Abstract
Heart failure (HF) is a devastating condition characterized by poor quality of life, numerous complications, high rate of readmission and increased mortality. HF is the most common cause of hospitalization in the United States especially among people over the age of 64 years. The number of people grappling with the ill effects of HF is on the rise as the number of people living to an old age is also on the increase.
Several factors have been attributed to these high readmission and mortality rates among which are; poor adherence with therapy, inability to keep up with clinic appointments and even failure to recognize early symptoms of HF deterioration which may be a result of cognitive impairment.
Therefore, this review seeks to compile the most recent information about the links between HF and dementia or cognitive impairment. We also assessed the prognostic consequences of cognitive impairment complicating HF, therapeutic strategies among patients with HF and focus on future areas of research that would reduce the prevalence of cognitive impairment, reduce its severity and also ameliorate the effect of cognitive impairment coexisting with HF.
Keywords: Heart failure, dementia, cognitive impairment, elderly, mortality, patients
1. Introduction
Heart failure (HF) is a devastating condition characterized by poor quality of life, numerous complications, high rate of hospital readmissions and mortality [1-3]. HF is the most common cause of hospitalization in the United States especially among people over the age of 64 years [1-4]. The number of people grappling with the ill effects of HF is on the rise as the number of people living to old age is also on the increase. Hospital readmissions are still high among patients with HF at a rate of about 25% within 30 days after discharge [4]. This high readmission rate among patients with HF in the United States is also accompanied by a very high cost burden which is over 20 billion dollars [5]. Several factors have been attributed to both high readmission and mortality rates some of which include poor adherence with therapy, inability to keep up with clinic appointments and even failure to recognize early symptoms of HF deterioration which may be a result of cognitive impairment (CGI) [6, 7].
CGI is prevalent among patients with HF and about 30% to 80% of patients with HF have varying degree of CGI
[6-9]. CGI in its broadest definition ranges from mild CGI at one end of the spectrum to dementia at the other end [10]. Interest in understanding the associations between heart disease and cognitive performance started several decades ago after a report in the Lancet which described “cardiogenic dementia” and the findings of Wanless et al. that reported variabilities in cardiac output having a direct impact on cerebral blood flow (CBF) [11, 12].
This review seeks to compile the most recent relevant information about the link between HF and dementia or CGI. We also aim to assess the prognostic consequences of CGI complicating HF, therapeutic and preventative strategies among patients with HF and CGI as well as provide focus on future areas of research that would reduce the prevalence of CGI, reduce its severity and also ameliorate the effect of CGI coexisting with HF.
2. Search Methods
The search strategy for this review article was carried out using PubMed, Science Direct, Web of Science, Scopus, Cochrane Library databases and Google Scholar. The reference lists of relevant articles were searched for additional studies related to heart failure and cognitive impairment.
All articles were reviewed in detail and only the most relevant articles were referenced.
Several search terms were used such as; “cognitive impairment and heart failure“ “dementia and heart failure among elderly patients” “pathophysiology of cognitive dysfunction among elderly patients with heart failure” “prognosis of heart failure with cognitive impairment” “therapeutic considerations among patients heart failure and cognitive impairment”.
3. Cognitive Impairment
CGI in its most severe form can have untoward effects on how people function on a daily basis and its effects are even more deleterious in patients with co-morbidities such as HF [13]. Mild degrees of CGI may still alter an individual unfavorably especially if it is a sequel to a disease state such as HF with an underlying dismal prognosis [14]. As stated earlier, CGI is a spectrum with mild CGI at one end and dementia at the other end [10, 14]. Mild CGI has been described by some researchers as an intermediary stage between normal aging and dementia. Mild CGI describes a state characterized by objective reduction in cognitive function with preserved daily functioning with or without loss of memory which can progress to dementia [14].
Dementia on the other hand, is a chronic progressive cognitive disorder which usually primarily affects memory and is also associated with aphasia, apraxia, agnosia and disturbances of executive functioning [15]. The most common form of dementia is Alzheimer’s disease (AD), and one of the major risk factors for its development is increasing age [16]. AD is characterized by accumulation of amyloid-beta and neurofibrillary tangles in the absence of a cerebrovascular insult. However, it has been noted that 20-40% of dementia cases in elderly patients are mixed AD and vascular dementia (combination of ischemic lesions and AD pathology) [17, 18]. There are some reports that AD is preceded by cardiovascular disease [17, 19]. There are also reports that suggest cardiovascular disease trigger AD-specific pathology [17, 19]. HF has been singled out among the cardiovascular diseases to be the most common contributor to the development of AD [20]. Some evidence also suggests that HF is an independent risk factor for AD [20].
4. Pathophysiology of Cognitive Impairment among Patients with Heart Failure
Distorted autoregulation with regards to cerebral blood flow (CBF) has been implicated in the occurrence of CGI among elderly patients as well as individuals with severe HF [21, 22]. CBF declines with increasing age and such gradual disruptions in cerebral hemodynamics appear to play a critical role in the pathogenesis of CGI [17, 19]. HF is another notable factor that may cause brain perfusion to drop below a critical threshold increasing the risk of CGI especially in elderly patients who as stated above also have intrinsically reduced CBF [17]. This line of thinking has been corroborated by some data indicating cognitive performance in patients with HF is closely related to the measurements of cerebral perfusion because decreased cardiac output was shown to be associated with lower brain volumes and the rate information is processed [7, 23]. In addition, some regions of the brain including the frontal cortex and para-hippocampal gyrus, which are implicated in cognition, appear to be more vulnerable in patients with HF [7, 24]. Imaging studies with magnetic resonance imaging (MRI) have revealed [7, 25] that patients with HF have increased frequencies of focal brain abnormalities ranging from multiple cortical or subcortical infarcts to small vessel disease with white-matter lesions and lacunar infarcts with cerebral embolism and hypo-perfusion being the most plausible pathophysiologic process [7, 26].
The domain of specific CGI is determined by the location of the lesions in each individual with HF and these differences among patients with HF may account for the inconsistencies between the studies regarding the impairment of specific cognitive domains [7]. In addition to location, severity of the lesion and the consequent cortical atrophy are also important determinants of CGI. Imaging techniques that integrate lesion location and severity of the lesion would be a valuable tool to fully evaluate patients with both HF and CGI [7].
It is also important to note that the reduction in CBF following HF may be further compounded by the ill effects of comorbid states such as hypertension, diabetes, sleep apnea, and depression [17]. In fact, concomitant increase in sympathetic tone associated with HF can cause chronic vasoconstriction in general and as well as in the cerebral circulation in response to physical and mental stress. These in turn can result in cerebrovascular damage and subsequent CGI.
Therefore, imbalance in hemodynamics can result in glucose and oxygen deprivation, which are critical for normal brain cell function. Disturbance of glucose and oxygen can in turn lead to a cascade of biochemical alterations that eventually lead to metabolic and tissue damage, including alterations to critical brain regions such as the hippocampus—a region highly sensitive to hypoxic episodes [24-32]. These biochemical changes can trigger neurodegeneration and cognitive decline.
5. Role of Ejection Fraction in Development of Cognitive Impairment
The correlations between different cardiac variables with CGI have been previously reported, although, with conflicting findings. In 1997, Zuccalà, et al. [33] suggested a positive correlation between left ventricular ejection fraction (LVEF) and cognition using the minimental state examination (MMSE) global score. The authors described a poorer cognitive performance in older adults with LVEF lower than 30% in a non-linear positive correlation. However, the degree of memory and CGI in presence of a reduced LVEF seems to be variable with age according with a study published by Festa et al. [34] which included younger adults with HF. This study showed that memory abilities in subjects younger than 63 years remained unchanged regardless of the level of LVEF while patients older than 63 years had a significant reduction in memory functions when LVEF was below 30% (P < 0.02). Furthermore, Bhattacharya et al. [35] reported how the volume of cerebral grey matter, measured with in-vivo brain MRI segmentation study, was inversely proportional to the LVEF levels, although, with a modest correlation (r=0.51, p=0.06).
However, the results of the findings by Jefferson et al. [36] was somewhat interesting. Their findings suggest a U-shaped association, rather than a linear relationship, between LVEF and markers of abnormal brain aging. Their findings also corroborated the fact that lower level of LVEF is related to abnormal brain aging but also noted an unexpected observation that participants with the highest (top quintile) LVEF values also had poorer cognitive performances [36]. The mechanism for their observation is unknown but they surmised that very high LVEF values may correspond to subtle cognitive impairment and that the U-shaped association between LVEF and cognitive aging requires further study [36].
LVEF at rest (i.e. without physical or mental stress) may be unrelated to severity of HF as reported by Franciosa et al. [37]. They noted that symptoms of HF occur most commonly during exertion, but cardiac evaluation is usually quantitated at rest. Franciosa et al. [37] also noted that the measures of left ventricular performance obtained at rest do not accurately reflect exercise tolerance and symptomatic status of patients with HF. Therefore, the potential relationship between LVEF and cognition probably has no physiopathological basis.
In spite of the unclear relationship between LVEF and cognitive dysfunction, it is still interesting to note that a significant improvement of cognitive function following heart transplantation was observed by some researchers [38]. Dixit et al. [39] also reported improvement in cognitive function after cardiac resynchronization suggesting that when hemodynamic compensation is achieved, the improvement in blood perfusion and specifically CBF leads to the recovery of cognition.
6. Role of Hypertension in Development of Cognitive Impairment
About 65% of individuals aged 65 years and over have hypertension [14, 40, 41]. Hypertension is a major risk factor for vascular dementia but it may also be involved in Alzheimer’s dementia which is also associated with some vascular abnormalities [19, 42]. As a result, mixed vascular and neurodegenerative dementia is the leading cause of age-related cognitive impairment [43].
