Abstract
Background:
A gut-microbial metabolite, trimethylamine N-oxide (TMAO) has been associated with coronary atherosclerotic burden. No previous prospective study has addressed associations of long-term changes in TMAO with coronary heart disease (CHD) incidence.
Objective:
To investigate whether 10-year changes in plasma TMAO levels were significantly associated with CHD incidence.
Methods:
This prospective nested case-control study included 760 healthy women at baseline. Plasma TMAO levels were measured both at the first (1989–90) and the second blood collections (2000–02); 10-year changes (Δ) in TMAO were calculated. Incident cases of CHD (n=380) were identified after the second blood collection through 2016 and were matched to controls (n=380).
Results:
Regardless of the initial TMAO levels, 10-year increases in TMAO from the first to second blood collections were significantly associated with an increased risk of CHD (relative risk [RR] in the top tertile: 1.58 [95% CI: 1.05, 2.38]; RR per 1 SD increment: 1.33 [1.06, 1.67]). Participants with elevated TMAO levels (the top tertile) at both time points showed the highest RR of 1.79 (1.08, 2.96) for CHD as compared with those with consistently low TMAO levels. Further, we found that the ΔTMAO-CHD relationship was strengthened by unhealthy dietary patterns (assessed by the Alternate Healthy Eating Index) and was attenuated by healthy dietary patterns (Pinteraction=0.008).
Conclusions:
Long-term increases in TMAO were associated with higher CHD risk, and repeated assessment of TMAO over 10 years improved the identification of people with a higher risk of CHD. Diet may modify the associations of ΔTMAO with CHD risk.
Keywords: Gut-microbial metabolites, Risk factors, Diet, Coronary heart disease, Prospective cohort study
Condensed Abstract:
This prospective nested case-control study newly indicates that 10-year changes in plasma levels of the atherogenic gut-microbial metabolite, trimethylamine N-oxide (TMAO), are significantly associated with the incidence of coronary heart disease (CHD) among women, and that a combined assessment of TMAO levels at two time points over 10 years contributes to identifying people at a higher risk for CHD. Further, adherence to healthy dietary patterns may modulate the adverse relationship between TMAO changes and CHD. Our results suggest that changes in TMAO by specific interventions, such as modification of dietary patterns, may contribute to CHD prevention.
Introduction
Growing evidence has implicated gut microbiota alterations in the development of atherosclerotic cardiovascular disease (CVD) (1). A gut-microbiota related metabolite, trimethylamine N-oxide (TMAO), has been related to risks of major adverse cardiovascular events including myocardial infarction (MI) and coronary heart disease (CHD) in epidemiological studies (2–6), although some studies did not support significant associations of circulating TMAO levels and cardiovascular outcomes (7–9).
The intestinal microbiota metabolizes nutrient precursors of TMAO, such as choline and L-carnitine that are abundant in animal foods, to produce trimethylamine (TMA) (2,10), which is further metabolized to TMAO by the flavin-containing enzyme monooxygenase 3 (FMO3) in the liver (11,12). Studies have also shown that circulating TMAO is a marker of coronary atherosclerotic burden (13–15); elevated TMAO levels are associated with carotid plaque burden and coronary plaque vulnerability (16,17).
Notably, prior studies (3,10,19) that investigated associations of plasma TMAO with CHD risk only used data obtained at a single time point, although analyzing dynamic changes in risk factors may provide more relevant information in terms of the translation of findings into prevention strategies (20,21). Assessment of temporal changes of TMAO would provide information on modifiability of circulating TMAO in relation to the risk of subsequent CHD incidence, and would contribute to the development of novel intervention strategies for the prevention of CHD events. Within-person changes over years may reflect longer term and more cumulative metabolite effects, which would contribute to a better understanding of the atherogenic impact of microbial metabolite alterations in the development of CHD (22–24). Also, changes to human gut microbiota and microbial metabolites can occur after dietary modifications (25–27); intakes of animal foods such as red meat as well as healthy plant foods have been found to modify the production of TMAO (10,18,28,29). However, no previous prospective cohort study has addressed whether long-term changes in TMAO are associated with CHD incidence and whether habitual dietary intakes can modify such associations.
We measured plasma concentrations of the atherogenic gut-microbial metabolite TMAO at two time points, roughly 10 years apart, among women in the Nurses’ Health Study (NHS), and we prospectively investigated whether the long-term changes in TMAO levels were associated with the subsequent incidence of CHD.
Methods
Study population
The NHS is a prospective cohort study of 121,701 female registered nurses aged 30–55 y when enrolled in 1976. Information on demographics, lifestyle factors, medical history, and disease status was collected through a self-administered questionnaire in 1976, and has been updated every 2 years through follow-up questionnaires. The follow-up rate was high with approximately 90% in each 2-year follow-up cycle. A total of 32,826 women provided blood samples at the first collection in 1989–1990, and 18,743 women provided a second blood sample in 2000–2002 (30). The second blood samples were collected using a protocol that was identical to that used at the first collection.
