INTRODUCTION
Apart from direct effects on the brain, excessive alcohol consumption is associated with increased risk for trauma (i.e., traumatic brain injury) (e.g., Alterman and Tarter, 1985; Chen et al., 2012), seizures (Eyer et al., 2011; Martindale et al., 2011), and stroke (de los Rios et al., 2012; Suzuki and Izumi, 2012), each of which can have effects on brain structure independently of alcohol or each other. Further, by affecting peripheral organs (including the alimentary tract (e.g., Bienia et al., 2002; Duell et al., 2012), liver (e.g., Cederbaum, 2012), heart (e.g., Roerecke et al., 2011), pancreas (e.g., Andersen et al., 2008), kidneys (e.g., Schaeffner and Ritz, 2012), and lungs (e.g., Yeligar et al., 2012)), alcohol can alter the brain. Mechanisms of indirect alcohol effects on brain via peripheral changes are likely mediated via soluble factors (e.g., de la Monte et al., 2012). Particularly well described are the effects of alcohol-related thiamine deficiency on the brain (Zahr et al., 2011). Malnutrition, vomiting, and diarrhea are common in chronic alcoholism and can contribute to thiamine deficiency (Morgan, 1982; Fields et al., 1994; Gloria et al., 1997; Ross et al., 2012). In addition, the ability of the gastrointestinal tract to absorb necessary quantities of thiamine is diminished in alcoholics (Hoyumpa, 1980; Thomson, 2000) and the liver, which stores a large part of the body’s supplies of thiamine, if diseased, can have a reduced capacity to store thiamine (Levy et al., 2002; Butterworth, 2009). Consequently, the function of essential thiamine requiring enzymes in the brain (e.g., transketolase, pyruvate dehydrogenase, and a-ketoacid dehydrogenase) is compromised, leading to oxidative stress, cellular energy impairment, and eventually neuronal loss (Thomson et al., 2012).
In order to evaluate its central nervous system (CNS) effects, researchers distinguish “uncomplicated alcoholism” from the various clinically diagnosable consequences of chronic alcohol consumption, including Wernicke’s encephalopathy (WE: i.e., the disease associated with thiamine deficiency), Korsakoff’s syndrome (KS), hepatic encephalopathy (HE), central pontine myelinolysis (CPM), alcoholic cerebellar degeneration (ACD), alcoholic dementia, and Marchiafava–Bignami disease (MBD). The radiologic evaluation of clinically defined syndromes associated with chronic alcoholism, each with relatively unique radiologic signatures (Table 17.1 and Fig. 17.1), provides guideposts for the interrogation of the brain in uncomplicated alcoholism. The aim of this chapter, then, is to review current macrostructural findings from magnetic resonance imaging (MRI) studies and microstructural findings from diffusion tensor imaging (DTI) studies of alcohol-related CNS disorders as a framework for findings in uncomplicated alcoholism, while additionally covering results in abstinence and relapse.
Table 17.1.
Primary and secondary brain regions that are targets of alcohol-related syndromes
| Alcoholism-related syndrome | Abbreviation | Primary targeted region(s) | Secondary targeted regions |
|---|---|---|---|
| Wernicke’s encephalopathy | WE | Mammillary bodies, periaqueductal gray matter, tissue surrounding third ventricle | Dorsal medulla, tectal plates, olivary bodies, pons |
| Korsakoff’s syndrome | KS | Mammillary bodies, hippocampus, thalamus, orbitofrontal cortices | Cerebellum, pons |
| Hepatic encephalopathy | HE | Globus pallidus, substantia nigra | Corticospinal tract, cortex |
| Central pontine myelinolysis | CPM | Pons | Basal ganglia, thalamus, cerebral gray–white-matter junctions |
| Alcoholic cerebellar degeneration | ACD | Cerebellum | |
| Alcohol-related dementia | ARD | Frontal cortex | |
| Marchiafava–Bignami disease | MBD | Corpus callosum | Cortex |
Fig. 17.1.
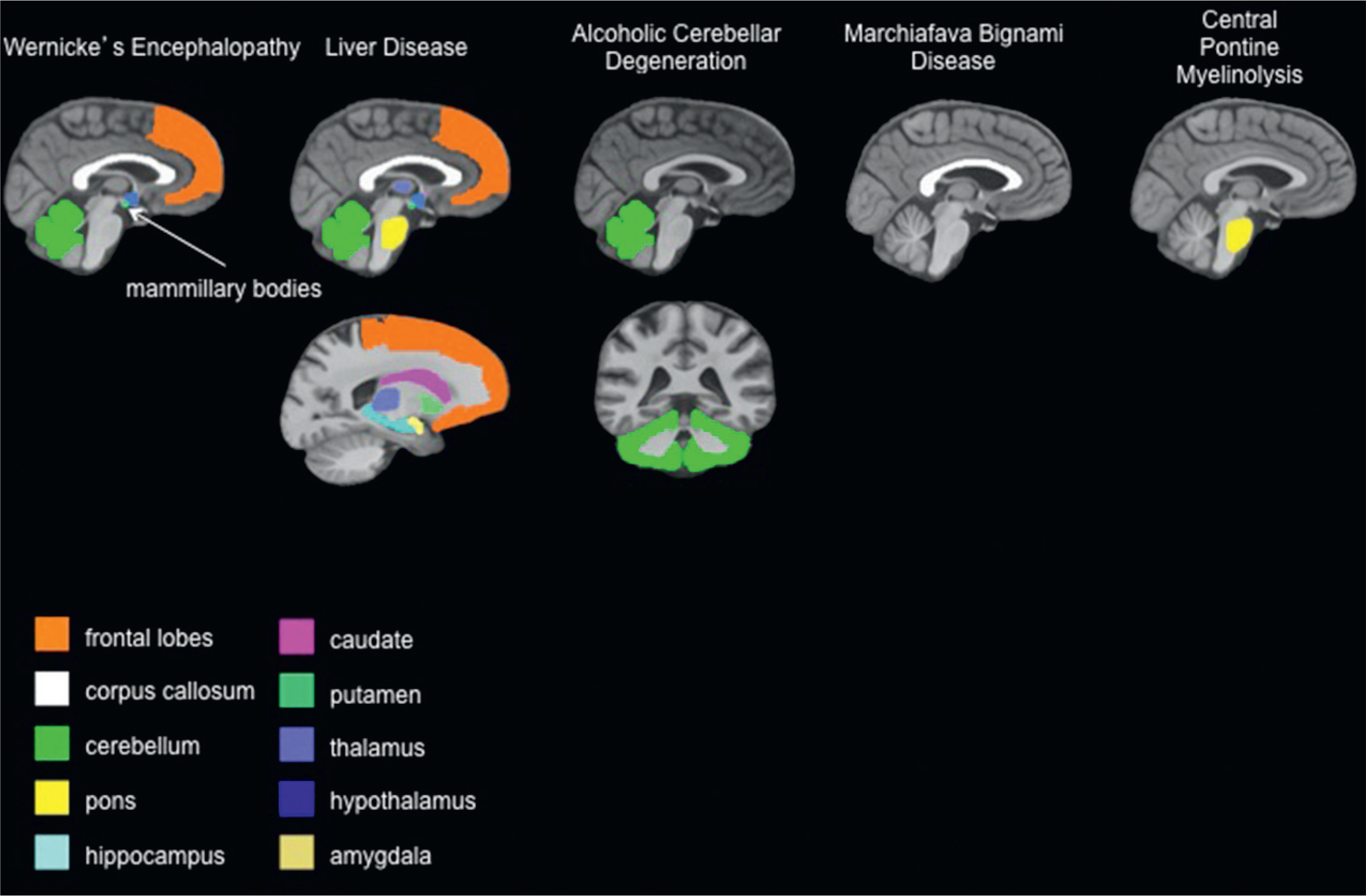
Brain regions targeted by alcohol-related disease. (Adapted from: Zahr et al., 2011.)
STRUCTURAL MAGNETIC RESONANCE IMAGING
Since the early 1980s, conventional structural MRI has made possible the visualization of the living human brain. MRI is possible because the nuclei of certain atoms exhibit nuclear magnetic resonance (NMR). When exposed to a strong magnetic field, nuclei with NMR potential spin at a particular rate (the resonant frequency, determined by the strength of the magnetic field), and align themselves with the field. If nuclei are then presented with radio waves at their resonant frequency, they absorb this energy, and their alignment in the magnetic field is disturbed. When the radio waves are turned off, the relaxation (i.e., free induction decay) of the nuclei as they return to thermodynamic neutrality induces a small current in the receiver coil, an “antenna” used to detect this MR signal. Magnetic field gradients can be used to vary the static magnetic field so that variable precession frequencies become associated with different spatial locations. Hydrogen is the most frequently imaged nucleus in MRI because it is present in great abundance in biologic tissues; detailed images of the brain are possible because the different brain tissue types (i.e., gray matter, white matter, cerebrospinal fluid (CSF)) contain different proportions of water (Rumboldt et al., 2012).
The magnetic resonance image is primarily determined by three variables: proton density, T1, and T2. Proton density reflects the number of hydrogen nuclei stimulated; T1 is an exponential time constant describing the time required for nuclei to realign with the external magnetic field (higher T1 values reflect a longer time required by nuclei to return to equilibrium after perturbation); and T2 is a tissue-specific time constant describing signal loss due to the exchange of energy between hydrogen protons and adjacent nuclei. T1 and T2 tend to reflect the proportion of free to bound water in tissue. The concentration of mobile hydrogen atoms (i.e., water) and therefore proton density, as well as T1 and T2, differs in various biologic tissue types, thereby providing the contrast necessary to visualize details of brain structure. The biologic significance of these variables, however, is not yet fully understood.
MRI, in contrast to computed tomography (CT), offers flexibility in the plane in which the brain is visualized. Because magnetic gradients can be applied in the three (orthogonal) directions to provide information about the spatial location of signals, the brain can be viewed from bottom to top (axial), as in conventional CT images, from front to back (coronal), from left to right (sagittal), or at any oblique angle to these planes. This flexibility also enables greater accuracy in aligning images with internal landmarks, an essential consideration for ensuring consistency of data from replicate images from the same individual (Rohlfing, 2006).
