Abstract
T cells shape immune responses in cancer, autoimmunity and infection, in which CD4+ T helper (Th) and CD8+ T cells mediate effector responses that are suppressed by regulatory T (Treg) cells. The balance between effector T cell and Treg cell function orchestrates immune homeostasis and functional programming, with important contributions to the onset and progression of cancer. Cellular metabolism is dynamically rewired in T cells in response to environmental cues and dictates various aspects of T cell function. In this review, we summarize recent findings on how cellular metabolism modulates effector T cell and Treg cell functional fitness in homeostasis and cancer immunity, and highlight the therapeutic implications of targeting immunometabolic pathways for cancer and other diseases.
Keywords: immunometabolism, cancer, Treg, effector T cell, homeostasis
Introduction
Conventional CD4+ or CD8+ αβ T cells that express T cell receptors (TCRs) recognizing tumor- and self-antigens play pivotal roles in shaping immune responses in cancer and autoimmune diseases. Upon cognate antigen stimulation, T cells are activated, proliferate, and undergo functional specialization in response to environmental cues. Antigen-inexperienced naïve CD8+ T cells differentiate into cytotoxic effector cells and long-lived memory cells. Naïve CD4+ T cells differentiate into Th1, Th2, Th17, and Tfh effector cells, which can also form long-term memory cells, as well as Foxp3-expressing immune-suppressive Treg cells [1].
One long standing goal of cancer immunotherapy is to identify factors that stimulate T cell responses to tumor antigens. Tumor antigens may be unique to tumors (tumor-specific antigens) or self-antigens expressed with temporally and spatially distinct patterns (tumor-associated antigens). Cancer development is frequently associated with an immunosuppressive tumor microenvironment (TME) wherein tumor-specific cytolytic CD8+ T cells are often underrepresented among T cells and T cells are frequently dysfunctional and unable to eradicate malignant cells. In addition, Treg cells accumulate and undergo functional maturation in tumors, supporting an immunosuppressive TME [2]. As a consequence, tumor antigens are often unable to elicit an effective antitumor response. While targeting immune checkpoint pathways has shown remarkable clinical success by reinvigorating tumor-specific T cell responses, one of the major challenges is the development of autoimmune-like immune-related adverse events (irAEs). Moreover, although Treg cell ablation can rapidly eradicate tumors, loss of Treg cell function promotes development of severe autoimmune and inflammatory complications [3,4]. Therefore, successful immunotherapies require an in-depth understanding of the mechanisms underlying immune homeostasis under steady state and antitumor immunity.
Although immune receptors, signaling proteins, and transcription factors dictate T cell responses, emerging data has identified cellular metabolism as a central regulator of T cell survival, proliferation, and function [5]. How metabolic rewiring impacts T cell functional adaptation in situ remains underexplored. Recently, extensive efforts have been made to fill this gap, by exploring immunometabolic changes underlying effector T cell and Treg cell functional fitness in the TME and other disorders, with important therapeutic implications. Given the availability of excellent reviews [6–9], we mainly discuss the recent progress, with a particular focus on immunometabolism in effector T cells and Treg cells in the TME.
T cell metabolism in immunity and homeostasis
Cell-intrinsic metabolic pathways direct the activation state of T cells (Figure 1a). In particular, aerobic glycolysis, glutaminolysis and mitochondria-associated functions, such as oxidative phosphorylation (OXPHOS) and one-carbon metabolism, support effector T cell responses by regulating their activation and differentiation. Overall, these pathways serve as bioenergenetic, biosynthetic, and signaling hubs to allow for the proliferative expansion and effector differentiation of T cells [10]. Aside from cell-intrinsic metabolic factors, metabolic rewiring in response to extracellular nutrients, metabolites, and growth factors also modulates effector T cell functional fitness. Indeed, glucose transport into cells is important for supporting aerobic glycolysis, while glucose, glutamine or fatty acid catabolism can drive flux via the tricarboxylic acid (TCA) cycle to support biosynthetic reactions and mitochondrial OXPHOS, both of which are essential for regulating T cell functionality [10,11]. Aside from glutamine, other amino acids have also been implicated in supporting T cell activation and immune responses [12]. For instance, extracellular methionine regulates epigenetic programming to tune CD8+ T cell fate decisions and cooperates with serine (which can be synthesized de novo from glucose or derived from extracellular sources) to promote one-carbon metabolism [13–15]. Further, serine metabolism is also essential for regulating cellular redox state through synthesis of glutathione (GSH), an important regulator of CD4+ and CD8+ T cell responses [16,17]. By integrating cell-intrinsic and extrinsic metabolic programs, T cells play a critical role for antitumor immunity and represent the cornerstone for successful immunotherapies.
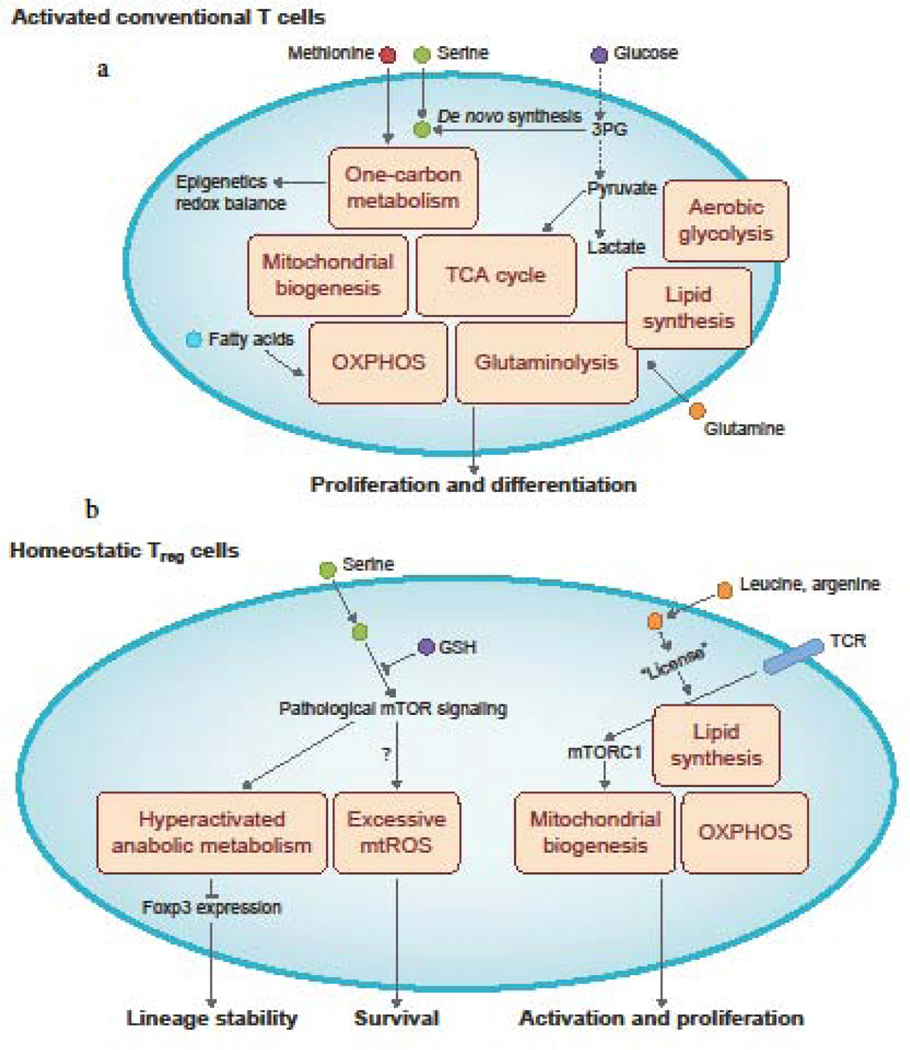
Figure 1: Cellular metabolism of activated conventional T cells and homeostatic Treg cells.
