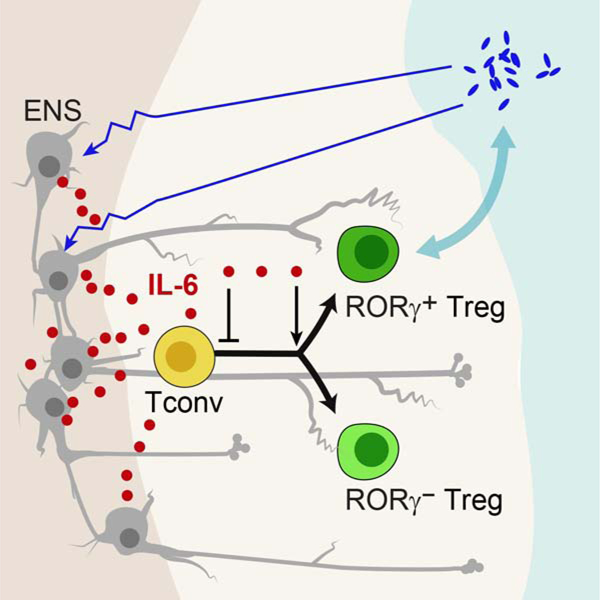
SUMMARY
The immune and enteric nervous (ENS) systems monitor the frontier with commensal and pathogenic microbes in the colon. We investigated whether FoxP3+ regulatory T cells (Treg) functionally interact with the ENS. Indeed, microbe-responsive RORγ+ and Helios+ subsets localized in close apposition to nitrergic and peptidergic nerve fibers in the colon lamina propria. Enteric neurons inhibited in vitro (i)Treg differentiation in a cell-contact independent manner. A screen of secreted factors revealed a role for interleukin (IL)-6 in modulating iTreg formation and their RORγ+ proportion. Colonization of germfree mice with commensals, especially RORγ+ Treg inducers, broadly diminished colon neuronal density. Closing the triangle, conditional ablation of IL-6 in neurons increased total Treg cells but decreased the RORγ+ subset, as did depletion of two ENS neurotransmitters. Our findings suggest a regulatory circuit wherein microbial signals condition neuronal density and activation, thus tuning Treg cell generation, with implications for tolerance in the gut.
Graphical Abstract
eTOC
Regulatory T (Treg) cells lie in proximity to nerve fibers in the colon lamina propria. Yan et al reveal a regulatory circuit wherein microbial signals condition neuronal density and activation, which in turn, via neuron-produced IL-6, tunes Treg cell generation, with implications for intestinal tolerance.
INTRODUCTION
The mammalian intestinal tract is one of the main ports of exchange between the organism and the outside world. It is not solely a barrier, but must maintain an intimate dialog with nutritional sources and with the microbial symbionts that assist in food processing. This frontier is closely monitored by both the immune and nervous systems, which together discriminate between nutritionally valuable vs harmful chemical entities and symbiotic vs pathogenic microbes. The enteric nervous system (ENS) is the largest neural organ outside the brain, can function largely autonomously, responding to and adapting to local challenges (Furness et al., 2014; Kulkarni et al., 2018; Zeisel et al., 2018). The ENS includes a full repertoire of sensory neurons, interneurons, and motor neurons that collectively detect luminal contents, drive secretory function and control intestinal motility (Furness et al., 2013; Yang and Chiu, 2017). Enteric neurons are organized within two sets of ganglionated plexuses, the submucosal plexus, which contains many neurons projecting to the mucosa, and the myenteric plexus located within the muscularis externa. The gut is a large lymphoid organ as well, with an architecture, cell composition and traffic patterns distinct from those of all other organismal locations; it also closely monitors luminal content through arrays of innate and adaptive receptors.
Perhaps predictably, there is cross-talk between the two systems (Margolis et al., 2016; Veiga-Fernandes and Mucida, 2016; Yoo and Mazmanian, 2017; Huh and Veiga-Fernandes, 2020). Macrophages influence the regulation of gut motility, are necessary for ENS homeostasis, and are reciprocally influenced by β2-adrenergic agonists or CSF1 released by neurons, all of which generally promote homeostasis and rapid tissue-protective responses (Muller et al., 2014; Gabanyi et al., 2016; De Schepper et al., 2018). Interactions between the ENS and innate like lymphocytes (ILC) have also been described: the neuropeptides NMU and CGRP regulate ILC2 maturation and activation, while neurotrophic factors of the GNDF family support IL22-producing ILC3s (Klose et al., 2017; Cardoso et al., 2017; Wallrapp et al., 2019; de Jong et al., 2015). Mucosal nerves occur adjacent to ILC2s, and glial cell projections are in close contact with ILC3s (Ibiza et al., 2016; Cardoso et al., 2017).
Regulatory T (Treg) cells that express the transcription factor Foxp3 Treg cellsare a subset of CD4+ T cells that control innate and adaptive immune responses (Josefowicz et al., 2012), and also have broader functions in maintaining tissue homeostasis in non-immunologic settings (Panduro et al., 2016). Treg cells are also key players in host-pathogen immunity (Schiering et al., 2014), in particular in the gut where their numbers and phenotypes are tuned by commensal microbes (Lathrop et al., 2011; Nutsch et al., 2016; Atarashi et al., 2011; Sefik et al., 2015; Ohnmacht et al., 2015; Al Nabhani Z. et al., 2019; Pratama et al., 2020). Two distinct subpopulations of intestinal Foxp3+ Treg cells have been distinguished. The first expresses the nuclear hormone receptor RORγ and the transcription factor (TF) c-Maf (Ohnmacht et al., 2015; Sefik et al., 2015; Yang et al., 2016; Yissachar et al., 2017; Xu et al., 2018; Neumann et al., 2019; Wheaton et al., 2017). They predominate in the colon, and are induced by commensal microbes, with highly varying efficacy between microbial species (Sefik et al., 2015), and through several potential mechanisms (Verma et al., 2018; Yissachar et al., 2017; Hang et al., 2019; Song et al., 2020). The second subset expresses Helios and Gata3, predominates in the small intestine (Wohlfert et al., 2011; Schiering et al., 2014; Sefik et al., 2015; Ohnmacht et al., 2015), and is less dependent on the microbiota but may respond more to tissue stress (Peine et al., 2016; Molofsky et al., 2015) mediated via the alarmin interleukin (IL)-33 (Schiering et al., 2014; He et al., 2017). RORγ+ and Helios+ Treg cells have non-redundant functions, as genetic inactivation of RORγ+ Treg cells influences disease severity in colitis models, food allergy, and colorectal tumors (Sefik et al., 2015; Ohnmacht et al., 2015; Neumann et al., 2019; Xu et al., 2018; Al Nabhani Z. et al., 2019; Neumann et al., 2019; Ye et al., 2017).
Beyond the canonical role of Treg cells in controlling neurological autoimmunity or inflammation, evidence for peripheral neuro-immune interactions involving Treg cells is somewhat limited. Interaction between CNS neurons and T cells promotes the conversion of potentially encephalitogenic T cells to Treg phenotypes (Liu et al., 2006). Treg cells in the muscle are associated with nerve fibers and muscle spindles, and nerve-associated stromal cells enhance Treg function via IL33 to promote muscle repair (Kuswanto et al., 2016). In a recent report, perturbations of vagal sensory afferents reduced the proportions of colonic Treg cells, via intestinal antigen-presenting cells (Teratani et al., 2020). While exploring the mechanism of RORγ+ Treg cell induction by gut commensals in germ free (GF) mice, we noted an intriguing relation between the ability of bacterial species to induce these Treg cells and their ability to trigger neuronal populations, manifest as changes in gene expression in the ENS or as induction of action potential firing of dorsal root ganglia (DRG) sensory neurons (Yissachar et al., 2017). In addition, colonic RORγ+ Treg cell frequencies were altered in mice fed a capsaicin-rich diet or lacking TAC1, the precursor protein for the neuropeptides substance P and neurokinin A.
These relationships suggest a direct crosstalk between the ENS and colonic Treg cells. Here, we examined the functional interactions between ENS and Treg cells, as well as the impact of microbiota given its role in driving Treg cell differentiation in the gut. Treg cells located in very close vicinity to neuronal fibers in the lamina propria. In vitro, enteric neurons modulated iTreg differentiation, reducing overall FoxP3 induction but promoting RORγ+ Treg cell proportions, an action that genomic and genetic explorations established as linked to IL-6 produced by neurons. In vivo, microbial colonization perturbed the ENS, reducing its ability to express IL6. Neuron-specific ablation of IL6 correspondingly impacted on Treg cell numbers and phenotypes. These data suggest a three-way interaction that controls tolerance at the microbial interface.
RESULTS
Colonic Treg cells localize closely to neurons
Our earlier results (Yissachar et al., 2017) evoked a crosstalk between neurons and Treg cells in the gut wall. To determine the possibility of a direct interaction, we performed immunofluorescence imaging on segments of mouse colon, asking whether Treg cells were located near neuronal bodies in enteric ganglia or their projections in the lamina propria (LP; Fig. 1A). On transverse sections of the colon, counterstained with neuronal (anti-βIII-Tubulin, Tuj1) and epithelial (EpCam1) markers, FoxP3+ Treg cells identified via the Foxp3-gfp reporter were seen at different levels of the LP (Fig. 1B), but were rare if ever detected in the myenteric plexus or muscularis externa. Whole-mount imaging of optically cleared tissue showed that Treg cells were located very close to, and organized along, the “honeycomb” of neuronal fibers projecting in the LP around colonic epithelial glands (Fig. 1C, S1A). For comparison, staining for F4/80+ macrophages showed them more continuously aligned with the nerve fibers, as reported (Gabanyi et al., 2016), less scattered than Treg cells (Fig. S1A). Unlike macrophages, Treg cells were rare or absent in the myenteric plexus, indicating that they do not contact the cell bodies of myenteric neurons (Fig. 1D, S1B). To define the category of nerve fibers contacted by Treg cells, we counterstained thin section of colonic LP for CGRP (Calcitonin Gene-Related Peptide, a product of Calca or Calcb genes in afferent neurons of both the myenteric plexus and the DRG) and NOS1 (nitric oxide synthase, predominantly identifies nitrergic inhibitory motor neurons from the myenteric plexus (Sang and Young, 1996)). Close apposition with Treg cells was observed for both types (Fig. 1E,F), but particularly for the NOS1+ fibers, somewhat surprisingly as these fibers are predominantly thought to innervate the muscularis externa (but NOS1+ fibers were clearly seen to radiate into the colonic LP – Fig. S1C).
Figure 1. Colonic Treg cells localize closely to neurons.
