Abstract
Although behavioral studies have demonstrated that executive function (EF) develops rapidly during early childhood, few studies have investigated neural systems supporting EF during the preschool years. These systems are sensitive to variations in children’s early life experiences, including preterm birth. The current study collected behavioral and event related potential (ERP) data during an EF task (directional Stroop) in a sample of 150 full-term and low-risk preterm children aged 4-years. Children’s IQ and processing speed (WPPSI-III), and parent report of EF (BRIEF-P), were also measured. Forty-nine children born full-term and 43 low-risk preterm children provided useable ERP data. Similar to prior studies with adults and older children, preschool-aged children showed modulation of ERP components (N2, P3) by cognitive conflict. Effects of trial type were also present for early attentional components (N1 and P2). Exploratory analyses demonstrated that ERP measures of EF were correlated with individual differences in cognitive and behavioral functioning in both full-term and low-risk preterm populations. Future research investigating the neural correlates of early measures of EF in low-risk preterm children and other at-risk groups is warranted to better understand how trajectories of EF development are altered in the first years of life.
Keywords: executive function, ERP, preterm birth, cognitive conflict, directional Stroop
A vast developmental psychology literature demonstrates that variations in children’s early environments have the potential to impact the development of prefrontal-dependent behaviors, including executive function (EF), across the lifespan. EF refers to a group of interrelated cognitive processes responsible for the regulation of thoughts, actions, and goal-directed behaviors (Miyake et al., 2000; Miyake & Friedman, 2012). In adults, this skill set has been classically decomposed into three main components (Miyake et al., 2000): working memory (holding information in mind and manipulating it), inhibitory control (refraining from executing a prepotent attentional or behavioral response), and cognitive flexibility (avoiding perseveration by shifting attention flexibily between cognitive tasks and responses). Although the underlying structure of EF emerges gradually over development (e.g. see Best & Miller, 2010 for review), early EF skills develop rapidly in infants, toddlers, and young preschoolers (Hendry, Jones, & Charman, 2016). Experiences that alter early trajectories of EF and prefrontal cortex development are relatively diverse (e.g. see Hodel, 2018 for review) including disruptions of the parent-infant caregiving relationship (e.g. maltreatment, institutionalization; e.g. Hanson et al., 2010; Hodel, Hunt, et al., 2015), deprivation or poverty (e.g. Lawson, Duda, Avants, Wu, & Farah, 2013), as well as perinatal risks, such as preterm birth (PT; birth before 37 weeks gestation; e.g. Nosarti, 2010).
Altered trajectories of EF development have been well-studied in high-risk PT populations, including children born very preterm (<32 weeks gestation) or extremely preterm (<28 weeks gestation). Collectively, this literature has demonstrated that individuals who are born PT show deficits in EF that are measurable during childhood (Aarnoudse-Moens & Smidts, 2009; Anderson, Doyle, & Victorian Infant Collaborative Study Group, 2004; Marlow, Hennessy, Bracewell, & Wolke, 2007) and persist into later adolescence (Luu, Ment, Allan, Schneider, & Vohr, 2011; Narberhaus, Segarra, Cald, & Gim, 2008).
Emerging evidence suggests that lower risk PT children may also demonstrate subtle impairments in EF. As many as 8% of children born annually in the United States are born within the moderate-to-late PT range (32–36 weeks gestation; Hamilton, Martin, & Osterman, 2016). While these children have significantly lower levels of neonatal morbidity than their higher risk, very PT peers, research suggests that moderate-to-late PT birth impacts children beyond the neonatal period (e.g. for recent reviews see Kugelman & Colin, 2013; Vohr, 2013). Population research has linked moderate-to-late PT birth to higher rates of academic, behavioral, and emotional problems during the school years (Chan & Quigley, 2014; Chyi, Lee, Hintz, Gould, & Sutcliffe, 2008; Kirkegaard, Obel, Hedegaard, & Henriksen, 2006; Lipkind, Slopen, Pfeiffer, & McVeigh, 2012; Morse, Zheng, Tang, & Roth, 2009; Quigley et al., 2012; van Baar, Vermaas, Knots, de Kleine, & Soons, 2009). Similarly, preschool-aged children born within the moderate-to-late PT range also show poorer EF development (Baron et al., 2009; Baron, Kerns, Muller, Ahronovich, & Litman, 2012; Brumbaugh, Hodel, & Thomas, 2014; Hodel, Brumbaugh, Morris, & Thomas, 2015), although impairments in this lower-risk PT population appear to be more subtle than those observed following very PT birth.
Neuroimaging evidence from older children, adolescents, and adults born PT indicates that behavioral deficits in EF are likely mediated by alterations in both prefrontal cortex structure (Ball et al., 2012; Bjuland, Rimol, Løhaugen, & Skranes, 2014; Kesler et al., 2008; Mullen et al., 2011; Nagy et al., 2009; Nosarti et al., 2008; Peterson et al., 2000; Thompson et al., 2007) and function (Griffiths et al., 2013; Mürner-Lavanchy et al., 2014; Lawrence et al., 2009; Nosarti, 2013; Nosarti et al., 2006)). However, to date there is little research delineating the brain bases of EF deficits in PT children early in development (i.e. prior to school age), likely due to the general paucity of research describing the neural correlates of EF in typically developing, preschool-aged children.
Neural Circuitry Supporting EF in Early Childhood
Neuroimaging studies with typically developing children, adolescents, and adults have clearly documented the role of prefrontal regulatory systems in supporting EF (Alvarez & Emory, 2006; Kane & Engle, 2002). Although the extended maturation of frontal lobe circuits during adolescence is well known in the developmental psychology literature, major changes in prefrontal cortex structure and function also occur earlier in development, including during the early childhood years. Frontal lobe white matter volume increases linearly across this age range (Giedd et al., 1999), along with significant expansion of cortical surface area during the preschool years (Brown & Jernigan, 2012). Functional near-infrared spectroscopy (fNIRS) studies have documented concomitant behavioral improvements and increases in frontal lobe activation during EF tasks in both cross-sectional and longitudinal studies of preschool-aged children (Moriguchi & Hiraki, 2011, 2013). In combination, this work suggests that structural and functional prefrontal cortex maturation during the preschool years supports EF development, or at least that these developmental changes are correlated in time.
Brain Measures of EF in Early Childhood
There are few studies examining functional brain changes related to EF during the preschool period, primarily because functional MRI measures are challenging within this age range due to participant motion and children’s limited abilities to complete EF tasks within the scanner environment. In contrast, electrophysiological measures such as event related potentials (ERPs) are better tolerated by preschool-aged children (e.g. see Karmiloff-Smith, 2010 for brief review of pediatric neuroimaging methods). The scalp-recorded ERP signal represents the synchronized activity of neurons time-locked to the onset of a specific event or stimulus and can also be used to measure neural correlates of EF.
Although ERP techniques have been readily used to understand the neural bases of cognitive processing in domains such as language and memory during early childhood, they have only more recently been applied to EF tasks. Across diverse cognitive tasks, preschool-aged children generally show longer ERP component latencies in comparison to older children and adults, especially for later components tied to higher-order cognitive processes (Brown & Jernigan, 2012). Developmental studies have provided some initial evidence that young children may show modulation of ERP components on EF tasks in a similar fashion to adults. For example, in adults, the N2 component, a negative-going waveform that peaks 200–350 ms post-stimulus, is linked to successful response inhibition (Downes, Bathelt, & Haan, 2017); it typically displays a greater (negative) amplitude for stimuli involving higher levels of cognitive conflict and is associated with activity in the anterior cingulate cortex (Folstein & Van Petten, 2008). Developmental studies have reported N2 modulation by task condition in preschool-aged populations on both inhibitory control (Lahat, Todd, Mahy, Lau, & Zelazo, 2009) and cognitive flexibility tasks (Espinet, Anderson, & Zelazo, 2012). However, these findings have been somewhat inconsistent and do not extend across all EF tasks, including versions of the classic Go/No-Go task and Attentional Networks Task (Abundis-Gutiérrez, Checa, Castellanos, & Rueda, 2014; Buss, Dennis, Brooker, & Sippel, 2011; Chevalier, Kelsey, Wiebe, & Espy, 2014; Rueda et al., 2005). The P3 component, a positive deflection that peaks 250–500 ms post-stimulus over frontocentral sites (Polich, 2007), has also been linked to EF related processing in adults, including attentional monitoring, stimulus evaluation, and working memory (Davis, Bruce, Snyder, & Nelson, 2003; Downes et al., 2017; Rueda, Posner, Rothbart, & Davis-Stober, 2004). The P3 is commonly divided into the P3a and P3b components, with the P3a more closely tied to orienting and novelty detection and the P3b linked to executive attention and working memory (Downes et al., 2017). Mature P3 amplitudes and latencies are not present until late adolescence and/or early adulthood, especially for the P3b component (Downes et al., 2017), suggesting cognitive processes associated with this component undergo protracted maturation. Although P3 amplitude is related to measures of EF in school-aged children (Wiersema & Roeyers, 2009) and is known to be sensitive to working memory demands in developmental samples (Polich, Ladish, & Burns, 1990), its relationship to EF has not been well-examined during the early childhood period.
Previous research has identified the predictive validity of early childhood EF measures for diverse measures of both short-term and long-term functioning (e.g. Casey et al., 2011; Eigsti et al., 2006). As such, there is a need for early measures that can detect even subtle differences in EF. Prior studies conducted with young children have demonstrated that ERP measures of EF can discriminate between children with varying levels of perinatal risk (Mayes, Molfese, Key, & Hunter, 2005), with early symptoms of attention deficit hyperactivity disorder (Spronk, Jonkman, & Kemner, 2008), with differing levels of temperamental anxiety (Lamm et al., 2014; Meyer et al., 2013), and with different cultural backgrounds (Lahat et al., 2009). ERP measures are ideal in that they may be more sensitive to individual differences than behavioral measures of EF during early childhood (Brydges, Fox, Reid, & Anderson, 2014) and may capture subtle deficits that impact preschool-aged children in lower risk populations, including children born moderate-to-late PT.
Current Study
In the current study, we tested low-risk moderate-to-late preterm (PT) and full-term (FT) preschoolers on a developmentally appropriate EF task (directional Stroop or Simon task) with both behavioral and ERP measures. The purpose of the study was threefold: (1) to establish that 4-year-old children show traditional modulation of ERP components on an EF task, (2) to determine whether a history of moderate-to-late PT birth was associated with altered patterns of neural processing early in development, and (3) to examine whether individual differences in ERP measures of processing (component amplitudes and latencies) were related to variation in everyday cognitive or behavioral function (measured via parent-reported behaviors and neuropsychological assessments).
As a variant of the classic Stroop task for pre-reading children, the directional Stroop task was selected to challenge a broad constellation of EF abilities including attention shifting, inhibitory control, and working memory. In this task, requiring children to inhibit the tendency to make a motor response on the same side as a visual stimulus generates cognitive conflict. The task uses multiple rules that must be held in mind, and children must switch flexibly between rules. Although the literature on the neural correlates of EF in preschoolers is relatively limited, existing studies and research with adults predict that trials with more cognitive conflict in this task should generate greater amplitudes and/or slower latencies in both the N2 and P3 components.
