Abstract
Objectives:
In recent decades, the diagnostic and therapeutic implications of the microbiome changes and the impact of probiotic supplementation have increased rapidly. However, the potential for clinical translation of microbiome research for children and adolescents with psychiatric disorders is unclear. This review examined available evidence related to gut microbiota as well as the impact of probiotic supplementation on psychiatric disorders in the pediatric population reported to date.
Methods:
We performed a literature search for the gut microbiota in child and adolescent population (0–18 years old) with mental health disorders from July 1999 through July 2019 in several databases: ClinicalTrials.gov, Ovid EBM Reviews, Ovid Embase, Ovid Medline, Ovid PsycINFO, Scopus, and Web of Science.
Results:
A total of 7 studies met inclusion criteria consisting of randomized controlled trials and cohort studies that examined various associations between psychiatric disorders and gut microbiota in youth. Six studies examined the effects of various treatment interventions such as probiotic supplementation on microbiota composition and behaviors. One study showed an increase in prosocial behavior in children with Autism Spectrum Disorder (ASD) and an increase in the Lachnospiraceae family following prebiotic supplementation. Another study suggested that prebiotic supplementation increased bifidobacterial populations for ASD and healthy controls. A study evaluating infant supplementation of prebiotics showed both a decreased likelihood of developing Attention Deficit Hyperactivity Disorder (ADHD) or ASD and decreased gut Bifidobacterium. One study did not find significant differences in microbiome composition after micronutrient treatment.
Conclusion:
The main goal of this systematic review was to comprehensively examine and summarize the current evidence focused on the potential effect of the relationship between microbiota gut composition as well as the effects of probiotic supplementation on psychiatric disorders in children and adolescents. This is a relatively new area of research and the number of included studies is limited. More studies are needed to determine whether gut dysbiosis leads to the development and/or contributes to the severity of mental disorders or whether gut dysbiosis is a result of other processes that accompany mental disorders.
Clinical significance:
A better understanding of the specific bacteria contributions, gut-brain pathways, and role in pathophysiological mechanisms in neuropsychiatric disorders in the child and adolescent populations can possibly provide alternative tools for a clinical psychiatrist. Moreover, it may ultimately aid the clinician with intervention strategies, or detect populations at risk for developing neuropsychiatric disorders.
Keywords: Probiotic supplementation, Gut microbiota, Infants, Teenagers, Children, Young adults, Autism, Depression, ADHD, Anxiety, Phobia, Neuropsychiatric disorder, Mental health
1. Introduction
Recently, the number of putative diagnostic and therapeutic implications of the gut microbiome has increased rapidly. Initially, the microorganisms that colonize humans were estimated to outnumber human cells by a factor of ten (Turnbaugh et al., 2007). However, Sender and colleagues published an updated analysis that reported bacteria colonization in the body is the same as the number of human cells, approximately 0.2 kg of the total mass (Sender et al., 2016). The microbiome has become an area of focus for research in the recent decade. Modern laboratory tools have helped facilitate microbiome research focused on developing insights into the microbiome’s interaction with the human body and brain in particular. Prior work has established that the gut microbiome likely has a key role in metabolism, immune defense, and behavior (Cresci and Bawden, 2015).
The phrase gut-brain-axis was established to describe the bidirectional processing of signals from the central nervous system (CNS) and the gut (Cryan and Dinan, 2012). This bidirectional association between the CNS and the gastrointestinal system relies on communication through neural, endocrine, and inflammatory mechanistic pathways (Martin et al., 2018). The CNS can impact the gut microbiome in the metabolic state through defined microbial-derived intermediates such as short-chain fatty acids (SCFAs), secondary bile acids (2BAs), and tryptophan metabolites (Tolhurst et al., 2012) (Islam et al., 2011; Tolhurst et al., 2012). Animal based studies and preclinical studies showed that the vagus nerve plays a crucial role in connecting microbiome in the gut with CNS. The gut microbiome is responsible for the regulation of major neurotransmitters such as serotonin (5HT) via alteration of plasma tryptophan levels.
Previous studies suggest that several factors have been identified that affect microbiome composition including preterm birth (Brennan et al., 2019), diet (Harmsen et al., 2000), obesity (Tun et al., 2018) probiotic/prebiotic use (Bagga et al., 2018; Umu et al., 2017), antibiotic exposure (Zou et al., 2018), vitamin A/D supplementation (Huda et al., 2019; Sordillo et al., 2017), allergies (Low et al., 2017), birthing method (Tun et al., 2018), geographic location (Boix-Amorós et al., 2019), and smoking (Huang and Shi, 2019).Moreover, the microbial imbalance called dysbiosis may change depending on the age and types of nutrients from which microbes extract energy, starting from infancy when microbes obtain energy from milk components, through childhood when solid foods are introduced. In females gut composition changes are also under the influence of cyclic hormones (Davenport et al., 2017). However, as highlighted by Taylor, there is limited understanding about what microbial profile is clearly associated with wellness (Taylor, 2019).
There is evidence that the gut microbiome influences the neurobiological underpinnings of psychiatric disorders (Anglin et al., 2015). The gut microbiome has been correlated with a substantial number of human psychiatric disorders including autism spectrum disorder (ASD) and depression (Knight et al., 2017). Gut microbiome are responsible for synthesis of other neurotransmitters associated with psychiatric disorders such as GABA (gamma-aminobutric acid), noradrenaline, dopamine and acetylcholine (Desbonnet et al., 2010; Lyte, 2013, 2014). The prospect of microbiome biomarkers for use in child and adolescent psychiatry also has practical appeal given that sample collection is noninvasive.
