Abstract
Natriuretic peptides, produced by cardiac myocytes, are regulators of the intravascular volume and blood pressure, and also exhibit neuroendocrine, metabolic and growth controlling effects. In heart failure, their synthesis increases exponentially as part of the neuroendocrine activation, but their beneficial effects are diminished. The paper reviews relevant data about their role as diagnosis and prognosis markers in heart failure, the hemodynamic and clinical benefits of their use as therapy in heart failure, together with the main adverse effects. Peptides non-specifically increase in extracardiac pathology and the literature reveals the mechanisms of increase, significance and threshold values to exclude cardiac dysfunction.
Keywords: Natriuretic peptides, heart failure, nesiritide, sacubitril- valsartan, cardiovascular diseases, cardiac dysfunction
1. Introduction
The importance of studying natriuretic peptides (NP) comes from their role in heart failure, a disease with an ever-increasing incidence, which significantly reduces the quality of life and survival. The prevalence of heart failure in Europe is 1-2% and increases by 10% in people over the age of 70 years [1]. NPs are useful for an early diagnosis of heart failure and their use reduces the time interval of diagnosis problems in patients with multiple comorbidities. They are also useful in monitoring the hemodynamic status and in heart failure therapy.
NP family has four compounds: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C type peptide (CNP) and D type peptide (DNP). The first three are present in significant concentrations in the human body and have multiple beneficial effects, while DNP is found in a small amount and limited data is known about its role.
2. History
First information about NP appeared 50 years ago, presented by Kisch who described a highly developed Golgi network inside atrial myocites, indicating a secretory function of the cells and later by Jamielson and Palade, who found spherical electron opaque granules inside the atrial
cells. These granules store NP precursors [2-4]. In 1981, de Bold showed that intravenous administration of atrial myocardium extracts in rats produced natriuresis and diuresis [2, 5]. In 1983, ANP was isolated from atrial myocardium; then, in 1988, Sudoh identified BNP in porcine brain and later in 1991, Mukoyama isolated BNP from human ventricular myocardium [2, 6, 7].
3. Physiology of natriuretic peptides
ANP and BNP are primarily produced by atrial and ventricular myocites and secondarily by cardiac fibroblasts as precursors that undergo proteolytic cleavage (under the effect of some enzymes like corin and furin) to release biologically active compounds [8]. In the normal heart, ANP and BNP are mainly produced in the atria, while the ventricles synthesize them in lower amounts. ANP is preferentially produced in the normal heart. Peptides are stored in granules in the atrial myocites and are released in response to hemodynamic stress, while ventricular myocites release them immediately [8].
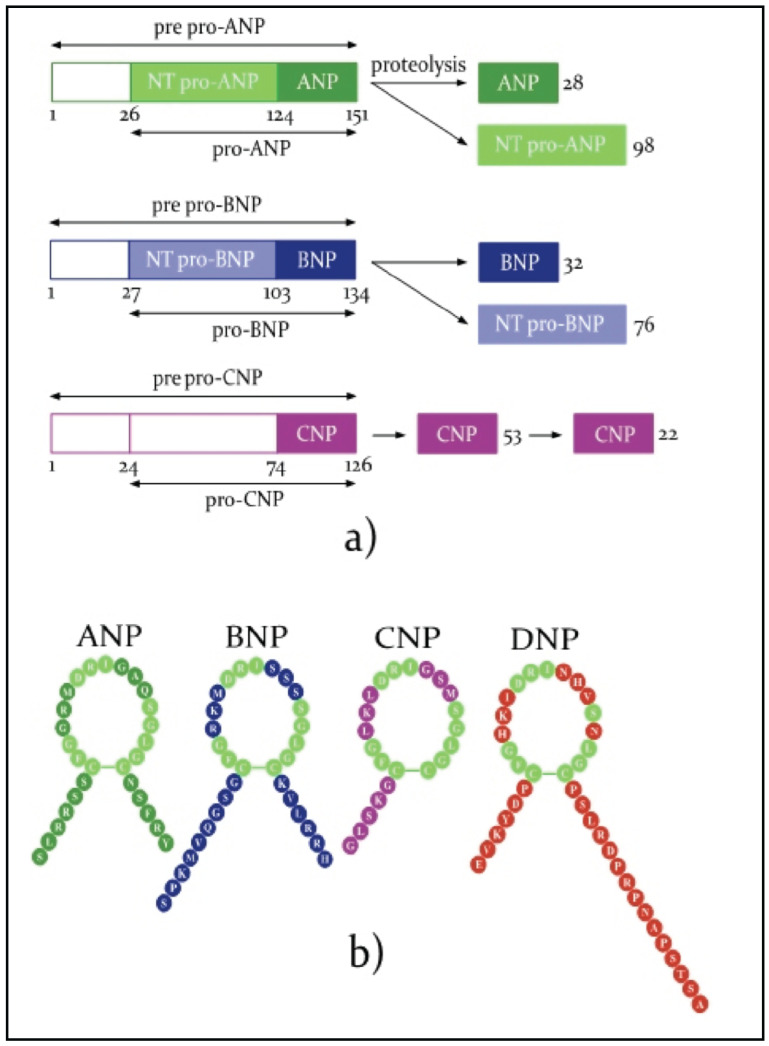
The representation of NP synthesis and a comparison of the chemical structure of active molecules are presented in Fig. (1).
Fig. (1).
