Abstract
Thymus vulgaris Linn. is a medicinal and culinary herb from the Southern European region known for its anti-infective, cardioprotective, gastroprotective, anti-inflammatory, and immunomodulatory activities since the Egyptian era. The reported pharmacological activities of T. vulgaris L. include antibacterial, antioxidant, anti-inflammatory, antiviral, and anti-cancerous activities. In this review, a comprehensive approach is put forth to scrutinize and report the available data on phytochemistry, ethnopharmacology, pharmacology, and toxicology of the plant. The different extracts and essential oil obtained from the plant have been assessed and reported to treat ailments like microbial infections, inflammation, non-communicable diseases like cancer, and sexually transmitted diseases like HIV-1 and Herpes. The literature review has also indicated the use of volatile oils, phenolic acids, terpenoids, flavonoids, saponins, steroids, tannins, alkaloids, and polysaccharides in pharmacotherapy. Applications of these compounds including antidiabetic, anti-Alzheimer's, cardio, neuro and hepatoprotective, anti-osteoporosis, sedative, immunomodulatory, antioxidant, anti-tyrosinase, antispasmodic, antinociceptive, gastroprotective, anticonvulsant, antihypertensive, antidepressant, anti-amnesia, and anti-helminthic activities have been mentioned. Further, based on research gaps, recommendations have been provided to evaluate T. vulgaris L. systematically to develop plant-based drugs, nutraceuticals, and to evaluate their clinical efficiency and safety.
Keywords: Thymus vulgaris L., Ethnopharmacology, Pharmacology, Phytochemistry, Toxicology
Thymus vulgaris L., ethnopharmacology, pharmacology, phytochemistry, toxicology
1. Introduction
T. vulgaris L. or thyme, known as “garden thyme” is an aromatic and perennial flowering plant belonging to the Lamiaceae family [1, 2]. The Greek form of the word ‘thyme’ depicts ‘to fumigate’, owing to its use as incense or for its balsamic odour or it belonged to the class of sweet-smelling herbs [1]. Being native to Southern Europe, T. vulgaris L. is reported to have a worldwide distribution [2]. The plant grows well in an arid climate and unshaded areas in coarse, rough, and well-drained soil that is generally unsuitable for many plants. It appears as a short and bushy plant with several small flowers [3]. It is usually grown for commercial purposes in several countries for its dried leaves, plant extracts, plant oil, and oleoresins [4, 5]. T. vulgaris L. is commercially used as aflavouring agent in food industries due to its extensive aromaticity [1]. It is also used for preserving meat, chicken, and fish [6], along with its use for flowering and ornamental purposes [7]. In addition, the perfumery and cosmetic industries also use T. vulgaris L. for its characteristic aroma [5].
T. vulgaris L. is reported with an array of ethnobotanical applications due to its extensive pharmacological properties. The plant was chiefly used for the treatment of wounds, as it possesses healing and antiseptic properties [4, 5]. Usage of its aerial parts for fumigation, treating skin and respiratory diseases in ancient Europe deliberates on the anti-infective property of the plant [5]. Besides, T. vulgaris L. was used in monasteries in food preparations. These properties depict its efficacy as a culinary and medicinal plant as well. It is believed that Romans and Greek fumigated their surroundings by burning the entire plant [8]. Treatment of skin diseases like Black Death during the 1340s and several foodborne illnesses was also done using the T. vulgaris L. aqueous extract [5, 9, 10]. This is supported by modern studies, which have proved the antibacterial efficacy using both normal and MDR strains of virulent bacteria and fungi [11]. In the 1980s, T. vulgaris L. was recommended for treating respiratory disorders raised due to the inflammation of upper respiratory tract mucous membranes, including whooping cough, bronchitis, and catarrh [9]. However, application of thyme (T. vulgaris L.) and primrose (Primula vulgaris) extract has demonstrated beneficiary in clinical trials against bronchitis and other respiratory disease related symptoms [12]. Experts have also recommended the use of T. vulgaris L. in cases of bacterial and fungal infections. The plant is expected to render a possible inhibition of bacterial adsorption and biofilm matrix formation [13]. In addition, T. vulgaris L. was believed to possess antiseptic, astringent, carminative, tonic, and anthelminthic properties. The plant has gained much popularity due to the profound activity shown towards intestinal infections and infestations caused by ascarids, hookworms, fungi, yeast, and bacteria. It has also found its importance in dermatological issues like acne, oily skin, dermatitis, bug bites as well as sciatica and rheumatic aches. In support of this, modern pharmacological investigations have come up with several findings that satisfactorily decipher the antifungal nature of different extracts and compounds of the plant [14, 15, 16]. It is reported that, T. vulgaris L. relieves bites and stings, and the neurological issues associated with it. In aromatherapy, red T. vulgaris L. oil and white T. vulgaris L. oil are used to treat skin disorders as well as body pains [17, 18]. Modern anti-inflammatory investigations have revealed the efficacy of T. vulgaris L. in the amelioration of oxidative stress and cell mediated immune response [19, 20, 21].
These initial findings prevail the significant therapeutic potential of the plant with respect to pharmacological properties, which may be the results of its active components. In recent years, T. vulgaris L. has been the centre of research interest and colossal amount literature is already available. Although a few small reviews have been carried out on limited aspects of T. vulgaris L. [3, 22], no comprehensive review has been published till date including details of the potential therapeutic aspects. Therefore, we deliberate on summing up the present and up-to-date information with respect to chemical composition, ethnopharmacology, pharmacology, and toxicology of T. vulgaris L. We also aim to document a few uncommon pharmacological activities that can completely change the present perspectives on the plant.
2. Materials and methods
2.1. Databases, softwares, e-sources, and keywords search
Literature survey was conducted to gather all the essential information surrounding T. vulgaris L. using electronic databases including Google Scholar, PubMed, SpringerLink, Wiley-Blackwell, and Web of Science. An extensive number of studies published in peer reviewed journals like Food Chemistry, International Journal of Molecular Sciences, Fitoterapia, Journal of Science in Food and Agriculture, Parasitology, Toxicology research and many others were collected. E-books “Thyme: the genus Thymus” and “Medicinal spices and vegetables from Africa” were utilized as well. Authors searched for the data using keywords including “Thymus vulgaris,” “T. vulgaris,” “Ethnopharmacology,” “Traditional uses,” “Ethnobotany,” “Chemical profiling,” “Pharmacological properties,” “Medicinal properties”, which resulted in the gathering of much literature. A special search was conducted to get the ethnomedicinal details of the plant using words “root”, “stem”, and “leaves”. Plant.org was used for the correct names of the plants used in the article. Also, chemical structures and IUPAC names were added using PubChem. The systematic arrangement of studies and their management till the end of the drafting was completed using the software Mendeley.
2.2. Literature management
Authors searched different databases using keywords to end up with a mammoth literature that included research articles, review articles, book chapters, and books. The literature was divided into different sections based on the title and abstract available along with the removal of non-relevant information and duplicates. Later, a second screening was carried out, where studies with unique methodology covering maximum research aspects were retained. Further, a few of the review articles with limited amount of information were removed. During the drafting of the review, a few research articles were removed to retain the articles with unique methodology and significant results. However, it was inevitable to retain a few of the articles despite their insignificant results and older methodology, due to lack of relevant and advanced information. From 2791 identified studies, a total of 118 studies were retained on completion of the different levels of screening. The outline was planned to meet up the PRISMA regulations (Figure 1). The article was primarily divided into different sections based on the previous work of authors on phytochemistry and pharmacological reviews. Further, a few additional changes were brought according to the amount and type of literature available.
Figure 1.
PRISMA outline followed for literature search.
3. Botanical background
3.1. Taxonomy
Being a member of the Lamiaceae family, the genus Thymus comprises a total of 928 species [23]. The most related genera are Origanum, Satureja, Micromeria and Thymbra [24]. The classification of Thymus species attributes to the chromosomal information, which is an important key for taxonomy. It is difficult to identify the chromosomes of Thymus species due to their small size and similar morphology [25].
3.2. Botanical description
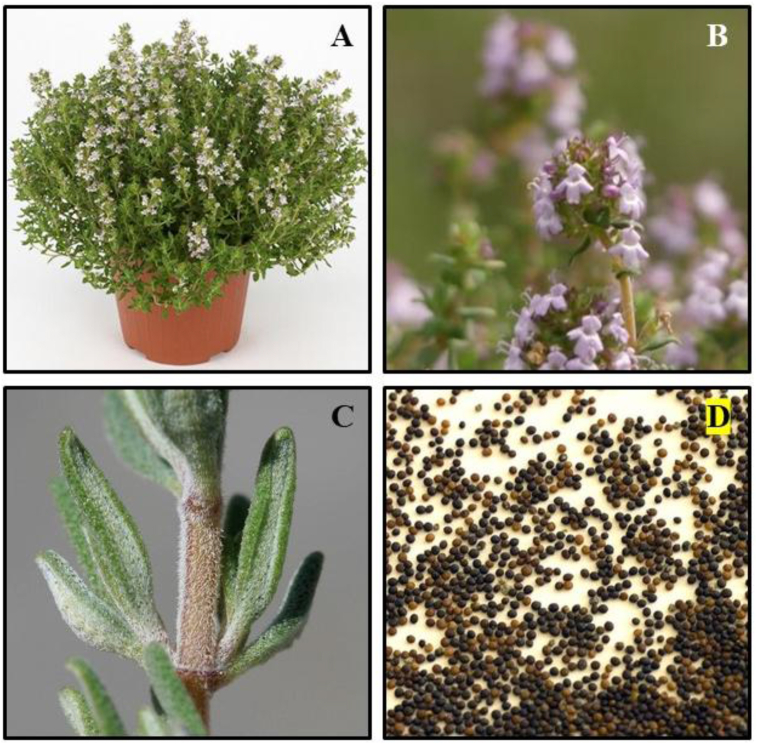
T. vulgaris L. is an aromatic, perennial, straight growing plant which measures up 10–30 cm in height with woody base [24, 26]. Leaves are small, opposite, greyish green coloured, oblong–lanceolate to linear, and are gland-dotted. They are measured up to 5–10 mm long and 0.8–2.5 mm wide with recurved margins. Flowers are light violet in colour, two-lipped, and possess a hairy glandular calyx. They measure up to 5 mm long with leaf-like bracts in loose whorls arranged in axillary clusters on the branchlets or in terminal oval or rounded heads [26]. Figure 2 details the different parts of T. vulgaris L. However, the morphological characters may vary according to environmental conditions. T. vulgaris L. grows well in arid, temperate, and unshaded areas. T. vulgaris L. grows well in hot, arid conditions with well-drained soil, and is usually planted in the spring. The plant can be propagated using seed, cuttings, or by dividing rooted sections. The plant also takes up deep freezes and can be found on mountain highlands [4].
Figure 2.
A) Plant B) flowers C) leaves D) seeds of Thymus vulgaris L.
3.3. Variations
T. vulgaris L. shows aneuploidy where the number of chromosomes varies in different plants of the same species. Among these, 2n = 28, 30, 56 and 60 are said to be the most frequent numbers for other plants belonging to the genus Thymus. Aneuploidy has played an important role during evolution and is responsible for varying numbers. This is true for T. vulgaris L. with 2n = 28, 58 [24]. Thus, it becomes evident that the plant has a diverse genetic constitution. This may also contribute to its morphological variation. However, there are not available data on the restriction of these genetically diverse species to a specific region or country. Several sub-species are present that further add to the complexities of genetic research. In addition, several chemotypes or chemovars of T. vulgaris L. exist. As the phytochemical profile of T. vulgaris L. depicts the presence of extensive amount of essential oil components, chemotypes vary based on the composition of the same [27]. There are as many as 13 different chemotypes have been identified based on the predominance of monoterpenes in the essential oil. Recently, a study identified 6 different chemotypes namely linalool, borneol, geraniol, sabinene hydrate, thymol, and carvacrol [10]. Pharmacological effects of different chemotypes vary due to their diverse essential oil composition [28, 29]. More research needs to be conducted in terms of chemotypes to identify the potent chemicals that can be used to target diseases at a molecular level. Based on the chemical profile and mode of employment of these chemotypes against specific diseases, significant results can be expected in phytotherapy.
3.4. Distribution
Native to Southern Europe, T. vulgaris L. has a worldwide distribution. It is indigenous to the Mediterranean region and other neighbouring countries. It is also found in Northern Africa including Egypt and Saharan countries like Morocco, Algeria, Tunisia, and Libya [9]. Also, the plant is cultivated in Nigeria, Cameroon, and South Africa. It is also cultivated in European countries, such as Spain, France, Bulgaria, Italy. Along the Mediterranean coastal region, it can be found growing up to 800 m from sea level. The plant can be cultivated by vegetative propagation, using seeds, cuttings, or divided root sections [2].
4. Ethnopharmacology
Thymus species have been used since ancient times for the treatment of different health aberrations. The plant possesses a variety of the medicinal properties that positively affect human health (Table 1). Authors were not able to find most of the specific parts of the plant used in traditional medicinal practices. Even though search was conducted using the words “root,” “stem,” or “leaves”, there was no available records for specific parts of the plant used for traditional medicine. Few available studies were able to depict the details limited to “aerial parts” of the plant, which includes stem, leaves, flowers, and buds. Also, these studies were not able to reveal the accurate use of the plant. Mechanisms of ethnopharmacological uses were not available. The information was limited to “whole plant” or “thyme water”. Investigations need to be done in this regard to decipher the unknown medicinal properties, which may benefit the pharmacological studies.
Table 1.
Summary of the ethnomedicinal uses of Thymus vulgaris L.
| Ethnomedicinal Use | Part of the Plant | Palnt Material | Mode of Application | References |
|---|---|---|---|---|
| Treatment of poisoning | Aerial parts/stem/leaves | Dried whole plant/water extract | Oral, Topical | [8] |
| Disinfection and wound healing | Aerial parts/stem/leaves | Dried whole plant/vapours, water extract | Topical | [8] |
| Plague-blistered skin, Acne, oily skin, dermatitis | Aerial parts/stem/leaves | Water extract, volatile oil | Topical | [9, 18] |
| Treatment of foodborne illnesses | Aerial parts/stem/leaves | Dried whole plant/Water extract | Oral | [10] |
| Asthma, bronchitis, whooping cough, pharyngitis | Aerial parts/stem/Leaves | Water extract | Oral | [37] |
| Cough, cold and sore throat | Aerial parts/stem/Leaves/Flower shoots | Water extract | Oral | [37] |
| Intestinal worms | Aerial parts/stem/Leaves | Dried whole plant/water extract | Oral | [37] |
| Rheumatic aches, body pain and sciatica | Aerial parts/stem/Leaves | Volatile oil | Topical | [17] |
| Emphysema, sedative, anthelmintic, antifungal, antispasmodic, diuretic | Not defined | Not defined | Not defined | [41] |
| Skin infections, high blood pressure, heart problems, fluid retention, cystitis, digestive system disorders, rheumatism and arthritis | Flower shoots | Not defined | Not defined | [38] |
4.1. Treatment for poisoning
The aqueous extract of the plant is reported to possess the properties of an antidote. It is believed that Romans used to consume the plant extract before or during meal to protect themselves from getting poisoned. Bathing in warm water dosed with T. vulgaris L. could stop the effects of poison, making it a favourite herb for the emperors [8]. These examples indicate the effectiveness of T. vulgaris L. as a natural antidote. In support of this, the present-day studies show presence of compounds like thymol and carvacrol, which can serve as a profound antidote [31, 32].
