Abstract
The life strategy of plants includes their ability to respond quickly at the cellular level to changes in their environment. The use of targeted fluorescent protein probes and imaging of living cells has revealed several rapidly induced organelle responses that create the efficient sub-cellular machinery for maintaining homeostasis in the plant cell. Several organelles, including plastids, mitochondria, and peroxisomes, extend and retract thin tubules that have been named stromules, matrixules, and peroxules, respectively. Here, I combine all these thin tubular forms under the common head of organelle extensions. All extensions change shape continuously and in their elongated form considerably increase organelle outreach into the surrounding cytoplasm. Their pleomorphy reflects their interactions with the dynamic endoplasmic reticulum and cytoskeletal elements. Here, using foundational images and time-lapse movies, and providing salient information on some molecular and biochemically characterized mutants with increased organelle extensions, I draw attention to their common role in maintaining homeostasis in plant cells.
Dynamic tubules extended from different organelles are integral components of the rapid subcellular response machinery involved in maintaining optimal working conditions within plant cells.
Introduction
The concept of an organelle in the eukaryotic cell is linked to functional specificity but does not place emphasis on the form of an organelle. Nevertheless, having been tutored on representative transmission electron microphotographs of ultrathin sections of fixed, dead tissue, often with the advice to “see 2D but think 3D” our minds readily associate each organelle with a typical shape. Interestingly, the shape that our mind conjures does not directly translate into an appreciation of the dynamic behavior and interactivity of different organelles. Recognition of subcellular dynamics started with observations of cytoplasmic streaming in living cells using transmitted light and differential interference contrast (DIC) microscopy. Recordings became possible using time-lapse cine-microphotography and the creation of video rate movies (Wildman et al., 1962; Gunning, 2005). Cell permeable dyes that directly stained or negatively highlighted specific organelles increased our appreciation of the living cell (Quader and Schnepf, 1986; Lichtscheidl and Url, 1990). A significant leap in our knowledge occurred following the cloning of the green fluorescent protein (GFP) and the quick targeting of numerous multicolored genetically encoded fluorescent proteins to specific organelles (Prasher et al., 1992; Haseloff et al., 1997). Imaging of fluorescently highlighted organelles in living plant cells is now considered a routine procedure (Mathur, 2007; Held et al., 2008; Griffiths et al., 2016; Renna et al., 2018).
The endoplasmic reticulum (ER), highlighted using an ER retained mGFP, was the first dynamic organelle to be visualized in living plant cells (Haseloff et al., 1997). The fluorescent highlighting of the plastid stroma was reported soon after (Köhler et al., 1997). The latter investigation revealed an interesting phenomenon wherein plastids sporadically extended and retracted thin tubules. Since the transiently formed tubules that extended into the neighboring cytoplasm had become apparent due to fluorescent highlighting of the stroma the phrase “stroma-filled tubule” was condensed to introduce a new term—“stromule” (Köhler and Hanson, 2000). Publications predating the term had also mentioned tubular extensions from plastids by describing them as “mobile protuberances” (Spencer and Wildman, 1962), “extensions” (Freeman and Duysen, 1975), “chloroplast tubules” (Menzel, 1994), and “stroma-containing protuberances” (Bourett et al., 1999).
Advances
Recognition that despite originating from different organelles under different conditions the transient and sporadic tubular extensions called stromules, peroxules, and matrixules share common features.
While appearing transiently, organelle extensions behave in a manner similar to tubular organelles.
The ER is a key modulator of the shape and dynamic behavior of all tubules.
Organelle extensions do not create an independent functional subunit but enhance the functional capability and exchange efficiency of the parent organelle by increasing its outreach into the cytoplasm.
Formation and dynamic behavior of extensions from different organelles involves similar molecular mechanisms and creates the rapid sub-cellular response machinery for reinstating homeostasis in plant cells following stress.
Soon after stromules had been observed similar tubular extensions were described from peroxisomes in transgenic Arabidopsis thaliana plants expressing peroxisome-targeted fluorescent proteins (Cutler et al., 2000; Jedd and Chua, 2002). Likewise, upon observing fluorescently highlighted mitochondria in mutants in two dynamin-related proteins ARABIDOPSIS DYNAMIN LIKE2A (ADL2A)/DYNAMIN-RELATED PROTEIN3A (DRP3A) and ADL2B/DRP3B, Logan et al. (2004) noticed an unusual mitochondrial morphology, rarely encountered in the wild-type Arabidopsis plants. Mitochondria in both mutants extended thin, several micrometers long protuberances (Logan et al., 2004; Logan, 2006b). Drawing on their similarity to stromules, the tubules extended by peroxisomes and mitochondria were named peroxules and matrixules, respectively (Scott et al., 2007). While the introduction of three new terms clearly differentiated between the tubules by associating them with the organelle from which they arose, the terms also indicated that the formation of transient and dynamic tubules is a common subcellular phenomenon. However, in the years since their introduction researchers have focused on the roles of the individually named extensions but not paid much attention to their common property of being tubular. Stromules have been investigated very thoroughly and been the subject of several concept-building reviews (Köhler and Hanson, 2000; Krenz et al., 2012; Gray, 2013; Schattat et al., 2014; Kumar et al., 2014; Erickson and Schattat, 2018; Hanson and Hines, 2018). Peroxules are still being discovered under different conditions and the information is largely descriptive (Barton et al., 2013, 2014; Mathur, 2009; Mathur et al., 2012, 2018; Gao et al., 2016; Rodriguez-Serrano et al., 2016). Matrixules, as pointed out by Logan et al. (2004) are most noticeable in the adl2a and adl2b mutants and appeared to be due to the constriction of elongated mitochondria. Indeed, matrixules resemble the extensions observed in mitochondria in animal cells that undergo fusion following treatment with cysteine-alkylators such as N-ethylmaleimide (Bowes and Gupta, 2008). Clearly, in both plant and animal cells, these extensions are associated with the continuous fission and fusion of mitochondria.
Tubules extended by other organelles, including the nuclei (Mathur et al., 2012) and vacuoles (Verbelen and Tao, 1998; Reisen et al., 2005; Wiltshire and Collings, 2009) have been reported but there is not much more information on them.
Here, in order to draw attention to the similarities between them I have grouped stromules, peroxules, and matrixules as “organelle extensions.” Despite all three transient extensions forming sporadically, and being tubular and dynamic, the organelles producing them are both functionally and structurally distinct. While plastids and mitochondria have two bounding membranes the peroxisomes have only one. Whereas plastids are directly involved in the harvesting of light energy and carbon fixation, the mitochondria utilize the fixed carbon and maintain the energy flow within a living cell. Peroxisomes largely scavenge the reactive radicals produced by plastids and mitochondria and also help in preventing cellular lipotoxicity through β-oxidation of fatty acids. The paradox posed by different organelles with different functions displaying a similar transient morphological feature raises interesting questions some of which have been addressed here.
Where are the extensions found and how are they different from other organelle forms?
Plant cells elongate/expand in response to turgor pressure, and the cytoplasm, bounded by the plasma membrane, is usually pressed against the relatively rigid cell wall. In a turgid cell, the cytoplasm thus forms a thin cortical sleeve. Dynamic cytoplasmic strands criss-cross the cell and connect the cortical areas. Consequently, at any given time an organelle may be located either in the thin cortical region or be part of a relatively fast-flowing cytoplasmic strand. In most observations involving confocal microscopy organelle extensions are best observed when viewed in the cell cortex from above.
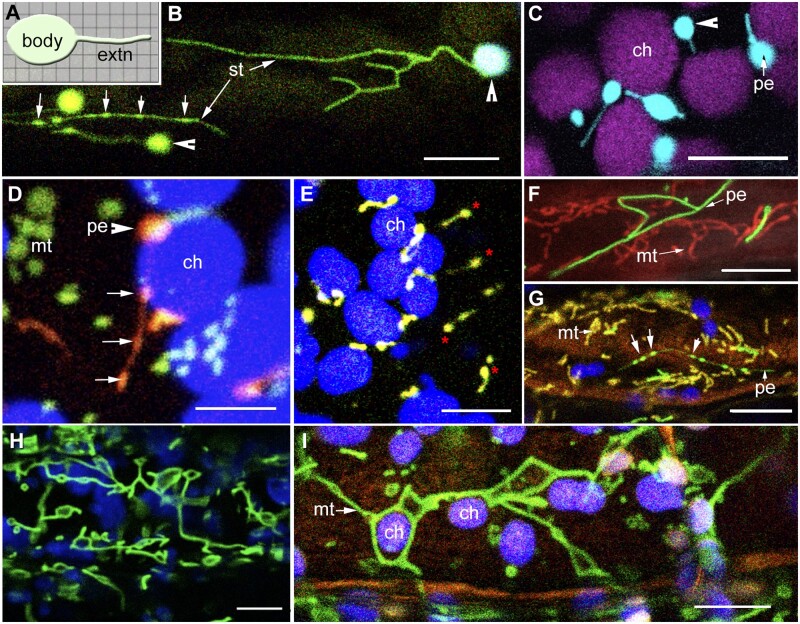
Time-lapse imaging of living plant cells shows that all organelles move around the cell and undulate continuously. They may appear almost spherical or tubular. In a spherical form, an organelle would present a minimal surface area-to-volume ratio while its tubular form would achieve the opposite. While the potential to transform from one form to the other always exists (Figure 1), under normal conditions of growth and development some organelles are observed predominantly in one form.
Figure 1.
