Abstract
A highly efficient method for controllable double CF2-insertion into pentafluorophenylcopper species using TMSCF3 as difluoromethylene source has been developed. The newly generated fluoroalkylcopper(i) species, C6F5CF2CF2Cu, shows good reactivity towards a myriad of structurally diverse aryl, heteroaryl and alkenyl iodides. This protocol is easy to handle, ready to scale up and applicable for the synthesis of relative complex molecules, thus providing a convenient method for facile access to tetrafluoroethylene-bridged structures.
Copper-mediated controllable double CF2 insertion into sp2 C–Cu bond and subsequent cross-coupling reaction open the door to the synthesis of an array of valuable tetrafluoroethylene-bridged molecules.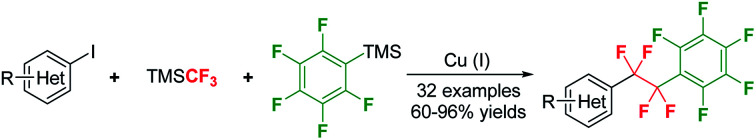
Introduction
Due to the unique physical, chemical and biological properties of organofluorine compounds, the introduction of fluorine atom(s) or fluorinated moieties into organic molecules has become a routine strategy in drug design and advanced material development.1–4 Among various fluorinated functionalities, the tetrafluoroethylene motif (–CF2CF2–) has attracted considerable attention because of its applications in agrochemicals5 and liquid-crystalline materials.6–8 Moreover, the introduction of –CF2CF2– group into liquid crystals often results in highly advantageous properties such as high clearing temperature, broad nematic phase range, low rotational viscosity and high dielectric anisotropy.6–8 Therefore, it is of strong demand to access tetrafluoroethylene-bridged molecules.
Current methods for the syntheses of tetrafluoroethylene-bridged structures are mainly based on (1) deoxofluorination of 1,2-dicarbonyl compounds with SF4 and DeoxoFluor;9–11 (2) fluorination of C–C triple bonds using F2;12–14 (3) 1,2-difunctionalization of tetrafluoroethylene (TFE);15–23 (4) difluoromethylene insertion using CF2Br2 as the CF2 source;24,25 and (5) using RCF2CF2Br as the build block.5 However, these methods suffer from several drawbacks such as (1) using toxic, highly reactive or hazardous reagents; (2) low functional group tolerance and/or (3) using explosive gaseous reagents or ozone-depleting substances (ODS). As such, developing a new method to incorporate –CF2CF2– structure motif into organic molecules with readily available, easy to handle and environmentally benign reagents under mild conditions is highly desired.
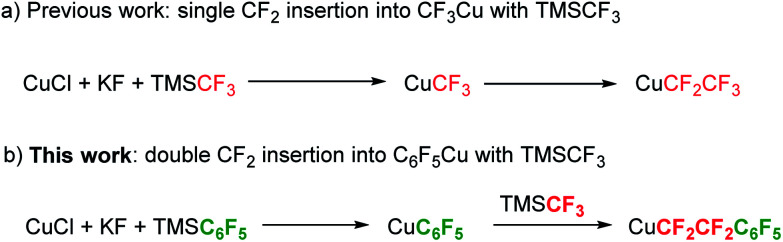
(Trifluoromethyl)trimethylsilane (TMSCF3), often called Ruppet–Prakash reagent, is arguably the most widely used trifluoromethylating agent.26–30 In 2011, our group, in collaboration with the Prakash group, revealed that TMSCF3 is a good difluorocarbene precursor, which can be used in the [2 + 1] cycloaddition reaction with alkenes and alkynes.31 Recently, our group reported that difluorocarbene generated from TMSCF3 could undergo dimerization to give tetrafluoroethene (TFE),32,33 which can be used for a variety of transformations.33 Very recently, our group demonstrated that, by using TMSCF3 as the difluoromethylene source, controllable CF2-insertion into CuCF3 to generate CuCF2CF3 could be realized.34 Inspired by this C1 to C2 process, we envisioned that it might be possible to insert CF2 into other C–M bonds. Herein, we report our latest progress in the fluorocarbon homologation reaction using TMSCF3 as the difluoromethylene source. By carefully tuning the reaction conditions, controllable double insertion of CF2 into C6F5–Cu gives rise to C6F5CF2CF2Cu, which can be applied to the preparation of a diverse range of tetrafluoroethylene-bridged compounds (Scheme 1).
