Summary
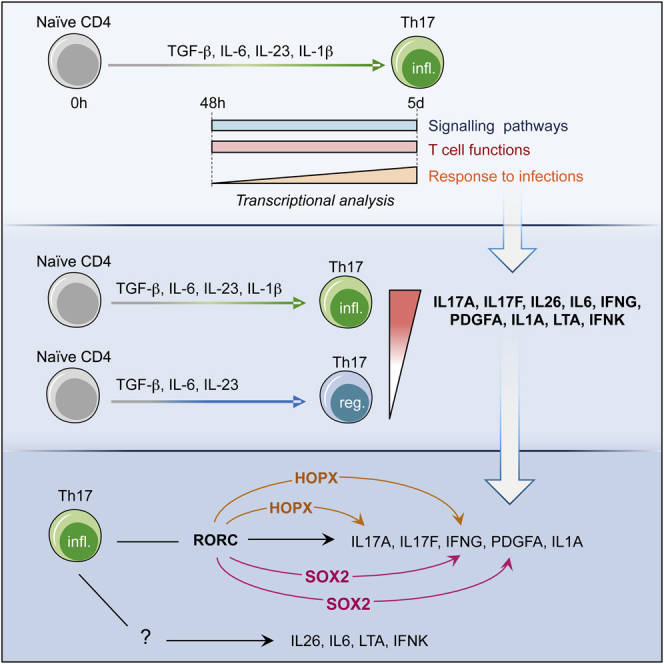
T helper (Th) 17 cells protect from infections and are pathogenic in autoimmunity. While human Th17 cell differentiation has been defined, the global and stepwise transcriptional changes accompanying this process remain uncharacterized. Herein, by performing transcriptome analysis of human Th17 cells, we uncovered three time-regulated modules: early, involving exclusively “signaling pathways” genes; late, characterized by response to infections; and persistent, involving effector immune functions. To assign them an inflammatory or regulatory potential, we compared Th17 cells differentiated in presence or absence of interleukin (IL)-1β, respectively. Most inflammatory genes belong to the persistent module, whereas regulatory genes are lately or persistently induced. Among inflammatory genes, we identified the effector molecules IL17A, IL17F, IL26, IL6, interferon (IFN)G, IFNK, LTA, IL1A, platelet-derived growth factor (PDGF) A and the transcriptional regulators homeodomain-only protein homeobox (HOPX) and sex-determining-region-Y-box (SOX)2, whose expression was independently validated. This study provides an integrative representation of the stepwise human Th17 differentiation program and offers new perspectives toward therapeutic targeting of Th17-related autoimmune diseases.
Subject areas: Systems Biology, Omics, Immunology
Graphical abstract
Highlights
-
•
Human Th17 cells are driven by early, late, and persistent transcriptional modules.
-
•
Human Th17 cells express IL17A, IL17F, IL26, IL6, IFNG, IFNK, LTA, IL1A, and PDGFA.
-
•
RORC regulates the IL17A, IL17F, IFNG, PDGFA, and IL1A expression in human Th17 cells.
-
•
HOPX and SOX2 contribute to the expression of IFNG by human Th17 cells.
Systems Biology; Omics; Immunology
Introduction
T helper (Th) 17 cells are mainly characterized by production of interleukin (IL)-17A and IL-17F and play a key role in the immune response against fungi and extracellular pathogens (Liang et al., 2007). The production of Th17-specific cytokines is regulated by a unique genetic program orchestrated by transcription factors, where the retinoic-acid-receptor-related orphan receptor (ROR)γt is a key regulatory factor in both human and mouse Th17 cells (Ivanov et al., 2006; Volpe et al., 2008; Manel et al., 2008; Capone and Volpe, 2020). Importantly, dysregulation of Th17 cell functions contributes to the pathogenesis of inflammatory and autoimmune diseases.
Since the discovery of Th17 cells as a distinct Th subset in 2005 (Park et al., 2005), significant progress has been made in the field, including the identification of cytokines, transcription factors and epigenetic modifications underlying Th17 differentiation (Veldhoen et al., 2006; Volpe et al., 2008; Manel et al., 2008; Capone and Volpe, 2020; Ciofani et al., 2012; Mukasa et al., 2010; Kanno et al., 2012), the discovery of typical effector cytokines regulating their functions (Annunziato et al., 2007; Volpe et al., 2009), and the involvement of Th17 cells in pathological conditions (Volpe et al., 2015; Maddur et al., 2012). In recent years, therapies directed against IL-17 have been developed and their efficacy has been proven. Secukinumab, a fully humanized antibody neutralizing IL-17A, is now approved as the first-line systemic treatment for moderate to severe plaque psoriasis (Garnock-Jones, 2015; McInnes et al., 2015). However, important questions on human Th17 cells, such as the sequence of molecular events occurring during the differentiation process and the identification of features that synergize with IL-17 and that contribute to the inflammatory properties of these cells, are still open. Elucidating the global transcriptional program of human Th17 cells is essential to answer these open questions and to further improve therapies directed against Th17 cells.
In this study, we have characterized the transcriptional program set in motion during human Th17 cell polarization and unveiled new genes characterizing their profile, which may contribute to their inflammatory potential. This study expands our knowledge on the nature and molecular regulation of human Th17 cells and reveals new potential therapeutic targets for the treatment of Th17-related diseases.
Results
Dynamic transcriptome changes underlie stepwise acquisition of early and late features of human Th17 cells
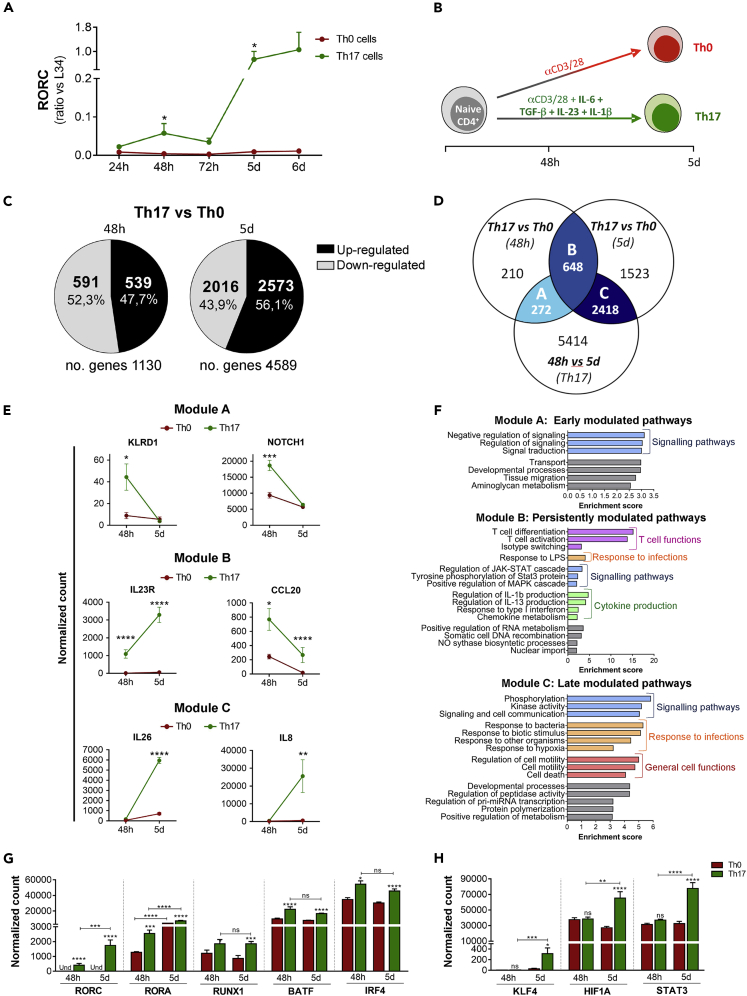
To exhaustively investigate the differentiation process of human Th17 cells, we performed a genome-wide transcriptome analysis of naive CD4+ T cells (Figure S1) differentiated under Th17 conditions, IL-1β, IL-6, transforming growth factor (TGF)-β, and IL-23, compared with unpolarized cells (Th0). First, we set out to determine key early and late time points of the human Th17 cell polarization process. Expression of RORC mRNA, encoding the transcription factor RORγt that is strictly required for terminal differentiation of Th17 cells (Ivanov et al., 2006), was induced at 48 hours (h) of culture under Th17 conditions compared with the Th0 condition (Figure 1A), suggesting that the polarization program started at 48 h. However, maximal RORC induction required 5 days of culturing, indicating acquisition of complete Th17 cell polarization at this later time point (Figure 1A). On this basis, we selected 48 h and 5 days as early and late stages, respectively, of the human Th17 polarization program (Figure 1B). To limit donor-associated variability, we performed high-throughput RNA-sequencing (RNA-seq) analyses of Th0 and Th17 cells obtained from 5 independent healthy donors (HD), at 48 h and 5 days of differentiation.
