Abstract
Vitamin D3 has been reported to protect liver against non-alcoholic fatty liver disease (NAFLD) by attenuating hepatic lipid dysregulation in type 2 diabetes mellitus (T2DM). However, the mechanism of vitamin D3 on hepatic lipid metabolism-associated autophagy in hyperglycemia-induced NAFLD remains yet to be exactly elucidated. C57BL/6J mice were intraperitoneally injected with 30 mg/kg of streptozotocin and fed a high-fat diet for induction of diabetes. All mice were administered with vehicle or vitamin D3 (300 ng/kg or 600 ng/kg) by oral gavage for 12 weeks. Histological demonstrations of the hepatic tissues were obtained by H&E staining and the protein levels related to lipid metabolism and autophagy signaling were analyzed by Western blot. Treatment with vitamin D3 improved insulin resistance, liver damage, and plasma lipid profiles, and decreased hepatic lipid content in the diabetic mice. Moreover, vitamin D3 administration ameliorated hepatic lipid dysregulation by downregulating lipogenesis and upregulating lipid oxidation under diabetic condition. Importantly, vitamin D3 treatment induced autophagy by activating AMP-activated protein kinase (AMPK), inactivating Akt and ultimately blocking mammalian target of rapamycin (mTOR) activation in the T2DM mice. Additionally, vitamin D3 was found to be effective in anti-apoptosis and anti-fibrosis in the liver of diabetic mice. The results suggested that vitamin D3 may ameliorate hepatic lipid dysregulation by activating autophagy regulatory AMPK/Akt-mTOR signaling in T2DM, providing insights into its beneficial effects on NAFLD in type 2 diabetic patients.
Keywords: Type 2 diabetes mellitus, non-alcoholic fatty liver disease, autophagy, mTOR protein, vitamin D3, lipid metabolism
Impact statement
Vitamin D3 supplementation has shown beneficial effects on hyperglycemia-induced hepatic damage by regulation of lipid metabolism with autophagy, apoptosis, and fibrosis in T2DM mice. In particular, vitamin D3 attenuated hepatic lipid accumulation by activation of AMPK/Akt-mTOR signaling-associated autophagy in T2DM mice. Furthermore, vitamin D3 administration prevented progression of steatosis by reduction of hepatic apoptosis and fibrosis in T2DM mice. Our findings suggested that vitamin D3 might have a beneficial effect to ameliorate hyperglycemia-induced hepatic damage under diabetic condition.
Introduction
Type 2 diabetes mellitus (T2DM) is one of the chronic metabolic diseases, with a growing prevalence worldwide and serious health problems. An estimated 425 million people had DM in the world in 2017, and the number is expected to increase to 629 million by 2045.1 In T2DM, high blood glucose triggers blood vessel damage and causes micro- and macro-vascular complications, contributing to increase of disability and mortality. Non-alcoholic fatty liver disease (NAFLD) is considered as a leading liver disorder in T2DM.2 Hepatic lipid dysregulation due to insulin resistance increases fat accumulation in the liver. The progression of NAFLD is faster in T2DM patients than in non-diabetic patients,3 consequently leading to severe complications such as cirrhosis and hepatocarcinoma.
Under insulin resistance, insulin signaling to repress gluconeogenesis is impaired, while signaling to stimulate de novo lipogenesis (DNL) is still activated through sterol response element-binding protein 1c (SREBP1c).4 Meanwhile, impairment of insulin signaling suppresses peroxisome proliferator-activated receptor (PPAR)-α and carnitine palmitoyltransferase 1 (CPT1) to decrease β-oxidation. The imbalance between lipid synthesis and degradation causes accumulation of excessive triglyceride (TG) in the hepatocytes. Furthermore, hyperglycemia-induced reactive oxygen species (ROS) increase hepatic apoptosis by disturbing the balance between pro- and anti-apoptotic molecules.5 As the hepatocyte death increases, activated hepatic stellate cells (HSCs) stimulate the production of pro-fibrogenic proteins including collagen and α-smooth muscle actin (α-SMA), to induce liver cirrhosis.6
Recent evidences have focused on protective effects of autophagy on NAFLD. Autophagy, a self-recycling system for cell survival, removes damaged proteins and cell organelles, and also degrades lipid droplets, thereby regulates cellular lipid stores.7 Impaired autophagy reduces hepatic β-oxidation and TG output and promotes lipid accumulation, inducing NAFLD.8 Evidence suggests9,10 that microtubule-associated protein 1 light chain-3B (LC3B)-II, an autophagy-related marker, was significantly reduced in obesity with insulin resistance, suggesting a negative link between autophagy and insulin resistance. Several evidences suggest that AMPK/Akt-mTOR signaling is important regulator of autophagy.8,11 Under stressful conditions such as deprivations of nutrients or growth factors, mTOR is repressed and autophagy is activated.12 AMP-activated protein kinase (AMPK) inhibits mTOR to increase autophagy, while Akt upregulates mTOR to reduce autophagy.13 In T2DM, hyperinsulinemia stimulates Akt and mTOR which also inhibits AMPK, resulting in a decrease in autophagy.14
Vitamin D is a well-known hormone precursor that regulates calcium-phosphate homeostasis and bone mineralization. Vitamin D also regulates inflammation by reducing the release of pro-inflammatory cytokines, and affects insulin action and lipid metabolism.15 Recent studies have shown that the vitamin D deficiency could cause insulin resistance through inflammation, as vitamin D deficiency is associated with increased inflammation.16 Furthermore, the accumulating data have suggested that 1,25(OH)2D3, a biological active form of vitamin D, may defend against liver injury by regulating lipogenesis or β-oxidation.17,18 It has been reported that the blood vitamin D concentration was generally decreased in NAFLD patients.19,20 In addition, a link between a low blood vitamin D3 level and a risk of T2DM has been reported.21 A previously reported study demonstrated that 1,25(OH)2D3 reduced hepatic triglyceride accumulation and glucose output under insulin-resistant conditions in patients with NAFLD.22 Furthermore, the benefits of vitamin D3 supplementation for improving insulin resistance depend on the baseline 25(OH)D3 status.22
However, no research has investigated the effect of vitamin D3 on autophagy pathway in hyperglycemia-induced hepatic complication. Therefore, we investigated whether vitamin D3 showed ameliorative effects on hepatic lipid dysregulation through activation of autophagy induced via AMPK/Akt-mTOR signaling in T2DM.
