Highlights
-
•
Critical analysis of the hybrid processes involving ultrasound, Fenton and ozone.
-
•
Guidelines for optimum operating conditions provided for maximum benefits.
-
•
Original study on treatment of two real industrial effluents.
-
•
US-Fenton approach followed by lime treatment established as the best approach.
-
•
Use of ozone does not add much benefits to US-Fenton treatment process.
Keywords: Wastewater treatment, Ultrasound, AOPs, Fenton’s oxidation, Lime treatment, Ozonation
Abstract
The present work demonstrates the significant role of ultrasound (US) in intensifying the efficacy of the combination with Fenton reagent and/or ozone for the treatment of real dye industry industrial effluent procured from the local industry. Initial part of the work focused on analysing the literature based on combination approaches of US with different oxidants applied for the treatment of real and simulated effluents focusing on the dyes. The work also provides guidelines for the selection of optimal operating parameters for maximizing the intensification of the degradation. The second part of the work presents an experimental study into combined approaches of ultrasound with ozone (O3) and Fenton’s reagent for treatment of real effluent. Under optimized conditions (100 W, 20 kHz and duty cycle of 70%), maximum COD reductions of 94.79% and 51% were observed using a combined approach of US + Fenton oxidation followed by lime treatment for the treatment of effluent-I and effluent-II respectively at H2O2 loading of 17.5 g/L, H2O2/Fe2+ ratio of 3, pH of 4, CaO dose of 1 g/L and an overall treatment time of 70 min. US + Fenton + O3 followed by lime was also applied for treatment under ozone loading of 1 g/h for the treatment of effluent-I and it was found that maximum COD reduction of 95.12% was obtained within 30 min of treatment time, indicating use of ozone did not result in significant value addition in terms of COD reduction but resulted in faster treatment. HC (inlet pressure: 4 bar) + Fenton + Lime scheme was successfully replicated on a pilot-scale resulting in maximum COD reduction of 57.65% within 70 min of treatment time. Overall, it has been concluded that the hybrid oxidative processes as US + Fenton followed by lime treatment is established as the best approach ensuring effective COD reduction at the same time obtaining final colourless/reusable effluent.
1. Introduction
The textile and dye processing industry is one of the dominant sources of wastewater and lower efficacy of the conventional treatment methods is resulting in a water scarcity issue [1]. When wastewater is discharged into the natural resources without proper treatment, organic compounds present in the wastewater consume dissolved oxygen in the natural water resulting in oxygen depletion and survival threat to aquatic life [2]. Inefficient wastewater disposal can lead to contamination of the water resources and this is a major problem worldwide. Several volatile or semivolatile oxygenated organic compounds (O-VOCs) containing oxygen atoms that are typically found in industrial or municipal wastewater are carcinogenic and hazardous. O-VOCs must be evaluated using sophisticated analytical techniques such as gas chromatography (GC) and high-performance liquid chromatography (HPLC), ion chromatography, and capillary electrophoresis in order to monitor the individual unwanted organic pollutant at the micro-level [3]. Conventional effluent treatment approaches are not capable of mineralizing all contaminants present in wastewater especially the toxic and refractory dyestuff molecules and emerging contaminants [4]. In order to combat the global water crisis, it is important to design and implement efficient effluent treatment technologies that comply with discharge norms. Cavitation based oxidation is one of the efficient and viable technologies that can be used for the degradation of toxic and complex compounds present in the effluent [5]. With increasing awareness and stringent environmental regulation, industries are also interested in adopting modern technology that is economically feasible and gives the desired outcome [6], [7]. The modern technologies can be based on the individual oxidation approaches involving cavitation or in general the advanced oxidation processes (AOPs) comprising of ozonation [8], hypochlorite [9], hydrogen peroxide (H2O2) [10], UV [11], Fenton oxidation [12], etc. or various combination approaches such as photo-Fenton oxidation [13], photocatalysis [14], [15], electrochemical process [16], peroxone [17] etc. The main governing mechanism is the generation of highly reactive species (HO•, HOO•) [18], [19], [20] as well as the direct attack of oxidants when chemical oxidants are used. In the case of cavitation, significant energy release locally creates conditions suitable for the oxidation of pollutants. Cavitation is the nucleation, subsequent growth, and the collapse of gas/vapour filled bubbles resulting in the formation of local hot spots in the low-pressure region. The energy released during cavity collapse generates conditions of high temperature (500 to 15000 K) and pressure (100–5000 atm) also leading to the production of extremely unstable or reactive radical species. The typical reactions taking place during the degradation of organic pollutant using cavitation can be represented by the following equations [5]:
| H2O+)))→HO•+H+ | (1) |
| HO•+HO•→H2O2 | (2) |
| R+HO•→Intermediates | (3) |
| Intermediates+HO•→CO2+H2O | (4) |
The production of oxidizing radicals is typically intensified in the hybrid processes and hence better efficacy can be obtained for the destruction of the complex molecules which is not possible through conventional processes [21]. The key objective of the hybrid process is to increase the efficiency of the effluent treatment process by incorporating the individual benefits and possibly reducing the drawbacks as well. Application of hybrid processes can give effective reuse/recycling of water based on the efficient treatment and help to accomplish the goal of environmental sustainability and also to generate a reliable source of water within the plant operation. The initial short review illustrates the significant benefits of applying cavitation based AOPs for wastewater treatment focusing on the field of dye processing. Table 1 depicts the overview of the important works dealing with the ultrasound based advanced oxidation technology for the treatment of real [22], [23], [24], [25] /simulated [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] effluent in terms of the dye studied for treatment, the operating conditions and also the important outcomes from the study. A critical analysis of the data summarized in Table 1 has been also presented with an objective of providing the guidelines for the operating parameters for the hybrid processes and subsequently research work is provided for treatment of real effluents using combined oxidation processes based on cavitation, which is not so common in the literature.
Table 1.
Studies based on combined approaches of US in the treatment of dye effluent.
| Sr. No. | Simulated/ real industrial dye effluent | Treatment scheme | Operating Conditions | Important Results | Ref. |
|---|---|---|---|---|---|
| 1 | C. I. Acid Orange 7 | US + O3, UV + O3, US + O3 + UV, US + UV | Co = 19.967 mg/L, US frequency = 520 kHz based on piezoelectric transducer, UV lamps (max emission at 253.7 nm), US power density = 0.073 W/L, O3 = 40 mg/L, Flow ratio of Ar(argon):O2 = 1:0.5, pH of solution = 5.5, Temperature = 25 °C, T = 60 min. | For US + O3, UV + O3, US + UV, US + O3 + UV, 27%, 33%, 6% and 40% of mineralization reported respectively, US + O3 + UV approach offered a biodegradable form of effluent (BOD5/TOC = 0.45) after treatment, Combined approach of US + O3 + UV as well as gas mixture (Ar/O2) presence improved degradation efficiency of dye. | [11] |
| 2 | Dye industrial wastewater | US (bath), EF, US + EF, EF + US | C0 = 15360 mg/L, US bath frequency = 33 kHz, US power = 100 W, Voltage = 3 V, Electrode area = 30 cm2, Electrode distance = 4 cm, FeSO4 = 100 mg/L, pH of solution = 3, Temperature = 38–40 °C, Electrolysis time = 120 min. | For US, EF, US followed by EF and EF followed by US, 10.4%, 79.2%, 81.3% and 85.4% of COD reduction obtained respectively, Sonication assisted electro-Fenton process was the best treatment process. | [22] |
| 3 |
|
US + H2O2, US + O3, US + Fenton + Lime, HC + Fenton + Lime, Only Fenton, HC + NaClO, HC + Fenton |
|
|
[23] |
| 4 |
|
|
|
|
[24] |
| 5 |
|
|
|
|
[25] |
| 6 | Eosin B | Sonocatalysis, US + ZnO | C0 = 5.08 mg/L, ultrasonic power = 250 W, ZnO dosage = 2170 mg/L, volume = 100 ml, Ambient temperature, T = 72 min. | For US + ZnO approach, 93.46% as dye degradation was obtained, US enhanced the catalytic performance of the ZnO nanoparticles in terms of the enhanced degradation of the dye | [26] |
| 7 | Malachite dye | US + MNPs | C0 = 200 mg/L, US frequency = 40 kHz, Magnetic nanomaterial (Fe3O4/HA) dose = 200 mg, volume = 100 ml, T = 35 min. | Almost complete degradation of malachite dye was obtained using US combined with nanomagnetic particles within 35 min of treatment, US enhanced the mineralization of the dye by enhancing the formation of OH• radicals | [27] |
| 8 | Direct Blue 15 (azo dye) | US + H2O2 + Fe0 | C0 = 43.61 mg/L, Fe0 = 1000 mg/L, H2O2 = 175.18 mg/L, US power density = 120 W/L, US frequency = 60 kHz, pH of solution = 3, T = 10 min. | For US + H2O2 + Fe0, almost 99% of decolorization of azo dye was obtained. US improved the synergetic mechanism by providing additional OH• radicals and also helped to produce more quantities of Fe2+ ions during the process. | [28] |
| 9 | Azure B dye | US, Fenton, Sono-electro-Fenton | C0 = 40 mg/L, COD = 152.9 mg/L, US power = 91 W, US frequency = 23 kHz, Fe2+= 44.68 mg/L, H2O2 = 81.64 mg/L, Platinum gauze counter and saturated calomel reference electrode, pH of solution = 2.6 to 3, T = 60 min, | US, Fenton, Sono-electro-Fenton resulted in 25%, 85% and 98% of dye removal and 21%, 68% and 85% of COD removal respectively, US enhanced the yield of hydroxyl radical in the process and hence the oxidation rates | [29] |
| 10 | C.I. Reactive Black 5 | US, O3, US + O3 | Co = 19.836 mg/L, US frequency = 520 kHz, US irradiation intensity = 1.63 W/cm2, O3 concentration = 3360 mg/L, temperature = 20 ± 0.5 °C, T = 60 min. | For US, O3 and US + O3, 2, 50, 76% of total mineralization was obtained respectively, US enhanced the mass transfer of O3 and also improved the rate of degradation in US + O3 as compared to that accomplished by O3 alone. | [30] |
| 11 |
|
US, EC, US + EC |
|
|
[31] |
| 12 | Acid Blue 113 | US + Fenton, UV + Fenton | C0 = 25 mg/L, H2O2 = 1.701 mg/L, FeVO4 = 1000 mg/L, US frequency = 37 kHz, UV intensity = 15 mW/cm2, US Power = 320 W, pH of solution = 6, T = 16 min. | US + Fenton, UV + Fenton resulted in 99% and 97% of decolorisation, 80% and 70% of TOC removal respectively, US more efficiently worked for the dye removal as compared to UV + Fenton scheme. | [32] |
| 13 | C.I. reactive yellow 145 | US + Fenton, Fenton | C0 = 50 mg/L, COD = 190 mg/L, temperature = 24 ± 2 0C, US type = US water bath (indirect irradiation), US bath frequency = 35 kHz, US bath power = 80 W, Fe2+= 20 mg/L, H2O2 = 20 mg/L (For Fenton process), H2O2 = 15 mg/L (For sono-Fenton process), pH of solution = 3, T = 60 min. | 91% and 47% of color and COD removal were obtained in sono-Fenton process respectively, 95% and 51% of color and COD removal were obtained in only Fenton process respectively, US improved decolorization at lower chemical consumption. | [33] |
| 14 | Acid green 50 textile dye | US, UV, US + Fenton, UV + Fenton | C0 = 23.064 mg/L, US frequency = 20 kHz, US amplitude = 60%, UV lamp power = 15 W, Fe2O3 = 100 mg/L, T = 30 min (for US + Fenton and UV + Fenton) and 40 min (US and UV). | US, UV, US + Fenton, UV + Fenton resulted in 48%, 10, 94% and 85% of decoloration respectively, US enhanced the mass transfer, resulted in additional hydroxyl radical production and improved catalyst activity (by continuous cleaning) in the Fenton process. | [34] |
| 15 | Azo dye Orange II | US, UV, US + UV, US + UV + Fenton | C0 = 49.04 mg/L, US power = 22.3 W, US frequency = 850 kHz, UV lamp (at 254 nm), H2O2 = 170.07 mg/L, Fe = 5.585 mg/L, Fe2+:H2O2 (molar ratio) = 1:50, pH of solution = Changed from 6.5 to 4 after treatment, T = 120 min. | US, UV, US + UV, US + UV + Fenton resulted in 6.5%, 28.9%, 47.8% and 80.8% of decolorization respectively, US enhanced the degree of decolorization in the US + Fenton system | [66] |
*C0 = initial dye concentration, TOC0 = initial total organic carbon, MNPs = magnetic nanoparticles, EC = Electrocoagulation, EF = electro-Fenton, T = treatment time.
