, somatosensory data, and genetic factors of individuals during the transition from acute to chronic LBP over time (Starkweather et al., 2016b; Starkweather et al., 2016c). Briefly, 220 English-speaking adults aged 18–50 with acute onset low back pain (>24 hours and <4 weeks, preceded by >1 pain-free month) were recruited through informational flyers placed around three university campuses, primary care clinics, and in the general public, and consented to enroll. Data were collected every six weeks for up to 24 weeks after the baseline visit if the subjects continued to meet study criteria for ongoing LBP (>2 out of 10 on numeric rating scale [NRS]). Subjects were considered to have resolved pain if they rated their LBP as < 2 at any of the six week follow-up visits, which would have triggered the end of study participation based on the study protocol (n=79). Subjects were considered to have persistent unresolved pain if they continued to experience pLBP at the 24-week visit (n=55). Subjects who continued to have pain, but did not attend follow-up appointments or were not able to be contacted for study continuation (i.e., attrition), were considered undefined unresolved (n=83). After the consenting procedure, three subjects were identified to be ineligible based on study parameters and were removed from further study participation, leaving 217 subjects for this analysis. Institutional Review Board approval was attained for the primary study as well as this secondary, ongoing analysis.
Measures
After enrollment in the primary study, all participants completed an initial visit in which demographics, LBP history, self-report measures (pain characteristics; mood state; coping strategies; reactivity; psychological stress; disability status; work history), and somatosensory data were collected. To meet this study’s objectives, the self-report measures included in this secondary data analysis were the Brief Pain Inventory-Short Form (BPI; worst pain, least pain, average pain, pain now, and pain interference) and the Perceived Stress Scale (PSS). The five BPI items assess pain severity and activity interference using 11-point, Likert-type scales with lower ratings indicating less pain severity or interference, and have been previously validated in chronic pain populations (Cleeland & Ryan, 1994; Keller et al., 2004). The PSS is a 10-item, self-report questionnaire that measures psychological stress (Cohen, Kamarck, & Mermelstein, 1983) with an observed Cronbach’s a = .92 reported in the parent study (Starkweather et al., 2016b). Scores for the PSS range from 0 to 40, with lower PSS scores indicating lower levels of psychological stress. Average values of the PSS in the general population of the US from 2009 have been reported as a mean of 15.52 for men and mean of 16.14 for women (Cohen & Janicki-Deverts, 2012).
Quantitative sensory testing (QST) was used to measure peripheral and central somatosensory changes over time, which assists in characterizing functional changes in sensory nerves (Belfer, & Dai, 2010). Understanding these changes during the course of developing pLBP informs an objective assessment of pain trajectories. In the parent study, a standardized protocol collected QST data on the dorsum of the non-dominant hand (control site) and three lumbar sites (painful site) (Starkweather et al., 2016a). In this secondary analysis, we included the QST measures of mechanical pain sensitivity (MPS), dynamic mechanical allodynia (ALL), cold pain threshold (CPT), heat pain threshold (HPT), and pressure pain threshold (PPT), as these measures have previously been shown to differentiate pain sensitization in subjects with pLBP (Starkweather et al., 2016c; Marcuzzi et al., 2018).
Analytic Strategy
Statistical analyses were all conducted using SPSS v25 and RStudio v1.1.453 software. We used descriptive statistics for categorical demographic variables. We computed the median and interquartile range for continuous demographic characteristics and primary study variables. A level of significance (a) of 0.05 was established a priori for all statistical testing.
In order to address the first aim of identifying if higher levels of psychological stress, self-reported pain scores, and pain sensitivity were independently predictive factors of pain trajectories, we created survival plots using a non-parametric maximum likelihood estimator (NPMLE) developed for use with interval censored data (Anderson-Bergman, 2017). The technique of interval censoring was implemented because the exact day of pain resolution (<2 on NRS) was not collected in the primary study that would allow for the creation of a continuous pain duration variable (Zhang & Sun, 2009). We established the visit number at which pain resolution was identified as the event, or hazard, in this scenario. Subjects that were lost to follow-up were also included in the analysis using the technique of right censoring, with the second time-point established as ∞ (Anderson-Bergman, 2017; Zhang & Sun, 2009).
Confounding demographic variables included in the analyses were age, sex, race, ethnicity, body mass index, income, and smoking (De Palma, Ketchum, & Saullo, 2012; Maly, & Vallerand, 2018; Shi, Weingarten, Mantilla, Hooten, & Warner, 2010; Shmagel et al., 2016). We divided all continuous variables into “high” and “low” groups based on the median. We collapsed race into 3 groups (White/Caucasian, Black/African American, and other/more than one race) based on frequencies reported in the parent study. All other demographic variables were categorically split into two groups based on the measure (e.g., smoker vs. non-smoker). The NPMLE plots allowed us to visualize if the groups differed in the probability of pain resolution over the 24-week study.
