Abstract
Interactions between plant hormones and environmental signals are important for the maintenance of root growth plasticity under ever-changing environmental conditions. Here, we demonstrate that arsenate (AsV), the most prevalent form of arsenic (As) in nature, restrains elongation of the primary root through transcriptional regulation of local auxin biosynthesis genes in the root tips of Arabidopsis (Arabidopsis thaliana) plants. The ANTHRANILATE SYNTHASE ALPHA SUBUNIT 1 (ASA1) and BETA SUBUNIT 1 (ASB1) genes encode enzymes that catalyze the conversion of chorismate to anthranilate (ANT) via the tryptophan-dependent auxin biosynthesis pathway. Our results showed that AsV upregulates ASA1 and ASB1 expression in root tips, and ASA1- and ASB1-mediated auxin biosynthesis is involved in AsV-induced root growth inhibition. Further investigation confirmed that AsV activates cytokinin signaling by stabilizing the type-B ARABIDOPSIS RESPONSE REGULATOR1 (ARR1) protein, which directly promotes the transcription of ASA1 and ASB1 genes by binding to their promoters. Genetic analysis revealed that ASA1 and ASB1 are epistatic to ARR1 in the AsV-induced inhibition of primary root elongation. Overall, the results of this study illustrate a molecular framework that explains AsV-induced root growth inhibition via crosstalk between two major plant growth regulators, auxin and cytokinin.
ARR 1 (ARR1) directly regulates ANTHRANILATE SYNTHASE ALPHA SUBUNIT 1 (ASA1) and BETA SUBUNIT 1 (ASB1) expression during arsenate-induced root growth inhibition in Arabidopsis.
Introduction
Root architecture plays important roles in plant water and nutrient acquisition, making the plasticity of root growth an important adaptive trait (Giehl et al., 2014; Tian et al., 2014; Rellan-Alvarez et al., 2016; Ogura et al., 2019). Through changing root architecture, plants adapt to various environmental cues. Arsenic (As), a class 1 carcinogen, is widely distributed in the environment and is a major threat to all life forms worldwide (Zhao et al., 2010; Naujokas et al., 2013). Arsenate (AsV) and arsenite (AsIII) are the two major forms of As present in the environment, and AsV is the predominant form found in soil under aerobic conditions (Zhao et al., 2010). Because of its structural similarity to phosphate (Pi), AsV is easily absorbed by plant roots through Pi transporters (Shin et al., 2004; Li et al., 2016). Upon entering the root system, AsV reductases rapidly reduce AsV to AsIII (Chao et al., 2014; Sanchez-Bermejo et al., 2014), which is either extruded from the cell or sequestered by phytochelatins and other related thiol-containing compounds and compartmentalized into vacuoles (Tripathi et al., 2007; Mohan et al., 2016). Excess AsV in the cell leads to toxicity, which limits plant root growth (Hartley-Whitaker et al., 2001). Tolerance to AsV stress in plants is achieved mainly through the combined action of two specific responses: restriction of AsV uptake by the suppression of ARABIDOPSIS PHOSPHATE TRANSPORTER1;1 (PHT1;1), which encodes the most active transporter of AsV and Pi (Castrillo et al., 2013), and detoxification of AsIII by the formation and sequestration of AsIII–thiol complexes into vacuoles (Schmoger et al., 2000; Tripathi et al., 2007; Mohan et al., 2016).
After taken up by plant roots, As accumulates in the edible parts of the plant, such as the seeds and fruits, which potentially threatens the health of humans and animals. Ingestion of As can lead to serious diseases, including cancers (Martinez et al., 2011; Bundschuh et al., 2012). In addition to being toxic, As limits the uptake of minerals essential for human health, such as iron and zinc, by plants (Duan et al., 2013; Brackhage et al., 2014). While a lot is known about the uptake, transport, and detoxification of As (Ciurli et al., 2014; Li et al., 2016; Cai et al., 2019), the mechanism responsible for As inhibition of root growth has received little or no attention, despite it being not only important for understanding root growth plasticity under various environmental cues, and also for crop design and breeding.
The phytohormone auxin plays a central role in root growth and development (Sabatini et al., 1999; Benkova and Hejatko, 2009; Vanneste and Friml, 2009; Overvoorde et al., 2010; Zhao, 2018). Auxin required for root development is partially synthesized in shoots and transported to the root tips via central vascular tissues (Grieneisen et al., 2007; Zhao, 2018). The auxin accumulated in root tips can be further transported in a basipetal manner toward the root transition and elongation zones (EZs), where it is essential for developmental processes such as cell elongation and lateral root initiation (Teale et al., 2006). These processes are dependent on auxin transport via the influx and efflux carriers of the polar auxin transport (PAT) system, directing auxin to specific cell types in the root apex (Swarup et al., 2001; Friml et al., 2002a, 2002b; Benkova et al., 2003; Blilou et al., 2005; Teale et al., 2006). The so-called fountain model of auxin transport has been used to account for root elongation and root gravitropic responses (Teale et al., 2006).
Auxin required for root development is obtained not only from the shoot but also from the root (Ljung et al., 2005; Chen et al., 2014; Zhao, 2018). Local auxin biosynthesis maintains optimal root growth in response to plant hormones and environmental signals. Mutations in the TRYPTOPHAN AMINOTRANSFERASE1 (TAA1) gene in Arabidopsis (Arabidopsis thaliana) alter the root response to gravity and 1-aminocyclopropane-1-carboxylic acid (ACC) because of the reduction in auxin level in roots (Teale et al., 2006; Stepanova et al., 2008; Tao et al., 2008). Aluminum (Al) promotes auxin biosynthesis by upregulating TAA1, YUCCA 8 (YUC8), and YUCCA 9 (YUC9) expression in the root apex transition zone (TZ), which leads to root growth inhibition under Al stress (Yang et al., 2014; Liu et al., 2016). ETHYLENE RESPONSE FACTOR1 (ERF1)- and ERF109-mediated ASA1 expression is involved in ethylene signaling- and jasmonate signaling-induced root growth regulation, respectively (Sun et al., 2009; Cai et al., 2014; Mao et al., 2016).
The crosstalk between auxin and cytokinin signaling plays important roles in root growth regulation. The AUXIN (AUX)/INDOLE-3-ACETIC ACID (IAA) protein, SUPPRESSOR OF HYPOCOTYL2 (SHY2), regulates root meristem activity by balancing auxin and cytokinin signaling. Auxin degrades SHY2 via the SKP1-CULLIN1-F-BOX (SCF)–TRANSPORT INHIBITOR RESISTANT1 (SCFTIR1) complex (Tian et al., 2002; Dharmasiri et al., 2003), whereas cytokinin induces SHY2 expression in the root TZ via type-B ARABIDOPSIS RESPONSE REGULATORS (ARRs), which directly activate SHY2 transcription, and SHY2 negatively regulates the transcription of PIN-FORMED (PIN) genes. In addition to modulating auxin responses, SHY2 also promotes cytokinin biosynthesis by elevating the expression of ISOPENTENYLTRANSFERASE5 (IPT5; Dello Ioio et al., 2008; Moubayidin et al., 2010). Cytokinin also interacts with auxin to modulate the activity of the quiescent center (QC) activity; ARR1 controls PAT by downregulating the auxin influx carrier LIKE AUXIN RESISTANT2 (LAX2), leading to cytokinin signaling, which attenuates auxin response and division in QC cells (Zhang et al., 2013). The suppression of ARR1 in the QC by SCARECROW (SCR) transcription factor affects auxin biosynthesis (Moubayidin et al., 2013). Auxin also antagonizes cytokinin signaling during zygotic embryogenesis, specifically during root stem cell specification, through the direct transcriptional activation of cytokinin signaling repressors, ARR7 and ARR15 (Muller and Sheen, 2008).
Root growth inhibition is a common symptom of As toxicity (Schmoger et al., 2000; Li et al., 2007; Shri et al., 2009; Yoon et al., 2015). Here, we show that AsV, the most prevalent As species in nature, restrains root growth by promoting ANTHRANILATE SYNTHASE A1 (ASA1) and B1 (ASB1)-mediated auxin biosynthesis in root tips. We also demonstrate that AsV activates cytokinin signaling in root tips, and ARR1, the main transcription factor in cytokinin signaling, directly modulates ASA1 and ASB1 expression in roots in response to AsV stress. Our results extend the view of how plants translate environmental stress cues into growth response, thus maintaining growth plasticity during postembryonic development.
