Abstract
Most anticancer molecules are administered in body-size-based dosing schedules, bringing up unsolved issues regarding pharmacokinetic data in heavy patients. The worldwide spread of obesity has not been matched by improved methods and strategies for tailored drug dosage in this population. The weight or body surface area (BSA)-based approaches may fail to fully reflect the complexity of the anthropometric features besides obesity in cancer patients suffering from sarcopenia. Likewise, there is a lack of pharmacokinetic data on obese patients for the majority of chemotherapeutic agents as well as for new target drugs and immunotherapy. Therefore, although the available findings point to the role of dose intensity in cancer treatment, and support full weight-based dosing, empirical dose capping often occurs in clinical practice in order to avoid toxicity. Thus a panel of experts of the Associazione Italiana Oncologia Medica (AIOM), Associazione Medici Diabetologi (AMD), Società Italiana Endocrinologia (SIE), and Società Italiana Farmacologia (SIF), provides here a consensus statement for appropriate cytotoxic chemotherapy and new biological cancer drug dosing in obese patients.
Key words: obesity, BSA, cancer drug dosing, chemotherapy dose, pharmacokinetic parameters
Highlights
-
•
The worldwide spread of obesity is an emerging challenge also in cancer patients
-
•
Weight or BSA-based approaches do not adequately address the critical issue of optimal dosing for cancer drugs under obesity
-
•
Empirical dose capping is often employed in clinical practice to avoid toxicities among overweight and obese patients
-
•
There is a lack of clinical and pharmacokinetic studies in this population
-
•
Clinical practice recommendations should guide suitable dosing of cytotoxic and biological cancer drugs in obese patients
Introduction
A direct link between excess body weight and both increased cancer risk and worse cancer outcomes has been seen to be rising globally over recent decades.1, 2, 3, 4 Obesity-related cancer accounts for 3.9% of all cancers worldwide, reaching between 7% and 8% in some high-income countries.3 Current evidence indicates that a 5 kg/m2 increase in body mass index (BMI) is associated with higher risk for several cancers, such as esophageal adenocarcinoma and endometrial, renal, colon and postmenopausal breast cancers.4, 5, 6 The International Agency for Research on Cancer has reviewed studies on the association between the amount of body fat and risk for 13 different cancer sites. The relative risk associated with a BMI ≥40 was up to 4.8 for esophageal adenocarcinoma and 7.1 for endometrial cancer, while physiological levels of adipose mass were associated with lower risk for most cancers.3
Poorer outcomes and the higher cancer mortality rates among obese patients are multifactorial, while it is often the case that empirically lower than full-weight-based chemotherapy dosage could be a potential explanation. Indeed, the clinical practice of dose capping to limit toxicities occurs in up to 40% of heavy cancer patients in the absence of outstanding clinical evidence.7 In 2012, therefore, the American Society of Clinical Oncology (ASCO) released clinical practice guidelines for clinicians to adjust chemotherapy dosage calculations to take account of actual body weight.8 No recommendations have however been provided for new target drugs and immunotherapy. There is moreover a lack of prospective randomized controlled clinical trials (RCTs) exploring the optimal dose of anticancer treatment in patients with excess body weight, who are also frequently under-represented in studies on novel anticancer drugs.
Anticancer agent doses are personalized according to the patient's weight or body surface area (BSA).9 Less common methods for determining dosing for adult cancer patients include ‘flat-fixed’ dosing10 and ‘dose banding’11 which help to avoid potential calculation errors. It is well known that anthropometric changes in obese individuals, as regards body proportions of water, fat and muscle mass, are accompanied by variations in district-specific blood flow, alterations of liver and renal functions and chronic, low-grade inflammatory state.12 All of these critical factors impact on pharmacokinetic (PK) parameters as well as pharmacodynamic (PD) endpoints of a given drug.13
Overall, the complex PK changes observed in obese individuals may, at least partially, explain why several limitations are observed when BMI and BSA are used to adapt treatments to overweight or obese patients.14 The assumption behind both scenarios is that the PK and PD of each drug increases in proportion to weight or BSA. This is however often not the case since BMI and BSA are not informative on body composition as they fail to distinguish between fat and lean tissue mass.
The poor reliability of currently employed dose adjustment strategies is a critical clinical issue for anticancer drugs whose narrow therapeutic index may result in drug overexposure (weight-based approach) or underexposure (BSA-based approach) in obese patients. This may mean an increased risk of toxicity on the one hand or risk of underdosing on the other.8,15 The question arises therefore of whether the adoption of personalized drug dosage in overweight/obese patients is really necessary.16
The Associazione Italiana Oncologia Medica (AIOM), the Associazione Medici Diabetologi (AMD), the Società Italiana Endocrinologia (SIE) and the Società Italiana Farmacologia (SIF) have gathered together here a panel of experts to review the current evidence on this topic and formulate a consensus for recommendations addressing dosages for cytotoxic chemotherapy, novel immunotherapies and targeted agents in overweight and obese adults.
Materials and methods
A web-based search of Medline/PubMed library data published for all relevant studies up to March 2021 was carried out using the following keywords: ‘obesity’ OR ‘obese’ OR ‘overweight’ OR ‘body weight’ AND ‘cancer’ OR ‘tumour’ OR ‘neoplasms’ AND ‘dose’ OR ‘dosing’ AND ‘chemotherapy’ OR ‘drug therapy’ OR ‘targeted therapy’ OR ‘target therapy’ OR ‘immunotherapy’ OR ‘immune checkpoint inhibitors’. The identified reports were independently screened by two investigators (A.A. and N.S.). Only papers written in English were included. Each paper was retrieved and its references were reviewed to identify additional studies. Most of the studies included in this consensus paper refer to retrospective analyses of RCTs and observational studies comparing full-weight and non-full-weight dose for antitumor therapy. ASCO guidelines for appropriate chemotherapy dosing in obese patients conveyed in 2012 were also taken into account and incorporated. Additional biological and clinical information, including drug metabolism, PK and PD parameters in overweight/obese patients was summarized by the panel of experts.
Body composition and conventional definitions of ‘overweight’ and ‘obesity’
According to the World Health Organization (WHO), ‘overweight’ and ‘obesity’ are defined as abnormal or excessive fat accumulation that presents a risk to health.17
In clinical practice, whether a person is overweight or obese is assessed by the BMI, calculated as weight (in kg) divided by height (in meters squared) and categorized using the following WHO classification (Table 1).
Table 1.
BMI classification according to the World Health Organization (WHO)
| WHO classification | BMI (kg/m2) |
|---|---|
| Underweight | BMI ≤ 19.9 |
| Normal weight | 20 ≤ BMI ≤ 24.9 |
| Overweight | 25 ≤ BMI ≤ 29.9 |
| Obesity grade I | 30 ≤ BMI ≤ 34.9 |
| Obesity grade II | 35 ≤ BMI ≤ 39.9 |
| Obesity grade III | BMI ≥ 40 |
BMI, body mass index; WHO, World Health Organization.
Unfortunately, BMI fails to take into account multiple important factors, including muscle mass, different distribution of adiposity and differences between races.18 In addition, BMI is not used for children and adolescents aged 2-18 years for whom a percentile scale based on the child's sex and age is recommended. In this population, overweight is defined as a BMI between the 85th to 94th percentile, and obesity is considered for a BMI ≥95th percentile.19 Despite these limitations, BMI is still the index most used in clinical practice for the categorization of overweight and obese patients (Figure 1).
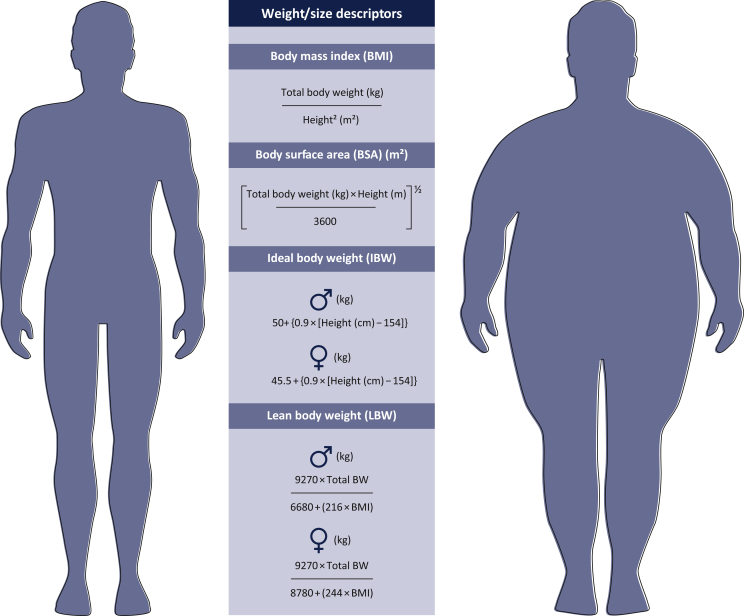
Figure 1.