Interestingly, diastolic blood pressure rather than systolic blood pressure has been reported to be predictive of cognitive impairment [44]. The association between blood pressure and poor cognitive performance has also been reported to be related by U-shaped curves such that both low and high blood pressures can result in CGI [45-47].
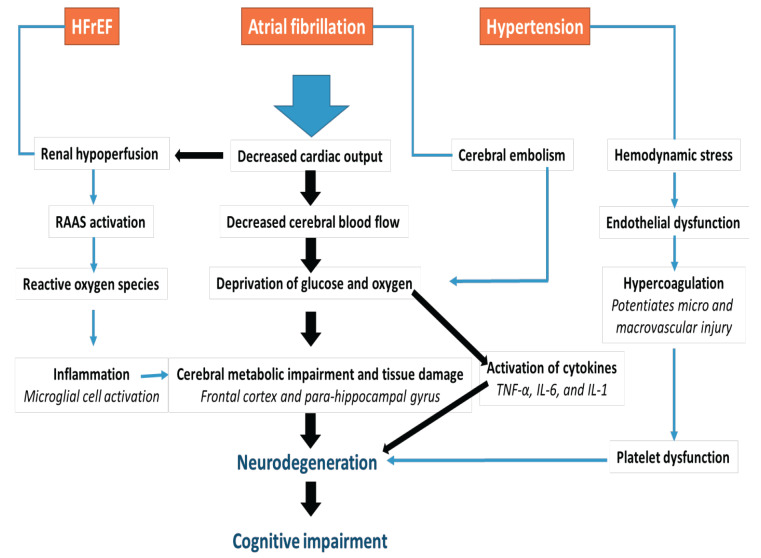
Systemic hypertension over an extended period induces hemodynamic stress on the cerebral blood vessels with resultant damage to the endothelium and consequent increase in vascular tone [48]. The role of hypertension and other factors in the pathophysiology of CGI among patients with HF is displayed in Fig. (1).
Fig. (1).
Pathophysiology of cognitive impairment in heart failure.
Abbreviations: TNF-α (tumor necrosis factor alpha), IL-6 (interleukin 6), IL-1(interleukin-1), RAAS (renin angiotensin aldosterone system).
Low blood pressure and orthostatic hypotension have been linked to CGI among elderly patients [48]. Orthostatic hypotension is especially common in elderly patients taking antihypertensive medications [48, 49].
Some reports indicate that the cerebrovascular system compensates for transient hypertension more efficiently as compared with hypotension [50, 51]. This was somewhat corroborated by a report that showed that the blood flow velocity in the middle cerebral artery was less affected by hypertension than by hypotension [52]. It therefore follows that, with the brain possibly being unable to compensate efficiently, low systemic blood pressure may result in a reduced cerebral blood flow, making the brain tissue susceptible to ischemic events [53].
Blood pressure abnormalities are common in patients with HF. Hypotension is particularly common in patients with a reduced ejection fraction [54]. Patients with a preserved ejection fraction on the other hand frequently exhibit elevated blood pressure [55, 56]. Therefore, patients with HF irrespective of the ejection fraction are predisposed to cerebrovascular damage and CGI because of blood pressure fluctuations.
7. Role of Coronary Artery Disease in Development of Cognitive Impairment
Patients with coronary artery disease (CAD) and CGI share many similar risk factors especially cardiovascular risk factors. The exact connection between CAD and CGI is still poorly understood. There are however some possible pathophysiologic mechanisms one of which is higher platelet activation among patients who have CAD and CGI in comparison with patients who have CAD without CGI [57]. Increased platelet activity may hasten the progression of CGI by increasing the adhesion of platelets on endothelial cells at the sites of vascular lesions by mechanisms involving Platelet-bound glycoprotein IIb–IIIa and P-selectin which could trigger perivascular inflammation in the brain [58]. Platelet activation is also associated with progression of carotid artery diseases and cerebral vasoconstriction which can further perpetuate cognitive decline [59].
8. Role of Atrial fibrillation in development of Cognitive Impairment
The prevalence of new atrial fibrillation (AF) is high in patients with HF and, ranges from 13 to 27% with increased prevalence as HF becomes more symptomatic [60-62]. Several mechanisms have been proposed for the association between AF and CGI. Ischemic brain lesions secondary to micro-embolism may be one of the mechanisms [63]. Zito et al. [64] also noted that elderly patients with non-valvular chronic AF, who used anticoagulants infrequently, had an inverse relationship between the number of lacunar lesions and MMSE value.
Another possible mechanism is related to consequent chronic hypoxic injury, secondary to reduced cardiac output among patients with AF and HF [48]. Cerebral hypoperfusion may result from elevated ventricular rate, reduced cardiac filling time and consequent reduced cardiac output [48, 65]. Therefore, patients with AF in the presence of HF have a reduced cardiac output which subsequently contributes to reductions in CBF to a critical point of causing neurocognitive dysfunction [66].
9. Role of Inflammation in Development of Cognitive Impairment
Systemic inflammation is an established accompaniment of HF [67-70]. Peripheral tissue hypoxia is one of the factors that activate cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-1 in patients with HF [67-70]. These cytokines have also been implicated in the neuronal inflammatory state associated with cognitive decline [67-70]. It is postulated that inflammatory mediators influence cognitive performance via various cytokine-mediated interactions between neurons and glial cells [69, 70]. Some in vitro and animal models appear to be in keeping with the hypothesis of a link between inflammation and cognitive decline [69].
The inflammatory state associated with HF is further potentiated by renal impairment as a complication of severe HF. Renal hypoperfusion is an important trigger for the activation of the renin aldosterone angiotensin system (RAAS), which then results in activation of the sympathetic nervous system and consequent release of reactive oxygen species (ROS) [71, 72]. ROS then escalate oxidative stress and result in a self-perpetuating inflammatory state characterized by constant release of cytokines and progressive cognitive decline [71, 72].
10. Role of Hypercoagulable State in Development of Cognitive Impairment
Patients with HF have an increased risk of developing a thrombus because the hemodynamic alterations lead to impaired blood flow and lower flow velocities. This in turn leads to stasis of blood and subsequent formation of thrombi in the left ventricle [73].
This state of hypercoagulability among patients with HF may also be potentiated by neuro-hormonal activation from HF related endothelial injury characterized by a cascade of platelet activation, platelet hyperreactivity, systemic inflammation, elevated levels of procoagulants and impaired fibrinolysis. All these act in concert to mediate hypercoagulability in patients with HF [73].
Hypercoagulability has been noted to be a feature of both HF and CGI resulting in the hypothesis that this may be an important pathophysiologic process linking HF and CGI [73, 74]. In line with this hypothesis, some studies have explored the effect of antithrombotic medication on cognitive decline and suggested a protective effect but some other studies could not demonstrate a very clear benefit [74, 75]. Therefore, further research may still be needed to corroborate the efficacy of antithrombotic therapy in reducing the risk of cognitive dysfunction.
11. Diagnosis of Cognitive Impairment
There are many screening tools used in the assessment of cognitive function. One of the most commonly used screening measure for cognitive assessment in clinical practice is the MMSE [76]. Other screening tools are the Montreal Cognitive Assessment (MoCA) and European Consortium Criteria (ECC). The ECC has been stated to be the gold standard but its application into clinical practice outside of specialized clinics may be somewhat cumbersome. Some researchers therefore opined that the use of the MoCA may be more universally applicable as it balances sensitivity and complexity issues [76-78].
Some comparative studies also revealed that MMSE was not a sensitive instrument in diagnosing mild CGI and that MoCA and ECC have far better sensitivity in diagnosing CGI [76-78]. They also pointed out appropriate use of screening tools with excellent sensitivities would increase the success rate in identifying patients at risk for developing dementia and also help with preventive strategies [76-78]. Interestingly, some researchers have used even easier tools such as the mini cog test to screen for mild cognitive impairment [79, 80]. The mini cog test combines a three-item memory test with a clock-drawing test.
Irrespective of what screen tool a physician decides to employ, the most important fact is that cognitive dysfunction among patients with HF should be screened because it may have far reaching consequences as discussed below.
12. Clinical and Prognostic Implications of Heart Failure Co-existing with Cognitive Impairment
Cognitive dysfunction is infrequently assessed among hospitalized patients [81, 82]. Cognitive dysfunction can impede management of HF as patients are likely to be oblivious of having a deficit in cognition [83]. CGI may result in non-adherence to dietary and medication management as well as follow-up clinic visits [76].
Agarwal et al. [79] reported that individuals with HF and CGI were readmitted at twice the rate of individuals who had HF without CGI and individuals with neither HF nor CGI. This result suggests that the relationship between CGI and hospital readmission may be different depending on the presence of comorbid conditions as well as other factors.
Mild CGI has also been noted to be related to higher cost of care as the direct cost of medical care was 44% higher for patients with mild CGI when compared to those without CGI [84].
Since CGI has been noted to be a marker for increased risk of readmission in HF, it may be useful to screen for CGI before discharge and follow up with modalities to ameliorate this condition [85-90].
In addition to increasing the risk of readmission, CGI has been shown to also increase the risk of death. Lan et al. [91] conducted a three-year follow up retrospective cohort study where CGI was shown to be an independent risk factor for mortality. The severity of CGI was noted to be a very important factor. When a comparison was made between patients with severe CGI and patients without CGI a worse outcome [hazard ratio (HR) of 2.710, (p=0.011)] was found among patients with HF and severe CGI [91]. These findings were similar to those reported by Byrne et al. [92].