Because the cognitive conflict present in the directional Stroop task was visual in nature, we also examined the N1 and P2 components, both of which are linked to early visual attentional processing. The N1 component, a negative-going waveform that onsets approximately 100 ms post-stimulus, and the P2 component, a positive deflection occurring at approximately 100–250 ms post-stimulus, are present across the scalp but are maximal at frontal sites; these early components are linked to basic visual attention processes, including orienting of attention and enhancement of attention at a selected spatial location (Luck, Heinze, Mangun, & Hillyard, 1990). These components are not typically evaluated in the context of EF tasks, although Rueda et al. (2004) reported that the N1 component was modulated by stimulus conflict on the Flanker task in preschool-aged children, but not adults. N1 and P2 latencies are delayed over frontal sites in older children with attention deficit hyperactivity disorder (Karayanidis, Robaey, Bourassa, & Koning, 2000); because PT birth is associated with altered attentional processing, latencies and/or amplitudes of these visual components may also be altered in young PT children in the context of an EF task.
Although much of this study was exploratory in nature, we predicted that: 1) typically developing preschool-aged children would show effects of task conflict for both the N2 and P3 components (i.e. greater amplitudes and/or slower latencies on trials with more cognitive conflict); 2) group differences would be measurable between typically developing full-term and low-risk PT children in both early attentional (N1, P2) and later cognitive components (N2, P3); and 3) across the whole sample of children, individual variations in ERP latencies and amplitudes for trials with greater cognitive conflict would be correlated with individual differences in measures of intelligence (IQ), processing speed, and/or parent-report of EF.
Method
Participants
Four–year-old children were recruited based on gestational age (32–42 weeks) from a database of families who endorsed interest in participating in child development research. A parent provided written informed consent, and each child participant gave verbal assent prior to participation. Children were provided a gift card and a paperback book for their participation. The University of Minnesota Institutional Review Board approved the study.
One hundred fifty children between 4.5 and 5.0 years of age participated, although not all children provided complete data sets (see Final sample sizes below). Seventy-four children were born moderate-to-late PT (32–36 weeks gestation; 38 males) and 76 full-term (37–42 weeks gestation; 39 males). Power analyses based on Brumbaugh et al. (2014), in which group differences in behavioral measures of EF were examined between FT and low-risk PT children, suggested 120 total participants would provide .80 power to detect a similarly sized group difference; although we assumed neural measures of EF would be more sensitive than behavioral, we also expected attrition from the ERP task, resulting in the final target number of 150 children.
Of the 150 children who participated, the majority of the children were white (93%), had mothers with a college education (81%), and were from two-parent households (94%) with a median income between $51,000-$100,000 (Table 1). Exclusion criteria included uncorrected hearing or vision impairment, neurological insult, complex congenital heart disease, and for FT children, admission to a special care or intensive care nursery for >24 hours. Exclusion criteria were assessed via parent interview; children’s birth hospitalization records were then obtained to confirm gestational age and to document perinatal history (Table 2).
Table 1.
Sample Demographic Characteristics
| Preterm (n = 74) | Full-Term (n = 76) | ||
|---|---|---|---|
| n (%) | n (%) | p | |
| Child’s Sex - # male | 38 (51.4) | 39 (51.3) | .99 |
| Child’s Ethnicity - # White | 69 (93.2) | 71 (93.4) | .99 |
| Maternal Education | .69 | ||
| High school degree or GED | 8 (10.8) | 7 (9.2) | |
| Associate degree | 7 (9.5) | 7 (9.2) | |
| Bachelor’s degree | 28 (37.8) | 36 (47.4) | |
| Graduate or professional degree | 31 (41.9) | 26 (34.2) | |
| Maternal Work | .01* | ||
| Full-time work for pay | 39 (52.7) | 21 (27.6) | |
| Part-time work for pay | 9 (12.2) | 30 (39.5) | |
| Student | 2 (2.7) | 0 (0) | |
| Stay at home parent | 24 (32.4) | 25 (32.9) | |
| Annual Household Income | .67 | ||
| ≤ $50,000 | 8 (11.0) | 8 (10.8) | |
| $51,000 – $100,000 | 32 (45.2) | 32 (43.2) | |
| $101,000 – $150,000 | 15 (20.5) | 21 (28.4) | |
| ≥ $151,000 | 17 (23.3) | 13 (17.6) | |
| Marital Status - # married | 67 (90.5) | 74 (97.4) | .10 |
Notes. Not all children contributed usable data for all tasks; information about the subsample of children who provided useable ERP data is presented in Table S1 in the Supplemental Methods & Results. Three families declined to provide household income. p represents the p-value corresponding to the independent samples t-test or Chi square test.
p < .05
Table 2.
Sample Perinatal Characteristics
| Preterm (n = 74) | Full-Term (n = 76) | ||
|---|---|---|---|
| M (SD) | M (SD) | p | |
| Birth History | |||
| Gestational age (weeks) | 35.14 (1.46) | 39.80 (.94) | < .01* |
| Birth weight (grams) | 2569.65 (526.09) | 3705.28 (487.56) | < .01* |
| Apgar at 1 minute | 7.76 (1.47) | 8.13 (1.10) | .10 |
| Apgar at 5 minutes | 8.65 (1.06) | 8.97 (.37) | .02* |
| Length of hospital stay (days) | 9.77 (10.02) | 1.93 (.78) | < .01* |
| Maternal age at delivery (years) | 31.32 (4.44) | 32.20 (4.71) | .25 |
| n (%) | n (%) | p | |
| Pregnancy Related Characteristics | |||
| Twin gestationa | 16 (21.6) | 0 (0) | < .01* |
| Cesarean delivery | 38 (52.1) | 13 (17.3) | < .01* |
| Preeclampsia or hypertension | 17 (23.0) | 2 (2.7) | < .01* |
| Diabetes mellitus | 9 (12.2) | 6 (8.0) | .40 |
| Neonatal Complications | |||
| Glucose treatment | 20 (27.0) | 1 (1.3) | < .01* |
| Phototherapy | 25 (33.8) | 2 (2.6) | < .01* |
| Respiratory distress | 19 (25.7) | 1 (1.3) | < .01* |
| Positive pressure ventilation | 9 (12.2) | 2 (2.7) | .03 * |
| Apnea | 12 (16.2) | 0 (0) | < .01* |
| Hypovolemia | 2 (2.7) | 0 (0) | .25 |
Notes. Not all children contributed usable data for all tasks; information about the subsample of children who provided useable ERP data is presented in Table S2 in the Supplemental Methods & Results. One full-term parent declined to provide access to medical records. p represents the p-value corresponding to the independent samples t-test or Chi square test.
Only one twin per pair was tested.
p < .05
Final sample sizes.
Not all of the 150 children provided complete data sets (parent-report of EF, IQ and processing speed measures, EF behavioral data, ERP data). Additional information about exclusionary criteria and their rationale are provided throughout the method section; this section briefly summarizes the sample sizes for each measure.
The final samples for group differences in neuropsychological assessments were generally large. The final sample size for group differences in parent-reported EF (n = 150) included all children. The final sample size for group differences in IQ (n = 145) contained 73 children born moderate-to-late PT (38 male) and 72 children born FT (37 male), indicating a drop out rate of 4%. Children were excluded due to refusal or failure to complete one of the subtests necessary to generate an estimated IQ score (1 PT, 4 FT). The final sample size for group differences in processing speed (n = 126) contained 64 children born moderate-to-late PT (32 male) and 62 children born FT (32 male), indicating a drop out rate of 16%. Children were excluded due to refusal or failure to complete one of the subtests necessary to estimate processing speed (10 PT, 14 FT).
The final sample size for EF behavioral task analyses (n = 129) contained 63 children born moderate-to-late PT (31 male) and 66 children born FT (34 male), indicating a drop out rate of 14%. Children were excluded due to failure to attempt the behavioral task (2 PT) and failure to meet task accuracy criteria (9 PT, 10 FT).
The final sample for ERP analyses (n = 92) contained 43 children born moderate-to-late PT (19 male) and 49 participants born FT (24 male), indicating a drop out rate of 39%. Children were excluded due to failure to attempt the behavioral task (2 PT), failure to meet task accuracy criteria (9 PT, 10 FT), technical errors in data collection (1 PT, 1 FT), refusal to wear the ERP net (6 PT, 6 FT), and insufficient valid trials per trial type (13 PT, 10 FT). Demographic and perinatal characteristics of the subsample of children that provided ERP data are provided in the Supplemental Methods & Results, Tables S1–S2; importantly, demographic and perinatal characteristics generally were not associated with exclusion from the ERP sample.
Executive Function ERP Task
We selected a developmentally appropriate version of the directional Stroop task (or Simon task) that has previously been used to index developmental (Davidson, Cruess, Diamond, O’Craven, & Savoy, 1999) and individual differences in EF skills (Molfese et al., 2010). The cognitive conflict in this variation of the Stroop task is visual in nature and challenges children’s prepotent perseverative behavior (responding on the same side as the visual stimulus). To maximize our ability to assess group and individual differences in the neural and behavioral correlates of cognitive conflict, we modified the design used by Molfese et al. (2010) by removing blocks of trials that only contained one trial type (and thus less cognitive conflict), and increasing the number of “mixed” blocks that required participants to switch flexibly between multiple rule sets while avoiding making perseverative responses; see Figure 1 for a visualization of the task design.
Figure 1.
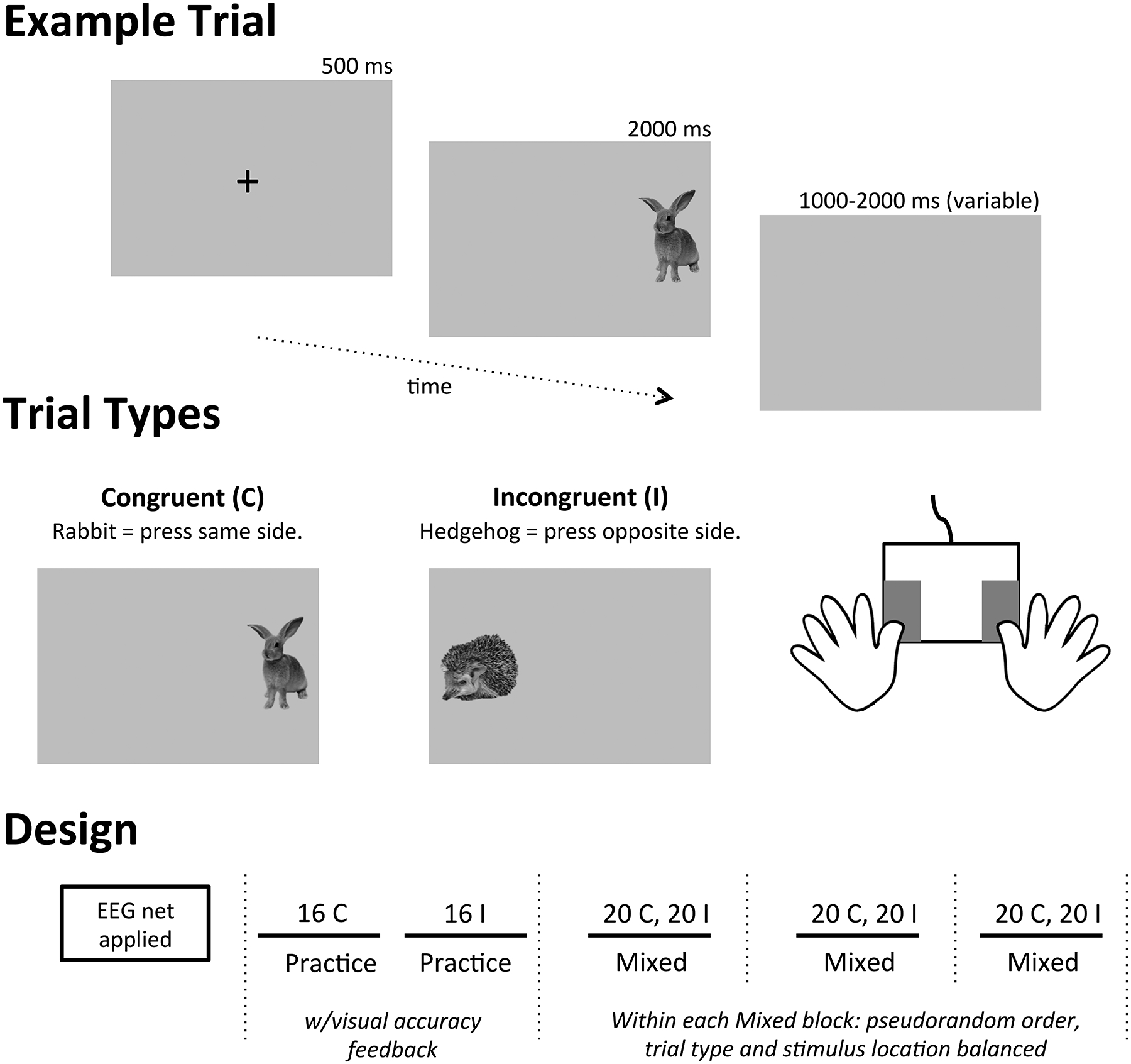
Directional Stroop task design. Congruent (C) trials (soft rabbit) required children to make a button press corresponding to the same side of the screen as the visual stimulus. Incongruent (I) trials (prickly hedgehog) required children to make a button press corresponding to the opposite side of the screen as the visual stimulus.