Summary of the most common weight and size descriptors and their limits.
Drug dose administration usually follows one of the three illustrated approaches: weight-based dosing, body-surface-area-based dosing, or fixed dosing. The first two strategies assume that drug PK parameters increase in proportion to increasing body size, whereas dosing drugs on a fixed basis presumes that body size does not influence drug PK parameters. Although commonly used to scale drug therapy in overweight or obese patients, each of these descriptors has important limitations. BMI and BSA are not informative as regards body composition and do not differentiate fat from lean tissue mass. IBW seems inappropriate as a dosing metric as it predicts the same dose for people of the same height, regardless of weight. LBW requires specialized equipment as it is measured with methods such as dual-energy X-ray absorptiometry, bioelectrical impedance analysis, underwater weighing and skinfold thickness.
BW, body-weight.
For several anticancer drugs, doses are defined according to BSA. A variety of algorithms has been proposed for estimating BSA, though none of the currently available methods amounts to a universal standard. Each algorithm is fundamentally based on the patient's height and weight, with somewhat different theoretical, empirical or pragmatic underpinnings.20 Among them, the ‘Mosteller’ formula is commonly used (Figure 1).21
Although chemotherapy efficacy and toxicity are still highly variable between individual patients despite normalization based on BSA dosing, this approach is still the most accepted in oncology.20 It should be noted that BSA, like BMI, is calculated on the basis of patient weight (kg) and height (cm), and is unable to distinguish between fat and lean tissue mass, so provides no information about body composition (Figure 1).
Alternative weight descriptors have been proposed to prevent drug overexposure with weight-based dosing, though each of these is associated with both benefits and limitations.22
Lean body weight (LBW) has also been recommended for scaling drug doses.23 LBW reflects the weight of all ‘non-fat’ body components, including muscle and vascular organs such as the liver and the kidneys. As LBW contributes to ∼99% of drugs clearance,24 it might be useful for guiding dosing in obesity. However, although recent approaches (such as dual-energy X-ray absorptiometry, magnetic resonance imaging and computerized tomography) offer accurate information about body composition, these measures are only used for research purposes as they are expensive and have difficulty in accommodating individuals with BMIs at ≥35.18 Predictive equations taking into account sex, body weight and height, have been proposed to quantify LBW, starting out from easily accessible patient characteristics (Figure 1).
Other convenient and affordable measures such as waist circumference, waist-to-hip ratio, bioelectrical impedance analysis (BIA) and skinfold thickness also have significant limitations.18
Finally, ideal body weight (IBW), which indirectly could represent LBW, is calculated as shown in Figure 1.25 However, IBW seems inappropriate as a dosing metric because it predicts the same dose for people of the same height, regardless of weight. Furthermore, since limited variability exists when we consider height, dosing on IBW has the net effect of estimating a very narrow range of doses.22
Overall, the availability of disparate weight/size descriptors highlights the lack of a unifying gold-standard index to define chemotherapy dose adjustment in obese subjects. More clinically meaningful indexes should carefully consider the complex scenario defined by obesity itself, whose intricate changes in both fat and non-fat components profoundly influence the PK and PD of anticancer drugs.
Effects of overweight and obesity on PK parameters
Obesity plays a significant impact on key organs engaged in drug PK, with subsequent potential changes in all main PK parameters13 (Figure 2).
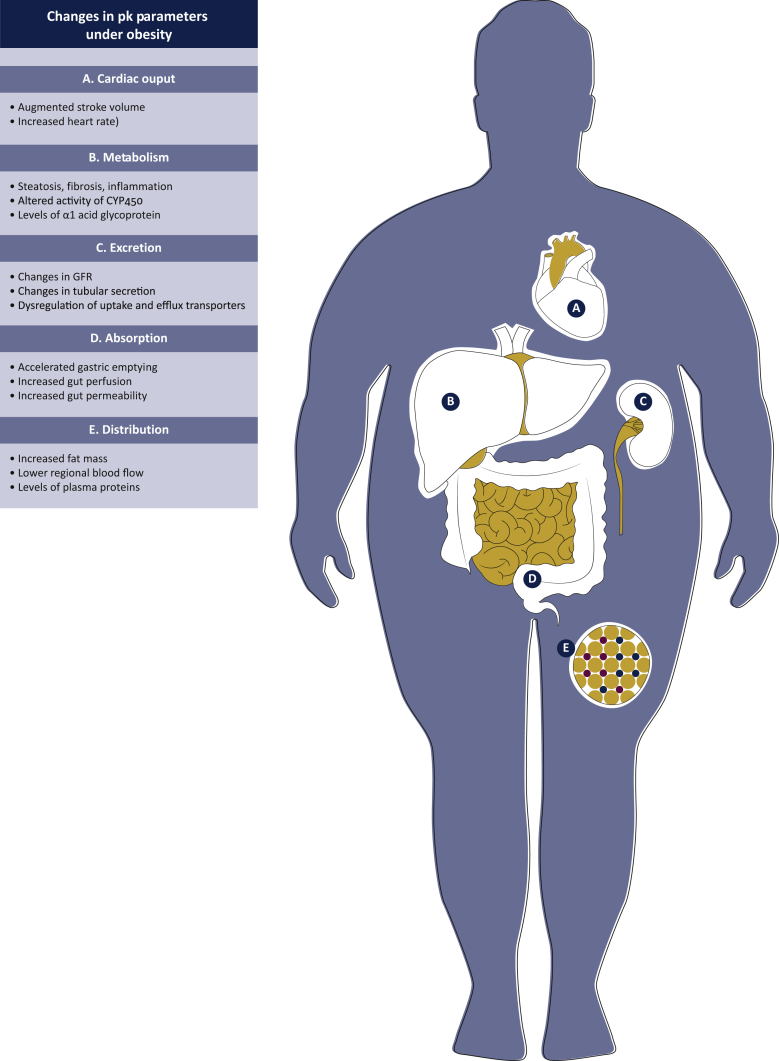
Figure 2.
Graphic summary of the complex changes induced by obesity in all pharmacokinetic (PK) parameters.
The dose of each drug is determined by the plasma concentration required to achieve the desired effect. The plasma concentration of each drug following administration is dependent on its absorption (if not administered via the intravenous route), distribution, metabolism and excretion from the body. The duration of administration will also affect drug plasma concentration. In obese individuals, anthropometric changes in body proportions of water, fat and muscle mass are accompanied by variations in cardiac output, regional blood flow, alterations in liver and renal function and a chronic, low-grade inflammatory state.
GFR, glomerular filtration rate.
