Abstract
Microbiota derived short chain fatty acids (SCFAs) are produced by fermentation of non-digestible fiber, and are a key component in intestinal barrier homeostasis. Since the microbiome has diurnal fluctuations, we hypothesized that SCFAs in humans have a diurnal rhythm and their rhythmicity would be impacted by the host central circadian misalignment (night shift work) which would make intestinal barrier more susceptible to disruption by alcohol. To test this hypothesis, we studied three groups of subjects: patients with alcohol use disorder, but no liver disease (AD), healthy day workers (DW), and night workers (NW). All subjects were studied at baseline and then in DW and NW subjects after moderate daily alcohol (0.5 g/kg) for seven days. Gut derived plasma SCFAs showed a significant circadian oscillation by cosinor analysis in DW; however, SCFA in the AD and NW subjects lost 24 hour rhythmicity. Decrease in SCFA correlated with increased colonic permeability. Both chronic and moderate alcohol consumption for one week caused circadian disruption based on wrist actigraphy and urinary melatonin. Our study shows that: (1) gut derived plasma SCFAs have a diurnal rhythm in humans that is impacted by the central clock of the host; (2) moderate alcohol suppresses SCFAs which was associated with increased colonic permeability; and (3) less invasive urinary 6-SM correlated and rest-activity actigraphy correlated with plasma melatonin. Future studies are needed to examine the role circadian misalignment on gut derived SCFAs as possible mechanism for loss of intestinal barrier resiliency to injurious agents like alcohol.
Keywords: SCFAs, alcohol, circadian, melatonin, actigraphy
Introduction
The human circadian clock is controlled by the central or master clock, located in the suprachiasmatic nucleus (SCN) which coordinates the timing and synchronization of peripheral clocks that are found in every organ in the body. As organisms evolved based on the 24-hour periodicity of the planet‟s natural light-dark cycle, pacemakers like the SCN have been tightly regulated across species and are essential for health.(1) The central clock controls sleep-wake cycle and the transcription of ~ 80% of protein coding genes in the primate brain and peripheral tissues.(2) Many of the genes that are under circadian control are involved in metabolism, inflammation, and oxidative stress; and therefore not surprisingly misalignment between central clock and environment has been associated with obesity,(3) metabolic syndrome,(4) chronic inflammatory conditions,(5–7) and cancer.(8–10)
The best-studied model of chronic circadian disruption is in shift work. Data from 2010 shows 29% of US workers worked an alternate or non-day shift,(11) and 15% of workers, approximately 23 million people, reported regularly working the night shift. In shift workers, exposure to artificial light at night causes misalignment of the circadian timing or the term „circadian disruption.”(12) In addition to shift work, other life style habits, light at night through TV and electronic devices (smart phones/PC/Laptop), and irregular eating and sleep/wake habits promote circadian misalignment. Thus, detailed mechanism of how circadian misalignment affect normal biology/physiology of the host is a critical unmet need because of its prevalence of circadian misalignment. In addition, shift work has been linked to breast cancer,(8) cardiovascular disease,(13) metabolic syndrome,(14) and irritable bowel syndrome(15). Combination of lifestyle factors may be important, as the Nurse‟s Health Study recently found that night shift work was associated with an increased risk of diabetes in those individuals with unhealthy lifestyle factors such as alcohol consumption.(16) The most reliable measure of central circadian timing in humans is the onset of melatonin secretion, when measured in dim light conditions (dim light melatonin onset, DLMO).(17) Melatonin typically begins to rise 2–3 h before the onset of sleep, and allows for frequent and precise sampling in saliva or plasma. Whereas the main urinary metabolite 6-sulphatoxymelatonin (6-SM) correlates well with salivary or plasma melatonin and can be measured at home but lack the precision of an in lab DLMO. Several studies have shown no or minimal impact on DLMO with alcohol.(18,19); however, in our previous work, we showed that disruption of circadian rhythms increased alcohol-induced colonic permeability and endotoxemia, (20) suppressed plasma melatonin,(21) and that night workers (NW) have phase delay compared to day workers (DW) after moderate alcohol consumption.(20) However, the mechanism of increased susceptibility to alcohol injurious effects on intestine in circadian disrupted host is not known, We and others have shown that alcohol consumption disrupts normal microbiota composition and function(22–24) and this alcohol-induced dysbiosis is characterized by increase abundance of putative pro-inflammatory bacteria and decreased abundance of putative anti-inflammatory bacteria like SCFA.(25) Several studies have shown that SCFAs, especially butyrate, is required for maintaining healthy intestinal barrier and low butyrate has been associated with disrupted intestinal barrier integrity.(26,27) It is now also well established that the microbiota exhibit a diurnal rhythm,(28) and the circadian rhythmicity of the host can influence the intestinal microbiota structure and function.(28–31) It is intriguing that dysbiosis in disrupted circadian rodents and human is also characterized by decreased abundance of SCFA producers like Lachnospiraceae (phylum Firmicutes, class Clostridia).(32) These data suggest that the increased susceptibility of the intestine of the disrupted circadian host to alcohol may be through dysbiotic intestinal microbiota.
Therefore, there is a clear rationale to assess plasma SCFAs, which correlate with stool SCFA,(33–35) over 24 hours in individuals with and without circadian misalignment and to determine the impact of alcohol consumption. We hypothesized that plasma SCFAs would have diurnal rhythmicity and would be impacted by central circadian disruption (night shift work) and disruption of SCFA diurnal oscillation might be a mechanism for increased susceptibility of shift workers to alcohol-induced hyperpermeability that we have previously reported.(20) Accordingly, we measured plasma SCFAs, wrist actigraphy, and urinary 6-SM in three groups of subjects: patients with alcohol use disorder (AD) who had no clinically significant liver disease, healthy, day (DW), and healthy night shift (NW) workers with the DW and NW subjects were studied before and after moderate alcohol (0.5 g/kg, red wine) for one week.
Methods
Participants
Participants were recruited through advertisements, posted flyers, or self-referral. All subjects gave written informed consent, and all procedures were approved by the Rush University IRB – #10012604, The study was designed to include three groups: AD, DW, and NW. The AD group was established via the Lifetime Drinking History (LDH) questionnaire and both the DW and NW groups did not fit criteria for AD by LDH. The DW cohort was comprised of healthy subjects on a stable work schedule of 7am-7pm for at least 3 shifts a week for a period longer than 3 months. The NW group consisted of healthy subjects on a stable work schedule of 7pm-7am for at least 3 shifts a week for a period longer than 3 months. All subjects completed blood tests and questionnaires at their initial visit and were excluded if they met any of the following criteria: 1) Any history of or blood tests consistent with liver disease (LFTs > 1.5 normal), renal impairment (Creatinine > 1.5), cardiovascular disease, diabetes (Hgb-A1 >8%), or thrombocytopenia (<80k); 2) Major depression (score ≥ 15 or any endorsement of suicidal intent on the Beck Depression Inventory);(36) 3) Sleep apnea (score high risk ≥ 2 or more categories on the Berlin Questionnaire);(37) 4) Restless leg syndrome IRLSSG consensus criteria for Restless Leg Syndrome;(38) 5) Regular use of medications that affect melatonin – including beta blockers, psychotropic medication (ie SSRI), and exogenous melatonin products.