Materials and methods
Animals
Four-week-old male C57BL/6J mice (16–18 g) were purchased from Raon Bio (Gyeonggi-do, South Korea). All mice were housed two or three per cage in a room maintained at 22 ± 1°C and 50 ± 5% humidity on a 12-h light/dark cycle, with free access to food and distilled water. After week-acclimation period, the mice were randomly assigned to two groups; a control group and a diabetic group. While the CON group was fed a control diet (AIN-93G; 10% kcal fat, Research Diets, New Brunswick, NJ, USA), the DM group was fed a high-fat diet (HFD; [D10110601] 40% kcal fat, Research Diets, New Brunswick, NJ, USA). After four weeks of diet treatment, 4 h-fasted mice were intraperitoneally injected with streptozotocin (30 mg/kg body weight, Sigma Aldrich) in citrate buffer (pH 4.5) twice at a week interval to induce T2DM or saline for the normal control mice, respectively23; 12-h fasting blood glucose (FBG) level was detected from the tail vein using a Onetouch Select glucometer (LifeScan Inc., Milpitas, USA) once a week. Mice with FBG ≥ 140.4 mg/dL (7.8 mmol/L) at least twice were considered diabetic. All protocols for the animal experiment were approved by Institutional Animal Care and Use Committee (IACUC) of Kyung Hee University, South Korea [KHUASP(SE)-16–005].
Experimental design
The diabetic mice were divided into three groups and treated with different levels of vitamin D3 supplement. The normal control mice (NC; n = 8) fed with the control diet was supplemented with a vehicle (olive oil). The diabetic control mice (DMC; n = 8) fed with the HFD were supplemented with the vehicle. Diabetic mice treated with vitamin D3 were fed HFD and supplemented with 300 ng/kg (low cholecalciferol, LC; n = 8) or 600 ng/kg (high cholecalciferol, HC; n = 8) of vitamin D3 (Sigma Aldrich, St. Louis, MO, USA) dissolved in olive oil, respectively. The vehicle and vitamin D3 were administrated everyday for 12 weeks by oral gavage. Food intake, body weight, and 10 h-FBG of the mice were measured every week.
After 12 weeks-treatment, 10 h-fasted mice were anesthetized with diethyl ether (Dukasn, Seoul, South Korea) to sacrifice. Blood was drawn from the cardiac puncture of the mice using a syringe coated with heparin (Sigma Aldrich, St. Louis, MO, USA). The collected blood was centrifuged at 845g for 15 min at 4°C to obtain plasma. The liver tissue was dissected out, rinsed with saline, immediately weighed and frozen with liquid nitrogen. The plasma supernatants and the liver tissue were stored at −80°C until analysis. Part of the liver was fixed with 10% formalin for paraffin embedding.
Oral glucose tolerance test
A week before sacrificing, 12 h-fasted mice were treated with 2 g/kg of glucose (Sigma Aldrich, St. Louis, MO, USA) by oral gavage to perform OGTT. After glucose administration, blood glucose level was determined at 0, 15, 30, 60, 90 and 120 min using Onetouch Select glucometer (LifeScan Inc., Milpitas, USA). The area-under-the-curve (AUC) of blood glucose was determined by sum of the trapezoidal area from 0 to 120 min. The AUC of each time point was as follows
AUC of each time point
= {(blood glucose) i+ (blood glucose) i-1}
× {(time) i – (time) i-1}/2 [i = time sequence]
Hepatic function test
Plasma aspartate transaminase (AST) and alanine transaminase (ALT) were measured by commercial kits (Asan pharmaceutical, Gyeonggi-do, South Korea). All analysis was performed in accordance with the instructions of manufacturer.
Plasma and hepatic lipid profiles and plasma vitamin D concentration
For obtainment of plasma and hepatic lipid profiles, triglyceride (TG), total cholesterol (TC), and high-density lipoprotein-cholesterol (HDL-C) were measured by using commercial kits (Asan pharmaceutical, Gyeonggi-do, South Korea). Analysis of plasma 25-OH vitamin D3 (EAGLE BIOSCIENCES, INC., NH, USA) was performed according to manufacturer protocols.
Hepatic lipid was extracted by the Folch method (2:1 chloroform/methanol mixture; CM mixture).24 All examinations were conducted with the instructions of manufacturer.
Western blot assay
To extract cytosolic protein, 0.5 g of liver tissue was homogenized in lysis buffer A (10 mM HEPES, 10 mM KCl, 0.5 mM DTT, 1.5 Mm MgCl2, 0.05% NP40 and distilled water; pH 7.9) with protease and phosphatase inhibitors and 0.5 M EDTA (Thermo Fisher, Waltham, Massachusetts, USA). The homogenates were incubated on ice for 1 h while gently shaking, and centrifuged at 845g for 10 min at 4°C. The supernatants were taken and re-centrifuged at 18,407g for 30 min at 4°C, then the final supernatants were used as cytosol extract. To prepare nuclear protein, the pelleted remnants were homogenized in lysis buffer B (5 mM HEPES, 0.5 mM DTT, 0.2 mM EDTA, 1.5 Mm MgCl2, 26% glycerol (v/v) and distilled water; pH 7.9) with 4.6 M NaCl. The homogenates were shaken on ice for 1 h followed by centrifugation at 18,407g for 20 min at 4°C, and the supernatants were used as nuclear extract.