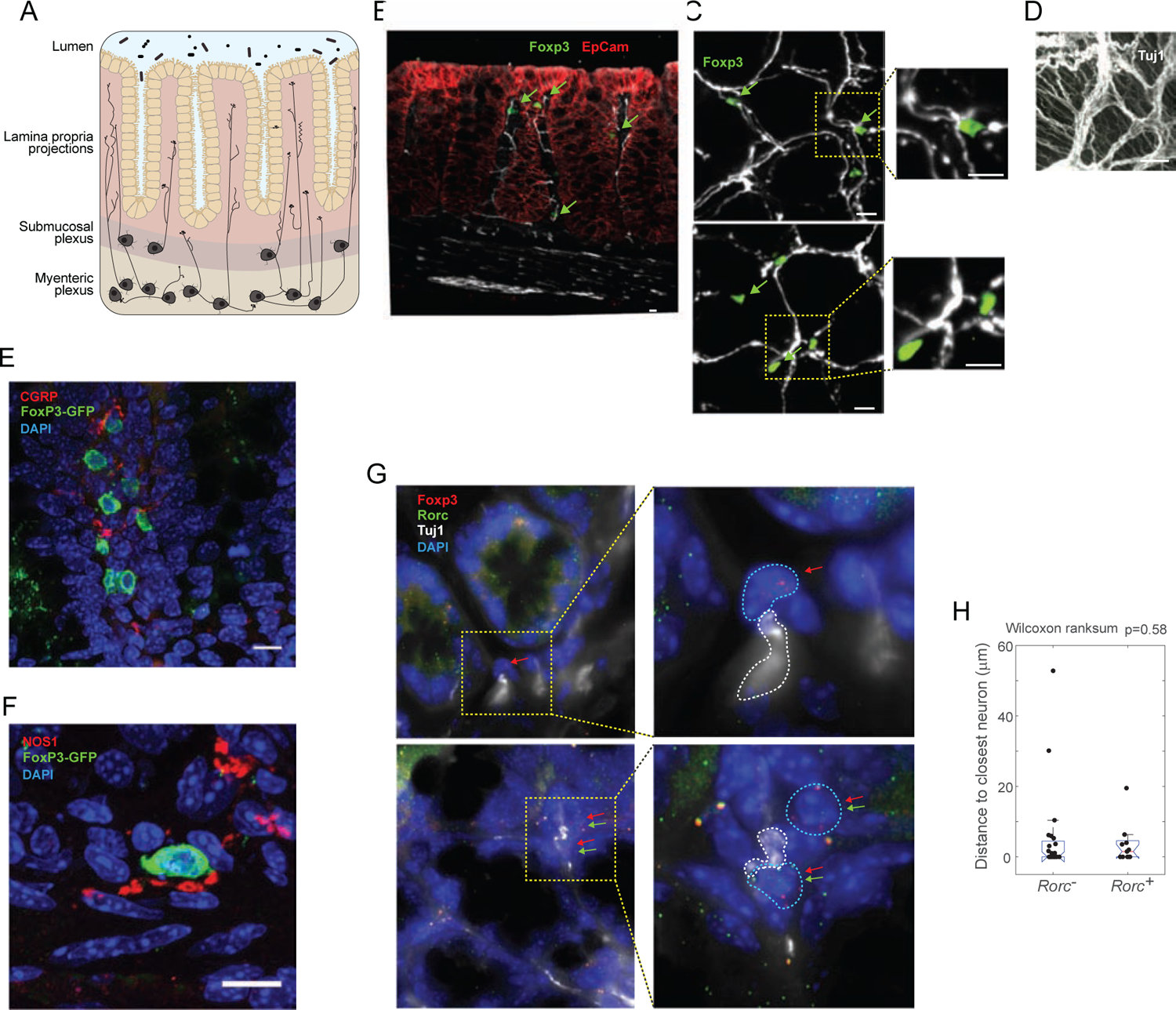
A. Schematic of the ENS in the colon.
B. Confocal images of colon cryosections from Foxp3-gfp reporter mice, immunostained for Tuj1 (white), GFP (green) and EpCAM (red). Arrows: GFP+ Treg cells scatted in the lamina propria.
C-D. Cleared whole-mount of the ENS in colon segments from Foxp3-gfp mice, stained for Tuj1 (white) and GFP (green). C: Z stacks images (0.5 μm) in the lamina propria, max projection to 5 mm total thickness; D: 35 mm max projections in the myenteric plexus.
E-F. Confocal images of colon cryosections from Foxp3-gfp reporter mice, immunostained for Tuj1 (green), CGRP or NOS1 (red), with DAPI nuclear stain.
G. smFISH of Foxp3 mRNAs (red dots) and Rorc mRNAs (green dots), counterstained with DAPI (blue, nuclei) and anti-Tuj1 (gray). Examples of Foxp3+Rorc− (i, ii) and Foxp3+Rorc+ (iii, iv) cells in close vicinity to nerve fibers. Images are max projections of 6 stacks (each 0.3 μm apart) for i,iii and 9 stacks for ii,iv.
H. Quantification, from smFISH such as G, of the distances to the closest Tuj1+ cell for 13 Foxp3+Rorc+ and 21 Foxp3+Rorc− cells (p=ns). All data (A-H) representative of 3 independent experiments.
As colonic Treg cells fall into major categories, RORγ+ and RORγ−, it was of interest to determine whether both populations were similarly close to neurons. While RORγ is readily visualized in ILCs and γδT cells, it proved impossible to detect in Treg cells by immunofluorescence or with reporters alleles, so we turned to single molecule fluorescence in-situ hybridization (smFISH (Itzkovitz et al., 2011)) with probe libraries detecting Foxp3 and Rorc, counterstained with anti-Tuj1 (Fig 1G). Within Foxp3+ cells, we identified those that contained or not Rorc transcripts, and measured their distance to the closest neuron. Both Foxp3+Rorc+ cells and Foxp3+Rorc− cells were observed at close distances to neurons, exhibiting distributions that were not significantly different (Fig. 1H; chi-sq. p=0.58). Foxp3+ cells therefore exhibited co-localization with neurons regardless of their Rorc expression.
Enteric neurons prevent iTreg induction
To explore more directly the functional crosstalk between ENS neurons and Treg cells, we performed co-culture experiments using as a proxy the “iTreg” system, in which FoxP3+ Treg-like cells are induced. The myenteric plexus was dissociated with the surrounding muscle layers (hereafter muscularis myenteric plexus, MMP), and cells were dissociated and cultured for 3–5 days in “progenitor cell differentiation medium” that supports ENS neurons in vitro (Zhang and Hu, 2013). CD4+ T conventional (Tconv) cells were added in a condition known to induce FoxP3 expression (supplementation with anti-CD3+CD28 beads, IL2 and TGFβ). In these co-cultures, enteric neurons inhibited iTreg induction in a dose-dependent manner (Fig. 2A). This inhibition was very sensitive, observed with only 1,000 neurons (~1/100 ratio relative to cultured CD4+ T cells). The inhibition was not due to neuron-induced death, as the total number of CD4+ T cells dropped only slightly with neuron addition (Fig. S2A), and neurons were not toxic to purified ex vivo Treg cells in similar cultures (Fig. S2B). We also noted that the inhibitory capacity required some degree of adaptation in culture, concurrent with the morphological maturation over 3–5 days observed in the cultures (Fig. 2B – there was no appreciable change in cell numbers over this period).
Figure 2. Enteric neurons inhibit iTreg induction.
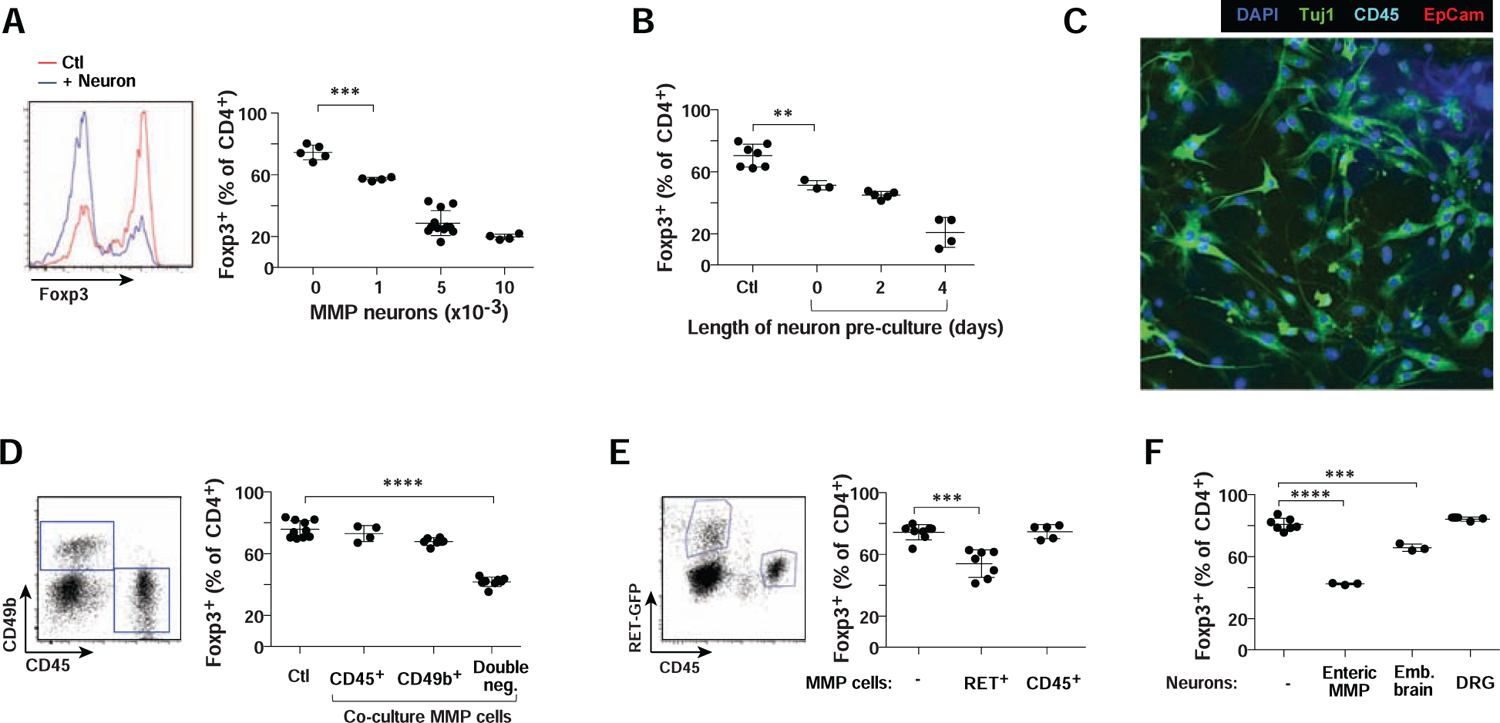
A. ENS neurons from colon MMP were adapted to culture, then co-cultured along with CD4+ T cells in 72 hr iTreg induction cultures, and the effect on induction of FoxP3 assessed by flow cytometry. Left: representative histograms; right: quantitation of FoxP3+ iTregs in the cultures. Each dot is an independent culture, composite of 3 experiments. Here and thereafter: *: p<0.05; **: p<0.01; ***: p<0.001; **** p<10−4 from unpaired Student’s t.test unless otherwise specified. Error bars mean ±SD.
B. As A, where MMP neurons were cultured for indicated times before addition to iTreg induction cultures. Composite of 3 experiments.
C. Confocal imaging of MMP neuron cultures immunostained for Tuj1, EpCAM (epithelial cells), CD45 (hematopoietic cells) and DAPI (nuclei). Representative of 2 or more fields in 3 independent cultures.
D. CD45+, CD49b+ and CD45−CD49b− cell fractions were sorted from dissociated colon MMP (left) and their ability to inhibit iTreg induction tested as in A. Composite of 3 experiments.
E. As in A, inhibitory cells tested were CD45+ hematopoietic cells or Ret+ neurons from the MMP of Ret-gfp reporter mice. Composite of 3 experiments.
F. As in A, inhibitory cells tested were cultured primary neurons from the MMP, embryonic brain or DRG. Composite of 3 experiments.
Several controls were performed to ensure that the inhibition of iTreg differentiation was due to neurons, and not to contaminating cells of another type, like myocytes, hematopoietic cells or glia. First, fluorescence microscopy showed that, after 3 days, these ENS cultures were positive for Tuj1, and none for markers of epithelial or hematopoietic cells (Fig. 2C - myocytes floated off after a day). Secondly, we purified the MMP cell suspension by flow cytometry, separating CD49b+ enteric glial cells (Joseph et al., 2011) and CD45+ hematopoietic cells. Only the double-negative fraction inhibited iTreg appearance (Fig. 2D). Third, we used Ret-gfp reporter mice, in which only neurons are fluorescent in the MMP (40%~60% of them) (Jain et al., 2006), sorting CD45−GFP+ neurons. These were inhibitory, while the CD45+ fraction was not (Fig. 2E). Thus, we concluded that it was enteric neurons, and not other cell types in these MMP cultures, that inhibited iTreg differentiation.
We then asked whether this inhibitory effect was a general characteristic of neurons. Cultures were prepared with the same numbers (5,000) of embryonic brain neurons, or adult DRG neurons (which include gut-innervating extrinsic sensory afferents). Brain neurons showed a relatively minor inhibitory effect, while DRG neurons had no effect, which indicated that the inhibition was not a generic property, ENS neurons having the highest capability (Fig. 2F).
Enteric neurons inhibit iTregs through cytokine-like large soluble factors
To determine if the inhibition by enteric neurons of iTreg required cell-cell contact, we used a Transwell chamber to separate neurons and T cells. Neurons were still inhibitory (Fig. 3A). Moreover, supernatants (SN) from the neuron cultures also had strong inhibitory effects (Fig. 3B). Thus, a soluble factor(s) released by neurons in culture played the inhibitory role.
Figure 3. Enteric neurons inhibit iTregs through cytokine-like large soluble factors.