After demonstrating verbal mastery of the instructions, children completed two practice blocks: one of congruent trials and the other of incongruent trials. Children then completed three mixed blocks that combined congruent and incongruent trials. Short breaks occurred between blocks (indicated by dashed lines) to maintain electrode impedances. Children were reminded of the two rules following each break. Accuracy and reaction time from children’s button presses were recorded, along with EEG data. ERP data was analyzed from artifact-free correct trials during the Mixed blocks only.
Stimuli were presented via E-Prime (Psychology Software Tools, Inc., Sharpsburg, PA) on a 17-inch color monitor positioned 25–30 inches in front of the seated child, and children’s responses were collected via a button press using both thumbs on a 2-button box (left thumb and right thumb presses). An experimenter sat adjacent to the child during the task to monitor the child’s behavioral performance and to encourage the child to remain still.
Children were asked to help animal characters find their home in the forest. For congruent trials, children were instructed to press the button on the same side of the monitor as the visual stimulus (a soft rabbit). For incongruent trials, children were instructed to press the button on the opposite side of the monitor as the visual stimulus (a prickly hedgehog). After demonstrating verbal mastery of these instructions, children completed two training blocks (one block congruent trials, one block incongruent trials) with visual accuracy feedback and coaching by the experimenter. Each block contained 16 trials, balanced by stimulus location (left or right side of the screen). Children then completed three mixed blocks combining congruent and incongruent stimuli; the experimenter encouraged the child to remain on task if necessary. The mixed blocks each contained 40 trials, pseudorandom in order, equally balanced by stimulus location and trial type. Throughout the task, all stimuli were preceded by a 500 ms fixation. The stimulus was then presented for 2000 ms, followed by a randomly jittered inter-stimulus interval ranging from 1000–2000 ms. Children’s accuracy and reaction times were recorded within a 2500 ms response window (entire length of the stimulus presentation and 500 ms of the following inter-stimulus interval); non-responses and/or responses outside of this window were considered incorrect.
For inclusion in behavioral analyses, children were required to have ≥50% accuracy on both the congruent and incongruent trials across the mixed experimental blocks. 2 PT participants refused to attempt the behavioral task. 9 PT participants and 10 FT participants did not meet the accuracy criteria. The final sample for behavioral task analyses thus consisted of 63 children born moderate-to-late PT (31 male) and 66 children born FT (34 male).
Electrophysiological Recording
Electrophysiological data were collected from a 128-electrode array (HydroCel Geodesic Sensor Net, Electrical Geodesics Inc., Eugene, OR) at a sampling rate of 250Hz and a gain of 1000. Electrophysiological data were recorded using a NetAmps 200 amplification system running under NetStation 4.1 (Electrical Geodesics Inc., Eugene, OR). Electrode-scalp impedances were maintained at less than 50kΩ throughout the experiment. Data were filtered online between 0.1 and 100Hz using an analog bandpass filter prior to digitization. Bandpass-filtered data were converted from analog to digital form using a 16-bit converter with a range of ±2,500μV, resulting in a precision of 0.076μV per conversion unit. Online electrodes were referenced to the vertex. The entire ERP session lasted less than 45 minutes, including familiarization with and application of the ERP net (during which children watched short videos and/or listened to music), practice blocks, and short breaks between each block to maintain electrode-scalp impedances.
Electrophysiological Data Processing
Raw data were exported from NetStation to MATLAB (version 8.0.0.783; The Mathworks Inc., Natick, MA), where they were processed using EEGLAB (version 9.0.4.4b; Delorme & Makeig, 2004), a MATLAB toolbox. Data were inspected for quality, and any electrodes with no variance or with abnormally high spectral power (greater than 2 SD 0–10 Hz or 5 SD 35–125 Hz) were interpolated. Participants with more than 5% of electrodes interpolated were removed from the data set. All data were then re-referenced to an average reference (Junghöfer, Elbert, Tucker, & Braun, 1999) and bandpass filtered between 0.1 and 30 Hz. Filtered data were segmented into epochs from 200 ms before to 1500 ms after the stimulus onset. Epochs containing incorrect responses were discarded. Eyeblink artifacts were then corrected using second-order blind identification (Tang, Sutherland, & McKinney, 2005). Surviving epochs were baseline corrected using the 200 ms period prior to stimulus onset. Within-epoch interpolation was applied to electrodes that exceeded ±200μV. Epochs (trials) with more than 8 interpolated electrodes were excluded from further analysis. In addition, epochs were excluded if the slope of the fitted line for any electrode exceeded 150μV with a coefficient of determination greater than R2=0.80. The remaining epochs were individually inspected by an experimenter blind to the children’s task performance and group status to confirm appropriate exclusion of bad trials. After artifact rejection, 20 epochs from each behavioral condition were randomly subsampled from each participant to ensure an equal number of trials for each behavioral condition for all participants. Average waveforms for each condition were created for each participant at each electrode. This subsampling procedure ensured that each participant had an equivalent number of trials contributing to their waveform average for each condition, resulting in similar signal to noise ratios across participants. Although 20 trials was selected as a practical threshold for retaining participants, adult ERP studies have demonstrated that 20 trials is sufficient to provide internally consistent measures of N2 and P3 in the context of a Go/No-Go task (Rietdijk, Franken, & Thurik, 2014).
ERP Components of Interest
We investigated task effects related to early attentional processing at frontal sites (N1 and P2 at Fz), and later components related to cognitive conflict at frontal central (N2 at electrode Fz and Cz) and central parietal (P3 at Cz and Pz) sites. See Molfese et al. (2010) for an alternative data analysis approach for this task. Measures of peak amplitude and latency to peak for components were set using visual inspection of the grand-averaged waveforms at midline sites across all participants and confirmed through comparison to individual participant data. See Figure 2 for grand-averaged waveforms in the FT participants. The N1 component was identified between 100–252 ms and the P2 component between 180–300 ms. The N2 component at Fz was identified between 380–600 ms and 352–520 ms at Cz.
Figure 2.
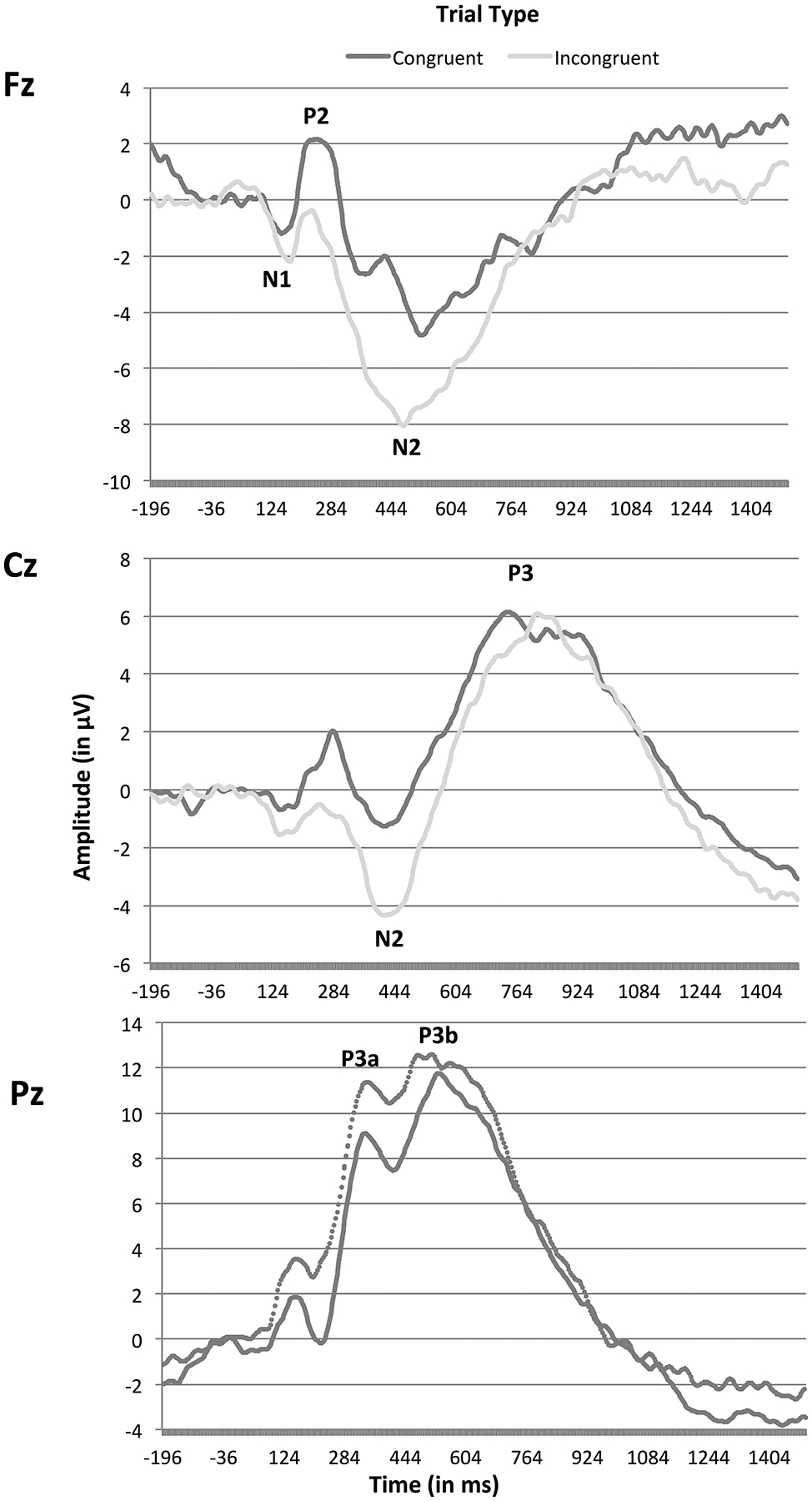
Effect of trial type on averaged ERP waveforms and components of interest. ERP waveforms and components of interest generated during correct trials on the directional Stroop task are depicted in the sample of full-term, preschool-aged children (n = 49) for congruent and incongruent trial types. Classic attention and cognitive control components were easily identifiable, although generally longer in latency than those observed in older children or adults.