4.2. Disinfection and wound healing
T. vulgaris L.is reported to be used as a disinfectant, where dried plant bundles were burned to purify the surroundings. Romans and Greeks evoked a spirit of courage by burning these bundles to purify their homes and temples [8]. This reminds us of the modern-day fumigation, where the disinfection is done with various antimicrobial substances. However, studies have proven the presence of γ-terpinene and p-cymene, which are the biochemical precursors of thymol and carvacrol, to be responsible for the observed antimicrobial properties [16, 33]. Nurses in the 19th century used to apply bandages soaked in thyme water, as this plant was believed to be a natural healer and an antiseptic. Also, the examination of the aqueous extract of T. vulgaris L. has been proved to improve immunomodulatory functions [34].
4.3. Treatment of skin diseases
T. vulgaris L. was also extensively used against plague in the late 1340s, an era that was known as the age of Black Death. T. vulgaris L. was directly applied on plague-blistered skin [5]. The beneficiary effects of such an application was later identified to be owing to the presence of a chemical compound known as thymol, which is widely used in hand sanitizers, mouthwashes, and acne medication in the present day. Also, several studies have proved the use of T. vulgaris L. for the treatment of skin problems like dermatitis [9]. These effects are observed due to the presence of volatile oils, which principally comprises linalool, α-pinene along with thymol. Other compounds like mono and sesquiterpenes (β-caryophyllene, germacrene-D and nerolidol) also reported to play an important role [33].
4.4. Treatment of foodborne illnesses
Apart from medicinal purpose, T. vulgaris L. has found its importance as a cooking herb alongside rosemary and sage in Europe. Monastries that served food, also used this plant as a food additive to wade away the microbial contamination as well as a part of the medicinal formulations. Food products like soups, roasts, and breads contained the plant formulation [10]. However, most of the studies have reported the antimicrobial activity of T. vulgaris L. extracts (ethanol and water) as well as its use in the form of essential oil against foodborne pathogens. In addition to thymol and carvacrol, phenolic compounds in the extracts are attributed for this property [35, 36].
4.5. Treatment of respiratory diseases
The whole plant extract was reported to be used against several respiratory disorders. It was believed to be an effective remedy for bronchitis, asthma, whooping cough, and pharyngitis. T. vulgaris L. tea was extensively used to treat cough and cold. They were used to treat sore throat [37]. In addition, flowering shoots were used to treat cold, chest infections and sore throat [38]. Recently, the phytochemical analysis of the plant extracts revealed the presence of carvacrol and γ-terpinene that possess antiviral and anti-inflammatory activities, which could be the reason for the observed effects during its traditional use [32, 39].
4.6. Other traditional uses
T. vulgaris L. has been an important medication in several other health maladies. The plant possesses the ability to cure gastrointestinal aberrations, as the extract was given to treat worms in children [37]. The modern-day studies have reported the anti-parasitic/anti-helminthic role of T. vulgaris L., which is attributed to the presence of monoterpenes and phenolic compounds [40]. Also, the topical use of T. vulgaris L. oil reduced rheumatic aches and sciatica, where the aromatherapy method used the oil to treat such body pains [17]. This property of reducing pain is mainly attributed to thymol, which possesses the greatest anti-inflammatory potential among all the reported constituents. Flower shoots were used to treat mouth and skin infections, high blood pressure, heart problems, fluid retention, cystitis, digestive system disorders, rheumatism, and arthritis [38]. However, no specific phytochemicals have been attributed to these medicinal properties. In addition, reports have revealed the anthelminthic, antifungal, antispasmodic, diuretic [41], intestinal anti-inflammatory, buccal antiseptic, ocular antiseptic, internal antiseptic, laxative, antiodontalgic, anticatarrhal, and urinary antiseptic [38] properties of the plant, yet without attributing the specific protogonistic phytochemicals.
5. Phytochemistry
T. vulgaris L. primarily consists of a myriad of chemical compounds categorised as phenolic compounds, terpenoids, flavonoids, steroids, alkaloids, tannins, and saponins. Most of them are volatile compounds extracted from plant oil. The studies have assessed the phytochemical composition mostly using gas chromatography-mass spectroscopy (GC-MS) and high-performance liquid chromatography/thin layer chromatography (HPLC/HPTLC) techniques [42, 43, 44]. The revelations depict the prevalence of essential oil components over the other metabolites.
Further, only a few studies could decipher the presence of steroids, alkaloids, tannins, and saponins, yet no information is available on individual compounds belonging into these categories [45, 46]. Apart from the plant oil characterisation, ethanolic and aqueous extracts need to be studied in detail to reveal information on the presence of various other important components. Several phytochemicals identified from this plant are of substantial pharmacological significance (Table 2). Though chemicals are present in T. vulgaris L., their isolation and identification has not been performed. Researchers have reported the activities of these phytochemicals from independent origin.
Table 2.
Summary of the individually reported phytochemicals and their pharmacological significance present in Thymus vulgaris Linn.
| Class | Compound Name | IUPAC Name | Pharmacological Activity | Reference |
|---|---|---|---|---|
| Phenolic compounds | Quinic acid | 6-methoxyquinoline-4-carboxylic acid | Anti-cancer, immunomodulatory, anti-fungal, antioxidant, neuroprotective | [47, 83, 84] |
| Rosmaric acid | (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl) prop-2-enoyl] oxypropanoic acid | Anti-alzheimer's, anti-cancer, antidiabetic, antimicrobial, cardioprotective, nephroprotective, anti-ageing, hepatoprotective, anti-inflammatory, anti-allergic, anti-depressant | [85, 86] | |
| Caffeic acid | (E)-3-(3,4-dihydroxyphenyl) prop-2-enoic acid | Antioxidant, antimicrobial, anticancer, adipogenetic, lipolytic, anti-alzheimer's, antiviral, antidiabetic, cardioprotective, hepatoprotective, anti-atherosclerotic | [87, 88, 89] | |
| p-coumaric acid | (E)-3-(4-hydroxyphenyl) prop-2-enoic acid | Immunomodulatory, anti-inflammatory, antioxidant, gastroprotective, antidiabetic, anti-hyperlipidemia, anti-tyrosinase, anticancer, hepatoprotective | [90, 91, 92] | |
| p-hydroxybenzoic acid | 4-hydroxybenzoic acid | Anticancer, antimicrobial, antiviral | [93, 94, 95] | |
| Gentisic acid | 2,5-dihydroxybenzoic acid | Anticancer, antioxidant, antimicrobial, cardioprotective, antimicrobial, anti-inflammatory, analgesic, nephroprotective, hepatoprotective, neuroprotective, muscle relaxant | [91,96,97] | |
| Syringic acid | 4-hydroxy-3,5-dimethoxybenzoic acid | Anticancer, antioxidant, antidiabetic, anti-inflammatory, neuroprotective, antimicrobial, antiendotoxic, hepatoprotective, anti-osteoporotic | [98, 99] | |
| Ferulic acid | (E)-3-(4-hydroxy-3-methoxyphenyl) prop-2-enoic acid | Anticancer, antidiabetic, antioxidant, cardioprotective, neuroprotective, anti-alzheimer's, | [100, 101] | |
| Terpenoids | Thymol | 5-methyl-2-propan-2-ylphenol | Antibacterial, antifungal, antispasmodic, antitussive, anxiolytic, neuroprotective, antihypertensive, antioxidant, antihyperlipidemic, anti-inflammatory, immunomodulatory, anti-cancerous, analgesic, growth promoting | [41] |
| Carvacrol | 2-methyl-5-propan-2-ylphenol | Antimicrobial, antimutagenic, antitumor, analgesic, anti-inflammatory, antihepatotoxic, antiparasitic, antispasmodic, and hepatoprotective | [32] | |
| Geraniol | (2E)-3,7-dimethylocta-2,6-dien-1-ol | Anti-cancerous, anti-inflammatory, antioxidant, hepatoprotective, antimicrobial, cardioprotective, antidiabetic, neuroprotective | [102] | |
| Linalool | 3,7-dimethylocta-1,6-dien-3-ol | Sedative, antiviral, anti-inflammatory, antioxidant, anti-nociceptive, analgesic, anesthetic, antimicrobial, anxiolytic, anti-hyperlipidemic, antinoceptive, antidepressive, neuroprotective | [41, 103] | |
| ρ-Cymene | 1-methyl-4-propan-2-ylbenzene | Antimicrobial, antinociceptive and anti-inflammatory, antioxidant, anxiolytic, anticancer, vasorelaxant, immunomodulatory, antinoceptive | [104, 105, 106] | |
| γ-terpinene | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene | Antibacterial, antioxidant, anti-inflammatory, antinociceptive. | [107, 108, 109] | |
| Limonene | 1-methyl-4-(1-methylethenyl)-cyclohexene | Antibacterial, antifungal, anti-inflammatory, antinociceptive, antioxidant, | [110, 111] | |
| β-Caryophyllene | (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo [7.2.0] undec-4-ene | Antimicrobial, cardioprotective, hepatoprotective, gastroprotective, neuroprotective, nephroprotective, antioxidant, anti-inflammatory, immunomodulatory | [48] | |
| β-Pinene | 6,6-dimethyl-2-methylidenebicyclo [3.1.1] heptane | Anti-cancerous, antimicrobial, antioxidant, anti-inflammatory, analgesic, cytogenetic, gastroprotective, anxiolytic, cytoprotective, anticonvulsant, neuroprotective | [49] | |
| α-Terpineol | 2-(4-methylcyclohex-3-en-1-yl) propan-2-ol | Anti-cancerous, antioxidant, antinociceptive, anticonvoluscent, gastroprotective, cardioprotective, antihypertensive, sedative | [112] | |
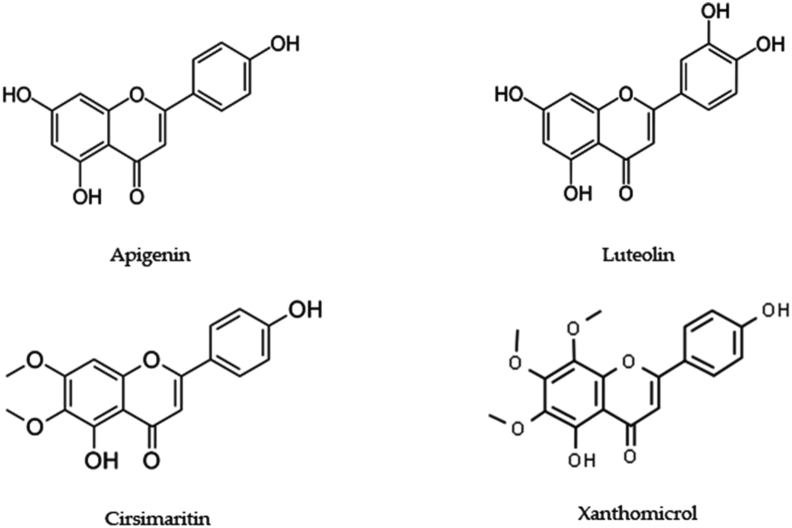
| Flavonoids | Apigenin | 5,7-dihydroxy-2-(4-hydroxyphenyl) chromen-4-one | Antidiabetic, anti-cancerous, antidepressive, anti-insomnia, anti-amnesia, anti-alzheimer's, antiviral | [113] |
| Luteolin | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one | Anti-alzheimer's, anti-cancerous | [114, 115] | |
| Cirsimaritin | 5-hydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxychromen-4-one | Antioxidant, anti-inflammatory, antimicrobial, antidiabetic, anticancer, neuroprotective, cardiovascular, hepatoprotective | [116] | |
| Xanthomicrol | 5-hydroxy-2-(4-hydroxyphenyl)-6,7,8-trimethoxychromen-4-one | Anti-inflammatory, anti-spasmodic, anti-platelet, anti-cancerous effects | [117, 118] |
5.1. Phenolic compounds
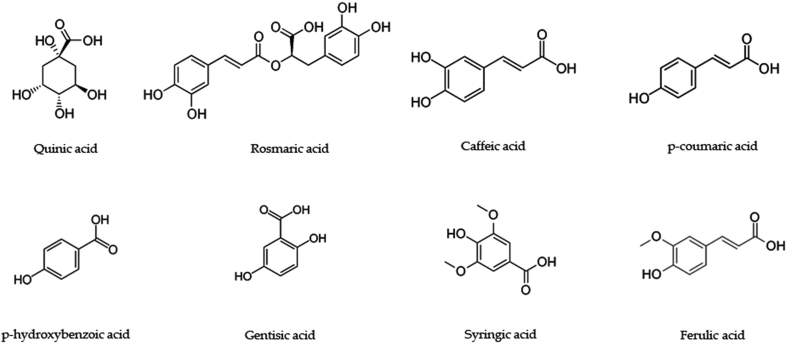
T. vulgaris L. is one of the principal sources of phenolic compounds. Heidari et al. (2018) identified new phenolic compounds using GC-MS technique, in which a few of the phenolic compounds were already reported. They include creosol 2-Methoxy-4-methylphenol), thiophenol (benzenethiol), quininic acid (6-methoxyquinoline-4-carboxylic acid), loliolide [(6S,7aR)-6-hydroxy-4,4,7a-trimethyl-6,7-dihydro-5H-1-benzofuran-2-one], phenol 4-(3- hydroxy-1- propenyl-2- methoxy), and 3-Methoxy-5- methylphenol [47]. Also, previous studies have identified the presence of rosmaric acid, caffeic acid, p-coumaric acid, geranic acid, p-hydroxybenzoic acid, gentisic acid, syringic acid, and ferulic acid [4]. Among the various others reported, the highest number of compounds with pharmacological significance were the phenolic compounds (Figure 3).
Figure 3.
Phenolic compounds with pharmacological activity in Thymus vulgaris L.
