Supplemental Digital Content is available in the text.
Keywords: acid-base equilibrium, balanced solutions, crystalloids, fluid therapy
Abstract
OBJECTIVE:
To summarize the evidence comparing various balanced crystalloid solutions.
DATA SOURCES:
We searched MEDLINE, EMBASE, PUBMED, and CENTRAL databases.
STUDY SELECTION:
We included randomized controlled trials that directly compared the IV administration of one balanced crystalloid solution with another.
DATA EXTRACTION AND ANALYSIS:
We examined metabolic and patient-important outcomes and conducted meta-analysis using random effects model. For comparisons or outcomes with insufficient data to allow for pooling, we describe results narratively. We assessed risk of bias for individual trials using the Cochrane risk of bias tool and certainty of evidence using Grading of Recommendations, Assessment, Development and Evaluations methodology.
DATA SYNTHESIS:
We included 24 randomized controlled trials comparing Plasmalyte, Ringer’s Lactate, Ringerfundin, Hartmann’s solution, Ringer’s Bicarbonate, Sterofundin, Kabilyte, Normosol, and novel balanced solutions. Of the included studies, 16 were performed in the perioperative setting, six in the ICU, one in the emergency department, and one in healthy volunteers. Administration of Plasmalyte resulted in a lower postinfusion serum chloride concentration (mean difference, 0.83 mmol/L lower; 95% CI, 0.03–1.64 mmol/L lower, low certainty), higher postinfusion base excess (mean difference, 0.65 mmol/L higher, 95% CI, 0.25–1.05 mmol/L higher, low certainty), and lower postinfusion serum lactate levels (mean difference, 0.46 mmol/L lower; 95% CI, 0.05–0.87 mmol/L lower, low certainty) compared with administration of any other balanced crystalloid. There were no important differences in postinfusion serum pH or potassium when comparing Plasmalyte with other balanced crystalloids. Data addressing other comparisons or examining the impact of different balanced crystalloids on patient-important outcomes were sparsely reported and too heterogeneous to allow for pooling.
CONCLUSIONS:
Administration of Plasmalyte results in lower serum concentrations of chloride and lactate, and higher base excess than other balanced crystalloids. The certainty of evidence is low and requires further study in large randomized controlled trials to inform the choice of balanced crystalloid in patients requiring volume replacement.
IV fluid (IVF) is a near ubiquitous practice in hospital-based medical care, especially in the critically ill. The most widely used IVF (1), 0.9% saline (i.e., normal saline [NS]), contains a chloride concentration that is higher than that of normal human serum (154 mmol/L in NS compared with 94–111 mmol/L in serum) (2). Some studies suggest large volume administration of NS may cause metabolic acidosis, decreased smooth muscle contractility, and renal blood flow (3, 4), although the evidence comparing the impact of administering NS compared with balanced crystalloids on patient-important outcomes remains unclear. A few studies suggest a potential benefit of balanced crystalloids compared with NS (5–9); however, uncertainty persists.
The term “balanced” is applied to IVF that has a lower chloride content more closely matching that of human plasma, accomplished through the substitution of chloride with an anion such as lactate or acetate (4). There are several commercially available balanced crystalloids with varying electrolyte concentrations including calcium, magnesium, and potassium. None of these solutions exactly matches that of human plasma and their comparative effects on clinical outcomes remain largely unknown. Although balanced IVF has several potential physiologic advantages (4) compared with unbalanced solutions, clinicians lack guidance when it comes to choosing between the available balanced fluids.
The objective of this systematic review and meta-analysis is to summarize all the randomized controlled trials (RCTs) that directly compared the effects of one balanced crystalloid with another examining physiochemical properties and patient-important outcomes.
MATERIALS AND METHODS
This systematic review is reported in accordance with the PRISMA guidelines (10) and was registered with PROSPERO (ID: CRD42019129267; submitted: March 23, 2019; registered: April 24, 2019).
Search Strategy and Study Selection
We searched the following electronic databases: MEDLINE, EMBASE, PUBMED, and CENTRAL from inception until October 4, 2020. We included RCTs that directly compared IVF of one balanced crystalloid with another. We checked the reference lists of included studies and those of review articles to identify other relevant studies. We did not limit our search based on population and included studies in children, adults, critically ill or hospitalized patients, and healthy volunteers. We limited results to English-language publications. We developed search terms using medical subject headings specific to each database platform in collaboration with a Health Information Specialist (11) (see Supplemental Digital Content, Table 1, http://links.lww.com/CCX/A585, for MEDLINE search strategy).
TABLE 1.
