Summary
Inflammatory bowel diseases (IBDs) are lifelong disorders in which an interaction between genetic and environmental factors is involved. IBDs include two entities: Crohn’s disease (CD) and ulcerative colitis (UC); these can be adequately diagnosed and distinguished with a correct methodological approach based on communicating exhaustive clinical, endoscopic and laboratory information to the pathologist and performing adequate bioptic sampling and precise morphological signs including crypt architecture, distribution of inflammation and granulomas, when present. IBD needs to be distinguished from non-IBD colitis, mostly at its onset. Moreover, IBDs are associated with an increased risk of developing colorectal adenocarcinoma. In daily pathological practice, correct diagnosis of IBD and its subclassification as well as a correct detection of dysplasia is imperative to establish the best therapeutic approach.
Key words: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, dysplasia, intestinal cancer
Introduction
Inflammatory bowel diseases (IBDs) are relapsing diseases that can involve any segment of the gastrointestinal tract and include two chronic gastrointestinal disorders: Crohn’s disease (CD) and ulcerative colitis (UC) 1. IBDs have a multifactorial pathogenesis, which includes genetic predisposition, epithelial barrier defects, dysregulated immune responses and environmental factors which contribute to the onset of the diseases and their progression 2,3. CD and UC differ from each other for epidemiological aspects, clinical presentation, endoscopic and histopathological findings, and disease course; the two entities have different complications and management 1. At the onset, however, the histopathological finding on biopsy samples can overlap between the two entities. Thus, for a correct interpretation of biopsy specimens from patients with suspected IBD, detailed clinical information is essential to make a correct differential diagnosis and to schedule appropriate therapy and follow-up 4,5. On the other hand, a correct pathological approach, both on adequate biopsy samples and surgical specimens is useful to better recognize histopathological findings of IBD, subclassify UC or CD and exclude a non-IBD colitis 4.
The aim of this paper is to provide an easy and ready-to-use guide for daily diagnostic practice for histopathological diagnosis of IBD, its subclassification in CD and UC and for dysplasia recognition. The main epidemiological, clinical, laboratory data and serological markers that the pathologist needs to know for a correct biopsy evaluation have also been reported. The differential diagnosis with non-IBD colitis has been discussed in the manuscript entitled “Histopathology of Non-IBD Colitis. A practical approach from the Italian Group for the study of the gastrointestinal tract (GIPAD)”, which is also included in this Special Issue of Pathologica 6.
Epidemiological and genetic features
The global prevalence of IBDs has been increasing since 2000; at present, IBD affects up to 1 of 200 individuals in Western countries 7. Genome-wide association studies have identified 200 risk loci for IBDs: together, these loci explain 13.1% and 8.2% of variance in disease liability in CD and UC, respectively, but their clinical significance is not clear 3,8. The incidence and prevalence of UC have been increasing worldwide over time, but no sex predominance has been described9. Between 8-14% of patients with UC have a family history of IBD, and first-degree relatives have four times the risk of developing the disease 10. Cigarette smoking, drugs such as oral contraceptives, hormone replacement therapy, and non-steroidal anti-inflammatory drugs, have also been associated with an increased risk of UC 11. The incidence of CD has surpassed that of UC in many Western regions; and the main risk factors are cigarette smoking, gut dysbiosis, and dietary changes3. Antibiotic exposure in childhood increases the risk of CD 11,12. Familiar inheritance of CD is well recognized, with a concordance rate among monozygotic twins higher for CD (50%) than for UC (15%) 13.
Recently, an increasing number of monogenic diseases has been described in association with IBDs14. For these patients, a genetic diagnosis is of much higher predictive value and clinical utility for correlate treatment options which are not similar such as in classical IBD 15. These monogenic defects can be grouped into the following pathogenic subtypes: epithelial barrier and epithelial response defects, neutropenia and defects in phagocyte bacterial killing, hyperinflammatory and autoinflammatory disorders, disorders affecting T and B lymphocyte selection and activation, immune dysregulation disorders with defects in negative control of innate and adaptative immune responses 15. These disorders, which represent key functional clues within inflammatory networks, can lead to IBD in children younger than 6 years of age, configuring the spectrum of Very-Early-Onset IBD (VEO-IBD) 16. VEO-IBDs are rare disorders representing a distinct genetic phenotype, but they also have some clinical stigmata, such as an increased resistance to conventional medical treatment and an high morbidity and even mortality 16.
Clinical and laboratory features
Increased stool frequency (6-8 discharges per day) and decreased stool consistency, i.e. diarrhea, protracted over time, at least for 4 weeks, are the most common presenting symptoms of IBDs, followed by abdominal pain. Tenesmus is a clue to the presence of proctitis, and will be more suggestive of UC as is the presence of blood with mucus in feces; conversely, fever is more often seen in CD than in UC, as is weight loss 17. The presence of perianal disease, such as skin tags, anal fissures, and perianal fistulae are among the most helpful clinical findings suggestive of CD 17. Besides intestinal symptoms, the so-called extraintestinal manifestations (EIM) are frequent, with a prevalence ranging from 6 to 47% which considerably affect morbidity and mortality in patients with IBDs 18. The pathophysiology of EIM remains elusive, although data from clinical trials demonstrating the efficacy of anti-tumour necrosis factor (TNF) agents suggest a common pathogenic link between intestinal and extraintestinal disease activity 19. EIMs are primarily associated with CD and are present in 43% of patients with CD; they can affect multiple organ systems, including the musculosketal (arthropathy, arthritis and ankylosing spondylitis), ocular (uveitis, scleritis and episcleritis), and hepatobiliary (primary sclerosing cholangitis) systems 20. Additional EIMs have also been described, such as metabolic bone disease and pulmonary or deep-venous thromboembolic disease 3. Cutaneous manifestations of IBDs include erythema nodosum (EN), pyoderma gangrenosum (PG), Sweet’s syndrome, and oral lesions 21. The association with erythema nodosum is more common in CD, whereas pyoderma gangrenosum prevails in UC 17. Treatment-induced manifestations have also been observed, such as skin reactions induced by anti-TNF therapy which can be misinterpreted as psoriasic lesions 22. However, no single IBD-related symptom is pathognomonic of IBD or specific for CD or UC; detailed questioning about the timing of the onset of symptoms is imperative to differentiate IBD from non-IBD colitis and to better subclassify IBDs 5,17.
Numerous hematological parameters, including white blood cell count, hemoglobin and hematocrit, platelet count, iron saturation, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) may cover the full spectrum from normal to markedly abnormal in IBDs, but these parameters are not specific for the diagnosis of IBD 17. Fecal calprotectin is highly specific in differentiating IBD from functional bowel disease and very useful in monitoring disease activity, relapse, and response to therapy 3. However, fecal calprotectin is not specific for IBD because this marker can be elevated in a variety of non-IBD conditions, such as enteric infections, drug therapy, and intestinal neoplasms 17. For this reason, clinical investigation is essential, and an integrated approach is of paramount importance. Every evaluation for suspected IBD should include a stool culture and a serological exam 17.
Serological markers
Antibody markers in IBDs can be classified into two main groups: autoantibodies, which are antibodies against intestinal and non-intestinal self-antigens, and microbial antibodies, which are antibodies related to microorganisms, including bacteria, yeasts and fungi. In addition, antibodies against some peptides, the origin of which remains unclear, have also been reported 23. In clinical practice, only two markers have demonstrated reasonable sensitivity and specificity in the diagnosis of IBD and its subclassification:
Anti-neutrophil cytoplasm antibody (ANCA): ANCA is an autoantibody, classified according to two staining patterns: cytoplasmic ANCA (cANCA) and perinuclear ANCA (pANCA). In IBD, pANCA is thought to be induced by a cross-reaction with intestinal bacterial antigens. pANCA is detected in 60-70% of UC cases, 10-15% of CD cases, and less than 5% of non-IBD colitis cases. Moreover, patients with pANCA-positive CD exhibit a clinical phenotype resembling UC 17,23.
Anti-Saccharomyces cerevisiae antibody (ASCA): ASCA is an anti-glycan antibody, a microbial antibody against the mannan found in the cell wall of baker’s yeast (S. cerevisiae) 23. The ASCA IgG-positive rate is 60-70% in patients with CD, 10-15% in patients with UC, and less than 5% in patients with non-IBD colitis 17,23.