Results
AsV inhibits elongation of the primary root
Growth of Arabidopsis ecotype Columbia (Col-0) seedlings in half-strength Murashige and Skoog (1/2 MS) medium supplemented with or without 250 µM AsV showed that AsV inhibited primary root elongation and lateral root development (Figure 1, A–D). To understand the basis of root growth inhibition by AsV, we examined the effect of different concentrations of AsV on the primary root length of Arabidopsis seedlings. The growth of Col-0 roots was inhibited by AsV in a dose-dependent manner (Figure 1, E). Because root length of Col-0 seedlings was significantly reduced over time in the presence of 250 μM AsV (Figure 1, F), based on a comparison between the two different treatments at the same time points, we used this AsV concentration in all subsequent experiments.
Figure 1.
Arsenate (AsV) inhibits root elongation in Arabidopsis. A–D, Six-day-old (A and B) and 9-d-old (C and D) seedlings of the wild type (Col-0) grown on MS medium containing no AsV (MS, control) or 250 μM AsV (AsV). DAG, days after germination. E, AsV-induced root growth inhibition. Col-0 seeds were germinated on medium containing different concentrations of AsV, and seedling root length was measured at 9 DAG. F, Time-course analysis of AsV-mediated root growth inhibition. Col-0 seeds were germinated on control medium (MS) or medium containing 250 μM AsV for the indicated time points. G and H, Effect of AsV on the size of the root MZ and EZ and epidermal cell length in the root DZ. Six-day-old Col-0 seedlings grown in the absence (G) or presence (H) of AsV are shown. DZ was defined as a region from root hair emergence to the root/hypocotyl junction. Insets show reduced length (marked with white rectangles) of epidermal cells in the DZ. Scale bar = 50 μm. Data represent mean ± standard deviation (sd) (n > 20) of at least three independent experiments.
Next, we investigated AsV-induced cellular changes in three morphologically distinct developmental zones along the longitudinal axis of the root: differentiation zone (DZ), EZ, and meristematic zone (MZ). Final cell length in the DZ, the sizes of EZ and MZ, and cell division activity in MZ were lower in Arabidopsis plants grown in presence of AsV than in those grown in medium without AsV (Figure 1, G and H and Supplemental Figure S1, A–C). Closer observation of the DZ and EZ of AsV-treated roots indicated that AsV reduced both the number and length of cells in these regions (Supplemental Figure S1, D–G), indicating that AsV reduces both cell proliferation and cell elongation, the two basic cellular processes affecting primary root elongation in plants (Scheres et al., 2002).
ASA1- and ASB1-mediated auxin biosynthesis is involved in AsV-induced root growth inhibition
To reveal the early molecular events in AsV-induced root growth inhibition, we carried out RNA-Seq experiments using 2-mm root tips of 5-d-old Col-0 seedlings treated with 250 μM AsV for 0, 1, 3, 6, 12, and 24 h. Significant differences in gene expression levels were detected between AsV treatments and mock (0 h) treatment. The number of differentially expressed genes (DEGs) varied between 4,000 and 9,000 at each time point (Supplemental Figure S2, A), implying that the root response to AsV stress is complex. Because auxin is a key player in root growth regulation (Vanneste and Friml, 2009), we mainly focused on genes involved in auxin signaling. The results showed that auxin biosynthesis-related genes were upregulated as an early response to AsV stress, and downregulated with 6 h onward (Supplemental Figure S2, B) which imply that –auxin biosynthesis-related genes regulated by AsV are tightly controlled.
Next, we examined that auxin signaling in root tips subjected to AsV treatment using the β-glucuronidase (GUS) reporter gene under the control of the auxin-inducible promoter DR5. In the AsV treatment, activity of the DR5 promoter was weakly but significantly increased in root tips at 3 h and further enhanced at 6 h compared with the 0 h time point (Figure 2, A, top panel). This suggests that auxin acts as an early AsV stress-responsive signal to regulate root growth inhibition.
Figure 2.
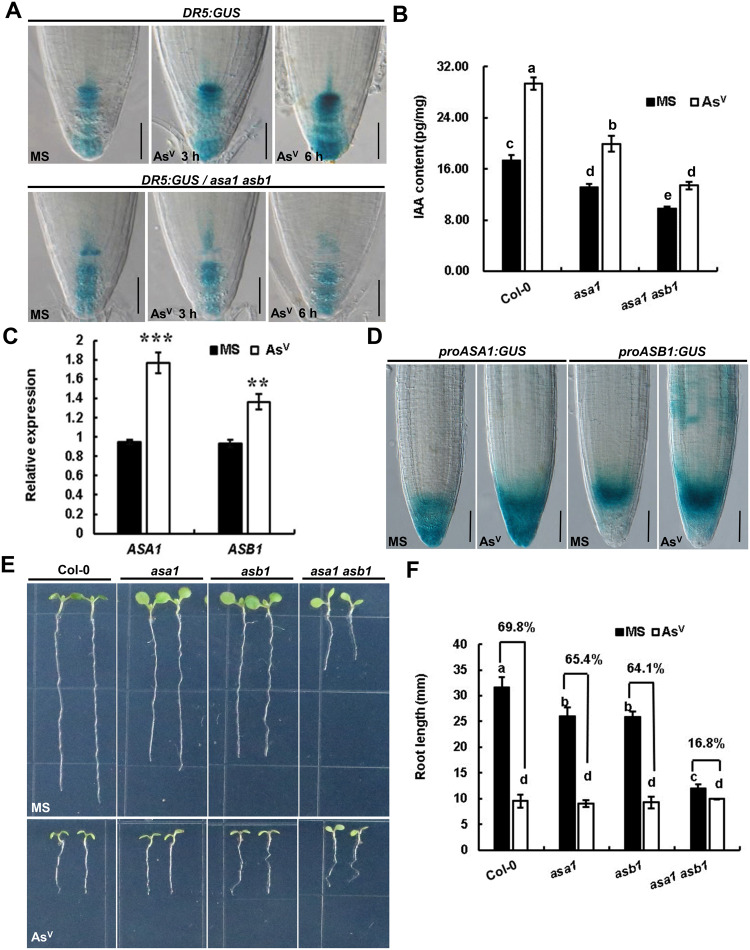
ASA1 and ASB1 are involved in arsenate (AsV)-induced root growth inhibition. A, Expression patterns of DR5:GUS in the root tips. Five-day-old wild-type Col-0 (top panel) and asa1 asb1 double mutant (bottom panel) seedlings germinated on control medium (MS) were transferred to MS medium or medium containing 250 μM AsV (AsV) for 3 or 6 h. Scale bar = 50 μm. B, Level of free IAA in the root tips of Col-0, asa1, and asa1 asb1. Six-day-old seedlings grown on control MS medium were transferred to medium without AsV (MS) or with 250 μM AsV (AsV) for 12 h. C, RT-qPCR analysis of ASA1 and ASB1 expression in root tips. Six-day-old Col-0 seedlings treated without AsV (MS) or with 250 μM AsV (AsV) for 6 h. Transcript levels of ASA1 and ASB1 were normalized to the ACTIN7 transcript level. D, AsV-induced proASA1:GUS and proASB1:GUS expression. Five-day-old seedlings grown on control medium (MS) were transferred to medium without AsV (MS) or with 250 μM AsV (AsV) for 6 h. Scale bar = 50 μm. E and F, Images (E) and root lengths (F) of the primary roots of Col-0, asa1, asb1, and asa1 asb1. Seedlings were grown on medium without AsV (MS) or with 250 μM AsV (AsV) for 6 d. Data in (B) and (F) represent mean ± standard error (se) and were examined by analysis of variance (ANOVA), followed by Fisher’s LSD mean separation test. Different lowercase letters indicate statistically significant differences at P < 0.01. Data in (C) represent mean ± sd of three replicates. Asterisks indicate statistically significant differences (**P < 0.01, ***P < 0.001; Student’s t test).
To test whether AsV-induced DR5:GUS signaling maximum in root tips reflected the endogenous auxin level, we measured the concentration of IAA in 2-mm Col-0 root tips. Consistent with the increased DR5:GUS expression in root tips, the level of endogenous auxin in root tips was significantly increased in AsV-treated root tips at 12 h (Figure 2, B). These results indicate that AsV-mediated auxin biosynthesis could potentially be involved in AsV-mediated inhibition of primary root elongation.