STRUCTURAL MRI FINDINGS IN SYNDROMES ASSOCIATED WITH ALCOHOLISM
The nutritional deficiency for thiamine in chronic alcoholism results in WE; if untreated, WE patients can develop KS, a severe neurologic disorder characterized by anterograde amnesia (Harper, 2006). In acute WE, MRI can detect symmetric, bilateral hyperintense foci, clearly visible on T2-weighted and fluid attenuation inversion recovery (FLAIR) images, in periaqueductal gray matter, mammillary bodies, and tissue surrounding the third ventricle (Lenz et al., 2002; Sullivan and Pfefferbaum, 2009) (Fig. 17.2). Additionally observed hyperintense areas in WE include the dorsal medulla, tectal plates (Ha et al., 2012), olivary bodies, and dorsal pons (Liou et al., 2012). MRI group analysis of KS patients compared with control subjects reveals substantial volume loss of the mammillary bodies in some, but not all, affected alcoholics (but see Victor et al., 1989; Shear et al., 1996; Sheedy et al., 1999; Sullivan et al., 1999b). In contrast to early MR studies suggesting that KS affects the mammillary bodies while sparing the hippocampi (Squire et al., 1990), more recent work demonstrates hippocampal volume deficits in KS (Sullivan and Marsh, 2003). Other regions affected by KS are the thalamus, orbitofrontal cortex (Jernigan et al., 1991a), cerebellum, and pons (Sullivan and Pfefferbaum, 2009).
Fig. 17.2.
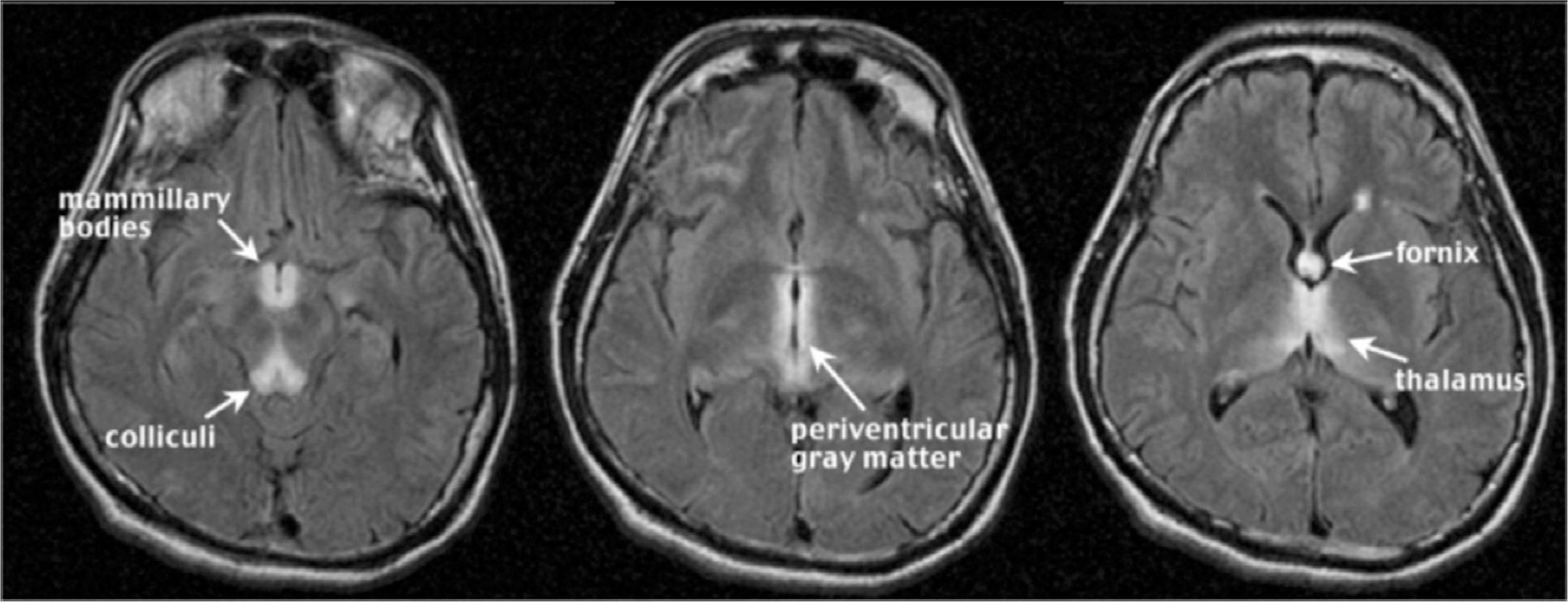
Wernicke’s encephalopathy. (Reproduced from Sullivan and Pfefferbaum, 2009.)
HE, occurring in acute or chronic liver disease, including acute liver failure and cirrhosis, is believed to arise, at least partially, from elevations in circulating levels of ammonia. HE presents with confusion, disorientation, and poor coordination (Vaquero et al., 2003; Prakash and Mullen, 2010). T1-weighted imaging in HE reveals bilateral, symmetric, high-intensity signals in basal ganglia structures, particularly the globus pallidus and substantia nigra (Naegele et al., 2000; Cordoba et al., 2002) (Fig. 17.3), probably due to manganese deposition and T1 shortening (Butterworth, 2013), while T2-weighted FLAIR shows hyperintense signals along the corticospinal tract and diffuse increases in white-matter signal intensities in the cerebral hemispheres (Rovira et al., 2002, 2008). Of note, although discriminating features of WE and HE have been outlined, these diseases can be difficult to differentially diagnose and distinguish, as they can present with similar clinical features and comparable changes on MRI, especially among alcoholics (Thorarinsson et al., 2011).
Fig. 17.3.
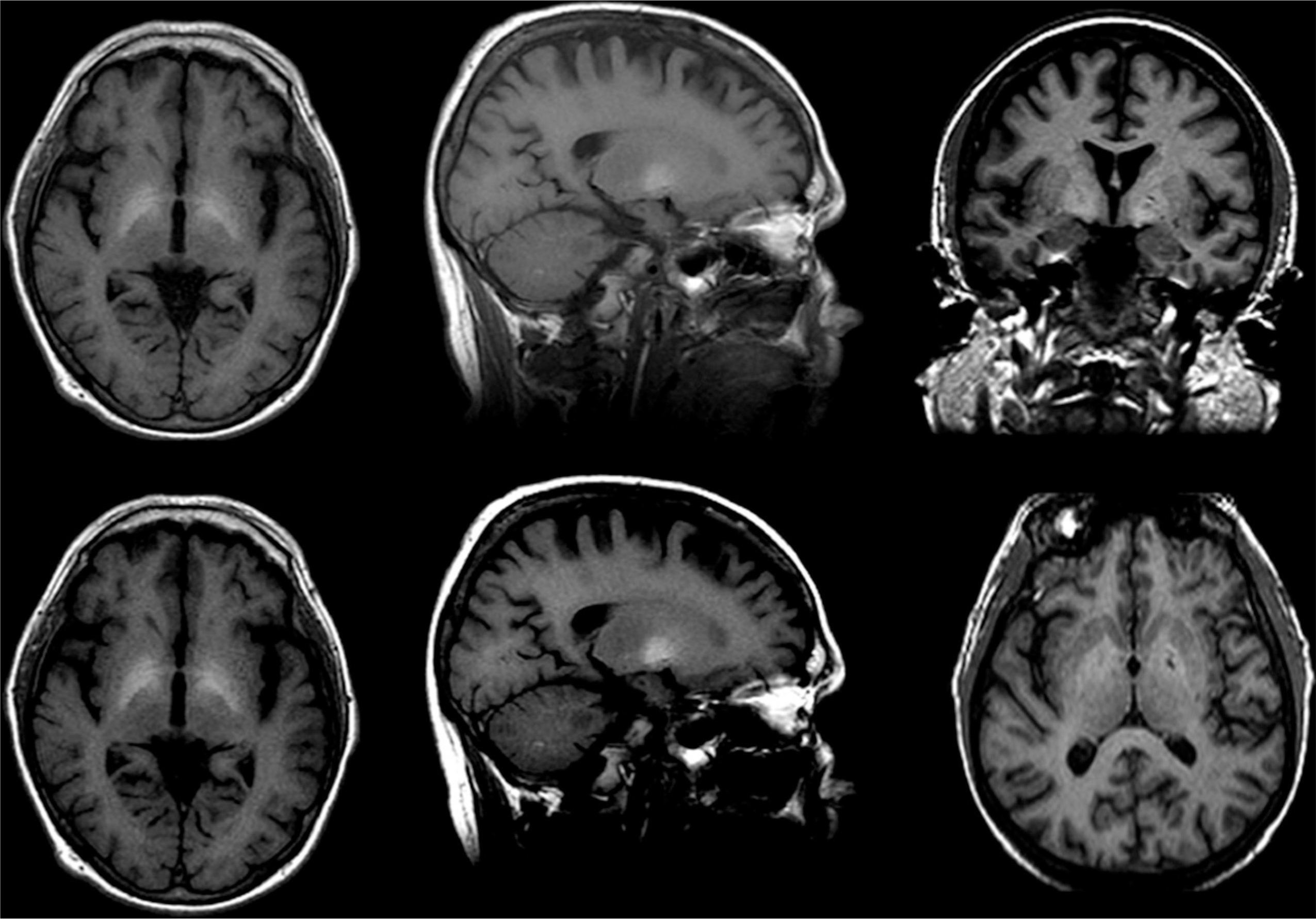
Hepatic encephalopathy. (Reproduced from Rosenbloom et al., 2010.)
CPM, associated with electrolyte disturbances, specifically with aggressive correction of hyponatraemia (Chua et al., 2002), can manifest with clinical features such as pseudobulbar palsy, ataxia, and acute changes in consciousness (Kumar et al., 2006). Classically, CPM was characterized by the presence of a symmetric triangular or “bat-wing” lesion in the pons, as visualized with hypointense T1 and hyperintense T2-weighted images (Kleinschmidt-Demasters et al., 2006) (Fig. 17.4) and reflecting demyelination (Goldman and Horoupian, 1981). Because extrapontine regions (e.g., basal ganglia, thalami, and cerebral gray–white-matter junctions) have also been reported as affected in CPM, the term “osmotic myelinolysis” has been coined (Chua et al., 2002), despite suggestions that pathology in extrapontine regions may not strictly represent demyelination (Kleinschmidt-Demasters et al., 2006; Kumar et al., 2006).
Fig. 17.4.