Each protein extract was separated by 6–15% SDS-polyacrylamide gels and transferred onto PVDF membranes (Millipore, Marlborough, MA, USA). After blocking with 3% BSA in PBS-Tween 20 (PBS-T), the membranes were incubated with primary antibodies; SREBP1c, CCAAT-enhancer-binding proteins (C/EBPα), PPARγ, acetyl-CoA carboxylase (ACC), phosphorylated ACC (p-ACC), PPARα, LC3B, mTOR, phosphorylated mTOR (p-mTOR), p53 (Cell Signaling Technology, Inc., Danvers, MA, USA, 1:2000), CPT1A, fatty acid synthase (FAS), AMPK, phosphorylated AMPK (p-AMPK), Akt, phosphorylated Akt (p-Akt), caspase-3, caspase-8, Bcl-2-associated X factor (Bax), Bcl-2, α-SMA, Lamine B1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:200), collagen 1 A (COL1A) (Abcame, Cambridge, MA, USA, 1:2000), α-tubluin (Sigma Aldrich, St. Louis, MO, USA, 1: 4000). Each membrane was washed with PBS-T followed by incubation with respective horseradish peroxide (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology, CA, USA, 1: 2000), and then washed with PBS-T again. For detection of protein, the membrane was treated with ECL luminol reagent (Biorad, CA, USA) and the protein band intensity was scanned using G:BOX Gel and Blot Imaging Systems (Syngene, Cambridge, UK).
Statistical analysis
Comparison of significant differences among the groups was analyzed by one-way analysis of variance (ANOVA) with post hoc Duncan’s multiple range test using SPSS (version 23 for Windows, SPSS Inc., IL, USA). Statistical significance was determined at a value of P-value < 0.05. The results were expressed as the mean ± standard error of mean (S.E.M.).
Results
Effect of vitamin D3 supplementation on food intake, body weight, and liver weight in T2DM mice
Food intake was not significantly different among all groups. Body weight gain was similar among the diabetic groups. However, low dose of vitamin D3 supplementation significantly decreased liver weight (% BW) compared to the DMC group. On the other hand, the HC group did not show any significant difference in liver weight (Table 1).
Table 1.
Effect of vitamin D3 supplementation on food intake, body weight, and liver weight in type 2 diabetic mice.
| Groups |
||||
|---|---|---|---|---|
| NC | DMC | LC | HC | |
| Body weight (g) | ||||
| Before treatment | 28.31 ± 0.69a | 33.42 ± 0.91b | 33.31 ± 1.70b | 32.76 ± 1.05b |
| After treatment | 30.41 ± 0.86a | 41.29 ± 1.23b | 42.30 ± 2.90b | 41.29 ± 1.60b |
| Weight gain | 2.11 ± 0.33a | 7.88 ± 0.94b | 8.99 ± 2.01b | 8.53 ± 1.14b |
| Liver weight (% BW) | 3.23 ± 0.28a | 5.51 ± 0.30c | 4.52 ± 0.35b | 4.93 ± 0.28bc |
Note: Data were presented as mean ± S.E.M (n = 8). Values with the different superscript letter were significantly different (P < 0.05; ANOVA with post hoc Duncan’s multiple range test).
NC: normal control; DMC: diabetic mellitus control; LC: low cholecalciferol; HC: high cholecalciferol; BW: body weight.
Effect of vitamin D3 supplementation on plasma vitamin D3 concentration, blood glucose, hepatic function, and lipid profiles of plasma and liver in type 2 diabetic mice
There was no statistically difference in plasma 25-OH vitamin D3 concentration between the CON and the DMC groups. However, high dose of vitamin D3 supplementation in the diabetic mice significantly increased the plasma 25-OH vitamin D3 level compared to the DMC group (Table 2).
Table 2.
Effect of vitamin D3 supplementation on plasma vitamin D3 concentration, blood glucose, hepatic function, and lipid profiles in type 2 diabetic mice.
| Groups |
||||
|---|---|---|---|---|
| NC | DMC | LC | HC | |
| Plasma vitamin D3 (ng/mL) | 80.00 ± 5.61a | 80.42 ± 3.74a | 90.64 ± 10.28ab | 100.96 ± 4.16b |
| FBG (mg/dL) | ||||
| Week 0 | 114.00 ± 8.35a | 165.20 ± 6.65b | 169.40 ± 6.69b | 170.80 ± 7.25b |
| Week 6 | 123.00 ± 4.95a | 163.80 ± 5.38b | 155.40 ± 13.67b | 170.20 ± 9.23b |
| Week 12 | 142.40 ± 8.59a | 179.80 ± 11.96b | 184.80 ± 14.24b | 167.00 ± 7.29ab |
| OGTT AUC (mg/dL×120 min) | 21363.40 ± 1679.21a | 35429.00 ± 1469.67c | 31308.20 ± 3229.82bc | 27825.40 ± 1828.19ab |
| Hepatic function (IU/L) | ||||
| Plasma AST | 91.73 ± 15.26 a | 153.04 ± 16.93b | 92.43 ± 12.72a | 88.57 ± 25.08a |
| Plasma ALT | 44.73 ± 6.95a | 72.24 ± 4.05b | 37.85 ± 3.91a | 52.00 ± 9.51a |
| Plasma lipid (mg/dL) | ||||
| TG | 52.00 ± 2.79a | 121.77 ± 39.23b | 54.13 ± 5.93a | 54.71 ± 5.46a |
| TC | 115.82 ± 4.51a | 159.00 ± 6.93b | 149.12 ± 7.77b | 149.26 ± 8.83b |
| HDL-C | 43.30 ± 2.93a | 52.64 ± 7.92ab | 58.43 ± 5.27ab | 71.33 ± 9.98b |
| Hepatic lipid (mg/g liver) | ||||
| TG | 9.18 ± 2.17a | 61.02 ± 7.89c | 44.78 ± 6.51bc | 39.89 ± 5.38b |
| TC | 0.68 ± 0.07a | 4.11 ± 0.87b | 3.56 ± 1.00b | 3.91 ± 0.93b |
Note: Data were presented as mean ± S.E.M. (n = 8). Values with the different superscript letter were significantly different (P < 0.05; ANOVA with post hoc Duncan’s multiple range test).