The expression of several auxin biosynthetic genes was upregulated by AsV in RNA-Seq samples (Supplemental Figure S2, C). The transcription of ASA1 and ASB1 genes was elevated in AsV-treated root tips compared with the mock treatment by reverse transcription quantitative PCR PCR (RT-qPCR; Figure 2, C). We also examined the effect of AsV on ASA1 and ASB1 expression by expressing the GUS reporter gene under the control of the ASA1 or ASB1 promoters (proASA1:GUS and proASB1:GUS) in 5-d-old Col-0 seedlings. The proASA1:GUS and proASB1:GUS constructs were expressed mainly in the root tips, and their expression was significantly enhanced by AsV (Figure 2, D). The initial upregulation of ASA1 and ASB1 expression was followed by downregulation with 6 h onward (Supplemental Figure S2, C), which imply that ASA1 and ASB1 expression mediated by AsV is tightly controlled by a sophisticated network. These data suggest that AsV promotes auxin biosynthesis by activating ASA1 and ASB1 transcription.
We also compared AsV-induced root growth inhibition among Col-0 (wild-type), asa1 and asb1 single mutants, and asa1 asb1 double mutant seedlings. AsV treatment reduced the root length of Col-0, asa1, asb1, and asa1 asb1 seedlings by 69.8%, 65.4%, 64.1%, and 16.8%, respectively, compared with the mock treatment. Compared with Col-0, root growth of asa1 and asb1 showed weak insensitivity to AsV whereas asa1 asb1 showed more insensitivity than asa1 or asb1 (Figure 2, E and F). Additionally, the effect of AsV on DR5:GUS expression and auxin biosynthesis was also greatly compromised in asa1 asb1 double mutant (Figure 2, A and B). These data imply that ASA1 and ASB1 are involved in the response of roots to AsV stress.
Because the double mutant asa1 asb1 showed severe developmental defects, it is reasonable to speculate that root growth insensitivity of asa1 asb1 to AsV is caused by the defects. To exclude this possibility, root growth inhibition by other stresses were compared between Col-0 and asa1 asb1. Root growth of asa1 asb1 showed hypersensitive phenotypes to sodium chloride, excess copper ion and phosphate deficiency stresses (Supplemental Figure S3). These data demonstrated that root growth inhibition by AsV does not attribute to the developmental defects of asa1 asb1.
All these lines of evidence demonstrate that AsV-induced root growth inhibition is partially dependent on ASA1- and ASB1-mediated auxin biosynthesis.
ARR1 promotes ASA1 and ASB1 expression through promoter binding
To identify transcription factors responsible for the AsV-mediated upregulation of ASA1 expression, we performed yeast one-hybrid (Y1H) screening using Arabidopsis cDNA libraries harboring the HISTIDINE3 (HIS3) reporter gene under the control of the ASA1 promoter. A total of 35 positive cDNA clones encompassing genes encoding seven transcription factors, including ARR1 and ARR10, were identified (Supplemental Figure S4). Analysis of the ASA1 promoter sequence using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) revealed several potential ARR1-binding sites (ABSs), suggesting the possibility that ARR1 is a transcriptional regulator of ASA1.
To confirm that ARR1 directly binds to the ABSs in the ASA1 promoter, electrophoretic mobility shift assays (EMSAs) were conducted using glutathione (GST)-tagged DNA-binding domain of ARR1 (ARR1-GST), and P1 and P2 regions of the ASA1 promoter harboring ABS1 (5′-CTTCGATCTT-3′) and ABS2 (5′-GAGGATTGTC-3′) motifs, respectively (Figure 3, A–C). Unlabeled DNA probes competed for binding to the ARR1-GST protein in a dose-dependent manner. Furthermore, ARR1-GST protein failed to bind to mutant probes. Parallel experiments indicated that ARR1-GST could also bind to the ASB1 promoter (proASB1, Supplemental Figure S5, A and B). These data demonstrate that ARR1 directly binds to ASA1 and ASB1 promoters in vitro.
Figure 3.
ARR1 promotes ASA1 and ASB1 expression through promoter binding. A, Schematic diagram of potential ABSs (red triangles) in ASA1 promoter (proASA1), DNA fragments (P1–P5) within 1-kb sequence upstream of the TSS of ASA1 used for ChIP-qPCR, and ABS1 and ABS2 probes used for EMSA. B and C, EMSA of the ARR1-GST fusion protein with ASA1 probe a (B) or ASA1 probe b (C). Biotin-labeled probes were incubated with ARR1-GST, and the free and bound probes were separated by polyacrylamide gel electrophoresis (PAGE). Unlabeled probes were used as competitors. Mutant probes (Mu) in which the 5′-GAGGATTGTC-3′ and 5′-CTTCGATCTT-3′ motifs were mutated to 5′-AAAAATAAAA-3′ and 5′-ATAATAAAAA-3′, respectively. D, Enrichment of ASA1 promoter fragments following ChIP using anti-Myc antibody. Chromatin of transgenic plants expressing 35S:ARR1-Myc was immunoprecipitated with anti-Myc antibody, and the presence of the indicated DNA in the protein–DNA complex was determined by quantitative real-time PCR (qRT-PCR). The Actin2 promoter fragment was used as a negative control. The experiment was repeated three times with similar results. E and F, Expression analysis of ASA1 (E) and ASB1 (F) by RT-qPCR in Col-0, arr1-3, arr10-5, and arr1-3 arr10-5. Five-day-old seedlings germinated on control MS medium were transferred to control medium (MS) or medium containing 250 μΜ AsV (AsV) for 6 h, and 2-mm long root tips were used for RNA extraction and qRT-PCR analysis. Transcript levels of ASA1 and ASB1 were normalized to ACTIN2 expression. Data in (D)–(F) represent mean ± sd of three replicates. Asterisks indicate statistically significant differences (***P < 0.001; Student’s t test).
Next, to test the interaction of ARR1 with ASA1 and ASB1 promoters in vivo, we performed chromatin immunoprecipitation (ChIP) assays using the 35S:ARR1-Myc transgenic plants and anti-Myc antibody. DNA fragments immunoprecipitated using anti-Myc antibody were significantly enriched in the P1 and P2 regions of the ASA1 promoter and P1 region of the ASB1 promoter (Figure 3, D and Supplemental Figure S5, C). These results confirm the direct physical association of ARR1 with the promoters of ASA1 and ASB1 in vivo.
Next, we verified the activation effect of ARR1 on ASA1 expression using the well-established Nicotiana benthamiana transient expression assay (Supplemental Figure S6). Infiltration of the construct expressing the firefly luciferase (LUC) reporter gene under the control of the ASA1 promoter (proASA1:LUC) into N. benthamiana leaves resulted in a substantial amount of LUC activity. Co-expression of proASA1:LUC with a construct expressing the ARR1 gene under the control of the constitutive 35S promoter (35S:ARR1) led to a further increase in LUC luminescence intensity, suggesting that 35S:ARR1 promotes proASA1:LUC expression. In a parallel experiment, co-expression of the proASA1-m:LUC construct, carrying mutant variants of ABS1 and ABS2 motifs, with 35S:ARR1 in N. benthamiana leaves resulted in highly attenuated LUC activity (Supplemental Figure S6). Additionally, ARR1 also activated the expression of proASB1:LUC in N. benthamiana leaves (Supplemental Figure S5, D and E). Together, our transient expression assays confirmed that ARR1 directly activates ASA1 and ASB1 expression in vivo.
The AsV-induced expression of ASA1 and ASB1 was further analyzed in Col-0, arr1-3, arr10-5, and arr1-10 arr10-5 seedlings by RT-qPCR. AsV promoted the expression of ASA1 and ASB1 in Col-0 but not in arr1-3, arr10-5, and arr1-3 arr10-5 mutants (Figure 3, E and F). Consistent with these results, the expression of proASA1:GUS and proASB1:GUS was not induced by AsV in the arr1-3 mutant (Supplemental Figure S7).
Taken together, these lines of evidence demonstrate that ARR1 promotes ASA1 and ASB1 expression through promoter binding.
