Abstract
Purpose:
Slight changes in urinary incontinence severity may be difficult to notice, so that even high functioning patients are unable to detect if urinary incontinence is improving or worsening. We describe a recently released free software app, iDry®, that enables individuals with urinary incontinence to document incontinence symptoms, view progress, evaluate effectiveness of interventions and report status to their health care provider.
Materials and Methods:
After 2 field trials, iDry was published as a free download from the Apple® App Store and was downloaded 1,231 times in the first 19 months. iDry also collects large quantities of anonymized usage data for research purposes.
Results:
Data analysis shows that long-term users had significantly more severe urinary incontinence symptoms (p ≤0.01) than short-term users. Short-term users reduced pad use by 20% but long-term users’ pad use remaining unchanged. Average leakage was reduced 14.6 mg per day for short-term vs 4.5 mg per day for long-term users, but this difference was not statistically significant (p=0.93) due to high data variability (SD 611). There was no significant difference between long-term and short-term users in severity of self-reported stress and urge incontinence. Bladder training positively correlated with a reduction in pad use (p=0.03) and leakage amount (p=0.02).
Conclusions:
Overall our findings suggest that iDry is a useful, accessible and convenient tool to document urinary incontinence symptoms and improvement, but controlled studies are needed to assess its effectiveness.
Keywords: urinary incontinence, software
Urinary incontinence is a serious problem affecting more than 13 million Americans, with annual direct health care costs of more than $30 billion.1 While UI is often associated with the elderly, a recent study showed that 15% of young women age 18 to 30 also reported urinary incontinence.2
Slight changes in UI severity, such as a 10% change in total leakage over a month, may be difficult to notice, leading even high functioning patients to be unable to tell physicians whether their UI is getting better or worse, and physicians being unable to determine the effectiveness of prescribed interventions. To our knowledge there are no other available tools that document incremental changes in UI symptoms while also assisting patients and physicians in evaluating the efficacy of interventions.
We describe a free software app, iDry, developed by Touchtown Inc., that addresses this problem in 2 ways. 1) iDry enables individuals with UI to conveniently and accurately document their incontinence symptoms, view progress in managing UI, evaluate effectiveness of interventions and report status to health care providers. Improved self-monitoring enhances the self-management of symptoms.3 2) iDry collects large quantities of anonymized usage data, assisting researchers in studying health behaviors and incontinence outcomes. iDry has several built-in features to help achieve these goals.
Incident Logging
A patient can record urine loss by selecting the time of a pad change (defaulting to “now”), selecting a pad from a database of 210 popular products and using a slider to indicate the degree of pad saturation (fig. 1). Data entry is quick, encouraging consistent data input and accuracy. The user selects the method, which can be as formal as weighing the wet pad or as simple as estimating the degree of pad saturation. A progress summary section shows overall progress by awarding 1 to 6 stars and showing an estimated date when, if trends continue, the user might be completely dry.
Figure 1.
