Abstract
Background
Leptomeningeal metastasis (LM) is a rare complication of metastatic breast cancer (MBC), with high morbidity/mortality rates. Our study aimed to describe the largest-to-date real-life population of MBC patients treated with intrathecal (IT) therapy and to evaluate prognostic models.
Methods
The Epidemiological Strategy and Medical Economics (ESME) MBC database (NCT03275311) includes all consecutive patients who have initiated treatment for MBC since 2008. Overall survival (OS) of patients treated with IT therapy was estimated using the Kaplan–Meier method. Prognostic models were constructed using Cox proportional hazards models. Performance was evaluated using C-index and calibration plots.
Results
Of the 22 266 patients included in the database between 2008 and 2016, 312 received IT therapy and were selected for our analysis. Compared with non-IT-treated patients, IT-treated patients were younger at MBC relapse (median age: 52 years versus 61 years) and more often had lobular histology (23.4% versus 12.7%) or triple-negative subtype (24.7% versus 13.3%) (all P < 0.001). Median OS was 4.5 months [95% confidence interval (CI) 3.8-5.6] and 1-year survival rate was 25.6%. Significant prognostic factors associated with poorer outcome on multivariable analysis were triple-negative subtype (hazard ratio 1.81, 95% CI 1.32-2.47), treatment line ≥3 (hazard ratio 1.88, 95% CI 1.30-2.73), ≥3 other metastatic sites (hazard ratio 1.33, 95% CI 1.01-1.74) and IT cytarabine or thiotepa versus methotrexate (hazard ratio 1.68, 95% CI 1.28-2.22), while concomitant systemic therapy was associated with better OS (hazard ratio 0.47, 95% CI 0.35-0.62) (all P < 0.001). We validated two previously published prognostic scores, the Curie score and the Breast-graded prognostic assessment, both with C-index of 0.57.
Conclusions
MBC patients with LM treated with IT therapy have a poor prognosis. We could identify a subgroup of patients with better prognosis, when concomitant systemic therapy and IT methotrexate were used.
Key words: breast cancer, leptomeningeal metastasis, intrathecal therapy, cohort study
Highlights
-
•
The outcome of BC patients with IT-treated LM is poor, with a median OS of 4.5 months.
-
•
Concomitant systemic therapy may improve the outcome in IT-treated patients.
-
•
Patients treated with IT methotrexate had better outcome than those treated with IT cytarabine/thiotepa.
-
•
The Curie and Breast-graded prognostic assessment scores were prognostic for IT-treated patients.
Introduction
Leptomeningeal metastasis (LM) is a rare yet dreaded cancer complication, occurring in 5%-10% of solid cancers, most frequently in breast cancer (BC), lung cancer and melanoma. The incidence of LM has been historically reported to be up to 5% in BC and is rising as patients live longer and with improvement of technologies and availability of neuroaxis imaging.1, 2, 3 This incidence could be underestimated due to the difficulty of diagnosis and the relatively high frequency of false-negative results.3
LM is associated with high morbidity and mortality. Patients usually develop debilitating neurological symptoms, affecting their quality of life and, although patients with LM from BC have longer survival relative to other solid malignancies, the median overall survival (OS) ranges between 1.5 and 4.5 months, even despite active multimodal treatment.2, 3, 4 Poor survival together with advanced progressive systemic disease in most patients5 and absence of validated response criteria6 make it difficult to implement prospective studies. To date, very few randomized trials have been conducted on patients with LM, mostly based on heterogeneous histologies and endpoints.1,7 Consequently, in the absence of strong evidence, treatment guidelines are based on expert opinion and consist of intrathecal (IT) therapy for most patients (methotrexate, cytarabine and thiotepa being the most commonly used drugs), systemic therapy, with a strong emphasis on regimen modification at LM diagnosis, and radiotherapy (RT) in the presence of symptomatic nodular disease.1,3,8 As neither of these treatment modalities has demonstrated significant benefit in OS in randomized trials,1 considerable heterogeneity of diagnostic and treatment strategies was observed among clinicians across Europe in a recent survey, reflecting the many unresolved controversies concerning these issues.9 However, only 10% of clinicians declared that IT therapy was never part of the treatment strategy.9 To assist clinicians' decisions in this challenging setting, previous efforts have been made to stratify patients into prognostic groups according to their characteristics and to identify those patients most likely to benefit from aggressive multimodal treatment,8,10, 11, 12, 13, 14 but, unfortunately, the performance of these models remains uncertain.
The objectives of our study were to report the characteristics and outcomes of patients with metastatic BC (MBC) receiving IT therapy for LM and to evaluate prognostic models in a nationwide real-life cohort. In addition to its large scale, this cohort has the enormous advantage of following all consecutive patients treated for MBC, enabling us to compare patients who developed LM and needed IT therapy, at some point in time, with the rest of the MBC population.
Materials and methods
Study design, patients and data collection
This was a retrospective analysis focusing on MBC patients with LM treated with IT therapy in the French nationwide Epidemiological Strategy and Medical Economics (ESME) MBC database (NCT03275311).15
The present analysis was approved by an independent ethics committee (Comité de Protection des Personnes Sud-Est II- 2015-79). No formal dedicated informed consent was required, but all patients had approved the reuse of their electronically recorded data. In compliance with French regulations, the ESME MBC database was authorized by the French data protection authority (Registration ID 1704113 and authorization No. DE-2013-117). Moreover, in compliance with the applicable European regulations, complementary authorization was obtained on 14 October 2019 regarding the ESME research data warehouse.