Figure 1.
Early, persistent, and late genes acquired during human Th17 cell polarization reflect specific functions
Naive CD4+ T cells cultured with anti-CD3/anti-CD28 alone (Th0) or with the addition of TGF-β, IL-6, IL-1β, and IL-23 (Th17) (n = 5) were analyzed by q-PCR for the expression of RORC transcript (A). RNA sequencing of Th17 and Th0 cells (n = 5) at 48 h and 5 days of differentiation (B): number of genes differentially modulated in Th17 versus Th0 at each time point (C); Venn's diagram shows the number of genes specifically modulated (either upregulated or downregulated) in Th17 profile (Th0 versus Th17) at 48 h (early) and 5 days (late) and persistently modulated (48 h Th17 versus 5 days Th17) (D); expression of selected Th genes included in A, B, and C modules reported as DEseq2 normalized count (E); bar graph representation of gene ontology for genes in A, B, and C modules (F); expression of selected Th17 transcription factors (G and H). Each replicate, including Th0_48h, Th17_48h, Th0_5d, Th17_5d, was performed in the same donor (paired cultures). Data are represented as mean ± SEM (Student's T-tests; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001).
Bioinformatics analyses highlighted a large extent of gene expression reprogramming associated with acquisition of the Th17 profile. By setting 1.5-fold difference and p value (p) ≤ 0.05 as filters, we identified 1130 genes differentially expressed between Th17 and Th0 cells at 48 h and 4589 genes at 5 days (Figure 1C; Tables S1 and S2). To rule out that the differential gene expression was related to the presence of mixed populations with varying degrees of activation, we evaluated the cell size and the CD25 expression in Th0 and Th17 cells at 48 h and 5 days. Although the activation state of both Th0 and Th17 cells increased over time and Th17 expressed higher levels of CD25 than Th0 cells, each Th profile was characterized by a unique cell population (Figure S2), indicating that the differential gene expression analysis was not affected by the presence of mixed subpopulations. Next, RNA-seq and bioinformatics analyses were verified by real-time quantitative polymerase chain reaction (q-PCR) on independent sets of human Th0 and Th17 cells. We found that 18 of 22 tested genes (81.8% of validation) were validated by q-PCR analysis (Figure S3A). In particular, the expression of Th17 signature genes (IL17A, IL17F, IL23R, IL26, RORA, RORC) mirrored the RNA-seq results (Figure S3B). Furthermore, analyses of other arbitrarily selected genes at either 48 h and 5 days of differentiation confirmed the reliability of the RNA-seq data (Figures S3C and S3D).
To identify genes relevant for the acquisition of specific Th17 features at the early and late time points of differentiation, we compared three datasets: Th17 versus Th0 at 48 h (early Th17 genes), Th17 versus Th0 at 5 days (late Th17 genes), and Th17 at 5 days versus Th17 at 48 h (genes that vary over time in Th17 cells). In particular, we identified the genes upregulated or downregulated in each pair of conditions. Overlap between these comparisons identified three modules that we named A, B, and C (Figure 1D). Interestingly, while in module A and B, the percentage of genes upregulated or downregulated was similar (Figures S4A and S4B), in module C, most genes were upregulated in Th17 versus Th0 conditions (Figure S4C). Moreover, module B contains a small percentage of genes upregulated at 48 h and downregulated at 5 days or downregulated at 48 h and upregulated at 5 days (Figure S4B). Module A (early) included genes specific of initial Th17 cell differentiation (i.e. Killer cell lectin-like receptor subfamily D and Notch homolog 1, translocation-associated) (Figure 1E) and was enriched in functional categories related to signaling pathways (Figure 1F). Module B (persistent) included genes that were constantly modulated during Th17 cell differentiation, such as the Th17 signature genes IL23R and C-C motif chemokine ligand (CCL)20 (Figure 1E). These genes were enriched in functional categories involved in “T cell differentiation/activation,” “cytokine production,” and “Janus kinase/signal transducer and activator of transcription (STAT) signaling,” which are all relevant for Th17 cell biology (Figure 1F). Module C (late) included genes of terminal differentiation, such as the Th17 effector cytokine genes IL26 and IL8 (Figure 1E), and it was enriched in functional categories of strong relevance for the effector functions of Th17 cells, such as “response to other organisms” and “cell motility” (Figure 1F).
Th17 cell differentiation and functions are finely regulated by the expression of specific transcriptional regulators. To study the timing of expression of transcription factors that are known to play a key role in Th17 biology (Capone and Volpe, 2020), we explored their presence in the early (A), persistent (B), and late (C) modules. We found that RORC, RORA, Runt-related transcription factor (RUNX1), basic leucine zipper ATF-like transcription factor (BATF), and interferon (IFN) regulatory factor (IRF)4 genes belonged to the persistent module and were constantly upregulated with respect to Th0 cells (Figure 1G), whereas Kruppel-like factor (KLF)4, hypoxia-inducible factor (HIF)1A, and STAT3 were upregulated only at later time in Th17 cells (C module; Figure 1H). Interestingly, none of these transcription factors was upregulated transiently only at the early time point, suggesting that their expression was required for early Th17 commitment as well as for maintenance of the transcriptional program involved in human Th17 cell polarization.