The underlying mechanism for increased mortality found among patients with HF and CGI compared to their cognitively intact peers is still nebulous. Some plausible theories are that CGI results in increased readmission due to poor self-care, nonadherence with therapy and diet. Some authors have also noted that readmission for HF is on its own a potent risk factor for HF associated mortality [93] implying that the CGI may have an indirect role in causing death among patients with HF.
An interesting study done in Australia revealed that patients with HF who were enrolled in special management programs after hospitalization for HF had a reduced likelihood of post-hospital adverse outcomes as compared to patients with HF who had regular care post discharge [94]. This also parallels findings in another study where patients with HF and CGI were noted to have better adherence with medications if they were discharged to a long term care facility as opposed to being discharged home [95]. It suffices to state that a comprehensive and integrated plan prior to discharge among patients with HF and CGI may improve outcomes.
13. Therapeutic Considerations among Patients Heart Failure and Cognitive Impairment: Non-pharmacologic Approach
The benefits of aerobic physical activity among healthy individuals on cognitive function are well established in the literature [96, 97]. Evidence shows that when aerobic physical activity is performed consistently over a long period of time, it has the potential to promote beneficial effects on cardiovascular health, mediated by increased vagal tone and decreased sympathetic activity in the sinus node [96, 97]. This consequently leads to improved vascular function, cardiac remodeling, and improved renal-adrenal functions [96, 97]. Regular aerobic physical activity also has an important role in the modulation of some regions of the brain related to cognitive functioning, through the increase of cerebral blood flow in the prefrontal cortex [98, 99] increased volume in the hippocampus [100], increased concentrations of vascular endothelial growth factors (VEGF) and brain-derived neurotrophic factor (BDNF) (strengthening of synaptic connections) [101], as well as angiogenesis in lobofrontal regions [102].
In the work of Tanne et al. [103], exercise improved the cognitive functions of selective attention and psychomotor speed among patients who had HF with New York Heart Association (NYHA) functional class III and ejection fraction ≤ 35%. It was an 18 week intervention (twice a week) of physical exercises performed as alternating between treadmill, cycle ergometer and stair simulator.
Some authors have also demonstrated a direct correlation between the six-minute walk test and the MMSE score indicating that the lower the functional cardiovascular capacity of the patient, the lower their cognitive function [104].
It therefore follows that supervised exercise training may be useful as it improves mental health, helps to prevent depression, and promotes or maintains positive self-esteem [97].
It is a known fact that adherence to guideline directed medical therapy (GDMT) for HF improves symptoms, reduces re-hospitalizations and mortality [105, 106]. However therapy for HF is only effective if patients take their medications regularly. Sadly, adherence to pharmacotherapy among patients with HF is appallingly low at only 50% and may even decline overtime among individuals who were initially adherent [105-108].
Therefore, cognitive training should be norm among patients with HF as some observational and experimental studies have shown that this improves memory and cognitive performance which in turn may be beneficial in improving medication adherence [105, 109-111].
14. Pharmacologic Considerations
It is concerning to note that prescription rates are relatively low for GDMT among patients with HF because of the contra-indications these medications may be fraught with [112-117]. More worrisome is the fact that, these medications even when administered fail to reach target dosages that are associated with improved clinical outcomes less than 50% of the time [112-117]. CGI has been implicated as one of the multiple factors contributing to low prescription rates of GDMT among patients with HF [112-117].
The role of RAAS in the pathophysiology of CGI among patients with HF has been described above. Some authors looked into the efficacy of RAAS modulation in altering the prevalence of cognitive dysfunction among patients with HF. Goh et al. [118] conducted an analysis of patients with HF treated with angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEIs) and followed up at the end of one year to identify the number of new diagnoses of dementia [118]. There was weak evidence of a decreased risk of dementia with exposure to angiotensin receptor blockers with a smaller reduction in dementia risk in comparison to ACEIs [118].
On the other hand, the relationship between ACEIs and cognition is equivocal. Hoth et al. [119] documented that patients with HF who were taking ACEIs did not perform differently than patients not taking ACEIs. Surprisingly, they noted that patients taking ACEIs performed significantly worse on a measure of attention than patients not taking ACEIs [119]. The lack of a significant correlation between ACEIs and cognitive function may be related to dosing as well as the ability to cross the blood brain barrier. This line of thinking is corroborated by two studies. The first is one by Sink et al. [120] who reported that ACEIs as a class do not appear to be independently associated with risk of dementia or cognitive decline in older hypertensive adults. However, centrally active ACEIs were noted to be associated with 65% less decline in three MMSE scores per year of exposure (p= 0.01) and non-centrally active ACEIs were associated with greater risk of incident dementia (adjusted HR 1.20 (1.00-1.43) per year of exposure) and greater odds of independent activities of daily living disability (adjusted odds ratio (OR) 1.16 (1.03-1.30) per year of exposure) compared to other antihypertensive drugs [120]. This was also corroborated in another study where centrally acting ACEIs (captopril and perindopril) were compared to those that do not (enalapril and imidapril) cross the brain barrier [121].
There are plausible reasons for why centrally active ACEIs may be useful in reducing the risk of cognitive decline. Firstly, in addition to the anti-inflammatory actions of ACEIs is its role in modulation of the increased concentration of angiotensin converting enzymes, angiotensin II, and angiotensin I (AT1) receptors in the cerebral cortex of patients with Alzheimer’s disease [121, 122]. Animal studies have also shown that angiotensin II inhibits acetylcholine release [123]. Therefore, centrally active ACE inhibitor could decrease angiotensin II levels, potentially reducing the inhibitory action on acetylcholine release and thereby increase acetylcholine concentration [121-123]. Indirect increase in cerebral blood flow by centrally active ACEIs by decreasing vasoconstriction may be another plausible mechanism [121-123].
The above stated mechanism was also extended to centrally acting ARBs such as losartan which was shown to improve cognitive function in patients with hypertension, including in those who were elderly (up to 73 years of age) [124].
In the study by Zuccala et al. [125] the beneficial effect of ACEIs was more or less a dose-response relationship. They studied over 1,200 hospitalized patients with HF and found that patients who were started on ACEIs experienced improved scores on measures of global cognitive function during their hospital stay. They also found that higher doses and longer duration of ACEIs were associated with greater improvement of cognition across hospitalization even after controlling for multiple confounders in linear regression models [112, 125].
Few studies have looked at the relationship between newer therapies such as the sacubitril/valsartan combination in relation to cognitive decline. There is a theoretical risk of increased incidence of Alzheimer type dementia because neprilsyn inhibition also reduces the breakdown of amyloid-𝛽 peptides in the central nervous system [126-128]. This same association with regards to amyloid-𝛽 peptides has also been noted with ACEIs as angiotensin converting enzyme has been noted to be important in converting amyloid--β 1-42 into Aβ1-40 and that ACEIs block this process and increase Aβ1-42 deposition in the brain of mice [120, 129].
This association was explored by Cannon et al. [126] in their study were the incidence of dementia-related adverse effects in patients treated with sacubitril/valsartan was similar to that in patients treated with enalapril in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial and in patients enrolled in other heart trials [126]. De Vecchis et al. [130] did a retrospective cohort study comparing patients with HF on sacubitril/valsartan vs patients with HF not on sacubitril/valsartan. They concluded that patients taking sacubitril/valsartan for at least 3 months had similar mean MMSE scores to control subjects [130].
More research may need to be done to ascertain in more definite terms the effect of the new HF medication sacubitril/valsartan on cognitive function.
15. Atrial fibrillation therapy and Cognitive Impairment
AF is the most common type of cardiac arrhythmia. Age is the single greatest risk factor for AF and the prevalence of AF also increases as people get older [131-134]. AF is also a major risk factor for ischemic stroke, thromboembolism, HF, myocardial infarction, death as well as cognitive decline independent of ischemic stroke [131-134]. Therefore, therapeutic strategies that target important dementia pathophysiologic processes associated with AF may be useful. These accompaniments of AF include the coagulation pathway, inflammation, cholesterol metabolism as well as tachycardia associated complications. These would be discussed in the sub-sections below.
16. Anticoagulation Therapy
Several studies have assessed the impact of different anticoagulation strategies for AF treatment on dementia [131, 135]. The efficacy of warfarin in preventing worsening of cognitive decline was assessed by Bunch et al. [136] after review of medical records of 10537 patients on warfarin therapy for varying indications. They found out that the presence of AF significantly increased the risk of dementia, when compared with matched patients receiving warfarin anticoagulation for other reasons [136]. Some other authors have reported that the incidence of dementia among patients with AF who are on warfarin is higher among those who were for prolonged periods in the sub-therapeutic range for the international normalized ratio (INR) [137, 138]. Chronic cerebral injury from silent cerebral ischemia has been implicated for the possible pathophysiology for the association between sub-therapeutic INR and dementia [137-139]. A corollary to this hypothesis, is that the rate of incident dementia has been noted to be significantly higher among patients with poor adherence to anticoagulation therapy compared to those with the excellent adherence to anticoagulation therapy [131, 135, 136].
Conversely, supratherapeutic INR also appears to increase the risk of dementia among patients with AF who are on anticoagulation with warfarin as reported by Jacob et al. [140]. Jacobs et al. [140] reported that these findings may be in part, related to the presence of microbleeds in patients with frequent supratherapeutic INR levels. It therefore suffices to say that the quality of anticoagulation may play an important role in preventing dementia among patients with AF. Do these findings favour other forms of anticoagulation as keeping the INR within the therapeutic range with warfarin may be an arduous task.
