Abstract
Introduction:
Colorectal cancer (CRC) is one of the most-deadly malignancies worldwide. Current therapeutic regimens for CRC patients are relatively generic, based primarily on disease type and stage, with little variation. As the field of molecular oncology advances, so too must therapeutic management of CRC. Understanding molecular heterogeneity has led to a new-found promotion for precision therapy in CRC; underlining the diversity of molecularly targeted therapies based on individual tumor characteristics.
Areas covered:
We review current approaches for the treatment of CRC and discuss the potential of precision therapy in advanced CRC. We highlight the utility of the intestinal protein guanylyl cyclase C (GUCY2C), as a multi-purpose biomarker and unique therapeutic target in CRC. Here, we summarize current GUCY2C-targeted approaches for treatment of CRC.
Expert opinion:
The GUCY2C biomarker has multi-faceted utility in medicine. Developmental investment of GUCY2C as a diagnostic and therapeutic biomarker offers a variety of options taking the molecular characteristics of cancer into account. From GUCY2C-targeted therapies, namely cancer vaccines, CAR-T cells, and monoclonal antibodies, to GUCY2C agonists for chemoprevention in those who are at high risk for developing colorectal cancer, the utility of this protein provides many avenues for exploration with significance in the field of precision medicine.
Keywords: Antibody Therapy, Biomarker, Cancer Vaccine, Colorectal Cancer, CAR-T Cell Therapy, Guanylyl Cyclase C, GUCY2C
1. Introduction
Colorectal cancer (CRC) remains the second most deadly cancer and the third most prevalent malignancy worldwide. In 2018, nearly 1.85 million new CRC cases were diagnosed [1], disproportionately affecting developed countries due to lifestyle habits [2]. Moreover, the incidence rate has declined over the past 30 years, which can be positively attributed to improvements in screening and treatment paradigms. However, alarmingly the incidence rates in individuals younger than 50 years have been steadily increasing, with a 2.2% annual overall incidence rate rise between 2012-2016 [3]. While etiology for this paradigm shift is unknown, it is attributed to changes in diet and other lifestyle risk factors [3].
Disease stage remains a defining element for prognosis and therapeutic management of CRC, profoundly affecting survival and therapeutic options [4]. Thus, while a patient in the United States diagnosed with CRC has an average 5-year survival of 64%, stage-specific survival profoundly affects this prognosis. For many localized cases (Stage I and II), surgery is first-line therapy, and resection of the tumor is often successful. Thus, the prognosis is highly positive, with a 5-year survival of 90%, when tumors remain restricted to the intestinal wall [3,5]. However, as the disease progresses to regional lymph nodes and metastasizes to distant organs, 5-year survival is less favorable – reduced to 70% and 14%, respectively. Moreover, despite a seemingly favorable prognosis in patients with localized disease, 30-65% will develop recurrence following resection [6,7]. Patients with ostensibly negative nodal involvement, by histological analysis, have up to a 25% chance of disease recurrence. Additionally, those with stage III diagnoses have up to a 50% chance of recurrence [8]. Thus, to reduce the risk of recurrence, surgical resection is often paired with neoadjuvant chemoradiotherapy which provides pre-operative downsizing with a complete response in approximately 20% of rectal cancers [8-10].The relevance of occult metastases in staging and prognosis is underscored by improved survival, when adjuvant chemotherapy is used for CRC patients with Stage III disease, and a subset of Stage II disease, as established by the MOSAIC [11] and NSABP C-07 trials [11,12]. Unfortunately, 25% of patients present with metastatic disease upon diagnosis and, therefore, are not candidates for surgical resection [6,7]. In patients where surgery is not an option, chemotherapy and radiotherapy are the chief strategies for controlling the growth and spread of CRC. Yet, despite advancements in medical treatments, the prognosis for patients with advanced CRC remains poor.
The current lack of effective therapies and non-specific cytotoxic limitations have led investigators to transition towards the development of predictive, preventative, and personalized medicine strategies to improve the treatment of CRC [1]. Targeted therapies, also referred to as precision medicine, interfere with cancer-specific molecules at a cellular level, working to slow or stop disease progression [13,14]. Rather than the generalized killing of replicating cells, targeted therapies are designed to selectively target unique biological features, exclusive to cancer cells, that inhibit growth, proliferation, or differentiation [15]. Precision therapy is characterized by treatment modalities tailored to target cancer-specific biomarkers [16]. Further, companion diagnostics are tools used to identify the population that will benefit from precision therapy, based on unique cancer-specific molecular characteristics, often by immunohistochemistry or next-generation sequencing [16]. While promising, only a few CRC-related pathways have been successfully exploited for CRC treatment—monoclonal antibodies targeting anti-epithelial growth factor (EGFR) and anti-vascular endothelial growth factor (VEGF) and most recently BRAF and HER-2 [17-22]. Furthermore, a small proportion of colorectal tumors harbor microsatellite instability (MSI) or deficient mismatch repair (dMMR) status, and respond well to treatment with checkpoint inhibitor-based immunotherapies [23-25].
Although a variety of molecular biomarkers (ALK [26-28], BRAF [14,21,29-33], HER2 [14,22,34-36], ROS1[28,37,38], and MSI status[23,24,39-41], KRAS/NRA [14,23,29,42-45]) have been identified in colorectal tumors, an abundantly expressed protein that can be used as both a diagnostic biomarker and a therapeutic target has yet to be fully clinically investigated. Guanylyl cyclase C (GUCY2C), a well-known CRC target antigen, is overexpressed in nearly 95% of colorectal tumors, as well as a subset of gastroesophageal and pancreatic tumors, and currently is being pursued as a novel target in precision therapy [46,47].
2. Metastatic Colorectal Cancer (mCRC)
The tandard of care for metastatic colorectal cancer (mCRC) is either a cytotoxic single-agent or multiple-agent chemotherapy for colon cancer patients, or a combination of chemoradiation therapy for rectal cancer patients [14,48]. Indeed, improvements in outcome for metastatic colorectal cancer (mCRC) have been achieved through both the advancements in surgical techniques which have enabled the resection of isolated metastases [9,10,49-51], as well as, adjuvant chemotherapy regimens[11,12,52,53]. Currently, the main chemotherapy agents used are fluoropyrimidine (intravenous or oral)-based as either single-agent treatments, such as intravenous 5-fluorouracil (5-FU) and oral capecitabine (CAP) or as multiple-agent regimens, including the combinations FOLFOX (5-FU and oxaliplatin), FOLFIRI (5-FU and irinotecan), XELOX/CAPOX (CAP and oxaliplatin), CAPIRI (CAP and irinotecan) [14,54-56]. FOLFOX and FOLFIRI combinations are the primary choices for first-line treatments in CRC, however, this is not because single-agent strategies have been proven inferior, nor is it based on cancer-specific qualifiers [57,58]. Rather, the regimen chosen for CRC patients to minimize adverse effects and achieve the maximum period of disease control, is determined by a patient’s overall performance and risk of deterioration [57]. In fact, efficacy appears similar between additive agents with the only differences being drug-related toxicities [59,60]. Thus, balancing drug tolerability with toxicity is the main criteria for chemotherapy selection and unfortunately, while chemotherapy has provided a higher overall survival time of up to 20 months, mCRC patients are rarely cured by this one-size-fits-all method [59-64].
Beyond traditional chemotherapy, the EGFR-targeted agents,cetuximab and panitumumab, are FDA-approved for first-line treatment of mCRC [65-69]. These anti-EGFR monoclonal antibodies interfere with EGFR signaling, blocking aberrant tumor cell proliferation, and effectively inducing cell apoptosis [70]. Similar efficacy was demonstrated between the two, with non-inferior overall survival benefits in the phase III ASPECCT trial [71]. However, as a fully-humanized monoclonal antibody, panitumumab has fewer side-effects compared with cetuximab [72]. Overall, combinations of cetuximab with FOLFIRI or panitumumab with FOLFOX have significantly enhanced therapeutic efficacy, not only improving overall survival but also reducing CRC disease progression [65-67].
