Highlights
-
•
Ageing is associated with declines in muscle mass, quality and insulin sensitivity.
-
•
These features of ageing quickly manifest in young exposed to physical inactivity.
-
•
The cellular mechanisms of inactivity-driven muscle dysregulation are unclear.
-
•
The precise contribution of inactivity to muscle decline with age is unknown.
-
•
Muscle ageing caused by lifestyle factors may be at least partly reversible.
Keywords: Physical inactivity, Ageing, Sarcopenia, Muscle, Metabolism
Abstract
In the United Kingdom (UK), it is projected that by 2035 people aged >65 years will make up 23 % of the population, with those aged >85 years accounting for 5% of the total population. Ageing is associated with progressive changes in muscle metabolism and a decline in functional capacity, leading to a loss of independence. Muscle metabolic changes associated with ageing have been linked to alterations in muscle architecture and declines in muscle mass and insulin sensitivity. However, the biological features often attributed to muscle ageing are also seen in controlled studies of physical inactivity (e.g. reduced step-count and bed-rest), and it is currently unclear how many of these ageing features are due to ageing per se or sedentarism. This is particularly relevant at a time of home confinements reducing physical activity levels during the Covid-19 pandemic. Current knowledge gaps include the relative contribution that physical inactivity plays in the development of many of the negative features associated with muscle decline in older age. Similarly, data demonstrating positive effects of government recommended physical activity guidelines on muscle health are largely non-existent. It is imperative therefore that research examining interactions between ageing, physical activity and muscle mass and metabolic health is prioritised so that it can inform on the “normal” muscle ageing process and on strategies for improving health span and well-being. This review will focus on important changes in muscle architecture and metabolism that accompany ageing and highlight the likely contribution of physical inactivity to these changes.
1. Introduction
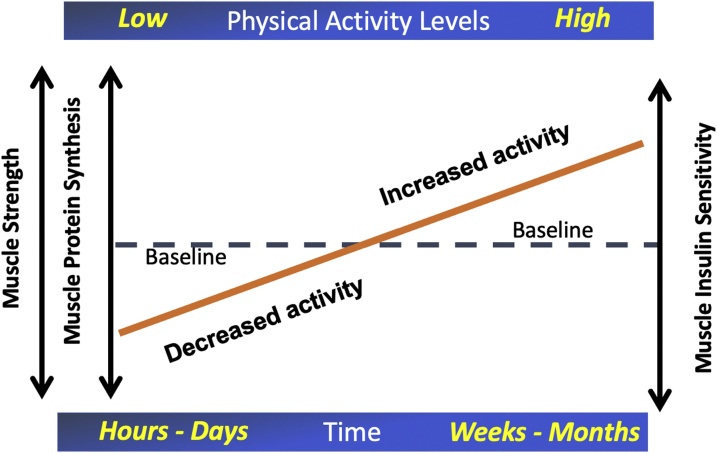
Ageing is associated with progressive changes in skeletal muscle mass, metabolism and functional capacity, which are associated with a decline in independence and increased total mortality (Srikanthan and Karlamangla, 2014). Given that by 2035, people aged >65 years will make up 23 % of the UK population, with those aged >85 years accounting for 5 % of the total population (Office for National Statistics), it is paramount that studies into the effects of aging on muscle mass and metabolism are investigated. Some features of ageing may in fact be partly reversible. Busse was the first to suggest the idea of primary and secondary ageing (Busse, 1969); primary ageing refers to the intrinsic time-related biological process not contingent on stress, trauma or disease, whilst secondary ageing is physiological decline that occurs secondary to environmental and lifestyle influences such as diet and physical activity. Factors contributing to secondary ageing by their nature therefore may be partly reversible. As people age physical activity levels tend to decline (Milanović, Pantelić et al. 2013). Studies in older people to date have not measured baseline physical activity levels in a detailed quantified manner. Similarly, studies comparing young and old have not matched groupings for habitual physical activity levels (Frontera et al., 2000) and it is also debatable whether the decline in muscle mass and quality in older people during inactivity follows the same trajectory as younger individuals, with studies reporting opposite responses (Kortebein et al., 2007; Suetta et al., 2009). This is currently of increased importance, given government-imposed restrictions of movement in a bid to curb viral transmission in the present global pandemic of Covid-19 has led to a significant increase in sedentary behaviour amongst the general population. Lockdown has had a detrimental effect on both the physical and mental health of older people, including cardiorespiratory deconditioning, weight gain and social isolation (Narici et al., 2020; Kirwan et al., 2020). Tailored guidance for older people is required to maintain their physical functioning and resilience during this time. The current World Health Organisation (WHO) recommendation for physical activity is set at a minimum of 150 min of moderate intensity aerobic activity per week in individuals aged 18–64, or at least 75 min of vigorous intensity aerobic physical activity (World Health Organisation (WHO), 2010). However, it was estimated that 17 % of adults worldwide failed to meet this guideline in 2009, and by 2012 this figure had increased to 31 %, pointing to a worsening world-wide public health problem (World Health Organisation (WHO), 2010). These responses in part are due to the expanding array of labour-saving devices or systems, present in occupational and domestic settings. Much research has been undertaken identifying beneficial effects of exercise intervention programs on muscle and metabolic health, as well as the mechanisms driving these adaptations. However, the mechanisms underlying inactivity-driven dysregulation has received far less attention. Considered as a theoretical construct, exercise will produce significant physiological stress on muscle to induce positive gains in muscle mass and metabolic health, and this stress will be relatively short lived. In comparison, physical inactivity will impose opposing physiological signals of smaller magnitude, but these signals will persist for considerably longer durations in sedentary individuals and will induce muscle level adaptations that persist as long as inactivity is present (Fig. 1). It is perhaps not surprising therefore that many of the features of skeletal muscle dysmetabolism seen in the elderly can be relatively quickly manifested in young people simply by exposure to inactivity.
Fig. 1.
A theoretical construct outlining the relationship between physical inactivity levels and muscle metabolic health. It is proposed exercise will produce significant physiological stress on muscle to produce positive gains in muscle strength, protein synthesis and insulin sensitivity, but this exercise stress will be relatively short lived. In comparison, physical inactivity will impose opposing physiological signals of smaller magnitude, but these signals will persist for considerably longer durations in sedentary individuals and will induce muscle level adaptations that persist as long as inactivity is present resulting in relatively quick (hours to days) negative adaptation.