Characteristic pleomorphy of organelle extensions and tubular organelles in plant cells. (A) Diagrammatic depiction of an organelle and its tubular extension (extn). The cytoplasm is represented by the grid in the background. Key features involve an independent organelle body from which tubules extend and retract. Cytoplasmic outreach of the organelle increases during tubule extension. (B) Snapshot showing shape diversity displayed by stroma filled tubules (st) extended from plastid bodies (arrowheads) including the presence of dilated regions (arrows) in two stromules (Supplemental Movie S1). (C) Tubular peroxules extended from the peroxisome (pe) body (arrowhead) in the cortical region lying above chloroplasts (ch; Supplemental Movie S2). (D) Snapshot from a time-lapse series showing punctate mitochondria (green; mt), peroxisomes (orange; pe), and chloroplasts (blue; ch). Dilated regions (arrows) appear in an extending peroxule (Supplemental Movie S3). (E) Peroxules (red asterisks) are formed in response to increased ROS in a cell and may be extended by peroxisomes that are not appressed to chloroplasts. (F) Snapshot of abnormally elongated peroxisomes (pe) and mitochondria (mt) resulting from lack of efficient fission in the Arabidopsis apm1/drp3a mutant. (G) Similar to their formation in different organelle extensions, dilations also appear in tubular organelles (large arrows) such as the elongated peroxisomes (green, pe) and mitochondria (yellow, mt) in the Arabidopsis apm1/drp3a mutant. (H) Under hypoxia mitochondria (green) transiently stop dividing and instead expand into assorted shapes. (I) Expanded mitochondria (green, mt) are able to fuse and frequently encompass large organelles like chloroplasts (ch). Scale bars in µm: D = 5; all others =10.
Plastids, known for their functional plasticity display the entire range of forms. In chloroplasts, stacks of membrane thylakoids (grana) are surrounded by the fluid stroma and bounded by the two membranes of the envelope. The grana create a dense, chlorophyll-enriched plastid body and impart a spheroidal form to the chloroplasts. By contrast, the lack of a well-defined body leads to the tubular form of leucoplasts in non-photosynthetic tissue. Intermediate forms are exhibited by the etioplasts and etio-chloroplasts where depending upon the degree of stacking of internal membranes a plastid may appear more rounded or more elongate (Solymosi and Schoefs, 2010). All plastids become completely spherical when they are osmotically compromised.
Peroxisomes are also usually considered as spherical organelles but display an elongate form that undergoes constriction and fission (Sinclair et al., 2009; Barton et al., 2014; Figure 1, C–E). In plant cells, mitochondria are typically considered as oval to sausage-like (Bereiter-Hahn, 1990; Logan and Leaver, 2000) but have also been described as elongate-reticulate in shoot meristems (Seguí-Simarro et al., 2008). However, mitochondrial form is very susceptible to physiological changes in the cell and they have been shown to elongate considerably under sugar starvation (Jaipargas et al., 2015) and to expand into giant flattened discs and interlinked polygons under hypoxic conditions (Van Gestel and Verbelen, 2002; Logan, 2006a; Jaipargas et al., 2015; Figure 1, H). Interestingly, during the interlinking of expanded mitochondria, small cytoplasmic pockets are formed where other organelles may appear entrapped (Figure 1, I).
Where do the organelle extensions fit in within the range of spherical to tubular forms? A generalized depiction presents an extension as a thin tubule protruding from a large independent body (Figure 1, A). Since the tubule extends and retracts, its length may vary considerably. While stromule lengths may sometimes exceed 30 µm, peroxules extending up to 15 µm have also been observed (Sinclair et al., 2009; Barton et al., 2013). Relatively, the matrixules are shorter extensions of 2–5 µm but as thin filamentous structures are still clearly distinguished from the broader mitochondrial body. Occasionally, all organelles exhibit small peripheral globular protuberances (Mulisch and Krupinska, 2013; Yamashita et al., 2016) that do not elongate into a tubule and usually break off as vesicles that move away from the parent organelle. Such globules are not considered as organelle extensions. As depicted in Figure 1, A, I have considered an “organelle extension” to contain the region delimited by a bounding organelle membrane. Tubular regions that stretch between two broader sections of a single organelle are not considered as organelle extensions.
Maintaining a clear definition for an extension is essential for avoiding confusion between an organelle extension and a tubular organelle. Indeed, in coining the term stromule, Köhler and Hanson (2000) considered stromules in freshly formed callus and cell suspension cultures and emphasized the presence of a distinct organelle body. This important parameter differentiated a stromule from a flexible and tubular leucoplast (Figure 1, B). While a distinct chlorophyll-containing plastid body is observed in green cell suspension cultures such as the tobacco (Nicotiana tabacum L.) cv Virginia Bright Italia 0 (VBI-0; Qiao et al., 2010) it is not readily apparent in the commonly used tobacco cv Bright Yellow (BY2) cell cultures, that contain spherical as well as tubular leucoplasts only (Kato et al., 1972; Nagata et al., 1992). It is noteworthy that irrespective of its form a plastid is called a leucoplast if it is devoid of chlorophyll pigmentation. True leucoplasts are found in roots that have developed in the dark in soil-grown plants and do appear spherical at the end of a night period (Barton et al., 2018). Their elongation occurs during the day as photosynthates become available. The flexibility of plastid form becomes very apparent since the change from a tubular to a spherical form in leucoplasts is strongly inhibited if the roots are provided with sucrose exogenously (Schattat et al., 2012b; Barton et al., 2018). Similarly, while plastids in epidermal pavement cells and guard cells in wild-type Arabidopsis and tobacco are chloroplasts, some Arabidopsis mutants lack chlorophyll and therefore should be considered leucoplasts (Barton et al., 2018).
In contrast to stromules, distinguishing between the dynamic peroxules (Figure 1, C) and the tubular peroxisomes appears relatively simple since a clear spherical body is apparent only at the time when peroxules are extended and retracted (Figure 1, D and E). Moreover, while peroxules retract quickly to the main body an elongated tubular form of the peroxisome progressively becomes constricted until it finally undergoes fission and results in more peroxisomes. The case of matrixules requires more consideration since the original observations clearly mention them as being unusual in wild-type plants but a consistent feature of Arabidopsis mutants with impaired mitochondrial fission (Logan et al., 2004; Logan, 2006a, 2006b).
Observations on the different extensions suggest that their transient presence creates an intermediate form that favors transition of a spherical organelle into a tubular form. This reasoning appears plausible since different organelles maintain extensions for varying periods that range from a few seconds in the case of peroxules and matrixules to several minutes or even hours in the case of stromules. Based on personal observations, a chloroplast may continue forming one or more stromules from the plastid body over several hours while remaining localized to a small cortical region. Similarly, a peroxisome may extend and quickly retract a peroxule and repeat this process intermittently for long durations.
While the tubular form confers some advantage to the organelle (discussed in subsequent sections), all extensions display continuous changes in their shapes (Supplementary Movies).
The dynamic behavior of organelle extensions and tubular organelles is similar
Time-lapse imaging shows that the continuous changes in shape of organelle extensions range from linear to curved, branched to polygonal with different angles, and sizes exhibited (Köhler and Hanson, 2000; Sinclair et al., 2009; Schattat et al., 2011a; Barton et al., 2014; Jaipargas et al., 2015; Supplementary Movie S1). When the organelles are a part of the rapidly flowing cytoplasmic strands, a linear form is most common, but the wider range of shape changes occurs when they are located in the cell cortex (Figure 1, A and B). Interestingly, the morphological changes shown by the extensions are similar to the pleomorphy exhibited by elongated mitochondria and leucoplasts (e.g. Figure 1, B and H).
The dynamic shape changes are very apparent in the long stromules formed in Arabidopsis mutants in the ACCUMULATION AND REPLICATION OF CHLOROPLASTS (ARC)5, ARC6, and PARALOG OF ARC6 (PARC6) genes that have aberrant chloroplasts (Holzinger et al., 2008; Fujiwara et al., 2018; Ishikawa et al., 2020). Similar shape changes are also observed in peroxisomes and mitochondria in several Arabidopsis mutants (Table 1) where the fission of these organelles is impaired (Arimura et al., 2004, 2008; Mano et al., 2004; Logan, 2006b, 2010; Zhang and Hu, 2008, 2010). Accordingly, the usually small, spheroidal peroxisomes and mitochondria elongate abnormally to form tubules that may sometimes extend through the entire length of a cell (Figure 1, F and G). While these long tubules now blur the difference between an organelle extension and a tubular organelle their presence once again reinforces the idea that extensions are intermediate forms between the spheroidal and tubular.
Table 1.
