Abstract
Thymosin alpha-1 (Tα1) is an immunomodulatory and antiviral agent with potential effects on chronic hepatitis B and liver cancer. Its impact on solitary hepatocellular carcinoma (HCC) remains controversial, so we aimed to investigate the efficacy of Tα1 in solitary HBV-related HCC patients after curative resection.
Between May 2010 and April 2016, 468 patients with solitary HBV-related HCC after curative resection were analyzed. Propensity score matching (PSM) was used to minimize confounding variables. Risk factors were identified by the Cox proportional hazards model. Recurrence-free survival (RFS) rates, overall survival (OS) rates, immunological, and virologic response were compared.
The median follow up was 60.0 months. Immunological response improved in the Tα1 group compared with the control group (P < .001) but the virologic response was similar between 2 groups after 24 months. Patients with Tα1 therapy had better RFS and OS before (P = .018 and P < .001) and after (P = .006 and P < .001) propensity matching. Multivariate analysis revealed that Tα1 therapy was an independent prognostic factor for both OS (P < .001, HR = 0.308, 95% CI: 0.175–0.541) and RFS (P < .001, HR = 0.381, 95% CI: 0.229–0.633).
Tα1 as an adjuvant therapy improves the prognosis of solitary HBV-related HCC patients after curative liver resection.
Keywords: chronic hepatitis B, hepatocellular carcinoma, propensity score matching, thymosin alpha-1
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth common solid tumor and the 3rd-leading cause of cancer-related death worldwide. It accounts for about 50% of the total number of death due to high hepatitis B virus (HBV) infection in China.[1,2] Curative treatment for HCC includes liver resection and transplantation. Liver resection remains a popular curative treatment especially for solitary HCC patients with well-preserved liver function regardless of tumor size.[3] Unfortunately, long-term prognosis after curative resection of HCC remains unsatisfactory.[4–7] Recent studies has reported that postoperative adjuvant therapy were available for prognosis of HCC patients such as chemotherapy and immunotherapy.[8,9] However, there is no universally accepted effective adjuvant treatment to prevent HCC recurrence. Serial studies showed that superior immune function of patients resulted in better prognosis after curative treatment.[10–12] Thymosin alpha-1 (Tα1, thymalfasin, ZADAXIN) is an immunomodulatory and antiviral agent which is approved in 35 countries worldwide. It is biologic peptide with immunomodulatory activities and therapeutic applicability in several diseases including depressed response to vaccination, chronic hepatitis B (CHB) and cancer. To date, over 3000 patients have received Tα1 therapy with significant benefit, especially for patients with CHB in China.[13,14] Previous investigations have shown that Tα1 could improve prognosis of HCC patients who underwent transarterial chemoembolization (TACE) or curative reseciton.[15,16] Additionally, a meta-analysis[17] of 8 randomized controlled trials showed Tα-1 and lamivudine combination therapy had superior effect than lamivudine monotherapy in terms of biochemical response, virologic response, and hepatitis B e antigen (HBeAg) seroconversion.[18] To the best of our knowledge, there is few study focusing on effect of Tα1 on the prognosis of solitary HBV-related HCC patients after curative treatment. The potent effect and mechanism of thymosin alpha-1 for solitary HBV-related HCC is still unclear. So we designed this research to evaluate the efficacy of Tα1 as adjuvant therapy in patients with solitary HBV-related HCC who underwent curative liver resection.
2. Materials and methods
2.1. Patients
The study was a retrospective, singlecenter trial. From May 2010 to April 2016, consecutive patients at our liver unit (West China Hospital) with newly diagnosed solitary HBV-related HCC who had received R0 liver resection were eligible for enrollment. The diagnosis of HCC was based on the histopathological study of the resected specimens. This study was approved by the Ethics Committee of West China Hospital, Sichuan University. As this study focused on long-term oncologic outcomes after curative resection, we excluded 12 patients who died in the hospital. Finally, 468 patients were enrolled in the study.
Inclusion criteria for this study were as follows:
-
1.
Primary solitary HCC without major vascular invasion or distant metastasis;
-
2.
Good liver function with Child-Pugh Class A;
-
3.
Received antiviral therapy postoperatively;
-
4.
Positive test for hepatitis B surface antigen and negative test for antibody to hepatitis C virus (HCV-Ab) or human immunodeficiency virus (HIV) before antiviral therapy;
-
5.
No previous treatment of HCC;
-
6.
Negative resection margin (R0 resection).
Exclusion criteria included the following:
-
1.
Extrahepatic malignancies;
-
2.
Previous resection, TACE, ablative therapies or liver transplantation;
-
3.
Poor liver reserve function with a Child–Pugh grade B or C;
-
4.
Simultaneous splenectomy.
Eligible patients were divided into Tα1 group and control group. Patients who accepted Tα1 as adjuvant therapy will be included in the Tα1 group (injected subcutaneously with 1.6 mg twice per week for at least 6 months as postoperative Tα1 therapy). Patients who refused Tα1 therapy will be included in the control group (long-term follow-up only). In 2015, the American Association for the Study of Liver (AASLD) adopted Entecavir (ETV) and tenofovir disoproxil fumarate (TDF) as the first-line antiviral treatment for hepatitis B.[19] All Patients received ETV tablets (RunZhong, CHIATAI TIANQING) 0.5 mg/d, TDF tablets (Viread, Aspen Port Elizabeth) 300 mg/d or adefovir disoproxil fumarate (ADV) tablets (Hepsera, GlaxoSmithKline) 10 mg/d orally. Patients who resistanted to ETV were recommended to add ADV or switch to TDF.