2. Age related muscle events
2.1. Muscle mass and metabolic resilience
Ageing is associated with insidious declines in skeletal muscle mass, termed sarcopenia, as well as a decline in strength and quality, termed dynapenia (Mitchell et al., 2012). However, there is little agreement on the rate of decline of muscle mass according to age, due to a paucity of longitudinal studies. Cross sectional studies comparing younger cohorts considered to be of peak muscle mass (age 18–45) with older cohorts (age 65–90) have highlighted differences in muscle mass between young and older individuals ranging from 8 to 45% (Tzankoff and Norris, 1977; Mitchell et al., 2012). However, cross-sectional studies assume that their young and old participants are fairly matched, when other factors such as intergenerational differences may also have an influence. Of the few longitudinal studies that have been conducted in this area, one showed a leg lean muscle mass loss of 0.7−0.8% per year, during a 7 year follow up of individuals in their 70 s (Koster et al., 2011). A large cross-sectional study has described a linear loss of muscle mass in later life, accelerated after the age of 60 years (Kyle et al., 2001), whilst others have reported a reduction in muscle mass starting as early as 30 years of age (Janssen et al., 2000). The rate of muscle mass loss has also been reported to be accelerated in the lower body; being twice as high as the upper body response (Janssen et al., 2000). A bed rest study in healthy older adults (mean age 67 years) found a greater loss of lean tissue after 10 days compared with young individuals after 28 days, particular of the lower extremities, associated with a negative urinary nitrogen balance (Kortebein et al., 2007), whilst another study showed greater loss of quadriceps muscle volume in the young volunteers in response to 14 days immobilization compared to older volunteers (mean age 67 years; Suetta et al., 2009).
Muscle mass is regulated by the balance of muscle protein synthesis (MPS) and muscle protein breakdown (MPB). This equilibrium has a diurnal variation and is predominantly stimulated by food intake (Wilkinson et al., 2015) and physical activity (Kumar et al., 2009). The anabolic effects of nutrition are primarily driven by amino acids from dietary proteins, which are incorporated into skeletal muscle (Atherton and Smith, 2012). One mechanism proposed to contribute to chronic muscle mass loss during ageing is anabolic resistance, or specifically, the inability to stimulate MPS or inhibit MPB following feeding or an exercise stimulus. Cuthbertson compared rates of MPS in 44 healthy young and old men in response to essential amino acids (EAA) and found that the elderly showed a blunted myofibrillar protein fractional synthetic rate in response to EAA compared with the young (Cuthbertson et al., 2005). In particular, they found the phosphorylation of mammalian target of rapamycin (mTOR) at Ser2448 and p706SK and downstream translational regulators increased less in the elderly in response to EAA compared to young, indicating a reduction in the ability of aged muscle to “sense” a nutrient signal. Similarly, Kumar found a blunted MPS response 1−2 h after resistance exercise training (RET) in elderly men compared to young as well as a reduction in phosphorylation of anabolic signalling molecules (Kumar et al., 2009). In a study measuring chronic MPS using “heavy water” (D2O) stable isotope tracer, Brook et al. found a blunted hypertrophic response to a 6 week RET programme which occurred secondary to reduced rates of chronic MPS in older individuals compared to young (Brook et al., 2016). This appeared to be multifactorial in nature, reflecting blunted ribosomal biogenesis and translational efficiency and lower anabolic hormone concentrations. Interestingly, a longitudinal study of 20 weeks RET in healthy young (18–28 years), middle aged (45–55 years) and older participants (65–75 years) demonstrated that only younger individuals increased muscle mass in response to RET (Phillips et al., 2017). This suggests an age-related failure of muscle hypertrophy which occurs earlier in life than previously reported. However, well controlled longitudinal studies are missing in order to conclusively determine whether anabolic resistance is a causative factor in sarcopenia.
Age related loss of quadriceps muscle cross-sectional area has been associated with loss of muscle mitochondrial ATP production capacity, due at least in part to a decline in mitochondrial volume density (Broskey et al., 2014). This will impact negatively muscle metabolic resilience, i.e. the ability of skeletal muscle metabolism to respond to and recover from a physiological stress, in particular muscle contraction. Muscle metabolic resilience can also be negatively affected by a reduction in intrinsic mitochondrial function, i.e. ATP production per mitochondrial unit caused by, for example, reactive oxygen species and defective autophagy (Gonzalez-Freire et al., 2015). Collectively, physiological ageing appears to be associated with muscle mass loss and declines in metabolic capacity and resilience which will limit functional ability, and in turn may accelerate further age-related decline.
2.2. Muscle strength and power
The magnitude of strength loss seen in ageing is consistently greater than the degree of muscle mass loss demonstrated in studies of hand-grip (Dey et al., 2009) and lower limb strength (Delmonico et al., 2009). In a 5-year follow-up of 1678 older participants, strength, measured as knee extensor torque, declined 2–5 times more than the loss of cross sectional area of the thigh (Delmonico et al., 2009). Similarly, Frontera noted a 20–30 % decline in strength of knee flexors and extensors with an average 16 % loss in quadriceps CSA in older men over 12 years follow up (Frontera et al., 2000). In this study however, there was a significant difference in weekly habitual physical activity levels between year 1 and year 12 in the participants (834 ± 1409 and 578 ± 489 kcal, respectively; P 0.05). Whilst the authors conclude that the longitudinal loss in muscle strength was secondary to ageing, it may have been confounded by a reduction in habitual activity levels that accompanied chronological age. Power (force x velocity) has been reported to decline at a more rapid rate than strength with chronological age (Skelton et al. 1994), especially in older adults with limitations in mobility and may be explained by a more preferential loss of fast twitch muscle fibres (see below). These changes in muscle mass, strength and power are clinically important, as lower muscle mass is associated with a higher mortality risk in older adults independent of fat mass and cardiovascular risk factors (Srikanthan and Karlamangla, 2014).
