Abstract
Objective
Chronic non-cancer pain (CNCP) is prevalent among individuals with opioid use disorder (OUD). However, the impact of CNCP on buprenorphine treatment outcomes is largely unknown. In this secondary analysis, we examined treatment outcomes among individuals with and without CNCP who received a low-barrier buprenorphine maintenance regimen during waitlist delays to more comprehensive opioid treatment.
Methods
Participants were 28 adults with OUD who received 12 weeks of buprenorphine treatment involving bimonthly clinic visits, computerized medication dispensing, and phone-based monitoring. At intake and monthly follow-up assessments, participants completed the Brief Pain Inventory, Beck Anxiety Inventory, Beck Depression Inventory (BDI-II), Brief Symptom Inventory (BSI), Addiction Severity Index, and staff-observed urinalysis.
Results
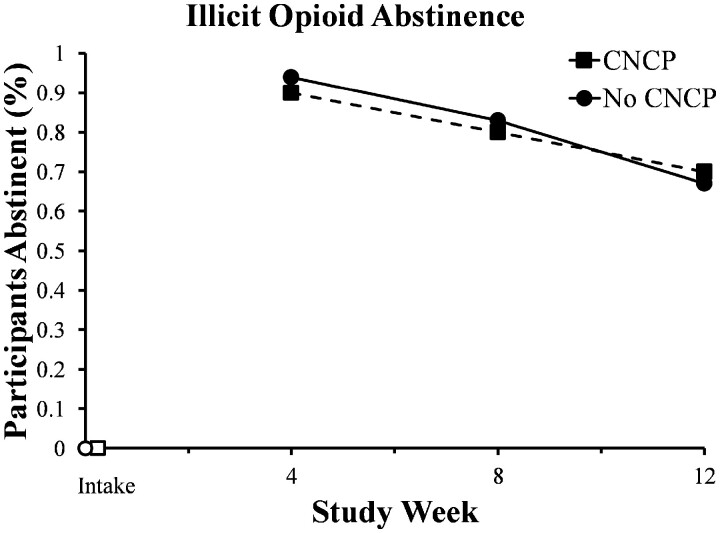
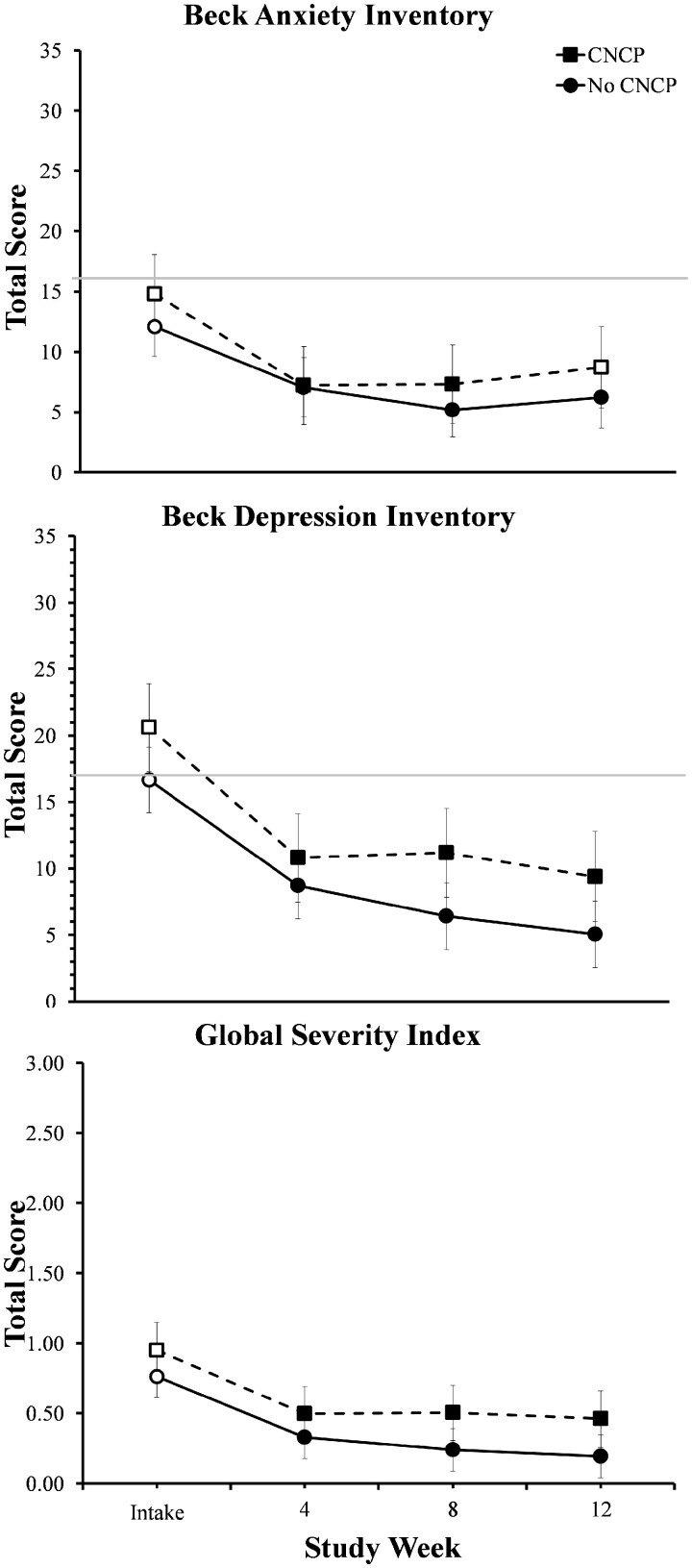
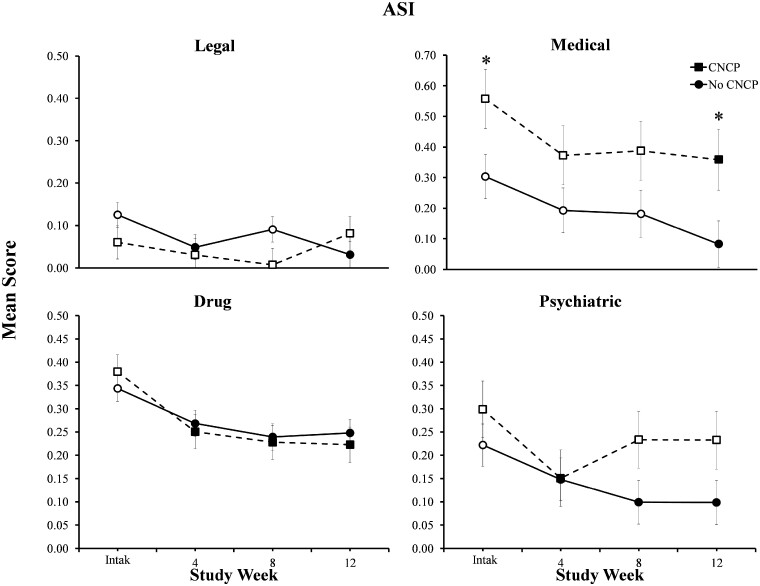
Participants with CNCP (n = 10) achieved comparable rates of illicit opioid abstinence as those without CNCP (n = 18) at weeks 4 (90% vs 94%), 8 (80% vs 83%), and 12 (70% vs 67%) (P = 0.99). Study retention was also similar, with 90% and 83% of participants with and without CNCP completing the 12-week study, respectively (P = 0.99). Furthermore, individuals with CNCP demonstrated significant improvements on the BDI-II and Global Severity Index subscale of the BSI (P < 0.05). However, those with CNCP reported more severe medical problems and smaller reductions in legal problems relative to those without CNCP (P = 0.03).
Conclusions
Despite research suggesting that chronic pain may influence OUD treatment outcomes, participants with and without CNCP achieved similar rates of treatment retention and significant reductions in illicit opioid use and psychiatric symptomatology during low-barrier buprenorphine treatment.