5.2. Terpenoids
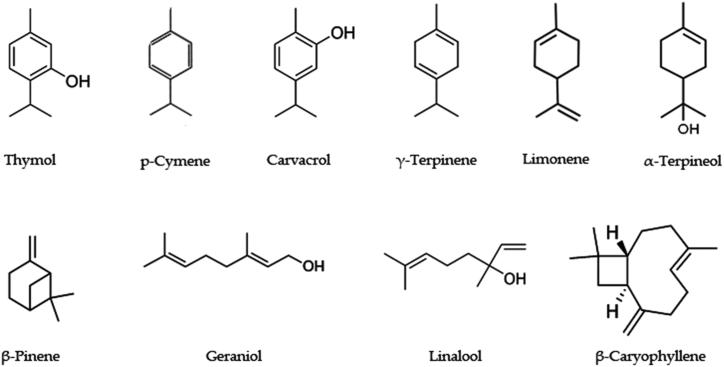
Characterization of the essential oil of T. vulgaris L. reveals a list of hydrocarbons, oxides, alcohols/esters, and aldehydes/ketones. Many of the studies have reported the uses of a variable number of compounds with different concentrations. Among all the reported volatile compounds, thymol, carvacrol, geraniol, linalool, α- and β-pinene, p-cymene, and γ-terpinene have been reported with major pharmacological activities [48, 49, 50]. In addition, hydrocarbons like 2,6-Octadienal, cis-Sabinene hydrate, germacrene D, limonene, β-ocimene, myrcene, β-caryophyllene, α-thujene, α-phellandrene and α-humulene are present. Oxides like 1,8-cineol, caryophyllene oxide; alcohol/esters including α-terpineol, borneol, 1-octen-3-ol, 3-octanol, p-cymen-8-ol, terpinen-4-ol, thymol methyl ether, carvacrol methyl ether; aldehydes/ketones like 3-octanone, camphor, thymoquinone, and geranial are present. The GC-MS analysis also showed the presence of esters including butanoic acid, 2-methyl-, methyl ester, bornyl acetate, and geranyl propanoate. The majority of these compounds need to be assessed individually to decipher their pharmacological potential [51]. However, some of the terpenoids have been reported with profound pharmacological significance (Figure 4).
Figure 4.
Terpenoids with pharmacological activity in Thymus vulgaris L.
5.3. Flavonoids
Flavones like 6-hydroxyluteolin, apigenin, luteolin, methyl-flavones including cirsimaritin or genkwanin, cirsilineol, 5-desmethylnobiletin, 8-methoxycirsilineol, 7-methoxyluteolin, gardenin B, salvigenin, thymonin, sideritoflavone, xanthomicrol and thymusin [4]. There have been no reports on pharmacological activities of these compounds except apigenin, luteolin, cirsimaritin, genkwanin, and xanthomicrol. Studies need to focus on the extraction and biosynthesis of individual compounds which can further become a basis for phytomedicines. Although only a few of the flavonoids are reported (Figure 5), they are found to exhibit profound pharmacological activities.
Figure 5.
Flavonoids with pharmacological activity in Thymus vulgaris L.
5.4. Steroids, tannins, alkaloids, saponins
A study that used HPTLC to assess the methanolic extract of T. vulgaris L. documented the presence of 9 alkaloids, 14 saponins, 12 steroids, and 8 tannins. The study revealed a good resolution with 10 bands of essential oils, along with bands corresponding to tannins (9.2%) and saponins (23.1%), where a remarkable antimicrobial activity is possessed by saponins [46]. However, it could only reveal the presence of a class of compounds but not decipher the presence and role of individual compounds. As most of the chemical components belong to essential oils and phenolic compounds, metabolites like steroids, tannins, alkaloids, and saponins have not been evaluated in detail. Therefore, it now becomes essential to focus on these compounds to reveal their biological significance.
5.5. Other phytochemical compounds
Banerjee et al. (2019) assessed polysaccharide content using aqueous extraction of the alcohol insoluble residue of T. vulgaris L. leaf and reported the presence of four types of polysaccharides. They include homogalacturonan, starch, cellulose and a unique type of polysaccharide known as rhamnogalacturonan I (RG-I). They also reported the association of RG-I with ester linked phenolic acids shows profound antioxidant activity [52]. However, much remains unknown about other biomolecules present in T. vulgaris L.. Identification of the other biomolecules like proteins and vitamins using the whole plant extracts could lead to the elucidation of nutritive profiling of the plant.
6. Pharmacology
6.1. Antibacterial activity
Bacteria obtained from the feces of the red deer (Cervus elaphus) including Escherichia coli, Klebsiella pneumoniae, Yersinia enterocolitica, Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis were found sensitive towards the ethanol extract of T. vulgaris L., where minimum inhibitory concentration (MIC) was observed as >6.25, 3.12,>6.25, 0.2, 0.39, 0.78 mm respectively at 100 μl concentration (p < 0.05). These bacteria showed mixed response towards antibiotics [53]. In another study, methanolic extract of T. vulgaris L. was found to be effective against methicillin-resistant S. aureus (MRSA), where bacteria isolated from the infected mice body parts were tested under in vivo conditions. It was found that for the bacteria isolated from throat and lungs, MIC was found to be 2.93 and 3.83 colony forming unit (CFU) (log10)/ml, respectively (p < 0.05) [54]. In case of Salmonella typhirium, thyme oil from T. vulgaris L. showed MIC of 25.5 mm at 100 μl concentration, in accordance with amoxicillin (23.0 mm) and cefotaxime (15.0 mm) [55]. Biofilm of Salmonella enteritidis (48 h) was inhibited by thyme oil at an MIC/MBC of 0.156/0.315 μl/ml, in accordance with that of the control. The oil also reduced the metabolic activity by 9.6–70.5%, where both biofilm and metabolic activity reductions were found to be significant (p < 0.05). The study also included the individual assessment of thymol and carvacrol, whose results were found to be strikingly similar in case of antibacterial assay [13].
Further, the essential oil from T. vulgaris L. was found to inhibit the growth of multi-drug resistant (MDR) variants of S. aureus. A significant inhibition was observed with a zone of 35–40 mm at 2.8–11.5 μg/ml for MDR variants, whereas cefotaxime showed MIC at 32 μg/ml concentration (p < 0.05) [11]. In addition, Pseudomonas aeruginosa swabs were taken from surgical wounds at the end of the hip implant surgery, where minimum bactericidal concentration (MBC) was found to be 8% at 100 μl concentration [56]. T. vulgaris L. essential oil or thyme oil was also found to be effective against porcine respiratory bacteria. Actinobacillu spleuropneumoniae, Streptococcus suis, Actinobacillus suis, Haemophilus parasuis, Pasteurella multocida, and Bordetella bronchiseptica were tested with thyme oil and considerable MIC values were obtained, ranging from 0.039% to 0.078% at dilutions 0.01–1.25% v/v (p < 0.05) [57].
These studies indicate the antibacterial potential of thyme oil and other extracts on bacteria isolated from various sources. A greater inhibitory activity was observed by the use of thyme oil against bacteria in comparison with that of the aqueous, ethanolic extracts, and even a few antibiotics [55]. Thyme oil is also capable of inhibiting biofilm formations, which are regarded to be highly infective [13]. Although both bacteriostatic and bactericidal values were discovered, dose-dependent activities need to be conducted using animal models and human cell lines. It is noteworthy that thyme oil has exhibited a significant bacteriostatic activity against MDR and MRSA strains [54, 56]. Though the oil serves as a better antibacterial agent owing to its facile extraction method and significant activity, studies need to focus on individual compounds to assess their effects either in single or in combination. Many of the studies have reported the synthesis as well as extraction of individual compounds present in thyme oil that are proved to have profound antibacterial effects [11, 13, 56, 57]. Further, mechanisms of the bacteriostatic and bactericidal functions of the fractions need to be elucidated with respect to physicochemical and physiological changes in bacterial cell.
6.2. Antioxidant activity
Antioxidant enzymes like catalase, glutathione, glutathione-S-transferase, and superoxide dismutase are primarily responsible for the reduction of free radicals in the body. Abdel-Gabbar et al. (2019) assessed the in vivo activity of these enzymes by the administration of T. vulgaris L. aqueous extract on the experimental rabbits, where the levels were found to be increased by 14.12%, 27.69%, 98.75% and 78.29%, respectively (p < 0.05) compared with that of the control (water). Total antioxidant capacity was increased at 100 mg/kg and 50 mg/kg concentration in rabbits, with no adverse effects on kidney and liver function parameters [58]. Similarly, the levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase were also significantly increased (2 units/ml) upon the administration of 500 mg/kg of T. vulgaris L. aqueous extract for 14 days. In combination with paracetamol, (200 mg/kg), the enzyme levels increased by 15–20 units/ml, compared with that of the control (p < 0.01) [59]. In addition, the antioxidant potential of the aqueous extract was also assessed using conventional methods. The aqueous extract of T. vulgaris L. leaf and stem was analysed with 2,2-diphenyl-1-picrylhydrazyl (DPPH) resulting in the free radical scavenging activity (92.0%) at a concentration of 1.5 mg/ml, in accordance with butylated hydroxyanisole (BHA-95.7%) and butylated hydroxytoluene (BHT-96.6%). The potential benefits observed in the study were attributed to the presence of polysaccharides like starch, homogalacturonan, rhamnogalacturonan I (RG-I) and cellulose in T. vulgaris L. leaves. The binding of RG-I with bovine serum albumin (BSA) indicated the formation of water-soluble complexes that further induces antioxidant activity [52].
In case of thyme oil, DPPH, Ferric reducing antioxidant power (FRAP), Ferrous ion-chelating ability assay (FIC), and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical cation scavenging activities were reported with the IC50 values 12.69, 13.29, and 6.46 mg/ml, respectively (p < 0.05) [60]. The unused plant material obtained after extracting the oil using steam distillation process was also found to be the reservoir of phenolic compounds. El-Guendouz et al. (2019) demonstrated the ability of the ethanolic extract obtained using the oil extraction by-product of T. vulgaris L. in preventing the formation of primary and secondary lipid oxidation products from oil in water (O/W) emulsions. Assessment of the antioxidant activity through DPPH showed an IC50 of 93 μg/ml, similar to that of BHT (89 μg/ml). But these values were found to be of lower activity compared with that of the pure aqueous and ethanol extracts [61].
The antioxidant potential of the oil is proved to be higher than those of the aqueous and ethanolic extracts [60, 61]. However, novel applications like silver and zinc nanoparticles could be used in place of pure extracts, as they possess lower amount of cytotoxicity. Usage of cell lines and animal models could provide a better insight to the studies, revealing the biochemical changes that could occur before and after the administration of the T. vulgaris L. fractions. It is noteworthy that the antioxidant enzymes have been focused rather than the conventional assays like DPPH, ABTS, and FRAP [58, 59]. However, it is advisable that these enzymatic analyses could be carried out using human cell lines with correlation in terms of its toxicity based on dose-dependent studies. In addition, studies have proved that different T. vulgaris L. extracts could alone be able to reduce the oxidative stress levels using the conventional antioxidant assays. But there are no available controls to compare its efficacy [58, 59]. In addition, the treatment with aqueous T. vulgaris L. extract alone has proved its potential to prevent or reverse the drug-induced toxic biochemical changes to normal via its antioxidant activity [58]. As a key finding, studies have revealed that an array of bioactive compounds showing antioxidant activities are also present in leaves and other parts of the plant, other than the oil alone [52].
6.3. Antifungal activity
Thyme essential oil was found to be effective against fungal species including Sclerotinia sclerotiorum, Botrytis cinerea, Phytophthora parasitica, Pythium aphanidermatum, Fusarium oxysporum, Alternaria brassicae, Trichoderma aggressivum f.sp. europaeum, and Cladobotryum mycophilum. The sensitivity of these fungi were assessed with the radial growth inhibition assay, which revealed an inhibitory value ranging from 13.9 to 41.4 mm at 5% concentration and ED50 values ranging from 9.3 to 18.0% for all the species tested (p < 0.05) [14]. Further, a group of 183 clinical isolates of Candida albicans and 76 isolates of C. glabrata species were tested for sensitivity towards T. vulgaris L. extract. Both MIC and minimum fungicidal concentration (MFC) were found to be in the range of 0.04–22.9 mg/ml for all the isolates. In the time kill assay, thyme oil reduced the fungal growth in the initial hours (4–8). Interestingly, thyme oil inhibited the fungal growth supplemented with sorbitol, an osmoprotectant with a much lower MIC (0.08 mg/ml) (p < 0.05) [15]. In addition, an in vivo assessment using thymol on C. albicans in a Caenorhabditis elegans nematode model resulted in the complete inhibition of the fungus at 64 mg/l and 128 mg/ml, better than that of the standard antibiotics used (p < 0.05). Thymol is also reported to enhance the expressions of pmk-1and sec-1 genes, thereby resulting in the enhancement of p38 MAPK signalling pathway, which exhibits signalling events to combat C. albicans [62].
Scalas et al. (2018) assessed the antifungal activity against Cryptococcus neoformans where MIC and MFC were found to be in the range of 56–1.12 mg/ml, in accordance with the standards fluconazole (FLC), itraconazole (ITC), and voriconazole (VRC). But individual antifungal assay showed profound results with thymol (0.02–0.08 mg/ml) (p < 0.05) [63]. Thyme oil in vapor and liquid phase was also found to be effective against fungi, where a total reduction in mycelial growth was detected at the concentrations of 20 and 400 μg/ml, respectively for Aspergillus flavus. Also, treatment with 10 μg/ml of thyme oil reduced production of aflatoxin by 97.0 and 56.4% through vapour and liquid phases, respectively (p < 0.05). In addition to this, the study also reported the downregulation of a few genes related to fungal development including brlA, abaA, and wetA and genes related to aflatoxin biosynthesis -aflR, aflD, and aflK [16].
It is noteworthy that a majority of the studies have performed both fungistatic and fungicidal assessments which determine the complete inhibition of fungi. A few of the studies have performed the time kill assay and in vivo inhibition assays to effectively determine the antifungal activity [15, 62]. The literature search was not able to find the antifungal studies completed using water and alcohol extracts. Also, there is a lack in antifungal studies performed, using the extracts and compounds on MDR species. The fungal resistance needs to be dealt with using variable number of doses of the extracts along with concerns regarding its cytotoxicity on the living cells. Furthermore, there is a lack of information on the fungicidal and fungistatic mechanism of the plant extracts and essential oil [15, 16, 62]. Owing to a lack of studies on host-pathogen interaction, advanced studies to decipher this mechanism should be carried out in order to identify the optimal dose. These studies must evaluate the cytotoxic effects as well as cell survivability so that clinical trials using plant fractions could be performed.