Natriuretic peptides (adapted from M. Volpe [10] and L.M.G. Meems [11]).
ANP synthesis starts from preproANP of 151 aminoacids (aa), which is cleaved first in proANP (126 aa), the major form stored in granules and then in ANP(28 aminoacids), the active form and in NT proANP (98 aa), an inactive compound [2]. BNP synthesis starts from preproBNP (134aa) which is cleaved first in proBNP (108 aa) and later in BNP (32 aa), the biologically active molecule, followed by NT proBNP(76aa), an inactive molecule used as a diagnosis marker in heart failure [2]. The main stimuli for ANP and BNP synthesis are an increase in cardiac wall tension, some hormones, cytokines and ischemia. According to Volpe, the mean serum concentrations of NP are 10 fmol/ml (20pg/ml) for ANP and 1fmol/l (3.5 pg/ml) for BNP [9, 10].
CNP is found in the brain and spinal cord, in the digestive tract and the reproductive system, in chondrocytes and endothelial cells. There are two biologically active forms, one of 38 aa, found mainly in tissues, and the other of 22 aa, found in the blood flow [2].
DNP, a 38 aa peptide, was first isolated from the venom of the green mamba snake or dendroaspis augusticeps and was later found in traceable amounts in humans in the atria, vascular walls and plasma [2, 9].
NP act on three types of receptors: type A (NPR-A), which uses GMPc as second messenger and is responsible for ANP and BNP effects; type B (NPR-B), which is activated by CNP; and type C receptor with a clearance role. NP are inactivated by enzymatic cleavage, through internalization by clearance receptors and are excreted by the kidneys [2].
The biological half-life of NP varies from 2 minutes for ANP, 2.6 minutes for CNP, 20 minutes for BNP and 25-70 minutes for NT proBNP [2, 12].
4. NP effects
ANP and BNP cause vasodilation, reduce water and salt reuptake in proximal convoluted tubule and collecting duct thereby increasing diuresis, reduce renin release and central sympathetic drive. They also prevent myocardial hypertrophy and fibrosis [2, 11].
The strongest blood pressure (BP) reducing effect belongs to ANP. Genetically modified mice lacking ANP or NPR-A gene have higher BP with an average value of 20-40 mmHg compared to control mice. Induction of arterial hypertension in NPR-A lacking mice leads to more severe left ventricular hypertrophy than in control rodents even if BP values are similar and hypertrophy persists irrespectively of an anti-hypertensive therapy. Transgenic re-expression of NPR-A decreases the myocite size [2].
In animal models, BNP at usual concentrations is not a blood pressure regulator but if its transcription is intensified 10-100 times, it reduces blood pressure at an average of 20 mmHg. Again in animal models, loss of BNP effect correlates with myocardial fibrosis [2].
CNP acting through NPR-B prevents cardiac hypertrophy and cardiac remodeling after myocardial infarction in animal models [12]. CNP causes vasodilation and inhibits proliferation of the vascular smooth muscle cells [12]. It does not have a direct role in BP regulation. It also stimulates the long bones growth [2]. NP, especially CNP, protect against endothelial dysfunction by reducing shear stress, modulating coagulation and fibrinolytic pathways and inhibiting platelet aggregation. NP have an anti-inflammatory and immune-modulating role. ANP, BNP and CNP are expressed in the central nervous system and act as neurotransmitters or neuromodulators [8].
DNP acts on the same receptors. It activates K channels (Ik) and increases prostaglandin synthesis, causing arterial and venous vasodilation. It is the strongest venous vasodilator, while its effect on the arteries is similar to that of BNP and weaker than ANP or CNP. In animal experiments, it inhibits myocardial L type Ca 2+ channels [9].
NP have metabolic effects: they decrease insulin resistance, activate lipolysis in the fatty tissue, increase lipid oxidation in the skeletal muscles and the liver [12, 13]. In the fatty tissue, NP inhibit the release of inflammatory cytokines and leptin (anorexigenic mediator) and increase the release of adiponectin (mediator that intensifies insulin sensitivity). Also, NP have direct effects on pancreas stimulating the release of insulin and pancreatic beta cell growth [12]. Insulin decreases NP synthesis and increases the proportion of clearance receptor while decreasing NPR-A in fat tissue [12].
5. Physiological variability
NP concentration increases in girls starting from adolescence and women have values twice higher than men [12]. This is because estrogen increases the transcription of NP and NPR-A genes and reduces NPR-C synthesis.
In healthy persons ANP, BNP and NT proBNP increase with age [14, 15]. One study found a mean value of ANP of 3±0.3 pmol/l in young people, while in the elderly, the value increased four times reaching 11.4± 1.1 pmol/l [15]. In another study, the BNP values were 4-73 pg/ml in people aged between 45-54 years and increased to 9-155 pg/ml in the group 75-83 years old [14]. Obese patients have NT proBNP values of 6-20% lower than people with normal weight [12].
BNP values can vary between 30-50% due to discontinuous synthesis and also a short biological half-life. So, only a decrease in BNP higher than 50% or a doubling of its value can be considered clinically relevant [16, 17].
There are ethnical differences of NT proBNP in healthy persons – the highest values are found in the Caucasians, followed by Hispanics, Chinese people and Afro-Americans. The NP value in Afro-Americans is 40% lower than in the Caucasians. The relative deficiency of NT proBNP and BNP in some populations could increase renal salt uptake and the prevalence of hypertension [12].