Additional targeted therapies in mCRC include bevacizumab, an anti-VEGF monoclonal antibody thath is FDA-approved for first-line treatment of mCRC in combination with chemotherapy [73]. This anti-angiogenic agent enhances the efficacy of chemotherapy delivery, by specifically targeting VEGF proteins thereby preventing blood vessel formation, in an attempt to break down the complex tumor vasculature created by blood vessel networks [13]. This was demonstrated in the phase III NO16966 trial, where an increase in progression-free survival and overall survival was observed when bevacizumab was added to chemotherapy regimens XELOX or FOLFOX4 [74]. Thus, in the adjuvant setting, VEGF-targeted agents have proven to be successful. however, the efficacy of bevacizumab remains dependent on chemotherapeutic regimens and inconsistent outcomes have been reported in various studies. [53]. While optimization in first-line treatment may be necessary, VEGF-targeted therapy also has been effective in second-line treatment for recurrent CRC demonstrated by the phase III E3200 trial [75,76], in which the addition of bevacizumab to FOLFOX4 improved patient survival time [53,74,76,77].
Overall, the efficacy of precision medicine for CRC patients is reliant on the identification of highly expressed cancer-associated molecular biomarkers [78], which guide the appropriate targeted therapy selection through genetic testing. Notably, the use of an extended RAS panel (KRAS, NRAS) [42,43], predictive for successful EGFR treatment, has impacted tumor response and overall survival in patients with wild-type tumors, as validated by both CRYSTAL [68] and PRIME [45] studies. Tumors with RAS wild-type oncogenes significantly benefit fromx the addition of cetuximab, while treatment in tumors with RAS mutation was highly ineffective [44]/ Thus, considering that 35-45% of CRCs possess mutations in RAS oncogenes, the status of RAS is a critical predictive marker to inform on treatment and management of CRC [78,79].
On the other hand, a mutation in BRAF (V600E) is a poor prognostic factor associated with significantly reduced survival in colorectal cancer patients [31]. Identification of BRAF mutations often prompts clinicians to choose a triplet cytotoxic chemotherapy regimen (FOLFOXIRI) with bevacizumab demonstrating modest improvement of survival [80,81]. More recently, precision medicine approaches improved survival in BRAF-mutated patients in the second-line setting. The combination of encorafenib (BRAFV600E inhibitor) and cetuximab improved outcomes (survival and quality of life) and has been FDA-approved for this indication [21,33]. However, BRAF mutations are only identified in ~10% of CRC cases [32].
MSI-H or dMMR status remains an area of intense research owing to its association with Lynch syndrome. Tumors deficient in MMR, including colorectal cancer, respond to anti-PD-1 immunotherapy with pembrolizumab [82,83], leading to its tumor-agnostic approval by the FDA. The Phase III Keynote-177 study compared outcomes of frontline pembrolizumab vs standard chemotherapy, which resulted in the doubling of progression-free survival with a median duration of response not reached vs. 10.7 months with chemotherapy [23]. However, only ~5% of metastatic CRC patients harbor MSI-H or dMMR tumors, limiting the application of this precision medicine approach [39].
Due to this molecular heterogeneity of CRC, nearly half of all CRCs are unresponsive to targeted therapies. Therefore, it stands to reason that a universally expressed molecular marker in CRC would provide superior therapy to a greater population of CRC patients. Not only are predictive biomarkers necessary for the selection of therapy, but they are essential for early detection, prognostic stratification, and tumor surveillance [84].
3. Guanylyl Cyclase C in Colorectal Cancer
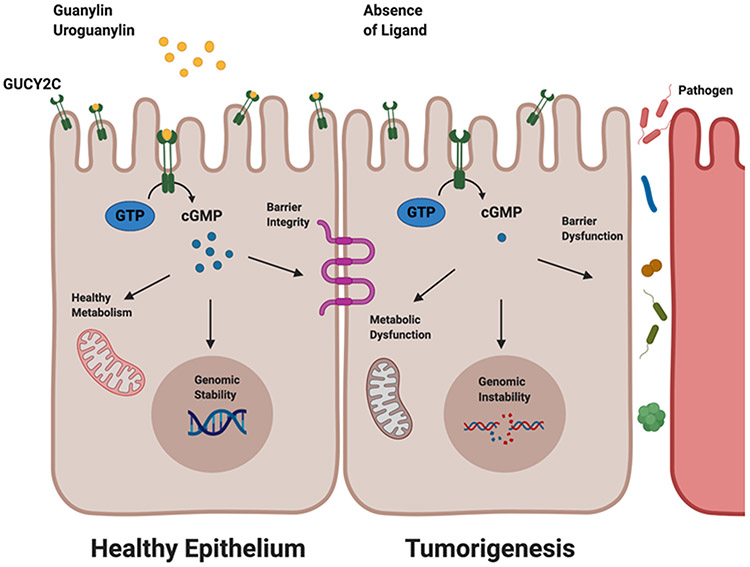
GUCY2C belongs to a family of mucosal cyclase receptors and is selectively expressed in intestinal epithelial cells [85-87]. It is a transmembrane receptor for the endogenous hormonal ligands:guanylin and uroguanylin. Ligand binding to the extracellular receptor catalyzes the conversion of GTP into cyclic GMP (cGMP) and initiates downstream cGMP-related signaling pathways, ultimately leading to the regulation of intestinal homeostatic processes such as epithelial cell proliferation, differentiation, and apoptosis [85,86,88]. Given the major role of GUCY2C in maintaining epithelial regeneration, which renews every 2-3 days, dysregulation of the GUCY2C-cGMP axis promotes pathologies that include inflammatory bowel disease and bowel transit disorder, in addition to colorectal cancer [89,90]. Importantly, silencing the GUCY2C signaling axis is associated with colorectal tumorigenesis through the loss of ligand binding [91-94]. Congruently, guanylin and uroguanylin are well-supported as a few of the most commonly silenced genes in colorectal carcinoma [91-93]. Thus, the canonical GUCY2C-cGMP signaling axis functions as a tumor suppressor only in the presence of its ligand [89]. Furthermore, at the earliest stages of tumor initiation, loss of ligand occurs universally in mice and humans, which subsequently results in the silencing of GUCY2C signaling and promotion of intestinal transformation [89,91,92,95-97]. Notably, while ligand loss occurs in the early stage of tumor initiation, GUCY2C expression is preserved throughout every stage of neoplastic transformation, from polyp to adenocarcinoma [47,87,94,98,99] (Figure 1).
Figure 1: GUCY2C downstream signaling pathway.
In the presence of ligands, guanylin and uroguanylin, the GUCY2C receptor converts GTP into cGMP maintaining healthy mitochondrial function, genomic stability, and epithelial barrier integrity. In the absence of ligands, GUCY2C silencing causes downstream metabolic dysfunction, genomic instability, and barrier dysfunction; Created with BioRender.com
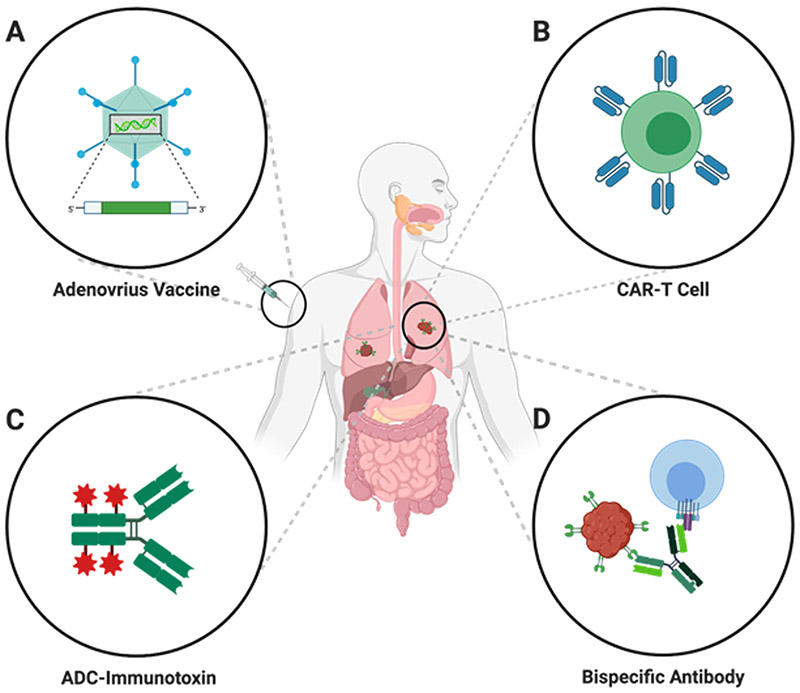
The endogenous expression pattern of the mucosa-specific antigen is perhaps what makes GUCY2C unique. Indeed, GUCY2C is found on the mucosal lining throughout the entire length of the gastrointestinal tract, from duodenum to rectum [46,100]. However unlike other markers, such as HER2 and CEA, GUCY2C is absent from all other extraintestinal tissues, except for the brain, where it plays a role in regulating satiety [46,101-106]. Moreover, endogenous intestinal GUCY2C expression is secluded to the apical side of the epithelial brush border facing the lumen, which is protected from systemic T cell-mediated immune responses, ostensibly removing the threat of autoimmunity in response to certain targeted-therapies [46,86,107]. Similarly, endogenous GUCY2C expression in the brain may be protected from antigen-specific immune responses by the blood-brain barrier [46]. In contrast to the normal intestine, colorectal cancer metastases emerge from intestinal epithelial mucosa into systemic compartments, such as lung and liver tissue, which thus allows immune cell accessibility. Importantly, during tumorigenesis cells undergo a loss of apical-basolateral polarization which leads to GUCY2C expression on the cancer cell surface [108,109]. Compartment-specific immune responses have been studied extensively in HIV vaccine development, which hasdemonstrated that while a response to immunization may be detected in systemic compartments such as the blood or spleen, effectively no response can be detected in mucosal tissues, like the intestine [110,111]. This suggests that certain mucosal antigens, like GUCY2C, which is restricted to the lumen by tight junctions of the epithelial wall, may not produce autoimmunity in response to mucosal antigen-targeted therapies [46,112]. Further, reduced tolerance caused by limited exposure of certain mucosal antigens, such as GUCY2C, to the systemic compartment may provide an opportunity to manufacture a stronger immune response to these tumor antigens [46,112]. Overexpression in cancer and decreased risk of autoimmunity are distinct qualities that provide an ability to induce powerful immune responses, identifying GUCY2C as a novel candidate for numerous targeted precision therapies (Figure 2).