Absorption
For the majority of tyrosine kinase inhibitors (TKIs) and for some cytotoxic molecules (e.g. capecitabine, vinorelbine and cyclophosphamide) administered orally, increases in gut perfusion and accelerated gastric emptying reported in obese subjects may contribute to enhancing their availability. Conversely, a decreased absorption rate may characterize drug treatments administered by subcutaneous and transdermal routes.26 This may in part be explained by dysregulated blood flow in subcutaneous adipose tissue of obese individuals as a result of physiological adaptation to the increased adipose tissue mass and the reduced metabolic needs in obese individuals.27,28
Distribution
Classical PK parameters such as volume of distribution (Vd), clearance (Cl) and protein binding depend both on the physico-chemical properties of a drug (lipophilicity, polarity, molecular size and degree of ionization) and body composition, blood supply and plasma protein levels.29, 30, 31
Compared with molecules with weak or moderate lipophilicity, whose distribution in lean tissue is quite predictable, the majority of anticancer drugs are partly distributed in adipose tissues, and their affinity for plasma proteins and/or tissue components may change significantly in obese subjects. Given the unique properties of each drug, it is not surprising that obese and non-obese patients may have significantly different drug plasma concentrations even in the presence of similar tissue concentrations. For example, although the Vd for lipophilic drugs is expected to be higher in obese subjects, decreased tissue perfusion and cardiac function may result in lower Vd values.29,32
Obesity is characterized by an increase in both lean and fat mass. However, while the increased lean mass is responsible for 20%-40% of the excess weight, the percentage of fat mass can almost double in obese subjects. The lean mass per kg of body weight is therefore decreased in obese patients and this affects drug tissue distribution.14 In addition, the potential role of the adipocytes on drug metabolism or on the specific activities that may characterize subcutaneous fat and visceral fat have not as yet been sufficiently investigated.33 Especially in the case of subcutaneous administration, the distribution of a drug to and from a target in adipose tissue may be modified since the blood flow per gram of fat is significantly lower in obese patients compared with lean individuals.34,35 For instance, in adipose tissue, the basal ethanol ratio was significantly higher and dialysate metabolite concentrations were significantly lower in obese than in non-obese men.36 Subcutaneous adipose tissue blood flow (ATBF) is downregulated in obesity, and its responsiveness to meal intake is reduced; the reduction in ATBF represents an adaptation to the increased fat mass, probably mediated by adrenergic stimulation.28 Furthermore, although plasma protein binding does not seem to be altered by body composition, the increased amount of alpha-1-acid-glycoprotein, linked to a chronic inflammatory state under conditions of obesity, may partially account for a prolonged half-life of some drugs.34
Cl is influenced by several factors including some that are unaffected by obesity (such as albumin binding and ionization status) and others potentially affected by obesity, including blood flow through the organ responsible for excretion (liver, kidney). In obese patients, liver steatosis may reduce blood flow through the liver and decrease Cl for several chemotherapy agents.37 Renal Cl is dependent on the glomerular filtration rate (GFR), which may increase due to the increased cardiac output and tubular excretion/reabsorption, which is likely to be independent of body mass. In patients with nonalcoholic fatty liver disease (NAFLD), the cytochrome P (CYP) 3A4 activity and its abundance in human liver tissue has been studied and it was that CYP3A4-dependent metabolism that was reduced significantly, suggesting a negative impact of hepatic steatosis on drug metabolism.38 Despite inconsistency on evaluation of creatinine, Cl does not seem to linearly correlate with total body weight in obese patients.32
Metabolism
The main site of metabolism is the liver, where drugs undergo transformation via phase I (biotransformation: oxidation, reduction and hydrolysis) and phase II (conjugation) reactions. Most parent drugs are active but a significant number of agents such as antimetabolites (e.g. antifolates, purines or pyrimidines derivatives) are prodrugs requiring activation in the liver to yield active metabolites. Liver abnormalities related to fatty infiltrations and steatosis combined with inflammation and fibrosis are proportionally correlated with the increasing BMI in obese subjects.39 Inflammation may decrease the activity of specific CYP isoforms,40,41 resulting in altered transformation and effectiveness of individual treatments. In addition, for drugs of high and moderate hepatic extraction, an increase in hepatic blood flow may enhance first-pass extraction in the liver as well as hepatic clearance.
Elimination
Drugs are excreted as metabolites or unchanged. The effects of obesity on hepatic and renal drug clearance are still incompletely understood. However, increased cardiac output and hepatic blood flow and increased GFR may contribute to changes in the elimination of drugs in obese patients,42 in particular since excess adipose tissue, blood volume, stroke volume and cardiac output are all increased to meet the metabolic demand of the excess adipose tissue. Concurrently, GFR may increase, although, in the long term, chronic renal dysfunction may occur with a resulting decline in GFR being observed.42 Similarly, obesity may interfere with biliary and renal secretion by dysregulating the expression and activities of tissue-specific uptake and efflux transporters.43 For example, the metabolism of morphine is not altered in obese patients; however, decreased elimination of glucuronate metabolites is found, while a rational explanation for this finding is alterations in membrane transporter function and/or expression in the liver.44 This suggests that pathophysiological changes associated with obesity may influence the activities of hepatic transporters and possibly contribute to an alteration in drug elimination rates.
Monoclonal antibodies (mAbs) differ significantly from chemical drugs in terms of PK parameters, showing low values for Cl and Vd, and a long half-life.45 Both absorption and metabolism phases are almost non-existent; their distribution is mainly in the blood and extracellular fluids due to their size and hydrophilicity, while elimination occurs by intracellular degradation subsequent to their Fc receptor binding and target recognition specificities and, less frequently, by proteolytic catabolism.46 The binding of mAbs to the target at the cell surface depends on tumor burden, expression levels of target and mAb affinity, while it is not affected by body weight. Conversely, proteolytic catabolism of mAbs targeting soluble targets taking place in endosomal space (accounting for 0.5% of the total tissue volume) is body-weight dependent, although its impact on total drug elimination is slight.46 These common features suggest lower patient variability, though more studies, especially conducted in the early clinical phases, are needed to show the impact of overweight or obesity on their PK/PD profile.46
The availability of antibody-drug conjugates (ADCs) enlightens additional complexities.47 The PK properties of ADCs should take into consideration the heterogeneity of these drugs and their composite structure, which gives rise to multiple active molecular species in systemic circulation and/or tissues of interest.
As briefly summarized, the question of obesity provides a tremendous challenge in the attempt to standardize dosing and for the achieving of consistent therapeutic effects while finding an acceptable or manageable level of toxicity in all patients. Clearly, in addition to the specific profile of each drug, the therapeutic intent, the type of tumor treated and the patient's age are all additional factors that must be taken into consideration.
Evidence-based chemotherapy dosing in overweight and obese patients
The determination of optimal doses in oncology is often challenging both for chemotherapeutic and for targeted agents. Based on the theory according to which the highest tolerated dose of a drug should be that given for the best therapeutic effect to be achieved,48 phase I and I/II clinical trials are currently designed to define the maximum tolerated dose (MTD) of novel molecules, whose schedules are further optimized in subsequent phase II-IV studies.20,49
By convention, chemotherapy unit dose administered per unit time is defined as ‘dose intensity’ (DI).49 The delivery of optimal DI in potentially curable cancer patients has been proposed as a major indicator of cancer care quality.20
Dose-dense chemotherapy protocols (i.e. regimens in which the standard drug dose is delivered at shorter time intervals)50 have been developed in recent years for some curable malignancies, such as early breast cancer,51 based on the hypothesis that increased treatment frequency might kill a higher proportion of rapidly proliferating cells.52
The magnitude of chemotherapy dosing variations is generally quantified in terms of relative DI (RDI), namely the ratio of the delivered dose intensity to the standard (or planned) DI for a chemotherapy regimen.49
The importance of DI maintenance in oncology first emerged from pre-clinical studies involving murine models of sarcomas or carcinomas, in which two- to three-fold chemotherapy dose reductions correlated with significant worsening of complete response rates.53
In the clinical setting, an early study by Bonadonna et al. randomized 386 women with lymph-node-positive breast cancer to undergo either systemic adjuvant chemotherapy with cyclophosphamide, methotrexate and 5-fluorouracil, or follow-up after radical mastectomy. At the 20-year analysis, women receiving at least 85% of the planned chemotherapy dose experienced the best clinical outcome.54 Additionally, a benefit of a higher chemotherapy dose was described by the Cancer and Leukemia Group B (CALGB) study 8541 and the French Adjuvant Breast Cancer Group,55,56 suggesting the existence of a strong correlation between treatment dose and outcome in early breast cancer patients, in terms of disease-free survival and overall survival (OS), regardless of body weight. Chemotherapy dose reduction and treatment delays have also been shown to negatively impact on OS in metastatic breast, ovarian and lung cancer settings.57, 58, 59, 60, 61
