Summary
The α7 nicotinic acetylcholine receptor plays critical roles in the central nervous system and in the cholinergic inflammatory pathway. This ligand-gated ion channel assembles as a homopentamer, is exceptionally permeable to Ca2+, and desensitizes faster than any other Cys-loop receptor. The α7 receptor has served as a prototype for the Cys-loop superfamily yet has proven refractory to structural analysis. We present cryo-EM structures of the human α7 nicotinic receptor in a lipidic environment in resting, activated, and desensitized states, illuminating the principal steps in the gating cycle. The structures also reveal elements that contribute to its function, including a C-terminal latch that is permissive for channel opening, and an anionic ring in the extracellular vestibule that contributes to its high conductance and calcium permeability. Comparisons among the α7 structures provide a foundation for mapping the gating cycle and reveal divergence in gating mechanisms in the Cys-loop receptor superfamily.
Keywords: ligand-gated ion channel, cryo-EM, nicotinic receptor, α7, acetylcholine receptor, Cys-loop receptor, ion channel
In brief
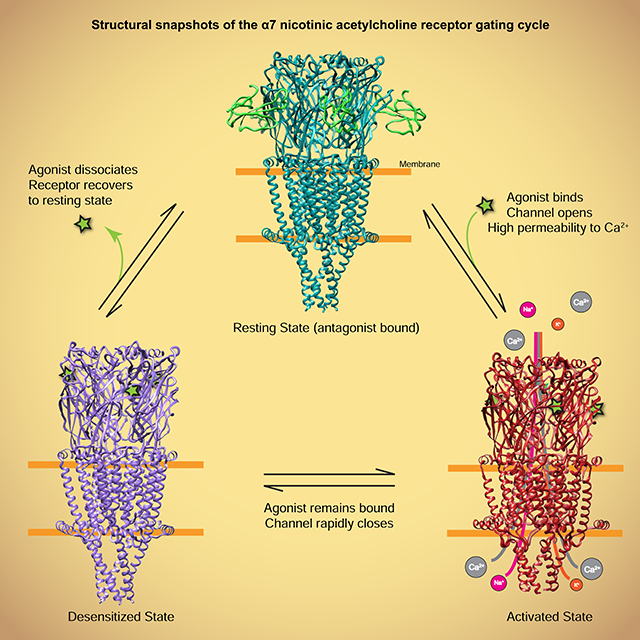
Cryo-EM structures of the α7 nicotinic acetylcholine receptor in three major conformational states of the gating cycle - resting, activated and desensitized - along with functional studies reveal components involved in calcium conductance and allosteric coupling of ligand binding to channel opening.
Graphical Abstract
Introduction
The α7 nicotinic receptor functions as a pentameric ligand-gated ion channel and plays diverse signaling roles in human physiology. In the central nervous system, α7 is usually found pre- or peri-synaptically. There it serves a largely modulatory function rather than directly mediating fast chemical neurotransmission like other Cys-loop receptor superfamily members (Berg and Conroy, 2002; Gharpure et al., 2020; Nemecz et al., 2016). Deficits in α7 function are linked to mental illnesses such as schizophrenia (Freedman et al., 1997), and degenerative conditions like Alzheimer’s disease (Leiser et al., 2009). In the cholinergic anti-inflammatory pathway, binding of ligands to α7 reduces inflammation (Wang et al., 2003) and may function in preventing the cytokine storm fatal during SARS-CoV-2 infection (Gonzalez-Rubio et al., 2020). In lung cell carcinoma, nicotine binding to α7 induces proliferation, angiogenesis, and metastasis (Wang and Hu, 2018).
The physiological roles of α7 are linked to its pharmacological and biophysical properties. It is the only nicotinic receptor to assemble physiologically as a homopentamer. α7 has relatively low affinity for the classic agonists acetylcholine and nicotine, and desensitizes much faster than other nicotinic receptor subtypes, a feature that may be balanced by its higher relative calcium ion permeability (Castro and Albuquerque, 1995; Seguela et al., 1993). The influx of calcium through α7 is associated with activation of voltage-dependent calcium channels in neurons (Dajas-Bailador et al., 2002) and stimulation of anti-inflammatory pathways in the immune system (Zdanowski et al., 2015). The rapid desensitization of α7 prevents excess calcium from entering the cells and allows fine-tuning of neuronal activity (Bouzat et al., 2008).
Here we present structures of the α7 nicotinic receptor in three conformational states: a resting-like closed-channel state in complex with the antagonist α-bungarotoxin (α-bgt); an activated, open-channel state stabilized by the agonist epibatidine and the positive allosteric modulator PNU-120596; and a desensitized, closed-channel state in complex with epibatidine alone. These structures provide a framework for probing α7’s unique biophysical and pharmacological properties and allow us to compare the gating mechanism of α7 with those in other Cys-loop receptors. We identify a structural element at the C-terminus that we call the “latch,” and pinpoint residues in this substructure required for coupling ligand binding to channel gating. We further discover an anionic residue in the extracellular domain required for the high unitary conductance and calcium permeability seen in α7 nicotinic receptors. Finally, we observe a structural interaction that may contribute to rapid desensitization inherent to α7.
Our structural understanding of gating mechanisms in Cys-loop receptors is far from complete. Mammalian Cys-loop receptors include the cation-selective nicotinic and 5-HT3 receptors, and the anion-selective GABAA and glycine receptors (Gharpure et al., 2020; Nemecz et al., 2016). Like most ligand-gated ion channels, Cys-loop receptors cycle among three major conformational states: an apo closed-channel resting state; an agonist-bound open channel activated state; and an agonist-bound closed-channel desensitized state. To date, high resolution structures of nicotinic receptors have only been obtained in desensitized and resting-like states (Gharpure et al., 2019; Rahman et al., 2020). Among Cys-loop receptors, only the anion-selective glycine receptor has been structurally characterized in the principal gating cycle conformations (Du et al., 2015; Kumar et al., 2020; Yu et al., 2021). The 5-HT3 family lacks a structural reference for a desensitized state and the two published open states are markedly different from each other (Basak et al., 2019; Basak et al., 2018a; Basak et al., 2018b; Basak et al., 2020; Hassaine et al., 2014; Polovinkin et al., 2018). The GABAA family lacks an activated-state structure (Kim et al., 2020; Masiulis et al., 2019; Miller and Aricescu, 2014; Phulera et al., 2018). As such, it is currently unclear whether gating mechanisms are conserved across the superfamily. This study on α7 provides structural touchstones for the gating cycle of the cationic branch of the superfamily. We find that conformational transitions underlying activation and desensitization in α7 diverge from those in the glycine receptor, suggesting distinct gating mechanisms in the Cys-loop receptor superfamily.
Results and Discussion
Biochemistry and Structure Determination
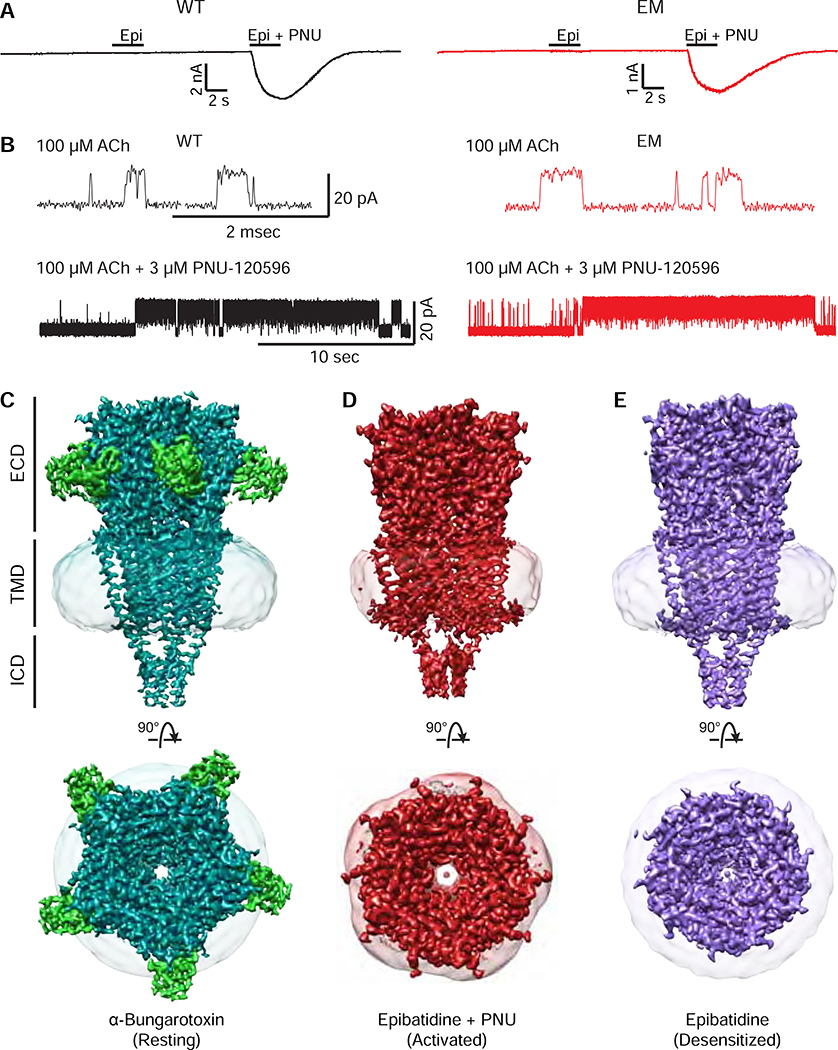
The homopentameric nature of the α7 nicotinic receptor made it a logical target for structural analysis. However, even after three types of heteropentameric nicotinic receptors were determined, α7 remained refractory to structure determination (Gharpure et al., 2019; Morales-Perez et al., 2016b; Rahman et al., 2020; Walsh et al., 2018). We modified WT α7 as described in Methods and Figures S1 and S4, and performed whole-cell and cell-attached single-channel electrophysiology experiments to measure channel function (Figure 1A, B and S2, refers to Figure 1). These experiments confirmed our electron microscopy (EM) α7 construct retains functional hallmarks of wild-type (WT) α7, including potentiation by PNU-120596 (Figure S2). The single channel conductance, though similar, was 14% higher for the EM construct.
Figure 1: α7 nicotinic receptor structures and function.
A, Whole-cell patch-clamp electrophysiology of WT α7 (black) vs. the EM construct (red). B, Single-channel electrophysiology, comparing responses of acetylcholine with and without PNU-120596. C, D, and E, cryo-EM maps of the α7 receptor (teal) bound to α-bungarotoxin (green), epibatidine + PNU-120596 (red), and epibatidine alone (purple). Top panels show the side view of maps normal to the plane of the cell membrane. Bottom panels show the top view looking down the central axis of the pore. The nanodisc shell is represented as a semitransparent surface.
We utilized high-affinity ligands to resolve different steps of the gating cycle by cryo-EM (Table S1, refers to Figures 1, S3, and S4). We selected the competitive antagonist, which yielded a map with an overall resolution of 3.0 A and a closed pore consistent with a resting state (Figure 1C; Figure S3A, refers to Figures 1C and S4A) (Moore and McCarthy, 1995). A combination of ligands was required to obtain the structure of an activated, open-channel state (Figure 1D). Epibatidine, one of the most potent α7 agonists (Gerzanich et al., 1995), and the positive allosteric modulator PNU-120596 (Hurst et al., 2005) produced a map of the receptor with an open pore at an overall resolution of 2.7 A (Figure 1D, Figure S4B, refers to Figure S3B and Figure 1D). Local resolution of this structure varied considerably between the extracellular domain (ECD), transmembrane domain (TMD), and intracellular domain (ICD) (Figure S3B, refers to Figures 1D and S4B). Epibatidine alone stabilized a desensitized state with an overall resolution of 3.6 A and a closed pore (Figure 1E; Figure S3C, refers to Figures 1E and S4C).
The three receptor structures adopt an overall cylinder shape in the ECD and TMD, with an intracellular domain (ICD) that tapers to a blunt point at its intracellular end. Cation permeation occurs down through the extracellular vestibule and generally narrower TMD pore before exiting through lateral portals framed by the ICD. The overall architecture is consistent with known structures in the Cys-loop receptor superfamily, though anion selective family members lack the ICD elements seen in nicotinic and 5-HT3 receptors. In the following sections, we first analyze architecture and conformational differences by receptor domain (Figure S4, refers to Figures 1–7 and Figures S5–6). We start with the ECD, where neurotransmitters and orthosteric ligands bind at subunit interfaces. We next move to the coupling region, which connects the ECD to the TMD, and test the functional role of the C-terminal latch. We then analyze conformational transitions in the TMD and ICD and functionally probe a determinant of ion permeation. Within each section we explore interactions at the amino acid level that contribute to the receptor’s movements during the gating cycle, concluding with an overall structure-based gating mechanism.
