Key Points
Question
Is adding a long-acting muscarinic antagonist (LAMA) to inhaled corticosteroids (ICS) and long-acting β2-agonists (LABAs) associated with differences in clinical outcomes and adverse events among individuals with moderate to severe persistent asthma?
Findings
In this systematic review and meta-analysis that included 20 randomized clinical trials and 11 894 patients, triple therapy (ICS, LABA, and LAMA), compared with dual therapy (ICS plus LABA), was significantly associated with fewer severe asthma exacerbations (risk ratio, 0.83) and slightly better asthma control, but no significant difference in quality of life or most adverse events.
Meaning
Among patients with moderate to severe asthma, triple therapy compared with dual therapy was significantly associated with fewer severe asthma exacerbations and modest improvements in asthma control but no significant difference in quality of life.
Abstract
Importance
The benefits and harms of adding long-acting muscarinic antagonists (LAMAs) to inhaled corticosteroids (ICS) and long-acting β2-agonists (LABAs) for moderate to severe asthma remain unclear.
Objective
To systematically synthesize the outcomes and adverse events associated with triple therapy (ICS, LABA, and LAMA) vs dual therapy (ICS plus LABA) in children and adults with persistent uncontrolled asthma.
Data Sources
MEDLINE, Embase, CENTRAL, ICTRP, FDA, and EMA databases from November 2017, to December 8, 2020, without language restriction.
Study Selection
Two investigators independently selected randomized clinical trials (RCTs) comparing triple vs dual therapy in patients with moderate to severe asthma.
Data Extraction and Synthesis
Two reviewers independently extracted data and assessed risk of bias. Random-effects meta-analyses, including individual patient-level exacerbation data, were used. The GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach was used to assess certainty (quality) of the evidence.
Main Outcomes and Measures
Severe exacerbations, asthma control (measured using the Asthma Control Questionnaire [ACQ-7], a 7-item list with each item ranging from 0 [totally controlled] to 6 [severely uncontrolled]; minimal important difference, 0.5), quality of life (measured using the Asthma-related Quality of Life [AQLQ] tool; score range, 1 [severely impaired] to 7 [no impairment]; minimal important difference, 0.5), mortality, and adverse events.
Results
Twenty RCTs using 3 LAMA types that enrolled 11 894 children and adults (mean age, 52 years [range, 9-71 years]; 57.7% female) were included. High-certainty evidence showed that triple therapy vs dual therapy was significantly associated with a reduction in severe exacerbation risk (9 trials [9932 patients]; 22.7% vs 27.4%; risk ratio, 0.83 [95% CI, 0.77 to 0.90]) and an improvement in asthma control (14 trials [11 230 patients]; standardized mean difference [SMD], −0.06 [95% CI, −0.10 to −0.02]; mean difference in ACQ-7 scale, −0.04 [95% CI, −0.07 to −0.01]). There were no significant differences in asthma-related quality of life (7 trials [5247 patients]; SMD, 0.05 [95% CI, −0.03 to 0.13]; mean difference in AQLQ score, 0.05 [95% CI, −0.03 to 0.13]; moderate-certainty evidence) or mortality (17 trials [11 595 patients]; 0.12% vs 0.12%; risk ratio, 0.96 [95% CI, 0.33 to 2.75]; high-certainty evidence) between dual and triple therapy. Triple therapy was significantly associated with increased dry mouth and dysphonia (10 trials [7395 patients]; 3.0% vs 1.8%; risk ratio, 1.65 [95% CI, 1.14 to 2.38]; high-certainty evidence), but treatment-related and serious adverse events were not significantly different between groups (moderate-certainty evidence).
Conclusions and Relevance
Among children (aged 6 to 18 years) and adults with moderate to severe asthma, triple therapy, compared with dual therapy, was significantly associated with fewer severe asthma exacerbations and modest improvements in asthma control without significant differences in quality of life or mortality.
This meta-analysis of 20 randomized trials reported in 6 clinical databases compares outcomes associated with dual therapy of inhaled corticosteroids (ICS) and long-acting β2-agonists (LABAs) vs triple therapy (ICS and LABA plus a long-acting muscarinic antagonist) in treating exacerbations in moderate to severe asthma.
Introduction
Asthma is the most prevalent chronic respiratory illness, and it affects all age groups.1 Exacerbations are burdensome to patients with moderate to severe disease (Global Initiative for Asthma [GINA] steps 4 and 5), the health care systems where they are treated, and the societies in which they live.1,2,3 International guidelines recommend medium- or high-dose inhaled corticosteroids (ICS) and long-acting inhaled β2-agonists (LABAs) combinations (dual therapy), delivered from the same inhaler, as the preferred controller options for patients aged 6 years or older with persistent, moderate to severe asthma.1,4,5 When patients’ symptoms remain uncontrolled despite this approach, the precise efficacy and adverse events of adding long-acting muscarinic antagonists (LAMAs) (ie, ICS, LABA, and LAMA, also described as triple therapy) is uncertain, contributing to existing weak (conditional) recommendations.1,4,5
LAMAs have a distinctly different mechanism of bronchodilation from LABAs,6 which makes them an attractive add-on therapy in persistent, uncontrolled asthma. In addition, if optimal asthma control can be achieved with the addition of LAMAs to the dual therapy of ICS and LABAs, patients may avoid escalation to other systemic therapies such as oral corticosteroids, which carry a higher risk of adverse effects,7,8,9 or to biologics, which substantially increase the costs of treatment.10
Despite being identified as a priority item to inform guideline development,11 the most recent systematic reviews addressing triple vs dual therapy in adults12,13 and children14 were limited by their search for studies until 2017, wide confidence intervals that spanned no effect, a small number of included studies using a single type of LAMA, lack of addressing harms, and inability to clarify the effects of triple therapy on asthma exacerbations. However, since 2017, tiotropium has been approved by the US Food and Drug Administration (FDA) in children, and a number of new trials on triple therapy for asthma, including LAMA preparations other than tiotropium, been completed. Therefore, this study synthesized the outcomes and adverse events of triple therapy vs dual therapy in children and adults with persistent, uncontrolled asthma.
Methods
The Cochrane15 and GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approaches16 were used to guide the conduct of this review. This review was prospectively registered (PROSPERO CRD42020172608). This study was reported in accordance to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.17 The Hamilton Integrated Research Ethics Board confirmed that institutional review board approval was not required for this systematic review and meta-analysis.
Data Sources and Selection
The following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), the World Health Organization (WHO) International Clinical Trials Registry, the FDA, and the European Medicines Agency (EMA) from November 2017, to December 8, 2020, without language restriction, were searched for randomized clinical trials (RCTs) of any duration and design comparing triple therapy (ICS,LABA, plus LAMA) with dual therapy (ICS plus LABA) in patients with moderate to severe asthma (eMethods 1 in the Supplement). Observational and preclinical studies and those limited to patients with chronic respiratory diseases other than asthma were excluded. Database searches were supplemented by using forward and backward citation analysis. Authors were contacted in all instances of missing or unclear data. Two investigators (CS and DKC) independently screened titles and abstracts using a prepiloted standardized data form (Covidence; Veritas Health Innovation). Similarly, 2 investigators (CS and LH-YK) independently reviewed full-text articles. Disagreements were resolved through consensus.
Outcomes
Outcomes of interest included asthma exacerbations, change in asthma control (measured using the Asthma Control Questionnaire [ACQ]18 or Asthma Control Test [ACT]19), change in asthma-related quality of life (measured using the Asthma Quality of Life Questionnaire [AQLQ]20 or Mini-AQLQ21), mortality, serious and nonserious adverse events (as defined by the FDA), and change in trough forced expiratory volume in 1 second (FEV1 [measured in liters]).
Severe asthma exacerbation was defined by a need for systemic steroids for 3 or more days, hospitalization, intensive care admission or intubation, or emergency department visits (based on the American Thoracic Society/European Respiratory Society recommendation).22 Asthma worsening was defined and reported in each trial, but it was generally defined as a progressive increase in 1 or more asthma symptoms or a decline in lung function for 2 or more consecutive days that did not meet the definition of severe asthma exacerbation.
