Abstract
Correlational studies of humans suggest that exposure to early life stress has long-term effects on neural circuits involved in vulnerability and resilience to mental health disorders. Stress-related mental health disorders are more prevalent in women than in men. Here, female squirrel monkeys are randomized to intermittently stressful (IS) social separations or a non-separated (NS) control condition conducted from 17 to 27 weeks of age. Nine years later in mid-life adulthood, resting-state functional magnetic resonance imaging was employed to parcellate prefrontal cortex (PFC). Resulting subdivisions were then used to characterize functional connectivity within PFC, and between PFC subdivisions and subcortical regions that are known to be altered by stress. Extensive hyper-connectivity of medial and orbitofrontal PFC with amygdala, hippocampus, and striatum was observed in IS compared to NS monkeys. Functional hyper-connectivity in IS monkeys was associated with previously reported indications of diminished anxiety-like behavior induced by prepubertal stress. Hyper-connectivity of PFC with amygdala and with hippocampus was also associated with increased ventral striatal dopamine D2 and/or D3 receptor (DRD2/3) availability assessed with positron emission tomography (PET) of [11C]raclopride binding in adulthood. Ventral striatal DRD2/3 availability has been linked to cognitive control, which plays a key role in stress coping as an aspect of emotion regulation. These findings provide causal support for enduring neurobiological effects of early life stress and suggest novel targets for new treatments of stress-related mental health disorders.
Subject terms: Stress and resilience, Predictive markers
Introduction
The deleterious effects of exposure to severe forms of early life stress are well-recognized across diverse disorders of human mental health [1–4]. Lesser known is evidence that mild, but not minimal or substantial, stress exposure promotes learning to cope and builds stress resilience [5, 6]. Beneficial effects of early life stress in humans have been reported in the context of family stress [7], work-related stress [8], childhood stress [9], social separation stress [10], and everyday stressful life events [11]. Studies of stress neurobiology have generally focused on deleterious outcomes instead of constructive effects.
The prefrontal cortex (PFC) is involved in stress coping and vulnerability or resilience to mental health disorders [3, 12–14]. Various forms of early life stress are associated with alterations in PFC–subcortical connectivity assessed by resting-state functional magnetic resonance imaging (rsfMRI) [15–20]. Severe forms of early life stress in humans are associated with diminished PFC–subcortical functional connectivity [15, 17, 19], whereas stress resilience has been linked to PFC hyper-connectivity [9, 18, 21]. These studies of humans are necessarily correlational and often rely on retrospective reports to identify prior stress exposure.
Here, we conduct a randomized controlled experiment to prospectively investigate the effects of early life stress on PFC–subcortical functional connectivity in adult female squirrel monkeys. Studies of females are important because stress-related mental health disorders are more prevalent in women than in men [22, 23]. Key aspects of PFC neurobiology are evolutionarily conserved in humans and monkeys [23–25], and rsfMRI is well-suited for interspecies comparisons because this approach does not impose task-specific demands [26].
First, we functionally parcellate adult squirrel monkey PFC and use resulting subdivisions to characterize functional connectivity within PFC, and between PFC subdivisions and subcortical regions that are known to be altered by stress [27]. Next, we determine whether PFC–subcortical functional connectivity in adulthood differs in squirrel monkeys randomized early in life to intermittently stressful (IS) social separations that elicit anxiety-like behavior and acutely increase plasma levels of cortisol [28–30] compared to non-separated (NS) monkeys. Differences in adult PFC–subcortical functional connectivity are then examined for associations with previously reported anxiety-like behavior induced by exposure to prepubertal stress [6]. Lastly, we examine whether PFC–subcortical functional connectivity is associated with ventral striatal DRD2/3 availability assessed previously by PET in adulthood [31]. This specific aim of the study is based on evidence that mesolimbic dopamine supports active stress coping in rodents [32], and previous findings that link mesolimbic DRD2/3 availability with PFC functional connectivity in humans [33–35].
Material and methods
Subjects
Overall, 17 randomly selected female squirrel monkeys (Saimiri sciureus) born and raised at the Stanford University Research Animal Facility served as subjects. These monkeys are part of a larger project designed to identify causal connections between early life stress and neurobiology [6]. Initially, all monkeys were housed in undisturbed mixed-sex groups through 16 weeks of age. Group composition was determined by birth dates to minimize developmental differences between groups. Seasonal synchronous breeding facilitated the generation of age-matched groups.
Groups were housed indoors in 1.8 × 1.2 × 1.8 m species-appropriate cages that were cleaned daily. Housing and testing occurred in climate-controlled rooms with an ambient temperature of 26 °C. Light/dark cycles were 12:12 h with lights on at 0700 h. All monkeys were provided unrestricted access to fresh drinking water and monkey chow with fruit and vegetable supplements. Various toys, swinging perches, and simulated foraging activities were provided for environmental enrichment. To facilitate husbandry-related activities and experimental manipulations, monkeys were trained to leave the home cage through a small door connected to a transport box. All procedures were approved by Stanford University’s Administrative Panel on Laboratory Animal Care, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental design
Monkeys were randomized to two treatment conditions that occurred between 17 and 27 weeks of age as described elsewhere [6]. Monkeys in the NS control condition (n = 8) were continuously maintained in undisturbed groups. Monkeys in the intermittently separated (IS) condition (n = 9) were briefly removed from groups with or without their mother once a week for ten total 1-h separations. At this stage of development, nearly weaned juvenile monkeys were approximately half their adult body size. After each separation, monkeys were returned to the group. All separations occurred between 1215 and 1745 h, and no more than one monkey from each group was separated on a given day.
Ten weeks after completion of the treatment conditions, prepubertal anxiety-like behavior was assessed in five daily 30-min sessions of involuntary exposure to a mildly stressful novel environment [6]. A trained observer seated in plain view of the monkeys used a computer-assisted data entry device (Observer XT, Noldus Information Technology; Leesburg, VA) to score four behavioral measures: (1) total duration of maternal clinging, i.e., time spent in the species-typical riding posture on the mother’s shoulders and upper back, (2) latency to first terminate maternal contact, (3) latency to first explore a toy-like object in the novel environment, and (4) total object exploration counts. Stress coping in humans has been linked to curiosity [36]. Inverse object exploration counts were used in all analyses below because counts were inversely correlated with all other measures of behavior.