Characteristics of Included Studies
| Reference | Country | Total No.of Participants | Population | Type of Balanced Crystalloid Compared | Cumulative Volume of Fluid, Mean (sd) or [Range] |
|---|---|---|---|---|---|
| Ramanathan et al (16) | United States | 68 | Pregnant women undergoing cesarean section | Ringer’s Lactate vs Plasmalyte A | 1,200 mL; 1,200 mL |
| Ratcliffe et al (17) | United Kingdom | 29 | Pediatric heart surgery patients | Plasmalyte vs Hartmann’s | 380 mL (222.6); 413 mL (220) |
| Attalla et al (18) | Egypt | 30 | Cholecystectomy patients | Ringer’s Lactate vs Ringer’s Acetate | 846.67 mL (109.33); 836.67 mL (106.01) |
| Shimada et al (19) | Japan | 20 | Elective aortic aneurysm repair patients | Ringer’s Acetate vs Ringer’s Bicarbonate | 4,061 mL (871); 4,480 mL (857) |
| Hadimioglu et al (20) | Turkey | 90 | Kidney transplant recipients | Plasmalyte vs Ringer’s Lactate | 2,756 mL (800); 2,770 mL (820) |
| Galas et al (21) | Brazil | 40 | Septic shock patients | Ringer’s Lactate vs Ringerfundin | Not reported |
| Zadák et al (22) | Czech Republic | 14 | Healthy volunteers | Plasmalyte vs Ringerfundin | 2,000 mL; 2,000 mL |
| Shin et al (23) | South Korea | 104 | Live liver donor patients | Plasmalyte vs Ringer’s Lactate | 3,302 mL (575); 3,407 mL (715) |
| Hasman et al (24) | Turkey | 90 | Dehydrated emergency department patients | Plasmalyte vs Ringer’s Lactate | 20 mL/kg/hr × 2 hr |
| Kiss et al (25) | Hungary | 102 | Pregnant women undergoing cesarean section | Ringer’s Lactate vs balanced Ringers | 572 mL (442); 617 mL (260) |
| Vichitvejpaisal et al (26) | Thailand | 90 | Endoscopy outpatients | Ringer’s Lactate vs Ringer’s Acetate | 1,140 mL (169); 1,046 mL (167) |
| Scotti et al (27) | Italy | 20 | Cardiac bypass patients | Ringer’s Lactate vs novel solution | 10 ± 5 mL/kg |
| Weinberg et al (28) | Australia | 60 | Liver resection patients | Plasmalyte vs Hartman’s | 2,000 mL [1,425–3,000]; 3,000 mL [1,800–4,000] |
| Benoit et al (29) | Belgium | 204 | Adult ICU patients | Plasmalyte vs Sterofundin | 1,000 mL; 1,000 mL |
| Kumar et al (30) | India | 80 | Adult surgical patients | Ringer’s Lactate vs Sterofundin vs Plasmalyte vs Kabilyte | Not reported |
| Rajan et al (31) | India | 60 | Major head and neck surgery patients | Ringer’s Lactate vs Sterofundin | 5,116.7 mL (1,744); 5,646.7 mL (1,295.0) |
| Uvizl et al (32) | Czech Republic | 112 | Postop ICU patients | Plasmalyte vs Ringerfundin | 1,000 mL; 1,000 mL |
| Omar and Mathivha (33) | South Africa | 86 | Adult ICU patients | Bicarbonate-balanced fluida vs conventional balanced fluidb | Not reported |
| Rawat et al (34) | India | 49 | Adult ICU patients | Ringer’s Lactate vs Acetate | 20 mL/kg/hr × 1 hr, 10 mL/kg/hr × 1 hr |
| Weinberg et al (35) | Australia | 50 | Cardiac surgery patients | Plasmalyte vs Hartman’s | 2,000 mL; 2,000 mL |
| Pfortmueller et al (36) | Switzerland | 148 | Cardiac surgery patients | Ringer’s Lactate vs Ringer’s Acetate | 6,104 mL [4,769–7,855]; 6,677 mL [5,325–8,479] |
| Chaussard et al (37) | France | 28 | Burn patients in ICU | Plasmalyte vs Ringer’s Lactate | 15,680 mL; 19,626 mL |
| Joseph et al (38) | India | 40 | Pediatric surgery patients | Ringer’s Lactate vs Sterofundin | Not reported |
| King et al (39) | United States | 59 | Adolescent spinal surgery patients | Ringer’s Lactate vs Normosol-R | 48 mL/kg (87); 35 mL/kg (23) |
aNa 143 mmol/L, Cl 99 mmol/L, Hco3 49 mmol/L.
bNa 130 mmol/L, Cl 110 mmol/L, Hco3 <27 mmol/L.
We included the following outcomes of interest: change in serum chloride and potassium concentrations, change in acid-base status (postinfusion serum pH, strong ion difference [SID], strong ion gap [SIG], and base excess), change in serum lactate, acute kidney injury (AKI) (using any validated scale), organ failure (using any validated scale), receipt of life support modalities (e.g., invasive mechanical ventilation, renal replacement therapy [RRT], and vasopressor use), ICU and hospital length of stay (LOS), and hospital mortality. If multiple time points were reported for postinfusion concentration, we used the time point closest to 1 hour following fluid administration. For the clinical outcomes, if multiple time points were reported, we used the time point of the longest follow-up.