Baseline Measures
After a baseline questionnaire assessment and blood tests, all subjects were asked to keep their usual sleep schedule during the week prior to the 24-hour circadian phase assessment in the Clinical Chronobiology Center (CCC). After the first phase assessment, DW or NW subjects were given red wine, a 2013 Bota Box Malbec, alcohol 13.5%, origin Mendoza, Argentina, and instructed to drink 0.5 gram of Etoh/kg of body weight each day for the 7 days prior to the second 24-hour phase assessment – for the majority of subjects, this meant 2–3 glasses of wine a day. Subjects were instructed to drink the wine after finishing work and prior to bedtime. Subjects in the NW and DW cohort were asked to keep a diary the week of alcohol consumption so as to record the time they drank the alcohol. The AD subject cohort was not given alcohol and only underwent the initial circadian phase assessment. During each phase assessment, subjects were kept in dim light (<5 lux, measured every 2 hours with a Minolta TIL-1 light meter), and seated in a reclining chair at a constant posture. Subjects were given small, nutritionally balanced snacks instead of meals and not allowed to exercise during the phase assessments to limit the impact of these factors on circadian timing (constant routine).
Plasma Melatonin and Intestinal Permeability
Plasma melatonin was collected hourly for 24 h between 1300 and 1200, for a total of 24 samples. Patients were kept in dim light (<5 lux, measured every 2 h with a Minolta TIL-1 light meter) and seated in a recliner chair in the CCC as previously published.(20) DLMO was calculated by the “3k” method.(39) Intestinal permeability was measured as 24 hour urinary sucralose represents “primarily” colonic or whole gut permeability as previously described.(40)
Short Chain Fatty Acids
Plasma was collected at 6 times points four hours apart during each phase assessment: (16:00, 20:00, 00:00, 04:00, 08:00, and 12:00) under dim light conditions and constant routine. Gut derived plasma SCFAs – Acetate (Ac), Propionate (Pr), and Butyrate (Bu) we measured by GC-MS assay as previously described.(34)
Rest-Activity Rhythms
All subjects wore a wrist actigraphy monitor (Philips Respironics) for the seven days prior to each phase assessment at 30 s epocs. Actigraphy monitoring allows for assessment of gross motor activity in order to establish defined rest-activity cycles. See Statistical Analysis for further details.
Urinary Melatonin Assessment
Urine was collected for 24 hours for each phase assessment in the CCC. Urinary 6-sulfatoxymelatonin (6-SM), the main metabolite of melatonin, was measured by Bühlmann ELISA kit (ALPCO Diagnostics, Windham, NH) in urine collected in two 12 hour intervals.
Statistical Analysis
Chi square tests and Mann-Whitney U-test were used to compare the two subject groups or Analysis of Variance (ANOVA) when comparing three groups. Correlation was done by linear regression. AUC calculated via the trapezoid method. Statistical significance was determined by using a P value of <0.05. Statistics were performed using SPSS version 19.0 and rest-activity rhythms were caluculated in R (R v. 3.8.1) with via cosinor or nParACT package.(41) For SCFA analysis, a single cosinor method was used to analyize the data over time as previously described. (42,43) The equation for the cosinor fit is a follows:
Where M is the MESOR (Midline Statistic of Rhythm, a rhythm adjusted mean), A is the amplitude (a measure of half the extent of the variation within the cycle), ϕ is the acrophase (a measure of the time of overall highest value), and τ is the period. The fit of the model was the determined by the residuals of the fitted wave as previously described.(44) A 24 hour period was used for all analysis. A Multivariate Wald test were used to test the null hypothesis that the MESOR, amplitude, or acrophase differ by the covariate (alcohol or group). The circular-circular corrleation of DLMO and acrophase were computed on Oriana (Kovach Computing Services, Wales, UK) which uses with Fisher and Lee method.(45)
For actigraphy data, NparACT calculates 3 distinct variables each informing on different aspects of a circadian rhythm: Interdaily stability (IS), Intradaily variability (IV), and Relative Amplitude (RA). IS is the measure of circadian rhythmicity consistency. The strength of the circadian activity from one day to the next, derived from the 24 hour value from a chi-square periodogram, will range from 0 to 1, with a higher value indicating a stronger stability of the rhythm. IV measures disturbances in circadian rhythmicity; ie circadian rhythm fragmentation. Scores range from 0–2 with values approaching 2 representing a stronger degree to which active and resting states of a circadian rhythm are disrupted. RA is a value derived from the averaged values of the 5 least active hours (L5) over the course of experiment and the 10 most active hours (M10) over the course of the experiment. The L5 and M10 values inform on the restfulness of a defined rest period and the degree of activeness during defined periods of activity, respectively. RA values range from 0–1 with values approaching 1 indicating a greater amplitude difference in rest and active periods, thought to be a robust indicator of circadian rhythm health. To validate the viability of actigraphy data in measuring the extent to which a circadian rhythm may be disrupted, the variables derived from this methodology were compared both to patient reported data from sleep logs.
Results
Clinical Variables
A total of 57 subjects were screened for the study l, and 42 consented, and complete the study. The most common reason the subjects did not participate after being screened was they voluntarily withdrew usually due to scheduling conflicts (7), were lost to follow up (4), had a chronic medical disease (2), or could not get a reliable IV line to collect frequent blood samples over 24 hours (2). There was a statistical difference between the three groups in terms of age and gender with an older and more likely male subject in the AD group (Table 1).