The Neurotransmitter-binding Domain
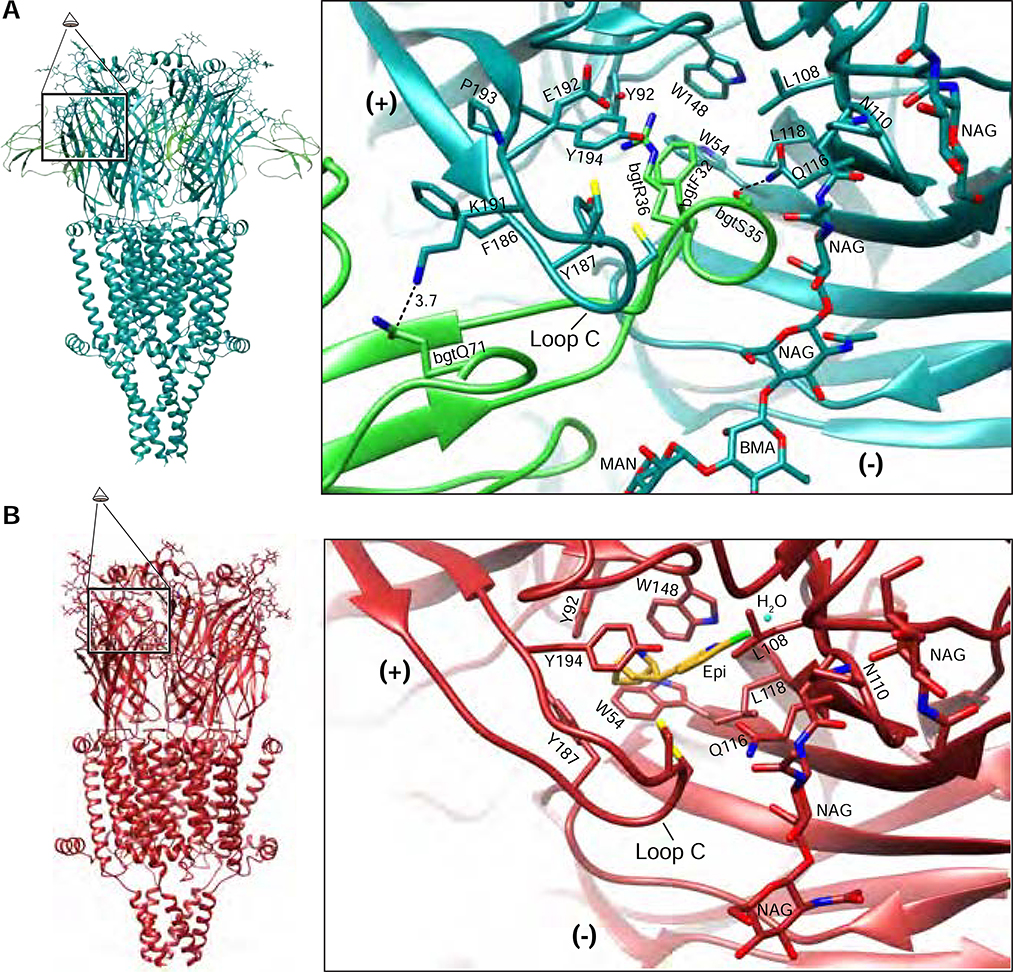
We determined the structure of the α7 receptor bound to α-bgt to probe the structure of a resting channel state (Figures 1C, 2A). α-Bgt is a venom component from Bungarus multicinctus, the Taiwanese banded krait. It contains three “fingers” formed by β-strands pinned together by five disulfide bonds. Finger II of α-bgt penetrates deeply into the neurotransmitter binding pocket (Figure 2A), with one flank against the complementary or (−) face of the binding site, and the other against Loop C of the principal or (+) face. Loop C in turn nestles between fingers I and II of α-bgt. Among nicotinic receptors, only the α7 and muscle subtypes bind α-bgt. Comparison of the α7-α-bgt structure to recent structures of α-bgt bound to the Torpedo nicotinic receptor (Rahman et al., 2020) or bound to a soluble α7-like ECD (Huang et al., 2013) provides insight into interactions that confer toxin affinity and subtype selectivity (Figure S5A–B, refers to Figure 2A). The toxin penetrates more deeply in the α7 neurotransmitter binding pocket than in that of Torpedo, due to the larger Loop F of Torpedo (Figure S5A, refers to Figure 2A). At the tip of toxin finger II, bgtR36 and bgtF32 form a cation-π stack to which Y187 aligns in an edge-to-face orientation (Figure 2A). This Loop C tyrosine is essential for high binding affinity (Huang et al., 2013). In the space between α-bgt fingers I and II, three residues, F186 and P193 from Loop C and G152 from Loop B, form a triad that confers toxin selectivity for α7 and muscle receptors (Sine et al., 2019). The α7 structure reveals an extensive hydrogen bond network between bgtQ71 and K191, E192, Y194 and G152 of the receptor that may further stabilize toxin binding. An N-linked glycan projects from N110 on the complementary subunit to form a hydrogen bond with the backbone of bgtS35, which also interacts electrostatically with Q116 of the receptor. Thus, the structure of the complex between α7 and α-bgt reveals interactions important for toxin affinity and subtype selectivity.
Figure 2: Neurotransmitter-binding pocket.
A, α-Bungarotoxin-bound (resting) α7 model on left; perspective magnified on right is indicated. α-Bgt is shown in green, receptor in teal. Key interacting residues from the toxin and the receptor are shown. B, Epibatidine + PNU-bound (activated) α7 model on left; perspective magnified on right is indicated. Epibatidine is shown in gold with coordinating residues shown. (+) indicates the principal subunit of the interface and (−) indicates the complementary subunit.
The activated and desensitized state structures of α7 are both bound by epibatidine and adopt similar conformations in the neurotransmitter binding pocket (Figure 2B). We discuss the higher resolution activated-state co-complex with PNU-120596 here. Within the neurotransmitter binding pocket, Loops A-F contribute aromatic and/or hydrophobic residues that nestle small ligands. Comparisons of epibatidine-bound α7 to other agonist-bound nicotinic receptors, such as α4β2 and α3β4 (Figure S5C–E, refers to Figure 2), show that positions of conserved aromatic residues are maintained. However, the bridged, basic nitrogen in epibatidine orients away from W148 on loop B and instead positions to form a cation-π interaction with the second tyrosine on loop C (Y198). This finding is consistent with unnatural amino acid substitutions, which showed that unlike most agonists bound to nicotinic receptors, epibatidine is predicted to form a strong cation-π interaction with Y194, and not with W148 (Puskar et al., 2011; Xiu et al., 2009). The conformational transition of the ECD from the resting state to the activated state involves closure of Loop C, as well as a larger-scale twist of the whole β sandwich (Movie S1). This twist is counterclockwise around an axis approximately normal to the membrane, which contributes to Loop C closure. A tilting of the apex of the ECD toward its complementary subunit adds to the twist; these conformational changes propagate toward the membrane. The conformational transition in the ECD from activated to desensitized involves a subtle global expansion of that domain (Movie S2).
The Coupling Region
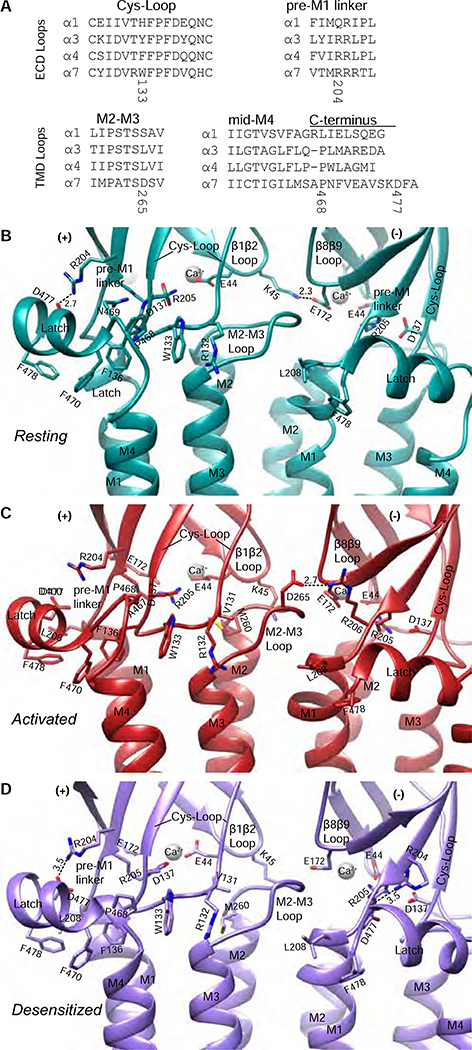
The movement of the ECD induced by agonist binding results in a reorganization of electrostatic and hydrophobic interactions in the coupling region at the ECD-TMD junction (Figure 3 and Movie S1). On the vestibule or channel-axis side of the coupling region, the β1-β2 loop and the β8-β9 loop form a subunit interface that changes binding partners during the gating cycle. The eponymous Cys-loop forms a hub connecting all coupling region elements, from the ECD vestibule loops to the M2-M3 loop of the TMD to the peripheral C-terminal latch. The flexible linker between β10 and the M1 helix, known as the pre-M1 linker, is positioned at the periphery of the receptor, where it also participates in coupling. Alignments and locations of the loops and linkers that compose the coupling region are shown in Figures 3 and S4.
Figure 3: Coupling region interactions and conformational differences.
A, Alignments of amino acids from α subunits in the nicotinic receptor family for coupling regions. B-D, The coupling region in resting (B), activated (C), and desensitized (D) conformations. Dashed lines indicate electrostatic interactions with distances in Angstrom (Å).
The vestibule side of the coupling region exerts direct effects upon the transmembrane pore during the gating cycle. In the resting state, K45 in the β1-β2 loop forms an inter-subunit salt bridge with E172 in the β8-β9 loop (Figure 3B). This contact is severed by the ECD tilt that occurs during activation and remains severed in desensitization. The K45 side chain liberated during activation pivots with its β1-β2 loop to form an intra-subunit interaction with the carbonyl oxygens of M260 and A257 at the extracellular end of M2 (Figure 4C, Movie S5). This helical capping remains intact during desensitization and may play a role in maintaining the extracellular end of M2 in a more open conformation during desensitization (Movie S5, Figure 4C). The importance of these coupling region interactions is underscored by mutations of K45 or E172 that abolish channel opening (Sala et al., 2005; Zhang et al., 2015).
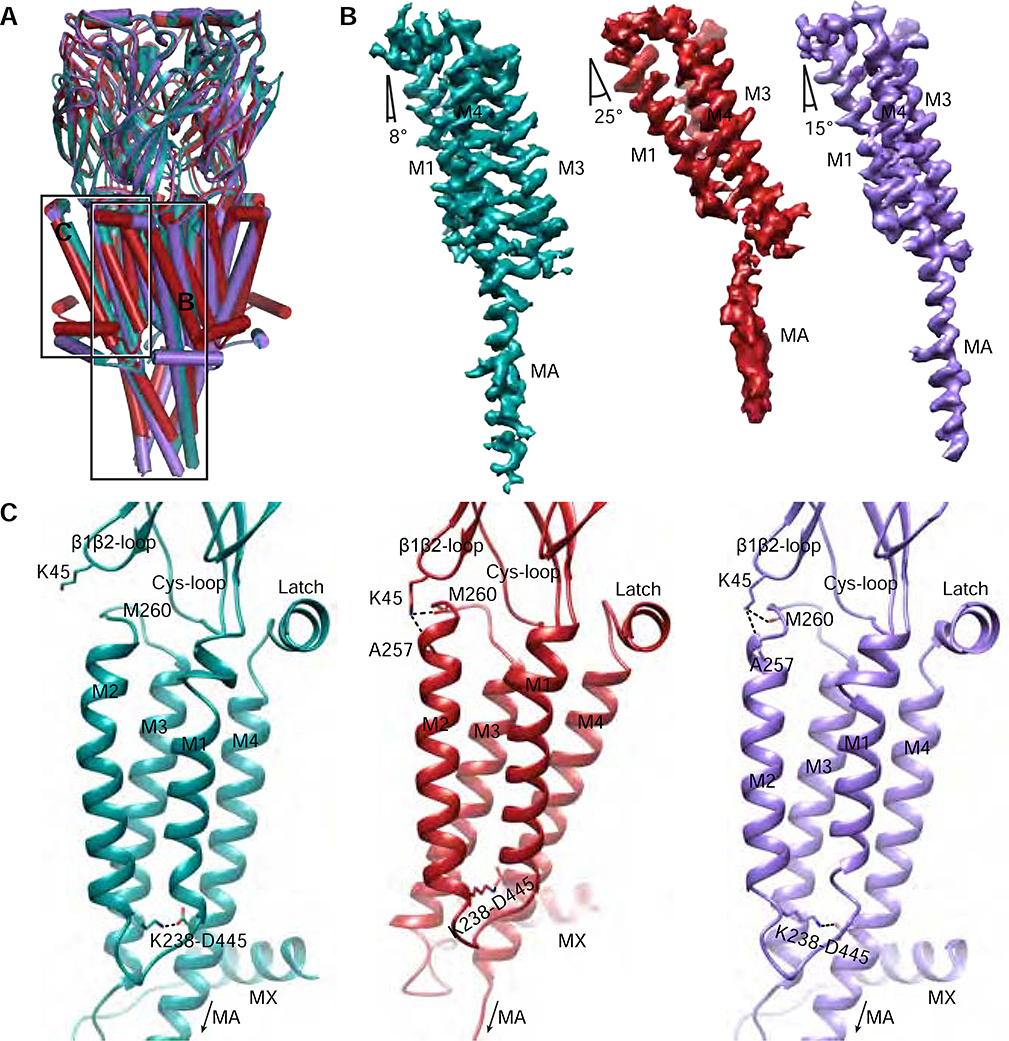
Figure 4: Conformational transitions in α7 transmembrane and intracellular domains.
A, Global superposition of the three α7 structures. α-Helices are represented as cylinders. Teal, resting. Red, activated. Purple, Desensitized. Boxed areas indicate regions in panels B-C. B, Experimental density maps aligned by their ECD showing tilting of M1, M3 and M4 helices from a single subunit from each structure. The Mx density has been removed for clarity. C, the TMD helix bundle as viewed from the pore. The M2-M4 salt bridge between K238 and D445 is indicated. K45 capping of the M2 helix (dashed lines) is shown in the activated (red) and desensitized (purple) states.
Interestingly, we observed a strong ball-shaped density coordinated by 4 carboxylate-containing side chains in the coupling region of each subunit: D41, E43 and E44 from the β1-β2 loop, and E172 from β8-β9 (Figure 3B–D). We modeled these densities as Ca2+ due to the strongly negative electrostatic environment and roles of E44 and E172 in the effects of Ca2+ on α7 agonist affinity and potentiation (Galzi et al., 1996). The five putative Ca2+ densities are present in all structures but are most prominent in the activated state.
The Cys-loop forms a central and conserved coupling element (Nemecz et al., 2016). A triad of electrostatic interactions provides architectural stabilization present in all three conformational states: D137 of the Cys-loop forms a salt bridge with the conserved R205 of the pre-M1 linker, which in turn forms a salt bridge with the same E44 that contributes to the Ca2+ site (Figure 3C–D, (Galzi et al., 1996; Lee and Sine, 2005; Mukhtasimova and Sine, 2013)). During activation, V131 of the Cys-loop shifts to make hydrophobic interactions with M260 in the M2-M3 loop. During desensitization, this interaction is maintained and may also contribute to keeping the extracellular end of M2 more open in concert with the K45-M2 helix capping.