NC: normal control; DMC: diabetic mellitus control; LC: low cholecalciferol; HC: fasting blood glucose; FBG: oral glucose tolerance test; OGTT: area-under-the-curve; AUC: high cholecalciferol; AST: aspartate transaminase; ALT: alanine transaminase; TG: triglyceride; TC: total cholesterol; HDL-C: high density lipoprotein-cholesterol.
The FBG level in the DMC group was significantly higher than the NC group. However, vitamin D3 administration showed no significant difference compared to that of the DMC group in FBG level during experiment (week 0 to 12).
OGTT was performed to determine glucose homeostasis. The OGTT AUC level in the DMC group was higher than that of the NC group. Simultaneously, the high dose of vitamin D3 treatment showed a significant decrease of AUC level compared with the DMC group.
Both plasma AST and ALT levels were significantly elevated in the DMC group compared with the NC group. However, vitamin D3 treatment in the diabetic mice significantly decreased the both of two levels to those of the NC group regardless of dose.
Plasma TG and TC levels were higher in the DMC group than the NC group, and vitamin D3 administration significantly reduced plasma TG level in the diabetic mice. While plasma TC level was not significantly altered after vitamin D3 administration, plasma HDL-C level was significantly elevated following treatment with high dose of vitamin D3 under type 2 diabetic condition.
Consistent with plasma lipid profiles, hepatic TG and TC levels were significantly higher in the DMC group than those of the NC group. However, the high dose of vitamin D3 supplementation reduced TG content in the liver compared to the DMC group. Meanwhile, hepatic TC level did not show the significant change after vitamin D3 treatment in diabetic mice (Table 2).
Effect of vitamin D3 supplementation on hepatic lipid metabolism in T2DM mice
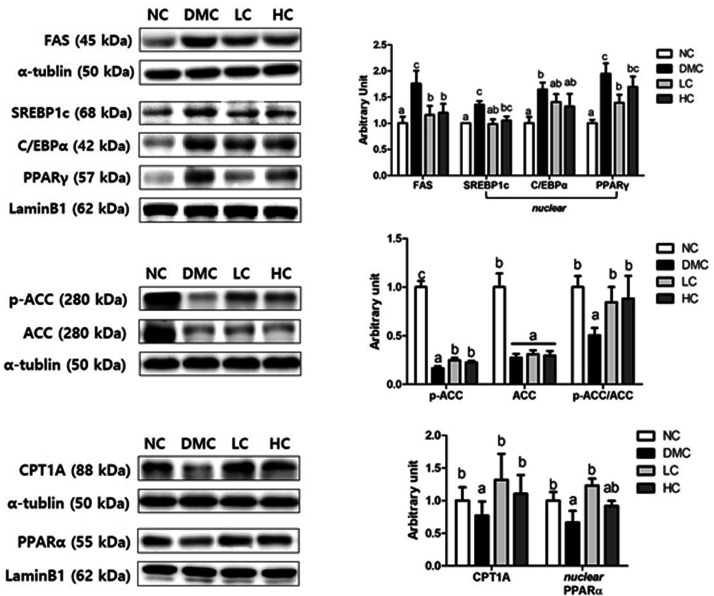
The protein levels of FAS, SREBP1c, C/EBPα, and PPARγ, which are involved in lipogenesis and lipid accumulation, were significantly upregulated in the DMC group than those in the NC group. However, the low dose of vitamin D3 treatment significantly reduced FAS, SREBP1c, and PPARγ levels in the diabetic mice. Similarly, the high dose of vitamin D3-treatment significantly reduced FAS level compared to the DMC group. In case of C/EBPα, no significant difference was observed between the DMC group and the vitamin D3 groups. Moreover, vitamin D3 supplementation regardless of dose reversed the decreased p-ACC/ACC ratio, indicating higher activity of ACC in vitamin D3 groups. Comparing with the NC group, the DMC group showed a significant decrease in the levels of CPT1A and PPARα, which involved in lipolysis. However, both levels of vitamin D3 supplementation in the diabetic mice significantly increased CPT1A level. PPARα level was increased only in the LC group (Figure 1).
Figure 1.
Effect of vitamin D3 supplementation on hepatic lipid metabolism in type 2 diabetic mice. The protein levels of fatty acid synthase (FAS), sterol response element-binding protein 1c (SREBP1c), CCAAT-enhancer-binding proteins (C/EBPα), peroxisome proliferator-activated receptor (PPAR)-γ, phosphorylation of acetyl-CoA carboxylase (ACC), carnitine palmitoyltransferase 1 A (CPT1A) and PPARα in hepatic tissue from NC, DMC, and vitamin D3-treated mice (LC, 300 ng/kg of vitamin D3; HC, 600 ng/kg of vitamin D3) were analyzed by Western blot. The band level of each marker was densitometrically quantified and normalized to that of α-tubulin (cytosol) and LaminB1 (nucleus). Data were presented as fold change relative to the NC group of mean ± S.E.M for each group (n = 6). Values with the different superscript letter were significantly different (P < 0.05; ANOVA with post hoc Duncan’s multiple range test).
