Abstract
Schizophrenia is a devastating mental illness and its effective treatment is among the most challenging issues in psychiatry. The symptoms of schizophrenia are heterogeneous ranging from positive symptoms (e.g., delusions, hallucinations) to negative symptoms (e.g., anhedonia, social withdrawal) to cognitive dysfunction. Antipsychotics are effective at ameliorating positive symptoms in some patients; however, they are not reliably effective at improving the negative symptoms or cognitive impairments. The inability to address the cognitive impairments is a particular concern since they have the greatest long-term impact on functional outcomes. While decades of research have been devoted to the development of pro-cognitive agents for schizophrenia, to date, no drug has been approved for clinical use. Converging behavioral, neurobiological, and genetic evidence led to the identification of the α7-nicotinic acetylcholine receptor (α7-nAChR) as a therapeutic target several years ago and there is now extensive preclinical evidence that α7-nAChR ligands have pro-cognitive effects and other properties that should be beneficial to schizophrenia patients. However, like the other pro-cognitive strategies, no α7-nAChR ligand has been approved for clinical use in schizophrenia thus far. In this review, several topics are discussed that may impact the success of α7-nAChR ligands as pro-cognitive agents for schizophrenia including the translational value of the animal models used, clinical trial design limitations, confounding effects of polypharmacy, dose-effect relationships, and chronic versus intermittent dosing considerations. Determining the most optimal pharmacologic strategy at α7-nAChRs: agonist, positive allosteric modulator, or potentially even receptor antagonist is also discussed.
Keywords: Cholinergic, pro-cognitive, psychosis, cognition, executive function, attention
1. Introduction
Schizophrenia is a debilitating mental illness characterized by positive symptoms (e.g., hallucinations, delusions), negative symptoms (e.g., depressed mood, anhedonia, social withdrawal) and cognitive impairments (e.g., deficits in information processing, attention, working memory, executive function, Green and Braff, 2001). Among these diverse symptoms, cognitive impairment is a core feature of schizophrenia that often appears prior to the onset of psychotic symptoms, it persists throughout the course of the illness, and it has the greatest long-term impact on functional outcomes (reviewed, Kahn and Keefe, 2013; Green and Harvey, 2014, Kahn, 2019). Unfortunately, the most commonly prescribed treatments for schizophrenia, the antipsychotics, while effective at improving positive symptoms in some patients, are not reliably effective at improving cognitive function. This unmet medical need has been an important focus of drug discovery efforts in both academia and the pharmaceutical industry for several decades; however, to date no pro-cognitive agent has been approved for clinical use in schizophrenia.
A key challenge to developing novel treatments for the cognitive dysfunction in schizophrenia is the complex and poorly understood etiology and pathophysiology of the illness. Multiple neurotransmitter systems have been implicated in the illness (Goff and Wine, 1997; Kapur and Mamo, 2003) including dopaminergic, serotoninergic, glutamatergic, adrenergic, and cholinergic pathways and accordingly, new compounds designed to target these various systems have been developed and evaluated. Cholinergic targets, particularly nicotinic acetylcholine receptors (nAChRs) have been a focus of a number of drug discovery programs over the last 20–25 years based on multiple lines of behavioral, neurobiological, and genetic evidence. From the behavioral perspective, a remarkable observation in schizophrenic patients is their especially heavy abuse of tobacco products. According to the National Institute on Drug Abuse (NIDA, 2020) tobacco smoking rates in schizophrenia patients range as high as 70–85%, which is dramatically higher than the general population (~19–20%) and significantly higher than in any other mental illness (George and Krystal, 2007). Smokers with schizophrenia have also been documented to extract more nicotine per cigarette and to smoke a higher number of cigarettes per day compared to smokers in the general population (Olincy et al., 1997; Strand and Nybäck, 2005). It has been suggested that this high level of nicotine consumption may represent an attempt of schizophrenia patients to self-medicate the cognitive symptoms, particularly deficits of information processing and attention (Olincy et al., 1997; Leonard et al., 2007).
2. Focus on α7-nAChRs
In drug discovery programs for neuropsychiatric illnesses, the heteromeric α4β2 and homomeric α7-nAChRs have been the most commonly targeted nAChR subtypes to date since they are the most predominant subtypes found in the mammalian brain. However, the α7-nAChR has been more commonly targeted for the cognitive deficits in schizophrenia. This is likely based, in part, on several factors including postmortem evidence of α7-nAChR deficits in the frontal cortex and hippocampus of schizophrenic patients (Guan et al., 1999) and linkage analysis implicating chromosome 15q14 (the region that includes the α7-nAChR gene). Polymorphisms in the core promoter of the α7-nAChR gene (CHRNA7; GeneBank accession no. Z23141) have been associated with reduced inhibition of the P50 evoked response to repeated auditory stimuli in schizophrenic patients, which is indicative of sensory gating abnormalities (reviewed, Freedman et al., 2003). α7-nAChR deficits may also contribute to abnormalities of smooth pursuit eye movements, sustained attention, and other domains of cognition in schizophrenia (reviewed Martin et al., 2004). In addition to schizophrenia, the CHRNA7 gene is also linked to multiple disorders where cognitive deficits are present including bipolar disorder, autism spectrum disorders, attention deficit hyperactivity disorder, Alzheimer disease, epilepsy, and sensory processing deficits (reviewed in Corradi and Bouzat, 2016).
The information in the paragraph above regarding the importance of α7-nAChR as a potential therapeutic target for the cognitive defects in schizophrenia is also supported by extensive preclinical evidence. From a neurobiological and neuropharmacological perspective, α7-nAChRs modulate multiple (cognition-related) processes in neurons that are calcium-dependent including neurotransmitter release (McGehee et al., 1995; Gray et al., 1996), postsynaptic signaling (Chang and Berg, 1999; Hefft et al., 1999) and neuronal survival (Messi et al., 1997; Berger et al., 1998). Moreover, α7-nAChRs are also abundant in regions of the brain that are important for learning and memory and executive function such as the hippocampus and prefrontal cortex (Gotti et al., 2007). In addition, agonists of α7-nAChRs have been shown to increase the phosphorylation of ERK and CREB (signaling pathways linked to long-term potentiation and memory formation) in the rodent brain (Bitner et al., 2007, 2010). There is also extensive evidence that α7-nAChR ligands improve behavioral processes that are relevant to schizophrenia (see Fig 1) such as auditory-evoked gating and prepulse inhibition in rodents, as well as multiple domains of cognition including attention, working memory, reference memory, social cognition, and executive function in rodent models as well as non-human primates (see reviews, Young and Geyer, 2013; Freedman, 2014; Bertrand and Terry, 2018). Table 1 provides a list of representative α7-nAChRs ligands that have been developed as pro-cognitive agents for potential use in schizophrenia and other disorders of cognition. Although not all-inclusive, Table 1 includes α7-nAChR agonists, partial agonists, and positive allosteric modulators (PAMs), a summary of some of the positive behavioral properties associated with each compound, and representative references.
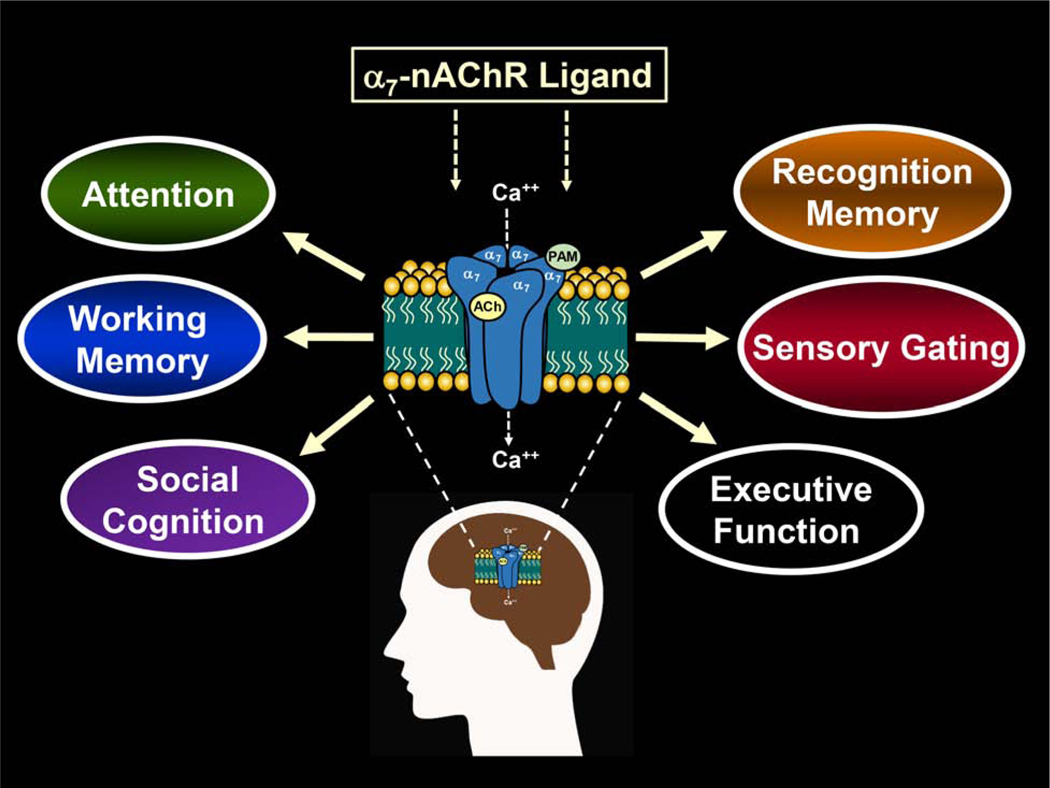
Fig 1.