Table 1:
Subject Characteristics
| AUD (N= 20) | DW (N= 11) | NW (N=11) | p-value (<) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age, Mean | 45.9 | 12.2 | 34.7 | 11.1 | 27.7 | 6.9 | 0.01 |
| Gender (male n; female n) | 15M;5F | 10F;1M | 11 F | 0.01 | |||
| BMI | 28.9 | 5.6 | 27.7 | 7.1 | 25.0 | 4.7 | 0.22 |
| Employment | 10U;10E | 11 E | 11 E | 0.01 | |||
| Years of Heavy Drinking | 27.5 | 11.3 | 0 | 0 | 0 | 0 | 0.01 |
| Ethnicity | 4C;16AA | 10C;1L | 10C;1L | 0.01 | |||
| Average Drinks/week | 55.0 | 41.3 | 0.25 | 4.3 | 0.01 | ||
| Maximum Drinks/day | 10.5 | 4.9 | 1 | 0.55 | 0.01 | ||
| Days Drink/Month | 18.1 | 8.9 | 2 | 4.65 | 0.01 | ||
| Adverse Life Events Related to Alcohol; n, % | 13, 70% | 0 | 0 | 0.01 | |||
| Tobacco Use, n, % | 9, 45% | 2, 18% | 1, 9% | 0.01 | |||
| Illicit Drug Use, n, % | 8,40% | 0, 0% | 0, 0% | 0.01 | |||
Short Chain Fatty Acids
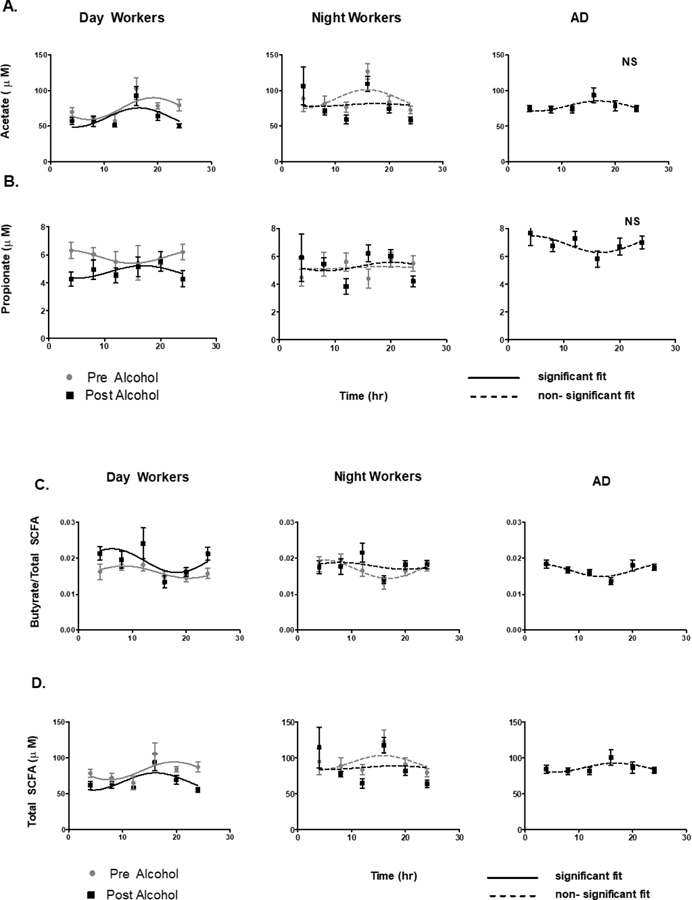
Acetate, propionate, and butyrate showed a significant diurnal oscillation by cosinor analysis in DW for acetate (F=4.5, p < 0.01), propionate (F= 2.9, p<0.01), Butyrate/Total (F=3.1, p<0.01), and Total SCFA (F=4.6, p<0.01). In the NW group, with central circadian misalignment, SCFA did not have a significant cosinor fit for acetate (F=1.1,p=0.35), propionate (F=0.1,p=0.94), Butyrate/Total (F=1.9, p=0.16), and Total SCFA (1.0, p=0.37). Similar to central circadian misalignment in NW, SCFA in the AD group also did not have a significant fit by cosinor analysis for acetate ( F=1.8, p=0.17), propionate (F=1.5. p=0.24), Total SCFA (F=1.3, p=0.28), oor butyrate/Total ratio (F=1.6, p<0.43) (Fig 1).
Figure 1: Cosinor Rhythmicity of plasma SCFAs.
The cosinor rhythm of plasma SCFAs was test at 6 timepoints over 24 hours under constant routine in all three groups: DW, NW, and AD in A) Acetate; B) Propionate; C) Butyrate/Total SCFAs; and D) Total SCFAs. In DW and NW two 24 hour phase assessments were completed before and after 0.5 g/kg of alcohol. A solid line represents a significant 24 hour cosinor curve fit and a dahsed line represents an insignificant 24 hour cosinor curve fit.
In the day worker (DW) group, moderate alcohol for one weFek had a significant decrease in the MESOR, amplitude, and acrophase of Total SCFA at 89.5 ± 5.3 vs 82.0 ± 2.4, 15.6 ± 7.4 vs 12.4 ± 4.7, and −0.9 ± 0.5 vs −1.2 ± 0.4; respectively all with p<0.01. There was a similar decrease in Acetate in MESOR, amplitude, and acrophase at 81.8 ± 5.1 vs 74.9 ± 2.3, 15.4 ± 7.1 vs 12.5 ± 4.5, −0.9 ± 0.5 vs −1.2 ± 0.4; respectively all with p<0.01 For propionate and Bu/Total SCFA ratio alcohol only significantly decreased the MESOR. In the night work group (NW) and alcohol use disorder group (AD), 7 days of daily wine consumption did not did not have a significant cosinor fit (Fig 1). Although SCFAs were not quantitatively different at individual time points by group,one week of moderate alcohol also had a significant suppression in total SCFAs as assessed by the area under the curve (AUC) of SCFAs which decreased from 4358 ± 905.56 to 3803.54 ± 707.61 in the DW and NW (p < 0.05, paired analysis).
Correlation of SCFAs with DLMO and Intestinal Permeability
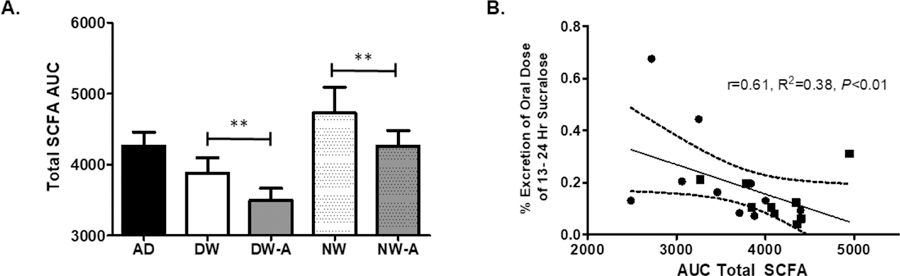
The timing of the central clock (DLMO) had a negative correlation to time of peak expression of SCFAs before and after alcohol in DW (r=−0.30, p<0.05 and r=−0.35, p<0.05), but this correlation with the central and peripheral clocks was decreased in the NW group and lost in the NW group after alcohol consumption for one week. (Fig 2A) A decrease in total SCFA AUC correlated with increased colonic permeability (% excretion of sucralose 13–24 h) r=0.61, p<0.01). (Fig 2B)
Figure 2: Total SCFA AUC compared to Colonic Permeability.
Total SCFA as calculated by area under the curve (AUC) in day workers (DW) and night workers (NW). A) Moderate alcohol for one week, 0.5 g/kg, suppressed total SCFAs as measured by Total SCFA area under the curve (AUC) in day workers (DW) and night workers (NW). B) A decrease in Total SCFA was correlated with an increase in primarly colonic permeability as measured by urinary sucralose collected 13–24 hours after ingestion. ** denotes a P < 0.05 by Wilcox Signed Rank Test. A linear regression was done to compare Total SCFA to urinary sucralose.