We used Covidence online platform (www.covidence.org) for screening. We combined search results from all sources and removed duplicates. Screening was performed in duplicate by three reviewers (J.D.C., P.M., K.T.) working independently and in parallel using a two-stage screening approach. In stage 1, we screened titles and abstracts and any potentially eligible citation was advanced to stage 2; the same reviewers evaluated full texts with disagreements resolved through consensus or third-party adjudication (J.D.C. or B.R.).
Data Extraction, Risk of Bias, and Certainty Assessment
We extracted data from the included studies in duplicate using a prepiloted data collection form and Covidence online platform including author, journal, year of publication, population characteristics, volume and rate of fluid administration, cointerventions (e.g., surgical procedure), and outcomes of interest. Life support modalities included the use of invasive mechanical ventilation, RRT, and vasopressor use.
We assessed risk of bias (ROB) for included studies using the Cochrane ROB Tool (12). Three reviewers (J.D.C., P.M., K.T.) assessed ROB independently and in parallel with disagreements resolved by discussion and involvement of a third reviewer (J.D.C. or B.R.) where necessary.
We assessed overall certainty of evidence in pooled estimates using Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methodology (13). We present final pooled results and certainty of effects using an Evidence Profile made with www.gradepro.org.
Data Analysis
When possible, we conducted meta-analyses using the inverse variance strategy in the method of DerSimonian and Laird (14) and using random effects model. We performed all analyses using the RevMan software (www.revman.cochrane.org; The Cochrane Collaboration, London, United Kingdom). We present results as risk ratios (RRs) for dichotomous outcomes or mean difference (MD) for continuous outcomes, both with associated 95% CIs. When sd or other related information (e.g., se and CIs) were not reported, we used an average measure of variance observed in the other studies and performed sensitivity analysis of imputed value using the smallest and largest variances observed. We assessed heterogeneity using the chi-square test, the I2 statistic, and visual inspection of the forest plot. Although we had planned to use Egger test (15) to assess for publication bias, this was not performed as less than 10 trials were identified for each comparison of interest. For comparisons or outcomes with insufficient data to allow for pooling, we describe narratively.
RESULTS
Search Strategy and Study Characteristics
A total of 24,877 citations were identified by the search; 137 underwent full-text review (Supplemental Digital Content, Fig. 1, http://links.lww.com/CCX/A584) and a total of 24 RCTs (n = 1673 participants) proved eligible. Table 1 summarizes the characteristics of the included studies. The balanced crystalloids that were studied include Plasmalyte (Plasma-Lyte 148/Plasma-Lyte A), Kabilyte, Normosol-R, Ringer’s Lactate, Ringerfundin/Ringer’s Acetate, Hartmann’s solution, Ringer’s Bicarbonate, Sterofundin, and novel balanced solutions (see Table 2 for a summary of commercially available fluids and their electrolyte concentrations). Of the included studies, 16 were performed in the perioperative setting (n = 1142 participants) (16–20, 23, 25, 26, 28, 30–32, 35, 36, 38, 39), six in the ICU (n = 427 participants) (21, 27, 29, 33, 34, 37), one in the emergency department (ED; n = 90 participants) (24), and one in healthy volunteers (n = 14 participants) (22). Five studies involved pediatric patients (n = 298 participants) (16, 17, 25, 38, 39), whereas the remainder focused on adult participants. Volumes of study fluid received ranged from 380 mL (17) to 19,626 mL (37) (median, 2,000 mL and interquartile range, 2,812.5 mL). The duration of study fluid administration also varied widely from 1 hour (29) to 5 days (37). Most studies (n = 19) reported only short-term metabolic outcomes (16–22, 24–27, 29–32, 34, 37–39), while a few (n = 5) reported longer term clinical outcomes (23, 28, 33, 35, 36). Six studies were available as abstract only (21, 25, 27, 33, 34, 38). A summary table of main outcomes of interest is available in Supplemental Digital Content, Table 2 (http://links.lww.com/CCX/A585).
TABLE 2.
Comparison of Electrolyte Composition of Commercially Available Fluid Types and Human Plasma (17, 19, 20, 22, 24, 28, 40–42)
| Fluid | Electrolytes (mmol/L) | Buffer (mmol/L) | pH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Cl | K | Lactate | Acetate | Malate | Gluconate | Bicarbonate | ||
| Plasmalyte | 140 | 98 | 5 | — | 27 | — | 23 | — | 5–7.4 |
| Kabilyte | 140 | 98 | 5 | — | 27 | — | 23 | — | 6.5–8 |
| Normosol-R | 140 | 98 | 5 | — | 27 | — | 23 | — | 6.6–7.4 |
| Ringer’s Lactate | 130 | 110–115 | 4 | 28 | — | — | — | — | 6–7.5 |
| Hartmann’s solution | 129–131 | 109–111 | 5 | 29 | — | — | — | — | 6.5 |
| Ringerfundin (Ringer’s Acetate) | 145 | 127 | 4 | — | 24 | 5 | — | — | 5.1–5.9 |
| Sterofundin | 140 | 106 | 4 | 45 | — | — | — | — | 4.5–7.5 |
| Human plasma | 134–146 | 98–108 | 3.4–5 | — | — | — | — | 22–32 | 7.4 |
ROB was judged to be high in most studies (n = 21) due to lack of blinding (n = 19), incomplete outcome data (n = 16), and lack of intention to treat analysis (n = 10). ROB summary table is available in Supplemental Digital Content, Table 3 (http://links.lww.com/CCX/A585).