Keywords: Chronic Pain, Opioid Use Disorder, Buprenorphine, Interim Treatment
Introduction
Approximately 20% of Americans experience chronic pain [1], and the majority of these 50 million individuals live with chronic non-cancer pain (CNCP), which is defined as pain that is not caused by a malignancy and lasts longer than 3 months [1, 2]. The annual economic costs associated with CNCP are estimated at $635 million, exceeding the combined annual costs associated with cancer and diabetes [3]. CNCP is particularly prevalent among individuals with opioid use disorder (OUD), with more than one-third of methadone or buprenorphine patients reporting pain that has been present for at least 3 months [4, 5]. Indeed, in one study of 244 individuals seeking office-based buprenorphine treatment, 36% reported CNCP, and a substantial proportion of those reported using illicit opioids during the past week to alleviate pain [5].
Opioid agonist treatment (OAT) with buprenorphine and methadone represents the most widely used and efficacious approach for treating OUD [6, 7]. However, relative to OAT patients reporting no pain, patients with co-occurring CNCP often present with significantly greater impairments in physical, psychosocial, and psychiatric functioning [4, 8–10]. Concomitant chronic pain may also undermine patients’ therapeutic response to OAT, with prior studies demonstrating more illicit opioid use and a need for higher OAT doses among patients with CNCP [11, 12]. In contrast, other studies have found no association between pain and illicit opioid use in OAT populations [13–17]. Furthermore, few studies have examined the association between CNCP and other important psychiatric and psychosocial outcomes (e.g., comorbid mood and anxiety disorders; legal, employment, and psychosocial problems) that may also negatively impact treatment retention and response [18–21].
