Abstract
Background
Reference standard indices of iron deficiency and iron overload are generally invasive, expensive, and can be unpleasant or occasionally risky. Ferritin is an iron storage protein and its concentration in the plasma or serum reflects iron stores; low ferritin indicates iron deficiency, while elevated ferritin reflects risk of iron overload. However, ferritin is also an acute‐phase protein and its levels are elevated in inflammation and infection. The use of ferritin as a diagnostic test of iron deficiency and overload is a common clinical practice.
Objectives
To determine the diagnostic accuracy of ferritin concentrations (serum or plasma) for detecting iron deficiency and risk of iron overload in primary and secondary iron‐loading syndromes.
Search methods
We searched the following databases (10 June 2020): DARE (Cochrane Library) Issue 2 of 4 2015, HTA (Cochrane Library) Issue 4 of 4 2016, CENTRAL (Cochrane Library) Issue 6 of 12 2020, MEDLINE (OVID) 1946 to 9 June 2020, Embase (OVID) 1947 to week 23 2020, CINAHL (Ebsco) 1982 to June 2020, Web of Science (ISI) SCI, SSCI, CPCI‐exp & CPCI‐SSH to June 2020, POPLINE 16/8/18, Open Grey (10/6/20), TRoPHI (10/6/20), Bibliomap (10/6/20), IBECS (10/6/20), SCIELO (10/6/20), Global Index Medicus (10/6/20) AIM, IMSEAR, WPRIM, IMEMR, LILACS (10/6/20), PAHO (10/6/20), WHOLIS 10/6/20, IndMED (16/8/18) and Native Health Research Database (10/6/20). We also searched two trials registers and contacted relevant organisations for unpublished studies.
Selection criteria
We included all study designs seeking to evaluate serum or plasma ferritin concentrations measured by any current or previously available quantitative assay as an index of iron status in individuals of any age, sex, clinical and physiological status from any country.
Data collection and analysis
We followed standard Cochrane methods. We designed the data extraction form to record results for ferritin concentration as the index test, and bone marrow iron content for iron deficiency and liver iron content for iron overload as the reference standards. Two other authors further extracted and validated the number of true positive, true negative, false positive, false negative cases, and extracted or derived the sensitivity, specificity, positive and negative predictive values for each threshold presented for iron deficiency and iron overload in included studies.
We assessed risk of bias and applicability using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)‐2 tool. We used GRADE assessment to enable the quality of evidence and hence strength of evidence for our conclusions.
Main results
Our search was conducted initially in 2014 and updated in 2017, 2018 and 2020 (10 June). We identified 21,217 records and screened 14,244 records after duplicates were removed. We assessed 316 records in full text. We excluded 190 studies (193 records) with reasons and included 108 studies (111 records) in the qualitative and quantitative analysis. There were 11 studies (12 records) that we screened from the last search update and appeared eligible for a future analysis. We decided to enter these as awaiting classification.
We stratified the analysis first by participant clinical status: apparently healthy and non‐healthy populations. We then stratified by age and pregnancy status as: infants and children, adolescents, pregnant women, and adults.
Iron deficiency
We included 72 studies (75 records) involving 6059 participants.
Apparently healthy populations
Five studies screened for iron deficiency in people without apparent illness. In the general adult population, three studies reported sensitivities of 63% to 100% at the optimum cutoff for ferritin, with corresponding specificities of 92% to 98%, but the ferritin cutoffs varied between studies. One study in healthy children reported a sensitivity of 74% and a specificity of 77%. One study in pregnant women reported a sensitivity of 88% and a specificity of 100%. Overall confidence in these estimates was very low because of potential bias, indirectness, and sparse and heterogenous evidence. No studies screened for iron overload in apparently healthy people.
People presenting for medical care
There were 63 studies among adults presenting for medical care (5042 participants). For a sample of 1000 subjects with a 35% prevalence of iron deficiency (of the included studies in this category) and supposing a 85% specificity, there would be 315 iron‐deficient subjects correctly classified as having iron deficiency and 35 iron‐deficient subjects incorrectly classified as not having iron deficiency, leading to a 90% sensitivity. Thresholds proposed by the authors of the included studies ranged between 12 to 200 µg/L. The estimated diagnostic odds ratio was 50.
Among non‐healthy adults using a fixed threshold of 30 μg/L (nine studies, 512 participants, low‐certainty evidence), the pooled estimate for sensitivity was 79% with a 95% confidence interval of (58%, 91%) and specificity of 98%, with a 95% confidence interval of (91%, 100%). The estimated diagnostic odds ratio was 140, a relatively highly informative test.
Iron overload
We included 36 studies (36 records) involving 1927 participants. All studies concerned non‐healthy populations. There were no studies targeting either infants, children, or pregnant women.
Among all populations (one threshold for males and females; 36 studies, 1927 participants, very low‐certainty evidence): for a sample of 1000 subjects with a 42% prevalence of iron overload (of the included studies in this category) and supposing a 65% specificity, there would be 332 iron‐overloaded subjects correctly classified as having iron overload and 85 iron‐overloaded subjects incorrectly classified as not having iron overload, leading to a 80% sensitivity. The estimated diagnostic odds ratio was 8.
Authors' conclusions
At a threshold of 30 micrograms/L, there is low‐certainty evidence that blood ferritin concentration is reasonably sensitive and a very specific test for iron deficiency in people presenting for medical care. There is very low certainty that high concentrations of ferritin provide a sensitive test for iron overload in people where this condition is suspected. There is insufficient evidence to know whether ferritin concentration performs similarly when screening asymptomatic people for iron deficiency or overload.
Plain language summary
How accurate are tests to measure the level of ferritin (a protein that stores iron) in the blood at diagnosing iron deficiency and overload?
Key messages
‐ Tests that measure the level of ferritin in the blood may be reasonably accurate for diagnosing iron deficiency (low iron levels) in people:
‐ seeking medical care; and
‐ whose doctors suspect iron deficiency.
‐ The accuracy of ferritin blood tests for diagnosing iron overload (high iron levels) is unclear, due to a lack of robust evidence.
‐ To strengthen the evidence, we need future studies to:
‐ investigate a wider range of populations; and
‐ identify the levels of ferritin in the blood that are the best indicators of iron deficiency and overload.
Why is it important to diagnose iron deficiency and overload?
Iron is a mineral found in every cell of the body. It comes from iron‐rich foods like red meat, beans and fortified cereal (to which iron has been added artificially), among others, or from supplements (iron tablets, micronutrient powders, drops or syrups). The body needs iron to make:
‐ red blood cells; and
‐ haemoglobin, a protein in blood that carries oxygen from the lungs to the rest of the body.
An iron test can show if someone has too little iron (iron deficiency) or too much iron (iron overload). It is important to test iron levels because:
‐ iron deficiency can cause anaemia (low levels of red blood cells or haemoglobin), tiredness and weakness. It can be a sign that someone has a serious health problem, such as internal bleeding; while
‐ iron overload can damage the liver, heart and other organs permanently.
What tests can be used to diagnose iron deficiency and overload?
There are several different tests available to check the level of iron in the body. The most accurate tests involve using a needle to collect a small sample of:
‐ bone marrow fluid (to diagnose iron deficiency); or
‐ tissue from the liver (to diagnose iron overload).
However, these tests are expensive and can be risky for people in poor health.
A simpler test involves measuring the level of ferritin (a protein that stores iron) in the blood, to estimate the amount of iron in the body.
What did we want to find out?
We wanted to find out if ferritin blood tests accurately diagnose iron deficiency and iron overload.
What did we do?
We searched for studies that compared ferritin blood tests against:
‐ tests of iron levels in the bone marrow, to diagnose iron deficiency; and
‐ tests of iron levels in the liver, to diagnose iron overload.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors like study methods and sizes.
What did we find?
We included 72 studies of 6059 people investigating the ability of ferritin blood tests to diagnose:
‐ iron deficiency in people who sought medical care and whose doctor suspected iron deficiency (70 studies, 5709 people);
‐ iron deficiency in people without any sign of disease (five studies, 350 people); and
‐ iron overload suspected by a doctor (36 studies, 1927 people).
Evidence suggests that ferritin blood tests may be reasonably accurate for diagnosing iron deficiency in people seeking medical care. For example, in studies where people with fewer than 30 micrograms of ferritin in one litre of blood were diagnosed with iron deficiency, ferritin blood tests correctly identified:
‐ iron deficiency in four out of five people who did have iron deficiency; and
‐ no iron deficiency in 19 out of 20 people who had normal levels of iron.
The evidence was not robust enough to determine if ferritin blood tests accurately diagnose:
‐ iron deficiency in people without any sign of disease; or
‐ iron overload suspected by a doctor.
What are the limitations of the evidence?
The studies were:
‐ small;
‐ conducted in ways that may have introduced errors into their results; and
‐ focused on specific populations (like children, young people and pregnant women).
For these reasons, we have limited confidence in the evidence.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to June 2020.
Summary of findings
Background
In 2020, anaemia is estimated to affect 29.6% of non‐pregnant women, 36.5% of pregnant women, and 60.2% of children 6‐59 months of age worldwide, equivalent to 269 million children with anaemia (WHO 2021). Iron deficiency is one of the major causes of anaemia (Chaparro 2019) and can also exist in the absence of anaemia (WHO 2011a). Specific assessment of iron status is therefore critical to defining the burden of iron deficiency in a population, and identifying the proportion of anaemia that is likely to be responsive to interventions that aim to improve iron status (Garcia‐Casal 2019). Conversely, iron overload due to genetic aberrations in genes regulating iron absorption can result in serious clinical consequences including cardiomyopathy, endocrinopathies and hepatic cirrhosis and cancer (Brissot 2017). Clinical detection of iron overload is thus of crucial importance, whilst ensuring public health iron intervention programmes are not contributing to the burden of iron loading is an emerging concern. Serum and plasma ferritin is a commonly utilised assay for evaluation of iron stores in individuals across the life course. Ferritin is an iron storage protein and measurement in the plasma or serum reflects iron stores in healthy individuals; low ferritin indicates iron deficiency while elevated ferritin reflects iron overload. However, ferritin is also an acute‐phase protein and its levels are elevated in inflammation and infection. Ferritin is a continuous index and, as such, thresholds below which to define iron deficiency and above which to define iron overload are required.
Reference standard measures of iron deficiency (i.e. bone marrow aspirate, which requires intramedullary sampling of the hematopoietic cells) and iron overload (liver biopsy) are generally invasive, and are inappropriate for widespread community screening in epidemiologic studies and clinical use in unselected patients (Pasricha 2010). Thus, if measurement of serum ferritin in the peripheral blood can indicate iron deficiency or iron overload, it can save the individual considerable cost and distress. At the population level, having a biomarker that can be used as an indicator of iron deficiency or iron overload can be useful for understanding the prevalence, magnitude and distribution of the problem. Nonetheless, there is considerable uncertainty and variability concerning the optimal thresholds of ferritin to use, thus making it of importance to determine relevant thresholds for clinical practice and epidemiological assessments.
A previous systematic review included 55 studies that compared laboratory tests with histologic examination of the bone marrow and concluded that serum ferritin was a useful test for the diagnosis of iron‐deficiency anaemia with an area under the receiver operating characteristic curve (AUCROC), a plot of true positives versus false positives, of 0.95 (Guyatt 1992), that is, high accuracy because the agreement with the reference standard is almost perfect. The authors found that a ferritin < 15 ng/mL had a positive likelihood ratio for iron deficiency of 51.9. However, this review is over 20 years old and newer data are likely to be available; furthermore, the authors did not provide specific analysis of the utility of ferritin by itself in different populations or contexts. The authors included only anaemic adults (i.e. excluded studies in non‐anaemic individuals and children). The authors also did not assess the diagnostic value of a particular threshold for ferritin, or include in their analysis evaluation of the currently used thresholds (i.e. lower than 12 µg/L or lower than 15 µg/L). Methodologies for measurement of ferritin have evolved over the past two decades. The above‐mentioned review did not address the diagnostic value of ferritin in other important conditions, namely iron deficiency in the absence of anaemia, and iron overload, thus thresholds to identify these conditions have not been evaluated. Furthermore, it did not report ferritin thresholds for use in clinically relevant subgroups such as in children and in women during pregnancy.
Development of modern guidelines and recommendations for use in public health policy, clinical laboratories and, hence, routine practice requires a complete, up‐to‐date appraisal of the literature, including recent studies. It is important to ensure that evidence covers the full range of potential conditions. It is thus essential to now undertake a new systematic review to identify the diagnostic test accuracy of serum and/or plasma ferritin as a diagnostic indicator in both iron deficiency and iron overload.
Target condition being diagnosed
Iron is an essential element with important physiologic functions in processes including oxygen transport (as a key component of haemoglobin), mitochondrial function, enzymatic activity (especially cytochromes), and muscle metabolism, particularly myoglobin (Ordway 2004; Netz 2013).
Iron deficiency
Iron deficiency exists when body iron stores are inadequate to meet the needs for metabolism. Progressive iron deficiency can result in iron‐deficient erythropoiesis and eventually, iron deficiency anaemia (BloodSafe eLearning Australia 2011). However, even in the absence of anaemia, iron deficiency appears to be associated with clinical impairments including fatigue (Verdon 2003), impaired physical performance (Pasricha 2014), decreased work productivity (Li 1994), and sub optimal brain development (Lozoff 2007).
Iron deficiency involves inadequate iron intake, excess iron (i.e. blood) loss, and increased iron utilisation, and may occur due to physiologic, environmental, pathologic, drug‐related, genetic or iron‐restricted erythropoietic causes (Camaschella 2015). Inadequate iron intake may result from a diet with a low iron content, and/or which contains iron in a biologically inaccessible form or a diet containing high concentrations of iron absorption inhibitors. Iron may also fail to be absorbed in individuals with intestinal disorders such as celiac disease, and perhaps Helicobacter pylori infection (Papagiannakis 2013). Inflammation can also impair iron absorption, which may mediate iron deficiency in athletes (Peeling 2008). The most common causes of blood loss is menstruation, which is the chief reason iron deficiency is more common in females; in low‐income settings other important causes include chronic blood loss from hookworm and schistosomiasis. In high‐income settings, blood losses from blood donation (Spencer 2013), and bleeding from intestinal lesions (Bull‐Henry 2013) must be considered. Iron requirements are increased during rapid growth (especially in infants and preschool children) and during adolescence when growth accelerates again and coincides with the onset of menarche in females (Pasricha 2010; Pasricha 2013), while iron requirements during pregnancy are increased due to iron needs for maternal and foetal erythropoiesis and foetal growth (Pasricha 2010; Pasricha 2013).
When considering these factors, it is unsurprising that iron deficiency and iron deficiency anaemia are most common in preschool children and women of reproductive age, and that overall, iron deficiency is most common in low‐income settings where dietary iron content and availability is low, and where parasitic infections are highly prevalent (Pasricha 2013). As such, it is estimated that approximately about a third of the world’s population is anaemic, with iron deficiency the leading cause, and that anaemia accounts for almost 9% of the world's years lived with a disability burden (Kassebaum 2014). Since 2000, the global prevalence of anaemia in children under five has slowly decreased over the years, from 48.0% (95% UI 45.1%, 51.0%) to 39.8% (95% UI 36%, 43.8%), and from 2010, it has been stagnant. The global prevalence of anaemia in women of reproductive age has been stagnant, while the prevalence of anaemia in pregnant women has decreased slightly (WHO 2021).
Iron overload
Because elemental iron is toxic to the body (due to its propensity to initiate redox reactions and generate free radicals, causing tissue damage), it must be chaperoned and stored in the body by binding proteins (i.e. transferrin, ferritin). There is no physiologic mechanism to excrete iron, hence homeostatic regulation of iron stores is entirely mediated by changes in iron absorption (chiefly via modulation of the hepatic hormone, hepcidin) (Ganz 2013). Iron overload results from excess iron absorption caused by generally autosomal recessive genetic conditions (hereditary haemochromatosis, caused chiefly by mutations in the High FE2+ (HFE) gene, but also less commonly by mutations in genes that encode haemojuvelin (HJV), transferrin receptor 2 (TFR2) and ferroportin (SLC40A1)), conditions associated with ineffective erythropoiesis (for example, thalassaemia intermedia and haemoglobin E‐beta thalassaemia), and iron accumulation from repeated red cell transfusions, usually to treat inherited (e.g. thalassaemia and other congenital conditions) or acquired (e.g. aplastic anaemia, myelodysplasia and other causes of bone marrow failure) anaemia (Brissot 2009; Piga 2009). We can distinguish two types of iron overload syndromes: inherited conditions ‐ primary iron overload, and acquired conditions ‐ and secondary iron overload.
Over time, iron overload results in excess iron accumulation in organs, especially the liver (resulting in cirrhosis, liver failure and hepatocellular carcinoma), endocrine organs (causing pituitary and gonadal failure), pancreas (causing diabetes), skin (causing pigmentation), and heart (resulting in cardiomyopathy, heart failure and arrhythmia) (Allen 2008). Untreated, patients with severe iron overload succumb to cardiac or hepatic complications. Thus, early diagnosis and noninvasive tests for monitoring of treatment, are essential to optimal management of iron loading (Bacon 2011).
The global burden of iron overload is uncertain and varies by population (ethnicity, age and sex) screened, as well as the methodology used to make the estimate (screening with ferritin, transferrin saturation or genetic testing). The prevalence of hereditary haemochromatosis in Caucasian populations has been estimated to be perhaps 3.5 to 4.5 per 1000 population, greater in males (Baer 1995; Phatak 1998). Worldwide, approximately 21,000 children are born with haemoglobin E‐beta thalassaemia (about half of whom are transfusion dependent) and approximately 23,000 are born with thalassaemia major annually; a further 14,000 are born with Haemoglobin H disease (HbH), a form of alpha thalassaemia. Thus, genetic conditions associated with risk of iron overload are prevalent conditions worldwide (Weatherall 2011).
Index test(s)
Ferritin can be measured in the serum or plasma, as well as in the erythrocyte. Ferritin is an iron storage protein, chiefly found in the liver, and comprises two isoforms of poli peptidic chains (L‐light, and H‐heavy). Assays for ferritin generally do not distinguish between these forms. Synthesis of ferritin is regulated in part by iron‐responsive proteins, which bind to iron responsive elements in the ferritin messenger ribonucleic acid (mRNA). Increased iron levels suppress binding of iron binding proteins to the iron responsive element, facilitating translation and thus increasing the quantity of ferritin synthesised; conversely, iron deficiency suppresses ferritin synthesis. However, ferritin is also an acute‐phase protein and is increased in acute and chronic inflammation due to infection, inflammatory illnesses and malignancy. As such, coexistent iron deficiency and inflammation can result in relative increases in ferritin when compared to similar body iron stores without inflammation. Elevated ferritin can reflect either iron overload or inflammation (or coexistence of both). Marked elevations in ferritin may also reflect hepatic damage with release of intracellular ferritin. This circulating form does not carry iron (apoferritin) and is predominantly glycosylated, extending its circulating half life compared with non‐glycosylated forms (Ferraro 2012). The small fraction of ferritin in the serum contributes little to overall iron storage, but is in equilibrium with the body’s depot iron and hence acts as an indicator for the level of the iron stores.
Measurement of ferritin can be performed in commercial laboratories using a range of automated methods including immuno‐turbidimetry and latex agglutination. Assays require separation of serum or plasma from the cellular component of whole blood. The numerous platforms and the widespread availability of ferritin assays, raise the potential for discrepancies between results in different laboratories. This problem is addressed through calibration of instruments using standards provided by the manufacturer which have themselves been calibrated against international reference standards; furthermore, accredited laboratories are generally required to participate in external quality assurance activities (which ensure that results for samples fall within a similar range to those reported by other laboratories), and also undergo regular inspections. A recent systematic review concluded that the laboratory methods most used to determine ferritin concentrations have comparable accuracy and performance (Garcia‐Casal 2018).
The World Health Organization (WHO) currently defines iron deficiency in adults as ferritin lower than 15 μg/L, and in children under five years of age when ferritin is lower than 12 µg/L, unless inflammation is coexistent, in which case iron deficiency may exist when ferritin is lower than 30 μg/L (WHO 2011b). Iron overload is considered to exist when ferritin exceeds 200 μg/L in males and 150 μg/L in females (WHO 2001). These thresholds were not derived from up‐to‐date systematic reviews and meta‐analyses, and various authorities and individual laboratories recommend different thresholds for defining pathology without clearly explaining their rationale; for example, the Royal College of Pathologists of Australasia has recommended ferritin thresholds below 30 μg/L to identify iron deficiency and a ferritin level exceeding 200 μg/L and 300 μg/L in females and males, respectively, as indicating iron overload (RCPA 2010).
Clinical pathway
Evaluation of iron status may be performed clinically, for individual patients, or across a population. Measurement of iron status in individuals is important to correctly define iron status and provide appropriate treatment for iron deficiency, to prompt further testing and management if iron overload is suspected, and to monitor interventions for both iron deficiency and iron overload. Measurement of iron status in populations is important to determine the prevalence and distribution of iron deficiency and overload, and thus to decide appropriate interventions, and to monitor and evaluate the impact and safety of implemented public health programmes.
A clinical pathway for investigation of iron deficiency is proposed in Figure 1.
1.

Clinical pathway for iron deficiency
The clinical pathway for iron overload with ferritin is depicted in Figure 2. In both cases, false negative cases may see patients undiagnosed, resulting in an ongoing undetected risk of disease, whilst false positive results may result in unnecessary, expensive further testing and treatment.
2.

Clinical pathway for iron overload
In a population, measurement of iron indices may be undertaken together with measurement of haemoglobin concentrations. Often, field testing of haemoglobin is measured in isolation from red cell indices using instruments for point‐of‐care testing with a small sample of capillary blood. In population‐based testing, strategies for timely transport and separation of samples must be considered, as point‐of‐care tests for ferritin are not presently appropriate for these uses.
Ferritin determinations are widely used for monitoring renal anaemia when iron utilisation and distribution disorders are present during therapy with erythropoietin.
Prior tests
Individuals may undergo measurement of ferritin with no prior tests, with concurrent testing of other iron and haematologic parameters (i.e. haemoglobin, red cell indices), or following measurement of haematologic indices that have prompted further investigation into the iron status of the individual.
In a population, ferritin testing to ascertain the prevalence of (and risk factors for) iron deficiency is usually performed together with measurement of haemoglobin to assess anaemia prevalence. Where possible, concomitant measurement of an inflammatory marker (for example, C‐reactive protein) is recommended (WHO 2001). Additional iron indices such as soluble transferrin receptor may also be performed (WHO/CDC 2007).
Role of index test(s)
Ferritin measurements are presently undertaken as the key test to define iron status in an individual or to establish the prevalence of iron deficiency (or overload) in a population, as serum or plasma ferritin concentrations are thought to reflect body iron stores (WHO 2011b). It is, therefore, important to determine if serum or plasma ferritin concentrations accurately reflect iron statuses of relevance to population health (deficiency and overload) and to identify the thresholds that define iron deficiency and overload in clinical care.
Alternative test(s)
Other indices of iron status can be measured in addition to ferritin for iron status, although they evaluate different components of iron metabolism (Pasricha 2010). Ideally, the final diagnosis of iron status needs to consider the whole clinical picture incorporating all laboratory results, rather than relying on single test findings (Camaschella 2015).
Iron indices
Serum iron: reduced in iron deficiency and inflammation
Transferrin: increased in iron deficiency, reduced in inflammation
Transferrin saturation: increased in iron overload, reduced in iron deficiency and inflammation. In cases where elevated ferritin is due to inflammation or liver disease (for example, hepatitis or fatty liver disease), transferrin saturation will not be raised
Total iron binding capacity: reduced in iron overload, increased in iron deficiency and normal to increased in inflammation
Soluble transferrin receptor: increased in iron deficiency (reflecting tissue iron), increased in raised erythropoiesis (i.e. physiologic states such as pregnancy, childhood; disease states including thalassaemia and haemolysis)
Haematologic indices
Haemoglobin – falls in intermediate to late stages of iron deficiency, but may only cross the threshold for anaemia in late stages of iron deficiency. Numerous alternative causes for anaemia
Red cell indices (particularly: mean cell volume – reduced in iron deficiency, mean cell haemoglobin – reduced in iron deficiency, red cell distribution width – increased in iron deficiency)
Reticulocyte haemoglobin – reduced in iron deficiency
Other indices
Zinc protoporphyrin – increased in iron deficiency, haemoglobinopathy and anaemia of inflammation
Hepcidin: reduced in iron deficiency and erythropoiesis, increased in iron repletion and inflammation, pathologically reduced (or in the normal range despite iron overload) in hereditary haemochromatosis
Inflammatory indices
Because ferritin (as well as other iron indices, especially serum iron) is influenced by inflammation, concurrent measurement of an inflammatory marker (for example, C‐reactive protein, alpha‐1 glycoprotein) may be necessary to identify inflammation and enable interpretation of ferritin in clinical settings where individuals may have concurrent inflammatory conditions, and in population studies in low‐income settings where infection burden is high.
Liver function tests
Ferritin concentrations are elevated in both acute and chronic liver disease due to hepatic cell necrosis (the liver is the primary storage organ for ferritin). Hepatocellular damage can be reflected by measurement of alanine transaminase and aspartate transaminase levels.
Rationale
Current WHO threshold values for ferritin concentrations to indicate depleted iron stores are provided for individuals younger than five years of age and five years of age or older, for males and females, and for individuals under five years of age with concurrent infection. These are based on consultations in 1987 and 2004, neither of which included a systematic review and meta‐analysis. A 1987 consultation by the International Nutritional Anaemia Consultative Group concluded that, at all ages, a serum ferritin value below 10‐12 μg/L was indicative of depletion of iron stores (WHO 1989). The thresholds for adults were derived largely from the clinical literature, specifically from studies examining the highest ferritin concentration among patients with microcytic iron deficiency anaemia who also either show a therapeutic response to iron or who have no stainable iron in the bone marrow (WHO 2011b). These thresholds were therefore adopted by WHO and revised in 1993.
In 2004, WHO and the United States Centers for Disease Control and Prevention (CDC) held a technical consultation on the assessment of iron status at the population level, and selected five out of 16 indicators: haemoglobin, zinc protoporphyrin, mean cell volume, transferrin receptor, serum ferritin along with C‐reactive protein (due to effects on ferritin concentration of inflammation) (WHO/CDC 2007). In preparation for that consultation, a non‐systematic review sought to identify the most efficient indicators to evaluate the impact of interventions to control iron deficiency and detect a true change in iron status of a population using the fewest and simplest tests. Serum ferritin and haemoglobin were determined to be the most efficient indicators of population response to iron interventions (Mei 2005). This review included data from nine randomised controlled trials in seven countries to evaluate the performance of haemoglobin, ferritin, transferrin receptor, zinc protoporphyrin, mean cell volume, transferrin saturation and total body‐iron stores in measuring a change due to an iron intervention. They expressed the change in each biomarker as response to the iron intervention in standard deviation units for the intervention group compared with the control group, concluding that haemoglobin and ferritin showed the biggest changes.
However, WHO policy now requires more rigorous processes to ensure that recommendations are informed by the best available evidence, including formal evaluation of its quality. Reviews of evidence must include the most recent available literature.
Following the EURopean micronutrient RECommendations Aligned Network of Excellence methods for assessment of micronutrient status (Hooper 2009), the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (GRADE Working Group 2004; Guyatt 2008), and the previous WHO/CDC meeting (WHO/CDC 2007), the group concluded that “There is an urgent need for better information on the iron status of populations to enable the right interventions to be chosen for combating both iron deficiency and anaemia, and then, once programmes are in place, to have the right indicators to monitor their impact”.
This review therefore seeks to evaluate the accuracy of ferritin as an index of iron deficiency (where iron deficiency is defined by a reference standard method), and to assess the quantity and quality of evidence available to support the selection of thresholds of ferritin that define iron deficiency in outpatients, otherwise well individuals without inflammation, as well as individuals with (or at risk of) inflammation, We are also seeking to identify thresholds for iron overload in individuals with hereditary haemochromatosis or non‐transfusion dependent thalassaemia (i.e. mediated by excess iron absorption), and those with transfusion‐induced iron overload.
Objectives
To determine the diagnostic accuracy of ferritin concentrations (serum or plasma) for detecting iron deficiency;
To determine the diagnostic accuracy of ferritin concentrations (serum or plasma) for detecting iron overload in primary and secondary iron‐loading syndromes.
Secondary objectives
To determine the diagnostic accuracy of ferritin to determine iron deficiency at current WHO threshold values (WHO 2011b): < 12 µg/L; < 15 µg/L and < 30 µg/L; as well as other commonly utilised thresholds;
To assess the diagnostic accuracy of ferritin to determine iron overload at putative threshold values such as 150 µg/L in females and 200 µg/L in males (Bacon 2011; WHO 2001).
Methods
Criteria for considering studies for this review
Types of studies
We included all study designs seeking to evaluate ferritin as an index of iron status where it had been compared against an eligible reference standard (Bossuyt 2008). We expected most studies to be cross‐sectional (where recruited individuals with and without the target condition are included in proportion to their prevalence in the overall sample), with essentially concurrent measurement of the index tests (ferritin).
Ferritin could have been assessed alone or together with (and/or in comparison with) other tests. Studies must have measured the index (ferritin) and reference test simultaneously, or at the very least before any treatment (for iron deficiency, defined as oral iron supplementation or other iron treatment e.g. intravenous iron) was initiated, to ensure the tests being compared reflected the same status. We did, however, include studies where this was not explicitly stated.
We included both prospective and retrospective studies in the analysis. We assessed the effect of these studies as outlined in Sensitivity analyses.
We also included case studies that measured simultaneously ferritin and the reference standard and summarised the distribution of ferritin concentrations at different stages of iron deficiency and iron overload.
We included studies of participants where recruitment was based in the laboratory (i.e. a consecutive series of bone marrow examinations were reviewed, with concurrent or retrospective measurement of serum ferritin) as well as studies where recruitment occurred in the clinic or in the population. We anticipated clinical studies were likely to be prospective and laboratory‐based studies retrospective; in this case, we evaluated the effects of clinic and laboratory‐based studies using sensitivity analysis.
Iron deficiency
We included studies that measured the diagnostic accuracy of ferritin as an index of iron deficiency defined by the reference standard of bone marrow iron stores, as measured by assessing marrow macrophage iron content on the aspirate via an iron stain.
Iron overload
We included studies that measured the diagnostic accuracy of ferritin against a reference standard of hepatic iron content, where the hepatic tissue was obtained via liver biopsy, and the iron content assessed using either:
liver tissue iron measurements (generally via measurement of non‐heme iron content), or
liver iron overload (defined by Perl’s (Prussian blue) stain) using an accepted scoring system.
Participants
Iron deficiency
We included participants of any sex, age (i.e. adults, children and infants), pregnancy status, hospitalisation status, living in any country. We stratified the populations according to apparently healthy and non‐healthy status. We aimed to assess the differences between infants and children, adolescents, pregnant women and adults. This latter category included all nonpregnant adults, since studies on pregnant women were analysed separately.
Iron overload
We included patients at risk of iron overload from primary (non‐transfusion‐induced or IV iron‐induced) causes, including participants of any sex, age, pregnancy status, hospitalisation status, living in any country.
Specific exclusion criteria
Renal failure causes very specific changes to iron homeostasis and interpretation of ferritin levels in this context is a specialised skill, with a different set of accepted conventions. We thus considered studies exclusively recruiting participants with end‐stage renal failure to be outside the scope of this review. We therefore excluded participants with end‐stage renal failure (as defined by study authors, or with a glomerular filtration rate reported among the study population < 60 mL/min), or in patients receiving haemodialysis.
We also excluded studies exclusively recruiting patients who had undergone chronic red cell transfusions. Such patients are likely to have undergone specific, complex investigations and management (including iron chelation). Thus, clinicians caring for these patients were likely to already have a high clinical suspicion of iron overload, and would not rely on a ferritin alone to diagnose liver iron loading.
Index tests
Serum or plasma ferritin concentration measured by any current or previously available quantitative assay i.e. enzyme‐linked immunosorbent assay, radioimmunoassay, chemiluminescence immunoassay or nephelometry.
Target conditions
Iron deficiency (as diagnosed by bone marrow iron stores);
Iron overload (as diagnosed by liver biopsy and measured by tissue iron quantification or histology).
Reference standards
Iron deficiency
The definitive diagnosis of iron deficiency is by demonstration of absent iron stores on a Perl's (Prussian blue) stain of the bone marrow. Bone marrow aspirates are invasive but generally safe procedures (Melempati 2009). Aspirated bone marrow particles are spread on a slide and stained. Siderotic material (haemosiderin) is a water‐insoluble complex of ferric iron, lipid, protein and carbohydrate found chiefly in macrophages. The ferric ions in haemosiderin react with potassium ferrocyanide to form a blue compound, ferriferrocyanide (the Prussian blue, or Perl's reaction). In a normal bone marrow, iron can be detected. However, in iron deficiency, staining is markedly reduced or absent. The absence of stainable iron on a bone marrow aspirate that contains particles is diagnostic of iron deficiency. Bone marrow examination for iron deficiency is not a quantitative test although reporting systems often utilised categories to indicate absent, marginal, normal and excess iron (Beutler 1958; Riley 2009). Table 5 and Table 6 present two examples of grading systems for bone marrow iron.
1. Bone marrow iron content (Perl's Prussian blue stain method).
| Grade | Features of Prussian blue stain |
| 0 | No stainable iron |
| + | Small intracellular iron stores using oil objective |
| ++ | Small, sparse intracellular iron particles at low power |
| +++ | Numerous small intracellular iron particles |
| ++++ | Larger particles with a tendency to aggregate into clumps |
| ++++++ | Dense, large clumps |
| ++++++ | Very large clumps and extracellular iron |
From: Riley 2009
2. Bone marrow iron content (other classification).
| Grade | Interpretation |
| 0 | No iron observed |
| Trace | Rare intracellular iron granules |
| + | Iron granules seen, but fewer than normal |
| ++ | Normal iron content (10% of fields) |
| +++ | Considerable amounts of iron in every field |
| ++++ | Considerable degree of iron excess |
From: Beutler 1958
Methodologic problems may limit interpretation of bone marrow iron stores (Bain 2011; Riley 2009). To diagnose iron deficiency, at least five to seven bone marrow particles should be examined and found negative for stainable iron. This can limit this method in hypocellular bone marrows. Technical limitations (i.e. few particles included on the slide), operator experience, interobserver error and stain quality can also limit accuracy of this examination (Bain 2011). These problems may be exacerbated where stainable iron is only very slightly detectable, but not completely absent. Despite these limitations, identification of absent bone marrow iron stores remains the most commonly accepted reference standard index of iron deficiency.
We defined iron deficiency as absent iron stores as determined by bone marrow examination.
Iron overload
Iron overload can be determined by quantitatively or qualitatively measuring iron accumulation in tissues. Quantitative iron measurement can be achieved by direct measurement of iron content in biopsied tissues, particularly the liver which is the main iron storage organ. Hepatic iron stores have been shown to correlate closely with overall body iron stores and thus measurement of liver iron is satisfactory as an indicator of overall body iron (Angelucci 2000). We used the reference standard definition of iron overload used by individual study authors.
Liver biopsy
Liver biopsy can be performed percutaneously or via the transjugular route; it is invasive, painful and carries a considerable risk of bleeding and infection. As such, it is reserved for patients in whom concomitant histologic evaluation (for example, for cirrhosis) is of additional clinical value, in particular in patients diagnosed with haemochromatosis who have marked elevations in ferritin (for example, higher than 1000 µg/L) and/or elevation in liver enzymes (Bacon 2011), and in individuals with evidence of iron overload together with multiple causes of liver impairment for which the specific contribution of iron cannot be fully determined.
Tissue iron can be measured on liver samples obtained by biopsy, and provides an accurate estimation of total body iron stores (Angelucci 2000). Measurement can be undertaken using atomic absorption spectrophotometry or calorimetry following acid digestion (Beilby 1999). Values lower than 1.8 to 2.0 mg iron/g dry weight are considered to represent the upper limit of the normal range, whereas values exceeding this indicate iron overload: specifically, 2 to 7 mg iron/g dry weight indicate mild iron overload, 7 to 15 mg iron/g dry weight moderate iron overloading, while values exceeding this are considered to represent severe iron loading (St Pierre 2014).
Liver iron loading can also be estimated semi‐quantitatively by histology using Perl's (Prussian blue) staining, and examination for iron in Kupffer cells and hepatocytes. A limitation to this approach is the element of subjectivity associated with decisions regarding stage; however, histologic grading and tissue iron measurements have been shown to broadly correlate (Barry 1974). Table 7 presents the interpretation on liver biopsies using a histological gradient for iron content.
3. Interpretation of iron content of liver biopsies: histological grade of iron storage.
| Grade | Ease of observation and magnification required |
| 0 | Granules absent or barely discernible at x 400 |
| 1 | Granules barely discernible at x 250 and easily confirmed at x 250 |
| 2 | Discrete granules resolved at x 100 |
| 3 | Discrete granules resolved at x 25 |
| 4 | Masses visible at x 10, or naked eye |
Search methods for identification of studies
We searched the following international and regional sources (10 June 2020). For the models, we used a preliminary search (April 2017) and kept eligible studies in the updated search as awaiting classification for an updated future version.
Electronic searches
International databases
Database of Abstracts of Reviews of Effects (DARE) (http://www.cochrane.org/editorial-and-publishing-policy-resource/database-abstracts-reviews-effects-dare)) Issue 2 of 4 2015 (10/6/20)
Health Technology Assessment (HTA) database (http://www.cochrane.org/editorial-and-publishing-policy-resource/health-technology-assessment-database-hta) HTA (Cochrane Library) Issue 4 of 4 2016 (10/6/20)
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 6 of 12 2020 (10/6/20)
MEDLINE & MEDLINE In Process (OVID) 1946 to June 9 2020 (10/6/20)
Embase (OVID) 1947 to week 23 2020 (10/6/20)
CINAHL (Ebsco) 1982 to June 2020 (10/6/20)
Web of Science (ISI) SCI, SSCI, CPCI‐exp & CPCI‐SSH to June 2020 (10/6/20)
POPLINE (www.popline.org) (16/8/18)
Open Grey (10/6/20)
TRoPHI (10/6/20)
Bibliomap (10/6/20)
ClinicalTrials.gov (clinicaltrials.gov) (18 September 2019)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/) (18 September 2019).
Regional databases
IBECS (http://ibecs.isciii.es/) (10/6/20)
SCIELO (http://www.scielo.br/) (10/6/20)
WHO Global Index Medicus (GIM): African Index Medicus (AIM); Index Medicus for South‐East Asia Region IMSEAR (10/6/20), Western Pacific Region Index Medicus (WPRIM) (10/6/20), IMEMR (10/6/20)
LILACS (lilacs.bvsalud.org/en) (10/6/20)
Pan American Health Library (PAHO) (10/6/20)
WHO Library (WHOLIS) (10/6/20)
Indexing of Indian Medical Journals (IndMED) (http://indmed.nic.in/) (16/8/18)
Native Health Research Database (https://hscssl.unm.edu/nhd/) (10/6/20)
We used keywords and controlled vocabulary (when available). Our MEDLINE search strategy is presented in Appendix 1 and we adapted this as appropriate for each database. We did not apply language or time restrictions for any database.
If we identified articles written in a language other than English, we commissioned their translations into English. If this was not possible, we stored such articles in the 'Studies awaiting classification' section of the review until a translation became available.
Searching other resources
For assistance in identifying ongoing or unpublished studies, we contacted the headquarters and the regional offices from the World Health Organization, as well as the nutrition section of the Centers for Disease Control and Prevention (CDC), Nutrition International, National Institutes of Health (NIH), International Life Sciences Institute Europe and WHO collaborating centres.
We handsearched the five journals with the highest number of included studies in the last 12 months to capture any article that might not have been indexed in the databases at the time of the search. We contacted authors of included studies and checked reference lists of included papers for identification of additional records.
Data collection and analysis
Selection of studies
Two review authors (MNG, SRP) and (MNG, JPR) independently screened the titles and abstracts of articles retrieved by each search to assess eligibility. When a title or abstract could not be excluded with certainty, we obtained the full text of the article for further evaluation. We aimed to retrieve full copies of all eligible papers.
The full text was then reviewed by two authors (MNG, SRP) and (MNG, JPR) and compared against inclusion/exclusion criteria. If full articles could not be obtained or when information regarding any aspect of study design or results was unclear, we attempted to contact the authors of the original reports to obtain further details of the study. Failing this, we classified such studies as "studies awaiting classification" until further information was published or made available.
We resolved disagreements at any stage of the eligibility assessment process through discussion or by consulting a third author (JPR, SRP) and together we made a final decision.
Data extraction and management
Two authors (MNG, SRP) independently extracted the following data using an extraction form tested and approved by all review authors in order to enhance consistency amongst reviewers. We collected information on:
General information: title, journal, year, publication status and study design;
Sample size: number of participants meeting the criteria and total number screened;
Participant characteristics: age, sex, race, and concurrent medications used;
Disease characteristics: prevalence of inflammation, infection or malignancy, indications for bone marrow studies, causes of iron overload;
Reported ferritin threshold points used to define iron deficiency or overload in studies;
Clinical reference standard test: methodological details on bone marrow determinations and records of procedural or analyst errors; methodological details on liver biopsies and imaging; reports on inter‐observer error if included;
Prevalence of iron deficiency or iron overload as defined by the reference standard;
Number of true positive, true negative, false positive, false negative, sensitivity, specificity, positive and negative predictive values, likelihood ratios, and AUCROC. We extracted these data for each threshold presented, for iron deficiency and iron overload;
Ferritin assay method (enzyme‐linked immunosorbent assay, radioimmunoassay, chemiluminescence immunoassay, nephelometry, inductively coupled plasma‐mass spectrometry, protein micro array or Western blot with quantum dots technology; commercial or non‐commercial kit; automated or manual; manufacturer; instrument).
We designed the data extraction form to enable recording of results for both the index test (ferritin) as well as for other alternative tests (also prespecified). We also extracted additional items related to recruitment and methodological details of studies, including the number and characteristics of study settings (presence of chronic or seasonal infection episodes, prevalence of chronic inflammation conditions such as hypertension, obesity and diabetes), whether recruitment was similar at different sites, levels of compliance and methods used for ferritin determinations. Two authors (LL, RXM) further extracted and validated the number of true positive, true negative, false positive, false negative, sensitivity, specificity, positive and negative predictive values, and likelihood ratios for each threshold presented and for iron deficiency and iron overload in included studies.
Two authors (JPR, LL) entered data into Cochrane's statistical software, Review Manager 2014, and a third author (RXM) carried out checks for accuracy. We resolved any discrepancies through discussion.
We included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart of study selection (Moher 2009).
Assessment of methodological quality
We assessed risk of bias and applicability using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)‐2 tool. We rated each of the four key domains (participant selection, index test, reference standard, flow and timing) using the signalling questions as developed by the QUADAS‐2 group (Whiting 2003; Whiting 2006; Whiting 2011). We have adapted the criteria for each signalling question for this review (Appendix 2).
Two review authors (MNG, SRP) independently assessed the included studies and resolved any disagreement by discussion or by consulting a third author (JPP). We scored all items in the QUADAS‐2 tool as ‘yes’, ‘no’ or ‘unclear’, and used graphs to present overall scores of risk of bias and applicability for each domain. We considered the quality of each study within the context of each study. We also considered the applicability of the data to support the review using the QUADAS‐2 applicability questions.
We appraised the overall quality of the evidence within the 'Summary of findings' tables. We incorporated an assessment of the effects of lower‐quality studies on the overall results (see Sensitivity analyses). We used GRADE assessment (based on the principal domains of risk of bias, directness, consistency, and precision, publication bias, dose response association, plausible unmeasured confounders that would decrease an effect, and strength of association) to enable the quality of evidence and hence strength of evidence for our conclusions (Singh 2012).
Statistical analysis and data synthesis
Definitions of key criteria
Iron deficiency and overload were defined using reference standards. For iron deficiency, we used bone marrow iron stores. For iron overload, we used liver biopsy by tissue iron quantification or histology).
We included true positives, false positives, false negatives and true negatives of each study in a 2 x 2 table and calculated test sensitivity and specificity with corresponding 95% confidence intervals (CIs). In some cases, we collected several sets of data from one study. For example, this occurred if the study contained multiple patient populations or used multiple reference criteria (bone marrow aspirates with different definitions of iron deficiency, liver biopsies using different thresholds for iron overload).
Descriptive plots
We presented coupled forest plots to depict sensitivity and specificity for each age group, and also generated pairs of sensitivity and specificity in ROC‐space to undertake exploratory initial analysis using Review Manager 2014.
Handling of multiple thresholds
We used current WHO thresholds for ferritin that define iron deficiency together with prespecified thresholds commonly used (ferritin lower than 12, 15 or 30 µg/L for iron deficiency; higher than 150 µg/L and 200 µg/L for females and males, respectively; and higher than 500 µg/L for iron overload (WHO 2011b) (Table 8). We anticipated that thresholds would vary. We expected studies to use multiple thresholds, both between studies and within individual studies. As other threshold values were identified during the extraction, we only included the threshold that was reported by the authors as the best fit. We used Review Manager 2014 to graphically present coupled forest plots, showing the pairs of sensitivity and specificity of each study and age group.
4. Relative extent of iron stores on the basis of serum ferritin concentration.
| Interpretation | Serum ferritin (µg/L) | |||
| Less than five years of age | Five years of age or older | |||
| Male | Female | Male | Female | |
| Depleted iron stores | < 12 | < 12 | < 15 | < 15 |
| Depleted iron stores in the presence of infection | < 30 | < 30 | ‐ | ‐ |
| Severe risk of iron overload (adults) | ‐ | ‐ | > 200 | > 150 |
From WHO 2011b
We handled the issue of multiple thresholds by estimating the performance of ferritin tests by age group, as well as assessing the underlying ROC curve to appreciate the interaction between the sensitivity and specificity. First, we estimated the sensitivity and specificity by age group and near corresponding critical thresholds currently used to define altered iron reserves: ferritin concentrations < 15 µg/L and < 30 µg/L for iron deficiency, and > 150 µg/L and 200 µg/L in females and males, respectively, for risk of severe iron overload.
When we had the same threshold in a specific target group, we used bivariate analysis to estimate the summary operating point and find the optimal sensitivity and specificity at the given the threshold (Reitsma 2005). In case of several thresholds for the same target group, we estimated overall test accuracy of ferritin as an index of iron deficiency/overload by evaluating the underlying summary ROC curves, using hierarchical summary ROC (HSROC) curves.
We used the diagnostic odds ratio (DOR), which summarises in a single number the accuracy of the index test. It is defined as DOR = (sens x spec)/(1 ‐ sens) x (1 ‐ spec), and it is used to build multi‐threshold models such as HSROC and interpret its results.
We used Review Manager 2014 to list and store retrieved studies and tabulate and present data. We exported data for the corresponding bivariate and/or HSROC analysis into SAS software (SAS 2015), using the procedure NLMIXED through the Nishimura and Metadas macro (Takwoingi 2010). We derived the summary operating point and/or the summary ROC curve, as well as their corresponding CIs, and a 95% prediction region.
Results were presented in a 'Summary of findings' table. The same analysis was performed for iron deficiency and iron overload, highlighting the differences in thresholds.
Investigations of heterogeneity
We expected heterogeneity to exist and consequently used random‐effects meta‐analysis. Both clinical and statistical heterogeneity were addressed.
We analysed the effects of study level covariates with the hierarchical HSROC model to explore potential heterogeneity. We assessed the effects of covariates on shape and position of the curve. Subsets of studies with a fixed covariate value that reduces variability were studied using the HSROC model. Sets of studies using fixed thresholds from WHO's iron deficiency definition were studied using the bivariate model. Depending on the number of included studies, separate coupled forest plots were developed for each subset.
We expected to have the following age groups:
Age groups: infants (less than two years old), children (two to less than 12 years old), adolescents (12 to less than 19 years old), women aged 15 to 49 years old, adults (19 years of age or older).
We expected to have the following covariates:
Inflammation/infection, sub classified by aetiology and duration;
Physiologic condition: healthy, non‐healthy, mixed;
Anaemia status: anaemic, non‐anaemic, mixed;
Pregnancy status: pregnant versus non‐pregnant versus mixed/unreported;
Place of study: hospitalised, outpatient, mixed;
Body mass index (BMI): low weight below 18.5 kg/m2, normal 18.5‐24.9 kg/m2, overweight 25‐29.9 kg/m2, obesity above 30 kg/m2);
Elderly (> 62 years old);
Sample test: serum, plasma;
Ferritin assay method: e.g. enzyme immunoassay, radiometric assays, turbidimetric assays;
Sex (male, female, mixed/unreported);
Publication year.
For the analysis, unless the inflammation status was explicit by the authors, we considered a study to contain a population at risk of inflammation if it was:
undertaken in hospitalised individuals;
undertaken in patients with a high prevalence of inflammatory, malignant or infective conditions (for example, a case series of bone marrow samples taken from patients with a mixed burden of disease);
undertaken in an outpatient community at high risk of infectious or inflammatory conditions (for example, an area endemic to malaria or HIV infection).
We used the same analyses approach for both iron deficiency and iron overload, although some of the covariates available on the studies were expected to be different.
Sensitivity analyses
We planned to perform sensitivity analyses on the key domains scored on the QUADAS‐2 tool, in order to explore the influence of the quality of the included studies. We also planned to undertake a sensitivity analysis to evaluate the influence of unclear decisions i.e. decisions that had to be taken without enough information to do so, such as classification of studies by target population, or by covariates when it was originally missing in the study. If in any of these cases there was uncertainty regarding eligibility of a study or allocation of covariates, we planned to analyse the totality of the studies to explore sensitivity of our results while changing the decisions made.
Assessment of reporting bias
In order to assess publication bias, we used an alternative approach to detecting funnel plot asymmetry in reviews of diagnostic studies (Deeks 2005). As indicated in this methodology, we tested for association between the natural logarithm (ln) of the diagnostic odds ratio ln(DOR) and the effective sample size (ESS).
Results
Results of the search
Our search strategy (Appendix 1) was conducted initially in 2014 and updated in 2017, 2018 and 2020 (10 June) and identified 21,217 records, while 11 additional references were identified through other sources. After removing duplications and initial screening, we examined 14,244 records; 316 full‐text articles (n = 316 records) were retained for further assessment: 176 records for iron deficiency and 130 records for iron overload. We excluded 190 studies (193 records) with reasons and we placed 11 studies (12 records) as awaiting classification.
We included 108 studies (n = 111 records) in the qualitative and quantitative synthesis. Of these, 72 studies (n = 75 records) focused on iron deficiency and 36 studies (n = 36 records) focused on risk of iron overload.
The detailed study flow diagram for iron deficiency and overload is presented in Figure 3.
3.

Study flow diagram for iron deficiency and risk of iron overload
Iron deficiency
Participants, and target populations
Only five studies were conducted in apparently healthy populations (Hallberg 1993; Jonker 2014; Milman 1983; Puolakka 1980; Sorbie 1975) and 70 in individuals or groups of patients presenting different pathological conditions (n = 75 records).
Concerning the non‐healthy population: 19 studies (26.4%) were in participants with diverse chronic or acute diseases, mainly over 50 year old outpatients and hospitalised individuals (Baillie 2003; Bârsan 2015; Guyatt 1990; Harju 1984; Holyoake 1993; Joosten 2002; Karlsson 2010; Karlsson 2015; Kis 1998; Martin‐Cabrera 2015; Nanas 2006; Nadeem 2011; Patterson 1985; Sorbie 1975; Sharma 1984; Terrovitis 2011; Thompson 1988; Van Tellingen 2001; Van Zeben 1990); 11 studies (15.3%) were on men and women with rheumatoid arthritis (Baumann Kurer 1995; Hansen 1983; Kim 2000; Mulherin 1996; Nielsen 1990; Porter 1994; Ravindran 2008; Shroff 1991; Smith 1977; Suominen 2000; Vreugdenhil 1990); 10 studies (13.9%) were on participants with blood disorders (Barron 2001; Brown 1988; Coenen 1991; Forman 1980; Mast 2002; Oluboyede 1980; Rao 1984; Ruivard 2000; Solomon 1981; Witte 1986); seven studies (9.7%) were in participants with infectious diseases (Aguilar 2012; Jonker 2013; Kotru 2004;Lewis 2007; Meira 2005; Phiri 2009; Van den Broek 1998); seven studies (9.7%) were in populations with alcoholism/liver cirrhosis/renal failure (Balaban 1993; Intragumtornchai 1998; Isa 1988; Kalantar‐Zadeh 1995; Krause 1980; Milman 1983; Nelson 1978); and 16 studies (22.2%) were on non‐healthy individuals without details about the origin and condition of their sickness or mixed non‐healthy and healthy populations who underwent bone‐marrow examination (Ali 1978; Brink 1982; Burns 1990; Chang 2007; Lindstedt 1980; Lough 1989; Mast 1998; Mazza 1978; Means 1999; North 1997; Ong 2005; Punnonen 1994; Punnonen 1997; Punnonen 1998; Puolakka 1980; Witte 1988).
Three of the studies performed in non‐healthy populations also contained separated and classified healthy groups that were used in the analysis of healthy individuals (Milman 1983; Puolakka 1980; Sorbie 1975), i.e. from 72 included studies, we obtained 75 records (data entries).
In relation to other subgroups of target populations, we found only five studies that were performed in children, of which four were in non‐healthy individuals (Aguilar 2012; Jonker 2013; Meira 2005; Phiri 2009), and one in healthy children (Jonker 2014). Studies during pregnancy with bone marrow data was found in four studies; three of them in non‐healthy women (Oluboyede 1980; Puolakka 1980; Van den Broek 1998), and one in 32 healthy pregnant women attending maternity centres in Finland (Puolakka 1980).
Covariates distribution in iron deficiency
In regard to sex, we found six studies (8.3%) related to females, four studies (5.3%) related to males, and 62 studies (86.1%) with mixed/unknown sex. Concerning age, we found five studies (6.7%) for ages between six and 59 months, 11 studies (14.7%) for ages between 20 to 49 years old, 31 studies (41.3%) studies for ages of 50 years or older, and 28 studies (37.3%) with mixed/unknown/unclear age.
In regard to inflammation/infection status, 37 studies (51.4%) reported inflammation, only three studies (4.2%) reported no inflammation, and 32 studies (44.4%) had unknown/unreported inflammation status. A total of 52 studies (72.2%) reported anaemia, only one study (1.4%) reported that the population was non‐anaemic, and 19 studies (26.4%) had mixed/unknown/unreported anaemia status.
In regard to the site of the study, 36 studies (50%) were set in an outpatient clinic, 26 studies (36.1%) were in hospitalised patients; only one study (1.4%) was in a community population, and nine studies (12.5%) were done in a mixed/unknown/unreported setting.
The studies included used different ferritin measurement laboratory methods; 18 studies (25%) used enzyme‐linked immunosorbent assay (ELISA), 35 studies (48.6%) used either RIA or IRMA (radioimmunoassay techniques), only five studies (6.9%) used CLIA (chemiluminescence immunoassay), and 14 studies (19.4%) used other/unknown/unreported techniques.
Types of studies, and settings
All studies were observational studies in which participants or, in some cases, healthy individuals were scheduled for a bone marrow biopsy for haematological assessment. In most of the studies, the group included a heterogeneous mixture of participants with haematological malignancies and chronic inflammation. Only five studies were conceived to determine bone marrow biopsy from healthy individuals.
In relation to the level of income of the countries where the study was performed, the majority (58, 81%) were high‐income countries and only six (8%) low‐income, five (7%) lower‐middle income, and three (4%) upper‐middle income countries, respectively (income classification source: World Bank 2019‐2020 FY‐20). Concerning the fourteen studies from non‐high‐income countries: seven of them (50%) took as the target population participants from malaria, TB or HIV infection endemic settings; and in the remaining seven studies (50%), the participants did not have infectious diseases but rather diverse chronic diseases, or were healthy (even if they came from countries with malaria, TB or HIV infection endemic settings).
The studies were performed across a 41‐year time span, between 1975 to 2015, chiefly between 1986 to 1999 (19 studies (26.4%) from 1975 to 1985; 29 studies (40.3%) from 1986 to 1999 and 24 studies (33.3%) from 2000 to 2015).
Reference standards
Bone marrow iron content was determined by bone marrow aspirate and stained using Perl's method. Iron stores were graded on the basis of visual impression of the stained granules. In all included studies, iron deficiency was diagnosed when iron content in bone marrow was 0. Classification of the degree of iron deficiency as moderate and severe could vary between authors, but the diagnosis of iron deficiency when iron in bone marrow was undetectable (0), was common in all publications.
Risk of iron overload
Participants
All 36 eligible studies were conducted in non‐healthy/mixed adult populations. From these, 32 studies (78%) used a unique threshold for males and females to classify the presence of iron overload (Chapman 1982; Cippa 2014; Halliday 1977; Harada 1992; Holmström 2002; Jensen 1994; Kaltwasser 1990; Lawrence 1996; Leggett 1990; Lim 2004; Lombard 1989; Macfarlane 1995; Maliken 2012; Niederau 1998; Olynyk 1999; Ortega 2005; Pascoe 1999; Phatak 1998; Pietrangelo 1999; Rowe 1977; Schöniger‐Hekele 2002; Sham 1997; Smith 1997; Summers 1990; Szurowska 2010; Thomsen 1992; Thorburn 2002; Valberg 1978; Villeneuve 1996; Walsh 2006; Wands 1976; Wong 2006), and nine studies (22%) used one threshold for males and another threshold for females to diagnose iron overload (Guyader 2007; Hagström 2016; Halliday 1977; Jensen 1994; Kaltwasser 1990; Lim 2004; Lombard 1989; Sebastiani 2006; Sebastinani 2012). Five studies contained data and analysis for a single and dual threshold points for diagnosis of iron overload based on sex (Halliday 1977; Jensen 1994; Kaltwasser 1990; Lim 2004; Lombard 1989), i.e. from 36 included studies, we obtained 41 records (data entries).
Covariates distribution in iron overload
We looked into each of the covariates distribution for the 36 included studies.
With regard to sex, four studies (11.1%) were performed in males and 32 (88.9%) studies had mixed, unknown, or unreported sex. No studies reported data only on women. Most of the studies, 33 out of 36 (91.6%), were in participants older than 18 years of age, and around half of this group (17 out of 33) were older than 35 years. For the remaining (three, 8.3%) included studies, the age group was not reported or unknown.
The pathologies associated with iron overload in the eligible studies were mainly haemochromatosis in 20 out of 36 studies (55.6%), chronic hepatitis, liver cirrhosis, hepatic fibrosis, or alcoholic liver disease in seven studies (19.4%), and mixed or unknown diseases in nine studies (25.0%).
As for the setting of the study, 25 studies (69.4%) were outpatient‐based, two studies (5.9%) were in hospitalised patients; six studies (16.7%) were in the community, and three studies (8.3%) were performed in a mixed, unknown, or unreported place.
Index test
With respect to serum ferritin measurement methodology, 25 studies (69.4%) used unreported techniques, five studies (13.9%) used CLIA (chemiluminescence immunoassay), three studies (8.3%) used either RIA or IRMA (radioimmunoassay techniques), only one study (2.8%) used nephelometry, and two studies (5.6%) used ELISA (enzyme‐linked immunosorbent assay).
Reference standards
Regarding the type of liver biopsy analysis method, 16 studies (44.4%) used liver iron content measurement, 12 studies (33.3%) used histology (Perl's Staining) methodology, and eight studies (22.2%) presented both liver iron content and Perl’s Staining methodologies.
Regarding the threshold of the liver biopsy method, 18 studies (50%) used liver iron concentrations of 1.8 mg/g dry tissue to define iron overload, and six studies (16.7%) used liver iron concentrations slightly different to 1.8 mg/g dry tissue. As to the histology technique, six studies (16.7%) used the Perl's staining liver iron score of more than or equal to one to declare iron overload, three studies (8.3%) used the Perl's staining of more than one, two studies (5.6%) used the Perl's staining of more than or equal to 2, and one study (2.8%) used more than two.
Types of studies
The studies we found were in the time span of 38 years between 1976 to 2014, where 17 studies (47.2%) were from 1986 to 1999, 14 studies (38.9%) were from 2000 to 2014, and only five studies (13.9%) were from 1976 to 1985.
Methodological quality of included studies
Iron deficiency
Study quality is summarised in Figure 4 and Extra Figure I (Figure 5). We found around one‐fifth (n =14, 19%) of the studies on iron deficiency had low risk of bias, and 43 (60%) were without concerns regarding applicability. Fifty‐eight (81%) of studies were classified as either at unclear or high risk of bias: forty‐one (57%) of them were classified as being at high risk of bias, and seventeen (24%) of them as having unclear risk of bias. Moreover 24 (33%) of the articles were at high concern regarding applicability because of participant selection. Concerning the participant selection bias corresponding signalling questions, 34 (47%) did not use a consecutive or random sample of participants enrolled, 23 (32%) did not avoid case‐control design, and 24 (33%) did not avoid inappropriate exclusions.
4.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
In the majority of the studies classified as having unclear to high risk of bias in the selection of participants, we found retrospective, prospective, and ambispective studies where the selection of participants was generally unclear or they were not randomly selected, that often did not avoid inappropriate exclusions, and sometimes that did not avoid the case‐control design.
Most of the studies (n = 60, 81%) had neither risk of bias or concerns regarding the applicability for the index test or reference standard domains. However 20 (28%) studies did not use prespecified thresholds, in contrast to 51 (71%) studies that used prespecified thresholds.
Approximately 14 (19%) of the studies had high risk of bias due to flow and timing; most of these cases were because only a sub selection of participants were included in the analysis.
Iron overload
We found that 29 (81%) of iron‐overload studies had a high risk of bias because of participant selection, and more than half (20 studies, 56%) had participant selection concerns regarding applicability to the review question. Concerning the participant selection bias corresponding signalling questions, 19 (53%) did not use a consecutive or random sample of participants enrolled, 21 (58%) did not avoid case‐control design, and 20 (56%) did not avoid inappropriate exclusions. One‐quarter of them were either case‐control studies, or retrospective studies.
Half of the studies (n = 18, 50%) had unclear risk of bias for the index and reference standard domains. However, most studies (n = 27, 75%) had low concerns regarding the applicability of either the index test or reference standard. Furthermore, 12 (33%) studies did not use prespecified thresholds, six (17%) studies were unclear, and half of them 18 (50%) used prespecified thresholds.
Seventeen (47%) of the studies had high risk of bias due to flow and timing. The majority of these cases were either because only a subsection of participants was included in the analysis or not all the participants received the same reference standard.
In the majority of these studies (n = 27, 75%), the participants had either haemochromatosis or a particularly severe liver disease. Furthermore, participants for liver sampling in these studies were generally selected from a larger participant population, based on disease severity or even risk of iron overload, causing high risk of bias regarding flow and timing (half of the included studies). None of the studies were conceived as diagnostic test accuracy studies, and half had either small sample sizes or only true positives or true negative cases.
The reference standard threshold for liver biopsy concentration to separate iron overloaded tissue from non‐iron overloaded tissues was different between studies compromising comparability. We thus considered that iron‐overload studies were at high risk of bias and we also had concerns regarding their applicability to the review question.
Findings
Meta‐analyses results are presented corresponding to the target populations that allowed for their making. However, for some of them, there were not enough studies to obtain convergence or meaningful results; for those target groups, we discuss the characteristics of the studies found by the search. We also present a heterogeneity analysis of the inflammation covariate for the iron deficiency ferritin test, as well as a bivariate model pooled estimation for threshold of 30 µg/L in non‐healthy adults. The section closes with the sensitivity analysis and the assessment of reporting bias. From now on, we will refer to prevalence of the included studies in each corresponding target's population category as **prevalence.
Ferritin as a marker of iron deficiency in apparently healthy populations
All populations and ages
Accuracy estimations of the test on the five studies (n = 350 participants) of healthy populations described above were computed using the HSROC framework; results are shown in the Table 1. For instance, for a sample of 1000 subjects with a 41% **prevalence of iron deficiency and supposing either a 60%, or 75% or 90% specificity, there would be 206, 298, and 378, respectively, iron‐deficient subjects incorrectly classified as not having iron deficiency. Thus, in all three cases,, the negative predictive value was approximately 60%, which means that from all negative serum ferritin test results, around 40% would be false negatives. On the other hand, there would be 237, 148, and 59 subjects, respectively, without iron deficiency incorrectly classified as having iron deficiency. Thus, the positive predictive value varied from 46% to 34%, and around 54% to 66% from all positive serum ferritin results would be false positives.These results predict poor sensitivity and poor negative, and positive predictive values in the three scenarios. The GRADE quality of evidence of these results is very low due to risk of bias, serious indirectness, and imprecision as shown in Table 1.
Summary of findings 1. Estimates of accuracy of serum ferritin to assess iron deficiency for apparently healthy (all).
| Patients/population: | Healthy persons at risk of iron deficiency. | |||||
| Settings | Community, outpatient, primary health care. | |||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | |||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay, etc. | |||||
| Reference standard | Aspirated bone marrow examination to identify the iron stores presence or absence on a Perl's (Prussian blue) stain. | |||||
| Importance | Avoid the challenges of an invasive, and occasionally risky bone marrow procedure, as well as elevated costs, and distress. | |||||
| Studies | 5 prospective/ambispective cohorts. | |||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 1.6 |
Certainty of Evidence (CoE):
|
|||
| Effect per 1000 participants for **prevalence of iron deficiency 41% | ||||||
| Outcome | Specificity = 60% | Specificity = 75% | Specificity = 90% | |||
| Sensitivity | 5 (143) | TPs | 203 | 111 | 30 | ⊕⊝⊝⊝ Very Low1,2,3 |
| FNs | 206 | 298 | 378 | |||
| Specificity | 5 (207) | FPs | 237 | 148 | 59 | |
| TNs | 355 | 444 | 532 | |||
**Prevalence of included studies in this category.
1 Downgraded one level due to risk of bias.Limitations in participants enrolment, selection and flow for most of the studies.
2 Downgraded one level due to indirectness. Studies done in children, young people, and pregnant women. High prevalence of iron deficiency in participants of included studies.
3 Downgraded one level due to imprecision. There was unexplained heterogeneity probably caused by very diverse populations and settings. All thresholds were proposed by authors were different, and ranged between 16 to 70 µg/L.
4There were very few studies, and the variability was high.
*Adults (group composed of adults excluding those studies focused exclusively on pregnant women)
Three studies assessed this age group (Hallberg 1993; Milman 1983; Sorbie 1975). One of the studies included 38‐year‐old women (n = 203) with known iron status based on absence/presence of stainable iron in bone‐marrow smears. The distributions in iron‐replete and iron‐deficient women showed less overlap (diagnostic efficiency 91%) for serum ferritin than for other haematological parameters. The best discrimination was obtained at serum ferritin < 16 µg/L (specificity 98%, sensitivity 75%). Absence of iron stores was associated with signs of an iron‐deficient erythropoiesis, starting at serum ferritin 25 to 40 µg/L (Hallberg 1993). Importantly, this otherwise well‐designed study was limited by the long duration of sample storage; although bone marrow and serum samples were collected concurrently, the serum samples were stored at ‐20 degrees celsius for several decades, and the authors acknowledged that sample deterioration had likely occurred.
In a study on participants with chronic renal failure, a subset of 53 healthy (i.e. control) individuals (30 male, 23 female, mean = 39 years (range 20‐90 years)) were studied and it was found that serum ferritin correlated to marrow iron. Healthy subjects with serum ferritin < 15 µg/L had absent or reduced marrow iron, while those with serum ferritin > 30 µg/L had normal marrow iron. Using a critical serum ferritin value of < 20 µg/L, the diagnostic efficiency in terms of diagnosing absent or reduced marrow iron was 0.90 (PPV = 0.85, NPV = 0.91) (Milman 1983). The other study reported various groups of iron‐related illnesses and included a subgroup of 20 healthy students (18 males, two females, aged 17 to 30 years) that were analysed by bone marrow iron content. Absent iron (bone marrow = 0) individuals (five males, two females) had a mean ferritin concentration of 18 µg/L (range 7‐38), intermediate iron (bone marrow = 1+) individuals (four male) had a ferritin concentration of 58 µg/L (range 19‐102) and plentiful iron (bone marrow = 2‐3+) individuals (9 male) had a ferritin concentration of 92 µg/L (range 59‐170) (Sorbie 1975).
With three studies available for meta‐analysis with high specificity values (> 92%), one study had a 100% sensitivity value, as seen in the corresponding forest plot in Extra Figure II. This set of studies was not fitted for a HSROC analysis, which we attempted but did not present here since we did not believe it would be meaningful. As there were very few studies which were relatively homogeneous in accuracy, they were not fitted for hierarchical models (see Taikwogi 2017).
Pregnant women
One included study assessed this group (Puolakka 1980). Thirty‐two healthy pregnant women were followed up in the antenatal clinic of Oulu University Central Hospital, Finland. They were evaluated longitudinally during pregnancy and six months postpartum by serum ferritin assay and by bone marrow iron content. It was found that a serum ferritin concentration below 70 µg/L was associated with absent iron stores at the 16th week of pregnancy. The correlation between the iron‐depleted group i.e. bone‐marrow = 0 and serum ferritin was statistically significant (P < 0.001), as well as the correlation between the iron‐repleted group i.e. bone‐marrow > 0 and serum ferritin (P < 0.05).
Infants and children
One included study assessed this age group (Jonker 2014). The study recruited eighty‐seven healthy Malawian children (6–66 months, mean age 37 months) scheduled for elective surgery at Hospital in Malawi. Thirty‐nine children (44.8%) had depleted bone marrow iron stores and 64% were anaemic, with a mean ferritin concentration of 19 (12–36) µg/L. Using optimised thresholds, ferritin (< 18 µg/L) and sTfR‐F (> 1.85) best predicted depleted iron stores with a sensitivity/specificity of 73.7/77.1% and 72.5/75.0%, respectively. We contacted the author who stated that the participants were selected in order to have a suitable sample to assess the accuracy of the serum ferritin test, avoiding participants with other inflammatory conditions.
Adolescents
No study assessed this target population.
Ferritin as a marker of iron deficiency in non‐healthy populations
All populations and ages
Accuracy estimations of the test on the seventy studies (n = 5709 participants) of non‐healthy populations described above were computed using the HSROC framework; results are shown in Table 9. Since there was no common threshold used in the studies of this estimation, the result of the analysis yielded a sensitivity/specificity trade‐off that was simulated for specific values of **prevalence of iron deficiency in Table 9 in terms of the test's expected outcomes. For instance, for a sample of 1000 subjects with a 36% **prevalence of iron deficiency and supposing a 85% specificity (leading to a 89% sensitivity), it would be expected to have 338 iron‐deficient subjects correctly diagnosed, 21 iron‐deficient subjects incorrectly classified as not having iron deficiency, 481 subjects without iron deficiency correctly diagnosed, and 160 subjects without iron deficiency incorrectly classified as having iron deficiency. For this particular case, the negative predictive value is 93%, that is, 93% of participants with negative serum ferritin test results will not have disease but 7% will (i.e. they will be false negatives), and the positive predictive value is 77%, which means that 77% of participants with positive serum ferritin test results will actually have disease but 23% will not (i.e. they will be false positives). The GRADE quality of evidence of these results is low due to risk of bias and serious inconsistency, as shown in Table 9.
5. Estimates of accuracy of serum ferritin to assess iron deficiency for non‐healthy (All).
| Patients/population | Non‐healthy patients at risk of iron deficiency. | |||||
| Settings | Community, outpatient, primary health care. | |||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | |||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay, etc. | |||||
| Reference standard | Aspirated bone marrow examination to identify the iron stores presence or absence on a Perl's (Prussian blue) stain. | |||||
| Importance | Avoid the challenges of an invasive, and occasionally risky bone marrow procedure, as well as elevated costs, and distress. | |||||
| Studies | 66 prospective/ambispective cohorts, 3 retrospective cohorts, and one case‐control. | |||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 49.2 |
Certainty of Evidence (CoE):
|
|||
| Effect per 1000 participants for **prevalence of iron deficiency 36% | ||||||
| Outcome | Specificity = 75% | Specificity = 85% | Specificity = 95% | |||
| Sensitivity | 70 (2051) | TPs | 349 | 338 | 300 | ⊕⊕⊝⊝ Low1,2 |
| FNs | 10 | 21 | 59 | |||
| Specificity | 70 (3658) | FPs | 256 | 160 | 64 | |
| TNs | 384 | 481 | 577 | |||
**Prevalence of included studies in this category
1Limitations in patient's enrolment, selection and final inclusion (ones with all available data) for at least half of the studies sufficient to rate down one level
2There was unexplained heterogeneity maybe caused by multiple and very diverse diseases. A large range of thresholds was used (from 12 to 273 µg/L).
*Adults
Sixty‐three studies assessed this group including 5042 participants (see forest plot in Extra Figure III). Patients (*adults, both sexes, with a wide range of ages) were from primary healthcare centres, outpatients, or hospitalised. Illnesses were varied and were classified into: chronic or acute diseases, rheumatoid arthritis, blood disorders, infectious diseases, alcoholism/liver cirrhosis/renal failure, and mixed or unknown. Distribution details per category can be seen in the Results section for iron deficiency participants and the target population description above.
Accuracy estimations of the test in the sixty‐three studies on non‐healthy *adults were computed using the HSROC framework; results are shown in the Table 2 and Extra Figure IV. Since there was no common threshold used in the studies of this estimation, the result of the analysis yielded a sensitivity/specificity trade‐off that was simulated for specific values of **prevalence of iron deficiency in Table 2 in terms of the test's expected outcomes. For instance, for a sample of 1000 subjects with a 35% **prevalence of iron deficiency and supposing a 85% specificity (leading to a 90% sensitivity), it would be expected to have 315 iron‐deficient subjects correctly diagnosed, 35 iron‐deficient subjects incorrectly classified as not having iron deficiency, 552 subjects without iron deficiency correctly diagnosed, and 97 subjects without iron deficiency incorrectly classified as having iron deficiency. For this particular case, the negative predictive value is 94%, that is, 94% of participants with negative serum ferritin test results will not have disease but 6% will (i.e. they will be false negatives), and the positive predictive value is 76%, which means that 76% of participants with positive serum ferritin test results will actually have disease but 24% will not (i.e. they will be false positives). The estimated DOR was 50, a moderately informative test. The GRADE quality of evidence of these results is low due to risk of bias, and serious inconsistency as shown in Table 2.
Summary of findings 2. Estimates of accuracy of serum ferritin to assess iron deficiency for non‐healthy (*adults).
| Patients/population: | Non‐healthy *adults at risk of iron deficiency. | |||||
| Settings | Community, outpatient, primary health care. | |||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | |||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay. | |||||
| Reference standard | Aspirated bone marrow examination to identify the iron stores presence or absence on a Perl's (Prussian blue) stain. | |||||
| Importance | Avoid the challenges of an invasive, and occasionally risky bone marrow procedure, as well as elevated costs, and distress. | |||||
| Studies | 59 prospective/ambispective cohorts + 3 retrospective cohorts + 1 case‐control. | |||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 49.8 |
Certainty of Evidence (CoE):
|
|||
| Effect per 1000 participants for **prevalence or iron deficiency of 35% | ||||||
| Outcome | Specificity = 75% | Specificity = 85% | Specificity = 95% | |||
| Sensitivity | 63 (1765) | TPs | 331 | 315 | 250 | ⊕⊕⊝⊝ Low1,2 |
| FNs | 19 | 35 | 100 | |||
| Specificity | 63 (3277) | FPs | 162 | 97 | 32 | |
| TNs | 487 | 552 | 617 | |||
*adults: This group is composed of adults excluding those studies focused exclusively on pregnant women.
**Prevalence of included studies in this category.
1 Downgraded one level due to limitations in patient's enrolment, selection and final inclusion (ones with all available data) for at least half of the studies.
2 Downgraded one level due to indirectness.Unexplained heterogeneity may be caused by multiple and very diverse diseases or conditions. Thresholds ranged between 12 to 200 µg/L.
Pregnant women
Three studies assessed this age group (Oluboyede 1980; Puolakka 1980; Van den Broek 1998).The first one studied 22 pregnant women attending the University Teaching Hospital antenatal clinic in Ibadan, Nigeria. All participants had blood disorders: 10 had haemoglobin SS (the most common type of sickle cell disease) and 12 had haemoglobin SC disease (a type of sickle cell disease). The mean ferritin values in both blood disorder groups were not significantly different from each other (P > 0.05). The ferritin levels increased progressively with greater amounts of marrow haemosiderin. Thus, the women with no visible marrow had a mean ferritin of 44.8 µg/L that was significantly lower (P < 0.001) than the group with normal iron (mean = 101.8 µg/L ) or the group with excess iron (355.6 µg/L).
Puolakka 1980 studied 33 anaemic pregnant females which were followed up in the antenatal clinic of Oulu University Central Hospital, Finland. Seventy‐five per cent of them had iron‐deficiency anaemia, and approximately 25% of them had anaemia due to an infectious disease. It was found that serum measurements (taken at the sixteenth week of pregnancy) are a useful addition to detect iron deficiency in pregnant women at a threshold of 35 µg/L (sensitivity 66%, and specificity 100%). Furthermore, they showed a significant correlation (P < 0.05) with bone marrow that was better than that obtained by serum iron or transferrin measurements.
Van den Broek 1998 studied 93 pregnant women attending the Queen Elizabeth antenatal clinic in Blantyre, Malawi. Half of the women were HIV positive, and 76% were in the third trimester. The author proposed the use of 30 µg/L as a threshold resulting in a relative high sensitivity of 91%, and a specificity of 86%.
Accuracy estimations of the test on the three studies on non‐healthy pregnant women (148 participants) were computed using the HSROC framework; results are shown in Table 10. Since there was no common threshold used in the studies of this estimation, the result of the analysis yielded a sensitivity/specificity trade‐off that was simulated for specific values of **prevalence of iron deficiency in Table 10 in terms of the test's expected outcomes. For instance, for a sample of 1000 subjects with a 53% **prevalence of iron deficiency and supposing a 85% specificity (leading to a 88% sensitivity), it would be expected to have 464 iron‐deficient subjects correctly diagnosed, 66 iron‐deficient subjects incorrectly classified as not having iron deficiency, 400 subjects without iron deficiency correctly diagnosed, and 71 subjects without iron deficiency incorrectly classified as having iron deficiency. The estimated DOR was 58, a relatively moderate informative test. This result agrees with the findings of each of the three studies that found significant correlation between ferritin measurements and bone marrow, however, the studies proposed three different ferritin thresholds: 100 µg/L, 35 µg/L, and 30 µg/L, respectively.The GRADE certainty of evidence of these results is very low due to risk of bias, serious indirectness, inconsistency, and serious imprecision as shown in Table 10.
