Abstract
Neurobiological markers of future susceptibility to posttraumatic stress disorder (PTSD) may facilitate identification of vulnerable individuals in the early aftermath of trauma. Variability in resting-state networks (RSNs), patterns of intrinsic functional connectivity across the brain, has previously been linked to PTSD, and may thus be informative of PTSD susceptibility. The present data are part of an initial analysis from the AURORA study, a longitudinal, multisite study of adverse neuropsychiatric sequalae. Magnetic resonance imaging (MRI) data from 109 recently (i.e., ~2 weeks) traumatized individuals were collected and PTSD and depression symptoms were assessed at 3 months post trauma. We assessed commonly reported RSNs including the default mode network (DMN), central executive network (CEN), and salience network (SN). We also identified a proposed arousal network (AN) composed of a priori brain regions important for PTSD: the amygdala, hippocampus, mamillary bodies, midbrain, and pons. Primary analyses assessed whether variability in functional connectivity at the 2-week imaging timepoint predicted 3-month PTSD symptom severity. Left dorsolateral prefrontal cortex (DLPFC) to AN connectivity at 2 weeks post trauma was negatively related to 3-month PTSD symptoms. Further, right inferior temporal gyrus (ITG) to DMN connectivity was positively related to 3-month PTSD symptoms. Both DLPFC-AN and ITG-DMN connectivity also predicted depression symptoms at 3 months. Our results suggest that, following trauma exposure, acutely assessed variability in RSN connectivity was associated with PTSD symptom severity approximately two and a half months later. However, these patterns may reflect general susceptibility to posttraumatic dysfunction as the imaging patterns were not linked to specific disorder symptoms, at least in the subacute/early chronic phase. The present data suggest that assessment of RSNs in the early aftermath of trauma may be informative of susceptibility to posttraumatic dysfunction, with future work needed to understand neural markers of long-term (e.g., 12 months post trauma) dysfunction. Furthermore, these findings are consistent with neural models suggesting that decreased top-down cortico-limbic regulation and increased network-mediated fear generalization may contribute to ongoing dysfunction in the aftermath of trauma.
Subject terms: Amygdala, Predictive markers, Human behaviour
Introduction
Traumatic experiences are unfortunately common within the United States with lifetime prevalence estimates ranging from ~60 to 90% [1, 2]. Trauma exposure can lead to acute and potentially chronic dysfunction in the form of posttraumatic stress disorder (PTSD) [3]. However, there is significant individual variability in susceptibility to PTSD, such that not all trauma exposed individuals will develop PTSD [4, 5]. Given the significant social, emotional, and financial burdens endured by individuals with PTSD, there is a pressing need for early biosignatures of PTSD vulnerability. Such markers may both advance our understanding of PTSD biology as well as guide predictive tools for identifying susceptible individuals. Further, these findings may impact early interventions and treatments to ultimately attenuate the risk and debilitating consequences of the disorder.
Neuroimaging-based markers of PTSD susceptibility have begun to emerge as a potential avenue for the expedited development of novel early identification and intervention tools. Although many individuals self-report heightened stress symptoms in the acute aftermath of trauma, a substantial proportion of traumatized individuals do not go on to develop PTSD, such that self-reports in the acute phase are not always predictive of future PTSD [6, 7]. Although neuroimaging cannot currently replace traditional subjective markers of stress, quantifiable neural markers, such as those identified with neuroimaging, may provide relevant alternative information for identifying future PTSD risk when assessed during the peritraumatic period. Furthermore, understanding neural markers of individual variation may provide biological targets for stratification of heterogeneous symptom variation, advancing both research and clinical approaches to the clinical heterogeneity seen with PTSD [8].
Prior task-based functional magnetic resonance imaging (fMRI) studies have observed that activity within the amygdala, hippocampus, and prefrontal cortex (PFC)—when assessed acutely following trauma—can be predictive of future PTSD. These previous findings are consistent with the view that the development of PTSD reflects disruptions in fear processing and fear inhibition, which is supported by PFC—hippocampal—amygdala circuitry [9–13]. Although task-based fMRI has been used to probe cognitive processes potentially related to PTSD susceptibility, resting-state (i.e., task independent) fMRI provides another avenue towards quantifying neural markers of PTSD susceptibility. Specifically, resting-state fMRI (rs-fMRI) allows for the identification of resting-state networks (RSNs) which represent spatial distributions of synchronized fluctuations in blood oxygen level dependent fMRI responses over time. RSNs reflect spatial patterns of temporal coherence in brain activity and can be identified using standardized and well-validated procedures [14, 15]. In contrast to task-based fMRI, rs-fMRI has several advantages for clinical use given that it does not require external stimuli or presentation equipment and does not have any task demands on patients [16]. Given that trauma exposure may have acute effects on cognitive and neural function during tasks [13], rs-fMRI may have important benefits over task-based fMRI for imaging PTSD susceptibility acutely after trauma. Therefore, imaging of RSNs through rs-fMRI may be a useful tool for generating neural signatures of PTSD susceptibility.