Plasmalyte
Twelve RCTs compared Plasmalyte versus another balanced crystalloid including Hartmann’s solution (n = 3), Ringer’s Lactate (n = 6), Sterofundin (n = 2), or Ringerfundin (n = 2). Of these 12 RCTs, eight were performed in the perioperative setting (16, 17, 20, 23, 28, 30, 32, 35), two in ICU (29, 37), one in ED (24), and one in healthy volunteers (22).
Compared with any of the other balanced crystalloids, administration of Plasmalyte resulted in a lower postinfusion serum chloride concentration (MD, 0.83 mmol/L lower; 95% CI, 0.03–1.64 mmol/L lower, low certainty). Comparing Plasmalyte with specific fluids, this finding was consistent with Hartmann’s solution (MD, 1.69 mmol/L lower; 95% CI, 0.80–2.59 mmol/L lower, low certainty) and Ringer’s Lactate (MD, 0.80 mmol/L lower; 95% CI, 2.02 lower to 0.42 mmol/L higher, very low certainty) with an uncertain effect compared with Sterofundin (MD, 0.74 mmol/L higher; 95% CI, 3.07 lower to 4.54 mmol/L higher, very low certainty) (Fig. 1).
Figure 1.
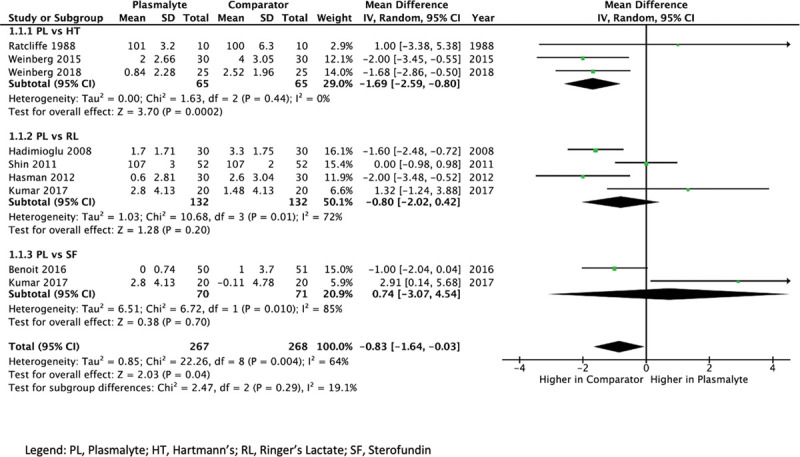
Forest plot of mean difference of chloride concentration postinfusion of Plasmalyte (PL) versus any comparator. HT = Hartmann’s, RL = Ringer’s Lactate, SF = Sterofundin.
Administration of Plasmalyte resulted in a higher postinfusion base excess (MD, 0.65 mmol/L higher; 95% CI, 0.25–1.05 mmol/L higher, low certainty) compared with all other balanced crystalloids. This finding was consistent when compared only with Ringer’s Lactate (MD, 0.84 mmol/L higher; 95% CI, 0.52–1.16 mmol/L higher, moderate certainty), whereas there was a similar effect on base excess compared with Sterofundin (MD, 0.09 mmol/L higher; 95% CI, 0.17 lower to 0.35 mmol/L higher, low certainty) and an uncertain effect compared with Hartmann’s (MD, 0.63 mmol/L higher; 95% CI, 1.24 lower to 2.49 mmol/L higher, very low certainty) (Fig. 2). The values for base excess from one RCT (24) comparing Plasmalyte with Ringer’s Lactate were calculated from reported values of bicarbonate and pH, which required imputation for sd; however, sensitivity analysis using both extremes of sd did not change the results.
Figure 2.
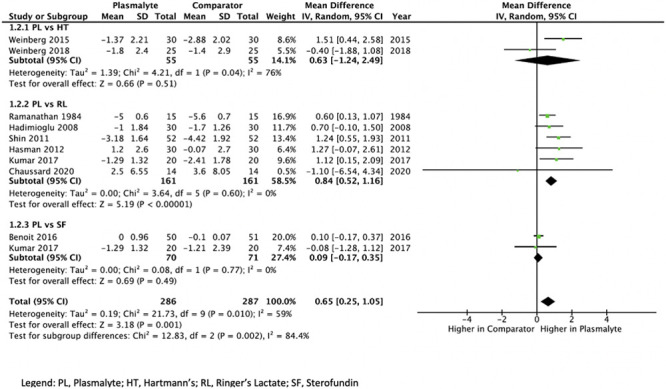
Forest plot of mean difference of base excess postinfusion of Plasmalyte (PL) versus any comparator. HT = Hartmann’s, RL = Ringer’s Lactate, SF = Sterofundin.