6. Estimates of accuracy of serum ferritin to assess iron deficiency for non‐healthy (adults, pregnant women).
| Patients/population | Non‐healthy (adults, pregnant women) at risk of iron deficiency. | |||||
| Settings | Community, outpatient, primary health care. | |||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | |||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay, etc. | |||||
| Reference standard | Aspirated bone marrow examination to identify the iron stores presence or absence on a Perl's (Prussian blue) stain. | |||||
| Importance | Avoid the challenges of an invasive, and occasionally risky bone marrow procedure, as well as elevated costs, and distress. | |||||
| Studies | 3 prospective/ambispective cohorts. | |||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 57.9 |
Certainty of Evidence
(CoE)
|
|||
| Effect per 1000 participants for **prevalence of iron deficiency 53% | ||||||
| Outcome | Specificity = 80% | Specificity = 85% | Specificity = 90% | |||
| Sensitivity | 3 (79) | TPs | 503 | 464 | 352 | ⊕⊝⊝⊝ Very Low1,2,3 |
| FNs | 27 | 66 | 178 | |||
| Specificity | 3 (69) | FPs | 94 | 71 | 47 | |
| TNs | 376 | 400 | 423 | |||
**Prevalence of included studies in this category
1Limitations in patient's enrolment, selection and final inclusion (ones with all available data) for at least half of the studies sufficient to rate down one level
2Studies done in very different settings with one study including half of the patients with HIV in Malawi, another including pregnant women in Finland, and the last study including blood disorder patients in Nigeria. Very high prevalence of ID in included studies
3There was unexplained heterogeneity probably due to the different conditions. Very different thresholds were used: one study used a 100 μg/L threshold, and two studies used 35 μg/L and 30 μg/L thresholds, respectively.
4There were very few studies and the variability was high.
Infants and children
Four studies assessed this age group (Aguilar 2012; Jonker 2013; Meira 2005; Phiri 2009). The first one studied 173 children of less than 60 months of age, attending the Manhic rural district hospital in Mozambique. Participants had infectious diseases (pneumonia, HIV‐related, and malaria), as well anaemia and malnutrition. Eighty per cent of the children had iron deficiency by bone marrow, 88% had an inflammatory process, and all had anaemia (66% moderate, 25% severe and 9% very severe). The best markers of iron deficiency included did not identify around a quarter of iron‐deficient children. A ferritin threshold equal to 30 µg/L, a sensitivity of 15%, specificity of 100%, and an area under the ROC curve equal to 0.70 was found. A mixed threshold of 12 µg/L (C‐reactive protein (CRP) lower than 10 mg/L) and 30 µg/L (CRP 10 mg/L or higher) was presented but the sensitivity found was of 11% with the same specificity 100%.
Jonker 2013 studied 237 anaemic (87% severely anaemic who were hospitalised) Malawian children of less than or equal to 60 months of age taken from a malaria endemic setting. Sixty per cent presented with malaria, 12.6% had bacteraemia, 7.7% were HIV infected, and 81.2% had CRP higher than 40 mg/L. At a ferritin threshold equal to 30 µg/L, a sensitivity of 41.7%, specificity of 98.1%, and an area under the ROC curve equal to.855 was found.
Meira 2005 studied eight anaemic children with HIV aged from 11 to 72 months who presented with peripheral cytopenias and were referred to the AIDS Children division of the city of Campinas, Brazil. On a one‐to‐one basis, there was no apparent correlation between iron in bone marrow, and serum ferritin measurements. At a ferritin threshold equal 30 µg/L, a sensitivity of 33%, and specificity of 100% was found.
Phiri 2009 studied 103 severely anaemic children aged less than 60 months of age who lived in a malaria‐endemic area of Malawi, with a mean age of 20 months. Sixty per cent of the participants had malaria, and around 90% had raised CRP when they were presented at the hospital. At a ferritin threshold equal to 273 µg/L, a sensitivity of 75%, specificity of 76%, and an area under the ROC curve equal to 0.82 was found.
Accuracy estimations of the test on the four studies of non‐healthy infants and children (519 participants) were computed using the HSROC framework and results are shown in Table 11. Since there was no common threshold used in the studies of this estimation, the result of the analysis yielded a sensitivity/specificity trade‐off that was simulated for specific values of **prevalence of iron deficiency in Table 11 in terms of the test's expected outcomes. For instance, for a sample of 1000 subjects with a 40% **prevalence of iron deficiency and supposing a 70% specificity (leading to a 75% sensitivity), it would be expected that 300 iron‐deficient subjects were correctly diagnosed, 100 iron‐deficient subjects incorrectly classified as not having iron deficiency, 420 subjects without iron deficiency correctly diagnosed, and 180 subjects without iron deficiency incorrectly classified as having iron deficiency. The estimated DOR was 7.3, a relatively low to moderate informative test. This result agreed with the findings of each of the four studies that found around a quarter of participants were misdiagnosed when using the thresholds recommended by the authors. The GRADE quality of evidence of these results is very low due to risk of bias, serious indirectness, inconsistency, and imprecision as shown in Table 11.
7. Estimates of accuracy of serum ferritin to assess iron deficiency for non‐healthy (infants and children).
| Patients/population | Non‐healthy (infants and children) less than 5 years old with infection/inflammation at risk of iron deficiency. | |||||
| Settings | Community, outpatient, and primary health care. | |||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | |||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay, etc. | |||||
| Reference standard | Aspirated bone marrow examination to identify the iron stores presence or absence on a Perl's (Prussian blue) stain. | |||||
| Importance | Avoid the challenges of an invasive, and occasionally risky bone marrow procedure, as well as elevated costs, and distress. | |||||
| Studies | 4 prospective/ambispective cohorts. | |||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 7.3 |
Certainty of Evidence
(CoE)
|
|||
| Effect per 1000 participants for **prevalence of iron deficiency 40% | ||||||
| Outcome |
*** Spec = 60% |
*** Spec = 70% |
*** Spec = 80% |
|||
| Sensitivity | 4 (207) | TPs | 355 | 300 | 193 | ⊕⊝⊝⊝ Very Low1,2,3,4 |
| FNs | 45 | 100 | 207 | |||
| Specificity | 4 (312) | FPs | 240 | 180 | 120 | |
| TNs | 360 | 420 | 480 | |||
**Prevalence of included studies in this category
1Limitations in patient's enrolment, selection and final inclusion (ones with all available data) for at least half of the studies sufficient to rate down one level
2The studies were done in different settings, and with very high severity of illness: three studies, in respective emergency hospitals in Mozambique and Malawi, included patients with malaria/TB and other infectious diseases, and with severe anaemia; one study included outpatients in a clinic in Malawi, and another study assessed only six outpatients of a hospital in Brazil with HIV and blood disorders. High prevalence of ID in most of the included studies
3 There was unexplained heterogeneity probably due to the different conditions. Very different thresholds were used: one study used a 273 μg/L threshold, and three studies used 30 μg/L.
4There were very few studies and the variability was high.
Adolescents
Ferritin as a marker of iron deficiency for fixed thresholds
Fixed threshold 30 µg/L for non‐healthy *adults
Nine studies assessed this group including 512 participants (Baumann Kurer 1995; Kotru 2004; Mast 1998; Nelson 1978; North 1997; Rao 1984; Sharma 1984; Thompson 1988; Van Zeben 1990) (see forest plot Extra Figure V). Patients (*adults, both sexes, wide range of ages) were from primary healthcare centres, outpatients, or were hospitalised. Illnesses that were presented within the nine studies were varied: three chronic or acute diseases, one rheumatoid arthritis, one blood disorder, one infectious disease, one alcoholism/liver cirrhosis/renal failure, and two mixed or unknown.
A pooled estimate of the specificity and sensitivity of serum ferritin as an index of iron deficiency for non‐healthy subjects using a fixed threshold of 30 µg/L was obtained using the bivariate model for the studies shown in Table 3. As shown in Table 3 and Extra Figure VI, the pooled estimate for sensitivity was 79% (95% CI: 58% to 91%) and specificity of 98% (95% CI: 91% to 100%). The corresponding pair estimate (sensitivity, specificity) implies that the serum ferritin test would detect 79 out of every 100 with disease but 21 would be missed (i.e. would be false negatives), and it would detect 98 out of every 100 without disease but two would be wrongly diagnosed as having it (i.e. would be false positives). For example, for a sample of 1000 subjects with a 40% **prevalence of iron deficiency and supposing the previous estimate (79%, 98%), it would be expected that 315 iron‐deficient subjects were correctly diagnosed, 85 iron‐deficient subjects incorrectly classified as not having iron deficiency, 588 subjects without iron deficiency correctly diagnosed, and 12 subjects without iron deficiency incorrectly classified as having iron deficiency. In this case, the serum ferritin test has high specificity, so it rules in iron deficiency, which means that if the result was positive, we would be almost sure the participant had iron deficiency. The estimated DOR was 140, a highly informative test. The GRADE certainty of evidence of these results is low due to risk of bias, and serious imprecision as shown in Table 3.
Summary of findings 3. Estimates of accuracy of serum ferritin to assess iron deficiency for non‐healthy (*adults): threshold = 30 µg/L.
| Patients/population | Non‐healthy *adults at risk of iron deficiency. | ||||
| Settings | Community, outpatient, primary health care. | ||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | ||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay. | ||||
| Reference standard | Aspirated bone marrow examination to identify the iron stores presence or absence on a Perl's (Prussian blue) stain. | ||||
| Importance | Avoid the challenges of an invasive, and occasionally risky bone marrow procedure, as well as elevated costs, and distress. | ||||
| Studies | Eight prospective/ambispective + 1 retrospective cohort. | ||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 139.9 |
Certainty of Evidence (CoE):
|
||
| Test summary pooled estimation (CI 95%) | Effect per 1000 participants for ID **prevalence of 40% | ||||
| Outcome | Specificity = 98% | ||||
| Sensitivity | 9 (203) | 79% (57.6, 90.9) |
TPs | 315 (230, 364) | ⊕⊕⊝⊝ Low1,2 |
| FNs | 85 (62, 99) | ||||
| Specificity | 9 (309) | 98% (90.6, 99.6) |
FPs | 12 (11, 12) | |
| TNs | 588 (543, 598) | ||||
*adults: This group is composed of adults excluding those studies focused exclusively on pregnant women.
**Prevalence of included studies in this category.
1Limitations in patient's enrolment, selection and final inclusion (ones with all available data) for at least half of the studies sufficient to rate down one level.
2The pooled‐estimate for the sensitivity confidence interval was relatively large, around 33%.
For other commonly used thresholds, estimations of accuracy were not possible due to the lack of a sufficient number studies for other categories.
Ferritin as a marker of iron deficiency for selected covariates
Non‐healthy *adults with or without the inflammation covariate
We compared studies of *adults with inflammation (32 studies with 2538 participants) versus studies of participants without inflammation (five studies with 269 participants), excluding 29 studies that were mixed/unknown concerning the inflammation covariate. We used the HSROC framework because of the variation in thresholds; the forest plot is shown in Extra Figure VII.
Three models were fitted: the first assuming different accuracy, shape and threshold parameters (mean DOR = 12.42), the second removing the covariate for a different shape (mean DOR = 17.33) and the last removing the covariate for a different shape and accuracy (mean DOR = 36.16). Whereas the SROC plot (Extra Figure VIII) did suggest a relationship, the statistical inferences did not yield a significant result (P = 0.2 for removal of the covariate for a different shape and P = 0.4 for removal of the covariate for a different shape and accuracy) but gave an indication in this direction (results are found in Table 12). The GRADE quality of evidence of this result is low due to risk of bias, and serious inconsistency (Table 12).
8. Heterogeneity analysis (covariate: inflammation): iron deficiency *adults.
| Patients/population | *Adults with and without inflammation at risk of iron deficiency. | ||||||
| Settings | Community, outpatient, hospital. | ||||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | ||||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay, etc. | ||||||
| Reference standard | Aspirated bone marrow examination to identify the iron stores presence or absence on a Perl's (Prussian blue) stain. | ||||||
| Importance | Avoid the challenges of an invasive, and occasionally risky bone marrow procedure, as well as elevated costs, and distress. | ||||||
| Studies | 32 studies (2538 participants) with inflammation and 5 studies (269 participants) without inflammation. | ||||||
| Between‐Study level Covariate: inflammation vs non‐inflammation association | |||||||
| HSROC Model and assumptions over parameters |
Mean DOR inflammation |
Mean DOR no inflammation |
Mean threshold parameter inflammation | Mean threshold parameter no inflammation |
P value
for difference in the fit of the model |
Certainty of Evidence
(CoE)
|
|
| Different accuracy, shape and threshold parameters | 0 | 12.42 | ‐0.72 | ‐0.07 | ⊕⊕⊝⊝ Low 1,2 | ||
| Same shape | 0 | 17.33 | ‐0.60 | ‐0.99 | 0.17 ( > 0.05 ) | (removal of covariate for different shape) | |
| Same shape and accuracy | 0 | 36.16 | ‐0.59 | ‐1.07 | 0.4 ( > 0.05) | (removal of covariate for different shape and accuracy) | |
*adults: This group was composed of adults excluding those studies focused exclusively on pregnant women.
1Limitations in patient's enrolment, selection and final inclusion (ones with all available data) for at least half of the studies sufficient to rate down one level
2We found there was unexplained heterogeneity, that probably caused the use of multiple thresholds (ranged between 12 to 200 µg/L).
Non‐healthy *adults by disease group and other covariates
Most included studies that focused on the diagnosis of iron deficiency related to specific conditions found in hospitalised patients, resulting in a very heterogenous set. Because of this, the analysis of other covariates seemed difficult in terms of the many possible confounders and the limited number of studies available for each subgroup.
We performed an analysis of the laboratory assessment method as a covariate for studies on non‐healthy subjects without significant results. The corresponding SROC plot is shown in Extra Figure IX.
As explained in the Differences between protocol and review section, we performed a subgroup analysis for the disease group covariate. In Table 13, we summarised the corresponding estimations per disease group that gave some insight into the heterogeneity of the non‐healthy *adults group. Fifteen studies with unknown/mixed diseases were not used in this analysis. All DOR estimates were produced using the HSROC model, with the exception of the infectious diseases groups composed of only two studies, where the bivariate model was used.
9. Iron deficiency for non‐healthy (*adults) ‐ by disease group.
| Disease group | N of studies | Estimated mean DOR | 95% LCI | 95% UCI |
| Alcoholism/liver cirrhosis/renal failure | 7 | 78.38 | 13.91 | 441.69 |
| Blood disorders (SS and/or SC or leukaemia) | 9 | 36.71 | 8.35 | 161.33 |
| Diverse chronic or acute diseases | 19 | 44.48 | 15.00 | 131.90 |
| Infectious diseases | 2 | 9.91 | 1.462 | 67.21 |
| Rheumatoid arthritis | 11 | 59.18 | 21.02 | 166.67 |
| All five disease groups of non‐healthy *adults | 48 | 52.30 | 30.36 | 90.1 |
*adults: This group was composed of adults excluding those studies focused exclusively on pregnant women.
15 studies with unknown/mixed diseases of the non‐healthy *adult group were left out of this analysis.
All DOR estimates have been produced using the HSROC model, with the exception of the infectious diseases groups composed of 2 studies where the bivariate model was used.
The subgroup analysis of the five disease subgroups yielded moderately informative tests for the following subgroups: alcoholism/liver cirrhosis/renal failure (DOR = 78, n = 7), diverse chronic or acute diseases (DOR = 44, n = 19) and rheumatoid arthritis (DOR = 59, n = 11). We found a relatively low to moderate informative test for the blood disorders subgroup (DOR = 37, n = 10), and a low informative test for the infectious diseases subgroup (DOR = 10, n = 2). Furthermore, the blood disorders subgroup had the largest confidence interval, and the infectious disease subgroup had only two studies and contained one (i.e. no informative test) within its corresponding confidence interval (see Table 13).
Ferritin as a marker of iron overload in apparently healthy populations
No study assessed this target population.
Ferritin as a marker of iron overload in non‐healthy populations
No study assessed infants, children, or adolescent populations.
All (one threshold for males and females)
Thirty‐six studies including 1927 participants assessed this group. Participants (adults, both sexes, wide range of ages) were generally outpatients or were hospitalised. Illnesses were varied and were classified into: haemochromatosis, alcoholism/liver cirrhosis/hepatic fibrosis, and mixed or unknown. Distribution details per category can be seen in the Results section in the risk of iron‐overload participants and target population description above.
Accuracy estimations of the test on the 36 studies of non‐healthy populations described above were computed using the HSROC framework and results are shown in Table 4. Since there was no common threshold used in the studies of this estimation, the result of the analysis yielded a sensitivity/specificity trade‐off that was simulated for specific values of **prevalence of iron deficiency in Table 4 in terms of the test's expected outcomes. For instance, for a sample of 1000 subjects with a 42% **prevalence of iron overload and supposing a 65% specificity (leading to a 80% sensitivity), it would be expected that 332 iron‐overloaded subjects were correctly diagnosed, 85 iron‐overloaded subjects incorrectly classified as not having iron overload, 379 subjects without iron overload correctly diagnosed, and 204 subjects without iron overload incorrectly classified as having iron overload. For this particular case, the negative predictive value was 82% i.e. 82% of negative serum ferritin test results would not have disease but 18% would (i.e. would be false negatives), and the positive predictive value was 62%: 62% of positive serum ferritin test results would actually have disease but 38% would not (i.e. would be false positives). The estimated DOR was 8, relatively low to moderate informative test. The GRADE certainty of evidence of these results is very low due to study design, very serious risk of bias, serious inconsistency, and indirectness, as shown in Table 4.
Summary of findings 4. Estimates of accuracy of serum ferritin to assess iron overload (all).
| Patients/population | Non‐healthy patients at risk of iron overload. | |||||
| Settings | Community, outpatient, primary health care. | |||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | |||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay. | |||||
| Reference standard | Liver biopsy test measured either using atomic absorption spectrophotometry, calorimetry, similar, or by using Perl's (Prussian blue) staining. | |||||
| Importance | Avoid the challenges of an invasive, and occasionally risky biopsy procedure, as well as elevated costs, and distress. | |||||
| Studies | 27 prospective/ambispective cohorts + 2 retrospective cohorts + 7 case‐controls1. | |||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 8.1 |
Certainty of Evidence (CoE):
|
|||
| Effect per 1000 participants for **prevalence of iron overload 42% | ||||||
| Outcome | Specificity = 65% | Specificity = 75% | Specificity= 85% | |||
| Sensitivity | 36 (803) | TPs | 332 | 304 | 258 | ⊕⊝⊝⊝ Very Low1,2,3,4 |
| FNs | 85 | 113 | 159 | |||
| Specificity | 36 (1124) | FPs | 204 | 146 | 87 | |
| TNs | 379 | 437 | 496 | |||
**Prevalence of included studies in this category.
1 Downgraded one level for study design. One‐third of the studies (n = 9) were case‐control or retrospective, and 20% (n = 7 studies) had only non‐healthy populations leading to a very high prevalence of included studies.
2 Downgraded one level for risk of bias due to patient's enrolment, flow, selection and final inclusion (ones with all available data) for at least half of the studies
3 Downgraded one level for indirectness. Around half of the studies (n = 18) focused on hereditary haemochromatosis, most of them on an outpatient basis. Very high prevalence of iron deficiency in participants in all included studies
4 We found there was unexplained heterogeneity.
Extra Figure X shows the summary ROC plot of the studies on iron overload. Statistical analysis has been done on the overall set but convergence was not achieved (perhaps because of high bias and many studies lay on the borders of the ROC); moreover, the interpretation of the results would have been compromised because of the applicability concerns of most of the studies.
All (threshold per sex: males/females)
Nine studies including 1292 participants assessed this group. Participants (adults, both sexes, with a wide range of ages) were generally outpatients were hospitalised. Illnesses were varied and were classified into: haemochromatosis, alcoholism/liver cirrhosis/hepatic fibrosis, and mixed or unknown. Distribution details per category can be seen in the Results section in the risk of iron‐overload participants and target population description above.
Accuracy estimations of the test of the nine studies of non‐healthy populations described above were computed using the HSROC framework and results are shown in Table 14. Since there was no common threshold used in the studies of this estimation, the result of the analysis yielded a sensitivity/specificity trade‐off that was simulated for specific values of **prevalence of iron deficiency in Table 14 in terms of the test's expected outcomes. For instance, for a sample of 1000 subjects with a 27% **prevalence of iron overload and supposing a 70% specificity (leading to a 76% sensitivity), it would be expected that 201 iron‐overloaded subjects were correctly diagnosed, 65 iron‐overloaded subjects incorrectly classified as not having iron overload, 514 subjects without iron overload correctly diagnosed, and 220 subjects without iron overload incorrectly classified as having iron overload. For this particular case, the negative predictive value was 89% i.e. 89% of negative serum ferritin test results would not have disease but 11% would (i.e. would be false negatives), and the positive predictive value was 48%: 48% of positive serum ferritin test results would actually have disease but 52% would not (i.e. would be false positives). The estimated DOR was 10, a relatively low to moderate informative test. The GRADE certainty of evidence of these results is very low due to study design, serious risk of bias, and indirectness, as shown in Table 14.
10. Estimates of accuracy of serum ferritin to assess iron overload (All: threshold per gender: males/females).
| Patients/population | Non‐healthy patients at risk of iron overload. | |||||
| Settings | Community, outpatient, primary health care. | |||||
| Prior testing | No prior test needed with concurrent testing of other iron and haematologic parameters or following measurement of haematologic indices. | |||||
| Index test | Serum or plasma ferritin concentration measured by any available quantitative assay: i.e. enzyme‐linked immunosorbent assay, radioimmunoassay, etc. | |||||
| Reference standard | Liver biopsy test measured either using atomic absorption spectrophotometry, calorimetry, similar, or by using Perl's (Prussian blue) staining. | |||||
| Importance | Avoid the challenges of an invasive, and occasionally risky biopsy procedure, as well as elevated costs, and distress. | |||||
| Studies | 5 prospective/ambispective cohorts + 1 retrosp cohort + 3 case‐controls1. | |||||
| Test Accuracy | # of studies (# of participants) | Mean DOR = 9.8 |
Certainty of Evidence
(CoE)
|
|||
| Effect per 1000 participants for **prevalence of iron overload 27% | ||||||
| Outcome | Specificity = 60% | Specificity = 70% | Specificity = 80% | |||
| Sensitivity | 9 (344) | TPs | 210 | 201 | 189 | ⊕⊝⊝⊝ Very Low1,2,3 |
| FNs | 56 | 65 | 77 | |||
| Specificity | 9 (948) | FPs | 294 | 220 | 147 | |
| TNs | 440 | 514 | 587 | |||
**Prevalence of included studies in this category
1Almost half of the studies (4/9) were case‐control or retrospective. For study design, we rated these down one level.
2Serious risk of bias due to patient's enrolment, flow, selection and final inclusion (ones with all available data) for at least half of the studies sufficient to rate down one level
3More than the half of the studies (5) focused on hereditary haemochromatosis, most of them on an outpatient basis. High prevalence of ID in all included studies
Comparisons of serum ferritin by bone marrow status (iron‐deplete or replete)
In order to look in more detail at the relationship between serum ferritin measures and the iron status of populations or groups of participants in this review, we developed tables containing the serum ferritin per iron status, measured as absence or presence of bone marrow (as stated by the authors of the studies) (see Tables 3.1 to 3.11 and 3.16 in Appendix 3).
We found differences in mean serum ferritin values between iron‐deficient/replete individuals in all comparable included studies. In the case of healthy individuals with iron deficiency, the mean ferritin was 15.2 μg/L (n = 46) versus 70.4 μg/L (n = 70) among iron‐replete individuals (three studies) (see Table 3.11 in Appendix 3). In addition, three studies (Milman 1983; Puolakka 1980; Sorbie 1975) showed a significant correlation between serum ferritin and bone marrow values (see Table 3.1 in Appendix 3).
In the case of non‐healthy individuals, the mean serum ferritin for iron deficiency was 66.9 μg/L (n = 1037) versus 317.2 μg/L (n = 1557) for iron‐replete individuals (37 studies), and within all disease groups with at least three studies per group, the mean serum ferritin differences ranged from 135 μg/L to 482 μg/L (see Table 3.11 in Appendix 3). Furthermore, 23 studies (Bârsan 2015; Baumann Kurer 1995; Brink 1982; Hansen 1983; Harju 1984; Intragumtornchai 1998; Joosten 2002; Kotru 2004; Means 1999; Milman 1983; Mulherin 1996; Nanas 2006; Nelson 1978; Oluboyede 1980; Puolakka 1980; Ravindran 2008; Sharma 1984; Shroff 1991; Sorbie 1975; Van den Broek 1998; Van Tellingen 2001; Witte 1986; Witte 1988) found a good Pearson’s correlation (r > 0.70) and/or significant correlation (P < 0.05), between serum ferritin and bone marrow values (see Tables 3.2 to 3.10 in Appendix 3).
Concerning the non‐healthy pregnant females, one study (Oluboyede 1980), with available mean serum ferritin concentrations, reported a value of 44.8 μg/L (n = 9) for iron‐deficient versus 129.7 μg/L (n = 13) for iron‐replete individuals, with a considerably low sample size for both groups. The three included studies in this category (Oluboyede 1980; Puolakka 1980; Van den Broek 1998) found a significant correlation (P < 0.05), between serum ferritin and bone marrow values (see Table 3.3 in Appendix 3).
The mean ferritin concentration in different pathologies varied widely between study categories and also showed a wide variation in dispersion measures depending on the study group. For blood disorders, the mean serum ferritin concentration for iron‐deficient individuals was 158.3 μg/L (n = 41) versus 510.3 μg/L (n = 85) for non‐iron‐deficient individuals (three studies) (see Table 3.11 in Appendix 3). Even though there was a difference of three times in ferritin concentration between replete and depleted individuals, a relative high variability in serum ferritin concentrations existed for both groups, especially in the iron‐deficient group where the relative number of false negatives was high leading to a poor sensitivity, as seen in six studies where the sensitivity was lower than 54% (Brown 1988; Coenen 1991; Forman 1980; Mast 2002; Rao 1984; Solomon 1981) (see Table 3.4 in Appendix 3).
For rheumatoid arthritis, the mean ferritin concentration for iron‐deficient individuals was 33.6 μg/L (n = 182) versus 168.9 μg/L (n = 184) for non‐iron‐deficient individuals (nine studies) (see Table 3.11 in Appendix 3). For this group, concentration was five times lower in iron‐deficient subjects compared to iron‐replete individuals. In addition, five studies found a significant correlation (P < 0.05), between serum ferritin and bone marrow values (Baumann Kurer 1995; Hansen 1983; Mulherin 1996; Ravindran 2008; Shroff 1991) (see Table 3.5 in Appendix 3).
For alcoholism, liver cirrhosis, renal failure or malignancies, the mean ferritin concentration for iron‐deficient individuals was 108.9 μg/L (n = 205) versus 392.6 μg/L (n = 368) for iron‐replete individuals (seven studies) (see Table 3.11 in Appendix 3). For this group, concentration was more than three times lower in iron‐deficient individuals compared to iron‐replete individuals. However, as in blood disorders, a relative high variability in serum ferritin concentrations for both groups was found, especially in the iron‐deficient group where the relative number of false negatives was high, leading to a poor sensitivity, as seen in four studies where the sensitivity was lower than 61% (Balaban 1993; Intragumtornchai 1998; Isa 1988; Kalantar‐Zadeh 1995). By contrast, in three studies, a significant correlation (P < 0.05) was found between serum ferritin and bone marrow values (Intragumtornchai 1998; Milman 1983; Nelson 1978) (see Table 3.4 in Appendix 3).
For diverse chronic diseases (mixed or unknown) in an elderly population (age > 62 years old), the mean ferritin concentration for iron‐deficient individuals was 38.9 μg/L (n = 133) versus 412.8 μg/L (n = 163) for iron‐replete individuals (five studies) (see Table 3.11 in Appendix 3). For this group, concentration was more than ten times lower in iron‐deficient individuals compared to iron‐replete individuals. In addition, in three studies, a significant correlation (P < 0.05) was found between serum ferritin and bone marrow values (Joosten 2002; Sharma 1984; Van Tellingen 2001) (see Table 3.8 in Appendix 3).
For diverse chronic diseases (mixed or unknown) in a not exclusively elderly population (age > 50 years or sample mean age > 50 years), the corresponding mean ferritin concentration for iron‐deficient individuals versus iron‐replete individuals was around five times lower in iron‐deficient participants compared to iron‐replete participants for both disease‐groups (see Tables 3.11 in Appendix 3). Furthermore, there was a relatively high variability in serum ferritin concentrations with a wide range of values for both groups, especially in the iron‐deficient individuals. Therefore, in nine of the 24 studies for both disease groups, there was a poor sensitivity (lower than 61%) (Baillie 2003; Lindstedt 1980; Lough 1989; Means 1999; Nanas 2006; North 1997; Punnonen 1998; Thompson 1988; Witte 1988). By contrast, in seven studies a significant correlation (P < 0.05) was found between serum ferritin and bone marrow values (Bârsan 2015; Brink 1982; Harju 1984; Means 1999; Nanas 2006; Sorbie 1975; Witte 1988) (see Tables 3.9 and 3.10 in Appendix 3).
A total of eight excluded studies (Hanif 2005; Jacobs 1972; Jacobs 1982; Leyland 1975; Lipschitz 1974; Luxton 1977; Vreugdenhil 1989; Vreugdenhil 1990b) were selected because they had valuable data comparing ferritin and bone marrow iron, even if they have not enough extractable data to be considered as included. They demonstrated a clear separation in ferritin values for depleted iron stores (bone marrow = absent) and replete iron stores (bone marrow = present). In populations with absent bone marrow iron, mean values ranged from 5 to 18.45 μg/L (n = 171 with range 1 to 105 μg/L) versus populations with replete iron stores where mean values ranged from 181.2 to 5464.2 μg/L (n = 192 with range 10 to 14,352 μg/L) (four studies: Hanif 2005; Leyland 1975; Lipschitz 1974;Luxton 1977). Due to the heterogeneity of the target population, the ranges overlapped. Furthermore, two studies (Lipschitz 1974, Luxton 1977) showed a moderate Pearson’s correlation between serum ferritin and bone marrow values (r = 0.53 and r = 0.64), and two studies (Vreugdenhil 1989, Vreugdenhil 1990b) indicated a “good correlation”, and a significant correlation (P < 0.002), respectively. In addition, we identified an unclear to high risk of bias in all articles accompanied with low concerns of applicability in relation to our review question (see Table 3.16 in Appendix 3).
These results are to be considered with caution, because of the scarcity of studies in healthy outpatient populations, whilst among non‐healthy populations there was considerable clinical heterogeneity because of the different sets of diseases/conditions in the target populations.
Comparisons of serum ferritin per liver concentration status (iron‐overloaded or not)
In order to look in more detail at the relationship between serum ferritin and iron overload, we compared ferritin measures to liver iron concentration (as reported by the corresponding authors) (see Tables 3.12 to 3.15 and 3.17 in Appendix 3).
There were differences in mean serum ferritin concentrations between non‐iron‐overload versus iron‐overload participants for all studies, including in each disease group. The non‐iron‐overload group had a mean ferritin concentration ranging from 219.6 to 1244.3 μg/L (n = 718 individuals) versus the iron‐overload group with mean ferritin concentrations varying from 492.6 to 1671.3 μg/L (25 studies, n = 717 individuals) (see Tables 3.12 to 3.15 in Appendix 3).
The mean ferritin concentration in different pathologies varied widely between study categories and also showed a wide variation in dispersion measures depending on the study group. For adult participants with haemochromatosis, the mean serum ferritin concentration for non‐iron‐overload individuals was 409.6 μg/L (n = 78) versus 908.7 μg/L (n = 192) for iron‐overload individuals (15 studies) (see Table 3.15 in Appendix 3). Even though there was more than two times the difference in ferritin concentration between non‐iron‐overload and iron‐overload individuals, a relative high variability in serum ferritin concentrations for both groups was found that was reflected in a poor sensitivity (lower than 51%) for four studies (Halliday 1977; Holmström 2002; Rowe 1977; Wands 1976), and poor specificity for two studies (Jensen 1994; Sham 1997) (see Table 3.12 in Appendix 3).
For adult participants with chronic hepatitis, alcoholic cirrhosis, alcoholic liver disease, and hepatic fibrosis, the mean ferritin concentration for non‐iron‐overloaded individuals was 219.6 μg/L (n = 629) versus 492.6 μg/L (n = 501) for iron‐overload individuals (six studies) (see Table 3.15 in Appendix 3). Although there was more than two times the difference in ferritin concentration between non‐iron‐overload and iron‐overload individuals, there was a relative high variability in serum ferritin concentrations for both groups that was reflected in a poor sensitivity (lower than 51%) for two studies (Sebastiani 2006; Sebastinani 2012), and poor specificity (lower than 51%) for one study (Sebastiani 2006) (see Table 3.13 in Appendix 3).
For adult participants with unknown and mixed diseases (haemochromatosis, chronic hepatitis, nonalcoholic fatty liver disease), the mean ferritin concentration for non‐iron‐overloaded individuals was 1244.3 μg/L (n = 11) versus 1671.3 μg/L (n = 24) for iron‐overload individuals (four studies) (see Table 3.15 in Appendix 3). Three of these studies (Harada 1992; Maliken 2012; Walsh 2006) presented a poor specificity (lower than 51%) (see Table 3.14 in Appendix 3).
Concerning the selected excluded studies with valuable data comparing ferritin and liver biopsy concentration, even if they have not enough extractable DTA data to be considered as included, results were variable. In Cecchin 1990, there was a clear division between individuals with iron overload (mean serum ferritin of 1015 μg/L) versus individuals without iron overload (mean serum ferritin of 137.1 μg/L). In Karam 2008, mean ferritin concentrations were lower (940 μg/L) in individuals without iron loading versus 3021.5 μg/L in those with iron loading. Kowdley 2012 found a significant difference (OR = 2.2 (1.78 to 2.76)) between serum ferritin and liver iron content comparing iron overload with non‐iron‐overloaded groups. Smith 2014 also found a clear differentiation between individuals with iron overload (mean serum ferritin of 3542.9 μg/L) versus individuals without iron overload (mean serum ferritin of 292 μg/L) (see Table 3.17 in Appendix 3).
Importantly, from a diagnostic test accuracy perspective, the quality of these studies was heterogeneous with a medium to high risk of bias and applicability concerns for Hellerbrand 2003 and Kowdley 2012, while there was a low risk of bias and no applicability concerns in Hofer 2004 and Karam 2008.
Sensitivity Analyses
In order to assess the sensitivity to the quality of the studies, we first made estimations by excluding from the analyses those studies considered as low quality, and we also made estimations excluding those labelled as 'retrospective'.
Concerning the sensitivity analysis to investigate the effects of the inclusion of diagnostic case‐control studies, only one case‐control study was included among the 70 studies of the non‐healthy All group, so analysis was not feasible.
We did not judge that it was useful to further investigate sensitivity for iron overload, since this would not change in any way the weakness of the conclusions. .
Excluding low‐quality studies
We conducted a sensitivity analysis based on the quality of the studies by removing all the low‐quality studies within the QUADAS‐2 table (Figure 4; Extra Figure I) based on the following classification.
On the one hand, we considered a study to be of low quality either if it was assessed as having a high risk of bias (four domains) or high concerns of applicability (three domains) in at least four of the seven possible domains (four risk of bias and three concerns of applicability) or with any high risk of bias or any high concern of applicability (indicated by the reviewers as low quality with proper justification). On the other hand, we considered a study to be of high quality if it was nor assessed as having high risk of bias (four domains) or high concerns of applicability (three domains) in any of the seven possible domains, it had at least seven out of 10 signalling questions with the answer “Yes”, and it was assessed as being of high quality by the reviewers. The studies that were not classified as high‐ or low‐quality were classified as having unclear quality.
By applying this classification, no low‐quality studies were found in the following groups:
ID healthy all
ID healthy *adults
ID non‐healthy pregnant women
But, there were low‐quality studies in the following target groups:
ID non‐healthy all: nine studies (Barron 2001;Brink 1982; Jonker 2013; Kim 2000; Lindstedt 1980; Meira 2005; Nanas 2006; Solomon 1981; Van Zeben 1990)
ID non‐healthy infants and children: two studies (Jonker 2013; Meira 2005)
ID non‐healthy adults: seven studies (same as ID non‐healthy All minus ID non‐healthy infants and children)
The infants and children’s estimations could not be carried out once the low‐quality studies were excluded since there would not be enough studies for the model to converge (the number of studies included decreased from four to two).
We then investigated sensitivity for the analyses concerning non‐healthy adults by re computing estimations, excluding the retrospective studies for the following analyses: ID non‐healthy adults cutoff = 30 µg/L, ID non‐healthy all (all thresholds), and ID non‐healthy *adults.
Excluding retrospective and unclear studies
We considered excluding retrospective and unclear studies with the following characteristics: retrospective, ambispective (judged more retrospective than prospective), and unclear studies.
There were no retrospective or unclear studies in the ID apparently healthy All, ID apparently healthy adults, ID non‐healthy pregnant women, and ID non‐healthy infants and children groups, and therefore there would be no change in the results for these groups. The following groups included retrospective studies:
ID non‐healthy all: seven studies (Baumann Kurer 1995, Brink 1982, Kis 1998, Krause 1980, North 1997, Punnonen 1998, Witte 1988)
ID non‐healthy *adults: seven studies (same as above)
We then investigated sensitivity for the analyses concerning non‐healthy *adults by re computing estimations, excluding the retrospective studies for the following analyses: ID non‐healthy *adults cutoff = 30 µg/L, ID non‐healthy all (all thresholds), and ID non‐healthy *adults (all thresholds).
Results
Iron deficiency cutoff 30 µg/L (bivariate model)
Initially, nine studies were included, from which we eliminated one study labelled as low‐quality in one case (Van Zeben 1990), and one study labelled as retrospective in the other (North 1997). We ran again a bivariate model using the remaining eight studies in each case (see additional Table 15).
11. Iron deficiency for non‐healthy (*adults): threshold = 30 µg/L (sensitivity analysis bivariate model results).
| Analysis | N (studies) | Sensitivity and 95% confidence Interval (%) |
Specificity and 95% confidence Interval (%) |
| Non‐healthy *adults | 9 | 78.7 (57.6, 90.9) | 98.0 (90.6, 99.6) |
| Non‐healthy *adults excluding low quality | 8 | 81.7 (65.0, 91.5) | 97.5 (91.6, 99.3) |
| Non‐healthy *adults excluding retrospectives | 8 | 78.24 (57.9, 90.4) | 96.92 (90.3, 99.1) |
*adults: This group was composed of adults excluding those studies focused exclusively on pregnant women.
Non‐healthy individuals (HSROC model)
Nine studies were excluded from the non‐healthy set as they were considered as low quality (Barron 2001, Brink 1982, Jonker 2013, Kim 2000, Lindstedt 1980, Meira 2005, Nanas 2006, Solomon 1981, Van Zeben 1990).
Seven studies were excluded from the non‐healthy set as they were considered as retrospective or unclear but mainly retrospective (Baumann Kurer 1995, Brink 1982, Kis 1998, Krause 1980, North 1997, Punnonen 1998, Witte 1988).
Seven studies were excluded from the non‐healthy adults set as they were considered as low quality (Barron 2001, Brink 1982, Kim 2000, Lindstedt 1980, Nanas 2006, Solomon 1981, Van Zeben 1990).
Seven studies were excluded from the non‐healthy adults set as they were considered as retrospective or unclear but mainly retrospective (Baumann Kurer 1995, Brink 1982, Kis 1998, Krause 1980, North 1997, Punnonen 1998, Witte 1988).
As we can observe from the additional tables, Table 15 and Table 16, the accuracy results did not vary considerably, either in the case of the estimation of the sensitivity and specificity of the studies using a 30 µg/L threshold, or in the case of the HSROC estimations.
12. Iron deficiency for non‐healthy (*adults) (sensitivity analyses HSROC).
| Analysis | N (studies) | Estimate of the mean DOR |
| Non‐healthy | 70 | 49.16 |
| Non‐healthy excluding low quality | 61 | 47.56 |
| Non‐healthy excluding retrospectives | 63 | 46.92 |
| Non‐healthy *adults | 63 | 49.80 |
| Non‐healthy *adults excluding low quality | 56 | 50.70 |
| Non‐healthy *adults excluding retrospectives | 56 | 51.28 |
*adults: This group was composed of adults excluding those studies focused exclusively on pregnant women.
We should note that the analyses where we had enough studies to undertake sensitivity analyses were the group of studies where we were able to present estimations worth being presented as findings and, that even though we excluded low‐quality studies, many included studies were considered of unclear quality.
Assessment of reporting bias
Iron deficiency
In order to analyse the publication bias within the included studies on iron deficiency (n = 72: with apparently healthy and non‐healthy participants) we used the Deeks regression funnel plot asymmetry test (Deeks 2005) that yielded a P of 0.044 (at a significance level α = 5% ) suggesting publication bias (see Figure 5).
5.
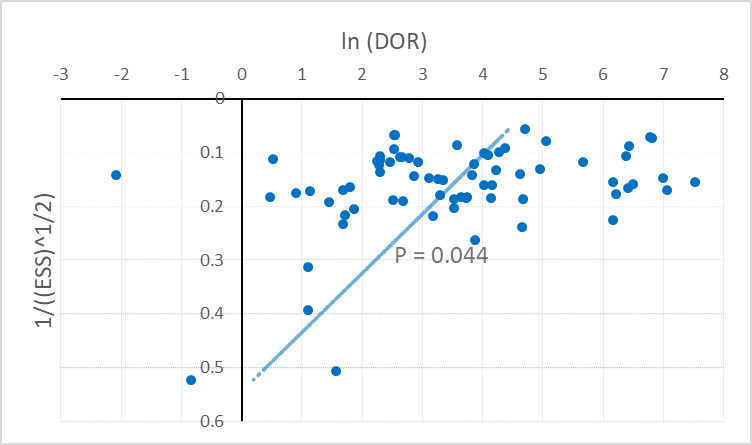
Funnel plot for all iron‐deficiency included studies (n = 72) and the corresponding effective sample size (ESS) weighted regression test of asymmetry
This result was partly influenced by the fact that the three studies with the lowest sample size happened to report a relatively low DOR (Meira 2005, Punnonen 1998, Solomon 1981); additionally there were some outliers with negative ln(DOR) (Karlsson 2010, Punnonen 1998). Most of these studies were also identified with a high risk of selection bias, and high concern in the three QUADAS domains: included participants and setting, index test, and the target condition as defined by the reference standard which did not match the review question (Barron 2001, Meira 2005, Solomon 1981).
Nevertheless, we found a relatively high DOR for the ensemble of studies (75% of studies had a DOR > 10), 70% of the studies had more non‐iron deficiency than iron deficiency subjects (more than half of them with a ratio 2:1), and there was heterogeneity between studies. All these reasons lower the power of the test and lessen our confidence on the result of the analysis. Because of this, we cannot confidently reach conclusions on the existence of publication bias.
Iron overload
Similarly, we performed the test with the same parameters for all included iron‐overload studies (36 studies). We obtained a P of 0.074, (see Figure 6), thus suggesting there was no evidence of publication bias for this set of studies.
6.

Funnel plot for all iron‐overload included studies (n = 36) and the corresponding effective sample size (ESS) weighted regression test of asymmetry
Discussion
Summary of main results
Our systematic review of ferritin thresholds to define iron deficiency and iron overload revealed very limited data to yield a reliable estimation of the accuracy of serum ferritin as a test for iron deficiency in otherwise healthy populations (i.e. without inflammation and comorbid disease), and essentially no data from applicable studies as a test for iron overload.
Iron deficiency
From the five studies in apparently healthy populations, the relative low DOR of 1.6 indicated an almost uninformative test. Moreover, there was no clear consensus on appropriate thresholds to detect iron deficiency (thresholds ranged from 16 µg/L to 70 µg/L).
Estimations for other target groups in this category were not possible due to the scarce number of included studies on children and infants, pregnant women, and *adults.
Non‐healthy *adults (n = 63) was the group with more included studies and the HSROC found an estimated DOR of 50 points, suggesting a moderately informative test. A lot of heterogeneity was suspected concerning the inflammation status and disease group.
Concerning the non‐healthy infants and children group (n = 4), the estimated DOR of 7.3 indicates a low to moderate informative test. Furthermore, three out four of the authors used a threshold of 30 µg/L. However, the GRADE judgement of very low‐quality as well as the use of a very high non‐prespecified threshold of 273 µg/L in Phiri 2009 diminished the validity of the estimate.
Similarly, for the group of non‐healthy pregnant women (n = 3), an estimated DOR of 58 was found indicating a moderately informative test but scarcity in the number of studies and a judgement of very‐low quality of the studies by GRADE diminishes the validity of the estimate.
The pooled estimates for the studies on non‐healthy *adults using a 30 µg/L threshold indicate a very specific test (sensitivity of 79% and specificity of 98% with DOR = 134), although the quality of the evidence was low and did not allow us to reach strong conclusions (see Table 3). No accuracy estimates have been pooled for thresholds of 12 µg/L or 15 µg/L since there were not enough studies available for each target population.
The analysis of inflammation as a covariant does point in the direction of different test accuracy for participants with and without inflammation, as would be expected from a priori knowledge of the biology, but again the evidence is limited by the quality of the studies and the inconclusive statistical inference.
the statistical inferences did not yield a significant result (P = 0.2 for removal of the covariate for a different shape and P = 0.4 for removal of the covariate for a different shape and accuracy) but gave an indication in this direction (results are found in Table 12). The GRADE quality of evidence of this result is low due to risk of bias, and serious inconsistency (Table 12).
Iron overload
Concerning iron overload, either for one threshold for males and females (36 studies) or one threshold per gender (nine studies), meta‐analyses found a low to moderate mean DOR of 8 and 10, respectively. For both cases, the very low quality of the studies by GRADE assessment diminishes the validity of the estimates.
It seems unlikely that a single serum ferritin threshold could discriminate with high accuracy between either iron‐replete/non‐iron‐overload versus iron‐deficient/iron‐overload participants across all disease groups. So, it is necessary to develop corresponding trade‐offs for each specific condition.
Strengths and weaknesses of the review
This is the first Cochrane diagnostic test accuracy review evaluating ferritin levels (measured in serum or plasma) for diagnosis of iron deficiency or iron overload.
The strengths of this review are as follows:
We undertook a very extensive search for relevant studies, including studies written in languages other than English.
We retrieved and screened a large number of potentially eligible studies, ensuring the relevant literature was included in the analysis.
We used the modified QUADAS‐2 tool to perform quality assessments of retrieved studies, and thus could summarise both the evidence and its quality; through these analyses, we have been able to show that the quality of evidence was very limited.
We adopted an approach to include a wide range of studies so that any relevant data, even if not collected in a formal diagnostic test accuracy study, could potentially be included in the review with appropriate determination of study quality. Because we included studies regardless of whether the participants belonged to a healthy or non‐healthy population, we were able to ensure our data encompassed the full spectrum of clinical settings in which ferritin may be used as a biomarker.
In the iron deficiency analysis, a suitable number of studies focused on non‐healthy adults and used a fixed threshold of 30 μg/L which allowed us to pool an estimate with sensitivity of 79% (with 95% CI of 58% to 91%), and specificity of 98% (with 95% CI of 91% to 100%). This allowed us to measure the corresponding accuracy of the serum ferritin test for this specific target group.
The limitations of this review are explained here below.
For iron deficiency analysis:
Although we identified a large number of studies comparing ferritin with bone marrow iron, we found relatively few studies undertaken in non‐inflamed, 'healthy', outpatient populations. Most studies were retrospective and/or based on recruitment not of participants, but of laboratory samples, and were undertaken in clinically unwell populations. This is important because ferritin is elevated in patients with inflammation (e.g. infection, cancer, autoimmune disorders) which distorts interpretation of the index test.
There were insufficient studies and available studies were too small to provide data in pregnancy and young children.
There were few studies undertaken either in low‐income countries or in malaria,TB or HIV infection endemic settings.
In the largest study, that was of high quality from a study design perspective, samples were measured many years after collection raising the likelihood of degradation of the samples, hindering interpretation of the results.
There was very low or low quality of evidence for most of the target groups. About half of the studies discussed more than one threshold but most of them provided data for the one they preferred. For 18% of the studies, we used the threshold proposed by the authors for coherence, since that reflected the available data, leading to more optimistic diagnostic accuracy estimates.
For iron‐overload analysis:
No diagnostic test accuracy studies undertaken in the population of interest were available and included studies had not been undertaken to address this specific set of questions. This limited subsequent analysis and estimation of appropriate thresholds to detect iron overload. Most studies were undertaken in populations which had been heavily preselected based on the index test (ferritin), or which were clinically inappropriate (e.g. patients with hepatitis, liver disease).
Definitions of iron overload (elevated liver iron or iron on Perl's staining of histologic samples of the liver) were defined differently between different authors. Where possible, we tried to use a single definition of iron loading (liver iron content > 1.8 mg/g dry weight or Perl's stain able to detect some iron in the liver '+'). This generated uncertainty in the reference standards being used in the studies.
There was very low quality of evidence.
In many of the iron deficiency and overload included studies, the source of funding was not explicitly disclosed or reported, indicating in many reports that funding was provided by internal sources or by competitive funding agencies. Thus, publication bias was unclear in most of the cases. The assessment of bias analysis did suggest a possible publication bias, but we need to be cautious and cannot strongly conclude this because of other sources of bias.
Applicability of findings to the review question
Although we undertook an extensive search of the literature and examined studies, we found that very few studies have assessed the diagnostic properties of ferritin to diagnose iron deficiency in otherwise apparently healthy populations, and no such studies exist for defining the properties and thresholds of ferritin to diagnose iron overload.
Our results for iron deficiency do not adequately indicate the most appropriate thresholds, but provide a rationale for the range of thresholds often used in practice (i.e. 15 to 30 µg/L) and insight into the accuracy of the test. In areas of widespread infection or inflammation, serum ferritin should be assessed with the concurrent measurement of two acute‐phase response proteins, since inflammation can distort the interpretation of ferritin as an index test. For iron overload, the picture is very unclear and our data do not advance understanding of the use of ferritin alone to diagnose this condition. Based on QUADAS‐2 analyses, all studies assessing iron overload have significant problems of quality and applicability. This is not surprising when considering that essentially none of these studies were designed for this purpose.
Authors' conclusions
Implications for practice.
Ferritin concentrations appear consistently lower in patients with iron deficiency compared with iron overload, and low ferritin concentrations therefore indicate iron deficiency. A ferritin threshold below 15 to 30 µg/L appears to indicate absent bone marrow iron stores in healthy populations, but there are too few studies to confidently recommend a particular threshold. Data in critical patient subgroups (e.g. pregnancy, children) are too sparse to be sure that the same broad conclusion holds in all these groups.
The sensitivity of ferritin for diagnosis of iron overload is around 80%, meaning that a negative test correctly excludes iron overload in most people. The specificity appears low, meaning that positive tests do not definitively indicate iron overload.
Implications for research.
We need diagnostic test accuracy studies to establish the accuracy and threshold value for ferritin. These studies should be designed to apply to clinically relevant populations and follow methodological standards described by the QUADAS‐2 checklist.
Likewise, we need appropriately conducted studies to confirm the diagnostic properties of ferritin in different populations.
History
Protocol first published: Issue 7, 2015
Acknowledgements
The World Health Organization and Lucero Lopez‐Perez, Ricardo X Martinez and Sant‐Rayn Pasricha retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
We would like to thank the Cochrane Tobacco Addiction Group for hosting the title and for support in the preparation of this review. We would like to thank Paul Good and the Cochrane Diagnostic Test Accuracy Review Unit for their support in the preparation of this review. As part of the pre‐publication editorial process, this review is commented on by external peer referees and we are grateful for their thoughtful feedback.
We would like to thank Ms Joanne Abbott (http://www.abbottinformation.co.uk/) for her technical support in the search strategy and updates of the searches in the electronic databases, and Ms Lucia Jimenez for her support to prepare the final version of this review.
Appendices
Appendix 1. Search strategies
CENTRAL, DARE, HTA (Cochrane Library)
#1 MeSH descriptor: [Iron Metabolism Disorders] explode all trees
#2 MeSH descriptor: [Iron, Dietary] this term only
#3 ((iron or ferrous* or ferric* or fe) near/5 (deficien* or overload* or excess*)):ti,ab,kw (Word variations)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Ferritins] explode all trees
#6 ferritin*:ti,ab,kw (Word variations)
#7 isoferritin*:ti,ab,kw (Word variations)
#8 #5 or #6 or #7
#9 MeSH descriptor: [Biological Markers] explode all trees
#10 marker*:ti,ab,kw (Word variations)
#11 biomarker*:ti,ab,kw (Word variations)
#12 screen*:ti,ab,kw (Word variations)
#13 detect*:ti,ab,kw (Word variations)
#14 accura*:ti,ab,kw (Word variations)
#15 predict*:ti,ab,kw (Word variations)
#16 diagnos*:ti,ab,kw (Word variations)
#17 MeSH descriptor: [Immunoassay] this term only
#18 (immunoassay* or assay*):ti,ab,kw (Word variations)
#19 immunoturbidimetric:ti,ab,kw (Word variations)
#20 immuno turbidimetric:ti,ab,kw (Word variations)
#21 MeSH descriptor: [Latex Fixation Tests] this term only
#22 latex agglutination:ti,ab,kw (Word variations)
#23 (SPECIFICITY or SPECIFICITIES):ti,ab,kw (Word variations)
#24 (SENSITIVITY or SENSITIVE or SENSITIVITIES):ti,ab,kw (Word variations)
#25 MeSH descriptor: [Sensitivity and Specificity] this term only
#26 MeSH descriptor: [Plasma] this term only
#27 plasma*:ti,ab,kw (Word variations)
#28 #25 and (#26 or #27)
#29 MeSH descriptor: [ROC Curve] this term only
#30 #29 and (#26 or #27)
#31 ROC:ab (Word variations)
#32 #31 and (#26 or #27)
#33 MeSH descriptor: [Area Under Curve] this term only
#34 AUC:ab (Word variations)
#35 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #28 or #30 or #32 or #33 or #34
#36 MeSH descriptor: [Ferritins] explode all trees and with qualifier(s): [Blood ‐ BL]
#37 #4 and #8 and (#35 or #36)
MEDLINE and MEDLINE in Progress (OVID)
1. exp Iron Metabolism Disorders/
2. Iron, Dietary/
3. ((iron or ferrous* or ferric* or fe) adj5 (deficien* or overload* or excess*)).tw.
4. or/1‐3
5. exp Ferritins/
6. ferritin*.tw.
7. isoferritin*.tw.
8. or/5‐7
9. exp Biological Markers/
10. marker*.tw.
11. biomarker*.tw.
12. screen*.tw.
13. detect*.tw.
14. accura*.tw.
15. predict*.tw.
16. diagnos$.ti,ab.
17. Immunoassay/
18. (immunoassay* or assay*).tw.
19. immunoturbidimetric.tw.
20. immuno turbidimetric.tw.
21. Latex Fixation Tests/
22. latex agglutination.tw.
23. (SPECIFICITY or SPECIFICITIES).tw.
24. (SENSITIVITY or SENSITIVE or SENSITIVITIES).tw.
25. "Sensitivity and Specificity"/ and (Plasma/ or plasma*.tw.)
26. ROC Curve/ and (Plasma/ or plasma*.tw.)
27. ROC.ab. and (Plasma/ or plasma*.tw.)
28. Area Under Curve/
29. AUC.ab.
30. or/9‐29
31. 4 and 8 and (30 or exp Ferritin/bl)
32. limit 31 to humans
Embase (OVID)
1. exp iron metabolism disorder/
2. iron intake/
3. ((iron or ferrous* or ferric* or fe) adj5 (deficien* or overload* or excess*)).tw.
4. or/1‐3
5. exp ferritin/
6. ferritin*.tw.
7. isoferritin*.tw.
8. or/5‐7
9. exp biological marker/
10. marker*.tw.
11. biomarker*.tw.
12. screen*.tw.
13. detect*.tw.
14. accura*.tw.
15. predict*.tw.
16. diagnos*.ti,ab.
17. immunoassay/
18. (immunoassay* or assay*).tw.
19. immunoturbidimetric.tw.
20. immuno turbidimetric.tw.
21. latex agglutination test/
22. latex agglutination.tw.
23. (SPECIFICITY or SPECIFICITIES).tw.
24. (SENSITIVITY or SENSITIVE or SENSITIVITIES).tw.
25. "Sensitivity and Specificity"/ and (Plasma/ or plasma*.tw.)
26. ROC Curve/ and (Plasma/ or plasma*.tw.)
27. ROC.ab. and (Plasma/ or plasma*.tw.)
28. Area Under Curve/
29. AUC.ab.
30. or/9‐29
31. 4 and 8 and 30
32. limit 31 to (human and embase)
CINAHL (EBSCO)
S35 S3 AND S7 AND S34
S34 (S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S27 OR S29 OR S31 OR S32 OR S33)
S33 AB area under curve
S32 AB AUC
S31 S26 AND S30
S30 AB ROC
S29 S26 AND S28
S28 (MH "ROC Curve")
S27 S23 AND S26
S26 S24 OR S25
S25 plasma
S24 (MH "Plasma")
S23 (MH "Sensitivity and Specificity")
S22 (SENSITIVITY or SENSITIVE or SENSITIVITIES)
S21 (SPECIFICITY or SPECIFICITIES)
S20 latex agglutination
S19 immuno turbidimetric
S18 immunoturbidimetric
S17 (immunoassay* or assay*)
S16 (MH "Immunoassay")
S15 AB diagnos* OR TI diagnos*
S14 predict*
S13 accura*
S12 detect*
S11 screen*
S10 biomarker*
S9 marker*
S8 (MH "Biological Markers+")
S7 S4 OR S5 OR S6
S6 isoferritin*
S5 ferritin*
S4 (MH "Ferritin")
S3 S1 OR S2
S2 ((iron or ferrous* or ferric* or fe) N5 (deficien* or overload* or excess*))
S1 (MH "Iron Metabolism Disorders+")
Web of Science (SCI, SSCI, CPCI & CRCI‐SSH)
#16 #15 AND #2 AND #1
DocType=All document types; Language=All languages;
#15 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3
DocType=All document types; Language=All languages;
#14 TS=(AUC or "area under curve")
DocType=All document types; Language=All languages;
#13 #12 AND #11
DocType=All document types; Language=All languages;
#12 TS=(ROC)
DocType=All document types; Language=All languages;
#11 TOPIC: (plasma*)
DocType=All document types; Language=All languages;
#10 TOPIC: ((SENSITIVITY or SENSITIVE or SENSITIVITIES))
DocType=All document types; Language=All languages;
#9 TOPIC: ((SPECIFICITY or SPECIFICITIES))
DocType=All document types; Language=All languages;
#8 TOPIC: (latex agglutination)
DocType=All document types; Language=All languages;
#7 TOPIC: (immunoturbidimetric or "immuno turbidimetric")
DocType=All document types; Language=All languages;
#6 TOPIC: ((immunoassay* or assay*))
DocType=All document types; Language=All languages;
#5 TOPIC: (detect* or accura* or predict* or diagnos*)
DocType=All document types; Language=All languages;
#4 TOPIC: (screen*)
DocType=All document types; Language=All languages;
#3 TOPIC: (marker* or biomarker*)
DocType=All document types; Language=All languages;
#2 TOPIC: (ferritin* or isoferritin*)
DocType=All document types; Language=All languages;
#1 TOPIC: (((iron or ferrous* or ferric* or fe) near/5 (deficien* or overload* or excess*)))
DocType=All document types; Language=All languages;
POPLINE and OpenGrey
(ferritin OR isoferritin) AND (iron* OR ferrous* OR ferric* OR fe) AND (deficien* OR overload* OR excess*) AND (marker* OR biomarker* OR screen* OR detect* OR accura* OR predict* OR diagnos* OR immunoassay* OR assay* OR immunoturbidimetric OR "immuno turbidimetric" OR "latex agglutination" OR SPECIFICITY OR (SPECIFICITIES OR SENSITIVITY OR SENSITIVE OR SENSITIVITIES AND plasma*))
TRoPHI and Bibliomap
1. Freetext: "marker*" OR "biomarker*" OR "screen*" OR "detect*" OR "accura*" OR "predict*" OR "diagnos*" OR "immunoassay*" OR "assay*" OR "immunoturbidimetric" OR "immuno turbidimetric" OR "latex agglutination" OR ("SPECIFICITY" OR "SPECIFICITIES" OR "SENSITIVITY "OR "SENSITIVE" OR "SENSITIVITIES" AND "plasma*")
2. Freetext: "ferritin" OR "isoferritin"
3. Freetext: "iron*" OR "ferrous*" OR "ferric*" OR "fe"
4. 1 AND 2 AND 3
IBECS, PAHO, WHOLIS, EMRO, AFRO and LILACS (BIRME)
ferritin$ or isoferritin$ [Words] and (iron or ferrous$ or ferric$ or fe) and (deficien$ or overload$ or excess$) [Words] and marker$ or biomarker$ or screen$ or detect$ or accura$ or predict$ or diagnos$ or immunoassay$ or assay$ or immunoturbidimetric or immuno turbidimetric or latex agglutination or SPECIFICITY or SPECIFICITIES or SENSITIVITY or SENSITIVE or SENSITIVITIES or ROC or AOC [Words]
SCIELO
(ferritin$ or isoferritin$) AND (iron or ferrous$ or ferric$ or fe) AND ((deficien$ or overload$ or excess$) and (marker$ or biomarker$ or screen$ or detect$ or accura$ or predict$ or diagnos$ or immunoassay$ or assay$ or immunoturbidimetric or immuno turbidimetric or latex agglutination or SPECIFICITY or SPECIFICITIES or SENSITIVITY or SENSITIVE or SENSITIVITIES or ROC or AOC))
WPRO, IMSEAR (GLOBAL INDEX MEDICUS)
(ferritin* or isoferritin*) AND (iron or ferrous* or ferric* or fe) AND ((deficien* or overload* or excess*) and (marker* or biomarker* or screen* or detect* or accura* or predict* or diagnos* or immunoassay* or assay* or immunoturbidimetric or immuno turbidimetric or latex agglutination or SPECIFICITY or SPECIFICITIES or SENSITIVITY or SENSITIVE or SENSITIVITIES or ROC or AOC))
INMED
ferritin or isoferritin AND iron or ferrous or ferric or fe AND marker or biomarker or screen or detect or accuracy or predict or diagnose or immunoassay or assay or immunoturbidimetric or immuno turbidimetric or latex agglutination or SPECIFICITY or SPECIFICITIES or SENSITIVITY or SENSITIVE or SENSITIVITIES or ROC or AOC
Native Health Research database
ferritin
Clinicaltrials.gov
"ferritin AND liver" and "ferritin AND bone marrow"
International Clinical Trials Registry Platform
"ferritin AND liver" and "ferritin AND bone marrow"
Appendix 2. QUADAS‐2 signalling questions for bias
| Domain | Yes | No | Unclear |
| Subjects selection | |||
| 1. Consecutive or random sample enrolled? | If the study specifically states that consecutive patients or a random sample was selected. | If the study clearly states that the selection of patients was not consecutive or random, or if this can be easily inferred from the design. | If not reported or cannot be determined. |
| 2. Was a case‐control design avoided? | If the study is cross‐sectional, prospective or retrospective using consecutive patients or a random selection. | If the study specifically states that it used a case‐control design or this can be easily inferred from the description. | If not reported or cannot be determined. |
| 3. Did the study avoid inappropriate exclusions? | If there are no subjects inappropriately excluded | If there is inappropriate exclusion (e.g. suspicious ferritin or bone marrow results) | If not reported or cannot be determined. |
| Applicability: Is there concern that the included patients do not match the review question? | |||
| Index test | |||
| 1. Were the index tests results interpreted without knowledge of the results of the reference standard? | If the study states that the ferritin concentration was performed independently from the assessment of bone marrow aspirates and liver biopsies OR the study specifically states that the interpretation of the index test was blinded to the reference test. | If the study clearly states that interpretation of the index test was not blinded to the reference test. | If not reported or cannot be determined. |
| 2. If a threshold was used, was it prespecified? | If the thresholds were prespecified. | If the thresholds were not prespecified. | If not reported or cannot be determined. |
| Applicability: Is there concern that the index test, its conduct, or interpretation differ from the review question? | |||
| Reference standard | |||
| 1. Is the reference standard likely to correctly classify the target condition? | If bone marrow aspirates were used to classify iron depletion and repletion AND liver biopsies were used to classify iron overload. | If other markers of iron status were used OR bone marrow aspirates and liver biopsies were not used. | If not reported or cannot be determined. |
| 2. Were the reference standard results interpreted without knowledge of the index test? | If the study clearly states that the index test was not used as part of the reference standard interpretation AND the assessment of the reference criteria was blinded to the index test result. | If the study clearly states that the index test was used as part of the reference criteria OR the assessment of the reference criteria was not blinded to the index test result. | If not reported or cannot be determined. |
| Applicability: Is there concern that the target condition as defined by the reference standard does not match the review question? | |||
| Flow and timing | |||
| 1. Was there an appropriate time interval between the index test and reference standard? | If the index test for each subject was obtained from the same sampling moment as the reference test. | If the index test for each subject was performed from different sampling moments than the reference test. (subclassify if the time interval between samplings varies widely). | If not reported or cannot be determined. |
| 2. Did all subjects receive a reference standard? | If all subjects (or a random subset) who received the index test were tested for the reference standard. | If not all subjects received a reference standard OR a nonrandom subset of subjects were evaluated by the reference test. | If not reported or cannot be determined. |
| 3. Did all subjects receive the same reference standard? | If bone marrow aspirates or liver biopsies were performed in all patients. | If other tests were performed OR bone marrow aspirates or liver biopsies were not performed in all patients. | It is not clear if bone marrow aspirates or liver biopsies were performed in all patients. |
| 4. Were all subjects included in the analysis? | If all the subjects enrolled were included in the analysis. | If not all the subjects enrolled were included in the analysis. | It is not clear if all subjects enrolled were included in the analysis. |
Appendix 3. Serum ferritin measures, correlations, and data description for diseased and non‐diseased groups per study
Here we present the corresponding serum ferritin measures and correlations per diseased and non‐diseased groups, as well as the data description for the 72 included studies of iron deficiency, 36 of iron overload, and 7 excluded but with important data in iron overload studies. The tables are divided by physiological status, age/state‐specific group, and disease group.
3.1 Serum ferritin measures per bone marrow status for healthy populations
| Studies | Study characteristics: Target population / Data Specifications / Serum Ferritin used threshold (SFth) | Bone marrow equal to 0 group (BM = 0) (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group (BM = 1+) (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Jonker 2014 | Healthy, both sexes, 6 to 66 months. Sens, spec, D+, and D‐ given for the authors proposed threshold. Sens and spec for other two alternative thresholds was given. SFth=18. |
32+11=43 | 19 | interquartile range:
12 to 36; median: 19 |
10+34=44 | r = 0.39 (Kendall rank r) | |||
| Hallberg 1993 | Healthy, 38‐year‐old women, and randomly selected from the population in Goteborg. TP, FN, FP, and TN given for the authors proposed threshold. Table of sens and spec for other 10 alternative thresholds was presented. SFth=16. |
52+17=69 | 12.5 (11.2) | geometric mean: 9.4 | 2+103=105 | 54.1 (40.5) | geometric mean: 44.1 | 41.6 | |
| Milman 1983 | Healthy, both sexes, aged from 20 to 90 years, Danish medical students randomly selected. PPV, NPV, D+, and D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=20. |
10+6=16 | 15 | 4 to 30 | 2+35=37 | 65 | 50 | significantly correlated (P < 0.001) | |
| Puolakka 1980 | Healthy, females, mean age of 26 years old, and in the second semester of pregnancy. Tables with D+, D‐, TP, FN, FP, and TN values for the authors proposed threshold. SFth=70. |
7+1=8 | geometric mean: 111 | 0+8=8 | geometric mean: 115 | significantly correlated (P < 0.05) | |||
| Sorbie 1975 | Healthy, both sexes, 17 to 30 years old, Canadian medical students selected randomly. TP, FN, FP, and TN extracted from figure, and tables given for the authors proposed unique threshold (among more than one threshold mentioned). SFth=40. |
7+0=7 | 18 | 7 to 38 | 1+12=13 | 92 | 59 to 170 | 74 | significantly correlated (P < 0.05) |
| *Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.2 Serum ferritin measures per bone marrow status for non‐healthy children
| Studies | Study characteristics: Target population / Data Specifications / Serum Ferritin used threshold (SFth) | Bone marrow equal to 0 group (BM = 0) (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group (BM = 1+) (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Aguilar 2012 | Non‐healthy with Infectious diseases (malaria, tuberculosis, and other), and anaemia, both sexes, infants 6 to 59 months, attending the MDH emergency department in a rural hospital. Sens, spec, TP, FN, FP, and TN given for WHO threshold value. Sens and spec presented for two alternative thresholds. SFth=30. |
21+117=138 | 0+35=35 | ||||||
| Jonker 2013 | Non‐healthy with high incidence of infectious diseases (malaria, tuberculosis, and other), and severely anaemic, both sexes, children from 6 to 60 months. Specificity, sensitivity, D+, and D‐ for WHO threshold. SFth=30. |
18+24=42 | 4+191=195 | ||||||
| Meira 2005 | Non‐healthy with HIV and blood disorders, both sexes, children from 6 to 59 months. Patient's data available to compute TP, FN, FP, and TN for WHO threshold. SFth=30. |
1+2=3 | 32.8 (15.2) | 17 to 47; median: 34.5 | 0+3=3 | 117.7 (89.1) | 51 to 219; median: (82.6) | 84.9 | |
| Phiri 2009 | Non‐healthy and severely anaemic children in high infection pressure area (malaria endemic), both sexes, children with mean age of 20 months. Specificity, sensitivity, TP, FN, FP, and TN given for WHO threshold value, and specificity, sensitivity, D+, D‐, and accuracy for authors proposed threshold. SFth=273. |
18+6=24 | 19+60=79 | ||||||
| *Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.3 Serum ferritin measures per bone marrow status for non‐healthy pregnant females
| Studies | Study characteristics: Target population / Data Specifications / Serum Ferritin used threshold (SFth) | Bone marrow equal to 0 group: B = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Oluboyede 1980 | Non‐healthy, pregnant females, blood disorders: haemoglobin SS or SC disease. TP, FN, FP, and TN extracted from figure for the authors proposed threshold (among more than one threshold mentioned). SFth=100. |
7+2=9 | 44.8 (2.7) | 5+8=13 | 129.7 | 84.9 | significantly correlated (P < 0.001) | ||
| Puolakka 1980 | Non‐healthy, pregnant females, before iron treatment in the second semester of pregnancy. Tables with D+, D‐, TP, FN, FP, and TN values for the authors proposed threshold (among more than one threshold mentioned). SFth=35. |
18+9=27 | geometric mean: 52 | 0+6=6 | geometric mean: 157 | significantly correlated (P < 0.05) | |||
| Van den Broek 1998 | Non‐healthy, pregnant females, almost half with HIV. Table with Sens, Spec, Acc, PPV, NPV, and LR for two thresholds: WHO and the authors proposed corresponding thresholds. SFth=30. |
39+4=43 | 7+43=50 | significantly correlated (P < 0.005) | |||||
| *Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.4 Serum ferritin measures per bone marrow status for non‐healthy *adults with blood disorders
| Studies | Study characteristics: Target population / Data Specifications / Serum Ferritin used threshold (SFth) | Bone marrow equal to 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Barron 2001 | Non‐healthy, both sexes, 50 years or older, with blood disorders. D+, and D‐, and tables given for the authors proposed threshold. Sens and spec given for two alternative thresholds. SFth=100. |
29+6=35 | 2 to 544; median: 29 | 0+0=0 | |||||
| Rao 1984 | Non‐healthy, both sexes, mixed age with 56.8 years as mean age, with blood disorders. D+, D‐, TP, TN values for the authors unique proposed threshold. SFth=30. |
5+12=17 | 112 | 15 to 1056 | 1+42=43 | 326 | 43 to 1821 | 214 | |
| Ruivard 2000 | Non‐healthy (mixed with healthy), both sexes, 60 years as mean age, with diverse chronic mainly blood disorders. Table with Sens, Spec, Acc, PPV, NPV, and LR for the authors proposed threshold (among more than one threshold mentioned). SFth=60. |
16+5=21 | 85 (165) | 1+32=33 | 778 (2012) | 693 | |||
| Witte 1986 | Non‐healthy, both sexes, 60 years as mean age, with blood disorders. Figure, D+, D‐ data for the authors unique proposed threshold. SFth=12. |
11+1=12 | 12 to 126 | 0+85=85 | 8 to 205 | r = 0.7752 | |||
| Brown 1988 | Non‐healthy, both sexes, mixed age with diverse chronic mainly blood disorders. Figure for the authors unique proposed threshold. SFth=15. |
1+7=8 | 12 to 100 | 0+51=51 | 20 to 1500 | ||||
| Forman 1980 | Non‐healthy, both sexes, mixed age with blood disorders. Figure, D+, D‐ data for the authors unique proposed threshold. SFth=15. |
4+7=11 | 4 to 500 | 4+38=42 | 11 to 850 | "good correlation" | |||
| Solomon 1981 | Non‐healthy, both sexes, 50 years and older with blood disorders. Patient's data available to compute TP, FN, FP, and TN at the authors unique proposed threshold. SFth=40. |
0+3=3 | 278 (212) | 136 to 522; median: 175 | 0+9=9 | 427 (418) | 45 to 1170; median: 238 | 149 | |
| Mast 2002 | Non‐healthy, both sexes, over 20 years old with blood disorders. Sens, Spec, PPV, NPV for the authors unique proposed threshold (among more than one threshold mentioned). SFth=50. |
12+16=28 | 3+47=50 | ||||||
| Coenen 1991 | Non‐healthy, both sexes, mixed age with blood disorders. Figure, D+, D‐ data for the authors proposed threshold (among more than one threshold mentioned). SFth=70. |
14+12=26 | 5+42=47 | ||||||
| *Adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. **Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.5 Serum ferritin measures per bone marrow status for non‐healthy *adults with rheumatoid arthritis
| Studies | Study characteristics: Target population / Data Specifications / Serum Ferritin used threshold (SFth) | Bone marrow 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Kim 2000 | Non‐healthy, both sexes, mixed age (mean 50.1 years old) with rheumatoid arthritis. Patient's data available to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=12. |
4+1=5 | 17.7 (19.5) | 1+12=13 | 72.2 (66.3) | 54.5 | correlated (P < 0.118) | ||
| Baumann Kurer 1995 | Non‐healthy, both sexes, mixed age (mean 57.5 years old) with rheumatoid arthritis. Sens, spec, D+, and D‐ for the authors unique proposed threshold. SFth=30. |
12+2=14 | interquartile range: 4 to 28; median: 12.5 | 3+28=31 | interquartile range: 46.5 to 165.5; median:99 | R2 = 0.721 (P < 0.0001) | |||
| Shroff 1991 | Non‐healthy, both sexes, 20 to 49 years old with rheumatoid arthritis. Sens, spec, D+, and D‐ for the authors unique proposed threshold. SFth=32. |
15+2=17 | 23.9 (11.5) | 2+11=13 | 69.9 (24.7) | 46 | r = 0.802 (P < 0.01) | ||
| Mulherin 1996 | Non‐healthy, both sexes, 50 years and older with rheumatoid arthritis. Sens, spec, D+, and D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=40. |
20+2=22 | 24 | 4 to 101 | 10+6=16 | 181 | 12 to 451 | 157 | significantly correlated (P < 0.0001) |
| Vreugdenhil 1990 | Non‐healthy, both sexes, 50 years and older (mean 63.4 years old) with rheumatoid arthritis. Sens, spec, PPV, D+, and D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=50. |
20+4=24 | 10 to 36; median: 17 | 3+17=20 | 10 to 246; median: 75.6 | ||||
| Hansen 1983 | Non‐healthy, both sexes, 50 years and older with rheumatoid arthritis. Table, figure, sens, spec, and D+, D‐ at the authors unique proposed threshold. SFth=60. |
12+2=14 | 29.9 (24.1) | 8 to 98; median: 22 | 2+14=16 | 189.7 (124.7) | 38 to 410; median 125 | 159.8 | r = 0.8358 |
| Nielsen 1990 | Non‐healthy, both sexes, 50 years and older with rheumatoid arthritis. Figure, D+, D‐ for the authors unique proposed threshold. SFth=60. |
19+5=24 | 51.6 (57.7) | 1+10=11 | 257.4 (228.1) | 205.8 | |||
| Suominen 2000 | Non‐healthy, both sexes, mixed age (26 to 79 years old) with rheumatoid arthritis. Table, figure, and D+, D‐ for the authors unique proposed threshold. SFth=60. |
11+1=12 | 24.5 (16.7) | 10 to 70 | 8+9=17 | 120.1 (123.4) | 20 to 200 | 95.6 | |
| Porter 1994 | Non‐healthy, both sexes, 50 years and older (mean age 58 years old) with rheumatoid arthritis. Sens, spec, D+, D+ for the authors proposed threshold Table of sens, and spec given for other 6 alternative thresholds. SFth=75. |
45+4=49 | 29 (33) | 7+45=52 | 95 (37) | 66 | |||
| Ravindran 2008 | Non‐healthy, both sexes, mixed age (mean age 56 years old) with rheumatoid arthritis. Sens, spec, D+, D+, LR for the authors proposed threshold (among more than one threshold mentioned). SFth=82. |
17+1=18 | 57.9 (20.1) | 0+32=32 | 201.5 (104.6) | 143.6 | iron‐depleted r = ‐0.09 (P = 0.57) iron‐repleted r = 0.79 (P < 0.0001) | ||
| Smith 1977 | Non‐healthy, both sexes, mixed age (mean age 55.3 years old) with rheumatoid arthritis. Figure, and D+, D‐, for the authors unique proposed threshold. SFth=100. |
21+0=21 | 43.6 | 0+14=14 | 333 | 289.4 | "poor correlation" | ||
| *adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. **Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.6 Serum ferritin measures per bone marrow status for non‐healthy *adults with Infectious diseases
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) | Bone marrow equal to 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Lewis 2007 | Non‐healthy, both sexes, 20 to 49 years old with HIV. Table with Sens, Spec, Acc, PPV, NPV, and LRs for the authors proposed threshold. Sens, and spec, given for two alternative thresholds. SFth= 150. |
2+8=10 | 4+50=54 | ||||||
| Kotru 2004 | Non‐healthy, both sexes, mixed age with infectious diseases. Table with Sens, Spec, PPV, and NPV for the authors unique proposed threshold. SFth=30. |
35+4=39 | 17.7 (16.7) | 10 to 100 | 4+12=16 | 91.9 (104.1) | 20 to 230 | 74.2 | significantly correlated (P < 0.012) |
| *Adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. **Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.7 Serum ferritin measures per bone marrow status for non‐healthy *adults with alcoholism, liver cirrhosis, renal failure or malignancies
| Studies | Study characteristics: target pospulation / data pecifications / serum ferritin used threshold (SFth) | Bone marrow equal to 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Balaban 1993 | Non‐healthy, males, 50 and older or mixed with several diseases, mainly malignancies (carcinoma, lymphoma, myeloma), and with chronic infections around 10% of the total. Only a few of the total were anaemic too. Sens, Spec, PPV, NPV, Figure, and D+, D‐ for the authors unique proposed threshold. SFth=70. |
17+11=28 | 63.7 | 22 to 258 | 9+83=92 | 212 | 31 to 2000 | 148.3 | r = 0.58 (P < 0.001) |
| Intragumtornchai 1998 | Non‐healthy, both sexes, mixed age (> 15 years) with alcoholism or liver cirrhosis or renal failure. Figure, and D+, D‐ for the authors unique proposed threshold. SFth=50. |
15+13=28 | 169.6 (324.4) | 1+41=42 | 682.1 (508.6) | 512.5 | significantly correlated (P < 0.00007) | ||
| Isa 1988 | Non‐healthy (mixed with healthy), both sexes, mixed age (30 to 69 years old) with alcoholism, liver cirrhosis or renal failure. Figure, and D+, D‐ for the authors unique proposed threshold. SFth=50. |
0+8=8 | 367.5 | 0+34=34 | 469 | 101.5 | |||
| Krause 1980 | Non‐healthy, both sexes, mixed age with diverse chronic diseases: iron deficiency, liver/renal disease, malignancies, and chronic inflammation. Figure, and D+, D‐ for the authors unique proposed threshold. SFth=20. |
87+17=104 | 17.7 (32.9) | 2 to 165; median: 7 | 0+89=89 | 90.0 (140.6) | 50 to 450; median:85 | 72.4 | |
| Kalantar‐Zadeh 1995 | Non‐healthy, both sexes, mixed age (44 to 84 years) with alcoholism or liver cirrhosis or renal failure. Sens, Spec, figure, and D+, D‐ for the authors unique proposed threshold. SFth=200. |
5+5=10 | 83.0 (9) | 2+13=15 | 795.0 | 712 | iron‐repleted significantly correlated (spearman rank) | ||
| Milman 1983 | Non‐healthy (mixed with healthy), both sexes, mixed age with alcoholism, liver cirrhosis or renal failure. PPV, NPV, D+, and D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=60. |
14+1=15 | 23.7 | 1+34=35 | 158.2 | 134.5 | significantly correlated (P < 0.001) | ||
| Nelson 1978 | Non‐healthy, males, mixed age with chronic inflammatory conditions including alcohol‐related conditions, liver disease, and malignancies. Figure, and D+, D‐ for the authors unique proposed threshold. SFth=30. |
11+1~11** | 37.2 (85.5) | 4 to 280; median: 9 | 1+60=61 | 341.7 (324.2) | 20 to 1500; median:260 | 304.5 | r = 0.75 (P < 0.00005) |
| *Adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. **Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.8 Serum ferritin measures per bone marrow status for non‐healthy *adults with diverse chronic diseases, mixed or unknown in elderly population (age > 62 years old)
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) | Bone marrow equal to 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Guyatt 1990 | Non‐healthy, both sexes, 65 years and older with diverse chronic diseases, mixed, or unknown. Sens, Spec, LR, Figure, and D+, D‐ for the authors proposed threshold. Alternative two thresholds were presented in with spec, and sens respectively. SFth=45. |
70+15=85 | 40+110=150 | ||||||
| Holyoake 1993 | Non‐healthy, both sexes, women with mean age 82 years and men with mean age 79 years (range between 62 and 101 years old) with diverse chronic diseases, mixed, or unknown. TP,TN, D+, D‐ for the authors unique proposed threshold. SFth=45. |
27+0=27 | 5+0=5 | ||||||
| Joosten 2002 | Non‐healthy, both sexes, mean age 83 years old with diverse chronic diseases, mixed, or unknown. Table, Figure, D+, D‐ for the authors unique proposed threshold. SFth=50. |
32+2=34 | 23 | 2+36=38 | 473.6 | 450.6 | r = 0.71 (P < 0.0001) | ||
| Karlsson 2010 | Non‐healthy, both sexes, 62 years and older with diverse chronic diseases. Sens, Spec, Accuracy, D+, D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=40. |
0+27=27 | 3+20=23 | ||||||
| Karlsson 2015 | Non‐healthy, both sexes, 65 years and older with diverse chronic diseases, and anaemia. Sens, Spec, Accuracy, D+, D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=41.5. |
8+3=11 | 65 (75) | 0+20=20 | 535 (856) | 470 | |||
| Kis 1998 | Non‐healthy, males, 68 years and older with diverse chronic diseases, mixed, or unknown. Sens, Spec, PPV, NPV, D+, D‐ for the authors proposed threshold. Another four thresholds were presented in an ROC figure. SFth=100. |
27+14=41 | 53.0 | 9 to 518 | 2+58=60 | 397.5 | 1 to 5811 | 344.5 | "good correlation" |
| Martin‐Cabrera 2015 | III, both sexes,mean age 74.5 years old with diverse chronic diseases. Figure, D+, D‐ for a WHO threshold. SFth=15. |
5+2=7 | 3+41=44 | ||||||
| Patterson 1985 | Non‐healthy, both sexes, 64 years and older with diverse chronic diseases mixed, or unknown. Sens, Spec, PPV, NPV, Accuracy, D+, D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=18. |
3+6=9 | 1+54=55 | ||||||
| Sharma 1984 | Non‐healthy, both sexes, 65 years and older with diverse chronic diseases mixed, or unknown. Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=30. |
19+6=25 | 18.7 (23.7) | 5 to 115; median:11 | 0+10=10 | 213.6 (179.0) | 35 to 525; median:95 | 194.9 | r = 0.737 |
| Van Tellingen 2001 | Non‐healthy, both sexes, mean age 70 years old with diverse chronic diseases. Sens, Spec, and D+, D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=32. |
19+5=24 | 35.0 (51) | 1+37=38 | 444.3 (300.5) | 409.3 | significantly correlated (P < 0.001) | ||
| *Adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. **Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.9 Serum ferritin measures per bone marrow status for non‐healthy *adults with diverse chronic diseases, mixed or unknown strictly over 50 years old (or mean age > 50 years, but not strictly older persons).