The periphery of the coupling region is coordinated by interactions between the pre-M1 linker, the Cys-loop, and a C-terminal latch that follows M4. The extracellular C-termini of Cys-loop receptors are poorly conserved in sequence (Figure 3A) and are usually structurally disordered. The α7 receptor cryo-EM maps revealed that just after M4 exits the membrane, the helical structure breaks and makes a 90° turn. It then forms a short C-terminal amphipathic helix running parallel to the membrane. We call the sub-structure encompassing the C-terminal cap of M4, the 90° turn, and the following helix “the latch” as it fastens the C-terminus to the coupling region.
The Cys-loop – latch interactions change dramatically during the gating cycle (Figure 3B–D). In the resting state, W133 of the Cys-loop interacts with P468 of the latch via CH-π interactions; this corresponds to an interaction proposed between F167 and P617 in the α4 subunit (α4P617 corresponds to α7 S466 in Figure 3A) that is associated with allosteric potentiation (Deba et al., 2018). W133 is stabilized on its other side by cation-π interactions with R132. In the middle of the latch helix, F470 forms aromatic interactions with F136, which is part of the Cys-loop FPF motif (Jha et al., 2007; Lee et al., 2009). The backbone carbonyl of F136 also makes an electrostatic contact with N469 of the latch in the resting state. During activation, the Cys-loop responds by moving away from the complementary subunit in the interface. This transition disrupts interactions between the Cys-loop and the latch; A467 forms weaker hydrophobic interactions with W133 in place of P468, and R132 rotates down to cap the M3 helix. During desensitization, the Cys-loop moves back toward its position in the resting state, reestablishing the strong hydrophobic interactions with the C-terminal latch. Unsurprisingly, all of the residues of the Cys-loop mentioned here have been implicated in achieving maximal response to agonists (Aldea et al., 2007; Grutter et al., 2005; Jha et al., 2007; Lee et al., 2009; Yan et al., 2015; Zhang et al., 2011). Toward the end of the latch, there is a hydrophobic interaction between L208 of the M1 helix and F470 of the latch that appears to be maintained in all states. This latter interaction is intriguing, as mutation of the corresponding leucine in the muscle nicotinic receptor subunits increased open-channel dwell times (Lee et al., 2009).
The pre-M1 linker is the final component of the coupling region, and comprises a trio of consecutive arginines (R204-R206) that play distinct roles during activation (Alves et al., 2011; Lee et al., 2009; Lee and Sine, 2005; Mukhtasimova and Sine, 2013; Vicente-Agullo et al., 2001; Zhang et al., 2015). R204 orients to form an electrostatic interaction with D477 near the end of the latch; this contact is disrupted during activation and then restored during desensitization. R205 is a major contributor to architectural stability, as noted above, and is the most conserved of the triad. It interacts with three anionic residues (E44, E172 and D137). These contacts are part of the principal coupling pathway and are required for efficient activation (Mukhtasimova and Sine, 2013); the structures show that the contacts with E44 and D137 are maintained in the resting, active and desensitized states. Finally, R206 forms a strong electrostatic interaction with D265 of the M2-M3 loop of the neighboring subunit during activation (Figure 3C). As mutation of D265 is known to abolish channel activity, this inter-subunit interaction may contribute to efficient channel gating (Campos-Caro et al., 1996; Zhang et al., 2011).
The Latch Turn is Essential for Gating
To investigate the functional significance of the Cys-loop-latch interaction, we designed point mutants and truncations and monitored assembly, trafficking, ligand binding and macroscopic currents. We analyzed these mutations in WT α7 that has an M3-M4 loop YFP insertion (Wang et al., 2014) to rapidly screen for assembly by fluorescence-detection size-exclusion chromatography (Kawate and Gouaux, 2006). We assayed total expression by binding to radiolabeled epibatidine, which can cross the cell membrane. Cell-surface expression was measured by binding of radiolabeled α-bgt, which cannot cross the membrane. We tested responses to nicotine alone, and nicotine + PNU-120596, via whole-cell patch-clamp electrophysiology. Finally, we performed radioligand binding assays measuring the ability of acetylcholine to compete against α-bgt binding. The results are discussed below and summarized in Table S2.
We made six C-terminal truncations and five point mutations for residues on both sides of the Cys-loop – latch interaction (Figure S1B, Table S2, both refer to Figure 3). Mutation of P468 to alanine or deletion of A467 abolished function of the α7 nicotinic receptor without a loss of expression or trafficking to the cell surface. Binding analysis of P468A and ΔA467 revealed decreases in both agonist affinity and Hill slope (Table S2, last two columns). The P468A mutant was shown to be functional in previous work done in the context of a pore mutation that slows desensitization and increases open channel probability (da Costa Couto et al., 2020). Our results are consistent with P468 and A467 in the latch turn being essential for coupling agonist binding to channel gating; when the energetic barrier to gating is lowered, the loss of function can be overcome (Zhang et al., 2011). Replacement of the α7 C-terminal helix (all residues after P468) with the α4 subunit C-terminus prevented pentamer formation (Figure S1B, refers to Figure 3 and Table S2), suggesting a role for the C-terminal latch in assembly. Indeed, replacement of the C-terminal helix of α7 with the 8 amino acid Strep-tag diminished pentamer expression, while removal of the affinity tag, as well as all amino acids following the P468 turn, resulted in functional receptors and relieved the block on expression (ΔC4 in Table S2 removes all of the C-terminus after P468). Surprisingly, if P468 in the latch turn and its preceding sequence are maintained, the following C-terminal helix appears dispensable for function.
Transmembrane and Intracellular Domains
To visualize movements of the TMD during the gating cycle, we first aligned the models globally and conducted structural comparisons (Figure 4A, Movies S1, 3–4). Map and model comparisons suggest that the M1, M3 and M4 helices tilt in concert during activation. To quantify large movements that give rise to activation and desensitization, we aligned the maps of individual subunits by their ECD to observe relative displacements in the TMD (Figure 4B). We then measured the tilt angle of each helix individually relative to the membrane normal (see Methods). While approximate, the measurements revealed important trends. M1, M3, and M4 tilt from 8° degrees in the resting state to 25° degrees in the activated state, and then relax to 15° degrees in the desensitized state (Figure 4B, Movie S3). The influence of the coupling region during these movements affects both M1, which is pushed by the pre-M1 linker, and M4, which tracks the Cys-loop position through its interaction with the latch. During activation the TMD tilt results in M4 sliding up 4 residues, or approximately one helical turn, along M3 (Movie S3). The consequence of this bundle tilt and M4 slide is a shift of the tethered M2 helix away from the central axis, opening the pore (Movie S4).
The M2 helix movements are regulated by interactions at its intracellular and extracellular ends. At the intracellular end, a strong intramembrane salt bridge between K238 on M2 and D445 on M4 is maintained in all three conformations, linking the movement of these helices (Figure 4C). D445 and the corresponding residue from the 5-HT3A receptor were shown to be important for assembly (da Costa Couto et al., 2020; Mesoy et al., 2019). Tilting of the M1-M3-M4 bundle during activation pulls M2 outward via the M2-M4 salt bridge. The extracellular end of M2 is secured in the activated and desensitized states through its intra-subunit interaction with K45 of the β1-β2 loop (Figures 3C,D, 4C and Movie S5). The intracellular end of M2 moves inward during desensitization, tracking the other helices of the TMD in returning to a more resting-like conformation. The M2 capping interaction with the β1-β2 loop remains intact (Figure 4C), preventing closure of the extracellular end of M2 until agonist leaves the binding pocket, allowing the ECD to rotate back to its resting position.
The TMD is directly connected to the ICD via the M3 and M4 helices. The ICD comprises two helices per subunit, not conserved in anionic Cys-loop receptors, termed MX and MA, connected by a large disordered loop (Figure S4, refers to Figures 1–5 and Figures S5–6). The ICD is involved in protein expression and trafficking (Rudell et al., 2014; Srinivasan et al., 2011; Xu et al., 2006). The cytosolic apex of MA acts as a structural anchor for the ICD, and contains residues important for upregulation of α7 by anti-apoptotic Bcl-2 proteins (Dawe et al., 2019). The ICDs in cation-conducting members of the Cys-loop superfamily are also involved in tuning conductance (Gharpure et al., 2019; Hales et al., 2006; Kelley et al., 2003). In the resting and desensitized states, the ICD MX and MA helices adopt conformations similar to those in the resting state of the Torpedo receptor and the desensitized state of α3β4 (Gharpure et al., 2019; Rahman et al., 2020). During activation, however, the upward shift of the M4 helix places a strain on the MA helix, resulting in a partial unwinding at the MA-M4 junction (Figure S4B, refers to Figure 1D, and Movie S3). This shift in M4 was also seen with 5-HT3A receptors (Basak et al., 2018a; Polovinkin et al., 2018). In sync with these changes, the M3-MX linker and MX helix tilt up into the inner leaflet of the membrane as well. These changes result in ion-conducting portals that are more widely open during activation, and then constrict again during desensitization.
Figure 5: Ion permeation pathway.
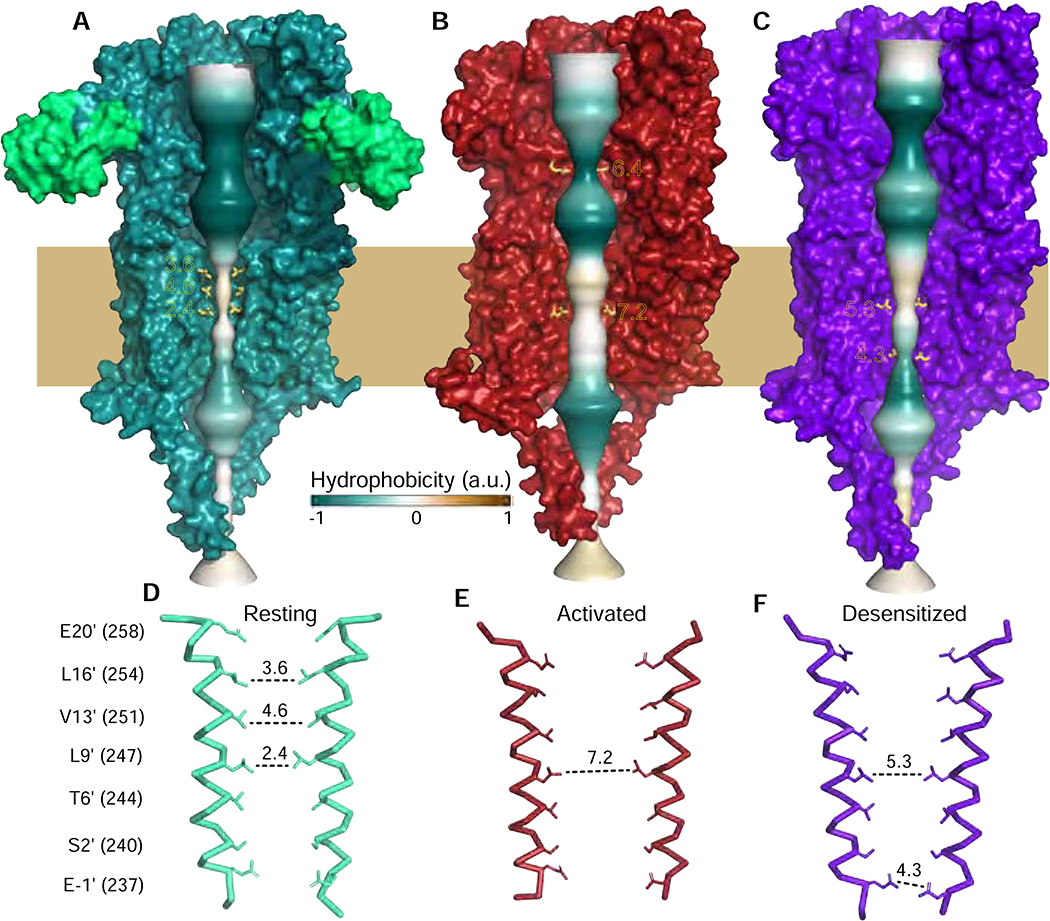
A-C, permeation pathways for the three conformational states represented by solid surface colored by hydrophobicity. Constriction points are indicated with distances in A. D-F, Two M2 helices are shown for each of the conformational states with side chains shown for pore-lining residues, numbering on the left. Diameters (A) are indicated with dashed lines.
The Permeation Pathway in the Gating Cycle
The permeation pathway in Cys-loop receptors extends from the vestibule of the ECD, to the intrinsic membrane channel of the TMD formed by the M2 helices, and through the lateral ICD portals. Examination of this pathway in the gating cycle steps revealed several surprising discoveries that were not predictable from homology modeling.
The α-Bgt-bound α7 structure reveals a widely-open ECD vestibule, 11.2 Å in diameter at its narrowest point. Within the extracellular half of the TMD a series of hydrophobic residues produces a constriction sufficiently narrow to prevent ion flux. This hydrophobic gate comprises residues on successive helical turns of M2 (16′ leucine, 13′ valine and 9′ leucine; Figure 5A, D; prime numbering refers to position above the cytosolic end of the pore). L9′ is highly conserved among eukaryotic Cys-loop receptors and is thought to form the resting-state gate (Figure S6A, refers to Figure 5, and (Labarca et al., 1995)). However, both α7 and the Torpedo receptor are additionally constricted above L9′ at the 13′ and 16′ positions, similar to findings from simulations of the α4β2 nicotinic receptor (Yu et al., 2019). Early studies on residues lining the channel identified polar mutations at 9′, 13′, and 16′ that allow the receptor to adopt a “high-affinity open state” that desensitizes more slowly than the wild-type α7 receptor (Bertrand et al., 1993; Galzi et al., 1992; Revah et al., 1991; Zhang et al., 2011). These residues likely contribute to stabilization of the resting channel conformation. Thus, the α7 + α-bgt structure represents a resting, activatable state in the α7 gating cycle.