2.3. Muscle fibre number, composition and motor unit size and number
Skeletal muscle fibre and composition changes have also shown to be associated with ageing. Skeletal muscle is composed of a range muscle fibre types which have been categorised in humans to: type I (slow twitch), type IIa and type IIx (fast twitch), with different biochemical and physiological characteristics (Peter et al., 1972; Schiaffino et al., 1989). Ageing is associated with the loss of type II muscle fibre area, affecting mostly IIx fibres. An early study by Lexell and Taylor (1991) associated a 35 % loss of type II fibre cross sectional area with age, which was not apparent in type I fibres (Lexell and Taylor, 1991), and has been corroborated by others (Frontera et al., 2000). Other studies have reported lower muscle fibre numbers in older individuals compared with young when measured in the quadriceps (McPhee et al., 2018). These changes have been proposed to occur, at least in part, secondary to age-related remodelling of motor units resulting in denervation of type II muscle fibres and subsequent collateral formation of type I muscle fibres (Lexell and Taylor, 1991). In contrast, some have contested fibre type differences between young and old individuals (Kostek and Delmonico, 2011). Human studies using EMG have demonstrated a decline in motor unit number in the tibialis anterior of older individuals when compared with young (Power et al., 2014). In addition to this loss of motor unit number, ageing has been associated with an increase in size of surviving motor units via reinnervation of denervated fibres (Power et al., 2014; Piasecki et al., 2016). Indeed, it has been proposed that healthy older men can reinnervate large numbers of muscle fibres to compensate for declining motor neuron numbers, and that a failure to do so contributes to sarcopenia (Piasecki et al., 2018). Thus a distinguishing feature in pre-sarcopenic vs sarcopenic muscles of older individuals is the lack of compensatory reinnervation of muscle fibres. However, the ability of exercise to preserve motor unit number in ageing or improve reinnervation of denervated fibres is currently unclear.
2.4. Adiposity and insulin resistance
Aging is associated with a decline in muscle insulin sensitivity, defined as a reduction in whole body glucose disposal in response to insulin (Fink et al., 1983). There has been debate about whether ageing per se is a cause of this metabolic change or whether age related deconditioning (Goodpaster et al., 2001), increased central and muscle adiposity (Kohrt et al., 1993), mitochondrial dysfunction (Petersen et al., 2003; Short et al., 2005), inflammation (Shoelson et al., 2006; Houmard et al., 1995) or changes in cellular signalling mechanisms governing glucose transport (Houmard et al., 1995) are responsible. Clearly there may be multiple causative and or additive factors. There remains a degree of uncertainty about how each of these elements interact, and the time scale or relative contribution of each; not least because controlled longitudinal intervention studies are missing. Nevertheless, the fact that exercise training intervention can increase whole-body and leg insulin sensitivity (Dela et al., 2019; Søgaard et al., 2018) and oxidative capacity (Broskey et al., 2014) in older volunteers strongly points to muscle deconditioning, occurring secondary to age related declines in physical activity levels, being an important contributor.
As people age, they inherently become less active, which may predispose to increases in adiposity and a reduction in whole-body lean mass (Dela et al., 1996). However, changes in muscle mass are complex as obesity has been shown to preserve muscle mass due to the greater muscle loading required to ambulate (Murton et al., 2015). There is a large body of evidence that adiposity is linked with insulin resistance (Lanza et al., 2008), visceral adiposity (Ross et al., 2002) and subcutaneous adiposity having the most significant associations with insulin resistance (Kelley et al., 2000 and Smith et al., 2001). Studies utilising the hyperinsulinaemic clamp technique in healthy older volunteers have shown that whole body insulin-stimulated glucose disposal in healthy individuals is not reduced by age per se but is probably explained by a relative increase in body fat compared with young (Dela et al., 1996). At a muscle level, intra-myocellular lipid (IMCL) accumulation also appears to have a significant negative impact on muscle insulin sensitivity, with the size, location and composition of the lipid droplets all appearing to diminish insulin sensitivity (Gemmink et al., 2017). Some suggest that ageing combined with increasing adiposity exacerbates insulin resistance (Kohrt et al., 1993; Seals et al., 1984; Basu et al., 2003), but it is very difficult to control for confounding variables in such studies. Indeed, recent evidence showing older lean volunteers displayed IMCL content and insulin sensitivity to that of younger volunteers (Chee et al., 2016), strongly suggests muscle insulin resistance and lipid accumulation often observed in older individuals are likely due to lifestyle factors rather than inherent aging of skeletal muscle per se. Indeed, obese older individuals were markedly insulin resistant and had more than twofold greater IMCL in the sub-sarcolemmal region compared to their older lean counterparts.
Mitochondrial dysfunction has been linked to lipid induced insulin resistance through the comparison of young healthy volunteers and older healthy volunteers. Petersen et al. (2003) identified that a reduction in mitochondrial respiratory capacity was associated with a greater muscle fat content (Petersen et al., 2003). However, this could simply reflect the impact of reduced habitual physical activity on muscle deconditioning and energy balance with ageing, rather than a causative effect of lipid accumulation on mitochondrial function. Nevertheless, evidence suggests that an increase in IMCL content has detrimental effects on mitochondrial fuel selection resulting in the inability of mitochondria to switch from lipid to glucose oxidation during insulin stimulation (Petersen et al., 2015). Importantly, much of the existing research that has been undertaken looking at the link between insulin resistance and mitochondrial dysfunction has not been definitive. For example, some studies have not normalised mitochondrial function measurements for the decline in muscle mitochondrial content that accompanies age. Whatever the drivers, adiposity is associated with alterations of both muscle lipid stores and mitochondrial dynamics. Furthermore, research showing changes in lipid droplet morphology, mitochondrial dynamics and improvements in insulin sensitivity after gastric bypass surgery suggests an association between these events (Kristensen et al., 2018), but the mechanistic links are not yet resolved (Dela and Helge, 2013) and may not be age related per se.