Scheme 1. Fluorocarbon homologation with TMSCF3. TMS = trimethylsilyl.
Results and discussion
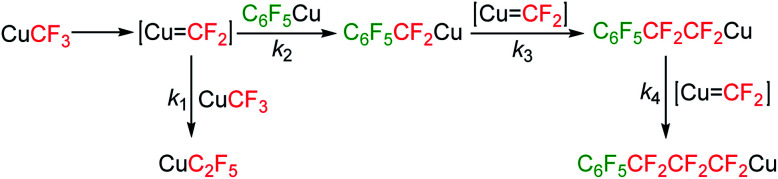
Our investigation commenced with the preparation of C6F5CF2CF2Cu from TMSC6F5 and TMSCF3. Initially, we used 1 equivalent of TMSC6F5 as the C6F5Cu precursor, 1 equivalent of TMSCF3 as the difluorocarbene precursor, 2 equivalents of KF as the desilylating reagent and 2.5 equivalents of CuCl as the copper source. All these components were added simultaneously with DMF as the solvent, and the resulting mixture was stirred at room temperature for 12 hours. Analysis of the mixture by 19F NMR spectroscopy revealed that C6F5CF2CF2Cu (21%), C6F5Cu (57%) and CuCF3 (37%) were formed; no single CF2-insertion product C6F5CF2Cu could be detected (Table 1, entry 1). When adding 2 equivalents of difluorocarbene source TMSCF3, we found that the desired product C6F5CF2CF2Cu was formed in 79% yield, in conjunction with C6F5Cu (3%), CuCF3 (10%) and CuC2F5 (5%) (entry 2).35 If 3 equivalents of TMSCF3 was used, C6F5CF2CF2Cu was formed in 83% yield, along with CuCF3 (16%) and CuC2F5 (12%) being formed; neither C6F5Cu nor triple CF2-insertion product C6F5CF2CF2CF2Cu could be detected (entry 3). These results (entries 1–3) clearly indicate that the TMSCF3-derived difluorocoppercarbene (Cu CF2) species34 could selectively undergo double CF2-insertion into C6F5Cu, regardless of the amount of TMSCF3 used. This behaviour is in accord with previous reports.24 The high selectivity may be attributed to the intrinsic reactivity of different fluoroalkylcopper species toward Cu CF2 (Scheme 2). As to the possible intermediate, C6F5CF2Cu, its benzylic C–Cu bond is highly reactive and tended to insert another CF2 unit to give C6F5CF2CF2Cu;24,25 the resulting C6F5CF2CF2Cu has lower reactivity than CuCF3 because of its longer fluoroalkyl chain.24,25 Therefore, even in the presence of excess of TMSCF3, triple CF2-insertion into C6F5Cu could not be observed; in that case, the CF2-insertion into CuCF3 to generate CuC2F5 would be favoured. Altogether, the relative reaction rate of each step is k3 > k2 > k1 > k4.
Optimization of reaction conditions for the double CF2 insertion into C6F5Cu with TMSCF3a.

| |||||
|---|---|---|---|---|---|
| Entry | TMSC6F5 : CuCl : KF : TMSCF3 | t (h) | T (°C) | Yield (%) | |
| C6F5C2F4Cu | C6F5Cu/CuCF3/CuC2F5 | ||||
| 1b | 1 : 2.5 : 2 : 1 | 12 | rt | 21 | 57/37/n.d. |
| 2b | 1 : 4 : 3 : 2 | 12 | rt | 79 | 3/10/5 |
| 3b | 1 : 5.5 : 4 : 3 | 12 | rt | 83 | n.d./16/12 |
| 4b | 1 : 4 : 3 : 2 | 20 | rt | 87 | 4/2/4 |
| 5b | 1 : 3 : 3 : 2 | 20 | rt | 1 | 87/82/n.d. |
| 6b | 1 : 4 : 3 : 2 | 10 | 50 | 75 | 1/n.d./23 |
| 7 | 1 : 4 : 3 : 2 | 28 | rt | 91 | <1/<1/4 |
| 8 | 1 : 4 : 2 : 2 | 28 | rt | 76 | n.d./2/5 |
| 9c | 1 : 4 : 3 : 2 | 28 | rt | 91 | n.d./6/2 |
| 10d | 1 : 4 : 3 : 2 | 28 | rt | 93 | n.d./8/2 |
| 11e | 1 : 4 : 3 : 1.9 | 28 | rt | 92 | 2/4/2 |
| 12e | 1 : 4 : 3 : 1.9 | 36 | rt | 86 | 3/4/2 |
| 13e,f | 1 : 4 : 3 : 1.9 | 28 | rt | 89 | n.d./1/2 |
Reactions were performed on 0.2 mmol TMSC6F5 (1.0 equiv.) scale. Yields were determined by 19F NMR spectroscopy using PhOCF3 as an internal standard. n.d. = not detected.