The activated conformation of the receptor pore is also straightforward to interpret (Figure 5B, E). The diameter of the transmembrane pore near L9’ increases to 7.2 Å (Figure 5B, E), which is close to the 7.4 Å - 8.4 Å range of open channel diameters estimated for muscle-type nicotinic receptors (Cohen et al., 1992; Dwyer et al., 1980; Wang and Imoto, 1992). However, the side chain of L9′ is not well ordered in the activated state. We suspect L9′ rotates in and out of the pore during ion flux, as suggested by MD simulations of the 5-HT3A receptor (Polovinkin et al., 2018). The conformation of the M2 helices in the α7 activated-state structure more closely resembles that of the 5-HT3A receptor 6HIN and glycine receptor 6UD3 than of the 5-HT3A receptor 6DG8 (all assigned as open states; PDB accession codes indicated; Figure S6B, refers to Figure 5). In the ECD, we were surprised to observe a constriction at the position of E97 (6.4 A) that was narrower than that seen in the transmembrane pore. We examine the contributions of this residue to the α7 permeation pathway below.
The permeation pathway of the desensitized-state structure is not as constricted in the ECD as it is in the open state. Its TMD pore is funnel-shaped, with a polar constriction at the −1′ position at the intracellular end (Figure 5C, F). In addition to this constriction, which was observed in α3β4 and α4β2 (Figure S5C, (Gharpure et al., 2019; Morales-Perez et al., 2016b; Walsh et al., 2018)), the pore is partially constricted at the 9′ position (Figure S6C, refers to Figure 5). The channel diameter and hydrophobicity at L9′ present a potential barrier for hydrated cations. While the L9′ residue contributes to the resting gate in Cys-loop receptors studied so far, it contributes uniquely to the desensitization gate in α7; hydrophobic substitutions may destabilize both states, thus favoring the active state (Revah et al., 1991). As this constriction at L9′ is not seen in other desensitized-state structures, we suggest that one component of the uniquely rapid desensitization of α7 may involve partial closure of this L9′ gate, with additional contributions from the ECD (Gay et al., 2008; McCormack et al., 2010) and coupling regions (Bouzat et al., 2008).
E97 in the Extracellular Vestibule Regulates Calcium Permeation
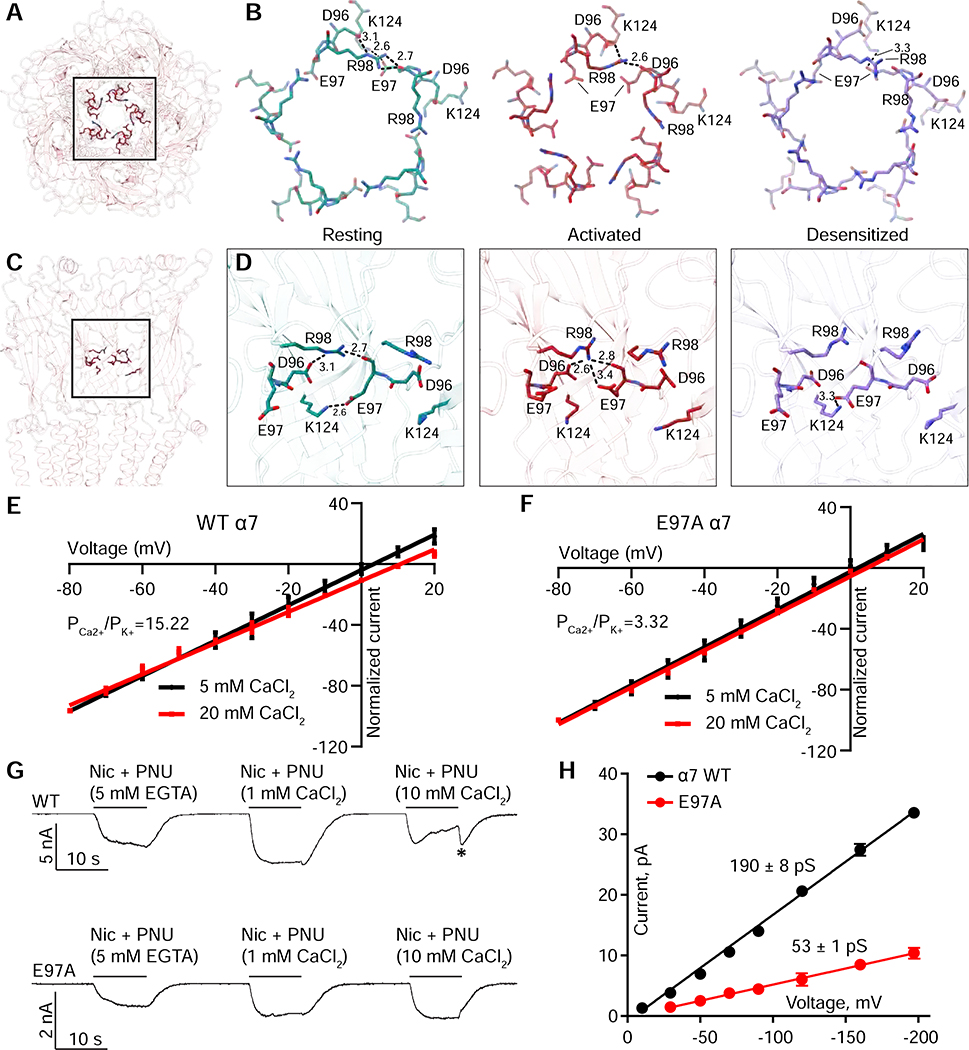
A striking feature in the activated α7 structure is a 6.4 Å constriction in the ECD formed by the side chains of E97 (Figures 5B, 6A,B, Figure S7A, refers to Figures 5–6). E97 is not conserved; it is either an aspartate (in α9) or a glycine in all other nicotinic receptor α subunits. Mutation of this residue in the bovine α7 nicotinic receptor modestly decreased agonist binding and peak macroscopic current (Criado et al., 2008). The preceding aspartate (D96) is highly conserved and contributes to ion selectivity in muscle nicotinic and 5-HT3 receptors (Hansen et al., 2008; Livesey et al., 2011); however, in all three α7 structures, D96 is engaged in a strong electrostatic interaction with R98 (Figure 6B,D).
Figure 6: Structural and functional interrogation of ECD vestibule constriction.
A, Top view of α7 in an activated conformation, highlighting residues involved in ECD constriction. B, amino acids related to the ECD constriction shown from the three receptor conformations. Residues and distances (A) are shown for one subunit interface. C, Side view showing three subunits from the perspective within the ECD vestibule, highlighting the same residues as in A and B. D, Orientation from C, focusing on interactions at one subunit interface and their differences as a function of conformational state. E, F, Current-Voltage (IV) relationships for two concentrations of CaCl2 are shown. G, Whole cell recordings in the absence and presence of calcium. Asterisk (*) indicates tail current seen from calcium-induced channel block. H, Single channel current-voltage relationships for WT and E97A. Lines are least squares fits with slope conductance in units of pS indicated.
In the resting state, E97 forms an inter-subunit salt bridge with K124 (Figure 6B,D). Tilting of the ECD during activation ruptures this interaction and E97 re-orients toward the central axis, ideally positioned to affect ion permeation. In the desensitized state, E97 resumes its interaction with K124. Thus, we sought to determine if E97 plays a role in the remarkably high calcium permeability of α7 (Seguela et al., 1993), and whether it contributes to ion permeation.
To investigate contributions of E97 to calcium permeability, we measured current-voltage (IV) relationships and calculated reversal potentials for WT and mutant receptors at two different calcium concentrations (Figure 6E–F). At both concentrations, WT receptors exhibited higher reversal potentials than E97A, suggesting that the mutant was less permeable to calcium. We used the change in reversal potentials to calculate permeability ratios (PCa2+/ PK+) and found that the E97A mutation resulted in a >75% reduction in calcium permeability. To further probe the effect of this mutation, we used a calcium-free extracellular solution and co-applied agonist/PAM with different concentrations of calcium (Figure 6E). In WT channels, the application of high calcium concentrations resulted in substantial channel block, an effect that was abolished by the E97A mutant (Figure 6G). Furthermore, the mutation E97A reduced the amplitudes of the unitary currents, corresponding to a decrease in conductance of 72% compared to WT (Figure 6H). Together, these experiments demonstrate that E97 facilitates access of both calcium and monovalent cations to the pore.
To test whether the narrowest constriction in the open channel is formed by E97, we measured IV relationships for both WT and the E97A mutant for a series of organic cations that varied in size (Figure S7, refers to Figure 6). For cations whose ionic diameters are predicted to be much smaller than the constriction seen at E97 (Cohen et al., 1992), there were no differences in reversal potential between WT and E97A receptors, nor were there differences in the calculated permeability ratio of the test cations relative to K+ (Figure S7C–E, refers to Figure 6). However, for larger cations with predicted diameters > 6.4 Å, WT and E97A receptors showed qualitative differences (Figure S7F–H, refers to Figure 6). At low voltages, there was a distinct non-linearity in the IV relationship for WT that was less pronounced for the E97A mutant. While these experiments do not allow us to definitively conclude that E97 contributes to size discrimination in the α7 pore, they do further support the idea that this residue interacts with and modulates the permeation pathway.
α7 Structural Gating Mechanism
Stepping back from the detailed atomic-scale interactions and how they reorganize during state transitions, here we summarize the large-scale changes in the basic gating cycle of α7. We encourage readers to view Supplementary Movies S1–2 and S5, which illustrate the process more effectively than 2D images.
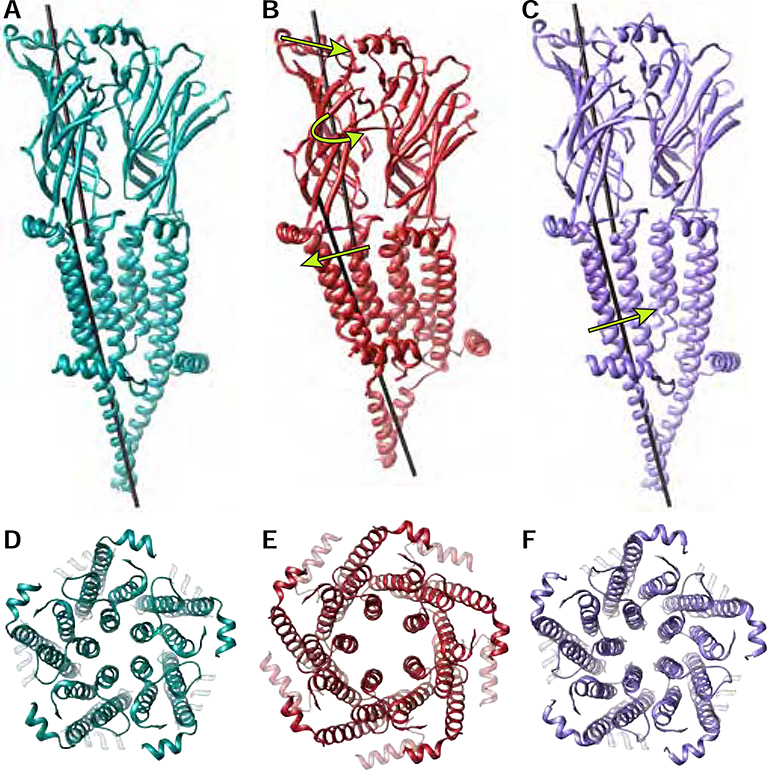
The agonist epibatidine binds to subunit interfaces in the α7 receptor ECD, which induces a closure of Loop C and a twisting of each subunit’s ECD β-sandwich (Figure 7A–B). This twist combines with a tilting of the apex of each subunit toward its complementary neighbor. The overall effect is a more compact ECD, transitioning from resting to an activated state. The twist and tilt produce conformational changes in the coupling region at the ECD-TMD junction, forcing the hydrophobic TM helices to tilt in the plane of the membrane and M4 to slide along M3 (Figure 7B). The collective motions result in expansion of the TMD, with the M2 helices translating away from the channel axis to open the pore (Figure 7D–E). The slide of M4 causes a local unwinding of the helix at the M4-MA junction that is present only in the open state. (Figure 7B, Movie S3). This partial unwinding at the M4-MA junction, reminiscent of a stretched spring, allows for widening of the intracellular portals through which ions permeate in the activated state.
Figure 7: Global conformational changes during the nicotinic receptor gating cycle.
A-C, Two-subunit interfaces of the resting, activated and desensitized states are shown with black rods drawn down the axis of the center of mass of the ECD or TMD of the principal subunit. Arrows indicate motions undergone from resting to activated (B) and activated to desensitized (C) states. D-F, top down view of the TMD pore of resting (D), activated (E) and desensitized (F) states.
α7 nicotinic receptors are unusual in that the open state is rarely populated in the presence of agonist. At the single channel level, channel openings appear as brief pulses with durations of several hundred microseconds flanked by much longer closed periods due to desensitization. Thus, the open state of α7 can be considered an unstable intermediate on the pathway toward desensitization. During desensitization, the TMD relaxes back partially toward a resting state-like conformation, with a return of the TM helices to a more vertical orientation in the membrane (Figure 7C). The unwound MA-M4 junction recovers its stable helical configuration. The cytosolic ends of M2 and M4 remain pinned together in all states in part by a conserved salt bridge (Figure 4C and Movie S5), and as such, reorientation of the helices brings the cytosolic end of M2 toward the channel axis to form a desensitization gate (Figure 5F, Figure 7F). While the hydrophobic activation gate in the middle of the pore closes partially, the extracellular ends of M2 do not come together. A contact made during activation, between the β1β2 loop and the M2M3 loop, remains intact during desensitization, holding the top of the pore open. The movements of the TMD push against the coupling loops, resulting in a slight expansion of the ECD in the desensitized state (Movie S2). This concerted conformational change may contribute to the increased affinity for agonists in the desensitized state (Neubig et al., 1982). Transition to the resting state is favored by agonist dissociation and results in a further expansion of the ECD, unlocking of the β1β2 – M2M3 loop interaction, and closure of the extracellular half of the TMD pore.