A recently completed series of studies by our group provided a unique opportunity to evaluate buprenorphine treatment response among participants with and without CNCP. Across two outpatient studies, we investigated the initial feasibility and efficacy of a low-barrier interim buprenorphine treatment (IBT) regimen for reducing illicit opioid use and other risk behaviors among participants with OUD who were waitlisted with an opioid treatment program or office-based buprenorphine provider in the community [22, 23]. The IBT intervention involved 12 weeks of buprenorphine treatment. As part of the treatment protocol, participants attended bimonthly clinic visits to ingest their dose under nurse observation and provide staff-observed urine specimens. Remaining doses were provided through a computerized dispenser that permitted buprenorphine administration at home to reduce the risk of nonadherence. This low-barrier treatment approach adheres to the philosophy that buprenorphine should be provided to individuals with OUD with minimal delays or barriers. Accordingly, the IBT treatment regimen does not require participation in individual or group counseling or daily clinic attendance for supervised dosing. Furthermore, this intervention aligns with recommendations to prescribe buprenorphine even if people use non-opioid substances or relapse to illicit opioid use [24]. In the present secondary analysis, we examined whether baseline characteristics and treatment response (e.g., illicit opioid abstinence, physical health, psychiatric symptoms, psychosocial functioning) varied as a function of CNCP status.
Method
Study Design and Participants
Participants were 28 adults with OUD who received IBT in one of two recent studies [22, 23] and provided complete data on the Brief Pain Inventory—Short Form (BPI) [25], which was the measure used at study intake to assess the presence of CNCP. Inclusion criteria were identical for both studies and required that participants be 18 years or older, meet Diagnostic and Statistical Manual of Mental Disorders, 5th edition [26] criteria for OUD, provide an opioid-positive urine specimen at intake, and be waitlisted for opioid treatment in the community. Individuals who were pregnant or nursing were excluded, as were those who had significant psychiatric or medical conditions that could interfere with consent or participation and those who were physically dependent on sedative hypnotics or alcohol.
The IBT intervention consisted of buprenorphine maintenance for 12 weeks. Following stabilization, participants presented for scheduled bimonthly clinic visits and ingested their medication under nurse observation, provided a urine specimen, and completed questionnaires. The remaining doses for each 2-week interval were ingested at home and administered via a portable, disk-shaped computerized medication dispenser (Med-O-Wheel Secure, Addoz, Finland). Participants received daily calls from an automated interactive voice response system that assessed opioid use, craving, and withdrawal as well as other illicit drug or alcohol use. The interactive voice response system also contacted participants twice monthly and instructed them to return to the clinic for a random call-back visit. At each random call-back, participants presented their device for inspection and pill count, provided a staff-observed urine specimen, and ingested that day’s dose under nurse observation. At intake and study weeks 4, 8, and 12, participants completed self-report and staff-administered follow-up assessments (described below) and urinalysis. The parent studies were approved by the University of Vermont institutional review board, and all participants provided written informed consent prior to participation.
Measures
At study intake, participants completed a Demographic and Drug History Questionnaire developed by our group, the BPI [25], Beck Anxiety Inventory (BAI) [27], Beck Depression Inventory (BDI-II) [28], Brief Symptom Inventory (BSI) [29], Addiction Severity Index (ASI), 5th edition [30], and a Time-Line Followback interview [31]. Each participant also provided a staff-observed urine specimen under observation of a same-sex research staff member. An abbreviated version of the intake battery that did not include the Demographic and Drug History Questionnaire or BPI-SF was completed at study weeks 4, 8, and 12. A Demographic and Drug History Questionnaire was used to assess participant demographics (and lifetime and recent history of opioid and other drug use).
Baseline pain severity was assessed using the BPI. The BPI is a reliable and valid measure of pain intensity and interference in function during the past week [25]. The BPI uses the primary question, “Throughout our lives, most of us have had pain from time to time (such as minor headaches, sprains, and toothaches), have you had pain beyond these everyday kinds of pain?” to determine whether respondents are currently experiencing any pain. Participants who endorsed the presence of pain were also asked whether the pain persisted for 3 months or more. The pain intensity section of the BPI includes four intensity ratings (e.g., pain at its worst, pain right now) that are scored from 0 (no pain) to 10 (worst pain); whereas, the functional interference section consists of seven items (e.g., general activity, enjoyment of life) that are scored from 0 (no interference) to 10 (complete interference). Respondents answer each item based on the pain they experienced in the past 7 days.