TMSC6F5 and TMSCF3 were added simultaneously without the pre-preparation of C6F5Cu.
TMSCF3 was added in three portions for every 4 hours.
TMSCF3 was added in three portions for every 6 hours.
TMSCF3 was added in two portions for every 6 hours.
After reacted at rt for 28 hours, the reaction mixture was stirred at 60 °C for another 2 hours.
Scheme 2. Proposed reaction mechanism.
With this understanding in mind, we went on to optimize the reaction conditions in order to increase the yield of C6F5CF2CF2Cu and minimize those of CuCF3 and CuC2F5. By using 2 equivalents of TMSCF3 and prolonging reaction time to 20 hours, C6F5CF2CF2Cu was formed in 87% yield (entry 4). When we decreased the amount of CuCl from 4 equivalents to 3 equivalents, only trace of C6F5CF2CF2Cu was observed, with >80% C6F5Cu and CuCF3 being recovered (entry 5). This result revealed that the presence of excess amount of CuCl is crucial for the Cu CF2 generation, which is consistent with our previous report.34 As CuCF3 was always observed, we tried to speed up the decomposition of CuCF3 at elevated temperatures. However, when the reaction was carried out at 50 °C, although no CuCF3 was observed, a larger amount of CuC2F5 was detected, and C6F5CF2CF2Cu was obtained in relatively lower yield (entry 6 vs. entry 4). Next, we attempted to add TMSCF3 into the reaction mixture after the preparation of C6F5Cu. Gratifyingly, the yield of C6F5CF2CF2Cu was increased slightly (entry 7). In light of the decomposition of CuCF3 to Cu CF2 would release fluoride ions, we surmised that the amount of externally added KF could be reduced. However, lowering down KF to 2 equivalents gave inferior result (entry 8). To further decrease the yield of CuC2F5, we envisaged that adding TMSCF3 in batches to decrease the concentration of CuCF3 might be helpful. After some brief optimizations and decreasing the amount of TMSCF3 to 1.9 equivalents (entries 9–11), C6F5CF2CF2Cu was formed in 92% yield, together with 2% of C6F5Cu and 4% of CuCF3 being formed (entry 11). Prolonging the reaction time to 36 hours did not have any beneficial effect (entry 12). Finally, when the reaction was conducted at room temperature for 28 hours, then stirred at 60 °C for 2 hours, no C6F5Cu and little amounts of CuCF3 (1%) and CuC2F5 (2%) could be detected, with C6F5CF2CF2Cu being formed in 89% yield (entry 13).
