Abstract
Introduction
Bone mineral density (BMD) measured with dual-energy X-ray absorptiometry (DXA) can be used to predict fractures, but its clinical utility has not been fully established in chronic kidney disease (CKD) patients. Magnesium is an essential trace element. Although magnesium is associated with the risk of fractures in non-CKD populations, the relationship is unknown in CKD patients.
Methods
BMD and serum magnesium levels were measured in 358 stable outpatients undergoing maintenance hemodialysis therapy. The primary outcome was fragility fracture. Patients were divided into groups according to the median level of magnesium and the normal threshold value of lumbar spine BMD.
Results
During the median follow-up period of 36 months, 36 (10.0%) fractures occurred. The cumulative incidence rates of fractures were 17.6% and 5.2% [adjusted hazard ratio (aHR) 2.31, 95% confidence interval (CI) 1.03–5.17, P = 0.030] in the lower (<2.6 mg/dL) and higher (≥2.6 mg/dL) magnesium (Mg) groups, respectively, and 21.2% and 7.3% (aHR 2.59, 95% CI 1.09–6.16, P = 0.027) in the low- and high-BMD groups, respectively. The lower-Mg and low-BMD group had a 9.21-fold higher risk of fractures (95% CI; 2.35–47.00; P = 0.0010) than the higher-Mg and high-BMD group. Furthermore, adding both magnesium levels and lumbar spine BMD levels to the established risk factors significantly improved the prediction of fractures (C-index: 0.784 to 0.830, p = 0.041).
Discussion/Conclusions
The combination of serum magnesium and lumbar spine BMD can be used for fracture risk stratification and synergistically improves the prediction of fractures in CKD patients.
Introduction
Fractures are a major source of morbidity and mortality in patients undergoing hemodialysis [1,2]. The risk of any fracture is higher in patients undergoing dialysis than in the general population [3–7]. Although low bone mineral density (BMD) is a strong risk factor for fractures among healthy men and women, the clinical utility of measuring BMD by dual-energy X-ray absorptiometry (DXA) might be limited in patients with chronic kidney disease mineral bone disorder (CKD-MBD), particularly those with stages 5 and 5D disease, because there are alterations in the bone microarchitecture that are not observable on DXA imaging [8].
Numerous factors, such as older age, female sex, low body mass index and muscle weakness, are known to increase the fracture risk in patients undergoing dialysis [5,9–11]. However, investigations of the effects of hormonal factors and nutrition on bone health have mostly focused on calcium and vitamin D deficiencies and altered parathyroid hormone (PTH) levels [12].
Magnesium (Mg) is an essential trace element that plays a key role in cellular processes and is an important component of bone; 67% of the magnesium in the body is found in bone tissue [13]. Several animal studies have shown that magnesium deficiency is associated with decreased osteoblast function, increased osteoclast function and skeletal fragility [14,15]. A recent meta-analysis of 12 studies reported that a higher magnesium intake level was positively associated with the BMD of the femoral neck and total hip [16]. Some cohort studies in non-CKD populations have reported that lower serum magnesium levels were associated with an increased risk of fractures, including hip fractures [17,18]. Although magnesium might affect the risk of fractures, little is known about the relationship between magnesium and the risk of fractures in patients with CKD. Recently, one cohort study of patients undergoing hemodialysis found that lower serum magnesium levels were associated with a higher risk of hip fractures [19]. In this study, our objective was to assess the association of serum magnesium and BMD with the risk of incident fractures and to determine whether the prediction of the risk of fractures in patients undergoing hemodialysis based on the combination of magnesium level and lumbar spine BMD is more accurate than the prediction based on either variable alone.
Materials and methods
Study population
A total of 358 patients who had undergone hemodialysis for at least 2 months and had baseline measurements of serum magnesium and BMD at Masuko Memorial Hospital between May 2016 and November 2017 were enrolled. Patients undergoing combination therapy with peritoneal dialysis were excluded. This study was performed in accordance with the Helsinki Declaration and was approved by Masuko Memorial Hospital Ethics Committee (Ethics approval number: MR2-19). Due to the retrospective nature of the study, written informed consent was waived. All data were anonymized before analysis and the data range was from May 2016 to July 2020.
Baseline covariates
The following data were collected: demographics (age, sex, body mass index, hemodialysis vintage, duration of hemodialysis treatment); laboratory measurements (predialysis albumin, urea nitrogen, calcium, phosphate, magnesium, C-reactive protein [CRP], hemoglobin, alkaline phosphatase [ALP], and intact parathyroid hormone [iPTH]; prescriptions (phosphate binders, cinacalcet hydrochloride, active vitamin D analogues and proton pump inhibitors); and past history of parathyroidectomy, cardiovascular diseases (myocardial infarction, cerebral infarction, and cerebral hemorrhage) and incident fractures. For determination of biochemical parameters, serum was obtained immediately before the first weekly dialysis treatment at baseline. The dialysate magnesium concentration was 1.2 mg/dL.
Measurements of BMD
For the assessment of BMD, we used dual-energy X-ray absorptiometry (DXA) because it is the most widely used tool for the assessment of bone mass and fracture risk in the general population. BMD of the lumbar spine (L2-L4), 1/3 distal radius and total hip is reported in g/cm2 or the T-score (calculated using the mean and SD for Japanese young adults). DXA was performed with the Lunar iDXA system (GE Health Care Japan, Tokyo, Japan). According to the World Health Organization classification system, osteoporosis was defined as a BMD T-score of -2.5 or less, osteopenia as a T-score between -1 and -2.5, and normal BMD as a T-score of -1 or more.
Follow-up period
The follow-up period was censored on July 2020. Patients were followed for up to 3 years. The study endpoint was defined as any type of new fragility fracture. We defined fragility fractures as low energy fractures occurring in falls from standing height or less. We identified the asymptomatic fractures with annual whole-body skeletal X-ray survey and detected the symptomatic fractures diagnosed with radiological examinations from the hospital chart.