The findings of the influence of the gut microbiome on psychiatric disorders has sparked interest in evaluating the impact of dietary supplements and psychotropic medications on the microbiome and health outcomes. Dietary supplement such as probiotics and probiotic fermented foods, omega-3 fatty acids, vitamin D, magnesium, and zinc supplementation have been studied and demonstrated benefits (Simkin, 2019).Psychotropic medications such as neuroleptics are known to increase the risk of weight gain and are associated with altering the gut microbiome (Chen et al., 2020). New studies are trying to address ways to mitigate weight gain related to neuroleptics medication use. Animal research shows promising data suggesting that pre-treatments with betahistidine as well as combination of antibiotics (neomycin, metronidazole and polymyxin) might slow weight gain in patients on neuroleptic medications (Chen et al., 2020). Changing the microbiome using dietary supplements or psychotropic medications may impact side effects of medications and psychiatric disorder outcomes.
Previous research demonstrated various experimental approaches to explore the role of gut microbiome and potentially manipulate the microbiome to impact psychiatric health outcomes. As the evidence base for the microbiome’s role in psychiatric disorders develops, there are many potential clinical implications. We sought to systematically review the current evidence base regarding human gut microbiota and its role in neuropsychiatric disorders.
2. Methods and materials
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Liberati et al., 2009).
2.1. Literature search strategy
The search strategy was designed and conducted by a medical reference librarian with input from the study authors for the concepts of gut microbiota in child and adolescent psychiatry. The search strategies were created using a combination of keywords and standardized index terms. Searches were run in July 2019 in ClinicalTrials.gov, Ovid EBM Reviews, Ovid Embase (1974+), Ovid Medline (1946+ including epub ahead of print, in-process & other non-indexed citations), Ovid PsycINFO (1806+), Scopus (1970+) and Web of Science (1975+). Results were limited to English language from 1999 to 2019. All results were exported to Endnote where 970 obvious duplicates were removed leaving 1563 citations. Search strategies and keywords are provided in the appendix.
2.2. Selection criteria
Studies were eligible if they a) included human gut microbiota as an outcome and individuals with anxiety, depression, psychosis, autism spectrum disorder (ASD), obsessive-compulsive disorder (OCD), anorexia nervosa, b) studies were limited based on the participants age 0–18 years c) only randomized-controlled trials and cohort studies were included d) only publications available in English were included, e) studies examining microbiota in both children and adults were included only if outcomes were investigated independently. Papers that did not address human gut microbiota as an outcome, pre-clinical studies and studies in humans above 18 years old, case reports, systematic reviews, narrative review, commentaries, editorials, conference abstracts were excluded.
2.3. Data extraction
Two reviewers independently [A.N.L., A.I⋅S] screened the titles and abstract in order to evaluate study eligibility. Conflicts of opinion were resolved through consensus with another author [M.R]. Full text screening and data extraction were completed by two reviewers [A.N.L., P.M.C.]. The agreement between two reviewers was good (Kappa = 0.73). Two reviewers [A.N.L., P.M.C.] independently identified, extracted and synthesized the data. Third reviewer [M.R.] was consulted when necessary.
2.4. Risk of bias analysis
Risk of bias was determined by two reviewers independently [A.N.L., A.I.S.] for the cohort and randomized control studies following Cochrane risk of bias tool (Higgins et al., 2011). A risk of bias graphs and summary figure were generated by using the Cochrane Software Review Manager (RevMan, 2014).
3. Results
Systematic review identified 2503 articles for screening. 7 studies met our inclusion criteria. 5 of them were randomized controlled trials (Partty et al., 2015; Stevens et al., 2019; Grimaldi et al., 2018; Parracho et al., 2010; Sanctuary et al., 2019) and 2 of them were cohort studies (; Grimaldi et al., 2017; Bahr et al., 2015). The review included randomized controlled trials and cohort studies that investigated various associations between mental health disorders and gut microbiota composition (Fig. 1). Overall, there were 184 participants reported on in included studies who provided stool samples. The results of the risk of bias assessment are available in supplementary materials.
Fig. 1.
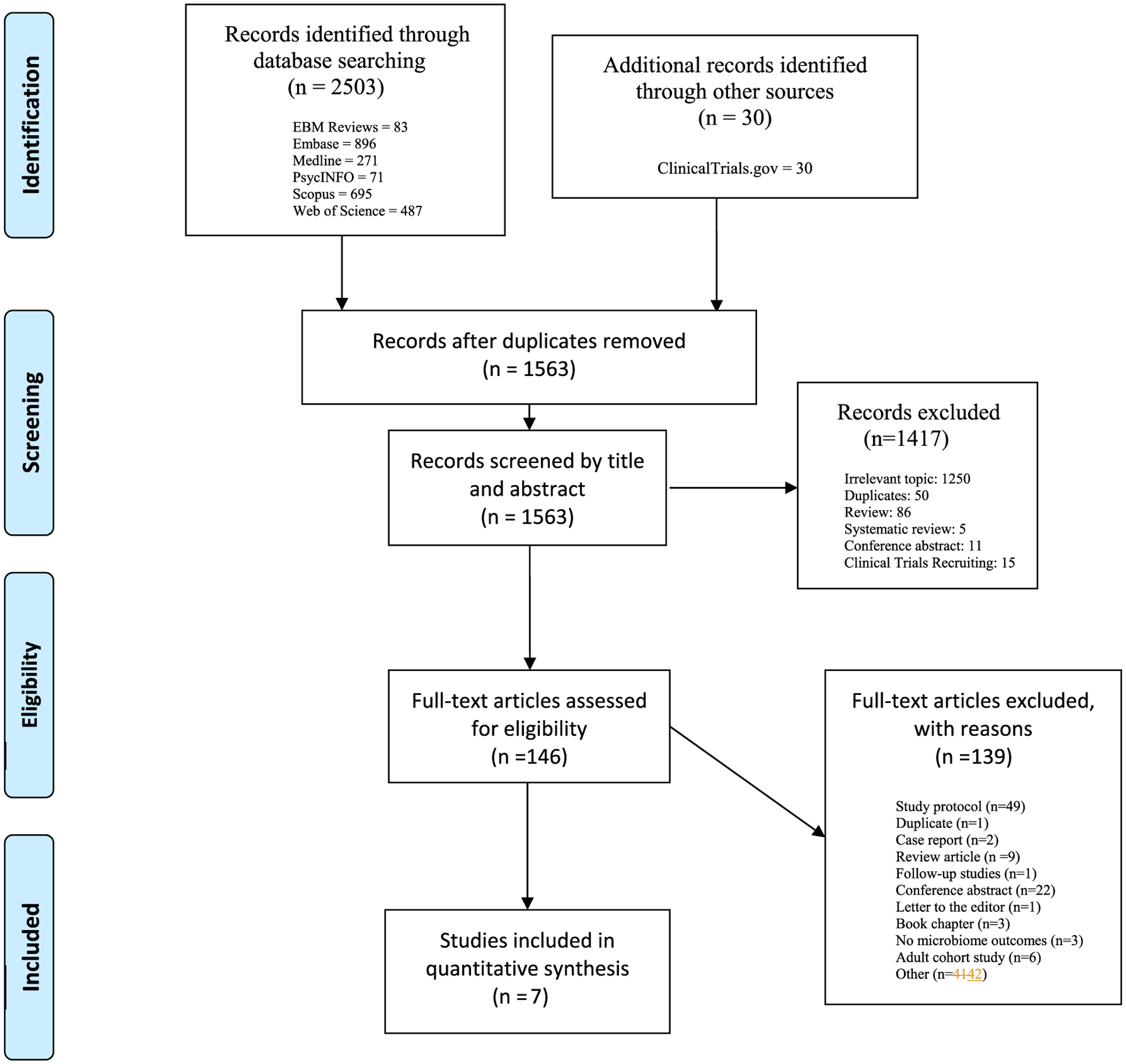
PRISMA flow diagram illustrating the systematic search procedure.