The P3 component is typically maximal at central and parietal electrode sites. Since visual inspection indicated that the latencies for the P3 component were strikingly different at Cz versus Pz in our population of preschool-aged children, we elected to examine them separately. The P3 component at Cz was identified between 652–952 ms. The P3 component at Pz was characterized by a complex form (separate P3a and P3b components were clearly visible), so the component was separated into its two sub-components (P3a: 300–412 ms; P3b: 412–612 ms) that were analyzed separately.
For inclusion in ERP analyses, children were required to meet the pre-specified behavioral accuracy criterion (≥50% accuracy on both the congruent and incongruent trials across the mixed experimental blocks) and to have a minimum of 20 valid trials per trial type (congruent, incongruent). Technical errors in ERP data collection occurred for 1 PT and 1 FT participant. 6 PT participants and 6 FT participants declined to wear the ERP net. 13 PT participants and 10 FT participants provided insufficient valid trials per trial type. The final sample for ERP analyses thus consisted of 43 children born moderate-to-late PT (19 male) and 49 participants born FT (24 male).
Parent-report of Executive Function
Parents completed the Behavior Rating Inventory of Executive Function-Preschool version (BRIEF-P; Isquith, Crawford, Espy, & Gioia, 2005), which measured their perceptions of their child’s executive function within everyday contexts. The clinical scales on the BRIEF-P form three broad indices (inhibitory self-control, flexibility, and emergent metacognition) and one overall composite score (global executive composite).
IQ Measures
Children completed four subtests of the Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III; Weschler, 2002) to estimate IQ (vocabulary and matrix reasoning) and processing speed (symbol search and coding). Of the original 150 children, 5 children (1PT, 4 FT) did not complete one of the subtests necessary to generate an estimated IQ score. Similarly, of the original sample, 10 PT and 14 FT children refused or were unable to complete one of the subtests necessary to estimate processing speed.
Statistical Analyses
Statistical analysis was performed using SPSS version 21 (IBM corporation, Armonk, NY). Two-tailed p-values of p < .05 were considered statistically significant. Given the exploratory nature of our group difference and correlational analyses, we elected not to correct for multiple comparisons for two primary reasons. First, we used multiple dependent measures of neural processing (multiple ERP components, both latency to peak and component amplitudes). There was no prior literature documenting the use of our ERP task in a preschool-aged population and studies with other EF tasks in this age group have provided mixed evidence about how components are modulated by EF task demands. Therefore, we did not have specific hypotheses in many cases regarding how components would be affected by cognitive conflict (e.g. would conflict be best detected in amplitude vs. latency) and/or predictions about which dependent measures would be most likely to show a group difference. Second, previous studies with preschool-aged children have found relatively subtle cognitive effects of moderate-to-late PT birth on varying EF tasks; as such, we did not want to miss potentially small, but meaningful, differences between groups (committing a type II error) or associations with neuropsychological measures by using an overly restrictive significance threshold.
ERP analyses.
Electrophysiological effects of the ERP task (i.e. cognitive conflict or incongruency effect as reflected in component amplitudes and/or latencies) were initially analyzed only within the FT children as there was no prior literature documenting the use of this task in a preschool-aged population. These analyses utilized repeated measures ANOVA models to investigate whether predicted task effects were detected for a priori components of interest at midline sites. Models included trial type (congruent, incongruent) and electrode site (when multiple sites were tested) as within-subjects factors.
To investigate potential effects of prematurity, analyses from the FT cohort were re-run as mixed effects models using the previously described factors, but now also including group (PT versus FT) and the interaction between group and trial type as additional predictors. Within the PT group, the association between gestational age as a continuous variable and ERP latencies and amplitudes were also evaluated using Pearson’s correlations.
Behavioral measure analyses.
Potential group differences in behavioral measures of EF and IQ were assessed via independent samples t-tests. Within the PT group, the association between gestational age as a continuous variable and EF and IQ outcome measures was also evaluated using Pearson’s correlations.
ERP-behavior correlational analyses.
To examine whether individual differences in ERP measures of processing were related to variation in everyday cognitive or behavioral function (measured via parent-reported behaviors and neuropsychological assessments), we conducted exploratory correlational analyses using Pearson’s correlations. ERP-behavior correlational analyses were restricted to latency and amplitudes of the components of interest described previously. We examined the relationship between variation in these electrophysiological effects and behavioral measures of EF, IQ, and processing speed across the entire sample (both PT and FT) of children. As these correlational analyses were exploratory, they are also not corrected for multiple comparisons and should be interpreted with some caution.
Results
ERP Measures of EF in Full-Term Preschoolers
We first examined whether the directional Stroop task was associated with expected modulation of ERP components in this younger, preschool-aged population. These analyses were conducted with FT children only as there was no prior literature documenting the use of this ERP task in a preschool-aged population.
Task behavior.
Repeated measures ANOVAs indicated that accuracy was equivalent for FT children on the congruent and incongruent trials, F(1, 65) = .04, p < .84, a likely side effect of the performance-based inclusion criterion. Cognitive conflict effects were present in reaction time measures, such that FT children were slower to correctly respond on incongruent versus congruent trials, F(1, 65) = 21.31, p < .01.
Summary of ERP results.
ERP analyses demonstrated that FT preschool-aged children showed similar modulation of components on this EF task as would be predicted by the adult literature. Figure 2 includes raw ERP waveforms across electrodes of interest for the FT participants. Classic attention (N1, P2) and cognitive control components (N2, P3 complex) were easily identifiable and followed expected distributions across the scalp (e.g. N1 maximal at frontal sites), although components were generally longer in latency than those observed in older children or adults. Importantly, ERP effects of task conflict were measurable in both early attentional processing components and later components indicative of cognitive conflict.
ERP results: Early attentional components.
N1.
A repeated measures ANOVA with trial type (congruent, incongruent) as the within-subjects factor indicated there was a trend toward an effect of trial type on amplitude for the N1 component at Fz, F(1, 48) = 3.83, p < .06, such that children showed more negative amplitude for incongruent than congruent trials. Similarly, there was an effect of trial type on N1 latency, F(1, 48) = 7.20, p <.01, where children showed a longer latency to peak on incongruent trials.
P2.
There was an effect of trial type on amplitude for the P2 component at Fz, F(1, 48) = 10.52, p < .02, such that children showed more positive amplitude for congruent than incongruent trials. However, there was no effect of trial type on P2 latency, F(1, 48), = .25, p < .61. This pattern of N1 and P2 trial type effects may reflect an overall change in the combined N1-P2 complex rather than separable impacts of trial type on these two components; see Figure 2.
ERP results: Later cognitive components.
N2.
A 2 × 2 repeated measures ANOVA with trial type (congruent, incongruent) and electrode site (Fz, Cz) as within-subjects factors indicated main effects of both trial type, F(1, 48) = 18.42, p < .00, and electrode site, F(1, 48) = 22.43, p < .00, with no significant interaction, F(1, 48) = .47, p < .50. Children showed a more negative amplitude for the N2 component on incongruent than congruent trials at both Fz and Cz; overall the N2 component had a more negative amplitude at Fz than Cz. There was also an effect of trial type on N2 latency, F(1,48) = 45.85, p < .00, with no effect of electrode site, F(1, 48) = .64, p < .39, or interaction, F(1, 48) = 1.71, p < .20. Children showed a longer latency to peak on the congruent trials relative to incongruent trials, although this unexpected difference in latency by condition was quite minimal; see Figure 2.
P3 complex.
There was no effect of trial type on P3 amplitude, F(1, 48) = .22, p < .64, or latency, F(1, 48) = 1.05, p < .31, at Cz. However, there was a significant effect of trial type on amplitude for both the P3a, F(1, 48) = 6.10, p < .02, and P3b, F(1, 48) = 5.89, p < .02, components at Pz. Children showed a greater positive amplitude for incongruent vs. congruent trials at both P3a and P3b. These differences in amplitude occurred in the absence of any effects of trial type on latency for either the P3a, F(1, 48) = .36, p < .55, or P3b, F(1, 48) = 2.86, p < .10, subcomponents.
Impact of Prematurity on Behavioral and Neuropsychological Measures
To address our second objective, we subsequently investigated the potential impact of low-risk, moderate-to-late PT birth on behavioral and ERP measures of EF and standardized neuropsychological assessments; see Table 3. In addition to examining between group differences (PT versus FT), we also examined potential linear associations between gestational age and EF and IQ measures within the PT children. As described below, in this sample of both low medical and low environmental (i.e. middle to high socioeconomic status) risk PT children, there were limited impacts of prematurity on behavioral and ERP measures of EF and IQ.
Table 3.
Behavioral and Neuropsychological Performance Measures by Group
| Preterm | Full-Term | ||
|---|---|---|---|
| M (SD) | M (SD) | p | |
| n = 74 | n = 76 | ||
| BRIEF-P, parent-report of EF | |||
| Inhibitory self control | 50.82 (10.18) | 50.13 (9.95) | .67 |
| Flexibility | 49.86 (9.13) | 48.84 (8.97) | .49 |
| Emergent metacognition | 51.62 (11.92) | 51.29 (11.02) | .86 |
| Composite | 50.81 (10.29) | 50.11 (10.40) | .68 |
| n = 73 | n = 72 | ||
| WPPSI-III, IQ | |||
| Estimated IQ | 114.58 (12.38) | 114.50 (11.92) | .97 |
| n = 64 | n = 62 | ||
| WPPSI-III, PSQ | |||
| PSQ | 110.03 (13.13) | 111.81 (13.16) | .45 |
| n = 63 | n = 66 | p | |
| Directional Stroop Task behavior* | |||
| Accuracy: Congruent trials (%) | 89.21 (8.19) | 87.41 (9.93) | .40 |
| Accuracy: Incongruent trials (%) | 88.17 (10.57) | 87.62 (10.63) | |
| RT: Congruent trials (ms) | 1191.48 (160.84) | 1188.88 (167.17) | .47 |
| RT: Incongruent trials (ms) | 1251.79 (155.20) | 1237.76 (171.02) |
Notes. Not all children contributed usable data for all tasks; n represents the sample size in each group for given analyses. p represents the p-value corresponding to the independent samples t-test.
For inclusion in behavioral analyses on the Directional Stroop Task, children were required to have ≥50% accuracy on both the congruent and incongruent trials across the mixed experimental blocks. RT (reaction time) gives values for correct trials only. The corresponding p gives the p-value for the F-test of the effect of group status on accuracy or reaction time.
Parent-report of EF.
There were no differences by group (FT versus PT) in the inhibitory self-control, flexibility, or emergent metacognition indices of the BRIEF-P, nor in overall EF composite scores, p’s > .49; see Table 3. Scores on all subscales of the BRIEF-P were at the population mean for both groups of children. However, within the PT group, higher gestational age at birth was associated with higher levels of parent-reported difficulties on the inhibitory self-control index, r(74) = .29, p < .01.
IQ and processing speed.
Estimated IQ scores did not differ by group, t(143) = .03, p < .97; MPT = 114.58 ± 10.76, MFT = 114.50 ± 11.92; see Table 3. Processing speed quotient also did not differ by group, t(124) = −.76, p < .45; MPT = 110.03 ± 13.13, MFT = 111.81 ± 13.16. Scores were approximately one standard deviation above the population mean for both groups in this low-risk, highly resourced PT sample. Within the PT group, higher gestational age at birth was correlated with higher processing speed scores, r(64) = .35, p < .01.