Age related insulin resistance has been linked to an age-related decline in insulin stimulated muscle GLUT4 content in both males and females (Houmard et al., 1995), and this relationship persisting despite adjusting for whole-body adiposity, regional adiposity, and cardiorespiratory fitness. This age-related decline in muscle GLUT4 content was also observed in the fast twitch fibres of the vastus lateralis, but not the slow twitch fibres, when comparing older and younger participants (Gaster et al., 2000). In contrast, Dela and colleagues (Dela et al., 1994) and Cox and colleagues (Cox et al., 1985) found no age-related differences in muscle GLUT4 protein abundance. Furthermore, Cox et al. identified similar relative increases in muscle GLUT4 protein content, irrespective of age, following a short-term exercise programme (Cox et al., 1985). The current evidence linking GLUT4 protein content with the age-related decline in insulin sensitivity is inconclusive and may simply be explained by habitual physical activity levels given exercise training is able to stimulate muscle GLUT4 protein content in young and older volunteers.
3. Inactivity related muscle events
3.1. Models of inactivity
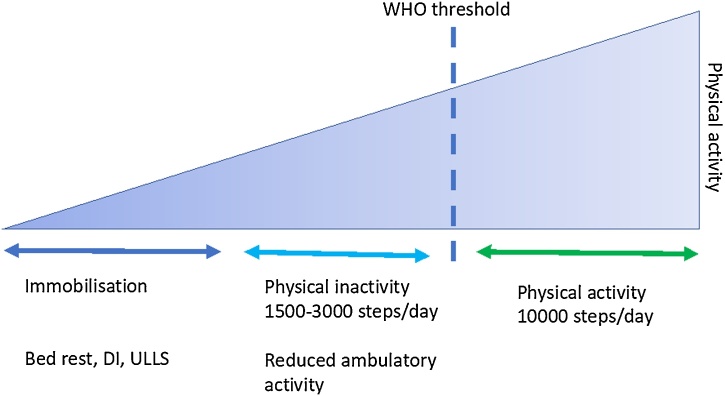
Mechanistic insight into the rate and magnitude of decline in muscle mass, muscle architecture and glucose disposal in response to physical inactivity is lacking. A recent upper limb immobilization study suggests changes in glucose disposal in response to immobilization is rapid and specific to the inactive limb (Burns et al., 2021), but precise mechanisms are unclear. This is a major gap in our understanding that needs to be addressed in controlled human volunteer studies given the poor generalisation from rodents that possess poor metabolic stability compared with humans (Demetrius, 2005). An appreciation of the different models of inactivity used to study this gap is therefore important. Several different models of physical inactivity, ranging in severity, have been developed to study the effects of inactivity in healthy human volunteers. These models allow the exploration of short and long-term physical inactivity and energy balance and the effects that these have metabolic health, providing useful insight into their physiological mechanisms (Fig. 2).
Fig. 2.
Models of inactivity/immobilisation against the spectrum of physical activity. DI = dry water immersion, ULLS = unilateral lower limb suspension, WHO = World Health Organisation.
Spaceflight, or a microgravity environment, is an extreme model of inactivity; the lack of load bearing activity results in reduced muscular contraction leading to substantial functional and metabolic adaptations. However, the opportunity to study physiological changes during spaceflight is limited due to the cost and complexity of studies; the number of studies is few and often of varying duration. Therefore, data remains hard to interpret because of the small sample size, the variations in flight duration and the lack of regulation in diet and exercise.
Bed rest is a widely accepted model of physical inactivity; generally, all activities are performed in a horizontal or -6 degree head-down tilt position, minimising use of all muscles and bringing about significant physiological adaptations similar to those observed during spaceflight. A substantial number of bed rest studies have been performed ranging from 3 to 120 days duration (LeBlanc et al., 1992; Ferrando et al., 1996; Smorawiński et al., 2000). These studies on the whole have shown, to a varying degree, a reduction in skeletal muscle mass, strength and whole body insulin sensitivity, with this effect being demonstrated after just 7 days (Dirks et al., 2016). Bed rest is an extreme stimulus when compared to the degree of physical inactivity encountered by the general population. However, they allow the impact of an intervention such as dietary energy content and composition, exercise or a pharmacological agent, to be examined. An alternative model of whole-body physical inactivity is ‘dry’ water immersion, which is often used as a model of weightlessness. It involves immersing the subject in thermoneutral water; however subjects are protected from water contact with a thin elastic waterproof fabric. ‘Dry’ water immersion produces similar physiological effects to those observed during bed rest, but the effects are often of greater magnitude and occur at a faster rate (Shenkman et al., 1997; Navasiolava et al., 2010).
Limb immobilisation and unilateral lower limb suspension extensively restricts motion in a targeted limb but allows maintenance of everyday activities. Limb immobilisation studies use a cast to fully immobilise the targeted limb, whilst in unilateral lower limb suspension studies, as originally described by Berg et al, the treatment limb is flexed and suspended above the ground with the use of a shoulder harness (Berg et al., 1991). Both models evoke muscular adaptations similar to those induced by bed rest; but these adaptations are mostly confined to the immobilised limb, allowing the localised effects of physical inactivity to be studied. The benefit of limb immobilisation is that the participant’s contralateral limb can serve as an internal control, drastically reducing a number of potential inter-participant variables. The use of limb immobilisation in research has more clinical relevance than bed rest due to it being a commonly used technique in the treatment of fractures, which are common throughout all age groups.
Studies involving reduced ambulatory activity normally achieved through a reduction in daily step counts is a model most realistic to the free-living state (Tudor-Locke and Bassett, 2004). There have been several examples of well controlled experiments of reduced step count which have demonstrated reductions in muscle mass, postprandial MPS and insulin sensitivity after 14 days of <1500 steps per day (Breen et al., 2013; Krogh-Madsen et al., 1985).
3.2. Many of the physiological features of ageing also appear to be major features of inactivity
Declines in muscle mass and strength attributed to ageing have been reproduced in young volunteers subjected to reduced step count or immobilisation. This implies that you can make a young person physiologically age simply by making them immobile or inactive using the models of physical inactivity outlined above. Two-weeks of full leg immobilisation in young men (aged 18–30) led to a -4.7 % decline in quadriceps lean mass and a -27 % reduction in isometric strength from the pre-immobile state (Jones et al., 2004). Similarly, 3-days dry immersion in 12 young participants (mean ± SD age 32 ± 5 years), resulted in an 11 % decline in maximal voluntary contraction and 2.4 % loss in quadriceps cross-sectional area (Demangel et al., 2017). This greater magnitude of voluntary strength loss was associated with some evidence of muscle fibre denervation in the form of increased neural cellular adhesion molecule positive fibre staining. The authors also reported a decrease in the proportion of IIa fibres and an increase in the proportion of hybrid fibres I/IIX.