3.1. ADHD and microbiome
Two studies measured the microbiome composition and the manifestation of ADHD (Attention Deficit Hyperactivity Disorder) (Partty et al., 2015; Stevens et al., 2019). Partty et al. compared results of the microbiome samples from infants followed up to 13 years old (Partty et al., 2015). In this study, 75 subjects were randomly allocated into two groups: 40 received Lactobacillus rhamnosus GC (probiotic group) and 35 subjects received placebo. Results showed that bacterial numbers (analyzed by FISH) of children with neuropsychiatric disorders was not significantly different during the first 3 months of life as compared to healthy controls. However an assessment by qPCR showed differences in numbers of Bifidobacetrium longum between the groups at the age of 3 mo. Children with diagnosed neuropsychiatric disorders had a significantly lower median number of B. longum than healthy controls. Moreover, only children from the group of participants that received a placebo (6 out 35 participants) instead of probiotic Lactobacillus rhamnosus GG (ATCC 53103) were diagnosed with ADHD or Asperger syndrome (AS) or both by age 13 years. The data suggest that early probiotic supplementation may have an impact on decreasing the risk of ADHD and AS. In another study, Stevens et al. performed the investigation of how micronutrient treatment is associated with the dynamic microbial changes in ADHD diagnosed children (Stevens et al., 2019). The results did not show a significant alteration in the microbiome taxa. Nevertheless, a significant reduction in the abundance of Bifidobacterium genus (gram-positive bacteria that belongs to the Actinobacteria phylum) in post-micronutrients treatment individuals was reported.
3.2. Autism and microbiome
A total of 4 studies were identified about the microbiome and autism spectrum disorder. They consisted of clinical trials evaluating the effect of probiotic/prebiotics on behavior scores or faecal microbial composition. The following interventions were studied: Lactobacillus plantarum (Parracho et al., 2010) Bifidobacterium infantis (Sanctuary et al., 2019), galactooligosaccharide (B-GOS) (Grimaldi et al., 2017) and galactooligosaccharide (B-GOS) +/− exclusion diet (mainly gluten and casein free) (Grimaldi et al., 2018). The summary of studies can be found in Table 1.
Table 1.
Summary of study characteristics and findings.
| Author | Question ID | Population | Health condition | Intervention | Intestinal microbiome outcomes | Mental health outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methodologic differences to analyze gut microbiome | Summary of findings | During or post intervention outcomes | Measure(s) used to assess psychological outcomes | Psychological or neuropsychiatric outcomes | Association between gut microbiome and psychological outcomes | |||||
| Partty et al. (2015) | Is the gut-brain-axis is involved in the manifestation of ADHD and AS? Is there is any association of compositional development of the gut microbiota, the blood group secretor type, and impact of the specific probiotic intervention on ADHD and AS? | Infants followed-up to 13 years old; 40 in the probiotic group and 35 in the placebo group (n = 75). | ADHD and AS | ADHD infants (53.3% received Lactobacillus rhamnosus GG, and 35 (46.7%) placebo during the first 6 mo of life were followed-up for 13 y. Children’s gut microbiota were evaluated at 3wk, 3, 6, 12, 18, 24 mo, and 13 years old. | Fluorescein in situ hybridization (FISH) and qPCR, and indirectly by determining the blood group secretor type at the age of 13 y. | No significant difference in gut microbiota in participants after intervention. | 3 mo affected children had significantly lower median (IQR) with Bifidobacterium longum to compare with healthy children, (P = 0.045). 6 mo diagnosed children the number of Bifidobacteria were lower (P = 0.03). 18 mo the mean of Bacteroides and Lactobacillus-Enterococcus were lower in children with ADHD/AS (P = 0.0008, P = 0.01) 24 mo the numbers of Clostridium histolyticum cells were lower in affected children (P = 0.04). No significant difference in gut microbiota in 13 year old children. |
Parent Assessment of Early Behavior Patterns: crying, sleeping, awake time, content, fussing, colic-type cry, and another cry across the study. | 6 of 35 children who received a placebo had been diagnosed with ADHD (3 male, 4.0%) or AS (1 male 1.3%) or both ADHD and AS (2 male 2.7%). All of them received a placebo, and none of them were in the probiotic group by the age 13 y. Logistic regression was significant when it was controlled for gender (P = 0.02) | At the 6 month age, the SD of Bifidobacterium species in feces was higher in healthy children 9.12 (0.64) log cells/g than in diagnosed children 8.26 (1.24) log cells/g |
| Stevens et al. (2019) | How is the impact of the micronutrient treatment on the relative abundance of microbial diversity in children with ADHD symptoms? | Children between the 7–12 years old; 10 in the treatment group and 7 in the placebo group (n = 17). | ADHD | 18 children diagnosed with ADHD enrolled in the 10-week pilot study: at 2, 4, 6, 8, and 10 weeks (or end of study). 10 children received a micronutrient treatment containing a formulation of vitamins, minerals, amino acids, and antioxidants, and 8 children received a placebo. | 16S rRNA gene sequencing. | Micronutrient treatment did not drive large-scale changes in the composition or structure of the microbiome. Operational Taxonomic Unit’s (OTUs) significantly increased in the treatment group, and showed no meaningful difference in the placebo group. |