Mothers were then permanently removed at 1 year of age and their offspring were housed with peers. Cognitive control of behavior was assessed in a subset of monkeys at 1.5 and 3.5 years of age [37, 38], and novelty-seeking was examined in a different subset of monkeys at 2.5 years of age [39]. Beginning around puberty at 3 years of age, females were housed separately from males to prevent breeding.
Whole brain structural magnetic resonance imaging was conducted at 9 years of age (range = 8.5–9.8), followed immediately by rsfMRI described below. Ventral striatal DRD2/3 availability was assessed 2 years later by [11C]raclopride binding determined with PET as described elsewhere [31]. All neuroimaging procedures were conducted during nonbreeding seasons when sex hormones remain stable at non-detectable levels in seasonally breeding squirrel monkeys [40]. Lifespan in captivity is ~20 years [41].
Data acquisition
Noninvasive rsfMRI data were acquired using a General Electric 3T Signa MR scanner (Milwaukee, WI) with protocols developed for squirrel monkeys. Monkeys were scanned under anesthesia induced by a subcutaneous injection of 20 mg/kg ketamine hydrochloride, 4 mg/kg xylazine hydrochloride, and 0.04 mg/kg atropine sulfate, followed by 0.5–1.0% isoflurane gas delivered through a face mask to maintain anesthesia. This low level of isoflurane preserves relative differences in rsfMRI measures of functional connectivity in squirrel monkeys [42]. Body temperatures were maintained using a cushioned water heating pad (Gaymar-Stryker T/Pump, Kalamazzo, MI) and earplugs provided protection from scanner noise.
Initially, T1-weighted whole brain images were acquired in the sagittal plane with a three-dimensional inversion recovery-prepared fast spoiled gradient pulse sequence: TR = 12 ms, TE = 3 ms, TI = 300 ms, flip angle = 15, NEX = 4, matrix = 160 × 160, FOV = 8 cm, voxel size = 0.5 × 0.5 × 0.5 mm, slice thickness = 0.5 mm, gap = 0 mm, scan time = 18 min. Immediately thereafter, whole brain rsfFMI data were acquired with a protocol adapted from a T2*-weighted gradient echo task-free spiral in-out pulse sequence [43]: TR = 2100 ms, TE = 36 ms, flip angle = 77°, one interleave, NEX = 2, matrix 64 × 64, FOV = 8 cm, in-plane spatial resolution = 1.25 × 1.25 mm, slice thickness 1.2 mm, scan time = 12 min.
Data preprocessing
Data were preprocessed using ANTs 1.4 [44] and AFNI [45]. Because of small sample sizes, a group template based on individual T1-weighted images was generated using the large deformation diffeomorphic metric mapping algorithm implemented in ANTs. This template was linearly aligned to squirrel monkey VALIDATe29 atlas space [46]. The first four volumes of rsfMRI data were discarded to allow stabilization of signal, and remaining data were realigned to the mean image for head motion correction. Aligned images were then co-registered with each monkey’s own T1-weighted images. The deformation field for individual T1-weighted images and the group template were linearly aligned to the template and VALIDATe29 space [46]. Six head motion parameters were regressed out from all of the time series, and a temporal bandpass filter ranging from 0.01 to 0.1 Hz was applied [47]. Finally, images were spatially smoothed by a 4 mm FWHM Gaussian kernel.
PFC and subcortical regions of interest
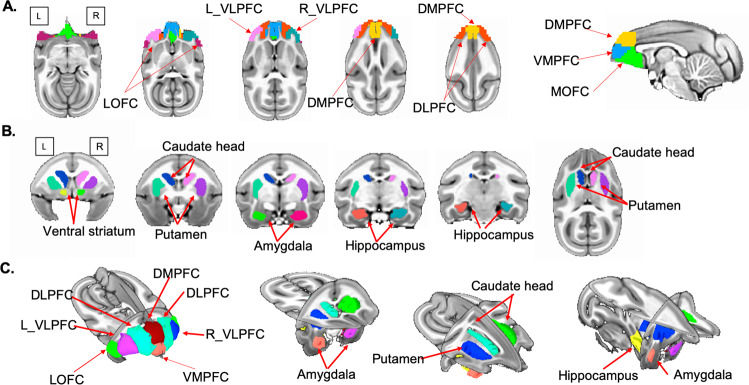
Based on previous studies of stress coping [13, 14, 27], we focused on PFC and subcortical regions that included amygdala, hippocampus, caudate, putamen, and ventral striatum on each brain side. Squirrel monkey VALIDATe29 defines only major cortical regions [46]. Therefore, we delineated subcortical regions based on previously described anatomical landmarks [31, 48]. To minimize human bias, subcortical regions were hand-drawn independently by two trained raters, and the overlap between raters was used to identify subcortical regions (Fig. 1).
Fig. 1. Functional parcellation of squirrel monkey prefrontal cortex and brain regions of interest.
A Squirrel monkey prefrontal cortex (PFC) is parcellated into seven subdivisions using resting-state fMRI. Axial and sagittal planes depict left and right ventrolateral PFC (VLPFC), ventromedial PFC (VMPFC), dorsomedial PFC (DMPFC), dorsolateral PFC (DLPFC), medial orbitofrontal cortex (MOFC), and lateral orbitofrontal cortex (LOFC) subdivisions. B Subcortical regions are depicted in coronal and axial planes: ventral striatum, caudate, putamen, amygdala, and hippocampus. C PFC subdivisions and subcortical regions are depicted in three dimensions.
