Abstract
Objectives:
To characterize abdominal visceral adipose tissue (VAT) trajectory relative to the final menstrual period (FMP), and to test whether menopause-related VAT accumulation is associated with greater average (cIMT), common (CCA-IMT), and/or internal (ICA-IMT) carotid artery intima-media thickness.
Methods:
Participants were 362 women (at baseline: age was (mean±SD) 51.1±2.8 years; 61% White, 39% Black) with no cardiovascular disease (CVD) from the SWAN Heart study. Women had up to two measurements of VAT and cIMT overtime. Splines revealed a non-linear trajectory of VAT with two inflection points demarcating 3 time segments: segment 1: >2 years before FMP, segment 2: 2 years before FMP to FMP, and segment 3: after FMP. Piecewise-linear random-effects models estimated changes in VAT. Random-effects models tested associations of menopause-related VAT with each cIMT measure separately. Estimates were adjusted for age at FMP, body mass index, and sociodemographic, lifestyle, and CVD risk factors.
Results:
VAT increased significantly by 8.2% (95% CI: 4.1% to 12.5%) and 5.8% (3.7% to 7.9%) per year in segments 2 and 3, respectively, with no significant change in VAT within segment 1. VAT predicted greater ICA-IMT in segment 2, such that a 20% greater VAT was associated with a 2.0% (0.8% to 3.1%) greater ICA-IMT. VAT was not an independent predictor of ICA-IMT in the other segments or of the other cIMT measures after adjusting for covariates.
Conclusions:
Women experience accelerated increase in VAT starting 2 years before menopause. This menopause-related increase in VAT is associated with greater risk of subclinical atherosclerosis in the internal carotid artery.
Keywords: Abdominal visceral adipose tissue, central adiposity, menopause, menopause transition, carotid intima-media thickness, subclinical cardiovascular disease
INTRODUCTION
Waist circumference predicts excess risk of cardiovascular disease (CVD) mortality after menopause irrespective of having normal body weight.1 With more than 70% of postmenopausal women having central obesity,2 namely waist circumference ≥88 cm, previous analyses have sought to determine whether the menopause transition (MT), independent of chronological aging, puts women at greater risk of accumulating more abdominal fat.3,4
Analyses of regional body fat have suggested that increases in waist circumference during midlife might be related to the MT.3,4 Waist circumference is an overall measure of abdominal subcutaneous (SAT) and visceral (VAT) adipose tissues. When matching for SAT level, greater VAT is associated with greater risk of carotid artery atherosclerosis.5,6 VAT-secreted adipocytokines are hypothesized to be the culprit of the heightened atherosclerotic risk.7 Studies that examined whether the MT is related to midlife VAT accumulation showed inconsistent results and were limited by cross-sectional design,8,9 small sample size,10,11 or insufficient covariate adjustment.11 Moreover, none of these studies assessed whether increases in VAT during the MT contributed to greater risk of subclinical markers of atherosclerosis known to be influenced by the MT.12,13
Midlife women experience increases in average carotid artery intima-media thickness (cIMT) during the late perimenopause stage,12 which typically starts 1–3 years before the final menstrual period (FMP) and extends to 1 year after the FMP.14 Averaged cIMT across all carotid artery segments is a strong predictor of CVD events.15 However, the internal carotid artery (ICA-IMT) has a greater predictive power for CVD risk compared with the common carotid artery (CCA-IMT).16 Since patterns of carotid artery remodeling are segment-specific,17 and in young adults greater waist circumference predicts greater progression in ICA-IMT but not the other segments,18 it is expected that VAT may have distinctive pathological effects on different carotid artery segments. Whether potential menopause-related increases in VAT are associated with measures of subclinical carotid atherosclerosis is unknown.
The Study of Women’s Health Across the Nation (SWAN) is a unique cohort study of midlife women followed longitudinally with careful measures of the MT and concurrent assessment of VAT and subclinical carotid atherosclerosis overtime enabling assessing the following objectives: 1) to characterize VAT trajectory over time relative to the FMP, independent of chronological aging, and 2) to test whether menopause-related increase in VAT was associated with subclinical carotid measures of atherosclerosis (average cIMT, CCA-IMT, and ICA-IMT). We hypothesized a non-linear trajectory of VAT over time relative to the FMP, with a larger change in VAT around the FMP, compared to prior to the FMP. We further hypothesized that menopause-related increases in VAT are associated with greater risk of subclinical carotid atherosclerosis.