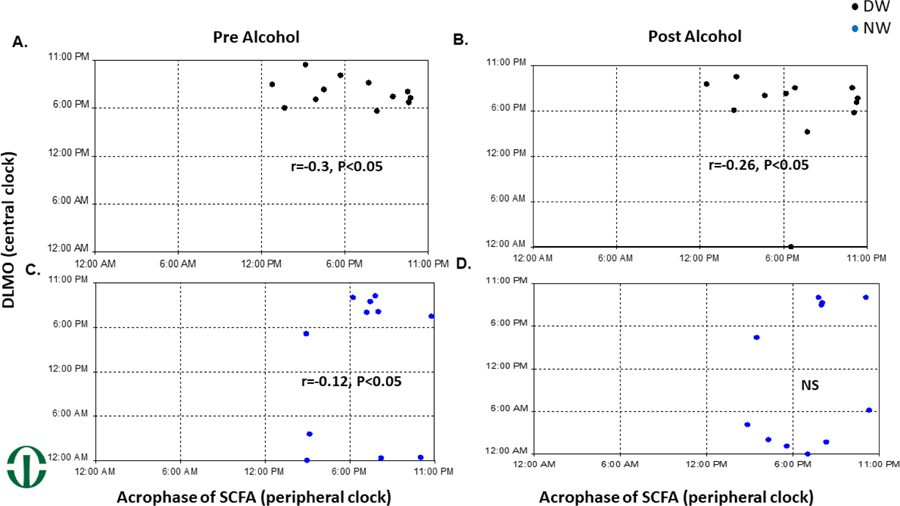
Acrophase of SCFAs compared to DLMO
The timing of peak expression of SCFA (acrophase) was compared to the timing of the central clock (DLMO) in both the DW and NW group. In the DW group there was a significant circular-circular correlation at r=–0.3, p<0.05 that was slightly decreased with alcohol, r=–0.26, p<0.05. (Fig 3A&B) In the NW group there was a significant correlation between DLMO and acrophase at r= –0.12, p<0.05, that became non-significant after alcohol. (Fig 3C&D)
Figure 3: Disrupted circadian host loses synchronization between central and peripheral timing.
The time of dim light melatonin onset (DLMO) marker of the central clock was compared to the time of peak expression of SCFAs (the acrophase). By a circular to circular correlation, there was a significant correlation with DLMO and SCFA Acrophase in A) Day Workers (DW) pre alcohol, B) DW post one week of alcohol (0.5g/kg), and C) NW pre alcohol. In NW after one week of alcohol (0.5g/kg) that correlation was not significant.
Comparison of Circadian Rest-Wake Activity by Wrist Actigraphy
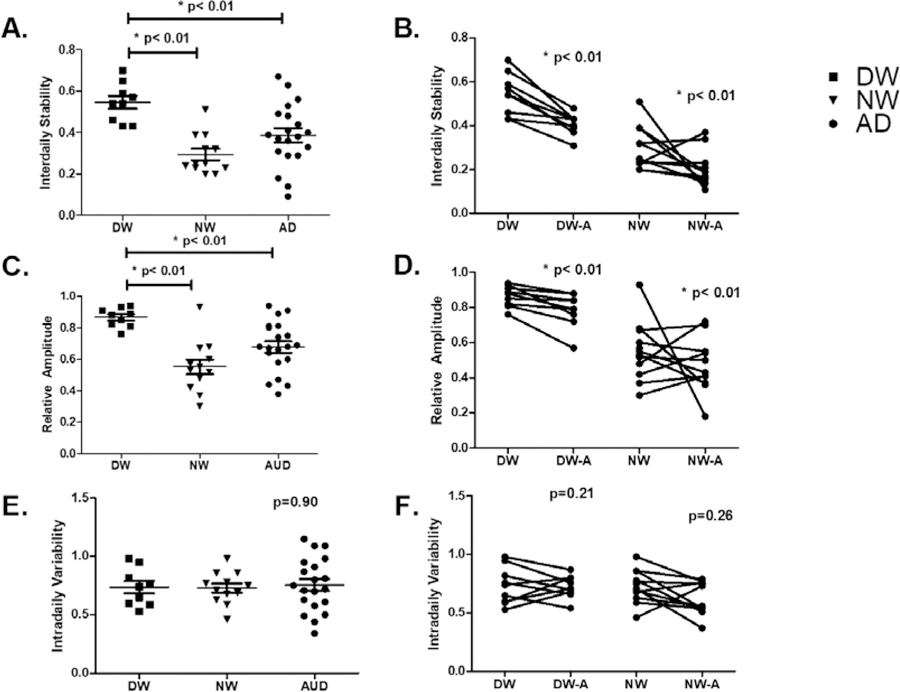
Mean interdaily stability values, prior to alcohol consumption, for the DW, NW and AD cohorts were .55, .29, and .38, respectively. (Fig. 4A). Upon consuming alcohol in the second week of experimentation, the interdaily stability scores dropped in the DW cohort from .55 to .40 (p<.02) and in the NW cohort from .29 to .20 (p<.01) (Fig. 4B). The mean baseline relative amplitude scores for the DW and NW cohorts were .86 and .55, respectively while the AD cohort had a mean relative amplitude value of .67. relative amplitude scores in the NW cohort post-alcohol consumption dropped from .55 to .47 (Fig 4C&D).
Figure 4: Circadian Rest-Activity Disruption with Alcohol.
Non-parametric analysis of rest-activity rhythms for one week prior to each phase assessment in all three groups was used to calculate the interdaily stability (IS), intradaily variability (IV), relative amplitude (RA). A) and C) At baseline, there was a significant differenat in IS and RA between DW and both NW and AD (P<0.01). E) There was not a significant difference in IV. B) and D) After one week of moderate alcohol there as a significant decrease in IS and RA by paired analysis. F) There was not a significant change in IV by paired analysis.
Mean intradaily variability scores at baseline were .74, .73, and .76 for the DW, NW and AD cohorts, respectively. Post-alcohol consumption, the intradaily variability scores in the DW cohort shifted from .74 to .73 and in the NW cohort they shifted from .73 to .62 (Fig 4E&F).
