Abstract
Human immunodeficiency virus (HIV) infection is associated with increased systemic microbial translocation, neuroinflammation, and occasionally, neuronal injury. Whether systemic lipopolysaccharide (LPS) penetrates into the brain and contributes to neuroinflammation remain unknown in HIV. Here, we measured plasma and cerebrospinal fluid (CSF) LPS levels along with biomarkers of neuroinflammation (white blood cell counts and 40 soluble markers) and neurofilament light chain (NfL). Notably, CSF LPS was undetectable in all samples, including 3 HIV-infected individuals with dementia. Increased plasma LPS, neuroinflammation, and blood-brain barrier (BBB) dysfunction were found in untreated HIV-infected individuals, but not in healthy or treated HIV-infected individuals. Plasma LPS levels were directly correlated with various markers of inflammation in both plasma and CSF, as well as with degree of BBB permeability but not with CSF NfL in HIV-infected subjects. These results suggest that the magnitude of microbial translocation associates with neuroinflammation and BBB permeability in HIV without direct penetration into the central nervous system.
Keywords: cerebrospinal fluid, lipopolysaccharide, HIV-1 infection, neuroinflammation, blood-brain barrier permeability, neuronal injury
In this study, the magnitude of microbial translocation (lipopolysaccharide) associates with neuroinflammation and blood-brain barrier permeability in HIV without direct penetration into the central nervous system.
Human immunodeficiency virus (HIV) infection is associated with a “permeable” gut, increased systemic microbial translocation, and persistent inflammation even in the presence of viral-suppressive antiretroviral therapy (ART) [1]. HIV-associated neurocognitive disorder (HAND) is more frequently found in patients with abundant systemic and central nervous system (CNS) immune activation, potentially a consequence of microbial translocation [2–4]. While neuroinflammatory responses are primarily a protective mechanism in the brain, persistent inflammation may accelerate neural injury [5]. Indeed, diseases other than HIV infection (eg, inflammatory bowel diseases) with increased systemic microbial translocation may also associate with accelerated neurocognitive impairment [6, 7]. Furthermore, the plasma levels of markers of monocyte activation (eg, soluble CD14 [sCD14] and soluble CD163 [sCD163]) through microbial Toll-like receptor (TLR) 4 agonists are associated with neurocognitive impairment in HIV [3, 4]. Monocytes and macrophages are considered to play a key role in HAND, and CD14+CD16+ monocytes are increased in HIV-infected (HIV+) patients with dementia and may preferentially migrate through the blood-brain barrier (BBB) [8].
Lipopolysaccharide (LPS) is the major component of the gram-negative bacterial outer membrane, which binds to TLR4 expressed on the cell surface. Notably, TLR2 and TLR4 are expressed on the human and rat BBB [9], suggesting that ligation of TLR2 and TLR4 may alter the permeability of BBB and induce proinflammatory cytokines to the CNS. Indeed, systemic LPS exposure resulted in increased BBB permeability both in vitro, using human brain microvascular endothelial cells (BMECs), and in vivo in animals [10–13]. Moreover, intraperitoneal injection of LPS resulted in neuroinflammation and behavioral changes [14–16]. However, whether LPS can directly enter and impact the CNS remains controversial.
We undertook this exploratory cross-sectional study measuring plasma and cerebrospinal fluid (CSF) LPS to examine the questions of whether systemically increased LPS is accompanied by increased CSF LPS and whether plasma LPS, as an indicator of microbial translocation, associates with CNS inflammation and CNS injury in HIV infection.
MATERIALS AND METHODS
Study Subjects
This was a cross-sectional exploratory study using paired plasma and CSF samples from 16 healthy individuals; 32 untreated HIV+ individuals, including 3 who had presented clinically with subacute HIV-associated dementia (HAD); and 27 ART-treated, virally suppressed HIV+ individuals. These samples were collected from 2 academic centers: Sahlgrenska University Hospital (Gothenburg, Sweden) and San Francisco General Hospital (University of California, San Francisco), in the context of research protocols approved by the local institutional review boards with informed consent obtained by all participants.
CSF and Blood Sampling
CSF was obtained according to standard protocols as previously described [17–20]. Subjects also underwent phlebotomy for concurrent blood sampling along with general medical and neurological assessments at the study visit as previously described [21]. CSF was placed immediately on wet ice and subsequently subjected to low-speed centrifugation to remove cells, aliquoted, and stored within 2 hours of collection at ≤ –70°C until the time of HIV-1 RNA and biomarker assays. Blood was collected either in ethylenediaminetetraacetic acid or as serum, aliquoted, and stored in parallel with CSF for later batch assays.