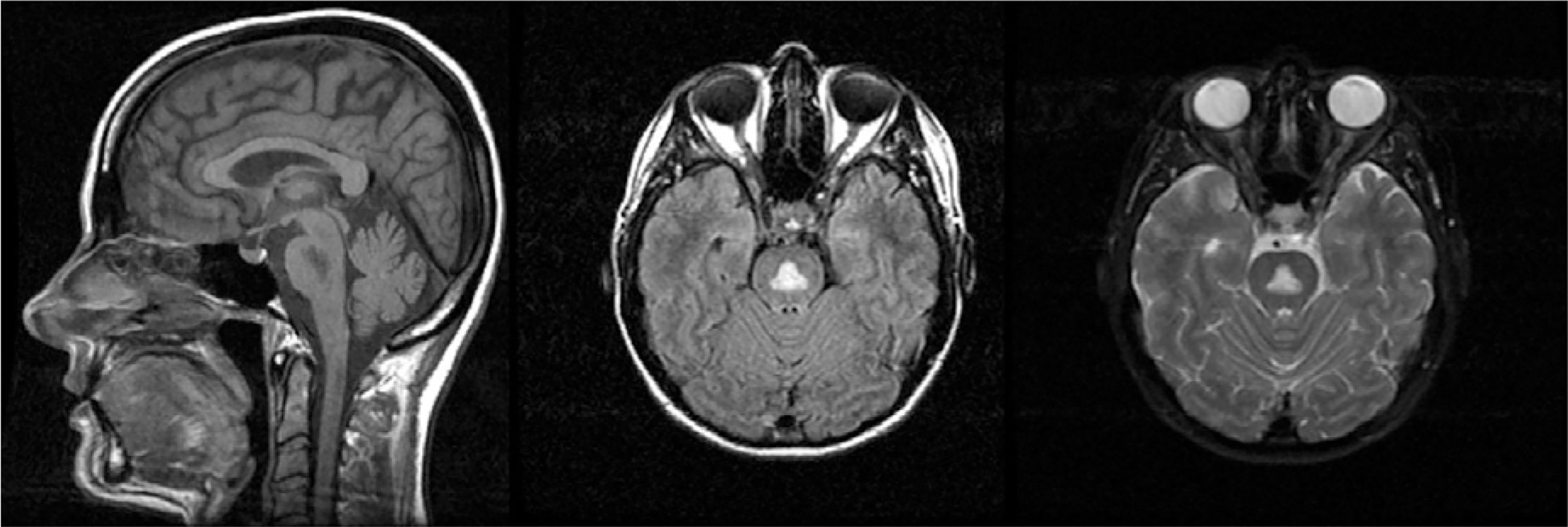
Central pontine myelinolysis.
ACD presents most frequently with ataxia, although clinical signs can include nystagmus and dysarthria (Fitzpatrick et al., 2012). Neuroimaging in ACD demonstrates damage disproportionately apparent in anterior superior portions of the cerebellar vermis (Sullivan et al., 2000c), with postmortem pathology indicating loss of cerebellar Purkinje cells (Feuerlein, 1977).
Alcoholic dementia, or alcohol-related dementia (ARD), a currently preferred term, remains a controversial diagnosis because of confounding syndromes such as WE and HE. Nevertheless, certain clinically distinguishing features of ARD include its presence in socially isolated men at a younger age of onset than other types of dementia (Draper et al., 2011; Ridley et al., 2013), deficits in visuospatial, executive, and mnemonic functions (Schmidt et al., 2005), slower progress compared with other types of dementia (Gupta and Warner, 2008), and partial reversibility (Oslin and Cary, 2003). ARD is considered a frontal dementia (Stewart, 2006); in support of such categorization, forensic evaluation of a sample of alcoholic brains noted a consistent pattern of synapse loss in the superior laminae of the frontal cortex (i.e., Brodmann’s area 10), not related to liver disease (Brun and Andersson, 2001).
The pathogenesis of MBD, a disease marked by mildly impaired mental status (e.g., confusion) and sometimes dysarthria (Lee et al., 2011) or ataxia (Arbelaez et al., 2003), is poorly understood but may be related to nutritional deficiencies in addition to chronic alcohol consumption (Kawamura et al., 1985). Although traditionally characterized by demyelination and necrosis of the corpus callosum, a number of reports identify cortical lesions in so-called MBD (Johkura et al., 2005; Khaw and Heinrich, 2006; Ihn et al., 2007; Yoshizaki et al., 2010; Namekawa et al., 2013). Such data, however, represent single case studies and may reflect inaccurate MBD diagnoses.
Given the aforementioned findings in clinically differential and diagnosable alcohol-related syndromes, the question whether similar brain insults also appear in alcoholics who do not manifest the full spectrum of symptoms present in these clinically recognized conditions is posed; that is, what is observed in brains of uncomplicated alcoholics? Quantitative MRI has shown that relatively mild yet significant structural deficits characteristic of alcoholic syndromes can occur in uncomplicated alcoholics.
STRUCTURAL MRI FINDINGS IN UNCOMPLICATED ALCOHOLISM
Although less severe than observed in KS, mild volume deficits in the mammillary bodies (Shear et al., 1996; Sullivan et al., 1999a), hippocampi, and thalami are reported in uncomplicated alcoholics (Sullivan, 2003; De Bellis et al., 2005; Chanraud et al., 2007; Pitel et al., 2012; van Holst et al., 2012). These structures show a graded effect of volume deficits (KS > uncomplicated alcoholism > normal controls; Fig. 17.5). That atrophic changes are not unique to amnesic KS alcoholics implies that mammillary body damage is not a prerequisite for the development of amnesia in alcoholism (Shear et al., 1996). MR findings also show hippocampal volume deficits in alcoholics compared with controls (Sullivan et al., 1995b; Agartz et al., 1999; Laakso et al., 2000; Kurth et al., 2004; Beresford et al., 2006; Wilhelm et al., 2008). Hippocampal volume deficits in alcoholism are influenced by age (Sullivan et al., 1995a), even though age-related decline is difficult to detect in cross-sectional study (Sullivan et al., 2005a; Raz et al., 2010; Pfefferbaum et al., 2013). While deficits in hippocampal volume are not related to seizure incidence (Sullivan et al., 1996; Bleich et al., 2003), temporal lobe white matter may be sensitive to alcohol withdrawal seizures (Sullivan et al., 1996). Hippocampal volume shrinkage in alcoholism is attributed to loss of white matter and decreased axonal diameter (Harding et al., 1997). Glial cell loss (Korbo, 1999) or reduced incorporation of newly formed neurons to the dentate gyrus (Nixon and Crews, 2004; He et al., 2005), however, could also affect hippocampal volume in alcoholism.
Fig. 17.5.
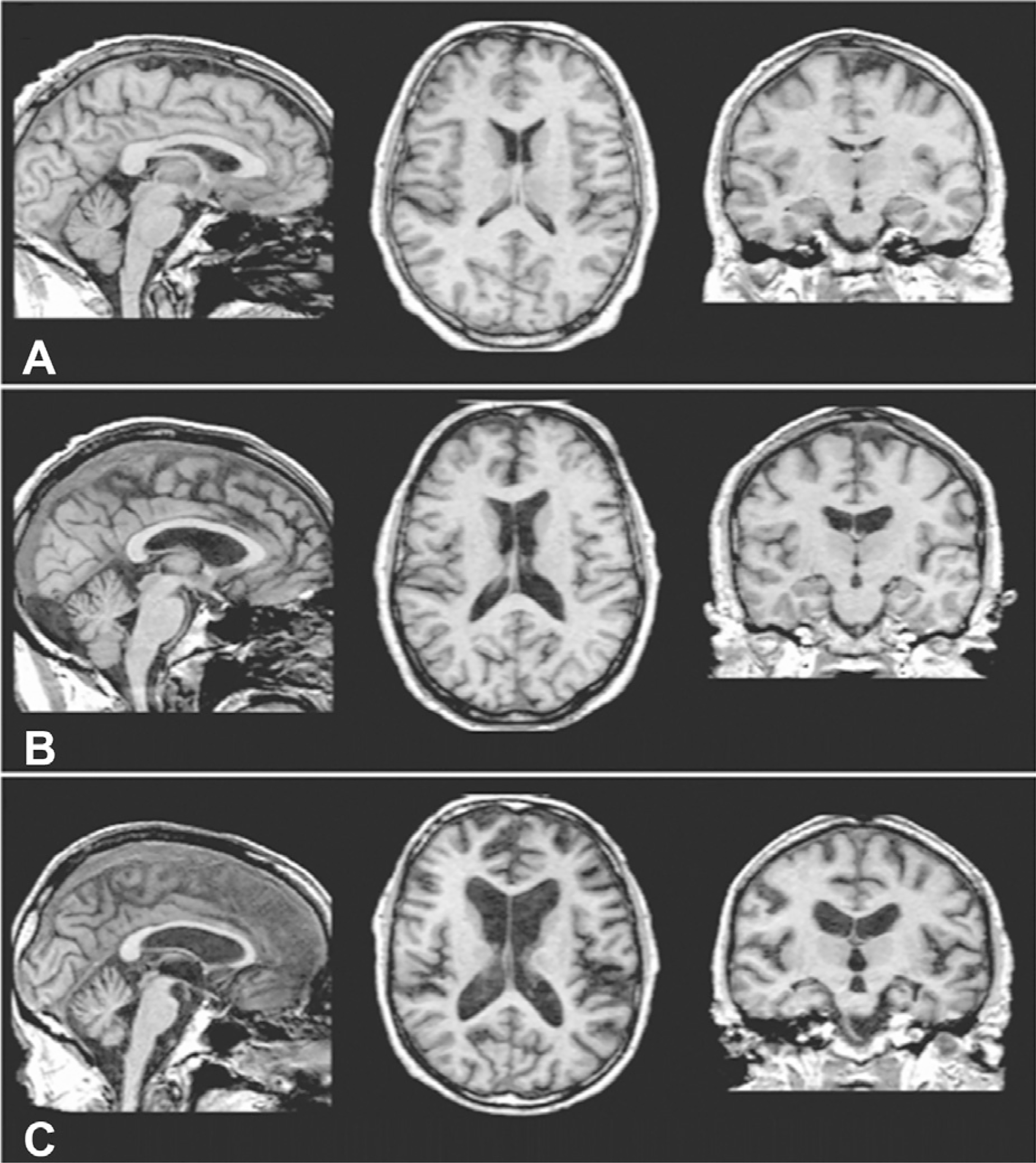
Brain volume deficits in Korsakoff’s syndrome compared with uncomplicated alcoholism. (Reproduced from Zahr et al., 2011.)
Other regions selectively affected in WE and KS include the orbitofrontal cortices (KS), periaqueductal gray matter, and tissue surrounding the third ventricle (WE). With respect to uncomplicated alcoholics, reports suggest that propensity to relapse following sobriety is related to pronounced atrophy in bilateral orbitofrontal cortices (Cardenas et al., 2011; Durazzo et al., 2011; Beck et al., 2012) and the third ventricle can be further enlarged with resumption of chronic alcohol consumption (Sullivan et al., 2000b; Pfefferbaum et al., 2001). There are currently no studies regarding periaqueductal gray-matter volume in uncomplicated alcoholics. Acute alcohol exposure to mice, however, results in robust enhancement of glutamatergic synaptic transmission and increased firing rate of glutamatergic neurons in ventral periaqueductal gray matter (Li et al., 2013). In rats, anxiety-like behavior following alcohol withdrawal also involves glutamatergic transmission in periaqueductal gray matter (Ezequiel Leite and Nobre, 2012). The key regions affected in HE include the globus pallidus and substantia nigra. Volume effects on these two structures have not been reported in uncomplicated alcoholics, but in children with fetal alcohol syndrome, globus pallidus volume is reduced in size compared with unaffected children (Nardelli et al., 2011). By contrast, other basal ganglia nodes of reward circuitry have been described as affected in uncomplicated alcoholism (Makris et al., 2008; Durazzo et al., 2011): MRI studies have revealed smaller volumes of caudate (Boutte et al., 2012), putamen (Jernigan et al., 1991b), amygdala (Fein et al., 2006), and nucleus accumbens, especially in more recently sober alcoholics compared with controls (Sullivan et al., 2005b). Given the role of the amygdala in emotional regulation and behavioral control (for review, see McBride, 2002), however, there is speculation that premorbid amygdala volume deficits put individuals at heightened risk for developing alcohol use disorders (Kamarajan et al., 2006; Benegal et al., 2007; Clarke et al., 2008).