Correlation of Circadian Rest-Wake Activity and DLMO
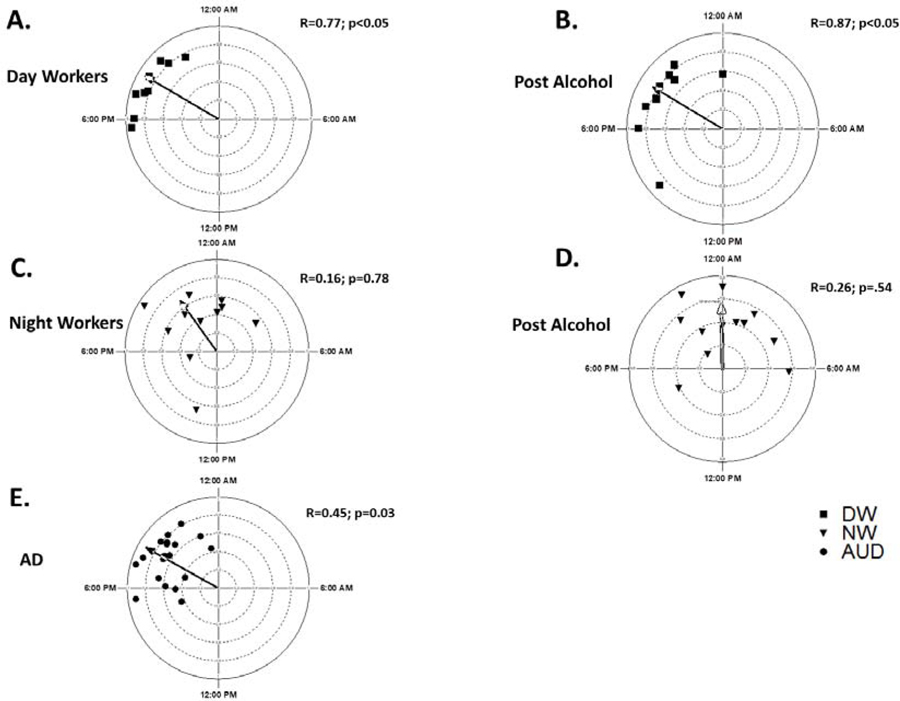
To access the correlation between the rest-wake rhythmicity by the gold standard, DLMO, and wrist actigraphy (Relative amplitude) a circular –linear regression analysis was performed. There was a significant correlation between RA and DLMO in the DW group pre and post alcohol at r=0.77, P<0.05 and r=0.87, p<0.05. (Fig 5A&B) In the NW group the circular-linear correlation between DLMO and relative amplitude was not significant. (Fig 5C&D) The mean DLMO for the AD cohort was 19 h 58 min ± 1 h 18 min. In the AD group, there was a significant correlation between DLMO and relative amplitude at r+0.45, p<0.05). (Fig 5E)
Figure 5: Circadian Rest-Activity Relative Amplitude Correlated to DLMO.
Comparing the timing of dim light melatonin onset (DLMO) to circadian rest-activity rhythms by wrist actigraphy there was a significant circular to circular correlation in A) Day Workers (DW) pre alcohol and B) DW post alcohol. In Night Workers (NW), C) and D) RA did not correlate with DLMO. In Alcohol use disorder (AD) E) there was a less robust but significant correlation between DLMO and RA.
Urinary Melatonin
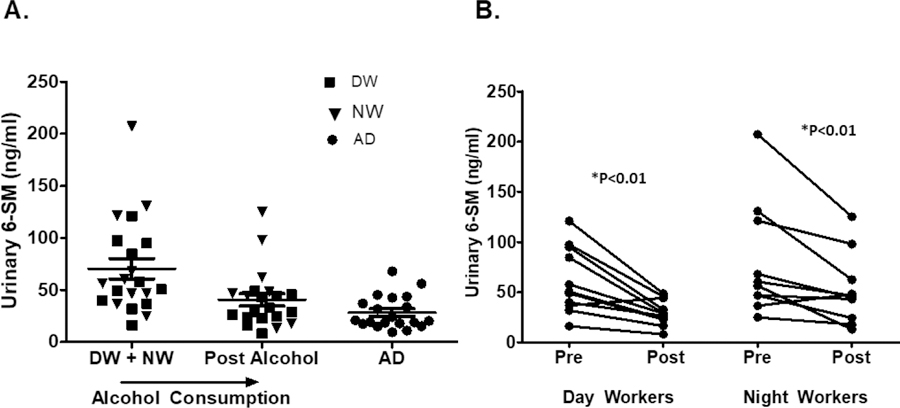
Urinary 6-SM was measured in each group after a 24-hour urine collection. At baseline, urinary 6-SM was 28.3 ± 15.9, 61.8 ± 32.8, and 79.5 ± 56.4 in the AD, DW, and NW groups respectively, with a significantly lower urinary melatonin value in the AD group (p<0.01). After moderate red wine consumption for 7 days, urinary 6-SM was 29.7 ± 12.5 and 52.4 ± 35.23 in DW and NW, respectively; a significant decrease in urinary 6-SM by paired analysis (p<0.01). Fig 6A and 6B.
Figure 6: Effect of Alcohol Consumption of Urinary 6-sulfatoxymelatonin.
Urinary 6-sulfatoxymelatonin (6-SM) was collected in all 3 groups over 24 hours during each phase assessment. A) Moderate alcohol for one week in Day Workers (DW) and Night Workers (NW) caused a significant decrease in and chronic alcohol in the alcohol use disorder (AD) group. B) By paired analysis, urinary 6-SM decrease in both Day workers and Night Workers by Wilcox Signed Rank Test.
Comparison of Urinary Melatonin to Plasma Melatonin AUC
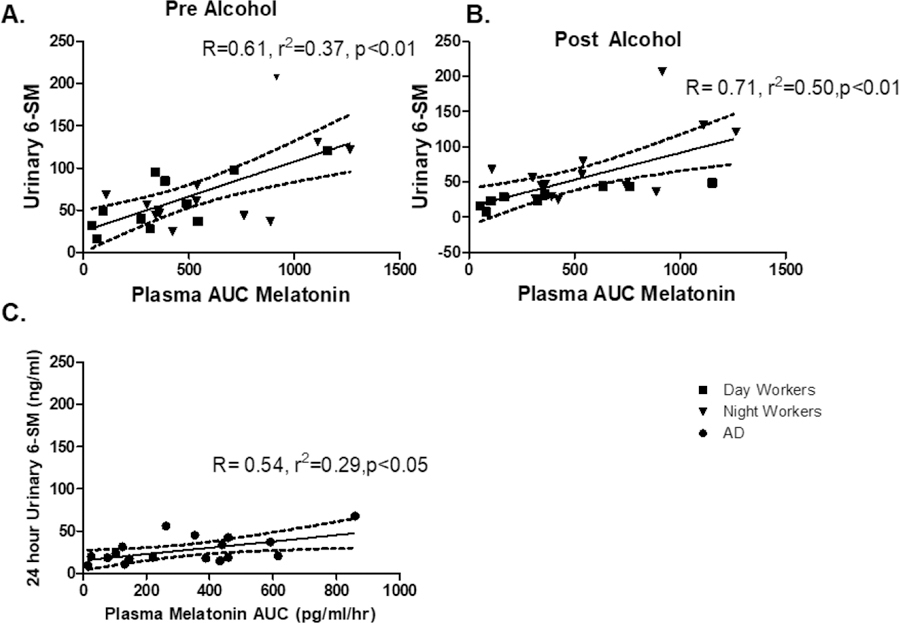
Plasma AUC, showed a significant linear correlation to urinary 6-SM in DW pre and post alcohol, NW pre and post alcohol (P<0.05), and AD subjects. Fig 7.