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) | Bone marrow equal to 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Baillie 2003 | Non‐healthy, both sexes, aged from 30 to 89 years old with at least a 6‐month history of chronic disease, and anaemia (ACD). Sens, Spec, Accuracy, D+, D‐ for the authors proposed unique threshold. SFth=12. |
0+11=11 | 112 | 26 to 261 | 0+27=27 | 209.0 | 50 to 1500 | 97 | |
| Bârsan 2015 | Non‐healthy, both sexes, median age 64.5 years (from 30 to 90 years old), with chronic kidney disease, most of them with anaemia: 26 with iron‐deficiency anaemia, 21 anaemia of chronic disease, and 7 without anaemia. Sens, Spec, Figure, and D+, D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=166. |
20+6=26 | 60 to 156; median:96 | 7+21=28 | 166 to 493; median:256 | significantly correlated (P < 0.01) | |||
| Chang 2007 | Non‐healthy, patients with several chronic diseases, and blood disorders with normal sTfR (< 2 mg/L). Sens, Spec, D+, D‐, and Table for the authors proposed threshold (among more than one threshold mentioned). SFth=100. |
12+2=14 | 13+49=62 | 5th to 95th percentiles: 56 to 2330; median:451 | |||||
| Lindstedt 1980 | Non‐healthy, both sexes, mean age 62 years (from 18 to 94 years old), and several diseases, specially iron‐deficiency anaemia. Sens, Spec, Figure and Table for the authors proposed threshold. Alternative three thresholds were presented in a Table with spec, and sens, respectively. SFth=40. |
20+13=33 | 81.3 (93.6) | 2 to 357; median 47 | 6+54=60 | 672.9 (1490.6) | 50 to 8150; median:262.5 | 591.6 | r = 0.3737 "poor correlation" |
| Nanas 2006 | Non‐healthy, both sexes, 50 years and older at the end stage of heart failure. TP, TN, D+, D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=17. |
2+25=27 | 75.3 (59.1) | 0+10=10 | 211.9 (99.9) | 136.6 | significantly correlated (P < 0.00001) | ||
| Punnonen 1997 | Non‐healthy, both sexes, 50 years and older with several diseases and anaemia. FP, FN, TP, TN, Sens, Spec, PPV, NPV, D+, D‐, and Table for the authors proposed threshold (among more than one threshold mentioned). SFth=41. |
59+6=65 | 21 (55) | 1+63=64 | 342 (385) | 321 | |||
| Punnonen 1994 | Non‐healthy, both sexes, 50 years and older with several diseases and anaemia. Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=25. |
19+0=19 | 9 (6) | 1+16=17 | 288 (274) |
279 | |||
| Terrovitis 2011 | Non‐healthy, both sexes, 50 years and older with anaemia and heart failure. Sens, Spec, PPV, D+, D‐ for the authors proposed threshold (among more than one threshold mentioned). SFth=150.5. |
61+14=75 | 6+22=28 | ||||||
| Thompson 1988 | Non‐healthy, both sexes, 50 years and older with diverse chronic diseases. Sens, Spec, D+, D‐, and Table for the authors proposed threshold (among more than one threshold mentioned). SFth=30. |
3+2=5 | 0+35=35 | ||||||
| Van Zeben 1990 | Non‐healthy, both sexes, mixed age (females with mean age 63 years, males with mean age 56 years) with diverse chronic diseases. Sens, Spec, Figure, D+, D‐ for the authors unique proposed threshold. SFth=30. |
65+7=72 | 22.5 (47.3) | 5 to 300; median 12 | 0+32=32 | 154.6 (144.2) | 32 to 670; median:110 | 132.1 | |
| *Adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. **Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.10 Serum ferritin measures per bone marrow status for non‐healthy *adults with diverse chronic diseases, or mixed/unknown diseases but not strictly 50 years or older
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) | Bone marrow equal to 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| TP+FN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | FP+TN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Ali 1978 | Non‐healthy, both sexes, mixed age with common diseases in a hospital. Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=12. |
49+20=69 | 10.3 (10.2) | 3 to 61; median:7 | 0+179=179 | 259.6 (211.1) | 12 to 1200; median:175 | 249.4 | r = 0.60 |
| Brink 1982 | Non‐healthy, both sexes, mixed age with several diseases. D+, D‐, and organigram for the authors unique proposed threshold. SFth=99. |
46+19=65 | 54+281=335 | significantly correlated (P < 0.000001) | |||||
| Burns 1990 | Non‐healthy, males, mixed age with several diseases and anaemic. Sens, Spec, Accuracy, PPV, NPV, D+, D‐ at the authors proposed threshold. Sens, and Spec, given for three alternative thresholds. SFth=50. |
16+5=21 | 20+116=136 | ||||||
| Harju 1984 | Non‐healthy, both sexes, mixed age with digestive chronic diseases (gastritis and ulcers). Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=20. |
62+12=74 | 19.5 (36.4) | 4 to 300; median:10 | 3+46~52** | 134.9 (95.5) | 4 to 372; median:118 | 115.5 | r = 0.701 "good correlation" |
| Mazza 1978 | Non‐healthy, both sexes, mixed age with several diseases. Sens, Spec, Accuracy, PPV, NPV, D+, D‐, Table and Figure for the authors proposed threshold (among more than one threshold mentioned). SFth=18 |
11+4=15 | 25.3 (46.7) | 2 to 220; median:8 | 4+67=71 | 404.2 (544.5) | 9 to 2700; median:220 | 378.8 | |
| Mast 1998 | Non‐healthy, both sexes, 20 to 49 years old with several diseases and anaemia. Sens, Spec, PPV, NPV, D+, D‐, and Table for the authors proposed threshold, and an alternative WHO threshold. SFth=30. |
5+0=5 | 24.6 (11.1) | 9 to 37; median:28 | 1+48=49 | 1769.4 (2793.9) | 8 to 14000; median:736 | 1744.8 | |
| Punnonen 1998 | Non‐healthy, females, mixed age who may have a disease except anaemia. Patient's data extracted from figure to compute TP, FN, FP, and TN the authors unique proposed threshold. SFth=12. |
3+7=10 | 36.5 (21.3) | 8 to 75; median:37.5 | 0+0=0 | ||||
| Sorbie 1975 | Non‐healthy (mixed with healthy), both sexes, 18 to 72 years old with diverse chronic diseases. Figure, and tables given for the authors proposed threshold where TP, FN, FP, and TN could be extracted (among more than one threshold mentioned). SFth=40. |
12+0=12 | 12.3 | 1+21=22 | 297.3 | 285.0 | significantly correlated (P < 0.05) | ||
| Witte 1988 | Non‐healthy, both sexes, mixed age with several diseases. PPV, NPV, D+, D‐, Table and Figure for the authors unique proposed threshold specified threshold. SFth=12. |
3+6=9 | 27.6 (35.5) | 2 to 118; median:20 | 0+34=34 | 135 (37) | median:154 | 107.5 | r = 0.775 "good correlation" |
| Ong 2005 | Non‐healthy, both sexes, range 13 to 90 years old (median age 63 years) with several diseases. Sens, Spec, PPV, L+, D+, D‐, and Table for the authors proposed threshold. Table given for two alternative thresholds. SFth=60. |
17+8=25 | 1+32=33 | ||||||
| Lough 1989 | Non‐healthy (mixed with healthy), both sexes, mixed age with several diseases. Sens, Spec, Accuracy, PPV, and Table at the authors proposed threshold. Table given for two alternative thresholds. SFth=10. |
25+78=103 | 1+343=344 | ||||||
| Nadeem 2011 | Non‐healthy (mixed with healthy), both sexes, mixed age with diverse chronic diseases. Sens, Spec, PPV, NPV, D+, D‐, and Table for the authors proposed threshold (among more than one threshold mentioned). SFth=150. |
40+7=47 | 12+21=33 | ||||||
| Means 1999 | Non‐healthy, both sexes, mixed age (mean 48 years) with several diseases (HIV, infections, leukaemia, and several chronic diseases) and anaemia. Sens, Spec, D+, D‐, and Table for the authors proposed threshold (among more than one threshold mentioned). SFth=25. |
10+14=24 | 937 (3006) | 6 to 14600; median:42 | 36+85=121 | 1433 (2262) | 18 to 13775; median:404 | 496 | significantly correlated (P < 0.001) |
| North 1997 | Non‐healthy, both sexes, mixed age with several diseases (frozen specimens). TP, TN, D+, D‐, and table for the authors unique proposed threshold based on assay's manufacturer recommendation. SFth=30. |
7+7=14 | 0+32=32 | ||||||
| *Adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. **Iron‐depleted sample size (n1) could be different to the sum of TP + FN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐repleted sample size (n2) and the corresponding sum FP + TN. | |||||||||
3.11 Mean serum ferritin for healthy and non‐healthy adults iron deficiency and non‐iron deficiency groups, by disease/condition
| Disease/Condition | Mean serum ferritin for iron‐deficient individuals (μg/L) | Mean serum ferritin for non‐iron‐deficient individuals (μg/L) | Means difference (μg/L) | Studies sample size (with available mean serum ferritin values for iron deficiency and non‐iron‐deficient groups) | Sample size (n) for iron‐deficient individuals | Sample size (n) for non‐iron‐deficiency individuals |
| Non‐healthy *adults with blood disorders | 158.3 | 510.3 | 352 | 3 | 41 | 85 |
| Non‐healthy *adults with rheumatoid arthritis iron deficiency | 33.6 | 168.9 | 135.3 | 9 | 182 | 184 |
| Non‐healthy *adults with Infectious diseases | 17.7 | 91.9 | 74.2 | 1 | 39 | 16 |
| Non‐healthy *adults with alcoholism or liver cirrhosis or renal failure or malignancies | 108.9 | 392.6 | 283.7 | 7 | 205 | 368 |
| Non‐healthy *adults (older adults population mean age > 62 years old) with diverse chronic diseases, mixed or unknown | 38.9 | 412.8 | 373.9 | 5 | 135 | 166 |
| Non‐healthy *adults (50 years or older or mean age > 50 years, but not older persons) with diverse chronic diseases, mixed or unknown | 53.5 | 313.1 | 259.5 | 6 | 227 | 210 |
| Non‐healthy *adults with diverse chronic diseases, or mixed/unknown | 150.9 | 633.3 | 482.4 | 7 | 208 | 528 |
| All non‐healthy adults | 66.871 | 317.195 | 250.3 | 37 | 1037 | 1557 |
| All healthy adults | 15.167 | 70.367 | 55.2 | 3 | 46 | 70 |
| *Adults: this group was composed of adults excluding those studies focused exclusively on pregnant women. | ||||||
3.12 Serum ferritin measures per liver iron concentration status in non‐healthy adults with haemochromatosis
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) / Iron‐overload threshold mg/g dry liver weight (dLw) or Perl's staining (Ps) grade: 0, 1, etc. |
Non‐iron‐overload group: Non‐iron‐overloaded
(liver iron concentration equal or lower than author's threshold, or Perl's staining author's threshold) |
Iron‐overload group: IO
(liver iron concentration higher than author's threshold, or Perl's staining author's threshold) |
Non‐IO vs IO comparison | Serum ferritin vs reference standard association | ||||
| FP+TN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | TP+FN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐overload group mean minus the non‐iron‐overload group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| **Halliday 1977 | Non‐healthy patients within families with hereditary haemochromatosis (mean age: 41.1 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors proposed threshold among more than 1 threshold. SFth=500. dLw=1.8. |
2+3=5 | 347 (317) | 40 to 709; median:180 | 4+8=12 | 597.1 (551.2) | 56 to 1460; median:312 | 250.1 | r = 0.63 |
| Holmström 2002 | Non‐healthy patients with hereditary haemochromatosis (mean age: 47.7 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. Ps=Grade 1 or more. |
0+1=1 | 364 | 3+3=6 | 411.2 (202.6) | 205 to 671; median:393.5 | 47.2 | ||
| **Jensen 1994 | Non‐healthy patients with hereditary haemochromatosis (26 to 52 years old). Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors proposed threshold Three alternative thresholds were available too. SFth=500. dLw=3.2. |
2+1=3 | 1241.6 (1279.1) | 310 to 2700; median:715 | 11+4=15 | 2604.7 (2241.7) | 226 to 6560; median:1430 | 1363.1 | r = 0.64 |
| **Kaltwasser 1990 | Non‐healthy patients with hereditary haemochromatosis (aged between 22 and 70 with mean 39 years old). Patient's data extracted from table and figure to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. dLw=1.8. |
0+2=2 | 37 | median:37 | 6+2=8 | 1480 (1643) | 57 to 5133; median:934.5 | 1443 | r = 0.85 |
| Lawrence 1996 | Non‐healthy patients with hereditary haemochromatosis within an army medical centre (aged between 26 and 70 with mean 55 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. dLw=1.8. |
0+0=0 | 9+1=10 | 1542.6 (1019.2) | 807 to 3635; median:1047.5 | r = 0.08 | |||
| Leggett 1990 | Non‐healthy patients with hereditary haemochromatosis selected from one banking and one insurance company (large corporations) (aged between 21 and 45 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=300. dLw=2. |
0+1=1 | 215 | 9+1=10 | 778.2 (411.06) | 237 to 1550; median:690 | 563.2 | r = 0.357 | |
| **Lim 2004 | Non‐healthy patients with hereditary haemochromatosis (aged between 27 and 75 with mean 51 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors proposed threshold (among more than one threshold mentioned). SFth=500. dLw=1.8 |
1+2=3 | 357 (257.0) | 123 to 632; median:316 | 10+5=15 | 1121.2 (1347.7) | 209 to 5730; median:8066 | 764.2 | "poor correlation" r = 0.286 |
| **Lombard 1989 | Non‐healthy patients with hereditary haemochromatosis (aged between 27 and 73 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. Ps=Grade 2 or more. |
0+9=9 | 54.4 (58.4) | 9 to 200; median:36 | 6+5=11 | 1080.6 (1008.6) | 70 to 3250; median:560 | 1026.2 | r = 0.744 |
| Macfarlane 1995 | Non‐healthy patients with idiopathic haemochromatosis (mean age 50 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. dLw=1.8 |
0+0=0 | 7+1=8 | 1263.9 (895.3) | 330 to 3069; median:987.5 | "poor correlation" r = 0.560 | |||
| Olynyk 1999 | Non‐healthy patients with hereditary haemochromatosis (aged between 35 and 74 with mean 55.8 years old). Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors proposed threshold Three alternative thresholds were available too. SFth=150. dLw=3.2 |
0+0=0 | 7+0=7 | 914.1 (678.7) | 187 to 2290; median:731 | r = 0.944 | |||
| Ortega 2005 | Non‐healthy patients with haemochromatosis (mean age 50.48 years old). Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors proposed threshold. Three alternative thresholds were available too. SFth=500. dLw=2. |
21+1=22 | 1131.3 (588.4) | 500 to 2600; median:910 | 23+1=24 | 1414.6 (723.5) | 500 to 3000; median:1050 | 283.3 | r = 0.34 |
| Phatak 1998 | Non‐healthy patients with haemochromatosis (aged between 23 and 75). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold (among more than one threshold mentioned). SFth=200. dLw=1.8. |
0+0=0 | 25+0=25 | 810.9 (542.4) | 213 to 2160; median:583 | r = 0.353 | |||
| Pietrangelo 1999 | Non‐healthy patients, and their relatives with haemochromatosis (aged between 20 and 85 years). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors proposed threshold (among more than one threshold mentioned). SFth=500. dLw=1.8. |
0+0=0 | 13+0=13 | 2671.2 (1913.5) | 650 to 5846; median:2165 | r = 0.490 | |||
| Rowe 1977 | Mixed individuals (relatives of a dead serious case of IO) and their relatives with haemochromatosis (aged over 14 years old). Patient's data extracted from table, and figure to compute TP, FN, FP, and TN for the authors proposed threshold (among more than one threshold mentioned). SFth=200. Ps=Grade 1 or more |
0+10=10 | 21.14 (13.5) | 8.1 to 45; median:16.1 | 1+18=19 | 72.8 (43.4) | 22 to 202; median:57.6 | 51.66 | r = 0.38 |
| Schöniger‐Hekele 2002 | Non‐healthy patients with hereditary haemochromatosis (mean age 48.3 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors proposed threshold (among more than one threshold mentioned). SFth=200. Ps=Grade 1 or more. |
0+3=3 | 40.3 (29.5) | 10 to 69; median:42 | 8+1=9 | 738 (616.8) | 110 to 1970; median:573 | 697.7 | r = 0.701 |
| Sham 1997 | Non‐healthy patients with hereditary haemochromatosis (between 28 and 80 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors proposed threshold among more than 1 threshold. SFth=300. dLw=1.8 |
3+0=3 | 1122.3 (419.3) | 640 to 1400; median:1327 | 31+2=33 | 802.9 (477.1) | 248 to 2246; median:634 | ‐319.4 | r = 0.46 |
| Smith 1997 | Non‐healthy patients with hereditary haemochromatosis (mean age 46 with range between 27 and 57 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. dLw=1.8. |
1+3=4 | 550 (679.7) | 41 to 1539; median:310 | 5+0=5 | 1187.4 (985.5) | 638 to 2933; median:706 | 637.4 | r = 0.025 |
| Summers 1990 | Non‐healthy patients with hereditary haemochromatosis (mean age 34 with range between 12 and 78 years old). Patient's data extracted from table, and figure to compute TP, FN, FP, and TN for the authors proposed threshold (among more than one threshold mentioned). SFth=300. dLw=1.8. |
0+3=3 | 173 | 3+0=3 | 653.2 (721.4) | 60 to 1856; median:319 | 480.2 | r = 0.471 | |
| Thomsen 1992 | Non‐healthy blood donors with haemochromatosis (aged between 20 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. dLw=1.8. |
1+4=5 | 402.6 (88.3) | 300 to 513; median:382 | 7+4=11 | 617.6 (226.4) | 316 to 1031; median:599 | 215 | r = 0.700 (P < 0.01) |
| Wands 1976 | Non‐healthy patients with hereditary haemochromatosis (mean age 34.2 with range between 19 and 67 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=200. Ps=Grade 2 or more. |
1+4=5 | 87.6 (69.7) | 23 to 202; median:55.5 | 0+13=13 | 70.9 (29.8) | 28 to 130; median:75.5 | ‐16.7 | r = 0 |
| *Non‐iron‐overload sample size (n1) could be different to the sum of FP + TN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐overload sample size (n2) and the sum TP + FN. **Studies that had two proposed thresholds: one for both sexes, and another one for each sex (males, females). Here we present the data as one threshold for both sexes. | |||||||||
3.13 Serum ferritin measures per liver iron concentration status for non‐healthy adults with chronic hepatitis, alcoholic cirrhosis, alcoholic liver disease, and hepatic fibrosis
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) / Iron‐overload threshold mg/g dry liver weight (dLw) or Perl's staining (Ps) grade: 0, 1, etc. | Non‐iron‐overload group: Non‐IO (liver iron concentration equal or lower than author's threshold, or Perl's staining author's threshold) | Iron‐overload group: IO (liver iron concentration higher than author's threshold, or Perl's staining author's threshold) | Non‐IO vs IO comparison | Serum ferritin vs reference standard association | ||||
| FP+TN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | TP+FN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐overload group mean minus the non‐iron‐overload group mean and/or **OR |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Chapman 1982 | Non‐healthy patients alcoholics with hepatitis and/or cirrhosis (mean age 44.7 years old). Patient's data extracted from figure to compute TP, FN, FP, and TN for the authors proposed threshold. Three alternative thresholds were available too. SFth=500. dLw=1.8. |
13+28=41 | 509.5 (387.5) | 50 to 1800; median:387 | 9+4=13 | 785.4 (391.6) | 50 to 1800; median:700 | 275.9 | r = 0.28 |
| Guyader 2007 | Non‐healthy patients with chronic hepatitis C (mean age 41.6 years old). D+, D‐, TP, TN for the authors unique proposed threshold. SFth=300 males and 250 females. Ps=Grade 1 or more. |
85+378=463 | 130 | 73+50=123 | 370 | median:406 | 240 | r = 0.461 (P < 0.0001) | |
| Pascoe 1999 | Non‐healthy patients with alcoholic liver disease (aged between 32 and 66). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors proposed threshold (among more than one threshold mentioned). SFth=200. dLw=1.8. |
9+12=21 | 264.9 (308.5) | 24 to 1090; median:83 | 16+0=16 | 838.8 (720.6) | 230 to 2980; median:570 | 573.91 | r = 0.53 |
| Sebastiani 2006 | Non‐healthy patients with chronic hepatitis C (mean age 47.8). Sens, Spec, PPV, NPV, D+, D‐, and Table for the authors unique proposed threshold. SFth=300 males and 200 females. Ps=Grade 2 or more. |
45+171=216 | 124.7 (122.8) | 17+9=26 | 346.4 (284.6) | 221.7 and **OR = 13.2 (P < 0.00001) | |||
| Sebastinani 2012 | Non‐healthy patients with hepatitis C, alcoholic liver disease (mean age 43.8). Sens, Spec, PPV, NPV, D+, D‐, and Table for the authors unique proposed threshold. SFth=300 males, and 200 females. Ps=Grade1 or more. |
19+114=133 | 135.7 (121.2) | 22+50=72 | 280.3 (200.2) | 144.6 and **OR = 4.3 (P < 0.008) | |||
| Szurowska 2010 | Non‐healthy patients with liver cirrhosis (mean age 64 years with range between 22 and 81 years old). Patient's data extracted from figure to compute TP, FN, FP and TN for the authors proposed threshold. Three alternative thresholds were available. SFth=400. Ps=Grade 1 or more. |
1+14=15 | 10+8=18 | ||||||
| Thorburn 2002 | Non‐healthy patients with viral hepatitis infections (mean age 35 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=300. dLw=1.8. |
1+2=3 | 153 (159.7) | 6 to 323; median:130 | 1+1=2 | 334.5 (222.7) | 177 to 492; median:334.5 | 181.5 | r = 0.297 |
| *Non‐iron‐overload sample size (n1) could be different to the sum of FP + TN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐overload sample size (n2) and the sum TP + FN. **Odds Ratio (OR) | |||||||||
3.14 Serum ferritin measures per liver iron concentration status for non‐healthy adults with unknown and mixed diseases (haemochromatosis, chronic hepatitis, nonalcoholic fatty liver disease,... ).
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) / Iron‐overload threshold mg/g dry liver weight (dLw) or Perl's staining (Ps) grade: 0, 1, etc. |
Non‐iron‐overload group: Non‐IO
(liver iron concentration equal or lower than author's threshold, or Perl's staining author's threshold) |
Iron‐overload group: IO
(liver iron concentration higher than author's threshold, or Perl's staining author's threshold) |
Non‐IO vs IO comparison | Serum ferritin vs reference standard association | ||||
| FP+TN=n1* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | TP+FN=n2* | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Mean difference (μg/L) in serum ferritin concentration: iron‐overload group mean minus the non‐iron‐overload group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Cippa 2014 | Non‐healthy patients with mean age of 51 years old with liver disease caused by haemochromatosis, chronic hepatitis, alcoholic liver disease, NASH, blood disorders, and other conditions. D+, D‐, TP, FN for the authors unique proposed threshold. SFth=500. dLw=1.395. |
0+0=0 | 91+56=147 | ||||||
| **Hagström 2016 | Non‐healthy patients from 30 to 60 years old with Nonalcoholic Fatty Liver Disease (NAFLD). D+, D‐, TP, TN for the authors unique proposed threshold. SFth=300 males and 150 females. Ps=**Grade 1 or more. |
37+76=113 | interquartile range: E‐system: 96 to 320 Dxl‐system: 83 to 170; medians: E‐system: 176 Dxl‐system: 123 | 34+28=62 | interquartile range: E‐system:140 to 342 Dxl‐system:107 to 299; medians: E‐system:249 Dxl‐system:177 | significant correlated E‐system: (P < 0.09) Dxl‐system: (P < 0.001) | |||
| Harada 1992 | Non‐healthy patients with secondary haemochromatosis, and other diseases (mean age: 58.2 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=1000. Ps=Grade 1 or more. |
1+0=1 | 1950 | 8+1=9 | 2238.5 (1723.4) | 720 to 6000; median:1510 | 288.5 | r = 0.082 | |
| Maliken 2012 | Non‐healthy patients with several diseases, nonalcoholic fatty liver disease, chronic viral hepatitis, and haemochromatosis (mean age 50 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. dLw=1.8. |
4+0=4 | 1356.2 (315.9) | 823 to 2071; median:1378 | 6+0=6 | 1425.5 (532.7) | 823 to 2071; median:1404 | 69.3 | r = 0 |
| Niederau 1998 | Non‐healthy patients taken from two large industries, several with alcoholism, hepatitis diseases, and haemochromatosis (aged between 28 and 60 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=300. dLw=1.8. |
0+0=0 | 19+1=20 | 2094.1 (1626.6) | 88 to 5800; median:2183.5 | r = 0.392 | |||
| Valberg 1978 | Non‐healthy patients with cirrhosis, haemochromatosis, and liver disease (aged between 17 and 74 years old). Sens, Spec, D+, D‐, and Table for the authors unique proposed threshold. SFth=500. Ps=Grade 3 or more. |
5+15=20 | 18+2=20 | ||||||
| Villeneuve 1996 | Non‐healthy patients (2 of them after autopsy) with alcoholic cirrhosis and hereditary haemochromatosis (mean age 57 with range between 39 and 79 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors proposed threshold (among more than one threshold mentioned). SFth=500. dLw=1.8. |
0+2=2 | 386.0 (24.0) | 369 to 403; median:386 | 6+0=6 | 1325.3 (353.9) | 879 to 1920; median:1236 | 939.3 | r = 0.88 |
| Walsh 2006 | Non‐healthy patients with haemochromatosis, and half of them alcoholic (mean age 44 with range between 27 and 58 years old). Patient's data extracted from table to compute TP, FN, FP, and TN for the authors unique proposed threshold. SFth=500. dLw=1.8. |
2+0=2 | 1285.0 (304.1) | 1070 to 1500; median:1285 | 5+0=5 | 1696.0 (594.0) | 1110 to 2500; median:1700 | 411 | r = 0 |
| Wong 2006 | Non‐healthy patients with liver disease including haemochromatosis, alcoholism, and nonalcoholic liver diseases. Patient's data extracted from table, and text to compute TP, FN, FP, and TN for the authors unique proposed threshold (among more than one threshold mentioned). SFth=400. dLw=1.95. |
0+11=11 | 1+0=1 | ||||||
| *Non‐iron‐overload sample size (n1) could be different to the sum of FP + TN because the studies generally reported the numbers separately (some reasons were exclusion of values, subgroups etc.). Similarly, for iron‐overload sample size (n2) and the sum TP + FN. | |||||||||
| **Liver iron assessed by the Deugnier’s Total Iron Score (TIS): made up of the addition of the hepatocyte (HIS = 0–36), sinusoidal (SIS = 0–12), and mesenchymal (MIS = 0–12) iron scores | |||||||||
3.15 Mean serum ferritin concentration for non‐healthy adults iron‐overload and non‐iron‐overload groups, by state and disease/condition (where serum ferritin data was available)
| State or disease/condition | Mean serum ferritin for non‐iron‐overload individuals (μg/L) | Mean serum ferritin for iron‐overload individuals (μg/L) | Means difference (μg/L) | Studies sample size (with available mean serum ferritin values for iron‐overload and non‐iron‐overload groups) | Sample size (n) for non‐iron‐overload individuals | Sample size (n) for iron‐overload individuals |
| Non‐healthy with haemochromatosis | 409.6 | 908.7 | 499.1 | 15 | 78 | 192 |
| Non‐healthy with chronic hepatitis, alcoholic cirrhosis, alcoholic liver disease, and hepatic fibrosis. | 219.6 | 492.6 | 272.9 | 6 | 629 | 501 |
| Non‐healthy mixed/unknown: hereditary haemochromatosis, alcoholic disease, chronic hepatitis, nonalcoholic fatty liver disease | 1244.3 | 1671.3 | 427 | 4 | 11 | 24 |
| All non‐healthy | 497.6 | 930.8 | 433.3 | 25 | 718 | 717 |
3.16 Serum ferritin measures per bone marrow status for excluded studies but with valuable findings
| Studies | Study characteristics: target population / data specifications / serum ferritin used threshold (SFth) | Bone marrow equal to 0 group: BM = 0 (or as defined by author as iron‐depleted or diseased) | Bone marrow 1 or more group: BM = 1+ (or as defined by author as iron‐repleted or non‐diseased) | BM = 0 vs BM = 1+ comparison | Serum ferritin vs BM association | ||||
| Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Sample size (n) | Mean (Standard deviation) serum ferritin (μg/L) | Range value for serum ferritin; other measures (μg/L) | Sample size (n) | Mean difference (μg/L) in serum ferritin concentration: iron‐repleted group mean minus the iron‐depleted group mean |
Pearson's correlation (r), Regression coefficient R2, P‐value |
||
| Hanif 2005 | Non‐healthy and/or anaemic, several with one or various chronic diseases pertaining to Pakistan Armed Forces (age not mentioned). SFth=90. | 18.4 (13.8) | 7.8 to 54.2 | 86 | 181.2 (88.7) | 88.9 to 370 | 90 | 162.8 | |
| Jacobs 1972 | 18 to 65 year old untreated IDA patients. SFth=12 | 5 (0.66) | 1 to 12; 95% CI: (4.34 to 5.66) | 21 | |||||
| Jacobs 1982 | Iron‐deficient patients previously pregnant, females, 18 to 45 years old. SFth=<20. | 13.21 (22.41) | 3 to 95; 95% CI: (3.14 to 23.29) | 19 | |||||
| Leyland 1975 | 18 to 65 year old untreated IDA patients, and IO states (including some with chronic diseases and unknown age). SFth=15. | 18.4 | 2 to 100 | 42 | 5464.2 | 97 to 14352 | 37 | 5445.8 | |
| Lipschitz 1974 | ID individuals selected because of suspicion of anaemia or a disorder in iron metabolism (presenting with inflammation and, in some cases, liver or renal disease). SFth=15. | 16.4 (28.00) | 1 to 105; median: 6 | 43 | 530.9 (511.8) | 29 to 3239; median: 410 | 65 | 514.45 | r = 0.53 |
| Luxton 1977 | No more details: only abstract available. SFth=12. | 10.6 (11.01) | 1 to 61; median: 7 | 69 | 265.1 (206.7) | 12 to 1150; median: 220 | 179 | 254.5 | r = 0.64 |
| Vreugdenhil 1989 | Mean age 64 years (range between 48 and 79), anaemic rheumatoid arthritis patients. SFth=50. | 10 to 58; median: 18 | 8 | 10 to 247; median: 71 | 20 | "good correlation" | |||
| Vreugdenhil 1990b | Rheumatoid arthritis patients, 75% anaemic, 5/13 males, aged between 54 and 65 years. SFth=50. | 10 to 98; median: 26 | 13 | 45 to 221; median: 90 | 12 | significantly correlated (P < 0.002) | |||
3.17 Serum ferritin concentration per liver iron concentration status for excluded studies but with valuable findings
| Studies | Study characteristics: target population |
Non‐iron‐overload group: Non‐IO (liver iron concentration <= 3.2 µmol/100 mg dry liver weight or as defined by author) |
Iron‐overload group: IO (liver iron concentration > 3.2 µmol/100 mg dry liver weight or as defined by author) |
Non‐IO vs IO comparison | Serum ferritin vs reference standard association | ||||
| *Cecchin 1990 | Patients receiving haemodialysis and intravenous iron infusions, and patients with hereditary haemochromatosis (mean age 48 years old). | 137.4 (95.82) | 40 to 411; median: 131.5 | 18 | 1015.1 (509) | 315 to 2120; median: 985 | 30 | 877.7 | r = 0.9473 |
| Hellerbrand 2003 | Patients with hereditary haemochromatosis, or cirrhosis and/or pathogenesis of hepatocellular carcinoma (mean age: 61.2 years old). | r = 0.35 | |||||||
| Hofer 2004 | Patients with chronic hepatitis C (mean age: 40.8 years old). | r = 0.335 | |||||||
| Karam 2008 | 38 sickle‐cell disease patients, and one with thalassaemia, all with chronic transfusion, 6 to 23 years old with mean of 12.7 years old. | 940 (358) | 515 to 1270; median: 987.5 | 4 | 3021.5 (1482) | 975 to 6076; median: 2930 | 35 | 2081.5 | r = 0.46 |
| Kowdley 2012 | Patients with nonalcoholic fatty liver disease with mean age of 40 years old. | ***OR =2.2 95% CI = (1.78 to2.76) | |||||||
| **Kreeftenberg 2000 | Patients at risk of iron overload with clinical suspicion and risk of haemochromatosis. | r = 0.759 | |||||||
| Smith 2014 | Sickle‐cell patients who underwent chronic transfusion. | 292 (150.6) | 64 to 480; median: 315 | 7 | 3542.9 (2573.30) | 430 to 13576; median: 3150 | 252 | 3250.9 | r = 0.74 |
| *Iron‐overload threshold = liver iron concentration > 3.6 µmol/100 mg dry liver weight. **Iron‐overload threshold = liver iron concentration > 7 µmol/100 mg dry liver weight. ***Odds Ratio (OR). | |||||||||
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Iron deficiency apparently healthy (All) | 5 | 350 |
| 2 Iron deficiency apparently healthy (*adults) | 3 | 247 |
| 3 Iron deficiency non‐healthy (All) | 70 | 5709 |
| 4 Iron deficiency non‐healthy (infants and children) | 4 | 519 |
| 5 Iron deficiency non‐healthy (*adults) | 63 | 5042 |
| 6 Iron deficiency non‐healthy (adults, pregnant women) | 3 | 148 |
| 7 Iron overload (All) | 36 | 1927 |
| 8 Iron overload (All: threshold per gender: males/females) | 9 | 1292 |
1. Test.

Iron deficiency apparently healthy (All)
2. Test.

Iron deficiency apparently healthy (*adults)
3. Test.

Iron deficiency non‐healthy (All)
4. Test.

Iron deficiency non‐healthy (infants and children)
5. Test.

Iron deficiency non‐healthy (*adults)
6. Test.

Iron deficiency non‐healthy (adults, pregnant women)
7. Test.

Iron overload (All)
8. Test.

Iron overload (All: threshold per gender: males/females)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aguilar 2012.
| Study characteristics | |||
| Patient Sampling | The study was undertaken as part of a case‐control study on the aetiology and risk factors of anaemia in children less than 5 years of age. Consecutive children aged 1 to 59 months, attending the Manhiça District Hospital (MDH) emergency department between October 2008 to August 2010 with anaemia (haemoglobin lower than 110 g/L), and with no history of blood transfusion in the preceding 4 weeks were recruited (n = 443). A subsample of 173 had their parents consent, and completed their corresponding bone marrow and serum ferritin measurements. | ||
| Patient characteristics and setting | The study was carried out at the Centro de Investigacao em Saude de Manhica (CISM) in Manhica District, southern Mozambique, an area where malaria transmission is of moderate intensity most of the year with some seasonality. More than 95% of the malaria infections are due to Plasmodium falciparum. Adjacent to the CISM is the Manhica District Hospital (MDH), a 110‐bed health facility. The main causes of hospital attendance and admission among children in the area are pneumonia, malaria, anaemia, malnutrition and HIV‐related diseases. HIV prevalence in pregnant women was 29% in 2010. Patients who entered the study were anaemic infants of both sexes, and aged between 6 to 59 months with several infectious diseases (malaria, tuberculosis, HIV, and other), attending the MDH emergency department in a rural region, southern Mozambique. | ||
| Index tests | Ferritin was measured in an enzyme immunoassay ADVIA Centaur analyser (Siemens Healthcare, Barcelona, Spain). Serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow iron content was semi‐quantitatively estimated classifying the amount of blue stained haemosiderin Perls in bone marrow fragments (aggregates of bone marrow cells) according to 4 categories: 0 (absent), 1 (diminished), 2 (normal) and 3 (abundant). The categories 0 and 1 were considered indicative of iron store deficiency. | ||
| Flow and timing | A bone marrow aspiration was performed after complete clinical examination was performed and the information was entered onto standardised questionnaires together with demographic data. Venous blood was collected by venipuncture for malaria parasitaemia examination, bacterial culture, full blood count and biochemical and molecular determinations. However, the flow and timing of these three types of tests was not described. | ||
| Comparative | |||
| Notes | Ferritin and other biomarkers of iron status were compared to bone marrow findings in 180 anaemic children attending a rural hospital in southern Mozambique. Eighty per cent (144/180) of the children had iron deficiency by bone marrow examination, 88% (155/176) had an inflammatory process, 66% (119/180) had moderate anaemia, 25% (45/180) severe anaemia, and 9% (16/180) very severe anaemia. The findings of this study showed that the majority (80%) of the anaemic children were iron deficient by direct assessment of iron stores, and that sTfR and TfR‐F index adjusted by CRP are the most sensitive markers with specificities of at least 50% to identify ID in this study population. However, even with these markers, 17% and 25% of children, respectively, will not be diagnosed with ID and, therefore, adequately treated. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ali 1978.
| Study characteristics | |||
| Patient Sampling | 248 adequate bone marrow specimens sequentially collected over a 18‐month period from hospital patients with pathologies representative of common pathologies in a general hospital in Canada | ||
| Patient characteristics and setting | The specimens were from hospital patients of both sexes, mixed ages, and with clinical diagnosis ranging from thrombocytopenia, liver disease, hypochromic anaemia, renal disease, myeloma, macrocytic anaemia, collagen disease, pyrexia of unknown origin, myeloproliferative disorder, acute leukaemia and lymphoproliferative disorder. The specimens came from St. Joseph's General Hospital in Hamilton, Ontario, Canada. | ||
| Index tests | Serum ferritin concentrations were measured by radioimmunoassay using ferritin labelled with iodine‐125 and rabbit anti‐ferritin antibody. Goat anti‐rabbit y‐globulin antibody and polyethylene glycol were used as separating agents. The working range of the method used was up to 500 µg ferritin per litre and required a sample of 75 µL of serum or plasma for the assay at two dilutions. Serum ferritin concentration lower than 12 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Air dried films of bone marrow aspirates were fixed in methanol for 10 to 20 minutes, then stained by May‐Grimwald‐Giemsa's stain for morphologic study and by the Prussian blue reaction for evaluation of iron stores. Iron stores were graded as absent, present, or increased by one observer who was not aware of the serum ferritin value. Iron was considered to be absent when no iron could be demonstrated in any of the bone marrow fragments and increased when every fragment contained iron deposits covering more than 50% of the area of the fragment. Grading of the iron content between these two extremes was not attempted. The absence of iron in BM was classified as iron deficiency. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, the flow and time of these three types of tests was not described. However, it was noted that blood samples were collected at the time of the bone marrow aspiration. | ||
| Comparative | |||
| Notes | Serum ferritin prospectively correlated with bone marrow iron stores in a mixed hospital population. 248 adequate bone marrow specimens from patients with pathologies representative of the clinical and haematologic problems likely to be encountered in a general hospital, were sent to St. Joseph's Hospital's haematology laboratory for evaluation over 18 months. Of the 248 patients, 69 were found to have no iron in the bone marrow, 116 had normal iron stores and 63 had increased iron stores. Of the 69 patients with no iron in the bone marrow, 20 had a serum ferritin value of more than 12 µg/L. Of these 20 patients, 2 were receiving iron therapy and 2 had received a blood transfusion, 6 had alcoholic liver disease, 2 had chronic active hepatitis, 5 had renal failure due to chronic glomerulonephritis, 2 had active Hodgkin's disease and 1 had multiple myeloma. All the patients with normal or increased iron stores had a serum ferritin value of more than 12 µg/L. The data reported in this study confirm the usefulness of taking measurements. Therefore, a low serum ferritin value probably indicates iron depletion, while an elevated value does not exclude that possibility. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Baillie 2003.
| Study characteristics | |||
| Patient Sampling | 120 patients with iron‐deficiency anaemia (n =40), anaemia of chronic disease (n = 40) and anaemic rheumatoid arthritis patients (n = 40) were selected for study. | ||
| Patient characteristics and setting | Iron‐deficiency anaemia (n = 40) was diagnosed when the ferritin level was < 12 µg/L. The patients with chronic disease anaemia (n = 40) and rheumatoid arthritis had at least a 6‐month history of chronic illness with elevated erythrocyte sedimentation rates to confirm inflammation. The 40 patients with rheumatoid arthritis were selected as a model group of patients with an inflammatory condition. The study was conducted at the Department of Haematology, Southern General Hospital, Glasgow, Scotland. As a source of bone marrow, aspirates were collected from 18 patients with rheumatoid arthritis undergoing total hip replacement at the Southern General Hospital and Victoria Infirmary, Glasgow, Scotland. | ||
| Index tests | Serum ferritin levels were performed using the Access® Immunoassay System analyser (Beckman Coulter Inc, Chaska, USA). | ||
| Target condition and reference standard(s) | Bone marrow aspirations were assessed as the gold standard in 20 of the patients with chronic disease anaemia. Seven patients with chronic disease anaemia had no stainable marrow iron [mean sTfR 7.43 mg/L (3.26–12.23), mean ferritin 112 µg/L (26–261)]. Of the seven patients with iron‐deficiency anaemia, six of these had elevated sTfR concentrations (> 3.3 mg/L), and one had a sTfR concentration in the upper normal range (sTfR higher than 3.26 mg/L). Thirteen patients with anaemia of chronic disease had marrow iron present [mean sTfR 3.41 mg/L (3.26–12.23), mean ferritin 209 µg/L (50–1500)]. The marrow smears were examined for the presence of iron using Perl’s Prussian blue stain. Iron stores were graded as 0 to 6+, grades of 1+ to 3+ being considered normal. The normal control range (n = 40) was generated using a group of 10 healthy volunteers and 30 non‐anaemic patients. | ||
| Flow and timing | Over a period of 5 months, patients had haematological measurements, including Hb, serum ferritin and sTfR along with other diagnostic methods including bone marrow aspirates. An elevated erythrocyte sedimentation rate of at least 6 month's duration was used to confirm inflammation. | ||
| Comparative | The aim of the study was to evaluate the usefulness of the IDeA sTfR immunoenzymometric assay (Orion Diagnostica, Espoo, Finland) in the differential diagnosis between iron‐deficiency anaemia and anaemia of chronic disease. The sensitivity, specificity and efficiency of the parameters at detecting iron deficiency when compared with bone marrow iron stains in 20 patients with anaemia of chronic disease and at detecting iron deficiency when compared with bone marrow iron stains in 18 patients with rheumatoid arthritis. | ||
| Notes | The study had ethical approval and all data collected followed the standard practice for confidentiality. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Balaban 1993.
| Study characteristics | |||
| Patient Sampling | 120 anaemic (Hb < 140 g/L) male patients with diagnosis of neoplasm inflammation, infection, or hepatic disease in Dallas, Texas, USA. No more details about the sampling procedure were described. | ||
| Patient characteristics and setting | Hospitalised male patients aged 50 years or older with several diseases, mainly malignancies (carcinoma, lymphoma, myeloma), and chronic infections (fewer than 10%) | ||
| Index tests | Serum ferritin (SERFER) was determined by radioimmunoassay (RAMCO, Houston, TX). Red blood cell ferritin (RBCFER) was determined by centrifuging blood samples to obtain the packed red cell fraction and diluting to the original volume with 0.9% saline. Red cell suspensions were then prepared by isotonic/percoll gradient density separation and ferritin determined by the same radioimmunoassay. A serum ferritin concentration lower than 70 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow examinations were obtained by iliac crest trephine aspiration and biopsy and evaluated by acid ferrocyanide (Prussian Blue) staining of aspirate smears. Zero iron was defined as no identified iron staining after examination under oil immersion (X 100). Trace staining was defined as iron stain seen only after examination under oil immersion (X 100). One to 4+ staining was based on the observed intensity of iron stain after examination under low power lens (X 40), with 1+ being less intense and 4+ being the most intense. A marrow was considered to have decreased iron stores (iron deficiency) if the averaged score was zero or trace. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records reviewing were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | 120 anaemic (Hb < 140 g/L) male patients, mainly with a diagnosis of neoplasm inflammation, infection, or hepatic disease. The correlation between SERFER and bone marrow iron staining was r = 0.58 (P < 0.001). This correlation was better than that between RBCFER and bone marrow iron content (r = 0.36; P < 0.001). The potential to predict an iron deficient state (positive predictive value) with this combination (0.82) was better than the positive predictive value of having either a low SERFER or RBCFER. The overall accuracy of knowing both the SERFER and RBCFER (0.94) was better than the accuracy of either test alone. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Barron 2001.
| Study characteristics | |||
| Patient Sampling | Of 108 consecutive bone marrow specimens that had a report of absent bone marrow hemosiderin and that were re‐reviewed, 19 (18%) did not allow an accurate assessment of iron stores and hence were deemed inadequate. An additional 15 specimens (14%) revealed decreased or increased, but not absent, stainable iron and were also excluded from further analysis. Therefore, only 74 specimens (69%) were thought to be accurately reported. Of these, only 37 had adequate laboratory and historical information. Two of them were excluded and two groups of 17 and 18, respectively, were made. No further details about the sampling procedure were described. | ||
| Patient characteristics and setting | 35 patients with blood disorders underwent bone marrow aspirations and blood tests at the Mayo Clinic, Rochester, Minnesota, United States. They were classified in two groups: group A (clinically not consistent with iron deficiency) and group B (clinically consistent with iron deficiency). Group A consisted of 17 patients (median age: 65 years; 12 of them female) and group B of 18 patients (median age: 60 years; 8 of them female). Group A had lymphomas, leukaemia, myelomas, myeloproliferative disorders, and inflammatory disorders, etc. and group B presented mainly with bleeding. | ||
| Index tests | Serum ferritin concentrations (method not specified). Normal ranges: ferritin 20–300 μg/L. Serum ferritin concentration lower than 100 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow hemosiderin was measured on the amount of stainable iron in the bone marrow. Iron stains (Prussian blue) were performed on one slide. For each batch of iron studies examined, a quality control of the reagents was performed on a smear previously read as showing increased iron stores. The slides were fixed with methyl alcohol for 1 min, decanted, and allowed to air‐dry. Afterward, they were placed into a Coplin jar containing one part (2%) hydrochloric acid to two parts potassium ferrocyanide, followed by incubation in an incubator at 37°C for 20 mins. After being washed with distilled water, the slides were placed into a Coplin jar and counterstained with Nuclear Fast Red for 15 mins. The slide was then washed with distilled water, fan‐dried, and examined by a pathologist. All the test slides in the current study were re‐reviewed by one of the authors. Patients in whom the absence of bone marrow hemosiderin was confirmed (iron deficient) were classified into two groups on the basis of an extensive historical and laboratory review. | ||
| Flow and timing | It was unclear. A review of the pathologic reports revealed 19 inadequate specimens and 15 with decreased, but not absent, iron stores. Thus, only 74 of the 108 cases had been accurately reported. In 35 of these cases, adequate clinical and laboratory data were available and allowed further analysis of two groups, divided according to the participants' clinical history. Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | We suspected the inaccuracy of bone marrow hemosiderin staining in the assessment for IDA in an unselected group of patients with suspected haematologic disease. In addition to potential inaccuracies attributable to methodology, we discovered that visual inspection for bone marrow hemosiderin by experienced haematopathologists was not always reproducible. In the current study, only 69% of the reports were believed to be accurate on re‐review. An interobserver difference of 31% is unacceptably high for a supposed 'gold standard', and further consequences in patient convenience and cost may not be trivial. Furthermore, subclassification of the study population according to serum ferritin concentration revealed seven patients (20%) with values above 100 μg/L, which confirmed the clinical evidence that IDA was doubtful. At the same time, the finding that 85% of the patients with low serum ferritin were believed clinically to have IDA suggests the potential superiority of measuring serum ferritin over assessing bone marrow hemosiderin in detecting IDA in certain circumstances. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Bârsan 2015.
| Study characteristics | |||
| Patient Sampling | Prospective observational single‐centre study | ||
| Patient characteristics and setting | 54 patients were selected from those admitted to a tertiary nephrology centre. Inclusion criteria were age over 18 years, chronic kidney disease of stages 3–5 with non‐dialysis, and anaemia (Hb under 110 g/L 2 times, 2 weeks apart). Patients with anaemia of a specific cause (e.g. vitamin B12 or acid folic deficiency, haemolytic anaemias), acute inflammatory conditions, recent bleeding, neoplasm, active liver disease (transaminases 2 times normal), severe secondary hyperparathyroidism (PTH higher than 800 pg/mL), or previous iron and epoetin therapy were excluded. Participants had a median age of 64.5 (range from 30 to 90) years, 56% males, 19% diabetes mellitus (but none with diabetic glomerular nephropathy as cause of chronic kidney disease), 50% stage 5, 20% stage 4, and 30% stage 3 chronic kidney disease with moderate anaemia (mean 99 (95% CI 94–102) g/L). | ||
| Index tests | Serum ferritin and serum transferrin were measured by immuno turbidimetric methods (Good Biotech – Taiwan; Giesse Diagnostics – Italy) on an autoanalyzer Olympus® AU400. | ||
| Target condition and reference standard(s) | Bone marrow was collected by aspiration from the iliac crest. The smears were prepared from bone marrow aspirate stained with Perls’ Prussian blue. The occurrence of siderotic granules in macrophages was graded on a scale from 0 to 6. The percentage of sideroblasts, e.g. erythroblasts with green blue particles, was also calculated. The pattern of bone marrow iron distribution was classified as normal (macrophage iron 2–4, sideroblasts in a normal percentage), iron deficiency (macrophage iron 0 or 1, sideroblasts absent or present in a very low percentage), and anaemia of chronic conditions (macrophage iron ≥ 5, sideroblasts absent or present in a very low percentage). According to bone marrow iron distribution of the 54 non‐dialysis chronic kidney disease patients, with investigations of epoetin and iron‐naive, only 7 had normal iron distribution, 26 had iron deficiency and 21 had anaemia of chronic disorders. | ||
| Flow and timing | Bone marrow was first used to classify patients, then measurements were made of hepcidin and ferroportin expression, and peripheral parameters of iron status and inflammation. The flow and timing were not described in detail. | ||
| Comparative | Using bone marrow iron distribution, the patients were classified as having normal iron distribution, iron deficiency or anaemia of chronic disorders. Comparisons were made between iron‐deficiency anaemia and anaemia of chronic disorders groups. | ||
| Notes | The local Ethical Committee (of the ‘Dr Carol Davila’ Teaching Hospital of Nephrology, Bucharest, Romania) approved the study protocol. ANEMIAWORKING GROUP ‐ Romania supported the costs of some lab reagents and publishing. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Baumann Kurer 1995.
| Study characteristics | |||
| Patient Sampling | Between December 1992 and August 1993, blood tests, bone marrow smears and trephine biopsies (posterior iliac crest) were prospectively collected from 46 anaemic (haemoglobin concentrations < 120 g/L for women and < 140 g/L for men) patients with chronic inflammatory rheumatic diseases in Zürich University Hospital. A subsample of 45 patients had available clinical history and serum ferritin measurements. | ||
| Patient characteristics and setting | 45 anaemic patients (37 women, 8 men) with mean age of 57.5 years old (aged between 26 and 87 years old). 28 of them had rheumatoid arthritis according to the American Rheumatism Association criteria, nine had connective tissue disease (systemic lupus erythematosus, Sjogren syndrome, mixed connective tissue disease, polymyalgia rheumatica), seven had seronegative spondathropathies (psoriasis, Crohn’s disease and Reiter syndrome) and one woman was suffering from gout arthritis. All patients took NSAIDs and most of them were also on a second‐line drug (gold, methotrexate, sulphasalazine. d‐Penicillamin and chloroquine) with or without prednisone. No patient had received either iron supplementation or blood transfusions in the preceding 6 weeks. The study included patients from the Department of Rheumatology of the University Hospital of Zurich, Switzerland. |
||
| Index tests | Serum ferritin was measured by a radioimmunoassay (Bio‐Rad, Glattbrugg, Switzerland). Serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | A May Gruewald Giemsa stain was carried out on four smears, an iron stain (Prussian blue) was used in addition to a nonspecific esterase (a‐naphthyl butyrate) reaction. The trephine biopsies were embedded in methacrylate, cut at 1 pm thickness and stained with May Griinwald Giemsa and ‘kemecht rot nach Romeis’. The smears as well as the biopsies were examined by three experienced observers and reviewed blindly by two of them. The iron stores in the smears were classified semi‐quantitatively from 0 to 4+. The iron grading of the biopsies was adapted from the smears. Zero and 0.5 (trace) iron was classified as iron deficiency and 1 to 4 as replete iron stores. | ||
| Flow and timing | Blood tests, bone marrow smears and trephine biopsies (posterior iliac crest) were prospectively collected from 45 patients. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | 45 anaemic patients (37 women, 8 men, mean age 57.5 years (range 26‐87)) with chronic inflammatory rheumatic diseases from the Department of Rheumatology of the University Hospital of Zurich, Switzerland. The combination of serum ferritin and CRP (as well as ESR) in its predictive capacity for bone marrow iron stores was examined. The relationship between iron in bone marrow and serum ferritin and CRP, transferrin, transferrin saturation, soluble transferrin receptor, erythrocyte porphyrins and percentage of hypochromic/microcytic erythrocytes was investigated. Stainable bone marrow iron was taken as standard to separate iron‐deficient from iron‐replete patients. 14 patients (31%) were lacking bone marrow iron. Regression analysis showed a good correlation between ferritin and bone marrow iron (adjusted R2 = 0.721, P < 0.0001). The combination of ferritin and CRP (ESR) did not improve the predictive power for bone marrow iron (adjusted R2 = 0.715) in this cohort of patients with low systemic inflammatory activity. With respect to the bone marrow iron content, the best predictive cut‐off value of ferritin was 30 µg/L (86% sensitivity, 90% specificity). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Brink 1982.
| Study characteristics | |||
| Patient Sampling | 1000 consecutive patients were referred to Tygerberg Hospital for bone marrow examination as part of the diagnostic evaluation of their disease between July 1978 and January 1981. The initial clinical diagnosis was obtained either from the hospital records or from the Haematology Clinic records at Tygerberg Hospital. A subsample of 400 patients had available bone marrow and serum ferritin data. No more details about the sampling procedure were given. | ||
| Patient characteristics and setting | 1000 patients of mixed age with anaemic conditions, malignant lesions, infective conditions, or general systemic disorders, (285 white males, 227 white females, 260 non‐white males and 228 non‐white females), referred to the Tygerberg Hospital, in South Africa, underwent bone marrow examination and diagnostic tests. A subsample of 400 of them had available bone marrow and serum ferritin data. | ||
| Index tests | Serum ferritin evaluated with a Gamma Dab ferritin radioimmunoassay kit (Travenol Laboratories). The upper cut‐off point of the test is 500 µg/L. Serum ferritin concentration lower than 99 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Smears of the bone marrow aspirates were evaluated with a Prussian blue reaction, according to the method of Dacie. The bone marrow smears had values ranging from 0 to 6. The corresponding findings in the bone marrow were carried out on an IBM 370/158 computer. Participants graded with absent or traces of their corresponding bone marrow smears staining were classified as iron deficient (the specific grades were not specified). | ||
| Flow and timing | 1000 consecutive patients were referred to Tygerberg Hospital for bone marrow examination as part of the diagnostic evaluation of their disease between July 1978 and January 1981. Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Case series of bone marrow examinations in South African hospitals. When the ability of serum ferritin to predict bone marrow smears values was tested statistically, it was found that a good estimate could often be made, especially in iron deficiency. It was demonstrated that with the use of readily available clinical information and SF values, it is frequently unnecessary to perform bone marrow aspiration for the assessment of BMS only. The prediction of absence of BMS can be made with an extreme degree of statistical assurance when the serum ferritin level is below 99 µg/L. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Brown 1988.
| Study characteristics | |||
| Patient Sampling | 59 consecutive patients undergoing bone marrow biopsy for haemotological assessment (no more details about the sampling procedure were given) | ||
| Patient characteristics and setting | 59 patients of both sexes and mixed age, with diverse chronic disorders (RA, liver disease, carcinomas, hyperthyroidism etc.) and mainly blood disorders (leukaemia, myelodysplastic syndromes, etc.) | ||
| Index tests | Serum ferritin by micro‐ELISA technique. Serum ferritin concentration lower than 15 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspirates were collected into EDTA and spread onto iron‐free, acid washed glass slides and then stained for iron by Perk's stain. Each slide was examined by the same 3 observers with final consensus undertaken. Marrow iron grade was recorded as either: 0 = absent, + = reduced, ++ = normal, + + + = moderate increase, or + + + + = marked increase. Absence of iron stores was classified as iron deficient. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | 59 patients undergoing bone marrow biopsy for haematological assessment. The group included a heterogeneous mixture of patients with haematological malignancies and chronic inflammation. There was very little correlation between serum and red cell ferritin (r = 0.53). Although serum ferritin increased in relation to increased bone marrow iron stores, only 1 out of 8 patients with absent marrow iron stores and none of 8 patients with reduced marrow iron stores had a decreased serum ferritin. In contrast, 6 of 8 patients with absent iron stores had a reduced red cell ferritin concentration. There was no significant difference between the mean red cell ferritin of the patients with reduced, normal and mild to moderately increased marrow iron stores (30, 26 and 34 ag/ red cell where is Azami‐Green fluorescent). Red cell ferritin was decreased in 78% of a group of 32 patients with a low mean cell volume. In the patients studied, red cell ferritin was a better indicator of absent iron stores than serum ferritin. However, red cell ferritin did not detect a reduction in the iron status until the marrow iron stores were completely depleted. Apparently, during normal erythropoiesis, the primitive erythroblasts continue to take up iron irrespective of the amount of iron available in the stores. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Burns 1990.
| Study characteristics | |||
| Patient Sampling | 301 anaemic hospitalised patients (157 males and 144 females) in Bronx, New York. From those, 157 male patients had bone marrow and haematological/biochemical examinations. No more details of the sampling procedure were described. | ||
| Patient characteristics and setting | 301 anaemic hospitalised patients attended affiliated hospitals of the Albert Einstein College of Medicine during a 5‐year period, in Bronx, New York. From those, 157 anaemic adult male patients with mixed age and one or more chronic diseases were selected for the study. | ||
| Index tests | Serum ferritin was measured using an immunoradiometric asssay (Quantimune, biorad, Richmond, California). Levels greater than 2500 µg/L were diluted with blank buffer to within the range of linearity of the test before final analysis (CV = 4.9%, normal range 25 to 300 µg/L for males, and 13 to 150 µg/L for females). Serum ferritin concentration lower than 50 µg/L was classified as iron deficiency (optimal value). | ||
| Target condition and reference standard(s) | All marrow slides reported as lacking stains were re‐evaluated at the time of this study. The stability of the iron stain and reproducibility of quantifying iron stores were prospectively evaluated by staining series of ten replicate thiick marrow smears at weekly intervals with a single batch of ferricyanide stain. These replicates were coded, sorted at random, and simultaneously examined at the end of the month. Marrow samples were scored for iron content using a scale that ranged from 0 to 4+. Patients whose marrow specimens contained no stainable iron were considered to have iron deficiency. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. Aspirates and haematological studies were performed at most within one week of each other. However, we did not find further descriptions about the flow and timing of these three types of tests. | ||
| Comparative | |||
| Notes | Records of all bone marrow examinations performed at affiliated hospitals of the Albert Einsten College of Medicine during a 5‐year period were reviewed along with haematological data. 301 anaemic hospitalised patients (157 males and 144 females) were included with measurements of bone marrow aspirates, serum ferritin, serum iron, TIBC and percentage of transferrin saturation data. Forty patients (13.3%) had absent bone marrow iron. Diagnosis of iron deficiency was accepted on the basis of the following: iron less than 11 µmol/L, total iron‐binding capacity (TIBC) greater than 45 µmol/L, transferrin saturation (% Sat) less than 0.20, and ferritin less than 13 µg/L for females and less than 25 µg/L for males. Using these criteria, iron deficiency was correctly diagnosed by serum iron in 41%, TIBC in 84%, % Sat in 50%, and ferritin in 90% of the patients. Serum ferritin was the most useful serum test for diagnosing iron deficiency in hospitalised patients but was limited by a low sensitivity. The bone marrow examination was the most sensitive test for diagnosing iron deficiency in hospitalised patients. For patients without stainable iron in bone marrow aspirates, diagnosis of iron deficiency was made when ferritin concentration was < 13 µg/L for females and < 25 µg/L for males. Using this cut‐off, iron deficiency was correctly diagnosed in 90% of the patients. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chang 2007.
| Study characteristics | |||
| Patient Sampling | The initial patient population consisted of 111 adult patients who had bone marrow aspirates/trephines reported at the Queensland Health Pathology Service Laboratories at Princess Alexandra Hospital and Royal Brisbane and Women’s Hospital, Australia in 2003–2006. | ||
| Patient characteristics and setting | In the final sample of 76 patients, 33 subjects had haematological malignancies, six had biochemical iron deficiency, two had solid tumours and 35 subjects had miscellaneous conditions, e.g. infection, autoimmune diseases. These 76 patients were assigned to three groups based on the iron status of the bone marrow and sTfR level: patients with normal sTfR and normal bone marrow iron stores (n = 49), patients with an elevated sTfR and normal bone marrow iron stores (n = 13) and patients with reduced or absent bone marrow iron stores (n = 14). All tests were performed at the Department of Chemical Pathology, Queensland Health Pathology Service Laboratories. | ||
| Index tests | Ferritin was measured using the ADVIA Centaur® Ferritin assay (Siemens, USA) which was a two‐site sandwich immunoassay using direct chemiluminometric technology. The inter‐assay coefficient of variation was 4.37% at 20.8 µg/L, 4.21% at 200 µg/L and 4.75% at 384 µg/L. The reference interval for adult females aged 20–28 years was 10–130 µg/L and aged > 29 years was 10–200 µg/L. The reference interval for adult males was 30–300 µg/L. | ||
| Target condition and reference standard(s) | Bone marrow biopsies were performed at public hospitals in Queensland and referred to Queensland Health Pathology Service metropolitan laboratories for reporting by a haematologist. The iron stain was performed on aspirated marrow fragments using Perl’s stain. Iron stores were reported as normal/replete (grades 2+ to 6+), reduced/trace (grade 1+) or absent (grade 0). Iron stains were reviewed by a senior haematologist to achieve consistency in the grading of bone marrow iron stores. | ||
| Flow and timing | All patients had serum iron studies (iron, transferrin and ferritin) and sTfR performed within 1 week of bone marrow aspiration/trephine. These 76 patients were assigned to three groups based on the iron status of the bone marrow and sTfR level: patients with normal sTfR and normal bone marrow iron stores (n = 49), patients with an elevated sTfR and normal bone marrow iron stores (n = 13) and patients with reduced or absent bone marrow iron stores (n = 14). | ||
| Comparative | This retrospective study was conducted to evaluate the bone marrow iron status and the clinical utility of sTfR in detecting iron‐deficiency anaemia in a heterogeneous group of patients, with emphasis on the evaluation of restricted iron supply for erythropoiesis in the presence of stainable bone marrow iron by combining red cell indices, serum sTfR measurements and bone marrow aspirate examination. | ||
| Notes | Serum ferritin higher than 100 µg/L was chosen as the cut‐off to exclude iron deficiency, and sTfR/log ferritin ratio of 2.5 was chosen as the cut‐off for iron deficiency. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Chapman 1982.
| Study characteristics | |||
| Patient Sampling | 60 patients with alcoholic liver disease, 15 idiopathic haemochromatosis patients, and 16 controls with biliary disease were included in the study. A subsample of 55 alcoholic patients had liver biopsy and serum ferritin available data. No further details about the sampling procedure were given. | ||
| Patient characteristics and setting | Ill hospitalised alcoholic patients with hepatitis and/or cirrhosis (mean age 44.7 years old), from London, UK | ||
| Index tests | Serum ferritin measurement technique not specified. Serum ferritin concentration higher than 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Percutaneous liver biopsy in the patients was achieved. Liver tissue was examined by light microscopy and the iron quantitated both by Perl's stain and by direct chemical measurement using the method of Barry and Sherlock. The histological findings in the alcoholics were classified as normal, fatty change, alcoholic hepatitis, or cirrhosis. Patients with alcoholic hepatitis and with alcoholic cirrhosis were considered to have significant liver disease. Liver iron content measurement with a threshold of > 2 mg/g dry weight was classified as iron overload. |
||
| Flow and timing | Haematological tests for measuring iron status (serum‐iron concentration, tranferrin saturation, and serum ferritin), liver biopsy, and other histocompatibility measurements. However, the flow and timing of these three kind of tests was not described. | ||
| Comparative | |||
| Notes | In normal subjects and in patients with idiophathic haemochromathosis, serum ferritin concentrations correlate well with total body iron stores. However, in alcoholic patients with liver disease, the correlation between serum ferritin and liver iron concentrations is relatively low (r = 0.2835). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Cippa 2014.
| Study characteristics | |||
| Patient Sampling | Liver biopsies, including the measurement of hepatic iron concentration performed in patients with suspected liver disease and hyperferritinaemia (serum ferritin higher than 300 µg/L) at the University Hospital of Zurich, Switzerland from 1997 to 2010, were analysed to identify predictors of tissue iron overload (defined by hepatic iron concentration > 25 mmol/kg). | ||
| Patient characteristics and setting | The study population consisted of 147 patients with mean age of 51 years and 80% of whom were male (3 patients were excluded because of severe malnutrition, fulminant toxic liver necrosis and liver transplantation). A total of 32 patients (22%) had hereditary haemochromatosis, 40 patients (27%) had nonalcoholic steato‐hepatitis, 6 patients (4%) had chronic hepatitis B, 25 patients (17%) had chronic hepatitis C, 20 patients (14%) had alcoholic liver disease, 14 patients (10%) had iron overload secondary to haematologic disorders, and 10 patients (7%) had other conditions. | ||
| Index tests | Serum ferritin, but method not described | ||
| Target condition and reference standard(s) | Hepatic iron concentration from liver biopsy | ||
| Flow and timing | The flow and timing was unclear. | ||
| Comparative | The diagnostic challenge in the evaluation of elevated serum ferritin in patients with liver disease was to identify patients with true iron overload assessed by liver biopsy. | ||
| Notes | Hyperferritinaemia was predictive of iron overload only in patients with a high level of serum ferritin (higher than 2000 µg/L). In patients with moderate hyperferritinaemia, liver transaminases inversely correlated with hepatic iron concentration. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Coenen 1991.
| Study characteristics | |||
| Patient Sampling | 73 anaemic patients from the Department of Internal Medicine and Rheumatology (no more details about the sampling procedure were given) | ||
| Patient characteristics and setting | Adult patients (30 men and 43 women of mixed age) with anaemia and one or more chronic diseases including blood disorders from the Department of Internal Medicine and Rheumatology, De Wever Hospital, The Netherlands | ||
| Index tests | Serum ferritin was measured with the immunoenzymometnc assay Tandem‐E Fer (cat. no. 14143; Hybritech, La Jolla, California). Serum ferritin concentration lower than 70 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspirates were obtained from the sternum; all smears were stained with May Grünwald‐Giemsa and Perls iron stain. The grade of staining was classified by microscopic evaluation of macrophage staining (absent, trace, or positive) and sideroblast staining (> 20% is positive). Absent and traces of iron stores were classified as iron deficiency, | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Bone marrow aspirates and serum samples were taken during the same period. Nomograms of CRP, ESR (i.e. acute‐phase reactants not influenced by changes in iron metabolism), or fibrinogen vs ferritin concentrations were constructed and used to estimate the iron stores in bone marrow. Iron stores estimated from the nomograms were compared with the results of staining cytological bone marrow smears for iron, the reference method for evaluating iron in bone marrow. Nomograms of CRP, fibrinogen, or ESR vs ferritin were not suitable to correct for the acute‐phase component of changes in ferritin concentrations. For ferritin concentrations < 70 µg/L, iron deficiency, as judged from the bone marrow iron stain, was always present. The idea of correcting the serum ferritin for increases attributable to the acute‐phase response, by using an acute‐phase protein that is not influenced by changes in iron metabolism, is attractive. However, as our investigations have shown, one cannot eliminate the acute‐phase component of serum ferritin changes by using the ESR, CRP, or fibrinogen as independent acute‐phase markers. Hence, we conclude that the use of a “corrected value” for serum ferritin to evaluate iron deficiency in patients with anaemia of chronic diseases appears to not be possible. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Forman 1980.
| Study characteristics | |||
| Patient Sampling | 48 hospitalised patients undergoing bone marrow examination (no more details about the sampling procedure were provided) | ||
| Patient characteristics and setting | 48 hospitalised adult patients of both sexes with mixed ages, and with one or more chronic conditions, mainly blood disorders (metaplasia, leukaemia, thrombocytosis, etc.) | ||
| Index tests | Serum ferritin by two‐site immunoradiometric, originally introduced by Addison 1972, and further modified in the Ramco Kit (Fer‐Iron; Ramco Labs., Inc., Houston, TX 77098), two‐stage reaction assay. Serum ferritin concentration lower than 15 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow iron was evaluated on a 0 to 4+ scale from smears of bone marrow aspirates stained with Prussian Blue. In five cases in which smears were not available, bone biopsies were substituted. All bone marrow evaluations were carried out without prior knowledge of the corresponding serum ferritin concentrations. However, we recognise and acknowledge the great subjectivity of the bone marrow evaluation. No detectable iron on smears of bone marrow was classified as iron deficient. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Correlation between serum ferritin and bone marrow stainable iron, measured in 48 hospitalised patients with varied haematological pathologies. Comparative studies indicated good correlation between iron content in the marrow and serum ferritin, except possibly in patients with leukaemia and metastatic tumors to the bone marrow. The clinical value of the assay in relation to other indicators of iron status (i.e. haemoglobin, erythrocyte indices, serum iron and iron‐binding capacity, and erythrocyte protoporphyrin) is in its specificity and sensitivity for assaying physiological iron stores. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Guyader 2007.
| Study characteristics | |||
| Patient Sampling | Patients with chronic hepatitis C from a hospital in Rennes were consecutively included in the study if they had liver biopsy performed prior to antiviral treatment (to accurate assess for liver fibrosis) It should be noted that clinically, not all patients with Hepatitis C underwent liver biopsy. The study subsample size was 586 patients. No more details of the sampling procedure were given. |
||
| Patient characteristics and setting | Patients with chronic hepatitis C who attended the University Hospital of Rennes, France (both sexes, with mean age 41.6 years old), with a mean age of infection of 16 years, and 36% of whom were excessive drinkers. | ||
| Index tests | Serum ferritin, method not reported. Elevated serum ferritin concentration (males > 300, females > 250 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver biopsy: liver iron was assessed by the Deugnier’s Total Iron Score (TIS), ranging from 0 to 60, which is the addition of the hepatocyte (HIS = 0–36), sinusoidal (SIS = 0–12), and mesenchymal (MIS = 0–12) iron scores, for assessment of overall stainable iron. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), liver biopsy, and reviews of medical records were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | There was a statistical correlation between serum ferritin and total iron score (r = 0.461; P < 0.0001) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Guyatt 1990.
| Study characteristics | |||
| Patient Sampling | Prospective study of 259 consecutive eligible and consenting anaemic (men: haemoglobin levels less than 12 g/dL; women: haemoglobin levels less than 11.0 g/dL) elderly patients presenting to Chedoke Hospital between January 1984 and March 1988. All underwent blood tests and bone marrow aspiration. A subsample of them (n = 235 patients) had serum ferritin and bone marrow measurements correctly done. | ||
| Patient characteristics and setting | Consecutive anaemic elderly inpatients and outpatients (both sexes over the age of 65 years) presenting to Chedoke Hospital in Hamilton, Ontario, Canada between January 1984 and March 1988. The anaemic patients had iron‐deficiency anaemia (n = 94), anaemia of chronic diseases (n = 113), megaloblastic anaemia (n = 21), multiple myeloma (n = 4), sideroblastic anaemia (n = 3), dysmyeloplastic anaemia (n = 3), or other diseases such as leukaemia, hemolytic anaemia, renal failure etc. (n = 21). | ||
| Index tests | Serum ferritin assessed by radioimmunoassay. Serum ferritin concentration lower than 45 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | A bone marrow aspiration was undertaken and the findings were interpreted by a haematologist who was unaware of the results of the laboratory tests. The bone marrow slides were air‐dried, fixed with methanol, and stained with Prussian blue. Results of the first 65 marrow aspirations were also interpreted by a second haematologist (also unaware of the laboratory test findings), and discrepancies resolved by consensus. The results of the aspiration were classified as iron absent, reduced, present, or increased. Bone marrow grading to determine iron deficiency (absent and reduced, or only absent) was not precisely specified . |
||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, precise flow and timing was unclear. It was only indicated that, after interpreting the bone marrow aspiration results, the haematologist reviewed all relevant clinical information and made a final decision regarding the causes of the anaemia. | ||
| Comparative | |||
| Notes | 259 elderly and anaemic patients were included in the study. 36% had no demonstrable marrow iron and were classified as iron‐deficient. The likelihood ratios associated with the serum ferritin level were as follows: greater than 100 µg/L, 0.13; greater than 45 µg/L but less than or equal to 100 µg/L, 0.46; greater than 18 µg/L but less than or equal to 45 µg/L, 3.12; and less than or equal to 18 µg/L, 41.47. These results indicate that values up to 45 µg/L increase the likelihood of iron deficiency, whereas values over 45 µg/L decrease the likelihood of iron deficiency. 72% of those who were not iron‐deficient had serum ferritin values greater than 100 pg/L, and in populations with a prevalence of iron deficiency of less than 40%, values of greater than 100 µg/L reduce the probability of iron deficiency to under 10%. 55% of the iron‐deficient patients had serum ferritin values of less than 18 µg/L, and in populations with a prevalence of iron deficiency of greater than 20%, values of less than 18 µg/L increase the probability of iron deficiency to over 95%. The serum ferritin concentration was the best test for distinguishing those with iron deficiency from those who were not iron‐deficient. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hagström 2016.