To meet the first aim, we also examined the statistical differences between groups visualized in the NPMLE plots by conducting bivariate analyses using a semi-parametric regression modeling procedure for interval-censored data, as established by Anderson-Bergman (2017) for R software. We used bootstrap sampling (n = 100) to adjust for bias. All variables were evaluated to ensure they met the proportional hazards assumption prior to fitting a proportional hazards model. If this assumption was not met, then a proportional odds ratio was used instead (Anderson-Bergman, 2017). A ratio less than one indicates that the risk factor had a decreased likelihood of pain resolution, while a ratio greater than one denotes that the risk factor had an increased likelihood of pain resolution (Bradburn, Clark, Love, & Altman, 2003).
To address the second aim of this study - identifying if perceived stress, pain score, and pain sensitivity together predicted LBP trajectories - we created a Cox proportional hazards regression model using a multistep selection process. First, all variables, including confounding factors, which had significant findings in the initial NPMLE bivariate analyses were included in order to build a preliminary model using multivariate analysis. Once the dominant predictor variable was identified in this model, we completed further analysis using the proportional hazards procedure to determine possible covariates to include in the final model. A variable was considered to be a covariate if it changed the hazard ratio of the dominant variable by greater than 25% in comparison to the model without the target variable included. This process was repeated until all potential covariates (i.e., age, race, smoking, PSS, BPI, QST variables) were considered and the final model was set. Lastly, correlation coefficients were calculated to examine associations between the dominant predictor variable and alternative factors.
Results
Participants Characteristics
In the total sample, the majority (51.2%) of subjects identified as male with a median age of 26 years old. Most participants had completed some post-secondary education and 71% of subjects had a household income of less than $60,000 per year. Although the majority of subjects (73.7%) of subjects had a previous history of low back pain, 74.2% expected their current pain to resolve after the baseline visit. Overall, most participants (76.5%) considered themselves to be in good or excellent health. Table 1 displays descriptive statistics of the demographics and pain related characteristics at the initial study visit.
Table 1.
Participant Demographic and Pain Characteristics (n = 217)
| Characteristic | n | % | Med | IQR |
|---|---|---|---|---|
| Age | 26 | 19 | ||
| Sex, Male | 111 | 51.2 | ||
| Race | ||||
| Caucasian | 88 | 40.6 | ||
| Black/African American | 90 | 41.5 | ||
| More than one race, Other | 39 | 17.9 | ||
| Ethnicity | ||||
| Hispanic/Latino | 16 | 7.4 | ||
| Household Income | ||||
| <$60K per year | 154 | 71 | ||
| ≥$60K per year | 63 | 29 | ||
| BMI | 26.5 | 7.94 | ||
| Current smoker | 63 | 29 | ||
| Prior history of LBP | 160 | 73.7 | ||
| Expected pain to resolve | 161 | 74.2 | ||
| Perceived Stress Scale (0–40) | 17 | 7.39 | ||
| Brief Pain Inventory, (NRS 0–10) | ||||
| Worst | 6 | 3 | ||
| Least | 2 | 3 | ||
| Average | 4 | 3 | ||
| Now | 4 | 4 | ||
| Interference | 3.14 | 3.9 | ||
| QST, Painful site | ||||
| MPS, rating 0–10 | 2.66 | 3.67 | ||
| ALL, rating 0–10 | 0.33 | 1.83 | ||
| CPT, °C | 21.86 | 13.55 | ||
| HPT, °C | 39.53 | 5.30 | ||
| PPT, kPa | 210.36 | 211.03 | ||
| QST, Control site | ||||
| MPS, rating 0–10 | 1.33 | 3 | ||
| ALL, rating 0–10 | 0 | 0.92 | ||
| CPT, °C | 19.83 | 14.93 | ||
| HPT, °C | 40.63 | 5.78 | ||
| PPT, kPa | 213.63 | 147.33 |
Note. n = Number, count; Med = Median; IQR = Interquartile Range; BMI =Body Mass Index; LBP = Low Back Pain; NRS = Numeric Rating Scale; QST = Quantitative Sensory Testing; MPS = Mechanical Pain Sensitivity; ALL = Dynamic Mechanical Allodynia; CPT = Cold Pain Threshold; HPT = Heat Pain Threshold; PPT = Pressure Pain Threshold; C = Celsius; kPa = Kilopascal.