Diagram illustrating several domains of cognition and other behaviors often targeted in drug discovery programs for schizophrenia and other neuropsychiatric disorders. The homomeric, low affinity α7-nicotinic acetylcholine receptor (α7-nAChR) is abundant in regions of the brain (e.g., hippocampus, prefrontal cortex) that are important for cognitive function. The receptor consists of five subunits arranged around a central channel that opens when endogenous ligands such as acetylcholine or exogenous ligands (nicotine) bind at the orthosteric site allowing cations (e.g., Ca++) to flow through the channel into the neuron causing depolarization. Allosteric sites are the target of positive allosteric modulators (PAMs) and they are located at a site which is distinct from the orthosteric where they serve to indirectly influence (modulate) the effects of the agonist.
Table 1.
In vivo Pharmacological Effects of α7-nAChR Ligands
| Compound Name | α7 nAChR activity & other actions | Cognitive Domain Enhanced | Additional Behavioral Improvements | References |
|---|---|---|---|---|
| ABBF | α7 full agonist & 5-HT3 antagonist | object and social recognition, working memory | Boess et al., 2007 | |
| ABT-107 | α7 full agonist | attention, working memory, social recognition | auditory gating | Bitner et al., 2010; Bordia et al., 2015; Radek et al., 2012 |
| A-582941 | α7 full agonist | working memory, social recognition, inhibitory avoidance | sensory gating | Tietje et al., 2008 |
| AQW051 | α7 partial agonist | object and social recognition, spatial reference memory | Feuerbach et al., 2015 | |
| AVL-3288 (Compound 6, CCMI or XY4083) | α7 Type I PAM | object recognition, social recognition, working memory, executive function | auditory gating, conditioned avoidance responding | Ng et al., 2007; Nikiforuk et al., 2015 |
| BMS-933043 | α7 partial agonist | object recognition, working memory, executive function | auditory gating | Bristow et al., 2016 |
| BMS-902483 | α7 partial agonist & 5-HT3 antagonist | object recognition, executive function | auditory gating | Pieschl et al., 2017 |
| Compound 7z | α7 Type I PAM | object recognition | Hogenkamp et al., 2013 | |
| DMXB-A (GTS-21) | α7 partial agonist & 5-HT3 antagonist | attention, object recognition, spatial reference memory, working memory | sensorimotor gating | Meyer et al, 1997; Callahan et al., 2014; Jones et al., 2014 |
| EVP-6124 (encenicline) | α7 partial agonist & 5-HT3 antagonist | object recognition | Prickaerts et al., 2012 | |
| EVP-5141 | α7 full agonist & 5-HT3 antagonist | object and social recognition, working memory | Boess et al., 2013 | |
| JNJ-39393406 | α7 Type I, Type II PAM | object recognition, executive function | auditory gating | Winterer et al., 2013 |
| Lu AF58801 | α7 Type I PAM | object recognition | Eskildsen et al., 2014 | |
| NS1738 | α7 Type I PAM | object and social recognition, spatial reference memory | Marcus et al., 2016; Timmermann et al., 2007 | |
| PAM-2 | α7 Type II PAM | object recognition, executive function | social interaction model | Potasiewicz et al., 2017 |
| PNU 120596 | α7 Type II PAM | recognition memory, spatial learning, working memory, executive function | auditory gating | Dunlop et al., 2009; Nikiforuk et al., 2015; Stevens et al., 2015 |
| PNU-282987 | α7 full agonist | recognition memory | conditioned avoidance responding | McLean et al., 2016; Marcus et al., 2016 |
| RG 3487 | α7 partial agonist & 5-HT3 antagonist | attention, object recognition, spatial reference memory, executive function | sensorimotor gating | Wallace et al., 2011 |
| SEN-12333 | α7 full agonist & histamine H3 antagonist | object recognition | sensorimotor gating | Roncarati et al., 2009 |
| SSR-180711 | α7 partial agonist | recognition memory, working memory, novelty discrimination | latent inhibition | Pichat et al., 2007; Barak et al., 2009 |
| TC-5619 | α7 full agonist | object recognition | sensorimotor gating | Hauser et al., 2009 |
| Tropisetron | α7 partial agonist & 5-HT3 antagonist | attention, object recognition, working memory, spatial reference memory | sensorimotor gating | Callahan et al., 2017; Hashimoto et al., 2005, 2006; Kohnomi et al., 2010; Pitsikas et al., 1997 |
3. Clinical Trial Failures
Despite extensive preclinical evidence to support the pro-cognitive effects of α7-nAChR ligands noted above (and summarized in Table 1) and positive results in some early (Phase I and II) clinical trials, to date, no compound has met the primary objective of cognitive improvement in schizophrenic patients in a large phase III, double blind, placebo controlled clinical trial or unanticipated side effects emerged (see review, Tregellas and Wylie, 2019). The failure of pro-cognitive agents in schizophrenia clinical trials have reduced the enthusiasm of pharmaceutical companies and, unfortunately, many have abandoned this line of research. While it is possible that the lack of robust (pro-cognitive) efficacy or side effect burden may represent real limitations of α7-nAChR ligands, it should be noted, that given the number of compounds that have been developed, only one agent have been evaluated in a large scale Phase III clinical trial in schizophrenia patients (Encenicline-EVP-6124, see Table 2). Moreover, there is an increasing amount of discussion about possible limitations of both the preclinical and clinical studies conducted to date that may have resulted in the so-called “treatment failures” of pro-cognitive agents. Some of these potential limitations are listed in Table 3. See also several recent reviews (Bertrand and Terry, 2018, Terry and Callahan, 2019; Tregellas and Wylie, 2019) on this subject.
Table 2.
α7-nAChR ligands that have been evaluated in human clinical trials for pro-cognitive effects
| Compound Name | α7 nAChR activity & other actions | Clinical Stage | Clinical Endpoints | References |
|---|---|---|---|---|
| ABT-126 | Partial agonist | Phase II | Cognition, Negative Symptomology | Haig et al., 2016; Haig et al., 2016; Haig et al., 2018 |
| AQW051 | Partial agonist | Phase II | Cognition, fMRI Brain Activation | Barch et al., 2016 |
| AVL-3288 | PAM | Phase Ib | Cognition, Auditory Sensory Gating Negative Symptomology | Gee et al., 2017; Kantrowitz et al., 2020 |
| Cytidine 5’-diphosphocholine (CDP-choline) | Full agonist | Phase II | Cognition, Auditory Sensory Gating | Knott et al., 2015; Aidelbaum et al., 2018; Choueiry et al., 2019 |
| DMXB-A (GTS-21) | Partial agonist | Phase II | Cognition, Auditory Sensory Gating, Negative Symptomology | Olincy et al., 2006; Freedman et al., 2008 |
| Encenicline (EVP-6124) | Partial agonist/5-HT3 antagonist | Phase III | Cognition, Negative Symptomology | Keefe et al., 2015 |
| Galantamine | PAM/acetylcholinesterase inhibitor | Phase II | Cognition, Auditory Sensory Gating, Negative Symptomology | Choueiry et al., 2019; Buchanan et al., 2017 |
| JNJ-39393406 | PAM | Phase Ib | Cognition,Auditory Sensory Gating, Smoking Cessation | Winterer et al., 2013; Perkins et al., 2018 |
| RG3487 | Partial agonist/5-HT3 antagonist | Phase II | Cognition, Negative Symptomology | Umbricht et al., 2014 |
| Tropisetron | Partial agonist/5-HT3 antagonist | Phase II | Cognition, Auditory Sensory Gating, Negative Symptomology | Shiina et al., 2010; Zhang et al., 2012; Noroozian et al., 2013 |
| TC-5619 | Full agonist | Phase II | Cognition, Negative Symptomology | Lieberman et al., 2013 |
Table 3.