Figure 2: GUCY2C as a target for precision therapy in the treatment of colorectal cancer.
CAR – chimeric antigen receptor; ADC – Antibody Drug Conjugate; Created with BioRender.com
4. GUCY2C Precision Therapy for Metastatic Colorectal Cancer
4.1. GUCY2C-Targeted Cancer Vaccines
Various types of therapeutic cancer vaccines have been investigated for the treatment of colorectal cancer. However, no such vaccines have been approved for clinical treatment [113]. The basic tenet of cancer vaccine therapy is to stimulate the immune system in response to a cancer-specific antigen, destroying existing cancer cells and maintaining long-term memory to protect against possible recurrence [114]. However, the task of identifying suitable targets along with employing a mechanism that can elicit an effective immune response remains a challenge [115,116].
GUCY2C-targeted cancer vaccines offer a unique clinical approach, compared to other vaccines tested in CRC. Most CRC vaccine studies to date have been tested in patients with advanced (stage IV) CRC, and have yielded disappointing results (<1% response rate) [117]. In contrast, the focus of GUCY2C cancer vaccines is to immunize patients with minimum residual disease (pN0) colon cancer and protect against possible recurrence [118-120].
The Ad5-hGUCY2C-PADRE cancer vaccine is comprised of a recombinant, replication-deficient adenovirus serotype 5 (Ad5) encoding the human GUCY2C extracellular domain (Ad5-hGUCY2C) fused to the universal CD4+ T-helper cell epitope, Pan DR Epitope (PADRE) [108,121,122]. The inclusion of the T-helper epitope Pan DR Epitope (PADRE) in clinical studies, is a direct response to a “split” tolerance observed in preclinical studies [118,122,123]. Murine studies containing an Ad5-GUCY2C murine analog, but lacking the T-helper epitope, effectively eliminated colon cancer cells expressing GUCY2C from liver and lung metastases without toxicity [121,124]. However, compared with Gucy2c−/− mice, which were capable of producing strong responses from CD4+ T cells, CD8+ T cells, and B cells, Gucy2c+/+ mice produced only moderate CD8+ T cell responses [121,122]. While GUCY2C-specific CD8+ T cells and B cells were not directly impacted by self-tolerance, they were indirectly affected by the loss of CD4+ T helper cell function, which bolsters CD8+ T cell and B cell responses [122]. Further, CD4+ T cell elimination is likely a reflection of tolerance to self-antigens. Thus, the “split” tolerance in which CD8+ T and B cell functionality is preserved creates a paradigm in which CD8+ T cells and B cells can be fully activated by GUCY2C-independent CD4+ T cell help [118,122,123]. Indeed, vaccine modification with the GUCY2C-independent PADRE antigen, known to elicit immunogenic T helper responses, restored CD4+ T cell, CD8+ T cell, and memory immunogenic responses without autoimmunity in mice [122,123,125].
A phase I clinical trial evaluated safety and immunological efficacy of the first-in-man GUCY2C-based cancer vaccine, Ad5-hGUCY2C-PADRE, in early-stage colorectal cancer patients (NCT01972737) [118]. Results of the phase I clinical trial demonstrated GUCY2C immunogenicity and safety. Moreover, GUCY2C “split” tolerance observed in mice was recapitulated in human studies; GUCY2C-specific T cell responses were restricted to cytotoxic CD8+ T cells and not CD4-helper T cells. Notably, the study called attention to the pre-existing titers of Ad5 neutralizing antibodies (NAbs) within patients, which correlated with the failure to produce GUCY2C-specific immunity post-vaccination [118,126]. As a solution to Ad5 seroprevalence, a chimeric adenoviral vector (Ad5.F35) was designed, which resisted neutralization associated with pre-existing Ad5 immunity in mice [126]. The Ad5.F35 vector utilizes the adenovirus capsid from serotype 5 (Ad5) and fiber from the rare serotype 35 (F35) [126]. Overall, the results from this Phase I clinical trial suggested that the cancer vaccine Ad5-GUCY2C-PADRE was safe and effective at inducing GUCY2C-specific CD8+ T cell responses in early-stage colorectal cancer patients [118].
4.2. GUCY2C-Targeted Chimeric Antigen Receptor T cells
Chimeric antigen receptor (CAR)-T cells are a type of adoptive cell therapy (ACT) first traced over thirty years ago. In CAR-T cell therapy, a single chain chimeric receptor is engineered with the extracellular antibody domain together with intracellular T cell motifs [127-129]. The CAR design takes advantage of monoclonal antibodies antigen-binding capacity and the lytic ability of T cells [129]. The intracellular T-cell portion contains motifs required for downstream T cell signaling, conferring T cell activation independent of MHC/HLA T cell molecules [121,128,129]. Thus, T lymphocytes are manipulated to express the uniquely designed CAR receptor on their surface, expanded ex vivo, and therapeutically re-infused into the patient [128,129]. Recently, cancer research using this sophisticated technology has been revitalized [130], arguably triggered by the FDA approval of the first CAR-T cell therapy in 2017 treating patients with refractory B cell malignancies [131]. CAR-T cells have a powerful ability to induce durable antitumor responses in treatment-refractory metastases that are often highly vascularized and bulky masses [46,132]. However, although there are nearly twenty active clinical trials to date employing CAR-T cell treatments in CRC, success has been elusive [133].
Toxicity has been one of the most significant challenges with CAR-T cell therapy across all disease targets. The most common toxicity induced by CAR-T cells is a systemic inflammatory response known as cytokine release syndrome (CRS) [101,134-138]. CRS is triggered by overstimulation from cytokines that are released by CAR-T cell recognition of target antigen [132]. It is likely that bystander immune cells, such as macrophages, are subsequently activated by CAR-T cells to release inflammatory cytokines, overstimulating the inflammatory response [139]. While CRS pathophysiology can be mild in some cases, such as loss of appetite or fatigue, early recognition and prompt treatment can prevent severe cases that lead to multiorgan failures [140,141]. Indeed, like CRS, other toxicities occur from CAR-T cell treatments, including neurotoxicity syndrome, macrophage activation syndrome, and tumor lysis syndrome.. In addition, on-target off-tumor antigen toxicity; a phenomenon caused by CAR-T cells responding to target antigens expressed on normal tissues. In patients treated with anti-HER2-targeted CAR-T cells, off-tumor detection of endogenous HER2 in lung epithelium led to complications of acute respiratory failure; a recurrent theme in CART-T cell studies [34,101,103]. This inadvertently reinforces the importance of selecting a target antigen that is sequestered from the systemic immune response, to circumvent these dangerous toxicities [133].
