Abstract
Mitochondrial dysfunction contributes to the pathogenesis and prognosis of many common disorders, including neurodegeneration, stroke, myocardial infarction, tumor, and metabolic diseases. Ginsenosides, the major bioactive constituents of Panax ginseng (P. ginseng), have been reported to play beneficial roles in the molecular pathophysiology of these diseases by targeting mitochondrial dysfunction. In this review, we first introduce the types of ginsenosides and basic mitochondrial functions. Then, recent findings are summarized on different ginsenosides targeting mitochondria and their key signaling pathways for the treatment of multiple diseases, including neurological disorders, cancer, heart disease, hyperglycemia, and inflammation are summarized. This review may explain the common targets of ginsenosides against multiple diseases and provide new insights into the underlying mechanisms, facilitating research on the clinical application of P. ginseng.
Keywords: ginsenosides, mitochondria, neurological disorder, cancer, heart disease
Graphical abstract
1. Introduction
Panax ginseng (P. ginseng) has tonifying effects on important organs, maintaining their function and strengthens vitality and body resistance to inhibit physiological aging and withstand pathological stresses [1]. P. ginseng contains various pharmacological ingredients, such as ginsenosides, polysaccharides, polyphenols, and polyacetylenes [2]. Among them, ginsenosides are recognized as the major bioactive ingredients. The beneficial effects of ginsenosides have been extensively investigated, and various bioactivities have been identified, such as neuroprotection, cardioprotection, anti-tumor, anti-diabetes, and anti-inflammation and so on [3]. However, it remains unclear whether the beneficial effects of ginsenosides on various diseases are mediated by a common functional mechanism.
The mitochondria, a cytoplasmic double-membrane organelle, plays a crucial role in cell physiological processes, including energy homeostasis, autophagy, oxidative stress balance, and apoptotic signaling cascades [4]. Mitochondrial homeostasis dysfunction or disruption also contributes to the pathogenesis of many disorders, including neurodegeneration, stroke, myocardial infarction, cancer, and metabolic diseases [5]. Importantly, a large number of results from in vitro and in vivo studies have confirmed the beneficial effects of ginsenosides for preventing and treating various diseases are related to their interference with mitochondrial dysfunction [[6], [7], [8]].
To better understand how ginsenosides regulate mitochondrial function and its related signaling pathways to elicit their multiple pharmacological effects in mammalian cells, we summarize recent findings with respect to ginsenosides targeting mitochondrial function in multiple disorders. First, we briefly introduce ginsenosides and the biological functions of mitochondria. Then, the roles of different ginsenosides in regulating mitochondrial biogenesis, bioenergetics, autophagy, motility, and apoptosis for the treatment of different diseases are summarized. For example, different ginsenosides have been reported to prevent acute and chronic diseases by influencing the opening of mitochondrial permeability transition pores (mPTPs), balancing reactive oxygen species (ROS), modulating mitochondrial fission and fusion, or mediating mitophagy [9]. A thorough understanding of the effects of ginsenosides on mitochondrial function and its key signaling pathways is necessary to explain how the main components of P. ginseng treat multiple diseases and to comprehensively explore the molecular mechanisms by which of P. ginseng treats multiple diseases.
2. Ginsenosides
Ginsenosides, a type of steroidal saponins, have a dammarane-type triterpenoid structure and a four-ring hydrophobic steroid-like structure conjugated with sugar moieties, which are divided into three different structural classes: protopanaxadiol (PPD-type), protopanaxatriol (PPT-type), and oleanolic acid (OA-type) groups [10]. PPD-type ginsenosides, including ginsenosides Rb1 (Rb1), ginsenosides Rb2 (Rb2), ginsenosides Rb3 (Rb3), ginsenosides Rc (Rc), ginsenosides Rd (Rd), ginsenosides Rg3 (Rg3), and ginsenosides Rh2 (Rh2), have conjugated sugar moieties attached to the 3-position of the triterpenoid dammarane structure, whereas PPT-type ginsenosides, including ginsenosides Re (Re), ginsenosides Rf (Rf), ginsenosides Rg1 (Rg1), and ginsenosides Rh1 (Rh1), have sugar moieties attached to the 6-position of the triterpenoid dammarane structure [11]. Quantitative and qualitative analysis for ginsenosides in vivo revealed that PPD-type ginsenosides exhibit higher plasma concentrations and longer half-lives than PPT-type ginsenosides [12]. Meanwhile, the absorption rate of intact ginsenosides in the intestines is as low as 1% to 3.7%, and most of them are metabolized and converted into other ginsenosides through acid hydrolysis in the stomach and bacterial hydrolysis in the intestine [12]. To date, more than 200 different natural and transformed ginsenosides and their metabolites have been identified and studied in a variety of pathological conditions [8,9].
3. Mitochondrial dysfunction
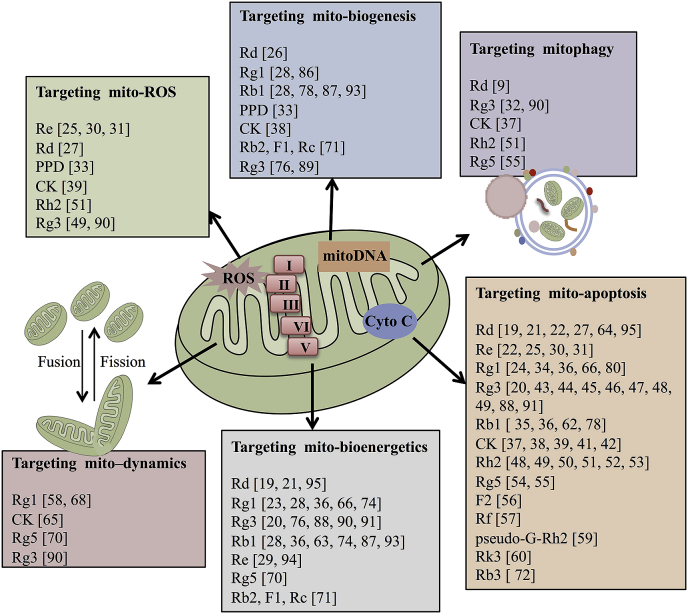
Mitochondria are responsible for the physiological and pathological processes of aerobic respiration-based cells in mammalian, including oxidative phosphorylation (OXPHOS), the biosynthesis of intermediates for cell growth, and the metabolism of three major nutrients (glucose, amino acids, and fatty acids), and also mediate the essential processes that determine cell function and status [13]. Mitochondrial dysfunction is found in many diseases, including neurodegeneration, cancer, metabolic diseases, heart failure, and ischemia-reperfusion (I/R) injury, which have been reported in some reviews [14]. Numerous studies have shown that ginsenosides can regulate mitochondrial (mito)-ROS, mito-apoptosis, mito-bioenergetics, mito-biogenesis, mito-dynamics, and mitophagy to exert pharmacological effects, which have been summarized in Fig. 1.
Fig. 1.
Summary of different ginsenoside monomers targeting mito-ROS, mito-apoptosis, mito-bioenergetics, mito-biogenesis, mito-dynamics, and mitophagy.
4. Ginsenosides target mitochondria, treating different diseases
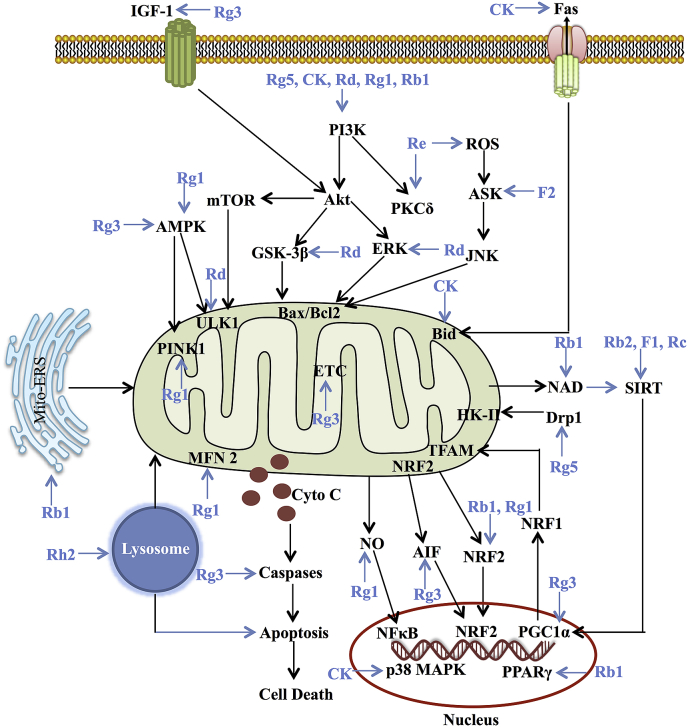
Some studies have demonstrated that ginsenosides act as protective agents of mitochondrial function, mainly by direct or indirect enhancement of mitochondrial biosynthesis, mitophagy, or electron transport chain (ETC) efficiency or inhibition of mito-ROS [8,15]. Conversely, ginsenosides also can treat cancer and other diseases by promoting mito-apoptosis and mitophagy [9,16]. In the models of different diseases, including nervous system disorders, cardiovascular system disorders, and tumors, different ginsenoside monomers cause mitochondrial function alterations by regulating transcription factors, the expression of mitochondria-related genes, and mitochondrial dysfunction pathway networks, which are discussed here (Fig. 2).