a. Upon their activation, conventional T cells upregulate the metabolic pathways of aerobic glycolysis, glutaminolysis, and lipid synthesis. They also increase mitochondria biogenesis to upregulate mitochondria-associated metabolic processes, including oxidative phosphorylation (OXPHOS), the tricarboxylic acid (TCA) cycle, and the methionine- and serine-dependent one-carbon metabolism pathway that is necessary for epigenetic programming and redox balance. Extracellular nutrients, including glucose, glutamine, methionine, and serine, are crucial for the induction of these different metabolic programs. Glucose metabolism can also support serine synthesis via its metabolic 3-phosphoglycerate (3PG). Fatty acids can also be used as a fuel source for OXPHOS in subsets of activated T cells. b. Mitochondrial biogenesis and OXPHOS, as well as lipid synthesis, are crucial for maintaining Treg cell functional activation and proliferation. These metabolic processes are activated downstream of mechanistic target of rapamycin complex 1 (mTORC1) signaling, which is induced by TCR engagement in the presence of the amino acids, leucine and arginine. Notably, excessive mitochondrial ROS (mtROS) production reduces Treg cell survival. Furthermore, excessive levels of anabolic metabolism (e.g. mitochondrial oxidative respiration or aerobic glycolysis), as a consequence of increased mTOR signaling, dampens Treg cell lineage stability. Treg cell stability can be counteracted by certain metabolites, including glutathione (GSH) that limits serine uptake and serine-induced mTORC1 activation in Treg cells.
Treg cells exert potent immunosuppressive function and thereby maintain self-tolerance and control autoimmunity and tissue inflammation [3]. Recent results reveal novel roles for cellular metabolic processes in regulating Treg cell functional integrity (Figure 1b). Treg cells have distinct glycolytic and mitochondrial metabolism from effector CD4+ T cells [18,19]. Moreover, mitochondrial metabolism is crucial for supporting Treg cell function, self-tolerance, and immune homeostasis, as evidenced by the development of autoimmune diseases in Treg-specific deletion of mitochondrial respiratory chain components or mitochondrial transcription factor A (Tfam), which is important for mitochondrial respiratory chain activity [20–23]. Treg cells also require anabolic processes, such as lipid synthesis, for their activation and function [10]; however, excessive aerobic glycolysis or mitochondrial respiration that produces ROS is detrimental to Treg cell lineage stability [24–27]. Recent studies have uncovered important roles of nutrient availability in modulating Treg cell functional fitness. Specifically, amino acids, especially arginine and leucine, signal through RagA/B and Rheb1/2 to license TCR-induced mTORC1 activation and subsequent mitochondrial metabolic changes in Treg cells [28,29]. Additionally, aberrant serine uptake and metabolism caused by GSH loss in Treg cells leads to increased mTORC1 activation, proliferation, impaired Foxp3 expression, and suppressive function, resulting in the development of lethal autoimmune inflammation that can be rescued by a serine/glycine-deficient diet [30]. Lastly, intestinal immune homeostasis is maintained by Treg cells, and microbial bile acid metabolites are essential for colonic Treg cell generation and suppression of intestinal inflammation [31–33]. These studies collectively indicate that Treg cell functional integrity and suppression of autoimmune responses requires proper cellular metabolic programming.
Metabolic activation of tumor-specific T cells
The immunosuppressive TME is one of the hallmarks of cancer and underlies the basis for tumor immune evasion [4]. Effector T cells play a pivotal role in controlling cancer; conversely, Treg cells promote immunosuppression in the TME that is often associated with tumor progression. Therefore, the functional balance between effector T cells and Treg cells is a key determinant of the effectiveness of immune control of tumor progression. Recent studies have made significant progress in elucidating the roles of immunometabolism in dictating the functional fitness of effector T cells (Figure 2) and Treg cells (Figure 3) in the TME, and hence this effector-regulatory balance.
Figure 2: Cellular metabolism shapes tumor-infiltrating effector T cell responses and differentiation.
a. Effector T cell expansion and function are controlled by glucose metabolism downstream of the AGK–PTEN–PI3K-mTOR signaling axis, which can be antagonized by PD-1 signaling. Further, mitochondrial respiration and metabolism support the function of tumor-infiltrating effector T cells, with metabolites BH4 (synthesized via the enzyme GCH1) and extracellular L-arginine (whose levels are antagonized by methylglyoxal produced by tumor cells) acting as important metabolite regulators of mitochondrial function. Bhlhe40 and BATF are crucial transcriptional regulators of mitochondrial function in tumor-infiltrating T cells, which are inhibited by PD-1 and Regnase-1 signaling, respectively. Cholesterol can also induce the ER stress response–XPB1 signaling axis that suppresses mitochondrial function. Methionine metabolism induced by methionine uptake is crucial to support the effector programming of tumor-infiltrating T cells. b. Stem-like programs that promote T cell longevity and persistence in the TME are programmed downstream of selective metabolic programs. Specifically, the acquisition of stem-like programs is associated with an increase of mitochondrial respiration and spare respiratory capacity (SRC), which can be mediated by glutamine-dependent glutamine metabolism, potassium-induced autophagy, leptin signaling, or fatty acid oxidation (FAO) that is antagonized by MEK signaling. In addition, a stem-like state is adopted upon mitigation of oxidative stress, such as via engaging mitophagy to clear damaged mitochondria or transient glucose restriction.