6.4. Anti-inflammatory activity
Though nitric oxide (NO) radicals act as intracellular messengers under normal conditions, their elevated numbers can bring up the cytotoxicity and inflammation issues. A study has evaluated the anti-inflammatory potential of T. vulgaris L. aqueous extract. Significant scavenging of NO radicals with 80.3% of the activity at 16 μg/ml concentration was observed in accordance with the values obtained using dexamethasone (p < 0.05) in murine macrophage cell line J774A.1 [19]. In case of inflammation, immune cells generally express the genes encoding for 5-lipoxygenease (5-LOX) production, which in turn activates the synthesis of leukotrienes. Tsai et al. (2011) targeted the inhibition of 5-LOX, showing an inhibition at 0.005 μg/ml (IC50) of thyme oil concentration. This was more effective than α-bisabolol (0.049 μg/ml). They also assessed the effect of essential oil on lipopolysaccharide (LPS)-induced TNF-α, interleukins IL-1β, and IL-8 secretions using THP-1 cells at 0.01 μg/ml [20].
In an in vivo study, Abdelli et al. (2017) analysed thyme oil in a dose dependent manner (100, 200 and 400 mg/kg) against mice with carrageenan-induced paw edema to determine the anti-inflammatory activity. Paw thickness was found reducing at a dose of 400 mg/kg. Results were in accordance with both the controls Tween 80 and diclofenac (p < 0.001). The study also determined the toxic level of thyme oil (4500 mg/kg), where sedation was observed at 5000 mg/kg [43]. Similarly, in the carrageenan-induced pleurisy model thyme oil at 250, 500 and 750 mg/kg reduced inflammatory exudates as well as migrated leucocytes in ear edema. Individual assessments revealed that thymol (34.2%) and carvacrol (47.3%) are attributable for the anti-inflammatory activity (p < 0.05). This study also reported the toxicity level of thyme oil as 4000 mg/kg, which stands in accordance with the findings of Abdelli et al. (2017) [64].
It was reported that anti-inflammatory response can be effectively generated by both extracts and essential oil from T. vulgaris L [19, 20, 43, 62]. It is a laudable approach by the researchers to assess the levels of inflammatory cytokines and tumour necrosis factor (TNF) [20]. A study was even able to determine the toxicity level of the thyme oil as 4500 mg/kg in mice models [43]. However, a few notable parameters like cyclooxygenase-2 (COX-2), cholestasis, vascular lesions, c-reactive protein (CRP) in chronic hepatitis, hepatic fibrosis, drug-induced autoimmunity could be assessed, which can support the antioxidant potential. In addition, autoimmune models like rheumatoid arthritis could be focused to assess the effect of T. vulgaris L. fractions.
6.5. Anti-cancerous activity
Cytotoxic activity of thyme oil was analysed against MCF7 (breast adenocarcinoma), HCT15 (colon carcinoma), HeLa (cervical carcinoma), HepG2 (hepatocellular carcinoma), and NCI-H460 (non-small cell lung cancer) cell lines. T. vulgaris L. oil showedgrowth inhibition for all the cell lines tested at 76.02–180.40 μg/ml concentration (GI50). However, thyme oil did not show any effect on non-tumour liver PLP cells, even at a high concentration of 400 μg/ml (p < 0.05) [65]. In case of THP-1 leukemia cell line, at a concentration of 100 μg/ml and >200 μg/ml, thyme oil prevented the proliferation [66]. Apart from the oil, thymus extracts also proved to be effective against lung cancer cells. In a study, H460 lung cancer cell line was found to be sensitive at 0.11% of hydroalcoholic extract (p < 0.05) and downregulated NF-κB p65 and NF-κB p52 proteins along with the reduction of IL-1β and IL-8 gene expression in LPS model. However, there was no cytotoxicity reported within a concentration range of 0.04–0.60% [21].
In an in vivo study, administration of dried T. vulgaris L. powder to mammary carcinoma rat and 4T1 mouse models led to a remarkable reduction in the volume of 4T1 tumours by 85% at 1% concentration. In the rat model, the same concentration decreased the tumour frequency by 53% compared with that of the control, besides suppressing the genes associated with tumour inducing properties. This property is attributed to the resultant upregulation of caspase-2 and caspase-3 enzymes, along with bcl-2 and Bax proteins that result in cell apoptosis. Results were in accordance with the controls used, and these results were significant (p < 0.05 [67].
It was noteworthy to find that thyme oil and extracts show a notable cytotoxic effect on tumour cells, but not on normal human cells. A study conducted by Deb et al. (2011) showed the effect of thymol on HL-60 acute promyelotic leukemia cells. Thymol showed no cytotoxic effect on human peripheral blood mononuclear cell (PBMC) at 5 and 25μM concentrations. However, extensive cytotoxicity was observed at >50 μM, after 24 h [68]. Heidari et al. (2018) employed a different approach, where both the plant extract and synthesized silver nanoparticles from the plant extract were evaluated against T47D human breast cancer cells. T47D cells showed a high sensitivity towards nanoparticles (90%) when compared with that of the extract (75%). T47D cells treated with nanoparticles showed 18.40% early and 0.69% late apoptosis with a varying IC50 concentrations (12.5–100 μg/ml) (p < 0.05), whereas in case of plant extract, 15.67% early and 1.70% late apoptosis was observed [47].
The selectiveness of thyme oil and other extracts towards tumour cells needs to be studied, for the possible presence of molecular pathways that mediate the anti-cancerous effect. The mechanisms of T. vulgaris L. fractions on different cell lines need to be elucidated with respect to biochemical and physiological changes occurring within the proliferating cell. These findings may serve as key sources during drug development process. Most of the studies have attributed the anti-proliferative activity to the monoterpenes, carvacrol and thymol that are predominantly present in thyme oil [66, 68]. According to Aazza et al. (2014) carvacrol and thymol possess higher anti-cancerous potential than p-cymene and borneol [66]. Thus, isolation and identification of individual components for anti-cancerous potential needs to be done. Although Deb et al. (2011) have deduced the mechanism of thymol in causing damage to the cancer cell via inducing the activity of apoptosis inducing factor (AIF) [68], much remains unknown about the other compounds. In a new approach, nanoparticles have induced better cell apoptosis compared with that of the plant extract, but with more cytotoxic effect [47]. Therefore, studies need to decipher the appropriate doses for consumption, prior to the complete decoding of the mechanism of action. Accurate determination of these doses using human cell lines and animal models could be useful to conduct clinical trials for drug development.
Also, effect of the plant fractions needs to be correlated with the expression of oncogenes, which may deduce druggable targets ensuring a check on its toxicity. For example, thymol showed higher cytotoxic effects in animal models at >50 μM, compared with the other extracts [68]. It becomes essential to focus on the other compounds present in the essential oil with lower toxicity level. Minimum toxicity levels have been reported with plant extracts and dried powder, which can be utilized for further analysis. Furthermore, much remains unknown about cancers like Hodgkin and non-Hodgkin's lymphomas, Kaposi sarcoma and leukemia. These cancer models along with the other rare cancer types also need to be evaluated.
6.6. Antiviral activity
Thyme oil in the form of vapour and liquid was found to be effective against the influenza virus. However, partial activity was observed in vapour phase, whereas the liquid phase at 3.1 μl/ml concentration completely inhibited the viral growth, which was better than that of control used (canola oil). In addition, the researchers also evaluated the effects on the principle external proteins of the virus, namely the hemagglutinin (HA) and neuraminidase (NA), where significant inhibition of HA was observed. In addition, the TC50 value, which is 50% reduction of the culture, was found to be 14.34 μl/ml (p < 0.05) [33].
Apart from influenza, thyme oil was found to be effective on causative viruses of sexually transmitted diseases (STDs) like herpes simplex virus (HSV) and human immunodeficiency virus 1 (HIV-1). HSV possesses two antigenic types, type 1 (HSV-1) and type 2 (HSV-2), resulting in flu like symptoms in humans. Thyme oil along with the major monoterpene compounds α-terpinene, terpinen-4-ol, α-terpineol, α-pinene, p-cymene, thymol, citral and 1,8-cineole were analysed for their antiviral activity. RC-37 kidney cells were used for non-cytotoxic dose determination, which ranged between 20 μg/ml for citral and 1250 μg/ml for 1,8-cineole. IC50 values for 1,8-cineole was 1200 μg/ml. Thyme oil proved to reduce the viral load by >96%, whereas all monoterpenes by >80% (p < 0.05) [69].
The human immuno deficiency virus is another STD which has no available vaccine yet. A study conducted by Feriotto et al. (2018) targeted the Tat protein that aids in the transcription of the viral genome. This study evaluated the interaction of the essential oil from T. vulgaris L. with the transcription of the Tat/TAR-RNA complex as well as the Tat-induced HIV-1 long terminal repeat (LTR) [70]. An electrophoretic mobility shift assay (EMSA) for this complex showed a notable inhibitory potential (3–6 μg/ml), compared with that of the control. Similarly, a reduction activity test against the Tat-induced HIV-1 LTR transcription resulted in RT50 = 0,83 μg/ml, a notable inhibitory potential which reduced viral transcription to 52% (p < 0.05) [70]. Similarly, the methanol extract of the plant was evaluated on the infected PBMC cells with HIV-1 subtype A. The cytotoxicity value (CC50) on PBMC was found to be 200 μg/ml. Further, the antiviral assay revealed EC50 value of >500 μg/ml. The study also focused on CD4+ expressions, where mean fluorescent intensity (MFI) of the cells was found to be 22.72 in PBMC (p < 0.05) [71].
The studies have primarily focused on determining cytotoxicity values using animal models, which may not give an accurate account in case of human trials [69, 71]. Thus, it becomes essential to conduct experiments using human cell lines. Human cells can be infected in vitro, subsequently treated with different extracts, oil, and individual compounds to provide conclusive evidence of its effects on human system. Effect of plant fractions on viral genome and protein synthesis needs to be studied along with the gene expression levels. Using bioinformatic tools like molecular docking to study the binding efficiency of plant-based compounds to viral proteins could be a feasible approach. For example, Feriotto et al. (2018) have deduced the role of TAT/TAR-RNA complex in the virulence of HIV-1. The same complex could be assessed in terms of its interaction with the plant phytochemicals [70]. Including HIV-1, diseases like influenza and Herpes are often difficult to identify because they are asymptomatic and have an extended incubation period. Herpes has a substantial effect on skin, which could be evaluated by the application of thyme oil. As the viruses affect targeted organs, an organ-targeted delivery of the modified plant fraction could be studied in a dose-dependent manner.
6.7. Other pharmacological activities
Apart from major pharmacological properties, T. vulgaris L. is reported to comprise a few other properties which are yet be focused on. Studies are yet to be conducted to assess the antidiabetic and anti-hyperglycemic activities regarded as the indispensable properties of a medicinal plant. A study by Aljarah and Hameed (2018) depicts the in vitro inhibition of α-glucosidase and α-amylase enzymes known to be the potential targets of antidiabetic drugs. Out of aqueous, methanol and ethanol extracts at different concentrations (4, 8, 15, and 20 μg/ml), methanol extract resulted in maximum inhibition of α-glucosidase (IC50 4.35, 22.04, 30.77, 43.13) and α-amylase (IC50 6.39, 11.47, 17.01, 22.93), yet lesser than the standard drug acarbose [72]. As stress and anxiety levels reported to play a crucial role in diabetes [73], T. vulgaris L. extract was also evaluated for its impact on anxiety levels in T. vulgaris L. on elevated plus-maze (EPM) rat model. The aqueous extract exhibited a significant increase in rat movement into the open arms at 100 mg/kg (p < 0.05) and 200 mg/kg (p < 0.01) [74].
Further, thyme oil and extracts were evaluated for their protectant ability against UV-A and UV-B rays, by inhibiting the proliferation of skin cells leading to cancer. Aqueous extract of T. vulgaris L. leaf (1.82 μg/ml) and thymol (1 μg/ml) reduced the release of lactic acid dehydrogenase (LDH), in cultured skin cells treated with UV rays. Significant cell proliferation was observed in T. vulgaris L. pre-treated skin cells in accordance with that of the control used, along with the reduction in DNA damage (p < 0.01) [75]. In addition, an in vitro evaluation of thyme oil showed a significant anti-helminthic activity against 4 species of Eimeria spp. at IC50 53.42 mg/ml for 5×104 oocysts (p < 0.05). These parasites affect poultry and cattle, reducing the yield [40].
Cholinergic affliction is found in Alzheimer's disease, a progressive neurodegenerative disorder involving the death of cholinergic neurons. Administration of thyme oil to C. elegans led to an enhancement of the neurotransmission by regulating synaptic acetylcholine levels [76]. Enhancement of the nicotinic acetylcholine receptor activity is also observed because of the upregulation of unc-17, unc-50, and cho-1 and genes at 40 and 60 parts per million (ppm). p-cymene, a monoterpene present in thyme extract, was attributed for the gene upregulation activity along with a corresponding downregulation of ace-1 and ace-2 at 20 and 100 ppm (p < 0.05). Interestingly, thymol and γ-terpinene enhanced synaptic acetylcholine levels in combination (40 ppm) but failed to do the so when administered individually [76].
Aging, a condition characterized by a reduced bone density, can be treated by increasing calcium uptake in the body. Elbahnasawy et al. (2019) conducted a study depicting the effect of T. vulgaris L. powder on rats with low calcium intake. The study resulted in a significant increase in the bone mass (2.93 g/kg), length 32.8 mm), and density (0.13 g/cm2), compared with that of the low calcium diet control (2.46 g/kg, 32.2 mm, and 0.09 g/cm2, respectively) (p < 0.05). This study proved the potential of T. vulgaris L.in the treatment of osteoporosis and other bone related diseases [77]. T. vulgaris L. is also effective on dental caries and proved its efficacy over the formed ocresolpulpotomy, due to profound antibacterial properties. T. vulgaris L. ethanolic extract along with zinc oxide was used in patients with dental caries. Clinical and radiographic evaluations showed 94.4% and 88.2% improvement, respectively with no statistical significance compared to that of the standard drug formocresol 88.2% (p > 0.05) [78].