For this study, we selected female patients included in the ESME MBC cohort between 2008 and 2016, who received IT therapy with methotrexate, thiotepa or cytarabine (either standard or liposomal) at any time during the course of their disease, as a proxy for LM, as our data did not allow the distinction between LM and brain metastases among central nervous system (CNS) lesions. Other details on study design, patients and data collection can be found in Supplementary Materials and methods, available at https://doi.org/10.1016/j.esmoop.2021.100150.
Objectives
The primary objective of this study was to assess the outcomes of MBC patients treated with IT therapy for LM and the primary endpoint was the median OS, defined as the time (in months) from the start date of IT therapy to the date of death from any cause. Secondary objectives and endpoints are detailed in Supplementary Materials and methods, available at https://doi.org/10.1016/j.esmoop.2021.100150.
Statistical analysis
Cox proportional hazards models were used to identify prognostic factors for OS and were expressed as hazard ratios with their 95% confidence intervals (CIs). Two other models were fitted using previously published prognostic scores.10,16
The ‘simplified’ Curie score10 was developed on a single-institution population that included all BC patients with LM between 2000 and 2007. Negative prognostic factors were hormone receptor (HR)-negative status (versus positive), Eastern Cooperative Oncology Group (ECOG) performance status (PS) 3-4 (versus 0-2) and previous chemotherapy lines >3 (versus ≤3 lines). For the purposes of validation, missing data for PS were imputed to PS 3-4 because patients with missing PS had similar survival to those with PS 3-4. Patients could have a score of 0, 1, 2 or 3, according to the sum of negative prognostic factors present (+1 each). Because of the small number of patients with score = 3 (n = 8), we stratified our patients into three risk groups, with scores of 0, 1 and 2-3, respectively.
The Breast-graded prognostic assessment (GPA) score16 was initially constructed for BC patients with brain metastases based on Karnofsky Performance Status (KPS), ‘genetic subtype’ and age. Missing data for PS were imputed to KPS 60, as these patients had an intermediate univariate OS, between those of patients with KPS ≤50 and KPS 70-80. Patients with missing data for BC subtype (n = 21) had Breast-GPA NA (not available). Details on the methods of conversion of ECOG PS to KPS and allocation of points according to prognostic factors are described in Supplementary Materials and methods (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100150). Because only two patients had a Breast-GPA score of 3.5-4.0, they were grouped with those with a Breast-GPA score of 2.5-3.0 and patients were therefore stratified into three classes, with Breast-GPA scores of 0.0-1.0, 1.5-2.0 and 2.5-4.0, respectively.
The performance of all models was assessed in terms of discrimination and calibration. Risk group stratification was also evaluated for the two previously published scores.
The significance level alpha was fixed at two-sided 5% and all analyses were carried out using R software (version 3.6.1; R Foundation for Statistical Analysis, Vienna, Austria). Other statistical details are presented as Supplementary Materials and Methods, available at https://doi.org/10.1016/j.esmoop.2021.100150.
Results
Patient characteristics
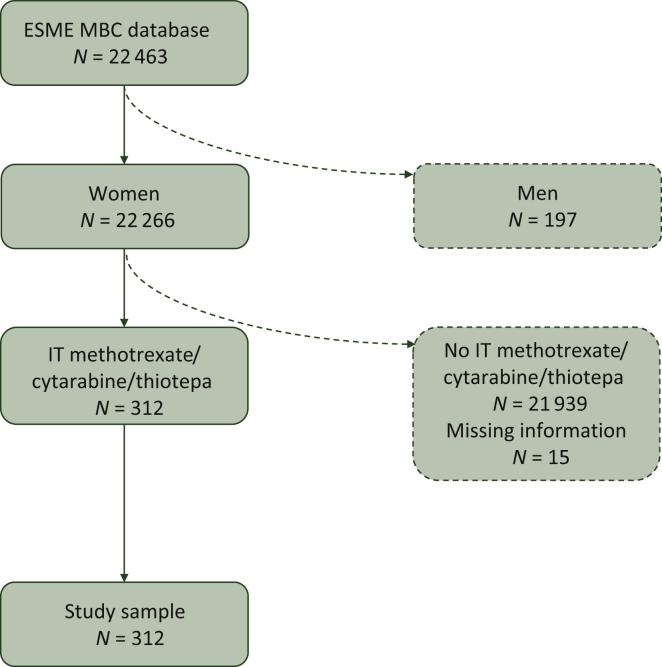
Between 2008 and 2016, 22 266 female MBC patients were included in the ESME database. The median follow-up of the whole population was 24.6 months (range 3.1-100.3). Among them, 327 patients (1.47%) received IT therapy at some time during the course of their disease. Fifteen patients had missing data concerning the treatment line and were excluded. Our final study sample therefore consisted of 312 patients (Figure 1).
Figure 1.
Flowchart of patients who received IT therapy in the ESME MBC database.
ESME, Epidemiological Strategy and Medical Economics; MBC, metastatic breast cancer; IT, intrathecal.
Patients included in our study sample, compared with nonselected patients, were younger (median age: 52 years versus 61 years at MBC diagnosis, P < 0.001) and more often had lobular histology (23.4% versus 12.7%, P < 0.001) and triple-negative (TN) subtype (24.7% versus 13.3%, P < 0.001; Table 1).
Table 1.