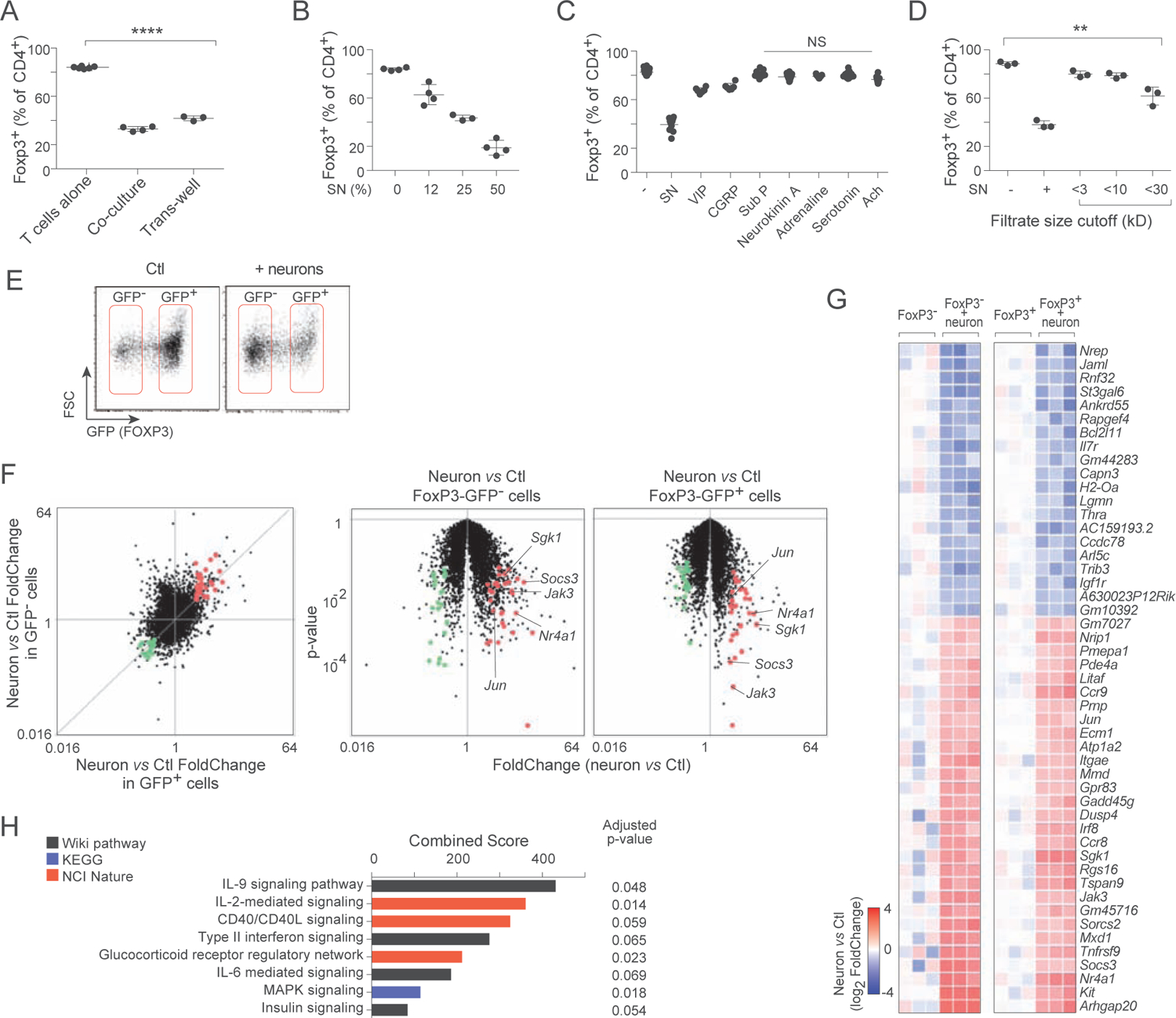
A. Frequency of Foxp3+ cells in 72 hr iTreg induction cultures, supplemented with neurons in direct contact or separated in a Transwell chamber. Each dot is an independent culture, composite of 3 experiments.
B. As in A, cultures supplemented with increasing dose of supernatant from 5 day cultures of primary MMP neurons. Composite of 2 experiments.
C. As in B, cultures supplemented with varying doses of neuromediators. Composite of 4 experiments. See Fig. S3 for detailed titration curves.
D. As in B, cultures supplemented with fractions from molecular weight cutoff filtration. Composite of 3 experiments.
E. Representative flow cytometry sorting gates for 72 hr iTreg cultures, alone or supplemented with neurons.
F. RNAseq profiling of cells shown in E. Left: changes in gene expression induced by the presence of neurons, shown on a “FoldChange/FoldChange” plot, relating in GFP+ iTregs and in non-converted GFP− cells. Right: FoldChange vs p.value “Volcano plot” showing the significance of neuron-induced changes in Foxp3-GFP− and Foxp3-GFP+ cells. Transcripts up- or down-regulated in T cells by MMP neurons in both cell states (at FC>2 and p.value<0.01) are highlighted (red and green).
G. Heatmap of changes in the geneset selected in F (log2 of FoldChange relative to the mean of controls, calculated independently for Foxp3−GFP+ and Foxp3−GFP− cells; each column is a biological replicate.
H. Pathway and GeneOntology analysis of the up-regulated transcripts in the signature from F.
T cells express receptors and are influenced by a number of neurotransmitters or neuropeptides, and these were attractive candidates as secreted inhibitors released by cultured neurons. When such mediators were added to iTreg induction cultures (summarized in Fig. 3C, full titrations and cell viability in Fig. S3A), most had no detectable inhibitory action, or only at doses that began to induce T cell death (VIP, CGRP, diethylenetriamine). These data suggested that classic neurotransmitters or neuropeptides might not be the mediators. We then performed a sizing analysis using centrifugal filters to fractionate the neuronal SN. Inhibition was absent in small molecular weight fractions, and started to be recovered in the filtrates > 30kD, suggesting that large molecules secreted by enteric neurons, but not small neuropeptides, inhibited iTreg induction (Fig. 3D).
For clues to determine the identity of the mediator(s), we analyzed by gene expression profiling the impact of the neurons on differentiating iTreg cells. Co-cultures were set up as above with Tconv cells from Foxp3-gfp reporter mice, with or without neurons. After 72 hrs we sorted and profiled by RNAseq both neurons and T cells from the cultures. For the latter, we analyzed independently both the GFP+ and GFP− populations, in order to factor out shifting frequencies of iTregs (Fig. 3E) and to focus on the signals received by the T cells rather than on iTreg differentiation itself. A common set of transcripts induced or repressed by neurons was observed in both GFP− and GFP+ T cells (Fig. 3F, G). These induced transcripts included Jak3 and Sgk1 (encoding kinases associated with cytokine signaling), Jun and Nr4a1 (encoding early-response TFs) and Socs3, which encodes a member of the Suppressor of Cytokine Signaling family typically induced upon cytokine exposure. These findings suggested that neurons were affecting T cells in these cultures through cytokine-like signals. Indeed, pathway and Gene Ontology analysis revealed that the gene-set affected by neurons overlapped with cytokine signaling (Fig. 3H).
Enteric neurons prevent Treg induction through IL6
Since the data above suggested the involvement of cytokine-like molecules, we performed an interaction analysis and searched these transcriptomes for interacting pairs, where the secreted ligand would be expressed by cultured neurons and the receptors by the T cells (Fig. 4A). Several such interactions appeared in the heatmaps ranked by expression intensity: IL6, LIF, the chemokines CCL2, CCL17 and CXCL12, and the TNF family member TL1A (Tnfsf15) were expressed by the cultured neurons, while their receptors were present on the CD4+ T cells. Within the nervous system, Il6, Lif and Ccl2 are mostly expressed by cells of the peripheral nervous system, as evidenced by single-cell RNAseq data (Fig. 4B; (Zeisel et al., 2018), www.mousebrain.org)., Il6 was expressed by several enteric neurons, especially the ENT2 nitrergic motor neurons (Fig. 4B, inset) whose termini were seen in close contact with LP Treg cells (Fig. 1F). To assess the relevance of those candidates, we added antibodies against these protein mediators to SN-supplemented T cell cultures. Of these, only anti-IL6 was able to revert the inhibition of iTreg differentiation (Fig. 4C). We verified that IL6 was indeed released by enteric neurons in culture (Fig. 4D), with an accentuation after a few days of culture that matched the neuronal maturation noted in Fig. 2B. We verified that the IL6 was produced by neuronal cells, using the fractionation strategy of cells from the MMP from Ret−gfp reporter mice described above. IL6 was detected in cultures of GFP+CD45− cells, but not of CD45+ cells (Fig. 4E). Together, these data confirm that the enteric neurons were the source of IL6 in this system.
Figure 4. Enteric neurons inhibit iTreg induction through IL6 pathway.
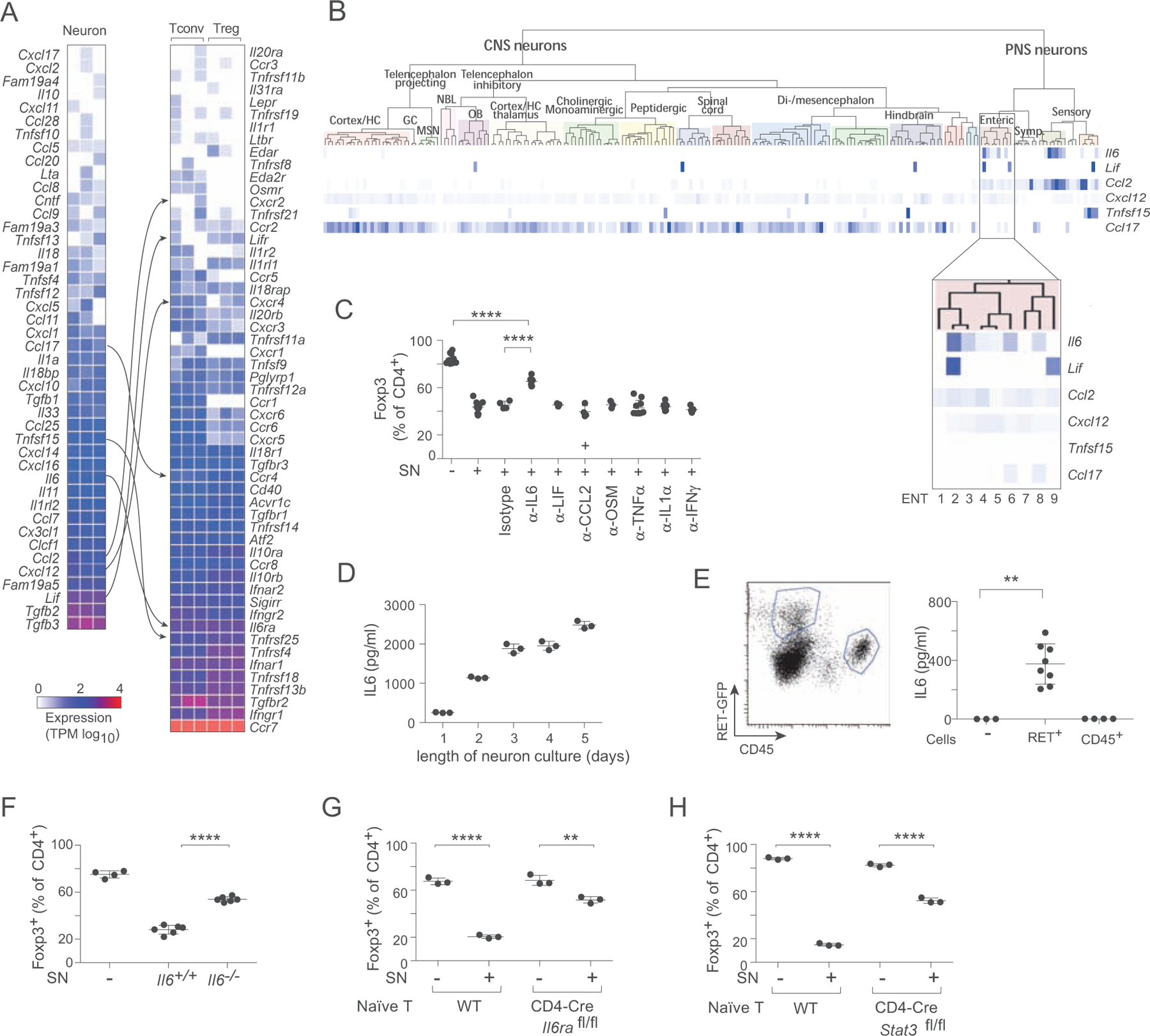
A. Gene expression results from the neuron/iTreg co-culture from 3E, representing expression values of transcripts encoding large secreted molecules by MMP neurons (left) and surface receptors by T cells (right). Interaction pairs are indicated. Each column is a biological replicate.