The research work presented in the work is focused on the comparative study of the cavitation based advanced oxidation processes for the degradation of complex organic dye compounds present in the two real industrial effluents (effluent-I and effluent-II) obtained from S.S. Techno. Pvt. Ltd., Pune. These effluents were a mixed stream of the various dyes used in the textile processing plant with high chemical oxygen demand (COD) and high total dissolved solids (TDS) content. The objective of the research work was to degrade the complex compound present in the effluent and ultimately convert the waste effluent into recyclable effluent in order to provide the source of water to the plant. The combinations of US + Fenton, US + ozone and US + Fenton + ozone have been investigated in this study. Individual approaches of only US, only Fenton (at optimum Fenton loading), only ozone (at optimum ozone loading) were also studied to check the synergism of the combined approach. Coagulation has also been applied after the Fenton oxidation to remove the intense color and solid sludge produced during the Fenton oxidation and to transform the dark brown color solution to colorless water. Finally the use of hydrodynamic cavitation has been demonstrated as a scale up option under optimum conditions established through detailed study in the first part involving ultrasound.
2. Literature analysis of the cavitation based hybrid treatment system
2.1. Overview of research studies carried out in past years
Several attempts have been made to understand the remediation of industrial toxic dye containing wastewater, either by mineralizing dye-containing synthetic effluent or by treating actual industrial effluent with the help of different oxidative technologies in recent years. Some of the studies have been analysed now to provide criteria for the selection of operating conditions and appropriate effluent treatment approach, focusing on the effluents related to the dye industry.
Harichandran and Prasad [37] investigated the US + Fenton system for the degradation of direct Red 81 (DR81) using different conditions of various operational parameters such as pH of the solution (2–10), initial DR81 concentration (20–120 mg/L), US frequency (20, 35 and 120 kHz), H2O2 loading (25–200 mM) and Ferrous sulfate loading (0–0.3 g/L). It was reported that almost 99% of decolorization of DR81 was obtained using US + Fenton at the optimal condition of pH as 3, Fe2+ loading of 0.2 g/L, H2O2 loading of 5.1 × 10−3 mol/L, US frequency of 120 kHz and US power 60 W within 75 min of reaction time.
Bharadwaj et al. [38] also studied the use of US + Fenton system for the decolorization of reactive blue 19 dye effluent illustrating the role of various operating parameters like dose of H2O2, dose of Fe2+ and pH. It was reported that US + Fenton was effective in terms of giving COD reduction of 93.3% at optimum conditions of dose of H2O2 as 50 mg/L, pH value of 3 and 360 min as treatment time. US + Fenton system was also implemented for the treatment of real industrial fabric dyeing effluent and it was reported to give 84% color removal after 540 min of treatment.
Menon et al. [22] studied the use of ultrasound in assistance with the electro-Fenton (EF) process for the removal of organic compound present in the dye intermediate containing industrial wastewater. The performance of the US assisted EF batch system for the removal of ammoniacal nitrogen and COD of the effluent was analysed under different values of various operating conditions such as pH of the solution (2 to 4), applied voltage (1 to 4 V), Fenton loading (50 to 200 ppm), and treatment time (15 to 180 min). It was reported that US combined with EF process resulted in maximum efficacy in terms of 65.5% of ammonical nitrogen removal and 85.4% of COD removal at optimal conditions i.e. pH of 3, an applied voltage of 3 V, Fenton loading of 100 ppm, electrolysis time of 120 min.
Mahdavi and Talesh [26] explored the degradation of Eosin B dye using US in the presence of ZnO as a sonocatalyst. Various experimental parameters like ultrasonic power (50–250 W), treatment time (10–70 min), initial Eosin B concentration (5–25 mg/L) and catalyst loading (1–3 g/L) were varied in the investigation and it was concluded that sonocatalytic degradation of Eosin dye was more efficient resulting in 93.46% removal of the dye at the optimum conditions of parameters, i.e. ultrasonic power, treatment time, initial dye concentration and catalyst loading as 250 W, 72 min, 5.08 mg/L and 2.17 g/L respectively. It was also demonstrated that the catalytic performance in the degradation process was considerably improved with the help of ultrasonic irradiation.
Weng et al. [39] studied the application of US assisted advanced Fenton process (sono-AFP) in the decolorization of real textile industrial effluent (initial American Dye Manufacturing Institute decolorization value of 1638 ADMI) also presenting the kinetic study. The effect of different parameters such as pH, the dosage of Fe0 (zero-valent iron), dosage of H2O2 and US frequency on the extent of decolorization was studied. Under the optimized condition, it was found that the desired outcome in terms of decolorization of the dye effluent with actual value as 74 ADMI (lower than the discharge limit of 550 ADMI) was obtained using sono-AFP process under the treatment conditions of pH as 3, Fe0 dose as 1 g/L, the dosage of H2O2 as 1.03 × 10−2 M and US frequency as 47 kHz. It was also reported that experimental data followed the zero-order kinetics with 6.9 as a synergetic index for the sono-AFP treatment option.
A similar study was reported by Cetinkaya et al. [40] based on the application of US in combination with classic Fenton oxidation for the textile industrial effluent treatment with a detailed parameter study for understanding the effect of pH of effluent, the dosage of Fe2+ ion, the dosage of H2O2 and treatment time. Almost complete decolorization (99%) was obtained by US (35 kHz as frequency) in combination with Fenton’s reagent (H2O2:1.65 g/L, Fe2+:0.05 g/L) at pH of 3 and in 60 min of treatment.
Zhang et al. [41] explored the use of combined US and Fenton oxidation process for the removal of carcinogenic polycyclic aromatic hydrocarbons (CPAHs) from the textile dyeing sludge. It was reported that at optimal parameters of H2O2 loading as 152 mmol/L, US power density as 408 W/L, pH of the solution as 3.7 and the molar ratio of H2O2/Fe2+ as 1.3, the synergetic index and the degradations of five CPAHs were obtained as 30.4 and around 81% to 85% respectively within 43 min of the treatment.
Johin et al. [42] reported that US played a significant role in the treatment of reactive black 5 (RB5) dye-based synthetic effluent using electrochemical process (Na2S2O8/MnSO4 as electrolyte) and the combined system was also successfully applied for the treatment of RB5 containing real textile effluent. It was reported that maximum decolorization of RB5 based synthetic effluent as 89% was achieved using US + electrolyte approach under the optimum conditions as initial dye concentration of 100 ppm, loading of Na2S2O8 as 50 ppm, loading of MnSO4 as 75 ppm, pH of the solution as 2, US power as 44 W and voltage of 20 V. For the real textile effluent, application of similar experimental conditions was reported to yield 90% of TOC removal matching the performance for simulated effluent.
Kiran et al. [43] studied the use of nickel nanoparticles (Ni-NPs) for the decolorization of reactive yellow 160 dye containing effluent and reported around 91.4% of decolorization at an optimum dye concentration of 0.02%, Ni-NPs dose of 9 mg/L, pH of 7 and temperature of 40 °C. The approach implemented by Maheshwari et al. [44] for the treatment of congo-red (CR) dye effluent involved using a combination of US, microwave and activated carbon (AC). The maximum degradation efficiency of 97.73% was demonstrated at a dye concentration of 10 mg/L, AC dose of 200 mg, pH of 7, and room temperature within 2 h of contact time. The hybrid US + microwave approach was also reported to be energy efficient (treatment cost of Rs. 2.11/50 ml batch).
Song et al. [35] reported a maximum degradation of Acid Red 88 dye as 98.6 % using a combined approach of US and Fenton whereas lower efficacy was seen for the individual approaches of Fenton oxidation (38.9%) and US (8.2%) at optimum conditions. Rehorek et al. [36] reported the degradation of various azo dyes (acid Direct Blue 71, Orange 5 and 52, Reactive Orange 16 and 107, Reactive Black 5) to the nontoxic end products using only ultrasound within 3 to 15 h at 90 W and within 1 to 4 h at 120 W of US power respectively. Karnjkar et al. [45] also studied the effect of US in combination with TiO2 and air for the degradation of magenta dye and reported 98.8% and 94% as the extent of degradation and COD reduction respectively in the presence of 1 g/L of TiO2.
There have also been several studies on sulfate-related AOPs that can be used as an alternative to traditional AOPs [46] based on the ability to produce sulfate radicals (SO4•−) with a high oxidation potential (2.6 V). Due to its higher solubility and a longer half-life than HO• radicals, sulfate radicals can be used for the degradation of a wide range of organic dye pollutants. Generally, sulfate radicals are activated thermally, by cavitation or by use of the catalyst in order to enhance the efficacy of the process [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58].
2.2. Important guidelines for the optimal parameters
Suitable recommendations for the selection of optimal ranges of operating parameters have been now discussed for the ultrasound assisted oxidation process based on the analysis of the existing research studies.
2.2.1. pH
The pH is an important parameter in the effluent treatment that is required to be optimized. Final treated water need to be neutralized after treatment and hence the optimal value of pH should be as near as to neutral value i.e. 7. Cavitation effectively works in the acidic condition in terms of giving maximum degradation as per the literature works and extent of degradation is lower in alkaline condition. The optimal value also depends on the pKa value of the specific compound to be degraded in the treatment. It is recommended to select the pH value of the effluent in the range of 4 to 6 so as to yield the best treatment efficacy with cost optimization in terms of the neutralization requirements.
2.2.2. US coupled with hydrogen peroxide
Hydrogen peroxide is an external source of hydroxyl radical which can enhance the degradation efficiency of the oxidation process. As per the earlier works, hydroxyl radical formation is typically enhanced with the addition of hydrogen peroxide during the treatment but beyond an optimal value of H2O2 loading, the extent of the degradation is minimized due to the scavenging effect and excess loading also contributes to the additional cost of the operation as well as COD. The requirement of H2O2 dosing to intensify the process again depends on the specific compound present in the effluent and hence it is recommended to optimize the H2O2 dosage based on the degradation of a specific compound in order to get the desired intensification results.
2.2.3. US coupled with Fenton loading
Fenton addition generally enhances the extent of degradation of complex pollutants present in the industrial wastewater. It is suggested that the Fenton loading should be at optimal value giving the desired outcome with the minimum cost of operation. The optimum value for the Fenton loading again depends on the specific compound present in the effluent or type of industrial effluent. It is therefore essential to optimize the dosing of Fenton oxidants based on the requirement of the particular effluent treatment. In many research works, it has been proved that the complete degradation of an organic compound can be possible using US in combination with Fenton oxidation with minimum treatment time. Fenton ratio i.e. ratio of H2O2 to Fe2+ is also an important parameter in US assisted Fenton oxidation which also affects the amount of the sludge generated during the Fenton oxidation in addition to the efficacy of treatment. The sludge generated can be minimized with the use of optimal loading of Fenton reagents or/and replacement of ferrous sulfate with zero-valent iron. The synergism of the combined process is likely to depend on the degree of intensity required to degrade the complex compound present in the effluent.
2.2.4. US coupled with ozone
US + ozone scheme is also an useful approach in disinfecting water or effluent treatment. In the literature works, it has been reported that the combined approach of US + ozone offers a dual mechanism for the effluent treatment, i.e. the direct attack of ozone molecule and the degradation of the compound by hydroxyl radicals produced in the process. In some cases, US + ozone is found to be effective in terms of giving desired results in minimum treatment time and no sludge generation as compared to US + fenton oxidation. US + ozone gives effective degradation of the compound but beyond the optimal value of ozone loading, the extent of degradation does not significantly impact further degradation. It can be said that the ozone loading is needed to be optimum so as to yield effective intensification of the specific compound based effluent. It is also important to check the reactivity of the contaminants present in the effluent toward molecular ozone. If the compounds are highly reactive and hence effectively oxidized using only ozone, then the combination with cavitation may not be effective as molecular ozone is usually converted to hydroxyl radicals which may have lower reactivity toward the contaminants.
Overall, it can be said that the process intensification and chemical dosing criteria for each organic compound are different and therefore process optimization is important for the degradation of a particular compound or real industrial effluent containing specific compounds.