The present nested case-control study included women who were free of the primary outcome (non-fatal MI or fatal CHD) at the time of second blood collection. We prospectively identified incident cases of CHD (non-fatal MI or fatal CHD) from the date of second blood collection through 2016. We used risk-set sampling to randomly select 1 control for each case from whom remained free of CHD events at the time of the case diagnosis. Cases and controls were matched on age at blood draw (± 1 y), smoking status (never, former, current), fasting status at blood draw, and date of blood draw at the two collections. The present analysis included participants with available plasma TMAO levels at both collections and those without missing a case-control pair; a total of 760 participants (380 incident cases of CHD and 380 matched-controls) were included in the present analysis. Almost all of the present study participants were free of self-reported chronic kidney failure (99.9%) and cancer (96.6%) at the second blood collection. The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital and by the Harvard T.H. Chan School of Public Health Human Subjects Committee Review Board. Informed consent was obtained from the study participants.
Outcome assessment
Incident CHD included non-fatal MI and fatal CHD; details of ascertainment of CHD cases are fully described in Online Methods. Deaths were reported by the next of kin or the postal system or identified by searching the National Death Index. Causes of death were primarily confirmed by review of autopsy reports, medical records, and death certificates.
Measurements of TMAO and its precursors
Blood samples were centrifuged and aliquoted into cryotubes as plasma, buffy coat, and erythrocyte fractions, which were then stored in liquid nitrogen freezers at −130 °C or colder until analysis. Blood concentrations of TMAO at the first and second blood collections were measured at the Cleveland Clinic using an established stable isotope dilution high-performance liquid chromatography with electrospray ionization tandem mass spectrometry (2,3). Samples of the case-control pairs were shipped in the same batch and analyzed in the same run. Both technicians and laboratory personnel were blinded to the case-control status of the samples. Changes (Δ) in plasma TMAO concentrations from the first collection to the second blood collection were calculated.
Assessment of covariates and dietary habits
Participants were asked to report data on height, weight, smoking habit, physical activity, history of physician-diagnosed diseases, medication use, and other characteristics at baseline and on biennial questionnaires (Supplemental Appendix). Self-reported weight was highly correlated with technician-measured weight (r=0.97) in a validation study (31). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Previous studies have confirmed the validity of self-reported histories of physician-diagnosed hypertension, high cholesterol, and diabetes (32,33); use of medication for the respective disease (such as antihypertensive medications, statin or other cholesterol-lowering medications, and insulin or oral hypoglycemic medications) was also considered to define the metabolic diseases in the present study. Diet and nutrient intake were assessed using validated semiquantitative food frequency questionnaires. As described previously (34,35), we calculated the Alternate Healthy Eating Index (AHEI) (34) and the healthful plant-based diet index in which healthy plant foods received positive scores, and the other foods received negative scores (35) (Supplemental Appendix). The AHEI and the healthful plant-based diet index are predictive of chronic diseases and cardiovascular mortality risks in the NHS cohort (21,34,35). The TMAO synthesis depends on dietary intake (2,3,10,18,28,29,36); we investigated whether these two diet quality indices modified associations of TMAO with CHD.
Statistical analysis
Data on TMAO were log-transformed before the analyses to improve data normality; log-transformed values were used for calculating changes and SD. We first analyzed associations of TMAO at the second collection with the risk of CHD to validate whether TMAO was a risk factor for CHD in this study population. The primary exposure was ΔTMAO in this study, and we calculated the risk of CHD by changes in TMAO. Conditional logistic regression was used to estimate the relative risk (RR) and 95% CI for CHD incidence. Tertile categories of the metabolite exposure were created using values among controls. To further examine whether sustained higher metabolite levels were associated with a significantly increased risk, we calculated RRs for CHD according to a combination of TMAO levels at the first and second collections. We performed spline regression (37) with 4 knots to model the dose-response relationship, and examined possible nonlinear relationships between TMAO levels and CHD risk. Participants with the highest 1% or the lowest 1% of each metabolite exposure were excluded in the dose-repose analysis to minimize the potential impact of outliers. Covariates included in the multivariate-adjusted model were addressed in Supplemental Appendix.
To examine whether habitual dietary patterns (assessed by the AHEI including alcohol as a component, or the plant-based diet index) can modify the associations of ΔTMAO and CHD risk, we tested interactions between ΔTMAO and high/low adherence to the healthy dietary patterns for CHD incidence by including multiplicative interaction terms in the model. Higher or lower adherence to dietary habits was based on the median value of the AHEI or the plant-based diet index. We calculated the risk of ΔTMAO for CHD incidence stratifying participants by high or low adherence to the healthy dietary patterns. Unconditional logistic regression was used in the stratified analyses to preserve statistical power since matched cases and controls were not necessarily in the same strata. We used cumulative averaged diet quality at the time of the first blood collection to examine whether habitual dietary patterns at the first blood collection could modify the associations of ΔTMAO with the CHD risk. Analyses were performed using SAS version 9.4 (SAS Institute); P values were 2-sided, and P values <0.05 were considered statistically significant.
Results
Characteristics of CHD cases and controls are shown in Online Table 1. As expected, the CHD cases had higher BMI and were more likely to have a family history of MI and metabolic risk factors (hypertension, dyslipidemia, and diabetes) at the first and second time points. There were no differences in median values of plasma TMAO levels at the first collection between cases and controls (P=0.56), whereas plasma TMAO levels at the second collection were higher in CHD cases than controls (median [25th, 75th] values of TMAO: 4.9 [3.3, 7.6] µM in cases; 4.3 [3.2, 6.4] µM in controls; P=0.04). Characteristics of the participants according to tertile categories of TMAO levels are presented in Online Table 2. Higher levels of TMAO at the second collection were associated with older age (P=0.015) and higher prevalence of hypertension (P=0.002) among controls. Similarly, higher TMAO levels at the second collection were associated with higher prevalence of hypertension (P=0.028) among cases.
