Abstract
Aims
Sudden death and aborted sudden death have been observed in patients with biallelic variants in TECRL. However, phenotypes have only begun to be described and no data are available on medical therapy after long-term follow-up.
Methods and results
An international, multi-centre retrospective review was conducted. We report new cases associated with TECRL variants and long-term follow-up from previously published cases. We present 10 cases and 37 asymptomatic heterozygous carriers. Median age at onset of cardiac symptoms was 8 years (range 1–22 years) and cases were followed for an average of 10.3 years (standard deviation 8.3), right censored by death in three cases. All patients on metoprolol, bisoprolol, or atenolol were transitioned to nadolol or propranolol due to failure of therapy. Phenotypes typical of both long QT syndrome and catecholaminergic polymorphic ventricular tachycardia (CPVT) were observed. We also observed divergent phenotypes in some cases despite identical homozygous variants. None of 37 heterozygous family members had a cardiac phenotype.
Conclusion
Patients with biallelic pathogenic TECRL variants present with variable cardiac arrhythmia phenotypes, including those typical of long QT syndrome and CPVT. Nadolol and propranolol may be superior beta-blockers in this setting. No cardiac disease or sudden death was present in patients with a heterozygous genotype.
Keywords: Sudden death, Ventricular fibrillation, Long QT syndrome, Catecholaminergic polymorphic ventricular tachycardia, Paediatric
What’s new?
Biallelic TECRL variants are associated with sudden death, including phenotypes typical of both long QT syndrome and catecholaminergic polymorphic ventricular tachycardia.
Different patients presented with divergent phenotypes, despite identical homozygous variants.
All patients on metoprolol, bisoprolol, or atenolol were transitioned to nadolol or propranolol due to failure of therapy.
No heterozygous carriers had sudden death or life-threatening events.
Introduction
Sudden death and aborted sudden death have been reported in patients with homozygous or compound heterozygous variants in the trans-2,3-enoyl-CoA reductase-like gene (TECRL), which is a cardiac and skeletal muscle protein hypothesized to have a role in calcium homeostasis.1 However, to date, only nine families have been described in five publications.1–5 The first family was reported by Bhuiyan et al.1,2 in 2007; the specific genetic defect in TECRL was described in 2016. Presentations of patients with TECRL variants have ranged in age from a cardiac arrest in a homozygous 4 years old to reports of multiple syncopal events without cardiac arrest in a heterozygous 42 years old.1,3 Similarly, a normal QTc has been described in some patients1,4,5; whereas others have had a distinctly prolonged QTc.1,4 In most marked contrast, one publication reported two family members with left ventricular dilation, one heterozygous, and one homozygous.4
In summary, while multiple case reports describe life-threatening arrhythmias associated with TECRL variants, the phenotype has only begun to be described. In addition, there are no reports of long-term follow-up to assess therapy. We present new cases and present long-term follow-up from cases reported previously.1,2,5 Our specific goals included the following: to more fully describe the clinical features of patients affected by TECRL variants; to report on a series of unaffected, heterozygous cases; and finally, to establish an initial clinical approach until a multi-centre prospective registry can be established to provide more definitive clinical recommendations.