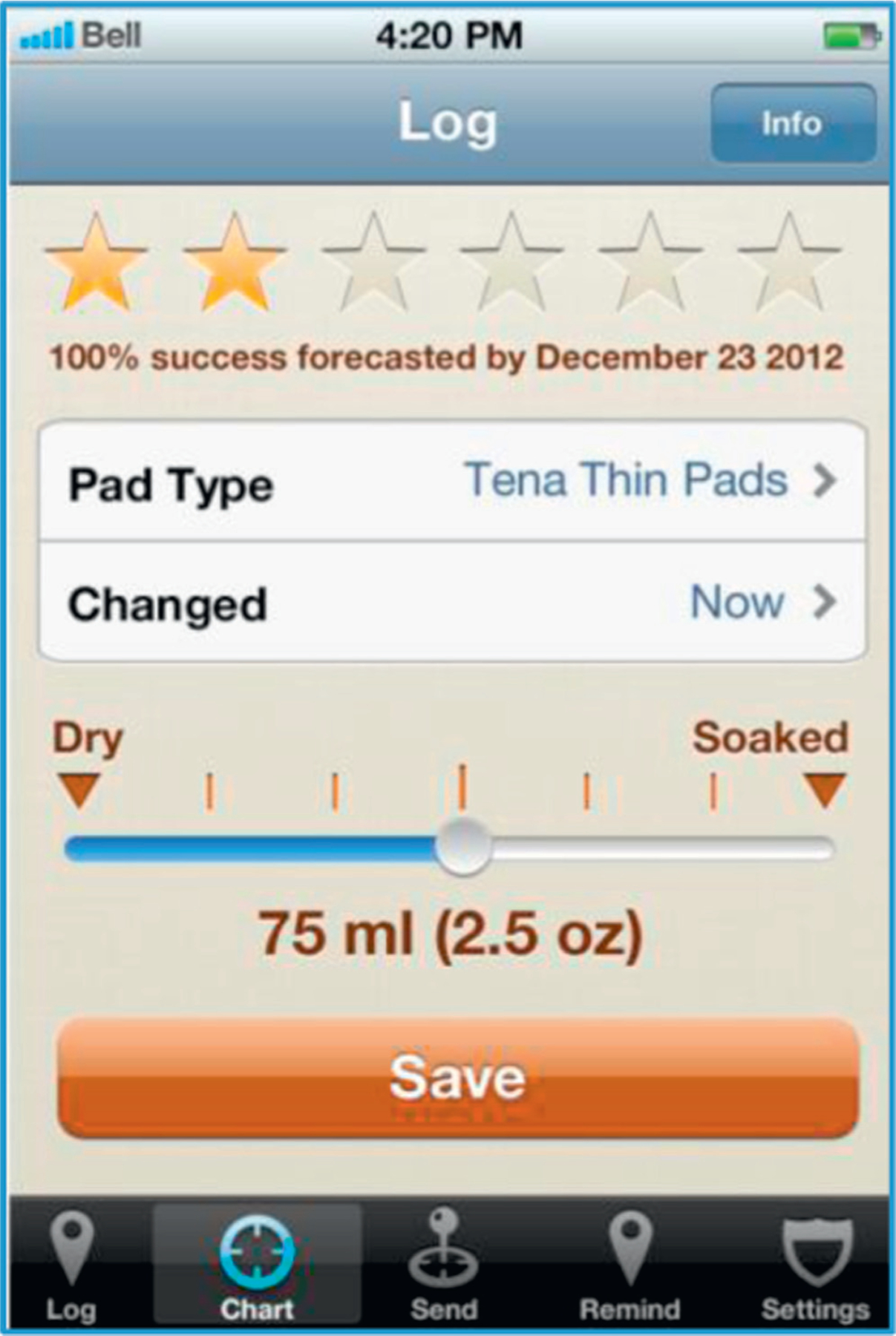
Main iDry log screen, used to record pad changes and display summary of user changes in UI with time.
Charting
Based on automated analysis of logged data, iDry shows the number of pad changes and the self-reported urine loss by day, week, month, quarter and year (fig. 2). Other charts show number of pads used, volume of urine loss and rate of urine retention over time. All charts show percentage change over time (for example, “15% improved vs previous month”).
Figure 2.