Growing research demonstrates that chronic PTSD is associated with alterations in RSNs such as the default mode network (DMN), salience network (SN), and central executive network (CEN, also referred to as the frontoparietal control network) [17, 18]. The DMN spans the ventromedial PFC, the inferior parietal lobe, the posterior cingulate cortex, and the precuneus and is thought to reflect self-referential or mind-wandering aspects of cognition [19–21]. The SN spans the dorsal anterior cingulate cortex and the anterior insula, and supports attentional processes toward biologically relevant stimuli [22, 23]. Finally the CEN consists predominately of the dorsolateral PFC (DLPFC) with notable extension to inferior parietal lobule, and is thought to support high-level cognitive and executive function [24]. PTSD is associated with disruptions across all three of these functional networks (for review see [18]). For example, individuals with PTSD show greater within-network connectivity of the SN and treatment appears to reverse this increase [25–27]. Notably, it is not entirely clear whether associations between PTSD and the DMN, SN, and CEN are specific to PTSD or reflect broader stress-related psychopathology. Depression emerges equally often as PTSD after trauma, and the two disorders are highly comorbid [28]. Similar to PTSD, depression has been associated with alterations in functional connectivity of important RSNs such as the DMN and SN [29], as well as alterations in subcortical connectivity [30]. This raises the possibility that neural correlates of PTSD susceptibility post-trauma may overlap with correlates of depression susceptibility. Characterizing disorder-specific or psychopathology-general circuits is critical for a more complete understanding of the neurobiology of psychiatric disorders. However, limited research to date has investigated how these RSNs may be related to or predict susceptibility to either PTSD or depression following trauma.
Notably, limited prior work has investigated RSNs in the early aftermath (~2 weeks) of trauma to determine their subsequent relationship with future PTSD symptoms. Previous research in individuals scanned within 2–84 days after trauma has found that variation in region-of-interest-based DMN connectivity with brain regions such as the amygdala and medial PFC is predictive of later PTSD [31–33]. The findings have been mixed such that some have observed greater DMN and amygdala/mPFC coupling associated with greater PTSD [31] while others have observed positive coupling is associated with reduced PTSD [32, 33]. These previous investigations utilized region of interest seeds and thus did not model the entire spatial extent of the DMN or other RSNs which may contribute to the mixed results. The lack of such investigations is a critical gap in our understanding of the neurobiology of PTSD development and more work is needed to better understand how variations in cognitive brain networks may play a role in susceptibility to the disorder. Specifically, although initial relevant evidence suggests that altered within-network connectivity of the DMN, SN, and CEN is associated with early PTSD, investigations assessing network-based susceptibility in the early acute posttrauma period, and among a well-powered and representative participant cohort, are needed.
It is therefore still an open question as to whether RSN alterations occurring early after trauma exposure are predictive of future PTSD development. Therefore, in the present study, we investigated rs-fMRI markers of posttraumatic stress symptom development. We hypothesized that RSN connectivity assessed acutely (~2 weeks) post trauma would predict subsequent PTSD symptom severity assessed at 3 months post trauma. Based on prior findings in chronic PTSD, we predicted greater PTSD symptom severity (at 3 months post trauma) would be associated with decreased connectivity between top-down regulatory regions (e.g., CEN and DMN). In addition to the cortical RSNs that have predominated prior work, we predicted that functional connectivity of subcortical regions, such as the amygdala and hippocampus, may be important to PTSD susceptibility. The amygdala and hippocampus are critical for fear learning and expression processes and show dysfunctional activity [34–36] and disrupted functional connectivity with regulatory regions in individuals with PTSD [37–39]. We therefore predicted greater connectivity among regions supporting threat-related attention and responses (e.g., SN and subcortical regions), and less connectivity between subcortical regions and CEN. Finally, we anticipated that these relationships would be specific to PTSD symptoms and the same relationship would not be observed with depressive symptoms.
Methods and materials
Participants
Participants were recruited from emergency departments (EDs) across the United States as part of the AURORA study, an ongoing multisite longitudinal study of adverse neuropsychiatric sequalae (U01 MH110925, [40]). For study inclusion, participants were required to have experienced a traumatic event that brought them to the ED. Participants were automatically qualified for study enrollment if exposed to: motor vehicle collision, physical assault, sexual assault, fall >10 feet, or mass casualty incidents. Other trauma exposures were also qualifying if: (a) the individual responded endorsed the exposure as involving actual or threatened serious injury, sexual violence, or death, either by direct exposure, witnessing, or learning about it and (b) the assessing research assistant agreed that the exposure was a plausible qualifying event. Participants with a moderate or severe traumatic brain injury were not included in the present study. rs-fMRI data from 161 participants were available. Eleven participants were excluded for MRI issues (one excluded for incomplete data, one excluded due to falx calcification, and nine excluded due to motion criteria described in the Supplementary Information). The analysis focused on acute rs-fMRI data as predictive of future PTSD symptoms. Thus, of the remaining 150 participants, a total of 109 participants who had complete 3-month PTSD assessments (the furthest available timepoint) were retained. Participants were recruited from twenty-two EDs within the Northeast, Southern, mid-Atlantic, or Midwest region of the United States. Participants completed MRI within ~2 weeks of recruitment (M = 17.91 days, SD = 5.82 days) at Emory University, McLean Hospital, Temple University, or Wayne State University. General exclusion criteria for the study are detailed in a prior report [40]. Additional MRI exclusion criteria included metal or ferromagnetic implants, unwillingness to complete MRI, history of seizures or epilepsy, history of Parkinson’s disease, dementia, or Alzheimer’s disease, and/or current pregnancy. Participant demographic information is presented in Table 1. Participants were largely admitted to the ED for a motor vehicle collision (78%) and additional information is available in the Supplementary Materials (Tables S1, S2). All participants gave written informed consent as approved by each study site’s Institutional Review Board.
Table 1.
