Abstract
Emerging evidence suggest association of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with the development of many liver abnormalities. The overarching aim of this study was therefore to assess the available evidence on the clinical effects of SARS-CoV-2 on the profiles of liver chemistries and coagulation in COVID-19 diagnosed patients. We considered all study designs including epidemiological and observational that reported liver function test abnormalities in patients confirmed with SARS-CoV-2 infection. Medline, Embase databases and Google Scholar as well as relevant reviews were searched to identify appropriate studies from inception to 31st of August 2020. We calculated the pooled mean with 95% confidence intervals (95% CI) through a random-effect model meta-analysis. A total of 35 studies with 10,692 participants were considered for the review from which 23 studies with sufficient quantitative data were included in the meta-analysis. The pooled mean for liver enzymes and coagulation parameters did not significantly change in patients diagnosed with COVID-19 and remained within normal range. Notwithstanding potential bias from confounding factors in interpretation of data in this review, findings from the observational studies and case reports suggest that COVID-19 does not appear to have a significant impact on the transaminases or total bilirubin levels of patients with confirmed SARS-CoV-2 infection. Further controlled studies and larger sample size observational studies are needed with adequate reporting of other liver function parameters are warranted.
Subject terms: Gastrointestinal diseases, Liver diseases
Introduction
During December 2019, an outbreak of a novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in the Wuhan City, China1. Initially, SARS-CoV-2 was reported as clusters of pneumonia with an unknown etiology2. Since then, the SARS-CoV-2 infection has rapidly and exponentially spread across the globe with majority of the infected cases reported first in Europe (Italy, Spain, UK) followed closely by the US and South America3. On 12th of March 2020, the World Health Organization (WHO) officially recognized and announced COVID-19 as a global pandemic disease. Globally by first week of September 2020, over 26 million people affected with SARS-CoV-2 infection and nearly 865,000 confirmed COVID-19 related deaths have been reported4. Unfortunately, no health care systems were prepared to combat this pandemic effectively resulting in major distress among all governments, health care professionals, researchers and communities worldwide5.
Available evidence suggest that SARS-CoV-2 primarily invades the upper respiratory system and progressively infects the lower respiratory tract6. This virus is thought to enter the respiratory system through infected droplets or mucosal contact by binding to the Angiotensin Converting Enzyme 2 (ACE2) receptor7. While some affected individuals remain asymptomatic8, majority of the individuals diagnosed with the SARS-CoV-2 infection experience fatigue, fever, dry cough as well as shortness of breath, nasal congestion and muscular pain9. Despite these symptoms, more than three-quarters of cases affected with SARS-CoV-2 resolve within 10–14 days of onset. However, the infection results in an estimated 3% mortality rate due to irreversible alveolar damage and progression to the fatal Acute Respiratory Distress Syndrome10. Furthermore, aged populations, individuals at high risk or those with cardiovascular comorbidities such as hypertension, diabetes, Chronic obstructive pulmonary disease and cancer develop a very severe form of the disease with significantly higher fatality rates11. At the time of our study there were no robust evidence for an effective treatment for COVID-19 other than preliminary data indicating dexamethasone therapy with ventilation support reduces mortality in hospitalized COVID-19 patients with severe respiratory complications12. Current preventive strategies to control spread of the SARS-CoV-2 infection include early diagnosis, quarantine and supportive treatment of infected patients13.
SARS-CoV is believed to be a systemic infection with multiorgan involvement including the heart, kidney, pancreas and liver. Nearly half of the SARS-CoV-2 infected individuals exhibit some degree of liver impairment which becomes more evident with the increasing severity of the disease14. In addition to this, RNA from the SARS-CoV-2 has been detected in blood and hepatic cells of the affected patients15–17. The high number of ACE2 receptors on the surface of the cholangiocytes in the liver18,19 bile duct cells20 presumably facilitate entry of SARS-COV-2 to the cells where they replicate leading to dysregulation of the liver function21,22. There is also evidence to suggest that most of the viruses which affect respiratory system are harmful to the liver cells through the CD8+ mediated immune response23.
Although data from a number of studies suggest an association between COVID-19 and abnormal liver function tests (LFTs) irrespective of health care setting24–27, however the evidence from recent epidemiological and clinical studies on LFTs in patients with SARS-CoV-2 infection is largely inconsistent and contradictory24,28–31. The prevalence of liver injury biomarker, alanine transferase (ALT) has been reported up to 32%, 38% and 39% in patients with COVID-19 from China, UK and USA, respectively27,32,33. On the other hand, some studies did not find any significant difference in ALT levels of COVID-19 patients based on the severity of the diseases30,34. Thus, as a result of reported inconsistency in findings for profile of liver chemistries in patients affected by COVID-19, a number of systematic reviews and meta-analyses have recently been published with a focus on the prevalence of abnormalities in liver biochemistry profile among patients affected by COVID-19 based on clinical severity and mortality of the disease26,29,35–38. A recent review of the evidence for the impact of COVID-19 on liver biochemistry profile concluded that despite reports on transient transaminase elevations, most laboratory changes in liver function test profile were mild-moderate and their clinical significance to COVID-19 related liver injuries remains unclear39. Another recent systematic review of available evidence on liver function tests found that although most of studies report significantly higher prevalence of liver test abnormalities in more severe hospitalized or non-surviving COVID-19 patients than milder cases, and given that other studies do not report significant changes in liver function tests of COVID-19 patients irrespective of severity, therefore in COVID-19 patients liver function abnormalities may not be a major characteristic35.
To our knowledge at the time of this publication, the reviews of the evidence for SARS-CoV-2 infection specific impact on the pooled estimate of liver functions is scarce. Therefore, we conducted a comprehensive systematic review with meta-analysis on the pooled mean of LFTs to provide an overview of the available evidence on the impact of SARS-CoV-2 infection on the liver function abnormalities.
Methods
This SR and meta-analysis was followed and conducted according to the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) reporting checklist (see Additional File 1)40.
Search strategy
We searched Medline (via PubMed) and Embase databases without any language restrictions from inception to 31st of August, 2020. Key search terms related to COVID-19 and liver were used (see full list in Table 1). We also searched Google Scholar as well as performed manual searches of citations together with cross-checking of the references of recent reviews published until the 31st of August, 2020.
Table 1.
Search strategies.
| PubMed (Medline) |
1. COVID-19.mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2. Corona.mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 3. exp Coronavirus/ 4. 1 or 2 or 3 5. Liver Diseases/or Liver/or liver abnormalities.mp. 6. exp Liver Cirrhosis/or exp Liver Failure, Acute/or exp Acute-On-Chronic Liver Failure/or exp Liver Function Tests/or exp Liver Failure/ 7. 5 or 6 8. 4 and 7 |
| EMBASE |
1. COVID-19.mp. 2. Exp SARS coronavirus/ 3. Corona.mp. 4. Corona virus.mp. 5. 1 or 2 or 3 or 4 6. exp liver function test/or exp liver dysfunction/or exp liver enzymes or exp liver cell damage or exp liver function/ 7. 5 and 6 |
Inclusion and exclusion criteria
The current analysis included all types of study designs, patients of any age or gender with a confirmed diagnosis of the COVID-19 disease. We excluded letters, editorials, reviews and commentaries. Outcomes of interest were LFTs and coagulation parameters reported for patients hospitalized with SARS-CoV-2 infection in any setting, those who had recovered or non-survivors. We assessed abnormalities in liver chemistry profiles of alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), total bilirubin, albumin, globulin and total protein. We also assessed changes in coagulation profiles of prothrombin time (PT) and activated partial thromboplastin time (aPTT). Studies that reported on patients with a history of liver disorders such as non-alcoholic fatty liver disease, acute/chronic liver injury, cirrhosis, liver failure, fibrosis and other liver related diseases were excluded. We also excluded non-English language studies due to lack of resources for accurate translation.
Two review authors independently assessed all identified studies for removal of duplication as well as initial eligibility assessment of title/abstract of all articles based on the eligibility criteria. Relevant studies were then subjected to full-text screening by the same reviewers. Disagreements were resolved by consensus or discussions with a third review author. The study characteristic and all outcomes of interest, including trial year, study setting and design, number of participants, hospitalization days and death-related information were extracted independently by two review authors.
