Rubella virus (RUBV), a rubivirus, is an airborne human pathogen that generally causes mild measles-like symptoms in children or adults. However, RUBV infection of pregnant women can result in miscarriage or congenital rubella syndrome (CRS), a collection of long-term birth defects, including incomplete organ development and mental retardation.
KEYWORDS: Matonaviridae, rubella virus, Rubivirus, virus assembly, virus entry, virus structure
ABSTRACT
Rubella virus (RUBV), a rubivirus, is an airborne human pathogen that generally causes mild measles-like symptoms in children or adults. However, RUBV infection of pregnant women can result in miscarriage or congenital rubella syndrome (CRS), a collection of long-term birth defects, including incomplete organ development and mental retardation. Worldwide vaccination campaigns have significantly reduced the number of RUBV infections, but RUBV continues to be a problem in countries with low vaccination coverage. Furthermore, the recent discovery of pathogenic rubiviruses in other mammals emphasizes the spillover potential of rubella-related viruses to humans. In the last decade, our understanding of RUBV has been significantly increased by virological, biochemical, and structural studies, providing a platform to begin understanding the life cycle of RUBV at the molecular level. This review concentrates on recent work on RUBV, focusing on the virion; its structural components; and its entry, fusion, and assembly mechanisms. Important features of RUBV are compared with those of viruses from other families. We also use comparative genomics, manual curation, and protein homology modeling to highlight distinct features of RUBV that are evolutionarily conserved in the nonhuman rubiviruses. Since rubella-like viruses may potentially have higher pathogenicity and transmissibility to humans, we also propose a framework for utilizing RUBV as a model to study its more pathogenic cousins.
INTRODUCTION
Rubella virus (RUBV) is a positive-strand RNA virus that belongs to the genus Rubivirus in the newly established Matonaviridae family (1). The Matonaviridae currently includes two categories of Rubivirus; the first category includes RUBV which uses humans as a reservoir, and the second category uses nonhumans as reservoirs. The nonhuman rubiviruses include Guangdong Chinese water snake rubivirus, a snake virus (2); Fedallah virus, a fish virus (3); and the recently reported ruhugu virus (RUHV) and rustrela (RUSV) virus (4), which use bats and mice, respectively, as reservoirs. The complete genomic sequences of RUHV and RUSV (4) and a partial genomic sequence of the snake rubivirus (accession no. MG600129.1) (2) are available. The GenBank accession number for the fish rubivirus (3) was not available as of the preparation of the manuscript. Phylogenetic analysis suggests that the snake and fish rubiviruses form a clade with only 35% amino acid (aa) similarity and are rather distantly related to RUBV (3). In contrast, the bat-hosted RUHV forms a clade with RUBV while RUSV forms a close out-group to this clade, suggesting a possible zoonotic origin for RUBV (4). Currently, the spillover host(s) for RUHV are unknown. The known RUSV spillover hosts are donkey, capybara, and wallaby, in all of which the virus can cause lethal encephalitis (4). While RUSV RNA was detected in the brain tissue of yellow-necked field mice, the natural reservoir of RUSV, the mice remain apparently healthy (4). Contact with oral secretions and/or feces may play a role in the transmission of RUSV from the natural reservoir to the spillover hosts (4).
RUBV is a highly contagious airborne pathogen with a basic reproductive rate (R0) between 3 and 12 (5). Humans are the only known host in nature (4). RUBV infection generally causes mild symptoms including fever and rash (5). However, infection of pregnant women particularly within the first trimester can lead to spontaneous miscarriage or congenital rubella syndrome (CRS) (5, 6). CRS includes inadequate organ development and systemic inflammation of the neonate (5) and several possible long-term sequelae, including cardiac abnormalities, deafness, intellectual disability, and developmental delays (6). Maternal circulating infected monocytes are proposed to disseminate RUBV to lymphatic vessels in the basal decidua at the uterine-placental interface (6), but it is not yet clear how RUBV crosses the placental barrier and disseminates into the fetal compartment (6, 7). Effective RUBV vaccines were licensed in 1969 to 1970 (8), and vaccination has dramatically reduced CRS cases. However, an estimated 100,000 cases still occur each year, of which only a fraction are reported to the WHO, thus contributing to an apparent gross underestimation of the actual number of CRS cases worldwide (9–11). While the Americas are essentially free of endemic cases, CRS remains a concern in the African and Southeast Asian regions due to low vaccination coverage (12).
RUBV strains are divided into two clades. Clade 1 is further divided into 10 genotypes (1a and 1B to 1J) while clade 2 has three (2A to 2C) genotypes. Currently, four genotypes, namely, 1E, 1G, 1J, and 2B, are reported to be commonly circulating in different regions of the world (13). Three attenuated strains (HPV77/DE5, RA27/3, and Cendehill) and four wild-type (wt) strains (M33, Therien, Thomas, and IB2) have been used for comparative studies (14).
This review focuses on the life cycle of RUBV with emphasis on recent findings on the structures of the virus particle and its component proteins and on the mechanisms of virus entry, fusion, assembly, and budding. Comparative genomics and protein homology modeling were used to analyze and compare the structural features of RUBV with those of RUHV and RUSV (4). For a more comprehensive coverage of the older literature and other topics such as RUBV’s clinical and immunological aspects, readers are directed to previously published articles and book chapters (5, 6, 15–17).
BRIEF OVERVIEW OF RUBV AND ITS LIFE CYCLE
RUBV is a pleomorphic enveloped virus with a single-stranded, positive-sense RNA genome (16, 18). The nucleocapsid core (NC) is comprised of the capsid protein and the genomic RNA (Fig. 1) (18, 19). The NC is enveloped by the viral membrane which contains heterodimers of the E2 and E1 envelope glycoproteins (Fig. 1) (16–18, 20, 21). RUBV particles enter target cells via clathrin-mediated endocytosis (Fig. 1) (22–24). Fusion of the viral and endosome membranes occurs in a low pH- and calcium-dependent reaction and is mediated by the E1 membrane fusion protein (23–26). Viral RNA replication occurs in the cytoplasm. The nascent NC, E2, and E1 assemble on and bud into the Golgi complex to form progeny virions, which are transported through the secretory pathway and released at the plasma membrane (Fig. 1) (16, 17, 27).
FIG 1.
Model of the RUBV life cycle. At the top: E2 (blue), E1 (red), and nucleocapsid (NC; cyan) are indicated in the RUBV cartoon, which shows virus binding to plasma membrane receptors. (a) RUBV enters the host cell by clathrin-mediated endocytosis. (b, c) In the early endosome, low pH and Ca2+ activate virus membrane fusion. (d) NC or the genome is released into the cytoplasm. (e, f) The genomic RNA is translated to the nonstructural polyprotein p200, which synthesizes negative-strand RNAs. (g, h) p200 is processed to p150 and p90; transcription of positive polarity genomic and subgenomic RNAs then takes place (102). (i) The structural polyprotein precursor p110 is translated from subgenomic RNA and translocated into the ER. Signal peptidase in the ER cleaves p110 to the capsid (cyan), E2 (blue), and E1 (red) proteins. (j) The capsid is suggested to assemble with genomic RNA into NC on the RER and then to be transported to the Golgi by interactions with E2 and/or the E2-E1 dimer. (k, l) E2-E1 heterodimers are transported to the Golgi, where RUBV assembly and budding take place (27). (m, n) Transport vesicles deliver mature RUBV from the Golgi to the cell surface. During the process of maturation, immature particles with a uniformly dense core are believed to mature into particles with a defined internal core well separated from the virus membrane (77), while the smooth exterior adopts a spiky appearance (18). For clarity, only pertinent cell organelles are diagrammed.
THE RUBV RNA GENOME
The RUBV 40S genomic RNA is ∼10 kb long (Fig. 2B) (15, 17). It is capped at the 5′ end and polyadenylated at the 3′ end (Fig. 2B) (20). The GC content of the RUBV and RUSV genomes is ∼70% and that of RUHV is ∼64%; these levels are the highest reported among RNA viruses (4, 28, 29). The RUBV genome contains two open reading frames (ORFs), namely, a 5′ ORF of ∼6.3 kb encoding the nonstructural proteins and a 3′ ORF of ∼3.2 kb encoding the structural proteins (Fig. 2B). cis-Acting elements are reported to be present at the 5′ and 3′ ends of the genome and at the junction region between the 5′ and 3′ ORFs (15–17). The incoming genomic RNA is translated into the p200 nonstructural polyprotein, which is processed by viral proteases to form p150 and p90 (Fig. 1 and 2C). The nonstructural proteins synthesize a minus-sense RNA intermediate that acts as the template for both progeny genomic RNA and a 24S subgenomic RNA encoding the p110 structural polyprotein (Fig. 1 and 2C) (16, 17, 30, 31).
FIG 2.
RUBV particles, genomes, and proteins. (A) Pleomorphic shapes of RUBV particles showing glycoprotein organization (18). Cylindrical/tube-like (left) and irregular shaped (right, top and bottom) particles. The range of helical pitch varies as follows: 53.3 nm (for tube shape), 39 nm (for top right), and 0 nm (for bottom right). The length of the scale bars is 10 nm. (B, C) Numbering of nucleotides and amino acids is shown in blue and black, respectively. (B) Schematics of the 40S RUBV genome (accession no. JN635281.1) and 24S subgenome encoding the nonstructural (p200) and structural (p110) polyproteins, respectively. The start sites of the subgenomic promoter (103) and ORFs are shown with green and black arrows, respectively. Untranslated regions are displayed in cyan. (C) Cleavage products of p200 (31) and p110. p150 viral protease and signal peptidase processing sites are shown with black and red arrows, respectively. (D) Putative arrangement of individual structural proteins on ER membranes. Capsid (umber) with E2SS (fuchsia), E2 (blue) with TM domain (yellow) and E1SS (orange), and E1 (red) with TM domain (green) are depicted. An arginine-rich sequence (RRACRRR) between E2TM and E1SS, and the E1 endodomain are shown as dotted-blue and -red lines, respectively. Sizes are not to scale. A is reproduced from reference 18.