With the studies depicting different pharmacological potentials of T. vulgaris L., it becomes evident that more studies need to be conducted using different approaches. For example, antidiabetic study conducted by Aljarah and Hammed (2019) using in vitro enzyme inhibition studies lack the usage of animal models and human cell lines [72]. In addition, a dose-dependent evaluation of the plant extracts is essential in order to understand the optimum dosage and toxicity. Analysis of binding of individual compounds could be done using molecular docking, which can become a pavement for drug development process. Also, studies need to focus on feasible experimental designs, which can give better yields, less toxicity and more pharmacological effect. If more studies with novel pharmacological models are available, one can compare these studies and suitable methods can be assayed to develop a specific dosage and formulation. The anxiolytic EPM model by Komaki et al. (2016) has highlighted the need for further evaluation of the mechanism behind the observed effect [73]. More elucidations need to be done with respect to the brain activity, secretion of hormones, and blood pressure upon the administration of different doses of T. vulgaris L. extracts. Further, a study conducted by Elbahnasawy et al. (2019) has discussed the anthelminthic activity of T. vulgaris L. in vitro that could be better represented using turkey as an animal model, instead of isolating the parasite from its feces [74]. Furthermore, the evaluation of T. vulgaris L. activity on dental caries need to be conducted using anti-infective or antibacterial assay primarily because the caries is principally caused by bacteria. In total, these studies depict T. vulgaris L.as a storehouse of different pharmacological properties attributed to the presence of various phytochemical compounds. Thus, isolation, identification and determination of the pharmacological properties of individual compounds needs to be focused. A summary of the pharmacological properties of different extracts and isolated compounds of T. vulgaris L. is given (Table 3). With this, one can understand the pharmacological evaluations done so far, and can further refine the research methodologies.
Table 3.
Summary of the pharmacological properties of Thymus vulgaris L.
| Pharmacological Activity | Type of Study | Models used | Plant part/material | Type of extract/compound | Doses used | Controls | Possible/reported mechanisms | Results | References |
|---|---|---|---|---|---|---|---|---|---|
| Antibacterial activity | in vitro | Escherichia coli, Klebsiella pneumoniae, Yersinia enterocolitica, Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis | Aerial parts | Ethanol | 100 μl of 6.25–0.025% serial dilutions | Solvent ethanol | Not defined | MIC was observed as >6.25, 3.12,>6.25, 0.2, 0.39, 0.78 mm respectively at 100 μl concentration (p < 0.05). These bacteria showed mixed response towards antibiotics. | [53] |
| in vivo | Methicillin-resistant Staphylococcus aureus (MRSA) in mice | Not defined | Methanol | 200 mg/ml per kg of body weight | Positive control-infected, negative control-normal mice, Antibiotics | Not defined | For the bacteria isolated from throat and lungs, MIC was found to be 2.93 and 3.83 CFU (log10)/ml, respectively (p < 0.05). | [54] | |
| in vitro | Salmonella typhirium | Aerial parts | Essential oil | 100 μl of 1/20–1/200 v/v serial dilutions | Amoxicillin, cefotaxime | Not defined | MIC of 25.5 mm at 100 μl concentration, in accordance with amoxicillin (23.0 mm) and cefotaxime (15.0 mm) (p < 0.05). | [55] | |
| in vitro | Salmonella Enteritidis Biofilm | Dried plant | Essential oil | 5–0.0024 μl/ml. | Growth control (broth + microbe), negative control (broth + propylene glycol + microbe), sterility control (broth + test oil), positive control (broth + streptomycin + microbe) | Possible inhibition of bacterial adsorption and biofilm matrix formation | Biofilm inhibition at MIC/MBC 0.156/0.315 μl/ml by oil, thymol, and carvacrol. Oil reduced the metabolic activity by 9.6–70.5%, (p < 0.05). | [13] | |
| in vitro | Staphylococcus aureus (MDR) | Not defined | Essential oil | 10 μl of 2.87–11.5 μg/ml | Cefotaxime | Not defined | A significant inhibition with 35–40 mm inhibition zone at 2.8–11.5 at μg/ml was observed for MDR variants, whereas cefotaxime showed MIC at 32 μg/mL concentration (p < 0.05) | [11] | |
| in vitro | Pseudomonas aeruginosa | Leaves and branches | Essential oil | 100 μl oil of different concentrations | Not defined | Not defined | Minimum bactericidal concentration (MBC) was found to be 8% at 100 μl concentration | [56] | |
| in vitro | Actinobacillus pleuropneumoniae, Streptococcus suis, Actinobacillussuis, Haemophilus parasuis, Pasteurella multocida, and Bordetella bronchiseptica | Not defined | Essential oil | 100 μl of 1.25 to 0.01% (v/v) | Media + microbes + PBS | Not defined | MIC values ranging from 0.039% to 0.078% at dilutions 0.01–1.25% v/v (p < 0.05) | [57] | |
| Antioxidant activity | in vivo | Antioxidant enzyme levels in rabbits | Not defined | Aqueous extract | 50 mg/kg of body weight | Water | Not defined | Levels of antioxidant enzymes catalase, glutathione, glutathione-S-transferase, and superoxide dismutase increased by 14.12%, 27.69%, 98.75% and 78.29%, respectively (p < 0.05) | [58] |
| in vivo | Antioxidant enzyme levels in rats | Dried leaves | Aqueous extract | 500 mg/kg body weight | Paracetamol (200 mg/kg) | Not defined | Alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase content increased by 2 units/mL. In combination with paracetamol, the enzyme levels increased by 15–20 units/ml (p < 0.01) | [59] | |
| in vitro | Radical scavenging activity using DPPH | Leaf and stem | Aqueous extract | 0.0125–3.0 mg/ml | Not defined | Polysaccharide biding with BSA brings out the radical scavenging | Radical free scavenging activity of 92.0% at the concentration of 1.5 mg/mL, in accordance with butylated hydroxyanisole (BHA-95.7%) and butylated hydroxytoluene (BHT-96.6%). | [52] | |
| in vitro | FRAP, ABTS, and FIC | Not defined | Essential oil | 0.23–30 mg/ml | Not defined | Not defined | FRAP, FIC, ABTS assays showed IC50 values 12.69, 13.29, and 6.46 mg/ml respectively (p < 0.05) | [60] | |
| in vitro | Primary and secondary lipid oxidation products in oil in water (O/W) emulsions through DPPH | Dry waste plant | Ethanolic extract | 100 μl of different concentrations | Not defined | Not defined | IC50 of 93 μg/ml compared to BHT (89 μg/mL) (p > 0.05) | [61] | |
| Antifungal activity | in vitro | Sclerotinia sclerotiorum, Botrytis cinerea, Phytophthora parasitica, Pythium aphanidermatum, Fusarium oxysporum, Alternaria brassicae, Trichoderma aggressivumf.sp. europaeum, Cladobotryum mycophilum | Not defined | Essential oil | 5,10,15,20,30% (v/v) | Media + Tween 20 | Not defined | Mycelial growth inhibition values were found to be ranging between 13.9 to 41.4 mm at 5% concentration with ED50 values ranging from 9.3-18.0% for all the species (p < 0.05) | [14] |
| in vitro | Clinical isolates of Candida albicans and C. glabrata species | Not defined | Essential oil | 0.005–2.5% (v/v) | Amphotericin B | Possible ergosterol binding | MIC and MFC were in the range of 0.04–22.9 mg/ml for all the isolates. Thyme oil reduced the fungal growth in the initial hours (4–8). It inhibited the growth with sorbitol at lower MIC (0.08 mg/ml) (p < 0.05) | [15] | |
| in vivo | C. albicans in a Caenorhabditis elegans nematode model | Not defined | Thymol | 32, 64, and 128 mg/l | Kanamycin (45 μg/ml), ampicillin (100 μg/mL), and streptomycin (100 μg/ml) | Enhancing pmk-1and sec-1 gene expressions, which in turn enhance p38 MAPK signalling pathway | Complete inhibition of fungi and biofilm at 64 mg/l and 128 mg/ml, compared to control used (p < 0.05). Growth reduction at 12 h, compared to control (36 h). Thymol enhances the expressions of pmk-1and sec-1 genes, in turn p38 MAPK signalling pathway | [62] | |
| in vitro | Cryptococcus neoformans | Not defined | Essential oil | 0.07–10 mg/ml | FLC (0.06–128 μg/mL), ITC (0.0078–2 μg/ml), VRC (0.0078–32 μg/mL) | Possible membrane deterioration by thymol, Possible ergosterol binding | MIC and MFC were found to be in 0.56–1.12 mg/ml in accordance with controls. Thymol showed better activity 0.02–0.08 mg/ml (p < 0.05) | [63] | |
| in vitro | Aspergillus flavus | Not defined | Essential oil vapour and liquid phases | 0, 1, 5, 10, and 20 μg/ml | Aflatoxin B | Downregulating of fungal development genes brlA, abaA, wetA and aflatoxin biosynthesis genes aflR, aflD, and aflK | Vapor and liquid phases reduced growth at 20 and 400 μg/ml, respectively. Thyme oil 10 μg/mL of reduced production of afltoxin by 97.0 and 56.4% through vapour and liquid phases, respectively. (p < 0.05). | [16] | |
| Anti-inflammatory activity | in vitro | NO radical scavenging in murine macrophage cell line J774A.1 | Flowering tops | Aqueous extract | 8.5, 16, 50.4, 84 μg/ml | Dexamethasone | Possible cellular mechanisms of suppression of iNOS induction by flavonoids | Significant scavenging of NO radicals with 80.3% of the activity at 16 μg/ml concentration was observed in accordance with control (p < 0.05) | [19] |
| in vitro | 5-lipoxygenease (5-LOX) production, lipopolysaccharide (LPS) induced TNF-α, IL-1β, and IL-8 secretions using THP-1 cells | Dried plant | Essential oil | 30 μl of different concentrations | α-bisabolol | Not defined | 5-LOX got inhibited at 0.005 μg/ml (IC50) of thyme oil, compared to α-bisabolol (0.049 μg/mL). TNF-α, IL-1β, and IL-8 got inhibited at 0.01 μg/ml. | [20] | |
| in vivo | Mice with carrageenan-induced paw edema | Aerial parts and dried leaves | Essential oil | 100, 200 and 400 mg/kg | Tween 80 and diclofenac | Not defined | Paw thickness was found reducing at a dose of 400 mg/kg. Results were in accordance with both the controls Tween 80 and diclofenac (p < 0.001). Toxic level of thyme oil was found (4500 mg/kg), where sedation was observed at 5000 mg/kg. | [43] | |
| in vivo | Mice with carrageenan-induced pleurisy | Leaves | Essential oil | 250, 500 and 750 mg/kg | Croton oil | Carvacrol may act by inhibiting cytokines and leukotrienes, and these mediators are likely not involved in the mechanism of action of thymol | All the concentrations reduced inflammatory exudates as well as migrated leucocytes in ear edema. Individual assessment showed thymol (34.2%) and carvacrol (47.3%) are attributable for the anti-inflammatory activity (p < 0.05) | [64] | |
| Anti-cancerous activity | in vitro | MCF7 (breast adenocarcinoma), HCT15 (colon carcinoma), HeLa (cervical carcinoma), HepG2 (hepatocellular carcinoma), and NCI-H460 (non-small cell lung cancer) cell lines | Dried aerial parts | Essential oil | 10–100 μg/ml | Ellipticine (0.24–65.2 μg/ml) | Possible involvement of thymol in the stimulation of active proliferation of pulp fibroblasts | T. vulgaris L. oil showed inhibition of growth at 76.02–180.40 μg/ml concentration (GI50). It did not show any effect on non-tumour liver PLP cells, even at a high concentration of 400 μg/ml (p < 0.05) | [65] |
| in vitro | THP-1 leukemia cell line | Not defined | Essential oil | 10–500 μg/ml | DMSO | Not defined | At a concentration of 100 μg/ml and >200 μg/ml, thyme oil prevented the proliferation of THP-1 leukemia cells | [66] | |
| in vitro | H460 lung cancer cell line | Not defined | Hydroalcoholic extract | 0.04–0.6% | Glyceraldehyde 3-phosphate dehydrogenase | Possible interference in pro-inflammatory cytokines | H460 lung cancer cell line was found to be sensitive at 0.11% of hydroalcoholic extract (p < 0.05) and downregulated NF-κB p65 and NF-κB p52 proteins along with the reduction of IL-1β and IL-8 gene expression in LPS model | [21] | |
| in vivo | Mammary carcinoma rat and 4T1 mouse models | Dried plant | Thyme powder | 50 mg/kg body weight | Untreated models | Possible interference with pro-inflammatory cytokines, Possible upregulation of caspase genes at epigenetic level | Thyme powder reduced the volume of 4T1 tumours by 85% at 1% concentration. In rat model, the same concentration decreased the tumour frequency by 53% (p < 0.05). Upregulation of caspase-2 and caspase-3 enzymes, along with bcl-2 and Bax proteins | [67] | |
| in vitro | HL-60 acute promyelotic leukemia cell line, human peripheral blood mononuclear cell (PBMC) | Not defined | Thymol | 5, 25, 50, 75 and 100 μM for 24 h | Camptothecin (5 μM) | Apoptosis induced by thymol in HL-60 cells was associated with ROS production, increase in mitochondrial H2O2 production, decrease in Bcl-2 protein, increase in Bax protein levels, enhancing apoptosis inducing factor (AIF) in mitochondria and caspase activation | Thymol showed no cytotoxic effect on human peripheral blood mononuclear cell (PBMC) at 5 and 25μM concentrations. However, extensive cytotoxicity was observed at >50 μM, after 24 h | [68] | |
| in vitro | Synthesized silver nanoparticles against T47D human breast cancer cells | Dried leaves | Silver nanoparticles and ethanol extract | 12.5–200 μg/ml | Untreated cells | Nanoparticles could trig- ger translocation of phosphatidylserine (PS) from the inner membrane indicating apoptosis pathway rather than necrosis | T47D cells showed high sensitivity towards nanoparticles (90%) compared to the extract (75%). T47D cells treated with nanoparticles showed 18.40% early and 0.69% late apoptosis with varying IC50 concentrations (12.5–100 μg/mL). Same was observed in case of plant extract, where 15.67% early and 1.70% late apoptosis was found (p < 0.05) | [47] | |
| Antiviral activity | in vitro | Influenza virus | Not defined | Essential oil vapour and liquid phases | 3.12–100 μl/ml | Canova oil | Possible interaction with hemagglutinin (HA) | Liquid phase at 3.1 μl/ml concentration completely inhibited the viral growth, which was better than that of control used (canola oil). Significant inhibition of HA was observed. Also, 50% of the culture was reduced depicted as TC50 14.34 μl/ml (p < 0.05) | [33] |
| in vitro | Herpes simplex virus (HSV) on RC-37 (African green monkey kidney cells) | Not defined | Essential oil | 10–750 μg/ml | Untreated cells | Not defined | Cytotoxicity ranged between 20 μg/ml for citral and 1250 μg/ml for 1,8-cineole. IC50 values for1,8-cineole was 1200 μg/ml. Thyme oil proved to reduce the viral load by >96%, whereas all monoterpenes by >80% (p < 0.05) | [69] | |
| in vitro | HIV-1 in HeLa HL3T1 cell line | Not defined | Essential oil | 7.5–240 μg/ml |
Neomycin, cisplatin | Possible alteration in the structure of Tat/TAR-RNA complex | EMSA showed a notable inhibitory potential of oil (3–6 μg/ml), compared to the control in case of Tat/TAR-RNA complex inhibition. Reduction activity test against Tat-induced HIV-1 LTR transcription resulted in RT50 = 0,83 μg/ml, a notable inhibitory potential which reduced viral transcription to 52% (p < 0.05) | [70] | |