Structural mapping of the α7 gating cycle provides an opportunity to delineate conserved versus divergent mechanisms among members of the Cys-loop receptor superfamily. While structures of invertebrate and mammalian Cys-loop receptors and prokaryotic receptor orthologs have been obtained in different conformations (Nemecz et al., 2016), only the α1 glycine receptor has been characterized in resting, activated and desensitized states, and notably, the recent structures are in lipid nanodiscs (Kumar et al., 2020; Yu et al., 2021). The results on α7 support a broadly conserved structural observation that the extracellular domain becomes more compact during activation, and this compact ECD is largely maintained in desensitized states (Nemecz et al., 2016). However, comparison of α7 with the glycine receptor reveals a striking divergence in the nature of TMD conformational changes. The dramatic tilting of the α7 helical bundle and sliding of M4 are absent in the glycine receptor. In contrast, the conformational changes leading to glycine receptor channel opening appear as a more subtle subunit TMD twist and tilt outward. Desensitization of the glycine receptor involves a conformational change localized principally to the cytosolic end of the pore to close the gate at the −2′ position only (Kumar et al., 2020; Yu et al., 2021).
The 5-HT3 receptor provides another important point of comparison, as several full-length structures have been determined in different conformational states. A desensitized state for this receptor remains missing from the gating cycle ensemble, but resting, intermediate and activated states have been determined by two groups (Basak et al., 2018a; Basak et al., 2018b; Polovinkin et al., 2018). All 5-HT3 receptor structures are in detergent, some with lipid additives. The resting to activated transition in 5-HT3 reveals important consistencies with α7 in the TMD tilting, and in the M4 helix sliding; these similarities between receptors that share just 26% amino acid sequence identity provide confidence that the rather dramatic changes we observe are physiologically meaningful. Functional analysis of 5-HT3 further supports conservation in the observed widening of portals in the activated state (Stuebler and Jansen, 2020). While some aspects of the transition from resting to active appear conserved, the two open state structures for 5-HT3 (PDB 6HIN and 6DG8) differ dramatically in the rotation of M2, the position of the pore constriction, and the position of the M2M3 loop. The α7 activated-state structure is similar to 6HIN but slightly wider in pore diameter.
These comparisons of cationic and anionic Cys-loop receptor structures reveal that the family does not share a single, conserved gating mechanism. The structural nexus of this mechanistic divergence may be the intracellular domain. The anion selective glycine and GABAA receptors, and further, the invertebrate GluCl and the bacterial orthologs, lack the MA and MX helices found in nicotinic and 5-HT3 receptors (Nemecz et al., 2016). The tight inter-subunit interactions at the cytosolic apex of MA in mammalian cation channels restrict the ability of M4 to rotate freely with the rest of the TMD bundle. M4 must then slide relative to M3 during channel activation and then relax back during desensitization.
Conclusions
We present here structures of the homopentameric human α7 nicotinic acetylcholine receptor in the three foundational gating cycle conformations: a closed, resting state; an open, activated state; and a closed, desensitized state. In addition to providing touchstones for steps in the gating cycle, the structures generated hypotheses that we tested using electrophysiology and binding assays to understand channel function. We report a component located in the ECD that contributes to α7’s high calcium permeability and may regulate relative permeability in the broader Cys-loop receptor family. The discovery of the C-terminal latch expands the role of the C-terminus in coupling agonist binding to channel opening. The presence of a hydrophobic constriction at the 9′ position of the pore in the desensitized state may contribute to the rapid onset of α7 desensitization. These provide insight into the gating cycle of nicotinic receptors, reveal how they contrast mechanistically with the glycine receptor, and open new paths of exploration to study this important family of signaling proteins.
Limitations of the Study
Although we have determined a structural framework for understanding α7 nicotinic receptor gating and permeation, several outstanding issues remain; we highlight a few of interest to us here. First, the conformation observed in the presence of agonist and PNU-120596 is used as a proxy for the physiological open state of the receptor. This assumption was a necessary starting point as the open state is transient in the absence of the PAM; however, this conformation may not perfectly match the open state when only the neurotransmitter acetylcholine is bound. Second, any sample preparation introduces caveats in structural interpretation. In the case of α7, the receptor construct was modified to enable purification of enough stable sample for structural elucidation, and detergent was used during membrane extraction and purification. Third, despite including a high concentration of PNU-120596 in the cryo-EM sample, we were unable to confidently position this modulator, either in a proposed binding site in the TMD (daCosta et al., 2011; Young et al., 2008), or elsewhere. We suspect that the locally dynamic nature of the TMD in the activated state, which correlates with its lower local resolution, precludes visualization of the small molecule, similar to what was observed in a recent study of the 5-HT3A receptor (Polovinkin et al., 2018). Lastly, while we identified functionally essential residues in the latch turn, the functional role of the C-terminal helix remains mysterious.
STAR Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ryan E. Hibbs (ryan.hibbs@utsouthwestern.edu).
Materials Availability
All unique reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
Cryo-EM maps and atomic model coordinates have been deposited in the RCSB and EMDB, respectively, under the accession codes 7KOO/EMD-22979, 7KOX/EMD-22983, and 7KOQ/EMD-22980.
Experimental Model and Subject Details
Cell lines
The Sf9 insect cells were obtained from the ATCC (Cat# CRL-1711) and cultured at 27°C at 130 rpm in Erlenmeyer flasks with smooth bottoms with Sf900 III media (Thermo Fisher Scientific), in an InnovaR shaking incubator. HEK293S GnTI− suspension cells were obtained from the ATCC (Cat# CRL-3022) and cultured at 37°C in baffle-bottomed Erlenmeyer flasks with 8% CO2 at 130 rpm, in a Thermo Scientific Reach-In CO2 Incubator. All cell lines used in this study are female in origin.
Method Details
Protein Expression Constructs
The human α7 receptor expression construct was modified from the CHRNΑ7-YFP plasmid deposited in Addgene by Henry Lester (plasmid #62630) (Wang et al., 2014). This construct includes YFP inserted into a region of the M3-M4 loop predicted to be structurally disordered. For functional analyses including binding, trafficking, and whole-cell electrophysiology unless noted otherwise, mutations and deletions were made on this parent construct with the YFP present. In the optimized expression construct used for structural analysis, YFP and 42 ICD amino acids were replaced with the thermostable protein bRIL (Chun et al., 2012), plus the linker sequence AGA (Figures S1 and S4, refer to Figures 1–7). Finally, the construct includes a Strep-tag II (Schmidt and Skerra, 1994) at its C-terminus. This construct exhibited vastly improved biochemical behavior compared to the WT human receptor, and was sub-cloned into the pEZT-BM vector for small-scale transient transfection tests and large scale expression for purification (Morales-Perez et al., 2016a). The NACHO gene (Gu et al., 2016; Matta et al., 2017) was similarly subcloned into pEZT-BM for expression. The Saposin A expression plasmid was provided by Salipro Biotech AB.
Receptor expression and purification
Bac-mam viruses were made in Sf9 cells (Vaughn et al., 1977). Viruses for the α7 receptor and NACHO were titered as previously described (Morales-Perez et al., 2016a). Briefly, viruses were serially diluted by 10-fold and added to Sf9 cells in a 96-well plate. Cells were then incubated at 27°C for 48 h and examined by eye for GFP fluorescence on an inverted microscope. Each focal point of GFP positive cells is counted as one infectious unit, and titers are calculated by multiplying an average of three wells by the dilution factor. The α7 and NACHO viruses were transduced at an MOI of 1:1 into HEK293S GnTI− suspension cells (Reeves et al., 2002) at a density of 3–4 × 106/ml. The cells were cultured at 37 °C for 72 h before harvesting by centrifugation. Cells were washed once in 20 mM Tris, pH 7.4, 150 mM sodium chloride (TBS) and recentrifuged. After resuspension in TBS + 1 mM phenylmethanesulfonyl fluoride (PMSF; Sigma), cells were lysed by passage through an Avestin Emulsiflex at 5,000–15,000 psi. Lysed cells were first centrifuged at low speed (10,000 g) to remove nuclei and unlysed cells, then centrifuged at 186,000 g for 2 h to pellet membranes. Membranes were stored at −80 °C until used. To purify proteins, approximately 5 g of membranes were Dounce homogenized in TBS + 1 mM PMSF. The detergent n-dodecyl-β-D-maltoside (DDM; Anatrace) was added to a final concentration of 40 mM, and solubilization was performed at 4 °C while nutating. Insoluble material was removed by centrifugation at 186,000 g for 40 min. The supernatant containing solubilized protein and its native lipids was bound to Strep-Tactin Superflow high capacity resin (IBA Life Sciences) via gravity flow. The resin was washed with TBS plus 1 mM DDM, supplemented with 10 μM Soy Polar Lipids (Avanti) and 0.25% w/vol cholesterol (Sigma) to promote receptor stability. The α7 receptor protein was eluted in the same wash buffer supplemented with 5 mM desthiobiotin (Sigma).
Cryo-EM Sample Preparation
The α7 receptor protein was concentrated to ~500 μl, and then saposin (Lyons et al., 2017) and soy lipids/25% cholesterol (w/w) were added to the purified protein at a molar ratio of 1 α7 protein: 50 saposin protein: 230 lipid. This mixture nutated at 4°C for 30 minutes before the removal of detergent was begun by the addition of bio-beads. The removal of detergent was continued overnight at 4 °C, after which incorporation was assayed by fluorescence-detection size-exclusion chromatography (Kawate and Gouaux, 2006). The receptor-lipidic disc mixture was further polished by size exclusion chromatography using only TBS as a mobile phase. The best fractions, as assessed by FSEC, were pooled and concentrated to ~4–6 mg/ml before addition of 200 μM α-bungarotoxin (Tocris) and subsequent freezing for cryo-electron microscopy (cryo-EM). In the case of the epibatidine dataset, 20 μM (±)-epibatidine (Tocris) was included throughout the purification along with 5 mM EGTA and increased to 200 μM epibatidine; otherwise all conditions were identical. For the epibatidine + PNU-120596 dataset, PNU-120596 was added at 50 μM during SEC and increased to 200 μM before freezing grids. No EGTA was present and an additional 5 mM CaCl2 was included with the ligands.
For preparation of cryo-EM grids, 200-mesh Cu 1.2/1.3 grids from Quantifoil were glow-discharged in a PELCO easiGlow glow discharge apparatus for 30 mA/80s on top of a metal grid holder (Ted Pella). To induce random particle orientations, protein was supplemented with 0.5 mM fluorinated fos-choline-8 (Anatrace) immediately before freezing. Grids were frozen with a Mark IV Vitrobot (FEI/ThermoFisher). Three μl of protein were applied to grids before blotting for 3.5 s at 100% humidity and 4 °C. Frozen grids were stored in liquid nitrogen until used.
Cryo-EM data collection and processing
The electron microscopy was performed on a 300kV Titan Krios (FEI/Thermo-Fisher) equipped with a K3 direct electron detector and a GIF energy filter (20 e−V) using super-resolution mode (Gatan) and collected via SerialEM (Mastronarde, 2005). The total dose was 36–86 e−/Å2. All data were processed within the Relion 3.0.8 suite of software (Zivanov et al., 2018).
For the bungarotoxin dataset, 3,245 movies were collected and aligned with MotionCor2 (Zheng et al., 2017). Contrast transfer function (CTF) estimation was performed with GCTF (Zhang, 2016). Autopicking was performed with Relion and yielded ~800,000 particles, which through iterations of 2D classification and 3D classification were pruned to 25,656 particles in the final refinement. These particles were polished and subjected to further rounds of refinement and beam-tilt refinement. The map was then subjected to B-factor sharpening in Relion, which produced a map with an overall resolution of 3.0 Å. However, this level of sharpening obscured the weaker density of the MX helix. The MX helix backbone was built using the unsharpened map. Local resolution was estimated using ResMap (Kucukelbir et al., 2014).
For the epibatidine/PNU dataset, 16,745 movies were collected and aligned with MotionCor2 (Li et al., 2013). After CTF estimation with GCTF (Zhang, 2016), particles were picked using crYOLO (Wagner et al., 2019). Particle box files were imported into Relion. After 2D and 3D classification and refinement, heterogeneity in the TMD required further classification with fine local angular searches to identify particles with strong density in the TMD. The final refinement used 710,549 particles from an original pool of 2,004,527 particles and reached an overall resolution of 2.7 Å. However, local resolution shows large differences between the ECD, which is closer to 2.5 Å, and the TMD, which is closer to 3.5 Å.
For the epibatidine dataset, 26,952 movies were collected and processed as per the epibatidine + PNU dataset. Several iterations of 3D classification with fine local angular searches were required to improve density of the M4 and MX helices, which appear to be the most mobile for this receptor. The final refinement was performed with only ~77,000 particles from a starting pool of ~1.8 million particles. We attempted to refine other 3D classes that had good signal for the ECD, but they did not lead to high resolution structures, or led to maps with no TMD visible. Nonetheless, the final map was estimated to be at a resolution of 3.77 Å in Relion, and further processing by density modification with the ResolveCryoEM program (Terwilliger et al., 2020) improved the map quality and resolution to 3.6 Å. Structural biology applications used in this project were compiled and configured by SBGrid (Morin et al., 2013)).
Model building and validation
We generated an α7 receptor homology model as a starting point for manual building and refinement. We used PROMALS3D (Pei et al., 2008) to generate a sequence alignment between α7 and the human α3 nicotinic receptor subunit (Gharpure et al., 2019) (RCSB 6PV7), then SWISS-MODEL (Waterhouse et al., 2018) to make the homology model of a single subunit. We expanded this α7 subunit into a pentamer model in Coot (Emsley et al., 2010). The resulting model was docked into the density map using Chimera (Pettersen et al., 2004). The position of Loop C and the α-bungarotoxin molecule were copied from the α1 subunit of Torpedo (Rahman et al., 2020). After initial real-space refinement was performed in Coot, further real space global refinement and B-factor refinement with stereochemistry restraints were performed in Phenix, with non-crystallographic symmetry restraints applied (Afonine et al., 2018). Model validation was performed using MolProbity (Davis et al., 2004).