Patient characteristics
| Feature | Group of patients |
P value | |
|---|---|---|---|
| Nonselected N = 21 939 |
Selected N = 312 |
||
| Age at primary cancer diagnosis, years | |||
| Median (interquartile range) | 54 (45-64) | 47.5 (40-56) | <0.001 |
| Age at MBC diagnosis, years | |||
| Median (interquartile range) | 61 (50-71) | 52 (44-60) | <0.001 |
| Time to MBC diagnosis, n (%) | |||
| <6 months (de novo) | 6543 (29.8) | 87 (27.9) | 0.052 |
| 6-24 months | 2728 (12.4) | 52 (16.7) | |
| 24-60 months | 4339 (19.8) | 69 (22.1) | |
| ≥60 months | 8275 (37.7) | 102 (32.7) | |
| NA | 54 (0.3) | 2 (0.6) | |
| Histologic type, n (%) | |||
| Invasive carcinoma of no special type | 16 217 (73.9) | 206 (66.0) | <0.001 |
| Invasive lobular carcinoma | 2777 (12.7) | 73 (23.4) | |
| Other | 2521 (11.5) | 23 (7.4) | |
| NA | 424 (1.9) | 10 (3.2) | |
| BC subtype, n (%) | |||
| HR+/HER2− | 13 562 (61.8) | 168 (53.8) | <0.001 |
| HER2+ | 3995 (18.2) | 47 (15.1) | |
| Triple negative | 2908 (13.3) | 77 (24.7) | |
| NA | 1474 (6.7) | 20 (6.4) | |
| Hormone receptor status, n (%) | |||
| HR− | 4382 (20.0) | 94 (30.1) | <0.001 |
| HR+ | 17 131 (78.0) | 208 (66.7) | |
| NA | 426 (2.0) | 10 (3.2) | |
| The following characteristics are reported for our selected population at initiation of IT therapy | |||
| Previous CNS local therapy, n (%) | |||
| WBRT | 64 (20.5) | ||
| Stereotactic RT | 19 (6.1) | ||
| Surgery | 14 (4.5) | ||
| Number of metastatic sites (excluding CNS), n (%) | |||
| <3 | 160 (51.3) | ||
| ≥3 | 152 (48.7) | ||
| Liver metastasis, n (%) | |||
| No | 176 (56.4) | ||
| Yes | 136 (43.6) | ||
| Treatment line, n (%) | |||
| Line 1 | 62 (19.9) | ||
| Line 2 | 88 (28.2) | ||
| Line 3 | 58 (18.6) | ||
| Line 4 | 37 (11.9) | ||
| Line 5 and more | 67 (21.5) | ||
| ECOG performance status, n (%) | |||
| PS 0-1 | 49 (15.7) | ||
| PS 2 | 42 (13.5) | ||
| PS 3-4 | 48 (15.4) | ||
| PS NA | 173 (55.4) | ||
| Intrathecal agent, n (%) | |||
| Methotrexate | 206 (66) | ||
| Cytarabine | 91 (29.2) | ||
| Thiotepa | 15 (4.8) | ||
| Concomitant systemic therapya, n (%) | |||
| Yes | 172 (55.1) | ||
| No | 140 (44.9) | ||
| Type of systemic therapyb, n (%) | |||
| Chemotherapy or targeted therapy backbone | 151 (48.4) | ||
| Endocrine therapy alone | 21 (6.7) | ||
| Concomitant radiotherapy for LMc, n (%) | |||
| WBRT | 32 (10.3) | ||
| Stereotactic RT | 7 (2.2) | ||
| No RT | 273 (87.5) | ||
| IT therapy initiation period, n (%) | |||
| 2008-2012 | 74 (23.7) | ||
| 2012-2016 | 238 (76.3) | ||
BC, breast cancer; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IT, intrathecal; LM, leptomeningeal metastases; MBC, metastatic breast cancer; NA, not available; PS, performance status; RT, radiotherapy; WBRT, whole-brain radiotherapy.
As defined in the ‘Materials and methods’ section.
Patients treated with ‘chemotherapy or targeted therapy backbone’ may have received more than one type of systemic therapy; percentages were calculated from the total number of our selected IT-treated patients.
Concomitant radiotherapy for LM was defined as RT administered in the 2 months before or after initiation of IT therapy.
The characteristics of our selected patients and details regarding therapies received for LM are also reported in Table 1. The median time to MBC was 30.5 months [interquartile range (IQR) 1.7-85.5], while 27.9% of patients had de novo MBC. The great majority of patients had extensive systemic disease, and almost half (48.7%) of them had at least three other metastatic sites in addition to leptomeningeal metastases, and 43.6% had liver metastases. The majority (52%) had received at least two previous treatment lines, and 21.5% of patients were receiving at least their fifth treatment line at initiation of IT therapy.
The IT agents most frequently used were methotrexate (in 66% of patients), followed by cytarabine (29.2%) and thiotepa (4.8%). The median interval between MBC diagnosis and initiation of IT therapy was 15.7 months (IQR 5.7-35.9). Patients received IT therapy for a median duration of 8.6 weeks [IQR 3.3-20.5; these data were available for 182 patients (58.3%)]. Fifty-five percent of patients received concomitant systemic therapy, which consisted of chemotherapy, targeted therapy (using, in the decreasing order of frequency, trastuzumab, lapatinib, bevacizumab and palbociclib), endocrine therapy or combinations of these systemic agents. Only a minority (12.5%) of patients received RT for LM, whole-brain RT (10.3%) or stereotactic RT (2.2%).