Muscle protein turnover is also altered secondary to immobilisation in the young. In a study of 9 healthy males undergoing 23 days of unilateral lower limb immobilisation, post-absorptive rates of MPS halved from day 0 to day 10 (0.047 % vs 0.022 %) and remained depressed at this rate until day 21 (de Boer et al., 2007). Apart from a fall in phosphorylation of focal adhesion kinase of 30 % (p < 0.01) by 10 days, there were no alterations in any of the proteins associated with protein anabolic signalling, implying that the signalling potential of skeletal muscle was not diminished at least in the fasted state. Furthermore, anabolic resistance to protein or amino acid administration, the characteristic finding shown in physiological studies of ageing, can be replicated in the young volunteers secondary to immobilisation irrespective of the dose administered (Glover et al., 2008). Kilroe and colleagues reported that changes in MPS are rapid, occurring after 2 days following lower limb immobilisation in young healthy volunteers using deuterated water to quantify cumulative myofibrillar protein synthesis rates, with greater reductions in the immobilised leg MPS when compared with the control leg after 7 days of immobilisation (Kilroe et al., 2020). This was associated with a reduction of 6.7 +/- 0.6 % in MRI derived quadriceps volume in the immobilised leg when compared to the non-immobilised leg.
Similarly, immobilisation and inactivity have been shown to induce deficits in whole body and leg glucose uptake under insulin stimulated conditions in young healthy adults. For example, whole-body and leg glucose uptake measured under hyperinsulinaemic euglycaemic clamp conditions declined following 7 days bed rest in young men, with the decline being of greatest magnitude in the legs (Mikines et al., 1991). In addition, bed rest had no impact upon hepatic insulin sensitivity in the same study. A less severe model involving a reduction in step count from ∼10,000 to <1500 per day for 2 weeks was sufficient to reduce whole-body glucose uptake under insulin clamp conditions in young healthy men (Krogh-Madsen et al., 1985). The mechanism(s) responsible for immobilisation induced reductions in glucose disposal is currently unresolved but candidates include alterations in the signalling and/or translocation of GLUT4 to the plasma membrane (Zierath et al., 1996), immobilisation induced IMCL accumulation (Manini et al., 2007; Cree et al., 2010) through excessive free fatty acid supply secondary to positive energy balance and finally a decrease in mitochondrial content and/or mitochondrial function with subsequent alterations in carbohydrate metabolism (Abadi et al., 2009). A recent upper limb immobilisation study reported a decline in forearm glucose uptake within 24 h that was specific to the immobilised limb and was not accounted for by increased lipid availability (Burns et al., 2021). It is thought that the reduction in muscle contraction per se drives the cellular and molecular changes described however the specific mechanistic basis remains poorly understood.
Despite the apparent evidence of detrimental effects on muscle and metabolic health occurring secondary to ageing, we are not aware of any studies that have measured baseline levels of habitual physical activity in a precise quantifiable manner in volunteers and used these data to match study volunteers for habitual physical activity levels. Indeed, in studies where older individuals recruited have been highly physically active, age related muscle differences have been less apparent (Pollock et al., 2015). For example, in a cross-sectional study (Wroblewski et al., 2011) involving 40 high level master athletes aged 40–81 years, variation in muscle cross sectional area, lean mass and strength did not appear to be age related. Some have suggested the magnitude of adiposity in older adults predicts the decline in muscle mass and quality associated with poor metabolic health (Koster et al., 2011). This so called “sarcopenic obesity” has been questioned in studies demonstrating that obesity in older individuals is not associated with reduced in absolute lean tissue volume or strength (Murton et al., 2015), which the authors suggested was a logical outcome due to the increased contractile work performed by obese individuals in locomotor activities of daily living. Indeed, sarcopenic obesity may simply reflect that the relative increase in fat mass with obesity is greater than the relative increase in lean mass, but in absolute terms both increase.
4. Conclusion
Despite a large body of cross-sectional evidence detailing differences in muscle mass, muscle quality, body composition and insulin sensitivity associated with ageing, without accurate measurements of habitual physical activity levels it is difficult to conclude these differences are definitively due to ageing processes. Furthermore, in studies subjecting young healthy adults to inactivity and immobilisation, many of the muscle level responses attributed to ageing can be manifested. Current knowledge gaps therefore include the relative contribution that physical inactivity plays in the development of many of the features associated with poor muscle metabolic health in older age; including muscle centric mechanisms linking physical inactivity and/or sedentary time to impaired metabolic health. Similarly, data demonstrating positive effects of government recommended physical activity guidelines (or indeed any other physical in/activity interventions relevant to preservation of health) on muscle specific health, the decline in which is strongly associated with functional deterioration in older adults, are largely missing. These are important knowledge gaps to address, as mitigation of some of the negative effects of secondary ageing due to inactivity or diet may be possible. Such an approach will also help identify the primary drivers of muscle ageing. Therefore, longitudinal intervention studies in humans to elucidate the contribution of both ageing and inactivity, and whether metabolic dysregulation with inactivity is additive to age and disease are warranted.
Funding
NFS salary is funded by the Versus Arthritis Centre for Sport, Exercise and Osteoarthritis. SS was funded by a BBSRC DTP studentship.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors contributions
NFS and LC drafted the first draft of the manuscript. All authors revised subsequent drafts and approved the final version of the manuscript before submission.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
This research was supported by the Medical Research Council [grant number MR/K00414X/1] and Arthritis Research UK [grant number 19891]. The National Institute of Health Research (NIHR) Nottingham Biomedical Research Centre and Nottingham University Hospitals Charities also supported the work.