Effect of vitamin D3 supplementation on hepatic autophagy and autophagy regulatory AMPK/akt-mTOR signaling in T2DM
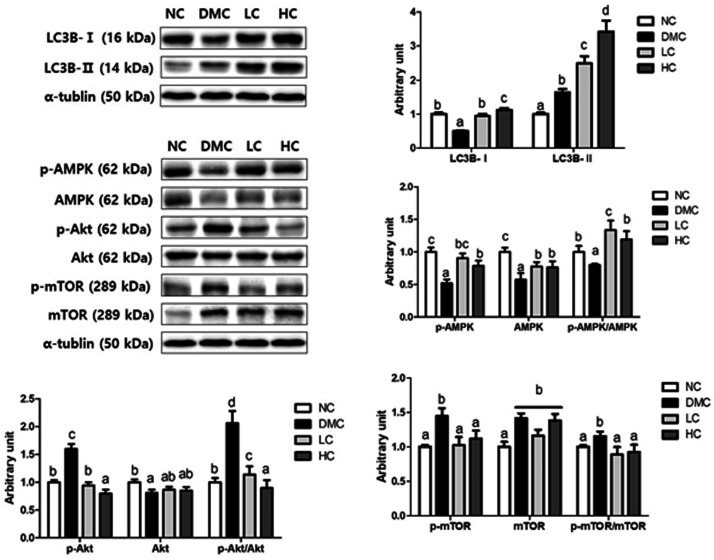
LC3B-I and LC3B-II levels were measured to determine whether the protection against hepatic lipid deposit by vitamin D3 treatment is dependent on autophagy in T2DM. As demonstrated in Figure 3, LC3B-I level was significantly lower in the DMC group than the NC group, and both low and high doses of vitamin D3 treatment in the diabetic mice significantly upregulated LC3B-I level. On the contrary, LC3B-II level was greater in the DMC group than the NC group. Importantly, vitamin D3 supplemented groups dramatically increased LC3B-II level compared to that of the DMC group, regardless of dose, effectively in the HC group.
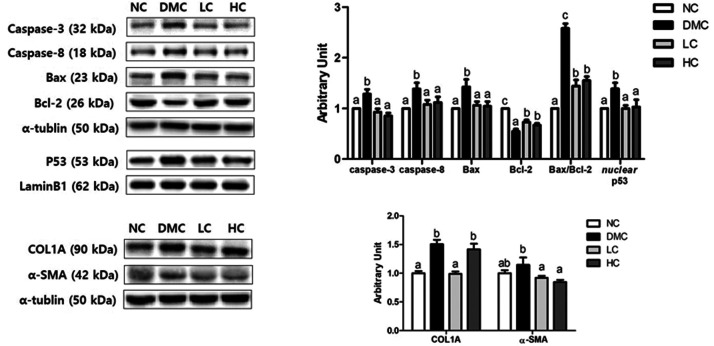
Figure 3.
Effect of vitamin D3 supplementation on hepatic apoptosis and fibrosis in type 2 diabetic mice. Representative images of Western blot for caspase-3, caspase-8, Bcl-2-associated X factor (Bax), Bcl-2, collagen 1 A (COL1A), and α-smooth muscle actin (α-SMA) in hepatic tissue from NC, DMC, and vitamin D3-treated mice (LC, 300 ng/kg of vitamin D3; HC, 600 ng/kg of vitamin D3). The band level of each marker was densitometrically quantified and normalized to that of α-tubulin (cytosol) and LaminB1 (nucleus). Data were presented as fold change relative to the NC group of mean ± S.E.M for each group (n = 6). Values with the different superscript letter were significantly different (P < 0.05; ANOVA with post hoc Duncan’s multiple range test).
One of the well-known autophagy regulatory proteins is mTOR, which interacts with AMPK and Akt during autophagy. To explore the potential role of vitamin D3 on autophagy regulatory signaling, we identified the protein levels of AMPK, Akt, mTOR and their phosphorylated forms. In the DMC group, the level of p-AMPK was lower and the levels of p-Akt and p-mTOR were higher than those of the NC group. Interestingly, vitamin D3 administration regardless of dose elevated phosphorylation of AMPK and reduced phosphorylation of Akt, contributing to a decrease in p-mTOR level compared to the levels of the DMC group. Therefore, vitamin D3 supplementation significantly increased p-AMPK/AMPK ratio, and decreased p-Akt/Akt ratio and p-mTOR/mTOR ratio, indicating that vitamin D3 would activate AMPK/Akt-mTOR signaling in the diabetic mice (Figure 2).
Figure 2.
Effect of vitamin D3 supplementation on hepatic autophagy and autophagy regulatory AMPK/Akt-mTOR signaling in type 2 diabetic mice. The protein levels of Western blot for microtubule-associated protein 1 light chain-3B (LC3B)-I and II, phosphorylation of AMP-activated protein kinase (AMPK), phosphorylation of Akt and phosphorylation of mammalian target of rapamycin (mTOR) in hepatic tissue from NC, DMC, and vitamin D3-treated mice (LC, 300 ng/kg of vitamin D3; HC, 600 ng/kg of vitamin D3) were analyzed by Western blot. The band level of each marker was densitometrically quantified and normalized to that of α-tublin. Data were presented as fold change relative to the NC group of mean ± S.E.M for each group (n = 6). Values with the different superscript letter were significantly different (P < 0.05; ANOVA with post hoc Duncan’s multiple range test).