Fig. 2.
Summary for the functional effects of ginsenoside monomers on mitochondria via multiple links across regulatory mechanisms and multi-target effects.
4.1. Neurological disorders
In the resting state of the human body, the brain consumes 20% of body energy, although it only has 2% of the total body mass [17]. Mitochondrial function can directly influence neuronal function, such as synaptic plasticity, axonal transport, and the release of neurotransmitters, and abnormal mitochondrial function in neurons precedes neurological changes and neuronal loss [18]. Multiple neurological diseases have been associated with mitochondrial dysfunction, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and ischemic stroke. Ginsenoside monomers can target different functions of mitochondria to preventing and treating neurological disorders [[19], [20], [21]].
4.1.1. Parkinson’s disease
Mitochondrial dysfunction plays key roles in PD initiation and progression. In 1-methyl-4-phenylpyridinium-induced human neuroblastoma SH-SY5Y cells and Parkinsonian toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse PD models, Rd shows a significant neuroprotective effect by enhancing antioxidant enzymatic activities, preserving the activity of ETC complex I, stabilizing the mitochondrial membrane permeabilization (MMP), and increasing intracellular ATP levels [19]. Additionally, the PI3K/Akt signaling pathway is involved in the protective effect of Rd against PD. In a rotenone-induced PD neuronal model with mitochondrial dysfunction, the combination of Rd with Re attenuates the extent of depolarization of MMP and restores calcium levels by modulating the Bcl2/Bax ratio and inhibiting the release of Cyto C and caspase-3 activation [22]. These findings highlight the efficacies of Rd and Re in the prevention and treatment of PD through improving mitochondrial integrity and ETC efficiency, and inhibiting mito-apoptosis.
4.1.2. Alzheimer’s disease
In Aβ peptide-treated neuroblastoma cells, proteomic analysis shows that Rg1 can alter the expression of 49 mitochondrial proteins, such as 3-hydroxyacyl-CoA dehydrogenase type-2, alanine-tRNA ligase, mitochondrial import receptor subunit TOM40 homolog, and Cyto C oxidase subunit 5A [23]. Moreover, the pretreatment of primary cultured cortical neurons with Rg1 (20 μM) for 24 h and exposure to 10 μM Aβ for 72 h can elevate the Bcl2/Bax ratio, reduce Cyto C release from mitochondria into the cytoplasm, and then block mitochondrial apoptotic cascades after Aβ insult [24].
Metabolomic and apoptotic analysis for Rg3-administrated AD rats shows that Rg3 could prevent cognitive impairment by regulating the abnormality of energy metabolism, ETC efficiency, amino acid metabolism, and purine metabolism and regulating mito-apoptosis [20]. Re, another ginsenoside, inhibits the ROS/ASK-1-dependent mito-apoptotic pathway in Aβ-triggered SH-SY5Y cells through the inhibition of the apoptosis-related pathway (the elevation of the Bcl2/Bax ratio and the reduction of Cyto release) and ROS production [25]. These results indicate that multiple ginsenoside monomers promote energy metabolism and inhibit the mitochondrial apoptosis by increasing ETC function in AD.
4.1.3. Ischemic stroke
In middle cerebral artery occlusion (MCAO) rat models, Rd administration reduces mtDNA damage and the cleavage of caspase-3, improving the survival rate and neurological function 7 days after MCAO [26]. Rd also protects against mitochondrial damage after reperfusion by preserving ETC activity and aconitase activity, which decreasing mitochondrial hydrogen peroxide production and depolarizing MMP in the aged MCAO mice [21]. In addition, the results from in vitro and in vivo models of cerebral ischemia demonstrate that Rd markedly decreases the secretion of lactate and attenuates mitochondrial swelling by preserving MMP and reducing the mitochondrial release of Cyto C and ROS production to minimize mitochondria-mediated apoptosis following ischemia [27].
In an oxygen–glucose deprivation/reoxygenation-induced primary mouse astrocyte injury model, Rb1 and Rg1 co-treatment decreases the mtDNA copy number and the MMP depolarization, and increases the activities of ETC complexes I, II, III, and V, promoting the production of ATP [28]. The results above show that ginsenoside monomers reduce ischemic stroke damage by enhancing ETC activity and inhibiting mitochondrial apoptosis.
4.1.4. Neuronal injury
The neuroprotective effect of ginsenosides is also closely related to mitochondrial function. Re treatment significantly attenuates phencyclidine-induced mitochondrial oxidative stress and ETC dysfunction by regulating NADPH oxidase activity in mouse dorsolateral cortex neurons [29]. Re also protects methamphetamine-induced mitochondrial burdens (i.e., decrease of MMP, Cyto C release from mitochondria, and activation of caspase-3) via the inhibition of protein kinase C δ (PKCδ) in SH-SY5Y cells and in PKCδ knockout (+/−) mice [30,31]. These Re-mediated protective effects are comparable to those observed in PKCδ knockout (−/−) or knockdown mice [30].
Mitochondrial quality control is regulated by successive rounds of fusion, fission, and mitophagy with the dynamic exchange of necessary components. Moon et al. reported that Rg3 can induce autophagy flux, attenuating human prion protein-mediated neurotoxicity and mitochondrial dysfunction in neuronal cells [32]. Furthermore, PPD inhibits mitochondrial morphological changes, scavenges the mito-ROS, improves MMP, and enhances mitochondrial counts compared with the cells exposed to glutamate only [33]. In the neurotoxicity model of PC12 cells induced by colistin, Rg1 also exerts neuroprotective effects via multiple mito-apoptosis-related pathways, including the decreases of Cyto C release and caspase-3/9 cleavages [34]. In primary cultured rat hippocampal neurons, Rb1 inhibits the burst of intracellular ROS and the depolarization of MMP induced by high glucose [35]. In addition, Rb1 and Rg1 protect neuronal cells against MMP loss, mito-apoptosis, and aconitase inhibition induced by mitochondrial complex I inhibitor, rotenone, in an in vitro neuronal model of neurotoxicity, which may be mediated by NRF-2 activation [36].
Based on these studies, we found that in AD, PD, ischemic stroke, and various chemically induced neuronal injury models, ginsenoside monomers not only inhibit mito-apoptosis and mito-ROS, but also enhance mtDNA replication, NADPH oxidase, and mitophagy. The beneficial effects of different ginsenoside monomers on neurological disorders are summarized in Table 1.
Table 1.
Summary of Mitochondria-Related Neurological Protective Effects of Ginsenosides
| Ref. | Ginsenoside | Model | Inducer | Experimental model | Effects |
|---|---|---|---|---|---|
| [19] | Rd | PD | 1-Methyl-4-phenylpyridinium | SH-SY5Y cell | COXI, ATP, MMP |
| [20] | Rg3 | AD | Senescence | Rat | ATP, ETC |
| [21] | Rd | Ischemic stroke | MCAO | Aged mice | ETC, MMP |
| [22] | Rd, Re | PD | Rotenone | SH-SY5Y cell | MMP, Cyto C, Bax/Bcl2, Caspase-3 |
| [24] | Rg1 | AD | Aβ | Primary neuron | Bax/Bcl2, Cyto C |
| [25] | Re | AD | Aβ | SH-SY5Y cell | Cyto C, Bax/Bcl2 |
| [26] | Rd | Ischemic stroke | MACO | Rat | mtDNA |
| [28] | Rb1, Rg1 | Ischemic stroke | OGD/R | Primary astrocyte | mtDNA, MMP, COX I–V, ATP |
| [29] | Re | Neuronal injury | Phencyclidine | Mice | ETC, NADPH oxidase |
| [30] | Re | Neuronal injury | Methamphetamine | SH-SY5Y cell | Mito-ROS; Cyto C, PKCδ |
| [31] | Re | Neuronal injury | Methamphetamine | Mice | Mito-ROS, PKCδ |
| [32] | Rg3 | Neuronal injury | Human prion protein | Primary neuron | Mitophagy |
| [33] | PPD | Neuronal injury | Glutamate | PC12 cell | MMP, mtDNA, mito-ROS |
| [34] | Rg1 | Neuronal injury | Colistin sulfate | PC12 cell | Cyto C, Caspase-3, Caspase-9 |
| [35] | Rb1 | Neuronal injury | High glucose | Primary neuron | MMP, Bcl2 |
| [36] | Rb1, Rg1 | PD | Rotenone | SH-SY5Y cell | MMP, Cyto C, Bax/Bcl2, Caspase-3, NRF-2 |
4.2. Cancer
Many studies have shown that ginsenoside monomers can promote tumor cell death by activating mitochondrial apoptosis and autophagy (Table 2).