Methods
Investigators contributed clinical cases of TECRL variants to an international, multi-centre retrospective review. After each centre had acquired appropriate human subjects permission or received a waiver of permission, a standardized data entry sheet was provided by the co-ordinating centre. Standardized data acquisition was supplemented with primary phenotypic data and additional clinical information as needed. A haemodynamic symptom was defined as syncope, seizure, or cardiac arrest. A cardiac arrest was any event requiring cardiopulmonary resuscitation, defibrillation, or any sudden event culminating in death with no known extracardiac cause. Data were gathered on all available cardiac tests, including electrocardiograms (ECG), echocardiograms, cardiac magnetic resonance imaging, exercise stress tests, cardiac catheterization, electrophysiology studies, and provocative drug infusion studies. QT values were corrected for heart rate by Bazett’s formula (QTc). QTc values were not tabulated in ECGs obtained in the first 2 weeks after a cardiac arrest. In cases where phenotypic uncertainty existed, primary data were reviewed by authors G.W. or A.A.M.W. Pedigrees were provided by each contributor and were simplified into a standardized format at the co-ordinating centre. Drug doses were tabulated in milligrams per kilogram per day (mg/kg/day) in children and as milligram (mg) doses in adults. Data were gathered for invasive therapies, including implantable cardioverter-defibrillators (ICDs), pacing, and cardiac sympathectomy. Data on appropriate shocks, inappropriate shocks, and complications were obtained for all ICDs. All DNA coding and protein effects were called against transcript NM_001010874. Variant classification was performed using American College of Medical Genetics standards by a single, independent clinical laboratory.6 Genes previously associated with phenotypes of long QT syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT), and calmodulinopathy were evaluated to ensure that other potentially pathogenic variants were not present (Supplementary material online, Table S1). The gnomAD database was accessed on 14 April 2020 from gnomad.broadinstitute.org.
Results
Ten cases were identified with a median age of 8 years (range 1–22) at the time of the first haemodynamic symptom (Table 1). The mean duration of clinical follow-up from the date of first symptom was 10.3 years (SD 8.3), right censored by death in 3/10 cases. The median age at first cardiac arrest was 12.5 years (range 4–31). An ICD was implanted in 5/10 cases, with 3/5 ICDs delivering appropriate shocks and 2/5 delivering inappropriate shocks. Families reported Canada (2 families), Inner Mongolia, Iraq, Sudan, and Turkey as their location of origin.
Table 1.
Summary of clinical presentations
| Case number | Family number | Age at presentation (years) | Clinical presentation | ECG | Provocative testing | Number of asymptomatic, heterozygous relatives |
|---|---|---|---|---|---|---|
| 1 | 1 | 2.5 | VF/TdP with exertion | Normal | Not performed | 24 (same family) |
| 2 | 1 | 3.5 | VF/TdP with exertion | QTc 494 ms | EST: PVCs | |
| 3 | 2 | 9 | AVR, NSVT, and TdP with syncope | QTc 475 ms | Isoproterenol: polymorphic PVCs | 2 |
| 4 | 3 | 12 | VF while playing outside | Low voltages and flat t-waves with normal QTc | EST: monomorphic PVCs from RVOT | 2 |
| 5 | 4 | 22 | VF/TdP while walking and after an argument | QTc 464 ms | Isoproterenol: prolonged QTc | 2 |
| 6 | 5 | 31 | VF after an argument; history of recurrent syncope since age <15 | QTc 464 ms with notched T-waves on one ECG | Isoproterenol: prolonged QTc from normal to 520 ms with subsequent polymorphic VT | N/A |
| 7 | 6 | 12 | Recurrent syncope | QTc 460 ms | EST: PVCs and bi-directional couplets | N/A |
| 8 | 7 | 7 | Sudden death | Not performed | Not performed | 7 (same family) |
AVR, accelerated ventricular rhythm; EST, exercise stress test; N/A, no data available; NSVT, non-sustained ventricular tachycardia; QTc, corrected QT interval; TdP, torsade de pointes; VF, ventricular fibrillation.
Therapy with nadolol or propranolol is currently prescribed in all seven living patients. Two patients are also treated with a second agent (flecainide and propafenone in one case each). Therapy with metoprolol, bisoprolol, or low-dose atenolol was attempted in 4/7 cases but was changed to nadolol or propranolol due to lack of efficacy in all four cases. Three children, all siblings, died prior to genotyping or treatment of their disease (Cases 8–10).
Three patients had secondary neurologic injury after cardiac arrest and one patient had a mild pre-existing developmental delay. None of the remaining patients had any known neurologic phenotype.