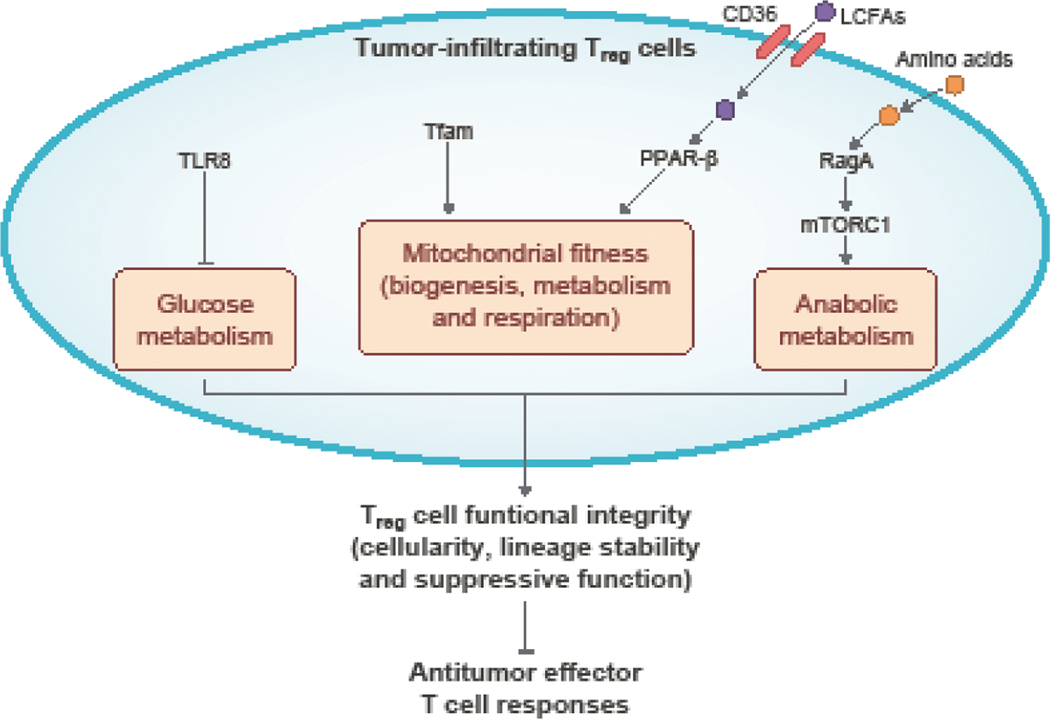
Figure 3: Cellular metabolism modulates Treg cell functional fitness in tumors.
Treg cells accumulate in tumors and inhibit antitumor effector T cell responses to establish an immune suppressive TME. The functional integrity (cellularity, lineage stability, and suppressive function) of Treg cells is mediated by metabolic adaptations. Tfam-dependent mitochondrial respiration supports tumor-infiltrating Treg cell accumulation and lineage stability. Treg cells also upregulate CD36 expression and uptake long-chain fatty acids to activate PPAR-β signaling and support mitochondrial fitness, supporting Treg cell survival and suppressive function. Amino acids, by signaling through RagA–mTORC1 that induces anabolic programming, are also required for tumor-infiltrating Treg cell suppressive function. In contrast, TLR8 activation dampens Treg cell functionality in tumors by inhibiting glucose metabolism.
Glucose metabolism is an essential regulator of T cell function in tumors, a site where competition for nutrients, including glucose, often occurs [5]. Accordingly, restriction of glucose or the glycolytic metabolite phosphoenolpyruvate (PEP) in effector CD8+ T cells dampens antitumor responses [34–36]. However, it was recently reported that the antitumor activity of adoptively transferred CD8+ T cells can be improved by acute glucose restriction, which enables them to more efficiently shuttle glucose-derived carbons into anabolic programs upon glucose refeeding [37]. CD8+ T cells can also utilize inosine as a carbon source to support cell proliferation and effector function under glucose restriction conditions in vitro [38], and inosine can promote effector T cell expansion and function in the TME [38,39]. How T cell adaptations to alternative nutrient sources impact effector function in the TME remains to be fully explored. PI3K-mTOR signaling has a well-established role in glucose metabolism [40], but its upstream regulators in primary T cells are less well understood. Recently, acylglycerol kinase (AGK), an enzyme involved in lipid and glycolipid metabolism, was identified as an upstream activator for mTORC1 and subsequent metabolic reprogramming by interacting with and inhibiting PTEN (the lipid phosphatase that antagonizes PI3K signaling) upon following antigen recognition, thereby promoting CD8+ T cell proliferation and antitumor functions [41]. Thus, glucose metabolism regulates cellular signaling and anabolic programs to support tumor-specific T cell function.
T cells also require mitochondria to fulfill the metabolic requirements for rapid proliferation, activation, and function [5]. Accordingly, impaired mitochondrial fitness underlies effector CD8+ T cell dysfunction in tumors [42–44], while restoring mitochondrial metabolism rescues tumor-infiltrating effector CD8+ T cell function [45]. Recent studies have uncovered important factors supporting mitochondrial metabolism, thereby fueling effector T cell expansion and function in the TME. Transcription factors have well-established roles in shaping effector T cell responses [46], and transcription factor–mediated metabolic programming and its contribution to antitumor T cell responses is just beginning to be explored. The transcription factor BATF has been identified to be a limiting factor for tumor-specific CD8+ T cell expansion and effector function in tumors, in part by supporting mitochondrial fitness [47]. Interestingly, unlike in tumors, BATF overexpression does not boost effector CD8+ T cell expansion in a viral infection model [48], suggesting unexpected context specificity. Additionally, the transcription factor Bhlhe40 helps sustain mitochondrial metabolism that drives acetyl-coenzyme A (CoA) synthesis and acetyl-CoA-associated functional epigenetic programing in tumor-infiltrating CD8+ T cells that promotes their accumulation and effector function [49]. CD8+ T cell dysfunction in tumors is, in part, driven by downregulation of Bhlhe40 downstream of PD-1 signaling. Indeed, anti-PD-L1 blockade-dependent reinvigoration of tumor-infiltrating CD8+ T cell requires Bhlhe40 re-expression [49]. Therefore, transcription factor control can act by modulating metabolic functions during antitumor immunity, and transcription factor activity intersects with key checkpoint blockade pathways, including PD-1–PD-L1.
Recent studies have uncovered additional metabolites that modulate effector T cell function in the TME. Specifically, mitochondrial respiration is supported by the intracellular metabolite BH4, which is generated by the rate-limiting enzyme GTP cyclohydrolase 1 (GCH1). Deleting GCH1 in T cells leads to defective proliferation and mitochondrial respiration; in contrast, enhancing production of BH4 by overexpressing GCH1 enhances the proliferation of both tumor-infiltrating CD4+ and CD8+ T cells, thereby inhibiting tumor growth [50]. Furthermore, in the TME, methylglyoxal from myeloid-derived suppressor cells (MDSCs) dampens CD8+ T cell mitochondrial respiration, activation, and proliferation, partially by depleting L-arginine [51]. This observation is in line with the crucial role of L-arginine in supporting antitumor effector CD8+ T cell function [52]. These results highlight the indispensable roles of cellular metabolites in programming effector T cell mitochondrial metabolism and effector function in the TME.
