Abstract
Objective
Due to high rates of obesity and alcohol consumption, the prevalence of fatty liver disease is increasing. There is no widely adopted approach to proactively screen for liver disease in the community. We aimed to assess the burden of potentially undiagnosed liver disease in individuals attending for colonoscopy to develop a pathway to identify and manage individuals with undiagnosed liver disease.
Design
The OSCAR Study was a cross-sectional study recruiting patients attending for colonoscopy. Patients’ metabolic and liver risk factors were measured. The prevalence of undiagnosed significant fatty liver disease was measured using the Fatty Liver Index (FLI) and Fibrosis-4 score (FIB-4).
Results
1429 patients (mean age 59±14 years; 48.8% men) were recruited. 73.3% were overweight/obese, 12.7% had diabetes and 17.9% had metabolic syndrome. 19% were consuming more than recommenced alcohol levels (<14 units/week) and 41% had an AUDIT-C score ≥5. After excluding those with known liver disease, 43.2% of the cohort had a high FLI (high likelihood of fatty liver). 5.3% of these had a high FIB-4 score (>2.67, high probability of advanced fibrosis) and 90% of these were previously undiagnosed. 818 patients had a predicted 10-year cardiovascular event risk of ≥10%, however only 377 (46.1%) were on statin therapy.
Conclusion
High levels of obesity, metabolic dysfunction and undiagnosed fatty liver disease were found in individuals attending for colonoscopy. Clinical encounters in the endoscopy unit may represent an opportunity to risk assess for liver and metabolic disease and provide an environment to develop targeted interventions.
Keywords: endoscopy, chronic liver disease, screening
Summary box.
What is already known about this subject?
Liver disease is commonly diagnosed when advanced fibrosis/cirrhosis has developed. Though liver disease risk factors are identifiable, there is no established ‘screening’ for patients for liver disease in primary or secondary care.
What are the new findings?
This study reports high levels of obesity, diabetes and metabolic risk factors among a population attending colonoscopy. More importantly, by using non-invasive markers, the potential of undiagnosed liver disease is high.
How might it impact on clinical practice in the foreseeable future?
The endoscopy unit seems a suitable setting to screen patients for liver disease. Further research needs to be undertaken to validate the proposed approach for its yield and clinical utility in screening patients at risk of liver disease.
Introduction
Chronic liver disease (CLD) is common with non-alcoholic fatty liver disease (NAFLD) and alcohol-related liver disease (ARLD) now the two major causes of CLD globally.1 2 Rates of liver disease are increasing in many countries due to rising obesity rates and high prevalence of harmful alcohol consumption. Moreover, deaths from CLD have increased in many countries and it is now one of the main causes of premature death.3 4 However, despite the high prevalence, the majority of affected individuals are undiagnosed and thus opportunities to intervene early and alter the natural history of the disease may be missed.
Liver disease is usually asymptomatic at early stages and patients frequently present once advanced liver disease has developed. A previous study showed that a staggering 73% of patients presenting with their first admission with cirrhosis or liver failure had never been referred to a liver clinic, indicating a clear lack of early detection of liver disease.5 Unfortunately, despite the main risk factors for liver disease being easily identifiable (obesity, alcohol), there is no widely used approach to proactively look for liver disease in the community. Strong evidence shows that early identification and a structured brief intervention in harmful drinkers are both successful and cost-effective in primary care6 with up to 65% of patients with early liver disease stopping drinking at harmful or dependent levels simply as a result of being informed of the diagnosis.7 Lifestyle change including weight loss, improved diet quality and exercise can reduce hepatic inflammation and fibrosis in patients with NAFLD.8 Therefore, there is a need to develop programmes to identify individuals with significant liver disease at an asymptomatic stage to introduce lifestyle changes to prevent disease progression to cirrhosis.
Endoscopy units could offer a viable place to identify individuals at risk of liver disease and then deliver appropriate interventions because patients typically spend a few hours in the department. We therefore aimed to measure the burden of potentially undiagnosed liver disease in individuals attending for colonoscopy to inform the development of pathways to identify and manage individuals with undiagnosed liver disease attending endoscopy units.
The specific aims of the study were to: (1) determine the prevalence of individuals with risk factors for liver disease (obesity, metabolic syndrome and hazardous alcohol consumption); (2) determine the prevalence of known liver disease; (3) determine the prevalence of potentially undiagnosed fatty liver disease using non-invasive markers.
Methods
Study design
The Obesity related Colorectal Adenoma Risk Study (OSCAR) was a prospective cross-sectional study recruiting patients who were referred for colonoscopy across 12 UK centres to assess the relationship between colonic neoplasia and fatty liver disease.