Standardized self-report questionnaires were used to assess psychiatric symptoms. The BAI and BDI-II [27, 28] are 21-item self-report measures of anxiety and depression, respectively. Scores for items on both measures range from 0 to 3 and are summed to yield a total score, with higher scores reflecting greater severity. The BSI is a 53-item self-report measure of psychiatric symptoms that consists of three indices (i.e., Global Severity Index [GSI], Positive Symptom Distress Index, Positive Symptom Total) [29]. Because the GSI represents the most sensitive and widely used indicator of psychiatric distress, it was the focus of our BSI analyses in the present study.
Staff-administered structured interviews were used to assess recent drug use, drug use history, and psychosocial functioning. The ASI assesses the severity of past-month and lifetime problems in seven areas of psychosocial functioning (medical, employment/support, drug, alcohol, legal, family/social relationships, and psychiatric problems) [30]. Severity in each area is reflected by a composite score that ranges from 0 to 1 with higher values indicating greater severity. The Time-Line Followback is a calendar-based tool that was used to identify instances of alcohol, illicit drug, and prescribed drug use in the 30 days prior to the study assessment [31].
Statistical Analysis
Similar to previous studies [32, 33], CNCP was operationalized as 1) endorsement of the first question of the BPI [25] (i.e., whether one has pain other than everyday kinds of pain) and 2) duration of pain lasting longer than 3 months. Participants with CNCP and without CNCP were compared on baseline characteristics using t tests for continuous measures and χ2 or Fisher exact tests for categorical measures. Treatment outcomes included illicit opioid abstinence and scores on the BAI, BDI-II, BSI, and ASI subscales. With regard to illicit opioid abstinence, urine specimens were submitted under staff observation at study weeks 4, 8, and 12, and missing urine specimens were considered to be positive for illicit opioids. Participants with and without CNCP were compared on illicit opioid abstinence across weeks 4, 8, and 12 using a repeated measures analysis based on general estimating equation (GEE) methodology for correlated dichotomous data. χ2 tests were used to compare abstinence between groups at each timepoint as well as treatment retention at week 12. Mixed model repeated measures analyses were used to compare temporal changes between the two groups on continuous outcomes assessed at intake and weeks 4, 8, and 12. All means presented are least square means derived from mixed model repeated measures analyses, which account for missing data due to incomplete follow-up. Linear contrasts were used to compare time-specific changes between and within the two groups. Power calculations were performed based on detecting a difference in our primary outcome measure—illicit opioid abstinence—between participants with and without CNCP. It is estimated that the GEE methodology results in power (1-β) = 0.80 using α = 0.05 for detecting an average difference of 35% (i.e., 85% vs 50%) in abstinence across weeks 4, 8, and 12 assessments. Statistical analyses were conducted with SPSS 24.0 (IBM, Inc, Chicago, IL, USA) and SAS Statistical Software, V9.4 (SAS Institute, Cary, NC, USA) with statistical significance based on P < 0.05.
Results
Baseline Characteristics
Participants with (n = 10) and without (n = 18) CNCP were generally similar with regard to baseline characteristics (Table 1). However, those with CNCP were less likely to be employed full-time compared with those without CNCP (20% vs 83%, respectively; P = 0.003). Participants with CNCP also presented with more severe medical problems at study intake than participants without CNCP (0.56 ± 0.35 vs 0.30 ± 0.34, respectively; t[26] = 2.11, P = 0.04).
Table 1.
Baseline participant characteristics
| Characteristics | CNCP (n = 10) | No CNCP (n = 18) | P Value |
|---|---|---|---|
| Age | 37.2 (11.8) | 31.6 (7.8) | 0.14 |
| Male, No. (%) | 6 (60.0) | 11 (61.1) | 0.99 |
| White, No. (%) | 10 (100) | 17 (94.4) | 0.99 |
| Employed full-time, (%) | 2 (20.0) | 15 (83.3) | 0.003 |
| Education, y | 13.0 (2.7) | 11.92 (1.8) | 0.21 |
| Duration of regular opioid use, y | 6.1 (6.3) | 7.8 (5.2) | 0.44 |
| Past-month opioid use, days | 27.6 (5.1) | 28.1 (4.7) | 0.79 |
| Use began with valid prescription, No. (%) | 8 (80.0) | 8 (44.4) | 0.11 |