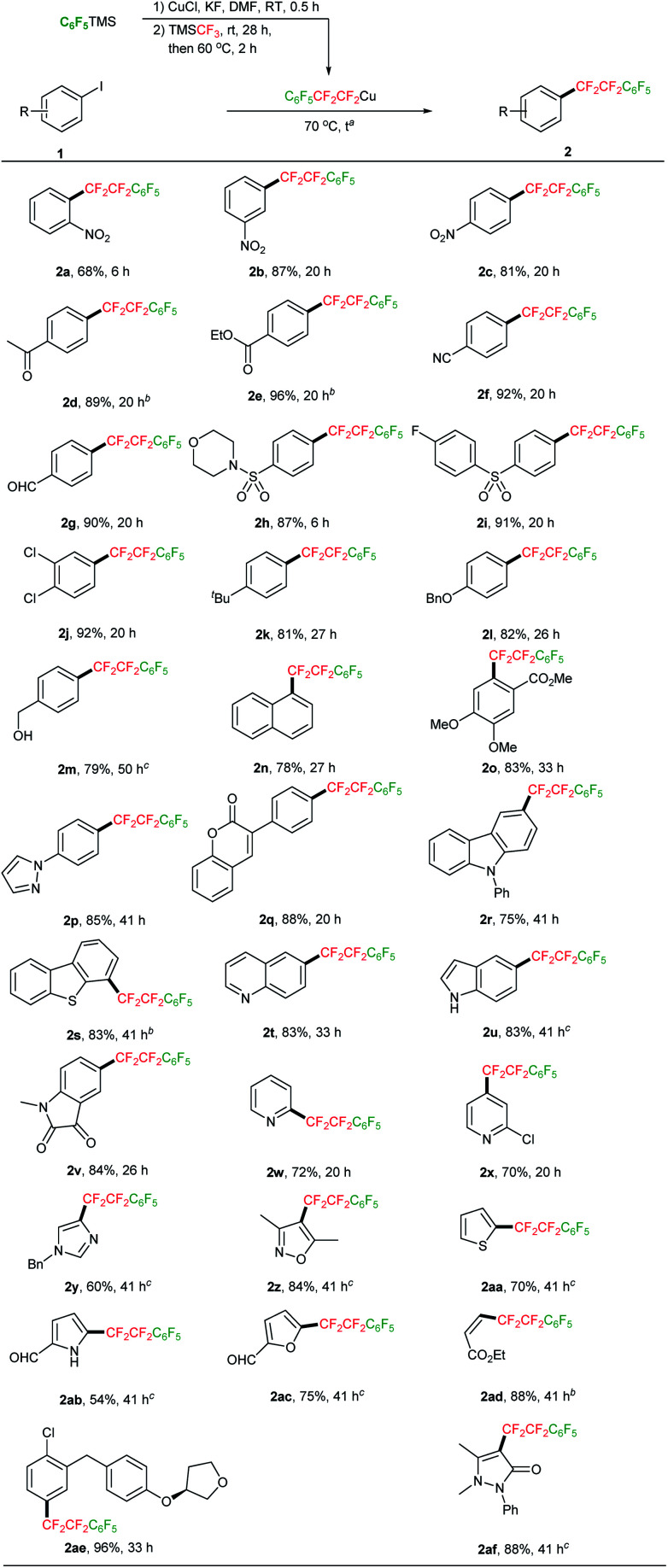
With the optimized conditions (Table 1, entry 13) in hand, the reactivity of this TMSCF3-derived C6F5CF2CF2Cu towards aryl iodides was studied. A variety of structurally diverse (hetero)aryl and alkenyl iodides are all viable substrate, giving the desired tetrafluoroethylene-bridged products in moderate to good yields (Scheme 3). The electronic character of aryl iodides do not have obvious influence on the reaction efficiency, and both electron-deficient (2a–i) and electron-rich (2k–p, 2r–s) substrates were readily transformed to the desired products in good yields. Common functional groups such as nitro (2a–c, in ortho, meta and para positions), acetyl (2d), ester (2e), cyano (2f), sulfonamide (2h) and sulfone (2i) were compatible with the reaction conditions, and good yields of products were observed. Notably, because of the mildness of the reaction conditions, some sensitive functionalities including aldehyde (2g, 2ab–ac), alcohol (2m) and unprotected NH group (2u, 2ab), were also tolerated. Heterocycles, such as pyrazole (2p), coumarin (2q), carbazole (2r), benzothiophene (2s), quinoline (2t) and indole (2u) were competent under the reaction conditions, as demonstrated by the formation of tetrafluoroethylene-bridged products in high yields. Moreover, heteroaryl iodides, including iodopyridine (2w–x), iodoimidazole (2y), iodoisoxazole (2z), iodothiophene (2aa), iodopyrrole (2ab) and iodofuran (2ac), participated in this perfluorophenylethylation to afford corresponding products in moderate to good yields (54–84%). Iodoalkene 1ad also showed good reactivity towards C6F5CF2CF2Cu, furnishing the desired product 2ad in 88% yield. This protocol is also effective for the perfluorophenylethylation of relatively complex compounds and pharmaceutical intermediates 1ae and 1af, giving the corresponding products 2ae and 2af in 96% and 88% yields, respectively. The broad scope of this reaction underscores the great potential of its application in the synthesis of a raft of valuable –CF2CF2– bridged molecules.