As detailed in the data analysis section, scores for ACQ versions 5, 6, 7, and the ACT were pooled together using standardized mean difference (SMD).15 To facilitate interpretability, we then converted these pooled SMDs to the most clinically familiar scale, which was the ACQ-7. The ACQ-7 is a 7-item list that contains 5 symptom-based questions, 1 question on rescue bronchodilator use, and 1 question about FEV1 assessment, with each item scored on a 7-point scale (0 [no impairment] to 6 [maximum impairment]).18 ACQ-6 contains all items of the ACQ-7 except FEV1 assessment.18,23 ACQ-5 contains only the 5 symptom-based questions.23 The ACQ score is the mean of the equally weighted included questions ranging from 0 (totally controlled) to 6 (severely uncontrolled),18 with minimal important difference for a change in ACQ of 0.5 for all 3 questionnaires.18 The ACT is a 5-item list with each item scored on a 5-point scale (score range for symptoms and activity-related rating: 1 [all the time] to 5 [not at all]; for asthma control rating: 1 [not controlled at all] to 5 [completely controlled]).19 The total score ranges from 5 (poor control of asthma) to 25 (complete control of asthma).19 Minimal important difference for a change in ACT score is 3.19 ACT was multiplied by −1 to correct for the difference in direction of the scale from ACQ. For quality-of-life measures, the AQLQ and Mini-AQLQ were pooled using SMD.15 The pooled SMDs were then converted back to AQLQ scale to aid in clinical interpretability. The AQLQ is a 32-item list with each item scored on a 7-point scale (1 [severely impaired] to 7 [not impaired at all]).20 The Mini-AQLQ is a shorter 15-item version of the AQLQ (also scored on a 7-point scale with the same range interpretations).21 The higher score on both questionnaires correlates with better quality of life.21,24 The minimal important difference for a change in both AQLQ and Mini-AQLQ is 0.5.24 For FEV1, although the minimal important difference is not definitively established, generally accepted minimal important difference in the literature is 100 to 200 mL25,26 or change of 15% to 20%.26 For additional details on these instruments, see the eMethods 2 in the Supplement.
Data Extraction and Risk of Bias Assessment
Two investigators (CS and LH-YK) independently extracted data using a prepiloted standardized form with disagreements resolved by consensus. Multiple records reporting on the same trial (eg, at 2 different time points of follow-up or subgroup analysis) were considered as a single trial for all analyses. Conversely, records presenting 2 different trials or comparisons in a single report were treated as 2 separate individual trials for all analyses, supplemented by sensitivity analyses pooling these reports together. Crossover trials were accounted for according to Cochrane guidance by using the first period to analyze as parallel group studies to reduce the risk of carryover effects (see eMethods 2 in the Supplement).15
Paired investigators (CS and AWB) independently assessed risk of bias using version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2).27 Each investigator assigned the risk of bias for each domain as low, some concerns, or high. We used a modified version of some concerns to stratify as probably high or probably low risk of bias.28 Following the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2 (chapter 8.2.4.; table 8.2.b.) guidance for reaching an overall risk-of-bias judgement for a result,15 overall risk of bias was rated as high if there was high risk of bias in any single domain or some concerns for multiple domains; some concerns of bias if there were some concerns in at least 1 domain for that result but no high risk of bias for any domain; and low if there was low risk of bias for all domains. Disagreements were resolved by consensus and, as needed, discussion with a third investigator. Because giving equal weight to each component of risk of bias may be problematic and instead focusing on the key domains associated with bias29 (ie, the randomization process [sequence generation and allocation concealment] and blinding), an alternate approach of analysis by risk of bias, focusing on the association of these key domains (high or some concerns vs low) on the outcomes was also used. Both approaches accounted for risk of bias, as described according to Cochrane guidance (handbook version 6.2; chapter 7.6.2),15 focusing on only those with low risk of bias and through the GRADE approach.30
To evaluate the certainty (quality) of evidence for each outcome, the GRADE approach16 was used with trial sequential analysis31 as an objective measure of optimal information size and imprecision. The domains considered in the GRADE approach include risk of bias, imprecision, inconsistency, indirectness, publication bias, and others (see eMethods 1, 2, and 3 and eTable 1 in the Supplement).
Data Synthesis and Analysis
DerSimonian and Laird random-effects models were used for the primary meta-analyses. Analyses were performed according to patients’ randomization group and the complete case scenario. Responder analyses of continuous outcomes were performed according to the GRADE approach by calculating the proportion of patients in the intervention and control groups who achieved the minimally important difference or greater.32,33 Heterogeneity was assessed by visual inspection according to GRADE34 and quantified with I2. For unclarified missing data, case analyses, including worst complete-case and most plausible scenario35 were completed. Publication bias was assessed using the GRADE approach,36 including visually inspecting funnel plots for small study effects.
Absolute risks were calculated by multiplying the relative risk and its 95% CI with the baseline risk. Two data sources were used to estimate baseline risk37: (1) the total event rate in the control group in the meta-analysis; and (2) risk-specific estimates from observational studies.2 Prespecified subgroup analyses with tests for interaction (see eMethods 2 in the Supplement for details) included stratification by study risk of bias, asthma severity (based on a history of exacerbations), smoking history, population age, dose, type of triple therapy and comparator, and inflammatory phenotype (type-2–high vs type-2–low asthma, as defined by peripheral blood eosinophil count). Post hoc analysis was by baseline FEV1. Sensitivity analyses to test the robustness of the findings included (1) worst-case or various plausible scenarios for missing participants; (2) reweighing trials using fixed-effect analysis; (3) excluding unpublished trials; (4) excluding crossover trials; (5) analyzing different doses of the intervention independently rather than collapsing them; (6) using the more conservative Knapp-Hartung-Sidik-Jonkman random-effects meta-analytic method; and (7) using different correlation coefficients (0.5 and 0.7) and mean SD for change from baseline continuous outcomes. For details on prespecified subgroup and sensitivity analyses, see the eMethods 2 in the Supplement. The credibility of subgroup analyses was judged using ICEMAN.38 To compare meta-analysis of aggregate severe exacerbation outcome data with patient-level time-to-event data, we digitized Kaplan-Meier curves and extracted patient-level data,39,40 confirmed fidelity to values in original study reports, validated proportional hazards assumptions, fitted a shared frailty Cox regression model with the study as a random-effects variable, and reported hazard ratios (HRs) with 95% CIs.
Analyses were completed using STATA versions 14.2 and 16.1 (StataCorp) and RevMan version 5.3 (Cochrane Collaboration). Though the statistical approach in this study focuses on estimating summary measures,41 2-sided P values less than .05 were considered statistically significant with safeguards against false-positive findings using the GRADE approach and trial sequential analysis, but there is potential for type I error due to multiple comparisons. Because the protocol did not distinguish between multiple primary, secondary, and exploratory outcomes, findings for the analyses should be interpreted as exploratory. GRADEpro GDT (Hamilton: McMaster University, 2015 [developed by Evidence Prime]) was used to create the summary of findings table following standardized GRADE terminology42 and to display all relative and absolute point estimates with associated 95% CIs.
Results
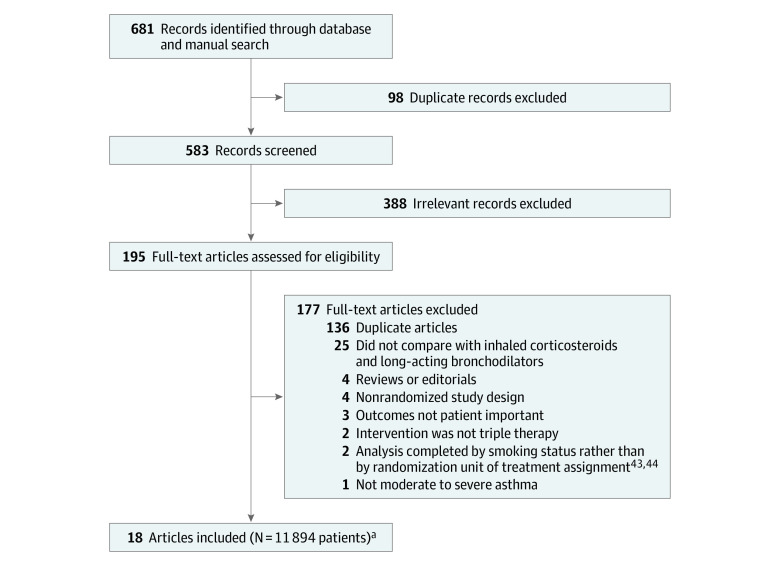
This review identified 514 unique records, assessed 195 full-text articles, and included 18 records representing 20 RCTs (Figure 1)43,44,45,46 in which a total of 11 894 patients were enrolled. For 16 records that had unpublished results or required clarification of data, multiple attempts through various modes of communication (e-mail and social media) were made, and 5 responses43,45,46,47,48,49 were received. Only 1 record provided additional data43 pertaining to 2 trials. Two trials (54 patients)45,46 could not contribute to the meta-analyses because although the studies randomized patients to triple vs dual therapy, the analyses compared smokers vs nonsmokers rather than according to their assigned treatment groups. The study authors did not provide data or analyses according to treatment assignment upon request.