Identification of a specific inflammatory Th17 effector program
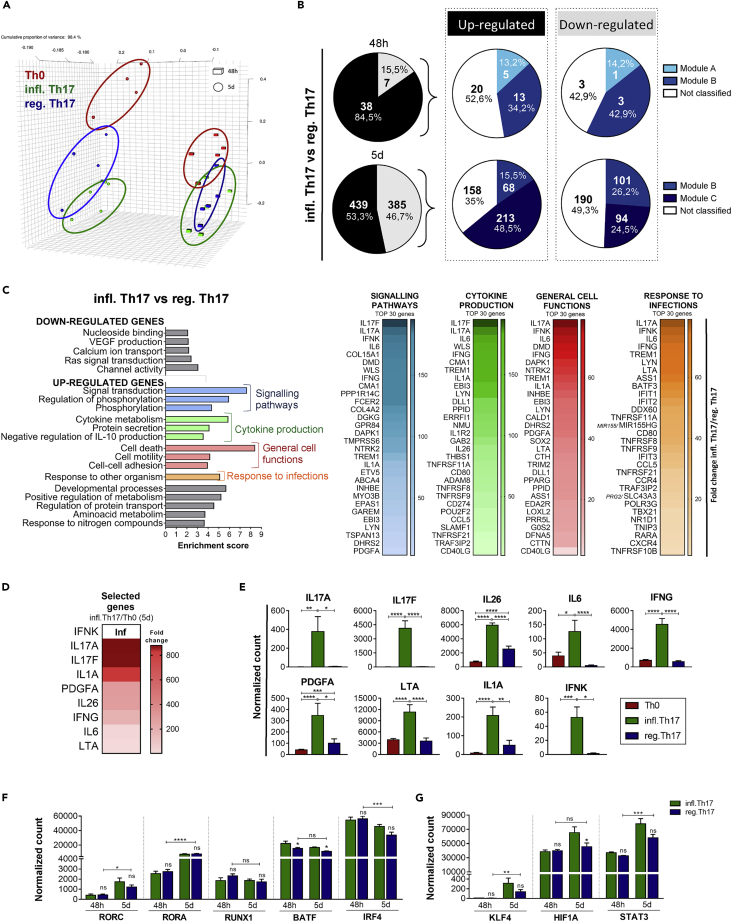
IL-1β is crucially involved in the pathology of Th17-related diseases (Chung et al., 2009; Sutton et al., 2006), whereas Th17 cells differentiated in the absence of IL-1β (IL-6, IL-23 and TGF-β) produce the anti-inflammatory IL-10 cytokine, which is generally produced by T regulatory cells (Treg) (Volpe et al., 2008; Zielinski et al., 2012). Thus, to delineate the inflammatory or regulatory potential of Th17 genes, we compared the transcriptome of T cells differentiated in presence of IL-1β, IL-6, IL-23, and TGF-β (“inflammatory” Th17 condition) or in presence of IL-6, IL-23, and TGF-β (“regulatory” Th17 condition). Principal component analysis (PCA) of RNA-seq data obtained from 5 independent donors revealed that absence or presence of IL-1β did not segregate samples at 48 h (Figure 2A). In contrast, at 5 days, inflammatory and regulatory Th17 cells segregate as two distinct profiles (Figure 2A). Importantly, the differential gene expression observed between inflammatory and regulatory Th17 cells was not related to a differential activation state (Figure S5). In line with the PCA profile, only 45 genes were differentially modulated in the absence of IL-1β at 48 h, whereas more than 800 genes were affected at 5 days (Figure 2B; Tables S3 and S4).
Figure 2.
Transcriptional analysis of inflammatory Th17 cells reveals crucial genes for the effector functions of Th17 cells
Naive CD4+ T cells cultured with anti-CD3/anti-CD28 alone (Th0) or with the addition of TGF-β, IL-6, IL-1β, and IL-23 (infl. Th17) or in the absence of IL-1β (reg. Th17) were analyzed by RNA sequencing (n = 5). Each replicate, including Th0_48hr, Th17_48hr, Th17 without IL-1β_48hr, Th0_5d, Th17_5d, Th17 without IL-1β_5d, was performed in the same donor (paired cultures). One sample Th0_48hr was omitted from analysis because it did not pass the quality control. Global gene expression of cells is represented by principal component analysis (A). Number of genes upregulated (black) and downregulated (gray) in infl. Th17 versus reg. Th17 at each time point of analysis, and segregation of those genes in modules A, B, and C is defined in Figure 1D B. Gene ontology of downregulated and upregulated genes in infl. Th17 versus reg. Th17 at 5 days, and heatmap of genes upregulated in infl. Th17 versus reg. Th17, belonging to the most represented categories (C). Heatmap (D) and expression profile (E) of nine selected genes upregulated in infl. Th17 versus reg. Th17 and in infl. Th17 versus Th0. Expression of persistent (F) and late (G) genes encoding for Th17 transcription factors in infl. Th17 and reg. Th17 profiles. Gene expression in E, F, and G are reported as DEseq2 normalized count (n = 5). Data are represented as mean ± SEM. (Student's T-tests; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001). Inflammatory Th17 = infl. Th17; regulatory Th17 = reg. Th17.
T cells differentiated under regulatory Th17 conditions displayed a profile characterized by decreased expression of some Th17 signature genes, such as low levels of IL26 and RORC, but similar expression of others, such as RORA and IL23R (Figure S6). This analysis allowed defining an inflammatory Th17 program (upregulated in presence of IL-1β) and a regulatory Th17 program (downregulated in presence of IL-1β). We found that most of IL-1β-regulated genes showed increased expression at 48 h under the inflammatory condition. This observation suggested that their upregulation may affect subsequent steps in Th17 polarization and cause the broader gene expression differences seen at 5 days (Figure 2B). Moreover, most part of the inflammatory program belongs to the persistent module B at 48 h (34.2%, Figure 2B) and to the late module C at 5 days (48.5%, Figure 2B). In contrast, few regulatory genes are induced at 48 h, while at 5 days, an equal number of genes are classified in persistent and late modules (Figure 2B). These results indicate that genes lately or persistently expressed during human Th17 cell differentiation are crucial for discriminating the commitment toward the inflammatory or regulatory profile of these cells.
Several transcriptomic studies have highlighted the molecular signature of inflammatory mouse (Lee et al., 2012; Gaublomme et al., 2015; Ghoreschi et al., 2010; Yosef et al., 2013) and human (Hu et al., 2017) Th17 cells. These studies were carried out by using in vitro polarized Th17 cells and memory Th1/17 cells. The analysis of genes associated with Th17 phenotype in these previous studies (Table S5) indicated that some of them, including IL17A, IFNG, T-box transcription factor 21 (TBX21), Epstein-Barr Virus-Induced 3 (EBI3), IRF8, tumor necrosis factor (TNF) receptor superfamily (RSF) 9, TNFRSF14, CCL5, CD40 ligand, BATF, and TNF (11 of 76 genes [14.5%]) were upregulated in inflammatory human Th17 cells at 5 days of differentiation (Figure S7), while other genes were not modulated.
To elucidate the global molecular signature that characterizes the acquisition of the inflammatory profile by human Th17 cells, we analyzed in more detail the genes differentially expressed in inflammatory versus regulatory Th17 conditions at 5 days. Gene ontology clustering revealed that IL-1β caused a significant upregulation of functional categories related to “signaling pathways,” “cytokine production,” and “response to other organisms,” which are all relevant for the effector functions of Th17 cells (Figure 2C). Moreover, genes involved in general cell functions, such as cell death, adhesion, and motility, were also upregulated. By contrast, genes downregulated under inflammatory conditions did not cluster in functional categories that are specific for Th17 cells (Figure 2C). Interestingly, top-ranking genes upregulated in Th17 versus Th0 (Figure 2D) and in the presence of IL-1β compared with the absence of IL-1β (Figure 2E) encode Th17-specific (IL17A, IL17F, and IL26) and nonspecific (IL6, IFNG, and IL1A) inflammatory cytokines, and other soluble proteins associated to immune functions, including IFNK, platelet-derived growth factor (PDGF)A, and LTA, may potentially alter functional features of human Th17 cells.
Next, we explored whether the expression of transcription factors driving the Th17-specific signature was modulated in inflammatory versus regulatory Th17 condition. The expression of BATF, which was described as an early determinant of the mouse Th17 cell signature (Ciofani et al., 2012), was slightly upregulated in the inflammatory human Th17 cell condition at both time points. However, expression of the other transcription factors, with the exception of HIF1A at 5d, was not significantly affected (Figures 2F and 2G). These results suggest that polarization of human inflammatory Th17 cells might rely on additional transcriptional regulators.