Table 2.
Summary of Mitochondria-Related Antitumor Effects of Ginsenosides
| Reference | Ginsenoside | Experimental model | Effects targeting mitochondia |
|---|---|---|---|
| [37] | CK | Neuroblastoma cells | MMP, mito-ROS |
| [38] | CK | Hepatocellular carcinoma cells (MHCC97-H) | mtDNA, MMP, Bax/Bcl2, Caspase-3/9, Cyto C |
| [39] | CK | Colon cancer cells (HT-29) | MMP, Bax/Bcl2, Caspase-3 |
| [42] | CK | Bladder cancer cells (T24) | Cyto C, Caspase-3/9, Bax/Bcl2 |
| [43] | Rg3 | HeLa cells | MMP |
| [44] | Rg3 | Myeloma cells (U266, RPMI8226, SKO-007) | MMP, Cyto C, Bax/Bcl2, Caspase-3 |
| [45] | Rg3 | Breast cancer cells (MDA-MB-231) | MMP, Cyto C, Bax/Bcl2, Caspase-3 |
| [46] | Rg3 | Colon cancer cells (HT-29) | Cyto C, Bax/Bcl2, Caspase-3/9 |
| [47] | Rg3 | Gallbladder cancer cells | Mitochondrial-mediated intrinsic caspase pathway |
| [48] | Rg3, Rh2 | Hepatocellular carcinoma cells (Hep3B) | MMP, Cyto C, Bax/Bcl2, Caspase-3 |
| [49] | Rh2, Rg3 | Leukemia cells (Jurkat) | Mito-ROS, mitophagy, MMP, mito-apoptosis |
| [50,52] | Rh2 | Leukemia cells (lymphoblastic, Reh) | Mito-apoptosis, MMP, Cyto C, Caspase-3/9 |
| [51] | Rh2 | Hepatoma cells (HepG2) | mitochondrial apoptosis |
| [53] | Rh2 | Breast cancer cells (MCF-7, MDA-MB-231) | Bcl2, Bcl-xL, Bak, Bax, Bim |
| [54] | Rg5 | Human esophageal cancer cells (Eca109) | MMP |
| [55] | Rg5 | Breast cancer cells | PI3K-mediated mitochondria apoptosis |
| [56] | F2 | Gastric carcinoma cells | MMP, Cyto C |
| [57] | Rf | Osteosarcoma cells (MG-63) | MMP, Cyto C, Bax/Bcl2, Caspase-3/9 |
| [58] | Rg1 | Leukemia cells (K562) | Mitochondria morphology |
| [59] | pseudo-Rh2 | Gastric cancer cells (SGC-7901) | MMP, Cyto C, Caspase-3/9, Bax/Bcl2 |
4.2.1. Compound K on tumors
Compound K (CK), a main intestinal bacterial metabolite of protopanaxadiol saponin, can inhibit various cancers by inducing mito-apoptosis, mito-ROS, and autophagy [37]. A recent study has shown that CK promotes autophagosome accumulation by inducing early-stage autophagy, and inhibits late-stage autophagy inducing mitochondria damage in in vitro and in vivo neuroblastoma models [37]. In human hepatocellular carcinoma MHCC97-H cells, CK treatment induces the reductions in MMP, mtDNA copy number, Bcl2/Bax ratio, and caspase-3/9 expression in an Akt phosphorylation-dependent manner [38]. Similarly, in human colon cancer HT-29 cells, CK induces a mito-apoptotic pathway by affecting Bax and Bcl2 expression, resulting in the depolarization of the MMP [39]. During cytotoxic apoptosis, the translocation of full-length Bid from cell nuclei to mitochondria directly causes the release of Cyto C from mitochondria, indicating that full-length Bid is necessary to activate mito-apoptotic pathways [40]. In subcutaneous tumor and liver metastasis tissues, immunohistochemical staining revealed that Bid expression is obviously decreased by CK treatment, suggesting that a Bid-mediated mitochondrial apoptotic pathway is induced by CK [41]. In addition, in bladder cancer T24 cells, Western blot analysis demonstrated that CK treatment enhances the release of Cyto C, activates procaspases-3 and procaspases-9, and increases the Bax/Bcl2 ratio by ROS generation and p38-MAPK activation [42].
4.2.2. Rg3 on tumors
Pretreatment with Rg3 decreases MMP and mito-apoptosis in HeLa cells by regulating autophagy [43]. In myeloma cells, Rg3 also induces apoptosis by altering the Bcl2/Bax ratio, caspase activation, and the release of Cyto C from the mitochondria into the cytoplasm through the IGF-1/Akt/mTOR pathway [44]. In human breast cancer, MDA-MB-231 cells, treatment with Rg3 (30 μM) increases the Bax/Bcl2 ratio, the depolarization of MMP, and the release of mitochondrial Cyto C and also induces the cleavage of caspase-3 and poly-(ADP-ribose) polymerase (PARP), which is attenuated by a caspase-3 inhibitor, Z-VAD-FMK, indicating that Rg3 induces breast cancer cell apoptosis via classical mitochondrial-dependent caspase activation [45]. Furthermore, in HT-29 colon cancer cells, Rg3 downregulates the expression of Bcl2 and upregulates the expression of the pro-apoptotic protein, p53 or Bax, thereby inducing the release of mitochondrial Cyto C and the cleavages of PARP, caspase-9, and caspase-3 through the AMPK signaling pathway [46]. Similarly, Rg3 exposure suppresses the survival of gallbladder cancer via the activation of the mitochondrial-mediated intrinsic apoptosis pathway in NOZ cells, GBC-SD cells, and nude mice [47]. In addition, in human hepatocellular carcinoma Hep3B cells, the combination of Rg3 and 20(S)–Rh2 decreases MMP, enhances the release of Cyto C, and activates the cleavage of caspase-3, which suggests that they may induce apoptosis by direct activation of the mitochondrial pathway [48]. Another study reported that Rg3 combined with Rh2 and 20(S)–Rh2 can induce mito-ROS and mito-apoptosis in human leukemia Jurkat cells [49].
4.2.3. 20(S)–Rh2 on tumors
Mitophagy inhibition could aggravate mito-ROS generation and mitochondrial function and accelerate mito-apoptosis. Xia et al. have shown that mitophagy plays a protective role in 20(S)–Rh2-induced apoptosis in human acute lymphoblastic leukemia (ALL) cell lines and primary ALL cells via mito-apoptosis and autophagy without cytotoxic effects on normal blood cells [50]. In liver cancer -HepG2 cells, 20(S)–Rh2 incubation induces apoptosis by increasing the accumulation of ROS and the activation of the lysosomal-mitochondrial apoptotic pathway [51]. 20(S)–Rh2 can also induce MMP of human leukemia Reh cells in the shift in JC-1 fluorescence from red to green and cause the release of mitochondrial Cyto C and activation of cleaved caspase-9 and caspase-3 [52]. In human breast cancer cell lines and in vivo xenograft models, 20(S)–Rh2-induced apoptosis is accompanied by the downregulation of anti-apoptotic proteins, Bcl2, Bcl-xL, and Mcl-1 and the induction of the pro-apoptotic members, Bak, Bax, and Bim, which leads to mito-apoptosis [53].
4.2.4. Rg5 on tumors
The exposure to various concentrations of ginsenosides Rg5 (Rg5) for 24 h in esophageal cancer Eca109 cells decreases MMP via the downregulation of the PI3K-Akt signaling pathway [54]. Moreover, Rg5 remarkably suppresses breast cancer cell proliferation through mito-apoptosis and autophagic cell death by targeting the PI3K signaling pathway [55].
4.2.5. Other ginsenosides on tumors
In addition to the abovementioned ginsenosides, other ginsenosides can also target mitochondria to inhibit tumorigenesis and development. In humor gastric carcinoma in vivo and in vitro models, ginsenoside F2 (F2) decreases MMP, and accelerates the release of Cyto C, which induces mitochondria-dependent apoptosis [56]. In human osteosarcoma MG-63 cells, Rf induces G2/M phase cell cycle arrest and apoptosis through the mito-apoptotic pathway [57]. In human leukemia K562 cells, Rg1 treatment at a concentration of 20 μM for 48 h inhibits dramatic morphological alterations, such as larger mitochondria and increased numbers of lysosomes [58]. In human gastric cancer SGC-7901 cells, pseudo-Rh2-induced apoptosis is associated with a decrease in MMP, downregulation of the Bcl2/Bax ratio, and cleavages of caspase-9 and caspase-3 [59]. Furthermore, in a non-small cell lung cancer mouse xenograft model, ginsenosides Rk3 (Rk3) induces the activation of caspase-8, -9, and -3, promotes the change in MMP, decreases the Bcl2/Bax ratio and causes the release of Cyto C, which indicates that the apoptosis-inducing effect of Rk3 is triggered via mitochondria-mediated pathways [60].