| Study characteristics | |||
| Patient Sampling | Longitudinal cohort study, setting the outcome variable as overall mortality. All liver biopsies performed at the Karolinska University Hospital, Huddinge and the University Hospital of Linkőping, both in Sweden, were stored in local pathology archives. | ||
| Patient characteristics and setting | 222 patients between 1979 and 2009 with biopsy‐proven non‐alcoholic fatty liver disease and available serum ferritin concentrations. The cohort was divided into ‘high’ (n = 89) and ‘normal’ (n = 133) ferritin values, using a cut‐point of 350 µg/L in males, and 150 µg/L in females, and stratified by iron overload status. | ||
| Index tests | Quantitative analysis of ferritin in serum was carried out utilising either DxI/Access® with the Access Ferritin Reagent Packs (Beckman‐Coulter AB, Bromma, Sweden), or Modular E/Cobas e602®, with the Elecsys® Ferritin Reagent Kit (Roche Diagnostics GmbH, Mannheim, Germany). Serum ferritin in 82.0% of patients was measured using the Modular E method, with an upper limit value of normal of 350 µg/L in males and 150 µg/L in females. | ||
| Target condition and reference standard(s) | We identified all liver biopsies performed at our clinics with the particular systemised nomenclature of medicine code for hepatic steatosis (M50080) between 1971 and the end of 2009. All charts were scrutinised from the earliest record, through the time of biopsy, and to the death of the patient or to the end of the follow‐up period (Jan 30, 2015) to exclude liver diseases other than non‐alcoholic fatty liver disease. Iron overload was determined for all patients semi‐quantitatively on histopathological examination of Perls’ stained liver biopsy samples as 0–4 for hepatocytes iron content and 0–2 for reticuloendothelial system iron content. | ||
| Flow and timing | In all cases, the ferritin value analysed closest in time to the liver biopsy was used. | ||
| Comparative | This study evaluated if high levels of ferritin, with or without iron overload, were associated with an increased mortality in non‐alcoholic fatty liver disease. | ||
| Notes | This study was approved by the regional ethical committee at the Karolinska Institutet (dnr 2011/905‐31/2). Because of the retrospective nature of the study and no contact with study participants, informed consent was not required. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hallberg 1993.
| Study characteristics | |||
| Patient Sampling | 203 women all aged 38 years and randomly selected from the population in Goteborg, Sweden | ||
| Patient characteristics and setting | Healthy, 38‐year‐old women from the population in Goteborg, Sweden. In the group, special investigations were undertaken to study the prevalence of iron deficiency and its causes. | ||
| Index tests | The study was undertaken in 1968‐69. Serum ferritin was determined in 1978 by frozen sera Ramco (RIA) and IRMA assay techniques. In 1992, samples were re‐analysed using an RIA calibrated with the International Standard 80/602 for serum ferritin determination. Serum ferritin concentration lower than 16 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | In the 1968 study, one experienced observer classified all smears without a knowledge of the other laboratory results. In a separate study, smears from 53 subjects were examined blind by this observer and four other experienced observers to evaluate the procedure. There was complete agreement in 48 of the 53 subjects. Only one smear with a grade I iron stain was falsely classified as absence of iron stain. Further details of the staining technique and other details have been published previously (Rybo 1985 and Rybo 1985a). Classification was done in 4 categories where a grade 0 iron stain indicated iron deficiency, and grade 1 to 3 indicated iron‐replete status. | ||
| Flow and timing | In the 1968 study, bone marrow examination was made without knowledge of the blood results. In 1978, in frozen sera, Ramco (RIA) and IRMA assay techniques were applied to determine serum ferritin. In 1992, samples were re‐analysed using an RIA calibrated with the International Standard 80/602 for serum ferritin determination. | ||
| Comparative | |||
| Notes | The efficacy of different methods of screening for iron deficiency was re‐examined in a randomly selected sample of 38 year old women (n = 203) with known iron status based on bone marrow smears. The study was undertaken in 1968‐69. The distributions of iron‐replete and iron‐deficient women showed less overlap (diagnostic efficiency, 91%) for serum ferritin than for other haematological parameters. The best discrimination was obtained with a value of serum ferritin < 16 µg/L (specificity 98%; sensitivity 75%). Absence of iron stores was associated with signs of an iron‐deficient erythropoiesis, starting at serum ferritin 25‐40 µg/L. Use of multiple criteria to diagnose iron deficiency falsely reduces prevalence figures for iron deficiency. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Halliday 1977.
| Study characteristics | |||
| Patient Sampling | Study of characteristics of haemochromatosis in first‐degree relatives of probands, in an era prior to the availability of genetic testing. Liver biopsy only done in those with abnormal serum measures, hence, there is a high risk of bias. 43 families with I.H.C. were studied, with 41 probands and 199 first or second‐degree relatives. | ||
| Patient characteristics and setting | Patients with haemochromatosis referred to a clinic corresponding to members of families with first‐degree relatives with hereditary haemochromatosis. Ill patients, of both genders, were adults with a mean age of 41.1 years old, from Brisbane, Australia. | ||
| Index tests | Serum ferritin by radioimmunoassay. Elevated serum ferritin concentration (males > 200, females > 150 µg/L), and both sexes. Values > 500 µg/L were classified as iron overload (each one leading to a different test, one threshold for both sexes, and one threshold per sex). | ||
| Target condition and reference standard(s) | Liver iron was assessed via liver biopsy. Several measurements concerning iron grading, iron content, and liver architecture were obtained. Specifically, participants with a liver iron content measurement over (>) the threshold of 1.8 mg/g dry weight were classified as having iron overload. | ||
| Flow and timing | Not all patients/relatives underwent biopsy; it was unclear how participants for biopsy were selected but it is possible that those with abnormal biochemistry were selected. | ||
| Comparative | |||
| Notes | In all 41 probands, the serum ferritin concentration was grossly elevated (range 670‐4100 µg/L). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Hansen 1983.
| Study characteristics | |||
| Patient Sampling | 38 anaemic patients (24 females; 14 males) 34‐80 years of age (median 65 years of age) with classical or definitive rheumathoid arthritis according to the American Rheumatism Association criteria. Three patients were receiving gold treatment, 10 penicillamine and 4 were receiving cyclophosphamide or azathioprine. | ||
| Patient characteristics and setting | All patients were ill, of both sexes and 50 years and older, with rheumatoid arthritis from Denmark. No patients were receiving iron treatment or had received blood transfusions within six months of the start of the study.. | ||
| Index tests | Serum ferritin was measured using a radioimmunoassay kit (Clinical Assays, Cambridge, USA). This test has a coefficient of variation of 8%. A serum ferritin concentration lower than 60 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | All participants had inflammation as they were all cases of rheumathoid arthritis. Iron deficiency was estimated by bone marrow examination. Posterior iliac crest bone marrow aspirations were performed on all patients. Serial sections were stained for iron with Perls Prussian Blue. The stained material was graded semiquantitatively for iron content as: 0 none; 1: minimal or very small content; 2 slight, small and patchy content; 3: moderate and diffuse; 4 strong, extensive and diffuse content. Grades 0 and 1 were regarded as indicating iron deficiency. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | 38 patients (24 females, 14 males) 34 to 80 years old, were examined to study the importance of serum ferritin and p‐lactoferrin in the anaemia of rheumatoid arthritis. 21 out of 38 randomly selected anaemic patients with classical or definite RA had iron deficiency, as estimated from the iron content in stained bone marrow aspiration. Serum ferritin concentrations below 60 µg/L had sensitivity and specificity for iron deficiency of 86% and 88%, respectively, which was much better than such commonly used variables as s‐iron, p‐transferrin, MCV, and MCHC. Although this cut‐off level is higher than in patients without inflammatory disease, serum ferritin was not correlated to disease activity. In 7 out of 8 patients, the ferritin level rose during iron therapy. P‐lactoferrin values were within the normal range and did not vary with the anaemia or with disease activity. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Low risk | ||
Harada 1992.
| Study characteristics | |||
| Patient Sampling | Study of liver iron measured by MRI and biopsy among patients with ferritin levels above 500 µg/L. Japanese patients with ferritin > 500 µg/L were recruited. The source of patients was unclear. | ||
| Patient characteristics and setting | Ill with patients with chronic hepatitis, liver cirrhosis, hepatic fibrosis (mean age: 58.2 years old) from Kurume, Japan | ||
| Index tests | Serum ferritin measurement approach not mentioned. Elevated serum ferritin concentration (both sexes 1000 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron by histology for risk of iron loading. Liver iron content was quantitatively estimated classifying the amount of blue stained haemosiderin perls deposit in liver biopsy according to 3 categories: ‐ = negative, + = weakly positive, and ++ = strongly positive. The categories + and ++ indicated an iron overload condition. | ||
| Flow and timing | Time scale between liver biopsy and imaging was 1‐2 months; time between ferritin and biopsy not reported | ||
| Comparative | |||
| Notes | Magnetic resonance imaging was useful in determing liver iron overload when the serum ferritin level was over 2000 µg/L. However, a histological study of the liver appears to still be the most accurate examination for the early detection of iron overload in the liver. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Harju 1984.
| Study characteristics | |||
| Patient Sampling | 123 symptomatic patients with gastritis, gastric ulcer and duodenal ulcer (no more details about the sampling were described) | ||
| Patient characteristics and setting | Outpatients, both sexes, of mixed age, with with digestive chronic diseases (gastritis, and ulcer), from Oulu, Finland | ||
| Index tests | Venous blood samples were taken between 8 to 9 am, and serum ferritin concentrations were assayed by an immunoradiometric method using reagent kits from Farmos Diagnostica (Oulunsalo, Finland). The results were standardised against a ferritin standard (human liver ferritin, code 80/602). The coefficient of intra‐assay variation was 3.5‐5.6% and of inter‐assay variation, 10%. A serum ferritin concentration lower than 20 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Sternal bone marrow aspirates were stained by the Prussian blue method. The amount of microscopically visible iron was estimated and graded as ‘absent’, ‘sufficient’ and ‘plenty’. The smears were interpreted by only one examiner, who was not aware of the serum ferritin levels of the patients. If iron in bone marrow was graded absent, it was classify as iron deficiency, and as iron replete, otherwise. | ||
| Flow and timing | The blood and serum ferritin were measured first, and then the sternal bone marrow aspirates were done (period between both tests not mentioned). However, the bone marrow smears were done by one person blinded to the serum ferritin results. | ||
| Comparative | |||
| Notes | There was good parallelism between serum ferritin levels and the amount of bone marrow stainable iron in 123 symptomatic outpatients with gastritis, gastric ulcer and duodenal ulcer. A serum ferritin concentration of about 20‐25 µg/L is the approximate level below which stainable iron could not be demonstrated in the bone marrow. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Low risk | ||
Holmström 2002.
| Study characteristics | |||
| Patient Sampling | 250 healthy controls (hospital staff, students, and their relatives), and 296 patients that were genotypes for novel haemochromatosis HFE mutations after referral due to iron loading to the Division of Clinical Chemistry, Huddinge University Hospital from 1st October 1997 to 19 September 2000 | ||
| Patient characteristics and setting | Adult patients, of both genders (mean age: 47.7 years old) with a rare subtype of HFE haemochromatosis (HFE S65C) from Stockholm, Sweden. Mutation analysis was also done to detect amino acid exchanges C282Y, H63D,and S65C in gene HFE. The setting was the Huddinge University Hospital, Stockholm, Sweden. | ||
| Index tests | Serum ferritin levels were measured by turbidimetry using dye binding reagents (Boehringer Mannheim or Dako) on a Hitachi 917 analyser. Elevated serum ferritin concentration for both sexes > 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron loading by staining. The degree of iron accumulation was graded from 0 (absent), to 1+ indicating weakly to strong iron staining. All cases with iron stains graded as 1+ were classified as iron overload. | ||
| Flow and timing | 296 patients were genotypes for novel haemochromatosis HFE mutations after referral due to iron loading from 1 October 1997 to 19 September 2000. Data from liver biopsy, staining, and serum ferritin measures were collected retrospectively. At the end, only a minority of patients were included in the analysis because of missing data either of serum ferritin or liver biopsy measures. For the sake of the review, only 7 of 296 patients had serum ferritin and liver biopsy measures. | ||
| Comparative | |||
| Notes | The haemochromatosis HFE S65C mutation may lead to mild to moderate hepatic iron overload but neither clinically manifest haemochromatosis or iron associated extensive liver fibrosis was encountered in any of the patients carrying this mutation. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Holyoake 1993.
| Study characteristics | |||
| Patient Sampling | 472 consecutive new referrals to a geriatric medical unit, 330 inpatients and 142 outpatients, were studied prospectively. Of those, bone marrow examination was performed in only 32 of 78 patients with serum ferritin of 12‐45 µg/L. | ||
| Patient characteristics and setting | 472 new referrals to a geriatric medical unit, 330 inpatients and 142 outpatients. 333 women (mean age 82 years) and 139 men (mean age 79 years) with diverse chronic diseases or who were elderly | ||
| Index tests | A standard venous blood sample was taken from all patients for a full blood count (Coulter S Plus IV) and plasma ferritin measured (immunoradiometric assay, Ciba‐Corning). Serum ferritin concentration lower than 45 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow examination was performed in only 32 of 78 patients with serum ferritin of 12‐45 µg/L. Absence of iron in Perls staining indicated iron deficiency, and presence of iron, otherwise. | ||
| Flow and timing | A bone marrow aspiration after complete clinical examination was performed but only for patients with serum ferritin of 12‐45 µg/L. | ||
| Comparative | |||
| Notes | 333 women (mean age 82 years) and 139 men (mean age 79 years) were studied prospectively. For those with ferritin of 12‐45 µg/L, bone marrow aspirates were performed and examined for the presence of stainable iron. When possible, a trial of oral iron was given to those with ferritin of < 45 µg/L and response was determined by remeasurement of the full blood count and ferritin after a minimum of three weeks of treatment. Bone marrow examination was performed in 32 patients with ferritin of 12‐45 µg/L, of whom 27 (84%) had absent stainable iron, suggesting that most elderly patients with ferritin in this range have iron deficiency. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Intragumtornchai 1998.
| Study characteristics | |||
| Patient Sampling | 72 consecutive anaemic outpatients with hepatic cirrhosis assisted at a hospital in Bangkok between January 1992 and December 1994 | ||
| Patient characteristics and setting | Consecutive anaemic patients with liver cirrhosis (age > 15 years, mean 49 years), both sexes; 55% were alcoholic, 44% with spontaneous bacterial perotonitis, 44% with other infections. Patients admitted to the Chulalongkorn University Hospital, Bangkok, between January 1992 and December 1994 | ||
| Index tests | Serum ferritin concentration was measured by the immunoradiometric assay using 125 I‐labeled antiferritin monoclonal and polyclonal antibody (Diagnostic Products Corporation, Los Angeles). Cut‐off of 50 µg/L was recommended. Serum ferritin concentration lower than 50 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow specimens were obtained by aspiration from the posterior iliac crest at the time the blood sample was collected. The air‐dried smears were stained by Wright’s and Prussian blue. Iron deficiency was diagnosed when the Prussian blue study revealed no stainable iron in the bone marrow. | ||
| Flow and timing | 72 consecutive anaemic outpatients seen between January 1992 and December 1994. From an initial selection of 75 patients with liver cirrhosis, three patients were excluded from the analysis, one suspected with malignancy with a hepatic focal lesion, one with haemoglobin H disease and another with an associated renal impairment. All measurements were made the same day. | ||
| Comparative | |||
| Notes | By using absence of bone marrow iron as the standard criterion, the diagnostic powers of mean corpuscular volume, transferrin saturation, serum ferritin and the presence of hypochromic red cells in the diagnosis of iron deficiency were compared by analysing the likelihood ratios, the area under the receiver operating curves and the stepwise logistic regression associated with each test. Twenty‐nine patients (40.3%) demonstrated no stainable iron in the bone marrow. The likelihood ratios associated with the serum ferritin levels was 22.3 for ferritin concentrations < 50 µg/L indicating that a serum ferritin level < 50 µg/L depicts a very high probability of iron deficiency anaemia (0.83–0.99) irrespective of the patient’s pre‐test probability. The likelihood ratios, the area under the ROC and the stepwise logistic regression indicated that serum ferritin was the most powerful test predictive of iron deficiency. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Low risk | ||
Isa 1988.
| Study characteristics | |||
| Patient Sampling | 42 hospitalised male patients with liver damage; 20 healthy controls were chosen but no bone marrow or liver biopsy was performed on them. | ||
| Patient characteristics and setting | 42 hospitalised male patients aged 39‐69 years with alcoholism or liver damage | ||
| Index tests | Serum ferritin in ill patients, determined in duplicate using a commercial immunoradiometric assay (Magic Ferritin I Ciba‐Corning Diagnostic Medfield, Mass, USA). No cut‐off mentioned | ||
| Target condition and reference standard(s) | Bone marrow smears, obtained by puncture of the posterior ilian spine, were stained for iron by Pearls reaction. The amount of stainable iron was assessed as described by Ploem 1966 in 5 grades of stainable iron: grade 0 with absent or reduced bone marrow iron stores, grade 1 and 2 with normal iron stores, and grade 3 and 4 to increased iron stores. | ||
| Flow and timing | The study included 42 hospitalised male patients with established diagnosis of alcoholism from the Ospidale di Gorgonzola in Italy. No more information was given about the timing or flow. | ||
| Comparative | |||
| Notes | Serum iron and ferritin, total iron‐binding capacity levels were unrelated to iron deposits, whereas RCF concentration was a good index of iron stores detected by direct assessment on bone marrow and liver biopsy specimens. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Jensen 1994.
| Study characteristics | |||
| Patient Sampling | Patients with hereditary haemochromatosis, of whom 16 were homozygotes and four heterozygotic siblings | ||
| Patient characteristics and setting | lll, adults, both genders, from Aarhus, Denmark with hereditary haemochromatosis | ||
| Index tests | Serum ferritin was by a commercially available immunometric technique, based on enhanced luminescence (Amerlite Ferritin assay, monoclonal). Specimens with a value greater than 230 pg/L were re‐analysed after dilution. Determinations of standards and samples were performed in duplicate. Quality control experiments revealed coefficients of variation within and between, varying from 5.1% to 8.1%. The sensitivity of the assay was < 0.3 pg/L and the recovery after addition of a known amount of analysate to the sample ranged from 105.7% to 114.9%. | ||
| Target condition and reference standard(s) | Histology of liver biopsy, MRI and Perls staining was performed. Liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | Histological liver biopsy and MRI examinations were undertaken in parallel with serum ferritin concentration measurements. | ||
| Comparative | |||
| Notes | The decrease in serum ferritin concentration per month as an index of the mobilisation rate of iron from the total body iron stores had a wide range (1‐252 µg/L per month). In comparison, the iron mobilisation rate from the liver, when assessed as the decrease of the MRI‐determined liver iron concentration per month, varied from 4 to 42 µmol Fe/g dry weight per month. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Jonker 2013.
| Study characteristics | |||
| Patient Sampling | 381 children (6–60 months old) presenting with severe anaemia (haemoglobin 5.0 g per decilitre) were enrolled as ‘cases’ if they had not been transfused in the previous 4 weeks. Bone marrow was performed on those whose clinical condition allowed it, yielding 207 patients. | ||
| Patient characteristics and setting | Male and female, mean age 14 months, severe anaemia, with 60% suspected of malaria, 30% iron deficient, 7.7% HIV, and 81.2% high CRP (> 40 mg/L). | ||
| Index tests | Serum ferritin for ill children, cut‐off for ID was 30 mg/L. Ferritin was determined using electro‐chemiluminescence immunoassay (Modular Analytics E170, Roche Diagnostics, Switzerland). | ||
| Target condition and reference standard(s) | Bone marrow slides were stained with Hematognost Fe (Merck, Darmstadt, Germany) and graded for intracellular iron content using a histological grading method which classifies iron status into six grades (0–6). In addition, all marrow smears were also assessed using an alternative grading method where utilisable iron was specifically assessed through determination of iron in the erythroblasts. At high power magnification (*1000), 20 fields surrounding the marrow fragments and a hundred erythroblasts were examined to enumerate the percentage containing iron granules in their cytoplasm. Deficiency of bone marrow iron stores was defined as grade 0 or 1. | ||
| Flow and timing | This study formed part of a large research programme conducted between 2002 and 2004 investigating the aetiology, pathogenesis and outcome of severe anaemia in Malawian children. At recruitment, a venous blood sample was obtained and, in the cases where the clinical condition allowed, a bone marrow aspiration was performed. | ||
| Comparative | |||
| Notes | 207 severely anaemic children were assessed for levels of hepcidin, ferritin, serum transferrin receptor, erythropoietin, haematological indices, C‐reactive protein, interleukin‐6, malaria parasites and HIV infection. Deficiency of bone marrow iron stores was graded in 139 cases and erythroblast iron incorporation estimated. Interaction of covariates was assessed by structural‐equation‐modelling. Hepcidin was a poor predictor of bone marrow iron deficiency (sensitivity 66.7%; specificity 48.5%), and of iron incorporation (sensitivity 54.2%; specificity 61.8%), and therefore would have limitations as a point‐of‐care test in this category of children. The study evaluated hepcidin as a predictor of iron deficiency and also had analysis on ferritin. For the hepcidin study, all cases with available bone marrow aspirates were included. In order to compare hepcidin levels between severely anaemic and non‐severely anaemic children, hepcidin was also assessed in a subsample of controls (without available bone marrow aspirates). |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Jonker 2014.
| Study characteristics | |||
| Patient Sampling | Participants were prospectively identified from children aged 6–66 months scheduled for elective surgery. | ||
| Patient characteristics and setting | 87 healthy Malawian children (6–66 months, mean age 37 months); 39 children (44.8%) had depleted bone marrow iron stores and 64% were anaemic with mean ferritin concentration 19 (12–36) µg/L. Forty‐two and a half per cent were female, 6% presented with HIV and there was no malaria. | ||
| Index tests | Ferritin was assessed following the manufacturer's procedure using a Beckman Coulter Chemistry System DxL880i with reagent kits from Beckman Coulter. An optimised cut‐off of ferritin < 18 µg/L was proposed for this population; levels of 12 µg/L and 30 µg/L were also studied. | ||
| Target condition and reference standard(s) | Bone marrow smears were prepared, fixed with methanol, and stored in a dry place at room temperature. To minimise diagnostic variation, fixed smears were all stained in one batch at the end of the study period (Hematognost Fe, Merck, Darmstadt, Germany) and graded for iron content. Histological grading classified iron status into six grades. Depleted bone marrow iron stores were defined as grade 0 or 1. | ||
| Flow and timing | Participants were prospectively identified from children scheduled for elective surgery at Queen Elizabeth Central Hospital and Beit Cure Orthopedic Hospital, in Blantyre, Malawi. The recruitment procedure included a detailed history and physical examination, a venous blood sample and a bone marrow aspiration made the same day. Both samples were collected during generalised anaesthesia and prior to surgical intervention. Between March and October 2011, a total of 93 children were recruited. In four children, the bone marrow aspiration resulted in a ‘dry tap’. In two children, bone marrow sample quality was insufficient (no marrow fragments found); these children were excluded from analysis. | ||
| Comparative | |||
| Notes | Exclusion criteria: blood transfusion within the previous 4 weeks, signs of infection (axillary temperature > 37.5°C or current infectious diagnosis, (suspected) neoplasm, known haemoglobinopathy, or a haemoglobin level below 8.0 g/dL (local guidelines for elective surgery). The generally accepted cut‐off to detect iron deficiency in preschool children in areas of low‐infection exposure is < 12 µg/L. In this population, this cut‐off had poor sensitivity despite a low prevalence of current infection in the study population (5.7%). A raised cut‐off of 30 µg/L is recommended for use in malaria endemic areas. Applying this latter cut‐off in the present population increased sensitivity (81.6%), but specificity was low (37.5%). This suggests that a cut‐off of 30 µg/L is too high for use in an otherwise healthy African population; the cut‐off with maximal accuracy in predicting iron‐deficient stores was < 18 µg/L, which gave a sensitivity of 73.7% and specificity of 77.1%. We contacted the authors, who assured us that the selection of the children was under the assumption of healthy children meant for orthopaedic surgery. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Joosten 2002.
| Study characteristics | |||
| Patient Sampling | 83 prospectively identified elderly patients with anaemia | ||
| Patient characteristics and setting | 83 elderly patients with anaemia (mean age 83 years; 49 females, 34 males) admitted to an acute geriatric ward of the University Hospitals of the K. U. Leuven. 22 were diagnosed with a benign gastrointestinal lesion, 5 had cancer, 2 pneumonia, 5 inflammatory diseases and 2 no clear aetiology. 23 were classified as ACD‐chronic inflammation. | ||
| Index tests | Serum ferritin was determined using AxSym analyzer from Abbott, in morning specimens, collected after an overnight fast. Reference values used were 16‐330 μg/L in males and 12‐230 μg/L in females. | ||
| Target condition and reference standard(s) | Bone marrow smears were stained using the May‐Grunwald‐Giemsa method, and the iron stores in marrow were investigated by the Prussian blue method. | ||
| Flow and timing | No mention of flow and timing, except for the overnight fast for the s‐ferritin samples. | ||
| Comparative | |||
| Notes | The aim of the study was to evaluate in an elderly hospitalised population the diagnostic value of sTfR in distinguishing IDA from ACD as compared to s‐ferritin. The conclusion was that serum ferritin is a more sensitive and specific parameter than the sTfR assay to predict the bone marrow status in an elderly anaemic population. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Kalantar‐Zadeh 1995.
| Study characteristics | |||
| Patient Sampling | Anaemic patients with chronic renal failure that underwent bone marrow examination to clarify the cause of the anaemia | ||
| Patient characteristics and setting | 25 anaemic patients (13 men, 12 women aged 44 to 84 y) with chronic renal failure were evaluated at the Fourth Medical Clinic, University of Erlangen‐Nurnberg. Nine patients had type II diabetes mellitus, six had interstitial nephritis with suspected analgesic nephropathy, one had polycystic disease, one had multiple myeloma, five had glomerulonephritis, and three had hypertensive nephrosclerosis. | ||
| Index tests | The serum ferritin value was measured by an immunoradiometric assay with polyclonal reagents. The study had a cut‐off of 200 ng/mL. | ||
| Target condition and reference standard(s) | Iron status was evaluated by bone marrow examination. Posterior iliac crest bone marrow aspirations and/or bone marrow biopsies were performed in all 25 patients within 72 hours of the haematologic investigations. The bone marrow was fixed in phosphate‐buffered saline, 4% formaldehyde, and then embedded in paraffin. Serial sections were stained for iron with Perl’s Prussian blue stain, as described elsewhere. The slides were graded as follows: 0, no iron; +1, slight amount of iron; +2, patchy iron stores present; +3, patchy to diffuse staining; +4, diffuse iron staining; and +5, extensive iron staining (iron overload). Normal values for bone marrow iron content for iron staining ranged from +3 to +4 according to this scale. | ||
| Flow and timing | From January to August 1993, 25 patients with chronic renal failure were evaluated at the Fourth Medical Clinic in Erlangen‐Numberg. Each patient underwent a bone marrow examination to clarify the anaemia after history, physical examination, haematologic values, folate and B12 concentrations left doubt as to the cause of the anaemia. | ||
| Comparative | |||
| Notes | The purpose of the study was to evaluate the accuracy of laboratory methods in the diagnosis of posterythropoietin‐era ID in chronic renal failure patients. Authors concluded: "On the basis of our findings we recommend both measurements of serum ferritin and determination of the transferrin saturation. The former test offers excellent specificity, while the latter offers acceptable sensitivity. Patients with transfer‐tin values of less than 150 mg/dL should be considered separately. Those patients deserve an increased index of suspicion with respect to iron deficiency. We believe that in this fashion, iron therapy in patients with chronic renal insufficiency can be improved. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kaltwasser 1990.
| Study characteristics | |||
| Patient Sampling | Twelve patients with HLA‐linked haemochromatosis, one patient with transfusion‐acquired thalassaemia. Nine subjects were healthy volunteers, among them three blood donors. Biopsy was taken from 2 healthy subjects and 9 patients with haemochromatosis. | ||
| Patient characteristics and setting | 22 persons: four females, and 18 males (aged betweetn 22 to 70 with a mean of 39 years old) from Frankfurt, Germany. Nine subjects were healthy volunteers, among them three blood donors. 12 patients suffered from hereditary HLA‐related haemochromatosis and one patient had thalassaemia major and haemochromatosis secondary to transfusion‐induced iron overload. | ||
| Index tests | Ferritin was determined using a commercially available enzyme‐immunoassay (Spectro‐ Ferritin. Ramco, Houston, Texas, USA). | ||
| Target condition and reference standard(s) | Liver iron was measured with liver biopsy. Non‐haem (not precursive to haemoglobin) hepatic iron concentration dry weight was measured by the chemical method described by Torrance 1980. Liver iron concentration measurement with a threshold over ( > ) 1.8 mg/g dry weight corresponds to iron overload. | ||
| Flow and timing | Serum ferritin was measured in parallel with MR‐imaging and liver biopsy. Liver tissue was obtained by needle biopsy only for two healthy subjects and nine patients with iron overload. Serum ferritin was measured for 21 persons. | ||
| Comparative | |||
| Notes | Ferritin ranged from 4 to 151 µg/L in the nine control subjects and from 37 to 5133 µg/L in the 13 patients with iron overload syndromes. Six of the male patients with hereditary haemochromatosis had ferritin concentrations within the normal range, due to previous treatment by phlebotomy. Quantitative liver iron determinations in liver needle biopsies in parallel to SF measurements were performed in two healthy control subjects and in nine patients with various degrees of iron overload. Three of the nine patients with iron overload showed otherwise unchanged liver histology, four had fibrosis and two showed complete cirrhosis of the liver. The limited number of biopsies is due to the ethical constraints in healthy subjects and contraindications due to coagulation disorders in several subjects with iron overload. SF and chemically determined liver iron were correlated with the transversal (spin spin) relaxation time of liver tissue. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Karlsson 2010.
| Study characteristics | |||
| Patient Sampling | 50 consecutive anaemic inpatients, cross‐sectional, unselected | ||
| Patient characteristics and setting | Fifty consecutive newly diagnosed anaemic patients 60 years of age or older (mean 80 years) admitted to the hospital were included in this study. Patients receiving iron supplementation or who had received red blood cell transfusions before testing were excluded. Patients presented with: haematological/lymphatic malignancies, breast, ovarian, colon and prostate cancer, infection (5 cases), renal failure, benign gastrointestinal haemorrhage, hairy cell leukaemia, acute arthitis and congestive heart failure. | ||
| Index tests | Serum ferritin. Standard methods were used to determine blood count and biochemical iron status (no method reported). Analyses were performed at the Department of Clinical Chemistry, Sankt Gorans Hospital, Stockholm, Sweden. A cut‐off of 40 mg/L was used. | ||
| Target condition and reference standard(s) | Bone marrow smears were stained by means of the May‐Grunwald‐Giemsa method. Bone marrow iron stores were investigated using Prussian blue staining. Patients with no readily stainable iron were diagnosed as having IDA, whereas the diagnoses for those with readily stainable iron were ACD or other non‐iron deficiency anaemia. | ||
| Flow and timing | A complete blood count, biochemical iron status (serum iron, TIBC, TSAT, ferritin), sTfR and bone marrow iron stores were analysed in every patient. No details on timing were reported. | ||
| Comparative | |||
| Notes | This study was a letter to the editor of the Journal of Internal Medicine. Authors concluded that "the serum soluble transferrin receptor assay is inferior to serum ferritin (when using a clinical cut‐off point of 40 ng/L) with respect to sensitivity and specificity, and to the TfR‐F Index with respect to specificity using a cut‐off point of 3.0, during screening for iron deficiency in an unselected population of elderly anaemic patients". | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Karlsson 2015.
| Study characteristics | |||
| Patient Sampling | Forty patients, 65 years of age or older, who were admitted to the Department of Haematology for anaemia (Hb < 130 g/L in men and < 120 g/L in women) with evaluation from their Family Physicians, from the Emergency Department or from the Department of Rheumatology at the Hospital were screened for this study. The screening period was from June 2012 to January 2014. | ||
| Patient characteristics and setting | Patients who had received iron supplementation or red blood cell transfusions within 1 month before testing were excluded from the study, as were patients with B12 or folate deficiency, haemolytic anaemia or any haematologic malignancy. Nine of the forty screened patients suffered from myelodysplastic syndrome, lymphoma in the bone marrow or acute haemorrhage and were excluded. This study was performed at the Department of Haematology, Uppsala University Hospital, Uppsala, Sweden. | ||
| Index tests | Ferritin was analysed using ARCHITECT Plus® ci 16200 (Abbot Diagnostics). | ||
| Target condition and reference standard(s) | Bone marrow smears were stained using the May‐Grunwald‐Giemsa method, and bone marrow iron stores were investigated using Prussian blue staining. All patients had elevated levels of C‐reactive protein. They were divided into two groups. Patients with no stainable bone marrow iron (n = 11) were diagnosed as having iron deficiency with concurrent inflammation. The diagnosis of those with stainable iron (n = 20) was anaemia of inflammation. | ||
| Flow and timing | All analyses, with the exception of bone marrow and hepcidin‐25 analyses, were performed at the Department of Clinical Chemistry, Uppsala University Hospital. The bone marrow analyses were performed at the Department of Pathology, Uppsala University Hospital. The flow and timing was unclear. | ||
| Comparative | In this study, mass spectrometry was used to evaluate the hepcidin‐25 assay in the differential diagnosis of iron deficiency anaemia with concurrent inflammation and anaemia of inflammation in elderly patients using the absence of stainable bone marrow iron as the gold standard criterion for iron deficiency. | ||
| Notes | The study was approved by the Regulatory Ethics Committee of Uppsala and performed in accordance with the Declaration of Helsinki. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Kim 2000.
| Study characteristics | |||
| Patient Sampling | Fifty‐five anaemic patients who visited a rheumatology clinic between March 1999 and February 2000 were randomly enrolled in this study. A subsample of those patients (18) underwent a bone marrow iron stain procedure. | ||
| Patient characteristics and setting | All 55 patients met the 1987 American College of Rheumatology revised criteria for the classification of rheumatoid arthritis (both sexes, 7 males, and 48 females with a mean age of 50.1 +/‐ 13.4 years old). The patients were attending the Department of Rheumatology/Medicine, Inha University School of Medicine, Republic of Korea. A subsample of 18 patients underwent a bone marrow procedure (both sexes, mixed age with mean 50.1 years old). | ||
| Index tests | Serum ferritin was measured by radioimmunoassay (Fer‐Iron II, Ramco, Houston, Tex., USA). Serum ferritin concentration lower than 12 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow specimens were obtained from the iliac bone. A clinical pathologist blinded to the clinical information determined the marrow iron status using Prussian blue staining. On the basis of the bone marrow study, the patients were divided into two subgroups, those with (iron non‐depleted) and without (iron‐depleted) signs of stainable iron in the marrow. | ||
| Flow and timing | Only 18 patients who consented underwent the bone marrow study and all patients had the haematologic markers. The order of the tests was not reported. | ||
| Comparative | |||
| Notes | 55 anaemic patients (7 men, 48 women with a mean age of 50.1 y), with rheumatoid arthritis underwent the anaemia study. Bone marrow iron stain was performed in 18 patients. sTfR and serum ferritin levels were compared with bone marrow iron stores. Mean sTfR concentration was 2.63 +/‐ 1.91 mg/L and correlated with most indicators of anaemia, except for CRP and ESR. Mean ferritin concentration for the whole group was 56.02 +/‐ 60.19 ug/L. 18 patients (16 women and 2 men) agreed to bone marrow aspiration. 5 patients had no iron in bone marrow and 13 had detectable iron in their bone marrow. Ferritin concentration was 7.80 +/‐ 3.16 for the deficient group and 19.93 +/‐ 11.04 for the replete group. For serum ferritin, a cut‐off point of 12 µg/L, had a 100% sensitivity and specificity to detect iron deficiency in the study group. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Kis 1998.
| Study characteristics | |||
| Patient Sampling | Retrospective chart review after a computer search of 101 anaemic veterans with any medical condition who underwent bone marrow aspiration and serum iron studies. | ||
| Patient characteristics and setting | Retrospective chart review of 101 anaemic veterans (99 males, 2 females, 68 years and older) with any medical condition who underwent bone marrow aspiration and serum iron studies in hospitals of the Department of Veterans Affairs in Madison and Milwaukee, Wisconsin. | ||
| Index tests | Serum ferritin measured by automated machine (method not specified). Serum ferritin concentration lower than 100 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The bone marrow aspirates were prepared and examined in the clinical laboratories at the two hospitals. All were obtained from the iliac crest placed in EDTA acid, concentrated, smeared on glass slides, and cover‐slipped. Stains for morphology were prepared in Wright‐Giemsa, and iron was stained with Prussian blue. Bone marrow iron was examined under oil immersion to look for ringed sideroblasts. Iron was quantified using a grade of 0 to 6. Iron deficiency was defined as the total absence of bone marrow hemosiderin (grade 0). | ||
| Flow and timing | Study of 101 anaemic veterans with any medical condition who underwent bone marrow aspiration and serum iron studies. Haematological and biochemical analyses (such as serum ferritin), and reviews of medical records were undertaken. However, the flow and timing of these three kind of tests was not described. It was specified that in the 13 cases where bone marrow examination indicated absence of iron, this was revised by a haematologist blinded to SF, and other clinical history results. Furthermore, all the tests were performed in a period of no more than 30 days. | ||
| Comparative | |||
| Notes | In this study of anaemic veterans with concomitant medical illnesses, it was found that a threshold of 100 µg/L outperformed MCV, transferrin saturation, and TIBC, either alone or in combination in correctly diagnosing iron deficiency. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Kotru 2004.
| Study characteristics | |||
| Patient Sampling | Fifty‐five anaemic patients with tuberculosis attending a large tertiary health care provider in Delhi were selected for the study (no further details in the sampling method were given). | ||
| Patient characteristics and setting | 55 adult anaemic patients with tuberculosis (31 females, 24 males) attending a large tertiary health care provider in Delhi, India | ||
| Index tests | Serum ferritin levels were taken with ELISA method (Diagnostic Automation, USA, Microwell ELISA). A serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow iron content was semi‐quantitatively estimated classifying the amount of blue stained haemosiderin perls in bone marrow fragments (aggregates of bone marrow cells) according to 6 categories (absent = 0). BM was assessed and graded by two independent observers. The category 0 was considered indicative of iron stores deficiency, and 1 or more iron replete. | ||
| Flow and timing | Haematologic and biochemical analyses (such as serum ferritin), and bone marrow aspiration measurements, were undertaken. However, the flow and time of these two kind of tests were not described. While performing analysis, first the TB patients were classified according to ID and iron replete BM iron staining results, and afterwards all biochemical analyses were compared between the two groups for any statistical significant difference. | ||
| Comparative | |||
| Notes | 55 adult anaemic patients with tuberculosis (31 females, 24 males), grouped as 39 iron deplete (ID) and 16 iron replete (IR) based on bone marrow iron (BMI). At SF levels of < 10 µg/L, only 23/39 patients (58%) were classified as ID. However, at values < 30 µg/L, 36 out of 39 patients (92%) were correctly classified as ID. A cut‐off value of < 30 µg/L was considered best as it had maximum sensitivity (90%) as well as the highest negative predictive power (75%) with a reasonable specificity (75%) and positive predictive power (90%). It was observed that the sensitivity of SF decreased with higher cut‐off values, whereas when lower cut‐off values were used, specificity decreased dramatically. Therefore, raising cut‐off SF levels between > 10 µg/L and < 30 µg/L was most effective in predicting absent BMI, especially in a population where ID was highly prevalent. The combination of SF < 30 µg/L with mean corpuscular volume (MCV), erythrocyte sedimentation rate (ESR) and total iron binding capacity (TIBC) did not improve the predictive power of SF further. Also, 89.5% of cases could be correctly classified by logistic regression equations using SF with ESR and C‐reactive protein (CRP). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Krause 1980.
| Study characteristics | |||
| Patient Sampling | Retrospective study with 1900 trephine biopsies (out of 2,400 biopsies performed during 2 years (1988‐1989) at the Presbyterian‐University Hospital in Pittsburgh) that could be evaluated for iron. A specimen was included only if an adequate core of marrow was present. A subsample of 1400 who had marrow biopsies had been concurrently drawn for haematological iron store indicators including serum ferritin. A smaller subsample of 193 biopsies was selected on the basis of low serum iron (below 65 µg/dL) and percentage serum iron ratios Fe/(total iron binding capacity) below 15%. | ||
| Patient characteristics and setting | 193 bone marrow specimens of low serum iron and percentage serum iron ratios below 15% were taken from patients who attended at the Presbyterian‐University Hospital in Pittsburgh. Patients of both sexes and mixed age with diverse chronic diseases: ID, liver/renal disease, malignancies, and chronic inflammation. | ||
| Index tests | Serum ferritin was performed by a two‐stage immunoassay modified from technic of Miles and associates of an original method developed by Addison 1972. A serum ferritin concentration lower than 20 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Neddle biopsy specimens were taken from the posterior superior iliac spine with a Westermman‐Jensen or Jasmine needle. A specimen was included only if an adequate core of marrow was present. The Prussian blue method was used for iron staining. Bone marrow biopsy was graded as absent or markedly decreased, present or increased iron. The iron state classification was made without previous knowledge of the clinical status or laboratory information. The category of absent or marked decrease was considered indicative of iron store deficiency. | ||
| Flow and timing | Retrospective study with 1900 trephine biopsies (out of 2400 biopsies) performed during 2 years (1988‐1989) at the Presbyterian‐University Hospital. A subsample of 1400 who had marrow biopsies had been drawn for haematological iron store indicator measurements. A smaller subsample of 193 biopsies was selected on the basis of low serum iron (below 65 µg/dL) and percentage serum iron ratios Fe/(total iron binding capacity) below 15%. Bone marrow aspirates and haemotologic indicator measurements were done over the 1400 biopsies without previous knowledge of the results of each of them. | ||
| Comparative | |||
| Notes | Serum ferritin levels proved to be useful in distinguishing the uncomplicated iron‐deficient state from other disorders associated with low serum iron and low percentage saturation of transferrin. However, a small percentage of ID persons may have liver disease or haematopoietic or lymphoreticular neoplasms. In these cases, assessment of the bone marrow biopsy iron would still be necessary to evaluate the body iron stores. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Lawrence 1996.
| Study characteristics | |||
| Patient Sampling | Patients referred for evaluation of haemochromatosis and liver biopsy using standard serologic criteria: fasting transferrin saturation > 55% or fasting serum ferritin > 400 ng/mL in males or > 300 ng/mL in females | ||
| Patient characteristics and setting | Patients with hereditary haemochromatosis were prospectively studied at Fitzsimons Army Medical Center located in Arizona, United States (aged between 26 to 70, with mean of 55 years old). They were referred for iron overload, including a subset with demonstrated iron overload. | ||
| Index tests | Serum ferritin, method not described | ||
| Target condition and reference standard(s) | Tissue iron analysis was performed at the Mayo Metals Laboratory (Rochester, MN, USA) by graphite atomic absorption spectrophotometry. Hepatic iron/age index >= 2.0, was obtained by dividing the tissue iron concentration, in micrograms per gram, by (55.8 × age). Liver iron was measured with liver biopsy. Liver iron content measurement with a threshold of > 1.8 mg/g dry weight corresponds to iron overload. | ||
| Flow and timing | Forty‐three patients without a prior diagnosis of HC were prospectively studied between 1991 and 1993. Patients were referred for evaluation of HC and liver biopsy using standard serologic criteria (including fasting transferrin saturation and fasting serum ferritin). Tissue iron analysis was performed at the Mayo Metals Laboratory (Rochester, MN, USA). Specimens were taken from selected patients, and were sent for analysis in an iron‐free container (the period of time after the serologic measures were taken was not specified). | ||
| Comparative | |||
| Notes | Hepatic iron concentrations of the liver biopsy specimens ranged from 441 to 22,400 µg/g, with normal values of 200‐2000 µg/g in men and 200‐1600 µg/g in women. Twenty‐six patients (60%) had quantitative iron levels within the normal range. Of those with homozygous HC, hepatic iron ranged from 6570 to 22,400 µg/g (mean, 13,007 µg/g), with six men and four women in this group. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Leggett 1990.
| Study characteristics | |||
| Patient Sampling | Participants selected from large corporations (one banking and one insurance) (age between 21 and 45 years old). However, only a minority of high‐risk participants were included. Subjects who had an initial transferrin saturation > 45% were recalled. Transferrin saturation and serum ferritin concentration measurements were repeated and if transferrin saturation had fallen below 45%, no further follow‐up was undertaken. If the transferrin saturation remained above 45%, subjects were interviewed and examined. Those with a consistently elevated serum ferritin concentration (males > 200, females > 150 µg/L) underwent percutaneous needle biopsy of the liver. | ||
| Patient characteristics and setting | The overall population was appropriate (i.e. apparently healthy individuals). Selection done from two large corporations from Brisbane, Australia (both genders, age between 21 and 45 years old). In the bank population, 1332 individuals (97.4%) were Caucasian, 23 (1.7%) Australian Aboriginal and 12 (0.9%) Asian, and in the insurance population 569 individuals (94.9%) were Caucasian, 12 (2.0%) Australian Aboriginal and 19 (3.1%) Asian. However, liver biopsy was only done in a very small number of high‐risk individuals with hereditary haemochromatosis. |
||
| Index tests | Serum ferritin. Venous blood was drawn from the non‐fasting subjects between 9 and 11 before midday, and serum was stored at ‐20°C until analysis. Serum ferritin concentration was measured by immunoradiometric assay using the Magic Fer kit (Ciba‐Corning) or standardised reagents suggested by Halliday 1975. Elevated serum ferritin concentration (males > 300, females > 200 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron loading defined by elevated liver iron content. Hepatic iron concentrations were measured by atomic absorption spectrophotometry and the hepatic iron index (hepatic iron concentration/age in years) was calculated as shown in Basset 1986. Liver iron content measurement with a threshold of > 2 mg/g dry weight corresponds to iron overload. | ||
| Flow and timing | Only a minority of participants (those with elevated biochemical iron indices) had liver iron measured. After a first selection of subjects with constant elevated transferrin saturation > 45%, a second selection of subjects with a consistently elevated serum ferritin concentration (males > 200, females > 150 µg/L) was made. Those underwent percutaneous needle biopsy of the liver. | ||
| Comparative | |||
| Notes | We concluded that approximately 1 in 300 individuals in the Australian population are likely to have iron overload due to homozygosity for haemochromatosis and that these individuals can be detected in the early asymptomatic phase by screening. If transferrin saturation is consistently elevated and serum ferritin concentration is also high, the diagnosis should be confirmed by liver biopsy with measurement of the hepatic iron concentration and therapy commenced. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Lewis 2007.
| Study characteristics | |||
| Patient Sampling | Adult patients consecutively admitted to the medical wards of Queen Elizabeth Central Hospital (approximately 800 patients per month) in Malawi. From those, 81 severely anaemic adults (haemoglobin < 70 g/L) were selected for the study. All of them had bone marrow aspirates and haemotological iron tests. | ||
| Patient characteristics and setting | A subsample of 81 severely anaemic adults received in the medical wards of Queen Elizabeth Central Hospital, the main provider of secondary care in Blantyre, the largest city in Malawi, was selected for the study. There were 47 women and 34 men aged from 18 to 88 years old (mean age of 37.6 years old). 59% of them had AIDS (defined by a CD4+ count < 200 cells/L.). Others had tuberculosis (23 patients (28%)), and 17 (21%) had positive bacterial blood cultures of predominantly non‐typhoidal salmonella (9 patients). The setting of Blantyre has a very high HIV seroprevalence. | ||
| Index tests | Serum ferritin was measured by immunoenzymometric assay (Ramco, Houston, TX, USA). Serum ferritin concentration lower than 150 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspirates were all examined by a haematologist blinded to clinical and laboratory details and were confirmed by a second independent haematologist. Marrow films were stained for iron using Prussian Blue (Perl's method) and classified as: 0, no iron present; 1, trace of iron; 2, normal iron; 3, plentiful iron; and 4, excessive iron. The categories 0 and 1 were considered indicative of iron store deficiency. |
||
| Flow and timing | Adult patients consecutively admitted to the Queen Elizabeth Central Hospital in Malawi. From those, a selection of 81 severely anaemic adults underwent bone marrow aspirates and haematologic iron status indicators. Both test types were undertaken blinded from the results of the other tests. | ||
| Comparative | |||
| Notes | 81 severely anaemic adults entering a hospital in Blantyre, Malawi, with satisfactory bone marrow aspirates. Ferritin levels were high in this population (n = 81), with a mean of 1370 µg/L. The main outcome measures were the validity of each test (sensitivity, specificity, and positive and negative predictive values) and likelihood ratios (LR) for iron deficiency. Twenty patients (25%) were iron deficient and 64 (79%) were HIV‐positive. Iron deficiency was more common in HIV‐negative compared with HIV‐positive patients (59% vs 16%; P < 0.001). In HIV‐positive patients, the optimal ferritin cut‐off was 150 ug/L (sensitivity 20%, specificity 93%, LR 2.7), but no test was accurate enough to be clinically useful. In HIV‐negative patients, ferritin was the single most accurate test (cut‐off < 70 ug/L, 100% specificity, 90% sensitivity, LR if positive ∞, LR if negative 10). In HIV‐negative patients, ferritin was the best blood test for iron deficiency, using a higher cut‐off than usual. For HIV‐positive patients, it was difficult to diagnose iron deficiency without bone marrow aspirates. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lim 2004.
| Study characteristics | |||
| Patient Sampling | A total of 246 patients were referred to the Hepatology Clinic at a tertiary hospital in Western Australia for HFE genotyping and further assessment of elevated serum transferrin saturation and ferritin. A total of 19 patients who were compound heterozygous for HFE were the subjects of the study. All had liver biopsy, quantitative liver iron estimation and liver histopathology. Liver biopsy was undertaken only when there was marked iron loading and evidence of liver impairment/transaminitis. | ||
| Patient characteristics and setting | Ill patients (18 males, and 1 female from Perth, Australia) with hereditary haemochromatosis referred to the Hepatology Clinic at a tertiary hospital (age betweetn 27 to 75 with mean of 51 years old). | ||
| Index tests | Serum ferritin was measured by chemiluminescence immunoassay (ACS‐180, Chiron, Norwood, MA). Elevated serum ferritin concentration (males > 300, females > 200 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver biopsy was performed in patients with abnormalities in biochemical iron studies and/or liver function tests, together with a relevant clinical picture. Quantitative liver iron stores were determined by graphite furnace atomic absorption on needle biopsy specimens and expressed as hepatic iron concentrations (µmol/g dry weight). The following classification for staging iron overload defined by the International Consensus Conference on Haemochromatosis was adopted: minimal iron overload: hepatic iron concentration 30–99; moderate: 100–199; and severe, 4200 mmol/g dry weight. Thus, liver iron content measurement with a threshold of > 1.68 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | Not reported | ||
| Comparative | |||
| Notes | Of our 18 C282Y/H63D patients who had serum iron indices available, nine (50%) had elevations in both serum transferrin saturation (45%) and serum ferritin (300 µg/L), compared with four of 65 (6%) asymptomatic C282Y/H63D subjects participating in a local community survey. Only one C282Y/H63D patient who was referred for evaluation of abnormal liver function tests had normal serum transferrin saturation and serum ferritin results, compared with 33/65 (51%) of community survey participants. Although 15 (83%) of our C282Y/H63D patients had elevated serum ferritin levels, this did not equate to clinically relevant iron overload as nonspecific elevations of ferritin are often because of an acute‐phase response, hepatic inflammation or necrosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Lindstedt 1980.
| Study characteristics | |||
| Patient Sampling | Consecutive sampling of 93 hospital patients attending the department of Medicine (Sahlgren Hospital, Sweden), regardless of patient disorder on whom sternal bone marrow puncture was done (collected from January to March of 1978). | ||
| Patient characteristics and setting | 93 patients attending the department of Medicine (Sahlgren Hospital, Sweden), regardless of patient disorder. Patients were both sexes aged between 18 and 94 years old (with a mean age of 62 years), and several diseases, specially iron deficiency anaemia. | ||
| Index tests | Serum ferritin was analysed in duplicate by an immunoradiometric method (Ramco reagent set) with intra‐assay coefficients of variation of 8.2% (mean 10.4, range 0 to 20 µg/L; n = 35), 4.9% (mean 44.9 µg/L, range 20‐100; n = 54), and 5.8% (mean 227 µg/L, range 100‐500; n = 51) for the three ranges, as calculated from the differences between the duplicates. Serum ferritin concentration lower than 40 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The hemosiderin content of the bone marrow was graded following the method of Lundin 1964. No more details about the grading were given. | ||
| Flow and timing | 93 patients attending the department of Medicine of Sahlgren Hospital, Sweden, regardless of patient disorder, underwent bone marrow extraction to measure their corresponding iron staining (collected from January to March of 1978). Afterwards, the corresponding sera for ferritin was analysed. All assays were done in a few days. | ||
| Comparative | |||
| Notes | Of the 93 consecutive patients studied, 33 had iron deficiency defined as absence or only traces of haemosiderin in the bone‐marrow smears. Values for sensitivity and specificity of the serum ferritin assay for the diagnosis of iron deficiency were based on cut‐off limits of 10, 20, 40, and 55 ug/L. With a cut‐off limit of 40 ug/L, specificity was 90% but sensitivity was 60%. These findings illustrate that the method used for ferritin assay may be useful for the evaluation of iron stores in healthy individuals such as blood donors and pregnant women. However, the situation seems quite different in unselected patients in general internal medicine wards. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Lombard 1989.
| Study characteristics | |||
| Patient Sampling | Study of 19 patients with hereditary haemochromatosis. Nine patients were studied before treatment was commenced, nine after a course of iron‐depleting venesection therapy and one patient before and during treatment. Recruitment strategy not reported | ||
| Patient characteristics and setting | Ill patients from Dublin, and London (Ireland and UK, respectively) with hereditary haemochromatosis (age between 27 and 73 years old) | ||
| Index tests | Serum ferritin was measured by a commercial radioimmunoassay kit (Becton Dickinson, United Kingdom), in which the antigen was purified I‐human liver ferritin and the antibody was raised in rabbits against purified human liver ferritin. Two thresholds were chosen independently to classify IO: elevated serum ferritin concentration (males > 200, females > 150 µg/L), and both sexes with levels > 500 µg/L were classified as having iron overload (each one leading to different tests, one threshold for both sexes, and one threshold per sex). | ||
| Target condition and reference standard(s) | All patients underwent diagnostic percutaneous liver biopsy, which was stained with potassium ferrocyanide (Perl's) and graded for siderosis. Liver iron content was by atomic absorption spectrophotometry. Liver iron was measured with liver biopsy and stained with Perl's. The degree of iron accumulation was graded: values of II or more were classified as iron overload. | ||
| Flow and timing | All 19 patients underwent serum ferritin, serum iron, transferritin saturation, and liver biopsy measures. The serum ferritin and serum iron were done first in order to select them, and then the subjects underwent transferrin saturation, and the corresponding liver biopsy. | ||
| Comparative | |||
| Notes | Total iron and ferritin levels in this study were consistent with previous reported series. A possible wider interpretation of the results of the present study is that the mechanism(s) which maintain iron homeostasis in the parenchymal cells of the liver, as reflected by transferrin receptor expression and ferritin synthesis, are normal and iron accumulates passively as a result of the gut lesion. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lough 1989.
| Study characteristics | |||
| Patient Sampling | Consecutive sampling of patients with iron deficiency only, iron deficiency with other diseases, other diseases without iron deficiency and healthy individuals | ||
| Patient characteristics and setting | 447 hospital patients attending the regional hospital of Galway, Scotland (175 females and 272 males of mixed age). Patients had several diseases, including ID and some were healthy individuals. | ||
| Index tests | Serum ferritin was determined using a radioimmunoassay kit (Femtin GammaDab‐ Travenol Laboratories Ltd,Thetford, Norfolk, England). Serum ferritin concentration lower than 10 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspiration was stained with Prussian Blue and it was examined microscopically by two of the authors to determinate iron stores. These were reported as present or absent, patients with absent iron stores being defined as iron deficient. | ||
| Flow and timing | 447 consecutive sampling of patients attending the regional hospital of Galway, Scotland, underwent bone marrow aspirates to detect iron stores. Ten millilitres of clotted blood were taken at the time of marrow aspiration for estimation of haemoglobin, serum iron, TIBC and serum ferritin and five millilitres of blood were aspirated into potassium EDTA containers for haemoglobin concentration, MCV, MCH and MCHC. No more details about the timing of bone marrow and SF tests were provided. | ||
| Comparative | |||
| Notes | From 447 hospital patients (175 females and 272 males), 103 (23 %) were iron deficient on bone marrow examination. The group included patients with iron deficiency only, iron deficiency with other diseases, other diseases without iron deficiency and healthy individuals. The laboratory tests had a low diagnostic efficiency, with either a low diagnostic rate or a high false positive rate. However, serum ferritin, despite a diagnostic rate of 25% did establish the diagnosis with near certainty, avoiding the need for invasive and costly investigations. Serum iron with TIBC, serum ferritin and MCV with MCH and MCHC were compared to assess their ability to diagnose iron deficiency when compared to bone marrow examination. The sensitivity, specificity, predictive value and efficiency of the three test groups were evaluated singly and in various combinations. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Macfarlane 1995.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study of 8 patients with symptomatic haemochromatosis. All patients were suspected of having idiopathic haemochromatosis (IH), 7 on the basis of arthralgias and one because of infertility. All had an abnormal iron spectrum (> 90% transferrin saturation). None had evidence of diabetes or of clinical liver disease. | ||
| Patient characteristics and setting | Ill adult patients both sexes (4 females, 4 males) with idiopathic haemochromatosis (mean age 50 years old with age range between 31 and 63 years old) from Leiden, Netherlands | ||
| Index tests | Serum ferritin, method not reported. Elevated serum ferritin concentration for both sexes > 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron loading measured by spectroscopy and histologically by Perl's staining. Liver biopsy was performed on all patients at a mean interval of 15 days after MR imaging. Specimens were obtained for both histological and toxicological analysis. All histological specimens were routinely examined by different pathologists and, in every case, there was excessive iron deposition, primarily in the hepatocytes. The same specimens were later scored blindly by one pathologist, using the Timann‐Schmelzer technique. The liver biopsy specimen intended for toxicological analysis was carefully placed in a previously weighed iron‐free test tube and dried until a constant weight was achieved. The amount of iron was determined by atomic absorption spectrometry using an acetylene flame. The actual absorption was measured using a hollow cathode lamp operated at a wave length of 248.3 nm and a slit width of 0.2 mm. The results were expressed as mg of iron/g dry weight. Liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | Serum ferritin and transferrifin were computed first with corresponding blood samples. Liver biopsy was performed on all patients at a mean interval of 15 days after MR imaging. Specimens were routinely examined by different pathologists and later scored blindly by one pathologist, using the Timann‐Schmelzer technique and Perls’s staining method. | ||
| Comparative | |||
| Notes | In keeping with other studies, the serum ferritin concentration was not a good predictor of the amount of iron in the liver biopsy, however useful it might be in the screening of possible IH patients. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Maliken 2012.
| Study characteristics | |||
| Patient Sampling | Patients with elevated ferritin (males > 300, females > 200 µg/L) referred for liver biopsy, and biochemical hepatic iron content. Sampling design not clarified | ||
| Patient characteristics and setting | Ten ill patients both sexes from Seattle, United States, with several diseases: nonalcoholic fatty liver disease, chronic viral hepatitis, and haemochromatosis (mean age 50 years old) | ||
| Index tests | Serum ferritin (measurent assay technique not mentioned). Elevated serum ferritin concentration for both sexes > 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron content measured with liver biopsy and room temperature susceptometry (RTS). Liver biopsy was performed according to standard clinical protocols. Hepatic iron content was determined by atomic absorption spectrophotometry of fresh or formalin‐fixed paraffin‐embedded liver tissue at the Mayo clinic laboratory. Liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | First serum ferritin was measured in order to classify the subjects with hyperferritinaemia. Then those with hyperferritinaemia underwent liver biopsy and had biochemical measurement of hepatic iron content. | ||
| Comparative | |||
| Notes | The ten studied patients with liver biopsy showed a wide range of SF (823‐2071 µg/L), and HIC measured by liver biopsy (631‐18,323 mg/g dry weight). However SF and HIC measurement did not correlate well, r = ‐0.021. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Martin‐Cabrera 2015.
| Study characteristics | |||
| Patient Sampling | Prospective and observational cohort study from 2012 to 2013 where 200 anaemic cardiac surgical patients were recruited over a 12‐month period. A subset of 165 patients had a complete dataset for evaluation, and were subsequently studied. Bone marrow aspiration was done for all, however, only 78 bone marrow biopsies were suitable for evaluation of iron stores. | ||
| Patient characteristics and setting | 200 anaemic cardiac surgical patients were recruited, and 165 were studied where 78 bone marrow biopsies were suitable for evaluation of iron stores. Those 78 anaemic adult patients included males and females with a mean age 74.5 years old with diverse chronic diseases. | ||
| Index tests | Serum ferritin (measurement approach not mentionned). Serum ferritin concentration lower than 15 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspirates were performed by two experienced haematologists who were blinded to laboratory results. Samples were stained by the Wright–Giemsa method for morphological evaluation and Perl’s Prussian blue reaction was used to assess bone marrow iron stores, as previously reported by Hughes 2004: staining enough bone marrow aspirate slides in order to assess iron stores in at least seven bone marrow particles. Iron stores were then reported according to the method of Rath 1948. We found no further description of the grading system to classify iron deficiency state. | ||
| Flow and timing | 200 anaemic cardiac surgical patients were recruited, and 165 were studied where 78 bone marrow biopsies were suitable for evaluation of iron stores. The 165 underwent haematologic and biochemical analyses (such as serum ferritin), and bone marrow aspiration measurements. However, the precise order of these two types of tests was not described. First, the anaemia state assessment was undertaken, then the bone marrow iron state classification, and then the haematological evaluation, which led to the full classification of different anaemia types into iron deficient and iron replete groups. | ||
| Comparative | |||
| Notes | Aside from bone marrow biopsy not being performed frequently in the clinical setting, we suggest that this study contributes minimal value to the assessment of anaemia. Assessing stainable iron stores, considered the gold standard in assessing a patient’s iron status, can be confusing and misleading as demonstrated here, even provided samples are adequate enough to give a diagnostic orientation. Iron restriction in anaemia of chronic diseases is a dynamic phenomenon that is only partly reflected in laboratory assays such as ferritin and Perl’s staining despite the latter being considered the gold standard. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mast 1998.
| Study characteristics | |||
| Patient Sampling | The study was conducted in five populations consecutively selected (total of 267 patients) but only a subsample (the bone marrow group) included adult anaemic patients who had bone marrow aspirates and ferritin concentrations (a serum or plasma sample was available within 5 days of the BM aspiration). The bone marrow group (54 patients) underwent bone marrow aspiration from the Washington University School of Medicine, Divisions of Laboratory Medicine and Hematology, in St Louis, United States of America. | ||
| Patient characteristics and setting | The bone marrow group of 54 anaemic patients included males and females from 20 to 49 years old with several diseases. This study was undertaken by the School of Medicine, Divisions of Laboratory Medicine and Hematology, in St Louis, United States of America, and approved by the Washington University Human Studies Committee. Serum or plasma was obtained from samples received by the Barnes‐Jewish Hospital laboratories for clinical testing. | ||
| Index tests | Ferritin values were determined using the Chiron automated chemiluminescence system ferritin assay or the Access immunoassay system ferritin from Beckman. Prior evaluation in the institution of the study indicated that they yielded similar ferritin values (unpublished data). Serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The bone marrow was assessed in 54 anaemic adult patients who were undergoing their first bone marrow aspiration. Samples for bone marrow aspirates were prepared and stained for iron with Prussian blue. Stainable iron was determined to be absent or present by a haematopathologist blinded to the results of ferritin. Absence of iron stores was considered iron deficiency. | ||
| Flow and timing | The study was conducted in five populations consecutively selected (total of 267 patients) but only a subsample of them (54 patients, the bone marrow group) underwent marrow aspiration, and ferritin concentration assessment (a serum or plasma sample was available after BM aspiration within the following 5 days of the BM aspiration). | ||
| Comparative | |||
| Notes | In 54 anaemic patients who had a bone marrow aspiration performed, the study showed that ferritin (< 12 mg/L) had a sensitivity of 25% and a specificity of 98% to detect iron deficiency compared to bone marrow results. However, the sensitivity and specificity of ferritin could be improved to 92% and 98%, respectively, by using a diagnostic cut‐off value of < 30 mg/L, resulting in a positive predictive value of 92%. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mast 2002.
| Study characteristics | |||
| Patient Sampling | Seventy‐eight patients undergoing bone marrow examination were enrolled in this study after informed consent. Peripheral blood samples from 34 medical students were obtained anonymously during a pathology course to establish reference limits. No student was anaemic, as defined by a haematocrit less than 36% for women and less than 39% for men. There was no further description about the selection of the sample. | ||
| Patient characteristics and setting | Seventy‐eight patients undergoing bone marrow examination within the Research and Pathology Services, Department of Veterans Affairs, Memphis, US. They were enrolled in this study after informed consent. Patients included both sexes, over 20 years old, with anaemia and/or blood disorders and other malignancies. | ||
| Index tests | Serum ferritin was measured in serum by using an Access Immunoanalyzer (Beckman‐Coulter, Brea, CA). A serum ferritin concentration lower than 50 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspirates were collected in K3EDTA and stained for iron with Prussian blue. Stainable iron was determined to be absent or present in aspirates containing marrow spicules by 2 pathologists who were kept blinded to the peripheral blood test results. Discrepant samples were referred to a third pathologist for interpretation. The Cohen coefficient K for the 2 pathologists initially interpreting the specimens was 0.78, indicating substantial agreement. No further details were given in the grading systems to determine absence in iron stores (iron deficiency). | ||
| Flow and timing | Peripheral blood samples from 34 medical students were obtained anonymously during a pathology course to establish reference limits. 78 patients undergoing bone marrow examination were enrolled in this study after informed consent. Peripheral blood samples from the patients were obtained on the day of the bone marrow examination and were submitted for routine analysis at the Memphis Veterans Affairs Medical Center clinical laboratories. The study used different people to carry out the haematological measurements and the bone marrow measurements, blinded to the results of the corresponding measurement. | ||
| Comparative | |||
| Notes | The reticulocyte haemoglobin content (CHr) in 78 patients undergoing bone marrow examination was measured. Reasons for biopsy included anemia work‐up (34%), benign haematologic disorders such as thrombocytopenia or monoclonal gammopathy (22%), haematologic malignancy (19%), lymphoma or multiple myeloma (19%), and other malignancies (6%). As determined by the absence of stainable iron in the bone marrow aspirate, 28 (36%) of the 78 patients were classified as iron deficient, based on the lack of stainable iron in the aspirate. The diagnostic power of CHr is limited in patients with high mean cellular volume (MCV) or red cell disorders such as thalassaemia. However, when patients with MCV of more than 100 fl are excluded, the receiver operator curve analysis of CHr, ferritin, transferrin saturation, and MCV demonstrates that CHr has the highest overall sensitivity and specificity of these peripheral blood tests for predicting the absence of bone marrow iron stores. The study also showed 42.3% sensitivity, 93.6% specificity, 78.6% PPV and 74.6% NPV for ferritin < 50 µg/L when stainable iron in bone marrow is absent. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mazza 1978.
| Study characteristics | |||
| Patient Sampling | Adult patients were sampled consecutively during a 4‐month period at University Hospital, London (100 patients). They required bone marrow aspiration for appraisal of their condition. | ||
| Patient characteristics and setting | 100 patients underwent bone marrow examination. They were males and females of mixed age with several diseases (blood disorders, chronic inflammation, uncomplicated disorders, solid tumours, portal cirrhosis, renal failure, and miscellaneous conditions), from London, UK. | ||
| Index tests | A radioimmunoassay with labelled ferritin and determined by the Lowry method was used for measurement of the serum ferritin concentration assigned to the ferritin standards. In accordance with the recommendations of Luxton 1977, the lower limit of normal for both men and women was taken as 18 µg/L, i.e. all the values below that limit were classified as iron deficient. | ||
| Target condition and reference standard(s) | Smears of bone marrow aspirates stained with Prussian blue were evaluated by one of the authors: R.M.B. The reports issued to the attending physicians were reviewed, and the grade of bone marrow hemosiderin given in the report was used for this study. Lack of hemosiderin was classified as iron deficient. | ||
| Flow and timing | 100 adult patients were sampled consecutively during a 4‐month period at University Hospital, London (100 patients). Blood samples were obtained within 48 hours of aspiration for measurement of haemoglobin concentration, serum iron concentration, per cent transferrin saturation and serum ferritin concentration. The diagnoses of the patients conditions were obtained from a review of the hospital charts. BM aspirates and SF assessment were undertaken by different persons, blinded to the results of the corresponding measurement. | ||
| Comparative | |||
| Notes | When iron deficiency is virtually the only identifiable factor responsible for anaemia, determination of the serum ferritin concentration has considerable advantage over determination of the serum iron concentration and the transferrin saturation because the results of the latter are often within normal limits in persons with mild iron deficiency. Nevertheless, a low serum ferritin concentration in these circumstances indicates iron depletion and a high serum ferritin concentration excludes it. In complicated clinical situations, determination of the grade of bone marrow hemosiderin is the most reliable test for iron deficiency anemia. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Means 1999.
| Study characteristics | |||
| Patient Sampling | 145 consecutive patients recruited to the study were at least 18 years of age, anaemic (haemoglobin 14 g/dL in males, 12 g/dL in females) and undergoing bone marrow examination for any diagnostic purpose (i.e. no bone marrow examinations were performed specifically for the purpose of this study). | ||
| Patient characteristics and setting | 145 anaemic patients who underwent BM examination previously. Patients were males and females of mixed age (mean 48 years) from Minneapolis, US, with various diseases: HIV, infectious diseases, leukaemia, and several chronic conditions. | ||
| Index tests | Serum was separated from the remaining blood and a portion was sent to a reference laboratory (Laboratory Corporation of America, Raritan, NJ, USA) for assays of serum ferritin, serum iron, TIBC and transferrin saturation (no more details of the assay method for SF were given). A serum ferritin concentration lower than 25 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Duplicate smears were prepared from the bone marrow aspirate and fixed with methanol. The smears were stained for iron with potassium ferrocyanide and counterstained with Nuclear Fast Red. Presence or absence of stainable iron was recorded by the one of the investigators. At the end of the study, 1 smear per subject was examined in a blinded manner (i.e. without any other information concerning the subject) by an independent assessor, who graded the iron content on a semi‐quantitative scale (no further details abaout the grading system was mentioned). | ||
| Flow and timing | 145 consecutive anaemic patients who underwent bone marrow examination for any diagnostic purpose were chosen (i.e. no bone marrow examinations were performed specifically for the purpose of this study). After the selection of anaemic patients with BM examinations, a blood sample to measure SF and other iron stores indicators was taken. No more details of the flow were described. | ||
| Comparative | |||
| Notes | Serum sTfR concentration is a sensitive predictor of the results of bone marrow aspirate iron studies in a heterogeneous population of patients with complicated medical presentations. When combined with serum ferritin concentration in a simple algorithm and applied to this same population, the sensitivity of the sTfR for the prediction of iron status is preserved and its specificity is enhanced. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Meira 2005.
| Study characteristics | |||
| Patient Sampling | Participants comprised all vertically infected children (n = 8), referred to the AIDS Children Division between June 2000 and December 2001 and presenting with peripheral cytopenias as the first sign of infection. Bone marrow aspirates and SF measures were available for 6 of them. | ||
| Patient characteristics and setting | A sample of 8 HIV‐infected children received medical care in the outpatient service (area of Campinas, Sao Paolo, Brazil) at least once a month on a routine basis. HIV infection was classified according to the Center for Disease Control (CDC) 1994 guidelines. The median age of the 4 boys and 4 girls was 13.5 months (4 months – 7 years). Six of them had anaemia: 3 with thrombocytopenia, 1 with neutropenia, and 2 cytopenia, and two without anaemia: 1 had pancytopenia, and 1 had neutropenia. | ||
| Index tests | Serum ferritin was determined by Stratus ferritin fluorimetric enzyme immunoassay using Stratus I‐EIA Fluorimetric equipment. A serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow (BM) examination was indicated in all patients for the elucidation of peripheral cytopenias of unclear origin. Aspiration was made from the anterior tibia in patients 2 and 8 (4 months old), and from the posterior iliac crest in the other patients. Cytologic smears were stained by May‐Gruenewald‐Giemsa, and Perl's stains. Haemosiderin grading was divided in three: normal, decreased, and increased. The category of decreased haemosiderin was considered indicative of iron stores deficiency. | ||
| Flow and timing | A sample of 8 HIV‐infected children received medical care in the outpatient service. Clinical data, peripheral blood counts, peripheral blood (including SF), bone marrow lymphocyte subsets, and viral load were measured for all of them. No further description of the timing of the different measurements was given and bone marrow and serum ferritin markers were available for only 6 children. | ||
| Comparative | |||
| Notes | Eight anaemic children (11 to 72 months old, 4 girls, 4 boys) with HIV were studied for peripheral cytopenias as the first sign of HIV infection. Five children were naıve to treatment (group 1) and three were under HAART (group 2). . Bone marrow cytology showed cell abnormalities mainly in granulocytic precursors and megakaryocytes. All except two (taking HAART) patients had a high viral load. There was a straight correlation between viral load in PB and bone marrow. Bone marrow examination was useful for determining the aetiology of the cytopenias and for detection of opportunistic infection. Although there were no data on ferritin related to bone marrow iron content, values for ferritin and bone marrow were available for each patient. On a one‐to‐one basis, there was no apparent correlation between iron in bone marrow and iron content in bone marrow. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Milman 1983.
| Study characteristics | |||
| Patient Sampling | The study comprised 50 non‐dialysis patients with a mean 24‐h endogenous creatinine clearance of 10 mL/min (range 3‐20 mL/min). The control group consisted of 53 healthy subjects (majority were medical students or members of the hospital staff). No further details about the sampling method were described. |
||
| Patient characteristics and setting | The study comprised 50 non‐dialysis patients (21 male, 29 female) with a mean 24‐h endogenous creatinine clearance of 10 mL/min (range 3‐20 mL/min) and a mean age of 64 years (range 32‐90 years). The control group consisted of 53 healthy subjects (30 male, 23 female) with a mean age of 39 years (range 20‐90 years). The majority were medical students or members of the hospital staff. 16 of the females had regular menstruation and none received hormonal treatment. Both sets of subjects worked, studied or were treated at the Bispebjerg Hospital in the Copenhagen area, Denmark. | ||
| Index tests | Serum ferritin was measured by a 2‐site immunoradiometric assay (Phadebas Ferritin PRISP, Pharmacia Diagnostics AB, Uppsala, Sweden), employing antibody‐coupled paper discs as solid phase. Serum ferritin concentration lower than 60 µg/L was classified as iron deficiency in the group of patients, and SF concentration lower than 20 µg/L was classified as iron deficiency in the control group of healthy subjects. | ||
| Target condition and reference standard(s) | Bone marrow aspirates were obtained by sternal puncture, and sections of the marrow clot stained for iron with Prussian blue, whereafter the haemosiderin iron content was graded semi‐quantitatively into 4 classes by one of the authors as previously described: 0 = no stainable iron; 1+ = trace, (often difficult to determine whether intracellular or not normal); 2+ = increased. Thus, grade 0 was classified as iron deficiency. | ||
| Flow and timing | The study comprised 50 non‐dialysis patients, and a control group of 53 healthy subjects. Haematologic and biochemical analyses (such as serum ferritin), bone marrow and aspiration measurements were undertaken for both groups separately. However, the flow and time of these two types of tests were not described. | ||
| Comparative | |||
| Notes | Serum ferritin (SF) was correlated to marrow iron both in patients with renal failure and in healthy subjects (P < 0.001). The geometric mean SF in patients with 0‐1+ marrow iron was 33 µg/L, 1+ marrow iron 166 µg/L, and 2+ marrow iron 519 µg/L. Healthy subjects with 0‐1+ marrow iron had a mean SF of 16 µg/L and those with 1+ marrow iron a value of 65 pg/L. SF levels were higher in patients than in healthy subjects at all marrow iron grades (P < 0.001).Healthy subjects with SF < 15 µg/L had absent or reduced marrow iron, while those with SF > 30 µg/L had normal marrow iron. Using a critical SF value of < 20 µg/L, the diagnostic efficiency in terms of diagnosing absent or reduced marrow iron was 0.90 (PPV = 0.85, NPV = 0.91). In patients with renal failure SF < 60 µg/L indicated absent or reduced marrow iron, while values > 80 pg/L were associated with normal marrow iron. The diagnostic efficiency of SF using a critical value of SF equal to 60 µg/L was 0.94 (PPV = 0.93, NPV = 0.97). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Mulherin 1996.