Experimental validation and characterization of homeodomain-only protein homeobox and sex-determining-region-Y-box 2 in human Th17 cells
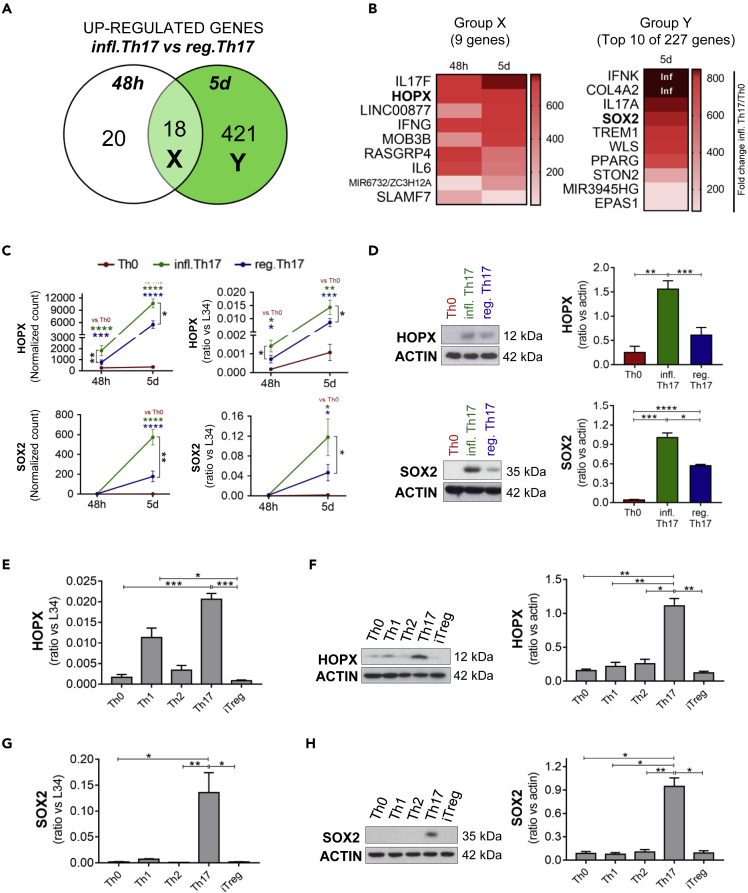
To search for novel factors possibly involved in the acquisition of an inflammatory Th17 phenotype, we further dissected the pool of genes that were lately or persistently upregulated in Th17 cells differentiated in the presence of IL-1β (inflammatory Th17) compared with those differentiated in the absence of IL-1β (regulatory Th17). Group Y comprised genes upregulated in the presence of IL-1β only at 5 days (late regulation), whereas group X included genes upregulated at both 48 h and 5 days (persistent regulation) (Figure 3A). Next, we further selected genes that were significantly induced in Th17 versus Th0 conditions (Figure S8) and focused our attention on two genes encoding transcriptional regulators: homeodomain-only protein homeobox (HOPX) (group X, persistent regulation) and sex-determining-region-Y-box 2 (SOX2) (group Y, late regulation) (Figure 3B).
Figure 3.
HOPX and SOX2 are specifically expressed in human Th17 cells
Naive CD4+ T cells cultured with anti-CD3/anti-CD28 alone (Th0) or with the addition of TGF-β, IL-6, IL-1β, and IL-23 (infl. Th17) or in the absence of IL-1β (reg. Th17) were analyzed by RNA sequencing. The Venn's diagram between genes upregulated in inflammatory Th17 versus regulatory Th17 at 48 h and 5 days unveils 18 genes regulated at both time points (group X) and 421 genes specifically upregulated at 5 days (group Y) (A). All the annotated genes included in group X and 10 of 421 genes in group Y are listed in panel (B) HOPX and SOX2 expression obtained by RNA sequencing (reported as DEseq2 normalized count) (n = 5) and by q-PCR (n = 4) (C) at 48 h and 5 days and by Western blot (n = 5) at 5 days (D) were analyzed in Th0, inflammatory Th17 (TGF-β, IL-6, IL-1β and IL-23), and regulatory Th17 (TGF-β, IL-6, and IL-23) cells. Expression of HOPX transcript (n = 5) (E) and protein (n = 4) (F), SOX2 transcript (n = 6) (G) and protein (n = 4) (H) in Th0, Th1, Th2, Th17, and Treg profiles differentiated in vitro from naive CD4+ T cells of HD. Data are represented as mean ± SEM (For pairwise conditions: Student's T-tests; For three or more conditions: One-way ANOVA test; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001). Th1: IL-12; Th2: IL-4; Th17: IL-1β, IL-6, TGF-β, and IL-23; iTreg: IL-2 and TGF-β; Th0: no cytokines. Inflammatory Th17 = infl. Th17; regulatory Th17 = reg. Th17.
HOPX is a transcriptional cofactor that was previously described to be involved in murine Th1 and Treg cell functions (Albrecht et al., 2010; Hawiger et al., 2010). RNA-seq and q-PCR analyses showed progressive and persistent upregulation of HOPX expression during Th17 cell differentiation and confirmed its dependency on IL-1β signaling (Figure 3C). Western blot analysis at 5 days of differentiation confirmed these results at the protein level (Figure 3D). Comparative analysis of human naive T cells differentiated into different Th or Treg subsets indicated that Th17 cells expressed the highest level of HOPX, followed by Th1 cells, whereas expression of this transcriptional regulator was barely detectable in the other subsets (Figure 3E). The specific expression of HOPX in human Th17 cells was even more evident at the protein level (Figure 3F), further suggesting its functional implication in these cells.
SOX2 is a transcription factor involved in stemness, a feature associated with the Th17 phenotype (Muranski et al., 2011; Kryczek et al., 2011). Interestingly, in silico analyses of chromatin immunoprecipitation-sequencing experiments to search for potential regulators of the genes modulated by IL-1β at early and late time points identified SOX2 as the most significantly enriched transcription factor (Figure S9). We confirmed that SOX2 expression in Th17 cells was dependent on IL-1β signalling by q-PCR and Western blot analyses (Figures 3C and 3D). Furthermore, comparative analyses of human T cell subsets revealed that SOX2 transcript and protein were highly specific of the Th17 profile (Figures 3G and 3H).