Survival analysis
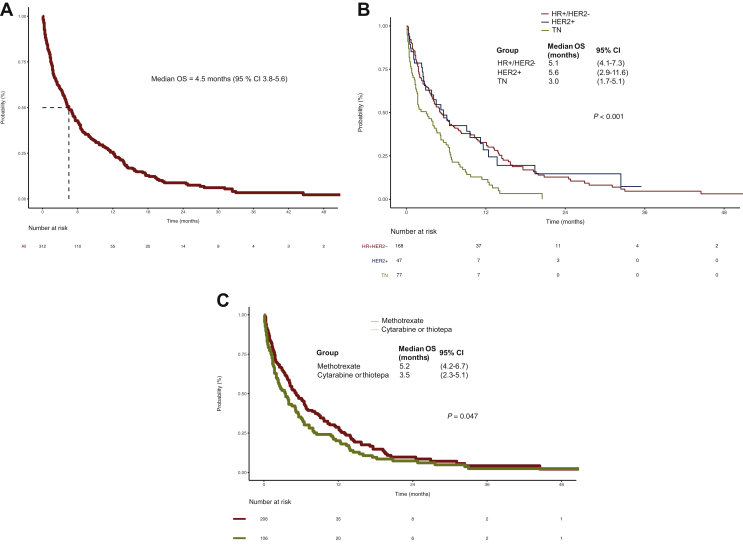
With a median follow-up of 24.6 months (min = 3.1/max = 100.3), 59/312 patients (18.9%) were still alive. Median OS was 4.5 months (95% CI 3.8-5.6), with estimated survival rates of 42.4% (95% CI 37.1-48.6) at 6 months and 25.6% (95% CI 20.8-31.5) at 1 year (Figure 2A). During follow-up, 90.1% of patients experienced an event.
Figure 2.
Kaplan–Meier plots for overall survival (A) in all patients treated with IT therapy; (B) according to BC subtype (HR+/HER2− versus HER2+ versus TN, log-rank P < 0.0001); (C) according to IT agent (methotrexate versus cytarabine or thiotepa, log-rank P = 0.047).
BC, breast cancer; CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IT, intrathecal; OS, overall survival; TN, triple negative.
In multivariable analysis, compared with HR+/HER2− subtype as reference, TN subtype was associated with a significantly poorer OS (hazard ratio 1.81, 95% CI 1.32-2.47, P < 0.001), while HER2+ subtype was equivalent (hazard ratio 0.92, 95% CI 0.62-1.38; Table 2). Median OS was 5.1 months (95% CI 4.1-7.3) for HR+/HER2−, 5.6 months (95% CI 2.9-11.6) for HER2+ and 3.0 months (95% CI 1.7-5.1) for TN BC patients, respectively, P < 0.0001 (Figure 2B).
Table 2.
Univariate and multivariable analyses of overall survival in patients treated with IT therapy
| Categories | N | Univariate Cox model (N = 312) |
Multivariable Cox model (N = 312) |
||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| IT therapy initiation period | |||||||
| 2008-2012 | 74 | 1 | 0.947 | ||||
| 2012-2018 | 238 | 0.99 | 0.74-1.32 | ||||
| Age at MBC, years | |||||||
| <55 | 175 | 1 | 0.618 | ||||
| ≥55 | 137 | 0.94 | 0.73-1.20 | ||||
| Time to MBC, months | |||||||
| <6 | 87 | 1 | 0.302 | ||||
| 6-24 | 52 | 1.03 | 0.71-1.50 | ||||
| 24-60 | 69 | 0.85 | 0.60-1.22 | ||||
| ≥60 | 102 | 0.77 | 0.56-1.07 | ||||
| ECOG performance status at initiation of IT therapy | |||||||
| PS 0-1 | 49 | 1 | 0.014 | 1 | 0.2 | ||
| PS 2 | 42 | 0.95 | 0.58-1.55 | 0.95 | 0.58-1.56 | ||
| PS 3-4 | 48 | 1.84 | 1.18-2.88 | 1.47 | 0.93-2.34 | ||
| PS NA | 173 | 1.39 | 0.97-1.98 | 1.3 | 0.9-1.88 | ||
| BC subtype | |||||||
| HR+/HER2− | 168 | 1 | <0.001 | 1 | <0.001 | ||
| HER2+ | 47 | 0.96 | 0.65-1.42 | 0.92 | 0.62-1.38 | ||
| TN | 77 | 1.87 | 1.39-2.51 | 1.81 | 1.32-2.47 | ||
| NA | 20 | 1.43 | 0.88-2.32 | 1.12 | 0.68-1.83 | ||
| Number of metastatic sites (excluding CNS) at initiation of IT therapy | |||||||
| <3 | 160 | 1 | 0.088 | 1 | <0.001 | ||
| ≥3 | 152 | 1.24 | 0.97-1.59 | 1.33 | 1.01-1.74 | ||
| Treatment line at initiation of IT therapy | |||||||
| Line 1 | 62 | 1 | 0.004 | 1 | <0.001 | ||
| Line 2 | 88 | 1.52 | 1.06-2.18 | 1.44 | 0.99-2.1 | ||
| Line ≥3 | 162 | 1.74 | 1.24-2.45 | 1.88 | 1.30-2.73 | ||
| Concomitant systemic therapy | |||||||
| No | 140 | 1 | <0.001 | 1 | <0.001 | ||
| Yes | 172 | 0.50 | 0.38-0.64 | 0.47 | 0.35-0.62 | ||
| Intrathecal agent | |||||||
| Methotrexate | 206 | 1 | 0.051 | 1 | <0.001 | ||
| Cytarabine/Thiotepa | 106 | 1.29 | 1-1.67 | 1.68 | 1.28-2.22 | ||
| Concomitant RT | |||||||
| No | 273 | 1 | 0.748 | ||||
| Yes | 39 | 0.94 | 0.66-1.35 | ||||
| Univariate Cox model for the scores evaluated | ||||
|---|---|---|---|---|
| N | Hazard ratio | 95% CI | P value | |
| Curie scorea | ||||
| 0 | 40 | 1 | <0.001 | |
| 1 | 123 | 1.31 | 0.87-1.98 | |
| 2-3 | 149 | 2.13 | 1.43-3.19 | |
| Breast-GPAa,b | ||||
| 0.0-1.0 | 74 | 1 | <0.001 | |
| 1.5-2.0 | 154 | 0.6 | 0.45-0.82 | |
| 2.5-4.0 | 63 | 0.46 | 0.31-0.68 | |
BC, breast cancer; CI, confidence interval; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; GPA, graded prognostic assessment; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IT, intrathecal; MBC, metastatic breast cancer; NA, not available; PS, performance status; RT, radiotherapy; TN, triple negative.