NP increase after intense physical exercise and correlate with the effort time. The NP increase is due to increased cardiac wall tension and is also part of the neuroendocrine response to stress which includes activation of the hypothalamus- pituitary- adrenal axis and of the sympathetic system [18].
6. NP in heart failure
In heart failure (HF), myocite elongation, increased wall tension and neuroendocrine mediators (angiotensin II, endothelin I, arginin vasopressin) stimulate the transcription of precursor NP genes. The concentration of peptides in heart failure increases up to 10-100 times compared to healthy subjects. The ventricles become the main site of synthesis and BNP is preferentially produced [10]. The molar ratio of BNP/ANP is below 1 in normal persons and increases progressively with heart failure severity reaching the value of 3 in NYHA IV class [8].
The beneficial hemodynamic and neuroendocrine effects of peptides are reduced. Some studies found that the diuretic and natriuretic response to NP is lower in HF than in healthy subjects when NP are administered at the same doses. The vasodilator response measured in forearm arteries and veins is reduced, as well as the GMPc concentration in plasma and urine. NP reduce plasmatic renin activity and aldosterone synthesis in healthy subjects and the response is lower in HF [19].
A reduced effectiveness of NP in HF has several reasons:
BNP concentration in blood is not really as high as indicated by usual immunological tests because they measure various related molecules found in higher amounts like: proBNP 1-108 (6-8 times lower biological activity), NT proBNP (inactive) and peptides derived from BNP1-32 lysis (like BNP 3-32, BNP 5-32, BNP 8-32), with some of them being inactive. Also in HF, the conversion of proBNP to BNP is delayed due to a reduced corin activity [19].
In HF, the natriuretic peptides are degraded faster because of increased activity of NEP and increased percentage of clearance receptors.
The target organs response to NP is diminished due to a decrease in NPR-A, desensitization of receptors and reduced synthesis of intracellular GMPc. Some of these effects are produced by angiotensin II [19].
ProBNP molecules can be glycosylated at seven serine/threonine residues found in the N terminal part. This reaction is a physiological process that increases the molecules’ stability. Recent research found that glycosylation of certain areas makes the molecule resistant to cleavage by corin and furin thus preventing the release of BNP [19]. In HF, up to 70% of proBNP can be glycosylated. The same process can block molecule detection by usual immunological tests.
7. NP use in heart failure
7.1. The Role of Diagnosis in Heart Failure
Besides the hemodynamic role, NP are useful diagnosis markers in heart failure. A high NP value in patients with shortness of breath constitutes an important argument for heart failure and helps physicians in differentiating from other causes of breathlessness- e.g. pulmonary diseases. BNP, NT proBNP and mid-regional proANP (MR proANP) are usually measured in serum/plasma and have a similar diagnosis accuracy. NT proBNP is more stable than BNP in biological samples [16]. ANP dosing is less reproducible and is replaced by proANP, a more stable compound and current immunological tests target the middle part of this molecule [18].
The 2016 ESC guideline for the diagnosis of heart failure indicates threshold values of 100pg/ml for BNP and 300 pg/ml for NT proBNP in patients with acute onset of dyspnea and lower values (35 pg/ml for BNP and 125 pg/ml for NT proBNP) in patients with chronic symptoms, values below which heart failure can be excluded. For MR proANP, the threshold value is 120 pmol/l [1]. E. Roberts’s meta-analysis indicates that the peptides have similar sensitivities for the diagnosis of HF: 0.95 for BNP and 0.99 for NT proBNP without statistically significant difference between them. In absolute values, NT proBNP has higher accuracy. For mid-regional proANP, sensitivity varied between 0.95-0.97. All the three peptides have an excellent ability to to exclude heart failure. Specificity has lower values: 0.63 for BNP, 0.43 for NT proBNP and 0.56-0.6 for MR-proANP, implying that echocardiography is necessary to confirm cardiac dysfunction [20].
7.2. NP Correlate with Functional Parameters
NP values correlate with structural and functional cardiac parameters and contribute to a better stratification of cardiovascular risk in all stages of HF [21-23]. NP increase in both forms of HF (HF with reduced ejection fraction and HF with preserved ejection fraction), with NP values being higher in the first form due to larger ventricular chambers and increased wall tension.
Echocardiographic studies found a correlation between BNP or NT proBNP values and left ventricle (LV) volumes, while the correlation with LV mass gave discordant results [24-27]. NP have a weak inverse correlation with ejection fraction (EF) and correlate strongly with variables of diastolic function like- relaxation parameters (septal e’), compliance parameters (DT) or markers of increased filling pressure (E/e’, E/Vp). The strongest correlation is with diastolic wall tension and LV filling pressure. NP values increase with the severity of diastolic dysfunction and left atrium volume [26, 27]. Peptides also reflect the right ventricle (RV) function and increase with RV pressure overload, dilation and systolic dysfunction and with the severity of tricuspid regurgitation [24, 27].
NP are good predictors of LV and RV moderate and severe dysfunction, but can miss mild dysfunction [27, 28].
Combined evaluation by NP dosing and echo parameters offers a better prediction of adverse events including mortality in HF. The greatest risk of unfavorable evolution is found in patients with BNP value > 250pg/ml and E/e’>15 [24].
7.3. The Prognosis role of NP in Heart Failure
NP reflect the severity of ventricular dysfunction and their concentration increases with NYHA class [20]. Many studies confirmed the prognosis role of BNP, NT proBNP and MR proANP in HF, the three peptides having similar sensitivities [18].