Scheme 3. Perfluorophenylethylation of (hetero)aryl iodides with TMSCF3-derived C6F5CF2CF2Cu. a Unless otherwise noted, reactions were performed on 0.5 mmol of 1 (1.0 equiv.) scale, and TMSCF3 was added in two portions every 6 h; 1.5 equivalents of TMSC6F5 was used; the molar ratio of TMSC6F5 : CuCl : KF : TMSCF3 = 1 : 4 : 3 : 1.9. b 1.6 equivalent of TMSC6F5 was used. c 1.8 equivalent of TMSC6F5 was used.
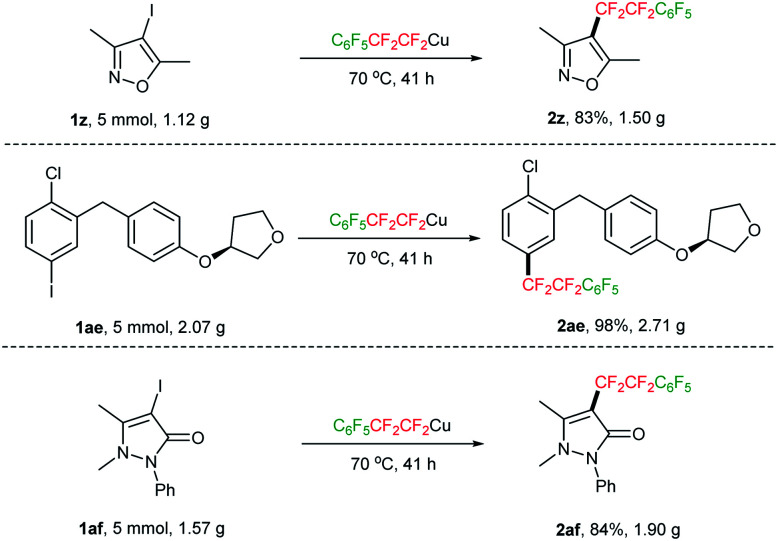
The inherent value of our controllable double CF2-insertion strategy with TMSCF3 for the introduction of tetrafluoroethylene bridge is further demonstrated by its applicability to gram-scale synthesis. For example, when iodoisoxazole 1z was scaled up to 5 mmol (1.12 g), the desired product 2z was obtained in 83% yield (1.50 g). Analogously, pharmaceutical intermediates 1ae and 1af were also successfully scaled up to 5 mmol, with the yields comparable to that on 0.5 mmol scale (Scheme 4).
Scheme 4. Gram-scale synthesis.
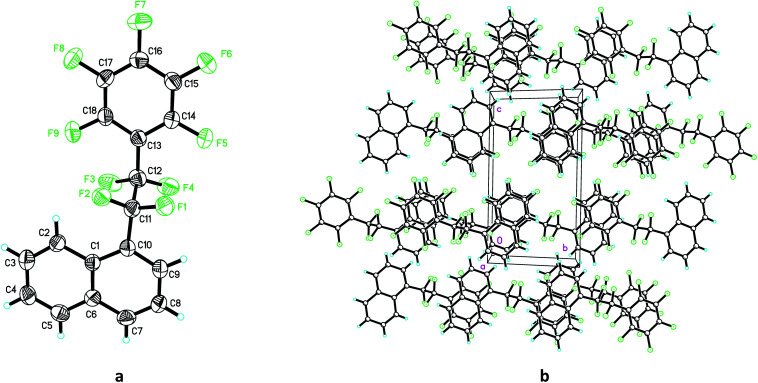
It is worthwhile to note that the tetrafluoroethylene-bridged product 2n possesses interesting conformation and intermolecular interaction. As shown in Fig. 1,36 the single crystal structure of product 2n shows that the dihedral angle of C10–C11–C12–C13 is 174.1°, and two aromatic (the naphthalenyl and pentafluorophenyl) rings in 2n are almost parallel to each other (see Fig. 1a, also see ESI†). The packing diagram shows there are extensive intermolecular π–π stackings between naphthalenyl and pentafluorophenyl rings of 2n (Fig. 1b), which might find useful applications in crystal engineering and materials science.
Fig. 1. (a) The single crystal structure and (b) packing diagram of 2n.36.