Map quality varied by receptor domain in a state-dependent manner. For the resting state structure, as in one of the α subunits of the Torpedo receptor, the disulfide bond at the tip of Loop C is broken, likely due to radiation damage (Hattne et al., 2018; Rahman et al., 2020). In agonist-bound structures the disulfide bond at the tip of Loop C appears to be protected from radiation damage by virtue of its interaction with agonist, analogous to its state-dependent protection from reducing reagents (Damle and Karlin, 1980). The desensitized-state map showed stronger density in the ICD region, and we used this model as the starting point for the other structures. The MX helix and the flexible linkers on either side of it are more poorly resolved in the resting state, and we utilized an unsharpened map to model the helix. In the activated state, the unsharpened map allowed placing of the backbone of MA and MX; side chains of MA and MX are positioned as an average of possible conformations. Density likely corresponding to bRIL could be seen in unsharpened maps of the desensitized and resting-state complexes at high contour. However, it was not of sufficient quality to model the bRIL insertion.
Whole Cell Electrophysiology
Whole-cell voltage-clamp recordings were made from adherent HEK293S GnTI− cells transiently transfected with 0.4 μg NACHO and α7 DNA each at 37 °C. 24 hours post-transfection, cells were replated on 35 mm dishes and allowed to settle for 24 hours. For all whole-cell recordings, borosilicate pipettes were used after being pulled and polished to an initial resistance of 2–4 MΩ. Recordings were made with an Axopatch 200B amplifier, sampled at 10 kHz, low-pass filtered at 1kHz using a Digidata 1440A (Molecular Devices), and analyzed with pClamp 11 software (Molecular Devices). Solution exchange was done using a gravity-driven RSC-200 rapid solution changer (Bio-Logic).
To test for functionality of wild type, EM, and mutant constructs, the bath solution contained (in mM): 140 NaCl, 2.4 KCl, 4 MgCl2, 4 CaCl2, 10 HEPES-NaOH pH 7.5, and 10 glucose. The pipette solution contained (in mM): 110 CsCl, 40 CsF, 10 EGTA, and 20 HEPES-NaOH pH 7.5. Cells were held at −75 mV and responses were evoked with either 100 μM nicotine or 2 μM epibatidine in the presence and absence of 3 μM PNU-120596. We noted a delay in trafficking of our EM construct to the cell surface for electrophysiology experiments; incubation of 1–2 days longer was required to obtain robust current responses from the EM construct.
To test for calcium permeability, the bath solution contained (in mM): 150 KCl, 5 or 20 CaCl2, 10 HEPES-KOH pH 7.4, and 10 glucose. The pipette solution contained (in mM): 150 KCl, 10 EGTA, and 20 HEPES-KOH pH 7.4. Cells were initially held at −80 mV and responses were evoked with 100 μM nicotine and 3 μM PNU-120596. Once currents reached a steady state value, a voltage step protocol was applied by increasing the holding potential by 10 mV every 500 ms from −80 to +60 mV. Current amplitudes were normalized to the −80 mV value. Data points between −80 and +20 mV (due to nonlinearity at higher voltages) were used to calculate reversal potentials using linear regression in Prism 8 (GraphPad). Reversal potentials are as follows: WT α7 (Vrev5mM = 3.36, n=5, [95%CI=1.93, 4.84]; Vrev20mM=10.65, n=5, [95%CI=9.17, 12.20]). The E97A mutant (Vrev5mM = 1.97 [95%CI=0.54, 3.47], n=6; Vrev20mM=4.49, [95%CI=3.47, 5.53), n=4).
To calculate relative calcium permeability (PCa/ PK), the following equation derived from GHK was used, where R, T, and F are standard thermodynamic parameters, [K+]0 represents the extracellular K+ activity, [Ca2+5]0 and [Ca2+20]0 represent extracellular Ca2+ activities at 5 mM and 20 mM respectively (ionic activities were determined using the Debye–Hückel equation), and ΔVrev = Vrev20 – Vrev5, where Vrev20 and Vrev5 represent the reversal potentials determined at 20 mM and 5 mM Ca2+ respectively:
(Castro and Albuquerque, 1995; Colon-Saez and Yakel, 2014; Ragozzino et al., 1998)
To test for calcium block, the bath solution contained (in mM): 150 KCl, 5 EGTA, 10 HEPES-KOH pH 7.4, and 10 glucose. The pipette solution contained (in mM): 150 KCl, 10 EGTA, and 20 HEPES-KOH pH 7.4. Cells were held at −75 mV and responses were evoked with 100 μM nicotine and 3 μM PNU-120596 in the presence of either 5 mM EGTA, 1 mM CaCl2, or 10 mM CaCl2.
To test for organic cation permeability, the bath contained (in mM): 150 KCl (or 75 KCl and 75 of the chloride salt for the cation being tested), 10 HEPES-KOH pH 7.4, and 10 glucose. The pipette solution contained (in mM): 150 KCl, 10 EGTA, and 20 HEPES-KOH pH 7.4. Cells were initially held at −80 mV and responses were evoked with 100 μM nicotine and 3 μM PNU-120596. Once currents reached a steady state value, a voltage step protocol was applied by increasing the holding potential by 10 mV every 500 ms from −80 to +60 mV. Current amplitudes were normalized to the −80 mV value. Data points between −80 and +10 mV (due to non-linearity at higher voltages) were used to calculate reversal potentials using linear regression in Prism 8 (GraphPad). To calculate relative cation permeability ratios (PX/ PK), the following equation derived from GHK was used, where R, T, and F are standard thermodynamic parameters, [K+]r represents the extracellular K+ activity in the reference solution (150 mM KCl), [K+]t represents the extracellular K+ activity in the test solution (75 mM KCl and 75 mM test cation chloride), and [X+] represents the extracellular activity of the organic cation in the test solution (ionic activities were determined using the Debye–Hückel equation), and ΔVrev = Vt − Vr, where Vt and Vr represent the reversal potentials for the test and reference solutions respectively:
Single Channel Recordings
Recordings from BOSC-23 cells, transfected with cDNAs encoding the α7 AChR or mutants thereof, were obtained in the cell-attached patch configuration at a temperature of 21 °C. Patch pipettes were pulled from glass capillary tubes (No.7052, King Precision Glass) and coated with Sylgard (Dow Corning). The bath solution contained (in mM): 142 KCl, 5.4 NaCl, 1.8 CaCl2, 1.7 MgCl2, and 10 HEPES, with the pH adjusted to 7.4 by addition of KOH. The pipette solution contained (in mM): 142 NaCl, 5.4 KCl, 1.8 CaCl2, 1.7 MgCl2, and 10 HEPES, with the pH adjusted to 7.4 by addition of NaOH. Acetylcholine chloride, with or without PNU-120596, was added to the pipette solution. Single channel currents were recorded using an Axopatch 200B patch clamp amplifier (Molecular Devices) with the gain set at 100 mV/pA and the internal Bessel filter at 100 kHz. Before establishing a cell-attached patch, the pipette offset potential was manually set to zero, and after forming a giga-ohm seal, a command voltage was applied to the interior of the patch pipette to establish the membrane potential. The current output was sampled at intervals of 0.2 μs using a National Instruments model BNC-2090 A/D converter with a PCI 6111e acquisition card and recorded to the hard disk of a PC computer using the program Acquire (Bruxton).
Single channel analysis was performed using the program TAC 4.2.0 (Bruxton). To determine the single channel current amplitude, the Gaussian digital filter within TAC was set at 5 kHz, an all-points histogram was generated for each sweep of data from a given patch, and two Gaussian functions, one for the baseline and the other for the open channel current, were fitted to the histogram. The difference between the means of the baseline and open channel currents yielded the current amplitude for each sweep. The current amplitude for at least twenty sweeps was averaged to yield the overall mean current amplitude. Currents are displayed in Figure 1 at a bandwidth of 10 kHz. To analyze single channel dwell times, histograms were plotted using a logarithmic abscissa and a square root ordinate, with a uniformly imposed dead time of 8 μs, and were fitted by the sum of exponentials by maximum likelihood using the program TACFit 4.3.3 (Bruxton).
Radioligand Binding
BOSC-23 cells, transfected with cDNAs encoding the α7 AChR or mutants thereof, were harvested by gentle agitation, centrifuged at 2500 rpm for 1 min, and re-suspended in extracellular bathing solution containing (mM): 140 KCl, 5.4 NaCl, 1.8 CaCl2, 1.7 MgCl2, HEPES, pH 7.4. The radioligands 125I-epibatidine (2 nM; a generous gift by Dr. Vanda Lennon, Neuroimmunology Laboratory, Mayo Clinic) or 125I-α-bgt (10 nM) (PerkinElmer) were added to replicate aliquots of cells, and after incubation for 45 minutes at 21 °C, bound radioligand was determined by filtration using a cell harvester. Specific binding was determined after subtracting the amount of radioligand bound to mock transfected cells. Triplicate measurements for each mutant were normalized to the mean value determined for wild type α7.
Binding of ACh was determined by competition against the rate of 125I-α-bgt binding. Briefly, replicate aliquots of cells, two per ACh concentration, were incubated with a series of increasing concentrations of ACh for 30 min, 125I-α-bgt (10 nM) was added, and after 10 minutes of incubation, radioligand bound to the cells was determined by filtration using a cell harvester. Under these conditions approximately 20 % of the total sites were occupied by 125I-α-bgt. After subtracting binding in the presence of 10 mM ACh, the ratio of binding in the presence of ACh divided by that in its absence, which equals 1-Fraction bound, was determined. The following form of the Hill equation was fitted to the data using non-linear regression analysis in GraphPad Prism: where KI is the apparent dissociation constant and nH is the Hill coefficient.
Structural analysis
UCSF Chimera was used to split and align maps in Figure 4. First, each map was split based on the amino acids of a single subunit ECD (residues 1–204). This created a reference to align the ECD maps of each conformation. The single-subunit ECDs were fitted into the bungarotoxin map to align them. Then the original map was split into a single subunit map, which was then aligned with its partnered ECD map. The TMD maps displayed are thus aligned via their ECD and reflect movement of the TMD only relative to the ECD.
Tilt angles of the models shown in Figure 4 were measured by adding pointer atoms in a line perpendicular to the axis of each helix, in Coot. Then the angle was measured between this line and one from the upper pointer atom to a reference Cα position at the intracellular end of M3. For the nanodisc density shown in Figure 1, the unsharpened maps were low-pass filtered to 8 Å using Relion image handler. To measure the diameter of the nanodisc, pointer atoms were placed at the edge of the low-pass filtered map in Coot, and the distance tool was used. PyMOL (Schrodinger), Chimera and ChimeraX were used to generate structural figures and ChimeraX was used to create the movies (Pettersen et al., 2021). CHAP was used to assess the hydrophobicity of the permeation pathway (Klesse et al., 2019) and HOLE was used to estimate pore diameters (Smart et al., 1996).
Quantification and Statistical Analysis
For the whole cell and single channel electrophysiology experiments, regression analysis was performed using GraphPad Prism 8, with error bars representing standard error of the mean and bracketed values indicating the 95% confidence interval. For the single channel experiments, individual recordings were analyzed in TAC to determine mean current at a given voltage before linear regression analysis of current amplitude versus voltage. Details of replicates are given in the individual methods section or figure legend, where n is the number of cells or patches that the measurements were taken from. For radioligand binding experiments to determine the total number of binding sites, specific binding was determined after subtracting the amount of radioligand bound to mock transfected cells, and details of the analysis and replicates are present in the individual methods section. For radioligand binding experiments to determine the apparent dissociation constant for ACh, data were analyzed by non-linear regression using Prism. All the values are included in the analysis. No methods were used to determine whether the data met assumptions of the statistical approach.
In Supplemental Table 2, ns is given for some mutants to indicate not significantly different from wild type. These were mutants for which an initial competition assay was done with a single determination per acetylcholine concentration. There was no statistical test of whether the mutants were different from wild type (the curves overlapped). However, for the three mutants for which we give Kd and nH values in the table, the initial assay revealed qualitative differences from wild type, and a subsequent assay was done with duplicate determinations of initial rate of binding for each ACh concentration, along with measurements of equilibrium binding to insure initial rate conditions were met. Results from these experiments are given in the table.
Supplementary Material
Supplemental Figure 1: Protein sequence alignments for cryo-EM construct and mutational analyses, related to Figures 1–7. A, Alignment of mature coding sequences of WT human α7 receptor and cryo-EM constructs. Helical boundaries (cylinders) are based on activated state structure; in resting and desensitized states, MA and M4 form one continuous helix. B, C-terminal modification constructs tested functionally in Supplemental Table 2.
Supplemental Figure 2: Comparison of acetylcholine (ACh)-elicited single channel currents for wild type α7 and the EM α7 construct, related to Figure 1. A, Single channel currents recorded in the presence of 100 μM ACh at a membrane potential of −100 mV are displayed at a bandwidth of 10 kHz. Channel openings are upward deflections. Representative histograms of open and burst dwell times, displayed on a logarithmic abscissa and square root ordinate, are fitted by the sum of exponentials; smooth curves for each component (blue) and their sum (black) are displayed. Bursts are defined as a series of openings flanked by closings briefer than 0.3 ms, as determined by the point of intersection between the major brief closed time component and the succeeding component. Bandwidth for analysis of dwell times was 25 kHz. B, Single channel currents recorded in the presence of 100 μM ACh plus 3 μM PNU-120596, and representative histograms of open and burst dwell times, are displayed as in (A). C, Single channel current amplitude in the presence of 100 μM ACh plus 3 μM PNU-120596 is plotted against membrane potential. Bandwidth for analysis of current amplitude was 5 kHz. Straight lines, determined by linear least squares fitting, reveal slope conductance values of 163 ± 4 pS for wild type α7 and 186 ± 4 pS for the α7 EM construct. D-G, Each table displays results from fitting the sum of exponentials to open or burst durations; values are mean determinations from two to four patches ± SD. Bursts are defined as a series of openings flanked by closings briefer than 0.3 ms. Bandwidth for analysis 25 kHz.