HOPX and SOX2 contribute to the expression of specific soluble factors in human Th17 cells
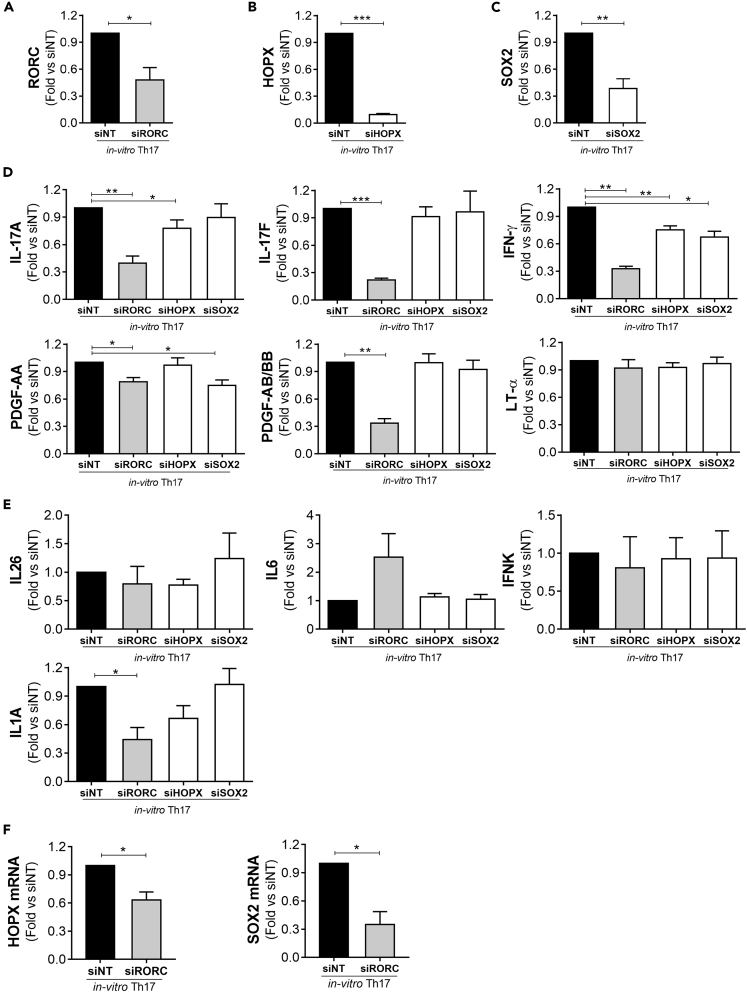
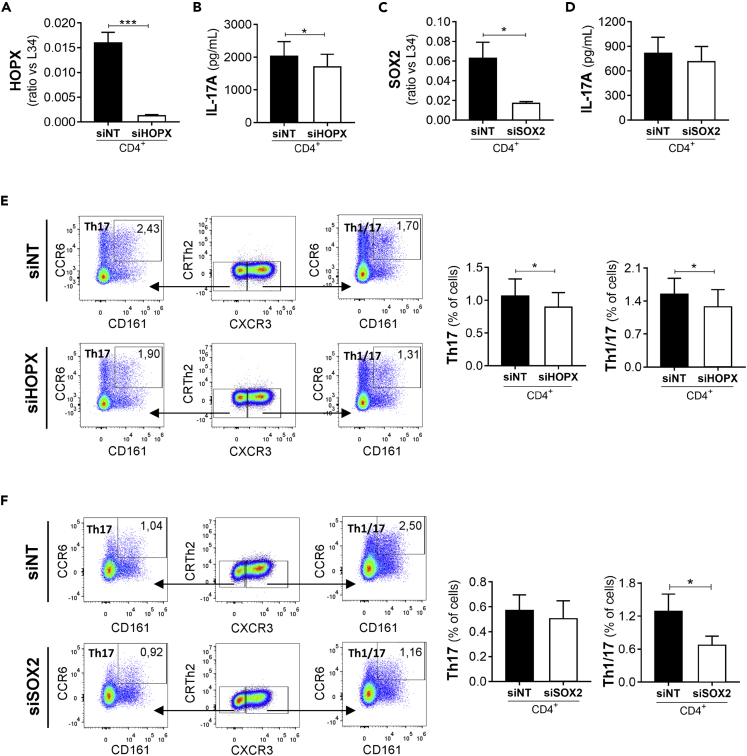
To test whether HOPX and SOX2 expression had an impact on human Th17 differentiation, we set out to silence their expression in human naive CD4+ T cells undergoing Th17 differentiation (Figures 4B and 4C; Figure S10A) without altering the viability and proliferation potential of differentiating T cells (Figures S10B and S10C). Next, we analyzed the expression of soluble factors previously identified as expressed in inflammatory Th17 condition and used RORC-silenced cells as reference control for a master regulator of Th17 cell profile (Figure 4A). As expected, immunoassays by enzyme-linked immunosorbent assay revealed that knockdown of RORC significantly reduced the IL-17A and IL-17F protein expression by polarized Th17 cells (Figure 4D). RORC silencing also modulated the production of IFN-γ, PDGF-AA, and PDGF-AA/BB (Figure 4D), as well as IL1A mRNA expression (Figure 4E), by human Th17 cells. By contrast, knockdown of RORC did not interfere with the expression of LT-α (Figure 4D), IFNK, IL6, and IL26 (Figure 4E).
Figure 4.
HOPX and SOX2 contribute to the expression of Th17 cytokines
Expression levels of RORC (A), HOPX (B,F), and SOX2 (C,F) transcripts; IL-17A, IL-17F, IFN-γ, PDGF-AA, PDGF-AB/BB, LT-α proteins (D); and IL26, IL6, IL1A, IFNK transcripts (E), were analyzed by q-PCR (transcript) or ELISA and Luminex (protein) at 5 days of cell differentiation in Th17 cells (TGF-β, IL-6, IL-1β and IL-23) treated with RORC (siRORC; n = 4), HOPX (siHOPX; n = 8), SOX2 (siSOX2; n = 6) siRNA. Each set was compared with Th17 cells treated with non targeting siRNA (siNT) (n = 4 for RORC experiments, n = 8 for HOPX experiments, n = 6 for SOX2 experiments). Transcriptional results were normalized on RPL34 expression. All data are presented as fold versus siNT. Data are represented as mean ± SEM. (Student's T-tests; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001).
Interestingly, silencing of HOPX (Figure 4B) also impaired the production of IL-17A and IFN-γ (Figure 4D), albeit at lower levels than RORC, whereas the expression of other soluble factors (LT-α, IL-17F, PDGF-AA, PDGF-AA/BB, IL26, IL6, IL1A, IFNK) was not affected (Figures 4D and 4E). On the other hand, knockdown of SOX2 expression in human polarized Th17 cells (Figure 4C) specifically modulated IFN-γ and PDGF-AA production (Figure 4D). The common regulation of IL-17A and IFN-γ by RORγt and HOPX and PDGF-AA and of IFN-γ by RORγt and SOX2 lead us to hypothesize the involvement of RORγt in regulating HOPX and SOX2 expression. Indeed, silencing of RORC in human Th17 cells reduced the expression of both HOPX and SOX2 (Figure 4F). These results suggest that the transcriptional regulators HOPX and SOX2 act downstream of RORγt signaling in human Th17 cells.
HOPX and SOX2 contribute to the regulation of Th1/17 cell features
T cells that concomitantly produce IL-17A and IFN-γ, named Th1/17 cells, play a pathogenic role in autoimmune diseases (Kebir et al., 2009; Reinert-Hartwall et al., 2015). Because HOPX and SOX2 contribute to regulate IFN-γ and IL-17A production by human Th17 cells, we next investigated their role in memory Th1/17 cells. To this end, peripheral memory CD4+ T cells were silenced for HOPX or SOX2, and their features were analyzed by flow cytometry. In line with the effect observed in Th17 cells polarized in vitro, HOPX silencing in peripheral memory CD4+ T cells (Figure 5A; Figure S11) leads to a minimal but significant reduction of the IL-17A expression (Figure 5B). This result indicates that HOPX contributes to the optimal production of IL-17A also in human memory Th1/17 cells. On the other hand, as expected from in vitro data, silencing of SOX2 in these cells did not affect IL-17A production (Figures 5C and 5D). The reduction of IL-17A upon HOPX silencing was also observed by evaluating the frequency of Th17 cells, which were identified by the expression of specific receptors (CXCR3-, CD161+, CCR6+) in total CD4+ T cells (Figure 5E). Furthermore, in line with the effect on IFN-γ production observed in vitro, HOPX depletion caused a reduced frequency of circulating Th1/17 cells, which were identified as CXCR3+, CD161+, and CCR6+ (Figure 5E).
Figure 5.
HOPX and SOX2 contribute to the regulation of Th1/17 cell features
Expression of HOPX transcript (A) and IL-17A protein (B) in total CD4+ T cells treated with nontargeting (siNT) or HOPX (siHOPX) siRNA was analyzed by q-PCR and ELISA, respectively (n = 8). Expression of SOX2 transcript (C) and IL-17A protein (D) in total CD4+ T cells treated with nontargeting (siNT) or SOX2 (siSOX2) siRNA was analyzed by q-PCR and ELISA, respectively (n = 6). Frequencies of Th17 cells (CXCR3- CD161+ CCR6+) and Th1/17 cells (CXCR3+ CD161+ CCR6+) (E) were analyzed by flow cytometry in total CD4+ T cells treated with non targeting (siNT) (n = 8) or HOPX (siHOPX) siRNA (n = 8). Frequencies of Th17 cells (CXCR3- CD161+ CCR6+) and Th1/17 cells (CXCR3+ CD161+ CCR6+) (F) were analyzed by flow cytometry in total CD4+ T cells treated with non targeting (siNT) (n = 6) or SOX2 (siSOX2) siRNA (n = 6). Data are represented as mean ± SEM (Student's T-tests; ∗p ≤ 0.05; ∗∗∗p ≤ 0.001).