Given its restricted expression among endogenous tissues and universal expression in CRC, GUCY2C has been investigated as a target for CAR-T cell therapy. Pre-clinical studies demonstrated the ability of GUCY2C CAR-T cells to eliminate CRC cells without inducing colitis, likely due to the presence of anatomical tight junctions sequestering endogenous intestinal GUCY2C to the luminal surface and acting as a barrier to immune cell accessibility [127]. In this study, animals treated with control CAR succumbed to disease within 50 days post-treatment; whereas the majority of animals treated with GUCY2C-specific CAR-T cells, survived about 100 days and 25% survived at least 200 days [127]. Importantly, safety was demonstrated in a syngeneic model, where mouse CAR-T cells directed to the murine GUCY2C homolog produced no toxicity in the intestinal epithelium of immunodeficient mice; moreover, GUCY2C-CAR T cell treatment provided mice with reduced morbidity and increased the chance of survival, compared to control [127]. In addition, GUCY2C CAR-T cells were absent from intestinal tissues upon histopathological examination supporting the concept of mucosal antigen protection. Likewise, extra-intestinal tissue demonstrated no evident immune-mediated damage in this study [127].
The efficacy of a human-specific GUCY2C construct (known as 5F9.m28BBz) was subsequently designed and evaluated against lung and peritoneal metastases in mice [109]. Here, a syngeneic tumor model for CRC lung metastases was employed to evaluate GUCY2C-targeted murine CAR-T cell anti-tumor responses. In this study, GUCY2C CAR-T, but not control, cells eradicated lung metastases and produced long-term protection against re-challenge with cancer cells [109]. Further, GUCY2C-specific CAR-T cells eliminated peritoneal metastases established by human colorectal cancer cells with endogenously expressed GUCY2C [109]. Overall, these studies established the power of GUCY2C CAR-T cells to eliminate human cancer metastases providing long-term survival, without the on-target/off-tumor toxicities characteristic of other prominent antigens being studied as targets for CAR-T cell therapy.
Despite previous challenges in CAR-T cell therapy, the immune-protected mucosal antigen, GUCY2C, may expand the reach of precision therapy to provide a tremendous therapeutic opportunity for mCRC patients. GUCY2C-targeted CAR-T cell therapy has the potential to safely and effectively redirect the patients’ immune system to overcome tumor cell immune-evasion [142].
4.3. GUCY2C-Targeted Therapeutic Antibodies
Therapeutic antibody targeting has brought forward a new generation of cancer treatments in which therapies are highly targeted and tumor-specific. Modifications of antibody domains have broadened the potential for these therapies, by the inclusion of toxins or recruitment of immune cells in the therapeutic design.
Antibody-drug conjugates (ADC) offer a more focused alternative to traditional chemotherapies, which broadly expose cancer and healthy cells to non-specific cytotoxic agents [143]. ADCs are monoclonal antibodies possessing a targeting domain that confers binding specificity as well as a “carrier” domain purposed with holding cytotoxic agents [143,144]. Thus, ADCs combine the potency of cytotoxic small molecules, with the antigen specificity of monoclonal antibodies to offer a potentially more tolerable and efficacious treatment paradigm for colorectal cancer.
GUCY2C-targeted ADCs offer a uniquely potent possibility for precision-targeted therapy [145]. In addition to its restricted endogenous expression to insulated compartments, GUCY2C is expressed in high numbers in colorectal cancer metastases which is a major requirement for inducing the tumor-suppressive function of ADCs [143,144,146]. Moreover, preclinical studies testing GUCY2C-ADCs demonstrated the ability of GUCY2C-ADCs to be rapidly internalized into the lysosome of colorectal cancer cells [147,148]. Thus, GUCY2C may be an amenable target for the delivery of ADC immunotoxins through innate internalization mechanisms [148].
A phase I clinical trial examined the ability of a GUCY2C-targeted ADC TAK-264 to deliver antitumor efficacy to patients with gastrointestinal malignancies [NCT01577758] [149]. TAK-264 consists of a human IgG1 monoclonal antibody to GUCY2C conjugated using a linker molecule to the cytotoxic agent monomethyl auristatin E (MMAE) [145]. Though results were preliminary, the ADC reportedly had a manageable safety profile; however, neutropenia was experienced by four out of nineteen patients likely caused by MMAE cytotoxicity [149]. While the results for colorectal patients required better elucidation, phase II clinical trials were initiated for gastric [NCT02202759] and pancreatic [NCT02202785] cancer patients [149]. These trials were subsequently terminated after failure to meet stage I efficacy and the clinical development program for the drug was discontinued [150,151]. As a follow up, a phase I clinical trial was recently initiated, to evaluate the potential of second-generation TAK-164 for therapeutic efficacy in gastrointestinal malignancies [NCT03449030]. Similar to the previous generation, this novel ADC is a fully human antibody to GUCY2C, however, a peptide linker is utilized for conjugation to DGN549, a highly cytotoxic DNA alkylator [152]. Based on preclinical study data evaluating TAK-164 in primary human xenograft models of metastatic CRC, the novel ADC is expected to produce highly selective binding and tumor uptake, with a positive therapeutic response that has a tight correlation with GUCY2C expression. TAK-164 is poised for a first-in-man phase I clinical trial, evaluating safety and tolerability with preliminary efficacy in participants with CRC or GUCY2C-positive GI cancers [NCT03449030] [149,152,153].
Bispecific antibody therapy is similar to ADCs, except the cytotoxicity is derived from re-directing conventional T cells by a CD3 bispecific antibody [154]. CD3 bispecific antibodies (bsAb) are well-established in the clinic for the treatment of hematologic malignancies with over 25 clinically approved bsAb therapeutics [155]. However, the attempt to translate this model to solid tumors has been clinically ineffective thus far, given the limitations caused by normal tissue expression of many prominent target antigens [155]. Preclinical studies employing PF-07062119, a CD3 bispecific antibody to GUCY2C (anti-GUCY2C/anti-CD3 bispecific), support the recognition of GUCY2C as a target antigen. Indeed, evaluation with human xenograft tumor models demonstrated the highly selective therapeutic efficacy of PF-07062119 exclusively in GUCY2C-expressing colorectal cancer tumors regardless of KRAS/BRAF mutation status; thus, further validating antigen-restricted expression to the apical intestinal epithelium [156]. Interestingly, when combined with either PD1/PDL1 or anti-angiogenic targets, the activity of PF-07062119 was enhanced with manageable toxicity in cynomolgus monkeys [156]. An ongoing phase I clinical will evaluate the clinical tolerability and safety of PF-07062119 when combined with either anti-PD1 or anti-VEGF agents in patients with advanced gastrointestinal cancer [NCT04171141].
5. GUCY2C: A Powerful Molecular Biomarker
The scalability of GUCY2C as a biomarker for precision medicine is not only in a therapeutic sense but also as a diagnostic, predictive, and prognostic application for disease detection and management [4,47,86,157]. Not only does GUCY2C presence in CRC tumors inform treatment eligibility, but also characterizes disease stage when detected outside of extra-intestinal tissues, providing more sensitive and accurate predictive and prognostic elements regarding the scope of disease [46,86,158].
GUCY2C retention through all stages of CRC transformation makes it a highly suitable prognostic biomarker in mCRC staging and management. GUCY2C has been identified as a marker for recurrent CRC by mRNA detection in ostensibly negative lymph nodes [8,159]. Multiple studies have recapitulated the utility of GUCY2C RT-qPCR as a tool to determine disease prognosis. A prospective study identified its association with the risk of recurrence by comparing GUCY2C and standard histopathological analysis. In a population of 250 CRC patients with stage 0-II tumors histologically identified lymph node-negative (pN0), RT-qPCR revealed that over 85% of cases harbored GUCY2C positive occult metastases [158,160]. Interestingly, GUCY2C molecular staging indicated that occult metastases with GUCY2C detection, had an increased risk of recurrence. Under-staging remains an unfortunate risk due to insensitive standard staging methods, reliant on histopathological evaluation, in which occult lymph node metastases may be missed. Indeed, 25% of cases diagnosed as lacking nodal involvement (pN0) by histopathological evaluation may result in recurrence [8]. Taken together, these data suggest that GUCY2C biomarker examination may provide a more accurate prognosis than traditional histopathological analysis [8,160]. Moreover, GUCY2C molecular staging has been confirmed across multiple institutions and may represent an improvement over current approaches to staging and treatment of colorectal cancers [161-163].