The Influence of Individual Risk Factors on Pain Trajectories
The results from the bivariate and multivariate analyses are shown in Table 2. In the bivariate analyses, psychological stress was found to be a significant risk factor as individuals with a self-reported PSS score of greater than 17 were 44.5% less likely (Hazard ratio [HR] = 0.555, 95% Confidence Interval [CI] [0.36 – 0.85], p = 0.02) to experience pain resolution. Additionally, as reported in Table 2, all of the pain scores reported in the BPI were significantly associated with longer pain trajectories, each indicating that reporting higher pain at the initial visit was associated with decreased likelihood of pain resolution. PPT was the only QST variable with a significant finding between groups, with higher pain thresholds at the painful (low back) site (HR = 1.885, 95% CI [1.14 – 3.11], p = 0.01) and at the control (dominant arm) site (HR = 2.159, 95% CI [1.31 – 3.35], p = 0.001) demonstrating a greater likelihood of pain resolution. Figure 1 displays examples of the NPLME survival plots created for PSS, the current pain severity score taken at the baseline study visit (BPI now), and PPT for the painful LBP site.
Table 2.
Hazard Ratios to Pain Resolution
| Bivariate Analysis |
Multivariate Analysisa |
|||||
|---|---|---|---|---|---|---|
| Variable | PE | HR [95% CI] | P-value | PE | HR [95% CI] | P-value |
| Primary Risk Factor | ||||||
| PSS (0–40) | ||||||
| >17 | −0.5878 | 0.555 [0.36 – 0.85] | 0.02* | −0.2658 | 0.766 [0.41 – 1.42] | 0.39 |
| BPI (NRS 0–10) | ||||||
| Worst, >6 | −1.068 | 0.343 [0.19 – 0.6] | < 0.001* | −0.5426 | 0.581 [0.25 – 1.33] | 0.2 |
| Least, >2 | −1.018 | 0.361 [0.21 – 0.61] | < 0.001* | −0.3307 | 0.718 [0.27 – 1.85] | 0.49 |
| Average, >4 | −0.5217 | 0.593 [0.36 – 0.96] | 0.01* | 0.4726 | 1.604 [0.68 – 3.74] | 0.27 |
| Now, >4 | −1.015 | 0.362 [0.19 – 0.65] | < 0.001* | −0.5512 | 0.576 [0.2 – 1.58] | 0.28 |
| Interference, >3.14 | −0.6886 | 0.502 [0.3 – 0.83] | 0.007* | 0.16120 | 1.175 [0.63 – 2.16] | 0.6 |
| QST, painful site | ||||||
| MPS, NRS >2.66 | −0.4382 | 0.645 [0.4 – 1.01] | 0.06 | |||
| ALL, NRS 0–10 | −0.3315 | 0.717 [0.43 – 1.17] | 0.18 | |||
| CPT, >21.86°C | −0.3976 | 0.671 [0.42 – 1.05] | 0.08 | |||
| HPT, >39.53°C | 0.2009 | 1.222 [0.76 – 1.95] | 0.41 | |||
| PPT, >210.336 kPa | 0.6337 | 1.885 [1.14 – 3.11] | 0.01* | −0.07417 | 0.384 [0.43 – 1.97] | 0.84 |