Data synthesis and statistical analysis
The data on the proportion of patients who had the abnormal (higher or lower) liver function tests were expressed as a percentage in narrative synthesis. The meta-analysis was performed using Review Manager Software. Case reports were excluded from the quantitative synthesis. The Mean and standard deviation (SD) were extracted from the included studies with the conversion of median and IQR to Mean and SD using the method described by Wan et al.41. The outcomes are presented as pooled mean together with the 95% confidence intervals (CI). Statistical heterogeneity of data was assessed using the I2 statistics with the random effect model applied when substantial heterogeneity was present (I2 > 50% or P ≤ 0.10)42. Subgroup analysis was planned where sufficient data from eligible studies was available. A funnel plot was used for visual inspection of publication bias43. A sensitivity analysis was performed by removing the studies with higher outliers to validate the robustness of the analyzed meta-data.
Results
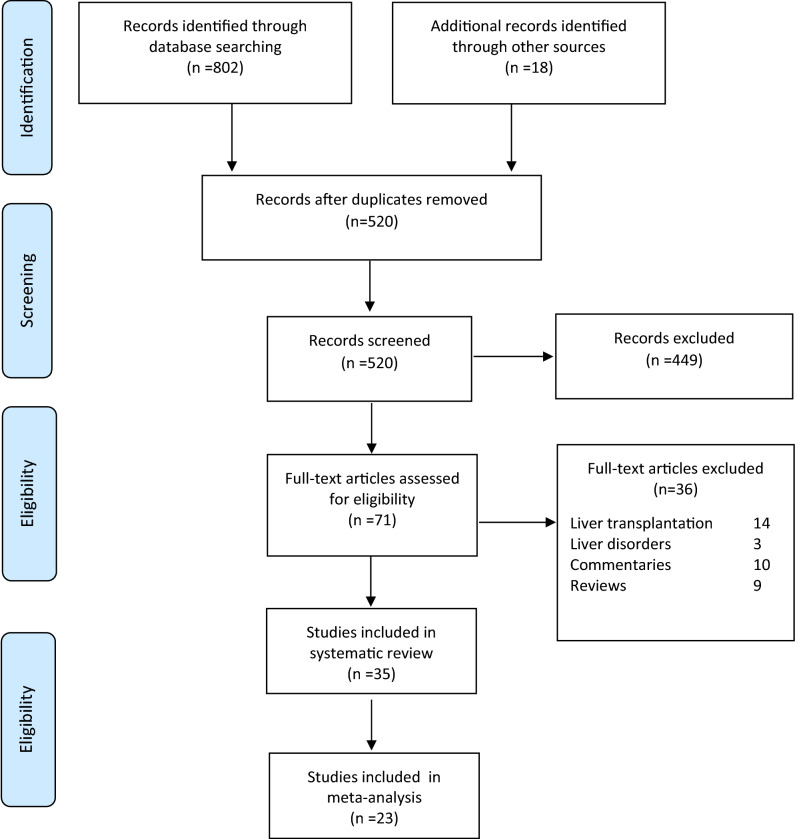
A total of 820 potential studies were identified through database and manual reference searches. After deduplication and screening a total of 35 studies were deemed eligible for this review with only 23 studies considered for the meta-analysis. A detailed study selection process based on PRISMA flow chart is presented in Fig. 1. The degree of agreement between review authors was 94% (33 studies agreed/35 studies) for inclusion of eligible studies and 92% for data extraction.
Figure 1.
Study protocol according to PRISMA flow chart.
Characteristics of the included studies
From the 35 included studies: retrospective studies (n = 25), case series (n = 6), case reports (n = 2) and cross sectional study (n = 2), majority of studies were from China (n = 31; 89%), followed by one study each from Singapore, USA, Germany and France14,25,27,31,44–73. The period of studies spanned from 11th of December 2019 to the 31st of August, 2020. The presence of SARS-COV-2 was confirmed using the molecular based technique of Reverse Transcriptase polymerase chain reaction (RT-PCR), while the diagnosis was based on the Chinese Center for Disease Prevention and Control (CDC) guidelines or WHO interim guidance. Table 2 summarizes the main characteristics of eligible studies.
Table 2.
Characteristics of all included studies.
| Study Author, Year | Country | Study design | Study duration | Diagnostic method | References |
|---|---|---|---|---|---|
| Cai et al. (2020) | China | Cross-sectional study | Jan 11 to Feb 21, 2020 | RT-PCR and recommendations of CDC in China | 46 |
| Schattenberg et al. (2020) | Germany | Case series | March 3 and April 30, 2020 | PCR positive for SARS-CoV-2 by throat swab | 72 |
| Meszaros et al. (2020) | France | Retrospective cohort study | March 10 to April 18, 2020 | Reverse transcription polymerase chain reaction | 69 |
| Qi Wang et al. (2020) | China | Retrospective study | January 12, and March 17, 2020 | New Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Edition 4–6) | 73 |
| Rui Hao et al. (2020) | China | Retrospective, cross-sectional study | January 17 to February 12, 2020 | WHO interim guidance | 71 |
| Shasha Li et al. (2020) | China | Clinical trial | NA | Pneumonia Treatment Plan for the Novel Coronavirus Infection, National Health and Health Commission of the People’s Republic of China (Version 1–6) | 70 |
| Fan et al. (2020) | China | Retrospective study | January 20 through January 31, 2020 | SARS-CoV-2 RNA by reverse transcriptase polymerase chain reaction | 74 |
| Cui et al. (2020) | China | Case report | 17 daysa | RT-PCR | 44 |
| Fan et al. (2020) | China | Retrospective, single-centre study | Jan 20 to 31, 2020 | RT-PCR | 45 |
| Li et al. (2020) | China | Case series | Jan 14 to Feb 13, 2020 | RT-PCR | 47 |
| Qain et al. (2020) | China | Retrospective study | Jan 20, to Feb 24, 2020 | NR | 66 |
| Wang et al. (2020) | China | Retrospective study | Jan 1 to Feb 6, 2020 | RT-PCR | 48 |
| Wu et al. (2020) | China | Retrospective study | Jan 22 to Feb 14, 2020 | RT-PCR | 49 |
| Xie et al. (2020) | China | Retrospective study | Feb 2, to Feb 23, 2020 | According to WHO interim guidance with laboratory-identified COVID-19 | 27 |
| Yang et al. (2020) | China | Retrospective study | Jan 6 to Feb 25, 2020 | RT-PCR and diagnosed by CDC China | 50 |
| Yang et al. (2020) | China | Single-centred, retrospective, observational study | Late-December 2019, to Jan 26, 2020 | According to WHO interim guidance | 31 |
| Yao et al. (2020) | China | Retrospective study | NR | NR | 67 |
| Zhang et al. (2020) | China | Retrospective, single-centre study | Jan 8 to Feb 22, 2020 | Commission of China | 52 |
| Guan et al. (2020) | China | Retrospective study | Dec 11, 2019, to Jan 29, 2020 | By RT-PCR and diagnosis according to WHO interim guidance | 25 |
| Cai et al. (2020) | China | Retrospective study | Jan 11 to Feb 6, 2020 | Based on WHO interim guidance | 53 |
| Xu et al. (2020) | China | Retrospective case series | Jan 10 to 26, 2020 | By RT-PCR and diagnosis according to WHO interim guidance | 54 |
| Mo et al. (2020) | China | Retrospective single-centre study | Jan 1 to Feb 5, 2020 | By RT-PCR | 55 |
| Wang et al. (2020) | China | Case series | Jan 16 to 29, 2020 | By RT-PCR | 56 |
| Zhu et al. (2020) | China | Retrospective study | Jan 24 to Feb 20, 2020 | Nucleic acid amplification | 58 |
| Wan et al. (2020) | China | Case Series | Jan 23 to Feb 8, 2020 | By RT-PCR | 59 |
| Shi et al. (2020) | China | Retrospective study | Dec 20, 2019 to Jan 23, 2020, | Next-generation sequencing or RT-PCR | 60 |
| Huang et al. (2020) | China | Retrospective study | Dec 16, 2019 to Jan 2, 2020 | Next-generation sequencing or RT-PCR | 61 |
| Xu et al. (2020) | China | Case report | Jan 21, 2020 | RT-PCR | 62 |
| Lie et al. (2020) | China | Multicentre retrospective cohort | Dec 20, 2019 to March 8, 2020 | RT-PCR | 75 |
| Wang et al. (2020) | China | Retrospective, single-centre case series | January 1 to Feb 3, 2020 | WHO interim guidance | 64 |
| Yang et al. (2020) | China | Retrospective cohort study | January 17th to February 10th, 2020 | By RT-PCR and diagnosis according to CDC China | 63 |
| Huang et al. (2020) | China | Retrospective study | December 2019 to January 2020 | NR | 68 |
| Qian et al. (2020) | China | Retrospective study | 20 January 2020 to 11 February 2020 | RT-PCR | 65 |
| Young et al. (2020) | Singapore | Descriptive case series | Jan 23 to Feb 3, 2020 | PCR | 51 |
| Arentz et al. (2020) | US | Case series | Feb 20 to March 5, 2020 | By RT-PCR | 57 |
RT-PCR real time polymerase chain reaction, CDC centre for disease control, WHO World Health Organization.
aThe follow-up period.