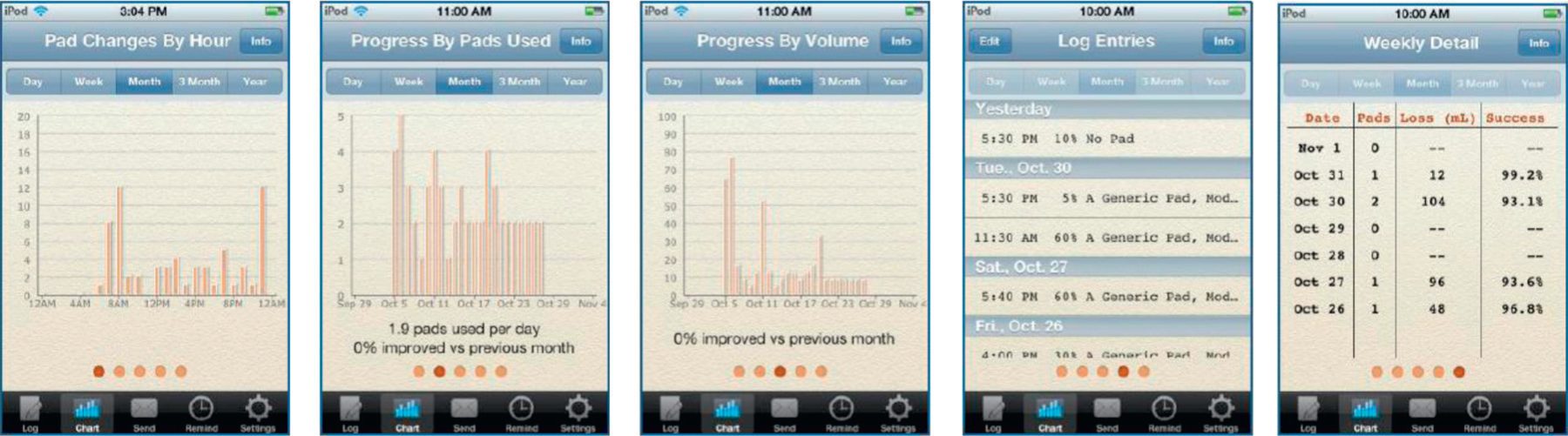
Sample iDry charts showing summaries and analysis of user UI with time. All charts can also be emailed.
Evaluating Interventions
iDry’s database contains 48 interventions for managing UI, including lifestyle management (alcohol control, managing fluid intake etc), physical therapy (biofeedback, bladder training etc), medications (imipramine etc), holistic and alternative therapies (yoga and herbs), physical devices, and surgical and medical procedures. Users can select an intervention, read a brief summary and research further by clicking a hyperlink. They can also activate an intervention, causing iDry to track the user’s progress since the start of that intervention, for example, “Alcohol control: 32% improvement since Jan 14, 2014.” iDry separately tracks progress for each intervention, allowing users to evaluate the effectiveness of each.
Reminders
Users can use this feature to set reminders for activities such as pelvic floor muscle exercises and scheduled voiding. They can specify the days when reminders are sent, start and end times, frequency of reminders and reminder texts.
Settings
Various settings allow users to report demographic information such as birth year, gender, height, weight, race, UI cause, UI start date, severity of symptoms and interventions used (fig. 3). User privacy is maintained as none of the entered information in iDry can be used to identify individual users.
Figure 3.