Demographic information.
| Variable | Count (%)/mean (SD) |
|---|---|
| Sex | |
| Male | 33 (30%) |
| Female | 76 (70%) |
| Age | |
| Years | 35.31 (12.97) |
| Race/ethnicity | |
| Hispanic/Latin American | 17 (16%) |
| White-American | 34 (31%) |
| Black-American | 53 (49%) |
| “Other” American | 5 (4%) |
| Employment | |
| Employed | 69 (63%) |
| Retired | 3 (3%) |
| Homemaker | 3 (3%) |
| Student | 5 (4%) |
| Unemployed/disabled/other | 23 (21%) |
| No response | 6 (6%) |
| Income | |
| <$19,001 | 28 (25%) |
| $19,001–$35,000 | 29 (27%) |
| $35,001–$50,000 | 16 (15%) |
| $50,001–$75,000 | 11 (10%) |
| $75,001–$100,000 | 6 (6%) |
| >$100,000 | 11 (10%) |
| No response | 8 (7%) |
| PCL-5 total scores | |
| 2 Weeks (n = 100) | 28.71 (17.10) |
| 3 Months (n = 109) | 23.30 (16.83) |
| PROMIS depression scores | |
| 2 Weeks (n = 104) | 54.18 (9.81) |
| 3 Months (n = 109) | 53.19 (10.88) |
PCL-5 PTSD Symptom Checklist for DSM-V, PROMIS Patient-Reported Outcomes Measurement Information System.
Psychometric assessment
PTSD symptoms were assessed using the PTSD Symptom Checklist for DSM-V (PCL-5). The PCL-5 is a 20 item self-report questionnaire that assesses the presence and severity of various posttraumatic stress symptoms [41]. Participants rated symptoms on a scale of 0 (not at all) to 4 (extremely) for the severity of each symptom. Depression symptoms were assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS) Depression instrument [42]. The PROMIS questionnaire (short form 8b) has eight-items evaluating depressive symptom frequency scored from 1 (never) to 5 (always). A raw total score was computed from summing the individual items and then converted to a T-score. Both the PCL-5 and the PROMIS were administered at ~2 weeks (i.e., about the time of the MRI) and 3 months post trauma to assess symptoms over the past 2 weeks and past 30 days respectively. Further, although not the focus of the present report, participants also provided medication usage at ~2 weeks post trauma, as well as completed assessments of substance use frequency in the past 2 weeks through the PhenX toolkit [43] (Table S3).
Magnetic resonance imaging
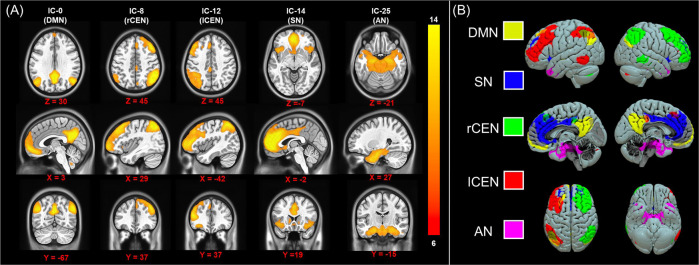
Full details on image acquisition and processing are available in the Supplementary Material. Briefly, rs-fMRI data (TR = 2.36 s, 230 volumes, 9:05 min scan time, eyes open) were acquired on four separate Siemens 3-Tesla MRI systems using largely harmonized scan sequences (Table S4) and were preprocessed using a standardized pipeline via the FMRIPREP software package (detailed in the Supplementary Material, sitewise quality control metric comparisons detailed in Fig. S1). The processed rs-fMRI data were included in a group-level independent components analysis using FSL’s Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) framework to identify RSNs [44]. A total of 28 RSNs were automatically estimated through MELODIC. Five RSNs including the DMN, SN, both left (l) and right (r) CEN, and a network comprising a priori subcortical regions of interest that spanned the amygdala, hippocampus, mamillary bodies, midbrain, and pons—regions thought to be critical to the pathology of PTSD—that we refer to here as the “arousal network (AN)” were included in our analyses (Fig. 1). Participant-specific RSN timeseries and associated RSN spatial maps were obtained using dual regression [45, 46]. Briefly, each group-level RSN spatial map was regressed into each participant’s 4D dataset to obtain participant-specific RSN timeseries. The timeseries were then used as regressors in a general linear model for each participants 4D dataset to derive participant-specific spatial maps for each RSN. The participant-level spatial maps describe the connectivity strength (as a parameter estimate) between each voxel and the participant RSN timeseries. The resultant voxelwise maps can be used to investigate “network-to-node” connectivity patterns [46]. Between-network connectivity was indexed using z-transformed Pearson correlation coefficients between each of the five a priori networks (ten total pairs).
Fig. 1. Resting-state networks.
Group-level independent components (IC) analysis was completed to identify resting-state networks (RSNs) of interest. We identified components that reflect the default mode network (DMN), salience network (SN), left (l) and right (r) central executive networks (CEN), and an arousal network (AN). The spatial maps for each IC/RSN are shown in the left panel (A) and resampled to 1 mm3 resolution for visualization. The right panel (B) shows a 3D visualization of each IC/RSN.