Effect of vitamin D3 supplementation on hepatic apoptosis and fibrosis in T2DM mice
The caspase-3 and caspase-8 levels were increased in the DMC group, but those levels were significantly reduced following treatment with vitamin D3 in a dose-independent manner. Furthermore, the vitamin D3-treated groups significantly decreased Bax level and increased Bcl-2 level compared to the DMC group, thereby reduced Bax/Bcl-2 ratio. Simultaneously, p53 level was higher in the DMC group compared to that of the NC group, but was significantly lower in the vitamin D3 groups.
The positive effect of vitamin D3 on hepatic fibrosis in T2DM was demonstrated by the protein levels of COL1A and α-SMA. Significant increases in COL1A and α-SMA were found in the DMC group compared with the NC group. However, only COL1A level was downregulated in the LC group, whereas α-SMA was upregulated in both LC and HC groups (Figure 3).
Discussion
The current study demonstrated the ameliorative effect of vitamin D3 supplementation on NAFLD by induction of autophagy through stimulation of AMPK/Akt-mTOR signaling pathway under type 2 diabetic condition. In addition, vitamin D3 improved insulin resistance and hepatic damage associated with apoptosis and fibrosis in T2DM mice.
Although it is known that vitamin D3 could improve insulin signaling,25,26 previous studies reported that vitamin D3 administration showed no significant difference of FBG in the diabetic animals.16,27,28 The current study also supported that vitamin D3 treatment had no direct effect on FBG level. In a recent study, however, Benetti E et al.29 confirmed that vitamin D3 administration ameliorated glucose tolerance in non-diabetic obese mice. Our result reported that increased plasma vitamin D3 concentration was correlated with reduced OGTT AUC. The result suggests that high dose of vitamin D3 supplementation would be effective to enhance insulin sensitivity in T2DM.
Increases in blood AST and ALT levels indicate hepatic damage such as hepatocellular necrosis.30 Previous studies showed the positive effect of vitamin D3 administration on lowering AST and ALT levels in diabetic animals.31,32 Furthermore, treatment with 2100 IU vitamin D3 over 48 weeks was well tolerated and decreased serum ALT levels in non-alcoholic steatohepatitis patients.33 The similar results shown in this study suggest that vitamin D3 treatment could improve hyperglycemia-induced hepatic malfunction by attenuating liver damage.
Insulin resistance stimulates over-secretion of VLDL from the liver to blood, causing hypertriglyceridemia.33 In hypertriglyceridemia, reduced plasma HDL-C level but increased small dense LDL particles results in dyslipidemia, which accelerates hepatic lipid deposit. Previous studies reported that vitamin D3 normalized serum TG, TC, and HDL-C levels in HFD-induced NAFLD rats34 and lowered hepatic TG level in diabetic rats16 According to the result of recent systematic reviews and meta-analysis on cross-sectional and case-control studies, patients with NAFLD had significantly lower levels of 25(OH) D3 than controls.11,26 Scientists believe that lower levels of 25(OH) D3 in patients with NAFLD might contribute to the progression of NAFLD. The exact mechanism of vitamin D3 deficiency and NAFLD is not fully indicated. Compared with the placebo, reductions in triglyceride and an increase in HDL cholesterol were seen over the 12 weeks of intervention in the 25 µg of calcitriol group.35
In our research, vitamin D3 treatment significantly reduced plasma TG level regardless of dose. Furthermore, vitamin D3 supplementation decreased hepatic TG level and elevated plasma HDL-C level depending on the concentration of plasma vitamin D3. Our results provide the evidence that vitamin D3 could improve dyslipidemia and prevent NAFLD in T2DM by inhibiting excess deposit of lipid in the liver.
How could vitamin D3 supplementation improve hepatic lipid accumulation in T2DM mice? In a previous study,16 vitamin D3 enhanced β-oxidation by upregulating PPARα and CPT1 expressions in a diabetic rat model. Further, vitamin D repressed SREBP1c and FAS and enhanced PPARα and CPT1 in the liver of obese rodents.15 Our study showed that vitamin D3 treatment significantly lowered lipogenesis-related proteins such as FAS, SREBP1c, PPARγ and p-ACC/ACC ratio, but increased β-oxidation-associated proteins including PPARα and CPT1A in diabetes. The current data support that vitamin D3 supplementation would inhibit lipid accumulation in the liver by lowering lipogenesis and improving β-oxidation under diabetic condition.
Autophagy, a cellular system to control turnover of unwanted and long-lived proteins, is regarded as another mechanism to attenuate fatty liver disease. LC3B, which involves in autophagosome formation and maturation, is proteolytically cleaved and converted to cytosolic form of LC3B-I. During autophagy, LC3B-I is conjugated to the lipophilic phosphatidylethanolamine (PE) and converted to LC3-II.36 Li et al.37 showed that vitamin D3 elevated hepatic LC3B-II activity and attenuated liver steatosis in HFD-fed mice. In our study, the conversion of LC3B-I to LC3B-II in the NC group was remarkably lowered, implying that the activation of autophagy was not changed. It is thought that cellular stress was not enough to induce autophagy in the NC group. However, LC3B-II was significantly increased after vitamin D3 treatment compared to the DMC group, indicating that increased autophagy would lead to removal of lipids in the liver. In particular, the result was consistent with the decreases in hepatic lipid deposition and morphological changes. Therefore, vitamin D3 supplementation might have an ameliorative effect on hepatic lipid accumulation by inducing autophagy in T2DM.