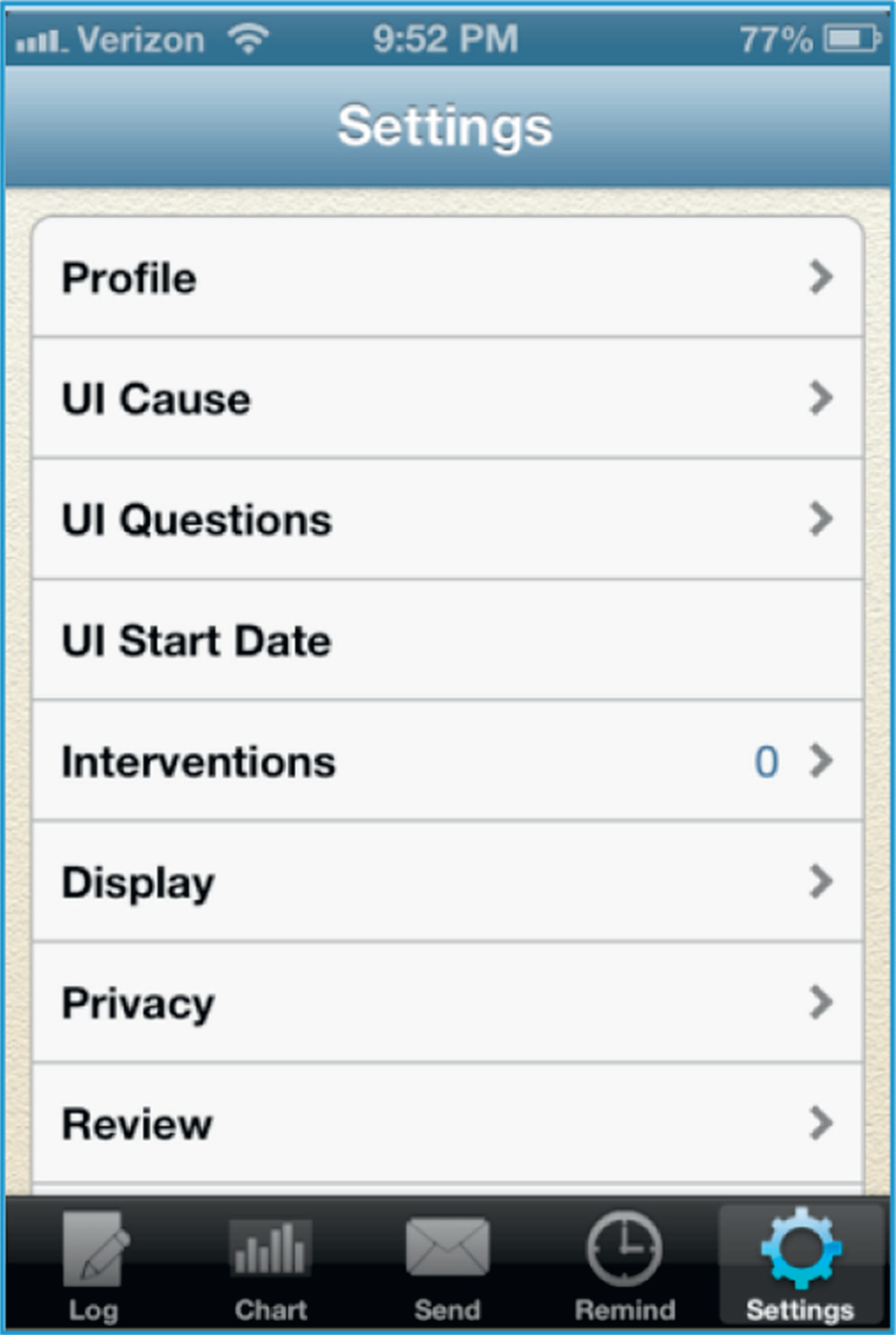
iDry Settings menu. Users are prompted to visit pages within menu (for example, “UI Cause”) to provide detailed demographics and information about their UI.
Data Sharing
There are 2 data sharing features. Users can email a chart or raw data for any period. The Share Data feature automatically downloads anonymized data to a HIPAA compliant database for research use. Users can easily disable automated data sharing if desired.
Feedback
Users can send feedback to the app’s development team and can submit reviews to the Apple App Store. Overall iDry presents a novel tool to benefit patients, physicians and the scientific community. Patients can enter data in a few seconds, visualize their improvement through graphs and charts, and see the impact of interventions. Physicians can make better decisions by viewing their patients’ data on a mobile device or via emails. Researchers can access an unprecedented source of raw data aggregated from iDry users worldwide. Thus, iDry can help improve clinical care, patient continence and overall understanding of the efficacy of UI interventions. Using this data set we expect that iDry users’ UI symptoms will improve with time, that the duration of iDry use positively correlates with a reduction in UI symptoms, and that a reduction in UI symptoms positively correlates with use of interventions.