In terms of tumor inhibition, CK and Rg3 are the most widely studied among ginsenoside monomers. Ginsenoside basically exerts its tumor-inhibitory effects by reducing MMP, increasing the release of Cyto C, and reducing the proportion of mitochondrial apoptotic pathways.
4.3. Heart disease
Heart diseases are characterized by persistent mitochondrial dysfunction. For example, during myocardial infarction, the supply of oxygen and energy is significantly compromised, and the mitochondrial structure in cardiomyocytes undergoes rapid changes, which cause heart functional damage: the suppression of OXPHOS, mainly due to the inhibition of complex I and/or ATP synthase, the loss of mitochondrial membrane integrity, due to the opening of mPTPs, and the release of Cyto C from mitochondria [61]. Numerous studies have shown that ginsenosides can target mitochondrial function, suppressing heart diseases (Table 3).
Table 3.
Summary of Mitochondria-Related Heart Protective Effects of Ginsenosides
| Ref. | Ginsenoside | Model | Inducer | Experimental model | Effects |
|---|---|---|---|---|---|
| [62] | Rb1 | I/R | Hypoxia-reoxygenation | H9c2 cells | MMP, Cyto C, Caspase-3 |
| [63] | Rb1 | I/R | Palmitate and high-fat diet | Isolated rat hearts, mice | Succinate, PDH |
| [64] | Rd | I/R | Ligature of left anterior descending heart | Rat model | Caspase-3/9, MMP, Cyto C |
| [65] | CK | I/R | Ligature of left anterior descending heart | Mice | Mitochondrial swelling |
| [66] | Rg1 | Nutritional stress | Glucose deprivation | Mice | ATP, MMP |
| [68] | Rg1 | I/R | Hypoxia-reoxygenation | Cardiomyocyte | Mitochondrial dynamics |
| [70] | Rg5 | Myocardial damage | PA, Ligature of left anterior descending heart | H9c2 cells, mice | HK-II, Drp1, ATP, MMP |
| [71] | Rb2, F1, Rc | Oxidative damage | Tert-butyl hydroperoxide | H9c2 cells | ATP, mtDNA, OCR |
| [72] | Rb3 | Heart failure | Myocardial infarction and I/R | Mice and H9c2 cells | MMP |
Mito-apoptosis plays a key role during myocardial I/R injury. In I/R-induced H9c2 cardiomyocytes, Rb1 can reduce mPTP by stabilizing MMP, leading to mito-apoptosis by reducing the release of Cyto C and the expression of cleaved caspase-3 in the cytoplasm, ultimately reducing programmed cell death [62]. In isolated rat hearts perfused with palmitate, high-fat diet-induced mice, and cardiomyocyte models, Rb1 prevents hypoxic succinate accumulation and improves pyruvate dehydrogenase (PDH) activity by blocking succinate-associated HIF-1α activation and GPR91 signaling, which ameliorates mitochondrial dysfunction and thereby reduces apoptosis during I/R [63]. In vivo and in vitro rat cardiomyocyte I/R injury models show that Rd pretreatment significantly stabilizes MMP, and attenuates cytosolic translocation of mitochondrial Cyto C and the activation of caspase-3/9, and increases the p-Akt or GSK-3β levels and the Bcl2/Bax ratio [64]. CK significantly inhibits mitochondrial swelling by partly mediating the activation of the PI3K pathway and phosphorylation of Akt in in vivo I/R injured mouse models [65]. Under conditions with insufficient nutrients, Rg1 rescues ATP levels and MMP in nutrient-starved cells induced by glucose deprivation and in mice with nutritional stress via aldolase/AMPK/PINK1 signaling [66].
The dysfunction of mitochondrial dynamics (fusion/fission) is a prominent feature of ischemia heart disease [67]. Rg1 moderates glutamate dehydrogenase dysregulation, increases mitochondrial length, and Mfn2 expression to reduce the number of cells with fragmented mitochondria, and prevents the imbalance of mitochondrial dynamics following I/R [68]. Hexokinase-II (HK-II) and Drp1 differently regulate mitochondrial glucose metabolism and fission [69]. In cardiomyocyte I/R injury models and isoproterenol-induced ischemic mouse hearts, Rg5 improves PDH activity and ATP production, preventing the opening of mPTPs and mitochondrial fission via Drp1 recruitment and HK-II activation [70].
Sirtuin-1 (SIRT1), an NAD+-dependent histone deacetylase, plays a critical role in cellular metabolism, ETC function, and the response to oxidative stress in mitochondria [71]. Rb2, F1, and Rc can enhance the deacetylated activity of SIRT1 and increase ATP content, the oxygen consumption rate (OCR), and mtDNA copy number in tert-butyl hydroperoxide-induced H9c2 cardiomyocytes, suggesting that these ginsenosides attenuate oxidative stress-induced mitochondrial damage through the activation of SIRT1 [71]. In a myocardial infarction-induced heart failure mouse model and an I/R-induced H9c2 injury model, Rb3 treatment upregulates the levels of the mitochondrial deacetylase SIRT3 and peroxisome proliferator-activated receptor α, protecting mitochondrial membrane integrity [72].
4.4. Hyperglycemia
When the concentration of glucose in the blood exceeds normal levels, the mitochondria in muscle cells and neurons can undergo excessive fragmentation, leading to mito-ROS burst and damaging the integrity of mtDNA [73]. Furthermore, mitochondrial fission transmits mito-apoptotic signaling that increases the sensitivity of cells to undergo apoptosis, whereas the fusion of mitochondria can enhance cell survival [73]. In oral glucose (0.5 g/kg body weight)-treated rats, Rb1 (0.01 and 0.1 mg/kg body weight) increases postprandial glucose levels, citrate synthase activity, and glycogen content, whereas Rg1 (0.01 mg/kg body weight) decreased these indices in the red gastrocnemius muscle [74]. Meanwhile, both ginsenosides can influence mitochondria turnover dynamics and alter muscle metabolism [74]. Type II diabetes mellitus and metabolic syndrome are characterized by mitochondrial dysfunction accompanied by insulin resistance in skeletal muscle [75]. Kim et al. have shown that Rg3 treatment increases ATP production and the OCR and upregulates the levels of mitochondrial biosynthesis-related proteins, including PGC1α, NRF-1, and complex IV/V, leading to an improvement of insulin resistance in skeletal muscle C2C12 cells [76]. Diabetic encephalopathy is a severe diabetic complication with characteristic symptoms of cognitive dysfunction and neuropsychiatric disability, which can be aggravated by mitochondrial dysfunction [77]. Rb1 could enhance the ratio of Bcl2/Bax, inhibit the expression of cleaved caspase-3/9, and alleviate mitochondrial damage and ROS production in the methylglyoxal-induced damage of SH-SY5Y cells through the activation of PI3K/Akt signaling pathway [78].
4.5. Inflammation
Oxidized mtDNA and other damage-associated factors are released from impaired mitochondria and act as pro-inflammatory molecules [79]. In an IL-1β-induced chondrocyte cell model of osteoarthritis, treatment with Rg1 for 2 h decreased IL-1β activity, reducing Bcl2 levels and Akt phosphorylation, and increased Bax activity, Cyto C release, and caspase-3 activation through PI3K/Akt signaling [80]. In contrast, Rb1 showed an inhibition of MMP permeability and caspase-3 activity and increased Bcl-xL/Bax ratio in H2O2-induced rat articular chondrocytes [81]. Moreover, Rd remarkably inhibits the activation of the NLRP3 inflammasome, which is dependent on the mitochondrial translocation of p62 and mitophagy via regulation of the AMPK/ULK1 signaling pathway in a dextran sulfate sodium-induced murine colitis model [9].
4.6. Other disorders
Other diseases, such as obesity, myotube atrophy, and sepsis, have also been associated with the disruption of mitochondrial functions and homeostasis [[82], [83], [84]]. Mitochondria play a key role in energy homeostasis in adipose tissues. White adipose tissue primarily stores excess energy, whereas brown adipose tissue predominantly dissipates energy by non-shivering thermogenesis [85]. In an animal model of subcutaneous white adipose cells isolated from C57BL/6 mice and 3T3-L1 adipocytes, UCP1 expression and mitochondrial biogenesis are upregulated by Rg1 via AMPK signaling pathway, which suggests that Rg1 exerts its anti-obesity effect by promoting adipocyte browning [86]. In addition, Rb1 promotes the browning of 3T3-L1 adipocytes by increasing basal glucose uptake and energy production, and upregulating the expression of brown fat-related transcriptional factors, including UCP-1, PGC-1α, and zinc finger protein 16 [87].