First, we confirmed previously reported associations that higher levels of TMAO at the second collection were associated with a higher risk of CHD (RR per 1 SD increment: 1.26 [95% CI: 1.10, 1.46] in model 1 adjusted for matched factors) (Online Table 3). The positive association remained significant after controlling for demographic, diet, and lifestyle factors (RR 1.26 [95% CI: 1.09, 1.46] per 1SD in model 2), but was attenuated after further adjusting for obesity and metabolic risk factors (model 3). Every 1 SD increment in TMAO at the second collection was associated with a 23% increased risk [RR 1.23; 95% CI: 1.05, 1.42], model 3). In spline regression analysis (Figure 1), we observed a positive linear relationship between TMAO levels and CHD risk. The linear trend was attenuated after adjusting for obesity and metabolic risk factors (hypertension, dyslipidemia, and diabetes).
Figure 1: Dose-response relationship between TMAO levels at the second collection and the risk of coronary heart disease (CHD).
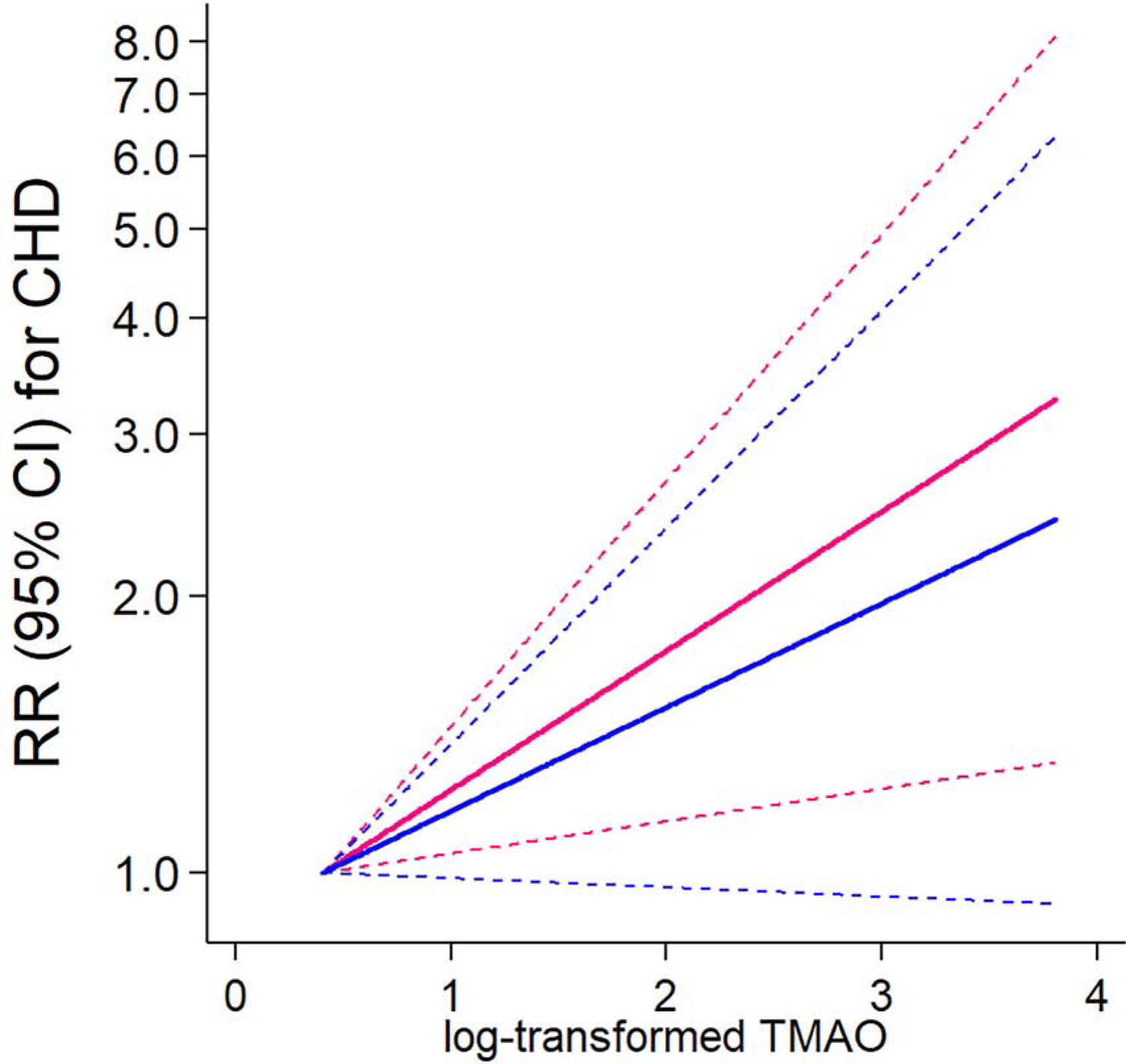
Reference: a minimum value. Dotted lines indicate lower and upper 95% confidence intervals. Pink line (Plinear =0.01) shows the relative risk (RR) in a model adjusted for matched factors (age, smoking habit, fasting status, and date of blood collection), family history of myocardial infarction, postmenopausal hormone use, aspirin use, alcohol, physical activity, and the Alternative Healthy Eating Index (without alcohol). All covariates were based on assessments at the second time point. Blue line (Plinear =0.07) shows the RR in a model with an additional adjustment of BMI, hypertension, dyslipidemia, and diabetes at the second time point.