Limitations of Preclinical and Clinical Studies of pro-cognitive agents to date
| Preclinical | Clinical | |
|---|---|---|
| Translational challenges due to the poor understanding of the etiology and pathophysiology of schizophrenia | Studies often underpowered due to the inclusion of cognitively “normal” patients | |
| Overreliance on rodent models | Polypharmacy and drug exposure history not properly addressed | |
| Overreliance on acute dose-effect analyses | Unanticipated practice effects masking a positive outcomes | |
| Pro-cognitive agents not evaluated in test subjects that have been chronically treated with antipsychotic. | Chronicity of the illness not taken into account. Recently diagnosed and first episode patients should be evaluated. | |
| Pro-cognitive agents not evaluated in test subjects that have been chronically treated with nicotine or nicotine plus antipsychotics | Inclusion of inexperienced trial sites and focus on volume of patients enrolled as opposed to the quality of the recruitment sites |
4. Translational Gaps and Overreliance on Rodent Models
The unfavorable results of clinical trials for pro-cognitive agents in schizophrenia described above have led to questions about the “translational validity” of animal models used in preclinical studies (see Lewis et al., 2018). In drug discovery research for schizophrenia and other neuropsychiatric disorders, addressing the translational elements, face, construct, and predictive validity in animal models is particularly challenging. In this context, multiple challenges include the subjective nature of many of the human symptoms, the lack of biomarkers and objective diagnostic tests, and our relatively poor understanding of the neurobiology and genetics of neuropsychiatric disorders (see review Nestler and Hyman, 2010; Monteggia et al., 2018). One of the goals of the Research Domain Criteria (RDoC) paradigm launched in 2010 by the National Institute of Mental Health (NIMH) was to improve translation between animal experiments and clinical studies in psychiatry research. The basic concept in the preclinical realm was to encourage basic scientists to identify molecular or neural mechanisms (or neural circuitry) that contributes to specific domains of a mental function rather than creating animal models of diseases. In the clinical realm, researchers were encouraged to conceptualize normal human behavior, emotion, and cognition as dimensional, with mental illnesses as dimensional extremes as opposed to being restricted by DSM diagnostic categories (see Morris and, Cuthbert, 2012; Ross and Margolis, 2019). However, more that 10 years after the introduction of the RDoC paradigm, it is unclear if it has improved research progress in psychiatry especially in the drug discovery arena and its clinical relevance is increasingly being questioned (see Carpenter, 2016; Ross and Margolis, 2019).
Regarding translational validity, rodent models are undoubtedly important in basic research for testing disease-related hypotheses and the early evaluations of novel therapeutic agents, however, it is likely they have been relied upon too much in neuropsychiatric drug discovery especially at the later preclinical stages of drug development. Compared to humans, the behavioral repertoire of rodents is quite limited and, while debated (see, Laubach et al., 2018), there are major anatomical differences in their brains, most notably, the development of cortical regions of the forebrain, particularly the dorsolateral prefrontal cortex (DLPFC). This portion of the brain of humans and more advanced non-human primates (e.g., macaques) has been implicated specifically in the most complex cognitive processes such as working memory, sustained attention, decision taking, and executive function (reviewed, Barbey et al., 2013).
Regarding, α7-nAChRs, there are also significant differences in the genetics, pharmacology, biophysical properties, and neuronal localization of α7-nAChRs between rodents and humans that could underlie different outcomes in preclinical and clinical trial evaluations of α7-nAChRs ligands (reviewed Bertrand and Terry, 2018). From a genetics standpoint, the recent findings of Yin et al., 2017 are particularly notable. In humans with 15q13.3 microdeletion syndrome, caused by heterozygous deletions involving the CHRNA7 gene, behavioral abnormalities often observed in neuropsychiatric conditions such as schizophrenia and autism were observed, whereas Chrna7 knockout mice did not exhibit similar neurobehavioral phenotypes. From a pharmacological perspective, the effects of specific agonists in vitro differ between human and rodent sequences coding for α7-nAChRs. For example, the partial agonist DMXB-A (GTS-21) activates the rat α7-nAChR to a maximal response greater than twice that of the human α7-nAChR, and the Ki of GTS-21 at the rat receptor is roughly an order of magnitude less than at the human receptor (Meyer et al., 1998), suggesting that similar serum levels might have disparate effects between the species. Finally, differences in the synaptic receptor expression between rodents and humans may be relevant as suggested by a recent study in nonhuman primates where postsynaptic localization of α7-nAChRs on spines were demonstrated in glutamatergic synapses of layer III dorsolateral prefrontal cortex (Yang et al., 2013). Most physiological studies in rodent frontal cortex, in contrast, have demonstrated presynaptic α7-nAChR actions and it has been suggested that spine α7-nAChRs are not prevalent or only have subtle effects on neuronal physiology in rodents compared to primates. Collectively, the information discussed here would appear to justify a renewed interest in the use of nonhuman primate species (see Monteggia et al., 2018) in neuropsychiatry and drug discovery research, given their richer behavioral repertoire and more homologous brain anatomy with humans compared to rodents.
5. Polypharmacy and Drug Exposure History
In both preclinical and clinical evaluations of α7-nAChR ligands, it is uncommon for the subject of polypharmacy and chronic antipsychotic drug history to be adequately addressed. Clearly, there are practical limitations in clinical trials, but the patient’s treatment history (which in many cases consists of multiple years of antipsychotic treatment) should be more carefully considered, not just concomitant antipsychotic treatment at the time of the clinical trial. Antipsychotics are well documented to have a variety of chronic effects on the mammalian brain including alterations of neurotransmitter receptor expression and neural plasticity (reviewed, Morrison and Murray, 2018), i.e., effects that could influence the response to a novel α7-nAChR ligand. Interestingly, guidelines related to polypharmacy and concomitant drug exposure have been developed for studies designed to evaluate potential pro-cognitive agents in schizophrenia trials. For example, a workshop on clinical trial design for evaluating cognitive enhancing drugs for schizophrenia was held in 2004 and it included experts from the FDA, NIMH, and scientists from academia and the pharmaceutical industry (see Buchanan et al., 2005). Among the various guidelines developed, it was recommended that polypharmacy (treatment with multiple antipsychotics) and combining a putative cognitive-enhancing agent with an antipsychotic with high affinity for the targeted receptor be avoided. However, it is unclear how closely such policies have been followed.
In preclinical evaluations of potential pro-cognitive agents for potential use in schizophrenia, the concomitant administration of antipsychotics has only rarely been done and when it has, the antipsychotic has most commonly been administered acutely (e.g., Marquis et al., 2011). We recently conducted a series of experiments in rats where the α7-nAChR partial agonist tropisetron was administered to rats that had been exposed to either risperidone or quetiapine for 30 or 90 days then tested them in a novel object recognition task (Poddar et al., 2018). Tropisetron markedly improved NOR performance in rats treated with either antipsychotic for 30 or 90 days indicating that in this particular case, the antipsychotic treatment history did not interfere with the pro-cognitive effect of tropisetron. Thus, α7-nAChR ligands like tropisetron may have potential as adjunctive medications in schizophrenia since the pro-cognitive effect was maintained in the presence of chronic antipsychotic treatment. However, in this study, tropisetron was administered acutely and future studies would need to be conducted to determine if this pro-cognitive effect of tropisetron is lasting.