There were no important differences in postinfusion pH when comparing Plasmalyte with all other balanced crystalloids (MD, 0.00; 95% CI, 0.01 lower in Plasmalyte to 0.01 higher, low certainty). This finding was consistent across comparisons with Hartmann’s solution (MD, 0.00; 95% CI, 0.03 lower in Plasmalyte to 0.02 higher, very low certainty), Ringerfundin (MD, 0.01 higher in Plasmalyte; 95% CI, 0.01 lower to 0.02 higher, low certainty), Sterofundin (MD, 0.00; 95% CI, 0.02 lower in Plasmalyte to 0.02 higher, low certainty), and Ringer’s Lactate (MD, 0.01 higher in Plasmalyte; 95% CI, 0.01 lower to 0.02 higher, low certainty).
Administration of Plasmalyte resulted in a lower postinfusion serum lactate level when compared with all other balanced crystalloids (MD, 0.46 mmol/L lower in Plasmalyte; 95% CI, 0.05–0.87 mmol/L lower, low certainty). This finding was consistent when compared with Ringer’s Lactate (MD, 0.69 mmol/L lower; 95% CI, 0.05–1.32 mmol/L lower, low certainty). However, Plasmalyte resulted in a similar postinfusion lactate compared with Sterofundin (MD, 0.07 mmol/L higher in Plasmalyte, 0.38 lower to 0.51 mmol/L higher, low certainty) and had an uncertain effect compared with Hartmann’s solution (MD, 0.23 mmol/L lower; 95% CI, 0.89 lower to 0.42 mmol/L higher, very low certainty).
Plasmalyte resulted in similar postinfusion serum potassium level when compared with all other balanced crystalloids; however, certainty of this finding was very low (MD, 0.04 mmol/L higher in Plasmalyte; 95% CI, 0.07 lower to 0.15 mmol/L higher). This finding was consistent across comparisons with Ringer’s Lactate (MD, 0.08 mmol/L higher; 95% CI, 0.04 lower to 0.2 mmol/L higher, very low certainty) and Sterofundin (MD, 0.02 mmol/L higher; 95% CI, 0.19 lower to 0.23 mmol/L higher, low certainty). However, Plasmalyte resulted in a lower postinfusion potassium level when compared with Hartmann’s solution (MD, 0.20 mmol/L lower; 95% CI, 0.44 lower to 0.04 mmol/L higher, low certainty). Forest plots of MDs in pH, lactate, and potassium are available in Supplemental Digital Content, Figures 2–3 (http://links.lww.com/CCX/A584). GRADE evidence summaries for Plasmalyte versus any other balanced crystalloid and individual fluid types are also available in Supplemental Digital Content, Tables 4–8 (http://links.lww.com/CCX/A585).
Data addressing patient-important outcomes were sparsely reported and too heterogeneous to allow for pooling. In a single study of patients undergoing liver resection (n = 60), investigators found fewer cardiorespiratory and surgical complications (e.g., pneumonia, myocardial infarction, and wound infections), less AKI and blood transfusions (20% vs 56%; RR, 2.8; 95% CI, 1.3–6.1) and shorter duration of hospital stay (median LOS, 5.9 vs 7.8 d) in those receiving Plasmalyte compared with Hartmann’s solution intraoperatively (28). Another RCT (n = 50) of cardiac surgery patients found similar ICU LOS (mean 41 hr [sd 29] vs mean 72 hr [sd 135]) and hospital LOS (mean 238 hr [sd 69] vs mean 277 hr [sd 222]) and no difference in rates of complications (arrhythmia, AKI, and cardiopulmonary complications) between those randomized to cardiac bypass prime solution containing Plasmalyte or Hartmann’s solution (35).
Ringer’s Lactate
Eleven RCTs compared Ringer’s Lactate with another balanced crystalloid other than Plasmalyte (including Ringer’s Acetate/Ringerfundin, Sterofundin, Normosol-R, Kabilyte, Balanced Ringer’s, and a novel solution). Given most of these studies were small and with significant clinical heterogeneity between the studies, we summarize the results narratively as meta-analyses would be too imprecise to allow for meaningful conclusions.
Ringer’s Lactate Compared With Ringer’s Acetate
Five RCTs compared Ringer’s Lactate with Ringer’s Acetate/Ringerfundin (18, 21, 26, 34, 36). One small RCT involving cholecystectomy patients (n = 30) found that infusion with Ringer’s Lactate resulted in higher serum lactate than Ringer’s Acetate (p < 0.001), and no difference in pH, bicarbonate, or base excess between the groups (18). One single-center RCT in cardiac surgery patients (n = 148) found no difference in rates of metabolic acidosis, hospital LOS, or AKI between these two fluids (36). Another RCT (n = 90) of elective endoscopy patients found no difference in SID comparing those that were randomized with either Ringer’s Lactate or Ringer’s Acetate (26). A small RCT (n = 40) of critically ill patients with septic shock found that Ringer’s Lactate resulted in lower postinfusion base excess (p < 0.0001) and higher postinfusion serum chloride (p < 0.002) and lactate (p < 0.002) concentrations compared with Ringerfundin; however, there were no differences in SIG or SID (21). One RCT involving adult ICU patients with metabolic acidosis (n = 49) found no difference in the rate of correction of metabolic acidosis between Ringer’s Lactate compared with Acetate (34).