Jacobs et al. [141] reported that there was significantly lower rates of incident dementia associated with use of direct oral anticoagulant (55.3% of participants were on rivaroxaban, 22.5% on apixaban, and 22.2% on dabigatran) for AF when compared to warfarin use for AF (0.3% with direct oral anticoagulants vs. 0.7% with warfarin, p=0.03) during a median follow-up of 243 days. However, some patients may have contra-indications to taking the direct oral anticoagulants and it may suffice to state that whatever anticoagulation modality is employed if used effectively may be associated with a lower risk of incident dementia.
17. Statin Therapy
On large-scale cohort study involving patients with AF older than 60 years of age receiving statin therapy compared with age- and sex-matched control participants with AF and no statin therapy revealed that statins were associated with a lower risk of nonvascular dementia (1.89% per year vs. 2.20% per year; P < 0.001) [135, 142]. This may be related to the pleotropic role of statins as well as its effect on the cholesterol metabolism which has been implicated in the pathophysiology of dementia. Further studies would be needed to corroborate these findings.
18. Catheter Ablation
There are conflicting reports on the effect of rhythm or rate control treatment strategy for AF on neurocognitive decline. It is however an established fact that catheter ablation is a well-established option for symptomatic AF that is resistant to drug therapy [143-145]. The recent report from the CASTLE-AF (Catheter Ablation versus Standard Conventional Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) trial indicates that patients with HF and AF who undergo catheter ablation have better outcomes [144-146].
The long term effect of catheter ablation for AF in preventing cognitive decline remains unclear. One prospective study compared 4212 consecutive patients who underwent AF ablation with 16,848 control participants with AF (matched for age and sex) [147]. The risk of dementia was significantly lower in patients with AF ablation compared with control participants (P < 0.0001).
The report of the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST), is still being awaited. They aim to randomize a total of 2745 patients with AF to early rhythm control therapy (antiarrhythmic drugs and AF ablation) or usual care [148, 149]. Cognitive function is a secondary end point that will be assessed after 24 months of follow-up. It would be exciting to see what the results are but preliminary results as mentioned above from one study appear to show a favorable response of early rhythm control therapy in preventing cognitive decline among patients with HF and AF.
19. Cardiac Resynchronization Therapy and Cognitive Function in Patients with Heart Failure
For patients with HF who meet the criteria for cardiac resynchronization therapy (CRT) improvement in survival, quality of life, and reduction in HF symptoms have been reported [150-152]. Duncker et al. [153] carried out a prospective study of patients with HF assigned to CRT in combination with an implantable cardioverter defibrillator (ICD) compared with ICD therapy alone. Cognitive functioning, concentration ability besides reduction in depressive symptoms and quality of life were noted to be significantly better after CRT-ICD compared with ICD alone in patients with HF. The exact reason is not fully understood but may be related to the fact that the benefit may result from secondary effects of both device therapies combined in reducing morbidity and quality of life.
One study looked specifically at the direct effect of CRT on cognitive dysfunction [39]. This study demonstrated that cognitive functioning associated with chronic HF significantly improved in patients responding to CRT, specifically in the domains of attention, working memory, and speed of processing [39]. This improvement may be related to the improvement of left ventricular ejection, reversal of cardiac remodeling, reduction of mitral regurgitation which ultimately results in less stretch of the myocardium and secondary reduction in incidence of malignant arrhythmias [154-157].
Large scale studies are still required to fully explore these potential secondary beneficial effects of CRT. However, since few of the preliminary studies in this regard have only proven these beneficial effects on cognition among CRT responders, more work needs to be done on increasing the response rate among patients with HF who are on CRT.
20. Implantable Cardioverter Defibrillator and Cognitive Function in Patients with Heart Failure
The ICDs though noted to be pivotal to improving survival among patients with severe HF has been noted to also have some complex trade-offs [152, 158]. ICDs come with a host of potential adverse events including a lower quality of life if shocked, increased number of hospitalizations and possible suffering at the end of life if they are not properly deactivated [158-160]. It would be interesting to note the effect of ICDs in improving cognitive function among patients with HF as well as to identify the effect of dementia on the efficacy of ICDs among patients with HF.
In line with the above, Green et al. [161] reported that more than 10% of patients receiving primary prevention ICDs are frail or have dementia. Frailty and dementia were also reported to be more strongly associated with mortality within the first year after ICD implantation than are traditional comorbidities [161]. Apparently patients with HF who have dementia do not appear to have long term benefits of ICDs in improving survival which has made some researchers to opine that it behooves on physicians to screen patients with HF for dementia before placing ICDs [161, 162]. Another study looked at a head to head comparison among patients with HF and CGI who had ICDs compared to a cohort of patients with HF and ICDs without cognitive dysfunction [163]. They found out that the 5-year survival of patients with CGI or dementia with pacemaker or ICD insertion was significantly lower than that of matched controls without cognitive impairment or dementia [163].
There are very few reports on the effect of ICDs on cognitive function among patients with HF. One single-center observational study demonstrated that ICD implantation for primary prevention among patients with HF did not show a worsening in anxiety and depression of mood and demonstrated similar cognitive performance in comparison with HF patients without ICD implantation [164].
More studies are required to explore the complex inter-relationship between cognitive function, ICD placement and HF prognosis.
21. Dementia Medications and Heart Failure
It would be great know the potential outcome of patients with HF taking both GDMT for HF with medications for dementia.
Two main pharmacological treatments are available for treating dementia which are; cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and N-methyl-D-aspartate (NMDA) receptor antagonists (memantine). Both drug classes can lead to statistically significant but clinically marginal improvements in cognition and overall assessment of dementia [165, 166]
Cholinesterase inhibitors are however, the most commonly prescribed medications for dementia and have been noted by some authors to have some cardio-protective properties [5, 167, 168].
The mechanism of this beneficial effect may be due to the fact that cholinesterase inhibitors have a negative chronotropic effect on the heart and increase vagal tone by augmenting acetylcholine release [169, 170]. Cholinesterase inhibitors have also been noted to have anti-inflammatory properties which positively impact the inflammation underlying atherosclerosis [171].
Some other reports have opined that there may be some potential deleterious effects of cholinesterase inhibitors as excessive cholinergic stimulation, may cause arrhythmias, such as bradycardia, sick sinus syndrome and torsades de pointes [172].
Not much has been studied about memantine on the other hand. However, some authors reported that memantine may cause adverse cardiovascular events [173].
Fosbol et al. [165] conducted a comparative study between donepezil and memantine to assess their cardiovascular safety across populations in Denmark and the United States. They concluded that cardiovascular safety was similar for both medications but memantine was associated with higher rates of myocardial infarction and cardiac death in the Danish publication which was however attributed to the fact that they had sicker cohorts in their own population.
Very few studies have been done to fully establish the relationship above and would potentially be a good line of research to further explore.
Conclusion
CGI is prevalent among patients with HF and can have dire consequences in terms of mortality, readmissions, length of hospital stay as well as health care cost. Screening for cognitive dysfunction among patients with HF is not yet routine and even simplistic modes of assessment may be beneficial as early detection may help in curbing further deterioration and a vicious cycle of a poor outcome among patients with HF. Some of the therapeutic options for HF such as GDMT, device therapy as well as non-pharmacology therapy appear to be useful on the whole in preventing and halting the progression of cognitive dysfunction, however, more studies would be needed to fully substantiate this evidence.
Future Direction of Research
Exploration of important markers and predictors of CGI among patients with HF which detects patients at risk of developing CGI would be very useful. Prospective cohort studies to assess the role of poor prognostic markers such as hormone sensitive troponin T, brain natriuretic peptide, uric acid among others in predicting the occurrence of CGI among patients with HF would be useful. This is because some of these biomarkers are easy to measure and may help to easily stratify patients at high risk for developing CGI. Also, the effect of modifying other identified predictors and markers in reducing the prevalence and severity of CGI among patients with HF would be very beneficial.
The potential impact of lifestyle modification (i.e. physical training, cessation of cigarette smoking, diet etc.) should be explored as potential aids to improving cognition among patients with HF. Randomized control trials taking these into consideration may be beneficial.
Acknowledgements
Declared none.