References
- Abadi A., Glover E.I., Isfort R.J., Raha S., Safdar A., Yasuda N., Kaczor J.J., Melov S., Hubbard A., Qu X., Phillips S.M., Tarnopolsky M. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One. 2009;4(8):e6518. doi: 10.1371/journal.pone.0006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton P.J., Smith K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012;590(5):1049–1057. doi: 10.1113/jphysiol.2011.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R., Breda E., Oberg A.L., Powell C.C., Dalla Man C., Basu A., Vittone J.L., Klee G.G., Puneet A., Jensen M.D., Toffolo G., Cobelli C., Rizza R.A. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- Berg H.E., Dudley G.A., Häggmark T., Ohlsén H., Tesch P.A. Effects of lower limb unloading on skeletal muscle mass and function in humans. J. Appl. Physiol. (1985) 1991;70(4):1882–1885. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- Breen L., Stokes K.A., Chirchward-Venne T.A., Moore D.R., Baker K.S., Smith K., Atherton P.J., Phillips S.M. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013;98(6):2604–2612. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- Brook M.S., Wilkinson D.J., mitchell W.K., Lund J.N., Phillips B.E., Szewczyk N.J., Greenhaff P.L., Smith K., Atherton P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016;594(24):7399–7417. doi: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broskey N.T., Greggio C., Boss A., Boutant M., Dwyer A., Schlueter L., Hands D., Gremion G., Kreis R., Boesch C., Canto C., Amati F. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J. Clin. Endocrinol. Metab. 2014;99(5):1852–1861. doi: 10.1210/jc.2013-3983. [DOI] [PubMed] [Google Scholar]
- Burns A.M., Nixon A., Mallinson J., Cordon S.M., Stephens F.B., Greenhaff P.L. Immobilisation induces sizeable and sustained reductions in forearm glucose uptake in just 24 hours but does not change lipid uptake in healthy men. J. Physiol. 2021 doi: 10.1113/JP281021. In press. [DOI] [PubMed] [Google Scholar]
- Busse E. In: Theories of Aging, in Behavior and Adaptation in Late Life. Brown Little., editor. 1969. pp. 11–31. Boston. [Google Scholar]
- Chee C., Shannon C.E., Burns A., Selby A.S., Wilkinson D., Smith K., Greenhaff P.L., Stephens F.B. Relative contribution of intramyocellular lipid to whole-body fat oxidation is reduced with age but subsarcolemmal lipid accumulation and insulin resistance are only associated with overweight individuals. Diabetes. 2016;65(4):840–850. doi: 10.2337/db15-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.H., Cortright R.N., Dohm G.L., Houmard Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J. Appl. Physiol. (1985) 1999;86(6):2019–2025. doi: 10.1152/jappl.1999.86.6.2019. [DOI] [PubMed] [Google Scholar]
- Cree M.G., Paddon-Jones D., Newcomer B.R., Rosen O., Aarsland A., Wolfe R.R., Ferrando A. Twenty-eight-day bed rest with hypercortisolemia induces peripheral insulin resistance and increases intramuscular triglycerides. Metabolism. 2010;59(5):703–710. doi: 10.1016/j.metabol.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., Wackerhage H., Taylor P.M., Rennie M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- de Boer M.D., Selby S., Atherton P., Smith K., Seynnes O.R., Maganaris C.N., Maffulli N., Movin T., Narici M.V., Rennie M.J. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J. Physiol. 2007;585(1):241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela F., Helge J.W. Insulin resistance and mitochondrial function in skeletal muscle. Int. J. Biochem. Cell Biol. 2013;45(1):11–15. doi: 10.1016/j.biocel.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Dela F., Ploug T., Handberg Petersen L.N., Larsen J.J., Mikines K.J., Galbo H. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43(7):862–865. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- Dela F., Mikines K.J., Larsen J.J., Galbo H. Training-induced enhancement of insulin action in human skeletal muscle: the influence of aging. J. Gerontol. 1996;51(4):B247–B252. doi: 10.1093/gerona/51a.4.b247. [DOI] [PubMed] [Google Scholar]
- Dela F., Ingersen A., Andersen N., Nielsen M.B., Petersen H.H.H., Hansen C.N., Larsen S., Wojtaszewski J., Helge J.W. Effects of one-legged high-intensity interval training on insulin-mediated skeletal muscle glucose homeostasis in patients with type 2 diabetes. Acta Physiol. (Oxf.) 2019;226(2):e13245. doi: 10.1111/apha.13245. [DOI] [PubMed] [Google Scholar]
- Delmonico M.J., Harris T.B., Visser M., Park S.W., Conroy M.B., Velasquez-Mieyer P., Boudreau R., Manini T.M., Nevitt M., Newman A.B., Goodpaster B.H. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demangel R., Treffel L., Py G., Brioche T., Pagano A.F., Bareille M.-P., Beck A., Pessemesse L., Candau R., Gharib C., Chopard A., Millet M. Early structural and functional signature of 3-day human skeletal muscle disuse using the dry immersion model. J. Physiol. 2017;595(13):4301–4315. doi: 10.1113/JP273895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetrius L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;(Suppl. 1):39–44. doi: 10.1038/sj.embor.7400422. Jul;6 Spec No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D.K., Bosaeus I., Lissner L., Steen B. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Goteborg. Sweden. Nutr. 2009;25(6):613–619. doi: 10.1016/j.nut.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Dirks M.L., Wall B.T., van de Valk B., Holloway T.M., Holloway G.P., Chabowski A., Hoossens G.H., van Loon L.J.C. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- Ferrando A.A., Lane H.W., Stuart C.A., Davis-Street J., Wolfe R.R. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am. J. Physiol. 1996;270(4 Pt 1):E627–33. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Fink R.I., Kolterman O.G., Griffin J., Olefsky J.M. Mechanisms of insulin resistance in ageing. J. Clin. Invest. 1983;71(6):1523–1535. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera W.R., Hughes V.A., Fielding R.A., Fiaterone M.A., Evans W.J., Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J. Appl. Physiol. (1985) 2000;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Gaster M., Poulsen P., Handberg A., Schroder H.D., Beck-Nielsen H. Direct evidence of fiber type-dependent GLUT-4 expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000;278(5):E910–6. doi: 10.1152/ajpendo.2000.278.5.E910. [DOI] [PubMed] [Google Scholar]