Because only eight patients had a Curie score of 3 and only four patients had a Breast-GPA score of 3.5-4.0, these patients were grouped with patients with a Curie score of 2 and a Breast-GPA score of 2.5-3.0, respectively.
Twenty-one patients had Breast-GPA NA due to missing data for BC subtype.
Patients with poor PS of 3-4 had significantly worse survival than patients with PS of 0-1 on univariate analysis (median OS of 4.1 months versus 7.3 months, unadjusted hazard ratio 1.84, 95% CI 1.18-2.88; P = 0.014). In multivariable analysis that also incorporated the type of IT therapy and the presence of concomitant systemic therapy, PS did not reach statistical significance (P = 0.20). More extensive systemic disease (≥3 metastatic sites except for CNS) was also associated with worse OS (hazard ratio 1.33, 95% CI 1.01-1.74; P < 0.001).
Multivariable analysis of the impact of specific IT agents showed that administration of cytarabine or thiotepa resulted in significantly worse OS of 3.5 months, versus 5.2 months for methotrexate (adjusted hazard ratio 1.68, 95% CI 1.28-2.22; P < 0.001; Table 2, Figure 2C).
Concomitant systemic therapy was associated with significantly better OS, increasing from 2.3 months (95% CI 1.7-3.8) without systemic therapy to 6.9 months (95% CI 6.0-10.3) with at least one systemic therapy, and was a strong prognostic factor in our final multivariable model (hazard ratio 0.47, 95% CI 0.35-0.62; P < 0.001; Table 2). Although patients with systemic therapy had longer OS regardless of the BC subtype, this difference was not statistically significant in the specific case of TN subtype (P = 0.24; Supplementary Figure S1A-C, available at https://doi.org/10.1016/j.esmoop.2021.100150). A longer OS with systemic therapy was observed regardless of the type of systemic therapy (data not shown). No statistically significant difference in OS was observed between patients with HR+/HER2− tumours treated with endocrine therapy versus chemotherapy backbone (hazard ratio 1.07, 95% CI 0.62-1.86; P = 0.811; Supplementary Figure S1D, available at https://doi.org/10.1016/j.esmoop.2021.100150). By contrast, administration of brain RT in the 2 months preceding or following IT therapy was not significantly associated with better survival (unadjusted hazard ratio 0.94, 95% CI 0.66-1.35; P = 0.748). This was also true for IT therapy administration after 2012 versus before 2012 [unadjusted hazard ratio 0.99, 95% CI 0.74-1.32; P = 0.947; median OS for patients treated between 2008 and 2012 of 4 months (95% CI 2.4-6.1) versus 4.5 months (95% CI 3.8-6.3) for patients treated between 2012 and 2016; Table 2].
In terms of performance, our multivariable model (including PS) had an acceptable bootstrap-corrected concordance index (C-index) of 0.65 (the area under the receiver operating characteristic curve plots presented in Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2021.100150). The performance of the model was also evaluated by plotting calibration curves at different timelines, showing a good predictive ability (Supplementary Figure S3A, available at https://doi.org/10.1016/j.esmoop.2021.100150).
Validation of previously published prognostic scores
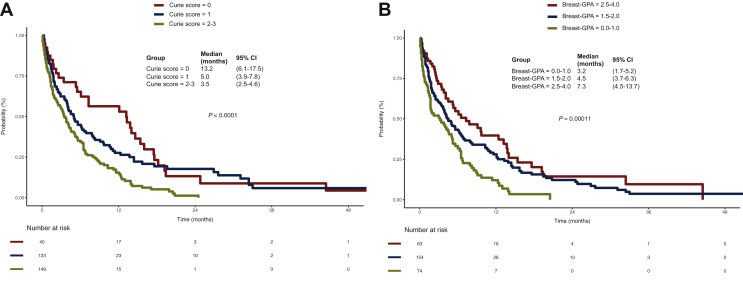
In the present cohort, the simplified Curie score10 had a modest discriminative ability, with a C-index of 0.57 (Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2021.100150), good calibration at 3 months and generally acceptable calibration (Supplementary Figure S3B, available at https://doi.org/10.1016/j.esmoop.2021.100150). When applied to the study patients, the Curie score was significantly prognostic for OS. In our cohort, 12.8% of patients (n = 40) had a Curie score = 0 and median OS of 13.2 months (95% CI 6.1-17.5), 39.4% of patients (n = 123) had score = 1 and median OS of 5.0 months (95% CI 3.9-7.8) and 47.8% of patients (n = 149) had score = 2-3 and median OS = 3.5 months (95% CI 2.5-4.6), P < 0.001 (Figure 3A, Table 2). Patients with a Curie score = 2-3 had significantly longer OS than those with a Curie score = 0 (unadjusted hazard ratio 2.13, 95% CI 1.43-3.19; P < 0.001; Table 2).
Figure 3.
Kaplan–Meier plots for overall survival according to (A) Curie score (0 versus 1 versus 2-3, log-rank P < 0.0001) and (B) Breast-GPA-designated risk groups (0.0-1.0 versus 1.5-2.0 versus 2.5-4.0, log-rank P = 0.00011). Patients were grouped in classes according to each score, as described in the ‘Materials and methods’ section.
CI, confidence interval; GPA, graded prognostic assessment.