We observed that the CHD cases had a significant 10-year increase in TMAO concentrations from the first collection to the second collection (P=0.009 by the paired t-test among the CHD cases) (Online Table 4). Table 1 shows RRs for CHD according to changes (Δ) in TMAO levels. As compared to participants with stable TMAO levels (T2 group: median value of ΔTMAO: 0.1 µM), those with the largest increases in TMAO (T3 group: median value of ΔTMAO: 3.7 µM) had a 1.67 (95% CI: 1.13, 2.44; P=0.009) times higher risk of CHD after controlling for matched factors, TMAO levels at the first collection, demographic and diet/lifestyle factors assessed at the first time point, and concurrent changes in diet/lifestyle factors (model 2). In the fully adjusted model with further including obesity and metabolic risk at the first time point and concurrent changes (model 3), women with the largest increases in TMAO (T3) had a 1.58 (95% CI: 1.05, 2.38; p=0.029) times higher risk of CHD. Every 1 SD increase in ΔTMAO was associated with a 33% increased risk for CHD (RR per 1 SD: 1.33 [1.06, 1.67]; P=0.013). These models concurrently included both initial TMAO and its changes; circulating levels of TMAO at the first collection also showed a positive association with the CHD risk (Online Table 5). These results showed the initial TMAO levels and its 10-y changes were independently associated with subsequent CHD events.
Table 1:
Risk of coronary heart disease (CHD) according to tertile categories of changes (Δ) in trimethylamine N-oxide (TMAO) or per 1 SD increment
| Tertile (T) categories of ΔTMAO | Per 1 SD increment | |||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| ΔTMAO, median [25th, 75th] µM | –3.8 [–7.9, – 2.0] | 0.1 [–0.4, 0.7] | 3.7 [2.0, 6.6] | |
| N of cases/controls | 114/126 | 104/127 | 162/127 | 380/380 |
| Model 1, RR (95% CI) | 0.83 (0.55, 1.26) | 1.00 (Ref.) | 1.72 (1.19, 2.49) | 1.40 (1.14, 1.72) |
| Model 2, RR (95% CI) | 0.82 (0.54, 1.26) | 1.00 (Ref.) | 1.67 (1.13, 2.44) | 1.39 (1.13, 1.73) |
| Model 3, RR (95% CI) | 0.91 (0.57, 1.43) | 1.00 (Ref.) | 1.58 (1.05, 2.38) | 1.33 (1.06, 1.67) |
Abbreviation: Trimethylamine N-oxide, TMAO; Reference, Ref.; Relative risk, RR.
Model 1: “matched factors” (age, smoking habit, fasting status, and date of blood collection) and TMAO levels at the first collection.
Model 2: model 1 + “demographic and diet/lifestyle factors assessed at the first time point” (family history of myocardial infarction, aspirin use, peri/postmenopausal status and hormone use, the Alternative Healthy Eating Index [AHEI] without alcohol, physical activity, and alcohol intake) + “changes in diet/lifestyle factors between the first and second time points” (changes in AHEI without alcohol, changes in physical activity, and changes in alcohol intake).
Model 3: model 2 + “obesity and metabolic status at the first time point and changes between the first and second time points” (BMI at the first time point, changes in body weight, hypertension (none [no at both time points], new incidence [no at the first time point and yes at the second time point], or prevalent [yes at the first or second time point except for new incidence]), dyslipidemia (none/new incidence/prevalent) diabetes (none/new incidence or prevalent).
In results of analyzing dose-response associations of changes in TMAO with CHD risk (Central Illustration), there was a strong linear relationship (P=0.016) between ΔTMAO and the CHD risk in the fully adjusted model. In the spline analysis, we used “no change” of metabolite (i.e., Δ=0) as a reference value to estimate RRs and 95% CIs for CHD; we observed that both increases and decreases in TMAO showed significant associations with either an increased or a decreased CHD risk, as compared to the reference.
Central Illustration: Risk for coronary heart disease (CHD) by changes (Δ) in trimethylamine N-oxide (TMAO).