Statistical analyses
Statistical analyses were completed using IBM SPSS version 24 and the Analysis of Functional NeuroImages (AFNI) software package [47]. Network-to-node connectivity analyses were conducted using multiple linear regressions in AFNI’s 3dttest++ to assess voxelwise functional connectivity for each a priori RSN as a function of PCL-5 scores at 3 months post trauma (five models total). Initial analyses focused on parsimonious models and only included dummy-coded covariates to model site/scanner effects. A gray matter mask that included subcortical areas was applied to the data. Cluster-based thresholding implemented in 3dttest++ was used to correct for multiple comparisons. Specifically, we completed permutation testing (10,000 permutations) from the residuals of each multiple linear regression to derive autocorrelation function parameters and define the minimum cluster extent at a cluster forming threshold of p = 0.001 (p = 0.005/5 comparisons) to maintain α = 0.05. Between-network connectivity was included in multiple linear regressions with dummy-coded covariates for scanner within SPSS (one model per connection). Sensitivity analyses for significant associations were completed to determine if these effects held after controlling for 2-week PCL-5 scores, or 3-month PROMIS depression scores respectively using both cluster-restricted and whole-brain analyses. Additional analyses were completed to assess the sensitivity of the effects and to examine if the observed effects also differed by subject characteristics and are detailed in the Supplementary Information.
Results
Participant demographics and psychological characteristics
PCL-5 scores at 3 months were correlated with PCL-5 scores at 2 weeks (r = 0.62, p < 0.001), PROMIS depression scores at 2 weeks (r = 0.80, p < 0.001), and PROMIS depression scores at 3 months (r = 0.79, p < 0.001). These results suggest that posttraumatic outcomes were highly comorbid in the relatively early post-trauma stages.
Network-to-node connectivity and posttraumatic dysfunction
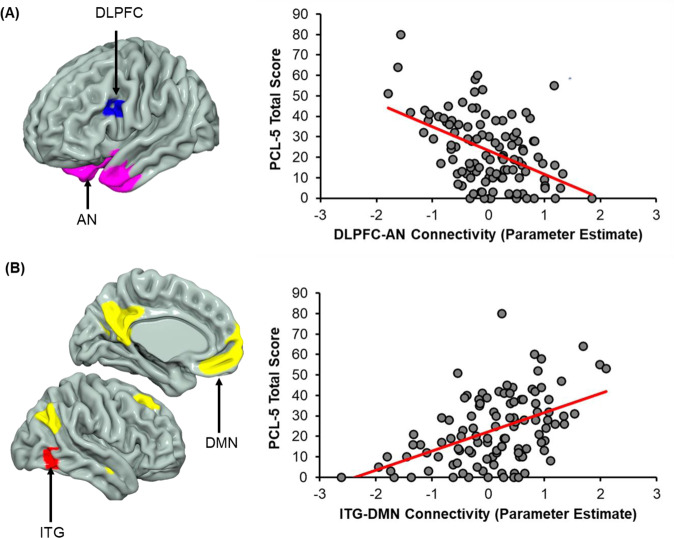
The AN and the DMN showed significant network-to-node connectivity relationships with 3-month PCL-5 scores (Table 2). Greater dorsolateral PFC (DLPFC) to AN connectivity was associated with reduced PCL-5 scores (Fig. 2A). Further, inferior temporal gyrus (ITG) to DMN connectivity was associated with higher PCL-5 scores (Fig. 2B). We further assessed if the present relationships were specific to PTSD symptoms or were also predictive of later depression using the functional connectivity values extracted from the significant clusters in the whole-brain analysis. Both DLPFC-AN connectivity [t (104) = −3.99, β = −0.37, p < 0.001] and ITG-DMN connectivity [t (104) = 3.75, β = 0.35, p < 0.001] significantly predicted PROMIS depression scores at 3 months post trauma.
Table 2.
Network-to-node connectivity associated with 3-month posttraumatic stress severity.
| Structure (Network) | Hemisphere | Z-statistic | Volume | Coordinates (MNI) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
|
Inferior temporal gyrus (Default mode network) |
Right | 4.69 | k = 39 (312 mm3) | 47 | −63 | −9 |
|
Dorsolateral prefrontal cortex (Arousal network) |
Left | −4.79 | k = 30 (240 mm3) | −47 | 11 | 27 |
Location, Z-statistic, cluster extent (k) (23 mm grid spacing), volume, and Montreal Neurological Institute (MNI) coordinates of the peak voxel for clusters that showed a significant (α = 0.05; corrected) relationship with 3-month PCL-5 (PTSD CheckList for DSM-V) scores.
Fig. 2. Network-to-node connectivity of the default mode and arousal networks vary with 3-month posttraumatic stress severity.
A Multiple regression analyses revealed connectivity between the left dorsolateral prefrontal cortex (DLPFC; blue) and the arousal network (AN; pink) varied inversely with 3-month PCL-5 scores. B Conversely, connectivity between the right inferior temporal gyrus (ITG; red) and the default mode network (DMN; yellow) was positively related to PCL-5 scores assessed 3 months post trauma. Scatterplots are not inferential but are included to illustrate the relationship between network-to-node connectivity and PCL-5 scores. Dots represent individual participant scores for connectivity (average of the cluster) and PCL-5 total score. Solid red lines represent the linear line of best fit.
Sensitivity analyses were completed to assess if the observed relationships between RSN connectivity and 3-month PCL-5 scores persisted when controlling for PCL-5 scores at 2 weeks or PROMIS depression scores at 3 months (described in the Supplementary Material). The cluster-restricted analyses (from the prior whole-brain analyses) revealed that DLPFC-AN connectivity was associated with 3-month PCL-5 scores when controlling for 2-week symptoms, whereas ITG-DMN connectivity was not. Additionally, ITG-DMN connectivity was associated with 3-month PCL-5 scores when controlling for 3-month depression symptoms, but DLPFC-AN connectivity was not. Exploratory whole-brain analyses that controlled for 2-week PCL-5 symptoms revealed positive associations between AN connectivity to the postcentral and visual gyri and 3-month PCL-5 symptoms (Table S5).