| QST, control site | ||||||
| MPS, NRS >1.33 | 0.1122a | 1.119 [0.59 – 2.09] | 0.72 | |||
| ALL, NRS >0.1 | −0.2598 | 0.771 [0.5 – 1.18] | 0.23 | |||
| CPT, >19.83°C | −0.2101 | 0.810 [0.53 – 1.23] | 0.32 | |||
| HPT, >40.63 °C | 0.4021 | 1.495 [0.93 – 2.39] | 0.09 | |||
| PPT, >213.63 kPa | 0.7695 | 2.159 [1.31 – 3.35] | 0.001* | 0.4545 | 1.575 [0.65 – 3.77] | 0.307 |
| Confounding Factor | ||||||
| Sexb | ||||||
| Male | −0.166 | 0.846 [0.45 – 1.58] | 0.56 | |||
| Age | ||||||
| >26 years old | −0.802 | 0.448 [0.26 – 0.74] | 0.006* | −0.19560 | 0.822 [0.36 – 1.87] | 0.64 |
| Racec | ||||||
| More than one race, | 1.521 | 4.577 [2.01 – 10.41] | <0.001* | 0.7074 | 2.029 [0.48 – 8.42] | 0.33 |
| White/Caucasian Other | 1.689 | 5.413 [2.59 – 11.27] | <0.001* | 0.9005 | 2.461 [0.76 – 7.93] | 0.13 |
| Ethnicityb | ||||||
| Non-Hispanic/Latino | −0.02409 | 0.976 [0.27 – 3.46] | 0.97 | |||
| Household Income | ||||||
| ≥$60K per year | 1.101 | 3.008 [1.88 – 4.79] | < 0.001* | 0.7532 | 2.124 [1.06 – 4.24] | 0.03* |
| BMI | ||||||
| >26.5 | −0.5367 | 0.584 [0.34 – 0.99] | 0.02* | |||
| Smoking | ||||||
| Current smoker | −1.198 | 0.301 [0.14 – 0.64] | 0.001* | −0.1194 | 0.887 [0.31 – 2.51] | 0.82 |
Note. Variable values split categorically or by median value in the sample. PE = Parameter estimate; HR = Hazard ratio; CI = Confidence interval; PSS = Perceived Stress Scale; BPI = Brief Pain Inventory; NRS = Numeric rating scale; QST = Quantitative sensory testing; MPS = Mechanical pain sensitivity; ALL = Dynamic mechanical allodynia; CPT = Cold pain threshold; C = Celsius; HPT = Heat pain threshold; PPT = Pressure pain threshold; kPa = Kilopascal; BMI = Body mass index.
Level of significance set at α = 0.05
Hazard ratios shown for preliminary Cox regression model, prior to identifying covariates.
Proportional odds model used.
Compared to identifying as Black/African American.
Figure 1 –
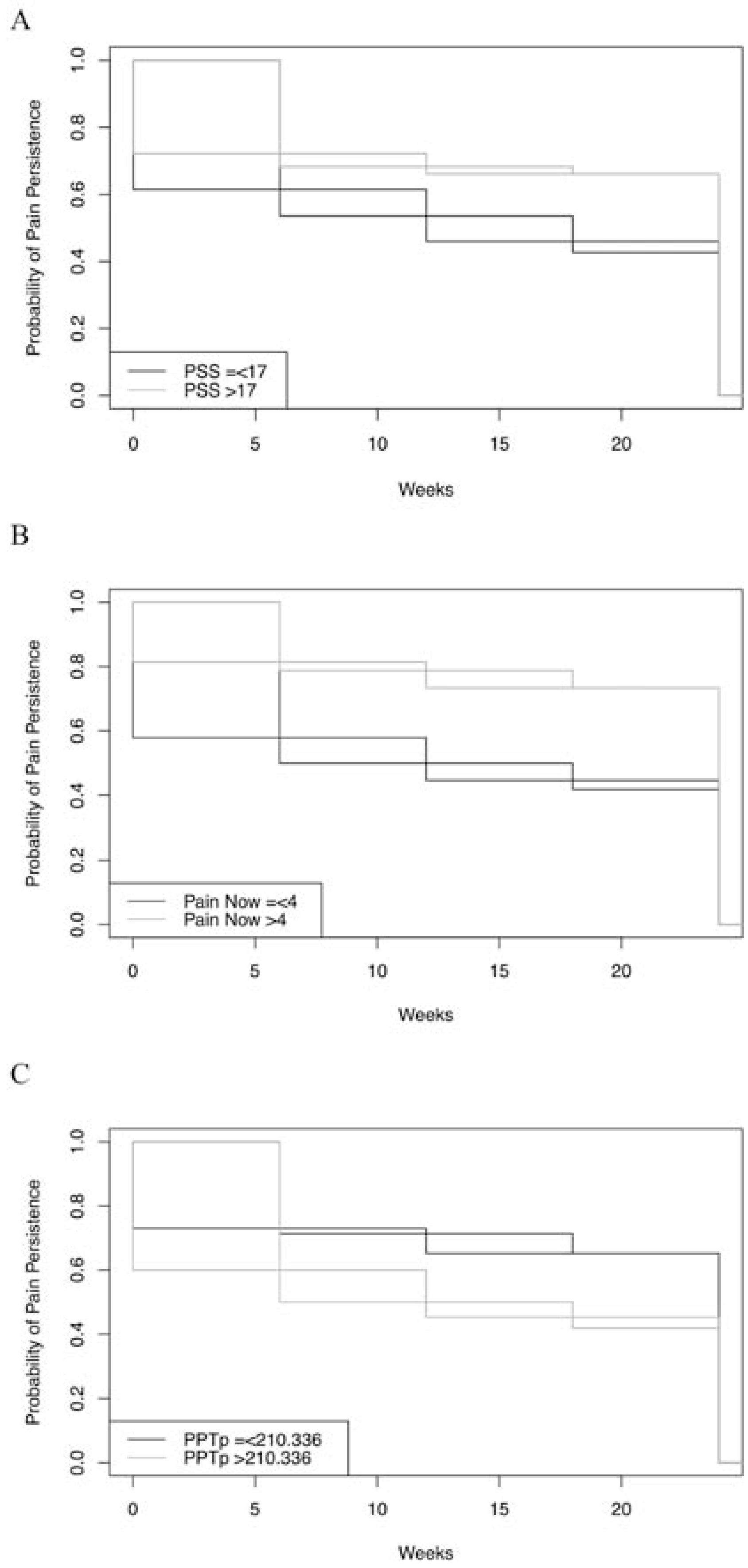
Example Plots of Survival Analysis for Three Individual Risk Factors. Note. Panel A: Non-parametric maximum likelihood estimator (NPMLE) plot for Perceived Stress Scale (PSS) scores, p = 0.02. Panel B: NPMLE plot for Brief Pain Inventory (BPI) score at time of baseline study visit, p < 0.001. Panel C: NPMLE plot for pressure pain threshold (PPT) at painful back site, p = 0.01.