Figure 1. Study Identification and Review for the Comparison of Triple vs Dual Therapy for Moderate to Severe Asthma.
aThe 18 articles indicated 20 unique randomized clinical trials. Two publications included 2 trials each.43,44
The included RCTs (Table)43,44,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62 enrolled patients with uncontrolled persistent asthma who received dual therapy with ICS plus LABA (See eTable 2 in the Supplement for detailed inclusion/exclusion criteria). Two studies52,53 (38 patients) included patients with asthma–chronic obstructive pulmonary disease overlap syndrome. Seven trials used a crossover study design (448 patients).47,48,52,53,54,55,56 Six of the 20 trials (9032 patients)43,44,57,58 required patients to have had an asthma exacerbation in the previous 12 months. Eleven trials (2334 patients)44,47,48,49,50,51,54,56,60,61 included tiotropium in the intervention group, 2 trials (2455 patients)53,58 included umeclidinium, 5 trials (45 391 patients)43,53,55,56,57 included glycopyrronium, and 2 trials (2514 patients)43,59 included both tiotropium and glycopyrronium. All included trials used medium-to-high ICS dose with LABAs as maintenance and short-acting β-agonist rescue (Table). Median study duration across all studies was 19.5 weeks (range, 6-58 weeks; interquartile range [IQR], 8-55 weeks), though most statistical information came from studies 24 to 54 weeks in duration.
Table. Study and Population Baseline Characteristics.
| Sourcea | Treatment durationb | No. of patients | Age, mean (SD), y | Female, No. (%) | Smoking, % | Baseline FEV1, mean (SD) | Baseline questionnaire symptom scores, mean (SD)c | Inhaled corticosteroid dose, µg/dd | LABA dose, µg/dd | Intervention and comparisons, µg/dd | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liters | % Predicted | ||||||||||
| Crossover trials | |||||||||||
| Ishiura et al (UMIN000021086),52 2019g | Four-week treatment periods, no washout | 17 | 70.1 (9.0) | 0 | Prior, 100 | NR | 46.8 (12.8) | ACT, 22.5 (7.0) |
|
|
|
| Ishiura et al (UMIN000026651),53 2020g | Four-week treatment periods, no washout | 19 | 70.7 (7.6) | 0 | Prior, 100 | 1.42 (0.51) | NR | ACQ-5, 6.4 (5.1) |
|
|
|
| Jabbal et al (NCT02039011),47 2017 | Two-week run-in, two 4-wk treatment periods, 2-wk washout | 18 | 46 (NR) | NR |
|
2.56 (0.39) | 87 (9.5) | ACQ-7 0.72 (0.24) |
|
|
|
| Jabbal et al (NCT02682862),54 2020 | Two- to 4-wk run-in, two 2- to 4-wk treatment periods, 2- to 4-wk washout | 17 | 44 (NR) | NR | Current, 100 | 2.41 (0.52) | 84 (NR) | ACQ 1.69 (NR) |
|
|
|
| Kerstjens et al (NCT00365560),48 2011f | Two-week run-in, three 8-wk treatment periods, no washout | 107 | 54.8 (11.7) | 58 (54.2) |
|
NR | 58.0 (11.6) | Mini-AQLQ 4.8 (1.1) |
|
|
|
| Singh et al (NCT02127866),55 2014f,h | Three 2-wk treatment periods | 211 | 50.8 (12.8) | 135 (64.0) | NR | NR | NR | NR |
|
|
|
| Watz et al (NCT03063086),56 2020f,h | Three 3-wk treatment periods, no washout | 116 | 49.5 (14.0) | 55 (47.4) | NR | 62.2 (NR) | 2.2 (NR) | NR |
|
|
|
| Parallel trials | |||||||||||
| Hamelmannet al (NCT01277523),50 2017e,f | 12 wk | 392 | 14.2 (1.7) | 159 (38.3) | Never, 100 | 2.41 (0.57) | 75.7 (8.00) | ACQ-7, 2.10 (0.40) |
|
|
|
| Hoshino et al (UMIN000019042),51 2018f | Four-week run-in, 48-wk treatment | 59 | 53.4 (14.1) | 31 (57.6) | Prior or never, 100 | NR | 71.4 (9.88) | AQLQ, 5.67 (1.05) |
|
|
|
| Kerstjens et al (NCT00772538 [PrimoTinA-asthma]),44 2012 #1f | 48-wk | 459 | 53.4 (12.6) | 289 (63.0) |
|
1.58 (0.54) | 54.6 (12.2) | ACQ-7 2.7 (0.70) |
|
|
|
| Kerstjens et al (NCT00776984 [PrimoTinA-asthma]),44 2012 #2f | 48-wk | 453 | 52.5 (12.1) | 262 (57.8) |
|
1.63 (0.54) | 55.0 (12.7) | ACQ-7 2.6 (0.7) |
|
|
|
| Kerstjens et al (NCT02571777 [IRIDIUM]),57 2020f | Two-week run-in, 52-wk treatment | 3092 | 52.2 (12.7) | 1918 (62.0) |
|
1.60 (0.58) | 54.8 (13.4) | ACQ-7 2.5 (0.57) |
|
|
|
| Lee et al (NCT02924688 [CAPTAIN]),58 2020f | Three-week run-in, 2-wk stabilization, 24- to 52-wk treatment | 2436 | 53.2 (13.1) | 1514 (62.2) |
|
2.02 (0.68) | 68.18 (14.76) | ACQ-7 2.12 (0.70) ACQ-6 1.87 (0.73) |
|
|
|
| Pearl Therapeutics (NCT03358147),59 2017f,h,i | 24-wk | 1077 | 47.7 (15.9) | 660 (61.6) | Prior or never, 100 | NR | NR | NR |
|
|
|
| Ohta et al (NCT01340209),60 2015f | 52-wk | 285 | 44.5 (12.7) | 176 (61.8) |
|
2.28 (NR) | 80.2 (NR) | ACQ-7 1.95 (0.39) |
|
|
|
| Szefler et al (NCT01634152 [VivaTinA-Asthma]),49 2017e | 12-wk | 401 | 9.0 (1.6) | 122 (30.4) | Exposed to second-hand smoke, 7.8 | 1.57 (0.35) | 81.6 (11.5) | ACQ-IA 1.97 (0.37) |
|
|
|
| Virchow et al (NCT02676076 [TRIMARAN]),43 2019 #1f | Parallel, 2-wk run-in, 52-wk treatment | 1155 | 52.6 (12.3) | 708 (61.6) | Prior, 16 Never, 84 |
1.70 (0.56) | 55.4 (12.2) | ACQ-7 2.3 (0.52) |
|
|
|
| Virchow et al (NCT02676089 [TRIGGER]),43 2019 #2f | Two-week run-in, 52-wk treatment | 1437 | 53.2 (12.1) | 871 (60.9) |
|
1.60 (0.57) | 51.9 (13.4) | ACQ-7 2.4 (0.54) |
|
|
|
| Wang et al (trial No. not reported),61 2015 | 12-wk | 63 | 36.4 (5.92) | 29 (46.0) | NR | NR | NR | NR |
|
|
|
| Zhang et al (trial No. not reported),62 2018 | 8-wk | 80 | 38.5 (8.71) | 36 (45) | NR | 2.0 (0.46) | NR | ACT 13.7 (2.28) |
|
|
|
Abbreviations: ACQ, Asthma Control Questionnaire; ACQ-IA, Asthma Control Questionnaire-Interviewer Administered; ACT, Asthma Control Test; AQLQ, Asthma-related Quality of Life; CFC, chlorofluorocarbon; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; FDC, fixed-dose combination; HFA, hydrofluoroalkane; LABA, long-acting β2-agonist; NR, not reported.
Sources include the clinical trial number and trial name (if reported).
Durations listed without run-in, washout, or stabilization information indicate that there was a single duration period for the number of weeks shown.
Asthma Control Questionnaire-7 is a 7-item list that contains 5 symptoms-based questions, rescue bronchodilator use, and FEV1 assessment with each item scored on a 7-point scale (0 = no impairment to 6 = maximum impairment). ACQ-6 contains the items of ACQ-7 with the exception of FEV1 assessment. ACQ-5 only contains 5 symptoms-based questions. The ACQ score is the mean of the included questions ranging from 0 [totally controlled] to 6 [severely uncontrolled]. Minimal important difference for a change in ACQ is 0.5 for all three questionnaires. Asthma Control Test (ACT) is a 5-item list with each item scored on a 5-point scale (for symptoms and activity-related rating: 1 = all the time to 5 = not at all; for asthma control rating: 1 = not controlled at all to 5 = completely controlled). The total score ranges from 5 (poor control of asthma) to 25 (complete control of asthma). Minimal important difference for a change in ACT is 3. Asthma-related Quality of Life (AQLQ) is a 32-item list with each item scored on a 7-point scare (1 = severely impaired to 7 = not impaired at all). Mini-AQLQ is a shorter 15-item list version of AQLQ. The higher score on both questionnaires correlates with better quality of life. The minimal important difference for a change in both AQLQ and Mini-AQLQ is 0.5.21
Doses are reported as micrograms per day unless otherwise noted.
Included pediatric patients only (aged <18 years).
Indicates trial protocols that excluded current smokers.
Included asthma-COPD overlap only.
Indicates an unpublished trial.
Included pediatric and adult patients.