Between-network connectivity and posttraumatic stress
Multiple regression analyses were completed to assess the relationship among between-network connectivity strengths (i.e., network to network connectivity) and PCL-5 scores. No significant associations were observed. These results suggest that connectivity strengths between full networks are not reflective of susceptibility to heightened PTSD symptoms at 3 months posttrauma.
Participant-specific factors, connectivity, and posttraumatic stress
Consistent with recent calls to consider demographic differences in the neural substrates of psychiatric disorders [48, 49], we completed exploratory analyses to investigate if the observed relationships varied between sex/gender, race/ethnicity, and site (described in the Supplementary Information). However, we note limited samples were available for some of the stratified analyses and the sample was unbalanced with regards to gender (70% women) and race/ethnicity (49% Black-American, 31% White-American) which may impact these exploratory analyses (see Table 1). We observed potential sex differences such that, although ITG-DMN connectivity predicted PCL-5 scores in men and women separately, DLPFC-AN connectivity did not predict PTSD symptoms in men. Further, both DLPFC-AN and ITG-DMN connectivity were generally predictive of PTSD symptoms across racial groups (i.e., Hispanic-American, White-American, and Black-American) assessed. Additionally, despite ~50% of participants having reported hitting their head during the trauma, the DLPFC-AN and ITG-DMN with 3-month PTSD associations were observed in both potentially concussed versus non-concussed individuals.
Discussion
Individual variability in PTSD susceptibility is a significant barrier to enacting early treatment approaches for the disorder. Specifically, it is difficult to predict which individuals are most likely to show long-term dysfunction following trauma. Several clinical studies of early interventions have proved unfruitful, partially due to such variability [50–53]. Identification of neural markers associated with variability in PTSD susceptibility may facilitate development of predictive analytics to quickly identify at-risk individuals who might benefit from intervention. Given the relative ease of data acquisition and standardization in data processing, RSNs may be well-suited to yield potential neural signatures of risk for PTSD. In the current investigation of RSNs in recently traumatized individuals, greater positive coupling of the right ITG to the DMN, and of the left DLPFC to the AN, were predictive of later PTSD symptom severity at 3 months post trauma. Contrary to our hypotheses, these connectivity patterns were not specific to PTSD and were also tied to posttraumatic depression symptom severity which suggests that disruptions in functional connectivity may be related to general posttraumatic dysfunction. The present findings shed important new light on the neural basis of the development of posttraumatic pathology and provide critical insight into the utility RSNs for early assessment of PTSD susceptibility.
Our analyses revealed that positive coupling of the left DLPFC to AN connectivity was associated with reduced PTSD/depression symptoms at 3 months post trauma. These findings are consistent with a recent prospective investigation showing DLPFC-amygdala connectivity in the acute phase following trauma was tied to PTSD symptoms [54]. The DLPFC is also a part of the CEN, and thus these connectivity patterns may potentially reflect some variation in between-network connectivity or communication. In the present study, the AN was comprised of the amygdala, hippocampus, and mamillary bodies, midbrain, and pons, which are regions necessary for the acquisition, behavioral expression, and extinction of fear [55–58]. Importantly, the DLPFC is thought to support cognitive-affective processes to aide in the top-down regulation of the emotional response through functional connections with regions of the AN [59, 60]. In fact, recent neuromodulation studies have noted increased DLPFC activation and connectivity with the amygdala following neurofeedback in individuals with PTSD [61]. Further, the DLPFC is a common site for neurostimulation studies (i.e., transcranial magnetic stimulation) of depression [62] and may be a promising candidate for anxiety and trauma-related disorder treatment due to concurrent attenuation of amygdala activity following DLPFC stimulation [63–65]. Taken together with the prior literature, the inverse relationship between DLPFC-AN connectivity and PTSD severity may suggest that reductions in PTSD symptoms after trauma are partially driven by top-down regulation of the amygdala and arousal-related networks by the DLPFC. Future translational work may be able to test whether neuromodulation of left DLPFC—given its connections with arousal-related regions—in the early aftermath of trauma may promote resilience to outcomes such as PTSD or depression. Importantly, our data show that lowered DLPFC-AN connectivity is not associated with 2-week posttraumatic stress symptoms and thus selecting “high risk” individuals for neuromodulatory interventions may benefit from concurrent assessments of neural circuit features. Future work assessing the generalizability of these connections across multiple samples and trauma types would be beneficial for determining optimal targets for neuromodulation.