While the overwhelming majority of colorectal cancers retain GUCY2C expression throughout tumorigenesis, differences in expression for a minority of CRCs have a direct impact on therapeutic utility. Indeed, 95% of colorectal cancers retain GUCY2C expression, particularly those arising through conventional transformation associated with the Adenomatous Polyposis Coli (APC)-B-catenin mutational pathway and microsatellite instability pathway [89,94,164]. However, a minority of CRCs originate from precursor lesions emerging from molecular alterations in the CDX2-BRAF pathway, giving rise to serrated adenomas [94,165,166]. Early events initiating the serrated pathway include mutational alterations in BRAF which is coupled with the silencing of CDX2, a tumor suppressor, and GUCY2C transcription factor [30,94]. Thus, silencing of CDX2 nearly eliminates GUCY2C expression in serrated adenomas. Importantly, the molecular alterations occurring in the serrated pathway are rarely identified in the conventional pathway of neoplasia [164]. Conversely, the conventional pathway of neoplasia hinges on the loss of GUCY2C ligand, guanylin, rather than the GUCY2C transcription factor, CDX2 [8,167]. In turn, loss of GUCY2C ligand silences the GUCY2C receptor and the tumor-suppressive GUCY2C-cGMP signaling axis, thereby creating a niche for tumorigenesis [94,95,98]. The differences in GUCY2C expression in molecularly diverse CRCs, underscores the diagnostic utility of GUCY2C and the need for a GUCY2C companion diagnostic in GUCY2C-directed colorectal cancer treatment.
6. Discussion
Curative therapy has historically been a challenge in colorectal cancer, with standard therapy relying on a non-specific, “one-size-fits-all,” model for disease control. GUCY2C-based precision therapy for colorectal cancer offers a promising new outlook in CRC management through improved therapeutic efficacy and enhanced diagnostic accuracy. In contrast to other target antigens, GUCY2C is distinct due to its near-universal expression in CRC and its restricted pattern of expression in endogenous tissues. Collectively, the use of GUCY2C as a therapeutic target shows great potential in cancer vaccine development, CAR-T cell therapy, and the use of monoclonal antibodies.
7. Expert opinion
A universal challenge in the treatment of cancer remains the identification of attractive therapeutics capable of balancing efficacy and tolerability to confer benefit with minimal discomfort. Moving forward, attention to the highly heterogeneous nature of cancer should strongly influence medical oncology, approaching therapy with a molecular lens. Rather than treating cancer in a generic formulaic fashion, cancer therapy should bebased on its unique molecular mosaic. Targeted and precision-based therapies have conferred many antitumor advantages for CRC patients; however, the pharmaceutical cost of development, as well as cost to the patient insurers has been a limiting factor. If pharmaceutical companies determine that investment costs out-weight the net return, we may see a decline in translational research reaching the clinic. In that context, GUCY2C as a precision molecular target may provide an umbrella of therapeutic utility including cancer vaccines, adoptive transfer therapies, and antibody therapy. Moreover, the use of GUCY2C as a therapeutic target covers nearly all stages of CRC – from occult metastases to highly vascularized tumors (Stage II – Stage IV) [47,87,94,98,99]. Of significance, GUCY2C-based precision therapy is not limited to CRC patients but could be expanded to treat other GUCY2C-expressing tumors such as gastric, esophageal, and pancreatic cancersectopically expressing GUCY2C during carcinogenesis [47,100,156,168]. Additionally, not only does GUCY2C have potential as a therapeutic target, but it can play an important role in diagnostic disease staging and therapeutic selection [157,158,160,169]. While GUCY2C may have a strong clinical impact as a stand-alone biomarker, the inclusion of GUCY2C within a panel of CRC biomarkers has the potential to design an even more powerful clinical tool. The relationship between GUCY2C expression and currently used clinical biomarkers has yet to be elucidated and may be informative in assessing responsiveness to GUCY2C-based as well as other targeted therapies. For example, nearly half of all colorectal cancers are currently unresponsive to targeted therapy, due to the presence of KRAS/NRAS mutations [29,42,45,71,79]; thus, GUCY2C detection may improve the otherwise poor prognosis for these patients. Conversely, detection of BRAF mutations in tumors combined with absence of GUCY2C and CDX2 expression may confer greater specificity for the identification of patients that will in fact benefit from anti-EGFR thearpy.[4,30,94] The development of a companion diagnostic to identify GUCY2C expression will be critical for patient eligibility especially for those cancers where GUCY2C expression may be less compared to CRC [4]. An effective companion diagnostic for GUCY2C-based therapies will require highly specific and sensitive detection, an area that our laboratory has taken a special interest in, to accompany GUCY2C-targeted immunotherapies in the treatment of all GUCY2C-expressing GI cancers [160,161,169]. The success of GUCY2C-targeted therapies will be tested over the coming years as GUCY2C-directed immunotherapies enter clinical testing. Recently, a phase IIa trial testing GUCY2C-based vaccines [NCT04111172] has been initiated and has the potential to impact CRC burden, along with GUCY2C expressing pancreatic, gastric, or esophageal tumors, by preventing advanced disease metastases, reducing the risk of recurrence, and thus, hopefully altering the overall mortality of CRC [118,126]. Moreover, advancements in clinical testing of GUCY2C-specific bsAbs and CAR-T cells will define the ability of GUCY2C-targeted therapeutics to treat patients with advanced malignancies. Results from these studies will be highly informative in evaluating the therapeutic potential and future of GUCY2C-directed therapies [NCT04171141] [156]. Over the next decade, translation of GUCY2C-targeted therapies from bench to clinic should be possible, as we unfold a deeper understanding of tolerance mechanisms, enhancing GUCY2C vaccine efficacy and demonstrate safety and efficacy of GUCY2C-targeted human T cells.
GUCY2C has the potential to not only impact cancer diagnostics and therapeutics but also as a chemo-preventative via GUCY2C agonists. The endogenous ligands (guanylin and uroguanylin), heat-stable enterotoxins, and synthetic peptides (such as linaclotide and plecanatide) activate GUCY2C signaling and increase cGMP production, suppressing tumor formation, and maintaining epithelial homeostasis and fluid secretion [86,170-172]. Synthetic peptides, linaclotide, and plecanatide are FDA-approved to treat chronic idiopathic constipation and constipation-type irritable bowel syndrome, for which these peptides act as endogenous ligands to agonize GUCY2C and induce cGMP [86,170,171,173-177]. In that context, they reduce symptom severity in chronic constipation syndromes and have the potential to prevent cancer development [86,176,177]. Investigative efforts are underway to further elucidate the GUCY2C gut-brain axis and its correlation with obesity and CRC [88,104,106,178]. Pathophysiological exploration into the underlying mechanisms of the GUCY2C hormonal axes may provide significant advancements in reversing obesity and reducing the risk for colorectal cancer [88,104,106,178]. These considerations provide significance in the study and development of a multi-faceted utility for GUCY2C in both prevention and therapy, not only in colorectal cancer but in a wide variety of gastrointestinal diseases.
Article highlights:
The gastrointestinal receptor, guanylyl cyclase c (GUCY2C) has been explored as a reliable diagnostic biomarker and therapeutic target for colorectal cancer.
GUCY2C-based precision therapies overcome some of the molecular heterogeneity of colorectal cancer and provide more targeted approaches to cancer treatment compared to the standard generic approach of chemotherapy.
The unique protective compartmentalization of GUCY2C allows for a variety of GUCY2C-based therapeutic applications, which have demonstrated efficacy without causing systemic toxicity in preclinical models.
The wide number of applications for GUCY2C-based therapy presents options that cover nearly all stages of colorectal cancer, carrying the potential to reduce the risk of recurrence and debulk large metastatic colorectal tumors.
Acknowledgments
Funding
This work was supported in part by the National Institutes of Health (R01 CA204881, R01 CA206026, and P30 CA56036), the Defense Congressionally Directed Medical Research Program W81XWH-17-PRCRP-TTSA, and Targeted Diagnostic & Therapeutics, Inc. to S.A.W. A.E.S received a Research Starter Grant in Translational Medicine and Therapeutics from the PhRMA Foundation and was supported by the Defense Congressionally Directed Medical Research Programs (W81XWH-17-1-0299, W81XWH-19-1-0263, and W81XWH-19-1-0067). S.A.W. and A.E.S. also were supported by a grant from The Courtney Ann Diacont Memorial Foundation. J.C.F. is supported by the Alfred W. and Mignon Dubbs Fellowship Fund and a PhRMA Foundation Pre-Doctoral Fellowship In Pharmacology/Toxicology. M.C. was supported by the NIH institutional award T32 GM008562 for Postdoctoral Training in Clinical Pharmacology. S.A.W. is the Samuel MV Hamilton Professor of Thomas Jefferson University.