STRUCTURAL PROTEINS
The p110 structural polyprotein has two in-frame initiation codons separated by 21 nucleotides, and both are reported to support translation (20, 32). p110 is translocated into the endoplasmic reticulum (ER), where it is processed by signal peptidase into the individual structural proteins capsid, E2, and E1 (Fig. 1 and 2C) (16, 17, 20). After cleavage, the E2 signal sequence (SS) is retained at the capsid C terminus and confers membrane binding of the capsid (Fig. 2D) (15–17). The E1SS remains at C terminus of E2, as discussed below. E2 and E1 are both type I membrane proteins with transmembrane (TM) anchors close to their C termini (Fig. 2D) (33–35). An arginine-rich sequence between the E2TM and the E1SS is required for efficient processing by the signal peptidase and appears to maintain the proper topological arrangement of E2-E1 on the ER membrane (Fig. 2D) (34). Coexpression of all three structural proteins leads to the assembly and secretion of virus-like particles (VLPs) (35–37). Although VLPs are noninfectious, their morphology and buoyant density are comparable to those of authentic RUBV particles (35). While RUBV VLPs lack the viral 40S genomic RNA, their similar density suggests the presence of cellular nucleic acids or the mRNA encoding the structural polyprotein (35, 37). As RUBV VLPs have not been extensively characterized structurally, it is not clear how the absence of the genomic RNA might influence their NC structure (35).
Summaries of our current understanding of the individual structural proteins follow.
Capsid.
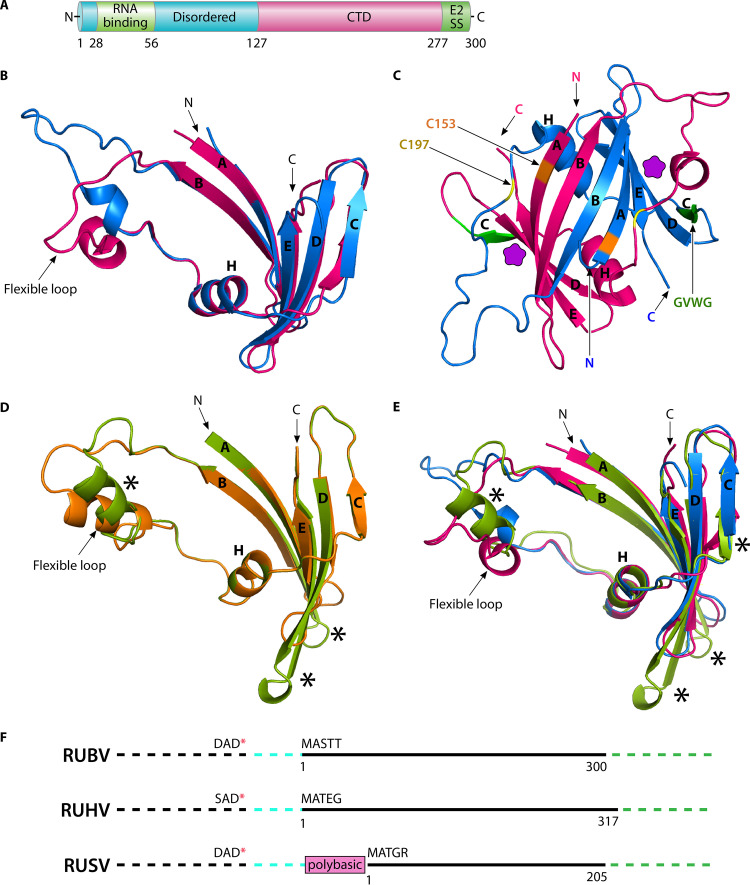
The capsid (∼33 kDa) is located at the N terminus of the p110 polyprotein precursor (20) (Fig. 2C). The capsid has three regions, as follows: an N-terminal region (amino acid [aa] 1 to 126) enriched in basic amino acids, a C-terminal structured domain (CTD) (aa 127 to 277), and the final 23-aa E2SS that also acts as the capsid membrane anchor (Fig. 2D and 3A) (19, 33, 38, 39). A specific arginine-rich peptide (aa 28 to 56) in the N-terminal region has been reported to interact with nucleotides 347 to 375 in the genomic RNA and may thus promote genomic RNA packaging (Fig. 3A) (40). The RUBV capsid also has important interactions with viral and host proteins, as summarized in reference 39. The nascent capsid is initially localized to the ER through the E2SS (Fig. 2D) and when coexpressed with either E2 or E2+E1 is transported to the Golgi, the primary site of RUBV budding (16, 41).
FIG 3.
Rubivirus capsid protein. (A) Linear diagram of RUBV capsid, showing the disordered N-terminal domain (cyan) (19) that contains the RNA binding region (green) (40), the C-terminal domain (CTD) (19), and the E2SS. Amino acid residues are numbered in black. (B) Structural alignment of two CTD monomers of capsid in two different conformations. N and C termini, strands A to E, helix H, and flexible loop are indicated. (C) Dimeric structure of CTD of capsid formed from individual CTD monomers, colored blue or magenta. N and C termini, strands, and helix are depicted as in B. Residues involved in disulfide bonding are indicated with long black arrows for one monomer and are color coded on both C153 (orange) and C197 (yellow). The GVWG peptides involved in interdimer interactions in orthorhombic crystals are highlighted in green for both and labeled for one monomer (19). Putative E2 endodomain binding sites are shown with purple pentagons. (D) Alignment of the structural model of RUHV CTD (aa 161 to 255 from accession no. MN547623.1 [orange]) and RUSV CTD (aa 45 to 148 from accession no. MN552442.1 [pea green]) generated by Phyre2 (43). The portions of the structures where the two models do not align are marked by black asterisks. (E) Structural alignment of RUSV CTD model (pea green) with RUBV CTDs (blue or magenta). Parts of the RUSV CTD model structure deviating from the RUBV CTD are highlighted with black asterisks. (F) Comparison of capsid across rubiviruses. The linear schematic of capsid (solid black line), nonstructural (NS) region (dotted black line), intergenic region between NS and structural ORFs (dotted cyan line), and E2 region (dotted green line) are shown. For each rubivirus (RUBV [accession no. Q86500 and P08563], RUHV [accession no. MN547623.1], and RUSV [accession no. MT274725.1]) the last three residues of the NS ORF with stop codon (red asterisk) and first five residues of capsid, start site, and end site of each capsid (black number) are indicated. A polybasic region (pink box) having similarity to aa ∼1 to 110 of the capsid of RUBV and RUHV was found in frame with MATGR, the proposed start site RUSV capsid (4, 44). B to E were prepared by PyMOL using PDB files 4HAR and 4HBE (19, 104), and the models of RUHV and RUSV capsid were generated by Phyre2 (43). The quality of all structural models were independently assessed by TM-align (71).
The structure of the capsid CTD (aa 127 to 277) has been solved by X-ray crystallography and displays a unique polypeptide fold (19). In the structure, the capsid forms a homodimer linked by disulfide bonds between C153 and C197 (Fig. 3C) (19). Although the role of disulfide bonds in infectious RUBV production is unknown, it is known that capsid disulfide bond formation is not required for RUBV VLP assembly (42). In the structure, each monomer consists of five antiparallel beta strands (A to E) and a two-turn alpha helix H located between strands B and C (Fig. 3B). An ∼20-residue loop between strands B and H was found to be in various configurations in different crystal forms, suggesting that this loop might be flexible (Fig. 3B). Two monomers interact with each other through hydrogen bonds between beta-strand B in an antiparallel manner (Fig. 3C). Furthermore, the dimer is stabilized by the insertion of beta strand A and B of one monomer into the long BH loop of the other monomer (Fig. 3C). Lateral interaction between capsid dimers form rows in which each dimer is oriented in such a way that the C terminus of the CTD remains perpendicular to the membrane, possibly interacting with cytoplasmic tail/endodomain peptide (RRACRRR) of E2 (Fig. 2D and 3C), while the N termini of both capsid monomers could interact with the genomic RNA (19). The observed lateral dimer-dimer interactions occur through capsid residues 219 to 222 (GVWG) (Fig. 3C), and mutations within these residues block the production of infectious virus and VLP formation, supporting an important role of the CTD dimer-dimer interactions in the virus (Fig. 3C) (19).
The capsid proteins of the recently discovered RUHV and RUSV share ∼51% and ∼46% aa sequence similarity, respectively, with the RUBV capsid (4). The capsid of RUSV was reported to be shorter than those of RUBV and RUHV (4). Protein homology modeling by Phyre2 (43) followed by structural alignment of the CTD of the RUHV and RUSV capsid proteins suggested that both share a similar fold with only occasional divergence from the alignment (Fig. 3D). A comparison of the CTD model of the RUSV capsid with the corresponding region of the RUBV capsid (Fig. 3E) suggested that the RUSV CTD deviates from RUBV CTD at comparable positions as it deviates from the RUHV CTD (compare asterisks in Fig. 3D and E). This analysis suggests that the CTD of the RUHV capsid is more closely related to the RUBV CTD than to the RUSV CTD. Further sequence alignment of the RUSV capsid with the RUBV and RUHV capsid proteins revealed that the N-terminal polybasic region (∼ aa 1 to 110) (Fig. 3A) that interacts with the genomic RNA is missing from the RUSV capsid. Comparative genomics and bioinformatic analysis suggested the presence of a polybasic region in frame with MATGR (4), the proposed start site of the RUSV capsid (Fig. 3F) (44). Although this newfound N-terminal ORF shares an amino acid sequence conservation with the corresponding regions of the RUHV and RUBV capsid proteins, it is not known if or how this fragment might be translated in RUSV-infected cells (44).