For myotube atrophy, the decreases of MMP and ATP synthesis, and the increase of ROS levels are reversed by Rg3 pretreatment in TNF-α-treated C2C12 myoblasts [88]. Furthermore, Rg3 upregulates the activity and expression of PGC1α, NRF-1, and mitochondrial transcription factor A [88].
Mitochondrial function also influences virus propagation and adaptation through morphology changes mediated by mitochondrial fusion and fission [89]. Kim et al. demonstrated that Rg3 inhibits hepatitis C virus (HCV) propagation by restoring the Drp1-mediated aberrant mitochondrial fission and mitophagy, thereby suppressing HCV infection [89]. In addition, in in vitro and in vivo sepsis models, Rg3 shows beneficial functions, such as OCR promotion, ROS attenuation, and the maintenance of GSH pools, by regulating AMPK-mediated mitophagy [90].
Multiple stimulations, such as UV, elevated glucose, particulate matter of 2.5 μm and smaller (PM2.5), and H2O2 can increase mito-ROS production and oxidative stress damage, which are associated with the depressed activity of the mitochondrial respiratory chain. In UV-irradiated normal human dermal fibroblast cells, Rg3 restores mitochondrial ATP levels and MMP, which leads to an increase in proteins linked with extracellular matrix, cell proliferation, and antioxidant activity [91]. In high glucose-induced rat retinal capillary endothelial cells, treatment with Rb1 significantly increases cell viability and mtDNA copy number, and inhibits ROS burst through the up-regulation of SIRT1 and SIRT3 expression [92]. In PM2.5-induced human HaCaT keratinocytes and normal dermal fibroblasts, Rb1 restores the production of ATP and blocks ER stress via the mitochondrial pathway [93]. Proteomic analysis has shown that 23 proteins spots are upregulated by Re pretreatment, which are mainly involved in the restoration of mitochondrial functions in H2O2-induced human umbilical vein endothelial cells [94]. In particular, in isolated spinal cord mitochondria, Rd regulates mPTP formation, Cyto C release, mitochondrial swelling, and NAD(P)H matrix content through Akt and ERK pathways [95].
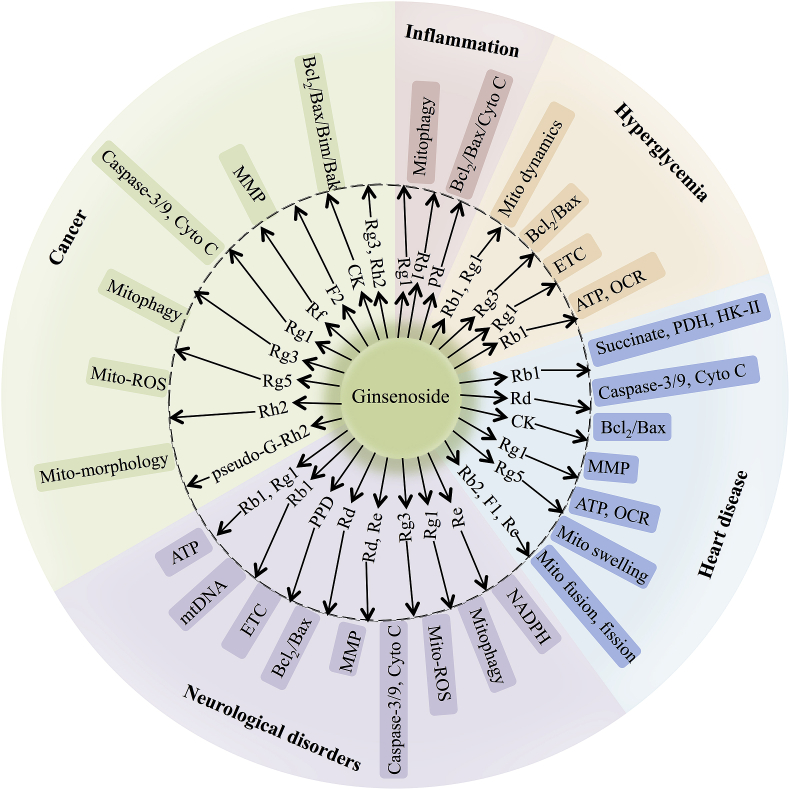
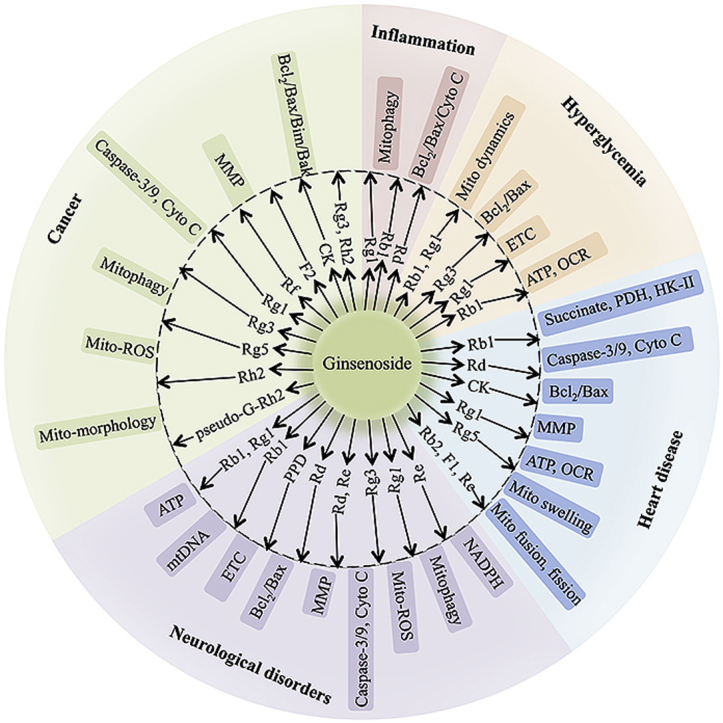
Overall, emerging evidences has shown that several ginsenosides can target mitochondria to treat different diseases through multiple mitochondria-related signaling pathways (Fig. 3).
Fig. 3.
Summary of the effects of different ginsenoside monomers on mitochondria function during neurological disorders, cancer, heart disease, hyperglycemia, and inflammation.
5. Conclusion and perspective
Ginsenosides have attracted much attention based on a wide range of pharmacological effects and medical applications. In the present review, we summarized recent findings about major ginsenosides from P. ginseng targeting mitochondrial function, hoping to partially explain the common targets of ginsenosides against multiple diseases. Currently, a huge body of pre-clinical evidences focuses on therapeutic role of ginsenosides in the cell and animal models. There are only a few clinical studies aim to evaluate the protective/therapeutic effects of several ginsenosides, such as Rd, and Rb1, which cannot explore the molecular mechanism of these ginsenosides [96,97]. In the future, our missions mainly contain two directions as below: one is to explore the potential targets or molecular networks of ginsenosides, and the other is to investigate the synergistically function of various ginsenosides as a mixture from ginseng extracts against a variety of diseases. Extensive and deep studies for ginsenosides targeting mitochondrial function could provide new insights into the clinical therapeutic application of P. Ginseng against multiple disorders.
Conflicts of interest
The authors have declared no conflict of interest.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2017YFC1702103, 2019YFC1709901), Regional Innovation and Development Joint Fund (U19A2013), National Natural Science Foundation of China (81602257), the Science and Technology Development Plan Project of Jilin Province (No. 20190101010JH, 202002053JC)), Jilin Provincial Administration of Traditional Chinese Medicine (2020168) and the Project for Science and Technology Bureau of Changchun (No. 18YJ013).
Contributor Information
Daqing Zhao, Email: zhaodaqing1963@163.com.
Xiangyan Li, Email: xiangyan_li1981@163.com.