Other factors related to polypharmacy and drug exposure history that have not been adequately addressed are how chronic nicotine exposure or the combination of chronic nicotine exposure and antipsychotic treatment might affect the efficacy of a pro-cognitive agent. Given the well-documented chronic effects of nicotine on nAChR expression (see Lewis and Picciotto 2013 for review), and the aforementioned high smoking rates in schizophrenia, this could certainly be an important consideration when evaluating an α7-nAChR ligand for pro-cognitive effects. Levin and colleagues (see review, Levin and Rezvani, 2007) have performed some experiments in rodents to investigate nicotinic interactions with antipsychotic drugs and cognitive function. For example, they have shown that nicotine and some nicotine agonists can reduce cognitive impairments caused by some antipsychotic drugs. In other studies, they have shown that nicotine-induced cognitive improvements were attenuated by the some antipsychotics (e.g., clozapine). However, the specific questions raised above (i.e., how chronic nicotine exposure or the combination of chronic nicotine exposure and antipsychotic treatment might affect the efficacy of a pro-cognitive agent including an α7-nAChR ligand) have not been rigorously investigated either in animal models or in clinical studies.
6. Pro-cognitive drug dose, frequency of administration, and duration of treatment
Another potential limitation of many clinical studies conducted to date to evaluate novel pro-cognitive agents was the choice of dose, the frequency of administration, and the duration of treatment. Most of the rodent studies (where robust cognitive effects were observed) have employed acute or sub-acute dosing of α7-nAChR ligands, which contrasts with most of the clinical trials that were conducted over several weeks or months. All neuronal nAChRs (including α7-nAChRs) become temporarily inactive after prolonged exposure to an agonist (Quick and Lester 2002), thus the repeated administration of α7-nAChR agonists in the clinical studies may have resulted in receptor desensitization, or potentially even functional antagonism. To support this argument are the disparate effects of the immediate and slow-release versions of DMXB-A (GTS-21) observed in clinical trials. Whereas, the immediate release formulation (which was rapidly absorbed, but quickly cleared) improved cognition and P50 sensory gating in schizophrenic patients, the slow-release version was not effective (Olincy et al., 2006; Freedman et al., 2008; Kem et al., 2018). Another emerging hypothesis is that low concentrations of α7-nAChR ligands may be more effective than higher concentrations, as the latter will maintain receptors in a desensitized and unresponsive state (see Tregellas and Wylie, 2019). In many of the more recent animal studies, the greatest response to a nAChR agonist was observed with a low drug dose and increasing the dose often produced a decreasing effect, in an inverted U-shaped response curve, which is thought to be due to receptor desensitization. A particularly notable example was a non-human primate study where, a low dose of an α7-nAChR agonist (PHA543613) facilitated neuronal activity in the prefrontal cortex and improved performance of a spatial working memory task, whereas higher doses were not effective (Yang et al., 2013).
The ability to select the proper dose and frequency of administration of an α7-nAChR ligand for optimal target engagement could possibly be improved if functional biomarkers were identified for use in both preclinical and clinical studies. Noninvasive neuroimaging methods such as functional magnetic resonance imaging (fMRI) may be able to facilitate the identification of biomarkers since they can be used to investigate neural circuitry alterations that underlie symptoms of schizophrenia as well as how medications affect this neural circuitry. Here, a biomarker that can be linked to a disease mechanism is categorized as a type I biomarker and a biomarker that can be linked to a treatment response is categorized as a type II biomarker (see Wylie et al., 2016 for review). Interestingly, Tregellas and colleagues, using fMRI, linked hippocampal hyperactivity to smooth pursuit eye movement (SPEM) deficits in schizophrenia patients. The findings revealed a link between eye-tracking abnormalities and a hypothesized disease mechanism, thereby potentially qualifying hippocampal hyperactivity during SPEM as a type I biomarker. Additional studies in schizophrenia patients demonstrated that nicotine and the α7-nAChR partial agonist DMXB-A could normalize hippocampal hyperactivity during SPEM, suggesting that SPEM during fMRI could also represent a potential type II biomarker of treatment response (see Wylie et al., 2016; Tregellas and Wylie, 2019). In summary, while the use of fMRI for the development of biomarkers for schizophrenia and other neuropsychiatric disorders is in the early stages, it has the potential to facilitate drug development by improving the translation from animal models to the clinical realm as well to inform investigators as to the best dosing strategies to optimize target engagement.
7. Optimizing α7-nAChR activity
A large-scale effort to overcome the challenges related to nAChR desensitization and the administration of orthosteric agonists and partial agonists has been the development of positive allosteric modulators (PAMs). PAMs are thought to bind to sites that are distinct from the well-conserved (orthosteric) agonist binding domains and they require the presence of the endogenous ligand to increase receptor activity. Two date, two types of PAMs have been developed, designated Type I and Type II. Type I PAMs are defined as molecules that predominately affect the apparent peak current, agonist sensitivity, and Hill coefficient, but not the receptor desensitization profile. Type II PAMs possess the aforementioned properties described for Type I PAMs as well as the ability to modify the desensitization profile of agonist responses (see Bertrand and Gopalakrishnan, 2007). It has been argued that Type II PAMs (compared to Type I PAMs) are less likely to induce tolerance, which may occur after the chronic administration of nAChR agonists, whereas, Type I PAMs, may have the advantage (compared to Type II PAMs) of minimizing the potential for calcium induced cytotoxicity (Ng et al., 2007, see also Nikiforuk et al., 2015). A large number of PAMs from both classes have been developed with pro-cognitive properties in animal models (see Table 1); however, to date only a few clinical trials have been conducted (or are underway) with α7-nAChR PAMs (i.e., AVL-3288, galantamine, JNJ-39393406).
Finally, the classical view that nAChR stimulation is the key action responsible for the pro-cognitive effects of α7-nAChRs ligands may require additional consideration given the observations that low doses of the selective α7-nAChR antagonist methyllycaconitine (MLA) can (in some cases) improve cognition in animal models (Hahn et al, 2011; Burke et al., 2014). and facilitate LTP induction in hippocampal region CA1 in rats (Fujii et al., 2000). More recently, low concentrations/doses of MLA exerted surprising (positive) effects in several model systems. Specifically, in electrophysiological experiments, low concentrations of MLA potentiated receptor responses to acetylcholine in human α7-nAChR-transfected oocytes, enhanced long term potentiation (LTP) in rat hippocampal slices, increased hippocampal glutamate efflux in microdialysis experiments in rats, and improved the acquisition of a novel object recognition task in rats (van Goethem et al., 2019). Interestingly, the nonselective nAChR antagonist mecamylamine (in some cases) has also been found to exert pro-cognitive effects in working memory tasks in both rodents and monkeys as well as a recognition memory task in humans with attention deficit hyperactivity disorder (ADHD). For details of these studies, see Buccafusco et al., 2009; Bertrand and Terry 2018.
8. Additional Clinical Trial Design Issues
There is growing evidence that schizophrenia patients whose cognitive performance is comparable to healthy controls (i.e., up to 25% of patients) may not benefit from pro-cognitive agents (Granger et al., 2018; DeTore et al., 2019). Therefore, the inclusion of such individuals in a clinical trial designed to determine a compound’s pro-cognitive efficacy may limit the power of the study. Unfortunately, a recent analysis of 87 randomized, double-blind, placebo-controlled, clinical trials listed on ClinicalTrials.gov indicated that the vast majority of such clinical trials may have been underpowered due to the inclusion of cognitively “normal” patients (Cotter et al., 2019).
The argument that pro-cognitive strategies (specifically α7-nAChR-based approaches) should target subgroups of individuals who exhibit lower levels of cognitive function is supported by recent clinical studies with the selective α7-nAChR agonist CDP-choline in both healthy subjects and patients with schizophrenia. In healthy study participants showing relatively lower cognitive and sensory gating scores at baseline, CDP-choline was found to enhance multiple domains of cognition (Knott et al., 2015a, 2015b) and to improve sensory gating (Knott et al., 2014b). The same laboratory also reported CDP-choline-mediated improvements of P50 sensory gating scores in schizophrenia patients who exhibited impaired gating (Aidelbaum et al., 2018). They also demonstrated that combining CDP-choline with galantamine (an acetylcholinesterase inhibitor and nAChR PAM) improved sensory gating to speech stimuli in schizophrenia patients who expressed low baseline suppression (Choueiry et al., 2019).