Chemotherapy dose capping nevertheless often occurs in clinical practice, particularly among overweight and obese patients, in order to avoid toxicities. The use of idealized body weight or a maximum of 2.0 m2 or 2.2 m2 BSA instead of actual body weight in chemotherapy dose calculations is often planned from the start of treatment and based on empirical underpinnings.8 Several retrospective studies in early-stage cancer patients reported that adjuvant chemotherapy dosage was often reduced in obese patients, with a subsequent negative impact on the clinical outcome.7,9,62,63 Stocker et al.,64 in an exploratory analysis of a PETACC 3 study, showed that dose reduction negatively affected relapse-free survival (RFS) [hazard ratio (HR): 0.48, 95% confidence interval (CI), 0.27-0.85; P = 0.01] with a strong trend toward better OS (HR: 0.53, 95% CI, 0.28-1.01; P = 0.052) in patients with BMI ≥30 kg/m2 and BSA ≥2 m2 receiving adjuvant chemotherapy for colon cancer.9 Similarly, the CALGB study 8541 supports the use of full-dose chemotherapy compared with a reduced initial dose due to the improved failure-free survival in obese women (overall adjusted failure risk ratio of 0.73, 95% CI, 0.53-1.00).63
The likelihood of receiving a first-cycle dose reduction (<90% of the expected dose) increased in step with the obesity grade, being 11%, 20% and 37% in overweight, obese and severely obese patients, respectively, in a retrospective cohort study of 9672 breast cancer women treated with doxorubicin hydrochloride and cyclophosphamide.7 However, the use of standard full-weight-based doses does not seem to be associated with a greater risk of adverse events in obese patients with respect to normal-weight patients in several retrospective analyses and observational studies.7,63,65, 66, 67, 68, 69, 70, 71, 72, 73, 74 Furthermore, a reduced risk of toxicity for events, such as leukopenia, neutropenia, thrombocytopenia and stomatitis, has been reported in some case series of weighty patients receiving full-dose chemotherapy, suggesting a BSA-related PK effect of BSA over drug elimination.7,75, 76, 77 In particular, Wright et al. reported grade 3-4 leukopenia in 44% and 70% (P = 0.0001), and any grade thrombocytopenia in 27% and 50% (P = 0.0004) of ovarian cancer patients receiving carboplatin with BMI >30 kg/m2 and BMI <25 g/m2, respectively.77 Likewise, Meyerhardt et al. showed lower rates of grade 3-4 leukopenia in heavier- compared with normal-weight patients (6% versus 11%, P = 0.0036) and any severe grade adverse events (45% versus 53%, P = 0.04).75,76
On the other hand, retrospective data from the randomized German Adjuvant Intergroup Node-positive (GAIN) study showed that dose-dense regimens (epirubicin, docetaxel and cyclophosphamide or epirubicin and cyclophosphamide followed by docetaxel plus capecitabine) at full dose according to the actual BSA in obese breast cancer patients correlated with a higher risk of severe toxicities, such as febrile neutropenia, high-grade thrombocytopenia and thromboembolic events, as compared with obese patients receiving an adjusted dose (16% versus 6%, P = 0.003; 9% versus 3%, P = 0.002; 17% versus 10%, P = 0.017, respectively). The authors therefore suggested a dose adjustment of intense dose-dense chemotherapy in obese patients to avoid the occurrence of life-threatening complications.78
A systematic review and meta-analysis attempted to reveal the risks and benefits of full-dose chemotherapy in obese patients.79 Twelve studies involving 9314 patients with colorectal cancer (55%), breast cancer (29%) or other types of tumors were analyzed to compare toxic effects and survival in obese and normal-weight patients treated according to the actual BSA. In most of these studies, toxicity and outcome did not statistically differ between the two groups. Quantitative pooling of the available data showed that the rates of toxic effects were similar or lower in obese patients [any grade 3/4 toxic effect: odds ratio (OR) 0.75, CI 0.65-0.87]. Among eight studies comparing progression-free survival and OS, Jones et al. showed that obese patients with B-cell non-Hodgkin's lymphoma and treated with seven different chemotherapy regimens (mostly, CHOP backbone) reported longer survival compared with normal-weight subjects.80 Conversely, Meloni et al. reported a benefit in normal-weight patients undergoing conditioning regimens with busulfan/cyclophosphamide for autologous stem cell transplantation.81
BSA however, taking into account weight and height, has not been deemed a proficient parameter for determining the optimal dose of chemotherapy.82 Indeed, body composition, considering muscle and adipose tissue distribution, seems to better reflect the complexity of the anthropometric features of cancer patients (such as obesity, sarcopenia and myopenia). A body of evidence suggests that lower LBW was associated with higher toxicity, while greater adiposity was associated with lower anthracycline and taxane-based chemotherapy adherence (RDI), irrespective of BSA.83,84 The lower muscle mass reduced the clearance of hydrophilic drugs, such as doxorubicin, resulting in a risk of overdose and increased toxicity.85,86 On the other hand, lipophilic compounds, such as paclitaxel and docetaxel, could accumulate in fat tissue with resulting delayed toxicity.87 Overall, body composition may be useful for predicting higher toxicity risk. Large prospective clinical trials with planned analysis of body composition and pharmacokinetic data are needed to provide evidence both as regards adverse events and efficacy.
ASCO guidelines for chemotherapy dosage in overweight/obese patients
To date, the only recommendations available for the management of overweight patients derive from ASCO guidelines published in 2012, which do not consider novel molecular-targeted therapies and immunotherapies. ASCO carried out a systematic literature search and review providing clinical practice guidelines on appropriate chemotherapy dosing in obese cancer patients. The expert panel recommended full-weight-based chemotherapy doses in the treatment of the obese cancer patient, particularly when cure is the goal of treatment. Moreover, the guidelines suggested treatment-related toxicities in obese cancer patients should be treated as in normal-weight patients. If a dose reduction is needed to limit toxicity, consideration should be given to the resumption of full-weight-based doses for subsequent cycles, especially if the putative cause of the observed toxicity (e.g. impaired renal, hepatic function) has been resolved.8
Targeted therapies and immune checkpoint inhibitors in overweight and obese patients
Compared with chemotherapy drugs, mAbs are generally not influenced by body size and composition in terms of their distribution and elimination, requiring fewer dosage variations unless clinically meaningful toxicities occur.88 Indeed, the change in Vd and in blood volume (BV) of mAbs is less significant than the change in body weight. In underweight patients, however, the reduction in BV could result in lower plasma levels of body-size-based dosing mAbs, while the greater BV in obese patients could result in higher plasma levels of the drug. Conversely, in flat-fixed dosing strategies, the underweight and obese patients could receive a relatively higher and lower dose, respectively.88 This interpatient variability gave rise to the comparison between body-size-based or fixed dosing of mAbs.
In particular, immune checkpoint inhibitors (ICIs) are characterized by a wide therapeutic index, for which fixed dosing has been introduced in clinical practice to reduce both errors and preparation costs.89,90
Nevertheless, the limited number of PK/PD studies on ICIs means there remain doubts about the existence of a potential relationship between the dose required and body weight for some of them.91 For instance, the clearance of ipilimumab increases with increasing body weight, making a body-weight normalized dosing regimen more appropriate than a fixed dose for this anti-CTLA-4.92 Similarly, the clearance of nivolumab might be influenced by high body weight resulting in lowest drug exposures.93,94 However, Bajaj et al. reported that nivolumab steady-state exposure seems to be comparable over the evaluated body weight ranges (from 34.1 to 168.2 kg). Thus the variation is not expected to be clinically relevant.93 According to a population PK analysis, total systemic clearance of avelumab also increases with body weight, whereas age, gender, race, programmed death-ligand 1 (PD-L1) status, tumor burden, renal impairment and mild or moderate hepatic impairment do not.95 Similarly, body weight seems to be significantly associated with varying clearance also for pembrolizumab, cemiplimab, atezolizumab and durvalumab even if the clearance variation does not appear clinically significant for all of them (effect on PK parameter does not exceed 30%).96 Thus, weight-based dosing seems to be appropriated for anti-programmed cell death protein 1 (PD-1) and anti-PD-L1 even in overweight and obese patients.
On the other hand, the flat dose regimens are approved for nivolumab and pembrolizumab, considering the former body-weight-based doses for 80 kg and 100 kg patients, respectively. The recommended dosages were approved according to population PK modeling showing a substantial overlap of exposure between body-weight-based and fixed dose with a comparable efficacy and safety profile.89,97,98 However, to date, the risk of reduced exposure cannot be ruled out for heavier patients, legitimizing questions as to the generalization of flat doses as opposed to body-weight-normalized doses.92,96 Even if some data published in the literature show a dependence of the PK of ICIs on the characteristics of patients, their consistency is not sufficiently robust to justify dose adjustment of ICIs in overweight/obese subjects.