References
- 1.Qi H.Y., Li L., Ma H. Cellular stress response mechanisms as therapeutic targets of ginsenosides. Medicinal Research Reviews. 2018;38:625–654. doi: 10.1002/med.21450. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Q., Bao X.-Y., Zhu P.-C., Tong Q., Zheng G.-Q., Wang Y. Ginsenoside Rb1 for myocardial ischemia/reperfusion injury: preclinical evidence and possible mechanisms. Oxidative Medicine and Cellular Longevity. 2017;(2017):1–14. doi: 10.1155/2017/6313625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Liu J., Deng J., Wang J., Weng W., Chu H. Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. Journal of Ginseng Research. 2020;44:14–23. doi: 10.1016/j.jgr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyman L., Karbowski M., Lederer W.J. Regulation of mitochondrial ATP production: Ca(2+) signaling and quality control. Trends in Molecular Medicine. 2020;26:21–39. doi: 10.1016/j.molmed.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock F.J., Tait S.W.G. Mitochondria as multifaceted regulators of cell death. Nature Reviews Molecular Cell Biology. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 6.Rajabian A., Rameshrad M., Hosseinzadeh H. Therapeutic potential of Panax ginseng and its constituents, ginsenosides and gintonin, in neurological and neurodegenerative disorders: a patent review. Expert Opinion on Therapeutic Patents. 2019;29:55–72. doi: 10.1080/13543776.2019.1556258. [DOI] [PubMed] [Google Scholar]
- 7.Jin X., Zhou J., Zhang Z., Lv H. The combined administration of parthenolide and ginsenoside CK in long circulation liposomes with targeted tLyp-1 ligand induce mitochondria-mediated lung cancer apoptosis. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46:S931–S942. doi: 10.1080/21691401.2018.1518913. [DOI] [PubMed] [Google Scholar]
- 8.Zhou P., Xie W., Sun Y., Dai Z., Li G., Sun G. Ginsenoside Rb1 and mitochondria: a short review of the literature. Molecular and Cellular Probes. 2019;43:1–5. doi: 10.1016/j.mcp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Liu C., Wang J., Yang Y., Liu X., Zhu Y., Zou J. Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochemical Pharmacology. 2018;155:366–379. doi: 10.1016/j.bcp.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Ong W.Y., Farooqui T., Koh H.L., Farooqui A.A., Ling E.A. Protective effects of ginseng on neurological disorders. Frontiers in Aging Neuroscience. 2015;7:129. doi: 10.3389/fnagi.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek S.H., Bae O.N., Park J.H. Recent methodology in ginseng analysis. J Ginseng Res. 2012;36:119–134. doi: 10.5142/jgr.2012.36.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Anderson G.A., Fernandez T.G., Dore S. Efficacy and mechanism of Panax ginseng in experimental stroke. Frontiers in Neuroscience. 2019;13:294. doi: 10.3389/fnins.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfanner N., Warscheid B., Wiedemann N. Mitochondrial proteins: from biogenesis to functional networks. Nature Reviews Molecular Cell Biology. 2019;20:267–284. doi: 10.1038/s41580-018-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy M.P., Hartley R.C. Mitochondria as a therapeutic target for common pathologies. Nature Reviews Drug Discovery. 2018;17:865–886. doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- 15.Lin J., Huang H.F., Yang S.K., Duan J., Qu S.M., Yuan B. The effect of Ginsenoside Rg1 in hepatic ischemia reperfusion (I/R) injury ameliorates ischemia-reperfusion-induced liver injury by inhibiting apoptosis. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2020;129:110398. doi: 10.1016/j.biopha.2020.110398. [DOI] [PubMed] [Google Scholar]
- 16.Hou J., Yun Y., Xue J., Jeon B., Kim S. Doxorubicin-induced normal breast epithelial cellular aging and its related breast cancer growth through mitochondrial autophagy and oxidative stress mitigated by ginsenoside Rh2. Phytotherapy Research : PTR. 2020;34:1659–1669. doi: 10.1002/ptr.6636. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Garcia C.M., Mongeon R., Lahmann C., Koveal D., Zucker H., Yellen G. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metabolism. 2017;26:361–374 e4. doi: 10.1016/j.cmet.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui S.A., Dutta S., Tang A., Liu L., Ross C.A., Baldo M.A. Magnetic domain wall based synaptic and activation function generator for neuromorphic accelerators. Nano Letters. 2020;20:1033–1040. doi: 10.1021/acs.nanolett.9b04200. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Zhang R.Y., Zhao J., Dong Z., Feng D.Y., Wu R. Ginsenoside Rd protects SH-SY5Y cells against 1-Methyl-4-phenylpyridinium induced injury. International Journal of Molecular Sciences. 2015;16:14395–14408. doi: 10.3390/ijms160714395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Yang X., Wang S., Song S. Ginsenoside Rg3 prevents cognitive impairment by improving mitochondrial dysfunction in the rat model of alzheimer’s disease. Journal of Agricultural and Food Chemistry. 2019;67:10048–10058. doi: 10.1021/acs.jafc.9b03793. [DOI] [PubMed] [Google Scholar]
- 21.Ye R., Kong X., Yang Q., Zhang Y., Han J., Zhao G. Ginsenoside Rd attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology. 2011;61:815–824. doi: 10.1016/j.neuropharm.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Burgos E., Fernandez-Moriano C., Lozano R., Iglesias I., Gomez-Serranillos M.P. Ginsenosides Rd and Re co-treatments improve rotenone-induced oxidative stress and mitochondrial impairment in SH-SY5Y neuroblastoma cells. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association. 2017;109:38–47. doi: 10.1016/j.fct.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Shim J.S., Song M.Y., Yim S.V., Lee S.E., Park K.S. Global analysis of ginsenoside Rg1 protective effects in beta-amyloid-treated neuronal cells. Journal of Ginseng Research. 2017;41:566–571. doi: 10.1016/j.jgr.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J., Yang H., Zhao Q., Zhang X., Lou Y. Ginsenoside Rg1 exerts a protective effect against Abeta(2)(5)(-)(3)(5)-induced toxicity in primary cultured rat cortical neurons through the NF-kappaB/NO pathway. International Journal of Molecular Medicine. 2016;37:781–788. doi: 10.3892/ijmm.2016.2485. [DOI] [PubMed] [Google Scholar]
- 25.Liu M., Bai X., Yu S., Zhao W., Qiao J., Liu Y. Ginsenoside Re inhibits ROS/ASK-1 dependent mitochondrial apoptosis pathway and activation of nrf2-antioxidant response in beta-amyloid-challenged SH-SY5Y cells. Molecules. 2019;24:25. doi: 10.3390/molecules24152687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L.X., Zhang X., Zhao G. Ginsenoside Rd attenuates DNA damage by increasing expression of DNA glycosylase endonuclease VIII-like proteins after focal cerebral ischemia. Chinese Medical Journal. 2016;129:1955–1962. doi: 10.4103/0366-6999.187851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye R., Zhang X., Kong X., Han J., Yang Q., Zhang Y. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Xu M., Ma Q., Fan C., Chen X., Zhang H., Tang M. Ginsenosides Rb1 and Rg1 protect primary cultured astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via improving mitochondrial function. International Journal of Molecular Sciences. 2019;20:23. doi: 10.3390/ijms20236086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran T.V., Shin E.J., Dang D.K., Ko S.K., Jeong J.H., Nah S.Y. Ginsenoside Re protects against phencyclidine-induced behavioral changes and mitochondrial dysfunction via interactive modulation of glutathione peroxidase-1 and NADPH oxidase in the dorsolateral cortex of mice. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association. 2017;110:300–315. doi: 10.1016/j.fct.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Shin E.J., Shin S.W., Nguyen T.T., Park D.H., Wie M.B., Jang C.G. Ginsenoside Re rescues methamphetamine-induced oxidative damage, mitochondrial dysfunction, microglial activation, and dopaminergic degeneration by inhibiting the protein kinase Cdelta gene. Molecular Neurobiology. 2014;49:1400–1421. doi: 10.1007/s12035-013-8617-1. [DOI] [PubMed] [Google Scholar]
- 31.Nam Y., Wie M.B., Shin E.J., Nguyen T.T., Nah S.Y., Ko S.K. Ginsenoside Re protects methamphetamine-induced mitochondrial burdens and proapoptosis via genetic inhibition of protein kinase C delta in human neuroblastoma dopaminergic SH-SY5Y cell lines. Journal of Applied Toxicology : JAT. 2015;35:927–944. doi: 10.1002/jat.3093. [DOI] [PubMed] [Google Scholar]