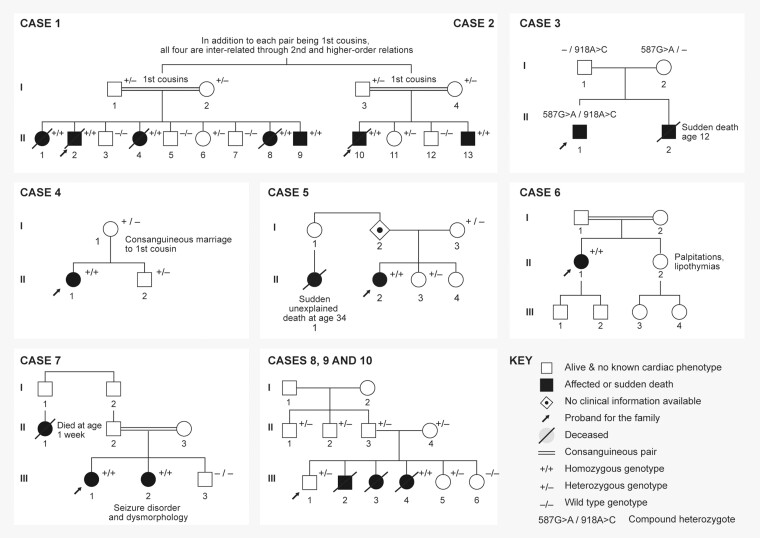
Homozygous variants in TECRL were present in 9/10 cases, annotated in Supplementary material online, Table S1. The remaining case had compound heterozygous TECRL variants (Table 2). Family history and cascade screening results are presented in Figure 1 and ECG tracings of family members, when available, are provided in Supplementary material online, Figure S2.
Table 2.
Summary of TECRL genotypes
| Case number | Family number | TECRL variant | Protein effect | Zygosity |
|---|---|---|---|---|
| 1 | 1 | c.331 + 1G>A | Splice donor | Homozygous |
| 2 | 1 | c.331 + 1G>A | Splice donor | Homozygous |
| 3 | 2 | c.918 + 3A>C and c.587G>A | Splice donor and p. Arg196Gln | Compound heterozygote |
| 4 | 3 | c.591C>A | p.Tyr197* | Homozygous |
| 5 | 4 | c.587G>A | p.Arg196Gln | Homozygous |
| 6 | 5 | c.587G>A | p.Arg196Gln | Homozygous |
| 7 | 6 | (234 + 1_235-1)_(286 + 1_287-1)del | Deletion, including Exon 2 | Homozygous |
| 8 | 7 | c.331 + 1G>A | Splice donor | Homozygous |
Splice donor = abolishment of a canonical splice donor site.
Figure 1.
Pedigree summaries for seven affected families.
Case reports
Case 1
A 2-year-old boy presented with syncope and ventricular fibrillation (VF) while running. His subsequent ECGs had normal T-wave morphology with QTc values between 400 and 440 ms. He developed cerebral palsy and epilepsy, both thought to be secondary to cardiac arrest. At last follow-up, he was 7 years old and had been event-free on propranolol monotherapy (0.6 mg/kg/day). Genotype: homozygous c. 331 + 1 G>A splice variant; Figure 1, Case 1.
Four of his siblings had previously suffered sudden death. Three siblings had structurally normal hearts and died at ages 8, 10, and 12; a fourth had double-outlet right ventricle with pulmonary atresia. She received a surgical repair, but died at age 8, while dancing. These four siblings have been previously described,1 so their case reports are not repeated here, except to note that one sibling died while being treated with metoprolol and one died while being treated with atenolol (both homozygous for c.331 + 1G>A).
Case 2
Separately, a 3-year-old boy presented with VF while running in the park (Figure 2A). He was later discovered to be the cousin of Case 1. After full recovery, his QTc was between 450 and 494 ms. Initial therapy was atenolol 1.5 mg/kg/day and flecainide 3 mg/kg/day, plus an epicardial ICD. Both antiarrhythmics were discontinued after recurrent arrhythmias. At last follow-up (age 12), he was treated with nadolol 2.4 mg/kg/day, without subsequent events. Despite the nadolol therapy, he continued to have pre-mature ventricular contractions (PVCs), occasional couplets, and occasional triplets on exercise stress testing (peak heart rate 144 b.p.m., 66% predicted for age). Genotype: homozygous c. 331 + 1 G>A splice variant; Figure 1, Case 2.