Patients
Eligible patients were aged 18 years and above, and were undergoing colonoscopy as part of the Bowel Cancer Screening Programme (BCSP) or due to standard care for colonic symptoms. Written informed consent was obtained from each patient.
Clinical and demographic data
Clinical and demographic data were collected at the time of enrolment including age, gender, ethnicity, alcohol consumption, smoking habits, medical history and medication history. Patients’ height, weight and waist circumference were measured. Waist circumference was measured horizontally from the narrowest point between the lowest rib and the iliac crest or the midpoint between these by research nurses who have received training by the study team. Blood tests were taken following a pre-colonoscopy fast including full blood count, liver blood tests (alkaline phosphatase, alanine transferase (ALT), albumin, aspartate aminotransferase (AST), gamma glutamyltransferase (GGT)), lipid profile, haemoglobin A1c (HbA1c) (pre-diabetes: 43–48 mmol/mol, diabetes: ≥48 mmol/mol), fasting glucose and IgA. Normal laboratory values for each site were recorded (online supplemental table 1: Upper limit of normal for liver enzymes).
bmjgast-2021-000638supp001.pdf (39.5KB, pdf)
For alcohol consumption history, patients completed the AUDIT-C questionnaire, an alcohol screening questionnaire where a score of ≥5 indicates potential hazardous or harmful intake.9 Current consumption of >14 units per week for both men and women was considered potentially harmful in accordance with the UK 2016 guidelines.10 Previous excessive alcohol consumption was defined by historical criteria as those who regularly drank in excess of >21 units for men and >14 units for women weekly for more than 1 year.
Known diagnosis of hypertension or type 2 diabetes was ascertained by asking patients, checking records and reviewing prescribed medication. Metabolic syndrome was defined using the harmonised criteria, whereby patients were considered to have metabolic syndrome if they had three of five of the following factors: waist circumference ≥94 cm (men) or 80 cm (women); impaired fasting glucose >5.4 mmol/L or on treatment; systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or on treatment; triglyceride ≥1.7 mmol/L or on treatment; high-density lipoprotein (HDL)-cholesterol <1.0 mmol/L (men) or <1.3 mmol/L (women) or on treatment.11
The Fatty Liver Index (FLI)12 and the Fibrosis-4 (FIB-4) score13 were calculated from the information gathered above and used to determine whether individuals had fatty liver or advanced fibrosis, respectively. FLI was selected over other scores such as the Hepatic Steatosis Index (HSI)14 as this was developed and validated among European individuals, whereas the HSI was developed in Asian individuals who typically have a different metabolic phenotype. We also report the prevalence of fatty liver disease using these scores. An FLI ≥60 was considered to indicate the presence of steatosis, whereas a score <30 was considered to exclude steatosis, with scores 30–59 considered indeterminate.12 The FIB-4 score was used to non-invasively stage liver fibrosis. FIB-4 score was chosen over other fibrosis staging scores such as NAFLD fibrosis score (NFS)15 or APRI score16 as the NFS is only validated among patients with NAFLD (where the study population in this study is not limited to patients with NAFLD), and that the APRI score underperforms compared with the FIB-4 score.17 Age-adjusted FIB-4 score was used to reduce false positive rates of advanced fibrosis among patients aged >65 years, a FIB-4 score <1.3 for those ≤65 years or <2.0 for those >65 years was used to exclude advanced fibrosis, while a score >2.67 was used to indicate the presence of advanced fibrosis irrespective of age.13 Scores between these values were considered indeterminate. QRISK2,18 a validated online tool, was used to estimate an individual’s predicted 10-year cardiovascular (CV) event risk. A CV event risk ≥10% is a trigger for formal assessment of individual CV risk factors, lifestyle modification and consideration of lipid-lowering therapy in the UK.19
To develop a potential algorithm to identify patients with significant fatty liver disease using a risk-based approach, patients were retrospectively categorised into three groups based on their number of risk factors for fatty liver disease including potentially harmful alcohol intake (AUDIT-C ≥5), and type 2 diabetes or obesity (body mass index ≥30). These factors were selected because they are the major risk factors for fatty liver disease and they independently indicate an increased risk of liver disease20 as well as an incremental risk of liver fibrosis with greater number of metabolic risk factors.21 Group 1 had no risk factors, group 2 had one risk factor and group 3 had two or more risk factors. The prevalence of fatty liver disease and liver fibrosis was assessed within each group using FLI and FIB-4 score.