At present, no single validated serological antibody exists for the diagnosis of IBD, and the use of combinations of several markers has been attempted. In patients with a diagnosis of IBD, the predictive value of a pANCA-negative/ASCA-positive result was 95% for CD, while that of a pANCA-positive/ASCA-negative result was 90% for UC 23.
Gross features
UC classically shows diffuse and continuous chronic inflammation involving the rectum and spreading proximally, with gradually decreasing severity 4. The transition between adjacent involved and healthy mucosa is sharp. The mucosa has a friable, granular appearance which can progress to mucosal denudation or deep penetration; the initially superficial ulcers, in severe or longstanding disease, may reach the muscularis mucosae 24. Extensive ulceration with sparing of mucosal islands may give rise to inflammatory pseudopolyps. Unusual macroscopic distribution patterns, such as rectal sparing, caecal patch and backwash ileitis, need to be recognized to avoid a misdiagnosis of CD 25. The rectum may be spared in children (30%), in adults with fulminant colitis (13%) or in patients receiving topical and/or systemic treatment (44%) 4. Another therapy-related finding is patchiness, i.e. discontinuous rather than continuous inflammation. For these reasons, clinical correlation is mandatory. Caecal patch consists in inflammation surrounding the appendiceal orifice associated with left-sided colitis, diagnosed in up to 75% of patients with distal disease 26.
The localization of CD usually remains stable over time, with approximately one third of patients presenting with colonic disease, one third with ileo-colic and one third with small-bowel limited disease 3. Upper gastro-intestinal involvement in CD refers to esophageal, gastric, duodenal and jejunal involvement, either isolated or together with other localizations, with a prevalence of 35-75%, as reported in different studies 27. Characteristically, macroscopic examination of a resection specimen shows a discontinuous pattern of inflammation, with diseased segments frequently and abruptly separated by areas of uninvolved bowel (skip lesions). The serosal surface of an involved bowel segment is often hyperemic and may be covered with inflammatory exudate, and in longstanding disease, serosal adhesions may occur 4. Mainly in small bowel CD, but less frequently also in large bowel CD, adipose tissue expands towards the antimesenteric surface, a finding termed “fat wrapping” or “creeping fat’’. The earliest grossly visible mucosal lesions are small aphthous ulcers that typically develop along the mesenteric margin of the bowel wall (over lymphoid follicles) and are bordered by normal mucosa 28. As aphthous ulcers coalesce, they form large deep serpiginous or linear ulcers with overhanging edematous mucosal edges giving rise to the classic “cobblestone” appearance 4. As in UC, inflammatory pseudopolyps can occur. Fistulae are more frequent in the small bowel but, though relatively rare, can also occur in the colon. Free perforation, however, is exceptional. The bowel wall becomes thickened and increasingly rigid as a consequence of transmural inflammation with fibrosis and fibromuscular proliferation 4.
Endoscopic sampling
The histological examination of endoscopic biopsies is a crucial step in the diagnostic work-up of suspected IBD patient and it is crucial in making a final diagnosis, particularly in differentiating between UC and CD and other forms of non-IBD colitis 4,29. In patients with suspected IBD, histological examination should be performed before initiation of treatment because drugs can induce changes in morphology 4. All the different segments of the ileo-colic tract should be extensively sampled, regardless of the endoscopic extension of the disease 2,30. Endoscopic sampling is required also during follow-up to assess the therapeutic efficacy in mucosal healing and for cancer prevention 29. Optimal sampling for newly diagnosed IBDs requires at least two biopsies in the terminal ileum and in each segment of the large bowel (caecum, ascending, transverse, descending, sigmoid colon and rectum), even if the mucosa appears endoscopically normal 2,31. If the upper digestive tract is suspected to be involved, esophageal, gastric and duodenal biopsies are indicated 31. The minimum sampling during follow-up consists in at least two biopsies in all large bowel tracts showing significant lesions and two biopsies in the rectum 2,30. In case of discordance between the initial histological diagnosis and the clinical course during follow-up, it is mandatory to repeat a complete sampling to settle the question. It is also strongly recommended that previous biopsies be critically re-evaluated and that such review be commented on in the final histological report 29. Finally, for the detection of dysplasia during follow-up in longstanding disease, it is recommended using high-definition chromoendoscopy with targeted biopsies, with two biopsies every 10 cm in separate vials, submitting any suspicious lesion or mass separately 32. If white-light endoscopy is used, the recommendation is to take four biopsies for every 10 cm of the entire colon as well as targeted biopsies from any visible lesion 2.
Biopsy handling
To better evaluate all histological parameters required for a diagnosis of IBD and to distinguish UC and CD, the use of appropriate cellulose acetate filters is highly recommended 29,30. In detail, after sampling the mucosa, the endoscopist can place each fragment upright in a straight line with proximal-to-distal orientation onto the cellulose acetate filter, which relies on a clarinet beak-shaped cut for orientation (Bio-Optica, Milan, Italy). All tissue samples should be fixed immediately by immersion in 10% buffered formalin and, after adequate fixation, processed and paraffin-embedded with 90°-rotation by the technician, in order to ensure the best trans-sectional cut 30. Since lesions may be focal, multiple sections from each tissue sample should be examined 2,29. However, the optimal number of sections to be examined in daily practice has not been established yet; presently, the numbers reported in different studies vary between two and six sections 4. For daily practice, step-sections with two or three tissue levels, each consisting of five or more sections, are recommended 2,4. Staining with hematoxylin and eosin (H&E) is sufficient for diagnosis 29. If a cellulose acetate filter is not available, it is imperative to use separate vials for each intestinal biopsy site 2.
Sampling of surgical resection
A surgical specimen requires systematic scheduled gross examination. Specimens, generally small-bowel segmentectomy or right hemicolectomy for CD, total colectomy for UC, should be opened longitudinally along the antimesenteric side. Macroscopic aspects that constitute potential diagnostic features, such as mucosal alterations, pseudopolyps, erosions/ulcerations and fistulae, should be recorded. Special attention should be paid to findings suspicious for neoplasm, such as elevated lesions or masses. Wall thickness and serosal findings should also be described. Tissue samples for histological examination should include resection margins, lymph nodes, terminal ileum, ileo-cecal valve and appendix, if present. Seriated sampling, both from affected mucosa and macroscopically normal mucosa, improves the diagnostic yield. The site of each tissue sample should be recorded 4. Details about the mesentery, such as size and eventual lesions, should also be recorded. A recent paper showed a reduction of surgical recurrence (over 40%) when the mesentery was included in an ileocolic resection 33.
KEY POINTS:
IBDs are mutifactorial diseases. Their diagnosis is clinical and requires integration of clinical information, endoscopic findings and morphological features as well as data from laboratory and imaging procedures;
no single serological test or antibody is specific for diagnosis of IBD; a combination of several markers is needed for correct management;
pANCA is detected in 60-70% of UC cases, 10-15% of CD cases, and less than 5% of non-IBD colitis cases; the IgG ASCA-positive rate is 60-70% in patients with CD, 10-15% in patients with UC, and less than 5% in patients with non-IBD colitis. Thus, the pANCA-negative/ASCA-positive phenotype is associated with CD, while the pANCA-positive/ASCA-negative combination is most often seen in UC;
standardized endoscopic sampling of intestinal mucosa and a correct handling of biopsies are mandatory to improve histological diagnosis of IBD. Similarly, a correct sampling of surgical specimens in patients without previous IBD diagnosis can yield insights.
Histopathological features of normal intestinal mucosa
The diagnosis of IBD relies on the identification of individual histopathological lesions. Particular attention should be placed on distinguishing true lesions from alterations caused by bowel preparation or trauma, and from minimal changes that fall within the spectrum of variability of the normal colonic mucosa. Therefore, we begin by briefly describing the features of normal colonic mucosa, based on the four major milestones of pathological changes 29.