Multiple confounding factors were also found to influence pain trajectories. The results indicate that being older than 26 (HR = 0.448, 95% CI [0.26 – 0.74], p = 0.006), having a BMI greater than 26.5 (HR = 0.584, 95% CI [0.34 – 0.99], p = 0.02), or identifying as a current smoker (HR = 0.301, 95% CI [0.14 – 0.64], p = 0.001) were each associated with decreased likelihood of pain resolution. In contrast, identifying as White/Caucasian (HR = 5.413, 95% CI [2.59 – 11.27], p <0.001) or another race (HR = 4.577, 95% CI [2.01 – 10.41], p <0.001), compared to identifying as Black/African American, significantly increased the likelihood of pain resolution. Additionally, subjects who reported having a household income greater than $60K per year were over 3 times more likely (p <0.001) to experience pain resolution.
The Influence of Multiple Primary Risk Factors on Pain Trajectories
When all variables with significant findings from the bivariate analysis were included in a Cox proportional hazards regression model, neither psychological stress, individual self-reported pain scores, nor pain sensitivity factors were indicated as dominant predictors of LBP trajectories. Alternatively, the preliminary model revealed household income to be the dominant predictor variable in this sample, as noted in Table 2. The covariates that changed the HR of household income greater than 25% included psychological stress, current pain severity score at baseline visit (BPI now), PPT at the control site, and age. After adjusting for covariates in the final model, the hazard ratio for household income was 2.79 (95% CI [1.63–4.67], p < 0.001).
Correlation results show that household income was significantly and negatively associated with worst pain score (BPI worst) (ϕ= -0.018, p = 0.007) and pain interference (BPI interference) (ϕ = -0.181, p = 0.01). Household income also demonstrated a weak positive correlation with PPT at the painful site (ϕ = 0.19, p < 0.01) and PPT at the control site (ϕ = 0.123, p < 0.01). Additionally, two confounding factors were correlated with household income, African American race (r = -0.422, p < 0.001) and smoking status (ϕ = -0.297, p < 0.001).
Discussion
The purpose of this study was to investigate if increased levels of psychological stress, self-reported pain, and pain sensitivity during an acute state of LBP were predictive of pLBP. Our findings demonstrate that, although these factors influenced the pain trajectories, household income was the dominant predictor in this sample. The final model suggests that subjects who had an annual household income greater than or equal to $60,000 and whose age was under 26 years with lower stress scores, lower reported pain severity, and higher pressure pain thresholds at the time of the baseline visit were most likely to experience resolution of their pain. These findings align with previous studies of individuals experiencing chronic pain conditions, including pLBP (Janevic, McLaughlin, Heapy, Thacker, & Piette, 2017; Mun et al., 2019; Shmagel et al., 2016).
The primary risk factors interest, baseline psychological stress and self-reported pain along with PPT, were individually indicated in the bivariate analyses as influencing factors on the LBP trajectories. Additionally, in the multivariate analysis, these three factors were also found to be key to characterizing LBP trajectories in this sample. However, the hypothesized grouping of these risk factors was not substantiated when accounting for the confounding factors in the final model. The results from this analysis indicated that external and non-modifiable factors primarily influenced the development of pLBP. These findings support the biopsychosocial model of pain (Gatchel et al., 2007), demonstrating the importance of examining biological, psychological, and social factors when accounting for mechanisms contributing to pLBP.