2.2. Follow-up and outcomes
All the patients received follow-up monitoring 1 month after the operation, every 3 months thereafter during the first 1 year and then every 6 months in subsequent years. Physical examination, blood cell and differential counts, liver function tests, alpha-fetoprotein levels (AFP), HBV markers, HBV-DNA levels, imaging examinations, and adverse events (AE) were included in the follow-up examinations when necessary. The primary outcome measures included both recurrence-free (RFS) and overall survival rates calculated from the date of the operation secondary outcome measures included an immune response (measured by neutrophil to lymphocyte ratio (NLR) which defined as absolute neutrophil counts divided by lymphocyte counts, virologic response, and liver function. The last follow-up date was the end of January 2019.
Patients prognosis and tumor recurrence in the study was according to the modified RECIST (mRECIST) criteria[20] and the European Association for the Study of the Liver (EASL) criteria.[21] Moreover, procedure of evaluation of tumor recurrence were followed by the guidelines of diagnosis and treatment of primary liver cancer in China.[22] Tumor recurrence was suspected on detection of new hepatic lesions on ultrasonograph or by a progressive and continuous elevation of serum AFP (>100 ng/mL). If the patients’ AFP level had fallen to normal level after operation or the patients had a normal AFP level before operation, the serum AFP levels of these patients were also regularly monitored. Patients with tumor recurrence were actively treated with salvage liver transplantation, repeat hepatic resection, radiofrequency ablation, TACE, sorafenib and/or chemotherapy, depending on the extent of the disease, the liver function, and the general condition of the patients.
2.3. Propensity score matching
To minimize the influence of confounders on the selection bias, propensity score matching was performed to balance these baseline differences and thereby simulate random group allocation.[23,24] Propensity scores were estimated using a logistic regression model based on age, gender, presence of HBeAg, serum AFP level, total bilirubin level, alanine aminotransferase (ALT) level, serum creatinine level (CREA), tumor size, presence of liver cirrhosis, microvascular invasion (MVI), and blood transfusion. Subsequently, 1:1 matching without replacement was performed using a 0.2 caliper width, and the resulting score-matched pairs were used in subsequent analyses, as previously reported.[25]
2.4. Statistical analysis
We used SPSS software version 24.0 (SPSS Company, Chicago, IL) for windows to perform statistical analysis. The continuous variables are expressed as the mean ± the standard deviation. The categorical variables are presented as numbers (percentages). Categorical data were compared by the Chi-Squared test or Fisher exact test. Continuous variables were compared by independent t test for normally distributed data or Mann–Whiney U test for skewness-distributed data. OS and RFS were analyzed by the Kaplan–Meier method, and the differences were analyzed by a log-rank test. Multivariate Cox proportional hazards regression analysis was used to evaluate the prognostic factors. Calculated P values were 2-sided, and a P value <.05 was considered statistically significant. In the study, neutrophil to lymphocyte ratio (NLR) was carried out to evaluate the immune response of patients, ΔNLR was defined as the NLR of follow-up visit minus preoperative NLR. Data of NLR were excluded if there were clinical symptoms or signs of sepsis at the time of blood sampling for NLR, or white blood cell counts >10 × 109/L.
3. Results
3.1. Characteristics of patients in the study
During this period, 468 patients with solitary HBV-related HCC received curative resection in our department, of whom 228 patients received Tα1 adjuvant therapy postoperatively (Tα1 group) while 240 patients did not (control group). During the follow-up, no patient in the Tα1 group were reported to have serious adverse events (SAE). No events were considered to be relevant to study drugs (Supplementary Fig. S1). Baseline characteristics, serologic parameters, tumor characteristics, and operative data were summarized in Table 1. The median follow-up was 60.0 months. The Tα1 and control groups were similar in the majority of baseline characteristics, but there were significant differences in age, gender, total bilirubin level, ALT level, tumor size, presence of liver cirrhosis and microvascular invasion (MVI). To reduce the risk of which the results were confounded by the these baseline difference, 1:1 propensity score matching was performed to generate 100 pairs that showed no significant differences in any of the baseline parameters. After propensity score matching, no variables exhibited a large imbalance (Fig. 1). There was no significant baseline difference in baseline parameters between the 2 patient groups (Table 1).