Characteristics of the included patients
The 35 studies included 10,692 patients diagnosed with SARS-CoV-2 of whom more than half consisted of females (n = 5469, 51.1%). The mean or median age of the patients ranged from 40 to 86 years. The most common comorbidities present in the included patients were diabetes, hypertension, kidney disease, acute cardiac injury, acute respiratory distress syndrome and shock. The major presenting symptoms of SARS-CoV-2 infection were fever, dry cough, expectoration, fatigue, anorexia, myalgia, dyspnea, pharyngalgia, diarrhea, nausea, breathlessness, chest tightness and dizziness. In addition to supportive and palliative care, the mainstay of treatment tailored to the presenting symptoms of the patients included antivirals, antibiotics, corticosteroids or immunoglobulin administration. The demographics, comorbidities, presenting symptoms and treatment modalities of patients diagnosed with COVID-19 are detailed in Table 3.
Table 3.
Characteristics of participants in the included studies.
| Study Author, Year | Sample size | Age (range) | Gender | Chronic diseases/comorbidities | Clinical severity n (%) |
Signs and symptoms (n %) | Treatment n (%) |
|---|---|---|---|---|---|---|---|
| Cai et al. (2020) | 417 | 47 years (33–59)a | Male: 81 (36%); Female: 336 (64%) | Diabetes: 12 (5%); Hypertension: 25 (11%); Liver disease: 4 (1.8%) | Mild: 326 (78.2); Severe: 91 (21.8) | Fever: 147 (65.3); Cough 73 (32.4) | NR |
| Qiu et al. (2020) | 1 | 56 years | Female | Decompensated alcoholic cirrhosis with a history of gastric varices | Severe | Abdominal pain, fever and diarrhoea | Empiric antibiotic (Zosyn and Vancomycin), intravenous hydration |
| Schattenberg et al. (2020) | 44 | 68 years (range 23–86)a | Male: 30 (68%); Female: 14 (32%); | NR | NR | Dyspnoea, fever and cough | Hydroxychloroquine, Protease inhibitor and Antibiotics |
| Meszaros et al. (2020) | 244 | 67 ± 14 years | Male: 149 (63.7%); Female: 95 (36.3%); | Arterial hypertension, Cardiovascular disease, diabetes mellitus, chronic liver disease, Malignancy Immunosuppression and Chronic alcohol consumption | NR | NR | ACEI/ARB B-blockers, Diuretics, Calcium channel blocking agent |
| Qi Wang et al. (2020) | 105 | 45 years (33.5–59.5)a | Male: 56 (53.3%); Female: 49 (46.7%); | Hypertension, Diabetes | Mild: 79 (48.1%); Severe: 26 (69.2%) | Fever and respiratory distress | Antipyretic, nutritional support, recombinant human interferon α-2b, lopinavir ritonavir tablet, reduced glutathione and compound glycyrrhizin |
| Shasha Li et al. (2020) | 159 | 43 years | Male: 90 (56.6%); Female: 69 (44.4%); | Chronic hepatitis B virus, HBV-related cirrhosis, Hypertension, Diabetes, Coronary heart disease and fatty liver | Mild: 125 (78.6%); Severe: 34 (21.4%); | Fever | lopinavir/ritonavir and hydroxychloroquine |
| Fan et al. (2020) | 148 | 50 years (36–64)a | Male: 73 (50.7%); Female: 75 (50.7%); | NR | Mild: 92 (62.2%); Severe: 10 (6.8%) |
Fever 127 (85.8%) Cough 67 (45.3%) Expectoration 38 (26.7%) Diarrhea 6 (4.1%) Nausea and vomiting 3 (2.0%) Asymptomatic 5 (3.4%) With other liver diseases 9 (6.1%) |
Antibiotics Antiviral Arbidol Oseltamivir Antipyretic analgesics |
| Cui et al. (2020) | 1 | 55 days old | Female: 1 | NR | NR | Rhinorrhoea and a dry cough | Inhaled interferon α-1b (15 μg, bid), amoxicillin potassium clavulanate (30 mg/kg, q8h, intravenous glucose tolerance test [IVGTT]), reduced glutathione, ursodeoxycholic acid, and traditional Chinese medicine lotus qingwen |
| Fan et al. (2020) | 148 | 50 yearsb | Male 73 (49.3%); Female 75 (50.7%) | NR | NR | Fever 127 (85.8%); Cough 67 (45.3%); Expectoration 38 (26.7%); diarrhoea 6 (4.1%); Nausea and vomiting 3 (2.0%); Asymptomatic 5 (3.4%); other liver diseases 9 (6.1%) | Antibiotics: 50 (68%); Antiviral: 39 (50.8%); Arbidol: 13 (15.8%); Oseltamivir: 27 (36.5%); Antipyretic and analgesics: 14 (17.3%); No medication: 69 (94.3%) |
| Li et al. (2020) | 25 | 73 years (55–100)a | Male: 10 (40%); Female: 15 (60%) | Hypertension: 16 (64%); Diabetes: 10 (40%); Heart diseases: 8 (32%); Kidney diseases: 5 (20%); Cerebral infarction: 4 (16%), Chronic obstructive pulmonary disease: 25 (8%); Malignant tumours: 2 (8%) and acute pancreatitis: 1 (4%) | NR | NR | NR |
| Qian et al. (2020) | 324 | 51 years (15–88)a | Males: 167 (51.5%) | HBsAg positive: 20 (6.2%); Fatty Liver: 70 (21.6%) | NR | NR | NR |
| Wang et al. (2020) | 339 | 69 years (65–76)a | Male: 166 (49%); Female: 173 (51%) | Hypertension: 138 (40.8%); Diabetes 54 (16.0%); Cardiovascular disease: 53 (15.7%); Cerebrovascular disease: 21 (6.2%); Chronic kidney disease: 13 (3.8%); Chronic liver disease: 2 (0.6%); COPD: 21 (6.2%); Malignancy: 15 (4.4%); Autoimmune disease: 5 (1.5%); Bacterial Infection 143 (42.8%); AKI 27 (8.1%); ARDS 71 (21.0%); Liver Enzyme Abnormalities 96 (28.7%); Acute cardiac injury 70 (21.0%); Arrhythmia 35 (10.4%); Cardiac insufficiency 58 (17.4%); Shock 8 (2.4%) | Moderate: 100 (29.5); Severe: 159 (46.9); Critical: 80 (23.6); | Fever 311 (92.0); Dry cough 179 (53.0); Expectoration 93 (27.5); Fatigue 135 (39.9); Anorexia 94 (27.8); Myalgia 16 (4.7); dyspnoea 138 (40.8); Pharyngalgia 13 (3.9); Diarrhoea 43 12.7); Nausea 13 (3.8); Chest tightness 88 (26.0); Dizziness 13 (3.8); Headache 12 (3.5); | NR |
| Wu et al. (2020) | 80 | 46.10 ± 15.42b | Male: 39 (48.75%); Female: 41 (51.25%) | Cardiovascular and cerebrovascular diseases: 25 (31.25%); Endocrine system diseases: 5 (6.25%); Digestive system disease 3 (3.75%): Respiratory system diseases: 1 (1.25%): Malignant tumor: 1 (1.25%); Nervous system diseases: 1 (1.25%); Chronic kidney disease: 1 (1.25%); Chronic liver disease: 1 (1.25%) | NR | NR | |
| Xie et al. (2020) | 79 | 60 years (27–87)a | Male: 44 (55.7%); Female: 35 (44.3%) | Hypertension: 14 (17.7%); Diabetes mellitus: 8 (10.1%); CHD: 7 (8.9%) | NR | NR | NR |
| Yang et al. (2020) | 92 | 69.8 ± 14.5b 30–97c | Male 49/92 (53.3%); Female 43/92 (46.7%) |
All 65/92 (70.7%); Hypertension 51/92 (56.1%); Heart disease 16/92 (20.7%); Diabetes 13/92 (18.3%); Cerebrovascular disease 10/92 (10.9%); Malignancy 4/92 (4.3%) Chronic liver disease 3/92 (3.3%); Chronic renal insufficiency 2/92 (2.2%); Haematological system disease 2/92 (2.2%); Chronic obstructive pulmonary disease 1/92 (1.1%) |