| Ever used IV, No. (%) | 8 (80.0) | 13 (72.2) | 0.99 |
| Ever used heroin, No. (%) | 9 (90.0) | 16 (88.2) | 0.99 |
| Primary route of opioid administration | 0.82 | ||
| Oral/sublingual, No. (%) | 8 (80.0) | 12 (66.7) | |
| Intranasal, No. (%) | 1 (10.0) | 2 (11.1) | |
| Intravenous, No. (%) | 1 (10.0) | 4 (22.2) | |
| Past month primary opioid of abuse* | 0.99 | ||
| Prescription opioid, No. (%) | 8 (80.0) | 15 (83.3) | |
| Buprenorphine, No. (%)† | 6 (75.0) | 13 (86.7) | |
| Mean daily dose, mg | 10.5 | 9.62 | |
| Oxycodone, No. (%)† | 1 (12.5) | 2 (13.3) | |
| Mean daily dose, mg | 100 | 150 | |
| Morphine, No. (%)† | 1 (12.5) | 0 (0) | |
| Mean daily dose, mg | 80 | N/A | |
| Heroin, No. (%)† | 2 (20.0) | 3 (16.7) | |
| Mean daily amount, bags | 6.45 | 6.33 | |
| Non-opioid substance use, any kind, No. (%) | 5 (50.0) | 13 (72.2) | 0.41 |
| Beck Anxiety Inventory (BAI) | 14.8 (16.6) | 12.1 (12.8) | 0.63 |
| Beck Depression Inventory (BDI-II) | 20.6 (16.1) | 16.7 (11.8) | 0.47 |
| Brief Symptom Inventory—Global Severity Index (BSI-GSI) | 0.95 (1.1) | 0.76 (0.85) | 0.61 |
| Addiction Severity Index (ASI)‡ | |||
| Medical | 0.56 (0.35) | 0.30 (0.34) | 0.04 |
| Employment | 0.40 (0.32) | 0.40 (0.34) | 0.93 |
| Alcohol | 0.10 (0.15) | 0.04 (0.06) | 0.23 |
| Drug | 0.38 (0.23) | 0.34 (0.10) | 0.57 |
| Legal | 0.06 (0.13) | 0.13 (0.16) | 0.29 |
| Family/social | 0.13 (0.20) | 0.16 (0.21) | 0.72 |
| Psychiatric | 0.30 (0.23) | 0.22 (0.22) | 0.39 |
Values represent mean (SD) unless otherwise indicated. Bold type indicates significant difference between groups. CNCP = chronic non-cancer pain.
Between-group comparison of individuals who reported prescription opioids vs heroin as primary opioid of abuse.
The percentage of participants endorsing each opioid prescription subtype is based on the total number of individuals who reported primary prescription opioid use within each group.
ASI subscale scores range from 0 to 1.
Illicit Opioid Abstinence
Participants with and without CNCP did not differ in the percentage of urine specimens that were biochemically verified as negative for illicit opioids across the three assessments based on GEE repeated measures analyses (Χ2 [1] = 0.06; P = 0.80) (Figure 1). No significant difference in abstinence was observed at weeks 4 (90% vs 94%; P = 0.99), 8 (80% vs 83%; P = 0.99), or 12 (70% vs 67%; P = 0.99). There were also no significant differences in treatment retention, with 90% and 83% of participants with and without CNCP completing the 12-week study, respectively (P = 0.99).
Figure 1.
Data represent changes over time in illicit opioid abstinence for participants with chronic non-cancer pain (CNCP) (dashed line) and participants without CNCP (solid line). Solid symbols indicate a significant within-group change from intake.
Psychiatric Symptoms
With respect to changes in psychiatric symptoms during buprenorphine treatment, significant main effects of time were observed on the BAI (F[3,72] = 5.90; P < 0.01), BDI-II (F[3,74] = 19.75; P < 0.001), and GSI subscale of the BSI (F[3,71] = 10.53; P < 0.001), with no differences in these changes between groups. On the BAI, both groups reported significant reductions in anxiety with mean scores significantly lower at weeks 4 and 8 compared with intake (P < 0.05; Figure 2, top panel). At the final 12-week assessment, BAI scores for participants without CNCP remained significantly lower than intake (P < 0.05); whereas, anxiety scores among participants with CNCP no longer differed significantly from intake. A similar pattern was seen on the BDI-II and GSI subscale of the BSI, with participants in both groups reporting mean scores that were significantly lower at the 4-, 8-, and 12-week assessments relative to intake (P < 0.05; Figure 2, middle and bottom panels) and no significant group differences at any timepoint.
Figure 2.
Changes over time in Beck Anxiety Inventory (BAI), Beck Depression Inventory (BDI-II), and Brief Symptom Inventory (BSI) scores for participants with and without chronic non-cancer pain (CNCP). Y axes are presented on a smaller scale to permit visual inspection of the data. The horizontal grey lines represent the cutoff score for moderate anxiety on the BAI (total score ≥ 16) and the commonly used cutoff indicating clinically significant depression on the BDI-II (total score ≥17). Error bars represent standard error of the mean. Solid symbols indicate a significant within-group change from intake.