MUCOSAL ARCHITECTURE
Normal terminal ileal mucosa is characterized by the ratio between the height of the villi and of the crypts, which is always in favor of the villus (2:1 or more). The irregularity of the profile of the villi may be observed in correspondence of the lymphoid follicles. The normal number of intraepithelial T lymphocytes in the villi is fewer than 4 per 100 of the enterocytes 34.
The normal colonic mucosa is exemplified by the “test tubes in a rack” analogy: a flat luminal surface with crypts that are parallel, straight, homogenously sized, regularly spaced, and with the bottom reaching the muscularis mucosae. No more than 10% of the crypts should be branching. The average crypt density is 7-8 per millimeter of mucosal length although this value may decrease in the cecum and distal rectum. Irregular architecture can be observed at the level of the ileo-cecal valve, near the appendiceal orifice and in proximity lymphoid follicles.
SUPERFICIAL EPITHELIAL FEATURES
The superficial epithelium is composed of absorptive and goblet cells located in a continuous layer covering the basement membrane, without any interruption or erosion. Pseudo-detachment of the epithelium from the basal membrane or occasional neutrophils in the surface can be observed as an effect of bowel preparation.
EPITHELIAL CELLULAR COMPONENTS
Other than absorptive and goblet cells, Paneth cells can be observed in the right colon and transverse colon. In the upper and middle third of the crypts, occasional apoptosis can be seen; in the lower third, mitoses are common, and immature cells can sometimes be seen.
LAMINA PROPRIA CELLULARITY
Lymphocytes and plasma cells are normally present in the lamina propria and are more abundant in the cecum, decreasing in density from the surface epithelium to the muscularis mucosae (i.e. top-heavy). Throughout the right colon, eosinophils are normally present. Primary and even secondary lymphoid follicles can be observed, sometimes extending to the submucosa. Acute hemorrhagic foci and pseudolipomatosis are alterations caused by endoscopic trauma; edema can be a consequence of bowel preparation.
Hallmarks of UC
The diagnosis of UC is based on elementary histological lesions that fall into the four main categories described below 4,29,30:
Mucosal architecture: Crypt architectural abnormalities are a consequence of chronic inflammation. Crypt distortion consists in crypts that are no longer parallel, vary in diameter and/or are dilated. Crypt branching represents regeneration through fission: branching of more than 10% of crypts or presence of more than two branched crypts in a well-oriented biopsy specimen with at least 2 mm of muscularis mucosae is abnormal. Crypt atrophy is defined as shortened crypts, accompanied by an increased layer of lamina propria stroma between the crypt base and the muscularis mucosae. In UC, distorted crypt architecture with crypt branching and atrophy is present in 57-100% of cases, increasing with the duration of disease; this is an important concept because in very early onset and, in particular, in children, the architecture of the crypts can be nearly normal.
Superficial epithelial features and
Epithelial cellular components: Abnormalities include surface epithelial damage, such as flattening, focal cell loss, erosions, and ulcers which reflect the activity of the disease. Mucin depletion, defined as reduction in the number of goblet cells and/or reduced quantity of intracellular mucin, is a weak diagnostic feature in UC as it also occurs in other diseases, such as infectious colitis and CD. Paneth cell metaplasia in the left colon, inflammatory polyps, hypertrophy of the muscularis mucosae, and the much less frequently observed submucosal fibrosis are additional features of chronicity.
Lamina propria cellularity: The inflammatory infiltrate in untreated disease is limited to the mucosa, and is diffuse and continuous without any variations in intensity or skip lesions. Its severity increases characteristically towards the rectum and particularly its lower third, which has the lowest lamina propria cell density in normal conditions. Plasma cells are predominantly observed between the base of the crypts and the muscularis mucosae (basal plasmacytosis). This feature is helpful in differentiating between a first episode of UC (63%) and infectious colitis (6%). Neutrophils, which reflect disease activity, are present in the lamina propria and/or invade the surface or crypt epithelium, resulting in cryptitis or crypt abscesses (presence of neutrophils within crypt lumina). Crypt abscesses are more common in UC (41%) than in CD (19%). The number of eosinophils is variable, but their coexistence with basal plasmocytosis (more than 3 at the base and lateral part of crypts) make a diagnosis of UC more likely, especially at first endoscopic sampling 35. Moreover, at first diagnosis, a large number of eosinophils (over 60 for 10 HPF), in particular in the left colon, can be predictive of non-reponse to medical therapy 36,37. Granulomas are generally not observed and need to be distinguished from those generated in response to foreign bodies or to mucin from ruptured crypts. These histopathological modifications are time-related and do not necessarily match the endoscopic aspect (Fig. 1).
Figure 1.
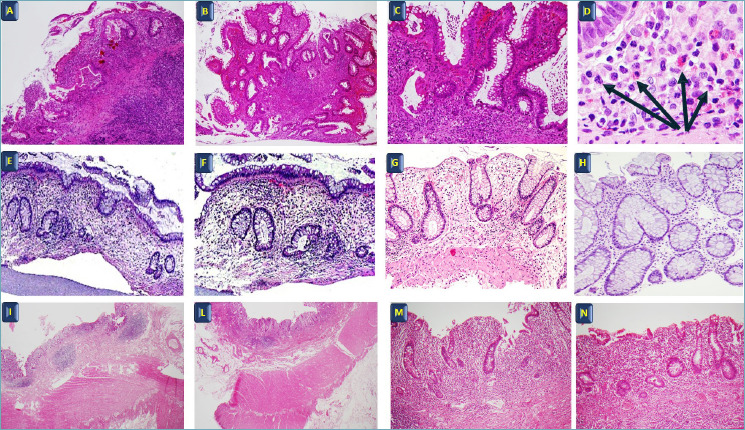
(A, B, C) Ulcerative colitis with moderate-severe activity. (D) Basal plasmocytosis with plasma cells and eosinophils (arrows). H&E A-B: 4x, C: 20x, D: 60x. (E, F, G, H) Ulcerative colitis in remission (quiescence of disease) H&E E: 10x, F-G-H: 20x. (I, L, M, N) Ulcerative colitis: surgical specimen with inflammation located in the mucosa. H&E I-L: 4x, M-N: 20x.
In early stage disease, the diagnosis of UC can be challenging. Preserved crypt architecture and absence of a transmucosal inflammatory infiltrate do not rule out early stage UC, in which the distinction from infectious colitis (acute self-limiting colitis) is a major concern. Focal or diffuse basal plasmacytosis is the earliest diagnostic feature with the highest predictive value for diagnosis and can be identified in 38% of patients within 2 weeks after presentation 2,4. The association between basal plasmocytosis and eosinophils raises sensivity for the diagnosis of UC 35. Strong immunohistochemical expression of TNFα in plasma cells has been recently described in UC patients at early stage disease, with possible implications for treatment approaches 38.
In long-standing disease, there is usually widespread architectural crypt distortion with Paneth cells metaplasia in the left colon and rectum, and decreased cellularity of the lamina propria. In this situation, confusing features such as patches of normal mucosa, discontinuous inflammation and rectal sparing, may occur leading to a misdiagnosis of CD. Under medical treatment, the extent of colon involvement tends to decrease, resulting in complete restoration of the rectal mucosa in 34-44% of patients. Similarity between UC and CD may result in diagnostic dilemmas, in particular when clinical information is not available 39. Backwash ileitis occurs in approximately 20% of patients with active ulcerative pancolitis and consists in a mild degree of neutrophilic inflammation in the lamina propria, which is often patchy in distribution and occasionally associated with cryptitis, crypt abscesses and a mild degree of villous atrophy and regenerative epithelial changes 25.