NR | NR | NR |
| Yang et al. (2020) | 52 | 59·7 ± 13·3b | Female: 17 (33%); Male: 35 (67%) | Chronic medical illness: 21 (40%); Chronic cardiac disease: 5 (10%); Chronic pulmonary disease: 4 (8%); Cerebrovascular disease: 7 (13·5%); Diabetes: 9 (17%); Malignancy: 2 (4%); Dementia: 1 (2%); Malnutrition: 1 (2%); Acute respiratory distress syndrome: 35 (67%); Acute kidney injury: 15 (29%); Cardiac injury: 12 (23%); Liver dysfunction: 15 (29%); Hyperglycaemia: 18 (35%); Gastrointestinal haemorrhage: 2 (4%); Pneumothorax: 1 (2%); Hospital-acquired pneumonia: 6 (11·5%); Bacteraemia: 1 (2%); Urinary tract infection: 1 (2%) | NR | Fever: 51 (98%); Cough: 40 (77%); Dyspnoea: 33 (63·5%); Myalgia: 6 (11·5%); Malaise: 18 (35%); Rhinorrhoea: 3 (6%); Arthralgia: 1 (2%); Chest pain: 1 (2%); Headache: 3 (6%); Vomiting: 2 (4%) | High flow nasal cannula: 33 (63·5%); Mechanical ventilation: 37 (71%); Non-invasive MV: 29 (56%); Invasive MV: 22 (42%); Prone position ventilation: 6 (11·5%); Extracorporeal membrane oxygenation: 6 (11·5%); Renal replacement therapy: 9 (17%); Vasoconstrictive agents: 18 (35%); Antiviral agents: 23 (44%); Antibacterial agents: 49 (94%); Glucocorticoids: 30 (58%); Immunoglobulin: 28 (54%) |
| Yao et al. (2020) | 40 | 22–83c; 53.87 ± 15.84b | Male: 25 (62.5%); Female: 15 (37.5%) | NR | NR | NR | NR |
| Young et al. (2020) | 18 | 47 years (31–73)a | Male: 9 (50%); Female: 9 (50%) | Any: 5 (28%) | NR | Fever: 13 (72); Cough: 15 (83); Shortness of breath: 2 (11); Rhinorrhoea: 1 (6); Sore throat: 11 (61); diarrhoea 3 (17) | Supplemental oxygen: 6 (33%); Admission to ICU: 2 (11%); Mechanical ventilation: 1 (6%); Antiviral: 5 (27.8) |
| Zhang et al. (2020) | 115 | 49.52 ± 17.06b | Male: 49 (42.6%); Female: 66 (57.4%) | NR | Mild: 84 (73%); Severe: 31 (27%) | NR | NR |
| Guan et al. (2020) | 1099 | 47.0 (35.0–58.0)a | Female: 459/1096 (41.9); Male: 637/1096 (58.1) | Any: 261 (23.7); Chronic obstructive pulmonary disease: 12 (1.1); Diabetes: 81 (7.4); Hypertension: 165 (15.0); Coronary heart disease: 27 (2.5); Cerebrovascular disease: 15 (1.4); Hepatitis B infection: 23 (2.1); Cancer: 10 (0.9); Chronic renal disease: 8 (0.7): Immunodeficiency: 2 (0.2) | NR | Fever: 473/1081 (43.8); Conjunctival congestion: 9 (0.8); Nasal congestion 53 (4.8); Headache: 150 (13.6); Cough: 745 (67.8); Sore throat: 153 (13.9); Sputum production: 370 (33.7); Fatigue: 419 (38.1); Haemoptysis: 10 (0.9); Shortness of breath: 205 (18.7); Nausea or vomiting: 55 (5.0); diarrhoea: 42 (3.8); Myalgia or arthralgia: 164 (14.9); Chills: 126 (11.5); Throat congestion: 19 (1.7); Tonsil swelling: 23 (2.1); Enlargement of lymph nodes: 2 (0.2); Rash: 2 (0.2) | NR |
| Cai et al. (2020) | 298 | 47.5 (33–61)a | Male: 145 (48.66); Female: 155 (51.44) | T2DM: 18 (6.04); Hypertension: 47 (15.8); Cardiovascular diseases: 25 (8.39); Liver disease: 28 (9.4); Cancer: 4 (1.3) | Non-severe: 240 (80.5%); Severe: 58 (19.5%) | No symptoms: 30 (10.1); Fever: 218 (73.15); Cough: 105 (35.23); Fatigue: 13 (4.36); Headache: 5 (1.68); diarrhoea: 9 (3.02); Sore throat: 2 (0.67); Nasal congestion: 3 (1.01) | Lopinavir/ritonavir: 236 (79.2); NSAID: 121 (40.6); Corticosteroid: 91 (30.5); Gamma-globulin: 94 (31.5); Need ICU care: 30 (10.1); Invasive mechanical ventilation: 30 (10.1); Extracorporeal membrane oxygenation: 3 (1.0) |
| Xu et al. (2020) | 62 | 41 (32–52)a | Male: 35 (56); Female 27 (44) | Any: 20 (32); Hypertension: 5 (8); Diabetes: 1 (2); Chronic obstructive pulmonary disease: 1 (2); Cerebrovascular disease: 1 (2); Renal diseases: 1 (2); Liver disease: 7 (11) | NR | Fever: 48 (77); Cough: 50 (81); Myalgia or fatigue: 32 (52); Expectoration: 35 (56); Haemoptysis: 2 (3); Headache: 21 (34); Diarrhoea 3 (8) | Antiviral treatment: 55 (89); Interferon alpha inhalation: 8 (13); Lopinavir/ritonavir: 4 (6); Arbidol + interferon alpha inhalation: 1 (2); Lopinavir/ritonavir + interferon alpha inhalation: 21 (34); Arbidol + lopinavir/ritonavir: 17 (28); Arbidol + lopinavir/ritonavir + interferon alpha inhalation: 4 (6); Antibiotics: 28 (45); Corticosteroid and gamma globulin: 16 (26) |
| Mo et al. (2020) | 155 | 54 (42–66)a | Male 86 | Hypertension: 37 (23.9), Diabetes: 15 (9.7), Cardiovascular diseases: 15 (9.7), Cerebrovascular diseases: 7 (4.5), Malignancy: 7 (4.5), Chronic liver diseases: 7 (4.5), Chronic renal diseases: 6 (3.9) | Stable: 63 (40.6); Serious: 55 (35.5); Critical: 37 (23.9) | Fever: 126 (81.3); Cough: 97 (62.6); Chest distress: 61 (39.4); Fatigue: 60 (73.2); Breath shortness: 50 (32.3); Myalgia or arthralgia: 50 (61.0); Anorexia: 26 (31.7) | Oxygen: 102 (65.8); Mechanical ventilation: 36 (23.2); Expectorant: 87 (56.1); Corticosteroid: 79 (51.0); Antiviral treatment: 45 (29.0); Arbidol: 31 (20.0); Lopinavir and ritonavir: 27 (17.4); Interferon inhalation: 30 (19.4); Immune enhancer: 14 (9.0); Thymalfasin: 11 (7.1); Immunoglobulin: 9 (5.8) |
| Wang et.al. (2020) | 69 | 42 (35–62)a | Male: 32 (46%); Female 37 (54%) | Hypertension: 9 (13%); Cardiovascular disease: 8 (12%); Diabetes: 7 (10%); Malignancy: 4 (6%); Asthma: 2 (3%); Chronic hepatitis: 1 (1%) | NR | Sputum production 20 (29%); Dyspnoea 20 (29%); Oppression in chest 14 (20%); diarrhoea 10 (14%); Headache 10 (14%); Anorexia 7 (10%); Chest pain 6 (9%); Pharyngalgia 6 (9%); Dizziness 5 (7%); Palpitation 5 (7%); Vomiting 3 (4%); Cough 38 (55%); Fatigue 29 (42%); Myalgia 21 (30%) | Antiviral therapy: 66 (98.5%); Antibiotic therapy: 66 (98.5%); Antifungal therapy: 8 (11.9%); Use of corticosteroids: 10 (14.9%); Arbidol: 36 (53.7%) |
| Arentz et al. (2020) | 21 | 70b; 43-92c | Male: 11 (52%); Female 10 (48%) | Asthma: 2 (9.1); Chronic obstructive pulmonary disease: 7 (33.3); Congestive heart failure: 9 (42.9); Diabetes: 7 (33.3); Rheumatologic disease: 1 (4.8); Obstructive sleep apnea: 6 (28.6); Chronic kidney disease: 10 (47.6); End-stage kidney disease: 2 (9.5); History of solid organ transplant: 2 (9.5); Cirrhosis 1 (4.8); Immunosuppression: (14.3) | Acute respiratory distress syndrome (ARDS): none: 1 (4.8); Mild: 2 (9.5); Moderate: 6 (28.6); Severe: 12 (57.1) | Cough:11 (47.6); Shortness of breath: 17 (76.2); Fever: 11 (52.4); Temperature (range) °C: 37.6 (35.3–39.2) | Use of non-invasive positive pressure ventilation: 4 (19.0): Use of high-flow oxygen therapy > 15 L/min: 1 (4.8): Required mechanical ventilation: 15 (71.0): Use of prone positioning for ARDS: 8 (50.0); Use of inhaled epoprostenol for ARDS: 5 (31.3); Use of vasopressors: 14 (67.0) |