The median of mean ages across studies was 52 years (range, 9-71 years; IQR, 44-53 years) with 57.7% of patients being female (range, 0%-64.0%; IQR, 45%-61.8%). Three trials included patients aged 6 to 18 years (1870 patients).49,50,59 Two trials included patients who were active smokers (302 patients).54,60 Across the included trials, the median trough prebronchodilator FEV1 was 1.85 L (range, 1.42-2.56 L; IQR, 1.6-2.28 L) or 62.2% (range, 46.8%-87%; IQR, 54.8%-80.2%), and baseline ACQ score of patients was 2.12 (range, 0.72-2.7; IQR, 1.96-2.45). Eighteen trials were deemed to be at low risk of bias, and 2 (122 patients)51,61 were at high risk of bias (eFigure 1 in the Supplement) because of deviations from the intended intervention, missing outcome data, and inadequate randomization. One study63 (80 patients) had some concerns in 2 key domains (randomization and deviations from intended interventions), and according to an alternate approach to rating risk of bias, could be considered as having high risk of bias, although either classification did not materially change the overall findings according to risk of bias. No strong evidence of publication bias nor asymmetries in the funnel plots was detected (eFigure 2 in the Supplement). A summary of findings with GRADE ratings and their rationales is found in eTable 1 in the Supplement.
Severe Asthma Exacerbations
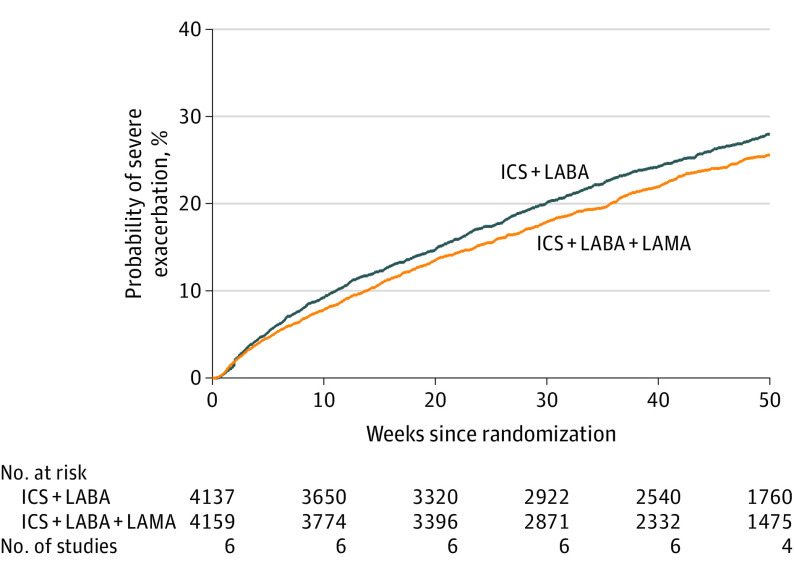
Ten studies (10 109 patients) reported severe asthma exacerbations.43,44,48,49,50,57,58 In 7 trials (10 109 patients),43,57,58,59 triple therapy compared with dual therapy was significantly associated with a reduction in severe exacerbation incidence rate (0.35 vs 0.41 per year; incidence rate ratio [IRR], 0.85 [95% CI, 0.78 to 0.92]; I2 = 0%; Figure 2), risk of at least 1 severe exacerbation was reported in 9 trials (9932 patients; 22.7% with triple therapy vs 27.4% with dual therapy; risk ratio [RR], 0.83 [95% CI, 0.77 to 0.90]; I2 = 17% [high-certainty evidence]),43,44,48,49,50,57,58 and time to first severe exacerbation was reported in 6 trials (8296 patients; 28.6% with triple therapy vs 24.7% with dual therapy; HR, 0.84 [95% CI, 0.77 to 0.92])43,44,57,58 (Figure 3 and eFigure 3 in the Supplement). Similar findings were seen in milder exacerbations or with asthma worsening as an outcome (eFigure 4 in the Supplement).
Figure 2. Severe Asthma Exacerbations in Randomized Trials of Triple vs Dual Therapy.
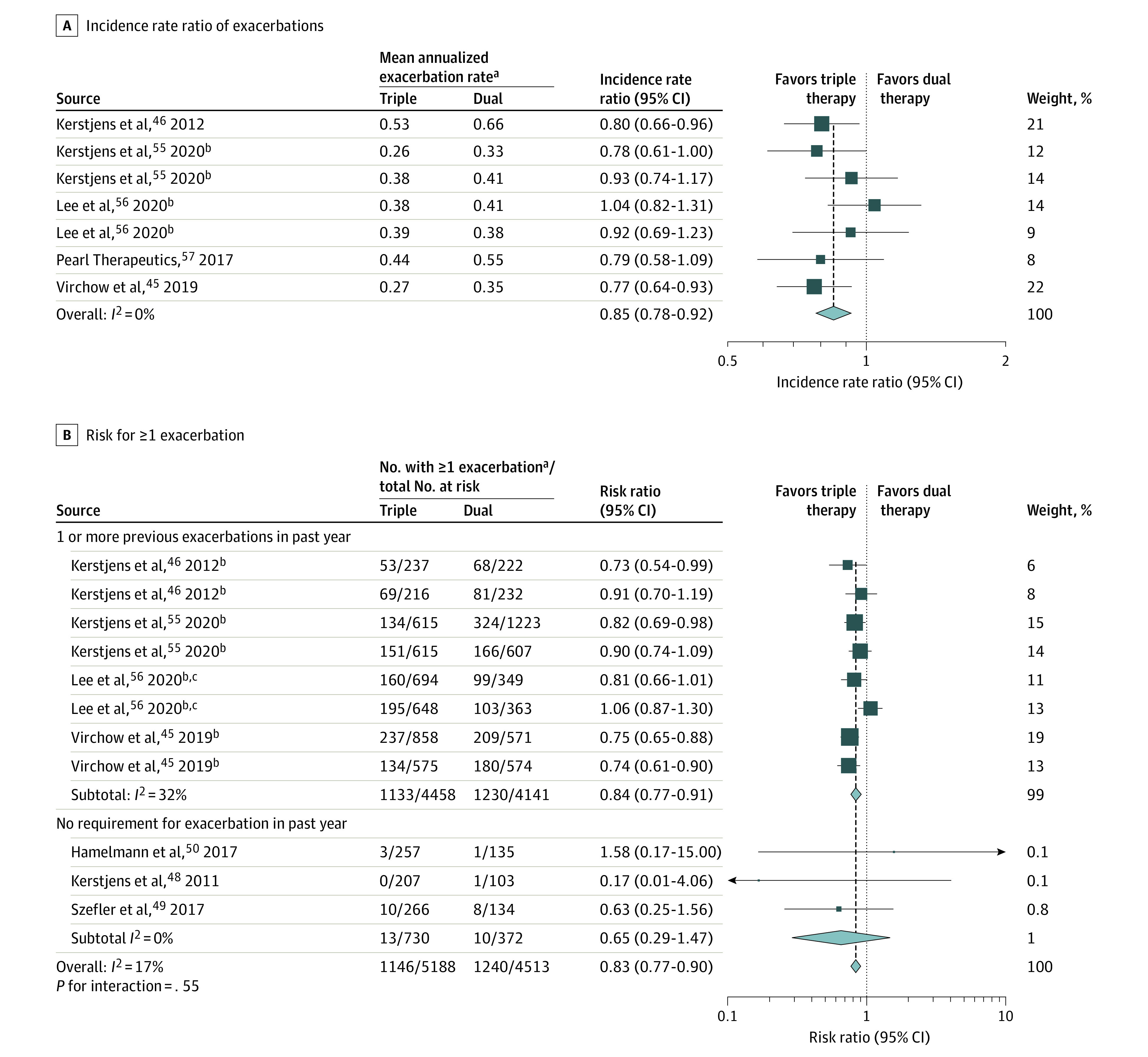
Error bars indicate 95% CI of the rate ratio estimates.
aSevere asthma exacerbation was defined by a need for systemic steroids for ≥3 days, hospitalization, intensive care admission or intubation, or emergency department visits. The mean annualized exacerbation rate indicates the mean number of exacerbations per patient per year (the total number of exacerbations in a group divided by the total follow-up time of the group).
bRepeated source names are not duplicates; they indicate 2 comparisons within a single report.
cEstimated from life table.
Figure 3. Kaplan-Meier Failure Curves of Time to First Severe Exacerbation in Patients Assigned to Triple vs Dual Asthma Inhaler Therapy.
Severe asthma exacerbation was defined by a need for systemic steroids for ≥3 days, hospitalization, intensive care admission or intubation, or emergency department visits. Triple therapy indicates inhaled corticosteroids (ICS), long-acting β2-agonists (LABAs), and long-acting muscarinic antagonists (LAMAs), and dual therapy indicates ICS with a LABA. Hazard ratio (0.84 [95% CI, 0.77-0.92]) was calculated from Cox regression model with shared frailty by study. Per-patient summary statistics on observation time: mean (SD), 37.8 (16.5) weeks; median, 46.4 weeks (range, 0.2-52 weeks [interquartile range [IQR], 35.3-52.0 weeks, calculated under the assumption that IQR = 1.35 × SD).