Ringer’s Lactate Compared With Sterofundin
One RCT (n = 60) of head and neck surgery patients compared Ringer’s Lactate with Sterofundin and found higher intraoperative lactate levels (mean 3.5 mmol/L [sd 1.8] vs mean 1.7 mmol/L [sd 0.5]; p < 0.001) and lower pH (mean 7.42 [sd 0.1] vs mean 7.4 [sd 0.1]; p = 0.027) in those receiving Ringer’s Lactate (31). There were no important differences in serum potassium, chloride, or bicarbonate concentration between the groups (31). One RCT involving pediatric surgery patients (n = 40) found no difference in postinfusion serum pH, lactate, base excess, chloride, or potassium concentration between Ringer’s Lactate and Sterofundin (38).
Ringer’s Lactate Compared With Others
One RCT (n = 102) compared Ringer’s Lactate with Balanced Ringer’s in pregnant women undergoing cesarean section (25) and found no difference in postinfusion lactate or pH between the groups. Another RCT (n = 20) compared administration of Ringer’s Lactate with a novel balanced solution (created to have an SID equal to individual patient’s serum bicarbonate concentration) in those with acute respiratory failure requiring extracorporeal membrane oxygenation (27). Investigators in this study found lower base excess (MD, 1.9 mEq/L lower; sd, 1.3), higher serum chloride (MD, 2 mEq/L higher; sd, 7), and higher serum lactate (MD, 0.8 mEq/L higher; sd, 0.82) in those randomized to Ringer’s Lactate compared with the novel solution group.
A single RCT compared infusion of Ringer’s Lactate with Normosol-R in adolescent spinal surgery patients (n = 59) and found higher postinfusion potassium concentration in those treated with Ringer’s Lactate (p = 0.007), and no difference in pH, base excess, or lactate between the groups (39).
Other Comparisons
One RCT (n = 20) compared Ringer’s Acetate with Ringer’s Bicarbonate in patients scheduled for elective aortic aneurysm repair (19). The Ringer’s Bicarbonate solution used in the study contained chelated calcium citrate and chelated magnesium citrate. Investigators found no differences in the postinfusion pH or base excess between groups.
A single RCT (n = 86) compared two different balanced solutions in adult ICU patients (33); however, the solutions are not named. Their composition was reported as bicarbonate-balanced solution (Na 143 mmol/L, Cl 99 mmol/L, and Hco3 49 mmol/L) and conventional balanced fluid (Na 130 mmol/L, Cl 110 mmol/L, Hco3 <27 mmol/L). The study reported a decline in serum creatinine in the bicarbonate-balanced fluid group, with no difference in receipt of RRT, ICU LOS, or mortality.
DISCUSSION
This systematic review and meta-analysis found that administration of Plasmalyte results in lower postinfusion serum concentrations of chloride and lactate, and higher base excess compared with other balanced crystalloids. The certainty of these results is low as studies included heterogeneous populations leading to issues with inconsistency for some outcomes and ROB among the included studies. We found no important differences in postinfusion pH or potassium concentration with Plasmalyte, again with low or very low certainty. The changes in base excess that do not correlate with differences in pH may reflect changes within compensatory respiratory mechanisms of the body. Studies rarely evaluated the impact of using various balanced crystalloids on patient-important outcomes such as mortality, hospital LOS, and receipt or duration of life support, and we were, therefore, unable to pool data evaluating these outcomes. The results suggest that Plasmalyte may be most beneficial in terms of impact on electrolyte levels postinfusion; however, we are uncertain whether this translates into improved patient outcomes. Furthermore, RCTs examining clinical outcomes would be needed using large volumes of fluid, perhaps focused on hospitalized or critically ill patients.
There are other considerations for clinicians and hospital administrators when deciding between the fluids. The cost of Plasmalyte varies between region and country although is generally higher than other balanced solutions, for example, the acquisition cost of Plasmalyte compared with Hartmann’s solution can be 2–2.5 times higher (43). A cost minimization analysis comparing 24-hour fluid resuscitation with Plasmalyte or NS found that use of Plasmalyte resulted in higher initial fluid acquisition costs; however, there was a net cost benefit with Plasmalyte due to decreased usage of magnesium supplementation (44). Cost-effectiveness analysis should be a part of any future studies comparing balanced crystalloids. There is also ongoing debate regarding the metabolic effects and safety of added anion buffers such as acetate, lactate, and gluconate, which are found in various balanced crystalloids (45).