3. Materials and methodology
3.1. Materials
Effluents (Effluent-I and Effluent-II) used in the study were obtained from SS Techno Pvt Ltd., Pune. COD, TDS and pH of the dark brown colored effluent-I were 12595 mg/L, 18800 mg/L and 8 respectively whereas the COD, TDS and pH values of dark yellow-colored effluent-II were 27000–27200 mg/L, 6200 mg/L and 6.5 respectively. Hydrogen peroxide (H2O2, 30% W/V, obtained from Loba chemicals), ferrous sulfate heptahydrate (FeSO4·7H2O, Thomas baker), silver sulfate (Ag2SO4, Thomas baker), mercury sulfate (HgSO4, Thomas baker), sulphuric acid (H2SO4, Thomas baker), sodium hydroxide pellets (NaOH, Thomas baker), and calcium oxide (CaO, Thomas baker) were used in this work. All the chemicals used in this work were analytical grade reagents and received from the supplier freshly for immediate use. Fresh distilled water used in the experiments was collected from the distilled water plant in the laboratory.
3.2. Methodology
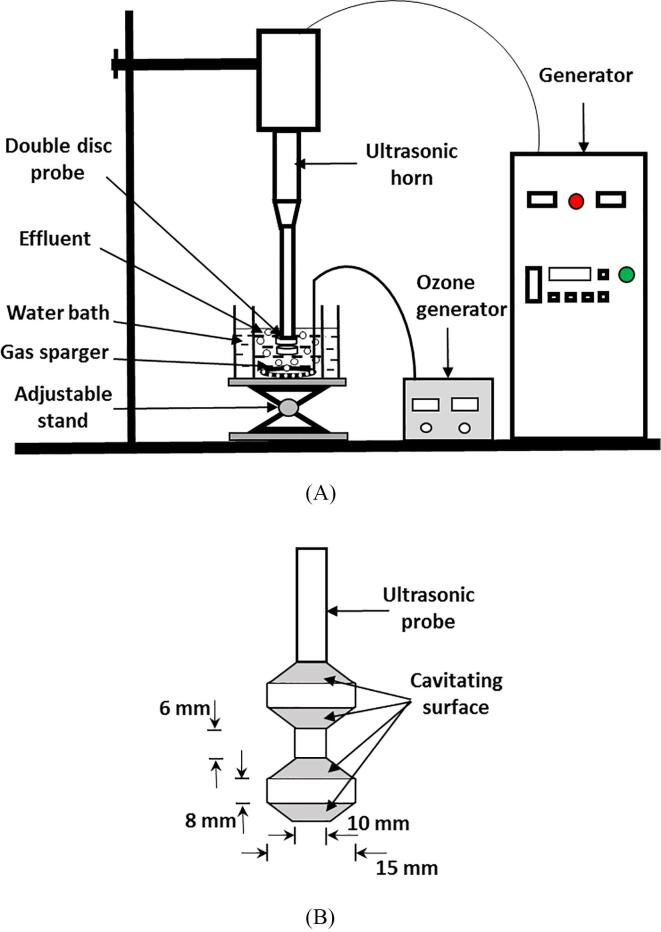
An ultrasonic horn procured from M/s Dakshin, Mumbai was used to perform the sonolysis experiments. Ultrasonic conditions such as power dissipation, frequency, and duty cycle were kept constant at 100 W, 20 kHz, and 70% (duty cycle decides the on time for ultrasound affecting both the intensity for degradation and also the performance of the equipment. 70% duty cycle means ON time of 7 sec followed by OFF time of 3 sec in the given cycle) respectively. All the experiments were performed using diluted fresh effluent using tap water by a dilution factor of 1.5. Initially, the pH of the effluent-I and effluent-II was kept constant at 4 (the pH was selected based on the higher efficacy observed for both cavitation and Fenton chemistry as also confirmed based on the analysis of literature presented earlier) for all the experimental studies using 1 N of sulphuric acid. COD of the diluted acidic effluent (at pH of 4) was analyzed and considered as the initial COD of the effluent. All the sonolysis experiments were conducted with 250 ml of effluent taken in a glass reactor (500 ml) placed in the water bath in order to maintain the temperature of the effluent at ambient conditions. With the help of a metal stand, the height of the ultrasonic horn tip was maintained in such a way that the cavitating surface of the horn was completely submerged in the liquid. In the ozonation experiments, an ozone generator (flow rate: 0 to 3 g/h) was used for the introduction of the ozone using a silicone pipe whose tip was attached to silicone based sparger and submerged at the bottom of the liquid. The experimental setup of the ultrasonic horn and the geometrical design of the horn have been illustrated in Fig. 1(A) and Fig. 1(B) respectively. All the sonolysis experiments based on the use of ozone were carried out in the fume hood for safety purposes. During the Fenton experiments, FeSO4·7H2O was previously dissolved in the effluent samples and the reactions were triggered by the addition of H2O2 and switching on US simultaneously.
Fig. 1.
Schematic diagram of (A) experimental setup based on ultrasonic horn (B) geometrical design of double-disc US horn.
The schematic representation of HC setup has been shown in Fig. 2. HC is a slit venturi based closed-loop reactor which consists of a holding tank (15 L of capacity), positive displacement pump (1.5 kW), control valves (V1, V2, V3), and pressure gauges (P1, P2). The geometrical configuration of the cavitating device with a detailed description of the flow system and working methodology have been reported in our earlier work [23].
Fig. 2.
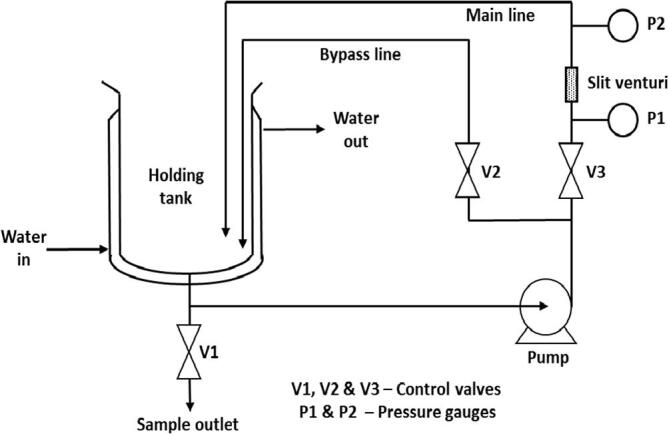
Schematic diagram of experimental setup based on hydrodynamic cavitation (HC) reactor.
Lime treatment was carried out using calcium oxide (CaO loading kept constant as 1 g/L) to remove the suspended solid particles typically formed in the Fenton process. The time required for the lime treatment and settling of the solid sludge were fixed at 5 min each. The solid sludge particles settled during the lime treatment were removed using filtration with a Whatman filter paper. Samples were taken out periodically during the treatment to monitor the process in terms of reduction in COD. All the results are reported by taking an average of three values based on the experimental sets performed in triplicates.
All the withdrawn samples were analyzed using the standard EPA approved COD estimation method in order to monitor the progress of treatment using different approaches. The suspended solid particles were separated by simple filtration using Whatman filter paper before the COD analysis, as applicable depending on the approach. During the COD analysis, 2.5 ml of the filtered sample was transferred to the cleaned glass vials and subsequently, both the COD reagents i.e. sulphuric acid and potassium dichromate were carefully added. The sample vials were tightly packed and placed in the COD digestor (model: HI839800) at 150 °C for 2 h of digestion. After digestion, the samples were cooled to ambient temperature. The standard titration process was applied to evaluate the COD of the samples using 0.01 N of aqueous ferrous ammonium sulfate. 1 to 2 drops of the ferroin indicator were used to detect the change in color from greenish-yellow to red wine. COD of the samples was evaluated using the following equation:
Where, A: burette reading of blank sample (ml); B: burette reading of the unknown sample (ml); N: normality of aqueous ferrous ammonium sulfate and C: volume of the sample taken for the COD analysis (ml)
A digital TDS meter (model HI 99301) was used to evaluate the content of total dissolved solids (TDS) of the samples. A calibrated digital pH meter procured from Hanna Equipments India Pvt. Ltd., Navi Mumbai was used to evaluate the pH of the samples. An ozone generator (Model el-oz-O-10 g/h) working on the corona discharge principle was used to produce ozone gas from oxygen.
4. Results and discussion
4.1. Case study of real industrial textile effluent (effluent-I)
4.1.1. US alone
The US horn used in this study was with double disc arrangement in order to enhance the effective cavitating zone as well as to achieve proper utilization of the acoustic power dissipation in the effluent. Individual approach of US was applied for the COD reduction of effluent-I at pH of 4 and it was observed that the maximum COD reduction of 27% was obtained within 60 min of treatment and further treatment using only US did not affect the COD reduction of the effluent-I. Lime treatment was not found to be effective for giving any further COD reduction or reducing the color of the effluent-I after the US process.
4.1.2. US + Fenton
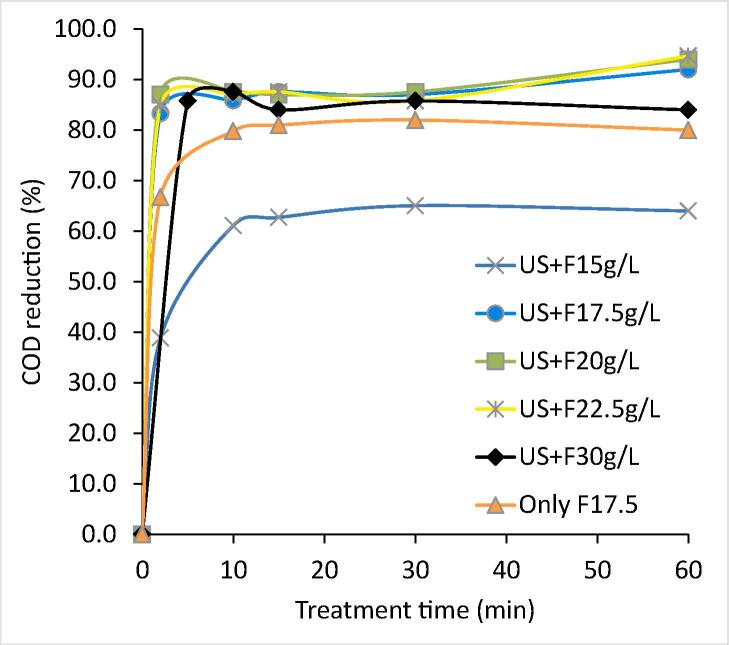
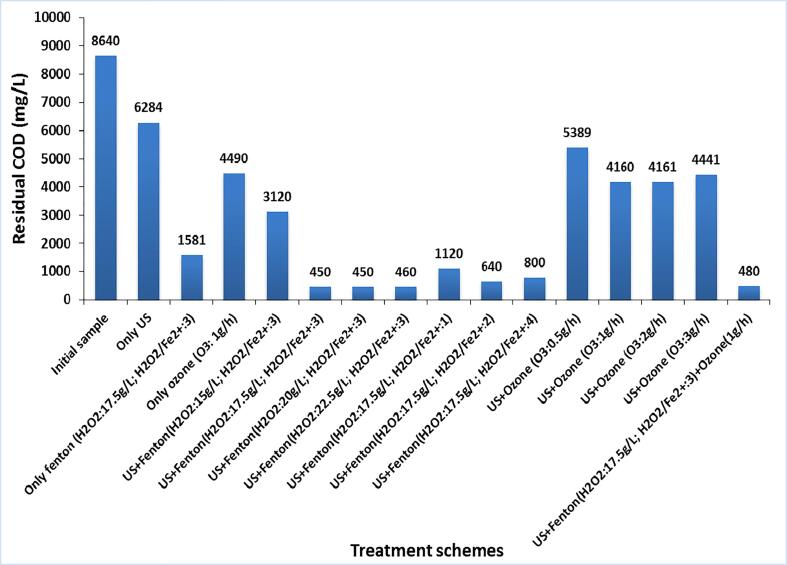
The combined approach of US and Fenton oxidation was explored for the treatment of the industrial textile effluent-I at different loadings of Fenton reagent (H2O2: 15 g/L, 17.5 g/L, 20 g/L, and 22.5 g/L; H2O2/Fe2+:3) with an objective to establish the optimum conditions to achieve the maximum COD reduction. The obtained results are shown in Fig. 3 and Table 2. The COD reduction using US + Fenton oxidation at various H2O2 loading of 15 g/L, 17.5 g/L, 20 g/L, 22.5 g/L and 30 g/L were 64%, 92%, 94%, 94.7% and 84.7% respectively. The approach of US + Fenton at 15 g/L of H2O2 loading was generally found to be inadequate for the effective generation of hydroxyl radicals so as to mineralize the dye compound due to a lower concentration of hydrogen peroxide. Conversely, the combined approaches of US + Fenton at H2O2 loading of 17.5 g/L, 20 g/L, and 22.5 g/L were successful in yielding the desired COD reduction of 92%, 94%, and 94.7% respectively. It was also observed that the COD removal increased spontaneously in less than 2 min of treatment time in the case of US + Fenton oxidation at 17.5 g/L, 20 g/L and 22.5 g/L of H2O2 loadings and conversely marginal/lower dye degradation was observed as treatment time increased to 60 min. Further enhancement in the H2O2 dosage to 30 g/L was again not effective in terms of giving significant COD reduction above 95% due to higher loading of H2O2 resulting in hydroxyl radical scavenging [59], [60].