Many researchers have focused on mTOR as the major regulatory protein of autophagy. A previous research demonstrated that vitamin D3 induces autophagy by downregulation of mTOR through AMPK activation in breast cancer cells and monocytes.38 In the current study, AMPK/Akt-mTOR signaling was significantly improved in the LC and HC groups, supporting that vitamin D3 would inactivate mTOR by normalizing the levels of AMPK and Akt in T2DM mice. Furthermore, the significant decrease in p-mTOR/mTOR ratio was accompanied by autophagy activation, demonstrated by the significant increase in LC3B-II in the vitamin D3 groups. Based on our data, vitamin D3-induced autophagy might be associated with the enhancement of AMPK/Akt-mTOR signaling pathway under T2DM condition.
Hyperglycemia-induced oxidative stress breaks balance of pro-apoptotic Bax and anti-apoptotic Bcl-2 to activate caspase-related apoptosis, which triggers hepatic morphological changes by excess cytoskeletal protein.39 Wang et al.40 showed that vitamin D3 suppressed apoptosis, whereas it induced autophagy as demonstrated by increase in Bcl-2 and LC3 levels in STZ-injected β-cells. Yang et al.41 reported that vitamin D3 treatment in high glucose-treated β-cells reduced p-Bcl-2/Bcl-2 ratio and caspase-3 by regulating mTOR activity. In our study, similarly, vitamin D3 treatment attenuated hepatic apoptosis verified by suppressed nuclear p53, caspase-3, caspase-8, and Bax/Bcl-2 ratio in the T2DM mice. Based on the previous studies,40,41 enhanced autophagy and repressed mTOR activity in our vitamin D3 groups might contribute to inhibition of pro-apoptotic proteins activity. Concerning fibrosis, Xie et al.42 reported that vitamin D3 treatment reduced the expression level of type III and type IV collagens in the diabetic kidney. Our results reported that vitamin D3 treatment decreased type I collagen (COL1A) and α-SMA levels except for COL1A in the HC group. Collectively, vitamin D3 could be effective to reduce hepatic apoptosis and fibrosis in diabetic mice, thus inhibit progression of steatosis in T2DM.
Our results demonstrated that vitamin D3 supplementation had ameliorative effects on hepatic lipid dysregulation in T2DM mice. Vitamin D3 supplementation suppressed de novo lipogenesis and enhanced β-oxidation, consequently preventing hepatic lipid accumulation in T2DM mice. In particular, vitamin D3 treatment reduced lipid deposit in the liver by inducing autophagy through enhancement of AMPK/Akt-mTOR signaling under T2DM. In our previous study, vitamin D3 supplementation reduced lipogenesis and increased mTOR-related autophagy in diabetic kidney. The findings from this study supported that vitamin D3 supplementation attenuated pro-fibrosis as well as apoptosis in diabetic hepatic tissue, which was not in renal tissue. Our findings suggest that vitamin D3 might have beneficial effects to prevent or ameliorate hyperglycemia-induced NAFLD under diabetic condition.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the design, interpretations of the study, data analysis, and review of the manuscript; YL conceived and designed the experiments, HL and HL performed the experiments and analyzed the data, HL, HL, and YL were involved in the writing and editing. All authors have read and agreed to the published version of the manuscripts.
DECLARATION OF CONFICTING INTERSETS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grant [2015R1D1A1A01061452] from the Ministry of Education, Science and Technology.
ORCID iDs: Heaji Lee https://orcid.org/0000-0003-0829-9249
Yunsook Lim https://orcid.org/0000-0002-3408-8595
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018; 138:271–81 [DOI] [PubMed] [Google Scholar]
- 2.Bril F, Cusi K. Nonalcoholic fatty liver disease: the new complication of type 2 diabetes mellitus. Endocrinol Metab Clin North Am 2016; 45:765–81 [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012; 56:943–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc 2016; 91:452–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci 2019; 19:994–1003 [DOI] [PubMed] [Google Scholar]
- 6.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 2008; 28:370–9 [DOI] [PubMed] [Google Scholar]
- 7.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature 2009; 458:1131–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong J, Gong W, Lu L, Chen J, Lu Z, Li H, Liu W, Liu Y, Wang M, Hu R, Long H, Wei L. Irbesartan ameliorates hyperlipidemia and liver steatosis in type 2 diabetic db/db mice via stimulating PPAR-gamma, AMPK/akt/mTOR signaling and autophagy. Int Immunopharmacol 2017; 42:176–84 [DOI] [PubMed] [Google Scholar]
- 9.Codogno P, Meijer AJ. Autophagy: a potential link between obesity and insulin resistance. Cell Metab 2010; 11:449–51 [DOI] [PubMed] [Google Scholar]
- 10.Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z, Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem 2009; 284:31484–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan M, Shi Y, Yang K. 4-Nonylphenol induces apoptosis, autophagy and necrosis in sertoli cells: involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology 2016; 341:28–40 [DOI] [PubMed] [Google Scholar]
- 12.Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 2014; 112:24–49 [DOI] [PubMed] [Google Scholar]
- 13.Yao F, Zhang M, Chen L. 5'-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharm Sin B 2016; 6:20–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin X, Xu Z, Zhang Z, Li L, Pan Q, Zheng F, Li H. Association of PI3K/AKT/mTOR pathway genetic variants with type 2 diabetes mellitus in Chinese. Diabetes Res Clin Pract 2017; 128:127–35 [DOI] [PubMed] [Google Scholar]