Functional parcellation of PFC
The VALIDATe29 atlas [46] demarcates only left and right hemisphere PFC, but squirrel monkey PFC is comprised of 7–10 subdivisions based on Brodmann’s classification of cytoarchitecture [24, 25]. Because cytoarchitecture-based subdivisions cannot be mapped directly onto neuroimaging data, we employed a multistep functional consistency clustering approach [49, 50]. First, we extracted time series from each voxel in each monkey and formed matrix X, where the number of rows (M) equaled the number of voxels in VALIDATe29 PFC, and the number of columns (N) equaled the number of time frames. Second, Pearson correlations were computed for each pair of rows in matrix X resulting in an M × M correlation matrix. Third, using each monkey’s correlation matrix, k-means clustering with random initialization was used to generate 1000 partitions in each monkey, with the number of clusters (k) ranging from 2 to 14. Fourth, we estimated the averaged adjacency matrix across 1000 partitions for each k. By averaging the adjacency matrix across monkeys, we obtained a consensus matrix for each k at the group level. Fifth, we applied k-means clustering with 1000 random initializations to obtain group-level consensus matrices. We then quantified the stability of partitions for each k across 1000 clusters using variation of information [51] and adjusted rank index [52]. Clustering metrics were employed to determine the optimal number of clusters (Supplementary Material Fig. S1), and the highly converged adjacency matrix from the previous step was used to generate the final parcellation, which contained seven PFC subdivisions (Fig. 1).
Comparison of functional connectivity between IS and NS groups
Functional connectivity within and between subdivisions of PFC and subcortical regions were estimated by Pearson correlation between the average time series across all voxels in each region. To control for age effects, normalized age was regressed out from functional connectivity measures. Nonparametric permutation tests were employed to evaluate differences in connectivity between IS and NS groups, and Pearson correlations were used to assess associations between functional connectivity and ventral striatal DRD2/3 availability. False discovery rate (FDR) corrected p values were determined to adjust for multiple comparisons in all tests of statistical significance.
Distinguishing connectivity in IS and NS monkeys using GLMnet with Lasso regression
To characterize group differences in patterns of PFC–subcortical functional connectivity, we employed GLMnet (https://cran.r-project.org/web/packages/glmnet/index.html), a widely used least absolute shrinkage and selection operator (Lasso) regression analysis method that performs variable selection and regularization to enhance prediction accuracy and interpretability of multivariate group differences [53, 54]. The L1 norm regularization of coefficients was used. GLMnet estimated the model so that independent variables which do not contribute to the model were set to zero. This approach produced sparse and interpretable solutions. Leave-one-out cross validation was used to evaluate the accuracy with which IS and NS groups could be distinguished, and to determine the most distinguishing connectivity features.
Partial least squares path modeling of the relation between functional connectivity and behavior
Multivariate relationships between functional connectivity and previously reported [6] anxiety-like behavior induced by prepubertal stress were determined for IS monkeys using partial least squares path modeling (PLS-PM) [55–57]. Missing measures of behavior precluded PLS-PM analysis of NS monkeys. Model fit was determined using R2, the proportion of variance explained by the mode, and statistical significance of model weights, loadings, and path coefficients was determined using bootstrapping, in which data were repeatedly sampled with replacement to create 2000 surrogate datasets. As recommended elsewhere [58], all model features with loadings greater than 0.7 and weights with 95% confidence intervals that did not include zero were considered to be significant. To illustrate the underlying structure of weights and loadings, hierarchical cluster analysis was performed with Ward’s method [59]. For each cluster, averaged weights and loadings were computed, and all statistics were evaluated with two-tail probabilities.
Results
Functional parcellation of PFC
The recently released VALIDATe29 neuroimaging atlas for squirrel monkeys [46] provides a novel tool for investigating connectivity of circuits in brain. However, squirrel monkey PFC in VALIDATe29 is parcellated only into left and right hemispheres. To investigate patterns of connectivity associated with finer-grained parcellation, we first subdivided PFC based on whole brain functional connectivity of each voxel using a consistency clustering approach [49] with both IS and NS monkeys. Our functional parcellation yielded seven subdivisions (Fig. 1A) consisting of left and right ventrolateral PFC (VLPFC), ventromedial PFC (VMPFC), dorsomedial PFC (DMPFC), dorsolateral PFC, medial orbitofrontal cortex (MOFC), and lateral orbitofrontal cortex. The fMRI-based parcellation of PFC is well-aligned with cytoarchitectonic boundaries of squirrel monkey cortex (Supplementary Materials Fig. S2). (Note: PFC functional parcellations will be made publicly available via NITRC and github).
IS alters PFC–subcortical connectivity patterns
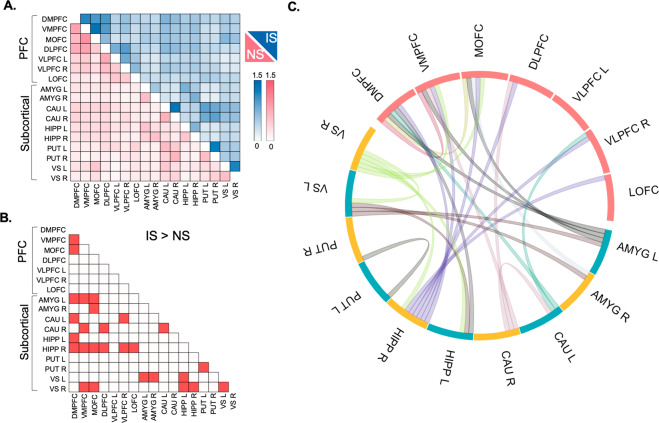
Our next goal was to determine whether patterns of PFC functional connectivity differed in IS vs. NS groups. We used PFC subdivisions described above (Fig. 1A), along with five subcortical regions associated with emotional reactivity and stress coping: amygdala, hippocampus, caudate, putamen, and ventral striatum on each brain side (Fig. 1B). Multivariate analysis and permutation tests (see “Methods” section) were employed to determine whether patterns of PFC–subcortical functional connectivity differed in IS vs. NS groups (Fig. 2). Analyses identified multiple intra-PFC, PFC–subcortical, and intra-subcortical links that contributed significantly to IS vs. NS differences (FDR-corrected p < 0.05).
Fig. 2. PFC–subcortical functional hyper-connectivity in the IS group.
A Connectivity within and between PFC subdivisions and subcortical regions are presented separately for intermittently separated (IS) and non-separated (NS) groups with light-to-dark scale bars signifying the range of functional connectivity. B Bright red-colored squares depict links that differ significantly between IS and NS groups (FDR-corrected p < 0.05). C Links that show significant hyper-connectivity in IS monkeys are illustrated in the circular plot.