Asthma Control
Fourteen trials (11 230 patients)43,44,47,49,50,52,53,55,57,58,59,61 measured asthma control using ACQ-5, ACQ-6, ACQ-7, or ACT scores. Median improvement from baseline across trials in ACQ-7 scores in the control group was a mean (SD) of 0.72 (0.66) units. Triple therapy was significantly associated with an improvement in asthma control scores compared with dual therapy (standardized mean difference [SMD], −0.06 SD units, [95% CI, −0.10 to −0.02 SD units]; I2 = 0%; high-certainty evidence; Figure 4A). This estimate on the ACQ-7 scale represents a mean difference of −0.04 units (95% CI, −0.07 to −0.01 [65% vs 63% achieving minimal important difference]; RR, 1.04 [95% CI, 1.01 to 1.06]).
Figure 4. Asthma Control and Asthma-Related Quality of Life in Randomized Trials of Triple vs Dual Therapy.
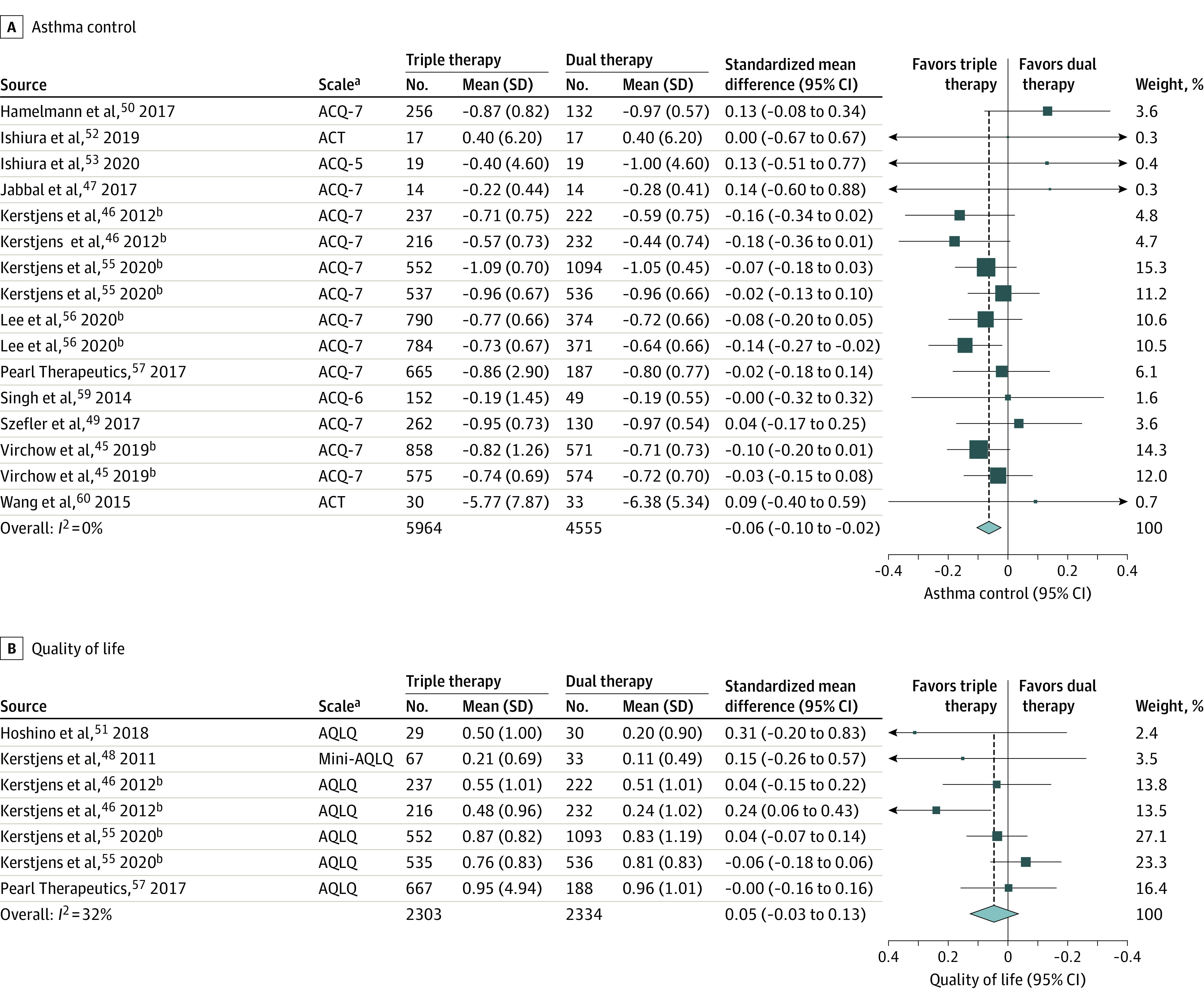
aThe Asthma Control Questionnaire (ACQ) has 3 versions: ACQ-7 is a 7-item list that contains 5 symptom-based questions, 1 question about rescue bronchodilator use, and 1 question about forced expiratory volume in the first second (FEV1, reported in liters), with each item scored on a 7-point scale (0 [no impairment] to 6 [maximum impairment])18; ACQ-6 contains all items of the ACQ-7 except FEV1 assessment18,23; and ACQ-5 contains only the 5 symptom-based questions.23 The questions are equally weighted and the ACQ score is the mean of the included questions (range, 0 [totally controlled] to 6 [severely uncontrolled]).18 Minimal important difference for a change in ACQ is 0.5 for all 3 questionnaires.18 The Asthma Control Test (ACT) is a 5-item list with each item scored on a 5-point scale (for symptoms and activity-related rating: 1 [all the time] to 5 [not at all]; for asthma control rating: 1 [not controlled at all] to 5 [completely controlled]).19 The total score ranges from 5 (poor control of asthma) to 25 (complete control of asthma).19 Minimal important difference for a change in ACT is 3.19 The Asthma-related Quality of Life (AQLQ) and Mini-AQLQ versions are as follows: AQLQ score is a 32-item list with each item scored on a 7-point scale (1 [severely impaired] to 7 [not impaired at all])20; and the Mini-AQLQ is a shorter 15-item list version of AQLQ.21 The higher score on both questionnaires correlates with better quality of life.21,24 The minimal important difference for a change in both AQLQ and Mini-AQLQ is 0.5.24 The ACQ-5, ACQ-6, ACQ-7, and ACT were pooled together using standardized mean difference (SMD). To facilitate interpretability, these pooled SMDs were converted to the most familiar scale, which was the ACQ-7 (see eFigures 4, 5, 6, 7, and 8 in the Supplement for asthma worsening [defined as a progressive increase in ≥1 asthma symptoms or a decline in lung function for ≥2 consecutive days that does not meet the definition of severe asthma exacerbation), serious and nonserious adverse events, treatment-related adverse events, breakdown of adverse events, and all-cause mortality.
Quality of Life
Seven trials (5247 patients)44,48,51,57,59 reported asthma-related quality of life, measured by AQLQ and Mini-AQLQ. Median improvement from baseline across trials in AQLQ scores in the control group was mean (SD) of 0.66 (1.01) units. Triple therapy was not significantly associated with an improvement in asthma-related quality of life (SMD, 0.05 [95% CI, −0.03 to 0.13]; I2 = 32%; moderate-certainty evidence; Figure 4B). As measured by AQLQ, this represented a mean difference of 0.05 units (95% CI, −0.03 to 0.13 [58% vs 56% achieving minimal important difference]; RR, 1.03 [95% CI, 0.98 to 1.09]).
Mortality
Seventeen trials reported mortality outcomes, with 14 of these reporting no deaths in either group. There was no significant difference in all-cause mortality between dual and triple therapy (0.12% vs 0.12%; risk difference, 0.02% [95% CI, −0.16% to 0.21%]; RR, 0.96 [95% CI, 0.33 to 2.75]; I2 = 0%; high-certainty evidence; eFigure 6 in the Supplement). Post hoc sensitivity analyses using different meta-analytic approaches to include all available trials also found no significant association with mortality (eTable 3 and eFigure 6 in the Supplement). None of the deaths were suspected to be treatment related or asthma related.