The Breast-GPA score16 showed a similar performance in this cohort, with a C-index also of 0.57 (Supplementary Figure S2C, available at https://doi.org/10.1016/j.esmoop.2021.100150) and good calibration at 3 months, better than that observed at 6 or 12 months (Supplementary Figure S3C, available at https://doi.org/10.1016/j.esmoop.2021.100150). The Breast-GPA risk group stratification was also prognostic in our patients. Patients with a Breast-GPA score = 2.5-4.0 had significantly better OS than those with a Breast-GPA score = 0.0-1.0 [median OS of 7.3 months (95% CI 4.5-13.7) versus 3.2 months (95% CI 1.7-5.2), unadjusted hazard ratio 0.46, 95% CI 0.31-0.68; P < 0.001]. Similarly, patients with a Breast-GPA score = 1.5-2.0 had a better OS [median OS of 4.5 months (95% CI 3.7-6.3)] than those with a Breast-GPA score = 0.0-1.0 [unadjusted hazard ratio 0.6, 95% CI 0.45-0.82; P < 0.001; Figure 3B, Table 2].
Discussion
IT therapy is recommended by current guidelines for the vast majority of patients with LM3,8 and is widely used in clinical practice.4,9 We conducted a retrospective analysis of the largest-to-date cohort of BC patients with LM treated with IT therapy. This population was extracted from a contemporary real-life nationwide cohort of MBC patients followed from the time of metastatic relapse, which allowed us to compare the characteristics of these patients with those of the general MBC population, confining however our main analysis exclusively to patients treated with IT.
The predisposition of BC with lobular histology to metastasize to the leptomeninges has been described in the literature10,17 as a specificity of LM, as opposed to brain metastases. Consistent with these data, we also found a higher prevalence of lobular histology in our selected population compared with the general population of MBC patients18 or even with the MBC population with any type of CNS metastases in the ESME database.19 BC subtypes also have different propensities to metastasize to the leptomeninges compared with brain parenchyma. We observed a higher prevalence of LM in TN BC, but not in HER2+ tumours (despite the higher prevalence of brain metastases20), in line with previous reports.10,21 Interestingly, ∼20% of our selected patients had LM at the time of metastatic relapse, while the majority of patients had been heavily pretreated and had extensive systemic disease at LM diagnosis, confirming previous data.5,10
Survival in our population, with a median OS of 4.5 months, was in the range or even better than that previously reported in other studies (e.g. 7 weeks,12 3.5 months,22 3.9 months,23 17 weeks,21 4.5 months,10 or pooled median OS of 14.9-18.1 weeks in the review by Scott et al.4). Although, in most of the cited studies, IT therapy was used for the majority of patients, it should be noted that a limitation in this comparison is our selection of only those patients treated with IT therapy. Besides, in our study, the median OS was defined as starting from the date of IT therapy initiation and not from the date of LM diagnosis. Nevertheless, the outcome of these patients remains disappointingly poor. We also did not find any significant difference in the outcome of patients treated between 2008 and 2012 compared with that of patients treated between 2012 and 2016. However, 25% patients in our study survived for >1 year, a higher percentage than that usually reported4,21,22 and only equal to the rate reported in patients treated with a high-dose IT methotrexate regimen in the study by Gauthier et al.10 This finding suggests a relatively consistent group of patients with a potential for better survival, who might benefit from more intensive therapy.
Significant prognostic factors in multivariable analysis were BC subtype, the number of treatment lines at initiation of IT therapy, the number of other non-CNS metastatic sites, the presence of concomitant systemic therapy and the IT agent used. The respective model (with the addition of ECOG PS) showed a relatively good performance in terms of discriminative and predictive abilities, better than those of the two previously published scores validated in our cohort. Current guidelines recommend patient stratification before choosing the treatment strategy and acknowledge that more individualized prognostic tools remain an unmet need for these patients.3,8 We chose to validate in our cohort two previously published prognostic scores, the simplified Curie score, developed in a population with BC and LM, the majority of them treated with IT methotrexate,10 and the Breast-GPA, initially developed for BC patients with brain metastases,16 but which has also been recently evaluated for BC patients with LM.14 We confirmed in our cohort the prognostic role of the two scores, which mainly differ in terms of the number of previous treatment lines and age. However, these scores both had a low C-index (<0.6) in our cohort. This is the first time that the C-index of the Curie score has been evaluated,10,11 while a higher C-index has been observed with the Breast-GPA in a smaller cohort.14 These low C-indexes for these scores, when applied to our cohort, could be explained by the limitations of this study, as many patients presented missing data for PS at the time of initiation of IT therapy and no data were available concerning concomitant brain metastases. It should also be noted that the PS in our patients was evaluated by the ECOG scale and not the Karnofsky scale and, in order to evaluate the Breast-GPA score, we had to convert one scale to the other, with the inherent limitations. Another major limitation of this study is that other prognostic factors previously shown to have an impact on survival were not available, such as cerebrospinal fluid biochemistry10,12,23 or cytology,13 magnetic resonance imaging aspect,8,13 neurological symptoms8,12 or response to treatment10 (reviewed in4).
The present study suggests that OS could be improved by concomitant systemic therapy (except in TN subtype) and the use of IT methotrexate (rather than cytarabine or thiotepa). There is little strong evidence from randomized trials concerning the benefit of a specific treatment modality in this population.24,25 However, data from many retrospective studies underpin the correlation of systemic therapy23,26 or a combination of IT and systemic therapies4,21,27 with better outcomes, and systemic therapy is recommended by EANO-ESMO guidelines.3 Interestingly, we found no significant difference in terms of survival between patients treated exclusively with endocrine therapy versus patients who received a chemotherapy backbone. To date, evidence for the activity of endocrine agents in LM is mainly based on case reports.28 Most of the previous randomized or observational studies (reviewed in2,5) did not find any significant difference in terms of survival between the various IT agents, except for two older reports of better neurological progression-free survival and quality-of-life-adjusted survival with liposomal cytarabine compared with standard methotrexate in patients with LM from solid tumours.29,30 Nevertheless, we should mention as caveats of our analysis the absence of information on treatment regimens (i.e. standard versus high-dose methotrexate), cytarabine formulation and of course the retrospective design that limited our analysis of all possible confounders. However, these data need to be validated in an independent cohort.