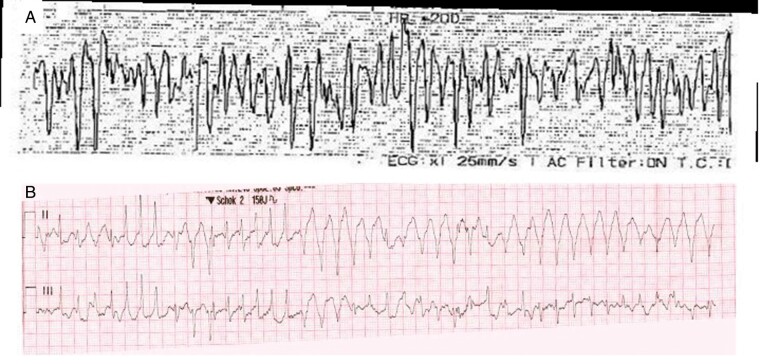
Figure 2.
Ventricular arrhythmias at the time of resuscitation. (A) VF in Case 2. (B) VF in Case 4 with failed defibrillation, delivered in early repolarization. A second defibrillation attempt was transiently successful, but VF recurred and sinus rhythm was eventually re-established after sedation was administered for rapid sequence intubation. VF, ventricular fibrillation.
Extended family of Cases 1 and 2
Since the prior publications, 24 unaffected heterozygous family members have been genotyped in the family. The mean age of affected heterozygous family members was 23.2 years (SD 17.2, range 2–59 years). No deaths or life-threatening events have occurred in the heterozygous relatives. All available cardiac evaluations in heterozygous relatives have been normal.
Case 3
A 13-year-old boy presented with recurrent syncopal episodes starting at age 9, without clear triggers. His longest QTc was 475 ms. A 24-h ambulatory cardiac monitor showed PVCs and bi-directional/polymorphic VT. An isoproterenol stress test with ventricular stimulation (coupling interval 300 ms) induced polymorphic PVCs. An ICD was placed at age 14. One year after the initial evaluation and device placement, an appropriate shock was delivered during metoprolol monotherapy. His most recent therapy was propranolol 4 mg/kg/day and propafenone 5 mg/kg/day. He continued to have non-sustained ventricular arrhythmias on this regimen, but no sustained arrhythmias or ICD shocks. Genotype: compound heterozygous c.918 + 3A>C splice variant and c.587G>A, p. Arg196Gln; Figure 1, Case 3. This case was originally published in 2019.5
Case 4
A 12-year-old girl presented with a history of two syncopal events while playing outside, beginning at age 9. Three syncopal events were diagnosed as epilepsy due to seizure activity during the events. At age 12, she had an out-of-hospital cardiac arrest with VF. After two defibrillation attempts (Figure 2B), there was return of spontaneous circulation, however polymorphic ventricular tachycardia and VF relapsed, despite escalating amiodarone, magnesium, and esmolol therapy. The rhythm normalized consistently when midazolam was administered intravenously for rapid sequence intubation. She was started on propranolol 3.2 mg/kg/day and flecainide 2 mg/kg/day. She received an endocardial ICD and the device has had neither shocks nor arrhythmia detection through her most recent follow-up at age 15. Her ECGs were unremarkable with a longest QTc of 426 ms. She had a pre-existing mild cognitive delay, assessed by neurocognitive testing, that was not thought to be secondary to cardiac arrest. Genotype: homozygous TECRL c.591C>A, p. Tyr197*; Figure 1, Case 4. An additional rare heterozygous variant was identified in SCN5A (Supplementary material online, Table S1); however, the SCN5A variant occurs in a non-conserved cytoplasmic domain and has not been reported to be associated with disease. No functional data have been published. This patient’s phenotype was not consistent with long QT or Brugada syndrome. The SCN5A variant was adjudicated as variant of uncertain significance. Our current interpretation is that the SCN5A VUS is not a monogenic cause of arrhythmia in this case. This case has not been previously published.