Some genes whose expression levels affect organelle extensions and tubular organelles in Arabidopsis thaliana
| Gene | Altered expression level due to | Organelle with major change in morphology | Suggested mechanism | References |
|---|---|---|---|---|
| ARC3, ARC6, ARC5 | Lower expression in mutants | Greatly increased stromules from enlarged chloroplasts | Incomplete chloroplast division apparatus | |
| ATG5 | Mutant. Lower expression | Increased stromule abundance and length during starvation conditions | Autophagosome pathway disrupted; causes stress/loss of membrane sequestration | Ishida et al. (2008) |
| BIGYIN 1 | Mutant | Enlarged mitochondria | Component of the mitochondrial division apparatus, a FIS1 homolog | Logan (2006a); Zhang and Hu (2008) |
| CHUP1 | Overexpression | Ectopic protrusions some of which are stromules | OEM localization. Altered protein:lipid ratio | Oikawa et al. (2008) |
| DRP3A and DRP3B | Mutants | Abnormally longated mitochondria and peroxisomes | Components of mitochondrial and peroxisomal fission machinery | Arimura et al. (2004); Logan et al. (2004); Mano et al. (2004); Fujimoto et al. (2009) |
| DRP5B (ARC5) | Mutation | Clustered peroxisomes showing membrane constriction but impaired division | Involved in the peroxisome and chloroplast division apparatus | Zhang and Hu (2010) |
| FIS1A, FIS1B | FIS1A mutation combined with FIS1B RNAi | Enlarged peroxisomes and mitochondria | Required for peroxisome and mitochondrial division apparatus | Zhang and Hu (2008; 2009) |
| LACS9 | Transient overexpression | Ectopic chloroplast extensions | Plastidial acyl coenzyme A synthetase involved in processing of fatty acids produced by chloroplasts | Schnurr et al. (2002); Breuers et al. (2012) |
| MSL2, MSL3 | Double mutant | Enlarged spherical plastids without stromules but the extensions appear upon plant dehydration. | Osmoregulatory mechanism involving plastid mechanosensitive ion channels | Veley et al. (2012) |
| NMT1/ELM1 | Mutant | Elongated mitochondria with strong dilated regions | Required for localization of a fission associated dynamin-related protein to the mitochondrial division site | Logan (2006a); Arimura et al. (2008) |
| OEP7 | Overexpression | Ectopic chloroplast extensions | OEM localized. Altered protein:lipid ratio | Lee et al. (2001) |
| PARC6 | Mutant | Epidermal plastids abnormally elongated and clustered | Impaired division apparatus | Ishikawa et al. (2020) |
| PEX11C, PEX11D | Overexpression | Elongated peroxisomes | Peroxisome membrane proteins that promote elongation | Lingard and Trelease (2006); Orth et al. (2007) |
| PEX3-1 PEX3-2i | Double knockdown RNAi | Elongated peroxisomes | Required to maintain the structure of the peroxisome. | Nito et al. (2007) |
| PGM1 | Mutant | Elongated chloroplasts with loose grana. Tubular form more apparent during growth under short day conditions. | Defective in sugar—starch metabolism. Accumulate sucrose during diurnal cycle. | Caspar et al. (1985); Schattat et al. (2012a) |
| AtTOC64 | Transient overexpression | Ectopic protrusions from chloroplasts | OEM localized protein. Altered protein:lipid ratio | Breuers et al. (2012) |
Another feature common to all extensions was first described in fluorescently labeled stromules as “packets” of GFP (Pyke and Howells, 2002). This feature involves the sporadic and transient appearance of one to more dilations of different sizes (Figure 1, A, C, and G) along the length of a tubule and often occurs during tubule extension. The dilation may persist for several seconds before its position on the tubule changes or it disappears completely. Dilated regions have now been routinely observed in long peroxules and in tubular peroxisomes and mitochondria in the aberrant peroxisome morphology1 (apm1)/dynamin-related protein3 (drp3)/Arabidopsis dynamin like2 (adl2; Mano et al., 2004; Fujimoto et al., 2009; Barton et al., 2013, 2014; Figure 1, G) and the network mitochondria1 (nmt1)/elongated mitochondria1 (elm1) mutants, respectively (Arimura et al., 2008; Logan et al., 2010).
The similarity in shape changes, including the presence of transient dilations, as features shared between organelle extensions and tubular organelles strongly suggests that all tubular forms might interact with a common subcellular element.
Do organelle extensions and tubular organelles interact with a common subcellular element?
Cytoskeletal elements and motor proteins are recognized as major interactors for a large number of organelles (Jedd and Chua, 2002; Mathur et al., 2002; Doniwa et al., 2007; Kadota et al., 2009; Sparkes et al., 2011). Consequently, investigations focused on the relation between stromules and actin filaments (Kwok and Hanson, 2003, 2004a, 2004b), myosin motors (Natesan et al., 2009; Sattarzadeh et al., 2013), and microtubules (Kwok and Hanson, 2003; Erickson et al., 2018; Kumar et al., 2018). Similarly, correlations between peroxules and the actin cytoskeleton have been presented (Sinclair et al., 2009; Barton et al, 2013, 2014; Mathur et al., 2018). The accumulated information suggests that treatment with cytoskeletal inhibitors rapidly affects the dynamic behavior of the extensions, mainly along cytoplasmic strands. While cytoskeletal elements are considered as major tracks for organelle motility, associated proteins that may directly link the formation as well as the extension–retraction behavior of organelle extensions have not been identified.
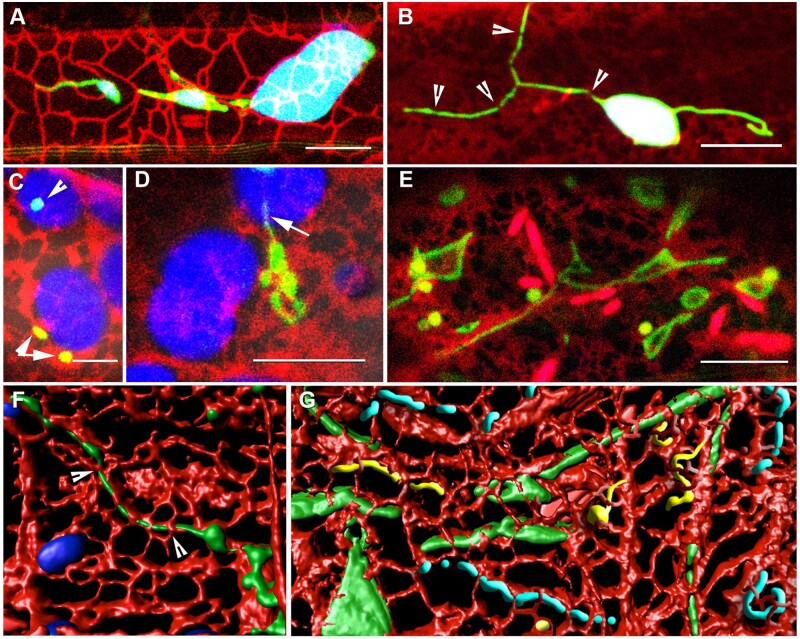
In this context, the ER suggests an interesting alternative. It forms a major membranous interactor within the plant cell and its dynamic behavior is strongly dependent upon the actin–myosin system as well as the microtubules (Sparkes et al., 2011; Hamada et al., 2012, 2014; Griffing et al., 2017). Moreover, chloroplasts, peroxisomes, and mitochondria are all surrounded by the ER (Figure 2, A–E). In the cell cortex, the assorted circular, branched, and looped forms of extensions from independent organelles as well as organelles that are predominantly in a tubular form have been found to align with and change in tandem with cortical ER reorganization (Sinclair et al., 2009; Schattat et al., 2011a, 2011b; Barton et al., 2013; Jaipargas et al., 2015; Figure 2, A–C). Interestingly, peroxisomes that often appear closely appressed to chloroplasts, when co-visualized with an ER probe are found embedded in the peri-plastid ER (Figure 2, C). Moreover, peroxules extend along paths defined by ER tubules that create the loose ER-cage (Figure 2, D). A similar intimate ER–mitochondria relationship has been observed and is responsible for mitochondrial fission (Jaipargas et al., 2015). The link between the state of the ER and mitochondrial morphology is reinforced during hypoxic conditions when the ER cisternae expand and become more prominent compared with the ER tubules. Concomitantly, mitochondrial fission slows down and giant, expanded mitochondria of assorted shapes are formed (Figures 1, H and I, and 2, E).
Figure 2.
The ER plays a major role in modulating the form and behavior of tubules. (A) Plastids surrounded by a loose ER cage (white outlined tubules against chlorophyll [blue] background) and stromules (green) aligned with red fluorescent protein-labeled ER (Supplemental Movie S4). (B) Dilations formed on a stromule due to constrictions (arrowheads) created by the ER mesh. The stromule extended on the other side remains free of constrictions and dilations. (C) Peroxisomes near chloroplasts (arrowheads) are actually embedded in the loose peri-plastidic ER mesh (red) suggesting that peroxisomal response to ROS generated by chloroplasts might be mediated by through the ER. (D) The ER–peroxule relationship illustrated by a peroxule (arrow) extended along an ER tubule (red) that is part of the ER cage around a chloroplast. (E) Expanded ER cisternae (red) and giant mitochondria (green) formed in epidermal cells in 7-d-old Arabidopsis seedlings after being kept in water under a coverslip for 45 min to create hypoxic conditions. (F) 3-D volume rendering of a confocal x, y, z image stack of an elongated peroxisome from the apm1 mutant and the surrounding ER (and blue rendered chloroplasts) suggests that tubular organelles are interspersed through the ER mesh. (G) A composite diagrammatic rendition suggests that tubular extensions from chloroplasts (green), peroxisomes (yellow), and mitochondria (blue) are all enmeshed in the ER (red). The dynamic behavior of the ER is thus responsible for the continuous shape changes of these organelles. Scale bars in µm: A, B, D, and E = 10; C = 5.
The presence of transient dilations has also been explained by the observations that all tubular forms appear to be threaded through the ER-mesh (Schattat et al., 2011b; Barton et al., 2013, 2014; Figure 2, F and G). Consequently, it has been suggested that the changes in the shape and size of the polygons of the ER-mesh that occur during ER reorganization may constrict the threaded tubule at one or more locations. While impeded protein flow within a tubule can create a dilated region subsequent ER reorganization may relieve the constriction completely and allow a tubule to revert to a smooth form. Alternatively, fresh constrictions may quickly alter the size and number of dilated regions in the tubule.
The ER, dependent on cytoskeletal elements for its dynamic behavior may also be the common membrane interactor and regulator of the shape and behavior of all other tubular elements in the cell.
Which mechanisms might be involved in the formation of organelle extensions?