Statistical analysis
Baseline characteristics are presented as the means (standard deviations) or medians (interquartile range) for continuous variables and percentages for categorical variables. For continuous variables, the differences between two groups were evaluated using the Mann-Whitney U test or Student’s t-test, and the differences among three or more groups were assessed with ANOVA or the Kruskal-Wallis test. For categorical variables, the chi-squared test was used. Kaplan–Meier cumulative event curves were constructed to describe the frequency of events among the groups with various risk factors and were compared with the log-rank test. The cut-off level for BMD was defined based on the normal reference range (T-score ≥ -1.0) in the system established by the World Health Organization, and the cut-off level for serum magnesium level was defined based on the median value. Hazard ratios and 95% confidence intervals (CIs) were calculated using Cox regression models to estimate the relationships between the variables and the outcome. To identify the independent predictors of the endpoints, all baseline variables with P < 0.05 in univariate analysis were entered into a multivariate model. We also calculated the C-index, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to assess whether the predictive ability for fractures was improved after the addition of Mg, BMD or both to a baseline model with established risk factors. The C-index was defined as the area under receiver-operating characteristic curves between individual predictive probabilities for fracture and the incidence of fracture, and was compared among each predicting model [20]. The NRI relatively indicates how many patients improve their predicted probabilities for fracture, while the IDI represents the average improvement in predicted probabilities for fracture after adding variables into the baseline model [21].
All reported P values were 2-sided, and P values < 0.05 were considered statistically significant. All statistical analyses were performed using JMP® 11 (SAS Institute Inc., Cary, NC, USA) and R version 3.4.1.
Results
Patient characteristics
The baseline characteristics are shown in Tables 1 and 2. During a median (interquartile range) follow-up of 3.0 (2.8–3.0) years, 36 incident fractures (hip fractures: 10, vertebral fractures: 18, other fractures: 8) occurred.
Table 1. Baseline characteristics of the groups stratified by serum magnesium levels.
| Groups based on the serum Mg level | Total n = 358 | Lower Mg (≤ 2.5 mg/dL) n = 190 | Higher Mg (≥ 2.6 mg/dL) n = 168 | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (yrs) | 65.6 ± 14.3 | 69.1 ± 14.1 | 61.6 ± 13.4 | < 0.0001 |
| Sex (%), male | 74.0 | 74.6 | 73.1 | 0.74 |
| BMI (kg/m2) | 21.4 ± 4.2 | 21.3 ± 4.6 | 21.6 ± 3.7 | 0.60 |
| DM (%) | 44.1 | 43.2 | 45.2 | 0.69 |
| HD vintage (yrs) | 6.2 (1.8, 12.0) | 5.6 (1.5, 11.6) | 6.7 (2.1, 12.5) | 0.49 |
| HD duration (hours/week) | 13.5 ± 3.4 | 13.3 ± 3.2 | 13.6 ± 3.5 | 036 |
| Laboratory data | ||||
| BUN (mg/dL) | 56.4 ± 12.8 | 54.4 ± 13.1 | 58.7 ± 12.0 | 0.0014 |
| Adj. Calcium (mg/dL) | 9.0 ± 0.5 | 9.0 ± 0.6 | 9.0 ± 0.5 | 0.71 |
| Phosphate (mg/dL) | 5.2 ± 1.2 | 5.0 ± 1.2 | 5.4 ± 1.1 | 0.0013 |
| Magnesium (mg/dL) | 2.5 ± 0.3 | 2.2 ± 0.2 | 2.8 ± 0.2 | < 0.0001 |
| CRP (mg/dl) | 0.13 (0.05, 0.32) | 0.19 (0.06, 0.46) | 0.09 (0.05, 0.21) | < 0.0001 |
| Alb (g/dL) | 3.5 ± 0.3 | 3.4 ± 0.4 | 3.7 ± 0.2 | < 0.0001 |
| Hb (g/dL) | 11.1 ± 1.2 | 10.9 ± 1.2 | 11.4 ± 1.1 | < 0.0001 |
| ALP (IU/L) | 267 ± 133 | 280 ± 125 | 252 ± 140 | 0.047 |
| iPTH (pg/mL) | 142 (92, 214) | 141 (99, 212) | 144 (86, 219) | 0.85 |
| Total hip DXA BMD (g/cm2) | 0.75 ± 0.17 | 0.75 ± 0.18 | 0.76 ± 0.15 | 0.49 |
| L2-L4 DXA BMD (g/cm2) | 1.12 ± 0.24 | 1.13 ± 0.25 | 1.11 ± 0.23 | 0.36 |
| 1/3 Radius DXA BMD (g/cm2) | 0.74 ± 0.19 | 0.72 ± 0.20 | 0.76 ± 0.18 | 0.10 |
| Past history | ||||
| CVD (%) | 31.8 | 38.9 | 23.8 | 0.0022 |
| Parathyoidectomy (%) | 3.0 | 3.2 | 3.0 | 0.9 |
| Prevalent fracture (%) | 13.4 | 19.5 | 6.5 | 0.0003 |
| Medication | ||||
| CaCO3 (%) | 47.4 | 41.1 | 54.8 | 0.0095 |
| Phophate binders (%) | 55.5 | 49.5 | 62.5 | 0.013 |
| Vitamin D (%) | 75.1 | 75.8 | 74.4 | 0.76 |
| Cinacalcet (%) | 28.4 | 28.4 | 28.6 | 0.9 |
| PPI (%) | 55.8 | 61.5 | 49.4 | 0.020 |
Data are expressed as the mean ± SD or median (interquartile range).