- Gemmink A., Goodpaster B.H., Schrauwen P., Hesselink M.K.C. Intramyocellular lipid droplets and insulin sensitivity, the human perspective. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2017;1862(10 Pt B):1242–1249. doi: 10.1016/j.bbalip.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Glover E.I., Phillips S.M., Oates B.R., Tang J.E., Tarnopolsky M.A., Selby A., Smith K., Rennie M.J. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J. Physiol. 2008;586(24):6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Freire M., de Cabo R., Bernier M., Sollott S.J., Fabbri E., Navas P., Ferrucci L. Reconsidering the role of mitochondria. Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70(11):1334–1342. doi: 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster B.H., Carlson C.L., Bisser M., Kelley D.E., Scherzinger A., Harris T.B., Stamm E., Newman A.B. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J. Appl. Physiol. (1985) 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Houmard J.A., Weidner M.D., Dolan P.L., Leggett-Frazier N., Gavigan K.E., Hickey M.S., Tyndall G.L., Zheng D., Alshami A., Dohm G.L. Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes. 1995;44(5):555–560. doi: 10.2337/diab.44.5.555. [DOI] [PubMed] [Google Scholar]
- Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. (1985) 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Jones S.W., Hill R.J., Krasney P.A., O-Conner B., Peirce N., Greenhaff P.L. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18(9):1025–1027. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- Kelley D.E., Thaete F.L., Troost F., Huwe T., Goodpaster B.H. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2000;278(5):E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- Kilroe S.P., Fulford J., Holwerda A.M., Jackman S.R., Lee B.P., Gijsen A.P., van Loon L.J.C., Wall B.T. Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates. Am. J. Physiol. Endocronol. Metab. 2020;318(February (2)):E117–E139. doi: 10.1152/ajpendo.00360.2019. [DOI] [PubMed] [Google Scholar]
- Kirwan R., McCullough D., Butler T., Perez De Heredia Benedicte F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-timer health effects of short-term muscle loss. Geroscience. 2020;42(6):1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt W.M., Kirwan J.P., Staten M.A., Bourey R.E., King D.S., Holloszy J.O. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42(2):273–281. [PubMed] [Google Scholar]
- Kortebein P., Ferrando A., Lombeida J., Wolfe R., Evans W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1769–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- Kostek M.C., Delmonico M.J. Age-related changes in adult muscle morphology. Curr. Aging Sci. 2011;4(3):221–233. doi: 10.2174/1874609811104030221. [DOI] [PubMed] [Google Scholar]
- Koster A., Ding J., Stenholm S., Caserotti P., Houston D.K., Nicklas B.J., You T., Lee J.S., Visser M., Newman Ab., Schwartz A.V., Cauley J.A., Tylavsku F.A., Goodpaster B.H., Kritchevsky S.B., Harris T.B. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J. Gerontol. A Biol. Sci. Med. Sci. 2011;66(8):888–895. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M.D., Petersen S.M., Moller K.E., Lund M.T., Hansen M., Hansen C.N., Courraud J., Helge J.W., Dela F., Prats C. Obesity leads to impairments in the morphology and organization of human skeletal muscle lipid droplets and mitochondrial networks, which are resolved with gastric bypass surgery‐induced improvements in insulin sensitivity. Acta Physiol. 2018;224(4):e13100. doi: 10.1111/apha.13100. [DOI] [PubMed] [Google Scholar]
- Krogh-Madsen R., Thyfault J.P., Broholm C., Mortensen O.H., Olsen R.H., Mounier R., Plomgaard P., van Hall G., Booth F.W., Pedersen B.K. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J. Appl. Physiol. (1985) 2010;108(5):1034–1040. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- Kumar V., Selby A., Rankin D., Patel R., Atherton P., Hildebrand W., Williams J., Smith K., Seynnes O., Hiscock N., Rennie M.J. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009;587(1):211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle U.G., Genton L., Hans D., karsegard L., Solsman D.O., Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur. J. Clin. Nutr. 2001;55(8):663–672. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- Lanza I.R., Short D.K., Short K.R., Raghavakaimal S., Basu R., Joyner M.J., McConnell J.P., Naid K.S. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57(11):2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc A.D., Schneider V.S., Evans H.J., Pientok C., Rowe R., Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J. Appl. Physiol. (1985) 1992;73(5):2172–2178. doi: 10.1152/jappl.1992.73.5.2172. [DOI] [PubMed] [Google Scholar]
- Lexell J., Taylor C.C. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J. Anat. 1991;174:239–249. [PMC free article] [PubMed] [Google Scholar]
- Manini T.M., Clark B.C., Nalls M.A., Goodpaster B.H., Ploutz-Snyder L.L., Harris T.B. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am. J. Clin. Nutr. 2007;85(2):377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- McPhee J.S., Cameron J., Maden-Wilkinson T., Piasecki M., Yap M.P., Jones D.A., Degens H. The contributions of fiber atrophy, fiber loss, in situ specific force, and voluntary activation to weakness in sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73(September (10)):1287–1294. doi: 10.1093/gerona/gly040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikines K.J., Richter E.A., Dela F., Galbo H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J. Appl. Physiol. (1985) 1991;70(3):1245–1254. doi: 10.1152/jappl.1991.70.3.1245. [DOI] [PubMed] [Google Scholar]
- Milanović Z., Pantelić S., Trajković N., Sporiš G., Kostić R., James N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging. 2013;8:549–556. doi: 10.2147/CIA.S44112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W.K., Williams J., Atherton P., Larvin M., Lund J., Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murton A.J., Marimuthu K., Mallinson J.E., Selby A.L., Smith K., Rennies M.J., Greenhaff P.L. Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes. 2015;64(9):3160–3171. doi: 10.2337/db15-0021. [DOI] [PubMed] [Google Scholar]
- Narici M., De Vito G., Franchi M., Paoli A., Moro T., Marcolin G., Grassi B., Baldessarre G., Zuccarelli L., Biolo G., dr Girolamo F.G., Fiotti n., Dela F., Greenhaff P.L., Maganaris C. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2020;12:1–22. doi: 10.1080/17461391.2020.1761076. [DOI] [PubMed] [Google Scholar]
- Navasiolava N.M., Dignat-George F., Sabartier F., Larina I.M., Demiot C., Fortrar J.-O., Gauquelin-Koch G., Kizlovskaya I.B., Custaud M.-A. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am. J. Physiol. Heart Circ. Physiol. 2010;299(2):H248–56. doi: 10.1152/ajpheart.00152.2010. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics, 2012. https://webarchive.nationalarchives.gov.uk/20160106100801/http://www.ons.gov.uk/ons/dcp171776_258607.pdf.