In seeking commonality between the molecular mechanisms involved in the formation of stromules and peroxules, an important role is suggested for membrane localized proteins (Table 1). For example, the plastidial LONG CHAIN ACYL CO-A SYNTHETASE enzyme in Arabidopsis AtLACS9 (Schnurr et al., 2002) has been shown to considerably increase the formation of extensions from plastids (Breuers et al., 2012). Following observations on transient over-expression of AtLACS9, Arabidopsis TRANSLOCON AT THE OUTER MEMBRANE OF CHLOROPLASTS 64-III (AtTOC64-III) and using an inner envelope membrane (IEM) localized Arabidopsis TRANSLOCON AT THE IEM OF CHLOROPLASTS 40 (AtTIC40) fused to GFP as a control, Breuers et al. (2012) suggested that stromules are formed due to a change in the protein-to-lipid ratio in the outer envelope membrane (OEM) of plastids. The idea is appealing and points to a general phenomenon since similar ectopic tubules have been observed upon over-expression of the Arabidopsis OEM-localized CHLOROPLAST UNUSUAL POSITIONING1 (CHUP1; Oikawa et al., 2008; Schmidt von Braun and Schleiff, 2008; Delfosse et al., 2016) and the Arabidopsis OEM PROTEIN7 (OEP7; Lee et al., 2001). While overexpression of OEM proteins does lead to tubular extensions it is worth noting that not all of the extensions qualify as stromules. Many of the tubules are completely devoid of the stroma or the IEM while in some the stroma-filled portion extends only for a portion of the tubule (Delfosse et al., 2016). Similar observations on ectopic OEM extensions have been made for mitochondria and named as mitochondrial outer-membrane protrusions (MOPs; Yamashita et al., 2016).
The idea of the protein:lipid ratio as a major factor in the formation of tubular organelle extensions is also strongly reinforced by observations of increased peroxule formation in response to augmented expression of peroxisomal membrane proteins (PMPs) such as the FISSION1A, FISSION1B, and PEROXIN11 (Kobayashi et al., 2007; Orth et al., 2007; Zhang and Hu, 2008, 2009; Ruberti et al., 2014; Rodriguez-Serrano et al., 2016) and other peroxins (Nito et al., 2007). These PMPs are integral components of the normal peroxisome proliferation machinery and interestingly, the transient appearance of peroxules occurs just prior to the formation and progressive fission of tubular peroxisomes.
A role of fission-associated proteins such as the mechanochemical GTPases DRP3A, DRP3B that form spirals to aid membrane tubulation and constriction (Praefcke and McMahon, 2004; Hoppins et al., 2007) is especially noteworthy. As mentioned earlier, the frequency of both peroxule and matrixule formation increases in the apm1/drp3A and drp3B/adl2A, adl2B mutants (Logan et al., 2004; Logan, 2006a; Barton et al., 2013, 2014) that lack the GTPase activity. Another mechanochemical enzyme, DRP5B/ARC5 is shared between peroxisomes and chloroplasts and affects both organelles during photorespiratory stress (Zhang and Hu, 2010).
The purported role of membrane-associated proteins in creating organelle extensions is also quite appealing since the insertion of a particular protein into a membrane bilayer can alter the regional membrane topography and curvature to produce small protuberances (McMahon and Boucrot, 2015; Jarsch et al., 2016; Bayer and Rosado, 2021). It has been speculated that when juxtaposed to ER membranes, the small protruding regions of an organelle membrane may lead to transient membrane contact sites (MCSs) between the two organelles (Mehrshahi et al., 2014; Michaud and Jouhet, 2019; Petit et al., 2019). Taking the speculation one step further, it is possible that the subsequent ER dynamics can account for the pulling out, stretching, and morphing of the tubular organelle extensions. In such a scenario, a break in connectivity at the MCS would result in organelle separation and an extension snapping back to the parent body.
Proteins that can foster MCS and simultaneously affect the interactions and behavior of two organelles have remained largely unidentified so far. However, a beginning seems to have been made with the demonstration that a GFP-fusion of CHLOROPLAST LOCALIZED LIPASE1 from Brassica napus (BnCLIP1-GFP; Tan et al., 2011) localizes to both the chloroplast and the ER membranes. BnCLIP1 is closely related to PHOSPHOLIPASE A1 (PLA1) proteins (Seo et al., 2009; Tan et al., 2011) and it will be interesting to experimentally test if small, lipase-enriched domains on organelles might be involved in MCS formation. With respect to peroxisomes, it is noteworthy that another lipase, SUGAR-DEPENDENT1 (SDP1) localizes to thin extensions and facilitates intimate connectivity with oil bodies (Thazar-Poulot et al., 2015).
Several putative mitochondrial MCS proteins have been identified (Michaud et al., 2016) and it will be interesting to see whether their overexpression leads to a better understanding of mitochondrial morphology and matrixule formation in relation to the ER.
Does each extension have a unique function, or does it only serve to extend the existing functions of the parent organelle further into the cytoplasm?
While the transient but eye-catching morphology and behavior of tubular extensions allows them to be easily recognized among hundreds of similar organelles, it is important to note that under normal conditions of growth, at any given time, only a small subset of organelles displays extensions. For example, in leaves of Arabidopsis plants grown under short-day conditions only 2%–3% of epidermal pavement cell chloroplasts and less than 1% of mesophyll chloroplasts may actually exhibit stromules at the beginning of the day. While the frequency of chloroplasts extending dynamic stromules increases after several hours of daylight it also increases considerably in excised and injured leaf tissue and under sucrose treatment (Schattat and Klösgen, 2011; Brunkard et al., 2015; Barton et al., 2018). The fact that a majority of chloroplasts do not exhibit stromules (Barton et al., 2018) suggests that the non-extended state represents a basal level of chloroplast functioning while the formation of stromules is a condition-specific response. Nevertheless, numerous speculations have been made about the roles that the transient extensions from a small subset of plastids might play in the cell. Several reviews have already presented these views and carefully dissected the evidence (Gray, 2013; Mathur et al., 2013; Kumar et al., 2014; Schattat et al., 2014; Erickson and Schattat, 2018; Hanson and Hines, 2018). Besides their interactions with the cytoskeleton and the ER described earlier, it has been suggested that stromules interact with peroxisomes and mitochondria (Kwok and Hanson, 2004a, 2004b, 2004c), and the nucleus (Kwok and Hanson, 2004c; Erickson et al., 2017; Kumar et al., 2018). However, reports pointing to stromule interactivity with these organelles as being coincidental and non-specific have also been published (Barton et al., 2018; Ding et al., 2019). To add yet another new facet, a singular set of observations draws attention away from the whole-cell context entirely by demonstrating that stromules can also form in isolated chloroplasts (Brunkard et al., 2015). Notably, this study draws attention to the important role that internal redox generation and its regulation may play in modulating the behavior of chloroplasts (Brunkard et al., 2015).
For peroxules, an increased frequency of formation in a cell has been clearly demonstrated as a response to higher levels of reactive oxygen species (ROS; Sinclair et al., 2009; Barton et al., 2014; Rodriguez-Serrano et al., 2016). A role of peroxules in the quick neutralization of ROS and preventing widespread membrane breakdown is strongly suggested by their acting as interaction platforms for ROS-distressed mitochondria formed during exposure to high intensity light (Jaipargas et al., 2016; Mathur et al., 2018). Again, as with stromules, the peroxules appear only under certain conditions.
Our views on the interactivity between mitochondria, peroxisomes, and chloroplasts are based on observations obtained by using techniques as diverse as transmission electron microscopy (Frederick and Newcomb, 1969) and optical tweezers (Oikawa et al., 2015, 2019; Gao et al., 2016). However, time-lapse imaging of unchallenged, uninjured young seedlings and leaves shows these three organelles moving quite independently of each other in the cell. Increased clustering of the three organelles is observed in senescent tissue and in injured cells but most often such proximity appears coincidental and is not sustained for more than a few seconds (Jaipargas et al., 2016; Barton et al., 2018). In the few instances where it has been possible to observe them simultaneously, chloroplast extensions appear distanced in both space and time from peroxules in their neighborhood.
A perusal of reports on extensions strongly suggests their formation as a conditional response (Table 2). High-light intensity, osmotic stress, and tissue injury caused by abiotic or biotic factors appear to be the leading causes. Notably, all of these conditions trigger rapid changes in cellular ROS levels (Caplan et al., 2008, 2015; Gray et al., 2012; Brunkard et al., 2015; Rodriguez-Serrano et al., 2016; Exposito-Rodriguez et al., 2017; Table 2). A change in cellular ROS levels is also known to rapidly affect ER architecture and dynamics (Margittai et al., 2008; Jaipargas et al., 2016), and upregulate the molecular machinery involved in peroxisome proliferation and mitochondrial fission (Reumann and Bartel, 2016; Costello et al., 2018). As suggested by Jaipargas et al. (2016), the state of the ER might be a major influencer on the dynamic behavior of an organelle extension and in altering the proximity between organelles.
Table 2.