The pons is targeted by CPM and the cerebellum by ACD. Total infratentorial volume (including pons, cerebellar hemispheres, vermis, fissures, cisterns, and fourth ventricle) is significantly smaller in uncomplicated alcoholics than controls. The volume of the pons (Pfefferbaum et al., 2002b; Sullivan, 2003; Chanraud et al., 2009c) and cerebellum (i.e., hemispheres: Sullivan et al., 2000a, c; De Bellis et al., 2005; Chanraud et al., 2007, 2009a; Boutte et al., 2012) (Fig. 17.6). is smaller in uncomplicated alcoholics than controls. Alcoholism-related volume deficits are also prevalent in gray and white matter (Shear et al., 1996; Sullivan et al., 2003) of the cerebellar vermis (Antunez et al., 1998; Piguet et al., 2006; Sullivan et al., 2006, 2010a), predominantly in anterior superior but not posterior inferior regions (Sullivan et al., 2000c).
Fig. 17.6.
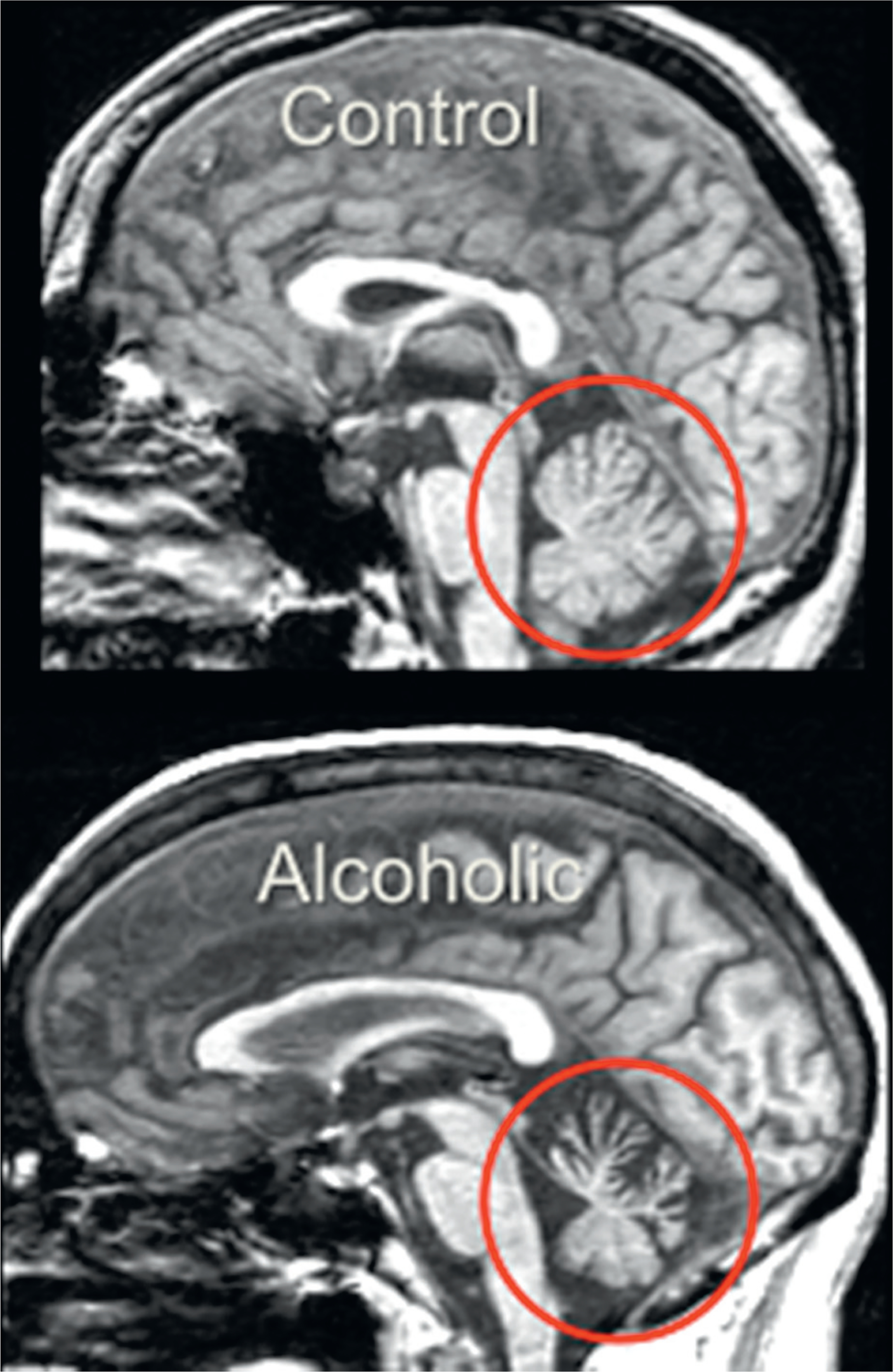
Cerebellar volume deficits in uncomplicated alcoholism. (Reproduced from Sullivan et al., 2010b.)
The frontal cortex is selectively damaged in ARD. With respect to cortical regions in uncomplicated alcoholism, various methods, including semiautomated procedures for brain tissue segmentation (Pfefferbaum, 1992), voxel-based morphometry (Jang et al., 2007; Mechtcheriakov et al., 2007), and deformation-based morphometry (Cardenas et al., 2007), report significant widespread shrinkage of both cortical gray and white matter with corresponding increases in CSF-filled spaces (Jernigan et al., 1991b; Pfefferbaum et al., 1992). In particular, older, but not younger, adult alcoholics show disproportionate deficits in both gray- and white-matter cortical volume, especially in the frontal lobes when volumes are statistically adjusted for brain tissue decline associated with normal aging (Pfefferbaum et al., 1997; Cardenas et al., 2005, 2007). This is the case even in comparisons made in groups selected on alcohol consumption, where older alcoholics have drunk equivalent amounts over their lifetime as younger alcoholics.
Thinning of the corpus callosum occurs in uncomplicated alcoholics and is more prominent in the anterior than posterior regions (Pfefferbaum et al., 1996; Estruch et al., 1997) (Fig. 17.7). As with WE and KS, evidence for MBD-like pathology in uncomplicated alcoholism raises the possibility of a continuum of graded brain dysmorphology.
Fig. 17.7.
Corpus callosum microstructural compromise in uncomplicated alcoholism. (Reproduced from Pfefferbaum et al., 2010.)
STRUCTURAL MRI FINDINGS IN RECOVERY FROM ALCOHOLISM
Longitudinal MRI investigations show reduction of ventricular dilatation following weeks (Schroth et al., 1988; Zipursky et al., 1989) or months (Shear et al., 1994) of drinking cessation. Reduction of lateral ventricles precedes reduction of third ventricular volume (Pfefferbaum et al., 1995) and may be related to improvement in hematocrit, hemoglobin, and red blood cell counts (Pfefferbaum et al., 2004). Structures showing volume gains with abstinence include the entire cerebral cortex (Liu et al., 2000), temporal, insular, and anterior cingulate cortices (Cardenas et al., 2007), the amygdala (Wrase et al., 2008) (a finding which would argue against a premorbid volume deficit), thalamus (Cardenas et al., 2007), hippocampus (Liu et al., 2000; Wrase et al., 2008), brainstem, and cerebellar cortex (Liu et al., 2000; Cardenas et al., 2007).
Sober alcoholics reveal several associations between brain volume gain as determined by MRI and improvement in neuropsychologic test performance: reduction in the volume of the lateral ventricles is related to improved memory performance (Rosenbloom et al., 2007), third ventricle to improved non-verbal short-term memory performance (Sullivan et al., 2000b), and fourth ventricle to improvement in measures of ataxia (Rosenbloom et al., 2007).
The brain’s capacity to return to “normal” following long-term sobriety is unknown. Short-term (6 weeks) abstinence seems sufficient to observe some brain volume recovery, but does not result in equivalent brain volumes between recovering chronic alcoholics and controls (Mann et al., 2005). Whether recovery is complete is difficult to determine. Aging is a factor since older compared with younger alcoholics exhibit reduced capacity for recovery (Fein et al., 1990; Reed et al., 1992; Rourke and Grant, 1999; Munro et al., 2000); longer periods of abstinence may be required for follow-up investigations; some brain damage, such as neuronal loss (Harper, 2007), may be irreversible, even with extended abstinence; and alcohol-dependent subjects may have premorbid brain volume deficits (Schottenbauer et al., 2007).
Despite evidence for recovery of brain volume with abstinence, the mechanisms accounting for recovery remain unclear. The hypothesis of brain rehydration was not supported by evidence for increased T2, which reflects fluctuation in brain free water levels (Schroth et al., 1988). An alternative explanation, neurogenesis (e.g., Mandyam and Koob, 2012), is not likely to be substantial enough to replace the volume loss observed in chronic alcoholism, nor is it clear that new neurons can migrate from neurogenic zones to distant areas of volume loss (Rakic, 2002). On the other hand, adequate volume recovery may be explained by white-matter regeneration since oligodendrocytes have the capacity to repair myelin and remyelinate neurons (Kipp et al., 2012) and oligodendrocyte progenitor cells have the potential to migrate long distances (Tirotta et al., 2010). Indeed, alcoholics who relapse show decrease of white matter (Pfefferbaum et al., 1995), while continued abstinence is associated with increased white matter (Shear et al., 1994), notably in the corpus callosum and subcortical white matter (Cardenas et al., 2007). DTI, by allowing microstructural examination of white matter, has begun to help answer questions such as whether alcohol predominantly affects white more than gray matter and whether brain volume recovery in abstinence can be accounted for by recovery of white-matter volume.
DIFFUSION TENSOR IMAGING
A number of books and papers provide extensive descriptions of the principles of DTI (Chien et al., 1990; Pierpaoli et al., 1996; Poupon et al., 1999; Le Bihan et al., 2001; Basser and Jones, 2002; Le Bihan, 2003; Gerig et al., 2005; Jones, 2005; Sullivan and Pfefferbaum, 2010). Here, only a brief description of DTI and its metrics is provided. DTI takes advantage of the fact that MR images of the brain are predominantly maps of water protons with contrast created by their immediate environment and their motility. In regions with few or no constraints imposed by physical boundaries, such as CSF in the ventricles, water movement is random and uniform in every direction and is therefore isotropic. In contrast to CSF, the path of a water molecule along a white-matter fiber is constrained by physical boundaries such as the axon sheath causing movement to be greater along the long axis of the fiber than across it. This movement is called anisotropic; diffusion along the long axis of a fiber (axial or longitudinal diffusion) is greater than diffusion across the fiber (radial or transverse diffusion) (Song et al., 2002).