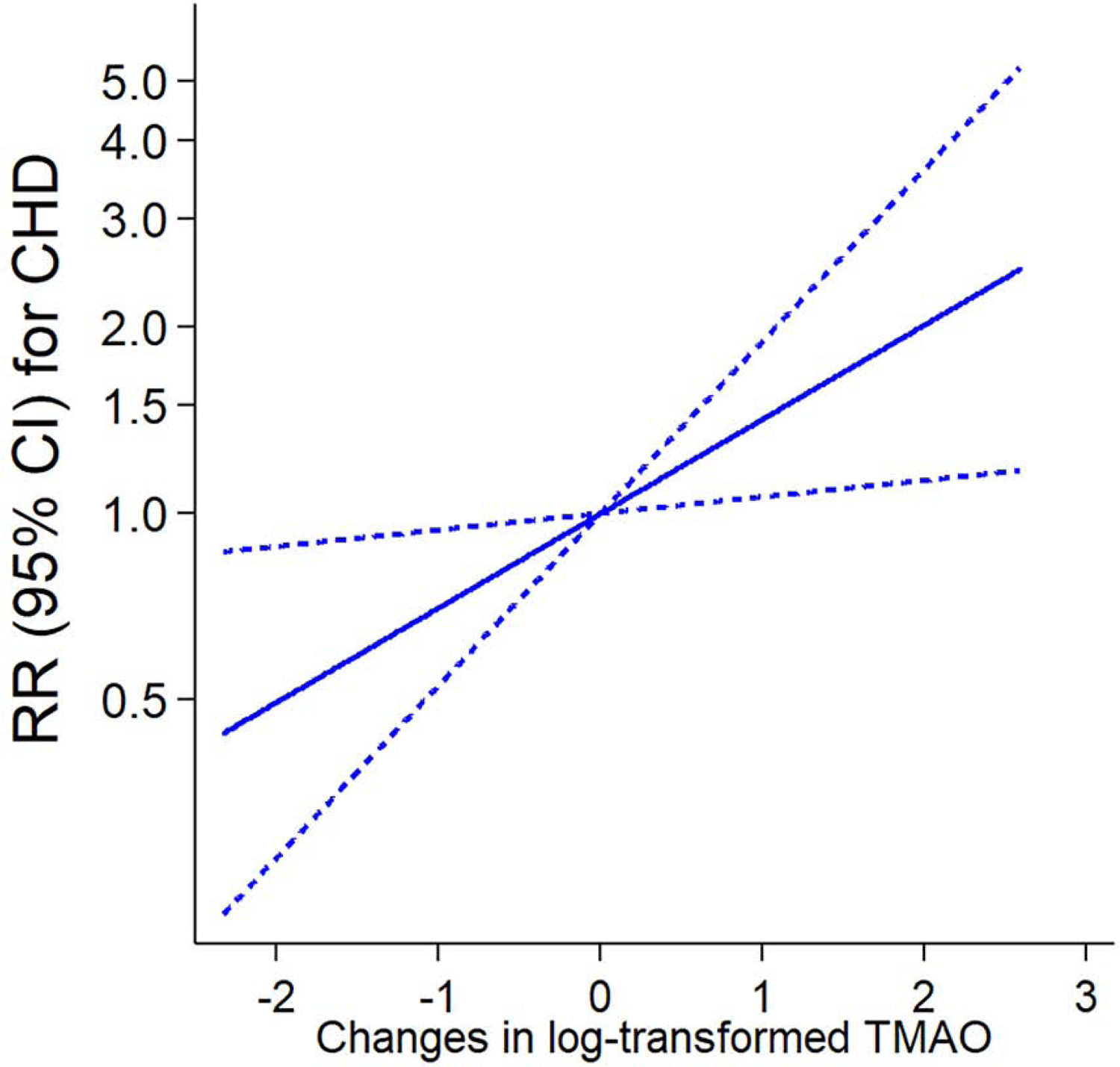
Reference: no change. Dotted lines indicate lower and upper 95% confidence intervals. RRs and 95% CIs were adjusted for the same covariates of model 3 in Table 1. P value of testing for linear association (Plinear)=0.016. Abbreviation: Trimethylamine N-oxide, TMAO; Reference, Ref.; Relative risk, RR.
We then analyzed whether sustained higher TMAO levels were associated with a significantly increased risk by calculating the risk of CHD according to a combination of TMAO levels at the first and second collections (Table 2). We categorized participants into 4 groups using cut-offs of the highest tertile (T3) vs. lower two tertiles (T1–T2) of TMAO. As compared to women with sustained low TMAO levels (T1–T2 at both time), those with sustained high TMAO levels (in the highest TMAO tertile, T3, at both time points) had a significantly increased risk of CHD with multivariable-adjusted RR of 1.79 (95% CI: 1.08, 2.96) (model 3); the RR was greater than women with elevated TMAO levels at a single time point.
Table 2:
Risk of coronary heart disease (CHD) according to a combination of TMAO levels at the first collection and second collection
| aGroup | Cases/controls | Model 1, RR (95% CI) | P | Model 2, RR (95% CI) | P | Model 3, RR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| 1) Low TMAO−first and low TMAO−second | 140/170 | 1.00 (Ref.) | - | 1.00 (Ref.) | 1.00 (Ref.) | - | |
| 2) Low TMAO−first and high TMAO−second | 92/82 | 1.40 (0.96, 2.04) | 0.08 | 1.42 (0.96, 2.11) | 0.08 | 1.27 (0.83, 1.94) | 0.26 |
| 3) High TMAO−first and low TMAO−second | 68/83 | 1.02 (0.68, 1.54) | 0.91 | 1.05 (0.69, 1.60) | 0.82 | 0.95 (0.61, 1.49) | 0.82 |
| 4) High TMAO−first and high TMAO−second | 80/45 | 2.29 (1.45, 3.62) | 0.0004 | 2.08 (1.30, 3.33) | 0.002 | 1.79 (1.08, 2.96) | 0.023 |
Abbreviation: Trimethylamine N-oxide, TMAO; Reference, Ref.; Relative risk, RR.
Participants were categories into 4 groups based on low (median [25th, 75th]: 3.3 [2.5, 4.1] µM) or high (8.2 [6.4, 12.0] µM) TMAO levels at the first collection (TMAO−first), and low (3.5 [2.8, 4.3] µM) or high (8.0 [6.4, 11.5] µM) TMAO levels at the second collection (TMAO−second).
Model 1: “matched factors” (age, smoking habit, fasting status, and date of blood collection).