Case 5
A 22-year-old woman suffered a cardiac arrest (VF/TdP) while walking. Her QTc has ranged from 433 to 464 ms and her QTc prolonged further during isoproterenol challenge. An ICD was placed and she was treated initially with nadolol, then bisoprolol at various doses. She had an arrhythmic storm at age 23 in the context of mild hypokalaemia (3.0 mmol/L) and received seven appropriate shocks from her ICD. She developed mild anoxic encephalopathy after the arrhythmia storm. At age 37, she had VT, VF, and recurrent paroxysmal atrial tachycardia (150 b.p.m.), triggered by an argument with her family, receiving six appropriate shocks. At age 38, she developed right bundle branch block of unclear aetiology (Supplementary material online, Figure S2). Transthoracic echocardiography and coronary angiography were both unremarkable. At last follow-up, she was maintained on nadolol 120 mg daily, spironolactone 12.5 mg daily, potassium 20 mEq/day, and magnesium citrate 870 mg twice daily, without events on this regimen. Genotype: homozygous c.587G>A, p. Arg196Gln; Figure 1, Case 5. This case was originally published in 2016.1
Case 6
A 31-year-old woman had a history of multiple episodes of syncope, both at rest and with exercise, starting before age 15. At the time, she had non-sustained ventricular tachycardia during an exercise stress test and was prescribed nadolol. Despite taking the nadolol, at age 31 she suffered cardiac arrest (VF) precipitated by a heated argument with a family member and an ICD was placed. Her QTc was between 426 and 464 ms, and there was notching of her T waves on one ECG. With isoproterenol challenge, she had prolongation of her QTc to 520 ms and developed polymorphic ventricular tachycardia. During follow-up, she also developed subsequent supraventricular arrhythmias. Her ICD eventually delivered 11 appropriate shocks for ventricular arrhythmias on metoprolol, despite serial dose escalations. She transitioned to nadolol 80 mg/day and she was subsequently asymptomatic. She had cerebral anoxia after the initial cardiac arrest accompanied by memory loss. Genotype: homozygous c.587G>A, p. Arg196Gln; Figure 1, Case 6. This case was originally published in 2016.1
Case 7
A 12-year-old girl presented with two episodes of syncope, including during peak exertion in gym class. During an exercise stress test, she developed isolated PVCs starting at a heart rate of 125 b.p.m. The PVCs became multi-focal with bi-directional couplets and 3–4 beats of multi-focal non-sustained ventricular arrhythmias. Her resting ECGs showed QTc values from 416 to 460 ms. She was started on nadolol at 0.5 mg/kg/day and has been asymptomatic for 2 years. She had a normal neurologic status. Genotype: homozygous for a deletion (234 + 1_235 − 1)_(286 + 1_287 − 1), which included Exon 2; Figure 1, Case 7. This case has not been previously published.
Cases 8–10
After the sudden death of three of his siblings, a 14-year-old boy presented with excessive sweating, anxiety, and panic attacks due to concern that he might die. He was treated empirically with beta-blockers, which improved his symptoms. His ECG and exercise stress test were normal. His first sibling (male) had convulsions during breast-feeding at a year of age and recurrent syncope between ages 5 and 6, ultimately dying at age 12 while playing. His second sibling (female) had her first episode of syncope at age 7, followed by five additional syncopal events in the ensuing years. On the sixth episode occurring at age 15, she died while making her bed. His third sibling (female) had her first episode of syncope at 7 years of age. She had a second episode of syncope during swimming and a third episode during fruit picking. The family reports that she had become emotionally depressed due to her sister’s death. At age 13, as she was standing quietly by a pond, she had syncope and died. No cardiac work-up had been performed on any of the three siblings.