Fig. 3.
Effect of Fenton loadings on COD reduction achieved in US + Fenton oxidation (H2O2/Fe2+:3) scheme (Effluent-I).
Table 2.
Comparison of different advanced oxidation approaches in term of COD reduction (%) and cavitational yield (mg/J).
| Type of effluent | Treatment scheme | Oxidant/chemical loading | Treatment time (min) | COD reduction (%) | Cavitational yield (10−3 mg/J) |
|---|---|---|---|---|---|
| Effluent-I | Only US | CaO:1g/L | 130 | 27.3 | 1.17 |
| Only Fenton | H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 81.7 | – | |
| Only ozone | O3: 1 g/h | 70 | 47.6 | – | |
| US + Fenton + Lime | H2O2:15 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 63.9 | 5.49 | |
| US + Fenton + Lime | H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 94.8 | 7.89 | |
| US + Fenton + Lime | H2O2:20 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 94.0 | 8.06 | |
| US + Fenton + Lime | H2O2:22.5 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 94.7 | 8.11 | |
| US + Fenton + Lime | H2O2:30 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 92.9 | 7.18 | |
| US + Fenton + Lime | H2O2:17.5 g/L, H2O2/Fe2+:1, CaO:1g/L | 70 | 87.1 | 7.38 | |
| US + Fenton + Lime | H2O2:17.5 g/L, H2O2/Fe2+:2, CaO:1g/L | 70 | 92.6 | 7.52 | |
| US + Fenton + Lime | H2O2:17.5 g/L, H2O2/Fe2+:4, CaO:1g/L | 70 | 90.8 | 7.48 | |
| US + Ozone | O3: 0.5 g/h | 70 | 37.3 | 0.77 | |
| US + Ozone | O3: 1 g/h | 70 | 51.6 | 1.06 | |
| US + Ozone | O3: 2 g/h | 70 | 51.6 | 1.02 | |
| US + Ozone | O3: 3 g/h | 70 | 48.4 | 0.99 | |
| US + Fenton + O3 | H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L, O3:1g/h | 30 | 95.1 | 5.23 | |
| Effluent-II | Only US | CaO:1g/L | 130 | 29 | 2.44 |
| US + Fenton + Lime | H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 51 | 8.50 | |
| HC + Fenton + Lime | H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L | 70 | 58 | 564.36 |
The use of only Fenton process at H2O2 loading of 17.5 g/L (in absence of ultrasound) was also efficient in giving COD reduction but was not able to meet the required discharge limits set by the pollution control boards. Using ultrasound in combination at the same loading of 17.5 g/L indeed enhanced the COD reduction to 92% which allowed meeting the discharge criteria. The comparison clearly allows understanding the role played by the use of ultrasound.
Lime coagulation as a finishing step was also applied subsequent to Fenton oxidation in order to remove the solid sludge and color of the effluent. It was observed that lime treatment (CaO: 1 g/L) was effective in removing solid sludge and changing the color of the effluent from dark brown to colorless. Without the use of lime, settling of sludge generated during Fenton oxidation took more than 12 h, which may not be the practical approach in commercial operation directing the importance of lime treatment. After the lime treatment (CaO:1g/L), final COD reduction values achieved using US + Fenton at different loading of H2O2 (15 g/L, 17.5 g/L, 20 g/L, 22.5 g/L and 30 g/L) were 63.9%, 94.8%, 94.8%, 94.7% and 92.9% respectively, which were only marginally different as compared to that obtained in the absence of lime treatment. Based on this analysis, it can be said that the use of lime is just to enhance the separation of solid sludge formed during the Fenton oxidation treatment and decolorize the effluent.
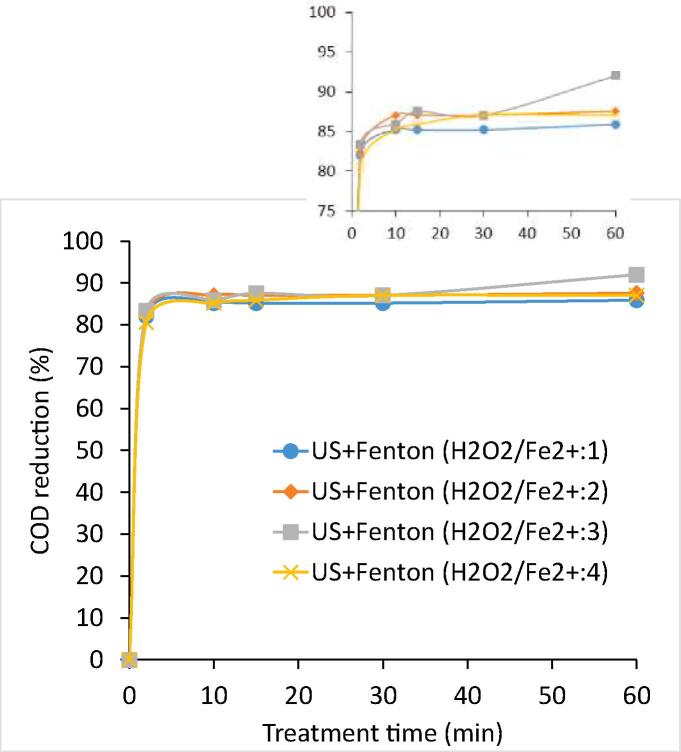
The experiments were also conducted using various Fenton ratios (H2O2/Fe2+) as 1, 2, 3 and 4 achieved by varying the FeSO4 quantity at the optimum dose of H2O2 as 17.5 g/L in the US + Fenton process for the treatment of industrial dye effluent-I. The amount of sludge generated during the Fenton process mainly depends on the amount of Fe2+ ion used. Hence, the amount of Fe2+ ion needs to be optimized so as to reduce the additional process cost required for the removal of sludge directing the importance of this work. US + Fenton scheme at Fenton ratio of 3 was found to be optimum in terms of yielding maximum COD reduction of 92% with minimum consumption of ferrous ion (Fe2+) amongst all the investigated Fenton ratios as shown in Fig. 4. The residual COD after US + Fenton oxidation (w/o use of lime treatment) was found to be minimum at 691 ppm at Fenton ratio (H2O2/Fe2+) of 3 as compared to Fenton ratio of 1 (residual COD as 1220 ppm), 2 (1075 ppm), and 4 (1120 ppm). It is important to note that the obtained residual COD are indeed statistically different even if the net COD reduction is appearing to be higher (>90%) for all the cases.
Fig. 4.
Effect of Fenton ratio (H2O2/Fe2+) on COD reduction achieved in US + Fenton oxidation (H2O2:17.5 g/L) scheme (Effluent-I).
In summary, it was established that US + Fenton (H2O2: 17.5 g/L) followed by lime treatment (CaO: 1 g/L) was found to be an optimum scheme amongst different versions of US + Fenton approaches in achieving a maximum COD reduction based on minimum chemical consumption and giving colorless effluent. The underlying mechanism for the improved processing using US + Fenton approach has been now discussed. In acoustic cavitation, ultrasonic irradiation splits water molecules into two species i.e. hydroxyl radical (OH•) and a hydrogen atom (H). Oxidation of organic compounds with the available hydroxyl radicals present in the bulk solution is mainly responsible for the COD reduction or the decolorisation of the effluent [61]. In addition, Fenton oxidation also substantially impacts the degradation of the dye compound by providing catalytic activity of the ferrous ion (Fe2+) to give additional hydroxyl radicals. The classical Fenton mechanism describes the generation of strong hydroxyl radicals (HO•) based on the following reaction sequence (Equations 6–12) [62]:
| Fe2++H2O2→Fe3++HO•+HO− k=63.5M−1s−1 | (6) |
| Fe2++HO•→Fe3++OH− k=3.2×108 M−1s−1 | (7) |
| RH+HO•→H2O+R•→further oxidation | (8) |
| R•+Fe3+→Fe2++R+ | (9) |
| Fe3++H2O2→Fe2++HOO•+H+ k=0.001–0.01M−1s−1 | (10) |
| HO•+H2O2→HOO•+H2O k=3.3×107 M−1s−1 | (11) |
| HO•+HO•→HOO•+HO− k=6.0×109 M−1s−1 | (12) |
Based on the above reactions, it can be said that Fe2+ is spontaneously oxidized to Fe3+, while Fe2+ is generated from the Fenton reaction between Fe3+ and H2O2 at a very slow rate. Equation 6 is known as the heart of the Fenton reaction. Fe3+ ions produced in Equations 6 and 7 also react with hydroxide ions to form ferric hydroxide [Fe(OH)2], which precipitates as sludge at the end of the treatment. The combined approach of US and Fenton oxidation is effective in the lowering of the mass transfer resistance by the physical effects of cavitation phenomena such as microjets, microstreaming and high-intensity shock waves [62] and the regeneration of the ferrous ions (Fe2+) through the breakage of the complex formed in the Fenton oxidation [63]. The dissociation of added hydrogen peroxide due to the action of ultrasound also helps in giving an enhanced generation of hydroxyl radicals and hence higher COD reduction. At much higher content of hydrogen peroxide, typically the undissociated hydrogen peroxide plays a scavenging action leading to lower availability of hydroxyl radicals and hence lower extents of COD reduction, thus directing the selection of an optimum ratio.
Fenton ratio plays an important role in the Fenton oxidation or US + Fenton system. If the Fenton ratio is less than 1 then Fe2+ acts as a coagulating agent and Fenton process is dominated by a simple coagulation process. Conversely, if the Fenton ratio is greater than 1 then the Fe2+ catalyses the H2O2 to produce hydroxyl radicals [64]. The Fenton ratio must therefore be optimized to yield an effective generation of hydroxyl radicals and also to minimize the sludge generation. In addition, there exists an option available to tackle the generated sludge during the Fenton oxidation. For example, one of the recent composting technology based on the rotary drum was demonstrated to convert sludge generated in common effluent treatment plants (CETPs) to stabilized, matured compost that can be used to boost soil fertility or for soil amendment [65]. Such technology can replace the conventional approaches of sludge disposal such as landfilling and incineration to transform the sludge generated in the Fenton based oxidation process into highly nutritious materials. Neyens and Baeyens [64] also reported the importance of Fenton ratio (H2O2/Fe2+) in the US + Fenton system by performing study at various Fenton ratios of 1 to 9. It was reported that maximum COD removal of 86.4% was obtained using an optimum Fenton ratio of 3. A similar study was performed by Dükkancı et al. [66] for the decolorization of azo dye Orange II using combined approach of US + UV + Fenton at various molar ratios of Fe2+:H2O2 viz. 1:60, 1:50, 1:30 and 1:10. It was reported that maximum decolorization using US + UV + Fenton (H2O2 = 5 mM) system was obtained at 1:50 molar ratio of Fe2+:H2O2 within 2 h of treatment. Similar studies were also reported for the degradation of C.I. Reactive Blue 181 [67], Reactive Yellow 16 [68], and Reactive Blue 19 [69] using a combined approach of US and Fenton. It is important to note that the extent of degradation of each compound and the optimal loading of the oxidant was different directing the need for establishing the best treatment conditions so as to maximize the degree of intensification.