The RUBV capsid is unique compared with those of the alphaviruses and flaviviruses (19, 45, 46). The alphavirus capsid (∼35 kDa) has a C-terminal domain with a beta-sheet structure that has a chymotrypsin-like fold and acts as an autoprotease (45, 47). There is no membrane anchor, and the alphavirus NC is a highly ordered icosahedral shell. The flavivirus capsid is considerably smaller, ∼12 kDa, with a basic N-terminal unstructured region of ∼22 aa followed by an alpha-helical structure that associates to form homodimers but does not form an icosahedral shell (46). The most C-terminal flavivirus capsid helix (helix 5) serves as the SS for the subsequent prM protein and is retained on a proportion of capsid where it appears to play an important role in virus assembly (48). Thus, while all three of these capsid proteins have an N-terminal basic unstructured region and a C-terminal structured domain, their overall length, the structures of their C-terminal domains, and their arrangement in the NC are quite different.
Envelope glycoprotein 2.
E2 is a type I membrane glycoprotein with its TM domain and the E1SS located toward the C terminus (Fig. 2D and 4A). It has a predicted molecular weight of 31 kDa, the smallest among the RUBV structural proteins, but due to posttranslational modifications, the observed molecular weight in RUBV particles ranges from 42 to 47 kDa (27, 49). E2 forms a heterodimeric complex with E1 in the ER (50). E2 and E1 associate with each other within 5 min of expression, but the folding kinetics of E2 are faster (half-life [t1/2], ∼15 min) than those of E1 (t1/2, ∼60 min), perhaps reflecting differences in disulfide bond formation (the E2 ectodomain has 12 cysteine residues versus 20 for the E1 ectodomain) (50). Individually expressed E1 or E2 proteins are inefficiently transported to the Golgi, indicating the importance of heterodimerization to promote proper folding to their correct conformations (36, 41, 50, 51). The E2-E1 heterodimer is targeted to the Golgi through a Golgi retention signal that maps to the TM domain of E2 (Fig. 2D) (27).
FIG 4.
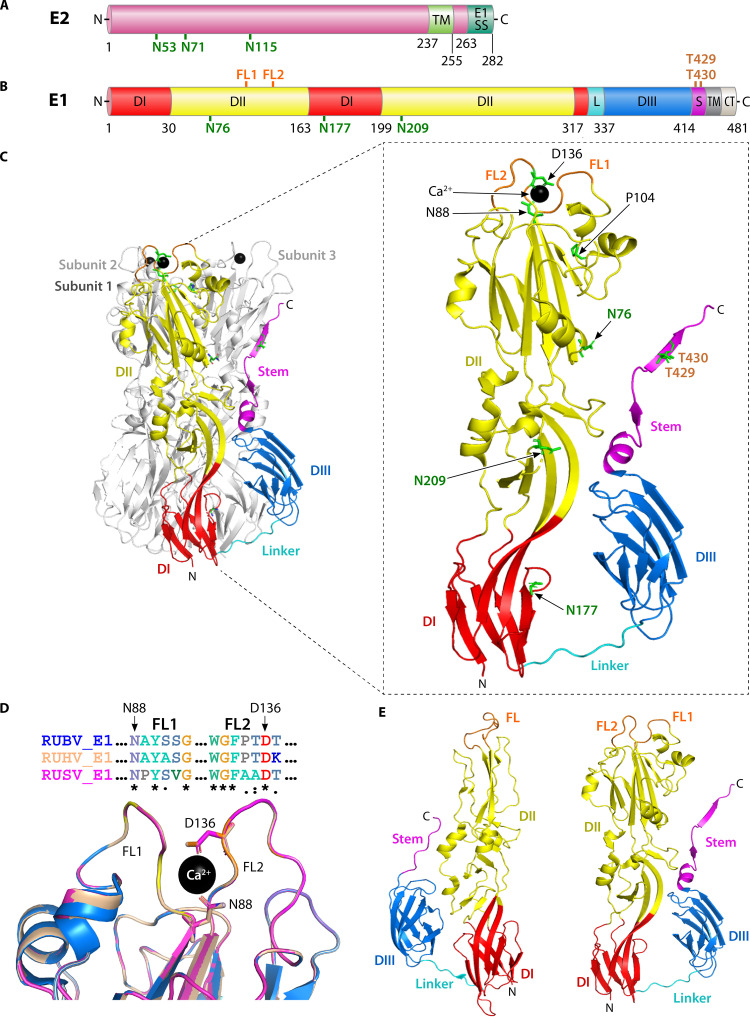
RUBV glycoproteins. (A) Linear diagram of E2 indicating the TM and E1SS. Residues are numbered in black, and N-linked glycosylation sites are shown in green. Although O-linked glycosylation of E2 has been reported, the specific sites remain unknown. (B) Linear diagram of E1 with domain boundary residues numbered in black (25). DI (red), DII (yellow), DIII (blue), linker (L; cyan), stem (S; magenta), fusion loops 1 and 2 (FL; orange), TM, and cytoplasmic tail/endodomain (CT) are indicated. N- and O- linked glycosylation sites are highlighted in green and sepia, respectively. (C) Structure of the postfusion E1 homotrimer complexed with Ca2+. Three E1 subunits are shown, but subunit 1 is highlighted with the color code as in B. Note how domain III is packed against the central DI-DII core region, while the stem region projects toward the FL of the neighboring subunit. Right: zoomed-in view highlighting the features as in B. Ca2+ ion (black sphere), proline seal residue P104, and Ca2+ coordinating residues N88 of FL1 and D136 of FL2 are shown. (D) Amino acid sequence and structural alignment of the E1 FL region of the members of the rubivirus genus. Top: sequence alignment of FL1 (aa 88 to 93) and FL2 (aa 131 to 137) of RUBV E1 (accession no. P08563 [blue]) with corresponding FL1 (aa 87 to 92) and FL2 (aa 130 to 136) of RUHV E1 (accession no. MN547623.1 [wheat]) and FL1 (aa 88 to 93) and FL2 (aa 132 to 138) of RUSV (accession no. MN552442.1 [magenta]) (4) using Bioedit (105). The conserved Ca2+ coordinating residues N88 and D136 (RUBV E1 numbering) (26) are indicated with black arrows. Conserved residues and residues with strongly or weakly similar properties are marked by asterisks (*), colon (:), or period (.), respectively. Flanking amino acid sequences are shown as dotted lines. Bottom: models of RUHV E1 and RUSV E1 were generated by Phyre2 (43) using the E1 sequences as in the top alignment, and aligned with the structure of chain B of the RUBV E1 trimer (PDB: 4ADJ) using PyMOL (104). For clarity, from the complete aligned E1 ectodomain structures (not shown), the portions representing the E1 FL region are shown using colors as in the top alignment. The FL1 (light yellow), FL2 (light orange), and Ca2+ (black sphere) coordinating residues N88 and D136 (RUBV E1 numbering) are indicated as in the top alignment. (E) Comparison of postfusion structures of SFV E1 (left) versus RUBV E1 (right). Single-subunit view of postfusion alphavirus E1 (SFV [PDB: 1RER; 106]) versus rubivirus E1 (RUBV [PDB: 4ADG; 25]). Domains and FLs are highlighted as in B. The DII of RUBV E1 is extensive compared with that of DII of SFV E1, with a larger membrane-interacting surface. The direction of DIII rotation is approximately opposite in SFV versus RUBV E1. The glycoprotein structures and/or model displayed in C, D, and E were prepared using PyMOL (104), and the PDB files are listed in the parentheses.
E2 undergoes extensive posttranslational modifications, including N- and O-linked glycosylation and fatty acylation (49, 50, 52). The commonly studied M33 strain of RUBV contains three N-linked glycosylation sites at positions N53, N71, and N115 of E2 (Fig. 4A). Mutation at these sites affected the formation of intra-E2 disulfide bonds, Golgi targeting, and E2 antigenicity (49). For the JR23 strain, mutations abrogating N-linked glycosylation at E2 position N53, N71, and N129 appear to moderately decrease membrane fusion, perhaps through indirect effects on E1 (53). While sensitivity to alkaline borohydride supports the presence of E2 O-linked carbohydrates, the sites of these modifications are yet to be determined (54). The location of the palmitoylation sites is also unclear (52).
The structure of E2 has not yet been determined. Homology modeling using Phyre2 and I-TASSER (43, 55) produced only low-confidence structural models (P. K. Das, unpublished data), suggesting that E2 may have a unique structural fold or may be disordered without its E1 dimeric partner. Secondary structural analysis using PSIPRED (56) predicted that the N-terminal 50-aa region is unstructured (P. K. Das, unpublished data). Manual curation of the E2 sequence revealed several patches of histidine residues within the N-terminal unstructured region. His residues have been reported to affect the pH dependence of alphavirus membrane fusion (57), but their role in RUBV fusion is unknown.
By analogy with the alphavirus E2-capsid interaction (58, 59), the internal/endodomain of RUBV E2 was suggested to interact with capsid to promote virus budding (36). An arginine-rich peptide (aa 256 to 262, RRACRRR) between the RUBV E2TM and E1SS (Fig. 2D) was proposed to interact with a pocket on the capsid that is enriched in hydrophobic and acidic residues and contains detergent molecules in one of the capsid crystal forms (Fig. 3C) (19). Interestingly, Phyre2 (43) predicts only a single transmembrane domain (aa 235 to 255) in the E2 protein (P. K. Das, unpublished data). It is not yet clear whether the E1SS remains in the membrane leaving only an RRACRRR loop in the virus interior or if it is released to form a longer E2 tail (Fig. 2D). Such signal peptide reorientation appears to occur for the C-terminal sequence of the alphavirus E2 protein (47, 60).
Envelope glycoprotein 1.