Conclusions
In conclusion, a controllable double CF2-insertion into C6F5Cu was realized using TMSCF3 as the difluoromethylene source. The resulting C6F5CF2CF2Cu species showed high reactivity towards various (hetero)aryl iodides and alkenyl iodides, providing an easy access to a variety of –CF2CF2– bridged molecules. Compared with previous methods for the construction of –CF2CF2– unit, this approach owns several merits such as utilizing commercially available and environmentally benign reagents as the CF2 source, easy to handle, broad substrate scope and mild conditions. This double CF2-insertion strategy represents the second generation of fluorocarbon homologation reactions via difluoromethylene insertion using TMSCF3 (the first generation is single CF2-insertion into CuCF3). Further efforts to seek after novel CF2-insertion reactions using TMSCF3 are currently underway in our laboratory.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program of China (No. 2015CB931900, 2016YFB0101200), the National Natural Science Foundation of China (No. 21632009, 21421002), the Key Programs of the Chinese Academy of Sciences (No. KGZD-EW-T08), the Key Research Program of Frontier Sciences of CAS (No. QYZDJ-SSW-SLH049), and Shanghai Science and Technology Program (No. 18JC1410601).
Electronic supplementary information (ESI) available. CCDC 1957757. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc05018c
Notes and references
- Uneyama K., Organofluorine Chemistry, Blackwell, Oxford, 2006 [Google Scholar]
- Kirsch P., Modern Fluoroorganic Chemistry: Synthesis. Reactivity, Applications, Wiley-WCH, Weinheim, 2nd edn, 2013 [Google Scholar]
- Ojima I., Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, U.K., 2009 [Google Scholar]
- Berger R. Resnati G. Metrangolo P. Weber E. Hulliger J. Chem. Soc. Rev. 2011;40:3496–3508. doi: 10.1039/C0CS00221F. [DOI] [PubMed] [Google Scholar]
- Zhu J. Ni C. Gao B. Hu J. J. Fluorine Chem. 2015;171:139–147. doi: 10.1016/j.jfluchem.2014.08.011. [DOI] [Google Scholar]
- Kirsch P. Bremer M. ChemPhysChem. 2010;11:357–360. doi: 10.1002/cphc.200900745. [DOI] [PubMed] [Google Scholar]
- Kirsch P. Huber F. Lenges M. Taugerbeck A. J. Fluorine Chem. 2001;112:69–72. doi: 10.1016/S0022-1139(01)00488-2. [DOI] [Google Scholar]
- Kirsch P. Bremer M. Huber F. Lannert H. Ruhl A. Lieb M. Wallmichrath T. J. Am. Chem. Soc. 2001;123:5414–5417. doi: 10.1021/ja010024l. [DOI] [PubMed] [Google Scholar]
- Chang Y. Tewari A. Adi A.-I. Bae C. Tetrahedron. 2008;64:9837–9842. doi: 10.1016/j.tet.2008.08.009. [DOI] [Google Scholar]
- Singh R. P. Majumder U. Shreeve J. n. M. J. Org. Chem. 2001;66:6263–6267. doi: 10.1021/jo0157674. [DOI] [PubMed] [Google Scholar]
- Hasek W. R. Smith W. C. Engelhardt V. A. J. Am. Chem. Soc. 1960;82:543–551. doi: 10.1021/ja01488a012. [DOI] [Google Scholar]
- Gatenyo J. Rozen S. J. Fluorine Chem. 2009;130:332–335. doi: 10.1016/j.jfluchem.2008.12.009. [DOI] [Google Scholar]
- York C. Prakash G. K. S. Olah G. A. J. Org. Chem. 1994;59:6493–6494. doi: 10.1021/jo00100a068. [DOI] [Google Scholar]
- Zupan M. Pollak A. J. Org. Chem. 1974;39:2646–2647. doi: 10.1021/jo00931a050. [DOI] [Google Scholar]