As psychological stress was identified as a covariate in the final model, the present findings suggest that household income is closely associated with perceived stress and may explain the relationship between perceived stress and pain persistence in the bivariate model. Poor adaptation to chronic stress can increase allostatic load, the impact of which is seen can be seen in associations between low socioeconomic status and increased rates of chronic disease, including persistent pain conditions (Buscemi et al., 2019; Fliesser, De Witt Huberts, & Wippert, 2017; McEwen, & Gianaros, 2010; Maly & Vallerand, 2018; Vachon-Presseau et al., 2013). It is also possible the PSS measure captured a sense of financial insecurity in the framework of general or chronic stress. This finding is in agreement with prior evidence using the PSS, describing increased levels of reported stress for individuals with lower income (Cohen & Janicki-Deverts, 2012). Investigations of psychological stress as a mechanism in developing pain trajectories continues to be an important area of research (Lunde & Seiberg, 2020).
Subjective pain severity and interference scores and one measure of pain sensitivity (PPT) were also included in the final model as covariates. It is interesting that the pain severity captured at the time of the baseline visit (BPI now) was the strongest covariate out of all subjective pain scores in this analysis. Bias should be considered since subjects were participating in a study that pulls attention to the experience of pain when considering these findings. Contrary to our study, a large systematic review found that baseline pain was not strongly predictive of chronic pain trajectories (Chou & Shekelle, 2010).
Differential baseline PPTs were also found in subjects who experienced pain persistence and confirms previous findings (Starkweather et al., 2016c; Marcuzzi et al., 2018; Yildiz et al., 2017). An enhanced pressure pain sensitivity at the control (LBP) site could indicate early central sensitization in pain pathways (Woolf, 2011). As the biological mechanisms of persistent stress are closely related to the biological mechanisms of pain, examining the mechanisms of stress as they relate to those of chronic pain continues to be an important consideration for future studies and nursing practice (Abdallah, & Geha, 2017; McEwen, 2013; Vachon-Presseau et al., 2013; Vachon-Presseau, 2018).
Taken together, these results suggest that psychological stress, pain severity, and pain sensitivity during acute LBP are influential factors in pLBP trajectories, supporting previous work on stress and pain trajectories (Allegri et al., 2016; Jennings et al., 2014; Mun et al., 2019; Vachon-Presseau, 2018). Moreover, the findings from this analysis add to the ongoing evidence that social factors affect pain outcomes and persistence (Janevic et al., 2017; Maly & Vallerand, 2018). These results reinforce the critical need for an improved recognition of personal and social factors in pain-related assessments. Building nursing practice standards which include evaluations of these factors will help bring light to how situational contexts and illness experiences can influence the biopsychosocial patterns of disease, including pLBP. Assessing an individual’s perception of stress will further help nurses to advance integration of social contexts into illness and pain care.
Study Limitations
As described in the methods, over 30% of the subjects included in the analysis did not complete follow-up visits and, therefore, their low back pain trajectory remains undetermined. Although we attempted to account for this by applying the methods of right censoring and a bootstrapping technique, bias still may have been introduced. Additionally, we only included variables in the model that were found to be significant in the bivariate analysis. Due to this approach, the final model did not include sex or BMI, which have both been shown to impact pLBP outcomes (De Palma et al., 2012; Larsson, Björk, Börsbo, & Gerdle, 2012). Examining perceived stress as a baseline predictive factor does not account for changes in levels of stress as pain continues or distinguish if the level of stress measured correlates with ongoing pain changes, which limits our ability to make conclusions regarding long-term associations between psychological stress and chronic pain. Lastly, alternative variables were included in the parent study that should be considered in subsequent investigations, including coping strategies, pain-related disability, and mood state.
Future Directions
Our findings support prior evidence that multiple factors play a major role in the development of persistent pain states. This study points to the need for the thorough evaluation of social and clinical factors during pain care consultations and the increased recognition of how pain-related health care disparities may evolve. Alone, nurses cannot identify and address every factor which impacts a painful experience or limits access to holistic pain care. Therefore, the focus should concentrate on collaborating with other team members that have an active role in caring for a patient with pain, including physicians, psychologists, social workers, and family members. Through their relationships with patients, nurses can be leaders in helping to drive these teams and the identification of key factors that may contribute to persistent pain conditions. Interdisciplinary team collaboration and the use of social support strategies that integrate assessment of pain, stress and social determinants for patients experiencing pain will help to advance nursing practice. Future research that focuses on the impact that nurse led social support strategies have on changing pain trajectories can provide further evidence of the value of advancing this practice model.