Figure 7: Correlation of Urinary 6-SM and plasma Melatonin AUC.
Hourly plasma melatonin taken for 24 hours was compared to a 24 hour collection of urinary 6-sulfatoxymelatonin (6-SM). In all three groups there was a significant correlation with plasma melatonin and urinary 6-SM in A) Day Workers, B) Night Workers, and C) Alcohol Use Disorder (AD). Linear correlation was used to compare plasma and urinary melatonin.
Discussion
Recently, there has been an increased understanding of how SCFAs may also acts as a zeitgeber in the peripheral tissues,(46) unlike the central circadian clock where the main zeitgeber is light. However, food timing is the zeitgeber for the intestinal epithelial cells, and contributes to the diurnal oscillation of intestinal microbiota and microbiota derived SCFAs.(47) Thus, plasma SCFA should be impacted in circadian misaligned host. Indeed, in the present study we found that: (1) gut derived SCFAs have a diurnal rhythm in humans which is impacted by the central clock of the host; (2) shift work and excessive chronic alcohol consumption, as in patients with alcohol use disorder, disrupts the oscillation of plasma SCFAs and (3) moderate alcohol consumption for one only week suppresses total SCFAs levels and this effect appears to have important functional impact because it correlates with increased colonic permeability in night shift workers who had disrupted circadian rhythms. Previously we published that central circadian misalignment increased colonic permeability with moderate alcohol.(20) These findings suggest SCFAs as potential mechanism for loss of resiliency on the colonic barrier in a disrupted circadian host.
Microbiota derived SCFAs are produced by fermentation of non-digestible fiber, and are a key component in intestinal barrier homeostasis. For example, butyrate induces epithelial cell proliferation, increases mucin production, is anti-inflammatory, and has protective effects on the epithelial barrier including tight junction proteins (TJPs) integrity.(48),(49) Butyrate enhances intestinal barrier function by facilitating TJPs assembly of ZO-1 and occludin in vitro,(49) and can decrease bacterial translocation.(50) Pretreatment with Butyrate, acetate, or propionate prevents TJP disruption and ameliorates alcohol-induced hyperpermeability in a dose dependent manner in vitro.(26) Therefore, circadian misalignment and alcohol both appear to impact gut derived SCFAs and colonic barrier homeostasis likely mediated via disruption of TJPs. The impact of central circadian disruption dissociating the diurnal variation of peripheral SCFAs is of particular interest as SCFAs could be a key signal through which the central and peripheral clocks communicate as either a peripheral tissue zeitgetber (46) or the marker of SCFA producing intestinal bacteria.
Previously, we have also published that either environmental or genetic disruption of central circadian clock can decrease resiliency of the intestinal barrier by disruption of TJPs.(51) Gut derived SCFA interact with receptors on the intestinal epithelial cells through G-protein coupled receptors (GPCR) like Gpr41, Gpr43, Gpr109a, and Olfr78. SCFA have been shown to activate signal transduction through GPCR with alcohol to cause intestinal tissue inflammation via effector T cells,(52,53) and impact other key gastrointestinal functions such as diurnal colonic contractility.(54) It is interesting to note that diurnal variation of acetate had higher concentrations during the night, where propionate and butyrate had higher concentrations during the day. (Figure 1). Previously, we reported decreased intestinal barrier resiliency to an alcohol binge during the rest phase in mice,(55) and since propionate and butyrate are more potent ligands for GPR41 and GPR43 than acetate,(56,57) this further suggests disrupted diurnal oscillation of SCFA production by gut microbiota may impact intestinal barrier resiliency. Butyrate or propionate, are also both potent inhibitors of histone deacetylase (HDAC), and an increase in HDAC1 and HDAC2 has been shown to prevent intestinal inflammation and colitis.(53,58) Furthermore, circadian regulation of intestinal microbiota may be mediated through HDAC.(59) Further studies are needed to further examine the mechanisms through which gut derived SCFAs maintain intestinal barrier homeostasis in conjunction with changes in intestinal microbiota to determine their relationship with the timing of central and peripheral circadian rhythms.
A second emphasis of this study was to demonstrate the viability of less invasive, more practical means by which to assess circadian rhythm disruption; i.e. wrist actigraphy and urinary melatonin. We found that: (1) chronic and moderate alcohol disrupt rest-activity rhythms and suppress urinary melatonin; and (2) rest-activity rhythms and urinary melatonin significantly correlate with the gold standard measure of central circadian rhythms in humans – DLMO. In humans, measurement of the central clock has been extensively studied, with a timing of ~24.2 hours,(60) and the gold standard of measurement is by plasma or salivary melatonin onset under dim light conditions to calculate the dim light melatonin onset (DLMO).(61–64) A secondary marker of circadian misalignment is melatonin amplitude or area under the curve (AUC).(65) Measurement of DLMO is tedious and requires hourly blood or saliva sampling in a controlled laboratory setting for up to 24 hours or longer. This makes DLMO impractical for large-scale human trials and the development of less invasive and at home biomarkers of circadian rhythms are critical. The most widely used biomarker of circadian misalignment is the main metabolite of melatonin, 6-sulfatoxymelatonin (6-SM), which has been used in large scale studies and has been associated with obesity and the onset of diabetes(66,67). Rest-activity patterns by wrist actigraphy have also been used to access central circadian rhythms in cancer treatment,(68,69) and by varying statistical approaches.(41,70) Although urinary 6-SM, overall correlates well with plasma melatonin,(71,72) and rest-activity rhythms in adolescents by wrist actigraphy correlation with salivary melatonin in small groups of subjects (.38 to .82);(73,74) however, comparisons of 6-SM to plasma melatonin and comparison of wrist actigraphy newer statistical methods has not been done.(41) Further establishment of these biomarkers to a gold standard will allow for a better understanding of chronobiology in health and disease.
In the present study, relative amplitude by wrist actigraphy had the best correlation with DLMO in day worker (DW) group, had a significant but less robust correlation in the patients with alcohol use disorder (AD) group, and was significantly correlated in night shift worker (NW) group. Plasma melatonin correlated well with urinary melatonin in all three groups when measured over 24 hours. Alcohol consumption (either chronic alcohol consumption in the AD group or moderate alcohol consumption in the DW and NW group) had a significant disruption in the stability and amplitude of circadian rhythms as measured by 24 hour urinary 6-SM or by rest-activity rhythms as evidenced by moderate alcohol significantly decreasing interdaily stability and relative amplitude but not impacting intradaily variability These finding highlight the feasibility on noninvasive markers of circadian rhythms in human subjects, the significant correlation with a gold standard (DLMO), and the impact of established human behaviors (shift work and alcohol consumption) that disrupt circadian alignment. In short, these noninvasive markers of circadian rhythmicity show great potential in future larger population studies.