Another factor that may be important to consider in the evaluation of pro-cognitive agents in schizophrenia is the chronicity of the illness. The duration of the disease and the efficacy of antipsychotics has been the subject of several reviews (e.g., Leucht et al., 2008), but the concept could also certainly apply to pro-cognitive drug evaluations. Most participants in antipsychotic clinical trials have been chronically ill having experienced multiple episodes and hospitalizations. While these patients may represent the “typical” cases of schizophrenia, there is increasing interest in the effects of medications on patients who have been recently diagnosed (e.g., first episode patients). As reviewed by Leucht et al., 2008, there are multiple differences between recent onset and chronic schizophrenic patients that could result in different outcomes in clinical trials. These differences include the level of cognitive impairment, the level of treatment compliance, the sensitivity to treatment side effects, and changes in brain morphology (Molina et al., 2005; Rabinowitz et al., 2006; Mori et al., 2007). Thus, studies explicitly recruiting recent onset or first episode patients should be conducted and in large clinical trials, these patients could be included along with chronically ill patients and the study outcomes stratified by group.
Another observation that has been commonly made in failed studies of pro-cognitive agents in neuropsychiatric patients is an improvement in symptoms across all treatment arms once patients are randomized, suggesting that being in a study itself may have a powerful therapeutic effect. It has been suggested that efforts to simplify the studies by reducing the number of interactions of patients with study staff prior to the drug evaluation phase be considered. Alternatively, efforts to ensure that all study procedures, staff interactions, and assessments are included in any run-in period prior to actual randomization of patients might also help to reduce this apparent practice or placebo effect (Marder et al., 2017).
From a meta-analytic review of placebo-controlled trials of antidepressant drugs, Undurraga and Baldessarini, 2012 argued that when drug evaluations progress to Phase III and additional testing sites are recruited to increase the number of study subjects, an unanticipated result is that the quality of the sites diminish (a factor that may contribute to the study failure). Thus, new policies should be developed for neuropsychiatric drug evaluations to focus more on the quality of the recruitment and site conduct rather than on the volume of patients enrolled, which may result in fewer but more productive study sites. Such policies would necessitate continual monitoring of the conduct of the study sites and contract research organizations and the termination of sites that do not perform properly (Marder et al., 2017).
9. Conclusions and Future Directions
There are now decades of preclinical evidence to support the argument that α7-nAChRs should be viable therapeutic targets for schizophrenia and other disorders of cognition. A wide variety of molecules developed to modulate α7-nAChRs exhibited pro-cognitive activity in animals and some have shown positive effects in early (Phase I and II) clinical trials. However, to date, there has been no large phase III clinical trial in schizophrenia where an α7-nAChR ligand has shown clear efficacy as pro-cognitive agent without untoward side effects. A thorough review of the literature indicates that multiple factors can potentially affect the success of pro-cognitive agents in schizophrenia (including α7-nAChR ligands) such as the translational value of the animal models used, clinical trial design limitations, confounding effects of polypharmacy, and complex dose-effect and dose frequency considerations.
From the preclinical perspective, more studies should be conducted where the pro-cognitive agent is evaluated in animals that have chronically been treated with an antipsychotic drug. Moreover, the number of chronic dosing paradigms in animal studies should be increased to parallel chronic administration in clinical populations. In both the preclinical and clinical evaluations of α7-nAChR ligands, a wider dose range (to include low doses) and the frequency of administration (repeated versus intermittent administration) should be evaluated. In the later phases of preclinical drug discovery, a greater emphasis should be placed on non-human primates (as opposed to rodents) given their more complex behavioral repertoire and brain homology with humans. From the clinical perspective, patients whose cognitive performance is comparable to healthy controls should be excluded and efforts should be made to reduce apparent practice or placebo effects associated with multiple interactions of patients with study staff prior to the drug evaluation. Recent onset or first episode patients should be included along with chronically ill patients and the study outcomes stratified by group. Finally, new policies should also be developed to focus more on the quality of recruitment sites rather than on volume of patients enrolled.
Highlights.
Cognitive impairment is a core feature of schizophrenia that is debilitating.
Currently, there are no clinically effective treatments for these impairments.
α7-nAChRs are considered viable therapeutic targets for cognition in schizophrenia.
However, to date no α7-nAChR ligand has been approved for schizophrenia.
This review discusses the relevant α7-nAChR literature and future directions.
Acknowledgements
The authors would like to thank Ms. Ashley Davis for her administrative assistance in preparing this manuscript. The author’s laboratories and/or salary are supported in part by the following funding sources, National Institutes of Health grants, MH097695, MH083317, and NS099455, and Prime Behavior Testing Laboratories, Evans, Georgia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors do not declare any conflict of interest.
References
- Aidelbaum R, Labelle A, Baddeley A, Knott V. Assessing the acute effects of CDP-choline on sensory gating in schizophrenia: A pilot study. J Psychopharmacol. 2018. May;32(5):541–551. doi: 10.1177/0269881117746903 [DOI] [PubMed] [Google Scholar]
- Barak S, Arad M, De Levie A, Black MD, Griebel G, Weiner I. Pro-cognitive and antipsychotic efficacy of the alpha7 nicotinic partial agonist SSR180711 in pharmacological and neurodevelopmental latent inhibition models of schizophrenia. Neuropsychopharmacology. 2009. June;34(7):1753–63. doi: 10.1038/npp.2008.232. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49(5):1195–1205. doi: 10.1016/j.cortex.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Marder SR, Harms MP, Jarskog LF, Buchanan RW, Cronenwett W, Chen LS, Weiss M, Maguire RP, Pezous N, Feuerbach D, Lopez-Lopez C, Johns DR, Behrje RB, Gomez-Mancilla B. Task-related fMRI responses to a nicotinic acetylcholine receptor partial agonist in schizophrenia: A randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2016. November 3;71:66–75. doi: 10.1016/j.pnpbp.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998. September 1;18(17):6871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007. October 15;74(8):1155–63. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Terry AV Jr. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2018. May;151:214–225. doi: 10.1016/j.bcp.2017.12.008. Review. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, Li J, Malysz J, Markosyan S, Marsh K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmermann D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007. September 26;27(39):10578–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, Marsh KC, Nikkel AL, Browman K, Radek R, Anderson DJ, Buccafusco J, Gopalakrishnan M. In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer’s disease. J Pharmacol Exp Ther. 2010. September 1;334(3):875–86. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ. Van Kampen M, Wiese WB, Koenig G. (2007). The novel alpha7 nicotinic acetylcholine receptor agonist N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-(7-(2-methoxyphenyl)-1-benzofuran)-2-carboxamide improves working and recognition memory in rodents. J Pharmacol Exp Ther 321:716–725. [DOI] [PubMed] [Google Scholar]
- Boess FG, de Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, König G. (2013). Pharmacological and behavioral profile of N-((3R)-1-azabicyclo(2.2.2)oct-3-yl)-6-chinolincarboxamide (EVP-5141), a novel α7 nicotinic acetylcholine receptor agonist/serotonin 5-HT3 receptor antagonist. Psychopharmacology 227:1–17. doi: 10.1007/s00213-012-2933-4. [DOI] [PubMed] [Google Scholar]