The RUBV E1 protein mediates both virus receptor binding and virus membrane fusion (16, 25). E1 is a type I membrane protein with a predicted molecular weight of 51 kDa; posttranslational modifications result in a molecular weight of ∼58 kDa (Fig. 4B) (16, 25, 27, 52). E1 has a predicted TM domain of ∼27 aa followed by a 13-residue-long endodomain (Fig. 2D and 4B) (16, 25, 61), both of which are required for retention of E1 in the ER and promotion of subsequent transport of the properly folded E2-E1 heterodimer to the Golgi (Fig. 4B) (62). There are N-linked glycosylation sites at E1 N76, N177, and N209 and O-linked glycosylation sites at E1 T429 and T430 (Fig. 4B) (25, 63). In the absence of E2, E1 accumulates in juxtanuclear smooth membrane domains that are post-ER but pre-Golgi compartment (51, 52). Mutation of the E1 N-linked sites did not appear to affect the targeting of E1 to these membrane subdomains (63).
The structure of the RUBV E1 ectodomain was solved in its postfusion conformation at a resolution of 1.8 Å (25). The structure reveals that RUBV E1 is a member of the class II membrane fusion proteins, a group that includes alphavirus E1 and flavivirus E as well as other viral and cellular fusion proteins (23, 25, 64). Class II proteins have a typical beta-sheet structure with 3 domains, as described below. The viral class II proteins form heterodimers with companion/chaperone proteins that are located immediately N-terminal to them in the precursor polyprotein (64, 65). In the case of the alphavirus E1 and flavivirus E proteins, folding and heterodimer formation occur with their respective companion proteins pE2 and prM. The companion proteins protect the E1/E fusion proteins from low pH in the secretory pathway. Proteolytic maturation of pE2 and prM by cellular furin primes the virus for subsequent low pH-dependent membrane fusion during entry into the host cell (23, 64, 66). While RUBV E2 is located N-terminal to E1 in the polyprotein and acts as an E1 chaperone, E2 does not appear to be proteolytically processed after the initial cleavage by signal peptidase. Thus, the mechanism of RUBV pH protection during virus biogenesis is unknown (25, 50).
Similar to other class II virus fusion proteins, the RUBV E1 ectodomain has three domains, namely, DI, DII, and DIII, and is linked to the TM by a stem region (Fig. 4C). The central DI is connected to an elongated DII on one side and via a short flexible linker to DIII on the other side. During low pH-triggered fusion, the tip of DII inserts into the target membrane, and a core trimer is formed by DII and DI. DIII and the stem region then fold back and pack against this trimer core (Fig. 4C), generating the class II trimeric hairpin conformation with the tip of DII and the TM domain at the same end of the stable trimer (Fig. 4B) (23, 25, 66). Interestingly, in the currently known structures of cellular and arboviral class II trimers, DIII rotates to the left, while in the nonarboviral fusion proteins such as RUBV E1, DIII rotates in the opposite direction (Fig. 4E) (64). However, irrespective of the DIII rotational direction, class II fusion is mediated by refolding to the postfusion homotrimer with both domain III and the stem region extending along the core of the trimer toward the fusion loop (23, 64, 65). The difference in the rotation may represent an evolutionarily distinct (64) but functionally similar feature of different class II fusion proteins.
The DII membrane-interacting region presents the highest difference versus the structures of other viral class II fusion proteins (Fig. 4C and E). Due to unique DII sequence insertions, the RUBV trimeric membrane-interacting surface has a considerably larger area (∼8,000 Å2 versus ∼4,000 Å2 in other class II proteins) (25). This hydrophobic surface is enriched with 15 aromatic residues and stabilized at the 3-fold axis by a “proline seal” formed by the interaction of P104 from the three subunits (Fig. 4C). Unlike the alphavirus and flavivirus fusion proteins, RUBV E1 has two fusion loops (FLs) rather than a single FL (Fig. 4B, C and E), at ∼residues 88 to 93 and 131 to 137, respectively (Fig. 4C) (25). Mutagenesis of the RUBV E1 region between 81 to 109, which includes FL1, impairs E1 interaction with E2 and membrane fusion activity (67). In the postfusion E1 structure, it was observed that the two FLs coordinated either a calcium (Ca2+) ion or, at lower affinity, a sodium (Na+) ion. Coordination was mediated by N88 in FL1 and D136 in FL2 (Fig. 4C). Ca2+ binding caused changes in the conformation of FL2, suggesting that Ca2+ could stabilize the optimal conformation of FL2 for membrane insertion (25). While such bipartite FLs are observed in the class II fusion proteins of the Bunyavirales (68) and the vesicular stomatitis virus G and herpes simplex virus gB class III fusion proteins (69, 70), there are no apparent Ca2+ binding sites in these fusion proteins.
The E1 proteins of RUHV and RUSV share >50% aa sequence similarity with RUBV E1 (4). Sequence analysis revealed that the Ca2+ coordinating residues of RUBV E1 (N88 and D136) (25) are conserved in RUHV E1 (N87 and D135) and RUSV E1 (N88 and D137) (Fig. 4D, top). Phyre2-generated (43) models of the RUHV and RUSV E1 proteins aligned well with the structure of RUBV E1 (25), with a TM align score of >0.95 for both E1 models (data not shown) (71). FL2 of the RUSV E1 protein had been hypothesized to have an altered conformation due to several amino acid replacements (4). However, manual analysis of the FL area in the aligned structures revealed that their conformations and the orientations of the Ca2+ coordinating residues in RUHV E1 and RUSV E1 are similar to those of RUBV E1 (Fig. 4D, bottom). Taken together, these bioinformatic analyses suggest the evolutionary conservation of the Ca2+ requirement for membrane fusion in these nonhuman relatives of RUBV.
Neutralizing antibodies from RUBV patients target the glycoproteins. Convalescent sera contain primarily antibodies against E1, suggesting that they are important for long-term immunity (72, 73) and that on the virus particle E1 may be more exposed and/or more immunogenic than E2 (74). An E1 region from residues 223 to 239 is the binding site for a potent neutralizing antibody (75). While both vaccinated individuals and RUBV convalescent patients produce significant levels of antibodies to this E1 peptide, patients with CRS and persistent RUBV infection show reduced levels and instead show an increase in antibodies to the E2 glycoprotein (73). The E1 223 to 239 site is buried in the postfusion trimer, suggesting that the antibody neutralizes infection by blocking E1 trimerization and fusion (25). A commercially available mouse monoclonal antibody, E1-20, has also been reported to bind RUBV E1 peptide from 223 to 239 and to neutralize infectivity (75, 76). This peptide is highly conserved between E1 of RUBV and RUHV, with only one amino acid substitution (R237Q) and shows five amino acid substitutions in RUSV. This finding suggests that E1-20 and other RUBV antibodies could potentially be exploited for the diagnosis of RUHV and RUSV (4, 73, 75).
STRUCTURE OF RUBV
Virus particle structure.
RUBV particles are pleomorphic and range from predominantly spherical to elongated tube-like structures. The average diameter is ∼70 nm, and tube-like particles can be up to 150 nm long (Fig. 2A) (18, 21). Unlike alphaviruses, RUBV particles are not organized with icosahedral symmetry (21). Cryo-electron tomography studies show that the particles contain an inner NC layer composed of capsid and RNA that is ∼6 nm thick. The NC center is relatively sparse. The NC is surrounded by an outer membrane layer, which including the glycoprotein spikes is ∼9 to 12 nm thick (18, 21). In the mature virus, these two layers are separated by a low-density region that is ∼7 nm thick. The E2-E1 glycoprotein spikes are organized in sets of 4 to 6 parallel rows on the surface of the virus (Fig. 2A) (18, 21). These parallel rows wrap around in a helical pattern with a pitch that varies between 0 for the spherical particles up to ∼53 nm for tubular particles (Fig. 2A) (18, 21). This is to date the only known helical arrangement of spikes on an enveloped virus (21). Individual glycoprotein spikes display a narrow tip distal to the lipid membrane with a bulge at the base of the spike near the lipid membrane (18). The spike density was fitted with the crystal structure of the postfusion E1. The results suggest that the tip of the spike is composed of E1, while the bulge at the base reflects glycosylated E2 (18). The spikes show variation in orientation, ranging from a 30° to 90° angle versus the virus membrane. A comparison with the structure of intracellular immature particles shows that the intracellular particles do not have the spiky appearance of the mature RUBV, and have a more uniformly dense core without a low-density region between the nucleocapsid and the membrane (18). This finding is in keeping with earlier electron microscopy visualization of RUBV in the Golgi of infected cells (77), as discussed in the section “Particle morphogenesis and maturation” below.
Nucleocapsid structure.
Based on the volume of the RUBV genome and size of a capsid, it was proposed that the NC contains ∼300 copies of monomeric capsid (21). To obtain further insights into the structure of NC, the RUBV capsid was expressed in bacteria and found to assemble into NC-like structures of various sizes (∼40 to 90 nm in diameter) (18). Digestion with benzonase removed the nucleic acid components without affecting the stability of these capsid assemblies (18). In contrast, while the isolated alphavirus NC is also permeable to RNase digestion, the RNA is needed to maintain NC stability (78, 79). Cryo-electron tomography of the RUBV NC-like structures reveals pseudotetrameric arrays of capsid dimers, which are also observed in RUBV particles (18). Each unit of the tetramer displays a dumbbell-shaped electron density. This was fitted with the structure of the C-terminal region of the capsid dimer (aa 127 to 277) which occupies one of the lobes, while the other lobe of the dumbbell can accommodate the RNA-interacting N-terminal domain of the capsid dimer (aa 1 to 126) (Fig. 3A) (18, 19). The E2SS at the C terminus of the capsid (aa 278 to 300) would be integrated into the virus membrane (18), while the C-terminal capsid structured region could potentially form a 1:1 interaction with the E2 endodomain (Fig. 2D and 3C) (18, 19, 64).
RUBELLA VIRUS ENTRY
Virus receptors.