BMI, body mass index; DM, diabetes mellitus; BUN, blood urea nitrogen; Adj. Calcium, albumin-adjusted calcium; CRP, C-reactive protein; Alb, albumin; Hb, hemoglobin; ALP, alkaline phosphatase; iPTH, intact parathyroid hormone; CVD, cardiovascular disease.
Table 2. Baseline Characteristics of the groups according to L2-L4 BMD (T-score).
| Groups based on L2-L4 BMD | Low L2-L4 BMD n = 109 | High L2-L4 BMD n = 246 | P-value |
|---|---|---|---|
| Demographics | |||
| Age (yrs) | 69.2 ± 15.1 | 63.9 ± 13.6 | 0.0010 |
| Sex (%) male | 42.2 | 88.6 | < 0.0001 |
| BMI (kg/m2) | 18.9 ± 2.5 | 22.6 ± 4.3 | < 0.0001 |
| DM (%) | 32.1 | 49.5 | 0.0022 |
| HD vintage (yrs) | 7.8 (3.4, 13.6) | 4.9 (1.4, 11.2) | 0.0019 |
| HD duration (hours/week) | 12.8 ± 2.9 | 13.7 ± 3.6 | 0.0022 |
| Laboratory data | |||
| BUN (mg/dL) | 56.6 ± 13.2 | 56.4 ± 12.6 | 0.9 |
| Adj. Calcium (mg/dL) | 9.1 ± 0.6 | 9.0 ± 0.5 | 0.20 |
| Phosphate (mg/dL) | 5.1 ± 1.1 | 5.2 ± 1.2 | 0.21 |
| Magnesium (mg/dL) | 2.5 ± 0.3 | 2.5 ± 0.3 | 0.18 |
| CRP (mg/dl) | 0.09 (0.05, 0.27) | 0.14 (0.06, 0.33) | 0.077 |
| Alb (g/dL) | 3.5 ± 0.4 | 3.6 ± 0.3 | 0.080 |
| Hb (g/dL) | 11.1 ± 1.0 | 11.1 ± 1.2 | 0.9 |
| ALP (IU/L) | 302 ± 164 | 251 ± 111 | 0.0007 |
| iPTH (pg/mL) | 144 (98, 224) | 144 (91, 213) | 0.45 |
| Total hip DXA BMD (g/cm2) | 0.57 ± 0.17 | 0.82 ± 0.14 | < 0.0001 |
| L2-L4 DXA BMD (g/cm2) | 0.85 ± 0.09 | 1.24 ± 0.19 | < 0.0001 |
| 1/3 Radius DXA BMD (g/cm2) | 0.61 ± 0.12 | 0.82 ± 0.14 | < 0.0001 |
| Past history | |||
| CVD (%) | 28.4 | 33.7 | 0.32 |
| Parathyoidectomy (%) | 4.5 | 2.4 | 0.28 |
| Prevalent fracture (%) | 18.3 | 10.9 | 0.058 |
| Medication | |||
| CaCO3 (%) | 42.2 | 50.0 | 0.17 |
| Phosphate binders (%) | 56.8 | 55.6 | 0.83 |
| Vitamin D (%) | 71.5 | 76.8 | 0.28 |
| Cinacalcet (%) | 33.9 | 26.4 | 0.14 |
| PPI (%) | 62.3 | 52.4 | 0.082 |
Data are expressed as the mean ± SD or median (interquartile range).
BMI, body mass index; DM, diabetes mellitus; BUN, blood urea nitrogen; Adj. Calcium, albumin-adjusted calcium; CRP, C-reactive protein; Alb, albumin; Hb, hemoglobin; ALP, alkaline phosphatase; iPTH, intact parathyroid hormone; CVD, cardiovascular disease; Low L2-L4 BMD, L2-L4 BMD T-score < -1.0; High L2-L4 BMD, L2-L4 BMD T-score ≥ -1.0.
Predictive value of serum magnesium and BMD for fractures
The multivariate Cox proportional hazards analysis identified serum magnesium level (adjusted HR 0.29; 95% CI 0.10–0.83, P = 0.021) and baseline L2-L4 BMD per 0.1 g/cm2 (adjusted HR 0.70; 95% CI 0.52–0.93, P = 0.011) as independent predictors of incident fractures after adjustment for age, body mass index, hemodialysis duration, serum magnesium level, past incident fracture, use of phosphate binders, total hip DXA BMD per 0.1 g/cm2, L2-L4 DXA BMD per 0.1 g/cm2 and distal 1/3 radius DXA BMD per 0.1 g/cm2 as covariates with P values < 0.05 in univariate analysis (Table 3). The patients were divided into two groups based on the median serum Mg level, and no significant differences were observed between the lower-Mg group and the higher-Mg group for total hip DXA BMD, L2-L4 DXA BMD or 1/3 radius DXA BMD (total hip DXA: 0.75 ± 0.18 g/cm2 versus 0.76 ± 0.15 g/cm2; P = 0.49, L2-L4 DXA: 1.13 ± 0.25 g/cm2 versus 1.11 ± 0.23 g/cm2; P = 0.36, 1/3 radius DXA: 0.72 ± 0.20 g/cm2 versus 0.76 ± 0.18 g/cm2; P = 0.10). The patients were divided into two groups based on the normal threshold value of L2-L4 DXA BMD, and there was no significant difference in serum magnesium levels between the low-BMD group and the high-BMD group (2.5 ± 0.3 mg/dL versus 2.5 ± 0.3 mg/dL; P = 0.18). There was no significant difference in iPTH values between the lower- and higher-Mg groups (141 (99, 212) versus 144 (86, 219); P = 0.85) (Tables 1 and 2).