Exome sequencing was performed on the 14-year-old who presented for cardiac care and on both of his parents. All three were found to be heterozygous carriers for TECRL c.331 + 1G>A. This led to the extraction of DNA from a blood stain on a cloth that remained from the third sibling. A site-specific assay was performed for the c.331 + 1G>A variant and the DNA was homozygous for the variant. The two other deceased siblings could not be tested because no DNA was available.
The 14-year-old boy who originally presented was reassured by the benign nature of his ECG and exercise stress test and his heterozygous status for the c.331 + 1G>A variant. His anxiety and panic attacks resolved and the beta-blockers were discontinued without subsequent symptoms (Figure 1, Cases 8–10). These cases have not been previously published.
Summary of unaffected carriers
Genotyping within the families revealed 37 family members with known heterozygous genotypes. These include 10 parents (Cases 1, 2, 3, 4, 5, and 8–10), 7 siblings (Cases 1, 2, 4, 5 and 8–10), and 20 higher-degree relatives. All 37 cases were asymptomatic. All seven siblings had normal ECGs and four of seven had exercise stress tests that were unremarkable. None of the adult carriers had been symptomatic or had a history of syncope or cardiac arrest (Figure 1, Case 6).
Summary of affected cases
In summary, all affected cases had a homozygous or compound heterozygous TECRL variant (Table 2). While most cases presented with syncope during exertion or emotion, Cases 3, 6, and 10 all had at least one event at rest. Ages at presentation ranged from 1 to 31 (Table 1), although many had suffered previous syncopal events that had not previously been recognized as arrhythmia equivalents. In a recurring theme in the literature, one case had been previously diagnosed as epilepsy (Case 4). Consanguinity was present in four of the seven reported families, as occurs in many early reports of autosomal recessive disease (Figure 1). None of the heterozygous carriers identified in our study had cardiac disease.
Discussion
Variable clinical features in TECRL variants
Clinical features among patients with TECRL genotypes are not uniform. The probands in Cases 1 and 2 presented with cardiac arrest during exercise. Both were homozygous for the c.331 + 1G>A genotype. However, the maximum QTc in one patient was ∼500 ms and the QTc remained between 400 and 440 ms in another, demonstrating one ‘long QT’ phenotype and one ‘typical’ CPVT phenotype, despite having the same homozygous TECRL variant. These cases demonstrate phenotypic heterogeneity associated with a single genetic locus. Looking more broadly across all ten cases, phenotypes ranged from recurrent syncope to early sudden death. Neither QTc prolongation nor a bi-directional ectopy/VT response to exercise was universal.
Therapy
In addition to the newly reported cases, an advantage of this study is that it extends the follow-up for cases reported early in the TECRL literature and provides long-term data on the newly reported cases. Cardiac rhythm events are probabilistic, not inevitable and so we cannot conclude that therapy with beta-blockers is always preventing arrhythmia nor recommend a specific dose. However, the anecdotal data in this study suggest that nadolol and propranolol were superior to metoprolol, bisoprolol, and low-dose atenolol. This observation is congruent with the larger data that are available in the long QT literature and CPVT literature.7,8 Several mechanisms have been proposed to suggest why this difference exists in patients with channelopathies, including a ‘membrane stabilizing’ effect secondary to sodium current blockade.9,10 While preliminary in vitro evidence and mechanistic supposition supports the use of flecainide in patients with TECRL variants,1 neither flecainide nor propafenone was used widely enough in our cases to draw conclusions about their clinical utility. Given the limited data available in the literature, we continue to recommend aggressive evaluation of all patients with potentially pathogenic, bilallelic TECRL variants, and careful work-up in the face of any arrhythmia symptoms, including syncope, palpitations, seizures, or alterations of consciousness.