Active and inactive (quiescent) disease: To differentiate between histologically active and inactive (quiescent) disease, many histological scores have been proposed to evaluate therapeutic efficacy, because endoscopic remission is not always paralleled by histologically quiescent disease 2. The histological scores reported in the literature have been elaborated for scientific purposes and are affected by low inter-observer reproducibility and by complexity in their daily practice application 2. The Nancy Index (NI) is the recommended histological score 2. In the NI, grade 4 means that ulceration is present; grade 3 refers to a moderate or severe neutrophil infiltrate with no ulceration; grade 2 indicates that there are only a few neutrophils and no ulceration; grade 1 refers to a moderate or severe chronic inflammation (plasma cells, lymphocytes and/or eosinophils) with no neutrophil activity or ulceration; and grade 0 represents little or no chronic inflammation with no neutrophils and no ulceration 40. Moreover, a simplified score has been proposed to asses mucosal healing in IBDs 41. This score takes into consideration only three well-defined histological variables: a) crypt abscesses; b) presence of granulation tissue or aggregates of inflammatory elements in the superficial parts of the mucosa, indicative of erosions or ulcers; c) neutrophils in the lamina propria and their anatomic localization, thus greatly limiting the subjective interpretation by the pathologist. In particular, the presence of neutrophils was considered the hallmark to differentiate the active from the remission/quiescent phase, and the expression of therapeutic efficacy, i.e. histological mucosal healing. Though this simplified score needs validation, it has been was shown to be easier and less time-consuming in daily practice, and can be proposed as a valid alternative 41.
Hallmarks of CD
A variety of microscopic features support the diagnosis of CD. The features with the highest diagnostic value are discontinuous chronic inflammation, focal crypt architectural distortion and granulomas not related to crypt injury, which are observed in the small and large bowel as well as in esophageal, gastric and duodenal localizations 4,29,30. The main alterations are described below.
Superficial epithelial alterations: Irregular villous architecture in ileal biopsy sample, for the discontinuous inflammatory infiltrate, is the most frequent and characteristic alteration. Aphthous ulcers, i.e. loss of tissue up to the underlying lymphoid follicle, are frequently observed.
Mucosal architecture alteration: Crypt distortion and atrophy are less pronounced than in UC and, like inflammation, show a variable distribution.
Mucosal components alteration: These are generally less prominent than in UC and focal or discontinuous. Pyloric gland metaplasia is related to mucosal ulceration and repair and is observed in 2-27% of ileal biopsies from patients with CD, while is more common in ileal resection specimens.
Lamina propria cellularity: Focal (discontinuous) chronic inflammation of variable density throughout the biopsy specimen and not confined to the superficial mucosa is characteristic of CD. One or more circumscribed foci of increased mononuclear cell density with or without infiltration of neutrophils is generally found, on a background of normal mucosa. Normal lymphoid aggregates are not considered as focal inflammation. As in UC, the presence of neutrophils in the lamina propria or in the epithelium, including cryptitis and crypt abscesses, is an hallmark of active disease. Another usual finding in CD is epithelioid cell granuloma, a discrete collection of at least five epithelioid cells, with or without multinucleated giant cells, not related to crypt injury, and often poorly delimited. Notably, non-caseating granulomas, small collections of epithelioid histiocytes with giant cells or isolated giant cells, occur also in infectious colitis (acute self-limiting colitis) and parasitic infections, in particular Yersinia enterocolitica or pseudotuberculosis and intestinal tuberculosis. When the ileitis is in continuity with proximal colitis, caution is needed, because these lesions may also occur in backwash ileitis. Neural hypertrophy (nerve fiber hyperplasia) can be present. Infiltration of eosinophils or T lymphocytes into the submucosal or intramuscular plexus (ganglionitis) should be reported on surgical specimens, such as transmural nodular inflammation. When present at the resection margins, this finding is prognostic of disease recurrence 42-46. The mucosal lesions of early CD are found primarily inthe ileum. The lesions usually have a focal distribution over a regular morphological background of villi. They are represented by the focal penetration of neutrophils and eosinophils into the surface epithelium 47,48 (Fig. 2).
Figure 2.
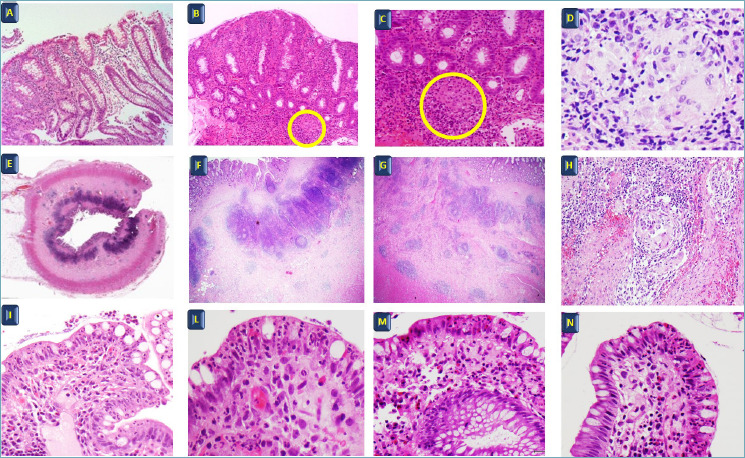
Crohn’s disease. (A) Patchy inflammation, (B-C) granuloma, (D) granuloma. H&E A-B: 20x, C: 40x, D: 60x. (E, F, G, H) Crohn’s disease surgical specimen, inflammation penetrating the different layers of the terminal ileum H&E E: 4x, F: 10x, G: 20x, H: 40x. (I, L, M, N) Early Crohn’s disease, focal superficial inflammation in the terminal ileum. H&E I: 10x, L: 40x, M: 20x; N 40x.
IBDs in children and adolescents
IBDs are an important cause of gastrointestinal pathology in children and adolescents and about 10-15% of IBDs are diagnosed before the age of 18 years. Pediatric histological features are distinctly different from those found in adults, a finding which may complicate the diagnosis and thus delay the onset of adequate therapy. Children with untreated UC commonly present with subtotal or extensive colitis, but with less severe inflammation, less epithelial injury and less architectural abnormality than UC in adults. Backwash ileitis occurs with similar frequency, but children more often present with unusual histological inflammation patterns, such as patchiness (21%) and rectal sparing (30%) 4.
At onset, CD in children less frequently affects ileum and is often characterized by colitis with rectal involvement which complicates the distinction between CD and UC. In such a case, the presence of areas with inflammation alternating with areas with less (or without) inflammation in each of the multiple colonic biopsies should be noted, since focal distribution of inflammation is highly suggestive of CD. The frequency of granulomas is higher in children than in adults 4.
In addition, in pediatric patients with CD, upper gastrointestinal involvement is more frequent than in adults, and focal inflammation may be found in the esophagus, stomach, and/or duodenum 4.
Peculiar and unusual histological patterns can be observed before 6 years age configuring VEO-IBDs, such as the apoptotic pattern, plasma-cells depletion, and moderate inflammation. The clinical manifestations and histopathological features of each VEO-IBD are very peculiar and specific and, for more details, we refer the reader to a selected bibliography 16.
Indeterminate colitis
In approximately 5% of clinically suspected IBDs, a definite diagnosis of UC or CD cannot be established, most often due to either insufficient clinical, radiologic, endoscopic or pathological data, or overlapping features of both disorders. Labels such as “indeterminate colitis” (IC), “inflammatory bowel disease unclassified (IBDU)”, “chronic inflammatory bowel disease unclassified” and “chronic idiopathic inflammatory bowel disease not otherwise specified” are in use for such cases 4. The term IC should be restricted to cases in which comprehensive histological examination of surgical specimen is possible. The term IC should be avoided on endoscopic biopsies, on which the term IBDU is preferred when a patient with chronic colitis clearly has IBD based upon the clinical history, but endoscopy and/or endoscopic biopsies show no definitive features of either UC or CD 4,30.
Intestinal superinfection in IBD
In active IBD, a superimposed bacterial or viral infection should always be excluded, especially in patients using immunosuppressive drugs, with colonic involvement, or with a sudden exacerbation. Cytomegalovirus (CMV) is an opportunistic pathogen having tropism for inflamed tissue 49. Testing for CMV detection should be performed in all patients with severe colitis refractory to immunosuppressive therapy and on biopsies with prominent granulation tissue associated with large ulcers 4,30. In UC, the risk of CMV reactivation is significantly higher than in CD (10-56.7% vs 0-29.6%) and pouchitis, and also depends on the type of immunosuppressive drugs used 49. The histological diagnosis is currently considered the gold standard 50. Although inclusion bodies indicative of a CMV infection can be detected in H&E-stained slides, immunohistochemistry or molecular techniques are more sensitive and have a higher diagnostic accuracy 4,50. On H&E-stained slides, CMV typically presents as isolated cytomegalic endothelial cells and fibroblasts in granulation tissue. These are typically two to four times larger than normal, with large amphophilic intranuclear inclusions, surrounded by a clear halo, and smaller cytoplasmic inclusions. However, some infected cells are morphologically less characteristic and the morphology of ganglion cells and necrotic or apoptotic cells may resemble CMV inclusions 4. Moreover, due to sampling error, false-negative biopsies are common 50. Immunohistochemistry increases the diagnostic yield in comparison with H&E staining, and its sensitivity reaches 93% 4,50.