B. Expressions of candidate ligands identified in A in different types of enteric neurons defined by (Zeisel et al., 2018) (picture generated at www.mousebrain.org).
C. The frequency of FoxP3+ cells was assessed in 72 hr iTreg cultures supplemented from the start with enteric neuron SN, alone or with antibodies (5 ug/ml) against the candidate cytokines identified in B. Each dot is an independent culture, composite of 4 experiments.
D. IL6 levels (ELISA) in neuronal SN after different times in culture. Each dot is an independent culture, composite of 2 experiments.
E. IL6 levels (ELISA) in SN of sorted RET+ and CD45+ populations cultured for 5–7 days. Each dot is an independent culture, composite of 3 experiments.
F. Frequency of Foxp3 in iTreg cultures supplemented with neuronal SN from Il6−/− or control littermates. Each dot is an independent culture, composite of 3 experiments.
G. Frequency of Foxp3 in iTreg cultures with Il6ra-deficient CD4+ T cells (from Cd4-Cre Il6rafl/fl mice) or control littermates supplemented with neuronal SN. Each dot is an independent culture, composite of 3 experiments.
H. As G, with input T cells from Stat3 deficient mice or control littermates. Composite of 3 experiments.
The effect of IL6 was not surprising per se, given its known impact on iTreg differentiation (Bettelli et al., 2006; Zhou et al., 2008), but its origin was more surprising as it is commonly thought of as an inflammatory cytokine produced by myeloid or mesenchymal cells (Hunter and Jones, 2015). We used genetic approaches to further ascertain the role of IL6. First, ENS neurons were prepared from IL6-deficient mice (Kopf et al., 1994). These neurons elicited less inhibition of iTreg induction than did SN from wild-type littermates (Fig. 4F). Conversely, T cells harboring deficiencies in the alpha chain of the IL6 receptor, or in the STAT3 signal transducer, were largely refractory to inhibition (Fig. 4G, H). These data confirmed that IL6 was the major factor through which enteric neurons prevent iTreg induction. In both of these experiments, however, some degree of iTreg inhibition was still present in spite of the elimination of the IL6-Il6R-STAT3 axis, indicating that, in addition to IL6, a secondary mediator made by neurons also downregulates iTreg differentiation.
Enteric neurons modulate RORγ+ Treg induction via IL6
IL6 is involved in the differentiation of RORγ+ Treg in the gut (Sefik et al., 2015; Eberl and Littman, 2004; Yissachar et al., 2017; Pratama et al., 2020). This major gut Treg subset is influenced by microbes, and we showed previously that the ability of bacterial strains to induce RORγ+ Treg cells correlated with their ability to trigger neuronal activity (Yissachar et al., 2017). It was thus of interest to determine whether the effect of neurons on iTreg differentiation also influenced the proportion of RORγ+ Treg cells in these cultures. The iTreg induction protocol used in the previous experiments did not lead to any detectable expression of RORγ (not shown), so we used an alternative protocol (Wheaton et al., 2017) (anti-CD3 presented by splenic presenting cells) in which RORγ+ Treg cells can differentiate. Added neurons led to a strong inhibition of total FoxP3 induction, as in the previous cultures, but also to the appearance of RORγ+ Treg cells at intermediate neuron numbers (Fig. 5A). Neuronal SN had similar effects (Fig. S4). Wheaton et al showed that IL6 influences the outcome in their cultures (Wheaton et al., 2017). To assess whether the effect of neurons could be entirely explained by IL6, we measured by ELISA the concentration of IL6 in neuron SN and in a batch of recombinant IL6, and performed parallel cultures with matching doses of IL6. Addition of recombinant IL6 and matched neuronal SN showed mirror-like trends (Fig. 5B), indicating that IL6 was the dominant driver of the neuronal effect, with a dose-dependent relationship between its effects on total Treg cells vs RORγ+ proportions (both shut down at higher doses, but RORγ+ Treg cells numerically increased in the intermediate dose range; Fig. 5C). Moreover, quenching of IL6 in the neuronal SN by antibodies (Fig. 5D) or using SN prepared from IL6-deficient mice (Fig. 5E) abrogated the effects on both total and RORγ+ Treg cells. The inhibitory effect was not completely eliminated by either genetic ablation or antibody blockade of IL6, confirming that a second molecule released by neurons can also inhibit iTreg differentiation, albeit far less efficiently than IL6, and apparently without the RORγ-inducing capacity.
Figure 5. Enteric neurons modulate RORγ+ Treg induction through IL6.
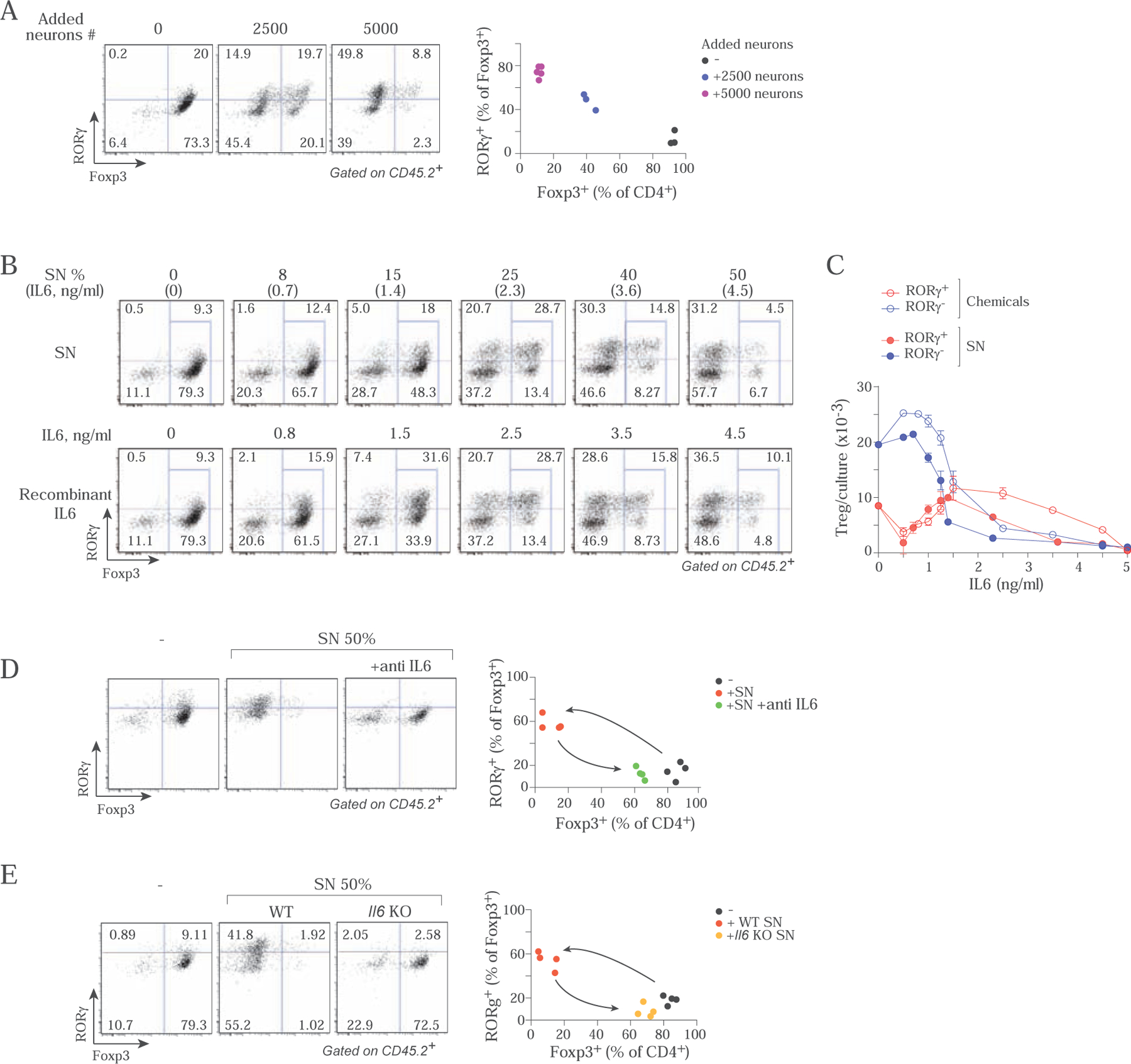
A. Representative flow cytometry profiles after 72 hrs of iTreg cultures (soluble αCD3 + splenic APCs) stained for FoxP3 and RORγ. Each dot is an independent culture. Plot at right compiled from 3 independent experiments.
B. Comparison of MMP neuron SN and recombinant IL6. Top panels: iTreg cultures (soluble αCD3 + splenic APCs) supplemented with titrated neuronal SN in which IL6 concentration had been pre-determined by ELISA. Bottom panels: parallel cultures supplemented with matching concentrations of rIL6 (after ELISA determination). Representative of two independent experiments.
C. Data from B, plotting numbers of RORγ+ and RORγ- Treg cells in iTreg cultures (soluble αCD3 + splenic APCs) supplemented with titrated amounts of neuron supernatant or recombinant IL6. Each dot is the average value of 2 or more independent preparations, plot compiled from 2 independent experiments.
D. Representative flow cytometry profiles of iTreg cultures (soluble αCD3 + splenic APCs) supplemented with MMP neuronal SN and anti-IL6 (Each dot is an independent culture, compiled data from 3 independent experiments plotted at right).
E. As D, where the neuron culture SN was from Il6-deficient mice or control littermates. (Compiled data from 3 independent experiments plotted at right).
Microbial impact on the neuron-Treg axis
Commensal gut microbes like Clostridium ramosum are potent inducers of RORγ+ Treg cells, in a manner that correlates with their ability to trigger neurons in vitro (Yissachar et al., 2017), suggesting that these actions could be linked. To connect these dots, we analyzed the effect of mono-colonization of GF mice on ENS structure and composition. Two weeks after mono-colonization with the high-Treg inducer C. ramosum or the non-inducer Peptostreptococcus magnus, we performed immunofluorescent imaging on whole-mount colon segments as described above (neuron cell bodies identified by antibodies of the pan-neuronal marker HuC/D, nerve fibers by anti-Tuj1). Bacterial colonization induced a strong reduction in the number of neuronal cell bodies and caliber of nerve fiber tracts in the myenteric plexus Fig. 6A,B), and the density of nerve projections to the LP (Fig. 6C,D). These changes were strongest after colonization with C. ramosum, more modest with P. magnus.
Figure 6. Commensal microbes affect neurons and their phenotypes.