Model 2: model 1 + “demographic and diet/lifestyle factors assessed at the first time point” (family history of myocardial infarction, aspirin use, peri/postmenopausal status and hormone use, the Alternative Healthy Eating Index [AHEI] without alcohol, physical activity, and alcohol intake) + “changes in diet/lifestyle factors between the first and second time points” (changes in AHEI without alcohol, changes in physical activity, and changes in alcohol intake).
Model 3: model 2 + “obesity and metabolic status at the first collection and changes between the first and second time points” (BMI at the first time point, changes in body weight, hypertension (none [no at both time points], new incidence [no at the first time point and yes at the second time point], or prevalent [yes at the first or second time point except for new incidence]), dyslipidemia (none/new incidence/prevalent) diabetes (none/new incidence or prevalent).
Finally, we tested whether the positive relationship between ΔTMAO and incident CHD was modified by adherence to healthy dietary patterns (Figure 2). We found significant interactions between ∆TMAO and adherence to healthy dietary habits assessed by the AHEI (Pinteraction=0.008 in panel A) or the plant-based diet index (Pinteraction=0.04 in panel B) in CHD risk; such positive relationship between ΔTMAO and incident CHD was significantly strengthened by unhealthy dietary patterns and were attenuated by healthy dietary patterns. Greater increases in TMAO were significantly associated with the CHD risk among women with lower adherence to these dietary habits, whereas the TMAO-CHD associations were not significant among those with higher adherence.
Figure 2: Risk of CHD incidence according to TMAO changes stratified by adherence to healthy dietary habits.
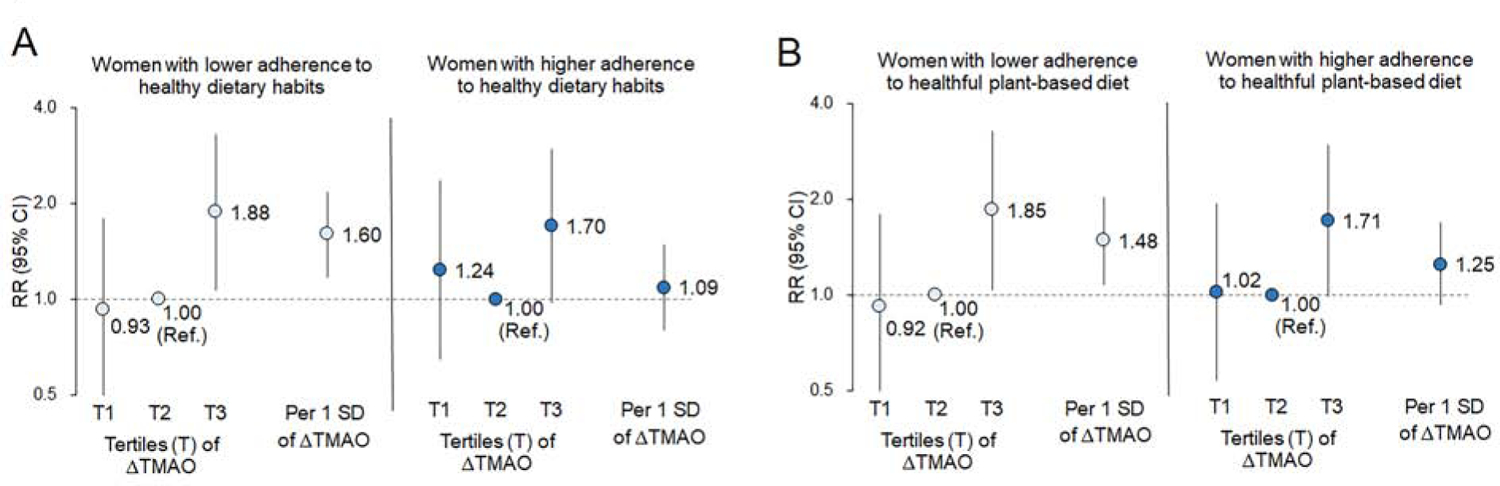
Risk of coronary heart disease (CHD) according to tertile (T) categories of changes (∆) in trimethylamine N-oxide (TMAO) or per 1 SD increment of ∆TMAO among women with lower or higher adherence to healthy dietary habits assessed by the Alternative Healthy Eating Index (panel A), or adherence to plant-based diet (panel B). Relative risks (RRs) were adjusted for total energy intake and the same covariates (including matched factors, demographic/lifestyle factors, obesity, and metabolic risk factors at the first time point and concurrent changes between two time points) in model 3 of Table 1 (details of covariates in the model in the Supplemental Appendix). Higher or lower adherence to dietary habits was based on the median value of the Alternative Healthy Eating Index (panel A) or the plant-based diet index (panel B). Test for interactions between ∆TMAO and diet: Pinteraction=0.008 in panel A; Pinteraction=0.04 in panel B.
Discussion
The present study newly showed that long-term changes in gut microbial metabolite TMAO were significantly associated with the subsequent CHD events, regardless of the initial TMAO levels. Our findings underscore the importance of repeated measurements in the gut microbial metabolites in predicting the risk of CHD among women at ‘usual’ risk. Further, we found that adherence to healthy dietary patterns modified the unfavorable effect of TMAO increases with the CHD risk.