- Bordia T, McGregor M, Papke RL, Decker MW, McIntosh JM, Quik M. The α7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions. Exp Neurol. 2015. January;263:277–84. doi: 10.1016/j.expneurol.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow LJ, Easton AE, Li YW, Sivarao DV et al. (2016). The novel, nicotinic alpha7 receptor partial agonist, BMS-933043, improves cognition and sensory processing in preclinical models of schizophrenia. PLoS One 11:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV Jr. (2009) Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. February;328(2):364–70. doi: 10.1124/jpet.108.145292. Review. PMID: 19023041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005. January;31(1):5–19 [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Kelly DL, Weiner E, Gold JM, Strauss GP, Koola MM, McMahon RP, Carpenter WT. A Randomized Clinical Trial of Oxytocin or Galantamine for the Treatment of Negative Symptoms and Cognitive Impairments in People With Schizophrenia. J Clin Psychopharmacol. 2017. August;37(4):394–400. doi: 10.1097/JCP.0000000000000720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DA, Heshmati P, Kholdebarin E, Levin ED. Decreasing nicotinic receptor activity and the spatial learning impairment caused by the NMDA glutamate antagonist dizocilpine in rats. Eur J Pharmacol. 2014. October 15;741:132–9. doi: 10.1016/j.ejphar.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, Terry AV Jr, Tehim A. Effects of the nicotinic α7 receptor partial agonist GTS-21 on NMDA-glutamatergic receptor related deficits in sensorimotor gating and recognition memory in rats. Psychopharmacology (Berl). 2014. September;231(18):3695–706. doi: 10.1007/s00213-014-3509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, Bertrand D, Bertrand S, Plagenhoef MR, Terry AV Jr. (2017). Tropisetron sensitizes α7 containing nicotinic receptors to low levels of acetylcholine in vitro and improves memory-related task performance in young and aged animals. Neuropharmacology, 117:422–433. [DOI] [PubMed] [Google Scholar]
- Carpenter WT Jr. The RDoC Controversy: Alternate Paradigm or Dominant Paradigm? Am J Psychiatry. 2016. June 1;173(6):562–3. doi: 10.1176/appi.ajp.2016.16030347. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. (1999) Nicotinic acetylcholine receptors containing alpha7 subunits are required for reliable synaptic transmission in situ. J Neurosci. May 15;19(10):3701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiry J, Blais CM, Shah D, Smith D, Fisher D, Labelle A, Knott V. Combining CDP-choline and galantamine, an optimized α7 nicotinic strategy, to ameliorate sensory gating to speech stimuli in schizophrenia. Int J Psychophysiol. 2019. November;145:70–82. doi: 10.1016/j.ijpsycho.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Corradi J, Bouzat C. Understanding the Bases of Function and Modulation of α7 Nicotinic Receptors: Implications for Drug Discovery. Mol Pharmacol. 2016. September;90(3):288–99. doi: 10.1124/mol.116.104240. Review. [DOI] [PubMed] [Google Scholar]
- Cotter J, Barnett JH, Granger K. The Use of Cognitive Screening in Pharmacotherapy Trials for Cognitive Impairment Associated With Schizophrenia. Front Psychiatry. 2019. September 6;10:648. doi: 10.3389/fpsyt.2019.00648. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTore NR, Mueser KT, Byrd JA, McGurk SR. Cognitive functioning as a predictor of response to comprehensive cognitive remediation. J Psychiatr Res. 2019. June;113:117–124. doi: 10.1016/j.jpsychires.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, Lock T, Jow B, Sitzia F, Grauer S, Jow F, Kramer A, Bowlby MR, Randall A, Kowal D, Gilbert A, Comery TA, Larocque J, Soloveva V, Brown J, Roncarati R. Old and new pharmacology: positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor by the 5-hydroxytryptamine(2B/C) receptor antagonist SB-206553 (3,5-dihydro-5-methyl-N-3-pyridinylbenzo[1,2- b:4,5-b’]di pyrrole-1(2H)-carboxamide), J. Pharmacol. Exp. Ther 328 (2009) 766–776. [DOI] [PubMed] [Google Scholar]
- Eskildsen J, Redrobe JP, Sams AG, Dekermendjian K, Laursen M, Boll JB, Papke RL, Bundgaard C, Frederiksen K, Bastlund JF. (2014). Discovery and optimization of Lu AF58801, a novel, selective and brain penetrant positive allosteric modulator of alpha-7 nicotinic acetylcholine receptors: attenuation of subchronic phencyclidine (PCP)-induced cognitive deficits in rats following oral administration. Bioorg Med Chem Lett 24:288–293. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Pezous N, Weiss M, Shakeri-Nejad K, Lingenhoehl K, Hoyer D, Hurth K, Bilbe G, Pryce CR, McAllister K, Chaperon F, Kucher K, Johns D, Blaettler T, Lopez Lopez C. (2015). AQW051, a novel, potent and selective α7 nicotinic ACh receptor partial agonist: pharmacological characterization and phase I evaluation. Br J Pharmacol 172:1292–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, Leonard S. The genetics of sensory gating deficits in schizophrenia. Curr. Psychiatry Rep 2003;5:155–161. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008. August;165(8):1040–7. doi: 10.1176/appi.ajp.2008.07071135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. (2014) α7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu. Rev. Med, 65 (2014), pp. 245–261, 10.1146/annurev-med-092112-142937. Review [DOI] [PubMed] [Google Scholar]
- Fujii S, Jia Y, Yang A, Sumikawa K. Nicotine reverses GABAergic inhibition of long-term potentiation induction in the hippocampal CA1 region. Brain Res. 2000. April 28;863(1–2):259–65. [DOI] [PubMed] [Google Scholar]
- Gee KW, Olincy A, Kanner R, Johnson L, Hogenkamp D, Harris J, Tran M, Edmonds SA, Sauer W, Yoshimura R, Johnstone T, Freedman R. First in human trial of a type I positive allosteric modulator of alpha7-nicotinic acetylcholine receptors: Pharmacokinetics, safety, and evidence for neurocognitive effect of AVL-3288. J Psychopharmacol. 2017. April;31(4):434–441. doi: 10.1177/0269881117691590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Krystal JH. Comorbidity of psychiatric and substance abuse disorders. Curr Opin Psychiatry. 2000;13(3):327–331. [Google Scholar]
- Goff DC, Wine L. (1997) Glutamate in schizophrenia: clinical and research implications. Schizophr Res. October 30;27(2–3):157–68. doi: 10.1016/S0920-9964(97)00079-0 [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007. October 15;74(8):1102–11. [DOI] [PubMed] [Google Scholar]
- Granger G, Cotter J, Baker E, Evenden J, Barnett J, Sand M. Exploring participant-level trajectories of cognitive performance among patients with schizophrenia in a multi-national trial. Schizophr Bull (2018) 44:S353. 10.1093/schbul/sby018.861 [DOI] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996. October 24;383(6602):713–6. [DOI] [PubMed] [Google Scholar]
- Green MF, Braff DL. (2001) Translating the basic and clinical cognitive neuroscience of schizophrenia to drug development and clinical trials of antipsychotic medications. Biol Psychiatry. February 15;49(4):374–84. [DOI] [PubMed] [Google Scholar]
- Green MF, Harvey PD. (2014) Cognition in schizophrenia: Past, present, and future. Schizophr Res Cogn. March;1(1):e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. (1999) Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. June 3;10(8):1779–82. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Selective nicotinic receptor antagonists: effects on attention and nicotine-induced attentional enhancement. Psychopharmacology (Berl). 2011. September;217(1):75–82. doi: 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Haig G, Wang D, Othman AA, Zhao J. The α7 Nicotinic Agonist ABT-126 in the Treatment of Cognitive Impairment Associated with Schizophrenia in Nonsmokers: Results from a Randomized Controlled Phase 2b Study. Neuropsychopharmacology. 2016. November;41(12):2893–2902. doi: 10.1038/npp.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig GM, Bain EE, Robieson WZ, Baker JD, Othman AA. A Randomized Trial to Assess the Efficacy and Safety of ABT-126, a Selective α7 Nicotinic Acetylcholine Receptor Agonist, in the Treatment of Cognitive Impairment in Schizophrenia. Am J Psychiatry. 2016. August 1;173(8):827–35. doi: 10.1176/appi.ajp.2015.15010093 [DOI] [PubMed] [Google Scholar]
- Haig GM, Wang D, Zhao J, Othman AA, Bain EE. Efficacy and Safety of the α7-Nicotinic Acetylcholine Receptor Agonist ABT-126 in the Treatment of Cognitive Impairment Associated With Schizophrenia: Results From a Phase 2b Randomized Controlled Study in Smokers. J Clin Psychiatry. 2018. May-Jun;79(3). pii: 16m11162. doi: 10.4088/JCP.16m11162. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M, Freedman R, Stevens KE. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of alpha 7 nicotinic acetylcholine receptors, Psychopharmacology 183 (2005) 13–19. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Ishima T, Hagiwara H, Iyo M (2006). Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of α7 nicotinic receptors. Eur J Pharmacol 553:191–195. [DOI] [PubMed] [Google Scholar]