METHODS AND APP DEVELOPMENT
iDry was developed between 2011 and 2012 by Touchtown Inc., a technology company specializing in medical and senior living applications, and it underwent 2 field tests before its public release. A 9-patient field trial was conducted to assess user satisfaction with the app. These patients had all undergone radical prostatectomy at the University of Pittsburgh Medical Center, were age 56 to 70, had leakage for 9.2 months (SD 11.8) and used an average of 4.7 pads per day (SD 3). During the 3-week trial most subjects used iDry daily and made data entries immediately after or within a day of each pad change. Subjects reviewed their charts and reports daily. In a post-trial survey all subjects agreed that iDry was “useful in helping manage incontinence,” was “easy to use” and “charting feature was helpful.” Of these subjects 8 (89%) agreed that “ability to see progress for each intervention was helpful” and they would be “likely to recommend iDry to others.”
A beta test was also conducted by soliciting participants online, primarily from the www.healingwell.org prostate cancer forum. Sixteen people including 2 women downloaded and evaluated iDry. Overall 75% of all pad changes were entered immediately and the remainder were entered within a day. All testers reviewed their charts daily to track progress. They unanimously indicated that iDry was a useful tool, offered easy data entry, and was helpful for understanding and managing incontinence.
In November 2012 iDry was published as a free download from the Apple App Store.4 Between November 2012 and May 2014 iDry was downloaded 1,231 times (65 downloads per month), which is a typical app download rate. One analyst estimates that two-thirds of all apps are downloaded fewer than 1,000 times in their first year.5 The heaviest download frequency, 157 per month, was during the initial 3 months when the app was promoted through a press release and several online articles and interviews. For the most recent 6 months the download rate stabilized at 36 per month. All downloads resulted from App Store search, online promotion and personal recommendations, and no efforts were made to engage clinicians in promoting the app. Half (50.3%) of people who downloaded iDry only used it once. There were 75 individuals who upgraded to the Premium version for $4.99 (or an equivalent price in non-U.S. currencies).
User data were collected automatically and sent anonymously to the development team’s servers. Users consented by default unless they opted out by touching the “Share Data” button. Data were collected from November 2012 through June 2013. From June 2013 until May 2014 logging was temporarily disabled due to a software problem and those data are not included in this analysis.
To test the study hypotheses we analyzed the data to determine the distribution and reduction of UI symptoms based on the 4 key variables of pads used per day, the amount of daily leakage (calculated by multiplying the pad fluid capacity by the user self-reported degree of saturation), and self-reported severity of stress incontinence and urge incontinence measured by the QUID.6 Users were prompted but not required to complete the QUID within the first week of using the app.
We performed t-tests to compare long-term users who used iDry for at least 30 days to short-term users who used iDry more than once but discontinued it within 30 days, to examine group differences in the 4 variables. Pearson’s correlation analysis was performed to examine correlations among the 4 variables and interventions. Descriptive statistics were used to explore user demographics.
RESULTS
A total of 878 users reported the number of pads used per day and the amount of daily leakage (table 1). This number consisted of 29 long-term users (3.3%), 407 short-term users (46.4%) and 442 users (50.3%) who only used iDry once. Long-term and short-term users on average used 1 pad per day and had daily leakage of 229 mg. However, long-term users had significantly more severe UI symptoms (p ≤0.01), using 2.5 pads per day and leaking 1,200 mg per day. We found no significant difference between long-term and short-term users with regard to self-reported UI severity using the QUID questionnaire, with 19 long-term and 220 short-term participants reporting an average mild to moderate severity for stress (mean 6.6) and urge (mean 6.1) incontinence.
Table 1.