Fresh microbiome samples were collected at baseline and ten weeks of study. OTUs significantly increased in the micronutrient children and presented no meaningful change in the placebo children. | Children’s Global Assessment Scale (CGAS)39 and ADHD Rating Scale IV (ADHD-RS-IV) -clinician | – | An overall trend supporting the observation that greater Bifidobacterium associated with a lower ADHD-IV-RS score, excepting post-micronutrient treatment where a low Bifidobacterium abundance was linked with a low ADHD-IV-RS score. Although, there was no significant association with C-GAS and ADHD-RS-IV score and low abundance of bacteria. |
| Grimaldi et al. (2017) | The main goal of this study was to investigate the impact of a prebiotic galactooligosqacchari (B-GOS) on gut microbial ecology metabolic end products of microbial fermentation. The in vitro gut model systems were inoculated with faecal samples. | 3 male children with ASD and 3 non-autistic children (in age 5–10 years old) provided samples used in an in vitro gut model system (n = 6). | ASD | Supplementation of B-GOS to the in vitro gut model systems inoculated with faecal samples from autistic and non-autistic children. | Bacteriology composition analysis was assessed by using flow cytometry with fluorescence in situ hybridization FISH-FCM. Metabolic activity was examined by HPLC and H-NMR. |
B-GOS implication significantly increased the number of Bifidobacterium spp. In autistic and non-autistic models. | The feacal samples from autistic group contained a greater number of Clostridium spp. but lower number of bifidobacteria in comparison to non-autistic models. B-GOS supplementation significaly increased number of bifidobacteria in both groups, and lactobacilli in the final vessel from non-autistic samples. | – | – | – |
| Grimaldi et al. (2018) | Evaluation of the impact of 6-week Bimuno galactooligosaccharide (B-GOS) prebiotic intervention and exclusion diets on children with ASD. | Children in range 4–11 years with formal diagnosis of Autism Spectrum Disorder completed the 10-week study (n = 26). | ASD | Participants (14 with un-restricted diet and 12 with exclusion diet) were divided to group who: I received placebo and II received B-GOS as a prebiotic intervention. | Bacterial enumeration was assessed by FISH analysis, 16sRNA gene amplicon sequencing, and metabolomics analysis by H-NMR to evaluate the urinary and faecal metabolomics profile in the different diet and after 6-weekB-GOS intervention. | After the intervention the number of Bifidobacterium spp. was greater but there was no significant difference after intervention and interactions between treatments and exclusion diet. | Participants with exclusion diet showed the significantly lower number of Bifidobacterium spp. and Veillonellaceae, and greater number of Faecalibacterium prausnitzii, Bacterioides spp. Moreover, in this group the significant correlation between types of bacteria and faecal amino acids has been found to compare with children on unrestricted diet. Group that received the B-GOD prebiotics presented significantly greater number of Lachnospiraceae family and changes in faecal and urine metabolomics profile. |
Anxiety and ASD-related behavior questionnaires: Autism Treatment Evaluation Checklist (ATEC), Autism spectrum quotient (AQ), Empathy and systemising quotient (EQ-SQ), Spence’s Children Anxiety Scale-Parent version SCAS-, parents completed 5-day sleep diaries at baseline and after intervention. | Children with exclusion diet showed the significant lower scores of abdominal pain and bowel movement. Subjects received the B-GOD prebiotics were observed with improvements in anti-social behavior. | – |
| Parracho et al. (2010) | The aims of the study was to asses in children with Autism the effects of the probiotic (L. plantarum WCFS1) on the intestinal microbiota and gut function and to examine the effects on the behavior. | Children between 4 and 16 years of age finished the 12-week feeding study (n = 17). | ASD | A cross over study that included a 3-week placebo-feeding period, followed by a 3 week washed out period and 3 week probiotic feeding period. | Bacterial population levels were examined using fluorescence in situ hybridization (FISH) | No statistically significant sequence effect was observed in the groups. | The probiotic significantly increased numbers of lactobacilli/enterococci and decreased the clostridium cluster in the faecal microbiota of ASD children compared to the placebo. | Development Behavior Checklist (DBC) before the feeding study and at the end of each feed and washout period. | No significant difference was observed in the median scores for the five sub-scales between probiotic and placebo feeding. The baseline median scores were significantly higher (P < 0.05) for the probiotic feeding for disruptive/antisocial behavior, self-absorbed behavior, communication disturbance and anxiety problems. |
The probiotic L. plantarum WCFS1 in ASD population, with modulation of the faecal microbiota observed, had a potential benefit in improved stool consistency and overall behavior scores (compared to baseline). |
| Sanctuary et al. (2019) | Objectives of this study include the assessment of tolerability of combination treatment with probiotic Bifidobacterium infantis with a bovine colostrum product (BCP) to compare BCP alone and assess the impact on GI symptoms, microbiome composition and immune factors in autistic children. | Children with ASD diagnosis and GI symptoms in age 2–11 completed both treatments (n = 8). | ASD | 12-week cross-over study participants received during the first 5 weeks the probiotic B. infantis and prebiotic BCP supplementation, Followed by two-week washout period, and 5 weeks of supplementation only with BCP. | 16S sequencing analysis and metabolomics measurement of faecal sample. | – | No significant differences in treatment effect on microbiota composition. Results suggest that probiotic-probiotic treatment (B. infantis and BCP) vs. only BCP treatment is well-tolerated in the study participants. Some of the participant from both groups reported improvement in chronic GI symptoms. |