To further characterize these differences, we employed GLMNet Lasso Regression, which generated a classification accuracy of 72%, specificity of 78%, and sensitivity of 67%. Differences between IS vs. NS groups determined by GLMNet feature selection (Supplementary Materials Fig. S3) overlapped with results from permutation tests (Fig. 2). Results suggest that IS vs. NS groups differed significantly in patterns of PFC–subcortical connectivity.
Functional PFC–subcortical circuits are hyper-connected in IS monkeys
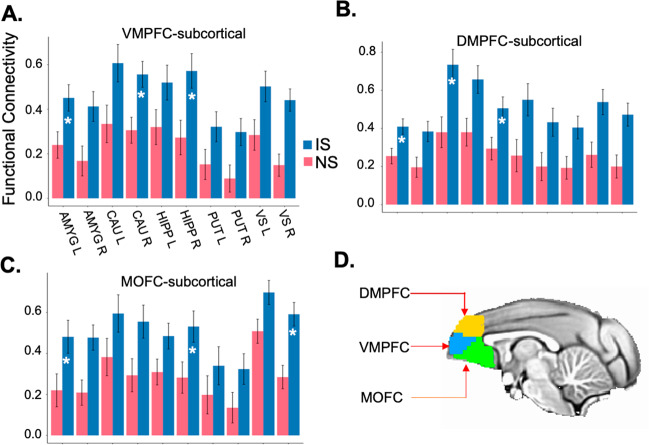
Our third goal was to identify specific links of functional connectivity that differentiated IS and NS groups. Three of seven PFC subdivisions showed extensive links with subcortical regions that differed significantly between IS vs. NS groups (FDR-corrected p < 0.05). Specifically, DMPFC (Fig. 3A), VMPFC (Fig. 3B), and adjoining MOFC (Fig. 3C) showed extensive functional hyper-connectivity with amygdala, hippocampus, caudate, and ventral striatum in IS vs. NS groups. To a lesser extent, other PFC subdivisions also showed functional hyper-connectivity with subcortical regions in IS vs. NS groups (Supplementary Materials Fig. S4). Subcortical hyper-connectivity was likewise evident in IS vs. NS groups for inter-hemispheric links between caudate, putamen, and ventral striatum (Fig. 2). No links showed significantly reduced functional connectivity in IS compared NS groups (FDR-corrected p > 0.05). Results indicate that the IS group shows extensive functional hyper-connectivity between PFC and subcortical regions, and within subcortical regions, compared to the NS group.
Fig. 3. Three regions of hyper-connectivity in the IS group.
Links between A ventromedial PFC (VMPFC), B dorsomedial PFC (DMPFC), and C medial orbitofrontal cortex (MOFC) and subcortical regions that show functional hyper-connectivity in IS compared to NS groups. Asterisks signify IS vs. NS group differences that are statistically significant (FDR-corrected p < 0.05). D Three regions of interest are illustrated on the group template.
We then conducted control analyses to examine the specificity of PFC functional hyper-connectivity in IS vs. NS groups. Measures of PFC functional connectivity with visual cortex areas identified using squirrel monkey VALIDate29 [46] were assessed (Supplementary Materials Fig. S5). IS vs. NS comparisons did not show hyper-connectivity of PFC subdivisions with visual cortex areas V1 and V2 (FDR-corrected p > 0.05). These control analyses demonstrate the specificity of PFC hyper-connectivity with subcortical regions in IS vs. NS groups.
Functional hyper-connectivity is associated with ventral striatal DRD2/3 availability
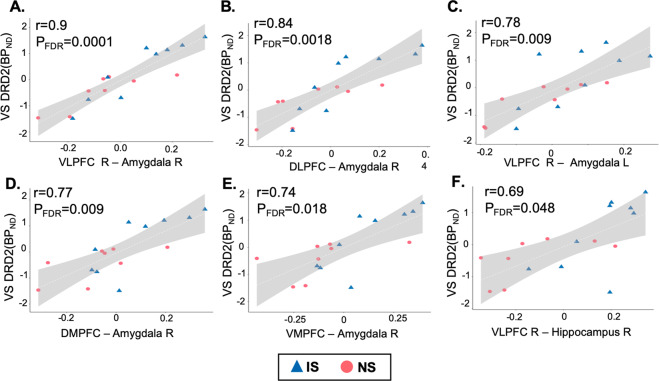
Our fourth goal was to determine whether functional hyper-connectivity correlated with previously reported [31] ventral striatal DRD2/3 availability in IS vs. NS monkeys. No significant correlations were found in NS monkeys, but functional connectivity between right VLPFC and right amygdala correlated significantly with ventral striatal DRD2/3 availability in IS monkeys (r = 0.93, FDR-corrected p < 0.05; Supplementary Materials Fig. S6). The same measure of connectivity, as well as five additional measures of PFC connectivity with amygdala and with hippocampus correlated significantly with ventral striatal DRD2/3 availability when IS and NS monkeys were analyzed together (FDR-corrected p < 0.05; Fig. 4). Correlations between functional connectivity and DRD2/3 availability did not differ significantly between IS and NS groups (FDR-corrected p > 0.05). Results suggest that differences between IS and NS monkeys in PFC–subcortical functional connectivity are correlated with differences in ventral striatal DRD2/3 availability assessed by PET in adulthood.
Fig. 4. PFC–subcortical functional connectivity is correlated with ventral striatal DRD2/3 availability.
PFC functional connectivity (x-axis) with amygdala (A–E) and with hippocampus (F) is correlated with ventral striatal DRD2/3 availability (y-axis) in IS and NS monkeys.