The application of several magnetic gradients during image acquisition allows the detection of microscopic water movement. Freely diffusing particles will move more during image acquisition than those with physical restrictions. To characterize the orientation of the diffusion motion in three-dimensional space, observations are made by applying the diffusion gradients in at least six non-collinear orientations. For each voxel, the amount of diffusion is quantified by calculating the ratio of the signals with and without the diffusion gradients for each of the six or more gradient directions, resulting in at least six different diffusion-weighted images, each comprising signal decrease due to the movement of water protons in the orientation of that particular gradient application.
DTI quantification requires computation of a tensor, which is a mathematic description of a three-dimensional ellipsoid depicting the magnitude and orientation of diffusion in individual voxels. The tensor is associated with three corresponding orientational vectors (eigenvectors, λ1, λ2, λ3), describing the diffusion ellipsoid by its major axes. The eigenvalue average, or trace, reflects the magnitude of diffusion, referred to as mean diffusivity (MD) or the apparent diffusion coefficient (ADC). The extent to which one eigenvalue, λ1, dominates the other two, λ2 and λ3, determines the degree of anisotropy, that is, the degree of orientational preference within a voxel, typically measured as fractional anisotropy (FA), ranging between 0 and 1 on a normalized scale (Pierpaoli et al., 1996). The largest eigenvalue, λ1, is the axial (or longitudinal) diffusivity, λL, and reflects axonal integrity, whereas λ2 and λ3 quantify radial (or transverse) diffusivity, λT = (λ2 + λ3)/2, and reflect myelin integrity (Song et al., 2002; Sun et al., 2006). Thus, disruption of white-matter microstructure detectable with DTI can reflect compromise of myelin, cytoskeletal structure, or axonal density (Basser, 1995; Basser and Pierpaoli, 1996; Spielman et al., 1996).
FA is the most commonly reported DTI metric, varying in magnitude with the characteristics of the tissue microstructure. For example, FA of the ventricular system, which contains mostly CSF, is near 0, whereas FA of the corpus callosum, where fibers are arranged in a regular and parallel fashion, can approach 0.8–0.9. Lower than expected FA (and the typically associated higher MD/ADC) in a region of fully volumed white matter can be an index of compromised white-matter integrity. FA, however, is quite sensitive to tissue inhomogeneity from crossing fibers within a voxel (Pierpaoli et al., 2001) and partial voluming (Pfefferbaum et al., 2003). Thus, if the fully volumed white-matter voxels are in a region where multiple fiber tracts cross in different directions, such as adjacent to the corpus callosum, FA will be lower, not necessarily because of reduced fiber integrity but because no single orientation predominates within a voxel (Virta et al., 1999; Pierpaoli et al., 2001).
Several approaches have been used to quantify DTI metrics, including identification of regions on FA maps or voxel-by-voxel comparison with statistical parametric mapping (http://www.fil.ion.ucl.ac.uk/spm/) of brains normalized to a common space or template. One of the more desirable approaches is the use of quantitative fiber tracking to depict selective commissures (e.g., corpus callosum), projection fibers, and association fibers.
Whole-brain analysis
One of the approaches to DTI quantification is voxel-based analysis of whole-brain DTI data, which is useful for identifying regions that differ with respect to diffusion metrics between groups when researchers do not have an a priori hypothesis regarding specific brain region. In voxel-by-voxel analysis, each subject’s diffusion images are registered into standard space, and then voxel-wise statistics are carried out to detect regional differences between populations or to find areas that correlate with a covariate of interest. The voxel-based approach is less sensitive than other methods of DTI quantification because spatial normalization processes can be imperfect (see Snook et al., 2007 for a further description of whole-brain DTI quantification methodologies), and a certain amount of residual morphometric differences might remain. A new approach named tract-based spatial statistics (TBSS), however, aims to remove such differences completely by using a “mean FA skeleton” (Smith et al., 2006), representing the center of fibers that is common to all subjects. Then, in the individual images, FA values can be assessed in the “heart” of the fiber of interest (Douaud et al., 2007). An advantage of TBSS over region of interest (ROI)-based methods is that it provides an automated, observer-independent method of analyzing DTI data. However, TBSS is prone to partial volume effects, is sensitive to crossing fibers in areas of multiple fiber orientation, and is inappropriately named as it is not a depiction of tracts, but a sample-based method, and the “FA skeleton” does not necessarily represent meaningful anatomic tracts.
Region of interest analysis
When a study is focused on a particular brain region, ROI analysis that involves operator-dependent manual outlining or automated parcellation routines is often employed. ROI analysis is time consuming and requires practice to achieve adequate measurement reliability. Inaccurate ROI segmentation can also result in partial voluming of tissue or CSF unintended for inclusion. Another problem is the choice of images on which to draw the ROIs. One study overcame this problem by using a tissue segmentation procedure for distinguishing different brain tissue types in the tensor images and then applying ROIs based on anatomic images to the segmented DTI data (Pfefferbaum et al., 2000a). Overall, while manual ROI placement has its own challenges, it can provide complementary information and convergent validity to whole-brain analysis and furthermore allows relevant correlational analyses with cognitive measures.
Quantitative fiber tracking and tractography
The degree to which the diffusion orientation of a voxel is similar to its neighbors, that is, shows orientational coherence between voxels (Jones et al., 1999; Pfefferbaum et al., 2000b), serves the conceptual basis for quantitative fiber tracking (e.g., Fillard and Gerig, 2003; Gerig et al., 2005; Mori et al., 2005; Le Bihan, 2007) and provides exquisite visual modeling of fiber systems. Analogous to following the linear trajectory of the longitudinal axis of bricks in a path, intervoxel coherence requires that neighboring eigenvectors do not vary by more than a set criterion and that intravoxel FA reaches a minimum value. An advantage of quantitative fiber tracking over other methods of DTI quantification is its ability to measure the diffusion properties of isolated fiber tracts along their full extent. A number of methods are now available for fiber identification and tracking quantification (e.g., Gerig et al., 2005; Mori et al., 2005; Le Bihan, 2007).
DTI FINDINGS IN SYNDROMES ASSOCIATED WITH ALCOHOLISM
Relatively few DTI studies have been conducted in the syndromes associated with alcoholism. Diffusion-weighted imaging in a 31-year-old alcoholic man with WE revealed high diffusivity specific to tissue in the mammillary bodies that resolved following 2 weeks of thiamine treatment. The authors concluded that the high diffusion was indicative of extracellular edema rather than cellular damage, which would not have resolved at follow-up (Bergui et al., 2001). Another case study indicated that, although diffusion-weighted imaging was useful in visually identifying thalamic signal abnormalities in an alcoholic with WE, diffusivity values were normal (Ducreux et al., 2002). In a final case study of WE resulting from alcohol abuse, diffusivity was low, paralleling the pattern observed in the chronic phase of ischemic stroke (Doherty et al., 2002).
HE due to alcoholism compared to other forms of HE (e.g., as a result of viral infection or primary biliary cirrhosis) appears to have different effects on DTI parameters (Miese et al., 2006). ROI (Kale et al., 2006) and voxel-based (Kumar et al., 2008) analysis in alcohol-related cirrhosis with or without HE showed that MD was elevated, while FA was not affected, in corpus callosum, internal capsule, and frontal white matter; the ROI-based study found only MD effects, while the voxel-based study found effects on both FA and MD of occipital white matter. These findings suggest an increase in the interstitial brain water in alcoholic HE patients compared with controls.
A DTI study of a single case of presumed CPM showed elevated MD in the middle cerebellar peduncles and no effects on the corticospinal tract (Min et al., 2012). FA values of seven segments of the corpus callosum in a single MBD patient compared with a normal control were diminished progressively in an anteroposterior fashion (Sair et al., 2006). Another DTI study of a single case of MBD similarly found that the corpus callosum fibers most affected were closest to the frontal horn of the lateral ventricles, presumably affecting fibers destined for the prefrontal regions of the formal lobe, while fibers to the cingulate region and from anterior corona radiata seem to be relatively spared (Pacheco et al., 2012).
DTI FINDINGS IN UNCOMPLICATED ALCOHOLISM
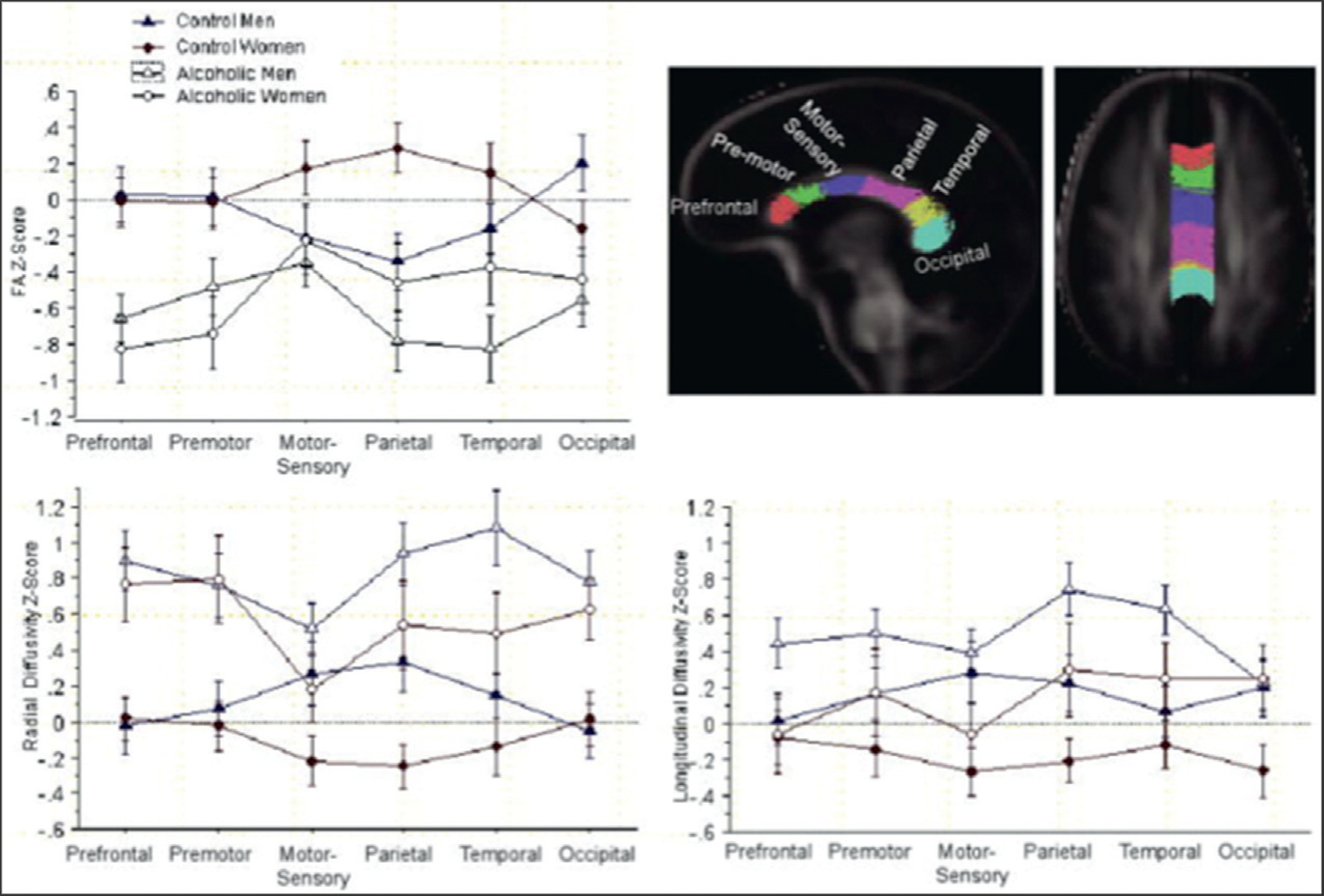
DTI has revealed microstructural damage related to alcoholism in cerebral areas that appear intact in structural MRI analyses (e.g., Pfefferbaum and Sullivan, 2002; Sullivan et al., 2003). Corpus callosum findings in uncomplicated alcoholics are common and, as observed for MBD, show greater anterior than posterior effects (e.g., Schulte et al., 2005; Arnone et al., 2006; Liu et al., 2010; Pitel et al., 2010; Konrad et al., 2012). A study using both voxel-based and ROI approaches in alcoholics found that FA was diminished in right but not left superior longitudinal fasciculus, orbitofrontal white matter, and cingulum bundles (Harris et al., 2008). Quantitative fiber tracking has demonstrated in alcoholics compared with controls greater FA deficits in anterior than in posterior fibers of supratentorial and infratentorial white-matter bundles as well as diminished FA in tracts of the corpus callosum, centrum semiovale (Pfefferbaum et al., 2000b, 2002a; Pfefferbaum and Sullivan, 2005), internal and external capsules, fornix, superior cingulate, longitudinal fasciculi (Pfefferbaum et al., 2009a), corticopontine bundles (Chanraud et al., 2009c), and frontolimbic fibers (Harris et al., 2008).