Adverse Events
In 10 trials (7395 patients), aside from triple vs dual therapy being significantly associated with increased dry mouth and dysphonia (3.0% vs 1.8%; RR, 1.65 [95% CI, 1.14 to 2.38]; I2 = 0%; high-certainty evidence), the overall incidence of serious adverse events was not significantly different between the triple vs dual therapy groups (5.2% vs 5.6% in 12 trials [11 505 patients]; RR, 0.92 [95% CI, 0.73 to 1.16]; I2 = 42%; moderate-certainty evidence; eFigure 5 in the Supplement). Triple therapy was significantly associated with fewer nonserious adverse events (44.7% vs 50.2% in 13 trials [8565 patients]; RR, 0.89 [95% CI, 0.81 to 0.97]; I2 = 68%; moderate-certainty evidence) compared with dual therapy (eFigure 5 in the Supplement). Between-group difference was not significant for treatment-related adverse events (5.9% vs 5.2% in 9 trials [10 078 patients]; RR, 1.13 [95% CI, 0.95 to 1.34]; I2 = 0; moderate-certainty evidence), serious pulmonary infections (0.8% vs 0.9% in 8 trials [10 394 patients]; RR, 0.87 [95% CI, 0.50 to 1.50]; I2 = 0%; high-certainty evidence), nonserious pulmonary infections (6.6% vs 6.9% in 12 trials [10 491 patients]; RR, 0.96 [95% CI, 0.83 to 1.11]; I2 = 3%; high-certainty evidence), eye-related adverse events (0.23% vs 0.24% in 9 trials [10 864 patients]; RD, −0.01% [95% CI, −0.21% to 0.01%]; I2 = 0%; moderate-certainty evidence), nonserious cardiovascular adverse events (0.5% vs 0.5% in 14 trials [7226 patients]; RD, 0.01% [95% CI, −0.27% to 0.29%]; I2 = 0%; moderate-certainty evidence), and serious cardiovascular adverse events (eg, arrhythmia, myocardial infarction, 0.5% vs 0.5% in 17 trials [11 458 patients]; RD, 0.006% [95% CI, −0.2% to 0.2%]; high-certainty evidence) (eFigure 7 in the Supplement).
Spirometry Parameters by FEV1 Measurement
Triple therapy was significantly associated with an improvement in spirometric indices measured by trough FEV1 (18 trials [11 715 patients]; mean difference, 0.08 L [95% CI, 0.07 to 0.10]; I2 = 0%; high-certainty evidence [Figure 5]). Median improvement across trials from baseline in FEV1 among the control group was mean (SD) 0.10 L (0.32 L). Assuming a minimal important difference of 200 mL, triple therapy was significantly associated with a higher percentage of patients achieving a 200-mL increase from baseline compared with dual therapy (47% vs 37%; RR, 1.27 [95% CI, 1.22 to 1.32]).
Figure 5. Lung Function as Measured by FEV1 in Randomized Trials of Triple vs Dual Therapy.
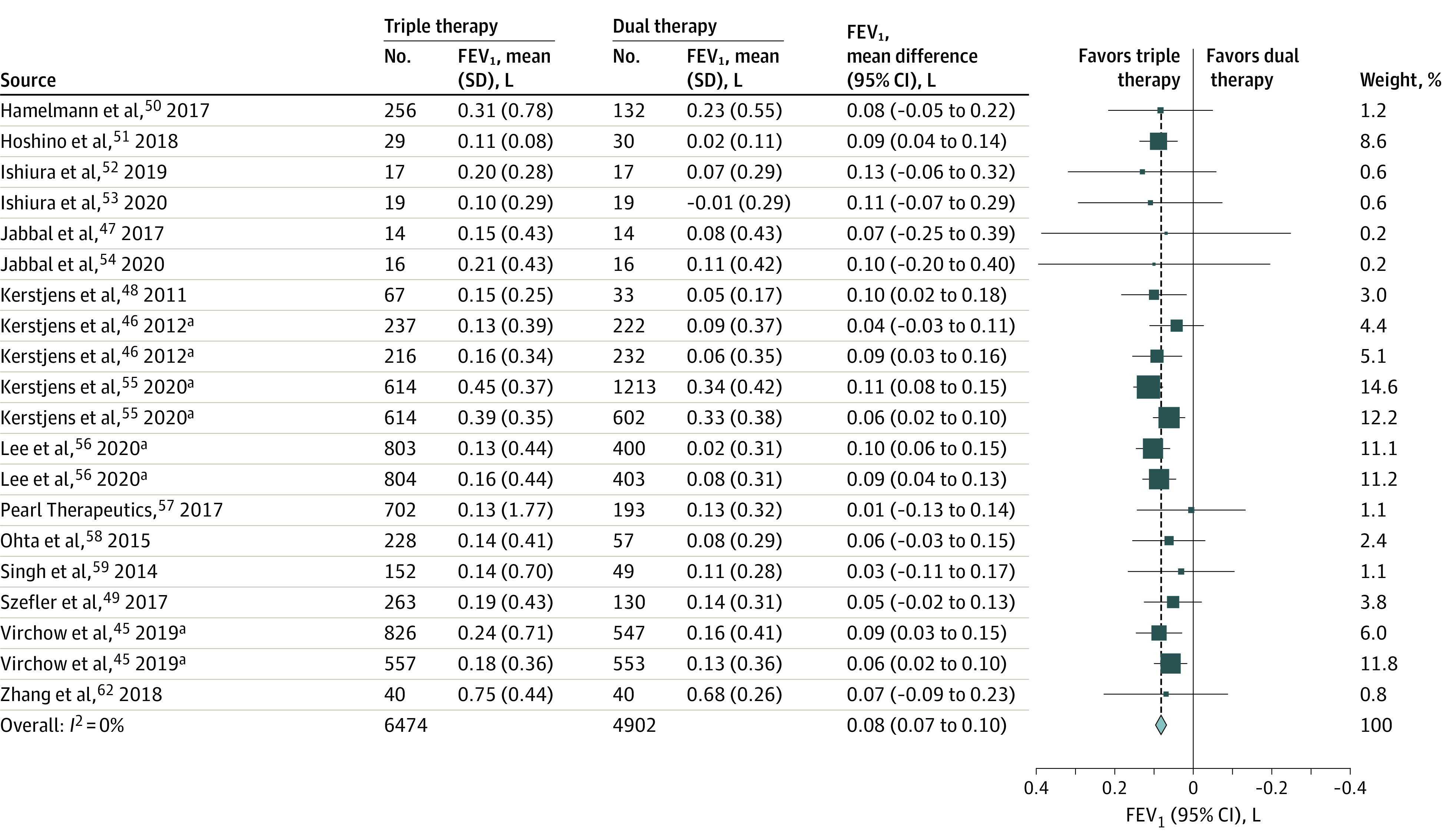
FEV1 indicates forced expiratory volume in the first second of expiration.
Subgroup and Sensitivity Analyses
Subgroup analyses for severity of asthma by exacerbation frequency (<1 previous exacerbation vs ≥1 previous exacerbations), age (<18 years vs ≥18 years), smoking history, ICS dose and type of either the intervention or comparator, type and dose of LAMAs, or inflammatory phenotype (type-2–high vs type-2–low asthma defined by peripheral blood eosinophil count) did not reveal any credible effect modifiers. Analyses limited to only published trials found consistent results (exacerbations RR, 0.83 [95% CI, 0.77 to 0.90]; FEV1 mean difference, 0.08 [95% CI, 0.06 to 0.10]; ACQ for asthma control SMD, −0.05 [95% CI, −0.09 to −0.01]; AQLQ for quality of life SMD, 0.06 [95% CI, −0.03 to 0.14]; mortality RD, 0.02% [95% CI, −0.02% to 0.02%]; and serious adverse events RR, 0.92 [95% CI, 0.73 to 1.16). Analyses limited to trials at low risk of bias, using the Cochrane Risk of Bias Tool approach and considering the key domains of randomization and blinding,29 also added consistent results (exacerbations RR, 0.83 [95% CI, 0.77 to 0.90]; FEV1 mean difference, 0.08 [95% CI, 0.07 to 0.10]; ACQ for asthma control SMD, −0.07 [95% CI, −0.10 to −0.03]; AQLQ quality of life SMD, 0.05 [95% CI, −0.03 to 0.13]; mortality RD, 0.02% [95% CI, −0.02% to 0.02%]; and serious adverse events RR, 0.90 [95% CI, 0.70 to 1.17; eTable 3 in the Supplement).
Trial Sequential Analysis
The findings were robust to trial sequential analyses (eFigure 8 in the Supplement).
Discussion
In this systematic review and meta-analysis of 20 RCTs including 11 894 children (aged 6 to 18 years) and adults with uncontrolled moderate to severe asthma, triple therapy compared with dual therapy was significantly associated with fewer severe exacerbations and modest improvements in asthma control without significant differences in quality of life or mortality. No significant difference was observed in treatment-related or serious adverse events.
These findings are consistent with multiple lines of evidence supporting antimuscarinic protective effects against exacerbations. In preclinical and clinical studies, short-acting antimuscarinics improved bronchodilation of airways64 and were associated with lower risk of hospitalization during acute asthma,65,66 and LAMAs protected against bronchoprovocation.67 This is also consistent with the study results demonstrating no subgroup differences across 3 types of LAMAs in association with exacerbations supporting a class effect. In clinical practice, LAMAs may have additional benefits besides reducing exacerbations. The complementary bronchodilation properties of a LAMA to a LABA allows its use in patients who have developed tachyphylaxis to LABAs68 or in patients limited by the adverse effects of LABAs, such as tachycardia. Compared with biologic therapies, LAMAs may be favorable in terms of independence of inflammatory phenotype, cost,10 and other contextual factors.