MATERIALS AND METHODS
Study Participants
SWAN is an ongoing multi-ethnic longitudinal study of the MT. Detailed methods are presented elsewhere.19 In brief, 3 302 women were recruited between 1996–1997 from: Detroit, MI; Boston, MA; Chicago, IL; Oakland, CA; Los Angeles, CA; Newark, NJ; and Pittsburgh, PA. To be eligible for SWAN, women had to be 42–52 years of age at enrollment, had an intact uterus and at least one ovary with menstrual bleeding within the past three months, not be pregnant or breast-feeding, and not had used hormones therapy within the past three months. The institutional review board at each participating site approved the study protocol, and all participants signed informed consent before participation.
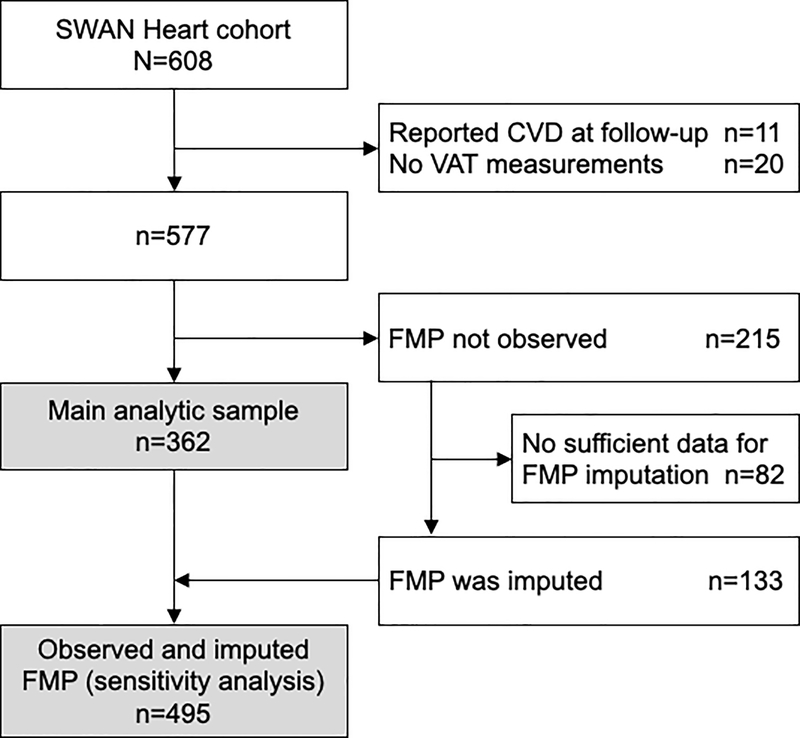
SWAN Heart was an ancillary study to SWAN designed to evaluate women’s midlife changes in subclinical atherosclerosis. Between 2001–2003, SWAN Pittsburgh and Chicago women enrolled to SWAN Heart Ancillary study (N=608) if they had no self-reported clinical CVD. By design, Pittsburgh and Chicago sites recruited only White or Black women. After a mean±SD of 2.3±0.5 years following the SWAN Heart baseline visit, women came for a follow-up visit. Because carotid atherosclerosis is a predictor of CVD events, we excluded women who reported CVD during the follow-up period (n=11). We additionally excluded women who did not have VAT measurements (n=20) or did not have an observed FMP date (n=215) as described below. The main analytic sample included 362 women who contributed 595 observations with each woman having one or two measurements over time (Figure 1). Women in the analytic sample were older, more likely to be smokers, had higher systolic blood pressure, had larger waist circumference, and were less likely to have ever used hormone therapy at baseline compared with the excluded participants; otherwise, they shared similar baseline clinical characteristics including cIMT measures and VAT.
Figure 1. Flowchart of study participants (the SWAN Heart study).
CVD = cardiovascular disease; FMP = final menstrual period; VAT = abdominal visceral adipose tissue.
Time anchored to FMP
At each visit, women provided the date of their most recent menstrual period which enabled retrospectively assigning the FMP date as the date of the participant’s last menstrual period before 12 consecutive months of amenorrhea. However, we did not observe the FMP in 215 SWAN Heart women due to hormone therapy, hysterectomy, or bilateral oophorectomy. Applying established methods,20 133 (62%) of women without an observed FMP had sufficient data available to allow multiple imputation of their FMP date and were combined with the women who have an observed FMP in a sensitivity analysis (Figure 1). The imputation is described in the Supplemental Digital Content.