- Hauser TA, Kucinski A, Jordan KG, Gatto GJ, Wersinger SR, Hesse RA, Stachowiak EK, Stachowiak MK, Papke RL, Lippiello PM, Bencherif M. TC-5619: an alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia, Biochem. Pharmacol. 78 (2009) 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. (1999) Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol. March 15;515 (Pt 3):769–76. doi: 10.1111/j.1469-7793.1999.769abx [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp DJ1, Ford-Hutchinson TA, Li WY, Whittemore ER, Yoshimura RF, Tran MB, Johnstone TB, Bascom GD, Rollins H, Lu L, Gee KW. Design, synthesis, and activity of a series of arylpyrid-3-ylmethanones as type I positive allosteric modulators of alpha7 nicotinic acetylcholine receptors, J. Med. Chem 56 (2013) 8352–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KM, McDonald IM, Bourin C, Olson RE, Bristow LJ, Easton A. Effect of alpha7 nicotinic acetylcholine receptor agonists on attentional set-shifting impairment in rats. Psychopharmacology (Berl). 2014. February;231(4):673–83. doi: 10.1007/s00213-013-3275-6. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Keefe RS. (2013) Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. October;70(10):1107–12. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Kahn RS. On the Specificity of Continuous Cognitive Decline in Schizophrenia. Am J Psychiatry. 2019. October 1;176(10):774–776. doi: 10.1176/appi.ajp.2019.19080794 [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC, Freedman R, Sehatpour P, Kegeles LS, Carlson M, Sobeih T, Wall MM, Choo TH, Vail B, Grinband J, Lieberman JA. Double blind, two dose, randomized, placebo-controlled, cross-over clinical trial of the positive allosteric modulator at the alpha7 nicotinic cholinergic receptor AVL-3288 in schizophrenia patients. Neuropsychopharmacology. 2020. February 3. doi: 10.1038/s41386-020-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Mamo D. (2003) Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. October;27(7):1081–90. Review. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Meltzer HA, Dgetluck N, Gawryl M, Koenig G, Moebius HJ, Lombardo I, Hilt DC. Randomized, Double-Blind, Placebo-Controlled Study of Encenicline, an α7 Nicotinic Acetylcholine Receptor Agonist, as a Treatment for Cognitive Impairment in Schizophrenia. Neuropsychopharmacology. 2015. December;40(13):3053–60. doi: 10.1038/npp.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem WR, Olincy A, Johnson L, Harris J, Wagner BD, Buchanan RW, Christians U, Freedman R. Pharmacokinetic Limitations on Effects of an Alpha7-Nicotinic Receptor Agonist in Schizophrenia: Randomized Trial with an Extended-Release Formulation. Neuropsychopharmacology. 2018. February;43(3):583–589. doi: 10.1038/npp.2017.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V, Smith D, de la Salle S, Impey D, Choueiry J, Beaudry E, Smith M, Saghir S, Ilivitsky V, Labelle A. CDP-choline: effects of the procholine supplement on sensory gating and executive function in healthy volunteers stratified for low, medium and high P50 suppression. J Psychopharmacol. 2014. December;28(12):1095–108. doi: 10.1177/0269881114553254. [DOI] [PubMed] [Google Scholar]
- Knott V, de la Salle S, Smith D, Choueiry J, Impey D, Smith M, Beaudry E, Saghir S, Ilivitsky V, Labelle A. Effects of acute CDP-choline treatment on resting state brain oscillations in healthy volunteers. Neurosci Lett. 2015. March 30;591:121–5. doi: 10.1016/j.neulet.2015.02.032 [DOI] [PubMed] [Google Scholar]
- Knott V, de la Salle S, Choueiry J, Impey D, Smith D, Smith M, Beaudry E, Saghir S, Ilivitsky V, Labelle A. Neurocognitive effects of acute choline supplementation in low, medium and high performer healthy volunteers. Pharmacol Biochem Behav. 2015. April;131:119–29. doi: 10.1016/j.pbb.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Kohnomi S, Suemaru K, Goda M, Choshi T, Hibino S, Kawasaki H, Araki H. Ameliorating effects of tropisetron on dopaminergic disruption of prepulse inhibition via the alpha(7) nicotinic acetylcholine receptor in Wistar rats, Brain Res. 1353 (2010) 152–158. [DOI] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro. 2018. October 25;5(5). doi: 10.1523/ENEURO.0315-18.2018. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Mexal S, Freedman R. (2007) Smoking, Genetics and Schizophrenia: Evidence for Self Medication. J Dual Diagn. November 1;3(3–4):43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Heres S, Hamann J, Kane JM. Methodological issues in current antipsychotic drug trials. Schizophr Bull. 2008;34(2):275–285. doi: 10.1093/schbul/sbm159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007. October 15;74(8):1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Picciotto MR. High-affinity nicotinic acetylcholine receptor expression and trafficking abnormalities in psychiatric illness. Psychopharmacology (Berl). 2013. October;229(3):477–85. doi: 10.1007/s00213-013-3126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CC, Mettert KD, Dorsey CN, Martinez RG, Weiner BJ, Nolen E, Stanick C, Halko H, Powell BJ. An updated protocol for a systematic review of implementation-related measures. Syst Rev. 2018. April 25;7(1):66. doi: 10.1186/s13643-018-0728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS, Duan N, Hosford DA. A randomized exploratory trial of an α−7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013. May;38(6):968–75. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus MM, Björkholm C, Malmerfelt A, Möller A, Påhlsson N, Konradsson-Geuken Å, Feltmann K, Jardemark K, Schilström B, Svensson TH. Alpha7 nicotinic acetylcholine receptor agonists and PAMs as adjunctive treatment in schizophrenia. An experimental study. Eur Neuropsychopharmacol. 2016. September;26(9):1401–1411. doi: 10.1016/j.euroneuro.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Marder SR, Laughren T, Romano SJ. Why are innovative drugs failing in phase III? Am J Psychiatry. 2017;174(9):829–831 [DOI] [PubMed] [Google Scholar]
- Marquis KL, Comery TA, Jow F, Navarra RL, Grauer SM, Pulicicchio C, Kelley C, Brennan JA, Roncarati R, Scali C, Haydar S, Ghiron C, Terstappen GC, Dunlop J. Preclinical assessment of an adjunctive treatment approach for cognitive impairment associated with schizophrenia using the alpha7 nicotinic acetylcholine receptor agonist WYE-103914/SEN34625. Psychopharmacology (Berl). 2011. December;218(4):635–47. doi: 10.1007/s00213-011-2357-6. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl). 2004. June;174(1):54–64. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995. September 22;269(5231):1692–6. [DOI] [PubMed] [Google Scholar]
- McLean SL, Grayson B, Marsh S, Zarroug SH, Harte MK, Neill JC. Nicotinic α7 and α4β2 agonists enhance the formation and retrieval of recognition memory: Potential mechanisms for cognitive performance enhancement in neurological and psychiatric disorders. Behav Brain Res. 2016. April 1;302:73–80. doi: 10.1016/j.bbr.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Messi ML, Renganathan M, Grigorenko E, Delbono O. 1997 Activation of alpha7 nicotinic acetylcholine receptor promotes survival of spinal cord motoneurons. FEBS Lett. 1997. July 7;411(1):32–8. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Papke RL, Meyers C, Huang GL, de Fiebre CM. 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat alpha7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997. September 12;768(1–2):49–56. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 3-(4-hydroxy, 2-Methoxybenzylidene)anabaseine selectivity and activity at human and rat alpha-7 nicotinic receptors. J Pharmacol Exp Ther. 1998. December;287(3):918–25 [PubMed] [Google Scholar]
- Molina V, Sánchez J, Reig S, Sanz J, Benito C, Santamarta C, Pascau J, Sarramea F, Gispert JD, Misiego JM, Palomo T, Desco M. N-acetyl-aspartate levels in the dorsolateral prefrontal cortex in the early years of schizophrenia are inversely related to disease duration. Schizophr Res. 2005. March 1;73(2–3):209–19. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Heimer H, Nestler EJ. Meeting Report: Can We Make Animal Models of Human Mental Illness? Biol Psychiatry. 2018. October 1;84(7):542–545. doi: 10.1016/j.biopsych.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H, Nakabayashi T, Hori H, Harada S, Saitoh O, Matsuda H, Kunugi H. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007;154:133–145. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012. March;14(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PD, Murray RM. The antipsychotic landscape: dopamine and beyond. Ther Adv Psychopharmacol. 2018. April;8(4):127–135. doi: 10.1177/2045125317752915. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010. October;13(10):1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW. Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci U S A. 2007. May 8;104(19):8059–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Potasiewicz A, Popik P. Positive allosteric modulation of alpha 7 nicotinic acetylcholine receptors enhances recognition memory and cognitive flexibility in rats, Eur. Neuropsychopharmacol. 2015. August;25(8):1300–13. doi: 10.1016/j.euroneuro.2015.04.018 [DOI] [PubMed] [Google Scholar]