Clinical Evaluations
All subjects underwent routine clinical bedside screening for symptoms or signs of CNS opportunistic infections or other conditions that might impact CSF biomarker concentrations; individuals with CNS opportunistic infections or other conditions confounding these analyses were omitted. Designation as AIDS dementia complex (ADC)/HAD was based on clinicians’ assessment at the time of diagnostic presentation, characteristically with subacute onset and progression of cognitive and motor symptoms and signs. This met American Academy of Neurology criteria in place at the time [22]. Most of these subjects were studied before publication of the more formal Frascati criteria [23] and were diagnosed with ADC stages 2–4 [24] but met the functional criteria for the Frascati diagnosis of HAD without the requisite extensive formal neuropsychological assessment.
Background Laboratory Methods
The salient clinical characteristics of these individuals are shown in Table 1. HIV-1 RNA levels were measured in cell-free CSF and plasma using the ultrasensitive Amplicor HIV Monitor assay (versions 1.0 and 1.5; Roche Molecular Diagnostic Systems, Branchburg, New Jersey), Cobas TaqMan RealTime HIV-1 (version 1 or 2; Hoffmann-La Roche, Basel, Switzerland), or the Abbott RealTime HIV-1 assay (Abbott Laboratories, Abbott Park, Illinois). All recorded viral loads that were below a LLQ of 20 copies/mL were standardized to a defined “floor” value of 19 copies/mL (log10 value of 1.279) for descriptive purposes. Each study visit included assessments by local clinical laboratories using routine methods to measure CSF white blood cell (WBC) count, CSF, and blood albumin in order to assess BBB integrity [25], and blood CD4+ and CD8+ T-lymphocyte counts by flow cytometry. BBB permeability was evaluated by the CSF to serum albumin quotient [26, 27]. CSF (mg/L) and serum (g/L) albumin levels were analyzed by nephelometry (Behring Nephelometer Analyzer; Behringwerke, Marburg, Germany). CSF neurofilament light chain (NfL) concentration was measured by a sensitive immunoassay using an enzyme-linked immunosorbent assay (ELISA) kit (NF-light ELISA kit, UmanDiagnostics, Umeå, Sweden) in the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital (Gothenburg, Sweden); intra-assay coefficients of variation were below 10% [26]. Since CSF NfL changes with age, CSF NfL levels were age-adjusted to 50 years for comparisons across subjects, and considered normal if <967 ng/L [28].
Table 1.
Clinical Characteristics
| Characteristic | HIV-Negative Control | HIV+/Untreated | HIV+/ART-Treated/Sup | P Value (HIV+/Untreated vs HIV+/ART-Treated Sup) |
|---|---|---|---|---|
| Total No. of subjects | 16 | 32 | 27 | |
| Age, y | 54 (37–62) | 49 (35–55) | 42 (36–53) | |
| CD4+ T-cell count, cells/μL | 799 (690–914) | 255 (98–490) | 570 (500–670) | <.0001 |
| Plasma HIV RNA load, log10 copies/mL | … | 4.9 (4.3–5.5) | 1.3 (1.3-1.3) | <.0001 |
| CSF HIV RNA load, log10 copies/mL | … | 3.9 (2.9–5.1) | 1.3 (1.3-1.3) | <.0001 |
| CSF WBCs, cells/uL | 1.5 (1.0–2.8) | 10 (1.5–19) | 0 (0–2.0) | <.0001 |
| BBB permeability, ratio | 4.65 (3.9–6.6) | 6.3 (4.5–7.3) | 4.9 (3.5–5.8) | .004 |
| QNZP4, score | –0.06 (–0.42 to 0.45) | –0.4 (–2.85 to 0.16) | 0.29 (–0.31 to 0.88) |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BBB, blood-brain barrier; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; HIV+, human immunodeficiency virus infected; QNZP4, global cognition score.
Evaluation of LPS
Plasma and CSF LPS levels were measured by endpoint chromogenic limulus amebocyte lysate assays according to the manufacturer’s protocol (Lonza, Basel, Switzerland), described previously [29]. In brief, CSF samples were from 1:1 to 1:40, and plasma samples were 1:10 diluted with endotoxin-free water and subsequently heated to 85°C for 15 minutes to inactivate inhibitory proteins. LPS levels were calculated after subtracting the background values. The limit of LPS detection in both CSF and plasma was 10 pg/mL.