List of Abbreviations
- ACEIs
Angiotensin-Converting Enzyme Inhibitors
- AD
Alzheimer’s Disease
- AF
Atrial Fibrillation
- ARBs
Angiotensin Receptor Blockers
- AT1
Angiotensin I
- BDNF
Brain-Derived Neurotrophic Factor
- CAD
Coronary Artery Disease
- CASTLE-AF
Catheter Ablation versus Standard Conventional Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation
- CBF
Cerebral Blood Flow
- CGI
Cognitive Impairment
- CRT
Cardiac Resynchronization Therapy
- EAST
Early Treatment of Atrial Fibrillation for Stroke Prevention Trial
- ECC
European Consortium Criteria
- GDMT
Guideline Directed Medical Therapy
- HF
Heart Failure
- HR
Hazard Ratio
- ICD
Implantable Cardioverter Defibrillator
- IL-1
Interleukin-1
- IL-6
Interleukin 6
- INR
International Normalized Ratio
- LVEF
Left Ventricular Ejection Fraction
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- MRI
Magnetic Resonance Imaging
- NYHA
New York Heart Association
- OR
Odds Ratio
- PARADIGM-HF
Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure
- RAAS
Renin Angiotensin Aldosterone System
- ROS
Reactive Oxygen Species
- TNF-α
Tumor Necrosis Factor Alpha
- VEGF
Vascular Endothelial Growth Factors
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Liu L. Changes in cardiovascular hospitalization and comorbidity of heart failure in the United States: findings from the National Hospital Discharge Surveys 1980–2006. Int. J. Cardiol. 2011;149(1):39–45. doi: 10.1016/j.ijcard.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M., Vaduganathan M., Fonarow G.C., et al. Rehospitalization for heart failure: Problems and perspectives. J. Am. Coll. Cardiol. 2013;61(4):391–403. doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Jhund P.S., MacIntyre K., Simpson C.R., et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: A population study of 5.1 million people. Circulation. 2009;119(4):515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 4.Yancy C.W., Jessup M., Bozkurt B., et al. ACCF/AHA Guideline for the Management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009;360(14):1418. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 6.Malik A.S., Giamouzis G., Georgiopoulou V.V., et al. Patient perception versus medical record entry of health-related conditions among patients with heart failure. Am. J. Cardiol. 2011;107(4):569–572. doi: 10.1016/j.amjcard.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dardiotis E., Giamouzis G., Mastrogiannis D., et al. Cognitive impairment in heart failure. Cardiol. Res. Pract. 2012;2012:9. doi: 10.1155/2012/595821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogels R.L., Scheltens P., Schroeder‐Tanka J.M., et al. Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Heart Fail. 2007;9(5):440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Gure T.R., Blaum C.S., Giordani B., et al. Prevalence of cognitive imp airment in older adults with heart failure. J. Am. Geriatr. Soc. 2012;60(9):1724–1729. doi: 10.1111/j.1532-5415.2012.04097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon J.A., Moffitt P., Perez-Moreno A.C., et al. Cognitive impairment and heart failure: Systematic review and meta-analysis. J. Card. Fail. 2017;23(6):464–475. doi: 10.1016/j.cardfail.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Dementia C. Cardiogenic dementia. Lancet. 1977;1(8001):27–28. [PubMed] [Google Scholar]
- 12.Wanless R.B., Anand I.S., Gurden J., et al. Regional blood flow and hemodynamics in the rabbit with adriamycin cardiomyopathy: Effects of isosorbide dinitrate, dobutamine and captopril. J. Pharmacol. Exp. Ther. 1987;243:1101–1106. [PubMed] [Google Scholar]
- 13.Cermakova P., Lund L.H., Fereshtehnejad S.M., et al. Heart failure and dementia: Survival in relation to types of heart failure and different dementia disorders. Eur. J. Heart Fail. 2015;17(6):612–619. doi: 10.1002/ejhf.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abete P., Della-Morte D., Gargiulo G., et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart–brain continuum hypothesis. Ageing Res. Rev. 2014;18:41–52. doi: 10.1016/j.arr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Cermakova P., Eriksdotter M., Lund L.H., et al. Heart failure and Alzheimer′ s disease. J. Intern. Med. 2015;277(4):406–425. doi: 10.1111/joim.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallin K., Bostrom G., Kivipelto M., et al. Risk factors for incident dementia in the very old. Int. Psychogeriatr. 2013;25:1135–1143. doi: 10.1017/S1041610213000409. [DOI] [PubMed] [Google Scholar]
- 17.Alosco M.L., Hayes S.M. Structural brain alterations in heart failure: A review of the literature and implications for risk of Alzheimer’s disease. Heart Fail. Rev. 2015;20(5):561–571. doi: 10.1007/s10741-015-9488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zekry D., Hauw J.J., Gold G. Mixed dementia: Epidemiology, diagnosis, and treatment. J. Am. Geriatr. Soc. 2002;50:1431–1438. doi: 10.1046/j.1532-5415.2002.50367.x. [DOI] [PubMed] [Google Scholar]
- 19.De la Toree J.C. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 20.Qiu C., Winblad B., Marengoni A., et al. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch. Intern. Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 21.Montagne A., Barnes S.R., Sweeney M.D., et al. Blood‐brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferson A.L., Liu D., Gupta D.K., et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology. 2017;89:2327–2334. doi: 10.1212/WNL.0000000000004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferson A.L., Himali J.J., Beiser A.S., et al. Cardiac index is associated with brain aging: The Framingham Heart Study. Circulation. 2010;122(7):690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo M.A., Macey P.M., Fonarow G.C., et al. Regional brain gray matter loss in heart failure. J. Appl. Physiol. 2003;95(2):677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sila C.A. Cognitive impairment in chronic heart failure. Cleve. Clin. J. Med. 2007;74:S132–S137. doi: 10.3949/ccjm.74.suppl_1.s132. [DOI] [PubMed] [Google Scholar]
- 26.Pullicino P.M., Hart J. Cognitive impairment in congestive heart failure? Embolism vs hypoperfusion. Neurology. 2001;57(11):1945–1946. doi: 10.1212/wnl.57.11.1945. [DOI] [PubMed] [Google Scholar]
- 27.Austin B.P., Nair V.A., Meier T.B., et al. Effects of hypoperfusion in Alzheimer’s disease. J. Alzheimers Dis. 2011;26:123–133. doi: 10.3233/JAD-2011-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreisman N.R., Soliman S., Gozal D. Regional differences in hypoxic depolarization and swelling in hippocampal slices. J. Neurophysiol. 2000;83:1031–1038. doi: 10.1152/jn.2000.83.2.1031. [DOI] [PubMed] [Google Scholar]
- 29.Meguro T., Meguro Y., Kunieda T. Atrophy of the parahippocampal gyrus is prominent in heart failure patients without dementia. ESC Heart Fail. 2017;4(4):632–640. doi: 10.1002/ehf2.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Torre J.C. Critically attained threshold of cerebral hypoperfusion: Can it cause Alzheimer’s disease? Ann. N. Y. Acad. Sci. 2000;903:424–436. doi: 10.1111/j.1749-6632.2000.tb06394.x. [DOI] [PubMed] [Google Scholar]
- 31.Liebeskind D.S. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 32.Choi Y.H., Park H.K., Paik N.J. Role of the posterior temporal lobe during language tasks: A virtual lesion study using repetitive transcranial magnetic stimulation. Neuroreport. 2015;26:314–319. doi: 10.1097/WNR.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 33.Zuccalà G., Cattel C., Manes-Gravina E., et al. Left ventricular dysfunction: A clue to cognitive impairment in older patients with heart failure. J Neurol Neruosurg Psych. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Festa J.R., Jia X., Cheung K., et al. Association of low ejection fraction with impaired verbal memory in older patients with heart failure. Arch. Neurol. 2011;68(8):1021–1026. doi: 10.1001/archneurol.2011.163. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharya P., Bao F., Shah M., et al. Left ventricular dysfunction is associated with cerebral grey matter injury: An in-vivo brain MRI segmentation study. J. Neurol. Sci. 2012;321:111–113. doi: 10.1016/j.jns.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson A.L., Himali J.J., Au R., et al. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am. J. Cardiol. 2011;108:1346–1351. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franciosa J.A., Park M., Levine T.B. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am. J. Cardiol. 1981;47(1):33–39. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 38.Gruhn N., Larsen F.S., Boesgaard S., et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 39.Dixit N.K., Vazquez L.D., Cross N.J., et al. Cardiac resynchronization therapy: A pilot study examining cognitive change in patients before and after treatment. Clin. Cardiol. 2010;33:84–88. doi: 10.1002/clc.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu C., Winblad B., Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 41.Kearney P.M., Whelton M., Reynolds K., et al. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 42.Launer L.J. Demonstrating the case that AD is a vascular disease: Epidemiologic evidence. Ageing Res. Rev. 2002;1(1):61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 43.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cacciatore F., Abete P., Ferrara N., et al. The role of blood pressure in cognitive impairment in an elderly population. J. Hypertens. 1997;15(2):135–142. doi: 10.1097/00004872-199715020-00003. [DOI] [PubMed] [Google Scholar]
- 45.Waldstein S.R., Giggey P.P., Thayer J.F., et al. Nonlinear relations of blood pressure to cognitive function: The Baltimore Longitudinal Study of Aging. Hypertension. 2005;45(3):374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- 46.Kahonen-Vare M., Brunni-Hakala S., Lindroos M., et al. Left ventricular hypertrophy and blood pressure as predictors of cognitive decline in old age. Aging Clin. Exp. Res. 2004;16(2):147–152. doi: 10.1007/BF03324544. [DOI] [PubMed] [Google Scholar]