Average daily severity and reduction of incontinence symptoms
| No. | Mean | SD | T | p Value | |
|---|---|---|---|---|---|
| Av of all data entered | |||||
| No. pads used daily: | 878 | 0.7 | 1.3 | 7.1 | 0.001 |
| Long-term users | 29 | 2.4 | 1.3 | ||
| Short-term users | 849 | 0.7 | 1.3 | ||
| Daily leakage (mg): | 878 | 228.9 | 726.6 | 3.0 | 0.01 |
| Long-term users | 29 | 1,183 | 1,785.1 | ||
| Short-term users | 849 | 196.3 | 639.2 | ||
| Stress incontinence severity: | 239 | 6.6 | 3.9 | 0.5 | 0.59 |
| Long-term users | 19 | 6.2 | 3.4 | ||
| Short-term users | 220 | 6.7 | 3.9 | ||
| Urge incontinence severity: | 239 | 6.1 | 4.8 | 1.0 | 0.34 |
| Long-term users | 19 | 5.1 | 4.3 | ||
| Short-term users | 220 | 6.2 | 4.8 | ||
| Changes in data entered | |||||
| Daily reduction in pads used: | 436 | 0.2 | 1.0 | 3.3 | 0.001 |
| Long-term users | 29 | 0 | 0 | ||
| Short-term users | 407 | 0.2 | 1.0 | ||
| Daily reduction in leakage (mg): | 436 | 13.9 | 591.2 | 0.1 | 0.93 |
| Long-term users | 29 | 4.5 | 9.3 | ||
| Short-term users | 407 | 14.6 | 612 | ||
| Change in stress incontinence severity: | 50 | −0.3 | 2.2 | 0.3 | 0.77 |
| Long-term users | 8 | −0.1 | 0.4 | ||
| Short-term users | 42 | −0.3 | 2.4 | ||
| Change in urge incontinence severity: | 50 | −0.2 | 3.2 | 0.2 | 0.86 |
| Long-term users | 8 | 0 | 0.2 | ||
| Short-term users | 42 | −0.2 | 3.5 | ||
Results from one-time users are combined with those of short-term users.
We also calculated the average reduction in leakage per day, excluding the 442 users who provided only a single day of data entry. Of the remaining 436 users 407 short-term users reduced pads per day by 20%, but the 29 long-term users were significantly different (p ≤0.001) in that their daily pad use unchanged with time. The 407 short-term users reported an average daily leakage reduction of 14.6 mg while 29 long-term users reported a reduction of only 4.5 mg. This is a large difference but due to the high variability in the data (SD 611) the difference was not statistically significant (p=0.93). Of 239 users 50 reported severity of stress and urge incontinence but we did not observe any significant group difference between 8 long-term users and 42 short-term users.
Of the 878 users 125 (14.2%) reported using interventions including pelvic floor muscle exercises (52), bladder training such as a voiding schedule (21), dietary interventions such as limiting caffeine or alcohol intake (67) and various medical treatments (36). Table 2 shows that the average number pads used daily and the average amount of daily leakage significantly positively correlated with all 4 interventions (p ≤0.001, mostly). The severity of urge incontinence also significantly positively correlated with bladder training (p ≤0.01). In addition, we found that the use of bladder training significantly positively correlated with the reduction of pads used per day (p=0.03) and reduction of leakage amount (p=0.02) with time among those who used iDry for more than 7 days.
Table 2.
| No. Pads Used Daily | Daily Leakage (mg) | Stress Incontinence Severity | Urge Incontinence Severity | |
|---|---|---|---|---|
| Muscle | ||||
| exercise: | ||||
| r Value | 0.12 | 0.09 | 0.04 | −0.05 |
| p Value | 0.001 | 0.01 | 0.54 | 0.46 |
| Bladder | ||||
| training: | ||||
| r Value | 0.18 | 0.22 | 0.08 | 0.16 |
| p Value | 0.001 | 0.001 | 0.22 | 0.01 |
| Dietary: | ||||
| r Value | 0.22 | 0.22 | 0.08 | 0.12 |
| p Value | 0.001 | 0.001 | 0.25 | 0.07 |
| Medical: | ||||
| r Value | 0.10 | 0.16 | 0.07 | 0.11 |
| p Value | 0.001 | 0.001 | 0.27 | 0.1 |
Demographic information was provided by 201 users (table 3). Mean age was 51 years (SD 17.6). Overall 73.1% of users were male and 25.4% were female. Most were white (80.6%), 4.5% were African-American and 14.9% other or unknown, with 5.5% reporting Hispanic ethnicity. Average BMI was 26.4 (SD 7.3) and mean UI duration was 2.4 years (SD 3.7). The most common UI causes were prostate cancer/diseases (23%), bladder diseases (eg overactive bladder, bladder stone 18.4%), aging (8%), spinal injury (5%), childbirth or hysterectomy (2.5%), followed by other causes including cardiovascular diseases, brain tumor, multiple sclerosis and obesity (total 16.5%). Long-term users were an average of 10 years older than the others (p=0.02).