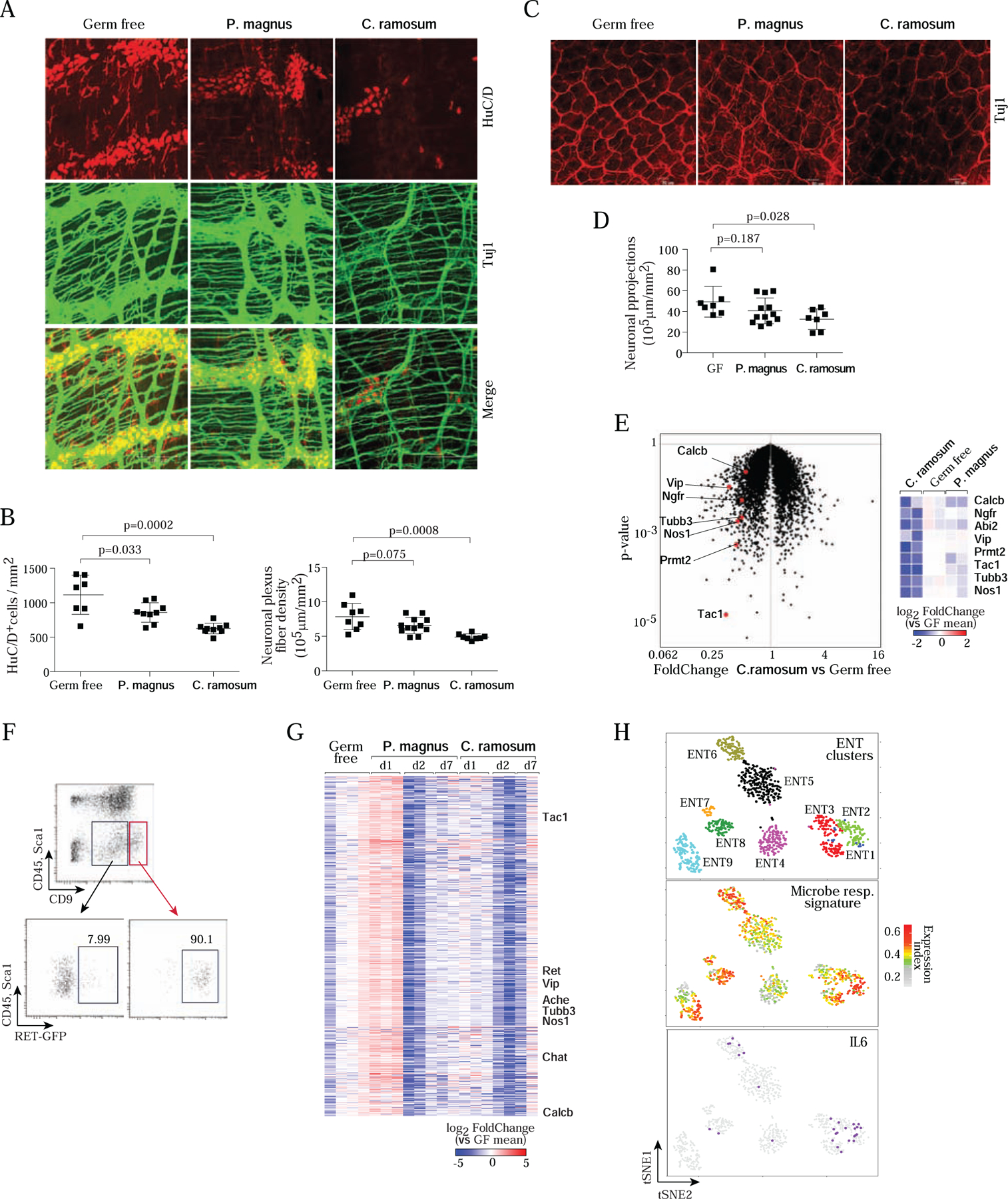
A. Whole-mount staining and quantification of the myenteric plexus in colon segments in GF, C. ramosum- or P. magnus-monocolonized mice, immunostained for Tuj1 (neuronal bodies and fibers, green) and HuC/D (neuron cell bodies, red). Images are max projections of 50–100 stacks (each 0.5 μm apart). Images representative of 4 independent experiments, and 5 or more monocolonized mice.
B. Quantitation (Imaris software) of neuronal body (left) and fiber (right) densities in myenteric plexus from images as in A. Each dot represents a mouse, composite from 4 imaging experiments.
C. Whole-mount immunostaining as in A, but focused on projections in the lamina propria.
D. Quantitation of fiber density in images as in C.
E. GF mice were mono-colonized with C. ramosum or P. magnus for 1 day, and RNA was prepared from whole MMP (neuronal and muscle layers) for RNAseq profiling. Left: changes in gene expression induced by C. ramosum vs GF controls, with characteristic neuronal transcripts highlighted. Right: heatmap representation of changes (log2) vs mean of GF controls, in all replicates.
F. Gating strategy to sort MMP neurons (CD45−Sca1−CD9hi) from C.ramosum or P.magnus mono-colonized GF mice for RNAseq. Bottom panels displayed RET-GFP expression in MMP from Ret GFP/+ mice used to establish the strategy then applied to wild-type GF mice.
G. Changes in gene expression in sorted neurons (gated as in F) for transcripts of the major co- regulated cluster, at different times after mono-colonization with P.magnus and C. Ramosum. (log2 of FoldChange relative to the mean of GF; clustered by gene-gene correlation within the cluster; each column represents a different mouse). Hallmark neuronal genes indicated.
H. Differential expression across enteric neurons of the cluster of genes affected by bacterial colonization. Upper panel: single-cell RNAseq of small intestinal ENS from (Zeisel et al., 2018), neurons clustered and positioned using tSNE coordinates from that study. Middle panel: normalized expression index for the microbe-responsive cluster (genes from G, expression of each gene normalized to its mean expression across all cells, then all genes summed for each cell) on those cells. Lower panel: superimposition of Il6- positive cells on the same tSNE.
We turned to RNAseq to confirm and analyze temporally the changes to enteric neurons imparted by mono-colonization with these commensal microbes. First, whole MMP from GF and microbe-colonized colons (24 hrs post-colonization) were dissected and profiled by RNAseq (Fig. 6E). Changes were relatively limited (only 18 transcripts induced or repressed at FC>2 and t.test pval<0.01), but there was a clear decrease in Tubb3 and in a set of neurotransmitter-encoding transcripts that we had previously found to be downregulated in whole colon tissue after microbial exposure in organotypic cultures (Yissachar et al., 2017): Nos1 (nitric oxide synthase), Calcb (encodes CGRP-β), Vip (Vasoactive Intestinal Peptide, VIP) and Tac1 (Substance P and Neurokinin A). These early effects confirmed in vivo the significance of our prior in vitro data, were concordant with the reduction in neural density seen by imaging, and suggested that various types of neurons were affected.
To profile the neuronal component more specifically, we then established a flow cytometric protocol for ENS neuron purification. The colonic MMP was dissected, cells were released by digestion and were stained with antibodies to CD45 (to exclude muscularis macrophages and hematopoietic cells), Sca1 (to exclude mensenchymal cells) and CD9 (which stains many enteric neurons, consistent with small intestine data in mousebrain.org). In pilot experiments performed on RetGFP/+ mice, RET+ neurons were found mainly in the CD45−Sca1− CD9hi component (Fig. 6F). We used this gating strategy to sort for RNAseq profiling neurons from C. ramosum or P. magnus mono-colonized GF mice at days 1, 2 and 7 after colonization. Parsing the temporal evolution of the transcripts identified one major coherent cluster of co-varying transcripts. This cluster showed a marked downregulation in response to both bacteria after 2 days, with some recovery after one week, and included many of the neurotransmitter transcripts mentioned above (Fig. 6G). This cluster included a number of transcripts typical of different neuron classes, suggesting a widespread effect. To further this point, we mapped the normalized and integrated expression of genes of this cluster onto the single-cell RNAseq profiles of small intestinal neurons from (Zeisel et al., 2018). The tSNE projection of Fig. 6H, upper panel demarcates the different ENT subsets. The microbe-responsive gene cluster was differentially expressed in ENT subsets, strongest in ENT2 nitrergic neurons (Fig. 6H, middle panel), superimposing well with IL6 production in ENT2, 3, 6 (Fig. 6H, bottom). Thus, gene expression affected by microbes affects a wide variety of effectors in ENS neurons, but with some specificity as well.
In these profiles, Il6 transcripts did not vary at the early time points when neurotransmitters were affected, but were markedly reduced 7 days after colonization by both microbes, whether or not they are RORγ+ Treg inducers (Fig.7A). This delay suggested that microbes did not alter neuron-secreted IL6 directly, but as an outcome of neuronal perturbation. We tested this relationship by comparing colonic Treg populations in genetically engineered mice. First, with mice deficient in several of the affected neurotransmitters, increases in the proportions of total colonic Treg cells were observed in Vip- and Calca-deficient mice relative to control littermates, with little or no impact for Calcb deficiency (consistent with its low expression in our ENS neuron profiling data) (Fig. 7B). None of the mutations affected RORγ+ Treg proportions, however (Fig. S5). Accordingly, culture SNs of myenteric neurons from Vip- and Calca-deficient mice were less inhibitory to Treg differentiation than those of littermates, with no effect of Calcb deficiency (Fig. 7C), reflecting their capacity to produce IL6 in culture (Fig. 7D). Secondly, we generated mice with neuron-specific deficiency in IL6, crossing the Il6fl/fl allele to Nestin-Cre and Syn1-Cre driver transgenes (Tronche et al., 1999; Zhu et al., 2001). Specificity was shown by reduced IL6 production in MMP neuron cultures, but not in LPS-stimulated spleen and muscle used as a control for potential inactivation in myeloid cells (Fig. 7E,F), and in the ability to repress iTreg differentiation in vitro (Fig. 7G,H). Most importantly, and providing cell cell-intrinsic validation of the importance of neuronal IL6, both crosses showed partial but significant increases in colonic Treg proportions, and decreases in the fraction of RORγ+ Treg (Fig. 7I,J). Together, these results established a connection between gut microbes, VIP and CGRP signaling within the ENS, and Treg differentiation in vivo and in vitro, one that revolves around IL6.
Figure 7. Genetic or microbial changes in neuronal IL6 affect Treg differentiation in vivo.
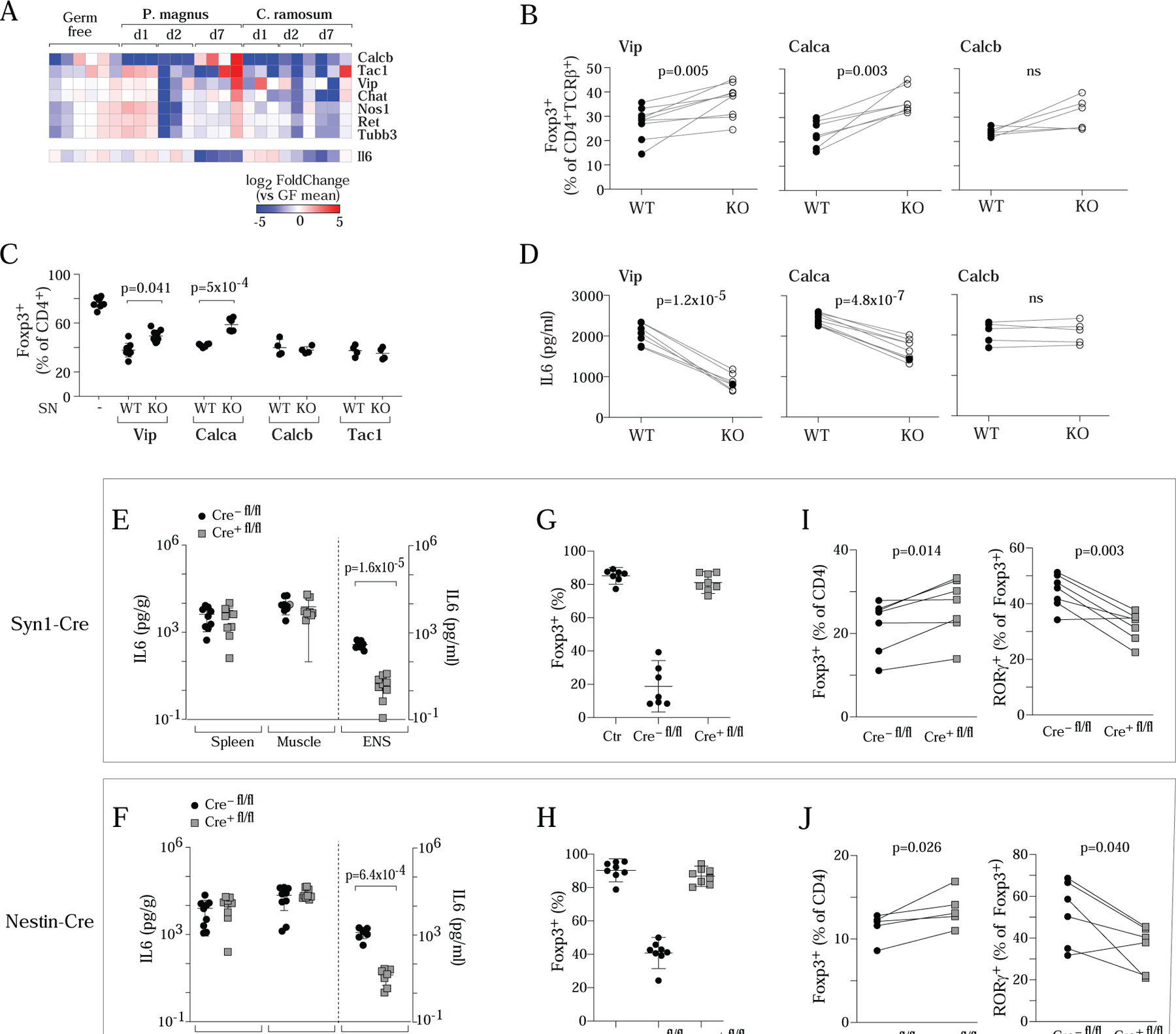
A. Expression of Il6 in sorted neurons during the monocolonization time-course experiment of Fig. 6G. Neurotransmitter transcripts repeated for reference.