There are several limitations to the present study. First, we could not standardize the time of alcohol consumption due to work schedules. However, we aligned time of drinking to sleep/wake cycle and thus controlled the impact of time of drinking to key circadian output- sleep/wake cycle. The day workers (DW) drank the wine in the evening after work before they went to sleep; while the night shift workers (NW) drank their wine in the morning after work before they went to sleep or the evening before they went to sleep during the days they did not work. Second, measures of rest-wake activity and urinary melatonin in this study examined patients who were at home in their natural environments during the two weeks of the study, and not done in lab under controlled conditions; however, subjects were asked to maintain their typical sleep and wake schedule to minimize variability. Third, we could not completely control for the number of night shifts worked each week due to varying work schedules in this population of nurses. We had no significant difference in the number of night shifts worked each week before and after alcohol, but it was not identical in every study subject. Finally, our sample size is limited, due in part to our decision to do more intensive 24 hour in lab phase assessments with plasma melatonin (taken through an indwelling IV), and in this limited sample size we had a significant difference in age and gender between groups. The AD group had more males and was older and the DW or NW group both factors which can affect circadian timing.(79)
In summary, the main findings of this study are: (1) that gut derived SCFAs have a robust diurnal rhythmicity that is disrupted by night shift work and acute or chronic alcohol consumption; (2) loss of resiliency of the colonic barrier is associated with a decrease in SCFAs; and (3) noninvasive makers of circadian rhythms by both rest-wake activity and urinary melatonin correlate well with the gold standard (DLMO), which has exciting potential for future large scale human studies. Further studies are needed to assess the mechanisms through which circadian misalignment and alcohol consumption impact the intestinal microbiota, bacterial products such as SCFAs, and colonic barrier homeostasis. Identifying factors associated with colonic barrier dysfunction and alcohol induced tissue injury could offer not only risk stratification for clinicians but could also lead to the development of novel targets for future investigations.
Acknowledgments:
The authors have read the journal’s policy on disclosure of conflicts of interest and have none to declare. The authors have read the journal’s authorship agreement and have reviewed and approved the manuscript.
G.R.S is funded by K23 AA019966 and R24 AA026801–01, and A.K is funded by R24 AA026801–01 form the National Institute of Alcohol Abuse and Alcoholism. In addition, G.R.S and A.K. were funded by The Alvin Baum Family fund.
Abbreviations
- SCFAs
short chain fatty acids
- AD
alcohol use disorder
- DW
day workers
- NW
night workers
- DLMO
dim light melatonin onset
- 6-SM
6-sulfatoxymelatonin
- SCN
suprachiasmatic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lucassen EA, Coomans CP, van Putten M, et al. Environmental 24-hr Cycles Are Essential for Health. Curr Biol 2016;26(14):1843–1853. [DOI] [PubMed] [Google Scholar]
- 2.Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018;359(6381): 10.1126/science.aao0318. Epub 2018 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broussard JL, Van Cauter E. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes 2016;23(5):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308(5724):1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hand LE, Hopwood TW, Dickson SH, et al. The circadian clock regulates inflammatory arthritis. FASEB J 2016;30(11):3759–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakradeo PS, Keshavarzian A, Singh S, et al. Chronotype, Social Jet Lag, Sleep Debt and Food Timing in Inflammatory Bowel Disease. Sleep Medicine 2018. [DOI] [PMC free article] [PubMed]
- 7.Cao Q, Zhao X, Bai J, et al. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci U S A 2017;114(47):12548–12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen J, Stevens RG. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer 2012;48(11):1722–1729. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Sun H, Zhang S, Yang X, Zhang G, Su T. Overexpression of PER3 Inhibits Self-Renewal Capability and Chemoresistance of Colorectal Cancer Stem-Like Cells via Inhibition of Notch and beta-Catenin Signaling. Oncol Res 2017;25(5):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan RW, Zhang C, Murugan S, et al. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol 2012;188(6):2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. Prevalence rates of work organization characteristics among workers in the U.S.: data from the 2010 National Health Interview Survey. Am J Ind Med 2013;56(6):647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smolensky MH, Hermida RC, Reinberg A, Sackett-Lundeen L, Portaluppi F. Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int 2016;33(8):1101–1119. [DOI] [PubMed] [Google Scholar]
- 13.Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ 2012;345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol 2013;23(5):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nojkov B, Rubenstein JH, Chey WD, Hoogerwerf WA. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol 2010;105(4):842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan Z, Li Y, Zong G, et al. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ 2018;363:k4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess HJ, Wyatt JK, Park M, Fogg LF. Home Circadian Phase Assessments with Measures of Compliance Yield Accurate Dim Light Melatonin Onsets. Sleep 2015;38(6):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiol Int 2012;29(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess HJ, Rizvydeen M, Fogg LF, Keshavarzian A. A Single Dose of Alcohol Does Not Meaningfully Alter Circadian Phase Advances and Phase Delays to Light in Humans. Am J Physiol Regul Integr Comp Physiol 2016:ajpregu.00001.2016. [DOI] [PMC free article] [PubMed]
- 20.Swanson GR, Gorenz A, Shaikh M, et al. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol Gastrointest Liver Physiol 2016:ajpgi.00087.2016. [DOI] [PMC free article] [PubMed]
- 21.Swanson GR, Gorenz A, Shaikh M, et al. Decreased Melatonin Secretion is Associated with Increased Intestinal Permeability and Marker of Endotoxemia in Alcoholics. Am J Physiol Gastrointest Liver Physiol 2015:ajpgi.00002.2015. [DOI] [PMC free article] [PubMed]
- 22.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 2012;302(9):G966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res 2009;33(10):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CCC M, LL N, MF C, et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol 2014;14:240-014-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol Res 2015;37(2):223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr 2013;143(12):1872–1881. [DOI] [PubMed] [Google Scholar]
- 27.Cresci GA, Glueck B, McMullen MR, Xin W, Allende D, Nagy LE. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol 2017;32(9):1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaiss CA, Zeevi D, Levy M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014;159(3):514–529. [DOI] [PubMed] [Google Scholar]
- 29.Leone V, Gibbons SM, Martinez K, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015;17(5):681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voigt RM, Forsyth CB, Green SJ, et al. Circadian disorganization alters intestinal microbiota. PLoS One 2014;9(5):e97500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaiss CA, Levy M, Korem T, et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016;167(6):1495–1510.e12. [DOI] [PubMed] [Google Scholar]
- 32.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A 2015;112(33):10479–10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoving LR, Heijink M, van Harmelen V, van Dijk KW, Giera M. GC-MS Analysis of Short-Chain Fatty Acids in Feces, Cecum Content, and Blood Samples. Methods Mol Biol 2018;1730:247–256. [DOI] [PubMed] [Google Scholar]
- 34.Yao L, Davidson E, Broeckling C, Prenni J. A Novel GC-MS Assay For Quantitation Of Short Chain Fatty Acids In Human Plasma 2017;228261.