Visceral Adipose Tissue
VAT area was assessed using electron beam computed tomographic scans. A 6-mm transverse image was obtained between L4 and L5 during breath hold with a C-150 Ultrafast CT Scanner (GE Imatron, San Francisco, CA). The scans were read by a single trained reader at the University of Pittsburgh. The area of adipose tissue was defined using image analysis (AccuImage Diagnostics, South San Francisco, CA) with fat structure determined using a pixel range of −30 to −190 Hounsfield units. A region-of-interest line was drawn at the interior of abdominal musculature along the fascial plane. Adipose tissue within the drawn area was considered to be VAT area. VAT readers were blinded to cIMT reads. Interobserver reliability was determined by repeat reads on 10 VAT scans, with an intraclass correlation coefficient of 0.94.21
Carotid Artery Intima-Media Thickness
cIMT was assessed using a Toshiba SSA-270A scanner (Toshiba American Medical Systems, Tustin, CA) and a Hewlett-Packard 5500 scanner (Hewlett-Packard, Andover, MA). B-mode images were obtained from the following 4 locations in the left and right carotid arteries: the near and far walls of the distal common carotid artery (1 cm proximal to the carotid bulb); the far wall of the carotid bulb (from the point where the near and far walls of the common carotid artery are no longer parallel and extending to the flow divider); and the far wall of the internal carotid artery (distal 1 cm from the flow divider). The lumen-intima interface and the media-adventitia interface across a 1-cm segment of each of these locations were electronically traced. A computer-assisted measurement of each pixel over the traced area was generated for a total of 140 data points in each location. Average of the readings in each location was calculated, and then readings were averaged across all locations to obtain the average cIMT value. The readings of the common and internal carotid artery were averaged to obtain the CCA-IMT and ICA-IMT values, respectively. cIMT readers were blinded to VAT reads. Interobserver reliability was determined by repeat reads on 20 cIMT scans, with an intraclass correlation coefficient of 0.98.21
Covariates
At SWAN visits concurrent with VAT assessment date, participants completed self- and interviewer-administered questionnaires that included assessment of sociodemographic and lifestyle factors and medical history. Participants had their physical and blood pressure measurements and a fasting blood sample obtained at each SWAN visit using standardized protocols. A detailed description of covariates and blood assays measurement is presented in the Supplemental Digital Content. Age at FMP, race, study site, and financial strain were time-fixed variables. For all other covariates, time-varying values that coincided or where the closest in time with each participant’s VAT assessment date were used.
Statistical Analysis
VAT and cIMT were log transformed due to skewed distributions. Repeated measures of VAT as a function of time relative to the FMP were plotted using locally weighted scatter-plot smoothing (LOWESS). The plot suggested a non-linear trajectory of VAT and thus piecewise linear random-effect models were used to estimate and compare annual changes of VAT across the identified time segments. Segment-specific annual percentage change of VAT was calculated as (eestimated annual change in log-VAT−1)×100. Multivariable analysis adjusted for factors affecting VAT including body mass index (BMI). To visualize VAT trajectory, we plotted annual means of VAT over time relative to the FMP, as well as VAT estimates from the piecewise linear random-effects model. We tested effect modification of race on VAT trajectory by including an interaction term of race with each of the three time-segments separately to avoid collinearity.
To assess whether menopause-related increases in VAT are associated with cIMT measures, we created an indicator variable of the time segments identified via LOWESS. Because VAT accelerated significantly 2 years before the FMP, we defined a menopause-related VAT increase as the VAT value between 2 years before the FMP to the FMP (segment 2, see results). We separately modeled the repeated measures of averaged cIMT, CCA-IMT, and ICA-IMT as a function of the repeated measures of VAT, the indicator variable of the time segments, and their interaction (model 1), with segment 1 as the reference level. For more meaningful estimates, we calculated percentage change in each cIMT measure per 20% greater VAT by time segments using (erespective log-VAT beta estimate × log(1.2)−1)×100. Multivariable analysis adjusted for factors affecting atherosclerosis.
As sensitivity analyses, we reran the previous analyses after combining women with imputed and observed FMP and on women for whom VAT was measured at both SWAN Heart visits. As additional analyses, we adjusted for estradiol in model 3 of the VAT trajectory analysis to explore whether it explains the significant inflection point in VAT trajectory at 2 years before the FMP. Additionally, because we observed a significant association between VAT and ICA-IMT in segment 2, we hypothesized that this association maybe modified by estradiol. For simplicity, we explored this effect modification using linear regression while limiting the analysis to women in segment 2 who do not have repeated measures. All analyses were conducted using SAS 9.4 with a significance level set at 0.05.
RESULTS
Study population
The baseline VAT was measured 0.8±3.2 years before the FMP, and the follow-up VAT 1.3±3.1 years after the FMP. Of the 362 women in our study, 233 (64%) had VAT and cIMT measured at baseline and follow-up visits, 114 (32%) at baseline visit only, and 15 (4%) at follow-up visit only. Characteristics of the study sample are shown in Table 1. Compared with women who had imputed FMP dates, women with an observed FMP were more likely to be smokers, had higher systolic blood pressure, and were less likely to have ever used hormone therapy at baseline (Supplemental Table I).
Table 1.