Similar to previous studies,2,23,26 RT for LM was not associated with increased survival, but it was used considerably less frequently than previously described. However, the role of RT in LM consists of alleviating symptoms, mostly in nodular or bulky disease,1 parameters that could not be assessed from our database.
In conclusion, we described and compared the characteristics and outcomes of the largest cohort of MBC with LM treated with IT therapy, associated with poor OS. However, we identified a subgroup of patients with survival >1 year and showed that concomitant systemic therapy may offer a survival advantage that was maintained in HR+/HER2− patients, regardless of whether chemotherapy or endocrine therapy was used. Patients treated with IT methotrexate may also have a better outcome than those treated with IT cytarabine or thiotepa. We also validated two previously published prognostic scores that might help to guide oncologists in the indication for IT and/or systemic therapy.
Acknowledgements
We thank the 18 French Comprehensive Cancer Centers for providing data and each ESME local coordinator for managing the project at the local level. We also thank the ESME Scientific Group and Strategic Committee for their ongoing support.
18 Participating French Comprehensive Cancer Centers (FCCC): I. Curie, Paris/Saint-Cloud, G. Roussy, Villejuif, I. Cancérologie de l'Ouest, Angers/Nantes, C. F. Baclesse, Caen, ICM Montpellier, C. L. Bérard, Lyon, C. G-F Leclerc, Dijon, C. H. Becquerel, Rouen; I. C. Regaud, Toulouse; C. A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C. E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C. J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C. P. Strauss, Strasbourg; I. J. Godinot, Reims; C. O. Lambret, Lille.
ESME central coordinating staff: Head of Research and Development: Claire Labreveux. Program director: Mathieu Robain. Data Management team: Coralie Courtinard, Emilie Nguyen, Olivier Payen, Irwin Piot, Dominique Schwob and Olivier Villacroux. Operational team: Nathalie Bouyer, Michaël Chevrot, Daniel Couch, Patricia D'Agostino, Pascale Danglot, Tahar Guesmia, Elodie Kupfer, Gaëtane Simon, Toihiri Said and Julie Tort. Software designer Team: Matou Diop, Blaise Fulpin, Fréja Messo, José Paredes and Alexandre Vanni.
ESME local coordinators: Thomas Bachelot, Delphine Berchery, Etienne Brain, Mathias Breton, Loïc Campion, Emmanuel Chamorey, Sandrine Dabakuyo, Valérie Dejean, Stéphanie Delaine, Anne-Valérie Guizard, Anne Jaffré, Lilian Laborde, Carine Laurent, Marie-Paule Lebitasy, Agnès Loeb, Damien Parent, Geneviève Perrocheau, Marie-Ange Mouret-Reynier, Michel Velten.
Funding
The Epidemiological Strategy and Medical Economics (ESME) metastatic breast cancer database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai and Daiichi Sankyo). Data collection, analysis and publication are managed entirely by UNICANCER independently of the industrial consortium (no grant number).
Disclosure
M Capone reports personal fees (consulting, advisory) from Lilly, Novartis, GT1, Daiichi Sankyo and fees to the institution from AstraZeneca, Sanofi, Servier and AbbVie, outside the submitted work. All other authors have declared no conflicts of interest.
Supplementary data
Kaplan–Meier plots for overall survival according to the presence or not of concomitant systemic therapy in (A) HR+/HER2− (log-rank P = 0.00095), (B) HER2+ (log-rank P = 0.00046), (C) TN (log-rank P = 0.24) subgroups. (D) Kaplan-Meier plot for overall survival in patients with HR+/HER2− tumours treated with ET alone versus chemotherapy backbone (log-rank P = 0.8). CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; TN, triple negative, ET, endocrine therapy; CT, chemotherapy.
Plots for area under the receiver operating characteristic curve at 1, 3, and 6 months for (A) our multivariate model; (B) Curie score; (C) Breast-GPA. AUC of ROC, area under the receiver operating characteristic curve; TP, true-positive rate; FP, false-positive rate; OS, overall survival; GPA, graded prognostic assessment.
Plots of calibration curves at 3 and 6 months for (A) our multivariable model; (B) score Curie; (C) Breast-GPA score. The grey line represents the ideal model, the black line represents the predicted survival, and the blue line represents the predicted survival computed from bootstrapping (overfitting-corrected). GPA, graded prognostic assessment.
References
- 1.Le Rhun E., Preusser M., van den Bent M., Andratschke N., Weller M. How we treat patients with leptomeningeal metastases. ESMO Open. 2019;4(suppl 2):e000507. doi: 10.1136/esmoopen-2019-000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saadeh F., Boire A. Leptomeningeal disease and the role of intrathecal therapy. In: Ramakrishna R., Magge R.S., Baaj A.A., Knisely J.P.S., editors. Central Nervous System Metastases: Diagnosis and Treatment. Springer International Publishing; Manhattan, New York: 2020. pp. 169–186. [Google Scholar]
- 3.Le Rhun E., Weller M., Brandsma D. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28:iv84–iv99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 4.Scott B.J., Oberheim-Bush N.A., Kesari S. Leptomeningeal metastasis in breast cancer – a systematic review. Oncotarget. 2015;7(4):3740–3747. doi: 10.18632/oncotarget.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taillibert S., Chamberlain M.C. Leptomeningeal metastasis. Handb Clin Neurol. 2018;149:169–204. doi: 10.1016/B978-0-12-811161-1.00013-X. [DOI] [PubMed] [Google Scholar]
- 6.Le Rhun E., Devos P., Boulanger T. The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol. 2019;21(5):648–658. doi: 10.1093/neuonc/noz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain M., Soffietti R., Raizer J. Leptomeningeal metastasis: a response assessment in neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16(9):1176–1185. doi: 10.1093/neuonc/nou089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Central Nervous System Cancers (Version 3.2020) 2020. https://www.nccn.org/professionals/physician_gls/pdf/cns_blocks.pdf Available at. Accessed July 5, 2020.