Alcoholics demonstrate an age–alcoholism interaction in which older alcoholics have higher diffusivity in callosal genu than would be expected based on age (Pfefferbaum et al., 2006) and women appear to have more severe white-matter fiber damage than men, despite having similar lifetime alcohol consumption (Pfefferbaum et al., 2007). Inverse correlations between FA and diffusivity in genu and centrum semiovale suggest that damage of white matter in alcoholism is attributable, at least in part, to the accumulation of intracellular and extracellular fluid in excess of that occurring in aging, and that the differential influence of these fluid compartments can vary across brain regions (Pfefferbaum and Sullivan, 2005). Exercise might attenuate the effects of heavy alcohol consumption on white-matter damage, as suggested by a report evaluating both heavy drinking and aerobic exercise (Karoly et al., 2013).
Regarding DTI–function relationships in alcoholism, FA in right anterior cingulate and left motor areas correlates with executive and psychomotor performance (Konrad et al., 2012) and splenium correlates with working memory (Pfefferbaum et al., 2000b). A double dissociation was found, showing that higher diffusivity in sensorimotor and parietal bundles was associated with poorer balance but not psychomotor speed, whereas higher diffusivity in prefrontal and temporal bundles was associated with slower psychomotor speed but not balance (Pfefferbaum et al., 2010). DTI changes in multiple supratentorial and infratentorial fiber systems in alcoholics correlate with impairment in speeded performance and postural stability (Pfefferbaum et al., 2009b), frontal fibers connecting left and right hemispheres predict performance on a coordinated psychomotor task (Rosenbloom et al., 2008), and number of reconstructed fibers running between the pons and the midbrain is related to cognitive flexibility performance (Chanraud et al., 2009c). Gray-matter diffusivity in hippocampus, which is lower in alcoholics than in controls, is related to episodic memory impairment (Chanraud et al., 2009b).
DTI FINDINGS IN RECOVERY FROM ALCOHOLISM
Similar to structural MRI findings demonstrating pronounced atrophy of orbitofrontal cortices in abstinent alcoholics who were likely to resume drinking (i.e., Cardenas et al., 2011; Durazzo et al., 2011; Beck et al., 2012), DTI identified alcoholic individuals more likely to resume drinking 6 months following initial evaluation based on lower FA and higher diffusivity in frontal white matter at baseline (Sorg et al., 2012). Increases in FA and decreases in diffusivity have been interpreted as evidence for white-matter recovery with abstinence. Substantiation for recovery has been shown in corpus callosum genu and body at 1 year compared with 2 weeks of abstinence (Alhassoon et al., 2012), and in frontal white matter at 1 month compared with 1 week of abstinence, at least in non-smoking, sober alcoholics (Gazdzinski et al., 2010).
CONCLUSION
Despite evidence for damage, alcoholics who maintain sobriety over extended periods show improvements in both brain volume and white matter integrity, potentially reflecting fiber reorganization and myelin restoration, indicative of a neural mechanism explaining recovery and perhaps enhancing chances for sustained sobriety.
References
- Agartz I, Momenan R, Rawlings RR et al. (1999). Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry 56: 356–363. [DOI] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ et al. (2012). Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res 36: 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman AI, Tarter RE (1985). Relationship between familial alcoholism and head injury. J Stud Alcohol 46: 256–258. [DOI] [PubMed] [Google Scholar]
- Andersen AM, Novovic S, Ersboll AK et al. (2008). Mortality in alcohol and biliary acute pancreatitis. Pancreas 36: 432–434. [DOI] [PubMed] [Google Scholar]
- Antunez E, Estruch R, Cardenal C et al. (1998). Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. AJR Am J Roentgenol 171: 1131–1137. [DOI] [PubMed] [Google Scholar]
- Arbelaez A, Pajon A, Castillo M (2003). Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol 24: 1955–1957. [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Abou-Saleh MT, Barrick TR (2006). Diffusion tensor imaging of the corpus callosum in addiction. Neuropsychobiology 54: 107–113. [DOI] [PubMed] [Google Scholar]
- Basser PJ (1995). Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8: 333–344. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK (2002). Diffusion-tensor MRI: theory, experimental design and data analysis – a technical review. NMR Biomed 15: 456–467. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996). Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor MRI. J Magn Reson B 111: 209–219. [DOI] [PubMed] [Google Scholar]
- Beck A, Wustenberg T, Genauck A et al. (2012). Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry 69: 842–852. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G et al. (2007). Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol 12: 122–132. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J et al. (2006). Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res 30: 1866–1870. [DOI] [PubMed] [Google Scholar]
- Bergui M, Bradac GB, Zhong JJ et al. (2001). Diffusion-weighted MR in reversible Wernicke encephalopathy. Neuroradiology 43: 969–972. [DOI] [PubMed] [Google Scholar]
- Bienia A, Sodolski W, Luchowska E (2002). The effect of chronic alcohol abuse on gastric and duodenal mucosa. Ann Univ Mariae Curie Sklodowska [Med] 57: 570–582. [PubMed] [Google Scholar]
- Bleich S, Sperling W, Degner D et al. (2003). Lack of association between hippocampal volume reduction and first-onset alcohol withdrawal seizure. A volumetric MRI study. Alcohol Alcohol 38: 40–44. [DOI] [PubMed] [Google Scholar]
- Boutte D, Calhoun VD, Chen J et al. (2012). Association of genetic copy number variations at 11 q14.2 with brain regional volume differences in an alcohol use disorder population. Alcohol 46: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Andersson J (2001). Frontal dysfunction and frontal cortical synapse loss in alcoholism – the main cause of alcohol dementia? Dement Geriatr Cogn Disord 12: 289–294. [DOI] [PubMed] [Google Scholar]
- Butterworth RF (2009). Thiamine deficiency-related brain dysfunction in chronic liver failure. Metab Brain Dis 24: 189–196. [DOI] [PubMed] [Google Scholar]
- Butterworth RF (2013). Parkinsonism in cirrhosis: pathogenesis and current therapeutic options. Metab Brain Dis 28: 261–267. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ et al. (2005). Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res 138: 115–130. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S et al. (2007). Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S et al. (2011). Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry 70: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI (2012). Alcohol metabolism. Clin Liver Dis 16: 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F et al. (2007). Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32: 429–438. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Leroy C, Martelli C et al. (2009a). Episodic memory in detoxified alcoholics: contribution of grey matter microstructure alteration. PLoS One 4: e6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M et al. (2009b). Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subject. Neuropsychopharmacology 34: 1223–1232. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M et al. (2009c). Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology 34: 1223–1232. [DOI] [PubMed] [Google Scholar]
- Chen CM, Yi HY, Yoon YH et al. (2012). Alcohol use at time of injury and survival following traumatic brain injury: results from the National Trauma Data Bank. J Stud Alcohol Drugs 73: 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien D, Buxton RB, Kwong KK et al. (1990). MR diffusion imaging of the human brain. J Comput Assist Tomogr 14: 514–520. [DOI] [PubMed] [Google Scholar]
- Chua GC, Sitoh YY, Lim CC et al. (2002). MRI findings in osmotic myelinolysis. Clin Radiol 57: 800–806. [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US et al. (2008). HPA-axis activity in alcoholism: examples for a gene–environment interaction. Addict Biol 13: 1–14. [DOI] [PubMed] [Google Scholar]
- Cordoba J, Sanpedro F, Alonso J et al. (2002). 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis 17: 415–429. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL et al. (2005). Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res 29: 1590–1600. [DOI] [PubMed] [Google Scholar]
- de la Monte S, Derdak Z, Wands JR (2012). Alcohol, insulin resistance and the liver-brain axis. J Gastroenterol Hepatol 27 (Suppl 2): 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Rios F, Kleindorfer DO, Khoury J et al. (2012). Trends in substance abuse preceding stroke among young adults: a population-based study. Stroke 43: 3179–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MJ, Watson NF, Uchino K et al. (2002). Diffusion abnormalities in patients with Wernicke encephalopathy. Neurology 58: 655–657. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M et al. (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130: 2375–2386. [DOI] [PubMed] [Google Scholar]
- Draper B, Karmel R, Gibson D et al. (2011). Alcohol-related cognitive impairment in New South Wales hospital patients aged 50 years and over. Aust N Z J Psychiatry 45: 985–992. [DOI] [PubMed] [Google Scholar]
- Ducreux D, Petit-Lacour MC, Benoudiba F et al. (2002). Diffusion-weighted imaging in a case of Wernicke encephalopathy. J Neuroradiol 29: 39–42. [PubMed] [Google Scholar]
- Duell EJ, Sala N, Travier N et al. (2012). Genetic variation in alcohol dehydrogenase (ADH1A, ADH1B, ADH1C, ADH7) and aldehyde dehydrogenase (ALDH2), alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Carcinogenesis 33: 361–367. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S et al. (2011). Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res 35: 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M et al. (1997). Atrophy of the corpus callosum in chronic alcoholism. J Neurol Sci 146: 145–151. [DOI] [PubMed] [Google Scholar]
- Eyer F, Schuster T, Felgenhauer N et al. (2011). Risk assessment of moderate to severe alcohol withdrawal – predictors for seizures and delirium tremens in the course of withdrawal. Alcohol Alcohol 46: 427–433. [DOI] [PubMed] [Google Scholar]
- Ezequiel Leite L, Nobre MJ (2012). The negative effects of alcohol hangover on high-anxiety phenotype rats are influenced by the glutamate receptors of the dorsal midbrain. Neuroscience 213: 93–105. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S et al. (1990). Cognitive impairments in abstinent alcoholics. West J Med 152: 531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H et al. (2006). Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage 32: 1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerlein W (1977). Neuropsychiatric disorders of alcoholism. Nutr Metab 21: 163–174. [DOI] [PubMed] [Google Scholar]
- Fields JZ, Turk A, Durkin M et al. (1994). Increased gastrointestinal symptoms in chronic alcoholics. Am J Gastroenterol 89: 382–386. [PubMed] [Google Scholar]
- Fillard P, Gerig G (2003). Analysis tool for diffusion tensor MRI. In: Proceedings of Medical Image Computing and Computer-assisted Intervention, Lecture Notes in Computer Science. Saint-Malo, Springer, France, pp. 967–968. [Google Scholar]
- Fitzpatrick LE, Jackson M, Crowe SF (2012). Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res 36: 1942–1951. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A et al. (2010). Cerebral white matter recovery in abstinent alcoholics – a multimodality magnetic resonance study. Brain 133: 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerig G, Corouge I, Vachet C et al. (2005). Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study. 13th Proceedings of the International Society for Magnetic Resonance in Medicine, Abstract no. 1337, International Society for Magnetic Resonance in Medicine, Miami, FL. [Google Scholar]