Aberrant Behavior Checklist (ABC), the Repetitive Behavior Scale-Revised (RBS-R), and the Adaptive Behavior Assessment System-Second Edition (ABAS-II) | Behavioral assessments: No difference in the adaptive behaviors. Significant decrease score were found in certain aberrant behaviors based on ABS questionnaire as: irritability (P = 0.003), stereotypy (P = 0.006), hyperactivity (P = 0.007), and total scores (P = 0.006 in BCP group, and for probiotic-probiotic group only in lethargy (P = 0.0499). No differences in adaptive behaviors were observed based on the ABAS-II questionnaire or repetitive behaviors based on the RBS-R. |
The absence of global changing of the microbiome indicates there were no relationship between microbiome and outcomes. |
| Bahr et al. (2015) | The aim of this study was to asses if the use of risperidone (RSP) and secondary weight gain is associated with an altered gut microbiota. | Children between 9 and 15 years with chronic RSP treatment. Five new users of RSP and 10 psychiatric controls (n = 18). |
Pediatric psychiatrically ill controls and users of RSP. | Follow up of risperidone taking patients up to 10 months. | 16S rRNA sequencing | Chronic treatment with RSP was associated with an increase in body mass index and a significantly lower ratio of Bacteroidetes: Firmicutes as compared controls (ratio = 0.15 vs 1.24, respectively. P < 0.05) | There was a gradual decrease of the ratio Bacteroidetes: Firmicutes, in association with BMI gain. | – | – | – |
Parracho and colleagues reported a significantly increased number of lactobacilli/enterococci and a significantly reduced Clostridium cluster after receiving the probiotic L. plantarum as compared to placebo in the faecal microbiota of children with ASD (Parracho et al., 2010). Additionally, the authors observed differences in stool consistency. Probiotic feeding resulted in a different consistency of stool samples. The probiotic group has significantly more stool samples classes as” formed” (P < 0.01) and lower “hard” (P < 0.01) with probiotic therapy compared to group taking placebo feeding (64.8%); however, the median scores for behavior scales were not significantly different between probiotic and placebo groups after receiving the probiotic intervention. An important limitation was a very high dropout rate in recruited subjects. The authors highlighted the inherent difficulties (ie. 12 weeks, comprising collection of six faecal samples, completion of detailed questionnaires) of microbiome studies in this population (Parracho et al., 2010).
Sanctuary et al. studied effect of the probiotic Bifidobacterium infantis in combination with bovine colostrum product (BCP), compared with the bovine colostrum alone in children with ASD and their gastrointestinal symptoms (Sanctuary et al., 2019). Their findings were a significant reduction in some aberrant behaviors (irritability, stereotypy, hyperactivity, and total scores) based on the Aberrant Behavior Checklist (ABC) questionnaire during a bovine colostrum product only treatment.All participants reported a reduction of at least one GI symptom after the treatment. A significant reduction in lethargy was observed solely in the combination treatment. No differences in adaptive behaviors were observed based on the Adaptive Behavior Assessment System–Second Edition ABAS-II questionnaire or repetitive behaviors based on the Repetitive Behavior Scale-Revised RBS-R in either group. As to the faecal microbial composition there was no treatment effect on any particular genera. The study did not have a placebo comparison group of healthy controls and the sample size was small (N = 8).
Furthermore, Grimaldi and colleagues assessed the prebiotic galactooligosaccharide (B-GOS) on a gut microbial ecology and metabolic function using faecal samples from children with and without autism in an in vitro gut model system (Grimaldi et al., 2017). They found that after the administration of B-GOS there was a significant increase of bifidobacterial population in both patients with ASD and healthy controls, and a significant increase of lactobacilli in healthy controls.
Finally, another study assessed the impact of exclusion diets and galactooligosaccharide (B-GOS) prebiotic in 30 children with autism (Grimaldi et al., 2018). This study identified several genera present in higher abundance in children with ASD, such as Bacteroides spp, Rikenellaceae, Roseburia spp, F. prausnitzii, and Clostridiaceae measured at baseline in the exclusion diet group. The unrestricted diet group had a higher abundance of Eggerthella lenta, Bifidobacterium spp., B. fragilis, Akkermansia muciphila, Streptococcus anginosus, Lactococcus spp., and Dehalobacterium spp. measured at baseline. There was an increase in the Bifidobacterium after B-GOS intervention, but there was no significant difference between the treatments and the interaction between treatments versus diet groups. Results showed a consistent increase over time in prosocial behavior in children when the combination of the exclusion diet and B-GOS intervention was used. Moreover, they were improvements in anti-social behavior in this group. The authors concluded that the addition of the prebiotic could be more beneficial in the ASD individuals than the exclusion diet alone.
These studies showed inconsistencies in their outcome measurements in the gut microbiome. Overall, all of the studies that assessed changes in behavior scales after probiotic treatment reported some kind of improvement.
3.3. Microbiome and medications
Only one study investigated the effect of psychotropic medications on microbiome (Bahr et al., 2015). The study observed that chronic treatment with risperidone (RSD) was associated with an increased BMI and a lower ratio of Bateriodetes:Firmicutes as compared with controls. In patients who received long term treatment with RSD and who did not experience weight gain, the family of Actinobacteria was the most prevalent.