Prognosis data is provided by the baseline NP value, by the amount of change under therapy or by the values at hospital discharge [22].
In patients with HF, an increase with 100 pg/ml of baseline BNP is associated with a 33% increase of the mortality risk [22]. An NT proBNP value higher than 550 pg/ml increases by 4.7 times the relative risk of mortality and hospitalization compared to lower values [22, 23]. After 4 months of therapy, patients with 30% increase of BNP had a relative risk for cardiovascular events and death, being 1.9 times higher than the group in which NP decreased by 45% [22]. Various clinical and biological parameters with a prognosis role (like NYHA class, creatinine, blood pressure and heart rate) lose statistical significance in models that include NP [22].
NP are useful for guiding therapy in HF. Neuroendocrine inhibitors used in HF - angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), aldosterone receptor antagonists- and other diuretic classes reduce NP values due to improved hemodynamic status. Beta blockers, with the exception of compounds with vasodilator effect, do not cause a decrease of NP values [29, 30]. A ‘’physiological” approach to therapy in HF seeks to titrate neuroendocrine inhibitors targeting a greater reduction of NP.
Murdoch and Troughton’s studies were the first to show good results. In the first study, titrating vasodilator therapy in patients with mild- moderate severity HF guided by BNP produced a stronger inhibition of renin-angiotensin- aldosterone axis [29, 31]. In the second study, intensive therapy targeting an NT proBNP value≤1600 pg/ml led after 6 months to significant reductions of death and hospitalizations [29-32]. A recent study on HF which compared intensive therapy (targeting NT proBNP below 1000pg/ml) with a more liberal therapy failed in increasing ACEIs/beta blockers to more than 55% of maximal doses and did not reduce mortality or hospitalizations [33].
7.4. NP as Therapy in Heart Failure
NP have an important therapeutic potential in HF. The first compound used was synthetic ANP (anaritide, carperitide). ANP causes vasodilation and diuresis and is used with good results in HF therapy in Japan [12]. Nesiritide, the recombinant form of BNP, received FDA approval for use in acute HF in 2001 [34]. It reduces pulmonary capillary wedge pressure (PCWP), left ventricle (LV) diastolic pressure, systemic vascular resistance and BP. It significantly improves breathing in acute heart failure (AHF) in the first 6h and 24 h of therapy compared with standard care [35]. It is administered as a bolus of 2 µg/kg followed by continuous infusion at 0.01µg/kg/min for 24h, which can be continued for up to 7 days [35]. Results from early studies were less encouraging. In Sackner Bernstein analysis, nesiritide raised mortality to 30 days and affected kidney function [36]. Other studies focusing on the kidney function suggested a neutral effect. In Chen study which enrolled patients with AHF and renal dysfunction (with GFR between 15-60 ml/min/1.73 m2), BNP and dopamine infusion in small doses did not increase diuresis or changed cystatin C value in the first 72h [37]. Similarly, in Witteles study, BNP was similar to placebo on renal parameters [38]. Gong meta analysis tracked the efficiency and safety of BNP in AHF across 22 studies and 38.064 patients and compared BNP with placebo, nitroglycerin and dobutamine [34]. Hemodynamic effects were confirmed as: BNP reduced systemic vascular resistance by 95-305 dyne/s/cm5, it reduced systolic and diastolic BP by a mean value of 6 mmHg, decreased right atrium pressure by 5.6 mmHg versus placebo and decreased PCWP by 2 mmHg versus nitroglycerin. BNP did not affect the kidney function and did not increase mortality on short, medium and long term [34]. The adverse effects often encountered were a decrease in blood pressure and bradycardia, due to the inhibitory effect on sympathetic tonus.
Inhibition of neutral endopeptidase or neprilysin (NEP) provides another way to increase the NP values. The first NEP inhibitors were candoxatril and sinorphan. They significantly increased the concentration of ANP and of other peptides like bradykinin, ANG II and endothelin I. They increased BP in hyperreninemic patients [39]. Omapatrilat, a common inhibitor of ACE and NEP, has beneficial hemodynamic effects but it significantly increases the risk of angioedema compared with enalapril(0.8% versus 0.5%) due to a stronger inhibition of bradykinin cleavage and it is therefore no longer used [39].
The newest compound used in HF (with reduced EF) therapy is a combination of angiotensin receptor blocker and neprilysin inhibitor (ARNI) known as sacubitril – valsartan or LCZ696. NEP inhibition increases the concentration of NP and other vasodilator peptides. PARADIGM HF study included 8442 pts with HF, with reduced EF (EF mean value 30%) and compared ARNI with enalapril on top of HF therapy. ARNI reduced mortality and hospitalization rate by 20%- 21% compared to enalapril [40]. Hypotension and angioedema were more often encountered in ARNI‘s arm, while kidney dysfunction and hyperkalemia were less common.
One concern about ARNI involves Alzheimer’s disease [39]. NEP is one of the proteases that cleaves amyloid β protein, a precursor of amyloid plaques. In healthy human volunteers, administration of sacubitril/ valsartan did not increase the cerebrospinal fluid concentration of species of amyloid β protein- like Aβ 1-40 and Aβ 1-42(components of amyloid plaque) but increased by 42% the concentration of amyloid β 1-38, found in a familial form of dementia [41-42]. In PARADIGM HF trial, the incidence of dementia/cognitive decline did not differ significantly between ARNI and enalapril (0.29% versus 0.36% after 2.25 years follow up) and the figure is similar to other modern trials in heart failure [42].