- 32.Moon J.H., Lee J.H., Lee Y.J., Park S.Y. Autophagy flux induced by ginsenoside-Rg3 attenuates human prion protein-mediated neurotoxicity and mitochondrial dysfunction. Oncotarget. 2016;7:85697–85708. doi: 10.18632/oncotarget.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bak D.H., Kim H.D., Kim Y.O., Park C.G., Han S.Y., Kim J.J. Neuroprotective effects of 20(S)-protopanaxadiol against glutamate-induced mitochondrial dysfunction in PC12 cells. International Journal of Molecular Medicine. 2016;37:378–386. doi: 10.3892/ijmm.2015.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang G.Z., Li J.C. Protective effects of ginsenoside Rg1 against colistin sulfate-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2014;34:167–172. doi: 10.1007/s10571-013-9998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D., Zhang H., Gu W., Liu Y., Zhang M. Neuroprotective effects of ginsenoside Rb1 on high glucose-induced neurotoxicity in primary cultured rat hippocampal neurons. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Moriano C., Gonzalez-Burgos E., Iglesias I., Lozano R., Gomez-Serranillos M.P. Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model. PloS One. 2017;12 doi: 10.1371/journal.pone.0182933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh J.M., Kim E., Chun S. Ginsenoside compound K induces ros-mediated apoptosis and autophagic inhibition in human neuroblastoma cells in vitro and in vivo. International Journal of Molecular Sciences. 2019;20:21. doi: 10.3390/ijms20174279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Z.Z., Ming Y.L., Chen L.H., Zheng G.H., Liu S.S., Chen Q.X. Compound K-induced apoptosis of human hepatocellular carcinoma MHCC97-H cells in vitro. Oncol Rep. 2014;32:325–331. doi: 10.3892/or.2014.3171. [DOI] [PubMed] [Google Scholar]
- 39.Lee I.K., Kang K.A., Lim C.M., Kim K.C., Kim H.S., Kim D.H. Compound K, a metabolite of ginseng saponin, induces mitochondria-dependent and caspase-dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. International Journal of Molecular Sciences. 2010;11:4916–4931. doi: 10.3390/ijms11124916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maryanovich M., Gross A. A ROS rheostat for cell fate regulation. Trends in Cell Biology. 2013;23:129–134. doi: 10.1016/j.tcb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Hu C., Song G., Zhang B., Liu Z., Chen R., Zhang H. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosis via Bid-mediated mitochondrial pathway. Journal of Cellular and Molecular Medicine. 2012;16:96–106. doi: 10.1111/j.1582-4934.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., Jiang D., Liu J., Ye S., Xiao S., Wang W. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biotherapy & Radiopharmaceuticals. 2013;28:607–614. doi: 10.1089/cbr.2012.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bian S., Zhao Y., Li F., Lu S., Wang S., Bai X. 20(S)-Ginsenoside Rg3 promotes HeLa cell apoptosis by regulating autophagy. Molecules. 2019;24:20. doi: 10.3390/molecules24203655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Yang T., Li J., Hao H.L., Wang S.Y., Yang J. Inhibition of multiple myeloma cell proliferation by ginsenoside Rg3 via reduction in the secretion of IGF-1. Molecular Medicine Reports. 2016;14:2222–2230. doi: 10.3892/mmr.2016.5475. [DOI] [PubMed] [Google Scholar]
- 45.Kim B.M., Kim D.H., Park J.H., Na H.K., Surh Y.J. Ginsenoside Rg3 induces apoptosis of human breast cancer (MDA-MB-231) cells. Journal of Cancer Prevention. 2013;18:177–185. doi: 10.15430/JCP.2013.18.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan H.D., Quan H.Y., Zhang Y., Kim S.H., Chung S.H. 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol Med Rep. 2010;3:825–831. doi: 10.3892/mmr.2010.328. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F., Li M., Wu X., Hu Y., Cao Y., Wang X. 20(S)-ginsenoside Rg3 promotes senescence and apoptosis in gallbladder cancer cells via the p53 pathway. Drug Design, Development and Therapy. 2015;9:3969–3987. doi: 10.2147/DDDT.S84527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park H.M., Kim S.J., Kim J.S., Kang H.S. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association. 2012;50:2736–2741. doi: 10.1016/j.fct.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 49.Xia T., Wang Y.N., Zhou C.X., Wu L.M., Liu Y., Zeng Q.H. Ginsenoside Rh2 and Rg3 inhibit cell proliferation and induce apoptosis by increasing mitochondrial reactive oxygen species in human leukemia Jurkat cells. Molecular Medicine Reports. 2017;15:3591–3598. doi: 10.3892/mmr.2017.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia T., Wang J., Wang Y., Wang Y., Cai J., Wang M. Inhibition of autophagy potentiates anticancer property of 20(S)-ginsenoside Rh2 by promoting mitochondria-dependent apoptosis in human acute lymphoblastic leukaemia cells. Oncotarget. 2016;7:27336–27349. doi: 10.18632/oncotarget.8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen F., Deng Z., Xiong Z., Zhang B., Yang J., Hu J. A ROS-mediated lysosomal-mitochondrial pathway is induced by ginsenoside Rh2 in hepatoma HepG2 cells. Food & Function. 2015;6:3828–3837. doi: 10.1039/c5fo00518c. [DOI] [PubMed] [Google Scholar]
- 52.Xia T., Wang J.C., Xu W., Xu L.H., Lao C.H., Ye Q.X. 20S-Ginsenoside Rh2 induces apoptosis in human Leukaemia Reh cells through mitochondrial signaling pathways. Biological & Pharmaceutical Bulletin. 2014;37:248–254. doi: 10.1248/bpb.b13-00667. [DOI] [PubMed] [Google Scholar]
- 53.Choi S., Oh J.Y., Kim S.J. Ginsenoside Rh2 induces Bcl-2 family proteins-mediated apoptosis in vitro and in xenografts in vivo models. Journal of Cellular Biochemistry. 2011;112:330–340. doi: 10.1002/jcb.22932. [DOI] [PubMed] [Google Scholar]
- 54.Zhang D., Wang A., Feng J., Zhang Q., Liu L., Ren H. Ginsenoside Rg5 induces apoptosis in human esophageal cancer cells through the phosphoinositide3 kinase/protein kinase B signaling pathway. Molecular Medicine Reports. 2019;19:4019–4026. doi: 10.3892/mmr.2019.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Fan D. The preparation of ginsenoside Rg5, its antitumor activity against breast cancer cells and its targeting of PI3K. Nutrients. 2020;12 doi: 10.3390/nu12010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao Q., Zhang P.H., Wang Q., Li S.L. Ginsenoside F(2) induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology. 2014;21:515–522. doi: 10.1016/j.phymed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Shangguan W.J., Li H., Zhang Y.H. Induction of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in human osteosarcoma MG63 cells through the mitochondrial pathway. Oncology Reports. 2014;31:305–313. doi: 10.3892/or.2013.2815. [DOI] [PubMed] [Google Scholar]
- 58.Liu J., Cai S.Z., Zhou Y., Zhang X.P., Liu D.F., Jiang R. Senescence as a consequence of ginsenoside rg1 response on k562 human leukemia cell line. Asian Pacific Journal of Cancer Prevention : APJCP. 2012;13:6191–6196. doi: 10.7314/apjcp.2012.13.12.6191. [DOI] [PubMed] [Google Scholar]
- 59.Qu X., Qu S., Yu X., Xu H., Chen Y., Ma X. pseudo-G-Rh2 induces mitochondrial-mediated apoptosis in SGC-7901 human gastric cancer cells. Oncol Rep. 2011;26:1441–1446. doi: 10.3892/or.2011.1442. [DOI] [PubMed] [Google Scholar]
- 60.Duan Z., Deng J., Dong Y., Zhu C., Li W., Fan D. Anticancer effects of ginsenoside Rk3 on non-small cell lung cancer cells: in vitro and in vivo. Food & Function. 2017;8:3723–3736. doi: 10.1039/c7fo00385d. [DOI] [PubMed] [Google Scholar]
- 61.Murphy M.P., Hartley R.C. Mitochondria as a therapeutic target for common pathologies. Nature Reviews Drug Discovery. 2018;17:865–886. doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H., Wang X., Ma Y., Shi Y. The effect of ginsenoside RB1, diazoxide, and 5-hydroxydecanoate on hypoxia-reoxygenation injury of H9C2 cardiomyocytes. Evidence-based Complementary and Alternative Medicine : eCAM. 2019;(2019) doi: 10.1155/2019/6046405. 6046405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J., Yang Y.L., Li L.Z., Zhang L., Liu Q., Liu K. Succinate accumulation impairs cardiac pyruvate dehydrogenase activity through GRP91-dependent and independent signaling pathways: therapeutic effects of ginsenoside Rb1. Biochimica et biophysica acta Molecular basis of disease. 2017;1863:2835–2847. doi: 10.1016/j.bbadis.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y., Li X., Wang X., Lau W., Wang Y., Xing Y. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3beta signaling and inhibition of the mitochondria-dependent apoptotic pathway. PloS One. 2013;8 doi: 10.1371/journal.pone.0070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsutsumi Y.M., Tsutsumi R., Mawatari K., Nakaya Y., Kinoshita M., Tanaka K. Compound K, a metabolite of ginsenosides, induces cardiac protection mediated nitric oxide via Akt/PI3K pathway. Life Sciences. 2011;88:725–729. doi: 10.1016/j.lfs.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Xu Z., Li C., Liu Q., Yang H., Li P. Ginsenoside Rg1 protects H9c2 cells against nutritional stress-induced injury via aldolase/AMPK/PINK1 signalling. Journal of Cellular Biochemistry. 2019;120:18388–18397. doi: 10.1002/jcb.29150. [DOI] [PubMed] [Google Scholar]