| in vitro | HIV-1 subtype A in PBMC cell line | Dried plant | Methanol extract | 10, 100, 200, 800 and 1600 μg/ml | DMSO, Zidovudine | Not defined | The cytotoxicity value (CC50) on PBMC was found to be 200 μg/ml. Antiviral assay revealed EC50 value of >500 μg/ml. Mean fluorescent intensity (MFI) of the CD4+ expressions were found to be 22.72 in PBMC (p < 0.05) | [71] | |
| Antidiabetic activity | in vitro | Inhibition of α-glucosidase and α-amylase enzymes | Not defined | Aqueous, methanol and ethanol extracts | 4, 8, 15, and 20 μg/ml | Acarbose | Not defined | Methanol extract resulted in maximum inhibition of α-glucosidase (IC50 4.35, 22.04, 30.77, 43.13), though less compared to Acarbose (IC50 16.11, 44.6, 53.03, 63.70). Similarly, α-amylase got reduced maximally by the same extract (IC50 6.39, 11.47, 17.01, 22.93), less compared to Acarbose (IC50 12.37, 25.16, 36.08, 44.97) | [72] |
| Anxiolytic activity | in vivo | Elevated plus-maze (EPM) rat model | Dried plant | Aqueous extract | 50 mg/kg, 100 mg/kg, and 200 mg/kg | Saline fed groups | Possible relation with antioxidant activity of phytochemicals | The aqueous extract exhibited a significant increase in rat movement into the open arms at 100 mg/kg (p < 0.05) and 200 mg/kg (p < 0.01). | [74] |
| UV-protective activity | in vitro | Human skin cells | Not defined | Aqueous extract, thymol | 1.82 μg/ml extract and 1 μg/ml thymol | Normal cells without UV treatment, but with extract treatment | Reduction of ROS induced DNA damage, Possible involvement of polyphenols in protectivity | Aqueous extract of thyme leaf (1.82 μg/ml) and thymol (1 μg/ml) reduced the release lactic acid dehydrogenase (LDH), in cultured skin cells treated with UV rays. Cell proliferation was observed in thyme pre-treated skin cells in accordance with control, along with the reduction in DNA damage (p < 0.01) | [75] |
| Anthelminthic activity | in vitro | Eimeria spp. oocysts from Turkey fowls | Not defined | Essential oil | 0, 1, 2, 4, 8, 10, 20, 40, 80, and 800 mg/ml | Ammonia and diclazuril | Not defined | Thyme oil showed significant anti-helminthic activity against 4 species of Eimeria spp. at IC50 53.42 mg/ml for 5×104 oocysts (p < 0.05) | [40] |
| Anti-anti-alzheimer's activity | in vivo | Acetylcholine esterase and nicotinic acetylcholine receptor in C. elegans nematode model | Aerial parts and leaves | Essential oil | 10, 20, 40, 60, 80, and 100 ppm | 10% DMSO | Upregulation of unc-17, unc-50, and cho-1 genes by ρ-Cymene | Enhancement of the nicotinic acetylcholine receptor activity, upregulation of unc-17, unc-50, and cho-1 genes at 40 and 60 ppm ρ-Cymene was attributed for gene upregulation activity along with downregulating ace-1 and ace-2 at 20 and 100 ppm (p < 0.05). Thymol and γ-terpinene enhanced synaptic acetylcholine levels in combination (40 ppm) | [76] |
| Anti-osteoporotic activity | in vivo | Rat model with low calcium intake | Dried leaves | Leaf powder | 5% w/w | Standard diet + normal calcium (Ca 0.5% w/w), standard diet + low calcium (Ca 0.1% w/w), Thyme powder (5% w/w) + low calcium (Ca 0.1% w/w) | Possible promotion of calcium resorption in the gut | Significant increase in the bone mass (2.93 g/kg), length 32.8 mm), and density (0.13 g/cm2), compared to low calcium diet control (2.46 g/kg, 32.2 mm, and 0.09 g/cm2, respectively) (p < 0.05) | [77] |
| Anti-pulpotomy activity | in vivo | Formocresolpulpotomy in humans | Not defined | Ethanolic extract | Suitable consistency | Formocresol | Not defined | Thyme ethanolic extract along with zinc oxide reduced pain and tenderness, Enhanced bone and root resorption. Clinical and radiographic evaluations showed 94.4% and 88.2% success, respectively with no statistical significance compared to the control, formocresol 88.2% (p > 0.05) | [78] |
7. Clinical trials of T. vulgaris L. preparations
Despite many explorations in the aspects of pharmacological efficiency, very little data are available on the clinical trials of T. vulgaris L.-based products. Our literature survey could find only few studies evaluating the pharmacological potential of T. vulgaris L.-based products using humans as study models [79, 80]. These clinical trials used different forms of T. vulgaris extracts and have resulted in the amelioration of the patients'/volunteers' health conditions (Table 4). Chemical characterization of these herbal products is yet to be done, which could decipher the specific action of phytochemicals involved in the pharmacological activity. Recently, a study by Caverzan et al. (2020) prepared a gel named ThymLec gel 2%, which was used as a phytocosmetic to improve facial health conditions [79]. A preparation from leaf and flower extract (1.0%–3.0%) + water, propanediol, glycerine +5.0%–13.4%) lecithin (additive) + benzyl alcohol, potassium sorbate, tocopherol (preservatives), application of this gel2 mg/cm2 on female facial skin with 2% concentration (20 mg/g w/w) twice a day (morning and evening) resulted in the reduction of area (7.0%), depth, and length (10.2%) of the perioral wrinkles on day 60, whereas the benchmark produced a reduction of 5.4% and 7.5% of the same parameters, respectively. The length of the nasolabial lines was also decreased (8.9%). Similarly, area (8.9%), breadth (3.9%), and length (11.1%) of the crow's feet wrinkles were decreased with a corresponding reduction in the smile lines by 6.9%, 4.1%, and 10.1%, respectively. Face oval remodelling evaluation using ThymLec 2% resulted in the reduction of total face volume (4.9 fold) in comparison with benchmark on day 60. In vitro adiponectin synthesis was significantly increased in cultured 3T3-L1 embryonic fibroblasts treated with ThymLec 2% (122% at 0.0195% concentration) and (137% at 0.039% concentration). This was 87% higher than the benchmark used. A dose-dependent treatment of ThymLec 2% resulted in the expression of PPAR-γ mRNA levels, in comparison with that of the control groups. ThymLec topical application led to adipogenesis and lipid production, further augmenting cell volume and better remodelling of face oval features. ThymLec modulates the PPAR-γ signalling pathway, increasing adiponectin production and adipocyte lipid accumulation [79.
Table 4.
Summary of clinical trials of T. vulgaris preparations.
| Pharmacological activity | Type of study | Models | Plant part/material | Type of Extract/compound | Doses | Controls | Mechanisms | Results | References |
|---|---|---|---|---|---|---|---|---|---|
| Adipogenetic and anti-aging | in vitro | Humans | Leaves and flowers | ThymLec gel 2% prepared from leaf and flower extract (1.0%–3.0%) + water, propanediol, glycerine +5.0%–13.4%) lecithin (additive) + benzyl alcohol, potassium sorbate, tocopherol (preservatives) | 2 mg/cm2 of the facial skin applied with 2% concentration (20 mg/g w/w), gently spreading, twice a day (morning and evening). | Placebo (formulation containing the same vehicle of ThymLec gel) and Benchmark 2% | ThymLec topical application leads into the adipogenesis and lipid production, further augmenting cell volume and better remodelling of face oval features. ThymLec modulates the PPAR-γ signalling pathway, increasing adiponectin production and adipocyte lipid accumulation. | Reduction of area (7.0%), depth, and length (10.2%) of the perioral wrinkles on day 60, whereas benchmark produced a reduction of 5.4% and 7.5%, respectively in case of humans. Length of the nasolabial lines (8.9%), Crow's feet wrinkles' area (8.9%), breadth (3.9s%), and length (11.1%) were also decreased. Face oval remodelling evaluation resulted in the reduction of total face volume (4.9 fold) in comparison with benchmark on day 60. In vitro adiponectin synthesis increased in 3T3-L1 embryonic fibroblasts treated with ThymLec 2% (122% at 0.0195% concentration) and (137% at 0.039% concentration). | [79] |
| Anti-dysmenorrhea | in vitro | Humans | Not defined | Essential oil | 25 drops of essential oil | Ibuprofen, Placebo (Ibuprofen 200mg + oil 25 drops) | Anti-prostaglandin and antispasmodic activity of T. vulgaris L. | Pain intensity mean values reached lowest point (6.57–1.14 VAS) in case of oil consumers' group in comparison with placebo (6.13–3.45) and ibuprofen (5.3–1.48) (p < 0.05). No significant results were obtained in case of bleeding control by both oil and placebo. Oil was able to reduce clinical symptoms like lower abdominal pain, nausea, mood swing, and fainting after 48 h of consumption (p < 0.05). | [80] |
| Anti-bronchitis | in vitro | Humans | Whole plant extract | Dry powder extract | 160 mg oral dose along with 60 mg primrose (P. vulgaris) root extract for 11 days | Placebo tablet without ingredients | Not defined | The extract reduced the cough symptoms on day 9 with an efficacy of 73.7% in comparison with placebo (day 11; 57.8%) (p < 0.0001). Reduction of bronchitis severity score was observed only after 4 days in case of extract (-6.2) in comparison with placebo (-4.1) (p < 0.05). The extract also reduced minor symptoms like disturbance in sleeping, rising body temperature, chest pain, and difficulty in breathing. | [12] |
In another trial, which was focused on the amelioration effects of T. vulgaris L. on dysmenorrhea (painful menstruation in females), essential oil (25 drops) was investigated for its pain relieving properties [80]. Pain intensity during menstruation was measured in visual analogue scale (VAS) on a 10 cm line before and after administration of T. vulgaris L. essential oil. Pain intensity mean values reached the lowest point (6.57–1.14) in case of oil consumers’ group in comparison with that of the placebo (6.13–3.45) and ibuprofen (5.3–1.48) (p < 0.05). However, no significant results were obtained in case of bleeding control by both oil and placebo (Ibuprofen 200mg + oil 25 drops). In addition, the oil was able to reduce clinical symptoms like lower abdominal pain, nausea, mood swing, and fatigue after 48 h of consumption (p < 0.05). Around 71.4% of the volunteers marked T. vulgaris L. oil as excellent whereas ibuprofen (28.0%) and placebo (14.3) were found to be underscored. Here, anti-prostaglandin and antispasmodic activity of T. vulgaris L. was attributed [80].
The use of T. vulgaris L. in the treatment of respiratory ailments [37], was supported by a clinical trial investigating a combination of T. vulgaris and Primula vulgaris (primrose, common thyme) dry powder extract on bronchitis patients. The combination (oral dose of 160 mg thyme and 60 mg primrose root extract for 11 days) reduced the cough symptoms on day 9 with an efficacy of 73.7% in comparison with that of the placebo (day 11; 57.8%) (p < 0.0001). Reduction of bronchitis severity score was observed only after 4 days in case of thyme and primrose treatment group (-6.2) in comparison with the placebo (-4.1) (p < 0.05). Extract was well tolerated in most of the patients (183) with only 1.7% adverse effects reported with 97.8 % patients rating this combination as preferable. The extract also reduced minor symptoms like disturbance in sleep, rising body temperature, chest pain, and difficulty in breathing [12]. However, no underlying mechanisms were decoded during this study. Therefore, understanding the mode of action along with product formulation with lesser adverse effect and affordability are to be focused. Research also needs to be focused on revealing the chemical components of such herbal products made.
8. Toxicology
T. vulgaris L. is considered as a culinary herb and has been extensively used since time immemorial in Europe and Mediterranean. In all the records of ethnomedicinal properties, there is no mention of toxic effects. Moreover, it has been described as the most used herb by the physician Dioscorides in the 2nd century [8]. In support of this, present-day studies report no cytotoxic activities of either the extracts or oil. A 28-day oral dose toxicity assessment of thyme oil in rats was conducted in single (2,000 mg/kg) and repeated dose (100, 250, and 500 mg/kg/day) methods, where no signs of toxicity were detected. Even with the administration of 500 mg/kg/day, the body weight was found to be altered, yet with no toxicity observed even on 28th day [81]. In support to this, a study conducted by Benourad et al. (2014) assessed the activity of thyme essential oil and found no histopathological changes in the rat liver and kidney, except a few metabolic changes including increase in urea nitrogen, creatinine and uric acid levels. Besides this, alterations in glycolysis, β-oxidative pathways, Kreb's cycle were also observed. In total, this study revealed that injection of repeated doses of thyme oil results in a few intermediary metabolic disturbances with no cytotoxic activities [82]. Several studies conducted on cancer cell lines reported the cytotoxic effect on tumour cells. However, thyme oil did not show any effect on non-tumour liver PLP cells, even at the high concentration of 400 μg/ml [66]. Even thymol is reported with no cytotoxic effect on normal human cells except >50 μM [68]. From these studies, it becomes evident that the T. vulgaris L. plant extracts and oil possess no cytotoxic activity though there are reported cases of mild metabolic changes.
9. Perspectives and projections
Based on the available literature and gaps present with respect to ethnopharmacology, pharmacology, and phytochemistry of T. vulgaris L., a few perspectives and future projections could be drawn out. Though a tremendous amount of ethnobotanical literature is present, it lacks specifications in terms of plant material, plant parts, and extracts used in ancient times. With these foundational evidences, concrete modern-day pharmacological approaches could be used, thereby supporting the vital claims by the previous generation that in turn could be used in drug discovery. Pharmacological findings need to be supported by the mechanisms. The action of plant extracts and individual compounds need to be defined at the molecular level to find more druggable targets, which can further be used to design a specific drug. Moreover, these studies have used multicomponent assays with varying concentrations, which complicate the determination of specific dose. Thus, qualitative and quantitative assays using individual compounds in a dose-dependent manner and a combination approach could reduce the burden of multifactorial results. Isolated compounds from the plant extracts could be further modified according the requirement based on cytotoxic evaluations done in primary screening. Such compounds could be analysed in silico for effective binding and inhibitory effect on specific component or any part of the identified target. Further, the bioavailability of the compounds could be dealt to modify the compound to match the physiological conditions of the target organ. Usage of in silico methods like molecular modelling and docking can save a lot of animals, which are used in laboratories.