Our initial analyses revealed that right ITG to DMN connectivity at 2 weeks post trauma was predictive of PTSD severity at 3 months post trauma, and the same connectivity pattern was also predictive of 2-week PTSD severity (described in the supplement). These data suggest that high ITG-DMN connectivity is predictive of a persistent aspect of PTSD symptoms such that individuals who have relatively high PTSD symptoms in the early phase after trauma are likely to show high symptoms in the later stages of trauma [6, 66]. The DMN is a highly replicable spatial pattern of intrinsic functional connectivity between the ventromedial PFC, posterior cingulate cortex, and inferior parietal lobule [15, 67, 68]. Each of these regions are implicated in important affective processes such as fear learning [69], and the DMN itself is thought to play a significant role in emotional memory and regulation processes [70, 71]. Further, the ITG lays along the ventral visual processing stream and may play a role in visual recognition and memory processes (for review, see [72]). Some data suggests the ITG may also be involved in envisioning future emotional events [73]. One interpretation of the present findings is that greater ITG-DMN connectivity in the early weeks after trauma exposure supports formation, consolidation, and retrieval of emotional, traumatic memories. The facilitation of trauma memory retrieval may then contribute to the pattern of persistently high PTSD symptoms, consistent with “overconsolidation” theories [74]. An alternative possibility is that these patterns are contributing to overgeneralization processes that are observed in individuals with PTSD [75–77]. Prior work suggests ITG activity, as part of the ventral visual stream, may reflect broad, high-level representations of stimuli (e.g., “objects” or “scenes”) [78]. Recent findings indicate fear overgeneralization may occur acutely following trauma exposure [13] and greater neural activity within regions of the DMN, AN, and ventral visual stream have been associated with fear generalization in individuals with chronic PTSD [76, 79]. Future work is needed to further assess whether ITG to DMN connectivity may be related to overconsolidation, overgeneralization, or another disrupted cognitive process relevant to trauma.
Several limitations to the current study should be noted. We note that the current results do not suggest neuroimaging markers can replace typical assessments of psychiatric symptoms, but instead that the findings illustrate that key brain networks partially underlie variability in future expression of posttraumatic symptoms. Relatedly, we further note that it is difficult to infer the specific functions of the observed RSNs. Importantly, it remains somewhat unclear whether the RSN patterns observed in the present study develop early after the trauma or may be true pre-trauma vulnerability factors. Without collection of pre-trauma brain imaging data, although often difficult or infeasible, we cannot state definitively if the present results could be used to identify susceptibility to posttraumatic dysfunction in non-traumatized groups. However, the potential of the present findings to be a trait-like marker of PTSD susceptibility warrants further investigation. Future work should seek to determine if these RSN patterns may be trait markers for posttraumatic dysfunction that may represent a signature of risk for development of PTSD following trauma exposure. In addition, although our results demonstrate the utility of RSNs for susceptibility, the observed patterns are not specific to any one type of posttraumatic outcome (e.g., PTSD or depression). This may be due in part to the high comorbidity of symptoms or shared neural substrates of symptoms in the early aftermath of trauma (~3 months). Further, identifying individuals with high comorbid symptoms may be of great benefit as these individuals may be at the most risk for long-term, chronic posttraumatic dysfunction. Nevertheless, the observed results cannot be said to be specific to a disorder in and of itself, and instead represent general cognitive-affective posttraumatic dysfunction. Finally, limited data for the present analysis was available on RSN associations with later chronic presentations (i.e., 12 months) of posttraumatic symptoms. It remains unclear if the observed associations between rs-fMRI patterns and posttraumatic symptoms is constant in the chronic phase, or if perhaps these findings diverge along different types of posttraumatic symptoms. For example, ITG-DMN connectivity may be associated with posttraumatic dysfunction in general at 12 months while DLPFC-AN connectivity may only be predictive of PTSD at 12 months. Future analyses of the growing AURORA dataset will utilize data collected at later timepoints in the chronic period to investigate these potential outcomes. Further, future research may also consider utilizing longer rs-fMRI sequences. Although ~10 min of rs-fMRI data may be sufficient to reliably measure functional connectivity [80, 81], emerging research suggests longer scans (~60 min) allow for more reliable estimates of individual brain networks [82].
In conclusion, the present study investigated the relationship between resting-state connectivity in the acute aftermath of trauma and future PTSD symptoms. We found that DLPFC-AN and ITG-DMN connectivity was related to future (i.e., 3-month) PTSD symptom severity. Notably, these connectivity patterns were also tied to 3-month depression symptoms. The present findings suggest that resting-state connectivity, assessed in the early aftermath of trauma, is related to later posttraumatic dysfunction. Further, these data suggest rs-fMRI and assessment of RSNs may provide for useful neural signatures of trauma and stress-related dysfunction.
Funding and disclosure
This research was supported by the National Institute of Mental Health K00 MH119603, K01 MH118467, and U01 MH110925, the US Army Medical Research and Material Command, The One Mind Foundation, and The Mayday Fund. DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Posit Science, and Takeda Pharmaceuticals, as well as an honorarium from Alkermes for activities unrelated to the current project. KJR has received consulting income from Alkermes and Takeda, research support from NIH, Genomind and Brainsway, and he is on scientific advisory boards for Janssen and Verily, all of which are unrelated to the present work. RCK has received support for his epidemiological studies from Sanofi Aventis; was a consultant for Datastat, Inc., Sage Pharmaceuticals, and Takeda. CWJ has received funding from Roche Diagnostics, AstraZeneca, Janssen, and Hologic Inc. JME reports support from the National Institutes of Health (NIH) through Grant Numbers R01HD079076 and R03HD094577: Eunice Kennedy Shriver National Institute of Child Health & Human Development; National Center for Medical Rehabilitation Research. SS has received funding from the Florida Medical Malpractice Joint Underwriter’s Association Dr. Alvin E. Smith Safety of Healthcare Services Grant; the NIH/NIA-funded Jacksonville Aging Studies Center (JAX-ASCENT; R33AG05654); and the Florida Blue Foundation. The authors declare no competing interests.
Supplementary information
Acknowledgements
The authors would like to thank Rebecca Price, Adam Hively, Saswati Data, Suraj Oomman, and the other members of the UNC Institute for Trauma Recovery for their efforts and aide in this research. We would also like to thank research staff at McLean Hospital, Emory University, Temple University, and Wayne State University for their efforts and aide. We further express our gratitude to the participants and their families for their willingness to participate in this research.