- 67.Bagur R., Tanguy S., Foriel S., Grichine A., Sanchez C., Pernet-Gallay K. The impact of cardiac ischemia/reperfusion on the mitochondria-cytoskeleton interactions. Biochimica et biophysica acta. 2016;1862:1159–1171. doi: 10.1016/j.bbadis.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Dong G., Chen T., Ren X., Zhang Z., Huang W., Liu L. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion. 2016;26:7–18. doi: 10.1016/j.mito.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Bi J., Zhang J., Ren Y., Du Z., Li Q., Wang Y. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biology. 2019;20:296–306. doi: 10.1016/j.redox.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y.L., Li J., Liu K., Zhang L., Liu Q., Liu B. Ginsenoside Rg5 increases cardiomyocyte resistance to ischemic injury through regulation of mitochondrial hexokinase-II and dynamin-related protein 1. Cell Death & Disease. 2017;8:e2625. doi: 10.1038/cddis.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y., Liang X., Chen Y., Zhao X. Screening SIRT1 activators from medicinal plants as bioactive compounds against oxidative damage in mitochondrial function. Oxidative Medicine and Cellular Longevity. 2016;2016:4206392. doi: 10.1155/2016/4206392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X., Wang Q., Shao M., Ma L., Guo D., Wu Y. Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARalpha pathway. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2019;120:109487. doi: 10.1016/j.biopha.2019.109487. [DOI] [PubMed] [Google Scholar]
- 73.Zorov D.B., Vorobjev I.A., Popkov V.A., Babenko V.A., Zorova L.D., Pevzner I.B. Lessons from the discovery of mitochondrial fragmentation (fission): a review and update. Cells. 2019;8:2. doi: 10.3390/cells8020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaunchaiyakul R., Leelayuwat N., Wu J.F., Huang C.Y., Kuo C.H. Contrasting actions of ginsenosides Rb1 and Rg1 on glucose tolerance in rats. The Chinese Journal of Physiology. 2019;62:267–272. doi: 10.4103/CJP.CJP_61_19. [DOI] [PubMed] [Google Scholar]
- 75.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiological Reviews. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim M.J., Koo Y.D., Kim M., Lim S., Park Y.J., Chung S.S. Rg3 improves mitochondrial function and the expression of key genes involved in mitochondrial biogenesis in C2C12 myotubes. Diabetes & Metabolism Journal. 2016;40:406–413. doi: 10.4093/dmj.2016.40.5.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quansah E., Peelaerts W., Langston J.W., Simon D.K., Colca J., Brundin P. Targeting energy metabolism via the mitochondrial pyruvate carrier as a novel approach to attenuate neurodegeneration. Molecular Neurodegeneration. 2018;13:28. doi: 10.1186/s13024-018-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nan F., Sun G., Xie W., Ye T., Sun X., Zhou P. Ginsenoside Rb1 mitigates oxidative stress and apoptosis induced by methylglyoxal in SH-SY5Y cells via the PI3K/Akt pathway. Mol Cell Probes. 2019;48:101469. doi: 10.1016/j.mcp.2019.101469. [DOI] [PubMed] [Google Scholar]
- 79.Zhong F., Liang S., Zhong Z. Emerging role of mitochondrial DNA as a major driver of inflammation and disease progression. Trends in Immunology. 2019;40:1120–1133. doi: 10.1016/j.it.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Huang Y., Wu D., Fan W. Protection of ginsenoside Rg1 on chondrocyte from IL-1beta-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Molecular and Cellular Biochemistry. 2014;392:249–257. doi: 10.1007/s11010-014-2035-1. [DOI] [PubMed] [Google Scholar]
- 81.Na J.Y., Kim S., Song K., Lim K.H., Shin G.W., Kim J.H. Anti-apoptotic activity of ginsenoside Rb1 in hydrogen peroxide-treated chondrocytes: stabilization of mitochondria and the inhibition of caspase-3. Journal of Ginseng Research. 2012;36:242–247. doi: 10.5142/jgr.2012.36.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palmer C.J., Bruckner R.J., Paulo J.A., Kazak L., Long J.Z., Mina A.I. Cdkal1, a type 2 diabetes susceptibility gene, regulates mitochondrial function in adipose tissue. Molecular Metabolism. 2017;6:1212–1225. doi: 10.1016/j.molmet.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Y.N., Yang Z.X., Ren F.Z., Fang B. FGF19 alleviates palmitate-induced atrophy in C2C12 cells by inhibiting mitochondrial overload and insulin resistance. International Journal of Biological Macromolecules. 2020;158:401–407. doi: 10.1016/j.ijbiomac.2020.04.186. [DOI] [PubMed] [Google Scholar]
- 84.Minter B.E., Lowes D.A., Webster N.R., Galley H.F. Differential effects of MitoVitE, alpha-tocopherol and trolox on oxidative stress, mitochondrial function and inflammatory signalling pathways in endothelial cells cultured under conditions mimicking sepsis. Antioxidants. 2020;9:3. doi: 10.3390/antiox9030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee J.H., Park A., Oh K.J., Lee S.C., Kim W.K., Bae K.H. The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. International Journal of Molecular Sciences. 2019;20:19. doi: 10.3390/ijms20194924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu H., Wang J., Liu M., Zhao H., Yaqoob S., Zheng M. Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients. 2018;10:7. doi: 10.3390/nu10070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mu Q., Fang X., Li X., Zhao D., Mo F., Jiang G. Ginsenoside Rb1 promotes browning through regulation of PPARgamma in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2015;466:530–535. doi: 10.1016/j.bbrc.2015.09.064. [DOI] [PubMed] [Google Scholar]
- 88.Lee S.J., Bae J.H., Lee H., Lee H., Park J., Kang J.S. Ginsenoside Rg3 upregulates myotube formation and mitochondrial function, thereby protecting myotube atrophy induced by tumor necrosis factor-alpha. Journal of Ethnopharmacology. 2019;242:112054. doi: 10.1016/j.jep.2019.112054. [DOI] [PubMed] [Google Scholar]
- 89.Kim S.J., Jang J.Y., Kim E.J., Cho E.K., Ahn D.G., Kim C. Ginsenoside Rg3 restores hepatitis C virus-induced aberrant mitochondrial dynamics and inhibits virus propagation. Hepatology. 2017;66:758–771. doi: 10.1002/hep.29177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xing W., Yang L., Peng Y., Wang Q., Gao M., Yang M. Ginsenoside Rg3 attenuates sepsis-induced injury and mitochondrial dysfunction in liver via AMPK-mediated autophagy flux. Bioscience Reports. 2017;37:4. doi: 10.1042/BSR20170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee H., Hong Y., Tran Q., Cho H., Kim M., Kim C. A new role for the ginsenoside RG3 in antiaging via mitochondria function in ultraviolet-irradiated human dermal fibroblasts. Journal of Ginseng Research. 2019;43:431–441. doi: 10.1016/j.jgr.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan C., Ma Q., Xu M., Qiao Y., Zhang Y., Li P. Ginsenoside Rb1 attenuates high glucose-induced oxidative injury via the NAD-PARP-SIRT Axis in rat retinal capillary endothelial cells. International Journal of Molecular Sciences. 2019;20:19. doi: 10.3390/ijms20194936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piao M.J., Kang K.A., Zhen A.X., Fernando P., Ahn M.J., Koh Y.S. Particulate matter 2.5 mediates cutaneous cellular injury by inducing mitochondria-associated endoplasmic reticulum stress: protective effects of ginsenoside Rb1. Antioxidants. 2019;8:9. doi: 10.3390/antiox8090383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang G.D., Zhong X.F., Deng Z.Y., Zeng R. Proteomic analysis of ginsenoside Re attenuates hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells. Food & Function. 2016;7:2451–2461. doi: 10.1039/c6fo00123h. [DOI] [PubMed] [Google Scholar]
- 95.Zhou J.S., Wang J.F., He B.R., Cui Y.S., Fang X.Y., Ni J.L. Ginsenoside Rd attenuates mitochondrial permeability transition and cytochrome C release in isolated spinal cord mitochondria: involvement of kinase-mediated pathways. International Journal of Molecular Sciences. 2014;15:9859–9877. doi: 10.3390/ijms15069859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X., Wang L., Wen A., Yang J., Yan Y., Song Y. Ginsenoside-Rd improves outcome of acute ischaemic stroke - a randomized, double-blind, placebo-controlled, multicenter trial. European Journal of Neurology. 2012;19:855–863. doi: 10.1111/j.1468-1331.2011.03634.x. [DOI] [PubMed] [Google Scholar]
- 97.Xu X., Lu Q., Wu J., Li Y., Sun J. Impact of extended ginsenoside Rb1 on early chronic kidney disease: a randomized, placebo-controlled study. Inflammopharmacology. 2017;25:33–40. doi: 10.1007/s10787-016-0296-x. [DOI] [PubMed] [Google Scholar]