Evaluation of Biomarkers of Inflammation in CSF and Plasma Samples
Levels of sCD14 and sCD163 were assessed by ELISA (R&D Systems, Minneapolis, Minnesota). CSF concentrations were measured at the Vitalant Research Institute (San Francisco, California), and plasma measurements were performed in the Wei Jiang Laboratory. Neopterin was analyzed in Innsbruck, Austria, using an ELISA kit (BRAHMS, Berlin, Germany) [30]. Additionally, plasma and CSF levels of 37 inflammatory markers were evaluated using Neuroinflammation Panel 1 kits following the manufacturer’s instruction (MESO Scale Discovery, Rockville, Maryland). The 37 markers are listed in Supplementary Table 1.
Statistical Analysis
The differences in continuous measurements were analyzed by nonparametric Mann–Whitney U tests for 2-group comparisons and by the analysis of variance and Kruskal–Wallis tests for >2-group comparisons. Correlations were analyzed by Spearman correlation tests. Age-adjusted P values were calculated by analysis of covariance (ANCOVA) using SAS version 9.3 software (SAS Institute, Cary, North Carolina). All tests were 2-sided, and P values of ≤ .05 were considered statistically significant.
RESULTS
LPS Was Increased in the Plasma of Viremic Subjects With HIV Compared to Uninfected Subjects but Was Not Detected in the CSF of Any Individual
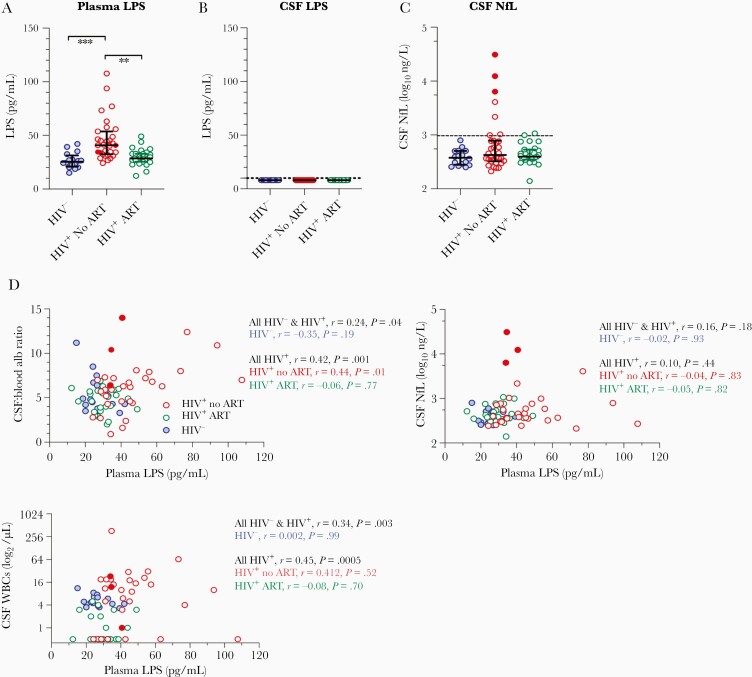
Lipopolysaccharide was detected in all 64 plasma samples assayed. By contrast, LPS was not detected in any of the matching CSF samples, including the 3 individuals with HAD (Figure 1A and 1B). Notably, plasma LPS levels were increased in ART-naive HIV+ individuals compared to ART-treated patients and HIV-uninfected (HIV–) controls (Figure 1A). Because age is the key factor associated with CNS function, we calculated P values after adjusting for age by ANCOVA. The differences of plasma LPS between HIV– controls and untreated patients (adjusted P = .0002), as well as between the 2 HIV+ groups (adjusted P = .0004), were still significant after adjusting for age. The difference between ART-treated patients and controls was not significant. Plasma LPS levels of the 3 HAD patients were not different from the other untreated patients (n = 28), sitting within the middle of the range (Figure 1A).
Figure 1.
Increased plasma level of lipopolysaccharide (LPS) in human immunodeficiency virus–infected (HIV+) individuals and its direct correlations with the magnitude of blood-brain barrier permeability, cerebrospinal fluid (CSF) levels of neopterin, and white blood cell (WBC) counts. A, Increased plasma level of LPS was found in HIV+ individuals even after adjusting for age compared to controls. **P < .01, ***P < .0001. B, Central nervous system LPS was undetectable in all samples. C, CSF levels of age-adjusted neurofilament light chain (NfL). Three HIV+ individuals with dementia are shown in filled red circles. D, Correlations between plasma LPS and albumin ratio in CSF vs serum, CSF age-adjusted NfL level, and CSF WBC counts in HIV-negative controls and HIV+ individuals. Abbreviations: alb, albumin; ART, antiretroviral therapy; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; LPS, lipopolysaccharide; NfL, neurofilament light chain; WBC, white blood cell.