In this systematic review and meta-analysis, triple therapy was associated with statistically significant but potentially clinically unimportant benefits in asthma symptoms, and there was no significant difference between triple therapy vs dual therapy with regard to asthma-related quality of life. Changes in FEV1 were also small, but the clinical significance of these changes is difficult to qualify without established minimal important difference thresholds in patients with moderate to severe disease and who receive treatment using more than 1 class of bronchodilator. The dissociation between indices of asthma control and exacerbations is consistent with the findings from previous studies in severe asthma. For instance, several trials with biologic therapies69 have found a clinically important reduction in severe exacerbations with improvements in asthma symptoms below the minimal important difference. These findings might reflect different pathophysiology underlying exacerbations vs symptom control. More broadly, the results of this meta-analysis in asthma parallel that of chronic obstructive pulmonary disease,62 showing comparable direction and magnitude of association on exacerbations, quality of life, FEV1, and adverse events with triple therapy (ICS, LABA, and LAMA) vs dual therapy (ICS plus LABA).
The results of this study are qualitatively distinct from the small evidence base (2-3 trials; 1304 patients)12 that informed the recently released National Asthma Education and Prevention Program Expert Panel 4 guidelines4,70 and concluded that triple therapy improves asthma control and quality of life but has no effect on asthma exacerbations. This meta-analysis, instead, showed that add-on LAMA therapy is associated with a reduction in exacerbations, small improvements in spirometry, and little to no change in symptoms and quality of life. The apparent juxtaposition of the risk of exacerbation and day-to-day clinical symptoms of asthma has important implications for the management of persistently uncontrolled asthma. One of the largest risk factors for future exacerbation is a history of a severe exacerbation in the past year.1 In a large cohort study enrolling more than 400 000 patients across the US and UK,2 the risk of exacerbation in patients with vs without a history of exacerbation in the past 12 months was 25% compared with 8%. Thus, clinicians should also consider the baseline risk of exacerbation among patients being considered for triple vs dual therapy. Therefore, although no effect modification in patients at high vs low risk for severe exacerbations was detected, this study’s findings strengthen the recommendation to add a LAMA to medium- to high-dose ICS plus LABA therapy for individuals at high risk of exacerbations and to rewrite the underlying rationale to do so. These findings raise the notion that patient selection and consideration of risk for future exacerbation are imperative to identify those who may benefit the most from addition of a LAMA to ICS plus LABA as triple therapy.
All included studies in this review compared triple therapy against ICS plus a LABA as maintenance with short-acting β-agonist as reliever. Although ICS–rapid-onset LABA as single controller and maintenance reliever therapy (ICS-LABA MART) has also been shown to reduce severe exacerbations in moderate to severe asthma (RR, 0.68 [95% CI, 0.58 to 0.80]),71 a direct efficacy comparison between the triple therapy with short-acting β-agonist reliever vs ICS-LABA MART with or without add-on LAMA is limited without additional studies. Clarifying the role of each strategy will inform optimal treatment approaches for patients with moderate to severe asthma.
The strengths of this review include its comprehensive and up-to-date search leading to 17 more captured trials, including 2 unpublished trials and a larger sample size of more than 10 000 patients compared with the last systematic review of 3 trials12 and assessment of adverse events and children. The broad eligibility criteria enhance generalizability across different LAMA types and doses and delivery of triple therapy as a single or a combination of inhalers. The criteria also include comparisons of triple vs dual therapy with similar or higher doses of ICS. The analyses were robust to multiple sensitivity analyses and consistent regardless of methods of exacerbation analyses, including incidence rate, risk, or individual patient-level survival data.
Limitations
This study has several limitations. First, although many trials reported the composite outcome of severe asthma exacerbation, defined as exacerbation requiring hospitalization, an emergency department visit, or more than 3 days of treatment with a systemic steroid, only 3 trials reported the breakdown of each category of severe exacerbation. Therefore, it was not feasible to identify with high certainty which outcome component of severe exacerbation could be driving the difference in association observed in the review.
Second, although no subgroup effects were detected by type-2 inflammatory status (type-2–high vs type-2–low asthma defined by peripheral blood eosinophil count), it is possible that there are other biomarkers, including sputum inflammatory indices,72,73 that could influence treatment effects.
Third, there is potential for type I error due to multiple comparisons.
Fourth, crossover RCTs were included in this review and were analyzed as parallel trials. However, they comprised a small portion of the overall analyses (448 of 11 894 total included patients), and the findings were robust to subgroup and sensitivity analyses accounting for these.
Conclusions
Among children (aged 6 to 18 years) and adults with moderate to severe asthma, triple therapy, compared with dual therapy, was significantly associated with fewer severe asthma exacerbations and modest improvements in asthma control without significant differences in quality of life or mortality.
eMethods 1. Search Strategies
eMethods 2. Additional Methods Details
eMethods 3. PRISMA Checklist
eTable 1. Summary of Findings
eTable 2. Inclusion/Exclusion criteria
eTable 3. Subgroup and Sensitivity Analyses
eFigure 1. Assessment of Risk of Bias Using Cochrane Collaboration Tool
eFigure 2. Funnel Plots
eFigure 3. Pooled Reported Hazard Ratios
eFigure 4. Asthma Worsening
eFigure 5. Serious and Non-serious Adverse Events
eFigure 6. All-cause Mortality – Frequentist Analysis
eFigure 7. Components of Adverse Events
eFigure 8. Trial Sequential Analyses
References
- 1.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020 update. Accessed December 16, 2020. https://ginasthma.org
- 2.Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. doi: 10.1186/s12890-017-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhl R, Heaney LG, Loefroth E, et al. One-year follow up of asthmatic patients newly initiated on treatment with medium- or high-dose inhaled corticosteroid-long-acting β2-agonist in UK primary care settings. Respir Med. 2020;162:105859. doi: 10.1016/j.rmed.2019.105859 [DOI] [PubMed] [Google Scholar]
- 4.Cloutier MM, Baptist AP, Blake KV, et al. ; Expert Panel Working Group of the National Heart, Lung, and Blood Institute . 2020 Focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. doi: 10.1016/j.jaci.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF Jr, Pace W, Schatz M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program. JAMA. 2020;324(22):2301-2317. doi: 10.1001/jama.2020.21974 [DOI] [PubMed] [Google Scholar]
- 6.Buels KS, Fryer AD. Muscarinic receptor antagonists: effects on pulmonary function. Handb Exp Pharmacol. 2012;(208):317-341. doi: 10.1007/978-3-642-23274-9_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre P, Duh MS, Lafeuille M-H, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488-1495. doi: 10.1016/j.jaci.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 8.Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi: 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canonica GW, Colombo GL, Bruno GM, et al. ; SANI Network . Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019;12(1):100007. doi: 10.1016/j.waojou.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson WC III, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122(4):367-372. doi: 10.1016/j.anai.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 11.National Heart, Lung, and Blood Institute . Needs assessment report for potential update of the Expert Panel Report-3(2007): guidelines for the diagnosis and management of asthma, April 2014. Accessed December 16, 2020. https://www.nhlbi.nih.gov/sites/default/files/media/docs/NHLBAC-Asthma-WG-Report-2-2015.pdf
- 12.Sobieraj DM, Baker WL, Nguyen E, et al. Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1473-1484. doi: 10.1001/jama.2018.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst Rev. 2016;(1):CD011721. doi: 10.1002/14651858.CD011721.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28(6):573-578. doi: 10.1111/pai.12759 [DOI] [PubMed] [Google Scholar]
- 15.Higgins J, Thomas J, eds et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2, 2021. Accessed April, 25, 2021. http://www.training.cochrane.org/handbook
- 16.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902-907. doi: 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 19.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59-65. doi: 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 20.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76-83. doi: 10.1136/thx.47.2.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32-38. doi: 10.1034/j.1399-3003.1999.14a08.x [DOI] [PubMed] [Google Scholar]
- 22.Reddel HK, Taylor DR, Bateman ED, et al. ; American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations . An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59-99. doi: 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, Svensson K, Mörk A-C, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553-558. doi: 10.1016/j.rmed.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 24.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115(5):1265-1270. doi: 10.1378/chest.115.5.1265 [DOI] [PubMed] [Google Scholar]
- 25.Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14(1):23-27. doi: 10.1034/j.1399-3003.1999.14a06.x [DOI] [PubMed] [Google Scholar]