| Study characteristics | |||
| Patient Sampling | Consecutive patients wiih rheumatoid arthritis (RA) attending St. Vincent Hospital (Dublin, Ireland) had haemoglobin (Hb) estimation performed if thought appropriate by their rheumatoIogist as part of normal clinical care. If anaemia (male <= 11 g/dL and female <= 10.5 g/dL) was identified, the patient was invited to participate in the study requiring them to undergo bone marrow sampling. 45 anaemic patients with RA underwent marrow sampling in addition to a complete blood count and serum ferritin and iron saturation measurements. | ||
| Patient characteristics and setting | 45 anaemic patients with rheumatoid arthritis (RA) attending St. Vincent Hospital (Dublin, Ireland). 36 patients were women, with mean age 62 years old (aged between 20 and 80 years old), and 36 were RA seropositive (mean RA particle agglutination titer was 2700‐1). Thirty‐three patients were taking regular nonsteroidal drugs, 26 disease‐modifying therapy, and 23 corticosteroid therapy. Nonspecific abdominal symptoms were recorded in 5 patients. | ||
| Index tests | Serum ferritin was measured by a commercial radioimmunoassay (Magic. Ciba Corning Diagnostics Corp., Medfield. MA). A serum ferritin concentration lower than 40 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Marrow was obtained under local anaesthesia by needle aspiration or trephine biopsy from the posterior superior iliac spine by an experienced operator. Marrow iron stores were assessed, following Perl’s Prussian Blue staining, as present (ACD) or absent (iron deficiency) by 2 experienced haematologists working independently and blinded to clinical and laboratory details. The observers agreed on 81% of samples (i.e. estimate of interobserver agreement was 0.63); where observers disagreed, if either identified intracellular marrow iron, the patient was classified as having ACD. | ||
| Flow and timing | Rheumatologists suggested that patients wiih rheumatoid arthritis (RA) attending St. Vincent Hospital should take the anaemia test. If the patientswas proven to be anaemic, he or she was invited to undergo bone marrow aspiration. At the time of marrow sampling, blood was taken to measure anaemia and iron store indicators (including SF). No more details of the flow were provided. | ||
| Comparative | |||
| Notes | 45 anaemic patients with rheumatoid arthritis (RA) and marrow sampling. 47% of patients had iron deficiency. These patients had significantly lower mean corpuscular volume (MCV), serum ferritin, and iron saturation. A 3‐step algorithm was developed using these laboratory variables to identify iron deficiency. This algorithm correctly classified 94% patients with iron deficiency and 85% with ACD. After exclusions for inadequate bone marrow results, 38 patients (36 women, 2 men) were included and 18 (47%) had iron deficiency anaemia based on absent iron stores. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Nadeem 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional comparative study conducted at Department of Haematology in Lahore, Pakistan from 2007 to 2009. 116 adult subjects (80 anaemic and 36 controls) who already have had their bone marrow examination done for various reasons were included. | ||
| Patient characteristics and setting | 116 adult subjects, both sexes with age not mentioned, attending the Department of Haematology, Sheikh Zayed Medical Complex, Lahore, Pakistan from 2007 to 2009. From those, 80 were anaemic presenting with diverse chronic diseases (who underwent bone marrow examination), and 36 were apparently healthy controls. | ||
| Index tests | Serum ferritin was estimated by Ferritin Enzyme immunoassay (Biocheck). SF concentration lower than 150 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Assessment of bone marrow iron stores was done by staining the already available marrow slides with Perl’s stain. Absence of these stores denoted iron deficiency anaemia (IDA) whereas increased macrophage iron with decreased siderocytes and sideroblasts was diagnostic of anaemia of chronic disease (ACD). | ||
| Flow and timing | Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were made apparently simultaneously. However, the flow and timing of these three kind of tests were not described. | ||
| Comparative | |||
| Notes | The sTfR and sTfR‐F indices have a more diagnostic efficacy than serum ferritin and other conventional laboratory iron parameters in diagnosing and distinguishing iron deficiency anemia from anaemia of chronic disease and are also as reliable as bone marrow aspirate in detecting iron stores in patients with iron deficiency anemia. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Nanas 2006.
| Study characteristics | |||
| Patient Sampling | Prospectively screening of all patients in a given period of time who had been hospitalised for acute decompensated advanced heart failure and had responded to intravenous dobutamine infusion. To guide their treatment, all patients were submitted to baseline right heart catheterisation. From those, a subsample of 37 consecutive hospitalised patients suffering from congestive heart failure and anaemia were selected. They had been treated with an optimal medical regimen and were hospitalised for management of acute cardiac decompensation, which did not respond to oral medical treatment and which required continuous intravenous infusions of dobutamine 10 g/kg/min for not more than 72 h. | ||
| Patient characteristics and setting | 37 consecutive hospitalised patients suffering from end‐stage chronic heart failure (median duration 60 months) and anaemia. They included 35 males and 2 females aged 50 years or older (mean age 57.9 years old). All were hospitalised and followed up at the Department of Hematology and Nuclear Cardiology, Alexandra Hospital in Athens, Greece. | ||
| Index tests | Serum ferritin (no further details about the measurement approach were given). A serum ferritin concentration lower than 17 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Iron deficiency on bone marrow biopsy was defined as absence of iron stores (no more details of the methodology and grading of the BM procedure were given). | ||
| Flow and timing | Prospective screening of all patients in a given period of time who had been hospitalised for acute decompensated advanced heart failure and had responded to intravenous dobutamine infusion. From those, a subsample of 37 consecutive hospitalised patients suffering from congestive heart failure and anaemia were selected. Haematologic and biochemical analyses (such as serum ferritin), bone marrow biopsy measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Serum ferritin concentrations were significantly lower among the iron‐deficient patients than among patients with anaemia not due to iron deficiency (mean difference of 136.6 µg/L). However, iron deficiency anemia in our patients was not associated with the expected decrease in ferritin concentrations. This relative increase in ferritin concentrations might be the result of the inflammation that accompanies the congestive heart failure syndrome. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Nelson 1978.
| Study characteristics | |||
| Patient Sampling | Selection of 73 consecutive anaemic patients for whom bone marrow and serum ferritin levels were available (no more details about the sampling were given). | ||
| Patient characteristics and setting | 73 anaemic (haematocrit level less than 40%) male medical inpatients, of mixed age attending the Hematology Oncology Department of Medicine, West Side Veterans Hospital, Chicago, United States. The patients had either chronic inflammatory conditions including alcohol‐related disease, liver disease, or malignancies. | ||
| Index tests | Serum ferritin was determined by a commercially available kit (Ramco Laboratories, Houston, Texas). A serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Smeared bone marrow aspirates or biopsy sections stained with prussian blue were graded for iron content by a BM technician with twenty years of experience. The grading scale was: 0: absent iron content (IC), 1: marked decrease in IC, 2: moderate decrease in IC, 3: mild decrease in IC, 4: normal IC, 5: mild increase in IC, 6: moderate increase in IC, 7: marked increase in IC. Thus, levels 0 to 3 were classified as iron deficiency. To test reproducibility of grading, a random sample of 10 unmarked slides was resubmitted to the technician, and the second grading differed from the first by only one point on 2 slides. | ||
| Flow and timing | The sample was a selection of 73 consecutive anaemic patients for whom bone marrow and serum ferritin levels were available. Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described, although the grading of bone marrow was done without knowledge of serum ferritin levels. | ||
| Comparative | |||
| Notes | If iron defiency is defined by absent bone marrow iron stores, then iron deficiency can be excluded if the ferritin levels are greater than 100 µg/L. Only one exception to this rule in our group was found: a patient with severe alcoholic cardiomyopathy and hepatomegaly with a SGOT value of 160 IU. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Niederau 1998.
| Study characteristics | |||
| Patient Sampling | A cross‐sectional study of 3012 workers (2515 men and 497 women from two industrial companies in Düsseldorf) and 3027 outpatients (1228 men and 1799 women from the Düsseldorf University Hospital). Only a minority went on to undergo liver biopsy. The healthy participants were sequentially asked to participate in the study (during twelve months) as part of the routine checkup examination while entering the company. The outpatients were patients of nine primary care physicians (not specialists). For practical reasons, selection in the primary care setting involved only every third patient from a list at the front desk. Approximately 95% of the invited patients agreed to participate. Further studies, including liver biopsy, were only done when both the ferritin level and the transferrin saturation level were abnormal. From 6039 initial participants, only 55 had both SF, and transferritin elevated levels. | ||
| Patient characteristics and setting | Outpatients from the Düsseldorf University Hospital, and employees from two industrial companies in Düsseldorf (6039 in total). Both groups were adults of mixed gender aged between 28 and 60 years old. After selection, the remaining 55 subjects were diagnosed with several diseases such as alcoholism, hepatitis, and haemochromatosis. Although the underlying population was appropriate, the selected population was at very high risk of selection bias as they all had elevated peripheral blood indices. | ||
| Index tests | Serum ferritin levels were measured by using a commercial enzyme‐linked immunosorbent assay (Enzymun, Boehringer Mannheim, Mannheim, Germany). An elevated serum ferritin concentration (males > 350, females > 250 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | All participants who had two abnormal values for both ferritin level and transferrin saturation were asked to undergo liver biopsy (55 subjects). A second blood test for both parameters led to a selection of 42 subjects. Participants who refused to have liver biopsy received phlebotomy (13 subjects). There were no details of the metholodology used to measure the liver iron content by liver biopsy. However, the liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | Only a minority of screened participants underwent liver biopsy ‐ these were at very high risk of iron loading based on peripheral blood parameters. 6039 selected subjects underwent transferrin and serum ferritin measures. From those, only 55 patients had high levels of both serum ferritin and transferrin. From those 55 patients, both blood tests were repeated and distinguished 42 participants with high levels of both indicators. Of the remaining 42 patients, 29 underwent liver biopsy, and 13 phlebotomy. Thus, only a minority of screened participants underwent liver biopsy ‐ these were at very high risk of iron loading based on peripheral blood parameters. | ||
| Comparative | |||
| Notes | In male employees, gross iron overload was almost as common as iron deficiency (0.4% compared with 0.5%) and was more common than iron‐deficient anaemia (0.4% compared with 0.3%). In female employees, iron deficiency and iron‐deficient anaemia were more common than iron overload. Considering the high prevalence of haemochromatosis found in this and other recent studies, it is important to ask why the disease is not diagnosed. Ferritin levels should be checked in persons at risk of iron overload annually. Whether it is necessary to measure both ferritin levels and transferrin saturation during screening remains to be discussed. It is well documented that transferrin saturation is a more sensitive marker for haemochromatosis than ferritin levels are; ferritin levels become elevated only in the presence of grossly increased body iron stores. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Nielsen 1990.
| Study characteristics | |||
| Patient Sampling | 35 anaemic (Hb below 114 g/L in women, and below 130 g/L in men) patients with classical or definite rheumatoid arthritis (as per the American Rheumatoid Association criteria). Active rheumatoid arthritis (RA) was defined as a condition with at least three of the following four criteria: swelling in at least three joints; tenderness in at least six joints; an erythrocyte sedimentation rate (a nonspecific measure of inflammation) of 28 mm/h or more; and morning stiffness of at least 45 min. | ||
| Patient characteristics and setting | Thirty of the 35 patients (23 female and 12 male), aged between 39 and 80 years (median age 66 years), with classical or definite rheumatoid arthritis, were treated with a disease‐modifying antirheumatic drug. The drugs used were (penicillamine (n = 10), sodium aurothiomalate (n = 6), hydroxychloroquine sulphate (n = 7) and sulphasalazine (n = 7). Thirty‐four patients were treated with a nonsteroidal anti‐inflammatory drug, and eight patients were treated with prednisolone systemically (5‐15 mg/day). The medical treatment was not significantly different between the iron replete and the iron‐depleted patients. Patients were recruited at the Department of Haematology, Herlev University Hospital, Herlev, Denmark. | ||
| Index tests | Serum ferritin concentrations were analysed by a competitive radioimmunoassay (Medicinsk Lab, Copenhagen, Denmark). The normal range was 15‐300 µg/L in men and 10‐120 µg/L in women. An iron depleted group was defined by a) a bone marrow iron grade of 0‐1, and b) a serum ferritin 60 µg/L or lower. | ||
| Target condition and reference standard(s) | Iron status was evaluated by bone marrow examination with posterior iliac crest bone marrow aspirations fixed in phosphate buffered saline, 4% formaldehyde and embedded in paraffin. Serial sections were stained for iron with Perl's Prussian blue. The test was graded as: 0 = no iron; 1 = minimal or very small amounts; 2 = slight and patchy; 3 = moderate and diffuse; 4 = strong, extensive and diffuse content. Iron grades 0‐1 were regarded as indicating iron deficiency. | ||
| Flow and timing | Patients were separated into two groups after bone marrow assessment and serum ferritin assessment, but the flow and timing is unclear. | ||
| Comparative | |||
| Notes | All patients were anaemic with haemoglobin concentrations below 114 g/L in women, and below 130 g/L in men. Patients were separated into two groups according to iron status: I) an iron replete group defined by a) a bone marrow iron grade 2‐4 and b) a serum ferritin higher than 60 µg/L; 2) an iron depleted group defined by a) a bone marrow iron grade 0‐1, and b) a serum ferritin 60 µg/L or lower. In 19/35 (54%) of the patients, iron stores were empty (marrow iron grade 0‐1) and serum ferritin concentrations lower than 60 µg/L, while only 10/35 (29%) of the patients had adequate marrow iron stores (bone marrow iron grade 2‐4) and serum ferritin 60 µg/L. Six patients were excluded owing to discrepancies between bone marrow iron grade and corresponding serum ferritin concentrations. Serum ferritin concentrations were significantly higher in patients with adequate marrow iron (MIG 2‐4) than in patients with depleted iron stores (MIG 0‐1) (mean (SD) 257.4 (228.1) µg/L vs 51.6 (57.7) µg/L. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
North 1997.
| Study characteristics | |||
| Patient Sampling | The specimens were obtained from 308 bone marrow aspirates performed on adults at the University of Cincinnati Medical Center. Specimens submitted to the Diagnostic Haematology Laboratory from 77 patients were frozen and stored and measured for ferritin. | ||
| Patient characteristics and setting | The sample number reflects specimens containing sufficient volume for analysis of different biomarkers, particularly ferritin. Also the characteristics of the patients were not available for all subjects. Only 46 samples included both ferritin and bone marrow results in participants from Cincinnati in the US, of both sexes, mixed age and with several diseases. | ||
| Index tests | Serum ferritin levels were determined by an automated microparticle enzyme immunoassay method (IMx; Abbott Laboratories). A serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | 308 bone marrow aspirations were conducted at the University of Cincinnati Medical Center. A total of 203 specimens were stained using the Prussian blue method. Biopsy reports of patients classified as iron deficient (absence of stainable iron) on aspirate examination were fully reviewed to support the diagnosis. | ||
| Flow and timing | Only patients who had ferritin concentrations measured around the time of the bone marrow examination were considered. | ||
| Comparative | |||
| Notes | During the period June 1994 – May 1995, 308 bone marrow aspirations were performed on adults at the University of Cincinnati Medical Center. Two‐hundred and three specimens were stained for iron stores with Prussian blue. The stained specimens were then reviewed by the responsible faculty physician and by another faculty physician. Biopsy reports of patients classed as iron deficient (absence of stainable iron) on aspirate examination were reviewed to support the diagnosis. Patients who had had ferritin determinations at or near the time of the specimen time of marrow examination were identified by review of the computerised hospital laboratory data base. Forty‐three iron‐replete and 18 iron deficient patients were identified, and their laboratory parameters correlated with marrow iron status. These results were used to estimate the number of iron deficient patients expected in groups with different serum ferritin values. A serum ferritin < 30 ug/L in this laboratory identified absence of stainable marrow iron. Seven patients with serum ferritin > 220 ug/L, presented with anaemia of chronic disease (hypoferraemic anaemia, elevated serum ferritin, and an underlying infectious, inflammatory, or neoplastic disease). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Oluboyede 1980.
| Study characteristics | |||
| Patient Sampling | Sample of 22 pregnant women attending the antenatal clinic of Ibadan, and 18 non‐pregnant women attending the Haematology Clinic. Both groups had blood disorders: haemoglobin SS, and haemoglobin SC. No further details about the sampling procedure were given. | ||
| Patient characteristics and setting | Sample of 40 women aged between 20 to 49 years old (all taking 5 mg of folic acid and 100 mg of paludrine each day) from Ibadan, Nigeria. The sample was made up of:
|
||
| Index tests | Serum ferritin was measured by the radioimmunoassay method of Jones 1978. A serum ferritin concentration lower than 70 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Smears of bone marrow aspirates were stained for iron using the Prussian blue reaction. The slides were numbered and read independently by two haematologists. The presence of iron was graded from 0 to 3, where 0 = no stainable iron; 1+ = trace of stainable iron (that is, stainable iron present in 1 to 25 per cent of high power fields); 2+ = stainable iron present in 26 to 75 per cent of high power fields and 3+ = stainable iron present in all the marrow fragments (Sorbie 1975). Grade 0 was classified as iron deficiency. | ||
| Flow and timing | Sample of 40 women with blood disorders (22 pregnant and 18 non‐pregnant). Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | IIron studies were performed in 22 pregnant and 18 non‐pregnant women with haemoglobinopathies. Serum ferritin values in the haemoglobin SS and SC pregnant patients were not significantly different (P > 0.05). There was a strong correlation between serum ferritin levels and transferrin saturation in the pregnant group (r = 0.71; P < 0.001). Fourteen of the 22 pregnant women (63 per cent) and 9 of the 18 non‐pregnant women (50 per cent) had scanty or no iron in the bone marrow; the serum ferritin levels increased progressively with greater amount of haemosiderin in the bone marrow. There was evidence of iron deficiency in both the pregnant and non‐pregnant women with haemoglobinopathies and this suggests the need for further study of the routine administration of iron in the management of patients with sickle cell disease. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Olynyk 1999.
| Study characteristics | |||
| Patient Sampling | Population‐based study with a randomly selected sample of 3011 unrelated white adults (age between 20 and 79 years old) who lived in Bussleton in WA, Australia. Clinical, biochemical, and genotypic information was collected for all the subjects. Patients with Tf sat > 45% or ferritin > 300 µg/L (continuously during 4 years of follow‐up) or absence of the C282Y mutation and the H63D mutation subsequently underwent liver biopsy for confirmation of iron overload diagnosis. At the end of the study in 1998, only 11 accepted and met the criteria for liver biopsy. | ||
| Patient characteristics and setting | Population‐based study with a randomly selected sample of 3011 unrelated white adults (age between 20 and 79 years old) who lived in Bussleton in WA, Australia. The eleven patients who went liver biopsy were found to have hereditary haemochromatosis (age between 35 and 74 with mean age of 55.8 years old). | ||
| Index tests | Serum ferritin levels were measured by chemiluminescence immunoassay (ACS‐180, Chiron Diagnostics, Norwood, Mass). Elevated serum ferritin concentration for both sexes > 150 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron measured with liver biopsy. Liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. There was no description of the methodology that was followed to perform the liver iron content measurements. | ||
| Flow and timing | 3011 randomly selected subjects from Busselton, Australia, with transferrin sat > 45% or serum ferritin > 300 µg/L (continuously from 1994 to 1998 i.e. 4 years of follow‐up) or absence of the C282Y mutation and the H63D mutation subsequently underwent liver biopsy in 1998 for confirmation of iron overload diagnosis. At the end of the study in 1998 only 11 accepted and met the criteria for liver biopsy. | ||
| Comparative | |||
| Notes | If hemochromatosis is detected before the age of 40 years and if the serum ferritin level is less than 1000 µg/L, then hepatic fibrosis is very likely to be absent and treatment can be initiated without the need for liver biopsy. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Ong 2005.
| Study characteristics | |||
| Patient Sampling | From October 1997 to April 2002, bone marrow aspirate samples from patients on whom concurrent full blood count and iron studies had been done were analysed. Samples were included for analysis only if the bone marrow aspirate and iron studies were done within 1 week of each other. A subsample of those (n = 92 patients) achieved the selection terms. | ||
| Patient characteristics and setting | A subsample of 92 patients (54 females and 38 males, with median age 63 years and range of 13 to 90 years old) with concurrent iron studies, were hospitalised, and followed up at the Department of Pathology and Laboratory Medicine, Tan Tock Seng Hospital, Singapore. Hospitalisation was due to different diseases, some of which had evidence of inflammation. | ||
| Index tests | Serum ferritin was measured using a microparticle enzyme immunoassay on the Abbott AxSym chemistry analyzer (Abbott Laboratories, Abbott Park, Illinois, USA). A serum ferritin concentration lower than 60 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | All bone marrow aspirate samples were stained for iron using the conventional Perl’s reaction with Prussian blue. The amount of iron stores was graded by a single assessor unaware of the results of the other iron studies. The amount of iron stores was graded on a scale of 0 (absent) to 5+ (marked increase), with 1+ to 3+ being normal iron stores and 4+ to 5+ increased iron stores. Bone marrow aspirates which had a score of 0 were considered iron‐deficient. With each iron stain, a positive control was concurrently performed. | ||
| Flow and timing | A sample of 92 patients with concurrent iron studies. Haematologic and biochemical analyses (such as serum ferritin), and bone marrow aspiration measurements were undertaken, and the analyses of the corresponding types of test were made by experts who were blinded to the other results. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Discordant results between different iron parameters are common and make up about 15% of our analysed samples (if the strict criteria, as proposed above, is applied – iron saturation < 7% or serum ferritin < 60 ng/mL). Although the absolute number of discordant results is few (n = 7), the discordance in the results is reflective of the high specificity of serum ferritin and the low sensitivity of percentage iron saturation at their respective levels. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Ortega 2005.
| Study characteristics | |||
| Patient Sampling | Study comparing liver iron measurements with ferritin and non‐invasive measurements. Samples selected at the level of the liver biopsy. Only biopsies with stainable liver iron were included in the analysis ‐ i.e. all patients had liver iron loading. | ||
| Patient characteristics and setting | Samples from patients who underwent liver biopsy on suspicion of iron loading, all of whom had liver iron loading. 56 samples were from patients who underwent liver biopsy on suspicion of iron loading (51 male and 5 female, mean age of 50.48 years, SD 14.1). From them, 46 samples/patients had the HFE genotype, and serum ferritin measures. The study was done in the Hospital Clinico San Carlos in Madrid, Spain. | ||
| Index tests | Serum ferritin, method not reported. Elevated serum ferritin concentration for both sexes > 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron was measured with liver biopsy. Measurements were made by iron Perl's staining, and iron content. Biopsies were blindly reviewed by two pathologists, who applied two grading systems. The first one, described by Rowe 1977 and the second one, described by Deugnier 1992. Besides, the amount of iron was quantitatively studied in the same sections by an Olympus semiautomatic image analysis system using Microimage (ver. 4.0) software. Liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | In 52 of 64 cases, the serum ferritin values had been measured within 1 month prior to biopsy. At the end of the study only 46 biopsies/patients had the corresponding liver iron content and serum ferritin measurements. | ||
| Comparative | |||
| Notes | The correlation between serum ferritin levels and liver iron content was not too good for the 46 biopsies/patients, r = 0.338 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Pascoe 1999.
| Study characteristics | |||
| Patient Sampling | Study of patients pre‐liver transplantation and iron measurement in explanted livers. Patients had alcoholic liver disease but had been abstinent for the previous 6 months. | ||
| Patient characteristics and setting | Patients with liver disease who underwent transplantation. The study selected 37 unwell, male adults (aged between 32 and 66 years old), from Brisbane, Australia with a diagnosis of alcoholic liver disease (history of chronic excessive alcohol intake with median consumption of 120 g per day), who underwent liver transplantation. From the 37 male patients, 36 were white and one Japanese. | ||
| Index tests | Serum ferritin was measured prospectively by the chemiluminescence technique. Elevated serum ferritin concentration for both sexes > 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron was measured by liver biopsy. Three measures were obtained: histological iron grading, hepatic iron content, and hepatic iron index of the liver explant. Explant liver iron concentration was measured by spectroscopy of a portion of representative liver removed from a wax block, and, from this value, the hepatic iron index was calculated. Liver iron content measurement with a threshold of > 1.9 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | The selected 37 patients with pre‐orthotopic liver transplantations (OLTs) and diagnosis of alcoholic liver disease underwent haemotological tests, and completed questionnaires including serum ferritin, transferrin saturation, Child‐Pugh Grade, spur cell anaemia status, and alcohol intake. Then the liver explant was measured for histological iron grade, hepatic iron concentration, and hepatic iron index. | ||
| Comparative | |||
| Notes | In this article it was shown that a likely cause of the gross iron loading in end‐stage alcoholic liver disease is haemolysis due to spur cell anaemia. The correlation between serum ferritin measurements and liver iron concentration measures was moderate, r = 0.535. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Patterson 1985.
| Study characteristics | |||
| Patient Sampling | From patients attending the inpatient geriatric ward, inpatient medical/surgical ward, and the geriatric outpatient clinic of the Geriatric Service of Ontario, Canada, 66 anaemic elders (64 years or older) with no obvious reason for anaemia were chosen (no further details about the sample process were given). A subsample of 64 patients had both measurements for iron stores: SF and BM. | ||
| Patient characteristics and setting | 66 anaemic elders (64 years or older) who were either inpatient or outpatients of clinics and hospitals within the secondary, and tertiary care of patients in the Hamilton‐Wentworth region of Ontario, Canada | ||
| Index tests | Serum ferritin by a radioimrnunoassay method. A serum ferritin concentration lower than 18 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The bone marrow aspirations were performed from the iliac crest under local anesthesia, and were submitted through the regular channels to the Department of Hematology. They were reported on the basis of hemosiderin stains present, absent, or doubtful, and in the doubtful cases, the slide was independently reviewed by the study haematologist (A. M. Benger), blinded from clinical information. The absence of hemosiderin stains were considered indicative of iron store deficiency. | ||
| Flow and timing | From in‐ and outpatients attending a Geriatric Service of Ontario, Canada, 66 anaemic elders with no obvious reason for anaemia were chosen. A subsample of 64 patients had both measurements for iron stores: SF and BM. Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records reviewing were undertaken. However, the flow and timing of these three types of tests were not described. |
||
| Comparative | |||
| Notes | Serum ferritin has been a most valuable addition to assessment of iron stores, and in the uncomplicated iron deficient state, it is both highly specific and sensitive. Unfortunately, numerous influences tend to elevate the serum ferritin level in an older population. Thus, if one takes the lower cutoff level of 18 µg/L, it remains an extremely specific test for iron deficiency, but its sensitivity in this population studied remains unacceptably low. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Phatak 1998.
| Study characteristics | |||
| Patient Sampling | Population screening study; only individuals at high risk of haemochromatosis and iron overload underwent liver biopsy. Patients with a fasting serum transferrin saturation of 55% or more and a serum ferritin level of 200 ug/L or more who had no apparent secondary cause of abnormal iron status, such as iron‐loading anaemias or other causes of chronic liver disease, were offered liver biopsy with quantitative iron estimation to confirm the diagnosis of hereditary haemochromatosis. | ||
| Patient characteristics and setting | Patients subselected from large cross‐sectional population for further evaluation. 16,031 adult patients (both sexes (42% male), 77% white, 14% African‐American, and 9% other or unknown) were subselected from a large cross‐sectional population screening study taken from 22 primary health care centres from Rochester, New York. These urban and suburban practices included 63 physicians, nurse practitioners, and physician assistants and ranged in type from solo practices to large clinics. Practice size varied from 1500 to 13,000 patients. The selected sample consisted of adult patients of both sexes with high risk of hereditary haemocromatosis (aged between 23 and 75 years old). | ||
| Index tests | Serum ferritin levels were measured on the Ciba‐Corning Automated Chemiluminescence System (Ciba‐Corning, Medfield, Massachusetts) by using a two‐site chemiluminometric sandwich immunoassay with acridinium ester as the luminescent tag. | ||
| Target condition and reference standard(s) | Liver iron measured with liver biopsy. Investigators obtained hepatic iron index scores and hepatic iron concentrations. A liver iron concentration measurement with a threshold of > 2.8 mg/g dry weight was classified as iron overload (no description of the methodology to measure the liver iron concentration was found). | ||
| Flow and timing | 16,031 adult Individuals screened for iron overload via iron indices (such as serum ferritin and transferrin saturation). From those, only the individuals with elevated indices and family history of haemochromatosis underwent liver biopsy. | ||
| Comparative | |||
| Notes | The cost‐effectiveness of screening for haemochromatosis was shown following a clever decision model (as shown in this article), where serum ferritin was a support measure of transferrin saturation to find hereditary haemochromatosis. Liver iron concentrations and serum ferritin measures had a low correlation of r = 0.35. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Phiri 2009.
| Study characteristics | |||
| Patient Sampling | 381 infants and children 6‐59 months of age with severe anaemia from Blantyre and Chikwawa, Malawi, presenting to the hospital with severe anaemia (Hb less than 50 g/L). These areas are malaria‐endemic areas. | ||
| Patient characteristics and setting | Patients were 381 infants and children (both sexes, aged from 6 to 59 months, with mean age of 20 months) with severe anaemia seeking medical care at hospitals located in the malaria‐endemic areas of Blantyre and Chikwawa, Malawi. This study formed part of a large case–control study investigating the aetiology of severe anaemia conducted between July 2002 and July 2004. | ||
| Index tests | Ferritin measurements, along with other haematological measurements, were measured on recruitment. The laboratory assay for ferritin was not reported in the article but the provider was Roche Diagnostics, Switzerland. A serum ferritin concentration lower than 273 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The data corresponded to severely anaemic infants and children diagnosed through the use of a commercial kit for bone marrow aspirates higher than 2 with 60% having malaria parasites in their blood. Under local anaesthesia, a bone marrow commercial kit was used for aspiration from the anterior or posterior iliac crest according to manufacturer guidelines (HematoGnost Fe, Darmstadt, Germany). Smears were graded for iron and defined as positive when the fragment iron was < 2 (Phiri 2009).Absent stains were considered indicative of iron store deficiency. | ||
| Flow and timing | Children received a clinical examination, and then venous blood was withdrawn for haematological tests. Later, under local anaesthesia, bone marrow aspiration was conducted. | ||
| Comparative | |||
| Notes | 381 Malawian children aged 6–59 months, severely anaemic in malaria‐endemic areas of Malawi. Biochemical iron markers were compared to bone marrow iron findings. TfR‐F index incorporated the high sensitivity of sTfR, a proxy for cellular iron need, and the high specificity of ferritin, a proxy for iron stores. In areas with a high infection pressure, the TfR‐F index best predicted iron deficiency. However, in settings where diagnostic tests are limited, MCHC may be an acceptable alternative screening test. Ferritin, sTfR, TfR‐F index and MCHC cut‐off levels, with an optimal combination of sensitivity and specificity, were determined from the ROC curves. The ability of sTfR or ferritin to predict iron stores deficiency (accuracy) was similar and above 75% using the derived cut‐offs of 273 µg/L and 15.2 µg/mL, respectively. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Pietrangelo 1999.
| Study characteristics | |||
| Patient Sampling | The study began in 1983 with the evaluation of a patient (the proband) who had a primary iron overload condition and was undergoing repeated phlebotomy at the University of Modena, in Modena, Italy. Between 1983 and 1998, serum iron (i.e. transferrin saturation and ferritin) was measured in the proband and 52 members of his family. Fifteen selected family members had abnormal blood iron values (serum ferritin > 500 µg/L and transferrin saturation > 50%) and underwent liver biopsy. | ||
| Patient characteristics and setting | A proband with primary iron‐overload condition and who underwent repeated phlebotomy, and 52 of his family members (both sexes from 9 to 85 years old) from Modena, Italy. 15 had abnormal blood iron values and underwent liver biopsy (6 females and 9 males aged between 14 and 61 years old). | ||
| Index tests | Serum ferritin was measured by standard methods in samples obtained after an overnight fast from all 53 family members (no further details of the SF measurement technique was found). An elevated serum ferritin concentration for both sexes > 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Hepatic iron concentration was determined with the use of an atomic‐absorption spectrophotometer (model S2380, Perkin–Elmer, Norwalk, Conn.), as described Rocchi 1986. Liver iron concentration measurement with a threshold of > 3.2 mg/g dry weight was classified as iron overload. In fact, all 13 patients had values over 4 mg/g dry weight. | ||
| Flow and timing | The study began in 1983 with the evaluation of a patient (the proband) who had a primary iron overload condition and was undergoing repeated phlebotomy. Between 1983 and 1998, serum iron (i.e. transferrin saturation and ferritin) was measured in the proband and 52 members of his family. 15 selected family members who had abnormal blood iron values (serum ferritin > 500 µg/L and transferrin saturation > 50%) underwent liver biopsy. | ||
| Comparative | |||
| Notes | The 15 patients' SF values ranged from 650 to 5846 µg/L, and the hepatic iron concentrations from 4.2 to 58.6 mg/g dry weight with a moderate correlation between them of 0.43. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Porter 1994.
| Study characteristics | |||
| Patient Sampling | All rheumatoid arthritis patients who were admitted to the Centre for Rheumatic Diseases, Glasgow Royal infirmary between 1959 and 1991 and had serum ferritin and bone marrow aspiration performed | ||
| Patient characteristics and setting | 101 patients (18 male, 83 female with mean age of 58 years old) with rheumatoid arthritis and suspected anaemia | ||
| Index tests | Serum ferritin (measurement method not described). A serum ferritin concentration lower than 75 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow procedure not described. However, the iron store smears grading was absent, scanty or very reduced to classify as iron deficient, and normal to increased otherwise. Another group in between iron deficiency and iron replete, described as 'reduced', was also studied. | ||
| Flow and timing | 101 anaemic patients with rheumatoid arthritis admitted to the Centre for Rheumatic Diseases, Glasgow Royal infirmary between 1959 and 1991. Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | 101 adult patients (18 male, 83 female) with rheumatoid arthritis and anaemia undergoing investigation in a teaching hospital rheumatology unit. 49 patients were iron deficient, 42 had anaemia of chronic disorder, and 10 patients had 'reduced' iron stores on bone marrow examination. The 'reduced' group had significantly lower ferritin compared to the anaemia of chronic disorder group (median 99 vs 217 ng/mL, P < 0.03) and significantly higher ferritin than the iron deficient group (median 99 vs 15 ng/mL, P < 0.0001). Patients with anaemia of chronic disorder had significantly higher serum ferririn (P < 0.0001), mean corpuscular volume (P < 0.05), and acute phase reactants (P < 0.001). The sensitivity, predictive value and validity of measuring serum ferritin to predict the absence of bone marrow iron stores was studied. Maximum validity (89%) was achieved by defining iron deficiency as occurring when serum ferritin was < 75 ng/mL. 93% of patients with ferritin < 50 ng/mL were iron deficient on bone marrow examination. 91% of patients with ferritin > 100 ng/mL were iron replete on bone marrow examination. 86% of patients had ferritin < 50 or > 100 ng/mL. Age was not a significant confounding factor. Diagnostic test accuracy data was provided as: sensitivity, specificity, and predictive values for different cut‐off points. Thus, it was possible to build the 2 X 2 tables. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Punnonen 1994.
| Study characteristics | |||
| Patient Sampling | 36 anaemic adult patients (no more details of the sampling procedure were described) | ||
| Patient characteristics and setting | 36 anaemic adults (both sexes, 50 years or older with several diseases ‐ mainly chronic) attending University Central Hospital of Turku, Finland. | ||
| Index tests | Ferritin was measured with an IRMA assay (Spectria; Orion Diagnostica). Reference range 15‐306 µg/L for men, 5‐103 µg/L for women, according to the manufacturer. Serum ferritin concentration lower than 25 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow was aspirated from the sternum, and smears were stained by the May‐Grilnwald‐Giemsa method (stain provided by Orion Diagnostica, Helsinki, Finland). The iron stores were stained by the Prussian blue method. The category of absent iron stores was considered indicative of iron deficiency. | ||
| Flow and timing | 36 anaemic adults. Haematologic and biochemical analyses (such as serum ferritin) and bone marrow aspiration measurements were undertaken. However, the flow and timing of these two types of tests were not described. | ||
| Comparative | |||
| Notes | The mean serum ferritin concentration was lower in patients with iron‐deficiency anemia (9 ± 6 µg/L, mean ± SD) than in the controls (72 ± 89 µg/L, mean ± SD). However, it was significantly increased in the patients with anaemia of chronic disease (288 ± 274 µg/L) Thus, the use of ferritin as a marker of iron deficiency was complicated by the acute‐phase responses associated with chronic disease. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Punnonen 1997.
| Study characteristics | |||
| Patient Sampling | 129 consecutive anaemic adult patients at the University Hospital of Turku who underwent a bone marrow examination because of anaemia (no more details about patient sampling were described). | ||
| Patient characteristics and setting | 129 consecutive anaemic adult patients (both sexes, aged 50 years or older) at the University Hospital of Turku, Finland. Patients had several diseases, mainly chronic and anaemia. | ||
| Index tests | Serum ferritin (reference range, 15 to 306 µg/L for men, 5 to 103 µg/L for women, according to the manufacturer) was measured using a radioimmunoassay (Spectria, Orion Diagnostics). A serum ferritin concentration lower than 41 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow was aspirated from the sternal bone or iliac crest. The smears were stained using the May‐Gruenwald‐Giemsa method (Orion Diagnostica, Helsinki, Finland), and the iron stores were stained by the Prussian blue method. Absent smear staining on bone marrow was classified as iron deficient. | ||
| Flow and timing | 129 consecutive anaemic adult patients. Haematologic and biochemical analyses (such as serum ferritin), and bone marrow aspiration measurements were undertaken. However, the flow and timing of these two types of tests were not described. | ||
| Comparative | |||
| Notes | In this study population, ferritin measurements (AUC_ROC 0.98) distinguished effectively between patients with uncomplicated IDA and those with ACD, but the optimal decision limit for the interpretation of SF measurements was found to be considerably above the conventional reference limits, which are based on the evaluation of apparently healthy populations. It is evident that the ability of ferritin to distinguish between IDA and ACD is due not only to the decrease in serum ferritin level in IDA patients but, to a considerable extent, to the increase in serum ferritin caused by the acute‐phase responses associated with chronic inflammatory disease. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Punnonen 1998.
| Study characteristics | |||
| Patient Sampling | 10 non‐anaemic adult women who had no stainable iron in bone marrow, at the University Hospital of Turku, Finland. Patients with disease conditions known to interfere with serum TfR concentrations (haematological malignancies, haemolytic anaemia or defined deficiency of vitamin B12 or folate) were excluded. |
||
| Patient characteristics and setting | This study evaluated non‐anaemic adult females with latent iron deficiency at the University Hospital of Turku, Finland. | ||
| Index tests | Ferritin was measured using radioimmunoassay (Spectria, orion Diagnostics, Helsinki, Finland). The lower reference limit for ferritin with this method was 5 µg/L. A serum ferritin concentration lower than 12 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow was aspirated from the sternal bone or iliac crest. The smears were stained using the May‐Gruenwald‐Giemsa method (Orion Diagnostica, Helsinki, Finland). Absent smear staining on bone marrow was classified as iron deficient. | ||
| Flow and timing | This study evaluated the ferritin and other haematological measurements from 10 non‐anaemic patients who had no stainable iron in bone marrow (latent iron deficiency). Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records reviewing were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | All 10 non‐anaemic patients that were considered to have latent iron deficiency were females, suggesting that in male patients latent iron deficiency is much less common. In different laboratories, the reported lower reference limits for serum ferritin in female subjects vary between 5 and 15 µg/L and, apparently, in latent iron deficiency serum ferritin concentration is likely to be still within the reference range. This result underlines the importance of using valid reference limits for ferritin, and it may also be questioned whether, in female subjects, the ferritin concentration that is indicative of iron deficiency is truly different from that of male subjects. The difference in the male and female reference limits might merely reflect the difference in iron status between male and female subjects.This part of the study reported on the use of 5 µg/L as a threshold. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Puolakka 1980.
| Study characteristics | |||
| Patient Sampling | 32 healthy pregnant women attending the maternity centers of Oulu, Finland volunteered for the study | ||
| Patient characteristics and setting | 32 healthy pregnant women attending maternity centers of Oulu University, Finland. They were between 20 and 49 years old, and they were followed during their pregnancy. Inclusion criteria for this review was accomplished for 12 women where serum ferritin and bone marrow was measured and available before iron treatment in the second semester of pregnancy. | ||
| Index tests | Blood samples were obtained according to the recommendations of the Committee of Reference Values of the Scandinavian Society for Clinical Chemistry and Clinical Physiology. Serum ferritin measurement method was not specified. A serum ferritin concentration lower than 70 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The smears of the bone marrow aspirates from the sternum were stained by the Prussian blue method and the amount of iron graded as described earlier in Puolakka 1980. Participants who had smears with absent iron were classified as iron deficient. | ||
| Flow and timing | 32 healthy pregnant women were evaluated longitudinally during pregnancy and 6 months postpartum by serum ferritin assay and by bone marrow iron content. Both measures were taken at the 16th and 32nd gestational weeks, and 6 months postpartum. Because one group received iron complementation, only a small subsample of females with SF and BM measurements taken at the 16th gestational week was used in this review. The flow and timing of these two types of tests, that occurred three times per woman at specific stages of pregnancy and postpartum, were not described. | ||
| Comparative | |||
| Notes | Measurements of serum ferritin were correlated with bone marrow iron content and other laboratory hematological indices in 44 anaemic pregnant women. About 72% of patients classified as iron deficient by the absence of bone marrow iron, had diagnostically low serum ferritin concentrations; the percentage was about double the findings revealed by measurements of serum transferrin, TIBC, transferrin saturation, serum iron, blood haemoglobin and red cell indices. Serum ferritin was the only measurement which was able to differentiate between iron deficiency and anaemia due to infection. Seven out of 8 patients with anaemia due to infection had elevated serum ferritin concentrations. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Rao 1984.
| Study characteristics | |||
| Patient Sampling | Sixty adult patients with sickle cell anaemia from the haematological clinic of the Cook County Hospital (no further details about the sampling were described). | ||
| Patient characteristics and setting | 60 adult patients with sickle cell anaemia, of mixed age (mean 56.8 years) attended the haematological clinic of the Cook County Hospital, Chicago (Chicago, Illinois, USA). | ||
| Index tests | Serum ferritin determinations were performed by a two‐site immunoradiometrtc assay using a commercial kit (Ramco Laboratories, Inc., Houston, Tex.). A serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspiration was performed from the posterior superior iliac spine using an Osgood bone marrow aspiration needle. Smears of the aspirate were stained for iron utilising Perl's Prussian blue reaction. A bone marrow smear known to contain iron was stained simultaneously in the same bath to serve as a control. Smears were examined using both the lower power and the oil immersion objectives. Marrow readings were confirmed by at least two individuals working independently; marrow iron was graded as absent, decreased, normal, or increased. Absent and decreased iron in BM smears was classifed as iron deficiency. | ||
| Flow and timing | 60 adult patients with sickle cell anaemia consented to enter the study. Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | The log SF values in the 60 patients studied were normally distributed, similar to that described in the general population. This was also true for the log SF values in the iron‐deficient and iron‐replete groups. Highly significant differences between the means were noted between the two groups with respect to log SF, serum transferrin, transferrin saturation, and the MCV. The correlation of SF with the clinical and laboratory parameters using linear regression analysis and the chi square test showed that log serum ferritin had a significant negative correlation with transferrin (P < 0.001 ), and a positive correlation with transferrin saturation (P < 0.005), history of prior blood transfusion (P = 0.005), and bone marrow iron stores (P < 0.001). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Ravindran 2008.
| Study characteristics | |||
| Patient Sampling | 50 consecutive anaemic adults with rheumatoid arthritis, fulfilling the American College of Rheumatology classification criteria for RA and attending the rheumatology outpatients, who had not received iron treatment or blood transfusion in the past 6 weeks were included (no further details about the samplig procedure were given). | ||
| Patient characteristics and setting | 50 consecutive anaemic adults with RA (33 females, 17 males, with a mean age of 56 years old). They were patients of the Department of Internal Medicine, Sawai Man Singh Medical College and Hospitals, Jaipur, India. | ||
| Index tests | Serum ferritin was assessed by enzyme linked immunosorbent assay (Ferrizyme, Abbott Labs, USA) using a method described by Conradie 1980. A serum ferritin concentration lower than 82 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Posterior iliac crest bone marrow aspiration was performed on each patient and fixed smears were stained for iron with Perl’s Prussian blue. A minimum of two smears per patient were evaluated and graded for iron content according to the following scale: 0: no stainable iron, I: minimal or very small content, II: slight, small and patchy content, III: moderate and diffuse, IV: strong, extensive and diffuse content. Grade 0 and I were regarded as indicating iron deficiency and the rest (grade II–IV) were considered iron replete, hence, as having ACD. | ||
| Flow and timing | 50 consecutive anaemic adults with RA underwent haematologic and biochemical analyses (such as serum ferritin), and bone marrow aspiration measurements. However, the flow and timing of these two types of tests were not described. | ||
| Comparative | |||
| Notes | 50 anaemic patients with RA were selected for the study. 18 patients (36%) had IDA and 32 (64%) had ACD. Correlation between the bone marrow iron stores and serum ferritin was poor in the IDA group (r = ‐0.09, P = 0.57) and significant in the ACD group (r = 0.79, P < 0.0001). Both the area under the ROC curve [0.98, 95% CI (0.94, 0.99)] and the negative predictive value (97%) were highest when the cut‐off for serum ferritin was < 82 µg/L. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Rowe 1977.
| Study characteristics | |||
| Patient Sampling | 50 family members of a proband in which autopsy had revealed severe haemochromatosis were screened for haemochromatosis in order to prevent the development of the disease. Fifty members over the age of 13 years spanning three generations were examined by medical history, alcohol intake, physical examination, and several haematological indices. A subset of them (31 subjects) underwent percutaneous liver biopsy (it was not specified how the subset was selected). | ||
| Patient characteristics and setting | 50 relatives of a severe case of haemochromatosis and with a history of death in family members because of severe liver disease (both sexes, more than 14 years old, from Baltimore, United States). Only a subset of 31 family members were selected for liver biopsy (13 females, 18 males, aged from 14 to 67 years old). | ||
| Index tests | Serum ferritin concentrations were determined by radioimmunoassay. Elevated serum ferritin concentration for both sexes > 200 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Percutaneous liver biopsy was performed in 31 family members. Each liver biopsy was studied by light microscopy to determine iron deposition, fat content, the extent of fibrosis, and other morphological changes. Each biopsy was stained, and Perl's reaction for iron concentration was examined by at least two reviewers. Liver iron assessed by Perl's staining. Iron deposition were graded on a scale ranging from 1+ to 4+ where 1+ or more indicated iron overload and 0 otherwise. | ||
| Flow and timing | 50 family members were examined by medical history, alcohol intake, physical examination, and several haematological indices. A subset of them (31 subjects) underwent percutaneous liver biopsy (it was not specified how the subset was selected). | ||
| Comparative | |||
| Notes | All serum ferritin values were under the normal range, and only one had a level superior to 200 µg/L corresponding to IO. By contrast, they were 19 family members with an iron deposition grading equal or bigger than 1 (diagnosed with iron overload by liver biopsy). The correlation between SF and liver iron grading was low, r = 0.38. Thus, SF was not related to serum iron, iron binding capacity, transfusion saturation, and it was a poor indicator of iron overload. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Ruivard 2000.
| Study characteristics | |||
| Patient Sampling | Prospective study undertaken during eight months, with the selection of 103 patients who where hospitalised in the departments of Internal Medicine and Clinical Haematology for several reasons and required a bone marrow aspirate examination. A subsample of 82 patients fit the inclusion criteria. From those, two groups were formed: 21 iron deficient patients and a control group of 33 (from the remaining 61 patients on the basis of having similar ages (no further details about the sampling were given). | ||
| Patient characteristics and setting | The 54 patients included 21 patients who were iron deficient (17 females, 4 males with a mean age of 52 years), and 33 control subjects (20 females, 13 males with a mean age of 60 years). Both groups were hospitalised in the departments of Internal Medicine and Clinical Haematology for several reasons and they required a bone marrow aspirate examination, and haematological iron store indicators. The patients were from Clermont‐Ferrand, France. | ||
| Index tests | Serum ferritin was measured by immunoassay enzymatic (Kit IMX Ferritine, Abott). A SF concentration lower than 60 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow was obtained by sternus or iliac aspiration. It was coloured by Perl's Prussian blue. The grading was focused on the absence or presence of iron in smears. Thus, the absence of iron in Perl's stained samples was classified as iron deficient, and the presence as iron replete. | ||
| Flow and timing | Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | 21 patients with iron deficiency and 33 control subjects were included in the study. The ratio of serum transferrin receptor/serum ferritin had the best diagnostic efficiency (78%) with a sensitivity of 81% and a specificity of 97%. Serum ferritin alone with a cut‐off value of 60 μg/L had the same specificity (97%) but a lower sensitivity (76%). The diagnostic value of all other analysed tests was below 66% (transferrin alone, mean corpuscular volume, transferrin saturation, iron, serum transferrin receptor alone, red cell distribution width). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Schöniger‐Hekele 2002.
| Study characteristics | |||
| Patient Sampling | 61 patients referred to the Gastroenterology and Hepatology department for suspicion of iron loading from January 1997 to December 2000. 37 of them had elevated liver enzymes and were selected for liver biopsy. From those, only 25 patients consented to the liver biopsy procedure. | ||
| Patient characteristics and setting | Study of liver iron and ferritin as well as fibrosis in the population (individuals with heterozygous HFE), along with controls (cadaveric liver donors). A sample of the 25 selected patients sample (adults, both sexes, with a mean age of 48.3 years, from Vienna, Austria) were divided in two groups: 15 Caucasians with compound heterozygosity for the mutations C282Y and H63D in the HFE gene without known underlying liver disease, and 10 heterozygous patients with known underlying liver disease. In addition, 11 cadaveric liver donors served as controls with 10 wild types for C282Y and H63D, with one heterozygous for H63D. | ||
| Index tests | Serum ferritin by turbidometry on a BM‐Hitachi automatic analyser (Boehringer Mannheim, Germany) with normal values of 18‐440 µg/L in men as well as 8‐120 µg/L and 30‐300 µg/L in pre‐ and postmenopausal women, respectively. | ||
| Target condition and reference standard(s) | Liver tissue was obtained either by biopsies using a disposable biopsy set (Hepafix; Braun, Melsungen, Germany) or at surgery. For histological examination, paraffin‐embedded sections were routinely stained with Perl's Prussian blue. The hepatic‐iron deposition was graded according to Rowe 1977 and Di Bisceglie 1992. This grading is based on the magnification at which iron granulates are readily discernible: grade 0: no detectable iron; grade 1: 400; grade 2: 100; grade 3: 25; grade 4: 10 or could be seen by the naked eye. Liver iron content by Perl's grading of 1 or more was classified as iron overload. Note: liver iron concentration was measured, however, these data do not appear to have been presented. |
||
| Flow and timing | 61 patients referred to a specialised hospital unit with suspicion of iron loading from January 1997 to December 2000. These patients had liver enzyme tests and genetic inspections for mutations. 37 of them had elevated liver enzymes and were selected for liver biopsy. From those, only 25 patients consented to the liver biopsy procedure. Haematological tests for measuring iron status (serum‐iron concentration, tranferrin saturation, and serum ferritin), liver biopsy, and serological determination of Hepatitis B virus markers were undertaken. The flow and timing of these three kind of tests were not described. | ||
| Comparative | |||
| Notes | This study documented the variability in histologic findings and iron parameters in compound heterozygous patients. Severe fibrotic changes might be present which are found predominantly in patients with high serum ferritin levels. Liver iron content and serum ferritin measures had a relatively high correlation of r = 0.70. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Sebastiani 2006.
| Study characteristics | |||
| Patient Sampling | This study included a consecutive series of 242 patients with chronic hepatitis C who underwent a diagnostic liver biopsy in a Hepatology unit in Padova, Italy. Cross‐sectional study of patients with the chronic hepatitis C virus. |
||
| Patient characteristics and setting | 242 patients with the hepatitis C virus (adults, both sexes, with a mean age of 47.8 +/‐ 12.3 years old) attended a Hepatology unit in Padova, Italy. | ||
| Index tests | Serum ferritin (measurements assay technique not mentioned). A SF concentration (males > 300, females > 200 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | All liver biopsy specimens were obtained by the Menghini technique after the patients had given written informed consent. Slides were stained with haematoxylin–eosin, trichrome, acid‐Schiff stain and Perl’s Prussian blue method. Semi‐quantitative histological evaluation of iron load was assumed by means of a five‐class grading system according to Basset 1986 as follows: grade 0: absent stainable iron; grade I: minimal amount of iron; grade II: mild; grade III: moderate; and grade IV: heavy hemosiderosis. Thus, grade hepatic iron deposits of I to IV indicated an iron overload state. | ||
| Flow and timing | Haematological tests for measuring iron stauts (serum‐iron concentration, tranferrin saturation, and serum ferritin), liver biopsy, and serological determination of Hepatitis C were made. It was not described the flow and time of these three kind of tests. | ||
| Comparative | |||
| Notes | Patients with hepatic iron deposits (HIDs) and normal serum ferritin had a lower grade of hepatic siderosis, most having grade I. Presence or absence of HIDs and its grade correlated significantly with serum ferritin levels (r = 0.55, P < 0.00001). Serum ferritin is not very sensitive for the prediction of HIDs but it has got a high specificity in excluding more advanced grades of hepatic iron, thus excluding the need of venesection without a liver biopsy. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sebastinani 2012.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study of risk of iron loading in patients with chronic hepatitis B. Consecutive patients with CHB who underwent a diagnostic percutaneous liver biopsy at Dello Angelo Hospital of Venice between March 2005 and June 2009 | ||
| Patient characteristics and setting | Consecutive patients (both sexes, with mean age of 43.8 +/‐ 11.3 years old) with chronic hepatitis B who underwent liver biopsy at Dello Angelo Hospital, Venice | ||
| Index tests | Serum ferritin (measurements assay technique not mentioned). A SF concentration (males > 300, females > 200 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | All liver biopsies were obtained from patients who had given written informed consent. Slides were stained with Perl’s Prussian blue method. Semi‐quantitative histological evaluation of iron load was assumed by means of a five‐class grading system according to Basset 1986 as follows: grade 0: absent stainable iron; grade I: minimal amount of iron; grade II: mild; grade III: moderate; and grade IV: heavy hemosiderosis. Thus, grade hepatic iron deposits of I to IV indicated an iron overload state. | ||
| Flow and timing | Haematological tests for measuring iron status (serum‐iron concentration, tranferrin saturation, and serum ferritin), liver biopsy, and serological determination of Hepatitis B were undertaken. The flow and timing of these three kind of tests were not described. Retrospective study. | ||
| Comparative | |||
| Notes | Serum ferritin measurements were significantly associated with hepatic iron deposits (HIDs) in both univariate analysis: OR = 4.3, 95% CI: 1.41 to 13.3, P = 0.008, and multivariate analysis: b = 0.008, OR = 1.2, 95% CI 1.05 to 1.4, P = 0.002 (in both cases, the corresponding groups were HIDs+ and HIDs‐ meaning presence or absence of iron overload). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sham 1997.
| Study characteristics | |||
| Patient Sampling | 140 patients with biopsy‐proven or clinically diagnosed hereditary haemochromatosis currently were treated in Rochester Hospital centre. From those, 61 patients with HFE genotype consented to be part of the study. A sample of 37 patients underwent liver biopsy and hepatic iron determination. | ||
| Patient characteristics and setting | Patients attending for therapeutic phlebotomy at hospital in Rochester, NY. Only patients proven with hereditary haemochromatosis were included in the study (both sexes, adults between 28 and 80 years old). | ||
| Index tests | Serum ferritin (measurements assay technique not mentioned). An elevated serum ferritin concentration for both sexes > 300 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | The liver biopsies were performed using a 17‐gauge Jamshidi needle, a standard transthoracic approach employing a Menghini aspiration technique to obtain the liver tissue. The specimens were sent for routine histological study and stained with haematoxylin and eosin, Massons trichome, reticulin and Prussian blue. The stainable iron was characterised with a semi‐quantitative grading system from grade 0‐IV, and a portion of the liver biopsy sample was used to measure the total iron in liver biopsy specimens spectrophotometrically. Thus, grade hepatic iron deposits of I to IV indicated an iron overload state. | ||
| Flow and timing | 140 patients with biopsy‐proven or clinically diagnosed hereditary haemochromatosis. From those, 61 patients with HFE genotype consented to be part of the study. The 61 patients underwent haematological tests for measuring iron status (serum‐iron concentration, tranferrin saturation, and serum ferritin). Fifty‐one of the 61 patients underwent therapeutic phlebotomy. Afterwards, a smaller sample of 37 patients underwent liver biopsy and hepatic iron determination. | ||
| Comparative | |||
| Notes | Serum ferritin measurements were moderately correlated with hepatic iron deposits (HIDs): r = 0.463 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Sharma 1984.
| Study characteristics | |||
| Patient Sampling | Prospective study of 35 anaemic patients, presenting to general medical and geriatric wards (no further details about the sampling procedure were given). | ||
| Patient characteristics and setting | 35 anaemic patients (16 males and 19 females) aged 65 years and over, presenting to general medical and geriatric wards. | ||
| Index tests | Serum ferritin was estimated using a commercial kit (supplied by Becton Dickinson Immunodiagnostics) involving a radioimmunoassay technique modified from the original technique described by Addison 1972. A serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspirate was stained using Perl's method and iron stores were graded on the basis of visual impression of the stained granules as: absent bone marrow iron stores (BMIS) graded as 0; low BMIS graded as + ; normal BMIS as + + ; increased BMIS as + + + ; and abundant BMIS as + + + +. Differentiation of three and four plus conditions may be difficult due to the variation in staining of granules when increased iron is present. Grade 0 or absence of iron stained granules were classified as iron deficiency. | ||
| Flow and timing | Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records reviewing were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Of the 35 patients studied, 25 had iron depletion on bone marrow iron stores estimation. Of these iron‐depleted patients, 19 (76%) had low serum ferritin and six (24%) had normal serum ferritin. As a single factor, low serum ferritin in the elderly appears to be a very good index of iron deficiency. Low serum ferritin in elderly anaemic patients invariably indicates iron depletion, and iron store estimation in bone marrow is not necessary. Normal serum ferritin does not exclude iron deficiency and, if clinically indicated, a bone marrow examination is essential to establish a diagnosis of iron deficiency. In these patients a raised ESR may indicate a concurrent condition affecting serum ferritin. This may be the case in chronic inflammatory conditions, liver disorder and malignant conditions. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Shroff 1991.
| Study characteristics | |||
| Patient Sampling | 30 patients (16 to 55 years) with rheumatoid arthritis were evaluated in a prospective and controlled study for iron status with special reference to serum ferritin levels. | ||
| Patient characteristics and setting | There were 16 classical and 14 definite patients of RA (participants had RA diagnosed based on the 1958 American Rheumatism Association). The mean age of these patients was 35 +/‐ 10 years with a range of 16 to 55 years old. The mean duration of illness was 3.8 +/‐ 2.4 years. 80% of patients had mild to moderate anaemia which in most cases was normocytic and normochromic on peripheral blood smears. The patients were measured and treated at the Department of Pathology, Maulana Azad Medical College and LNJPN Hospital, New Delhi, India. | ||
| Index tests | Serum ferritin assessed by immunoradiometric assay using IRMA kit (Biclone, Australia). This test measures ferritin concentrations from 2.5 to less than 1000 µg/L in serum or plasma. It is a sandwich assay employing constant amounts of two antibodies: 1) covalently coupled to magnetisable polystyrene particles and 2) a radio‐iodinated anti‐ferritin contained goat anti‐human liver ferritin. A serum ferritin concentration lower than 32 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The marrow iron stores were assessed by bone marrow aspiration after Perl's staining and were graded semi‐quantitatively as follows: grade 0 to 1: diminished to absent, grade 2+ to 3+: normal, grade 4+ to 6+: increased. Grades 0 and 1 were classified as iron deficient. | ||
| Flow and timing | All patients were evaluated clinically and then subjected to blood tests and investigations of the bone marrow. The flow and timing was not fully described in the manuscript. | ||
| Comparative | |||
| Notes | 30 patients (16 to 55 years) with rheumatoid arthritis were evaluated in a prospective and controlled study for iron status with special reference to serum ferritin levels. Serum ferritin levels were estimated by RIA technique and marrow iron status was ascertained by semi‐quantitative estimation after Perl's staining of marrow aspirate (G 0‐6). Marrow iron stores were found absent to decreased in 17 patients (56.7%), normal in 2 (6.7%) and increased in 11 patients (36.6%). The serum ferritin levels in the iron depleted rheumatoid arthritis patients were significantly lower in comparison to patients with normal to increased marrow iron stores (23.91 +/‐ 11.45 ug/L vs 69.94 +/‐ 24.7 ug/L, P < 0.001). There was a strong positive correlation between serum ferritin levels and marrow iron stores (r = +0.08, P < 0.001). A serum ferritin value of less than or equal to 32 ug/L was a good predictor of decreased iron stores in the bone marrow, with a sensitivity of 88.2% and specificity of 84.5%. The test had a predictive value of 83.33%. There was no correlation between marrow iron stores and conventional indicators or iron status i.e. serum iron, TIBC, transferrin saturation and MCHC. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Smith 1977.
| Study characteristics | |||
| Patient Sampling | 35 anaemic patients with rheumatoid arthritis | ||
| Patient characteristics and setting | 35 anaemic patients with rheumatoid arthritis (28 females, 7 males; ages between 19 to 73 years old with a mean age of 55.3 years). The patients were treated and followed up in the University of Alberta Hospital, Edmonton, Alberta, Canada. | ||
| Index tests | Serum ferritin by two‐site immunoradiometric assay. A serum ferritin concentration lower than 100 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Posterior iliac crest bone marrow aspiration was performed, stained for iron with Perl’s Prussian blue. Graded from 0‐4+, where: 0 = no stainable iron; 1+ = traces; 2+ = stainable iron present in 26‐75% of high power fields; 3+ = stainable iron present in 75‐100% of high power fields; 4+ = definite blue staining visible on naked eye inspection of the slide. Grades 0 and 1 were classified as iron deficient. | ||
| Flow and timing | Not reported | ||
| Comparative | |||
| Notes | 35 anaemic patients with rheumatoid arthritis (28 females, 7 males; ages 19 to 73 years with a mean of 55.3 years) were studied to determine the relationship between serum ferritin levels and body iron status, as assessed by the grading of bone marrow iron stores. The incidence of greatly reduced or absent marrow iron stores was 60% (grade 0 and1 in bone marrow). Peripheral blood smear. RBC indices, serum iron, and iron binding capacity correlated poorly with stainable marrow iron. There was also a poor correlation between bone marrow iron stores and the serum ferritin concentrations. The serum ferritin levels fell into two distinct groups. On the one hand, anaemic patients with a marrow grading of 0 and 1+ had a serum ferritin less than 100 ng/mL, whereas those with a marrow grading of 2+ or higher had serum ferritin levels greater than 100 ng/mL. The mean ± SD of the serum ferritin for grade 0 and 1 was 35 ± 27 ng/mL and 50 ± 29 ng/mL, respectively. The mean ± SD serum ferritin for grades 2+ and 3+ were 223 ± 88 ng/mL and 316 ± 203 ng/mL. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Smith 1997.
| Study characteristics | |||
| Patient Sampling | Adults from Massachusetts general population. The study population was derived from two sources: 963 frozen serum samples remained from a 1993 Polaroid study of prostate‐specific antigen values in male employees over age 50 years, and an additional 1331 nonduplicate serum samples were collected through a corporation‐wide informational and promotional campaign offering free screening for hereditary haemochromatosis. | ||
| Patient characteristics and setting | Selected sample of 15 unwell patients with hereditary haemochromatosis (14 men, 1 woman, with a mean age of 46 years and a range between 27 and 57 years old) | ||
| Index tests | Serum ferritin levels were measured by fluorometric enzyme immunoassay. An elevated SF (women, 300 µg/L; men, 400 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Diagnosis was based on liver biopsy findings of parenchymal loading, increased hepatic iron concentration, and hepatic iron stores. Liver biopsy sections were stained with hematoxylin‐eosin, Mallory’s trichrome, and Perl's Prussian blue stain for iron. Hepatic iron concentrations were measured by atomic absorption spectrophotometry. A liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | A total of 2294 patients were screened. Then haematological tests for measuring iron status (serum‐iron concentration, tranferrin saturation, and serum ferritin). Thus, 15 subjects met the criteria for liver biopsy, but only 8 liver biopsies were finally performed, and 5 Caucasian employees found to have HHC. 963 of the initial samples came from frozen samples that were 5 years old. | ||
| Comparative | |||
| Notes | This study supports the concept of a Celtic origin for the HHC gene. Serum ferritin levels showed no correlation with hepatic iron concentrations in the five patients found with hereditary haemochromatosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Solomon 1981.
| Study characteristics | |||
| Patient Sampling | 13 subjects with idiopathic refractory anaemia. | ||
| Patient characteristics and setting | 13 subjects with idiopathic refractory anaemia studied in the Clinical Research Center of the University of Washington. Patients were 7 females and 6 males aged from 31 to 84 years old with the following blood disorders: 7 idiopathic ring sideroblastic anaemia (2 of them with leukaemia), and 6 idiopathic anaemia with abnormal sideroblasts (2 of them with leukaemia). | ||
| Index tests | Serum ferritin was assayed by the method of Miles 1974. A serum ferritin concentration lower than 40 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow aspiration with Wright's and Prussian blue stain preparations. Sideroblasts normal classification was defined as a normoblast with 10 or more perinuclear iron granules encircling at least one‐third of the circumference of the nucleus. An abnormal sideroblast was defined as a cell with more than 10 unusually large iron granules in a diffuse distribution in the cytoplasm. Reticuloendothelial iron stores were graded on a scale of 0 to 6+ as described in Rath 1948. Absence of iron stores was classified as iron deficiency. | ||
| Flow and timing | Haematologic and biochemical analyses (such as serum ferritin), and bone marrow aspiration measurements. However, the flow and timing of both types of tests were not described. | ||
| Comparative | |||
| Notes | The relationship between the level of erythropoiesis and iron balance was evaluated in 13 subjects with idiopathic refractory anaemias. Serum ferritin levels and bone marrow iron stores were increased only in those patients with ring sideroblasts, erythroid hyperplasia and ineffective erythropoiesis. The magnitude of the increase correlated with the duration of anemia and the degree of increase in the erythron iron turnover. Ferritin levels were not related to the severity of the anaemia, indicating that increased iron stores did not represent a shift of iron from the erythron or an absorption response to anaemia per se. It did suggest that the level of erythroid proliferation directly affects gastrointestinal iron absorption, which in time leads to iron overload. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sorbie 1975.
| Study characteristics | |||
| Patient Sampling | 64 subjects where 44 patients had a variety of disorders including iron overload, and 20 were a control group of healthy students (no further details about the sampling procedure were given). A subsample of them (n = 54) underwent liver biopsy and haematological/biochemical analyses. | ||
| Patient characteristics and setting | 64 subjects (of both sexes from 18 to 72 years old) within the Kingston General Hospital (Canada) were divided into 3 groups. Group 1: 34 patients including 16 with and 18 without liver disease, but other conditions. Group 2: the 10 patients with iron overaload where five had iron overload secondary to alcoholic cirrhosis, one had taken oral iron with large amounts of brandy for 30 years, one had primary sideroblastic anaemia, and three patients had idiopathic haemochromatosis. Group 3: 20 healthy students, 18 men and 2 women (ages 17 to 30 years) | ||
| Index tests | Serum ferritin was measured by a two‐site immunoradiometric assay. A serum ferritin concentration lower than 40 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The amount of stainable iron in smears of a bone marrow aspirate was estimated by one of the authors, without knowledge of the clinical status of the subjects. The iron in reticuloendothelial cells was graded from 0 (no stainable iron) to 4+ (stainable iron visible in 100% of high power fields). The absence of iron in smears of grade 0 indicated iron deficiency. Liver biopsy sections stained with Prussian blue were graded on a scale of 0 to 4+ for hemosiderin. Grades 0 to 3+ were considered normal. A grade of 4+ represented iron in 100% of the cells in massive amounts; subjects with this grade were considered to have iron overload. |
||
| Flow and timing | Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration and liver biopsy measurements, as well as reviews of medical records reviewing were undertaken. However, the flow and timing of these tests were not described. | ||
| Comparative | |||
| Notes | Absent Fe (BM = 0) (12 subjects: 3M, 9F) had a ferritin concentration of 9 (5‐15), intermediate Fe (BM = 1+) (7 subjects: 5M, 2F) had a ferritin concentration of 126 (30‐267) and plentiful Fe (BM = 2‐3+) (15 subjects: 13M, 2F) had a ferritin concentration of 441 (105‐1063). The third group consisted of 20 healthy students (18M, 2F, aged 17 to 30 years) and was also analysed according to bone marrow iron content. Absent Fe (BM = 0) (7 subjects: 5M, 2F) had a ferritin concentration of 18 (7‐38), intermediate Fe (BM = 1+) (4 subjects: 4M) had a ferritin concentration of 58 (19‐102) and plentiful Fe (BM = 2‐3+) (9 subjects: 9M) had a ferritin concentration of 92 (59‐170). We extracted data from groups 1 and 3 for purposes of iron deficiency. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Summers 1990.
| Study characteristics | |||
| Patient Sampling | 63 Australian patients selected because of suspected iron overload and hereditary haemochromatosis on the basis of serum fenitin or transferrin saturation levels which were elevated above the normal range for age on two successive occasions. After haemotological tests were done, 48 patients qualified for the liver biopsy. After diagnosis of HLA genetic type 6, patients were followed for further diagnosis. | ||
| Patient characteristics and setting | Subjects who had liver biopsy for suspected iron overload at a tertiary centre. A final sample was chosen of 6 selected patients with suspicion of hereditary haemochromatosis from Brisbane, Australia (both sexes, mean age of 34 years and range between 12 and 78 years old). The selection was unclear, therefore, all participants were at increased suspicion of iron loading. | ||
| Index tests | Serum ferritin (method not mentioned). An elevated serum ferritin concentration (both sexes, > 300 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Hepatic iron concentration was measured by atomic absorption spectrophotometry mg/g dryweight. Liver iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | Liver iron only performed in a minority of cases. | ||
| Comparative | |||
| Notes | If the results are equivocal at the initial assessment, serial studies should be made estimating serum ferritin and transferrin saturation levels every 6 to 12 months. Repeat liver biopsy with hepatic iron concentration determination should be performed when the serum ferritin level rises significantly, since homozygous subjects will demonstrate a progressive increase in hepatic iron concentration. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Suominen 2000.
| Study characteristics | |||
| Patient Sampling | 30 anaemic patients with rheumatoid arthritis (RA) who were admitted to the hospital because of active disease and who underwent bone marrow aspirate examination in order to determine the cause of mild‐to‐moderate anaemia. All patients were willing to be followed up for 4 months, and met the American College of Rheumatology (formerly, the American Rheumatism Association) 1987 criteria for RA. | ||
| Patient characteristics and setting | 30 anaemic RA patients (5 men and 25 women, age range 26–79 years) followed up in the Central Hospital of Turku, Finland. Eighteen of the patients were receiving both disease‐modifying antirheumatic drugs (DMARDs) and low‐dose oral glucocorticoids, 6 were receiving only DMARDs, and 1 was receiving only glucocorticoid treatment. All patients were also taking nonsteroidal anti‐inflammatory drugs during the study. | ||
| Index tests | Serum ferritin (reference range at our hospital 20–240 µg/litre for men, 10–100 µg/litre for women) was measured using an automated time resolved immunofluorimetric assay (Autodelfia; Wallac,Turku, Finland). A serum ferritin concentration lower than 60 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | The amount of stainable iron in smears of a bone marrow aspirate was estimated without knowledge of the clinical status of the subjects. The iron in smears was graded from 0 (no stainable iron) to 3+ (stainable iron visible in 100% of high power fields). The absence of iron in smears grade 0 indicated iron deficiency. | ||
| Flow and timing | At the beginning of the study, the patients underwent both blood tests and bone marrow aspirate examination. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | All patients with diminished or exhausted iron stores (n = 18) received oral iron supplementation with 100 mg of elemental iron as ferrous sulfate for 16 or 12 weeks depending on iron content in bone marrow (exhausted or low bone barrow stores respectively). Haemoglobin, haematocrit, mean corpuscular haemoglobin, mean corpuscular volume, erythrocyte count, reticulocyte count, sTfR, TfR–F Index, erythrocyte sedimentation rate, serum C‐reactive protein level, white blood cell count, alanine aminotransferase, g‐glutamyltransferase, and alkaline phosphatase were measured. The same blood test protocol was repeated 4 weeks and 18 weeks later. The patients with BMI 0 (exhausted bone marrow stores) or BMI +/++ (low to low‐normal iron stores) were then classified as being either iron‐deplete or iron‐replete by the positive or negative responses to the supplementation. Patients with BMI +++ (normal or elevated bone marrow iron stores) were considered to be iron‐replete, and did not receive supplementation treatment. The patients with BMI 0 or BMI +/++ were then classified as being either iron‐deplete or iron‐replete by the positive or negative responses to the supplementation. An increase in the Hgb level of >= 8 gr/liter was deemed to represent a positive response. 15 patients had a positive response (11 of 12 with BMI 0 and 4 of 6 with BMI +/++). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Szurowska 2010.
| Study characteristics | |||
| Patient Sampling | 300 Polish patients with hepatic pathology underwent liver biopsy and they were examined with MRI in the Department of Radiology of the Medical University of Gdansk between 2004 and 2008. A subsample of 44 consecutive patients with compensated (Child Pugh A) liver cirrhosis of different aetiologies was chosen for the study. | ||
| Patient characteristics and setting | Consecutive Polish cirrhotic patients of varying aetiology (27 men and 17 women aged from 22 to 81 years, with median age of 64 years) | ||
| Index tests | Serum ferritin, method not reported. An elevated serum ferritin concentration (both sexes > 400 µg/L were classified as iron overload. | ||
| Target condition and reference standard(s) | Iron deposition in the liver oligobiopsy specimen was evaluated according to Scheuer’s grading scale on tissue sections stained with Perl’s method. Thus, grades of hepatic iron deposits of 1 to 3 indicated an iron overload state. There was haemosiderin staining in the liver (note all patients had cirrhosis). | ||
| Flow and timing | 300 Polish patients with hepatic pathology underwent liver biopsy. A subsample of 44 consecutive patients with compensated liver cirrhosis of different aetiologies was chosen for the study. The delay between MRI and liver biopsy measurements was less than 31 days. Biochemical tests of iron (serum‐iron concentration, tranferrin saturation, and serum ferritin) and histopathologically proved iron deposition within the hepatic tissue were undertaken. However, it the precise flow and timing of these two kind of tests were not described. | ||
| Comparative | |||
| Notes | There was a statistical correlation between serum ferritin level and Scheuer’s grading scale for liver iron concentration (f = 0.62, P < 0.001). Very high values of ferritin correlate with liver iron deposits mainly in liver cirrhosis that is a consequence of hereditary hemochromatosis (HH). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Terrovitis 2011.
| Study characteristics | |||
| Patient Sampling | 103 consecutive patients with anaemia (Hb <= 13 gr/dL in men and Hgb < =12 gr/dL), and advanced heart failure. No further details about the sampling procedure were given. | ||
| Patient characteristics and setting | Adults patients (both sexes) with anaemia and heart failure attending the Alexandra University Hospital in Athens, Greece. | ||
| Index tests | A serum ferritin concentration lower than 150.5 µg/L was classified as iron deficiency (no further details about the SF measurement approach were given). | ||
| Target condition and reference standard(s) | Assessment of iron stores in bone marrow where absence of iron in smears was classified as iron deficiency (no further details about the measurement approach or grading scale were given). | ||
| Flow and timing | Haematologic and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | ROC analysis showed that ferritin < 150.5 µg/L diagnosed iron deficiency with a sensitivity of 81.3%,and specificity of 76.9% (PPV = 85.7%). An algorithm based exclusively on an easily obtainable laboratory test can provide rapid, practical but valid diagnostic evaluation of iron deficiency in anaemic patients with heart failure and thus facilitate treatment selection in everyday clinical practice. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Thompson 1988.
| Study characteristics | |||
| Patient Sampling | 600 patients with anaemia were admitted to Bellevue Hospital (New York) between July and November of 1985. 403 patients had either ferritin and/or bone marrow examination. 319 of them were hospitalised, and a subsample of 40 patients had serum ferritin and bone marrow measurements. | ||
| Patient characteristics and setting | 247 anaemic hospitalised patients at Bellevue Hospital (New York), with ferritin or bone marrow data between July and November of 1985. Bone marrow examinations were performed on 40 patients who previously underwent serum ferritin blood tests. These patients were both sexes, 50 years and older and with diverse chronic diseases. | ||
| Index tests | Serum ferritin was measured by immunoradiometric assay (RAMCO Laboratories Inc., Houston). A serum ferritin of 30 μg/L or less was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Iron in bone marrow examination. The absence of iron in BM was classified as iron defiency (no further details about the measurement technique or grading scale were given). | ||
| Flow and timing | 600 patients with anaemia were admitted tot Bellevue Hospital (New York) between July and November of 1985. Bone marrow examinations were performed on 40 patients who had ferritin levels. | ||
| Comparative | |||
| Notes | Using serum ferritin or bone marrow examinations, the data provide strong evidence that RDW, MCV and transferrin saturation are not sufficiently sensitive to be of clinical utility in screening for iron deficiency in hospitalised patients with anaemia. If the ferritin is between 30 and 175 μg/L and especially if the patient has a condition causing elevated ferritin levels (e.g. chronic liver disease), a bone marrow examination for iron (or a short course of iron watching for increased haemoglobin or reticulocytosis) will be necessary to make a diagnosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Thomsen 1992.
| Study characteristics | |||
| Patient Sampling | During a period of four months, 4302 consecutive blood donors were selected for iron overload tests. From those, 18 met one of the following three criteria: 1.TS > 0.70, 2. TS > 0.50 and SF > 150 µg/L or 3. SF > 300 µg/L. Eleven accepted liver biopsy, and 5 relatives of the already chosen patients were selected and underwent liver biopsy too (N = 16). | ||
| Patient characteristics and setting | Blood donors (4302), in Copenhagen, Denmark for a period of 4 months. 16 were selected for the study (adults of both sexes). | ||
| Index tests | Serum ferritin; method not reported. An elevated serum ferritin concentration (both sexes > 500 µg/L) were classified as iron overload. | ||
| Target condition and reference standard(s) | Risk of bias: only patients at high pre‐test risk due to elevated ferritin underwent subsequent biopsy. Liver iron measured with liver biopsy, and MRI. Liver iron concentration measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload (no description of the methodology to measure the liver iron concentration was found). | ||
| Flow and timing | During a period of four months 4302 consecutive blood donors were selected for iron overload tests. From those, 18 met one of the following three criteria: 1.TS > 0.70, 2. TS > 0.50 and SF > 150 µg/L or 3. SF > 300 µg/L. 16 patients were selected and underwent liver biopsy. | ||
| Comparative | |||
| Notes | Ferritin had a much lower relaxivity 0.132 sec‐1 .µmol‐1.g than FeCl3, and even lower than iron in the liver 0.714 sec‐1 .µmol‐1.g. It was, therefore, concluded that ferritin could only account for a fraction of the observed changes in the transverse relaxation rate in vivo (R2). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Thorburn 2002.
| Study characteristics | |||
| Patient Sampling | A total of 164 consecutive patients with evidence of chronic hepatitis C (HCV) infection were prospectively studied. All patients were anti‐HCV positive with abnormal liver function tests, and they underwent liver biopsy. Only a minority of 5 patients had data presented, all of whom had genetic haemochromatosis. | ||
| Patient characteristics and setting | 164 consecutive patients from Scotland with evidence of HCV infection. They were divided into two groups: group one with HFE genotype: 157 (both sexes, aged between 23 and 70 years old, with mean alcohol consumption per week ranging between 8 to 15 units of alcohol ‐ the range corresponds to four different HFE genotype sub‐groups ‐), and group two with special mutations: 7 (both sexes aged between 22 and 35 years old). | ||
| Index tests | Serum ferritin was measured by turbidimetry using a Chiron ACS18 automatic analyser (Bayer, USA). An elevated serum ferritin concentration (both sexes > 300 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Two cores of liver tissue were obtained from each patient: one formalin fixed for histological analysis and one stored immediately at ‐20°C for LIC estimation. Formalin fixed specimens were paraffin embedded and sections stained with haematoxylin and eosin and Perl’s Prussian blue stain. Slides were then evaluated by two experienced pathologists (RMacS and KO) blinded to the clinical and laboratory information. Histological assessment of hepatocyte and macrophage iron stores were graded on a scale of 0–4 on Perl’s Prussian blue stained liver sections. Thus, grades of hepatic iron deposits of 1 to 4 indicated an iron overload state. | ||
| Flow and timing | A total of 164 consecutive patients with evidence of chronic hepatitis C (HCV) infection were prospectively studied. All patients were anti‐HCV positive with abnormal liver function tests, and they underwent Iron status assessments (serum iron concentration, tranferrin saturation, and serum ferritin), histological grading of liver iron content, and measurement of liver iron concentration (LIC). The flow and timing of these three kind of tests were not described. However, only a minority of 5 patients had data presented, all of whom had genetic haemochromatosis. | ||
| Comparative | |||
| Notes | Patients with elevated LICs were older (P = 0.05), acquired HCV infection earlier (P = 0.02), had higher serum ferritin (P = 0.003), and had more severe necro‐inflammatory activity on liver biopsy (P= 0.02). By stepwise regression analysis with a backwards approach, LICs could be predicted by a combination of serum ferritin, transferrin saturation, and hepatocyte staining with iron on liver biopsy (all, P < 0.001). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Valberg 1978.