- 9.Le Rhun E., Rudà R., Devos P. Diagnosis and treatment patterns for patients with leptomeningeal metastasis from solid tumors across Europe. J Neuro Oncol. 2017;133(2):419–427. doi: 10.1007/s11060-017-2452-6. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier H., Guilhaume M.N., Bidard F.C. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21(11):2183–2187. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 11.Bidard F.-C., Lossignol D., Larsimont D., Piccart M., Awada A. Validation of the Institut Curie simplified prognostic score for breast cancer meningeal carcinomatosis. Ann Oncol. 2011;22(2):480–482. doi: 10.1093/annonc/mdq689. [DOI] [PubMed] [Google Scholar]
- 12.Lara-Medina F., Crismatt A., Villarreal-Garza C. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. 2012;18(3):233–241. doi: 10.1111/j.1524-4741.2012.01228.x. [DOI] [PubMed] [Google Scholar]
- 13.Le Rhun E., Devos P., Weller J. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol. 2020:noaa298. doi: 10.1093/neuonc/noaa298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratosa I., Znidaric T. Breast cancer patients with leptomeningeal carcinomatosis: treatment results and validation of prognostic indexes. Eur J Cancer. 2020;138:S65. [Google Scholar]
- 15.Pérol D., Robain M., Arveux P. The ongoing French metastatic breast cancer (MBC) cohort: the example-based methodology of the Epidemiological Strategy and Medical Economics (ESME) BMJ Open. 2019;9(2):e023568. doi: 10.1136/bmjopen-2018-023568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperduto P.W., Kased N., Roberge D. The effect of tumor subtype on survival and the graded prognostic assessment (GPA) for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niwińska A., Rudnicka H., Murawska M. Breast cancer leptomeningeal metastasis: propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med Oncol. 2013;30(1):408. doi: 10.1007/s12032-012-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deluche E., Antoine A., Bachelot T. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Darlix A., Louvel G., Fraisse J. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000. doi: 10.1038/s41416-019-0619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koniali L., Hadjisavvas A., Constantinidou A. Risk factors for breast cancer brain metastases: a systematic review. Oncotarget. 2020;11(6):650–669. doi: 10.18632/oncotarget.27453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwińska A., Pogoda K., Michalski W., Kunkiel M., Jagiełło-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM) J Neuro Oncol. 2018;138(1):191–198. doi: 10.1007/s11060-018-2790-z. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa A., Jordan L., Rozner R. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–28. doi: 10.1016/j.clbc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griguolo G., Pouderoux S., Dieci M.V. Clinicopathological and treatment-associated prognostic factors in patients with breast cancer leptomeningeal metastases in relation to tumor biology. Oncologist. 2018;23(11):1289–1299. doi: 10.1634/theoncologist.2018-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boogerd W., van den Bent M.J., Koehler P.J. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40(18):2726–2733. doi: 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Le Rhun E., Wallet J., Mailliez A. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro Oncol. 2020;22(4):524–538. doi: 10.1093/neuonc/noz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwińska A., Rudnicka H., Murawska M. Breast cancer leptomeningeal metastasis: the results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin Breast Cancer. 2015;15(1):66–72. doi: 10.1016/j.clbc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Le Rhun E., Taillibert S., Zairi F. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neuro Oncol. 2013;113(1):83–92. doi: 10.1007/s11060-013-1092-8. [DOI] [PubMed] [Google Scholar]
- 28.Almajed M.M., Esfahani K., Pelmus M., Panasci L. Complete response and long-term survival of leptomeningeal carcinomatosis from breast cancer with maintenance endocrine therapy. Case Reports. 2016;2016 doi: 10.1136/bcr-2016-215525. bcr2016215525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glantz M.J., Jaeckle K.A., Chamberlain M.C. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 30.Cole B.F., Glantz M.J., Jaeckle K.A., Chamberlain M.C., Mackowiak J.I. Quality-of-life-adjusted survival comparison of sustained-release cytosine arabinoside versus intrathecal methotrexate for treatment of solid tumor neoplastic meningitis. Cancer. 2003;97(12):3053–3060. doi: 10.1002/cncr.11449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan–Meier plots for overall survival according to the presence or not of concomitant systemic therapy in (A) HR+/HER2− (log-rank P = 0.00095), (B) HER2+ (log-rank P = 0.00046), (C) TN (log-rank P = 0.24) subgroups. (D) Kaplan-Meier plot for overall survival in patients with HR+/HER2− tumours treated with ET alone versus chemotherapy backbone (log-rank P = 0.8). CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; TN, triple negative, ET, endocrine therapy; CT, chemotherapy.
Plots for area under the receiver operating characteristic curve at 1, 3, and 6 months for (A) our multivariate model; (B) Curie score; (C) Breast-GPA. AUC of ROC, area under the receiver operating characteristic curve; TP, true-positive rate; FP, false-positive rate; OS, overall survival; GPA, graded prognostic assessment.
Plots of calibration curves at 3 and 6 months for (A) our multivariable model; (B) score Curie; (C) Breast-GPA score. The grey line represents the ideal model, the black line represents the predicted survival, and the blue line represents the predicted survival computed from bootstrapping (overfitting-corrected). GPA, graded prognostic assessment.