- 15.Garbossa SG, Folli F. Vitamin D, Sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev Endocr Metab Disord 2017; 18:243–58 [DOI] [PubMed] [Google Scholar]
- 16.Lips P, Eekhoff M, van Schoor N, Oosterwerff M, de Jongh R, Krul-Poel Y, Simsek S. Vitamin D and type 2 diabetes. J Steroid Biochem Mol Biol 2017; 173:280–5 [DOI] [PubMed] [Google Scholar]
- 17.Yin Y, Yu Z, Xia M, Luo X, Lu X, Ling W. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest 2012; 42:1189–96 [DOI] [PubMed] [Google Scholar]
- 18.Ning C, Liu L, Lv G, Yang Y, Zhang Y, Yu R, Wang Y, Zhu J. Lipid metabolism and inflammation modulated by vitamin D in liver of diabetic rats. Lipids Health Dis 2015; 14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barchetta I, Angelico FD, Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med 2011; 9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Targher G, Scorletti E, Mantovani A, Byrne CD. Nonalcoholic fatty liver disease and reduced serum vitamin D(3) levels. Metab Syndr Relat Disord 2013; 11:217–28 [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2013; 5:1422–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grober U, Holick MF. Diabetes prevention: vitamin D supplementation may not provide any protection if there is no evidence of deficiency. Nutrients 2019; 11:2651 [CrossRef[ 10.3390/nu11112651] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res 2008; 2008:704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lie-Injo L. Simple method for the isolation and purification of hemoglobin components. J Chromatogr 1976; 117:53–8 [DOI] [PubMed] [Google Scholar]
- 25.Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct 2002; 20:227–32 [DOI] [PubMed] [Google Scholar]
- 26.Nelson JE, Roth C, Wilson LA, Yates KP, Aouizera B, Morgan-Stevenson V, Whalen E, Hoofnagle A, Mason M, Gersuk V, Yeh MM, Kowdley KV. Vitamin D deficiency is associated with increased risk of non-alcoholic steatohepatitis in adults with non-alcoholic fatty liver disease: possible role for MAPK and NF-kappaB? Am J Gastroenterol 2016; 111:852–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Y, Das SK, Li M. Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing NF-kappaB-mediated inflammatory genes. Biosci Rep 2018; 38:BSR2017129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Lee TI, Kao YH, Chen YC, Tsai WC, Chung CC, Chen YJ. Cardiac metabolism, inflammation, and peroxisome proliferator-activated receptors modulated by 1,25-dihydroxyvitamin D3 in diabetic rats. Int J Cardiol 2014; 176:151–7 [DOI] [PubMed] [Google Scholar]
- 29.Benetti E, Mastrocola R, Chiazza F, Nigro D, D'Antona G, Bordano V, Fantozzi R, Aragno M, Collino M, Minetto MA. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS One 2018; 13:e0189707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr 2007; 74:663–71 [DOI] [PubMed] [Google Scholar]
- 31.Elattar S, Estaphan S, Mohamed EA, Elzainy A, Naguib M. The protective effect of 1alpha, 25-dihydroxyvitamin d3 and metformin on liver in type 2 diabetic rats. J Steroid Biochem Mol Biol 2017; 173:235–44 [DOI] [PubMed] [Google Scholar]
- 32.Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, Ayadi F, Elfeki A. 1Alpha,25 dihydroxyvitamin D3: therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J Nutr Sci Vitaminol 2009; 55:215–22 [DOI] [PubMed] [Google Scholar]
- 33.Subramanian S, Chait A. Hypertriglyceridemia secondary to obesity and diabetes. Biochim Biophys Acta 2012; 1821:819–25 [DOI] [PubMed] [Google Scholar]
- 34.El-Sherbiny M, Eldosoky M, El-Shafey M, Othman G, Elkattawy HA, Bedir T, Elsherbiny NM. Vitamin D nanoemulsion enhances hepatoprotective effect of conventional vitamin D in rats fed with a high-fat diet. Chem Biol Interact 2018; 288:65–75 [DOI] [PubMed] [Google Scholar]
- 35.Lorvand Amiri H, Agah S, Mousavi SN, Hosseini AF., Shidfar F. regression of non-alcoholic fatty liver by vitamin D supplement: a double-blind randomized controlled clinical trial. Arch Iran Med 2016; 19:631–8 [PubMed] [Google Scholar]
- 36.Barth S, Glick D, Macleod KF. Macleod Autophagy: assays and artifacts. J Pathol 2010; 221:117–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Guo E, Yang J, Li A, Yang Y, Liu S, Liu A, Jiang X. 1,25(OH)2 D3 attenuates hepatic steatosis by inducing autophagy in mice. Obesity 2017; 25:561–71 [DOI] [PubMed] [Google Scholar]
- 38.Hoyer-Hansen M, Nordbrandt SP, Jaattela M. Autophagy as a basis for the health-promoting effects of vitamin D. Trends Mol Med 2010; 16:295–302 [DOI] [PubMed] [Google Scholar]
- 39.Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta 2006; 364:33–60 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, He D, Ni C, Zhou H, Wu S, Xue Z, Zhou Z. Vitamin D induces autophagy of pancreatic beta-cells and enhances insulin secretion. Mol Med Rep 2016; 14:2644–50 [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Liu F, Qu H, Wang H, Xiao X, Deng H. 1, 25(OH)2D3 protects beta cell against high glucose-induced apoptosis through mTOR suppressing. Mol Cell Endocrinol 2015; 414:111–9 [DOI] [PubMed] [Google Scholar]
- 42.Xie X, Li Z, Pi M, Wu J, Zeng W, Zuo L, Zha Y. Down-regulation of p38 MAPK and collagen by 1, 25-(OH)2-VD3 in rat models of diabetic nephropathy. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016; 32:931–5 [PubMed] [Google Scholar]