- 47.Pandav R., Dodge H.H., DeKosky S.T., et al. Blood pressure and cognitive impairment in India and the United States: A cross-national epidemiological study. Arch. Neurol. 2003;60(8):1123–1128. doi: 10.1001/archneur.60.8.1123. [DOI] [PubMed] [Google Scholar]
- 48.de La Torre J.C. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc. Psychiatry Neurol. 2012;2012:367516. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewington S., Clarke R., Qizilbash N., et al. Age-specific relevance of usual blood pressure to vascular mortality. Lancet. 2003;361(9366):1391–1392. doi: 10.1016/s0140-6736(03)13063-2. [DOI] [PubMed] [Google Scholar]
- 50.Rickards C.A., Tzeng Y.C. Arterial pressure and cerebral blood flow variability: Friend or foe? A review. Front. Physiol. 2014;5:120. doi: 10.3389/fphys.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Numan T., Bain A.R., Hoiland R.L., et al. Static autoregulation in humans: A review and reanalysis. Med. Eng. Phys. 2014;36:1487–1495. doi: 10.1016/j.medengphy.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Brassard P., Ferland-Dutil H., Smirl J.D., et al. Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H701–H704. doi: 10.1152/ajpheart.00790.2016. [DOI] [PubMed] [Google Scholar]
- 53.Sabayan B., van Buchem M.A., Sigurdsson S., et al. Cardiac hemodynamics are linked with structural and functional features of brain aging: The age, gene/environment susceptibility (AGES)-Reykjavik study. J. Am. Heart Assoc. 2015;4:e001294. doi: 10.1161/JAHA.114.001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erkelens C.D., van der Wal H.H., de Jong B.M., et al. Dynamics of cerebral blood flow in patients with mild non-ischaemic heart failure. Eur. J. Heart Fail. 2017;19:261–268. doi: 10.1002/ejhf.660. [DOI] [PubMed] [Google Scholar]
- 55.Yancy C.W., Lopatin M., Stevenson L.W., et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J. Am. Coll. Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 56.Quiroz R., Doros G., Shaw P., et al. Comparison of characteristics and outcomes of patients with heart failure preserved ejection fraction versus reduced left ventricular ejection fraction in an urban cohort. Am. J. Cardiol. 2014;113:691–696. doi: 10.1016/j.amjcard.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Stellos K., Panagiota V., Kögel A., et al. Predictive value of platelet activation for the rate of cognitive decline in Alzheimer’s disease patients. J. Cereb. Blood Flow Metab. 2010;30(11):1817–1820. doi: 10.1038/jcbfm.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akiyama H., Barger S., Barnum S., et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Velpen I.F., Yancy C.W., Sorond F.A., Sabayan B. Impaired cardiac function and cognitive brain aging. Can. J. Cardiol. 2017;33(12):1587–1596. doi: 10.1016/j.cjca.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Lip G.Y., Heinzel F.R., Gaita F., et al. European heart rhythm association/heart failure association joint consensus document on arrhythmias in heart failure, endorsed by the Heart rhythm society and the Asia Pacific heart rhythm society. Ep Europace. 2015;18(1):12–36. doi: 10.1093/europace/euv191. [DOI] [PubMed] [Google Scholar]
- 61.Staerk L., Sherer J.A., Ko D., et al. Atrial fibrillation: Epidemiology, pathophysiology, and clinical outcomes. Circ. Res. 2017;120(9):1501–1517. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mene-Afejuku T.O., López P.D., Akinlonu A., et al. Atrial fibrillation in patients with heart failure: Current state and future directions. Am. J. Cardiovasc. Drugs. 2018;18(5):347–360. doi: 10.1007/s40256-018-0276-1. [DOI] [PubMed] [Google Scholar]
- 63.Kwok C.S., Loke Y.K., Hale R., et al. Atrial fibrillation and incidence of dementia A systematic review and meta-analysis. Neurology. 2011;76(10):914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 64.Zito M., Muscari A., Marini E., et al. Silent lacunar infarcts in elderly patients with chronic non valvular atrial fibrillation. Aging Clin. Exp. Res. 1996;8(5):341–346. doi: 10.1007/BF03339591. [DOI] [PubMed] [Google Scholar]
- 65.Cacciatore F., Testa G., Langellotto A., et al. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: A 10-year study. Dement. Geriatr. Cogn. Disord. 2012;34(3-4):143–148. doi: 10.1159/000342195. [DOI] [PubMed] [Google Scholar]
- 66.Alosco M.L., Spitznagel M.B., Sweet L.H., et al. Atrial fibrillation exacerbates cognitive dysfunction and cerebral perfusion in heart failure. Pacing Clin. Electrophysiol. 2015;38(2):178–186. doi: 10.1111/pace.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anker S.D., von Haehling S. Inflammatory mediators in chronic heart failure: An overview. Heart. 2004;90:464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Candia A.M., Villacorta H., Jr, Mesquita E.T. Immune-inflammatory activation in heart failure. Arq. Bras. Cardiol. 2007;89:183–190, 201-208. doi: 10.1590/s0066-782x2007001500009. [DOI] [PubMed] [Google Scholar]
- 69.Athilingam P., Moynihan J., Chen L., et al. Elevated Levels of Interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure. Congest. Heart Fail. 2013;19:92–98. doi: 10.1111/chf.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cannon J.A., McMurray J.J., Quinn T.J. ‘Hearts and minds’: Association, causation and implication of cognitive impairment in heart failure. Alzheimers Res. Ther. 2015;7(1):22. doi: 10.1186/s13195-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Florea V.G., Cohn J.N. The autonomic nervous system and heart failure. Circ. Res. 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 72.Viswanathan G., Gilbert S. The cardiorenal syndrome: Making the connection. Int. J. Nephrol. 2010;2011:283137. doi: 10.4061/2011/283137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J.H., Shah P., Tantry U.S., et al. Coagulation abnormalities in heart failure: Pathophysiology and therapeutic implications. Curr. Heart Fail. Rep. 2016;13(6):319–328. doi: 10.1007/s11897-016-0308-6. [DOI] [PubMed] [Google Scholar]
- 74.Gupta A., Watkins A., Thomas P., et al. Coagulation and inflammatory markers in Alzheimer’s and vascular dementia. Int. J. Clin. Pract. 2005;59(1):52–57. doi: 10.1111/j.1742-1241.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 75.Moffitt P., Lane D.A., Park H., et al. Thromboprophylaxis in atrial fibrillation and association with cognitive decline: Systematic review. Age Ageing. 2016;45(6):767–775. doi: 10.1093/ageing/afw104. [DOI] [PubMed] [Google Scholar]
- 76.Alagiakrishnan K., Mah D., Dyck J.R., et al. Comparison of two commonly used clinical cognitive screening tests to diagnose mild cognitive impairment in heart failure with the golden standard European Consortium Criteria. Int. J. Cardiol. 2017;228:558–562. doi: 10.1016/j.ijcard.2016.11.193. [DOI] [PubMed] [Google Scholar]
- 77.Cameron J., Worrall-Carter L., Page K., et al. Screening for mild cognitive impairment in patients with heart failure: Montreal Cognitive Assessment versus Mini Mental State Exam. Eur. J. Cardiovasc. Nurs. 2013;12(3):252–260. doi: 10.1177/1474515111435606. [DOI] [PubMed] [Google Scholar]
- 78.Athilingam P., King K.B., Burgin S.W., Ackerman M., Cushman L.A., Chen L. Montreal Cognitive Assessment and Mini-Mental Status Examination compared as cognitive screening tools in heart failure. Heart Lung. 2011;40(6):521–529. doi: 10.1016/j.hrtlng.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal K.S., Kazim R., Xu J., et al. Unrecognized cognitive impairment and its effect on heart failure readmissions of elderly adults. J. Am. Geriatr. Soc. 2016;64(11):2296–2301. doi: 10.1111/jgs.14471. [DOI] [PubMed] [Google Scholar]
- 80.Scanlan J., Borson S. The Mini-Cog: Receiver operating characteristics with expert and naive raters. Int. J. Geriatr. Psychiatry. 2001;16:216–222. doi: 10.1002/1099-1166(200102)16:2<216::aid-gps316>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 81.Dodson J.A., Truong T.T., Towle V.R., et al. Cognitive impairment in older adults with heart failure: Prevalence, documentation, and impact on outcomes. Am. J. Med. 2013;126(2):120–126. doi: 10.1016/j.amjmed.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boustani M., Baker M.S., Campbell N., et al. Impact and recognition of cognitive impairment among hospitalized elders. J. Hosp. Med. 2010;5:69–75. doi: 10.1002/jhm.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riegel B., Carlson B., Glaser D. Development and testing of a clinical tool measuring self-management of heart failure. Heart Lung. 2000;29(1):4–15. doi: 10.1016/s0147-9563(00)90033-5. [DOI] [PubMed] [Google Scholar]
- 84.Zhu C.W., Sano M., Ferris S.H., et al. Health‐related resource use and costs in elderly adults with and without mild cognitive impairment. J. Am. Geriatr. Soc. 2013;61(3):396–402. doi: 10.1111/jgs.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cameron J., Worrall‐Carter L., Page K., et al. Does cognitive impairment predict poor self‐care in patients with heart failure? Eur. J. Heart Fail. 2010;12(5):508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 86.Alosco M.L., Spitznagel M.B., Cohen R., et al. Cognitive impairment is independently associated with reduced instrumental ADLs in persons with heart failure. J. Cardiovasc. Nurs. 2012;27(1):44. doi: 10.1097/JCN.0b013e318216a6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stilley C.S., Bender C.M., Dunbar-Jacob J., et al. The impact of cognitive function on medication management: Three studies. Health Psychol. 2010;29(1):50. doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakal J.A., McAlister F.A., Liu W., Ezekowitz J.A. Heart failure re-admission: Measuring the ever shortening gap between repeat heart failure hospitalizations. PLoS One. 2014;9(9):e106494. doi: 10.1371/journal.pone.0106494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huynh Q.L., Negishi K., Blizzard L., et al. Mild cognitive impairment predicts death and readmission within 30 days of discharge for heart failure. Int. J. Cardiol. 2016;221:212–217. doi: 10.1016/j.ijcard.2016.07.074. [DOI] [PubMed] [Google Scholar]