- Noroozian M, Ghasemi S, Hosseini SM, Modabbernia A, Khodaie-Ardakani MR, Mirshafiee O, Farokhnia M, Tajdini M, Rezaei F, Salehi B, Ashrafi M, Yekehtaz H, Tabrizi M, Akhondzadeh S. A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology (Berl). 2013. August;228(4):595–602. doi: 10.1007/s00213-013-3064-2. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. (1997) Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. July 1;42(1):1–5. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006. June;63(6):630–8. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Roy Chengappa KN, Karelitz JL, Boldry MC, Michael V, Herb T, Gannon J, Brar J, Ford L, Rassnick S, Brunzell DH. Initial Cross-Over Test of A Positive Allosteric Modulator of Alpha-7 Nicotinic Receptors to Aid Cessation in Smokers With Or Without Schizophrenia. Neuropsychopharmacology. 2018. May;43(6):1334–1342. doi: 10.1038/npp.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichat P, Bergis OE, Terranova JP, Urani A, Duarte C, Santucci V, Gueudet C, Voltz C, Steinberg R, Stemmelin J, Oury-Donat F, Avenet P, Griebel G, Scatton B. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007. January;32(1):17–34. [DOI] [PubMed] [Google Scholar]
- Pieschl RL, Miller R, Jones KM, Post-Munson DJ, Chen P, Newberry K, Benitex Y, Molski T, Morgan D, McDonald IM, Macor JE, Olson RE, Asaka Y, Digavalli S, Easton A, Herrington J, Westphal RS, Lodge NJ, Zaczek R, Bristow LJ, Li YW. (2017). Effects of BMS-902483, an α7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents. Eur J Pharmacol 807:1–1 doi: 10.1016/j.ejphar.2017.04.024.1. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Borsini F. Different effects of tropisetron and ondansetron in learning and memory paradigms, Pharmacol. Biochem. Behav 56 (1997) 571–576. [DOI] [PubMed] [Google Scholar]
- Poddar I, Callahan PM, Hernandez CM, Yang X, Bartlett MG, Terry AV Jr. Tropisetron enhances recognition memory in rats chronically treated with risperidone or quetiapine. Biochem Pharmacol. 2018. May;151:180–187. doi: 10.1016/j.bcp.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potasiewicz A, Holuj M, Kos T, Popik P, Arias HR, Nikforuk A (2017). 3-Furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the α7 nicotinic receptor, reverses schizophrenia-like cognitive and social deficits in rats. Neuropharmacology 113:188–197. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel C, Reneerkens OA, Flood DG, Hilt D, Gawryl M, Bertrand S, Bertrand D, König G. EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors, Neuropharmacology 62 (2012) 1099–1110. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002. December;53(4):457–78. Review. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Harvey PD, Eerdekens M, Davidson M. Premorbid functioning and treatment response in recent-onset schizophrenia. Br J Psychiatry. 2006. July;189:31–5. [DOI] [PubMed] [Google Scholar]
- Radek RJ, Robb HM, Stevens KE, Gopalakrishnan M, Bitner RS. Effects of the novel alpha7 nicotinic acetylcholine receptor agonist ABT-107 on sensory gating in DBA/2 mice: pharmacodynamic characterization, J. Pharmacol. Exp. Ther 343 (2012) 736–745. doi: 10.1124/jpet.112.197970 [DOI] [PubMed] [Google Scholar]
- Roncarati R, Scali C, Comery TA, Grauer SM, Aschmi S, Bothmann H, Jow B, Kowal D, Gianfriddo M, Kelley C, Zanelli U, Ghiron C, Haydar S, Dunlop J, Terstappen GC. Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders, J. Pharmacol. Exp. Ther 329 (2009) 459–468. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL. Research Domain Criteria: Strengths, Weaknesses, and Potential Alternatives for Future Psychiatric Research. Mol Neuropsychiatry. 2019. October;5(4):218–236. doi: 10.1159/000501797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina A, Shirayama Y, Niitsu T, Hashimoto T, Yoshida T, Hasegawa T, Haraguchi T, Kanahara N, Shiraishi T, Fujisaki M, Fukami G, Nakazato M, Iyo M, Hashimoto K. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry. 2010. June 24;9:27. doi: 10.1186/1744-859X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Zheng L, Floyd KL, Stitzel JA. Maximizing the effect of an α7 nicotinic receptor PAM in a mouse model of schizophrenia-like sensory inhibition deficits. Brain Res. 2015. June 22;1611:8–17. doi: 10.1016/j.brainres.2015.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand JE, Nybäck H. (2005) Tobacco use in schizophrenia: a study of cotinine concentrations in the saliva of patients and controls. Eur Psychiatry. January;20(1):50–4. doi: 10.1016/j.eurpsy.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Terry AV, Callahan PM. Nicotinic Acetylcholine Receptor Ligands, Cognitive Function, and Preclinical Approaches to Drug Discovery. Nicotine Tob Res. 2019. February 18;21(3):383–394. doi: 10.1093/ntr/nty166. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietje KR, Anderson DJ, Bitner RS, Blomme EA, Brackemeyer PJ, Briggs CA, Browman KE, Bury D, Curzon P, Drescher KU, Frost JM, Fryer RM, Fox GB, Gronlien JH, Håkerud M, Gubbins EJ, Halm S, Harris R, Helfrich RJ, Kohlhaas KL, Law D, Malysz J, Marsh KC, Martin RL, Meyer MD, Molesky AL, Nikkel AL, Otte S, Pan L, Puttfarcken PS, Radek RJ, Robb HM, Spies E, Thorin-Hagene K, Waring JF, Ween H, Xu H, Gopalakrishnan M, Bunnelle WH. Preclinical characterization of A-582941: a novel α7 neuronal nicotinic receptor agonist with broad spectrum cognition-enhancing properties. CNS Neurosci. Ther 2008;14:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, Nielsen EØ, Dam E, Jørgensen TD, Ahring PK, Peters D, Holst D, Christensen JK, Malysz J, Briggs CA, Gopalakrishnan M, Olsen GM. An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo, J. Pharmacol. Exp. Ther 323 (2007) 294–307. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Wylie KP. Alpha7 Nicotinic Receptors as Therapeutic Targets in Schizophrenia. Nicotine Tob Res. 2019. February 18;21(3):349–356. doi: 10.1093/ntr/nty034. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Keefe RS, Murray S, Lowe DA, Porter R, Garibaldi G, Santarelli L. A randomized, placebo-controlled study investigating the nicotinic α7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014. June;39(7):1568–77. doi: 10.1038/npp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012. March;37(4):851–64. doi: 10.1038/npp.2011.306. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goethem NP, Paes D, Puzzo D, Fedele E, Rebosio C, Gulisano W, Palmeri A, Wennogle LP, Peng Y, Bertrand D, Prickaerts J. Antagonizing α7 nicotinic receptors with methyllycaconitine (MLA) potentiates receptor activity and memory acquisition. Cell Signal. 2019. October;62:109338. doi: 10.1016/j.cellsig.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Callahan PM, Tehim A, Bertrand D, Tombaugh G, Wang S, Xie W, Rowe WB, Ong V, Graham E, Terry AV Jr, Rodefer JS, Herbert B, Murray M, Porter R, Santarelli L, Lowe DA. RG3487, a novel nicotinic alpha7 receptor partial agonist, improves cognition and sensorimotor gating in rodents, J. Pharmacol. Exp. Ther 336 (2011) 242–253. [DOI] [PubMed] [Google Scholar]
- Winterer G, Gallinat J, Brinkmeyer J, Musso F, Kornhuber J, Thuerauf N, Rujescu D, Favis R, Sun Y, Franc MA, Ouwerkerk-Mahadevan S, Janssens L, Timmers M, Streffer JR. Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study, Neuropharmacology 64 (2013) 197–204. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Smucny J, Legget KT, Tregellas JR. Targeting Functional Biomarkers in Schizophrenia with Neuroimaging. Curr Pharm Des. 2016;22(14):2117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AF, Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci U S A. 2013. July 16;110(29):12078–83. doi: 10.1073/pnas.1307849110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Chen W, Yang H, Xue M, Schaaf CP. Chrna7 deficient mice manifest no consistent neuropsychiatric and behavioral phenotypes. Sci Rep. 2017. January 3;7:39941. doi: 10.1038/srep39941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013. October 15;86(8):112–232. doi: 10.1016/j.bcp.2013.06.031. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Liu L, Liu S, Hong X, Chen DC, Xiu MH, Yang FD, Zhang Z, Zhang X, Kosten TA, Kosten TR. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. 2012. September;169(9):974–81. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]