Task behavior.
Task performance was equivalent for PT children and their FT peers; see Table 3. A 2 × 2 mixed model ANOVA with trial type (congruent, incongruent) and group indicated that accuracy was equivalent in PT and FT children, F(1, 127) = .72, p < .40. Similarly, overall reaction time on both congruent and incongruent trials did not differ by group, F(1, 127) = .53, p < .47; see Table 3. Within the PT group, there was no relationship between gestational age at birth and accuracy or reaction time on congruent or incongruent trials.
ERP measures of EF.
To investigate potential effects of prematurity on ERP component amplitude and latency, analyses from the FT cohort were re-run as a mixed model with the previously described factors, now also including group (PT versus FT) as a between-subjects factor and the interaction between group and trial type as additional predictors. These results, along with figures illustrating the average waveforms by condition in each group (Figures S1–S3), are presented in the Supplemental Methods & Results.
In general, we did not detect significant between group differences for component amplitudes or latencies for either early attentional or later cognitive components. See Supplemental Methods & Results for statistical details. However, there was a trend-level effect of group status on P2 amplitude at Fz, F(1, 90) = 3.06, p < .08, where PT children showed smaller P2 amplitudes than children born FT. Similarly, PT children also showed greater P2 latency differences by trial type than their FT peers, F(1, 90) = 3.14, p < .08. This greater latency difference by trial type in the PT group was also detected for the P3a component at Pz, F(1, 90) = 3.86, p < .05.
Within the PT group, there were limited relationships between individual variation in component amplitude and/or latency and gestational age at birth. Although there was no effect of group on P3b amplitude at Pz, F(1, 90) = .03, p < .87, within the PT group, higher gestational age at birth was related to a faster latency to peak for incongruent trials, r(43) = −.33, p <.03; see Figure 3 and Supplemental Methods & Results.
Figure 3.
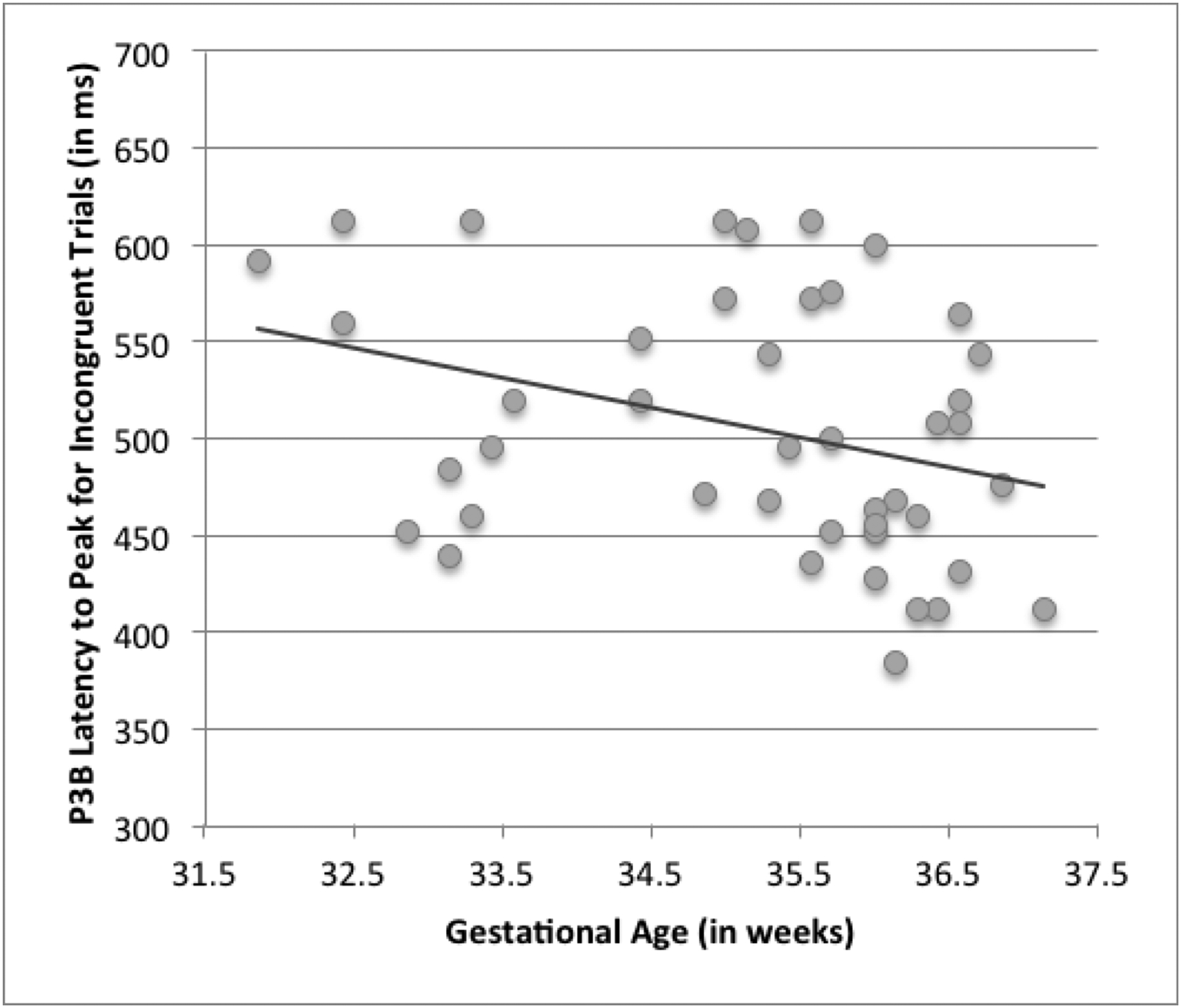
Relationship between gestational age and P3 latency. For children born moderate-to-late preterm, higher gestational age at birth was linearly associated with faster latency to peak of the P3b component for incongruent trials at Pz, r(43) = −.33, p <.03.
Relating Individual Differences in ERP Measures to Behavioral Measures of Executive Function and IQ
Our final objective was to examine if individual differences in ERP measures of attentional processing and cognitive conflict were related to variation in measures of cognitive and behavioral function in the full sample (both PT and FT) of children. These exploratory correlational analyses were restricted to latency and amplitudes of the components of interest described previously.
ERP-task behavior correlations.
Due to the reduction of variability in accuracy associated with the use of an accuracy criterion, reaction time (for correct trials) was utilized as the primary individual difference measure of children’s behavioral task performance. Individual differences in children’s reaction times on correct congruent trials were not correlated with amplitude or latency for any ERP components of interest. However, there were significant relationships between children’s reaction times on the more difficult, incongruent trial type and N2 amplitude and latency at Cz. Specifically, slower reaction times on incongruent trials were correlated with larger amplitude of the N2 component, r(92) = .229, p < .03, and longer latency to peak, r(92) = .25, p < .02.
ERP-parent-report of EF correlations.
There were no significant relationships between parent-report of children’s EF skills and early attentional components (N1, P2). However, individual differences in N2 and P3 component amplitude and latencies were correlated with parent-report of EF at Cz. Specifically, increased difficulties on the inhibitory self-control index, r(92) = .26, p <.01, emergent metacognition index, r(92) = .23, p < .03, and overall EF composite scores, r(92) = .25, p < .02, were associated with longer latency to peak for the N2 component on incongruent trials; see Figure 4. Similarly, parental report of increased difficulties on the emergent metacognition index also was correlated with both N2, r(92) = −.25, p < .02, and P3, r(92) = −.22, p < .03, amplitudes on incongruent trials at Cz.
Figure 4.
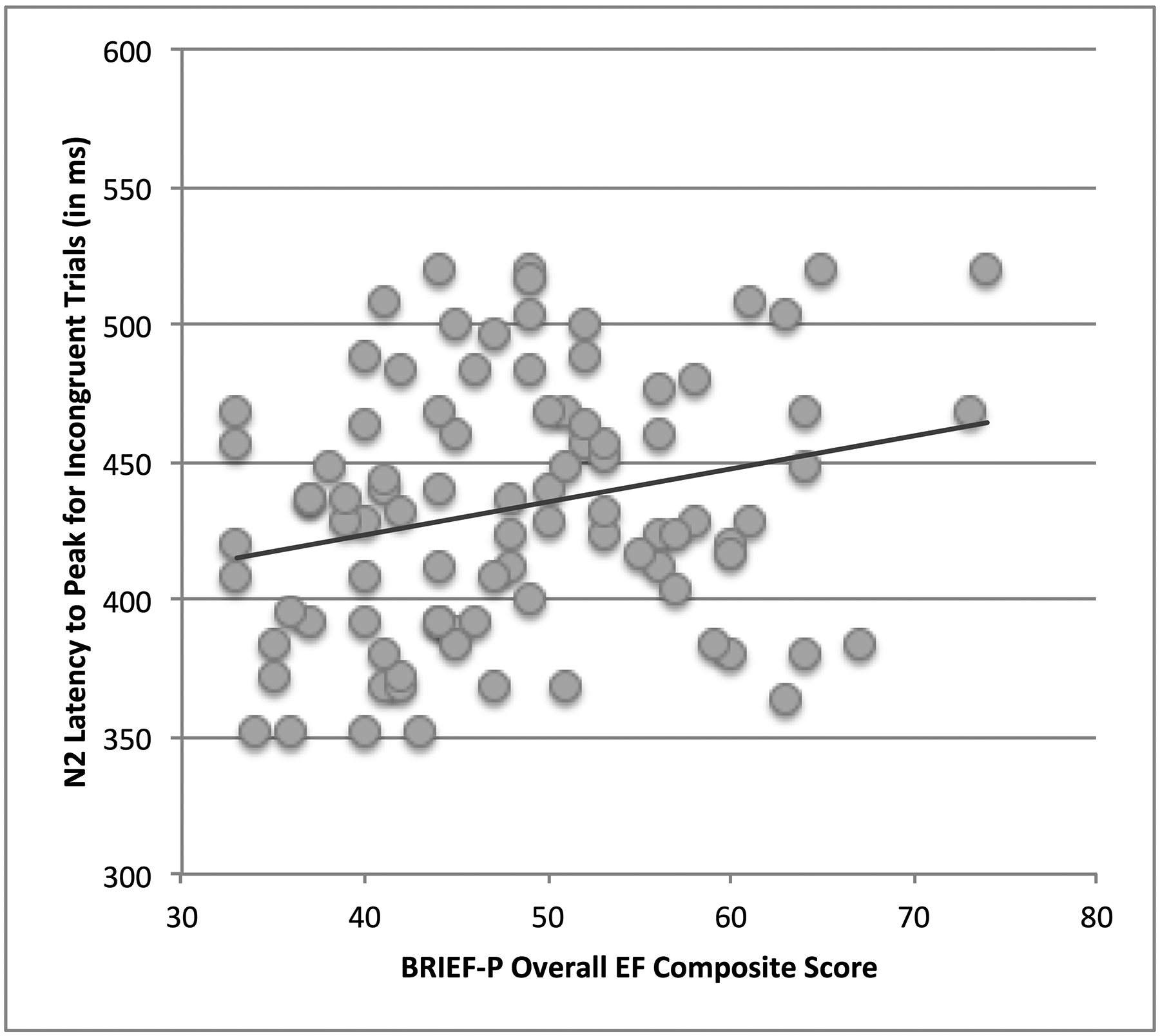
Relationship between parent-report of EF and N2 latency. Lower parent-reported EF composite scores (representing fewer EF problems) were linearly associated with faster latency to peak for the N2 component on incongruent trials at Cz across all children.