Characteristics of Study Participant at Baseline (n=362)
| Variables a | Values |
|---|---|
| Age (years) | 51.13 ± 2.77 |
| Race, N (%) | |
| White | 222 (61) |
| Black | 140 (39) |
| Financial strain, N (%) | 117 (36) |
| Alcohol (drinks/month), N (%) | |
| 1 or less | 152 (42) |
| Between 2 and 4 | 125 (34) |
| 5 or more | 85 (24) |
| Current Smoker, N (%) | 70 (19) |
| Physical activity score b | 7.85 ± 1.77 |
| Total daily calorie intake (kcal), median (Q1, Q3) | 1731.9 (1355.5, 2204.7) |
| BMI (kg/m2) | 29.66 ± 6.63 |
| Waist circumference (cm) | 89.97 ± 14.79 |
| Systolic blood pressure (mmHg) | 120.92 ± 16.89 |
| Diastolic blood pressure (mmHg) | 118.41 ± 17.00 |
| Total Cholesterol (mg/dl) | 201.86 ± 38.67 |
| High-density lipoprotein cholesterol (mg/dl) | 57.39 ± 14.50 |
| Low-density lipoprotein cholesterol (mg/dl) | 121.57 ± 33.83 |
| Triglycerides (mg/dl), median (Q1, Q3) | 98.5 (75.0, 135.0) |
| Diabetes, N (%) | 13 (4) |
| Age at FMP (years) | 51.97 ± 2.87 |
| Ever used hormone therapy, N (%) | 49 (14) |
| Estradiol (pg/mL), median (Q1, Q3) | 27.2 (15.0, 76.7) |
| Abdominal VAT area (cm2), median (Q1, Q3) | 113.0 (78.1, 166.4) |
| Mean of average carotid IMT (mm), median (Q1, Q3) | 0.66 (0.60, 0.73) |
| Mean of common carotid IMT (mm), median (Q1, Q3) | 0.66 (0.62, 0.73) |
| Mean of internal carotid IMT (mm), median (Q1, Q3) | 0.57 (0.50, 0.65) |
Mean ± standard deviation is presented unless specified.
Modified Baecke Scores of Habitual Physical Activity, with higher scores indicating more physical activity.
BMI = body mass index; FMP = final menstrual period; IMT = intima-media thickness; VAT = visceral adipose tissue.
VAT Trajectory over the FMP
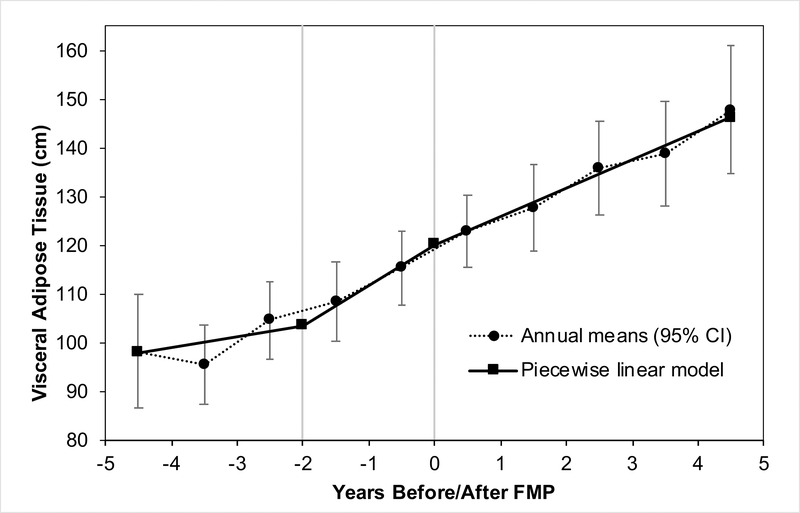
LOWESS suggested a non-linear trajectory of VAT with inflection points at 2 years before the FMP and at the FMP, which divided the VAT trajectory into 3 time segments: segment 1: before 2 years before the FMP, segment 2: between 2 years before the FMP to the FMP, and segment 3: after the FMP. In segments 2 and 3, VAT increased 8.2% and 5.8% per year, respectively, and these estimates remained significant after adjusting for study covariates (Table 2; Figure 2). There was no significant change in VAT within segment 1. VAT increase in segment 2, but not segment 3, was significantly greater than VAT change in segment 1 adjusting for study covariates (model 3). The annual increase in VAT in segments 2 and 3 were not statistically different from each other. Further adjustment for estradiol attenuated the difference between segments 1 and 2, P=0.09 (data not shown). VAT trajectory over the FMP did not differ between White and Black women (P of interaction terms >0.74).
Table 2.