The study suggested that the alterations of Firmicutes and Actinobacteria phyla may drive the alterations in the gut microbiota in relation to long-term RSD treatment. The difference with alterations in the composition of Formicates and Proteobacteria may be associated to weight gain following long-term RSD treatment. It is also important to note that this research showed the possibility of using Actinobacteria in development of prebiotics/probiotics to prevent or reverse weight gain associated with RSP treatment (Bahr et al., 2015). Desbonnet and colleagues provided the encouraging evidence that probiotic administration showed the influence of tryptophan which suggest that manipulation of microbiota composition might be strategy in modulation of neurotransmitters activity in CNS (Desbonnet et al., 2008). Remarkably, previous preclinical research reported the supports that gut flora alteration might be a determinant in pathophysiology of obesity and is linked to host energy homeostasis (Turnbaugh et al., 2006; Bäckhed et al., 2007).
4. Discussion
This systematic review summarized clinical studies investigating the impact of gut microbiome composition as well as probiotic supplementation through the different developmental periods starting from infancy through the early adulthood. The literature explored the relation between microbial compositions with various mental health disorders. Overall, the investigators suggest variability in the bacterial species associated with mental health disorders. The data from five publications that were included in the review described modulation of the intestinal microbiome using different types of probiotics (L. rhamnosus, bimuno galactooligosaccharide B-GOS, L.plantarum WCFS1, Bifidobacterium infantis) and prebiotic (L. plantarum) (Partty et al., 2015; Grimaldi et al., 2017; Grimaldi et al., 2018; Parracho et al., 2010; Sanctuary et al., 2019). A previous pre-clinical study shed some light on underlying mechanism of action and showed neurochemical alterations in gut microbiome using L. rhamnosus (JB-1) in healthy male mice (Bravo et al., 2011). L. rhamnosus could mediate a direct impact on the GABAergic system, which is thought to be involved in the development of psychiatric symptoms related to stress. Moreover, this study demonstrated that probiotic supplementation may have an association with behavioral and physiological reactions connected to vagus nerve (Bravo et al., 2011).
Anxiety and gastrointestinal problems are frequently comorbid. Animal models are helpful with explaining underlying mechanisms of actions. Most of the studies discuss activation of the vagus nerve in the gut-brain axis. Nevertheless, some studies in animal models are investigating the gut-brain pathways through the vagus nerve by using vagotomy surgery. For instance, Bercik et al. performed a study on animal models with the vagotomy surgery and gastrointestinal inflammation. After the daily administration of probiotic Bifidobacterium longum, they observed a reduction of anxiety-like behavior in mice. The study demonstrated that gut-brain signals are not limited to the mediation of the vagus nerve. The data suggest impact of other biological pathways (Bercik et al., 2014). There were no studies that addressed issues of pediatric mood disorder or psychosis.
Many studies are investigating the effect of microbiome colonization in children with autism. ASD is characterized by a significant heterogeneity of behaviors, medical comorbidities, and cognitive capability, therefore, gastrointestinal (GI) issues can be challenging to identify and study in patients with ASD (Saurman et al., 2020). Sanctuary et al. was able to investigate the impact of supplementation of probiotic Bifidobacterium infantis and prebiotic a bovine colostrum product (BCP) in autistic children and their gastrointestinal symptoms. In his study all participants reported a reduction of at least one GI symptom after the treatment (Sanctuary et al., 2019). Unfortunately the study did not have a placebo comparison group, they also did not have healthy controls which was a limitation of the study. Their sample size was small (N = 8).
One of the included studies also examined the effect of probiotic on the microbiome. The reported results showed that administration of probiotic Lactobacillus plantarum WCSD1 reduced the number of Clostridium cluster XIVa and increased Lactobacilli and enterococci species (Parracho et al., 2010) in children with ASD. Moreover, other previous clinical work demonstrated improvement in oppositional and defiant behaviors in children with ASD after the application of Lactobacillus plantarum PS128 (Liu et al., 2019). Considering the complexity of phenotypes in ASD future studies should focus on identifying the probiotic intervention to ASD phenotype and specific comorbid symptoms.
Prior systematic review by Barbosa and Vieira-Coelho summarized the existing clinical evidence of impact of probiotics and prebiotics use in patients with neuropsychiatric disorders. The study demonstrated that probiotics might offer some benefits for other psychiatric disorders like major depressive disorder and Alzheimer’s disease, but results for ASD were inconsistent (Barbosa and Vieira-Coelho et al., 2020). In contrast to that review our paper specifically focused on pediatric population and psychiatric disorders.
In terms of weight gain associated with neuroleptic medications and its impact on microbiome the study by Bahr and colleagues reported preliminary evidence that in patients chronically treated with RSD that in addition to the weight gain there is also alteration of gut microbiota composition with significantly lower ration of Bacterioidetes:Firmicutes (Bahr et al., 2015). Le Bastard et al. summarized the findings and reported the association between proton pump inhibitors and antipsychotic medications as reducers of α diversity of the gut microbiome (Le Bastard et al., 2018). Davey and Morgan and their colleagues proposed mechanisms for antipsychotics medication pathway and their impact on intestinal microbiome as a directly effecting the growth of organisms of the gut (Davey et al., 2012; Morgan et al., 2014). It is important that we have a better understanding of neuroleptics mechanisms of action as it may lead to improved treatments with potentially less side effects.
Understanding of microbiota could be significant both for prediction of susceptibility for development of psychiatric symptoms and disorders such as ADHD or ASD. In a small study by Partty lower number of Bifidobacteria genus in participants seemed to predispose them to development of ADHD or ASD later in life (Partty et al., 2015). Results of that study have to be taken with caution due to very low numbers, however, the design of the study is interesting and worth replication.