| Zhu et al. (2020) | 116 | 40 (27‐53)a | Male: 56 (46%); Female: 65 (54%) | Hypertension: 22 (19); Diabetes: 10 (9); Chronic obstructive pulmonary disease: 6 (5); Cerebrovascular disease: 5 (4); Mental disorder: 4 (3); Coronary heart disease: 5 (4); Tumour: 4 (3); Liver disease: 5 (4); Renal diseases: 2 (2) | NR | Fever: 84 (72); Cough: 73 (63); Myalgia or fatigue: 11 (9); Expectoration: 22 (19); Chest stuffiness: 5 (4); Haemoptysis: 1 (1); Headache: 3 (3); diarrhoea: 2 (2) | NR |
| Wan et al. (2020) | 135 | 47 (36‐55)a | Male: 72 (53.3%); Female: 63 (46.7%) | Diabetes: 12 (8.9%); Cardiovascular disease: 7 (5.2%); Hypertension: 13 (9.6); Malignancy: 4 (3.0%); Pulmonary disease: 1 (0.7%); Chronic liver disease: 2 (1.5%); Acute respiratory distress syndrome: 21 (15.6%); Acute cardiac injury: 10 (7.4%); Acute kidney injury: 5 (3.7%); Secondary infection: 7 (17.5%); Shock: 1 (0.7%) | Severe: 40 (29.6%); mild: 95 (70.4%) | Fever: 120 (88.9%);Cough: 102 (76.5′%); Myalgia or fatigue: 44 (32.5%);Headache: 34 (32.5%); Pharyngalgia: 24 (17.7%); diarrhoea: 18 (13.3%); dyspnoea: 18 (13.3%); Chest tightness and shortness of breath:12 (8.8%); Sputum production: 12 (8.8%); Fear of cold: 14 (10.3%); Loss of appetite: 6 (4.4%); Palpitation: 5 (3.7%); Haemoptysis: 4 (3.0%); Retching: 4 (3.0%) | Antiviral therapy: 135 (100%); Antibiotic therapy: 59 (43.7%); Use of corticosteroid: 36 (26.7%); Traditional Chinese medicine: 124 (91.8%); Continuous renal replacement therapy: 5 (3.7%); Oxygen support: 90 (66.7%); Non-invasive ventilation or high‐flow nasal cannula: 34 (25.2%); Invasive mechanical ventilation: 1 (0.7%) |
| Shi et al. (2020) | 81 | 49.5 (11.0)a | Male: 42 (52%), Female: 39 (48%) | Chronic pulmonary disease 9 (11%); Diabetes 10 (12%); Hypertension 12 (15%); Chronic renal failure 3 (4%); Cardiovascular disease 8 (10%); Cerebrovascular disease 6 (7%); Malignancy 4 (5%); Hepatitis or liver cirrhosis 7 (9%) | NR | Fever 59 (73%); Dyspnoea 34 (42%); Chest tightness 18 (22%); Cough 48 (59%); Sputum 15 (19%); Rhinorrhoea 21 (26%); Anorexia 1 (1%); Weakness 7 (9%); Vomiting 4 (5%); Headache 5 (6%); Dizziness 2 (2%); Diarrhoea 3 (4%) | NR |
| Huang et al. (2020) | 41 | 49.0 (41.0–58.0)a | Male: 30 (73%); Female: 11 (27%) | Diabetes 8 (20%); Hypertension 6 (15%); Cardiovascular disease 6 (15%); Chronic obstructive pulmonary disease 1 (2%); Malignancy 1 (2%); Chronic liver disease 1 (2%) | NR | Fever 40 (98%); Cough 31 (76%); Myalgia or fatigue 18 (44%); Sputum production 11/39 (28%); Headache 3/38 (8%); Haemoptysis 2/39 (5%); Diarrhoea 1/38 (3%); Dyspnoea 22/40 (55%); | Antibiotic therapy 41 (100%); Antiviral therapy 38 (93%); Use of corticosteroid 9 (22%) |
| Xu et al. (2020) | 1 | 50 | Male | NR | NR | Fever, chills, cough, fatigue and shortness of breath | Interferon alfa-2b (5 million units twice daily, atomisation inhalation) and lopinavir plus ritonavir (500 mg twice daily, orally) as antiviral therapy, and moxifloxacin (0·4 g once daily, intravenously |
| Lie et al. (2020) | 5771 | 56 (43–65)a | Male: 2,724 (47.2%) | NR | NR | NR | NR |
| Wang et al. (2020) | 138 | 56 (42–68)a | Male: 75 (54.3%); Female: 63 (45.7% | Hypertension 43 (31.2); Cardiovascular disease 20 (14.5); Diabetes 14 (10.1); Malignancy 10 (7.2); Cerebrovascular disease 7 (5.1); COPD 4 (2.9); Chronic kidney disease 4 (2.9); Chronic liver disease 4 (2.9); HIV infection 2 (1.4), | NR | Fever 136 (98.6); Fatigue 96 (69.6); Dry cough 82 (59.4); Anorexia 55 (39.9); Myalgia 48 (34.8); dyspnoea 43 (31.2); Expectoration 37 (26.8); Pharyngalgia 24 (17.4); diarrhoea 14 (10.1); Nausea 14 (10.1); Dizziness 13 (9.4); Headache 9 (6.5); Vomiting 5 (3.6); Abdominal pain 3 (2.2) | Antiviral therapy 124 (89.9); Glucocorticoid therapy 62 (44.9); invasive mechanical ventilation 17 (12.32); extracorporeal membrane oxygenation 4 (2.9) |
| Yang et al. (2020) | 149 | 45.11 ± 13.35b | Male: 81; Female: 68 | Cardio-cerebrovascular disease 28 (18.79%); Digestive system diseases 8 (5.37%); Endocrine diseases 9 (6.04%); Malignant tumour 2 (1.34%); Neural system diseases 0 (0%); Respiratory system diseases 1 (0.67%); Others 4 (2.68%) | NR | Fever 114 (76.51%); cough 87 (58.39%); Expectoration 48 (32.21%); dyspnoea 2 (1.34%); Muscle pain 5 (3.36%); Headache 13 (8.72%); Sore throat 21 (14.09%); Snotty 5 (3.36%); Chest pain 5 (3.36%); Chest tightness 16 (10.74%); Chill 21 (14.09%); diarrhoea 11 (7.38%); Nausea and vomiting 2 (1.34%) | Antibiotic treatment 34 (22.82%); Antifungal treatment 0 (0.0%); Antiviral treatment 140 (93.96%); Interferon administration 144 (96.64%); Glucocorticoids 5 (3.36%); Immunoglobulin therapy 19 (12.75%) |
| Huang et al. (2020) | 34 | 56.24 ± 17.14b | Male: 14 (41.2%); Female: 20 (58.8%) | Diabetes 4 (11.8%); Hypertension: 8 (23.5%); Cardiovascular disease: 6 (17.6%); Chronic obstructive: 1 (2.9%); Pulmonary disease: 2 (5.9%); Malignancy: 3 (8.8%); Chronic liver disease: 1 (2.9%); Hyperuricemia: 1 (2.9%); Hypothyroidism: 2 (5.9%); HIV infection: 2 (5.9%) | NR | Fever: 32 (94.1%); Cough: 17 (50.0%); Myalgia or fatigue: 22 (64.7%); Sputum production: 8 (23.5%); Headache: 2 (5.9%); diarrhoea: 5 (14.7%); dyspnoea: 5 (14.7%) | Antibiotic therapy: 31 (91.2%); Antiviral therapy (other drugs but not lopinavir/ritonavir): 32 (94.1%); Antiviral therapy (switch to lopinavir/ritonavir later): 9 (26.5%); Use of corticosteroid 21 (61.8%) |
| Qian et al. (2020) | 91 | 50 (36.5–57)a | Female: 54 (59.34%); Male: 37 (40.66%) | Hypertension: 15 (16.48%); Diabetes Mellitus: 8 (8.79%); Cardiovascular and cerebrovascular disease: 3 (3.30%) | NR | Fever 65 (71.43); unknown 2 (2.2); cough 55 (60.44%); Fatigue 40 (43.96%); Expectoration 30 (32.97%); Anorexia 23 (25.27%); diarrhoea 21 (23.08%); Chest distress: 17 (18.68%); Nausea: 11 (12.09%); Shortness of breath: 10 (10.99%); dyspnoea: 3 (3.3%); Headache7 (7.69%); Vomiting 6 (6.59%); Myalgia: 5 (5.49%); Back discomfort 3 (3.3%) | NR |
T2DM type 2 diabetes mellitus, ACEi/ARBi Angiotensin II Receptor Blockers and angiotensin-converting enzyme inhibitor, NR not reported.