Many of the studies quoted in this review need to be modified in terms of their methodology. Antimicrobial evaluations need a reformation, where the zone of inhibition could be excluded. Metabolic approaches like enzyme inhibition, protein inactivation, and interference in DNA and protein biosynthesis could be carried out. These mechanisms support the specific drug discovery process. In case of antioxidant activity, conventional assays like DPPH, ABTS, and FRAP need to be excluded, as advancements including evaluation of cytokine factors and antioxidant enzymes have been a major concern in recent years. Herein, the use of in vivo approach in support to the in vitro findings needs to be focused. Evaluations require animal model and cell lines with drug-induced autoimmunity. As the inflammation is concerned with several signalling pathways and immune cells, the fate of these cells and pathways need to be studied upon the introduction of plant fractions. Anti-cancerous studies need to include animal model and more in vivo approaches than cell lines, along with a dose-dependent approach using individual compounds.
These suggestions are utmost necessary to enhance the pharmacological studies associated with the plant products. Following up these protocols will help in the generation of data from phytochemicals, which can be transferred into clinical trials to get a completely processed plant product. Furthermore, high quality, advanced phase, randomized, placebo-controlled, multi-centred, randomized, and double-blind clinical trials could be done.
10. Conclusion
The present-day proximate analysis of T. vulgaris L. supports the use of the plant as an important ingredient in food since the Egyptian era. Alongside biomolecules, the rich diversity of phytochemicals present in the T. vulgaris L. plant may be accountable for major health benefits. The ethnomedicinal uses of the plant have revealed the importance in treating various diseases. Several studies conducted in vitro and in vivo using cell lines and animal models with induced pathological conditions have proved the efficiency of the plant as a therapeutic agent. T. vulgaris L. is found to be effective against both infectious and foodborne pathogens. Both the oil and extract of the plant were evaluated for their effects on inflammation and cancer. Along with the proven pharmacological aspects, there are several activities that are yet to be reported. For example, the literature survey could find only 2 studies with significant results on anti-diabetic potential of the plant. Furthermore, inhibition of acetylcholine esterase could be assessed for more physiological aspects, especially with diabetes. The extensive inhibitory effect on foodborne and oral pathogens has proved its possible use as a preservative agent and an important drug for dental caries, respectively. Meanwhile, T. vulgaris L. also possesses anti-helminthic property, which can make it a prominent therapeutic agent against parasitic infections. Also, a great amount of work needs to be done with respect to its skin protection activity. The promising evidence on the cell proliferation post-exposure to UV rays suggests its clinical trials for establishing a potent product. Pre-clinical studies at a large scale including in vivo cytotoxicity assays are suggested for the evaluation of accurate dosage levels of different plant fractions. Pharmacokinetics and pharmacodynamics of the compounds need to be focused. In addition, interactions with dietary molecules and drugs need to be assessed for the development of nutraceuticals. The pharmacological analysis at the genetic and proteomic level, along with commercially available drugs would determine the beneficial and harmful effects of the plant extracts or individual compounds. In total, T. vulgaris L. can open the doors to the development of many prominent therapeutic agents with diverse nature of applications to resolve several health maladies.
Declarations
Author contribution statement
Ramith Ramu; Shashank M Patil; Prithvi S Shirahatti: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Raghavendra G. Amachawadi; Chandan Shivamallu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
All the authors thank JSS Academy of Higher Education and Research, Mysore for constant support and encouragement to carry out this work.
Contributor Information
Ramith Ramu, Email: ramithramu@jssuni.edu.in.
Raghavendra G. Amachawadi, Email: agraghav@vet.k-state.edu.
References
- 1.DebMandal S.M. Thyme (Thymus vulgaris L.) oils. In: Preedy V., editor. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; London, UK: 2016. pp. 825–834. [Google Scholar]
- 2.Hosseinzadeh S., Kukhdan A.J., Hosseini A. The application of Thymus vulgaris in traditional and modern medicine: a review. Global J. Pharmacol. 2015;9:260–266. [Google Scholar]
- 3.Javed H., Erum S., Tabassum S. An overview on medicinal importance of thymus vulgaris. J. Asian Sci. Res. 2013;3:974–982. [Google Scholar]
- 4.Kuete V. Thymous vulgaris. In: Kuete V., editor. Medicinal Spices and Vegetables from Africa. first ed. Elsevier Inc.; 2017. pp. 599–609. [Google Scholar]
- 5.Basch E., Ulbricht C., Hammerness P. From natural standard thyme (Thymus vulgaris L.) Thymol. J. Herb. Pharmacother. 2004;4:49–68. [PubMed] [Google Scholar]
- 6.Mogoşanu G.D., Grumezescu A.M., Bejenaru C. Food Preservation. Academic Press; London, UK: 2017. Natural products used for food preservation; pp. 365–411. [Google Scholar]
- 7.Vouillamoz J.F., Christ B. Thymus vulgaris L.: thyme. In: Novak J., Blüthner W.D., editors. Medicinal, Aromatic and Stimulant Plants. Handbook of Plant Breeding. Springer; 2020. pp. 547–557. [Google Scholar]
- 8.Jarić S., Mitrović M., Pavlović P. Review of ethnobotanical, phytochemical and pharmacological study of Thymus serpyllum L. Evid-Based. Compl. Alt. Med. 2015;101978:1–10. doi: 10.1155/2015/101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl B.E., Saez F. first ed. CRC Press/Taylor and Francis Group; New York, USA: 2002. Thyme: the Genus Thymus. [Google Scholar]
- 10.Satyal P., Murray B., McFeeters R. Essential oil characterization of thymus vulgaris from various geographical locations. Foods. 2016;5:70–75. doi: 10.3390/foods5040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benameur Q., Gervasi T., Pellizzeri V. Antibacterial activity of Thymus vulgaris essential oil alone and in combination with cefotaxime against blaESBL producing multidrug resistant Enterobacteriaceae isolates. Nat. Prod. Res. 2019;33:2647–2654. doi: 10.1080/14786419.2018.1466124. [DOI] [PubMed] [Google Scholar]
- 12.Kemmerich B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of thyme herb and primrose root in adults suffering from acute bronchitis with productive cough. Arzneim. Forsch. 2007;57:607–615. doi: 10.1055/s-0031-1296656. [DOI] [PubMed] [Google Scholar]
- 13.Čabarkapa I., Čolović R., Đuragić O. Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella Enteritidis. Biofouling. 2019;35:361–375. doi: 10.1080/08927014.2019.1610169. [DOI] [PubMed] [Google Scholar]
- 14.Diánez F., Santos M., Parra C. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018;67:400–410. doi: 10.1111/lam.13053. [DOI] [PubMed] [Google Scholar]
- 15.Gucwa K., Milewski S., Dymerski T. Investigation of the antifungal activity and mode of action of thymus vulgaris, citrus limonum, pelargonium graveolens, cinnamomum cassia, ocimumbasilicum, and eugeniacaryophyllus essential oils. Molecules. 2018;23:1116. doi: 10.3390/molecules23051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian F., Lee S.Y., Chun H.S. Comparison of the antifungal and antiaflatoxigenic potential of liquid and vapor phase of Thymus vulgaris essential oil against Aspergillus flavus. J. Food Protect. 2019;82:2044–2048. doi: 10.4315/0362-028X.JFP-19-016. [DOI] [PubMed] [Google Scholar]
- 17.Prasanth R.V., Ravi V.K., Varsha P.V. Review on Thymus vulgaris traditional uses and pharmacological properties. Med. Aromat. Plants. 2014;3:1–3. [Google Scholar]
- 18.Soković M., Glamočlija J., Marin P.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vigo E., Cepeda A., Perez-Fernandez R. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris : nitric oxide inhibition in J774A.1 murine macrophages. J. Pharm. Pharmacol. 2004;56:257–263. doi: 10.1211/0022357022665. [DOI] [PubMed] [Google Scholar]
- 20.Tsai M.L., Lin C.C., Lin W.C. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci. Biotechnol. Biochem. 2011;75:1977–1983. doi: 10.1271/bbb.110377. [DOI] [PubMed] [Google Scholar]
- 21.Oliviero M., Romilde I., Beatrice M.M. Evaluations of thyme extract effects in human normal bronchial and tracheal epithelial cell lines and in human lung cancer cell line. Chem-Biol. Int. 2016;256:125–133. doi: 10.1016/j.cbi.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Miraj S., Kiani S. Study of pharmacological effect of Thymus vulgaris: a review. Der. Pharmacia, Lettre. 2016;8:315–320. [Google Scholar]
- 23.Nabavi S.M., Marchese A., Izadi M. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015;173:339–347. doi: 10.1016/j.foodchem.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 24.Morales R. The History, botany and taxonomy of the genus Thymus. In: Stahl-Biskup E., Sáez F., editors. The Genus Thymus. first ed. Taylor and Francis Inc.; London, UK: 2002. pp. 1–43. [Google Scholar]
- 25.Ghasemi P.A., Emami Z.A., Malekpoor F. An overview on genus Thymus. J. Herb. Drug. 2015;6:93–100. [Google Scholar]
- 26.Stahl B.E., Venskutonis R.P. Thyme. In: Peter K.V., editor. Handbook of Herbs and Spices. second ed. Woodhead Publishing; London, UK: 2012. pp. 499–525. [Google Scholar]
- 27.György Z., Incze N., Pluhár Z. Differentiating Thymus vulgaris chemotypes with ISSR molecular markers. Biochem. Syst. Ecol. 2020;19:104118. [Google Scholar]
- 28.Schmidt E., Wanner J., Höferl M. Chemical composition, olfactory analysis and antibacterial activity of Thymus vulgaris chemotypes geraniol, 4-thujanol/terpinen-4-ol, thymol and linalool cultivated in southern France. Nat. Prod. Commun. 2012;7 1934578X1200700833. [PubMed] [Google Scholar]
- 29.Borugă O., Jianu C., Mişcă C. Thymus vulgaris essential oil: chemical composition and antimicrobial activity. J. Med. Life. 2014;7:56–60. [PMC free article] [PubMed] [Google Scholar]
- 31.Meeran M.F.N., Javed H., Al Taee H. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017;8:380–389. doi: 10.3389/fphar.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baser K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008;14:3106–3119. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 33.Vimalanathan S., Hudson J. Anti-influenza virus activity of essential oils and vapors. Amer J. Essential. Oils. Nat. Prod. 2014;2:47–53. [Google Scholar]
- 34.Amirghofran Z., Ahmadi H., Karimi M.H. Immunomodulatory activity of the water extract of hymus vulgaris, thymus daenensis, and zataria multiflora on dendritic cells and T cells responses. J. Immun. Immunochem. 2012;33:388–402. doi: 10.1080/15321819.2012.655822. [DOI] [PubMed] [Google Scholar]
- 35.Gonçalves C., Zapelini A.P., Sganzerla W.G. Application in situ of zeinnanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food. Hydrocol. 2020;99:105339. [Google Scholar]
- 36.Gonelimali F.D., Lin J., Miao W. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018;9:1–9. doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preedy V.R. first ed. Acad Press; New York: 2015. Essential Oils in Food Preservation, Flavor and Safety. [Google Scholar]
- 38.Li X., He T., Wang X. Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem. Biodivers. 2019;9 doi: 10.1002/cbdv.201900254. [DOI] [PubMed] [Google Scholar]
- 39.Ramalho T.R., Oliveira M.T., Lima A.L. Gamma-terpinene modulates acute inflammatory response in mice. Planta Med. 2015;81:1248–1254. doi: 10.1055/s-0035-1546169. [DOI] [PubMed] [Google Scholar]
- 40.Isakakroudi N., Talebi A., Allymehr M. Effects of essential oils combination on sporulation of Turkey (Meleagrisgallopavo) Eimeria oocysts. Arch. Razi. Inst. 2018;73:113–120. doi: 10.22092/ARI.2017.109255.1102. [DOI] [PubMed] [Google Scholar]
- 41.Salehi B., Mishra A.P., Shukla I. Thymol, thyme, and other plant sources: health and potential uses. Phytother Res. 2018;32:1688–1706. doi: 10.1002/ptr.6109. [DOI] [PubMed] [Google Scholar]
- 42.Al-Asmari A.K., Athar M.T., Al-Faraidy A.A. Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian. Pac. J. Trop. Biomed. 2017;7:147–150. [Google Scholar]
- 43.Abdelli W., Bahri F., Romane A. Chemical composition and anti-inflammatory activity of algerian thymus vulgaris essential oil. Nat. Prod. Commun. 2017;12:611–614. [PubMed] [Google Scholar]
- 44.Stefanis I., Hadjipavlou-Litina D., Bilia A.R. LC-MS- and NMR-guided isolation of monoterpene dimers from cultivated thymus vulgaris varico 3 hybrid and their antityrosinase activity. Planta Med. 2019;85:941–946. doi: 10.1055/a-0927-7041. [DOI] [PubMed] [Google Scholar]
- 45.Fayad N.K., Hamad O., Al-Obaidi S. Water and alcohol extraction of thyme plant (thymus vulgaris) and activity study against bacteria, tumors and used as anti-oxidant in margarine manufacture. Innov. Sys. Des. Engg. 2013;4:1727. [Google Scholar]
- 46.Asha D., Lizzy M. Chemical profiling of Thymus vulgaris L. using HPTLC. J. Pharmacogn. Phytochem. 2017;6:1017–1023. [Google Scholar]
- 47.Heidari Z., Salehzadeh A., Sadat Shandiz S.A. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech. 2018;8:1–14. doi: 10.1007/s13205-018-1199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma C., Al Kaabi J.M., Nurulain S.M. Polypharmacological properties and therapeutic potential of β-caryophyllene: a dietary phytocannabinoid of pharmaceutical promise. Curr. Pharma. Des. 2016;22:3237–3264. doi: 10.2174/1381612822666160311115226. [DOI] [PubMed] [Google Scholar]
- 49.Salehi B., Upadhyay S., Erdogan Orhan I. Therapeutic potential of α- and β-pinene: a miracle gift of nature. Biomolecules. 2019;9:738–742. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aprotosoaie A.C., Hăncianu M., Costache I.I. Linalool: a review on a key odorant molecule with valuable biological properties. Flav. Frag. J. 2014;29:193–219. [Google Scholar]
- 51.Tschiggerl C., Bucar F. Influence of saponin plants on the volatile fraction of thyme in herbal teas. Fitoterapia. 2011;82:903–910. doi: 10.1016/j.fitote.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee P., Mukherjee S., Bera K. Polysaccharides from Thymus vulgaris leaf: structural features, antioxidant activity and interaction with bovine serum albumin. Int. J. Biol. Macromol. 2019;125:580–587. doi: 10.1016/j.ijbiomac.2018.11.117. [DOI] [PubMed] [Google Scholar]