Similar CSF Levels of Age-Adjusted NfL in HIV-Infected Individuals and HIV-Negative Individuals
To investigate active neuronal injury in HIV+ individuals, we evaluated age-adjusted NfL levels in the CSF samples. Overall, there was not a difference in age-adjusted NfL levels in CSF among the 3 study groups, though the 3 individuals with HAD had notably elevated levels (Figure 1C).
Correlations Between Plasma LPS Levels and the Magnitude of BBB Permeability as Well as CSF WBC Counts
To investigate the link between systemic microbial translocation and neuronal injury, we analyzed the correlations between plasma LPS levels and CSF NfL levels as well as the ratio of albumin levels in the CSF vs serum, a marker of BBB permeability [26, 27]. Notably, the levels of BBB permeability were increased in HIV+ untreated individuals compared to ART-treated HIV+ individuals or HIV– individuals (Table 1). There was no difference of the degree of BBB permeability between ART-treated HIV+ individuals and healthy individuals (Table 1). Plasma LPS levels were correlated with the magnitude of BBB permeability in all individuals (r = 0.24, P = .04), all HIV+ subjects (r = 0.42, P = .001), and untreated HIV+ subjects (r = 0.44, P = .01) but not in ART-treated HIV+ subjects or healthy controls (Figure 1D). Plasma LPS levels were not correlated with CSF NfL levels in any study group (Figure 1D). Consistently, CSF WBC counts were increased in untreated patients compared to treated patients or HIV– individuals, but no difference was shown between treated patients and healthy individuals (Table 1). Plasma levels of LPS were correlated with CSF WBC counts in all subjects (r = 0.34, P = .003) and all HIV+ subjects (r = 0.45, P = .0005), but not in any single HIV study group or healthy controls (Figure 1D).
Correlations Between Plasma LPS Levels and Levels of Proinflammatory Cytokines or Chemokines in the Blood and CSF Samples
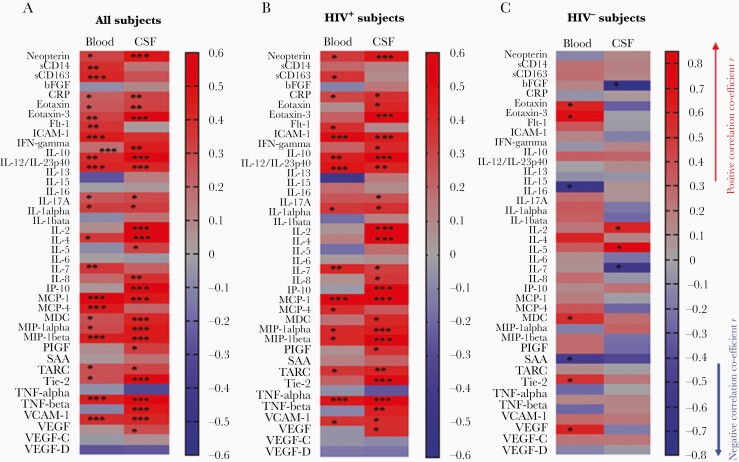
Increased neuroinflammation has been observed in HIV+ individuals that was associated with neurocognitive impairment [2, 31]. We further evaluated 40 soluble markers related to neuroinflammation. Notably, most inflammatory markers were higher in plasma than in CSF, with the exception of interleukin (IL) 6, monocyte chemoattractant protein (MCP) 1, IL-8, and placenta growth factor (PIGF) (Supplementary Table 1). HIV infection was associated with increases of various inflammatory markers in plasma and CSF; however, both plasma and CSF levels of Fms-like tyrosine kinase 1-1, bFGF, IL-13, IL-15, IL-5, and vascular endothelial growth factor (VEGF) D, and plasma levels of IL-1α, IL-1β, IL-4, MIP-1β, serum Amyloid A, thymus- and activation- regulated chemokine (TARC), Tie-2, tumor necrosis factor beta (TNF-β), and VEGF were similar in HIV+ subjects and controls (Supplementary Table 1). Compared to untreated HIV, ART was associated more significantly with decreased CSF inflammation compared to that in plasma (Supplementary Table 1). In contrast, compared to untreated HIV, ART was associated with decreases in the plasma sCD14 and sCD163 levels but not those in the CSF (Supplementary Table 1); both plasma sCD14 and sCD163 levels, but not CSF levels, were correlated with plasma LPS in all subjects (Figure 2).
Figure 2.