aThe Median with IQR.
bMean with SD.
cThe range.
Abnormalities of the plasma proteins
Albumin
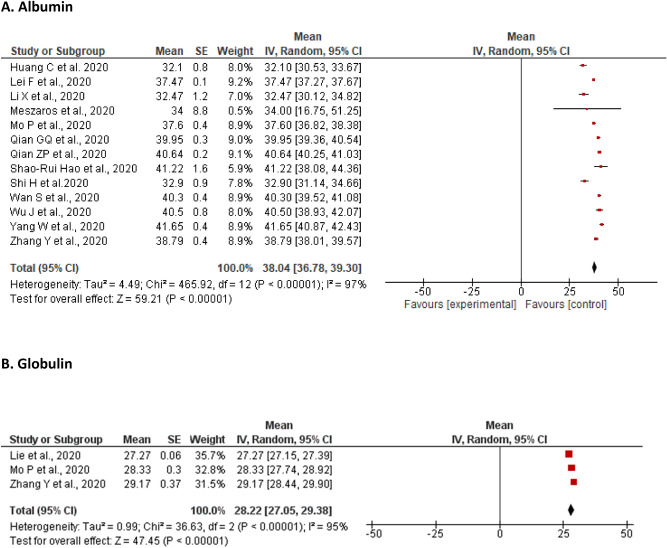
A meta-analysis of 13 studies revealed that the pooled mean of albumin levels was 38.04 g/L [36.78, 39.30] with a significant level of heterogeneity (I2 = 97%, P < 0.00001). Majority of the studies did not report the number of patients who showed changes in albumin levels. However, Yang et al. and Huang et al. reported elevation of albumin in 2% (n = 3) and 20.6% (n = 7) of affected patients, respectively (Fig. 2A). In contrast, lower level of albumin were reported widely ranging from 6% (n = 9)63 to 100% (n = 5)47.
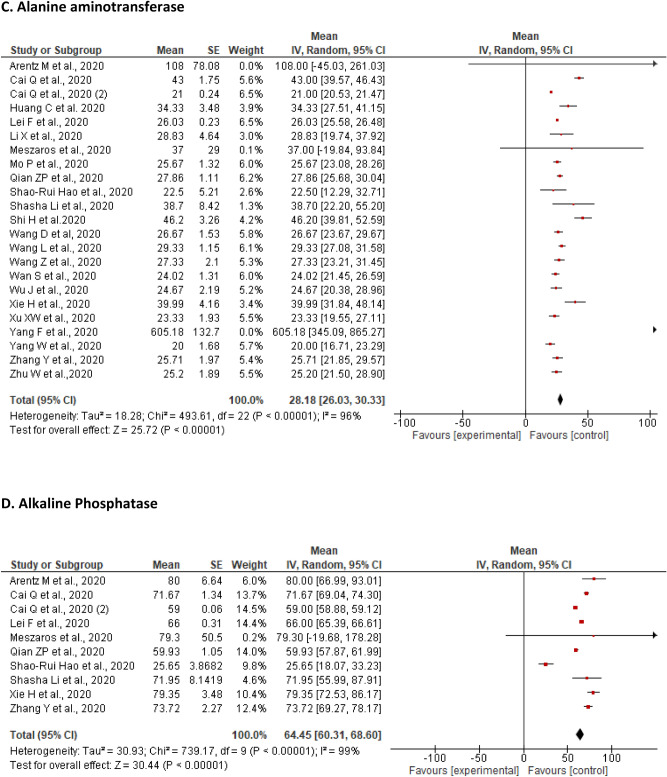
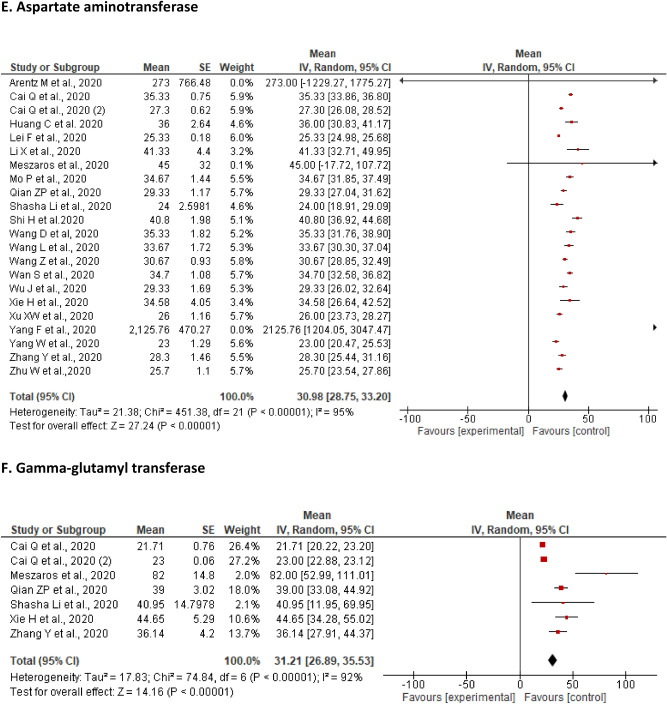
Figure 2.
Meta-analyses of the pooled mean of Liver enzymes. (A) Albumin; (B) Globulin; (C) Alanine aminotransferase; (D) Alkaline phosphatase; (E) Aspartate aminotransferase; (F) Gamma-glutamyl transferase.
Globulin
The pooled mean of globulin level from three studies was found to be 28.22 g/L [27.05, 29.38] with a significant level of heterogeneity (I2 = 95%, P < 0.00001). Only Zhang et al. reported an elevation of globulin in a third of the included patients (n = 42; 36.5%) (Fig. 2B).
Total protein
Total protein was only reported by Xu et al. and estimated to be 56 g/L in the patients with SARS-CoV-2 infection.
Alanine aminotransferase (ALT)
A total of 23 studies reported on changes in ALT levels of the included patients. The pooled mean of ALT was found to be 28.18 U/L [26.03, 30.33] with a significant level of heterogeneity (I2 = 96%, P < 0.00001). The proportion of patients with an elevated ALT level ranged from 3.75% (n = 3)49 to 36.79% (n = 117)48,50,53,64, while a decrease from the lower limit of ALT levels varied from 2 (1.34%) to 67% (n = 46)56 (Fig. 2C).
A sensitivity analysis performed by removing two studies50,57 with outlier ALT values did not show a noticeable change in the pooled results revealing mean of 28.65 U/L [26.47, 30.83] with a significant heterogeneity (I2 = 92%, P < 0.00001) (Additional File 2, Supplementary Data Fig. S1).
Alkaline phosphatase (ALP)
A total of ten studies reported on changes in levels of ALP of the included patients. The pooled mean for ALP was found to be 64.45 U/L [60.31, 68.60] with a significant level heterogeneity (I2 = 99%, P < 0.00001). The proportion of patients with an elevated ALP levels ranged from 0.3% (n = 1) to 6.6% (n = 21)46,53, while a decrease from the lower limit of ALP levels was not reported by any study (Fig. 2D).