Table 1.
Patient demographics and preoperative laboratory analysis before and after propensity score matching.
| Before matching | After matching | |||||
| Factor | Control Group(n = 240) | Tα1 Group(n = 228) | P | Control Group(n = 100) | Tα1 Group(n = 100) | P |
| Basic characteristics | ||||||
| Age, yr | 52.8 ± 11.7 | 49.7 ± 13.2 | <0.001 | 52.8 ± 11.7 | 39.6 ± 11.0 | .160 |
| Gender (male/female), n | 157/83 | 190/38 | <0.001 | 79/21 | 80/20 | .861 |
| Total Bilirubin, umol/L | 15.4 ± 5.9 | 16.6 ± 6.6 | 0.036 | 16.3 ± 6.4 | 16.5 ± 7.2 | .858 |
| ALT, IU/L | 38.0 ± 22.1 | 48.5 ± 33.7 | <0.001 | 42.9 ± 26.7.7 | 47.1 ± 43.2 | .413 |
| Albumin, g/L | 41.9 ± 5.5 | 41.5 ± 4.4 | 0.373 | 41.5 ± 4.7 | 41.3 ± 4.2 | .693 |
| Platelet count, 109/L | 109.6 ± 63.7 | 106.7 ± 62.6 | 0.620 | 114.6 ± 76.9 | 107.6 ± 59.6 | .476 |
| PT, s | 12.6 ± 5.0 | 12.5 ± 6.0 | 0.888 | 12.4 ± 2.4 | 13.0 ± 7.0 | .393 |
| Neutrophil count, 109/L | 3.93 ± 9.4 | 4.14 ± 11.3 | 0.824 | 4.74 ± 14.2 | 4.13 ± 13.6 | .758 |
| Lymphocyte count, 109/L | 2.06 ± 8.5 | 2.68 ± 11.4 | 0.505 | 2.86 ± 13.1 | 2.81 ± 13.7 | .979 |
| Creatinin, umol/L | 73.8 ± 16.1 | 73.5 ± 17.1 | 0.843 | 72.8 ± 14.5 | 72.8 ± 16.2 | .993 |
| Virologic characteristics | ||||||
| HbeAg (Positive/Negative), n | 37/203 | 31/197 | 0.577 | 17/83 | 13/87 | .428 |
| HBV-DNA (<2 × 103/≥2 × 103), n, IU/mL | 134/106 | 118/110 | 0.376 | 58/42 | 54/46 | .569 |
| Antiviral Therapy (ADV/TDF/ETV), n | 124/5/111 | 112/12/108 | 0.182 | 60/2/38 | 52/0/48 | .154 |
| Tumor characteristics | ||||||
| Tumor size, cm | 4.04 ± 3.85 | 3.65 ± 2.32 | <0.001 | 4.62 ± 3.2 | 3.68 ± 2.3 | .067 |
| MVI (Yes vs No), n | 29/211 | 56/172 | <0.001 | 15/85 | 18/82 | .568 |
| Differentiation (Low/Moderate/High), n | 89/142/9 | 76/146/6 | 0.503 | 37/61/2 | 39/60/1 | .821 |
| Cirrhosis (Yes vs No), n | 149/91 | 186/42 | <0.001 | 79/21 | 79/21 | 1.000 |
| AFP (<400/≥400), n, ng/mL | 174/66 | 156/72 | 0.331 | 65/35 | 64/36 | .883 |
| Operation data | ||||||
| Hospital stay, d | 7.25 ± 1.52 | 8.82 ± 2.23 | 0.087 | 8.77 ± 3.17 | 8.13.25 ± 5.52 | .795 |
| Transfusion (Yes vs No), n | 26/214 | 27/201 | 0.731 | 13/87 | 14/86 | .836 |
| Complication (Yes vs No), n | 31/240 | 20/228 | 0.150 | 9/91 | 7/93 | .602 |
Figure 1.
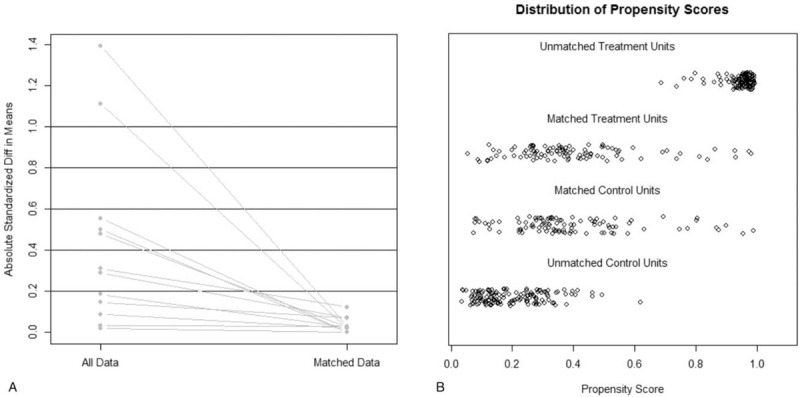
Minimized difference between Tα1 and control group after propensity score matching. A, Lineplot of standardized differences before and after matching; B, Even distribution of propensity score in matched patients.
3.2. Overall and recurrence-free survival analysis
Before propensity matching, the 1-, 3-, and 5-year OS were 96.5%, 87.1%, and 74.0% in the Tα1 group and 94.2%, 70.4%, and 52.6% in the control group, respectively (Fig. 2A); The 1-, 3-, and 5-year RFS were 93.6%, 76.3%, and 61.1% in the Tα1 group and 84.8%, 59.9%, and 30.4% in the control groups, respectively (Fig. 2B). The OS (HR: 0.512, 95% CI: 0.363–0.721, P < .001) and RFS (HR: 0.463, 95% CI: 0.337–0.637, P < .001) of patients who received Tα1 treatment were both significantly better than those who did not in the entire cohort. Univariable and multivariable Cox regression analyses of OS and RFS in the entire cohort were shown in Tables 2. On multivariable Cox regression analyses with robust estimator, Tα1 treatment was an independent risk factor associated with both OS (HR: 0.370, 95% CI: 0.260–0.528, P < .001) and RFS (HR: 0.345, 95% CI: 0.242–0.492, P < .001) in the entire cohort as well as low differentiation (HR: 0.378, 95% CI: 0.267–0.534, P < .001), MVI (HR: 5.249, 95% CI: 3.589–7.676, P < .001) and high AFP level (HR: 2.049, 95% CI: 1.411–2.977, P < .001).
Figure 2.
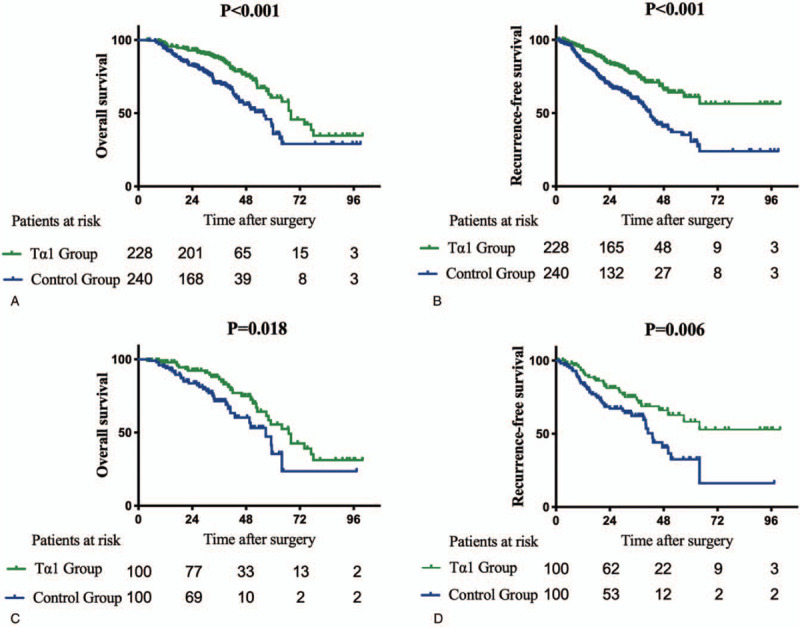
Survival curves of the control groups and Tα1 groups in the entire cohort and propensity matched cohort. (A-B) Overall survival and Recurrence-free survival of control group and Tα1 group in the entire cohort. (C-D) Overall survival and Recurrence-free survival of control group and Tα1 group in the propensity matched cohort.