ARNI therapy increases BNP value and reduces NT proBNP and the last one is recommended to monitor therapy. ARNI stimulates NT proBNP glycosylation making the molecule less recognizable by current tests [39].
New indications for NP in therapy could be HF with preserved ejection fraction (as ARNI improves myocardial strain in these patients) and diabetes mellitus (to improve insulin sensitivity) [39, 43, 44].
Liraglutide, an agonist of GLP-1R already used in diabetes mellitus, combines metabolic effects with a vasodilator action similar to ANP [12].
7.5. Limitations of NP- its Increase in Various Pathologies
NP, considered specific markers for HF, increase in other cardiac and extra cardiac diseases like- left ventricular hypertrophy, acute myocardial infarction, pulmonary thromboembolism and pulmonary hypertension, atrial fibrillation, valvular heart disease, conditions with increased circulating volume like liver cirrhosis, kidney disease and anemia, sepsis, stroke,and endocrine diseases (hyperthyroidism, Cushing, hyperaldosteronism) [16]. NP increase in extracardiac pathology is the main limitation of their use. Still, they are markers of fluid overload and have a prognosis role.
7.6. NP in Pulmonary Thromboembolism
An increase in troponin and NP values indicates RV dysfunction due to pulmonary hypertension. A BNP value higher than 75-100 pg/ml increases by 7.63 times the in-hospital mortality and similarly, an NT proBNP above 600pg/ml increases the risk 7 times. NP together with clinical and echo parameters are useful for risk stratification and choosing the appropriate therapy [45].
7.7. NP in Acute Myocardial Infarction (AMI)
NP increase is due to ischemia which intensifies BNP transcription and increased filling pressure and neuroendocrine activation, closely related to infarct size. NP reflect diastolic and systolic dysfunction and have a prognosis role. Persistent high values of NT proBNP during admission for AMI and 1 month later correlate with more frequent hospitalizations and increased mortality. In general, these patients have many cardiovascular risk factors, higher Grace score and an EF below 40% [46]. A BNP ≥ 80ng/l or an NT proBNP ≥ 1170 ng/l in men and ≥ 2150 ng/l in women correlate with higher mortality at 1 year [16, 47]. Even in patients treated with PCI, a high baseline NT proBNP value remains a predictor of death and reinfarction in the following 12-14 months [47].
7.8. NP in Atrial Fibrillation
In atrial fibrillation, increased NP synthesis takes place in the atria as a result of abnormal contraction and stretching of groups of atrial myocytes and can be due to increased atrial pressure [48]. NT proBNP can raise to 800-1100 pg/ml in asymptomatic patients [48]. NP have lower accuracy as diagnosis markers in atrial fibrillation (AF) and higher threshold values are necessary to exclude heart failure. In the BACH trial, optimal cutoff values for BNP/NT proBNP were 490 and 3460 pg/ml for patients with AF, while the values were 200 and 1075 pg/ml in the absence of AF. In patients with heart failure, the mean NP values do not differ between the groups with and without AF [49]. NT proBNP value does not correlate with the arrhythmia duration or left atrial volume and decreases after conversion to sinus rhythm. It can predict arrhythmia recurrence independent of other variables [48].
7.9. NP in Chronic Kidney Disease
NP increase in chronic kidney disease (CKD) is due to reduced renal excretion, to an increase in circulating volume and cardiac wall tension and also to subclinical heart dysfunction. NP are considered as mediators of response to functional renal loss. In animal models, after unilateral nephrectomy, an increase in NP value is seen with up-regulation of NPR-A and a decrease in NRP-C. NP have a modest natriuretic effect in CKD and their increase could compensate for the decrease in functional renal mass [50].
In kidney dysfunction, NT proBNP increases exponentially compared to BNP, because of the longer half-life and because its main elimination route is renal. As renal extraction is similar for both peptides in all stages of CKD [51], peptides have a modest inverse correlation with GFR, with the correlation being slightly better for NT proBNP [52].
Both peptides have similar diagnosis sensitivity for cardiac dysfunction in CKD.
De Filippi proposed threshold values of 200ng/l for BNP and 1200 ng/l for NT proBNP below which one can exclude HF in patients with GFR≤ 60ml/min/1.73 m2. In patients with GFR >60ml/min/1.73 m2, cut off values for BNP are 100 ng/l and for NT proBNP are 450 ng/l under the age of 50 years and 900 ng/l over the age of 50 years [53]. NP values correlate with the risk of mortality and hospitalization in CKD [52, 53].
Hemodialysis decreases BNP by 20-40% while peritoneal dialysis does not change its value. NP could be used to monitor circulating volume in patients with hemodialysis [50].
The NP threshold values for diagnosis of heart failure in patients with comorbidities and for prognosis assessment are presented in Table 1.
Table 1.