It remains unclear whether CMV reactivation exacerbates the course of IBD, but subclassifying patients based on the number of CMV-positive cells can improve therapeutic management 51. Five or more viral inclusions constitute the high-grade CMV density group 50, in which colectomy rates differed significantly by the antiviral therapeutic status (44% of patients on therapy vs 83% of patients without therapy) 51. Fewer than five inclusions constitute the low-grade CMV density group 50, the colectomy rate of which did not differ between patients who underwent antiviral therapy and those who did not 51.
The second most frequent infection is caused by Clostridium difficile, especially in patients being treated with steroids and/or immunosoppressors 52. Histology does not allow reliable detection of bacterial superinfection of the small or large intestine, because the histologically characteristic pseudomembranous colitis is usually not present in IBD patients 4. Such evaluation, therefore, should include assessment of three sets of stool samples for all patients. Finally, specific testing for sexually transmitted diseases, such as Neisseria gonorrheae, Herpes simplex virus, Chlamydia trachomatis and Treponema pallidum, should be considered in specific instances, and particularly in patients with severe rectal symptoms 52.
Pouchitis
Proctocolectomy with ileal pouch anal anastomosis (IPAA) is the procedure of choice for most patients with UC refractory to treatment. Usual histological findings, present in up to 87% of biopsies from healthy pouches, consist of chronic inflammatory changes, such as architectural distortion, villous atrophy, crypt hyperplasia and infiltration of the lamina propria by mononuclear cells, eosinophils and histiocytes. Neutrophils are rarely present. Villous atrophy and crypt hyperplasia are considered adaptive changes (termed colonic metaplasia). Mild ischemic changes occasionally occur, while some patients develop features of mucosal prolapse, often in the anterior wall 4. The term pouchitis refers to active inflammation of the IPAA mucosa and is considered as a primary, non-specific, idiopathic inflammation of the neorectal ileal mucosa 4. The etiology of pouchitis is unknown; however, it probably results from an immune-mediated injury that occurs in genetically predisposed individuals. Specific pathological predictors of pouchitis include the presence of colonic superficial fissuring ulcers, severe appendiceal inflammation, and severe pancolitis 25. The diagnosis of pouchitis is based on a combination of clinical symptoms, endoscopic and histological findings 25. Histological changes may be patchy and more prominent in the lower and posterior regions of the pouch. Consequently, multiple biopsies from these sites are essential for the diagnosis 4. In pouchitis, patchy intraepithelial neutrophil infiltrates become more numerous and cryptitis ensues, with crypt abscesses and ulcerations. In this case, other possible causes such as infections (particularly CMV) should be considered. While in patients with acute pouchitis antibiotic therapy is usually effective, approximately 10-15% of these patients develop chronic pouchitis, with CD-like complications including perianal fistulas and inflammation, stenosis or fistulas in the pre-pouch ileum and/or in the pouch itself. Approximately 12% of patients develop medically refractory pouchitis that may require immunosuppressive therapy or, ultimately, surgical resection of the pouch. Histology of pouches excised for these complications may show deep submucosal lymphoid aggregates and granulation tissue-lined fistulous tracts. Similar changes, and even granulomas, have been observed in blind-ending rectal stumps left in situ after total colectomy for UC. The occurrence of CD-like complications and the presence of deeply located lymphoid aggregates do not refute a diagnosis of UC 25. CD should only be diagnosed after IPAA surgery when re-examination of the original proctocolectomy specimen shows typical features of CD. The presence of inflammation and/or ulceration in the afferent limb proximal to the pouch often represents recurrent CD, but, interestingly, proximal limb ulcers have also been described in UC patients who had been taking NSAIDs 25.
Differential diagnosis of IBD colitis
At first diagnosis, IBD needs to be differentiated from non-IBD colitis which can have similar clinical and histopathological findings. For the differential diagnosis between IBD and non-IBD colitis, we refer the reader to the manuscript entitled “Histopathology of Non-IBD Colitis. A practical approach from the Italian Group for the study of the gastrointestinal tract (GIPAD)”, which is also included in this special issue of Pathologica 6.
KEY POINTS:
A systematic approach in the histopathological evaluation of biopsies is the first step to establish the diagnosis of IBD. The four histological parameters are: mucosal architecture, superficial epithelial findings, epithelial cellular component and lamina propria cellularity;
a combination of specific alterations of each of the four parameters can suggest a diagnosis of UC or CD;
in the early stage, it is important to differentiate IBD from non-IBD colitis;
the pathological report in the diagnosis of IBD requires a detailed description of mucosal alterations as well as a definition of “active” or “inactive” disease and the CMV immunoistochemical assessment;
IBDU is the preferred definition when endoscopic biopsies show no definitive features of either UC or CD.
IBD-associated dysplasia
Patients with IBD carry an increased risk of colorectal cancer (CRC) 53. Dysplasia is the best and most reliable marker of increased risk of malignancy, and it is defined as “histologically unequivocal neoplastic epithelium without evidence of tissue invasion” 54. The identification and grading of dysplasia is the cornerstone of management of IBD 55. Dysplasia related to IBD develops only in areas with chronic inflammation in any part of the colorectum, and is often multifocall 53. In IBD, “flat dysplasia” refers to lesions that are endoscopically undetectable, whereas “elevated dysplasia” refers to endoscopically detectable lesions. Flat dysplasia is generally detected in random biopsies from endoscopically unremarkable mucosa, and carries a high risk for cancer and, in this case, a synchronous adenocarcinoma is found in 42-67% of cases 4,53. Depending on the endoscopic appearance, the elevated lesions/dysplasia are subclassified as “adenoma-like” and “non-adenoma-like”. The latter is termed “colitis-associated dysplasia (CAD)”; the term “dysplasia-associated lesion or mass (DALM)” is no longer recommended 56.
To simplify the matter, we define as ‘‘adenoma-like mass’ (ALM) the condition in which the dysplastic lesion is outside the area affected by the disease, and CAD as a dysplastic lesion localized in the context of the disease 57.
Microscopic features are used to categorize the dysplasia, including both architectural and cytological abnormalities, leading to four distinct categories: negative for dysplasia, indefinite for dysplasia, and positive for low-grade (LGD) or high-grade dysplasia (HGD) 4,53. Conventional (or intestinal type) dysplasia is the more recognized form of dysplasia 53,55 (Fig. 3).
Figure 3.
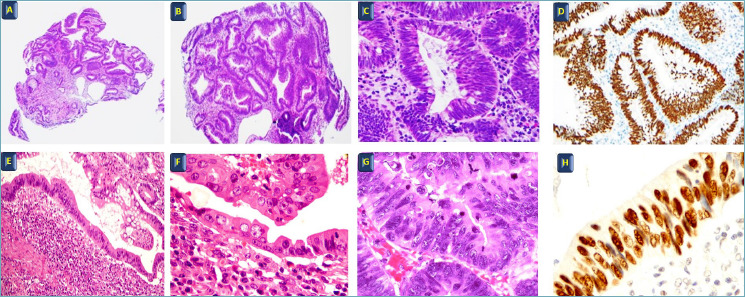
Dysplasia in IBD. (A, B, C, D) Low grade dysplasia A-B-C H&E A: 10x, B: 20x, C: 40x; D: immunostain for P53 20x. (E, F, G, H) High grade dysplasia E-F-G H&E E: 10x, F-G: 40x; H: immunostain for P53 40x.