Notably, SOX2 was also important for the Th1/17 phenotype, as its depletion caused a reduction of their frequency, but not of the frequency of Th17 cells (CXCR3-, CD161+, and CCR6+ cells) (Figure 5F).
These results indicate that HOPX and SOX2 are two transcriptional regulators that contribute to insure the optimal polarization of inflammatory human Th17 cells. Moreover, our results indicate that in vitro differentiated Th17 cells share common mechanisms with ex vivo memory Th17 cells, such as the regulation of IL-17A and IFN-γ by HOPX and of IFN-γ by SOX2.
Discussion
To our knowledge, this is the first transcriptional study of human Th17 cells that proposes a system-level analysis followed by experimental validation of testable hypotheses. Previous transcriptomic studies did not exhaustively analyze the differentiation process of human Th17 cells (Cosmi et al., 2008; Tuomela et al., 2012, 2016; Aijo et al., 2014; Tripathi et al., 2017) because they were either performed on Th17 cells isolated from peripheral blood (Cosmi et al., 2008; Ranzani et al., 2015) or with Th17 cells differentiated in vitro in a medium that lacked IL-23 (Tuomela et al., 2012, 2016; Aijo et al., 2014; Tripathi et al., 2017), which plays a crucial role in human Th17 cell differentiation (Volpe et al., 2008; Manel et al., 2008) as well as in Th17-related diseases (McGeachy et al., 2007). Thus, a comprehensive transcriptional analysis of human Th17 cells undergoing differentiation in optimal Th17 conditions (TGF-β, IL-6, IL-1β, and IL-23) (Volpe et al., 2008; Manel et al., 2008) had not been performed to date.
In this work, we dissected the gene expression program underlying early and late time points of the polarization process of human Th17 cells. Based on RORC expression, we found that the differentiation process requires 5 days in human Th17 cells, unlike mouse cells where optimal differentiation is achieved in 3 days (Gaublomme et al., 2015; Veldhoen et al., 2006). Moreover, our study revealed that the largest modulation of the transcriptome occurs at late stages of differentiation. In fact, while cells cultured under Th17 conditions are already distinguishable from cells cultured in absence of cytokines (Th0) at 48 h, the complete segregation between Th17 cells differentiated under inflammatory or regulatory culture conditions is obtained only after 5 days. The delay in differentiation of human with respect to mouse Th17 cells may rely on the different cytokine requirements: while administration of TGF-β and IL-6 is sufficient to induce murine Th17 cell polarization in vitro, human cells also require the presence of IL-1β and IL-23. Because naive CD4+ T cells do not express IL-1β and IL-23 receptors, the timing requested for their upregulation may contribute to the delay. However, also in murine Th17 cells, IL-6 and TGF-β are the early triggers of differentiation, whereas IL-23, IL-1β, and IL-21 are involved in the late stabilization and amplification steps (Veldhoen et al., 2006; Bettelli et al., 2008).
We propose that the human Th17 cell differentiation process may be divided in steps. Our transcriptomic analysis highlighted genes that were modulated at either early or late time points together with others that were constantly and progressively modulated throughout the whole cell polarization process. Interestingly, we found a strong association between the timing of expression and the function of the genes undergoing modulation. Genes associated with signaling pathways were always modulated, indicating that each time point of the polarization process requires proper response to polarizing cytokines or soluble factors released in the milieu. However, only late genes were enriched in pathways associated to Th17 effector functions, such as response to bacteria and other organisms, which is consistent with the antimicrobial activity of these cells (Veldhoen, 2017). Persistently modulated genes were enriched in functional categories involved in specific transduction pathways, such as the phosphorylation of STAT3, which is activated by IL-23 and IL-6 (Taga and Kishimoto, 1997; Heinrich et al., 2003; Yang et al., 2007), and general T cell functions, such as cell activation and differentiation, but also cytokine production and response to infections. Most part of Th17 signature genes were persistently or lately expressed in human Th17 cells, indicating that late steps are crucial for their inflammatory commitment. This result implicates that an efficient therapeutic inhibition of inflammatory Th17 cells should target persistent or late genes.
In this context, we selected the top-ranking genes induced at the late stage of differentiation in inflammatory versus regulatory Th17 conditions. Among others, we found known factors, such as IL17A, IL17F, IL6, IFNG, IL26, and other effector molecules, such as LTA, PDGFA, IL1A, IFNK, and LTA, encoding for LT-α and formerly known as TNF-β, are involved in the induction of adhesion molecules and chemokines by endothelial cells (Calmon-Hamaty et al., 2011). Notably, LT-α produced by Th17 cells is involved in the formation of tertiary lymphoid tissues in the meninges of patients affected by multiple sclerosis (Grogan and Ouyang, 2012), a typical Th17-mediated disease. In turn, LT-α contributes to amplify the expression of adhesion molecules and soluble factors that potentiate the ability of the extracellular matrix-stromal-cell network to recruit, retain, and activate lymphocytes within the meninges (Pikor et al., 2015). In this regard, we previously observed that Th17 cells from patients with multiple sclerosis produce higher amount of LT-α than those from HD (Capone et al., 2019). IL-1α expressed by CD4+ T cells (van Rietschoten et al., 2006) could contribute to the amplification of the inflammatory response by binding to IL-1 receptor 1 and activating the downstream signaling pathway (Kurt-Jones et al., 1985; Kaplanski et al., 1994; Di Paolo and Shayakhmetov, 2016; Dinarello, 2018). Consistent with our observations, IL-1α is produced in response to a variety of stimuli, including exposure to IL-1β (Di Paolo and Shayakhmetov, 2016). Thus, IL-1α could contribute to the inflammatory environment generated by IL-1β in Th17 cells. We also observed that Th17 cells produce PDGF-AA and PDGF-AB/BB. These growth factors are known to direct the differentiation of a variety of cell types (Hoch and Soriano, 2003), but their function in immune cells is not known. In addition, we found that human Th17 cells express IFNK, encoding for a member of the type I IFN family of cytokines, which play an important role in host defense against viral infections (Nardelli et al., 2002). Studies concerning the role of Th17 cells in the defense against viruses have generated conflicting results (Ma et al., 2019). Thus, further investigation of the expression and function of type I IFN by Th17 cells could highlight new antiviral mechanisms of these cells.
Collectively, our study revealed a partial overlap between mouse and human Th17 genes, in line with the partial overlap observed for selected mouse and human Th17 proteins (Tripathi et al., 2019). Moreover, transcriptional regulators previously indicated as relevant for mouse Th17 cells, such as RORC, RORA, HIF1A, BATF, IRF4, STAT3, RUNX1, and KLF4, were also induced in human Th17 cells. However, the expression of most of them was not differentially expressed in inflammatory and regulatory Th17 conditions, suggesting that other transcriptional regulators contribute to the acquisition of the inflammatory human Th17 profile. In this context, we identified HOPX and SOX2 as transcriptional regulators that act downstream to RORγt signaling and are involved in the differentiation program of inflammatory Th17 cells. To date, HOPX has been mainly studied for its role in cardiac and lung development (Chen et al., 2002; Shin et al., 2002; Yin et al., 2006) and in skeletal muscle differentiation (Kee et al., 2007). Moreover, HOPX was previously reported to be expressed in mouse Treg and Th1 cells (Albrecht et al., 2010; Hawiger et al., 2010). In Treg cells, HOPX participates to anergy induction on effector T cells, while in Th1 cells, it promotes cell expansion by modulating proapoptotic and antiapoptotic genes. However, we detected low levels of HOPX in human Th1 and Treg cell subsets, whereas it was expressed at much higher levels in Th17 cells, where HOPX contributes to the IL-17A and IFN-γ expression.