- Shirataki H. Ono T. Ohashi M. Ogoshi S. Org. Lett. 2019;21:851–856. doi: 10.1021/acs.orglett.8b03674. [DOI] [PubMed] [Google Scholar]
- Kawashima T. Ohashi M. Ogoshi S. J. Am. Chem. Soc. 2018;140:17423–17427. doi: 10.1021/jacs.8b11671. [DOI] [PubMed] [Google Scholar]
- Shirataki H. Ohashi M. Ogoshi S. Eur. J. Org. Chem. 2019:1883–1887. doi: 10.1002/ejoc.201801721. [DOI] [Google Scholar]
- Kawashima T. Ohashi M. Ogoshi S. J. Am. Chem. Soc. 2017;139:17795–17798. doi: 10.1021/jacs.7b12007. [DOI] [PubMed] [Google Scholar]
- Ohashi M. Ishida N. Ando K. Hashimoto Y. Shigaki A. Kikushima K. Ogoshi S. Chem. –Eur. J. 2018;24:9794–9798. doi: 10.1002/chem.201802415. [DOI] [PubMed] [Google Scholar]
- Ohashi M. Adachi T. Ishida N. Kikushima K. Ogoshi S. Angew. Chem., Int. Ed. 2017;56:11911–11915. doi: 10.1002/anie.201703923. [DOI] [PubMed] [Google Scholar]
- Ohashi M. Shirataki H. Kikushima K. Ogoshi S. J. Am. Chem. Soc. 2015;137:6496–6499. doi: 10.1021/jacs.5b03587. [DOI] [PubMed] [Google Scholar]
- Saijo H. Ohashi M. Ogoshi S. J. Am. Chem. Soc. 2014;136:15158–15161. doi: 10.1021/ja5093776. [DOI] [PubMed] [Google Scholar]
- Li L. Ni C. Xie Q. Hu M. Wang F. Hu J. Angew. Chem., Int. Ed. 2017;56:9971–9975. doi: 10.1002/anie.201705734. [DOI] [PubMed] [Google Scholar]
- Yang Z.-Y. Wiemers D. M. Burton D. J. J. Am. Chem. Soc. 1992;114:4402–4403. doi: 10.1021/ja00037a057. [DOI] [Google Scholar]
- Yang Z.-Y. Burton D. J. J. Fluorine Chem. 2000;102:89–103. doi: 10.1016/S0022-1139(99)00247-X. [DOI] [Google Scholar]
- Liu X. Xu C. Wang M. Liu Q. Chem. Rev. 2015;115:683–730. doi: 10.1021/cr400473a. [DOI] [PubMed] [Google Scholar]
- Roy S. Gregg B. T. Gribble G. W. Le V.-D. Roy S. Tetrahedron. 2011;67:2161–2195. doi: 10.1016/j.tet.2011.01.002. [DOI] [Google Scholar]
- Singh R. P. Shreeve J. n. M. Tetrahedron. 2000;56:7613–7632. doi: 10.1016/S0040-4020(00)00550-0. [DOI] [Google Scholar]
- Ma J.-A. Cahard D. J. Fluorine Chem. 2007;128:975–996. doi: 10.1016/j.jfluchem.2007.04.026. [DOI] [Google Scholar]
- Prakash G. K. Yudin A. K. Chem. Rev. 1997;97:757–786. doi: 10.1021/cr9408991. [DOI] [PubMed] [Google Scholar]
- Wang F. Luo T. Hu J. Wang Y. Krishnan H. S. Jog P. V. Ganesh S. K. Prakash G. K. Olah G. A. Angew. Chem., Int. Ed. 2011;50:7153–7157. doi: 10.1002/anie.201101691. [DOI] [PubMed] [Google Scholar]
- Lee G. M. Harrison D. J. Korobkov I. Baker R. T. Chem. Commun. 2014;50:1128–1130. doi: 10.1039/C3CC48468H. [DOI] [PubMed] [Google Scholar]
- Li L. Ni C. Xie Q. Hu M. Wang F. Hu J. Angew. Chem., Int. Ed. 2017;56:9971–9975. doi: 10.1002/anie.201705734. [DOI] [PubMed] [Google Scholar]
- Xie Q. Li L. Zhu Z. Zhang R. Ni C. Hu J. Angew. Chem., Int. Ed. 2018;57:13211–13215. doi: 10.1002/anie.201807873. [DOI] [PubMed] [Google Scholar]
- The chemical shifts of these fluoroalkylcopper/fluoroarylcopper species were assigned as follows (in ppm): C6F5CF2CF2Cu, −101.1 (t, 2F), −106.6 (s, 2F), −139.4 (m, 2F), −153.1 (t, 1F), −163.9 (t, 2F); C6F5Cu, −111.0 (d, 2F), −163.3 (t, 1F), −164.3 (t, 2F); CuCF3, −26.7 (s, 3F); CuC2F5, −84.0 (s, 3F), −112.4 (s, 2F)
- CCDC 1957757† contains the supplementary crystallographic data for compound 2n
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.