Table 3.
Demographics and reporting behaviors
| Mean age (SD) | 50.8 | (17.6) |
| Mean kg/m2 BMI (SD) | 26.4 | (7.3) |
| Mean yrs incontinent (SD) | 2.4 | (3.7) |
| No. gender (%): | ||
| F | 51 | (25.4) |
| M | 147 | (73.1) |
| Unknown | 3 | (1.5) |
| No. race (%): | ||
| American Indian/Alaskan | 3 | (1.5) |
| Asian | 6 | (3.0) |
| African-American | 9 | (4.5) |
| Hawaiian or Pacific Islander | 3 | (1.5) |
| White | 162 | (80.6) |
| Unknown | 18 | (9.0) |
| No. ethnicity (%): | ||
| Hispanic/Latino: yes | 11 | (5.5) |
| Hispanic/Latino: no | 171 | (85.1) |
| Unknown | 19 | (9.5) |
| No. primary cause of incontinence (%): | ||
| Aging | 16 | (8.0) |
| Bladder disease | 37 | (18.4) |
| Prostate disease/surgery | 46 | (22.9) |
| Pregnancy/hysterectomy | 5 | (2.5) |
| Spinal injury | 10 | (5.0) |
| Other | 33 | (16.5) |
| Unknown | 54 | (26.9) |
| No. use behavior (%): | ||
| Long-term (30 days or more) | 29 | (3.3) |
| Short-term (1–29 days) | 407 | (46.4) |
| One-time | 442 | (50.3) |
DISCUSSION
The data collected from iDry provided clinical and demographic profiles of users who experienced UI for a variety of reasons. The findings suggest that patients with more leakage and more frequent pad changes tended to use iDry longer (more than 30 days). The type of incontinence (stress vs urge) did not affect app use. Interestingly short-term users showed signs of improvement, using 20% fewer pads per day. However, contrary to our expectation, long-term iDry users did not show any improvement in daily pad use and they improved less than others in the amount of leakage. Because this was not a controlled study, it remains unclear whether the observed improvement with time is attributable to the improved self-monitoring or is simply a natural recovery of continence function with time.
Importantly, the data indicate that patients with more severe symptoms have improvement more slowly and to a lesser degree than others, despite continuous self-monitoring using iDry. This is likely due to the underlying disease conditions that require professional management and interventions.
Furthermore, the findings show that the use of interventions is significantly associated with UI severity. Not surprisingly, those leaking more and using more pads were more likely to use interventions for managing incontinence symptoms. In particular, patients with urge incontinence were more likely to use bladder training techniques. Bladder training correlated with symptom reduction among users who used iDry more than a week, but other interventions did not significantly correlate with any symptom reduction. Although self-reported use of interventions may be incomplete or inaccurate, only 14% of users reported using any interventions. The implication is that it is important to educate patients and public about the value of self-management and intervention in overcoming UI.