Aspartate aminotransferase (AST)
A total of 22 studies reported on the changes in AST levels of the included patients. The pooled mean of AST was found to be 30.98 U/L [28.75, 33.20] with a significant level of heterogeneity (I2 = 95%, P < 0.00001). The proportion of patients with an elevation of AST ranged from 3.75% (n = 3)49 to 36% (n = 9)47. In contrast, a decrease from the lower limit of AST level (n = 50; 72%) was reported only by Wang et al. (Fig. 2E).
The sensitivity analysis performed by removing two studies with outliers did not alter the findings of original analysis of the mean 32.01 U/L [29.61, 34.42] with the heterogeneity of I2 = 96%; P < 0.00001 (Additional File 2, Supplementary Fig. S2).
Total bilirubin (TB)
A total of 17 studies reported on the changes in levels of TB of the included patients. The pooled mean of TB was found to be 11.36 μmol/L [10.35, 12.38] with a significant level of heterogeneity (I2 = 96%, P < 0.00001). The proportion of patients with an elevated TB level ranged from 1.25% (n = 1)49 to 44.02% (n = 140)46. Whereas, only Yang et al., 2020 reported a decrease in TB levels among seven patients (4.70%) (Supplementary Fig. S3).
A sensitivity analysis by removing two studies demonstrated no noticeable change in the overall results (11.25 μmol/L [10.27, 12.22]) with the heterogeneity (I2 = 96%, P < 0.00001) (Additional File 2, Supplementary Data Fig. S4).
Gamma-glutamyl transferase (GGT)
A total of seven studies reported on the levels of GGT in the included patients. The pooled mean of GGT was found to be 31.21 U/L [26.89, 35.53] with a significant level of heterogeneity (I2 = 96%, P < 0.00001). The proportion of patients with an elevated level of GGT ranged from 0.9% (n = 3)66 to 28.61% (n = 91)53. However, none of the studies reported a decreased level of GGT among the affected patients (Fig. 2F).
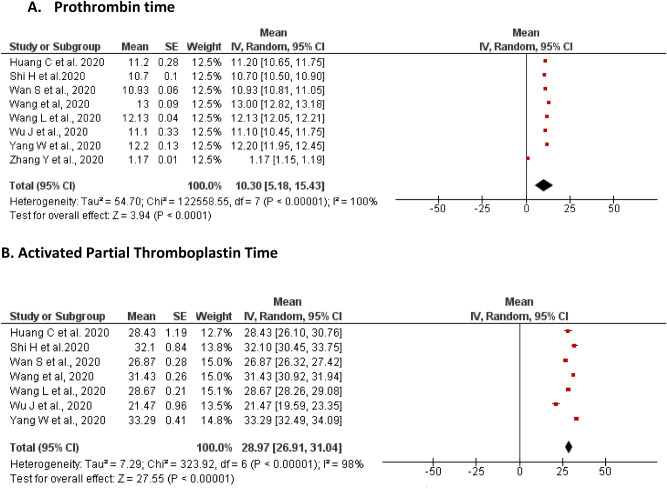
Prothrombin time (PtT)
A total of eight studies reported on changes in the levels of PtT of the included patients. The pooled mean of PtT was found to be 10.30 s [5.18, 15.43] with a significant level of heterogeneity (I2 = 100%, P < 0.00001). An elevation of PtT was reported by Zhang et al. and Yang et al. among 52.2% (n = 60) and 11.41% (n = 17) of the patients, respectively. In contrast, a decrease from the lower limit in PtT levels was reported by Yao et al. (7.5%, n = 3), Wu et al. (3.75%, n = 3) and Yang et al. (2.68%, n = 4) (Fig. 3A).
Figure 3.
Meta-analyses of the pooled mean of coagulation parameters. (A) prothrombin time; (B) Activated Partial Thromboplastin time.
Activated partial thromboplastin time (aPTT)
A total of seven studies reported on the levels of aPTT in the included patients. The pooled mean of aPTT was found to be 28.97 s [26.91, 31.04] with a significant level of heterogeneity (I2 = 100%, P < 0.00001). An elevation of aPTT was reported by Yang et al., among quarter of the patients (n = 40; 26.85%). In contrast, a decrease from the lower limit of aPTT level was reported by Wu et al., in only two patients (Fig. 3B).
Publication bias
The visual inspection of the funnel revealed that all studies concentrated on a single point which could represent a potential publication bias in the studies.
Discussion
We have shown in this systematic review with meta-analysis that most of the liver enzymes and coagulation parameters in patients diagnosed with SARS-CoV-2 infection are not significantly impacted by COVID-19. We have presented an in depth analysis of pooled mean data for liver chemistries and each of the liver function test markers in patients diagnosed with SARS-CoV-2 infection upon hospital admission. The same findings were observed with the prothrombin and activated prothrombin time. In these patients, the enzymatic liver function tests for albumin, globulin, ALT, ALP, AST, GGT and TB together with the coagulation profile were not significantly associated with COVID-19 at initial presentation.
In this review, all the mean values of liver function tests were found to be within the normal range and not associated with disease progression consistent with the findings of Wang et al.38 and Mantovani et al.31 meta-analyses. Although Wang et al. conducted a meta-analysis on gastrointestinal symptoms in patients with COVID-19, however they only reviewed evidence of liver injury qualitatively. Their review of evidence on liver injury in patients with COVID-19 revealed that 2.6–53% of the affected patients had abnormal levels of ALT, AST and TB based on the severity of the disease38. A recent meta-analysis of observational studies on the prevalence of liver injuries in patients with SARS-CoV-2 infection further showed a relatively low association at initial presentation but reported important changes in liver enzymes of the patients with severe form of the disease37. However, similar to the systematic review by Wang et al.38, the study by Mantovani et al.31 did not include a meta-analysis of liver function tests whereas we analysed pooled means of liver enzymes as well as the profiles of coagulative and fibrinolytic pathways in patients with COVID-19.
A meta-analysis conducted by Mao et al. on the prevalence of abnormal liver enzyme levels in confirmed COVID-19 patients, reported the pooled prevalence of elevated ALT, AST, and TB among patients with COVID-19 as 18%, 21% and 6% respectively26. Our meta-analysis also revealed reduced ALT and albumin levels in patients with COVID-19 albeit with wide ranges. However, only one study reported reduced AST and TB levels (72% and 4.70%, respectively)56. Although we did not find any increase in the coagulation time, nevertheless the physicians need to be vigilant and closely monitor the coagulation profile of the patients with COVID-19. Additionally, we found elevated levels of GGT, PtT, and ALP in several studies. Of note, none of the studies evaluated the proportion and mean changes in total protein.
The underlying mechanism of liver injury in patients with SARS-CoV-2 infection is poorly understood35. Several potential mechanisms have been proposed. There is evidence to suggest that liver functional impairment in the COVID-19 patients could have resulted from drug-induced hepatotoxicity76. Another mechanism for liver injury involves the inflammatory response of immune system, especially cytokine storms which can lead to the damage of the liver cells77. While contribution from other viral infections or drug-induced toxicity cannot be ruled out, given the propensity of data on abnormal liver tests in severe cases of COVID-19 it is plausible that liver injury may also occur from the direct effects of SARS-CoV-2 as part of a complex multifactorial mechanism but further focused research is needed to clarify the contribution of abnormal liver tests to the pathogenetic mechanism of COVID-1924,35,36.
Limitations
There are several limitations and confounding factors that my present as potential bias and therefore results should be interpreted with caution. The major imitation of this review was the presence of a high level of heterogeneity across all included studies. We could not assess sources of heterogeneity in our included studies, due to lack of reporting of subgroup data such as comorbidities, hospitalization (ICU setting) and asymptomatic patients. There was also a significant publication bias noted in this meta-analysis. Moreover, we did not conduct quality assessment of included studies due to the low number of studies reporting assessment of liver function tests as the primary outcome, hence assessing the quality of assessment was deemed not appropriate. In addition, most studies were retrospective with small sample sizes and we only identified one controlled trial.