Cytological alterations include nuclear enlargement with increased nuclear/cytoplasmic ratio, mucin depletion, nuclear localization within the cells, LGD being characterized by nuclei confined to the basal half of the cells and HGD by nuclei distributed haphazardly between the basal and apical halves. With progression from LGD to HGD, the nuclei become markedly enlarged, pleomorphic, hyperchromatic with loss of nuclear polarity, round, and oval-shaped. Mitoses are frequent and, in HGD, sometimes atypical 53-55. Architectural features include abnormal growth pattern including glandular crowding, tubular or villiform architecture, and the absence of normal base-to-surface maturation 53-55. The LGD-HGD transition is characterized by greater architectural complexity, as well as glandular crowding and cribriform or significant papillary architecture. In crypt dysplasia, the dysplasia is limited to the crypts and does not extend to the surface epithelium 53. The indefinite for dysplasia category is appropriate for cases in which a definite distinction between non-neoplastic changes and dysplasia cannot be made, for deep and active inflammation. Indeed, regenerating epithelium in active IBD often shows changes overlapping with those observed in true dysplasia, e.g. enlarged and variably sized nuclei, nuclear hyperchromasia, stratification and pleomorphism, prominent nucleoli, and an increased number of mitoses 53-55. One of the hallmarks of a regenerative process is the maturation of epithelial cell nuclei towards the luminal surface in contrast with dysplasia, which extends uniformly along the crypt axis and surface epithelium with little or no surface maturation 53. Confirmation of dysplasia by an expert gastrointestinal pathologist has been recommended, and even the problem of poor reproducibility persists even among experts 4,53,55. p53 immunostain overexpression may help in distinguishing dysplasia from regenerative alterations. The “all or nothing” concept means that dysplastic epithelium should stain either strongly positive (due to impaired protein degradation) or completely negative (due to protein truncation); however, this is currently a very controversial issue 53,58.
In addition to the adenomatous lesions, even serrated lesions can occur in IBD patients. These are characterized by a saw-toothed glandular contour and usually lack classic cytologic dysplasia, and include sessile serrated lesion without cytological dysplasia (SSL) and traditional serrated adenoma (TSA). Their prevalence, anatomic distribution and rate of cytologic dysplasia development are similar to general population 59. However, other forms of serrated lesions that do not meet the histomorphological criteria of SSP or TSA have been frequently observed in patients with IBD, and their association with IBD-CRC has been noticed 60. Finally, several less common non-adenomatous and non-serrated epithelial changes/lesions have been increasingly found prior or adjacent to IBD-CRC, and have been considered to be putative neoplastic changes and likely variants of CAD, including villous hypermucinous, goblet cell-depleted or eosinophilic changes/lesions, in which no frank adenomatous cytologic dysplasia is appreciated 61. The latest WHO classification of Tumours of the Digestive System briefly mentions an IBD-associated dysplastic lesions classification with seven subtypes: (1) conventional adenoma-like (intestinal, see above), (2) hypermucinous, (3) SSP-like, (4) TSA-like, (5) dysplasia with terminal epithelial differentiation, (6) goblet cell deficient/depleted and (7) serrated, NOS 54. Each of these categories has a distinct morphological and biological profile and has been shown to be associated with a distinct CRC subtype 62. However, there is no clinical utility to further subtype IBD-associated dysplasia or IBD-associated CRC 55.
IBD-associated adenocarcinoma
Compared with the general population, IBD patients are at increased risk of developing some cancers, particularly intestinal and biliary tract adenocarcinoma 53,63. Chronic inflammation is believed to promote carcinogenesis, also in the extra-intestinal sites and with non-epithelial neoplasms as lymphomas, acute myeloid leukemia, myelodisplastic syndromes, skin cancers, and urinary tract cancers, thought to be at least partly imputable to immunosuppressive therapies 53. CRC is the most frequent neoplasm in both UC and CD, arising through dysplasia. The most important risk factor is duration of disease, with the incidence of CRC being relatively rare within the first 8 years of diagnosis. Minor risk factors are age at disease onset, extent, severity of disease, inflammatory complications, family history of CRC and associated primary sclerosing cholangitis 63. Compared with sporadic CRC, IBD-related CRC has several clinical and pathological features: it arises on average 20 years earlier, and in CD tends to have a more proximal distribution, while in UC it shows a predominance of sigmoid and right colonic localization. Moreover, cancers complicating CD are often detected in bypassed or excluded bowel segments. From a morphological point of view, IBD-associated CRC develops from more polymorphous dysplastic lesions than sporadic CRC, and is often multifocal. IBD-associated CRC is characterized by Crohn’s like inflammatory reaction, tumour heterogeneity, presence of mucin and signet ring cell differentiation, and well-differentiated tumours and lack of tumor necrosis 53. The molecular pathway in IBD-related CRC involves alterations in key regulatory genes in the intestinal epithelium that are also found in sporadic CRC; however, the timing of these changes is different, because of the chronic inflammation that characterizes long-standing IBD 63. Unlike sporadic CRC, loss of p53 tumor suppressor function and microsatellite instability (MSI) are early events in IBD-associated CRC, while adenomatous polyposis coli (APC) loss of function is less frequent and appears later 63. Metaplastic changes showing gastric and/or pancreatobiliary immunophenotype have frequently been observed in dysplastic or non-dysplastic mucosa adjacent to CD-associated carcinomas, suggesting a precancerous role of such lesions 64. Finally, Epstein-Barr virus (EBV)-positive carcinomas have been described in the ileum of CD patients and are characterized by increased tumor-infiltrating lymphocytes (TILs), absence of MSI, and a better prognosis compared to sporadic cases 65.
KEY POINTS:
IBD is associated with an increased risk of neoplasia, most frequently CRC, of which dysplasia is the best and most reliable marker;
conventional/intestinal type dysplasia is the more recognized form of dysplasia and it is subclassified into indefinite for dysplasia, LGD and HGD;
the key features of LGD are: (i) abrupt transition with a distinct area of glands that demonstrate a lack of surface maturation, (ii) mucin depletion, (iii) nuclear enlargement and hyperchromasia, and (iv) increase in mitosis;
the key features of HGD are: (i) greater architectural complexity, (ii) full-thickness nuclear stratification with loss of nuclear polarity, (iii) deep nuclear enlargement and pleomorphism and (iv) atypical mitoses;
CRC in IBD patients has peculiar histological and molecular features compared to sporadic CRC.
Pathological report
The pathology report should give an indication of disease localization (i.e. specify the intestinal side of disease localization) and activity, as reflected by the extent of neutrophil granulocyte infiltration and epithelial damage 2,4. In cases of UC, a distinction should be made between inactive disease and active disease; and the latter should be graded 2,29,40,41. In CD, there is less evidence supporting the validity of the histological grading of the disease activity, but data from drug trials indicate that patients showing mucosal healing have a better outcome 53. In CD, inactivity in a biopsy may not reflect inactivity of IBD because of the discontinuous and transmural character of the disease, which induces sampling error, and also because, as a rule, biopsy samples of the ileum are limited, whereas the ileum may be the only bowel segment involved 3. Presence/absence of both dysplasia and CMV inclusions needs to be reported 2.
In summary, it is important to not forget that gastrointestinal biopsies provide critical information for patient management. The pathologist not only assesses current biopsies but, in difficult cases, reviews all available biopsies at different time points and takes into account the clinical information and even the possibility of a second opinion from expert GI pathologists. The responsibility of the pathologist is to provide the optimum pathology report on these biopsies. Ideally a pathology report should give a diagnosis, not merely a description of the histologic findings 66.
Conclusion
Diagnosis of UC and CD requires an integrated clinical and pathological approach. Deep knowledge of histopathological lesions, differential diagnoses and dysplasia recognition are imperative in the daily pathological practice for the management of IBD patients.
Figures and tables
Acknowledgments
We are grateful to Ms. Chiara Di Giorgio (Scientific Direction of Foundation IRCCS Ospedale Casa Sollievo della Sofferenza, San Giovanni Rotondo) for proofreading the manuscript and to Dr. Stefano Zannella (Ospedale S. Anna ASST Lariana Como) for the kind permission to use some histological pictures of dysplasia in IBD. Special thanks to AITIC (Italian Association of Laboratory Technicians) for scientific support regarding methodology and handling of biopsy samples.