A non-comprehensive list of developmental conditions and exogenous treatments known to affect the formation of organelle extensions
| Treatment or condition | Phenotype | Postulated mechanism | Reference |
|---|---|---|---|
| Related to tissue/organ-specific development/cautious interpretation | |||
| Fresh callus and cell suspensions of Tobacco | Developmental condition: Chloroplast body present. Generally elongated plastids and stromules | High sucrose and phytohormone media. Possible effect on internal membranes in chloroplasts | Köhler and Hanson (2000) |
| In normal, mature leaves mesophyll chloroplasts | Developmental condition: Low stromule abundance | Normal phenomenon compared to epidermal chloroplasts. Plastids are fully differentiated. | Waters et al. (2004) |
| Normal non-photosynthetic cells of epidermis, petals, roots | Developmental condition: High stromule abundance. Stromules may be confused with leucoplasts. | Immature plastids, etioplasts, and non-green plastids are usually elongated. | Holzinger et al. (2007); Köhler et al. (1997); Köhler and Hanson (2000) |
| Ripe fruit (tomato) | Developmental condition: Increased stromule abundance | Mostly leucoplasts and chromoplasts. Might fall under tubular organelles. | Waters et al. (2004) |
| Temperature fluctuation | Undetectable stromules at 10°C, many stromules at 40°C | Higher temperatures are associated with more fluid membranes. | Holzinger et al. (2007) |
| Anoxia for more than 4 h | Elongated and fused mitochondria (Tobacco cell culture) | Mitochondrial fusion as a stress response in order to optimize recombination of mitochondrial DNA. | Van Gestel and Verbelen (2002) |
| Hypoxia resulting from been kept underwater ca. 45 min (Arabidopsis and tobacco tissue) | Elongation of mitochondria followed by their expansion into giant mitochondria | Mitochondrial expansion as a way of dealing with low O2. Could also involve compromised osmoregulation involving increased permeability of mitochondrial envelope membranes | Jaipargas et al. (2015) |
| Vacuum infiltration of 40 mM sucrose or glucose solution into leaves | Increased stromule abundance | Unclear mechanism. Speculated as an osmotic response. | |
| 500 mM mannitol solution (Wheat) | Increased stromules and some filamentous chloroplasts in leaves | Osmotic stress triggers stromule formation. | Gray et al. (2012) |
| ≥ 200 mM potassium chloride (tobacco) | Over 80% of chloroplasts with extended stromules | Salt stress triggers stromule formation. | Gray et al. (2012) |
| 100 µM abscisic acid (tobacco) | Over 80% of plastids with extended stromules | Mimics salt stress and dark growing conditions, dependent on cytosolic protein synthesis. | Gray et al. (2012) |
| 6% polyethylene glycol (tobacco) | Over 80% of plastids emitting stromules | Stimulated drought conditions at the plastid trigger stromules. | Gray et al. (2012) |
| Leaf exposed at room temperature for 4 h (tobacco) | Greater stromule abundance | Desiccation triggers stromule formation. | Gray et al. (2012) |
| Antimycin A (50 and 10 µM) | Extreme, reversible plastid elongation in root cortex | Treatment blocks the mitochondrial bc1 complex in the electron transport chain, triggering the alternative oxidase pathway. Could involve elongated leucoplast. | Itoh et al. (2010) |
| 0.08–0.8 M H2O2 | Significant increase in peroxules | Hydroxyl stress increases subcellular demand for peroxisomal catalase. Peroxules appear before the formation of completely tubular peroxisomes that undergo fission. | Sinclair et al. (2009) |
| 15–90 s UV-A treatment | Elongated peroxisomes | Produces subcellular H2O2, increases subcellular demand for peroxisomal catalase. Leads to peroxisomal fission. | Sinclair et al. (2009) |
| Cells undergoing arbuscule formation during mycorrhizae colonization (tobacco) | Increased localization of chloroplasts to the nucleus and the formation of stromules | Cell invasion triggers ROS accumulation and alters cellular sugar status, peroxisomes may respond. | Fester and Hause (2005); Fester et al. (2001) |
| 100 µM s-triazine | Results in elongated mitochondria | Produces superoxide by blocking the chloroplast electron transport chain at photosystem II. | Scott and Logan (2008); Bliek (2009) |
| Abutilon mosaic virus infected cells | Increased stromule formation | A combination of ROS-induced responses that affect chloroplast envelope | Krenz et al. (2012) |
| Viral/bacterial infection of N. benthamiana leaves |
Induced stromules that aggregated around the nucleus; Replicated upon salicylic acid and H2O2 treatment |
Implicate pro-defence molecules and innate immunity signaling pathways | Caplan et al. (2015) |
| 100 µM methyl viologen | Triggers mitochondrial elongation | Produces superoxide radicals that can trigger apoptosis. | Scott and Logan (2008); Bliek (2009) |
| 1-Aminocyclopropane-1-carboxylic acid (ACC) | Increased stromule abundance | Mimics pathogen attack conditions when the cell produces ethylene (ACC is the first precursor in the committed ethylene synthesis pathway). | Gray et al. (2012) |
| 1 µM silver nitrate | Decreases stromule abundance | Inhibits ethylene activity. May also link to ROS management. | Gray et al. (2012) |
| Mechanical wounding. Agrobacterium infiltration | Increased stromule abundance in cells near wound site | Altered ROS and sugar status in cell | Schattat et al. (2012b); Mathur (unpublished data) |
| Treatment with photosynthetic electron transport chain (pETC) inhibitors | Increased stromule frequency in chloroplasts | Implicate internal light-sensitive redox signaling pathways | Brunkard et al. (2015) |
| Isolated chloroplasts | Independent chloroplasts can form stromules | Internal redox signals suggested | Brunkard et al. (2015) |
| Senescent cotyledon and leaf tissue | Increased stromules and peroxules | Altered permeability of organelle membranes and major changes in ROS perception | Mathur (unpublished data) |
| Exposure to high light conditions | Increased peroxules and breakdown of mitochondria | Typical response to increased ROS levels in cell | Jaipargas et al. (2016); Mathur et al. (2018) |
Could organelle extensions achieve a common objective within the cell?
My grouping of stromules, peroxules, and matrixules under the common head of “organelle extensions” has been due to their tubular form and the fact that they all transiently extend a portion of an organelle into the cytoplasm. Their formation conforms to a fundamental biological pattern that repeatedly uses thin tubules to maximize the surface area and volume ratio of a body (Purves et al., 2001; Hamilton et al., 2007). Consequently, a tubular extension does not alter the primary function of an organelle but greatly augments an organelle’s efficiency by the increased outreach and bidirectional exchange capability. As discussed in the preceding sections, the extensions from different organelles have strong similarities. Two common features still need to be addressed; one, the need for increased exchange and two, the transient nature of all extensions.
While innumerable ionic and metabolic exchanges may occur simultaneously in a plant cell, a very simplified example is presented to illustrate the need for increased exchange. In the form of chloroplasts, plastids carry out photosynthesis, fix carbon, and produce basic sugar and fatty acid molecules. For photosynthesis to continue unimpeded, the primary products need to be removed from the chloroplast and specific ions, such as inorganic phosphate (Pi) to be replenished (Heldt et al., 1977; Iglesias et al., 1993). This is achieved by a variety of plastid envelope-localized transporters including specific antiporters that allow triose sugars to be exchanged for Pi (Facchinelli and Weber, 2011; Li et al., 2016). Based on observations (Brunkard et al., 2015; Barton et al., 2018), the large number of chloroplasts without any extensions at the beginning of a day suggests that at that time the basal level exchange is quite sufficient. However, as photosynthesis starts and continues during the day there is a gradual build-up of primary metabolites that necessitate increasingly efficient exchange between the chloroplast and the cytoplasm (Iglesias et al., 1993). A concomitant increase in stromule formation occurs (Schattat et al., 2012b; Brunkard et al., 2015). As stromules extend they also increase the cytoplasmic outreach of the stochastically dispersed transporters on the plastid envelope (Delfosse et al., 2018). It thus appears plausible to suggest that exchange efficiency between the cytosol and the plastid is increased due to the presence of the extensions. By contrast, heterotrophic plastids such as leucoplasts import the sugars and lipids from the surrounding cytoplasm and subsequently, after due processing some metabolites may be exported (Neuhaus and Emes, 2000). Again, the need to exchange primary metabolites from aerial, photosynthetic regions increases as they become available to the sink tissue. The tubular form may thus be expected to facilitate efficient exchange.
Another exchange process that is extremely important for cell survival involves the plastids and mitochondria as the major sources of ROS production in plant cells and the peroxisomes as the major scavengers of ROS (Corpas et al., 2017; Sies et al., 2017). The reactive species are known to diffuse rapidly over short distances and if not removed quickly can cause extensive damage to cellular membranes (Foyer and Noctor, 2003). Based on the reports, it appears likely that the formation of thin peroxules within seconds of exposure to ROS facilitates the up-take and neutralization of these cytotoxic radicals (Sinclair et al., 2009; Barton et al., 2013, 2014; Jaipargas et al, 2016).
It is tempting to link organelle extensions to retrograde signaling and response but the rapidity of their formation by only a small subset of organelles and the transient nature of the phenomenon do not suggest an adequate time frame for the complete molecular machinery to become involved. However, as observed during peroxisomal response to ROS (Sinclair et al., 2009), when the ROS stress persists peroxule formation progresses to a complete tubulation of the organelle. The tubular peroxisome ultimately undergoes fission and increases the peroxisomal population in a cell. The repetitiveness and persistence of a particular stress may thus be the most important factor in invoking retrograde signaling pathways.
Whereas a number of questions remain unanswered (see box—“Outstanding questions”), the transient nature of organelle extensions underscores their role in re-establishing an optimal physiological state within a cell. Clearly, the fluctuations in the cellular milieu created due to increased production of a specific metabolite or the depletion of a key resource create subtle imbalances that require quick correction before any negative effects get amplified. Stress, which I loosely consider as “SomeThing Requiring an Effective Solution Soon,” needs a boosted exchange efficiency. However, once an optimal state has been reinstated, the cell goes back to its normal functioning. Based on the high frequency of their formation under stress I suggest that all extensions transiently increase the exchange efficiency of their parent organelle and thus subscribe to the common goal of maintaining homeostasis in the plant cell.
Outstanding questions
Why is global stress perceived by only a few organelles in a cell? What determines localized responses at the sub-cellular level?
What distinguishes where a tubular extension is formed in a larger organelle body?
Considering that all organelles are surrounded by the ER, do the different organelles ever interact directly? Would organelle interactions not always involve ER mediation?
Considering that the ER is a common interactor, could there be a common set of ER-membrane-resident proteins that allow MCS formation with functionally diverse organelles?
Considering the concept of the protein:lipid ratio of an organelle membrane playing a pivotal role in the formation of extensions, what is the precise contribution of the lipids?
What role do diurnal fluctuations in cytosolic sugar levels play in regulating the formation of the different organelle extensions?