| Study characteristics | |||
| Patient Sampling | 8 patients with clinically manifest primary haemochromatosis, 12 patients with cirrhosis and iron overload, and 20 patients with liver disease and low or normal iron stores. 34 healthy volunteers were added to establish a normal range of values for serum ferritin. | ||
| Patient characteristics and setting | A sample of 40 adult Canadian patients aged from 21 to 74 years old was selected. They were divided in 3 groups:
There were 34 healthy volunteers aged from 17 to 38 years old too. |
||
| Index tests | Serum ferritin by two‐site radioimmunoassay with Luxton 1977 method. An elevated serum ferritin concentration (both sexes > 300 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron content reporting method: liver biopsy sections were stained for hemosiderin with Prussian blue and coded. Grading was at follows: 0 = no iron; 1+ = iron in occasional cells; 2+ = iron in fewer than 50% of the cells; 3+ = iron in more than 50% of the cells; and 4+ = iron in all the cells in massive amounts. In accordance with the experience of Barry 1974: grades of 0 to 2+ were considered as indicating low to normal hepatic iron stores and grades of 3+ to 4+ as indicating excessive hepatic iron. | ||
| Flow and timing | Haematological tests for measuring iron status (serum‐iron concentration, transferrin saturation, SGOT index, and serum ferritin), and liver biopsy. The flow and timing of these two types of tests were not described. | ||
| Comparative | |||
| Notes | The mean serum ferritin concentration was 2221 µg/L in the patients with primary haemochromatosis or cirrhosis with excessive hepatic iron, but only 264 µg/L in patients with liver disease and low to normal hepatic iron stores. In the first two groups, all the individual values were greater than 300 µg/L, but in the third group only 8 of the 20 patients had values greater than 300 µg/L. A comparable separation of the healthy controls from the patients with primary haemochromatosis was obtained when iron absorption was plotted against the serum ferritin. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Van den Broek 1998.
| Study characteristics | |||
| Patient Sampling | Anaemic pregnant women attending the antenatal clinic in Blantyre, Malawi. A subsample of 93 women consented to bone marrow aspiration, and were selected for the study (no further details about the sampling procedure were given). | ||
| Patient characteristics and setting | A subsample of 93 anaemic pregnant women (half of them with HIV) attending the antenatal clinic at the Queen Elizabeth Central Hospital in Blantyre, Malawi. Their mean gestational age was 31 weeks. 76% of the women were in the third trimester, 23% in the second and 1% in the first. | ||
| Index tests | Serum ferritin was determined by immunoenzymometric assay (Ramco USA, detection limit 0.6 µg/L). A serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | BM aspirates were obtained by sterile procedure under local anaesthesia from the anterior iliac crest. Bone marrow films were stained for iron using Prussian blue Perl’s method, Dacie 1995. The stained films were examined by a haematologist blinded to the biochemical and haematological results and classified as: 0 = no iron present; 1 = traces of iron only; 2 = moderate amounts of iron present; 3 = abundant iron present. Iron = 0 was classified as iron deficiency. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | The study examined the diagnostic accuracy of iron parameters including mean cellular volume (MCV), serum iron, transferrin, total iron binding capacity (TIBC) and its saturation, zinc protoporphyrin (ZPP), ferritin and serum transferrin receptor (TfR) for the assessment of iron status in a population of 93 anaemic pregnant women in Malawi, that consented for a bone marrow aspirate. Results showed that, for the purpose of screening, serum ferritin is the best single indicator of storage iron provided a cut‐off point of 30 µg/L is used. A number of other commonly used parameters of iron status were shown to have limited diagnostic accuracy. Logistic regression was used to obtain mathematical models for the prediction of bone marrow iron status using a combination of available parameters. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Van Tellingen 2001.
| Study characteristics | |||
| Patient Sampling | Adults anaemic inpatients in a hospital ward | ||
| Patient characteristics and setting | 62 anaemic elderly patients (with a mean age of 70 years old and with diverse chronic diseases), were admitted to the internal ward of St. Lucas Andreas Hospital, in Amsterdam, The Netherlands. | ||
| Index tests | Serum ferritin leves were measured by chemiluminescence immunometric assay (Immulite 2000 analyser, Diagnostic Products Corporation, Los Angeles, USA). Intra‐assay 2.6%, and inter‐assay variation was 4%. A reference interval 18 to 370 µg/L for men and 9 to 120 µg/L for women was used. A serum ferritin concentration lower than 32 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Smears were obtained from the posterior iliac crest and stained for iron with Prussian Blue. At least three spicules were examined in each sample by two independent methodologists blinded to the results of the laboratory parameters. In case of dry tap, patients were excluded and a histological bone marrow examination was performed to obtain a diagnosis. Stainable iron was determined to be absent, normal or overload, based on the amount of iron in the reticuloendothelial system (RES), the aspect of hemosideringranules, sideroblasts and the presence of siderocytes and/or macrophages. Based on these results, patients were divided into two study groups: IDA if stainable iron was absent and non‐IDA if stainable iron was normal or increased. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. Haematological biochemical analyses, and bone marrow measurements were made blinded to other results. However, further details about the flow and timing of these three types of tests were not given. | ||
| Comparative | |||
| Notes | Prospective study of 62 anaemic patients to determine the predictive value of ferritin, zinc protoporphyrin (ZPP), plasma transferrin receptor (PtrfR), PtrfR/ferritin ratio, for diagnosing iron deficiency anaemia. Bone marrow examination was used as a gold standard to discriminate between IDA and non‐IDA. 24 patients had depleted iron stores. Univariate analysis showed that ferritin, PtrfR/ferritin ratio, ZPP and PtrfR had significant predictive values for differentiating IDA from non‐IDA. The low sensitivity and specificity of ZPP, PtrfR and PtrfR/ferritin ratio render them insufficient to be used as a single ‘best’ test for the identification of IDA in a non‐selected group of anaemic patients and do not even add to the prediction if the value of ferritin is known. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Van Zeben 1990.
| Study characteristics | |||
| Patient Sampling | During a 12‐month period (May 1986‐May 1987), 119 consecutive patients with an mean corpuscular volume < 80 fl were evaluated either hospitalized or visiting the out‐patient clinic of the Department of Internal Medicine. A subsample of 104 patients met the criteria, and had both bone marrow and serum ferritin measurements done. | ||
| Patient characteristics and setting | 104 patients with a mean corpuscular volume < 80 fl, both those hospitalised and those visiting the outpatient clinic of the Department of Internal Medicine of Bronovo Hospital, The Hague, The Netherlands. Patients (66 females with a mean age of 63 years, and 38 males with a mean age of 56 years) presented with one or more chronic diseases (10 patients with haemoglobinopathy or thalassaemia). | ||
| Index tests | Serum ferritin measured by Abbott‐Ferrizyme®‐ELISA technique. Serum ferritin concentration lower than 30 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow smears were stained for iron by the Prussian blue method. The absence of bone marrow iron stores was classified as iron deficient. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | The absence of bone marrow iron stores (n = 15) or the response to iron supplementation were used to establish the diagnosis iron deficiency. Using data on response to iron treatment to define iron deficiency, and a cut‐off point of 30 µg/L, serum ferritin concentration is more suitable for assessment of iron deficiency (on the basis of sensitivity (90%) and specificity (100%)), than serum iron concentration, total iron‐binding capacity or % saturation of transferrin. The red cell distribution width is the parameter with the highest sensitivity for iron deficiency (94%). A red cell distribution width value within the reference interval can be used to exclude iron deficiency in those cases in which the serum ferritin concentration does not accurately reflect the iron stores owing to severe tissue damage, as in inflammation or malignancy. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Villeneuve 1996.
| Study characteristics | |||
| Patient Sampling | Liver explants from 8 Canadian patients with cirrhosis (6 at time of liver transplant, 2 at autopsy). The patients were selected on the basis of elevated serum ferritin. | ||
| Patient characteristics and setting | 8 patients from Quebec, Canada with decompensated liver disease due to alcoholism with alcoholic cirrhosis and hereditary haemochromatosis (mean age 57 years with a range between 39 and 79 years old) | ||
| Index tests | Serum ferritin; methods not reported. An elevated serum ferritin concentration for both sexes > 500 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Multiple biopsies were performed using a 14 gauge Menghini needle. An average of 21 specimens per liver were obtained, ranging in length from 2.5 to 40 mm; they were shipped to the analytical laboratory in test tubes for trace element determination (Vacutainer® 6527, Beckton Dickinson, USA). The hepatic iron content was measured by atomic absorption spectroscopy using a Perkin‐Elmer® model 5 100 spectrophotometer equipped with a HGA‐600® graphite tube atomiser, an iron hollow cathode lamp, pyrolytically coated partition tubes and an automatic sampler. A hepatic iron content measurement with a threshold of > 1.8 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | Haematological tests for measuring iron status (serum‐iron concentration, transferrin saturation, and serum ferritin), liver biopsy, and other hepatic measures were undertaken. However, the flow and timing of these three kind of tests were not described. | ||
| Comparative | |||
| Notes | Liver iron concentrations and serum ferritin measures had a very high correlation, r = 0.88. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Vreugdenhil 1990.
| Study characteristics | |||
| Patient Sampling | 44 anaemic rheumatoid arthithis patients in a clinic of Rotterdam, Netherlands | ||
| Patient characteristics and setting | 44 anaemic rheumatoid arthithis patients in a clinic of Rotterdam, Netherlands: both sexes, mean age 63.44 years. Patients had several diseases with mean disease duration 4.9 years; 79% were seropositive (Rose test) with a mean reciprocal titre of 221. Sixty‐nine per cent received long‐acting antirheumatic drugs and 78% used nonsteroidal anti‐inflammatory drugs. | ||
| Index tests | Serum ferritin (s‐ferritin) was measured by solid phase enzyme immunoassay (Ferrizyme®, Abbott Labs, Chicago, USA). A serum ferritin concentration lower than 50 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Patients were classified according to the stainable bone marrow iron content: group 1: (0; no haemosiderin granules), group 2: (1; very small amount of haemosiderin granules), group 3: (2; heavy granules in every second or third immersion field) and group 4: (3 or more; heavy granules in every immersion field or massive haemosiderin deposits and clumps). Groups 1 and 1 were considered iron deficient. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | The anaemia was normochromic normocytic in 60% and hypochromic normocytic in 30% of those with anaemia of chronic disease. Iron deficiency was present in 55% and the anaemia was hypochromic microcytic in 54% and hypochromic normocytic or normochromic normocytic in 21%. For the detection of iron deficiency among rheumatoid arthithis patients with anaemia of chronic disease, the mean corpuscular volume showed the highest specificity (90%) and predictive value (87%). Serum ferritin was the most sensitive (82%) and valid (86%) test. Combination of mean corpuscular volume, ferritin and transferrin resulted in 100% validity. It was concluded that iron deficiency can be detected accurately without bone marrow aspiration using combinations of blood parameters. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Walsh 2006.
| Study characteristics | |||
| Patient Sampling | Data were obtained from 91 C282Y/H63D probands referred for clinical assessment either at the Royal Brisbane and Women’s Hospital or the Canberra Hospital in Australia, and 483 C282Y homozygotes probands. Liver biopsy examination was performed because of increased serum ferritin levels (usually 500 µg/L), abnormal liver enzyme levels, hepatomegaly, or a combination of these. A subsample of 357 patients had a liver biopsy. | ||
| Patient characteristics and setting | 574 adult probands from Australia (both sexes with a mean age of 46 years old). Patients referred for assessment or identified through family screening with mutations in the human hemochromatosis protein gene. Australian tertiary referral centres; both referred probands and individuals identified from family screening. Only a minority of 10 patients with haemochromatosis had available liver concentrations and serum ferritin data (mean age 44 with a range between 27 and 58 years old). | ||
| Index tests | Serum ferritin, method not explicitly reported. An elevated serum ferritin concentration (for males > 350 µg/L, and females > 250 µg/L) was classified as iron overload. | ||
| Target condition and reference standard(s) | Iron stores were graded 0–4 according to Searle 1987 (Perls’ Prussian blue). Thus, a grade of hepatic iron deposits of 1 to 4 indicated an iron overload state. | ||
| Flow and timing | Haematological tests for measuring iron status (serum‐iron concentration, transferrin saturation, and serum ferritin), liver biopsy, and determination of human haemochromatosis protein mutations were undertaken. However, the flow and timing of these three kind of tests were not described. | ||
| Comparative | |||
| Notes | Although the increased serum ferritin level in compound heterozygous subjects may reflect increased iron stores, it also may be increased by inflammation and hepatocyte injury. In our study, all C282Y/H63D subjects with serum ferritin levels greater than 1000 µg/L and with clinical data available had associated diseases, notably 8 with steatosis on liver biopsy examination, 7 with excess alcohol consumption, 4 with diabetes, and 4 with an increased body mass index (kg/m2) > 30. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Wands 1976.
| Study characteristics | |||
| Patient Sampling | 33 individuals from 58 members of two families (where two individuals who died of severe haemochromatosis were assessed by blood tests). Those with serum iron values greater than 140 with µg/L per 100 mL were admitted to the hospital for diagnostic liver biopsy (18 in total). | ||
| Patient characteristics and setting | 33 family members of two different families from Boston, United States of America (with 1 patient from each family having died of severe haemochromatosis). From those, 18 individuals with high serum iron values were selected for liver biopsy on suspicion of hederitary haemochromatosis (mean age 34.2 with a range between 19 and 67 years old). | ||
| Index tests | Serum ferritin by radioimmunoassay approach. An elevated serum ferritin concentration for both sexes > 200 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver biopsies were processed for routine light microscopy and stained with eosin, Mallory's trichrome, and Perl's Prussian blue reaction for iron. The biopsies were coded and assessed by at least two of the specialists. The amount of stainable iron in hepatocytes was graded on a scale of 1 to 4+. 1+ deposition was assigned to biopsies with stainable iron barely recognisable but easily recognisable at higher magnification 430 X. 2+ could be easily detected at 250 X, 3+ recognisable at 50 X, and 4+ was easily detected by the naked eye. Thus, grades of hepatic iron deposits of 1 to 4+ indicated an iron overload state. | ||
| Flow and timing | Haematological tests for measuring iron status (serum‐iron concentration, transferrin saturation, and serum ferritin) were performed to select the patients that underwent liver biopsy. | ||
| Comparative | |||
| Notes | There was no correlation at all between serum ferritin levels and iron stores (P < 0.5). In addition, there was no relationship (P < 0.2) between serum ferritin values and serum iron, iron‐binding capacity and transferrin saturation. However, duplicate frozen samples were sent to Seattle to measure serum ferritin again, and most of the serum ferritin measurements were considerably higher than in the first measurement. These values had a better correlation with iron stores than the first ones. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Witte 1986.
| Study characteristics | |||
| Patient Sampling | Bone marrow data from 97 consecutive unselected anaemic patients attending specific hospitals in Iowa City region, United States of America. The study goal was to evaluate anaemia of unknown cause (no further details about the sampling procedure were given). | ||
| Patient characteristics and setting | 97 patients with a bone marrow examination ordered by the primary physicians to evaluate anaemia of unknown cause. Patients were attending either the University of Iowa hospitals or the Veterans Administration Medical Center at Iowa City, Iowa, United States of America. Anaemic patients (both sexes, with mean age of 60 years) had either chronic diseases, blood disorders or iron deficiency. | ||
| Index tests | Serum ferritin was measured using the RAMCO (Houston, TX, USA) two‐day procedure. A SF concentration lower than 12 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow iron was evaluated using the Prussian blue stain from iliac crest aspirate smears. The stains were examined without knowledge of historical information by two observers, and compared to published morphologic criteria for the amount of reticuloendothelial storage of iron and sideroblast iron. Authors defined abnormal sideroblasts as those with more than four or large siderotic granules and defined ring sideroblasts as more than half the perinuclear circumference filled with large iron‐positive granules. A normal sideroblast percentage was defined as 10% to 50%. The patient groups were defined by marrow results: group 1: iron deficient, when neither observer could identify storage iron; group 2: anaemia of chronic disease when normal to elevated storage iron and low to absent morphologically normal sideroblast iron was seen; group 3: abnormal sideroblast, where sideroblast percentage exceeded 50% and morphologically abnormal particles of iron‐positive material were seen peripherally in the cytoplasm of developing erythroid cells; group 4: ring sideroblasts, where sideroblast percentage exceeded 50% and iron‐positive perinuclear granules encircled at least half the nucleus in more than 15% of the normblasts; and group 5: had normal storage and sideroblast iron. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records reviewing were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Bone marrow data from 97 consecutive patients with anaemia who were divided into five marrow morphologic groups: (l) iron deficiency; (2) anaemia of chronic disease; (3) abnormal sideroblasts; (4) ring sideroblasts; and (5) other. Tests of peripheral blood included haemoglobin. haematocrit, red blood cell count and red blood cell indices, reticulocyte count, sedimentation rate, ferritin, iron, iron binding capacity, free erythrocyte protoporphyrin, and tests of hepatic and renal function. Cluster analysis, multidimensional scaling, and logistic discriminant analysis were used to derive a graph of serum ferritin with the sedimentation rate, allowing accurate confirmation or exclusion of iron deficiency in most patients. 14 of 97 patients had no stainable iron in the bone marrow. Percent saturation of serum transferrin and serum ferritin allowed identification of only 50 percent of patients with abnormal or ring sideroblasts, while excluding 100% percent of patients without abnormal or ring sideroblasts. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Witte 1988.
| Study characteristics | |||
| Patient Sampling | Retrospective review where 86 consecutive marrow examinations were performed in a 24‐month period in hospital, and 43 had complete data available. Cases included were bone marrow examinations for which concomitant serum ferritin, erytrocyte sedimentation rate, and marrow iron stains results were available (no further details about the sampling were given). | ||
| Patient characteristics and setting | 43 consecutive hospital cases from a community hospital in Iowa, United States, who underwent bone marrow examination previously. Adult patients were of both sexes and mixed age; the majority had one or more chronic diseases. | ||
| Index tests | Serum ferritin was measured by use of Abbott Ferrizyme®. A serum ferritin concentration lower than 12 µg/L was classified as iron deficiency. | ||
| Target condition and reference standard(s) | Bone marrow iron was evaluated by use of the Prussian blue stain from iliac crest marrow aspirate smears. The stains were interpreted by the practicing community pathologists and classified as iron present or iron absent by use of criteria similar to those in previous studies. Comparability of community and tertiary pathologist interpretation of iron stains was verified by previous review of cases not included in this study. Cases were classified as iron present or absent by the bone marrow iron stain. No specific details about the grading system were given. | ||
| Flow and timing | Haematological and biochemical analyses (such as serum ferritin), bone marrow aspiration measurements, and reviews of medical records were undertaken. However, the flow and timing of these three types of tests were not described. | ||
| Comparative | |||
| Notes | Serum ferritin values less than 12 µg/L confirmed iron deficiency. However, some iron‐deficient patients had ferritin values of more than 12 µg/L. Serum ferritin is an acute‐phase reactant. When ferritin values are between 13 and 150 µg/L, careful interpretation is required. Among the 43 bone marrow examinations of anaemic patients in a community hospital, the iron stores were correctly predicted in 38 cases, indeterminate in 4, and erroneously predicted in 1. The prediction of deficiency was 100% correct. The prediction of non‐deficiency was 97% correct. These data suggest the two‐dimensional graphic analysis of serum ferritin and Westergren erythrocyte sedimentation rate is useful in primary care. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Wong 2006.
| Study characteristics | |||
| Patient Sampling | Outpatient referrals for raised serum ferritin (n = 482 adults) to Canadian tertiary referral centre. All patients had serum ferritin concentrations greater than 300 µg/L. Of these, 146 had liver biopsies and hepatic iron concentration was measured only if there was significant iron staining on liver biopsy or if specified by the ordering physician. HIC was performed on 44 patients with serum ferritin > 1000 µg/L and an undetermined number of those with serum ferritin 300‐1000 µg/L. | ||
| Patient characteristics and setting | 482 outpatient referrals of adults to the haemochromatosis and liver disease clinic at the University Hospital in London, Ontario, Canada (both sexes, around 75% males and 25% females, with mean age of 50 years old and a range of from 49 to 55 years old). All patients had high ferritin (greater than 300 µg/L) and risk of iron loading. All patients with HFE‐linked haemochromatosis were Caucasian. The group of patients with iron overload from other causes included patients with Asian, Indian, Caribbean and Arab ancestry. | ||
| Index tests | Serum ferritin; method not reported. An elevated serum ferritin concentration for both sexes > 400 µg/L was classified as iron overload. | ||
| Target condition and reference standard(s) | Liver iron content by atomic absorption spectrophotometry. Liver tissue was removed from paraffin‐embedded blocks by washing in xylene and then drying to a constant weight before analysis. Liver iron measured with liver biopsy. Liver iron content measurement with a threshold of > 1.95 mg/g dry weight was classified as iron overload. | ||
| Flow and timing | 482 adults referred from outpatients had a first serum ferritin test. From those, 146 patients, where serum ferritin concentrations were greater than 300 µg/L, underwent liver biopsy. | ||
| Comparative | |||
| Notes | Mild ferritin elevations 300 µg/L to 1000 µg/L may be more difficult to diagnose definitively in the absence of useful, noninvasive testing. Elevated liver enzymes appear to decrease the probability of a diagnosis of HFE‐linked haemochromatosis. The clinical management of patients with mild hyperferritinaemia has not been well studied. Observation over time or a trial as a voluntary blood donor are useful approaches in this group. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
ACD: anaemia of chronic disorders AIDS: acquired immunodeficiency syndrome AUC_ROC: area under the receiver operating characteristic curve (ROC) curve BM: bone marrow BMI: body mass index BMIS: bone marrow iron stores BMS: bone marrow iron stores CD4: (lymphocytes CD4, cluster of cuadruple differentiation Meira 2005 ) CHB: chronic hepatitis B CHr: reticulocyte hemoglobin content CISM: Centro de Investigação em Saúde de Manhiça CRP: C‐reactive protein CV: coefficient of variation EDTA: ethylenediaminetetraacetic acid ELISA: enzime‐linked immunosorbent assay ESR: erythrocyte sedimentation rate F: Iron (serum iron concentration) Fe: Iron (serum iron concentration)
fl: femtoliters, equivalent to 10 exp (‐15) liters HAART: highly active antiretroviral therapies Hb: haemoglobin HFE: gene that provides instructions to produce the HFE protein (human homeostatic iron regulator). Mutations in this gene cause HFE hereditary haemochromatosis. HH: hereditary haemochromatosis. HHC: hereditary haemochromatosis; HIC: hepatic iron concentration HIS: hepatocyte iron score HIV: human immunodeficiency virus HLA: HLA‐linked hereditary haemochromatosis (where HLA is the corresponding haplotype of associated genes) IC: confidence interval ID: iron‐deficiency IDA: iron‐deficiency anaemia IH: idiopathic haemochromatosis I.H.C.: idiopathic haemochromatosis IO: iron overload IR: iron replete IRMA: Immunoradiometric assay LIC: Liver iron concentration LR: likelihood ratios MCH: major histocompatibility complex MCHC: mean corpuscular haemoglobin concentration MCV: (mean corpuscular volume; mean cell volume) MIG: marrow iron grade min: minutes
MIS: mesenchymal iron score MRI: magnetic resonance imaging
MR: medical resonance applied to imaging technique is MRI NPV: negative predictive values NSAIDs: nonsteroidal anti‐inflammatory drugs OLTs: orthotopic liver transplantations PB: peripheal blood pm: post meridiem or after noon. PPV: positive predictive values PTH: Parathyroid Hormone PtrfR: plasma transferrin receptor r: correlation coefficient
R2: transverse relaxation rate. RA: rheumatoid arthritis RBC: red blood cell RBCFER: red cell ferritin RCF: red cell ferritin RDW: red cell distribution width RIA: radioimmunoassay ROC: receiver operating characteristic RTS: room temperature susceptometry Sat: transferrin saturation SC: Haemoglobin sickle cell diseases are a type of sickle cell diseases where it appear the the following compound heterozygous disorders: Hb SC, Hb SD, Hb SG,...) SD: standar deviation SERFER: serum ferritin SF: serum ferritin SGOT: serum glutamic oxaloacetic transaminase found in several organs such as liver, heart, kidney, etc. SIS: sinusoidal iron scores SS: Haemoglobin SS disease is a type of sickle cell disease and occurs when people inherit copies of the hemoglobin S gene from both parents. STfR: soluble transferrin receptor TB: tuberculosis TfR(‐F): soluble transferrin receptor/log ferritin Tf sat: transferrin saturation TIBC: total iron binding capacity TIS: total iron score TS: transferrin saturation TSAT: transferrin saturation vs: versus ZPP: zinc protoporphyrin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ang 2017 | This was a retrospective study that assessed adult non‐transfusion‐dependent thalassaemia patients in the Department of Haematology, Singapore General Hospital, Singapore; who had serum ferritin levels assayed within three months of liver iron concentration measurements by MRI, before initiation of iron chelation. The study was excluded because liver iron content was determined by magnetic resonance. |
| Bafna 2016 | Magnetic resonance studies to measure iron content in thalassaemia patients undergoing bone‐marrow transplant and comparisons with liver biopsy determinations. The study was excluded because it is the abstract of a conference in a congress without usable data for this review. |
| Bardou‐Jacquet 2014 | The study described the long‐term evolution of iron overload after iron removal by venesection in 58 patients with dysmetabolic iron overload syndrome. The mean liver iron content at diagnosis was 80 ± 43 µmol/g and the mean amount of iron removed was 2.2 ± 1.2 g. The mean follow‐up time was 71 ± 23 months since end of treatment. The study was excluded because liver iron content was determined by magnetic resonance. Additionally, there were no data on iron content and ferritin that could be used for 2 x 2 tables or raw data for analysis. |
| Barsan 2015a | This study investigated the relationships between bone marrow iron distribution, hepcidin–ferroportin expression in bone marrow cells, and peripheral iron indices in 54 non‐dialysis chronic kidney‐disease patients. Bone marrow was collected by aspiration from the iliac crest and classified as having normal iron distribution, iron deficiency, or anemia of chronic disorders. Due to the low number of participants with normal iron distribution, comparisons were made between iron deficiency anaemia and anaemia of chronic disease groups. Although haemoglobin and mean corpuscular haemoglobin were comparable, the mean corpuscular volume was lower in iron‐deficiency anaemia than in anaemia of chronic disease. It appeared that the index test included both haemogobin and ferritin. Medullar hepcidin and ferroportin expression (immunofluorescence, semi‐quantitative scales) and serum hepcidin (Hep25 – ELISA) were the main studied parameters. |
| Braga 2014 | This was a literature review critically assessing the evidence to assess the diagnostic efficacy of sTfR in complicated anaemia, using bone marrow examination as a diagnostic gold standard for iron‐deficiency anaemia as well as evaluating sTfR versus ferritin concentrations. |
| Busti 2016 | Study factors involved in the development of iron overload in 17 subjects (14 males and 3 females, mean age 58 years) with β‐thalassaemia trait (βTT), and to evaluate feasibility and efficacy of mini‐phlebotomies to remove iron overload in this condition. Iron overload was documented through increased ferritin levels (in most cases higher than 1000 µg/L in repeated assays), and either MRI or liver biopsy, when indicated. Nine patients started treatment with “mini‐phlebotomies” and four of them reached iron depletion. The study was excluded because it is an abstract of a conference without usable data on iron content by liver biopsies or ferritin concentrations needed for this review. |
| Cancado 2018 | This multicentre, observational study assessed 175 transfusion‐dependent patients older than 10 years of age, with chronic anaemias in Latin American countries including Argentina, Brazil, Colombia, Mexico, and Venezuela. Patients were recruited at tertiary‐care haematology centres with 200 or more monthly consultations and/or located in cities with populations of 1 million people or more. Iron overload was defined when patients had liver R2 MRI > 2 mg Fe/g dry weight (dw) and/or cardiac T2* MRI < 20 ms, assessed by performing MRI (Ferriscan®) within 15 days of evaluation of serum ferritin. The study was excluded because liver iron content was determined by magnetic resonance and participants were receiving transfusions. |
| Cazzola 1983 | Red cell ferritin was measured in 68 normal subjects and patients with disorders of iron metabolism, inflammation, liver dysfunction, impaired haemoglobin synthesis and increased red cell turnover by means of radioimmunoassays (29 males, 39 females). The major factor determining the red cell ferritin content appeared to be the transferrin saturation. The determination of red cell ferritin, could have clinical application. It can be used for evaluating the adequacy of the iron supply to the erythroid marrow, particularly in patients with increased red cell turnover and to evaluate the body iron status in patients with haemochromatosis and liver disease. There are data on serum and erythrocyte ferritin, without bone marrow data and four cases with liver biopsy data. |
| Cecchin 1990 | Study of iron overload in renal haemodialysis patients. Patients were receiving serial intravenous iron infusions (as per clinical practice in renal medicine). The study was excluded as iron was infused (similar to transfusion) and did not reflect excess oral iron absorption induced‐iron loading. No extractable data for cross tabulation for diagnostic test accuracy (2 X 2 DTA table). |
| Chin 2019 | 202 consecutive subjects diagnosed with HFE (haemochromatosis), and who had undergone a liver biopsy at the Royal Brisbane and Women’s Hospital in Australia between 1972 and 2010, were measured for hepatic iron concentration by atomic absorption spectrophotometry on fresh liver biopsy specimens that were classifed by fibrosis stage according to the grading system of Scheuer. This study aimed to assess the relationship between hepatic iron concentration, age, serum ferritin and mobilisable iron in the prediction of advanced fibrosis. The study was excluded as no data were presented in the manuscript enabling 2 x 2 extraction of analysis between ferritin and liver‐iron loading. |
| Costa‐Matos 2013 | Cross‐sectional study of patients with alcoholic liver disease evaluating risk of iron overload. The study was excluded as no data were presented in the manuscript enabling 2 x 2 extraction of analysis between ferritin and liver‐iron loading. |
| Davidson 1984 | 62 patients with rheumatoid arthritis were studied to evaluate conventional laboratory indices for iron deficiency in the anaemia of rheumatoid arthritis. For 23 patients with rheumatoid arthritis and normocytic anaemia irrespective of plasma ferritin concentration, red cell ferritin content did not differ significantly from that for non‐anaemic patients with rheumatoid arthritis. For 27 patients with rheumatoid arthritis and microcytic anaemia, the mean red cell ferritin content for patients with a plasma ferritin concentration in the 13‐110 µg/L range was appreciably reduced. It was indistinguishable from that for patients with rheumatoid arthritis and classical iron deficiency anaemia, indicated by plasma ferritin concentrations of less than 12 µg/L. By contrast, the mean red cell ferritin content for patients with rheumatoid arthritis, microcytic anaemia, and plasma ferritin concentrations above 110 µg/L did not differ from that for patients with rheumatoid arthritis and normocytic anaemia. Oral treatment with iron in patients with rheumatoid arthritis, microcytic anaemia, and appreciably reduced red cell ferritin concentrations was accompanied by significant increases in haemoglobin concentration (P < 0.01), mean corpuscular volume (P < 0.001), and red cell ferritin contents (P < 0.05). This treatment, however, did not produce any appreciable change in haemoglobin concentration in patients with rheumatoid arthritis, normocytic anaemia, and normal red cell ferritin contents. There were data on serum and erythrocyte ferritin, without bone marrow data. |
| Deugnier 2010 | Data from 219 patients with b‐thalassaemia, collected from histologic analyses of biopsy samples taken at baseline and after at least 3 years of treatment with deferasirox. Treatment response was assessed from liver iron concentrations at baseline and the end of the study. Liver fibrosis, necroinflammation, and markers of iron overload and liver enzymes were recorded. Patients were also assessed, by serologic analysis at baseline, for hepatitis C virus infection. The study was excluded because it was an analysis of the long‐term effect of treatment on liver iron and there were no usable data to construct 2 x 2 tables on results from biopsies and ferritin. Some data could be used for pre‐treatment data on ferritin and iron content |
| Duport 2003 | 472 iron‐depleted menstruating women (ferritin lower than 15 µg/L) were compared with 393 iron‐sufficient (ferritin 30–80 µg/L) menstruating women (aged 35–51 years) in terms of health variables and quality of life using logistic regression and analysis of variance. The risk of any infection or of specific types of infections was not increased by iron deficiency. Regarding the health profile, no specific score was significantly different between the two groups. The only significant difference between iron‐depleted and iron sufficient women concerned memory disorders. The authors concluded that there was no conclusive evidence that an absence of iron stores has negative consequences; however, in case of a worsening of the iron balance, it may lead to a rapid decrease in the level of functional compounds. There were no data on bone marrow. |
| Elalfy 2015 | Comparison of the safety, efficacy, compliance, treatment satisfaction, and quality of life of two oral iron chelators: deferiprone and deferoxamine versus deferiprone and deferasirox for treatment of b‐thalassemia in 96 patients with severe iron overload for 1 year. Efficacy endpoints were the difference between two groups in the change of serum ferritin, liver iron concentration, cardiac MRI, and quality of life. For both treatments, liver iron content (by magnetic resonance) and serum ferritin at 12 months were significantly lower, and geometric mean cardiac T2* was higher compared to baseline. The study was excluded because liver iron content was determined by magnetic resonance and not by liver biopsies. |
| Feld 2015 | A cohort of sickle cell disease patients followed prospectively in a natural history protocol at the National Institutes of Health (NIH), USA. A total of 247 patients were followed prospectively in a natural history protocol on sickle cell disease for a median of 30 months (range 13–49). The average age was 36.2 years (range 18–74) and 40% were males. All were anaemic and had symptoms of sickle cell disease. Patients underwent standard therapies for sickle cell disease. Eighty‐five of 247 were treated with hydroxyurea and 186 of 247 with folic acid. Of those referred for evaluation, 17 of 80 (22%) were on chelation therapy. In those with iron overload (Deugnier > 20), only 11 of 24 (46%) were on chelation at the time of referral, including only two of four with iron overload and advanced fibrosis. Patients with suspected liver‐related complications of sickle cell disease were referred for complete hepatological evaluation by the hepatology consult service between January 2005 and July 2008. Reasons for referral included persistent elevation of alanine aminotransferase, alkaline phosphatase or direct bilirubin, elevation of ferritin or other iron parameters, or evidence of viral hepatitis. If clinically indicated, patients were offered liver biopsy. Liver biopsies were preferentially performed by the transjugular route which allowed for estimation of the hepatic venous pressure gradient. Hepatic iron load was evaluated and soluble CD14 was measured according to the manufacturer’s protocol (R & D systems, Minneapolis, USA). Because the focus was on chronic liver injury, all laboratory analyses were performed at least two weeks after or before any acute illness. No patients without iron overload were on chelation therapy. The type of participants is outside the scope of this review. |
| Fernandez Salazar 2004 | Determined epidemiological, biochemical, virological, and histological factors associated with liver steatosis in chronic hepatitis C. Medical histories of 53 patients biopsied for chronic hepatitis C diagnosis between June 2000 and December 2002 were retrospectively studied. Steatosis was identified in 52% of biopsies. No comparable data on ferritin or liver iron were available. |
| Finch 1986 | In normal persons, plasma ferritin reflects the size of iron stores. A decrease to less than 12 µg/L indicates iron deficiency. Increased iron stores are associated with an increased plasma ferritin level. Various other conditions, however, can increase the plasma ferritin concentration including increased metabolism, inflammation, tissue damage and neoplastic disease. The use of the plasma ferritin determination in diagnosing iron overload depends on excluding these other causes, leaving storage iron as the only explanation for the increased plasma ferritin. It is then necessary to establish the parenchymal nature of the iron overload by showing an elevated transferrin saturation and, if elevated, the more definitive liver biopsy should be done. Clinical review on the usefulness of ferritin as a diagnostic tool in the case of iron deficiency and overload. Very informative, but without usable data. |
| Fleming 1990 | Average weights of wet tissue used for hepatic iron estimation were about 20 mg from necropsy specimens and 5 mg from percutaneous biopsy specimens. These were processed and tissue iron assessed chemically by the Barry and Sherlock methods. Liver iron was measured with liver biopsy. Liver iron content measurement with a threshold of > 3.2 mg/g dry weight was classified as iron overload.There was no significant correlation between hepatic iron and plasma iron, ferritin, or transferrin. Serum ferritin concentrations in excess of 1000 µg/L were seen in six patients who had liver iron concentrations ranging from 345 to 1846 mg/100 mg dry weight. The correlation between serum ferritin and liver iron was less close in our patients than in other series. The correlation of serum ferritin and liver concentration values was r = 0.41 for the 22 patients that underwent liver biopsy. Some patients were deceased. Serum ferritin was taken up to 4 weeks before death, when the patient may have been very sick. Bone marrow examination was taken after the serum ferritin measurements. Each type of measurement was taken blinded to the results of the other test. The patients were in end‐stage kidney failure in haemodialysis. The type of participants is outside the scope of this review. |
| Gallego 2015 | The primary objective of this study was to determine the frequency of diagnosis of HFE‐related HH and to estimate the penetrance of clinically‐related variables in the Electronic Medical Records and Genomics Network, a national consortium organised by the National Human Genome Research Institute. In the cohort of approximately 39,000 individuals, 618 individuals had the p.Cys282Tyr homozygous or p.[Cys282Tyr];[His63Asp] compound‐heterozygous genotype and of these, 538 individuals had corresponding data from electronic medical records. The study was excluded because although there was reference to iron liver content, there was no detail about methodology and also because there were no data on iron content and ferritin that could be used for 2 x 2 tables or raw data for analysis. |
| Greni 2017 | This study analysed alleles and genotype frequencies and their relationship with iron parameters in a cohort of 298 HFE p.C282Y homozygotes of Italian ancestry (205 men and 93 women) and a group of 169 male healthy blood donors. Two‐hundred and twenty‐six patients (160 men and 66 women) underwent liver biopsy for diagnostic (before HFE testing) and/or prognostic aim. Liver iron concentration was measured on liver biopsy samples in fresh or deparaffinised specimens or by quantitative magnetic resonance. The study was excluded as the data was not presented separately by method for assessing liver iron concentration. |
| Gupta 2003 | 40 patients (21 males, 19 females, aged 22 to 63 years of age) with chronic renal failure not in haemodialysis were studied to evaluate iron deficiency by serum transferrin receptor and serum ferritin levels. There were no data on bone marrow. |
| Gupta 2009 | 102 patients with stage 5 chronic kidney disease were enrolled. 36 age‐ and sex‐matched anaemic patients without any known renal disease were taken as controls. sTfR, iron, TIBC, ferritin, and transferrin saturation levels were measured. The patients were followed up twice, at four weeks and six months. The authors concluded that, in chronic kidney disease, sTfR cannot be used as a reliable marker of iron deficiency anaemia. There were no bone marrow data. |
| Guyatt 1992a | Systematic review to determine the diagnostic value of laboratory tests used in the diagnosis of iron deficiency anaemia. In total, 55 studies included the results of laboratory tests and histological examination of the bone marrow for at least 50% of an identifiable patient group. Results showed that serum ferritin radioimmunoassay was the most powerful test with an area under the receiver operating characteristic curve of 0.95. Test properties differed for populations of patients with inflammatory, liver, or neoplastic disease and patients without these conditions. Likelihood ratio lines, which allow precise interpretation of results across the entire range of ferritin concentration values, were constructed for the individual populations. |
| Hagve 2013 | This is a non‐systematic literature review of searches in databases with a discretionary selection of articles. This review did not contain original data on bone marrow or ferritin. |
| Hallberg 1993b | The prevalence of iron deficiency was determined in Goteborg, Sweden, in a sample of 15‐ to 16‐year‐old girls (n = 220) and boys (n = 207) using serum ferritin. In a recent study on the relationship between serum ferritin and stainable bone marrow iron in women, it was established that, at a cut‐off value for serum ferritin of < 16 µg/L in 75% of women with no iron stores, the serum ferritin concentration was below this value (sensitivity 75%), whereas, in 98% of iron‐replete women, it was above this cut‐off value (specificity 98%). There were associations of serum ferritin with Tf, Hb, and mean corpuscular volume. The study did not provide bone marrow data although the abstract referred to bone marrow in a previous study. |
| Hallberg 2001 | This was a review on iron deficiency without usable data on ferritin or bone marrow. |
| Hallberg 2002 | This was a review on iron absorption and metabolism with graphs on ferritin with Tf, but not with concurrent bone marrow. Only one graph showed values of haemoglobin concentration and subjects with stainable iron in bone marrow smears. |
| Halonen 2003 | Comparison of liver iron loading with ferritin and sTfR in children recovered from acute lymphoblastic leukaemia. Finland. The study patients had acquired their iron loading due to transfusions. |
| Hamedani 1991 | Serum ferritin levels were determined using immunoassay and compared to blood films, serum iron levels and total iron‐binding capacity values in 300 apparently healthy Pakistanis, 2 to 35 years of age. No data on bone marrow. |
| Hamidieh 2015 | Ninety‐three paediatric haematopoietic stem cell transplantation candidates with thalassaemia major who underwent liver biopsy were included in this study. Hepatic T2* MRI values and serum ferritin concentrations were assessed to investigate and determine the useful method in detection of patients with TM class III who received different conditioning regimens, in comparison with class I and II. Twenty (21.5%) patients were categorised as class III. The study was excluded because there were no extractable data on iron content and ferritin that could be used for 2 x 2 tables or raw data for analysis. |
| Handelman 2008 | This was a review on iron metabolism with descriptions of mechanisms of anaemia and iron metabolism. No usable data on ferritin and bone marrow. |
| Hanif 2005 | No extractable data for cross tabulation for diagnostic test accuracy (2 X 2 DTA table). |
| Hankins 2010 | 32 children 7‐18 years of age with sickle cell anaemia and iron overload (serum ferritin lower than 1000 ng/mL or more than 18 lifetime transfusions) were eligible. Serum ferritin and hepatic iron content were measured and participants underwent non sedated T2* MRI of the heart, echocardiogram, electrocardiogram, and multi‐uptake gated acquisition scan. Age‐matched normative echocardiographic data were used for comparison. Relationships among transfusional iron burden, myocardial iron deposition, and diastolic ventricular dysfunction by T2* MRI and tissue Doppler echocardiography in iron‐overloaded children with sickle cell anaemia were assessed. |
| Haque 1996 | Analysis of liver iron loading in patients with chronic hepatitis C virus, with measurements of both serum ferritin and liver iron. No data available for extraction. |
| Harmatz 2000 | Comparison between liver iron with ferritin in patients with sickle cell disease. The study was excluded because all patients were transfusion dependent. |
| Heinrich 1977 | Correlations between iron absorption and ferritin concentration for determination of iron deficiency. Although the abstract mentioned iron content in bone marrow and the paper described the use of the traditional invasive method of Berlin‐Blue staining for assessing body iron stores in the bone marrow, the exam was refused by many patients and had the disadvantage of being at best a semi‐quantitative and subjective method with a limited reproducibility and capacity which required some experience for a relevant evaluation by the observer. The study focused on comparisons of serum ferritin determinations by an immunoradiometric assay and radioisotopic iron absorption studies. |
| Heinrich 1980 | Short communication without data on bone marrow. Correlation of ferritin concentration with iron absorption to diagnose iron deficiency. |
| Hellerbrand 2003 | Case‐control study of iron loading in patients with hepatocellular carcinoma. The study did not provide any extractable data for iron loading/ferritin. |
| Hernandez 1988 | Compared MRI with liver iron together with ferritin in patients with chronic sickle cell disease. The study was excluded as all patients were chronically transfused. |
| Ho 2001 | This study involved 147 subjects, divided into 4 groups: healthy controls (n = 50), patients with iron‐deficiency anaemia (n = 54); patients with anaemia of chronic disorders (n = 34), and patients with thalassaemia (n = 9). sTfR, ferritin, TIBC, unbound iron‐binding capacity and complete blood count were measured in all subjects. In 17 patients with iron‐deficiency anaemia, serum ferritin, sTfR, and haemoglobin were rechecked after iron therapy. The study contained data on ferritin, Tf, Hb and other indicators, but data on iron in bone marrow were used to classify subjects as iron deficient in one particular group. No comparisons with ferritin. |
| Hod 2012 | This was an animal study in mice. |
| Hofer 2004 | Liver biopsies were obtained from 169 interferon‐naïve patients with chronic hepatitis C. Biopsy specimens were evaluated according to the DiBisceglie scoring system and iron grading. Hepatic iron concentration was determined by atomic absorption spectroscopy. Ferritin and transferrin saturation and presence of HFE‐C282Y and H63D gene mutations were determined at baseline. The study was excluded because if was not possible to extract a 2 X 2 table. |
| Hussain 1998 | This study was conducted in patients on haemodialysis. |
| Hyman 1983 | Case study of a 39‐year‐old woman with severe iron‐deficiency anaemia. The patient had lost no blood and her serum iron level was high. Her bone marrow was hypercellular with a predominance of erythroid elements and had no stainable iron deposits, but it also showed dyserythropoiesis and an excess of apparently normal plasma cells. Serum ferritin was 225 µg/L. The study contained single data on bone marrow, ferritin, Tf, Hb and other indicators, but no data for comparisons in a 2 x 2 table or to determine a cut‐off point. |
| Infusino 2012 | This was a meta‐analysis to evaluate the diagnostic efficacy of sTfR and sTfR/log ferritin index, without data on ferritin and bone marrow. |
| Inthawong 2015 | The aim of this study was to determine the prevalence of pulmonary hypertension in patients with non‐transfusion‐dependent thalassaemia and to investigate its correlation with the clinical parameters, liver iron concentration and non‐transferrin‐bound iron. The study was excluded because liver iron content was determined by magnetic resonance imaging. |
| Invernizzi 1990 | 10 non‐haemopathic subjects and 34 patients suffering from various haematological disorders were studied. In all cases, bone marrow aspiration had been performed as part of the diagnostic procedures. The control group, five males and five females, presented with normal blood cell counts and normal body iron status; none of them had evidence of underlying bone marrow disease. The group of haemopathic patients included: eight subjects with iron deficiency anaemia, four with refractory hypoplastic anaemia and transfusional iron overload, four with anaemia of chronic disease, fourteen with myelodysplastic syndrome, and four with acute myeloid leukaemia. Although bone marrow aspiration and ferritin determinations were performed, the study was designed to identify the ferritin content in bone marrow in control and in haemopathic groups by immunohistochemistry. There were no data or analyses on iron content in bone marrow related to ferritin concentration in serum, plasma or red cells. |
| Isa 2013 | Ten patients (8 males and 2 females) were diagnosed with neonatal haemochromatosis. Two patients had intrauterine growth restriction and 6 were preterm. The median birth weight was 1700 grams. All patients had raised ferritin levels, prolonged prothrombin time, and 9 patients had high serum iron and serum AFP. Abdominal MRI showed iron overload in the liver (n = 8). Liver biopsies showed evidence of haemochromatosis (n = 3). Buccal biopsies stained positive for iron (n = 1). Eight patients received antioxidant therapy and survived. Two patients passed away. The study was excluded because liver iron content was determined in only 3 patients and there were no data on iron content and ferritin that could be used for 2 x 2 tables or raw data for analysis. |
| Isokawa 1997 | 22 patients (10 women, 12 men, mean age 48 years) with various haematological disorders including iron‐deficiency anaemia in two patients, one refractory anaemia with ringed sideroblasts, one refractory anaemia with excess of blasts (RAEB), four RAEB in transformation (RAEB‐t), haemochromatosis in one patient, chronic myelogenous leukaemia in one, myelofibrosis in two, aplastic anaemia in one, alcohol‐induced anaemia in one, non‐Hodgkin’s lymphoma in one, and lung cancer without marrow metastasis in seven. Bone marrow was biopsied from the right posterosuperior iliac crest within 3 days after MRI. Serum ferritin was determined by a radioimmunoassay (Ferritin kit, Daiichi Radio Assay Co, Tokyo, Japan). Although bone marrow aspiration and ferritin determinations were performed, only 2 cases of iron‐deficiency anaemia were included (without indications about how the diagnosis was made). Additionally, this study determined iron content in bone marrow chemically, not by microscopic observation. The study reported iron content in bone marrow in each patient, indicating presence of iron in bone marrow in all cases. There were detected and quantified levels of iron even in cases diagnosed as iron deficiency anaemia. It was not possible to identify true positives because the traces of bone marrow iron content were not clearly defined as diseases or not. |
| Jacobs 1972 | No extractable data for 2 x 2 DTA table |
| Jacobs 1975 | This was a review on the clinical and biochemical implications of determining serum ferritin, its distribution in populations and the methods for determining it. No usable data on ferritin or bone marrow. |
| Jacobs 1979 | This was an article with a sequence of strategies to diagnose and treat anaemia. No usable data on ferritin or bone marrow |
| Jacobs 1982 | No extractable data for 2 x 2 DTA table |
| Jankowska 2013 | This was a case‐control study with iron staining of bone marrow in 30 patients with ischaemic heart failure and 10 healthy controls, and an observational study at 3 tertiary cardiology centres of 791 patients with systolic heart failure. Main outcome measures: bone marrow specimens were stained for iron, and iron deficiency was diagnosed for 0‐1 grades according to Gale scale. Serum ferritin, iron, transferrin saturation, and sTfR were assessed as circulating iron biomarkers. It contained very limited information. Was an abstract for a congress. No usable data on ferritin or bone marrow |
| Jensen 1999 | This article presented case series of anaemic patients referred for bone marrow given an oral iron absorption test with serum ferritin measured. There was no 2 x 2 table data available. |
| Jitani 2011 | Abstract for a congress without numeric data. A 1‐year prospective hospital‐based study was conducted to correlate biochemical parameters with complete haemogram and bone marrow findings in 58 patients (aged 8 to 81 years) with haemoglobin ranging from 17 to 104 g/L, without prior treatment with haematinics. There was a complete correlation between the three parameters in 12/49 (24.5%) cases only. The authors concluded that bone marrow aspiration, though invasive, provided a rapid and cost‐effective investigation for confirming the diagnosis of dimorphic anaemia; there was no usable data on ferritin or bone marrow. No follow‐up publication in extenso |
| Jolobe 2005 | 365 patients aged 67 to 96 years of age with a mean corpuscular haemoglobin < 26 pg and/or a mean corpuscular volume < 80 fL, 201 patients proved to be iron deficient (serum ferritin level < 18 μg/L). No usable data on ferritin and no data on bone marrow |
| Junca 1998 | This study did not contain bone marrow or ferritin. |
| Junca 2001 | 37 anaemic patients: 10 hypoferritinaemic patients (serum ferritin < 25 μg/L), and 27 with hyperferritinaemia (serum ferritin higher than 200 μg/L) and clinical/analytical criteria of anaemia of chronic disorders, who were submitted to a bone marrow aspirate with iron stain. In these patients, the gold standard for iron stores evaluation continued to be bone marrow aspirate and Perl's stain. No usable data on ferritin in association with data on bone marrow for sensitivity or specificity analysis or 2 x 2 tables. The cases with bone marrow data had inflammation and hyperferritinaemia (serum ferritin higher than 200 μg/L). Data from this study could not be used in this review for iron deficiency because the 10 patients with low ferritin had no bone marrow data and were not included in the analysis. |
| Just 2011 | 59,651 pre‐donation samples were evaluated by complete blood count. Samples with a mean corpuscular volume equal or less than 80 fL were further investigated (serum iron, ferritin, transferrin and percentage of transferrin saturation). Iron deficiency was defined as a ferritin level below 20 μg/L in males and 15 μg/L in females or a transferrin saturation level of less than 15%. No data on ferritin or bone marrow for sensitivity or specificity analysis or 2 x 2 tables. Abstract for a congress with no follow‐up publication in extenso |
| Kaldara 2011 | 101 consecutive patients with end stage Heart Failure and anaemia (Hb < 130 g/L in men and < 120 g/L in postmenopausal women) were evaluated. Patients with diseases known to cause anaemia or creatinine > 3 mg/dL were excluded. All patients underwent complete diagnostic work‐up including bone marrow aspiration for iron store assessment. Data on sensitivity and specificity compared bone marrow with ferritin and mean corpuscular volume. Authors did not present data on bone marrow and ferritin alone. Abstract for congress that was not found published in extenso |
| Kaltwasser 1977a | This manuscript described methods for ferritin determination. No usable data on bone marrow |
| Kaltwasser 1977b | This was a study assessing the impact of iron therapy on ferritin concentrations. No data on bone marrow |
| Karam 2008 | Study of liver iron in patients with sickle cell disease. The study was excluded as patients were transfusion dependent. The study did not provide any extractable data comparing ferritin to liver iron loading. |
| Karimi 2017 | This cross‐sectional study was conducted among 108 patients with b‐thalassaemia intermediate, referred to a tertiary hospital at the Shiraz University of Medical Sciences, in Shiraz, Southern Iran. It aimed to determine an optimal cut‐off value of ferritin in proportion to T2 MRI of liver and measurement of liver iron concentration for early detection of hepatic iron overload in beta‐thalassaemia intermediate patients. The study was excluded because liver iron content was determined by magnetic resonance. |
| Karlsson 2015a | Forty anaemic patients, 65 yrs of age or older, who were admitted to the Department of Haematology at the Department of Haematology, Uppsala University Hospital, Uppsala, Sweden. Bone marrow smears were stained using the May‐Grunwald‐Giemsa method, and bone marrow iron stores were investigated using Prussian blue staining. Patients with no stainable bone marrow iron (n = 11) were diagnosed as having iron deficiency with concurrent inflammation. The diagnosis of those with stainable iron (n = 20) was anaemia of inflammation. Mass spectrometry was used to evaluate the hepcidin‐25 assay in the differential diagnosis of iron‐deficiency anaemia with concurrent inflammation and anaemia of inflammation in elderly patients using the absence of stainable bone marrow iron as the standard criterion for iron deficiency. The study was excluded as there were no usable data to construct 2 x 2 tables on results from biopsies and ferritin. |
| Karlsson 2017 | This study was conducted among 30 consecutive anaemia patients 65 years of age or older attending the Department of Haematology, Uppsala University Hospital, Uppsala, Sweden. Bone marrow smears were stained using the May‐Grünwald‐Giemsa method, and bone marrow iron stores were investigated using Prussian blue staining. Patients with no stainable bone marrow iron were diagnosed as having iron‐deficiency anaemia with or without concurrent inflammation based on C‐reactive protein measurements. The study was excluded as there were no usable data to construct 2 x 2 tables on results from biopsies and ferritin. |
| Kerlin 1979 | This was a 15‐month study to determine the cause of iron deficiency in 215 adult males and postmenopausal females attending a general hospital. The laboratory computer identified all anaemic subjects. The iron status of each patient was assessed by serum iron, serum ferritin or sternal marrow aspiration. No usable data on ferritin and bone marrow. The gold standard for iron depletion was not bone marrow in all cases. The criteria selected for a diagnosis of iron deficiency were one or more of the following: a) serum iron < 10 µmol/L or TIBC > 75 µmol/L or % saturation < 14%; b) serum ferritin < 10 µg/L in females or < 20 µg/L in males; c) bone marrow aspirate absent or markedly reduced stainable iron; d) a rise in haemoglobin of 2 g/L or greater if iron supplements had been commenced; it was not possible to separate out those based on bone marrow data. No data on sensitivity or specificity were available. |
| Kim 1984 | 153 Guatemalan children, ranging in age from 30 to 72 months, were assigned to one of three groups based on their initial venous haemoglobin values (< 105 g/L; 105‐115 g/L; > 115 g/L). The gold standard for iron depletion was not bone marrow, but the response of haemoglobin concentration to oral iron administration. No usable data on sensitivity or specificity |
| Kimber 1983 | This was a review on ferritin as indicator of iron stores, with experimental data showing low ferritin concentrations when no iron in bone marrow measurements were available. There were no additional details about the study. |
| Koca 2013 | 454 cases with bone marrow and simultaneous serum iron tests (ferritin and transferrin), 407 retrospectively and 47 prospectively. Serum iron tests were considered as compatible with iron‐deficiency anaemia if both serum ferritin level (< 15 µg/L) and transferrin saturation saTf (< 15%) were decreased. The tests were incompatible with iron deficiency if both of them were above specified thresholds, > 20 µg/L and > 20%, respectively. Analysis was undertaken of specificity, sensitivity and predictive values for iron deficiency defined by no iron in the bone marrow, and “serum‐iron tests” that included simultaneously serum ferritin level (< 15 µg/L) and transferrin saturation transferrin saturation saTf (< 15%). There were no analyses of bone marrow data with serum ferritin alone. |
| Koulaouzidis 2007 | This was a review without usable data on ferritin or bone marrow. |
| Koulaouzidis 2009 | This systematic review aimed to identify studies that compared sTfR measurement against bone marrow iron stain. Overall, 20 prospective studies were identified, of which nine fulfilled the inclusion criteria (sTfR measurement in anaemic adults alongside bone marrow iron staining). Analysis was undertaken of specificity, sensitivity and predictive values for iron deficiency defined by no iron in the bone marrow, and serum transferrin receptor studies. There were no analyses of bone marrow data with serum ferritin alone. |
| Kowdley 1997 | Study of the use of hepatic iron index to diagnose haemochromatosis. Hepatic iron concentration was measured in 509 patients undergoing liver biopsy. The diagnosis of haemochromatosis was made using clinical, biochemical, and histopathologic criteria. The study was excluded as there were no data comparing liver iron with ferritin. |
| Kowdley 2012 | Study of liver iron loading in three large cross‐sectional studies of non‐alcoholic steatohepatitis. The study did not report any extractable data comparing ferritin to hepatic iron loading. |
| Kreeftenberg 2000 | Patients with clinical suspicion of iron overload in the liver (arthralgias, infections and fatigue), suspicious laboratory findings and a family history of iron overload. The study did not provide any extractable data comparing ferritin to liver iron loading. |
| Krittayaphong 2018 | This study assessed the accuracy of serum ferritin in the diagnosis of iron overload compared to MRI in 405 patients (mean age of 18.8 ± 12.5 years of age) with transfusion‐dependent thalassaemia or non‐transfusion‐dependent thalassaemia who were referred for MRI study for assessment of cardiac iron overload and liver iron overload between 2011 and 2015 in eight centres across Thailand. The study was excluded because liver iron content was determined by magnetic resonance imaging. |
| Labbe 2004 | This is a review without usable data on ferritin or bone marrow that focuses on biochemical, analytical, and clinical features of serum transferrin receptor and the erythrocyte zinc protoporphyrin/haeme ratio. |
| Lebray 2004 | The study analysed liver histology findings in a large cohort of patients with chronic hepatitis C. No association was observed between HFE mutations and histological activity. Increased iron parameters were associated with liver disease severity by univariate analysis only. The study was excluded as ferritin and liver iron content were not compared. |
| Lee 2004 | This was a review without usable data on ferritin or bone marrow. Focused on non‐transferrin bound iron and the intracellular labile‐iron pool. |
| Legros 2015 | 77 patients (C282Y homozygotes) with serum ferritin levels higher than 1000 µg/L and/or increased serum transaminase levels are at risk of severe F3/F4 fibrosis. Current practical guidelines recommend liver biopsy in such individuals to determine the degree of fibrosis. This prospective observational cohort study aimed to evaluate non‐invasive alternative means such as hyaluronic acid and transient elastography for the assessment of severe fibrosis in patients with serum ferritin higher than 1000 µg/L or elevated transaminases. There were no data on iron content from liver biopsies. The method was used to study fibrosis and not for iron content. There were no data on iron content and ferritin that could be used for 2 x 2 tables or raw data for analysis. |
| Leitch 2009 | Case report of patient with myelodysplastic syndrome and iron overload receiving iron chelation therapy. No usable bone marrow or ferritin data. |
| Leitch 2012 | Retrospective analyses suggest iron overload is associated with inferior survival in lower risk myelodysplastic syndrome and iron chelation therapy with improvement (study presented as an abstract at a conference). No data on ferritin or bone marrow |
| Lewis 1982 | 76 Caucasian patients attending the gynaecological outpatient department St Mary's Hospital, Manchester, United Kingdom were included in the study. 42 patients (mean age of 38.5 years) were referred because of menorrhagia (group 1) and 34 patients (mean age of 30.1 years) were randomly selected from premenopausal patients referred because of various other gynaecological problems but who had normal menstrual periods (group 2). No usable data on ferritin and no data on bone marrow |
| Leyland 1975 | No extractable data for 2 x 2 DTA table |
| Li 2002 | Comparison of ferritin and liver iron in patients with thalassaemia major. All patients were transfusion dependent. |
| Licata 2009 | One hundred and twenty‐four consecutive patients with hyperferritinaemia (male > 300 µg/L, female > 200 µg/L) were evaluated; clinical, biochemical and serological data, iron status parameters, HFE gene mutations and homeostasis model assessment scores were obtained. Steatosis was graded by ultrasound as absent or present. Histology was available for 53 patients only. By logistic regression, ferritin and ‐glutamyltransferase were independent predictors of steatosis. The study did not provide any data comparing ferritin and liver iron. |
| Linkesch 1978 | This was a review on method and significance of ferritin determination. No usable data on ferritin and no data on bone marrow |
| Lipschitz 1974 | No extractable data for 2 x 2 DTA table |
| Lu 2013 | In this study, the haematopoietic colony‐forming capacity and reactive‐oxygen species bone marrow cells were tested before and after iron‐chelation therapy. After administering deferoxamine, reduced blood transfusion, increased neutrophilss, increased platelets, and improved pancytopenia were observed in 76.9%, 46.2%, 26.9%, and 15.4% of the patients, respectively. Results showed that iron overload injured the haematopoiesis by damaging the hematopoietic cell and hematopoietic microenvironment, which is mediated by reactive oxygen species‐related signalling proteins. |
| Luxton 1977 | No extractable data for 2 x 2 DTA table |
| Macdougall 1998 | This was a review on the significance of the percentage of hypochromic red cells. No usable data on ferritin and no data on bone marrow |
| Machado 2009 | Cross‐sectional study of iron loading in patients with alcoholic liver disease (vs alcoholics without liver disease). The study was excluded as no extractable data were included in the manuscript. |
| Maliken 2012a | Susceptometry was performed in 10 patients with hyperferritinaemia due to hereditary haemochromatosis (n = 2), secondary iron overload (n = 3), nonalcoholic fatty liver disease (n = 2), and chronic viral hepatitis (n = 3) within one month of liver biopsy in the absence of iron‐depletion therapy. Liver biopsy was performed according to standard clinical protocols. Hepatic iron content was determined by atomic absorption spectrophotometry. This study aimed to examine the relationship between room‐temperature susceptometry and biochemical hepatic iron content measured in liver biopsy specimens in a more varied patient cohort. The study was excluded as no data were presented in the manuscript enabling 2 x 2 extraction of analysis between ferritin and liver‐iron loading. |
| Martinelli 2000 | Study of the relationship between liver damage in patients with hepatitis C virus and haemochromatosis mutations. Included patients with hepatitis C virus. Patients with liver biopsy appeared to have required biopsy for clinical rationale. The study was excluded as there were no extractable data for a 2 x 2 table between liver iron loading and ferritin concentrations. |
| Martinelli 2004 | Cross‐sectional study evaluating risk factors for iron overload (measured by liver iron deposits) in patients with hepatitis B virus, including comparison with serum ferritin. The study was excluded as no data were extractable for 2 x 2 analysis between ferritin and liver iron deposits. |
| Massawe 1999 | 2235 pregnant women, booking for antenatal care at two clinics in Dar‐es‐Salaam, Tanzania, were screened for anaemia. Blood cell counts, microscopy of blood films, S‐ferritin, C‐reactive protein, HIV, stool parasites and bone marrow analysis were performed. Iron deficiency was present in 86% and malaria in 1/3 of anaemia cases. Using a WHO recommended cut‐off point of 50 µg/L for serum ferritin among women in tropical Africa, 76% of those assessed were iron deficient. Bone marrow was aspirated from the iliac crest from a subsample (n = 71) of anaemic women and stained by the Perl’s Prussian blue reaction for assessment of haemosiderin granules. Intracellular iron was recorded as positive or negative. Results from bone marrow were not correlated with ferritin. Ferritin and bone marrow results were correlated with mean corpuscular volume, C‐reactive protein and haemoglobin. There was no analysis of bone marrow data with serum ferritin alone. |
| Massey 1992 | This was a review on microcytic anaemia for diagnosis of iron‐deficiency anaemia. No usable data on ferritin and no data on bone marrow |
| Maximova 2017 | This retrospective study reported on clinical data from 42 pediatric patients 2‐17 years of age who had undergone oral chelation therapy with deferasirox in the Bone Marrow Transplant Unit, Institute for Maternal and Child Health in Trieste, Italy. All study patients had undergone allogeneic haematopoietic stem cell transplantation preceded by a myeloablative conditioning regimen. During the pre‐transplantation work‐up, all patients had undergone an abdominal MRI‐based evaluation of iron concentration in the liver, pancreas, spleen, and bone. The study was excluded because liver iron content was determined by magnetic resonance imaging. |
| Mazza 1995 | Comparison of liver iron measured by biopsy and MRI, along with serum ferritin, in patients with thalassaemia major. The study was excluded because all patients were transfusion‐dependent. |
| Mazzolenis 1992 | 23 patients receiving haemodialysis at Servicio de Nefrologia del Sanatorio Guemes, Argentina, were chosen for having haematocrit levels under 25% and/or transfusional requirements > 150 mL red blood cells/month. 17 men and 6 women (mean age, 48 years, mean haemodialysis time, 55.6 months) were included. Assays performed included complete blood counts, reticulocytes and reticulocyte production index, serum iron, total iron binding capacity and transferrin saturation, serum ferritin, haptoglobin, parathormone, and creatinine. Bone marrow aspiration and percutaneous biopsy were done on each patient for cytologic, histologic, cytochemical analysis of iron deposits and bone histomorphometry including aluminium deposits. Although there were data on bone marrow iron content correlated to ferritin concentration, the study was excluded, since the study group included 23 patients receiving haemodialysis. This condition was an exclusion criteria for this DTA systematic review. |
| Meredith 1999 | This review described iron deficiency anaemia, thalassaemia, anaemia of chronic inflammation, and sideroblastic anaemias, as well as the clinical and laboratory findings in each patient. No original data on bone marrow iron content or ferritin concentration |
| Milman 1991 | Description of 179 cases of hereditary haemochromatosis in Denmark. Clinical and biochemical phenotype reported. The study was excluded as there was no comparison between liver iron and biochemical iron indices. |
| Milman 1996 | This was a population study with data on ferritin but no data on bone marrow. |
| Minniti 2011 | Comparison between liver biopsy and MRI in 10 patients with sickle cell disease ‐ all biopsies were clinically indicated. The study was excluded because all patients were transfusion‐dependent. |
| Moicean 2004 | This was a case study of a 35 year old female with a severe anaemic syndrome. Bone marrow biopsy established the diagnosis of erythroblastopenia. No data on ferritin. Bone marrow biopsy established the diagnosis of erythroblastopenia. |
| Moirand 1997 | Study characterising the phenotype of iron loading in patients with a metabolic syndrome; patients derived from a referral base of high‐risk patients referred to a specialist clinic. The study was excluded as no 2 x 2 data for extraction were available. |
| Moreb 1983 | The diagnostic usefulness of bone marrow haemosiderin, serum ferritin, transferrin saturation, mean corpuscular volume and red cell protoporphyrin in the evaluation of iron status in 39 patients on chronic haemodialysis. Haemodialysis was an exclusion criterion for type of participants in this review. |
| Morrison 2003 | The study developed noninvasive criteria to predict the presence or absence of advanced hepatic fibrosis or cirrhosis in Americans with haemochromatosis. Liver histopathology and serum ferritin, aspartate aminotransferase, and alanine aminotransferase levels were measured in 182 participants with hereditary haemochromatosis. In a multivariate model, the probability of cirrhosis was 7.4% among patients with serum ferritin levels less than 1000 µg/L compared with 72% among patients with serum ferritin levels greater than 1000 µg/L after adjustment for age and elevated serum liver enzyme levels. The study was excluded as there was no comparison between ferritin and liver iron. |
| Mueller 2017 | 264 adult patients (66 females and 198 males) from Germany with or without signs of iron overload or liver disease. Thirty‐five patients underwent medically‐indicated liver biopsy with histological determination of semiquatntitative degree of iron (Prussian Blue staining). IN 33 of the patients liver uiron concentrations was cquantified by atomic absorption spectroscopy. In 15 patients hepatic iron was quantified by magnetic resonance imaging. Three methods for iron concentration were compared an ferritin was not possible to isolate. Excluded as no 2 x 2 data for extraction available. |
| Murphy 1997 | This was a review on suggested modest changes in the criteria used for the diagnosis of essential thrombocytopenia and allowed tentative recommendations concerning therapy. |
| Murphy 2006 | A raised percentage of hypochromic red cells was detected at diagnosis in 10 of 34 consecutive patients with low‐risk myelodysplastic syndrome, all of whom had normal or increased serum ferritin and bone marrow iron stores. Patients diagnosed with primary low‐risk MDS and complete blood counts, reticulocyte counts and reticulocyte Hb content analyses were recorded, along with serum levels of C‐reactive protein, lactic dehydrogenase, iron, total‐iron binding capacity, transferrin saturation and ferritin, within a month of the diagnostic bone marrow examination and prior to any blood transfusions and without evidence of acute illness. In a subset of these patients, serum lead and erythrocyte zinc protoporphyrin (normal range 0–3 μg/g Hb) were then measured and compared to the above results. Myelodysplastic patients with a raised percentage of hypochromic red cells had evidence of functional iron deficiency, all of whom had normal or increased serum ferritin and bone marrow iron stores. No iron deficiency and no usable data on iron in bone marrow and ferritin correlations |
| Musso 1991 | Study of 19 patients (7 women 22‐65 years and 12 men 22‐66 years of age) with chronic renal failure in haemodialysis for 1 to 108 months. A significant correlation between serum ferritin and the iron in bone marrow was observed. Five of the 19 patients studied had iron deficiency (26%), and 2 of them responded favourably to parenteral administration. Iron deficiency was defined by iron in bone marrow more than 2+, instead of 0 used as inclusion criteria. There were data on sensitivity and specificity, but all patients were on haemodialysis which was an exclusion criterion for this review. |
| Nagai 2005 | This was a case study of a 29‐year old woman with severe iron deficiency anaemia and marked thrombocytosis that was complicated by central retinal vein occlusion. Platelet count was over one million per microlitre and an increased number of megakaryocytes was observed in the bone marrow at the time of diagnosis of iron‐deficiency anaemia, features that resemble those of essential thrombocythaemia. Study case with no usable data on bone marrow and ferritin |
| Nagashima 2006 | The aim of this study was to generate some information about hepcidin in chronic hepatitis C. It measured serum pro‐hepcidin levels in patients with hepatitis C virus and hepatitis B virus infection and in healthy controls. Serum pro‐hepcidin and ferritin levels were negatively correlated (r = ‐0.182, P = 0.037) in hepatitis C virus patients and positively correlated in hepatitis B virus patients and in healthy controls. The study was excluded as there were no data comparing ferritin with liver iron. |
| Nathanson 1999 | This was a review on markers of iron status including their relationship with bone marrow iron content, without data on ferritin or bone marrow. |
| Navone 1988 | Comparison of ferritin content by an immunoperoxidase method with haemosiderin, stained with Perl's reaction in 340 bone marrow biopsies. Ferritin and haemosiderin showed the same distribution in reticuloendothelial cells. All the Perl's‐positive cases (n = 177) were ferritin‐positive too. None of the ferritin‐negative cases (n = 13) were Perl's‐positive. Of 163 cases with negative Perl's reaction in bone marrow, 13 (12.5%) were also ferritin‐negative: these patients were mainly affected by polycythaemia vera or by untreated iron deficiency anaemia. Thus, immunohistochemical assessment of bone marrow ferritin can be a more sensitive tool for the evaluation of body iron stores in iron deficiency than Perl's reaction. Comparison of data on iron content in bone marrow by Perl's reaction with ferritin content in bone marrow. The study did not present data on serum, plasma or erythrocyte content. |
| Nelson 2011 | The aim of this study was to validate a new room temperature magnetic susceptometry device for in vivo measurement of hepatic iron content in patients with chronic liver disease. Thirteen subjects with hyperferritinaemia were enrolled into the study as follows: five subjects had iron overload, including 2 with haemochromatosis and 3 with secondary iron overload; 4 subjects had viral hepatitis including 3 with hepatitis C virus and 1 with hepatitis B virus; 4 subjects had nonalcoholic fatty liver disease. No comparison between ferritin and liver iron presented |
| Nielsen 2000 | The iron content of serum ferritin was determined in groups of patients with normal or increased iron stores by using a technique of ferritin immunoprecipitation followed by iron quantitation with atomic absorption spectroscopy. The study was excluded as it was not a study of serum ferritin per se (a study of iron content of ferritin). |
| Nuwayri‐Salti 1991 | 31 patients (18 male, 13 female, ages between 20 and 69 years) and chronic renal failure in regular haemodialysis were included in this study. Care was taken not to include any patient with any factor that may produce false elevation or serum ferritin such as a history of hepatitis, recent infection or a blood transfusion received within the year preceding the study. Blood and bone marrow samples were obtained through venous and sternal punctures which were done immediately prior to a dialysis session. The control group included 28 apparently healthy adults (15 males and 13 females) aged between 20 and 50 years. The study was a comparison of data on iron content in bone marrow with serum, polymorphs, lymphocytes and monocytes ferritin content in patients with haemodialysis, which was an exclusion criterion for this review. |
| O'Brien 1990 | Thirty‐eight of 572 patients screened (6.6%) had a serum ferritin level greater than 324 µg/L. The diagnosis of haemochromatosis seemed certain in 1 of 3 patients who were not biopsied for technical reasons. Of 8 patients biopsied, two had haemochromatosis, four had fatty liver, one had haemosiderosis, and one had a chronic inflammatory cell infiltrate with no iron deposition. Measurement of liver iron not performed |
| O'Keeffe 2005 | This was a case study of a 83‐year‐old man with a 2‐year history of severe restless legs syndrome. The patient had a history of ischaemic heart disease and of bronchiectasis secondary to old tuberculosis. Haemoglobin was 12.7 g/dl mean corpuscular volume was 89, transferrin saturation was 25% and serum ferritin was 93 µg/L. Case study with bone marrow and ferritin data, but no data for sensitivity and specificity. The authors mentioned a pre‐test predictive value with data obtained from previous studies in elders. |
| Oduor 2017 | This was a report of a retrospective analysis of two patients with sickle cell disease with cardiac iron overload, describing the serum ferritin concentrations and the liver iron concentrations on magnetic resonance imaging. One of the cases also had a liver biopsy. The study was excluded as no 2 x 2 data for extraction available. |
| Olivieri 1995 | Study of effects of deferiprone on liver iron loading. All patients had thalassaemia major (i.e. transfusion dependent). |
| Olynyk 2009 | Study evaluating hepatic iron overload in patients with hyperferritinaemia. Iron overload due to haemochromatosis. Study excluded as iron overload was quantified using MRI techniques. |
| Özatli 2000 | This study did not contain a 2 x 2 table as data for analysis. |
| Ozkale 2014 | A total of 34 (11 females and 23 males) consecutive cases of severe protein‐energy malnutrition, with no underlying diseases aged 3–20 months. Ten of the patients were in the marasmic‐kwashiorkor group, 10 were in the kwashiorkor group, and 14 were in the marasmic group. Full blood count, protein, albumin, serum iron, iron‐binding capacity, ferritin, vitamin B12, folic acid, complement‐3 (C3), complement‐4 (C4), and bone marrow were investigated in all groups. 97% of infants were anaemic, low serum iron levels were detected in 67.6% of the patients, transferrin saturation levels were low in 76.4% of the patients and ferritin levels were low in 20.5%, while bone marrow iron was absent in 58.8% of the cases. The level of vitamin B12 was normal in all patients. Bone marrow analysis showed erythroid series hypoplasia in 28.5% of patients in the marasmic group, 50% in the kwashiorkor group, and 30% in the marasmic‐kwashiorkor group. Data on ferritin and iron bone marrow content but not correlated to obtain sensitivity or specificity outcomes. Ferritin data were separated by the malnutrition condition but not by iron content in bone marrow. It was not possible to correlate the ferritin concentration in cases of no iron in bone marrow. |
| Pakbaz 2005 | Ninety patients with thalassaemia major were prospectively assessed for adherence to chelation therapy with deferoxamine, liver iron concentration, and serum ferritin. Liver iron concentration was measured by a low‐temperature (LTc) SQUID biosusceptometer system (Ferritometer®, Model 5700; Tristan Technologies, San Diego, CA, USA), and not liver biopsy. Patients were on iron‐chelation therapy. |
| Parkash 2015 | A total of 22 male patients were identified as having haemochromatosis. There was a mean age of 53 years at the time of diagnosis. Liver biopsy was done in 10 (45%) and using Perl's stain histopathological features were consistent with haemochromatosis and none had carcinoma. All Perl's Prussian blue stain showed excessive (grade IV) iron deposition in all biopsy specimens. The study was excluded because liver iron content showed excessive (grade IV) iron deposition and there were no data on iron content and ferritin that could be used for 2 x 2 tables or raw data for analysis. |
| Pastrana 2007 | Abstract of a study on 18 patients (11 females, 7 males) who underwent ileal pouch with anal anastomosis for ulcerative colitis, and to compare the distribution of complications in patients with and without anaemia, especially pouchitis, after ileal pouch with anal anastomosis. 10 were anaemic and pouchitis was found in 77% (14/18). Reference was made to bone marrow aspirates being performed in this study, but there was no description of methods or data on iron content in bone marrow. |
| Pautas 2012 | Overall, 190 consecutive patients 70 years of age or older, admitted to a geriatric short stay unit over a 1‐year period. When the haemoglobin level was < 120 g/L, the following serum assays were performed routinely: iron, ferritin, transferrin saturation, folate, vitamin B12, C‐reactive protein, thyroid‐stimulating hormone, albumin, and haptoglobin. When these tests were normal, bone marrow aspiration was performed to look for myelodysplastic syndrome. Haemoglobin was lower than 120 g/L in 83 (43.7%) of 190 included patients. Patients with anaemia had a mean haemoglobin level of 105 ± 11 g/L. The most common potential causes of anaemia were inflammation, severe renal impairment, severe malnutrition, and iron deficiency; each of these causes was found in at least one‐third of patients with anaemia. Although with data on ferritin and bone marrow aspiration, this test was only used to diagnose myelodysplastic syndrome. No correlations with ferritin |
| Peters 2017 | This was a study that retrospectively collected and reviewed data on clinical presentation, biochemical tests and DNA analysis of four patients who were diagnosed with type 3 hereditary haemochromatosis at the Radboudumc Expertise Center for Iron Disorders, The Netherlands. Liver iron content in two patients was assessed by magnetic resonance imaging or liver biopsy. It was not possible to determine the method for liver iron concentration in all the sample and to correlate with ferritin. |
| Phatak 2003 | Data from 79 unrelated C282Y homozygotes from treatment centres in Rochester, NY and Birmingham, USA who had undergone liver biopsy with measurement of hepatic iron content and who had achieved iron depletion. The sample consisted of 57 men and 22 women; their median age at diagnosis was 47 years (range 23–76 years). Sixty‐three of 79 (79.7%) had hepatic iron index (HII; µmol/g dry weight of liver divided by age in years) > 1.9, a conventional phenotypic definition of haemochromatosis. Serum ferritin levels ranged from 135 to 5613 µg/L (arithmetic mean: 1182 µg/L). The study was excluded because results correlated hepatic iron content by biopsies with phlebotomy mobilised iron. There were no data on iron content and ferritin that could be used for 2 x 2 tables or raw data for analysis. |
| Phelps 1996 | This study was done in patients undergoing dialysis. |
| Pietrangelo 2006 | Magnetic resonance imaging was used to study ferroportin disorder in 22 patients from four different pedigrees carrying different ferroportin mutations: A77D, N144H, G80S and Val 162del. Based on the iron status of spleen and bone macrophages, two different forms of the disease could be identified: a classic, common form, characterised by hepatocyte, splenic macrophage and bone marrow macrophage iron retention in patients carrying the A77D, G80S and Val 162del ferroportin variants; and a rarer non‐classic form, associated with liver iron overload but normal spleen and bone marrow iron content in patients with the N144H mutation. Study on MRI and liver content. No data on ferritin or bone marrow |
| Porter 2016 | Data on liver iron concentration, transferrin saturation, predose labile plasma iron and their relationship to serum ferritin were collected before and after 1 year of deferasirox treatment in 1530 patients with thalassaemia major (n = 1114), myelodysplastic syndromes (n = 336), and sickle‐cell disease (n = 80). Baseline and end of study serum ferritin values showed a clear and similar relationship to liver iron concentration for all disease groups. The liver iron content was determined by magnetic resonance and not liver biopsy. |
| Porter 2017a | This study compared ferritin and liver iron content in 317 patients with transfusion‐dependent thalassaemia before and after 1 yr of deferasirox. Serum ferritin decreases occurred in 73% of patients, 80% of whom also had decreased LIC. Serum ferritin response was accompanied by decreased liver iron content in 89% and 70% of cases when serum ferritin was lower than 4000 and higher than 4000 µg/L, respectively. The liver iron content was determined by magnetic resonance and not liver biopsy. |
| Porter 2017b | Biomarkers of iron metabolism were examined in 166 non‐transfusion‐dependent thalassaemia patients with b thalassaemia intermedia (n = 95), haemoglobin (Hb) E/b thalassaemia (n = 49) and Hb H syndromes (n = 22). Liver iron concentration, serum ferritin, transferrin saturation and non‐transferrin‐bound iron were elevated and correlated across diagnostic subgroups. The liver iron content was determined by magnetic resonance and not liver biopsy. |
| Powell 2008 | Prevalence of bleeding gastrointestinal lesions in 100 anaemic patients without evidence of iron deficiency (non‐iron deficiency anaemia group) and 271 patients with confirmed iron‐deficiency anaemia. No data on bone marrow |
| Rao 1985 | In this abstract, the authors reported that clinical data and liver histology for iron overload were analysed in 74 renal allograft recipients. Of these, 20 had histological evidence of haemosiderosis and four patients had haemochromatosis. Of the two noninvasive diagnostic tests, the serum ferritin level was more reliable than percent saturation of transferrin in predicting the histological diagnosis of haemosiderosis. Of the 20 patients with haemosiderosis, 14 died either from liver failure or concomitant sepsis. The abstract did not contain extractable data. |
| Rao 2013 | This study included 60 patients with sickle‐cell anaemia from the adult haematology clinic of the Cook County Hospital, USA. Very limited information in the abstract available. No 2 x 2 table data available |
| Rehu 2011 | This study included 58 iron‐deficiency anaemia patients, 129 anaemia of chronic disease patients and 63 controls, on whom bone marrow examination and blood count with percentages of hypochromic red blood cells and cellular haemoglobin in reticulocytes had been performed. Receiver operating characteristic analyses with area under the curve were used as statistical tests. Data on bone marrow iron content related to blood count with percentages of hypochromic red blood cells and cellular haemoglobin in reticulocytes. No data on ferritin |
| Remacha 2012 | This was a review on diagnosis of iron status without usable data on ferritin or bone marrow. |
| Rimon 2002 | 49 consecutive male and female patients older than 80 years who were admitted to an acute geriatric department. Bone marrow aspirate confirmed iron‐deficiency anaemia in all 49 patients. Fourteen additional patients, also older than 80 years, with anaemia but without evidence of iron deficiency on results of bone marrow examination, served as a control group. All patients underwent evaluation by means a medical history and results of complete physical examination, routine blood tests and bone marrow aspirates. Only 8 patients could be diagnosed as having iron‐deficiency anaemia by means of routine blood test results (serum iron, ferritin, and transferrin saturation levels). Iron deficiency was diagnosed by “laboratory test results” when serum iron, transferrin saturation and ferritin levels were all abnormal. It was not possible to separate data from ferritin to correlate with bone marrow aspirates. |
| Rioja 1990 | Iliac crest (bone marrow) biopsies from patients with thalassaemia major to assess bone deformities. This study assessed hepatic iron overload. There was no 2 x 2 table with data available. |
| Rocchi 1993 | Comparison of ferritin and liver iron concentration in patients with porphyria cutanea tarda. However, no comparison between ferritin and liver iron concentration given and therefore there were no extractable data. |
| Rushton 2010 | This was a review on iron ferritin and haemoglobin with data on iron content in bone marrow and ferritin concentrations that was already individually extracted from the original source for most of the authors. |
| Sahoo 2012 | This was an abstract presenting limited data on 70 patients with chronic infection, autoimmune disorders or malignancy admitted to different departments of a hospital during 2010‐2012, who were included and tested for routine haematological profile, erythrocyte sedimentation rate, serum iron, serum ferritin and bone marrow aspiration. All cases showed either an increase or normal bone marrow iron store and increased values for serum ferritin. No complete publication yet and data unavailable |
| Saliba 2017 | This study of cross‐sectional design, from previously published studies, evaluated 71 patients with serum ferritin levels ranging between 300 and 800 ng/mL attending centers in Italy, Lebanon, Oman, and Thailand. The assessment of iron conccentrations in these studies was done by magnetic resonance imaging. |
| Shet 2010 | This study included 23 patients with iron‐deficiency anaemia (n = 23, 15 females and 8 males) and 15 healthy subjects with no previous medical and haematological disease and no history of medication or tobacco use. This was a study on glutathionyl haemoglobin in iron deficiency anaemia, without data on bone marrow or ferritin. |
| Siddappa 2007 | Serum ferritin and gestational age data at birth, from 457 low‐risk preterm and term infants of 23–41 weeks gestation obtained from 35 published studies reviewed from a period of 25 years and from recently collected data, were assessed by regression analysis. Umbilical cord serum ferritin concentrations increased with advancing gestational age, from a mean of 63 μg/L at 23 weeks to 171 μg/L at 41 weeks gestation (P < 0.001). Normative cord serum ferritin data may permit a more precise assessment of infants who are at risk for abnormal iron status at birth. |
| Silva 2005 | Serum markers of iron stores are frequently increased in chronic hepatitis C virus‐infected carriers but the real impact of the hepatic iron overload is poorly understood. Liver steatosis was associated with serum iron, transferrin saturation, and ferritin. Ferritin and liver iron data were not presented in direct comparison or association. |
| Sivgin 2016 | A total of 50 allogeneic haematopoietic stem cell transplant recipients, with transfusional iron overload in Erciyes Stem Cell Transplantation Hospital, Erciyes University, Turkey between 2004 and 2011, were enrolled in the study. The liver biopsy specimens had been obtained from the archives of Erciyes University, Department of Pathology and stained for iron content. |
| Smieja 1996 | 183 consecutive patients 65 years of age or older were admitted between April 1992 and March 1993 to the medical clinical teaching unit in a secondary care hospital in Ontario, Canada, to determine the proportion of anaemic patients for whom adequate investigations were performed (measurement of serum ferritin level, bone marrow and endoscopy of upper or lower gastrointestinal tract). Data on ferritin, but only 5 bone marrow aspirates were recorded and there was no correlation of ferritin values with bone marrow iron content. |
| Smith 1998 | Study comparing liver iron by biopsy (Perl's stain) to ferritin in patients with biopsy proven hepatitis C virus. Patients with secondary iron overload were excluded. Study excluded because no 2 x 2 data were available for extraction. |
| Smith 2014 | Investigated role of ferritin in detecting iron overload in children with sickle‐cell disease. The study was excluded as all patients were transfusion‐dependent, and no extractable data for cross tabulation for diagnostic test accuracy (2 X 2 DTA table). |
| Sokal 1986 | This study included 149 patients with chronic granulocytic leukaemia (n = 46), acute nonlymphocytic leukaemia (n = 61) and Hodgkin's disease (n = 42). Stainable iron was absent or decreased in 36 of 45 bone marrow biopsy specimens (80%) among 33 patients with chronic‐stage chronic granulocytic leukaemia. This was a retrospective study aimed at determining bone marrow iron content in patients with chronic granulocytic leukaemia, acute non‐lymphocytic leukaemia and Hodgkin's disease. Ferritin was determined in 8 out of 149 without correlation data with bone marrow. |
| Stancu 2010 | In this study, 100 non‐dialysis patients who had chronic kidney disease and anaemia, iron‐naive, and were taking erythropoiesis‐stimulating agents (medications which stimulate the bone marrow to make red blood cells). Bone marrow iron stores were evaluated by aspiration. Haemoglobin, transferrin saturation index and ferritin were measured at baseline and 1 month after 1000 mg of intravenous iron sucrose. Post‐test predictive values for the erythropoietic response of peripheral and central iron indices were calculated. This was a diagnostic study evaluating the value of iron reserves to predict the response to intravenous iron in non‐dialysis patients with chronic kidney disease and anaemia. No usable data correlating ferritin and bone marrow |
| Stein 2010 | In this study, 114 patients with type‐1 Gaucher disease were assessed for serum ferritin, transferrin saturation and HFE genotype. The results were correlated with the extent of hepatosplenomegaly, overall Gaucher disease severity score index, and response to enzyme replacement therapy. Liver biopsy was performed in a subset. The study was excluded as no data comparing ferritin and liver iron were presented. |
| Sucak 2008 | Investigated the role of liver biopsy in determining the cause of elevated liver enzymes and its impact on the management of patients in the post‐hematopoietic stem cell transplantation setting. All patients had transfusion‐induced liver iron overload. |
| Sumida 2007 | Insulin resistance and hepatic iron overload are frequently demonstrated in hepatitis C virus‐related liver diseases; the study, therefore, investigated the relationship between insulin resistance and hepatic iron deposition in 56 patients with chronic hepatitis C virus infection. Hepatic iron overload may be associated with insulin resistance in patients with chronic hepatitis C, especially in patients with mild to moderate fibrosis. The study was excluded as there were no data on comparison between ferritin and liver iron. |
| Sutor 2002 | This was a case study on a 69‐year‐old anaemic woman with refractory anaemia. There was an excessive elevation of serum ferritin. A bone marrow biopsy and aspirate led to the diagnosis of a myelodysplastic condition. In this case, there was an uncommon association between sideroblastic anaemia and secondary haemochromatosis. This report did not report usable data on ferritin or bone marrow. |
| Taher 2005 | Study of the effects of deferiprone (iron chelation) on liver iron loading. Excluded as all patients were transfusion dependent. |
| Tan 2011 | Investigated the clinical utility of directly measured serum hepcidin as a quantitative biomarker of 210 hepatic iron in patients with chronic liver disease. The study found that the ratio of hepcidin to ferritin could identify cases of hepatic iron in liver disease. Excluded as no comparison data presented comparing ferritin with liver iron |
| Tan 2012 | This study investigated the potential role of serum hepcidin as a biomarker of advanced liver disease. Serum hepcidin was measured in 332 adults with chronic liver disease of varying aetiologies, 45 healthy and 50 non‐liver disease patient controls. Liver biopsy data were available for 228 chronic liver disease subjects. Hepcidin was decreased in patients with chronic liver disease compared with non‐liver disease patient controls (P < 0.0001) but not healthy controls, and was lowest in those with cirrhosis (P < 0.0001). Excluded as no comparison between ferritin and liver iron presented. |
| Thomason 2009 | This was a report on six cases (3 women and 3 men aged 44 to 80 years) who received parenteral iron and, at one or more points in follow‐up, were found to have low or borderline low serum ferritin levels and/or serum iron levels, even though marrow aspirate smears revealed abundant stainable iron in a pattern characteristic of prior parenteral iron therapy (2 patients received iron dextran (INFeD), 2 sodium iron gluconate (Ferrlecit®), and 2 iron sucrose (Venofer®)). Ferritin was determined prior to iron administration and bone marrow biopsies were performed after receiving iron. No usable data correlating ferritin and bone marrow |
| Thorsteinsson 2010 | All patients (n = 89) undergoing bone marrow aspiration in the FSA Hospital, Akureyri, Iceland, in the period 1999 to 2003. The sensitivity, specificity, efficiency, and Youden index of ferritin, mean corpuscular volume, CHr, sTfR, sTfR‐ferritin‐index, and the iron saturation of transferrin were calculated by the Thomas‐Plot method. The complete absence of stainable iron in bone marrow was used as the definitive marker of iron depletion. There was no 2 x 2 table of data available. |
| Tsironi 2008 | Study of the effects of deferiprone on liver and cardiac iron. Excluded as all patients had thalassaemia major i.e. were transfusion‐dependent. |
| Valberg 1975 | In this abstract, authors reported assessing iron metabolism in patients with iron overload and in control subjects with cirrhosis but no excess body iron. In 4 patients with advanced iron overload studied late in the course of their illness, excess haemosiderin was present in both bone marrow and liver. In contrast, 2 patients with idiopathic haemochromatosis whose excess iron had been depleted by phlebotomy subsequently developed progressive hepatic parenchymal and reticuloendothelial deposition of iron, yet marrow haemosiderin remained sparse. There was no additional extractable data from this abstract. |
| Valberg 1980 | This was a review on the uses and clinical significance of ferritin. No original data on ferritin and bone marrow |
| Valenti 2003 | Aim to determine whether increased iron parameters/heterozygosity for the mutations of the HFE gene confer susceptibility to nonalcoholic fatty liver disease. 134 consecutive Italian patients were studied with clinical and ultrasonographic diagnosis of nonalcoholic fatty liver disease (82 with hyperferritinemia), half confirmed by liver biopsy. Excluded as no comparison between ferritin and liver iron presented |
| Valenti 2006 | The study evaluated the prevalence of the common alpha‐1 anti trypsin PiS/PiZ mutants in 353 patients with nonalcoholic fatty liver disease, 195 of whom had hyperferritinaemia versus 114 matched controls and their influence on iron metabolism and the severity of liver damage in the 212 patients submitted to biopsy. Excluded as no comparative data for liver iron and ferritin |
| Van Mook 2001 | Prospective and descriptive study of 16 cases of macrocytic anaemia with anisocytosis with erythrogram suggestive of sideroblastic changes in the bone marrow. This was a meeting presentation without usable data on bone marrow or ferritin. |
| Vassalle 2018 | A total of 369 thalassaemia major patients (187 men; 33 ± 6 years) were retrospectively studied, from the myocardial iron overload in thalassaemia electronic databank, to evaluate the relationship between uric acid, hepatic and cardiac iron overload (assessed using T2* magnetic resonance imaging), ferritin, endocrinological diseases and cardiac complications. |
| Velasco‐Rodriguez 2014 | Prospective and descriptive study of 16 cases of macrocytic anaemia with anisocytosis with erythrogram suggestive of sideroblastic changes in the bone marrow. Meeting presentation without usable data on bone marrow or ferritin |
| Voigt 2008 | This was a case study with no usable data on ferritin or bone marrow. |
| Vreugdenhil 1989 | Erythrocyte and serological parameters were assessed in 44 anaemic rheumatoid arthritis patients to detect iron deficiency as assessed by stainable bone marrow iron. The anaemia was normochromic normocytic in 60% and hypochromic normocytic in 30% of those with anaemia of chronic disease. Serum ferritin was the most sensitive (82%) and valid (86%) test. There was no 2 x 2 table of data available. |
| Vreugdenhil 1990b | Ferritin was measured by solid phase enzyme immunoassay (Ferrizyme, Abott Labs, Chicago, USA). Bone marrow was aspirated after sternal puncture in the anaemic patients. Iron content was measured by Perls' Prussian blue. A stainable iron content of 0‐1 on a semi‐quantitative scale was considered as iron deficiency. The iron deficiency of anaemia of chronic disease (ACD) was distinguished by ferritin concentration, which was higher in that group. No 2 X 2 table of sensitivity and specificity data. Data on ferritin concentration of non‐anaemic, iron deficient and ACD patients only |
| Walter 2008 | Compared liver iron in patients treated with deferasirox. However, excluded as all patients were transfusion dependent. |
| Walters 1973 | Twenty‐two apparently healthy normal adult subjects (12 males, 10 females; aged 19 to 46 years and 22 to 40 years, respectively) were included in this study. Serial phlebotomies of approximately 400 mL were performed at weekly intervals. After each phlebotomy, the volume of blood removed was measured and its haemoglobin concentration determined. Serum iron, total iron binding capacity, and ferritin concentrations were also determined. Serum ferritin concentration was measured by using the immunoradiometric assay and the amount of storage iron was calculated by the method of Haskins, Stevens, Finch, and Finch. Initial serum ferritin concentrations ranged from 2 to 83 µg/L in the females (mean 35.6 µg/L) and from 36 to 224 µg/L in the males (mean 103 µg/L). There was a significant difference between the male blood donors and non‐donors, with mean values of 64 and 132 µg/L, respectively. Storage iron in female subjects was 0‐340 mg (mean 210 mg) and in males 140‐1390 mg (mean 690 mg). The male blood donors again gave significantly lower results with mean stores of 400 mg compared with 900 mg in the non‐donors. There was a close correlation between the serum ferritin concentration and mobilisable iron stores expressed by the equation y = 11.8 + 0.13x, where y was the ferritin concentration in µg/L and x was the storage iron in mg. The correlation between the calculated total circulating ferritin and iron stores was also very similar (r = 0.843, P < 0.001). There were no data on bone marrow or liver biopsy. |
| Wang 2009 | Five patients (1 male and 4 females), with congenital dyserythropoietic anaemia type I, whose clinical and haematological features were analysed retrospectively and reported. One patient had congenital malformations, 3 jaundice and 4 hepatosplenomegaly. Bone marrow specimens invariably showed hypercellularity due to erythroid hyperplasia with megaloblastic changes. Serum ferritin levels were increased in 3/4 patients. There was no extractable 2 x 2 table of data available. |
| Wang 2014 | This study aimed to elucidate the role and mechanisms of hepcidin in hepatic iron deposition and liver injury in chronic hepatitis C. Serum hepcidin‐25 was positively correlated with hepatic hepcidin protein expression in the liver sections, and negatively correlated with serum ferritin, degree of hepatic iron deposition and fibrosis (P < 0.05). Serum levels and hepatic expressions of 8‐OHdG and IL‐6 were significantly increased, while STAT3 and INF‐alpha were decreased in chronic hepatitis C patients more than those in the controls (P < 0.05). This was a meeting abstract with no further data to establish whether data on ferritin/ liver iron comparisons were available. |
| Whiting 2002 | This study compared the iron indices of 35 C282Y homozygous and 35 C282Y heterozygous same‐sex sibling pairs. It found that concordance of iron indices exists in C282Y homozygote and heterozygote sibling pairs. Siblings expressing C282Y heterozygotes require phenotypic assessment. Excluded as no comparative data between ferritin and liver iron |
| Wickenhauser 2011 | 90 patients (48 males/42 females, median age 57 years) with bone marrow taken 24 days before allogeneic hematopoietic cell transplantation. Median serum ferritin was 2009 µg/L with 25% of patients having a serum ferritin higher than 3000 µg/L with transfusional iron overload, with iron deposited in the bone marrow both interstitially and in macrophages. Iron overload measured by an elevated serum ferritin was accompanied by augmented marrow iron stores but the correlation remained poor. No usable data comparing ferritin to bone marrow, and results indicating iron overload. |
| Wu 1989 | Serum ferritin, erythrocyte protoporphyrin, serum iron, total iron binding capacity and transferrin saturation were measured in 92 anaemic patients who were classified as having iron deficient anaemia and non‐iron deficient anaemia by their bone marrow iron status. There were three models of a combination of tests designed by varying the test combination and diagnostic levels in model 1 and 2 and multiple regression analysis in model 3. This abstract indicated only sensitivity/specificity analysis but of combinations of biomarkers. There was no 2 x 2 table of data available. |
| Wulfhekel 1999 | Assessment of iron in plasma cells rather than in macrophages which is the standard measure of bone marrow iron stores |
| Xian 2012 | 59 patients with haematologic malignant disease and 43 healthy volunteers were enrolled. Routine blood, routine bone marrow and iron bound on bone marrow cells and serum ferritin, serum transferrin, serum transferrin receptor before and after treatment were measured. The level of sTfR in the non‐remission group after chemotherapy was higher than that of control. Correlation analysis showed that there was positive correlation between the serum ferritin and the original and naive bone marrow cells (r = 0.347). And there was negative correlation between serum ferritin and haemoglobin (r = ‐0.207). However there was enough diagnostic test accuracy data to build the corresponding 2 x 2 table. Thus, exclude for lack of DTA data. |
| Yanardag 2002 | Study of bone marrow infiltration by granulomatous pathology, rather than diagnostic test accuracy study of iron stores vs ferritin. |
| Zadrazil 1991 | Patients on dialysis, hence excluded |
ACD:anaemia of chronic disorders AFP: alpha‐fetoprotein levels βTT: β‐thalassaemia trait CHr: reticulocyte hemoglobin content DTA: diagnostic test accuracy dw: dry weight ELISA: enzime‐linked immunosorbent assay Fe: Iron fL: femtoliters, equivalent to 10 exp (‐15) liters Hb: haemoglobin HFE: human haemochromatosis protein HH:chronic viral hepatitis HHC: hereditary haemochromatosis HII: hepatic iron index HIV:human immunodeficiency virus LIC: liver iron concentration LTc: low temperature MDS: myelodysplastic syndrome MRI: magnetic‐resonance imaging pg: picogram RAEB(‐t): Refractory anaemia with excess of blasts in transformation R2: transverse relaxation rate, process by which the transverse components of magnetization decay saTf: Transferrin saturation sTfR: soluble transferrin receptor T2*: spin relaxation time is a time constant characterizing the signal decay TIBC: total iron binding capacity Tf: transferrin TM: Thalassemia major vs: versus WHO: World Health Organization
Characteristics of studies awaiting classification [ordered by study ID]
Anupama 2017.