Supplemental Figure 3, related to Figures 1–7: Cryo-EM processing results and resolution. Local resolution and sharpened maps from A, α-bgt, B, epibatidine + PNU, and C, epibatidine datasets. FSC Curves in A and B are half-map (top) Fourier shell correlations, or model-map Fourier shell correlations. In C, maps and FSC curves are from Phenix ResolveCryoEM post processing. Top graph represents Fourier shell correlation of estimated CC to perfect, and bottom graph is unmasked Fourier shell correlation from half maps.
Supplemental Figure 4: α7 receptor models and architecture, related to Figures 2–7. A-C, Side views of each model. ECD, extracellular domain. TMD, transmembrane domain. ICD, intracellular domain. The coupling region is boxed. D, A subunit interface labeled to indicate loops, domains, helices and glycans. Deletion of disordered residues in the EM construct and insertion of bRIL are indicated.
Supplemental Figure 5: Nicotinic receptor neurotransmitter site comparisons, related to Figure 2. A, α7 – α-bungarotoxin complex compared to the Torpedo nicotinic receptor bound to the same toxin (PDB: 6UWZ). For α7, the receptor is in teal and the toxin is in green. For Torpedo, the receptor and toxin are in light gray. B, Similar comparison but for α7 vs. the α7-AChBP chimera plus α bungarotoxin complex (PDB: 4HQP), which are shown in light blue. C-E, α7-epibatidine complex compared to the α4:α4 interface of α4β2 (light gray, C), the α4:β2 interface (light gray, D), and the α3:β4 interface (gray, E). Key residues coordinating ligand binding are shown. In B, C and D, the ligand for the α4β2 (PDB: 6CNJ, 6CNK) and α3β4 (PDB: 6PV7) receptors is nicotine.
Supplemental Figure 6: Representative Cys-loop receptor pore conformations, related to Figure 5. A pair of opposing M2 helices are shown and pore diameters are indicated for the Closed/Resting (A), Open/Activated (B), and Desensitized (C) states. The PDB accession code is given below each structure name. α7, Torpedo, α4β2 and α3β4 are nicotinic family members. Experimental density for M2 helices is shown for α7 structures as a semitransparent surface.
Supplemental Figure 7: Relative permeabilities of organic cations in α7 vs. α7 E97A, related to Figure 6. A, Top view of activated-state structure showing experimental density map for E97 side chains with pore diameter indicated. Map shown was high-pass filtered to 5 Å. B, Table of diameters of cations as measured in (Cohen et al., 1992). C-H, IV relationships of indicated cations. n=5 independent cells for all experiments except E, where n=6. 95% confidence interval of Vrev is indicated in square brackets.
Movie S1, related to Figures 1–7: Side view of transitions from the resting, to activated, to desensitized states.
Movie S2, related to Figure 1–7: Top view of transitions from the resting, to activated, to desensitized states.
Movie S3, related to Figure 4: Side view of conformational transitions in the TMD and ICD from resting state to open state to desensitized state, then back to resting state. The unwinding of the M4-MA junction is highlighted.
Movie S4, related to Figures 4–5: Top view of conformational transitions in the TMD and ICD from resting state to open state to desensitized state, then back to resting state.
Movie S5, related to Figure 5 and S6: Conformational transitions of the ion channel pore highlighting interactions of specific residues of the M2 helix, the β1-β2 loop, the M2-M3 loop, and the M2-M4 salt bridge.
Highlights.
Structures of the human α7 nicotinic acetylcholine receptor
Structures of the three major conformations of the gating cycle
Identification of a C-terminal substructure involved in gating
Identification of an extracellular residue key to calcium permeability
Acknowledgements
We thank Richard Walsh and Jinfeng Teng for early contributions to α7 studies. Single-particle cryo-EM grids were screened at the University of Texas Southwestern Medical Center Cryo-Electron Microscopy Facility, which is supported by the CPRIT Core Facility Support Award RP170644. We thank Harry Scott for cryo-EM data collection at the PNCC under user proposal 50839. A portion of this research was supported by NIH grant U24GM129547 and performed at the PNCC at OHSU and accessed through EMSL (grid.436923.9), a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research. This work was supported by grants from the NIH (NS120496, NS077983, and NS095899 to R.E.H., and NS94124 to S.M.S.) and by the Friends of the Alzheimer’s Disease Center to R.E.H.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonine PV, Poon BK, Read RJ, Sobolev OV, Terwilliger TC, Urzhumtsev A, and Adams PD (2018). Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D Struct Biol 74, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldea M, Mulet J, Sala S, Sala F, and Criado M (2007). Non-charged amino acids from three different domains contribute to link agonist binding to channel gating in alpha7 nicotinic acetylcholine receptors. J Neurochem 103, 725–735. [DOI] [PubMed] [Google Scholar]
- Alves DS, Castello-Banyuls J, Faura CC, and Ballesta JJ (2011). An extracellular RRR motif flanking the M1 transmembrane domain governs the biogenesis of homomeric neuronal nicotinic acetylcholine receptors. FEBS Lett 585, 1169–1174. [DOI] [PubMed] [Google Scholar]
- Basak S, Gicheru Y, Kapoor A, Mayer ML, Filizola M, and Chakrapani S (2019). Molecular mechanism of setron-mediated inhibition of full-length 5-HT3A receptor. Nat Commun 10, 3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Gicheru Y, Rao S, Sansom MSP, and Chakrapani S (2018a). Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor. Nature 563, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Gicheru Y, Samanta A, Molugu SK, Huang W, Fuente M, Hughes T, Taylor DJ, Nieman MT, Moiseenkova-Bell V, et al. (2018b). Cryo-EM structure of 5-HT3A receptor in its resting conformation. Nat Commun 9, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Kumar A, Ramsey S, Gibbs E, Kapoor A, Filizola M, and Chakrapani S (2020). High-resolution structures of multiple 5-HT3AR-setron complexes reveal a novel mechanism of competitive inhibition. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DK, and Conroy WG (2002). Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol 53, 512–523. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, and Changeux JP (1993). Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci U S A 90, 6971–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat C, Bartos M, Corradi J, and Sine SM (2008). The interface between extracellular and transmembrane domains of homomeric Cys-loop receptors governs open-channel lifetime and rate of desensitization. J Neurosci 28, 7808–7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Caro A, Sala S, Ballesta JJ, Vicente-Agullo F, Criado M, and Sala F (1996). A single residue in the M2-M3 loop is a major determinant of coupling between binding and gating in neuronal nicotinic receptors. Proc Natl Acad Sci U S A 93, 6118–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro NG, and Albuquerque EX (1995). alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J 68, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun E, Thompson AA, Liu W, Roth CB, Griffith MT, Katritch V, Kunken J, Xu F, Cherezov V, Hanson MA, et al. (2012). Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 20, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BN, Labarca C, Davidson N, and Lester HA (1992). Mutations in M2 alter the selectivity of the mouse nicotinic acetylcholine receptor for organic and alkali metal cations. J Gen Physiol 100, 373–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Saez JO, and Yakel JL (2014). A mutation in the extracellular domain of the alpha7 nAChR reduces calcium permeability. Pflugers Arch 466, 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M, Mulet J, Castillo M, Aldea M, Sala S, and Sala F (2008). Interactions between loop 5 and beta-strand beta6’ are involved in alpha7 nicotinic acetylcholine receptors channel gating. J Neurochem 104, 719–730. [DOI] [PubMed] [Google Scholar]
- da Costa Couto A, Price KL, Mesoy S, Capes E, and Lummis SCR (2020). The M4 Helix Is Involved in alpha7 nACh Receptor Function. ACS Chem Neurosci 11, 1406–1412. [DOI] [PubMed] [Google Scholar]
- daCosta CJ, Free CR, Corradi J, Bouzat C, and Sine SM (2011). Single-channel and structural foundations of neuronal alpha7 acetylcholine receptor potentiation. J Neurosci 31, 13870–13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Mogg AJ, and Wonnacott S (2002). Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca2+ channels and Ca2+ stores. J Neurochem 81, 606–614. [DOI] [PubMed] [Google Scholar]
- Damle VN, and Karlin A (1980). Effects of agonists and antagonists on the reactivity of the binding site disulfide in acetylcholine receptor from Torpedo californica. Biochemistry 19, 3924–3932. [DOI] [PubMed] [Google Scholar]
- Davis IW, Murray LW, Richardson JS, and Richardson DC (2004). MOLPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res 32, W615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe GB, Yu H, Gu S, Blackler AN, Matta JA, Siuda ER, Rex EB, and Bredt DS (2019). alpha7 nicotinic acetylcholine receptor upregulation by anti-apoptotic Bcl-2 proteins. Nat Commun 10, 2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deba F, Ali HI, Tairu A, Ramos K, Ali J, and Hamouda AK (2018). LY2087101 and dFBr share transmembrane binding sites in the (alpha4)3(beta2)2 Nicotinic Acetylcholine Receptor. Sci Rep 8, 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Lu W, Wu S, Cheng Y, and Gouaux E (2015). Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer TM, Adams DJ, and Hille B (1980). The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol 75, 469–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, et al. (1997). Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A 94, 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi JL, Bertrand S, Corringer PJ, Changeux JP, and Bertrand D (1996). Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J 15, 5824–5832. [PMC free article] [PubMed] [Google Scholar]
- Galzi JL, Devillers-Thiery A, Hussy N, Bertrand S, Changeux JP, and Bertrand D (1992). Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature 359, 500–505. [DOI] [PubMed] [Google Scholar]
- Gay EA, Giniatullin R, Skorinkin A, and Yakel JL (2008). Aromatic residues at position 55 of rat alpha7 nicotinic acetylcholine receptors are critical for maintaining rapid desensitization. J Physiol 586, 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, and Lindstrom J (1995). Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol 48, 774–782. [PubMed] [Google Scholar]
- Gharpure A, Noviello CM, and Hibbs RE (2020). Progress in nicotinic receptor structural biology. Neuropharmacology 171, 108086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure A, Teng J, Zhuang Y, Noviello CM, Walsh RM Jr., Cabuco R, Howard RJ, Zaveri NT, Lindahl E, and Hibbs RE (2019). Agonist Selectivity and Ion Permeation in the alpha3beta4 Ganglionic Nicotinic Receptor. Neuron 104, 501–511 e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rubio J, Navarro-Lopez C, Lopez-Najera E, Lopez-Najera A, Jimenez-Diaz L, Navarro-Lopez JD, and Najera A (2020). Cytokine Release Syndrome (cRs) and Nicotine in COVID-19 Patients: Trying to Calm the Storm. Front Immunol 11, 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter T, de Carvalho LP, Dufresne V, Taly A, Edelstein SJ, and Changeux JP (2005). Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc Natl Acad Sci U S A 102, 18207–18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Matta JA, Lord B, Harrington AW, Sutton SW, Davini WB, and Bredt DS (2016). Brain alpha7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO. Neuron 89, 948–955. [DOI] [PubMed] [Google Scholar]
- Hales TG, Dunlop JI, Deeb TZ, Carland JE, Kelley SP, Lambert JJ, and Peters JA (2006). Common determinants of single channel conductance within the large cytoplasmic loop of 5-hydroxytryptamine type 3 and alpha4beta2 nicotinic acetylcholine receptors. J Biol Chem 281, 8062–8071. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Wang HL, Taylor P, and Sine SM (2008). An ion selectivity filter in the extracellular domain of Cys-loop receptors reveals determinants for ion conductance. J Biol Chem 283, 36066–36070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, et al. (2014). X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512, 276–281. [DOI] [PubMed] [Google Scholar]
- Hattne J, Shi D, Glynn C, Zee CT, Gallagher-Jones M, Martynowycz MW, Rodriguez JA, and Gonen T (2018). Analysis of Global and Site-Specific Radiation Damage in Cryo-EM. Structure 26, 759–766 e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Li SX, Bren N, Cheng K, Gomoto R, Chen L, and Sine SM (2013). Complex between alpha-bungarotoxin and an alpha7 nicotinic receptor ligand-binding domain chimaera. Biochem J 454, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, et al. (2005). A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25, 4396–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Cadugan DJ, Purohit P, and Auerbach A (2007). Acetylcholine receptor gating at extracellular transmembrane domain interface: the cys-loop and M2-M3 linker. J Gen Physiol 130, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, and Gouaux E (2006). Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, and Peters JA (2003). A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 424, 321–324. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gharpure A, Teng J, Zhuang Y, Howard RJ, Zhu S, Noviello CM, Walsh RM Jr., Lindahl E, and Hibbs RE (2020). Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 585, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesse G, Rao S, Sansom MSP, and Tucker SJ (2019). CHAP: A Versatile Tool for the Structural and Functional Annotation of Ion Channel Pores. J Mol Biol 431, 3353–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukelbir A, Sigworth FJ, and Tagare HD (2014). Quantifying the local resolution of cryo-EM density maps. Nat Methods 11, 63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Basak S, Rao S, Gicheru Y, Mayer ML, Sansom MSP, and Chakrapani S (2020). Mechanisms of activation and desensitization of full-length glycine receptor in lipid nanodiscs. Nat Commun 11, 3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, and Lester HA (1995). Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376, 514–516. [DOI] [PubMed] [Google Scholar]
- Lee WY, Free CR, and Sine SM (2009). Binding to gating transduction in nicotinic receptors: Cys-loop energetically couples to pre-M1 and M2-M3 regions. J Neurosci 29, 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, and Sine SM (2005). Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature 438, 243–247. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Bowlby MR, Comery TA, and Dunlop J (2009). A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther 122, 302–311. [DOI] [PubMed] [Google Scholar]
- Li X, Mooney P, Zheng S, Booth CR, Braunfeld MB, Gubbens S, Agard DA, and Cheng Y (2013). Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods 10, 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey MR, Cooper MA, Lambert JJ, and Peters JA (2011). Rings of charge within the extracellular vestibule influence ion permeation of the 5-HT3A receptor. J Biol Chem 286, 16008–16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JA, Boggild A, Nissen P, and Frauenfeld J (2017). Saposin-Lipoprotein Scaffolds for Structure Determination of Membrane Transporters. Methods Enzymol 594, 85–99. [DOI] [PubMed] [Google Scholar]