The present study is novel in terms of analyzing dynamic changes in the atherogenic microbial metabolite for CHD events, and we found that assessment of longitudinal changes in TMAO over 10 years improved identification of people with a higher risk of CHD. Viewed differently, we observed that women with sustained high levels of TMAO for many years based on the repeated assessment had a significantly elevated risk of CHD. Our observations are supported by previous findings from experimental studies such as that modulation of the TMAO-generating enzyme FMO3 impacts systemic TMAO levels (38), and the FMO3 is involved in atherosclerotic pathogenesis by regulating cholesterol metabolism and insulin resistance (39,40). TMAO-related increases in proinflammatory monocytes may be associated with elevated cardiovascular risk of patients with increased TMAO levels (41). One study suggested that increases in TMAO induced by dietary choline intake were associated with an enhanced prothrombotic effect in human subjects (22). Older age was related to higher TMAO among control subjects, which was also observed in previous studies (36,42); we speculated that aging-related gradual increases in TMAO over ten years among the CHD cases might have contributed to accelerating endothelial cell senescence and vascular aging (42) and vascular inflammation (43).
We observed a linear relationship between TMAO concentrations at the second time point and CHD risk, indicating that plasma TMAO is a biomarker for CHD incidence among women at “usual risk”, although the trend was attenuated after controlling for obesity and metabolic risk status. Further, our dose-response analysis on TMAO changes demonstrated that as compared with no change, both increases and decreases in TMAO showed associations with either an increased or a decreased risk of CHD after adjusting for traditional risk factors including obesity and metabolic diseases. These results support the importance of modulating TMAO levels by interventions in the prevention of subsequent CHD events. Regarding potential explanations for these findings, several possible mechanisms might be involved. A study reported that a nonlethal inhibitor that blocks the production of TMA (which in turn reduces the production of TMAO) from choline might prevent the formation of atherosclerotic lesions (23), suggesting a potential role of the gut microbial TMA lyases as a therapeutic target for atherosclerosis. Greater decrease of TMAO was related to the improvement of carotid intima-media thickness in a lifestyle intervention (44). Genetic variations may causally induce impaired TMA metabolism which can reduce TMAO production (45). These data, including our findings, support that decreasing plasma TMAO levels may contribute to reducing the risk of CHD, and suggest that atherogenic gut microbiota metabolites might be targets in novel interventions for CHD prevention.
Our main findings of the positive relationship between TMAO changes and CHD risk were significant among total participants, even after controlling for traditional risk factors and overall dietary patterns. On the other hand, our stratified analysis by dietary patterns also showed that the TMAO-CHD association was significantly strengthened by unhealthy dietary patterns, and was attenuated by healthy dietary patterns characterized by higher intake of vegetables and lower intake of animal foods. Previous studies show that omnivorous people produced more TMAO than did vegans or vegetarians following dietary L-carnitine intake through a microbiota-dependent mechanism (10,18). A more recent study reported similar associations that intake of red meat increased TMAO levels, and discontinuation of red meat intake reduced plasma TMAO levels within 4 weeks (29). Diet is one of the most important modifiable factors to modulate circulating TMAO levels and gut microbiome (3,10,18,25–29,46) and our study emphasizes the importance of better dietary quality and healthier eating patterns, as recommended by current dietary guidelines, to reduce the adverse effects of TMAO changes on the development of CHD. These findings suggest that TMAO is a biomarker of incident CHD and a potential intermediate endpoint in dietary interventions, as the modification of dietary patterns may significantly modulate the association of TMAO with CHD incidence. On the other hand, it is worth mentioning that healthy dietary habits may affect the host and the microbiome through unknown pathways independent of the TMAO metabolism; the interplays between adherence to healthy dietary patterns and gut microbiota/metabolites in the risk of CHD would warrant further investigations.
Our study has several strengths. Taking advantage of longitudinally repeated collections of blood samples in the NHS cohort, our study for the first time assessed the relations of long-term changes in the metabolite levels and subsequent risk of CHD. The study participants were initially free of major chronic diseases such as cancer and kidney failure that may affect TMAO levels; results of our traditional cohort at ”usual” risk contribute to understanding metabolomics precursors for CHD events. Comprehensive measurements available in the NHS allowed us to consider concurrent changes in major risk factors for CHD in the multivariate analysis. However, several potential limitations warrant consideration. Our study did not assess the timing or trajectories of the changes (e.g., rapid or gradual increases/decreases) in the metabolites contributing to the development of CHD. There might be unmeasured endogenous or exogenous confounding factors that might affect changes in TMAO levels; there also might be residual or unmeasured confounding factors for CHD incidence. The assessment of dietary patterns and other covariates included in the multivariate-analysis were based on self-reports, which might affect the estimated risk of CHD; however, previous validation studies have confirmed the validity of self-reported data such as diet and metabolic risk factors in the NHS. Our study included women only, and all were health professionals. Further research is necessary to confirm our findings, especially in male cohorts and a population that is more representative of the US population.
Conclusions
In conclusion, long-term changes in plasma TMAO levels are significantly associated with the CHD incidence among healthy women, and a repeated assessment of plasma TMAO levels over 10 years contributes to identifying people at high risk for CHD incidence. Adherence to healthy dietary patterns may modulate the adverse relationship between TMAO changes and CHD, suggesting that TMAO as a potential intermediate endpoint of interventions focusing on dietary modifications for CHD prevention.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
Longitudinal changes in the atherogenic gut-microbial metabolite, trimethylamine N-oxide (TMAO), improves identification of people at elevated risk of coronary heart disease (CHD). The relationship between increases in TMAO and CHD risk could be attenuated by a healthier diet.