ARR proteins play an important role in AsV-induced root growth inhibition
Because ARR1 regulates the expression of ASA1 and ASB1 (as shown above), we examined AsV-induced root growth inhibition in Col-0, arr1-3, arr10-5, and arr12-1 single mutants and various double mutants (Figure 4, A). Because of the redundancy of type-B ARR transcription factors, roots of arr1-3, arr10-5, and arr12-1 single mutant showed similar root growth inhibition rate as that of Col-0 roots in the presence of AsV. However, AsV reduced the root length of Col-0, arr1-3 arr10-5, and arr1-3 arr12-1 seedlings by 73.1%, 61.2%, and 63.6%, respectively, compared with the mock treatments (Figure 4, B). The roots of arr1-3 arr10-5 and arr1-3 arr12-1 seedlings showed significantly reduced sensitivity to AsV, compared with that of Col-0 seedlings. To overcome the functional redundancy of type-B ARRs, we generated transgenic Arabidopsis lines expressing the dominant negative forms of ARR1 and ARR10 by fusing them with the SUPERMAN repression domain X (SRDX; Hiratsu et al., 2002, 2003; Du et al., 2014), which resulted in their overexpression (Supplemental Figure S8). Compared with Col-0, root lengths of ARR1-SRDX and ARR10-SRDX transgenic lines showed reduced sensitivity to AsV (Figure 4). These data indicate that AsV-mediated root growth inhibition is partially dependent on ARR1 and ARR10.
Figure 4.
ARR proteins are important for arsenate (AsV)-induced root growth inhibition. A, Images of seedlings of the indicated genotypes grown on control medium (MS) or medium containing 250 μM AsV (AsV) for 6 d. Red boxes indicate the root length was significantly reduced by AsV. B, Quantification of the primary root length of seedlings shown in (A). Fisher’s LSD means separation test (SPSS) for multiple comparisons. Different lowercase letters indicate statistically significant differences (P < 0.01).
AsV promotes the accumulation of ARR proteins and activates cytokinin response
To visualize cytokinin signaling output in root tips in response to AsV stress, we employed a robust and sensitive cytokinin synthetic sensor, TWO COMPONENT SIGNALING SENSOR new (TCSn):GREEN FLUORESCENT PROTEIN (GFP), which is mainly expressed in root caps (Muller and Sheen, 2008; Zurcher et al., 2013). After a 3-h AsV treatment, an elevated TCSn:GFP signal was detected in root caps (Figure 5, A). More importantly, TCSn:GFP was activated earlier than DR5rev:GFP in the presence of AsV (Supplemental Figure S9). This demonstrates that AsV activates cytokinin signaling in root caps.
Figure 5.
AsV activates cytokinin signaling by stabilizing ARR proteins. A, Expression of the cytokinin sensor TCSn:GFP in root tips in response to AsV stress. Five-day-old seedlings grown in control MS medium were transferred to medium without AsV (MS) or with 250 μM AsV (AsV) for 6 h. Cell boundaries appear red following propidium iodide staining. Scale bar = 50 μm. B, Localization of ARR proteins in root tips under AsV stress. Five-day-old seedlings of the transgenic lines expressing type-B ARRs tagged with Ypet at their C-termini (+C-1xYpet) were grown in control MS medium and then transferred to MS medium, or MS medium containing 50 µm MG132, 250 μM AsV (AsV), or both MG132 and AsV, respectively, for 6 h. Fluorescence signals were detected in 10 plants selected from each treatment, and representative images are shown (n = 10). Scale bar = 50 μm. C, Quantification of the fluorescence intensities in (B). Data represent mean ± sd of three biological replicates. Fisher’s LSD means separation test (SPSS) for multiple comparisons. Different lowercase letters indicate statistically significant differences (P < 0.01). D, Expression analysis of ARR1, ARR10, and ARR12 in root tips by RT-qPCR. Five-day-old seedlings germinated on MS medium were transferred to control medium (MS) or medium containing 250 μΜ AsV (AsV) for 6 h, and 2-mm long root tips were used for RNA extraction and qRT-PCR analysis. Gene transcript levels were normalized to ACTIN2 expression. Data represent mean ± sd of three biological replicates.
To monitor the cellular distribution patterns of ARRs proteins in root tips, we used transgenic Arabidopsis lines expressing type-B ARRs tagged with a yellow fluorescent protein, Ypet, by a recombination-based gene tagging technique which reflect the nearest endogenous expression and localization of ARRs (Zhou et al., 2011; Xie et al., 2018). The Ypet-tagged ARR1, ARR10, and ARR12 proteins showed weak signals in root tips in the absence of AsV stress. Addition of the proteasome inhibitor MG132 or AsV separately to the transgenic seedlings led to increased intensity of fluorescence compared with that of seedlings in MS medium, and the fluorescence with MG132 was much stronger than that with AsV (Figure 5, B and C). Co-treatment with MG132 and AsV showed similar fluorescence intensities with MG132 treatment (Figure 5, B and C). AsV treatment did not induce the expression of ARRs, suggesting that AsV regulates ARR expression at the post-transcriptional level (Figure 5, D). These supported the AsV-induced accumulation of ARR proteins in root tips by repressing the activity of 26S proteasome.
These data demonstrate that cytokinin signaling is activated by stabilizing ARRs proteins in response to AsV stress in root tips.
Both ASA1 and ASB1 act genetically downstream of ARR1 to regulate AsV-induced root growth inhibition
To determine the genetic relationship of ARR1 with ASA1 and ASB1, we assessed the effect of AsV on root growth inhibition in Col-0, arr1-3 arr10-5 and asa1 asb1 double mutants, and arr1-3 arr10-5 asa1 asb1 quadruple mutant. The root length of Col-0, arr1-3 arr10-5, asa1 asb1, and arr1-3 arr10-5 asa1 asb1 was reduced by 71.9%, 63.4%, 17.7%, and 2.1%, respectively, compared with the mock treatment (Figure 6, A and B). Response of the quadruple mutant to AsV resembled that of the asa1 asb1 double mutant. These data elucidate that ASA1 and ASB1 act downstream of ARR1 in the root growth inhibition by AsV.
Figure 6.
ASA1 and ASB1 function downstream of ARRs to regulate arsenate (AsV)-induced root growth inhibition. A, Images of Col-0, arr1 arr10, asa1 asb1, and arr1 arr10 asa1 asb1 seedlings grown on control medium (MS) or medium containing 250 μM arsenate (AsV) for 6 d. B, Quantification of the primary root length of seedlings shown in (A). Data represent mean ± sd of three biological replicates. Fisher’s LSD means separation test (SPSS) for multiple comparisons. Different lowercase letters indicate statistically significant differences (P < 0.01). C, Schematic of the model explaining how arsenate (AsV) inhibits primary root elongation. When plants sense high levels of AsV in the soil, cytokinin signaling is activated in root tips, and ANTHRANILATE SYNTHASE ALPHA SUBUNIT 1 (ASA1)-mediated local auxin biosynthesis is enhanced in root caps. Basipetal auxin transport from root tips to the EZ by the auxin transporters AUXIN RESISTANT 1 (AUX1) and PIN-FORMED 2 (PIN2) results in excess auxin accumulation in the EZ, which inhibits cell elongation, thus leading to primary root elongation arrest.
Our results reveal that exogenous AsV treatments inhibit primary root elongation by cytokinin-mediated local auxin biosynthesis in root tips. Monitoring the expression dynamics of auxin and cytokinin signaling reporter lines in response to AsV stress revealed that AsV activates cytokinin signaling earlier than auxin signaling (Supplemental Figure S9). Auxin transporters, AUXIN RESISTANT 1 (AUX1) and PIN-FORMED 2 (PIN2), regulate the basipetal transport of auxin from root tips to the EZ along the root epidermal cells. In this study, roots of aux1 and pin2 mutant seedlings showed reduced sensitivity to AsV stress. AUX1 and PIN2 expression in root tips was not regulated by AsV which implies that basipetal auxin transport may not be affected by AsV stress (Supplemental Figure S10). Additionally, DII-VENUS, an auxin sensor rapidly degraded by auxin, was significantly downregulated in the root meristem and EZ by AsV, in ARR1- and ARR10-dependent manner (Supplemental Figure S11), suggesting that auxin signaling is activated in EZ by AsV. Based on these data, we propose a working model that explains how AsV inhibits primary root elongation (Figure 6, C). When plant roots sense high levels of AsV in the soil, cytokinin signaling is activated in the plant, which increases the accumulation of ARR proteins in root tips. Then, local auxin biosynthesis is stimulated by ARR1-mediated ASA1 and ASB1 expression in root tips. Excess auxin accumulation in the EZ due to AUX1- and PIN2-mediated basipetal transport from root tips to EZ inhibits cell elongation in the EZ, which leads to primary root growth arrest.