| Patient Sampling | All consecutive patients who had been diagnosed as having dimorphic anaemia, refractory anaemia, pancytopenia, unexplained thrombocytopenia, megaloblastic anaemia, myelodysplastic syndrome and myeloproliferative disorder were considered for bone marrow aspiration and included in our study. Patients with infections, inflammatory conditions, malignancy, hepatocellular diseases, chronic kidney disease and those undergoing blood transfusion, parenteral or oral iron supplementation one week prior to bone marrow aspiration were excluded. |
| Patient characteristics and setting | 39 study participants were patients aged 18–76 years of age (23 male), who had undergone bone marrow aspiration or biopsy as part of the diagnostic work up for their illness, attending a tertiary care centre in South India over a period of one year from September 2012 to September 2013. |
| Index tests | Serum ferritin analysis from blood sample collected by peripheral venipuncture, using ELISA |
| Target condition and reference standard(s) | Bone marrow samples were analysed with Prussian blue staining and scoring of Perl’s staining assessed by Gale’s method by an experienced pathologist at the tertiary care centre. Aspirate was obtained from the posterior superior iliac spine observing strict asepsis. |
| Flow and timing | Blood sample was collected on patients who had been selected for bone marrow aspirate. |
| Comparative | Receiver operating characteristic (ROC) curve analysis was done to justify a maximum ferritin value in the diagnosis of iron deficiency. |
| Notes | Median serum ferritin among those with iron sufficiency was 737.9 pmol/L and with iron deficiency 57.7 pmol/L. Sensitivity (65.4%) and specificity (92.3%) of a microcytic hypochromic picture in the smear was likewise evaluated for diagnosing iron deficiency, based on bone marrow iron stain results. |
Balan 1994.
| Patient Sampling | For a period of 12 consecutive weekdays in October 1990, serum iron levels were determined in 12,258 consecutive blood samples from patients attending Mayo Clinic, Rochester, Minnesota, USA for diagnostic laboratory studies. |
| Patient characteristics and setting | The majority were of European ancestry; relatively few were of Afro‐American, Asian, Middle Eastern, or Hispanic extraction. The male‐female ratio was nearly 1:1, with the mean age of 58 years of age for men and 56 years of age for women. |
| Index tests | Serum ferritin (Magic FER, Ferritin [125I] radioimmunoassay; Ciba‐ Corning Diagnostics Corp., Medfield, MA, USA) |
| Target condition and reference standard(s) | Liver biopsy was advised in patients whose transferrin saturation exceeded 62% and whose ferritin level exceeded 400 pg/L on the second determination, unless biopsy was contraindicated by the patient’s clinical condition. The procedure was performed transthoracically with a Tru‐cut needle (Travenol Laboratories, Deerfield, IL, USA). Each specimen was examined histologically after staining with H & E, Masson trichrome, and Perl's Prussian blue stains. Also, a portion of the biopsy specimen was used for measurement of hepatic iron concentration by atomic absorption spectrophotometry. |
| Flow and timing | Eight patients with elevated serum transferrin and ferritin levels on repeat determination were considered candidates for liver biopsy. In none of the 8, had iron overload been suspected before inclusion in this study. |
| Comparative | One hundred twenty‐seven patients had an initial serum iron concentration of 180 µg/dL or higher. |
| Notes | Eight patients (aged 38‐71 years; 7 men and 1 woman) had transferrin saturation 62% or higher (range, 84‐99) and serum ferritin value 400 µg/L (range, 457‐4004) with no other explanation for the abnormal iron test results. Three patients (2 male and 1 female) had markedly elevated hepatic iron concentration (range, 11080‐29719 µg/g dry wt) and hepatic iron index (range, 2.9‐8.4) indicative of homozygous haemochromatosis. |
Cai 2017.
| Patient Sampling | Adults attending the Peking Union Medical College Hospital and National Institute of Nutrition for Health, Chinese Center for Disease Control and Prevention, China |
| Patient characteristics and setting | 140 patients who needed to undergo a bone marrow aspiration for diagnosis in the Haematology Department were divided into three groups according to haemoglobin levels and bone marrow iron staining results: the iron‐deficiency anaemia group (bone marrow iron staining showed negative, Hb < 110 g/L (female) and Hb < 120 g/L (male)); the non‐iron deficiency anaemia group (bone marrow iron staining showed positive, Hb < 110 g/L (female) and Hb < 120 g/L (male)); and the control group (bone marrow iron staining showed positive, Hb ≥ 110 g/L (female) and Hb ≥ 120 g/L (male)). |
| Index tests | Serum ferritin and other biochemical assays of iron status and inflammation markers were performed using an automatic biochemical analyser (7180 Hitach, Ibarakiken, Japan), from venous blood samples. |
| Target condition and reference standard(s) | Bone marrow samples were obtained for the bone marrow iron staining. Prussian blue staining was used to observe the iron in the reticulocytes. The results for “+/++/+++” were defined as positive results and “‐” was defined as negative. |
| Flow and timing | Both tests appeared to be conducted during the same period, with bone marrow aspirates following the venous blood sample tests. |
| Comparative | In this study, bone marrow iron staining results were compared with the efficiency of serum ferritin and other indexes in the diagnosis of iron‐deficiency anaemia, and suitable threshold values were explored. |
| Notes | The iron‐deficiency anaemia group (n = 56) had significantly lower reticulocyte haemoglobin content, mean cellular volume, corpuscular haemoglobin concentration, haemoglobin content, and serum ferritin; and higher free erythrocyte protoporphyrin, unsaturated iron‐binding capacity, and serum transferrin receptor (P < 0.05) compared with the non‐iron deficiency anaemia group (n = 38) and control group (n = 46). Haematocrit, serum ferritin, and unsaturated iron‐binding capacity were significantly affected by inflammation while reticulocyte haemoglobin content and other parameters were not. |
Cippà 2014a.
| Patient Sampling | We analysed 147 liver biopsies, including measurement of hepatic iron concentration, performed at the University Hospital, Zurich, from 1997 to 2010 to identify clinical and laboratory predictors of iron overload in patients with elevated serum ferritin. |
| Patient characteristics and setting | The study population consisted of 147 patients (mean age, 51 years; 80% male) after 3 patients were excluded because of severe undernutrition, fulminant toxic‐liver necrosis, and liver transplantation. |
| Index tests | Hyperferritinaemia (serum ferritin higher than 300 µg/L). Aspartate transaminase and transferrin saturation were also measured. |
| Target condition and reference standard(s) | Authors indicated that elevated serum ferritin alone was not appropriate to estimate tissue iron overload in this patient population and that additional consideration of a surrogate of hepatocyte damage was critical to evaluate iron status in patients with liver disease and moderate hyperferritinaemia. |
| Flow and timing | Liver biopsies were done in patients with suspected iron overload by serum ferritin and transferrin saturation. |
| Comparative | Authors stated that "The aspartate transaminase was superior to alanine transaminase, presumably because of the different impact of haemochromatosis, alcoholic liver disease, and nonalcoholic steatohepatitis on aspartate transaminase and alanine transaminase elevation." |
| Notes | Authors concluded that "only high levels of serum ferritin (> 2000 µg/L) were predictive of iron overload, "and that elevated serum ferritin alone is not appropriate to estimate tissue iron overload in this patient population and that additional consideration of a surrogate of hepatocyte damage is critical to evaluate iron status in patients with liver disease and moderate hyperferritinaemia." |
Grote Beverborg 2018.
| Patient Sampling | 50 patients who were scheduled for coronary artery bypass graft surgery at the University Medical Center Groningen, Groningen, The Netherlands, with a history of heart failure with an N‐terminal pro‐B‐type natriuretic peptide of > 125 pg/mL and reduced left ventricular ejection fraction (45% or less) assessed by an echocardiogram (n = 49) or multigated acquisition scan (n = 1). |
| Patient characteristics and setting | 42 patients were included in the study. Patients were mostly male (76%) with mild‐to moderate heart failure and a mean age of 68 ± 10 years of age. |
| Index tests | Serum ferritin was measured using standard methods on a Roche modular Cobas 8000 (Roche Diagnostics, Indianapolis) from fresh venous blood with ethylenediaminetetraacetic acid. The detection method was electro chemiluminescence immunoassay (ECLIA). |
| Target condition and reference standard(s) | Bone marrow aspirates were taken from the sternum in patients, just before median sternotomy was performed. In a certified core laboratory, Prussian blue staining with potassium ferrocyanide was used on multiple slides per sample to assess the presence of nonheme‐bound iron in the erythroblasts and the extracellular space. The iron stores were assessed as the amount of iron present in the extracellular space and graded using the Gale histological grading method. |
| Flow and timing | It appeared as though the haematologic and blood biochemistry tests were done prior to surgery and to bone marrow aspirates. |
| Comparative | This study aimed to define and validate the biomarker‐based definition of iron deficiency in heart failure patients using bone marrow iron staining as the reference standard. |
| Notes | Bone marrow iron deficiency was found in 17 (40%) of the heart failure patients. The most commonly used definition of iron deficiency had a sensitivity of 82% and a specificity of 72%. |
Hagstrom 2016a.
| Patient Sampling | Longitudinal cohort study, setting the outcome variable as overall mortality. All liver biopsies performed at the Karolinska University Hospital, Huddinge and the University Hospital of Linkoping, both in Sweden, were stored in local pathology archives. |
| Patient characteristics and setting | All liver biopsies were performed at our clinics with the particular SNOMED code for hepatic steatosis (M50080) between 1971 and the end of 2009. All charts were scrutinised from the earliest record, through the time of biopsy, and to the death of the patient or to the end of the follow‐up period (Jan 30, 2015) to exclude liver diseases other than nonalcoholic fatty liver disease. |
| Index tests | Quantitative analysis of ferritin in serum was carried out utilising either DxI/Access with the Access Ferritin Reagent Packs (Beckman‐Coulter AB, Bromma, Sweden), or Modular E/Cobas e602, with the Elecsys Ferritin Reagent Kit (Roche Diagnostics GmbH, Mannheim, Germany). Serum ferritin was measured in 82.0% of patients using the Modular E method, with an upper limit normal value of 350 µL in males and 150 µL in females. |
| Target condition and reference standard(s) | Cases were classified as alcoholic‐liver disease if they consumed more than 30 g of alcohol per day for men, 20 g per day for women, or if binge‐drinking (defined as drinking equal to or more than five units of alcohol on the same occasion) was reported in the charts at any time. Iron overload was determined for all patients semi‐quantitatively on histopathological examination of Perl's stained liver biopsy samples as 0–4 for hepatocytes' iron content and 0–2 for reticuloendothelial system iron content. |
| Flow and timing | In all cases, the ferritin value analysed closest in time to the liver biopsy was used. Patients with a serum ferritin value above the respective cutoff were classified as high serum ferritin and patients with a value below the cutoff were classified as normal serum ferritin. |
| Comparative | Data were extracted from patient charts at the time of biopsy. Smoking at the time of biopsy was classified as ever or never. Diabetes mellitus type 1 or 2 was classified as present if the patient was taking any oral antidiabetic drug or insulin, or if there was an elevated, fasting or non‐fasting serum glucose level (> 180 mg/dL) within six months of the time of biopsy. |
| Notes | Of the 89 patients with high serum ferritin, iron staining was available in 71 cases (80%). Of these, 34 patients had positive iron staining and 37 patients had negative iron staining. Patients with high serum ferritin and positive iron staining were more likely to be men (62% vs 38%, P = 0.045) and have higher haemoglobin counts (15.2 vs 14.2 g/dL, P = 0.002). There was no significant difference in systemic inflammatory markers in patients with high serum ferritin and positive iron staining compared to high serum ferritin and negative iron staining, including C‐reactive protein (8.5 vs 6.7 µg/L, P = 0.13) and white blood cell count (6.7 vs 6.8 9 109/L, P = 0.97). |
Mehta 2016.
| Patient Sampling | Patients aged 15‐65 years, with newly diagnosed and untreated iron‐deficiency anaemia admitted to medicine wards and not suffering from any inflammatory disorders (excluded by C‐reactive protein). Patients having other forms of anaemia, haemoglobinopathies, any malignancy, having MCV > 80 fL and pregnant females were excluded from this study. |
| Patient characteristics and setting | A hospital‐based study was conducted among patients (aged 15‐65 years) with newly diagnosed and untreated iron‐deficiency anaemia admitted to medicine wards and not suffering from any inflammatory disorders (excluded by C‐reactive protein) in Jaipur, Rajasthan, India. |
| Index tests | Serum ferritin was measured on a IMMULITE 2000 Systems analyser using a solid phase, two‐site chemiluminescent immunometric assay. Serum iron was measured using colorimetric assay. TIBC was measured using the saturation‐precipitation method. Transferrin saturation was calculated as transferrin saturation = (serum iron/TIBC) x 100 and expressed as a percentage. |
| Target condition and reference standard(s) | A total of 144 patients were included in the study. Of these, 42 (29.2%) patients were excluded from final statistical analysis as their bone marrow aspirates were aparticulate and, therefore, their iron stores could not be assessed. From the bone marrow aspirates of the remaining 102 patients, Prussian blue stained films were examined and graded. These 102 study participants had a mean age of 35.63 ± 15.96 years (range 15 to 65 years) with a male:female ratio of 1:1.83. Of the 102 patients, 73 (71.6%) had no stainable iron in bone marrow (grade 0), 10 (9.8%) had grade 1 stainable iron, 8 (7.8%) had grade 2 stainable iron and 11 (10.8) had grade 3 stainable iron. |
| Flow and timing | This hospital‐based observational study was conducted at a tertiary care centre in Rajasthan, India, during September 2013 to December 2014. |
| Comparative | All the patients included in our study had microcytic hypochromic anaemia with an increased red blood cell distribution width‐curve variation, low serum ferritin and low transferrin saturation. Those with grade 0 and 1 were considered to have depleted iron stores and, therefore, represented absolute iron deficiency. Those with grades 2 and 3 in our study had functional iron deficiency. |
| Notes | Retyculocite haemoglobin was found to have significant positive correlation with serum ferritin. |
Pilo 2018.
| Patient Sampling | Retrospectively analysed data from patients with low risk, intermediate‐I myelodysplastic syndromes, who were diagnosed in our institution in Italy since 1998. |
| Patient characteristics and setting | Patients had undergone bone marrow aspiration as part of the diagnostic work‐up for their myelodysplastic syndromes. Two different experienced haematologists analysed all samples. |
| Index tests | Authors concluded in their abstract that "Perl's stain, together with ferritin and blood transfusional burden could be another marker at diagnosis of iron‐related toxicity that predicts overall survival", although there was no information on how ferritin was measured. |
| Target condition and reference standard(s) | Bone marrow aspiration slides with at least seven fragments were considered suitable. Perl's Prussian blue stain was used to stain bone marrow, assessed by modified Gale's grading. Marrow staining of 114 consecutive MDS patients was revised and analysed. Median age was 70 years (range 32‐93 years). Eighty‐three patients were IPSS low‐risk and 30 intermediate I. All patients were evaluated for bone marrow iron stores with Perl's stain. Twenty‐seven patients had grade 1 (+), 31 grade 2 (++) and 56 grade 3 (+++). |
| Flow and timing | Not reported |
| Comparative | None of these patients had received iron chelation before marrow examination. |
| Notes | Data presented in the abstracts were very limited. |
Rudnicka 2017.
| Patient Sampling | A cohort of 243 patients with confirmed chronic hepatitis C was diagnosed at the Department of Infectious Diseases Medical University of Gdansk, Poland. |
| Patient characteristics and setting | The mean patients age was 47.4 years (range 19‐77 years), with 155 males and 88 females. |
| Index tests | Serum ferritin was tested in all subjects as well as biochemical markers of liver function. Moreover several iron overload parameters were tested too. |
| Target condition and reference standard(s) | Percutaneous needle biopsy of the liver was performed in 225 cases. The histopathological evaluation of inflammatory activity, fibrosis, steatosis, and iron content was assessed in hepatocytes according to a modified Scheuer scale. |
| Flow and timing | Blood tests prior to liver biopsies were performed. |
| Comparative | The frequency of single nucleotide polymorphism and the expression levels of CYBRD1 were compared between the groups of patients with and without markers of iron overload. The groups were defined based on the threshold values of serum iron‐overload markers: iron concentration > 140 μg/dL; ferritin concentration, women > 150 ng/mL, men > 200 ng/mL; and transferrin saturation > 45%. Patients with elevated iron concentration and at least one from two measurements, ferritin concentration and transferrin saturation, were included in the iron‐overload group. |
| Notes | This study findings suggested that the influence of rs884409 on iron homeostasis may depend on the background of iron storage disease. |
Uhrig 2019.
| Patient Sampling | Between May 2015 and October 2018, 47 patients were screened for this prospective study in Germany. A set of 42 patients out of 47 screened patients with several chronic liver diseases underwent MRI examination at 1.5 T including R2‐measurements by single‐voxel high speed T2‐corrected multiecho spectroscopy, additional liver biopsy, abdominal ultrasound, controlled attenuation parameter, and room‐temperature susceptometer. |
| Patient characteristics and setting | The patients with several diffuse liver diseases underwent the study MRI examination and had additional liver biopsy, abdominal ultrasound, controlled attenuation parameter, and room‐temperature susceptometer measurements. |
| Index tests | For all patients, routine blood and serum parameters were determined, including ferritin (ng/mL). |
| Target condition and reference standard(s) | Liver biopsy was performed in the centre of the right liver lobe according to the Menghini technique. The diagnosed liver diseases included alcoholic liver disease (n = 12), nonalcoholic fatty liver disease (n = 7), haemochromatosis (n = 6), elevated ferritin or transaminase of unknown origin (n = 7), liver cirrhosis (n = 6), auto‐immune hepatitis (n = 3), and Morbus Wilson (n = 1). Authors defined moderate iron overload by any liver iron concentration in atomic absorption spectroscopy 2 mg/g or higher dry weight and severe iron overload by liver iron concentration in atomic absorption spectroscopy 4 mg/g or higher dry weight. |
| Flow and timing | Liver biopsy, controlled attenuation parameter, ultrasound, room‐temperature susceptometer, and MRI were performed within a one‐week interval. The order for the routine blood and serum parameters was not explicit in the report. |
| Comparative | The purpose of this study was to compare the susceptibility‐based methods single‐voxel MRI spectroscopy and room temperature susceptometer with ultrasound, controlled attenuation parameter, and ferritin measurements using liver biopsy as the reference standard. |
| Notes | Authors concluded that the optimal cutoff value for ferritin was 878 ng/mL, both for moderate and severe iron overload. |
Wood 2017.
| Patient Sampling | Subjects were identified at a single institution between 1964 and 2007 and were confirmed as being C282Y homozygous on the basis of genetic testing conducted prospectively or using stored samples collected prior to the identification of the HFE gene. Additional inclusion criteria included a liver biopsy with biochemical measurement of hepatic iron concentration. |
| Patient characteristics and setting | All patients were untreated at the time of their serum ferritin measurement, data collection and liver biopsy. Male subjects made up 67.4% of the study population. The mean age of the study cohort was 42.5 years (95% CI: 41‐44.1). These studies were approved by the human ethics committees of the Royal Brisbane and Women’s Hospital and the QIMR Berghofer Medical Research Institute, Brisbane, Australia. |
| Index tests | Serum ferritin and other biochemical markers of iron status were measured by standard biochemical techniques but the method was not specified. |
| Target condition and reference standard(s) | Hepatic iron concentration was measured by atomic absorption spectrophotometry on fresh specimens. Paraffin‐embedded sections were stained with hematoxylin andeosin, and Perl's Prussian blue. |
| Flow and timing | The flow and timing were unclear in the report. |
| Comparative | This study evaluated whether serum ferritin was a better predictor of hepatic fibrosis compared to variables previously associated with increased fibrosis risk in haemochromatosis. |
| Notes | Addition of serum ferritin in multivariate analysis substantially improved the predictive power of the model and was highly predictive of fibrosis stage. Inclusion of serum ferritin in this model rendered the effects of hepatic iron concentration, sex, alcohol and steatosis to non‐significance. |
ECLIA: electro chemiluminescent immunoassay ELISA: enzyme‐linked immunosorbent assay fL: femtoliters, equivalent to 10 exp (‐15) liters Hb: haemoglobin HFE: human homeostatic iron regulator protein IPSS: International Prognostic Scoring System MCV:mean corpuscular volume MPS: mucopolysaccharidoses MRI:magnetic resonance imaging R2: transverse relaxation rate, process by which the transverse components of magnetization decay ROC: receiver operating characteristic SNOMED: Systematized Nomenclature of Medicine T2: spin relaxation time is a time constant characterizing the signal decay TIBC: total iron binding capacity vs: versus wt: weight
Differences between protocol and review
We planned to include these databases in our original search strategy but we were unable to get access to them: MEDION (http://www.mediondatabase.nl); Evidence‐Based Laboratory Medicine Base (C‐EBLM) (http://www.ifcc.org/ifcc-education-division/emd-committees/c-eblm/evidence-based-laboratory-medicine-c-eblm-base/); Aggressive Research Intelligence Facility (ARIF) (http://www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx). These databases were not used in the search.
We changed the inclusion criteria for studies eligible for the iron‐overload analysis. After considering the goals of this review further, we decided that clinicians caring for patients who had undergone chronic red cell transfusions were likely to already have a high clinical suspicion of iron overload, and would not rely on ferritin alone to diagnose liver iron loading. Such patients are likely to have undergone specific complex investigations and management (including iron chelation). We thus decided to omit studies exclusively recruiting participants who had undergone chronic red cell transfusions from this review.
We stratified the analysis by participant clinical group (particularly for the iron deficiency analysis into age and pregnancy status): we found that the majority of studies were performed in patients who were at very high risk of inflammation (for example, patients with cancer, inflammatory conditions such as rheumatoid arthritis, or case series of bone marrow examinations from hospitalised patients) and, perhaps not unexpectedly, relatively few studies had recruited 'healthy' outpatient populations who were at low risk of inflammation. As discussed in the background section, as well as being a biomarker of iron status, ferritin is also an acute‐phase protein and is elevated in inflammation. As such, it was inappropriate to combine data from healthy and inflamed populations.
In addition, we realised that there are many MRI protocols used to detect liver iron overload (for example, T2/R2, signal intensity ratio). These methods have varying values used to define iron overload, and not all thresholds have been validated against tissue iron measures. As such, we decided to only include studies which measured liver iron by tissue sample, using either liver iron measurements or histology by Perl's staining.
Concerning the investigations of heterogeneity: When we wrote the protocol for the review, we proposed to study a high number of possible covariates, because we did not know which of them would be available and did not yet know that some of them would be used to stratify the target population. Thus, four covariates: physiological status, sex, age group, and pregnancy status were not further analysed as such because these parameters were used to stratify the target groups (as explained above). Many of the studies raised high concern on applicability to the review questions and selection bias. Because of this, there were not enough studies for most of the categories and covariates that we initially intended to assess in meta‐analysis and heterogeneity investigations. Also, it is considered good practice to concentrate on one or two covariates. Once we extracted the data for all the covariates from the included studies, we decided to analyse only the covariates that were consistent enough in most of the included studies to proceed to heterogeneity analysis. Hence, two covariates (place of study and anaemia status) were found to not be consistently defined in several included studies (several mixed or unknown places/status). Finally, we analysed two covariates for iron deficiency: laboratory assay and inflammation/infection. Concerning the heterogeneity analysis by disease group, because of the difficulty raised by the number of disease groups and all the possible combinations of alternative HSROC models and hypotheses, we decided instead to present a subgroup analysis.
Concerning the sensitivity analyses, in addition to the quality of evidence analyses, we had planned to perform sensitivity analyses to evaluate the influence of unclear decisions, but we realised that this was manageable only in the case of a greater proportion of clear decisions. As the low quality of the evidence and the quantity of unclear decisions was so prevalent, at the end, we only focused on sensitivity analyses of the quality of the evidence.
We had planned in our protocol to assess the diagnostic accuracy of ferritin to determine iron overload at putative threshold values other than WHO thresholds such as ferritin higher than 200 µg/L and 300 µg/L in males and females and 500 µg/L in mild iron overload (Brissot 2009). However, we decided that given the limited evidence, we made assessments only according to existing WHO values.
We compared ferritin concentrations between dichotomous reference standard states (iron deficiency/iron‐replete; iron‐overload/not iron‐overloaded). This comparison was added at the request of the WHO Guidelines Development Group.
In order to present the available data even when it was not detailed by the authors, we generated tables describing the relevant study, sample size, ferritin threshold used, number of true positives, false negatives, false positives, true negatives, mean and standard deviation of the ferritin, range of ferritin concentrations, difference between means for iron‐deficient and replete individuals, and correlations between ferritin concentrations and the reference standard. This was done for several iron‐depleted and repleted clinical groups: healthy populations, non‐healthy children, non‐healthy pregnant women, non‐healthy adults with blood disorders, non‐healthy adults with rheumatoid arthritis, non‐healthy *adults with infectious disease, non‐healthy adults with liver or renal disease or alcoholism, non‐healthy elderly adults with non specified or mixed non‐healthy conditions, non‐healthy adults with non specified or mixed diseases (specifically older than 50 or sample mean age more than 50 years old), or non‐healthy adults not otherwise specified (see tables 3.1 to 3.10 in Appendix 3). Similarly it was done for iron‐overload, and non‐overload groups (see tables 3.12 to 3.15 in Appendix 3). Note: *adults: This group was defined as adults, excluding those studies focused exclusively on pregnant women. **(P)prevalence was defined as prevalence of included studies in this category.
To incorporate relevant data from excluded studies either for iron deficiency or iron overload, we generated tables to report the sample size, ferritin thresholds, reference standards used in the paper, number of true positives, false negatives, false positives, true negatives, mean and standard deviation of ferritin, range of ferritin value reported, correlations between ferritin and the reference standard, and other available statistics. These data are shown in tables 3.16 to 3.17 in Appendix 3.
Contributions of authors
Maria Nieves Garcia‐Casal drafted the initial protocol with technical input from Juan Pablo Peña‐Rosas and Sant‐Rayn Pasricha. Ricardo X Martinez and Lucero D Lopez‐Perez prepared the methods for statistical analysis. Maria Nieves Garcia‐Casal extracted the data for iron deficiency and Sant‐Rayn Pasricha for iron overload. Ricardo X Martinez and Lucero D Lopez‐Perez rechecked the extracted data, carried out the statistical analysis, drafted the final manuscript and provided input into the interpretation of results. All authors provided input and contributed to drafting the final version of the review. All authors edited the final manuscript.
Juan Pablo Peña‐Rosas has overall responsibility for this review. This review has aimed to provide evidence‐based information to contribute to the WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations (WHO 2020).
Sources of support
Internal sources
Department of Nutrition and Food Safety, World Health Organization, Switzerland
External sources
-
US Centers for Disease Control and Prevention (CDC), USA
The World Health Organization gratefully acknowledges the financial contribution of the International Micronutrient Malnutrition Prevention and Control (IMMPaCt) Program, Division of Nutrition, Physical Activity and Obesity for this work.
-
The Bill & Mellinda Gates Foundation, USA
The World Health Organization gratefully acknowledges the financial contribution of the Bill & Mellinda Gates Foundation for the work on systematic reviews of the evidence in nutrition.
-
Department of Nutrition and Food Safety, World Health Organization, Switzerland
Dr Lucero Lopez, Dr Ricardo Martinez and Dr Sant‐Rayn Pasrisha received partial financial support from Department of Nutrition and Food Safety, World Health Organization for this work.
Declarations of interest
Disclaimer: Juan Pablo Peña‐Rosas and Maria Nieves Garcia‐Casal are full‐time staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization. WHO gratefully acknowledges the financial contribution of the Bill & Melinda Gates Foundation, Nutrition International, the Centers for Disease Control and Prevention (CDC), the US Agency for International Development (USAID), towards the work in the area of biomarkers of nutrition.
Sant‐Rayn Pasricha has received an unrestricted research grant as a co‐investigator from Vifor Pharma Ltd and has served as a consultant to the Australian Red Cross Blood Service. Dr Pasricha received partial financial support from the Department of Nutrition and Food Safety, World Health Organization for this work.
Ricardo X. Martinez is a consultant at WHO. He received partial financial support from the Department of Nutrition and Food Safety, World Health Organization for this work.
Lucero D. Lopez‐Perez is an independent consultant with no known conflicts of interest. She received partial financial support from the Department of Nutrition and Food Safety, World Health Organization for this work.
New
References
References to studies included in this review
Aguilar 2012 {published data only}
- Aguilar R, Moraleda C, Quintó L, Renom M, Mussacate L, Macete E, et al. Challenges in the diagnosis of iron deficiency in children exposed to high prevalence of infections. PLOS One 2012;7(11):e50584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 1978 {published data only}
- Ali M, Luxton A, Walker W. Serum ferritin concentration and bone marrow iron stores: a prospective study. Canadian Medical Association Journal 1978;118(8):945-6. [PMC free article] [PubMed] [Google Scholar]
Baillie 2003 {published data only}
- Baillie FJ, Morrison AE, Fergus I. Soluble transferrin receptor: a discriminating assay for iron deficiency. Clinical and Laboratory Haematology 2003;25(6):353-7. [DOI] [PubMed] [Google Scholar]
Balaban 1993 {published data only}
- Balaban EP, Sheehan RG, Demian SE, Cox JV, Frenkel EP. Evaluation of bone marrow iron stores in anemia associated with chronic disease: a comparative study of serum and red cell ferritin. American Journal of Hematology 1993;42(2):177-81. [DOI] [PubMed] [Google Scholar]
Barron 2001 {published data only}
- Barron BA, Hoyer JD, Tefferi A. A bone marrow report of absent stainable iron is not diagnostic of iron deficiency. Annals of Hematology 2001;80(3):166-9. [DOI] [PubMed] [Google Scholar]
Bârsan 2015 {published data only}
- Bârsan L, Stanciu A, Stancu S, Căpuşă C, Brătescu L, Mandache E, et al. Bone marrow iron distribution, hepcidin, and ferroportin expression in renal anemia. Hematology 2015;20(9):543-52. [DOI] [PubMed] [Google Scholar]
Baumann Kurer 1995 {published data only}
- Baumann Kurer SB, Seifert B, Michel B, Ruegg R, Fehr J. Prediction of iron deficiency in chronic inflammatory rheumatic disease anaemia. British Journal of Haematology 1995;91(4):820-6. [DOI] [PubMed] [Google Scholar]
Brink 1982 {published data only}
- Brink S, Van Schalkwyk DJ. Serum ferritin and mean corpuscular volume as predictors of bone marrow iron stores. South African Medical Journal 1982;61(12):432-4. [PubMed] [Google Scholar]
Brown 1988 {published data only}
- Brown RD, Benfatto J, Gibson J, Kronenberg H. Red cell ferritin and iron stores in patients with chronic disease. European Journal of Haematology 1988;40(2):136-41. [DOI] [PubMed] [Google Scholar]
Burns 1990 {published data only}
- Burns E, Goldberg N, Lawrence C, Wenz B. Clinical utility of serum tests for iron deficiency in hospitalized patients. American Journal of Clinical Pathology 1990;93(2):240-5. [DOI] [PubMed] [Google Scholar]
Chang 2007 {published data only}
- Chang J, Bird R, Clague A, Carter A. Clinical utility of serum soluble transferrin receptor levels and comparison with bone marrow iron stores as an index for iron-deficient erythropoiesis in a heterogeneous group of patients. Pathology 2007;39(3):349-53. [DOI] [PubMed] [Google Scholar]
Chapman 1982 {published data only}
- Chapman RW, Morgan MY, Laulicht M, Hoffbrand AV, Sherlock S. Hepatic iron stores and markers of iron overload in alcoholics and patients with idiopathic hemochromatosis. Digestive Diseases and Sciences 1982;27(10):909-16. [DOI] [PubMed] [Google Scholar]
Cippa 2014 {published data only}
- Cippà PE, Boucsein I, Adams H, Krayenbuehl PA. Estimating iron overload in patients with suspected liver disease and elevated serum ferritin. American Journal of Medicine 2014;127(10):1011.e1–1011.e3. [DOI] [PubMed] [Google Scholar]
Coenen 1991 {published data only}
- Coenen JL, Van Dieijen-Visser MP, Van Pelt J, Van Deursen CT, Fickers MM, Van Wersch JW, et al. Measurements of serum ferritin used to predict concentrations of iron in bone marrow in anemia of chronic disease. Clinical Chemistry 1991;37(4):560-3. [PubMed] [Google Scholar]
Forman 1980 {published data only}
- Forman D, Malcom V. Immunoradiometric serum ferritin concentration compared with stainable bone-marrow iron as indices to iron stores. Clinical Chemistry 1980;26(1):145-7. [PubMed] [Google Scholar]
Guyader 2007 {published data only}
- Guyader D, Thirouard AS, Erdtmann L, Rakba N, Jacquelinet S, Danielou H, et al. Liver iron is a surrogate marker of severe fibrosis in chronic hepatitis C. Journal of Hepatology 2007;46(4):587-95. [DOI] [PubMed] [Google Scholar]
Guyatt 1990 {published data only}
- Guyatt GH, Patterson C, Ali M, Singer J, Levine M, Turpie I, et al. Diagnosis of iron-deficiency anemia in the elderly. American Journal of Medicine 1990;88(3):205-9. [DOI] [PubMed] [Google Scholar]
Hagström 2016 {published data only}
- Hagström H, Nasr P, Bottai M, Ekstedt M, Kechagias S, Hultcrantz R, et al. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver International 2016;36(11):1688-95. [DOI] [PubMed] [Google Scholar]
Hallberg 1993 {published data only}
- Hallberg L, Bengtsson C, Lapidus L, Lindstedt G, Lundberg PA, Hulten L. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. British Journal of Haematology 1993;85(4):787-98. [DOI] [PubMed] [Google Scholar]
- Hallberg L, Hulthen L, Bengtsson C, Lapidus L, Lindstedt G. Iron balance in menstruating women. European Journal of Clinical Nutrition 1995;49(3):200-7. [PubMed] [Google Scholar]
Halliday 1977 {published data only}
- Halliday JW, Russo AM, Cowlishaw JL, Powell LW. Serum-ferritin in diagnosis of haemochromatosis. A study of 43 families. Lancet 1977;2(8039):621-4. [DOI] [PubMed] [Google Scholar]
Hansen 1983 {published data only}
- Hansen TM, Hansen NE, Birgens HS, Holund B, Lorenzen I. Serum ferritin and the assessment of iron deficiency in rheumatoid arthritis. Scandinavian Journal of Rheumatology 1983;12(4):353-9. [DOI] [PubMed] [Google Scholar]
Harada 1992 {published data only}
- Harada M, Hirai K, Sakisaka S, Ueno T, Abe H, Tanikawa K. Comparative study of magnetic resonance imaging, computed tomography and histology in the assessment of liver iron overload. Internal Medicine (Tokyo, Japan) 1992;31(2):180-4. [DOI] [PubMed] [Google Scholar]
Harju 1984 {published data only}
- Harju E, Pakarinen A, Larmi T. A comparison between serum ferritin concentration and the amount of bone marrow stainable iron. Scandinavian Journal of Clinical and Laboratory Investigation 1984;44(6):555-6. [DOI] [PubMed] [Google Scholar]
Holmström 2002 {published data only}
- Holmström P, Marmur J, Eggertsen G, Gåfvels M, Stål P. Mild iron overload in patients carrying the HFE S65C gene mutation: a retrospective study in patients with suspected iron overload and healthy controls. Gut 2002;51(5):723-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holyoake 1993 {published data only}
- Holyoake TL, Stott DJ, McKay PJ, Hendry A, MacDonald JB, Lucie NP. Use of plasma ferritin concentration to diagnose iron deficiency in elderly patients. Journal of Clinical Pathology 1993;46(9):857-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Intragumtornchai 1998 {published data only}
- Intragumtornchai T, Rojnukkarin P, Swasdikul D, Israsena S. The role of serum ferritin in the diagnosis of iron deficiency anaemia in patients with liver cirrhosis. Journal of Internal Medicine 1998;243(3):233-41. [DOI] [PubMed] [Google Scholar]
Isa 1988 {published data only}
- Isa L, Jean G, Silvani A, Arosio P, Taccagni GL. Evaluation of iron stores in patients with alcoholic liver disease: role of red cell ferritin. Acta Haematologica 1988;80(2):85-8. [DOI] [PubMed] [Google Scholar]
Jensen 1994 {published data only}
- Jensen PD, Peterslund NA, Poulsen JH, Jensen FT, Christensen T, Ellegaard J. The effect of iron overload and iron reductive treatment on the serum concentration of carbohydrate-deficient transferrin. British Journal of Haernatology 1994;88(1):56-63. [DOI] [PubMed] [Google Scholar]
Jonker 2013 {published data only}
- Jonker FAM, Calis JCJ, Phiri K, Kraaijenhagen RJ, Brabin BJ, Faragher B, et al. Low hepcidin levels in severely anemic Malawian children with high incidence of infectious diseases and bone marrow iron deficiency. PLOS ONE 2013;8(12):e78964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonker 2014 {published data only}
- Jonker FAM, Boele Van Hensbroek M, Leenstra T, Vet RJWM, Brabin BJ, Maseko N, et al. Conventional and novel peripheral blood iron markers compared against bone marrow in Malawian children. Journal of Clinical Pathology 2014;67(8):717-23. [DOI] [PubMed] [Google Scholar]
Joosten 2002 {published data only}
- Joosten E, Van Loon R, Billen J, Blanckaert N, Fabri R, Pelemans W. Serum transferrin receptor in the evaluation of the iron status in elderly hospitalized patients with anemia. American Journal of Hematology 2002;69(1):1-6. [DOI] [PubMed] [Google Scholar]
Kalantar‐Zadeh 1995 {published data only}
- Kalantar-Zadeh K, Höffken B, Wünsch H, Fink H, Kleiner M, Luft FC. Diagnosis of iron deficiency anemia in renal failure patients during the post-erythropoietin era. American Journal of Kidney Diseases 1995;26(2):292-9. [DOI] [PubMed] [Google Scholar]
Kaltwasser 1990 {published data only}
- Kaltwasser JP, Gottschalk R, Schalk KP, Hartl W. Non-invasive quantitation of liver iron-overload by magnetic resonance imaging. British Journal of Haematology 1990;74(3):360-3. [DOI] [PubMed] [Google Scholar]
Karlsson 2010 {published data only}
- Karlsson T. Plasma soluble transferrin receptor assay when screening for iron-deficiency in an unselected population of elderly anaemic patients. Journal of Internal Medicine 2010;267(3):331-4. [DOI] [PubMed] [Google Scholar]
Karlsson 2015 {published data only}
- Karlsson T. Mass spectrometry evaluation of the hepcidin-25 assay in the differential diagnosis of iron deficiency anaemia with concurrent inflammation and anaemia of inflammation in elderly patients. European Journal of Haematology 2015;95(5):467-71. [DOI] [PubMed] [Google Scholar]
Kim 2000 {published data only}
- Kim SS, Park W, Bae SK, Lee YH, Song JS, Choi JW, et al. The usefulness of serum transferrin receptor in anemia with rheumatoid arthritis: comparison with bone marrow iron store. Journal of the Korean Rheumatism Association 2000;7(4):360-9. [Google Scholar]
- Song JS, Park W, Bae SK, Kim SS, Lee YH, Choi JW, et al. The usefulness of serum transferrin receptor and ferritin for assessing anemia in rheumatoid arthritis: comparison with bone marrow iron study. Rheumatology International 2001;21(1):24-9. [DOI] [PubMed] [Google Scholar]
Kis 1998 {published data only}
- Kis AM, Carnes M. Detecting iron deficiency in anemic patients with concomitant medical problems. Journal of General Internal Medicine 1998;13(7):455-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotru 2004 {published data only}
- Kotru M, Rusia U, Sikka M, Chaturvedi S, Jain AK. Evaluation of serum ferritin in screening for iron deficiency in tuberculosis. Annals of Hematology 2004;83(2):95-100. [DOI] [PubMed] [Google Scholar]
Krause 1980 {published data only}
- Krause JR, Stolc V. Serum ferritin and bone marrow biopsy iron stores. II. Correlation with low serum iron and Fe/TIBC ratio less than 15%. American Journal of Clinical Pathology 1980;74(4):461-4. [DOI] [PubMed] [Google Scholar]
Lawrence 1996 {published data only}
- Lawrence SP, Caminer SJ, Yavorski RT, Borosky BD, Rak KM, Merenich JA, et al. Correlation of liver density by magnetic resonance imaging and hepatic iron levels. A noninvasive means to exclude homozygous hemochromatosis. Journal of Clinical Gastroenterology 1996;23(2):113-7. [DOI] [PubMed] [Google Scholar]
Leggett 1990 {published data only}
- Leggett BA, Halliday JW, Brown NN, Bryant S, Powell LW. Prevalence of haemochromatosis amongst asymptomatic Australians. British Journal of Haematology 1990;74(4):525-30. [DOI] [PubMed] [Google Scholar]
Lewis 2007 {published data only}
- Lewis DK, Whitty CJM, Epino H, Letsky EA, Mukiibi JM, Van den Broek NR. Interpreting tests for iron deficiency among adults in a high HIV prevalence African setting: routine tests may lead to misdiagnosis. Transactions of the Royal Society of Tropical Medicine & Hygiene 2007;101(6):613-7. [DOI] [PubMed] [Google Scholar]
Lim 2004 {published data only}
- Lim EM, Rossi E, De Boer WB, Reed WD, Jeffrey GP. Hepatic iron loading in patients with compound heterozygous HFE mutations. Liver International 2004;24(6):631-6. [DOI] [PubMed] [Google Scholar]
Lindstedt 1980 {published data only}
- Lindstedt G, Lundberg PA, Bjorn-Rasmussen E, Magnussen B. Serum-ferritin and iron-deficiency anaemia in hospital patients. Lancet 1980;1(8161):205-6. [DOI] [PubMed] [Google Scholar]
Lombard 1989 {published data only}
- Lombard M, Bomford A, Hynes M, Naoumov NV, Roberts S, Crowe J, et al. Regulation of the hepatic transferrin receptor in hereditary hemochromatosis. Hepatology 1989;9(1):1-5. [DOI] [PubMed] [Google Scholar]
Lough 1989 {published data only}
- Lough M, Egan EL, O'Cearbhaill HG. Diagnosing iron deficiency in a hospital population. Journal of Medical Science 1989;158(5):108-9. [DOI] [PubMed] [Google Scholar]
Macfarlane 1995 {published data only}
- Macfarlane JD, Vreugdenhil GR, Doornbos J, Van der Voet GB. Idiopathic haemochromatosis: magnetic resonance signal intensity ratios permit non-invasive diagnosis of low levels of iron overload. Netherlands Journal of Medicine 1995;47(2):49-53. [DOI] [PubMed] [Google Scholar]
Maliken 2012 {published data only}
- Maliken BD, Avrin WF, Nelson JE, Mooney J, Kumar S, Kowdley KV. Room-temperature susceptometry predicts biopsy-determined hepatic iron in patients with elevated serum ferritin. Annals of Hepatology 2012;11(1):77-84. [PMC free article] [PubMed] [Google Scholar]
Martin‐Cabrera 2015 {published data only}
- Martin-Cabrera P, Hung M, Ortmann E, Richards T, Ghosh M, Bottrill F, et al. Clinical use of low haemoglobin density, transferrin saturation, bone marrow morphology, Perl's stain and other plasma markers in the identification of treatable anaemia presenting for cardiac surgery in a prospective cohort study. Journal of Clinical Pathology 2015;68(11):923-30. [DOI] [PubMed] [Google Scholar]
Mast 1998 {published data only}
- Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clinical Chemistry 1998;44(1):45-51. [PubMed] [Google Scholar]
Mast 2002 {published data only}
- Mast AE, Blinder MA, Lu Q, Flax S, Dietzen DJ. Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood 2002;99(4):1489-91. [DOI] [PubMed] [Google Scholar]
Mazza 1978 {published data only}
- Mazza J, Barr RM, McDonald JW, Valberg LS. Usefulness of the serum ferritin concentration in the detection of iron deficiency in a general hospital. Canadian Medical Association Journal 1978;119(8):884-6. [PMC free article] [PubMed] [Google Scholar]
Means 1999 {published data only}
- Means RTJ, Allen J, Sears DA, Schuster SJ. Serum soluble transferrin receptor and the prediction of marrow aspirate iron results in a heterogeneous group of patients. Clinical & Laboratory Haematology 1999;21(3):161-7. [DOI] [PubMed] [Google Scholar]
Meira 2005 {published data only}
- Meira DG, Lorand-Metze I, Toro ADC, Silva MTN, Vilela MMS. Bone marrow features in children with HIV infection and peripheral blood cytopenias. Journal of Tropical Pediatrics 2005;51(2):114-9. [DOI] [PubMed] [Google Scholar]
Milman 1983 {published data only}
- Milman N, Bangsboll S, Strandberg Pedersen N, Visfeldt J. Serum ferritin in non-dialysis patients with chronic renal failure: relation to bone marrow iron stores. Scandinavian Journal of Haematology 1983;30(4):337-44. [DOI] [PubMed] [Google Scholar]
Mulherin 1996 {published data only}
- Mulherin D, Skelly M, Saunders A, McCarthy D, O'Donoghue D, Fitzgerald O, et al. The diagnosis of iron deficiency in patients with rheumatoid arthritis and anemia: an algorithm using simple laboratory measures. Journal of Rheumatology 1996;23(2):237-40. [PubMed] [Google Scholar]
Nadeem 2011 {published data only}
- Nadeem S, Shah S, Iqbal T, Iqbal Z, Hanif E. Serum transferrin receptor, serum ferritin and serum transferrin receptor-ferritin index in adults with iron deficiency anaemia. Journal of Ayub Medical College (Abbottabad) 2011;23(3):44-6. [PubMed] [Google Scholar]
Nanas 2006 {published data only}
- Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, et al. Etiology of anemia in patients with advanced heart failure. Journal of the American College of Cardiology 2006;48(12):2485-9. [DOI] [PubMed] [Google Scholar]
Nelson 1978 {published data only}
- Nelson R, Chawla M, Connolly P, LaPorte J. Ferritin as an index of bone marrow iron stores. Southern Medical Journal 1978;71(12):1482-4. [DOI] [PubMed] [Google Scholar]
Niederau 1998 {published data only}
- Niederau C, Niederau CM, Lange S, Littauer A, Abdel-Jalil N, Maurer M, et al. Screening for hemochromatosis and iron deficiency in employees and primary care patients in Western Germany. Annals of Internal Medicine 1998;128(5):337-45. [DOI] [PubMed] [Google Scholar]
Nielsen 1990 {published data only}
- Nielsen OJ, Andersen LS, Hansen NE, Hansen TM. Serum transferrin receptor levels in anaemic patients with rheumatoid arthritis. Scandinavian Journal of Clinical & Laboratory Investigation 1994;54(1):75-82. [DOI] [PubMed] [Google Scholar]
- Nielsen OJ, Andersen LS, Ludwigsen E, Bouchelouche P, Hansen TM, Birgens H, et al. Anaemia of rheumatoid arthritis: serum erythropoietin concentrations and red cell distribution width in relation to iron status. Annals of the Rheumatic Diseases 1990;49(6):349-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
North 1997 {published data only}
- North M, Dallalio G, Donath AS, Melink R, Means RT Jr. Serum transferrin receptor levels in patients undergoing evaluation of iron stores: correlation with other parameters and observed versus predicted results. Clinical and Laboratory Haematology 1997;19(2):93-7. [DOI] [PubMed] [Google Scholar]
Oluboyede 1980 {published data only}
- Oluboyede OA. Iron studies in pregnant and non-pregnant women with haemoglobin SS or SC disease. British Journal of Obstetrics & Gynaecology 1980;87(11):989-96. [DOI] [PubMed] [Google Scholar]
Olynyk 1999 {published data only}
- Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population-based study of the clinical expression of the hemochromatosis gene. New England Journal of Medicine 1999;341(10):718-24. [DOI] [PubMed] [Google Scholar]
Ong 2005 {published data only}
- Ong KH, Tan HL, Lai HC, Kuperan P. Accuracy of various iron parameters in the prediction of iron deficiency in an acute care hospital. Annals of the Academy of Medicine, Singapore 2005;34(7):437-40. [PubMed] [Google Scholar]
Ortega 2005 {published data only}
- Ortega L, Ladero JM, Carreras MP, Alvarez T, Taxonera C, Oliván MP, et al. A computer-assisted morphometric quantitative analysis of iron overload in liver biopsies. A comparison with histological and biochemical methods. Pathology, Research & Practice 2005;201(10):673-5. [DOI] [PubMed] [Google Scholar]
Pascoe 1999 {published data only}
- Pascoe A, Kerlin P, Steadman C, Clouston A, Jones D, Powell L, et al. Spur cell anaemia and hepatic iron stores in patients with alcoholic liver disease undergoing orthotopic liver transplantation. Gut 1999;45(2):301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patterson 1985 {published data only}
- Patterson C, Turpie ID, Benger AM. Assessment of iron stores in anemic geriatric patients. Journal of the American Geriatrics Society 1985;33(11):764-7. [DOI] [PubMed] [Google Scholar]
Phatak 1998 {published data only}
- Phatak PD, Sham RL, Raubertas RF, Dunnigan K, O'Leary MT, Braggins C, et al. Prevalence of hereditary hemochromatosis in 16031 primary care patients. Annals of Internal Medicine 1998;129(11):954-61. [DOI] [PubMed] [Google Scholar]
Phiri 2009 {published data only}
- Phiri KS, Calis JC, Siyasiya A, Bates I, Brabin B, Van Hensbroek MB. New cut-off values for ferritin and soluble transferrin receptor for the assessment of iron deficiency in children in a high infection pressure area. Journal of Clinical Pathology 2009;62(12):1103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pietrangelo 1999 {published data only}
- Pietrangelo A, Montosi G, Totaro A, Garuti C, Conte D, Cassanelli S, et al. Hereditary hemochromatosis in adults without pathogenic mutations in the hemochromatosis gene. New England Journal of Medicine 1999;341(10):725-32. [DOI] [PubMed] [Google Scholar]
Porter 1994 {published data only}
- Porter DR, Sturrock RD, Capell HA. The use of serum ferritin estimation in the investigation of anaemia in patients with rheumatoid arthritis. Clinical & Experimental Rheumatology 1994;12(2):179-82. [PubMed] [Google Scholar]
Punnonen 1994 {published data only}
- Punnonen K, Irjala K, Rajamäki A. Iron-deficiency anemia is associated with high concentrations of transferrin receptor in serum. Clinical Chemistry 1994;40(5):774-6. [PubMed] [Google Scholar]
Punnonen 1997 {published data only}
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997;89(3):1052-7. [PubMed] [Google Scholar]
Punnonen 1998 {published data only}
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor, ferritin and TfR-F index in identification of latent iron deficiency. European Journal of Haematology 1998;60(2):135-7. [DOI] [PubMed] [Google Scholar]
Puolakka 1980 {published data only}
- Puolakka J, Jänne O, Pakarinen A, Vihko R. Serum ferritin in diagnosis of anemia during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1980;95(Suppl):57-63. [DOI] [PubMed] [Google Scholar]
Rao 1984 {published data only}
- Rao KR, Patel AR, McGinnis P, Patel MK. Iron stores in adults with sickle cell anemia. Journal of Laboratory & Clinical Medicine 1984;103(5):792-7. [PubMed] [Google Scholar]
Ravindran 2008 {published data only}
- Ravindran V, Jain S, Mathur DS. The differentiation of anaemia in rheumatoid arthritis: parameters of iron-deficiency in an Indian rheumatoid arthritis population. Rheumatology International 2008;28(6):507-11. [DOI] [PubMed] [Google Scholar]
Rowe 1977 {published data only}
- Rowe JW, Wands JR, Mezey E, Waterbury LA, Wright JR, Tobin J, et al. Familial hemochromatosis: characteristics of the precirrhotic stage in a large kindred. Medicine 1977;56(3):197-211. [PubMed] [Google Scholar]
Ruivard 2000 {published data only}
- Ruivard M, Boursiac M, Mareynat G, Sapin AF, Gerbaud L, Derumeaux H, et al. Ratio serum transferrin receptor/serum ferritin in the diagnosis of iron deficiency [Diagnostic de la carence en fer: evaluation du rapport 'recepteur soluble de la transferrine/ferritine]. Revue de Medecine Interne 2000;21(10):837-43. [DOI] [PubMed] [Google Scholar]
Schöniger‐Hekele 2002 {published data only}
- Schöniger-Hekele M, Müller C, Polli C, Wrba F, Penner E, Ferenci P. Liver pathology in compound heterozygous patients for hemochromatosis mutations. Liver 2002;22(4):295-301. [DOI] [PubMed] [Google Scholar]
Sebastiani 2006 {published data only}
- Sebastiani G, Vario A, Ferrari A, Pistis R, Noventa F, Alberti A. Hepatic iron, liver steatosis and viral genotypes in patients with chronic hepatitis C. Journal of Viral Hepatitis 2006 Mar;13(3):199-205. [DOI] [PubMed] [Google Scholar]
Sebastinani 2012 {published data only}
- Sebastiani G, Tempesta D, Alberti A. Hepatic iron overload is common in chronic hepatitis B and is more severe in patients coinfected with hepatitis D virus. Journal of Viral Hepatitis 2012;19(2):170-6. [DOI] [PubMed] [Google Scholar]
Sham 1997 {published data only}
- Sham RL, Ou CY, Cappuccio J, Braggins C, Dunnigan K, Phatak PD. Correlation between genotype and phenotype in hereditary hemochromatosis: analysis of 61 cases. Blood Cells Molecules & Diseases 1997;23(2):314-20. [DOI] [PubMed] [Google Scholar]
Sharma 1984 {published data only}
- Sharma JC, Roy SN. Value of serum ferritin as an index of iron deficiency in elderly anaemic patients. Age & Ageing 1984;13(4):248-50. [DOI] [PubMed] [Google Scholar]
Shroff 1991 {published data only}
- Shroff K, Gaiha M, Singh T, Sharma SK. Iron status in patients of rheumatoid arthritis with special reference to serum ferritin levels. Journal of the Association of Physicians of India 1991;39(9):675-7. [PubMed] [Google Scholar]
Smith 1977 {published data only}
- Smith RJ, Davis P, Thomson AB, Wadsworth LD, Fackre P. Serum ferritin levels in anemia of rheumatoid arthritis. Journal of Rheumatology 1977;4(4):389-92. [PubMed] [Google Scholar]
Smith 1997 {published data only}
- Smith BN, Kantrowitz W, Grace ND, Greenberg MS, Patton TJ, Ookubo R, et al. Prevalence of hereditary hemochromatosis in a Massachusetts corporation: is Celtic origin a risk factor? Hepatology 1997;25(6):1439-46. [DOI] [PubMed] [Google Scholar]
Solomon 1981 {published data only}
- Solomon LR, Hillman RS, Finch CA. Serum ferritin in refractory anemias. Acta Haematologica 1981;66(1):1-5. [DOI] [PubMed] [Google Scholar]
Sorbie 1975 {published data only}
- Sorbie J, Valberg LS, Corbett WE, Ludwig J. Serum ferritin, cobalt excretion and body iron status. Canadian Medical Association Journal 1975;112(10):1173-8. [PMC free article] [PubMed] [Google Scholar]
Summers 1990 {published data only}
- Summers KM, Halliday JW, Powell LW. Identification of homozygous hemochromatosis subjects by measurement of hepatic iron index. Hepatology 1990;12(1):20-5. [DOI] [PubMed] [Google Scholar]
Suominen 2000 {published data only}
- Suominen P, Mottonen T, Rajamaki A, Irjala K. Single values of serum transferrin receptor and transferrin receptor ferritin index can be used to detect true and functional iron deficiency in rheumatoid arthritis patients with anemia. Arthritis & Rheumatism 2000;43(5):1016-20. [DOI] [PubMed] [Google Scholar]
Szurowska 2010 {published data only}
- Szurowska E, Sikorska K, Izycka-Swieszewska E, Nowicki T, Romanowski T, Bielawski KP, et al. The role of MR imaging in detection of hepatic iron overload in patients with cirrhosis of different origins. BMC Gastroenterology 2010;10(13):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terrovitis 2011 {published data only}
- Terrovitis J, Kaldara E, Ntalianis A, Kapelios C, Sventzouri S, Vakrou S, et al. An easily applicable in everyday clinical practice diagnostic algorithm for the detection of iron deficiency in heart failure. European Heart Journal 2011;32:918. [Google Scholar]
Thompson 1988 {published data only}
- Thompson WG, Meola T, Lipkin M Jr, Freedman ML. Red cell distribution width, mean corpuscular volume, and transferrin saturation in the diagnosis of iron deficiency. Archives of Internal Medicine 1988;148(10):2128-30. [PubMed] [Google Scholar]
Thomsen 1992 {published data only}
- Thomsen C, Wiggers P, Ring-Larsen H, Christiansen E, Dalhøj J, Henriksen O, et al. Identification of patients with hereditary haemochromatosis by Magnetic Resonance Imaging and spectroscopic relaxation time measurements. Magnetic Resonance Imaging 1992;10(6):867-79. [DOI] [PubMed] [Google Scholar]
Thorburn 2002 {published data only}
- Thorburn D, Curry G, Spooner R, Spence E, Oien K, Halls D, et al. The role of iron and haemochromatosis gene mutations in the progression of liver disease in chronic hepatitis C. Gut 2002;50(2):248-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valberg 1978 {published data only}
- Valberg LS, Ghent CN, Lloyd DA, Frei JV, Chamberlain MJ. Diagnostic efficacy of tests for the detection of iron overload in chronic liver disease. Canadian Medical Association Journal 1978;119(3):229-36. [PMC free article] [PubMed] [Google Scholar]
Van den Broek 1998 {published data only}
- Van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? British Journal of Haematology 1998;103(3):817-24. [DOI] [PubMed] [Google Scholar]
Van Tellingen 2001 {published data only}
- Van Tellingen A, Kuenen JC, De Kieviet W, Van Tinteren H, Kooi ML, Vasmel WL. Iron deficiency anaemia in hospitalised patients: value of various laboratory parameters. Differentiation between IDA and ACD. Netherlands Journal of Medicine 2001;59(6):270-9. [DOI] [PubMed] [Google Scholar]
Van Zeben 1990 {published data only}
- Van Zeben D, Bieger R, Van Wermeskerken RK, Castel A, Hermans J. Evaluation of microcytosis using serum ferritin and red blood cell distribution width. European Journal of Haematology 1990;44(2):106-9. [DOI] [PubMed] [Google Scholar]
Villeneuve 1996 {published data only}
- Villeneuve JP, Bilodeau M, Lepage R, Côté J, Lefebvre M. Variability in hepatic iron concentration measurement from needle-biopsy specimens. Hepatology 1996;25(2):172-7. [DOI] [PubMed] [Google Scholar]
Vreugdenhil 1990 {published data only}
- Vreugdenhil G, Baltus C, Van Eijk H, Swaak A. Anaemia of chronic disease: diagnostic significance of erythrocyte and serological parameters in iron deficient rheumatoid arthritis patients.. British Journal of Rheumatology 1990;29(2):105-10. [DOI] [PubMed] [Google Scholar]
Walsh 2006 {published data only}
- Walsh A, Dixon JL, Ramm GA, Hewett DG, Lincoln DJ, Anderson GH, et al. The clinical relevance of compound heterozygosity for the C282Y and H63D substitutions in hemochromatosis. Clinical Gastroenterology and Hepatology 2006;4(11):1403–10. [DOI] [PubMed] [Google Scholar]
Wands 1976 {published data only}
- Wands JR, Rowe JA, Mezey SE, Waterbury LA, Wright JR, Halliday JW, et al. Normal serum ferritin concentrations in precirrhotic hemochromatosis. New England Journal of Medicine 1976;294(6):302-5. [DOI] [PubMed] [Google Scholar]
Witte 1986 {published data only}
- Witte DL, Kraemer DF, Johnson GF, Dick FR, Hamilton H. Prediction of bone marrow iron findings from tests performed on peripheral blood. American Journal of Clinical Pathology 1986;85(2):202-6. [DOI] [PubMed] [Google Scholar]
Witte 1988 {published data only}
- Witte DL, Angstadt DS, Davis SH, Schrantz RD. Predicting bone marrow iron stores in anemic patients in a community hospital using ferritin and erythrocyte sedimentation rate. American Journal of Clinical Pathology 1988;90(1):85-7. [DOI] [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong K, Adams P. The diversity of liver diseases among outpatient referrals for elevated serum ferritin. Canada Journal of Gastroenterology 2006;20:467-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ang 2017 {published data only}
- Ang AL, Le TT, Tan RS. HbH Constant Spring disease has lower serum ferritin relative to liver iron concentration (LIC): importance of LIC measurement and potential impact on serum ferritin thresholds for iron chelation. British Journal of Haematology 2017;176(6):986-8. [DOI] [PubMed] [Google Scholar]
Bafna 2016 {published data only}
- Bafna V, Chandrashekhar B, Nataraj KS, Shobha B, Bhat S, Nataraj KS, et al. Quantification of liver iron overload: correlation of MRI versus liver tissue biopsy in thalassemia patients undergoing bone marrow transplant. Indian Journal of Hematology and Blood Transfusion 2016;32:S380. [DOI] [PMC free article] [PubMed]
Bardou‐Jacquet 2014 {published data only}
Barsan 2015a {published data only}
- Barsan L, Stanciu A, Stancu S, Capusa C, Bratescu L, Mandache E, et al. Bone marrow iron distribution, hepcidin, and ferroportin expression in renal anemia. Hematology 2015;20(9):543-52. [DOI] [PubMed] [Google Scholar]
Braga 2014 {published data only}
- Braga F, Infusino I, Dolci A, Panteghini M. Soluble transferrin receptor in complicated anemia. Clinica Chimica Acta 2014;431:143-7. [DOI] [PubMed] [Google Scholar]
Busti 2016 {published data only}
- Busti F, Campostrini N, Badar S, Ferrarini A, Xumerle L, Giuffrida AC, et al. Toward better pathophysiological characterization and therapeutic solution in beta-thalassemia trait patients with iron overload. Preliminary reports from an ongoing study (E1487). American Journal of Hematology (2015 BioIron Meeting Abstracts) 2016;91:E84. [Google Scholar]
Cancado 2018 {published data only}
- Cancado R, Watman NP, Lobo C, Chona Z, Manzur F, Traina F, et al. Assessment of liver and cardiac iron overload using MRI in patients with chronic anemias in Latin American countries: results from ASIMILA study. Hematology (Amsterdam, Netherlands) 2018;23(9):1-7. [DOI] [PubMed] [Google Scholar]
Cazzola 1983 {published data only}
- Cazzola M, Dezza L, Bergamaschi G, Barosi G, Bellotti V, Caldera D, et al. Biologic and clinical significance of red cell ferritin. Blood 1983;62(5):1078-87. [PubMed] [Google Scholar]
Cecchin 1990 {published data only}
- Cecchin E, De Marchi S, Querin F, Marin MG, Fiorentino R, Tesio F. Efficacy of hepatic computed tomography to detect iron overload in chronic hemodialysis. Kidney International 1990;37(3):943-50. [DOI] [PubMed] [Google Scholar]
Chin 2019 {published data only}
- Chin J, Powell LW, Ramm LE, Ayonrinde OT, Ramm GA, Olynyk JK. Utility of hepatic or total body iron burden in the assessment of advanced hepatic fibrosis in HFE hemochromatosis. Scientific Reports 2019;9(1):20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa‐Matos 2013 {published data only}
- Costa-Matos L, Batista P, Monteiro N, Henriques P, Girão F, Carvalho A. Hfe mutations and iron overload in patients with alcoholic liver disease. Arquivos de Gastroenterologia 2013;50(1):35-41. [DOI] [PubMed] [Google Scholar]
Davidson 1984 {published data only}
- Davidson A, Van der Weyden MB, Fong H, Breidahl MJ, Ryan PF. Red cell ferritin content: a re-evaluation of indices for iron deficiency in the anaemia of rheumatoid arthritis. British Medical Journal, Clinical Research Edition 1984;289(6446):648-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deugnier 2010 {published data only}