There is a huge body of evidence suggesting the potential link between obesity and prognosis in patients receiving ICIs, highlighting the role of proper dosing strategy to maximize drug efficacy.99
Indeed, chronic inflammatory state and consequent T-cell exhaustion observed in both obese murine models and humans have been shown to correlate with suppressed immune responses.100 On the other hand, leptin secretion, typically increased in obese subjects,101 has been associated with increased tumor cell proliferation and cancer infiltration by PD-1-expressing lymphocytes. In pre-clinical studies, administration of anti-PD-1 agents resulted in increased tumor shrinkage and reduced metastasis formation in obese versus control murine melanoma models.102
In the clinical setting, several retrospective studies explored the impact of BMI on the clinical outcome of cancer patients who underwent treatment with ICIs.103, 104, 105 Among these, Richtig et al. described a significantly higher response rate (RR) and lower incidence of brain metastases in patients with BMI ≥25 kg/m2 treated with 3 mg/kg ipilimumab, in the absence of significant differences in terms of side-effects, compared with the normal-weight group (P = 0.498, χ2 test).105 A wide multi-cohort analysis including data from 1918 patients receiving chemotherapy, immunotherapy or targeted treatment of metastatic melanoma confirmed the association between obesity and OS, although this correlation was restricted to males who underwent treatments other than chemotherapy.103 The authors suggested that such discrepancy between sexes might be explained, at least partially, by differences in the hormonal milieu and body composition. Notably, there was no safety profile difference between patients with normal and with high BMIs across different regimens. A recent retrospective study explored the correlation between obesity and clinical outcome not only in melanoma, but also in lung and renal cell carcinoma, confirming better clinical outcomes in terms of OS and progression-free survival (PFS) in patients with a BMI ≥25 kg/m2 treated with first-line PD-1/PD-L1 inhibitors.106 In this study, overweight/obese patients turned out to be more likely to experience any grade immune-related adverse events (irAEs) as against non-overweight patients (55.6% versus 25.2%, respectively; P < 0.0001), but no differences were observed between the two groups in terms of grade 3-4 irAEs (7.6% versus 5.3%, P = 0.1338). Two wider meta-analyses, including 13 and 16 studies on ICIs, respectively, have further confirmed the favorable prognostic significance of high BMI with respect to OS and PFS.107,108 The former study described no significant differences between obese/overweight patients and normal-weight patients for all-grade irAEs (overweight versus normal: pooled RR = 1.28, 95% CI 0.76-2.18, P = 0.356; obese versus normal: pooled RR = 1.36, 95% CI 0.85-2.17, P = 0.207),107 while the latter showed a significantly higher risk of adverse events in high- versus low-BMI subjects (OR = 2.91, 95% CI 1.39-6.11; P = 0.005).108 It is of note that none of the above mentioned studies reported ICI dose adjustment in overweight/obese subjects.
Such data led to the coining of the term ‘obesity paradox’, namely the apparently favorable correlation between overweight/class I obesity and prognosis in cancer patients undergoing treatment with ICIs,99,109 whose underlying biological mechanisms have, however, yet to be elucidated. In this regard, a putative explanation was proposed by Sanchez and coworkers who analyzed the gene expression profile of primary tumors and peritumoral fat derived from renal clear-cell carcinoma patients with variable BMI. Interestingly, the extent of immune cell infiltration did not significantly differ according to the patients' BMI, but up-regulation of Th1 and Th2 pathways, dendritic cell maturation and CD28 signaling were found in samples from obese versus normal-weight patients.110 Higher angiogenic scores on gene-set enrichment analyses were moreover found in the tumors of obese patients, suggesting a potential higher sensitivity of these malignancies to angiogenesis inhibitors.110
With respect to anti-angiogenic drugs, conflicting data emerged with regard to the correlation between obesity and treatment outcomes. For instance, a study from Miyamoto et al. on bevacizumab in metastatic colorectal cancer showed a significant correlation between increased visceral fat and longer OS (P = 0.03),111 whereas a BMI ≥25 kg/m2 was found to positively impact on both PFS and OS (P < 0.05 in both instances) in metastatic HER2-negative breast cancer patients treated with first-line bevacizumab plus paclitaxel.112 Other studies, however, showed a negative correlation between high BMI and clinical outcome in metastatic colorectal cancer patients,113,114 particularly in those with KRAS wild-type left-sided primary tumors, receiving bevacizumab in addition to chemotherapy.113
As for other mAbs, in non-metastatic HER2-positive breast cancer, a recent report from Gonzalez Garcia et al. has suggested that the administration of trastuzumab via subcutaneous injection allows the target concentration of 20 μg/ml to be reached in 87.5% of patients with BMI ≤30 kg/m2, compared with only 20% of women with BMI >30 kg/m2 (P < 0.001). By contrast, the proportion of patients reaching the target concentration after intravenous trastuzumab administration has been independent of their BMI. Though based on a small patient series (N = 50),115 this study highlights the need for further investigation on this topic to ensure adequate drug exposure in this population suffering from potentially curable cancer.
With respect to TKIs and other targeted agents, the impact of the patient being overweight or obese on the treatment outcome was investigated in 1975 patients from the International Metastatic Renal Cell Carcinoma Database Consortium. Interestingly, a BMI ≥25 kg/m2 was found to be associated with improved OS (25.6 months, 95% CI 23.2-28.6 versus 17.1 months, 95% CI 15.5-18.5) in advanced clear-cell renal cell carcinoma patients.116 The cumulative incidence of treatment failure due to toxicity did not differ between the overweight/obese (13%, 95% CI 10%-17%) and underweight/normal groups (15%, 95% CI 12%-19%).116 Bergerot et al. have recently described a similar trend on a smaller case series.117
In metastatic EGFR-mutated non-small cell lung cancer (NSCLC), however, no correlation has been found between patient nutritional status (defined by BMI, body weight and BSA) and response to gefitinib,118 whereas a potential higher risk of grade ≥2 hepatic dysfunction has been observed in overweight subjects.119
As for other targeted agents, BMI did not impact on molecular RR of nilotinib and dasatinib, while a delayed and low rate of molecular responses were observed for imatinib as frontline treatment in obese patients, probably due to the effect of the drug on signaling regulation of macrophages via platelet-derived growth factor (PDGF) receptors adipogenesis stimulation.120 Interestingly, blood levels of imatinib after bariatric surgery in an obese patient were 40%-60% lower than before operation.121
A study on renal cell carcinoma patients treated with cabozantinib, stratified by BMI, showed that a BMI ≥25 correlated with longer survival. Although the PK of cabozantinib was not examined, the study suggests that BMI may be considered a prognostic biomarker for advanced renal cell carcinoma.122
As regards regorafenib, a multi-kinase inhibitor administered to patients with several solid tumors, covariate analysis identified sex and BMI as impacting exposure to regorafenib; however, the changes observed in PK were rather limited and neither single nor combined covariates predicted exposures that would warrant a priori regorafenib dose adjustment.123
Finally, a case report found that in a patient with severe obesity, plasma levels of sunitinib were below clinical active level, and thus individual therapeutic drug monitoring is required for optimal guidance of treatment.124
Among the ADCs, a retrospective study included adult patients with breast cancer receiving T-DM1 and the primary endpoint was the incidence of T-DM1 treatment modifications secondary to an adverse event. Treatment modifications and delays due to toxicity were significantly more frequent in obese patients compared with non-obese subjects. Left ventricular ejection fraction decrease, bilirubin increase, thrombocytopenia and peripheral neuropathy were also significantly increased in the obese population compared with controls. This study suggests that obese patients receiving T-DM1 may require more accurate treatment monitoring for adverse events,125 although the data are not sufficiently robust to recommend interventions other than careful follow-up.
In conclusion, conflicting data are emerging on the onset of adverse events in overweight/obese patients treated with ICIs administered at standard doses, but there is a huge body of evidence suggesting the lack of a negative impact of high BMI on clinical outcome in this setting. By contrast, data on targeted molecules are much more heterogeneous, even within the same drug class, confirming the complexity of such a clinical condition and suggest the need for prospective clinical studies to uniquely define to what extent obesity can impact treatment choices, dosing and outcomes in cancer patients eligible for novel anticancer drugs.
Expert opinion on dosage of anticancer drug in overweight and obese patients
Ethical issues limit the carrying out of RCTs that compare full-weight-based versus adjusted dose of anticancer drugs in obese patients. These recommendations are thus based on observational studies and subgroup analyses of RCTs evaluating safety and efficacy profiles in heavy patients as compared with those of normal weight.
Question 1: is BSA the best approach for cytotoxic chemotherapy dosing?
BSA dosing is the most highly endorsed approach in clinical practice for chemotherapy drugs. The lack of excess toxicity in obese patients receiving BSA-based dose chemotherapy supports the reliability of this approach despite its acknowledged limitations. The BSA formula does not consider the patient's sex and body composition, leaving out the complexity of the cancer patient typified by increased fat mass associated with sarcopenia. Nevertheless, the actual tools of body composition analysis, such as anthropometry, are accurate. These alternative dosing methods are currently therefore limited to clinical studies.
Question 2: is a dose adjustment of cytotoxic chemotherapy required in obese patients?
To date, there has been no evidence that full-weight-based dosing of chemotherapeutic agents increases the toxicity profile for obese patients, while outstanding evidence indicates the role of DI on clinical outcome. The panel of experts therefore recommends avoiding empirical dose reduction of chemotherapy agents in the absence of other comorbidities associated with obesity. In patients receiving dose-dense regimens, careful clinical monitoring should be considered.
Question 3: is a dose adjustment of targeted therapy and ICIs required in obese patients?