Annual Percentage Change in VAT in Time Segments Relative to FMP
| Model a | Annual Percentage Change in VAT (95% CI) | P-Value for Pairwise Difference | ||||
|---|---|---|---|---|---|---|
| Segment 1 >2 Years Before FMP |
Segment 2 2 Years Before FMP to FMP |
Segment 3 After FMP |
Segment 1 vs. 2 |
Segment 1 vs. 3 |
Segment 2 vs. 3 |
|
| Unadjusted | 0.82 (−2.10 to 3.82) | 8.20 (4.10 to 12.46) | 5.77 (3.69 to 7.89) | 0.01 | 0.01 | 0.35 |
| Model 1 | 3.43 (0.29 to 6.68) | 8.94 (4.84 to 13.19) | 6.52 (4.42 to 8.65) | 0.07 | 0.11 | 0.35 |
| Model 2 | 3.21 (0.09 to 6.42) | 10.44 (6.23 to 14.81) | 5.59 (3.46 to 7.77) | 0.02 | 0.21 | 0.07 |
| Model 3 | 2.27 (−0.19 to 4.79) | 7.86 (4.33 to 11.51) | 4.53 (2.78 to 6.31) | 0.03 | 0.14 | 0.14 |
Model 1: age at FMP, race, study site, and hormone therapy. Model 2: model 1 + physical activity, alcohol consumption, daily calorie intake, and diabetes. Model 3: model 2 + body-mass index.
Bolded estimates indicate P-value < 0.05.
VAT = visceral adipose tissue; FMP = final menstrual period.
Figure 2. Means of Abdominal Visceral Adipose Tissue (VAT) in years around the final menstrual period (FMP).
Figure showing annual mean values compared with estimated values from piecewise linear model of VAT over time since FMP for women from the SWAN Heart Study. Model adjusted for age at FMP, race, study site, hormone therapy, physical activity, alcohol consumption, daily calorie intake, diabetes, and body-mass index (model 3)
Error bars represent 95% CI.
Menopause-related VAT Increase and cIMT
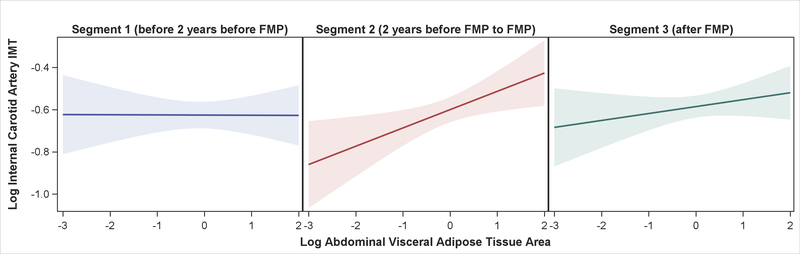
Menopause-related increases in VAT were associated with increases in ICA-IMT but with modest increases in averaged and CCA-IMT. Each 20% increase in VAT in segment 2 was associated with 2.0% increase in ICA-IMT, 0.9% increase in averaged cIMT, and 0.8% increase in CCA-IMT (Table 3). Only the estimate in ICA-IMT of segment 2 remained significant after adjusting for study covariates (Table 3, Figure 3). Associations between VAT and cIMT in segments 1 and 3 were not significant after adjusting for study covariates. Segment-specific difference between segments 1 and 2 in the ICA-IMT analysis was significant. No other segment-specific comparisons were statistically significant.
Table 3.
Percentage Change in Carotid IMT Measures Per 20% Greater VAT by Time Segments
| Carotid-IMT Measure | Model a | Estimate (95% CI) | P-Value for Pairwise Difference | ||||
|---|---|---|---|---|---|---|---|
| Segment 1 | Segment 2 | Segment 3 | Segment 1 vs. 2 | Segment 1 vs. 3 | Segment 2 vs. 3 | ||
| Average Carotid Artery IMT | 1 | 0.59 (−0.04 to 1.23) | 0.88 (0.20 to 1.56) | 0.94 (0.39 to 1.49) | 0.49 | 0.40 | 0.88 |
| 2 | 0.52 (−0.07 to 1.12) | 0.86 (0.21 to 1.51) | 0.72 (0.19 to 1.26) | 0.39 | 0.60 | 0.72 | |
| 3 | 0.08 (−0.62 to 0.79) | 0.41 (−0.37 to 1.19) | 0.25 (−0.43 to 0.95) | 0.44 | 0.68 | 0.70 | |
| Common Carotid Artery IMT | 1 | 0.98 (0.32 to 1.64) | 0.79 (0.08 to 1.51) | 0.79 (0.24 to 1.35) | 0.67 | 0.66 | 1.00 |
| 2 | 0.85 (0.22 to 1.48) | 0.69 (0.01 to 1.38) | 0.54 (0.01 to 1.08) | 0.71 | 0.44 | 0.70 | |
| 3 | 0.21 (−0.50 to 0.93) | 0.16 (−0.64 to 0.96) | −0.19 (−0.87 to 0.49) | 0.90 | 0.33 | 0.41 | |
| Internal Carotid Artery IMT | 1 | 0.54 (−0.47 to 1.55) | 1.95 (0.83 to 3.07) | 1.08 (0.22 to 1.95) | 0.04 | 0.41 | 0.18 |
| 2 | 0.52 (−0.44 to 1.50) | 2.06 (0.98 to 3.16) | 1.01 (0.17 to 1.85) | 0.02 | 0.44 | 0.10 | |
| 3 | −0.01 (−1.13 to 1.11) | 1.60 (0.33 to 2.87) | 0.60 (−0.48 to 1.69) | 0.02 | 0.34 | 0.13 | |
Model 1: main effects of VAT and time segment indicator and their interaction. Model 2: model 1 + age, race, and study site. Model 3: model 2 + smoking, systolic blood pressure, low-density lipoprotein cholesterol, diabetes, and body-mass index.