Author contributions
Design and conceptualization of study: RCK, KCK, SM, and KJR. Data acquisition, recruitment, and logistics: SBH, NMD, JBM, SEB, SLH, FLB, XA, DZ, TCN, GDC, SDL, KAB, SLR, CL, PLH, SS, ABS, PIM Jr., JPH, CWJ, BEP, RAS, MEM, JLP, MJS, KM, AMC, CP, DAP, RMD, NKR, and LDS. Data processing and statistical analyses: NGH, SVR, TDE, JSS. Data interpretation NGH, SVR, TDE, LAML, VPM, TJ, KJR, and JSS. Drafting of the paper: NGH, SVR, LAML, VPM, TJ, KJR, and JSS. All authors revised the paper critically for important intellectual context and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nathaniel G. Harnett, Email: nharnett@mclean.harvard.edu
Jennifer S. Stevens, Email: jennifer.stevens@emory.edu
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00946-8).
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–47. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association Press, Arlington, VA; 2013.
- 4.Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, et al. Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the jerusalem trauma outreach and prevention study (J-TOPS). PLoS ONE. 2013;8:e70084. [DOI] [PMC free article] [PubMed]
- 5.Bonanno GA, Mancini AD. Beyond resilience and PTSD: mapping the heterogeneity of responses to potential trauma. Psychol Trauma Theory Res Pract Policy. 2012;4:74–83. [Google Scholar]
- 6.Galatzer-Levy IR, Huang SH, Bonanno GA. Trajectories of resilience and dysfunction following potential trauma: A review and statistical evaluation. Clin Psychol Rev. 2018;63:41–55. doi: 10.1016/j.cpr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC, Forbes D. A comparison of the capacity of DSM-IV and DSM-5 acute stress disorder definitions to predict posttraumatic stress disorder and related disorders. J Clin Psychiatry. 2015;76:391–7. doi: 10.4088/JCP.13m08731. [DOI] [PubMed] [Google Scholar]
- 8.Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci. 2013;8:651–62. doi: 10.1177/1745691613504115. [DOI] [PubMed] [Google Scholar]
- 9.van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, et al. The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol Psychiatry. 2018;84:106–15. doi: 10.1016/j.biopsych.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 2017;81:1023–9. doi: 10.1016/j.biopsych.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, et al. Amygdala response to negative stimuli predicts ptsd symptom onset following a terrorist attack. Depress Anxiety. 2014;31:834–42. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, et al. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc Natl Acad Sci USA. 2009;106:14120–5. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harnett NG, Ference EW, Wood KH, Wheelock MD, Knight AJ, Knight DC. Trauma exposure acutely alters neural function during Pavlovian fear conditioning. Cortex. 2018;109:1–13. doi: 10.1016/j.cortex.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. [DOI] [PMC free article] [PubMed]
- 17.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. [DOI] [PubMed]
- 18.Akiki TJ, Averill CL, Abdallah CG. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep. 2017;19:81. [DOI] [PMC free article] [PubMed]
- 19.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 21.Poerio GL, Sormaz M, Wang HT, Margulies D, Jefferies E, Smallwood J. The role of the default mode network in component processes underlying the wandering mind. Soc Cogn Affect Neurosci. 2017;12:1047–62. doi: 10.1093/scan/nsx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdallah CG, Averill CL, Ramage AE, Averill LA, Goktas S, Nemati S, et al. Salience network disruption in U.S. army soldiers with posttraumatic stress disorder. Chronic Stress. 2019;3:247054701985046. doi: 10.1177/2470547019850467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdallah CG, Averill CL, Ramage AE, Averill LA, Alkin E, Nemati S, et al. Reduced salience and enhanced central executive connectivity following PTSD treatment. Chronic Stress. 2019;3:247054701983897. doi: 10.1177/2470547019838971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety. 2016;33:592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- 28.Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress. 2013;26:299–309. doi: 10.1002/jts.21814. [DOI] [PubMed] [Google Scholar]
- 29.Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–44. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71:1138–47. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Wang Z, Qin L-d, Wan JQ, Sun Y-W, Su S-S, et al. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS ONE. 2012;7:e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin L-D, Wang Z, Sun YW, Wan JQ, Su SS, Zhou Y, et al. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res. 2012;1484:50–6. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4:317–25. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- 37.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47:1469–78. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR, Neria Y. Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J Psychiatr Res. 2017;94:15–22. doi: 10.1016/j.jpsychires.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–9. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, et al. The AURORA study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry. 2020;25:283–96. [DOI] [PMC free article] [PubMed]
- 41.Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD checklist for DSM-5 (PCL-5) Natl Cent PTSD. 2013;5:2002. [Google Scholar]
- 42.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18:263–83. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, et al. The PhenX toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–60. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. [DOI] [PubMed]
- 45.Nickerson LD, Smith SM, Öngür D, Beckmann CF. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front Neurosci. 2017;11:115. [DOI] [PMC free article] [PubMed]
- 46.Beckmann C, Mackay C, Filippini N, Smith S. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47:S148. [Google Scholar]