Footnotes
Declaration of interests
SAW is the Chair of the Scientific Advisory Board and member of the Board of Directors of, and AES is a consultant for, Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6), 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Sharma R. An examination of colorectal cancer burden by socioeconomic status: evidence from GLOBOCAN 2018. EPMA J. 11(1), 95–117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 70(3), 145–164 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Koncina E, Haan S, Rauh S, Letellier E. Prognostic and predictive molecular biomarkers for colorectal cancer: updates and challenges. Cancers (Basel). 12(2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SEER*Stat Databases: November 2018 Submission [Internet]. Available from: https://seer.cancer.gov/data-software/documentation/seerstat/nov2018/.
- 6.van der Stok EP, Spaander MCW, Grünhagen DJ, Verhoef C, Kuipers EJ. Surveillance after curative treatment for colorectal cancer. Nat. Rev. Clin. Oncol 14(5), 297–315 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci. Rep 6, 29765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldman SA, Hyslop T, Schulz S, et al. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA. 301(7), 745–752 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 394(10207), 1467–1480 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Du D, Su Z, Wang D, Liu W, Wei Z. Optimal Interval to Surgery After Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Systematic Review and Meta-analysis. Clin Colorectal Cancer. 17(1), 13–24 (2018). [DOI] [PubMed] [Google Scholar]
- 11.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol 27(19), 3109–3116 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J. Clin. Oncol 25(16), 2198–2204 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Xie Y-H, Chen Y-X, Fang J-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther 5(1), 22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price TJ, Tang M, Gibbs P, et al. Targeted therapy for metastatic colorectal cancer. Expert Rev Anticancer Ther. 18(10), 991–1006 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 125(23), 4139–4147 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Krzyszczyk P, Acevedo A, Davidoff EJ, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 6(3–4), 79–100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan DLH, Segelov E, Wong RS, et al. Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer. Cochrane Database Syst. Rev 6, CD007047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vecchione L, Jacobs B, Normanno N, Ciardiello F, Tejpar S. EGFR-targeted therapy. Exp. Cell Res 317(19), 2765–2771 (2011). [DOI] [PubMed] [Google Scholar]
- 19.He K, Cui B, Li G, Wang H, Jin K, Teng L. The effect of anti-VEGF drugs (bevacizumab and aflibercept) on the survival of patients with metastatic colorectal cancer (mCRC). Onco. Targets. Ther 5, 59–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat. Rev. Cancer 13(12), 871–882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med 381(17), 1632–1643 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17(6), 738–746 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Andre T, Shiu K-K, Kim TW, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. JCO. 38(18_suppl), LBA4–LBA4 (2020). [Google Scholar]
- 24.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18(9), 1182–1191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenz H-J, Lonardi S, Zagonel V, et al. Nivolumab plus low-dose ipilimumab as first-line therapy in microsatellite instability-high/DNA mismatch repair deficient metastatic colorectal cancer: Clinical update. JCO. 38(4_suppl), 11–11 (2020). [Google Scholar]
- 26.Yakirevich E, Resnick MB, Mangray S, et al. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin. Cancer Res 22(15), 3831–3840 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Cesi G, Philippidou D, Kozar I, et al. A new ALK isoform transported by extracellular vesicles confers drug resistance to melanoma cells. Mol. Cancer 17(1), 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cremolini C, Pietrantonio F. How the lab is changing our view of colorectal cancer. Tumori. 102(6), 541–547 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J. Clin. Oncol 27(35), 5931–5937 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Landau MS, Kuan S-F, Chiosea S, Pai RK. BRAF-mutated microsatellite stable colorectal carcinoma: an aggressive adenocarcinoma with reduced CDX2 and increased cytokeratin 7 immunohistochemical expression. Hum. Pathol 45(8), 1704–1712 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One. 7(10), e47054 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest. Oncol 6(6), 660–667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopetz S, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab with or without binimetinib for BRAF V600E-mutant metastatic colorectal cancer: Quality-of-life results from a randomized, three-arm, phase III study versus the choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC). JCO. 38(4_suppl), 8–8 (2020). [Google Scholar]
- 34.Teng R, Zhao J, Zhao Y, et al. Chimeric Antigen Receptor-modified T Cells Repressed Solid Tumors and Their Relapse in an Established Patient-derived Colon Carcinoma Xenograft Model. J Immunother. 42(2), 33–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh D-Y, Bang Y-J. HER2-targeted therapies - a role beyond breast cancer. Nat. Rev. Clin. Oncol 17(1), 33–48 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Lee MKC, Loree JM. Current and emerging biomarkers in metastatic colorectal cancer. Curr. Oncol 26(Suppl 1), S7–S15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J. Natl. Cancer Inst 109(12) (2017). [DOI] [PubMed] [Google Scholar]
- 38.Guler I, Askan G, Klostergaard J, Sahin IH. Precision medicine for metastatic colorectal cancer: an evolving era. Expert Rev Gastroenterol Hepatol. 13(10), 919–931 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Amonkar M, Lorenzi M, Zhang J, Mehta S, Liaw K-L. Structured literature review (SLR) and meta-analyses of the prevalence of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in gastric, colorectal, and esophageal cancers. JCO. 37(15_suppl), e15074–e15074 (2019). [Google Scholar]
- 40.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol 23(3), 609–618 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N. Engl. J. Med 342(2), 69–77 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Kriegshäuser G, Auner V, Zeillinger R. New and potential clinical applications of KRAS as a cancer biomarker. Expert Opin. Med. Diagn 4(5), 383–395 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Al-Shamsi HO, Alhazzani W, Wolff RA. Extended RAS testing in metastatic colorectal cancer-Refining the predictive molecular biomarkers. J. Gastrointest. Oncol 6(3), 314–321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Cutsem E, Lenz H-J, Köhne C-H, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J. Clin. Oncol 33(7), 692–700 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Douillard J-Y, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med 369(11), 1023–1034 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Snook AE, Magee MS, Waldman SA. GUCY2C-targeted cancer immunotherapy: past, present and future. Immunol. Res 51(2–3), 161–169 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danaee H, Kalebic T, Wyant T, et al. Consistent expression of guanylyl cyclase-C in primary and metastatic gastrointestinal cancers. PLoS One. 12(12), e0189953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel R, Miller K, Jemal A. Colorectal Cancer Facts & Figures 2020-2022 . American Cancer Society, Inc., Atlanta, GA. [Google Scholar]
- 49.Lan Y-T, Chang S-C, Yang S-H, et al. Comparison of clinicopathological characteristics and prognosis between early and late recurrence after curative surgery for colorectal cancer. Am. J. Surg 207(6), 922–930 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Matsuda T, Yamashita K, Hasegawa H, et al. Recent updates in the surgical treatment of colorectal cancer. Ann. Gastroenterol. Surg 2(2), 129–136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow FC-L, Chok KS-H. Colorectal liver metastases: An update on multidisciplinary approach. World J Hepatol. 11(2), 150–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Gramont A, Van Cutsem E, Schmoll H-J, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 13(12), 1225–1233 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol 26(12), 2013–2019 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Comella P, Casaretti R, Sandomenico C, Avallone A, Franco L. Role of oxaliplatin in the treatment of colorectal cancer. Ther Clin Risk Manag. 5(1), 229–238 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br. J. Cancer 102(3), 500–505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol 25 Suppl 3, iii1–9 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 370(9582), 143–152 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 370(9582), 135–142 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J. Clin. Oncol 23(22), 4866–4875 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J. Clin. Oncol 22(2), 229–237 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J. Clin. Oncol 22(11), 2084–2091 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br. J. Cancer 94(6), 798–805 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J. Clin. Oncol 22(1), 23–30 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Grothey A, Sargent D, Goldberg RM, Schmoll H-J. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J. Clin. Oncol 22(7), 1209–1214 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Aranda E, García-Alfonso P, Benavides M, et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: Phase II randomised MACRO2 TTD study. Eur. J. Cancer 101, 263–272 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Douillard J-Y, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol 28(31), 4697–4705 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol 25(7), 1346–1355 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Van Cutsem E, Köhne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med 360(14), 1408–1417 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med 351(4), 337–345 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Mendelsohn J, Prewett M, Rockwell P, Goldstein NI. CCR 20th anniversary commentary: a chimeric antibody, C225, inhibits EGFR activation and tumor growth. Clin. Cancer Res 21(2), 227–229 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 15(6), 569–579 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Fakih M, Vincent M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr. Oncol 17 Suppl 1, S18–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med 350(23), 2335–2342 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Cassidy J, Clarke S, Díaz-Rubio E, et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br. J. Cancer 105(1), 58–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodman A E3200 trial of metastatic colorectal cancer. Oncology Times. 27(12), 17–18 (2005). [Google Scholar]
- 76.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol 25(12), 1539–1544 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J. Clin. Oncol 26(33), 5326–5334 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the american society for clinical pathology, college of american pathologists, association for molecular pathology, and the american society of clinical oncology. J. Clin. Oncol 35(13), 1453–1486 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J. Gastroenterol 18(37), 5171–5180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 16(13), 1306–1315 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med 371(17), 1609–1618 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 357(6349), 409–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med 372(26), 2509–2520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chand M, Keller DS, Mirnezami R, et al. Novel biomarkers for patient stratification in colorectal cancer: A review of definitions, emerging concepts, and data. World J Gastrointest Oncol. 10(7), 145–158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuhn M Molecular physiology of membrane guanylyl cyclase receptors. Physiol. Rev 96(2), 751–804 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Aka AA, Rappaport JA, Pattison AM, Sato T, Snook AE, Waldman SA. Guanylate cyclase C as a target for prevention, detection, and therapy in colorectal cancer. Expert Rev Clin Pharmacol. 10(5), 549–557 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carrithers SL, Barber MT, Biswas S, et al. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc. Natl. Acad. Sci. USA 93(25), 14827–14832 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodríguez A, Gómez-Ambrosi J, Catalán V, et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int. J. Obes 40(9), 1405–1415 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Pattison AM, Barton JR, Entezari AA, et al. Silencing the intestinal GUCY2C tumor suppressor axis requires APC loss of heterozygosity. Cancer Biol. Ther 21(9), 799–805 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rappaport JA, Waldman SA. The Guanylate Cyclase C-cGMP Signaling Axis Opposes Intestinal Epithelial Injury and Neoplasia. Front. Oncol 8, 299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shailubhai K, Yu HH, Karunanandaa K, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 60(18), 5151–5157 (2000). [PubMed] [Google Scholar]
- 92.Steinbrecher KA, Tuohy TM, Heppner Goss K, et al. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem. Biophys. Res. Commun 273(1), 225–230 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Wilson C, Lin JE, Li P, et al. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol. Biomarkers Prev 23(11), 2328–2337 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bashir B, Merlino DJ, Rappaport JA, et al. Silencing the GUCA2A-GUCY2C tumor suppressor axis in CIN, serrated, and MSI colorectal neoplasia. Hum. Pathol 87, 103–114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin JE, Li P, Snook AE, et al. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 138(1), 241–254 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li P, Schulz S, Bombonati A, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 133(2), 599–607 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Li P, Lin JE, Snook AE, Waldman SA. ST-Producing E. coli Oppose Carcinogen-Induced Colorectal Tumorigenesis in Mice. Toxins (Basel). 9(9) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blomain ES, Rappaport JA, Pattison AM, et al. APC-β-catenin-TCF signaling silences the intestinal guanylin-GUCY2C tumor suppressor axis. Cancer Biol. Ther 21(5), 441–451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carrithers SL, Parkinson SJ, Goldstein S, Park P, Robertson DC, Waldman SA. Escherichia coli heat-stable toxin receptors in human colonic tumors. Gastroenterology. 107(6), 1653–1661 (1994). [DOI] [PubMed] [Google Scholar]
- 100.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum. Pathol 36(2), 170–179 (2005). [DOI] [PubMed] [Google Scholar]
- 101.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther 18(4), 843–851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bos R, van Duikeren S, Morreau H, et al. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res. 68(20), 8446–8455 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther 19(3), 620–626 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valentino MA, Lin JE, Snook AE, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J. Clin. Invest 121(9), 3578–3588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gong R, Ding C, Hu J, et al. Role for the membrane receptor guanylyl cyclase-C in attention deficiency and hyperactive behavior. Science. 333(6049), 1642–1646 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Kim GW, Lin JE, Snook AE, et al. Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr. Diabetes 6, e211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am. J. Pathol 171(6), 1847–1858 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Snook AE, Li P, Stafford BJ, et al. Lineage-specific T-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 69(8), 3537–3544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Magee MS, Abraham TS, Baybutt TR, et al. Human GUCY2C-Targeted Chimeric Antigen Receptor (CAR)-Expressing T Cells Eliminate Colorectal Cancer Metastases. Cancer Immunol Res. 6(5), 509–516 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Belyakov IM, Moss B, Strober W, Berzofsky JA. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 96(8), 4512–4517 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belyakov IM, Ahlers JD, Brandwein BY, et al. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Invest 102(12), 2072–2081 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abraham TS, Flickinger JC, Waldman SA, Snook AE. TCR Retrogenic Mice as a Model To Map Self-Tolerance Mechanisms to the Cancer Mucosa Antigen GUCY2C. J. Immunol 202(4), 1301–1310 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wrobel P, Ahmed S. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis. 34(1), 13–25 (2019). [DOI] [PubMed] [Google Scholar]
- 114.Advani S, Kopetz S. Ongoing and future directions in the management of metastatic colorectal cancer: Update on clinical trials. J Surg Oncol. 119(5), 642–652 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Pernot S, Terme M, Voron T, et al. Colorectal cancer and immunity: what we know and perspectives. World J. Gastroenterol 20(14), 3738–3750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carlson RD, Flickinger JC, Snook AE. Talkin’ toxins: from coley’s to modern cancer immunotherapy. Toxins (Basel). 12(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nagorsen D, Thiel E. Clinical and immunologic responses to active specific cancer vaccines in human colorectal cancer. Clin. Cancer Res 12(10), 3064–3069 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Snook AE, Baybutt TR, Xiang B, et al. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. J. Immunother. Cancer 7(1), 104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mosolits S, Ullenhag G, Mellstedt H. Therapeutic vaccination in patients with gastrointestinal malignancies. A review of immunological and clinical results. Ann. Oncol 16(6), 847–862 (2005). [DOI] [PubMed] [Google Scholar]
- 120.Mosolits S, Nilsson B, Mellstedt H. Towards therapeutic vaccines for colorectal carcinoma: a review of clinical trials. Expert Rev. Vaccines 4(3), 329–350 (2005). [DOI] [PubMed] [Google Scholar]
- 121.Snook AE, Stafford BJ, Li P, et al. Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J. Natl. Cancer Inst 100(13), 950–961 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Snook AE, Magee MS, Schulz S, Waldman SA. Selective antigen-specific CD4(+) T-cell, but not CD8(+) T- or B-cell, tolerance corrupts cancer immunotherapy. Eur. J. Immunol 44(7), 1956–1966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Snook AE, Baybutt TR, Hyslop T, Waldman SA. Preclinical Evaluation of a Replication-Deficient Recombinant Adenovirus Serotype 5 Vaccine Expressing Guanylate Cyclase C and the PADRE T-helper Epitope. Hum. Gene Ther. Methods 27(6), 238–250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiang B, Baybutt TR, Berman-Booty L, et al. Prime-Boost Immunization Eliminates Metastatic Colorectal Cancer by Producing High-Avidity Effector CD8+ T Cells. J. Immunol 198(9), 3507–3514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alexander J, Sidney J, Southwood S, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1(9), 751–761 (1994). [DOI] [PubMed] [Google Scholar]