Correlation coefficient r and P values between plasma lipopolysaccharide (LPS) levels and inflammation in plasma and cerebrospinal fluid (CSF) samples. Plasma and CSF levels of 40 proinflammatory cytokines and neuropathologic markers were evaluated using MSD neuroinflammation kit or enzyme-linked immunosorbent assay. The Spearman correlation coefficient (r values in red: positive correlations; blue: negative correlations) and P values between plasma LPS levels and levels of inflammatory markers in the plasma and CSF in all subjects (A), human immunodeficiency virus–infected (HIV+) subjects (B), and HIV-negative subjects (C). *P < .05; **P < .001; ***P < .0001.
Among the LPS-related inflammatory biomarkers associated with neural injury or HAND [3, 32–35], CSF neopterin levels were increased in untreated HIV+ subjects compared to the other 2 groups, but no difference was observed between treated patients and healthy controls (Supplementary Table 1). Moreover, plasma sCD14 levels were increased in both treated and untreated HIV+ subjects compared to HIV– controls, but CSF sCD14 levels were similar among the 3 study groups (Supplementary Table 1). There was a correlation between plasma LPS and CSF neopterin (r = 0.47, P < .0001) in all subjects, as well as in HIV+ subjects (r = 0.47, P = .0002), but not in the healthy control group (Figure 2). There was a correlation between plasma LPS and plasma sCD14 in all subjects only (r = 0.38, P = .001; Figure 2). Furthermore, plasma sCD163 levels were increased in untreated HIV+ subjects compared to the other 2 groups (Supplementary Table 1); CSF sCD163 levels were increased in untreated HIV+ subjects compared to HIV-negative controls, but were similar to those in ART-treated HIV+ subjects (Supplementary Table 1). There was a correlation between plasma LPS and plasma sCD163 in all subjects (r = 0.41, P = .0002) and HIV+ subjects (r = 0.36, P = .005) (Figure 2). However, no correlation was observed between plasma LPS and CSF sCD14 or sCD163 (Figure 2).
Intriguingly, various direct correlations were observed between plasma LPS and levels of inflammatory markers in both plasma and CSF in all subjects (Figure 2A) and all HIV+ subjects (Figure 2B), but few in HIV– controls (Figure 2C). The correlations between plasma LPS and CSF levels of neopterin, eotaxin, eotaxin-3, IL-10, IL-1β, IL-2, IL-7, IL-8, MCP-4, MDC, MIP-1β, TARC, TNF-β, and VEGF were stronger than those between plasma LPS and blood levels of inflammation in both all subjects and all HIV+ subjects (Figure 2A and 2B). In contrast, some correlations between plasma LPS and plasma levels of sCD163, Flit-1, IL-6, and MCP-1, were stronger than those between plasma LPS and CSF inflammation in both all subjects and all HIV+ subjects (Figure 2A and 2B). These results show the associations between long-term repeated circulating microbial product translocation and inflammation in the blood and CNS in subjects with HIV disease.
DISCUSSION
A previous study showed that intravascular injection of LPS into the jugular vein of rats resulted in increased BBB permeability; however, LPS was undetectable in the rodents’ CNS [13]. Another study showed that systemic exposure of LPS resulted in BBB impairment, but LPS did not cross the BBB [12]. In contrast, LPS has been reported to infiltrate to the CNS under certain physiologic conditions in rats [36]. Also, 2 Alzheimer disease studies detected LPS in human primary brain tissues by immunohistochemistry [37, 38]. Moreover, a previous study showed that plasma levels of total bacterial 16S rDNA, a marker of microbial translocation, were correlated with more structural brain abnormalities in HIV patients [39]. This raises the question about cause or consequence of the link between plasma microbial translocation and neuroinflammation and cognitive performance. It remains unknown whether systemic microbial products can enter the CNS in humans. In the current study, we found increased plasma LPS in HIV+ individuals vs HIV– individuals; but LPS was undetectable in the CSF of all subjects including 3 dementia patients (Figure 1A and 1B).