Accession numbers
Genes/proteins mentioned: AtCHUP1—AT3G25690; ADL2A/DRP3A—AT4G33650; ADL2B/DRP3B—AT2G14120; ATG5—AT5G17290; AtLACS9—AT1g77590; AtTOC64 III—AT3g17970; AtTIC40—AT5g16620; ARC3—AT1G75010; ARC5/DRP5B—AT3G19720; ARC6—AT5G42480; ELM1/NMT1—AT5G22350; FIS1A/BIGYIN—AT3g57090; FIS1B—AT5G12390; MSL2—AT5G10490; MSL3—AT1g58200; OEP7—AT3G52420; PARC6—AT3G19180; PEX3—AT1G48635; PEX11C—AT1G01820; PEX11D—AT2G45740; PGM1—AT5G51820; SDP1—AT5G04040.
Supplemental data
Supplemental Movie S1. The typical behavior of plastid extensions.
Supplemental Movie S2. Dynamic behavior of peroxisome extensions.
Supplemental Movie S3. Proximity and suggested interactivity of organelles.
Supplemental Movie S4. Correlated dynamics of a plastid-extensions and the neighboring ER.
Supplementary Material
Acknowledgments
Helpful discussions with my laboratory members are acknowledged.
Funding
Work in the Mathur lab is funded by the Natural Science and Engineering Research Council (NSERC), Canada.
Conflict of interest statement. None declared.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys) is: Jaideep Mathur (jmathur@uoguelph.ca).
References
- Arimura S, Aida GP, Fujimoto M, Nakazono M, Tsutsumi N (2004) Arabidopsis dynamin-like protein 2a (ADL2a), like ADL2b, is involved in plant mitochondrial division. Plant Cell Physiol 45:236–242 [DOI] [PubMed] [Google Scholar]
- Arimura S, Fujimoto M, Doniwa Y, Kadoya N, Nakazono M, Sakamoto W, Tsutsumi N (2008) Arabidopsis ELONGATED MITOCHONDRIA1 is required for localization of DYNAMIN-RELATED PROTEIN3A to mitochondrial fission sites. Plant Cell 20:1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KA, Mathur N, Mathur J (2013) Simultaneous live-imaging of peroxisomes and the ER in plant cells suggests contiguity but no luminal continuity between the two organelles. Front Physiol 4:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KA, Jaipargas EA, Griffiths N, Mathur J (2014) Live-imaging of peroxisomes and peroxules in plants. In Brocard C, Hartig, eds A, Molecular Machines Involved in Peroxisome Biogenesis and Maintenance. Springer-Verlag, Wien, pp. 233–253 [Google Scholar]
- Barton KA, Wozny MR, Mathur N, Jaipargas EA, Mathur J (2018) Chloroplast behaviour and interactions with other organelles in Arabidopsis thaliana pavement cells. J Cell Sci 131:jcs202275. [DOI] [PubMed] [Google Scholar]
- Bayer E, Rosado A (2021) Geometry and cellular function of organelle membrane interfaces. Plant Physiol 185: 650–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J (1990) Behaviour of mitochondria in the living cell. Int Rev Cytol 122:1–63 [DOI] [PubMed] [Google Scholar]
- Bliek AM (2009) Fussy mitochondria fuse in response to stress. EMBO J 28:1533–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourett TM, Czymmek KJ, Howard RJ (1999) Ultrastructure of chloroplast protuberances in rice leaves preserved by high-pressure freezing. Planta 208:472–479 [Google Scholar]
- Bowes T, Gupta RS (2008) Novel mitochondrial extensions provide evidence for a link between microtubule-directed movement and mitochondrial fission. Biochem Biophys Res Commun 376(1):40–405. doi: 10.1016/j.bbrc.2008.08.120. [DOI] [PubMed] [Google Scholar]
- Breuers FK, Bräutigam A, Geimer S, Welzel UY, Stefano G, Renna L, Brandizzi F, Weber AP (2012) Dynamic remodeling of the plastid envelope membranes—a tool for chloroplast envelope in vivo localizations. Front Plant Sci 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkard JO, Runkel AM, Zambryski PC (2015) Chloroplasts extend stromules independently and in response to internal redox signals. Proc Natl Acad Sci U S A 112:10044–10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, Mamillapalli P, Burch-Smith TM, Czymmek K, Dinesh-Kumar SP (2008) Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 132:449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, Kumar AS,, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP (2015) Chloroplast stromules function during innate immunity. Dev Cell 34:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in a chloroplast phosphoglucomutase activity. Plant Physiol 79:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ,, Barroso JB, Palma JM, Rodriguez-Ruiz M (2017) Plant peroxisomes: a nitro-oxidative cocktail. Redox Biol 11:535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello JL, Passmore JB, Islinger M, Michael Schrader M (2018) Multi-localized proteins: the peroxisome–mitochondria connection. In del Río LD, Schrader M eds, Subcellular Biochemistry Series 89. Proteomics of peroxisomes: identifying novel functions and regulatory networks, Springer Nature Singapore Pte Ltd., pp.383–415. Available from: 10.1007/978-981-13-2233-4_17 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci U S A 97:3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse K, Wozny MR, Jaipargas EA, Barton KA, Anderson C, Mathur J (2016) Fluorescent protein aided insights on plastids and their extensions: a critical appraisal. Front Plant Sci 6:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse K, Wozny MR, Barton KA, Mathur N, Griffiths N, Mathur J (2018) Plastid envelope-localized proteins exhibit a stochastic spatiotemporal relationship to stromules. Front Plant Sci 9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Jimenez-Gongora T, Krenz B, Lozano-Duran R. (2019) Chloroplast clustering around the nucleus is a general response to pathogen perception in Nicotiana benthamiana. Mol Plant Pathol 20:1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniwa Y, Arimura S, Tsutsumi N (2007) Mitochondria use actin filaments as rails for fast translocation in Arabidopsis and tobacco cells. Plant Biotechnol 24:441–447 [Google Scholar]
- Erickson JL, Kantek M, Schattat MH (2017) Plastid–nucleus distance alters the behavior of stromules. Front Plant Sci 8:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JL, Adlung N, Lampe C, Bonas U, Schattat MH (2018) The Xanthomonas effector XopL uncovers the role of microtubules in stromule extension and dynamics in Nicotiana benthamiana. Plant J 93:856–870 [DOI] [PubMed] [Google Scholar]
- Erickson JL, Schattat MH (2018) Shaping plastid stromules—principles of in vitro membrane tubulation applied in planta. Curr Opin Plant Biol 46:48–54 [DOI] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM (2017) Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat Commun 8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchinelli F, Weber APM (2011) The metabolite transporters of the plastid envelope: an update. Front Plant Sci 2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester T, Hause G (2005) Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15:373–379 [DOI] [PubMed] [Google Scholar]
- Fester T, Strack D, Hause B (2001) Reorganization of tobacco root plastids during arbuscule development. Planta 213:864–868 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364 [Google Scholar]
- Frederick SE, Newcomb EH (1969) Microbody-like organelles in leaf cells. Science 163:1353–1355 [DOI] [PubMed] [Google Scholar]
- Freeman TP, Duysen ME (1975) The effect of imposed water stress on the development and ultrastructure of wheat chloroplasts. Protoplasma 83:131–145 [Google Scholar]
- Fujimoto M, Arimura S, Mano S, Kondo M, Saito C, Ueda T, Nakazono M, Nakano A, Nishimura M, Tsutsumi N (2009) Arabidopsis dynamin-related proteins DRP3A and DRP3B are functionally redundant in mitochondrial fission but have distinct roles in peroxisomal fission. Plant J 58:388–400 [DOI] [PubMed] [Google Scholar]
- Fujiwara MT, Yasuzawa M, Kojo KH, Niwa Y, Abe T, Yoshida S, Takeshi N, Itoh RD (2018) The Arabidopsis arc5 and arc6 mutations differentially affect plastid morphology in pavement and guard cells in the leaf epidermis. PLoS ONE 13:e0192380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Metz J, Teanby NA, Ward AD, Botchway SW, Coles B, Pollard MR, Sparkes I (2016) In vivo quantification of peroxisome tethering to chloroplasts in tobacco epidermal cells using optical tweezers. Plant Physiol 170:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, Hansen MR, Shaw DJ, Graham K, Dale R, Smallman P, Natesan SA, Newell CA (2012) Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J 69:387–398 [DOI] [PubMed] [Google Scholar]
- Gray JC (2013) Stromule formation. In Biswal B, Krupinska K, Biswal UC eds, Plastid Development in Leaves During Growth and Senescence, Springer Science Business Media Dordrecht, pp. 169–186 [Google Scholar]
- Griffing LR, Lin C, Perico C, White RR, Sparkes I (2017) Plant ER geometry and dynamics: biophysical and cytoskeletal control during growth and biotic response. Protoplasma 254:43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths N, Jaipargas EA, Wozny MR, Barton KA, Mathur N, Delfosse K, Mathur J (2016) Photo-convertible fluorescent proteins as tools for fresh insights on subcellular interactions in plants. J Microsc 263:148–157 [DOI] [PubMed] [Google Scholar]
- Gunning BES (2005) Plastid stromules: video microscopy of their out-growth, retraction, tensioning, anchoring, branching, bridging, and tip-shedding. Protoplasma 225:33–42 [DOI] [PubMed] [Google Scholar]
- Hamada T, Tominaga M, Fukaya T, Nakamura M, Nakano A, Watanabe Y, Hashimoto T, Baskin TI (2012) RNA processing bodies, peroxisomes, Golgi bodies, mitochondria, and endoplasmic reticulum tubule junctions frequently pause at cortical microtubules. Plant Cell Physiol 53:699–708 [DOI] [PubMed] [Google Scholar]