Table 2.
Univariable and multivariable Cox regression analyses with robust estimator of overall survival and recurrence-free survival after curative resection of solitary HBV-related hepatocellular carcinoma in the entire cohort.
| Overall survival | Recurrence-free survival | |||||||
| Factors | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | |
| Gender (male/female) | <.001 | 0.535 (0.381–0.753) | .439 | .004 | 0.626 (0.344–0.862) | |||
| Age (>60 vs ≤60, Month) | .009 | 0.579 (0.382–0.879) | .833 | .012 | 0.631 (0.442–0.902) | |||
| Total Bilirubin, umol/L | .215 | .409 | ||||||
| ALT, IU/L | .877 | .175 | ||||||
| Albumin, g/L | .583 | .555 | ||||||
| ΔNLR (>0 vs ≤0) | .124 | .301 | ||||||
| Differentiation (Low, Moderate, High) | <.001 | 0.416 (0.296–0.585) | <.001 | 0.378 (0.267–0.534) | .001 | 0.593 (0.434–0.809) | ||
| MVI (Yes vs No) | <.001 | 4.741 (3.382–6.646) | <.001 | 5.249 (3.589–7.676) | <.001 | 2.244 (1.599–3.147) | <.001 | 4.547 (3.103–6.662) |
| Tumor Size (<5 vs ≥5, cm) | .744 | .888 | ||||||
| Cirrhosis (Yes vs No) | <.001 | 1.832 (1.307–2.568) | .511 | <.001 | 1.769 (1.296–2.416) | .615 | ||
| AFP (≤400 vs >400, ng/mL) | <.001 | 3.074 (2.199–4.297) | <.001 | 2.049 (1.411–2.977) | <.001 | 2.858 (2.105–3.880) | <.001 | 2.136 (1.466–3.110) |
| HBeAg (Positive vs Negative)) | .543 | .152 | ||||||
| HBV-DNA (≥2 × 103 vs 2 × <103, IU/mL) | .237 | .090 | ||||||
| Antiviral Therapy (NtA vs NsA) | .103 | .032 | 0.847 (0.487–0.903) | .038 | 0.899 (0.247–0.935) | |||
| Transfusion (Yes vs No) | .398 | .789 | ||||||
| Complication (Yes vs No) | .013 | 0.551 (0.344–0.882) | 0.230 | .098 | ||||
| Immunotherapy (Tα1 vs none Tα1) | <.001 | 0.512 (0.363–0.721) | <0.001 | 0.370 (0.260–0.528) | <.001 | 0.463 (0.337–0.637) | <.001 | 0.345 (0.242–0.492) |
After propensity matching, the 1-, 3-, and 5-year OS rates were 98.0%, 86.4%, and 55.5% in the Tα1 group and 95.0%, 71.5%, and 47.2% in the control group, respectively (Fig. 2C); The 1-, 3-, and 5-year RFS rates were 92.2%, 73.1%, and 58.2% in the Tα1 group and 84.7%, 62.2%, and 32.6% in the control group, respectively (Fig. 2D). The OS (HR: 0.542, 95% CI: 0.324–0.908, P = .018) and RFS (HR: 0.517, 95% CI: 0.317–0.842, P = .006) of patients who received the Tα1 treatment were significantly better than those who did not in the propensity-matched cohort. Univariable and multivariable Cox regression analyses of OS and RFS in the propensity-matched cohort were shown in Tables 3. On multivariable Cox regression analyses with robust estimator, Tα1 treatment was an independent risk factor associated with both OS (HR: 0.308, 95% CI: 0.175–0.541, P < .001) and RFS (HR: 0.381, 95% CI: 0.229–0.633, P < .001) in the propensity-matched cohort.
Table 3.
Univariable and multivariable Cox regression analyses with robust estimator of overall survival and recurrence-free survival after curative resection of solitary HBV-related hepatocellular carcinoma in the propensity matched cohort.