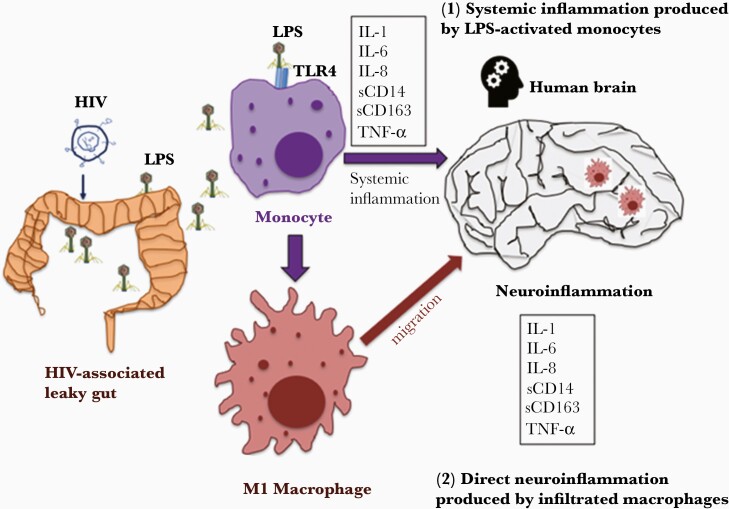
In our model (Figure 3), HIV-associated comprised gut mucosal barrier results in increased plasma LPS, a marker of systemic microbial translocation. The levels of neuroinflammation are low in the healthy individuals due to low plasma levels of LPS as well as an intact BBB barrier. In untreated HIV, high levels of plasma LPS promote a permeable BBB, systemic inflammation, and monocyte activation, which affect neuroinflammation. In virally suppressed ART-treated HIV, if the mucosal barrier is fully recovered and microbial translocation is limited to levels similar to those of healthy controls, then the neuroinflammation is likely low. However, some HIV+ subjects on virally suppressed ART may exhibit increased plasma levels of LPS, likely with poor CD4+ T-cell recovery (immune nonresponder) [40, 41]. Increased plasma LPS may contribute to neuroinflammation in the immune nonresponders, which deserves further investigation. Thus, mucosal barrier recovery may be a critical factor accounting for plasma LPS-mediated neuroinflammation.
Figure 3.
The mechanisms of plasma lipopolysaccharide (LPS)–mediated neuroinflammation in human immunodeficiency virus (HIV) disease. HIV infection is associated with a comprised mucosal barrier (eg, gut), which results in systemic microbial translocation (eg, bacterial LPS). There are at least 2 potential mechanisms accounting for plasma LPS-mediated neuroinflammation in HIV: (1) increased circulating microbial LPS persistently activates monocytes to produce proinflammatory cytokines in the circulation, which affect the brain as well; (2) increased circulating microbial LPS persistently activates monocytes to differentiate to M1 macrophages, which migrate to tissue sites such as brain and mediate neuroinflammation. Abbreviations: HIV, human immunodeficiency virus; IL, interleukin; LPS, lipopolysaccharide; sCD14, soluble CD14; sCD163, soluble CD163; TLR, Toll-like receptor; TNF, tumor necrosis factor.
Neuronal injury in HIV-infected individuals may result from persistent neuroinflammation, HIV viral replication, immune activation, oxidative stress, comorbidities, and other factors [42]. NfL is a structural component of axons that can be released from damaged neurons to the CSF; thus, increased CSF NfL levels indicate neuronal injury [28]. In the CSF of HIV+ subjects compared to controls, we found increased neuroinflammation and BBB permeability, but CSF NfL levels were similar among the 3 study groups (Figure 1 and Table 1). Previous studies show CSF NfL levels were increased in untreated HIV+ individuals compared to controls [31, 34]. In the other studies [20, 34, 43], HIV+ individuals with HAD had increased levels of CSF NfL, which was consistent with our results (Figure 1C). Nonetheless, our results suggest that increased neuroinflammation is not necessary to result in neural injury and neurodegeneration in HIV. The observation that elevated CSF NfL and HAD are not distinguished by plasma LPS is not consistent with the finding of LPS being associated with CNS injury [44, 45]. However, the cross-sectional nature of the study may limit the evaluation of duration of neuroinflammation and its effects on neuronal damage in HIV.
Systemic LPS exposure resulted in increased BBB permeability in animals in vivo, and in vitro treatment of LPS increased permeability of BMECs [10–13]. Consistently, we found a direct correlation between the plasma LPS level and the degree of BBB permeability in HIV+ subjects (Figure 1D). Furthermore, intraperitoneal injection of LPS in mice resulted in neuroinflammation [14–16]. LPS induced neopterin in human PBMCs and macrophages in vitro [46]. Consistently, we found that plasma LPS levels were correlated with blood and CSF levels of neopterin in HIV. In human studies, correlations were observed between the degree of BBB permeability and CSF neopterin levels and CSF WBC counts, suggesting that CSF neopterin and WBC infiltration may be a consequence of a permeable BBB in HIV [35]. Increased plasma LPS has been reported in HIV+ patients with dementia; plasma sCD14, produced by LPS stimulation in monocytes, is associated with neurocognitive impairment in HIV [32, 47, 48]. Plasma, but not CSF sCD14 or sCD163, is a better marker to associate with HAND [3, 32]. In the current study, significant correlations were observed between plasma LPS and plasma but not CSF levels of sCD14 and sCD163 in all subjects. The disruption of BBB barriers by systemic LPS exposure and TLR-downstream proinflammatory cytokines has been reported in both human and animal studies, but not in HIV [10–13, 49].