Table 3. Cox proportional hazard analysis of the associations of baseline variables with the risk of any fracture.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (yrs) | 1.09 (1.06–1.13) | < 0.0001 | 1.07 (1.03–1.12) | < 0.0001 |
| Sex, male | 0.64 (0.33–1.33) | 0.23 | ||
| BMI (kg/m2) | 0.89 (0.80–0.98) | 0.018 | 1.03 (0.93–1.12) | 0.42 |
| DM | 1.30 (0.67–2.51) | 0.43 | ||
| HD vintage (yr) | 1.01 (0.96–1.05) | 0.63 | ||
| HD duration (hours/week) | 0.74 (0.56–0.91) | 0.0011 | 0.92 (0.72–1.10) | 0.45 |
| BUN, mg/dL | 0.99 (0.97–1.02) | 0.87 | ||
| Adj. Ca, mg/dL | 0.92 (0.52–1.58) | 0.78 | ||
| P, mg/dL | 0.95 (0.71–1.24) | 0.73 | ||
| Mg, mg/dL | 0.18 (0.072–0.48) | 0.0006 | 0.29 (0.10–0.83) | 0.021 |
| CRP, mg/dL | 1.22 (0.69–1.72) | 0.41 | ||
| Alb, g/dL | 0.45 (0.22–1.02) | 0.058 | ||
| Hb, g/dL | 0.87 (0.65–1.15) | 0.33 | ||
| ALP, IU/L | 1.00 (0.99–1.00) | 0.52 | ||
| iPTH, pg/mL | 1.00 (0.99–1.00) | 0.88 | ||
| Past history of CVD | 1.14 (0.55–2.24) | 0.70 | ||
| Parathyroidectomy | 0.76 (0.043–3.54) | 0.79 | ||
| Prevalent fracture | 7.38 (3.76–14.27) | < 0.0001 | 3.82 (1.78–8.02) | 0.0008 |
| CaCO3 | 0.70 (0.35–1.35) | 0.30 | ||
| Phosphate binders | 0.44 (0.22–0.86) | 0.017 | 0.58 (0.28–1.17) | 0.12 |
| Vitamin D | 1.10 (0.52–2.60) | 0.79 | ||
| Cinacalcet | 0.91 (0.41–1.83) | 0.80 | ||
| PPI | 1.89 (0.95–4.00) | 0.068 | ||
| Total hip DXA BMD (per 0.1 g/cm2) | 0.72 (0.59–0.89) | 0.0026 | 1.21 (0.84–1.73) | 0.29 |
| L2-L4 DXA BMD (per 0.1 g/cm2) | 0.77 (0.65–0.90) | 0.0009 | 0.70 (0.52–0.93) | 0.011 |
| Distal 1/3 Radius DXA BMD (per 0.1 g/cm2) | 0.73 (0.61–0.86) | 0.0002 | 1.12 (0.83–1.52) | 0.43 |
CI, confidence interval; HR, hazard ratio.
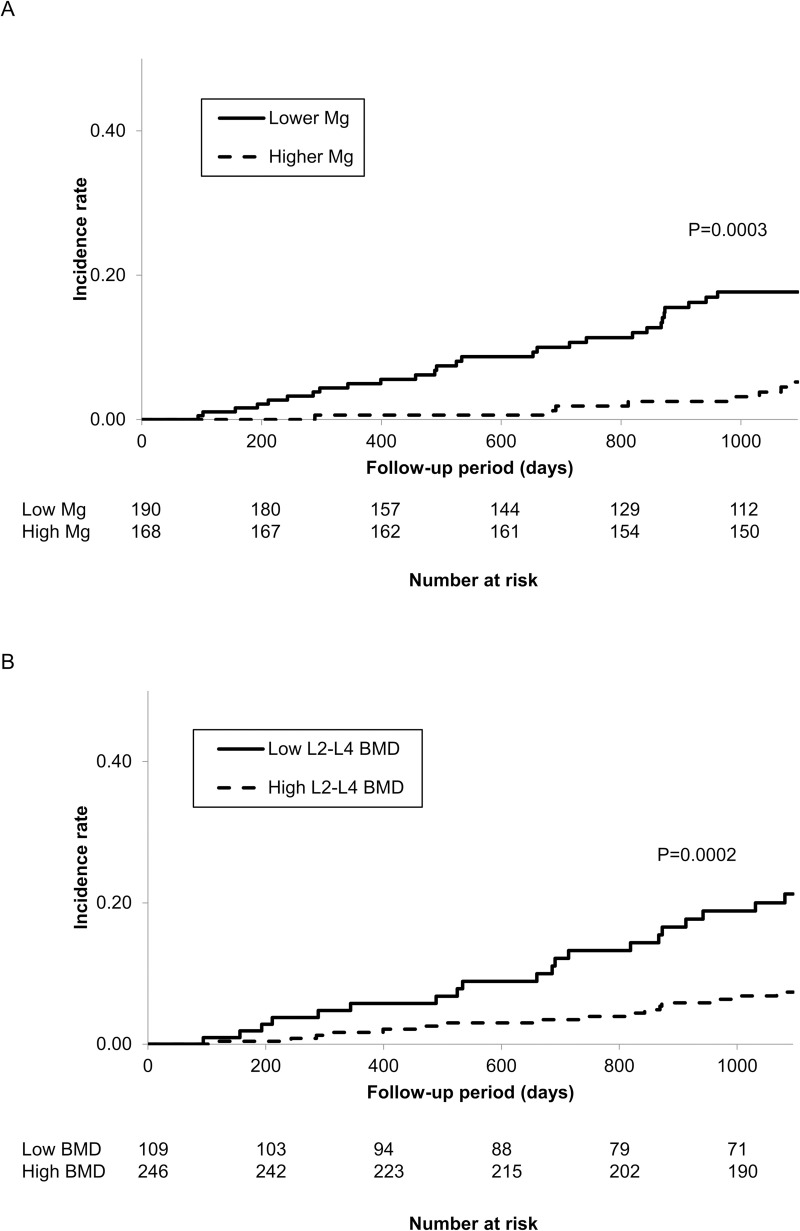
The 3-year cumulative incidence rate of fractures was significantly higher in the lower Mg group than in the higher Mg group (17.6% vs. 5.2%, adjusted HR 2.31; 95% CI 1.03–5.17, P = 0.030) and was higher in the low-BMD group than in the high-BMD group (21.2% vs. 7.3%, adjusted HR 2.59; 95% CI 1.09–6.16, P = 0.027) (shown in Fig 1 and Table 4).