SOX2 is a transcription factor associated with pluripotency of stem cells. For instance, SOX2 promotes self-renewal in embryonic stem cells (Feng and Wen, 2015) and is required for reprogramming of induced pluripotent stem cells (Takahashi and Yamanaka, 2006). Moreover, in some adult tissues, such as the central nervous system, SOX2 ensures homeostasis by supporting the stem-like features of progenitor cells (Feng and Wen, 2015). Herein, we found that SOX2 contributes to the expression of PDGF-AA, a growth factor that we recently associated to the Th17 profile in HD and patients with multiple sclerosis (Capone et al., 2019). PDGF-AA binds to PDGF receptor A, which is considered critical player in the disruption of the blood-brain barrier (Ma et al., 2011). Thus, Th17 cells could contribute to disruption of the blood-brain barrier in multiple sclerosis through production of PDGF-AA. Moreover, we found that both HOPX and SOX2 contribute to regulate the production of IFN-γ by invitro-differentiated human Th17 cells. Importantly, IFN-γ-secreting Th17 cells, called Th1/17 cells, are abundant in inflamed tissues of human autoimmune diseases and are considered highly pathogenic (Nistala et al., 2010; Kebir et al., 2009; Reinert-Hartwall et al., 2015; Annunziato et al., 2007). The Th1/17 cell population, which is characterized by the simultaneous production of IFN-γ and IL-17A and the expression of the CXCR3, CCR6, and CD161 receptors (Acosta-Rodriguez et al., 2007), is significantly reduced in memory CD4+ T cells depleted of HOPX or SOX2. These findings suggest that HOPX and SOX2 participate to regulate the gene expression underlying the acquisition of an inflammatory phenotype by human Th17 cells.
In conclusion, our study analyzes the kinetics of the differentiation process of human Th17 cells using the optimal experimental conditions. Our findings could open new perspectives for the pharmacological modulation of Th17 responses and help elucidate and/or predict the outcome of specific therapeutic intervention against Th17 cells.
Limitations of the study
This study identified key molecules expressed by human Th17 cells and discovered that RORC, HOPX, and SOX2 are involved in the regulation of those molecules. The current data are related to invitro-differentiated Th17 cells. However, the analysis of those mechanisms in invivo-differentiated IL-17-producing cells could have provided further insight into the Th17 cell biology. Follow-up studies in this direction are required to transfer these findings in clinical research.
Ethical aspects
The usage of blood of unknown donors was approved by the Ethics Committee of the San Camillo Hospital, Rome (Italy).
Resource availability
Lead contact
Information and requests for resources should be directed to and will be fulfilled by the lead contact, Elisabetta Volpe (e.volpe@hsantalucia.it).
Materials availability
Materials used or generated in this study will be available upon reasonable request, and a material transfer agreement may be required.
Data and code availability
The RNAseq data from this study is available to the Gene Expression Omnibus (GEO) with identifier GSE172317.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We acknowledge Pierre de la Grange, Genosplice (Paris, France) and the Next Generation Sequencing Core Facility LaBSSAH CIBIO (University of Trento, Italy) for their crucial support.
This study was supported by grants from the Italian Ministry of Health (GR-2016-02361163) and FISM-Fondazione Italiana Sclerosi Multipla (FISM 2016/R/31) to E.V., the Italian Ministry of Health (RF-2018-12366111) and FISM-Fondazione Italiana Sclerosi Multipla (FISM Progetto Speciale 2018/S/5) to L.B., and FISM-Fondazione Italiana Sclerosi Multipla (FISM 2017/R/24) to C.S.
Author contributions
A.C. designed research, performed research, analyzed data, and drafted the paper; M.B., C.N., M.D.B., F.N. performed research and analyzed data; P.M. contributed to data analysis; L.B., V.S. contributed to research design, writing and revising manuscript; C.S., E.V. designed research, analyzed data, and wrote the article.
Declaration of interests
The authors have no competing interests.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102492.
Contributor Information
Elisabetta Volpe, Email: e.volpe@hsantalucia.it.
Claudio Sette, Email: claudio.sette@unicatt.it.
Supplemental information
References
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Aijo T., Butty V., Chen Z., Salo V., Tripathi S., Burge C.B., Lahesmaa R., Lahdesmaki H. Methods for time series analysis of RNA-seq data with application to human Th17 cell differentiation. Bioinformatics. 2014;30:i113–i120. doi: 10.1093/bioinformatics/btu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht I., Niesner U., Janke M., Menning A., Loddenkemper C., Kuhl A.A., Lepenies I., Lexberg M.H., Westendorf K., Hradilkova K. Persistence of effector memory Th1 cells is regulated by Hopx. Eur. J. Immunol. 2010;40:2993–3006. doi: 10.1002/eji.201040936. [DOI] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Fili L., Ferri S., Frosali F. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Oukka M., Kuchroo V.K. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmon-Hamaty F., Combe B., Hahne M., Morel J. Lymphotoxin alpha revisited: general features and implications in rheumatoid arthritis. Arthritis Res. Ther. 2011;13:232. doi: 10.1186/ar3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone A., Bianco M., Ruocco G., De Bardi M., Battistini L., Ruggieri S., Gasperini C., Centonze D., Sette C., Volpe E. Distinct expression of inflammatory features in T helper 17 cells from multiple sclerosis patients. Cells. 2019;8:533. doi: 10.3390/cells8060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone A., Volpe E. Transcriptional regulators of T helper 17 cell differentiation in Health and autoimmune diseases. Front. Immunol. 2020;11:348. doi: 10.3389/fimmu.2020.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Kook H., Milewski R., Gitler A.D., Lu M.M., Li J., Nazarian R., Schnepp R., Jen K., Biben C. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M., Madar A., Galan C., Sellars M., Mace K., Pauli F., Agarwal A., Huang W., Parkhurst C.N., Muratet M. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L., De Palma R., Santarlasci V., Maggi L., Capone M., Frosali F., Rodolico G., Querci V., Abbate G., Angeli R. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo N.C., Shayakhmetov D.M. Interleukin 1alpha and the inflammatory process. Nat. Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R., Wen J. Overview of the roles of Sox2 in stem cell and development. Biol. Chem. 2015;396:883–891. doi: 10.1515/hsz-2014-0317. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones K.P. Secukinumab: a review in moderate to severe plaque psoriasis. Am. J. Clin. Dermatol. 2015;16:323–330. doi: 10.1007/s40257-015-0143-7. [DOI] [PubMed] [Google Scholar]
- Gaublomme J.T., Yosef N., Lee Y., Gertner R.S., Yang L.V., Wu C., Pandolfi P.P., Mak T., Satija R., Shalek A.K. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell. 2015;163:1400–1412. doi: 10.1016/j.cell.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., Yang X.P., Tato C.M., Mcgeachy M.J., Konkel J.E., Ramos H.L., Wei L., Davidson T.S., Bouladoux N. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan J.L., Ouyang W. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur. J. Immunol. 2012;42:2255–2262. doi: 10.1002/eji.201242656. [DOI] [PubMed] [Google Scholar]