4.1.3. US + O3
Ozone is one of the powerful chemicals widely accepted for industrial use as a chemical oxidant or as a disinfectant. The effect of using ozonation at different ozone loading of 0.5 g/h to 3 g/h to intensify the ultrasound process was checked in terms of COD reduction. Fig. 5 depicts the obtained results for the application of US + O3 system for the treatment of effluent-I. The obtained results depicted that US + ozone at 0.5 g/h of ozone loading was found to be inadequate for the expected outcome and resulted in only 37% of COD reduction within 60 min of treatment. A similar trend to that seen in US + Fenton was also observed in the case of the US + ozone scheme in terms of spontaneously increasing degradation rate within 5 min of treatment time followed by constant degradation up to 60 min of treatment time due to an increase in the rate of degradation via the initial direct attack or indirect generation and attack of hydroxyl radicals during the process [70]. The rate of O3 decomposition is accelerated by an increase in pH, resulting in the generation of various oxidants such as HO•, HO2•, and O2 through the following reactions [62], [71]:
| O3+OH−→O2+HO2− k=70M−1s−1 | (13) |
| O3+HO2−→HO•+O2+O2•− k=2.8×106M−1s−1 | (14) |
| O3+O2•−→O3•−+O2 k=1.6×109M−1s−1 | (15) |
| O3•−+H+↔HO3• k=5×1010M−1s−1 | (16) |
| HO3•→HO•+O2 k=1.1–1.4×105M−1s−1 | (17) |
| HO•+O3→HO2•+O2 k=1.0×108–3.0×109M−1s−1 | (18) |
Fig. 5.
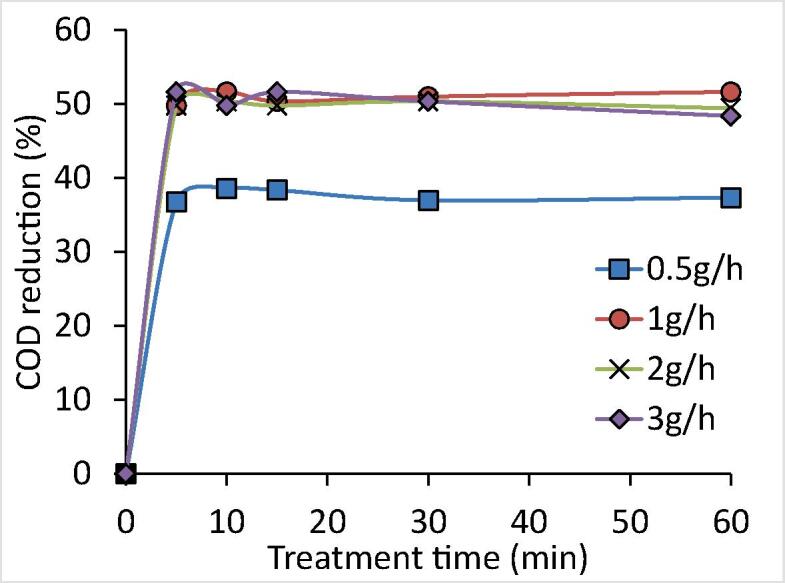
Effect of ozone loadings in US + ozone scheme on the obtained COD reduction (Effluent-I).
The extent of the COD reduction increased from 37% to 51.6% as ozone loading changed from 0.5 g/h to 1 g/h. Conversely, the extent of COD reduction was lower as ozone loading increased beyond 1 g/h with actual extent of reduction as 49.4% and 48.4% for the case of 2 and 3 g/h of ozone loadings respectively attributed to improper utilization of O3 in the treatment process [72], [73]. It was found that the combination of US and ozone at 1 g/h of ozone loading was effective and optimum in terms of obtaining a maximum COD reduction of 51.6% amongst all the ozone loadings and individual approaches of only US (27.3%) and only ozone (46.6%). It is important to note that the use of ultrasound is not giving a synergism in terms of observed COD reduction with respect to ozone though there is some reduction in the required time of treatment to reach the maximum COD reduction. A similar pattern was also reported for the degradation of CI Red 23 using US + ozone approach [73]. It was reported that the combined approach of US + O3 (3.2 g/h) was efficient in giving 98% of decolorization within 1 min of treatment time which was much higher as compared to only US (4% in 8 min) and marginally higher but much faster compared to only ozone (97% in 8 min). Shen et al. [74] also explored the use of US based ozonation process for the degradation of reactive red X-3B dye at various initial concentrations (50, 100, 125, 150 and 200 mg/L), pH of the sample (1 to 10), ozone flow rate (24 to 60 L/h), temperature (298 to 328 K), and US intensity (80 to 200 W/L). US + ozone scheme was reported to yield the maximum decolorization of 99.2% which was also higher as compared to US alone (19.1%) and ozone alone (84.55%) at optimum conditions as 100 mg/L of initial concentration, 6.52 of pH, 40 L/h of ozone flux, 200 W/L of US intensity, and 6 min of the treatment time. US + O3 system was effective to yield synergism achieving a synergetic index of 1.42. Thus, it can be said that observed effects are dependent on the system as in the current work synergism was not observed for the combination and only higher COD reduction was seen.
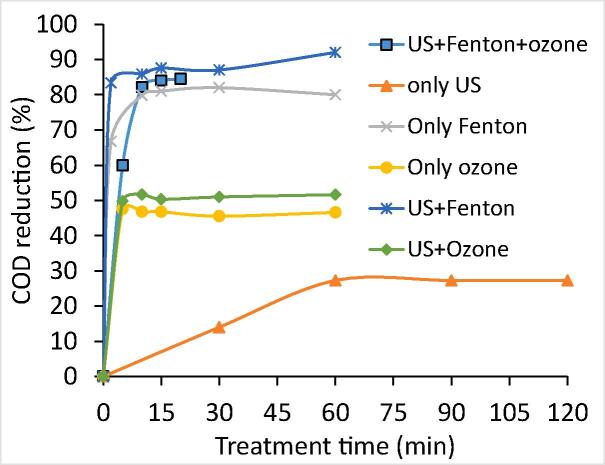
4.1.4. US + Fenton + O3
The combined approach of US coupled with Fenton reagent and ozone was also applied for the treatment of industrial dye effluent-I under conditions of H2O2 loading as 17.5 g/L, H2O2/Fe2+ ratio of 3 and ozone flow rate of 1 g/h. Fig. 6 depicts the obtained results for the application of US + Fenton + O3 system for the treatment of effluent-I. US + Fenton + O3 followed by lime treatment scheme using the optimal value of Fenton loading and ozone dose was applied for the treatment of effluent-I with an objective of enhancement in the efficiency of the process. The COD reduction was typically found to be increased with increased treatment time up to 10 min of treatment time and further treatment was not effective for additional COD reduction. Lime treatment (CaO:1g/L) was applied in the process as a finishing step for the US + Fenton + O3 scheme to remove the color as well as sludge particles formed during the Fenton process. It was observed that the US + Fenton + O3 followed by lime treatment was effective in achieving 95% of COD reduction in 30 min of treatment time. A similar result (95% of COD reduction) was also observed in the case of US + Fenton (H2O2: 17.5 g/L, H2O2/Fe2+: 3) followed by a lime treatment scheme within 70 min of treatment time, meaning that inclusion of ozone was making the treatment faster.
Fig. 6.
Effect of different approaches involving US, Fenton and O3 (H2O2: 17.5 g/L, H2O2/Fe2+: 3, O3: 1 g/h) on COD reduction (Effluent-I).
Typically ultrasonic irradiation enhances the turbulence in the ozonation system and breaks the bubbles of ozone that leads to creating high surface area. Due to these mechanical effects, US indirectly enhanced the mass transfer coefficient by reducing the liquid film thickness and breakage of gas bubbles [75]. Hence, the combined effect of US and ozone is most effective as compared to the individual approach of ozone and US. Application US + ozone system in the oxidation processes like Fenton oxidation reduces the activation energy required for the degradation of specific bio-refractory or recalcitrant compounds leading to efficient degradation with lower chemical consumption [76]. In addition, as higher amount of the hydroxyl radicals are generated in the US + Fenton + O3 process, higher COD reduction is seen and also reduced treatment time is required.
A similar trend in terms of best results for the US + O3 + Fe2++H2O2 process was also reported by Asaithambi et al. [77] for the removal of color and COD of the landfill leachate. A detailed study using different approaches as US, O3, US + H2O2, O3 + H2O2, US + Fe2++H2O2, O3 + Fe2++H2O2, and O3 + US + Fe2++H2O2 clearly revealed that US + O3 + Fe2++H2O2 process was most effective method amongst all the methods in terms of giving maximum color and COD removal of 100% and 95% respectively under the optimum conditions of H2O2 dosage of 60 mM, initial COD of 1500 ppm, Fe2+ dosage of 30 mM, O3 flow rate of 20 LPM, O3 mass flow rate of 3.5 g/hr, pH of 7, US power of 100 W, US frequency of 20 kHz and 180 min of treatment time. The synergy index and the energy required for the combination system were 18.5 and 1.20 kWh/m3 respectively. Ghanbari et al. [78] also reported the efficient use of US + Fenton + O3 for the degradation of benzotriazole (BTA) with an indepth study into the effect of various operating parameters such as pH, O3 loading, PMS loading, and US power. It was reported that maximum degradation of BTA as 54%, 72.3%, and 94.6% were observed using US + PMS + O3 process for the saline water, petrochemical effluent, and secondary effluent matrices respectively at fixed conditions of neutral pH, O3 loading of 6.8 mg/L, PMS loading of 1.5 mM, US power of 200 W and 60 min of treatment time.
4.2. Case study of real industrial effluent (effluent-II)
4.2.1. Only US
The effect of only US on the COD reduction of the effluent-II was studied to compare the results with the combined approaches. It was observed that 26% of COD reduction was obtained using the individual approach of US for the treatment of effluent-II within 120 min of treatment time and further lime treatment (CaO: 1 g/L) subsequent to only US enhanced the COD reduction to 29% within 130 min of overall treatment time.
4.2.2. US + Fenton
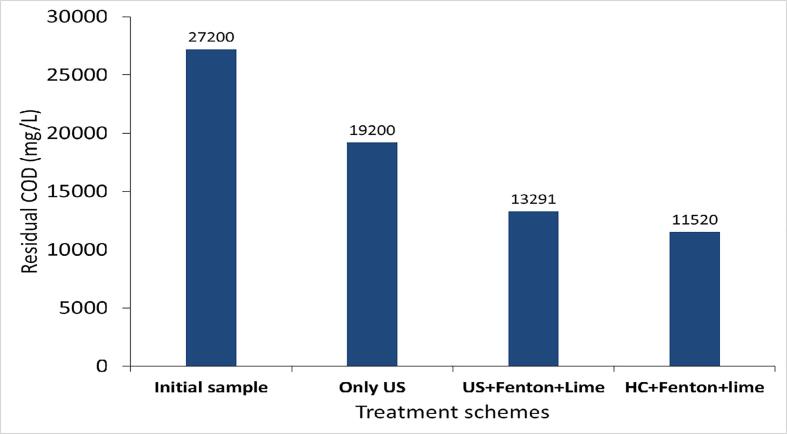
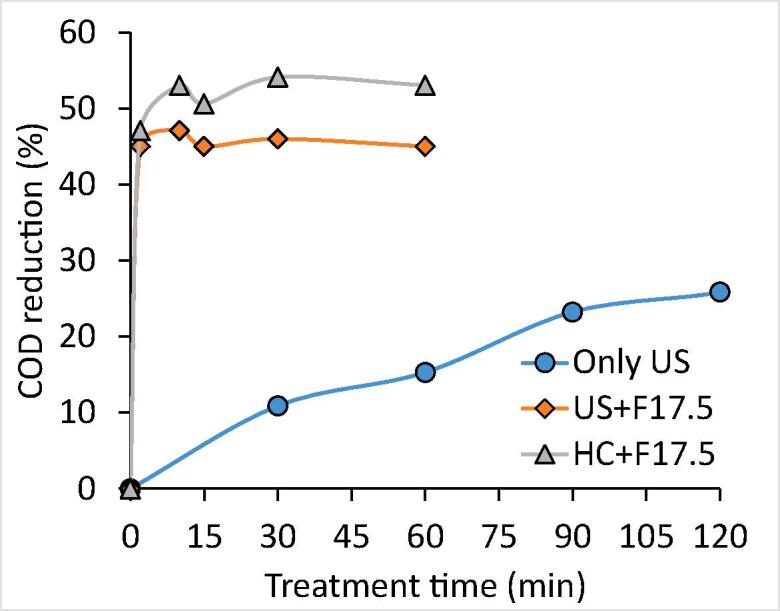
A similar approach of US + Fenton used in the treatment of effluent-I was also implemented in the treatment of effluent-II using the same optimum conditions. The result obtained using US + Fenton (H2O2: 17.5 g/L, H2O2/Fe2+: 3) followed by a lime treatment are depicted in Table 2 and Fig. 7. It was observed that extent COD reduction was maximum (45%) within 2 min of treatment time and further treatment was not significantly useful for the additional reduction of COD. Lime treatment played a significant role in increasing the additional COD reduction to 51%, also reducing the color from turbid brown (due to the presence of ferrous salt) to clear light yellow and allowed the removal of sludge generated in the Fenton process. The combined approach of US + Fenton followed by lime treatment was found to be efficient in yielding a maximum COD reduction of 51% which was much higher as compared to the individual approach of US alone (29%).