Discussion
Identification of cellular and molecular mechanisms that mediate the effect of environmental stresses on plant growth is challenging. The contamination of soil and water resources by As is a worldwide environmental problem. In recent years, studies have increased our knowledge of the absorption, transportation, and detoxification of As in plants; however, how As restrains plant growth, especially root growth, remains largely unknown. Here, we address the cellular and molecular mechanisms underlying AsV-induced inhibition of primary root growth in Arabidopsis. We show that AsV inhibits cell division activity in the root meristem, and reduces cell number and limits cell elongation in the root DZ and EZ. At the molecular level, we show that ARR1 directly activates the expression of ASA1 and ASB1 upon AsV stress, suggesting that ARR1-mediated promotion of ASA1 expression is important for AsV-induced modulation of primary root elongation. This study demonstrates crosstalk between auxin and cytokinin in roots in response to AsV. Our results provide further insights into how plants translate stress cues into growth response, thus retaining growth plasticity during postembryonic development.
Local auxin biosynthesis is involved in the root response to AsV
Auxin is a core regulator of root cell division, cell differentiation, and overall root growth. Other plant hormones and environmental stimuli can regulate root growth by interacting with auxin. Whether auxin is involved in AsV-induced root growth inhibition is not clear. In this study, we found that auxin signaling was activated at a very early stage in the root response to AsV stress (Figure 2, A and Supplemental Figures S2, B, S9), and that auxin biosynthesis was promoted by the root response to AsV (Figure 2, B). Together, these observations suggest that auxin serves as a regulator of the early root response to AsV stress. Genetic experiments further demonstrated that the auxin synthesis genes ASA1 and ASB1 were involved in root growth inhibition by AsV (Figure 2). Local auxin biosynthesis maintains optimal plant growth in response to environmental signals (Zhao, 2018). The increased auxin level in root tips and the changes in the expression patterns of ASA1 and ASB1 in response to AsV (Figure 2, B and D) suggest that local auxin biosynthesis is promoted by AsV in root tips, and that this facilitates root growth responses to high levels of As. ASA1 and ASB1 are mainly expressed in the root caps (Figure 2, D), and this suggested that root cap is a potential site for perception of AsV stress in root tips. The TZ in root apex is a critical site for the perception and response to both endogenous phytohormones and environmental cues (Baluška et al., 2010). In the root meristem, the antagonistic interaction of auxin and cytokinin regulates the balance between cell division in RAM and cell elongation in EZ by positioning the TZ, where mitotically active cells lose their capacity to divide and initiate their differentiation programs (Dello Ioio et al., 2008). Our data demonstrated that the sizes of EZ and RAM were reduced by AsV which means TZ in root apex moved rootward (Figure 1, G and H and Supplemental Figure S1). Expression of the auxin reporter DII-VENUS was rapidly repressed in TZ with AsV treatment for 6 h, and this demonstrated that auxin signaling or auxin synthesis was activated by AsV in a short time in TZ (Supplemental Figure S11). Our data also showed that cytokinin signaling was promoted in RAM and TZ (Figure 5, A and B). All these lines of evidence demonstrated that the cytokinin signaling and auxin signaling in TZ were regulated by AsV stress and TZ is another potential important site for root responses to AsV stress. Whether root caps or TZ or both of them are involved in perception of AsV stress signals in root tips requires further investigation. And the mechanism of how auxin signaling and cytokinin signaling are coordinated in response to AsV stress in root tips also needs further exploration.
Double mutant asa1 asb1 seedlings still showed some root growth inhibition and increased auxin synthesis after exposure to AsV stress (Figure 2, B and F), suggesting that other auxin biosynthesis genes are also involved in this process. Our data demonstrate that ASA1 and ASB1 act as important nodes for the transmission of environmental AsV signals to the mechanism regulating root growth. Excess auxin accumulation in the EZ induced by AsV stress inhibited cell elongation (Figure 1, G and H). Cell division in the MZ and cell elongation in the EZ collaborate during primary root elongation (Scheres et al., 2002). It should be noted that not only cell elongation in the EZ but also cell division in the MZ were decreased during the root response to AsV (Supplemental Figure S1). It will be important to clarify further how cell division activity in the root MZ is regulated by AsV.
Plant roots sense AsV stress partially through cytokinin signaling
Cytokinin is a multifunctional plant hormone, which plays momentous roles not only in plant growth and development but also in plant response to environmental stimuli. Current evidence suggest that the metabolism, transport, and signaling of cytokinin are involved in the tolerance to various environmental stresses, including drought, salt, extreme temperature, and nutrient deficiencies (Pavlu et al., 2018). This study uncovers at least a part of the molecular mechanism underlying AsV-mediated primary root growth arrest, which involves cytokinin signaling. We showed that AsV activates cytokinin signaling within 10 min (Supplemental Figure S9), which suggests that cytokinin signaling serves as an early AsV stress-responsive signal. Previously, Mohan et al. (2016) reported that AsV reduces the endogenous cytokinin level in whole plants, which enhances tolerance to AsV stress because of the accumulation of thiol compounds, such as phytochelatins and GST, essential for As sequestration. In this study, ASA1 and the cytokinin sensor TCSn:GFP showed enhanced expression in root caps (Figures 2, C and D, and 5, A), and ARR protein levels also increased significantly in the root cap under AsV stress (Figure 5, B). A previous study reported that the endogenous cytokinin level in whole plants is reduced upon AsV stress (Mohan et al., 2016); however, we found that cytokinin signaling was activated. This suggests that cytokinin synthesis may not be coupled to cytokinin signaling during the root response to AsV. In Arabidopsis, the root tip exhibits a cytokinin gradient, with a concentration maximum in the lateral root cap, columella, columella initials, and QC cells (Antoniadi et al., 2015). Together, these data demonstrate that the root cap is a potential site of As stress perception. Further investigation is needed to understand whether cytokinin content in the root tip is regulated under AsV stress, and how the latter induces cytokinin signaling in root caps.
Our data demonstrated that root growth of asa1 asb1 showed strong insensitivity to AsV stress than Col-0 (Figure 2, E and F). This showed that the auxin biosynthesis genes ASA1 and ASB1 are major regulators involved in AsV-mediated root growth inhibition. We further found that the B-type transcription factors ARR1 and ARR10 were direct modulators of ASA1 and ASB1 transcription (Figure 3 and Supplemental Figures S5, S6). But the roots of arr1-3 arr10-5, arr1-3 arr12-1, ARR1-SRDX, and ARR10-SRDX seedlings showed subtle but significantly reduced sensitivity to AsV, compared with that of Col-0 and asa1 asb1 seedlings (Figure 4). This weak phenotype may be caused by the functional redundancy among the B-type ARR transcription factors. And it is also possible that there are other signaling pathways parallel to the ARR1- and ARR10-mediated cytokinin signaling involved in root responses to AsV stress. And it will be interesting to explore other regulators involved in roots responses to AsV stress.
Materials and methods
Plant materials and growth conditions
Arabidopsis (A. thaliana) ecotype Columbia (Col-0) was used as the wild type in this study. Various transgenic lines used in this study have been described previously: proCYCB1;1:GUS (Colon-Carmona et al., 1999); DR5:GUS (Ulmasov et al., 1997); DR5rev:GFP (Benkova et al., 2003); DII:VENUS (Brunoud et al., 2012); TCSn::GFP (Zurcher et al., 2013); proASA1:GUS and proASB1:GUS (Stepanova et al., 2005); asa1 (wei2-1), asb1 (wei7-1), and asa1 asb1 (wei2-1 wei7-1; Alonso et al., 2003; Stepanova et al., 2005); arr1-3, arr10-5, arr12-1, arr1-3 arr10-5, arr1-3 arr12-1, arr10-5 arr12-1, and arr1-3 arr10-5 arr12-1 (Mason et al., 2005; Yokoyama et al., 2007; Argyros et al., 2008); and aux1-22 (Swarup et al., 2007). Seeds of pin2 (SALK_122916C), ARR1-C1×Ypet (CS71599), ARR10-C1×Ypet (CS71600), and ARR12-C1×Ypet (CS71601; Xie et al., 2018) lines were obtained from the Arabidopsis Biological Resource Center (ABRC, OH, USA; Alonso et al., 2003).