- Hawiger D., Wan Y.Y., Eynon E.E., Flavell R.A. The transcription cofactor Hopx is required for regulatory T cell function in dendritic cell-mediated peripheral T cell unresponsiveness. Nat. Immunol. 2010;11:962–968. doi: 10.1038/ni.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Muller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch R.V., Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- Hu D., Notarbartolo S., Croonenborghs T., Patel B., Cialic R., Yang T.H., Aschenbrenner D., Andersson K.M., Gattorno M., Pham M. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat. Commun. 2017;8:1600. doi: 10.1038/s41467-017-01571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Mckenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kanno Y., Vahedi G., Hirahara K., Singleton K., O'shea J.J. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu. Rev. Immunol. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G., Farnarier C., Kaplanski S., Porat R., Shapiro L., Bongrand P., Dinarello C.A. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994;84:4242–4248. [PubMed] [Google Scholar]
- Kebir H., Ifergan I., Alvarez J.I., Bernard M., Poirier J., Arbour N., Duquette P., Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- Kee H.J., Kim J.R., Nam K.I., Park H.Y., Shin S., Kim J.C., Shimono Y., Takahashi M., Jeong M.H., Kim N. Enhancer of polycomb1, a novel homeodomain only protein-binding partner, induces skeletal muscle differentiation. J. Biol. Chem. 2007;282:7700–7709. doi: 10.1074/jbc.M611198200. [DOI] [PubMed] [Google Scholar]
- Kryczek I., Zhao E., Liu Y., Wang Y., Vatan L., Szeliga W., Moyer J., Klimczak A., Lange A., Zou W. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E.A., Beller D.I., Mizel S.B., Unanue E.R. Identification of a membrane-associated interleukin 1 in macrophages. Proc. Natl. Acad. Sci. U S A. 1985;82:1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A., Wu C., Kleinewietfeld M., Kunder S., Hafler D.A. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.C., Long A.J., Bennett F., Whitters M.J., Karim R., Collins M., Goldman S.J., Dunussi-Joannopoulos K., Williams C.M., Wright J.F., Fouser L.A. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- Ma Q., Huang B., Khatibi N., Rolland W., 2nd, Suzuki H., Zhang J.H., Tang J. PDGFR-alpha inhibition preserves blood-brain barrier after intracerebral hemorrhage. Ann. Neurol. 2011;70:920–931. doi: 10.1002/ana.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W.T., Yao X.T., Peng Q., Chen D.K. The protective and pathogenic roles of IL-17 in viral infections: friend or foe? Open Biol. 2019;9:190109. doi: 10.1098/rsob.190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddur M.S., Miossec P., Kaveri S.V., Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am. J. Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Manel N., Unutmaz D., Littman D.R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., Mcclanahan T., Cua D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McInnes I.B., Mease P.J., Kirkham B., Kavanaugh A., Ritchlin C.T., Rahman P., Van Der Heijde D., Landewe R., Conaghan P.G., Gottlieb A.B. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- Mukasa R., Balasubramani A., Lee Y.K., Whitley S.K., Weaver B.T., Shibata Y., Crawford G.E., Hatton R.D., Weaver C.T. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Borman Z.A., Kerkar S.P., Klebanoff C.A., Ji Y., Sanchez-Perez L., Sukumar M., Reger R.N., Yu Z., Kern S.J. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli B., Zaritskaya L., Semenuk M., Cho Y.H., Lafleur D.W., Shah D., Ullrich S., Girolomoni G., Albanesi C., Moore P.A. Regulatory effect of IFN-kappa, a novel type I IFN, on cytokine production by cells of the innate immune system. J. Immunol. 2002;169:4822–4830. doi: 10.4049/jimmunol.169.9.4822. [DOI] [PubMed] [Google Scholar]
- Nistala K., Adams S., Cambrook H., Ursu S., Olivito B., De Jager W., Evans J.G., Cimaz R., Bajaj-Elliott M., Wedderburn L.R. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl. Acad. Sci. U S A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikor N.B., Prat A., Bar-Or A., Gommerman J.L. Meningeal tertiary lymphoid tissues and multiple sclerosis: a gathering place for diverse types of immune cells during CNS autoimmunity. Front. Immunol. 2015;6:657. doi: 10.3389/fimmu.2015.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzani V., Rossetti G., Panzeri I., Arrigoni A., Bonnal R.J., Curti S., Gruarin P., Provasi E., Sugliano E., Marconi M. The long intergenic noncoding RNA landscape of human lymphocytes highlights the regulation of T cell differentiation by linc-MAF-4. Nat. Immunol. 2015;16:318–325. doi: 10.1038/ni.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert-Hartwall L., Honkanen J., Salo H.M., Nieminen J.K., Luopajarvi K., Harkonen T., Veijola R., Simell O., Ilonen J., Peet A. Th1/Th17 plasticity is a marker of advanced beta cell autoimmunity and impaired glucose tolerance in humans. J. Immunol. 2015;194:68–75. doi: 10.4049/jimmunol.1401653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C.H., Liu Z.P., Passier R., Zhang C.L., Wang D.Z., Harris T.M., Yamagishi H., Richardson J.A., Childs G., Olson E.N. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110:725–735. doi: 10.1016/s0092-8674(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tripathi S.K., Chen Z., Larjo A., Kanduri K., Nousiainen K., Aijo T., Ricano-Ponce I., Hrdlickova B., Tuomela S., Laajala E. Genome-wide analysis of STAT3-mediated transcription during early human Th17 cell differentiation. Cell Rep. 2017;19:1888–1901. doi: 10.1016/j.celrep.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Välikangas T., Shetty A., Khan Mm, Moulder R., Bhosale Sd, Komsi E., Salo V., De Albuquerque R.S., Rasool O. Quantitative proteomics reveals the dynamic protein landscape during initiation of human Th17 cell polarization. iScience. 2019;21:334–355. doi: 10.1016/j.isci.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomela S., Rautio S., Ahlfors H., Oling V., Salo V., Ullah U., Chen Z., Hamalisto S., Tripathi S.K., Aijo T. Comparative analysis of human and mouse transcriptomes of Th17 cell priming. Oncotarget. 2016;7:13416–13428. doi: 10.18632/oncotarget.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomela S., Salo V., Tripathi S.K., Chen Z., Laurila K., Gupta B., Aijo T., Oikari L., Stockinger B., Lahdesmaki H., Lahesmaa R. Identification of early gene expression changes during human Th17 cell differentiation. Blood. 2012;119:e151–e160. doi: 10.1182/blood-2012-01-407528. [DOI] [PubMed] [Google Scholar]
- van Rietschoten J.G., Verzijlbergen K.F., Gringhuis S.I., Van Der Pouw Kraan T.C., Bayley J.P., Wierenga E.A., Jones P.A., Kooter J.M., Verweij C.L. Differentially methylated alleles in a distinct region of the human interleukin-1alpha promoter are associated with allele-specific expression of IL-1alpha in CD4+ T cells. Blood. 2006;108:2143–2149. doi: 10.1182/blood-2006-01-021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 2017;18:612–621. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Volpe E., Battistini L., Borsellino G. Advances in T Helper 17 Cell Biology: Pathogenic Role and Potential Therapy in Multiple Sclerosis. Mediators of Inflamm. 2015;2015:475158. doi: 10.1155/2015/475158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe E., Servant N., Zollinger R., Bogiatzi S.I., Hupe P., Barillot E., Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Volpe E., Touzot M., Servant N., Marloie-Provost M.A., Hupe P., Barillot E., Soumelis V. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114:3610–3614. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yin Z., Gonzales L., Kolla V., Rath N., Zhang Y., Lu M.M., Kimura S., Ballard P.L., Beers M.F., Epstein J.A., Morrisey E.E. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L191–L199. doi: 10.1152/ajplung.00385.2005. [DOI] [PubMed] [Google Scholar]
- Yosef N., Shalek A.K., Gaublomme J.T., Jin H., Lee Y., Awasthi A., Wu C., Karwacz K., Xiao S., Jorgolli M. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–468. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski C.E., Mele F., Aschenbrenner D., Jarrossay D., Ronchi F., Gattorno M., Monticelli S., Lanzavecchia A., Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNAseq data from this study is available to the Gene Expression Omnibus (GEO) with identifier GSE172317.