While humans are the only known RUBV host in nature, in cell culture RUBV can infect other species, including hamster and African green monkey, and a variety of cell types, including kidney epithelial cells, endothelial cells, fibroblasts, and keratinocytes (14, 80, 81). These results suggest that RUBV uses either a broadly expressed receptor or multiple receptors. Older studies suggest that RUBV infection is reduced by treatment of Vero cells with glycosidase and phospholipase but not protease (80, 82, 83), although such treatments can be difficult to interpret. A putative RUBV protein receptor was identified by tandem affinity purification and mass spectrometry using a recombinant E1 ectodomain as bait (84). This protein bait was shown to bind to permissive LLC-MK2 cells but not to the nonpermissive HEK 293T cells. Neither cell line bound a recombinant E2 ectodomain, and no specific retrieval was obtained with the E2 bait. In contrast, the recombinant E1 protein retrieved myelin oligodendrocyte glycoprotein (MOG), a type I integral membrane protein of the immunoglobulin superfamily that is expressed on the surface of oligodendrocytes (83, 84). Incubation of RUBV with purified MOG ectodomain or incubation of cells with antibody to MOG inhibited infection, while ectopic expression of MOG in the nonpermissive HEK 293T cells promoted RUBV binding and infection (84). E1 is normally associated in a heterodimeric complex with E2 on the RUBV surface (19), and it remains to be investigated how MOG engages with E1 on the virus particle. While it was initially thought that MOG might be acting as a molecular mimic of E2 (85), this does not appear to be the case, and the confusion was due to an early RUBV sequencing artifact (83). The limited expression pattern of MOG does not appear to explain the relatively wide cell tropism of RUBV, as discussed in reference (83 and 84). Indeed, a recent report demonstrated RUBV infection of a human keratinocyte cell line (HaCaT) and human umbilical vein endothelial cells, even though they were both negative for MOG protein and mRNA (81). Further work on the receptor(s) that mediate RUBV infection will be illuminating.
The effect of N-linked and/or O-linked glycosylation on RUBV-host cell interaction is poorly understood. Combinatorial mutations that abrogate N-linked glycosylation sites on the E2 and E1 glycoproteins of the RUBV JR23 strain decrease hemadsorption by cell surface-expressed E2 and E1 (53). However, the lack of glycosylation could also lead to E2 and E1 misfolding and indirect effects. Production of RUBV in glycosylation mutant cell lines may be able to circumvent such issues (86). Further investigations are needed to understand how the glycosylation of RUBV might affect its interaction with host cell/receptor(s).
Virus endocytosis.
RUBV utilizes endocytic uptake to enter host cells (87). Experiments with inhibitors such as chlorpromazine support clathrin-mediated endocytosis as the primary mode of internalization (Fig. 1) (22), while acidification inhibitors, such as NH4Cl, indicate that infection requires exposure to endosomal low pH (22, 24, 87). Detailed studies of the RUBV M33 strain show that internalization of virus prebound to Vero cells in the cold occurs very quickly after shifting to 37°C (t1/2 of ∼3 to 4 min) (24). This internalization is similar to the kinetics of endocytic uptake of alphaviruses and other viruses that are internalized by the clathrin-mediated endocytic pathway (88). Older studies suggested slower entry kinetics and may reflect differences in the experimental protocol (87). Dequenching of RUBV labeled with a lipid dye showed that initial lipid mixing occurs with a t1/2 of ∼9 min, while escape from NH4Cl inhibition has a t1/2 of ∼10 min (24). These kinetics argue that low pH-triggered RUBV fusion occurs within the early endosome compartment. In support of this finding, RUBV lipid mixing primarily colocalizes with Rab5, an early endosome marker. RUBV infection is efficiently blocked by dominant negative Rab5, which inhibits delivery to early endosomes, but not by dominant negative Rab7, which inhibits delivery to late endosomes (24). RUBV fusion has a pH optimum of ∼6.0 to 6.2, in keeping with its fusion in the early endosome (26).
VIRUS-MEMBRANE FUSION
Conformational changes in the RUBV spike during fusion.
On the virus, the RUBV spike complex must remain in a fusion-competent metastable state until fusion is triggered in the endosome (23). Trypsin digestion studies indicate that the virus spike undergoes significant rearrangements upon exposure to acid pH. In the prefusion form, the E1 glycoprotein is readily degraded, while E2 is relatively protease resistant. Upon acid treatment, E2 becomes trypsin sensitive, while E1 converts to a trypsin-resistant conformation (26, 89, 90). Similar to results with alphaviruses (91), these changes in protease sensitivity reflect the conformational changes in the RUBV spike complex that result in the formation of the postfusion E1 homotrimer (26, 90). The structure of the RUBV E2-E1 heterodimer remains unresolved to date. The cryo-electron tomography studies suggest that on the virus particle, E2 could prevent E1 trimerization by a location at the base of E1, potentially restraining the rotation of the linker between E1 DI and DIII (Fig. 4C) (18, 25). If this model is correct, then low pH exposure would be hypothesized to change the location of E2 and free the E1 protein for trimerization.
Calcium requirement.
Extensive data support a critical role for calcium during RUBV fusion and infection (24, 26). Calcium is not required for virus-receptor binding or endocytic uptake or for the formation of the E1 homotrimer, as assayed by its trypsin resistance, and its absence does not protect the virus from inactivation at low pH. However, in the absence of calcium, low pH-dependent virus membrane fusion is blocked due to a block in E1 membrane insertion, as discussed further below. Time course studies using the calcium chelator BAPTA-AM show that calcium is specifically required in the early endosome and that virus escape from BAPTA inhibition has a t1/2 of ∼13 min. Alanine substitution of either E1 N88 or D136, residues that mediate Ca2+ coordination in the E1 structure (see “Envelope glycoprotein 1” and reference 25), permits E2-E1 heterodimer formation, assembly of virus particles, virus-cell binding, and E1 conversion to trypsin resistance at low pH (26). However, these mutations completely block virus fusion and infection. The configuration of N88 and D136 is also critical, as the “swapping” mutations N88D and D136N are also lethal and only reversion of any mutations at these residues to the wild-type sequence restores virus infectivity (24). Ca2+ is specifically required and could not be substituted by the other tested cations (Na+, Mg2+, Mn2+, and Zn2+) (26). Based on the sequences and modeling of their fusion loops, it appears that the recently identified rubiviruses RUHV and RUSV also require Ca2+ for membrane fusion (4) (see “Envelope glycoprotein 1”).
Membrane insertion and fusion.
Based on other class II proteins, such as alphavirus E1 and flavivirus E, initial insertion of the RUBV fusion protein into the target membrane probably occurs as a monomer, and three proteins then trimerize through DI/DII interactions, followed by fold-back of DIII to complete the fusion reaction (23). The initial membrane insertion of RUBV E1 can be followed by monitoring the coflotation of virus with liposomes on sucrose gradients under various conditions. Such coflotation experiments demonstrate that RUBV E1 membrane interaction is strongly Ca2+ dependent (24–26, 92). Mutation of the Ca2+ coordinating residues E1 N88 or D136 blocked membrane interaction under any conditions, thus explaining the mutants’ fusion defect (24, 26). When maintained in the continued presence of Ca2+, RUBV could interact with liposomes at either neutral or acidic pH. However, subsequent Ca2+ removal by EDTA results in the loss of the RUBV-liposome interaction unless the samples had also been treated at acidic pH (24). Thus, E1 membrane insertion is triggered by the presence of calcium and its interaction with the E1 fusion loops. However, the stable virus-liposome interaction after calcium and low pH-treatment reflects the irreversible formation of the postfusion E1 homotrimer and virus-liposome fusion. These results indicate that RUBV fusion has two distinct triggers, namely, an initial calcium-dependent membrane insertion and the low pH-dependent E1 refolding to the postfusion form.
The cellular TIM family proteins and synaptotagmins show calcium-dependent binding of anionic lipids such as phosphatidylserine (93, 94). However, coflotation experiments of RUBV using liposomes of different lipid compositions demonstrated that the E1 membrane interaction is not dependent on anionic lipids, such as phosphatidylserine or lysobisphosphatidic acid or the zwitterionic phospholipid phosphatidylethanolamine (24). Membrane interaction is however promoted by cholesterol and sphingomyelin (24, 92).
The BAPTA experiments indicate that calcium is required within the endosome, arguing that even though the virus is exposed to extracellular calcium, it still needs to have or maintain calcium on the FLs during the fusion process at endosomal low pH (24). The time to escape from BAPTA inhibition is somewhat longer (t1/2 of ∼13 min) than the time required to escape from NH4Cl inhibition (t1/2 of ∼10 min) or for initial lipid mixing (t1/2 of ∼9 min) (24). While these kinetic measurements are imprecise, the apparent longer time course of the calcium requirement could also reflect its continued role in downstream fusion protein rearrangements.
VIRUS EXIT PATHWAY: ASSEMBLY AND BUDDING
Golgi targeting of structural proteins.
All three virus structural proteins are initially associated with or translocated into the endoplasmic reticulum (16). However, as RUBV assembles and buds into the Golgi compartment, the individual structural proteins need to be transported there for assembly and budding (16). Among the structural proteins, E2 carries a Golgi targeting/retention signal (27). Thus, E1 is transported to Golgi by dimerizing with E2 (50), while the capsid/nucleocapsid is targeted to the Golgi by interaction with E2 and/or the E2-E1 dimer (41, 95). It is not known what are the interactions that stabilize the E2-E1 heterodimer. For the capsid, experimental evidence suggests that an interaction with E2 and/or E2-E1 is mediated by the E2SS at the capsid C terminus, thus conferring Golgi localization (16, 41, 95).
Nucleocapsid assembly.