- Gloria L, Cravo M, Camilo ME et al. (1997). Nutritional deficiencies in chronic alcoholics: relation to dietary intake and alcohol consumption. Am J Gastroenterol 92: 485–489. [PubMed] [Google Scholar]
- Goldman JE, Horoupian DS (1981). Demyelination of the lateral geniculate nucleus in central pontine myelinolysis. Ann Neurol 9: 185–189. [DOI] [PubMed] [Google Scholar]
- Gupta S, Warner J (2008). Alcohol-related dementia: a 21st-century silent epidemic? Br J Psychiatry 193: 351–353. [DOI] [PubMed] [Google Scholar]
- Ha ND, Weon YC, Jang JC et al. (2012). Spectrum of MR imaging findings in Wernicke encephalopathy: are atypical areas of involvement only present in nonalcoholic patients? AJNR Am J Neuroradiol 33: 1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M et al. (1997). Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus 7: 78–87. [DOI] [PubMed] [Google Scholar]
- Harper C (2006). Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur J Neurol 13: 1078–1082. [DOI] [PubMed] [Google Scholar]
- Harper C (2007). The neurotoxicity of alcohol. Hum Exp Toxicol 26: 251–257. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM et al. (2008). Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res 32: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, McGough NN, Ravindranathan A et al. (2005). Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci 25: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyumpa AM Jr (1980). Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr 33: 2750–2761. [DOI] [PubMed] [Google Scholar]
- Ihn YK, Hwang SS, Park YH (2007). Acute Marchiafava-Bignami disease: diffusion-weighted MRI in cortical and callosal involvement. Yonsei Med J 48: 321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang DP, Namkoong K, Kim JJ et al. (2007). The relationship between brain morphometry and neuropsychological performance in alcohol dependence. Neurosci Lett 428: 21–26. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Schafer K, Butters N et al. (1991a). Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology 4: 175–186. [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G et al. (1991b). Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res 15: 418–427. [DOI] [PubMed] [Google Scholar]
- Johkura K, Naito M, Naka T (2005). Cortical involvement in Marchiafava-Bignami disease. AJNR Am J Neuroradiol 26: 670–673. [PMC free article] [PubMed] [Google Scholar]
- Jones DK (2005). Fundamentals of diffusion MR imaging. In: Gillard J, Waldman AD, Barker P (Eds.), Clincal MR Neuroimaging, Cambridge University Press, Cambridge. [Google Scholar]
- Jones D, Simmons A, Williams S et al. (1999). Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med 42: 37–41. [DOI] [PubMed] [Google Scholar]
- Kale RA, Gupta RK, Saraswat VA et al. (2006). Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology 43: 698–706. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K et al. (2006). Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry 59: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Stevens CJ, Thayer RE et al. (2013). Aerobic exercise moderates the effect of heavy alcohol consumption on white matter damage. Alcohol Clin Exp Res 37: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Shiota J, Yagishita T et al. (1985). Marchiafava-Bignami disease: computed tomographic scan and magnetic resonance imaging. Ann Neurol 18: 103–104. [DOI] [PubMed] [Google Scholar]
- Khaw AV, Heinrich A (2006). Marchiafava-Bignami disease: diffusion-weighted MRI in corpus callosum and cortical lesions. Neurology 66: 1286, author reply 1286. [DOI] [PubMed] [Google Scholar]
- Kipp M, Victor M, Martino G et al. (2012). Endogeneous remyelination: findings in human studies. CNS Neurol Disord Drug Targets 11: 598–609. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-Demasters BK, Rojiani AM, Filley CM (2006). Central and extrapontine myelinolysis: then. . .and now. J Neuropathol Exp Neurol 65: 1–11. [DOI] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Lorscheider M et al. (2012). Broad disruption of brain white matter microstructure and relationship with neuropsychological performance in male patients with severe alcohol dependence. Alcohol Alcohol 47: 118–126. [DOI] [PubMed] [Google Scholar]
- Korbo L (1999). Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res 23: 164–168. [PubMed] [Google Scholar]
- Kumar S, Fowler M, Gonzalez-Toledo E et al. (2006). Central pontine myelinolysis, an update. Neurol Res 28: 360–366. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gupta RK, Elderkin-Thompson V et al. (2008). Voxel-based diffusion tensor magnetic resonance imaging evaluation of low-grade hepatic encephalopathy. J Magn Reson Imaging 27: 1061–1068. [DOI] [PubMed] [Google Scholar]
- Kurth C, Wegerer V, Reulbach U et al. (2004). Analysis of hippocampal atrophy in alcoholic patients by a Kohonen feature map. Neuroreport 15: 367–371. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L et al. (2000). A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res 109: 177–186. [DOI] [PubMed] [Google Scholar]
- Le Bihan D (2003). Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4: 469–480. [DOI] [PubMed] [Google Scholar]
- Le Bihan D (2007). The ‘wet mind’: water and functional neuroimaging. Phys Med Biol 52: R57–R90. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin J, Poupon C et al. (2001). Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13: 534–546. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim SS, Kim SH et al. (2011). Acute Marchiafava-Bignami disease with selective involvement of the precentral cortex and splenium: a serial magnetic resonance imaging study. Neurologist 17: 213–217. [DOI] [PubMed] [Google Scholar]
- Lenz V, Vargas MI, Bin JF et al. (2002). Value of MRI findings in Gayet-Wernicke encephalopathy. J Neuroradiol 29: 153–160. [PubMed] [Google Scholar]
- Levy S, Herve C, Delacoux E et al. (2002). Thiamine deficiency in hepatitis C virus and alcohol-related liver diseases. Dig Dis Sci 47: 543–548. [DOI] [PubMed] [Google Scholar]
- Li C, McCall NM, Lopez AJ et al. (2013). Alcohol effects on synaptic transmission in periaqueductal gray dopamine neurons. Alcohol 47: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou KC, Kuo SF, Chen LA (2012). Wernicke encephalopathy with atypical magnetic resonance imaging. Am J Emerg Med 30 (2086): e2081–e2083. [DOI] [PubMed] [Google Scholar]
- Liu RSN, Lemieux L, Shorvon SD et al. (2000). Association between brain size and abstinence from alcohol. Lancet 355: 1969–1970. [DOI] [PubMed] [Google Scholar]
- Liu IC, Chiu CH, Chen CJ et al. (2010). The microstructural integrity of the corpus callosum and associated impulsivity in alcohol dependence: a tractography-based segmentation study using diffusion spectrum imaging. Psychiatry Res 184: 128–134. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK et al. (2008). Decreased volume of the brain reward system in alcoholism. Biol Psychiatry 64: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF (2012). The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci 35: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KF, Ackermann K, Croissan B et al. (2005). Neuroimaging of gender differences in alcoholism: are women more vulnerable? Alcohol Clin Exp Res 29: 896–901. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Goldstein JN, Pallin DJ (2011). Emergency department seizure epidemiology. Emerg Med Clin North Am 29: 15–27. [DOI] [PubMed] [Google Scholar]
- McBride WJ (2002). Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav 71: 509–515. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K et al. (2007). A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry 78: 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miese F, Kircheis G, Wittsack HJ et al. (2006). 1H-MR spectroscopy, magnetization transfer, and diffusion-weighted imaging in alcoholic and nonalcoholic patients with cirrhosis with hepatic encephalopathy. AJNR Am J Neuroradiol 27: 1019–1026. [PMC free article] [PubMed] [Google Scholar]
- Min Y, Park SH, Hwang SB (2012). Corticospinal tract and pontocerebellar fiber of central pontine myelinolysis. Ann Rehabil Med 36: 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MY (1982). Alcohol and nutrition. Br Med Bull 38: 21–29. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM et al. (2005). An atlas of human white matter, Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- Munro CA, Saxton J, Butters MA (2000). The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res 24: 1510–1516. [PubMed] [Google Scholar]
- Naegele T, Grodd W, Viebahn R et al. (2000). MR imaging and (1)H spectroscopy of brain metabolites in hepatic encephalopathy: time-course of renormalization after liver transplantation. Radiology 216: 683–691. [DOI] [PubMed] [Google Scholar]
- Namekawa M, Nakamura Y, Nakano I (2013). Cortical involvement in Marchiafava-Bignami disease can be a predictor of a poor prognosis: a case report and review of the literature. Intern Med 52: 811–813. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C et al. (2011). Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 35: 1404–1417. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT (2004). Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci 24: 9714–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Cary MS (2003). Alcohol-related dementia: validation of diagnostic criteria. Am J Geriatr Psychiatry 11: 441–447. [PubMed] [Google Scholar]
- Pacheco FT, Rego MM, do Rego JI et al. (2012). “Ears of the lynx” sign in a Marchiafava-Bignami patient: structural basis and fiber-tracking DTI contribution to the understanding of this imaging abnormality. J Neuroimaging 24: 205–207. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A (1992). Use of MRI to evaluate structural changes in addictive medicine. In: Proceedings of the American Society of Addiction Medicine’s 23rd Annual Medical-Scientific Conference. [Google Scholar]
- Pfefferbaum A, Sullivan EV (2002). Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage 15: 708–718. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV (2005). Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology 30: 423–432. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Rosenbloom MJ (1992). Structural imaging of the brain in chronic alcoholism. Imaging in Alcohol Research: NIAAA Research Monograph S Zakhari, Witt E (Eds.), 21: U.S. Dept Health and Human Services, Rockville, MD, pp. 99–120. [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH et al. (1995). Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 19: 1177–1191. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE et al. (1996). Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res 20: 752–757. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH et al. (1997). Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 21: 521–529. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M et al. (2000a). Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 44: 259–268. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M et al. (2000b). In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res 24: 1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A et al. (2001). Sex differences in the effects of alcohol on brain structure. Am J Psychiatry 158: 188–197. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Sullivan EV (2002a). Alcoholism and AIDS: magnetic resonance imaging approaches for detecting interactive neuropathology. Alcohol Clin Exp Res 26: 1031–1046. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Serventi K et al. (2002b). Corpus callosum, pons and cortical white matter in alcoholic women. Alcohol Clin Exp Res 26: 400–405. [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV (2003). Replicability of diffusion tensor imaging measurements of fractional anisotropy and trace in brain. J Magn Reson Imaging 18: 427–433. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Serventi KL et al. (2004). Brain volumes, RBC status, and hepatic function in alcoholics after 1 and 4 weeks of sobriety: predictors of outcome. Am J Psychiatry 161: 1190–1196. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV (2006). Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol Aging 27: 994–1009. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E et al. (2007). Diffusion tensor imaging with quantitative fiber tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130: 48–64. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T et al. (2009a). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 65: 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T et al. (2009b). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 65: 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R et al. (2010). Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcohol Clin Exp Res 34: 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ et al. (2013). Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage 65: 176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ et al. (1996). Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S et al. (2001). Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13: 1174–1185. [DOI] [PubMed] [Google Scholar]