Addiction Severity Index
Finally, in terms of changes in ASI scores during treatment, a significant group x time interaction was observed for the ASI legal subscale (F[3,71] = 3.06; P = 0.034) (Figure 3, top left panel). Specifically, participants with CNCP demonstrated no significant changes in legal problem severity; whereas, those without CNCP reported legal severity scores that were significantly lower than intake at weeks 4 and 12 (P < 0.01).
Figure 3.
Data represent changes over time in ASI legal, medical, drug, and psychiatric subscale scores for participants with and without chronic non-cancer pain (CNCP). Participants with CNCP reported mean scores that were significantly higher than participants without CNCP independent of assessment time. Error bars represent standard error of the mean. Solid symbols indicate a significant within-group change from intake; asterisks indicate that the change from intake to assessment timepoint significantly differed between participants with and without CNCP. Y axes are presented on a smaller scale to permit visual inspection of the data (full range of axis is 0–1).
Significant main effects of time were also observed on the medical (F[3,70] = 4.32, P < 0.01), drug (F[3,71] = 14.06; P < 0.001), and psychiatric (F[3,71] = 4.54; P < 0.01) subscales, with both groups demonstrating significant reductions during treatment. While both groups demonstrated significant reductions in medical problem severity over time (Figure 3, top right panel), mean scores remained significantly higher among participants with CNCP vs those without CNCP independent of assessment time (group main effect: F[1,26] = 5.55; P = 0.026; and group x time interaction: F[3,70] = 0.26; P = 0.85). Similarly, both groups reported reductions on ASI drug subscale scores at the 4-, 8-, and 12-week assessments relative to intake (P < 0.01) with no difference between groups. With regard to psychiatric problem severity, participants without CNCP reported significant reductions at weeks 8 and 12 relative to intake (P < 0.01; Figure 3, bottom right panel). In contrast, the CNCP group reported an initial decrease in psychiatric symptom severity at week 4 (P < 0.01) that did not persist at later timepoints. Finally, there were no significant group differences or changes over time on the employment, alcohol, or family/social subscales (P > 0.05).
Discussion
Because CNCP is prevalent among individuals with OUD, it is important to understand how this co-occurrence affects opioid treatment retention and response. In the present study, participants with CNCP achieved comparable rates of study retention and illicit opioid abstinence as those without CNCP. Participants with CNCP also demonstrated significant improvements on key measures of psychiatric severity. Despite these notable improvements, participants with CNCP reported more severe medical problems relative to those without CNCP.
Previous studies examining individuals with OUD reported that individuals with co-occurring CNCP often presented for treatment with more impaired social and psychiatric functioning compared with individuals without pain [4, 9, 10, 34]. In the present study, demographic characteristics and drug use histories among participants with and without CNCP were generally similar. However, individuals with CNCP were less likely to report full-time employment and reported greater medical problem severity. The finding that chronic pain may interfere with an individual’s ability to work has been reported elsewhere [34–37] and highlights the complex challenges experienced by individuals with OUD and CNCP. Participants in the present study with co-occurring CNCP also presented with numerically higher depression, anxiety, and psychiatric symptom severity scores than participants without CNCP. However, group differences were not statistically significant. Although power was limited due to the relatively small sample size, the absence of significant baseline differences may also be attributable to differences in study samples. Unlike previous studies, participants in this study were presenting for buprenorphine treatment and waitlisted for opioid treatment program in the community. Accordingly, these individuals may have been more motivated for treatment and less disabled by their pain compared with previous study samples.
Clinicians frequently report considerable treatment challenges associated with treating patients with co-occurring chronic pain and OUD in part due to a lack of evidence-based treatments and available providers for the concurrent treatment of OUD and pain [38–40]. Despite these challenges, participants with CNCP who received a brief low-barrier buprenorphine regimen in the present studies achieved illicit opioid abstinence and study retention outcomes that were generally similar to those achieved by those without CNCP. These findings stand in contrast to previous research demonstrating more illicit opioid use among individuals with CNCP [12]. However, our data are consistent with the majority of prior studies [13–17] that found no association of CNCP on illicit opioid use or intervention adherence during OAT. Although further investigation is needed to disentangle the complex association between OAT and pain, these data suggest that IBT and other low-barrier treatment approaches may hold promise for patients with CNCP.
Individuals with co-occurring chronic pain and OUD also present with high rates of psychiatric comorbidities [8]. Although prior data from our group has suggested that IBT may be associated with reductions in psychiatric distress [41], this is the first study to our knowledge that has examined the role of CNCP on psychiatric and psychosocial outcomes among individuals receiving buprenorphine treatment for OUD. In addition to achieving high rates of illicit opioid abstinence, participants with CNCP experienced significant improvements in psychiatric and psychosocial functioning during the IBT intervention. These findings are noteworthy given that the IBT intervention, unlike other forms of OAT, did not include formal psychosocial counseling and suggest that IBT may be associated with improvements in psychiatric symptoms even among patients with CNCP.