In addition to HIV, other diseases (eg, inflammatory bowel diseases) exhibit increased systemic microbial translocation, accompanied by accelerated neurocognitive disorders [7]. Notably, deletion of CD14+ monocytes attenuated Alzheimer disease pathology [50], suggesting that monocytes or macrophages play a role in neurocognitive impairment. In the current study, we found that plasma LPS was directly correlated with plasma and CSF levels of serial proinflammatory cytokines in all subjects and HIV+ subjects, but not in HIV– individuals. Some of these cytokines or chemokines can be released from monocytes or macrophages after LPS stimulation. The link between plasma microbial translocation and monocyte activation and migration, as well as their contribution to CNS inflammation and dysfunction, deserves further investigation.
There are several limitations to this exploratory study. These include the relatively small sample size, particularly with respect to the untreated HIV+ group with a limited range of systemic disease progression, and only 3 individuals with HAD. However, even with this small number, it is clear that LPS does not (or only rarely) enter the CSF in the absence of bacterial infection in the brain or sepsis, so any direct effect of microbial translocation is seemingly confined to its systemic impact. The correlations of plasma LPS with various inflammatory markers in CSF and plasma should likely be considered as preliminary and bear examination in a larger and more broadly constructed group of untreated HIV+ subjects and treated HIV+ immune nonresponders.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers R01NS094067 to R. W. P. and R01AI128864 to W. J.), as well as the Swedish government and county councils (Avtal om Läkarutbildning och Forskning i Västra Götalandsregionen agreement number ALFGBG-717531). H. Z. is a Wallenberg Scholar supported by the Swedish Research Council (grant number 2018–02532), the European Research Council (grant number 681712), Swedish State Support for Clinical Research (grant number ALFGBG-720931), the Alzheimer Drug Discovery Foundation (grant number 201809-2016862), and the United Kingdom Dementia Research Institute at University College London.
Potential conflicts of interest. H. Z. has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chung CY, Alden SL, Funderburg NT, Fu P, Levine AD. Progressive proximal-to-distal reduction in expression of the tight junction complex in colonic epithelium of virally-suppressed HIV+ individuals. PLoS Pathog 2014; 10:e1004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edén A, Marcotte TD, Heaton RK, et al. . Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 2016; 11:e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryan LA, Zheng J, Brester M, et al. . Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis 2001; 184:699–706. [DOI] [PubMed] [Google Scholar]
- 5. Kempuraj D, Thangavel R, Selvakumar GP, et al. . Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci 2017; 11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol 2013; 10:585–95. [DOI] [PubMed] [Google Scholar]
- 7. Morís G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol 2014; 20:1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fischer-Smith T, Croul S, Sverstiuk AE, et al. . CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 2001; 7:528–41. [DOI] [PubMed] [Google Scholar]
- 9. Nagyoszi P, Wilhelm I, Farkas AE, et al. . Expression and regulation of Toll-like receptors in cerebral endothelial cells. Neurochem Int 2010; 57:556–64. [DOI] [PubMed] [Google Scholar]
- 10. Banks WA, Gray AM, Erickson MA, et al. . Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation 2015; 12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dohgu S, Fleegal-DeMotta MA, Banks WA. Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF. J Neuroinflammation 2011; 8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology 2004; 201:197–207. [DOI] [PubMed] [Google Scholar]
- 13. Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun 2010; 24:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wendeln AC, Degenhardt K, Kaurani L, et al. . Innate immune memory in the brain shapes neurological disease hallmarks. Nature 2018; 556:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JW, Lee YK, Yuk DY, et al. . Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 2008; 5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodrigues FTS, de Souza MRM, Lima CNC, et al. . Major depression model induced by repeated and intermittent lipopolysaccharide administration: Long-lasting behavioral, neuroimmune and neuroprogressive alterations. J Psychiatr Res 2018; 107:57–67. [DOI] [PubMed] [Google Scholar]
- 17. Price RW, Paxinos EE, Grant RM, et al. . Cerebrospinal fluid response to structured treatment interruption after virological failure. AIDS 2001; 15:1251–9. [DOI] [PubMed] [Google Scholar]
- 18. Spudich SS, Nilsson AC, Lollo ND, et al. . Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis 2005; 5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andersson LM, Svennerholm B, Hagberg L, Gisslén M. Higher HIV-1 RNA cutoff level required in cerebrospinal fluid than in blood to predict positive HIV-1 isolation. J Med Virol 2000; 62:9–13. [DOI] [PubMed] [Google Scholar]
- 20. Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis 2007; 195:1774–8. [DOI] [PubMed] [Google Scholar]