- Hamada T, Ueda H, Kawase T, Hara-Nishimura I (2014) Microtubules contribute to tubule elongation and anchoring of endoplasmic reticulum, resulting in high network complexity in Arabidopsis. Plant Physiol 166:1869–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Kerr MC, Burrage K, Teasdale RD (2007) Analyzing real-time video microscopy: the dynamics and geometry of vesicles and tubules in endocytosis. Curr Protoc Cell Biol 4.16. doi. 10.1002/0471143030.cb0416s35 [DOI] [PubMed] [Google Scholar]
- Hanson MR, Hines KM (2018) Stromules: probing formation and function. Plant Physiol 176:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci U S A 94:2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held MA, Boulaflous A, Brandizzi F (2008) Advances in fluorescent protein-based imaging for the analysis of plant endomembranes. Plant Physiol 147:1469–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt HW, Chon CJ, Maronde D (1977) Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol 59:1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Buchner O, Lütz C, Hanson MR (2007) Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma 230:23–30 [DOI] [PubMed] [Google Scholar]
- Holzinger A, Kwok EY, Hanson MR (2008) Effects of arc3, arc5 and arc6 mutations on plastid morphology and stromule formation in green and nongreen tissues of Arabidopsis thaliana. Photochem Photobiol 84:1324–1335 [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J (2007) The machines that divide and fuse mitochondria. Annu Rev Biochem 76:751–780 [DOI] [PubMed] [Google Scholar]
- Iglesias AA, Plaxton WC, Podestá FE (1993) The role of inorganic phosphate in the regulation of C4 photosynthesis. Photosynth Res 35:205–211 [DOI] [PubMed] [Google Scholar]
- Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, Ohsumi Y, Hanson MR, Mae T. (2008) Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol 148(1):142–55. doi: 10.1104/pp.108.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Yasuzawa M, Koike N, Sanjaya A, Moriyama S, Nishizawa A, Matsuoka K, Sasaki S, Kazama Y, Hayashi Y, et al. (2020) Arabidopsis PARC6 is critical for plastid morphogenesis in pavement, trichome, and guard cells in leaf epidermis. Front Plant Sci 10:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh RD, Yamasaki H, Septiana A, Yoshida S, Fujiwara MT (2010) Chemical induction of rapid and reversible plastid filamentation in Arabidopsis thaliana roots. Physiol Plant 139:144–158 [DOI] [PubMed] [Google Scholar]
- Jaipargas EA, Barton KA, Mathur N, Mathur J (2015) Mitochondrial pleomorphy in plant cells is driven by contiguous ER dynamics. Front Plant Sci 6:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaipargas E-A, Mathur N, Bou Daher F, Wasteneys GO, Mathur J (2016) High light intensity leads to increased peroxule–mitochondria interactions in plants. Front Cell Dev Biol 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsch IK, Daste F, Gallop JL (2016) Membrane curvature in cell biology: an integration of molecular mechanisms. J Cell Biol 214:375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Chua NH (2002) Visualization of peroxisomes in living plant cells reveals acto-myosin-dependent cytoplasmic streaming and peroxisome budding. Plant Cell Physiol 43:384–392 [DOI] [PubMed] [Google Scholar]
- Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci U S A 106:13106–13111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Matsumoto T, Koiwai S, Mizusaki S, Nishida K, Nogushi M, Tamaki E (1972) Liquid suspension culture of tobacco cells. In Terui G, ed., Ferment Technology Today. Society of Fermentation Technology, Osaka, pp. 689–695 [Google Scholar]
- Kobayashi S, Tanaka A, Fujiki Y (2007) Fis1, DLP1, and Pex11p coordinately regulate peroxisome morphogenesis. Exp Cell Res 313:1675–1686 [DOI] [PubMed] [Google Scholar]
- Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR (1997) Exchange of protein molecules through connections between higher plant plastids. Science 276:2039–2042 [DOI] [PubMed] [Google Scholar]
- Köhler RH, Hanson MR (2000) Plastid tubules of higher plants are tissue-specific and developmentally regulated. J Cell Sci 113:81–89 [DOI] [PubMed] [Google Scholar]
- Krenz B, Jeske H, Kleinow T (2012) The induction of stromule formation by a plant DNA-virus in epidermal leaf tissues suggests a novel intra- and intercellular macromolecular trafficking route. Front Plant Sci 3:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AS, Dinesh-Kumar SP, Caplan JL (2014) Stromules. Adv Plant Biol 5:189 (doi: 10.1007/978-1-4939-1136-3_7) [Google Scholar]
- Kumar AS,, Park E, Nedo A, Alqarni A, Ren L, Hoban K, Modla S, McDonald JH, Kambhamettu C, Dinesh-Kumar SP, et al. (2018) Stromule extension along microtubules coordinated with actin-mediated anchoring guides perinuclear chloroplast movement during innate immunity. Elife 7:e23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2003) Microfilaments and microtubules control the morphology and movement of non-green plastids and stromules in Nicotiana tabacum. Plant J 35:16–26 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004a) Stromules and the dynamic nature of plastid morphology. J Microsc 214:124–137 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004b) In vivo analysis of interactions between GFP-labeled microfilaments and plastid stromules. BMC Plant Biol 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004c) Plastids and stromules interact with the nucleus and cell membranes in vascular plants. Plant Cell Rep 23:188–195 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim DH, Kim YW, Hwang I (2001) Identification of a signal that distinguishes between the chloroplast outer envelope membrane and the endomembrane system in vivo. Plant Cell 13:2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Xu C, Li-Beisson Y, Philippar K (2016) Fatty acid and lipid transport in plant cells. Trends Plant Sci 21:145–158 [DOI] [PubMed] [Google Scholar]
- Lichtscheidl IK, Url WG (1990) Organization and dynamics of cortical endoplasmic reticulum in inner epidermal cells of onion bulb scales. Protoplasma 157:203–215 [Google Scholar]
- Lingard MJ, Trelease RN (2006). Five Arabidopsis peroxin 11 homologs individually promote peroxisome elongation, duplication or aggregation. J Cell Sci 119:1961–1972 [DOI] [PubMed] [Google Scholar]
- Logan DC, Leaver CJ (2000) Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J Exp Bot 51:865–871 [PubMed] [Google Scholar]
- Logan DC, Scott I, Tobin AK (2004) ADL2a, like ADL2b, is involved in the control of higher plant mitochondrial morphology. J Exp Bot 55:783–785 [DOI] [PubMed] [Google Scholar]
- Logan DC (2006a) The mitochondrial compartment. J Exp Bot 58:1225–1243 [PubMed] [Google Scholar]
- Logan DC (2006b) Plant mitochondrial dynamics. Biochim Biophys Acta 1763:430–441 [DOI] [PubMed] [Google Scholar]
- Logan DC (2010) Mitochondrial fusion, division and positioning in plants. Biochem Soc Trans 38:789–795 [DOI] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E (2015) Membrane curvature at a glance. J Cell Sci 128:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano S, Nakamori C, Kondo M, Hayashi M, Nishimura M (2004) An Arabidopsis dynamin-related protein, DRP3A, controls both peroxisomal and mitochondrial division. Plant J 38:487–498 [DOI] [PubMed] [Google Scholar]
- Margittai E, Löw P, Szarka A, Csala M, Benedetti A, Bánhegyi G. ( 2008) Intraluminal hydrogen peroxide induces a permeability change of the endoplasmic reticulum membrane. FEBS J 582:4131–4136 [DOI] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Hulskamp M (2002) Simultaneous visualization of peroxisomes and cytoskeletal elements reveals actin and not microtubule-based peroxisome motility in plants. Plant Physiol 128:1031–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J (2007) The illuminated plant cell. Trends Plant Sci 12:506–513 [DOI] [PubMed] [Google Scholar]
- Mathur J (2009) Rapid peroxisomal responses to ROS suggest an alternative mechanistic model for post-biogenesis peroxisomal life cycle in plants. Plant Signal Behav 4:787–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Mammone A, Barton KA (2012) Organelle extensions in plant cells. J Int Plant Biol 54:851–867 [DOI] [PubMed] [Google Scholar]
- Mathur J, Barton KA, Schattat MH (2013) Fluorescent protein flow within stromules. Plant Cell 25:2771–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Sheikh A, Mathur N (2018) Peroxisome mitochondria inter-relations in plants In del Río LD, Schrader M, eds., Subcellular Biochemistry Series 89. Proteomics of Peroxisomes: Identifying Novel Functions and Regulatory Networks, Springer Nature Singapore Pte Ltd, pp. 417–436. Available from: 10.1007/978-981-13-2233-4_17 [DOI] [PubMed] [Google Scholar]
- Mehrshahi P, Johnny C, DellaPenna D (2014) Redefining the metabolic continuity of chloroplasts and ER. Trends Plant Sci 19:501–507 [DOI] [PubMed] [Google Scholar]
- Menzel D (1994) An interconnected plastidom in Acetabularia: implications for the mechanism of chloroplast motility. Protoplasma 179:166–171 [Google Scholar]
- Michaud M, Gros V, Tardif M, Brugiere S, Ferro M, Prinz WA, Toulmay A, Mathur J, Wozny M, Falconet D, et al. (2016) AtMic60 is involved in plant mitochondria lipid trafficking and is part of a large complex, Curr Biol 26:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Jouhet J (2019) Lipid trafficking at membrane contact sites during plant development and stress response. Front Plant Sci 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulisch M, Krupinska K (2013) Chapter 14: Ultrastructural analyses of senescence associated dismantling of chloroplasts revisited. In Biswal B, Krupinska K, Biswal UC, eds, Plastid development in leaves during growth and senescence, advances in photosynthesis and respiration, Springer Science + Business Media Dordrecht, pp. 307–335 [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “Hela” cell in the cell biology of higher plants. Int Rev Cytol 132:1–30 [Google Scholar]