Fig. 7.
Comparison of various treatment schemes in terms of the COD reduction (operating conditions = H2O2:17.5 g/L, H2O2/Fe2+:3, HC inlet pressure: 4 bar) (effluent-II).
Siddique et al. [69] studied the decolorization of reactive blue 19 dye effluent using US assisted Fenton oxidation approach under varying operating conditions of initial dye concentration, US power intensity, loading of H2O2, loading of ferrous sulfate, and pH value at fixed 20 kHz as the US frequency. It was reported that maximum decolorization of 78% was obtained using US + Fenton system under the optimized conditions of initial dye concentration as 25 mg/L, H2O2 loading of 0.5 mM, FeSO4 loading of 3 mg/L, pH of 3.5, US power of 8 W/cm2 and 30 min of treatment. Sun et al. [79] also explored the use of US to intensify the low concentration iron-based Fenton oxidation for the degradation of acid black 1 (AB1) dye, also exploring the effect of various operating parameters like initial dye concentration, US power density, US frequency, pH, H2O2 dosage, Fe2+ dosage, and temperature. It was reported that maximum AB1 degradation as 98.83% was obtained using combined approach of US + Fenton (higher as compared to individual process of Fenton oxidation and US) under the optimum conditions of initial dye concentration as 0.081 mM, 8.0 mM as the H2O2 loading, 0.025 mM as the quantity of Fe2+, US frequency of 40 kHz, US power density 50 W/L, pH of 3, temperature of 20 °C and 30 min as treatment time. It is thus established from the current study as well as literature comparison that US + Fenton approach is efficient in terms of intensifying the oxidative treatment of wastewater as compared to the individual oxidation approaches.
4.2.3. HC + Fenton
The optimized treatment scheme was demonstrated at pilot scale using a hydrodynamic cavitation reactor with a capacity of 4L. The inlet pressure of the HC reactor was kept constant at 4 bar (flow rate of effluent: 0.756 m3/h; cavitation number: 0.5722). The obtained results are shown in Fig. 7. The COD reduction spontaneously increased with the treatment time giving a maximum COD reduction of 53% within 10 min of treatment time and further, it was constant with increasing time to 60 min of treatment time. Lime treatment (CaO: 1 g/L) was also applied to the HC + Fenton scheme in order to remove the sludge particles and color. Lime also played some role in enhancing the COD reduction of HC + Fenton treated effluent from 53% to 58%. A combined approach of HC and Fenton (H2O2: 17.5 g/L, H2O2/Fe2+: 3) followed by lime treatment was thus found to be effective in terms of achieving a maximum COD reduction of 58%.
A similar study was reported by Rajoriya et al. [80] for the treatment of textile dyeing industrial effluent using HC + Fenton scheme at initial COD concentration range of 2560 to 4640 mg/L. It was reported that maximum decolorisation (97.7%), COD reduction (38% in 120 min) and TOC reduction (48% in 15 min) were obtained at an optimal Fenton ratio (Fe2+: H2O2) of 1:5 and 5 bar of inlet pressure. Askarniya et al. [81] explored the use of HC + Fenton scheme for the decolorization of Congo red (diazoic dye) with studies performed at various operating parameters viz. pH of the solution (3–10), H2O2 dosage (0–1 g/L) and FeSO4 loading (0–0.05 g/L). At optimal conditions i.e. pH of the solution as 3, H2O2 dosage as 1 g/L, and FeSO4 loading as 0.025 g/L, a maximum decolorization (70%) was obtained using combined HC and Fenton oxidation within 60 min of treatment time with a synergetic coefficient of 3.22 and yield efficiency of 8.3 × 10−3 mg/kJ.
4.3. Comparison of different combined approaches based on two case studies
A comparison of all the approaches implemented for the textile industrial effluent-I and II was performed in terms of effective COD reduction (%), cavitational yield (mg/J) and the cost of the process (Figs. 8, 9, Tables 2 and 3). The operational cost including electricity cost and chemical cost has been calculated based on the commercial price of the electricity and chemicals. US + Fenton (H2O2: 17.5 g/L; H2O2/Fe2+: 3) followed by lime treatment (CaO: 1 g/L) was found to be most efficient amongst all the studied approaches in terms of giving maximum COD reduction (94.8% and 51% for effluent I and II respectively), cavitational yield (7.89 10−3 mg/J and 8.50 10−3 mg/J) and the treatment cost (4.41 Rs/L and 9.49 Rs/L [approx. 0.059 USD/L and 0.126 USD/L considering conversion factor of 1 USD = 75 Rs]) for the treatment of effluent-I and effluent-II respectively at batch scale as depicted in Figs. 8, 9 and Table 2. The maximum cost of the operation was required for the individual approach of US (17.88 Rs/L [approx. 0.238 USD/L] for effluent-I and 27.06 Rs/L [approx. 0.361 USD/L] for effluent-II) in both the case studies due to the higher consumption of electricity required for the desired outcome. It can be also seen that HC + Fenton (H2O2: 17.5 g/L; H2O2/Fe2+: 3) was found to be the most efficient scheme amongst all other scheme involving US for the treatment of effluent-II in terms of cavitational yield (564.36 10−3 mg/J) and the treatment cost (1.93 Rs/L [approx. 0.0257 USD/L]). Importantly, HC + Fenton is demonstrated to be efficient at large scale operation which should give credence to possible commercial application.
Fig. 8.
Comparison of treatment schemes for the treatment of effluent-I.
Fig. 9.
Comparison of treatment schemes for the treatment of effluent-II.
Table 3.
Comparison of different advanced oxidation approaches in terms of cost of treatment.
| Type of effluent | Treatment scheme | Total Energy required for the treatment (kWh) | Total electricity cost, Rs/L (USD/L) | Chemical cost, Rs/L (USD/L) | Treatment cost, Rs/L (USD/L) |
|---|---|---|---|---|---|
| Effluent-I | Only US (CaO:1g/L) | 2.04 | 17.875 (0.238)* | 0.005 (6.67 × 10−5) | 17.880) (0.238) |
| Only Fenton (H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.54 | 4.756 (0.063) | 1.448 (0.019) | 6.204 (0.083) | |
| Only ozone (O3: 1 g/h) | 1.30 | 11.429 (0.152) | 0.000 (0.000) | 11.429 (0.152) | |
| US + Fenton + Lime (H2O2:15 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.47 | 4.096 (0.055) | 1.242 (0.017) | 5.338 (0.071) | |
| US + Fenton + Lime (H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.34 | 2.961 (0.039) | 1.448 (0.019) | 4.408 (0.059) | |
| US + Fenton + Lime (H2O2:20 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.33 | 2.906 (0.039) | 1.654 (0.022) | 4.560 (0.061) | |
| US + Fenton + Lime (H2O2:22.5 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.33 | 2.888 (0.039) | 1.860 (0.025) | 4.748 (0.063) | |
| US + Fenton + Lime (H2O2:30 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.37 | 3.215 (0.043) | 2.478 (0.033) | 5.693 (0.076) | |
| US + Fenton + Lime (H2O2:17.5 g/L, H2O2/Fe2+:1, CaO:1g/L) | 0.36 | 3.140 (0.042) | 1.448 (0.019) | 4.588 (0.061) | |
| US + Fenton + Lime (H2O2:17.5 g/L, H2O2/Fe2+:2, CaO:1g/L) | 0.35 | 3.087 (0.041) | 1.448 (0.019) | 4.535 (0.060) | |
| US + Fenton + Lime (H2O2:17.5 g/L, H2O2/Fe2+:4, CaO:1g/L) | 0.35 | 3.103 (0.041) | 1.448 (0.019) | 4.551 (0.061) | |
| US + Ozone (O3: 0.5 g/h) | 4.74 | 41.598 (0.555) | 0.000 (0.000) | 41.598 (0.555) | |
| US + Ozone (O3: 1 g/h) | 3.90 | 34.219 (0.456) | 0.000 (0.000) | 34.219 (0.456) | |
| US + Ozone (O3: 2 g/h) | 4.00 | 35.086 (0.468) | 0.000 (0.000) | 35.086 (0.468) | |
| US + Ozone (O3: 3 g/h) | 4.05 | 35.522 (0.474) | 0.000 (0.000) | 35.522 (0.474) | |
| US + Fenton + O3 (H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L, O3:1g/h) | 1.04 | 9.134 (0.122) | 1.448 (0.019) | 10.582 (0.141) | |
| Effluent-II | Only US (CaO:1g/L) | 3.08 | 27.055 (0.361) | 0.005 (6.67 × 10−5) | 27.060 (0.361) |
| US + Fenton + Lime (H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.92 | 8.041 (0.107) | 1.448 (0.019) | 9.489 (0.127) | |
| HC + Fenton + Lime (H2O2:17.5 g/L, H2O2/Fe2+:3, CaO:1g/L) | 0.05 | 0.481 (0.006) | 1.448 (0.019) | 1.929 (0.026) |
*Numbers in brackets give Cost in US Dollar using the conversion rate of 75 Rs = 1 USD.
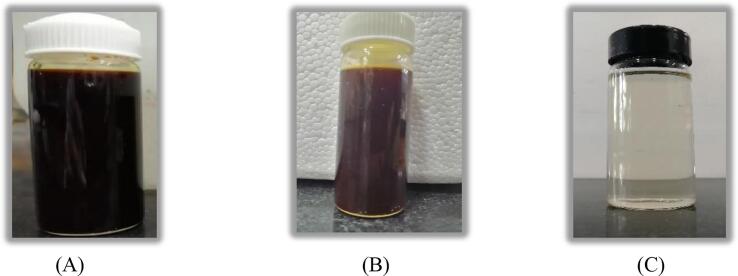
Lime treatment applied in both the schemes for removing the solid sludge particles from the effluent after the Fenton oxidation was indeed effective and able to convert the brown color and dark yellow effluents to colorless and transparent effluent respectively as shown in Figs. 10 and 11. The introduction of ozonation in the US + Fenton system was found to be beneficial in terms of minimizing the treatment time for getting the desired output. In the case of US + Fenton + O3 (O3: 1 g/h, H2O2: 17.5 g/L; H2O2/Fe2+: 3), a COD reduction of 95.1% was obtained within 30 min of treatment time as shown in Fig. 6. Hence, it was concluded that O3 played an important role to enhance the rate of destruction of the compounds and shortened the treatment time to obtain the desired outcome. It is also confirmed that combined approach is more efficient as compared to the individual approach due to less chemical and treatment time requirement as per the data shown in Table 2 and Table 3.
Fig. 10.
Photographic representation of (A) fresh effluent-I (B) effluent-I after US + Fenton (H2O2:17.5 g/L, H2O2/Fe2+:3) treatment (C) effluent-I after CaO (dose: 1 g/L) treatment subsequent to US + Fenton.
Fig. 11.
Photographic representation of (A) fresh effluent-II (B) effluent-II after US + Fenton (H2O2:17.5 g/L, H2O2/Fe2+:3) treatment (C) effluent-II after CaO (dose: 1 g/L) treatment subsequent to US + Fenton.
5. Conclusions
The current research demonstrated the application of an imperative hybrid approach based on cavitation for the treatment of textile industrial wastewater. The initial literature analysis highlighted the important guidelines for the selection of operating parameters for US and advanced oxidation based hybrid processes also highlighting the beneficial results in terms of the enhanced mineralization with synergism. Treatment studies of two commercial effluents have also been presented using various combined approaches of US with Fenton oxidation and ozone. The lab-scale system of US + Fenton was also demonstrated at pilot scale using HC reactor (volume: 4L). It was demonstrated that the US in combination with Fenton oxidation (H2O2: 17.5 g/L; H2O2/Fe2+: 3) followed by lime treatment (CaO: 1 g/L) was most effective amongst all approaches in terms of giving maximum COD reduction of 94.8% for the treatment of textile effluent-I. The maximum COD reduction of 51% for the treatment of effluent-II was obtained using US in combination with Fenton oxidation (H2O2: 17.5 g/L; H2O2/Fe2+: 3) followed by lime treatment (CaO: 1 g/L). The ozonation process also showed a beneficial effect in terms of reducing the required time (30 min) for the combined approach of US + Fenton + ozone as compared to only ozone (47.6% in 70 min), only US (27.3% in 130 min), a combined approach of US + Fenton (94.8% in 70 min), a combined approach of US + ozone (51.6% in 70 min) and also giving desired results in terms of COD reduction of 95.1%. The treatment cost for the best approach of US + Fenton + O3 under optimum treatment conditions of H2O2 loading of 17.5 g/L, H2O2/Fe2+ ratio of 3, CaO loading of 1 g/L and O3 flow rate as 1 g/h was observed to be 10.582 Rs/L (0.141 USD/L). Lime treatment applied after the Fenton based process played a significant role in terms of removing the sludge particles, giving additional COD reduction and also removing the color of the effluent such that the effluent turned from dark brown (effluent-I) to colorless and from dark yellow (effluent-II) to clear pale yellow. Overall, the hybrid approach of cavitation + Fenton system was most effective in terms of getting the desired COD reduction with minimum operating cost and lower chemical consumption.