- Petersen K.F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D., DiPietro L., Cline G.W., Shulman G.I. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K.F., Morino K., Alves T.C., Kibbey R.G., Dufour S., Sono S., Yoo P.S., Cline G.W., Shulman G.I. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc. Natl. Acad. Sci. U.S.A. 2015;112(36):11330–11334. doi: 10.1073/pnas.1514844112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B., Williams J.P., Greenhaff P.L., Smith K., Atherton P.J. Physiological adaptations to resistance exercise as a function of age. JCI Insight. 2017;2(17):e95581. doi: 10.1172/jci.insight.95581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki M., Ireland A., Coulson J., Stashuk D.W., Hamilton-Wright A., Swiecicka A., Rutter M.K., McPhee J.S., Jones D.A. Motor unit number estimates and neuromuscular transmission in the tibialis anterior of master athletes: evidence that athletic older people are not spared from age-related motor unit remodeling. Physiol. Rep. 2016;4(19):e12987. doi: 10.14814/phy2.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock R.D., Carter S., Velloso C.P., Duggal N.A., Lord J.M., Lazarus N.R., Harridge S.D.R. An investigation into the relationship between age and physiological function in highly active older adults. J. Physiol. 2015;593(3):657–680. doi: 10.1113/jphysiol.2014.282863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki M., Ireland A., Piasecki J., Stashuk D.W., Swiecicka A., Rutter M.K., Jones D.A., McPhee J.S. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older menJ. J. Physiol. 2018;596(9):1627–1637. doi: 10.1113/JP275520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power G.A., Dalton B.H., Behm D.G., Vandervoort A.A., Doherty T.J., Rice C.L. Motor unit number estimates in masters runners: use it or lose it? Med. Sci. Sports Exerc. 2014;42(9):1644–1650. doi: 10.1249/MSS.0b013e3181d6f9e9. [DOI] [PubMed] [Google Scholar]
- Ross R., Aru J., Freeman J., Hudson R., Janssen I. Abdominal adiposity and insulin resistance in obese men. Am. J. Physiol. Endocrinol. Metab. 2002;282(3):E657–E663. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J. Muscle Res. Cell Motil. 1989;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Seals D.R., Hagberg J.M., Allen W.K., Hurley B.F., Dalsky G.P., Ehsani A.A., Holloszy J.O. Glucose tolerance in young and older athletes and sedentary men. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984;56(6):1521–1525. doi: 10.1152/jappl.1984.56.6.1521. [DOI] [PubMed] [Google Scholar]
- Shenkman B.S., Kozlovskaya I.B., Nemirovskaya T.L., Tcheglova I.A. Human muscle atrophy in supportlessness: effects of short-term exposure to dry immersion. J. Gravit. Physiol. 1997;4(2):P137–P138. [PubMed] [Google Scholar]
- Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006;116(7):793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K.R., Bigelow M.L., Kahl J., Singh R., Coenen-Schimke J., Raghavakaimal S., Nair K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U.S.A. 2005;102(155):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.R., Lovejoy J.C., Greenway F., Ryan D., deJonge L., de la Bretonne J., Volafova J., Bray G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- Søgaard D., Lund M.T., Scheuer C.M., Dehlbaek M.S., Dideriksen S.G., Abildskov C.V., Christensen K.K., Dohlmann T.L., Larsen S., Vigelsø A.H., Dela F., Helge J.W. High-intensity interval training improves insulin sensitivity in older individuals. Acta. Physiol. (Oxf) 2018;222(4):e13009. doi: 10.1111/apha.13009. [DOI] [PubMed] [Google Scholar]
- Smorawiński J., Kaciuba-Uściłko H., Nazar K., Kubala P., Kamińska E., Ziemba A.W., Adrian J., Greenleaf J.E. Effects of three-day bed rest on metabolic, hormonal and circulatory responses to an oral glucose load in endurance or strength trained athletes and untrained subjects. J. Physiol. Pharmacol. 2000;51(2):279–289. [PubMed] [Google Scholar]
- Srikanthan P., Karlamangla A.S. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 2014;127(6):547–553. doi: 10.1016/j.amjmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetta L., Hvid L.G., Justesen L., Christensen U., Neergaard K., Simonsen L., Otrtenblad N., Magnusson S.P., Kjaer M., Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J. Appl. Physiol. 2009;107(4):1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C., Bassett D.R., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- Tzankoff S.P., Norris A.H. Effect of muscle mass decrease on age-related BMR changes. J. Appl. Physiol. 1977;43(6):1001–1006. doi: 10.1152/jappl.1977.43.6.1001. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.J., Cegielski J., Philips B.E., Boereboom C., Lund J.N., Atherton P.J., Smith K. Internal comparison between deuterium oxide (D2O) and L-[ring-13C6] phenylalanine for acute measurement of muscle protein synthesis in humans. Physiol. Rep. 2015;3(7):e12433. doi: 10.14814/phy2.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (WHO) 2010. Global Recommendation on Physical Activity for Health; pp. 23–32.https://www.who.int/publications/i/item/9789241599979 Geneva, Switzerland. [Google Scholar]
- Wroblewski A.P., Amati F., Smiley M.A., Goodpaster B., Wright V. Chronic exercise preserves lean muscle mass in masters athletes. Phys. Sportsmed. 2011;39(3):172–178. doi: 10.3810/psm.2011.09.1933. [DOI] [PubMed] [Google Scholar]
- Zierath J.R., He J., Guma A., Wahlström E.O., Klip A., Wallberg-Henriksson Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39(10):1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.