B. Frequency of colonic Treg cells within CD4+ T cells in Vip−/−, Calca−/−, Calcb−/− mice or control littermates.
C. iTreg induction cultures were supplemented with neuronal SN (25%) produced by cultured MMP from Vip−/−, Calca−/−, Calcb−/− or Tac1−/− mice or their wild-type littermates. Each dot is an independent culture, composite of 3 experiments.
D. IL6 levels (ELISA) in neuronal SN from Vip−/−, Calca−/−, or Calcb−/−or their wild type littermates.
E,F. IL6 levels in MMP neuronal SN from Syn1-CreIl6fl/fl (E) and Nestin-CreIl6fl/fl (F) or their control littermates (mostly cre-negative). LPS-stimulated (100ng/ml, overnight) whole spleen or muscle cultures used as a control.
G,H. MMP neurons from Syn1-CreIl6fl/fl (G) and Nestin-CreIl6fl/fl (H) or their wild type littermates were adapted to culture, then co-cultured along with CD4+ T cells in 72 hr iTreg induction cultures, and the effect on induction of FoxP3 assessed by flow cytometry. Each dot is an independent culture, composite of 3 or more experiments.
I,J. Frequency of colonic Treg cells and RORγ+Treg cells within CD4+ T cells in Syn1-CreIl6fl/fl (I) and Nestin-CreIl6fl/fl (J) or wild-type control littermates. Throughout, each dot represents an individual mouse unless mentioned, p-value from paired Student’s t test.
DISCUSSION
This work establishes the existence of a triangular crosstalk between gut microbiota, enteric neurons and Treg cells, an interaction that affects the vertices of the triangle and modulates homeostatic settings. Treg cells lined enteric neuron projections in the lamina propria; enteric neurons affected iTreg induction in vitro; commensal microbes strongly and widely affected ENS structure and transcriptome, and genetic perturbations of the ENS affected colonic Treg frequencies. A recurring theme proved to be the production of IL6 by neurons, a lead anchored by the conditional genetic deletion experiments.
A first argument for an interaction between Treg cells and neurons in the colon came from the close apposition of Treg cells with the fibers that innervate the LP – in contrast, Treg cells were virtually absent from the myenteric plexus, unlike macrophages, which are abundant there (Muller et al., 2014; Gabanyi et al., 2016; De Schepper et al., 2018). Neuronal fibers in the LP tend to follow the microvasculature, and the resolution of the whole-mount imaging or FISH sections cannot formally resolve whether these interactions are direct, or via an intermediate as in the case of the muscle, where IL33-producing mesenchymal stromal cells connect neurons and Treg cells (Kuswanto et al., 2016). Colonic RORγ+ and RORγ− Treg cells differ in their responsiveness to IL33 (only the latter expressing the IL33 receptor), but both are found in contact with nerve fibers, suggesting that IL33 is not as dominant an intermediate in the gut as it is in the muscle. Interestingly, the functional data showed the impact of neuronal IL6 on the differentiation of iTregs, while the images of close apposition between Treg cells and nerve fibers suggest interactions involving differentiated FoxP3+ Treg cells, suggesting neuron/Treg cross-talk at different stages.
Neurons, and especially enteric neurons, inhibited iTreg induction in two different iTreg induction protocols. They also increased the proportion of RORγ+ Treg cells, implying that neurons may have complex dose-dependent effects on the representation and balance of the two Treg populations in the colon. Rather than a non-specific effect of cultured neurons (e.g. Wallerian degeneration), a string of mechanistic and biochemical analyses proved that a defined cytokine, IL6, proved to be the major mediator of this effect. IL6 is one of the main inflammatory cytokines, with pleiotropic functions and effects on various immunocytes (Hunter and Jones, 2015). Discovering it in this context was both surprising and unsurprising. IL6 was unsurprising in this role because it is known to inhibit iTreg differentiation (Bettelli et al., 2006; Zhou et al., 2008), and is required for normal RORγ+ Treg frequencies in the colon (Sefik et al., 2015; Eberl and Littman, 2004; Yissachar et al., 2017; Pratama et al., 2020). But finding IL6 was surprising because it had not been previously considered as a mechanism used by neurons to influence the immune system. Documented instances of control of immune cells by the nervous system tend to involve small neuromediators like CGRP, NMU, serotonin or β-adrenergic agonists (de Jong et al., 2015; Wallrapp et al., 2017; Wallrapp et al., 2019; Klose et al., 2017; Pinho-Ribeiro et al., 2018; Cardoso et al., 2017; Gabanyi et al., 2016). One precedent is the cytokine CSF1 which is secreted by myenteric neurons and promotes the growth of local macrophages (Muller et al., 2014). IL6 has some recognized roles in the central nervous system (CNS), affecting synaptic transmission and plasticity, with behavioral and cognitive consequences (reviewed in (Gadient and Otten, 1997; Gruol, 2015)). Both glia and neurons in the CNS can secrete IL6 (März et al., 1998; Ringheim et al., 1995), but single-cell RNAseq revealed an enrichment of Il6 transcripts in peripheral neurons (Zeisel et al., 2018), especially in enteric and sensory neurons, and we confirmed here that Il6 is expressed in purified neurons of the myenteric plexus. While IL6 is the dominant mediator of neuronal inhibition of iTreg induction, one or more other molecules must also be involved: genetic inactivation of Il6ra in the T cells (or Il6 in the neurons) largely, but not completely, abolished the neurons’ inhibitory impact. Nor did antibody blockade. The IL6 family member LIF made a plausible candidate, as it is highly expressed in cultured neurons, but anti-LIF antibody failed to block iTreg inhibition, leaving the identity of this second mediator mysterious.
We found that the ENS neurons’ ability to produce IL6 was reduced by inactivation of the important neuropeptide-encoding genes, Vip and Calca (less so for Calcb), with corresponding effects on Treg frequencies in vivo. ENT2, nitrergic motor neurons that express Nos1 and Vip, might not have been expected to innervate the LP, but we did observe a strong representation of NOS1-positive fibers there, some in close contacts with Treg cells. There was thus consistency between the imaging and transcriptome data, which hints that ENS motor neurons might have additional and unrecognized roles related to immune cell cross-talk (IL6 was also detected in motor-neuron populations in a recent single-cell atlas of the ENS (Drokhlyansky et al., 2020)). On the other hand, Calca is primarily expressed in peptidergic sensory neurons of the DRG, and to a much lower extent in the ENS (Mulderry et al., 1988; Zeisel et al., 2018; Drokhlyansky et al., 2020). These DRG cells were naturally absent from our ENS cultures, suggesting that the effect of the Calca deficiency on IL6 secretion may be indirect. DRG neurons expressing CGRP send their projections to the myenteric plexus (Lai et al., 2020) and may regulate neuron function via RAMP1/CALCRL, the receptor complex for CGRP that is expressed by most enteric neurons (Zeisel et al., 2018). Thus, CGRP from sensory neurons may deliver signals in enteric neurons that are necessary for proper Il6 expression. More generally, an integrated perspective of the results might simply be that the neuronal release of IL6 in the colon requires an intact and fully connected ENS, and that perturbations in signals from the extrinsic (Calca in the DRG) or intrinsic (Vip in the ENS) nervous systems perturb this harmonious integration. In keeping, both Calca−/− and Vip−/− mice have increased susceptibility to colitis (Engel et al., 2012; Wu et al., 2015), which one might speculate to be linked to the neuronal control of Treg cells.
Finally, the impact of commensal microbes on the ENS provides the third side of the triangle. In keeping with the downregulation of neurotransmitter transcripts induced by commensals in the organotypic culture system in vitro (Yissachar et al., 2017), the introduction of commensals to the gut of adult GF mice, especially of the RORγ+ Treg inducer C. ramosum, led to a rapid decrease of neuronal transcripts in the whole myenteric plexus and to a diminution of the total ENS density over a two-week period. Neuronal Il6 expression also decreased, but later, consistent with the notion that it is tuned by ENS integrity. Neurons can directly sense bacteria through detection of LPS, bacterial N-formylated peptides, or pore-forming toxins (Chiu et al., 2013; Meseguer et al., 2014; Pinho-Ribeiro et al., 2018). We propose that the early downregulation upon microbial exposure results as a negative feedback from activation, through sensory receptors or the action of microbial metabolites, of an ENS previously “naïve” to microbial input. It is important to note that the loss seemed to affect all neuronal types, as evidenced by the reduction of several specific marker transcripts like Nos1, Tac1 or Chat, which together cover a large proportion of enteric neurons, indicating that the effects are not limited to sensory neurons. It might occur through cell death resulting from over-excitation, or a more subtle retuning of ENS homeostasis.
Both RORγ+ Treg-inducing and non-inducing species depressed IL6 production. Recall, however, that several signaling pathways from microbes may speak to RORγ+ Treg cells, whether lipid or polysaccharide components [e.g. (Arpaia et al., 2013; Hang et al., 2019; Verma et al., 2018)], as also modulated by IgA (Ramanan et al., 2020)]. Thus, while neuron-produced IL6 is a significant tuner of colonic Treg pools, it does not represent the totality of local Treg homeostasis control, and we hypothesize that P. magnus is missing one of the other components required for efficient Treg induction.
Neurons and IL6 showed a complex relationship between its effects on total Treg cells and on the RORγ+ fraction: lowering total iTregs and boosting the RORγ+ fraction at low concentrations, but blocking both at high concentrations, as relative proportions and absolute cell numbers. This dose-dependence may contribute to the different outcomes of colonization of GF mice with C.ramosum and P.magnus, which showed different abilities to induce total Treg cells and RORγ+ representations and to downregulate neurons. We should stress, though, that we do not suggest here that neuronal IL6 is the sole driver of the induction of RORγ+ Treg cells by gut commensals, as good evidence has been provided for a role of several bacterially derived products (Verma et al., 2018; Yissachar et al., 2017; Hang et al., 2019; Song et al., 2020). Rather, we propose that the neuronal influence modulates the outcome, amplifying the consequences of bacterially derived triggers, and providing a system-wide integration that the local effect of microbial molecules might not achieve.
Altogether, this study defines a triangular mode of interaction among enteric neurons, Treg cells and gut microbes, where the nervous system uses IL6 to tune immunoregulatory tone. One implication is that environmental or genetic perturbation in any one of these poles (microbial dysbiosis, extrinsic neuronal influences, or immune-modulators) may alter the 3-way equilibrium, and change how tolerance to food or microbes is enforced, modulating the host-microbe interface and the course of inflammatory bowel diseases.
LIMITATION OF STUDY
This study identifies neuronal IL6 as a strong mediator of the neuron-Treg crosstalk, but IL6 is not the sole molecular player: either in vitro where we could not identify the minor mediator of IL6-independent neuronal inhibition of iTreg differentiation; or in vivo where inactivation of neuronal Il6 expression only partially affects Treg numbers and phenotypic balance. The impact of Vip and Calca ablation were also partial, and it would be interesting to determine mechanistically how their loss affects neuronal IL6 production. While neuron-derived IL6 is clearly important, we do not know how it integrates with other sources of IL6 in the gut (conditional inactivation in all cells except neurons?), and whether its impact extends beyond Treg cells. It will be important to define the relevance of neuronal IL6 to inflammatory or autoimmune diseases, how its influence evolves in inflammatory backdrops, and whether it operates in other organismal locations.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Prof. Christophe Benoist (cbdm@hms.harvard.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The accession number for the RNA sequencing data reported in this paper on NCBI GSE164577 (RNA profilings of T cells and enteric neuron in T/neuron cocultures, related to Fig 3 FGH), GSE164576 (RNA profilings of MMP with microbe stimulations, related to Fig 6E) and GSE164575 (RNA profilings of enteric neurons with microbe stimulations, related to Fig 6 FGH).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6J (B6), B6.Cg-Foxp3tm2Tch/J (Foxp3-gfp), B6.129S4-Viptm1Clw/J (Vip−/−), B6.129S2-Il6tm1Kopf/J (Il6−/−), B6.SJL-Il6ratm1.1Drew/J (Il6rafl/fl), B6.129S1-Stat3tm1Xyfu/J (Stat3fl/fl), B6.Cg-Tg(Cd4-cre)1Cwi/BfluJ (CD4-Cre), B6.Cg-Tac1tm1Bbm/J (Tac1−/−), B6.Cg-Tg(Nes-cre)1Kln/J (Nestin-Cre), B6.Cg-Tg(Syn1-cre)617Jxm/J (Syn1-Cre) and B6.CD45,1 mice were obtained from the Jackson Laboratory and bred in the SPF facility at Harvard Medical School (HMS). Il-6lox/lox (Il6fl/fl) (Quintana et al., 2013). B6.129S6-Calcatm1Hku (Calca−/−), B6.129(Cg)-Rettm13.1Jmi/SjnJ (Ret-gfp) and Calcb−/− mice (Thompson et al., 2008) were bred in the specific-pathogen-free (SPF) facilities at HMS and BCH. Ret-gfp mice were used as hemizygotes. For constitutive genetic deletion, F1 heterozygotes were intercrossed to generate WT and KO littermates. To obtain conditional genetic deletion mice lacking IL6RA and STAT3 in T cells, Il6rafl/fl and Stat3fl/fl mice were first crossed with Cd4-Cre, then intercrossed to generate WT and Cd4-Cre Il6rafl/fl and Cd4-Cre Stat3fl/fl littermates. To obtain mice lacking IL6 in neurons, Il6fl/fl mice were first crossed with Nestin-Cre and Syn1-Cre, then intercrossed to generate WT and neuronal deficient littermates. Only matched littermates were used in control and experimental groups in all experiments. All experimentation was performed following animal protocols approved by the HMS Institutional Animal Use and Care Committee (protocols IS00001257and IS00000054).