Fig 1. Kaplan-Meier cumulative incidence curves for fractures according to the groups.
(A) Stratified by the magnesium level. (B) Stratified by L2-L4 BMD.
Table 4. Cox proportional hazard analysis of associations of the Mg level and BMD with the risk of total incident fractures after transforming the Mg levels and BMD into binary categories using the median value or official cut-off value.
| Variables | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Lower Mg (≤ 2.5 mg/dL) | 3.91 (1.86–9.22) | 0.0002 | 2.31 (1.03–5.17) | 0.030 |
| Total hip BMD T-score < -1.0 | 3.14 (1.12–13.10) | 0.026 | 1.49 (0.38–5.87) | 0.55 |
| L2-L4 BMD T-score < -1.0 | 3.13 (1.62–6.12) | 0.0007 | 2.59 (1.09–6.16) | 0.027 |
| 1/3 Radius BMD T-score < -1.0 | 2.14 (1.04–4.82) | 0.036 | 0.57 (0.21–1.52) | 0.27 |
Adjusted for age, BMI, HD duration, low serum Mg level (≤ 2.5 mg/dL), past incident fracture, use of phosphate binders, total hip BMD T-score < -1.0, L2-L4 BMD T-score < -1.0 and distal 1/3 radius BMD T-score < -1.0, which were the covariates with P<0.05 in the univariate analysis, as shown in Table 3.
Combined predictive value of Mg and BMD
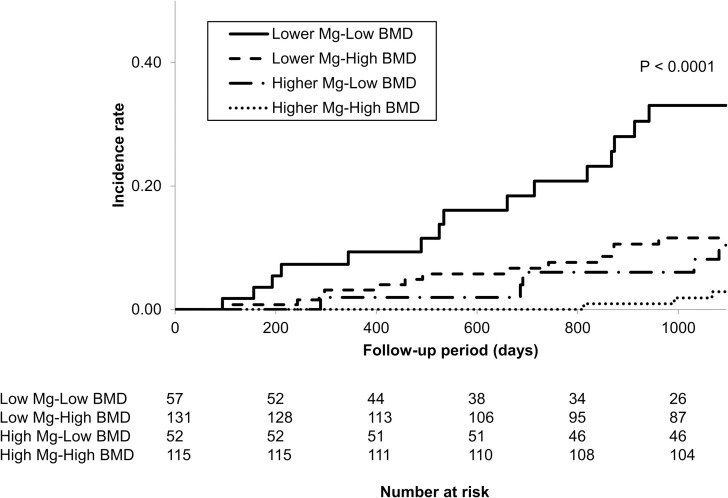
The 3-year cumulative incidence rates of fracture were 2.8%, 10.4%, 11.6% and 33.0% in the higher-Mg and high-BMD group, higher-Mg and low-BMD group, lower-Mg and high-BMD group and lower-Mg and low-BMD group, respectively (P < 0.0001, shown in Fig 2 and Table 5). Considering the higher-Mg and high-BMD group as the reference, the adjusted HRs (95% CIs) for fractures in the lower-Mg and low-BMD group and the higher-Mg and low-BMD group were 9.21 (2.35–47.00; P = 0.0010) and 5.09 (1.01–29.63; P = 0.048), respectively (Table 6).
Fig 2. Kaplan-Meier cumulative incidence curves for fractures stratified by both the magnesium level and L2-L4 BMD.
Table 5. Baseline characteristics according to the groups stratified by both the serum Mg level and BMD.
| Groups according to the serum Mg level and L2-L4 BMD level | Lower Mg-Low BMD n = 57 | Higher Mg-Low BMD n = 52 | Lower Mg-High BMD n = 131 | Higher Mg-High BMD n = 115 | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (yrs) | 76.0 ± 11.0 | 61.8 ± 15.6 | 66.0 ± 14.3 | 61.4 ± 12.2 | < 0.0001 |
| Sex (%), male | 38.5 | 46.1 | 91.6 | 85.2 | < 0.0001 |
| BMI (kg/m2) | 18.6 ± 2.4 | 19.2 ± 2.6 | 22.5 ± 4.8 | 22.6 ± 3.7 | < 0.0001 |
| DM (%) | 33.3 | 30.7 | 48.0 | 51.3 | 0.021 |
| HD vintage (yr) | 7.2 (3.4, 14.0) | 7.9 (3.2, 13.1) | 4.7 (1.4, 9.9) | 5.7 (1.4, 12.5) | 0.012 |
| HD duration (hours/week) | 12.6 ± 2.7 | 13.1 ± 3.2 | 13.6 ± 3.5 | 13.9 ± 3.7 | 0.10 |
| Laboratory data | |||||
| BUN (mg/dL) | 53.7 ± 14.9 | 59.7 ± 10.4 | 54.7 ± 12.4 | 58.3 ± 12.7 | 0.014 |
| Adj. Calcium (mg/dL) | 9.1 ± 0.5 | 9.0 ± 0.6 | 9.0 ± 0.5 | 9.0 ± 0.5 | 0.51 |
| Phosphate (mg/dL) | 4.9 ± 1.2 | 5.3 ± 0.9 | 5.0 ± 1.2 | 5.5 ± 1.3 | 0.0096 |
| Magnesium (mg/dL) | 2.2 ± 0.2 | 2.8 ± 0.2 | 2.2 ± 0.2 | 2.7 ± 0.1 | < 0.0001 |
| CRP (mg/dl) | 0.17 (0.05, 0.45) | 0.06 (0.05, 0.19) | 0.19 (0.07, 0.47) | 0.10 (0.05, 0.23) | 0.0002 |
| Alb (g/dL) | 3.3 ± 0.4 | 3.7 ± 0.3 | 3.5 ± 0.3 | 3.7 ± 0.2 | < 0.0001 |
| Hb (g/dL) | 10.9 ± 1.1 | 11.3 ± 0.9 | 10.8 ± 1.2 | 11.4 ± 1.2 | 0.0003 |
| ALP (IU/L) | 309 ± 119 | 294 ± 204 | 265 ± 122 | 234 ± 95 | 0.0015 |
| iPTH (pg/mL) | 138 (100, 214) | 165 (95, 232) | 149 (99, 213) | 140 (82, 213) | 0.65 |
| Total hip DXA BMD (g/cm2) | 0.59 ± 0.13 | 0.62 ± 0.11 | 0.82 ± 0.15 | 0.82 ± 0.13 | < 0.0001 |
| L2-L4 DXA BMD (g/cm2) | 0.84 ± 0.10 | 0.87 ± 0.08 | 1.26 ± 0.18 | 1.22 ± 0.20 | < 0.0001 |