Strengths of this study include enhanced generalizability with the inclusion of diverse populations of patients. We also performed a comprehensive search, duplicate screening and data abstraction, ROB evaluation for each included study, and have contextualized study results with overall certainty using GRADE methodology. This analysis has several limitations. First, the low to very low certainty of data for most comparisons and outcomes limits the strength of conclusions. The majority of included studies were judged to have a high ROB due to lack of blinding, incomplete data reporting, and lack of intention to treat analysis. The sparsity of data precluded the ability to perform pooled analysis for all comparisons, and heterogeneous populations and volumes of fluid between the studies contributed to important inconsistency. We were unable to find data addressing long-term patient-important outcomes. The certainty of outcomes may be enhanced as further RCT evidence becomes available, such as the Balanced Solutions and Plasma Electrolytes trial comparing Normosol to Ringer’s Lactate (ClinicalTrials.gov Identifier: NCT03537898).
CONCLUSIONS
Plasmalyte may result in less metabolic abnormalities compared with other balanced crystalloid solutions. There were insufficient data to examine the impact of different balanced crystalloids on patient-important outcomes such as mortality and length of hospitalization. If RCTs demonstrate balanced crystalloids are beneficial compared with NS, then future studies examining the comparative effectiveness among these balanced fluids will be crucial.
ACKNOWLEDGMENTS
We thank Sandy Culley and Jo-Anne Petropoulos for their valuable contribution to the search strategy.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013; 369:1243–1251 [DOI] [PubMed] [Google Scholar]
- 2.Self WH, Semler MW, Wanderer JP, et al. ; SALT-ED Investigators. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018; 378:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999; 90:1265–1270 [DOI] [PubMed] [Google Scholar]
- 4.Langer T, Santini A, Scotti E, et al. Intravenous balanced solutions: From physiology to clinical evidence. Anaesthesiol Intensive Ther. 2015; 47:s78–s88 [DOI] [PubMed] [Google Scholar]
- 5.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to plasma-lyte. Ann Surg. 2012; 255:821–829 [DOI] [PubMed] [Google Scholar]
- 6.Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med. 2014; 42:1585–1591 [DOI] [PubMed] [Google Scholar]
- 7.Shaw AD, Raghunathan K, Peyerl FW, et al. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014; 40:1897–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochwerg B, Alhazzani W, Sindi A, et al. ; Fluids in Sepsis and Septic Shock Group. Fluid resuscitation in sepsis: A systematic review and network meta-analysis. Ann Intern Med. 2014; 161:347–355 [DOI] [PubMed] [Google Scholar]
- 9.Semler MW, Self WH, Wanderer JP, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018; 378:829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson M, McGowan J, Cogo E, et al. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009; 62:944–952 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64:383–394 [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramanathan S, Masih AK, Ashok U, et al. Concentrations of lactate and pyruvate in maternal and neonatal blood with different intravenous fluids used for prehydration before epidural anesthesia. Anesth Analg. 1984; 63:69–74 [PubMed] [Google Scholar]
- 17.Ratcliffe JM, Wyse RK, Hunter S, et al. The role of the priming fluid in the metabolic response to cardiopulmonary bypass in children of less than 15 kg body weight undergoing open-heart surgery. Thorac Cardiovasc Surg. 1988; 36:65–74 [DOI] [PubMed] [Google Scholar]
- 18.Attalla HA, Abulkassem MS, Abo Elenine KM. Assessment of intraoperative use of Ringer acetate in patients with liver cirrhosis. AJAIC. 2005; 8:75–82 [Google Scholar]
- 19.Shimada Y, Kitamura A, Nakanishi K, et al. Effect of bicarbonated Ringer’s solution on the acid-base balance in patients undergoing abdominal aortic aneurysm repair. J Nippon Med Sch. 2005; 72:364–369 [DOI] [PubMed] [Google Scholar]
- 20.Hadimioglu N, Saadawy I, Saglam T, et al. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg. 2008; 107:264–269 [DOI] [PubMed] [Google Scholar]
- 21.Galas F, Hajjar L, Simoes C, et al. Effects of Ringer’s lactate or Ringerfundin resuscitation on the acid-base status and serum electrolytes in septic oncologic patients. Crit Care. 2009; 13(Suppl 1):P447 [Google Scholar]
- 22.Zadák Z, Hyspler R, Hronek M, et al. The energetic and metabolic effect of Ringerfundin (B. Braun) infusion and comparison with Plasma-Lyte (Baxter) in healthy volunteers. Acta Medica (Hradec Kralove). 2010; 53:131–137 [DOI] [PubMed] [Google Scholar]
- 23.Shin WJ, Kim YK, Bang JY, et al. Lactate and liver function tests after living donor right hepatectomy: A comparison of solutions with and without lactate. Acta Anaesthesiol Scand. 2011; 55:558–564 [DOI] [PubMed] [Google Scholar]