Data analysis
Continuous variables were reported as mean±SD, and categorical variables displayed as number and percentage (N, %). Univariate analysis was performed using Χ2 test for categorical variables and the unpaired t-test for continuous variables to assess the significance of the difference of frequency and means between each assessed group. Pearson’s correlation was used to assess the relationship between the FIB-4 score and NFS.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Clinical characteristics of the cohort
A total of 1429 patients were recruited from December 2017 to July 2019. Of these, 387 (27.1%) patients attended colonoscopy as part of BCSP and 1042 (72.9%) attended colonoscopy through standard symptomatic services. In total, 698 (48.9%) were men and mean age was 59±14 (range 18–87) years. The overwhelming majority (98.1%) of participants were white or white British. Detailed patient characteristics are described in table 1.
Table 1.
Study patient characteristics
| Variable | Study data | N |
| Sex | ||
| Male | 698 (48.9%) | 1429 |
| Age* | 59±14 (18–87) | 1429 |
| Age group | 1429 | |
| <40 | 170 (11.9%) | |
| 41–60 | 500 (35.0%) | |
| 61–80 | 733 (51.3%) | |
| ≥81 | 26 (1.8%) | |
| Ethnicity | 1429 | |
| White or white British | 1402 (98.1%) | |
| Other† | 27 (1.9%) | |
| Body weight (kg)* | 1425 | |
| All | 82.4±19.1 (38.1–203) | |
| Male | 89.0±18.4 (53.1–203) | |
| Female | 76.1±17.7 (38.1–171) | |
| BMI (kg/m2))* | 28.7±6.0 (15.1–61.5) | 1424 |
| BMI categories | 1424 | |
| Underweight (<18.5) | 18 (1.3%) | |
| Normal (18.5–25) | 362 (25.4%) | |
| Overweight (25–30) | 557 (39.1%) | |
| Obese class I (30–35) | 299 (21.0%) | |
| Obese class II (35–40) | 115 (8.1%) | |
| Obese class III (>40) | 73 (5.1%) | |
| Waist circumference (cm)* | 1413 | |
| All | 98.1±15.5 (29–200) | |
| Male | 102.3±13.8 (69–200) | |
| Female | 94.1±16.0 (29–148) | |
| Smoking status | 1428 | |
| Non-smoker | 648 (45.4%) | |
| Ex-smoker | 594 (41.6%) | |
| Current smoker | 186 (13.0%) | |
| Alcohol intake | 1400 | |
| Non-drinker | 298 (21.3%) | |
| Drinks ≤14 units | 830 (59.3%) | |
| Drinks >14 units | 272 (19.4%) | |
| Previous alcohol excess | 617 (43.5%) | 1419 |
| AUDIT-C score | 1421 | |
| Low risk (0–4) | 828 (58.3%) | |
| High risk (5–12) | 593 (41.7%) | |
| Hypertension‡ | 480 (33.6%) | 1429 |
| Type 2 diabetes‡ | 182 (12.7%) | 1428 |
| Metabolic syndrome | 595 (45.1%) | 1320 |
| Statin | 454 (31.8%) | 1429 |
| HbA1c (mmol/mol)* | 39.8±10.4 (21–112) | 1287 |
| Elevated triglyceride >1.7 mmol/L | 262 (20.2%) | 1297 |
| Reduced HDL-cholesterol (male <1 mmol/L, female <1.3 mmol/L) | 251 (19.3%) | 1302 |
| Elevated cholesterol >5 mmol/L | 600 (46.1%) | 1302 |
| Elevated ALT >1× ULN | 152 (11.8%) | 1291 |
| Elevated AST >1× ULN | 104 (8.7%) | 1196 |
| Elevated ALP >1× ULN | 59 (4.5%) | 1321 |
| Elevated GGT >1× ULN | 168 (13.1%) | 1284 |
| FLI | 1245 | |
| Low (0–30) | 393 (31.6%) | |
| Indeterminate (30–60) | 299 (24.0%) | |
| High (≥60) | 553 (44.4%) | |
| Age-adjusted FIB-4 | 1173 | |
| Low | 849 (72.4%) | |
| Intermediate | 267 (22.8%) | |
| High | 57 (4.9%) | |
| QRISK2 ≥10% | 818 (62.3%) | 1313 |
*Mean±SD (range).
†Asian (India, Pakistan, Bangladesh), Chinese, black/African/Caribbean, mixed ethnic groups.
‡Previous diagnosis.
ALP, alkaline phosphatase; ALT, alanine transferase; AST, aspartate aminotransferase; BMI, body mass index; FIB-4, Fibrosis-4; FLI, Fatty Liver Index; GGT, gamma glutamyltransferase; HbA1c, haemoglobin A1c; HDL, high-density lipoprotein; ULN, upper limit of normal.