Similar to previous studies [10, 34], participants with CNCP reported greater medical problem severity at intake compared with those without CNCP. Although the IBT intervention was associated with significant reductions in medical problems for both groups, participants with CNCP still had higher scores on ASI medical subscale at the end of the 12-week study relative to those without CNCP, suggesting that additional medical support (e.g., referral to a primary care physician or pain management practice) may be warranted to help some individuals with CNCP achieve even better physical health outcomes. Nonetheless, this study lends additional support to the utility of low-barrier treatment approaches for reducing the broader individual and societal costs associated with OUD among individuals who may experience treatment delays.
Several limitations are important to note. The present sample was racially homogenous, and thus, future research is needed to examine the relationship between pain and OAT among a more diverse sample of individuals with OUD. This secondary analysis also examined data from a small number of individuals over a relatively brief 12-week treatment duration. Accordingly, our ability to detect small effect sizes was limited. To address these limitations, a larger-scale, longer-duration randomized-controlled trial is currently underway. Finally, these findings are also limited by the fact that the BPI-SF was only administered at baseline. Prior studies indicate that intra-individual change in self-reported pain intensity is a better predictor of illicit opioid use than baseline pain severity [42, 43]. Furthermore, measures of functional interference from pain may also represent a potentially important treatment outcome measure [9]. Thus, future research should examine the effects of low-barrier buprenorphine treatment for OUD on measures of pain severity and functional interference from pain over time and whether these changes are associated with subsequent illicit opioid abstinence and psychosocial outcomes.
In summary, despite the considerable vulnerabilities experienced by individuals with co-occurring chronic pain and OUD, participants with CNCP responded favorably to interim buprenorphine dosing when more comprehensive treatment was unavailable. In addition to achieving high rates of illicit opioid abstinence, participants with CNCP also experienced significant improvements in psychiatric, psychosocial, and medical functioning. Further research is needed to better understand the unique treatment needs of this challenging clinical population and to identify and disseminate effective evidence-based treatments for patients with co-occurring OUD and chronic pain.
Funding sources: This work was supported in part by the National Institute on Drug Abuse (R01DA042790, 5T32DA007242-29), the Laura and John Arnold Foundation, and the National Institute of General Medical Sciences (5P20GM103644-06). The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the preparation of the manuscript; or in the decision to submit the paper for publication.
Conflicts of interest: None.
References
- 1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults–United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67(36):1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turk DC, Okifuji A.. Pain terms and taxonomies of pain. In: Fishman SM, Ballantyne JC, Rathmell JP, eds. Bonica’s Management of Pain . 4th ed. New York, NY: Lippincott Williams & Wilkins; 2009:13–23. [Google Scholar]
- 3. Gaskin DJ, Richard P.. The economic costs of pain in the United States. J Pain 2012;13(8):715–24. [DOI] [PubMed] [Google Scholar]
- 4. Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS.. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J Clin Psychiatry 2009;70(9):1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barry DT, Savant JD, Beitel M, et al. Pain and associated substance use among opioid dependent individuals seeking office-based treatment with buprenorphine-naloxone: a needs assessment study. Am J Addict 2013;22(3):212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattick RP, Breen C, Kimber J, Davoli M.. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014;16(2):CD002207. [DOI] [PubMed] [Google Scholar]
- 7. Gowing L, Ali R, White J, Mbewe D.. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev 2017;2:CD002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS.. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry 2016;77(10):1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK.. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 2003;289(18):2370–8. [DOI] [PubMed] [Google Scholar]
- 10. Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K.. Treatment needs associated with pain in substance use disorder patients: Implications for concurrent treatment. Drug Alchol Depend 2004;73(1):23–31. [DOI] [PubMed] [Google Scholar]
- 11. Peles E, Schreiber S, Gordon J, Adelson M.. Significantly higher methadone dose for methadone maintenance treatment (MMT) patients with chronic pain. Pain 2005;113(3):340–6. [DOI] [PubMed] [Google Scholar]