- Deugnier Y, Turlin B, Dong V, Giannone V, Zhang Y, Griffer L, et al. Deferasirox improves liver pathology in beta-thalassemia patients with transfusional iron overload. Blood 2010;116:4274. [Google Scholar]
- Deugnier Y, Turlin B, Ropert M, Cappellini D, Porter JB, Giannone V, et al. Improvement in liver pathology of patients with b-Thalassemia treated with deferasirox for at least 3 years. Gastroenterology 2011;141(4):1202–11. [DOI] [PubMed] [Google Scholar]
Duport 2003 {published data only}
- Duport N, Preziosi P, Boutron-Ruault M, Bertrais S, Galan P, Favier A, et al. Consequences of iron depletion on health in menstruating women. European Journal of Clinical Nutrition 2003;57(9):1169–75. [DOI] [PubMed] [Google Scholar]
Elalfy 2015 {published data only}
- Elalfy MS, Adly AM, Wali Y, Tony S, Samir A, Elhenawy YI. Efficacy and safety of a novel combination of two oral chelators deferasirox/deferiprone over deferoxamine/deferiprone in severely iron overloaded young beta thalassemia major patients. European Journal of Haematology 2015;95(5):411-20. [DOI] [PubMed] [Google Scholar]
Feld 2015 {published data only}
- Feld JJ, Kato GJ, Koh C, Shields T, Hildesheim M, Kleiner DE, et al. Liver injury is associated with mortality in sickle cell disease. Alimentary Pharmacology & Therapeutics 2015;42(7):912-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez Salazar 2004 {published data only}
- Fernandez Salazar LI, Alvarez Gago T, Aller de la Fuente R, Orduna Domingo A, Arranz Santos T, la Calle Valverde F, et al. Iron overload and genotype 3 are associated with liver steatosis in chronic hepatitis C. Revista Espanola de Enfermedades Digestivas 2004;96(12):818-28. [DOI] [PubMed] [Google Scholar]
Finch 1986 {published data only}
- Finch CA, Bellotti V, Stray S, Lipschitz DA, Cook JD, Pippard MJ, et al. Plasma ferritin determination as a diagnostic tool. Western Journal of Medicine 1986;145(5):657-63. [PMC free article] [PubMed] [Google Scholar]
Fleming 1990 {published data only}
- Fleming LW, Hopwood D, Shepherd AN, Stewart WK. Hepatic iron in dialysed patients given intravenous iron dextran. Journal of Clinical Pathology 1990;43(2):119-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallego 2015 {published data only}
- Gallego CJ, Burt A, Sundaresan AS, Ye Z, Shaw C, Crosslin D, et al. Penetrance of hemochromatosis in HFE genotypes resulting in p.Cys282Tyr and p.Cys282Tyr; His63Asp in the eMERGE Network. American Journal of Human Genetics 2015;97(4):512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greni 2017 {published data only}
- Greni F, Valenti L, Mariani R, Pelloni I, Rametta R, Busti F, et al. GNPAT rs11558492 is not a major modifier of iron status: study of Italian hemochromatosis patients and blood donors. Annals of Hepatology 2017;16(3):451-6. [DOI] [PubMed] [Google Scholar]
Gupta 2003 {published data only}
- Gupta M, Kannan M, Gupta S, Saxena R. Contribution of iron deficiency to anemia in chronic renal failure. Indian Journal of Pathology & Microbiology 2003;46(4):563-4. [PubMed] [Google Scholar]
Gupta 2009 {published data only}
- Gupta S, Uppal B, Pawar B. Is soluble transferrin receptor a good marker of iron deficiency anemia in chronic kidney disease patients. Indian Journal of Nephrology 2009;19(3):96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 1992a {published data only}
- Guyatt G. Transferrin as a predictor of iron deficiency. American Journal of Medicine 1992;92(4):453. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. Journal of General Internal Medicine 1992;7(2):145-53. [DOI] [PubMed] [Google Scholar]
Hagve 2013 {published data only}
- Hagve TA, Lilleholt K, Svendsen M. Iron deficiency anaemia - interpretation of biochemical and haematological findings [Jernmangelanemi – tolking av biokjemiske og hematologiske funn]. Tidsskrift for Den Norske Laegeforening 2013;133(2):161-4. [DOI] [PubMed] [Google Scholar]
Hallberg 1993b {published data only}
- Hallberg L, Hulten L, Lindstedt G, Lundberg PA, Mark A, Purens J, et al. Prevalence of iron deficiency in Swedish adolescents. Pediatric Research 1993;34(5):680-7. [DOI] [PubMed] [Google Scholar]
Hallberg 2001 {published data only}
- Hallberg L. Perspectives on nutritional iron deficiency. Annual Review of Nutrition 2001;21:1-21. [DOI] [PubMed] [Google Scholar]
Hallberg 2002 {published data only}
- Hallberg L, Hulthen L. Perspectives on iron absorption. Blood Cells Molecules & Diseases 2002;29(3):562-73. [DOI] [PubMed] [Google Scholar]
Halonen 2003 {published data only}
- Halonen P, Mattila J, Suominen P, Ruuska T, Salo MK, Mäkipernaa A. Iron overload in children who are treated for acute lymphoblastic leukemia estimated by liver siderosis and serum iron parameters. Pediatrics 2003;111(1):91-6. [DOI] [PubMed] [Google Scholar]
Hamedani 1991 {published data only}
- Hamedani P, Raza R, Bachand R, Manji M, Hashml K. Laboratory diagnosis of iron deficiency in a developing country, Pakistan. Journal of International Medical Research 1991;19(1):19-23. [DOI] [PubMed] [Google Scholar]
Hamidieh 2015 {published data only}
- Hamidieh AA, Moeininia F, Tayebi S, Shamshiri AR, Behfar M, Jalili M, et al. Efficacy of hepatic T2*MRI values and serum ferritin concentration in predicting thalassemia major classification for hematopoietic stem cell transplantation. Pediatric Transplantation 2015;19(3):301-6. [DOI] [PubMed] [Google Scholar]
Handelman 2008 {published data only}
- Handelman GJ, Levin NW. Iron and anemia in human biology: a review of mechanisms. Heart Failure Reviews 2008;13(4):393-404. [DOI] [PubMed] [Google Scholar]
Hanif 2005 {published data only}
- Hanif E, Ayyub M, Anwar M, Ali W, Bashir M. Evaluation of serum transferrin receptor concentration in diagnosing and differentiating iron deficiency anaemia from anaemia of chronic disorders. Journal of the Pakistan Medical Association 2005;55(1):13-6. [PubMed] [Google Scholar]
Hankins 2010 {published data only}
- Hankins JS, McCarville MB, Hillenbrand CM, Loeffler RB, Ware RE, Song R, et al. Ventricular diastolic dysfunction in sickle cell anemia is common but not associated with myocardial iron deposition. Pediatric Blood & Cancer 2010;55(3):495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haque 1996 {published data only}
- Haque S, Chandra B, Gerber MA, Lok AS. Iron overload in patients with chronic hepatitis C: a clinicopathologic study. Human Pathology 1996;27(12):1277-81. [DOI] [PubMed] [Google Scholar]
Harmatz 2000 {published data only}
- Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, et al. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 2000;96(1):76-9. [PubMed] [Google Scholar]
Heinrich 1977 {published data only}
- Heinrich HC, Bruggemann J, Gabbe EE, Glaser M. Correlation between diagnostic 59Fe2+-absorption and serum ferritin concentration in man. Zeitschrift fur Naturforschung (Section C Journal of Biosciences) 1977;32(11-12):1023-5. [DOI] [PubMed] [Google Scholar]
Heinrich 1980 {published data only}
- Heinrich HC. Limited value of serum ferritin in the differential diagnosis of hypoferremia. Deutsche Medizinische Wochenschrift 1980;105(49):1724. [PubMed] [Google Scholar]
Hellerbrand 2003 {published data only}
- Hellerbrand C, Pöppl A, Hartmann A, Schölmerich J, Lock G. HFE C282Y heterozygosity in hepatocellular carcinoma: evidence for an increased prevalence. Clinical Gastroenterology and Hepatology 2003;1(4):279-84. [DOI] [PubMed] [Google Scholar]
Hernandez 1988 {published data only}
- Hernandez RJ, Sarnaik SA, Lande I, Aisen AM, Glazer GM, Chenevert T, et al. MR evaluation of liver iron overload. Journal of Computer Assisted Tomography 1988;12(1):91-4. [DOI] [PubMed] [Google Scholar]
Ho 2001 {published data only}
- Ho CH. The differential diagnostic values of serum transferrin receptor, serum ferritin and related parameters in the patients with various causes of anemia. Haematologica 2001;86(2):206-7. [PubMed] [Google Scholar]
Hod 2012 {published data only}
- Hod EA, Francis RO, Spitalnik SL, Winters A, Cooke KS, Sasu BJ. Validation and preclinical correlation of a new sandwich ELISA for measuring murine hepcidin. Blood Journal by the American Society of Hematology, ASH 2012;120(21):2100. [Google Scholar]
Hofer 2004 {published data only}
- Hofer H, Osterreicher C, Jessner W, Penz M, Steindl-Munda P, Wrba F, et al. Hepatic iron concentration does not predict response to standard andpegylated-IFN/ribavirin therapy in patients with chronic hepatitis C. Journal of Hepatology 2004;40(6):1018–22. [DOI] [PubMed] [Google Scholar]
Hussain 1998 {published data only}
- Hussain A. Serum ferritin: an indicator of bone marrow iron stores in haemodialysed patient. Professional Medical Journal 1998;5(3):352-6. [Google Scholar]
Hyman 1983 {published data only}
- Hyman ES. Acquired iron-deficiency anaemia due to impaired iron transport. Lancet 1983;1(8316):91-5. [DOI] [PubMed] [Google Scholar]
Infusino 2012 {published data only}
- Infusino I, Braga F, Dolci A, Panteghini M. Soluble transferrin receptor (sTfR) and sTfR/log ferritin index for the diagnosis of iron-deficiency anemia. A meta-analysis. American Journal of Clinical Pathology 2012;138(5):642-9. [DOI] [PubMed] [Google Scholar]
Inthawong 2015 {published data only}
- Inthawong K, Charoenkwan P, Silvilairat S, Tantiworawit A, Phrommintikul A, Choeyprasert W, et al. Pulmonary hypertension in non-transfusion-dependent thalassemia: correlation with clinical parameters, liver iron concentration, and non-transferrin-bound iron. Hematology (Amsterdam, Netherlands) 2015;20(10):610-7. [DOI] [PubMed] [Google Scholar]
Invernizzi 1990 {published data only}
- Invernizzi R, Cazzola M, De Fazio P, Rosti V, Ruggeri G, Arosio P. Immunocytochemical detection of ferritin in human bone marrow and peripheral blood cells using monoclonal antibodies specific for the H and L subunit. British Journal of Haematology 1990;76(3):427-32. [DOI] [PubMed] [Google Scholar]
Isa 2013 {published data only}
- Isa HM, Mohamed AM. Neonatal hemochromatosis. Case series from Bahrain. Saudi Medical Journal 2013;34(12):1274-80. [PubMed] [Google Scholar]
Isokawa 1997 {published data only}
- Isokawa M, Kimura F, Matsuki T, Omoto E, Otsuka K, Kurokawa H, et al. Evaluation of bone marrow iron by magnetic resonance imaging. Annals of Hematology 1997;74(6):269-74. [DOI] [PubMed] [Google Scholar]
Jacobs 1972 {published data only}
- Jacobs A, Miller F, Worwood M, Beamish M, Wardrop C. Ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. British Medical Journal 1972;4(5834):206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 1975 {published data only}
- Jacobs A, Worwood M. Ferritin in serum. Clinical and biochemical implications. New England Journal of Medicine 1975;292(18):951-6. [DOI] [PubMed] [Google Scholar]
Jacobs 1979 {published data only}
- Jacobs J. Diagnosis and treatment of iron deficiency anaemia. South African Medical Journal 1979;56(4):123-5. [PubMed] [Google Scholar]
Jacobs 1982 {published data only}
- Jacobs P, Dommisse J. The plasma ferritin level as a reliable index of body iron stores following intravenous iron dextran. Journal of Medicine 1982;13(4):309-21. [PubMed] [Google Scholar]
Jankowska 2013 {published data only}
- Jankowska EA, Suchocki T, Wojtas K, Mazur G, Butrym A, Rybinska I, et al. High soluble transferrin receptor in patients with systolic heart failure: a measure of iron deficiency and a strong predictor of mortality. Journal of the American College of Cardiology 2013;61(10 (suppl S)):Poster E749. [Google Scholar]
Jensen 1999 {published data only}
- Jensen NM, Brandsborg M, Boesen AM, Yde H, Dahlerup JF. Low-dose oral iron absorption test in anaemic patients with and without iron deficiency determined by bone marrow iron content. European Journal of Haematology 1999;63(2):103-11. [DOI] [PubMed] [Google Scholar]
Jitani 2011 {published data only}
- Jitani A, Khonglah Y, Athar R, Raphae V, Pal A. Clinico-hematologic and biochemical profile of dimorphic anemia with bone marrow study. Indian Journal of Hematology and Blood Transfusion 2011;27(4):252. [Google Scholar]
Jolobe 2005 {published data only}
- Jolobe OMP. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clinic Proceedings 2005;80(10):1389-90. [DOI] [PubMed] [Google Scholar]
Junca 1998 {published data only}
- Junca J, Fernández-Avilés F, Oriol A, Navarro JT, Milla F, Sancho JM, et al. The usefulness of the serum transferrin receptor in detecting iron deficiency in the anemia of chronic disorders. Haematologica 1998;83(8):676-80. [PubMed] [Google Scholar]
Junca 2001 {published data only}
- Junca J. A diagnostic algorithm for ferropenia. Medicina Clinica 2001;116(4):146-9. [DOI] [PubMed] [Google Scholar]
Just 2011 {published data only}
- Just IM, Suner S, Schaumberger R, Renke C, Gabriel C. Evaluation of low red blood cell mean corpuscular volume is useful in the detection of iron deficiency in a whole blood donor population. Transfusion Medicine and Hemotherapy 2011;38:35-6. [Google Scholar]
Kaldara 2011 {published data only}
- Kaldara E, Terrovitis J, Ntalianis A, Kapelios C, Vakrou S, Sventzouri S, et al. A simple diagnostic and therapeutic algorithm for anemia in end stage heart failure patients. Circulation: American Heart Association 2011;124(Suppl 21):A14653. [Google Scholar]
Kaltwasser 1977a {published data only}
- Kaltwasser JP, Werner E. Ferritin. Radioimmunological determination in serum and clinical significance [Die radioimmunologische meassung von ferritin im serum und ihre klinishe bedeutung]. Klinische Wochenschrift 1977;55(22):1103-7. [DOI] [PubMed] [Google Scholar]
Kaltwasser 1977b {published data only}
- Kaltwasser JP, Werner E, Becker H. Serum ferritin as a control parameter for oral iron therapy. Deutsche Medizinische Wochenschrift 1977;102(32):1150-5. [DOI] [PubMed] [Google Scholar]
Karam 2008 {published data only}
- Karam LB, Disco D, Jackson SM, Lewin D, McKie V, Baker RD, et al. Liver biopsy results in patients with sickle cell disease on chronic transfusions: poor correlation with ferritin levels. Pediatric Blood & Cancer 2008;50(1):62-5. [DOI] [PubMed] [Google Scholar]
Karimi 2017 {published data only}
- Karimi M, Amirmoezi F, Haghpanah S, Ostad S, Lotfi M, Sefidbakht S, et al. Correlation of serum ferritin levels with hepatic MRI T2 and liver iron concentration in nontransfusion beta-thalassemia intermediate patients: a contemporary issue. Pediatric Hematology and Oncology 2017;34(5):292-7. [DOI] [PubMed] [Google Scholar]
Karlsson 2015a {published data only}
- Karlsson T. Mass spectrometry evaluation of the hepcidin-25 assay in the differential diagnosis of iron deficiency anaemia with concurrent inflammation and anaemia of inflammation in elderly patients. European Journal of Haematology 2015;95(5):467-71. [DOI] [PubMed] [Google Scholar]
Karlsson 2017 {published data only}
- Karlsson T. Evaluation of a competitive hepcidin ELISA assay in the differential diagnosis of iron deficiency anaemia with concurrent inflammation and anaemia of inflammation in elderly patients. Journal of Inflammation (London, England) 2017;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerlin 1979 {published data only}
- Kerlin P, Reiner R, Davies M, Sage RE, Grant AK. Iron deficiency anaemia - a prospective study. Australian & New Zealand Journal of Medicine 1979;9(4):402-7. [DOI] [PubMed] [Google Scholar]
Kim 1984 {published data only}
- Kim I, Pollitt E, Leibel RL, Viteri FE, Alvarez E. Application of receiver-operator analysis to diagnostic tests of iron deficiency in man. Pediatric Research 1984;18(9):916-20. [DOI] [PubMed] [Google Scholar]
Kimber 1983 {published data only}
- Kimber RJ, Rudzki Z, Blunden RW. Iron deficiency and iron overload: serum ferritin and serum iron in clinical medicine. Pathology 1983;15(4):497-503. [DOI] [PubMed] [Google Scholar]
Koca 2013 {published data only}
- Koca E, Cetiner DA, Buyukasik Y, Uner A, Sayinalp N, Haznedaroglu IC, et al. Bone marrow iron staining is a reliable test for elimination of iron deficiency anemia rather than its diagnosis [Kemik iligi Demir Boyasi Demir Eksikligi Anemisinin Teflhisinden Cok Difllanmasi Icin Guvenilir Bir Yontemdir]. Uluslararasi Hematoloji-Onkoloji Dergisi 2013;23(4):260-3. [Google Scholar]
Koulaouzidis 2007 {published data only}
- Koulaouzidis A, Tseung A. New diagnostic approaches to iron deficiency anaemia: from ferritin to ZnPP and sTfR and back to ferritin again. CME Journal Gastroenterology, Hepatology and Nutrition 2007;8(1):10-3. [Google Scholar]
Koulaouzidis 2009 {published data only}
- Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. Journal of Gastrointestinal & Liver Diseases 2009;18(3):345-52. [PubMed] [Google Scholar]
Kowdley 1997 {published data only}
- Kowdley KV, Trainer TD, Saltzman JR, Pedrosa M, Krawitt EL, Knox TA et al. Utility of hepatic iron index in American patients with hereditary hemochromatosis: a multicenter study. Gastroenterology 1997;113(4):1270-7. [DOI] [PubMed] [Google Scholar]
Kowdley 2012 {published data only}
- Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 2012;55(1):77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreeftenberg 2000 {published data only}
- Kreeftenberg HG Jr, Mooyaart EL, Huizenga JR, Sluiter WJ. Quantification of liver iron concentration with magnetic resonance imaging by combining T1-, T2-weighted spin echo sequences and a gradient echo sequence. Netherlands Journal of Medicine 2000;56(4):133–7. [DOI] [PubMed] [Google Scholar]
Krittayaphong 2018 {published data only}
- Krittayaphong R, Viprakasit V, Saiviroonporn P, Wangworatrakul W, Wood JC. Serum ferritin in the diagnosis of cardiac and liver iron overload in thalassaemia patients real-world practice: a multicentre study. British Journal of Haematology 2018;182(2):301-5. [DOI] [PubMed] [Google Scholar]
Labbe 2004 {published data only}
- Labbe RF, Dewanji A. Iron assessment tests: transferrin receptor vis-a-vis zinc protoporphyrin. Clinical Biochemistry 2004;37(3):165-74. [DOI] [PubMed] [Google Scholar]
Lebray 2004 {published data only}
- Lebray P, Zylberberg H, Hue S, Poulet B, Carnot F, Martin S, et al. Influence of HFE gene polymorphism on the progression and treatment of chronic hepatitis C. Journal of Viral Hepatitis 2004;11(2):175-82. [DOI] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Lee DH, Jacobs DR Jr. Serum markers of stored body iron are not appropriate markers of health effects of iron: a focus on serum ferritin. Medical Hypotheses 2004;62(3):442-5. [DOI] [PubMed] [Google Scholar]
Legros 2015 {published data only}
- Legros L, Bardou-Jacquet E, Latournerie M, Guillygomarc'h A, Turlin B, Le Lan C, et al. Non-invasive assessment of liver fibrosis in C282Y homozygous HFE hemochromatosis. Liver International 2015;35(6):1731-8. [DOI] [PubMed] [Google Scholar]
Leitch 2009 {published data only}
- Leitch HA, Chase J, Vickars L. Red blood cell transfusion independence following the initiation of Iron chelation therapy in myelodysplastic syndrome: a case report and review of the literature. Haematologica 2009;94:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leitch 2012 {published data only}
- Leitch HA, Chan C, Leger CS, Foltz LM, Ramadan KM, Vickars LM. Improved survival with iron chelation therapy for red blood cell transfusion dependent lower IPSS risk MDS may be more significant in patients with a non-RARS diagnosis. Leukemia Research 2012;36(11):1380-6. [DOI] [PubMed] [Google Scholar]
Lewis 1982 {published data only}
- Lewis GJ. Do women with menorrhagia need iron? British Medical Journal, Clinical Research Edition 1982;284(6323):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leyland 1975 {published data only}
- Leyland MJ, Ganguli PC, Blower D, Delamore IW. Immunoradiometric assay for ferritin in human serum. Scandinavian Journal of Haematology 1975;14(5):385-92. [DOI] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li CK, Chik KW, Lam CW, To KF, Yu SC, Lee BV, et al. Liver disease in transfusion dependent thalassaemia major. Archives of Disease in Childhood 2002;86(5):344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Licata 2009 {published data only}
- Licata A, Nebbia ME, Cabibbo G, Lo Iacono G, Barbaria F, Brucato V, et al. Hyperferritinemia is a risk factor for steatosis in chronic liver disease. World Journal of Gastroenterology 2009;15(17):2132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linkesch 1978 {published data only}
- Linkesch W. Serum ferritin - diagnostic and clinical significance [Serumferritin - diagnostische und klinische bedeutung]. Acta Medica Austriaca 1978;5(4-5):169-71. [PubMed] [Google Scholar]
Lipschitz 1974 {published data only}
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. New England Journal of Medicine 1974;290(22):1213-6. [DOI] [PubMed] [Google Scholar]
Lu 2013 {published data only}
- Lu W, Zhao M, Rajbhandary S, Xie F, Chai X, Mu J, et al. Free iron catalyzes oxidative damage to hematopoietic cells/mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. European Journal of Haematology 2013;91(3):249-61. [DOI] [PubMed] [Google Scholar]
Luxton 1977 {published data only}
- Luxton AW, Walker WH, Gauldie J, Ali AM, Pelletier C. A radioimmunoassay for serum ferritin. Clinical Chemistry 1977;23(4):683-9. [PubMed] [Google Scholar]
Macdougall 1998 {published data only}
- Macdougall IC. What is the most appropriate strategy to monitor functional iron deficiency in the dialysed patient on rhEPO therapy? Merits of percentage hypochromic red cells as a marker of functional iron deficiency. Nephrology Dialysis Transplantation 1998;13(4):847-9. [DOI] [PubMed] [Google Scholar]
Machado 2009 {published data only}
- Machado MV, Ravasco P, Martins A, Almeida MR, Camilo ME, Cortez-Pinto H. Iron homeostasis and H63D mutations in alcoholics with and without liver disease. World Journal of Gastroenterology 2009;15(1):106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maliken 2012a {published data only}
- Maliken BD, Avrin WF, Nelson JE, Mooney J, Kumar S, Kowdley KV. Room-temperature susceptometry predicts biopsy-determined hepatic iron in patients with elevated serum ferritin. Annals of Hepatology 2012;11(1):77-84. [PMC free article] [PubMed] [Google Scholar]
Martinelli 2000 {published data only}
- Martinelli AL, Franco RF, Villanova MG, Figueiredo JF, Secaf M, Tavella MH, et al. Are haemochromatosis mutations related to the severity of liver disease in hepatitis C virus infection? Acta Haematologica 2000;102(3):152-6. [DOI] [PubMed] [Google Scholar]
Martinelli 2004 {published data only}
- Martinelli AL, Filho AB, Franco RF, Tavella MH, Ramalho LN, Zucoloto S, et al. Liver iron deposits in hepatitis B patients: association with severity of liver disease but not with hemochromatosis gene mutations. Journal of Gastroenterology and Hepatology 2004;19(9):1036-41. [DOI] [PubMed] [Google Scholar]
Massawe 1999 {published data only}
- Massawe SN, Urassa EN, Mmari M, Ronquist G, Lindmark G, Nystrom L. The complexity of pregnancy anemia in Dar-es-Salaam. Gynecologic & Obstetric Investigation 1999;47(2):76-82. [DOI] [PubMed] [Google Scholar]
Massey 1992 {published data only}
- Massey AC. Microcytic anemia. Differential diagnosis and management of iron deficiency anemia. Medical Clinics of North America 1992;76(3):549-66. [DOI] [PubMed] [Google Scholar]
Maximova 2017 {published data only}
- Maximova N, Gregori M, Simeone R, Sonzogni A, Boz G, Fucile C, et al. Safety and tolerability of deferasirox in pediatric hematopoietic stem cell transplant recipients: one facility's five years' experience of chelation treatment. Oncotarget 2017;8(38):63177-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazza 1995 {published data only}
- Mazza P, Giua R, De Marco S, Bonetti MG, Amurri B, Masi C, et al. Iron overload in thalassemia: comparative analysis of magnetic resonance imaging, serum ferritin and iron content of the liver. Haematologica 1995;80(5):398-404. [PubMed] [Google Scholar]
Mazzolenis 1992 {published data only}
- Mazzolenis D, Scuteri R, Blasetti A, Sivak L, Rendo P, Cavalli N, et al. Study and treatment of anaemia among chronic hemodialysis patients. Nefrologia 1992;12:15-21. [Google Scholar]
Meredith 1999 {published data only}
- Meredith J, Rosenthal N. Differential diagnosis of microcytic anemias. Laboratory Medicine 1999;30(8):538-42. [Google Scholar]
Milman 1991 {published data only}
- Milman N. Hereditary haemochromatosis in Denmark 1950-1985. Clinical, biochemical and histological features in 179 patients and 13 preclinical cases. Danish Medical Bulletin 1991;38(4):385-93. [PubMed] [Google Scholar]
Milman 1996 {published data only}
- Milman N. Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy. International Journal of Hematology 1996;63(2):103-35. [DOI] [PubMed] [Google Scholar]
Minniti 2011 {published data only}
- Minniti CP, Abd-Bmoniem KZ, Wilson J, Heller T, Koh C, Jackson M, et al. Relationship of liver iron concentration with hepatic and cardiac R2* in adults with sickle cell anemia. American Journal of Hematology 2011;86(10):E13. [Google Scholar]
Moicean 2004 {published data only}
- Moicean A, Brabete A, Diculescu M, Puscariu T, Dobrea C, Ostroveanu D, et al. Primary biliary cirrhosis (PBC) and idiopathic hemochromatosis (IH) associated with erythroblastopenia (PRCA). Evolution on treatment with cyclosporin. Annals of Fundeni Hospital 2004;9(1-2):30-4. [Google Scholar]
Moirand 1997 {published data only}
- Moirand R, Mortaji AM, Loréal O, Paillard F, Brissot P, Deugnier Y. A new syndrome of liver iron overload with normal transferrin saturation. Lancet 1997;349(9045):95-7. [DOI] [PubMed] [Google Scholar]
Moreb 1983 {published data only}
- Moreb J, Popovtzer MM, Friedlaender MM, Konijn AM, Hershko C. Evaluation of iron status in patients on chronic hemodialysis: relative usefulness of bone marrow hemosiderin, serum ferritin, transferrin saturation, mean corpuscular volume and red cell protoporphyrin. Nephron 1983;35(3):196-200. [DOI] [PubMed] [Google Scholar]
Morrison 2003 {published data only}
- Morrison ED, Brandhagen DJ, Phatak PD, Barton JC, Krawitt EL, El-Serag HB, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Annals of Internal Medicine 2003;138(8):627-33. [DOI] [PubMed] [Google Scholar]
Mueller 2017 {published data only}
- Mueller J, Raisi H, Rausch V, Peccerella T, Simons D, Ziener CH, et al. Sensitive and non-invasive assessment of hepatocellular iron using a novel room-temperature susceptometer. Journal of Hepatology 2017;67(3):535-42. [DOI] [PubMed] [Google Scholar]
Murphy 1997 {published data only}
- Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Seminars in Hematology 1997;34(1):29-39. [PubMed] [Google Scholar]
Murphy 2006 {published data only}
- Murphy PT, Quinn JP, O'Donghaile D, Swords R, O'Donnell JR. Myelodysplastic patients with raised percentage of hypochromic red cells have evidence of functional iron deficiency. Annals of Hematology 2006;85(7):455-7. [DOI] [PubMed] [Google Scholar]
Musso 1991 {published data only}
- Musso A, Gonzalez E, Santos M, Dominguez M, Madaio M, Del Rio O, et al. Investigation of iron deficiency in patients with chronic kidney failure in hemodialysis [Investigación de la deficiencia de hierro en pacientes con insuficiencia renal crónica en hemodiálisis]. Revista de Nefrología, Diálisis y Transplante 1991;30:3-10. [Google Scholar]
Nagai 2005 {published data only}
- Nagai T, Komatsu N, Sakata Y, Miura Y, Ozawa K. Iron deficiency anemia with marked thrombocytosis complicated by central retinal vein occlusion. Internal Medicine 2005;44(10):1090-2. [DOI] [PubMed] [Google Scholar]
Nagashima 2006 {published data only}
- Nagashima M, Kudo M, Chung H, Ishikawa E, Hagiwara S, Hagiwara S, et al. Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatology Research 2006;36(4):288-93. [DOI] [PubMed] [Google Scholar]
Nathanson 1999 {published data only}
- Nathanson S, Deschenes G, Bensman A. The biochemical and hematological assessment of iron metabolism. Archives de Pediatrie 1999;6(11):1199-204. [DOI] [PubMed] [Google Scholar]
Navone 1988 {published data only}
- Navone R, Azzoni L, Valente G. Immunohistochemical assessment of ferritin in bone marrow trephine biopsies: correlation with marrow hemosiderin. Acta Haematologica 1988;80(4):194-8. [DOI] [PubMed] [Google Scholar]
Nelson 2011 {published data only}
- Nelson J, Mooney J, Avrin W, Kumar S, Kowdley K. Room temperature susceptometry for hepatic iron measurement. American Journal of Hematology 2011;86(9):E115. [Google Scholar]
Nielsen 2000 {published data only}
- Nielsen P, Gunther U, Durken M, Fischer R, Dullmann J. Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. Journal of Laboratory & Clinical Medicine 2000;135(5):413-8. [DOI] [PubMed] [Google Scholar]
Nuwayri‐Salti 1991 {published data only}
- Nuwayrisalti N, Jabre F, Saab G, Daouk M, Salem Z. Blood leukocyte contribution to serum ferritin levels in patients on chronic hemodialysis. Nephron 1991;57(2):144-8. [DOI] [PubMed] [Google Scholar]
O'Brien 1990 {published data only}
- O'Brien T, Barrett B, Murray DM, Dinneen S, O'Sullivan DJ. Usefulness of biochemical screening of diabetic patients for hemochromatosis. Diabetes Care 1990;13(5):532-4. [DOI] [PubMed] [Google Scholar]
O'Keeffe 2005 {published data only}
- O'Keeffe ST. Iron deficiency with normal ferritin levels in restless legs syndrome. Sleep Medicine 2005;6(3):281-2. [DOI] [PubMed] [Google Scholar]
Oduor 2017 {published data only}
- Oduor H, Minniti CP, Brofferio A, Gharib AM, Abd-Elmoniem KZ, Hsieh MM, et al. Severe cardiac iron toxicity in two adults with sickle cell disease. Transfusion 2017;57(3):700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olivieri 1995 {published data only}
- Olivieri NF, Brittenham GM, Matsui D, Berkovitch M, Blendis LM, Cameron RG, et al. Iron-chelation therapy with oral deferipronein patients with thalassemia major. New England Journal of Medicine 1995;332(14):918-22. [DOI] [PubMed] [Google Scholar]
Olynyk 2009 {published data only}
- Olynyk JK, Gan E, Tan T. Predicting iron overload in hyperferritinemia. Clinical Gastroenterology & Hepatology 2009;7(3):359-62. [DOI] [PubMed] [Google Scholar]
Özatli 2000 {published data only}
- Özatli D, Köksal AS, Haznedaroglu IC, Simsek H, Karakus S, Buyukasik Y, et al. Anemias in chronic liver diseases. Hematology 2000;5(1):69-76. [DOI] [PubMed] [Google Scholar]
Ozkale 2014 {published data only}
- Ozkale M, Sipahi T. Hematologic and bone marrow changes in children with protein-energy malnutrition. Pediatric Hematology and Oncology 2014;31(4):349-58. [DOI] [PubMed] [Google Scholar]
Pakbaz 2005 {published data only}
- Pakbaz Z, Fischer R, Treadwell M, Yamashita R, Fung EB, Calvelli L, et al. A simple model to assess and improve adherence to iron chelation therapy with deferoxamine in patients with thalassemia. Annals of the New York Academy of Sciences 2005;1054:486-91. [DOI] [PubMed] [Google Scholar]
Parkash 2015 {published data only}
- Parkash O, Akram M. Hereditary hemochromatosis. Journal of the College of Physicians and Surgeons - Pakistan 2015;25(9):644-7. [PubMed] [Google Scholar]
Pastrana 2007 {published data only}
- Pastrana RJ, Torres EA, Arroyo JM, Rivera CE, Sanchez CJ, Morales L. Iron-deficiency anemia as presentation of pouchitis. Journal of Clinical Gastroenterology 2007;41(1):41-4. [DOI] [PubMed] [Google Scholar]
Pautas 2012 {published data only}
- Pautas E, Siguret V, Kim TMA, Chaibi P, Golmard JL, Gouronnec A, et al. Anemia in the elderly: usefulness of an easy and comprehensive laboratory screen. Annales de Biologie Clinique 2012;70(6):643-7. [DOI] [PubMed] [Google Scholar]
Peters 2017 {published data only}
- Peters TMA, Meulders AFM, Redert K, Cuijpers MLH, Rennings AJM, Janssen MCH, et al. TFR2-related haemochromatosis in the Netherlands: a cause of arthralgia in young adulthood. Netherlands Journal of Medicine 2017;75(2):56-64. [PubMed] [Google Scholar]
Phatak 2003 {published data only}
- Phatak PD, Barton JC. Phlebotomy-mobilized iron as a surrogate for liver iron content in hemochromatosis patients. Hematology (Amsterdam, Netherlands) 2003;8(6):429-32. [DOI] [PubMed] [Google Scholar]
Phelps 1996 {published data only}
- Phelps KR. Diagnosis of iron deficiency in dialysis patients. American Journal of Kidney Diseases 1996;27(5):762-4. [DOI] [PubMed] [Google Scholar]
Pietrangelo 2006 {published data only}
- Pietrangelo A, Corradini E, Ferrara F, Vegetti A, De Jong G, Luca Abbati G, et al. Magnetic resonance imaging to identify classic and nonclassic forms of ferroportin disease. Blood Cells Molecules & Diseases 2006;37(3):192-6. [DOI] [PubMed] [Google Scholar]
Porter 2016 {published data only}
- Porter JB, El-Alfy M, Viprakasit V, Giraudier S, Chan LL, Lai Y, et al. Utility of labile plasma iron and transferrin saturation in addition to serum ferritin as iron overload markers in different underlying anemias before and after deferasirox treatment. European Journal of Haematology 2016;96(1):19-26. [DOI] [PubMed] [Google Scholar]
Porter 2017a {published data only}
- Porter JB, Elalfy M, Taher A, Aydinok Y, Lee SH, Sutcharitchan P, et al. Limitations of serum ferritin to predict liver iron concentration responses to deferasirox therapy in patients with transfusion-dependent thalassaemia. European Journal of Haematology 2017;98(3):280-8. [DOI] [PubMed] [Google Scholar]
Porter 2017b {published data only}
- Porter JB, Cappellini MD, Kattamis A, Viprakasit V, Musallam KM, Zhu Z, et al. Iron overload across the spectrum of non-transfusion-dependent thalassaemias: role of erythropoiesis, splenectomy and transfusions. British Journal of Haematology 2017;176(2):288-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 2008 {published data only}
- Powell N, McNair A. Gastrointestinal evaluation of anaemic patients without evidence of iron deficiency. European Journal of Gastroenterology & Hepatology 2008;20(11):1094-100. [DOI] [PubMed] [Google Scholar]
Rao 1985 {published data only}
- Rao KV, Anderson WR. Hemosiderosis and hemochromatosis in renal transplant recipients. Clinical and pathological features, diagnostic correlations, predisposing factors, and treatment. American Journal of Nephrology 1985;5(6):419-30. [DOI] [PubMed] [Google Scholar]
Rao 2013 {published data only}
- Rao PS, Khandelia B. Assessment of iron stores in bone marrow using an intensive grading method. Indian Journal of Hematology and Blood Transfusion 2013;29(4):370-1. [Google Scholar]
Rehu 2011 {published data only}
- Rehu M, Ahonen S, Punnonen K. The diagnostic accuracy of the percentage of hypochromic red blood cells (%HYPOm) and cellular hemoglobin in reticulocytes (CHr) in differentiating iron deficiency anemia and anemia of chronic diseases. Clinica Chimica Acta 2011;412(19-20):1809-13. [DOI] [PubMed] [Google Scholar]
Remacha 2012 {published data only}
- Remacha AF, Sarda MP, Fernandez MC, Murga MJ. Laboratory diagnosis of iron deficiency. Transfusion Alternatives in Transfusion Medicine 2012;12(3-4):95-102. [Google Scholar]
Rimon 2002 {published data only}
- Rimon E, Levy S, Sapir A, Gelzer G, Peled R, Ergas D, et al. Diagnosis of iron deficiency anemia in the elderly by transferrin receptor-ferritin index. Archives of Internal Medicine 2002;162(4):445-9. [DOI] [PubMed] [Google Scholar]
Rioja 1990 {published data only}
- Rioja L, Girot R, Garabedian M, Cournot-Witmer G. Bone disease in children with homozygous beta-thalassemia. Bone & Mineral 1990;8(1):69-86. [DOI] [PubMed] [Google Scholar]
Rocchi 1993 {published data only}
- Rocchi E, Cassanelli M, Borghi A, Paolillo F, Pradelli M, Casalgrandi G, et al. Magnetic resonance imaging and different levels of iron overload in chronic liver disease. Hepatology 1993;17(6):997-1002. [PubMed] [Google Scholar]
Rushton 2010 {published data only}
- Rushton DH, Barth JH. What is the evidence for gender differences in ferritin and haemoglobin? Critical Reviews in Oncology-Hematology 2010;73(1):1-9. [DOI] [PubMed] [Google Scholar]
Sahoo 2012 {published data only}
- Sahoo N, Dash AK, Sahu P, Mishra B, Rath G, Pradhan SP. Haematological profile, serum iron and ferritin level in anemia of inflammation: a prospective study. Indian Journal of Hematology and Blood Transfusion 2012;28(4):248-9. [Google Scholar]
Saliba 2017 {published data only}
- Saliba AN, Musallam KM, Cappellini MD, Graziadei G, Daar S, Viprakasit V, et al. Serum ferritin values between 300 and 800 ng/mL in nontransfusion-dependent thalassemia: a probability curve to guide clinical decision making when MRI is unavailable. American Journal of Hematology 2017;92(3):E35-7. [DOI] [PubMed] [Google Scholar]
Shet 2010 {published data only}
- Shet AS, Pinto S, Mitra G, Subramaniam P, Mandal A. Glutathionyl hemoglobin has potential as a biomarker of iron deficiency anemia. Blood 2010;116(21):3210. [Google Scholar]
- Shet AS, Pinto SM, Mitra G, Mandal AK. Glutathionyl hemoglobin is elevated in iron deficiency anemia. Acta Haematologica 2012;127(1):26-30. [DOI] [PubMed] [Google Scholar]
Siddappa 2007 {published data only}
- Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology 2007;92(2):73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2005 {published data only}
- Silva IS, Perez RM, Oliveira PV, Cantagalo MI, Dantas E, Sisti C, et al. Iron overload in patients with chronic hepatitis C virus infection: clinical and histological study. Journal of Gastroenterology & Hepatology 2005;20(2):243-8. [DOI] [PubMed] [Google Scholar]
Sivgin 2016 {published data only}
- Sivgin S, Baldane S, Deniz K, Zararsiz G, Kaynar L, Cetin M, et al. Increased hepatic iron content predicts poor survival in patients with iron overload who underwent allogeneic hematopoietic stem cell transplantation. Clinical Lymphoma, Myeloma & Leukemia 2016;16(Suppl):S10-8. [DOI] [PubMed] [Google Scholar]
Smieja 1996 {published data only}
- Smieja MJ, Cook DJ, Hunt DL, Ali MA, Guyatt GH. Recognizing and investigating iron-deficiency anemia in hospitalized elderly people. Canadian Medical Association Journal 1996;155(6):691-6. [PMC free article] [PubMed] [Google Scholar]
Smith 1998 {published data only}
- Smith BC, Gorve J, Guzail MA, Day CP, Daly AK, Burt AD, et al. Heterozygosity for hereditary hemochromatosis is associated with more fibrosis in chronic hepatitis C. Hepatology 1998;27(6):1695-9. [DOI] [PubMed] [Google Scholar]
Smith 2014 {published data only}
- Smith E, Lebensburger J, Hilliard L, Kelly D, Fineberg N, Bai S, et al. Ferritin and LIC: predicting liver injury in children with sickle cell. Journal of Pediatric Gastroenterology and Nutrition 2014;58(3):387-90. [DOI] [PubMed] [Google Scholar]
Sokal 1986 {published data only}
- Sokal JE, Sheerin KA. Decreased stainable marrow iron in chronic granulocytic leukemia. American Journal of Medicine 1986;81(3):395-9. [DOI] [PubMed] [Google Scholar]
Stancu 2010 {published data only}
- Stancu S, Bârsan L, Stanciu A, Mircescu G. Can the response to iron therapy be predicted in anemic nondialysis patients with chronic kidney disease? Clinical Journal of the American Society of Nephrology 2010;5(3):409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2010 {published data only}
- Stein P, Yu H, Jain D, Mistry PK. Hyperferritinemia and iron overload in type 1 Gaucher disease. American Journal of Hematology 2010;85(7):472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sucak 2008 {published data only}
- Sucak GT, Yegin ZA, Ozkurt ZN, Aki SZ, Karakan T, Akyol G. The role of liver biopsy in the workup of liver dysfunction late after SCT: is the role of iron overload underestimated? Bone Marrow Transplantation 2008;42(7):461-7. [DOI] [PubMed] [Google Scholar]
Sumida 2007 {published data only}
- Sumida Y, Kanemasa K, Fukumoto K, Yoshida N, Sakai K. Hepatic iron accumulation may be associated with insulin resistance in patients with chronic hepatitis C. Hepatology Research 2007;37(11):932-40. [DOI] [PubMed] [Google Scholar]
Sutor 2002 {published data only}
- Sutor GC, Ceconi C, Flemming P, Wysk J, Fabel H, Schuppert F. An older female patient with refractory anemia and hemochromatosis [Eine ältere patientin mit refraktärer anämie und hämochromatose]. Deutsche Medizinische Wochenschrift 2002;127(34-35):1754-8. [DOI] [PubMed] [Google Scholar]
Taher 2005 {published data only}
- Taher A, Sheikh-Taha M, Sharara A, Inati A, Koussa S, Ellis G, et al. Safety and effectiveness of 100 mg/kg/day deferiprone in patients with thalassemia major: a two-year study. Acta Haematologica 2005;114(3):146-9. [DOI] [PubMed] [Google Scholar]
Tan 2011 {published data only}
- Tan T, Jaskowski L, Murphy T, Lipka G, Franklin M, Taylor P, et al. The serum hepcidin-to-ferritin ratio is an accurate predictor of increased hepatic iron and liver cirrhosis in non-hemochromatosis subjects (Podium #86). American Journal of Hematology 2011;86(9):E45. [Google Scholar]
Tan 2012 {published data only}
- Tan TCH, Crawford DHG, Franklin ME, Jaskowski LA, MacDonald GA, Jonsson JR, et al. The serum hepcidin: ferritin ratio is a potential biomarker for cirrhosis. Liver International 2012;32(9):1391-9. [DOI] [PubMed] [Google Scholar]
Thomason 2009 {published data only}
- Thomason RW, Almiski MS. Evidence that stainable bone marrow iron following parenteral iron therapy does not correlate with serum iron studies and may not represent readily available storage iron. American Journal of Clinical Pathology 2009;131(4):580-5. [DOI] [PubMed] [Google Scholar]
Thorsteinsson 2010 {published data only}
- Thorsteinsson V, Yngvason FE. Evaluation of iron status by serum transferrin receptor level [Mat á járnbúskap með mælingu á transferrínviðtökum]. Laeknabladid 2010;96(1):11-8. [DOI] [PubMed] [Google Scholar]
Tsironi 2008 {published data only}
- Tsironi M, Assimakopoulos G, Polonofi K, Rigaki K, Aessopos A. Effects of combined deferiprone and deferoxamine chelation therapy on iron load indices in beta-thalassemia. Hemoglobin 2008;32(1-2):29-34. [DOI] [PubMed] [Google Scholar]
Valberg 1975 {published data only}
- Valberg LS, Simon JB, Manley PN, Corbett WE, Ludwig J. Distribution of storage iron as body stores expand in patients with hemochromatosis. Journal of Laboratory & Clinical Medicine 1975;86(3):479-89. [PubMed] [Google Scholar]
Valberg 1980 {published data only}
- Valberg LS. Plasma ferritin concentrations: their clinical significance and relevance to patient care. Canadian Medical Association Journal 1980;122(11):1240-8. [PMC free article] [PubMed] [Google Scholar]
Valenti 2003 {published data only}
- Valenti L, Dongiovanni P, Fracanzani AL, Santorelli G, Fatta E, Bertelli C, et al. Increased susceptibility to nonalcoholic fatty liver disease in heterozygotes for the mutation responsible for hereditary hemochromatosis. Digestive & Liver Disease 2003;35(3):172-8. [DOI] [PubMed] [Google Scholar]
Valenti 2006 {published data only}
- Valenti L, Dongiovanni P, Piperno A, Fracanzani AL, Maggioni M, Rametta R, et al. Alpha 1-antitrypsin mutations in NAFLD: high prevalence and association with altered iron metabolism but not with liver damage. Hepatology 2006;44(4):857-64. [DOI] [PubMed] [Google Scholar]
Van Mook 2001 {published data only}
- Van Mook WNKA, Bourass-Bremers IHDN, Bos LP, Verhoeven HMJM, Engels LGJB. The outcome of esophagogastroduodenoscopy (EGD) in asymptomatic outpatients with iron deficiency anemia after a negative colonoscopy. European Journal of Internal Medicine 2001;12(2):122-6. [DOI] [PubMed] [Google Scholar]
Vassalle 2018 {published data only}
- Vassalle C, Meloni A, Pistoia L, Gamberini MR, Spasiano A, Gerardi C, et al. Relationship between uric acid levels and cardiometabolic findings in a large cohort of β-thalassemia major patients. Biomarkers in Medicine 2018;12(4):341-8. [DOI] [PubMed] [Google Scholar]
Velasco‐Rodriguez 2014 {published data only}
- Velasco-Rodriguez D, Alonso-Dominguez JM, Munoz-Novas C, Masso P, Jimenez-Rolando M, Quiros V, et al. Dysplastic bone marrow features can be predicted using the erythrogram provided by ADVIA 2120I analyzer (poster 145). International Journal of Laboratory Hematology 2014;36(Suppl 1):90. [Google Scholar]
Voigt 2008 {published data only}
- Voigt W, Jordan K, Sippel C, Amoury M, Schmoll HJ, Wolf HH. Severe thrombocytosis and anemia associated with celiac disease in a young female patient: a case report. Journal of Medical Case Reports 2008;2(96):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vreugdenhil 1989 {published data only}
- Vreugdenhil G, Baltus JA, Van Eijk HG, Swaak AJ. Prediction and evaluation of the effect of iron treatment in anaemic RA patients. Clinical Rheumatology 1989;8(3):352-62. [DOI] [PubMed] [Google Scholar]
Vreugdenhil 1990b {published data only}
- Vreugdenhil G, Wognum AW, Van Eijk HG, Swaak AJ. Anaemia in rheumatoid arthritis: the role of iron,vitamin B12, and folic acid deficiency, and erythropoietin responsiveness. Annals of the Rheumatic Diseases 1990;49(2):93-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walter 2008 {published data only}
- Walter PB, Macklin EA, Porter J, Evans P, Kwiatkowski JL, Neufeld EJ, et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of the Novartis CICL670A0107 trial. Haematologica 2008;93(6):817-25. [DOI] [PubMed] [Google Scholar]
Walters 1973 {published data only}
- Walters G, Miller F, Worwood M. Serum ferritin concentration and iron stores in normal subjects. Journal of Clinical Pathology 1973;26(10):770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang HJ, Zhang L, Zhou K, Jing LP, Yang DL, Li HQ, et al. Clinical and hematological features of congenital dyserythropoietic anemia type I. Chung-Hua Hsueh Yeh Hsueh Tsa Chih (Chinese Journal of Hematology) 2009;30(6):377-80. [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang R, Zhao S, Du J, He H, Tan P, Nan Y. Hepcidin involves in hepatic iron overload and liver injury in chronic hepatitis C virus infection. Journal of Hepatology 2014;1:S327. [Google Scholar]
Whiting 2002 {published data only}
- Whiting PW, Fletcher LM, Dixon JK, Gochee P, Powell LW, Crawford DHG. Concordance of iron indices in homozygote and heterozygote sibling pairs in hemochromatosis families: implications for family screening. Journal of Hepatology 2002;37(3):309-14. [DOI] [PubMed] [Google Scholar]
Wickenhauser 2011 {published data only}
- Wickenhauser C, Hoeppner M, Krahl R, Jaekel N, Niederwieser D, Al-Ali HK. Iron distribution in pre-transplant bone marrow biopsies and outcome after allogeneic hematopoietic cell transplantation. Onkologie 2011;34:274. [Google Scholar]
Wu 1989 {published data only}
- Wu M, Lu YY, Lin YH. Diagnosis of iron deficiency anemia with an optimum combination of tests. Zhonghua Nei Ke Za Zhi 1989;28(3):145-9. [PubMed] [Google Scholar]
Wulfhekel 1999 {published data only}
- Wulfhekel U, Düllmann J. Storage of iron in bone marrow plasma cells. Ultrastructural characterization, mobilization, and diagnostic significance. Acta Haematologica 1999;101(1):7-15. [DOI] [PubMed] [Google Scholar]
Xian 2012 {published data only}
- Xian LI. Clinical significance of detection of iron metabolism indexes in patients with hematologic malignant disease before and after treatment [血液系统恶性肿瘤患者监测铁代谢指标的临床意义]. Chinese Journal of Postgraduates of Medicine 2012;z2:27-30. [Google Scholar]
Yanardag 2002 {published data only}
- Yanardag H, Pamuk GE, Karayel T, Demirci S. Bone marrow involvement in sarcoidosis: an analysis of 50 bone marrow samples. Haematologia 2002;32(4):419-25. [PubMed] [Google Scholar]
Zadrazil 1991 {published data only}
- Zadrazil J, Papajík T, Scudla V, Jezáková J, Budíková M, Slavícek J. The iron reserve status in patients on regular dialysis treatment [Stav zásobního zeleza u nemocných v pravidelném dialyzacním lécení]. Vnitrni Lekarstvi 1991;37(4):369-75. [PubMed] [Google Scholar]
References to studies awaiting assessment
Anupama 2017 {published data only}
- Anupama KV, Rao PS, Adappa S, Balanthimogru P, Mahabala C. Correlation between serum ferritin and bone marrow iron stores. Tropical Doctor 2017;47(3):217-21. [DOI] [PubMed] [Google Scholar]
Balan 1994 {published data only}
- Balan V, Baldus W, Fairbanks V, Michels V, Burritt M, Klee G. Screening for hemochromatosis: a cost-effectiveness study based on 12,258 patients. Gastroenterology 1994;107(2):453-9. [DOI] [PubMed] [Google Scholar]
Cai 2017 {published data only}
- Cai J, Wu M, Ren J, Du Y, Long Z, Li G, et al. Evaluation of the efficiency of the reticulocyte hemoglobin content on diagnosis for iron deficiency anemia in Chinese adults. Nutrients 2017;9(5):E450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cippà 2014a {published data only}
- Cippà PE, Boucsein I, Adams H, Krayenbuehl PA. Estimating iron overload in patients with suspected liver disease and elevated serum ferritin. American Journal of Medicine 2014;127(10):1011.e1-3. [DOI] [PubMed] [Google Scholar]
Grote Beverborg 2018 {published data only}
- Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, Van der Wal HH, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circulation. Heart Failure 2018;11(2):e004519. [DOI] [PubMed] [Google Scholar]
Hagstrom 2016a {published data only}
- Hagstrom H, Nasr P, Bottai M, Ekstedt M, Kechagias S, Hultcrantz R, et al. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver International 2016;36(11):1688-95. [DOI] [PubMed] [Google Scholar]
Mehta 2016 {published data only}
- Mehta S, Goyal LK, Kaushik D, Gulati S, Sharma N, Harshvardhan L, et al. Reticulocyte hemoglobin vis-a-vis serum ferritin as a marker of bone marrow iron store in iron deficiency anemia. Journal of the Association of Physicians of India 2016;64(11):38-42. [PubMed] [Google Scholar]
Pilo 2018 {published data only}
- Pilo F, Caocci G, Mele G, Serreli V, Brundu E, La Nasa G. Perl's stain grade in the bone marrow aspirate correlates with overall survival in low risk myelodysplastic patients (P127). Haematologica 2019;104 (Suppl 2):109. [DOI] [PubMed] [Google Scholar]
- Pilo F, Caocci G, Mele G, Serreli V, Brundu E, La Nasa G. Perls' stain grade in the bone marrow aspirate correlates with overall survival in low risk myelodysplastic patients. Blood 2018;132 (Supplement 1):5517. [DOI] [PubMed] [Google Scholar]
Rudnicka 2017 {published data only}
- Rudnicka A, Woziwodzka A, Wroblewska A, Rybicka M, Bielawski KP, Sikorska K, et al. Analysis of polymorphism and hepatic expression of duodenal cytochrome b in chronic hepatitis C. Journal of Gastroenterology and Hepatology 2017;32(2):482-6. [DOI] [PubMed] [Google Scholar]
Uhrig 2019 {published data only}
- Uhrig M, Mueller J, Longerich T, Straub BK, Buschle LR, Schlemmer HP, et al. Susceptibility based multiparametric quantification of liver disease: non-invasive evaluation of steatosis and iron overload. Magnetic Resonance Imaging 2019;63:114-22. [DOI] [PubMed] [Google Scholar]
Wood 2017 {published data only}
- Wood MJ, Crawford DHG, Wockner LF, Powell LW, Ramm GA. Serum ferritin concentration predicts hepatic fibrosis better than hepatic iron concentration in human HFE-Haemochromatosis. Liver International 2017;37(9):1382-8. [DOI] [PubMed] [Google Scholar]
Additional references
Addison 1972
- Addison GM, Beamish MR, Hales CN, Hodgkins M, Jacobs A, Llewellin P. An immunoradiometric assay for ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. Journal of Clinical Pathology 1972;25(4):326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2008
- Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. New England Journal of Medicine 2008;358(3):221-30. [DOI] [PubMed] [Google Scholar]
Angelucci 2000
- Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, et al. Hepatic iron concentration and total body iron stores in thalassemia major. New England Journal of Medicine 2000;343(5):327-31. [DOI] [PubMed] [Google Scholar]
Bacon 2011
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS, American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54(1):328-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baer 1995
- Baer DM, Simons JL, Staples RL, Rumore GJ, Morton CJ. Hemochromatosis screening in asymptomatic ambulatory men 30 years of age and older. American Journal of Medicine 1995;98(5):464. [DOI] [PubMed] [Google Scholar]
Bain 2011
- Bain B, Bailey K. Pitfalls in obtaining and interpreting bone marrow aspirates: to err is human. Journal of Clinical Patholology 2011;64:373-9. [DOI] [PubMed] [Google Scholar]
Barry 1974
- Barry M. Liver iron concentration, stainable iron, and total body storage iron. Gut 1974;15:411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basset 1986
- Bassett ML, Halliday JW, Powell LW. Value of hepatic iron measurements in early hemochromatosis and determination of the critical iron level associated with fibrosis. Hepatology 1986;6(1):24-9. [DOI] [PubMed] [Google Scholar]
Beilby 1999
- Beilby JP, Prins AW, Swanson NR. Determination of hepatic iron concentration in fresh and paraffin-embedded tissue. Clinical Chemistry 1999;45(4):573-4. [PubMed] [Google Scholar]
Beutler 1958
- Beutler E, Robson MJ, Buttenwieser E. A comparison of the plasma iron, iron-binding capacity, sternal marrow iron and other methods in the clinical evaluation of iron stores. Annals of Internal Medicine 1958;48(1):60-82. [DOI] [PubMed] [Google Scholar]
BloodSafe eLearning Australia 2011
- BloodSafe eLearning Australia. Iron deficiency anaemia. bloodsafelearning.org.au/course/iron-deficiency-anaemia-ida/ (accessed prior to 7 April 2021).
Bossuyt 2008
- Bossuyt PM, Leeflang MM. Chapter 6: Developing criteria for including studies. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.4 [updated September 2008]. The Cochrane Collaboration, 2008. Available from srdta.cochrane.org/handbook-dta-reviews.
Brissot 2009
- Brissot P, Le Lan C, Troadec MB, Ropert M, Gaboriau F, Lescoat G, et al. Diagnosis and treatment of HFE-haemochromatosis. In: Beaumont C, Béris P, Beuzard Y, Brugnara C, editors(s). Disorders of Erythropoiesis, Erythrocytes and Iron Metabolism. European School of Haematology, 2009. [Google Scholar]
Brissot 2017
- Brissot P, Cavey T, Ropert M, Guggenbuhl P, Loréal O. Genetic hemochromatosis: pathophysiology, diagnostic and therapeutic management. Presse Medicale 2017;46(12 part 2):e288-e295. [DOI] [PubMed] [Google Scholar]
Bull‐Henry 2013
- Bull-Henry K, Al-Kawas FH. Evaluation of occult gastrointestinal bleeding. American Family Physician 2013;87(6):430-6. [PubMed] [Google Scholar]
Camaschella 2015
- Camaschella C. Iron-deficiency anemia. New England Journal of Medicine 2015;372(19):1832-43. [DOI] [PubMed] [Google Scholar]
Chaparro 2019
- Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low‐ and middle‐income countries. Annals of the New York Academy of Sciences 2019;1450(1):15-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conradie 1980
- Conradie JD, Mbhele BE. Quantitation of serum ferritin by enzyme-linked immunosorbent assay (ELISA). South African Medical Journal 1980;57(8):282-7. [PubMed] [Google Scholar]
Dacie 1995
- Dacie JV, Lewis SM. Chapter 8: Red Cell Cytochemistry. In: Practical Haematology. 8th edition. London: Churchill Livingston, 1995. [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882-93. [DOI] [PubMed] [Google Scholar]
Deugnier 1992
- Deugnier YM, Loréal O, Turlin B, Guyader D, Jouanolle H, Moirand R, et al. Liver pathology on genetic hemochromatosis: a review of 135 homozygous cases and their bioclinical correlations. Gastroenterology 1992;102(6):2050-9. [DOI] [PubMed] [Google Scholar]
Di Bisceglie 1992
- Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon Br. Measurements of iron status in patients with chronic hepatitis. Gastroenterology 1992;102(6):2018-13. [DOI] [PubMed] [Google Scholar]
Ferraro 2012
- Ferraro S, Mozzi R, Panteghini M. Revaluating serum ferritin as a marker of body iron stores in the traceability era. Clinical Chemistry and Laboratory Medicine 2012;50(11):1911-6. [DOI] [PubMed] [Google Scholar]
Ganz 2013
- Ganz T. Systemic iron homeostasis. Physiological Reviews 2013;93(4):1721-41. [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2018
- Garcia-Casal MN, Peña-Rosas JP, Urrechaga E, Escanero JF, Huo J, Martinez RX, et al. Performance and comparability of laboratory methods for measuring ferritin concentrations in human serum or plasma: a systematic review and meta-analysis. PLOS One 2018;13(5):e0196576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Casal 2019
- Garcia‐Casal MN, Pasricha SR, Sharma AJ, Peña‐Rosas JP. Use and interpretation of hemoglobin concentrations for assessing anemia status in individuals and populations: results from a WHO technical meeting. Annals of the New York Academy of Sciences 2019;1450(1):5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 1992
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. Journal of General Internal Medicine 1992;7(2):145-53. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halliday 1975
- Halliday JW, Gera KL, Powell LW. Solid phase radioimmunoassay for serum ferritin. Clinica Chimica Acta 1975;58(3):207-214. [DOI] [PubMed] [Google Scholar]
Hooper 2009
- Hooper L, Ashton K, Harvey L, Decsi T, Fairweather-Tate S. Assessing potential biomarkers of micronutrient status by using a systematic review methodology: methods. American Journal of Clinical Nutrition 2009;89:1953S-9S. [DOI] [PubMed] [Google Scholar]
Hughes 2004
- Hughes DA, Stuart-Smith SE, Bain BJ. How should stainable iron in bone marrow films be assessed? Journal of Clinical Pathology 2004;57(10):1038-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1978
- Jones BM, Wormwood M. Immunoradiometricassay for the acidic ferritin of humanheart: application to human tissue, cells and serum.. Clinica Chimica Acta 1978;85:81-8. [DOI] [PubMed] [Google Scholar]
Kassebaum 2014
- Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123(5):615-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 1994
- Li R, Chen X, Yan H, Deurenberg P, Garby L, Hautvast JG. Functional consequences of iron supplementation in iron-deficient female cotton mill workers in Beijing, China. American Journal of Clinical Nutrition 1994;59(4):908-13. [DOI] [PubMed] [Google Scholar]
Lozoff 2007
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(4 Suppl):S560-71. [DOI] [PubMed] [Google Scholar]
Lundin 1964
- Lundin P, Persson E, Weinfeld A. Comparison of hemosiderin estimation in bone marrow sections and bone marrow smears. Acta Medica Scandinavica 1964;175(3):383-390. [DOI] [PubMed] [Google Scholar]
Mei 2005
- Mei Z, Cogswell M, Parvanta I, Lynch S, Beard J, Stolzfus R, et al. Hemoglobin and ferritin are currently the most efficient Indicators of population response to iron interventions: an analysis of nine randomized controlled trials. Journal of Nutrition 2005;135:1974-80. [DOI] [PubMed] [Google Scholar]
Melempati 2009
- Malempati S, Joshi S, Lai S, Braner D, Tegtmeyer K. Bone marrow aspiration and biopsy. New England Journal of Medicine 2009;361:e28. [DOI] [PubMed] [Google Scholar]
Miles 1974
- Miles LE, Lipschitz DA, Bieber CP, Cook JD. Measurement of serum ferritin by a 2-site immunoradiometric assay. Analytical Biochemistry 1974;61(1):209-24. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Netz 2013
- Netz DJ, Mascarenhas J, Stehling O, Pierik AJ, Lill R. Maturation of cytosolic and nuclear iron-sulfur proteins. Trends in Cell Biology 2014;24(5):303–12. [DOI] [PubMed] [Google Scholar]
Ordway 2004
- Ordway GA, Garry DJ. Myoglobin: an essential hemoprotein in striated muscle. Journal of Experimental Biology 2004;207(20):3441-6. [DOI] [PubMed] [Google Scholar]
Papagiannakis 2013
- Papagiannakis P, Michalopoulos C, Papalexi F, Dalampoura D, Diamantidis MD. The role of Helicobacter pylori infection in hematological disorders. European Journal of Internal Medicine 2013;24(8):685-90. [DOI] [PubMed] [Google Scholar]
Pasricha 2010
- Pasricha SR, Flecknoe-Brown SC, Allen KJ, Gibson PR, McMahon LP, Olynyk JK, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Medical Journal of Australia 2010;193(9):525-32. [DOI] [PubMed] [Google Scholar]
Pasricha 2013
- Pasricha SR, Drakesmith H, Black J, Hipgrave D, Biggs BA. Control of iron deficiency anemia in low- and middle-income countries. Blood 2013;121(14):2607-17. [DOI] [PubMed] [Google Scholar]
Pasricha 2014
- Pasricha SR, Low M, Thompson J, Farrell A, De-Regil LM. Iron supplementation benefits physical performance in women of reproductive age: a systematic review and meta-analysis. Journal of Nutrition 2014;144(6):906-14. [DOI] [PubMed] [Google Scholar]
Peeling 2008
- Peeling P, Dawson B, Goodman C, Landers G, Trinder D. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. European Journal of Applied Physiology 2008;103(4):381-91. [DOI] [PubMed] [Google Scholar]
Piga 2009
- Piga A, Roggero S, Longo F, Ernst O, Rose C. Chapter 25: Evaluation and treatment of secondary iron overload. In: Beaumont C, Béris P, Beuzard Y, Brugnara C, editors(s). Disorders of Erythrocytes, Erythropoiesis and Iron Metabolism. European School of Haematology, 2009. [Google Scholar]
Ploem 1966
- Ploem JE, Wael J, Verloop MC, Punt K. Sideruria following a single dose of desferrioxamine-B as a diagnostic test in iron overload. British Journal of Haematology 1966;12(4):396-408. [DOI] [PubMed] [Google Scholar]
Rath 1948
- Rath CE, Finch CA. Sternal marrow hemosiderin; a method for the determination of available iron stores in man. The Journal of Laboratory and Clinical Medicine 1948;33(1):81-6. [PubMed] [Google Scholar]
RCPA 2010
- Royal College of Pathologists of Australasia. Ferritin - Plasma or Serum. RCPA Manual. Sydney: Royal College of Pathologists of Australasia, 2010. [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982-90. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2009
- Riley R, Williams D, Ross M, Zhao S, Chesney A, Clark B, et al. Bone marrow aspirate and biopsy II. Interpretation of the bone marrow aspirate and biopsy. Journal of Clinical Laboratory Analysis 2009;23:259-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rocchi 1986
- Rocchi E, Gilbertini P, Cassanelli M, Pietrangelo A, Borghi A, Ventura E. Serum ferritin in the assessment of liver iron overload and iron removal therapy in porphyria cutanea tarda. The Journal of Laboratory and Clinical Medicine 1986;107(1):36-42. [PubMed] [Google Scholar]
Rybo 1985
- Rybo E, Bengstsson Calle, Hallberg L. The relative importance of various laboratory measurements in the diagnosis of iron deficiency. Scandinavian Journal of Haematology 1985;34(S43):57-75. [DOI] [PubMed] [Google Scholar]
Rybo 1985a
- Rybo E. Diagnosis of iron deficiency. Scnndinaviari Journal of Haematology 1985;34(Suppl. 43):1-39. [DOI] [PubMed] [Google Scholar]
SAS 2015 [Computer program]
- SAS Institute Inc SAS software. SAS Institute Inc, Version 9.4. Cary: SAS Institute Inc, 2015.
Searle 1987
- Searle JW, Kerr JFR, Hallida JW, Powell LW. Iron storage disease. In: MacSween RNM, Anthony PP, Scheuer PJ, editors(s). Pathology of the liver. Churchill Livingstone edition. Edinburgh: Churchill Livingstone, 1987. [Google Scholar]
Singh 2012
- Singh S, Chang SM, Matchar DB, Bass EB. Chapter 7: Grading a body of evidence on diagnostic tests. Journal of General Internal Medicine 2012;27(Suppl 1):47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2013
- Spencer B. Blood donor iron status: are we bleeding them dry? Current Opinion in Hematology 2013;20(6):533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
St Pierre 2014
- St Pierre TG, El-Beshlawy A, Elalfy M, Al Jefri A, Al Zir K, Daar S, et al. Multicenter validation of spin-density projection-assisted R2-MRI for the noninvasive measurement of liver iron concentration. Magnetic Resonance in Medicine 2014;71(6):2215-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taikwogi 2017
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Mathematical Research 2017;26(4):1896–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2010
- Takwoingi Y, Deeks J. MetaDAS: A SAS macro for meta-analysis of diagnostic accuracy studies. Quick reference and worked example. Version 1.3. srdta.cochrane.org 2010 (accessed prior to 7 April 2021).
Torrance 1980
- Torrance JD, Bothwell TH. Tissue iron stores. Iron. In: Cook JD, editors(s). Methods in Haematoloy. Vol. 1. Edinburgh: Churchill Livingstone, 1980:90-115. [Google Scholar]
Verdon 2003
- Verdon F, Burnand B, Stubi CL, Bonard C, Graff M, Michaud A, et al. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ 2003;326(7399):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weatherall 2011
- Weatherall DJ. The inherited disorders of haemoglobin: an increasingly neglected global health burden. Indian Journal of Medical Research 2011;134(4):493-7. [PMC free article] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt P, Keijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3:25-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2006
- Whiting PF, Weswood ME, Rutjes AWS, Reitsma JB, Bossuyt PNM, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology 2006;6:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
WHO/CDC 2007
- World Health Organization/Centers for Disease Control and Prevention. Assessing the Iron Status of Populations: Including Literature Reviews. Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April 2004, 2007. who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107.pdf (accessed prior to 7 April 2021).
WHO 1989
- World Health Organization. Preventing and Controlling Anaemia through Primary Health Care: a Guide for Health Administrators and Programme Managers 1989. who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9241542497.pdf (accessed prior to 7 April 2021). [ISBN: 92 4 154249 7]
WHO 2001
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control. A Guide for Programme Managers 2001. apps.who.int/iris/bitstream/10665/66914/1/WHO_NHD_01.3.pdf?ua=1 (accessed prior to 7 April 2021). [WHO/NHD/01.3]
WHO 2011a
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System 2011. apps.who.int/iris/bitstream/10665/85839/3/WHO_NMH_NHD_MNM_11.1_eng.pdf?ua=1 (accessed prior to 7 April 2021). [WHO/NMH/NHD/MNM/11.1]
WHO 2011b
- World Health Organization. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations. Vitamin and Mineral Nutrition Information System 2011. who.int/vmnis/indicators/serum_ferritin.pdf= (accessed prior to 7 April 2021). [WHO/NMH/NHD/MNM/11.2]
WHO 2020
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. Geneva: World Health Organization, 2020. [PubMed] [Google Scholar]
WHO 2021
- World Health Organization. WHO Global Anaemia estimates, 2021 Edition Global anaemia estimates in women of reproductive age, by pregnancy status, and in children aged 6-59 months. WHO Global Health Observatory (www.who.int/data/gho, accessed 9 May 2021) 2021.