| Overall survival | Recurrence-free survival | |||||||
| Factors | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | |
| Gender (male/female) | .049 | 0.586 (0.345–0.997) | .400 | .662 | ||||
| Age (>60 vs ≤60, Month) | .058 | .109 | ||||||
| Total Bilirubin, umol/L | .718 | .338 | ||||||
| ALT, IU/L | .940 | .159 | ||||||
| Albumin, g/L | .764 | .771 | ||||||
| ΔNLR (>0 vs ≤0) | .006 | 0.622 (0.445–0.871) | .024 | 0.678 (0.483–0.950) | .022 | 0.700 (0.515–0.951) | .050 | 0.736 (0.541–1.000) |
| Differentiation (Low, Moderate, High) | .001 | 0.420 (0.254–0.694) | .103 | .007 | 0.523 (0.325–0.840) | .002 | 0.467 (0.287–0.761) | |
| MVI (Yes vs No) | <.001 | 3.730 (2.242–6.208) | <.001 | 5.445 (2.842–10.012) | .004 | 2.218 (1.295–3.799) | .018 | 2.043 (1.130–3.692) |
| Tumor Size (<5 vs ≥5, cm) | .237 | .455 | ||||||
| Cirrhosis (Yes vs No) | .059 | .769 | ||||||
| AFP (≤400 vs >400, ng/mL) | <.001 | 2.448 (1.498–4.001) | .012 | 2.036 (1.168–3.548) | <.001 | 2.637 (1.649–4.217) | <.001 | 2.610 (1.575–4.326) |
| HBeAg (Positive vs Negative)) | .389 | .407 | ||||||
| HBV-DNA (≥2 × 103 vs 2 × <103, IU/mL) | .136 | .007 | 0.506 (0.307–0.834) | .208 | ||||
| Antiviral Therapy (NtA vs NsA) | .043 | 0.722 (0.217–0.996) | .045 | 0.859 (0.531–0.907) | .028 | 0.373 (0.307–0.698) | .041 | 0.768 (0.536–0.979) |
| Transfusion (Yes vs No) | .394 | .665 | ||||||
| Complication (Yes vs No) | .343 | .136 | ||||||
| Immunotherapy (none Tα1 vs Tα1) | .018 | 0.542 (0.324–0.908) | <.001 | 0.308 (0.175–0.541) | .006 | 0.517 (0.317–0.842) | <.001 | 0.381 (0.229–0.633) |
3.3. The effects of Tα1 on dynamic NLR change
Before propensity matching, the mean preoperative NLR level in the Tα1 and the control group were 3.26 ± 0.83 and 2.55 ± 0.37 (P = .4). ΔNLR in 1, 3, 6 months have decreased in 83 (34.5%), 72 (30%), and 90 (37.5%) patients in the control group, In the Tα1 group, 173 (75.9%), 180 (79.0%), and 189 (82.9%) patients had decreased ΔNLR level in 1, 3, 6 months (P < .001, P < .001, P < .001, respectively), as shown in Figure 3A. After propensity matching, the mean preoperative NLR level in the Tα1 and control group were 2.54 ± 1.27 and 2.19 ± 0.381 (P = .641). The percent of patients with a decreased ΔNLR in Tα1 group were significantly higher than those in the control group in 1, 3, and 6 months, respectively (65% vs 36%, P < .001; 64% vs 48%, P = .027; 78% vs 52%, P < .001, Fig. 3B).
Figure 3.
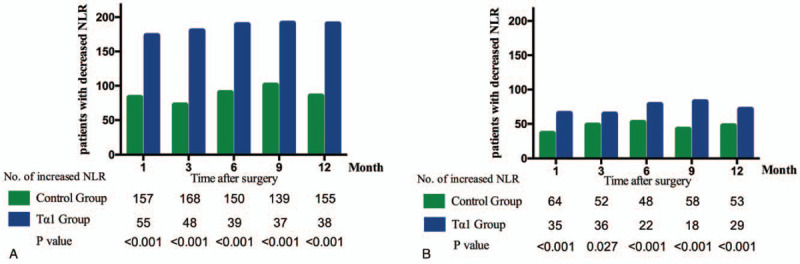
Dynamic NLR changes of patients after radical hepatectomy in 1 year follow-up in the entire cohort (A) and propensity matched cohort (B). NLR = neutrophil to lymphocyte ratio, Tα1 = thymosin alpha-1.
3.4. Virologic response and biochemical response
In the entire cohort, the HBeAg seroconversion and HBeAg loss rate were similar among the HBeAg positive patients in Tα1 and control group at month 3 (2.7% vs 6.4%, P = .453, 6.4% vs 0%, P = .117, respectively) and month 24 (29.2% vs 27.6%, P = .270, 12.0% vs 11.3%, P = .890, respectively, Fig. 4A). As for HBV-DNA level, undetectable HBV-DNA rate was similar in the control group and Tα1 group at 3, 6, and 24 months (62.3% vs 64.1%, P = .662; 69.0% vs 66.9%, P = .629; 96.2% vs 92.6%, P = .127) while HBV-DNA undetectable rate in the Tα1 group was higher than control group in 12 months (72.7% vs 87.4%, P = .001, Fig. 4C). In the propensity-matched cohort, the HBeAg seroconversion and HBeAg loss rate were also similar in Tα1 and control groups at month 3 (5.9% vs 0%, P = .791, 11.7% vs 7.7%, P = .712, respectively) and month 24 (29.2% vs 27.6%, P = .270, 12.0% vs 11.3%, P = .890, respectively, Fig. 4B). Undetectable HBV-DNA rate was similar in the control group and Tα1 group at 3, 6, 12, and 24 months (51% vs 60%, P = .200; 74% vs 69%, P = .434; 89% vs 90%, P = .818; 97% vs 95%, P = .471, Fig. 4D).
Figure 4.
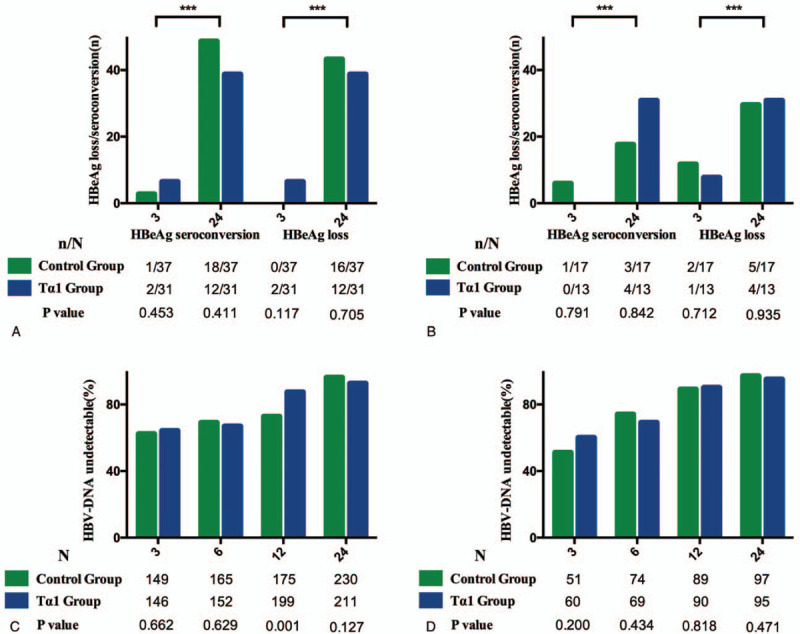
Viral response, HBeAg seroconversion in control and Tα1 group. (A-B) HBeAg loss and HBeAg seroconversion rate in entire and propensity matched cohort; (C-D) HBV-DNA undetectable rate in entire and propensity matched cohort (∗∗∗P < .0001). HbeAg = Hepatitis B surface antigen.