Proposed threshold values for heart failure diagnosis in various clinical conditions.
| Proposed Threshold Values for Heart Failure Diagnosis in Various Clinical Conditions (with Reference Number) | BNP (pg/ml) | NT proBNP (pg/ml) | Values below threshold exclude heart failure; patients with higher values need echocardiography to confirm the diagnosis. |
|---|---|---|---|
| Acute onset of dyspnea [1] | 100 | 300 | |
| Progressive onset of dyspnea [1] | 35 | 125 | |
| Atrial fibrillation [48] [49] |
- | 1100 | |
| 490 | 3460 | ||
| Chronic kidney disease GFR>60ml/min/1.73 m2 [53] |
100 | 450 for pts below 50 years 900 for pts above 50 years |
|
| GFR ≤60ml/min/1.73 m2 [53] | 200 | 1200 | |
| Septic shock [55] | - | 1200 | |
| COPD exacerbation [57] | - | 1077 | |
| Prognosis threshold values | |||
| Chronic heart failure [22] | Increase with 100 pg/ml | Above 550 | Higher values correlate with increased mortality |
| Pulmonary embolism [45] | 75-100 | 600 | |
| Acute myocardial infarction [47] | 80 | 1170 for men 2150 for women |
7.10. NP in Critically Ill Patients
NP values increase in patients with septic shock. Peptides do not correlate with pulmonary-artery pressure but rather with C reactive protein concentration and white cells number [54]. Bacterial lipopolysaccharides and inflammatory cytokines (IL6) increase BNP transcription but NP value could also reflect the cardiac dysfunction induced by sepsis [55].
According to ESC recommendation, if NT proBNP value is below 1200 pg/ml, one can exclude cardiogenic shock in critically ill patients [55]. If values are higher, echocardiography is necessary to confirm cardiac dysfunction. Patients with septic shock and high NT proBNP value have four times higher risk of death during the following 28 days [55].
Other pathologies According to BNP trial, hemoglobin value inversely correlates with log BNP in men without heart failure and in patients with heart failure with preserved EF, while anemia does not influence NP in patients with reduced EF [56].
Acute exacerbation of chronic obstructive pulmonary disease (COPD) will increase NT proBNP to an average value of 1077 pg/ml in 53-77% of patients. NP correlate with right ventricle pressure estimated non-invasively by echocardiography. A high NT proBNP is found in patients with longer hospital stay and need for intensive care [57].
Conclusion
Natriuretic peptides have important hemodynamic, neuroendocrine and metabolic effects. They are extensively used in heart failure (HF) for early diagnosis, for prognosis prediction and for guiding therapy. Apart from their beneficial actions, it is also important to understand their limitations. They have excellent sensitivity in diagnosing HF but a lower specificity implying that echocardiography is necessary to exclude false positive cases. NP correlate with structural and functional parameters of both ventricles and can identify moderate or severe ventricular dysfunction but still missing a mild dysfunction. Recent trials have confirmed the benefits of two synthetic compounds: nesiritide and the combination sacubitril/valsartan which are used as therapy in acute and chronic HF. They have good hemodynamic effects, with a neutral action on renal function and reduce mortality and hospitalizations (only sacubitril/valsartan). The main limitation of diagnosis is their increase in extracardiac diseases requiring different threshold values. The NP increase in extracardiac pathology is due to hypervolemia, neuroendocrine activation or inflammation which have detrimental effects on cardiac myocytes. This paper reviews important information about NP from the medical literature alongside with analyses of both benefits and limitations of their use.
Mustafa Roxana, Târtea Georgică and Florescu Cristina have equally contributed to this paper.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Ponikowski P., Voors A.A., Anker S.A., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure; The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology(ESC); developed with the special contribution of the Heart Failure Association of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Potter L.R., Yoder A.R., Dickey D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisch B. Electron microscopy of the atrium of the heart in guineo pig. Exp. Med. Surg. 1856;14:99–112. [PubMed] [Google Scholar]
- 4.Jamielson J.D., Palade G.E. Specific granules in atrial muscle cells. J. Cell Biol. 1964;23:151–172. doi: 10.1083/jcb.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bold A.J., Borenstein H.B., Veress A.T., Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 6.Sudoh T., Kangawa K., Minamino N., Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 7.Mukuyoma M., Nakao K., Hosada K., et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerico A., Recchia F.A., Passino C., Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: Physiological and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H17–H29. doi: 10.1152/ajpheart.00684.2005. [DOI] [PubMed] [Google Scholar]
- 9.Best P.J.M., Burnett J.C., Jr, Wilson S.H., et al. Dendroaspis natriuretic peptide relaxes isolated human arteries and veins. Cardiovasc. Res. 2002;55(2):375–384. doi: 10.1016/s0008-6363(02)00402-9. [DOI] [PubMed] [Google Scholar]