Unaffected heterozygous carriers
We report 37 heterozygous family members who received genotype and phenotype testing. None of the 37 patients with a heterozygous TECRL genotype had a demonstrated cardiac phenotype. Jaouadi et al.3 previously reported a homozygous (c.331 + 1G>A) proband with LV dilation and sudden death. The proband’s heterozygous mother also had LV dilation. His mother remains the only patient reported with a heterozygous TECRL variant and a cardiac phenotype. Jaouadi et al. note that the TECRL genotype and the LV dilated phenotype may not necessarily be related or may represent interactions with genetic modifiers. Our limited data support the conclusion that TECRL may be life-threatening when both alleles are affected by pathogenic variants. We did not find any phenotypically affected patients with a heterozygous TECRL genotype. Our sample is too small to draw a final conclusion. In the sudden death literature, cases of CASQ2 variants with a CPVT phenotype and convincing segregation data have been reported in a heterozygous state, despite a typical presentation requiring biallelic pathogenic variants.11 Thus, the prospect of a future case with a dominant negative effect should be considered. We find no evidence of that effect here.
Two variants occurred in unrelated families (c.331 + 1G>A and c.587G>A). The c.331 + 1G>A variant was also present in one of two reports not included in our working group.4 Neither variant is present in the gnomAD database, suggesting that they are ultrarare in the general population. Additional data will be required to determine whether these variants are over-represented in TECRL-associated cardiac disease.
Relationship of mechanism and clinical disease
The protein encoded by TECRL is expressed only in cardiac and skeletal muscles. It localizes to the endothelial reticulum. Pathway analysis suggests that TECRL has roles in fatty acid and lipid metabolism, which can alter expression and function of ion channels and other cellular proteins.12 An initial mechanistic study showed down-regulation of RYR2 and CASQ2 in some cell lines affected with TECRL abnormalities. These lines also had smaller Ca2+ transient amplitudes and elevated diastolic calcium.1 The driving mechanism of arrhythmia substrate in TECRL abnormalities may be its role in maintaining calcium homeostasis. While prolongation of the action potential was seen in some cell lines, this was not uniform. Not all patients in our current study had prolongation of the QTc. As has been described in other syndromes related to calcium homeostasis, such as calmodulinopathy,13 there appears to be overlap between LQTS and CPVT in the phenotypes associated with biallelic TECRL variants. Further study is needed to understand the cellular mechanism of each of those presentations.
Study limitations
This study is subject to the limitations of all retrospective data, including a potentially skewed population for ascertainment and the likelihood that early cases in the literature represent a more severe phenotype than the population of cases that will eventually be found. The c.331A>G variant was reported in Families 1 and 7. The c.587G>A variant was reported in Families 2, 4, and 5. By history, the families were not related, but we were not able to obtain data for haplotype analysis. Variant calling was performed at each collaborating centre; re-analysis of the variant call file was performed in four of eight cases (Supplementary material online, Table S1).
Conclusion
In summary, this case series reinforces that hypothesis that biallelic pathogenic TECRL variants are a cause of life-threatening arrhythmias. We present new evidence that TECRL variants cause variable phenotypes, neither exclusively long QT nor CPVT.13–15 Our data demonstrate that even gene locus is not sufficient to determine phenotype. Therefore, in family screening, a normal ECG in a homozygous family member is not sufficient to exclude penetrant disease. Further testing should be pursued, even if the initial family member’s ECG was abnormal. Finally, we offer preliminary data that nadolol and propranolol may be superior beta-blockers in patients with clinical arrhythmias and biallelic TECRL variants.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors thank Alfred L. George, Jr, MD, for his review of the initial manuscript draft, Mara Russo for graphic design, and Philippe Goyette, PhD, from the Montreal Heart Institute Foundation for genetic expertise.
Funding
This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health (grant number K23HL130554); the American Heart Association Mentored Clinical and Population Research Award (17MCPRP33660457) and the American Heart Association Strategic Focused Research Network grant for Sudden Death (Dallas, TX); the Netherlands CardioVascular Research Initiative; the Dutch Heart Foundation; Dutch Federation of University Medical Centres; the Netherlands Organisation for Health Research and Development; the Royal Netherlands Academy of Sciences (PREDICT-2); and the Swiss Heart Foundation (number: 29283).