Functional hyper-connectivity is associated with diminished anxiety-like behavior
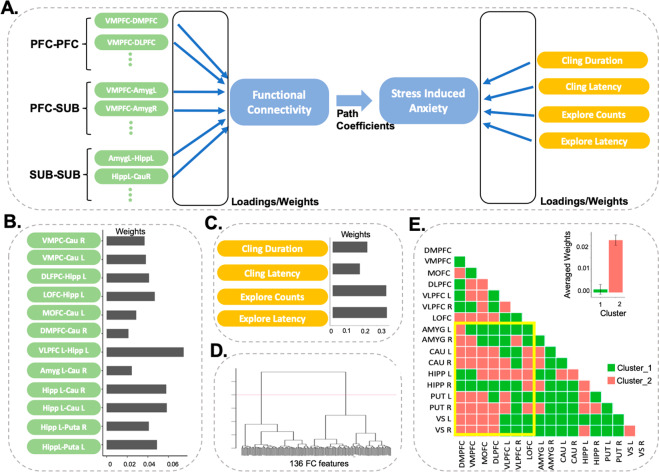
Lastly, we investigated multivariate relationships between functional hyper-connectivity in adulthood and previously reported [6] anxiety-like behavior induced by prepubertal stress. Only IS monkeys were examined because of missing measures of behavior in NS monkeys. Functional hyper-connectivity was significantly associated with diminished anxiety-like behavior (R2~0.39, path coefficient = −0.79, p = 0.01), as identified using the PLS-PM model depicted in Fig. 5A. Significant features of functional connectivity included PFC, hippocampus, and striatum (Fig. 5B), and the most prominent features of anxiety-like behavior included measures of exploration in mildly stressful novel environment (Fig. 5C). Hierarchical cluster analysis of weights and loadings of functional connectivity features revealed two major clusters (Fig. 5D). In the cluster with higher weights and loadings, the most predictive features are predominantly PFC–subcortical connections (Fig. 5E).
Fig. 5. Partial least squares path model (PLS-PM) showing that functional connectivity is linked to diminished anxiety-like behavior in the IS group.
A PLS-PM model grouped into intra-PFC, PFC–subcortical (SUB), and intra-subcortical connections. Measures of anxiety-like behavior include the duration of maternal clinging, latency to first terminate maternal clinging, inverse of object exploration counts, and latency to first explore an object. B, C Model weights are presented for significant functional connectivity and stress-induced anxiety-like behavioral measures, respectively. D Hierarchical cluster analysis of weights and loadings using all functional connectivity features reveals two major clusters. E In the cluster with higher weights and loadings (Cluster 2), predictive features are predominantly PFC–subcortical connections (yellow boundary).
To further delineate multivariate relationships between functional connectivity in adulthood and previously reported [6] anxiety-like behavior induced by prepubertal stress, alternative PLS-PM models were examined (Supplementary Material Fig. S7). Functional hyper-connectivity was associated with diminished anxiety-like behavior when only PFC–subcortical connections were analyzed alone (R2~0.39, path coefficient = −0.69, p = 0.04), or when only intra-subcortical connections were analyzed alone (R2~0.35, path coefficient = 0.89, p = 0.001). Functional connectivity was not significantly associated with anxiety-like behavior when intra-PFC connections were analyzed alone. Results indicate that diminished anxiety-like behavior induced by prepubertal stress in IS monkeys is associated with functional hyper-connectivity in PFC–subcortical circuits in adulthood.
Discussion
Long-term effects of early life stress on PFC–subcortical functional connectivity in female squirrel monkeys are examined using a randomized controlled experimental design. Novel quantitative methods are used to characterize functional connectivity between subdivisions of PFC, and between PFC subdivisions and subcortical regions known to be altered by stress [27]. Extensive functional hyper-connectivity in PFC–subcortical circuits is induced by early exposure to stressful IS compared to non-stressful NS conditions. Extensive hyper-connectivity in IS vs. NS groups is also observed within subcortical regions. Increased PFC–subcortical functional connectivity is associated with previously reported indications of diminished anxiety-like behavior induced by prepubertal stress [6], and with increased ventral striatal DRD2/3 availability assessed by PET in adulthood [31].
Attempts to parcellate squirrel monkey PFC based on Brodmann’s classification of cytoarchitecture have recognized 7–10 subdivisions [24, 25], but PFC subdivisions based on cytoarchitecture cannot be mapped precisely onto neuroimaging data. To address this challenge, we employed functional connectivity determined by rsfMRI to quantitatively parcellate squirrel monkey PFC into seven subdivisions which encompass medial, lateral, dorsal, ventral, and orbitofrontal regions that resemble those identified by cytoarchitecture [24, 25]. Medial and lateral subdivisions of PFC serve different roles in cognitive control and emotion regulation [60, 61]. Parcellation of PFC allowed us to assess functional connectivity within PFC, and between PFC and subcortical regions known to be altered by stress [27].
As a starting point for probing PFC–subcortical circuits, permutation tests and machine learning algorithms are used to identify patterns of connectivity that differ in IS vs. NS monkeys with high levels of accuracy, sensitivity, and specificity. Extensive functional hyper-connectivity was observed in IS compared to NS monkeys for medial and orbitofrontal PFC. This result aligns with evidence that PFC myelination is enhanced in IS compared to NS monkeys [62], and agrees with suggestions that medial PFC drives inhibitory control of subcortical regions [12, 13, 63]. IS relative to NS monkeys also showed inter-hemispheric functional hyper-connectivity in subcortical regions, including all striatal nuclei examined here: caudate, putamen, and ventral striatum.
We then leveraged a multimodal brain imaging approach to identify associations between PFC–subcortical functional connectivity determined here by rsfMRI and previously reported ventral striatal DRD2/3 availability assessed by PET in adulthood [31]. In IS monkeys, functional connectivity between right VLPFC and right amygdala is associated with ventral striatal DRD2/3 availability. Previously reported IS vs. NS differences in ventral striatal DRD2/3 availability [31] are also associated with multiple measures of PFC–subcortical functional connectivity. Increased ventral striatal DRD2/3 availability in IS vs. NS monkeys predicts cognitive control of behavior on a task that requires an intact PFC in monkeys [31, 64, 65].
Mesolimbic DRD2/3 availability has been linked with PFC functional connectivity in humans [33–35], and transcranial direct current stimulation of PFC induces dopamine release in striatal regions that express high levels of DRD2/3 in humans and rodents [66]. Increased ventral striatal DRD2/3 availability predicts cognitive control [31, 67], which plays a key role in emotion regulation [68–70], and mesolimbic dopamine supports active stress coping in rodents [32]. Taken together, these findings suggest that mesolimbic DRD2/3 availability considered in relation to PFC functional connectivity may offer novel opportunities to improve stress coping and enhance resilience. Administration of varenicline, for example, increases ventral striatal DRD2/3 availability [71], enhances PFC functional connectivity as a correlate of cognitive control [72], and reduces perceived stress in humans [73].