| 1/3 radius DXA BMD (g/cm2) | 0.53 ± 0.16 | 0.61 ± 0.18 | 0.82 ± 0.14 | 0.83 ± 0.14 | < 0.0001 |
| Past history | |||||
| CVD (%) | 35.0 | 21.1 | 41.2 | 25.2 | 0.014 |
| Parathyoidectomy (%) | 3.5 | 5.7 | 3.0 | 1.7 | 0.57 |
| Prevalent fracture (%) | 28.0 | 7.6 | 15.2 | 6.0 | 0.0005 |
| Medication | |||||
| CaCO3 (%) | 26.3 | 59.6 | 48.0 | 52.1 | 0.0025 |
| Phosphate binders (%) | 49.1 | 65.3 | 50.3 | 61.7 | 0.10 |
| Vitamin D (%) | 71.9 | 71.1 | 77.8 | 75.6 | 0.73 |
| Cinacalcet (%) | 33.3 | 34.6 | 26.7 | 26.0 | 0.54 |
| PPI (%) | 71.9 | 51.9 | 56.4 | 47.8 | 0.025 |
Low Mg, serum Mg ≤ 2.5 mg/dL; High Mg, serum Mg ≥ 2.6 mg/dL; Low L2-L4 BMD, L2-L4 BMD T-score < -1.0; High L2-L4 BMD, L2-L4 BMD T-score ≥ -1.0.
Table 6. Adjusted HRs for fractures in the groups stratified by both the serum Mg level and lumbar spine BMD.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Higher Mg and High L2-L4 BMD | Reference | ‐ | Reference | ‐ |
| Lower Mg and High L2-L4 BMD | 4.59 (1.47–20.02) | 0.0068 | 2.78 (0.86–12.37) | 0.088 |
| Higher Mg and Low L2-L4 BMD | 3.80 (0.93–18.55) | 0.062 | 5.09 (1.01–29.63) | 0.048 |
| Lower Mg and Low L2-L4 BMD | 14.86 (4.86–64.25) | < 0.0001 | 9.21 (2.35–47.00) | 0.0010 |
Adjusted for age, BMI, HD duration, past incident fracture, use of phosphate binders, total hip DXA BMD and 1/3 radius DXA BMD (significant variables in univariate Cox regression analysis for fractures).
High Mg, serum Mg level ≥ 2.6 mg/dL; Low Mg, serum Mg level ≤ 2.5 mg/dL.
Low L2-L4 BMD, L2-L4 BMD T-score < -1.0; High L2-L4 BMD, L2-L4 BMD T-score ≥ -1.0.
Regarding the discriminatory ability of the model with regard to the prediction of fractures, the addition of both magnesium levels and lumbar spine BMD levels to the prediction model based on established risk factors with P < 0.05 in univariate analysis, namely, age, BMI, hemodialysis duration, past incident fracture, use of phosphate binders, total hip DXA BMD and 1/3 radius DXA BMD, improved the C-index (0.784 to 0.830, P = 0.041), NRI (0.600, P = 0.00044) and IDI (0.041, P = 0.010) significantly more than each alone (Table 7).
Table 7. Discriminatory ability of each prediction model for any type of incident fracture based on the C-index, Net Reclassification Improvement (NRI), and Integrated Discrimination Improvement (IDI).
| Variables | C-index (95% CI) | P-value | NRI | P | IDI | P-value |
|---|---|---|---|---|---|---|
| Established risk factors | 0.784 (0.699–0.869) | Reference | Reference | Reference | ||
| Established risk factors + serum Mg | 0.789 (0.705–0.873) | 0.49 | 0.174 | 0.16 | 0.001 | 0.31 |
| Established risk factors + L2-L4 BMD | 0.825 (0.750–0.899) | 0.076 | 0.497 | 0.0028** | 0.039 | 0.012* |
| Established risk factors + serum Mg + L2-L4 BMD | 0.830 (0.759–0.901) | 0.041* | 0.600 | 0.00044** | 0.041 | 0.010* |
Established risk factors include age, BMI, HD duration, past incident fracture, use of phosphate binders, total hip DXA BMD and 1//3 radius DXA BMD as covariates with P<0.05 in the univariate analysis.
Discussion/Conclusion
In the present study, we demonstrated that a lower serum magnesium level and a lower lumbar spine BMD can be used to predict the incidence of fractures; furthermore, the combination of these variables can be used to stratify patients according to fracture risk and improve the prediction of fractures in patients undergoing hemodialysis. To our knowledge, this is the first study to show the combined predictive value of the serum magnesium level and BMD for the occurrence of fractures.