Metabolic control of T cell differentiation state in the TME
The antitumor activity of tumor-specific CD8+ T cells critically depends upon their differentiation state and longevity [53]. Recent findings have established the role of immunometabolism in controlling tumor-infiltrating effector T cell fate decisions. In the TME, most tumor-specific CD8+ T cells express high levels of inhibitory receptors, possess limited effector function, and often acquire a dysfunctional differentiation state termed exhaustion [53]. Recent work has begun to illuminate the metabolic drivers of tumor-infiltrating CD8+ T cell dysfunction. Although increased cholesterol level in the plasma membrane potentiates antitumor CD8+ T cell responses [54], cholesterol uptake by tumor-infiltrating CD8+ T cells in the TME activates ER stress response and inositol-requiring enzyme 1 alpha (IRE1α)–X-box binding protein-1 (XBP1) signaling to induce inhibitory receptor expression and CD8+ T cell exhaustion [55], indicating divergent roles of cholesterol metabolism in modulating T cell function. Additionally, methionine metabolism-dependent epigenetic programming is essential to establish CD8+ T cell effector function, but tumor cells often express high levels of the methionine transporter Slc43a2, leading to CD8+ T cell dysfunction by outcompeting CD8+ T cells for methionine in the TME [13]. High expression of Slc43a2 on tumor cells correlates with CD8+ T cell dysfunction in patients, and supplementation of methionine reverses CD8+ T cell dysfunction and restores antitumor responses [13]. It will therefore be important to explore how nutrient fluctuations in the TME drive CD8+ T cell dysfunction.
Although exhausted CD8+ T cells have limited effector function, a subset of tumor-infiltrating exhausted CD8+ T cells preserve a stem cell-like state, with preserved self-renewal capacity and reconstitution of effector subsets. The adoption of a stem-like state is essential for tumor-specific CD8+ T cell persistence and antitumor efficacy [56], and hence there has been great interest in understanding the metabolic programs that promote “stemness” of CD8+ T cells to improve tumor immunotherapy. In particular, pathways that improve mitochondrial fitness have emerged as crucial regulators of stem-like versus dysfunctional T cells in the TME. Indeed, in response to chronic antigen stimulation in the TME, mitochondrial oxidative stress increases in CD8+ T cells, resulting in reduced stemness and antitumor efficacy associated with increased T cell dysfunction [57]. These effects can, in part, be attributed to an accumulation of depolarized mitochondria in tumor-infiltrating CD8+ T cells, owing to reduced mitophagy in these cells [27]. It has recently been shown that altering redox metabolism in favor of a reduced state, either via acute glucose restriction or antioxidant treatment, can improve the antitumor activity of CD8+ T cells; this effect is associated with improved self-renewal capacity of the T cells [37,57,58]. Although high levels of potassium in tumor tissues activate starvation response-associated mitochondrial metabolism that inhibits effector differentiation-associated epigenetic remodeling, potassium-stimulated tumor-infiltrating CD8+ T cells also acquire stemness-associated programs that equip them with potent antitumor activity [59]. Mitochondrial respiratory capacity is also linked to the CD8+ T cell stem-cell-like state, with memory CD8+ T cells displaying high mitochondrial SRC [60–62]. Accordingly, blocking glutamine metabolism in the TME enhances oxidative metabolism and SRC, driving CD8+ T cells to adopt a long-lasting stem cell-like state that is associated with superior antitumor responses [63,64]. Additionally, the adipokine leptin induces metabolic reprogramming in tumor-infiltrating CD8+ T cells, resulting in increased SRC, stem-cell-like phenotypes, and antitumor function [65]. Finally, it was recently shown that inhibition of MEK signaling could improve mitochondrial OXPHOS driven by fatty acid oxidation and enhance the stemness of CD8+ T cells for antitumor immunity [66]. Thus, pathways that improve mitochondrial function, including SRC and fatty acid oxidation, and reduce oxidative stress represent promising targets to improve T cell longevity, thereby promoting better antitumor responses by T cells.
Negative control of T cell metabolic activity in antitumor immunity
In addition to these positive regulators of mitochondrial fitness, factors suppressing metabolism and effector T cell expansion and function during antitumor immunity have been revealed. As a result of their high rate of aerobic glycolysis, tumor cells often produce and secrete lactate, which has been shown to diminish T cell responses by limiting the activation of CD8+ T cells and aerobic glycolysis in CD4+ T cells [67,68]. Ovarian tumors have been found to suppress mitochondrial metabolism in CD4+ and CD8+ T cells by inducing ER stress and downstream XBP1 signaling in T cells; notably, targeting XBP1 restores tumor-infiltrating effector T cell mitochondrial respiration, function, and antitumor effects against ovarian tumors [69]. Additionally, although S-2-hydroxyglutarate produced by activated CD8+ T cells promotes the proliferation and antitumor activity of CD8+ T cells [70], its enantiomer R-2-hydroxyglutarate is produced by isocitrate dehydrogenase (IDH)-mutated tumors and accumulates in tumor tissues to dampen mitochondrial respiration and paralyze both CD4+ and CD8+ effector T cells in the TME [71]. Inhibiting R-2-hydroxyglutarate production in the TME restores antitumor effector CD8+ T cell response and impairs tumor growth [71]. Thus, external factors present in the TME can suppress the antitumor activity of T cells.
Cell-intrinsic negative regulators of metabolic reprogramming of T cells have also been reported. For example, signaling via the checkpoint blockade molecules PD-1 and CTLA4 can alter the metabolic status, including reducing glycolysis, of activated CD4+ and CD8+ T cells [72,73], suggesting that immunotherapies targeting these molecules may act, in part, by rewiring metabolic programs of tumor-infiltrating T cells. Moreover, the endoribonuclease Regnase-1 is a major negative regulator of effective antitumor CD8+ T cell responses via its suppression of BATF-dependent mitochondrial fitness, and Regnase-1 inhibition unleashes robust tumor-specific CD8+ T cell expansion in tumor sites [47]. Sirtuin-2 (Sirt2), a NAD+-dependent deacetylase, inhibits glucose, lipogenic, and mitochondrial metabolism by repressing expression of key metabolic enzymes, and inhibition of Sirt2 potentiates both CD4+ and CD8+ T cell proliferation and antitumor function. The repression of T cell metabolism and antitumor responses by SIRT2 is also observed in patients with cancer [74]. Collectively, these findings demonstrate that metabolism is an essential determinant of antitumor T cell expansion and effector function. Further studies are required to reveal how the various positive and negative regulatory factors of metabolic fitness are coordinated in the TME.