Bolded estimates indicate P-value < 0.05.
IMT = intima-media thickness; VAT = visceral adipose tissue.
Figure 3. Association Between Abdominal Visceral Adipose Tissue and Internal Carotid Artery IMT by Time Segments.
Figure showing association between abdominal visceral adipose tissue area and internal carotid artery intima-media thickness (both log-transformed) by time segments relative to the FMP for women from the SWAN Heart Study. Model 3: adjusted for age, race, study site, smoking, systolic blood pressure, low-density lipoprotein cholesterol, diabetes, and body-mass index.
Bands represent 95 % CI of the prediction.
FMP = final menstrual period; IMT = intima-media thickness.
Limiting the analysis to segment 2 (n=115), although the interaction term was not significant, the reported increase in ICA-IMT with greater VAT was found to be more pronounced at lower levels of estradiol such that a 20% increase in VAT was associated with a 2.5% (95% CI: 0.50% to 4.5%) increase in ICA-IMT for each one unit decrease in log-estradiol (data not shown).
Sensitivity Analyses
Analyses including women with imputed and observed FMP and women who had 2 VAT measurements resulted in the same conclusions (Supplemental Tables II–V).
DISCUSSION
Using precise data on the timing of the MT, we showed a non-linear increase in VAT as women traverse menopause that accelerated remarkably starting 2 years before the FMP. VAT acceleration was independent of aging, lifestyle factors and overall adiposity. Importantly, menopause-related increases in VAT within 2 years before and up to the FMP was associated with a significant increase in ICA-IMT independent of traditional CVD risk factors and overall adiposity. Results of the current study suggest that the VAT increase during midlife is indeed a menopause-related phenomenon that could contribute to increased risk of subclinical carotid atherosclerosis in women during the MT.
Previous studies showed that premenopausal women gained a significant amount of VAT as they transitioned to postmenopause,10 while others showed no gain mainly after adjusting for age.22 Analysis 11 grouped VAT measurements to the nearest year relative to the FMP and showed that only mean VAT at years 4 and 3 before the FMP differed from mean VAT at the FMP. However, this analytic approach has limited ability to estimate rate of change in VAT relative to FMP timing as we have done in our study. We provided adjusted estimates of change and tested differences in VAT trajectory over time relative to the FMP.
As women traverse menopause, they experience fluctuations in estradiol, a relative domination of testosterone, and increases in follicle-stimulating hormone.23 These hormonal changes favor greater deposition of body fat and greater central adiposity by influencing appetite, energy expenditure, whole-body thermogenesis, and lipoprotein lipase activity.24 Consistent with this, adjusting for estradiol in our analysis attenuated the inflection point of VAT trajectory at 2 years before the FMP suggesting that estradiol may mediate VAT acceleration. However, future studies should formally examine whether changes in estradiol would mediate VAT acceleration during the menopause transition.
As VAT accumulates, it acts as a metabolic organ that delivers excess free fatty acids into the portal circulation and secretes proinflammatory adipocytokines.25 These VAT-derived metabolites are thought to influence atherosclerosis through increasing insulin resistance, inflammation, and blood pressure and viscosity.26 Epidemiologic studies of the link between VAT and atherosclerosis measures report mixed results, with some showing an independent relationship between VAT and measures of subclinical carotid atherosclerosis,5,27 while others showing the relationship to weaken after adjusting for traditional CVD risk factors.28 However, none of these studies focused on ICA-IMT.