A quarter of iDry users reported demographic information so the reported demographic profile is not representative of all users but provides some important information. The study sample is relatively young (mean age 51 years), predominantly male (73%) and white (81%), with an incontinence history averaging 2.4 years and 23% caused by prostate diseases. Obviously prostate cancer and related diseases significantly impact urinary function. Furthermore, the high BMI (mean 26.4 kg/m2) alerts us to the comorbidity of obesity and incontinence and negative impact of obesity on continence. It calls for more social awareness and education of this public problem for reducing the burden of incontinence on patients and society.
Overall iDry users included men and women, ranged in age from 21 to 90 years, and included members of all racial and ethnic groups. This diverse profile of user groups indicates that iDry is well accepted and used across demographic boundaries. However, just half (49.7%) of people who downloaded iDry used it more than once, which is lower than the 77% reported average for all Apple iOS apps.7 User acceptance of iDry is supported by the positive user ratings submitted to the Apple App Store, where 22 of 23 submitted reviews gave iDry the highest possible rating.4
Mobile apps for health self-care are proliferating rapidly, with 500 million smartphone users worldwide expected to use a health care application by 2015, and 1.7 billion smartphone and tablet users by 2018.8 Our experience with iDry is useful for guiding other mobile health app development. Our findings strongly suggest that iDry is a useful, accessible and convenient tool to document UI symptoms and improvement. It can be used effectively to help patients manage this chronic condition. Nonetheless, it has yet to be used in combination with effective interventions to reduce the negative impact of UI, especially among patients experiencing more severe symptoms. Individuals who use no wetness protection may not find iDry attractive as iDry’s main feature is to track the total amount of urine captured in pads.
The data abstracted from iDry are anonymously self-reported and, thus, subject to self-selection and reporting inaccuracy due to concerns about personal privacy. The demographic information is incomplete and can be misleading. Therefore, the findings reported here should be taken with caution.
ACKNOWLEDGMENTS
The Department of Urology at the University of Pittsburgh Cancer Institute provided support for patient access. Additional in-kind support was provided by Touchtown Inc.
Supported by the National Institutes of Health/National Institute on Aging (Grant R43 AG042162-01; PI: Pepper).
Study received approval from University of Pittsburgh Institutional Review Board under 45 CFR 46.110, IRB # PRO12060202.
Abbreviations and Acronyms
- BMI
body mass index
- iOS
Internetwork Operating System
- QUID
Questionnaire for Urinary Incontinence Diagnosis
- UI
urinary incontinence
Footnotes
Financial interest and/or other relationship with Touchtown Inc.
REFERENCES
- 1.Dmochowski RR and Gomelsky A: Overactive bladder in males. Ther Adv Urol 2009; 1: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tremback-Bell A, Levine A and Dawson G: Young women’s self-efficacy in performing pelvic muscle exercises. J Womens Health Phys Therap 2012; 36: 158. [Google Scholar]
- 3.Lee KS, Lennie TA, Dunbar SB et al. : The association between regular symptom monitoring and self-care management in patients with heart failure. J Cardiovasc Nurs 2014; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.iDRY, iTunes Preview website. Available at https://itunes.apple.com/us/app/idry/id545087250?mt=8. Accessed July 25, 2014
- 5.Canalys: Top app stores risk losing control of app discovery. June 11, 2012. Available at http://www.canalys.com/newsroom/top-app-stores-risk-losing-control-app-discovery. Accessed September 18, 2014.
- 6.Bradley CS, Rahn DD, Nygaard IE et al. : The questionnaire for urinary incontinence diagnosis (QUID): validity and responsiveness to change in women undergoing non-surgical therapies for treatment of stress predominant urinary incontinence. Neurourol Urodyn 2010; 29: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Localytics: App Retention Improves e Apps Used Only Once Declines to 20%. June 11, 2014. Available at http://info.localytics.com/blog/app-retention-improves. Accessed September 18, 2014.
- 8.Jahns R: 500m people will be using healthcare mobile applications in 2015. Available at http://research2guidance.com/500m-people-will-be-using-healthcare-mobile-applications-in-2015/. Accessed September 18, 2014.