Since COVID-19 is a rapidly evolving disease and given majority of our included studies were from China, generalizability of these results are not possible and therefore ethnic subgroup analysis and epigenetics were not possible. One of the main drawbacks of the currently available literatures on COVID-19 is the lack of control group reporting in studies. Hence, there is a growing need to compare the laboratory liver function tests conducted in the primary studies with a comparator or a control group. Another limitation is the lack of sufficient data on the proportion of patients with changes in liver function parameters at discharge. Furthermore, most studies did not report on some of the variables that we investigated including globulin, GGT, total protein and coagulation profile. Therefore, to assess whether there is a causal association between SARS-CoV-2 infection and liver injury, it is important that future studies report other important liver function tests, the proportion of patients with altered levels of liver function parameters and number of patients who have recovered from COVID-19 at discharge.
Conclusion
The findings from the available evidence to date from observational studies and case reports indicate that at least the transaminases and total bilirubin levels in COVD-19 diagnosed patients appear not to have significantly changed. Future studies would benefit from inclusion of a control group and larger sample size observational studies are needed with reporting of the number of patients with changes in levels of liver function abnormalities.
Supplementary Information
Author contributions
K.B., M.A. and N.M. contributed to the conception and design of the review and critically revised the manuscript drafts until the final version was approved. N.J. performed the database searches. K.B. and N.J. conducted assessment of eligibility, data extraction, analysis and interpretation. K.B., D.B. and J.A. were responsible for the drafting and revisions of the manuscript. All authors have made substantive contributions to the article and assume full responsibility for its content. All authors have seen and approved the final version of the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-89513-9.
References
- 1.Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Diseases IJID Off. Publ. Int. Soc. Infect. Diseases. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun J, et al. COVID-19: Epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020;26:483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhikari SP, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Coronavirus disease (COVID-19): Weekly epidemiological update, 11 October 2020. (2020).
- 5.Kannan S, Ali PSS, Sheeza A, Hemalatha K. COVID-19 (novel coronavirus 2019—Recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- 6.Tian S, et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin, X. et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 69(6), 1002–1009. 10.1136/gutjnl-2020-320926 (2020). [DOI] [PMC free article] [PubMed]
- 10.Jiang F, et al. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J. Gen. Intern. Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo, W. et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev.10.1002/dmrr.3319 (2020). [DOI] [PMC free article] [PubMed]
- 12.Horby, P. et al. Dexamethasone in hospitalized patients with COVID-19-preliminary report. N. Engl. J. Med.383(21), 2030–2040 10.1056/NEJMoa2022926. (2020). [DOI] [PMC free article] [PubMed]
- 13.Pascarella, G. & Strumia, A. COVID-19 diagnosis and management: a comprehensive review. J Intern Med.288(2), 192–206 10.1111/joim.13091 (2020). [DOI] [PMC free article] [PubMed]
- 14.Qi X, et al. Multicenter analysis of clinical characteristics and outcome of COVID-19 patients with liver injury. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/s2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng G, et al. COVID-19 and liver dysfunction: Current insights and emergent therapeutic strategies. J. Clin. Transl. Hepatol. 2020;8:18–24. doi: 10.14218/jcth.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang, J. et al.Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020 May 26;117(21):11727-11734 10.1073/pnas.2003138117 (2020). [DOI] [PMC free article] [PubMed]
- 19.Chai, X. et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv (2020). 10.1101/2020.02.03.931766
- 20.Hamming I, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020;127:110195–110195. doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am. J. Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolini A, et al. Abnormal liver function tests in patients with COVID-19: Relevance and potential pathogenesis. Hepatology (Baltimore, MD) 2020;72:1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao R, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie, H., Zhao, J. & Lian, N. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020 Jun;40(6):1321–1326. 10.1111/liv.14449 (2020). [DOI] [PMC free article] [PubMed]
- 28.Kulkarni AV, et al. Systematic review with meta-analysis: Liver manifestations and outcomes in COVID-19. Aliment. Pharmacol. Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kukla M, et al. COVID-19, MERS and SARS with concomitant liver injury—Systematic review of the existing literature. J. Clin. Med. 2020;9:1420. doi: 10.3390/jcm9051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lok, J. & Gess, M. Liver dysfunction in COVID-19: A useful prognostic marker of severe disease? Frontline Gastroenterology (2020). 10.1136/flgastro-2020-101689 [DOI] [PMC free article] [PubMed]
- 33.Richardson S, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan S, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali N, Hossain K. Liver injury in severe COVID-19 infection: Current insights and challenges. Expert Rev. Gastroenterol. Hepatol. 2020;14:879–884. doi: 10.1080/17474124.2020.1794812. [DOI] [PubMed] [Google Scholar]
- 36.Ali N. Relationship between COVID-19 infection and liver injury: A review of recent data. Front. Med. 2020;7:458. doi: 10.3389/fmed.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantovani, A., Beatrice, G. & Dalbeni, A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020 Jun;40(6):1316-1320. 10.1111/liv.14465 (2020). [DOI] [PubMed]
- 38.Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2020 doi: 10.1016/j.clinre.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrido I, Liberal R, Macedo G. COVID-19 and liver disease—What we know on 1st May 2020. Aliment. Pharmacol. Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG, Group, P Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delgado-Rodríguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med. Intensiva. 2018;42:444–453. doi: 10.1016/j.medin.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui Y, et al. A 55-day-old female infant infected with 2019 novel coronavirus disease: Presenting with pneumonia, liver injury, and heart damage. J. Infect. Dis. 2020;221:1775–1781. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan, Z. et al. Clinical features of COVID-19-related liver damage. Clin. Gastroenterol. Hepatol.18(7), 1561-1566. 10.1016/j.cgh.2020.04.002 (2020). [DOI] [PMC free article] [PubMed]
- 46.Cai Q, et al. COVID-19: Abnormal liver function tests. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Diseases IJID Off. Publ. Int. Soc. Infect. Diseases. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: A multicenter descriptive study. Clin. Infect. Diseases Off. Publ. Infect. Diseases Soc. Am. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang F, et al. Analysis of 92 deceased patients with COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young BE, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, et al. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan City, China. Liver Int. Off. J. Int. Assoc. Study Liver. 2020 doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 53.Cai Q, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province. China. 2020 doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 54.Xu, X. W. et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. 368, m606. 10.1136/bmj.m606 (2020). [DOI] [PMC free article] [PubMed]
- 55.Mo P, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Diseases Off. Publ. Infect. Diseases Soc. Am. 2020 doi: 10.1093/cid/ciaa270. [DOI] [Google Scholar]
- 56.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Diseases Off. Publ. Infect. Diseases Soc. Am. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arentz M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu W, et al. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei. China. J. Med. Virol. 2020 doi: 10.1002/jmv.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan, S., Xiang, Y., Fang, W. & Zheng, Y. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol92(7), 797–806 10.1002/jmv.25783 (2020). [DOI] [PMC free article] [PubMed]
- 60.Shi H, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet. Infect. Dis. 2020;20:425–434. doi: 10.1016/s1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Z, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang W, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian GQ, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM Monthly J. Assoc. Phys. 2020 doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qian ZP, et al. Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area. Chin. J. Hepatol. 2020;28:229–233. doi: 10.3760/cma.j.cn501113-20200229-00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao N, et al. Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chin. J. Hepatol. 2020;28:234–239. doi: 10.3760/cma.j.cn501113-20200226-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y, et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med. Infect. Disease. 2020 doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meszaros M, et al. Abnormal liver tests in patients hospitalized with Coronavirus disease 2019: Should we worry? Liver Int. Off. J. Int. Assoc. Study Liver. 2020;40:1860–1864. doi: 10.1111/liv.14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S, et al. COVID-19 induced liver function abnormality associates with age. Aging (Albany NY) 2020;12:13895–13904. doi: 10.18632/aging.103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao SR, et al. Liver enzyme elevation in coronavirus disease 2019: A multicenter, retrospective, cross-sectional study. Am. J. Gastroenterol. 2020;115:1075–1083. doi: 10.14309/ajg.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schattenberg JM, et al. Patterns of liver injury in COVID-19—A German case series. United Eur. Gastroenterol. J. 2020;8:814–819. doi: 10.1177/2050640620931657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Q, et al. Pattern of liver injury in adult patients with COVID-19: A retrospective analysis of 105 patients. Mil. Med. Res. 2020;7:28. doi: 10.1186/s40779-020-00256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan Z, et al. Clinical features of COVID-19-related liver functional abnormality. Clin. Gastroenterol. Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lei, F. et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology72(2), 389–398. 10.1002/hep.31301 (2020). [DOI] [PMC free article] [PubMed]
- 76.Boeckmans J, et al. COVID-19 and drug-induced liver injury: A problem of plenty or a petty point? Arch. Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.