High prevalence of metabolic and liver risk factors in the cohort
Overall, 1044 (73.3%) of patients were overweight (39.1%) or obese (34.2%). Two hundred and seventy-two (19.4%) patients reported drinking >14 units of alcohol per week, while 593 (41.7%) scored ≥5 in the AUDIT-C questionnaire. One hundred and eighty-two (12.7%) individuals were previously diagnosed with type 2 diabetes, while among those with no prior diagnosis of diabetes in whom HbA1c was available (n=1107), 81 (7.3%) had an elevated HbA1c in keeping with pre-diabetes and 22 (2.0%) had a level in keeping with diabetes. Among 818 patients with a predicted 10-year CV event risk of ≥10%, only 377 (46.1%) patients were on statin therapy.
High prevalence of probable fatty liver disease in the cohort
A total of 71 (0.05%) patients had been previously diagnosed with liver disease (33 NAFLD, 5 ARLD, 4 fatty liver disease of mixed aetiologies, 16 viral hepatitis and 13 with other liver diseases).
A significant proportion of the cohort had raised liver enzymes with 12% having a raised ALT, 9% a raised AST and 13% a raised GGT. Of those with abnormal liver enzymes, depending on which enzyme was measured, 89%–92% had no prior diagnosis of liver disease. Individuals with a raised serum ALT (>1× upper limit of normal) were younger, more commonly men, obese, drank alcohol to excess, and had a greater proportion of patients at high risk of hepatic steatosis or advanced fibrosis than those with normal range ALT levels. There was no significant difference in the proportion of patients with hypertension, type 2 diabetes or metabolic syndrome between those with normal and raised ALT (online supplemental table 2).
bmjgast-2021-000638supp002.pdf (66.8KB, pdf)
Five hundred fifty-three (44.4%) patients had a high-risk FLI (FLI ≥60) indicating a high likelihood of hepatic steatosis. These individuals were more often men, obese, drank alcohol in excess, and had hypertension, diabetes or a previous diagnosis of fatty liver disease. They had a significantly higher ALT, GGT, IgA and triglyceride levels, lower HDL-cholesterol levels and had a greater proportion of those with an elevated QRISK2 score compared with those with a low or intermediate risk FLI (table 2). Nonetheless, 80% of patients with high likelihood of hepatic steatosis have a normal ALT.
Table 2.
Low, intermediate, high risk FLI
| Low risk | Intermediate risk | High risk (FLI ≥60) (N=553) | P value* | |
| (FLI ≤30) | (FLI 30–60) | |||
| (N=393) | (N=299) | |||
| Age | 57±16 | 60±14 | 60±12 | <0.01 |
| Gender | <0.01 | |||
| Male | 141 (35.9%) | 159 (53.4%) | 313 (56.6%) | |
| Female | 252 (64.1%) | 140 (46.8%) | 240 (43.4%) | |
| Obesity | <0.01 | |||
| BMI <30 | 387 (98.5%) | 256 (85.6%) | 182 (32.9%) | |
| BMI ≥30 | 6 (1.5%) | 43 (14.4%) | 371 (67.1%) | |
| WC (cm) | 83.1±8.9 | 95.6±6.8 | 109.8±12.3 | <0.01 |
| Alcohol | <0.01 | |||
| Non-drinker | 65 (16.9%) | 56 (19.2%) | 143 (26.2%) | |
| Within limits | 287 (74.7%) | 211 (72.3%) | 313 (57.3%) | |
| Above limits | 32 (8.3%) | 25 (8.6%) | 90 (16.5%) | |
| Hypertension | 81 (20.6%) | 93 (31.1%) | 247 (44.7%) | <0.01 |
| Type 2 diabetes | 19 (4.8%) | 27 (9.1%) | 112 (20.3%) | <0.01 |
| Prior diagnosis of fatty liver disease (NAFLD, fatty liver disease on imaging, alcohol-related fatty liver disease) | 3 (0.8%) | 4 (1.34%) | 30 (5.43%) | <0.01 |
| Statin | 84 (21.4%) | 96 (32.1%) | 214 (38.7%) | <0.01 |
| ALT | 21.8±14.9 | 24.0±11.1 | 32.8±22.2 | <0.01 |
| Elevated ALT >1× ULN | 18 (4.7%) | 16 (5.4%) | 108 (20.0%) | <0.01 |
| AST | 25.4±11.6 | 25.8±7.9 | 30.3±17.3 | 0.07 |
| Elevated AST >1× ULN | 18 (4.9%) | 10 (3.6%) | 72 (14.0%) | <0.01 |
| GGT | 21.8±14.0 | 31.5±21.5 | 75.9±177.1 | <0.01 |
| IgA | 2.3±1.0 | 2.5±1.2 | 2.7±1.3 | <0.01 |
| Triglyceride | 0.8±0.3 | 1.1±0.4 | 1.9±1.8 | <0.01 |
| HDL-cholesterol | 1.7±0.4 | 1.5±0.4 | 1.3±0.4 | <0.01 |
| HbA1c | 37.0±7.0 | 38.8±10.3 | 42.6±12.1 | <0.01 |
| Fasting glucose | 4.8±1.1 | 5.2±2.1 | 5.9±2.0 | <0.01 |
| Age-adjusted FIB-4 risk | 0.64 | |||
| Low | 272 (74.9%) | 194 (71.6%) | 361 (70.8%) | |
| Intermediate | 77 (21.2%) | 65 (24.0%) | 121 (23.7%) | |
| High | 14 (3.9%) | 12 (4.4%) | 28 (5.5%) | |
| Elevated QRISK | 178 (46.8%) | 184 (62.4%) | 398 (72.6%) | <0.01 |
*From Χ2 test/one-way ANOVA across the three groups.