- 90.Gelow J.M., Mudd J.O., Chien C.V., Lee C.S. Usefulness of cognitive dysfunction in heart failure to predict cardiovascular risk at 180 days. Am. J. Cardiol. 2015;115(6):778–782. doi: 10.1016/j.amjcard.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lan H., Hawkins L.A., Kashner M., Perez E., Firek C.J., Silvet H. Cognitive impairment predicts mortality in outpatient veterans with heart failure. Heart Lung. 2018;47(6):546–552. doi: 10.1016/j.hrtlng.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 92.Byrne C.J., Toukhsati S.R., Toia D., O’Halloran P.D., Hare D.L. Hopelessness and cognitive impairment are risk markers for mortality in systolic heart failure patients. J. Psychosom. Res. 2018;109:12–18. doi: 10.1016/j.jpsychores.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Lin A.H., Chin J.C., Sicignano N.M., et al. Repeat hospitalizations predict mortality in patients with heart failure. Mil. Med. 2017;182(9):e1932–e1937. doi: 10.7205/MILMED-D-17-00017. [DOI] [PubMed] [Google Scholar]
- 94.Huynh Q., Negishi K., De Pasquale C., et al. Effects of post-discharge management on rates of early re-admission and death after hospitalisation for heart failure. Med. J. Aust. 2018;208(11):485–491. doi: 10.5694/mja17.00809. [DOI] [PubMed] [Google Scholar]
- 95.Rattinger G.B., Dutcher S.K., Chhabra P.T., et al. The effect of dementia on medication use and adherence among medicare beneficiaries with chronic heart failure. Am. J. Geriatr. Pharmacother. 2012;10(1):69–80. doi: 10.1016/j.amjopharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rêgo M.L., Cabral D.A., Fontes E.B. Cognitive deficit in heart failure and the benefits of aerobic physical activity. Arq. Bras. Cardiol. 2018;110(1):91–94. doi: 10.5935/abc.20180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu Q., Levine B.D. Exercise and the autonomic nervous system. Handb. Clin. Neurol. 2013;117:147–160. doi: 10.1016/B978-0-444-53491-0.00013-4. [DOI] [PubMed] [Google Scholar]
- 98.Tempest G.D., Davranche K., Brisswalter J., Perrey S., Radel R. The differential effects of prolonged exercise upon executive function and cerebral oxygenation. Brain Cogn. 2017;113:133–141. doi: 10.1016/j.bandc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 99.Leeuwis A.E., Hooghiemstra A.M., Amier R., et al. Design of the ExCersion-VCI study: The effect of aerobic exercise on cerebral perfusion in patients with vascular cognitive impairment. Alzheimers Dement. (N. Y.) 2017;3(2):157–165. doi: 10.1016/j.trci.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Praag H. Neurogenesis and exercise: Past and future directions. Neuromol Med. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 101.Hillman C.H., Erickson K.I., Kramer A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9(1):58. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 102.Swain R.A., Harris A.B., Wiener E.C., et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 103.Tanne D., Freimark D., Poreh A., et al. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int. J. Cardiol. 2005;103(2):145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 104.Baldasseroni S., Mossello E., Romboli B., et al. Relationship between cognitive function and 6-minute walking test in older outpatients with chronic heart failure. Aging Clin. Exp. Res. 2010;22(4):308–313. doi: 10.1007/BF03324936. [DOI] [PubMed] [Google Scholar]
- 105.Salmoirago-Blotcher E., Carey M.P. Can mindfulness training improve medication adherence? Integrative review of the current evidence and proposed conceptual model. Explore (NY) 2018;14(1):59–65. doi: 10.1016/j.explore.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cubbon R.M., Gale C.P., Kearney L.C., et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: A study across therapeutic eras. Circ Heart Fail. 2011;4(4):396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 107.Wu J.R., Moser D.K., Lennie T.A., Burkhart P.V. Medication adherence in patients who have heart failure: A review of the literature. Nurs. Clin. North Am. 2008;43(1):133–153. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 108.Wu J.R., Corley D.J., Lennie T.A., Moser D.K. Effect of a medication-taking behavior feedback Theory–Based intervention on outcomes in patients with heart failure. J. Card. Fail. 2012;18(1):1–9. doi: 10.1016/j.cardfail.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Canter P.H., Ernst E. The cumulative effects of Transcendental Meditation on cognitive function-a systematic review of randomised controlled trials. Wien. Klin. Wochenschr. 2003;115(21-22):758–766. doi: 10.1007/BF03040500. [DOI] [PubMed] [Google Scholar]
- 110.Newberg A.B., Wintering N., Khalsa D.S., Roggenkamp H., Waldman M.R. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: A preliminary study. J. Alzheimers Dis. 2010;20(2):517–526. doi: 10.3233/JAD-2010-1391. [DOI] [PubMed] [Google Scholar]
- 111.Chiesa A., Calati R., Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin. Psychol. Rev. 2011;31(3):449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Bratzke L.C., Moser D.K., Pelter M.M., et al. Evidence-based heart failure medications and cognition. J. Cardiovasc. Nurs. 2016;31(1):62. doi: 10.1097/JCN.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Juilliere Y., Suty-Selton C., Riant E., et al. Prescription of cardiovascular drugs in the French ODIN cohort of heart failure patients according to age and type of chronic heart failure. Arch. Cardiovasc. Dis. 2014;107:21–32. doi: 10.1016/j.acvd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 114.Scrutinio D., Passantino A., Ricci V.A., Catanzaro R. Association between conformity with performance measures and 1-year postdischarge survival in patients with acute decompensated heart failure. Am. J. Med. Qual. 2013;28(2):160–168. doi: 10.1177/1062860612451049. [DOI] [PubMed] [Google Scholar]
- 115.Krantz M.J., Ambardekar A.V., Kaltenbach L., Hernandez A.F., Heidenreich P.A., Fonarow G.C. Patterns and predictors of evidence-based medication continuation among hospitalized heart failure patients. Am. J. Cardiol. 2011;107(12):1818–1823. doi: 10.1016/j.amjcard.2011.02.322. [DOI] [PubMed] [Google Scholar]
- 116.Kfoury A.G., French T.K., Horne B.D., et al. Incremental survival benefit with adherence to standardized heart failure core measures: A performance evaluation study of 2958 patients. J. Card. Fail. 2008;14(2):95–102. doi: 10.1016/j.cardfail.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 117.Calvin J.E., Shanbhag S., Avery E., Kane J., Richardson D., Powell L. Adherence to evidence-based guidelines for heart failure in physicians and their patients: Lessons from the heart failure adherence retention trial (HART). Congest. Heart Fail. 2012;18:73–78. doi: 10.1111/j.1751-7133.2011.00263.x. [DOI] [PubMed] [Google Scholar]
- 118.Goh K.L., Bhaskaran K., Minassian C., Evans S.J., Smeeth L., Douglas I.J. Angiotensin receptor blockers and risk of dementia: Cohort study in UK Clinical Practice Research Datalink. Br. J. Clin. Pharmacol. 2015;79(2):337–350. doi: 10.1111/bcp.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoth K.F., Poppas A., Moser D.J., Paul R.H., Cohen R.A. Cardiac dysfunction and cognition in older adults with heart failure. Cogn. Behav. Neurol. 2008;21(2):65–72. doi: 10.1097/WNN.0b013e3181799dc8. [DOI] [PubMed] [Google Scholar]
- 120.Sink K.M., Leng X., Williamson J., et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2009;169(13):1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ohrui T., Matsui T., Yamaya M., et al. Angiotensin‐converting enzyme inhibitors and incidence of Alzheimer’s disease in Japan. J. Am. Geriatr. Soc. 2004;52(4):649–650. doi: 10.1111/j.1532-5415.2004.52178_7.x. [DOI] [PubMed] [Google Scholar]
- 122.Savaskan E., Hock C., Olivieri G., et al. Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer’s dementia. Neurobiol. Aging. 2001;22(4):541–546. doi: 10.1016/s0197-4580(00)00259-1. [DOI] [PubMed] [Google Scholar]
- 123.Barnes N.M., Cheng C.H., Costall B., Naylor R.J., Williams T.J., Wischik C.M. Angiotensin converting enzyme density is increased in temporal cortex from patients with Alzheimer’s disease. Eur. J. Pharmacol. 1991;200(2-3):289–292. doi: 10.1016/0014-2999(91)90584-d. [DOI] [PubMed] [Google Scholar]
- 124.Tedesco M.A., Ratti G., Di Salvo G., Natale F. Does the angiotensin II receptor antagonist losartan improve cognitive function? Drugs Aging. 2002;19(10):723–732. doi: 10.2165/00002512-200219100-00001. [DOI] [PubMed] [Google Scholar]
- 125.Zuccalà G., Marzetti E., Cesari M., et al. Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. Am. J. Med. 2005;118(5):496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 126.Cannon J.A., Shen L., Jhund P.S., et al. Dementia‐related adverse events in PARADIGM‐HF and other trials in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2017;19(1):129–137. doi: 10.1002/ejhf.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nalivaeva N.N., Belyaev N.D., Kerridge C., Turner A.J. Amyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer’s disease. Front. Aging Neurosci. 2014;6:235. doi: 10.3389/fnagi.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Langenickel T.H., Tsubouchi C., Ayalasomayajula S., et al. The effect of LCZ696 (sacubitril/valsartan) on amyloid‐β concentrations in cerebrospinal fluid in healthy subjects. Br. J. Clin. Pharmacol. 2016;81(5):878–890. doi: 10.1111/bcp.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zou K., Yamaguchi H., Akatsu H., et al. Angiotensin-converting enzyme converts amyloid β-protein 1-42 (Aβ1-42) to Aβ1-40, and its inhibition enhances brain Aβ deposition. J. Neurosci. 2007;27(32):8628–8635. doi: 10.1523/JNEUROSCI.1549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Vecchis R., Ariano C., Di Biase G., Noutsias M. Cognitive performance of patients with chronic heart failure on sacubitril/valsartan. Herz. 2018 doi: 10.1007/s00059-018-4683-5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 131.Aldrugh S., Sardana M., Henninger N., Saczynski J.S., McManus D.D. Atrial fibrillation, cognition and dementia: A review. J. Cardiovasc. Electrophysiol. 