Relations between early life stress and vulnerability or resilience to mental health disorders are complex [74]. Considerable evidence has emerged for a nonlinear U-shaped relationship between stressful adversity and responses to subsequent adversity. Children growing up in conditions of high or low adversity display heightened stress responses relative to children growing up in conditions of moderate adversity [75]. People with a history of some lifetime adversity report better mental health and well-being than not only the people with a history of high adversity but also than the people with no history of adversity [76]. Indications of stress resilience are enhanced in squirrel monkeys randomized to one or two early life stressors compared to zero or three early life stressors [6].
Whether vulnerability or resilience is induced by exposure to early life stress depends, in part, on the type, frequency, duration, and developmental timing of stress exposure [74]. In natural [77] and semi-natural conditions [78], squirrel monkey mothers and other group members briefly leave nearly weaned juvenile offspring to forage for food on their own. Initially, brief intermittent separations studied in controlled experimental conditions elicit anxiety-like behavior and acutely increase plasma levels of cortisol [28–30]. Later in life, squirrel monkeys exposed to early intermittent separations show subsequent indications of resilience [5, 6, 31].
Resilience induced by early life stress is difficult to determine in humans because of challenges inherent in assessing early life stress in children. Consequently, conflicting results are reported in humans [15–20]. For example, PFC–subcortical functional connectivity correlates inversely with concurrent symptoms of anxiety, but correlates positively with depressive symptoms, suggesting that different neural pathways are involved in anxiety and depression [16]. Another study reports that early life stress is positively associated with emotional task-related functional connectivity in PFC–subcortical circuits that protect against human mental health disorders [9].
Our findings should be interpreted in the context of potential limitations. Subjective experiences cannot be determined in monkeys, and diminished anxiety-like behavior may reflect affective blunting instead of adaptive stress coping. Girls are more sensitive than boys to the effects of insensitive parenting on PFC–subcortical functional connectivity [18], and results from females may or may not hold true for males. Although low levels of isoflurane used to maintain anesthesia preserve relative differences in functional connectivity assessed by rsfMRI in squirrel monkeys [47], isoflurane is known to non-selectively dampen global brain activity. Studies of awake behaving subjects are needed to confirm that PFC–subcortical functional hyper-connectivity is causally induced by exposure to early life stress. Correlational findings that less sensitive parenting [18] and maternal deprivation [20] enhance PFC–subcortical functional connectivity in children are consistent with causal effects reported here in squirrel monkeys.
Conclusions
Early exposure of squirrel monkeys to IS compared to non-stressful NS conditions induces functional hyper-connectivity in adult PFC–subcortical circuits. Functional hyper-connectivity is associated with diminished anxiety-like behavior induced by exposure to prepubertal stress, and with increased ventral striatal DRD2/3 availability assessed by PET in adulthood. These findings provide causal support for enduring neurobiological effects of early life stress and suggest that DRD2/3 availability and PFC functional connectivity are potential targets for building stress resilience in humans.
Funding and disclosure
Supported by the Pritzker Neuropsychiatric Disorders Research Consortium Fund, Deutsche Forschungsgemeinschaft CRC1193 subproject Z02, and National Institutes of Health grants MH047573, MH066537, MH094094, DA035505, EB022907, and MH121069, and the Stanford Maternal Child Health Research Institute Transdisciplinary Initiatives Program. Yuan, Nechvatal, Buckmaster, Ayash, Parker, and Menon report no financial interests or potential conflicts of interest related to this work. Schatzberg has served as a consultant for Alkermes, Avanir, Bracket, Epiodyne, Jazz, Lundbeck/Takeda, McKinsey, Myriad Genetics, Neuronetics, Owl, and Sage; holds equity in Corcept (co-founder), Epiodyne, Gilead, Incyte, Intersect ENT, Merck, Owl, Seattle Genetics, Titan, and Xhale; and he is listed as an inventor on pharmacogenetic and mifepristone patents from Stanford University. Schatzberg and Lyons are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by Pritzker Neuropsychiatric Disorders Research Fund, LLC. A shared intellectual property agreement exists between the academic and philanthropic entities of the Consortium. The Pritzker Neuropsychiatric Disorders Research Fund had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript. The authors declare no competing interests.
Supplementary information
Acknowledgements
We gratefully acknowledge the assistance of Catie Chang and Dequiang Qui for help with neuroimaging protocols.
Author contributions
DML and AFS designed the research. JMN, CLB, and KJP collected and analyzed data. SA analyzed data. RY and VM designed the analysis, contributed the analytic tools, and analyzed the data. RY, DML, and VM wrote the paper. All authors read and approved the final manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rui Yuan, Jordan M. Nechvatal
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-021-00956-0).
References
- 1.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–11. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Shonkoff JP. Capitalizing on advances in science to reduce the health consequences of early childhood adversity. JAMA Pediatr. 2016;170:1003–07. doi: 10.1001/jamapediatrics.2016.1559. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Effects of stress on the developing brain. Cerebrum. 2011;2011:14. [PMC free article] [PubMed] [Google Scholar]
- 4.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons DM, Schatzberg AF. Resilience as a process instead of a trait. In: Chen A, editor. Stress resilience: molecular and behavioral aspects. San Diego: Academic Press/Elsevier; 2020. p. 33-44.