Arabidopsis seeds were surface-sterilized for 15 min in 10% bleach, washed four times with sterile water, and plated on 1/2 MS medium supplemented with or without 250 μM AsV (NaH2AsO4⋅7H2O) and 0.8% agar. Plants were stratified at 4°C for 2 d in darkness and then transferred to a growth chamber maintained at 22°C and illuminated for 16 h using white light (100 µmol m−2s−1 light intensity).
Plasmid construction and plant transformation
To construct 35S:ARR1-Myc, the coding sequence (CDS) of ARR1 was amplified using ARR1-Myc-F/-R primers and cloned into pDONR 221 (Invitrogen). To construct 35S:ARR1-SRDX and 35S:ARR10-SRDX plasmids, a 36-bp DNA sequence encoding the SRDX repression domain (LDLDLELRLGFA) was fused in frame to the 3′-end of ARR1 and ARR10 CDSs using ARR1-SRDX-F/-R and ARR10-SRDX-F/-R primers, respectively, and cloned into the pDONR 221 vector. The above-mentioned pDONR vectors were subcloned into pK7FWG2 using the MultiSite Gateway Three-Fragment Vector Construction Kit (Invitrogen) to generate 35S:ARR1-Myc, 35S:ARR1-SRDX, and 35S:ARR1-SRDX constructs (Hilson et al., 2004; Karimi et al., 2007). The mutants and reporter lines were crossed with the asa1 asb1 double mutant and arr1-3 single mutant. All primers are listed in Supplemental Table S1.
The above-mentioned constructs were transformed into Agrobacterium tumefaciens strain GV3101, which was used for the transformation of Arabidopsis plants by vacuum infiltration (Bechtold and Pelletier, 1998).
Phenotypic, statistical, and microscopy analyses
To analyze the root phenotype, Arabidopsis seedlings were photographed and root length was measured using ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij). Root meristem size was analyzed using seedlings mounted in chloroacetaldehyde:water:glycerol (HCG; 8:3:1) solution. Microscopy was performed on a ZEISS Axio Imager Z2 microscope, fitted with an Axiocam 506 color camera. Images were processed using Adobe Photoshop CS and ZEN 2.6 system. Data are presented as mean values ± standard deviation (sd) of at least three biological repeats. Statistical significance of the data was evaluated using Student’s t test for pairwise comparison and analysis of variance (ANOVA), followed by Fisher’s least significant difference (LSD) mean separation test, for multiple comparisons in SPSS at a probability level of P < 0.01 or P < 0.05.
GFP and propidium iodide fluorescence was detected at excitation wavelengths of 488 and 543 nm, respectively, and emission wavelengths of 510 and 620 nm, respectively, using a ZEISS LSM880 NLO. Fluorescence in the confocal images acquired using the same microscope settings (Ruzicka et al., 2007; Zhou et al., 2010) was quantified using the ZEN 2.3 SP1 program. At least 10 seedlings per sample were examined, and at least three independent experiments were performed. The statistical significance was evaluated using Student’s t test.
Whole seedlings or different tissues were cleared in HCG solution for several minutes before microscopy. Histochemical GUS staining was performed as described previously (Chen et al., 2015).
Measurement of IAA levels
Five-day-old wild-type (Col-0) and asa1 asb1 double mutant seedlings were treated with 250 μM AsV for 0 or 12 h. Two-millimeter root tips were excised from the seedlings and immediately frozen in liquid nitrogen. To extract IAA, the root tips were ground to a fine powder in liquid nitrogen and 1 mL of 80% (v/v) methanol was added to each sample and vortexed. The concentration of IAA in each sample was determined by gas chromatography–mass spectrometry (GC–MS), as described previously (Zhou et al., 2010). Data are presented as mean ± sd of at least three biological replicates.
Gene expression analysis by RT-qPCR
Five-day-old Arabidopsis seedlings were treated with 250 μM AsV for different durations, and 2-mm root tips were harvested for RNA extraction. Total RNA was extracted using the OminiPlant RNA Kit (CWbiotech), and cDNA was synthesized from 1 µg of total RNA using the SuperMix Kit (Transgen). Then, qRT-PCR was performed on a CFX Connect Real-Time PCR Detection System (Bio-Rad) using the TransStart Tip Green qPCR SuperMix (Transgen), according to the manufacturer’s instructions. Primers for qRT-PCR were designed using Primer 5 and are listed in Supplemental Table S1. All PCR reactions were performed as independent biological triplicates. Expression levels of genes were normalized to the expression of ACTIN2. Statistical significance of qRT-PCR data was evaluated by Student’s t test for pairwise comparisons and by Fisher’s LSD means separation test for multiple comparisons using SPSS.
ChIP-qPCR assay
ChIP was performed using roots of 8-d-old 35S:ARR1-Myc seedlings (1 g), as previously described (Gendrel et al., 2005; Kaufmann et al., 2010). Briefly, roots were crosslinked in 1% (w/v) formaldehyde, followed by chromatin isolation. Anti-Myc antibody (Abcam, ab32; 1:500) was used to immunoprecipitate protein–DNA complexes, and the precipitated DNA was purified using a PCR purification kit (Qiagen). ChIP experiments were performed in triplicate. Chromatin precipitated without any antibody was used as a negative control, while the isolated chromatin before precipitation was used as the input. The enrichment of DNA fragments was determined by quantitative real-time PCR (qPCR) using primer pairs listed in Supplemental Table S1. Data are presented as the mean ± sd of at least three biological replicates.
EMSA
The EMSAs were conducted using LightShift Chemiluminescent EMSA Kit (Thermo Scientific), according to the manufacturer’s protocol. DNA fragments encompassing ABSs (236–299 amino acids) were cloned into the pGEX-4T-1 vector digested with BamHI and XhoI. The resulting construct was then expressed in Escherichia coli BL21 (DE3) cells to produce GST-tagged ARR1 protein. The recombinant fusion protein was purified using BeaverBeads GSH, according to the manufacturer’s instructions. Annealed double-stranded oligos containing putative ABSs were labeled with biotin and detected using a Chemiluminescent Nucleic Acid Detection Module (Thermo Scientific). Fragments of ASA1 and ASB1 promoters were amplified by PCR using biotin-labeled or unlabeled primers. Unlabeled fragments of the identical sequence with or without the ABSs were used as competitors. Sequences of the probes and primers are listed in Supplemental Table S1.
Y1H screening
The Y1H assay was performed using the Matchmaker One-Hybrid System (Clontech; Mitsuda et al., 2010). To construct the reporter plasmid (bait), a 1,024-bp fragment upstream of the transcription start site (TSS) of ASA1 was amplified using proASA1-Y1H-F/-R primers and cloned into the EcoRI and XbaI sites of pHISi-1. The resulting construct was then linearized and introduced into Saccharomyces cerevisiae strain YM4271, according to the Matchmaker One-Hybrid System user manual (Clontech Laboratories, Inc.). The cDNA library was cloned in the pDEST22 vector. To perform Y1H screening, the strain YM4271 carrying the proASA1-HISi-1 reporter construct was transformed with 4 μg of the cDNA library and plated on synthetic defined (SD) medium lacking His and tryptophan (SD/-His/-Trp) and containing 4 mM 3-amino-1,2,4-triazole (3-AT). More than 10,000 small colonies were recovered and the large positive colonies were selected for further analysis.
Immunoblot assays
Five-day-old ARR1-C1×Ypet, ARR10-C1×Ypet, and ARR12-C1×Ypet seedlings were treated with or without 250 μM AsV for 6 h, and root tissues were harvested for protein extraction. The recombinant fusion proteins were visualized by immunoblotting using anti-GFP antibody (Abcam, ab290). The anti-Actin antibody was used as a loading control.