References
- 1.Loddenkemper C. Diagnostic standards in the pathology of inflammatory bowel disease. Dig Dis 2009;27:576-83. https://doi.org/10.1159/000233301 10.1159/000233301 [DOI] [PubMed] [Google Scholar]
- 2.Magro F, Doherty G, Peyrin-Biroulet L, et al. ECCO position paper: harmonisation of the approach to ulcerative colitis histopathology. J Crohns Colitis 2020;6:jjaa110. https://doi.org/10.1093/ecco-jcc/jjaa110 10.1093/ecco-jcc/jjaa110 [DOI] [PubMed] [Google Scholar]
- 3.Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers 2020;6:22. https://doi.org/10.1038/s41572-020-0156-2 10.1038/s41572-020-0156-2 [DOI] [PubMed] [Google Scholar]
- 4.Langner C, Magro F, Driessen A, et al. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Arch 2014;464:511-27. https://doi.org/10.1007/s00428-014-1543-4 10.1007/s00428-014-1543-4 [DOI] [PubMed] [Google Scholar]
- 5.Macaluso FS, Orlando A, Bassotti G, et al. How clinicians and pathologists interact concerning inflammatory bowel disease in Italy: an IG-IBD survey. Dig Liver Dis 2018;50:734-6. https://doi.org/10.1016/j.dld.2018.03.020 10.1016/j.dld.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 6.Villanacci V, Reggiani-Bonetti L, Leoncini G, et al. Histopathology of Non-IBD colitis. A practical approach from the Italian Group for the study of the gastrointestinal tract (GIPAD). Pathologica 2021;113:54-65. https://doi.org/10.32074/1591-951X-234 10.32074/1591-951X-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;23;390(10114):2769-78. https://doi.org/10.1016/S0140-6736(17)32448-0 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 8.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979-86. https://doi.org/10.1038/ng.3359 10.1038/ng.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42. https://doi.org/10.1053/j.gastro.2011.10.001 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 10.Halme L, Paavola-Sakki P, Turunen U, et al. Family and twin studies in inflammatory bowel disease. World J Gastroenterol 2006;12:3668-72. https://doi.org/10.3748/wjg.v12.i23.3668 10.3748/wjg.v12.i23.3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornish JA, Tan E, Simillis C, et al. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol 2008;103:2394-400. https://doi.org/10.1111/j.1572-0241.2008.02064.x 10.1111/j.1572-0241.2008.02064.x [DOI] [PubMed] [Google Scholar]
- 12.Piovani D, Danese S, Peyrin-Biroulet L, et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 2019;157:647-659.e4. https://doi.org/10.1053/j.gastro.2019.04.016 10.1053/j.gastro.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 13.Halfvarson J, Bodin L, Tysk C, et al. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 2003;124:1767-73. https://doi.org/10.1016/s0016-5085(03)00385-8 10.1016/s0016-5085(03)00385-8 [DOI] [PubMed] [Google Scholar]
- 14.Serra EG, Schwerd T, Moutsianas L, et al. Somatic mosaicism and common genetic variation contribute to the risk of very-early-onset inflammatory bowel disease. Nat Commun 2020;21;11:995. https://doi.org/10.1038/s41467-019-14275-y 10.1038/s41467-019-14275-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlig HH, Booth C. A spectrum of genetic variants contributes to immune defects and pathogenesis of inflammatory bowel diseases. Gastroenterology 2018;154:2022-4. https://doi.org/10.1053/j.gastro.2018.05.001 10.1053/j.gastro.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 16.Ouahed J, Spencer E, Kotlarz D, et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm Bowel Dis 2020;12;26:820-42. https://doi.org/10.1093/ibd/izz259 10.1093/ibd/izz259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology 2004;126:1518-32. https://doi.org/10.1053/j.gastro.2004.02.072 10.1053/j.gastro.2004.02.072 [DOI] [PubMed] [Google Scholar]
- 18.Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2016;10:239-54. https://doi.org/10.1093/ecco-jcc/jjv213 10.1093/ecco-jcc/jjv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vavricka SR, Gubler M, Gantenbein C, et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD Cohort Study. Inflamm Bowel Dis 2017;23:1174-81. 10.1097/MIB.0000000000001109 10.1097/MIB.0000000000001109 [DOI] [PubMed] [Google Scholar]
- 20.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 2011;106:110-9. https://doi.org/10.1038/ajg.2010.343 10.1038/ajg.2010.343 [DOI] [PubMed] [Google Scholar]
- 21.Greuter T, Navarini A, Vavricka SR. Skin manifestations of inflammatory bowel disease. Clin Rev Allergy Immunol 2017;53:413-27. https://doi.org/10.1007/s12016-017-8617-4 10.1007/s12016-017-8617-4 [DOI] [PubMed] [Google Scholar]
- 22.Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut 2020. 63:567-77. https://doi.org/10.1136/gutjnl-2012-302853 10.1136/gutjnl-2012-302853 [DOI] [PubMed] [Google Scholar]
- 23.Mitsuyama K, Niwa M, Takedatsu H, et al. Antibody markers in the diagnosis of inflammatory bowel diseas. World J Gastroenterol 2016;22:1304-10. https://doi.org/10.3748/wjg.v22.i3.1304 10.3748/wjg.v22.i3.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farmer M, Petras RE, Hunt LE, et al. The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am J Gastroenterol 2020;95:3184-8. https://doi.org/10.1111/j.1572-0241.2000.03199.x 10.1111/j.1572-0241.2000.03199.x [DOI] [PubMed] [Google Scholar]
- 25.Yantiss RK, Odze RD. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology 2006;48:116-32. https://doi.org/10.1111/j.1365-2559.2005.02248.x 10.1111/j.1365-2559.2005.02248.x [DOI] [PubMed] [Google Scholar]
- 26.Ladefoged K, Munck LK, Jorgensen F, et al. Skip inflammation of the appendiceal orifice: a prospective endoscopic study. Scand J Gastroenterol 2005;40:1192-6. https://doi.org/10.1080/00365520510023305 10.1080/00365520510023305 [DOI] [PubMed] [Google Scholar]
- 27.Kuriyama M, Kato J, Morimoto N, et al. Specific gastroduodenoscopic findings in Crohn’s disease: comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig Liver Dis 2008;40,468-75. https://doi.org/10.1016/j.dld.2008.01.008 10.1016/j.dld.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 28.Anthony A, Dhillon AP, Pounder RE, et al. Ulceration of the ileum in Crohn’s disease: correlation with vascular anatomy. J Clin Pathol 1997;50:1013-7. https://doi.org/10.1136/jcp.50.12.1013 10.1136/jcp.50.12.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornaggia M, Leutner M, Mescoli C, et al. Chronic idiopathic inflammatory bowel diseases: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S293-303. https://doi.org/10.1016/S1590-8658(11)60585-9 10.1016/S1590-8658(11)60585-9 [DOI] [PubMed] [Google Scholar]
- 30.Villanacci V, Reggiani-Bonetti L, Caprioli F, et al. Histopathology of inflammatory bowel disease - Position statement of the Pathologists of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) and Italian Group of Gastrointestinal Pathologists (GIPAD-SIAPEC). Dig Liver Dis 2020;52:262-7. https://doi.org/10.1016/j.dld.2019.11.005 10.1016/j.dld.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 31.Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827-51. https://doi.org/10.1016/j.crohns.2013.06.001 10.1016/j.crohns.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 32.Yantiss RK, Odze RD. Optimal approach to obtaining mucosal biopsies for assessment of inflammatory disorders of the gastrointestinal tract. Am J Gastroenterol 2009;104:774-83. https://doi.org/10.1038/ajg.2008.108 10.1038/ajg.2008.108 [DOI] [PubMed] [Google Scholar]
- 33. CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohn’s Colitis 2018;12:1139-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. S, Yantiss RK, Baker SP, et al. Normal variation in intraepithelial lymphocytes of the terminal ileum. Am J Clin Pathol 2007;127:816-9. [DOI] [PubMed] [Google Scholar]