Conflicting data on targeted molecules (even within the same class) do not at present permit univocal recommendations: in most cases, individual therapeutic drug monitoring is required for optimal guidance of treatment. mAbs have a wide therapeutic window, while body size contributes little to exposure variability. The high body weight increases the ICIs clearance without a clinically relevant effect. Therefore, no dosing variations are recommended for overweight or obese patients eligible for ICIs.
The unique properties of ADCs suggest the need for careful monitoring of obese patients undergoing treatment with these agents, but more specific recommendations are at present unattainable.
Question 4: what is the best schedule for dosing mAbs in obese patients?
To date, most approved mAbs are dosed at body-size-based schedules (milligram per kg or BSA-based), while only selected drugs are approved for flat-fixed dosing use. The molecules with a meaningful effect of body weight on Vd and Cl, have less interpatient variability using fixed-dose method than is the case with body-size-based dosing. Nevertheless, the risk of reduced exposure of anti-PD-1 and anti-PD-L1 cannot be ruled out for heavier patients. Therefore, PK/PD and dose-response clinical analyses are needed to support the wider use of fixed dose of mAbs thus reducing medication errors and health care costs.
Acknowledgments
Funding
None declared.
Disclosure
NS has served as consultant for Celgene and Isheo. GB has served as consultant for Roche, Servier, Celgene, Ipsen, Sanofi, Merck Serono. All other authors have declared no conflicts of interest.
References
- 1.Arnold M., Leitzmann M., Freisling H. Obesity and cancer: an update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Pandeya N., Byrnes G. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K. Body fatness and cancer – viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H., Siegel R.L., Torre L.A. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69:88–112. doi: 10.3322/caac.21499. [DOI] [PubMed] [Google Scholar]
- 5.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock G., Lewington S., Sherliker P. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griggs J.J., Sorbero M.E., Lyman G.H. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 8.Griggs J.J., Mangu P.B., Anderson H. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 9.Bouleftour W., Mery B., Chanal E. Obesity and chemotherapy administration: between empiric and mathematic method review. Acta Oncol. 2019;58:880–887. doi: 10.1080/0284186X.2019.1585942. [DOI] [PubMed] [Google Scholar]
- 10.Smorenburg C.H., Sparreboom A., Bontenbal M., Stoter G., Nooter K., Verweij J. Randomized cross-over evaluation of body-surface area-based dosing versus flat-fixed dosing of paclitaxel. J Clin Oncol. 2003;21:197–202. doi: 10.1200/JCO.2003.01.058. [DOI] [PubMed] [Google Scholar]
- 11.Chatelut E., White-Koning M.L., Mathijssen R.H., Puisset F., Baker S.D., Sparreboom A. Dose banding as an alternative to body surface area-based dosing of chemotherapeutic agents. Br J Cancer. 2012;107:1100–1106. doi: 10.1038/bjc.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das S.K. Body composition measurement in severe obesity. Curr Opin Clin Nutr Metab Care. 2005;8:602–606. doi: 10.1097/01.mco.0000171122.60665.5f. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency Reflection paper on investigation of pharmacokinetics and pharmacodynamics in the obese population. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-investigation-pharmacokinetics-pharmacodynamics-obese-population_en.pdf Available at. Accessed March 31, 2021.
- 14.Hall R.G., II, Jean G.W., Sigler M., Shah S. Dosing considerations for obese patients receiving cancer chemotherapeutic agents. Ann Pharmacother. 2013;47:1666–1674. doi: 10.1177/1060028013509789. [DOI] [PubMed] [Google Scholar]
- 15.Slawinski C.G.V., Barriuso J., Guo H., Renehan A.G. Obesity and cancer treatment outcomes: interpreting the complex evidence. Clin Oncol (R Coll Radiol) 2020;32:591–608. doi: 10.1016/j.clon.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Smit C., De Hoogd S., Bruggemann R.J.M., Knibbe C.A.J. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14:275–285. doi: 10.1080/17425255.2018.1440287. [DOI] [PubMed] [Google Scholar]
- 17.WHO Obesity. https://www.who.int/topics/obesity/en/ Available at. Accessed March 31, 2021.
- 18.Williams E.P., Mesidor M., Winters K., Dubbert P.M., Wyatt S.B. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. 2015;4:363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 19.Apovian C.M. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:s176–s185. [PubMed] [Google Scholar]
- 20.Lyman G.H., Sparreboom A. Chemotherapy dosing in overweight and obese patients with cancer. Nat Rev Clin Oncol. 2013;10:451–459. doi: 10.1038/nrclinonc.2013.108. [DOI] [PubMed] [Google Scholar]
- 21.Mosteller R.D. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 22.Pai M.P. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy. 2012;32:856–868. doi: 10.1002/j.1875-9114.2012.01108.x. [DOI] [PubMed] [Google Scholar]
- 23.Janmahasatian S., Duffull S.B., Ash S., Ward L.C., Byrne N.M., Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 24.Morrish G.A., Pai M.P., Green B. The effects of obesity on drug pharmacokinetics in humans. Expert Opin Drug Metab Toxicol. 2011;7:697–706. doi: 10.1517/17425255.2011.570331. [DOI] [PubMed] [Google Scholar]
- 25.Pai M.P., Paloucek F.P. The origin of the ‘ideal’ body weight equations. Ann Pharmacother. 2000;34:1066–1069. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 26.Cho S., Yoon I., Kim D. Obesity-related physiological changes and their pharmacokinetic consequences. J Pharm Invest. 2013;43:161–169. [Google Scholar]
- 27.Frayn K.N. Obesity and metabolic disease: is adipose tissue the culprit? Proc Nutr Soc. 2005;64:7–13. doi: 10.1079/pns2004403. [DOI] [PubMed] [Google Scholar]
- 28.Frayn K.N., Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond) 2014;38:1019–1026. doi: 10.1038/ijo.2013.200. [DOI] [PubMed] [Google Scholar]
- 29.Hanley M.J., Abernethy D.R., Greenblatt D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Oie S. Drug distribution and binding. J Clin Pharmacol. 1986;26:583–586. doi: 10.1002/j.1552-4604.1986.tb02953.x. [DOI] [PubMed] [Google Scholar]
- 31.Zuckerman M., Greller H.A., Babu K.M. A review of the toxicologic implications of obesity. J Med Toxicol. 2015;11:342–354. doi: 10.1007/s13181-015-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blouin R.A., Warren G.W. Pharmacokinetic considerations in obesity. J Pharm Sci. 1999;88:1–7. doi: 10.1021/js980173a. [DOI] [PubMed] [Google Scholar]
- 33.Longo M., Zatterale F., Naderi J. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20:2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith S.A., Waters N.J. Pharmacokinetic and pharmacodynamic considerations for drugs binding to alpha-1-acid glycoprotein. Pharm Res. 2018;36:30. doi: 10.1007/s11095-018-2551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summers L.K., Samra J.S., Humphreys S.M., Morris R.J., Frayn K.N. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci (Lond) 1996;91:679–683. doi: 10.1042/cs0910679. [DOI] [PubMed] [Google Scholar]
- 36.Hodson L. Adipose tissue oxygenation: effects on metabolic function. Adipocyte. 2014;3:75–80. doi: 10.4161/adip.27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ijaz S., Yang W., Winslet M.C., Seifalian A.M. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003;10:447–456. doi: 10.1038/sj.mn.7800206. [DOI] [PubMed] [Google Scholar]
- 38.Jamwal R., de la Monte S.M., Ogasawara K., Adusumalli S., Barlock B.B., Akhlaghi F. Nonalcoholic fatty liver disease and diabetes are associated with decreased CYP3A4 protein expression and activity in human liver. Mol Pharm. 2018;15:2621–2632. doi: 10.1021/acs.molpharmaceut.8b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan R., Wang J., Du J. Association between body mass index and fatty liver risk: a dose-response analysis. Sci Rep. 2018;8:15273. doi: 10.1038/s41598-018-33419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng P.Y., Morgan E.T. Hepatic cytochrome P450 regulation in disease states. Curr Drug Metab. 2001;2:165–183. doi: 10.2174/1389200013338676. [DOI] [PubMed] [Google Scholar]
- 41.Morgan E.T. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther. 2009;85:434–438. doi: 10.1038/clpt.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chagnac A., Herman M., Zingerman B. Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant. 2008;23:3946–3952. doi: 10.1093/ndt/gfn379. [DOI] [PubMed] [Google Scholar]