The present review also was able to underscore the need for future studies of the microbiome and mental health disorders in the child and adolescent population. Specifically there is a need for more longitudinal cohort studies that would allow us to dissect the complex relationships between the microbiota, patient, environment, and various psychiatric outcomes. Neuropsychiatric disorders are complex and multifactorial and it is unlikely that there is a direct correlation between them and microbiota. Luckily there have been significant advances in brain imaging, epigenomics as well as metabolomics studies that could help with our understanding of the dynamic interconnections between these system-wide data. The reviewed studies that discuss probiotic supplementation suggest that before moving forward with our research on the microbiome in children and adolescents with neuropsychiatric disorders, we should deepen our understanding of the meaning and impact of microbiome changes on psychiatric disorders.
The present review has a number of limitations that are important to consider in the synthesis and interpretation of findings. The small samples size across all the studies was low and this limited statistical power. There were differences in metrics and high heterogeneity across studies included in the review (Ioannidis et al., 2008). Conversely in general the risk of bias analysis of the studies was favorable. Inclusion/exclusion selection process did not specify the type of microbiota sequencing in this review. 16S rRNA sequencing versus shotgun metagenomics results in markedly different resolution at the species and strain levels and therefore better reliability. Notably strengths include randomization, allocation concealment, double blinding, minimal attrition, and a low risk of reporting bias. Nevertheless, a larger number of randomized-controlled trials and longitudinal cohort studies are urgently needed.
5. Conclusion
This systematic review investigated a link between differences in microbiome composition and neuropsychiatric disorders such as ADHD, ASD, and anxiety disorders in children. This is a relatively new area of research and the number of included studies is limited. More studies are needed to determine whether gut dysbiosis leads to the development and/or contributes to the severity of mental disorders or whether gut dysbiosis is a result of other processes that accompany mental disorders.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the determinations of Mayo Clinic Libraries, who helped in the development and completion of the literature search contained within the manuscript. The literature was searched by an experienced medical librarian Danielle J. Gerberi for the concepts of gut microbiota in child and adolescent psychiatry.
Financial support (presence or absence)
Dr. Romanowicz received grant funding from the Mayo Foundation Departmental Small Grant Program and the Palix Foundation. Dr. Croarkin was supported under NIH grant R01MH113700. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The supporters had no role in the design, analysis, interpretation, or publication of the manuscript.
Declaration of Competing Interest
Dr. Croarkin has received research grant support from Pfizer, Inc.; equipment support from Neuronetics, Inc.; and received supplies and genotyping services from Assurex Health, Inc. for investigator-initiated studies. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc. Dr. Croarkin is a consultant for Procter & Gamble Company and Myriad Neuroscience. The other authors have no disclosures or potential conflicts of interest to declare.
Footnotes
Ethical statement
This systematic review did not require local institutional review board approval but was conducted in accordance with PRISMA guidelines.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pnpbp.2020.110187.
References
- Anglin Rebecca, Surette Michael, Moayyedi Paul, Bercik Premysl, 2015. Lost in translation: the gut microbiota in psychiatric illness. Canad. J. Psychiatry / La Revue canadienne de psychiatrie 60, 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed Fredrik, Manchester Jill K., Semenkovich Clay F., Gordon Jeffrey I., 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci 104, 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga Deepika, Reichert Johanna Louise, Koschutnig Karl, Aigner Christoph Stefan, Holzer Peter, Koskinen Kaisa, Moissl-Eichinger Christine, Schöpf Veronika, 2018. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 9, 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, Oltman CL, Azcarate-Peril MA, Kirby JR, Calarge CA, 2015. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry Psychiatry 5, e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa RSD, Vieira-Coelho MA, et al. , 2020. Probiotics and prebiotics: focus on psychiatric disorders - a systematic review. Nutr. Rev 78 (6), 437–450. 10.1093/nutrit/nuz080.NutrRev.2020. [DOI] [PubMed] [Google Scholar]
- Bercik P, Collins SM, Lyte M, Cryan JF, 2014. The effects of inflammation, infection and antibiotics on the microbiota-gut-brain axis. Adv. Exp. Med. Biol [DOI] [PubMed] [Google Scholar]
- Boix-Amorós Alba, Puente-Sánchez Fernando, du Toit Elloise, Linderborg Kaisa M., Zhang Yumei, Yang Baoru, Salminen Seppo, Isolauri Erika, Tamames Javier, Mira Alex, Collado Maria Carmen, 2019. Mycobiome profiles in breast Milk from healthy women depend on mode of delivery, geographic location, and interaction with Bacteria. Appl. Environ. Microbiol 85 e02994–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Javier A., Forsythe Paul, Chew Marianne V., Escaravage Emily, Savignac Hélène M., Dinan Timothy G., Bienenstock John, Cryan John F., 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci 108, 16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan Patricia A., Dunlop Anne L., Smith Alicia K., Kramer Michael, Mulle Jennifer, Corwin Elizabeth J., 2019. Protocol for the Emory University African American maternal stress and infant gut microbiome cohort study. BMC Pediatr. 19, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Park TY, Li KJ, DeLisi LE, 2020. Antipsychotics and the microbiota. Curr Opin Psychiatry 33, 225–230. [DOI] [PubMed] [Google Scholar]
- Cresci Gail A., Bawden Emmy, 2015. Gut Microbiome. Nutr. Clin. Pract 30, 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG, 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci 13, 701–712. [DOI] [PubMed] [Google Scholar]