Treg cell metabolism and tumor immunosuppression
Treg cells often accumulate in tumors, where they establish an immunosuppressive TME and inhibit antitumor effector responses, making them both a major hurdle and a promising target for cancer immunotherapy [4]. Given the indispensable role of Treg cells in maintaining self-tolerance and immune homeostasis [3], the selective disruption of Treg cell function in tumors is a considerable challenge. As an example, Treg cell-specific deletion of RagA, a guanine nucleotide-binding protein that mediates amino acid-induced mTORC1 activation, reinvigorates antitumor T cell responses and inhibits tumor growth; however, loss of RagA also promotes development of delayed onset autoimmunity, and combined deficiency of RagA and RagB leads to Scurfy-like autoimmunity in mice, as is seen in mice lacking Treg cells [28,29]. Similarly, deletion of the mitochondrial transcription factor A (Tfam), which is important for mitochondrial respiratory chain activity, in Treg cells impairs tumor-infiltrating Treg cell accumulation and lineage stability and dampens tumor growth. However, Treg cells with mitochondrial respiratory chain deficiency also cannot maintain self-tolerance [20–23]. Therefore, the consequences of the rewiring of Treg cells metabolic programs are complex, and how to enforce functional adaptation selectively in tumors remains to be explored.
Treg cells exhibit a metabolic profile distinct from that of effector T cells [18,19,75], suggesting that the identification of pathways associated with their unique metabolic state, especially in the TME, could provide powerful insights for cancer immunotherapy. A recent study has made progress in this regard, demonstrating that glucose metabolism is important for supporting the suppressive function of human peripheral blood Treg cells, and inhibition of glucose metabolism through TLR8 activation dampens Treg cell activity in both lymphoid tissue and tumors [75]. The scavenger receptor CD36, which mediates the uptake of long-chain fatty acids, is upregulated on tumor-infiltrating Treg cells [76]. CD36 is specifically required for Treg cell accumulation in tumors by maintaining peroxisome proliferator-activated receptor-β (PPAR-β) signaling-dependent mitochondrial fitness [76]. In contrast, inhibition of fatty acid binding protein 5 (FABP5), a lipid chaperone that is required for lipid uptake, enhances the suppressive function of Treg cells, which is associated with altered mitochondrial fitness [77], suggesting that fatty acid uptake through FABP5 represses Treg cell suppressive activity. Accordingly, tumor-infiltrating Treg cells are found to take up fewer fatty acids than peripheral Treg cells, and display both the mitochondrial alterations observed in FABP5-deficient Treg cells and enhanced suppressive activity [77]. Further studies are required to uncover the requirements and underlying mechanisms by which lipid metabolism dictates Treg cell fitness in tumors, which may help reconcile these seemingly disparate findings. It is also urgent to investigate whether and how Treg cell functional fitness can be selectively shaped in the TME by modulating intracellular metabolic networks.
Conclusions and prospects
Although multiple cellular metabolic pathways control effector T cell and Treg cell functional fitness in the TME and inflamed tissues, it is unclear how inputs from various metabolic factors are coordinated in complex disease contexts. Several important questions remain to be addressed. First, context-specific functions of cellular metabolism in modulating T cell functional states need to be clarified. For example, Gpi1-dependent metabolism is indispensable for CD4+ Th17 cells in hypoxic inflammatory but not homeostatic conditions [78], illustrating the need to further explore immunometabolism in specific diseases. Identifying functionally relevant regulators and pathways in each physiological context will be necessary to define how immunometabolic pathways can be therapeutically modified in the TME, especially to avoid the development of autoimmunity or irAEs. The successful application of pooled CRISPR-Cas9 mutagenesis screening of metabolism-related factors, such as that employed in the antitumor CD8+ T cell response [47], will enable the systematic and unbiased discovery of key metabolism-related molecules and pathways controlling a given T cell function in defined conditions. In addition, the metabolic states of T cells need to be profiled in disease contexts, such as by using robust metabolic tracing methods in CD8+ T cells that can uncover fundamental differences between T cell metabolic phenotypes profiled in vivo and in vitro [15].
Second, given the heterogeneity of T cell functional populations in the TME and organs under steady state, the roles of metabolic factors in modulating T cell functions and fate decisions in these diseases should be investigated at the single-cell resolution. For example, recent single-cell RNA-sequencing results revealed the respective roles of Regnase-1 and mTORC1 in defining a memory cell-like subset among bulk tumor-specific CD8+ T cells and CD4+ Th17 cells [47,79]. The development of cutting-edge technologies, such as combining pooled CRISPR screening and single-cell transcriptome profiling [80–82], further enables the systematic identification of functionally-relevant factors in T cell functions and fate decisions at the single-cell resolution. Emerging single-cell metabolic profiling techniques that can reveal metabolic heterogeneity among T cell subsets or those in diverse tissue environments may also be useful in characterizing the cell populations with the highest antitumor efficacies, including certain stem-like populations [83–85].
Third, recent studies highlight that tumor cells can functionally paralyze T cells by modulating the availability of metabolites in the TME [13,71]. More studies are needed to further identify how nutrient-sensing processes influence T cell function in tumors. Indeed, there is emerging evidence that systemic nutritional status can impact antitumor immunity or cancer therapy [86,87]. Moreover, evidence from clinical trials support the notion that dietary alterations may improve clinical prognosis to cancer therapies [87], although the impacts on the immune system require additional investigation. Thus, understanding the mechanisms underlying how cell- or microenvironment-specific nutrient transport, sensing, and signaling occurs in conventional T cells or Treg cells may lead to novel therapies for targeting of immunometabolism in cancers without leading to adverse events, including autoimmunity or irAEs. Collectively, a detailed profile and comprehensive understanding of T cell metabolism in disease contexts will translate into innovative therapies for cancer and autoimmune diseases.
Highlights.