- Masiulis S, Desai R, Uchanski T, Serna Martin I, Laverty D, Karia D, Malinauskas T, Zivanov J, Pardon E, Kotecha A, et al. (2019). GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 565, 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152, 36–51. [DOI] [PubMed] [Google Scholar]
- Matta JA, Gu S, Davini WB, Lord B, Siuda ER, Harrington AW, and Bredt DS (2017). NACHO Mediates Nicotinic Acetylcholine Receptor Function throughout the Brain. Cell Rep 19, 688–696. [DOI] [PubMed] [Google Scholar]
- McCormack TJ, Melis C, Colon J, Gay EA, Mike A, Karoly R, Lamb PW, Molteni C, and Yakel JL (2010). Rapid desensitization of the rat alpha7 nAChR is facilitated by the presence of a proline residue in the outer beta-sheet. J Physiol 588, 4415–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesoy S, Jeffreys J, and Lummis SCR (2019). Characterization of Residues in the 5-HT3 Receptor M4 Region That Contribute to Function. ACS Chem Neurosci 10, 3167–3172. [DOI] [PubMed] [Google Scholar]
- Miller PS, and Aricescu AR (2014). Crystal structure of a human GABAA receptor. Nature 512, 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MA, and McCarthy MP (1995). Snake venom toxins, unlike smaller antagonists, appear to stabilize a resting state conformation of the nicotinic acetylcholine receptor. Biochim Biophys Acta 1235, 336–342. [DOI] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, and Hibbs RE (2016a). Manipulation of Subunit Stoichiometry in Heteromeric Membrane Proteins. Structure 24, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, and Hibbs RE (2016b). X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 538, 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A, Eisenbraun B, Key J, Sanschagrin PC, Timony MA, Ottaviano M, and Sliz P (2013). Collaboration gets the most out of software. Elife 2, e01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtasimova N, and Sine SM (2013). Nicotinic receptor transduction zone: invariant arginine couples to multiple electron-rich residues. Biophys J 104, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecz A, Prevost MS, Menny A, and Corringer PJ (2016). Emerging Molecular Mechanisms of Signal Transduction in Pentameric Ligand-Gated Ion Channels. Neuron 90, 452–470. [DOI] [PubMed] [Google Scholar]
- Neubig RR, Boyd ND, and Cohen JB (1982). Conformations of Torpedo acetylcholine receptor associated with ion transport and desensitization. Biochemistry 21, 3460–3467. [DOI] [PubMed] [Google Scholar]
- Pei J, Kim BH, and Grishin NV (2008). PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res 36, 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, and Ferrin TE (2021). UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci 30, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulera S, Zhu H, Yu J, Claxton DP, Yoder N, Yoshioka C, and Gouaux E (2018). Cryo-EM structure of the benzodiazepine-sensitive alpha1beta1gamma2S tri-heteromeric GABAA receptor in complex with GABA. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polovinkin L, Hassaine G, Perot J, Neumann E, Jensen AA, Lefebvre SN, Corringer PJ, Neyton J, Chipot C, Dehez F, et al. (2018). Conformational transitions of the serotonin 5-HT3 receptor. Nature 563, 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskar NL, Xiu X, Lester HA, and Dougherty DA (2011). Two neuronal nicotinic acetylcholine receptors, alpha4beta4 and alpha7, show differential agonist binding modes. J Biol Chem 286, 14618–14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Barabino B, Fucile S, and Eusebi F (1998). Ca2+ permeability of mouse and chick nicotinic acetylcholine receptors expressed in transiently transfected human cells. J Physiol 507 (Pt 3), 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Teng J, Worrell BT, Noviello CM, Lee M, Karlin A, Stowell MHB, and Hibbs RE (2020). Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PJ, Callewaert N, Contreras R, and Khorana HG (2002). Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A 99, 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi JL, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, and Changeux JP (1991). Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353, 846–849. [DOI] [PubMed] [Google Scholar]
- Rudell JC, Borges LS, Rudell JB, Beck KA, and Ferns MJ (2014). Determinants in the beta and delta subunit cytoplasmic loop regulate Golgi trafficking and surface expression of the muscle acetylcholine receptor. J Biol Chem 289, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F, Mulet J, Sala S, Gerber S, and Criado M (2005). Charged amino acids of the N-terminal domain are involved in coupling binding and gating in alpha7 nicotinic receptors. J Biol Chem 280, 6642–6647. [DOI] [PubMed] [Google Scholar]
- Schmidt TG, and Skerra A (1994). One-step affinity purification of bacterially produced proteins by means of the “Strep tag” and immobilized recombinant core streptavidin. J Chromatogr A 676, 337–345. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, and Patrick JW (1993). Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine SM, Strikwerda JR, and Mazzaferro S (2019). Structural basis for alpha-bungarotoxin insensitivity of neuronal nicotinic acetylcholine receptors. Neuropharmacology 160, 107660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart OS, Neduvelil JG, Wang X, Wallace BA, and Sansom MS (1996). HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J Mol Graph 14, 354–360, 376. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, and Lester HA (2011). Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol 137, 59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebler AG, and Jansen M (2020). Mobility of Lower MA-Helices for Ion Conduction through Lateral Portals in 5-HT3A Receptors. Biophys J 119, 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Ludtke SJ, Read RJ, Adams PD, and Afonine PV (2020). Improvement of cryo-EM maps by density modification. Nat Methods 17, 923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JL, Goodwin RH, Tompkins GJ, and McCawley P (1977). The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 13, 213–217. [DOI] [PubMed] [Google Scholar]
- Vicente-Agullo F, Rovira JC, Sala S, Sala F, Rodriguez-Ferrer C, Campos-Caro A, Criado M, and Ballesta JJ (2001). Multiple roles of the conserved key residue arginine 209 in neuronal nicotinic receptors. Biochemistry 40, 8300–8306. [DOI] [PubMed] [Google Scholar]
- Wagner T, Merino F, Stabrin M, Moriya T, Antoni C, Apelbaum A, Hagel P, Sitsel O, Raisch T, Prumbaum D, et al. (2019). SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun Biol 2, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RM Jr., Roh SH, Gharpure A, Morales-Perez CL, Teng J, and Hibbs RE (2018). Structural principles of distinct assemblies of the human alpha4beta2 nicotinic receptor. Nature 557, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, and Imoto K (1992). Pore size and negative charge as structural determinants of permeability in the Torpedo nicotinic acetylcholine receptor channel. Proc Biol Sci 250, 11–17. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. (2003). Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- Wang S, and Hu Y (2018). alpha7 nicotinic acetylcholine receptors in lung cancer. Oncol Lett 16, 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xiao C, Indersmitten T, Freedman R, Leonard S, and Lester HA (2014). The duplicated alpha7 subunits assemble and form functional nicotinic receptors with the full-length alpha7. J Biol Chem 289, 26451–26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, et al. (2018). SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46, W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Puskar NL, Shanata JA, Lester HA, and Dougherty DA (2009). Nicotine binding to brain receptors requires a strong cation-pi interaction. Nature 458, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, and Heinemann SF (2006). Identification of sequence motifs that target neuronal nicotinic receptors to dendrites and axons. J Neurosci 26, 9780–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Pan N, Xue F, Zheng Y, Li C, Chang Y, Xu Z, Yang H, and Zhang J (2015). The coupling interface and pore domain codetermine the single-channel activity of the alpha7 nicotinic receptor. Neuropharmacology 95, 448–458. [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, and Millar NS (2008). Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A 105, 14686–14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhu H, Lape R, Greiner T, Du J, Lu W, Sivilotti L, and Gouaux E (2021). Mechanism of gating and partial agonist action in the glycine receptor. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Tae HS, Xu Q, Craik DJ, Adams DJ, Jiang T, and Kaas Q (2019). Molecular dynamics simulations of dihydro-beta-erythroidine bound to the human alpha4beta2 nicotinic acetylcholine receptor. Br J Pharmacol 176, 2750–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdanowski R, Krzyzowska M, Ujazdowska D, Lewicka A, and Lewicki S (2015). Role of alpha7 nicotinic receptor in the immune system and intracellular signaling pathways. Cent Eur J Immunol 40, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xue F, Whiteaker P, Li C, Wu W, Shen B, Huang Y, Lukas RJ, and Chang Y (2011). Desensitization of alpha7 nicotinic receptor is governed by coupling strength relative to gate tightness. J Biol Chem 286, 25331–25340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K (2016). Gctf: Real-time CTF determination and correction. J Struct Biol 193, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Du Y, Zhang J, Xu X, Xue F, Guo C, Huang Y, Lukas RJ, and Chang Y (2015). Functional Impact of 14 Single Nucleotide Polymorphisms Causing Missense Mutations of Human alpha7 Nicotinic Receptor. PLoS One 10, e0137588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanov J, Nakane T, Forsberg BO, Kimanius D, Hagen WJ, Lindahl E, and Scheres SH (2018). New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Protein sequence alignments for cryo-EM construct and mutational analyses, related to Figures 1–7. A, Alignment of mature coding sequences of WT human α7 receptor and cryo-EM constructs. Helical boundaries (cylinders) are based on activated state structure; in resting and desensitized states, MA and M4 form one continuous helix. B, C-terminal modification constructs tested functionally in Supplemental Table 2.
Supplemental Figure 2: Comparison of acetylcholine (ACh)-elicited single channel currents for wild type α7 and the EM α7 construct, related to Figure 1. A, Single channel currents recorded in the presence of 100 μM ACh at a membrane potential of −100 mV are displayed at a bandwidth of 10 kHz. Channel openings are upward deflections. Representative histograms of open and burst dwell times, displayed on a logarithmic abscissa and square root ordinate, are fitted by the sum of exponentials; smooth curves for each component (blue) and their sum (black) are displayed. Bursts are defined as a series of openings flanked by closings briefer than 0.3 ms, as determined by the point of intersection between the major brief closed time component and the succeeding component. Bandwidth for analysis of dwell times was 25 kHz. B, Single channel currents recorded in the presence of 100 μM ACh plus 3 μM PNU-120596, and representative histograms of open and burst dwell times, are displayed as in (A). C, Single channel current amplitude in the presence of 100 μM ACh plus 3 μM PNU-120596 is plotted against membrane potential. Bandwidth for analysis of current amplitude was 5 kHz. Straight lines, determined by linear least squares fitting, reveal slope conductance values of 163 ± 4 pS for wild type α7 and 186 ± 4 pS for the α7 EM construct. D-G, Each table displays results from fitting the sum of exponentials to open or burst durations; values are mean determinations from two to four patches ± SD. Bursts are defined as a series of openings flanked by closings briefer than 0.3 ms. Bandwidth for analysis 25 kHz.
Supplemental Figure 3, related to Figures 1–7: Cryo-EM processing results and resolution. Local resolution and sharpened maps from A, α-bgt, B, epibatidine + PNU, and C, epibatidine datasets. FSC Curves in A and B are half-map (top) Fourier shell correlations, or model-map Fourier shell correlations. In C, maps and FSC curves are from Phenix ResolveCryoEM post processing. Top graph represents Fourier shell correlation of estimated CC to perfect, and bottom graph is unmasked Fourier shell correlation from half maps.
Supplemental Figure 4: α7 receptor models and architecture, related to Figures 2–7. A-C, Side views of each model. ECD, extracellular domain. TMD, transmembrane domain. ICD, intracellular domain. The coupling region is boxed. D, A subunit interface labeled to indicate loops, domains, helices and glycans. Deletion of disordered residues in the EM construct and insertion of bRIL are indicated.
Supplemental Figure 5: Nicotinic receptor neurotransmitter site comparisons, related to Figure 2. A, α7 – α-bungarotoxin complex compared to the Torpedo nicotinic receptor bound to the same toxin (PDB: 6UWZ). For α7, the receptor is in teal and the toxin is in green. For Torpedo, the receptor and toxin are in light gray. B, Similar comparison but for α7 vs. the α7-AChBP chimera plus α bungarotoxin complex (PDB: 4HQP), which are shown in light blue. C-E, α7-epibatidine complex compared to the α4:α4 interface of α4β2 (light gray, C), the α4:β2 interface (light gray, D), and the α3:β4 interface (gray, E). Key residues coordinating ligand binding are shown. In B, C and D, the ligand for the α4β2 (PDB: 6CNJ, 6CNK) and α3β4 (PDB: 6PV7) receptors is nicotine.
Supplemental Figure 6: Representative Cys-loop receptor pore conformations, related to Figure 5. A pair of opposing M2 helices are shown and pore diameters are indicated for the Closed/Resting (A), Open/Activated (B), and Desensitized (C) states. The PDB accession code is given below each structure name. α7, Torpedo, α4β2 and α3β4 are nicotinic family members. Experimental density for M2 helices is shown for α7 structures as a semitransparent surface.
Supplemental Figure 7: Relative permeabilities of organic cations in α7 vs. α7 E97A, related to Figure 6. A, Top view of activated-state structure showing experimental density map for E97 side chains with pore diameter indicated. Map shown was high-pass filtered to 5 Å. B, Table of diameters of cations as measured in (Cohen et al., 1992). C-H, IV relationships of indicated cations. n=5 independent cells for all experiments except E, where n=6. 95% confidence interval of Vrev is indicated in square brackets.
Movie S1, related to Figures 1–7: Side view of transitions from the resting, to activated, to desensitized states.
Movie S2, related to Figure 1–7: Top view of transitions from the resting, to activated, to desensitized states.
Movie S3, related to Figure 4: Side view of conformational transitions in the TMD and ICD from resting state to open state to desensitized state, then back to resting state. The unwinding of the M4-MA junction is highlighted.
Movie S4, related to Figures 4–5: Top view of conformational transitions in the TMD and ICD from resting state to open state to desensitized state, then back to resting state.
Movie S5, related to Figure 5 and S6: Conformational transitions of the ion channel pore highlighting interactions of specific residues of the M2 helix, the β1-β2 loop, the M2-M3 loop, and the M2-M4 salt bridge.
Data Availability Statement
Cryo-EM maps and atomic model coordinates have been deposited in the RCSB and EMDB, respectively, under the accession codes 7KOO/EMD-22979, 7KOX/EMD-22983, and 7KOQ/EMD-22980.