The usefulness of DXA to classify fracture risk in CKD patients has been controversial because the pathogenesis of renal osteodystrophy is not uniform and may be the result of multiple causes, and BMD measured by DXA does not provide information about the type of renal osteodystrophy [22]. Some past studies with small sample sizes showed that BMD measured by DXA could not be used to identify the fracture risk in hemodialysis patients [23,24]. Despite these limitations, some recent prospective studies found that BMD measured by DXA could be used to predict fractures in patients with CKD [25,26]. Based on these studies, the 2017 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines suggested the measurement of BMD by DXA to assess the fracture risk in advanced CKD patients [27]. The results of our study confirmed the findings of these prior studies with regard to the association between BMD measured by DXA and the fracture risk in end-stage renal disease (ESRD) patients on hemodialysis. To improve the specificity and sensitivity of BMD measured by DXA for the fracture risk, there has been interest in adding clinical risk factors. In this study, we investigated whether combining the BMD and serum magnesium level could increase the accuracy of the prediction of fractures.
A previous large-scale cohort study showed the relationship between magnesium and the risk of fracture among patients undergoing hemodialysis [19]. The serum magnesium level is considered a practical and easy-to-measure marker of the bone magnesium content because the serum magnesium level has been reported to correlate well with the bone magnesium content [28]. Some experimental studies have identified the effects of magnesium deficiency on bone metabolism. In vitro studies have shown that a low extracellular magnesium concentration stimulates osteoclastogenesis and inhibits osteoblast proliferation through the upregulation of inducible nitric oxide synthesis [29,30]. In animal studies, a magnesium-deficient diet has been shown to activate osteoclasts and cause excessive bone mineralization, leading to the impairment of bone strength [14,31,32]. The hydroxyapatite crystal size and perfection in bone were first suggested to contribute to the mechanical strength of bones in the early 1980s [33]. A past animal study showed that newly formed crystals of apatite were larger in magnesium-deficient animals than controls, and that was associated with increased bone fragility [34]. Magnesium may affect bone quality, independent of bone density. Lower magnesium has been associated with increased crystal size and reduced bone mass, that contribute to osteoporosis. Magnesium levels may supplement the ability of bone mineral densities to predict fractures. Consistent with these experimental studies, our study showed that lower serum magnesium levels were associated with an increased risk of fracture, and the combination of serum magnesium levels and lumbar spine BMD levels improved the prediction of fractures. Magnesium is an agonist of the calcium-sensing receptor [35]. A previous experimental study showed that magnesium could suppress PTH secretion from the parathyroid glands, particularly at moderately low extracellular calcium levels [36]. Although a lower serum magnesium level may increase the fracture risk through its effect on PTH, no significant difference in PTH values between the two magnesium groups was observed in our study.
Associations of calcium, phosphate and PTH levels with the risk of fractures were not demonstrated in our study. This could be because these factors are well-known treatment targets in the field of mineral and bone disorders, and therapeutic interventions for these factors had already been initiated in our patients. We should recognize the clinical importance of magnesium with regard to the risk of fractures, especially in patients with low lumbar spine BMD levels.
In hemodialysis patients, hypomagnesemia is commonly observed because of the complex effects of magnesium intake, drugs, and the dialysate magnesium concentration. The major factors that affect magnesium diffusion during dialysis are the concentration gradient across the dialysis membrane and the Gibbs-Donnan effect [37]. Because the dialysate magnesium concentration in Japan is 1.2 mg/dL, it is worth investigating whether high magnesium dialysates provide benefits with regard to reducing the risk of fractures.
There are some limitations of this study. First, this study was conducted in a single center and included a relatively small sample size. Second, our study had an observational retrospective design; therefore, no inferences about a causal relationship between predictive variables and fracture risk could be made. The results of our study need to be confirmed in prospective studies with larger sample sizes.
In conclusion, the combination of lower serum magnesium and low lumbar spine BMD was associated with a high risk of incident fractures in patients undergoing hemodialysis. Although magnesium has long been under recognized in the field of mineral and bone disorders in CKD patients, the combination of serum magnesium level and lumbar spine BMD may be useful for assessing the fracture risk in patients undergoing hemodialysis.
Supporting information
(XLS)
Acknowledgments
We are grateful for the generous contributions of the radiology technicians, nurses, other staff and patients at the Masuko Memorial Hospital. Thanks to them, we were able to complete this study.