Cell-intrinsic and -extrinsic factors influence the metabolic state of T cells
Glucose and mitochondrial metabolism regulate antitumor activity of T cells
Targeting Treg cell metabolism has therapeutic potential for tumor immunity
Future perspectives in immunometabolism and antitumor immunity are discussed
Acknowledgments
The authors acknowledge Joshua Stokes for figure preparation. This work was supported by ALSAC and by NIH grants AI105887, AI131703, AI140761, AI150241, AI150514, CA221290, and CA250533 (to H.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement
H. Chi is a consultant for Kumquat Biosciences, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
References
- 1.Saravia J, Chapman NM, Chi H: Helper T cell differentiation. Cell Mol Immunol 2019, 16:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Togashi Y, Shitara K, Nishikawa H: Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol 2019, 16:356–371. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez-Villar M, Hafler DA: Regulatory T cells in autoimmune disease. Nat Immunol 2018, 19:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A: Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168:707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geltink RIK, Kyle RL, Pearce EL: Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol 2018, 36:461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader JE, Voss K, Rathmell JC: Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol Cell 2020, 78:1019–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leone RD, Powell JD: Metabolism of immune cells in cancer. Nat Rev Cancer 2020, 20:516–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kono M, Yoshida N, Tsokos GC: Metabolic control of T cells in autoimmunity. Curr Opin Rheumatol 2020, 32:192–199. [DOI] [PubMed] [Google Scholar]
- 9.Patel CH, Leone RD, Horton MR, Powell JD: Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat Rev Drug Discov 2019, 18:669–688. [DOI] [PubMed] [Google Scholar]
- 10.Chapman NM, Boothby MR, Chi H: Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol 2020, 20:55–70. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Raynor J, Nguyen TL, Chi H: Nutrient and Metabolic Sensing in T Cell Responses. Front Immunol 2017, 8:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Zou W: Amino Acids and Their Transporters in T Cell Immunity and Cancer Therapy. Mol Cell 2020, 80:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian Y, Li W, Kremer DM, Sajjakulnukit P, Li S, Crespo J, Nwosu ZC, Zhang L, Czerwonka A, Pawlowska A, et al. : Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 2020, 585:277–282.●● This article highlights that tumor cells can inhibit T cells by modulating the availability of methionine in the TME. Tumor cells express high levels of methionine transporter Slc43a2 and outcompete CD8+ T cells for the methionine in tumors, and methionine metabolism is essential for the epigenetic programming and T cell effector function.
- 14.Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, Mainolfi N, Suri V, Guak H, Balmer ML, et al. : Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab 2017, 25:345–357. [DOI] [PubMed] [Google Scholar]
- 15.Ma EH, Verway MJ, Johnson RM, Roy DG, Steadman M, Hayes S, Williams KS, Sheldon RD, Samborska B, Kosinski PA, et al. : Metabolic Profiling Using Stable Isotope Tracing Reveals Distinct Patterns of Glucose Utilization by Physiologically Activated CD8(+) T Cells. Immunity 2019, 51:856–870 e855.●● This work argues that the metabolic states of T cells need to be profiled in disease contexts. By combining bioenergetic profiline and 13C-glucose infusion techniques, the work studies the metabolism of CD8+ T cells in a bacterial infection model and uncovers fundamental differences between the T cell metabolic phenotypes profiled in vitro and in vivo.
- 16.Lian G, Gnanaprakasam JR, Wang T, Wu R, Chen X, Liu L, Shen Y, Yang M, Yang J, Chen Y, et al. : Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mak TW, Grusdat M, Duncan GS, Dostert C, Nonnenmacher Y, Cox M, Binsfeld C, Hao Z, Brustle A, Itsumi M, et al. : Glutathione Primes T Cell Metabolism for Inflammation. Immunity 2017, 46:675–689. [DOI] [PubMed] [Google Scholar]
- 18.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC: Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 2011, 186:3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H: HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 2011, 208:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman NM, Zeng H, Nguyen TM, Wang Y, Vogel P, Dhungana Y, Liu X, Neale G, Locasale JW, Chi H: mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat Commun 2018, 9:2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu Z, Ye J, Dean JW, Bostick JW, Weinberg SE, Xiong L, Oliff KN, Chen ZE, Avram D, Chandel NS, et al. : Requirement of Mitochondrial Transcription Factor A in Tissue-Resident Regulatory T Cell Maintenance and Function. Cell Rep 2019, 28:159–171 e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saravia J, Zeng H, Dhungana Y, Bastardo Blanco D, Nguyen TM, Chapman NM, Wang Y, Kanneganti A, Liu S, Raynor JL, et al. : Homeostasis and transitional activation of regulatory T cells require c-Myc. Sci Adv 2020, 6:eaaw6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martinez-Reyes I, Gao P, Helmin KA, Abdala-Valencia H, Sena LA, et al. : Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature 2019, 565:495–499.● This article demonstrates that mitochondrial respiratory complex III is essential for epigenetic programming and suppressive molecule expression in Treg cells. This work also indicates that mitochondrial respiratory chain complexes play distinct roles in modulating Treg cell metabolism and gene expression.
- 24.Alissafi T, Kalafati L, Lazari M, Filia A, Kloukina I, Manifava M, Lim JH, Alexaki VI, Ktistakis NT, Doskas T, et al. : Mitochondrial Oxidative Damage Underlies Regulatory T Cell Defects in Autoimmunity. Cell Metab 2020, 32:591–604.● This paper examines Treg cells from patients with autoimmune diseases and finds they are featured with mitochondiral oxidative stress and increased cell death. Scavenging of mitochondrial ROS in Treg cells prevents cell death and restores suppressive acitivty, suggesting that elevated mitochondrial oxidative stress is a common feature underlying Treg cell dysfunction in autoimmunity.
- 25.Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, et al. : Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol 2016, 17:1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, Wu C, Vogel P, Neale G, Green DR, et al. : Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol 2016, 17:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X, Teng XL, Wang F, Zheng Y, Qu G, Zhou Y, Hu Z, Wu Z, Chang Y, Chen L, et al. : Metabolic control of regulatory T cell stability and function by TRAF3IP3 at the lysosome. J Exp Med 2018, 215:2463–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do MH, Wang X, Zhang X, Chou C, Nixon BG, Capistrano KJ, Peng M, Efeyan A, Sabatini DM, Li MO: Nutrient mTORC1 signaling underpins regulatory T cell control of immune tolerance. J Exp Med 2020, 217.● This work reveals a critical role of amino acids and downstream mTORC1 activation in sustaining Treg cell activation and suprressive activity, which maintains both self-tolerance and tumor immunosuppression.
- 29.Shi H, Chapman NM, Wen J, Guy C, Long L, Dhungana Y, Rankin S, Pelletier S, Vogel P, Wang H, et al. : Amino Acids License Kinase mTORC1 Activity and Treg Cell Function via Small G Proteins Rag and Rheb. Immunity 2019, 51:1012–1027 e1017.● This work highlights that amino acids signal through RagA/B and Rheb1/2 and serve as “signal 4” to license mTORC1 activation in response to TCR stimulation, which is crucial for maintaining Treg cell activation and suppressive function.