2017;28(8):958–965. doi: 10.1111/jce.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen L.Y., Lopez F.L., Gottesman R.F., et al. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: The atherosclerosis risk in communities study. Stroke. 2014;45:2568–2574. doi: 10.1161/STROKEAHA.114.005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Bruijn R.F., Heeringa J., Wolters F.J., et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72:1288–1294. doi: 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- 134.Thacker E.L., McKnight B., Psaty B.M., et al. Atrial fibrillation and cognitive decline A longitudinal cohort study. Neurology. 2013;81:119–125. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rivard L., Khairy P. Mechanisms, clinical significance and prevention of cognitive impairment in atrial fibrillation. Can. J. Cardiol. 2017;33(12):1556–1564. doi: 10.1016/j.cjca.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 136.Bunch T.J., May H.T., Bair T.L., et al. Atrial fibrillation patients treated with long‐term warfarin anticoagulation have higher rates of all dementia types compared with patients receiving long‐term warfarin for other indications. J. Am. Heart Assoc. 2016;5(7):e003932. doi: 10.1161/JAHA.116.003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jacobs V., Woller S.C., Stevens S., et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm. 2014;11:2206–2213. doi: 10.1016/j.hrthm.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 138.Kwok C.S., Loke Y.K., Hale R., Potter J.F., Myint P.K. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology. 2011;76:914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 139.Pettersen J.A., Sathiyamoorthy G., Gao F.Q., et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch. Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 140.Jacobs V., Woller S.C., Stevens S.M., et al. Percent time with a supratherapeutic INR in atrial fibrillation patients also using an antiplatelet agent is associated with long-term risk of dementia. J. Cardiovasc. Electrophysiol. 2015;26:1180–1186. doi: 10.1111/jce.12776. [DOI] [PubMed] [Google Scholar]
- 141.Jacobs V., May H.T., Bair T.L., et al. Long-term population-based cerebral ischemic event and cognitive outcomes of direct oral anticoagulants compared with warfarin among long-term anticoagulated patients for atrial fibrillation. Am. J. Cardiol. 2016;118:210–214. doi: 10.1016/j.amjcard.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 142.Liao J.N., Chao T.F., Liu C.J., et al. Risk and prediction of dementia in patients with atrial fibrillationea nationwide population-based cohort study. Int. J. Cardiol. 2015;199:25–30. doi: 10.1016/j.ijcard.2015.06.170. [DOI] [PubMed] [Google Scholar]
- 143.Andrade J.G., Macle L., Nattel S., Verma A., Cairns J. Contemporary atrial fibrillation management: A comparison of the current AHA/ACC/HRS, CCS, and ESC guidelines. Can. J. Cardiol. 2017;33:965–976. doi: 10.1016/j.cjca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 144.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 145.Shah S.R., Moosa P.G., Fatima M., et al. Atrial fibrillation and heart failure- results of the CASTLE-AF trial. J. Community Hosp. Intern. Med. Perspect. 2018;8(4):208–210. doi: 10.1080/20009666.2018.1495979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marrouche N.F., Brachmann J., Andresen D., et al. Catheter ablation for atrial fibrillation with heart failure. N. Engl. J. Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 147.Bunch T.J., Crandall B.G., Weiss J.P., et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011;22:839–845. doi: 10.1111/j.1540-8167.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 148.Kirchhof P., Breithardt G., Camm A.J., et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the early treatment of atrial fibrillation for stroke prevention trial. Am. Heart J. 2013;166:442–448. doi: 10.1016/j.ahj.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 149.Haegeli L. Cardiopulse articles. Eur. Heart J. 2015;36(5):255–264. [Google Scholar]
- 150.Cleland J.G., Daubert J.C., Erdmann E., et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 151.Tanner H. Cardiac resynchronisation therapy in Europe: Are Swiss CRT recipients different? Swiss Med. Wkly. 2018;148(3738):w14670. doi: 10.4414/smw.2018.14670. [DOI] [PubMed] [Google Scholar]
- 152.Butrous H., Hummel S.L. Heart failure in older adults. Can. J. Cardiol. 2016;32(9):1140–1147. doi: 10.1016/j.cjca.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Duncker D., Friedel K., König T., et al. Cardiac resynchronization therapy improves psycho-cognitive performance in patients with heart failure. Europace. 2015;17(9):1415–1421. doi: 10.1093/europace/euv005. [DOI] [PubMed] [Google Scholar]
- 154.Bartko P.E., Arfsten H., Heitzinger G., et al. Papillary muscle dyssynchrony-mediated functional mitral regurgitation: mechanistic insights and modulation by cardiac resynchronization. JACC Cardiovasc. Imaging. 2018 doi: 10.1016/j.jcmg.2018.06.013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 155.Sadeghian H., Lotfi-Tokaldany M., Montazeri M., et al. Early improvement in mitral regurgitation after cardiac resynchronization therapy in cardiomyopathy patients. J. Heart Valve Dis. 2017;26(5):557–563. [PubMed] [Google Scholar]
- 156.Hess P.L., Jackson K.P., Hasselblad V., Al-Khatib S.M. Is cardiac resynchronization therapy an antiarrhythmic therapy for atrial fibrillation? A systematic review and meta-analysis. Curr. Cardiol. Rep. 2013;15:330. doi: 10.1007/s11886-012-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Conti J.B., Sears S.F. Cardiac resynchronization therapy: Can we make our heart failure patients smarter? Trans. Am. Clin. Climatol. Assoc. 2007;118:153. [PMC free article] [PubMed] [Google Scholar]
- 158.Matlock D.D., Jones J., Nowels C.T., Jenkins A., Allen L.A., Kutner J.S. Evidence of cognitive bias in decision making around implantable-cardioverter defibrillators: A qualitative framework analysis. J. Card. Fail. 2017;23(11):794–799. doi: 10.1016/j.cardfail.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dunbar S.B., Dougherty C.M., Sears S.F., et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: A scientific statement from the American Heart Association. Circulation. 2012;126(17):2146–2172. doi: 10.1161/CIR.0b013e31825d59fd. [DOI] [PubMed] [Google Scholar]
- 160.Goldstein N.E., Kalman J., Kutner J.S., et al. A study to improve communication between clinicians and patients with advanced heart failure: Methods and challenges behind the working to improve discussions about defibrillator management trial. J. Pain Symptom Manage. 2014;48(6):1236–1246. doi: 10.1016/j.jpainsymman.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Green A.R., Leff B., Wang Y., et al. Geriatric conditions in patients undergoing defibrillator implantation for prevention of sudden cardiac death: prevalence and impact on mortality. Circ. Cardiovasc. Qual. Outcomes. 2016;9(1):23–30. doi: 10.1161/CIRCOUTCOMES.115.002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Setoguchi S., Nohria A., Rassen J.A., Stevenson L.W., Schneeweiss S. Maximum potential benefit of implantable defibrillators in preventing sudden death after hospital admission because of heart failure. CMAJ. 2009;180:611–616. doi: 10.1503/cmaj.080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jama A., Rabinstein A., Hodge D., et al. Cardiac device complications in the cognitively impaired. Pacing Clin. Electrophysiol. 2013;36:1061–1067. doi: 10.1111/pace.12205. [DOI] [PubMed] [Google Scholar]
- 164.Feola M., Vallauri P., Salvatico L., Vado A., Testa M. Neuropsychological impact of implantable cardioverter defibrillator in congestive heart failure patients. Int. J. Cardiol. 2013;166(1):275–276. doi: 10.1016/j.ijcard.2012.09.150. [DOI] [PubMed] [Google Scholar]
- 165.Fosbol E.L., Peterson E.D., Holm E., et al. Comparative cardiovascular safety of dementia medications: A cross‐national study. J. Am. Geriatr. Soc. 2012;60(12):2283–2289. doi: 10.1111/j.1532-5415.2012.04241.x. [DOI] [PubMed] [Google Scholar]
- 166.Raina P., Santaguida P., Ismaila A., et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: Evidence review for a clinical practice guideline. Ann. Intern. Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 167.Handa T., Katare R.G., Kakinuma Y., et al. Anti-Alzheimer’s drug, donepezil, markedly improves long-term survival after chronic heart failure in mice. J. Card. Fail. 2009;15:805–811. doi: 10.1016/j.cardfail.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 168.Sato K., Urbano R., Yu C., et al. The effect of donepezil treatment on cardiovascular mortality. Clin. Pharmacol. Ther. 2010;88:335–338. doi: 10.1038/clpt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Park-Wyllie L.Y., Mamdani M.M., Li P., Gill S.S., Laupacis A., Juurlink D.N. Cholinesterase inhibitors and hospitalization for bradycardia: A population-based study. PLoS Med. 2009;6:e1000157. doi: 10.1371/journal.pmed.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Okazaki Y., Zheng C., Li M., Sugimachi M. Effect of the cholinesterase inhibitor donepezil on cardiac remodeling and autonomic balance in rats with heart failure. J. Physiol. Sci. 2010;60:67–74. doi: 10.1007/s12576-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reale M., Iarlori C., Gambi F., Lucci I., Salvatore M., Gambi D. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp. Gerontol. 2005;40:165–171. doi: 10.1016/j.exger.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 172.Tanaka A., Koga S., Hiramatsu Y. Donepezil-induced adverse side effects of cardiac rhythm: 2 cases report of atrioventricular block and Torsade de Pointes. Intern. Med. 2009;48:1219–1223. doi: 10.2169/internalmedicine.48.2181. [DOI] [PubMed] [Google Scholar]
- 173.Gallini A., Sommet A., Montastruc J.L. Does memantine induce bradycardia? A study in the French PharmacoVigilance Database. Pharmacoepidemiol. Drug Saf. 2008;17:877–881. doi: 10.1002/pds.1620. [DOI] [PubMed] [Google Scholar]