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Devalla HD, Gelinas R, Aburawi EH, Beqqali A, Goyette P, Freund C. et al. TECRL, a new life-threatening inherited arrhythmia gene associated with overlapping clinical features of both LQTS and CPVT. EMBO Mol Med 2016;8:1390–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhuiyan ZA, Hamdan MA, Shamsi ET, Postma AV, Mannens MM, Wilde AA. et al. A novel early onset lethal form of catecholaminergic polymorphic ventricular tachycardia maps to chromosome 7p14-p22. J Cardiovasc Electrophysiol 2007;18:1060–6. [DOI] [PubMed] [Google Scholar]
- 3. Jaouadi H, Bouyacoub Y, Chabrak S, Kraoua L, Zaroui A, Elouej S. et al. Multiallelic rare variants support an oligogenic origin of sudden cardiac death in the young. Herz 2020. doi: 10.1007/s00059-019-04883-1. [DOI] [PubMed] [Google Scholar]
- 4. Moscu-Gregor A, Marschall C, Muntjes C, Schonecker A, Schuessler-Hahn F, Hohendanner F. et al. Novel variants in TECRL cause recessive inherited CPVT type 3 with severe and variable clinical symptoms. J Cardiovasc Electrophysiol 2020;31:1527–35. [DOI] [PubMed] [Google Scholar]
- 5. Xie L, Hou C, Jiang X, Zhao J, Li Y, Xiao T.. A compound heterozygosity of Tecrl gene confirmed in a catecholaminergic polymorphic ventricular tachycardia family. Eur J Med Genet 2019;62:103631. [DOI] [PubMed] [Google Scholar]
- 6. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J. et al. ; on behalf of the ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahn J, Kim HJ, Choi JI, Lee KN, Shim J, Ahn HS. et al. Effectiveness of beta-blockers depending on the genotype of congenital long-QT syndrome: a meta-analysis. PLoS One 2017;12:e0185680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leren IS, Saberniak J, Majid E, Haland TF, Edvardsen T, Haugaa KH.. Nadolol decreases the incidence and severity of ventricular arrhythmias during exercise stress testing compared with beta1-selective beta-blockers in patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2016;13:433–40. [DOI] [PubMed] [Google Scholar]
- 9. Chockalingam P, Crotti L, Girardengo G, Johnson JN, Harris KM, van der Heijden JF. et al. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2: higher recurrence of events under metoprolol. J Am Coll Cardiol 2012;60:2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Besana A, Wang DW, George AL Jr, Schwartz PJ.. Nadolol block of Nav1.5 does not explain its efficacy in the long QT syndrome. J Cardiovasc Pharmacol 2012;59:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gray B, Bagnall RD, Lam L, Ingles J, Turner C, Haan E. et al. A novel heterozygous mutation in cardiac calsequestrin causes autosomal dominant catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2016;13:1652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonnet D, Martin D, Pascale De L, Villain E, Jouvet P, Rabier D. et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 1999;100:2248–53. [DOI] [PubMed] [Google Scholar]
- 13. Crotti L, Spazzolini C, Tester DJ, Ghidoni A, Baruteau AE, Beckmann BM. et al. Calmodulin mutations and life-threatening cardiac arrhythmias: insights from the International Calmodulinopathy Registry. Eur Heart J 2019;40:2964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tester DJ, Arya P, Will M, Haglund CM, Farley AL, Makielski JC. et al. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing. Heart Rhythm 2006;3:800–5. [DOI] [PubMed] [Google Scholar]
- 15. Kimura H, Zhou J, Kawamura M, Itoh H, Mizusawa Y, Ding WG. et al. Phenotype variability in patients carrying KCNJ2 mutations. Circ Cardiovasc Genet 2012;5:344–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.