ALT, alanine transferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; BMI, body mass index; FIB-4, Fibrosis-4; FLI, Fatty Liver Index; GGT, gamma glutamyltransferase; HbA1c, haemoglobin A1c; HDL, high-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; ULN, upper limit of normal; WC, waist circumference.
Using FLI, 553 (44.4%) patients were at high risk of fatty liver disease compared with 682 (57.9%) patients using the HSI.
Applying fibrosis staging scores across the study cohort irrespective of liver disease aetiology or alcohol consumption, using FIB-4 score, 57 (4.9%) patients were at high risk of advanced fibrosis (FIB-4 >2.67), compared with 46 (3.9%) for NFS (>0.676). The correlation between FIB-4 score and NFS was good at r=0.71 (p<0.005).
Of the individuals with an available FLI and FIB-4 score with no prior diagnosis of liver disease (n=1088), 470 (43.2%) had a high FLI, with 25 (5.3%) having a high FIB-4 indicating a high likelihood of advanced liver fibrosis/cirrhosis. An additional 102 (21.7%) had an indeterminate age-adjusted FIB-4 score. After exclusion of individuals who consumed excess alcohol, there were 350 (39.7%) patients with potentially undiagnosed NAFLD.
Algorithm to identify significant liver disease at colonoscopy appointment
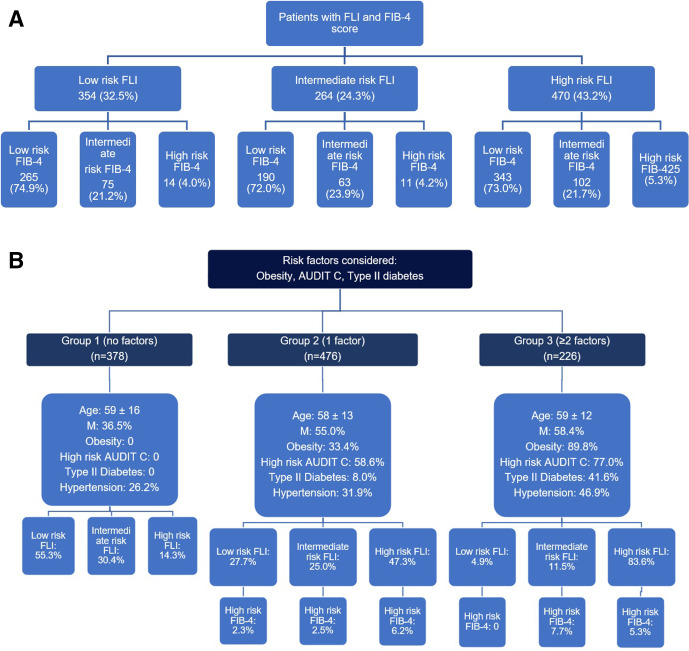
Figure 1A shows the categorisation of individuals with suspected fatty liver and advanced fibrosis if an algorithm using FLI and FIB-4 was used for all patients attending for colonoscopy. However, applying this to all patients attending for colonoscopy would be resource-intensive. Given that major risk factors for advanced fatty liver disease are obesity, type 2 diabetes and harmful alcohol consumption,1 2 a more focused approach while being resource-efficient was undertaken where these scores were applied to patients with one or more risk factors (figure 1B). Using this algorithm, there is a markedly higher proportion of patients with suspected hepatic steatosis (high FLI) in those with ≥2 risk factor than those with 1 risk factor or no risk factors (83.6% vs 47.3% vs 14.3%, respectively, p<0.01). Among patients with a high FLI with 1 or ≥2 risk factors, 6.2% and 5.3% of individuals, respectively, had a high-risk FIB-4 score suggesting they have fatty liver with associated advanced liver fibrosis/cirrhosis.