METHOD DETAILS
Germ-free mice (GF) and colonization
GF B6 mice (originally obtained from the National Gnotobiotic Rodent Resource Center of the University of North Carolina at Chapel Hill), were bred in the Kasper/Benoist/Mathis shared facility at Harvard Medical School in GF flexible film isolators (Class Biologically Clean). Bacteria (C.ramosum, P.magnus) were originally obtained from the ATCC or BEI as described (Geva-Zatorsky et al., 2017). Anaerobic bacteria were grown on Brucella 5% SB HEMIN VIT K1 plates (Thermo) under strict anaerobic conditions (80% N2, 10% H2, 10% CO2) at 37°C in an anaerobic chamber, scraped to PBS with 20% glycerol to reach a titer of 109 colonies/ml, and frozen in 100ul aliquots. For colonization, 4-week-old sex-matched GF littermates were divided into control and experimental groups, orally inoculated by gavage with vehicle (PBS plus 20% glycerol) or bacterial aliquots (1 aliquot per mouse), and kept in sterile cages under sterile conditions (sterile food and water).
Enteric neuron cultures
Colons from adult mice (7–12 weeks) were opened lengthwise, and the MMP was dissected completely under a binocular microscope using watchmaker forceps (Zhang and Hu, 2013), cut into pieces of approximately 2 to 5 mm2 and placed in a 1.5-ml tube containing 50U/ml penicillin and 50ug/ml streptomycin (Thermo Fisher), 0.1mg/ml Liberase TL (Roche) in neutral Hank’s Balanced Salt Solution (HBSS, Thermo Fisher) to digest for 0.5 h at 37°C, and then mechanically disrupted by mild shaking. Tissue was then further digested with 0.05% Trypsin -EDTA (Thermo Fisher) solution at 37 °C for 10 min. After trituration using a P1000 pipette, single cell suspensions were filtered through 70um filter, cleaned of debris by centrifugation through 1ml fetal bovine serum (110g for 10min), and captured by selective plating on Matrigel (Thermo Fisher)-coated cell culture dishes in B27 (Thermo Fisher) supplemented Neurobasal-A medium (Thermo Fisher) plus 10 ng/ml fibroblast growth factor-basic (FGF-b, Peprotech), 20ng/ml Epidermal growth factor (EGF, Peprotech), 50U/ml penicillin and 50ug/ml streptomycin (Zhang and Hu, 2013).
Embryonic brain neuron cultures
Papain dissociation (Worthington) were used to prepare neonatal neural cells. Briefly, neonatal mouse brains were minced, digested in Earle’s Balanced Salt Solution (EBSS) with papain (20 U/ml, Worthington) for 0.5h at 37°C and DNase (100U/ml) for 5 min at room temperature. The cell pellets were centrifuged over a 10% BSA at 70g for 6 min and plated in cell culture dishes in B27 supplemented Neurobasal-A medium plus 50U/ml penicillin and 50ug/ml streptomycin.
DRG neuron cultures
Primary lumber dorsal root ganglia (DRG, segments T7-L6) from adult mice (7–12 weeks) were dissected into Neurobasal-A medium, dissociated in 1 mg/ml collagenase A plus 2.4 U/ml dispase II (Thermo Fisher) in HBSS for 40 min at 37°C. After trituration with glass Pasteur pipettes of decreasing size, DRG cells were centrifuged over a 10% BSA gradient at 260g for 10 min, plated on Matrigel-coated cell culture dishes in B27 supplemented Neurobasal-A medium supplemented with 50 ng/ml nerve growth factor (NGF, Thermo Fisher), 2 ng/ml glial cell line-derived neurotrophic factor (GDNF, Sigma), 50U/ml penicillin and 50ug/ml streptomycin.
iTreg induction in culture and flow cytometry
Naïve CD4+ T splenocytes were sorted as GFP−CD4+CD44lowCD62Lhigh from Foxp3-gfp reporter mice (conjugated mAbs from BioLegend) on a Moflo sorter. Two different protocols were then used: in the first, T cells were activated with anti-CD3/CD28–coated beads (Thermo Fisher) at a concentration of two cells per bead in the presence of 2 U/ml of human rIL-2 and 10 ng/ml rTGF-β (Peprotech) in RPMI with 10% FCS (Chen et al., 2003). In the second, T cells were activated with 1μg/ml soluble anti-CD3ε (Thermo) presented by T-depleted Mitomycin C (Sigma)-inactivated splenocytes (1:3 ratio) in the presence of 2 U/ml rhIL-2 and 10 ng/ml rTGF-β in RPMI with 10% FCS (Wheaton et al., 2017). To prepare these presenting cells, spleen suspension from congenic B6.CD45.1 mice were depleted of T cells by incubation with Biotin-conjugated anti-CD3ε (Biolegend) and anti-TCRβ in Dynal buffer (1mg/ml BSA and 2 mM EDTA in PBS) for 20min on ice, washed and incubated with Dynabeads BiotinBinder (150ul per spleen, Thermo) for 30min at 4°C with rotation. T cells were removed using DynaMag magnet (Thermo), and the remaining cells blocked with 0.5mg/ml Mitomycin C at 37°C for 2hs. After 3 days in culture, cells were analyzed by flow cytometry.
T/neuron co-cultures
For T cell and neuron co-cultures, enteric neurons, DRGs or embryonic brain neurons were purified and cultured as above at 5000 cells per well in 96-well plates for 3 days (unless otherwise indicated) and then sorted naïve CD4+ T cells were added (100,000 cells per well) for 3 more days using iTreg induction protocols as above. For the co-culture of T cells and neuronal SN, enteric neurons were prepared as above and cultured at a concentration of 10,000 cells per well at 96-well plates for 5 days (unless otherwise indicated), the SN were collected and filtered through 0.22um filter (Millipore), and added to iTreg cultures at 1:4 dilution unless otherwise indicated.
RNAseq profiling
RNA-seq was performed with the standard ImmGen low-input protocol (www.immgen.org). A total of 1,000 cells were sorted directly into 5ul of lysis buffer (TCL Buffer (QIAGEN) with 1% 2-Mercaptoethanol). For cultured neurons, enteric neurons were prepared as above, cultured for 5 days, detached by Accutase (Innovative) and lyzed in TCL buffer (Qiagen) with 1% 2-Mercaptoethanol at 1000 cells in 5ul buffer. To profile whole MMP preparations, the fragments were peeled off from the colons, cut into 2cm segments, lyzed and homogenized in 400ul TCL buffer. For primary enteric neurons, the MMP neurons were prepared as above, stained with anti-CD45, Sca1, CD9, and the CD9high population were sorted into TCL buffer at 1000 cells in 5ul buffer. Smart-seq2 libraries were prepared as previously described (Picelli et al., 2014) with slight modifications. Briefly, total RNA was captured and purified on RNAClean XP beads (Beckman Coulter). Polyadenylated mRNA was then selected using an anchored oligo(dT) primer (50 –AAGCAGTGGTATCAACGCAGAGTACT30VN-30) and converted to cDNA via reverse transcription. First strand cDNA was subjected to limited PCR amplification followed by Tn5 transposon-based fragmentation using the Nextera XT DNA Library Preparation Kit (Illumina). Samples were then PCR amplified for 12 cycles using barcoded primers such that each sample carries a specific combination of eight base Illumina P5 and P7 barcodes for subsequent pooling and sequencing. Paired-end sequencing was performed on an Illumina NextSeq 500 using 2 × 38bp reads with no further trimming. Short reads were then mapped to mm10 genome using hisat2 [version2.0.4 (https://ccb.jhu.edu/software/hisat2/manual.shtml)] with–transcriptome-mapping-only–no-discordant options. Unmapped and low quality scoring (MAPQ < 5) reads were removed using samtools. Moreover, duplicated reads were removed using the Picard MarkDuplicates function. Properly paired reads were selected by samtools view -f 0×02 and counted for each gene using htseqcount (version0.6.1) with -s no option and a GTF file from UCSC mm10 refGene downloaded from UCSC table browser (https://genome.ucsc.edu/cgi-bin/hgTables). Genes with a minimum read count of 5 in all replicates of a population (17,535 genes) were retained. A pseudo count of 1 was added and log2-transformed prior to quantile normalization. Quantile-normalized counts were converted back to a linear scale and means of replicates were calculated for each population.
Flow cytometric analysis
For iTreg in culture, fluorescence from the GFP reporter was measured directly, together with viability detection (stained with 10ug/ml DAPI in PBS for 5min immediately prior to analysis). When required, cells were stained with anti-CD45.1 or anti-CD45, -CD4 and TCRβ for 0.5h on ice, incubated overnight in Fixation/Permeabilization buffer (Thermo Fisher), and stained with mAbs detecting transcription factors Foxp3, RORγ and Helios (Biolegend) for 40min on ice. Cells were analyzed with a BD LSRII flow cytometer and data were processed with FlowJo software.
Microscopy
For immunostained cryosections, colons from Foxp3-gfp mice were fixed with 4% paraformaldehyde (PFA) in PBS for 16 hours, equilibrated in 30% sucrose/PBS overnight, embedded in OCT medium (Sakura), and cut into 14um frozen sections using a Leica CM1950 cryostat. Section were washed twice in PBS (20min), blocked with serum (10% in PBS) of the same species as the secondary antibody for 1h at room temperature, rinsed in staining buffer (1% BSA, 0.3% Tween-20 in PBS), and incubated with primary antibodies against Tuj1 (Biolegend), EpCAM (Becton Dickinson), GFP (Abcam), NOS1 (Abcam) and CGRP (Sigma) for 1h at room temperature or overnight at 4°C. Tissue sections were then washed 3 times with staining buffer and incubated with secondary antibody (Cy5, Cy3 or FITC-conjugated donkey anti-rabbit/rat/chicken immunoglobulin) for 1h at room temperature. After washing, sections were mounted with Prolong diamond mounting medium. Images were acquired on a Nikon Ti inverted spinning disk Confocal microscope or a Zeiss LSM700 (63X objective) and processed with Image J (Schindelin et al., 2012). All images are either single planar images or maximum intensity projections of z-stacks, as detailed in the Figure Legends.
To immunostain cultured cells, cells attached to the glass bottom dishes (Cellvis) were fixed with 4% PFA in PBS overnight on ice, blocked with serum (10% in PBS) of the same species as the secondary antibody for 1h at room temperature, rinsed in staining buffer, and incubated with primary antibodies against Tuj1 and EpCAM overnight at 4°C. Cells were then washed 3 times with staining buffer, and incubated with Alexa647-conjugated anti-CD45 (Biolegend) and secondary antibody (FITC-conjugated donkey anti-rabbit or anti-rat immunoglobulin) for 1h at room temperature. After washing, sections were mounted with Prolong mounting medium.