- 10.Volpe M., Carnovali M., Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: From molecular basis to treatment. Clin. Sci. (Lond.) 2017;130(2):57–77. doi: 10.1042/CS20150469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meems L.M.G., Burnett J.C., Jr Innovative therapeutics designer natriuretic peptides. J. Am. Coll. Cardiol. 2016;1(7):557–567. doi: 10.1016/j.jacbts.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlueter N., de Sterke A., Willmes D.M., et al. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol. Ther. 2014;144(1):12–27. doi: 10.1016/j.pharmthera.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Sengenes C., Berlan M., De Glisezinski I., Lafontan M., Galitzky J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB J. 2000;14:40409–40415. [PubMed] [Google Scholar]
- 14.Redfiield M.M., Rodeheffer R.J., Jacobsen S.J., et al. Plasma brain natriuretic peptide concentration: Impact of age and gender. J. Am. Coll. Cardiol. 2002;40(5):976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 15.Davis K.M., Fish L.C., Minaker K.L., Elahi D. Atrial natriuretic peptide levels in the elderly: Differentiating normal aging changes from disease. J. Gerontol. A Biol. Sci. Med. Sci. 1996;51(3):M95–M101. doi: 10.1093/gerona/51a.3.m95. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K., Mair J., Mueller C., Huber K., Weber M. ESC Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the study group on biomarkers in cardiology of the ESC working group on acute cardiac care. EHJ. 2012;33(16):2001–2006. doi: 10.1093/eurheartj/ehq509. [DOI] [PubMed] [Google Scholar]
- 17.Clerico A., Zucchelli G.C., Pilo A., Emdin M. Clinical relevance of biological variation of B type natriuretice peptide. Clin. Chem. 2005;51(5):925–926. doi: 10.1373/clinchem.2004.046615. [DOI] [PubMed] [Google Scholar]
- 18.Hamasaki H. The effects of exercise on natriuretic peptides in individuals without heart failure. Sports (Basel) 2016;4(2):32. doi: 10.3390/sports4020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.1 Diez J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur. J. Heart Fail. 2017;19(2):167–176. doi: 10.1002/ejhf.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts E., Ludman A.J., Worzynski K., et al. The diagnostic accuracy of the natriuretic peptides in heart failure: Systematic review and diagnostic meta analysis in the acute setting. BMJ. 2015;350:h910. doi: 10.1136/bmj.h910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.C., Stevens T.L., Sandberg S.M., et al. The potential of brain natriuretic peptide as a biomarker for New York Heart Association class during the outpatient treatment of heart failure. J. Card. Fail. 2002;8(3):149–154. doi: 10.1054/jcaf.2002.125368. [DOI] [PubMed] [Google Scholar]
- 22.Doust J.A., Pietrzak E., Dobson A., Glaszion P. How well does B type natriuretic peptide predict death and cardiac events in patients with heart failure: Systematic review. BMJ. 2005;330(74921):625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards A.M., Doughty R., Nicholls M.G., et al. Plasma N terminal proBNP and adrenomedullin: Prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction, Australia- New Zealand Heart Failure Group. J. Am. Coll. Cardiol. 2001;37:1781–1787. doi: 10.1016/s0735-1097(01)01269-4. [DOI] [PubMed] [Google Scholar]
- 24.Troughton R.W., Richards M. B type natriuretic peptides and echocardiographic measures of cardiac structure and function. JACC Cardiovasc. Imaging. 2009;2(2):216–225. doi: 10.1016/j.jcmg.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Y. Iwanaga I B type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: Comparison between systolic and diastolic heart failure. J. Am. Coll. Cardiol. 2006;47(4):742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Troughton R.W., Prior D.L., Pereira J.J., et al. Plasma B type natriuretic peptide levels in systolic heart failure. J. Am. Coll. Cardiol. 2004;43(3):416–422. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 27.Lubien E., de Maria A., Krishnaswamy P., et al. Utility of B natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation. 2002;105:595–601. doi: 10.1161/hc0502.103010. [DOI] [PubMed] [Google Scholar]
- 28.Abhayaratna W.P., Marwick T.H., Becker N.G., et al. Population based detection of systolic and diastolic dysfunction with aminoterminal proB type natriuretic peptide. Am. Heart J. 2006;152(5):941–948. doi: 10.1016/j.ahj.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Krupicka J., Janota T., Kasalova Z., Hradec J. Natriuretic peptides- physiology, pathophysiology and clinical use in heart failure. Physiol. Res. 2009;58:171–177. doi: 10.33549/physiolres.931461. [DOI] [PubMed] [Google Scholar]
- 30.Krupicka J., Janota T., Hradec J. Natriuretic peptides in heart failure. Cor Vasa. 2013;75(4):e370–e396. [Google Scholar]
- 31.Murdoch D.R., McDonagh T.A., Byrne J., et al. Titration of vasodilator therapy in chronic heart failure according to plasma brain natriuretic peptide concentration – randomized comparison of the hemodynamic and neuroendocrine effects of tailored versus empirical therapy. Am. Heart J. 1999;138(6Pt1):1126–1132. doi: 10.1016/s0002-8703(99)70079-7. [DOI] [PubMed] [Google Scholar]