Figure 1.
(A) Patients by FLI and FIB-4 score risk groups. (B) Categorisation of advanced liver disease screening based on risk factors. FIB-4, Fibrosis-4; FLI, Fatty Liver Index.
Discussion
This study demonstrates an alarming picture of obesity and undiagnosed liver disease (some advanced) among a population attending for colonoscopy. In our cohort, liver disease risk factors were highly prevalent with 73% being overweight or obese, 15% having type 2 diabetes (13% known and 2% new diagnosis), while 19% reported currently drinking alcohol above recommended alcohol limits and 42% of patients had a positive AUDIT-C score (indicating potentially harmful levels of alcohol consumption). The prevalence of undiagnosed probable fatty liver disease was very high with 44% of the cohort having a high FLI indicating a high likelihood of hepatic steatosis. Worryingly, 5.3% of these individuals also had a high FIB-4 score indicating the presence of advanced fibrosis/cirrhosis. Despite the high rate of suspected liver disease in this study, less than 1% of the cohort had a formal diagnosis of liver disease. Our findings were in keeping with one study from primary care that found 42.9% of the adult population to be diagnosed with NAFLD.22 This suggests a failure of systems to identify patients with liver disease at an early stage in the community.
As well as having high rates of undiagnosed liver disease and metabolic disease, the cohort had a high prevalence of individuals with high CV risk. Overall, 818 (62.3%) of individuals with a QRISK2 score had a predicted 10-year risk of a major CV event of >10% by QRISK2, and only 377 (46%) of these individuals were treated with a statin. We did not assess why patients were not on a statin, but this low rate of statin prescribing in those at high CV risk is in keeping with those from previous studies.23 This suggests a clear need to improve CV risk assessment rates and subsequent initiation of statins in the community when there is overwhelming benefit of reduction in all-cause mortality.
Early diagnosis of CLD prior to onset of cirrhosis is vital because nearly all liver-related morbidity and mortality occurs due to complications of cirrhosis. Moreover, cirrhosis is associated with a significant reduction in quality of life and an increase in healthcare costs.24 25 There are currently no widely adopted programmes in primary care in the UK or other countries that aim to detect liver disease at an early stage but there is an increasing body supporting screening for liver disease in primary care. There are examples of good practice such as a pathway evaluated in Nottingham, UK where patients’ risk is assessed in primary care with specific criteria including harmful alcohol use, AST/ALT ratio ≥0.8 or an FLI ≥60 leads to a referral for transient elastography to formally stage their liver fibrosis, and this has increased the detection of significant liver disease, but this remains a local service and has not been adopted nationally.26
More than 2.5 million endoscopies are undertaken in the UK each year, including over 900 000 colonoscopies.27 During a patient’s endoscopy appointment, there is usually a waiting period before and after the procedure. This time is rarely used for healthcare promotion, but there is significant opportunity to do this. Given the high rates of undiagnosed liver disease and pre-existing metabolic disease among patients attending for colonoscopy, endoscopy appointments may offer an opportunity to actively ‘screen’ patients for liver and metabolic disease and deliver brief lifestyle interventions. Our findings indicate that use of the FLI and FIB-4, two inexpensive widely available biomarkers of fatty liver disease and liver fibrosis, may be a viable approach to identify individuals with probable fatty liver and advanced fibrosis. However, this would be relatively resource-intensive if all individuals attending were screened and there would be the likelihood of high false positive rates. The majority of individuals with significant fatty liver disease have recognisable liver risk factors (obesity, type 2 diabetes or harmful alcohol consumption). These three factors have been previously shown to confer the highest risk for cirrhosis and liver-related complications.20 28 In patients with NAFLD, the presence of type 2 diabetes is associated with the greatest risk of the development of advanced fibrosis.29 Moreover, the impact of coexistent obesity and excess alcohol consumption on liver-related mortality is additive (risk ratio 1.29 for obesity alone; 3.66 for alcohol alone; and 9.53 for the combination).28 Therefore, a more focused algorithm that assesses the FLI and FIB-4 in individuals with one or more of these three risks may be more effective. In our study, this method identified a high proportion of individuals with a high FLI. However, prospective evaluation of both algorithms using other confirmatory tests for fatty liver and fibrosis is warranted to confirm clinical utility.