It is not obvious why menopause-related VAT increase predicted increased cIMT mainly in the internal, but not the common carotid artery. cIMT seems to be impacted by blood flow velocity, with a faster flow through the carotid artery being associated with a thinner cIMT.29 A lower cerebral vascular resistance is associated with faster blood flow,30 and estrogen may contribute to decreasing this resistance via its vasodilatory effects.31 Interestingly, increase in circulating estrogen level in women receiving hormone therapy was associated with faster blood flow within the internal, but not the common carotid artery.32 Thus, it is possible that estrogen decline around FMP 23 may reduce flow velocity through the internal carotid artery creating a milieu where excess VAT can synergistically exert its atherosclerotic effects with a higher affinity predominantly on the internal carotid artery. Our exploratory analysis showed that VAT had a larger effect on ICA-IMT at lower levels of estradiol. However, this hypothesis needs to be rigorously tested in future studies. Additionally, using a larger sample size, future research should determine whether increases in central adiposity during the menopause transition predicts future atherosclerotic cerebrovascular disease.
By midlife, >80% of women have one or more traditional CVD risk factor.33 Moreover, adverse changes in CVD risk factors and vasculature begin to accumulate during the MT independent of aging.12,34–36 These results and our current findings highlight the importance of frequent monitoring of CVD risk factors early in the MT as women can be counseled to stress lifestyle changes.37 Results from meta-analyses showed that lifestyle intervention programs including aerobic exercise with or without hypocaloric diets reduced CT scan-measured VAT by 30 cm2.38–40 Importantly, healthy lifestyle during midlife is prospectively associated with less carotid atherosclerosis.41 Future research should assess whether lifestyle factors targeting central adiposity in midlife women is associated with favorable cardiovascular outcomes later in life.
Study Limitations
The excluded women were healthier compared with the women in the current analysis. It is therefore expected that our results may have been overestimated. However, sensitivity analyses run after adding back 62% of the excluded women through multiply imputing their FMP dates resulted in similar conclusions. Although we used repeated measures data in VAT-cIMT associations, the analysis was cross-sectional since VAT and cIMT were measured on the same occasions. Therefore, temporality in VAT-cIMT associations cannot be established. Moreover, our results may only be generalizable to populations similar to SWAN Heart women.
Conclusions
Using a well-characterized woman traversing menopause, we showed that women experience an accelerated increase in VAT starting 2 years before the FMP. Additionally, the increase in VAT between 2 years before the FMP up to the FMP may predispose women to subclinical carotid atherosclerosis. The results underscore the importance of frequent and timely monitoring of CVD risk factors including central adiposity and stressing intensive lifestyle modifications in women traversing menopause.
Supplementary Material
Acknowledgements:
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Sources of funding: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
SWAN Heart was supported by grants from the NHLBI (HL065581, HL065591).
Footnotes
Financial Disclosures/Conflict of interest: None reported.
An abstract related to this study was presented in the American Heart Association EPI|Lifestyle Scientific Sessions, 2019. The related abstract was published in Circulation: Samargandy S, Matthews Karen A, Brooks Maria M, et al. Abstract 028: Increase in Abdominal Visceral Adipose Tissue Accelerates Two Years Prior to Menopause: The Study of Women’s Health Across the Nation (SWAN) Heart. Circulation. 2019;139(Suppl_1):A028-A028.
REFERENCES
- 1.Sun Y, Liu B, Snetselaar LG, et al. Association of Normal-Weight Central Obesity With All-Cause and Cause-Specific Mortality Among Postmenopausal Women. JAMA Network Open. 2019;2(7):e197337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring, Md). 2007;15(1):216–224. [DOI] [PubMed] [Google Scholar]
- 3.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. The Journal of clinical endocrinology and metabolism. 2007;92(3):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Hong X, Wilker E, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196(2):590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gast KB, den Heijer M, Smit JW, et al. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: The NEO study. Atherosclerosis. 2015;241(2):547–554. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature Reviews Immunology. 2011;11(2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotani K, Tokunaga K, Fujioka S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1994;18(4):207–202. [PubMed] [Google Scholar]
- 9.van der Leeuw J, Wassink AM, van der Graaf Y, Westerveld HE, Visseren FL. Age-related differences in abdominal fat distribution in premenopausal and postmenopausal women with cardiovascular disease. Menopause. 2013;20(4):409–417. [DOI] [PubMed] [Google Scholar]
- 10.Abdulnour J, Doucet E, Brochu M, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause (New York, NY). 2012;19(7):760–767. [DOI] [PubMed] [Google Scholar]