As for biochemical response, there were not significant changes in the liver and renal function during 3-year follow-up between the control and Tα1 group before and after propensity score matching (Supplementary Table S1).
4. Discussion
Despite advance in surgical and multidisciplinary treatment, there is still few effective adjuvant treatment to prevent tumor recurrence after curative resection for HBV-related HCC.[1] The well-known risk factors of HCC recurrence included tumor characteristics, microvascular infiltration, and antiviral therapy.[26–28] Recent studies[29–31] have reported that impaired immunity after resection was considered to contribute to HCC recurrence both soon afterward and in the longer term. The immunotherapeutic strategy based on overcoming barriers within the tumor microenvironment was widely accepted. The results of this study showed that using the peptide Tα1 postoperatively may significantly increase overall and recurrence-free survival as well as immunological function in the patients with solitary HBV-related HCC.
Immunotherapy for HCC showed some potential efficacy, but the evidence was not strong enough.[14,16,32,33] Previous studies had investigated the tumor-inhibiting efficacy of Tα-1 in HCC patients.[14,34] Tα-1 treatment after transarterial chemoembolization (TACE) contributed to prevent the tumor recurrence and benefit the prognosis.[35] This strength maintained till the end of follow-up. A randomized controlled trial had reported that for patients with unresectable HCC, adding thymalfasin after TACE was generally well tolerated and may improve patients’ prognosis.[36] However, they also informed that this study lacked statistical significance for differences in response rates due to the small sample size. In our study, we used ΔNLR to evaluate the immune response of patients, the decline of NLR was associated with the reduction of neutrophils and increment of lymphocytes. In previous studies, NLR was considered as one of the systemic inflammation markers, high postoperative NLR was associated with poor prognosis of HCC, and reduction of postoperative NLR is associated with better prognosis,[11] which was in lines with the present results. Moreover, during the follow-up period, the ΔNLR level significantly decreased in patients treated with Tα1 postoperatively compared with those who did not. The potent mechanism of antitumor effect of Tα1 could be explained as follows. Tα1 could enhance the mitogen-triggered maturation of lymphocytes in the peripheral blood and increase the secretion of various T cell lymphokines.[37–39] Tα1 could also increase the expression of proteins such as MHC class I, MHC class II, β-2 microglobulin and tumor-specific antigens on the surface of tumor cells,[40] which might lead to tumor suppression. All in all, Tα1 could be useful for the significant survival benefits mainly by boosting the immune function of HCC patients following curative resection.
Additionally, the present results also showed that the combination therapy of Tα1 and nucleos(t)ide analog had a similar effect as antiviral monotherapy in terms of virologic response rate during the treatment of HBV-related HCC patients after curative liver resection. The outcomes was inconsistent with some other studies of Tα-1 combination antiviral therapy for CHB patients. A meta-analysis compared the efficacy of Interferon (IFN) and Tα-1 combination therapy with IFN monotherapy for CHB patients, in which 7 randomized controlled trials were included. It showed that combination therapy was remarkably more effective than monotherapy in terms of HBV-DNA suppression, ALT normalization, HBeAg loss, and HBeAg seroconversion.[41] In a recent randomized, open-label, multicenter study, Tα1 (1.6 mg twice a week) combined with entecavir was used in patients with HBV-compensated cirrhosis to evaluate the long-term efficacy of Tα1. The results showed that there was no statistically significant difference between the entecavir treatment and the combination therapy in terms of mortality, HCC incidence, and incidence of complications of cirrhosis, but Tα1 combination therapy tended to be effective in inhibiting the increase of HCC in HBV-related liver cirrhosis patients.[18] Also, the present study showed that patients treated with nucleotide analogs had better overall and recurrence-free survival than those treated with nucleoside analogs. A recent study showed that patients treated with nucleotide analogs (ADV and TDF) additionally had higher serum interferon λ3 levels than those treated with nucleoside analogs (lamivudine and entecavir).[42] The specific mechanism of antiviral effect of Tα1 still needed more studies.
There are several limitations to our study. First, it was a singlecenter, retrospective study though we used the propensity score matching to balance selection bias. In the propensity-matched cohort, the baseline difference between Tα1 and control was comparable. Second, as aggressive tumor characteristics might overshadow the effect of Tα1 treatment, we only studied patients with solitary tumor, clear microscopic resection margin after hepatectomy. Third, we did not analyzes the changes in the subgroup of lymphocytes in detail and we did not stratify recurrence as early or late recurrence because there was no consensus. However, we explored the role of Tα1 in different tumor diameter after hepatectomy. These indices should be included in future studies.
5. Conclusion
In conclusion, our study demonstrated that Tα1 as adjuvant therapy could delay recurrence and prolong overall survival for patients with solitary HBV-related HCC after curative resection. Tα1 therapy might be compatible with a wider range of HCC patients.
Author contributions
Data curation: He Linye, Xia Zijing, Peng Wei, Li Chuan, Tianfu Wen.
Formal analysis: He Linye, Xia Zijing, Peng Wei, He Chao, Li Chuan.