The RUBV RNA replication complexes are assembled inside membranous structures of endo-lysosomal origin termed cytopathic vacuoles (96, 97). Assembled NCs are frequently observed on the cytoplasmic face of these vacuoles (96, 98). Rough endoplasmic reticulum (RER) cisternae, mitochondria, and Golgi stacks are recruited around the cytopathic vacuoles (96). Given the proximity of the RER (96, 98), it is plausible to suggest NCs might start to assemble on the RER, while the capsid remains bound through the E2SS (36, 95). Although direct evidence is lacking, the ER-bound capsid could interact with genomic RNA through its polybasic N-terminal cytoplasmic region while concomitantly interacting with nascent E2 and/or E2-E1 within the membrane (36, 41, 95). Consistent with this notion, it was observed that a capsid lacking the E2SS forms distinct puncta of capsid oligomers and/or aggregates in the cytoplasm (41), suggesting that membrane anchoring may promote correct capsid interactions, localization, and NC assembly.
Particle morphogenesis and maturation.
Two classes of virus particles, termed immature and mature, are found in the Golgi compartment of RUBV-infected cells (77). These two forms are morphologically distinct, and the finding that only the mature form is observed in secretory vesicles and extracellularly supports a precursor-product relationship between them (77). The immature RUBV appears relatively uniform and smooth, with the viral membrane closely apposed to a dense core and the glycoproteins oriented close to the membrane rather than as spiky structures (18). In contrast, in the mature or extracellular virus particles, E1/E2 protrude outward, and the NC core layer becomes less dense in the center and also condensed, leading to the appearance of a noticeable gap between the NC and the virus membrane (18, 77). In mature RUBV, strips of fine electron density are observed to connect the NC with the glycoprotein layer (18). RUBV is not known to encode a protein equivalent to flavivirus pr or alphavirus E3, which are known to directly or indirectly protect their FL during low-pH transit through the secretory pathway. Instead, the structural rearrangement of the RUBV envelope proteins from a smooth to spiky appearance during morphogenesis has been proposed to be essential for avoiding the unintended interaction of the E1 FLs with the host membranes during transit through the low pH milieu of the TGN and secretory vesicles (18). Although the microscopy studies suggest that RUBV undergoes a maturation process, it is not known what might be the cause of such maturation events (64). Careful comparisons of the infectivity and pH resistance of the two RUBV forms have not been done and could help to determine the importance of virus maturation.
After RUBV buds into the Golgi, it is transported to the cell surface via the secretory pathway and released into the extracellular milieu. Virus infection alters the morphology of the Golgi complex, but the importance of these changes to virus production is unclear. The E1 TM and endodomain have been reported to be involved in postbudding events that affect virus release (36, 99). Deletion of the endodomain or replacement of the E1 (TM + endodomain) with the analogous vesicular stomatitis virus (VSV) G protein sequences results in VLP assembly but not secretion (36). Mutagenesis of Y472 and Y473 in the E1 endodomain causes a similar block in virus particle release (99). As the endodomain of budded virus particles would face the virus interior, it is unclear how it could be involved in RUBV release.
FUTURE RESEARCH DIRECTIONS
The recent report of the existence of RUBV-related viruses in small animals (4) argues that there are more rubiviruses in nature than previously appreciated. We currently cannot assess the pathogenicity and spillover potential of the new or as yet unknown rubiviruses to humans; nevertheless, RUBV can be used as a model to learn more about those pathogens under controlled laboratory conditions. Some possible future directions for research are suggested below.
RUBV is a model for other placenta-crossing and fetus-infecting viruses. It can be handled at a relatively low biosafety level due to the availability of effective vaccines. While at present the lack of a reliable animal model for RUBV presents a bottleneck, organoid cultures may be an effective system (100).
The pleomorphic nature of the RUBV particle and the helical spike arrangement are intriguing. The mechanisms that drive particle budding into the Golgi, the basis of these structural features, and their possible effects on infectivity and viral fitness remain unexplored.
The receptors that mediate RUBV infection in many different cell types are undefined. Do receptors explain RUBV’s broad cell tropism? Do receptors and/or other factors explain the exclusive human host range of the virus in nature versus its ability to infect different species in laboratory cell cultures?
The maturation of RUBV particles from a smooth to spiky exterior and the changes in the NC core are unexplored. Currently, it is not known what factors drive such changes in particle morphology. High-resolution structural analyses of immature and mature particles and of the prefusion form of the E2-E1 heterodimer can address some of these issues.
RUBV is not known to undergo proteolytic maturation or to express a viroporin like the hepatitis C virus p7 (101). What is the basis for pH protection and for the regulation of low pH-triggered fusion in RUBV?
Following membrane fusion, the mechanism of NC uncoating and the role of host factors in subsequent aspects of the RUBV life cycle are unknown.
As vaccination coverage increases, RUBV infections and CRS are expected to decline. However, the recent virus discoveries indicate the potential danger of human infection by zoonotic transmission of more pathogenic rubiviruses (4). RUBV remains a model system to study basic science questions in virology and to apply such research findings to develop useful antiviral strategies.
ACKNOWLEDGMENTS
We thank the members of the Kielian laboratory for their helpful comments and discussions. We thank Patrick Lane (ScEYEnce Studios) for his contributions to graphical enhancement of the figures.
Work from our lab described in this review was supported by NIH grant R01-AI075647. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies

Pratyush Kumar Das obtained his bachelor of science with honors from Utkal University, India, and master of science with Gold Medal from Fakir Mohan University, India. For doctoral studies, he moved to the laboratory of Dr. Andres Merits at the University of Tartu, Estonia, with a competitive scholarship from the European Regional Development Fund to study replicase proteins of alphaviruses. After finishing his Ph.D. studies, he moved to the laboratory of Dr. Jussi Taipale at the University of Helsinki, Finland, as a postdoctoral researcher where he studied the sequence specificity of human transcription factors using genomics and biochemical techniques. Currently, he works as a research fellow in the laboratory of Dr. Margaret Kielian at the Department of Cell Biology in Albert Einstein College of Medicine, NY. His research interests include viral and host factors driving virus entry, egress, and evolution.

Margaret Kielian received her undergraduate training at the University of Nebraska, where she obtained a B.A. in microbiology. She did her Ph.D. studies with Dr. Zanvil Cohn at The Rockefeller University and her postdoctoral studies with Dr. Ari Helenius at Yale University. She is currently Professor of Cell Biology and Samuel H. Golding Chair in Microbiology at the Albert Einstein College of Medicine in New York, NY. Her laboratory studies virus entry, membrane fusion, biogenesis, RNA packaging, and assembly and exit using alphavirus, flavivirus, and rubella virus systems.
REFERENCES
- 1.Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Dempsey DM, Dutilh BE, Harrach B, Harrison RL, Hendrickson RC, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert M, Rubino L, Sabanadzovic S, Simmonds P, Varsani A, Zerbini FM, Davison AJ. 2019. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch Virol 164:2417–2429. 10.1007/s00705-019-04306-w. [DOI] [PubMed] [Google Scholar]
- 2.Shi M, Lin X-D, Chen X, Tian J-H, Chen L-J, Li K, Wang W, Eden J-S, Shen J-J, Liu L, Holmes EC, Zhang Y-Z. 2018. The evolutionary history of vertebrate RNA viruses. Nature 556:197–202. 10.1038/s41586-018-0012-7. [DOI] [PubMed] [Google Scholar]
- 3.Geoghegan JL, Giallonardo FD, Wille M, Ortiz-Baez AS, Costa VA, Ghaly T, Mifsud JC, Turnbull OM, Bellwood DR, Williamson JE, Holmes EC. 2020. Host evolutionary history and ecology shape virome composition in fishes. bioRxiv 10.1101/2020.05.06.081505. [DOI]
- 4.Bennett AJ, Paskey AC, Ebinger A, Pfaff F, Priemer G, Höper D, Breithaupt A, Heuser E, Ulrich RG, Kuhn JH, Bishop-Lilly KA, Beer M, Goldberg TL. 2020. Relatives of rubella virus in diverse mammals. Nature 586:424–428. 10.1038/s41586-020-2812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. 2015. Rubella. Lancet Lond Engl 385:2297–2307. 10.1016/S0140-6736(14)60539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira L. 2018. Congenital viral infection: traversing the uterine-placental interface. Annu Rev Virol 5:273–299. 10.1146/annurev-virology-092917-043236. [DOI] [PubMed] [Google Scholar]
- 7.Coyne CB, Lazear HM. 2016. Zika virus—reigniting the TORCH. Nat Rev Microbiol 14:707–715. 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SA. 2006. The history of rubella and rubella vaccination leading to elimination. Clin Infect Dis 43:S164–S168. 10.1086/505950. [DOI] [PubMed] [Google Scholar]
- 9.Patel MK, Antoni S, Danovaro-Holliday MC, Desai S, Gacic-Dobo M, Nedelec Y, Kretsinger K. 2020. The epidemiology of rubella, 2007–18: an ecological analysis of surveillance data. Lancet Glob Health 8:e1399–e1407. 10.1016/S2214-109X(20)30320-X. [DOI] [PubMed] [Google Scholar]