- 6.Parker KJ, Buckmaster CL, Hyde SA, Schatzberg AF, Lyons DM. Nonlinear relationship between early life stress exposure and subsequent resilience in monkeys. Sci Rep. 2019;9:16232. doi: 10.1038/s41598-019-52810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagan MJ, Roubinov DS, Purdom Marreiro CL, Luecken LJ. Childhood interparental conflict and HPA axis activity in young adulthood: examining nonlinear relations. Dev Psychobiol. 2014;56:871–80. doi: 10.1002/dev.21157. [DOI] [PubMed] [Google Scholar]
- 8.Mortimer JT, Staff J. Early work as a source of developmental discontinuity during the transition to adulthood. Dev Psychopathol. 2004;16:1047–70. doi: 10.1017/s0954579404040131. [DOI] [PubMed] [Google Scholar]
- 9.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:326–34. doi: 10.1016/j.bpsc.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulton R, Milne BJ, Craske MG, Menzies RG. A longitudinal study of the etiology of separation anxiety. Behav Res Ther. 2001;39:1395–410. doi: 10.1016/s0005-7967(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 11.DiCorcia JA, Tronick E. Quotidian resilience: exploring mechanisms that drive resilience from a perspective of everyday stress and coping. Neurosci Biobehav Rev. 2011;35:1593–602. doi: 10.1016/j.neubiorev.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. PNAS. 2016;113:8837–42. doi: 10.1073/pnas.1600965113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei C, Han J, Zhang Y, Hannak W, Dai Y, Liu Z. Affective emotion increases heart rate variability and activates left dorsolateral prefrontal cortex in post-traumatic growth. Sci Rep. 2017;7:16667. doi: 10.1038/s41598-017-16890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. PNAS. 2013;110:19119–24. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park AT, Leonard JA, Saxler P, Cyr AB, Gabrieli JDE, Mackey AP. Amygdala-medial prefrontal connectivity relates to stress and mental health in early childhood. Soc Cogn Affect Neurosci. 2018;13:430–9. doi: 10.1093/scan/nsy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thijssen S, Muetzel RL, Bakermans-Kranenburg MJ, Jaddoe VW, Tiemeier H, Verhulst FC, et al. Insensitive parenting may accelerate the development of the amygdala-medial prefrontal cortex circuit. Dev Psychopathol. 2017;29:505–18. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- 19.Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. Eur Neuropsychopharmacol. 2013;23:24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. PNAS. 2013;110:15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi L, Sun J, Wei D, Qiu J. Recover from the adversity: functional connectivity basis of psychological resilience. Neuropsychologia. 2019;122:20–27. doi: 10.1016/j.neuropsychologia.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oikonomidis L, Santangelo AM, Shiba Y, Clarke FH, Robbins TW, Roberts AC. A dimensional approach to modeling symptoms of neuropsychiatric disorders in the marmoset monkey. Dev Neurobiol. 2017;77:328–53. doi: 10.1002/dneu.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis DA, Foote SL, Goldstein M, Morrison JH. The dopaminergic innervation of monkey prefrontal cortex: a tyrosine hydroxylase immunohistochemical study. Brain Res. 1988;449:225–43. doi: 10.1016/0006-8993(88)91040-2. [DOI] [PubMed] [Google Scholar]
- 25.Rosabal F. Cytoarchitecture of the frontal lobe of the squirrel monkey. J Comp Neurol. 1967;130:87–108. doi: 10.1002/cne.901300202. [DOI] [PubMed] [Google Scholar]
- 26.Guerra-Carrillo B, Mackey AP, Bunge SA. Resting-state fMRI: a window into human brain plasticity. Neuroscientist. 2014;20:522–33. doi: 10.1177/1073858414524442. [DOI] [PubMed] [Google Scholar]
- 27.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 28.Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–9. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 29.Hennessy MB. Multiple, brief maternal separations in the squirrel monkey: changes in hormonal and behavioral responsiveness. Physiol Behav. 1986;36:245–50. doi: 10.1016/0031-9384(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 30.Lyons DM, Martel FL, Levine S, Risch NJ, Schatzberg AF. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Horm Behav. 1999;36:266–75. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- 31.Lee AG, Nechvatal JM, Shen B, Buckmaster CL, Levy MJ, Chin FT, et al. Striatal dopamine D2/3 receptor regulation by stress inoculation in squirrel monkeys. Neurobiol Stress. 2016;3:68–73. doi: 10.1016/j.ynstr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabib S, Puglisi-Allegra S. Mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, et al. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex. 2012;22:2784–93. doi: 10.1093/cercor/bhr354. [DOI] [PubMed] [Google Scholar]
- 34.McCutcheon RA, Nour MM, Dahoun T, Jauhar S, Pepper F, Expert P, et al. Mesolimbic dopamine function is related to salience network connectivity: an integrative positron emission tomography and magnetic resonance study. Biol Psychiatry. 2019;85:368–78. doi: 10.1016/j.biopsych.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohno M, Okita K, Morales AM, Robertson CL, Dean AC, Ghahremani DG, et al. Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: link to impulsivity in methamphetamine users. Mol Psychiatry. 2016;21:1554–60. doi: 10.1038/mp.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denneson LM, Smolenski DJ, Bush NE, Dobscha SK. Curiosity improves coping efficacy and reduces suicidal ideation severity among military veterans at risk for suicide. Psychiatry Res. 2017;249:125–31. doi: 10.1016/j.psychres.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Parker KJ, Buckmaster CL, Lindley SE, Schatzberg AF, Lyons DM. Hypothalamic-pituitary-adrenal axis physiology and cognitive control of behavior in stress inoculated monkeys. Int J Behav Dev. 2012;36:45–52. doi: 10.1177/0165025411406864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–55. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Parker KJ, Rainwater KL, Buckmaster CL, Schatzberg AF, Lindley SE, Lyons DM. Early life stress and novelty seeking behavior in adolescent monkeys. Psychoneuroendocrinology. 2007;32:785–92. doi: 10.1016/j.psyneuen.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiml PA, Mendoza SP, Saltzman W, Lyons DM, Mason WA. Annual physiological changes in individually housed squirrel monkeys (Saimiri sciureus) Am J Primatol. 1999;47:93–103. doi: 10.1002/(SICI)1098-2345(1999)47:2<93::AID-AJP1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Brady AG. Research techniques for the squirrel monkey (Saimiri) Ilar J. 2000;41:10–18. doi: 10.1093/ilar.41.1.10. [DOI] [PubMed] [Google Scholar]
- 42.Wu TL, Mishra A, Wang F, Yang PF, Gore JC, Chen LM. Effects of isoflurane anesthesia on resting-state fMRI signals and functional connectivity within primary somatosensory cortex of monkeys. Brain Behav. 2016;6:e00591. doi: 10.1002/brb3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 44.Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS) Insight J. 2009;2:1–35. [Google Scholar]
- 45.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 46.Schilling KG, Gao Y, Stepniewska I, Wu TL, Wang F, Landman BA, et al. The VALiDATe29 MRI based multi-channel atlas of the squirrel monkey brain. Neuroinformatics. 2017;15:321–31. doi: 10.1007/s12021-017-9334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 48.Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–51. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- 49.Ryali S, Chen T, Padmanabhan A, Cai W, Menon V. Development and validation of consensus clustering-based framework for brain segmentation using resting fMRI. J Neurosci Methods. 2015;240:128–40. doi: 10.1016/j.jneumeth.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33:1914–28. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meila M. Comparing clusterings by the variation of information. In: Scholkopf B, Warmuth MK, editors. Learning theory and kernel machines. Berlin: Springer; 2003. p. 173–87.