Investigation: He Chao, Tianfu Wen.
Methodology: Peng Wei, He Chao, Tianfu Wen.
Resources: Li Chuan.
Writing – original draft: He Linye, Xia Zijing.
Writing – review & editing: He Linye, Li Chuan, Tianfu Wen.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ADV = adefovir, AFP = alpha fetoprotein, ALT = alanine aminotransferase, CHB = chronic hepatitis B, CREA = serum creatinine level, ETV = entecavir, HBeAg = hepatitis B e Antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, MVI = microvascular invasion, NLR = neutrophil to lymphocyte ratio, RFS = recurrence survival rate, Tα1 = thymosin alpha-1, TACE = transarterial chemoembolization, TDF = tenofovir disoproxil fumarate.
How to cite this article: Linye H, Zijing X, Wei P, Chao H, Chuan L, Tianfu W. Thymosin alpha-1 therapy improves postoperative survival after curative resection for solitary hepatitis B virus-related hepatocellular carcinoma: a propensity score matching analysis. Medicine. 2021;100:20(e25749).
This study was supported by grant from the State Key Scientific and Technological Research Programs (2017ZX10203207-003-0020 and 21ZDYF1542), the Postdoctoral Sustentation Fund of West China Hospital, Sichuan University, China (18HXBH074 and 2020HXBH057), the Science and Technological Supports Project of Sichuan Province (2018SZ0204, 2019YJ0149), the Health and Family Planning Commission of Sichuan Province (17PJ393), the Science and Technology Project of Chengdu (2018-YF05-01460-SN).
The authors have no conflicts of interests to declare.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
ADV = adefovir dipivoxil, AFP = alpha fetoprotein, ALT = alamine aminotransfera, ETV = entecavir, HbeAg = hepatitis B surface antigen, HBV = hepatitis B virus, MVI = Microvascular invasion, PT = prothrombin time, Tα1 = thymosin alpha-1, TDF = tenofovir disoproxil fumarate.
ALT = alamine aminotransfera, AFP = alpha fetoprotein, HbeAg = Hepatitis B surface antigen, HBV = hepatitis B virus, MVI = microvascular invasion, NtA = nucleotide analogue, NsA = nucleoside analogue, ΔNLR = neutrophil to lymphocyte ratio difference, Tα1 = Thymosin alpha-1.
ALT = alamine aminotransfera, AFP = alpha fetoprotein, HbeAg = hepatitis B surface antigen, HBV = hepatitis B virus, MVI = microvascular invasion, NtA = nucleotide analogue, NsA = nucleoside analogue, ΔNLR = neutrophil to lymphocyte ratio difference, Tα1 = thymosin alpha-1.
References
- [1].Aghemo A. Update on HCC management and review of the new EASL guidelines. Gastroenterol Hepatol (N Y) 2018;14:384–6. [PMC free article] [PubMed] [Google Scholar]
- [2].Chinese College of Interventionalists CMDA. Chinese Clinical Practice Guidelines for transarterial chemoembolization of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2019;27:172–81. [DOI] [PubMed] [Google Scholar]
- [3].Zhang DZ, Wei XD, Wang XP. Comparison of hepatic resection and transarterial chemoembolization for solitary hepatocellular carcinoma. World J Gastroenterol 2015;21:4635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Y, Kuang S, Shan Q, et al. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol 2019;29:5791–803. [DOI] [PubMed] [Google Scholar]
- [5].Yan WT, Quan B, Xing H, et al. Time to recurrence, but not recurrence-free survival, should be the endpoint used to predict early recurrence after HCC resection. J Hepatol 2019;70:570–1. [DOI] [PubMed] [Google Scholar]
- [6].Goldaracena N, Mehta N, Scalera I, et al. Multicenter validation of a score to predict prognosis after the development of HCC recurrence following liver transplantation. HPB (Oxford) 2019;21:731–8. [DOI] [PubMed] [Google Scholar]
- [7].Nishibatake Kinoshita M, Minami T, Tateishi R, et al. Impact of direct-acting antivirals on early recurrence of HCV-related HCC: comparison with interferon-based therapy. J Hepatol 2019;70:78–86. [DOI] [PubMed] [Google Scholar]
- [8].Abdel Ghafar MT, Morad MA, El-Zamarany EA, et al. Autologous dendritic cells pulsed with lysate from an allogeneic hepatic cancer cell line as a treatment for patients with advanced hepatocellular carcinoma: a pilot study. Int Immunopharmacol 2020;82:106375. [DOI] [PubMed] [Google Scholar]
- [9].Mandlik DS, Mandlik SK. Herbal and natural dietary products: upcoming therapeutic approach for prevention and treatment of hepatocellular carcinoma. Nutr Cancer 2020;01–25. [DOI] [PubMed] [Google Scholar]
- [10].Ayuso C, Rimola J, Vilana R, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol 2018;101:72–81. [DOI] [PubMed] [Google Scholar]
- [11].Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer 2016;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang D, Bai N, Hu X, et al. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ 2019;7:e7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liang YR, Guo Z, Jiang JH, et al. Thymosin alpha1 therapy subsequent to radical hepatectomy in patients with hepatitis B virus-associated hepatocellular carcinoma: a retrospective controlled study. Oncol Lett 2016;12:3513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Qiu SJ, Zhou ZG, Shen F, et al. A multicenter, randomized, observation-controlled clinical trial to evaluate the efficacy and safety of thymalfasin adjuvant therapy in patients with HBV-related HCC after curative resection - first announcement of the protocol. Expert Opin Biol Ther 2015;15: Suppl 1: S133–137. [DOI] [PubMed] [Google Scholar]