- 30.Kurniawan H, Franchina DG, Guerra L, Bonetti L, Baguet LS, Grusdat M, Schlicker L, Hunewald O, Dostert C, Merz MP, et al. : Glutathione Restricts Serine Metabolism to Preserve Regulatory T Cell Function. Cell Metab 2020, 31:920–936 e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante S, et al. : Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, et al. : Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019, 576:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C, et al. : Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature 2020, 577:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. : Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162:1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gemta LF, Siska PJ, Nelson ME, Gao X, Liu X, Locasale JW, Yagita H, Slingluff CL Jr., Hoehn KL, Rathmell JC, et al. : Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8(+) T cells. Sci Immunol 2019, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, et al. : Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162:1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein Geltink RI, Edwards-Hicks J, Apostolova P, O’Sullivan D, Sanin DE, Patterson AE, Puleston DJ, Ligthart NAM, Buescher JM, Grzes KM, et al. : Metabolic conditioning of CD8(+) effector T cells for adoptive cell therapy. Nat Metab 2020, 2:703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Gnanaprakasam JNR, Chen X, Kang S, Xu X, Sun H, Liu L, Rodgers H, Miller E, Cassel TA, et al. : Inosine is an alternative carbon source for CD8(+)-T-cell function under glucose restriction. Nat Metab 2020, 2:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, et al. : Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369:1481–1489. [DOI] [PubMed] [Google Scholar]
- 40.Liu GY, Sabatini DM: mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 2020, 21:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Z, Qu G, Yu X, Jiang H, Teng XL, Ding L, Hu Q, Guo X, Zhou Y, Wang F, et al. : Acylglycerol Kinase Maintains Metabolic State and Immune Responses of CD8(+) T Cells. Cell Metab 2019, 30:290–302 e295. [DOI] [PubMed] [Google Scholar]
- 42.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, Delgoffe GM: The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity 2016, 45:374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siska PJ, Beckermann KE, Mason FM, Andrejeva G, Greenplate AR, Sendor AB, Chiang YJ, Corona AL, Gemta LF, Vincent BG, et al. : Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu YR, Imrichova H, Wang H, Chao T, Xiao Z, Gao M, Rincon-Restrepo M, Franco F, Genolet R, Cheng WC, et al. : Disturbed mitochondrial dynamics in CD8(+) TILs reinforce T cell exhaustion. Nat Immunol 2020. [DOI] [PubMed] [Google Scholar]
- 45.Beckermann KE, Hongo R, Ye X, Young K, Carbonell K, Healey DCC, Siska PJ, Barone S, Roe CE, Smith CC, et al. : CD28 costimulation drives tumor-infiltrating T cell glycolysis to promote inflammation. JCI Insight 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Zander R, Khatun A, Schauder DM, Cui W: Transcriptional and Epigenetic Regulation of Effector and Memory CD8 T Cell Differentiation. Front Immunol 2018, 9:2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei J, Long L, Zheng W, Dhungana Y, Lim SA, Guy C, Wang Y, Wang YD, Qian C, Xu B, et al. : Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 2019, 576:471–476.●● This work incorporates in vivo pooled CRISPR screening of ~3,000 metabolic factors and single-cell RNA-sequencing to identify Regnase-1 as a major negative regulator of antitumor CD8+ T cell effector expansion and memory-like cell differentiation. Through secondary in vivo genome-scale CRISPR screening, BATF and mitochondrial respiration are demonstrated to be essential Regnase-1 functional targets.
- 48.Kurachi M, Barnitz RA, Yosef N, Odorizzi PM, DiIorio MA, Lemieux ME, Yates K, Godec J, Klatt MG, Regev A, et al. : The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol 2014, 15:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Zhu B, Son YM, Wang Z, Jiang L, Xiang M, Ye Z, Beckermann KE, Wu Y, Jenkins JW, et al. : The Transcription Factor Bhlhe40 Programs Mitochondrial Regulation of Resident CD8(+) T Cell Fitness and Functionality. Immunity 2019, 51:491–507 e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cronin SJF, Seehus C, Weidinger A, Talbot S, Reissig S, Seifert M, Pierson Y, McNeill E, Longhi MS, Turnes BL, et al. : The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature 2018, 563:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumann T, Dunkel A, Schmid C, Schmitt S, Hiltensperger M, Lohr K, Laketa V, Donakonda S, Ahting U, Lorenz-Depiereux B, et al. : Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat Immunol 2020, 21:555–566.● This paper uncovers that myeloid-derived suppressor cells have compromised metabolism and generate the metabolite methylglyoxal. Methylglyoxal inhibits CD8+ T cells, in part, by depleting L-arginine and impairing mitochondrial respiration.
- 52.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et al. : L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167:829–842 e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong M, Clubb JD, Chen YY: Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell 2020. [DOI] [PubMed] [Google Scholar]
- 54.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, et al. : Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature 2016, 531:651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J, et al. : Cholesterol Induces CD8(+) T Cell Exhaustion in the Tumor Microenvironment. Cell Metab 2019, 30:143–156 e145.● This work demonstrates that high levels of cholesterol in the TME is taken up by CD8+ T cells and induces ER stress and XBP1 activation. XBP1 induces the expressions of inhibitory receptors to drive CD8+ T cell exhaustion.
- 56.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al. : Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 2019, 20:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vardhana SA, Hwee MA, Berisa M, Wells DK, Yost KE, King B, Smith M, Herrera PS, Chang HY, Satpathy AT, et al. : Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat Immunol 2020, 21:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL, Peralta R, Wang Y, Wang Y, DePeaux K, et al. : Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol 2021, 22:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, Lee PH, Shin M, Patel SJ, Yu Z, et al. : T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019, 363.●● This work demonstrates that elevated extracellular potassium found in tumor interstitial fluid plays a dual role in shaping antitumor immunity by triggering T cell dysfunction while driving stem-cell-like cell differentiation. High levels of potassium activate starvation response-associated mitochondrial metabolism, which inhibits epigenetic remodeling and prevents progressive effector differentiation.
- 60.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL: Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012, 36:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klein Geltink RI, O’Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, Firat E, Zhu X, Niedermann G, Caputa G, et al. : Mitochondrial Priming by CD28. Cell 2017, 171:385–397 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O,Braas D, van der Windt GJ, et al. : Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 2016, 166:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, et al. : Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019, 366:1013–1021.●● This work uncovers that glutamine metabolism plays differential roles in tumor cells and CD8+ T cells in the TME. Glutamine blockade suppresses tumor cell oxidative and glycolytic metabolism and inhibits tumor cell growth. In contrast, glutamine blockade upregulates CD8+ T cell oxidative metabolism to drive CD8+ T cells to adopt a long-lived effector cell phenotype.
- 64.Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, Maseda D, Liberti MV, Paz K, Kishton RJ, et al. : Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell 2018, 175:1780–1795 e1719.●● Another study highlights the cell type-specific function of glutamine metabolism. Although deletion of glutaminase impairs T cell initial expansion and Th17 cell differentiation, Th1 and cytotoxic CD8+ T cell differentiation and function are enhanced in the absence of glutamine metabolism. Transient glutaminase inhibition elevates Th1 and cytotoxic CD8+ T cell numbers and antitumor immune respones.