ERP-IQ and processing speed correlations.
There were no significant relationships between children’s processing speed scores and amplitude or latency for ERP components of interest. Individual differences in IQ scores were related to variability in amplitude of cognitive conflict related components, but not early attentional components. Specifically, higher IQ scores were associated with smaller (less negative) N2 amplitudes for incongruent trials at Fz, r(89) = .30, p <.01, and smaller P3b amplitudes for incongruent trials at Pz, r(89) = −.22, p < .04.
Discussion
The current study examined the neural processes supporting EF during the early childhood period in a large sample of preschool-aged children. This study adds to the literature delineating the relationship between behavioral measures of EF and activity in supporting brain systems during the preschool years, a time when both EF and the prefrontal cortex experience rapid development. Our results indicated that ERP components that subserve neural processes related to EF in older children and adults are present in 4-year-old children during a directional Stroop task. Furthermore, individual variation in amplitude and latency of later cognitive ERP components was related to concurrent measures of children’s task behavior, neuropsychological functioning (i.e. IQ), and parent-report of EF in real-world contexts. However, despite their sensitivity to individual differences in EF, ERP measures detected relatively few differences in neurocognitive functioning between a low-risk PT sample (i.e. children born moderate-to-late PT) and typically developing FT children.
ERP Measures of EF in Early Childhood
To date, few studies have assessed whether ERP components indicative of cognitive conflict and inhibitory control (i.e. N2 and P3) are present in preschool-aged children in the context of an EF task (Abundis-Gutiérrez et al., 2014; Buss et al., 2011; Chevalier et al., 2014; Rueda et al., 2005). Our results indicated that on a directional Stroop task, preschool-aged children showed conflict-related modulation of the N2 component in both amplitude and latency over frontal and central sites. These results are consistent with both the adult literature and studies of school-aged children that report modulation of N2 amplitude and latency in relation to increasing conflict and response inhibition demands (Downes et al., 2017). The literature on N2 modulation by cognitive conflict in preschool-aged children is quite small and relatively inconsistent. Although some studies have detected modulation of this component (Espinet et al., 2012; Lahat et al., 2009), others have not, despite using versions of classical inhibition (e.g. Go/No-Go) and cognitive conflict (e.g. Attentional Networks Task) tasks (Abundis-Gutiérrez, Checa, Castellanos, & Rueda, 2014; Buss, Dennis, Brooker, & Sippel, 2011; Chevalier, Kelsey, Wiebe, & Espy, 2014; Rueda et al., 2005).
We suspect that the N2 effect we observed may be related to three factors of our study design. First, behavioral results demonstrated that the task induced a robust conflict effect in children’s reaction times. Additionally, reaction time on the more difficult, incongruent trial type was related to both N2 amplitude and latency. As such, because our task produced strong behavioral effects of conflict, this likely increased our ability to detect neural effects of conflict in the N2 component. Second, differences across developmental studies in the selection of timing windows for the N2 component may also explain inconsistencies in this literature. Like most ERP components, some developmental studies of school-aged children report decreases in both amplitude and latency of the N2 component with age, although this literature is somewhat mixed (Downes et al., 2017). Inconsistent effects of N2 modulation in preschool-aged children may therefore be related to differences across studies in the timing window utilized for this component. In the current study, timing windows for the N2 component were identified for Fz and Cz based on when the component was maximal (380–600 ms at Fz; 352–520 ms at Cz). Utilization of earlier timing windows, especially at anterior sites where the component may be quite delayed in young children, could reduce the likelihood of detecting modulation effects. Last, source localization studies indicate that the location of anterior cingulate generators for the N2 depends on individual differences in school-aged children’s EF performance (Lamm, David, & Lewis, 2006). For inclusion in our ERP analyses, children were required to meet an accuracy criterion of ≥50% accuracy across trial types and blocks. Overall, this resulted in high mean accuracy rates (85–90%) across trial types. High behavioral accuracy, within the context of conflict effects in reaction time, suggests our behavioral task was a developmentally appropriate and sensitive measure of conflict for young children, likely maximizing our ability to detect N2 modulation in this younger age group.
We also found that young children showed conflict-related modulation of the P3 component at parietal sites, although this effect occurred in the absence of any latency modulation. The P3 component in our preschool-aged sample was maximal and exhibited its complex form (separable P3a and P3b components) at posterior sites (Pz). In the directional Stroop task, inhibitory control processes occurred following the identification of task-based conflict. However, unlike many classic inhibitory control tasks that require participants to withhold a prepotent response (e.g. Go/No-Go task), children were required to make a behavioral response even on trials that required inhibition of their prepotent tendency. Task-related modulation of the P3 component by cognitive conflict has not been well-studied in young children, although this component is associated with working memory processes in older children (Polich et al., 1990). Previous studies have detected minimal P3 modulation by trial type in slightly older children during an inhibitory control task (Spronk et al., 2008); however, P3 activity has been documented in school-aged children during an affective-based EF task (Carlson, Zayas, & Guthormsen, 2009). Developmental studies of the P3 component in school-aged children, especially the P3b which is more closely tied to executive control and working memory in adults, have demonstrated that this component undergoes extended maturation in both latency and amplitude into the early adulthood years (Downes et al., 2017). Our results are consistent with this literature; P3 effects of cognitive conflict can be observed even in preschool-aged children, although component latencies and typology are immature, especially at more anterior sites.
Interestingly, the effects of task conflict were also present at early, exogenous components in our preschool-aged population. Specifically, preschool-aged children showed conflict-related modulation of both the N1 and P2 component amplitude, as well as an effect of trial type on N1 latency. Trials with higher conflict increased the N1 amplitude and decreased the P2 amplitude in comparison to the easier, congruent trial type. Previous developmental ERP studies have indicated that developmental differences in ERP components are not always present for early sensory components (Brown & Jernigan, 2012). Although adult ERP studies have documented effects of EF tasks on early attentional components (e.g. Lorist & Jolij, 2012), to our knowledge this has not been well-investigated in developmental studies. Based on the task design, visual stimuli in the current study differed for congruent versus incongruent trials, and this was not randomized across participants. Although it is possible that the modulation of these early ERP components by conflict is an effect of differential visual stimuli, the current results suggest that neural processing of conflict in the preschool-aged brain can occur as soon as 100–150 ms post-stimulus.
ERP and Individual Differences in EF
We found that both the amplitudes and latencies of later cognitive ERP components were related to individual differences in task behavior, neuropsychological measures of IQ, and parent-report of EF across the full sample of children. Importantly, although we did not correct for multiple comparisons in our analyses, these associations between electrophysiology and other measures of EF and global functioning were specific, in that they were restricted to the more difficult, incongruent, trial type and were not present for early attentional components. Ultimately, these relationships indicate that differences in neural processing measured at the electrophysiological level on the directional Stroop task are meaningful across other types of behavioral measurement.
Correlation analyses indicated that higher levels of conflict at the electrophysiological level (e.g. larger N2 and P3 amplitudes for incongruent trials) were associated with poorer behavioral performance (slower reaction times), lower IQ scores, and more EF problems as reported by parents. Reductions in N2 effects are often associated with improved task performance (Brydges et al., 2014; Buss et al., 2011; Espinet et al., 2012; Espinet, Anderson, & Zelazo, 2013), and age-related changes in the N2 component are inferred to represent maturation of the anterior cingulate cortex (Buss et al., 2011). The relationship between the P3 component, task behavior, and other concurrent measures of EF has not been well-investigated in developmental studies, although correlations between P3b component amplitudes and EF measures have previously been detected in older children (Brydges et al., 2014). Current results complement previous research in older children linking EF task behavior and ERPs, while extending into a younger age group and expanding measures to include parent-reported behavior.
Interestingly, neuropsychological measures of processing speed were related to behavioral EF task reaction times, but processing speed was not related to ERP component latency. This demonstrates that the neural measure of processing speed obtained via ERP is quite different from both behavioral (reaction time) and neuropsychological (processing speed quotient) measures of processing speed. Because ERP is able to measure the brain’s electrical response to a stimulus on the order of milliseconds, behavioral and neuropsychological measures of processing speed by necessity include a confounded motor component. However, even ERP measures of neural processing speed will likely vary by the cognitive demands of the task (see recent review in Cepeda, Blackwell, & Munakata, 2013). The heterogeneity of this construct is important to consider when characterizing individual and/or group differences in processing speed in young children, who are likely to show exaggerated effects of motor and attention demands on processing speed measures.
The association between later cognitive ERP components and parent-report and neuropsychological measures indicates that electrophysiological measures of EF are sensitive to differences observed both in other laboratory measures of neuropsychological functioning as well as in children’s real-world behaviors. This is significant given that behavioral measures of EF and related neuropsychological assessments, particularly for younger children, often lack the sensitivity to detect subtle differences in brain development in at-risk populations. For example, at-risk populations such as children born preterm may show equivalent performance on some behavioral measures of EF, such as non-verbal inhibitory control and spatial working memory, (e.g. Brumbaugh et al., 2014; Hodel, Brumbaugh et al., 2015) despite neuroimaging evidence that brain structure and/or function are altered (Kelly et al., 2015; Munakata et al., 2013; Schonhaut, Armijo, & Perez, 2015; Walsh, Doyle, Anderson, Lee, & Cheong, 2014). Furthermore, at-risk populations may experience reorganization of neural systems supporting EF, which could ultimately result in either equivalent or poorer behavioral performance. The ability to understand early changes in neural systems supporting EF, even when group differences in behavior are not detected, may help identify individuals at risk for atypical functioning later in development.
Impacts of Moderate-to-Late PT Birth
Neuropsychological assessments indicated there was a relationship between moderate-to-late PT birth and measures of processing speed. Specifically, within the group of children born moderate-to-late PT, higher gestational age at birth was associated with higher processing speed scores. However, it is important to note that the PT group in general did not differ from their FT peers, including on parental report of EF difficulties, and fell well within the normal range on all assessments.
Although ERP measures of EF provide increased sensitivity in comparison to behavioral measures, we found relatively few differences in EF task behavior and/or electrophysiology in our low-risk PT sample. Children born moderate-to-late PT showed trend level reductions in P2 amplitude over frontal sites in comparison to their FT peers. The P2 component has not been widely studied in the context of EF tasks, but likely reflects early attentional modulation of perceptual processing (e.g. Steinberg, Moeller, & Swann, 2009). While this marginally significant group difference should be interpreted with caution, a reduced P2 amplitude across both congruent and incongruent trial types could reflect poorer alertness to detect stimuli in the PT group, which may be an early sign of altered attention development. Although moderate-to-late PT and FT children did not differ in P3 amplitude or latency at anterior sites, degree of prematurity was correlated with P3b latency on incongruent trials within the PT group, suggesting immaturity of inhibitory control processing in earlier born PT children. Last, group by trial type interactions for the N2 and P3a components indicated greater conflict modulation of latency in the PT versus the FT group.