- 21. Price RW, Sidtis JJ. Evaluation of the AIDS dementia complex in clinical trials. J Acquir Immune Defic Syndr 1990; 3(Suppl 2):S51–60. [PubMed] [Google Scholar]
- 22. Research Criteria for Diagnosis of Chronic Inflammatory Demyelinating Polyneuropathy. Report from an ad hoc subcommittee of the American Academy of Neurology AIDS Task Force. Neurology 1991; 41:617–8. [PubMed] [Google Scholar]
- 23. Antinori A, Arendt G, Becker JT, et al. . Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price RW, Sidtis J, Rosenblum M. The AIDS dementia complex: some current questions. Ann Neurol 1988; 23(Suppl):S27–33. [DOI] [PubMed] [Google Scholar]
- 25. Spudich S, Gisslen M, Hagberg L, et al. . Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis 2011; 204:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaetani L, Höglund K, Parnetti L, et al. . A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther 2018; 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blennow K, Fredman P, Wallin A, et al. . Protein analysis in cerebrospinal fluid. III. Relation to blood-cerebrospinal fluid barrier function for formulas for quantitative determination of intrathecal IgG production. Eur Neurol 1993; 33:134–42. [DOI] [PubMed] [Google Scholar]
- 28. Yilmaz A, Blennow K, Hagberg L, et al. . Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn 2017; 17:761–70. [DOI] [PubMed] [Google Scholar]
- 29. Luo Z, Li M, Wu Y, et al. . Systemic translocation of Staphylococcus drives autoantibody production in HIV disease. Microbiome 2019; 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yilmaz A, Fuchs D, Price RW, et al. . Cerebrospinal fluid concentrations of the synaptic marker neurogranin in neuro-HIV and other neurological disorders. Curr HIV/AIDS Rep 2019; 16:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guha D, Mukerji SS, Chettimada S, et al. . Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS 2019; 33:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyons JL, Uno H, Ancuta P, et al. . Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weaver LK, Pioli PA, Wardwell K, Vogel SN, Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol 2007; 81:663–71. [DOI] [PubMed] [Google Scholar]
- 34. Jessen Krut J, Mellberg T, Price RW, et al. . Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One 2014; 9:e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anesten B, Yilmaz A, Hagberg L, et al. . Blood-brain barrier integrity, intrathecal immunoactivation, and neuronal injury in HIV. Neurol Neuroimmunol Neuroinflamm 2016; 3:e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vargas-Caraveo A, Sayd A, Maus SR, et al. . Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci Rep 2017; 7:13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016; 87:2324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Y, Cong L, Jaber V, Lukiw WJ. Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Front Immunol 2017; 8:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vera JH, Guo Q, Cole JH, et al. . Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology 2016; 86:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luo ZW, Li Z, Martin L, et al. . Pathological role of anti-CD4 antibodies in HIV-infected immunologic non-responders under viral suppressive antiretroviral therapy. J Infect Dis 2017; 216:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu W, Luo Z, Alekseyenko AV, et al. . Distinct systemic microbiome and microbial translocation are associated with plasma level of anti-CD4 autoantibody in HIV infection. Sci Rep 2018; 8:12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saylor D, Dickens AM, Sacktor N, et al. . HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peterson J, Gisslen M, Zetterberg H, et al. . Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 2014; 9:e116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vassallo M, Dunais B, Durant J, et al. . Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: the Neuradapt study. J Neurovirol 2013; 19:376–82. [DOI] [PubMed] [Google Scholar]
- 45. Monnig MA, Kahler CW, Cioe PA, et al. . Markers of microbial translocation and immune activation predict cognitive processing speed in heavy-drinking men living with HIV. Microorganisms 2017; 5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Tumour necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biol Chem Hoppe Seyler 1989; 370:1063–9. [DOI] [PubMed] [Google Scholar]
- 47. Ancuta P, Kamat A, Kunstman KJ, et al. . Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jespersen S, Pedersen KK, Anesten B, et al. . Soluble CD14 in cerebrospinal fluid is associated with markers of inflammation and axonal damage in untreated HIV-infected patients: a retrospective cross-sectional study. BMC Infect Dis 2016; 16:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anda T, Yamashita H, Khalid H, et al. . Effect of tumor necrosis factor-alpha on the permeability of bovine brain microvessel endothelial cell monolayers. Neurol Res 1997; 19:369–76. [DOI] [PubMed] [Google Scholar]
- 50. Reed-Geaghan EG, Reed QW, Cramer PE, Landreth GE. Deletion of CD14 attenuates Alzheimer’s disease pathology by influencing the brain’s inflammatory milieu. J Neurosci 2010; 30:15369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.