- 26.Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3)(suppl):S65-S87. doi: 10.1016/j.jaci.2011.12.986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 28.Akl EA, Sun X, Busse JW, et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol. 2012;65(3):262-267. doi: 10.1016/j.jclinepi.2011.04.015 [DOI] [PubMed] [Google Scholar]
- 29.Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA. 2014;312(6):623-630. doi: 10.1001/jama.2014.8166 [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407-415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 31.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659-663. doi: 10.2307/2336502 [DOI] [Google Scholar]
- 32.Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health-related quality of life outcomes in meta-analysis-a tutorial and review of methods for enhancing interpretability. Res Synth Methods. 2011;2(3):188-203. doi: 10.1002/jrsm.46 [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66(2):173-183. doi: 10.1016/j.jclinepi.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group . GRADE guidelines: 7. rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294-1302. doi: 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 35.Akl EA, Johnston BC, Alonso-Coello P, et al. Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PLoS One. 2013;8(2):e57132. doi: 10.1371/journal.pone.0057132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64(12):1277-1282. doi: 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 37.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 38.Schandelmaier S, Briel M, Varadhan R, et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192(32):E901-E906. doi: 10.1503/cmaj.200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. doi: 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei Y, Royston P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017;17(4):786-802. doi: 10.1177/1536867X1801700402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasserstein RL, Lazar NA. The ASA statement on p values: context, process, and purpose. Am Stat. 2016;70(2):129-133. doi: 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 42.Santesso N, Glenton C, Dahm P, et al. ; GRADE Working Group . GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126-135. doi: 10.1016/j.jclinepi.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 43.Virchow JC, Kuna P, Paggiaro P, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019;394(10210):1737-1749. doi: 10.1016/S0140-6736(19)32215-9 [DOI] [PubMed] [Google Scholar]
- 44.Kerstjens HAM, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198-1207. doi: 10.1056/NEJMoa1208606 [DOI] [PubMed] [Google Scholar]
- 45.Yoshida M, Nakano T, Fukuyama S, et al. Effects of tiotropium on lung function in severe asthmatics with or without emphysematous changes. Pulm Pharmacol Ther. 2013;26(2):159-166. doi: 10.1016/j.pupt.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 46.Yoshida M, Kaneko Y, Ishimatsu A, Komori M, Iwanaga T, Inoue H. Effects of tiotropium on lung function in current smokers and never smokers with bronchial asthma. Pulm Pharmacol Ther. 2017;42:7-12. doi: 10.1016/j.pupt.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Jabbal S, Manoharan A, Lipworth BJ. Bronchoprotective tolerance with indacaterol is not modified by concomitant tiotropium in persistent asthma. Clin Exp Allergy. 2017;47(10):1239-1245. doi: 10.1111/cea.12972 [DOI] [PubMed] [Google Scholar]
- 48.Kerstjens HAM, Disse B, Schröder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128(2):308-314. doi: 10.1016/j.jaci.2011.04.039 [DOI] [PubMed] [Google Scholar]
- 49.Szefler SJ, Murphy K, Harper T III, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017;140(5):1277-1287. doi: 10.1016/j.jaci.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 50.Hamelmann E, Bernstein JA, Vandewalker M, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017;49(1):1601100. doi: 10.1183/13993003.01100-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoshino M, Akitsu K, Ohtawa J. Comparison between montelukast and tiotropium as add-on therapy to inhaled corticosteroids plus a long-acting β2-agonist in for patients with asthma. J Asthma. 2019;56(9):995-1003. doi: 10.1080/02770903.2018.1514047 [DOI] [PubMed] [Google Scholar]
- 52.Ishiura Y, Fujimura M, Ohkura N, et al. Effect of triple therapy in patients with asthma-COPD overlap. Int J Clin Pharmacol Ther. 2019;57(8):384-392. doi: 10.5414/CP203382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishiura Y, Fujimura M, Ohkura N, et al. Triple therapy with budesonide/glycopyrrolate/ formoterol fumarate improves inspiratory capacity in patients with asthma-chronic obstructive pulmonary disease overlap. Int J Chron Obstruct Pulmon Dis. 2020;15:269-277. doi: 10.2147/COPD.S231004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jabbal S, Kuo CRW, Lipworth B. Randomized controlled trial of triple versus dual inhaler therapy on small airways in smoking asthmatics. Clin Exp Allergy. 2020;50(10):1140-1147. doi: 10.1111/cea.13702 [DOI] [PubMed] [Google Scholar]
- 55.Chiesi Farmaceutici . A multicentre, randomised, double-blind, active-controlled, 3-way cross-over study to evaluate the efficacy and safety of a free combination of 3 doses of CHF 5259 (glycopyrrolate) plus Foster 100/6µg (fixed combination of beclomethasone dipropionate plus formoterol) in a metered dose inhaler for the treatment of patients with uncontrolled asthma under medium doses of inhaled corticosteroids plus long-acting β2-agonists. EudraCT number: 2013-003043-36. Accessed December 16, 2020. https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-003043-36/results
- 56.Watz H, Hohlfeld JM, Singh D, et al. Letter to the editor: indacaterol/glycopyrronium/mometasone furoate compared with salmeterol/fluticasone propionate in patients with asthma: a randomized controlled cross-over study. Respir Res. 2020;21(1):87. doi: 10.1186/s12931-020-01349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerstjens HAM, Maspero J, Chapman KR, et al. ; IRIDIUM Trial Investigators . Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8(10):1000-1012. doi: 10.1016/S2213-2600(20)30190-9 [DOI] [PubMed] [Google Scholar]
- 58.Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69-84. doi: 10.1016/s2213600(20)30389-1 [DOI] [PubMed] [Google Scholar]
- 59.Pearl Therapeutics Efficacy and safety of PT001 to placebo and open-label Spiriva Respimat in subjects with persistant asthma. August 12, 2020. Accessed December, 16, 2020. https://clinicaltrials.gov/ct2/show/results/NCT03358147
- 60.Ohta K, Ichinose M, Tohda Y, et al. Long-term once-daily tiotropium Respimat is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS One. 2015;10(4):e0124109. doi: 10.1371/journal.pone.0124109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang K, Tian P, Fan Y, Wang Y, Liu C. Assessment of second-line treatments for patients with uncontrolled moderate asthma. Int J Clin Exp Med. 2015;8(10):19476-19480. [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y, Zhu J, Liu Y, et al. Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis. BMJ. 2018;363:k4388. doi: 10.1136/bmj.k4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, Huang G, Jin L, Han S. Therapeutic effects of a long-acting cholinergic receptor blocker, tiotropium bromide, on asthma. Med Sci Monit. 2018;24:944-950. doi: 10.12659/MSM.907950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donohue JF, Wise R, Busse WW, et al. Efficacy and safety of ipratropium bromide/albuterol compared with albuterol in patients with moderate-to-severe asthma: a randomized controlled trial. BMC Pulm Med. 2016;16(1):65. doi: 10.1186/s12890-016-0223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2013;(8):CD000060. doi: 10.1002/14651858.CD000060.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirkland SW, Vandenberghe C, Voaklander B, Nikel T, Campbell S, Rowe BH. Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma. Cochrane Database Syst Rev. 2017;1(1):CD001284. doi: 10.1002/14651858.CD001284.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blais CM, Davis BE, Cockcroft DW. Duration of bronchoprotection of the long-acting muscarinic antagonists tiotropium & glycopyrronium against methacholine-induced bronchoconstriction in mild asthmatics. Respir Med. 2016;118:96-101. doi: 10.1016/j.rmed.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 68.Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma: effect on bronchial responsiveness during and after treatment. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1170-1174. doi: 10.1164/ajrccm.152.4.7551366 [DOI] [PubMed] [Google Scholar]
- 69.Institute for Clinical and Economic Review . Biologic Therapies for Treatment of Asthma Associated With Type 2 Inflammation: Effectiveness, Value, and Value-Based Price Benchmarks. November 13, 2018. Accessed December 16, 2020. https://icer.org/wp-content/uploads/2020/10/Asthma-Revised-Report-FOR-PUBLICATION-11.13.2018.pdf
- 70.Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF, Pace W, Schatz M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program. 2020;324(22):2301-2317. doi: 10.1001/jama.2020.21974 [DOI] [PubMed] [Google Scholar]
- 71.Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1485-1496. doi: 10.1001/jama.2018.2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aziz-Ur-Rehman A, Dasgupta A, Kjarsgaard M, Hargreave FE, Nair P. Sputum cell counts to manage prednisone-dependent asthma: effects on FEV1 and eosinophilic exacerbations. Allergy Asthma Clin Immunol. 2017;13:17. doi: 10.1186/s13223-017-0190-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715-1721. doi: 10.1016/S0140-6736(02)11679-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search Strategies
eMethods 2. Additional Methods Details
eMethods 3. PRISMA Checklist
eTable 1. Summary of Findings
eTable 2. Inclusion/Exclusion criteria
eTable 3. Subgroup and Sensitivity Analyses
eFigure 1. Assessment of Risk of Bias Using Cochrane Collaboration Tool
eFigure 2. Funnel Plots
eFigure 3. Pooled Reported Hazard Ratios
eFigure 4. Asthma Worsening
eFigure 5. Serious and Non-serious Adverse Events
eFigure 6. All-cause Mortality – Frequentist Analysis
eFigure 7. Components of Adverse Events
eFigure 8. Trial Sequential Analyses