The associations of both P3a conflict modulation and P3b latency to peak on incongruent trials with low-risk PT birth, along with differential modulation of N2, suggest that multiple aspects of EF processing, including attentional orienting (P3a), executive attention and working memory (P3b), and cognitive conflict and inhibition (Downes et al., 2017; Polich, 2007) may be impacted by low-risk PT birth. Although behavioral accuracy was equivalent across the two groups, these increased neural conflict effects may reflect increased task difficulty for PT children. Again, these group differences should be interpreted with caution, as they would be non-significant following correction for multiple comparisons. However, our results are consistent with the few studies to date that have assessed EF in a laboratory context in lower-risk PT children. This work has documented relatively subtle impacts of low-risk PT birth on laboratory measures of EF development (Brumbaugh et al., 2014; Hodel, Brumbaugh et al., 2015), consistent with results from larger, population-based studies reporting increased rates of school difficulties (Lipkind et al., 2012; Morse et al., 2009) It is possible that the combination of EF abilities indexed by the directional Stroop task used here does not reflect those EF skills that are most impacted by prematurity during the preschool-age range. Additionally, as is common in developmental studies with young children, participants were excluded from ERP analyses due to excessive artifact and/or poor behavioral accuracy. This may have resulted in the exclusion of moderate-to-late preterm children with the highest levels of risk, minimizing our ability to detect an overall effect of moderate-to-late preterm birth on ERP measures of EF.
Equivalent EF task behavior and electrophysiology could also be reflective of the low-risk nature of our PT sample. Although PT children were intentionally selected to be of low medical risk (based on gestational age and study inclusion criteria), they were also from low environmental risk backgrounds (i.e. middle to high socioeconomic status families). A hospital and/or epidemiological cohort of moderate-to-late PT children with more diverse levels of both neonatal and environmental risk may result in more pronounced differences between groups. Because the health complications of moderate-to-late preterm birth are heterogeneous, an important future direction of this work is more careful consideration of how individual differences in perinatal characteristics (e.g. birth weight variation) and the post-natal environment (e.g. parental education and/or income) are related to long-term measures of neurobehavioral development in lower-risk PT children. Alternatively, our failure to detect group differences may be related to the fact that the early childhood period is also a period of rapid development for both behavioral measures of EF as well as underlying neural circuitry. Increased individual variability during this time of dramatic change may minimize the ability to detect relatively subtle group differences in functioning.
Large-scale epidemiological studies indicate that moderate-to-late PT birth has long-term impacts on educational and occupational attainment, even after controlling for familial socioeconomic status (Heinonen et al., 2013). Research in normative populations linking early differences in EF to such long-term outcomes suggests prefrontal development is likely altered in moderate-to-late PT children. However, the time point at which altered development of EF in this population is most easily observed is unknown. Future longitudinal work employing both behavioral and neural measures in a cohort of moderate-to-late PT children with more diverse medical and environmental risk is warranted to determine when EF difficulties become most salient (e.g. when structure and demands increase during the transition into formalized schooling).
Conclusion
In conclusion, we documented that cognitive ERP components are present and modulated by task conflict demands during the early childhood period. Furthermore, exploratory analyses indicated that individual differences in electrophysiological measures of cognitive conflict and inhibitory control processes were related to behavioral indices of task performance, neuropsychological measures of IQ, and parent-report of EF in children’s everyday lives. Finally, ERP measures of prefrontal-dependent behavior were able to detect some subtle differences in cognitive processing in at-risk children (i.e. children born moderate-to-late preterm), even in the context of equivalent task behavior. Although these group differences would not survive correction for multiple comparisons, they are consistent with a broader literature documenting altered EF development in this population.
Sensitive measurement of both group and individual differences in EF during the early childhood period is critical. This is especially relevant given recent interest in developing effective interventions to promote EF development in at-risk populations (Diamond & Lee, 2011). The current study demonstrates that ERP measures of EF are feasible and sensitive to individual differences within the early childhood period. Although ERP may be a measure ideally suited to investigating subtle impacts of early adversity on EF development, ERP techniques of course have their own methodological issues. Challenges in working with younger populations who are less compliant and cannot tolerate long ERP testing sessions include noisier data, small samples sizes, and reductions in the number of trials available for analysis, in comparison with older children and adults. Unfortunately, these data quality problems are likely heightened when working with at-risk children. Additionally, there is a lack of consensus on best practices in pediatric ERP research (Downes et al., 2017). This is particularly relevant for ERP studies of EF in younger populations, as this research remains quite limited. Techniques to improve data collection from young and at-risk children, along with standardization of reporting methodological and analysis details, will continue to improve our understanding of the neural correlates of EF in younger children.
The current study demonstrates that ERP measures are useful both for understanding the neural correlates of EF in young children and for detecting individual differences in EF development. Given that modulation of ERP components by task conflict was demonstrated in our preschool-aged sample of children, both N2 and P3 effects may serve as easily measurable biomarkers (Buss et al., 2011) for prefrontal cortex development, even in young children. ERP measures of EF represent an opportunity to understand the development of these neural systems during early childhood, and can be used to characterize how early prefrontal cortex development differs across individuals and in children who are at risk for atypical EF development.
Supplementary Material
Acknowledgments
This research was supported by pre-and post-doctoral training grants at the University of Minnesota (NIH T32-HD007151 and T32-DA022616), a University of Minnesota Graduate Fellowship and Doctoral Dissertation Fellowship, the Benjamin Walker Hanson Neonatology Fund, and the University of Minnesota Center for Neurobehavioral Development.
The authors thank members of the Cognitive Development and Neuroimaging Lab for their help and support, especially Alyssa Morris and Shelby Rentmeester, for their assistance with participant testing and recruitment. We also thank all the children and families who participated in this research.
References
- Aarnoudse-Moens CSH, & Smidts DP (2009). Executive function in very preterm children at early school age. Journal of Abnormal Child Psychology, 37, 981–993. 10.1007/s10802-009-9327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abundis-Gutiérrez A, Checa P, Castellanos C, & Rueda MR (2014). Electrophysiological correlates of attention networks in childhood and early adulthood. Neuropsychologia, 57(1), 78–92. 10.1016/j.neuropsychologia.2014.02.013 [DOI] [PubMed] [Google Scholar]
- Alvarez J. a., & Emory E (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16(1), 17–42. 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW, & Victorian Infant Collaborative Study Group. (2004). Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics, 114(1), 50–57. [DOI] [PubMed] [Google Scholar]
- Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, … Counsell SJ (2012). The effect of preterm birth on thalamic and cortical development. Cerebral Cortex, 22(5), 1016–1024. 10.1093/cercor/bhr176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron IS, Erickson K, Ahronovich MD, Coulehan K, Baker R, & Litman FR (2009). Visuospatial and verbal fluency relative deficits in “complicated” late-preterm preschool children. Early Human Development, 85(12), 751–754. 10.1016/j.earlhumdev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Baron IS, Kerns KA, Muller U, Ahronovich MD, & Litman FR (2012). Executive functions in extremely low birth weight and late-preterm preschoolers: Effects on working memory and response inhibition. Child Neuropsychology, 18(6), 586–599. [DOI] [PubMed] [Google Scholar]
- Best JR, & Miller PH (2010). A developmental perspective on executive function. Child Development, 81(6), 1641–1660. 10.1111/j.1467-8624.2010.01499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjuland KJ, Rimol LM, Løhaugen GCC, & Skranes J (2014). Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. European Journal of Paediatric Neurology, 18(5), 1–13. 10.1016/j.ejpn.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Brown TT, & Jernigan TL (2012). Brain development during the preschool years. Neuropsychology Review, 22(4), 313–333. 10.1007/s11065-012-9214-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh JE, Hodel AS, & Thomas KM (2014). The impact of late preterm birth on executive function at preschool age. American Journal of Perinatology, 31(4), 305–314. 10.1055/s-0033-1348950 [DOI] [PubMed] [Google Scholar]
- Brydges CR, Fox AM, Reid CL, & Anderson M (2014). Predictive validity of the N2 and P3 ERP components to executive functioning in children: a latent-variable analysis. Frontiers in Human Neuroscience, 8(February), 80. 10.3389/fnhum.2014.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss K. a., Dennis T. a., Brooker RJ, & Sippel LM (2011). An ERP study of conflict monitoring in 4–8-year old children: Associations with temperament. Developmental Cognitive Neuroscience, 1(2), 131–140. 10.1016/j.dcn.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Zayas V, & Guthormsen A (2009). Neural correlates of decision making on a gambling task. Child Development, 80(4), 1076–1096. 10.1111/j.1467-8624.2009.01318.x [DOI] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, … Shoda Y (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences of the United States of America, 108(36), 14998–15003. 10.1073/pnas.1108561108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Blackwell K. a., & Munakata Y (2013). Speed isn’t everything: Complex processing speed measures mask individual differences and developmental changes in executive control. Developmental Science, 16(2), 269–286. 10.1111/desc.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, & Quigley MA (2014). School performance at age 7 years in late preterm and early term birth: a cohort study. Archives of Disease in Childhood - Fetal and Neonatal Edition, 99(6), F451–F457. 10.1136/archdischild-2014-306124 [DOI] [PubMed] [Google Scholar]
- Chevalier N, Kelsey KM, Wiebe S. a., & Espy KA (2014). The Temporal Dynamic of Response Inhibition in Early Childhood: An ERP Study of Partial and Successful Inhibition. Developmental Neuropsychology, 39(8), 585–599. 10.1080/87565641.2014.973497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyi LJ, Lee HC, Hintz SR, Gould JB, & Sutcliffe TL (2008). School outcome in late preterm infants: Special needs and challenges for infants born at 32 to 36 weeks gestation. Journal of Pediatrics, 153, 25–31. [DOI] [PubMed] [Google Scholar]
- Davidson M, Cruess L, Diamond A, O’Craven KM, & Savoy RL (1999). Comparison of executive functions in children and adults using directional Stroop tasks. In Biennial Meeting of the Society for Research in Child Development, April; Albuquerque, NM. [Google Scholar]
- Davis EP, Bruce J, Snyder K, & Nelson C. a. (2003). The X-trials: neural correlates of an inhibitory control task in children and adults. Journal of Cognitive Neuroscience, 15(3), 432–443. 10.1162/089892903321593144 [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Diamond A, & Lee K (2011). Interventions shown to aid executive function development in children 4–12 years old. Science, 333(6045), 959–964. 10.1126/science.1204529.Interventions [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes M, Bathelt JOE, & Haan MDE (2017). Event-related potential measures of executive functioning from preschool to adolescence. Developmental Medicine & Child Neurology, 581–590. 10.1111/dmcn.13395 [DOI] [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, … Casey BJ (2006). Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science, 17(6), 478–484. 10.1111/j.1467-9280.2006.01732.x [DOI] [PubMed] [Google Scholar]