- 24.Hasman H, Cinar O, Uzun A, et al. A randomized clinical trial comparing the effect of rapidly infused crystalloids on acid-base status in dehydrated patients in the emergency department. Int J Med Sci. 2012; 9:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiss K, Zimanyi M, Agocs S, et al. Ringer’s lactate (RL) and balanced Ringer’s solution (BR) during elective caesarean delivery in spinal anaesthesia: effects on neonatal homeostasis. Eur J Anaesthesiol. 2012; 29:168 [Google Scholar]
- 26.Vichitvejpaisal P, Akaraviputh T, Chutipongtanate A, et al. Intravenous fluid administration in patients undergoing colonoscopy: Double blind, randomised clinical trial of underlying acid-base derangement. Eur J Anaesthesiol. 2013; 30:35 [Google Scholar]
- 27.Scotti E, Ferrari M, Chiodi M, et al. Acid-base effects of different crystalloid solutions for ECMO priming: preliminary report. Crit Care. 2015; 19(Suppl 1):P356 [Google Scholar]
- 28.Weinberg L, Pearce B, Sullivan R, et al. The effects of plasmalyte-148 vs. Hartmann’s solution during major liver resection: a multicentre, double-blind, randomized controlled trial. Minerva Anestesiol. 2015; 81:1288–1297 [PubMed] [Google Scholar]
- 29.Benoit D, Crivits M, Hemeryck M, et al. Small volume fluid infusion with balanced or un-balanced fluids: does it make a difference? J Intensive Care Soc. 2016; 17(3 Suppl):S41 [Google Scholar]
- 30.Kumar AK, Pratyusha AC, Kavitha J, et al. Comparative study of effect of intra-operative administration of Ringer’s lactate, sterofundin, plasmalyte-A and kabilyte on ionic and acid base status. Med Int Med J. 2017; 4:59–67 [Google Scholar]
- 31.Rajan S, Srikumar S, Tosh P, et al. Effect of lactate versus acetate-based intravenous fluids on acid-base balance in patients undergoing free flap reconstructive surgeries. J Anaesthesiol Clin Pharmacol. 2017; 33:514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uvizl R, Bohanes T, Urbanek K, et al. Acid-based disturbances due to perioperative fluid therapy with slightly alkalized and acid-based neutral balanced crystalloids: A comparative study. Signa Vitae. 2017; 13:65–69 [Google Scholar]
- 33.Omar S, Mathivha LR. A randomized trial comparing two balanced fluids in the critically ill. Crit Care. 2018; 22(Suppl 1):8232831123 [Google Scholar]
- 34.Rawat N, Sahni N, Yaddanapudi L. Effect of fluid resuscitation with Ringer lactate versus acetate solution on correction of metabolic acidosis in critically ill patients. Intensive Care Med Exp. 2018; 6(Suppl 2):4030341496 [Google Scholar]
- 35.Weinberg L, Chiam E, Hooper J, et al. Plasma-Lyte 148 vs. Hartmann’s solution for cardiopulmonary bypass pump prime: A prospective double-blind randomized trial. Perfusion. 2018; 33:310–319 [DOI] [PubMed] [Google Scholar]
- 36.Pfortmueller CA, Faeh L, Müller M, et al. Fluid management in patients undergoing cardiac surgery: Effects of an acetate- versus lactate-buffered balanced infusion solution on hemodynamic stability (HEMACETAT). Crit Care. 2019; 23:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaussard M, Dépret F, Saint-Aubin O, et al. Physiological response to fluid resuscitation with Ringer lactate versus plasmalyte in critically ill burn patients. J Appl Physiol (1985). 2020; 128:709–714 [DOI] [PubMed] [Google Scholar]
- 38.Joseph A, Dogra N, Rathore P, et al. To compare effect of sterofundin v/s Ringer’s lactate on intraoperative acid base and electrolyte status in children undergoing major surgery: A randomized, double blind, study. Indian J Anaesth. 2020; 64(Suppl 1):22–28 [Google Scholar]
- 39.King M, Martin D, Miketic R, et al. Impact of intraoperative fluid management on electrolyte and acid-base variables during posterior spinal fusion in adolescents. Orthop Res Rev. 2020; 12:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fresenius Kabi India Pvt Ltd: Blood volume substitutes/HES. 2021. Available at: https://www.fresenius-kabi.com/in/products/kabilyte. Accessed November 13, 2020
- 41.Normosol-R [package insert]. Lake Forest, IL: Hospira, 2009 [Google Scholar]
- 42.Rochwerg B, Włudarczyk A, Szczeklik W, et al. ; FISSH group. Fluid resuscitation in severe sepsis and septic shock: Systematic description of fluids used in randomized trials. Pol Arch Med Wewn. 2013; 123:603–608 [DOI] [PubMed] [Google Scholar]
- 43.Weinberg L, Collins N, Van Mourik K, et al. Plasma-lyte 148: A clinical review. World J Crit Care Med. 2016; 5:235–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CA, Duby JJ, Utter GH, et al. Cost-minimization analysis of two fluid products for resuscitation of critically injured trauma patients. Am J Health Syst Pharm. 2014; 71:470–475 [DOI] [PubMed] [Google Scholar]
- 45.Reddy S, Weinberg L, Young P. Crystalloid fluid therapy. Crit Care. 2016; 20:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