Highlights.
Baseline stress, self-reported pain, and pain sensitivity impact LBP trajectories
Higher household income increased the likelihood of pain resolution over 24 weeks
Income’s relation to LBP course influenced by baseline stress, pain and sensitivity
Consistent evaluation of biopsychosocial factors of pain could advance nursing care
Acknowledgement:
We would like to acknowledge and thank Dr. Stephen Walsh for provided support and guidance throughout the data analysis.
Funding acknowledgement:
The research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR013932. The time spent to develop this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number T32NR013456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have to conflicts of interest to disclose.
References
- Abdallah CG, & Geha P (2017). Chronic Pain and Chronic Stress: Two Sides of the Same Coin? Chronic Stress, 1. 10.1177/2470547017704763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegri M, Montella S, Salici F, Valente A, Marchesini M, Compagnone C, … Fanelli G (2016). Mechanisms of low back pain: A guide for diagnosis and therapy. F1000Research, 5. 10.12688/f1000research.8105.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Bergman C (2017). icenReg: Regression Models for Interval Censored Data in R. Journal of Statistical Software , Articles, 81(12), 1–23. 10.18637/jss.v081.i12 [DOI] [Google Scholar]
- Belfer I, & Dai F (2010). Phenotyping and genotyping neuropathic pain. Current Pain and Headache Reports, 14 (3),203–212. 10.1007/s11916-010-0110-1 [DOI] [PubMed] [Google Scholar]
- Bradburn MJ, Clark TG, Love SB, & Altman DG (2003). Survival analysis part II: multivariate data analysis--an introduction to concepts and methods. British Journal of Cancer, 89(3), 431–436. 10.1038/sj.bjc.6601119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi V, Chang W, Liston M, McAuley J, & Schabrun S (2019). The Role of Perceived Stress and Life Stressors in the Development of Chronic Musculoskeletal Pain Disorders: A Systematic Review. Journal of Pain Official Journal of the American Pain Society, 20(10), 1127–1139. 10.1016/j.jpain.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Chou R, & Shekelle P (2010). Will this patient develop persistent disabling low back pain? JAMA, 303 (13), 1295–1302. 10.1001/jama.2010.344 [DOI] [PubMed] [Google Scholar]
- Cleeland CS, & Ryan KM (1994). Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore, 23(2), 129–138. [PubMed] [Google Scholar]
- Cohen S, & Janicki- Deverts D (2012). Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. Journal of Applied Social Psychology, 42(6), 1320–1334. 10.1111/j.1559-1816.2012.00900.x [DOI] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Starkweather AR, Heineman A, Storey S, Rubia G, Lyon DE, Greenspan J, & Dorsey SG (2016a). Methods to measure peripheral and central sensitization using quantitative sensory testing: A focus on individuals with low back pain. Applied Nursing Research, 29, 237–241. 10.1016/j.apnr.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Starkweather AR, Lyon DE, Kinser P, Heineman A, Sturgill JL, Deng X, … Dorsey SG (2016b). Comparison of Low Back Pain Recovery and Persistence: A Descriptive Study of Characteristics at Pain Onset. Biological Research for Nursing, 18(4), 401–410. 10.1177/1099800416631819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather AR, Ramesh D, Lyon DE, Siangphoe U, Deng X, Sturgill J, … Greenspan J (2016c). Acute low back pain: Differential somatosensory function and gene expression compared with healthy no-pain controls. The Clinical Journal of Pain, 32(11), 933–939. 10.1097/AJP.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma MJ, Ketchum JM, & Saullo TR (2012). Multivariable analyses of the relationships between age, gender, and body mass index and the source of chronic low back pain. Pain Medicine, 13, 498–506. 10.1111/j.1526-4637.2012.01339.x [DOI] [PubMed] [Google Scholar]
- Edwards RR, Dworkin RH, Sullivan MD, Turk DC, & Wasan AD (2016). The role of psychosocial processes in the development and maintenance of chronic pain. The Journal of Pain, 17(9 Suppl), T70–T92. 10.1016/j.jpain.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff N, Hirschi A, & von Känel R (2013). Traumatization and chronic pain: a further model of interaction. Journal of Pain Research, 6, 765–770. 10.2147/JPR.S52264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesser M, De Witt Huberts J, & Wippert PM (2017). The choice that matters: The relative influence of socioeconomic status indicators on chronic back pain- a longitudinal study. BMC Health Services Research, 17(1), 800. 10.1186/s12913-017-2735-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, & Richard P (2012). The economic costs of pain in the United States. The Journal of Pain, 13(8), 715–724. 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, & Turk DC (2007). The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin, 133(4), 581–624. 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, & Senba E (2006). Stress-induced hyperalgesia: animal models and putative mechanisms. Frontiers in Bioscience, 11, 2179–2192. [DOI] [PubMed] [Google Scholar]