- 43.Ho R.H., Kim R.B. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78:260–277. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 44.de Hoogd S., Valitalo P.A.J., Dahan A. Influence of morbid obesity on the pharmacokinetics of morphine, morphine-3-glucuronide, and morphine-6-glucuronide. Clin Pharmacokinet. 2017;56:1577–1587. doi: 10.1007/s40262-017-0544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mir O., Broutin S., Desnoyer A., Delahousse J., Chaput N., Paci A. Pharmacokinetics/pharmacodynamic (PK/PD) relationship of therapeutic monoclonal antibodies used in oncology: what's new? Eur J Cancer. 2020;128:103–106. doi: 10.1016/j.ejca.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Paci A., Desnoyer A., Delahousse J. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 1, monoclonal antibodies, antibody-drug conjugates and bispecific T-cell engagers. Eur J Cancer. 2020;128:107–118. doi: 10.1016/j.ejca.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Malik P., Phipps C., Edginton A., Blay J. Pharmacokinetic considerations for antibody-drug conjugates against cancer. Pharm Res. 2017;34:2579–2595. doi: 10.1007/s11095-017-2259-3. [DOI] [PubMed] [Google Scholar]
- 48.Mathijssen R.H., Sparreboom A., Verweij J. Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol. 2014;11:272–281. doi: 10.1038/nrclinonc.2014.40. [DOI] [PubMed] [Google Scholar]
- 49.Lyman G.H. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7:99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 50.Reinisch M., Ataseven B., Kummel S. Neoadjuvant dose-dense and dose-intensified chemotherapy in breast cancer – review of the literature. Breast Care (Basel) 2016;11:13–20. doi: 10.1159/000444543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Early Breast Cancer Trialists' Collaborative Group Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393:1440–1452. doi: 10.1016/S0140-6736(18)33137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norton L., Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep. 1977;61:1307–1317. [PubMed] [Google Scholar]
- 53.Frei E., III, Canellos G.P. Dose: a critical factor in cancer chemotherapy. Am J Med. 1980;69:585–594. doi: 10.1016/0002-9343(80)90472-6. [DOI] [PubMed] [Google Scholar]
- 54.Bonadonna G., Valagussa P., Moliterni A., Zambetti M., Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 55.Bonneterre J., Roche H., Kerbrat P. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2005;23:2686–2693. doi: 10.1200/JCO.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 56.Budman D.R., Berry D.A., Cirrincione C.T. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90:1205–1211. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 57.Crawford J., Denduluri N., Patt D. Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer. Support Care Cancer. 2020;28:925–932. doi: 10.1007/s00520-019-04875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denduluri N., Lyman G.H., Wang Y. Chemotherapy dose intensity and overall survival among patients with advanced breast or ovarian cancer. Clin Breast Cancer. 2018;18:380–386. doi: 10.1016/j.clbc.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Hanna R.K., Poniewierski M.S., Laskey R.A. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2013;129:74–80. doi: 10.1016/j.ygyno.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 60.Loibl S., Skacel T., Nekljudova V. Evaluating the impact of relative total dose intensity (RTDI) on patients' short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer – a pooled analysis. BMC Cancer. 2011;11:131. doi: 10.1186/1471-2407-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luciani A., Bertuzzi C., Ascione G. Dose intensity correlate with survival in elderly patients treated with chemotherapy for advanced non-small cell lung cancer. Lung Cancer. 2009;66:94–96. doi: 10.1016/j.lungcan.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 62.Lyman G.H., Dale D.C., Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Rosner G.L., Hargis J.B., Hollis D.R. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and Leukemia Group B study 8541. J Clin Oncol. 1996;14:3000–3008. doi: 10.1200/JCO.1996.14.11.3000. [DOI] [PubMed] [Google Scholar]
- 64.Stocker G., Hacker U.T., Fiteni F. Clinical consequences of chemotherapy dose reduction in obese patients with stage III colon cancer: a retrospective analysis from the PETACC 3 study. Eur J Cancer. 2018;99:49–57. doi: 10.1016/j.ejca.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Carroll J.P., Protani M.M., Nguyen L. Toxicity and tolerability of adjuvant breast cancer chemotherapy in obese women. Med Oncol. 2014;31:881. doi: 10.1007/s12032-014-0881-z. [DOI] [PubMed] [Google Scholar]
- 66.Chambers P., Daniels S.H., Thompson L.C., Stephens R.J. Chemotherapy dose reductions in obese patients with colorectal cancer. Ann Oncol. 2012;23:748–753. doi: 10.1093/annonc/mdr277. [DOI] [PubMed] [Google Scholar]
- 67.Colleoni M., Li S., Gelber R.D. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet. 2005;366:1108–1110. doi: 10.1016/S0140-6736(05)67110-3. [DOI] [PubMed] [Google Scholar]
- 68.Georgiadis M.S., Steinberg S.M., Hankins L.A., Ihde D.C., Johnson B.E. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Natl Cancer Inst. 1995;87:361–366. doi: 10.1093/jnci/87.5.361. [DOI] [PubMed] [Google Scholar]
- 69.Hansen J., Stephan J.M., Freesmeier M., Bender D., Button A., Goodheart M.J. The effect of weight-based chemotherapy dosing in a cohort of gynecologic oncology patients. Gynecol Oncol. 2015;138:154–158. doi: 10.1016/j.ygyno.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 70.Jenkins P., Elyan S., Freeman S. Obesity is not associated with increased myelosuppression in patients receiving chemotherapy for breast cancer. Eur J Cancer. 2007;43:544–548. doi: 10.1016/j.ejca.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 71.Lote H., Sharp A., Redana S., Papadimitraki E., Capelan M., Ring A. Febrile neutropenia rates according to body mass index and dose capping in women receiving chemotherapy for early breast cancer. Clin Oncol (R Coll Radiol) 2016;28:597–603. doi: 10.1016/j.clon.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Lyman G.H., Dale D.C., Friedberg J., Crawford J., Fisher R.I. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: a nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 73.Poikonen P., Blomqvist C., Joensuu H. Effect of obesity on the leukocyte nadir in women treated with adjuvant cyclophosphamide, methotrexate, and fluorouracil dosed according to body surface area. Acta Oncol. 2001;40:67–71. doi: 10.1080/028418601750071082. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz J., Toste B., Dizon D.S. Chemotherapy toxicity in gynecologic cancer patients with a body surface area (BSA) > 2 m2. Gynecol Oncol. 2009;114:53–56. doi: 10.1016/j.ygyno.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Meyerhardt J.A., Catalano P.J., Haller D.G. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 76.Meyerhardt J.A., Tepper J.E., Niedzwiecki D. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 77.Wright J.D., Tian C., Mutch D.G. Carboplatin dosing in obese women with ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;109:353–358. doi: 10.1016/j.ygyno.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 78.Furlanetto J., Eiermann W., Marme F. Higher rate of severe toxicities in obese patients receiving dose-dense (dd) chemotherapy according to unadjusted body surface area: results of the prospectively randomized GAIN study. Ann Oncol. 2016;27:2053–2059. doi: 10.1093/annonc/mdw315. [DOI] [PubMed] [Google Scholar]
- 79.Hourdequin K.C., Schpero W.L., McKenna D.R., Piazik B.L., Larson R.J. Toxic effect of chemotherapy dosing using actual body weight in obese versus normal-weight patients: a systematic review and meta-analysis. Ann Oncol. 2013;24:2952–2962. doi: 10.1093/annonc/mdt294. [DOI] [PubMed] [Google Scholar]
- 80.Jones J.A., Fayad L.E., Elting L.S., Rodriguez M.A. Body mass index and outcomes in patients receiving chemotherapy for intermediate-grade B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51:1649–1657. doi: 10.3109/10428194.2010.494315. [DOI] [PubMed] [Google Scholar]
- 81.Meloni G., Proia A., Capria S. Obesity and autologous stem cell transplantation in acute myeloid leukemia. Bone Marrow Transplant. 2001;28:365–367. doi: 10.1038/sj.bmt.1703145. [DOI] [PubMed] [Google Scholar]
- 82.Laviano A., Rianda S., Rossi Fanelli F. Sarcopenia and chemotherapy dosing in obese patients. Nat Rev Clin Oncol. 2013;10:664. doi: 10.1038/nrclinonc.2013.108-c1. [DOI] [PubMed] [Google Scholar]
- 83.Cespedes Feliciano E.M., Chen W.Y., Lee V. Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol. 2020;6:264–270. doi: 10.1001/jamaoncol.2019.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shachar S.S., Deal A.M., Weinberg M. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res. 2017;23:3537–3543. doi: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prado C.M., Lima I.S., Baracos V.E. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67:93–101. doi: 10.1007/s00280-010-1288-y. [DOI] [PubMed] [Google Scholar]