- 10.Vynnycky E, Adams EJ, Cutts FT, Reef SE, Navar AM, Simons E, Yoshida L-M, Brown DWJ, Jackson C, Strebel PM, Dabbagh AJ. 2016. Using seroprevalence and immunisation coverage data to estimate the global burden of congenital rubella syndrome, 1996–2010: a systematic review. PLoS One 11:e0149160. 10.1371/journal.pone.0149160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 2020. Weekly epidemiological record, vol 95, 27, p 301–324. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.World Health Organization. Rubella. 2021. World Health Organization, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/rubella. [Google Scholar]
- 13.World Health Organization. 2013. Rubella virus nomenclature update: 2013. Wkly Epidemiol Rec 88:337–343. [PubMed] [Google Scholar]
- 14.Chantler JK, Lund KD, Miki NP, Berkowitz CA, Tai G. 1993. Characterization of rubella virus strain differences associated with attenuation. Intervirology 36:225–236. 10.1159/000150341. [DOI] [PubMed] [Google Scholar]
- 15.Lee J-Y, Bowden DS. 2000. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev 13:571–587. 10.1128/cmr.13.4.571-587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobman TC. 2013. Rubella virus, p 687–711. In Knipe DM, Howley PM. (ed), Fields virology, 6th ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 17.Frey TK. 1994. Molecular biology of rubella virus. Adv Virus Res 44:69–160. 10.1016/s0065-3527(08)60328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangala Prasad V, Klose T, Rossmann MG. 2017. Assembly, maturation and three-dimensional helical structure of the teratogenic rubella virus. PLoS Pathog 13:e1006377. 10.1371/journal.ppat.1006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangala Prasad V, Willows SD, Fokine A, Battisti AJ, Sun S, Plevka P, Hobman TC, Rossmann MG. 2013. Rubella virus capsid protein structure and its role in virus assembly and infection. Proc Natl Acad Sci U S A 110:20105–20110. 10.1073/pnas.1316681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oker-Blom C, Ulmanen I, Kääriäinen L, Pettersson RF. 1984. Rubella virus 40S genome RNA specifies a 24S subgenomic mRNA that codes for a precursor to structural proteins. J Virol 49:403–408. 10.1128/JVI.49.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battisti AJ, Yoder JD, Plevka P, Winkler DC, Prasad VM, Kuhn RJ, Frey TK, Steven AC, Rossmann MG. 2012. Cryo-electron tomography of rubella virus. J Virol 86:11078–11085. 10.1128/JVI.01390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kee S-H, Cho E-J, Song J-W, Park KS, Baek LJ, Song K-J. 2004. Effects of endocytosis inhibitory drugs on rubella virus entry into VeroE6 cells. Microbiol Immunol 48:823–829. 10.1111/j.1348-0421.2004.tb03614.x. [DOI] [PubMed] [Google Scholar]
- 23.Kielian M. 2014. Mechanisms of virus membrane fusion proteins. Annu Rev Virol 1:171–189. 10.1146/annurev-virology-031413-085521. [DOI] [PubMed] [Google Scholar]
- 24.Dubé M, Etienne L, Fels M, Kielian M. 2016. Calcium-dependent rubella virus fusion occurs in early endosomes. J Virol 90:6303–6313. 10.1128/JVI.00634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DuBois RM, Vaney M-C, Tortorici MA, Kurdi RA, Barba-Spaeth G, Krey T, Rey FA. 2013. Functional and evolutionary insight from the crystal structure of rubella virus protein E1. Nature 493:552–556. 10.1038/nature11741. [DOI] [PubMed] [Google Scholar]
- 26.Dubé M, Rey FA, Kielian M. 2014. Rubella virus: first calcium-requiring viral fusion protein. PLoS Pathog 10:e1004530. 10.1371/journal.ppat.1004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobman TC, Woodward L, Farquhar MG. 1995. Targeting of a heterodimeric membrane protein complex to the Golgi: rubella virus E2 glycoprotein contains a transmembrane Golgi retention signal. Mol Biol Cell 6:7–20. 10.1091/mbc.6.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez G, Wang CY, Frey TK. 1990. Sequence of the genome RNA of rubella virus: evidence for genetic rearrangement during togavirus evolution. Virology 177:225–238. 10.1016/0042-6822(90)90476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auewarakul P. 2005. Composition bias and genome polarity of RNA viruses. Virus Res 109:33–37. 10.1016/j.virusres.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JP, Strauss JH, Strauss EG, Frey TK. 1996. Characterization of the rubella virus nonstructural protease domain and its cleavage site. J Virol 70:4707–4713. 10.1128/JVI.70.7.4707-4713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Y, Yao J, Gillam S. 2000. Rubella virus nonstructural protein protease domains involved in trans- and cis-cleavage activities. J Virol 74:5412–5423. 10.1128/jvi.74.12.5412-5423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke DM, Loo TW, McDonald H, Gillam S. 1988. Expression of rubella virus cDNA coding for the structural proteins. Gene 65:23–30. 10.1016/0378-1119(88)90413-1. [DOI] [PubMed] [Google Scholar]
- 33.Suomalainen M, Garoff H, Baron MD. 1990. The E2 signal sequence of rubella virus remains part of the capsid protein and confers membrane association in vitro. J Virol 64:5500–5509. 10.1128/JVI.64.11.5500-5509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Z, McDonald HL, Chen J, Hobman TC, Gillam S. 1994. Mutational analysis of the arginine residues in the E2-E1 junction region on the proteolytic processing of the polyprotein precursor of rubella virus. Virology 200:821–825. 10.1006/viro.1994.1250. [DOI] [PubMed] [Google Scholar]
- 35.Hobman TC, Lundstrom ML, Mauracher CA, Woodward L, Gillam S, Farquhar MG. 1994. Assembly of rubella virus structural proteins into virus-like particles in transfected cells. Virology 202:574–585. 10.1006/viro.1994.1379. [DOI] [PubMed] [Google Scholar]
- 36.Garbutt M, Law LMJ, Chan H, Hobman TC. 1999. Role of rubella virus glycoprotein domains in assembly of virus-like particles. J Virol 73:3524–3533. 10.1128/JVI.73.5.3524-3533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu Z, Ou D, Hobman TC, Gillam S. 1994. Expression and characterization of virus-like particles containing rubella virus structural proteins. J Virol 68:4086–4091. 10.1128/JVI.68.6.4086-4091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzeng W-P, Frey TK. 2009. Functional replacement of a domain in the rubella virus P150 replicase protein by the virus capsid protein. J Virol 83:3549–3555. 10.1128/JVI.02411-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilkow CS, Willows SD, Hobman TC. 2010. Rubella virus capsid protein: a small protein with big functions. Future Microbiol 5:571–584. 10.2217/fmb.10.27. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Yang D, Qiu Z, Lim KT, Chong P, Gillam S. 1996. Identification of domains in rubella virus genomic RNA and capsid protein necessary for specific interaction. J Virol 70:2184–2190. 10.1128/JVI.70.4.2184-2190.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron MD, Ebel T, Suomalainen M. 1992. Intracellular transport of rubella virus structural proteins expressed from cloned cDNA. J Gen Virol 73:1073–1086. 10.1099/0022-1317-73-5-1073. [DOI] [PubMed] [Google Scholar]
- 42.Lee JY, Hwang D, Gillam S. 1996. Dimerization of rubella virus capsid protein is not required for virus particle formation. Virology 216:223–227. 10.1006/viro.1996.0051. [DOI] [PubMed] [Google Scholar]
- 43.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. 2015. The Phyre2 Web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das PK, Kielian M. 2021. The enigmatic capsid protein of an encephalitic rubivirus. J Virol 10.1128/JVI.02294-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jose J, Snyder JE, Kuhn RJ. 2009. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol 4:837–856. 10.2217/fmb.09.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byk LA, Gamarnik AV. 2016. Properties and functions of the dengue virus capsid protein. Annu Rev Virol 3:263–281. 10.1146/annurev-virology-110615-042334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuhn RJ. 2021. Togaviridae: the viruses and their replication, p 173–193. In Fields virology: emerging viruses, 7th ed. Wolters Kluwer, Philadelphia, PA. [Google Scholar]
- 48.Tan TY, Fibriansah G, Kostyuchenko VA, Ng T-S, Lim X-X, Zhang S, Lim X-N, Wang J, Shi J, Morais MC, Corti D, Lok S-M. 2020. Capsid protein structure in Zika virus reveals the flavivirus assembly process. Nat Commun 11:895. 10.1038/s41467-020-14647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu Z, Hobman TC, McDonald HL, Seto NO, Gillam S. 1992. Role of N-linked oligosaccharides in processing and intracellular transport of E2 glycoprotein of rubella virus. J Virol 66:3514–3521. 10.1128/JVI.66.6.3514-3521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hobman TC, Woodward L, Farquhar MG. 1993. The rubella virus E2 and E1 spike glycoproteins are targeted to the Golgi complex. J Cell Biol 121:269–281. 10.1083/jcb.121.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hobman TC, Woodward L, Farquhar MG. 1992. The rubella virus E1 glycoprotein is arrested in a novel post-ER, pre-Golgi compartment. J Cell Biol 118:795–811. 10.1083/jcb.118.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hobman TC, Lundstrom ML, Gillam S. 1990. Processing and intracellular transport of rubella virus structural proteins in COS cells. Virology 178:122–133. 10.1016/0042-6822(90)90385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu B, Liu X, Wang Z. 2009. Effects of E2 and E1 glycosylation on specific membrane fusion in rubella virus strain JR23. Intervirology 52:68–77. 10.1159/000214861. [DOI] [PubMed] [Google Scholar]
- 54.Lundström ML, Mauracher CA, Tingle AJ. 1991. Characterization of carbohydrates linked to rubella virus glycoprotein E2. J Gen Virol 72:843–850. 10.1099/0022-1317-72-4-843. [DOI] [PubMed] [Google Scholar]