For whole-mount staining, freshly dissected colons were immersed on ice for 20mins in 1uM nicardipine hydrochloride (calcium channel blocker) to maximize smooth muscle relaxation, cut open longitudinally, stretched flat on paper towels, and fixed with 4% PFA overnight on ice. The tissues were bleached by 6% hydrogen peroxide in Methanol in 4°C for 1 hour and blocked by Mouse BD Fc Bloc (0.5ug/ml, BD) and 1% donkey serum in PBSGT (0.5%Triton X-100, 0.2% Gelatin in PBS) for 2 days with shaking in 37°C incubator. After incubating with primary antibodies against Tuj1, HuC/D, EpCAM or F4/80 in PBSGT for 3 days and corresponding secondary anti-Ig antibodies for 2 days in the dark at 37°C incubator with shaking, samples were washed and cleared by BABB (1 volume Benzyl Alcohol to 2 volume Benzyl Benzoate) overnight. Tissues were then laid on slides and sealed with nail polish under a coverslip. Z stack images were acquired on an Olympus Fluoview Confocal microscope (0.5um intervals), and IMARIS software used to construct 3D images, Dot module in IMARIS were used to count neurons and Filament module to measure fiber length.
Fluorescence in situ hybridization
Probe libraries were designed using the Stellaris FISH Probe Designer Software (Biosearch Technologies). 7–15µm thick sections of fixed colon were sectioned onto poly L-lysine coated coverslips and used for smFISH staining. The colonic sections were hybridized with smFISH probe sets according to a previously published protocol (Itzkovitz et al., 2011). Foxp3 probe library was labelled with Cy5, Rorc library was labelled with Alexa594. Anti-Tuj1 primary antibody was added to the smFISH hybridization buffer and Cy2-conjugated Donkey anti rabbit IgG was added in GLOX buffer for 45 minutes after DAPI (Sigma-Aldrich, D9542) nuclear staining. All images were acquired with 100x magnification. Quantification of smFISH was done using Image. 37 cells from 2 mice were quantified. Laplacian of Gaussian filtering was used to enhance mRNA dots.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data were routinely presented as mean ± SD. Unless stated otherwise, significance was assessed by Student’s t-test using GraphPad Prism 8.0.
Supplementary Material
Highlights:
Treg cells in the colon lamina propria reside close to neuron projections
Neurons modulate the differentiation and phenotype of iTreg cells in culture via IL6
Neuron-specific ablation of Il6 increases the number of RORγ+ Treg cells in vivo
Microbial colonization affects a subset of neurons in the enteric nervous system.
ACKNOWLEDGMENTS
We thank Drs. J. Wheaton, A. Munoz-Rojas, M. Ciofani, D. Kasper, J, Huh, for insightful discussions and advice, L. Yang, A. Diallo and C. Yapp (HMS IDAC facility) for help with computational analyses, and A. Jacobson, K. Hattori, N. Lai, A. Louis and S. Soualhi for mice, advice and assistance. This study benefited from data posted at mousebrain.org. This work was supported by grants AI125603 (CBDM), DK110532 (MR), AI30019 and AT009499 (IC) from the NIH, by the JPB Foundation (CBDM), by the Broad-ISF exchange (SI, CBDM) and in part by SRAs from UCB and Evelo Biosciences. DR was supported by the Damon Runyon Cancer Research Foundation (DRG 2300–17, National Mah Jongg League Fellow).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al BS, Dejardin F, Sparwasser T, Berard M, Cerf-Bensussan N, and Eberl G (2019). A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 50, 1276–1288. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van d. V, Deroos P, Liu H, Cross JR, Pfeffer K, Coffer PJ et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, and Kuchroo VK (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238. [DOI] [PubMed] [Google Scholar]
- Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, and Veiga-Fernandes H (2017). Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, and Wahl SM (2003). Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198, 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR et al. (2013). Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PR, Takahashi N, Peiris M, Bertin S, Lee J, Gareau MG, Paniagua A, Harris AR, Herdman DS, Corr M et al. (2015). TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal. Immunol 8, 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx d.C. I et al. (2018). Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175, 400–415. [DOI] [PubMed] [Google Scholar]
- Drokhlyansky E, Smillie CS, Van WN, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T et al. (2020). The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G and Littman DR (2004). Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251. [DOI] [PubMed] [Google Scholar]
- Engel MA, Khalil M, Siklosi N, Mueller-Tribbensee SM, Neuhuber WL, Neurath MF, Becker C, and Reeh PW (2012). Opposite effects of substance P and calcitonin gene-related peptide in oxazolone colitis. Dig. Liver Dis 44, 24–29. [DOI] [PubMed] [Google Scholar]
- Furness JB, Callaghan BP, Rivera LR, and Cho HJ (2014). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med Biol 817, 39–71. [DOI] [PubMed] [Google Scholar]
- Furness JB, Rivera LR, Cho HJ, Bravo DM, and Callaghan B (2013). The gut as a sensory organ. Nat Rev Gastroenterol. Hepatol 10, 729–740. [DOI] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, and Mucida D (2016). Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadient RA and Otten UH (1997). Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog. Neurobiol 52, 379–390. [DOI] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D et al. (2017). Mining the human gut microbiota for immunomodulatory organisms. Cell 168, 928–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL (2015). IL-6 regulation of synaptic function in the CNS. Neuropharmacology 96, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang S, Paik D, Yao L, Kim E, Jamma T, Lu J, Ha S, Nelson BN, Kelly SP, Wu L et al. (2019). Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Chen L, Souto FO, Canasto-Chibuque C, Bongers G, Deshpande M, Harpaz N, Ko HM, Kelley K, Furtado GC et al. (2017). Epithelial-derived IL-33 promotes intestinal tumorigenesis in Apc (Min/+) mice. Sci Rep 7, 5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR and Veiga-Fernandes H (2020). Neuroimmune circuits in inter-organ communication. Nat. Rev. Immunol 20, 217–228. [DOI] [PubMed] [Google Scholar]
- Hunter CA and Jones SA (2015). IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448–457. [DOI] [PubMed] [Google Scholar]
- Ibiza S, Garcia-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ et al. (2016). Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van EJ, Lees J, Jacks T, Clevers H, and van OA (2011). Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol 14, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Golden JP, Wozniak D, Pehek E, Johnson EM Jr., and Milbrandt J (2006). RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J Neurosci 26, 11230–11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, and Rudensky AY (2012). Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NM, He S, Quintana E, Kim YG, Nunez G, and Morrison SJ (2011). Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin. Invest 121, 3398–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N et al. (2017). The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, and Kohler G (1994). Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Ganz J, Bayrer J, Becker L, Bogunovic M, and Rao M (2018). Advances in enteric neurobiology: The “brain” in the gut in health and disease. J Neurosci 38, 9346–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, and Mathis D (2016). Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 44, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Jacobson A, Ma P, Potts DE, Chen Z, Paik D, Soualhi S et al. (2020). Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 180, 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, and Hsieh CS (2011). Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Teige I, Birnir B, and Issazadeh-Navikas S (2006). Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med 12, 518–525. [DOI] [PubMed] [Google Scholar]
- Margolis KG, Gershon MD, and Bogunovic M (2016). Cellular Organization of Neuroimmune Interactions in the Gastrointestinal Tract. Trends Immunol 37, 487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- März P, Cheng JG, Gadient RA, Patterson PH, Stoyan T, Otten U, and Rose-John S (1998). Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci U S A 95, 3251–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T et al. (2014). TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun 5, 3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Savage AK, and Locksley RM (2015). Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 42, 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S et al. (1988). Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience 25, 195–205. [DOI] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D et al. (2014). Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C, Blume J, Roy U, Teh PP, Vasanthakumar A, Beller A, Liao Y, Heinrich F, Arenzana TL, Hackney JA et al. (2019). c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host-microbiota homeostasis. Nat Immunol 20, 471–481. [DOI] [PubMed] [Google Scholar]
- Nutsch K, Chai JN, Ai TL, Russler-Germain E, Feehley T, Nagler CR, and Hsieh CS (2016). Rapid and Efficient Generation of Regulatory T Cells to Commensal Antigens in the Periphery. Cell Rep 17, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M et al. (2015). The microbiota regulates type 2 immunity through RORγ+ T cells. Science 349, 989–993. [DOI] [PubMed] [Google Scholar]
- Panduro M, Benoist C, and Mathis D (2016). Tissue Treg cells. Annu. Rev Immunol 34, 609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peine M, Marek RM, and Lohning M (2016). IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends Immunol 37, 321–333. [DOI] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, and Sandberg R (2014). Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc 9, 171–181. [DOI] [PubMed] [Google Scholar]
- Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR et al. (2018). Blocking neuronal nignaling to immune cells treats streptococcal invasive infection. Cell 173, 1083–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratama A, Schnell A, Mathis D, and Benoist C (2020). Developmental and cellular age direct conversion of CD4+ T cells into RORγ+ or Helios+ colon Treg cells. J Exp. Med 217, e20190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Erta M, Ferrer B, Comes G, Giralt M, and Hidalgo J (2013). Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav. Immun 27, 162–173. [DOI] [PubMed] [Google Scholar]
- Ramanan D, Sefik E, Galvan-Pena S, Wu M, Yang L, Yang Z, Kostic A, Golovkina TV, Kasper DL, Mathis D et al. (2020). An immunologic mode of multigenerational transmission governs a gut Treg setpoint. Cell 181, 1276–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringheim GE, Burgher KL, and Heroux JA (1995). Interleukin-6 mRNA expression by cortical neurons in culture: evidence for neuronal sources of interleukin-6 production in the brain. J Neuroimmunol 63, 113–123. [DOI] [PubMed] [Google Scholar]
- Sang Q and Young HM (1996). Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res 284, 39–53. [DOI] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN et al. (2014). The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J et al. (2015). Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C et al. (2020). Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teratani T, Mikami Y, Nakamoto N, Suzuki T, Harada Y, Okabayashi K, Hagihara Y, Taniki N, Kohno K, Shibata S et al. (2020). The liver-brain-gut neural arc maintains the Treg cell niche in the gut. Nature 585, 591–596. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Washington MK, Kurre U, Singh M, Rula EY, and Emeson RB (2008). Protective roles of alpha-calcitonin and beta-calcitonin gene-related peptide in spontaneous and experimentally induced colitis. Dig. Dis. Sci 53, 229–241. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, and Schutz G (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet 23, 99–103. [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H and Mucida D (2016). Neuro-immune interactions at barrier surfaces. Cell 165, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Lee C, Jeun EJ, Yi J, Kim KS, Ghosh A, Byun S, Lee CG, Kang HJ, Kim GC et al. (2018). Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3(+) regulatory T cells. Sci Immunol 3. [DOI] [PubMed] [Google Scholar]
- Wallrapp A, Burkett PR, Riesenfeld SJ, Kim SJ, Christian E, Abdulnour RE, Thakore PI, Schnell A, Lambden C, Herbst RH et al. (2019). Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 51, 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D et al. (2017). The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton JD, Yeh CH, and Ciofani M (2017). Cutting Edge: c-Maf Is Required for Regulatory T Cells To Adopt RORgammat(+) and Follicular Phenotypes. J Immunol 199, 3931–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R et al. (2011). GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J. Clin. Invest 121, 4503–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Conlin VS, Morampudi V, Ryz NR, Nasser Y, Bhinder G, Bergstrom KS, Yu HB, Waterhouse CC, Buchan AM et al. (2015). Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PLoS One 10, e0125225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, Galan C, Belkaid Y, Bonneau R, and Littman DR (2018). c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, Fohse L, Prinz I, Pezoldt J, Suerbaum S et al. (2016). Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal. Immunol 9, 444–457. [DOI] [PubMed] [Google Scholar]
- Yang NJ and Chiu IM (2017). Bacterial signaling to the nervous system through toxins and metabolites. J Mol Biol 429, 587–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S, Jobin C, Chen ZE, and Zhou L (2017). The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep 21, 2277–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yissachar N, Zhou Y, Ung L, Lai NY, Mohan JF, Ehrlicher A, Weitz DA, Kasper DL, Chiu IM, Mathis D et al. (2017). An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 168, 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BB and Mazmanian SK (2017). The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 46, 910–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La MG et al. (2018). Molecular architecture of the mouse nervous system. Cell 174, 999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y and Hu W (2013). Mouse enteric neuronal cell culture. Methods Mol Biol 1078, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY et al. (2008). TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, and Parada LF (2001). Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev 15, 859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the RNA sequencing data reported in this paper on NCBI GSE164577 (RNA profilings of T cells and enteric neuron in T/neuron cocultures, related to Fig 3 FGH), GSE164576 (RNA profilings of MMP with microbe stimulations, related to Fig 6E) and GSE164575 (RNA profilings of enteric neurons with microbe stimulations, related to Fig 6 FGH).