- [15].Garaci E, Pica F, Matteucci C, et al. Historical review on thymosin alpha1 in oncology: preclinical and clinical experiences. Expert Opin Biol Ther 2015;15: Suppl 1: S31–9. [DOI] [PubMed] [Google Scholar]
- [16].Tuthill C, Rios I, McBeath R. Thymosin alpha 1: past clinical experience and future promise. Ann N Y Acad Sci 2010;1194:130–5. [DOI] [PubMed] [Google Scholar]
- [17].Zhang YY, Chen EQ, Yang J, et al. Treatment with lamivudine versus lamivudine and thymosin alpha-1 for e antigen-positive chronic hepatitis B patients: a meta-analysis. Virol J 2009;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu X, Shi Y, Zhou J, et al. Combination of entecavir with thymosin alpha-1 in HBV-related compensated cirrhosis: a prospective multicenter randomized open-label study. Expert Opin Biol Ther 2018;18(sup1):61–9. [DOI] [PubMed] [Google Scholar]
- [19].Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fricker J. European association for the study of the liver: international liver congress 2017 meeting. Lancet Oncol 2017;18:713. [DOI] [PubMed] [Google Scholar]
- [22].Xiao CLCLM. Clinical practice guidelines for liver transplantation of liver cancer in China (2018). Chin J Gen Surg 2019;190–2. [Google Scholar]
- [23].Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329–40. [DOI] [PubMed] [Google Scholar]
- [24].Zhu SL, Ke Y, Peng YC, et al. Comparison of long-term survival of patients with solitary large hepatocellular carcinoma of BCLC stage A after liver resection or transarterial chemoembolization: a propensity score analysis. PLoS One 2014;9:e115834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guo Z, Zhong JH, Jiang JH, et al. Comparison of survival of patients with BCLC stage A hepatocellular carcinoma after hepatic resection or transarterial chemoembolization: a propensity score-based analysis. Ann Surg Oncol 2014;21:3069–76. [DOI] [PubMed] [Google Scholar]
- [26].Fraum TJ, Cannella R, Ludwig DR, et al. Assessment of primary liver carcinomas other than hepatocellular carcinoma (HCC) with LI-RADS v2018: comparison of the LI-RADS target population to patients without LI-RADS-defined HCC risk factors. Eur Radiol 2019;30:996–1007. [DOI] [PubMed] [Google Scholar]
- [27].Rinaldi L, Perrella A, Guarino M, et al. Incidence and risk factors of early HCC occurrence in HCV patients treated with direct acting antivirals: a prospective multicentre study. J Transl Med 2019;17:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ayub A, Ashfaq UA, Haque A. HBV induced HCC: major risk factors from genetic to molecular level. Biomed Res Int 2013;2013:810461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fartoux L, Rosmorduc O. Treatment of the advanced HCC: a second revolution by using immunotherapy. Biol Aujourdhui 2018;212:85–7. [DOI] [PubMed] [Google Scholar]
- [30].Killock D. Immunotherapy: nivolumab keeps HCC in check and opens avenues for checkmate. Nat Rev Clin Oncol 2017;14:392. [DOI] [PubMed] [Google Scholar]
- [31].Buttner N, Schmidt N, Thimme R. Perspectives of immunotherapy in hepatocellular carcinoma (HCC). Z Gastroenterol 2016;54:1334–42. [DOI] [PubMed] [Google Scholar]
- [32].Hong YJ, Kim SH, Choi GH, et al. Long-term outcome after liver resection and clinicopathological features in patients with small hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg 2011;15:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stefanini GF, Foschi FG, Castelli E, et al. Alpha-1-thymosin and transcatheter arterial chemoembolization in hepatocellular carcinoma patients: a preliminary experience. Hepatogastroenterology 1998;45:209–15. [PubMed] [Google Scholar]
- [34].Garaci E, Pica F, Sinibaldi-Vallebona P, et al. Thymosin alpha(1) in combination with cytokines and chemotherapy for the treatment of cancer. Int Immunopharmacol 2003;3:1145–50. [DOI] [PubMed] [Google Scholar]
- [35].Shuqun C, Mengchao W, Han C, et al. Combination transcatheter hepatic arterial chemoembolization with thymosin alpha1 on recurrence prevention of hepatocellular carcinoma. Hepatogastroenterology 2004;51:1445–7. [PubMed] [Google Scholar]
- [36].Gish RG, Gordon SC, Nelson D, et al. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatol Int 2009;3:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Torabi Goudarzi S, Hajivalili M, Hosseini M, et al. Tetanus neurotoxin HCC protein commits T cells to IFN-gamma producing cells. Cell Mol Biol (Noisy-le-grand) 2016;62:20–4. [PubMed] [Google Scholar]
- [38].Teixeira AC, Mendes CT, Jr, Marano LA, et al. Alleles and genotypes of polymorphisms of IL-18, TNF-alpha and IFN-gamma are associated with a higher risk and severity of hepatocellular carcinoma (HCC) in Brazil. Hum Immunol 2013;74:1024–9. [DOI] [PubMed] [Google Scholar]
- [39].Vickers SM, Jhala NC, Ahn EY, et al. Tamoxifen (TMX)/Fas induced growth inhibition of human cholangiocarcinoma (HCC) by gamma interferon (IFN-gamma). Ann Surg 2002;235:872–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Garaci E, Pica F, Serafino A, et al. Thymosin alpha1 and cancer: action on immune effector and tumor target cells. Ann N Y Acad Sci 2012;1269:26–33. [DOI] [PubMed] [Google Scholar]
- [41].Mao HY, Shi TD. Treatment with interferon and thymosin alpha-1 versus interferon monotherapy for HBeAg positive chronic hepatitis B: a meta-analysis. Zhonghua Gan Zang Bing Za Zhi 2011;19:29–33. [DOI] [PubMed] [Google Scholar]
- [42].Murata K, Asano M, Matsumoto A, et al. Induction of IFN-lambda3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut 2018;67:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