CRediT authorship contribution statement
Swapnil K. Gujar: Methodology, Investigation, Writing - original draft. Parag R. Gogate: Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
SG and PRG gratefully acknowledge the SS Techno Limited, Pune for financial support for the research work.
Appendix I.
Sample calculation for treatment cost (Rs/L) and cavitational yield (mg/J):
Acoustic power dissipated by ultrasonic horn (250 ml of effluent) = 100 W
Acoustic power dissipated per unit volume = 100 W 4 = 400 W/L
Extent of COD reduction (mg/L) = (8640 27.27)/100 = 2356.13 mg/L
Cavitational yield (mg/J) = Extent of COD reduction / power dissipation
= 2356.13 / (400 120 60 0.7)
= 1.17 10−3 mg/J
Energy (E1) required for the COD reduction upto discharge limit (250 mg/L)
= 8690 / 1.17 10−3
= 1.994 kWh
Energy (E2) required for stirring in the coagulation treatment
= (Power × t) / (60 × 1000)
= (500 × 5) / (60 × 1000)
= 0.042 kWh
Total energy (E1 + E2) required for the treatment (kWh)
= 1.994 + 0.042
= 2.036 kWh
Considering, 1 kWh = Rs 8.78 (data from Maharashtra State Electricity Distribution Co. Ltd., Mumbai)
Operational cost = 2.036 8.78
= 17.876 Rs/L
Chemical cost considering commercial price of chemicals as (CaO: 5 Rs/kg),
Chemical cost = dose of CaO (kg/L) price of CaO (Rs/kg)
= 0.001 5
= 0.005 Rs/L
Overall treatment cost of the process = operational cost + chemical cost
= 17.876 + 0.005
= 17.88 Rs/L (0.238 USD/L considering 1 USD – 75 Rs)
References
- 1.Alinsafi A., Khemis M., Pons M.N., Leclerc J.P., Yaacoubi A., Benhammou A., Nejmeddine A. Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Process. 2005;44(4):461–470. [Google Scholar]
- 2.Misra O.P., Chaturvedi D. Fate of dissolved oxygen and survival of fish population in aquatic ecosystem with nutrient loading: a model. Model Earth Syst. Environ. 2016;2:1–14. [Google Scholar]
- 3.Makoś P., Przyjazny A., Boczkaj G. Methods of assaying volatile oxygenated organic compounds in effluent samples by gas chromatography-a review. J. Chromatogr. A. 2019;1592:143–160. doi: 10.1016/j.chroma.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Katheresan V., Kansedo J., Lau S.Y. Efficiency of various recent wastewater dye removal methods: a review. J. Environ. Chem. Eng. 2018;6(4):4676–4697. [Google Scholar]
- 5.Gore M.M., Saharan V.K., Pinjari D.V., Chavan P.V., Pandit A.B. Degradation of reactive orange 4 dye using hydrodynamic cavitation based hybrid techniques. Ultrason. Sonochem. 2014;21(3):1075–1082. doi: 10.1016/j.ultsonch.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Chakinala A.G., Gogate P.R., Burgess A.E., Bremner D.H. Industrial wastewater treatment using hydrodynamic cavitation and heterogeneous advanced Fenton processing. Chem. Eng. J. 2009;152(2-3):498–502. [Google Scholar]
- 7.Bhandari V.M., Sorokhaibam L.G., Ranade V.V. Industrial wastewater treatment for fertilizer industry-a case study. Desalin. Water Treat. 2016;57:27934–27944. [Google Scholar]
- 8.Liu Z., Yang Y., Shao C., Ji Z., Wang Q., Wang S., Guo Y., Demeestere K., Hulle S.V. Ozonation of trace organic compounds in different municipal and industrial wastewaters: kinetic-based prediction of removal efficiency and ozone dose requirements. Chem. Eng. J. 2020;387:123405. [Google Scholar]
- 9.Behin J., Akbari A., Mahmoudi M., Khajeh M. Sodium hypochlorite as an alternative to Hydrogen peroxide in Fenton process for industrial scale. Water Res. 2017;121:120–128. doi: 10.1016/j.watres.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Chen J.R., Xu X.-W., Lee A.S., Yen T.F. A feasibility study of dechlorination of chloroform in water by ultrasound in the presence of hydrogen peroxide. Environ. Technol. 1990;11(9):829–836. [Google Scholar]
- 11.Tezcanli-Güyer G., Ince N.H. Individual and combined effects of ultrasound, ozone and UV irradiation: a case study with textile dyes. Ultrasonics. 2004;42:603–609. doi: 10.1016/j.ultras.2004.01.096. [DOI] [PubMed] [Google Scholar]
- 12.Jessieleena A.A., Priyanka M., Saravanakumar M.P. Comparative study of Fenton, Fe2+/NaOCl and Fe2+/(NH4)2S2O8 on tannery sludge dewaterability, degradability of organics and leachability of chromium. J. Hazard. Mater. 2021;402:123495. doi: 10.1016/j.jhazmat.2020.123495. [DOI] [PubMed] [Google Scholar]
- 13.Heinz O.L., Cunha M.A., Amorim J.S., Barbosa-Dekker A.M., Dekker R.F., Barreto-Rodrigues M. Combined fungal and photo-oxidative Fenton processes for the treatment of wood-laminate industrial waste effluent. J. Hazard. Mater. 2019;379:120790. doi: 10.1016/j.jhazmat.2019.120790. [DOI] [PubMed] [Google Scholar]
- 14.Mahdavi R., Talesh S.S.A. Sol-gel synthesis, structural and enhanced photocatalytic performance of Al doped ZnO nanoparticles. Adv. Powder Technol. 2017;28(5):1418–1425. [Google Scholar]
- 15.Fernandes A., Makoś P., Wang Z., Boczkaj G. Synergistic effect of TiO2 photocatalytic advanced oxidation processes in the treatment of refinery effluents. Chem. Eng. J. 2020;391:123488. [Google Scholar]
- 16.Sathishkumar K., AlSalhi M.S., Sanganyado E., Devanesan S., Arulprakash A., Rajasekar A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B, Biol. 2019;200:111655. doi: 10.1016/j.jphotobiol.2019.111655. [DOI] [PubMed] [Google Scholar]
- 17.Boczkaj G., Gągol M., Klein M., Przyjazny A. Effective method of treatment of effluents from production of bitumens under basic pH conditions using hydrodynamic cavitation aided by external oxidants. Ultrason. Sonochem. 2018;40:969–979. doi: 10.1016/j.ultsonch.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Cuerda-Correa E.M., Alexandre-Franco M.F., Fernández-González C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water. 2020;12:102. [Google Scholar]
- 19.Gągol M., Przyjazny A., Boczkaj G. Effective method of treatment of industrial effluents under basic pH conditions using acoustic cavitation–a comprehensive comparison with hydrodynamic cavitation processes. Chem. Eng. Process. 2018;128:103–113. [Google Scholar]
- 20.Fernandes A., Gągol M., Makoś P., Khan J.A., Boczkaj G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019;224:1–14. [Google Scholar]
- 21.Gogate P.R., Kabadi A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009;44(1):60–72. [Google Scholar]
- 22.Menon P., Singh T.A., Pani N., Nidheesh P.V. Electro-Fenton assisted sonication for removal of ammoniacal nitrogen and organic matter from dye intermediate industrial wastewater. Chemosphere. 2021;269:128739. doi: 10.1016/j.chemosphere.2020.128739. [DOI] [PubMed] [Google Scholar]
- 23.Gujar S.K., Gogate P.R., Kanthale P., Pandey R., Thakre S., Agrawal M. Combined oxidation processes based on ultrasound, hydrodynamic cavitation and chemical oxidants for treatment of real industrial wastewater from cellulosic fiber manufacturing sector. Sep. Purif. Technol. 2021;257:117888. [Google Scholar]
- 24.Jaafarzadeh N., Takdastan A., Jorfi S., Ghanbari F., Ahmadi M., Barzegar G. The performance study on ultrasonic/Fe3O4/H2O2 for degradation of azo dye and real textile wastewater treatment. J. Mol. Liq. 2018;256:462–470. [Google Scholar]
- 25.R. Darvishi Cheshmeh Soltani, S. Jorfi, S. Alavi, P. Astereki, F. Momeni, Electrocoagulation of textile wastewater in the presence of electro-synthesized magnetite nanoparticles: simultaneous peroxi-and ultrasonic-electrocoagulation, Sep. Sci. Technol. 55 (2020) 945–954.
- 26.Mahdavi Reza, Ashraf Talesh S. Siamak. Enhancement of ultrasound-assisted degradation of Eosin B in the presence of nanoparticles of ZnO as sonocatalyst. Ultrason. Sonochem. 2019;51:230–240. doi: 10.1016/j.ultsonch.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Gautam R.K., Tiwari I. Humic acid functionalized magnetic nanomaterials for remediation of dye wastewater under ultrasonication: application in real water samples, recycling and reuse of nanosorbents. Chemosphere. 2020;245:125553. doi: 10.1016/j.chemosphere.2019.125553. [DOI] [PubMed] [Google Scholar]
- 28.Weng Chih-Huang, Lin Yao-Tung, Chang Cheng-Kuan, Liu Na. Decolourization of direct blue 15 by Fenton/ultrasonic process using a zero-valent iron aggregate catalyst. Ultrason. Sonochem. 2013;20(3):970–977. doi: 10.1016/j.ultsonch.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Martínez Susana Silva, Uribe Edgar Velasco. Enhanced sonochemical degradation of azure B dye by the electroFenton process. Ultrason. Sonochem. 2012;19(1):174–178. doi: 10.1016/j.ultsonch.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Ince Nilsun H., Tezcanlí Gökçe. Reactive dyestuff degradation by combined sonolysis and ozonation. Dyes Pigm. 2001;49(3):145–153. [Google Scholar]
- 31.Özyonar F., Gökkuş O., Sabuni M. Removal of disperse and reactive dyes from aqueous solutions using ultrasound-assisted electrocoagulation. Chemosphere. 2020;258:127325. doi: 10.1016/j.chemosphere.2020.127325. [DOI] [PubMed] [Google Scholar]
- 32.Shokoofehpoor Fatemeh, Chaibakhsh Naz, Ghanadzadeh Gilani Ali. Optimization of sono-Fenton degradation of Acid Blue 113 using iron vanadate nanoparticles. Sep. Sci. Technol. 2019;54(17):2943–2958. [Google Scholar]
- 33.Özdemir Celalettin, Öden Muhammed Kamil, Şahinkaya Serkan, Kalipçi Erkan. Color removal from synthetic textile wastewater by sono-fenton process. Clean-Soil Air Water. 2011;39(1):60–67. [Google Scholar]
- 34.K.E. Barrera-Salgado, G. Ramírez-Robledo, A. Álvarez-Gallegos, C.A. Pineda-Arellano, F.Z. Sierra-Espinosa, J.A. Hernández-Pérez, S. Silva-Martínez, Fenton process coupled to ultrasound and UV light irradiation for the oxidation of a model pollutant, J. Chem. 2016 (2016) Article ID 4262530.