- Natesan SKA, Sullivan JA, Gray JC (2009) Myosin XI is required for actin-associated movement of plastid stromules. Mol Plant 2:1262–1272 [DOI] [PubMed] [Google Scholar]
- Neuhaus HE, Emes MJ (2000) Non-photosynthetic metabolism in plastids. Annu Rev Plant Physiol 51:111–140 [DOI] [PubMed] [Google Scholar]
- Nito K, Kamigaki A, Kondo M, Hayashi M, Nishimura M (2007) Functional classification of Arabidopsis peroxisome biogenesis factors proposed from analyses of knockdown mutants. Plant Cell Physiol 48:763–774 [DOI] [PubMed] [Google Scholar]
- Oikawa K, Yamasato A, Kong S-G, Kasahara M, Nakai M, Takahashi F. , Ogura Y, Kagawa T, Wada M. (2008) Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol 148:829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa K, Matsunaga S, Mano S, Kondo M, Yamada K, Hayashi M, Kagawa T, Kadota A, Sakamoto W, Higashi S, et al. (2015) Physical interaction between peroxisomes and chloroplasts elucidated by in situ laser analysis. Nat Plants 1:15035. [DOI] [PubMed] [Google Scholar]
- Oikawa K, Hayashi M, Hayashi Y, Nishimura M (2019) Re‐evaluation of physical interaction between plant peroxisomes and other organelles using live‐cell imaging techniques. J Integr Plant Biol 61:836–852 [DOI] [PubMed] [Google Scholar]
- Orth T, Reumann S, Zhang X, Fan J, Wenzel D, Quan S, Hu J (2007) The PEROXIN11 protein family controls peroxisome proliferation in Arabidopsis. Plant Cell 19:333–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit JD, Immel F, Lins L, Bayer EM (2019) Lipids or proteins: who is leading the dance at membrane contact sites? Front Plant Sci 10:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke GJ, McMahon HT (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5:133–147 [DOI] [PubMed] [Google Scholar]
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ (1992) Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111:229–233 [DOI] [PubMed] [Google Scholar]
- Purves WK, Sadava D, Orians GH, Heller HC (2001) Life, the science of biology, 6th edn. Sinauer, Sunderland. [Google Scholar]
- Pyke KA (1999) Plastid division and development. Plant Cell 11:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Howells CA (2002) Plastid and stromule morphogenesis in tomato. Ann Bot 90:559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader H, Schnepf E (1986) Endoplasmic-reticulum and cytoplasmic streaming—fluorescence microscopic observations in adaxial epidermis cells of onion bulb scales. Protoplasma 131:250–252 [Google Scholar]
- Qiao F, Petrásêk J, Nick P (2010) Light can rescue auxin-dependent synchrony of cell division in a tobacco cell line. J Exp Bot 61:503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen D, Marty F, Leborgne-Castel N (2005) New insights into the tonoplast architecture of plant vacuoles and vacuolar dynamics during osmotic stress BMC Plant Biol 5:13 (doi: 10.1186/1471-2229-5-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna L, Stefano G, Brandizzi F (2018) Live cell imaging and confocal microscopy. Methods Mol Biol 1789:117–130 (doi: 10.1007/978-1-4939-7856-4_9) [DOI] [PubMed] [Google Scholar]
- Reumann S, Bartel B (2016) Plant peroxisomes: recent discoveries in functional complexity, organelle homeostasis, and morphological dynamics. Curr Opin Plant Biol 34:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Serrano M, Romero-Puertas MC, Sanz-Fernandez M, Hu J, Sandalio LM (2016) Peroxisomes extend peroxules in a fast response to stress via a reactive oxygen species-mediated induction of the peroxin PEX11a. Plant Physiol 171:1665–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti C, Costa A, Pedrazzini E, Lo Schiavo F, Zottini M (2014) FISSION1A, an Arabidopsis tail-anchored protein, is localized to three subcellular compartments. Mol Plant 7:1393–1396 [DOI] [PubMed] [Google Scholar]
- Sattarzadeh A, Schmelzer E, Hanson MR (2013) Arabidopsis myosin XI sub-domains homologous to the yeast myo2p organelle inheritance sub-domain target subcellular structures in plant cells. Front Plant Sci 4:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguí-Simarro JM, Coronado MJ, Staehelin LA (2008) The mitochondrial cycle of Arabidopsis shoot apical meristem and leaf primordium meristematic cells is defined by a perinuclear tentaculate/cage-like mitochondrion. Plant Physiol 148:1380–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Klösgen RB (2011) Induction of stromules formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana. BMC Plant Biol 11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat M, Barton K, Baudisch B, Klosgen RB, Mathur J (2011a) Plastid stromule branching coincides with contiguous endoplasmic reticulum dynamics. Plant Physiol 155:1667–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat M, Barton K, Mathur J (2011b) Correlated behavior implicates stromules in increasing the interactive surface between plastids and ER tubules. Plant Signal Behav 6:715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Griffiths S, Mathur N, Barton K, Wozny MR, Dunn N, Greenwood JS, Mathur J (2012a) Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell 24:1465–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Klösgen RB, Mathur J (2012b) New insights on stromules: stroma filled tubules extended by independent plastids. Plant Signal Behav 7:1132–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Barton KA, Mathur J (2014) The myth of interconnected plastids and related phenomena. Protoplasma 252:359–371 [DOI] [PubMed] [Google Scholar]
- Schmidt von Braun S, Schleiff E (2008) The chloroplast outer membrane protein CHUP1 interacts with actin and profilin. Planta 227:1151–1159 [DOI] [PubMed] [Google Scholar]
- Schnurr JA, Shockey JM, de Boer GJ, Browse JA (2002) Fatty acid export from the chloroplast. Molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arabidopsis. Plant Physiol 129:1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I, Logan DC (2008) Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytol. 177(1): 90–101. doi: 10.1111/j.1469-8137.2007.02255.x [DOI] [PubMed] [Google Scholar]
- Scott I, Sparkes IA, Logan DC (2007) The missing link: inter-organellar connections in mitochondria and peroxisomes? Trends Plant Sci 12:380–381 [DOI] [PubMed] [Google Scholar]
- Seo YS, Kim EY, Kim JH, Kim WT (2009) Enzymatic characterization of class I DAD1-like acylhydrolase members targeted to chloroplast in Arabidopsis. FEBS Lett 583:2301–2307 [DOI] [PubMed] [Google Scholar]
- Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86:715–748 [DOI] [PubMed] [Google Scholar]
- Sinclair AM, Trobacher CP, Mathur N, Greenwood JS, Mathur J (2009) Peroxule extension over ER-defined paths constitutes a rapid subcellular response to hydroxyl stress. Plant J 59:231–242 [DOI] [PubMed] [Google Scholar]
- Solymosi K, Schoefs B (2010) Etioplast and etio-chloroplast formation under natural conditions: the dark side of chlorophyll biosynthesis in angiosperms. Photosynth Res 105:143–166 [DOI] [PubMed] [Google Scholar]
- Sparkes I, Hawes C, Frigerio L (2011) FrontiERs: movers and shapers of the higher plant cortical endoplasmic reticulum. Curr Opin Plant Biol 14:658–665 [DOI] [PubMed] [Google Scholar]
- Spencer D, Wildman S (1962) Observations on the structure of grana-containing chloroplasts and a proposed model of chloroplast structure. Aust J Biol Sci 15:599–610 [Google Scholar]
- Tan X, Wang Q, Tian B, Zhang H, Lu D. , Zhou J (2011) A Brassica napus lipase locates at the membrane contact sites involved in chloroplast development. PLoS ONE 6:e26831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazar-Poulot N, Miquel M, Fobis-Loisy I, Gaude T (2015) Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies. Proc Natl Acad Sci U S A 112:4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gestel K, Verbelen JP (2002) Giant mitochondria are a response to low oxygen pressure in cells of tobacco (Nicotiana tabacum L.). J Exp Bot 53:1215–1218 [DOI] [PubMed] [Google Scholar]
- Veley KM, Marshburn S, Clure CE, Haswell ES (2012) Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol 22:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbelen JP, Tao W (1998) Mobile arrays of vacuole ripples are common in plant cells. Plant Cell Rep 17:917–920 [DOI] [PubMed] [Google Scholar]
- Waters MT, Fray RG, Pyke KA (2004) Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. Plant J 39:655–667 [DOI] [PubMed] [Google Scholar]
- Wildman SG, Hongladarom T, Honda SI (1962) Chloroplast and mitochondria in living cells: cinephotomicrographic studies. Science 138:434–436 [DOI] [PubMed] [Google Scholar]
- Wiltshire EJ, Collings DA (2009) New dynamics in an old friend: dynamic tubular vacuoles radiate through the cortical cytoplasm of red onion epidermal cells. Plant Cell Physiol 50:1826–1839 [DOI] [PubMed] [Google Scholar]
- Yamashita A, Fujimoto M, Katayama K, Yamaoka S, Tsutsumi N, Arimura S (2016) Formation of mitochondrial outer membrane derived protrusions and vesicles in Arabidopsis thaliana. PLoS ONE 11:e0146717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hu J (2008) FISSION1A and FISSION1B proteins mediate the fission of peroxisomes and mitochondria in Arabidopsis. Mol Plant 1:1036–1047 [DOI] [PubMed] [Google Scholar]
- Zhang X, Hu J (2009) Two small protein families, DYNAMIN-RELATED PROTEIN3 and FISSION1, are required for peroxisome fission in Arabidopsis. Plant J 57:146–159 [DOI] [PubMed] [Google Scholar]
- Zhang X, Hu J (2010) The Arabidopsis chloroplast division protein DYNAMIN-RELATED PROTEIN5B also mediates peroxisome division. Plant Cell 22:431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.