- Janevic MR, McLaughlin SJ, Heapy AA, Thacker C, & Piette JD (2017). Racial and Socioeconomic Disparities in Disabling Chronic Pain: Findings From the Health and Retirement Study. The Journal of Pain, 18(12), 1459–1467. 10.1016/j.jpain.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings EM, Okine BN, Roche M, & Finn DP (2014). Stress-induced hyperalgesia. Progress in Neurobiology, 121, 1–18. 10.1016/j.pneurobio.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, & Cleeland CS (2004). Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. The Clinical Journal of Pain, 20(5), 309–318. 10.1097/00002508-200409000-00005 [DOI] [PubMed] [Google Scholar]
- Larsson B, Björk J, Börsbo B, & Gerdle B (2012). A systematic review of risk factors associated with transitioning from regional musculoskeletal pain to chronic widespread pain. European Journal of Pain, 16(8), 1084–1093. 10.1002/j.1532-2149.2012.00117.x [DOI] [PubMed] [Google Scholar]
- Lunde CE, & Sieberg CB (2020). Walking the Tightrope: A Proposed Model of Chronic Pain and Stress. Frontiers in Neuroscience, 14, 270. 10.3389/fnins.2020.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly A, & Vallerand AH (2018). Neighborhood, socioeconomic, and racial influence on chronic pain. Pain Management Nursing, 19(1), 14–22. 10.1016/j.pmn.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuzzi A, Wrigley PJ, Dean CM, Graham PL, & Hush JM (2018). From acute to persistent low back pain: A longitudinal investigation of somatosensory changes using quantitative sensory testing-an exploratory study. Pain Reports, 3(2), e641. 10.1097/PR9.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2013). The Brain on stress: Toward an integrative approach to brain, body, and behavior. Perspectives on Psychological Science, 8(6), 673–675. 10.1177/1745691613506907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meints SM, & Edwards RR (2018). Evaluating psychosocial contributions to chronic pain outcomes. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 87(Pt B), 168–182. 10.1016/j.pnpbp.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun CJ, Davis MC, Molton IR, Karoly P, Suk HW, Ehde DM, . . . Jensen MP (2019). Personal resource profiles of individuals with chronic pain: Sociodemographic and pain interference differences. Rehabilitation Psychology, 64(3), 245–262. 10.1037/rep0000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Weingarten TN, Mantilla CB, Hooten WM, & Warner DO (2010). Smoking and pain: pathophysiology and clinical implications. Anesthesiology, 113(4), 977–992. 10.1097/ALN.0b013e3181ebdaf9 [DOI] [PubMed] [Google Scholar]
- Shmagel A, Foley R, & Ibrahim H (2016). Epidemiology of chronic low back pain in US adults: Data from the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care & Research, 68(11), 1688–1694. 10.1002/acr.22890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede IR-D, Rief BW, Barke BA, Aziz AQ, Bennett HM, Benoliel WSR, . . . Wang S-JWS (2019). Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain, 160(1), 19–27. 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E (2018). Effects of stress on the corticolimbic system: Implications for chronic pain. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 87, 216–223. 10.1016/j.pnpbp.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Roy M, Martel M-O, Caron E, Marin M-F, Chen J, … Rainville P (2013). The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain, 136(Pt 3), 815–827. 10.1093/brain/aws371 [DOI] [PubMed] [Google Scholar]
- Woolf CJ (2011). Central sensitization: Implications for the diagnosis and treatment of pain. Pain, 152(3 Suppl), S2–S15. 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz SH, Ulasli AM, Özdemir Erdogan M, Dikici Ö, Arikan Terzi ES, Dündar Ü, & Solak M (2017). Assessment of pain sensitivity in patients with chronic low back pain and association with HTR2A gene polymorphism. Archives of Rheumatology, 32(1), 3–9. 10.5606/ArchRheumatol.2017.5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, & Sun J (2009). Interval censoring. Statistical Methods in Medical Research, 19(1), 53–70. 10.1177/0962280209105023 [DOI] [PMC free article] [PubMed] [Google Scholar]