- 52.Lawrence H, Arabie P. Comparing partitions. J Classif. 1985;2.1:193–218. [Google Scholar]
- 53.Ryali S, Chen T, Supekar K, Menon V. Estimation of functional connectivity in fMRI data using stability selection-based sparse partial correlation with elastic net penalty. Neuroimage. 2012;59:3852–61. doi: 10.1016/j.neuroimage.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supekar K, Menon V. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol Autism. 2015;6:50. doi: 10.1186/s13229-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23:S250–63. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 56.Shaw DJ, Marecek R, Grosbras MH, Leonard G, Pike GB, Paus T. Co-ordinated structural and functional covariance in the adolescent brain underlies face processing performance. Soc Cogn Affect Neurosci. 2016;11:556–68. doi: 10.1093/scan/nsv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosley PE, Paliwal S, Robinson K, Coyne T, Silburn P, Tittgemeyer M, et al. The structural connectivity of discrete networks underlies impulsivity and gambling in Parkinson’s disease. Brain. 2019;142:3917–35. doi: 10.1093/brain/awz327. [DOI] [PubMed] [Google Scholar]
- 58.Benitez J, Henseler J, Castillo A, Schuberth F. How to perform and report an impactful analysis using partial least squares: guidelines for confirmatory and explanatory IS research. Inf Manag-Amster. 2020;57:103168. [Google Scholar]
- 59.Murtagh F, Legendre P. Ward’s hierarchical agglomerative clustering methods: which algorithms implement Ward’s criterion? J Classif. 2014;31:274–95. [Google Scholar]
- 60.Lacroix L, Spinelli S, Heidbreder CA, Feldon J. Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behav Neurosci. 2000;114:1119–30. doi: 10.1037//0735-7044.114.6.1119. [DOI] [PubMed] [Google Scholar]
- 61.Taren AA, Venkatraman V, Huettel SA. A parallel functional topography between medial and lateral prefrontal cortex: evidence and implications for cognitive control. J Neurosci. 2011;31:5026–31. doi: 10.1523/JNEUROSCI.5762-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz M, Liu C, Schaer M, Parker KJ, Ottet MC, Epps A, et al. Prefrontal plasticity and stress inoculation-induced resilience. Dev Neurosci. 2009;31:293–9. doi: 10.1159/000216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 64.Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann N. Y Acad Sci. 1990;608:637–69. doi: 10.1111/j.1749-6632.1990.tb48913.x. [DOI] [PubMed] [Google Scholar]
- 65.Walker SC, Mikheenko YP, Argyle LD, Robbins TW, Roberts AC. Selective prefrontal serotonin depletion impairs acquisition of a detour-reaching task. Eur J Neurosci. 2006;23:3119–23. doi: 10.1111/j.1460-9568.2006.04826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer B, Mann C, Gerlicher A, Saase V, Yuen KSL, Aedo-Jury F, et al. Increased neural activity in mesostriatal regions after prefrontal transcranial direct current stimulation and L-DOPA administration. J Neurosci. 2019;39:5326–35. doi: 10.1523/JNEUROSCI.3128-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jentsch JD, Pennington ZT. Reward, interrupted: inhibitory control and its relevance to addictions. Neuropharmacology. 2014;76:479–86. doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabrys RL, Tabri N, Anisman H, Matheson K. Cognitive control and flexibility in the context of stress and depressive symptoms: the cognitive control and flexibility questionnaire. Front Psychol. 2018;9:2219. doi: 10.3389/fpsyg.2018.02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Compton RJ, Hofheimer J, Kazinka R. Stress regulation and cognitive control: evidence relating cortisol reactivity and neural responses to errors. Cogn Affect Behav Neurosci. 2013;13:152–63. doi: 10.3758/s13415-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cogn Emot. 2010;24:281–98. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crunelle CL, Schulz S, de Bruin K, Miller ML, van den Brink W, Booij J. Dose-dependent and sustained effects of varenicline on dopamine D2/3 receptor availability in rats. Eur Neuropsychopharmacol. 2011;21:205–10. doi: 10.1016/j.euroneuro.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Wheelock MD, Reid MA, To H, White DM, Cropsey KL, Lahti AC. Open label smoking cessation with varenicline is associated with decreased glutamate levels and functional changes in anterior cingulate cortex: preliminary findings. Front Pharm. 2014;5:158. doi: 10.3389/fphar.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, et al. Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. J Gen Intern Med. 2009;24:563–9. doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev. 2011;35:1466–83. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shakiba N, Ellis BJ, Bush NR, Boyce WT. Biological sensitivity to context: a test of the hypothesized U-shaped relation between early adversity and stress responsivity. Dev Psychopathol. 2020;32:641–60. doi: 10.1017/S0954579419000518. [DOI] [PubMed] [Google Scholar]
- 76.Seery MD, Holman EA, Silver RC. Whatever does not kill us: cumulative lifetime adversity, vulnerability, and resilience. J Pers Soc Psychol. 2010;99:1025–41. doi: 10.1037/a0021344. [DOI] [PubMed] [Google Scholar]
- 77.Boinski S, Fragaszy DM. The ontogeny of foraging in squirrel monkeys, Saimiri oerstedi. Anim Behav. 1989;37:415–28. [Google Scholar]
- 78.Lyons DM, Kim S, Schatzberg AF, Levine S. Postnatal foraging demands alter adrenocortical activity and psychosocial development. Dev Psychobiol. 1998;32:285–91. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.