- 32.Troughton R.W., Frampton C.M., Yandle T.G., et al. Treatment of heart failure guided by plasma aminoterminal BNP (N-BNP) concentrations. Lancet. 2000;355:1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 33.Felker G.M., Anstrom K.J., Adams K.F., et al. Effect of natriuretic peptide guided therapy on hospitalization or cardiovascular mortality in high risk patients with heart failure and reduced ejection fraction: A randomized clinical trial. JAMA. 2017;318(8):713–720. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong B., Wu Z., Li Z. Efficacy and safety of nesiritide in patients with decompensated heart failure: a meta-analysis of randomized trials. BMJ Open. 2016;6(1):e008545. doi: 10.1136/bmjopen-2015-008545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’ Connor C.M., Starling R.C., Hernandez A.F., et al. ASCEND trial -Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 36.Sackner Bernstein J.D., Skopicki H.A., Aaronson K.D. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111(12):1487. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 37.Chen H.H., Anstrom K.J., Givertz M.M., et al. Low dose dopamine or low dose nesiritide in acute heart failure with renal dysfunction, the ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witteles R.M., Kao D., Christopherson D., et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and preexisting renal dysfunction a randomized double blind placebo controlled clinical trial. J. Am. Coll. Cardiol. 2007;50:1835. doi: 10.1016/j.jacc.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 39.Lee N.S., Daniels L.B. Current understanding of the compensatory actions of cardiac natriuretic peptides in cardiac failure a clinical perspective. Card. Fail. Rev. 2016;2(1):14–19. doi: 10.15420/cfr.2016:4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. www.acc.org/latest -in cardiology/clinical-trials
- 41.Langenickel T.H., Tsubouchi C., Ayalasomayajula S., et al. The effect of LCZ 696 (sacubitril/valsartan) on amyloid beta concentrations in cerebrospinal fluid of healthy subjects. Br. J. Clin. Pharmacol. 2016;81:878–890. doi: 10.1111/bcp.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannon J.A., Shen L., Jhund P.S., et al. On behalf of the PARADIGM- HF Investigators and Committees. Dementia related adverse events in PARADIGM- HF and other trials in heart failure with reduced ejection frcation. Eur. J. Heart Fail. 2017;19(1):129–137. doi: 10.1002/ejhf.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biering-Sorensen T., Shah A., Claggett B., et al. The angiotensin receptor blocker- neprilysin inhibitor sacubitril/valsartan improves left ventricle myocardial deformation in heart failure with preserved ejection fraction (PARAMOUNT trial). J. Am. Coll. Cardiol. 2018;71(11):A2665. [Google Scholar]
- 44.Solomon S.D., Rizkala A.R., Gong J., et al. Angiotensin receptor- neplilysin inhibition in heart failure with preserved ejection fraction: Rationale and design of the PARAGON –HF Trial. JACC Heart Fail. 2017;5(7):471–482. doi: 10.1016/j.jchf.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Cavallazzi R., Nair A., Vasu T., Marik P.E. Natriuretic peptides in acute pulmonary embolism: A systematic review. Intensive Care Med. 2008;34:2147–2156. doi: 10.1007/s00134-008-1214-5. [DOI] [PubMed] [Google Scholar]
- 46.Kontos M.C., Lanfear D.E., Spertus J.A., et al. Prognostic value of serial N terminal pro brain natriuretic peptide testing in patients with acute myocardial infarction. Am. J. Cardiol. 2017;120(2):181–185. doi: 10.1016/j.amjcard.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D. Farmakis G Natriuretic peptides in acute coronary syndromes prognostic value and clinical implications. Congest. Heart Fail. 2008;14(4) Suppl. 1:25–29. doi: 10.1111/j.1751-7133.2008.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 48.Marsiliani D., Buccelletti F., Carroccia A., et al. Natriuretic peptides and atrial fibrillation. Eur. Rev. Med. Pharmacol. Sci. 2010;14:855–860. [PubMed] [Google Scholar]
- 49.Richards M., Di Somma S., Mueller C., et al. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients. Results from the BACH Study (Biomarkers in Acute Heart Failure). J. Am. Coll. Cardiol. 2013;1:192–199. doi: 10.1016/j.jchf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Goetze J.P., Jensen G., Mollar S., Bendtsen F., Rehfeld J.F., Henriksen J.M. BNP and N terminal pro BNP are both extracted in the normal kidney. Eur. J. Clin. Invest. 2006;36:8–15. doi: 10.1111/j.1365-2362.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- 51.Austin WJ, Bhalla V. Correlation of prognostic utility of B type natriuretic peptide and its amino terminal fragment in patients with chronic kidney disease. Am. J. Clin. Pathol. 2006;126:506–512. doi: 10.1309/M7AAXA0J1THMNCDF. [DOI] [PubMed] [Google Scholar]
- 52.Santos Araujo C., Leite Moreira A., Pestana M. Clinical value of natriuretic peptides in chronic kidney disease. Nefrologia. 2015;35(3):227–233. doi: 10.1016/j.nefro.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 53.De Filippi C.R., Seliger S.L., Maynard S., Christenson R.H. Impact of renal disease on natriuretic peptide testing for diagnosing decompensated heart failure and predicting mortality. Clin. Chem. 2007;53:1511–1519. doi: 10.1373/clinchem.2006.084533. [DOI] [PubMed] [Google Scholar]
- 54.Rudiger A., Fischler M., Harpes P., et al. In critically ill patients, BNP and amino terminal – pro BNP correlate with C reactive protein values and leukocyte counts. Int. J. Cardiol. 2008;126(1):28–31. doi: 10.1016/j.ijcard.2007.03.108. [DOI] [PubMed] [Google Scholar]
- 55.Brueckmann M., Borggrefe M. Natriuretic peptide testing for risk stratification of critically ill patients. E J Cardiol Pract. 2006;5(14):3. [Google Scholar]
- 56.Wu A.H., Omland T., Wold-Knudsen C., et al. BNP Multinational Study Investigators Relationship of B type Natriuretic peptide and anemia in patients with and without heart failure: A substudy from Breathing Not Properly (BNP) multinational study. Am. J. Hematol. 2005;80(3):174–180. doi: 10.1002/ajh.20456. [DOI] [PubMed] [Google Scholar]
- 57.Adrish M., Nannaka V.B., Cano E.J., Bajantri B., Diaz-Fuentes G. Significance of NT proBNP in acute exacerbation of COPD patients without underlying left ventricular dysfunction. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:1183–1189. doi: 10.2147/COPD.S134953. [DOI] [PMC free article] [PubMed] [Google Scholar]