- Piguet O, Cramsie J, Bennett HP et al. (2006). Contributions of age and alcohol consumption to cerebellar integrity, gait and cognition in non-demented very old individuals. Eur Arch Psychiatry Clin Neurosci 256: 504–511. [DOI] [PubMed] [Google Scholar]
- Pitel A-L, Chanraud S, Sullivan E et al. (2010). Callosal microstructural abnormalities in Alzheimer disease and alcoholism: same phenotype, different mechanisms. Psychiatry Research: Neuroimaging 184: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Chetelat G, Le Berre AP et al. (2012). Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology 78: 1330–1333. [DOI] [PubMed] [Google Scholar]
- Poupon C, Clark CA, Frouin V et al. (1999). Tracking white matter fascicles with diffusion tensory imaging. Proceedings of the Seventh International Society for Magnetic Resonance in Medicine; 325. [Google Scholar]
- Prakash R, Mullen KD (2010). Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol 7: 515–525. [DOI] [PubMed] [Google Scholar]
- Rakic P (2002). Neurogenesis in adult primates. Prog Brain Res 138: 3–14. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM et al. (2010). Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage 51: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RJ, Grant I, Rourke SB (1992). Long-term abstinent alcoholics have normal memory. Alcohol Clin Exp Res 16: 677–683. [DOI] [PubMed] [Google Scholar]
- Ridley NJ, Draper B, Withall A (2013). Alcohol-related dementia: an update of the evidence. Alzheimers Res Ther 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, Greenfield TK, Kerr WC et al. (2011). Heavy drinking occasions in relation to ischaemic heart disease mortality – an 11–22 year follow-up of the 1984 and 1995 US National Alcohol Surveys. Int J Epidemiol 40: 1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T (2006). Transformation model and constraints cause bias in statistics on deformation fields. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv 9: 207–214. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O’Reilly A et al. (2007). Improvement in memory and static balance with abstinence in alcoholic men and women: selective relations with changes in regional ventricular volumes. Psychiatry Res Neuroimaging 155: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sassoon SA, Fama R et al. (2008). Frontal callosal fiber integrity selectively predicts coordinated psychomotor performance in alcoholism. Brain Imaging Behav 2: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sullivan EV, Pfefferbaum A (2010). Effect of neurotoxins on brain tissue and function revealed through in vivo MR imaging and spectroscopy. In: Harry GJ, Tilson HA (Eds.), Neurotoxicology, Informa Healthcare, New York. [Google Scholar]
- Ross LJ, Wilson M, Banks M et al. (2012). Prevalence of malnutrition and nutritional risk factors in patients undergoing alcohol and drug treatment. Nutrition 28: 738–743. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Grant I (1999). The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: a 2-year follow-up study. J Int Neuropsychol Soc 5: 234–246. [DOI] [PubMed] [Google Scholar]
- Rovira A, Cordoba J, Sanpedro F et al. (2002). Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 59: 335–341. [DOI] [PubMed] [Google Scholar]
- Rovira A, Alonso J, Cordoba J (2008). MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29: 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumboldt Z, Castillo M, Huang B et al. (2012). Brain Imaging with MRI and CT: An Image Pattern Approach, Cambridge University Press, Cambridge. [Google Scholar]
- Sair HI, Mohamed FB, Patel S et al. (2006). Diffusion tensor imaging and fiber-tracking in Marchiafava-Bignami disease. J Neuroimaging 16: 281–285. [DOI] [PubMed] [Google Scholar]
- Schaeffner E, Ritz E (2012). Alcohol and kidney damage: a Janus-faced relationship. Kidney Int 81: 816–818. [DOI] [PubMed] [Google Scholar]
- Schmidt KS, Gallo JL, Ferri C et al. (2005). The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord 20: 286–291. [DOI] [PubMed] [Google Scholar]
- Schottenbauer MA, Momenan R, Kerick M et al. (2007). Relationships among aging, IQ, and intracranial volume in alcoholics and control subjects. Neuropsychology 21: 337–345. [DOI] [PubMed] [Google Scholar]
- Schroth G, Naegele T, Klose U et al. (1988). Reversible brain shrinkage in abstinent alcoholics, measured by MRI. Neuroradiology 30: 385–389. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM et al. (2005). Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex 15: 1384–1392. [DOI] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N (1994). Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res 18: 172–176. [DOI] [PubMed] [Google Scholar]
- Shear PK, Sullivan EV, Lane B et al. (1996). Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res 20: 1489–1495. [DOI] [PubMed] [Google Scholar]
- Sheedy D, Lara A, Garrick T et al. (1999). Size of mamillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcohol Clin Exp Res 23: 1624–1628. [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Snook L, Plewes C, Beaulieu C (2007). Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage 34: 243–252. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ et al. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Sorg SF, Taylor MJ, Alhassoon OM et al. (2012). Frontal white matter integrity predictors of adult alcohol treatment outcome. Biol Psychiatry 71: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman D, Butts K, de Crespigny A et al. (1996). Diffusion-weighted imaging of clinical stroke. Intl J Neuroradiol 1: 44–55. [Google Scholar]
- Squire LR, Amaral DG, Press GA (1990). Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J Neurosci 10: 3106–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JT (2006). The frontal/subcortical dementias: common dementing illnesses associated with prominent and disturbing behavioral changes. Geriatrics 61: 23–27. [PubMed] [Google Scholar]
- Sullivan EV (2003). Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res 27: 1409–1419. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L (2003). Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology 61: 1716–1719. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A (2009). Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol 44: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A (2010). Diffusion tensor imaging in aging and age-related disorders. In: Jones DK (Ed.), Diffusion MRI: Theory, Methods and Applications, Oxford University Press, Oxford, pp. 624–643. [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH et al. (1995a). Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res 19: 110–122. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH et al. (1995b). Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res 19: 110–122. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH et al. (1996). Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin Exp Res 20: 348–354. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Lane B, Deshmukh A et al. (1999a). In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res 23: 1629–1636. [PubMed] [Google Scholar]
- Sullivan EV, Lane B, Rosenbloom MJ et al. (1999b). In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res 23: 1629–1636. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A (2000a). Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res 24: 611–621. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO et al. (2000b). Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology 14: 178–188. [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE et al. (2000c). Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology 14: 341–352. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R et al. (2003). Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res 27: 301–309. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A (2005a). Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging 26: 1093–1098. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E et al. (2005b). Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry 57: 768–776. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A (2006). Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex 16: 1077–1086. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A (2010a). Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women. Biol Psychiatry 67: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Harris A, Pfefferbaum A (2010b). Alcohol’s effects on brain and behavior. Alcohol Res Health 33: 1–2. [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K et al. (2006). Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55: 302–308. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Izumi M (2012). Alcohol is a risk factor not for thalamic but for putaminal hemorrhage: the Akita Stroke Registry. J Stroke Cerebrovasc Dis 22: 1064–1069. [DOI] [PubMed] [Google Scholar]
- Thomson AD (2000). Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl 35: 2–7. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Guerrini I, Marshall EJ (2012). The evolution and treatment of Korsakoff’s syndrome: out of sight, out of mind? Neuropsychol Rev 22: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorarinsson BL, Olafsson E, Kjartansson O et al. (2011). Wernicke’s encephalopathy in chronic alcoholics. Laeknabladid 97: 21–29. [DOI] [PubMed] [Google Scholar]
- Tirotta E, Carbajal KS, Schaumburg CS et al. (2010). Cell replacement therapies to promote remyelination in a viral model of demyelination. J Neuroimmunol 224: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, de Ruiter MB, van den Brink W et al. (2012). A voxel-based morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug Alcohol Depend 124: 142–148. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Chung C, Cahill ME et al. (2003). Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis 23: 259–269. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH (1989). The Wernicke–Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2nd edn F.A. Davis, Philadelphia. [Google Scholar]
- Virta A, Barnett A, Pierpaoli C (1999). Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MR. Magn Reson Imaging 17: 1121–1133. [DOI] [PubMed] [Google Scholar]
- Wilhelm J, Frieling H, Hillemacher T et al. (2008). Hippocampal volume loss in patients with alcoholism is influenced by the consumed type of alcoholic beverage. Alcohol Alcohol 43: 296–299. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF et al. (2008). Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry 165: 1179–1184. [DOI] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM et al. (2012). Ethanol induces oxidative stress in alveolar macrophag via upregulation of NADPH oxidases. J Immunol 188: 3648–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Hashimoto T, Fujimoto K et al. (2010). Evolution of callosal and cortical lesions on MRI in Marchiafava-Bignami disease. Case Rep Neurol 2: 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, Harper CG (2011). Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol 7: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Pfefferbaum A (1989). MRI study of brain changes with short term abstinence from alcohol. Alcohol Clin Exp Res 13: 664–666. [DOI] [PubMed] [Google Scholar]