- 126.Flickinger JC, Singh J, Carlson R, et al. Chimeric Ad5.F35 vector evades anti-adenovirus serotype 5 neutralization opposing GUCY2C-targeted antitumor immunity. J. Immunother. Cancer 8(2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Magee MS, Kraft CL, Abraham TS, et al. GUCY2C-directed CAR-T cells oppose colorectal cancer metastases without autoimmunity. Oncoimmunology. 5(10), e1227897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 168(4), 724–740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev 257(1), 107–126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 86(24), 10024–10028 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med 377(26), 2531–2544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 359(6382), 1361–1365 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Sur D, Havasi A, Cainap C, et al. Chimeric Antigen Receptor T-Cell Therapy for Colorectal Cancer. J Clin Med. 9(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 119(12), 2709–2720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 124(2), 188–195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frey N Cytokine release syndrome: Who is at risk and how to treat. Best Pract Res Clin Haematol. 30(4), 336–340 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med 371(16), 1507–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med 45(2), e124–e131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 37 Suppl 1, 48–52 (2019). [DOI] [PubMed] [Google Scholar]
- 140.Le RQ, Li L, Yuan W, et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 23(8), 943–947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med 6(224), 224ra25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baybutt TR, Aka AA, Snook AE. Immunotherapy in colorectal cancer: where are we now? Curr. Colorectal Cancer Rep 13(5), 353–361 (2017). [Google Scholar]
- 143.Nejadmoghaddam M-R, Minai-Tehrani A, Ghahremanzadeh R, Mahmoudi M, Dinarvand R, Zarnani A-H. Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna J Med Biotechnol. 11(1), 3–23 (2019). [PMC free article] [PubMed] [Google Scholar]
- 144.Casi G, Neri D. Antibody-drug conjugates: basic concepts, examples and future perspectives. J. Control. Release 161(2), 422–428 (2012). [DOI] [PubMed] [Google Scholar]
- 145.Almhanna K, Prithviraj GK, Veiby P, Kalebic T. Antibody-drug conjugate directed against the guanylyl cyclase antigen for the treatment of gastrointestinal malignancies. Pharmacol. Ther 170, 8–13 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Kim M, Pyo S, Kang CH, et al. Folate receptor 1 (FOLR1) targeted chimeric antigen receptor (CAR) T cells for the treatment of gastric cancer. PLoS One. 13(6), e0198347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gallery M, Zhang J, Bradley DP, et al. A monomethyl auristatin E-conjugated antibody to guanylyl cyclase C is cytotoxic to target-expressing cells in vitro and in vivo. PLoS One. 13(1), e0191046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marszalowicz GP, Snook AE, Magee MS, Merlino D, Berman-Booty LD, Waldman SA. GUCY2C lysosomotropic endocytosis delivers immunotoxin therapy to metastatic colorectal cancer. Oncotarget. 5(19), 9460–9471 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Almhanna K, Kalebic T, Cruz C, et al. Phase I Study of the Investigational Anti-Guanylyl Cyclase Antibody-Drug Conjugate TAK-264 (MLN0264) in Adult Patients with Advanced Gastrointestinal Malignancies. Clin. Cancer Res 22(20), 5049–5057 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Almhanna K, Miron MLL, Wright D, et al. Phase II study of the antibody-drug conjugate TAK-264 (MLN0264) in patients with metastatic or recurrent adenocarcinoma of the stomach or gastroesophageal junction expressing guanylyl cyclase C. Invest. New Drugs 35(2), 235–241 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Almhanna K, Wright D, Mercade TM, et al. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Invest. New Drugs 35(5), 634–641 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Abu-Yousif AO, Cvet D, Gallery M, et al. Preclinical Antitumor Activity and Biodistribution of a Novel Anti-GCC Antibody-Drug Conjugate in Patient-Derived Xenografts. Mol. Cancer Ther (2020). [DOI] [PubMed] [Google Scholar]
- 153.Bolleddula J, Shah A, Shadid M, Kamali A, Smith MD, Chowdhury SK. Pharmacokinetics and Catabolism of [3H]TAK-164, a Guanylyl Cyclase C Targeted Antibody-Drug Conjugate. Drug Metab. Dispos (2020). [DOI] [PubMed] [Google Scholar]
- 154.Shim H Bispecific Antibodies and Antibody-Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules. 10(3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benonisson H, Altıntaş I, Sluijter M, et al. CD3-Bispecific Antibody Therapy Turns Solid Tumors into Inflammatory Sites but Does Not Install Protective Memory. Mol. Cancer Ther 18(2), 312–322 (2019). [DOI] [PubMed] [Google Scholar]
- 156.Mathur D, Root AR, Bugaj-Gaweda B, et al. A Novel GUCY2C-CD3 T-Cell Engaging Bispecific Construct (PF-07062119) for the Treatment of Gastrointestinal Cancers. Clin. Cancer Res 26(9), 2188–2202 (2020). [DOI] [PubMed] [Google Scholar]
- 157.Mejia A, Schulz S, Hyslop T, Weinberg DS, Waldman SA. GUCY2C reverse transcriptase PCR to stage pN0 colorectal cancer patients. Expert Rev Mol Diagn. 9(8), 777–785 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gong JP, Schulz S, Hyslop T, Waldman SA. GUCY2C molecular staging personalizes colorectal cancer patient management. Biomark Med. 6(3), 339–348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cagir B, Gelmann A, Park J, et al. Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer. Ann. Intern. Med 131(11), 805–812 (1999). [DOI] [PubMed] [Google Scholar]
- 160.Hyslop T, Waldman SA. Molecular staging of node negative patients with colorectal cancer. J. Cancer 4(3), 193–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beaulieu M, Desaulniers M, Bertrand N, et al. Analytical performance of a qRT-PCR assay to detect guanylyl cyclase C in FFPE lymph nodes of patients with colon cancer. Diagn Mol Pathol. 19(1), 20–27 (2010). [DOI] [PubMed] [Google Scholar]
- 162.Sargent DJ, Resnick MB, Meyers MO, et al. Evaluation of guanylyl cyclase C lymph node status for colon cancer staging and prognosis. Ann. Surg. Oncol 18(12), 3261–3270 (2011). [DOI] [PubMed] [Google Scholar]
- 163.Haince J-F, Houde M, Beaudry G, et al. Comparison of histopathology and RT-qPCR amplification of guanylyl cyclase C for detection of colon cancer metastases in lymph nodes. J. Clin. Pathol 63(6), 530–537 (2010). [DOI] [PubMed] [Google Scholar]
- 164.Marisa L, de Reyniès A, Duval A, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med. 10(5), e1001453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Balbinot C, Armant O, Elarouci N, et al. The Cdx2 homeobox gene suppresses intestinal tumorigenesis through non-cell-autonomous mechanisms. J. Exp. Med 215(3), 911–926 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dalerba P, Sahoo D, Paik S, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N. Engl. J. Med 374(3), 211–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cohen MB, Hawkins JA, Witte DP. Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab. Invest 78(1), 101–108 (1998). [PubMed] [Google Scholar]
- 168.Debruyne PR, Witek M, Gong L, et al. Bile acids induce ectopic expression of intestinal guanylyl cyclase C Through nuclear factor-kappaB and Cdx2 in human esophageal cells. Gastroenterology. 130(4), 1191–1206 (2006). [DOI] [PubMed] [Google Scholar]
- 169.Frick GS, Pitari GM, Weinberg DS, Hyslop T, Schulz S, Waldman SA. Guanylyl cyclase C: a molecular marker for staging and postoperative surveillance of patients with colorectal cancer. Expert Rev Mol Diagn. 5(5), 701–713 (2005). [DOI] [PubMed] [Google Scholar]
- 170.Bryant AP, Busby RW, Bartolini WP, et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci. 86(19–20), 760–765 (2010). [DOI] [PubMed] [Google Scholar]
- 171.Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin. Gastroenterol. Hepatol 11(9), 1084–1092.e3; quiz e68 (2013). [DOI] [PubMed] [Google Scholar]
- 172.Blomain ES, Pattison AM, Waldman SA. GUCY2C ligand replacement to prevent colorectal cancer. Cancer Biol. Ther 17(7), 713–718 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shailubhai K, Comiskey S, Foss JA, et al. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig. Dis. Sci 58(9), 2580–2586 (2013). [DOI] [PubMed] [Google Scholar]
- 174.Shailubhai K, Palejwala V, Arjunan KP, et al. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther. 6(4), 213–222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N. Engl. J. Med 365(6), 527–536 (2011). [DOI] [PubMed] [Google Scholar]
- 176.Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am. J. Gastroenterol 107(11), 1714–24; quiz p.1725 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am. J. Gastroenterol 107(11), 1702–1712 (2012). [DOI] [PubMed] [Google Scholar]
- 178.Lin JE, Colon-Gonzalez F, Blomain E, et al. Obesity-Induced Colorectal Cancer Is Driven by Caloric Silencing of the Guanylin-GUCY2C Paracrine Signaling Axis. Cancer Res. 76(2), 339–346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]