- 47.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 48.Seligowski AV, Harnett NG, Merker JB, Ressler KJ. Nervous and endocrine system dysfunction in posttraumatic stress disorder: an overview and consideration of sex as a biological variable. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:381–91. doi: 10.1016/j.bpsc.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harnett NG. Neurobiological consequences of racial disparities and environmental risks: a critical gap in understanding psychiatric disorders. Neuropsychopharmacology. 2020. 10.1038/s41386-020-0681-4. [DOI] [PMC free article] [PubMed]
- 50.Maples-Keller JL, Post LM, Price M, Goodnight JM, Burton MS, Yasinski CW, et al. Investigation of optimal dose of early intervention to prevent posttraumatic stress disorder: a multiarm randomized trial of one and three sessions of modified prolonged exposure. Depress Anxiety. 2020;37:429–37. doi: 10.1002/da.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ressler KJ. Alpha-adrenergic receptors in PTSD—failure or time for precision medicine? N Engl J Med. 2018;378:575–6. doi: 10.1056/NEJMe1716724. [DOI] [PubMed] [Google Scholar]
- 52.Zohar J, Fostick L, Juven-Wetzler A, Kaplan Z, Shalev H, Schreiber G, et al. Secondary prevention of chronic PTSD by early and short-term administration of escitalopram: a prospective randomized, Placebo-Controlled, double-blind trial. J Clin Psychiatry. 2018;79:48–54. doi: 10.4088/JCP.16m10730. [DOI] [PubMed] [Google Scholar]
- 53.Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 54.Belleau EL, Ehret LE, Hanson JL, Brasel KJ, Larson CL, deRoon-Cassini TA. Amygdala functional connectivity in the acute aftermath of trauma prospectively predicts severity of posttraumatic stress symptoms: Functional connectivity predicts future PTSD symptoms. Neurobiol Stress. 2020;12:100217. doi: 10.1016/j.ynstr.2020.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Rooij SJH, Jovanovic T. Impaired inhibition as an intermediate phenotype for PTSD risk and treatment response. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:435–45. doi: 10.1016/j.pnpbp.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behav Neurosci. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- 58.Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proc Natl Acad Sci USA. 2008;105:8108–13. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Comte M, Schön D, Coull JT, Reynaud E, Khalfa S, Belzeaux R, et al. Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb Cortex. 2016;26:144–55. doi: 10.1093/cercor/bhu185. [DOI] [PubMed] [Google Scholar]
- 60.Wheelock MD, Sreenivasan KR, Wood KH, Ver Hoef LW, Deshpande G, Knight DC. Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage. 2014;102:904–12. doi: 10.1016/j.neuroimage.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wheelock MD, Sreenivasan KR, Wood KH, Ver Hoef LW, Deshpande G, Knight DC. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. 2017;38:541–60. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berlim MT, Van Den Eynde F, Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38:543–51. doi: 10.1038/npp.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baeken C, De Raedt R, Van Schuerbeek P, Vanderhasselt MA, De Mey J, Bossuyt A, et al. Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav Brain Res. 2010;214:450–5. doi: 10.1016/j.bbr.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 64.Philip NS, Barredo J, van’t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83:263–72. doi: 10.1016/j.biopsych.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, et al. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am J Psychiatry. 2017;174:1163–74. doi: 10.1176/appi.ajp.2017.16091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shalev AY, Gevonden M, Ratanatharathorn A, Laska E, van der Mei WF, Qi W, et al. Estimating the risk of PTSD in recent trauma survivors: results of the International Consortium to Predict PTSD (ICPP) World Psychiatry. 2019;18:77–87. doi: 10.1002/wps.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015. 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed]
- 68.Harrison BJ, Pujol J, López-Solà M, Hernández-Ribas R, Deus J, Ortiz H, et al. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA. 2008. 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed]
- 69.Goodman AM, Harnett NG, Knight DC. Pavlovian conditioned diminution of the neurobehavioral response to threat. Neurosci Biobehav Rev. 2018;84:218–24. doi: 10.1016/j.neubiorev.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan J, Zhan L, Hu CL, Yang J, Wang C, Gu L, et al. Emotion regulation and complex brain networks: Association between expressive suppression and efficiency in the fronto-parietal network and default-mode network. Front Hum Neurosci. 2018;12:70. [DOI] [PMC free article] [PubMed]
- 71.Satpute AB, Lindquist KA. The default mode network’s role in discrete emotion. Trends Cogn Sci. 2019;23:851–64. doi: 10.1016/j.tics.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci. 2013;17:26–49. doi: 10.1016/j.tics.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008. 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed]
- 74.Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectr. 2005;10:99–106. doi: 10.1017/s109285290001943x. [DOI] [PubMed] [Google Scholar]
- 75.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morey RA, Haswell CC, Stjepanović D, Brancu M, Beckham JC, Calhoun PS, et al. Neural correlates of conceptual-level fear generalization in posttraumatic stress disorder. Neuropsychopharmacology. 2020. 10.1038/s41386-020-0661-8. [DOI] [PMC free article] [PubMed]
- 77.Berg H, Ma Y, Rueter A, Kaczkurkin A, Burton PC, DeYoung CG, et al. Salience and central executive networks track overgeneralization of conditioned-fear in post-traumatic stress disorder. Psychol Med. 2020;1–10. [DOI] [PMC free article] [PubMed]
- 78.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–30. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry. 2015;5:e700. doi: 10.1038/tp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomasi DG, Shokri-Kojori E, Volkow ND. Temporal evolution of brain functional connectivity metrics: Could 7 min of rest be enough? Cereb Cortex. 2017;27:4153–65. doi: 10.1093/cercor/bhw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–29. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D. Influences on the test-retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb Cortex. 2017;27:5415–29. doi: 10.1093/cercor/bhx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.