- 12. Potter JS, Dreifuss JA, Marino EN, et al. The multi-site prescription opioid addiction treatment study: 18-month outcomes. J Subst Abuse Treat 2015;48(1):62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: A systematic review and meta-analysis. Subst Abuse 2015;10(9):59–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhingra L, Perlman DC, Masson C, et al. Longitudinal analysis of pain and illicit drug use behaviors in outpatients on methadone maintenance. Drug Alcohol Depend 2015;149:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox AD, Sohler NL, Starrels JL, Ning Y, Giovanniello A, Cunningham CO.. Pain is not associated with worse office-based buprenorphine treatment outcomes. Subst Abuse 2012;33(4):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ilgen MA, Trafton JA, Humphreys K.. Response to methadone maintenance treatment of opiate dependent patients with and without significant pain. Drug Alcohol Depend 2006;82(3):187–93. [DOI] [PubMed] [Google Scholar]
- 17. Tsui JI, Lira MC, Cheng DM, et al. Chronic pain, craving, and illicit opioid use among patients receiving opioid agonist therapy. Drug Alcohol Depend 2016;166:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB.. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction 1998;93(1):73–92. [PubMed] [Google Scholar]
- 19. Cox J, Allard R, Maurais E, Haley N, Small C.. Predictors of methadone program non-retention for opioid analgesic dependent patients. J Subst Abuse Treat 2013;44(1):52–60. [DOI] [PubMed] [Google Scholar]
- 20. Ferri M, Finlayson AJR, Wang L, Martin PR.. Predictive factors for relapse in patients on buprenorphine maintenance. Am J Addict 2014;23(1):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Proctor SL, Copeland AL, Kopak AM, Hoffman NG, Herschman PL, Polukhina N.. Predictors of patient retention in methadone maintenance treatment. Psychol Addict Behav 2015;29(4):906–17. [DOI] [PubMed] [Google Scholar]
- 22. Sigmon S, Meyer A, Hruska B, et al. Bridging waitlist delays with interim buprenorphine: Initial feasibility. Addict Behav 2015;51:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sigmon S, Ochalek T, Meyer A, et al. Interim buprenorphine vs. waiting list for opioid dependence. N Engl J Med 2016;375(25):2504–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin SA, Chiodo LM, Bosse JD, Wilson A.. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med 2018;169(9):628–35. [DOI] [PubMed] [Google Scholar]
- 25. Cleeland CS, Ryan KM.. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23(2):129–38. [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed.Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 27. Beck AT, Steer RA.. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 28. Beck AT, Steer RA, Brown GK.. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 29. Derogatis LR. Brief Symptom Inventory. Minneapolis, MN: National Computer Systems, Inc; 1993. [Google Scholar]
- 30. McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat 1992;9(3):199–213. [DOI] [PubMed] [Google Scholar]
- 31. Sobell LC, Sobell MB, Leo GI, Cancilla A.. Reliability of a timeline method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict 1988;83(4):393–402. [DOI] [PubMed] [Google Scholar]
- 32. Sigmon SC, Dunn KE, Saulsgiver K, et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry 2013;70(12):1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weiss RD, Potter JS, Provost SE, et al. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): Rationale, design, and methodology. Contemp Clin Trials 2010;31(2):189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jamison RN, Kauffman J, Katz NP.. Characteristics of methadone maintenance patients with chronic pain. J Pain Symptom Manage 2000;19(1):53–62. [DOI] [PubMed] [Google Scholar]
- 35. Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ.. Chronic pain in Australia: A prevalence study. Pain 2001;89(2):127–34. [DOI] [PubMed] [Google Scholar]
- 36. Campbell G, Nielsen S, Bruno R, et al. The Pain and Opioids IN treatment study: Characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain 2015;156(2):231–42. [DOI] [PubMed] [Google Scholar]
- 37. Saastamoinen P, Leino-Arjas P, Laaksonen M, Lahelma E.. Socio-economic differences in the prevalence of acute, chronic and disabling chronic pain among ageing employees. Pain 2005;114(3):364–71. [DOI] [PubMed] [Google Scholar]
- 38. Barry DT, Bernard MJ, Beitel M, Moore BA, Kerns RD, Schottenfeld RS.. Counselor’s experiences treating methadone-maintained patients with chronic pain: A needs assessment study. J Addict Med 2008;2(2):108–11. [DOI] [PubMed] [Google Scholar]
- 39. Barry DT, Irwin KS, Jones ES, et al. Opioids, chronic pain, and addiction in primary care. J Pain 2010;11(12):1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berg K, Arnsten J, Sacajiu G, Karasz A.. Providers’ experiences treating chronic pain among opioid-dependent drug users. J Gen Intern Med 2009;24(4):482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Streck JM, Ochalek TA, Badger GJ, Sigmon SC.. Interim buprenorphine treatment during delays to comprehensive treatment: Changes in psychiatric symptoms. Exp Clin Psychopharmacol 2018;26(4):403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Worley MJ, Heinzerling KG, Shoptaw S, Ling W.. Pain volatility and prescription opioid addiction treatment outcomes in patients with chronic pain. Exp Clin Psychopharmacol 2015;23(6):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griffin ML, McDermott KA, McHugh RK, Fitzmaurice GM, Jamison RN, Weiss RD.. Longitudinal association between pain severity and subsequent opioid use in prescription opioid dependent patients with chronic pain. Drug Alcohol Depend 2016;163:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]