- Davenport Emily R., Sanders Jon G., Song Se Jin, Amato Katherine R., Clark Andrew G., Knight Rob, 2017. The human microbiome in evolution. BMC Biol. 15, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF, 2012. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology 221, 155–169. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG, 2008. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiatr. Res 43, 164–174. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG, 2010. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Grimaldi R, Cela D, Swann JR, Vulevic J, Gibson GR, Tzortzis G, Costabile A, 2017. In vitro fermentation of B-GOS: impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol. Ecol 93, 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejia JL, Hansen LH, Gibson EL, Nielsen DS, Costabile A, 2018. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen Hermie, Veloo Alida, Raangs Gerwin, Wagendorp Arjen, Klijn Nicolette, Bindels Jacques, Welling Gjalt, 2000. Analysis of intestinal Flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr 30, 61–67. [DOI] [PubMed] [Google Scholar]
- Higgins Julian P.T., Altman Douglas G., Gøtzsche Peter C., Jüni Peter, Moher David, Oxman Andrew D., Savovic Jelena, Schulz Kenneth F., Weeks Laura, Sterne Jonathan A.C., Group Cochrane Bias Methods, and Group Cochrane Statistical Methods, 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed.) 343 d5928–d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Chunrong, Shi Guochao, 2019. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med 17, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda M. Nazmul, Ahmad Shaikh M., Kalanetra Karen M., Taft Diana H., Alam Md J., Khanam Afsana, Raqib Rubhana, Underwood Mark A., Mills David A., Stephensen Charles B., 2019. Neonatal vitamin A supplementation and vitamin A status are associated with gut microbiome composition in Bangladeshi infants in early infancy and at 2 years of age. J. Nutr 149, 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Patsopoulos NA, Rothstein HR, 2008. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ 336, 1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam KB, Saiful M, Fukiya Satoru, Hagio Masahito, Fujii Nobuyuki, Ishizuka Satoshi, Ooka Tadasuke, Ogura Yoshitoshi, Hayashi Tetsuya, Yokota Atsushi, 2011. Bile acid is a host factor that regulates the composition of the Cecal microbiota in rats. Gastroenterology 141, 1773–1781. [DOI] [PubMed] [Google Scholar]
- Knight R, Callewaert C, Marotz C, Hyde ER, Debelius JW, McDonald D, Sogin ML, 2017. The microbiome and human biology. Annu. Rev. Genomics Hum. Genet 18, 65–86. [DOI] [PubMed] [Google Scholar]
- Le Bastard Q, Al-Ghalith GA, Gŕegoire M, Chapelet G, Javaudin F, Dailly E, Batard E, Knights D, Montassier E, 2018. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther 47, 332–345. [DOI] [PubMed] [Google Scholar]
- Liberati Alessandro, Altman Douglas G., Tetzlaff Jennifer, Mulrow Cynthia, Gøtzsche Peter C., Ioannidis John P.A., Clarke Mike, Devereaux PJ, Kleijnen Jos, Moher David, 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6, e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, Yu Y, Mei L, Yang P, Tang Y, Zheng P, 2019. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep 9, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low JSY, Soh SE, Lee YK, Kwek KYC, Holbrook JD, Van der Beek EM, Shek LP, Goh AEN, Teoh OH, Godfrey KM, Chong YS, Knol J, Lay C, 2017. Ratio of Klebsiella/Bifidobacterium in early life correlates with later development of paediatric allergy. Benefic. Microbes 8, 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, 2013. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9, e1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, 2014. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol 817, 3–24. [DOI] [PubMed] [Google Scholar]
- Martin CR, Osadchiy V, Kalani A, Mayer EA, 2018. The brain-gut-microbiome Axis. Cell Mol Gastroenterol Hepatol 6, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF, 2014. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One 9, e115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho Hmrt, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL, 2010. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int. J. Probiot. Prebiot 5, 69–74. [Google Scholar]
- Partty A, Kalliomaki M, Wacklin P, Salminen S, Isolauri E, 2015. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr. Res 77, 823–828. [DOI] [PubMed] [Google Scholar]
- RevMan, Review Manager, 2014. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2020 The Cochrane Collaboration. [Google Scholar]
- Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, Yang HT, Tancredi DJ, Bruce German J, Slupsky CM, Ashwood P, Mills DA, Smilowitz JT, Angkustsiri K, 2019. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurman Virginia, Margolis Kara G., Luna Ruth Ann, 2020. Autism Spectrum disorder as a brain-gut-microbiome Axis disorder. Dig. Dis. Sci 65, 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender Ron, Fuchs Shai, Milo Ron, 2016. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin Deborah R., 2019. Microbiome and mental health, specifically as it relates to adolescents. Curr. Psychiatry Rep 21, 93. [DOI] [PubMed] [Google Scholar]
- Sordillo Joanne E., Zhou Yanjiao, McGeachie Michael J., Ziniti John, Lange Nancy, Laranjo Nancy, Savage Jessica R., Carey Vincent, O’Connor George, Sandel Megan, Strunk Robert, Bacharier Leonard, Zeiger Robert, Weiss Scott T., Weinstock George, Gold Diane R., Litonjua Augusto A.., 2017. Factors influencing the infant gut microbiome at age 3–6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J. Allergy Clin. Immunol 139, 482–491 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AJ, Purcell RV, Darling KA, Eggleston MJF, Kennedy MA, Rucklidge JJ, 2019. Human gut microbiome changes during a 10 week Randomised Control Trial for micronutrient supplementation in children with attention deficit hyperactivity disorder. Sci. Rep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Valerie H., 2019. The microbiome and mental health: Hope or hype? JPN 44, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM, 2012. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun Hein M., Bridgman Sarah L., Chari Radha, Field Catherine J., Guttman David S., Becker Allan B., Mandhane Piush J., Turvey Stuart E., Subbarao Padmaja, Sears Malcolm R., Scott James A., Kozyrskyj Anita L., Study, Investigators Canadian Healthy Infant Longitudinal Development, 2018. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 172, 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI, 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Turnbaugh Peter J., Ley Ruth E., Hamady Micah, Fraser-Liggett Claire M., Knight Rob, Gordon Jeffrey I., 2007. The human microbiome project. Nature 449, 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umu Özgün C.O., Rudi Knut, Diep Dzung B., 2017. Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb. Ecol. Health Dis 28, 1348886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Zhi-Hui, Dong Liu, Li Hong-Dong, Zhu Dan-Ping, Yu He, Hou Ting, Jia-Lin Yu., 2018. Prenatal and postnatal antibiotic exposure influences the gut microbiota of preterm infants in neonatal intensive care units. Ann. Clin. Microbiol. Antimicrob 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