Translational Outlook:
Pharmacological and lifestyle intervention studies could explain the biological mechanisms linking circulating levels of gut-microbial metabolites like TMAO to CHD and lead to more effective strategies for disease prevention.
Acknowledgments:
We appreciate all the participants of the Nurses’ Health Study (NHS) for their continued cooperation. We thank Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School for their assistance. The authors also thank the Prevention Research Laboratory and Laboratory Diagnostic Core, Cleveland Clinic for the measurements of metabolites.
Funding:
The study is supported by NIH grants from the National Cancer Institute (UM1 CA186107 and CA49449), the National Heart, Lung, and Blood Institute (R01 HL034594, R01 HL088521, HL071981, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616, DK115679), an NIH shared instrumentation grant (S10OD016346), the Boston Obesity Nutrition Research Center (DK46200), and United States– Israel Binational Science Foundation Grant 2011036. Yoriko Heianza was a recipient of a Grant-in-Aid for Scientific Research, and a Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science. Yoriko Heianza is a recipient of the 2019 AHA postdoctoral fellowship award (19POST34380035). The sponsors had no role in the design or conduct of the study.
Abbreviations
- CVD
cardiovascular disease
- TMAO
trimethylamine N-oxide
- CHD
coronary heart disease
- MI
myocardial infarction
- TMA
trimethylamine
- FMO3
flavin-containing enzyme monooxygenase 3
- NHS
Nurses’ Health Study
- BMI
Body Mass Index
- AHEI
Alternate Healthy Eating Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heianza Y, Ma W, Manson JE, et al. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc 2017;6. 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–56. [DOI] [PubMed] [Google Scholar]
- 6.Qi J, You T, Li J, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med 2018;22:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guasch-Ferre M, Hu FB, Ruiz-Canela M, et al. Plasma Metabolites From Choline Pathway and Risk of Cardiovascular Disease in the PREDIMED (Prevention With Mediterranean Diet) Study. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer KA, Benton TZ, Bennett BJ, et al. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc 2016;5. 10.1161/JAHA.116.003970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018;137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motika MS, Zhang J, Cashman JR. Flavin-containing monooxygenase 3 and human disease. Expert Opin Drug Metab Toxicol 2007;3:831–45. [DOI] [PubMed] [Google Scholar]
- 13.Senthong V, Li XS, Hudec T, et al. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, Is Associated With Atherosclerotic Burden. J Am Coll Cardiol 2016;67:2620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stubbs JR, House JA, Ocque AJ, et al. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J Am Soc Nephrol 2016;27:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Xie Z, Sun M, et al. Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. Int J Cardiol 2018;265:18–23. [DOI] [PubMed] [Google Scholar]
- 16.Bogiatzi C, Gloor G, Allen-Vercoe E, et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 2018;273:91–97. [DOI] [PubMed] [Google Scholar]
- 17.Fu Q, Zhao M, Wang D, et al. Coronary Plaque Characterization Assessed by Optical Coherence Tomography and Plasma Trimethylamine-N-oxide Levels in Patients With Coronary Artery Disease. Am J Cardiol 2016;118:1311–15. [DOI] [PubMed] [Google Scholar]
- 18.Koeth RA, Lam-Galvez BR, Kirsop J, et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest 2019;129:373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Z, Liang Z, Guo M, et al. The Association between Plasma Levels of Trimethylamine N-Oxide and the Risk of Coronary Heart Disease in Chinese Patients with or without Type 2 Diabetes Mellitus. Dis Markers 2018;2018:1578320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. Changes in Diet Quality Scores and Risk of Cardiovascular Disease Among US Men and Women. Circulation 2015;132:2212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. N Engl J Med 2017;377:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Wang Z, Tang WHW, et al. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation 2017;135:1671–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015;163:1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med 2018;24:1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heianza Y, Sun D, Li X, et al. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: the POUNDS Lost trial. Gut 2019;68:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science 2018;362:776–80. [DOI] [PubMed] [Google Scholar]
- 28.Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr 2017;68:488–95. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Bergeron N, Levison BS, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019;40:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao Y, Bertoia ML, Lenart EB, et al. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am J Public Health 2016;106:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 32.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 33.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med 1991;151:1141–7. [PubMed] [Google Scholar]
- 34.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satija A, Bhupathiraju SN, Spiegelman D, et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol 2017;70:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manor O, Zubair N, Conomos MP, et al. A Multi-omic Association Study of Trimethylamine N-Oxide. Cell Rep 2018;24:935–46. [DOI] [PubMed] [Google Scholar]
- 37.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W, Buffa JA, Wang Z, et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost 2018;16:1857–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warrier M, Shih DM, Burrows AC, et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep 2015;10:326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haghikia A, Li XS, Liman TG, et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler Thromb Vasc Biol 2018;38:2225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke Y, Li D, Zhao M, et al. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med 2018;116:88–100. [DOI] [PubMed] [Google Scholar]
- 43.Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J Am Heart Assoc 2016;5. 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randrianarisoa E, Lehn-Stefan A, Wang X, et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci Rep 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Y, Hwang LD, Li J, et al. Genetic analysis of impaired trimethylamine metabolism using whole exome sequencing. BMC Med Genet 2017;18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valdes AM, Walter J, Segal E, et al. Role of the gut microbiota in nutrition and health. BMJ 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