- 11.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. International journal of obesity (2005). 2008;32(6):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause (New York, NY). 2013;20(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen I, Powell LH, Jasielec MS, Kazlauskaite R. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity (Silver Spring, Md). 2015;23(2):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 2012;15(2):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness. Circulation. 2007;115(4):459–467. [DOI] [PubMed] [Google Scholar]
- 16.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB. Carotid-Wall Intima–Media Thickness and Cardiovascular Events. New England Journal of Medicine. 2011;365(3):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watase H, Sun J, Hippe DS, et al. Carotid Artery Remodeling Is Segment Specific: An In Vivo Study by Vessel Wall Magnetic Resonance Imaging. Arteriosclerosis, thrombosis, and vascular biology. 2018;38(4):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen QM, Toprak A, Xu JH, Srinivasan SR, Chen W, Berenson GS. Progression of segment-specific carotid artery intima-media thickness in young adults (from the Bogalusa Heart Study). The American journal of cardiology. 2011;107(1):114–119. [DOI] [PubMed] [Google Scholar]
- 19.Sowers MFR, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathology. 1st ed. USA: Academic Press; 2000:175–188. [Google Scholar]
- 20.Little RJ, Rubin DB. Statistical analysis with missing data. Vol 793: John Wiley & Sons; 2019. [Google Scholar]
- 21.Wildman RP, Janssen I, Khan UI, et al. Subcutaneous adipose tissue in relation to subclinical atherosclerosis and cardiometabolic risk factors in midlife women. The American journal of clinical nutrition. 2011;93(4):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trikudanathan S, Pedley A, Massaro JM, et al. Association of female reproductive factors with body composition: the Framingham Heart Study. The Journal of clinical endocrinology and metabolism. 2013;98(1):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randolph JF, Zheng H, Sowers MR, et al. Change in Follicle-Stimulating Hormone and Estradiol Across the Menopausal Transition: Effect of Age at the Final Menstrual Period. The Journal of Clinical Endocrinology & Metabolism. 2011;96(3):746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine reviews. 2013;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(6):1039–1049. [DOI] [PubMed] [Google Scholar]
- 26.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological reviews. 2013;93(1):359–404. [DOI] [PubMed] [Google Scholar]
- 27.Liu KH, Chan YL, Chan JC, Chan WB. Association of carotid intima-media thickness with mesenteric, preperitoneal and subcutaneous fat thickness. Atherosclerosis. 2005;179(2):299–304. [DOI] [PubMed] [Google Scholar]
- 28.Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB. Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke; a journal of cerebral circulation. 2007;38(9):2422–2429. [DOI] [PubMed] [Google Scholar]
- 29.Frauchiger B, Schmid HP, Roedel C, Moosmann P, Staub D. Comparison of carotid arterial resistive indices with intima-media thickness as sonographic markers of atherosclerosis. Stroke; a journal of cerebral circulation. 2001;32(4):836–841. [DOI] [PubMed] [Google Scholar]
- 30.Kang Y, Park HJ, Kang MI, et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15(6):R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santizo R, Baughman VL, Pelligrino DA. Relative contributions from neuronal and endothelial nitric oxide synthases to regional cerebral blood flow changes during forebrain ischemia in rats. Neuroreport. 2000;11(7):1549–1553. [PubMed] [Google Scholar]
- 32.Krejza J, Mariak Z, Huba M, Wolczynski S, Lewko J. Effect of Endogenous Estrogen on Blood Flow Through Carotid Arteries. Stroke; a journal of cerebral circulation. 2001;32(1):30–36. [DOI] [PubMed] [Google Scholar]
- 33.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54(17):1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Khoudary SR, Thurston RC. Cardiovascular Implications of the Menopause Transition: Endogenous Sex Hormones and Vasomotor Symptoms. Obstet Gynecol Clin North Am. 2018;45(4):641–661. [DOI] [PubMed] [Google Scholar]
- 35.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. The Journal of clinical endocrinology and metabolism. 2012;97(12):4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samargandy S, Matthews KA, Brooks MM, et al. Arterial Stiffness Accelerates Within 1 Year of the Final Menstrual Period: The SWAN Heart Study. Arteriosclerosis, thrombosis, and vascular biology. 2020;40(4):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown HL, Warner JJ, Gianos E, et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137(24):e843–e852. [DOI] [PubMed] [Google Scholar]
- 38.Verheggen RJHM, Maessen MFH, Green DJ, Hermus ARMM, Hopman MTE, Thijssen DHT. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obesity Reviews. 2016;17(8):664–690. [DOI] [PubMed] [Google Scholar]
- 39.Vissers D, Hens W, Taeymans J, Baeyens J-P, Poortmans J, Van Gaal L. The Effect of Exercise on Visceral Adipose Tissue in Overweight Adults: A Systematic Review and Meta-Analysis. PloS one. 2013;8(2):e56415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazlauskaite R, Karavolos K, Janssen I, et al. The association between self-reported energy intake and intra-abdominal adipose tissue in perimenopausal women. Journal of obesity. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Jackson EA, Karvonen-Gutierrez CA, et al. Healthy Lifestyle During the Midlife Is Prospectively Associated With Less Subclinical Carotid Atherosclerosis: The Study of Women’s Health Across the Nation. Journal of the American Heart Association. 2018;7(23):e010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.