Transient expression assays in N. benthamiana
Transient expression assays were performed in N. benthamiana leaves as described previously (Matsui et al., 2008; Guo et al., 2015). To generate the effector construct, ARR1 CDS was cloned into the pGreenII62-SK vector downstream of the 35S promoter. Then, 1,024- and 995-bp fragments upstream of the TSSs of ASA1 and ASB1 were amplified using proASA1:LUC-F/-R and proASB1:LUC-F/-R primers, respectively. The PCR products were sequenced and cloned into the pGreenII 0800-LUC vector digested with KpnI and BamHI to produce proASA1:LUC and proASA1:LUC constructs, respectively. To generate mutant variants of ASA1 and ASB1 promoter fragments, proASA1mu and proASB1mu were synthesized (Shanghai Sangon Biotechnology Incorporation, Shanghai, China) and cloned into the pGreenII 0800-LUC vector. The resulting proASB1mu:LUC and proASA1mu:LUC plasmids were used as reporter constructs. The pGreenII 0800-LUC vector harboring the Renilla luciferase (REN) gene under the control of the 35S promoter was used as an internal control. These experiments were performed in three independent biological replicates, each comprising three leaves from different plants. Primer and mutant promoter sequences are listed in Supplemental Table S1.
RNA-Seq and data analysis
Five-day-old, uniform Col-0 seedlings cultivated under normal conditions were selected and continually treated with or without 250 μM AsV for 0, 1, 3, 6, 12, and 24 h. A total of 18 root samples were harvested for transcriptome analysis. Total RNA was extracted using the OminiPlant RNA Kit (ComWin Biotech Co., Beijing, China), according to the manufacturer’s instructions. The quality of total RNA was assessed by a spectrophotometer (ThermoNanoDrop 2000, CA, USA) and Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). Subsequently, total RNA (3 μg) isolated from each sample (with A260/A280 > 2.0) was used for the construction of RNA-Seq libraries, which were sequenced on the Illumina HiSeq 2500 platform, according to the manufacturer’s instructions. Raw sequence reads containing adaptor sequences and low-quality reads were filtered to generate clean reads, which were used for further analysis. The clean reads were mapped onto the Arabidopsis reference genome downloaded from the Ensembl Plants (http://plants.ensembl.org/index.html) using TopHat (v2.0.12; Trapnell et al., 2009). Gene expression levels, expressed as Fragments Per Kilobase of transcript sequence per Millions of base pairs (FPKM), were calculated using Kallisto (v0.44.0), according to the gene length and read count (Bray et al., 2016). Genes showing significant difference in expression between treated and control groups (log2 fold-change > 1.5 and adjusted P-value < 0.05) were identified as DEGs using the DESeq R package (v1.18.0; Anders and Huber, 2012). Gene Ontology (GO) enrichment analysis was performed using AGRIGO (v2.0; http://systemsbiology.cau.edu.cn/agriGOv2/index.php).
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative (AGI) under the following accession numbers: AT5G05730 (ASA1), AT1G25220 (ASB1), At3G16857 (ARR1), At4G31920 (ARR10), At2G25180 (ARR12), AT2G38120 (AUX1), AT5G57090 (PIN2), and AT3G18780 (ACTIN2).
Supplemental data
Supplemental Figure S1. Arsenate (AsV) affects the MZ, EZ, and DZ in Arabidopsis roots.
Supplemental Figure S2. Transcriptome profiling of Col-0 roots in response to arsenate (AsV) stress.
Supplemental Figure S3. Wild type (Col-0) and asa1asb1 response to different stresses.
Supplemental Figure S4. Identification of ASA1 promoter (proASA1)-interacting transcription factors by Y1H screening.
Supplemental Figure S5. ARR1 binds to ASB1 promoter.
Supplemental Figure S6. ARR1 promotes ASA1 expression.
Supplemental Figure S7. Arsenate (AsV) induces ASA1 and ASB1 expression in an ARR1-dependent manner.
Supplemental Figure S8. Expression analysis of ARR1 and ARR10 in 35S:ARR1-SRDX and 35S:ARR10-SRDX root tips by RT-qPCR.
Supplemental Figure S9. Expression dynamics of DR5rev:GFP and TCSn:GFP in Arabidopsis roots in response to arsenate (AsV) stress.
Supplemental Figure S10. Reduced sensitivity of aux1 and pin2 roots to arsenate AsV stress.
Supplemental Figure S11. Effect of arsenate AsV stress on the auxin sensor DII-VENUS.
Supplemental Table S1. List of sequence-specific primers used in this study
Supplementary Material
Acknowledgments
The authors thank Joseph R. Ecker and Xiansheng Zhang for sharing research materials.
Funding
This work was supported by the National Key Research and Development Program of China [2019YFD1000300], the Basic Research Program of Shandong [Grant ZR2018ZC08N1], the Ministry of Agriculture-Chinese [2016ZX08009003-001-006], the Tai-Shan Scholar Program from the Shandong Provincial Government [tsqn20161021 and tsxk20150901], and the National Basic Research Program of China [Grant 2015CB942900].
Conflict of interest statement. The authors declare no competing financial interests.
Q.C., C.L., and J.Z. designed the research. T.T., S.Z., and P.R. performed the experiments. Q.C., C.L., P.R., J.Z., and X.M. analyzed the data. Q.C. and C.L. wrote the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys) is: Qian Chen (chenqiangenetics@163.com).
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci U S A 100:2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W (2012) Differential expression of RNA-Seq data at the gene level—the DESeq package [J]. European Molecular Biology Laboratory (EMBL), Heidelberg, Germany
- Antoniadi I, Plackova L, Simonovik B, Dolezal K, Turnbull C, Ljung K, Novak O (2015) Cell-type-specific cytokinin distribution within the Arabidopsis primary root apex. Plant Cell 27:1955–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyros RD,, Mathews DE, Chiang YH, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE (2008) Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20:2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Mancuso S, Volkmann D, Barlow PW (2010) Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 15:402–408 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82:259–266 [DOI] [PubMed] [Google Scholar]
- Benkova E, Hejatko J (2009) Hormone interactions at the root apical meristem. Plant Mol Biol 69:383–396 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433:39–44 [DOI] [PubMed] [Google Scholar]
- Brackhage C, Huang JH, Schaller J, Elzinga EJ, Dudel EG (2014) Readily available phosphorous and nitrogen counteract for arsenic uptake and distribution in wheat (Triticum aestivum L.). Sci Rep 4:4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L (2016) Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34:525–527 [DOI] [PubMed] [Google Scholar]
- Brunoud G, Wells DM, Oliva M, Larrieu A, Mirabet V, Burrow AH, Beeckman T, Kepinski S, Traas J, Bennett MJ, et al. (2012) A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482:103–106 [DOI] [PubMed] [Google Scholar]
- Bundschuh J, Nath B, Bhattacharya P, Liu CW, Armienta MA, Moreno Lopez MV, Lopez DL, Jean JS, Cornejo L, Lauer Macedo LF, et al. (2012) Arsenic in the human food chain: the Latin American perspective. Sci Total Environ 429:92–106 [DOI] [PubMed] [Google Scholar]
- Cai C, Lanman NA, Withers KA, DeLeon AM, Wu Q, Gribskov M, Salt DE, Banks JA (2019) Three genes define a bacterial-like arsenic tolerance mechanism in the arsenic hyperaccumulating fern Pteris vittata. Curr Biol 29:1625–1633.e1623 [DOI] [PubMed] [Google Scholar]
- Cai XT, Xu P, Zhao PX, Liu R, Yu LH, Xiang CB (2014) Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat Commun 5:5833. [DOI] [PubMed] [Google Scholar]
- Castrillo G, Sanchez-Bermejo E, de Lorenzo L, Crevillen P, Fraile-Escanciano A, Tc M, Mouriz A, Catarecha P, Sobrino-Plata J, Olsson S, et al. (2013) WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 25:2944–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DY, Chen Y, Chen J, Shi S, Chen Z, Wang C, Danku JM, Zhao FJ, Salt DE (2014) Genome-wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biol 12:e1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Dai X, De-Paoli H, Cheng Y, Takebayashi Y, Kasahara H, Kamiya Y, Zhao Y (2014) Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol 55:1072–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Liu Y, Maere S, Lee E, Van Isterdael G, Xie Z, Xuan W, Lucas J, Vassileva V, Kitakura S, et al. (2015) A coherent transcriptional feed-forward motif model for mediating auxin-sensitive PIN3 expression during lateral root development. Nat Commun 6:8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurli A, Lenzi L, Alpi A, Pardossi A (2014) Arsenic uptake and translocation by plants in pot and field experiments. Int J Phytoremediation 16:804–823 [DOI] [PubMed] [Google Scholar]
- Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20:503–508 [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S (2008) A genetic framework for the control of cell division and differentiation in the root meristem. Science 322:1380–1384 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Jones AM, Estelle M (2003) Auxin action in a cell-free system. Curr Biol 13:1418–1422 [DOI] [PubMed] [Google Scholar]