As well as being used to identify liver, metabolic and CV risk factors, endoscopy attendances could be used to deliver brief interventions and lifestyle advice to those identified at increased risk. A prior study showed that it was feasible to conduct an alcohol assessment using AUDIT questionnaire and deliver brief interventions to at-risk individuals by endoscopy nurses who had received specific training.30 Lifestyle advice can also be given to those with obesity in the endoscopy setting. One study showed that a 12-month programme with a lifestyle councillor initiated for those with overweight/obesity within a national bowel cancer screening programme led to significantly greater weight loss compared with provision of a weight loss booklet only.31
The current study has limitations. First, this was a population of patients attending for colonoscopy conducted predominantly in an area with high rates of obesity and harmful alcohol consumption and as such may not be representative of other areas or the general population. However, the ratio of individuals with previously diagnosed liver disease to those we identified with probable fatty liver disease was striking and it is likely that undiagnosed significant liver disease is highly prevalent in other areas as well. Second, we used simple non-invasive blood markers to indicate probable fatty liver disease and stage liver fibrosis rather than using more accurate techniques such as transient elastography, MRI or liver biopsy and as such may not give a definitive estimate of rates of significant liver disease. However, FLI has been externally validated in multiple studies for assessment of fatty liver disease and is reasonably accurate in identifying patients with fatty liver disease.32 Conversely, though FIB-4 score has not been validated in patients who consume excessive alcohol or as a ‘case finding’ setting, it has been well validated in patients with NAFLD13 17 with low and high scores excluding or diagnosing advanced fibrosis, respectively, with reasonable accuracy. The role of FIB-4 as a ‘screening tool’ needs to be further evaluated, with some studies suggesting a role for assessment among patients with diabetes33 but others suggest that a normal FIB-4 score does not exclude advanced fibrosis when compared with transient elastography in a general population ‘screening’ setting.34 Certainly, one study supports screening for liver fibrosis among patients with type 2 diabetes by transient elastography.35 Even though these tests may not provide a definitive estimate of prevalence rates of fatty liver and advanced fibrosis/cirrhosis, the strikingly high rates of probable undiagnosed liver disease are likely to be real. Third, alcohol history is often unreliable and under-reporting of excess alcohol consumption is common. However, the AUDIT-C screening questionnaire is more objective and validated, and within our study, over 40% of patients had a positive screen indicating a higher rate of potentially harmful alcohol consumption than was seen from the reported weekly alcohol consumption. Fourth, the medical history ascertained from the patients was, in many cases, self-reported so may not be entirely accurate with regard to history of diabetes, liver disease and other medical conditions.
Conclusion
Undiagnosed liver disease, obesity, metabolic dysfunction and potentially harmful alcohol consumption were highly prevalent among individuals attending for colonoscopy with many individuals at risk of significant morbidity. Given the rising rates of liver-related mortality and the lack of a widely adopted programme to identify liver disease in the community, development of a liver disease risk assessment and a targeted intervention programme in endoscopy may be a viable option to address this problem. Prospective evaluation and a targeted intervention programme are being developed.
Acknowledgments
We would like to thank the support from the OSCAR Study sites in recruitment of this study.
Footnotes
Twitter: @LauraJNeilson, @stumcp
Contributors: CJR, SM and MH were involved in the conception and design of the study. SK performed the research, collected the data, conducted initial analysis and drafted the manuscript. SK, SM and CJR analysed the data. LS, SR, LJN, MH, CJR and SM revised the manuscript. CJR is the guarantor of this article.
Funding: The research was funded by an unrestricted educational research grant from Norgine medical.
Competing interests: CJR has received grant funding from ARC medical, Norgine and Olympus medical. He was an expert witness for ARC medical.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The OSCAR study team, A Verma, R Bevan, Clair Gabriel, J Greenaway, M Hendrickse, S Hellier, Robert Hart, Amit Chattree, and Salil Singh
Data availability statement
No data are available. There is no data repository available for the dataset from this study.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval was received from the Research Ethics Committee (REC) of London Surrey Borders (REC reference: 17/LO/1746).