- 53.Gnat S., Majer-Dziedzic B., Nowakiewicz A. Antimicrobial activity of some plant extracts against bacterial pathogens isolated from faeces of red deer (Cervuselaphus) Pol. J. Vet. Sci. 2017;20:697–706. doi: 10.1515/pjvs-2017-0087. [DOI] [PubMed] [Google Scholar]
- 54.Arshad N., Mehreen A., Liaqat I. In vivo screening and evaluation of four herbs against MRSA infections. BMC Compl. Alt. Med. 2017;17:1–7. doi: 10.1186/s12906-017-2001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fadil M., Fikri-Benbrahim K., Rachiq S. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtuscommunis L. essential oils against Salmonella typhimurium: optimization of antibacterial activity by mixture design methodology. Eur. J. Pharm. Biopharm. 2018;126:211–220. doi: 10.1016/j.ejpb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Amorese V., Donadu M.G., Usai D. In vitro activity of essential oils against pseudomonas aeruginosa isolated from infected hip implants. J. Infect. Dev. Count. 2018;12:996–1001. doi: 10.3855/jidc.10988. [DOI] [PubMed] [Google Scholar]
- 57.LeBel G., Vaillancourt K., Bercier P. Antibacterial activity against porcine respiratory bacterial pathogens and in vitro biocompatibility of essential oils. Arch. Microbiol. 2019;201:833–840. doi: 10.1007/s00203-019-01655-7. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Gabbar M., Ahmed R.R., Kandeil M.A. Administration of ginger and/or thyme has ameliorative effects on liver and kidney functions of V-line rabbits: histological and biochemical studies. J. Anim. Physiol. Anim. Nutr. 2019;103:1758–1767. doi: 10.1111/jpn.13166. [DOI] [PubMed] [Google Scholar]
- 59.Abd El Kader M.A., Mohamed N.Z. Evaluation of protective and antioxidant activity of thyme (Thymus vulgaris) extract on paracetamol-induced toxicity in rats. Aust. J. Basic. Appl. Sci. 2012;6:467–474. [Google Scholar]
- 60.Ballester-Costa C., Sendra E., Fernández-López J. Assessment of antioxidant and antibacterial properties on meat homogenates of essential oils obtained from four thymus species achieved from organic growth. Foods. 2017;6:59–65. doi: 10.3390/foods6080059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Guendouz S., Aazza S., Dandlen S.A. Antioxidant activity of thyme waste extract in O/W emulsions. Antioxidants. 2019;8:1–14. doi: 10.3390/antiox8080243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shu C., Sun L., Zhang W. Thymol has antifungal activity against Candida albicans during infection and maintains the innate immune response required for function of the p38 MAPK signaling pathway in Caenorhabditis elegans. Immun. Res. 2016;64:1013–1024. doi: 10.1007/s12026-016-8785-y. [DOI] [PubMed] [Google Scholar]
- 63.Scalas D., Mandras N., Roana J. Use of Pinussylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Compl. Alt. Med. 2018;18:1–13. doi: 10.1186/s12906-018-2219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fachini-Queiroz F.C., Kummer R., Estevão-Silva C.F. Effects of thymol and carvacrol, constituents of thymus vulgaris L. essential oil, on the inflammatory response. Evid-Based. Compl. Alt. Med. 2012:211–220. doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikolić M., Glamočlija J., Ferreira I.C.F.R. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensisBoiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crop. Prod. 2014;52:183–190. [Google Scholar]
- 66.Aazza S., Lyoussi B., Megías C. Anti-oxidant, anti-inflammatory and anti-proliferative activities of moroccan commercial essential oils. Nat. Prod. Commun. 2014;9:587–594. [PubMed] [Google Scholar]
- 67.Kubatka P., Uramova S., Kello M. Anticancer activities of thymus vulgaris L. In experimental breast carcinoma in vivo and in vitro. Int. J. Mol. Sci. 2019;20:1749. doi: 10.3390/ijms20071749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deb D.D., Parimala G., Saravana Devi S. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem-Biol. Int. 2011;193:97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Astani A., Reichling J., Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res. 2010;24:673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feriotto G., Marchetti N., Costa V. Chemical composition of essential oils from thymus vulgaris, cymbopogoncitratus, and rosmarinus officinalis, and their effects on the HIV-1 Tat protein function. Chem. Biodivers. 2018;15 doi: 10.1002/cbdv.201700436. [DOI] [PubMed] [Google Scholar]
- 71.Farsani M.S., Behbahani M., Z Isfahani H. The effect of root, shoot and seed extracts of the Iranian Thymus L. (Family: Lamiaceae) species on HIV-1 replication and CD4 expression. Cell J. 2016;18:255–261. doi: 10.22074/cellj.2016.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aljarah A.K., Hameed I.H. In vitro anti-diabetic properties of methanolic extract of thymus vulgaris using α-glucosidase and α-amylase inhibition assay and determination of its bioactive chemical compounds. Indian J. P. Health. Res. Dev. 2018;9:388–392. [Google Scholar]
- 73.Weaver L.J., Madhu S.V. Type 2 diabetes and anxiety symptoms among women in New Delhi, India. Am. J. Pub. Health. 2015;105:2335–2340. doi: 10.2105/AJPH.2015.302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komaki A., Hoseini F., Shahidi S. Study of the effect of extract of Thymus vulgaris on anxiety in male rats. J. Trad. Compl. Med. 2016;6:257–261. doi: 10.1016/j.jtcme.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cornaghi L., Arnaboldi F., Calò R. Effects of UV rays and Thymol/Thymus vulgaris L. extract in an ex vivo human skin model: morphological and genotoxicological assessment, Cells. Tiss. Org. 2016;201:180–192. doi: 10.1159/000444361. [DOI] [PubMed] [Google Scholar]
- 76.Sammi S.R., Trivedi S., Rath S.K. 1-Methyl-4-propan-2-ylbenzene from thymus vulgaris attenuates cholinergic dysfunction. Mol. Neurobiol. 2017;54:5468–5481. doi: 10.1007/s12035-016-0083-0. [DOI] [PubMed] [Google Scholar]
- 77.Elbahnasawy A.S., Valeeva E.R., El-Sayed E.M. The impact of thyme and rosemary on prevention of osteoporosis in rats. J. Nutr. Metabol. 2019:1–10. doi: 10.1155/2019/1431384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alolofi H., El-Sayed M., Taha S. Clinical and radiographical evaluation of propolis and thymus vulgaris extracts compared with formocresolpulpotomy in human primary molars. BDJ Open. 2016;2:1–6. doi: 10.1038/bdjopen.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caverzan J., Mussi L., Sufi B. A new phytocosmetic preparation from Thymus vulgaris stimulates adipogenesis and controls skin aging process: in vitro studies and topical effects in a double-blind placebo-controlled clinical. J. Cosmet. Dermatol. 2020;1 doi: 10.1111/jocd.13818. [DOI] [PubMed] [Google Scholar]
- 80.Salmalian H., Saghebi R., Moghadamnia A.A. Comparative effect of Thymus vulgaris and ibuprofen on primary dysmenorrhea: a triple-blind clinical study. Caspian. J. Internal. Med. 2014;5:82–89. [PMC free article] [PubMed] [Google Scholar]
- 81.Rojas-Armas, Arroyo-Acevedo J., Ortiz-Sánchez J. Acute and repeated 28-day oral dose toxicity studies of thymus vulgaris L. Essential oil in rats. Toxicol. res. 2019;35(3):225–232. doi: 10.5487/TR.2019.35.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benourad F., Kahvecioglu Z., Youcef-Benkada M. Prospective evaluation of potential toxicity of repeated doses of Thymus vulgaris L. extracts in rats by means of clinical chemistry, histopathology and NMR-based metabonomic approach. Drug Test. Anal. 2014;6:1069–1075. doi: 10.1002/dta.1606. [DOI] [PubMed] [Google Scholar]
- 83.Baraya Y.S., Wong K.K., Yaacob N.S. The immunomodulatory potential of selected bioactive plant-based compounds in breast cancer: a review, anti-cancer. Agent. Med. Chem. 2017;17:770–783. doi: 10.2174/1871520616666160817111242. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L., Zhang J., Zhao B. Quinic acid could be a potential rejuvenating natural compound by improving survival of Caenorhabditis elegans under deleterious conditions. Rejuven. Res. 2012;15:573–583. doi: 10.1089/rej.2012.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nadeem M., Imran M., Gondal T.A. Therapeutic potential of rosmarinic Acid : a comprehensive review. Appl. Sci. 2019;9:3139. [Google Scholar]
- 86.Hase T., Shishido S., Yamamoto S. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-45168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Monteiro Espíndola K.M., Ferreira R.G., Mosquera Narvaez L.E. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019;9:3–5. doi: 10.3389/fonc.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duangjai A., Nuengchamnong N., Suphrom N. Potential of coffee fruit extract and quinic acid on adipogenesis and lipolysis in 3T3-L1 adipocytes. Kobe J. Med. Sci. 2018;64:E84–E92. [PMC free article] [PubMed] [Google Scholar]
- 89.Habtemariam S. Protective effects of caffeic acid and the alzheimer's brain: an update. Mini Rev. Med. Chem. 2017;17:667–674. doi: 10.2174/1389557516666161130100947. [DOI] [PubMed] [Google Scholar]
- 90.Pragasam S.J., Venkatesan V., Rasool M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013;36:169–176. doi: 10.1007/s10753-012-9532-8. [DOI] [PubMed] [Google Scholar]
- 91.Saibabu V., Fatima Z., Khan L.A. Therapeutic potential of dietary phenolic acids. Adv. Pharmacol. Sci. 2015:1–10. doi: 10.1155/2015/823539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen Y., Song X., Li L. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019;111:579–587. doi: 10.1016/j.biopha.2018.12.074. [DOI] [PubMed] [Google Scholar]
- 93.Cueva C., Moreno-Arribas M.V., Martín-Alvarez P.J. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010;161:372–382. doi: 10.1016/j.resmic.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Flausino O.A., Jr., Dufau L., Regasini L.O. Alkyl hydroxybenzoic acid derivatives that inhibit HIV-1 protease dimerization. Curr. Med. Chem. 2012;19:4534–4540. doi: 10.2174/092986712803251557. [DOI] [PubMed] [Google Scholar]
- 95.Seidel C., Schnekenburger M., Mazumder A. 4-Hydroxybenzoic acid derivatives as HDAC6-specific inhibitors modulating microtubular structure and HSP90α chaperone activity against prostate cancer. Biochem. Pharmacol. 2016;99:31–52. doi: 10.1016/j.bcp.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Abotaleb M., Liskova A., Kubatka P. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10:221–228. doi: 10.3390/biom10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abedi F., Razavi B.M., Hosseinzadeh H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother Res. 2020;34:729–741. doi: 10.1002/ptr.6573. [DOI] [PubMed] [Google Scholar]
- 98.Srinivasulu C., Ramgopal M., Ramanjaneyulu G. Syringic acid (SA) ‒ A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka T., Kawaguchi N., Zaima N. Antiosteoporotic activity of a syringic acid diet in ovariectomized mice. J. Nat. Med. 2017;71:632–641. doi: 10.1007/s11418-017-1105-6. [DOI] [PubMed] [Google Scholar]
- 100.Chaudhary A., Jaswal V.S., Choudhary S. Ferulic acid: a promising therapeutic phytochemical and recent patents advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019;13:115–123. doi: 10.2174/1872213X13666190621125048. [DOI] [PubMed] [Google Scholar]
- 101.Mancuso C., Santangelo R. Ferulic acid: pharmacological and toxicological aspects. Food Chem. Toxicol. 2014;65:185–195. doi: 10.1016/j.fct.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 102.Lei Y., Fu P., Jun X. Pharmacological properties of geraniol - a review. Planta Med. 2019;85:48–55. doi: 10.1055/a-0750-6907. [DOI] [PubMed] [Google Scholar]
- 103.Pereira I., Severino P., Santos A.C. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces. 2018;171:566–578. doi: 10.1016/j.colsurfb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Kiskó G., Roller S. Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiol. 2005;5:36. doi: 10.1186/1471-2180-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonjardim L.R., Cunha E.S., Guimarães A.G. Evaluation of the anti-inflammatory and antinociceptive properties of p-cymene in mice. Zeitschrift fur Naturforschung. C, J. Biosci. 2012;67:15–21. doi: 10.1515/znc-2012-1-203. [DOI] [PubMed] [Google Scholar]
- 106.Marchese A., Arciola C.R., Barbieri R. Update on monoterpenes as antimicrobial agents: a particular focus on p-cymene. Materials. 2017;10:947–995. doi: 10.3390/ma10080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid-Based. Compl. Alt. Med. 2016;3012462:1–21. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Passos F.F., Lopes E.M., de Araújo J.M. Involvement of cholinergic and opioid system in γ-terpinene-mediated antinociception. Evid-Based. Compl. Alt. Med. 2015;829414:1–9. doi: 10.1155/2015/829414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Foti M.C., Ingold K.U. Mechanism of inhibition of lipid peroxidation by gamma-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agri. Food. Chem. 2003;51:2758–2765. doi: 10.1021/jf020993f. [DOI] [PubMed] [Google Scholar]
- 110.Erasto P., Viljoen A.M. Limonene - a review: biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008;3:1193–1202. [Google Scholar]
- 111.Mukhtar Y.M., Adu-Frimpong M., Xu X. Biochemical significance of limonene and its metabolites: future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018;38 doi: 10.1042/BSR20181253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khaleel C., Tabanca N., Buchbauer G. α-Terpineol, a natural monoterpene: a review of its biological properties, Open. Inside Chem. 2018;16:349–361. [Google Scholar]
- 113.Salehi B., Venditti A., Sharifi-Rad M. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019;20:1305–1400. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ali F., Rahul, Jyoti S. Therapeutic potential of luteolin in transgenic Drosophila model of Alzheimer's disease. Neurosci. Lett. 2019;692:90–99. doi: 10.1016/j.neulet.2018.10.053. [DOI] [PubMed] [Google Scholar]
- 115.Lin Y., Shi R., Wang X. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adeem M., Hamad Z.A. Isolation, synthesis and pharmacological applications of cirsimaritin – a short review. Acad. J. Med. Plant. 2020;7:252–260. [Google Scholar]
- 117.Guo S., Wu X., Zheng J. Anti-inflammatory effect of xanthomicrol, a major colonic metabolite of 5-demethyltangeretin. Food. Funct. 2018;9:3104–3113. doi: 10.1039/c8fo00279g. [DOI] [PubMed] [Google Scholar]
- 118.Fattahi M., Cusido R.M., Khojasteh A. Xanthomicrol: a comprehensive review of its chemistry, distribution, biosynthesis and pharmacological activity. Min. Rev. Med. Chem. 2014;14:725–733. doi: 10.2174/1389557514666140820122818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.