Data Availability
All relevant data are contained in the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis. 2004;44: 672–679. [PubMed] [Google Scholar]
- 2.Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014;85: 166–173. 10.1038/ki.2013.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58: 396–399. 10.1046/j.1523-1755.2000.00178.x [DOI] [PubMed] [Google Scholar]
- 4.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36: 1115–1121. 10.1053/ajkd.2000.19812 [DOI] [PubMed] [Google Scholar]
- 5.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2006;70: 1358–1366. 10.1038/sj.ki.5001754 [DOI] [PubMed] [Google Scholar]
- 6.Wakasugi M, Kazama JJ, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, et al. Increased risk of hip fracture among Japanese hemodialysis patients. J Bone Miner Metab. 2013;31: 315–321. 10.1007/s00774-012-0411-z [DOI] [PubMed] [Google Scholar]
- 7.Maravic M, Ostertag A, Torres PU, Cohen-Solal M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int. 2014;25: 159–165. 10.1007/s00198-013-2435-1 [DOI] [PubMed] [Google Scholar]
- 8.West SL, Patel P, Jamal SA. How to predict and treat increased fracture risk in chronic kidney disease. J Intern Med. 2015;278: 19–28. 10.1111/joim.12361 [DOI] [PubMed] [Google Scholar]
- 9.Leinau L, Perazella MA. Hip fractures in end-stage renal disease patients: incidence, risk factors, and prevention. Semin Dial. 2006;19: 75–79. 10.1111/j.1525-139X.2006.00122a.x [DOI] [PubMed] [Google Scholar]
- 10.Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, et al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58: 2200–2205. 10.1111/j.1523-1755.2000.00394.x [DOI] [PubMed] [Google Scholar]
- 11.Jamal SA, Leiter RE, Jassal V, Hamilton CJ, Bauer DC. Impaired muscle strength is associated with fractures in hemodialysis patients. Osteoporos Int. 2006;17: 1390–1397. 10.1007/s00198-006-0133-y [DOI] [PubMed] [Google Scholar]
- 12.Babayev R, Nickolas TL. Bone disorders in chronic kidney disease: an update in diagnosis and management. Semin Dial. 2015;28: 645–653. 10.1111/sdi.12423 [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Luthringer BJ, Feyerabend F, Schilling AF, Willumeit R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014;10: 2843–2854. 10.1016/j.actbio.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 14.Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009;28: 131–141. 10.1080/07315724.2009.10719764 [DOI] [PubMed] [Google Scholar]
- 15.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Kilburn J. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone. 2005;37: 211–219. 10.1016/j.bone.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 16.Farsinejad-Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporos Int. 2016;27: 1389–1399. 10.1007/s00198-015-3400-y [DOI] [PubMed] [Google Scholar]
- 17.Hayhoe RP, Lentjes MA, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr. 2015;102: 376–384. 10.3945/ajcn.114.102723 [DOI] [PubMed] [Google Scholar]
- 18.Kunutsor SK, Whitehouse MR, Blom AW, Laukkanen JA. Low serum magnesium levels are associated with increased risk of fractures: a long-term prospective cohort study. Eur J Epidemiol. 2017;32: 593–603. 10.1007/s10654-017-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi Y, Hamano T, Wada A, Hoshino J, Masakane I. Magnesium and risk of hip fracture among patients undergoing hemodialysis. J Am Soc Nephrol. 2018;29: 991–999. 10.1681/ASN.2017080849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44: 837–845. [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27: 157–172; discussion 207–112. 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 22.Nickolas TL. The utility of circulating markers to predict bone loss across the CKD spectrum. Clin J Am Soc Nephrol. 2014;9: 1160–1162. 10.2215/CJN.04660514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamal SA, Chase C, Goh YI, Richardson R, Hawker GA. Bone density and heel ultrasound testing do not identify patients with dialysis-dependent renal failure who have had fractures. Am J Kidney Dis. 2002;39: 843–849. 10.1053/ajkd.2002.32006 [DOI] [PubMed] [Google Scholar]
- 24.Ureña P, Bernard-Poenaru O, Ostertag A, Baudoin C, Cohen-Solal M, Cantor T, et al. Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant. 2003;18: 2325–2331. 10.1093/ndt/gfg403 [DOI] [PubMed] [Google Scholar]
- 25.Iimori S, Mori Y, Akita W, Kuyama T, Takada S, Asai T, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant. 2012;27: 345–351. 10.1093/ndt/gfr317 [DOI] [PubMed] [Google Scholar]
- 26.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7: 1130–1136. 10.2215/CJN.12871211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7: 1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfrey AC, Miller NL, Trow R. Effect of age and magnesium depletion on bone magnesium pools in rats. J Clin Invest. 1974;54: 1074–1081. 10.1172/JCI107851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leidi M, Dellera F, Mariotti M, Banfi G, Crapanzano C, Albisetti W, et al. Nitric oxide mediates low magnesium inhibition of osteoblast-like cell proliferation. J Nutr Biochem. 2012;23: 1224–1229. 10.1016/j.jnutbio.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 30.Belluci MM, Schoenmaker T, Rossa-Junior C, Orrico SR, de Vries TJ, Everts V. Magnesium deficiency results in an increased formation of osteoclasts. J Nutr Biochem. 2013;24: 1488–1498. 10.1016/j.jnutbio.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 31.Creedon A, Flynn A, Cashman K. The effect of moderately and severely restricted dietary magnesium intakes on bone composition and bone metabolism in the rat. Br J Nutr. 1999;82: 63–71. 10.1017/s0007114599001130 [DOI] [PubMed] [Google Scholar]
- 32.Rude RK, Gruber HE, Wei LY, Frausto A, Mills BG. Magnesium deficiency: effect on bone and mineral metabolism in the mouse. Calcif Tissue Int. 2003;72: 32–41. 10.1007/s00223-001-1091-1 [DOI] [PubMed] [Google Scholar]
- 33.Chatterji S, Wall JC, Jeffery JW. Age-related changes in the orientation and particle size of the mineral phase in human femoral cortical bone. Calcif Tissue Int. 1981;33: 567–574. 10.1007/BF02409493 [DOI] [PubMed] [Google Scholar]
- 34.Castiglioni S, Cazzaniga A, Albisetti W, Maier JA. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5: 3022–3033. 10.3390/nu5083022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quitterer U, Hoffmann M, Freichel M, Lohse MJ. Paradoxical block of parathormone secretion is mediated by increased activity of G alpha subunits. J Biol Chem. 2001;276: 6763–6769. 10.1074/jbc.M007727200 [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Ortiz ME, Canalejo A, Herencia C, Martínez-Moreno JM, Peralta-Ramírez A, Perez-Martinez P, et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol Dial Transplant. 2014;29: 282–289. 10.1093/ndt/gft400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhosaini M, Leehey DJ. Magnesium and dialysis: the neglected cation. Am J Kidney Dis. 2015;66: 523–531. 10.1053/j.ajkd.2015.01.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are contained in the manuscript and its Supporting Information files.