- 35.van Eijk HM, Bloemen JG, Dejong CH. Application of liquid chromatography-mass spectrometry to measure short chain fatty acids in blood. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877(8–9):719–724. [DOI] [PubMed] [Google Scholar]
- 36.BECK AT, WARD CH, MENDELSON M, MOCK J, ERBAUGH J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 37.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 38.Hening WA, Allen RP. Restless legs syndrome (RLS): the continuing development of diagnostic standards and severity measures. Sleep Med 2003;4(2):95–97. [DOI] [PubMed] [Google Scholar]
- 39.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 1997;12(5):457–466. [DOI] [PubMed] [Google Scholar]
- 40.Arrieta MC. Alterations in intestinal permeability. Gut 2006;55(10):1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blume C, Santhi N, Schabus M. ‘nparACT’ package for R: A free software tool for the non-parametric analysis of actigraphy data. MethodsX 2016;3:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia 1979;6(4):305–323. [PubMed] [Google Scholar]
- 43.Cornelissen G Cosinor-based rhythmometry. Theor Biol Med Model 2014;11:16-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2013;97(6):1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.FISHER NI, LEE AJ. A correlation coefficient for circular data. biomet 1983;70(2):327–332. [Google Scholar]
- 46.Tahara Y, Yamazaki M, Sukigara H, et al. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci Rep 2018;8(1):1395-018-19836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukherji A, Kobiita A, Damara M, et al. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc Natl Acad Sci U S A 2015;112(48):E6691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ploger S, Stumpff F, Penner GB, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 2012;1258:52–59. [DOI] [PubMed] [Google Scholar]
- 49.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009;139(9):1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis K, Lutgendorff F, Phan V, Soderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis 2010;16(7):1138–1148. [DOI] [PubMed] [Google Scholar]
- 51.Summa KC, Voigt RM, Forsyth CB, et al. Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PLoS One 2013;8(6):e67102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013;145(2):396–406.e1–10. [DOI] [PubMed] [Google Scholar]
- 53.Turgeon N, Blais M, Gagne JM, et al. HDAC1 and HDAC2 restrain the intestinal inflammatory response by regulating intestinal epithelial cell differentiation. PLoS One 2013;8(9):e73785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segers A, Desmet L, Thijs T, Verbeke K, Tack J, Depoortere I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol (Oxf) 2019;225(3):e13193. [DOI] [PubMed] [Google Scholar]
- 55.Voigt RM, Forsyth CB, Shaikh M, et al. Diurnal variations in intestinal barrier integrity and liver pathology in mice: implications for alcohol binge. Am J Physiol Gastrointest Liver Physiol 2018;314(1):G131–G141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci 2013;34(4):226–232. [DOI] [PubMed] [Google Scholar]
- 57.Hong YH, Nishimura Y, Hishikawa D, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005;146(12):5092–5099. [DOI] [PubMed] [Google Scholar]
- 58.Yusta B, Baggio LL, Koehler J, et al. GLP-1R Agonists Modulate Enteric Immune Responses Through the Intestinal Intraepithelial Lymphocyte GLP-1R. Diabetes 2015;64(7):2537–2549. [DOI] [PubMed] [Google Scholar]
- 59.Kuang Z, Wang Y, Li Y, et al. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 2019;365(6460):1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms 2008;23(4):374–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res 2005;14(3):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int 2011;28(8):714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980;210(4475):1267–1269. [DOI] [PubMed] [Google Scholar]
- 64.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int 1989;6(1):93–102. [DOI] [PubMed] [Google Scholar]
- 65.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med 2008;4(1):66–69. [PMC free article] [PubMed] [Google Scholar]
- 66.Schernhammer ES, Kroenke CH, Dowsett M, Folkerd E, Hankinson SE. Urinary 6-sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res 2006;40(2):116–124. [DOI] [PubMed] [Google Scholar]
- 67.McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA 2013;309(13):1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levi F, Dugue PA, Innominato P, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int 2014;31(8):891–900. [DOI] [PubMed] [Google Scholar]
- 69.Natale V, Innominato PF, Boreggiani M, et al. The difference between in bed and out of bed activity as a behavioral marker of cancer patients: A comparative actigraphic study. Chronobiol Int 2015;32(7):925–933. [DOI] [PubMed] [Google Scholar]
- 70.Huang Q, Cohen D, Komarzynski S, et al. Hidden Markov models for monitoring circadian rhythmicity in telemetric activity data. J R Soc Interface 2018;15(139): 10.1098/rsif.2017.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang U, Kornemark M, Aubert ML, Paunier L, Sizonenko PC. Radioimmunological determination of urinary melatonin in humans: correlation with plasma levels and typical 24-hour rhythmicity. J Clin Endocrinol Metab 1981;53(3):645–650. [DOI] [PubMed] [Google Scholar]
- 72.Nowak R, McMillen IC, Redman J, Short RV. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: two non-invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol (Oxf) 1987;27(4):445–452. [DOI] [PubMed] [Google Scholar]
- 73.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep 1998;21(8):871–881. [DOI] [PubMed] [Google Scholar]
- 74.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms 1997;12(3):278–289. [DOI] [PubMed] [Google Scholar]
- 75.Bonmati-Carrion MA, Middleton B, Revell V, Skene DJ, Rol MA, Madrid JA. Circadian phase assessment by ambulatory monitoring in humans: correlation with dim light melatonin onset. Chronobiol Int 2014;31(1):37–51. [DOI] [PubMed] [Google Scholar]
- 76.Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry 2007;31(1):1–11. [DOI] [PubMed] [Google Scholar]
- 77.Markey SP, Higa S, Shih M, Danforth DN, Tamarkin L. The correlation between human plasma melatonin levels and urinary 6-hydroxymelatonin excretion. Clin Chim Acta 1985;150(3):221–225. [DOI] [PubMed] [Google Scholar]
- 78.Ortiz-Tudela E, Martinez-Nicolas A, Campos M, Rol MA, Madrid JA. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput Biol 2010;6(11):e1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Komarzynski S, Bolborea M, Huang Q, Finkenstadt B, Levi F. Predictability of individual circadian phase during daily routine for medical applications of circadian clocks. JCI Insight 2019;4(18): 10.1172/jci.insight.130423. [DOI] [PMC free article] [PubMed] [Google Scholar]