- 35.Canavese G, Villanacci V, Antonelli E, et al. Eosinophilia - associated basal plasmacytosis: an early and sensitive histologic feature of inflammatory bowel disease. APMIS 2017;125:179-83. https://doi.org/10.1111/apm.12639 10.1111/apm.12639 [DOI] [PubMed] [Google Scholar]
- 36. P, Patsiaoura K, Nakos A, et al. Severe eosinophilic infiltration in colonic biopsies predicts patients with ulcerative colitis not responding to medical therapy. Colorectal dis 2014;16:O420-30. [DOI] [PubMed] [Google Scholar]
- 37.Leoncini G, Villanacci V, Marin MG, et al. Colonic hypereosinophilia in ulcerative colitis may help to predict the failure of steroid therapy. Tech Coloproctol 2018;22:941-6. [DOI] [PubMed] [Google Scholar]
- 38.Villanacci V, Cadei M, Lanzarotto F, et al. Localization of TNF alpha in ileocolonic biopsies of patients with inflammatory bowel disease. Ann Diagn Pathol 2019;38:20-25. https://doi.org/10.1016/j.anndiagpath.2018.10.011 10.1016/j.anndiagpath.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 39.Canavese G, Villanacci V, Sapino A, et al. The diagnosis of inflammatory bowel disease is often unsupported in clinical practice. Dig Liver Dis 2015;47:20-3. https://doi.org/10.1016/j.dld.2014.09.007 10.1016/j.dld.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Magro F, Lopes J, Borralho P, et al. Comparison of the nancy index with continuous geboes score: histological remission and response in ulcerative colitis. J Crohns Colitis 2020;14:1021-5. https://doi.org/10.1093/ecco-jcc/jjaa010 10.1093/ecco-jcc/jjaa010 [DOI] [PubMed] [Google Scholar]
- 41.Villanacci V, Antonelli E, Lanzarotto F, et al. Usefulness of different pathological scores to assess healing of the mucosa in inflammatory bowel diseases: a real life study. Sci Rep 2017;7:6839. https://doi.org/10.1038/s41598-017-0733 10.1038/s41598-017-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. S, Boucher AL, Joubert J, et al. Myenteric plexitis is a risk factor for endoscopic and clinical postoperative recurrenceafter ileocolonic resection in Crohn’s disease. Dig Liver Dis 2016;48:753-8. [DOI] [PubMed] [Google Scholar]
- 43.Damen GM, van Krieken JH, Hoppenreijs E, et al. Overlap, common features, and essential differences in pediatric granulomatous inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2010;51:690-7. [DOI] [PubMed] [Google Scholar]
- 44.James SD, Wise PE, Zuluaga-Toro T, et al. Identification of pathological features associated with “ulcerative colitis-like” Crohn’s disease. World J Gastroenterol 2014;20:13139-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018;113:481-517. [DOI] [PubMed] [Google Scholar]
- 46.Gasche C, Sholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 2000;6:8-15. [DOI] [PubMed] [Google Scholar]
- 47.Sankey EA, Dhillon AP, Anthony A, et al. Early mucosal changes in Crohn’s disease. Gut 1993;34:375-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villanacci V, Bassotti G. Histopathological findings of extra-ileal manifestations at initial diagnosis of Crohn’s disease-related ileitis. Virchows Arch 2017;470:595-6. [DOI] [PubMed] [Google Scholar]
- 49.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:414-22.e5. https://doi.org/10.1016/j.cgh.2013.06.019 10.1016/j.cgh.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 50.McCurdy JD, Enders FT, Jones A, et al. Detection of cytomegalovirus in patients with inflammatory bowel disease: where to biopsy and how many biopsies? Inflamm Bowel Dis 2015;21:2833-8. https://doi.org/10.1097/MIB.0000000000000556 10.1097/MIB.0000000000000556 [DOI] [PubMed] [Google Scholar]
- 51.Park SC, Jeen YM, Jeen YT. Approach to cytomegalovirus infections in patients with ulcerative colitis. Korean J Intern Med 2017;32:383-92. https://doi.org/10.3904/kjim.2017.087 10.3904/kjim.2017.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonelli E, Baldoni M, Giovenali P, et al. Intestinal superinfections in patients with inflammatory bowel diseases. J Crohns Colitis 2012;6:154-9. https://doi.org/10.1016/j.crohns.2011.07.012 10.1016/j.crohns.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 53.Svrcek M, Borralho Nunes P, Villanacci V, et al. Clinicopathological and molecular specificities of inflammatory bowel disease-related colorectal neoplastic lesions: the role of inflammation. J Crohns Colitis 2018. 28;12:1486-98. https://doi.org/10.1093/ecco-jcc/jjy132 10.1093/ecco-jcc/jjy132 [DOI] [PubMed] [Google Scholar]
- 54.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of tumours of the digestive system. 5th ed. Lyon: IARC press; 2019. [Google Scholar]
- 55.Karamchandani MD, Zhang Q, Liao XY, et al. Inflammatory bowel disease- and Barrett’s esophagus-associated neoplasia: the old, the new, and the persistent struggles. Gastroenterol Rep(Oxf) 2019,13;7:379-95. https://doi.org/10.1093/gastro/goz032 10.1093/gastro/goz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu K, Riddell RH, Schaeffer DF. DALM, rest in peace: a pathologist’s perspective on dysplasia in inflammatory bowel disease in the post-DALM era. Mod Pathol 2018;31:1180-90. https://doi.org/10.1038/s41379-018-0068-9 10.1038/s41379-018-0068-9 [DOI] [PubMed] [Google Scholar]
- 57.Leoncini G, Donato F, Reggiani-Bonetti L, et al. Diagnostic interobserver variability in Crohn’s disease-and ulcerative colitis-associated dysplasia: a multicenter digital survey from the IG-IBD Pathologists Group. Tech Coloproctol 2020:1-8. [DOI] [PubMed] [Google Scholar]
- 58.Neumann H, Vieth M, Langner C, et al. Cancer risk in IBD: how to diagnose and how to manage DALM and ALM. World J Gastroenterol 2011;17:3184-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko HM, Harpaz N, McBride RB, et al. Serrated colorectal polyps in inflammatory bowel disease. Mod Pathol 2015;28:1584-93. https://doi.org/10.1038/modpathol.2015.111 10.1038/modpathol.2015.111 [DOI] [PubMed] [Google Scholar]
- 60.Shen J, Gibson JA, Schulte S, et al. Clinical, pathologic, and outcome study of hyperplastic and sessile serrated polyps in inflammatory bowel disease. Hum Pathol 2015;46:1548-56. https://doi.org/10.1016/j.humpath.2015.06.019 10.1016/j.humpath.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 61.Gui X, Köbel M, Ferraz JP, et al. Histological and molecular diversity and heterogeneity of precancerous lesions associated with inflammatory bowel diseases. J Clin Pathol 2020;73:391-402. https://doi.org/10.1136/jclinpath-2019-206247 10.1136/jclinpath-2019-206247 [DOI] [PubMed] [Google Scholar]
- 62.Choi WT, Yozu M, Miller GC, et al. Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: a multicenter clinicopathologic study. Mod Pathol 2020;33:933-43. https://doi.org/10.1038/s41379-019-0419-1 10.1038/s41379-019-0419-1 [DOI] [PubMed] [Google Scholar]
- 63.Nebbia M, Yassin NA, Spinelli A. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg 2020;33:305-17. https://doi.org/10.1055/s-0040-1713748 10.1055/s-0040-1713748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vanoli A, Di Sabatino A, Martino A, et al. Small bowel carcinomas in celiac or Crohn’s disease: distinctive histophenotypic, molecular and histogenetic pattern. Mod Pathol 2017;30:1453-66. https://doi.org/10.1038/modpathol.2017.40 10.1038/modpathol.2017.40 [DOI] [PubMed] [Google Scholar]
- 65.Vanoli A, Di Sabatino A, Martino A, et al. Epstein-Barr virus-positive ileal carcinomas associated with Crohn’s disease Virchows Arch 2017;471:549-52. https://doi.org/10.1007/s00428-017-2209-9 10.1007/s00428-017-2209-9 [DOI] [PubMed] [Google Scholar]
- 66.Rutgeerts P. The perfect pathologists for IBD. An endoscopist’s vision. Belgian Week of Gastroenterology; 2010. [Google Scholar]