- Du M, Zhai Q, Deng L, Li S, Li H, Yan L, Huang Z, Wang B, Jiang H, Huang T, et al. (2014) Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26:3167–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G, Liu W, Chen X, Hu Y, Zhu Y (2013) Association of arsenic with nutrient elements in rice plants. Metallomics 5:784–792 [DOI] [PubMed] [Google Scholar]
- Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jurgens G, et al. (2002a) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108:661–673 [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002b) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415:806–809 [DOI] [PubMed] [Google Scholar]
- Gendrel AV, Lippman Z, Martienssen R, Colot V (2005) Profiling histone modification patterns in plants using genomic tiling microarrays. Nat Methods 2:213–218 [DOI] [PubMed] [Google Scholar]
- Giehl RF, Gruber BD, von Wiren N (2014) It’s time to make changes: modulation of root system architecture by nutrient signals. J Exp Bot 65:769–778 [DOI] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449:1008–1013 [DOI] [PubMed] [Google Scholar]
- Guo D, Zhang J, Wang X, Han X, Wei B, Wang J, Li B, Yu H, Huang Q, Gu H, et al. (2015) The WRKY transcription factor WRKY71/EXB1 controls shoot branching by transcriptionally regulating RAX genes in Arabidopsis. Plant Cell 27:3112–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley-Whitaker J, Ainsworth G, Vooijs R, Ten Bookum W, Schat H, Meharg AA (2001) Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol 126:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilson P, Allemeersch J, Altmann T, Aubourg S, Avon A, Beynon J, Bhalerao RP, Bitton F, Caboche M, Cannoot B, et al. (2004) Versatile gene-specific sequence tags for Arabidopsis functional genomics: transcript profiling and reverse genetics applications. Genome Res 14:2176–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M (2003) Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 34:733–739 [DOI] [PubMed] [Google Scholar]
- Hiratsu K, Ohta M, Matsui K, Ohme-Takagi M (2002) The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett 514:351–354 [DOI] [PubMed] [Google Scholar]
- Karimi M, Depicker A, Hilson P (2007) Recombinational cloning with plant gateway vectors. Plant Physiol 145:1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Muino JM, Osteras M, Farinelli L, Krajewski P, Angenent GC (2010) Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP). Nat Protoc 5:457–472 [DOI] [PubMed] [Google Scholar]
- Li CX, Feng SL, Shao Y, Jiang LN, Lu XY, Hou XL (2007) Effects of arsenic on seed germination and physiological activities of wheat seedlings. J Environ Sci 19:725–732 [DOI] [PubMed] [Google Scholar]
- Li N, Wang J, Song WY (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol 57:4–13 [DOI] [PubMed] [Google Scholar]
- Liu G, Gao S, Tian H, Wu W, Robert HS, Ding Z (2016) Local transcriptional control of YUCCA regulates auxin promoted root-growth inhibition in response to aluminium stress in Arabidopsis. PLoS Genet 12:e1006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17:1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JL, Miao ZQ, Wang Z, Yu LH, Cai XT, Xiang CB (2016) Arabidopsis ERF1 mediates cross-talk between ethylene and auxin biosynthesis during primary root elongation by regulating ASA1 expression. PLoS Genet 12:e1005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL (2011) Arsenic exposure and the induction of human cancers. J Toxicol 2011:431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE (2005) Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17:3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Umemura Y, Ohme-Takagi M (2008) AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J 55:954–967 [DOI] [PubMed] [Google Scholar]
- Mitsuda N, Ikeda M, Takada S, Takiguchi Y, Kondou Y, Yoshizumi T, Fujita M, Shinozaki K, Matsui M, Ohme-Takagi M (2010) Efficient yeast one-/two-hybrid screening using a library composed only of transcription factors in Arabidopsis thaliana. Plant Cell Physiol 51:2145–2151 [DOI] [PubMed] [Google Scholar]
- Mohan TC, Castrillo G, Navarro C, Zarco-Fernandez S, Ramireddy E, Mateo C, Zamarreno AM, Paz-Ares J,, Munoz R, Garcia-Mina JM, et al. (2016) Cytokinin determines thiol-mediated arsenic tolerance and accumulation. Plant Physiol 171:1418–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L, Di Mambro R, Sozzani R, Pacifici E, Salvi E, Terpstra I, Bao D, van Dijken A, Dello Ioio R, Perilli S, et al. (2013) Spatial coordination between stem cell activity and cell differentiation in the root meristem. Dev Cell 26:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L, Perilli S, Dello Ioio R, Di Mambro R, Costantino P, Sabatini S (2010) The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr Biol 20:1138–1143 [DOI] [PubMed] [Google Scholar]
- Muller B, Sheen J (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453:1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Goeschl C, Filiault D, Mirea M, Slovak R, Wolhrab B, Satbhai SB, Busch W (2019) Root system depth in Arabidopsis is shaped by EXOCYST70A3 via the dynamic modulation of auxin transport. Cell 178:400–412.e416 [DOI] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2:a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlu J, Novak J, Koukalova V, Luklova M, Brzobohaty B, Cerny M (2018) Cytokinin at the crossroads of abiotic stress signalling pathways. Int J Mol Sci 19:2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellan-Alvarez R, Lobet G, Dinneny JR (2016) Environmental control of root system biology. Annu Rev Plant Biol 67:619–642 [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19:2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472 [DOI] [PubMed] [Google Scholar]
- Sanchez-Bermejo E, Castrillo G, del Llano B, Navarro C, Zarco-Fernandez S, Martinez-Herrera DJ, Leo-del Puerto Y, Munoz R, Camara C, Paz-Ares J, et al. (2014) Natural variation in arsenate tolerance identifies an arsenate reductase in Arabidopsis thaliana. Nat Commun 5:4617. [DOI] [PubMed] [Google Scholar]
- Scheres B, Benfey P, Dolan L (2002) Root development. Arabidopsis Book 1:e0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoger ME, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122:793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H,, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642 [DOI] [PubMed] [Google Scholar]
- Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, et al. (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17:2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191 [DOI] [PubMed] [Google Scholar]
- Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, Zhou W, Chen R, Li X, Tietz O, et al. (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21:1495–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15:2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19:2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133:164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7:847–859 [DOI] [PubMed] [Google Scholar]
- Tian H, De Smet I, Ding Z (2014) Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci 19:426–431 [DOI] [PubMed] [Google Scholar]
- Tian Q, Uhlir NJ, Reed JW (2002) Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14:301–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJ (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136:1005–1016 [DOI] [PubMed] [Google Scholar]
- Xie M, Chen H, Huang L, O’Neil RC, Shokhirev MN, Ecker JR (2018) A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat Commun 9:1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z (2014) TAA1-regulated local auxin biosynthesis in the root–apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 26:2889–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Yamashino T, Amano Y, Tajima Y, Imamura A, Sakakibara H, Mizuno T (2007) Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol 48:84–96 [DOI] [PubMed] [Google Scholar]
- Yoon Y, Lee WM, An YJ (2015) Phytotoxicity of arsenic compounds on crop plant seedlings. Environ Sci Pollut Res Int 22:11047–11056 [DOI] [PubMed] [Google Scholar]
- Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ (2013) Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol 23:1979–1989 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559 [DOI] [PubMed] [Google Scholar]
- Zhao Y (2018) Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol 69:417–435 [DOI] [PubMed] [Google Scholar]
- Zhou R, Benavente LM, Stepanova AN, Alonso JM (2011) A recombineering-based gene tagging system for Arabidopsis. Plant J 66:712–723 [DOI] [PubMed] [Google Scholar]
- Zhou W, Wei L, Xu J, Zhai Q, Jiang H, Chen R, Chen Q, Sun J, Chu J, Zhu L, et al. (2010) Arabidopsis tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. Plant Cell 22:3692–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurcher E, Tavor-Deslex D, Lituiev D, Enkerli K, Tarr PT, Muller B (2013) A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol 161:1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.