References
- 1.Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–LP - 568. 10.1136/gutjnl-2019-318813 [DOI] [PubMed] [Google Scholar]
- 2.Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718–35. 10.1016/j.jhep.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 2020;5:245–66. 10.1016/S2468-1253(19)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 2018;362:k2817. 10.1136/bmj.k2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazeldine S, Hydes T, Sheron N. Alcoholic liver disease - the extent of the problem and what you can do about it. Clin Med 2015;15:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gornall J. Alcohol and Public Health . Under the influence. BMJ 2014;348:f7646. [DOI] [PubMed] [Google Scholar]
- 7.Kaner EFS, Beyer F, Dickinson HO. Effectiveness of brief alcohol interventions in primary care populations. Cochrane database Syst Rev 2007;2:CD004148. [DOI] [PubMed] [Google Scholar]
- 8.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Wong W-S, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a Multi-National cohort study. Gastroenterology 2018;155:443–57. 10.1053/j.gastro.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 9.Public Health England . Alcohol use screening tests, Published 2017. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/684826/Alcohol_use_disorders_identification_test_for_consumption__AUDIT_C_.pdf [Accessed March 4, 2020].
- 10.Department of Health . UK Chief Medical Officers’ Alcohol Guidelines Review: Summary of the Proposed New Guidelines. [Google Scholar]
- 11.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes Federation Task force on epidemiology and prevention; National heart, lung, and blood Institute; American heart association; world heart Federation; international atherosclerosis Society; and international association for the study of obesity. Circulation 2009;120:1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 12.Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. 10.1186/1471-230X-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson S, Hardy T, Dufour J-F, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740–51. 10.1038/ajg.2016.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J-H, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503–8. 10.1016/j.dld.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 15.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 16.Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 17.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. 10.1136/gut.2010.216077 [DOI] [PubMed] [Google Scholar]
- 18.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475–82. 10.1136/bmj.39609.449676.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Insitute of Health and Care Excellence (NICE) . Cardiovascular disease: risk assessment and reduction, including lipid modification Clinical guideline [CG181], Published 2016. Available: https://www.nice.org.uk/guidance/cg181 [Accessed May 29, 2020].
- 20.Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med 2020;17:e1003100. 10.1371/journal.pmed.1003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong VW-S, Chu WC-W, Wong GL-H, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012;61:409–15. 10.1136/gutjnl-2011-300342 [DOI] [PubMed] [Google Scholar]
- 22.Alazawi W, Mathur R, Abeysekera K, et al. Ethnicity and the diagnosis gap in liver disease: a population-based study. Br J Gen Pract 2014;64:e694 LP–702. 10.3399/bjgp14X682273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson S, Gosrani S, Hogg S, et al. Increased cardiovascular risk and reduced quality of life are highly prevalent among individuals with hepatitis C. BMJ Open Gastroenterol 2020;7. 10.1136/bmjgast-2020-000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng J-K, Hepgul N, Higginson IJ, et al. Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat Med 2019;33:24–36. 10.1177/0269216318807051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petta S, Ting J, Saragoni S, et al. Healthcare resource utilization and costs of nonalcoholic steatohepatitis patients with advanced liver disease in Italy. Nutr Metab Cardiovasc Dis 2020;30:1014–22. 10.1016/j.numecd.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 26.Chalmers J, Wilkes E, Harris R. Development and implementation of a commissioned pathway for the identification and stratification of liver disease in the community. Frontline Gastroenterol 2020;11:86 LP–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenbagaraj L, Thomas-Gibson S, Stebbing J, et al. Endoscopy in 2017: a national survey of practice in the UK. Frontline Gastroenterol 2019;10:7–15. 10.1136/flgastro-2018-100970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. 10.1136/bmj.c1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. 10.1016/j.jhep.2014.11.034 [DOI] [PubMed] [Google Scholar]
- 30.O'Neill G, Masson S, Bewick L, et al. Can a theoretical framework help to embed alcohol screening and brief interventions in an endoscopy day-unit? Frontline Gastroenterol 2016;7:47–53. 10.1136/flgastro-2014-100519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson AS, Craigie AM, Caswell S, et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ 2014;348:g1823. 10.1136/bmj.g1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koehler EM, Schouten JNL, Hansen BE, et al. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol 2013;11:1201–4. 10.1016/j.cgh.2012.12.031 [DOI] [PubMed] [Google Scholar]
- 33.Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021;44:399–406. 10.2337/dc20-1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graupera I, Serra-Burriel M, Thiele M. Value of FIB-4 and NAFLD fibrosis scores for screening of liver fibrosis in the general population. J Hepatol 2020;73:S414–5. [DOI] [PubMed] [Google Scholar]
- 35.Ciardullo S, Monti T, Perseghin G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 2021;44:519–25. 10.2337/dc20-1778 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2021-000638supp001.pdf (39.5KB, pdf)
bmjgast-2021-000638supp002.pdf (66.8KB, pdf)
Data Availability Statement
No data are available. There is no data repository available for the dataset from this study.