- 35.Song Ya-Li, Li Ji-Tai, Chen Hua. Degradation of CI Acid Red 88 aqueous solution by combination of Fenton's reagent and ultrasound irradiation. J. Chem. Technol. Biotechnol. 2009;84(4):578–583. [Google Scholar]
- 36.Rehorek Astrid, Tauber Michael, Gübitz Georg. Application of power ultrasound for azo dye degradation. Ultrason. Sonochem. 2004;11(3-4):177–182. doi: 10.1016/j.ultsonch.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Harichandran G., Prasad S. SonoFenton degradation of an azo dye, Direct Red. Ultrason. Sonochem. 2016;29:178–185. doi: 10.1016/j.ultsonch.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Bharadwaj A., Saroha A.K. Decolorization of the textile wastewater containing reactive blue 19 dye by Fenton and photo-Fenton oxidation. J. Hazard. Toxic Radioact. Waste. 2015;19:04014043. [Google Scholar]
- 39.Weng Chih-Huang, Lin Yao-Tung, Liu Na, Yang Hong-Yang. Enhancement of the advanced Fenton process by ultrasound for decolorisation of real textile wastewater. Color. Technol. 2014;130(2):133–139. [Google Scholar]
- 40.Cetinkaya Semanur Giray, Morcali Mehmet Hakan, Akarsu Sümeyye, Ziba Cengiz Ayhan, Dolaz Mustafa. Comparison of classic Fenton with ultrasound Fenton processes on industrial textile wastewater. Sustain. Environ. Res. 2018;28(4):165–170. [Google Scholar]
- 41.Zhang Jian-Hao, Zou Hai-Yuan, Ning Xun-An, Lin Mei-Qing, Chen Chang-Min, An Tai-Cheng, Sun Jian. Combined ultrasound with Fenton treatment for the degradation of carcinogenic polycyclic aromatic hydrocarbons in textile dying sludge. Environ. Geochem. Health. 2018;40(5):1867–1876. doi: 10.1007/s10653-017-9946-1. [DOI] [PubMed] [Google Scholar]
- 42.J. Johin, P. V. Nidheesh, T. Sivasankar, Sono-electro-chemical treatment of reactive black 5 dye and real textile effluent using MnSO4/Na2S2O8 electrolytes, Arab. J. Sci. Eng. 44 (2019) 9987–9996.
- 43.Kiran Shumaila, Rafique Muhammad Asim, Iqbal Sarosh, Nosheen Sofia, Naz Saba, Rasheed Abdur. Synthesis of nickel nanoparticles using Citrullus colocynthis stem extract for remediation of Reactive Yellow 160 dye. Environ. Sci. Pollut. Res. 2020;27(26):32998–33007. doi: 10.1007/s11356-020-09510-9. [DOI] [PubMed] [Google Scholar]
- 44.Maheshwari K., Solanki Y.S., Ridoy M.S., Agarwal M., Dohare R., Gupta R. Ultrasonic treatment of textile dye effluent utilizing Microwave-assisted Activated carbon. Environ. Prog. Sustain. Energy. 2020;39:13410. [Google Scholar]
- 45.Karnjkar Y.S., Dinde R.M., Dinde N.M., Bawankar K.N., Hinge S.P., Mohod A.V., Gogate P.R. Degradation of magenta dye using different approaches based on ultrasonic and ultraviolet irradiations: Comparison of effectiveness and effect of additives for intensification. Ultrason. Sonochem. 2015;27:117–124. doi: 10.1016/j.ultsonch.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 46.G. Boczkaj, A. Fernandes, Wastewater treatment by means of Advanced Oxidation Processes at basic pH conditions: a review, Chem. Eng. J. 320 (2017) 608–633.
- 47.Fedorov K., Plata-Gryl M., Khan J.A., Boczkaj G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020;397:122804. doi: 10.1016/j.jhazmat.2020.122804. [DOI] [PubMed] [Google Scholar]
- 48.K. Fedorov, X. Sun, G. Boczkaj, Combination of hydrodynamic cavitation and SR-AOPs for simultaneous degradation of BTEX in water, Chem. Eng. J. (2020) Article ID 128081, In Press.
- 49.Gągol M., Cako E., Fedorov K., Soltani R.D.C., Przyjazny A., Boczkaj G. Hydrodynamic cavitation based advanced oxidation processes: studies on specific effects of inorganic acids on the degradation effectiveness of organic pollutants. J. Mol. Liq. 2020;307:113002. [Google Scholar]
- 50.Gągol M., Soltani R.D.C., Przyjazny A., Boczkaj G. Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs) Ultrason. Sonochem. 2019;58:104610. doi: 10.1016/j.ultsonch.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 51.Yuan R., Jiang Z., Wang Z., Gao S., Liu Z., Li M., Boczkaj G. Hierarchical MnO2 nanoflowers blooming on 3D nickel foam: a novel micro-macro catalyst for peroxymonosulfate activation. J. Colloid Interface Sci. 2020;571:142–154. doi: 10.1016/j.jcis.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 52.Yuan R., Jiang M., Gao S., Wang Z., Wang H., Boczkaj G., Liu Z., Ma J., Li Z. 3D mesoporous α-Co (OH) 2 nanosheets electrodeposited on nickel foam: a new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation. Chem. Eng. J. 2020;380:122447. [Google Scholar]
- 53.Soltani R.D.C., Mahmoudi M., Boczkaj G., Khataee A. Activation of peroxymonosulfate using carbon black nano-spheres/calcium alginate hydrogel matrix for degradation of acetaminophen: Fe3O4 co-immobilization and microbial community response. J. Ind. Eng. Chem. 2020;91:240–251. [Google Scholar]
- 54.Fernandes André, Makoś Patrycja, Boczkaj Grzegorz. Treatment of bitumen post oxidative effluents by sulfate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean. Prod. 2018;195:374–384. [Google Scholar]
- 55.Fernandes André, Makoś Patrycja, Khan Javed Ali, Boczkaj Grzegorz. Pilot scale degradation study of 16 selected volatile organic compounds by hydroxyl and sulfate radical based advanced oxidation processes. J. Clean. Prod. 2019;208:54–64. [Google Scholar]
- 56.Cako E., Gunasekaran K.D., Soltani R.D.C., Boczkaj G. Ultrafast degradation of brilliant cresyl blue under hydrodynamic cavitation based advanced oxidation processes (AOPs) Water Resour. Ind. 2020;24:100134. [Google Scholar]
- 57.Agarkoti C., Gogate P.R., Pandit A.B. Comparison of acoustic and hydrodynamic cavitation based hybrid AOPs for COD reduction of commercial effluent from CETP. J. Environ. Manage. 2021;281:111792. doi: 10.1016/j.jenvman.2020.111792. [DOI] [PubMed] [Google Scholar]
- 58.Roy K., Agarkoti C., Malani R.S., Thokchom B., Moholkar V.S. Mechanistic study of sulfadiazine degradation by ultrasound-assisted Fenton-persulfate system using yolk-shell Fe3O4@ hollow@ mSiO2 nanoparticles. Chem. Eng. Sci. 2020;217:115522. [Google Scholar]
- 59.Patil Amar L., Patil Pankaj N., Gogate Parag R. Degradation of imidacloprid containing wastewaters using ultrasound based treatment strategies. Ultrason. Sonochem. 2014;21(5):1778–1786. doi: 10.1016/j.ultsonch.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 60.Lim Myunghee, Son Younggyu, Khim Jeehyeong. The effects of hydrogen peroxide on the sonochemical degradation of phenol and bisphenol A. Ultrason. Sonochem. 2014;21(6):1976–1981. doi: 10.1016/j.ultsonch.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 61.Cai Mei Qiang, Wei Xiao Qin, Song Zhi Jun, Jin Mi Cong. Decolorization of azo dye Orange G by aluminum powder enhanced by ultrasonic irradiation. Ultrason. Sonochem. 2015;22:167–173. doi: 10.1016/j.ultsonch.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Gągol Michał, Przyjazny Andrzej, Boczkaj Grzegorz. Wastewater treatment by means of advanced oxidation processes based on cavitation–a review. Chem. Eng. J. 2018;338:599–627. [Google Scholar]
- 63.Pirsaheb M., Moradi N. Sonochemical degradation of pesticides in aqueous solution: investigation on the influence of operating parameters and degradation pathway–a systematic review. RSC Adv. 2020;10:7396–7423. doi: 10.1039/c9ra11025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neyens E., Baeyens J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003;98(1-3):33–50. doi: 10.1016/s0304-3894(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 65.Maheshwari S., Jethoo A.S., Vishvakarma V.K., Khwairakpam M., Kriplani P. Biodegradation of sludge produced from common effluent treatment plant (CETP) using drum composting technique. Nat. Environ. Pollut. Technol. 2019;18:231–236. [Google Scholar]
- 66.Dükkancı M., Vinatoru M., Mason T.J. The sonochemical decolourisation of textile azo dye Orange II: effects of Fenton type reagents and UV light. Ultrason. Sonochem. 2014;21:846–853. doi: 10.1016/j.ultsonch.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 67.Basturk Emine, Karatas Mustafa. Advanced oxidation of reactive blue 181 solution: a comparison between fenton and sono-fenton process. Ultrason. Sonochem. 2014;21(5):1881–1885. doi: 10.1016/j.ultsonch.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 68.Sundararaman T.R., Ramamurthi V., Partha N. Decolorization and COD removal of reactive yellow 16 by Fenton oxidation and comparison of dye removal with photo Fenton and sono Fenton process. Mod. Appl. Sci. 2009;3:15–22. [Google Scholar]
- 69.Siddique Maria, Farooq Robina, Price Gareth J. Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19. Ultrason. Sonochem. 2014;21(3):1206–1212. doi: 10.1016/j.ultsonch.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 70.WANG JIAN LONG, XU LE JIN. Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit. Rev. Env. Sci. Tec. 2012;42(3):251–325. [Google Scholar]
- 71.Ghumra Dishit P., Agarkoti Chandrodai, Gogate Parag R. Improvements in effluent treatment technologies in Common Effluent Treatment Plants (CETPs): review and recent advances. Process Saf. Environ. 2021;147:1018–1051. [Google Scholar]
- 72.Xiong Zhenglong, Cheng Xiang, Sun Dezhi. Pretreatment of heterocyclic pesticide wastewater using ultrasonic/ozone combined process. J. Environ. Sci. 2011;23(5):725–730. doi: 10.1016/s1001-0742(10)60465-2. [DOI] [PubMed] [Google Scholar]
- 73.Song Shuang, Ying Haiping, He Zhiqiao, Chen Jianmeng. Mechanism of decolorization and degradation of CI Direct Red 23 by ozonation combined with sonolysis. Chemosphere. 2007;66(9):1782–1788. doi: 10.1016/j.chemosphere.2006.07.090. [DOI] [PubMed] [Google Scholar]
- 74.Shen Yongjun, Xu Qihui, Wei Rongrong, Ma Jialing, Wang Yi. Mechanism and dynamic study of reactive red X-3B dye degradation by ultrasonic-assisted ozone oxidation process. Ultrason. Sonochem. 2017;38:681–692. doi: 10.1016/j.ultsonch.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Hui, Duan Lijie, Zhang Daobin. Decolorization of methyl orange by ozonation in combination with ultrasonic irradiation. J. Hazard. Mater. 2006;138(1):53–59. doi: 10.1016/j.jhazmat.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Lifen, Ye Xinqian, Ding Tian, Sun Xiaoyang, Xu Yuting, Liu Donghong. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013;20(1):222–231. doi: 10.1016/j.ultsonch.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 77.Asaithambi P., Sajjadi Baharak, Abdul Aziz Abdul Raman, Daud Wan Mohd Ashri Bin Wan. Ozone (O3) and sono (US) based advanced oxidation processes for the removal of color, COD and determination of electrical energy from landfill leachate. Sep. Purif. Technol. 2017;172:442–449. [Google Scholar]
- 78.Ghanbari F., Khatebasreh M., Mahdavianpour M., Lin K.Y. Oxidative removal of benzotriazole using peroxymonosulfate/ozone/ultrasound: synergy, optimization, degradation intermediates and utilizing for real wastewater. Chemosphere. 2020;244:125326. doi: 10.1016/j.chemosphere.2019.125326. [DOI] [PubMed] [Google Scholar]
- 79.Sun Jian-Hui, Sun Sheng-Peng, Sun Jing-Yu, Sun Rui-Xia, Qiao Li-Ping, Guo Hui-Qin, Fan Mao-Hong. Degradation of azo dye Acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation. Ultrason. Sonochem. 2007;14(6):761–766. doi: 10.1016/j.ultsonch.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Rajoriya Sunil, Bargole Swapnil, George Suja, Saharan Virendra Kumar. Treatment of textile dyeing industry effluent using hydrodynamic cavitation in combination with advanced oxidation reagents. J. Hazard. Mater. 2018;344:1109–1115. doi: 10.1016/j.jhazmat.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Askarniya Z., Sadeghi M.T., Baradaran S. Decolorization of Congo red via hydrodynamic cavitation in combination with Fenton’s reagent. Chem. Eng. Process. 2020;150:107874. [Google Scholar]