- 65.Rivadeneira DB, DePeaux K, Wang Y, Kulkarni A, Tabib T, Menk AV, Sampath P, Lafyatis R, Ferris RL, Sarkar SN, et al. : Oncolytic Viruses Engineered to Enforce Leptin Expression Reprogram Tumor-Infiltrating T Cell Metabolism and Promote Tumor Clearance. Immunity 2019, 51:548–560 e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verma V, Jafarzadeh N, Boi S, Kundu S, Jiang Z, Fan Y, Lopez J, Nandre R, Zeng P, Alolaqi F, et al. : MEK inhibition reprograms CD8(+) T lymphocytes into memory stem cells with potent antitumor effects. Nat Immunol 2021, 22:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. : LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab 2016, 24:657–671. [DOI] [PubMed] [Google Scholar]
- 68.Pucino V, Certo M, Bulusu V, Cucchi D, Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et al. : Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4(+) T Cell Metabolic Rewiring. Cell Metab 2019, 30:1055–1074 e1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, Raundhal M, Chaurio RA, Payne KK, Konrad C, et al. : IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 2018, 562:423–428.● This work demonstrates that ovarian tumor-derived cancer cells induce ER stress and IRE1α– XBP1 signaling in CD4+ and CD8+ T cells. IRE1α–XBP1 activation impairs T cell effector function under glucose-deprived conditions by suppressing glutamine transporter expression and mitochondrial metabolism.
- 70.Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, You J, Chia GS, Sim J, Doedens A, et al. : S-2-hydroxyglutarate regulates CD8(+) T-lymphocyte fate. Nature 2016, 540:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, Alansary D, Sonner JK, Green E, Deumelandt K, et al. : Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 2018, 24:1192–1203.● Although its enantiomer S-2-hydroxyglutarate has been reported to enhance CD8+ T cell antitumor activity, this article reveals that R-2-hydroxyglutarate is produced by IDH-mutated tumors to suppress CD4+ and CD8+ T cells by impairing their mitochondrial respiration.
- 72.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al. : PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 2015, 6:6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, Zhang Z, Li W, Lee H, Aftabizadeh M, et al. : STAT3 Activation-Induced Fatty Acid Oxidation in CD8(+) T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth. Cell Metab 2020, 31:148–161 e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamaidi I, Zhang L, Kim N, Wang MH, Iclozan C, Fang B, Liu M, Koomen JM, Berglund AE, Yoder SJ, et al. : Sirt2 Inhibition Enhances Metabolic Fitness and Effector Functions of Tumor-Reactive T Cells. Cell Metab 2020, 32:420–436 e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li L, Liu X, Sanders KL, Edwards JL, Ye J, Si F, Gao A, Huang L, Hsueh EC, Ford DA, et al. : TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell Metab 2019, 29:103–123 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, Mohmood SR, Fernandez-Garcia J, Tsai CH, Schulze I, et al. : CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol 2020, 21:298–308.●● This study uncovers a mechanism by which Treg cells undergo metabolic and functional adaptations in the TME. Tumor-infiltrating Treg cells upregulate CD36 expression specifically in tumors to increase long-chain fatty acids uptake to activate PPAR-β signaling and support Treg cell mitochondrial fitness and survival in the lactic acid-enriched TME.
- 77.Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, Hippen KL, Loschi M, Thangavelu G, Corrado M, et al. : Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab 2020, 31:422–437 e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu L, Hollinshead KER, Hao Y, Au C, Kroehling L, Ng C, Lin WY, Li D, Silva HM, Shin J, et al. : Niche-Selective Inhibition of Pathogenic Th17 Cells by Targeting Metabolic Redundancy. Cell 2020, 182:641–654 e620.●● This study highlights niche-specific requirements of Gpi1-dependent glycolytic metabolism in Th17 cell energy homeostasis, arguing for exploring immunometabolism in specific disease contexts. Gpi1-deficiency selectively impairs pathogenic Th17 cells but is dispensable for homeostatic Th17 cells, since under non-hypoxic conditions, the pentose phosphate pathway and increased mitochondrial respiration can compensate for the energy reduction caused by Gpi1-deficiency. In inflammatory, hypoxic conditions such as EAE, mitochondrial respiration is limited, and Gpi1-deficient Th17 cells cannot survive.
- 79.Karmaus PWF, Chen X, Lim SA, Herrada AA, Nguyen TM, Xu B, Dhungana Y, Rankin S, Chen W, Rosencrance C, et al. : Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature 2019, 565:101–105.●● This work demonstrates that Th17 cells in EAE mice are functionally and metabolically heterogeneous. EAE-derived Th17 cells contain a TCF-1-expressing population with low anabolic metabolism, high stemness features, and low pathogenesis capacity and a TCF-1-negative population with high metabolic activity and the ability to transdifferentiate into pathogenic Th1-like cells. mTORC1-dependent anabolic metabolism is essential for modulating Th17 cell stemness and plasticity.
- 80.Replogle JM, Norman TM, Xu A, Hussmann JA, Chen J, Cogan JZ, Meer EJ, Terry JM, Riordan DP, Srinivas N, et al. : Combinatorial single-cell CRISPR screens by direct guide RNA capture and targeted sequencing. Nat Biotechnol 2020, 38:954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dixit A, Parnas O, Li B, Chen J, Fulco CP, Jerby-Arnon L, Marjanovic ND, Dionne D, Burks T, Raychowdhury R, et al. : Perturb-Seq: Dissecting Molecular Circuits with Scalable Single-Cell RNA Profiling of Pooled Genetic Screens. Cell 2016, 167:1853–1866 e1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Datlinger P, Rendeiro AF, Schmidl C, Krausgruber T, Traxler P, Klughammer J, Schuster LC, Kuchler A, Alpar D, Bock C: Pooled CRISPR screening with single-cell transcriptome readout. Nat Methods 2017, 14:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahl PJ, Hopkins RA, Xiang WW, Au B, Kaliaperumal N, Fairhurst AM, Connolly JE: Met-Flow, a strategy for single-cell metabolic analysis highlights dynamic changes in immune subpopulations. Commun Biol 2020, 3:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arguello RJ, Combes AJ, Char R, Gigan JP, Baaziz AI, Bousiquot E, Camosseto V, Samad B, Tsui J, Yan P, et al. : SCENITH: A Flow Cytometry-Based Method to Functionally Profile Energy Metabolism with Single-Cell Resolution. Cell Metab 2020, 32:1063–1075 e1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartmann FJ, Mrdjen D, McCaffrey E, Glass DR, Greenwald NF, Bharadwaj A, Khair Z, Verberk SGS, Baranski A, Baskar R, et al. : Single-cell metabolic profiling of human cytotoxic T cells. Nat Biotechnol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ringel AE, Drijvers JM, Baker GJ, Catozzi A, Garcia-Canaveras JC, Gassaway BM, Miller BC, Juneja VR, Nguyen TH, Joshi S, et al. : Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183:1848–1866 e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanarek N, Petrova B, Sabatini DM: Dietary modifications for enhanced cancer therapy. Nature 2020, 579:507–517. [DOI] [PubMed] [Google Scholar]