- 86.Wong A.L., Seng K.Y., Ong E.M. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat. 2014;144:143–152. doi: 10.1007/s10549-014-2843-8. [DOI] [PubMed] [Google Scholar]
- 87.Sparreboom A., Wolff A.C., Mathijssen R.H. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol. 2007;25:4707–4713. doi: 10.1200/JCO.2007.11.2938. [DOI] [PubMed] [Google Scholar]
- 88.Hendrikx J., Haanen J., Voest E.E., Schellens J.H.M., Huitema A.D.R., Beijnen J.H. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22:1212–1221. doi: 10.1634/theoncologist.2017-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Long G.V., Tykodi S.S., Schneider J.G. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol. 2018;29:2208–2213. doi: 10.1093/annonc/mdy408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao X., Shen J., Ivaturi V. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann Oncol. 2020;31:302–309. doi: 10.1016/j.annonc.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 91.Pestine E., Stokes A., Trinquart L. Representation of obese participants in obesity-related cancer randomized trials. Ann Oncol. 2018;29:1582–1587. doi: 10.1093/annonc/mdy138. [DOI] [PubMed] [Google Scholar]
- 92.Feng Y., Masson E., Dai D., Parker S.M., Berman D., Roy A. Model-based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br J Clin Pharmacol. 2014;78:106–117. doi: 10.1111/bcp.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajaj G., Wang X., Agrawal S., Gupta M., Roy A., Feng Y. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6:58–66. doi: 10.1002/psp4.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Centanni M., Moes D., Troconiz I.F., Ciccolini J., van Hasselt J.G.C. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58:835–857. doi: 10.1007/s40262-019-00748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilkins J.J., Brockhaus B., Dai H. Time-varying clearance and impact of disease state on the pharmacokinetics of avelumab in Merkel cell carcinoma and urothelial carcinoma. CPT Pharmacometrics Syst Pharmacol. 2019;8:415–427. doi: 10.1002/psp4.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Desnoyer A., Broutin S., Delahousse J. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 2, immune checkpoint inhibitor antibodies. Eur J Cancer. 2020;128:119–128. doi: 10.1016/j.ejca.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Freshwater T., Kondic A., Ahamadi M. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi: 10.1186/s40425-017-0242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao X., Suryawanshi S., Hruska M. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28:2002–2008. doi: 10.1093/annonc/mdx235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gallo M., Adinolfi V., Barucca V. Expected and paradoxical effects of obesity on cancer treatment response. Rev Endocr Metab Disord. 2020:1–22. doi: 10.1007/s11154-020-09597-y. [DOI] [PubMed] [Google Scholar]
- 100.Woodall M.J., Neumann S., Campbell K., Pattison S.T., Young S.L. The effects of obesity on anti-cancer immunity and cancer immunotherapy. Cancers (Basel) 2020;12:1230. doi: 10.3390/cancers12051230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Izquierdo A., Crujeiras A., Casanueva F., Carreira M. Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients. 2019;11:2704. doi: 10.3390/nu11112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z., Aguilar E.G., Luna J.I. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McQuade J.L., Daniel C.R., Hess K.R. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naik G.S., Waikar S.S., Johnson A.E.W. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer. 2019;7:89. doi: 10.1186/s40425-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richtig G., Hoeller C., Wolf M. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study. PLoS One. 2018;13:e0204729. doi: 10.1371/journal.pone.0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cortellini A., Bersanelli M., Buti S. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.An Y., Wu Z., Wang N. Association between body mass index and survival outcomes for cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Transl Med. 2020;18:235. doi: 10.1186/s12967-020-02404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu H., Cao D., He A., Ge W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: a pooled analysis of 4090 cancer patients. Int Immunopharmacol. 2019;74:105745. doi: 10.1016/j.intimp.2019.105745. [DOI] [PubMed] [Google Scholar]
- 109.Lysaght J. The ‘obesity paradox’ in action with cancer immunotherapy. Nat Rev Endocrinol. 2019;15:132–133. doi: 10.1038/s41574-019-0161-2. [DOI] [PubMed] [Google Scholar]
- 110.Sanchez A., Furberg H., Kuo F. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2019;21:283–293. doi: 10.1016/S1470-2045(19)30797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miyamoto Y., Oki E., Emi Y. Low visceral fat content is a negative predictive marker for bevacizumab in metastatic colorectal cancer. Anticancer Res. 2018;38:491–499. doi: 10.21873/anticanres.12249. [DOI] [PubMed] [Google Scholar]
- 112.Pizzuti L., Sergi D., Sperduti I. Body mass index in HER2-negative metastatic breast cancer treated with first-line paclitaxel and bevacizumab. Cancer Biol Ther. 2018;19:328–334. doi: 10.1080/15384047.2017.1416938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Artac M., Korkmaz L., Coskun H.S. Bevacuzimab may be less effective in obese metastatic colorectal cancer patients. J Gastrointest Cancer. 2019;50:214–220. doi: 10.1007/s12029-017-0047-2. [DOI] [PubMed] [Google Scholar]
- 114.Patel G.S., Ullah S., Beeke C. Association of BMI with overall survival in patients with mCRC who received chemotherapy versus EGFR and VEGF-targeted therapies. Cancer Med. 2015;4:1461–1471. doi: 10.1002/cam4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gonzalez Garcia J., Gutierrez Nicolas F., Ramos Diaz R. Pharmacokinetics of trastuzumab after subcutaneous and intravenous administration in obese patients. Ann Pharmacother. 2020;54:775–779. doi: 10.1177/1060028020902318. [DOI] [PubMed] [Google Scholar]
- 116.Albiges L., Hakimi A.A., Xie W. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol. 2016;34:3655–3663. doi: 10.1200/JCO.2016.66.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bergerot P., Bergerot C., Philip E. Targeted therapy and immunotherapy: effect of body mass index on clinical outcomes in patients diagnosed with metastatic renal cell carcinoma. Kidney Cancer. 2019;3:63–70. [Google Scholar]
- 118.Imai H., Kuwako T., Kaira K. Evaluation of gefitinib efficacy according to body mass index, body surface area, and body weight in patients with EGFR-mutated advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;79:497–505. doi: 10.1007/s00280-016-3232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oda N., Hotta K., Yoshioka H. Potential influence of being overweight on the development of hepatic dysfunction in Japanese patients with EGFR-mutated non-small cell lung cancer undergoing gefitinib monotherapy: the Okayama Lung Cancer Study Group experience. Cancer Chemother Pharmacol. 2016;78:941–947. doi: 10.1007/s00280-016-3146-z. [DOI] [PubMed] [Google Scholar]
- 120.Molica M., Canichella M., Colafigli G. Body mass index does not impact on molecular response rate of chronic myeloid leukaemia patients treated frontline with second generation tyrosine kinase inhibitors. Br J Haematol. 2018;182:427–429. doi: 10.1111/bjh.14783. [DOI] [PubMed] [Google Scholar]
- 121.Pavlovsky C., Egorin M.J., Shah D.D., Beumer J.H., Rogel S., Pavlovsky S. Imatinib mesylate pharmacokinetics before and after sleeve gastrectomy in a morbidly obese patient with chronic myeloid leukemia. Pharmacotherapy. 2009;29:1152–1156. doi: 10.1592/phco.29.9.1152. [DOI] [PubMed] [Google Scholar]
- 122.Santoni M., Massari F., Bracarda S. Body mass index in patients treated with Cabozantinib for advanced renal cell carcinoma: a new prognostic factor? Diagnostics (Basel) 2021;11:138. doi: 10.3390/diagnostics11010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keunecke A., Hoefman S., Drenth H.J., Zisowsky J., Cleton A., Ploeger B.A. Population pharmacokinetics of regorafenib in solid tumours: exposure in clinical practice considering enterohepatic circulation and food intake. Br J Clin Pharmacol. 2020;86:2362–2376. doi: 10.1111/bcp.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Desar I.M., Burger D.M., Van Hoesel Q.G., Beijnen J.H., Van Herpen C.M., Van der Graaf W.T. Pharmacokinetics of sunitinib in an obese patient with a GIST. Ann Oncol. 2009;20:599–600. doi: 10.1093/annonc/mdn779. [DOI] [PubMed] [Google Scholar]
- 125.Lee A., Larck C., Moore D.C. Impact of obesity on safety outcomes and treatment modifications with ado-trastuzumab emtansine in breast cancer patients. J Oncol Pharm Pract. 2020 doi: 10.1177/1078155220982648. 1078155220982648. [DOI] [PubMed] [Google Scholar]