- 55.Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738. 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchan DWA, Jones DT. 2019. The PSIPRED protein analysis workbench: 20 years on. Nucleic Acids Res 47:W402–W407. 10.1093/nar/gkz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin Z, Zheng Y, Kielian M. 2009. Role of conserved histidine residues in the low-pH dependence of the Semliki forest virus fusion protein. J Virol 83:4670–4677. 10.1128/JVI.02646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee S, Owen KE, Choi HK, Lee H, Lu G, Wengler G, Brown DT, Rossmann MG, Kuhn RJ. 1996. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Struct Lond Engl 4:531–541. 10.1016/S0969-2126(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 59.Skoging U, Vihinen M, Nilsson L, Liljeström P. 1996. Aromatic interactions define the binding of the alphavirus spike to its nucleocapsid. Structure 4:519–529. 10.1016/s0969-2126(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 60.Harrington HR, Zimmer MH, Chamness LM, Nash V, Penn WD, Miller TF, Mukhopadhyay S, Schlebach JP. 2020. Cotranslational folding stimulates programmed ribosomal frameshifting in the alphavirus structural polyprotein. J Biol Chem 295:6798–6808. 10.1074/jbc.RA120.012706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao J, Gillam S. 1999. Mutational analysis, using a full-length rubella virus cDNA Clone, of rubella virus E1 transmembrane and cytoplasmic domains required for virus release. J Virol 73:4622–4630. 10.1128/JVI.73.6.4622-4630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hobman TC, Lemon HF, Jewell K. 1997. Characterization of an endoplasmic reticulum retention signal in the rubella virus E1 glycoprotein. J Virol 71:7670–7680. 10.1128/JVI.71.10.7670-7680.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hobman TC, Qiu Z, Chaye H, Gillam S. 1991. Analysis of rubella virus E1 glycosylation mutants expressed in COS cells. Virology 181:768–772. 10.1016/0042-6822(91)90915-x. [DOI] [PubMed] [Google Scholar]
- 64.Rey FA, Lok S-M. 2018. Common features of enveloped viruses and implications for immunogen design for next-generation vaccines. Cell 172:1319–1334. 10.1016/j.cell.2018.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kielian M. 2006. Class II virus membrane fusion proteins. Virology 344:38–47. 10.1016/j.virol.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 66.Harrison SC. 2015. Viral membrane fusion. Virology 479–480:498–507. 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang D, Hwang D, Qiu Z, Gillam S. 1998. Effects of mutations in the rubella virus E1 glycoprotein on E1-E2 interaction and membrane fusion activity. J Virol 72:8747–8755. 10.1128/JVI.72.11.8747-8755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guardado-Calvo P, Rey FA. 2017. The envelope proteins of the Bunyavirales. Adv Virus Res 98:83–118. 10.1016/bs.aivir.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220. 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 70.Roche S, Bressanelli S, Rey FA, Gaudin Y. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187–191. 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Skolnick J. 2005. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res 33:2302–2309. 10.1093/nar/gki524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katow S, Sugiura A. 1985. Antibody response to individual rubella virus proteins in congenital and other rubella virus infections. J Clin Microbiol 21:449–451. 10.1128/JCM.21.3.449-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell LA, Zhang T, Ho M, Décarie D, Tingle AJ, Zrein M, Lacroix M. 1992. Characterization of rubella virus-specific antibody responses by using a new synthetic peptide-based enzyme-linked immunosorbent assay. J Clin Microbiol 30:1841–1847. 10.1128/JCM.30.7.1841-1847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green KY, Dorsett PH. 1986. Rubella virus antigens: localization of epitopes involved in hemagglutination and neutralization by using monoclonal antibodies. J Virol 57:893–898. 10.1128/JVI.57.3.893-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolinsky JS, Sukholutsky E, Moore WT, Lovett A, McCarthy M, Adame B. 1993. An antibody- and synthetic peptide-defined rubella virus E1 glycoprotein neutralization domain. J Virol 67:961–968. 10.1128/JVI.67.2.961-968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolinsky JS, McCarthy M, Allen-Cannady O, Moore WT, Jin R, Cao SN, Lovett A, Simmons D. 1991. Monoclonal antibody-defined epitope map of expressed rubella virus protein domains. J Virol 65:3986–3994. 10.1128/JVI.65.8.3986-3994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Risco C, Carrascosa JL, Frey TK. 2003. Structural maturation of rubella virus in the Golgi complex. Virology 312:261–269. 10.1016/s0042-6822(03)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Acheson NH, Tamm I. 1970. Ribonuclease sensitivity of Semliki forest virus nucleocapsids. J Virol 5:714–717. 10.1128/JVI.5.6.714-717.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tellinghuisen TL, Hamburger AE, Fisher BR, Ostendorp R, Kuhn RJ. 1999. In vitro assembly of alphavirus cores by using nucleocapsid protein expressed in Escherichia coli. J Virol 73:5309–5319. 10.1128/JVI.73.7.5309-5319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mastromarino P, Cioè L, Rieti S, Orsi N. 1990. Role of membrane phospholipids and glycolipids in the Vero cell surface receptor for rubella virus. Med Microbiol Immunol 179:105–114. 10.1007/BF00198531. [DOI] [PubMed] [Google Scholar]
- 81.Trinh QD, Pham NTK, Takada K, Komine-Aizawa S, Hayakawa S. 2018. Myelin oligodendrocyte glycoprotein-independent rubella infection of keratinocytes and resistance of first-trimester trophoblast cells to rubella virus in vitro. Viruses 10:23. 10.3390/v10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mastromarino P, Rieti S, Cioè L, Orsi N. 1989. Binding sites for rubella virus on erythrocyte membrane. Arch Virol 107:15–26. 10.1007/BF01313874. [DOI] [PubMed] [Google Scholar]
- 83.Backovic M, Rey FA. 2012. Virus entry: old viruses, new receptors. Curr Opin Virol 2:4–13. 10.1016/j.coviro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cong H, Jiang Y, Tien P. 2011. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J Virol 85:11038–11047. 10.1128/JVI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duvanel CB, Honegger P, Matthieu J-M. 2001. Antibodies directed against rubella virus induce demyelination in aggregating rat brain cell cultures. J Neurosci Res 65:446–454. 10.1002/jnr.1173. [DOI] [PubMed] [Google Scholar]
- 86.Patnaik SK, Stanley P. 2006. Lectin‐resistant CHO glycosylation mutants. Methods Enzymol 416:159–182. 10.1016/S0076-6879(06)16011-5. [DOI] [PubMed] [Google Scholar]
- 87.Petruzziello R, Orsi N, Macchia S, Rieti S, Frey TK, Mastromarino P. 1996. Pathway of rubella virus infectious entry into Vero cells. J Gen Virol 77:303–308. 10.1099/0022-1317-77-2-303. [DOI] [PubMed] [Google Scholar]
- 88.Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu Rev Biochem 79:803–833. 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 89.Ho-Terry L, Cohen A. 1980. Degradation of rubella virus envelope components. Arch Virol 65:1–13. 10.1007/BF01340535. [DOI] [PubMed] [Google Scholar]
- 90.Katow S, Sugiura A. 1988. Low pH-induced conformational change of rubella virus envelope proteins. J Gen Virol 69:2797–2807. 10.1099/0022-1317-69-11-2797. [DOI] [PubMed] [Google Scholar]
- 91.Kielian M, Chanel-Vos C, Liao M. 2010. Alphavirus entry and membrane fusion. Viruses 2:796–825. 10.3390/v2040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Otsuki N, Sakata M, Saito K, Okamoto K, Mori Y, Hanada K, Takeda M. 2017. Both sphingomyelin and cholesterol in the host cell membrane are essential for rubella virus entry. J Virol 92:e01130-17. 10.1128/JVI.01130-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuo W, Herrick DZ, Ellena JF, Cafiso DS. 2009. The calcium-dependent and calcium-independent membrane binding of synaptotagmin 1: two modes of C2B binding. J Mol Biol 387:284–294. 10.1016/j.jmb.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim Y-LE, Lee H-H, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM. 2010. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol 184:1918–1930. 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Law LMJ, Duncan R, Esmaili A, Nakhasi HL, Hobman TC. 2001. Rubella virus E2 signal peptide is required for perinuclear localization of capsid protein and virus assembly. J Virol 75:1978–1983. 10.1128/JVI.75.4.1978-1983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fontana J, López-Iglesias C, Tzeng W-P, Frey TK, Fernández JJ, Risco C. 2010. Three-dimensional structure of Rubella virus factories. Virology 405:579–591. 10.1016/j.virol.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fontana J, Tzeng W-P, Calderita G, Fraile-Ramos A, Frey TK, Risco C. 2007. Novel replication complex architecture in rubella replicon-transfected cells. Cell Microbiol 9:875–890. 10.1111/j.1462-5822.2006.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee J-Y, Marshall JA, Bowden DS. 1999. Localization of rubella virus core particles in Vero cells. Virology 265:110–119. 10.1006/viro.1999.0016. [DOI] [PubMed] [Google Scholar]
- 99.Yao J, Gillam S. 2000. A single-amino-acid substitution of a tyrosine residue in the rubella virus E1 cytoplasmic domain blocks virus release. J Virol 74:3029–3036. 10.1128/jvi.74.7.3029-3036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lancaster MA, Huch M. 2019. Disease modelling in human organoids. Dis Model Mech 12:dmm039347. 10.1242/dmm.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wozniak AL, Griffin S, Rowlands D, Harris M, Yi M, Lemon SM, Weinman SA. 2010. Intracellular proton conductance of the hepatitis C virus p7 protein and its contribution to infectious virus production. PLoS Pathog 6:e1001087. 10.1371/journal.ppat.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liang Y, Gillam S. 2000. Mutational analysis of the rubella virus nonstructural polyprotein and its cleavage products in virus replication and RNA synthesis. J Virol 74:5133–5141. 10.1128/jvi.74.11.5133-5141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tzeng W-P, Frey TK. 2002. Mapping the rubella virus subgenomic promoter. J Virol 76:3189–3201. 10.1128/jvi.76.7.3189-3201.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DeLano WL. 2019. The PyMOL molecular graphics system, version 2.3.5. Schrödinger, LLC, New York, NY. [Google Scholar]
- 105.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser (Oxf) 41:95–98. [Google Scholar]
- 106.Gibbons DL, Vaney M-C, Roussel A, Vigouroux A, Reilly B, Lepault J, Kielian M, Rey FA. 2004. Conformational change and protein-protein interactions of the fusion protein of Semliki forest virus. Nature 427:320–325. 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]