- Espinet SD, Anderson JE, & Zelazo PD (2012). N2 amplitude as a neural marker of executive function in young children: An ERP study of children who switch versus perseverate on the Dimensional Change Card Sort. Developmental Cognitive Neuroscience, 2, S49–S58. 10.1016/j.dcn.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinet SD, Anderson JE, & Zelazo PD (2013). Reflection training improves executive function in preschool-age children: Behavioral and neural effects. Developmental Cognitive Neuroscience, 4, 3–15. 10.1016/j.dcn.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, & Van Petten C (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology, 45(1), 152–170. 10.1111/j.1469-8986.2007.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2(10), 861–3. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Griffiths ST, Gundersen H, Neto E, Elgen I, Markestad T, Aukland SM, & Hugdahl K (2013). fMRI: Blood oxygen level-dependent activation during a working memory-selective attention task in children born extremely preterm. Pediatric Research, 74(2), 196–205. 10.1038/pr.2013.79 [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, & Osterman MJK (2016). Births: Preliminary Data for 2015. National Vital Statistics Reports, 65(3), 1–15. [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff E. a, Gee JC, Davidson RJ, & Pollak SD (2010). Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30(22), 7466–72. 10.1523/JNEUROSCI.0859-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen K, Eriksson JG, Kajantie E, Pesonen A-K, Barker DJ, Osmond C, & Raikkonen K (2013). Late-Preterm birth and lifetime socioeconomic attainments: The Helsinki Birth Cohort Study. Pediatrics, 132(4), 647–655. 10.1542/peds.2013-0951 [DOI] [PubMed] [Google Scholar]
- Hendry A, Jones EJH, & Charman T (2016). Executive function in the first three years of life : Precursors, predictors and patterns. Developmental Review, 42, 1–33. 10.1016/j.dr.2016.06.005 [DOI] [Google Scholar]
- Hodel AS (2018). Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Developmental Review. 10.1016/j.dr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Brumbaugh JE, Morris AR, & Thomas KM (2015). Hot executive function following moderate-to-late preterm birth: Altered delay discounting at 4 years of age. Developmental Science, 19(2), 221–234. 10.1111/desc.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, & Thomas KM (2015). Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage, 105, 112–119. 10.1016/j.neuroimage.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isquith PK, Crawford JS, Espy KA, & Gioia G. a. (2005). Assessment of executive function in preschool-aged children. Mental Retardation and Developmental Disabilities Research Reviews, 11(3), 209–215. 10.1002/mrdd.20075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, & Braun C (1999). The polar average reference effect: A bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology, 110(6), 1149–1155. 10.1016/S1388-2457(99)00044-9 [DOI] [PubMed] [Google Scholar]
- Kane MJ, & Engle RW (2002). The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychonomic Bulletin & Review, 9(4), 637–671. 10.3758/BF03196323 [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Robaey P, Bourassa M, & Koning DDE (2000). ERP differences in visual attention processing between attention-deficit hyperactivity disorder and control boys in the absence of performance differences. Psychophysiology, 37, 319–333. [PubMed] [Google Scholar]
- Kelly CE, Cheong JLY, Gabra Fam L, Leemans A, Seal ML, Doyle LW, … Thompson DK (2016). Moderate and late preterm infants exhibit widespread brain white matter microstructure alterations at term-equivalent age relative to term-born controls. Brain Imaging and Behavior, 10(1), 41–49. 10.1007/s11682-015-9361-0 [DOI] [PubMed] [Google Scholar]
- Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, … Ment LR (2008). Brain volume reductions within multiple cognitive systems in preterm children at age twelve. Journal of Pediatrics, 152(4), 513–521. 10.1016/j.peds.2007.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard I, Obel C, Hedegaard M, & Henriksen TB (2006). Gestational age and birth weight in relation to school performance of 10-year-old children: a follow-up study of children born after 32 completed weeks. Pediatrics, 118(4), 1600–1606. 10.1542/peds.2005-2700 [DOI] [PubMed] [Google Scholar]
- Kugelman A, & Colin AA (2013). Late preterm infants: Near term but still in a critical developmental time period. Pediatrics, 132(4), 741–751. 10.1542/peds.2013-1131 [DOI] [PubMed] [Google Scholar]
- Lahat A, Todd RM, Mahy CEV, Lau K, & Zelazo PD (2009). Neurophysiological correlates of executive function: a comparison of European-canadian and chinese-canadian 5-year-old children. Frontiers in Human Neuroscience, 3(72), 1–10. 10.3389/neuro.09.072.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, David P, & Lewis MD (2006). Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia, 44, 2139–2148. 10.1016/j.neuropsychologia.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, Mcdermott JM, & Fox NA (2014). Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: An ERP study. Developmental Science, 5, 667–681. 10.1111/desc.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EJ, Rubia K, Murray RM, McGuire PK, Walshe M, Allin M, … Nosarti C (2009). The neural basis of response inhibition and attention allocation as mediated by gestational age. Human Brain Mapping, 30(3), 1038–1050. 10.1002/hbm.20564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science, 16(5), 641–52. 10.1111/desc.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind HS, Slopen ME, Pfeiffer MR, & McVeigh KH (2012). School-age outcomes of late preterm infants in New York City. American Journal of Obstetrics and Gynecology, 206(3), 222.e1–222.e6. 10.1016/j.ajog.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Lorist MM, & Jolij J (2012). Trial history effects in Stroop task performance are independent of top-down control. PLoS ONE, 7(6). 10.1371/journal.pone.0039802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, & Hillyard SA (1990). Visual event-related potentials index focused attention within bilateral stimuls arrays. II. Functional dissociation fo P1 and N1 components. Electroencephalography and Clinical Neurophysiology, 75, 528–542. [DOI] [PubMed] [Google Scholar]
- Luu TM, Ment L, Allan W, Schneider K, & Vohr BR (2011). Executive and memory function in adolescents born very preterm. Pediatrics, 127(3), e639–e646. 10.1542/peds.2010-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow N, Hennessy EM, Bracewell M. a, & Wolke D (2007). Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics, 120(4), 793–804. 10.1542/peds.2007-0440 [DOI] [PubMed] [Google Scholar]
- Mayes LC, Molfese DL, Key APF, & Hunter NC (2005). Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicology and Teratology, 27(6), 797–813. 10.1016/j.ntt.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, … Klein DN (2013). Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology, 41(8), 1257–1266. 10.1007/s10802-013-9762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differencesi n executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. 10.1177/0963721411429458.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki a H., Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Molfese VJ, Molfese PJ, Molfese DL, Rudasill KM, Armstrong N, & Starkey G (2010). Executive function skills of 6–8 year olds: Brain and behavioral evidence and implications for school achievement. Contemporary Educational Psychology, 35(2), 116–125. 10.1016/j.cedpsych.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, & Hiraki K (2011). Longitudinal development of prefrontal function during early childhood. Developmental Cognitive Neuroscience, 1(2), 153–162. 10.1016/j.dcn.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, & Hiraki K (2013). Prefrontal cortex and executive function in young children: a review of NIRS studies. Frontiers in Human Neuroscience, 7(December), 867. 10.3389/fnhum.2013.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse SB, Zheng H, Tang Y, & Roth J (2009). Early school-age outcomes of late preterm infants. Pediatrics, 123(4), e622–e629. 10.1542/peds.2008-1405 [DOI] [PubMed] [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, … Ment LR (2011). Preterm birth results in alterations in neural connectivity at age 16 years. NeuroImage, 54(4), 2563–2570. 10.1016/j.neuroimage.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata S, Okada T, Okahashi A, Yoshikawa K, Usukura Y, Makimoto M, … Okuhata Y (2013). Gray matter volumetric MRI differences late-preterm and term infants. Brain and Development, 35(1), 10–16. 10.1016/j.braindev.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Mürner-Lavanchy I, Steinlin M, Nelle M, Rummel C, Perrig WJ, Schroth G, & Everts R (2014). Delay of cortical thinning in very preterm born children. Early Human Development, 90(9), 443–450. 10.1016/j.earlhumdev.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Nagy Z, Ashburner J, Andersson J, Jbabdi S, Draganski B, Skare S, … Lagercrantz H (2009). Structural correlates of preterm birth in the adolescent brain. Pediatrics, 124(5), e964–e972. 10.1542/peds.2008-3801 [DOI] [PubMed] [Google Scholar]
- Narberhaus A, Segarra D, Cald X, & Gim M (2008). Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia, 46, 111–116. 10.1016/j.neuropsychologia.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Nosarti C (2010). Neurodevelopmental outcomes following very preterm birth. (Murray R & Maureen H, Eds.). Cambridge University Press. [Google Scholar]
- Nosarti C (2013). Structural and functional brain correlates of behavioral outcomes during adolescence. Early Human Development, 89(4), 221–227. 10.1016/j.earlhumdev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, … Murray RM (2008). Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain, 131, 205–217. 10.1093/brain/awm282 [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rubia K, Smith AB, Frearson S, Williams SC, Rifkin L, & Murray RM (2006). Altered functional neuroanatomy of response inhibition in adolescent males who were born very preterm. Developmental Medicine and Child Neurology, 48(4), 265–271. 10.1017/S0012162206000582 [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, … Ment LR (2000). Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Journal of the American Medical Association, 284(15), 1939–1947. 10.1001/jama.284.15.1939 [DOI] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neuorphysiology, 118(10), 2128–2148. 10.1016/j.clinph.2007.04.019.Updating [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Ladish C, & Burns T (1990). Normal variation of P300 in children: Age, memory span, and head size. International Journal of Psychophysiology, 9(3), 237–248. 10.1016/0167-8760(90)90056-J [DOI] [PubMed] [Google Scholar]
- Quigley M, Poulsen G, Kurinczuk J, Boyle E, Field D, Wolke D, & Alfirevic Z (2012). Early term and late preterm birth are associated with poorer school performance at age 5 years: A cohort study. Archives of Disease in Childhood - Fetal and Neonatal Edition, 97, 167–174. 10.1136/archdischild-2011-300888 [DOI] [PubMed] [Google Scholar]
- Rietdijk WJR, Franken IHA, & Thurik AR (2014). Internal consistency of event-related potentials associated with cognitive control : N2 / P3 and ERN / Pe. PLoS ONE, 9(7), 3–9. 10.1371/journal.pone.0102672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK, & Davis-Stober CP (2004). Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neuroscience, 5, 39. 10.1186/1471-2202-5-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, & Posner MI (2005). Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the United States of America, 102(41), 14931–6. 10.1073/pnas.0506897102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhaut L, Armijo I, & Perez M (2015). Gestational age and developmental risk in moderately and late preterm and early term infants. Pediatrics, 135(4), e835–41. 10.1542/peds.2014-1957 [DOI] [PubMed] [Google Scholar]
- Spronk M, Jonkman LM, & Kemner C (2008). Response inhibition and attention processing in 5-to 7-year-old children with and without symptoms of ADHD: An ERP study. Clinical Neurophysiology, 119(12), 2738–2752. 10.1016/j.clinph.2008.09.010 [DOI] [PubMed] [Google Scholar]
- Steinberg L, Moeller FG, & Swann AC (2009). P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology, 46(5), 1–20. 10.1111/j.1469-8986.2009.00845.x.P50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Sutherland MT, & McKinney CJ (2005). Validation of SOBI components from high-density EEG. NeuroImage, 25(2), 539–553. 10.1016/j.neuroimage.2004.11.027 [DOI] [PubMed] [Google Scholar]
- Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, … Inder TE (2007). Perinatal risk factors altering regional brain structure in the preterm infant. Brain, 130(3), 667–677. 10.1093/brain/awl277 [DOI] [PubMed] [Google Scholar]
- van Baar AL, Vermaas J, Knots E, de Kleine MJK, & Soons P (2009). Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics, 124(1), 251–257. 10.1542/peds.2008-2315 [DOI] [PubMed] [Google Scholar]
- Vohr B (2013). Long-term outcomes of moderately preterm, late preterm, and early term infants. Clinics in Perinatology, 40, 739–751. [DOI] [PubMed] [Google Scholar]
- Walsh JM, Doyle LW, Anderson PJ, Lee KJ, & Cheong JLY (2014). Moderate and late preterm birth: Effect on brain size and maturation at term-equivalent age. Radiology, 273(1), 132410. 10.1148/radiol.14132410 [DOI] [PubMed] [Google Scholar]
- Weschler. (2002). Wechsler Preschool and Primary Scale of Intelligence - Third Edition. Harcourt Assessment. [Google Scholar]
- Wiersema JR, & Roeyers H (2009). ERP correlates of effortful control in children with varying levels of ADHD symptoms. Journal of Abnormal Child Psychology, 37(3), 327–336. 10.1007/s10802-008-9288-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


